Title: The Avifauna of Micronesia, Its Origin, Evolution, and Distribution
Author: Rollin H. Baker
Release date: April 14, 2013 [eBook #42537]
Most recently updated: October 23, 2024
Language: English
Credits: Produced by Chris Curnow, Matthias Grammel, Joseph Cooper,
The Internet Archive for some images and the Online
Distributed Proofreading Team at http://www.pgdp.net
MUSEUM OF NATURAL HISTORY
VOLUME 3 · 1951
EDITORS
E. Raymond Hall, Chairman
A. Byron Leonard
Edward H. Taylor
Robert W. Wilson
Museum of Natural History
UNIVERSITY OF KANSAS
LAWRENCE 1951
Museum of Natural History
UNIVERSITY OF KANSAS
LAWRENCE
PRINTED BY
FERD VOILAND, JR., STATE PRINTER
TOPEKA, KANSAS
1951
24-1811
| 1. | The Avifauna of Micronesia, Its Origin, Evolution, and Distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June 12, 1951 |
| 2. | A Quantitative Study of the Nocturnal Migration of Birds. By George H. Lowery, Jr. Pp. 361-472, 47 figures in text. June 29, 1951 |
| 3. | Phylogeny of the Waxwings and Allied Birds. By M. Dale Arvey. Pp. 473-530, 49 figures in text, 13 tables. October 10, 1951 |
| 4. | Birds from the State of Veracruz, Mexico. By George H. Lowery, Jr. and Walter W. Dalquest. Pp. 531-649, 7 figures in text, 2 tables. October 10, 1951 |
| Index, Pp. 651-681. | |
The Avifauna of Micronesia,
Its Origin, Evolution, and Distribution
BY
ROLLIN H. BAKER
University of Kansas Publications
Museum of Natural History
Volume 3, No. 1, pp. 1-359, 16 figures in text
June 12, 1951
University of Kansas
LAWRENCE
1951
The Avifauna of Micronesia,
Its Origin, Evolution, and Distribution
BY
ROLLIN H. BAKER
University of Kansas Publications
Museum of Natural History
Volume 3, No. 1, pp. 1-359, 16 figures in text
June 12, 1951
University of Kansas
LAWRENCE
1951
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, A. Byron Leonard,
Edward H. Taylor, Robert W. Wilson
Volume 3, No. 1, pp. 1-359, 16 figures in text
Published June 12, 1951
University of Kansas
Lawrence, Kansas
PRINTED BY
FERD VOILAND, JR., STATE PRINTER
TOPEKA, KANSAS
1951
24-1811
The Avifauna of Micronesia,
Its Origin, Evolution, and Distribution
By
ROLLIN H. BAKER
| PAGE | |||
| Introduction | 5 | ||
| Description of Micronesia | 5 | ||
| Climate | 8 | ||
| Soils | 9 | ||
| Surface water | 9 | ||
| Vegetation | 10 | ||
| Gazetteer of the Islands of Micronesia | 11 | ||
| Mariana Islands | 11 | ||
| Palau Islands | 13 | ||
| Caroline Islands | 14 | ||
| Marshall Islands | 15 | ||
| Ornithological Exploration in Micronesia | 16 | ||
| Check-list of the Birds of Micronesia | 21 | ||
| Discussion of the Avifauna | 28 | ||
| Oceanic Birds | 28 | ||
| Inshore Oceanic Birds | 29 | ||
| Offshore and Pelagic Oceanic Birds | 30 | ||
| Faunal Components | 30 | ||
| Migratory Shore Birds | 32 | ||
| Original Homes of the Shore Birds that Visit Micronesia | 32 | ||
| Routes of Migration | 34 | ||
| Populations of Shore Birds in Micronesia | 37 | ||
| Land and Fresh-Water Birds | 42 | ||
| Polynesian Component | 44 | ||
| Melanesian Component | 44 | ||
| Moluccan and Celebesian Components | 45 | ||
| Philippine Component | 45 | ||
| Palearctic Component | 46 | ||
| Speciation | 48 | ||
| Time of Colonization | 50 | ||
| [Pg 4] | Factors Causing Dispersal | 52 | |
| Analysis of Speciation | 55 | ||
| Conservation of the Avifauna of Micronesia | 58 | ||
| The Future of Ornithological Research in Micronesia | 60 | ||
| Methods and Acknowledgments | 60 | ||
| Accounts of the Kinds of Birds in Micronesia | 63 | ||
| Summary and Conclusions | 340 | ||
| Bibliography | 343 | ||
| Figure | Page | |
| 1. | The islands of Micronesia. | 6 |
| 2. | The Mariana Islands. | 12 |
| 3. | The Palau Islands. | 13 |
| 4. | The Caroline Islands. | 15 |
| 5. | The Marshall Islands. | 16 |
| 6. | Routes of migration used by shore birds in the Pacific. | 34 |
| 7. | Avifaunal regions of the central Pacific. | 43 |
| 8. | Faunal areas from which Micronesian birds have been derived. | 46 |
| 9. | Routes of dispersal of rails in the Pacific area. | 120 |
| 10. | Variation in length of culmen of Sterna s. sumatrana. | 159 |
| 11. | Geographic distribution of Thalasseus bergii. | 164 |
| 12. | Geographic distribution of Gygis alba in the Pacific. | 177 |
| 13. | Distribution and dispersal of Ptilinopus porphyraceus. | 190 |
| 14. | Distribution and dispersal of Gallicolumba in the Pacific. | 204 |
| 15. | Distribution and dispersal of Acrocephalus in the Pacific. | 260 |
| 16. | Distribution and dispersal of Myzomela in the Pacific. | 316 |
Birds in Micronesia comprise the most outstanding animal life of the islands, as far as vertebrates are concerned. No fewer than 206 kinds, belonging to 37 families and 91 genera have been found there. Although this number upon first consideration may seem large, actually any large land mass in the same latitude has many more kinds of birds than does Micronesia. In this connection it is pertinent to recall that the islands of Micronesia are oceanic and have apparently been formed independently of any continental land mass. Thus, animal life found on these islands has reached them by overseas migration, either by some passive means or by individual effort. Zoogeographers have had some difficulty in explaining the presence of snails and other nonflying animals on isolated oceanic islands. Crampton, in his studies of the land snails of the genus Partula at Guam and Saipan (1925:10), writes, "Despite the geological difficulties, the biological findings strongly support the view that the dominant process in this part of Oceania has been one of subsidence and of insular dissection." Although there exists today some question as to how certain forms of life have reached these remote dots of land, the ornithologist has not been much in doubt as to the actual means of arrival of birds. With the exception of six kinds of birds which are definitely known to have been introduced by man, the birds have apparently reached these islands by flying there from somewhere else. The ornithologist is, therefore, concerned with learning from where, by what route, when, and why the various species of birds came and how they have become established on these islands of Micronesia. These birds exist in small populations; often less than 100 individuals of one kind may be found on a small island. How have such small numbers had the ability to survive and what environmental adaptations have occurred, are two additional questions which confront the student of Micronesian birds.
The vast expanse of the Pacific Ocean is dotted with numerous islands, most of which are concentrated in the central and western part and are known collectively as Oceania. Within Oceania three divisions are popularly recognized: Melanesia, Polynesia, and Micronesia. According to Krieger (1943:6), the Micronesia islands include the Mariana, Palau, Caroline, Marshall, and Gilbert islands; they may take in also the Volcano, Bonin, and Ellice islands (from [Pg 6] the standpoint of anthropology). Zoogeographically, according to Wallace (1876), Micronesia is to be included in the Polynesian Subregion of the Australian Region. Mayr (1941a:193), on the basis of the distribution of birds, ranks Micronesia as one of the four subdivisions of the Polynesian Subregion, and includes within Micronesia the Palau, Caroline, Mariana, Marshall, and Gilbert islands. Except in the discussion of distribution, this report does not treat of the avifauna of the Gilbert Islands, which straddle the equator south of the Marshall Islands. This report is concerned only with the birds in the Mariana, Palau, Caroline, and Marshall islands formerly mandated to Japan, and with the birds of the island of Guam, which is a possession of the United States.
The word Micronesia is, of course, derived from the Greek words mikros meaning small and nesos meaning island, and, as shown in figure 1 , this term is appropriate, for the islands of this area are small. For the most part they are too small even for inclusion on standard-sized maps of the world. There are thousands of these islands in an area some 2,400 miles long from east to west and some 1,200 miles broad from north to south. All of the islands of Micronesia are oceanic islands; that is to say, they have never been connected to the Asiatic continent or to other land masses by means of land bridges.
Geologists and oceanographers have shown (see descriptions by Hobbs, 1945), that islands of Micronesia are of two general types: arcuate and strewn. The Pacific Ocean is surrounded by rising mountain ranges which are arranged in elongated, near-circular arcs, [Pg 7] which form an extended series of scallops. In the western Pacific these sweeping arcs extend into the ocean, where the mountain ranges project upward from the bottom of the sea with only the crests showing above the waves to point out, in dotted outline, the position of the mountains. The easternmost of these arcs is marked by the islands of the Aleutians, Kuriles, Japan, Izo, Bonins, Volcanoes, Marianas, Yap, Palaus, and others continuing southward into Melanesia. These are characterized by igneous rocks of andesitic nature.
To the eastward of the arcuate islands in Micronesia, are numerous and irregularly distributed islands, making up all of the central and eastern Carolines and the Marshalls, which are known as strewn islands. Strewn islands mark the places of former volcanoes or volcanic peaks. If these volcanic peaks have been completely drowned and are now marked by a series of low islands edged by a protecting reef formed by coral growth enclosing a lagoon and with all exposures consisting of coral rock, the island is known as a coral atoll (example, Ulithi Atoll). Some of the coral exposures lack lagoons; they are known merely as coral islands (example, East Fayu). Some atolls become elevated by geologic activity and the lagoons may dry out or drain. The accumulation of guano of oceanic birds and the residue of fish and other organisms in the area of the lagoon remains as a rich phosphate deposit; these raised atolls have been called phosphate islands (example, Fais). Other strewn islands consist of igneous rocks which are exposed above the surface of the ocean. These are known as "high" or volcanic islands and may occur as a single mountain rising out of the ocean (example, Kusaie), or be partly drowned and surrounded by a coral reef (example, Truk). The igneous rocks found on these strewn islands are basaltic in nature.
The Mariana Islands consist of a chain of volcanic islands approximately 450 miles long. As shown in figure 2, there are 14 single islands and one group of three islands (Maug), from Uracas in the north to Guam in the south. The Palau Islands which are situated in the easternmost part of Micronesia have often been considered from a political standpoint as part of the Caroline Islands. As shown in figure 3, the Palau Islands are a chain of islands approximately 120 miles long from north to south. Sonsorol, Tobi, Merir, Pulo Anna, and Helen Island occur to the southward of the Palaus and may be considered as part of the Carolines or as part of the Palaus. The Palaus together with the Carolines, to the eastward, extend in an east-west direction for approximately 1,700 [Pg 8] miles. The Palaus and Carolines include (as shown in figures 3 and 4) 37 atolls, 34 banks, 11 coral islands without lagoons, 2 uplifted phosphate islands, 4 volcanic islands, and the Palau chain. The Marshall Islands to the extreme eastward extend approximately 700 miles from north to south and, as shown in figure 5, contain 29 atolls and five coral islands without lagoons. No volcanic exposures occur in the Marshall Islands.
There is a total land surface of approximately 846 square miles in the islands of Micronesia. The Palaus and Carolines have 525 square miles, the Marianas 247 square miles, and the Marshalls 74 square miles of land surface. Guam has the largest land surface of any of the islands of Micronesia with 225 square miles, Ponapé has 145 square miles, and Babelthuap has 143 square miles. Asuncion, in the northern Marianas, has the highest elevation, rising as an almost perfect cone to a height of 2,923 feet; Ponapé reaches a height of 2,579 feet above the sea level. The volcanic islands are known as "high" islands, and the coral atolls are known as "low" islands. The coral islands usually rise but a few feet above sea level.
In Micronesia there are two seasons: a wet summer and a dryer winter. Temperatures rarely go above 90° F. and rarely below 70° F. Rainfall in the Marianas averages approximately 85 inches per year, in the Palaus approximately 150 inches, in the Carolines it ranges from 129 to 185 inches, and in the Marshalls it goes up to 160 inches. The humidity is excessive, the average annual mean of relative humidity for selected islands in Micronesia being between 82 and 86 percent. The relative humidity is lower in the western Carolines and the Palaus, than in other parts of Micronesia.
The Mariana Islands lie between the area of the Asiatic monsoon and the belt of the northeast trade winds. At Saipan from November until March or April, winds usually are easterly or northeasterly and are strong and steady since the northeast trades and the winter monsoon reinforce each other. In April and May the directions of the winds shift toward the southeast, and they become weaker and more variable. In this period there may be some easterly winds in addition to the predominating southeasterly winds. Detailed information is not available on the winds which occur in the Marianas north of Saipan, but at Pagan easterly winds probably prevail from May to July and westerly winds prevail in the remainder of the year. The Carolines lie in the belt of alternating northeast trade winds and southwest monsoons. The northeast trades begin in October [Pg 9] and prevail until May or June. The southwest monsoon occurs from May to October and may be felt as far east as Truk. To the eastward, the winds of the summer are usually light and variable. In the Marshall Islands, the northeast trade winds predominate from about December to April, especially in the northern part of the Marshalls. In summer, winds are variable and weak; periods of calm may occur. Typhoons and squalls occur most frequently in the spring and summer in Micronesia. Some of the severe typhoons are known to engulf entire islands, as did the one at Woleai in 1907.
The soils of the islands of Micronesia have been derived from volcanic materials or from depositions of coralline limestone. Volcanic soils occur on the "high" islands of Micronesia. In many places, especially on the islands of the northern Marianas there is little soil; there are large areas of bare igneous rock, because the islands are geologically of relatively recent origin and little erosion has occurred. On islands where volcanic rocks have decomposed, the resulting soil may have a top layer of humus. The richest soils of the islands are along drainage areas and in alluvial deposits.
Coralline soils result from the decomposition of limestone, coral fragments, shells, and sand, and are overlain by some humus. Where the layer of humus is deep, the fertility is greatest. Coralline-volcanic soils occur on some "high" islands where coral rock and volcanic rock have become mixed in the decomposition process which forms soil. In parts of the Marianas and elsewhere, unwise practices of burning and overgrazing have allowed extensive erosion to occur, resulting in reduced fertility of the soil. On the island of Yap certain sedimentary rocks are exposed which are thought to have been elevated from the ocean bottom. Soils at Yap which have developed from this rock are considered more fertile than soils of coralline origin, although the fertility there also is dependent on the depth of the layer of humus.
There is little fresh water on the coral atolls, but brackish marshes are present on some islands, and many of these marshes are used for the cultivation of taro by the natives. Some volcanic islands, on the other hand, possess small streams and fresh water lakes, producing suitable habitat for certain rails, gallinules and ducks. On the "low" islands in the Marshalls, natural surface pools are rare.
The "high" islands of Micronesia support a heavy cover of vegetation. Typically the lowlands and stream courses are covered with dense jungle vegetation, and the slopes and higher hills are covered with grasses and brush. The vegetation of the "low" coral atolls and islands is, by comparison, much less dense. Many shorelines are covered with scant grasses and shrubs and the interior in many places is dominated by coconut, betel palms, breadfruit, papaya, and pandanus. References to papers dealing with plants in the islands of the Pacific may be obtained in Merrill (1945), who (1945:207) writes, "Botanically, the low islands are very uninteresting and monotonous. The flora of one is usually quite the same as that of another, although these islands and islets may be separated by many hundred and in some cases several thousand miles. The native vegetation may be scanty or reasonably well developed, depending on the size of the island, the quality of its soil, and whether or not it is permanently inhabitated." Of the vegetation on the "high" islands of the Pacific area, Merrill (1945:209) comments that the vegetation "is well developed, particularly within the forested areas, but for these high islands within the Pacific basin as a whole, the number of endemic genera is relatively small and most of them have definite relationships with those of Malaysia." Concerning the "high" islands of Micronesia, Merrill (1945:210) remarks that these islands are smaller and more isolated than some of the others in Oceania and have fewer individual species "as compared with what one finds on islands of a similar size located within limits of the Malay Archipelago. Thus with all of the islands under Japanese mandate, and including a number of high, but at the same time relatively small islands, less than 1,300 different species are known, of which 230 manifestly represent purposely or accidentally introduced ones. This relatively small flora includes representatives of approximately 620 genera in 192 families.... Specific endemism is relatively high, for approximately 460 species are confined to the islands within the area under consideration. The generic endemism is very low; about seven endemic genera only are involved for the whole group." The figures for endemism of plants are comparable to those for birds. Of endemic birds there are 5 genera, 35 species, and 73 subspecies. The total number of species of birds known from Micronesia is only 206 as compared with 1,300 plants. Yamada (1926:966) writes that the number of species of plants that Micronesia has in common with Japan may be due to the influence of the "Japan Stream."
Many land birds in Micronesia depend directly on the plant life for food. Possibly the soil (including its mineral content), upon which the plants themselves depend for development of fruits and other edible parts, may offer a limiting factor to the distribution of birds in Micronesia. Possibly the fruits and other edible parts of plants do not provide the necessary amounts of proteins, carbohydrates, minerals, vitamins, and other essential food items for species of plant-eating birds, which have not become established in Micronesia. Possibly some species of plant-eating birds have reached Micronesia but have failed to establish themselves because of some dietary deficiency caused by poverty of the soils on which the plants grow. If a comparison were made of soils and of the food values of fruits of plants in both the islands of Micronesia and similarly sized islands in the Malay region, a difference might be revealed which would partly explain why some plant-eating birds have not become established in Micronesia.
In the following list the name in current usage for each island or island group in Micronesia is followed by other names which have been used. There is no attempt made to list the names of the small islands of each atoll or those of the myriads of small islets that lie offshore from the larger volcanic islands. Collections have not been made on most of the smaller islands. For the few on which a species has been collected, the islet is adequately described in the account of the particular species concerned. The reader may refer to Brigham (1900) for a listing of the islands of the Pacific Ocean. Most of the islands included in the following list may be located on the map of Micronesia as shown in figures 2, 3, 4, and 5. These listings follow in order of arrangement those in the Civil Affairs Handbooks, published by the United States Navy Department (1943, 1944a, 1944b, and 1944c).
The Mariana Islands (also called Ladrone, Marianne, Marian) consist of 14 single islands and one group of three islands. The Marianas are all "high" or volcanic islands. The islands, shown in figure 2, are listed as follows:
Agrihan (also called Agrigan, Arijan, Francisco Xavier, Granger, Gregus, Grigan, San Francisco Javier).
Agiguan (also called Agaigan, Agiigan, Agiguan, Agigwan, Aguigan, Aguijan, Aguyan, Guigan, Saint Ange, Santa Angel).
Alamagan (also called Almagan, Aramagan, Concepcion).
Anatahan (also called Anatagen, Anatajen, Anataxan, San Joaquin).
Asuncion (also called Asonson, Assongsong, Assumption).
Guam (also called Guaham, Guahan).
Guguan (also called Guguwan, Guugwan, Piedras, San Felipe, St. Philippe).
Maug (also called Mang, Mangs, Mauga, Monjas, Mougu, Saint Laurent, San Lorenzo, Tunas).
Medinilla (also called Bade, Bird, Farallon de Medinilla, Rocher).
Pagan (also called Pagon, Paygan, St. Ignace, San Ignacio).
Rota (also called Luta, St. Anne, Santa Ana, Sarpan, Satpana, Suta, Zarpane).
Saipan (also called (Saepan, St. Joseph, San José, Saypan, Siepan, Serpan, Seypan).
Sarigan (also called St. Charles, San Carlos, Sariguan, Sarigwan).
Tinian (also called Bona Vista, Buenavista, Temean, Tenian, Tiniamou).
Uracas (also called Guy Rock, Farallon de Pájaros, Pájaros, Urakasu).
The Palau Islands (also called Arrecifos, Palaos, Paleu, Pally, Paloc, Pannog, Parao, Pelew) consist of 8 large islands, 18 smaller islands, and a large number of minute islets, all enclosed in a single reef system. The northern islands (Babelthuap and Koror) are of volcanic origin; the southern islands (Peleliu and others) are of coralline formation. Angaur, to the south of Peleliu, [Pg 14] may be included with the Palau Archipelago. From the standpoint of the avian zoogeography, the coral islands or atolls of Kayangel, Merir, Pulo Anna, Sonsorol, and Tobi are also included. The principal islands, shown in figure 3, are listed below:
Arakabesan (also called Ngarekobasang).
Aurapushekaru (also called Aburashokoru, Auluptagel, Oluksakel, Oropu-shakaru).
Babelthuap (also called Babeldzuap, Babel Taob, Babelthouap, Baberthaob, Baberudaobu, Babldaob).
Eil Malk (also called Amototi, Cogeal, Irakong, Makarakaru).
Garakayo
Koror (also called Coror, Goreor, Kororu).
Malakal (also called Amalakell, Malaccan, Marakaru, Nanalake).
Ngabad
Ngesebus (also called Guadokusu).
Peleliu (also called Pelelew, Periryu, Pililer, Peliliu, Uler).
Urukthapel (also called Cape, Kuapasungasu, Ngurukdapel, Ulugeang, Uruk-taaburu, Uruktapi).
Included with the Palau group because of proximity and relationships of the avifauna are the following:
Angaur (also called Angauru, Angyaur, Ngaur, Ngeour, N'Yaur).
Kayangel (also called Kadjangle, Kajanguru, Kazyanguru, Kianguel, Kreiangel, Moore, Ngajangel, Ngeiangel).
Merir (also called Marir, Meliel, Meriel, Meriru, Pulo Marier, Warren Hastings).
Pulo Anna (also called Anna, Bul, Bur, Current, Paola, Pul, Puru, Wull).
Sonsorol (also called St. Andrew, San Andreas, Sonesor, Songosor, Sonseron, Sonsol, Sonsoru, Tschontil).
Tobi (also called Codopuei, Johnstone, Kadogubi, Lectobis, Lord North, Nevil, Togobei, Tokobei).
The Caroline Islands consist of 41 island clusters or isolated islands (exclusive of submerged coral reefs). These are of coral formation. They are atolls or single islands except for Yap, which is of sedimentary rock, and Kusaie, Ponapé, and Truk, which are of volcanic rock. The principal islands are shown in figure 4 and are listed as follows:
East Fayu (also called Fajo, Faliao, Lutké, Rukutee).
Eauripik (also called Aurepik, Eourpyg, Iuripik, Kama, Low, Yorupikku, Yuripik).
Fais (also called Astrolabe, Feis, Feys, Fuhaesu, Huhaesu, Tromelin, Woaje).
Faraulep (also called Faraulip, Faroilap, Fattoilap, Foroilap, Furaarappu, Gardner, Huraarappu).
Ifalik (also called Evalook, Faloc, Furukku, Hurukku, Ifalouk, Ifelug, Two Sisters, Wilson).
Kapingamarangi (also called Bakiramarang, Constantine, Greenwich, Guriinitchi, Kabeneylon, Kapenmailang, Makarama, Pikiram, Tenuv).
Kusaie (also called Arao, Armstrong, Experiment, Hope, Kusai, Kuschai, Kushai, Kuthiu, Oualan, Quollen, Strong, Teyoa, Ualan, Walang).
Lamotrek (also called Lamorsu, Lamureck, Lamutrik, Low, Namotik, Namotikku, Manochikku, Namurrek, Swede).
Lukunor (also called Lemarafat, Lougoullos, Lougounor, Luganor, Lugunor, Lugunoz, Mortlock, Namonefeng, Rukunoru, Youngwilliam).
Namonuito (also called Anonyma, Baxos de San Bartolomeo, Bunkey, Las Hermanas, Livingstone, Lost Jardines, Lutké, Namenwita, Olol, Omun, Onon, Ororu, Remp, Ueito, Ulul).
Ngulu (also called Angegul, Anolul, Goulou, Kurru, Lamoliao, Lamoliork, Lamuliur, Lamuniur, Matelotas, Ngilu, Ngoli, Ngolog, Spencer Keys, Ulu).
Nukuoro (also called Dunkin, Matakema, Menteverde, Nugoru, Nukor, Nukuor).
Pikelot (also called Bigali, Biguela, Coquille, Lydia, Pigela, Pigerotto, Pigouelao, Pik, Pyghella).
Pingelap (also called Macaskill, Musgrave, Pelelap, Piigerappu, Punlap, Sailrocks, Tucks Reef).
Ponapé (also called Ascension, Bonabee, Bonybay, Faloupet, Faounoupei, Funopet, Niponpei, Painipete, Ponapi, Piunipet, Puynipet, Quirosa, Seniavin, William IV). Ponapé is the largest island of the Senyavin Islands.
Truk (also called Djuk, Hogoleu, Hogolu, Hoguleu, Lugulus, Ola, Rough, Ruck, Ruk, Torakku, Tuck, Ugulut). The Truk group includes approximately 100 islands.
Ulithi (also called Mackenzie, Mogmog, Mogumogu, Mokomok, Ouluthy, Uluthi, Uluti, Urushi).
West Fayu (also called Faiyao, Fajahu, Faliau, Huiyao, West Faiu).
Woleai (also called Anagai, Mereyon, Oleai, Ouleyai, Thirteen Islands, Uala, Ulea, Uola, Ulie, Wolea).
Yap (also called Eap, Guap, Heap, Jap, Ouap, Uap, Wuap, Yappu).
The Marshall Islands consist of 29 atolls and 5 coral islands without lagoons arranged in two chains, the Ralik and the Radak chains, which extend in a northwesterly to southeasterly direction. No volcanic rocks are exposed in these islands. The principal islands shown in figure 5 are as follows:
Ailuk (also called Ailu, Fisher, Krusenstern, Tindall, Watts).
Arhno (also called Arno, Aruno, Auru).
Bikar
Bikini
Ebon (also called Boston Atoll).
Elmore (also called Ailinglap, Ailinglapalap, Iringlob).
Eniwetok
Jaluit (also called Bonham, Taluit).
Kwajalein
Likieb (also called Likiep).
Majuro (also called Arrowsmith, Mezyuro).
Mejit
Maloelab
Mille (also called Mulgrave).
Namorik
Namu (also called Musquillo, Namo).
Rongelap
Wotje (also called Romanzov, Wotze, Wozzie).
The Micronesian islands were first explored and colonized by a a people who came from Malaysia. It is thought that these people spread into the Palau, Caroline, Mariana, Marshall, and Gilbert islands as a single wave of migration. Following this occupation, the people apparently underwent a normal process of cultural evolution [Pg 17] and differentiation. Remains of stone walls, dikes, fences, pillars, graves, and other structures which may be found today at various islands in Micronesia were constructed by the ancestors of the islanders of the present day. It is thought by archeologists that the Polynesians moved eastward into the Pacific islands by way of Micronesia. The date of this wave of migration is thought to have been approximately 1200 A. D. What kinds of birds may have been exterminated by this earliest of human colonization cannot be ascertained. Edible species, particularly megapodes, rails, and pigeons, probably were eliminated or reduced in numbers, as is indicated by later discussions.
The first Europeans to visit Micronesia, as far as the present writer can ascertain, left no accounts of the birds significant for the study here reported upon. Magellan, on his trip around the world, was the leader of the first party of Europeans who touched at Guam; this was on March 6, 1521. Rota, Agiguan, Saipan, and Tinian were also discovered by this Portuguese sea captain in the service of the king of Spain. Eltano, one of Magellan's lieutenants, revisited the Pacific and stopped at Rota in 1524. After the voyage of Magellan, other seafarers, mostly in the service of Spain, visited the Micronesian islands. The Caroline Islands were apparently first observed by the Portuguese captain, Diego de Rocha, in 1526. Loyasa and Saavdera, both Spaniards, visited the Marshall Islands in 1526 and 1529, respectively.
One of the first travelers to record observations on the bird life was Henry Wilson. Wilson was captain of the schooner "Antelope" which became grounded on a reef in the Palau Islands in August, 1783. He lived with the islanders while the ship was being repaired and kept a journal of his observations (Wilson, 1788). Wilson also visited several other islands in western Micronesia. Adelbert von Chamisso (1821), as naturalist with the Russian expedition in the ship "Rurick," made observations of the animal life in Micronesia in 1817 and 1818. Under the command of Otto von Kotzebue, this Russian expedition made the first detailed exploration of the Marshall Islands; visits were made also to Guam and Rota and to Yap, Fais, Ulithi, Palau, and other island groups in western Micronesia. Freycinet's famous expedition in the ships "Uranie" and "Physicienne," visited Guam, Rota, and Tinian in 1819. Quoy and Gaimard, the naturalists of the expedition, obtained birds, which were among the first to be described from Micronesia. These two naturalists revisited the Marianas in 1829 on board the ship "Astrolabe." [Pg 18] Scientific results of both of these expeditions (Quoy and Gaimard, 1824-'26 and 1830-'35) include texts and plates dealing with the birds obtained.
The French expedition in the corvette "La Coquille" visited Kusaie in June, 1824. Lesson (1829) wrote the zoology of this trip. Kittlitz (1836) of the expedition which sailed in the corvette "Le Seniavine" commanded by Lutké obtained birds at Kusaie in December and January, 1827-'28, at Guam in March, 1828, and at Lukunor and other islands of the Carolines. At Kusaie, Kittlitz found a rail (Aphanolimnas monasa) and a starling (Aplonis corvinus) which have not been obtained since his time. His specimens were deposited in St. Petersburg. He was one of the most competent of the early naturalists; his writings contain accounts of habits as well as descriptions and are accompanied by colored plates. The expedition which sailed on the "Astrolabe" and the "Zélée" in 1827-'40 under the command of Dumont d'Urville visited the Caroline Islands. The naturalists, Hombron and Jacquinot, obtained birds at Truk, including the interesting flycatcher, Metabolus rugensis, which they described (1841). The "Novara," in the course of its voyage around the world (1857-'59) visited the Caroline Islands in 1858. Birds were recorded from Ponapé, Lukunor and other islands by Pelzeln in his account of the birds of the expedition (1865).
In the years following the middle of the Nineteenth Century, Godeffroy and Sons, of Hamburg, opened branches of its trading firm in Micronesia. Representatives of the company including Heinsohn and Peters, who were ship captains, obtained collections of birds at Palau and Yap. These were deposited in the Godeffroy Museum at Hamburg and reported on by Hartlaub and Finsch (Hartlaub, 1868; Hartlaub and Finsch, 1868a and 1872). Tetens became representative of Godeffroy and Sons at Yap in 1869 and obtained birds. Perhaps the most famous collector in this period was Johann Kubary. He went to Ponapé at the age of nineteen and traveled in Micronesia for many years for Godeffroy and Sons. He obtained birds at many of the islands of the Carolines, spending fourteen months at Truk. In 1873, one of his collections of some 200 birds was lost in a shipwreck. Hartlaub and Finsch, (Hartlaub and Finsch, 1872; Finsch, 1876a) described much of his material; Nehrkorn (1879) reported on nests and eggs which he obtained. Hartlaub and Finsch (1868b) also reported on birds obtained at Palau by Doctor Semper, which were deposited in the museum at Altona. Otto Finsch (1880b, 1880d, 1881b, 1881c) traveled in Micronesia about 1880, observing birds in the eastern Carolines and in the Marshalls.
One of the largest collections from Micronesia was made by Alfred Marche in the Marianas. He arrived there on April 22, 1887, and stayed until May, 1889. He obtained approximately 732 specimens of birds, nests, and eggs at Guam, Rota, Tinian, Saipan, Pagan, and Alamagan, which were deposited in the Paris Museum and reported on by Oustalet (1895-'96). Shortly after Marche's visit, Japanese collectors in the hire of Alan Owston, a professional collector of Yokahama, obtained birds in the Marianas and at Truk in the years 1894-'97. These went to the Rothschild collection at Tring and were reported on by Hartert in 1898 and 1900.
At the turn of the Twentieth Century, several ornithologists were visiting Micronesia. Alvin Seale (1901) obtained a collection of birds at Guam in the summer of 1900 which was deposited in the Bernice P. Bishop Museum in Honolulu. The U. S. Fish Commission steamer "Albatross" visited Micronesia from August, 1899, to March, 1900; birds obtained by the expedition were reported on by Townsend and Wetmore (1919). Paul Schnee (1901) spent approximately one year, 1899-1900, at Jaluit in the Marshalls and obtained records of birds. In 1899, Brandeis, on board the German ship "Kaiserland" visited many of the islands in the Marshalls and recorded birds. William Safford (1905) resided at Guam in the early part of this century and reported on the bird life in the course of his studies of the botany and native life. Bartsch (Mearns, 1909) also obtained a small collection of birds at Guam, this is in the United States National Museum.
In the first World War when the Japanese gained a mandated control over the islands of Micronesia, the Japanese ornithologists promptly visited the area, obtained collections, and published works concerning the birds. In 1922, Momiyama and Kuroda prepared a list of the birds of Micronesia. The work was published under the auspices of the Ornithological Society of Japan. Subsequent editions appeared in 1932 and 1942.
The Whitney South Sea Expedition of the American Museum of Natural History visited Micronesia from October, 1930, to December, 1931, with William F. Coultas as collector. Although experiencing some difficulty and being restricted somewhat in his travels by the Japanese officials, he managed to obtain collections at Ponapé (October 26, 1930, to January 1, 1931), Kusaie (January 15 to June 11, 1931), Guam (June 24 to August 30, 1931), Saipan and Tinian (September 1 to 26, 1931), and Palau (October 2 to December, 1931). Many of the species which he obtained are represented by [Pg 20] large series of fine skins. Only part of his collections have been reported on by Mayr and his associates.
Other than the work of Coultas and that of the Japanese, there was little ornithological work done in the period between the two world wars, probably, at least in part, because of the "iron curtain," which Japan had thrown about her mandate. Bryan (1936) did visit Guam in the middle 1930's and published an account of the birds in the newspaper, Guam Recorder.
When the Micronesian islands were taken by the American forces in 1944, personnel attached to various units made observations on the bird life. The first reports, published or unpublished, were from the Marshalls, which were taken at the beginning of the campaign. Gleise, Genelly, Wallace, and others made contributions. In the Marianas considerably more observing and collecting were done by service personnel including Marshall, Stott, Borror, Strophlet, Buss, Watson, Arvey, Downs, and others. Marshall (1949) obtained also a collection of birds in the Palaus in 1945. The Laboratory of Mammalogy, United States Naval Medical Research No. 2, to which I was attached, collected at Guam (January to October, 1945), at Rota (October 17 to November 2, 1945), at Ulithi (August 11 to 23, 1945), at Palau (August 24 to September 24, 1945), and at Truk (November 24 to December 18, 1945). Following the end of the war, Harvey I. Fisher visited Micronesia and obtained a collection of birds at Yap, which is to be reported on in the near future. Larry P. Richards obtained 33 birds at Ponapé and 4 at Truk in the period from August 28, 1947, to February 10, 1948.
Descriptions of birds in Micronesia began with the naming of Halcyon c. cinnamomina in 1821; the most recent description is that of Rhipidura rufifrons mariae in 1946. In all, 131 descriptions have designated type localities in Micronesia. Table 1 lists the dates (on the basis of ten-year intervals) when names of birds (synonyms or otherwise) were proposed. In the period from 1821 to 1860, twenty-five birds were made known to science by the earliest workers, including Kittlitz, Lesson, Bonaparte, and Pelzeln. In the period from 1861 to 1880, thirty-four birds were newly named, mostly by Hartlaub and Finsch, from the collections which the Godeffroy Museum obtained through the efforts of Kubary, Tetens, Peters, and Heinsohn. Nineteen original descriptions were published from 1881 to 1900, principally by Oustalet and Hartert, who studied the material of Marche and Owston, respectively. From 1901 to 1910, only four birds were described, but from 1911 to 1940, forty-seven descriptions were published, mostly by the Japanese following World War I. [Pg 21] From 1931 to 1940, the number of known birds was increased by the efforts of Mayr, who studied the material of the Whitney South Sea Expedition. From 1941 to date only two original descriptions have appeared—only one was postwar. Except for possible undescribed subspecies in the northern Marianas, I think that the heyday of the taxonomist in ornithology in Micronesia is over. The field of avian ecology in Micronesia has barely been scratched.
| Years | No. of descriptions |
Years | No. of descriptions |
| 1821-1830 | 8 | 1881-1890 | 9 |
| 1831-1840 | 8 | 1891-1900 | 10 |
| 1841-1850 | 4 | 1901-1910 | 4 |
| 1851-1860 | 5 | 1911-1920 | 10 |
| 1861-1870 | 11 | 1921-1930 | 15 |
| 1871-1880 | 23 | 1931-1940 | 22 |
| 1941-1949 | 2 |
The 206 kinds of birds of 150 full species known to occur in Micronesia belong to 91 genera of 37 families of 13 orders. In the following list, nonresident birds are marked with an *; birds introduced by man are marked with a [+].
| Class AVES—birds | ||||
| Page | ||||
| Order Procellariiformes—albatrosses, petrels, and allies | ||||
| Family Diomedeidae—albatrosses | ||||
| Diomedia nigripes Audubon* | Black-footed Albatross | 63 | ||
| Family Procellariidae—petrels and shearwaters | ||||
| Puffinus pacificus chlororhynchus Lesson | Wedge-tailed Shearwater | 64 | ||
| Puffinus pacificus cuneatus Salvin | Wedge-tailed Shearwater | 65 | ||
| Puffinus tenuirostris (Temminck)* | Short tailed Shearwater | 66 | ||
| Puffinus nativitatus Streets | Christmas Shearwater | 66 | ||
| Puffinus lherminieri dichrous Finsch and Hartlaub | Dusky Shearwater | 66 | ||
| Pterodroma rostrata rostrata (Peale)* | Tahiti Petrel | 69 | ||
| Pterodroma hypoleuca hypoleuca Salvin | Stout-billed Gadfly Petrel | 70 | ||
| Order Pelecaniformes—tropic birds, boobies, cormorants, frigate birds and allies | ||||
| [Pg 22] | ||||
| Family Phaëthontidae—tropic birds | ||||
| Phaëthon aethereus mesonauta Peters* | Red-billed Tropic Bird | 70 | ||
| Phaëthon rubricauda rothschildi (Mathews) | Red-tailed Tropic Bird | 71 | ||
| Phaëthon lepturus dorotheae Mathews | White-tailed Tropic Bird | 72 | ||
| Family Sulidae—boobies and gannets | ||||
| Sula dactylatra personata Gould | Masked Booby | 75 | ||
| Sula sula rubripes Gould | Red-footed Booby | 75 | ||
| Sula leucogaster plotus (Forster) | Brown Booby | 76 | ||
| Family Phalacrocoracidae—cormorants | ||||
| Phalacrocorax melanoleucus melanoleucus (Vieillot) | Little Pied Cormorant | 78 | ||
| Family Fregatidae—frigate birds or man-o'-war birds | ||||
| Fregata minor minor (Gmelin)* | Pacific Man-o'-War | 79 | ||
| Fregata ariel ariel (Gray) | Least Man-o'-War | 80 | ||
| Order Ciconiiformes—herons, storks, and allies | ||||
| Family Ardeidae—herons and bitterns | ||||
| Butorides striatus amurensis Schrenck* | Amur Green Heron | 81 | ||
| Bubulcus ibis coromandus (Boddaert)* | Cattle Egret | 82 | ||
| Egretta intermedia intermedia (Wagler)* | Plumed Egret | 82 | ||
| Demigretta sacra sacra (Gmelin) | Reef Heron | 84 | ||
| Nycticorax nycticorax nycticorax (Linnaeus)* | Black-crowned Night Heron | 87 | ||
| Nycticorax caledonicus pelewensis Mathew | Rufous Night Heron | 87 | ||
| Gorsachius goisagi (Temminck)* | Japanese Bittern | 89 | ||
| Gorsachius melanolophus melanolophus (Raffles)* | Malay Bittern | 90 | ||
| Ixobrychus sinensis (Gmelin) | Chinese Least Bittern | 93 | ||
| Ixobrychus eurhythmus (Swinhoe)* | Shrenck's Least Bittern | 93 | ||
| Dupetor flavicollis flavicollis (Latham)* | Black Bittern | 94 | ||
| Order Anseriformes—ducks, geese, swans, and allies | ||||
| Family Anatidae—ducks, geese, and swans | ||||
| Anas oustaleti Salvadori | Marianas Mallard | 94 | ||
| Anas poecilorhyncha pelewensis Hartlaub and Finsch | Australian Gray Duck | 98 | ||
| Anas querquedula Linnaeus* | Garganey Teal | 100 | ||
| Anas crecca crecca Linnaeus* | European Teal | 100 | ||
| Anas crecca carolinensis Gmelin* | Green-winged Teal | 100 | ||
| Anas acuta acuta Linnaeus* | Pintail | 101 | ||
| Anas acuta tzitzihoa Vieillot* | Pintail | 101 | ||
| Anas penelope Linnaeus* | Widgeon | 102 | ||
| Anas clypeata Linnaeus* | Shoveller | 102 | ||
| Aythya fuligula (Linnaeus)* | Tufted Duck | 103 | ||
| Aythya valisineria (Wilson)* | Canvas-back | 103 | ||
| [Pg 23] | ||||
| Order Falconiformes—vultures, hawks, falcons | ||||
| Family Accipitridae—hawks, harriers, and allies | ||||
| Accipiter soloënsis (Horsfield)* | Chinese Goshawk | 104 | ||
| Accipiter virgatus gularis (Temminck and Schlegel)* | Asiatic Sparrow Hawk | 104 | ||
| Pandion haliaetus melvillensis Mathews | Osprey | 105 | ||
| Family Falconidae—falcons and caracaras | ||||
| Falco peregrinus japonensis Gmelin* | Peregrine Falcon | 105 | ||
| Order Galliformes—megapodes, pheasants, and allies | ||||
| Family Megapodidae—megapodes | ||||
| Megapodius lapérouse senex Hartlaub | Micronesian Megapode | 106 | ||
| Megapodius lapérouse lapérouse Gaimard | Micronesian Megapode | 109 | ||
| Family Phasianidae—quails, pheasants, and allies | ||||
| Coturnix chinensis lineata (Scopoli)[+] | Painted Quail | 113 | ||
| Gallus gallus (Linnaeus)[+] | Red Jungle Fowl | 114 | ||
| Phasianus colchicus Linnaeus[+] | Ring-necked Pheasant | 115 | ||
| Order Gruiformes—cranes, rails, and allies | ||||
| Family Rallidae—rails, gallinules, and coots | ||||
| Rallus philippensis pelewensis (Mayr) | Banded Rail | 116 | ||
| Rallus owstoni (Rothschild) | Guam Rail | 118 | ||
| Rallina fasciata (Raffles)* | Malay Banded Crake | 120 | ||
| Rallina eurizonoïdes eurizonoïdes (Lafresnaye)* | Philippine Banded Crake | 121 | ||
| Aphanolimnas monasa (Kittlitz) | Kusaie Black Rail | 121 | ||
| Poliolimnas cinereus micronesiae Hachisuka | White-browed Rail | 123 | ||
| Gallinula chloropus subsp. near orientalis Horsfield | Gallinule | 126 | ||
| Gallinula chloropus guami Hartert | Gallinule | 127 | ||
| Porphyrio porphyrio pelewensis Hartlaub and Finsch | Purple Swamphen | 129 | ||
| Fulica atra atra Linnaeus* | Common Coot | 131 | ||
| Order Charadriiformes—shorebirds, gulls, and auks | ||||
| Family Charadriidae—plovers, turnstones, and allies | ||||
| Squatarola squatarola (Linnaeus)* | Black-bellied Plover | 131 | ||
| Pluvialis dominica fulva (Gmelin)* | Pacific Golden Plover | 132 | ||
| Charadrius hiaticula semipalmatus Bonaparte* | Semipalmated Plover | 134 | ||
| Charadrius dubius curonicus Gmelin* | Ring-necked Plover | 135 | ||
| Charadrius alexandrinus nihonensis Deignan* | Kentish Plover | 135 | ||
| [Pg 24] | Charadrius mongolus stegmanni Stresemann* | Mongolian Dotteral | 135 | |
| Charadrius leschenaultii Lesson* | Large Sand Dotteral | 137 | ||
| Family Scolopacidae—snipe, sandpipers, and allies | ||||
| Numenius phaeopus variegatus (Scopoli)* | Whimbrel | 137 | ||
| Numenius tahitiensis (Gmelin)* | Bristle-thighed Curlew | 139 | ||
| Numenius madagascariensis (Linnaeus)* | Long-billed Curlew | 140 | ||
| Limosa lapponica baueri Naumann* | Pacific Godwit | 140 | ||
| Tringa nebularia (Gunnerus)* | Greenshawk | 141 | ||
| Tringa melanoleuca (Gmelin)* | Greater Yellow-legs | 142 | ||
| Tringa glareola Linnaeus* | Wood Sandpiper | 142 | ||
| Actitus hypoleucos Linnaeus* | Common Sandpiper | 143 | ||
| Heteroscelus brevipes (Vieillot)* | Gray-tailed Tattler | 144 | ||
| Heteroscelus incanus (Gmelin)* | Amer. Wandering Tattler | 145 | ||
| Arenaria interpres interpres (Linnaeus)* | Turnstone | 147 | ||
| Gallinago megala Swinhoe* | Marsh Snipe | 149 | ||
| Gallinago gallinago gallinago (Linnaeus)* | Common Snipe | 150 | ||
| Crocethia alba (Pallas)* | Sanderling | 150 | ||
| Calidris tenuirostris (Horsfield)* | Asiatic Knot | 151 | ||
| Erolia minuta ruficollis (Pallas)* | Little Stint | 151 | ||
| Erolia subminuta (Middendorff)* | Least Sandpiper | 152 | ||
| Erolia melanotos (Vieillot)* | Pectoral Sandpiper | 152 | ||
| Erolia acuminata (Horsfield)* | Sharp-tailed Sandpiper | 152 | ||
| Erolia ferruginea (Pontoppidan)* | Curlew Sandpiper | 153 | ||
| Limicola falcinellus sibirica Dresser* | Broad-billed Sandpiper | 154 | ||
| Family Phalaropidae—phalaropes | ||||
| Phalaropus lobatus (Linnaeus)* | Northern Phalarope | 154 | ||
| Family Laridae—gulls and terns | ||||
| Larus argentatus vegae Palmén* | Herring Gull | 154 | ||
| Chlidonias leucopterus (Temminck)* | White-winged Black Tern | 155 | ||
| Sterna hirundo longipennis Nordmann* | Black-billed Com. Tern | 155 | ||
| Sterna sumatrana sumatrana Raffles | Black-naped Tern | 156 | ||
| Sterna lunata Peale | Spectacled Tern | 160 | ||
| Sterna anaetheta anaetheta Scopoli | Bridled Tern | 160 | ||
| Sterna fuscata oahuensis Bloxham | Sooty Tern | 161 | ||
| Sterna albifrons sinensis Gmelin* | Least Tern | 161 | ||
| Thalasseus bergii pelecanoides (King) | Crested Tern | 162 | ||
| Procelsterna cerulea saxatilis W. E. Fisher* | Blue-gray Tern | 164 | ||
| Anoüs stolidus pileatus (Scopoli) | Common Noddy | 165 | ||
| Anoüs tenuirostris marcusi (Bryan) | White-capped Noddy | 170 | ||
| Gygis alba candida (Gmelin) | White Tern | 174 | ||
| Gygis alba pacifica (Lesson) | White Tern | 180 | ||
| [Pg 25] | ||||
| Order Columbiformes—pigeons, doves, and allies | ||||
| Family Columbidae—pigeons and doves | ||||
| Columba livia Gmelin[+] | Blue Rock Pigeon | 182 | ||
| Ptilinopus porphyraceus ponapensis (Finsch) | Crimson-crw'd Fruit Dove | 182 | ||
| Ptilinopus porphyraceus hernsheimi (Finsch) | Crimson-crw'd Fruit Dove | 184 | ||
| Ptilinopus porphyraceus pelewensis Hartlaub and Finsch | Crimson-crw'd Fruit Dove | 185 | ||
| Ptilinopus roseicapillus (Lesson) | Marianas Fruit Dove | 186 | ||
| Ducula oceanica monacha (Momiyama) | Micronesian Pigeon | 190 | ||
| Ducula oceanica teraokai (Momiyama) | Micronesian Pigeon | 193 | ||
| Ducula oceanica townsendi (Wetmore) | Micronesian Pigeon | 194 | ||
| Ducula oceanica oceanica (Lesson and Garnot) | Micronesian Pigeon | 195 | ||
| Ducula oceanica ratakensis (Takatsukasa and Yamashina) | Micronesian Pigeon | 197 | ||
| Streptopelia bitorquata dusumieri (Temminck)[+] | Philippine Turtle Dove | 198 | ||
| Gallicolumba canifrons (Hartlaub and Finsch) | Palau Ground Dove | 201 | ||
| Gallicolumba xanthonura xanthonura (Temminck) | White-thrt'd Ground Dove | 203 | ||
| Gallicolumba xanthonura kubaryi (Finsch) | White-thrt'd Ground Dove | 207 | ||
| Caloenas nicobarica pelewensis Finsch | Nicobar Pigeon | 209 | ||
| Order Psittaciformes—lories and parrots | ||||
| Family Psittacidae—lories, parrots, and allies | ||||
| Trichoglossus rubiginosus (Bonaparte) | Ponapé Lory | 211 | ||
| Order Cuculiformes—cuckoos, plantain-eaters | ||||
| Family Cuculidae—cuckoos, anis, and allies | ||||
| Cuculus canorus telephonus Heine* | Common Cuckoo | 213 | ||
| Cuculus saturatus horsfieldi Moore* | Oriental Cuckoo | 214 | ||
| Eudynamis taitensis (Sparrman)* | Long-tailed New Zealand Cuckoo | 214 | ||
| Order Strigiformes—owls | ||||
| Family Strigidae—owls | ||||
| Otus podarginus (Hartlaub and Finsch) | Palau Scops Owl | 215 | ||
| Asio flammeus flammeus (Pontoppidan)* | Short-eared Owl | 217 | ||
| Asio flammeus ponapensis Mayr | Short-eared Owl | 218 | ||
| Order Caprimulgiformes—goatsuckers and allies | ||||
| Family Caprimulgidae—goatsuckers | ||||
| Caprimulgus indicus jotaka Temminck and Schlegel* | Jungle Nightjar | 219 | ||
| Caprimulgus indicus phalaena Hartlaub and Finsch | Jungle Nightjar | 219 | ||
| [Pg 26] | ||||
| Order Apodiformes—swifts and hummingbirds | ||||
| Family Apodidae—swifts | ||||
| Collocalia inexpectata pelewensis Mayr | Edible Nest Swiftlet | 221 | ||
| Collocalia inexpectata bartschi Mearns | Edible Nest Swiftlet | 222 | ||
| Collocalia inquieta inquieta (Kittlitz) | Carolines Swiftlet | 224 | ||
| Collocalia inquieta rukensis Kuroda | Carolines Swiftlet | 225 | ||
| Collocalia inquieta ponapensis Mayr | Carolines Swiftlet | 226 | ||
| Order Coraciiformes—kingfishers, rollers, and allies | ||||
| Family Alcedinidae—kingfishers | ||||
| Halcyon cinnamomina cinnamomina Swainson | Micronesian Kingfisher | 227 | ||
| Halcyon cinnamomina pelewensis Wiglesworth | Micronesian Kingfisher | 229 | ||
| Halcyon cinnamomina reichenbachii (Hartlaub) | Micronesian Kingfisher | 230 | ||
| Halcyon chloris teraokai Kuroda | White-collared Kingfisher | 233 | ||
| Halcyon chloris orii Takatsukasa and Yamashina | White-collared Kingfisher | 235 | ||
| Halcyon chloris albicilla (Dumont) | White-collared Kingfisher | 235 | ||
| Halcyon chloris owstoni Rothschild | White-collared Kingfisher | 237 | ||
| Family Coraciidae—rollers | ||||
| Eurystomus orientalis connectens Stresemann* | Dollar Bird | 238 | ||
| Order Passeriformes—perching birds | ||||
| Family Hirundinidae—swallows | ||||
| Hirundo rustica gutteralis Scopoli* | Eastern Barn Swallow | 239 | ||
| Family Campephagidae—cuckoo-shrikes | ||||
| Edolisoma tenuirostre monachum (Hartlaub and Finsch) | Cicada Bird | 239 | ||
| Edolisoma tenuirostre nesiotis (Hartlaub and Finsch) | Cicada Bird | 241 | ||
| Edolisoma tenuirostre insperatum (Finch) | Cicada Bird | 242 | ||
| Family Dicruridae—drongos | ||||
| Dicrurus macrocercus harterti S. Baker[+] | Black Drongo | 244 | ||
| Family Corvidae—crows, magpies, and jays | ||||
| Corvus kubaryi Reichenow | Marianas Crow | 244 | ||
| Family Turdidae—thrushes | ||||
| Luscinia calliope calliope (Pallas)* | Siberian Rubythroat | 248 | ||
| Monticola solitaria philippensis (Müller)* | Chinese Blue Rock Thrush | 248 | ||
| Turdus obscurus obscurus Gmelin* | Dusky Thrush | 248 | ||
| [Pg 27] | ||||
| Family Sylviidae—Old World warblers | ||||
| Psamathia annae Hartlaub and Finsch | Palau Bush-warbler | 249 | ||
| Acrocephalus luscinia luscinia (Quoy and Gaimard) | Nightingale Reed-warbler | 251 | ||
| Acrocephalus luscinia syrinx (Kittlitz) | Nightingale Reed-warbler | 254 | ||
| Acrocephalus luscinia yamashinae (Takatsukasa) | Nightingale Reed-warbler | 256 | ||
| Acrocephalus luscinia nijoi (Yamashina) | Nightingale Reed-warbler | 257 | ||
| Family Muscicapidae—Old World flycatchers | ||||
| Rhipidura rufifrons uraniae Oustalet | Rufous-fronted Fantail | 261 | ||
| Rhipidura rufifrons saipanensis Hartert | Rufous-fronted Fantail | 262 | ||
| Rhipidura rufifrons mariae R. H. Baker | Rufous-fronted Fantail | 263 | ||
| Rhipidura rufifrons versicolor Hartlaub and Finsch | Rufous-fronted Fantail | 264 | ||
| Rhipidura rufifrons kubaryi Finsch | Rufous-fronted Fantail | 265 | ||
| Rhipidura lepida Hartlaub and Finsch | Palau Fantail | 266 | ||
| Metabolus rugensis (Hombron and Jacquinot) | Truk Monarch | 269 | ||
| Monarcha godeffroyi Hartlaub | Yap Monarch | 272 | ||
| Monarcha takatsukasae (Yamashina) | Tinian Monarch | 274 | ||
| Myiagra oceanica erythrops Hartlaub and Finch | Micronesian Broadbill | 275 | ||
| Myiagra oceanica freycineti Oustalet | Micronesian Broadbill | 277 | ||
| Myiagra oceanica oceanica Pucheran | Micronesian Broadbill | 279 | ||
| Myiagra oceanica pluto Finsch | Micronesian Broadbill | 280 | ||
| Muscicapa narcissina narcissina Temminck* | Narcissus Flycatcher | 282 | ||
| Muscicapa griseisticta (Swinhoe)* | Chinese Gray-spotted Flycatcher | 282 | ||
| Colluricincla tenebrosa (Hartlaub and Finsch) | Palau Morning Bird | 282 | ||
| Family Artamidae—wood-swallows | ||||
| Artamus leucorhynchus pelewensis Finsch | White-breasted Wood-swallow | 284 | ||
| Family Sturnidae—starlings | ||||
| Aplonis opacus opacus (Kittlitz) | Micronesian Starling | 286 | ||
| Aplonis opacus ponapensis Takatsukasa and Yamashina | Micronesian Starling | 288 | ||
| Aplonis opacus angus Momiyama | Micronesian Starling | 289 | ||
| Aplonis opacus kurodai Momiyama | Micronesian Starling | 291 | ||
| Aplonis opacus orii (Takatsukasa and Yamashina) | Micronesian Starling | 292 | ||
| Aplonis opacus guami Momiyama | Micronesian Starling | 293 | ||
| Aplonis opacus aeneus (Takatsukasa and Yamashina) | Micronesian Starling | 297 | ||
| Aplonis pelzelni Finsch | Ponapé Mountain Starling | 299 | ||
| Aplonis corvinus (Kittlitz) | Kusaie Mountain Starling | 301 | ||
| Sturnus philippensis (Forster)* | Violet-backed Starling | 302 | ||
| Sturnus cineraceus Temminck* | Ashy Starling | 302 | ||
| [Pg 28] | ||||
| Family Meliphagidae—honey-eaters | ||||
| Cleptornis marchei (Oustalet) | Golden Honey-eater | 302 | ||
| Myzomela cardinalis rubratra (Lesson) | Cardinal Honey-eater | 304 | ||
| Myzomela cardinalis dichromata Wetmore | Cardinal Honey-eater | 307 | ||
| Myzomela cardinalis major Bonaparte | Cardinal Honey-eater | 307 | ||
| Myzomela cardinalis saffordi Wetmore | Cardinal Honey-eater | 309 | ||
| Myzomela cardinalis kurodai Momiyama | Cardinal Honey-eater | 312 | ||
| Myzomela cardinalis kobayashii Momiyama | Cardinal Honey-eater | 313 | ||
| Family Zosteropidae—white-eyes | ||||
| Zosterops conspicillata conspicillata (Kittlitz) | Bridled White-eye | 316 | ||
| Zosterops conspicillata saypani Dubois | Bridled White-eye | 318 | ||
| Zosterops conspicillata rotensis Takatsukasa and Yamashina | Bridled White-eye | 319 | ||
| Zosterops conspicillata semperi Hartlaub | Bridled White-eye | 320 | ||
| Zosterops conspicillata owstoni Hartert | Bridled White-eye | 321 | ||
| Zosterops conspicillata takatsukasai Momiyama | Bridled White-eye | 322 | ||
| Zosterops conspicillata hypolais Hartlaub and Finsch | Bridled White-eye | 323 | ||
| Zosterops cinerea cinerea (Kittlitz) | Micron. Dusky White-eye | 326 | ||
| Zosterops cinerea ponapensis Finsch | Micron. Dusky White-eye | 327 | ||
| Zosterops cinerea finschii (Hartlaub) | Micron. Dusky White-eye | 328 | ||
| Rukia palauensis (Reichenow) | Palau Greater White-eye | 330 | ||
| Rukia oleaginea (Hartlaub and Finsch) | Yap Greater White-eye | 331 | ||
| Rukia ruki (Hartert) | Truk Greater White-eye | 332 | ||
| Rukia sanfordi (Mayr) | Ponapé Greater White-eye | 333 | ||
| Family Ploceidae—weaver-finches | ||||
| Erythrura trichroa trichroa (Kittlitz) | Blue-faced Parrot-finch | 336 | ||
| Erythrura trichroa clara Takatsukasa and Yamashina | Blue-faced Parrot-finch | 337 | ||
| Erythrura trichroa pelewensis Kuroda | Blue-faced Parrot-finch | 338 | ||
| Lonchura nigerrima minor (Yamashina) | Black-faced Weaver-finch | 339 | ||
| Lonchura punctulata cabanisi (Sharpe)[+] | Phil. Nutmeg Mannikin | 340 | ||
Of the 206 kinds of birds found in Micronesia, 30 kinds are classed as sea birds, 29 kinds as migratory shore birds, and 147 kinds are classed as land and fresh-water birds. For purposes of discussion these birds are arranged in these three categories, following the system used by Mayr (1945a).
Oceanic birds found in Micronesia belong to the following families: Diomedeidae, Procellariidae, Phaëthontidae, Pelecanidae, [Pg 29] Fregatidae, and Laridae. Following Wynne-Edwards (1935:240) and Murphy (1936:326), these birds may be grouped as inshore birds (Laridae and others), offshore birds (Pelecanidae, Fregatidae and others), and pelagic birds (Diomedeidae, Procellariidae, Phaëthontidae). As shown in table 2 there are 30 kinds of oceanic birds in Micronesia, 18 kinds that are resident and 12 kinds that are regarded as visitors to the area. Records of nestings are few; field work in the future probably will yield evidence that more kinds of oceanic birds are actually resident in the Micronesian islands.
| Genera | Resident kinds |
Nonresident kinds |
| Diomedea | 0 | 1 |
| Puffinus | 4 | 1 |
| Pterodroma | 1 | 1 |
| Phaëthon | 2 | 1 |
| Sula | 3 | 0 |
| Fregata | 1 | 1 |
| Larus | 0 | 1 |
| Chlidonias | 0 | 1 |
| Sterna | 2 | 4 |
| Thalasseus | 1 | 0 |
| Procelsterna | 0 | 1 |
| Anoüs | 2 | 0 |
| Gygis | 2 | 0 |
The inshore zone, according to Wynne-Edwards (1935:240), "extends from high-water mark to a maximum of four or five miles out to sea, including islands and reefs within sight of shore." In Micronesia the majority of the Laridae occur in this zone including such residents as Sterna sumatrana, S. anaetheta, Thalasseus bergii, Anoüs stolidus, A. tenuirostris, Gygis alba. These birds, especially S. anaetheta, Thalasseus, and Anoüs, may venture into the offshore zone. Visitors to Micronesia include several terns which probably [Pg 30] normally range in the inshore (as well as in offshore) zones, such as Childonias leucopterus and Sterna hirundo. These birds feed to a considerable extent inside the outer reefs surrounding the lagoons, coming to shore frequently in small or large groups. Gygis alba probably spends considerable time on shore; stomachs examined contained fish, crustaceans and insects, indicating that they obtain some of their food ashore.
Wynne-Edwards (1935:241) defines the offshore zone as extending to the continental edge; however, in Micronesia where small islands rise abruptly out of the ocean's depths, there is no useful way to separate the offshore zone from the pelagic zone. Since certain species go farther from the land than others, the two zones may be combined as a single zone extending beyond the sight of land. Birds which frequent this area beyond the inshore zone but may not range extensively at sea include Fregata, Sula, Sterna fuscata, S. hirundo, S. anaetheta, and others. The Herring Gull (Larus argentatus), which has been taken in the northern Marianas, may be classed with this group although it probably ranges widely in the open sea. Birds which spend considerable time at sea and may seldom approach land include Diomedea nigripes, the petrels (Puffinus and Pterodroma), and possibly the tropic birds (Phaëthon).
In numbers of individuals the birds inhabiting the inshore zones are relatively more numerous than those preferring the offshore and pelagic zones, although 12 of the 18 resident kinds of oceanic birds apparently prefer the offshore zone, while only 6 kinds appear to be restricted primarily to the inshore areas.
The oceanic birds were probably among the earliest birds to reach the islands of Micronesia. The presence of phosphate deposits on islands (Fais, Angaur), denoting deposition of guano by oceanic birds (possibly boobies, noddies, sooty terns), indicates long time residence by these birds. A person is prone to think that these deposits must have been made by larger concentrations of oceanic birds than are found in these islands today. Whether there were actually more individuals present during the period of deposition of phosphate in the lagoons of these islands is not known, although the elevation of the lagoons (forming the raised islands of Fais and Angaur) with the resulting freshening of the water probably was a great attraction to oceanic birds, especially to those which prefer to drink fresh water. According to Leonard P. Schultz (in litt.), [Pg 31] the abundance of fish in the areas about these Pacific islands has been approximately the same since Pleistocene times, so that there was apparently no greater concentration of fish near these islands to attract large populations of fish-eating sea birds. Probably the time element is of sufficient magnitude to account for such deposition by birds with a population similar to that found there today.
The oceanic avifauna of Micronesia contains birds which are apparently from ancestral homes in the Palearctic Region, in the North and Central Pacific, in Polynesia, in Melanesia and Malaysia, and from homes the positions of which are uncertain because of the widespread circumtropical occurrence of the birds. There are no sea birds that are endemic in Micronesia.
Oceanic birds whose range is in the Northern Hemisphere (especially Palearctica) reach the northern and western edges of Micronesia as winter visitors. These include Larus argentatus, Chlidonias leucopterus, and Sterna hirundo. Another northern gull, Larus ridibundus, has been reported in the Marianas.
One bird of the North and Northcentral Pacific, Diomedea nigripes, reaches the northern Marianas where it has been taken at Agrihan. It is not unlikely that other birds of the North Pacific reach northern Micronesia as occasional visitors.
Species of oceanic birds which are restricted in their distribution to Polynesia and some adjacent islands and which range to Micronesia, either as visitors or residents, include Puffinus tenuirostris, P. nativitatis, Pterodroma rostrata, P. hypoleuca, Sterna lunata, and Procelsterna cerulea. The islands of the vast Pacific basin offer havens for many kinds of oceanic birds. Apparently there has been considerable speciation among sea birds in Polynesia, especially in its marginal areas. Micronesia has received only a small part of this avifauna.
Two terns, Sterna sumatrana and Thalasseus bergii, have reached Micronesia, either directly or indirectly, each from a dispersion point somewhere in the Melanesian or the Malayan area. These two birds are restricted in their ranges to the western Pacific and the Indian oceans.
Many of the species of oceanic birds found in Micronesia have circumtropical ranges. These include Puffinus pacificus, P. lherminieri, Phaëthon, Sula, Fregata, Sterna anaethetus, S. fuscata, Anoüs stolidus, A. tenuirostris, and Gygis alba. Some of these kinds range along continental shores as well as in island archipelagoes. Others, like Gygis alba, are rarely found along the shores of continents or even at coastal islands.
Twenty-eight species of shore birds of the families Charadriidae and Scolopacidae have been recorded from Micronesia, and one other of the family Phalaropodidae apparently occurs in the area, making a total of 29 kinds. From the entire Southwest Pacific, Mayr (1945a:28-47) lists 31 species and subspecies of shore birds and mentions six other species which may occur there. Thus, of a possible 37 kinds of shore birds in this large area (which includes Micronesia), 29 are present in the islands of Micronesia. For purposes of discussion, shore birds are here placed in one of two groups: regular visitors or uncommon visitors. A regular visitor is one which has been recorded in the literature or in unpublished field accounts as being frequently observed in Micronesia in periods of migration. An uncommon visitor is one which has been infrequently observed in Micronesia. Of the 28 kinds of shore birds recorded from Micronesia, 17 are classed as regular visitors and 11 are classed as uncommon visitors.
| Class | Number | Circum- polar[A] |
Asiatic | American |
| Regular visitors | 17 | 5 | 10 | 2 |
| Uncommon visitors | 11 | 2 | 8 | 1 |
| Totals | 28 | 7 | 18 | 3 |
Part B. Location of wintering grounds
| Class | Number | Circum- tropical |
Asiatic | American | Oceanic |
| Regular visitors | 17 | 2 | 13 | 1 | 1 |
| Uncommon visitors | 11 | 1 | 8 | 2 | 0 |
| Totals | 28 | 3 | 21 | 3 | 1 |
[A] Denotes birds which breed on both American and Asiatic sides of the Pacific Ocean.
The shore birds which are known to visit Micronesia breed in the Northern Hemisphere. Table 3 summarizes the data concerning the [Pg 33] breeding and wintering areas of these birds. As shown in part A of table 3, 18 of the 28 species which visit Micronesia come from Asiatic breeding grounds. Seven have circumpolar breeding ranges and three (two are regular visitors) come from American breeding grounds. As shown in part B of table 3, 21 of the 28 waders have their winter ranges on the Asiatic side of the Pacific with eastern extensions to Micronesia and other parts of Oceania. Of the 7 remaining species, the winter ranges of three are circumtropical; the winter range of a fourth is restricted to Oceania; and the winter ranges of the remaining three (two classed as uncommon visitors) are American.
Bryan and Greenway (1944:109-115) record 14 species of shore birds from the Hawaiian Islands. One of these, Himantopus himantopus knudsoni, is a resident, probably of New World origin, according to Mayr (1943:56). The others, listed in table 7, include three species unknown in Micronesia. One of these, Phalaropus fulicarus, apparently winters at sea off the west coast of South America. The other two species (Charadrius vociferus vociferus and Gallinago delicata) are classified by Bryan and Greenway as "accidental" and "occasional" visitors from North America. The ten species common to both the Hawaiian Islands and Micronesia include seven whose breeding grounds are circumpolar, two whose breeding grounds are in Arctic America and one whose breeding ground is in Arctic Asia. The winter ranges of these ten species include four which are circumtropical, three which are Asiatic, one which is restricted to Oceania, and only two which are American.
The ability of the shore birds to migrate almost as well over water as over land may explain their spread into Oceania. The likelihood that shore birds, when migrating may have ventured to Micronesia and Polynesia initially from the Asiatic side of the Pacific is strongly suggested by the data given in the paragraph above. Also, on the Asiatic side of the Pacific there are large numbers of islands, which form several archipelagoes extending from Kamchatka south to Malaysia. Once accustomed to migrating along these chains of islands from the Arctic to Australia, birds would probably have to make only minor adjustments to extend the breadth of their migratory routes eastward into the islands of the Pacific Ocean. In contrast, on the Pacific coast of North America there are few coastal or offshore islands and there is a vast area of open water separating the Hawaiian Islands from the American mainland. Probably the vastness of this area of water offers little stimulus to birds to expand [Pg 34] their migratory ranges westward, and in part accounts for the small North American contingent in the population of shore birds of the Central Pacific. Some North American shore birds do visit the Pacific. The brisk trade winds from the northeast might be an aid to the birds in their flights from Nearctica to Hawaii.
The long flight now made by shore birds going from the Aleutians to the Hawaiian Islands may have commenced as a gradual expansion from the west, or perhaps such a route was initiated by birds flying northward through the Hawaiian Chain to the Arctic in migrating to their breeding grounds, and then later returning via the same route to reach their wintering grounds.
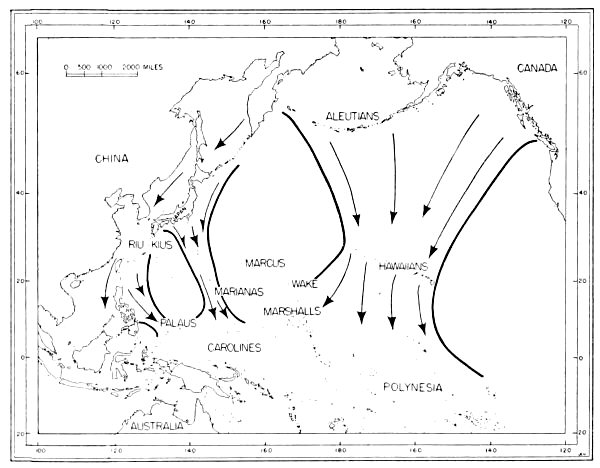 Fig. 6. Routes of migration used by shore birds in the Pacific area. From west to east these are: The Asiatic-Palauan Flyway, the
Japanese-Marianan Flyway, the Nearctic-Hawaiian Flyway.
Fig. 6. Routes of migration used by shore birds in the Pacific area. From west to east these are: The Asiatic-Palauan Flyway, the
Japanese-Marianan Flyway, the Nearctic-Hawaiian Flyway.The small and isolated islands of Oceania might, upon first inspection, seem to offer but little attraction to shore birds. Hesse, Allee and Schmidt (1937:172, 173) point out that the "open southeastern Pacific" being least supplied with water from land sources, which is an important means of fertility, is known to have one of the poorest faunas found anywhere in the oceans. However, there are extensive tidal flats, especially on the leeward sides of the islands, and these [Pg 35] flats apparently afford extensive feeding grounds for these birds. Also, the absence of competition from resident birds as well as the virtual absence of predatory animals (native man and his domesticated animals excepted) are other factors which may help to make the islands attractive wintering grounds for shore birds.
Only a few birds have been banded in the Pacific, and the knowledge which comes from the recovery of banded birds gives but little aid to the student of movements of birds in the Pacific. The probable flyways for migratory shore birds there have to be deduced from sight records, data from specimens collected, known stations of breeding and wintering (summarized by Peters, 1934:234-293), and from a study of maps of the region. Analysis of information from the above-mentioned sources indicates that there are three routes taken by shore birds which migrate from Micronesia to and from their northern breeding grounds (see figure 6): (1) Asiatic-Palauan Flyway; (2) Japanese-Marianan Flyway; (3) Nearctic-Hawaiian Flyway.
1. Asiatic-Palauan Flyway. For shore birds, there appears to be a migration route extending almost due south from the Riu Kiu and the Japanese islands to the Palau Islands. Some birds may migrate via the Philippines and others may pass to the east of the Philippines. This route is considered to be distinct from that used by birds which follow the Asiatic Coast and coastal islands, because the Palau Islands are situated approximately 600 miles east of the Philippines. Moreover, there are fewer species—only 20 recorded from the Palaus as compared with the number recorded from islands closer to the mainland of Asia. Delacour and Mayr (1946:68-74) list 46 species of shore birds from the Philippines; the Hand-list of Japanese Birds (Hachisuka et al, 1942) lists 34 species from the Riu Kiu Islands.
The information available indicates that migrant shore birds which utilize this flyway move east into the Carolines (examples, Tringa nebularia, Charadrius leschenaultii); however, the recording of 20 species from the Palaus as compared with only 12 species in the western Carolines (table 4) indicates that this spread eastward may not be very pronounced. Migrants in autumn probably move from the Palaus in a southerly direction toward the New Guinea area. Eight species of shore birds which reach the Palaus (and adjacent islands in the western Carolines), are not recorded from other parts of Micronesia. Species which apparently utilize the Asiatic-Palauan Flyway are listed in table 5.
2. Japanese-Marianan Flyway. Shore birds from Asiatic, and probably Aleutian and Alaskan, breeding grounds may follow the Asiatic Coast or the adjacent island chains southeast to the Japanese Archipelago. From there some of the birds apparently fly south through the Bonin and Volcano islands to the Marianas, from where they may spread in fanlike fashion to the southeast, south and southwest, even reaching to the Palau Islands (example, Heteroscelus incanus). The number of species of shore birds recorded from the Marianas (see table 4) is greater than that found in the Carolines, but it must be remembered that more intensive investigations have been made by ornithologists in the Marianas, which might account for the recording of more species (especially stragglers, such as Gallinago gallinago). Species which apparently use this flyway are named in table 6.
3. Nearctic-Hawaiian Flyway. Shore birds from breeding grounds in western Canada, Alaska, the Aleutians, the Bering Sea area, and probably northeastern Asia may fly in a southerly direction along a broad front to the Hawaiian Islands. This flyway is probably the one which supplies to central and eastern Oceania the largest wintering populations of shore birds. From the Hawaiian Islands birds may fly directly south through the scattered islands to southern Polynesia, or they may fly in a southwesterly direction and reach the Marshall Islands. The shore birds which visit the Marshall Islands apparently move south through the Gilbert, Ellice and other more southern island groups rather than west into the Carolines as exemplified by the fact that Numenius tahitiensis, a characteristic migrant through the Marshalls from the Hawaiian Islands, is rarely found west of the Marshall Islands in Micronesia. Species which apparently use this flyway are listed in table 7.
Flyways additional to the three suggested above may be utilized by some shore birds on their southward (and northward) migrations. Species reaching Wake and the Marcus Islands may fly directly south from the islands of the North Pacific. Bryan (1903:115, 116) lists four species of shore birds from Marcus (Erolia acuminata, Heteroscelus incanus, Pluvialis dominica, Arenaria interpres).
| Palaus | Western Carolines |
Marianas | Central Carolines |
Eastern Carolines |
Marshalls | |
| Number of species | 20 | 12 | 17 | 11 | 10 | 10 |
| Regular Visitors | Uncommon? Visitors | |
| Pluvialis dominica fulva | Charadrius dubius curonicus | |
| Charadrius mongolus stegmanni | Charadrius alexandrinus | |
| Charadrius leschenaultii | Calidris tenuirostris | |
| Numenius phaeopus variegatus | Erolia ferruginea | |
| Numenius madagascariensis | Erolia subminuta | |
| Limosa lapponica baueri | Limicola falcinellus sibirica | |
| Tringa nebularia | ||
| Tringa glareola | ||
| Actitis hypoleucos | ||
| Heteroscelus brevipes | ||
| Arenaria i. interpres | ||
| Gallinago megala | ||
| Erolia minuta ruficollis | ||
| Erolia acuminata |
| Regular Visitors | Uncommon? Visitors | |
| Pluvialis dominica fulva | Squatarola squatarola | |
| Charadrius mongolus stegmanni | Numenius tahitiensis | |
| Numenius phaeopus variegatus | Numenius madagascariensis | |
| Limosa lapponica baueri | Tringa glareola | |
| Actitis hypoleucos | Gallinago gallinago gallinago | |
| Heteroscelus brevipes | Erolia minuta ruficollis | |
| Heteroscelus incanus | ||
| Arenaria i. interpres | ||
| Gallinago megala | ||
| Crocethia alba | ||
| Erolia acuminata |
| Regular Visitors | Uncommon? Visitors | |
| Pluvialis dominica fulva* | Squatarola squatarola* | |
| Numenius tahitiensis* | Charadrius hiaticula semipalmatus[+] | |
| Heteroscelus incanus* | Charadrius v. vociferus | |
| Arenaria i. interpres* | Limosa lapponica baueri* | |
| Crocethia alba* | Tringa melanoleuca*[+] | |
| Phalaropus fulicarius | Gallinago delicata | |
| Phalaropus lobatus*? | Erolia melanotos* | |
| Erolia acuminata* |
* Indicates species which are found in Micronesia.
[+] Indicates species not recorded from the Hawaiian Islands; see Bryan and Greenway (1944:109-115).
Populations of Shore Birds in Micronesia
Although shore birds have been observed in Micronesia on many occasions, actual counts of numbers of individuals of the different birds have rarely been made. Kubary, Finsch, Marche, Seale and other early collectors and observers record some data of this kind [Pg 38] as have the Japanese investigators in later times. William Coultas of the Whitney South Sea Expedition obtained considerable information of this nature at Guam, Saipan, Kusiae, Ponapé, and the Palaus, but it is unpublished. His records were made in fall, winter and spring, when migrants were present in large numbers and these observations offer evidence that many of the migrants are comparatively numerous, especially in the Carolines, throughout the winter months. McElroy's observations made on his trip for NAMRU2 to Truk in December, 1945, offer further evidence of this.
| Chara- drius mongolus |
Pluvialis dominica |
Numenius phaeopus |
Actitis hypo- leucos |
Hetero- scelus spp. |
Hetero- scelus incanus[+] |
Hetero- scelus brevipes[+] |
Limosa lapponica |
Arenaria interpres |
Uniden- tified |
Total No. of indivi- duals |
Total No. of species |
|
| March 11 | x | x | 1 | |||||||||
| March 17 | 10 | 1 | 2 | 13 | 3 | |||||||
| March 19 | x | x | x | x | x | 5 | ||||||
| April 24 | x | x | 1 | |||||||||
| April 26 | 1 | 1 | 1 | |||||||||
| May 19 | 3 | 2 | 5 | 1 | ||||||||
| May 21 | 4 | 4 | 1 | |||||||||
| May 26 | x | 2 | x | 1 | ||||||||
| June 1 | 1 | 1 | 1 | |||||||||
| June 6* | 1 | x | x | 1 | x | x | 4 | |||||
| June 11 | 1 | 1 | 1 | |||||||||
| June 12 | 12 | 2 | 14 | 2 | ||||||||
| June 22 | 2 | 1 | 3 | 2 | ||||||||
| June 30 | 2 | 2 | 1 | |||||||||
| July 7 | 2 | 2 | 1 | |||||||||
| July 8 | 3 | x | 1 | x | 3 | |||||||
| July 16* | 6 | 3 | 3 | 4 | 1 | 17 | 4 | |||||
| July 19 | x | x | x | x | 3 | |||||||
| July 24* | 10 | 6 | 3 | 2 | 3 | 5 | 29 | 5 | ||||
| July 26 | 8 | 8 | 1 | |||||||||
| August 2 | x | x | x | 2 | ||||||||
| August 3 | 1 | 1 | 1 | |||||||||
| August 6* | 6 | 12 | 18 | 2 | ||||||||
| September 29 | x | x | x | x | 2 | |||||||
| October 3* | x | x | x | 2 | ||||||||
| October 10 | x | x | x | 2 | x | x | 4 | |||||
| October 11 | 2 | 2 | 1 | |||||||||
| October 23* | x | x | x | 1 | 1 | x | x | 5 | ||||
| October 24 | x | x | 1 |
* Observations made on beach at Agfayan Bay area.
x Observed but numbers not recorded.
[+] Figures based on identified skins.
None of the above workers, however, obtained very much information on comparative numbers of species.
Tables 8, 9, and 10 present the writer's findings on populations of migratory shore birds in Micronesia in 1945. At Guam, as shown in table 8, the records for March, April and early May are few, owing to a limited amount of field observation. Beginning in late May and until October 24 a greater amount of time was spent in the field and more regular records were obtained. No observations were made by the author at Guam in the period from August 11 to September 25. The dates marked with an asterisk are those on which observations were made on the extensive tidal flats at Agfayan Bay and vicinity. These flats, at low tide, present excellent feeding grounds for waders and in 1945 were undisturbed by parties of service personnel, because the area was "off-limits."
Table 8 shows that Pluvialis dominica, Numenius phaeopus, and Heteroscelus spp. were the shore birds most frequently found at Guam in this period. Pluvialis dominica was the most numerous of the three species. Of Heteroscelus there was approximately equal representation of H. incanus and H. brevipes as indicated by specimens collected. These birds were not identified to species in the field.
Although records were made only infrequently in the spring migration, such information as was obtained indicates that the populations were largest in March and early April. On April 24, Pluvialis dominica was the only bird observed on beaches and in upland openings. On April 26, a single Limosa lapponica was recorded. On May 15, no shore bird was seen on a trip along several beaches. In late May and early June, single individuals of Heteroscelus were found. Of this genus, those collected in May were in nuptial plumage, and those collected in June were in winter plumage and probably should be classed as non-migrants. Numenius phaeopus was occasionally recorded beginning in early June, but waders were totally absent from beaches at Agfayan Bay and vicinity on June 18 and 19. Few shore birds were seen in early August. In late September, birds, especially Pluvialis dominica, Numenius phaeopus, and Heteroscelus spp., were numerous. These species were numerous until October 24, when observations were discontinued.
Of the 17 species of migratory shore birds recorded from the Mariana Islands, eight were identified. Of these eight, three species, Limosa lapponica, Actitis hypoleucos, and Charadrius mongolus, were found on only one occasion. Never more than four species [Pg 40] were identified on a single field trip. These data give an idea of the lack of variety of species that may be observed on Micronesian islands.
| Species | Island and Date | |||||||
| Potangeras | Fas- sari |
Mange- jang |
Pau | Losiep | ||||
| Aug. 14 |
Aug. 15 |
Aug. 16 |
Aug. 17 |
Aug. 19 |
Aug. 20 |
Aug. 21 |
Aug. 22 |
|
| Pluvialis dominica | 6 | 5 | 4 | 10 | 5 | |||
| Charadrius mongolus | x | 2 | ||||||
| Numenius phaeopus | 1 | 4 | 1 | 1 | 2 | |||
| Actitus hypoleucos | 2 | 2 | ||||||
| Heteroscelus spp. | 2 | 6 | 3 | |||||
| H. incanus* | 1 | 2 | ||||||
| Crocethia alba | 30 | 5 | ||||||
| Total No. of Individuals | 1 | 4 | 6 | 6 | 6 | 1 | 49 | 21 |
| Total No. of Species | 1 | 1 | 1 | 2 | 2 | 1 | 6 | 6 |
* Figures based on identified skins.
x Observed but numbers not recorded.
Table 9 lists the shore birds seen at Ulithi Atoll, Caroline Islands, on eight field excursions in the period from August 14 to August 22, 1945. Of seven species of shore birds known to visit the atoll, six were taken in this period. As observed at Guam, Pluvialis dominica and Numenius phaeopus were the species most frequently found. Heteroscelus was seen on three occasions; those collected were identified as H. incanus. Most of the shore birds were seen at Pau and Losiep, islands unoccupied by man. Similar tidal flats are present at most of the other small islands in the atoll, but these islands (Asor, Fallalop, Potangeras, Fassarai and Mangejang were visited) were occupied by small detachments of service personnel or by natives, which may have tended to keep many of the shore birds away. At the more populated islands of Asor and Fallalop, no shore birds [Pg 41] were seen. Almost as many species were recorded at Ulithi on the eight field trips as were found by the author at Guam in eight months of observations.
| Species | Peleliu | Angaur | |||||||
| August | September | Sept. 21 |
|||||||
| 24 | 28 | 1 | 6* | 8* | 9[+] | 16* | 20* | ||
| Pluvialis dominicaa | x | x | x | 25 | 20 | x | x | ||
| Charadrius mongolu | x | 25 | 5 | x | x | ||||
| C. leschenaultii | x | 25 | 5 | x | x | ||||
| Numenius phaeopus | 3 | x | 30 | 20 | x | x | |||
| N. madagascariensis | 1 | 1 | 15 | ||||||
| Limosa lapponica | 3 | 4 | |||||||
| Tringa nebularia | 6 | 3 | |||||||
| T. glareola[++] | 1 | ||||||||
| Actitis hypoleucos | 2 | ||||||||
| Heteroscelus sp. | x | x | 75 | x | x | x | |||
| H. brevipes[++] | 3 | 2 | 2 | ||||||
| Arenaria interpres | 20 | ||||||||
| Capella megala | 4 | ||||||||
| Calidris tenuirostris | 15 | 20 | |||||||
| Erolia minuta | x | 50 | 50 | x | x | ||||
| E. acuminata[++] | 3 | ||||||||
| E. ferruginea[++] | 1 | ||||||||
| Limicola falcinellus[++] | 1 | ||||||||
| Unidentified | x | x | x | x | x | x | |||
| Total No. of Individuals | x | 6 | x | x | 271+ | 3 | 129+ | x | x |
| Total No. of Species | 1 | 1 | 3 | 7 | 10 | 2 | 9 | 7 | 10 |
* Observations made on beaches at Akarakoro Point, Peleliu.
x Observed but numbers not recorded.
[+] Observations made at fresh water ponds.
[++] Figures based on identified skins.
Table 10 presents field counts at the Palau Islands in the period from August 24 to September 21, 1945. Of 20 species of shore birds known from the Palaus, 17 species were collected or identified on this trip. It was apparent that the fall migration was at its height at this time. Birds were numerous at inland openings and ponds, air field strips, and on the extensive tidal flats at Akarakoro Point. The latter area is between Peleliu and the adjacent island of Ngesebus to the north. Several observations were made at this area (as indicated by the dates marked with asterisks in the table); on September 8, 271+ shore birds were counted; on September 16, [Pg 42] 129+ were counted. Six species were observed to be abundant. The majority of the birds found at these beaches were in small flocks which consisted of several birds of one or more species.
The birds observed at Angaur on September 21 were seen at several fresh and brackish ponds. Four species (Tringa glareola, Erolia acuminata, Limicola falcinellus, Gallinago megala), which were not taken on the tidal flats or elsewhere at Peleliu, were found at these ponds.
The abundance, and more especially the variety, of shore birds at the Palau Islands during this period was in marked contrast to the smaller and less diversified populations of shore birds in rather similar insular environments at Ulithi and Guam. These differences offer support for the supposition that the Asiatic-Palauan Migratory Shore Bird Flyway is distinct from the Japanese-Marianan Migratory Shore Bird Flyway.
The land and fresh-water avifauna of Micronesia consists of 147 kinds of birds. Of these, 37 kinds are non-residents, 104 kinds are residents, and 6 kinds have been introduced by man. The 104 resident birds include 98 kinds (94 percent) which are found only within the confines of Micronesia. Included in these 98 kinds which are restricted to Micronesia are 5 endemic genera, 31 endemic species and 76 endemic subspecies.
Gulick (1932: 407, 413) stresses that the fauna and flora of the oceanic islands may be "disharmonic" (he uses Easter Island as his example) and says, "It is evident that mature groups of islands will attain an internal harmony, from the standpoint of the systematist. But this harmony, instead of reflecting the pre-existing harmony of some continental source (as in the case of the continental islands or land-bridge remnants) will be recognizably derivable by descent from a quite limited number of original importations, at the start distinctly miscellaneous and 'disharmonic'." Analysis of the land and fresh-water avifauna of Micronesia supports Gulick's view.
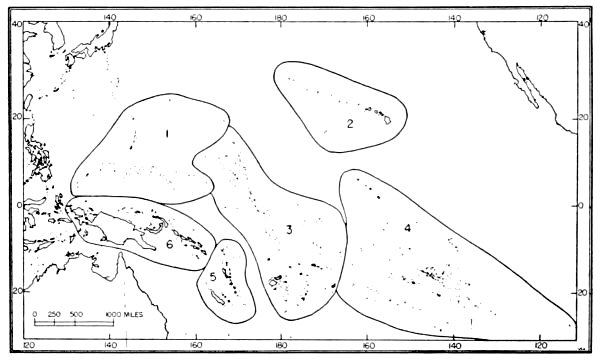 Fig. 7. Divisions of the islands of part of the Pacific Basin from the standpoint of the distribution of land birds and
fresh-water birds: (1) Micronesia; (2) Hawaii; (3) Central Polynesia; (4) Eastern Polynesia; (5) Southern Melanesia; (6) Melanesia.
Fig. 7. Divisions of the islands of part of the Pacific Basin from the standpoint of the distribution of land birds and
fresh-water birds: (1) Micronesia; (2) Hawaii; (3) Central Polynesia; (4) Eastern Polynesia; (5) Southern Melanesia; (6) Melanesia.As mentioned previously, the islands of Micronesia, from the zoogeographical viewpoint, have been regarded as a part of the Polynesian Subregion of the Australian Region. Mayr (1941a: 192) defines the Polynesian Subregion as comprising "all the tropical and subtropical islands of the Pacific Basin which indicate by their impoverished fauna that they have had no recent continental connection (after early Tertiary) and which derived the major part of [Pg 43] their fauna directly or indirectly from the Papuan Region or jointly from Australia and the Papuan Region." As based on the distribution of the resident avifauna, Mayr (1941a:193) subdivides the Polynesian Subregion into the following districts: Micronesia ("including Palau, the Marianne, Caroline, Marshall, and Gilbert islands"); Central Polynesia ("including Fiji, Tonga, Samoa, Phoenix, Ellice, Union islands, and a number of small islands, such as Rotuma, Fotuna, Keppel, Niue, Niouafu, and Uvea"); Eastern Polynesia ("all the islands east of 165° W"); and Southern Melanesia ("including the Santa Cruz group, Banks Islands, New Hebrides, Loyalty Islands, and New Caledonia"). He considers that the Hawaiian Islands, Solomon Islands, and possibly New Caledonia are bordering districts to the Polynesian Subregion. Figure 7 shows the divisions of the islands of the Pacific Basin from the standpoint of the distribution of the land and fresh-water birds. I have placed the Gilbert and Marshall islands in the Central Polynesian rather than in the Micronesian District. For purposes of discussion in this report, however, I am considering the Marshalls to be a part of Micronesia. The birdlife of the Bonin and Volcano islands northward of the Marianas is regarded as having its closest affinities to the Japanese avifauna. The Papuan or Melanesian Subregion of the Australian Region includes the districts of New Guinea and [Pg 44] Northern Melanesia, including the Bismarck Archipelago, the Admiralty Islands, and the Solomon Islands.
The resident land and fresh-water birds of Micronesia have been derived from several sources. Studies of these birds and their closest relatives in adjacent areas indicate that the avifauna has been derived from five different sources: Polynesia, Melanesia, the Moluccas and Celebes, Philippines, and Palearctica.
Aphanolimnas monasa (extinct?), Ptilinopus porphyraceus, and Ducula oceanica are the only species of birds which have reached Micronesia directly from Polynesia. There are in Micronesia, as Mayr (1941b: 204) points out, eight species "which are members of typically Polynesian species or genera" and six species which are either Papuan or Polynesian. The relationships between Polynesian and Micronesian birds is evident, but insofar as the pathways of colonization are concerned the majority of these Micronesian species listed by Mayr have come from elsewhere than Polynesia and the birds of these two areas are thought to have arisen from common ancestors. Aphanolimnas, Ptilinopus, and Ducula apparently invaded Micronesia from Central Polynesia via the Marshall Islands through a rather continuous chain of islands and atolls. Aphanolimnas is known only from Kusaie in the extreme eastern part of the Carolines while Ptilinopus and Ducula are known from the Marshalls, Carolines, and Palaus.
The Papuan or Melanesian Region (New Guinea, Bismarck Archipelago, Solomon Islands) has supplied to Micronesia its greatest number of endemic land and fresh-water residents. Fifty kinds of birds belonging to the following species reached Micronesia from Melanesia: Nycticorax caledonicus, Megapodius lapérouse, Ptilinopus roseicapillus, Gallicolumba xanthonura, G. canifrons, Caloenas nicobarica, Halcyon cinnamomina, Trichoglossus rubiginosus, Collocalia inquieta, Edolisoma tenuirostre, Rhipidura rufifrons, Metabolus regensis, Monarcha godeffroyi, M. takatsukasae, Colluricincla tenebrosa, Aplonis opacus, A. pelzelni, A. corvinus (extinct?), Cleptornis marchei, Myzomela cardinalis (probably by way of Southern Melanesia), Rukia palauensis, R. oleaginea, R. ruki, R. sanfordi, Erythrura trichroa. The colonization of Micronesia by these species has probably extended over a considerable period of time. Megapodius, Trichoglossus, and Aplonis corvinus may represent older [Pg 45] colonizations which have become well differentiated from the ancestral forms; Nycticorax, Myzomela, and Erythrura may have become established later and have had "less time" to become modified from the ancestral forms. Birds from Melanesia have reached Micronesia probably by direct flight to the Caroline Islands. Aided by favorable winds which blow from the southwest, south and southeast during the period from May to November, birds, particularly the young of the year, might conceivably be blown in the direction of the Carolines, where 57 percent of the birds derived from Melanesia reside. The Palaus are populated with 15 percent, the Marianas with 28 percent, and the Marshalls (lacking "high" islands) with none; these may be secondary colonizations from the Carolinas excepting Ptilinopus, Megapodius, Gallicolumba canifrons, Cleptornis, and Colluricincla. The Marshall Islands have received no avian components from Melanesia. The absence of "high" islands in the Marshalls and the possible inability of birds accustomed to life on the luxuriant islands of Melanesia to become established on relatively barren atolls are logical reasons for this. Instead of New Guinea itself, the outlying islands of Melanesia (Bismarck Archipelago, Solomons, Southern Melanesia) probably have been the principal "taking-off" places for birds invading Micronesia.
Birds which reached Micronesia by way of the islands of Celebes and the Moluccas may have been derived originally from Melanesia. The following birds appear to have used this route: Porphyrio porphyrio, probably Halcyon chloris, Rhipidura lepida, Myiagra oceanica, Zosterops conspicillata, and Z. cinerea. These birds apparently became established initially in the Palaus; Porphyrio and Rhipidura lepida have not been recorded elsewhere in Micronesia, but Myiagra and the two species of Zosterops have spread to the Carolines and Marianas, although not into the Marshall Islands. Wind from the southeast in summer and fall has probably been a factor aiding these colonizations. The population of Gallinula chloropus resident at Palau may also have arrived by this route.
Ten of the kinds of birds of Micronesia have come from or by way of the Philippine area. These are known principally from the Palaus and the Marianas and include: Rallus philippinus, R. owstoni, Poliolimnas cinereus, Caprimulgus indicus, Corvus kubaryi, Psamathia annae, Artamus leucorhynchus, possibly Lonchura nigerrima, and [Pg 46] Collocalia inexpectata. The Philippines may have been the actual point of dispersal of the birds (example, Psamathia), or may have been used as a stepping stone to Micronesia by birds coming from Melanesia (examples, Rallus and Artamus), by birds from Malaysia (example, Collocalia), and by birds from Asia (example, Caprimulgus). Two birds of this component have reached the islands of eastern Micronesia. A subspecies of Lonchura nigerrima is endemic at Ponapé, and a subspecies of Poliolimnas cinereus occurs on several islands in the Carolines and has even been recorded at Bikini in the Marshall Islands. Three species are known only from the Palaus; two are known only from the Marianas.
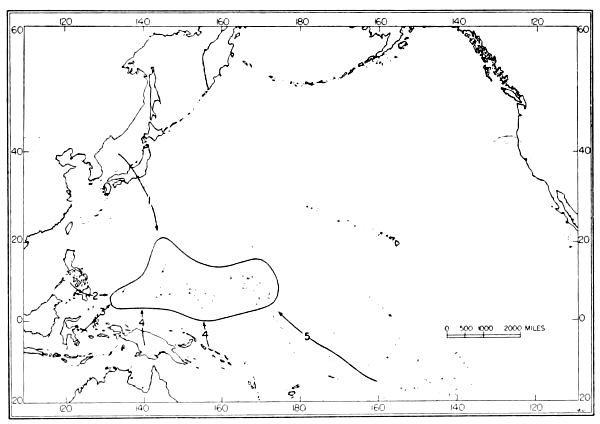 Fig.
8. Faunal areas from which the resident land birds and fresh-water birds of Micronesia have been derived. (1) Palearctica; (2) Philippines; (3) Moluccas and Celebes (Malaysia); (4)
Melanesia (New Guinea and northern Melanesia); (5) Polynesia.
Fig.
8. Faunal areas from which the resident land birds and fresh-water birds of Micronesia have been derived. (1) Palearctica; (2) Philippines; (3) Moluccas and Celebes (Malaysia); (4)
Melanesia (New Guinea and northern Melanesia); (5) Polynesia.Birds of Micronesia which have been derived directly from Palearctica are Gallinula chloropus guami, Otus podarginus, Asio flammeus, Acrocephalus luscinia and Anas oustaleti. Apparently Gallinula, Asio, and Acrocephalus arrived in Micronesia by way of the chain of islands from Japan southward to the Bonins, Volcanoes, and Marianas. Otus reached Palau from Asia, possibly by way of the Philippines. The smallness of the representation of this component may result partly from lesser ability of the northern birds [Pg 47] to adapt themselves to, and to establish themselves on, the semi-tropical and tropical islands of Micronesia as compared with birds from Melanesia where the climate and ecologic conditions resemble more closely those found in Micronesia. Evidence supporting this possibility is the large number of Palearctic residents in the Bonin and Volcano islands as compared with fewer in the Marianas; the Bonins and Volcanoes are less tropical and more temperate in climate.
Table 11 lists the birds concerned, by faunal areas from which the birds have been derived and shows the number of kinds of birds which are present as a result of these colonizations. There is some overlap in the numbers since some endemics may be found in more than one area in Micronesia. Figure 8 shows the faunal areas from which the endemic land and fresh-water birds of Micronesia have been derived. Melanesia (Papua) supplied 52 percent of this population. Birds reaching Micronesia by way of the Moluccas and Celebes include 21 percent of the total population. The Philippines have supplied 10 percent; Polynesia, 9 percent; and Palearctica, 8 percent. This population of endemic land birds and fresh-water birds has seemingly evolved from 46 colonizations, of which 27 have been derived from Melanesia, 6 from the Philippines, 5 from the Moluccan and Celebean areas, 5 from Palearctica, and 3 from Polynesia.
The Palaus have received a large part of their avifauna from the west (Moluccas, Philippines, Palearctica). Their Melanesian component is mostly the result of secondary colonization from the Carolines. The Carolines have received a greater share of their land birds and fresh-water birds from Melanesia and a smaller share from Polynesia. The Marshalls are definitely associated with the Polynesian element. The Marianas exhibit a considerable amount of secondary colonization from other Micronesian islands, as well as some unique components from the Philippines, Melanesia, and Palearctica. Thus, the number of endemics in Micronesia provides little information concerning the actual number of successful colonizations by birds from other areas. Many of the endemics probably have resulted in this way: Individuals of an endemic subspecies flew to another island and there underwent further differentiation, producing another endemic subspecies. Such secondary colonization probably is going on now.
This analysis of the avifauna shows that Micronesia, with the exception of the Marshall Islands (and the Gilbert Islands), has [Pg 48] but little affinity to Polynesia. It has greater affinity, from the zoogeographical standpoint, with the Papuan Region (Melanesia).
| Faunal Component | Palau | Western and central Carolines |
Eastern Carolines |
Marianas | Marshalls |
| Polynesian | 2 | 3 | 5 | 0 | 3 |
| Melanesian | 11 | 14 | 16 | 12 | 0 |
| Moluccan-Celebean | 6 | 3 | 4 | 7 | 0 |
| Philippine | 6 | 2 | 2 | 4 | 1 |
| Palearctic | 2 | 1 | 2 | 5 | 0 |
| Totals | 27 | 23 | 29 | 28 | 4 |
Of the 104 native fresh-water birds and land birds which are resident in Micronesia, only 7 kinds or 6.5 percent remain undifferentiated from populations elsewhere. These birds are Phalacrocorax melanoleucus, Pandion haliaetus, Demigretta sacra, Ixobrychus sinensis, Anas poecilorhyncha, and possibly Lonchura punctulata (may be an introduction by man). Another bird, Gallinula chloropus, a resident at Palau, may or may not be distinct from the gallinule of Malaysia, G. c. orientalis. Of the 104 resident birds, 97 kinds or 93.5 percent have become differentiated and can be separated taxonomically from populations elsewhere. Of the kinds of birds which are found only in Micronesia, there are 5 endemic genera (16 percent), 31 endemic species (32 percent) and 76 endemic subspecies (75 percent). If we consider the avifauna of Micronesia as a single element, the endemism is high as compared with that on larger and less isolated islands. For example, Mayr (1944a:174) found 137 resident birds on Timor including 22 endemic species (16 percent) and 67 endemic subspecies (47.5 percent). Stresemann (1939b:313) found 220 species including 84 endemic species (38.2 percent) on Celebes. Mayr (1944a:174) also writes that on Java, of 337 breeding species, 16 (4.8 percent) are endemic, and on New Caledonia, of 68 species 19 (27.9 percent) are endemic. Speciation in Micronesia has not progressed much farther than that at New [Pg 49] Caledonia and not so far as at Celebes, but subspeciation has progressed considerably more than at the island of Timor. The avifauna of the Hawaiian Islands, as recorded by Bryan and Greenway (1944), has 73 resident land birds and fresh-water birds, all of which are endemic, including one family, 23 genera and 36 species. The North American night heron, Nycticorax n. hoactli, may be included in this list as the only resident which is undifferentiated. The development of full specific differentiation within the resident avifauna is greater in the more isolated Hawaiian chain where 49 percent of these birds are regarded as endemic species, while in Micronesia, which is less remote from other bodies of land, the specific endemism is only 32 percent.
| Family | Residents | Endemic genera |
Endemic species |
Endemic subspecies |
Total endemic |
| Phalacrocoracidae | 1 | 0 | 0 | 0 | 0 |
| Ardeidae | 3 | 0 | 0 | 1 | 1 |
| Anatidae | 2 | 0 | 1 | 0 | 1 |
| Accipitridae | 1 | 0 | 0 | 0 | 0 |
| Megapodidae | 2 | 0 | 1 | 2 | 2 |
| Rallidae | 7 | 1* | 2 | 4 | 6 |
| Columbidae | 13 | 0 | 4 | 11 | 13 |
| Psittacidae | 1 | 0 | 1 | 0 | 1 |
| Strigidae | 1 | 0 | 1 | 1 | 2 |
| Caprimulgidae | 1 | 0 | 0 | 1 | 1 |
| Apodidae | 5 | 0 | 1 | 5 | 5 |
| Alcedinidae | 7 | 0 | 1 | 7 | 7 |
| Apodidae | 3 | 0 | 0 | 3 | 3 |
| Corvidae | 1 | 0 | 1 | 0 | 1 |
| Sylviidae | 5 | 1 | 2 | 4 | 5 |
| Muscicapidae | 14 | 1 | 6 | 9 | 14 |
| Artamidae | 1 | 0 | 0 | 1 | 1 |
| Sturnidae | 9 | 0 | 3[+] | 7 | 9 |
| Meliphagidae | 7 | 1 | 1 | 6 | 7 |
| Zosteropidae | 14 | 1 | 6 | 10 | 14 |
| Ploceidae | 5 | 0 | 0 | 4 | 4 |
| Totals | 104 | 5 | 31 | 76 | 97 |
* Aphanolimonasa is included but may be extinct.
[+] Aplonis corvinus is included but may be extinct.
Table 12 lists the families of land birds and fresh-water birds which have resident members as part of the avifauna of Micronesia. It can be observed from the table that only two families are represented by no endemic kinds, several families are represented by one or two endemic kinds, and others are represented by as many as 14 [Pg 50] endemic kinds. Endemism has reached its greatest development in the families Rallidae (6), Columbidae (13), Apodidae (5), Alcedinidae (7), Sylviidae (5), Muscicapidae (14), Sturnidae (9), Meliphagidae (7), and Zosteropidae (14). Generic endemism is greatest in the Sylviidae where one endemic genus occurs among 5 endemic species and subspecies (20 percent), in Rallidae one in 6 (17 percent), in Meliphagidae one in 7 (14 percent). Specific endemism is greatest in Psittacidae and Corvidae where the single representative of each family in Micronesia is considered specifically distinct (100 percent), in Megapodidae and Strigidae one in 2 (50 percent), in Muscicapidae and Zosteropidae 6 in 14 (43 percent) in Sylviidae 2 in 5 (40 percent), in Rallidae 2 in 6 (33 percent), in Sturnidae 3 in 9 (33 percent) in Columbidae 4 in 13 (31 percent). Subspeciation within species which are endemic in Micronesia has occurred in 8 families, occurring within two species in each of the families Columbidae and Zosteropidae and once in each of the families Megapodidae, Apodidae, Alcedinidae, Sylviidae, Muscicapidae, and Sturnidae.
In summary, the families of land and fresh-water birds found in Micronesia which have the greatest number of endemic forms are Muscicapidae (14), Zosteropidae (14), Columbidae (13), and Sturnidae (9). Speciation has occurred in the single representative of the families Psittacidae (Trichoglossus rubiginosus) and Corvidae (Corvus kubaryi). Where family representation is large, speciation has occurred most frequently, as in the Muscicapidae (6 in 14 = 43 percent), in the Zosteropidae (6 in 14 = 43 percent), and in the Columbidae (4 in 13 = 31 percent). Subspeciation has occurred in 8 families, in two species in the Columbidae and Zosteropidae and in one species in each of 6 other families.
Previously (and in the accounts of the species to follow), comments are made concerning the subjects of from where and by what route the various kinds of birds have arrived at Micronesia. The problem of when these birds arrived is a difficult and usually unanswerable one. Although geology provides some evidence on the relative age of the islands, and although deposits of bird guano on now elevated coral islands show that oceanic birds have inhabited these islands for a long time, there is no evidence to show the time of the first colonization by land birds. No fossil remains of land birds or fresh-water birds have been found in Micronesia. The relative extent of differentiation in color and structure, which has [Pg 51] taken place between different birds, offers one means for estimating the relative length of residence in the area, provided all other factors are equivalent. Concerning the birds of the Galapagos, Lack (1947:113) writes "That Darwin's finches are so highly differentiated suggests that they colonized the Galapagos considerably ahead of the other land birds." Evidence from this source actually is of little value, because the speed of evolution is unknown and its rate may be different in different species, even though they live under the same circumstances. Dobzhansky (1941) says that evolution is a modification of the genetic equilibrium, which, if true, may not result in similar manifestations in different kinds of birds living under the same conditions of life. Relative antiquity of the birds might be ascertained by measuring their ecologic adaptations. The Guam Rail (Rallus owstoni) and the Micronesian White-browed Rail (Poliolimnas) can be examined in this way. R. owstoni has the ability to live in both brackish and fresh water swamps, as well as in the scrub and grass of the uplands and in the virtually barren, rocky areas in the dense jungles. Poliolimnas, on the other hand, appears to be restricted to swampy areas in Micronesia. If the swampy areas were removed this rail probably would become extinct. R. owstoni appears to have been resident in Micronesia longer than Poliolimnas. However, ability to live in a variety of habitats might be acquired by R. owstoni in a relatively short time.
Another possibility is that the birds, which are less differentiated from their ancestral stocks, may be less differentiated because of suppression of newly evolved characters by dilutions, which result from interbreeding with new birds, which may be arriving at irregular intervals from the ancestral home. Interbreeding of the resident population with newcomers may overshadow any modifications which might have appeared as a result of insular isolation, especially modifications which have little adaptive significance. One would suspect, from their modifications, that Rallus owstoni, Metabolus rugensis, Corvus kubaryi, and other endemic forms have experienced less of this "dilution," than such birds as Rallus philippensis pelewensis, Artamus leucorhynchus pelewensis, Myzomela cardinalis, and others. Murphy (1938) mentions this "dilution" effect in his discussion of "strong" and "weak" subspecies among warblers of the Marquesas. He writes that "strong" subspecies may develop if the birds are present on islands which are upwind from islands containing related subspecies. The wind acts to block interisland migration in these weak-flyers. On the other hand, "weak" subspecies may show the effect of "dilution," being situated on islands [Pg 52] downwind from islands containing related subspecies. The direction of the wind acts to aid the weak flyers to move to the downwind islands and continually "dilute" the resident subspecies. Similar examples can be cited for Micronesian birds. Hesse, Allee, and Schmidt (1937:87) write, "Endemism on islands is most frequent in forms for which the difficulty of reaching the island is most extreme, so that new increments of the parent form are unlikely to follow."
Employing the criteria mentioned above, the birds of Micronesia can be tentatively divided into four groups as regards the relative time when they arrived at the islands:
1. Birds of ancient colonizations which reached certain individual islands, became modified, and dispersed no farther. Examples are Aphanolimnas, Rallus owstoni, Aplonis corvinus, Metabolus rugensis, and Corvus kubaryi.
2. Birds of ancient colonizations which reached or dispersed through a number of islands but are now restricted to relatively few islands. Examples are Ducula oceanica, Ptilinopus porphyraceus, Megapodius lapérouse, Asio flammeus, and Acrocephalus luscinia.
3. Birds of ancient, or possibly more recent, colonizations which initially reached or subsequently dispersed to many of the islands of Micronesia possessing habitat suitable for them. Examples are Myzomela cardinalis, the two species of Halcyon, Aplonis opacus, and Zosterops conspicillata.
4. Birds of rather recent colonizations, which may have reached only a few islands and are relatively unmodified from their parental stocks. Examples are Artamus leucorhynchus, Caprimulgus indicus, Poliolimnas cinereus, and Nycticorax caledonicus.
Darlington (1938:274) in discussing the origin of the fauna of the Greater Antilles uses the term "over-water dispersal" in referring to the spread of terrestrial animals across water. He is against the use of the term "accidental dispersal" since many factors besides accident are involved. He contends, as do others, that certain forms of organisms, owing to their "nature and behavior" cross water barriers more successfully than others. These observations may be applied to the "over-water dispersal" of birdlife to the islands of Micronesia. Certain groups of birds are more evident in Micronesia than others. Certain groups of birds which are found on other islands of the Pacific basin are found in Micronesia only in small [Pg 53] numbers or may not be represented; Mayr (1945a:284) writes, "Remarkable is the almost complete absence of parrots and honey-eaters, the small number of pigeons and the absence of such widespread genera as Lalage, Turdus, and Pachycephala." The absence of some species and the presence of others produces the characteristic insular effect termed "disharmonic" by Gulick (1932:407), as compared with the continental area or island which derived its avifauna by way of a land bridge. One would think from looking at table 12 that members of the families Rallidae, Columbidae, Muscicapidae, Sturnidae, and Zosteropidae were the most successful colonizers in Micronesia on the basis of the number of successful colonizations (not necessarily on the number of endemics developed from a single colonization). Of these families, Sturnidae and Zosteropidae and possibly Columbidae contain species which often move in flocks. Furthermore, these families as well as the Muscicapidae feed on either fruits, seeds, or insects, any one of which is a type of food which might "give out" suddenly, stimulating a migratory behavior within the birds. From a flock embarking seaward in "search" of more food, a part or even all of the birds might survive in a chance flight to an isolated island in Micronesia. If a flock containing both males and females reaches an island, the species has a good chance of becoming established. Evidence that such a rapid colonization by flocks of birds can take place is found in the remarkable colonization of New Zealand by Zosterops lateralis from the Australian area. The bird was first seen as a winter migrant in New Zealand in 1856 and records of nestings were obtained at North Island in 1862, according to Oliver (1930:489). In the case of rails there is no evidence that they move in flocks; however, they are among the most successful colonizers and are on many of the oceanic islands in the tropical and subtropical oceans. Representatives of several species of the family Rallidae have invaded Micronesia and have successfully established 6, or possibly 7, "colonies."
Darlington (1938:274) further writes that "it is no accident that some islands, because of their nature and position, the direction of winds and currents, and the nature of the neighboring land, receive more organisms than other islands do." Semper (1881:294) writes that the distribution of flying creatures "must be in a great degree dependent on the direction and strength of atmospheric currents." These statements are applicable to the history of the avifauna of Micronesia. The Caroline Islands, for example, present a "broad front" for wanderers from the Melanesian islands. As mentioned [Pg 54] previously, the prevailing winds in the late spring, summer, and early fall are from the south, southwest, and southeast and would favor bird flight to the northward towards the Carolines. In addition, the breeding season of many of the birds in Melanesia is from November to February, and in the spring and summer, restless young birds seeking living space might fly seaward and aided by the winds fly northward towards Micronesia. Adults, which may have well-established home territories, may be less likely to attempt such a movement.
One could conclude from the above discussion that the Micronesian islands, especially the Carolines, might be well populated with a large variety of birds from Melanesia, a scant 500 or more miles away. As it turns out, there are only a few islands in this extensive archipelago possessing proper vegetation, fresh water, and other qualities which make them capable of supporting the land and fresh-water birds of Melanesia. The few islands which have these qualities are the so-called "high" islands, including the entire Mariana chain, the Palaus, and four widely separated islands in the Carolines: Yap, Truk, Ponapé, and Kusaie. The other islands of Micronesia are "low" coral islands, which often lack fresh water and have a meager variety of fruits, insects and other foods. Thus, if birds do reach Micronesia but arrive at the atolls instead of the "high" islands, these birds may be doomed. It is noteworthy that the Micronesian islands are small compared with the Solomons, Fijis, and others. The smaller the island, the fewer the number of ecologic niches and the fewer the kinds of birds present.
Mayr (1941b:215) writes that the distance from the nearest land mass and the climatic conditions are important factors controlling dispersal. With regard to the degree of remoteness of the islands, table 13 lists the number of resident land and fresh-water birds present in the Palaus and the "high" islands of the Carolines. Also, the approximate distance from the nearest large land mass and the area in square miles are given. There is some correlation between the distance from the nearest land mass and the number of resident land birds and fresh-water birds. For example, Palau, with 32 resident birds, is only 410 miles from the nearest land mass whereas Kusaie, with only 11 resident birds, is 720 miles from the nearest land mass. The comparative size of the land mass must also be taken into account, as shown by the fact that the large island of Ponapé contains more kinds of birds but is more remote from large land masses than either Yap or Truk.
| Island | No. of Birds |
Approximate distance from nearest land mass (statute miles) |
Nearest land mass | Area in square miles |
| Palau | 32 | 410 | Approximately equal distance from Mindanao, Morotai, New Guinea |
171 |
| Yap | 13 | 580 | New Guinea | 83 |
| Truk | 17 | 525 | New Ireland | 50 |
| Ponapé | 20 | 630 | New Ireland | 145 |
| Kusaie | 11 | 720 | Malaita (Solomons) | 42 |
Climatic factors are important in the dispersal of bird life; Micronesia, where the climate is tropical to subtropical, is better suited for colonization by birds from the tropics (Melanesia) than by birds from the temperate or cold climates (Palearctica). The climatic factor may be one of the principal reasons why birds from Palearctica make up only a small part of the avifauna of Micronesia.
The process of speciation within insular populations has been discussed by many authors. Hesse, Allee, and Schmidt (1937:517) list the motives for differentiation as, "Special character of insular faunae rests on the conditions common to all islands—isolation, freedom from competition, space restriction, and special insular climates." This combination of characteristics is seldom found elsewhere in nature, and as Murphy (1938:357) points out, an island is the nearest approach to a "man-controlled laboratory." Isolation of small populations is probably the most influential factor in the process of speciation in insular organisms. Lack (1947:134) writes that "in all organisms the isolation of populations is an essential preliminary to the origin of new species." Buxton (1938:265) also stresses this point with regard to the formation of species of insects in Samoa and emphasizes that evolution may occur more quickly in small populations. When mutations appear in such small and isolated populations, they have a greater chance to become fixed than do mutations in less restricted populations in a larger land mass, where such a mutation might be lost by the swamping effects [Pg 56] of outbreedings. In addition, Wright (1931 and elsewhere) suggests the possibility of change by accidental elimination and recombination of hereditary characters in micropopulations. This mechanism could well be a factor in Micronesian bird populations, many of which possess no more than a few hundred individuals. Huxley (1938:256) emphasizes that "accidental" mutations may be perpetuated in small, isolated groups. It might be added that such changes might be either advantageous or disadvantageous to the organism concerned. Huxley (1938:263) states also that geographic isolation may promote nonadaptive differentiation, which may be caused by "colonization by a random sample" or by subsequent "preservation of nonadaptive mutations in numerically small isolated groups." Mayr (1942b:237) cites the importance of the "founder" principal for reduced variability in small populations. He points out that if the "founders" of the population carried with them only "a very small proportion of the variability of the parent population," one would expect to see divergence from the ancestral stock.
Freedom from competition, especially interspecific strife, is an important factor in differentiation; this is especially true in the early period of colonization. Lack (1947:113) points to the absence of food competitors, especially in the initial period of colonization, as an important influence in the evolution of Darwin's finches at the Galapagos Islands. Once a population has become established and "adjusted" to a given environment on a small island, intraspecific competition might bring about adaptative selection. Subsequent colonists might be eliminated by the competition brought about by these previously adapted organisms, especially if both organisms were adapted for life in the same ecologic niche. Space restriction may be important in such Micronesian birds as Rhipidura and Myiagra, which appear to possess recognizable territories. A new colonist entering the territory of one of these birds might be forced out. This competition might not play such an important part among birds, which live in flocks and do not range in closely guarded territories; birds in this group include some pigeons, starlings, and white-eyes.
Freedom from the pressure of predation probably exerts a direct influence on formation of species. Aside from a few migrant hawks and two kinds of resident owls, most of the avifauna feeds on vegetable and invertebrate foods. The large lizard Varanus may be classed as the only native predator on many of the islands. Man has been responsible for the introduction of rats, house cats, and other [Pg 57] mammals, which may be destructive to birds. Thus, before the advent of man the factor of predation may not have been of great consequence. As mentioned previously, nonadaptive modifications may be perpetuated where the "weeding-out" process by predation is not an influence. Flightless rails have apparently developed in the absence of predation.
The absence of the pressure of predation should remove a certain amount of control on the population turn-over. As Hesse, Allee, and Schmidt (1937:521) write, a characteristic of the faunas of oceanic islands is the fact that they are distinguished by the occurrence of "disproportionately developed taxonomic groups in which one or a few basic types have undergone adaptative radiation and come to fill unduly large proportions of the population as compared with conditions that obtain on neighboring continents." Lack (1947:114) writes, "that the absence of predators may well have accelerated the adaptative radiation" in the Galapagos finches. In Micronesia, the starling (Aplonis opacus) dominates much of the available habitat on some of the Caroline atolls, and even on "high" islands, where other land birds are present. There appears to be no tendency towards selective adaptations occurring, or towards ecologic isolation.
Available data indicate that the life spans of individual birds in Micronesia may be short. For example, it was obvious on many of the islands visited by the NAMRU2 party that starlings (Aplonis opacus) in immature plumage outnumbered starlings in adult plumage, although it is possible that immature plumages are retained longer in these island birds than in others. Similar observations were made by Coultas, who noted the ratio of birds in immature plumage to birds in adult plumage at Kusaie to be 5 to 1. If the life span is shorter in these insular forms as compared with that of the ancestral stocks, the higher annual population turn-over would allow for the speed of genetic changes to be accelerated.
The origin of species by hybridization between different kinds of organisms has been a subject of frequent discussion. Lack (1947:100) concludes that it is improbable that hybridization has played an important part in the origin of new kinds of birds. Nevertheless, the absence of sufficient mates in the confines of a small island probably stimulates the crossbreeding between two species of birds. Fertile offspring of such a cross might conceivably account for some of the populations, the origins of which are puzzles to present day taxonomists. Such Micronesian forms as Metabolus and Cleptornis could conceivably have been derived in such a manner. Yamashina (1948) has described the origin of Anas oustaleti as a result of [Pg 58] hybridization between A. platyrhynchos and A. poecilorhyncha. It might be difficult to explain every case of the formation of other insular species on the basis of the effects of isolation and paucity alone. However, Mayr (1942b:236) includes the development of questionable and unusual kinds of insular forms in a general statement: "The potentiality for rapid divergent evolution in small populations explains also why we have on islands so many dwarf or giant races, or races with peculiar color characters (albinism, melanism), or with peculiar structure (long bills in birds), or other peculiar characters (loss of male plumage in birds)."
Nutrition may be also a factor influencing speciation in bird life. The types of food plants (coconut, papaya, breadfruit, pandanus, etc.) might be similar on a Micronesian island and on a continental island in the Philippine region; however, the value of these plants as foods might vary and might reflect differences in mineral content of the soils. For example, if the soils on an island lack, or by leaching out have lost, sufficient amounts of potassium and other elements, plants may store foods, not as proteins, but possibly as carbohydrates, simple sugars, or alkaloids. Whether nutritional influences might have a selective effect on the bird populations, has not been ascertained.
In summary, it may be said that genetic change altering the phenotypic expression of avian characteristics is no more apt to happen in insular populations than in continental populations but genetic change may have a greater chance of being perpetuated in small insular populations where isolation, limited competition, freedom from the selective influences of predation, and other factors exert influences.
The islands of Micronesia are small and their occupation by man often produces serious effects on the endemic animal life of the islands. The vulnerability of insular bird populations is well attested by the fact that the majority of birds, which have become extinct in the past two hundred years, have been insular forms. Two birds in Micronesia, the Kusaie Rail (Aphanolimnas) and the Kusaie Mountain Starling (Aplonis corvinus), are known to be either extinct or so rare that they have not been taken since the time of Kittlitz, who visited the island of Kusaie in December, 1827, and January, 1828. Other birds (Anas oustaleti, Caloenas nicobarica, Megapodius l. lapérouse, and Metabolus rugensis) have become reduced in numbers and may be threatened with extermination.
Nelson (1921:270-274) has described the following agencies destructive to island life of the Pacific: fire, volcanic eruptions, tidal waves, hurricanes, clearing of the land, introduction of domestic animals and grazing, introduction of wild animals and birds. Mayr (1945c) also presents a discussion of conservation problems in these islands.
Fire is a serious hazard to island life, especially to the land birds. It destroys both food and cover, these two habitat requirements being most essential to the birds. The firing of open lands to improve grazing conditions was a practice which persisted in the Marianas during the time of the Spanish. This practice has declined, but the resultant vegetational changes and erosion have adversely affected the avifauna. Tidal waves and hurricanes (typhoons) are occasionally of such intensity as to flood low coral atolls. Such events are damaging to, or might even exterminate populations of land birds (Aplonis, Acrocephalus and others), and prevent colonizations which might otherwise occur. Clearing of the land for agricultural use probably has affected the avifauna, especially on the island of Tinian where much of the island has been placed in cultivation. The occurrence of domestic stock, especially feral hogs and cats, has affected the birds. Hogs, apparently, have been in the islands for a long time. The English privateer, Lord Anson, visited Tinian in October, 1742, and noted a large number of hogs present at that time. At Guam, in 1945, the NAMRU2 party found both hogs and cats moving freely in all parts of the island. Stomachs of cats examined showed that they had been feeding principally on rodents.
Introduction of wild animals and plants have not been so extensive as in the Hawaiians or other islands. There have been at least five importations of land birds to Micronesia as well as several mammals, other vertebrates and invertebrates. The effect of these established colonies on the native bird life has not been studied.
The late world war has brought changes to the population of bird life in Micronesia. The author (1946b) has elsewhere described some of the effects of the bombing, invasion, and occupation of small islands. Some islands, like Peleliu, suffered severely from bombing and invasion operations. Some islands, especially smaller ones like Kwajalein and Ulithi, were partly or almostly entirely cleared of vegetation by occupation forces. Other effects were caused by "recreational" shooting of birds by garrison forces; introductions of pests in materials unloaded; and pest control by clearing, draining, and spraying with DDT and other insecticides to the detriment of inoffensive species.
It is obvious that a well-planned program of conservation should be placed in operation to insure survival of the endemic avifauna of Micronesia.
Collections of birds have been made at most of the major islands of Micronesia, and it is thought that there are but few if any unnamed birds in the region. The distribution of several species has not been completely investigated, especially those land birds (Ducula, Ptilinopus, and Aplonis) which inhabit coral atolls in the Carolines and Marshalls. The bird life of the northern Marianas is also incompletely known. Continued observations in the Micronesian islands will increase our knowledge of the kinds of migratory shore birds and migratory land birds which reach the island as winter visitors. Further information is needed concerning the breeding activities of sea birds in Micronesia, especially in the Marshalls and Carolines.
The systematic status of most of the birds in Micronesia is already established. It is hoped that the present account advances our knowledge of the methods of colonization. Although these fundamental investigations have been nearly completed in Micronesia the field of avian ecology has been relatively untouched. In the past, expeditions have visited Micronesia with the aim of obtaining within a short time collections of the animal life as large and as representative as possible. Many of the collectors made few or no field notes on the bird life; some, like Finsch, Kubary, Marche, and Coultas, made valuable observations on the habits of the birds. Intensive ecological researches may be accomplished more thoroughly by resident investigators, who can devote full time to such pursuits.
My own opportunity to study the bird life of Micronesia came as a member of the scientific staff of the Laboratory of Mammalogy of United States Naval Medical Research Unit No. 2 (NAMRU2) in the late war. The primary duty of this laboratory was to obtain examples of the vertebrate fauna for examination for ectoparasites by the Laboratory of Acarology and to preserve specimens for identification. As a result sizeable collections of mammals, birds, and other vertebrates were obtained. In addition, ecological data were obtained (as time permitted), especially as an aid in studying the distribution of ectoparasites which affected man. In 1945, I spent [Pg 61] eleven months in Micronesia; for most of this time I was stationed at Guam, the headquarters of the Unit, although one month was spent in the Palau Islands, two weeks were spent at Ulithi Atoll, and short stop-overs were made at Eniwetok and Kwajalein atolls. Other members of the laboratory staff visited Rota and Truk islands.
Subsequent to the field studies in the Pacific, I was sent to Washington and spent approximately eight months at the United States National Museum studying the collections of birds and preparing several reports for publication. In this period other material was studied, both in the United States National Museum and at the American Museum of Natural History, New York, and the literature dealing with the birds of Micronesia was explored and a bibliography of Micronesian birds was prepared. At the University of Kansas, I continued the bibliographic work, borrowed and studied some specimens, and completed accounts of the avifauna of Micronesia.
Under the account of each bird, all known references in the literature, which mention the scientific name of the bird and its distribution in Micronesia, are listed. The references are arranged as follows: (1) citation to the original description, and (2) citations to names in literature in the order of their first appearance. When a name is a pure synonym, it may be recognized as such by the fact that the type locality is given immediately following the citation. In compiling these references the writer made use of the invaluable work by Wiglesworth (1891) and of Utinomi's "Bibliographica Micronesia," made available through the translation by Fisher (1947). The arrangement of the families follows that of Peters (1931-1945) and Wetmore (1940).
Specimens examined are designated as to collection in which catalogued by the following abbreviations: USNM, the United States National Museum; AMNH, the American Museum of Natural History; MCZ, the Museum of Comparative Zoölogy; and KMNH, the University of Kansas Museum of Natural History. Average and extreme measurements of specimens are usually listed in tables; unless otherwise indicated, measurements are in millimeters, and are of adult specimens. The wings have been measured by flattening them on a ruler. Weights are in grams. Unless otherwise indicated, descriptions of the birds have been written by the author. Descriptions of shore birds are not given; for these the reader may refer to Mayr (1945a:28-47) where characters useful for identification of the birds in the field also are given. The writer is especially [Pg 62] indebted to Dr. Ernst Mayr for making available the descriptions of Micronesian birds made by Miss Cardine Bogert, especially those dealing with color of the irides, feet, and bill. Color terms in quotation marks refer to those in Ridgway (1912).
In dealing with insular forms the criterion of intergradation as indicative of subspecies cannot be applied as it can in kinds of birds on the mainland which have geographically continuous distributions. Instead, degree of difference in combination with geographic position plus other factors such as degree of variation in the geographic races of the same species or a related species on continental areas are used in deciding whether two closely related kinds are subspecies or full species. Many kinds of birds in the islands are modified but little from island to island (examples, Rhipidura rufifrons, Aplonis opacus, Ducula oceanica, and Myzomela cardinalis), and can be treated as subspecies. Others show much variability from island to island and it is uncertain whether they should be treated as subspecies or as separate species (examples, Myiagra oceanica, Zosterops cinerea, Rukia, and possibly Acrocephalus luscinia). Decisions on generic status are equally difficult to make. In many cases the experience and judgment of the taxonomist may be the only criteria by which he can decide whether a bird is different enough to be considered as a distinct genus. This "human element" has caused some disagreement. Knowing whether the bird is to be considered as a distinct genus or instead merely as a species may not be as important as knowing its correct phylogenetic relationship. The circumstance that variation in these insular birds is in general less predictable than in mainland birds adds, I think, to the pleasure inherent in the classification of the variations.
First, I thank Commodore Thomas N. Rivers (MC) USNR, then commanding officer of NAMRU2, for the opportunity to join the Unit, for his interested cooperation in seeing that the plans for field trips were successful, and for his thoughtfulness in obtaining for me the orders for duty at the United States National Museum subsequent to our field investigations. Greatly appreciated also is the help rendered by my former colleagues of NAMRU2, including Dr. David H. Johnson, Dr. George W. Wharton, Dr. Aaron B. Hardcastle, Mr. Odis A. Muennik, Mr. L. P. McElroy, Mr. Charles O. Davison, Mr. Merle H. Markley, Mr. Walter L. Necker, Dr. Wilbur G. Downs, Dr. Bernard V. Travis, and Mr. E. W. Coleman. Other personnel, then stationed in Micronesia, who contributed data used in this report include: Dr. Joe T. Marshall, Jr., (who generously [Pg 63] loaned some of the specimens taken by him in Micronesia), Dr. C. K. Dorsey, Dr. George Hensel, Mr. Tom Murray, Dr. Irwin O. Buss, Mr. James O. Stevenson, Dr. Wilfred D. Crabb, Mr. Herbert Wallace, and Dr. M. Dale Arvey. Authorities of the United States National Museum provided generously for using the collections there, and I am especially grateful to Dr. Alexander Wetmore, Dr. Herbert Friedman, and Mr. Herbert G. Deignan for their cooperation and assistance. Doctor Wetmore kindly made available many of the birds collected at Bikini during the atomic bomb experiments. Dr. Robert Cushman Murphy, Dr. Ernst Mayr, and Dr. Dean Amadon of the American Museum of Natural History made available the collections in their charge. Doctor Murphy allowed me to examine some of the heretofore unstudied collections of sea birds of the Whitney South Sea Expedition. Doctor Mayr generously helped me with taxonomic and evolutionary problems and made available to me some of his own unpublished taxonomic notes, the unpublished field accounts of Mr. William F. Coultas and a partly completed manuscript on the birds of Micronesia by Miss Cardine Bogert. Mr. James L. Peters generously loaned specimens from the Museum of Comparative Zoölogy. The use of unpublished field notes made by Mr. Larry P. Richards at Ponapé and Truk in 1947 and 1948 is also gratefully acknowledged. I am grateful also to my colleagues at the Museum of Natural History of the University of Kansas and would single out for special mention Dr. E. Raymond Hall who gave critical assistance with the manuscript, Drs. Edward H. Taylor and Herbert B. Hungerford who made helpful suggestions, and Mrs. Virginia Cassell Unruh who drew the distributional maps.
Diomedea nigripes Audubon, Ornith. Biog., 5, 1839, p. 327. (Type locality, Pacific Ocean, lat. 30°44´N., long. 146°W.)
Diomedea fuliginosa Oustalet, Le Nat., 1889, p. 261 (Mariannes).
Diomedea nigripes Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris (3), 8, 1896, p. 51 (Agrigan); Hartert, Novit. Zool., 5, 1898, p. 68 (Marianne); Seale, Occ. Papers Bernice P. Bishop Mus. 1, 1901, p. 22 (Marianas); Safford, Osprey, 1902, p. 70 (Mariannes); idem, The Plant World, 7, 1904, p. 268 (Guam?); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 32 (Marriane); Peters, Check-list Birds World, 1, 1931, p. 43 (Marshalls); Hand-list Japanese Birds, rev., 1932, p. 188 (Marianas); Hand-list Japanese Birds, 3rd ed., 1942, p. 210 (Marianas); Mayr, Birds Southwest Pacific, 1945, p. 5 (Marshalls).
Geographic range.—North Pacific Ocean. Breeds on islands northwest of Hawaii. In Micronesia: Mariana Islands—Agrigan.
Characters.—A large oceanic bird with sooty-brown coloration; darker on nape, wings and tail; lighter on forehead, sides of head, and abdomen; area surrounding bill whitish; tail whitish at base; bill dark reddish-brown; feet black.
Remarks.—This albatross has been recorded from waters near the Mariana Islands. Quoy and Gaimard (1824:145) observed "albatross" between the Mariana and the Hawaiian Islands. The only actual specimens obtained from the islands were reported on by Oustalet (1896:51). These were eight Black-footed Albatrosses which were taken on the coast of Agrigan by Marche in December, 1888, and January, 1889. Oustalet gives the following measurements: total length, 680-785; wing, 485-525; tail, 180-225; tarsus, 80-90; culmen, 108-125. The specimens are apparently in the Paris Museum.
Peters (1931:43) lists the Marshall Islands as part of the range of D. nigripes.
In the period of the late war Gleise (1945:221) observed eight Short-tailed Albatrosses (D. albatrus Pallas) "off Saipan." Specimens of D. albatrus have not been taken in Micronesia. According to Austin (1948b:32) this albatross "is now virtually extinct," and this record may be questioned.
Puffinus chlororhynchus Lesson, Traité d'Ornith., 8, 1931, p. 613. (Type is from Shark's Bay, West Australia.)
Puffinus sphenurus Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 299 (Mortlock).
Puffinus chlororhynchus Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 80 (Luganor?); Godman, Monogr. Petrels, pt. 2, 1908, p. 88 (Carolines); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 33 (Luganor or Ruk?).
Puffinus pacificus chlororhynchus Hand-list Japanese Birds, rev., 1932, p. 187 (Lukunor or Truk?, Kusaie); Hand-list Japanese Birds, 3d ed., 1942, p. 209 (Lukunor or Truk?, Kusaie).
Geographic range.—Breeds at the Seychelles, Australia, Lord Howe, Norfolk, and other islands in the Australian area. Ranges throughout most of the warmer parts of the Indian and Pacific oceans. In Micronesia: Mariana Islands—Guam; Caroline Islands—Lukunor or Truk?, Kusaie.
Characters.—A large shearwater with long wedge-shaped tail; upper parts sooty-brown with crown, neck, and wings darker and forehead paler; under parts paler than upper parts; bill dark; feet flesh-colored.
Remarks.—This shearwater was taken by Kubary either at Lukunor or at Truk in the Caroline Islands. At a later date, apparently between 1922 and 1932, the Japanese recorded the bird at Kusaie. In using this subspecific name, I am following the Hand-list of Japanese Birds (Hachisuka et al., 1932:187).
At Guam on August 10, 1931, Coultas obtained a male shearwater, which is tentatively placed in this subspecies. Its measurements are as follows: wing, 290; tail, 128; exposed culmen, 39; tarsus, 47. Coultas (field notes) writes that he was told by natives that petrels nest and roost on the high cliffs behind the city of Agaña on Guam. At sea south of the eastern Caroline islands, Coultas obtained five other birds which appear to be the same as the bird from Guam. All specimens are in the collections of the American Museum of Natural History.
Puffinus cuneatus Salvin, Ibis, 1888, p. 353. (Type locality, Krusenstern Island==Ailuk, Marshall Islands, fide Fisher, Auk, 63, 1946, pp. 587-588.)
Puffinus cuneatus Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 80 (Krusenstern); Salvin, Cat. Birds British Mus., 25, 1896, p. 371 (Krusenstern); Godman, Monogr. Petrels, pt. 2, 1908, p. 76 (Marshalls).
Puffinus pacificus cuneatus Mathews, Birds Australia, 2, 1912, p. 84 (Marshall Group); Peters, Check-list Birds World, 1, 1931, pp. 55-56 (Krusenstern); Hand-list Japanese Birds, 3d ed., 1942, p. 209 (Krusenstern); Fisher, Auk, 63, 1946, pp. 587-588 (Ailuk).
Thyellodroma cuneata cuneata Mathews and Iredale, Ibis, 1915, p. 597 (Krusenstern); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 113 (Marshall Group).
Thyellodroma cuneata Oberholser, Auk, 34, 1917, p. 474 (Krusenstern).
Thyellodroma pacificia cuneata Mathews, Novit. Zool., 39, 1934, p. 186 (Caroline Islands).
Geographic range.—Pescadores east to the Hawaiian Islands and south to eastern Micronesia. In Micronesia: Marshall Islands—Ailuk.
Remarks.—Osbert Salvin received two specimens of this shearwater from H. J. Snow, who got them at the Krusenstern Islands in 1883. In describing them, Salvin (1888:353) comments that the locality is seemingly in the Marshall Islands at approximately 10°17´ N. and 190° W. This locality was confusing to Seebohm (1891:191) who thought it was between the Hawaiians and the Marshalls, while Hartert (1926:352) decided it was really Krusenstern Rocks in the Hawaiian Group. To clear the matter up, Fisher (1946:587-588) writes that Salvin was correct and suggests that the name of the island should be the better established one, Ailuk, rather than the little used one, Krusenstern.
P. p. cuneatus resembles P. p. chlororhynchus but is whiter on the underparts, especially the breast. These two subspecies are inseparable according to the twenty-fourth supplement to the American Ornithologists' Union Check-list of North American Birds (Auk, vol. 66, 1949:281).
Procellaria tenuirostris Temminck, Pl. Col., livr. 99, 1835, text to pl. 587. (Type locality, Seas north of Japan and shores of Korea.)
Puffinus tenuirostris tenuirostris Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam).
Puffinus tenuirostris Yamashina, Tori, 10, 1940, p. 677 (Kinajon, Marshall Islands); Hand-list Japanese Birds, 3d ed., 1942, p. 210 (Kinajon, Marshall Islands).
Geographic range.—Breeds in Tasmania, southeastern Australia, islands in Bass Straits, and Bounty Islands. Ranges north to the Bering Sea. In Micronesia: Mariana Islands—Guam?; Marshall Islands—Kinajon.
Character.—A rather large shearwater with short, rounded tail; upper parts sooty brown; underparts paler and more grayish than back; throat may be occasionally whitish; bill lead-gray; feet grayish, browner on outer side.
Remarks.—On migration this shearwater probably reaches most parts of Micronesia. It has been recently recorded by the Japanese at Kinajon in the Marshall Islands. Bryan (1936:15) includes this species as a "chance arrival" in his list of the birds of Guam.
Puffinus (Nectris) nativitatis Streets, Bull. U. S. Nat. Mus., 7, 1877, p. 29. (Type locality, Christmas Island, Pacific Ocean.)
Puffinus nativitatis Salvin, Cat. Birds British Mus., 25, 1896, p. 389 (Krusenstern); Lister, Proc. Zool. Soc. London, 1891, pp. 295-300 (Krusenstern); Godman, Monogr. Petrels, pt. 3, 1908, p. 153 (Marshalls).
Geographic range.—Breeds at Wake and Laysan Islands south to Christmas, Phoenix, Marquesas, Tuamotu, and Austral Islands. In Micronesia: Marshall Islands—Ailuk.
Characters.—Upper parts chocolate brown; underparts resemble upper parts but throat may be slightly grayer; bill and feet black. P. nativitatis resembles P. pacificus but is similar with black feet.
Remarks.—The only specimens of this bird known from Micronesia, are those taken in the spring of 1883 by H. J. Snow at Krusenstern (Ailuk) in the Marshall Islands. For two birds from this island in the collections of the British Museum, Godman (1908:154) gives the following measurements: wing, 9.6 and 10.0; tail, 3.35 and 3.4; culmen, 1.15 and 1.2; tarsus, 1.7 and 1.8; middle toe and claw, 2.0 and 2.1.
Puffinus dichrous Finsch and Hartlaub, Fauna Centralpolynesiens, 1867, p. 244. (Type locality, McKean Island, Phoenix Group.)
Puffinus dichrous Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 90, 108 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 6, 44 (Palau).
Puffinus opisthomelas var. minor Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 832 (Type locality, Pelew); Finsch, Journ. f. Ornith., 1872, p. 57 (Pelew).
Puffinus opisthomelas Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 9, 118 (Pelew); Finsch, Journ. f. Ornith., 1870, p. 371 (Pelew).
Puffinus tenebrosus Pelzeln, Ibis, 1873, p. 47, fig. 1 (Type locality, unknown==Pelew Islands, ex Mathews); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 55 (Rota); Hartert, Novit. Zool., 5, 1898, p. 69 (Marianne); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 23 (Marianas?); Safford, The Plant World, 7, 1904, p. 268 (Guam).
Puffinus obscurus Finsch, Journ. Mus. Godeffroy, 12, 1876, pp. 18, 40 (Ponapé, Palau); idem, Proc. Zool. Soc. London, 1877, p. 786 (Palau); idem, Proc. Zool. Soc. London, 1877 (1878), p. 782 (Ponapé); idem, Journ. f. Ornith., 1880, pp. 295, 309 (Ponapé, Kuschai); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); idem, Ibis, 1881, p. 109 (Kuschai); idem, Ibis, 1881, pp. 113, 115 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 353 (Ruk); Salvin, Ibis, 1888, p. 357 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 79 (Ruk, Ponapé, Pelew); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 54 (Saypan, Palaos); Salvin, Cat. Birds British Mus., 25, 1896, p. 382 (Carolines, Pelews); Hartert, Novit. Zool., 5, 1898, p. 68 (Marianne); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 23 (Marianas?); Safford, Osprey, 1902, p. 70 (Marianas); Dubois, Syn. Avium, 2, 1904, p. 1031 (Pelew, Carolines); Godman, Monogr. Petrels, pt. 2, 1908, pp. 126, 127 (Pelew, Ruk, Ponapé).
Puffinus obscurus obscurus Hartert, Novit. Zool., 7, 1900, p. 10 (Ruk); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 32 (Saipan, Ruk, Ponapé, Pelew).
Puffinus lherminieri minor Mathews, Birds Australia, 2, 1912, p. 70 (Pelew, Carolines).
Puffinus assimilis minor Mathews, Syst. Avium Australasianarum, 1, 1927, p. 111 (Pelew).
Puffinus lherminieri dichrous Murphy, Amer. Mus. Novit., no. 276, 1927, p. 10 (Pelews, Carolines); Peters, Check-list Birds World, 1, 1931, p. 60 (Pelew); Yamashina, Tori, 7, 1932, p. 408 (Arakabesan); Hand-list Japanese Birds, rev., 1932, p. 188 (Saipan, Truk, Ponapé, Palaus); Hand-list Japanese Birds, 3rd ed., 1942, p. 209 (Saipan, Truk, Ponapé, Palau); Mayr, Birds Southwest Pacific, 1945, p. 10 (Carolines, Palaus); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 37 (Palau).
Alphapuffinus lherminieri minor Mathews, Novit. Zool., 39, 1934, p. 182 (Pelew Islands).
Puffinus obscura Bryan, Guam Rec., vol. 13, No. 2, 1936, p. 15 (Guam).
Geographic range.—Known from Phoenix, Nauru, Micronesia, and south to the Samoan, Society, Tuamotu, and Marquesas islands. In Micronesia: Mariana Islands—Guam, Rota, Saipan; Palau Islands—Babelthuap, Koror, Arakabesan; Caroline Islands—Truk, Ponapé, Kusaie.
Characters.—A small shearwater with upper parts sooty-black; under parts white except for sides of breast grayish and under tail-coverts blackish; bill blackish; feet yellowish, outer toe black.
Measurements.—Measurements of 17 adult birds (9 males, 7 females, 1 unsexed) from Micronesia (Palau, Truk, Ponapé, Kusaie) and 10 adult birds (6 males, 4 females) from the Phoenix Group (Enderbury, Canton) are listed in table 14.
| Locality | Wing | Tail | Exposed culmen |
Tarsus |
| Micronesia | 203 (197-211) | 83.6 (77-89) | 27.9 (26-30) | 38.5 (37.5-40) |
| Phoenix | 197 (193-203) | 82.2 (79-85) | 26.3 (25-28) | 37.2 (36-39) |
Specimens examined.—Total number, 72 (44 males, 19 females, 9 unsexed), as follows: Palau Islands, AMNH—exact locality not given, 64 (Oct., Nov., Dec.); Caroline Islands, AMNH—Truk, 4 (June 15, 16)—Ponapé, 3 (undated)—Kusaie, 1 (April 25).
Nesting.—The Dusky Shearwater in Micronesia nests in holes on high, and usually isolated, coral cliffs. Owston's collectors, according to Hartert (1900:10), found a nest with one egg at Truk on June 16. The nest was in a hole four feet deep in the side of a cliff. The egg is white and measures 42 × 35. Yamashina (1932a:408) records the taking of one egg at Arakabesan, Palau Islands, on May 26. Coultas (field notes) gives an interesting account of nesting activities of this shearwater at the Palau Islands. He found the bird nesting on small islands of the group from October to December, 1931; however, he states that the natives told him that the bird nests throughout the year. Land crabs and shearwaters were often found together in the same burrow. Apparently the adult birds did not remain in the burrow with the young during the day. At Kusaie, Coultas was told by the natives that the adult birds were caught by tying the mandibles of the young together. When the parent birds approached and hovered over the young birds expecting their mouths to open, the natives had the opportunity to strike them down with clubs. Coultas collected six downy nestlings at Palau in November and December.
Remarks.—The first published account of this shearwater in Micronesia was apparently by Kittlitz (1858, pt. 1:358) when he recorded his "Schwärzlicher Sturmvogel" at Kusaie, according to Wiglesworth (1891a:79). Finsch (1875:44 and 1881b:113, 115) studied specimens taken by Tetens, Heinsohn, and Kubary at the Palau Islands and those taken by Kubary at Ponapé. Earlier, Hartlaub (1868:832) used some of these specimens from the Palau Islands to describe his Puffinus opisthomelas var. minor, which was destined to be placed in synonymy (Murphy, 1927:10). Oustalet (1896:54, 55) recorded specimens taken by Marche at Saipan in May, 1887, and at Rota in July, 1888. Oustalet referred to them as P. obscuras and P. tenebrosus, respectively. T. W. Gulick obtained undated skins at Ponapé. Hartert (1900:10) reported on specimens taken by Owston's collectors at Truk. In 1931, Coultas with the Whitney South Sea Expedition took one shearwater at Kusaie and a series of 64 skins at the Palau Islands. He failed to find birds at Ponapé and wrote that their scarcity there may have been due to persistent hunting of them by the inhabitants of the island. The NAMRU2 party obtained no information concerning the birds at Guam, Rota, or Truk, but at the Palau Islands observed shearwaters at sea approximately 6 miles east of Babelthuap Island on September 2, 1945.
Murphy (1927:6-15) revised the shearwaters of the Puffinus lherminieri group, and recognized several subspecies. P. l. dichrous was assigned a range consisting of Micronesia, the Phoenix Islands, and Nauru Island. The breeding range of P. l. polynesiae was given [Pg 69] as the Samoan, Society, Tuamotu and Marquesas islands. Color differences between the two subspecies are very slight, and he separated them on the basis of the length of the exposed culmen as follows: P. l. dichrous 22.6-27 (26) in P. l. polynesiae 25.5-30 (28.9). In other measurements they closely resembled one another. At the time of his study, Murphy did not have the shearwaters from Micronesia collected by Coultas and actually did not have a large series from these islands. On studying this new material, I find the length of the exposed culmen of 17 adult birds from Micronesia (including 12 from the Palaus) to be 26-30 (27.9). In comparison with Murphy's findings, my measurements of Micronesian birds fall almost midway between the measurements which he recorded as characteristic of P. l. dichrous (from the Phoenix Islands) and P. l. polynesiae. The intermediate position of the measurements of the Micronesian birds, together with the absence of other distinguishing characters, suggests that these shearwaters belong to only one subspecies which consists of a group of isolated and variable populations. Unless the old specific name, obscuras of Gmelin, is revived, the name for the entire group in Micronesia and Polynesia would be P. l. dichrous. I agree with Murphy that the Bonin form, P. l. bannermani, is a well-defined subspecies.
Procellaria rostrata Peale, U. S. Expl. Exp., 8, 1848, p. 296. (Type locality, Mountains about 6,000 feet on Tahiti, Society Islands.)
Procellaria desolata Pucheran, Voy. Pôle Sud, 3, 1853, p. 138 (des îles Carolines); Hartlaub, Journ. f. Ornith., 1854, p. 168 (Carolinen).
Procellaria (Aestrelata) desolata Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 55 (Caroline Islands).
Oestrelata rostrata Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 82 (Caroline Is.); Godman, Monogr. Petrels, pt. 3, 1908, p. 190 (Caroline Is.).
Pterodroma rostrata Kuroda, in Momiyama, Birds Micronesia, 1922, p. 33 (Carolines).
Pterodroma rostrata subsp. (?) Hand-list Japanese Birds, rev., 1932, p. 188 (Carolines); Hand-list Japanese Birds, 3d ed., 1942, p. 210 (Carolines).
Geographic range.—Known to breed on the Society and Marquesas Islands. In Micronesia: Caroline Islands—exact locality unknown.
Characters.—A large petrel with blackish-brown plumage except for belly and under tail-coverts white and throat, upper breast and flanks pale brown; bill black; legs yellowish; feet black. This oceanic bird differs from other petrels and shearwaters of Micronesia by the presence of a white abdomen in contrast with dark plumage on upper parts, throat, and breast.
Remarks.—A petrel which is referred to this subspecies has been taken once in Micronesia, by Hombron and Jacquinot in the Caroline Islands. It may be pointed out that the subspecies P. r. becki [Pg 70] Murphy is known from the sea east of the Bismarck Archipelago and might range into Micronesian waters.
Oestrelata hypoleuca Salvin, Ibis, 1888, p. 359. (Type locality, Krusenstern Island = Ailuk, Marshall Islands, fide Fisher, Auk., 63, 1946, pp. 587-588).
Oestrelata hypoleuca Salvin, Cat. Birds British Mus., 25, 1896, p. 409 (Krusenstern); Godman, Monogr. Petrels, pt. 3, 1908, p. 212 (Krusenstern).
Cookilaria hypoleuca hypoleuca Mathews, Syst. Avium Australasianarum, 1, 1927, p. 122, (Marshall Group).
Pterodroma leucoptera hypoleuca Hand-list Japanese Birds, rev., 1932, p. 188 (Marshalls); Hand-list Japanese Birds, 3d ed., 1942, p. 210 (Krusenstern); Fisher, Auk, 63, 1946, pp. 387-388 (Ailuk).
Pterodroma hypoleuca hypoleuca Mayr, Birds Southwest Pacific, 1945, p. 11 (Micronesia).
Geographic range.—Ranges from the Bonins east to the Hawaiians and south to Micronesia. In Micronesia: Marshall Islands—Ailuk.
Characters.—Upper parts grayish except for forehead whitish, crown and nape sooty-black; underparts whitish except for sides of breast sooty-black; legs and feet flesh color except for tips of toes and webs which are black.
Remarks.—In Micronesia, this petrel is known only from the type locality, Krusenstern or Ailuk, Marshall Islands. Fisher (1946: 587-588) has corrected the confusion regarding the exact position of this type locality.
Phaëthon aethereus mesonauta Peters, Occ. Papers Boston Soc. Nat. Hist., 5, 1930, p. 261. (Type locality, Swan Key, Almirante Bay, Panama.)
Phaeton aethereus Finsch, Ibis, 1880, pp. 329, 333, (Ratak Chain, Marshalls); idem, Journ. f. Ornith., 1880, p. 310 (Kuschai); idem, Ibis, 1881, p. 109 (Kuschai); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 73 (Kushai, Marshalls); Ogilvie-Grant, Cat. Birds British Mus. 26, 1898, p. 457 (Kushai, Marshalls); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall Inseln); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 34 (Kusaie, Marshall Islands).
Phaethon aethereus [?mesonauta] Hand-list Japanese Birds, rev., 1932, p. 187 (Kusaie, Marshalls); Hand-list Japanese Birds, 3d ed., 1942, p. 208 (Kusaie, Marshall Islands).
Geographic range.—Tropical parts of Atlantic and eastern Pacific from Cape Verde Islands west to Panama and Galapagos Islands. In Micronesia: Caroline Islands—Kusaie; Marshall Islands—Ratak Chain.
Characters.—Adult: A large, white sea bird with a long white tail; dorsal surface marked with blackish, transverse vermiculations; bill red; tarsus and foot flesh-colored with a yellowish hue, with plantar surface grayish. Immature: Resembles adults but dark transverse bars are broader; crown blacker; bill yellow.
Remarks.—No specimens have been examined. The Red-billed Tropic-bird is placed in the list of birds known from Micronesia on [Pg 71] the basis of two observations by the German ornithologist, Otto Finsch. It has not been reported since his time, and may be considered as an unusual record for the area. I am following the Hand-list of Japanese Birds (Hachisuka et al., 1942:208) in assigning the bird to the subspecies, P. a. mesonauta.
Scaeophaethon rubricauda rothschildi Mathews, Birds Australia, 4, 1915, p. 303. (Type locality, Laysan and Niihau.)
Phaeton rubricaudus Finsch, Journ. f. Ornith., 1880, p. 296 (Carolines); idem, Ibis, 1881, p. 115 (Ponapé).
Phaeton rubricauda Finsch, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 73 (Ruk, Ponapé, Marshalls).
Phaeton rubricauda Ogilvie-Grant, Cat. Birds British Mus., 26, 1898, p. 451 (Caroline Islands); Hartert, Novit. Zool., 7, 1900, p. 11 (Ruk); Hand-list Japanese Birds, rev., 1932, p. 187 (Pagan, Truk, Ponapé, Marshalls).
Scaeophaethon rubricauda Kuroda, in Momiyama, Birds Micronesia, 1922, p. 34 (Mariannes, Ruk, Ponapé, Marshalls).
Phaethon rubricauda rothschildi Yamashina, Tori, 7, 1932, p. 406 (Pagan); idem, Tori, 10, 1940, p. 676 (Maug).
Phaethon rubricaudus rothschildi Hand-list Japanese Birds, 3d ed., 1942, p. 209 (Maug, Pagan, Truk, Ponapé, Marshalls).
Geographic range.—Bonin and Hawaiian islands south to Micronesia. In Micronesia: Mariana Islands—Maug, Pagan; Caroline Islands—Truk, Ponapé; Marshall Islands—exact locality unknown.
Characters.—Adult: Long-tailed sea bird white with pinkish tint except for black lores and eye streak; black shafts on feathers of secondaries, flanks, and tail coverts; black bases on feathers of head; central tail feathers elongate with black shafts and bright red webs; bill orange-red with black nasal streak; tarsus and foot bluish-yellow, distal part blackish. Immature: Resembles adult but barred with black above; bill blackish.
Measurements.—Yamashina (1940:676) lists the measurements for seven adult birds from Maug in the northern Marianas as wing 304-319 and exposed culmen 55-62.
Nesting.—Yamashina (1932a:406) reports the taking of one egg at Pagan in the Marianas on February 15, 1931.
Remarks.—The Red-tailed Tropic Bird has been recorded from the Mariana, Caroline, and Marshall Islands. On the basis of our present knowledge it appears to be uncommon in most of Micronesia and may be established as a resident bird only in the northern Marianas, as shown by Yamashina (1932a:406 and 1940:676), Coultas obtained an immature male at 3° N and 158° E, which is at a point in the ocean south of the eastern Carolines. Possibly this bird and others obtained in the Carolines are representatives of the subspecies, P. r. melanorhynchos Gmelin, which is known from the Palmerston, Society and Turtle islands.
Phoethon lepturus dorotheae Mathews, Austr. Avium. Rec., 2, 1913, p. 7. (Type locality, Queensland.)
Phaeton candidus Kittlitiz, Denkw. Reise russ. Amer. Micron. und Kamchat., 1, 1858, p. 382 (Ualan); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 9, 118 (Pelew); Finsch, Journ. f. Ornith., 1872, p. 57 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 90, 114 (Pelew, Ualan); Finsch, Journ. Mus. Godeffroy, 1875, pp. 6, 47 (Palau); idem, Proc. Zool. Soc. London, 1877 (1878), p. 782 (Ponapé); idem, Journ. f. Ornith., 1880, pp. 296, 309 (Ponapé, Kuschai); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); Schmeltz and Krause, Ethnogr. Abth. Mus., Godeffroy, 1881, pp. 281, 299, 330, 353 (Ponapé, Mortlock, Nukuor, Ruk); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 52 (Kuschai); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 73 (Pelew, Ruk, Luganor, Nukuor, Ponapé, Ualan, Marshalls); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 62 (Agrigan, Palaos, Ruk, Kushai, Marshalls); Hartert, Novit. Zool., 5, 1898, p. 68 (Marianne).
Phaeton flavirostris Finsch, Ibis, 1880, pp. 329, 333 (Ratak Chain); idem, Ibis, 1881, pp. 105, 109, 115 (Kuschai, Ponapé).
Phaethon candidus Salvadori, Ornith. Papuasia, 3, 1882, p. 426 (Pelews, Carolines, Marshalls); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 23 (Agrigan); Safford, Osprey, 1902, p. 70 (Mariannes); Takatsukasa and Kuroda, Tori, 1, 1915, p. 50 (Pelew, Ponapé); Uchida, Annot. Zool. Japon., 9, 1918, pp. 489, 492 (Palau).
Phaëthon lepturus Ogilvie-Grant, Cat. Birds British Mus., 26, 1898, p. 453 (Pelew, Carolines, Marshalls); Hartert, Novit. Zool., 7, 1900, p. 10 (Ruk); Safford, The Plant World, 7, 1904, p. 268 (near Guam); idem, Contr. U. S. Nat. Herb., 9 1905, p. 80 (northern Marianas); Mayr, Birds Southwest Pacific, 1945, p. 17 (Palau); Strophlet, Auk, 63, 1946, p. 535 (Guam); Borror, Auk, 64, 1947, p. 416 (Agrihan); Stott, Auk, 64, 1947, p. 524 (Saipan).
Phaeton lepturus Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall Inseln).
Leptophaethon lepturus dorothea Mathews, Birds Australia, 4, 1915, p. 309 (Pelew).
Phaethan lepturus Cox, Island of Guam, 1917, p. 22 (northern Marianas).
Leptophaethon lepturus lepturus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 33 (Agrigan, Saipan, Pelew, Ruk, Luganor, Nukuor, Ponapé, Kusaie, Marshalls).
Phaethon lepturus dorotheae Yamashina, Tori, 7, 1932, p. 407 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 187 (Agrigan, Pagan, Saipan, Agiguan, Palaus, Truk, Luganor, Nukuor, Ponapé, Kusaie, Marshalls); Hand-list Japanese Birds, 3d ed., 1942, p. 209 (Agrigan, Pagan, Saipan, Agiguan, Babelthuap, Koror, Urukthapel, Angaur, Unusuto, Truk, Luganor, Nukuor, Ponapé, Kusaie, Namorik); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 38 (Guam, Peleliu, Ulithi, Truk).
Geographic range.—Islands in the southwestern Pacific area. In Micronesia: Mariana Islands—Agrigan, Pagan, Saipan, Agiguan, Rota, Guam; Palau Islands—Babelthuap, Koror, Urukthapel, Peleliu, Anguar, Unusuto; Caroline Islands Truk, Ulithi, Luganor, Nukuor, Ponapé, Kusaie; Marshall Islands—Namorik.
Characters.—Adult: White often with pinkish shade but lores and eye streak black; feathers of head, flanks and under tail-coverts with bases black; black on outer and subterminal part of inner webbing of primaries; black, subterminal coloring on scapulars and secondaries; black on shafts of elongated tail plumes; bill horn yellow, dark basally; tarsus dark yellow; feet blackish.
Immature: Resembles adult but upper parts barred with black, bill black on terminal part.
Measurements.—Measurements of adult birds from Micronesia are given in table 15.
Weights.—The NAMRU2 party recorded weights of five adult males from Guam as 294 (267-321) grams.
| No. | Wing | Tail | Exposed culmen |
Tarsus | |
| Marianas: Asuncion, Guam | 6 | 264 | 107 | 47 | 21 |
| 256-287 | 97-117 | 44-50 | 20-21 | ||
| Palaus: Peleliu | 11 | 257 | 108 | 45 | 21 |
| 242-270 | 98-122 | 40-49 | 19-21 | ||
| Carolines: Ponapé, Kusaie | 11 | 261 | 105 | 47 | 21 |
| 252-271 | 97-114 | 44-49 | 21-22 | ||
| Total: Micronesia | 28 | 260 | 107 | 46 | 21 |
| 242-287 | 97-122 | 40-50 | 19-22 |
Specimens examined.—Total number, 37 (22 males, 10 females, 5 unsexed), as follows: Mariana Islands, USNM—Guam, 5 (June 11, July 21); AMNH—Asuncion, 1 (June?); Palau Islands, USNM—Peleliu, 5 (Aug. 29, 31, Sept. 5, 6); AMNH—exact locality not given, 7 (Oct. 13, 26, Nov. 15, 23, Dec. 18); Caroline Islands, AMNH—Ponapé, 9 (Dec. 8, 9, undated)—Kusaie, 10 (March 1-8, April).
Nesting.—The NAMRU2 party observed nests of the White-tailed Tropic Bird at Peleliu in August and September, 1945. Several nests were seen in hollows of the Australian pine (Casuarina equisetifolia) between 20 and 30 feet above the ground. Birds could be seen in the nest hollows because the plumes of their long tail usually extended well out of the entrance. One nest was found in a dead tree in a battle-cleared area; others were observed in jungle habitat. Coultas observed nesting at Ponapé between November 1 and December 30, 1930, and found nests in the tops of trees and in hollow trees; a few were observed in holes in cliffs. Yamashina (1932a:407) records the taking of one egg at Ponapé on August 18, 1931. At Guam the NAMRU2 party found birds along the high cliffs which edge the beach. There was no evidence that they were nesting from May to July; nevertheless males taken in June had enlarged gonads. The bird is known to breed at Namorik in the Marshall Islands, according to the Hand-list of Japanese Birds (Hachisuka et al., 1942:209).
Food habits.—The NAMRU2 party found small fish in the stomachs of these birds taken at Peleliu.
Parasites.—Uchida (1918:489, 492) records the bird lice (Mallophaga), Colpocephalum epiphanes and Menopon eulasius, from the White-tailed Tropic Bird from Palau.
Remarks.—Birds taken in Micronesia differ only slightly from those from other areas in Oceania. Within Micronesia (see table 15) the birds from the Palaus have the shortest wing and shortest exposed culmen.
The White-tailed Tropic Bird appears more numerously in western and northern Micronesia than in the Marshall Islands. This distribution may be correlated with a preference for the "high" islands; especially those which have rocky cliffs, including Guam, Rota, [Pg 74] Peleliu, Angaur, and Truk. Reports were received in 1945 that the birds were only infrequently seen at Ulithi, a low atoll. Stott (1947:524) observed birds flying into rocky crevices at Saipan on December 18. Gleise (1945:221) also recorded the bird in the vicinity of Saipan. Borror (1947:416) reports seeing birds at Agrigan on July 29, August 5 and 6, 1945. Coultas (field notes) found tropic birds common at Ponapé in November and December, 1930, in forested regions and along the cliffs. He made similar observations at Kusaie and Palau. At Ponapé and Palau, Coultas noted the use of the eggs, young and adults as food by the natives. At Palau the plumes are used in headdresses worn by the natives, the birds being taken with the blowgun.
Murphy (1936:807) states that the principal enemy of the White-tailed Tropic Bird at Bermuda is the introduced rat (Rattus rattus). Introduced rats, particularly Rattus mindanensis on Guam, may prey on the nesting birds. Baker (1946c:404) writes that this rat is a good climber and may spend considerable time in trees. The rat was trapped also in rough coral jungle at the edge of the cliffs, where tropic birds, Micronesian Starlings and other species, may have been nesting.
Little has been recorded concerning the post-breeding season wanderings of these tropic birds in Micronesia. They seemingly spend considerable time at sea, but whether they move as far from their breeding areas as do birds in the Atlantic, as reported by Murphy (1936:803), Baker (1947a:253) and others, is not known.
Murphy (1936:796) notes that the northward distribution of the tropic birds in the Atlantic is dependent on the warm currents of water. In the western Atlantic, the poleward-flowing, warm currents of the Gulf Stream allow for the northern extension of the range of these birds to Bermuda. In the eastern Atlantic, cool currents flowing toward the equator restrict the northern range. The same condition prevails in the eastern Pacific where warm current flowing toward the pole enable the birds to range north to the Bonins and other islands.
The three species of tropic birds known from Micronesia overlap very little in their ranges in this area. The White-tailed Tropic Bird has become firmly established in the western part of Micronesia, but there are only a few records from the extreme eastern part. The Red-tailed Tropic Bird appears to be resident only in the northern Marianas although it has been recorded in the Carolines and Marshalls. Interspecific competition may prevent considerable intermingling [Pg 75] of breeding populations in Micronesia, or it may be that each species requires different ecologic conditions.
Sula personata Gould, Proc. Zool. Soc. London, 1846, p. 21. (Type locality, North and northeast coasts of Australia = Raine Island.)
Sula cyanops Finsch, Ibis, 1880, p. 219 (Taluit); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 72 (Marshalls); Ogilvie-Grant, Cat. Birds British Mus., 26, 1898, p. 430 (Marshalls).
Parasula dactylatra personata Kuroda, in Momiyana, Birds Micronesia, 1922, p. 35 (Marshall Islands); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 232 (Marshall Islands).
Sula dactylatra personata Yamashina, Tori, 7, 1932, p. 407 (Medinilla); Hand-list Japanese Birds, rev., 1932, p. 187 (Medinilla, Marshall Islands); Hand-list Japanese Birds, 3d ed., 1942, p. 208 (Medinilla, Marshall Islands).
Geographic range.—Central and western Pacific from the Hawaiian Islands south to Australia, probably also in the Indian Ocean. In Micronesia: Mariana Islands—Medinilla; Marshall Islands—Jaluit?
Characters.—Adult: A large, white sea bird, with brown wings and tail; face dark blue; bill horn-colored with base orange-yellow in males and pink or light red in females; feet olive in males and lead gray in females.
Immature: Resembles adult, but head, wings, tail, chin and throat dark brown; some white mottling may be present on back and rump; bill dark; feet lead colored.
Nesting.—Yamashina (1932a:407) reports the taking of 12 eggs on February 19, 1931, at Medinilla Island in the Marianas.
Remarks.—No specimen has been examined by me from the area reported upon. Little is known regarding the distribution of the Masked Booby in Micronesia. It is found on the island groups which surround Micronesia and future field observations probably will add to our knowledge of its occurrence in this area. It is known to be resident only in the northern Marianas.
Sula rubripes Gould, Syn. Birds Australia, pt. 4, 1838, app., p. 7. (Type locality, New South Wales = Raine Island.)
Pelecanus piscator Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 296, 299 (Lougounor = Lukunor); idem, Denkw. Reise russ. Amer. Micron. und Kamchat., 1, 1858, p. 351 (Lugunor).
Dysporus piscator Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 831 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 9, 118 (Pelews); idem, Proc. Zool. Soc. London, 1872, p. 90 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 6, 47 (Palau).
Sula piscatrix Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 72 (Pelew, Luganor); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 64 (Rota, Palaos, Carolines); Hartert, Novit. Zool., 5, 1898, p. 68 (Marianne); Safford, Osprey, 1902, p. 70 (Rota); idem, The Plant World, 7, 1904, p. 267 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); idem, Guam, 1912, p. 19 (Guam); Cox, Island of Guam, 1917, p. 22 (Guam).
Sula piscator Ogilvie-Grant, Cat. Birds British Mus., 26, 1898, p. 432 (Pelew); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 24 (Guam).
Piscatrix sula rubripes Kuroda, in Momiyana, Birds Micronesia, 1922, p. 34 (Pelew, Luganor, Rota).
Sula sula rubripes Hand-list Japanese Birds, rev., 1932, p. 185 (Medinilla, Saipan, Rota, Palau, Lukunor, Likieb); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam); Yamashina, Tori, 10, 1940, p. 676 (Maug, Bikar); Hand-list Japanese Birds, 3d ed., 1942, p. 208 (Maug, Medinilla, Saipan, Rota, Palau, Lukunor, Bikar, Likieb).
Geographic range.—Indian Ocean east to central Pacific islands. In Micronesia: Mariana Islands—Maug, Medinilla, Saipan, Rota; Palau Islands—exact locality unknown; Caroline Islands—Lukunor; Marshall Islands—Bikar, Likieb, Bikini, Eniwetok.
Characters.—Adult: A large sea bird with plumage of variable color, mainly white or partly buff with black primaries and black-tipped secondaries, or grayish or brownish with white or grayish tail; throat blackish; face blue or green; bill bluish and lighter at tip; legs and feet red.
Immature: Resembles adult, but often wholly brownish, lighter ventrally; bill blackish; feet yellowish red. Immature resembles that of S. leucogaster.
Nesting.—Morrison obtained a male nestling at Bikini on May 3, 1946.
Specimens examined.—Total number, 10 (3 males, 7 females) from Marshall Islands, USNM—Bikini (April 28, May 1, 2, 3).
Remarks.—The writer saw several birds approximately 20 miles east of Eniwetok on January 7, 1945. Morrison obtained a series of birds at Bikini in April and May, 1946. Murphy (1936:861-870) presents a wealth of information concerning the bird. He points out the need for a better understanding of the plumages of the adult birds and gives evidence that the birds of different colors may occur within the same population. He describes the Red-footed Booby as nesting in trees and shrubs. This type of nesting environment is present at many of the islands in Micronesia.
Pelecanus Plotus Forster, Descr. Anim., ed. Licht., 1844, p. 278. (Type locality Near New Caledonia.)
Dysporus sula Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 831 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 9, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, p. 90 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 6, 47 (Palau); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); Hartert, Novit. Zool., 7, 1900, p. 11 (Ruk).
Sula fusca Finsch, Ibis, 1880, p. 218 (Taluit).
Sula leucogastra Salvadori, Ornith. Papuasia, 3, 1882, p. 423 (Pelew, Carolinis); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 72 (Pelew, Ruk, Marshalls); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 63 (Palaos, Mariannes, Marshalls, Carolines); Hartert, Novit. Zool., 5, 1898, p. 68 (Marianne).
Sula sula Ogilvie-Grant, Cat. Birds British Museum, 26, 1898, p. 436 (Asuncion, Pelew); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 24 (Guam); Safford, Osprey, 1902, p. 66 (Mariannas); idem, The Plant World, 7, 1904, p. 267 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); idem, Guam, 1912, p. 19 (Guam); Prowazek, Die deutschen Marianen, 1913, p. 100 (Marianen); Takatsukasa and Kuroda, Tori, 1, 1915, p. 50 (Marianne); Cox, Island of Guam, 1917, p. 22 (Guam); Uchida, Annot. Zool. Japon., 9, 1918, pp. 487, 493 (Sea off Mariana Islands).
Sula leucogaster plotus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 34 (Pelew, Ruk, West Faiu, Uracas, Saipan, Marshalls); Yamashina, Tori, 7, 1932, p. 407 (Medinilla); Hand-list Japanese Birds, rev., 1932, p. 185 (Uracas, Pagan, Medinilla, Saipan, Truk, West Fayu, Grimes, Marshalls); Hand-list Japanese Birds, 3d ed., 1942, p. 208 (Uracas, Pagan, Medinilla, Saipan, Grimes, West Fayu, Truk, Marshalls); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 41 (Rota, Guam, Truk).
Geographic range.—Throughout tropical Pacific area and south to Australia. In Micronesia: Mariana Islands—Asuncion, Uracas, Pagan, Medinilla, Saipan, Rota, Guam; Palau Islands—exact locality unknown; Caroline Islands—Grimes, West Fayu, Truk, Kusaie; Marshall Islands—Jaluit, Eniwetok.
Characters.—Adult: A heavy sea bird dark brown except for white lower breast, belly, under tail, and auxillars; bill heavy and light bluish; face, gular pouch and feet greenish yellow.
Immature: Resembles adult, but lower breast, belly and under tail mottled with brown; feet light yellow.
Measurements.—Two adult males (Rota, Guam) measure: wing 386, 408; tail 194; exposed culmen 93, 98; tarsus 45, 49; two adult females (Rota, Kusaie): wing 380, 487; tail 193, 217; exposed culmen 94, 99; tarsus 45, 50.
Weights.—The author (1948:41) records one immature female from Rota weighing 1042 grams.
Specimens examined.—Total number, 6 (3 males, 3 females), as follows: Mariana Islands, USNM—Rota, 3 (Oct. 24); AMNH—Guam, 1 (July 23); Palau Islands, AMNH—exact locality not given, 1 (Dec. 1); Caroline Islands, AMNH—Kusaie, 1 (April 19).
Nesting.—Few records have been published concerning nesting of the Brown Booby in Micronesia. Yamashina (1932a: 407) reports the taking of 12 eggs at Medinilla in the Mariana Islands on February 19, 1931. At Palau, Coultas (field notes) obtained reports that the bird nests at Kiangat, a small islet north of Babelthuap.
Parasites.—Uchida (1918:487, 493) obtained bird lice (Mallophaga), Menopan brevipalpe and Lipeurus potens, from the Brown Booby from the "sea off Mariana Islands."
Remarks.—The Brown Booby has not been found abundantly by observers in the Micronesian area. Coultas and Kubary, who spent considerable time in this region, observed the bird at only a few of the islands. Probably the bird does not nest abundantly in Micronesia, although small colonies may be present. The NAMRU2 party observed a flock of twelve brown boobies on high cliffs at Taipingot Peninsula at Rota on October 24, 1945. Birds were seen also at Guam in May, July and November, 1945, and at Truk in December of the same year. Coultas obtained a single specimen at Kusaie; the natives told him that it was not a resident of the island. The writer observed several Brown Boobies approximately twenty miles east of Eniwetok in the Marshall Islands on January 7, 1945. These were in the company of other sea birds.
Hydrocorax melanoleucos Vieillot, Nouv. Dict. Hist. Nat., 8, 1817, p. 88. (Type locality, "Australasie," restricted to New South Wales.)
Carbo melanoleucus Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 9, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 90, 114 (Pelew).
Graculus melanoleucus Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 48 (Pelew).
Microcarbo melanoleucus Salvadori, Ornith. Papuasia, 3, 1882, p. 410 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 72 (Pelew).
Phalacrocorax melanoleucus Ogilvie-Grant, Cat. Birds British Mus., 26, 1898, p. 398 (Pelew); Nehrkorn, Kat. Eiers., 1899, p. 235 (Palau); Takatsukasa and Kuroda, Tori, 1, 1915, p. 50 (Pelew); Uchida, Annot. Zool. Japon., 9, 1918, p. 486 (Palau).
Ph[alacrocorax] melanoleucos Reichenow, Die Vögel, 1, 1913, p. 127 (Palauinseln).
Microcarbo melanoleucus melanoleucus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 35 (Pelew).
Microcarbo melanoleucus melvillensis Mathews, Syst. Avium Australasianarum, 1, 1927, p. 228 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 186 (Babelthuap, Koror).
Haliëtor melanoleucos melanoleucos Peters, Check-list Birds World, 1, 1931, p. 93 (Pelew).
Phalacrocorax melanoleucus melanoleucus Mayr, Amer. Mus. Novit., no. 486, 1931, p. 5 (Pelew); Amadon, Amer. Mus. Novit., no. 1175, 1942, p. 2 (Palau); Mayr, Birds Southwest Pacific, 1945, pp. 50, 284 (Palau, Marianas); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 41 (Palau).
Phalacrocorax melanoleucos melvillensis Hand-list Japanese Birds, 3d ed., 1942, p. 207 (Pagan, Babelthuap, Koror, Angaur).
Geographic range.—Tasmania, Australia, Lesser Sunda north through Melanesia to Palau Islands. In Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Ngabad, Peleliu, Anguar.
Characters.—Adult: A small cormorant with upper parts black with dull greenish gloss; under parts white except vent and under tail-coverts which are sooty-black.
Measurements.—The author (1948: 41) gives the following measurements of two adult females from Peleliu: wing, 220 and 222; tail, 153 and 157; culmen from notch of suture between maxilla and quadratojugal bones, 35 and 36.
Specimens examined.—Total number, 15 (1 male, 12 females, 2 unsexed), as follows: Palau Islands, USNM—Peleliu, 6 (Aug. 27, Sept. 7, 10, 16); AMNH—exact locality not given, 9 (Nov. part).
Nesting.—Nehkorn (1899:235) recorded eggs taken at Palau. Some of the specimens obtained by Coultas in November, 1931, had swollen gonads. The author found no evidence of nesting in August and September, 1945, in the southern Palaus.
Food habits.—The author (1948: 41) found small fish in the stomachs of birds taken in August and September. The contents of each stomach averaged approximately 3 cc. in volume.
Parasites.—Uchida (1918:486) found the bird louse (Mallophaga), Lipeurus subsetosus, on the Little Pied Cormorant from Palau.
Remarks.—The Palaus mark the northernmost point of range of the Little Pied Cormorant. It does not occur in the Philippines and must have reached Palau from the New Guinea region. It is [Pg 79] unknown at Yap and other "high" islands in the Carolines. A sight record of this species at Pagan in the northern Marianas, made by Orii and reported in the Hand-list of Japanese Birds (Hachisuka et al., 1942:207), may be questioned. Amadon (1942:1) has studied the races of this species and points out that there is little geographic variation in the species; it is divisible into three subspecies. One of these is confined to New Zealand. Another occurs only on Rennell Island, Solomons. The six specimens taken by the NAMRU2 party at Peleliu included only two adults, whose measurements are within the range of those studied by Amadon.
The NAMRU2 party found the birds numerously in the southern Palaus in 1945. Birds were concentrated in the areas of mangrove swamp and on the tidal flats. In August and September, they were observed frequently in groups of 10 to 15, either sitting on the ground or perched on low mangroves or dead snags sunning themselves. Coultas (field notes) received reports that they nested at a freshwater lake on the "main island" (Babelthuap?)
Ripley (1948) reports the occurrence of "about a dozen anhingas (presumably Anhinga melanogaster)" at Babelthuap on 12 November 1946.
Pelecanus minor Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 572. (No type locality = Christmas Island, Indian Ocean.)
Pelecanus aquila? Quoy and Gaimard, Voy. "Uranie," Zool., 1824, p. 154 (Carolines).
Pelecanus aquilus? Lesson, Man. d'Ornith., 2, 1828, p. 354 (Carolines).
Atagen aquilus Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 61 (Ladrone or Marian Islands).
Tachypetes aquila Finsch, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); Hartert, Novit. Zool., 7, 1900, p. 11 (Ruk); Prowazek, Die deutschen Marianen, 1913, p. 100 (Marianen).
Tachypetes aquilus Finsch, Ibis, 1880, p. 333 (Taluit); idem, Journ. f. Ornith., 1880, pp. 296, 310 (Ponapé, Kuschai); idem, Ibis, 1881, pp. 109, 115 (Kuschai, Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 299, 353 (Mortlock, Ruk).
Fregata aquila Salvadori, Ornith. Papuasia, 3, 1882, p. 403 (Carolines, Marshalls); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, (1890-1891), p. 71 (Ruk, Luganor, Ponapé, Ualan, Marshalls); Ogilvie-Grant, Cat. Birds British Mus., 26, 1898, p. 443 (Carolines, Marshalls); Finsch, Deut. Ver. zum Schultze der Vogelwelt, 25, 1900, p. 452 (Ponapé, Kuschai, Marshalls); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 24 (Guam); Safford, The Plant World, 7, 1904, p. 267 (Guam); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall Inseln); Safford, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Cox, Island of Guam, 1917, p. 22 (Guam).
Fregata aquila palmerstoni Kuroda, in Momiyama, Birds Micronesia, 1922, p. 35 (Carolines, Marshalls).
Fregata minor peninsulae Mathews, Syst. Avium Australasianarum, 1, 1927, p. 233 (Carolines, Marshalls); Peters, Check-list Birds World, 1, 1931, p. 96 (Carolines?, Marshalls?).
Fregata minor palmerstoni Hand-list Japanese Birds, rev., 1932, p. 186 (Yap, Faraulep, Truk, Lukunor, Ponapé, Kusaie, Namu, Likieb); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam); Yamashina, Tori, 10, 1940, p. 676 (Maug, Bikar).
Fregata minor minor Hand-list Japanese Birds, 3d ed., 1942, p. 207 (Maug, Yap, Faraulep, Truk, Lukunor, Ponapé, Kusaie, Namu, Bikar, Likieb).
Fregata minor Borror, Auk, 64, 1947, p. 416 (Agrihan).
Geographic range.—Eastern Indian Ocean to western Pacific Ocean. Limits of range not certainly known. In Micronesia: Mariana Islands—Agrigan, Maug, Saipan, Guam; Caroline Islands—Yap, Faraulep, Truk, Lukunor, Ponapé, Kusaie; Marshall Islands—Namu, Bikar, Likieb, Kwajalein, Bikini.
Characters.—Adult male: Large sea bird with deeply forked tail; blackish but wing-coverts paler; head and back glossy purple and blue; breast lighter than belly. Adult female: Resembles adult male, but head blacker; chin and throat grayer; breast more whitish. Immature: Resembles adult, but head and throat whitish washed with buff; breast dark brown; belly whitish.
Measurements.—Two adult males measure: wing, 572; tail, 354, 396; exposed culmen, 98, 103; two adult females; wing, 583, 604; tail, 365; exposed culmen, 119, 127. These four specimens are from Bikini.
Specimens examined.—Total number, 10 (3 males, 7 females), from Marshall Islands, USNM—Bikini (March 11, 22, 29, 30, April 13, 29, May 3, 14).
Remarks.—The systematic position of the subspecies of Fregata minor in the Pacific area is not well established. I am following the committee who prepared the Hand-list of Japanese Birds (Hachisuka et al., 1942:207) in using the name F. m. minor, although a thorough study may show that these birds have closer relationships to one of the other subspecies of the Pacific area.
Fregata minor has been reported only occasionally in the Marianas and probably is not resident there. Borror (1947:416) reports the bird at Agrihan on August 11, 1945, and Seale (1901:24) mentions one taken at Guam in November, 1889. No records are known from the Palaus. In the Carolines the birds are probably resident, especially in the eastern part. In the Marshalls the species is a conspicuous member of the bird colonies on the coral atolls. Wallace (field notes) observed two birds at Loi Island in Kwajalein Atoll on May 7, 1944. Morrison obtained ten specimens at Bikini in the period from March through May in 1946.
Atagen (sic) Ariel Gray, Gen. Birds, 3, 1845, col. pl. [185]. (Type locality, Raine Island, Queensland.)
Pelecanus minor Lesson, Traite d'Ornith., 1831, p. 607 (Mariannes, Carolines).
Tachypetes minor Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 831 (Mackenzie Group); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 90 (Uap); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap).
Fregata minor Salvadori, Ornith. Papuasia, 3, 1882, p. 405 (Mariannes, Mackenzie); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 71 (Uap, Ngoli or Matelotas).
Tachypetes aquila var. minor Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 65 (Rota, Carolines, Marshalls); Hartert, Novit. Zool., 5, 1898, p. 68 (Marianne).
Fregata ariel Ogilvie-Grant, Cat. Birds British Mus., 26, 1898, p. 447 (Marianas, Carolines); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 25 (Guam?); Safford, Osprey, 1902, p. 70 (Marianas); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam).
Fregata ariel ariel Mathews, Birds Australia, 4, 1914-15, p. 285 (Carolines, Marshalls); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 36 (Yap, Ngoli, Rota); Hand-list Japanese Birds, rev., 1932, p. 186 (Rota, Yap, Ngulu, Uluthi); Hand-list Japanese Birds, 3d ed., 1942, p. 208 (Rota, Yap, Ngulu, Uluthi).
Geographic range.—China coast and Philippines south to Australia and east to Pacific islands. In Micronesia: Mariana Islands—Guam?, Rota; Caroline Islands—Yap, Ngulu, Ulithi.
Characters.—Adult male: Resembles F. m. minor, but smaller and blacker with upper parts lustrous greenish-blue and white patch on lower flank.
Adult female: Resembles adult male, but browner with paler nape and white breast. Immature: Resembles adult, but with head, chin, throat, and belly white washed with rufous.
Remarks.—Like F. minor, the Least Man-o'-War has not been observed often in Micronesia. Marche obtained one female at Rota in June, 1888. D. H. Johnson saw a bird thought to be of this species at Agfayan Bay, Guam, on 4 June 1945. Records from the western Carolines are few. There are no reports of this bird from the Palaus and the Marshalls. It may breed on some of the atolls in the Carolines.
The two species of man-o'-war birds may be difficult to distinguish in the field. The smaller size of Fregata ariel is perhaps the most useful character although it may be easily recognized also by the presence of the white flank patch, if it can be observed.
Both of the species of Fregata discussed in this report have representatives in the Atlantic, Indian and Pacific oceans. Murphy (1936:920) has shown that the man-o'-war birds are able to cross the Isthmus of Panamá between the Pacific and Atlantic oceans. This route may also be the means of dispersal for other species. The irregular distribution of these birds as well as of other sea birds in the oceanic islands of the Pacific may be caused by their remaining over waters which contain preferred foods and their avoidance of waters which lack preferred foods.
Ardea (Butorides) virescens var. amurensis Schrenck, Reise Amur Lande, 1, pt. 2, 1860, p. 441. (Type locality, Amurland.)
Butorides striatus javanicus Hand-list Japanese Birds, rev., 1932, p. 183 (Koror, Babelthuap).
Butorides striatus amurensis Hand-list Japanese Birds, 3d ed., 1942, p. 204 (Babelthuap, Koror); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Breeds in northeastern Asia, China, Japan, Bonins. Winters south to Philippines and Malaysia. In Micronesia: Palau Islands—Babelthuap, Koror.
Specimens examined.—Total number, 2 females, from Palau Islands, AMNH—exact locality not given (Nov. 13, Dec. 17-18).
Remarks.—The Amur Green Heron has been recorded as a winter visitor to the Palau Islands. Two females taken by Coultas in November and December, 1931, are immature. He comments (field notes) that he saw, in all, three birds in taro patch and mangrove swamp habitat.
Cancroma Coromanda Boddaert, Table Pl. enlum., 1783, p. 54. (Type locality, Coromandel.)
Ardeola ibis coromanda Hand-list Japanese Birds, rev., 1932, p. 183 (Koror).
Bubulcus ibis coromandus Hand-list Japanese Birds, 3d ed., 1942, p. 204 (Koror, Babelthuap); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—India, Ceylon, east to China and Japan and south to Malaysia. In Micronesia: Palau Islands—Babelthuap, Koror.
Remarks.—The Japanese ornithologists have recorded the Cattle Egret from Babelthuap and Koror in the Palau Islands. It is a winter migrant.
Ardea intermedia Wagler, Isis, 1829, p. 659. (Type locality, Java.)
Egretta intermedia intermedia Hand-list Japanese Birds, rev., 1932, p. 183 (Koror); Hand-list Japanese Birds, 3d ed., 1942, p. 203 (Koror); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 42 (Rota, Guam, Peleliu, Angaur, Ulithi).
Egretta intermedia Wharton and Hardcastle, Journ. Parasitology, 32, 1946, pp. 306, 310 (Ulithi); Baker, Ecol. Monogr., 16, 1946, p. 408 (Guam).
Geographic range.—India and Ceylon east to Malaysia, Philippines, China and Japan. In Micronesia: Mariana Islands—Saipan, Rota, Guam; Palau Islands—Koror, Peleliu, Angaur; Caroline Islands—Ulithi.
Characters.—Adult: A large white heron with green facial skin; black legs, feet and toes. In breeding plumage: Head with crest; neck and back with ornamental plumes; bill black. Winter plumage: Without crest or plumes; bill yellow with blackish tip. Immature: Resembles adult in winter plumage, but feathers soft and downy.
Measurements.—Five males from Saipan, Rota, Guam, and Angaur measure: wing, 295-321 (308); tail, 112-127 (119); culmen, 85-87 (87); tarsus, 111-118 (114); three females from Saipan, Ulithi, Angaur: wing, 294-301 (297); tail, 101-116 (110); culmen, 77-83 (80); tarsus, 108-115 (107).
Weights.—The author (1948:43) records the weights of two males from Guam as 445 and 463.
Specimens examined.—Total number, 8 (5 males, 3 females), as follows: Mariana Islands, USNM—Saipan, 2 (Sept. 29, Oct. 2)—Rota, 1 (Oct. 31)—Guam, 2 (June 13); Palau Islands, USNM—Angaur, 2 (Sept. 21); Caroline Islands, USNM—Ulithi, 1 (Aug. 15).
Food habits.—The NAMRU2 party found grasshoppers, other insects, spiders and lizards in the stomachs of egrets taken at Guam, Ulithi, and Angaur.
Parasites.—Wharton and Hardcastle (1946:306, 310) obtained the chiggers (Acarina), Neoschöngastia egretta and N. ewingi, from this egret from Ulithi.
Remarks.—The NAMRU2 party obtained Plumed Egrets at Rota, Guam, Ulithi, and Angaur in 1945. Previously, the only known record was from Koror, as reported in the Hand-list of Japanese Birds (Hachisuka et al., 1932:183). In addition, in 1945, Joe T. Marshall, Jr., obtained two birds at Saipan, and Gleise (1945:220) reported seeing "white herons" at Tinian, which probably were egrets. Gleise estimated the number of these birds at Tinian to be fifty; he found them in swampy areas. At Rota, the NAMRU2 party found a flock of sixteen birds in a cultivated field on October 31. At Guam, egrets were first observed on February 25, 1945, when a flock of fourteen was found in a fallow rice paddy near Piti. This flock remained in this area and were seen occasionally until as late as June 13, when two were taken as specimens. A short time later (June 30) the entire area was cleared for military use and the birds were seen no more. At Agfayan Bay a flock of sixteen birds was found on the beach on July 24 and on August 6. These birds kept apart from Reef Herons which were also in the area. In June, 1946, M. Dale Arvey observed egrets in swamps along the Ylig River at Guam. At Ulithi Atoll, three egrets were seen on August 15 at Potangeras Island, feeding in grassy areas adjacent to the beach. In the southern Palaus, the NAMRU2 party found egrets in August and September on tidal flats and open grasslands at Peleliu and Angaur. At Peleliu, a flock of twenty-five birds was seen on September 8 and a flock of eight birds on September 16. At Angaur approximately twenty birds were seen in groups of five or more on September 21. These birds, unlike the Reef Herons, preferred grasslands to beach areas for feeding and were usually seen in sizeable flocks.
There was no evidence of breeding; specimens examined were either immatures or adults in winter plumage, since they had yellow bills tipped with black and slight or no development of ornamental plumes. Birds taken at Guam in June and at Angaur in September had no ornamental plumes, while birds taken at Ulithi in August, at Saipan in September and October, and at Rota in late October show some development of the back plumes. Wharton and Hardcastle (1946:306) found the same species of chigger on Plumed Egrets from Ulithi and from Okinawa in the Riu Kiu Islands. The [Pg 84] NAMRU2 party observed the birds in Micronesia from February until October in 1945, and although the Plumed Egret may be considered as merely a visitor to Micronesia, it would not be surprising to find nests there. The fact that several new distributional records were obtained for Micronesia in 1945 may indicate that the birds have been overlooked by ornithologists in the past or that the birds are increasing the breadth of their winter (or breeding?) range.
Ardea sacra Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 640. (Type locality, Tahiti.)
Ardea jugularis Kittlitz, Observ. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 286, 299, 304 (Ualan, Lougounor, Guahan); Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen); Kittlitz, Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 63 (Ualan); Pelzeln, Reise "Novara," Vögel, 1865, pp. 118, 162, 120, 121 (Puynipet, Ualan).
Ardea (Herodias) atra Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 48 (Ladrone or Marian Islands, Caroline Islands).
Ardea sacra Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 831 (Matelotas Islands); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 8, 118 (Pelews); Finsch and Hartlaub, Journ. f. Ornith., 1870, p. 137 (Pelews, Matelotas); Gray, Hand-list Birds, 3, 1871, p. 28 (Marian, Carolines, Pelews, Matelotas); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 104 (Pelew, Uap, Ualan); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 32 (Palau); idem, Journ. Mus. Godeffroy, 12, 1876, pp. 18, 38 (Ponapé, Ualan); idem, Proc. Zool. Soc. London, 1877 (1878), p. 781 (Ponapé); idem, Journ. f. Ornith., 1880, pp. 294, 306 (Ponapé, Kuschai); idem, Ibis, 1880, pp. 220, 330, 332 (Taluit); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); idem, Ibis, 1881, pp. 105, 106, 109, 115 (Kushai, Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 299, 353 (Mortlocks, Ruk); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 51 (Jaluit, Kuschai); Oustalet, Le Nat., 1889, p. 261 (Mariannes); Wiglesworth, Ibis, 1893, p. 211 (Marshalls); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 36 (Guam, Marshalls, Palaos, Carolines); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall-Inseln).
Demiegretta sacra Salvadori, Ornith. Papuasia, 3, 1882, p. 348 (Marshalls, Ualan, Ponapé, Ruck, Pelew, Mariannis); Wiglesworth, Abhandl. Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 67 (Marianne, Pelews, Luganor, Ruk, Ponapé, Ualan, Taluit); Hartert, Novit. Zool., 5, 1898, p. 64 (Saipan); Sharpe, Cat. Birds British Mus., 26, 1898, p. 137 (Pelew, Carolines, Marshalls); Hartert, Novit. Zool., 7, 1900, p. 11 (Ruk); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 29 (Guam); Safford, Osprey, 1902, p. 67 (Marianas); idem, The Plant World, 7, 1904, p. 266 (Guam); Kuroda, Avifauna Riu Kiu, 1925, p. 129 (Micronesia); Bryan, Guam, Rec., vol. 13, no. 2, 1936, p. 15 (Guam); Bequaert, Occ. Papers Bernice P. Bishop Mus., 16, 1941, p. 266 (Kusaie).
Demigretta sacra Safford, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Prowazek, Die deutschen Marianen, 1913, p. 101 (Saipan, Tinian); Cox, Island of Guam, 1917, p. 21 (Guam); Bequaert, Mushi, 12, 1939, p. 81 (Kusaie); Warton, Ecol. Monogr., 16, 1946, p. 175 (Guam); Warton and Hardcastle, Journ. Parasitology, 32, 1946, pp. 306, 316 (Ulithi, Guam).
Demiegretta jugularis Takatsukasa and Kuroda, Tori, 1, 1915, p. 50 (Truk, Ponapé, Pelew).
Demiegretta jugularis grayi Uchida, Annot. Zool. Japon., 9, 1918, pp. 484, 488, 490 (Ponapé).
Demiegretta sacra sacra Kuroda, in Momiyama, Birds Micronesia, 1922, p. 36 (Guam, Saipan, Angaur, Luganor, Yap, Ngoli, Ruk, Ponapé, Kusaie, Taluit).
Demigretta sacra sacra Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 171 (Kusaie); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 198 (Carolines); Yamashina, Tori, 7, 1932, p. 406 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 183 (Saipan, Guam, Babelthuap, Peliliu, Angaur, Ngulu, Yap, Truk, Lukunor, Ponapé, Kusaie, Jaluit, Majuro); Mayr and Amadon, Amer. Mus. Novit., no. 1144, 1941, p. 10 (Guam, Saipan, Palau, Ponapé, Kusaie, Ruk, Tah); Hand-list Japanese Birds, 3d ed., 1942, p. 203 (Saipan, Rota, Babelthuap, Peliliu, Angaur, Ngulu, Yap, Ulithi, Truk, Lukunor, Ponapé, Kusaie, Jaluit, Arhno, Majuro, Moloclab, Wotze, Likieb, Ailuk); Mayr, Birds Southwest Pacific, 1945, pp. 51, 284 (Micronesia); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 90 (Tinian); Strophlet, Auk, 63, 1946, p. 535 (Guam); Borror, Auk, 64, 1947, p. 417 (Agrihan); Stott, Auk, 64, 1947, p. 524 (Saipan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 42 (Rota, Guam, Peleliu, Ulithi, Truk).
Demigretta sacra micronesiae Momiyama, Tori, 5, no. 22, 1926, p. 110 (Type locality, Caroline Islands; Pelew, Yap, Truk, Ponapé, Kusaie).
Geographic range.—Coasts of Asia and adjacent islands from Korea and Japan south to Malaysia, Australia, Melanesia, Polynesia and Micronesia. In Micronesia: Mariana Islands—Agrigan, Tinian, Saipan, Rota, Guam; Palau Islands—Babelthuap, Koror, Garakayo, Ngesebus, Peleliu, Ngabad, Anguar; Caroline Islands—Ulithi, Yap, Ngulu, Truk, Lukunor, Ponapé, Kusaie; Marshall Islands—Jaluit, Arhno, Majuro, Maloclab, Wotze, Likieb, Ailuk, Bikini, Eniwetok, Kwajalein.
Characters.—A medium-sized heron with three color phases: in gray phase color of body varies from "deep blackish-slate" to light bluish-slate, particularly on the breast, with a white gular stripe; wear and fading causes the color of the body to change to brownish-slate; bluish-gray ornamental plumes may be present on adult; in white phase color of body is pure white in adult stage; plumage of immature may be mottled; in mottled phase there may be a variable amount of gray and white (for complete study of plumages of Demigretta sacra see Mayr and Amadon, 1941:4).
Measurements.—Mayr and Amadon (1941:1) record the length of the wing of thirty adults from the Marianas and Carolines as 268-309 (284). Seven adult males obtained by the NAMRU2 party at Rota, Guam and Peleliu measure: wing, 287-307 (294); tail, 95-114 (101); culmen, 91-101 (96); tarsus, 78-87 (82); seven adult females, from Rota and Guam: wing, 265-285 (275); tail, 87-96 (91); culmen, 86-92 (89); tarsus, 72-79 (76).
Weights.—The author (1948:42) lists the following weights: four adult males from Guam (gray phase) 590-667 (614); two adult males from Guam (white phase) 600 and 662; five adult females from Guam and Rota (gray phase) 477-553 (506).
Specimens examined.—Total number, 80 (38 males, 40 females, 2 unsexed) as follows: Mariana Islands, USNM—Rota, 3 (Oct. 18, Nov. 2, 5)—Guam, 21 (May 11, June 6, 18, July 6, 8, 16, 24, 27, Aug. 6, 8, 27); AMNH—Saipan, 2 (July 22)—Guam, 9 (Feb. 11, Mar. 6, 7, April 11, Aug. 15, Sept. 14, 16, Nov. 27, Dec. 20); Palau Islands, USNM—Peleliu, 3 (Sept. 10, 16); AMNH—exact locality not given, 5 (Nov. 8, 21, 23); Caroline Islands, USNM—Ulithi, 1 (Aug. 15)—Kusaie, 1 (Feb. 8); AMNH—Truk, 3 (Feb. 18, May 20, Nov. 5)—Tah, 2 (Oct. 18)—Ponapé, 2 (Nov. 21, undated)—Kusaie, 26 (Jan. 25, 26, Feb., Mar. 10-20, 20-30, April 1-10, 18); Marshall Islands, USNM—Bikini, 2 (March 29, April 2).
Nesting.—The Reef Heron apparently nests on most of the islands in Micronesia. The eggs are laid in a nest of grass and twigs on or near the ground. Hartert (1898:64) records a nest found in grass at Saipan on July 28, 1895. Yamashina (1932a:406) reports on one egg taken at Ponapé on July 23, 1931. Marshall (1949:219, fig. 37) found a breeding bird in April at Tinian. Coultas (field notes) learned from the natives at Ponapé that the Reef Heron builds a nest of small sticks near the ground in the mangrove thickets. Two or three eggs are laid, and nests can be found at various times of the year. Mayr and Amadon (1941:4) comment on the prolonged breeding season and report six sets of eggs from Polynesia taken in January, March, April, September, October, and November.
Food habits.—The author (1948:42) found fish and crabs in the stomachs of birds taken at Guam, Ulithi and Peleliu.
Parasites.—Uchida (1918:484, 488, 490) found the following bird lice (Mallophaga) on the Reef Heron at Ponapé: Nirmus orarius, Colpocephalum importunum, and Myrsidea teraokai. Bequaert (1939:81 and 1941:266) found the fly (Hippoboscidae), Ornithoctona plicata, on the heron at Kusaie. Wharton (1946:175) and Wharton and Hardcastle (1946:306, 316) obtained chiggers (Acarina), Neoschöngastia egretta and N. carveri, from the Reef Heron at Guam and Ulithi.
Remarks.—The species Demigretta sacra contains two subspecies, the widespread D. s. sacra and a larger form, D. s. albolineata (Gray), known from New Caledonia and the Loyalty Islands. The latter subspecies is surrounded by the former, a distribution which closely parallels that in each of the species Phalacrocorax melanoleucus and Gygis alba of Oceania. Recently Delacour (in Delacour and Mayr, 1945b:105) has dropped the name Demigretta placing all of the forms of this genus in Egretta. He says, "We cannot accept the genus Demigretta, which is based on the more extended feathering of the tibia, the different length and texture of the feathers of the trains, the shortness of the tarsus and the presence of a dark gray color phase. The latter exists in the Madagascan and African subspecies of Egretta garzetta."
The Reef Heron is a conspicuous member of the bird life of Micronesia, being recorded from most of the island groups. It prefers the placid and shallow waters of the lagoons and tidal beaches where it obtains the littoral animal life as food. The birds are seldom seen inland and usually frequent the beaches and rocky coasts. In this respect there is little opportunity for competition with the migratory Plumed Egret, which prefers the grassy upland and marsh areas and inland ponds. The Reef Heron is a quiet, usually solitary, and retiring bird, being exceedingly difficult to approach, especially when found on the open tidal flats.
The problem of plumages and color phases in the Reef Heron has been treated by Mayr and Amadon (1941:4-10). Specimens which they examined from Micronesia were found to be 54 percent gray, 40 percent white, and 6 percent mottled. Of the birds obtained by NAMRU2 field parties, fewer than 40 percent were white. Field counts showed a considerable variation in the ratio of grays to [Pg 87] whites: Guam—6 grays to 4 whites; Ulithi—4 grays, 6 whites, 1 mottled; Palau—equal number of grays and whites; Truk—2 whites, 1 gray, 1 mottled. For some unknown reason, the gray birds were more easily approached than the white birds. Gleise and Genelly (1945:221) saw one white Reef Heron at Eniwetok. Wallace (field notes) found white herons more numerous than gray ones at Kwajalein in 1944 and 1945. Borror (1947:417) observed gray birds at Agrigan. Stott (1947:524) saw one blue heron on December 24, at Saipan. The 150 birds seen by him at Lake Susupe in December probably were Plumed Egrets.
In discussing the variation in the color phases of the Reef Heron throughout its range, Mayr (1924b:237) suggests that the reduced variability of small populations may not be due to accidental gene loss, but instead to the population having descended from a single pair or from one fertilized female. The descendents would naturally possess only those characters provided for in the genetic make-up of the parents. Reef Herons on New Zealand and in the Marquesas Islands all are gray, while at other island groups different proportions of gray and white individuals occur; such phenomena may result because of the genetic constitution of the "founders."
Ardes Nycticorax Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 142. (Type locality, Southern Europe.)
Nycticorax griseus Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 105 (Uap); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 69 (Uap).
Nycticorax nycticorax Sharpe, Cat. Birds British Mus., 26, 1898, p. 146 (Yap).
Nycticorax nycticorax nycticorax Kuroda, in Momiyama, Birds Micronesia, 1922, p. 36 (Mackenzie, Yap); Hand-list Japanese Birds, rev., 1932, p. 183 (Yap, Uluthi); Hand-list Japanese Birds, 3d ed., 1942, p. 204 (Yap, Uluthi); Mayr, Birds Southwest Pacific, 1945, p. 302 (Marianas, Yap).
Geographic range.—Europe and Africa east to Japan and Malaysia. In Micronesia: Mariana Islands—Tinian; Palau Islands—Koror; Caroline Islands—Yap, Ulithi, Truk.
Specimens examined.—Total number, 2 immature females, as follows: Palau Islands, USNM—Koror, 1 (Nov. 27); Caroline Islands, AMNH—Truk, 1 (June 18).
Remarks.—The Black-crowned Night Heron is a winter visitor to western Micronesia. Marshall (1949:221) records six of these birds on Tinian on April 4, 1945, and one on Koror on November 27.
Nycticorax caledonicus pelewensis Mathews, Bull. British Ornith. Club, 46, 1926, p. 60. (Type locality, Pelew Islands.)
Nycticorax caledonicus Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 117, 118 (Pelew); Sharpe, Cat. Birds British Mus., 26, 1898, p. 158 (Pelew); Hartert, Novit. Zool., 7, 1900, p. 10 (Ruk); Reichenow, Die Vögel, 1, 1913, p. 255 (Palauinseln); Takatsukasa and Kuroda, Tori, 1, 1915, p. 50 (Pelew); Uchida, Annot. Zool. Japon., 9, 1918, p. 486 (Palau); Wetmore, in Towsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 172 (Uala, Truk Atoll); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 37 (Pelew, Ruk).
Nycticorax manillensis Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 105 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 33 (Palau); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 353 (Ruk); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 69 (Pelew, Ruk).
Nycticorax caledonicus pelewensis Mathews, Syst. Avium Australasianarum, 1, 1927, p. 200 (Pelew, Carolines); Peters, Proc. Boston Soc. Nat. Hist., 39, 1930, p. 271 (Pelew, Carolines); Peters, Check-list Birds World, 1, 1931, p. 115 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 183 (Palau, Truk); Hand-list Japanese Birds, 3d ed., 1942, p. 204 (Babelthuap, Koror, Coracel, Truk); Amadon, Amer. Mus. Novit., no. 1175, 1942, p. 6 (Palau, Ruk); Mayr, Birds Southwest Pacific, 1945, p. 285 (Palau, Truk); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 43 (Angaur, Peleliu, Garakayo, Truk).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Coracel, Garakayo, Peleliu, Ngabad, Angaur; Caroline Islands—Truk.
Characters.—Adult: Size medium; head and nape dark slaty-black; occipital plumes white with dark tips and shafts; back dark reddish-brown, lighter on sides of neck, wings, wing coverts, rump, and tail; under parts whitish with light reddish-brown on sides of neck extending to throat and upper breast; tibia with some brownish feathers; underwing pinkish; feet yellowish-brown; bill black.
Immature: Resembles adult, but upper parts mottled black with reddish-brown; underparts with lighter streaks of brown and whitish on breast; feet yellowish; bill black above, yellowish below.
Adult resembles N. c. manillensis Vigors, but is duller above.
Measurements.—Two adult males from Peleliu measure: wing, 293, 299; tail, 105, 107; culmen, 82, 89; tarsus, 79, 81; seven adult females from Peleliu: wing, 269-286 (280); tail, 101-106 (104); culmen, 76-84 (80); tarsus, 78-83 (80); one adult female from Truk: wing, 280; tail, 97; culmen, 83; tarsus, 79.
Specimens examined.—Total number, 27 (5 males, 18 females, 4 unsexed), as follows: Palau Islands, USNM—Peleliu, 9 (Aug. 31, Sept. 1, 5, 6, 8, Dec. 6); AMNH—exact locality not given, 16 (Nov. 7, 8, 13, 23, 25, Dec. 1, undated); Caroline Islands, USNM—Truk, 1 (Feb. 16); AMNH—Truk, 1 (May 25).
Nesting.—The NAMRU2 party observed a nesting colony of these night herons at Peleliu on August 29, 1945. Approximately eight nests were observed in a grove of saplinglike trees at the edge of a mangrove swamp. These nests were 15 to 20 feet above the ground; most of them contained one or two nestling birds. Two subadults and three nestlings in postnatal molt were obtained; no eggs were found. Marshall (1948:219) records breeding in August, September and December.
Food habits.—Baker (1948:43) reports that stomachs of night herons obtained by the NAMRU2 party at Peleliu contained a great variety of animal foods, including eels, fish, lizards (skinks), crabs, shrimp, and insects. The stomach of one adult contained 14 large grasshoppers and four fish, totaling about 15 cc. in volume. The nestlings had eels, skinks, and insects in their stomachs.
Parasites.—Uchida (1918:486) found the bird louse (Mallophaga), Lipeurus baculus, on the night heron at Palau.
Remarks.—Amadon (1942:4-8) has made the most recent study of the species Nycticorax caledonicus and recognizes eight subspecies from Australia and New Calendonia north to the Caroline and Bonin islands. This is one of the few tropical and subtropical species which has extended its range to the Bonin islands. The discontinuous distributions of this species prevents an accurate estimation of the route by which it reached the Bonins. The presence of the bird at Palau and at Truk makes it difficult to account for its absence at Yap and other intervening, and seemingly suitable, islands. Populations at Palau and Truk appear to be similar and are placed in the same subspecies, but when adequate material is available from Truk, further study may reveal that the populations on the two islands (Truk and Palau) are recognizably different.
At the southern Palau Islands, night herons were found by the NAMRU2 party in mangrove swamps, lagoons and on beaches. I found them to be inactive during the daytime; the birds were usually perched singly in trees or at the edge of the water. The birds appeared to have special roosting places and were observed sitting in the same place on several different occasions. McElroy of the NAMRU2 party reported seeing three night herons at Truk in December, 1945.
Nycticorax goisagi Temminck, Pl. Col., livr. 98, 1835, pl. 582. (Type locality, Japan.)
Gorsakius goisagi Hand-list Japanese Birds, rev., 1932, p. 184 (Koror); Hand-list Japanese Birds, 3d ed., 1942, p. 204 (Koror); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Eastern China, Japan, Riu Kius, Formosa, and Philippine Islands. In Micronesia: Palau Islands—Koror.
Remarks.—Gorsachius goisagi has been recorded from Koror in the Palau Islands. It may be classed as a rare migrant to western Micronesia.
Ardea melanolopha Raffles, Trans. Linn. Soc. London, 13, 1822, p. 326. (Type locality, Western Sumatra.)
Nycticorax goisagi Hartlaub and Finsch, Proc. Zool. London, 1868, pp. 8, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, p. 89 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 68 (Pelew).
Nycticorax melanolophus Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 35 (Palau).
Gorsachius melanolophus Sharpe, Cat. Birds Brit. Mus., 26, 1898, p. 166 (Pelew).
Gorsahius melanolophus melanolophus Hand-list Japanese Birds, rev., 1932, p. 184 (Pelew); Hand-list Japanese Birds, 3d ed., 1942, p. 204 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Gorsachius melanolophus melanolophus Mathews, Syst. Avium Australasianarum, 1, 1927, p. 200 (Pelew).
Geographic range.—India, Ceylon, southern China, Formosa, Indochina, Malaysia. In Micronesia: Palau Islands—exact locality unknown.
Remarks.—Captain Tetens obtained a specimen of this bittern at the Palau Islands which was reported on by Hartlaub and Finsch (1868a:8, 1868b:118). It is probably a rare straggler to western Micronesia. The specimen has not been seen by me; it may be of the subspecies G. m. kutteri (Cabanis), which is known from the Philippine Islands.
Ardea Sinensis Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 642. (Type locality, China.)
Ardea lepida Lesson, Traité d'Ornith., 1831, p. 573 (Marianne); Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen).
Ardea sinensis Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 305 (Guahan); Gray, Hand-list Birds, 3, 1871, p. 31 (Marian); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 105 (Uap); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 33 (Palau, Yap); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 353 (Ruk).
Ardea (Ardetta) sinensis Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 49 (Ladrone or Marian Islands).
Ardetta Sinensis Salvadori, Ornith. Papuasia, 3, 1882, p. 364 (Pelew, Carolines, Mariannis); Oustalet, Le Nat., 1889, p. 261 (Mariannes); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 68 (Marianne, Uap, Ruk, Pelew); Oustalet, Nouv. Arch, Mus. Hist. Nat. Paris, (3), 8, 1896, pp. 38, 39 (Guam, Saypan, Ponapi, Ruk, Palaos); Hartert, Novit. Zool., 5, 1898, p. 65 (Guam); Sharpe, Cat. Birds British Mus., 26, 1898, p. 227 (Marianne, Carolines, Pelew); Hartert, Novit. Zool., 7, 1900, p. 11 (Ruk); Safford, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Prowazek, Die deutschen Marianan, 1913, p. 100 (Saipan); Cox, Island of Guam, 1917, p. 21 (Guam).
Ardetta bryani Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 27 (Type locality, Guam); Safford, Osprey, 1902, p. 66 (Guam); idem, The Plant World, p. 266 (Guam).
Ardetta sinensis sinensis Takatsukasa and Kuroda, Tori, 1, 1915, p. 50 (Ruk, Pelew).
Ixobrychus sinensis bryani Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, pp. 173, 175 (Guam); Kuroda, in Momoyama, Birds Micronesia, 1922, p. 37 (Guam,?Yap,?Mackenzie,?Pelew); idem, Avifauna Riu Kiu, 1925, p. 134 (Guam,?Yap,?Pelew); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 202 (Guam,?Pelew); Peters, Check-list Birds World, 1, 1931, p. 121 (Guam); Hand-list Japanese Birds, rev., 1932, p. 184 (Saipan, Tinian, Rota, Guam); Oberholser, Bull. U. S. Nat. Mus., 159, 1932, p. 18 (Guam); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 205 (Saipan, Tinian, Rota, Guam); Amadon, Bull. Bernice P. Bishop Mus., 186, 1945, p. 25 (Guam); Stott, Auk, 64, 1947, p. 525 (Saipan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 44 (Rota, Guam).
Ixobrychus sinensis moorei Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 173 (Type locality, Uala, Truk group); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 38 (Ruk); Kuroda, Avifauna Riu Kiu, 1925, p. 134 (Ruk); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 202 (Middle Carolines); Peters, Check-list Birds World, 1, 1931, p. 121 (Truk); Hand-list Japanese Birds, rev., 1932, p. 184 (Palaus, Yap, Truk); Oberholser, Bull. U. S. Nat. Mus., 159, 1932, p. 17 (Carolines, ?Pelews); Hand-list Japanese Birds, 3d ed., 1942, p. 205 (Babelthuap, Koror, Yap, Truk); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 44 (Truk, Peleliu).
Ixobrychus sinensis Hartert, Vogel pal. Fauna, 10, 1920, p. 1260 (Truk, Palau, Guam); Mayr, Birds Southwest Pacific, 1945, p. 285 (Marianas, Palau, Yap, Truk); Watson, The Raven, 17, 1946, p. 41 (Guam); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 91 (Tinian); Wharton, Ecol. Monogr., 16, 1946, p. 174 (Guam); Delacour and Mayr, Birds Philippines, 1946, p. 29 (Guam); Strophlet, Auk, 63, 1946, p. 536, (Guam); Baker, Condor, 49, 1947, p. 125 (Guam).
Ixobrychus sinensis sinensis Hachisuka, Birds Philippines, 1, 1932, p. 365 (Guam, Truk); Robinson and Chasen, Birds Malay Peninsula, 3, 1936, p. 195 (Marianne).
Ixobrychus sinensis palewensis Momiyama, Bull. Biogeogr. Soc. Japan, 2, 1932, p. 333 (Type locality, Pelew); Mathews, Ibis, 1933, p. 88 (Pelew).
Ixobrychus sinensis yapensis Momiyama, Bull. Biogeogr. Soc. Japan, 2, 1932, p. 333 (Type locality, Yap); Mathews, Ibis, 1933, p. 89 (Yap).
Geographic range.—Northeastern China and Japan south to Micronesia, Malaysia, Burma, India and Ceylon. Winter visitor to Papuan region. In Micronesia: Mariana Islands—Saipan, Tinian, Rota, Guam; Palau Islands—Babelthuap, Koror, Peleliu; Caroline Islands—Yap, Truk.
Characters.—Adult male: A small bittern with crown and short occipital crest slaty-black; mantle light buffy-brown; back and rump gray; tail black; wing-coverts brownish-buff; primaries and secondaries slaty-black; underparts yellowish buff; chin and throat whitish; sides of head and neck and a line of feathers across chest blackish edged with buff; bill yellowish green; feet greenish yellow.
Adult female: Resembles adult male, but with upper parts mottled brown and golden chestnut; underparts deep buff streaked with pale brown on neck.
Immature: Resembles adult, with upper parts heavily streaked with blackish-brown, and underparts streaked with chestnut and dark brown.
Measurements.—Measurements of specimens from Micronesia are given in table 16.
| Location | Sex | No. | Wing | Tail | Full culmen |
Tarsus |
| Yap, Truk | males | 6 | 132 | 43 | 56 | 44 |
| 130-134 | 41-47 | 54-59 | 42-47 | |||
| Guam | males | 11 | 134 | 47 | 57 | 46 |
| 127-138 | 45-50 | 55-60 | 45-47 | |||
| Guam | females | 10 | 130 | 46 | 57 | 45 |
| 127-134 | 44-49 | 55-59 | 43-47 |
Weights.—The author (1948: 44) records the weights of eight adult males from Guam as 82-103 (92) and eight adult females from Guam as 84-109 (95).
Specimens examined.—Total number, 69 (34 males, 27 females, 8 unsexed), as follows: Mariana Islands, USNM—Saipan, 1 (Sept. 30)—Tinian, 1 (Oct. 13)—Guam, 29 (May 16, June 4, 6, 7, 8, 14, 18, 19, July 10, 16, 18, 24, 27, Aug. 4); AMNH—Saipan, 1 (Aug. 6)—Tinian, 3 (Sept. 13)—Guam, 14 (Feb. 1, Mar. 13, 29, July 11, 13, 25, Aug. 1, 7, 13, Sept. 4, 10, Dec. 8); Palau Islands, AMNH—exact locality not given, 6 (Nov. 19, 21, 23, 25, Dec. 1, 18); Caroline Islands, USNM—Truk, 1 (Feb. 16); AMNH—Yap, 1 (not dated)—Truk, 12 (Feb. 9, Mar. 5, 17, May 7, June 13, 14, 15, Oct. 3, Nov. 1, 5, Dec. 20).
Nesting.—The author (1948:44) records a nest found by the NAMRU2 party near Achang Bay on Guam on June 6, 1945. It was found in a cane thicket at the edge of a fallow rice paddy, approximately four feet from the ground and was constructed of about three quarts of reeds and cane. Two eggs found in the nest are oval, white with a greenish cast and measure 33 by 24 and 34 by 24. On February 1, 1945, the writer found two recently occupied nests of the Chinese Least Bittern at Oca Point, Guam. These nests were in dense inkberry brush approximately five feet above the ground. The area was not marshy, the nearest water being at the beach some 300 yards away. Nearby one of the nests was found a young bittern, which apparently had only recently left the nest. The pin feathers were growing. A parent bird remained in the vicinity with the young bird until it left the area after March 9.
Food habits.—The Chinese Least Bittern feeds on animal foods obtained along waterways, marshes and beaches as well as in forests and fields. The NAMRU2 party observed several types of insects in the stomachs of birds taken at Guam. Seale (1901:27) found black crickets in stomachs of bitterns taken at Guam. Coultas (field notes) learned from the natives of the Palau Islands that the bittern feeds on land mollusks.
Parasites.—Wharton (1946:174) obtained the chigger (Acarina), Trombicula acuscutellaris, from the Chinese Least Bittern at Guam.
Remarks.—The Chinese Least Bittern has been regarded by many workers as consisting of several geographic races; as many as eight have been recognized. Other workers have concluded that I. sinensis is made up of highly variable populations and that it lacks well-fined geographic variation. Hartert (1920:1260), Hachisuka (1932:365), and Mayr (1945a:285) have reached the latter conclusion. As yet this problem has not been satisfactorily solved; a thorough study is needed, but may not be possible until additional material, especially from the continental areas, can be obtained. In coloration there appears to be little difference between birds from the various localities in Micronesia. These birds may average slightly paler than populations from the continental areas, but on this basis I doubt that a person could recognize the Micronesian birds in a group of skins from many other localities. Birds in fresh plumage may show geographic differences better than slightly worn specimens. Measurements made by the author offer no clear-cut differences either.
I. sinensis was first recorded in Micronesia by Quoy and Gaimard (1824:536), whose ship, the "Uranie," stopped at Guam. They called the bird "Petit Héron aux ailes noires." Most of the ornithological collectors in the years following Quoy and Gaimard obtained this bittern in Micronesia. At Guam, its abundance and the ease with which it may be approached and shot is attested by the large series obtained by collectors: Seale (1901:27) took eight birds; [Pg 93] Marche (Oustalet, 1896:36) took eighteen skins; the NAMRU2 party took twenty-nine skins.
The Chinese Least Bittern is found in habitats associated with both salt water and fresh water, as well as in upland habitat in Micronesia. The bird appears to be well adapted to areas of open forest and coconut groves. Coultas (field notes) found the birds in taro patches in the Palaus. Although a considerable amount of field observing was done in the southern Palaus, the NAMRU2 party saw only one bird (September 13, 1945, at Peleliu). Perhaps the birds prefer Babelthuap and other large islands farther north in the chain. McElroy found bitterns in taro patches at Truk in December, 1945. The NAMRU2 party did not find any birds at Rota in October and November, 1945. Downs (1946:91) found the birds in upland sugar cane and beach habitats on Tinian.
Regarding the bittern in the Palaus, Coultas (field notes) writes, "Always found alone, never a pair. A bird that is not easily frightened. In the heat of the day, one finds it standing in the shade of a taro leaf quietly viewing the intruder and very reluctant about moving. I have tossed pieces of earth and sticks at the bird to encourage him to fly so that I would not blow him to pieces when I shot, but my efforts at dislodgement have been rewarded by harsh scolding squawks. It became necessary for me to move into proper gun range. I have also found them perched in low trees at the edge of taro swamps. In flight they are atrociously awkward. They can't keep a course and their legs dangle every-which way. Their jerky, slow flight usually ends abruptly when the bird becomes entangled in weeds or the branches of trees. Extracting himself from his predicament he is soon in another and invariably resorts to blasphemy."
Ardetta eurhythma Swinhoe, Ibis, 1873, p. 74, pl. 2. (Type locality, Amoy Shanghai.)
Ixobrychus eurythmus Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Southeastern Siberia and Japan south to India and Malaysia. In Micronesia: Palau Islands—exact locality unknown.
Specimens examined.—Total number, 3 (2 males, 1 female), from Palau Islands, AMNH—exact locality not given (Nov. 19, 21, Dec. 3).
Remarks.—Coultas obtained three immature specimens at Palau in November and December, 1931.
Ardea flavicollis Latham, Ind. Ornith., 2, 1790, p. 701. (Type locality, India.)
Dupetor flavicollis Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 26 (Guam); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam).
Dupetor f. flavicollis Mayr, Birds Southwest Pacific, 1945, p. 302 (Guam).
Geographic range.—Central China south to Malaysia and India. In Micronesia: Mariana Islands—Guam.
Remarks.—Seale (1901:26) records a female shot at the Agaña River on Guam on June 11, 1900. The skin probably is in the Bernice P. Bishop Museum in Honolulu.
Anas oustaleti Salvadori, Bull. British Ornith. Club, 4, 1894, p. 1. (Type locality, Mariannis Islands.)
Anas oustaleti Salvadori, Cat. Birds British Mus., 27, 1895, p. 189 (Guaham); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 49 (Guam); Hartert, Novit. Zool., 5, 1898, p. 66 (Guam, Saipan); Wheeler, Report Island of Guam, 1900, p. 13 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 25 (Guam, Saipan); Matschie, Journ. f. Ornith., 1901, pp. 110, 113 (Guam, Saipan); Safford, Osprey, 1902, p. 66 (Mariannas); idem, Amer. Anthro., 4, 1902, p. 711 (Guam); idem, The Plant World, 7, 1904, p. 267 (Guam); Dubois, Syn. Avium, 2, 1904, p. 990 (Mariannes); Safford, Contr. U. S. Nat. Herb., 9, 1905, pp. 80, 126 (Guam); Prowazek, Die deutschen Marianen, 1913, pp. 47, 100 (Marianen); Cox, Island of Guam, 1917, p. 22 (Guam); Phillips, Nat. Hist. Ducks, 2, 1923, p. 53 (Guam, Saipan); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 214 (Guam, Saipan); Berlioz, Bull. Mus. Hist. Nat. Paris, 2d ser., 1, 1929, p. 67 (Guam); Peters, Check-list Birds World, 1, 1931, p. 159 (Guam, Tinian, Saipan); Hand-list Japanese Birds, rev., 1932, p. 184 (Guam, Tinian, Saipan); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam); Kuroda, Tori, 11, 1941-42, pp. 99, 443 (Marianas); Hand-list Japanese Birds, 3d ed., 1942, p. 205 (Guam, Tinian, Saipan); Amadon, Amer. Mus. Novit., no. 1237, 1943, p. 1 (Marianne); Mayr, Birds Southwest Pacific, 1945, p. 285 (Marianas); idem, Audubon Mag., 47, 1945, p. 282 (Marianas); Baker, Trans. 11th N. Amer. Wildlife Conf., 1946, p. 208 (Guam); Stott, Auk. 64, 1947, p. 525 (Saipan); Baker, Smithson, Misc. Coll., vol. 107, no. 15, 1948, p. 45 (Saipan, Tinian); Momiyama, Pacific Science, 2, 1948, p. 121 (Saipan, Tinian, Guam).
Polionetta oustaleti Kuroda, in Momiyama, Birds Micronesia, 1922, p. 39 (Guam, Saipan).
Anas superciliosa oustaleti Hartert, Novit. Zool., 36, 1930, p. 112 (Guam, Saipan).
Anas platyrhynchos oustaleti Delacour and Mayr, Wilson Bull., 57, 1945, pp. 21, 39 (Marianas).
Geographic range.—Micronesia: Mariana Islands—Guam, Tinian, Saipan.
Characters.—From study of a large series of specimens of Anas oustaleti, Yamashina (1948) described two types of plumages: one type resembles that of A. platyrhynchos and another type resembles that of A. poecilorhyncha. He based his conclusions on both a study of prepared skins and observations of the molt of living specimens as reported by Kuroda (1941-1942). The following descriptions are quoted from Yamashina (1948:122).
Adult male in nuptial plumage of A. platyrhynchos type: "Whole head is dark green, except at the sides where buff feathers are plentifully intermingled, a dark brown streak through the eye, and faint white ring on the lower neck. Feathers on scapulars and sides of body are as those of Anas poecilorhyncha. Sides of body are vermiculated but some brown feathers are found even in the full nuptial plumage. Upper breast is dark reddish chestnut with dusky spots. Upper and under tail-coverts are as in Anas platyrhynchos. Speculum is as that of Anas platyrhynchos, but the tips of the greater coverts are buff instead of white. Central tail feathers are more or less curled upward. Base of bill is black, tip is olive color. Iris is dark brown. Feet, reddish-orange, webs darker." Eclipse plumage of adult male resembles that of A. platyrhynchos.
Adult male in nuptial plumage of A. poecilorhyncha type: "Resembles Anas poecilorhyncha pelewensis from the Palau Islands and Truk Island, but sides of head are browner, superciliary stripes and ground color of cheeks are more buffy. Feathers on upper breast and sides of body are more broadly edged with brown. Speculum is usually violet-purple as in the platyrhynchos type, but in two specimens from Saipan and Tinian, respectively, it is dark green as in Anas poecilorhyncha pelewensis. Tips of the secondaries are usually white, but sometimes very faint as in Anas poecilorhyncha pelewensis, and in one specimen from Saipan they are buffy. Bill is olive color with a black spot in the center of the upper mandible. Iris, dark brown. Feet, dark orange, darker in joints and webs." Eclipse plumage of adult male resembles the nuptial plumage.
Measurements.—Measurements of nine ducks from Guam and Saipan are: wing, 238-266 (252); tail, 75-84 (81); exposed culmen, 49-53 (51); tarsus, 41-43 (42).
Specimens examined.—Total number, 9 (5 males, 2 females, 2 unsexed), as follows: Mariana Islands, USNM—Saipan, 2 (Oct. 2, 3)—Guam, 1 (June 6); AMNH—Saipan, 2 (Aug. 7, 11)—Guam, 4 (Jan. 10, April 6, Dec. 11, 16).
Nesting.—At Guam, Seale (1901:25) found nests of the Marianas Mallard "among the reedy swamps and streams of the island." He obtained two downy young in June. Kuroda (1941-1942) reports nesting at Lake Challankanoa, Saipan, in July. He writes that nests contained 7 to 12 eggs. Ducklings and incubated eggs were obtained in June and July, but he is of the opinion that the breeding season may be longer. He notes that adults exhibit both nuptial plumage and eclipse plumage at the same time, suggesting that breeding may occur at various times in the year. A nest with seven eggs taken on July 4, 1941, at Hagoi Lake, Tinian, is described by Kuroda as having been found among rushes and constructed of dead leaves, stems, and roots and lined with down. He describes the eggs as being grayish-white with a pale greenish tinge, and measuring 61.6 by 38.9. Marshall (1949:202) saw a family of ducklings in April.
Remarks.—The Marianas Mallard is rare; probably it never has been very abundant in the small chain of islands to which it is restricted, because fresh water marshes and swamps are not extensive. The bird was first recorded by Bonaparte as Anas boschas a. Freycineti in 1865. This name was a nomen nudum and later the same specimen in the Paris Museum was named by Salvadori (1894) as Anas oustaleti. In 1888, Marche obtained six specimens at Guam; these were reported on by Oustalet (1896:49). Later collecting [Pg 96] showed that the duck inhabited also the islands of Saipan and Tinian. There have been no records of this duck in the more northern islands of the Marianas. According to Yamashina (1948:121) in the period from 1931 to 1940, the Japanese obtained 38 specimens of the Marianas Mallard at Tinian and Saipan. In 1940, four birds from Tinian were shipped alive to Japan and kept in an aviary by Kuroda. At Tinian in 1940, one of the collectors observed two flocks of A. oustaleti, each containing 50 or 60 individuals. The Japanese took specimens at a lagoon area and at fresh water lakes. Yamashina describes one of the localities, Lake Hagoi on Tinian, as "a small body of fresh water surrounded by about 40 acres of marsh." During the war, servicemen reported the presence of the Marianas Mallard at both Saipan and Tinian. Moran (1946:261) counted twelve ducks at Saipan. Stott (1947:525) saw seven birds at Lake Susupe on Saipan in December, 1945. He writes that the birds were gentle and easily approached and that they preferred winding channels in reed beds to open water. Marshall obtained two ducks at Lake Susupe in early October, 1945. These specimens are in the United States National Museum. He (1949:202) found ducks at both Saipan and Tinian; twelve was the greatest number seen at any one time. Gleise (1945:220) estimated that there were twelve birds on Tinian in 1945, remarking that their habitat was swamp area.
At Guam and Rota, the NAMRU2 party failed to obtain any specimens but received reports of the presence of ducks on both islands. At Guam, reports were obtained of ducks of unknown species at a fallow rice paddy in August, 1944, and in a marsh near Agat on June 13, 1945. The presence of Japanese soldiers in the interior of Guam made it inadvisable to investigate marshes and swamps of the interior and the upper courses of streams. H. G. Hornbostel, as quoted by Phillips (1923:54), reported that ducks were found at Guam only in the Tolofofo River Valley. The NAMRU2 field parties investigated the lower reaches of this valley and found no evidence of the ducks. The upper part of this valley was used as an artillery range in 1945. Probably the firing of field guns was a disturbing influence to any birds that might have been there. If the ducks were on Guam at that time, they must have been secretive and restricted in their movements. At Rota, two ducks which might have been A. oustaleti were seen by the NAMRU2 party on October 20, 1945, in a cultivated field.
These recent reports indicate that the Marianas Mallard is secure for the present on the islands of Saipan and Tinian, but thoughtful [Pg 97] conservation practices need to be placed in operation to insure its survival in the future.
Evolutionary history of Anas oustaleti.—In the past, most of the studies have pointed to a northern ancestry for A. oustaleti. Bryan (1941:187) has noted a relationship between A. oustaleti and the Laysan Duck (A. laysanensis Rothschild) and the Hawaiian Duck (A. wyvilliana Sclater). Amadon (1943:1) suggests that these three species of ducks are rather recent derivatives of the Common Mallard (A. platyrhynchos) and postulates the evolution of A. wyvilliana from migrants from North America. He further states that A. laysanensis and A. oustaleti may have been derived from A. wyvilliana or may represent independent colonizations. Delacour and Mayr (1945:21) go a step further and make these forms subspecies of A. platyrhynchos, saying that they are "dull-colored editions" of the Common Mallard, that because of isolation they have become reduced in size and have lost many of the characteristics of their ancestors. Recently, however, Yamashina (1948) has concluded that the Marianas Mallard has evolved as the result of hybridization between the two species, A. platyrhynchos and A. poecilorhyncha. His conclusions are based on a study of a large number of specimens, both museum skins and captive birds, in which he has been able to detect plumages of the A. platyrhynchos type and of the A. poecilorhyncha type (see Characters). He has noted specimens which have ninety percent of the characteristics of A. platyrhynchos and ten percent of the A. poecilorhyncha type. These percentages are reversed in specimens favoring the A. poecilorhyncha type. In his series of skins he finds the A. poecilorhyncha type of plumage most frequently, in forty-four specimens out of fifty examined, while only six specimens have the A. platyrhynchos type of plumage. Yamashina cites also as evidence favoring his conclusion that hybridization has taken place the results obtained from the crossing of captive A. platyrhynchos and A. poecilorhyncha. It is his assumption that there has been a resident form of A. poecilorhyncha in the Marianas, apparently resembling closely that which occurs in the Palaus and at Truk (A. p. pelewensis), and that stragglers of A. platyrhynchos from the north occasionally reach the Marianas where hybridization between the two species occurs. Yamashina remarks (1948:123): "The opportunity for hybridization should occur more rarely in the south, and thus more frequent back-crossing of the hybrid with the indigenous Anas poecilorhyncha on Tinian and Guam explains the superabundance there of the poecilorhyncha type. As the hybridization [Pg 98] should have taken place more frequently to the north in Saipan, the ratio of the occurrence of the platyrhynchos type is logically higher there." The Common Mallard (A. p. platyrhynchos) has not been recorded in Micronesia, but according to Yamashina (1948:123) "winters frequently just north of the Marianas in the Bonin and Volcano Islands."
This remarkable explanation for the development of the Marianas Mallard is not questioned by this author, who feels that hybridization may be found to be the cause for other unusual forms of life in island habitats whose ancestry has not been explained. As Yamashina comments, the special environments of islands together with small and restricted populations of animals are factors which could favor such development.
Anas superciliosa var. pelewensis Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 108. (Type locality, Pelew Islands.)
Anas superciliosa Hartlaub and Finsch, Proc. Zool. London, 1868, pp. 8, 118 (Pelew); Sclater, Proc. Zool. Soc. London, 1869, p. 659 (Pelew); Gray, Hand-list Birds, 3, 1871, p. 82 (Pelew); Salvadori, Ornith. Papuasia, 3, 1882, p. 395 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 70 (Pelew); Salvadori, Cat. Birds British Mus., 27, 1895, p. 206 (Pelew); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 50 (Palaos).
Anas superciliosa pelewensis Dubois, Syn. Avium, 2, 1904, p. 990 (Pelew); Mathews, Birds Australia, 4, 1915, p. 90 (Pelew); Phillips, Nat. Hist. Ducks, 2, 1923, p. 113 (Pelew); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 215 (Pelew); Hartert, Novit. Zool., 36, 1930, p. 112 (Pelew); Peters, Check-list Birds World, 1, 1931, p. 160 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 184 (Palaus, Truk); Hand-list Japanese Birds, 3d ed., 1942, p. 205 (Babelthuap, Peliliu); Amadon, Amer. Mus. Novit., no. 1237, 1943, p. 3 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 286 (Palaus, Truk); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 45 (Peleliu, Truk).
Anas pelewensis Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 40 (Palau); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 71 (Palau).
Polionetta superciliosa pelewensis Kuroda, in Momiyama, Birds Micronesia, 1922, p. 38 (Pelew).
Anas superciliosa rukensis Kuroda, "Gan to Kamo" (Geese and Ducks), 1939, page not numbered, description between pls. 52 and 53 (Type locality, Ruk); Hand-list Japanese Birds, 3d ed., 1942, p. 206 (Truk).
Anas poecilorhyncha superciliosa Delacour and Mayr, Wilson Bull., 57, 1945, pp. 21, 39 (no locality given); Yamashina, Pacific Science, 2, 1948, p. 122 (Palau, Truk).
Geographic range.—Islands of Micronesia, Polynesia, and Melanesia. In Micronesia: Palau Islands—Babelthuap, Peleliu; Caroline Islands—Truk.
Characters.—Adult: A medium-sized duck with upper parts dark brown, feathers edged with buff; top of head blackish merging into gray on hind neck with narrow buff line below; eye-stripe broad and blackish; lower parts uniformly dark brown to gray brown, feathers edged with buff; face, chin and throat light buff with some dark streakings; under wing white; speculum green; bill plumbeous with nail black; legs yellow-brown to yellowish, webs dusky. A. p. pelewensis resembles A. p. rogersi Mathews, but is smaller with a wing length averaging as much as 20 mm. shorter.
Measurements.—As given by Amadon (1943:4) seven unsexed skins from the Palaus, studied by Finsch (1875:40), have wing lengths of 207, 212, 212, 214, 223, 235, 230. For an adult male taken by Coultas at Palau, the exposed culmen measures 45 and the tarsus 37.
Specimens examined.—Total number, 3 males from Palau Islands, AMNH—exact locality not given (Oct. 26, Nov. 25).
Remarks.—A. p. pelewensis is apparently rare in the Palau Islands. Coultas, who visited the Palaus in October to December, 1931, writes (field notes) that he received reports that the birds were present and nested in numbers on fresh water lakes. He took specimens in taro patches and comments that the ducks probably feed at night and have retiring habits during the day. At Peleliu in 1945, the NAMRU2 party received several reports of ducks but failed to find the birds. At Truk, in December, 1945, McElroy of the NAMRU2 party found ducks to be fairly numerous in rice paddies, marshes, and swamps. He observed that the birds roosted at Moen Island at night but that they apparently flew to outlying islands to spend the day. Richards observed ducks on Moen Island on August 28 and 29, 1947, and again in the period from January 19 to February 10, 1948. He saw several flocks of ducks including one containing "about a dozen ducks" at ponds along a roadway and at an airstrip. Kuroda named the population at Truk as distinct in 1939. I have not been able to examine his description and no specimens are available for study, but if the birds at Truk represent an independent colonization (different from that of the birds at Palau) they might exhibit recognizable variation. Amadon (1943:5) has already pointed out that the shortness of the wing of specimens in the Palaus may merit subspecific status for the population. Delacour and Mayr (1945:21) propose that the Palau Gray Duck is a subspecies of A. poecilorhyncha; this treatment is followed in the present work.
Evolutionary history.—A. p. pelewensis, as Amadon (1943:1) has stated, represents a population of mallards which became separated from the ancestral stock in the Australian or Malayan area and when once differentiated, invaded New Zealand and other parts of Polynesia, Melanesia, and southwestern Micronesia. Amadon points out that its range in the Pacific islands is more or less complimentary to that of A. oustaleti in the Marianas and the Philippine Mallard (A. poecilorhyncha luzonica Fraser), as well as to the Hawaiian forms (A. wyvilliana Sclater and A. laysanensis Rothschild). [Pg 100] The range of A. p. pelewensis gives one the impression that its present distribution may be only a stage in a gradual spreading of the species, for it certainly has not yet occupied all habitats suitable for it in southern Micronesia nor elsewhere in Oceania. As in the case of A. oustaleti, A. p. pelewensis appears to prefer areas of fresh, and possibly brackish, water on the larger islands.
A. p. luzonica is a near relative of A. p. pelewensis but has rufous-brown instead of buffy-brown coloring on the chin, throat, sides of head, and superciliary region. The underparts of the Philippine Mallard are much less mottled. The specula are similar. Both of these forms were probably derived from a mallard of the A. p. poecilorhyncha type.
Anas Querquedula Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 126. (Type locality, Europe, restricted to Sweden.)
Anas querquedula Marshall, Condor, 51, 1949, p. 221 (Tinian).
Geographic range.—Breeds in Europe and Asia. Winters from northern Africa to New Guinea. In Micronesia: Mariana Islands—Tinian.
Remarks.—Marshall (1949:221) obtained one of a pair of these ducks which he observed "daily in April on Lake Hagoi" at Tinian.
Anas Crecca Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 126. (Type locality, Europe, restricted to Sweden.)
Querquedula crecca crecca Hand-list Japanese Birds, rev., 1932, p. 185 (Pagan).
Anas crecca crecca Hand-list Japanese Birds, 3d ed., 1942, p. 206 (Pagan).
Anas crecca Mayr, Birds Southwest Pacific, 1945, p. 302 (Micronesia).
Geographic range.—Breeds in Iceland, northern Europe, Asia, and Aleutians. Winters south to northern Africa, Asia and Philippines. In Micronesia: Mariana Islands—Pagan.
Remarks.—The European Teal has been recorded by the Japanese at Pagan in the northern Marianas. It appears to be an uncommon winter visitor to Micronesia.
Anas carolinensis Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 533. (Type locality, Carolina to Hudson Bay.)
Anas carolinensis Reichenow, Ornith. Monatsber., 1901, p. 17 (Jaluit); Schnee, Ornith. Monatsber., 1901, p. 131 (Marshalls); idem, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall Inseln); Phillips, Nat. Hist. Ducks, 2, 1923, p. 235 (Marshall Islands).
Querquedula crecca carolinensis Hand-list Japanese Birds, rev., 1932, p. 185 (Marshall Islands).
Anas crecca carolinensis Hand-list Japanese Birds, 3d ed., 1942, p. 206 (Marshall Islands).
Geographic range.—Breeds in northwestern and northcentral North America. Winters to West Indies, Central America and Mexico. In Micronesia: Marshall Islands—Jaluit.
Remarks.—Reichenow (1901:17) and Schnee (1901:131) record the Green-wing Teal in the Marshall Islands. It is the only record known for Micronesia. Bryan and Greenway (1944:104) record the teal as a migrant to the Hawaiian Islands.
Anas acuta Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 126. (Type locality, Europe, restricted to Sweden.)
Dafila acuta acuta Hand-list Japanese Birds, rev., 1932, p. 185 (Pagan).
Anas acuta acuta Hand-list Japanese Birds, 3d ed., 1942, p. 206 (Pagan).
Anas acuta Mayr, Birds Southwest Pacific, 1945, p. 302 (Micronesia).
Geographic range.—Breeds in Iceland, northern Europe and Asia. Winters south to northern Africa, Asia and Philippines. In Micronesia: Mariana Islands—Pagan, Guam; Palau Islands—exact locality unknown.
Remarks.—The Pintail has been recorded from Pagan and Guam in the northern Marianas and from the Palau Islands and is thought to be an uncommon visitor to Micronesia. At Guam, Flavin (field notes) recorded one female on October 27, 1945, and three females and two drakes on January 19, 1946. Marshall (1949:221) saw a flock of fifteen Pintails at Saipan on February 7, 1945.
Anas tzitzihoa Vieillot, Nouv. Dict. Hist. Nat., 5, 1816, p. 163. (Type locality, Mexico, ex Hernandez.)
Anas acuta americana Reichenow, Ornith. Monatsber., 1901, p. 17 (Jaluit); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall Inseln).
Anas acuta Schnee, Ornith. Monatsber., 1901, p. 131 (Marshalls); Phillips, Nat. Hist. Ducks, 2, 1923, p. 316 (Jaluit).
Anas acuta tzitzihoa Hand-list Japanese Birds, 3d ed., 1942, p. 206 (Marshall Islands).
Geographic range.—Breeds in northwestern and northcentral North America. Winters south to West Indies, Panamá, and west to Hawaiian Islands. In Micronesia: Marshall Islands—Jaluit.
Remarks.—Reichenow (1901:17) and Schnee (1901:131) reported that flocks of ducks belonging to this and other American species were observed in the Marshall Islands in October, 1899, and May, 1900. This species may winter in the Hawaiian Islands, according to Peters (1931:167). If so it is not surprising that occasional visitors reach eastern Micronesia.
Anas penelope Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 126. (Type locality, Europe, restricted to Sweden.)
Anas penelope Finsch, Ibis, 1880, pp. 332, 333 (Taluit); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall Inseln); Phillips, Nat. Hist. Ducks, 2, 1923, p. 175 (Taluit); Hand-list Japanese Birds, 3d ed., 1942, p. 206 (Tinian, Yap, Jaluit); Mayr, Birds Southwest Pacific, 1945, p. 302 (Micronesia).
Mareca penelope Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 56 (Jaluit); Wiglesworth, Abhandl. und Ber Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 71 (Taluit); Finsch, Deut. Ver. zum Schultze der Vogelwelt, 18, 1893, p. 458 (Marshalls); Kuroda, in Momoyama, Birds Micronesia, 1922, p. 38 (Taluit); Hand-list Japanese Birds, rev., 1932, p. 185 (Tinian, Yap, Jaluit).
Geographic range.—Breeds in Iceland, northern Europe and Asia. Winters south to Africa, southern Asia and Philippines; casual to eastern North America. In Micronesia: Mariana Islands—Tinian; Caroline Islands—Yap; Marshall Islands—Jaluit.
Remarks.—The Widgeon may be an occasional winter visitor to Micronesia. The record at Jaluit in the Marshall Islands may be questioned.
Anas clypeata Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 124. (Type locality, Coasts of Europe, restricted to southern Sweden.)
Spatula clypeata Hand-list Japanese Birds, rev., 1932, p. 185 (Pagan); Yamashina, Tori, 10, 1940, p. 676 (Pingelap); Hand-list Japanese Birds, 3d ed., 1942, p. 206 (Pagan, Pingelap).
Anas clypeata Mayr, Birds Southwest Pacific, 1945, p. 302 (Micronesia).
Geographic range.—Breeds in northern Europe, Asia, North America and adjacent islands. Winters to northern Africa, southern Asia, Philippines, Hawaiians, southern United States to Central America. In Micronesia: Mariana Islands—Pagan, Tinian; Caroline Islands—Ponapé, Pingelap.
Specimens examined.—One female from Mariana Islands, USNM—Tinian (Oct. 12).
Remarks.—The Shoveller is known from localities in the Marianas and in the Carolines. In the collections of the American Museum of Natural History there is a female taken by Rollo Beck at Kauehi, Tuamotu Archipelago, on March 6, 1923. A specimen examined from Tinian was taken there by Joe T. Marshall, Jr., at Lake Hogoya on October 12, 1945. Richards obtained two Shovellers (one immature male and one immature female) at Ponapé on December 21, 1947, and January 6, 1948, respectively. He found them in a pond in a bomb crater. This duck appears to be a casual winter visitor to Micronesia and other parts of Oceania.
Anas fuligula Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 128. (Type locality, Europe, restricted to Sweden.)
Fuligula cristata Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 9, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, p. 90 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 40 (Palau); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 71 (Pelew); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 50 (Mariannes, Palaos).
Fuligula fuligula Salvadori, Cat. Birds British Mus., 27, 1895, p. 363 (Pelew); Hartert, Novit. Zool., 5, 1898, p. 68 (Marianne); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 26 (Micronesia); Safford, Osprey, 1902, p. 70 (Mariannes); idem, The Plant World, 7, 1904, p. 268 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 38 (Mariane, Pelew, Yap); idem, Avifauna Riu Kiu, 1925, p. 143 (Pelew, Marianne).
Marila fuligula McGregor, Man. Philippine Birds, 1909, p. 199 (Marianne, Pelew).
Nyroca fuligula Phillips, Nat. Hist. Ducks, 3, 1925, p. 234 (Guam); Hand-list Japanese Birds, rev., 1932, p. 185 (Pagan, Saipan, Palau, Yap); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam); Mayr, Birds Southwest Pacific, 1945, p. 302 (Micronesia).
Aytha fuligula Hand-list Japanese Birds, 3d ed., 1942, p. 207 (Pagan, Saipan, Tinian, Guam, Yap, Palau).
Geographic range.—Breeds in Iceland, Europe, northern Asia. Winters in Europe, Africa, Asia, Malaysia, and parts of Oceana. In Micronesia: Mariana Islands—Pagan, Saipan, Tinian, Guam; Palau—exact locality unknown; Caroline Islands—Yap.
Remarks.—The Tufted Duck is a winter migrant to western Micronesia. It has been recorded only a few times and may be an irregular visitor. Flavin observed a duck, which he thought to be of this species, at Guam on January 19, 1946. Marshall (1949:221) reports that two Tufted Ducks were seen at Lake Hagoi in April 1945.
Anas valisineria Wilson, Amer. Ornith., 8, 1814, p. 103, pl. 70, f. 5. (Type locality, Eastern United States.)
Nyroca valilisineria Reichenow, Ornith. Monatsber., 1901, p. 17 (Jaluit); Schnee, Ornith. Monatsber., 1901, p. 131 (Marshalls); idem, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall Inseln).
Nyroca valisineria Phillips, Nat. Hist. Ducks, 3, 1923, p. 124 (Marshall Islands).
Aythya valisineria Hand-list Japanese Birds, 3d ed., 1942, p. 207 (Marshall Islands).
Geographic range.—Breeds in northwestern and northcentral North America. Winters south to Gulf States, Florida and Mexico. In Micronesia: Marshall Islands—Jaluit.
Remarks.—Reichenow (1901:17) and Schnee (1901:131) reported three species of American ducks (Aythya valisineria, Anas acuta tzitzihoa and Anas crecca carolinensis) in the Marshalls in October, 1899, and May, 1900. These species may be stragglers to eastern Micronesia.
Falco Soloënsis Horsfield, Trans. Linn. Soc. London, 13, 1821, p. 137. (Type locality, Java.)
Accipiter soloënsis Hand-list Japanese Birds, rev., 1932, p. 182 (Yap); Hand-list Japanese Birds, 3d ed., 1942, p. 203 (Yap, Rota); Mayr, Birds Southwest Pacific, 1945, p. 302 (Yap).
Geographic range.—Breeds in northern China south to Kwangtung. Winters to Malaysia. In Micronesia: Mariana Islands—Rota; Caroline Islands—Yap.
Remarks.—The Chinese Goshawk is a winter visitor to Micronesia and has been recorded at Rota and Yap. The NAMRU2 party saw several unidentified hawks in Micronesia in 1945. At Mt. Tenjo, Guam, Muennink saw a small hawk, resembling an accipiter, darting at swiftlets on June 8, 1945. At Angaur, the writer saw a small hawk flying through heavy vegetation along the rugged coast line on September 21, 1945. A hawk "Butio(?)" was reported at Saipan in 1945 by Moran (1946:262); this hawk may have been Butastur indicus (Gmelin). Marshall (1949:221) reports seeing "three kinds of hawks" on Palau in November, 1945. Obviously, further observations and collecting will increase our knowledge of the known number of kinds of hawks which visit Micronesia.
Astur (Nisus) gularis Temminck and Schlegel, in Siebold, Fauna Japon., Aves, 1845, p. 5, pl. 2. (Type locality, Japan.)
Accipiter nisoides Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 166 (Guam); Hartert, Novit. Zool., 5, 1898, p. 51 (Marianne); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 44 (Guam); Safford, Osprey, 1902, p. 70 (Marianas).
Accipiter gularis Kuroda, in Momiyama, Birds Micronesia, 1922, p. 39 (Guam).
Accipiter virgatus gularis Hand-list Japanese Birds, rev., 1932, p. 182 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 203 (Guam); Mayr, Birds Southwest Pacific, 1945, p. 302 (Micronesia).
Accipiter virgatus nisoides Bryan, Guam. Rec., vol. 13, no. 2, 1936, p. 15 (Guam).
Geographic range.—Breeds in Japan and northern China. Winters south to Philippines and Malaysia. In Micronesia: Mariana Islands—Guam.
Remarks.—Oustalet (1895:166) records a male bird shot by Marche at Guam in October, 1887. Seale (1901:44) records a specimen taken at Guam by Owston's Japanese collectors. These are the only records found for Micronesia, and the hawk may be classed as a casual winter visitor. Strophlet (1946:535) observed "a small light-throated" falcon at Guam on November 7, 1945, which may have been of this species.
Pandion haliaëtus melvillensis Mathews, Australian Avium Rec., 1, 1912, p. 34. (Type locality, Melville Island.)
Pandion leucocephalus Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 49 (Palau).
Pandion haliaetus leucocephalus Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 1 (Pelew).
Pandion haliaëtus cristatus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 40 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 182 (Pelew); Hand-list Japanese Birds, 3d ed., 1942, p. 203 (Palau).
Pandion haliaëtus melvillensis Mayr, Birds Southwest Pacific, 1945, pp. 55, 286 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 46 (Guam, Palau).
Geographic range.—Malaysia, northern Australia, Melanesia. In Micronesia: Mariana Islands—Guam; Palau Islands—Peleliu.
Remarks.—The Osprey was first recorded at Palau by Finsch (1875:49). The author (1948:46) cites records obtained by C. K. Dorsey at Peleliu in 1944 and 1945. Dorsey saw the Osprey on several occasions; the NAMRU2 party did not find the bird while on their stay there in August and September, 1945. B. V. Travis of NAMRU2 saw an Osprey at Agaña Bay, Guam, in December, 1945. He observed the bird to be carrying a fish in its talons. Flavin observed the Osprey at Guam on January 28, 1945, and on December 23, 1945. Mayr (1945a:286) says that the Osprey apparently breeds at Palau. The bird seen in the Marianas may have been P. h. haliaetus (Linnaeus), a visitor from Asia, which is known to winter in the Philippines and adjacent areas.
The Osprey is the only resident member of the order Falconiformes, and it is principally a fish eater. The few records of mammal and bird eating hawks in Micronesia indicate that predation on insular vertebrate populations from this source is at a minimum. The absence of this predation may have a pronounced effect on the resident land birds, particularly from the standpoint of the perpetuation of nonadaptive mutations, which might be "weeded out" under what might be considered as normal predatory pressure in continental bird populations.
Falco japonensis Gmelin, Syst. Nat., 1, pt. 1, 1788, p. 257. (Type locality, Off the coast of Japan.)
Falco peregrinus Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 90 (Mackenzie); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 122 (Yap); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 8 (Palau); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 391 (Yap); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 1 (Yap, Pelew); Hand-list Japanese Birds, rev. 1932, p. 182 (Yap, Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 202 (Yap, Palau); Mayr, Birds Southwest Pacific, 1945, p. 302 (Yap, Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 46 (Guam).
?Falco peregrinus calidus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 40 (Yap, Pelew).
Geographic range.—Breeds in northern Asia. Winters to southern Asia, Malaysia and Melanesia. In Micronesia: Mariana Islands—Guam; Palau Islands—exact locality unknown; Caroline Islands—Yap.
Remarks.—The Peregrine Falcon may be classed as a casual winter visitor to Micronesia. It has been recorded by Hartlaub and Finsch at Yap and Palau. A specimen from Yap was taken by Kubary in November, 1870. On November 2, 1945, at Guam as previously recorded (Baker, 1948:46) Irvin O. Buss saw a falcon alight on the superstructure of his ship. He watched it catch and eat a Common Noddy (Anous stolidus). As the ship approached the island, the bird flew to the rugged cliffs near Facpi Point. Strophlet (1946:535) saw a large falcon, "presumed to be a Duck Hawk," at Guam on November 16, 1945. Possibly these two observers saw the same bird. In July, 1945, Flavin observed a Peregrine Falcon at Guam. F. p. fruitii Momiyama, which is known from the Volcano Islands, may occur in Micronesia.
Megapodius senex Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 820. (Type locality, Pelew Islands.)
Megapodius senex Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 7, 118 (Pelew); Gray, Hand-list Birds, 2, 1870, p. 256 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 103 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 29, pl. 5, fig. 2, 3 (Palau); Giebel, Thes. Ornith., 2, 1875, p. 547 (Pelew); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Oustalet, Ann. Sci. Nat., (6), art. 2, 1881, pp. 63, 140, 145, 171, 175 (Pelew); Tristram, Cat. Birds, 1889, p. 30 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 58 (Pelew); Oustalet, Ann. Sci. Nat. Zool., 11, 1891, p. 196 (Peleu); idem, Nouv. Arch Mus. Hist. Nat. Paris, (3), 8, 1896, p. 30 (Palaos); Ogilvie-Grant, Hand-book Game-birds, 2, 1897, p. 182 (Pelew); Hartert, Novit. Zool., 5, 1898, p. 62 (Pelew); Bolau, Mitteil, Naturhist. Mus. Hamburg, 1898, p. 69 (Palau); Finsch, Sammlung wissensch. Vorträge, 14 ser., 1900, p. 659 (Palau); Matschie, Journ. f. Ornith., 1901, p. 113 (Palau); Lister, Proc. Zool. Soc. London, 1911, p. 757 (Pelew).
Megapodius laperousii Ogilvie-Grant (part), Cat. Birds British Mus., 22, 1893, p. 460 (Pelew); Takastukasa and Kuroda, Tori, 1, 1915, p. 51 (Pelew); Kuroda, Dobutsu. Zasshi, 27, 1915, p. 390 (Pelew); idem, Dobutsu. Zasshi, 28, 1916, p. 69 (Pelew).
Megapodius laperousi Seale (part), Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 39 (Pelew); Safford (part), The Plant World, 7, 1904, p. 265 (Pelew); Uchida, Annot. Zool. Japon., 9, 1918, pp. 486, 487 (Palau).
Megapodius laperousii var. senex Dubois, Syn. Avium, 2, 1904, p. 787 (Pelew).
M[egapodius] lapeyrousei Reichenow (part), Die Vögel, 1, 1913, p. 273 (Palauinseln).
Megapodius laperousei senex Kuroda, in Momiyama, Birds Micronesia, 1922, p. 40 (Pelew).
Megapodius lapérouse senex Mathews, Syst. Avium Australasianarum, 1, 1927, p. 14 (Pelew); Takastukasa, Birds Nippon, vol. 1, pt. 1, 1932, p. 13, pl. 4, 5 (Pelew); Yamashina, Tori, 7, 1932, p. 412 (Ngesebus, Auror, Peliliu); Hand-list Japanese Birds, rev., 1932, p. 198 (Palau); Peters, Check-list Birds World, 2, 1934, p. 6 (Palau); Yamashina, Tori, 10, 1940, p. 679 (Gayangas, Arumidin); Amadon, Amer. Mus. Novit., no. 1175, 1942, p. 9 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 286 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 46 (Garakayo, Peleliu, Ngabad).
Megapodius la pérouse senex Hand-list Japanese Birds, 3d ed., 1942, p. 223 (Babelthuap, Koror, Auror, Ngesebus, Peliliu, Gayangas, Arumidin).
Megapodius laperouse Wharton and Hardcastle, Journ. Parasitology, 32, 1946, p. 294 (Garakayo).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Auror, Kayangel, Garakayo, Ngesebus, Peleliu, Ngabad, Gayangas, Arumidin.
Characters.—Adult: A small megapode with top of head near "mouse gray"; forehead, sides of face and neck, chin, and throat thinly covered with feathers of the same color; mantle and upper breast grayish-black becoming dark olive-brown on wings; lower back, rump and upper tail-coverts dark brown; tail blackish-brown; underparts grayish-brown, lighter on midline of belly; under wings dark brown; exposed skin of head reddish to yellowish-red; bill yellowish, basally blackish; legs yellowish; feet and claws black; iris tan.
Measurements.—Measurements of three adult males: wing, 178, 182, 188; tail, 55, 63; culmen, 22.7, 23.3; tarsus, 55, 56, 57; of seven adult females: wing, 171-189 (182); tail, 46-68 (58); culmen, 25-30 (27); tarsus, 45-60 (55). Takatsukasa (1932:14) lists the following measurements: males—wing, 176-181; tail, 59-67; culmen, 25.5-26.0; tarsus, 58-61; females—wing, 177-187; tail, 62-68; culmen, 24.0-26.0; tarsus, 55-58.
Specimens examined.—Total number, 23 (11 males, 8 females, 4 unsexed), as follows: Palau Islands, USNM—Koror, 1 (Nov. 28)—Garakayo, 5 (Sept. 17, 18, 19)—Peleliu, 2 (Aug. 31, Sept. 1)—Ngabad, 1 (Sept. 11); AMNH—Palau, 16 (Nov., Dec., not dated).
Nesting.—The megapodes do not incubate their eggs, but the female deposits them in a moundlike structure of sand, volcanic ash, and forest litter or some other type of soil in which there is warmth sufficient to hatch the eggs after an extended period (perhaps 40 days or more) without further attention from the parent bird. The young dig out and lead an independent existence. Several megapodes may utilize one nest site, which ordinarily is at a low elevation near a beach or lagoon.
The NAMRU2 party obtained two downy chicks at Gayakayo Island on September 18 and 19, 1945. A female taken on September 1 at Peleliu contained large eggs. Coultas obtained two chicks (one in postnatal molt) in November and December, 1931. Kubary, as quoted by Takatsukasa (1932:15), says that eggs may be found in the mounds throughout the year at Palau but are found most numerously in the south-east monsoon (April to November). Yamashina (1932a:412) reports on eggs taken in 1932 as follows: eight eggs from Auror Island on January 15; one egg from Ngesebus Island on January 16; and four eggs from Peleliu Island on January 16. Takatsukasa (1932:15) states that eggs are most numerous in the mounds in the months of May and June. The chicks obtained by NAMRU2 in September were of such a size as to suggest that they too had been laid in June.
Takatsukasa (1932:15) comments, "Whilst Dr. Yaichir[=o] Okada was in the Pelew Group, he found two nests on Kajangel Island, which is an uninhabited island about twelve sea-miles southeast of the island of Malacal. He says that he found two nests, one of which was obsolete and the other was in use.
The first one was oval in shape; the diameter of the largest part was twenty-four feet, and the smallest part was twenty feet, and it had a height of four feet. The second one was fan-shaped, as an obstacle existed at one side of the nest, and its diameter was twelve feet and the height was a little more than four feet, and the native whom he asked to dig out the eggs got three. One of the eggs contained a well-advanced embryo and the others were not so advanced as the first one. This distance from the top of the mound to the spot where the eggs were laid was about two and a half feet, and the natives made a great deal of effort to get these eggs. These nests were found in the bush by the natives." The NAMRU2 party observed a mound on Ngabad Island, a small islet near Peleliu, on September 11. It was much like those described by Takatsukasa, being approximately six feet high and some twelve or fifteen feet across. It was not excavated.
Molt.—Birds taken in August, September and November were molting body feathers. Birds taken in December were molting wing feathers.
Food habits.—Takatsukasa (1932:16) comments, "My collector reports to me that this bird diets on insects and tender shoots which it gets from under the soil by scratching with its large and powerful feet." According to Captain Tetens, as noted by Takatsukasa, the food of the bird consists of insects and berries. Birds taken by the NAMRU2 party had the following food items in their stomachs: adult female—2 cc. seeds, grit; adult female—3 cc. crab parts, grit; adult female—2 cc. seeds, sand; male chick—1 cc. ground food, grit; female chick—1 cc. ground food, grit, in crop 3 cc. small wood roaches (Blattidae).
Parasites.—Wharton and Hardcastle (1946:294) obtained the chigger (Acarina), Neoschöngastia yeomansi, from the megapode at Palau. Uchida (1918:486, 487) found the bird lice (Mallophaga), Goniocotes minor and Lipeurus sinuatus, on megapodes from the Palaus.
Remarks.—The NAMRU2 party arrived at the Palau Islands on August 23, 1945, with little notion that the megapode would be found on the war-torn island of Peleliu. As reported by the author (1946b:209 and 1948:46) we found birds in small numbers in the relatively undisturbed areas of rough coral covered by jungle and a few birds in the heavy matting of viny and brushy vegetation which was rapidly covering the battlefields. The finding of a higher population on the more isolated and relatively undisturbed offshore islets (Ngabad, Garakayo) by the NAMRU2 party was an observation similar to those of Takatsukasa (1932:15, 16) and Coultas (field notes). Takatsukasa (1932:16) remarks, "Dr. Finsch said that this Megapode frequents nearly all the islands of the Pelew Group ... but it is very noticeable that this bird has either disappeared, or only very rarely exists in the following islands: Koror, Ngarekobasanga, and especially the main island of Babelthuap." He quotes Otto Finsch as remarking that, "It seems that the bird occasionally moves from one island to another, as the bird is a good flier." Takatsukasa [Pg 109] continues, "According to Tetens, this Megapode runs very swiftly among the bushes, and when it is startled it takes to the nearest tree.... Captain Wilson says nothing about the Megapode, but Dr. Finsch wrote that Captain Wilson is probably referring to the egg of this bird under 'Wild Fowls,' when he said that the natives of the Palaus do not eat the flesh of the birds, but they go to the woods and bring back the eggs; they do not appreciate the newly laid eggs, but they consider it as a delicacy to swallow the well advanced embryo."
The NAMRU2 party found the birds to prefer rough, coral jungle where there was considerable heavy undergrowth and ground litter. The birds were located by their loud screeches and cackles but were difficult to stalk. It was best to remain quiet and let them approach within shooting distance. Young chicks were extremely active and wild. One of the two chicks taken at Garakayo was obtained by a fortunate shot as the bird was flying rapidly through the brush. The natives use them as food, and I learned of one serviceman who had worked out a technique for trapping the birds. He traded the live birds to the natives for island souvenirs. As Wilson and Takatsukasa note, the natives apparently prefer the eggs to the adults as food, and in normal times of food abundance they probably do not molest the adults but hunt for their eggs. This seems logical, since if a determined trapping program were in operation by the natives, it should not take many decades to eliminate completely the entire population. On four islands visited by the NAMRU2 party in August and September, 1945, I estimated the following populations: Garakayo—20 to 30; Ngabad—5 to 10; Peleliu—10 to 20; Angaur—less than 10.
Megapodius La Pérouse Gaimard, Bull. Gén. Univ. Annon. Nouv. Sci., 2, 1823, 451. (Type locality, Tinian, Archipel des Mariannes.)
Megapodius La Pérouse Quoy and Gaimard, Voy. "Uranie," Zool., 1824, pp. 127, 693, Atlas, pl. 33 (Tinian); idem, Ann. Sci. Nat. Paris, 6, 1825, p. 149 (Tinian).
Megapodius La Pérousii Quoy and Gaimard, Voy. "Uranie," Zool., 1824, p. 127, pl. 33 (Tinian); Wagler, Isis, 1829, p. 735 (Tinian, Guam, Rota); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 46 (Tinian); Oustalet, Ann. Sci. Nat., (6), art. 2, 1881, pp. 63, 138, 140, 143, 171, 175, 176, 177 (Tinian); idem, Le Nat., 1889, p. 261 (Mariannes); idem, Ann. Sci. Nat., Zool., 11, 1891, p. 196 (Tinian, Seypan, Pagon).
Megapodius La Peyrouse Lesson, Man. d'Ornith., 2, 1828, p. 221 (Tinian); idem, Compl. de Buffon, 2d ed., 2, Ois., 1838, p. 255 and accompanying plate (Tinian).
Megapodius laperousii Lesson, Traité d'Ornith., 1831, p. 478 (Mariannes); Gray, Hand-list Birds, 2, 1870, p. 256 (Marian); Ogilvie-Grant, Hand-book Game-birds, 2, 1897, p. 183 (Marianne); Dubois, Syn. Avium, 2, 1904, p. 787 (Mariannes); Lister, Proc. Zool. Soc. London, 1911, p. 757 (Marianne).
Megapodius Lapeyrousii Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen).
Megapodius La Peyrousii Reichenbach, Tauben, 1861, p. 5 (Marianen).
Megapodius la-perousi Gray, Proc. Zool. Soc. London, 1864, p. 43 (Guam, Botta, Tinian).
Megapodius laperousi Giebel, Thes. Ornith., 2, 1875, p. 547 (Marianae); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 39 (Marianas); Safford; Osprey, 1902, p. 68 (Tinian); idem, The Plant World, 7, 1904, p. 265 (Tinian); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 78 (Rota, Saipan, Pagan, Agrigan); Schnee, Zeitschr, f. Naturwisch., 82, 1912, p. 467 (Marianen); Prowazek, Die deutschen Marianen, 1913, pp. 47, 101 (Marianen); Linsley, Guam, Rec., vol. 12, no. 8, 1935, p. 249 (Rota, Saipan, Pagan, Agrigan).
Megapodius perousei Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 30 (Marianen); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 58 (Guam, Botta, Tinian, Pagon).
Megapodius laperousii Ogilvie-Grant (part), Cat. Birds British Mus., 22, 1893, p. 460 (Marianne).
Megapodius la perousei Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 26 (Saypan, Pagan, Rota, Agrigan, Tinian).
Megapodius laperouse Hartert, Novit. Zool., 5, 1898, p. 61 (Saipan, Tinian, Rota, Guam).
Megapodius laperousei Finsch, Sammlung wissensch. Vorträge, 14 ser., 1900, p. 660 (Marianen); Prowazek, Die deutschen Marianen, 1913, p. 87 (Marianen).
Megapodius lapeyrouse Matschie, Journ. f. Ornith., 1901, p. 113 (Guam, Saipan).
M[egapodius] lapeyrousei Reichenow (part), Die Vögel, 1, 1913, p. 273 (Mariannen).
Megapodius laperousei laperousei Kuroda, in Momiyama, Birds Micronesia, 1922, p. 40 (Guam, Saipan, Rota, Tinian, Pagan, Agrigan).
Megapodius lapérouse lapérouse Mathews, Syst. Avium Australasianarum, 1, 1927, p. 16 (Marianas); Takatsukasa, Birds Nippon, vol. 1, pt. 1, 1932, p. 6, pl. 4, 5 (Marianne); Yamashina, Tori, 7, 1932, p. 411 (Pagan Agrigan); Hand-list Japanese Birds, rev., 1932, p. 198 (Marianas); Peters, Check-list Birds World, 2, 1934, p. 7 (Marianne Islands); Yamashina, Tori, 10, 1940, p. 679 (Assongsong); Amadon, Amer. Mus. Novit., no. 1175, 1942, p. 9 (Asuncion, Saipan, Guam); Mayr, Birds Southwest Pacific, 1945, p. 286 (Marianas).
Megapodius laperousi laperousi Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam).
Megapodius la pérouse la pérouse Hand-list Japanese Birds, 3d ed., 1942, p. 223 (Assongsong, Agrigan, Pagan, Almagan, Saipan, Tinian, Agiguan, Rota, Guam).
Geographic range.—Micronesia: Mariana Islands—Asuncion, Agrihan, Pagan, Almagan, Saipan, Tinian, Agiguan, Rota, Guam. Probably extinct on Saipan, Tinian, Rota, Guam.
Characters.—Adult: Resembles M. l. senex, but crown slightly darker gray; back, wing-coverts and scapulars more heavily washed with olivaceous-brown; mantle less slate; underparts paler and more brownish, especially belly. (Note—The specimens examined from the Marianas are old and rather worn in appearance.)
Measurements.—Two males measure: wing 180?, 182?; tail 62, 63; tarsus 55, 55; three females: wing 181?, 181?; tail 55, 59, 62; tarsus 54, 54, 56. Takatsukasa (1932: 10) lists the following measurements: males—wing, 155-169; tail, 54-62; culmen, 22.5-24; tarsus, 51-54; females—wing, 158-170; tail, 56-65; culmen, 23-25; tarsus, 50-55.
Specimens examined.—Total number, 10 (3 males, 4 females, 3 unsexed), as follows: Mariana Islands, AMNH—Guam, 1 (June 6)—Saipan, 6 (1895)—Asuncion, 3 (1904).
Nesting.—Concerning the nest of the Micronesian Megapode in the Marianas, Takatsukasa (1932:10) writes: "The nest is a large mound of sand mixed with grass and is made in the wooded land along the seashore. The mound is over one hundred feet in circumference and a few yards in height, and is built by the united efforts of the male and female, by scratching sand and grass with their large feet. The eggs are laid in this mound and they are hatched by the heat of the sun and that produced by the fermentation of the grass, and they are never hatched by the parent birds. The egg is of a pale brown, but always stained by nesting materials."
Takatsukasa (1932:11) quotes Oustalet as follows: "Specimens collected by Mr. Marche have proved that the breeding season of La Pérouse's Megapode is rather long, like the other species of the same family, it begins to breed in January or February and ends in June. Accordingly, in this period the eggs just laid, the chicks, the young and adult can be seen at one place, but Mr. Marche did not obtain any egg." Hartert (1898:61) records a chick taken on July 17. Yamashina (1932a: 411) records eggs taken in 1931 as follows: two eggs from Pagan, February 17; three eggs from Pagan, May 15; four eggs from Agrihan, June 24. The breeding season for both of the incubator birds, M. l. senex and M. l. lapérouse, is apparently from about January to August.
Remarks.—The Micronesian Megapode was first taken in the Marianas by the expedition of the Uranie. Bérard, a member of the expedition, obtained the bird at Tinian in December, 1820. Quoy and Gaimard (1824:27), who studied the birds of this expedition, reported that according to native tradition the species was in former times widely distributed in the Marianas and domesticated by the ancient people of the islands, but that in 1819 and 1820 the birds were not numerous on Tinian and not found on Guam and Rota. Marche (in Oustalet, 1896:27) obtained twenty-three birds at Saipan, one from Rota, two from Agrihan, and five from Pagan in 1887, 1888, and 1889; it is apparent that Quoy and Gaimard missed the bird at Rota. Marche was of the opinion that the megapodes were never domesticated and that they would probably not last much longer at Saipan and Rota owing to the incessant hunting for them by the natives. As in the Palaus, the natives apparently prefer the eggs to the adults. The latest collections of these birds in the Marianas were made by the Japanese. Yamashina (1932:411) obtained eggs in 1931 at Pagan and Agrihan, and again in 1940. He (1940:679) reported birds at Assongsong (Asuncion). Takatsukasa (1932:12) says, "A collector, working for Marquis Yamashina and myself, lately procured many specimens in Saipan and Pagan." Linsley (1935:249, 250) in searching for the megapode at Guam found little evidence of the birds. He interviewed people between the ages of forty-five and eighty and only two or three remembered seeing the bird. He said he saw one or two cross the road; but I suspect that they might have been rails (Rallus owstoni). Service personnel stationed at various islands in the Marianas during the late war have not reported the birds. The NAMRU2 party found no trace of the bird at Guam or Rota. Joe T. Marshall, Jr. (1949:203), did not find the bird at Saipan, Tinian, [Pg 112] or Guam in 1945. Its status on Agiguan is unknown; isolated Japanese troops present on this small island from the time of the American invasion (1944) until the armistice (1945) may have used the birds for food and depleted the population seriously. At present the birds apparently still occur on islands in the northern Marianas. It seems that if these birds are to survive, they must be given some protection.
Evolutionary history.—The genus Megapodius consists, according to Peters (1934:1-7), of nine species which are distributed through the islands from the Philippines and Borneo to Australia and Melanesia. These have been redesignated under three specific names by Mayr (1938). Outlying forms occur in the Nicobar Group to the west and in Tonga (Niuafou Island) in the east and in the Palaus and Marianas to the extreme northeast. Lister (1911:757) is of the opinion that the megapodes may have reached these outlying islands by having been transported by the natives, by whom the eggs were highly valued as food. This idea is also maintained by Rutland (1896:29-30) and Christian (1926:260). Possibility and not factual evidence support this hypothesis. From their seeming ancestral stocks, M. pritchardii Gray of Niuafou Island and M. lapérouse of Micronesia are remarkably distinct which may indicate their early arrival at these islands and subsequent change from their ancestral stocks.
Like M. pritchardii, the Micronesian species is smaller than its relatives to the southwest and has short, rather rounded wings, although its feet are heavily built whereas those of M. pritchardii are lightly constructed. In comparing these birds with the species of megapode found in the Philippines, Celebes and Melanesia, it seems that both M. pritchardii and M. Lapérouse are closely related to the widespread species, M. freycinet, which may have been ancestral to both. The differences between M. prichardii and M. lapérouse indicate that they represent independent invasions. Nevertheless these megapodes may have had a wider range in Oceania in former times; man may have eliminated the birds from some islands by using their eggs. The eggs are laid in conspicuous mounds which are easily found by man.
M. lapérouse differs from M. freycinet of New Guinea and other parts of Melanesia and the Philippines; its small size, short wing and pearl gray head are distinctive characters. It shows greatest resemblance to the subspecies in Celebes (M. f. gilberti) in size and to the subspecies in the Moluccas (M. f. freycinet) in coloring; possibly M. [Pg 113] lapérouse represents stock from one of these regions. Apparently the group as a whole evolved from a center of dispersal in the New Guinea area. Mayr (1942b:167) regarded all the species of Megapodius as belonging to one polytypic species, except M. lapérouse and M. pritchardii, which are allopatric species.
Oriolus lineatus Scopoli, Del. Flor. et Faun. Insubr., fasc. 2, 1786, p. 87. (Type locality, Luzon, ex Sonnerat.)
Excalfactoria sinensis Hartert, Novit. Zool., 5, 1898, p. 61 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 37 (Guam); Safford, Osprey, 1902, p. 68 (Guam); idem, Amer. Anthro., 4, 1902, p. 711 (Guam); idem, The Plant World, 7, 1904, p. 265 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 78 (Guam); Cox, Island of Guam, 1917, p. 21 (Guam); Nelson, Proc. 1st Pan-Pacific Sci. Conf., 1921, p. 273 (Guam).
Excalfactoria chinensis lineata Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 176 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 41 (Guam); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 20 (Marianne); Hand-list Japanese Birds, rev., 1932, p. 198 (Guam); Peters, Check-list Birds World, 2, 1934, p. 96 (Guam); Bryan, Guam. Rec., vol. 13, no. 2, 1936, p. 15 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 223 (Guam); Mayr, Birds Southwest Pacific, 1945, p. 287 (Guam).
Excalfactoria chinensis Strophlet, Auk, 1946, p. 536 (Guam).
Coturnix chinensis lineata Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 47 (Guam).
Geographic range.—Philippines and parts of Malaysia. In Micronesia: Mariana Islands—Guam (introduced).
Characters.—Adult: A small quail with upper parts brown splotched with black and streaked with buff; males with face and throat black surrounded by white line, upper breast blue gray, lower breast, belly and under tail-coverts and tail near "burnt sienna"; females lighter than males, underparts pale brown, mottled with blackish on breast and sides of body; bill dark lead colored, feet yellow.
Measurements.—Three adult males from Guam measure: wing, 66, 67, 67; culmen, 9.2, 10.0, 10.3; tarsus, 18.1, 18.7, 22.6.
Weights.—Two adult males taken by NAMRU2 at Guam weigh 34.5 and 35.5 grams.
Specimens examined.—Total number, 3 males from Mariana Islands, USNM—Guam (Feb. 24, June 13, 28).
Remarks.—Seale (1901:37) writes that the Painted Quail was introduced to Guam from Manila, or the island of Luzon in the Philippine Islands, by Captain Pedro Duarty of the Spanish Army in 1894. It was a successful introduction; the bird is well adapted to the grasslands, open hillsides, and fallow rice paddies. The bird appears to offer no serious competition to native species, because there are few native birds which depend largely on this habitat. The NAMRU2 party obtained specimens at Mt. Santa Rosa and near Agat; others were seen as singles or pairs near Umatac and on Mount [Pg 114] Tenjo. Strophlet (1946:536) observed the birds in the southern part of Guam in 1945. He found them as singles or pairs in the months of September, October and November. Wilfred Crabb reported a covey of seven birds in June, 1945. Two males taken in June had enlarged testes. Seale (1901:37) obtained a nest of seven eggs.
Phasianus Gallus Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 158. (Type locality, "India orientali, Pouli condor etc.," restricted to Pulo Condor, off mouths of the Mekong.)
Phasianus Gallus Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 284 (Ualan = Kusaie).
Gallus bankiva Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 103 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 29 (Palau); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 281, 298, 353 (Ponapé, Mortlock, Ruk); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 59 (Pelew, Caroline, Marianne, Marshall); Oustalet, Nouv. Arch. Mus. Hist. Nat., Paris, (3), 8, 1896, p. 25 (Saypan, Palaos, Marshall); Hartert, Novit. Zool., 5, 1898, p. 61 (Saipan); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 38 (Marianas); Safford, Osprey, 1902, p. 70 (Marianas).
Gallus ferrugineus Finsch, Proc. Zool. Soc. London, 1877 (1878), p. 780 (Ponapé); idem, Ibis, 1881, p. 114 (Ponapé, Kushai).
Gallus gallus bankiva Kuroda, in Momiyama, Birds Micronesia, 1922, p. 41 (Saipan, Pelew, Ponapé, Marshall).
Gallus gallus Mathews, Syst. Avium Australasianarum, 1, 1927, p. 21 (Micronesia); Cram, Bull. U. S. Nat. Mus., 140, 1927, pp. 238, 328 (Guam); Bequaert, Mushi, 12, 1939, p. 81 (Kusaie); idem, Occ. Papers Bernice P. Bishop Mus., 16, 1941, p. 266 (Kusaie); Mayr, Birds Southwest Pacific, 1945, pp. 57, 286 (Marianas, Carolines, Palaus); Wharton and Hardcastle, Journ. Parasitology, 32, 1946, pp. 294, 310 (Ulithi, Garakayo); Stott, Auk, 1947, p. 525 (Saipan).
Gallus gallus domesticus Hand-list Japanese Birds, rev., 1932, p. 198 (Marianas, Palaus, Carolines, Marshalls).
Gallus gallus micronesiae Hachisuka, Tori, 10, 1939 (1940), p. 600 (Type locality, Truk, also from Pelew, Rota, Yap, Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 222 (Saipan, Rota, Babelthuap, Koror, Yap, Truk, Lukunor, Ponapé, Kusaie, Marshalls).
Gallus gallus gallus Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 47 (Peleliu, Ngabad, Garakayo, Ulithi, Truk).
Geographic range.—Southeastern Asia and Malaysia; introduced into many islands of Oceana. In Micronesia: Mariana Islands—Saipan, Rota; Palau Islands—Kayangel, Babelthuap, Koror, Garakayo, Peleliu, Ngabad, Angaur; Caroline Islands—Ulithi, Yap, Truk, Lukunor, Ponapé, Kusaie; Marshall Islands—exact locality not known.
Specimens examined.—Total number, 3 (1 male, 2 females) as follows: Palau Islands, USNM—Garakayo, 1 (Sept. 19)—Peleliu, 1 (Sept. 13)—Ngabad, 1 (Sept. 11).
Parasites.—Cram (1927:238, 328) found the round worms (Nematoda), Dispharnyx nasuta and Oxyspirura mansoni in birds from Guam. Bequaert (1939:81 and 1941:266) found the fly (Hippoboscidae) Ornithoctona plicata, on fowl from Kusaie. Wharton and Hardcastle (1946:294, 310) obtained the chiggers (Acarina), Neoschöngastia yeomansi and N. ewingi from fowl at Ulithi and Garakayo.
Remarks.—The Red Jungle Fowl has been introduced in Micronesia, [Pg 115] as it has been in other parts of Oceania. It is found on many of the islands of Micronesia, including the volcanic islands and the atolls. The NAMRU2 party did not find feral fowl at Guam but found the wary birds at Ulithi and in the Palaus. The birds at Ulithi were small and of a mixed breed. At Palau some fine examples of typical jungle fowl were observed. Coultas obtained similar specimens at Ponapé and Kusaie. The natives have apparently allowed these birds to go wild, but catch them for food. These wild stocks may represent the earlier "liberations" while domestic fowl kept by natives at present appear to include several other breeds probably obtained from Europeans.
The committee that prepared the Hand-list of Japanese Birds (Hachisuka et al., 1942:222) points out that although many ornithologists believe the Red Jungle Fowl to be introduced in Micronesia and other parts of Oceania, it is their opinion (based on a series of more than 100 skins before them) that the population in Micronesia is racially distinct. They further comment, as did Hachisuka (1939b:600), that one may find hybrids between these birds and the domestic fowl belonging to the native peoples; this is commonly seen on the more populated islands such as Koror and Saipan. I have no doubt that these skins show distinct features; nevertheless, I am reluctant to recognize these by subspecific name, since the birds may be a mixture of domestic strains introduced by man at different times after the jungle fowl was first brought by the early Micronesians. It seems that the production of hybrids between the feral and domestic fowl, which we find there today, may have been going on ever since the European colonists arrived with their fancy breeds of chickens.
Phasianus colchicus Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 158. (Type locality, Africa, Asia = Rion.)
Phasianus torquatus Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 47 (Guam).
Geographic range.—Eastern China and northeastern Tonkin. Widely introduced into North America, Europe, and New Zealand. In Micronesia: Mariana Islands—Guam (introduced).
Remarks.—On July 4, 1945, fifty-seven Ring-necked Pheasants (sixteen cocks and forty-one hens) were liberated at Guam by personnel of the U. S. Navy. The birds were eleven weeks old when released, having been brought by plane from the hatcheries of the State Division of Game and Fish in California. Twenty-four birds were liberated at the site of CincPoa headquarters near Mt. Tenjo. [Pg 116] Thirty-three were placed near the FEA dairy farm, approximately one and one-fourth miles west of Price School. One month after release the birds were present at the liberation sites, although there were reports that some had drifted as far away as a mile or more. The birds were not banded. This liberation has been reported on by Quinn (1946:32-33) and by the author (1946b:211 and 1948:47). In using the name P. colchicus, I am following Delacour (in McAtee, 1945:8) and the twenty-third supplement to the American Ornithologists' Union check-list of North American birds (Auk, 65, 1948:440).
Hypotaenidia philippensis pelewensis Mayr, Amer. Mus. Novit., no. 609, 1933, p. 3. (Type locality, Palau Islands.)
Rallus philippensis Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 831 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 37 (Palau); idem, Proc. Zool. Soc. London, 1877, p. 587 (Palau); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 59 (Pelew); Finsch, Deut. Ver. zum Schulze der Vogelwelt, 18, 1893, p. 459, Palau).
Rallus pectoralis Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 8, 117, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 89, 107 (Pelew).
Eulabeornis forsteri Gray (part), Hand-list Birds, 3, 1871, p. 57 (Pelew).
Hypotaenidia philippensis Salvadori (part), Ornith. Papuasia, 3, 1882, p. 261 (Pelew); Sharpe (part), Cat. Birds British Mus., 23, 1894, p. 39 (Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 42 (Pelew).
Eulabeornis philippensis? Mathews, Birds Australia, 1, 1910-1911, p. 199 (Pelew).
Hypotaenidia philippinensis philippensis Hand-list Japanese Birds, rev., 1932, p. 196 (Palau).
Rallus philippensis pelewensis Hand-list Japanese Birds, 3d ed., 1942, p. 220 (Babelthuap, Koror); Mayr, Birds Southwest Pacific, 1945, p. 287 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 48 (Peleliu, Garakayo).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Arakabesan, Garakayo, Peleliu, Angaur.
Characters.—Adult: A large, slender rail with black crown streaked with brown; superciliary stripe ashy-gray, lighter toward bill; eye stripe brown becoming more rufous behind eye and on nape; chin ashy-gray; throat near "mouse gray" tinged with olive especially toward breast; breast, belly and sides barred with black and white, with a broad "tawny" band on breast; posterior part of belly and vent buffy with some barring; under tail-coverts barred with black, white, and buff; mantle black with feathers subterminally barred with white; back, scapulars, inner wing-coverts, and rump black with white spotting and feathers edged with olive brown; outer wing-coverts, secondaries, and primaries barred with black and rufous with some buffy-white on outer webs; under wing barred black and white with some brownish markings; tail with both bars and blotches of black, white, and buffy-rufous; maxilla horn-colored; mandible yellowish; feet light brown.
R. p. pelewensis resembles R. p. philippensis Linnaeus of the Philippines, but is darker with nape more rufous-brown; upper parts marked with narrower and darker edgings to feathers and with pronounced whitish spotting.
Resembles R. p. chandleri (Mathews) of Celebes, but with wing shorter; more pronounced band on breast; bird darker above and below; rump and upper tail-coverts less spotted.
Measurements.—Specimens in the collection of the United States National Museum measure as follows: four adult males—wing, 130-134 (132); tail, 59-63 (61); full culmen, 30-37 (34); tarsus, 38-45 (43); four adult females—wing, 125-130 (127); tail, 54-61 (58); full culmen, 29-35 (32); tarsus, 38-42 (40). Mayr (1933c:4) lists the following measurements: twelve adult males—127-143 (134.6); tail, 54-65 (60); exposed bill, 25-28 (27.7); tarsus, 41-46 (43.5); three adult females—wing, 129, 136, 136; tail, 56, 57, 58; exposed bill, 23, 24, 25; tarsus, 40, 41, 42.
Specimens examined.—Total number, 27 (18 males, 9 females), as follows: Palau Islands, USNM—Garakayo, 4 (Sept. 18, 19, 20)—Peleliu, 4 (Aug. 27, 28, Sept. 16)—Arakabesan, 1 (Nov. 26); AMNH—exact locality not given, 18 (Oct., Nov., Dec.).
Nesting.—The condition of the gonads in specimens obtained indicates that the breeding season is principally in the fall and winter. Of adult rails taken by Coultas in October, November and December, 1931, 6 of 12 males and 3 or 4 females had enlarged gonads. In September, 1945, the NAMRU2 party obtained two adult males with swollen testes. Marshall (1949:219) recorded breeding in September and November.
Food habits.—Stomachs of rails obtained by the NAMRU2 party contained insects, seeds and small mollusks. Coultas (field notes) notes that the birds eat snails, roots and other vegetable matter.
Remarks.—Rallus philippensis is geographically widespread, being found from Tasmania and Australia north to Malaysia and the Philippines west to Cocos Keeling Island east to Melanesia and western Polynesia and north to the Palau Islands. The species is divisible into several subspecies. The one in the Palaus, although distinctive, does not appear to have undergone a higher degree of differentiation (even though isolated as a small population) than any of the subspecies in Malaysia or Melanesia. Perhaps the form on Palau as well as the relatively undifferentiated Poliolimnas cinereus are rather recent invaders of Micronesia, as compared with Rallus owstoni and Aphanolimnas monasa.
The Banded Rail is less secretive in habits than Rallus owstoni of Guam, and neither was seen to fly. At Angaur, Peleliu and Garakayo, the NAMRU2 party found the rail in areas of swamp and marsh as well as in the rocky uplands; it probably prefers the former habitats. Several rails were observed and shot in open places, but they probably prefer to remain in dense cover. Coultas found the birds at taro patches and swamps. I watched a rail feeding along an open trail on Garakayo. The bird was eating small mollusks and other items which were in the open area. Being a true skulker, the bird would make a quick dash to the feeding place, remain only a [Pg 118] few moments, hurriedly return to the protective cover, and then repeat the process. The best means that I found of obtaining these birds was using traps baited with peanut butter and oatmeal. The traps had to be visited frequently or the ants made short work of the captured birds.
Hypotaenidia owstoni Rothschild, Novit. Zool., 2, 1895, p. 481. (Type locality, Guam.)
?Rallus philippinus Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 51 (Marian or Ladrone Is.).
Rallus pectoralis Finsch and Hartlaub, Fauna Centralpolynesiens, 1867, p. 157 (Guam).
Eulabeornis forsteri Gray (part), Hand-list Birds, 3, 1871, p. 57 (Marian).
Hypotaenidia philippensis Pelzeln, Ibis, 1873, p. 41 (Marianne Isl.); Salvadori (part), Ornith. Papuasia, 3, 1882, p. 261 (Marianas); Sharpe (part), Cat. Birds British Mus., 23, 1894, p. 39 (Guam).
Rallus philippinus Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 59 (Guam).
Hypotaenidia owstoni Hartert, Novit. Zool., 5, 1898, p. 62 (Guam); Safford, Osprey, 1902, pp. 41, 67 (Guam); idem, The Plant World, 7, 1904, p. 265 (Guam); Dubois, Syn. Avium, 2, 1904, p. 961 (Mariannes); Safford, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Cox, Island of Guam, 1917, p. 21 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 42 (Guam); Hartert, Novit. Zool., 34, 1927, p. 22 (Guam); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 84 (Guam); Hand-list Japanese Birds, rev., 1932, p. 196 (Guam).
Hypotaenidia marchei Oustalet, Nouv. Arch. Mus. Hist. Nat., Paris, (3), 8, 1896, p. 32 (Type locality, Guam).
Hypotaenidia oustini Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 30 (Guam).
Rallus owstoni Peters, Check-list Birds World, 2, 1934, p. 166 (Guam); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 220 (Guam); Mayr, Birds Southwest Pacific, 1945, p. 287 (Guam); idem, Audubon Mag., 47, 1945, p. 279 (Guam); Watson, Raven, 17, 1946, p. 41 (Guam); Strophlet, Auk, 1946, p. 536 (Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 48 (Guam).
Geographic range.—Micronesia: Mariana Islands—Guam.
Characters.—Adult: A large rail with head, neck, and eye stripe near "mummy brown" with feathers on sides of neck tipped with "russet"; superciliary stripe to back of neck, throat and upper breast near "mouse gray"; mantle, back, scapulars, and some upper wing-coverts dark olive-brown becoming browner on rump and upper tail-coverts; wings dark with brownish spots and barred with white; lower breast, abdomen, under tail-coverts, and tail blackish with white barrings; bill lead colored; feet dark brown; tibia brown; iris red.
Measurements.—Four adult males measure: wing, 120-123 (121); tail, 46-54 (50); full culmen, 37-43 (41); tarsus, 47-51 (50); six adult females measure: wing, 108-118 (112); tail, 38-46 (42); full culmen, 36-39 (37); tarsus, 43-47 (45).
Weights.—The NAMRU2 party obtained specimens with the following weights: two adult males 256, 257; four females 147, 153, 210, 252 grams.
Specimens examined.—Total number, 13 (5 males, 6 females, 2 unsexed), from Mariana Islands, USNM—Guam (Jan. 29, May 8, June 19, 20, 23, 28, 30, July 14, 19, 23, Sept. 8).
Nesting.—A nest was found by McElroy of the NAMRU2 party at Guam on October 24, 1945, in dense grass on a hillside near Mount Santa Rosa. The nest contained three eggs, which the author (1948:48) describes as "white with a pinkish cast and a scattering of small spots of colors near 'russet' and near 'pear blue' which are concentrated at the large ends. They measure 37.5 by 29.1, 39.1 by 28.0, and 40.7 by 29.0." Downey, black chicks were found on April 1, May 16, and May 26. M. Dale Arvey found a chick on August 2, 1946, near Tumon Bay. A parent bird with young ones was seen near Merizo on October 2. A male taken on January 26 had enlarged gonads. Seale (1901:30) obtained a black chick in June or July. On the basis of the above observations it seems that the nesting season extends from spring to fall, although Marshall (1949:219) assumes that this rail breeds the year around.
Remarks.—The Guam Rail was first reported by Quoy and Gaimard who called it "Ralê tiklin," but was not described as new until 1895 by Rothschild. It appears to be equally at home in upland grassy areas and in jungle areas. The species was not seen frequently by the NAMRU2 party, although birds were occasionally observed crossing the roads. Few birds were shot; most of the specimens were taken in rat traps, which may be the most satisfactory method of obtaining them. Coultas took his specimens with the aid of a dog. On June 19, 1945, a small patch of woodland was being removed by a bulldozer. Four rails, which were hiding in this thicket, were surrounded and three were captured by hand. These birds tried to escape over the cleared ground by running with wings flapping but made no effort to fly. I am inclined to believe, as the natives do, that these birds are virtually incapable of actual flight.
The Guam Rail usually appeared to be a quiet bird, but at Tarague Point on July 12, 1945, I heard its loud penetrating cry; it was a series of rapid screeches. At the same time rapid movement made considerable noise in the undercover. The bird making the call suddenly appeared, either rapidly chasing, or being chased by, another rail. The birds had abandoned their usual skulking habits and had little concern for the observer. I took this to be breeding behavior, comparable to that of some of the North American rails during the mating period.
The Guam Rail is probably not in serious danger of extermination. It is utilized by the natives as food; they capture the bird, using dogs and trail snares. Its skulking habits and ability to inhabit most types of cover on the island should insure its existence for a long time to come.
Evolutionary history.—Rallus owstoni is endemic to the island of Guam with no closely related forms nearby. It is one of the several [Pg 120] rails found in the Pacific which live on isolated islands. In comparison with other species in the region, it has some resemblance to both R. torquatus and R. philippensis. In general, the underparts of R. owstoni resemble those of the R. philippensis group, although the upper parts resemble somewhat those of R. torquatus. Certain specimens of R. owstoni have a slight indication of a pale pectoral band. The bill is shorter and heavier than that of R. torquatus, possibly more like that of R. philippensis. The short rounded wing is a distinctive character. The bird came from an ancestral stock possibly resembling R. philippensis and probably originated in the Philippine or Papuan areas. It may have invaded Micronesia at an early date and may have had a wider distribution in the islands in former times. Perhaps this same invasion resulted in the establishment of R. wakensis (Rothschild) at Wake. The supposed route of colonization is shown in figure 9.
Rallus fasciatus Raffles, Trans. Linn. Soc. London, 13, pt. 2, 1822, p. 328. (Type locality, Benkulen, western Sumatra.)
Rallina fasciata Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 831 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 7, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 89, 106 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 37 (Palau); Salvadori, Ornith. Papuasia, 3, 1882, p. 264 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 60 (Pelew); Sharpe, Cat. Birds British Mus., 23, 1894, p. 75 (Pelew); Finsch, Deut. Ver. zum Schulze der [Pg 121] Vogelwelt, 18, 1893, p. 459 (Palau); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 41 (Pelew); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 88 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 196 (Palau); Peters, Check-list Birds World, 2, 1934, p. 171 (Pelew); Hand-list Japanese Birds, 3d ed., 1942, p. 221 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 287 (Palau); Delacour, Birds Malaysia, 1947, p. 77 (Palau).
Geographic range.—Burma east and south to Malaysia and the Philippines. In Micronesia: Palau—exact locality unknown.
Remarks.—The Malay Banded Crake is known in the Palau Islands from birds taken by captains Tetens, Heinsohn, and Peters and by Kubary according to Finsch (1875: 37). It has not been taken by later collectors. Two unsexed and undated skins are in the collection of the American Museum of Natural History; these are from the Kubary collection.
Gallinula eurizonoïdes Lafresnaye, Rev. Zool., 1845, p. 368. (No locality; the type agrees with specimens from the Philippine Islands.)
Rallina eurizonoides eurizonoides Hand-list Japanese Birds, rev. 1932, p. 196 (Koror); Hand-list Japanese Birds, 3d ed., 1942, p. 221 (Koror).
Rallina eurizonoides subsp. Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Philippine Islands. In Micronesia: Palau Islands—Koror.
Remarks.—This crake is apparently a straggler to western Micronesia from the Philippine area.
Rallus monasa Kittlitz, Denks. Riese russ. Amer. Micron. und Kamchat., 2, 1858, p. 30. (Type locality, Kushai.)
Rallus tabuensis? Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 286 (Ualan).
Ortygometra tabuensis Finsch, Journ. f. Ornith., 1880, pp. 297, 307 (Kuschai); idem, Ibis, 1881, pp. 106, 109 (Kushai); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 60 (Ualan).
Kittlitzia monasa Hartlaub, Abhandl. nat. Ver. Bremen, 12, 1892, p. 391 (Kuschai); Finsch, Mitth. Ornith. Ver. Wien, 17, 1893, p. 1 (Kuschai).
Aphanolimnas monasa Sharpe, Bull. British Ornith. Club, 1892, p. 20 (Kuschai); Finsch, Deut. Ver. zum Schulze der Vogelwelt, 18, 1893, p. 457, pl. 4 (Ualan); Wiglesworth, Ibis, 1893, p. 214 (Kushai); Sharpe, Cat. Birds British Museum, 23, 1894, p. 115 (Kushai); Matschie, Journ. f. Ornith., 1901, pp. 110, 113 (Ualan); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 93 (Caroline Islands); Hand-list Japanese Birds, rev., 1932, p. 197 (Kusaie); Peters, Check-list Birds World, 2, 1934, p. 189 (Kusaie); Hand-list Japanese Birds, 3d ed., 1942, p. 221 (Kusaie); Mayr, Birds Southwest Pacific, 1945, p. 288 (Kusaie); idem, Audubon Mag., 47, 1945, p. 280 (Kusaie).
Porzana tabuensis Sharpe, Cat. Birds British Mus., 23, 1894, p. 111 (Kushai).
Pennula monasa Dubois, Syn. Avium, 2, 1904, p. 969 (Kuschai).
Porzana tabuensis tabuensis Kuroda, in Momiyama, Birds Micronesia, 1922, p. 42 (Kusaie).
Geographic range.—Micronesia: Caroline Islands—Kusaie (probably extinct).
Characters.—Sharpe (1894:115) gives the following description: "Adult. Black with a bluish-grey reflexion; quills and tail somewhat browner; inner wing-coverts brownish with white spotting, outer edge of first primary dull brownish, chin and middle of the throat somewhat paler; bill blackish (Hartlaub.)."
Remarks.—Two specimens of this rail are known. The two were taken by Kittlitz on his visit to Kusaie in December and January of 1827-'28. Coultas made a search for the bird in 1931 and failed to obtain it; he suggested that the high population of introduced rodents may have been a factor contributing to its extinction. The bird is considered to be extinct by the authors of the Hand-list of Japanese Birds (Hachisuka et al., 1942:221).
The two known specimens are in Leningrad, and Mayr sent examples of Porzana tabuensis there for comparison. The following is a translation of the letter received by Mayr from Boris Stegmann dated at Leningrad, December 7, 1937.
"I have compared the two specimens of Porzana tabuensis with our specimens of Aphanolimnas monasa. The difference is in my opinion of generic value. Aphanolimnas is distinctly larger and more robust. The bill is not only absolutely but also relatively longer. Its length (measured from the forehead) reaches to the end of the second phalanx of the middle toe while it not nearly reaches it in tabuensis. The proportions of feet and toes are the same in both, but the feet are distinctly heavier in Aphanolimnas. The wings are relatively shorter in Aphanolimnas and the wing feathers are very soft. The wing is also much more rounded, the first primary is about 21 mm. shorter than the wing tip. The tail consists of very soft loose feathers which resemble only distantly true tail feathers. It is therefore questionable whether this bird was at all able to fly.
"The coloration is in general dull black, brownish black on head and wings, chin and upper throat are dark slate colored lighter in the middle. The under wing and tail-coverts are marked with scattered white spots (querflecken). The first primary has an irregular whitish brown margin on the outer web. The bill is dark and the feet yellowish."
Possibly this rail represents an ancient colonization of Kusaie from an ancestral stock of Porzana in Polynesia. Mayr (1941b:203) is also of this opinion, and if this is true there is no close relationship between Aphanolimnas and the rails at Guam and Wake, Rallus owstoni and R. wakensis, which are probably colonizers from the Philippines or the Papuan area. Mayr (1943:46) remarks further [Pg 123] that the Hawaiian flightless rail (Peuula) is of doubtful taxonomic position, but may be related to the "Aphanolimnas-Porzanoidea-Nesophylax stock," although there is no evidence that Pennula is not related to Rallus. Supposed colonization routes are shown in figure 9.
Poliolimnas cinereus micronesiae Hachisuka, Bull. British Ornith. Club, 59, 1939, p. 151. (Type locality, Yap.)
Ortygometra quadristrigata Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 8, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 90, 107 (Pelew, Uap).
Ortygometra cinerea Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 38 (Palau, Yap); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); Salvadori, Ornith. Papuasia, 3, 1882, p. 273 (Yap, Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 61 (Pelew, Yap, Ruk); Finsch, Deut. Ver. zum Schulze der Vogelwelt, 18, 1893, p. 459 (Palau).
Ortygometra cinerea = quadristrigata Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 353 (Ruk).
Poliolimnas cinereus Sharpe, Cat. Birds British Mus., 23, 1894, p. 130 (Pelew, Yap, Ruk); Hartert, Novit. Zool., 5, 1898, p. 64 (Guam); idem, Novit. Zool., 7, 1900, p. 9 (Ruk); Scale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 30 (Guam); Safford, Osprey, 1902, p. 67 (Mariannes); idem, The Plant World, 7, 1904, p. 265 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Cox, Island of Guam, 1917, p. 21 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 42 (Guam, Pelew, Yap, Ruk).
Porzana cinerea Stresemann, Novit. Zool., 21, 1914, p. 54 (Guam, Truk).
Porzana cinerea ocularis Hartert, Novit. Zool., 31, 1924, p. 264 (Ruk, Guam).
Poliolimnas cinereus collingwoodi Mathews, Syst. Avium Australasianarum, 1, 1927, p. 95 (Pelew, Marianne, Carolines); Hand-list Japanese Birds, rev., 1932, p. 197 (Guam, Koror, Yap, Truk); Hachisuka, Birds Philippine Islands, 1, 1932, p. 236 (Marianne, Pelew, Caroline); Peters, Check-list Birds World, 2, 1934, p. 198 (Marianne, Caroline, Pelew); Bryan, Guam Rev., vol. 13, no. 2, 1936, p. 15 (Guam); Mayr, Birds Southwest Pacific, 1945, p. 288 (Guam, Palau, Yap, Truk, Bikini); Delacour and Mayr, Birds Philippines, 1946, p. 64 (Micronesia); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 48 (Ulithi?, Truk).
Porzana cinerea collingwoodi Rensch, Mitt. Zool., 1931, p. 468 (Marianne, Karolinen, Palau).
Poliolimnas cinereus micronesiae Yamashina, Tori, 10, 1940, p. 679 (Bikini); Hand-list Japanese Birds, 3d ed., 1942, p. 221 (Guam, Babelthuap, Koror, Yap, Truk, Bikini).
Geographic range.—Micronesia: Mariana Islands—Guam; Palau Islands—Koror, Babelthuap; Caroline Islands—Yap, Ulithi?, Truk; Marshall Islands—Bikini.
Characters.—Adult: A slightly built, long-legged rail with forehead and anterior crown light gray with darker, slate-colored feather shafts; color more olive-brown on occiput and nape; eyestripe dark slate extending to occiput; superciliary from bill to eye, and stripe below eye, white; chin and throat ashy-white; sides of head, neck and breast ashy-gray, lighter on breast and whitish on abdomen; sides of abdomen ashy-brown becoming more buffy on tibia and under tail-coverts; mantle olive-colored becoming lighter and more brownish on back, rump, and scapulars; wing-coverts similar in color but feathers with broad dark brown shaft-marks; wings brown, first primary with whitish outer web; under wing gray with some lighter streaks; tail dark brown, lighter on edges; bill horn colored, tan below; feet brown; iris vermillion.
Immature: Resembles adult, but head more rufous, upper parts marked with buffy rufous; eye stripe light rufous-brown; underparts tinged with rufous.
P. c. micronesiae differs from P. c. collingwoodi Mathews of the Philippines by having more pale gray and less olivaceous-brown on the nape and shoulder; darker on the under tail-coverts; and having a shorter culmen. P. c. brevipes (Ingram) of the Volcano Islands differs from P. c. micronesiae by being paler on upper parts, particularly back and wing-coverts and more washed with buff below; by having a shorter, thicker culmen; and by having a shorter tarsus.
Measurements.—Measurements are shown in table 17.
| Location | No. | Wing | Tail | Full culmen |
Tarsus |
| Poliolimnas cinereus collingwoodi Philippines, Talaut. Celebes |
13 | 98 | 22.5 | 38.0 | |
| 92-108 | 21.0-24.0 | 35.0-41.0 | |||
| Poliolimnas cinereus micronesiae Guam |
10 | 95 | 51 | 21.0 | 37.0 |
| 91-102 | 50-53 | 20.0-22.5 | 34.5-39.0 | ||
| Palau |
10 | 93 | 51 | 21.0 | 37.0 |
| 89-95 | 51-53 | 20.0-23.0 | 34.0-38.0 | ||
| Truk |
5 | 95 | 51 | 21.0 | 36.0 |
| 94-97 | 51-53 | 20.5-22.5 | 35.0-37.0 | ||
| Poliolimnas cinereus brevipes S. Dionisio Island |
8 | 96 | 19.0 | 30.0 | |
| 94-97 | 17.0-20.0 | 29.0-32.0 |
Specimens examined.—Total number, 25 (11 males, 13 females, 1 unsexed), as follows: Mariana Islands, AMNH—Guam, 10 (July 13, Aug. 1, 5, 7, 13, 19, 23, 31); Palau Islands, AMNH—exact locality not given, 10 (Nov. 11, 13, 15, 23, 25); Caroline Islands, AMNH—Truk, 5 (June 3, 8, 16, 17, 18).
Nesting.—Hartert (1900:9) describes two nests found on swampy ground. One contained three eggs, the other four eggs. He writes, "The eggs are pale buff, or cream-colour, speckled all over with brownish rufous, more frequently near the broad end. In some eggs, these spots are larger, in others minute, and there are often some, underlying pale purplish gray spots."
Remarks.—Superficially, the White-browned Rail of Micronesia is distinct from its near relative, P. c. collingwoodi, but the differences are not so well marked as they are between insular populations of other species of rails. It is probably a comparatively recent addition to the Micronesian avifauna, and its pattern of distribution may represent an early stage in the development of endemism in contrast to the pattern of later stages in the development of insular forms shown by the isolated rails, Rallus owstoni and Aphanolimnas [Pg 125] monasa. The fact that Poliolimnas cinereus is found only on widely separated islands in Micronesia does not necessarily mean that it has become "extinct" on the intervening islands, but that it may be partial to the larger, "high" islands, or that it is actually present but remains to be discovered on these intervening islands when more intensive field investigations are made. Hachisuka (1939a:151), in naming the Micronesian form, comments that it has a shorter bill than P. c. collingwoodi of the Philippines and Celebes, and that it is intermediate between this subspecies and P. c. brevipes of the Volcano Island to the north. Within these three subspecies there are trends toward a shorter culmen and shorter tarsus and, less markedly, toward a shorter wing. From the evidence at hand, it can be concluded that Poliolimnas first colonized Micronesia probably from the Philippine area (or Papuan area) through the Palaus and Carolines, to the Marianas and north to the Volcano Islands. Further, this has probably been a relatively recent invasion, although the subspecies in the Volcano Islands shows marked change in length of tarsus and culmen. This extension of range to the islands north of the Marianas is unusual and resembles somewhat the distribution of Nycticorax caledonicus in the same general area.
The Micronesian White-browed Rail is a shy bird with the typical skulking habits of most rails. The NAMRU2 party did not find the bird at Guam, although reports were obtained that it was present in the marsh and swamp areas. Coultas (field notes) tells of observing the rail at Palau at a fresh water lake on Babelthuap, where it was difficult to obtain and apparently rare. Seale (1901:30) obtained a female specimen at Guam from native boys who snared it in a sweet potato patch near the Agaña River. This bird, taken in June or July, had eggs ready for laying. McElroy of the NAMRU2 party observed rails at Truk in brackish swamps, where he found them to be fairly common. A male which was taken in December had enlarged gonads. At Asor in the Ulithi Atoll, the NAMRU2 party learned that a small rail (possibly of this species) was found at taro patches in the early days of occupation, but that it was apparently eliminated by clearing operations. The taking of a bird at Bikini, as reported by Yamashina (1940:679), is further evidence that these birds may subsist on coral atolls as well as on the high volcanic islands; possibly the bird of the Marshalls may have been derived from the south rather than from the west. Unlike Rallus owstoni, this bird is apparently restricted to swampy areas, and may be eliminated from its habitat by drainage or clearing by man. It may [Pg 126] always persist, however, in the taro patches maintained by the natives.
Gallinula orientalis Horsfield, Trans. Linn. Soc. London, 13, 1821, p. 195. (Type locality, Java.)
Gallinula chloropus indicus Hand-list Japanese Birds, rev., 1932, p. 197 (Babelthuap); Takatsukasa and Yamashina, Dobutsu. Zasshi, 44, 1932, p. 266 (Pelew, Coror).
Gallinula chloropus indica Hand-list Japanese Birds, 3d ed., 1942, p. 221 (Babelthuap).
Gallinula chloropus subsp. Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 49 (Peleliu, Angaur).
Geographic range.—Malaysia from southern Malay Peninsula to Celebes. In Micronesia: Palau Islands—Babelthuap, Koror, Peleliu, Angaur.
Characters.—Adult: Resembles G. c. indica Blyth, G. c. lozanoi Lletget and G. c. guami Hartert, but smaller and paler; upper wing-coverts less olivaceous-brown and more slate-colored; back, rump, and scapulars less richly washed with olivaceous-brown. Resembles G. c. orientalis from Java in size, but much paler.
Measurements.—An unsexed adult bird from Angaur measures: wing, 150; bill from rictes, 27.1; bill from nostril, 13.4; tarsus, 46.
Specimens examined.—Total number, 3 (2 males, 1 unsexed) from Palau Islands, USNM—Angaur (Sept. 21).
Remarks.—Owing to the lack of sufficient material, I am unable to determine the exact status of the resident gallinule in the Palau Islands. On the basis of a single, unsexed adult and two immatures there is not very much that can be said. The adult is smaller and paler than G. c. indica, G. c. lozonoi, and G. c. guami. It resembles specimens of the subspecies G. c. orientalis in size but is also paler than the skins of this race which I have examined. It seems closest to this latter subspecies to which I tentatively refer it. If it is closest to this subspecies, it probably reached Palau from the Celebean region, rather than from the Philippines or some other route. Whether specimens taken by the Japanese at Babelthuap and Koror are G. c. indica is questionable, unless the skins were from migrants which may visit Palau from the west or northwest. The Hand-list of Japanese Birds (Hachisuka et al., 1942:177) records G. c. indica from the Bonin Islands.
The three Gallinules were taken by the NAMRU2 party at fresh and brackish water swamps at Angaur on September 21, 1945. Several Gallinules were seen in the area and several were observed also at Peleliu Island. One of the immatures was just growing its wing feathers, indicating that the birds must breed in the Palau Islands.
Gallinula chloropus guami Hartert, Novit. Zool., 24, 1917, p. 268. Type locality, Guam).
Fulica chloropus Quoy and Gaimard, Voy. "Uranie," Zool., 1824, p. 703 (Guam); Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 305 (Guahan).
Gallinula galeata var. sandwichensis Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 34 (Saypan, Tinian, Guam).
Gallinula chloropus Hartert, Novit. Zool., 5, 1898, p. 62 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 31 (Guam); Safford, Osprey, 1902, p. 67 (Marianas); idem, Amer. Anthro., 4, 1902, p. 711 (Guam); idem, The Plant World, 7, 1904, p. 265 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Prowazek, Die deutschen Marianen, 1913, p. 101 (Marianen); Cox, Island of Guam, 1917, p. 21 (Guam); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 177 (Guam); Strophlet, Auk, 63, 1946, p. 536 (Guam).
Gallinula chloropus guami Hartert, Vögel pal. Fauna, 15, 1921, p. 1843 (Guam); Kuroda, Avifauna Riu Kiu, 1925, p. 199 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 43 (Guam, Tinian, Saipan); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 99 (Mariana Islands); Takatsukasa and Yamashina, Dobutsu. Zasshi, 44, 1932, p. 226 (Pagan); Hand-list Japanese Birds, rev., 1932, p. 197 (Guam, Tinian, Saipan, Pagan); Hachisuka, Birds Philippine Islands, 1, 1932, p. 241 (Guam); Peters, Checklist Birds World, 2, 1934, p. 204 (Marianne Islands); Bryan, Guam. Rec., vol. 13, no. 2, 1936, p. 15 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 222 (Guam, Tinian, Saipan, Pagan); Mayr, Birds Southwest Pacific, 1945, p. 288 (Marianas); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 92 (Tinian); Stott, Auk, 1947, p. 525 (Saipan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 49 (Guam, Tinian, Saipan).
Geographic range.—Micronesia: Mariana Islands—Pagan, Saipan, Tinian, Guam.
Characters.—Adult: Head and neck sooty black; upper back dark, bluish slate-gray; lower back and wing-coverts brownish; tail blackish-brown; wings dark brown, outer edge of first primary white; breast and upper abdomen dark slate-gray, feathers on sides of breast with longitudinal white streak; under wing dark with white edges; lower abdomen grayish with white-tipped feathers; vent black; under tail-coverts white; bill and frontal shield red, tip of bill yellowish; legs and feet olive-green.
Adult female: Resembles adult male but usually with smaller frontal shield.
Immature: Resembles adult, but forehead mottled white and brown, with sides of head less distinctly speckled with brown; crown, neck and upper back dusky brown; back, scapulars and upper tail-coverts olivaceous-brown; chin and throat whitish; breast feathers pearly-gray tipped with white; abdomen white; sides gray, washed with buff. Older birds are darker above and more brownish-gray below; frontal shield small.
G. c. guami resembles G. c. indica, but upper wing-coverts darker and near "olivaceous black"; back, rump and scapulars darker and less olivaceous brown, although not so dark as in G. c. orientalis. From G. c. lozanoi, G. c. guami differs in: slightly darker upper wing-coverts; richer olivaceous-brown on back, scapulars and rump; thinner culmen with possibly less yellow coloring on tip. G. c. guami resembles G. c. sandvicensis Streets of the Hawaiian Islands, but has less olive wash on the feathers and a smaller frontal shield.
Measurements.—Measurements of Gallinula chloropus are presented in table 18. In general, females are smaller than males.
| Location | No. | Wing | Bill from rictus |
Bill from nostril |
Tarsus |
| G. c. indica | 15 | 164 | 27 | 14.4 | 48 |
| 158-173 | 24-29 | 13.1-18.1 | 44-50 | ||
| G. c. orientalis | 3 | 152 | 27 | 13.8 | 45 |
| 146-152 | 26-29 | 13.1-14.4 | 44-46 | ||
| G. c. lozanoi | 11 | 164 | 27 | 14.5 | 50 |
| 153-170 | 24-29 | 13.1-15.2 | 45-57 | ||
| G. c. guami | 11 | 164 | 27 | 14.7 | 49 |
| 156-171 | 24-28 | 13.1-16.2 | 47-56 | ||
| G. c. sandvicensis | 2 | 150-158 | 27 | 13.4 | 52-56 |
Weights.—From Guam an adult male weighed 291 grams and an adult female 256 (Baker, 1948:49).
Specimens examined.—Total number, 42 (16 males, 22 females, 4 unsexed), as follows: Mariana Islands, USNM—Guam, 5 (Feb. 24, May, June 5, 7, 18—Tinian, 3 (Oct. 12, 18)—Saipan, 3 (Sept. 28, 30); AMNH—Guam, 25 (Feb. 21, April 6, July 13, 28, 30, Aug. 1, 3, 6, 7, 13, 19, 23, 30, 31, Sept. 3, 17, Dec. 11—Tinian, 5 (June 11, Sept. 12, 13, 14).
Nesting.—Hartert (1898:63) reports nests of the Gallinule at Guam in grass and on swampy ground in December and March. A male with enlarged gonads was taken by the NAMRU2 party at Guam on June 7. Marshall (1949:219) is of the opinion that this bird breeds all year.
Food habits.—Seale (1901:31) found grass, insects, and larvae in stomachs obtained at Guam.
Remarks.—The subspecies G. c. indica, G. c. lozanoi, G. c. guami, and G. c. sandvicensis bear a close resemblance to one another in size and color. G. c. guami and G. c. lozanoi resemble each other so closely that it would be difficult to separate unlabeled specimens of the two subspecies. G. c. orientalis differs from all of the gallinules in smaller size and darker color. Study of these forms indicates that the Gallinule has colonized the Marianas from Asia probably by way of Japan and the Bonin and Volcano islands. The Hawaiian subspecies is probably of American origin, as pointed out by Mayr (1943:46), and is not a close relative of the Mariana subspecies. The fact that these insular subspecies have not undergone much differentiation does not necessarily mean that they are recent arrivals, but probably is a reflection of the lack of plasticity of the species; as a whole the species does not exhibit anywhere a great amount of geographic variation. A thorough study of all insular populations of this species (including specimens from the Azores, Seychelles, Réunion, [Pg 129] Mauritus, and the Greater and Lesser Antilles) might reveal the effect of isolation on the species in general. Its ability to become established on isolated islands is apparent, although it is indeed peculiar that the species has not reached the Caroline Islands.
The Gallinule in the Marianas is restricted to fresh water lakes, marshes and swamps on the islands of Guam, Tinian, Saipan and Pagan. Coultas (field notes), on visiting the island of Tinian in 1931, comments that the bird is rare and found at only one lake on the island. Downs (1946:92) noted the species in 1945, and Joe T. Marshall Jr. obtained three specimens at Lake Hagoya in October of the same year. Gleise (1945:220) estimated the population of Gallinules on Tinian in 1945 at 70 individuals. Stott (1947:525) reports that the birds were abundant at Lake Susupe, Saipan, in 1945. Seale (1901:31) found the Gallinule to be abundant at Guam in marshes and taro patches. In 1945, the NAMRU2 party found fairly large populations of the Gallinule in fresh water marshes and fallow rice paddies at Guam. The greatest concentration of birds appeared to be in the Agaña Swamp and along the Ylig River. They seldom ventured out into open water but preferred weedy edges into which they could suddenly dart when disturbed. It was interesting to note such wary behavior, for an observer would think that after the bird had been in an environment virtually devoid of birds of prey (except for an occasional migrant) for a number of generations, it would have lost such behaviorisms as a result of the absence of the selective processes involved in predation.
Porphyrio melanotus Temm. var. pelewensis Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 107. (Type locality, Pelew Islands.)
Porphyrio melanotus Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 8, 117, 118 (Pelew); Gray, Hand-list Birds, 3, 1871, p. 64 (Pelew).
Porphyrio melanotus pelewensis Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 61 (Pelew); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 70 (Palau); Dubois, Syn. Avium, 2, 1904, p. 976 (Pelew); Mathews, Birds Australia, 1, 1911, p. 241 (Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 43 (Pelew); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 100 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 197 (Palau); Hachisuka, Birds Philippines, 1, 1932, p. 245 (Pelew).
Porphyrio pelewensis Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 39 (Palau); Salvadori, Atti Accad. Sci. Torino, 14, 1879, p. 1169 (Pelew); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Finsch, Deut. Ver. zum Schutze der Vogelwelt, 18, 1893, p. 459 (Palau); Sharpe, Cat. Birds British Mus., 23, 1894, p. 206 (Pelew); Nehrkorn, Nat. Eiers., 1899, p. 205 (Palau-Inseln); Matschie, Journ. f. Ornith., 1901, p. 113 (Palau); Reichenow, Die Vögel, 1, 1913, p. 216 (Palauinseln); Takatsukasa and Kuroda, Tori, 1, 1915, p. 51 (Pelew).
Porphyrio cyanocephalus Elliot, Stray Feathers, 7, 1878, pp. 10, 13 (Palau).
Porphyrio poliocephalus pelewensis Peters, Check-list Birds World, 2, 1934, p. 208 (Pelew); Hand-list Japanese Birds, 3d ed., 1942, p. 222 (Koror).
Porphyrio porphyrio pelewensis Mayr, Birds Southwest Pacific, 1945, p. 288 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 49 (Angaur).
Geographic range.—Micronesia: Palau Islands—Koror, Angaur.
Characters.—Adult: A large, purplish-blue, marsh bird with crown and sides of head dusky-black; wing-coverts purplish-blue; rest of upper parts dark, washed with olivaceous-brown; outer webs of primaries and secondaries tinged with purplish-blue; chin, axillaries and under wing-coverts dusky; under tail-coverts whitish; rest of underparts purplish-blue, blacker on abdomen.
Porphyrio p. pelewensis resembles P. p. palliatus Bruggemann of Celebes and P. p. melanopterus Bonaparte of the Moluccas and New Guinea but upper parts paler and slightly less glossy; lesser and primary wing-coverts more purplish-blue and less greenish-blue; outer webs of primaries and secondaries lighter purplish-blue; underparts less blue with patch on throat and breast paler blue with less green (patch present on only one specimen from the Palaus).
Measurements.—Measurements of one male: wing, 227; tail, 81; culmen and shield, 62.5; tarsus, 77; of three females: wing, 212, 218, 227; tail, 77, 81, 86; culmen and shield, 57, 61, 64; tarsus, 75, 75, 77.
Specimens examined.—Total number, 6 (1 male, 3 females, 2 unsexed), as follows: Palau Islands, USNM—Angaur, 1 chick (Sept. 21) AMNH—exact locality not given, 5 (Nov. 13, 19, Dec. 17-19, undated).
Nesting.—A black, downy chick was captured on September 21, 1945, at the edge of a fresh-water lake on Angaur by Davidson of the NAMRU2 party (Baker, 1948:49). Two females taken by Coultas in December had enlarged gonads.
Remarks.—The Purple Swamphen in the Palaus stands out as one of the more distinctive subspecies of P. porphyrio. It also marks the most northeastern extension of the range of this species. The subspecies in the Palaus shows affinities to that found to the south and southwest and probably reached Micronesia via the Papuan area, Celebes or the Moluccas rather than from the Philippines. It is interesting that this bird, as well as several other species, has been able to establish itself at the Palau Islands, but has not extended its range farther into other islands of Micronesia. Perhaps, the bird is now in an early stage in its island occupation.
The Purple Swamphen is probably not abundant in the Palaus. It is a large and conspicuous bird, and its restriction to swamps and areas around lakes may allow native hunters to obtain it rather easily, particularly by snares or by organized drives. Coultas (field notes) obtained specimens in taro swamps; he saw only 4 individuals and remarks that the birds utter harsh cries at night. The NAMRU2 party flushed an adult from lake side vegetation at [Pg 131] Angaur on September 21, 1945. This bird was not taken, but a downy young was obtained in the area the same day.
Fulica atra Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 152. (Type locality, Europe, restricted to Sweden.)
Fulica atra Hartert, Novit. Zool., 5, 1898, pp. 64, 69 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 32 (Guam); Safford, Osprey, 1902, p. 70 (Marianas); idem, The Plant World, 7, 1904, p. 268 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 43 (Guam); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam).
Fulica atra atra Hartert, Vögel pal. Fauna, 15, 1921, p. 1852 (Guam); Hand-list Japanese Birds, rev., 1932, p. 197 (Tinian, Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 222, (Tinian, Guam); Mayr, Birds Southwest Pacific, 1945, p. 302 (Micronesia).
Geographic range.—Breeds in Europe, northern Africa, and Asia. Winters south to Africa, Malaysia, southern Asia. In Micronesia: Mariana Islands—Tinian, Guam.
Remarks.—The Common Coot is a straggler to Micronesia in winter. It has been recorded from Guam and Tinian. An unsexed specimen in the collections of the American Museum of Natural History was taken at Guam in the fall of 1896 by one of Owston's collectors.
Tringa Squatarola Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 149. (Type locality, Europe, restricted to Sweden.)
Charadrius squatarola Hartert, Novit. Zool., 5, 1898, p. 66 (Saipan); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 35 (Micronesia); Safford, Osprey, 1902, p. 67 (Marianas).
Squatarola squatarola Hartert, Novit. Zool., 7, 1900, p. 9 (Ruk); Safford, The Plant World, 7, 1904, p. 266 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Cox, Island of Guam, 1917, p. 22 (Guam); Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 72 (Ruk); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 15 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 216 (Saipan, Truk); Mayr, Birds Southwest Pacific, 1945, p. 36 (Truk); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 50 (Guam).
Squatarola helvetica Takatsukasa and Kuroda, Tori, 1, 1915, p. 61 (Marianas, Ruk).
Squatarola squatarola hypomelaena Kuroda, in Momiyama, Birds Micronesia, 1922, p. 43 (Ruk, Saipan); Hand-list Japanese Birds, rev., 1932, p. 193 (Saipan, Truk).
Geographic range.—Breeds in arctic regions of Holarctica. Winters in Southern Hemisphere. In Micronesia: Mariana Islands—Guam, Saipan; Caroline Islands—Truk; Marshall Islands—Eniwetok.
Specimens examined.—One female from Mariana Islands. USNM—Guam (Aug. 27).
Remarks.—The Black-bellied Plover is an uncommon visitor to Micronesia. One bird was obtained by Markley of the NAMRU2 party at Guam on August 27, 1945; Flavin recorded five of these birds from November, 1944, to January, 1946. Bryan and Greenway (1944:109) record this species as an occasional visitor to the [Pg 132] Hawaiian Islands. Gleise and Genelly (1945:221) observed the Black-bellied Plover at Eniwetok in 1945.
Charadrius fulvus Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 687. (Type locality, Tahiti.)
Charadrius pluvialis Kittlitz, Obser. Zool., in Lutké., Voy. "Le Séniavine," 3, 1836, pp. 287, 299, 304 (Ualan, Longounor, Guahan); idem, Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, pp. 32, 55 (Ualan).
Charadrius virginianus Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen, Carolinen).
Charadrius longipes? Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 47 (Ladrone or Marian Islands, Oualan).
Pluvialis fulvus Schlegel, Mus. Pays-Bas, 6, no. 29, 1865, p. 52 (Micronesie).
Charadrius fulvus Finsch and Hartlaub, Fauna Central-polynesiens, 1867, p. 196 (Marianen, Ualan); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 8, 117, 118 (Pelews); Finsch and Hartlaub, Journ. f. Ornith., 1870, p. 139 (Pelew); Finsch, Journ. f. Ornith., 1872, p. 52 (Pelew, Carolinen); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 104 (Pelew, Mackenzie, Uap); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 31 (Palau); idem, Journ. Mus. Godeffroy, 12, 1876, pp. 18, 38 (Ponapé); idem., Proc. Zool. Soc. London, 1877 (1878), p. 781 (Ponapé); idem, Proc. Zool. Soc. London, 1880, p. 576 (Ruk); idem, Journ. f. Ornith., 1880, pp. 293, 305 (Ponapé, Kuschai); idem, Ibis, 1880, pp. 220, 331, 332 (Taluit); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 281, 353 (Ponapé, Ruk); Finsch, Ibis, 1881, pp. 105, 106, 109, 113, 115 (Kushai, Ponapé); Salvadori, Ornith. Papuasia, 3, 1882, p. 395 (Carolines, Pelews, Marianas); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 55 (Jaluit, Milli, Kuschai); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 63 (Marshall Islands, Ualan, Luganor, Ponapé, Ruk, Uap, Pelew, Marianne); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 46 (Guam, Hogoleu, Marshalls, Palaos); Hartert, Novit. Zool. 5, 1898, p. 66 (Guam); idem, Novit. Zool., 7, 1900, p. 9 (Ruk); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 36 (Micronesia); Schnee, Ornith. Monatsber., 1901, p. 132 (Marshalls); Safford, Osprey, 1902, p. 68 (Marianas); idem, The Plant World, 7, 1904, p. 266 (Guam); Schnee, Zool. Jahrbücher, 20, 1904, p. 389 (Marschall-Inseln); Takatsukasa and Kuroda, Tori, 1, 1915, p. 51 (Ponapé).
Charadrius dominicus fulvus Safford, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Cox, Island of Guam, 1917, p. 22 (Guam).
Charadrius dominicus Sharpe, Cat. Birds British Mus., 24, 1896, p. 195 (Micronesia).
Pluvialis dominicus fulvus Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 89 (Kuschai, Pelew, Ruk, Marianas, Mackenzie, Ponapé); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 177 (Uala, Arhno, Rongelab); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 44 (Guam, Angaur, Ualan, Luganor, Ponapé, Ruk, Yap, Arhno); Hand-list Japanese Birds, 3d ed., 1942, p. 216 (Saipan, Tinian, Guam, Babelthuap, Koror, Peliliu, Angaur, Yap, Ulithi, Truk, Lukunor, Ponapé, Kusaie, Mille, Arhno, Majuro, Likieb).
Pluvialis apricarius fulvus Hand-list Japanese Birds, rev., 1932, p. 193 (Saipan, Tinian, Babelthuap, Koror, Pelilieu, Angaur, Yap, Uluthi, Truk, Lukunor, Ponapé, Kusaie, Mille, Arhno, Majuro, Likieb).
Pluvialis dominica fulva Peters, Check-list Birds World, 2, 1934, p. 244 (Oceania); Bryan, Guam, Rec., vol. 13, no. 2, 1936, p. 24 (Guam); Stickney, Amer. Mus. Novit., no. 1248, 1943, p. 3 (Saipan, Guam, Palau, Ponapé, Kusaie, Ruk, Tarawa); Mayr, Birds Southwest Pacific, 1945, p. 39 (Oceania); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 93 (Tinian); Strophlet, Auk, 1946, p. 536 (Guam); Borror, Auk, 1947, p. 417 (Agrihan); Stott, Auk, 1947, p. 525 (Saipan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 50 (Guam, Rota, Peleliu, Garakayo, Ulithi, Truk).
Pluvialis dominica Wharton and Hardcastle, Journ. Parasitology, 32, 1946, pp. 306, 310, 313, 316, 318 (Ulithi, Guam); Wharton, Ecol. Monogr., 16, 1946, pp. 174, 175 (Guam).
Geographic range.—Breeds from Siberia to western Alaska. Winters from India east to Oceania; stragglers occur west to Africa and east to Pacific coast of North America. In Micronesia: Mariana Islands—Guam, Rota, Tinian, Saipan, Agrihan, Asuncion; Palau Islands—Angaur, Peleliu, Ngabad, Garakayo, Koror, Babelthaup; Caroline Islands—Yap, Ulithi, Truk, Lukunor, Ponapé, Kusaie; Marshall Islands—Mille, Arhno, Rongelab, Majuro, Likieb, Bikini.
Specimens examined.—Total number, 69 (39 males, 26 females, 4 unsexed), as follows: Mariana Islands, USNM—Guam, 17 (July 8, 19, 24, Aug. 31, Sept. 4, 17, 19, 26, Oct. 5, 8, 23, 24)—Rota, 5 (Oct. 20, 25); AMNH—Guam, 6 (Mar. 7, 8, 27, Aug. 15)—Saipan, 1 (Sept. 8)—Asuncion, 2 (Feb. 16); Palau Islands, USNM—Peleliu, 9 (Sept. 6-20)—Garakayo, 1 (Sept. 20); AMNH—exact locality not given, 7 (Oct. 13, Nov. 13, 15); Caroline Islands, USNM—Ulithi, 4 (Aug. 16, 21); AMNH—Kusaie, 9 (Mar. 10-30)—Ponapé, 2 (Dec. 15)—Truk, 3 (Feb. 6); Marshall Islands, USNM—Bikini, 3 (Mar. 4, 7, May 3).
Parasites.—Wharton (1946:174, 175) records the following chiggers (Acarina) from Pluvialis taken by the NAMRU2 party at Guam: Acariscus pluvius, A. anous, Neoschöngastia carveri, and N. namrui; and at Ulithi: N. pauensis and N. ewingi.
Weights.—Birds taken at Guam and Rota weighed as follows: seven males, 107-125 (117); four females, 109-120 (114).
Remarks.—The Pacific Golden Plover is one of the most abundant migratory shore birds to visit Micronesia. So characteristic of Micronesia is this species that almost all ornithologists who have made observations in the area have recorded it. Finsch observed the plover in the Carolines and Marshalls. Coultas made notes on, and collected specimens of, it in the Marianas, Carolines, and Palaus. The Hand-list of Japanese Birds (Hachisuka et al., 1942:216) lists Pluvialis from 17 islands in Micronesia.
Stickney (1943:3, 4) discusses the migrations of the Pacific Golden Plover through Oceania, using as a basis for her remarks the data from the extensive collections made by the Whitney South Sea Expedition. She states that the northward migration begins in March from the southern islands (New Zealand and southern Australia). At Guam in 1945, the writer observed flocks of plover beginning on February 11. Birds were seen in small groups in March and April. In the latter month most of the birds seen were in nuptial plumage. For the year 1945, the latest spring record at Guam was April 28. In the same year, Gleise (1945:220) observed his last spring record at Tinian "between April 26 and 27." In 1946, Morrison obtained plover in nuptial plumage at Bikini on May 3.
In an effort to obtain dates when shore birds appeared at Guam, field parties of NAMRU2 made observations at several beaches in [Pg 134] late spring, summer, and early fall, as is shown in table 8. Pacific Golden Plovers in post-nuptial molt were first observed and collected on July 8. Following this date, small flocks and later large flocks were more numerous; by September 29, plover were abundant. Similar findings were obtained at Ulithi (see table 9) and in the Palau Islands (see table 10) in August and September. The birds collected by the NAMRU2 party at Guam, Ulithi, Peleliu, and Garakayo in July, August, September, and early October were in postnuptial molt. Birds taken at Rota on October 20 and 26 were in winter plumage. Downs (1946:93) observed plover in small flocks at Tinian in 1945, beginning after September 5. Borror (1947:417) saw two birds at Agrihan on August 10, 1945.
The flocks of plover seen by the NAMRU2 party varied in size from three to 30 birds, the average being less than ten. Coultas (field notes) noted "large flocks" at the Palaus from October to December, 1931. Although plover was often found on the same beach as other birds, the NAMRU2 observers rarely saw plover together with other shore birds. However, on air strips Pluvialis occasionally occurred with small numbers of Arenaria, Heteroscelus spp., and Numenius phaeopus. Pluvialis and N. phaeopus were the only shore birds found to use open grassy flats and other inland areas at Guam and Peleliu in 1945.
Stickney (1943) records Pluvialis in late spring and summer from Polynesia, indicating these to be birds remaining in the winter range during the breeding season. The NAMRU2 party observed no Pacific Golden Plovers at Guam which might be regarded as non-migrants, but other species of shore birds were found which might be considered as such. The lingering of individuals in the winter range is not unusual among migratory birds, and as Stickney points out, most of the non-migrants retain their winter dress or assume an incomplete breeding plumage.
Charadrius semipalmatus Bonaparte, Journ. Acad. Nat. Sci. Phila., 5, 1825, p. 98. New name for Tringa hiaticula Ord. not Charadrius hiaticula Linnaeus, in Wilson's Amer. Ornith., Ord. repr., 7, 1824, p. 65. (Type locality, Coast of New Jersey.)
Charadrius hiaticula Finsch, Ibis, 1880, p. 331 (Taluit); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 64 (Taluit or Bonham); Schnee, Zool. Jahrbücher, 20, 1904, p. 389 (Marschall-Inseln); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 45 (Taluit).
Geographic range.—Breeds from Arctic America south to coastal Canada. Winters from southern United States to South America. In Micronesia: Marshall Islands—Jaluit.
Remarks.—Finsch (1880d:331) reported this bird (sight record) at Jaluit in the Marshall Islands. Other than this observation, there is no history of the species in Micronesia.
Charadrius curonicus Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 692. (Type locality, Kurland.)
Charadrius dubius curonicus Hand-list Japanese Birds, rev., 1932, p. 194 (Yap); Hand-list Japanese Birds, 3d ed., 1942, p. 217 (Yap); Mayr, Birds Southwest Pacific, 1945, p. 37 (Micronesia).
Geographic range.—Breeds in northern Europe and Asia. Winters from Africa east to Malaysia and Melanesia. In Micronesia: Caroline Islands—Yap.
Remarks.—The Ring-necked Plover has been recorded at Yap by the Japanese collectors. Mayr (1945a:37) remarks that the bird is an occasional migrant through Micronesia. Gleise and Genelly (1945:221) observed four "Papuan" Ring-necked Plovers at Eniwetok in 1945. Apparently no specimen was obtained.
Charadrius alexandrinus nihonensis Deignan, Journ. Washington Acad. Sci., vol. 31, 1941, p. 106. (Type locality, Aomori, Hondo.)
Charadrius cantianus Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 117, 118 (Pelew); idem, Proc. Zool. Soc. London, p. 89 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 31 (Palau).
Aegialitis cantianus Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 64 (Pelew); Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Pelew).
Aegialitis alexandrinus dealbatus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 45 (Pelew).
Charadrius alexandrinus dealbatus Hand-list Japanese Birds, rev., 1932, p. 194 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 217 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 37 (Palau).
Geographic range.—Breeds in Japan and possibly on adjacent parts of the Asiatic mainland. Winters south to Malaya. In Micronesia: Palau Islands—exact locality unknown.
Remarks.—The Kentish Plover is known from a single record obtained by Semper in the Palau Islands. It is tentatively assigned to C. a. nihonensis, which breeds directly north of the Palau Islands on Japan. C. a. dealbatus (Swinhoe) breeds more to the west on the Asiatic mainland and adjacent islands south of Japan. Additional specimens are needed before the subspecific status of migrants to Micronesia can be accurately determined.
Charadrius mongolus stegmanni Stresemann, Ornith. Monatsb., 48, 1940, p. 55. New name for Charadrius mongolus littoralis Stegmann, 1937, preoccupied. (Type locality, Behring Island.)
Charadrius sanguineus Lesson, Man. d'Ornith., 2, 1828, p. 330 (No type locality = Mariana Islands, ex Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 48); idem, Traité d'Ornith., 1831, p. 544 (no locality = Mariana Islands); Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen).
Charadrius mongolicus Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 48 (Guam, Jaluit, Palaos, Carolines); Hartert, Novit. Zool., 5, 1898, p. 66 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 36 (Guam); Safford, Osprey, 1902, p. 68 (Guam).
Aegialitis mongolus Hartert, Novit. Zool., 7, 1900, p. 9 (Ruk).
Aegialis mongola Safford, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam).
Aegialites mongola Cox, Island of Guam, 1917, p. 22 (Guam).
Ochthodromus mongolicus Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Marianas, Ruk).
Charadrius mongolus Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 132 (Ruk); Mayr, Birds Southwest Pacific, 1945, p. 38 (Micronesia).
Charadrius mongolus mongolus Hartert, Vögel pal. Fauna, 11-12, 1920, p. 1543 (Marianen, Karolinen); Hand-list Japanese Birds, rev., 1932, p. 194 (Guam, Truk, Iuripik, Kusaie, Jaluit, Majuro); Peters, Check-list Birds World, 2, 1934, p. 253 (Carolines, Marianas); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 50 (Guam, Peleliu, Ulithi).
Cirrepidesmus mongolus mongolus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 44 (Guam, Ruk).
Charadrius mongolus stegmanni Hand-list Japanese Birds, 3d ed., 1942, p. 217 (Guam, Peliliu, Truk, Iuripik, Kusaie, Jaluit, Majuro).
Geographic range.—Breeds in northeastern Siberia and Bering Sea area. Winters south to eastern Malaysia, Melanesia, and Australia. In Micronesia: Mariana Islands—Guam; Palau Islands—Angaur, Peleliu; Caroline Islands—Ulithi, Truk, Iuripik, Kusaie; Marshall Islands—Jaluit, Majuro.
Specimens examined.—Total number, 10 (4 males, 5 females, 1 unsexed), as follows: Mariana Islands, USNM—Guam, 2 (June 7, Sept. 1); AMNH—Guam, 3 (Aug. 15, 18, Nov. 30); Palau Islands, USNM—Peleliu, 3 (Sept. 7-12); Caroline Islands, USNM—Ulithi, 1 (Aug. 22); AMNH—Truk, 1 (Feb. 8).
Remarks.—According to Oustalet (1896:48), Lesson used two specimens of this species, which were collected in the Marianas by the expedition in the "Uranie," as types for his Charadrius sanguineus.
The Mongolian Dotterel is a regular visitor to western Micronesia. It is recorded also from the Marshall Islands, which it probably reaches from the westward by way of the Carolines, since the species has not been recorded in the Hawaiian Islands.
A bird taken by the writer at Guam on June 7, 1945, was in winter plumage and probably nonmigratory. The species was recorded also at Guam in September. At Peleliu in September, 1945, the Mongolian Dotterel was seen frequently on tidal flats by the NAMRU2 party. On September 8 there was a flock of approximately fifty birds, in company with Charadrius leschenaultii, at Akarakoro Point. In August at Ulithi, birds were on the beaches in company with Crocethia alba. At Angaur on September 21, 1945, the species was with other shore birds in small groups at fresh water ponds.
I am tentatively referring all specimens examined to C. m. [Pg 137] stegmanni although at this writing (1948) I am inclined to the opinion that a critical reexamination of the referred specimens might reveal one or a few individuals of the subspecies C. m. mongolus Pallas.
Charadrius Leschenaultii Lesson, Dict. Sci. Nat., ed. Levrault, 42, 1826, p. 36. (Type locality, Pondichery, India.)
Charadrius griseus Lesson, Traité d'Ornith., 1831, p. 544 (Oulan).
Charadrius geoffroyi Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 117, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, p. 89 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 31 (Palau).
Aegialitis geoffroyi Salvadori, Ornith. Papuasia, 3, 1882, p. 299 (Ualan, Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 64 (Pelew, Ualan).
Ochthodromus geoffroyi Sharpe, Cat. Birds British Mus., 24, 1896, p. 217 (Pelew, Ualan); Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Pelew).
Pagoa leschenaultii Kuroda, in Momiyama, Birds Micronesia, 1922, p. 44 (Pelew, Kusaie, Yap).
Charadrius leschenaultii leschenaultii Hand-list Japanese Birds, rev., 1932, p. 193 (Yap, Kusaie, Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 216 (Yap, Kusaie, Palau).
Charadrius leschenaultii Mayr, Birds Southwest Pacific, 1945, p. 38 (Micronesia); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 51 (Peleliu).
Geographic range.—Breeds in Asia south to Persia. Winters from Malaysia east to Australia and Melanesia. In Micronesia: Palau Islands—Peleliu; Caroline Islands—Yap, Kusaie.
Specimens examined.—Total number, 9 (2 males and 7 females), as follows: Palau Islands, USNM—Peleliu, 7 (Sept. 6-12); AMNH—exact locality not given, 2 (Nov. 21, 25).
Remarks.—The Large Sand Dotterel is a regular visitor to the Palau Islands. It has been recorded also at Yap and Kusaie in the Carolines, where it may be considered as an uncommon visitor.
At Peleliu, the species was seen on several occasions in September, 1945, by the NAMRU2 party. The birds were found on tidal flats in company with Charadrius mongolus stegmanni in flocks of 10 to 30 individuals.
Tantalus variegatus Scopoli, Del. Flor. et Faun. Insubr., fasc. 2, 1786, p. 92. (Type locality, Luzon, ex. Sonnerat.)
Scolopax phaeopus Lesson, Traité d'Ornith., 1831, p. 566 (Marianas).
Numenius phaeopus Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 287, 304 (Ualan, Guahan), Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen); Kittlitz, Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 129 (Ualan); Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 831 (Pelew, Matelotas); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 8, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 89, 106 (Uap, Pelews); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 35 (Palau); idem, Journ. f Ornith., 1880, pp. 294, 307 (Ponapé, Kuschai); idem, Proc. Zool. Soc. London, 1880, p. 576 (Ruk); idem, Ibis, 1881, pp. 107, 109, 115 (Kushai, Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 281, 299, 353 (Ponapé, Mortlock, Ruk); Wharton and Hardcastle, Journ. Parasitology, 32, 1946, pp. 308, 316, 318, 320 (Ulithi, Guam); Wharton, Ecol. Monogr., 16, 1946, pp. 174, 175 (Guam).
Numenius tenuirostris Kittlitz, Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 55 (Marianas, Ualan).
Numenius uropygialis Gray, Hand-list Birds, 3, 1871, p. 43 (Pelew).
Numenius variegatus Salvadori, Ornith. Papuasia, 3, 1882, p. 332 (Pelew, Ponapé); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 66 (Marianne, Pelew, Matalotas, Luganor, Ruk, Ponapé, Ualan); Sharpe, Cat. Birds British Mus., 24, 1896, p. 361 (Micronesia); Safford, The Plant World, 7, 1904, p. 266 (Guam).
Numenius phaeopus variegatus Oustalet, Nouv. Arch. Mus. Hist. Nat Paris, (3), 8, 1896, p. 39 (Mariannes, Palaos, Carolines, Jaluit); Hartert, Novit. Zool., 5, 1898, p. 65 (Guam); idem, Novit. Zool., 7, 1900, p. 8 (Ruk); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 34 (Guam); Safford, Osprey, 1902, p. 67 (Marianas); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Marianas, Carolines, Pelews); Cox, Island of Guam, 1917, p. 21 (Guam); Hartert, Vögel pal. Fauna, 13-14, 1921, p. 1649 (Guam); Hand-list Japanese Birds, rev., 1932, p. 192 (Marianas, Carolines, Palaus, Marshalls); Peters, Check-list Birds World, 2, 1934, p. 261 (Caroline, Marianne, Pelew); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 215 (Guam, Koror, Babelthuap, Ngulu, Yap, Uluthi, Iuripik, Truk, Lukunor, Ponapé, Kusaie, Jaluit, Wotze); Mayr, Birds Southwest Pacific, 1945, p. 39 (Micronesia); Strophlet, Auk, 1946, p. 537 (Guam); Stott, Auk, 1947, p. 525 (Saipan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 51 (Guam, Angaur, Peleliu, Ulithi).
Phaeopus phaeopus variegatus Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 178 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 45 (Palaus, Carolines, Marians).
Geographic range.—Breeds in northeastern Asia. Winters from Malaysia east to Oceania. In Micronesia: Mariana Islands—Guam; Palau Islands—Angaur, Peleliu, Koror, Babelthuap; Caroline Islands—Ngulu, Yap, Ulithi, Truk, Lukunor, Iuripik, Ponapé, Kusaie; Marshall Islands—Jaluit, Wotze.
Specimens examined.—Total number, 26 (9 males, 17 females), as follows: Mariana Islands, USNM—Guam, 16 (June 4, 6, July 24, 26, 27, Sept. 1, 19, 25, Oct. 8); Palau Islands, USNM—Peleliu, 5 (Sept. 8, 12, 14)—Angaur, 4 (Sept. 21); Caroline Islands, USNM—Ulithi, 1 (Aug. 17).
Weights.—At Guam, the NAMRU2 party obtained the weights of two males, 373 and 435, and of six females, 295-426 (384).
Parasites.—Wharton (1946:174, 175) lists the following species of chiggers (Acarina) taken from the Whimbrel at Guam: Acariscus pluvius, A. anous, Neoschöngastia strongi, and N. carveri; and at Ulithi: N. namrui and N. atollensis.
Remarks.—The Whimbrel is an abundant visitor to western Micronesia. It was first taken by Quoy and Gaimard, who found it in the Marianas. It is recorded in the Marshall Islands (Jaluit and Wotze), but apparently reaches these islands from the west, since the species is unknown in the Hawaiian Islands.
As shown in table 8, the NAMRU2 party observed the Whimbrel at Guam on spring migration in March, 1945, the last record being on March 21. In June and July, single birds or small groups were occasionally seen on the tidal flats. Some of these birds may have been nonmigratory. Beginning on July 24, more birds were recorded as they began to migrate south after their nesting season. [Pg 139] Whimbrels were numerous from August until the conclusion of the observations in October. Birds were abundant at the Palaus in September; only a few were noted at Ulithi in late August. The Whitney South Sea Expedition of the American Museum of Natural History made collections of this species at several islands in Micronesia. At Ponapé, Coultas (field notes) writes that in November and December, 1930, a few birds were seen on the reefs and at the edges of mangrove swamps. At Peleliu in October to December, 1931, he found Whimbrels concentrated on a small islet between Koror and Babelthuap. At both Ponapé and Palau Coultas received reports that the birds remain at the islands throughout the year.
Scolopax tahitiensis Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 656. (Type locality, Tahiti, Society Islands, based on the Otaheiti Curlew of Latham, Gen. Syn., 3, pt. 1, 1785, p. 122, no. 4.)
Numenius femoralis Finsch, Ibis, 1880, pp. 220, 331, 332 (Jaluit, Arno).
Numenius tahitiensis Seebohm, Geogr. Dist. Charadriidae, 1887, p. 332 (Marshalls); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 66 (Marianne?, Marshalls); Sharpe, Cat. Birds British Mus., 24, 1896, p. 367 (Marianas, Marshalls); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall-Inseln); Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Marianas, Pelews); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 49 (Marianas, Marshalls); Bent, Bull. U. S. Nat. Mus., 146, 1929, p. 143 (Jaluit); Hand-list Japanese Birds, rev., 1932, p. 192 (Saipan, Marshalls); Peters, Check-list Birds World, 2, 1934, p. 261 (Marshalls); Yamashina, Tori, 10, 1940, p. 677 (Jarchi); Hand-list Japanese Birds, 3d ed., 1942, p. 215 (Saipan, Jaluit, Arhno, Maloelab, Wotze, Ailuk, Ringelab, Larchi); Stickney, Amer. Mus. Novit., no. 1248, 1943, p. 4 (Ponapé, Marshalls); Mayr, Birds Southwest Pacific, 1945, p. 39 (Marshalls, straggler to Carolines and Marianas).
Phaeopus tahitiensis Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 179 (Rongelab); Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 407 (Marianas, Marshalls).
Geographic range.—Breeds in western Alaska. Winters in eastern and central Polynesia. In Micronesia: Mariana Islands—Saipan; Caroline Islands—Ponapé; Marshall Islands—Jaluit, Arhno, Moloelab, Wotze, Ailuk, Rongelab, Larchi, Bikini.
Specimens examined.—Total number, 6 (3 males, 3 females), as follows: Caroline Islands, AMNH—Ponapé, 2 (Dec. 15); Marshall Islands, USNM—Bikini, 4 (Mar. 10, 14, April 2, 30).
Remarks.—The Bristle-thighed Curlew is a regular migrant through the Marshall Islands of eastern Micronesia. It is recorded as a straggler to the Caroline and Mariana islands. Stickney (1943:4, fig. 1) shows a map and discusses the breeding and wintering ranges of this curlew. As can be observed from her map, the principal wintering areas are east and south of Micronesia. She records the species from the Bonin Islands, which is the westernmost record.
It is difficult to offer plausible reasons for the present migratory [Pg 140] habits of the Bristle-thighed Curlew. It is related to both the Asiatic form, N. phaeopus, and to the American species, N. hudsonicus, but its origin is not understood. The characteristics of its route of migration resemble that of some continental migrants and might have come about by a slow adjustment of the species to its environment, probably through an expansion of range from the west.
Scolopax madagascariensis Linnaeus, Syst. Nat., ed. 12, 1, 1766, p. 242. (Type locality, Madagascar, error = Manila, Philippine Islands, fide Stresemann.)
Numenius cyanopus Hartert, Novit. Zool., 5, 1898, p. 65 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 35 (Micronesia); Safford, Osprey, 1902, p. 67 (Marianas); idem, The Plant World, 7, 1904, p. 266 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Cox, Island of Guam, 1917, p. 21 (Guam); Hartert, Vögel pal. Fauna, 13-14, 1921, p. 1645 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 45 (Guam); Hand-list Japanese Birds, rev., 1932, p. 192 (Guam).
Numenius madagascariensis Hand-list Japanese Birds, 3d ed., 1942, p. 214 (Guam); Mayr, Birds Southwest Pacific, 1945, p. 40 (Micronesia); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 51 (Guam, Ngesebus).
Geographic range.—Breeds in eastern Siberia. Winters from Malaysia east to Australia and Melanesia. In Micronesia: Mariana Islands—Guam; Palau Islands—Peleliu, Ngesebus.
Remarks.—The Long-billed Curlew is a regular visitor to western Micronesia, especially to the Palau Islands. It is apparently a less common migrant in the Marianas, although it has been recorded from Guam. At Guam, the NAMRU2 party observed a single bird on June 6 and two on October 3 at tidal beaches. At Peleliu these large curlews were seen on several occasions between September 9 and 16, 1945. They were found usually as singles feeding on tidal flats in company with other shorebirds.
Limosa Baueri Naumann, Naturg. Vög. Deutschl., 8, 1836, p. 429. (Type locality, New Holland = Victoria, apud Mathews; Novit. Zool., 18, 1912, p. 220.)
Limosa uropygialis Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 299 (Mortlock).
Limosa novae-sealandiae Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 66 (Luganor).
Limosa lapponica baueri Hartert, Novit. Zool., 5, 1898, p. 65 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 34 (Guam); Safford, Osprey, 1902, p. 67 (Marianas); idem, The Plant World, 7, 1904, p. 266 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Prowazek, Die deutschen Marianen, 1913, p. 101 (Marianen); Cox, Island of Guam, 1917, p. 21 (Guam); Hartert, Vögel pal. Fauna, 13-14, 1921, p. 1641, (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 46 (Carolines, Marianas); Hand-list Japanese Birds, rev., 1932, p. 191 (Marianas, Carolines); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam); Stickney, Amer. Mus. Novit., no. 1248, 1943, p. 5 (Guam, Palau); Mayr, Birds Southwest Pacific, 1945, p. 41 (Oceania); Strophlet, Auk, 1946, p. 537 (Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 52 (Guam, Peleliu).
Limosa lapponica novazealandiae Hartert, Novit. Zool., 7, 1900, p. 8 (Ruk); Hand-list Japanese Birds, 3d ed., 1942, p. 214 (Guam, Truk).
Limosa rufa uropygialis Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Marianas, Ruk).
Geographic range.—Breeds in northeastern Asia and northwestern North America. Winters from Malaysia east to Oceania. In Micronesia: Mariana Islands—Guam; Palau Islands—Peleliu; Caroline Islands—Truk.
Specimens examined.—Total number, 5 (2 males, 3 females), as follows: Mariana Islands, AMNH—Guam, 2 (Sept. 26); Palau Islands, USNM—Peleliu, 1 (Sept. 7); AMNH—exact locality not given, 2 (Nov. 21, 23).
Remarks.—The principal wintering grounds of the Pacific Godwit are probably in Australia and New Zealand according to Stickney (1943:5). The bird reaches these areas from Arctic breeding grounds by migrating to a great extent along the edge of the Asiatic Continent. It may also be considered as a regular migrant in western Micronesia, and probably reaches eastern Micronesia as an uncommon visitor, since it is occasionally recorded in the Hawaiian Islands.
At Guam in 1945, the NAMRU2 party found the Pacific Godwit at tidal beaches on April 26 and October 15. Strophlet (1946:537) recorded one bird from Guam on October 20, 1945. At Peleliu, the NAMRU2 party found birds at beaches on September 7 and 16. Coultas (field notes) reported that "a few" were seen at Peleliu from October to December, 1931. McElroy did not find any of these birds at Truk in December, 1945.
Scolopax nebularis Gunnerus, in Leem, Beskr. Finm. Lapper, 1767, p. 251. (Type locality, District of Trondhjem, Norway.)
Glottis nebularius Kuroda, in Momiyama, Birds Micronesia, 1922, p. 47 (Yap); Takatsukasa and Yamashina, Dobutsu. Zasshi, 44, 1932, p. 225 (Truk); Hand-list Japanese Birds, rev., 1932, p. 191 (Yap, Truk).
Tringa nebularis Hand-list Japanese Birds, 3d ed., 1942, p. 214 (Yap, Truk); Mayr, Birds Southwest Pacific, 1945, p. 41 (Yap, Truk); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 52 (Peleliu).
Geographic range.—Breeds in northern Eurasia. Winters in Mediterranean area, Africa, southern Asia, Malaysia, Australia and Melanesia. In Micronesia: Palau Islands—Peleliu; Caroline Islands—Yap, Truk.
Specimens examined.—Total number, 4 (1 male, 3 females) from Palau Islands, USNM—Peleliu (Aug. 28, Sept. 14, 15).
Remarks.—The Greenshank has been recorded at the Palau Islands and at Yap and Truk in the Caroline Islands. It is apparently a regular visitor to western Micronesia. It probably reaches the western Carolines as an occasional visitor from the region of the [Pg 142] Palaus to the westward, rather than from the northward, since the bird has not been observed in the Marianas.
The NAMRU2 party observed two small flocks of these birds at Peleliu in August and September, 1945. One group of six birds was found wading in the shallow water of a mangrove swamp on August 28. Another group of three birds was seen on a tidal beach on September 14 and 15, where they were observed feeding apart from other species of shore birds.
Scolopax melanoleuca Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 659. (Type locality, Sandy shores of Labrador = Chateau Bay, Labrador.)
Tringa melanoleuca Kuroda, Dobutsu. Zasshi, 46, 1934, p. 313 (Jaluit); Hand-list Japanese Birds, 3d ed., 1942, p. 214 (Jaluit).
Geographic range.—Breeds in Alaska and Canada. Winters from California east to the Gulf States and the West Indies and south to South America. In Micronesia: Marshall Islands—Jaluit.
Remarks.—Kuroda records one specimen of the Greater Yellow-legs from Jaluit Atoll in the Marshall Islands. It is a straggler to Oceania and has not been recorded in the Hawaiian Islands.
Tringa glareola Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 149. (Europe, restricted type locality, Sweden.)
Totanus glareola Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 43 (Guam); Hartert, Novit. Zool., 5, 1898, pp. 65, 69 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 34 (Guam); Safford, Osprey, 1902, p. 70 (Guam); idem, The Plant World, 7, 1904, p. 268 (Guam).
Rhyacophilus glareola Kuroda, in Momiyama, Birds Micronesia, 1922, p. 48 (Guam, Angaur).
Tringa glareola Hand-list Japanese Birds, rev., 1932, p. 191 (Guam, Angaur, Koror); Hand-list Japanese Birds, 3d ed., 1942, p. 213 (Guam, Anguar, Koror); Mayr, Birds Southwest Pacific, 1945, p. 41 (Guam, Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 52 (Anguar).
Tringa glariola Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam).
Geographic range.—Breeds in northern Eurasia from Norway and Germany east to Siberia, Sakhalin, and Kamchatka. Winters from Africa east to southern Asia, Malaysia, and Australia. In Micronesia: Mariana Islands—Guam; Palau Islands—Anguar, Koror.
Specimens examined.—Total number, 2 (1 male, 1 female), as follows: Palau Islands, USNM—Angaur, 1 (Sept. 21); AMNH—exact locality not given, 1 (October 26).
Remarks.—Marche, in 1877, first recorded the Wood Sandpiper in Micronesia (at Guam). In the Marianas it is apparently an uncommon migrant but it is considered to be a regular visitor in the Palau Islands. At the Palaus in September, 1945, the writer found [Pg 143] the bird at a fresh water pond on Angaur. It was not observed on the tidal beaches at Peleliu.
Tringa Hypoleucos Linnaeus, Syst. Nat., ed. 10, 1, 1858, p. 149 (Europe, restricted type locality, Sweden.)
Totanus hypoleucos Lesson, Traité d'Ornith., 1831, p. 552 (Marianas).
Totanus (Tringoides) hypoleucus Gray, Birds Trop. Is. Pacific Ocean, 1859, p. 51 (Marianas).
Actitis hypoleuca Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 8 (Pelew).
Actitis hypoleucus Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 89, 106 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 36 (Pelew); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 299, 353 (Ruk, Mortlock); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 64 (Luganor, Marianne, Pelew); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris (3), 8, 1896, p. 43 (Guam, Palaos, Luganor).
Tringoides hypoleucos Gray, Hand-list Birds, 3, 1871, p. 46 (Pelew, Ladrone); Salvadori, Ornith. Papuasia, 3, 1882, p. 318 (Pelew).
Tringoides hypoleucus Sharpe, Cat. Birds British Mus., 24, 1896, p. 456 (Micronesia); Takatsukasa and Kuroda, Tori, 1, 1915, pp. 51, 62 (Pelews, Marianas).
Totanus hypoleucus Hartert, Novit. Zool., 5, 1898, p. 65 (Saipan); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 34 (Guam); Safford, Osprey, 1902, p. 70 (Mariannes); idem, The Plant World, 7, 1904, p. 268 (Guam).
Actitis hypoleucos Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 372 (Micronesia); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 47 (Marianas, Carolines, Pelews); Peters, Check-list Birds World, 2, 1934, p. 269 (Micronesia); Bryan, Guam Rec., vol. 13, no. 1, 1936, p. 24 (Guam); Mayr, Birds Southwest Pacific, 1945, p. 42 (Micronesia); Strophlet, Auk, 1946, p. 537 (Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 52 (Guam, Peleliu, Ulithi).
Tringa hypoleucos Hand-list Japanese Birds, rev., 1932, p. 191 (Marianas, Carolines, Pelews); Hand-list Japanese Birds, 3d ed. 1942, p. 214 (Saipan, Babelthuap, Koror, Peleliu, Angaur, Ulithi, Truk).
Geographic range.—Breeds in Europe and Asia. Winters from Africa east to Polynesia. In Micronesia: Mariana Islands—Guam, Saipan; Palau Islands—Angaur, Peleliu, Koror, Babelthuap; Caroline Islands—Ulithi, Truk, Lukunor.
Specimens examined.—Total number, 12 (4 males, 7 females, 1 unsexed), as follows: Mariana Islands, USNM—Guam, 4 (July 16, Sept. 20); AMNH—Saipan, 1 (July 27); Palau Islands, USNM—Peleliu, 3 (Sept. 9, 14).—Koror, 1 (Nov. 7); AMNH—exact locality not given, 2 (Nov. 11, 19); Caroline Islands, USNM—Ulithi, 1 (Aug. 22).
Weights.—The present author (1948:52) recorded the weight of one male taken at Guam as 67 grams, and of two females as 57 and 63 grams. These were fall migrants taken by the NAMRU2 party.
Remarks.—The Common Sandpiper has been known from Micronesia since the time of Lesson. Tetens, Peters and Kubary obtained specimens in the Palaus; the latter collector obtained the bird at Lukunor and probably also at Truk. In recent years several collectors have taken the birds in western Micronesia, where the species appears to be a regular visitor. Field observations by the NAMRU2 party indicate that the birds are usually found as singles and remain apart from other species of migratory shorebirds which visit the islands. The margins of inland ponds and beaches consisting of [Pg 144] rocks and pebbles appear to be preferred over the sandy, tidal flats. At Peleliu on September 9, 1945, two birds were taken at a bare bank of coral at an inland pond. These were the only two Common Sandpipers seen at the island. A specimen taken by the NAMRU2 party at Ulithi on August 22 at a beach, piled with debris from ships, has its entire and underparts stained by fuel oil.
Totanus brevipes Vieillot, Nouv. Dict. Hist. Nat., 6, 1816, p. 410. (No locality given, the type is from Timor.)
Totanus pedestris Lesson, Traité d'Ornith., 1831, p. 552 (Marianne, Ualan).
Totanus brevipes Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 287, 299, 304 (Ualan, Lougounor, Guahan); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 51 (Ladrone or Marian Is.); Pelzeln, Reise "Novara," Vögel, 1865, p. 129, 162 (Puynipet, Ualan).
Totanus incanus Finsch and Hartlaub (part), Fauna Centralpolynesians, 1867, p. 187 (Mariannen, Ualan, Puynipet); Salvadori (part), Ornith. Papuasia, 3, 1882, p. 322 (Micronesia); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 65 (Mulgrave, Taluit, Ualan, Ponapé, Ruk, Luganor, Uap, Pelew, Marianas); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 41 (Saypan, Guam, Jaluit, Carolines, Palaos).
Heteractitis brevipes Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 35 (Marianas); Safford, Osprey, 1902, p. 67 (Marianas); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Cox, Island of Guam, 1917, p. 21 (Guam); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 180 (Uala = Truk); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 170 (Carolines).
Heteractitis brevis Prowazek, Die deutschen Marianen, 1913, pp. 47, 101 (Marianen).
Heteroscelus brevipes Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 367 (Western Pacific); Peters, Check-list Birds World, 2, 1934, p. 270 (Carolines).
Tringa incana brevipes Hartert, Vögel pal. Fauna, 13-14, 1921, p. 1623 (Guam, Truk); Hand-list Japanese Birds, rev., 1932, p. 191 (Palaus, Carolines); Hand-list Japanese Birds, 3d ed., 1942, p. 213 (Babelthuap, Koror, Angaur, Yap, Iuripik, Faraulep, Truk, Ponapé).
Heteroscelus incanus brevipes Kuroda, in Momiyama, Birds Micronesia, 1922, p. 47 (Pelew, Yap, Ruk); Kuroda, Avifauna Riu Kiu, 1925, p. 177 (Micronesia); Stickney, Amer. Mus. Novit., no. 1248, 1943, p. 5 (Saipan, Guam, Palau, Ruk, Kusaie); Mayr, Birds Southwest Pacific, 1945, p. 43 (Micronesia); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 52 (Guam, Peleliu, Truk).
Heteroscelus incanus Wharton and Hardcastle (part), Journ. Parasitology, 32, 1946, pp. 296, 316, 318 (Guam, Peleliu).
Geographic range.—Breeds in eastern Siberia and adjacent areas. Winters south to Malaysia and east to Australia and Oceania. In Micronesia: Mariana Islands—Guam, Saipan; Palau Islands—Angaur, Peleliu, Koror, Babelthuap; Caroline Islands—Yap, Truk, Iuripik, Faraulep, Ponapé, Kusaie.
Specimens examined.—Total number, 39 (11 males, 27 females, 1 unsexed), as follows: Mariana Islands, USNM—Guam, 16 (June 4, 6, July 16, 24, Aug. 6, 27, Sept. 4, 6, 27, Oct. 23); AMNH—Saipan, 1 (Sept. 8),—Guam, 5 (Feb. 11, Mar. 4, 13, Sept. 14, Dec. 5); Palau Islands, USNM—Peleliu, 7 (Sept. 6-8, 16); AMNH—exact locality not given, 4 (Nov. 8); Caroline Islands, USNM—Truk, 1 (Dec. 13); AMNH—Truk, 3 (Feb. 6, 26, Oct. 14),—Kusaie, 2 (Mar., April).
Weights.—Weights of birds obtained by the NAMRU2 party were as follows: three males from Guam, 90-104 (95); six females from Guam, 99-116 (104).
Remarks.—It is not clear whether some of the accounts cited above refer to this species or to the species, Heteroscelus incanus. Owing to the fact that specimens used in some of these early reports have not been examined by me, the identifications of the birds concerned cannot be verified and consequently it is impossible to be certain to which species some of the references pertain. In listing these accounts in the literature, I am following Sharpe (1896:455) whenever possible.
Tattlers were among the first birds observed and taken in Micronesia. Quoy and Gaimard found them in the Marianas, and Kittlitz and Kubary recorded the species in the Carolines. Kubary also reported the birds at the Palaus.
The Gray-tailed Tattler apparently does not reach the Marshall Islands but visits only the western part of Micronesia. Stickney (1943:2) shows a map of the known geographic range of this species in Micronesia. The separation of H. brevipes and H. incanus in the field is not always possible. For identification, the NAMRU2 party depended primarily on specimens collected. At Guam, specimens of H. brevipes, thought to be nonmigratory, were taken in early June. These were in winter plumage. Beginning in mid-July there was an increase in the number of tattlers seen; apparently fall migration had begun. At Peleliu in September, 1945, the NAMRU2 party found tattlers to be numerous. Apparently all were of this species; no H. incanus were taken there. On September 8, approximately 75 individuals in small and large flocks were counted at Akarakoro Point on the tidal flats. The birds remained apart from the other shorebirds which were feeding at the same locality.
Scolopax incana Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 658. (Type locality, Eimeo = Moorea, Society Islands and Palmerton Islands.)
Totanus oceanicus Lesson, Mamm. et Ois., 2, 1847, p. 244 (Kusaie); Hartlaub, Archiv f. Naturgesch., 1852, p. 135 (Carolinen); idem, Journ. f. Ornith., 1854, pp. 167, 168 (Carolinen, Mariannen).
Tryanga glareola Kittlitz, Denkw. Reise russ. Amer. Micron. und Kamchat., 1, 1858, p. 365, 2, pp. 55, 86 (Ualan).
Totanus incanus Schlegel, Mus. Pays-Bas, 5, no. 27, 1864, p. 74 (Micronésie); Salvadori (part), Ornith. Papuasia, 3, 1882, p. 322 (Ualan, Puynipet, Marshalls, Mariannis); Wiglesworth (part), Abhandl. und. Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 65 (Mulgrave, Taluit, Ualan, Ponapé, Ruk, Luganor, Uap, Marianne, Pelew); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 41 (Saypan, Guam, Jaluit, Carolines, Palaos); Hartert, Novit. Zool., 5, 1898, p. 64 (Guam); idem, Novit. Zool. 7, 1900, p. 8 (Ruk); Schnee, Zool. Jahrbücher, 20, 1904, p. 389 (Marschall-Inseln).
Actitis incanus Finsch and Hartlaub (part), Fauna Centralpolynesions, 1867, p. 187 (Mariannen, Ualan, Puynipet); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 106 (Uap, Ualan); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 36 (Palau); idem, Journ. Mus. Godeffroy, 12, 1876, pp. 18, 38 (Ponapé); idem, Journ. f. Ornith., 1880, pp. 294, 306 (Ponapé, Kuschai, Marshalls); idem, Ibis, 1881, pp. 105, 109, 115 (Kushai, Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 299 (Mortlock); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 55 (Jaluit, Arno, Kuschai).
Actitis incana Finsch, Proc. Zool. Soc. London, 1877 (1878), p. 781 (Ponapé); idem, Proc. Zool. Soc. London, 1880, p. 576 (Ruk); idem, Ibis, 1880, pp. 219, 220, 330, 332 Milli or Mulgrave, Taluit).
Heteractitis incanus Sharpe, Cat. Birds British Mus., 24, 1906, p. 455 (Oceania); Safford, The Plant World, 7, 1904, p. 268 (Guam); Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Yap, Ruk, Ponapé, Kusaie); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 179 (Kusaie); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 70 (westcentral Pacific).
Heteroscelus incanus Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 367 (Carolines, Marianas); Peters, Check-list Birds World, 2, 1934, p. 270 (Micronesia); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam): Watson, The Raven, 17, 1946, p. 42 (Guam); Wharton and Hardcastle (part), Journ. Parasitology, 32, 1946, pp. 296, 316, 318 (Guam, Peleliu); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 93 (Tinian); Strophlet, Auk, 1946, p. 537 (Guam); Wharton, Ecol. Monogr., 16, 1946, pp. 174, 175 (Guam); Borror, Auk, 1947, p. 417 (Agrihan).
Tringa incana incana Hartert, Vögel pal. Fauna, 13-14, 1921, p. 1623 (Guam); Hand-list Japanese Birds, rev., 1932, p. 191 (Marianas, Carolines, Marshalls, Palaus); Hand-list Japanese Birds, 3d ed., 1942, p. 214 (Saipan, Guam, Koror, Angaur, Yap, Faraulep, Lamatrek, Truk, Ponapé, Kusaie, Jaluit, Mille, Arhno, Majuro, Maloelab, Wotze, Likieb, Ailuk).
Heteroscelus incanus incanus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 46 (Kusaie, Ruk, Ponapé, Yap, Marianas, Mulgrave, Taluit, Pelew); Stickney, Amer. Mus. Novit., no. 1248, 1943, p. 7 (Guam, Palau, Ponapé, Ruk, Kusaie); Mayr, Birds Southwest Pacific, 1945, p. 42 (Palau, Marianas); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 53 (Guam, Rota, Ulithi).
Geographic range.—Breeds in Alaska south to Prince William Sound. Winters in North and South America and west in Oceania to Melanesia. In Micronesia: Mariana Islands—Guam, Rota, Saipan, Agrihan; Palau Islands—Angaur, Koror; Caroline Islands—Yap, Ulithi, Truk, Faraulep, Lamatrek, Ponapé, Kusaie; Marshall Islands—Jaluit, Mille, Arhno, Majuro, Maloelab, Wotze, Likieb, Ailuk, Bikini.
Specimens examined.—Total number, 47 (23 males, 20 females, 4 unsexed) as follows: Mariana Islands, USNM—Guam, 13 (May 21-29, Sept. 19-27, Oct. 10, 23),—Rota, 2 (Oct. 23, 25); AMNH—Guam, 4 (April 23, Aug. 16); Palau Islands, AMNH—exact locality not given, 1 (no date); Caroline Islands, USNM—Ulithi, 3 (Aug. 20, 22); AMNH—Truk, 1 (June 25),—Ponapé, 1 (Dec. 15),—Kusaie, 19 (Feb., Mar., April 1-10); Marshall Islands, USNM—Bikini, 3 (Feb. 26, 28, April 28).
Weights.—In 1948 (1948:53) I listed weights of two males from Guam as 175 (May) and 109 (September); weights of two females from Guam were 175 and 192 (both in May). These data were obtained by the NAMRU2 field party.
Parasites.—Wharton and Hardcastle (1946:296, 316, 318) list the following chiggers (Acarina) from tattlers taken by NAMRU2 collectors at Guam and Peleliu: Neoschöngastia bougainvillensis, N. ewingi, N. carveri, and N. namrui. Wharton (1946:174, 175) records the chiggers, Acariscus pluvius and A. anous, from tattlers from Guam. It is not certain from which species of Heteroscelus these chiggers were obtained.
Remarks.—Records indicate that the American Wandering Tattler is a regular visitor to eastern Micronesia, and that it only occasionally reaches the Palau Islands in western Micronesia.
The NAMRU2 field parties found H. brevipes as singles or in small groups of five or less. They remained apart from other species and appeared to prefer rocky beaches and coral-reef rocks to the sandy beaches. At Guam in 1945, the latest spring migrants were taken on May 29. These birds were in nuptial plumage. Birds taken at Bikini by Morrison on February 26 and April 28, 1946, were in worn, winter plumage. At Guam, the NAMRU2 observers obtained the first fall migrants on September 19. These observations in 1945, showed that H. incanus arrived at Guam on its southbound flight fully one month after the first individuals of H. brevipes began to appear (mid-July). This difference may partly result from the fact that the distance to the Asiatic breeding grounds of H. brevipes is not so great as that to the American breeding grounds of H. incanus.
Whether the two tattlers, H. brevipes and H. incanus, are distinct species (allopatric species insofar as breeding ranges are concerned), or whether they are mere subspecies (geographic races) is open to question. I failed to find evidences of intergradation in the few specimens which I examined critically; however, the final answer to the problem might be obtained by collecting series of birds from breeding grounds where ranges closely approach each other or overlap (if they do). Stickney (1943:6, 7) lists the distinctive differences in these two birds, particularly the character of the nasal groove, and does not mention having found any evidence of intergradation. Wetmore (in Townsend and Wetmore, 1919:180) gives evidence that they belong to two separate species.
Tringa Interpres Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 148. (Type locality, Europe and North America, restricted to Gotland, Sweden.)
Tringa interpres Quoy and Gaimard, Voy. "Uranie," Zool., 1824, p. 708 (Guam).
Strepsila collaris Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 287, 299, 304 (Ualan, Lougounor, Guahan); idem, Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 32 (Ualan).
Strepsilas interpres Kittlitz, Denk. Reise russ. Amer. Micron. und Kamchat., 2, 1858, pp. 32, 55, 86 (Ualan); Pelzeln, Reise "Novara," Vögel, 1865, p. 117 (Mariannen); Finsch and Hartlaub, Fauna Ornith. Centralpolynesian, 1867, p. 200 (Mariannen); Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 831 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 8, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 89, 104 (Pelew, Uap, Mackenzie); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 32 (Palau); idem, Proc. Zool. Soc. London, 1877 (1878), p. 781 (Ponapé); idem, Ibis, 1880, pp. 220, 330, 332 (Taluit); idem, Journ. f. Ornith., 1880, pp. 294, 306 (Ponapé, Kuschai); idem, Proc. Zool. Soc. London, 1880, p. 576 (Ruk); idem, Ibis, 1881, pp. 105, 109, 115 (Kushai, Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 281, 330, 353 (Ponapé, Nukuor, Ruk); Salvadori, Ornith. Papuasia, 3, 1882, p. 289 (Pelew, Mariannis); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 56 (Jaluit, Kuschai); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891); p. 63 (Ualan, Ponapé, Luganor, Nukuor, Ruk, Mackenzie, Pelew, Marianne); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 45 (Guam, Saypan, Hogoleu, Marshalls, Mackensie, Palaos); Hartert, Novit. Zool., 5, 1898, p. 66 (Guam); idem, Novit. Zool., 7, 1900, p. 9 (Ruk); Takatsukasa and Kuroda, Tori, 1, 1915, p. 51 (Ponapé); Uchida, Annot. Zool. Japon., 9, 1918, p. 489 (Ponapé).
Cinclus interpres Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 48 (Ladrones).
Arenaria interpres Sharpe, Cat. Birds British Mus., 24, 1896, p. 92 (Micronesia); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 37 (Guam); Safford, Osprey, 1902, p. 68 (Marianas); idem, The Plant World, 7, 1904, p. 266 (Guam); Schnee, Zool. Jahrbücher, 20, 1904, p. 389 (Marshall Islands); Safford, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam), Cox, Island of Guam, 1917, p. 22 (Guam); Wharton and Hardcastle, Journ. Parasitology, 32, 1946, pp. 316, 320 (Guam, Peleliu); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 105 (Tinian); Strophlet, Auk, 1946, p. 537 (Guam); Wharton, Ecol. Monogr., 16, 1946, pp. 174, 175 (Guam); Borror, Auk, 1947, p. 417 (Agrihan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 53 (Guam, Rota, Peleliu, Truk).
Arenaria interpres oahuensis Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 66, 1919, p. 177 (Jaluit, Rongelab, Uala); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 45 (Guam, Saipan, Pelew, Angaur, Kusaie, Ponapé, Luganor, Nukuor, Ruk, Yap, Mackenzie, Taluit, Rongelab).
Arenaria interpres interpres Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 45 (Micronesia); Hand-list Japanese Birds, rev., 1932, p. 194 (Guam, Saipan, Anguar, Kusaie, Ponapé, Luganor, Nukuor, Ruk, Yap, Mackenzie, Taluit, Rongelab, Mille, Majuro, Wotze, Likieb); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 217 (Guam, Saipan, Angaur, Kusaie, Ponapé, Luganor, Ruk, Yap, Mackenzie, Taluit, Rongelab, Mille, Majuro, Wotze, Likieb); Stickney, Amer. Mus. Novit., no. 1248, 1943, p. 8 (Guam, Palau, Ponapé, Kusaie).
Geographic range.—Breeds in northern parts of the Northern Hemisphere. Winters to Southern Hemisphere. In Micronesia: Mariana Islands—Guam, Rota, Saipan; Palau Islands—Angaur, Peleliu, Caroline Islands—Yap, Ulithi, Truk, Lugunor, Nukuor, Ponapé, Kusaie; Marshall Islands—Jaluit, Rongelab, Mille, Majuro, Wotze, Likieb, Bikini.
Specimens examined.—Total number, 36 (17 males, 16 females, 3 unsexed), as follows: Mariana Islands. USNM—Guam, 7 (Oct. 10-26)—Rota, 2 (Oct. 20, Nov. 2); AMNH—Guam, 4 (Mar. 22, 27, Aug. 18); Palau Islands, USNM—Peleliu, 1 (Sept. 8); AMNH—exact locality not given, 3 (Dec. 8); Caroline Islands, USNM—Truk, 1 (Dec. 22); AMNH—Ponapé, 4 (Dec. 16)—Truk, 4 (Feb. 5, 7, July 14)—Kusaie, 7 (Mar. 10-30); Marshall Islands, USNM—Bikini, 3 (Feb. 26, Mar. 4).
Weights.—The NAMRU2 party obtained the weights of four males taken at Guam and Rota as 77-99 (92) and one female from Guam as 90. These birds were obtained in October and November.
Parasites.—Wharton and Hardcastle (1946:316, 320) list the following chiggers (Acarina) from the Turnstone from Guam and Peleliu: Neoschöngastia carveri and N. strongi. Wharton (1946:174) records also Acariscus anous from the Turnstone at Guam. Uchida (1918:489) records the bird louse (Mallophaga), Colpocephalum pediculoides, from this bird at Ponapé.
Remarks.—The Turnstone is a regular visitor to Micronesia and to most other parts of Oceania. As pointed out by Stickney (1943:8), the material obtained by the Whitney South Sea Expedition yields evidence that the population which winters in Oceania is as widespread as that of Pluvialis dominica fulva but less abundant. The writer's observations at Guam, Ulithi and the Palaus are in agreement with this evidence. Stickney suggests that the reason the [Pg 149] Turnstone was not recorded by the Whitney South Sea Expedition in eastern Polynesia was because of "a tendency of the turnstone to hug the continental coasts more closely, avoiding extensive overseas migrations."
At Guam in 1945, the NAMRU2 party recorded the Turnstone on its northward migration as late as March 19; on its southward migration it was first seen at Guam on July 24. On its southward migration the bird was not numerous until September. Our observations indicated that in 1945, the principal waves of migration of the Turnstone appeared approximately two weeks after those of the Pacific Golden Plover and the Whimbrel. Stickney remarks that the spring migratory season in Oceania is completed in May and that the fall migratory season begins in August. Borror (1947:417) found small flocks on the beaches at Agrihan on August 10 and 11, 1945.
Bryan and Greenway (1944:112) indicate that the subspecies, Arenaria interpres morinella, which breeds in North America, east of Point Barrow, Alaska, may reach the Hawaiians. Careful examination of specimens from eastern Micronesia might reveal its presence there also. The name Areneria interpres oahuensis (Bloxham) may apply to specimens from eastern Micronesia but Peters (1934:271) considers oahuensis to be inseparable from Arenaria interpres interpres (Linnaeus).
Gallinago megala Swinhoe, Ibis, 1861, p. 343. (Type locality, Between Takoo and Pekin, China.
Gallinago heteroeaca Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 36 (Palau).
Gallinago megala Salvadori, Ornith. Papuasia, 3, 1882, p. 337 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 67 (Pelew); Sharpe, Cat. Birds British Mus., 24, 1896, p. 624 (Pelew); Hartert, Novit. Zool., 5, 1898, p. 65 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 33 (Mariannas); Safford, Osprey, 1902, p. 67 (Mariannas); idem, The Plant World, 7, 1904, p. 266 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Cox, Island of Guam, 1917, p. 21 (Guam); Hartert, Vögel pal. Fauna, 13-14, 1921, p. 1665 (Palau, Guam); Mayr, Birds Southwest Pacific, 1945, p. 44 (Guam, Palau); Strophlet, Auk, 63, 1946, p. 537 (Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 54 (Angaur).
Subspilura megala Kuroda, in Momiyama, Birds Micronesia, 1922, p. 49 (Guam, Pelew).
Capella megala Hand-list Japanese Birds, rev., 1932, p. 193 (Guam, Koror); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam); Robinson and Chasen, Birds Malay Peninsula, 3, 1936, p. 170 (Pelew, Marianne); Hand-list Japanese Birds, 3d ed., 1942, p. 316 (Guam, Koror).
Geographic range.—Breeds in east-central Asia. Winters south to Malaysia, Australia, and parts of Melanesia. In Micronesia: Mariana Islands—Guam; Palau Islands—Koror, Angaur.
Specimens examined.—One female from Palau Islands, USNM—Angaur (Sept. 21).
Remarks.—The Marsh Snipe is a regular visitor to western Micronesia, being recorded from the Mariana and Palau islands. At Angaur on September 21, 1945, the NAMRU2 party observed four birds at the edge of a brackish water swamp, which was margined with reeds and other vegetation. Birds were not seen on tidal beaches at Peleliu. Strophlet (1946:537) records the Marsh Snipe at Guam on October 21 and December 3, 1945.
Scolopax Gallinago Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 147. (Europe, restricted type locality, Sweden.)
Capella gallinago roddei Takatsukasa and Yamashina, Dobutsu. Zasshi, 44, 1932, p. 224 (Saipan).
Capella gallinago gallinago Hand-list Japanese Birds, rev., 1932, p. 193 (Saipan); Hand-list Japanese Birds, 3d ed., 1942, p. 216 (Saipan).
Gallinago gallinago Mayr, Birds Southwest Pacific, 1945, p. 44 (Saipan).
Geographic range.—Breeds in northern Eurasia. Winters in southern part of breeding range and south to Africa and east to Malaysia. In Micronesia: Mariana Islands—Saipan.
Remarks.—From Micronesia there is a single record of the taking of this bird at Saipan, apparently by Japanese collectors. It is probably an occasional straggler to the area, but owing to its similarity to Gallinago megala it may not often be recognized in the field.
Trynga alba Pallas, in Vroeg's Cat., 1764, Adumbr., p. 7. (Type locality, Coast of the North Sea.)
Calidris arenaria Finsch, Ibis, 1880, pp. 331, 332 (Taluit); idem, Mitth. Ornith. Ver. Wien, 1884, p. 56 (Jaluit); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 33 (Guam); Safford, Osprey, 1902, p. 70 (Mariannes); idem, The Plant World, 7, 1904, p. 268 (Guam); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall-Inseln).
Tringa arenaria Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 64 (Taluit); Hartert, Novit. Zool., 5, 1898, pp. 65, 69 (Guam).
Calidris alba Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 308 (Marshall Islands).
Crocethia alba Kuroda, in Momiyama, Birds Micronesia, 1922, p. 48 (Taluit, Guam); Hand-list Japanese Birds, rev., 1932, p. 193 (Taluit, Guam); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 215 (Jaluit, Guam); Stickney, Amer. Mus. Novit., no. 1248, 1943, p. 9 (Guam, Jaluit); Mayr, Birds Southwest Pacific, 1945, p. 44 (Marianas, Marshalls); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 54 (Ulithi).
Geographic range.—Breeds in Arctic regions of the Northern Hemisphere. Winters to Southern Hemisphere. In Micronesia: Mariana Islands—Guam; Caroline Islands—Ulithi; Marshall Islands—Jaluit.
Specimens examined.—Total number, 5 (2 males, 3 females), as follows: Mariana Islands, AMNH—Guam, 4 (Dec. 2-4); Caroline Islands, USNM, 1 (Aug. 21).
Remarks.—Stickney (1943:8, 9) summarizes the available information concerning the Sanderling in Oceania. The bird may be [Pg 151] classed as a regular visitor in eastern Micronesia; the most western record is from Ulithi in the western Carolines. It has been recorded also at Guam and Jaluit.
The NAMRU2 party secured one Sanderling from a flock of approximately thirty birds containing this species and Charadrius mongolus stegmanni at Pau Island, Ulithi Atoll, on August 21, 1945.
Totanus tenuirostris Horsfield, Trans. Linn. Soc. London, 13, pt. 1, 1821, p. 192. (Type locality, Java.)
Calidris tenuirostris Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 54 (Peleliu).
Geographic range.—Breeds in northeastern Siberia. Winters from India east to Malaysia and Australia. In Micronesia: Palau Islands—Peleliu.
Specimens examined.—Four males from Palau Islands, USNM—Peleliu (Sept. 16).
Remarks.—The Asiatic Knot was observed and obtained by the NAMRU2 party at Peleliu in September, 1945. Flocks containing fifteen to twenty birds were noted at the tidal flats of Akarakoro Point on September 8 and 16. The birds appeared to remain apart from other shore birds in this area.
Trynga ruficollis Pallas, Reise versch. Prov. Russ. Reichs, 3, 1776, p. 700. (Type locality, "Circa lacus salsos Dauriae campestris" = Kulussutai, southern Transbaikalia.)
Tringa minuta Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 8, 118 (Pelew); Gray, Hand-list Birds, pt. 3, 1871, p. 50 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 106 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 36 (Palau).
Tringa albescens Salvadori, Ornith. Papuasia, 3, 1882, p. 316 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 64 (Pelew).
Limonites minuta Takatsukasa and Kudora, Tori, 1, 1915, p. 62 (Pelew).
Pisobia ruficollis Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 290 (Pelew).
Pisobia minuta ruficollis Kuroda, in Momiyama, Birds Micronesia, 1922, p. 48 (Palau, Ulithi); Hand-list Japanese Birds, rev., 1932, p. 192 (Palau, Ulithi).
Calidris ruficollis ruficollis Hand-list Japanese Birds, 3d ed., 1942, p. 215 (Palau, Ulithi).
Calidris minuta ruficollis Mayr, Birds Southwest Pacific, 1945, p. 45 (Micronesia); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 54 (Rota, Peleliu).
Geographic range.—Breeds from northeastern Siberia to northwestern Alaska. Winters south from the Malay area to Australia. In Micronesia: Mariana Islands—Rota; Palau Islands—Angaur, Peleliu; Caroline Islands—Ulithi.
Specimens examined.—Total number, 16 (4 males, 12 females), as follows: Mariana Islands, USNM—Rota, 1 (Oct. 20); Palau Islands, USNM—Peleliu, 14 (Sept. 6-14)—Angaur, 1 (Sept. 21).
Remarks.—The Little Stint is apparently a regular visitor to the Palau Islands and a less common visitor to the Mariana Islands. At Peleliu and Angaur the NAMRU2 party found these birds in small [Pg 152] flocks of 10 to 15 at tidal flats and at inland ponds. On tidal flats the species appeared to remain apart from other kinds of shore birds, but at inland ponds the Little Stint was found in company with other species. On shooting into a mixed flock of shore birds at an island pond at Angaur, the writer secured specimens of this species and also of Erolia acuminata.
Tringa subminuta Middendorff, Reise Nord. und Ost. Siberien, 2, Th. 2, 1853, p. 222, pl. 19, fig. 6. (Type locality, Western slopes of the Stanovoi Mountains and mouth of the Udá.)
Pisobia minutilla subminuta Hand-list Japanese Birds, rev., 1932, p. 192 (Koror).
Calidris minutilla subminuta Hand-list Japanese Birds, 3d ed., 1942, p. 215 (Koror); Mayr, Birds Southwest Pacific, 1945, p. 45 (Palau).
Geographic range.—Breeds in northeastern Asia. Winters south to India and east to Malaysia. In Micronesia: Palau Islands—Koror.
Remarks.—The Least Sandpiper has been recorded in the Palau Islands by the Japanese investigators. It is probably an uncommon visitor to this area.
Tringa melanotos Vieillot, Nouv. Dict. Hist. Nat., 34, 1819, p. 462. (Type locality, Paraguay.)
Pisobia melanota Hand-list Japanese Birds, rev., 1932, p. 192 (Ponapé).
Calidris melanotos Hand-list Japanese Birds, 3d ed., 1942, p. 215 (Ponapé).
Calidris melanota Mayr, Birds Southwest Pacific, 1945, p. 45 (Ponapé).
Geographic range.—Breeds on the Arctic coast of northeastern Asia and eastward into Arctic America. Winters to South America. In Micronesia: Caroline Islands—Ponapé.
Remarks.—The Pectoral Sandpiper has been recorded from Ponapé. Bryan and Greenway (1944:114) list the species as an "accidental" visitor to the Hawaiian Islands from North America.
Totanus acuminatus Horsfield, Trans. Linn. Soc. London, 13, pt. 1, 1821, p. 192. (Type locality, Java.)
Tringa acuminata Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 8, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 89, 106 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 35 (Palau); Salvadori, Ornith. Papuasia, 3, 1882, p. 314 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 64 (Pelew); Hartert, Novit. Zool., 5, 1898, p. 65 (Marianne); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 33 (Guam); Safford, Osprey, 1902, p. 70 (Marianas); idem, The Plant World, 7, 1904, p. 268 (Guam).
Heteropygia acuminata Sharpe, Cat. Birds British Mus., 24, 1896, p. 566 (Pelew); Hartert, Novit. Zool., 7, 1900, p. 8 (Ruk); Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Marianas, Ruk, Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 48 (Pagan, Pelew, Ruk).
Tringa maculata var. acuminata Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3) 8, 1896, p. 44 (Pagan, Palaos).
Pisobia acuminata Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 276 (Caroline Islands).
Erolia acuminata Hartert, Vögel pal. Fauna, 11-12, 1920, p. 1586 (Palau, Karolinen); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam).
Pisobia acuminatus Hand-list Japanese Birds, rev., 1932, p. 192 (Ponapé, Truk, Pagan, Jaluit, Koror).
Calidris acuminata Hand-list Japanese Birds, 3d ed., 1942, p. 215 (Pagan, Jaluit, Koror, Truk, Ponapé); Mayr, Birds Southwest Pacific, 1945, p. 45 (Micronesia); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 54 (Guam, Angaur).
Geographic range.—Breeds in northeastern Siberia. Winters from the Malay Archipelago and Australia to the Southwest Pacific. In Micronesia: Mariana Islands—Guam, Pagan; Palau Islands—Angaur; Caroline Islands—Truk, Ponapé; Marshall Islands—Jaluit.
Specimens examined.—Total number, 4 (2 males, 2 females), as follows: Mariana Islands, USNM—Guam, 1 (Sept. 17); Palau Islands, USNM—Angaur, 3 (Sept. 21).
Remarks.—The Sharp-tailed Sandpiper is a regular visitor to western Micronesia and an uncommon visitor to eastern Micronesia. It was first recorded from the Palau Islands in 1868, where the bird was taken by Tetens, Heinsohn, and Kubary. In 1896 and 1898, records of this bird in the Mariana and Caroline islands were published by Oustalet and Hartert.
The NAMRU2 party obtained one specimen at Guam on September 17 and three at Angaur on September 21. At Angaur several birds of this species were seen at fresh water ponds in company with Erolia minuta ruficollis, Limicola falcinellus sibirica, Tringa glareola, and other shore birds.
Tringa ferrugineus Pontoppidan, Danske Atlas, 1, 1763, p. 624. (No type locality = Denmark.)
Calidris ferruginea Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 55 (Peleliu).
Geographic range.—Breeds in northern Asia. Winters from Africa east to Australia. In Micronesia: Palau Islands—Peleliu.
Specimens examined.—One female from Palau Islands, USNM—Peleliu (Sept. 6).
Remarks.—The NAMRU2 party obtained one female on September 6 at a tidal flat on Peleliu. The Curlew Sandpiper is seemingly a rare visitor to the Palau Islands from Asia. In using this specific name, I am following Mayr (in Delacour and Mayr, 1945:107).
Limicola sibirica Dresser, Proc. Zool. Soc. London, 1876, p. 674. (Type locality, Siberia and China.)
Limicola falcinellus sibirica Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 55 (Angaur).
Geographic range.—Breeds in northeastern Asia. Winters from India east to Australia. In Micronesia: Palau Islands—Angaur.
Specimens examined.—One male from Palau Islands, USNM—Angaur (Sept. 21).
Remarks.—A single male bird was taken by the NAMRU2 party at a fresh water pond on Angaur Island on September 21, 1945. This is the only known record for this bird from Micronesia.
Tringa lobata Linnaeus, Syst. Nat., ed. 10, 1, 1758, p. 148, in Emendanda, p. 824. (Type locality, Hudson Bay.)
Geographic range.—Breeds throughout Arctic region. Winters at sea in tropical and subtropical waters.
Remarks.—The Northern Phalarope has not been found in Micronesia. Mayr (1945a:46) records it in the pelagic areas north of the New Guinea region. The occurrence there suggests that migration is through the Micronesian area.
Larus argentatus Brünn. var. Vegae Palmén, in Nordenskiöld, Vega-Exped. Vetensk. Iakttag., 5, 1887, p. 370. (Type locality, Pidlin, northeastern Siberia.)
Larus vegae Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 56 (Agrigan); Hartert, Novit. Zool., 5, 1898, p. 68 (Marianne); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 20 (Marianas); Safford, Osprey, 1902, p. 70 (Marianas); idem, The Plant World, 7, 1904, p. 268 (Guam?).
Larus vegae Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Marianas).
Larus argentatus vegae Kuroda, in Momiyama, Birds Micronesia, 1922, p. 49 (Agrigan); Hand-list Japanese Birds, rev., 1932, p. 196 (Agrigan); Hand-list Japanese Birds, 3d ed., 1942, p. 220 (Agrigan).
Geographic range.—Breeds in northern Siberia. Ranges east to Alaska and south to the Philippines and the China coast. In Micronesia: Mariana Islands—Agrihan.
Remarks.—The Herring Gull is ascribed to Micronesia on the basis of one bird obtained by Marche in January, 1889, at Agrihan in the northern Marianas and reported on by Oustalet (1896:56). The gull is considered a straggler to the northern Marianas from the northward. Stott (1947:525) observed a gull, which was thought to be this species or Larus ridibundus, at Lake Susupe, Saipan, in 1945.
Sterna leucoptera Temminck, Man. d'Ornith., 1815, p. 483. (Type locality, Coasts of the Mediterranean.)
Hydrochelidon leucoptera Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 57 (Guam); Hartert, Novit. Zool., 5, 1898, p. 67 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 20 (Guam); Safford, Osprey, 1902, p. 70 (Marianas); idem, The Plant World, 7, 1904, p. 268 (Guam); Hartert, Vögel pal. Fauna, 13-14, 1921, p. 1686 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 51 (Guam).
Chlidonias leucoptera Hand-list Japanese Birds, rev., 1932, p. 194 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 217 (Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 55 (Angaur).
Geographic range.—Breeds in central and southern Eurasia. Winters from Africa east to Australia. In Micronesia: Mariana Islands—Guam; Palau Islands—Angaur.
Measurements.—One adult male has the following measurements: wing, 211; tail, 72; exposed culmen, 27; tarsus, 20; one adult female: wing, 210; exposed culmen, 25.5. These specimens were taken at the Palau Islands.
Specimens examined.—Total number, 6 (3 males, 3 females), as follows: Palau Islands, USNM—Angaur, 1 (Sept. 21); AMNH—exact locality not given, 5 (Oct. 13).
Remarks.—The White-winged Black Tern was first collected at Guam in October, 1887, by Marche and reported on by Oustalet (1896:57). It was later taken at the Palau Islands by Coultas in 1931, and by the NAMRU2 party at Angaur in 1945. The bird is seemingly an uncommon winter visitor to Micronesia.
At Angaur, the NAMRU2 party obtained one of four terns seen at a small fresh water lake. Coultas took five birds at the Palau Islands. He writes (field notes) that a flock of 14 of the terns appeared at the island following a heavy typhoon. All birds examined are in winter plumage (September and October).
Sterna longipennis Nordmann, in Erman's Verz. Thier. Pflanz., 1835, p. 17. (Type locality, Mouth of the Kutchui River, Sea of Okhotsk.)
Sterna longipennis Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 90, 112 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 41 (Palau); Salvadori, Ornith. Papuasia, 3, 1882, p. 440 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 74 (Pelew); Saunders, Cat. Birds British Mus., 25, 1896, p. 67 (Pelew); Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 21 (Pelew).
Sterna hirundo longipennis Hand-list Japanese Birds, rev., 1932, p. 195 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 218, (Palau); Mayr, Birds Southwest Pacific, 1945, p. 25 (Palau).
Geographic range.—Breeds in northeastern Asia. Winters south to Melanesia. In Micronesia: Palau Islands—exact locality unknown.
Remarks.—Finsch (1875:41) states that Heinsohn and Kubary obtained specimens of this tern from the Palau Islands for the [Pg 156] Godeffroy Museum. These are the only records for the occurrence of the Black-billed Common Tern in Micronesia.
Sterna Sumatrana Raffles, Trans. Linn. Soc. London, 13, pt. 2, 1822, p. 329. (Type locality, Sumatra.)
Sterna melanauchen Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 306, 308 (Guahan, Ouleai); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 90, 113 (Pelew, Uap); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 41 (Palau); idem, Ibis, 1880, pp. 220, 330, 332 (Taluit); idem, Journ. f. Ornith., 1880, p. 295 (Ponapé); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); idem, Ibis, 1881, pp. 113, 115 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 281, 299, 330, 353 (Ponapé, Mortlock, Nukuor, Ruk); Salvadori, Ornith. Papuasia, 3, 1882, p. 444 (Pelew, Mackenzie, Ruk, Ponapé, Marshalls); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 74 (Pelew, Uap, Ruk, Luganor, Nukuor, Ponapé, Taluit); Sanders, Cat. Birds British Mus., 25, 1896, p. 126 (Carolines, Pelews, Marshalls); Nehrkorn, Kat. Eiers., 1899, p. 222 (Palau); Hartert, Novit. Zool., 7, 1900, p. 10 (Ruk); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall-Inseln); Takatsukasa and Kuroda, Tori, 1, 1915, p. 52 (Ruk, Ponapé); Uchida, Annot. Zool. Japon., 9, 1918, pp. 483, 488 (Ponapé).
Sterna sumatrana Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 186 (Arhno).
Gygisterna sumatrana Kuroda, in Momiyama, Birds Micronesia, 1922, p. 52 (Pelew, Mackenzie, Yap, Ruk, Luganor, Nukuor, Ponapé, Taluit, Arhno).
Gygisterna sumatrana sumatrana Kuroda, Avifauna Riu Kiu, 1925, p. 192 (Carolines, Pelews).
Sterna sumatrana sumatrana Yamashina, Tori, 7, 1932, p. 410 (Aruno); Hachisuka, Birds Philippines, 2, 1932, p. 335 (Caroline, Pelew); Hand-list Japanese Birds, rev., 1932, p. 195 (Palau, Guam, Saipan, Yap, Truk, Lukunor, Nukuoro, Ponapé, Jaluit, Namu, Arhno, Majuro, Aurh); Peters, Check-list Birds World, 2, 1934, p. 336 (Caroline Islands); Mayr, List New Guinea Birds, 1941, p. 36 (Micronesia); Hand-list Japanese Birds, 3d ed., 1942, p. 218 (Babelthuap, Koror, Yap, Truk, Lukunor, Nukuoro, Ponapé, Jaluit, Namu, Arhno, Majuro, Aurh); Mayr, Birds Southwest Pacific, 1945, p. 24 (Micronesia); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 55 (Peleliu, Ulithi).
Geographic range.—Micronesia, central Polynesia, northern Australia, Malaysia, west to India, and north to the Riu Kiu Islands. In Micronesia: Palau Islands—Babelthuap, Koror, Peleliu; Caroline Islands—Yap, Ulithi, Truk, Lukunor, Nukuoro, Ponapé; Marshall Islands—Jaluit, Namu, Majuro, Aurh, Bikini.
Characters.—Adult: A small tern with a long, forked tail and white plumage often with pinkish cast except for mantle, back, rump, tail, wing-coverts, and scapulars which are pale pearl-gray; band across nape, spot in front of eye, and outer web of outer primary black; bill and feet black.
Immature: Resembles adult, but black and white mottling on upper parts.
Measurements.—Measurements are listed in table 19.
Specimens examined.—Total number, 15 (8 males, 6 females, 1 female?), as follows: Palau Islands, AMNH—exact locality not given, 4 (Oct.-Dec.); Caroline Islands, USNM—Ulithi Atoll, 6 (Aug. 15, 16, 20, 22); AMNH—Truk, 1 (Feb. 10); Marshall Islands, USNM—Bikini, 4 (March 26, April 30).
Nesting.—Nehrkorn (1899:222) recorded eggs taken at the Palau Islands. Yamashina (1932a:410) listed the finding of three nests containing one egg each on September 26, 1931, at Arhno in the Marshall Islands. The NAMRU2 party obtained no evidence of nesting at Ulithi or Palau in August and September, 1945. Coultas (field notes) obtained reports of the finding of two eggs at the Palau Islands in the period October to December, 1931.
Parasites.—Uchida (1918:483, 488) records the following Mallophaga taken at Ponapé from this tern: Docophorus albemarlensis, Colpocephalum milleri, and Colpocephalum impertunum.
Remarks.—There are no records for the Black-naped Tern from the Mariana Islands, although the species is known from the Palau, Caroline and Marshall Islands. At Ulithi Atoll, the NAMRU2 party observed these terns at the islands of Potangeras, Mangejang, Pau, and Losiep in August, 1945. They were found in groups of 4 to 15, either sitting on sandy beaches or rocky exposures or flying over the reefs. Unlike the Crested Tern, these birds appeared quite unafraid of man and would hover over a freshly killed or wounded individual of their own kind, making of themselves easy targets. The writer saw only one Black-naped Tern at the Palau Islands (Peleliu, on September 16, 1945). The birds seem to prefer the "low" atolls to the "high" volcanic islands of Micronesia.
Two subspecies of Sterna sumatrana are recognized by Peters (1934:336): Sterna sumatrana mathewsi known from islands of the western Indian Ocean and Sterna s. sumatrana from islands of Oceania, Australia, Malaysia, and China coast. There is a considerable area separating these subspecies. For populations in the Pacific area, other names which have been proposed are Sterna sumatrana kempi Mathews for birds from Torres Straits and Gygis decorata Hartlaub for birds from the Fiji Islands. A study of 201 specimens of this species from various parts of its range (in the collections of the American Museum of Natural History and the United States National Museum) shows that there is little color variation within the species. This observation is the same as that of Mathews (1912:372).
As listed in table 19, measurements of the length of the wing show little variation. The length of the tail of birds from localities more remote from the continent of Asia (Micronesia, Phoenix, Union, Fiji, Samoa, Tonga, and the islands of the Indian Ocean: Aldabra and Providence) is, on the average, shorter than the length of the tail of birds from islands nearer the Asiatic mainland. This shortness is reflected also in the measurement of the difference between the shortest and longest tail feather.
Location No. Wing Tail Difference:
Longest
and
shortest
tail
featherExposed
culmenTarsus S. s. sumatrana
Micronesia13 221 127 65 37 20.5 211-225 117-138 54-79 35-39 20.0-21.0 Phoenix and Union 5 228 113 66 37 19.5 36-38 18.5-20.0 Fiji, Samoa, Tonga 29 221 131 63 38 20.0 218-229 122-142 51-74 36-41 18.0-21.0 New Caledonia
Loyalty, New
Hebrides8 224 141 72 39 19.5 221-230 135-148 68-81 37-41 18.5-20.0 Queensland, Torres
Straits4 229 142 78 38 19.5 139-148 71-83 36-40 18.5-20.0 Solomons 52 227 144 77 36 19.0 220-232 129-162 66-95 34.0-38.5 18.5-20.5 New Guinea,
Bismarcks10 224 143 76 34 19.5 219-231 135-146 67-81 32.0-36.5 18.5-20.0 Malay area 49 228 141 74 34 20.0 220-234 125-153 63-84 32.0-37.0 19.0-20.5 China coast,
Riu Kiu21 223 144 77 35 19.5 212-234 130-151 67-85 31.5-38.0 19.0-20.0 S. s. mathewsi
Micronesia10 220 125 71 38 19.0 35.0-40.0 18.0-20.0 The differences in the length of the exposed culmen of these terns shows that birds from islands more remotely oceanic possess longer bills than do those from islands closer to the Asiatic continent. Murphy (1938:538) has written that this phenomenon is characteristic among some species which have both continental and insular populations (or subspecies). Figure 10 shows the southeastern part of the range of the subspecies, Sterna s. sumatrana, and gives the average measurements of the exposed culmen of birds from several localities. [Pg 159] These localities are given in table 19. Terns with longer bills (37-39) were taken in Micronesia, in the Polynesian islands, and in northern Australia. Terns with shorter bills (34-36) were taken in Melanesia, Malaysia, and the coastal region of China, but there appears to be no abrupt line of demarkation between them. Further evidence of this tendency may be obtained from the literature. Kuroda (1925:191) gives the measurements of the exposed culmen of seven males and five females from the Riu Kius as averaging 35 mm. (range 31-40.5). It is also of interest to note that the length of the exposed culmen of the males averages one to two mm. longer than that of the females. The status of Sterna sumatrana mathewsi may be questioned. I find no characters separating my series of mostly poor specimens. The systematic position of this subspecies from the Indian Ocean (and likewise the status of subspecies of other sea birds which range into the Indian Ocean) may not be known with certainty until additional material is obtained.
Fig. 10. Geographic variation in the average length of the exposed culmen of Sterna sumatrana sumatrana.
Sterna lunata Peale
Spectacled Tern
Sterna lunata Peale, U. S. Expl. Exped., 8, 1848, p. 277. (Type locality, Vincennes Island, Paumotu Group.)
Sterna lunata Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 831 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 9, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 90, 113 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 41 (Palau); Saunders, Cat. Birds British Mus., 25, 1896, p. 100 (Pelew); Takatsukasa and Kuroda, 1, 1915, p. 62 (Ruk, Pelew); Hand-list Japanese Birds, rev., 1932, p. 195 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 218 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 26 (Micronesia).
Onychoprion lunatus Salvadori, Ornith. Papuasia, 3, 1882, p. 451 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 76 (Pelew).
Melanosterna lunata Kuroda, in Momiyama, Birds Micronesia, 1922, p. 52 (Pelew).
Geographic range.—Breeds in Oceania from the Hawaiian Group south to Fiji and the Tuamotus and west to the Moluccas. In Micronesia: Palau Islands—exact locality not known.
Remarks.—Finsch (1875:41) recorded specimens taken by Tetens, Peters and Kubary at the Palau Islands. Coultas obtained one immature male at sea south of the eastern Caroline Islands at 1° 25´ N and 159° E on October 19, 1930. The Spectacled Tern ranges throughout the tropical Pacific, spending considerable time at sea, and probably reaches most parts of Micronesia in its travels.
Sterna anaetheta anaetheta Scopoli
Bridled Tern
Sterna Anaethetus Scopoli, Del. Flor. et Faun., Insubr., fasc. 2, 1786, p. 92. (Type locality, "In Guinea" = Panay, Philippine Islands, ex. Sonnerat.)
Sterna anaestheta Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Pelew).
Melanosterna anaestheta anaestheta Kuroda, in Momiyama, Birds Micronesia, 1922, p. 52 (Pelew).
Sterna anaethetus anaethetus Hand-list Japanese Birds, rev., 1932, p. 195 (Palau); Yamashina, Tori, 10, 1940, p. 678 (Bikar); Hand-list Japanese Birds, 3d ed., 1942, p. 218 (Palau, Bikar).
Sterna anaetheta anaetheta Mayr, Birds Southwest Pacific, 1945, p. 26 (Palau).
Geographic range.—Breeds from Malaysia to Australia and Oceania and north to Formosa. Ranges west to Ceylon and north to Japan. In Micronesia: Palau Islands—exact locality not known; Marshall Islands—Bikar.
Measurements.—Four adult males from the Palau Islands have the following measurements: wing 246-254, longest tail feather 147-177, shortest tail feather 71-72, exposed culmen 40-44, tarsus 21-23; one adult female: wing 266, exposed culmen 40.5, tarsus 22.5.
Specimens examined.—Total number, 7 (4 males, 3 females) from Palau Islands, AMNH—exact locality not given (Dec. 20).
Remarks.—The Bridled Tern is known from the Palau Islands and from Bikar in the Marshall Islands. In Micronesia, the species apparently reaches the northeastern extent of its range. In the Palaus, Coultas found the terns on small outlying islands. He [Pg 161] observed them to fly to sea early in the day and to return to the islands in the evening. Of the seven specimens obtained by him, two males and one female had enlarged gonads (Dec. 20).
Sterna fuscata oahuensis Bloxham
Sooty Tern
Sterna Oahuensis Bloxham, Voy. "Blonde," 1826, p. 251. (Type locality, Oahu, Hawaiian Islands.)
Sterna fuliginosa Finsch, Journ. Mus. Godeffroy, 12, 1876, pp. 18, 39 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 781 (Ponapé); idem, Journ. f. Ornith., 1880, p. 295 (Ponapé); Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Ponapé).
Onychoprion fuscata infuscata Kuroda, in Momiyama, Birds Micronesia, 1922, p. 51 (Ponapé).
Sterna fuscata nibilosa Hand-list Japanese Birds, rev., 1932, p. 195 (Ponapé); Yamashina, Tori, 10, 1940, p. 677 (Helen Reef); Hand-list Japanese Birds, 3d ed., 1942, p. 218 (Ponapé, Helen Reef).
Sterna fuscata oahuensis Mayr, Birds Southwest Pacific, 1945, p. 25 (Micronesia).
Geographic range.—Breeds from the Hawaiian, Marcus, and Bonin islands south to the Phoenix Islands and Micronesia. In Micronesia: Mariana Islands—Asuncion; Palau Islands—Helen Reef; Caroline Islands—Ponapé.
Specimens examined.—Total number, 1 unsexed from Mariana Islands, AMNH—Asuncion (Jan. 18).
Remarks.—The systematic position of the Sooty Tern in Micronesia is uncertain; in using this name I am following Peters (1934:338), who comments that the species "is badly in need of revision." Coultas obtained one immature female at O° 90´ S and 159° 50´ E, a position south of the eastern Caroline Islands. The bird is tentatively placed in the subspecies S. f. oahuensis. The Sooty Tern probably does not breed in large numbers in Micronesia, unless it be in the northern Marianas. Bryan (1903:97) reports that this species is very abundant at Marcus Island, which is north and east of the Marianas.
Sterna albifrons sinensis Gmelin
Least Tern
Sterna sinensis Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 608. (Type locality, China, ex Latham.)
Sterna albifrons Marshall, Condor, 51, 1949, p. 221 (Saipan).
Geographic range.—Found on coastal areas from Korea and China south to New Guinea. In Micronesia: Mariana Islands—Saipan.
Specimens examined.—One female from Mariana Islands, USNM—Saipan (Sept. 26).
Remarks.—Marshall (1949:221) took one of two Least Terns at Lake Susupe on Saipan on September 26, 1945. The specimen taken, a female, is in post juvenal molt.
Thalasseus bergii pelecanoides (King)
Crested Tern
Sterna pelecanoides King, Surv. Intertrop. and Western Coasts Australia, 2, 1827, p. 422. (Type locality, Torres Strait, northern Queensland.)
Sterna bergii Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 50 (Palau); idem, Proc. Zool. Soc. London, 1877 (1878), p. 781 (Ponapé); idem, Ibis, 1880, pp. 330, 332 (Ratak Chain); idem, Journ. f. Ornith., 1880, p. 295 (Ponapé); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); idem, Ibis, 1881, pp. 113, 115 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 281, 299, 330, 353 (Ponapé, Mortlock, Nukuor, Ruk); Salvadori, Ornith. Papuasia, 3, 1882, p. 434 (Ruk, Ponapé, Marshalls); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 51 (Jaluit); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 74 (Pelew, Luganor, Nukuor, Ruk, Ponapé, Marshall Islands); Hartert, Novit. Zool., 7, 1900, p. 10 (Ruk); Saunders, Cat. Birds British Mus., 25, 1896, p. 89 (Ponapé, Marshalls); Takatsukasa and Kuroda, Tori, 1, 1915, p. 52 (Ponapé); Uchida, Annot. Zool. Japon., 9, 1918, pp. 483, 488 (Ponapé).
Sterna bergeri Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall-Inseln).
Sterna bergii cristata Stresemann, Novit. Zool., 21, 1914, p. 58 (Truk).
Thalasseus bergii pelecanoides Oberholser, Proc. U. S. Nat. Mus., 49, 1915, p. 523 (Marshall Islands); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 51 (Luganor, Nukuor, Ruk, Ponapé, Marshall Islands); Kuroda, Avifauna Riu Kiu, 1925, p. 188 (Marshall Islands); Hand-list Japanese Birds, rev., 1932, p. 194 (Palau, Faraulep, Truk, Lukunor, Nukuoro, Ponapé, Jaluit, Mille, Aurh, Maloelab, Ailuk); Yamashina, Tori, 10, 1940, p. 677 (Helen Reef, Babelthuap); Hand-list Japanese Birds, 3d ed., 1942, p. 218, (Babelthuap, Helen Reef, Faraulep, Truk, Lukunor, Nukuoro, Ponapé, Jaluit, Mille, Aurh, Maloelab, Ailuk).
Thalasseus bergii cristatus Peters, Check-list Birds World, 2, 1934, p. 342 (Carolines, Marshalls); Mayr, Birds Southwest Pacific, 1945, p. 26 (Micronesia); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 55 (Peleliu, Ngajangel [Kayangel], Truk).
Geographic range.—Malaysia and east coast of Australia south to Tasmania, east to Melanesia and Polynesia, north to Phoenix Islands and Micronesia (see figure 11). In Micronesia: Palau Islands—Helen Reef, Peleliu, Babelthuap; Caroline Islands—Ulithi, Truk, Faraulep, Lukunor, Nukuoro, Ponapé; Marshall Islands—Jaluit, Mille, Aurh, Moloelab, Ailuk, Bikini.
Characters.—Adult: A large, white tern with back, rump, tail, wing-coverts, wing, and axillaries pearl gray; outer edges of primaries pearly grayish-black; crown black with crest; bill greenish-yellow with blackish base; feet black. Crown black, mottled with white and mantle paler in postnuptial plumage.
Immature: Resembles adult, but crown and back dark, mottled with white and crest small.
Measurements.—Measurements of Crested Terns of the Pacific area are listed in table 20.
Specimens examined.—Total number, 10 (6 males, 4 females), as follows: Caroline Islands, USNM—Ulithi, 1 (Aug. 21); AMNH—Truk, 2 (May 7, Dec. 5)—Ponapé, 3 (Nov. 1, 7); Marshall Islands, USNM—Bikini, 4 (March 4, 11, 12).
Parasites.—Uchida (1918:483, 488) obtained the following species of bird lice (Mallophaga) from the Crested Tern at Ponapé: Docophorus albemarlensis and Colpocephalum importunum.
Remarks.—Oberholser (1915:520-526, pl. 66) lists five subspecies (T. b. cristatus, T. b. halodramus, T. b. pelecanoides, T. b. rectirostris, and T. b. poliocercus) in the region including the coast of [Pg 163] China, the Riu Kiu Islands, Malaysia, Melanesia, eastern Australia, Polynesia, and Micronesia. Only one subspecies, T. b. cristatus, is recognized in this area by Stresemann (1914:58), Hartert (1921:1695-1696), and Peters (1934:341-342), who mention that there is much variation in size and coloring.
Location No. Wing Longest
tail
featherShortest
tail
featherExposed
culmenTarsus Thalasseus bergii pelecanoides
Palaus, Carolines,
Marshalls6 343 168 82 60 334-352 153-184 80-85 58-65 Christmas, Phoenix,
Tuamotus, Society,
Fiji, Loyalty,
New Hebrides48 344 170 83 58 27 329-362 145-198 77-92 54-64 25-29 Eastern Australia 14 345 165 88 58 27 338-349 152-174 84-92 55-63 26-29 New Guinea, Bismarck,
Archipelago, Moluccas18 342 168 81 59 27 332-361 144-194 75-87 53-64 26-28 Totals 86 344 169 83 58 27 329-362 144-198 75-92 53-65 25-29 Thalasseus bergii cristatus
Philippines, China,
Formosa, Riu Kius18 332 162 81 58 28 324-342 149-182 78-87 55-64 26-30 Thalasseus bergii gwendolenae
Western Australia14 354 171 86 58 27 339-369 162-182 81-91 53-65 25-29 Measurements, as shown in table 20, indicate a wide range of sizes but, in most series, the averages are nearly the same. Nevertheless, it is evident that birds from the coast of China, the Riu Kius, Formosa, and the Philippines have a distinctly shorter wing than birds from the Moluccas, Melanesia, eastern Australia, Polynesia, and Micronesia. Further evidence of this is presented by Kuroda (1925:186) who lists the measurements of the wing of eight Crested Terns from the Riu Kiu Islands as 322 to 340 (average 330). The occurrence of populations with [Pg 164] shorter wings has already been pointed out in the work of Oberholser (1915:520-526), who divided the short-winged birds into two subspecies. It seems advisable to recognize but one subspecies, T. b. cristatus, for the birds with short wings and another subspecies, T. b. pelecanoides, to include the birds with the longer wings (see figure 11). The average measurements of the length of wings of these two subspecies, 332, and 344, differ significantly, although there is some overlap in measurements. A few specimens at hand from the western part of Malaysia are in poor condition and not measurable.
Fig. 11. Geographic distribution of Thalasseus bergii. (1) T. b. bergii; (2) T. b. thalassinnus; (3) T. b. velox; (4) T. b. cristatus; (5) T. b. gwendolenae; (6) T. b. pelecanoides.
Most specimens of T. b. cristatus and T. b. pelecanoides have lighter-colored upper parts than specimens of T. b. velox, but not so light-colored as specimens of T. b. gwendolenae. Size probably is a better character than color to use in separating these groups.
In Micronesia, the NAMRU2 party observed Crested Terns at Ulithi, Peleliu and Truk, in August, September, and December, 1945, respectively. Birds were seen as singles or in small groups flying over the reefs. The birds were wary and difficult to approach, but they were conspicuous and easily identified.
Procelsterna cerulea saxatilis W. K. Fisher
Blue-gray Tern
Procelsterna saxatilis W. K. Fisher, Proc. U. S. Nat. Mus., 26, 1903, p. 559. (Type locality, Necker Island, Hawaiian Islands.)
Procelsterna cerulea saxatilis Yamashina, Tori, 10, 1940, p. 678 (Bikar); Hand-list Japanese Birds, 3d ed., 1942, p. 219 (Bikar); Mayr, Birds Southwest Pacific, 1945, p. 27 (Micronesia).
Geographic range.—Known from Marcus Island and the western Hawaiian Islands. In Micronesia: Marshall Islands—Bikar.
Remarks.—Yamashina (1940:678) recorded the taking of eight of these terns (5 adult males, 3 adult females) on July 10, 1932, at Bikar in the Marshall Islands. He gives the following measurements: wing, 180.5-188; tail, 104-113.5; exposed culmen, 24-26.5. This is the only known record for the species in Micronesia.
Anous stolidus pileatus (Scopoli)
Common Noddy
Sterna pileata Scopoli, Del. Flor. et Faun. Insubr., fasc. 2, 1786, p. 92. (No type locality = Philippines, ex. Sonnerat.)
Sterna stolida Chamisso, in Kotzebue's Voy. "Rurick," 3, 1821, pp. 150, 157 (Marshall Islands); Kittlitz, Kupfertaf. Naturgesch. Vögel, 3, 1833, p. 27, pl. 36, fig. 1 (Mordloks-Inseln); idem, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 286, 299, 308, 309 (Ualan, Lougounor, Ouleai); idem, Denkw. Reise russ. Amer. Micron. und Kamchat., 1, 1858, p. 364, 2, pp. 77, 86 (Ualan); Wiglesworth, Ibis, 1893, p. 212 (Marshalls).
Anous stolidus Hartlaub, Archiv f. Naturgesch., 18, 1852, p. 137 (Mortlock); idem, Journ. f. Ornith., 1854, p. 168 (Carolinen); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 59 (Carolines); Finsch and Hartlaub, Fauna Centralpolynesiens, 1867, p. 236 (Mordlocks, Puynipet = Ponapé); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 9, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 90, 112 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 6, 42 (Palau); idem, Journ. Mus. Godeffroy, 12, 1876, pp. 18, 40 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 781 (Ponapé); idem, Journ. f. Ornith., 1880, pp. 295, 307 (Ponapé, Ruck, Kuschai); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk, Ponapé, Kuschai); idem, Ibis, 1881, pp. 105, 109, 115, 246, 247 (Kuschai, Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 299, 330, 353 (Mortlock, Nukuor, Ruk); Salvadori, Ornith. Papuasia, 3, 1882, p. 455 (Pelews, Carolines, Marshalls); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 51 (Jaluit, Ponapé); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 76 (Pelew, Mortlock, Ruk, Nukuor, Ponapé, Ualan, Marshalls); Saunders, Cat. Birds British Museum, 25, 1896, p. 136 (Pelew, Carolines, Marshalls); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 59 (Saypan, Guam, Rota, Agrigan, Hogoleu = Truk, Kushai, Ponapi, Marshalls); Hartert, Novit. Zool., 5, 1898, p. 68 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 21 (Guam); Safford, Osprey, 1902, p. 66 (Mariannas); Bryan, Occ. Papers Bernice P. Bishop Mus., 2, 1903, p. 101 (Guam); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marshall-Inseln); Safford, The Plant World, 7, 1904, p. 267 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Prowazek, Die deutschen Marianen, 1913, p. 100 (Marianen); Takastukasa and Kuroda, Tori, 1, 1915, p. 51 (Ponapé, Ruk); Cox, Island of Guam, 1917, p. 22 (Guam); Uchida, Annot. Zool. Japon., 9, 1918, pp. 484, 488 (Palau, Ponapé); Wharton, Ecol. Monogr., 16, 1946, p. 174 (Guam); Wharton and Hardcastle, Journ. Parasitology, 32, 1946, pp. 292, 296, 306 (Guam, Ulithi).
Anous pileatus Pelzeln, Reise "Novara," Vögel, 1865, pp. 155, 162 (Puynipet = Ponapé).
Anous stolidus pileatus Hartert, Novit. Zool., 7, 1900, p. 9 (Ruk); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 183 (Kusaie); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 49 (Guam, Saipan, Pelew, Mortlock, Ruk, Wolea, Nukuoro, Ponapé, Kusaie, Marshalls); Hand-list Japanese Birds, rev., 1932, p. 195 (Koror, Urukthapel, Angaur, Saipan, Guam, Wolea, Truk, Mortlock, Lukunor, Nukuoro, Ponapé, Kusaie, Jaluit, Mille, Aurh, Wotze); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam); Yamashina, Tori, 10, 1940, p. 678 (Assongsong, Babelthuap); Hand-list Japanese Birds, 3d ed., 1942, p. 219 (Saipan, Assongsong, Guam, Babelthuap, Koror, Urukthapel, Peliliu, Angaur, Wolea, Truk, Mortlock, Lukunor, Nukuoro, Ponapé, Kusaie, Taluit, Mille, Aurh, Wotze); Borror, Auk, 1947, p. 417 (Agrihan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 56 (Rota, Guam, Peleliu, Ngabad, Ulithi, Truk).
Anous stolidus unicolor? Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 547 (Guam).
Location No. Wing Tail Exposed
culmenAnoüs stolidus ridgwayi
Isabella, Cocos, Clipperton Islands18 278 158 41 260-295 147-166 38-42 Anoüs stolidus galapagensis
Galapagos Islands11 277 151 40 274-282 142-160 38-42 Anoüs stolidus pileatus
Hawaiian Islands: Nihoa to Midway35 281 162 42 268-299 149-176 38-40 Wake Islands 8 278 159 41 273-285 152-170 39-43 Mariana Islands: Guam, Rota 12 280 167 41 275-288 159-187 39-43 Palau Islands 9 278 161 41 268-283 155-166 39-42 Caroline Islands 41 282 164 42 270-291 150-173 39-45 Marshall Islands 3 282 164 42 270-289 154-174 41-43 Ellice, Phoenix, Danger,
Suvarov Islands27 284 162 41 265-295 152-174 39-44 Christmas Island 13 287 162 43 280-292 152-174 40-46 Marquesas Islands 19 282 163 42 275-291 155-170 40-43 Tuamotu Archipelago 38 287 165 42 277-299 154-173 39-46 Society, Austral, Cook,
Rapa Islands16 290 164 43 280-301 155-173 40-45 Oeno, Henderson, Ducie,
Easter Islands6 293 164 44 285-298 154-175 41-45 Samoa, Fiji, Tonga 19 285 164 42 277-295 153-173 39-44 Kermadecs, Norfolk 23 276 158 41 269-289 148-173 38-43 New Hebrides, Solomons,
New Guinea area31 278 158 41 265-287 150-172 39-44 [Pg 167] Northwest Australia 9 263 145 40 258-267 138-152 38-42 South China Sea area, Strait of Malacca 4 271 153 39 262-278 148-257 37-40 Riu Kius, Japan 5 268 148 39 259-275 143-155 37-40 Indian Ocean area: Seychelles,
Aldebra, Providence, Somaliland20 276 154 41 270-286 146-164 39-42 Geographic range.—Islands in the Indian Ocean east to tropical parts of western and central Pacific. In Micronesia: Mariana Islands—Agrihan, Asuncion, Saipan, Rota, Guam; Palau Islands—Kayangel, Babelthuap, Koror, Urukthapel, Ngabad, Peleliu, Angaur; Caroline Islands—Ulithi, Truk, Wolea, Mortlock, Lukunor, Nukuoro, Ponapé, Kusaie; Marshall Islands—Jaluit, Mille, Aurh, Wotze, Bikini, Kwajalein.
Characters.—Adult: A large, dark-brown tern with grayish crown and whitish forehead; line above eye white; crescent of white on lower eyelid; lores blackish; bill black; feet brownish, iris dark.
Immature: Resembles adult, but lighter and browner and top of head grayish-brown.
A. s. pileatus resembles A. s. ridgwayi, but darker and less brownish, although not so dark as A. s. galapagensis; forehead and crown usually duller; length of wing and tail average larger (282 and 161) than in A. s. ridgwayi (278 and 158) and A. s. galapagensis (277 and 151).
Measurements.—Measurements of the Common Noddy of the Pacific area are listed in table 21.
Weights.—In 1948 (1948:56) I listed the weights of specimens from Guam and Rota as follows: four adult males 187-204 (197); three adult females 177-203 (189).
Specimens examined.—Total number, 92 (43 males, 39 females, 10 unsexed), as follows: Mariana Islands, USNM—Guam, 7 (May 24, June 15, July 6, 21)—Rota, 3 (Oct. 18, 24); AMNH—Guam, 4 (April 21, 27, Aug. 18)—Asuncion, 1 (Jan. 18); Palau Islands, USNM—Peleliu, 2 (Sept. 1)—Ngabad, 1 (Sept. 11); AMNH—exact locality not given, 6 (Nov. 3, 8); Caroline Islands, USNM—Ulithi, 3 (Aug. 15)—Kusaie, 1 (Feb. 8); AMNH—Truk, 15 (Feb. 1, 8, 25, March 10, May 6, June 12, 13, Nov. 25, Dec. 25)—Ponapé, 20 (Dec. 3, 5, 8, 12, 15)—Kusaie, 24 (Jan., March 10-30, April 1-10); Marshall Islands, USNM—Bikini, 5 (Feb. 28, March 2, 19).
Nesting.—Murphy (1936:1152) writes that the Atlantic subspecies, A. s. stolidus, breeds in tropical localities every month of the year, although there may be a part of the resident population away at sea at any given time. In the Pacific area, Kirby (1925:187) found nests "on platforms of sticks built on tufts of grass" at Christmas Island in August. In Micronesia, Coultas obtained young birds at Kusaie in January and April and commented (field notes) that they probably nest "spasmodically at all times of the year." At Ponapé, Coultas observed nests in high trees in December, and birds obtained by him in that month had enlarged gonads. At Bikini, Morrison obtained eggs on March 2 and 19, and young on March 19. At Palau, Coultas took one female tern in postnatal molt on November 8. Adults obtained by him in that month had enlarged gonads. At Ulithi, the NAMRU2 party recorded one nest containing a single egg on August 21. At the same atoll the NAMRU2 party received reports of a large colony of nesting noddys in May to July, 1945. In the following August few noddies were seen by the NAMRU2 party. McElroy found nests on cliffs and in coconut trees at Truk in December, 1945. Hartert (1900:10) reports on eggs taken at Truk in the period from March to July 1. The NAMRU2 party observed birds carrying nest materials at Peleliu on August 28 but failed to find the nests. At Guam, the writer found terns in numbers varying from 4 to 75 in May to July, 1945, along the rocky cliffs but no evidence of nesting activity was obtained. Strophlet (1946:537) reports that nests may have been present on Orote Peninsula at Guam on December 13, 1945. Coultas (field notes) is of the opinion that the birds do not nest at Guam but do nest farther north in the Marianas. Borror (1947:417) found two colonies at Agrihan on August 10, 1945. Thus, there are records of nesting in nine months of the year in Micronesia; although I suspect that the larger flocks of terns have more regular breeding habits correlated with their pelagic feeding activities. "Stragglers" probably nest irregularly.
Food habits.—The author (1948:56) records small fish and crustaceans in stomachs of terns taken at Ulithi and Peleliu. At Ypao Point, Guam, birds were seen to fly back and forth in the day from their roosts on the sea-cliffs. On one occasion I saw these birds feeding approximately a half mile from shore.
Parasites.—Wharton (1946:174) and Wharton and Hardcastle (1946:292, 296, 306) list the following species of chiggers (Acarina) from the Common Noddy from Guam and Ulithi: Neoschöngastia bougainvillensis, N. americana solomonis, N. egretta, Acariscus pluvius, and A. anous. Uchida (1918:484, 488) found the bird louse (Mallophaga), Nirmus separatus, on terms at Palau and at Ponapé he found Colpocephalum milleri on the bird. Bequaert (in litt.) has identified a fly (Hippoboscidae) as Olfersia aenescens from a tern from Rota.
Remarks.—Of the Common Noddy Tern of the Pacific area, three subspecies are recognized by Peters (1934:346-347). Anoüs stolidus ridgwayi is known from islands off the western coast of Mexico and Central America; A. s. galapagensis is recorded from the Galapagos Archipelago; and A. s. pileatus is found on tropical islands throughout the Pacific and west to Madagascar and the African coast in the Indian Ocean. These subspecies differ from one another principally [Pg 169] in color, as noted by Ridgway (1919:545); A. s. galapagensis is the darkest form, A. s. ridgwayi is less blackish and more brownish in color of body, and A. s. pileatus is between the two in coloring. A. s. pileatus averages larger in length of wing and tail, but these measurements do not appear to be significant from a taxonomic standpoint.
As shown in table 21, measurements of length of wing for specimens from throughout most of the Pacific area are almost the same. Length of tail is correspondingly uniform. There is a gradual increase in size of birds in the Tuamotus and Societies and east to Easter Island. In this region the average measurement for length of wing is 293 millimeters. The lengths of wing and tails are shorter in specimens from the Kermadecs and Norfolk Island, which may indicate relationships with the smaller birds of the Australian area, Western Melanesia and possibly Malaysia and the Riu Kiu Islands. I am unable to determine the subspecific status of the birds from the Kermadecs and Norfolk Island, because of the lack of sufficient material from the Australian region and Malaysia. Possibly Mathews' name, A. s. gilberti, is valid for the noddys of Australia and also for the birds at Norfolk and the Kermadecs. The small-sized birds of the Riu Kiu Islands have been designated as A. s. pullus by Bangs. When specimens from the type locality of A. s. pileatus in the Philippine Islands are available, the true relationships of the populations from Micronesia and the other areas in the Pacific can be ascertained.
The tern found in the Hawaiians has the palest body and the most chalky-white forehead of any of the birds of the Pacific. Bryan (1903:101) found terns from Marcus Island to agree with specimens from Guam and to be "slightly darker" than birds from Midway and Laysan in the Hawaiian chain. The birds from the Riu Kius are darker and thus similar to the few specimens seen from Malaysia. Birds from Polynesia and Melanesia possess the most sooty underparts while those from Micronesia are only slightly less pale. This condition also seems to be true for the birds in the Australian area and for specimens seen from islands in the Indian Ocean. With fading, or wear, or both, there is a change from dusky black to dusky brown in the plumage; effort was made by me to compare specimens with relatively similar conditions of plumage. In summary, the systematic position of the Common Noddy Terns of the Pacific seemingly depends on the characteristics of specimens from the type locality in the Philippines. When topotypes are available for study, [Pg 170] they may be found to be nearer the darker forms of Malaysia or may tend toward the paler, oceanic forms. The Hawaiian population probably is distinct.
In Micronesia the Common Noddy Tern is not a conspicuous bird except during its breeding period. Probably it spends most of its life at sea, being unlike Gygis alba in this respect. Large flocks seem less wary of man than are small groups and singles, which are often easily disturbed. Birds of this species appear to prefer the low atolls and offshore islets where both tall vegetation and bare ground are utilized for nesting or roosting. At Ponapé, Coultas (field notes) observed the birds to fly to sea at daybreak and to begin to return to their roosts by 4:00 pm. Wallace (field notes) observed similar activities at Kwajalein in May, 1944, where he saw approximately forty individuals in a flock with Gygis alba.
Anoüs stolidus is divided naturally into an Atlantic subspecies, which is distinguished by its browner color, and into several subspecies which are distinguished by their blacker color in the Pacific and Indian oceans. Whether the genus and species evolved in the Atlantic or in the Pacific region is not known. If it were the Pacific region, the center of differentiation may very well have been the islands of Oceania. There, relatively little variation is observable within populations covering a large area. To the eastward, birds along the American coast are darker or lighter, to the northward, the birds of Hawaii are paler, to the southward and southwestward, the birds are smaller and to the westward, the birds are smaller and darker. The virtual absence of ground-living, predatory animals which might prey on nesting colonies has probably been a reason for the lack of discrimination by this tern in selecting breeding sites. This is probably true of other birds which nest in colonies.
Anous tenuirostris marcusi (Bryan)
White-capped Noddy
Micranous marcusi Bryan, Occ. Papers Bernice P. Bishop Mus., 2, 1903, p. 101. (Type locality, Marcus Island.)
Sterna tenuirostris Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 286, 308 (Ualan, Ouleai); idem, Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 64 (Ualan).
Anous tenuirostris Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 90, 113 (Pelew, Carolines); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 6, 42 (Palau); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 299, 330 (Mortlock, Nukuor); Stott, Auk, 64, 1947, p. 526 (Saipan).
Anous melanogenys Finsch, Proc. Zool. Soc. London, 1877 (1878), p. 781 (Palau); idem, Journ. f. Ornith., 1880, pp. 295, 308 (Ponapé, Kuschai); idem, Ibis, 1880, pp. 219, 220, 332 (Taluit, Arno); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); idem, Ibis, 1881, pp. 107, 109, 115 (Kuschai, Ponape); Salvadori, Ornith. Papuasia, 3, 1882, p. 456 (Pelew, Ponapé, Marshalls); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 52 (Jaluit, Arno, Kuschai); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1901 (1891), p. 77 (Pelew, Ualan, Ponapé, Nukuor, Luganor, Ruk); Hartert, Katalog Vogelsamml. Senckenb., 1891, p. 238 (Ualan); Takatsukasa and Kuroda, Tori, 1, 1915, p. 62 (Ruk); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 50 (Pelew, Ruk, Wolea, Luganor, Nukuor, Ponapé, Kusaie, Marshalls).
Anous leucocapillus Finsch, Proc. Zool. Soc. London, 1877 (1878), p. 781 (Ponapé); Nehrkorn, Journ. f. Ornith., 1879, p. 410 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 281 (Ponapé); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 52 (Jaluit); Tristram, Cat. Coll. Birds, 1889, p. 10 (Pelew); Salvadori, Ornith. Papuasia, 3, 1882, p. 457 (Pelew); Wiglesworth, Abhandl. und Ber Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 77 (Pelew); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 60 (Saypan, Palaos, Ruk, Luganor, Nukuor, Ponapé, Kuschai, Bonham); Hartert, Novit. Zool., 5, 1898, p. 68 (Marianne); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 20 (Saipan?); Safford, Osprey, 1902, p. 66 (Marianas); idem, The Plant World, 7, 1904, p. 267 (Guam); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall-Inseln); Safford, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Cox, Island of Guam, 1917, p. 22 (Guam).
Micranous leucocapillus Saunders, Cat. Birds British Mus., 25, 1896, p. 145 (Pelew, Caroline Islands); Nehrkorn, Kat. Eiers., 1899, p. 222 (Kusai); Hartert, Novit. Zool., 7, 1900, p. 9 (Ruk); Takatsukasa and Kuroda, Tori, 1, 1915, p. 51 (Pelew).
Megalopterus minutus marcusi Mathews, Birds Australia, 2, 1912, p. 423 (Marianas?); Ridgway, Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 553 (Mariannes?); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 146 (Mariannes); Hachisuka, Birds Philippines, 2, 1932, p. 343 (Mariannes).
Megalopterus tenuirostris leucocapillus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 50 (Saipan, Pelew, Ruk, Ponapé, Kusaie).
Megalopterus minutus minutus Fisher and Wetmore, Proc. U. S. Nat. Mus., 79, 1931, p. 45 (Caroline Islands).
Anous minutus worcesteri Yamashina, Tori, 7, 1932, p. 409 (Coror, Namo, Iringlab); Hand-list Japanese Birds, rev., 1932, p. 195 (Saipan, Babelthuap, Koror, Truk, Ponapé, Kusaie, Ebon, Namorik, Jaluit, Elmore, Mille, Aurh, Wotze, Ailuk); Yamashina, Tori, 10, 1940, p. 678 (Assongsong, Saipan); Hand-list Japanese Birds, 3d ed., 1942, p. 219 (Assongsong, Saipan, Babelthuap, Koror, Peliliu, Truk, Ponapé, Kusaie, Ebon, Namorik, Jaluit, Elmore, Mille, Aurh, Wotze, Ailuk).
Anous minutus marcusi Peters, Check-list Birds World, 2, 1934, p. 347 (Caroline Islands).
Anous minutus Bequaert, Mushi, 12, 1939, p. 82 (Ponapé); idem, Occ. Papers Bernice P. Bishop Mus., 16, 1941, p. 253 (Ponapé, Palau).
Anous tenuirostris marcusi Mayr, Birds Southwest Pacific, 1945, p. 27 (Micronesia); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 56 (Peleliu, Ulithi, Truk).
Geographic range.—Marcus, Wake, and Micronesia. In Micronesia: Mariana Islands—Asuncion, Saipan, Guam?; Palau Islands—Babelthuap, Koror, Peleliu; Caroline Islands—Ulithi, Truk, Ponapé, Luganor, Nukuor, Wolea; Marshall Islands—Ebon, Namorik, Jaluit, Elmore, Mille, Aurh, Wotze, Ailuk.
Characters.—Adult: A small tern with sooty-black plumage, grayer on rump and tail; forehead and crown white becoming grayer on nape to merge with blackish on shoulder; narrow, black superciliary stripe; lores black, lower eyelid with white streak, upper eyelid with white spot. Resembles A. t. melanogenys but wing and tail longer and superciliary stripe narrower. Resembles A. t. minutus but with narrower, black superciliary stripe.
abruptly at nape, with mottling in some birds; plumage of body with brownish wash.
Measurements.—Measurements are listed in table 22.
Specimens examined.—Total number, 51 (27 males, 22 females, 2 unsexed), as follows: Mariana Islands, AMNH—Asuncion, 1 (Jan. 18); Palau Islands, USNM—Peleliu, 2 (Sept. 9, 12); AMNH—exact locality not given, 2 (Nov. 3); Caroline Islands, USNM—Ulithi, 4 (Aug. 20); AMNH—Truk, 5 (Nov. 16, 21, 22)—Ponapé, 15 (Dec. 15)—Kusaie, 17 (Jan. 10, March 10-30, April 1-10); Marshall Islands, USNM—Bikini, 4 (May 2, 14); AMNH—no locality given, 1 (Sept. 3).
Location No. Wing Tail Exposed
culmenAnoüs tenuirostris melanogenys
Hawaiian Islands29 222 113 41 210-229 105-120 41-48 Anoüs tenuirostris marcusi
Hawaiian Islands8 227 118 45 218-231 112-124 44-48 Mariana Islands 1 223 117 44 Palau Islands 3 228 122 43 227-228 117-126 41-45 Caroline Islands 32 229 120 44 220-240 113-127 40-47 Marshall Islands 5 224 118 44 222-229 114-123 41-46 Anoüs tenuirostris minutus
Hawaiian Islands13 227 120 44 220-234 108-128 41-46 Phoenix, Howland, Union, Danger,
Suvarov Islands9 229 119 46 226-233 113-124 42-48 Anoüs tenuirostris minutus
Marquesas Islands10 226 117 45 220-233 115-124 42-48 Tuamotu Archipelago 17 229 118 45 222-234 112-126 42-47 Society, Cook, Austral Islands 12 230 118 46 223-238 114-120 43-47 Samoa, Fiji, Tonga Islands 6 228 118 44 224-231 115-121 42-47 Kermadec, Norfolk Isl'ds,
New Zealand15 226 116 44 219-235 112-121 42-47 New Hebrides, Solomon, Bismarck,
Admiralty Islands, New Guinea34 229 117 43 222-237 109-130 40-46 Anoüs tenuirostris diamesus
Marquesas Islands14 230 120 44 224-237 114-127 41-47 Nesting.—Few reports have been obtained concerning the nesting of the White-capped Noddy in Micronesia. Finsch (1881b:107) recorded nests, and Nehrkorn (1899:222) reported on eggs taken at Kusaie. Yamashina (1932a:409) recorded the taking of eggs at Koror in the Palau Islands on January 19 and November 10 and in the Marshalls at Namo on October 19, and at Iringlab on October 21. No evidence of nestings was obtained by the NAMRU2 party in 1945, although a number of birds were seen at Ulithi in August. Coultas (field notes) writes that a colony of approximately 20 birds began nesting about Christmas time on a small offshore island near Ponapé. Nests were placed in the crotches of limbs of mangroves, 8 to 15 feet above the ground.
Food habits.—The NAMRU2 party found small fish in the stomachs of terns taken at Ulithi and Peleliu.
Parasites.—Bequaert (1939:82 and 1941:253) records the fly (Hippoboscidae), Alfersia aenescens, from the White-capped Noddy taken at Ponapé and Palau.
Remarks.—The subspecies of Anoüs tenuirostris are well differentiated by color and to a lesser extent by measurements. Table 22 lists measurements which show that the Hawaiian subspecies, A. t. melanogenys, has the shortest wing and the shortest tail whereas the subspecies from Cocos and Clipperton islands, A. t. diamesus, has the longest wing and the longest tail. The exposed culmen varies in length but little among the four subspecies. The systematic position of A. t. worcesteri from Cavilli Island in the Sula Sea has not been determined because of lack of material. In the third edition of the Hand-list of Japanese Birds (Hachisuka et al., 1942:219) the birds from Micronesia are referred to A. t. worcesteri as they are also in other recent publications by the Japanese. Specimens from the Philippines are needed for examination to determine satisfactorily the subspecies status of the birds under consideration.
Field observations indicate that the White-capped Noddy is not abundant in the Mariana Islands. According to Oustalet (1896:60), Marche obtained a female at Saipan in June, 1888, and Yamashina (1940:678) records five adults from Assongsong (Asuncion). Owston's collectors obtained a specimen at Asuncion on January 18, 1904. In the Palaus, Carolines, and Marshalls birds of this species are numerous and have been observed or collected at many of the islands. Coultas with the Whitney South Sea Expedition obtained specimens at Kusaie, Ponapé and Palau. He found them along the shores of the large islands and, especially, on the smaller offshore islets. At Ulithi Atoll in August, 1945, the NAMRU2 party observed small flocks of four to ten individuals flying offshore and feeding inside the reef. They were frequently observed in company [Pg 174] with Sterna sumatrana. Fewer birds were seen in September, 1945, at the Palau Islands by the NAMRU2 party.
Gygis alba candida (Gmelin)
White Tern
Sterna candida Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 607. (Type locality, Christmas Island.)
Gygis candida Finsch, Ibis, 1880, p. 220 (Taluit); Saunders (part), Cat. Birds British Mus., 25, 1896, p. 149 (Marshalls); Schnee, Zool. Jahrbücher, 20, 1904, p. 390 (Marschall-Inseln).
Gygis alba Finsch, Ibis, 1880, pp. 330, 332 (Taluit); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 78 (Marshalls); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 58 (Saypan, Pagan, Agrigan, Marshalls); Safford, Guam, 1912, p. 19 (Guam); Strophlet, Auk, 63, 1946, p. 537 (Guam); Baker, Condor, 49, 1947, p. 125 (Guam); Stott, Auk, 64, 1947, p. 525 (Saipan); Baker (part), Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 57 (Guam, Rota, Saipan).
Gygis alba kittlitzi Hartert, Novit. Zool., 5, 1898, p. 67 (Saipan, Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 21 (Guam); Safford, Osprey, 1902, 66 (Marianas); idem, The Plant World, 7, 1904, p. 267 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 80 (Guam); Mathews (part), Birds Australia, 2, 1912, p. 443 (Marianas); Prowazek, Die deutschen Marianen, 1913, p. 100 (Marianan); Cox, Island of Guam, 1917, p. 22 (Guam); Ridgway (part), Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 559 (Mariannes); Kuroda, Avifauna Riu Kiu, 1925, p. 193 (?Mariannes); Yamashina, Tori, 7, 1931, p. 410 (Saipan); Yamashina, Tori, 7, 1932, p. 409 (Iringlab, Namo, Aruno); Hand-list Japanese Birds (part), rev., 1932, p. 196 (Guam, Tinian, Saipan, Pagan, Agrigan, Jaluit, Mille, Aurh, Wotze, Likieb, Mejit); Yamashina (part), Tori, 10, 1940, p. 678 (Assongsong).
Gygys alba Wheeler, Report Island of Guam, 1900, p. 13 (Guam).
Gygis albus kittlitzi Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 50 (Guam, Saipan, Pagan, Agrigan, Marshalls).
Leucanous albus kittlitzi Mathews (part), Syst. Avium Australasianarum, 1, 1927, p. 143 (Marianne).
Gygis alba microrhyncha La Touche (part), Handbook Birds Eastern China, 2, 1933, p. 335 (Marianne).
Gygis alba candida Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam); Hand-list Japanese Birds (part), 3d ed., 1942, p. 219 (Guam, Tinian, Saipan, Pagan, Agrigan, Assongsong, Jaluit, Mille, Aurh, Wotze, Likieb, Mejit); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 94 (Tinian); Borror, Auk, 64, 1947, p. 417 (Agrihan).
Geographic range.—Northern Pacific from Bonins and Marianas east to Wake and Hawaiian Chain, south to Marshall, Phoenix, Christmas and Fanning islands (see figure 12). In Micronesia: Mariana Islands—Guam, Rota, Tinian, Saipan, Pagan, Agrihan; Marshall Islands—Jaluit, Mille, Aurh, Wotze, Likieb, Mejit, Eniwetok, Bikini, Kwajalein.
Characters.—Adult: A small tern with ivory-white plumage except for black, narrow, orbital ring; shafts of primary quills dark brown; shafts of tail feathers blackish; bill black with bluish base; tarsus dark bluish with yellowish webs; iris and skin black.
Immature: Resembles adult, but with light brown mottlings on upper parts, especially on the mantle; feathers softer, bill shorter.
Measurements.—Measurements are listed on table 23.
Weights.—The NAMRU2 party obtained weights of 11 adult males from Guam and Rota as 110 (97-124); weights of 6 adult females from Guam as 108 (100-116). These specimens were taken from May to October, 1945.
Specimens examined.—Total number, 41 (23 males, 14 females, 4 unsexed), as follows: Mariana Islands, USNM—Guam, 20 (May 24, 29, June 6, 8, 14, 15, 16, 18, 23, July 10, 19, 20)—Rota, 2 (Oct. 19, 27)—Saipan, 1 (Sept. 26); AMNH—Guam, 4 (March 7, 9, 20)—Tinian, 1 (Sept. 8)—Asuncion, 4 (Jan. 1, 18, 25); MCZ—Saipan, 3 (Jan. 7, March 20, April 17); Marshall Islands, USNM—Bikini, 6 (Feb. 27, March 2, 16, 19).
>Nesting.—Gygis alba does not construct a nest but places its single egg rather precariously in the crotch of a branch in a tree (or on rock). In Micronesia nesting activities have been observed at various times of the year. Yamashina (1932a:409, 410) reported on eggs taken in the Marianas at Saipan on February 2 and in the Marshalls at Arhno on September 26, at Iringlab on October 21 and at Namo on October 19. At Guam a pair of White Terns was seen in a large tree on March 27, 1945, by the NAMRU2 observers. Because of their behavior, it was suspected that they had an egg or young in the tree. Further inspection revealed, on March 31, a downy young sitting in the tree. The young bird was attended by the parents until it began to fly on April 17. Hartert (1898:68) reports that eggs of the White Tern were taken at Saipan on July 28 and August 11. Morrison obtained a male nestling on March 16 and eggs on March 22 at Bikini in 1946.
Remarks.—The White Tern is usually restricted to the remote islands in the Pacific, Indian and South Atlantic oceans; there, according to the latest treatment, which is that of Peters (1934:348, 349), six subspecies are recognized. In studying the geographical variation of the species, the writer has examined 595 adult specimens, including previously unstudied material collected by the Whitney South Sea Expedition, which is deposited in the American Museum of Natural History.
This ivory-white species presents an unusual problem in that there are few characters available to distinguish the subspecies. Measurements of taxonomic value include those of the wing, tail, exposed culmen, and depth and the shape of the culmen. There appears to be no significant secondary sexual difference between males and females, and measurements of the two sexes are combined. The chief problem within this species seems to hinge on how to classify isolated, but relatively similar, populations. The examination of the large series of specimens from the Whitney collections has yielded more complete information to assist in the solution of this problem.
Gygis alba alba (Sparrman) of the South Atlantic Ocean (Fernando de Noronha, South Trinidad, Ascension, and St. Helena islands) and G. a. monte Mathews of the Indian Ocean (Seychelles, Aldabra, Mascarene and Chagos islands) are isolated populations. Specimens examined are those which have previously been studied by other workers; measurements are shown in table 23.
With the exception of G. a. microrhyncha, G. a. monte has the smallest average length of wing of all of the subspecies of G. alba. [Pg 176] In G. a. alba the length of wing as well as most of the other measurements differ but slightly from those of some of the populations in the Pacific area although the slender bill of the Atlantic bird is a distinctive character, as pointed out by Murphy (1936:1166).
Subspecies No. Wing Longest
tail
featherShortest
tail
featherExposed
culmenDepth
culmenTarsus Gygis alba alba 24 246 99 71 40 8.0 14.5 239-256 93-111 68-77 35-44 7.5-9.0 13.0-16.5 Gygis alba monte 35 232 106 71 39 8.5 13.5 224-244 98-116 64-81 37-44 8.0-8.5 12.5-14.0 The taxonomic position of the White Terns of the Pacific area has been one of uncertainty for a long time; as Peters (1934:349) puts it, "It is obvious that the last word on the Pacific races of Gygis has not yet been said." A principal feature of the problem in this region is the presence in the Marquesas of a well-marked subspecies, G. a. microrhyncha, virtually surrounded by a wide-ranging and relatively undifferentiated form, G. a. pacifica (Lesson) (see figure 12). The small cormorant (Phalacrocorax melanoleucus brevicauda Mayr) from Rennell Island, Solomons, is another example of a distinct form surrounded by a widely distributed subspecies.
In all, 55 adult specimens of G. a. microrhyncha have been examined from the following islands in the Marquesas Group: Mukahiva, Eiau, Motane, Hivaoa, Uapu, Tahuata, Uahuka, Fatuhiva. The measurements are listed in table 24, and show that the White Tern in the Marquesas is a much smaller bird than the other subspecies and has a shorter bill, wing, and tail. The tail possesses a shallow fork as compared with the deeper fork of the tail of other subspecies. In addition, the depth of the culmen averages two millimeters less in the subspecies in the Marquesas. The presence of a wider, black eye-ring is also a distinguishing character in this subspecies.
Gygis a. microryhncha was for a long time treated as a species distinct from G. alba but has recently been considered as a subspecies G. alba by Peters and others. On the islands of Hatutu and Motane in the Marquesas, the Whitney South Sea Expedition obtained some birds which appear to be intergrades between the two [Pg 177] subspecies of White Terns in the area. The measurements of nine birds which show intergradation between G. a. microrhyncha and G. a. pacifica are listed in table 24. Probably the Marquesas population is tending toward complete reproductive isolation.
Fig. 12. Geographic distribution of Gygis alba in the Pacific area. (1) G. a. candida; (2) G. a. pacifica; (3) G. a. microrhyncha; (4) G. a. royana.
Peters (1934:348, 349) recognizes three other subspecies from the Pacific area: G. a. rothschildi Hartert from Laysan, Lisiansky, and Krusenstern islands; G. a. candida (Gmelin) from "the Carolines east to Christmas Island and south to the Tonga and Society Islands"; and G. a. royana Mathews from Norfolk and the Kermadec Islands. Birds from Revilla Gigedo, Cocos and Clipperton islands, although geographically isolated, are placed in G. a. candida. On the basis of a critical study of specimens at hand, the populations in the Pacific fit into three groups. Small birds, G. a. candida, are found in the North Pacific from the Bonins and Marianas east to Wake and the Hawaiian Chain and south to the Marshall, Phoenix, Christmas and the Fanning islands (see figure 12). Larger birds, G. a. pacifica, are found in the Central Pacific and South Pacific from the Carolines in the west southeastward through Melanesia and eastward through Samoa, to the Tuamotus and Easter to Cocos, Clipperton, and Revilla Gigedo islands. In the Southwest Pacific, at Norfolk and the Kermadec Islands, a longer-winged population occurs; it is separable as G. a. royana. The measurements of these birds are given in table 24.
Location No. Wing Longest
tail
featherShortest
tail
featherExposed
culmenDepth
culmenTarsus Gygis alba candida (Gmelin)
Japan, Bonins4 238 109 65 36 34-38 Mariana Islands 35 237 111 69 38 9.0 13.0 227-246 98-120 61-75 36-41 12.0-14.0 Wake Islands 10 236 109 69 38 13.0 232-243 101-118 64-77 37-41 13.0-14.0 Hawaiian Islands 36 235 109 68 37 8.5 13.0 220-246 102-118 64-74 33-40 8.0-9.0 12.0-14.0 Marshall Islands 4 234 111 71 39 231-238 107-115 70-73 38-40 Phoenix, Howland,
Hull, Canton Islands8 238 107 70 39 8.5 14.0 237-240 101-116 64-76 37-41 Fanning, Washington,
Christmas Islands19 238 107 68 38 8.0 13.5 227-242 97-119 65-72 37-42 7.5-9.0 12.0-15.0 Totals 116 236 109 69 38 8.5 13.0 220-246 107-120 61-77 33-42 7.5-9.0 12.0-15.0 Gygis alba pacifica (Lesson)
Caroline, Palau Islands33 245 116 73 42 8.5 13.5 236-253 112-125 67-76 38-44 13.0-13.5 Bismarck Arch.,
Solomon Islands12 247 116 74 42 242-256 105-129 68-78 39-45 Samoa, Wallis, Fiji,
Tonga, Niue Islands20 247 115 71 42 239-254 110-127 67-78 39-44 Line, Danger Islands 13 245 115 73 41 238-252 107-118 69-78 39-42 Cook, Austral Islands 29 247 114 73 42 241-255 104-124 65-78 40-45 Society Islands 37 249 113 71 42 8.5 13.5 241-257 107-126 62-76 40-45 8.0-9.0 12.0-14.0 Tuamotu Arch 118 245 114 72 42 236-252 107-127 62-82 38-46 Rapa, Bass Rocks, Oeno,
Henderson, Ducie, Pitcairn,
Easter Islands54 247 113 73 41 240-255 106-126 63-84 40-45 Clipperton, Cocos Islands 10 245 115 72 40 8.5 13.5 240-253 110-120 71-73 38-43 8.5-9.5 13.0-14.0 Totals 326 246 114 72 42 8.5 13.5 236-257 104-129 62-84 38-46 8.0-9.5 12.0-14.0 [Pg 179] Intergrades between
G. a. microrhyncha
and G. a. pacifica9 237 105 74 38 7.5 13.0 230-247 93-122 67-89 36-41 7.0-8.0 12.0-14.0 Gygis alba microrhyncha 55 218 78 64 36 6.5 12.0 211-235 72-96 60-75 32-39 6.0-8.0 11.0-12.5 Gygis alba royana Mathews
Norfolk Island16 250 113 73 42 242-257 105-124 68-79 41-44 Kermadec Islands 12 251 115 75 43 244-255 110-121 71-81 40-46 Totals 28 250 114 74 42 242-257 105-124 68-81 40-46 The measurements indicate that there is a gradient in size from small in the north to large in the south; however, there is a definite separation in average measurements—ten millimeters in length of wing and four millimeters in length of exposed culmen—between the two populations which are designated as G. a. candida and G. a. pacifica. In studying material from Micronesia and the Hawaiian Islands, I (1948:57) pointed out the similarities between birds of the Marianas and the Hawaiians and separated these from terns found in the Caroline Islands. The systematic position of the White Tern in the Gilbert and Ellice islands will remain in doubt until specimens are available for examination.
G. a. royana is provisionally retained as the name for the Fairy Tern of the Kermadecs and Norfolk Island; there is considerable overlap in measurements between G. a. royana and G. a. pacifica. Measurements have given evidence of the degrees of structural resemblance of the White Terns of the different islands, but it is not certain that the groupings made on this basis are natural; more data is needed on ecology and life history. Of particular importance is to learn whether these birds fly regularly from island to island. On the basis of eleven months of rather continuous observation in Micronesia, I suspect that the White Tern has little tendency to make inter-island migrations. This might account for the differences in size in the populations at Guam in the Marianas (G. a. candida) and at Ulithi in the Carolines (G. a. pacifica) where only approximately 400 miles of open water separate the two islands. The occurrence of the distinct G. a. microrhyncha in the Marquesas may be accounted for by such nonmigratory behavior. Mayr [Pg 180] (1945a:27), however, is of the opinion that White Terns found in the Bismarck Archipelago, the Solomons, Santa Cruz and New Hebrides islands may not breed there, which is another way of saying that they are migrants. Swarth (1934:221) and Murphy (1936:1268) record the wandering of the White Tern to the Galapagos Islands, probably from breeding grounds at Cocos Island. Swarth suggests that the tern is not established at the Galapagos because of the presence of colder water in the area. Murphy (1936:1166) is of the opinion that the South Atlantic White Terns are sedentary, but reports evidence of pelagic migration in the Pacific at the Kermadecs. The fact that G. alba is restricted in its distribution to widely separated groups of islands in tropical and subtropical areas of the South Atlantic, Indian and Pacific oceans may indicate that the birds at one time had a more extensive range than at present, probably including even coastal regions of the continents and large continental islands.
Gygis alba pacifica (Lesson)
White Tern
Sterna pacifica Lesson, Ann. Sci. Nat., 4, 1825, p. 101. (Type locality, Society Islands, Paumotu Islands, and Bora Bora.)
Sterna alba Kittlitz, Kupfertaf. Naturgesch. Vögel, 3, 1833, p. 28 (Carolinen); idem, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 286, 299, 308 (Ualan, Lougounor, Ouleai).
Gygis candida Hartlaub, Archiv f. Naturgesch., 18, 1852, p. 137 (Carolinen); Hartlaub, Journ. f. Ornith., 1854, p. 168 (Carolinen); Kittlitz, Denkw. Reise russ. Amer. Micron. und Kamchat., 1, 1858, p. 382, 2, 1858, pp. 39, 60 (Ualan); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 59 (Caroline Islands); Saunders (part), Cat. Birds British Mus., 25, 1896, p. 149 (Pelew, Carolines); Takatsukasa and Kuroda, Tori, 1, 1915, p. 51 (Ruk, Pelew).
Gygis alba Finsch and Hartlaub, Fauna Centralpolynesiens, 1867, p. 233 (Carolinen); Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 832 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 9, 118 (Pelew); Finsch and Hartlaub, Journ. f. Ornith., 1870, p. 140 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 90, 114 (Pelew, Uap, Ualan); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 6, 43 (Palau); idem, Journ. Mus. Godeffroy, 12, 1876, pp. 18, 40 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 782 (Ponapé); idem, Journ. f. Ornith., 1880, pp. 295, 309 (Ponapé, Kuschai); idem, Proc. Zool. Soc. London, 1880, p. 577 (Ruk); idem, Ibis, 1881, pp. 105, 106, 109, 115, 246, 247 (Kushai, Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 299, 330, 353 (Mortlock, Nukuor, Ruk); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 52 (Kuschai); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 78 (Pelew, Uap, Luganor, Nukuor, Ruk, Ponapé, Ualan); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 8, 1896, p. 58 (Palaos, Carolines); Baker (part), Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 57 (Peleliu, Ulithi, Truk, Kusaie).
Gygis alba kittlitzi Hartert, Katalog Vogelsamml. Senckenb., 1891, p. 237 (Type locality, Ulea = Wolea); idem, Novit. Zool., 7, 1900, p. 10 (Ruk); Dubois, Syn. Avium, 2, 1904, p. 1020 (Carolines); Mathews (part), Birds Australia, 2, 1912, p. 443 (Carolines); Ridgway (part), Bull. U. S. Nat. Mus., 50, pt. 8, 1919, p. 559 (Carolines); Kuroda (part), Avifauna Riu Kiu, 1925, p. 193 (Carolines); Hand-list Japanese Birds (part), rev., 1932, p. 196 (Pelew, Yap, Wolea, Luganor, Ruk, Ponapé, Kusaie); Yamashina (part), Tori, 10, 1940, p. 678 (Babelthuap).
Gygis albus kittlitzi Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 50 (Pelews, Yap, Wolea, Luganor, Nukuor, Ruk, Ponapé, Kusaie).
Leucanous albus kittlitzi Mathews (part), Syst. Avium Australasianarum, 1, 1927, p. 143 (Carolines).
Gygis alba candida Peters, Check-list Birds World, 2, 1934, p. 349 (Carolines); Hand-list Japanese Birds (part), 3d ed., 1942, p. 219 (Babelthuap, Koror, Angaur, Yap, Wolea, Truk, Lukunor, Nukuoro, Ponapé, Kusaie).
Geographic range.—Central and southern Pacific from Carolines southeast through Melanesia and east through Samoa to Tuamotus, Easter to Cocos and Clipperton (see figure 12). In Micronesia: Palau Islands—Angaur, Peleliu, Garakayo, Koror, Babelthuap, Kayangel; Caroline Islands—Yap, Ulithi, Wolea, Truk, Lukunor, Ponapé, Kusaie.
Characters.—Resembles G. a. candida, but size larger, wing length of adult males and females 236-253 (245); length of exposed culmen 38-44 (42).
Measurements.—Measurements are listed in table 24.
Specimens examined.—Total number, 36 (22 males, 12 females, 2 unsexed), as follows: Palau Islands, USNM—Peleliu, 1 (Sept. 1); AMNH—exact locality not given, 1 (Nov. 13);-Caroline Islands, USNM—Ulithi, 12 (Aug. 14, 15, 16, 20, 21)—Truk, 1 (Dec. 13); AMNH—Truk, 7 (Mar. 8, May 7, June 8, Nov. 11, 26)—Ponapé, 1 (undated)—Kusaie, 10 (Jan., Feb., March 20-30, April 1-10); MCZ—Yap, 3 (Jan. 13).
Nesting.—The NAMRU2 party learned that in May and June, 1945, several young White Terns were seen at Asor, Ulithi Atoll, by service personnel. These young were observed in breadfruit trees within a recreational area; the presence of the service personnel seemingly had little disturbing effect on the terns. At Bulubul, another island of this atoll, a downy young was obtained on August 22. Hartert (1900:10) reports that eggs of the White Tern were found on the ground and in forks of branches of trees at Truk in June.
Food Habits.—The author (1948:58) reports that stomachs of birds taken at Ulithi and Peleliu contained fish, insects and marine crustaceans. Probably the birds feed to a large extent along the edge of the tidal reef. They almost certainly obtain food also on the islands as indicated by the presence of insects in stomach contents; this is not surprising since the birds frequent woodland habitats.
Remarks.—Gygis alba is one of the most characteristic birds in Micronesia. It is seemingly more numerous at the coral atolls than at the high, volcanic islands. At the latter islands the birds prefer the coastal coconut grove environment. At Pau and Bulubul, two small islands in the Ulithi Atoll, the writer counted approximately 100 birds on August 21, 1945. Kittlitz was the first to publish an account of these birds in the Caroline Islands. Tetens, Peters, Semper and Kubary reported their presence in the Palaus. No doubt, these terns attract the attention of every traveler in the islands owing to their conspicuously white beauty and their seemingly friendly behavior toward man. Their habit of hovering in small flocks close over the head of the observer is indeed spectacular.
Columba livia Gmelin
Blue Rock Pigeon
Columba domestica [Greek: b] livia Gmelin, Syst. Nat., 1, pt. 2, 1789, p. 769. (No type locality = Europe.)
Columba livia Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam); Marshall, Condor, vol. 51, 1949, p. 221 (Tinian).
Geographic range.—Europe and Asia Minor. Introduced to many parts of the world. In Micronesia: Mariana Islands—Guam, Tinian.
Remarks.—In 1945, the NAMRU2 party observed pigeons about the towns on Guam, particularly at the town of Inarajan. Bryan (1936:24) writes that the birds were introduced by the United States Navy and Marine Corps at Guam; the stock originating from escaped carrier pigeons. Marshall (1949:221) records this bird from Tinian.
Ptilinopus porphyraceus ponapensis (Finsch)
Crimson-crowned Fruit Dove
Ptilinopus ponapensis Finsch, Proc. Zool. Soc. London, 1877 (1878), p. 779. (Type locality, Ponapé.)
Ptilinopus? fasciatus Finsch, Journ. Mus. Godeffroy, 12, 1876, pp. 18, 37 (Ponapé).
Ptilopus fasciatus Elliot, Proc. Zool. Soc. London, 1878, p. 536 (Ponapé); Tristram, Cat. Birds, 1889, p. 44 (Ponapé).
Ptilopus ponapensis Schmeltz, Verhandl. Ver. nat. Unterhaltung Hamburg, 1877 (1879), pp. 178, 179 (Ponapé); Finsch, Proc. Zool. Soc. London, 1880, p. 576 (Ruk, Ponapé); idem, Journ. f. Ornith., 1880, pp. 291, 303 (Ponapé); idem, Proc. Zool. Soc. London, 1880, p. 578 (Ruk, Ponapé); idem, Ibis, 1881, pp. 113, 115 (Ponapé); Wiglesworth, Ibis, 1891, p. 583 (Ponapé, Ruk); idem, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 50 (Ponapé, Ruk); Salvadori, Cat. Birds British Mus., 21, 1893, p. 93 (Ponapé, Ruk); Oustalet, Nouv. Arch. Mus. Hist. Nat., Paris, (3), 7, 1895, p. 222 (Ponapé); Nehrkron, Kat. Eiers., 1899, p. 180 (Ruk); Dubois, Syn. Avium, 2, 1904, p. 736 (Ruck, Ponapé); Reichenow, Die Vögel, 1, 1913, p. 354 (Ruk, Ponapé); Takatsukasa and Kuroda, Tori, 1, 1915, p. 52 (Ruk, Ponapé); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 189 (Uala, Ponapé).
Ptilinopus ponapensis Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 353 (Ruk); Hartert, Novit. Zool., 7, 1900, p. 7 (Ruk, Ponapé); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 42 (Ponapé); Matschie, Journ. f. Ornith., 1901, p. 113 (Ruck, Ponapé); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 32 (Ponapé); Bequaert, Mushi, 12, 1939, pp. 81, 82 (Ponapé); Mayr. Proc. 6th Pacific Sci. Congr., 4, 1939 (1941), p. 204 (Ponapé); Bequaert, Occ. Papers Bernice P. Bishop Mus., 16, 1941, pp. 266, 290 (Ponapé).
Ptilinopus Ponapensis Christian, The Caroline Islands, 1899, p. 357 (Ponapé).
Ptilinopus ponepensis ponapensis Kuroda, in Momiyama, Birds Micronesia, 1922, p. 57 (Ponapé, Ruk); Yamashina, Tori, 7, 1932, p. 408 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 190 (Ponapé, Ruk); Peters, Check-list Birds World, 3, 1937, p. 31 (Ruk, Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 213 (Ponapé, Truk).
Ptilinopus porphyraceus ponapensis Ripley and Birckhead, Amer. Mus. Novit., no. 1192, 1942, p. 7 (Ruk, Ponapé); Mayr, Birds Southwest Pacific, 1945, p. 289 (Truk, Ponapé); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 59 (Truk).
Geographic range.—Micronesia: Caroline Islands—Truk, Ponapé. Characters.—Adult male: A green fruit dove with forehead, anterior lores and crown near "pansy purple," faintly margined with yellow; occiput, sides of head, neck, upper breast grayish-green with bifid feathers of midbreast more olivaceous; chin and midthroat light yellow; breast, sides and tibia green; midpart of lower breast dark bluish-green, tinged with dark purple; lower abdomen, vent, and undertail yellow, under tail-coverts deeper yellow tinged with orange; upper parts dark green; wings metallic green on outer webs and tips, inner secondaries and some posterior scapulars with purple spots near tips; primaries and secondaries edged on outer webs with yellowish; underwing gray with yellow edges on hind, under wing-coverts; upper side of tail metallic green with terminal, broad yellow band; under side of tail gray; bill lead-colored, feet wine-brown, iris whitish to pale brown. Adult female resembles adult male, but slightly smaller and duller.
Immature: Resembles adult, but entirely green with yellow edgings on feathers and lacking crimson crown and colored breast patch.
Measurements.—Measurements of subspecies of P. porphyraceus in Micronesia are presented in table 25.
Subspecies Number Wing Exposed
culmenTarsus P. p. ponapensis 12 males
137 (133-141) 14 (13-15) 25 (24-27) 11 females
133 (126-137) 14 (13-15) 25 (24-26) P. p. hernsheimi 6 males
134 (130-138) 13 (12-14) 25 (24-26) 5 females
127 (125-130) 13 (12-13) 25 (24-25) P. p. pelewensis 10 males
133 (131-134) 15 (13-15) 25 (23-26) 4 females
133 (130-138) 15 (14-15) 24 (23-24) Specimens examined.—Total number, 81 (52 males, 26 females, 3 unsexed), as follows: Caroline Islands, USNM—Truk, 4 (Feb. 16, Dec. 24); AMNH—Truk, 24 (Jan., June, Oct.)—Ponapé, 53 (Nov., Dec).
Nesting.—Yamashina (1932a:408) reports on eggs taken at Ponapé on the following dates: July 10, 12, August 1, 12, 15, 21. Only one egg was found to a nest. Hartert (1900:8) records nests containing eggs in May and June at Truk. Coultas (field notes) describes the nest as a flimsy affair. At Ponapé in November and December he found nests on low branches (10 to 20 feet from the ground) each containing a single egg. Nests were found also in the tops of tree ferns. Females taken in these months had enlarged gonads.
Parasites.—Bequaert (1939:81, 82, and 1941:266, 290) records the two flies (Hippoboscidae), Ornithoctona plicata and O. pusilla, from the fruit dove at Ponapé.
Remarks.—McElroy of the NAMRU2 party found the birds in mountainous areas at Truk in December, 1945. At Ponapé in November and December, 1931, Coultas (field notes) comments that the bird is rapidly disappearing owing to persistent hunting by the natives and, at that time, by the Japanese. He found the birds to be strictly forest-living and to frequent the larger fruit-bearing trees of [Pg 184] the lowlands and the mountain sides. Coultas writes that the Japanese hunters attracted the doves by the use of calls. The natives catch the birds with a gum mixture obtained from bread-fruit gum and coconut oil.
Ptilinopus porphyraceus hernsheimi (Finsch)
Crimson-crowned Fruit Dove
Ptilopus Hernsheimi Finsch., Journ. f. Ornith., 1880, p. 303. (Type locality, Kuschai.)
Ptilopus hernsheimi Finsch, Proc. Zool. Soc. London, 1880, p. 577 (Kuschai); Reichenow and Schalow, Journ. f. Ornith., 1881, p. 75 (Kuschai); Finsch, Ibis, 1881, pp. 106, 107, 108 (Kushai); Wiglesworth, Ibis, 1891, p. 583 (Ualan); idem, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 51 (Ualan); Salvadori, Cat. Birds British Mus., 21, 1893, p. 94 (Ualan); Oustalet, Nouv. Arch. Mus. Hist. Nat., Paris, (3), 7, 1895, p. 222 (Oualan); Dubois, Syn. Avium, 2, 1904, p. 736 (Kuschai); Reichenow, Die Vögel, 1, 1913, p. 355 (Kuschai); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 189 (Kusaie).
Ptilinopus hernsheimi Matschie, Journ. f. Ornith., 1901, p. 113 (Ualan); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 33 (Kusaie).
Ptilinopus ponapensis hernsheimi Kuroda, in Momiyama, Birds Micronesia, 1922, p. 57 (Kusaie); Hand-list Japanese Birds, rev., 1932, p. 190 (Kusaie); Peters, Check-list Birds World, 3, 1937, p. 31 (Kusaie); Hand-list Japanese Birds, 3d ed., 1942, p. 212 (Kusaie).
Ptilinopus marshallianus Peters and Griscom, Proc. New England Zool. Club, 10, 1928, p. 104 (Type locality, Ebon); Hand-list Japanese Birds, rev., 1932, p. 190 (Ebon).
Ptilinopus ponapensis marshallianus Peters, Check-list Birds World, 3, 1937, p. 31 (Ebon); Hand-list Japanese Birds, 3d ed., 1942, p. 213 (Ebon).
Ptilinopus porphyraceus hernsheimi Ripley and Birckhead, Amer. Mus. Novit., no. 1192, 1942, p. 6 (Kusaie, Ebon); Mayr, Birds Southwest Pacific, 1945, p. 289 (Kusaie).
Geographic range.—Micronesia: Caroline Islands—Kusaie; Marshall Islands—Ebon (extinct?).
Characters.—Adult: Resembles P. p. ponapensis, but occiput, nape, sides of head more gray and less greenish-yellow; chin and midthroat paler; crown coloring very faintly margined with yellow; tail band brighter yellow; under tail-coverts more orange; abdominal spot may be present as a brownish-red tinge; abdomen slightly more yellowish.
Immature: Resembles immature of P. p. ponapensis.
Measurements.—Measurements are listed in table 25. Ripley and Birckhead (1942:7) give the measurements of the only known specimen from Ebon (Marshall Islands) as: wing, 124; tail, 74; bill from base, 15.
Specimens examined.—Total number, 11 (6 males, 5 females), as follows: Caroline Islands, USNM—Kusaie, 1 (Feb. 9); AMNH—Kusaie, 10 (Jan., Feb., March, April).
Remarks.—I am following Ripley and Birckhead (1942:6) in identifying the dove from Ebon Island as of the subspecies P. p. hernsheimi. This specimen from Ebon may, however, represent the final vestige of a formerly well-distributed population in the Marshall Islands. This distribution is of particular interest because it may show the pathway by which these small fruit pigeons invaded eastern Micronesia from Polynesia.
The small fruit dove at Kusaie has apparently the same habitat requirements as others of the species. Coultas (field notes) comments that in 1931 the birds were "quite common." He found them in the high trees on the mountain sides away from the native villages and gardens.
Ptilinopus porphyraceus pelewensis Hartlaub and Finsch
Crimson-crowned Fruit Dove
Ptilinopus pelewensis Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 7. (Type locality, Pelew Islands.)
Ptilinopus pelewensis Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 118 (Pelew); Gray, Hand-list Birds, 2, 1870, p. 225 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 101 (Pelew); Gräffe, Journ. Mus. Godeffroy, 1, 1873, pl. 7, fig. 5 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 24 (Palau); Finsch, Journ. Mus. Godeffroy, 12, 1876, p. 37 (Palau); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Matschie, Journ. f. Ornith., 1901, p. 113 (Palau); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 56 (Pelew); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 32 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 190 (Palau); Peters, Check-list Birds World, 3, 1937, p. 31 (Babeltop, Korror); Hand-list Japanese Birds, 3d ed., 1942, p. 213 (Babelthuap, Koror).
Ptilonopus pelewensis Finsch, Proc. Zool. Soc. London, 1874, p. 94 (Pelew).
Ptilopus pelewensis Giebel, Thes. Ornith., 3, 1877, p. 366 (Pelew); Elliot, Proc. Zool. Soc. London, 1878, p. 531 (Palau); Schmeltz, Verhandl. Ver. nat. Unterhatlung Hamburg, 1877 (1879), p. 178 (Pelew); Tristram, Cat. Birds, 1889, p. 44 (Pelew); Wiglesworth, Ibis, 1891, p. 584 (Pelew); idem, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 48 (Pelew); Salvadori, Cat. Birds British Mus., 21, 1893, p. 86 (Pelew); Dubois, Syn. Avium, 2, 1904, p. 736 (Pelew); Reichenow, Die Vögel, 1, 1913, p. 354 (Palau); Takatsukasa and Kuroda, Tori, 1, 1915, p. 52 (Pelew).
Ptilinopus porphyraceus pelewensis Ripley and Birckhead, Amer. Mus. Novit., no. 1192, 1942, p. 7 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 289 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 60 (Peleliu, Ngabad, Garakayo).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Peleliu, Ngabad, Anguar.
Characters.—Adult male: A green fruit pigeon with anterior lores and crown purple, margined with pale yellow; forehead paler than crown; chin and midthroat pale yellow; neck, sides of head, and breast greenish-gray, darker on occiput; feathers of upper breast cross-banded with partly concealed violet bands; abdomen orange, its lower part and region of vent yellow; sides greenish; tibia grayish; under tail-coverts near "Indian lake" with yellowish-orange edgings; upper parts green; wings metallic green, secondaries and primaries margined on outer webs with yellow; inner secondaries spotted with violet-blue near tips; under wing gray; upper side of tail green with pale yellow terminal band; under side of tail gray; bill lead-colored; feet dark blood-red.
Adult female: Resembles adult male, but upper parts greener with upper side of wing and upper tail-coverts washed with olivaceous-brown; breast duskier. Immature resembles adult, but lacks purple crown, violet breast spot, orange abdomen and maroon under tail-coverts; upper and lower parts margined with yellow; forehead pale green; supercillary stripe pale yellow.
P. p. pelewensis resembles P. p. ponapensis, but crown more purple; yellow tail-bar narrower; bifurcated, central breast feathers violet; abdomen orange; and under tail-coverts near "Indian lake".
Measurements.—Measurements are presented in table 25.
Specimens examined.—Total number, 14 (10 males, 4 females), as follows: Palau Islands, USNM—Koror, 3 (Nov. 14, Dec. 3)—Garakayo, 1 (Sept. 19)—Peleliu, 3 (Aug. 27, Sept. 1, 4)—Ngabad, 2 (Sept. 11)—Pelew, 2 (Mar. 1, 2); AMNH—Palau, 3 (Oct., Dec.).
Nesting.—At Ngabad Island on September 11, 1945, the NAMRU2 party found a nest in jungle in a low tree about six feet above the ground. It was loosely constructed and contained a single white egg, size 31 by 23 mm. Another nest was found at Ngabad the same day. It was on the branch of a tree approximately 20 feet from the ground. The nest was not examined other than to observe a parent bird on the nest. Three males obtained in August and in September had enlarged testes. Males taken in December by Coultas had enlarged testes.
Food Habits.—Stomachs examined by the NAMRU2 party contained fruit parts and seeds. This species seemingly obtains its foods from the large fruit-producing trees and to a lesser extent from the smaller shrubs or from ground berries.
Remarks.—P. p. pelewensis was found in small numbers at all islands visited in the southern Palaus by the NAMRU2 party in 1945. At Peleliu, the bird was restricted to undisturbed woodlands and thickets, although some were seen in the thickly growing vegetation covering over the battle areas. The bird evidently lives a solitary existence; it was only rarely observed in pairs. It was often located by its calls. Coultas (field notes) reports that in 1931 the species was becoming rare in the Palaus, owing to persistent hunting by the Japanese, who sold the bird for 25 sen each.
Ptilinopus roseicapillus (Lesson)
Marianas Fruit Dove
Columba roseicapilla Lesson, Traité d'Ornith., 6, 1831, p. 472. (Type locality, Marianne Islands.)
Columba roseicapilla Lesson, Compl. de Buffon, 2d ed., 2, Oiseaux, 1838, p. 278 (Mariannes).
Columba purpurata Kittlitz, Kupfertaf. Naturgesch. Vögel, 3, 1833, p. 25, pl. 23, fig. 2 (Guahan); idem, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 305 (Guahan).
Ptilinopus purpuratus Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen); Hartert, Katalog Vogelsamml. Senckenb., 1891, p. 190 (Guaham).
Ptilopus roseicapillus Bonaparte, Comptes Rendus Acad. Sci. Paris, 39, 1854, p. 877 (Mariannes); idem, Icon. Pigeons, 1857, pl. 23 and desc. letterpress (Mariannes); Schlegel, Mus. Pays-Bas, 6, no. 35, 1873, p. 8 (Guam); Giebel, Thes. Ornith., 3, 1877, p. 368 (Mariannae); Elliot, Proc. Zool. Soc. London, 1878, p. 537 (Marianne); Oustalet, Le Nat., 1889, p. 261 (Mariannes); Wiglesworth, Ibis, 1891, p. 584 (Marianne); idem, Abhandl. und Ber Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 48 (Marianne); Salvadori, Cat. Birds British Mus., 21, 1893, p. 108 (Marianne Islands); Oustalet, Nouv. Arch. Mus. Hist. Nat., Paris, (3), 7, 1895, p. 218 (Saypan, Guam, Rota); Safford, The Plant World, 7, 1904, p. 264 (Guam); Dubois, Syn. Avium, 2, 1904, p. 736 (Mariannes); Safford, Contr. U. S. Nat. Herb., 9, 1905, p. 78 (Guam); Schnee, Zeitschr. f. Naturwisch., 82, 1912, p. 465 (Marianen); Prowazek, Die deutschen Marianen, 1913, p. 101 (Marianen); Reichenow, Die Vögel, 1, 1913, p. 354 (Marianen); Cox, Island of Guam, 1917, p. 20 (Guam); Bryan, Guam Rec. vol. 13, no. 2, 1936, p. 24 (Guam); Thompson, Guam and its people, 1942, p. 23 (Guam).
Kurukuru roseicapillus Prévost and Des Murs, Voy. "Venus," Oiseaux, 1855, pp. 221, 231, 257, 259, 269 (Guam).
Ptilopus roseicapilla Bonaparte, Consp. Avium, 2, 1855, p. 21 (Mariannis).
Ptilonopus roseicapillus Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 31 (Guam); Reichenbach, Tauben, 1861, p. 96 (Mariannen); Finsch, Proc. Zool. Soc. London, 1874, p. 94 (Mariannes).
Ptilinopus roseicapillus Finsch and Hartlaub, Fauna Centralpolynesiens, 1867, pp. 122, 127 (Mariannen); Gray, Hand-list Birds, 2, 1870, p. 225 (Ladrones); Hartert, Novit. Zool., 5, 1898, p. 60 (Guam, Rota, Saipan); Wheeler, Report Island of Guam, 1900, p. 13 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 39 (Guam, Rota, Saipan); Matschie, Journ. f. Ornith., 1901, p. 113 (Guam, Saipan); Safford, Osprey, 1902, p. 68 (Marianas); idem, Amer. Anthro., 4, 1902, p. 711 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 56 (Guam, Rota, Saipan); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 33 (Marianne); Hand-list Japanese Birds, rev., 1932, p. 190 (Tinian, Saipan, Rota); Hand-list Japanese Birds, 3d ed., 1942, p. 212 (Guam, Rota, Tinian, Saipan); Mayr, Birds Southwest Pacific, 1945, p. 288 (Marianas); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 95 (Tinian); Watson, The Raven, 17, 1946, p. 42 (Guam); Strophlet, Auk, 63, 1946, p. 538 (Guam); Baker, Condor, 49, 1947, p. 125 (Guam); Stott, Auk, 64, 1947, p. 526 (Saipan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 59 (Guam, Rota).
Ptilopus diadematus Giebel, Thes. Ornith., 3, 1877, p. 363 (Marianae).
Ptilinopus roseicapilla Peters, Check-list Birds World, 3, 1937, p. 31 (Saipan, Tinian, Rota, Guam); Ripley and Birckhead, Amer. Mus. Novit., no. 1192, 1942, p. 3 (Guam, Rota, Tinian, Saipan).
Geographic range.—Micronesia: Mariana Islands—Guam, Rota, Tinian, Saipan.
Characters.—Adult male: A green dove with crown, forehead, anterior lores, and spot at base of mandible near "aster purple," margined with pale yellow especially on top of head; chin and throat pale yellow to white; sides of head greenish-gray, darker on occiput; breast green with pearly-gray tinge on feathers of middle part; lower breast with dark purple patch; abdomen orange with yellowish-green coloring at midline; anal region and lower tail-coverts yellow, tinged with orange on lower tail-coverts; sides and tibia greenish with yellow tinges; upper parts green, more yellowish-green on rump; wings glossy, upper wing-coverts brighter in middle and margined with yellow; under side of wing and under side of tail gray; upper side of tail green with broad grayish terminal band margined with yellow; iris pale yellow; bill grass-green; legs and feet reddish-black.
Adult female: Resembles male, but slightly smaller with neck greener. Immature resembles adult, but lacking colored crown; body feathers edged with yellow.
Birds from Guam, Rota, and Tinian exhibit no conspicuous differences. P. roseicapillus is closest to P. regina of southern Papua, Lesser Sunda Islands, and Australia being, according to Ripley and Birckhead (1942:3), "Similar to regina, but crown and abdominal band darker; malar apex concolorous with crown; hind neck more grayish; tail-bar wider and paler."
Measurements.—Measurements of P. roseicapillus are presented in table 26.
Weights.—In 1948 (1948:59) I listed the weights of 14 adult males as 81-103 (90), of 4 adult females as 85-99 (92), and of one nestling in post natal molt as 44 grams. These were taken at Guam.
Specimens examined.—Total number, 43 (32 males, 10 females, 1 unsexed), as follows: Mariana Islands, USNM—Guam, 28 (March 8, May 25, 27, June 3, 12, 14, July 2, 6, 10, 18, 19, 29, Aug. 21)—Rota, 3 (Oct. 28, 31, Nov. 2)—Tinian, 1 (Oct. 26); AMNH—Guam, 8 (Aug.)—Tinian, 3 (Sept.).
Number Wing Tail Exposed
culmenTarsus 32 adult males 127 (122-133) 80 (75-84) 14 (13-15.3) 25 (24-27) 10 adult females 124 (121-130) 76 (75-79) 13 (12-13.7) 24 (22-25.5) Nesting.—At Guam, I obtained records of nests of fruit doves on March 1, 1927, and May 7, 1945. David H. Johnson observed a pair of fruit doves in the act of copulation on May 26, 1945. Birds with enlarged gonads were taken by the NAMRU2 party in March and July. A nestling in post natal molt, just beginning to fly, was taken on July 6. Seale (1901:39) reports two nests, each containing one white egg, taken in the period from May to July. These nests were found in trees eight to ten feet above the ground.
Food habits.—The Marianas Fruit Dove feeds on fruits and seeds of trees and shrubs. The birds are apparently strictly tree dwellers; I saw no birds on the ground. A favorite fruit is that of a flowering shrub known as the "ink berry." Birds were collected which contained stomachs full of these small black berries. The fruit of the papaya is also a favorite food.
Remarks.—The NAMRU2 party found the Marianas Fruit Dove at Guam to be fairly numerous in undisturbed jungle, and more abundant in the heavy, second-growth, scrub-forest as was found on Amantes Point in 1945. The birds were secretive but were easily located by their calls. They were usually found as singles sitting quietly concealed in thick vegetation. Birds were seen flying rather infrequently, and then only for short distances. The removal of large tracts of jungle to provide space for the construction of air strips and installations in the late war has disturbed some of the habitat of these birds. Although vast tracts of forest were undisturbed, the birds probably have decreased at Guam. Coultas (field notes) found the birds common at the northern end of Guam in 1931. He commented that natives catch them with snares and bird lime for the local markets. At Tinian in 1931, Coultas found few birds. Downs (1946:95) and Stott (1947:526) record the birds at Tinian and Saipan, respectively, in 1945. At Rota, the NAMRU2 party found the dove to be numerous.
Evolutionary history of Ptilinopus in Micronesia.—Oceania is especially rich in species and subspecies of the genus Ptilinopus. Ripley and Birckhead (1942) have made the most recent and most thorough contribution concerning these birds. They state that the center of distribution for the genus lies in the Papuan region. Within the Oceanic region there are several species of Ptilinopus [Pg 189] which in one way or another are rather closely related; Rensch (1938:277) uses these as examples of species which have been formed by isolation. These include P. perousii from Samoa, Fiji, and Tonga; P. mercierii from the Marquesas; P. dupetithouarsii from the Marquesas; P. huttoni from Rapa; P. purpuratus from Henderson, Tuamotus, Societies; P. porphyraceus from Samoa, Fiji, Tonga, Carolines, Solomons, New Hebrides, New Caledonia, and adjacent areas; and P. roseicapillus from Marianas. In all of these birds the crown is wine-red except in P. dupetithouarsii in which it is whitish. P. porphyraceus appears to be more closely related to P. purpuratus than to any other species and is characterized by an often brightly washed spot of color of some shade of red or orange on the breast. These birds may have invaded Micronesia from the region of the Solomon Islands, although it appears more likely that they arose in the Samoa-Fiji-Tonga region and moved northward, probably by way of the Marshall Islands. P. p. hernsheimi from Kusaie and P. p. ponapensis from Ponapé and Truk resemble P. p. faciatus Peale from Samoa more closely than they do any other subspecies. P. p. pelewensis from Palau, on the other hand shows little relation to these other two Micronesian subspecies and appears to be closest to P. p. porphyraceus of Fiji and Tonga or possibly to P. grayi from Melanesia. Ripley and Birckhead (1942:7) suggest that the subspecies at Palau owes its marked divergence to its isolated position at the periphery of the range of the species. P. p. pelewensis probably represents an independent and an earlier colonization, possibly from a stock different from that from which the two subspecies in the Carolines arose. The presence in the Palaus of subspecies singularly different from subspecies in the Carolines can also be observed in other genera, as for example, Rhipidura, and Myiagra. Figure 13 shows the inferred routes of colonization of Ptilinopus to Micronesia.
P. roseicapillus seemingly represents a remnant, or perhaps a successful straggler, of an early invasion to Micronesia. Ripley and Birckhead (1942:2) classify this species as "Old Stock," along with P. monachus, P. coronulatus and P. regina. Its pathway of invasion to the Marianas was probably directly northward from the Papuan area and not by way of the Polynesian islands. Its resemblance to the species P. regina of southern Papua, Lesser Sundas, and Australia is most unusual, especially since there is a separation between the two species of some 1,400 miles; this is pointed out by Ripley and Birckhead (1942:4). As I have said [Pg 190] (1948:59) elsewhere, "On the basis of its characters the Mariana birds would merit only subspecific separation, but owing to the great distance between the two doves and the possibility of independent origin and subsequent convergence, it may be more advisable to continue to regard the two as separate species."
Fig. 13. Geographic distribution of Ptilinopus porphyraceus and routes of its dispersal. (1) P. p. porphyraceus; (2) P. p. fasciatus; (3) P. p. hernsheimi; (4) P. p. ponapensis; (5) P. p. pelewensis.
Ducula oceanica monacha (Momiyama)
Micronesian Pigeon
Globicera oceanica monacha Momiyama, Birds Micronesia, March, 1922, p. 4. (Type locality, Yap.)
Columba oceanica Lesson and Garnot (part), Dict. Sci. Nat., éd. Levrault, 40, 1826, p. 317 (Pelew); Lesson (part), Man. d'Ornith., 2, 1828, p. 166 (Pelew); idem (part), Voy. "La Coquille," Zool., 2, 1828, pp. 432, 709 (Pelew); idem, Compl. de Buffon, 2d ed., 2, Oiseaux, 1838, p. 292 (Pelew); Prévost and Knip, Les Pigeons, 2, 1838-43, p. 49 (Pelew).
Carpophaga oceanica Hartlaub (part), Archiv. f. Naturgesch., 18, 1852, p. 115 (Pelewinseln); idem, Proc. Zool. Soc. London, 1867 (1868), p. 830 (Pelew); Gray (part), Hand-list Birds, 2, 1870, p. 229 (Pelew); Hartlaub and Finsch (part), Proc. Zool. Soc. London, 1872, pp. 89, 101 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 26 (Palau); idem (part), Proc. Zool. Soc. London, 1877 (1878), pp. 775, 780 (Palau); Salvadori (part), Cronaca del R. Liceo-Ginnasio Cavour, 1878, pp. 3, 8 (Pelew); idem, Ibis, 1879, p. 364 (Pelew); Tristram, Cat. Birds, 1889, p. 42 (Pelew); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 52 (Pelew); Matschie (part), Journ. f. Ornith., 1901, p. 113 (Palau); Dubois (part), Syn. Avium, 2, 1904, p. 743 (Pelew); Reichenow (part), Die Vögel, 1, 1913, p. 351 (Palau).
Globicera oceanica Bonaparte (part), Consp. Avium, 2, 1855, p. 31 (Pelew); Reichenbach (part), Tauben, 1861, p. 120 (Pelew); Salvadori (part), Cat. Birds British Mus., 21, 1893, p. 176 (Pelew); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 52 (Pelew); Uchida, Annot. Zool. Japon., 9, 1918, pp. 486, 489 (Palau).
Carpophaga (Globicera) oceanica Gray (part), Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 41 (Pelew).
Carpophaga pacifica Finsch and Hartlaub (part), Fauna Centralpolynesiens, 1867, p. 145 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 7, 118 (Pelew); Finsch and Hartlaub, Journ. f. Ornith., 1870, p. 134 (Pelew).
Globicera oceanica monacha Kuroda, in Momiyama, Birds Micronesia, 1922, p. 55 (Yap); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 46 (Yap); Yamashina, Tori, 7, 1932, p. 408 (Yap); Hand-list Japanese Birds, rev., 1932, p. 189 (Yap, Palau, Current = Palo Anna).
Globicera oceanica momiyamai Kuroda, in Momiyama, Birds Micronesia, March, 1922, pp. 25, 56 (Type locality, Angaur); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 46 (Pelew); Kuroda, Ibis, 1927, p. 719 (Pelew).
Muscadivora oceanica winkleri Neumann, Verhandl. Ornith. Ges. Bayern, 25, Sept. 1, 1922, p. 234 (Type locality, Palau).
Ducula oceanica monacha Peters, Check-list Birds World, 3, 1937, p. 43 (Yap, Babelthuap, Koror, Angaur, Current); Hand-list Japanese Birds, 3d ed., 1942, p. 211 (Yap, Babelthuap, Koror, Angaur, Current); Amadon, Amer. Mus. Novit., no. 1237, 1943, p. 11 (Yap, Palau); Mayr, Birds Southwest Pacific, 1945, p. 289 (Palau, Yap); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 66 (Peleliu, Garakayo, Babelthuap).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Peleliu, Angaur, Palo Anna; Caroline Islands—Yap.
Characters.—Adult: Resembles D. o. oceanica from Kusaie but throat, breast, head, and neck light ashy-gray; feathers around bill grayish-white; abdomen and under tail-coverts tipped with light brown.
Immature: Resembles adult, but underparts paler; back lacking dark bluish spots; back feathers and wing feathers edged with light brown.
Measurements.—Measurements of D. oceanica are listed in table 27.
Subspecies Number Wing Exposed
culmenTarsus D. o. monacha 8 males
228 (219-233) 36 (34-37) 6 females
221 (214-228) 22.5 (22.0-23.0) 31 (29-33) D. o. teraokai 5 males
230 (225-237) 23.5 (23.0-25.0) 34 (33-35) 8 females
231 (221-238) 23.0 (21.5-24.5) 34 (33-35) D. o. townsendi 8 males
226 (211-234) 24.0 (23.5-25.0) 34 (32-35) 5 females
226 (215-233) 24.0 (23.0-24.5) 33 (32-34) D. o. oceanica 4 males
222 (217-228) 25.0 (24.5-26.0) 35 (34-36) 13 females
219 (213-226) 24.0 (23.0-25.0) 32 (30-34) D. o. ratakensis[B] 6 males
(211-217) (25.0-27. 3 females
(208-213) (25.0-26.0) [B] From Takatsukasa and Yamashina (1932:221).
Specimens examined.—Total number, 17 (9 males, 8 females), as follows: Palau Islands, USNM—Garakayo, 1 (Sept. 19)—Peleliu, 7 (Aug. 27, 28, 29, Sept. 4, 5); AMNH—Palau, 9 (Oct., Nov. 13, 15, 21, Dec. 1).
Nesting.—Yamashina (1932a:408) records the finding of one egg at Yap on December 3, 1930. The NAMRU2 party obtained no evidence of breeding activity of these pigeons at the Palaus in August and September, 1945. Coultas, in November and December of 1931, obtained birds with enlarged gonads at Palau. Probably the nesting season begins in November or December.
Food habits.—The pigeons feed on both fruits and green stuffs. The NAMRU2 party found berries, fruit parts and green plant materials in stomachs of birds taken in September, 1945. The birds were found to be exceedingly fat at this time.
Parasites.—Uchida (1918:486, 489) records the bird lice (Mallophaga), Goniocotes carpohagae and Colopocephalum unicolor, from this pigeon at Palau.
Remarks.—The Micronesian Pigeon at Palau was first observed in 1783, when Captain Henry Wilson of the packet "Antelope" was shipwrecked in these islands. In his account of the islands, as compiled by George Keate (Wilson, 1788), Wilson described the large pigeons, which were kept as pets by the natives and were eaten by only certain classes of people. In 1826, Lesson and Garnot made first reference to the birds found by Wilson. It was almost 100 years after Wilson's visit that the bird was again observed; this time it was obtained by the sea captains, Tetens and Heinsohn, and by Kubary, the collector for the Godeffroy Museum.
It is surprising that a pigeon as large and conspicuous as this one, has not already been exterminated by man on these small islands. Every traveller to the islands, who has made observations, writes that the pressure of hunting on these birds is severe. Coultas (field notes) reports that in 1931 the birds were "very scarce and wild." He comments that the Japanese hunters obtained the birds and received the market price of 35 sen for each one. He writes, "There is a group of Japanese hunters in the islands who vie with one another to see who can obtain the most birds. They are all atrocious shots but some employ natives and since so many of them are in the business they are inflicting considerable damage to the bird life. During my stay there one Japanese was sentenced to six weeks hard labor for hiring native hunters. The native hunter who preferred charges claimed that money was due him for having shot some 3,500 birds and the account had been standing over a year." Price (1936b:491) shows a picture of a captive pigeon at Palau. The natives used this bird as a calling decoy to attract others within range of their blowguns.
The NAMRU2 party observed pigeons at all islands visited in August and September, 1945. At Peleliu, the pigeons were found to be restricted to relatively undisturbed areas where tall trees [Pg 193] remained or where shrubs were present on the faces of overhanging cliffs. The shrubs on cliffs were favorite roosting places. Although the pigeons remained in these relatively inaccessible areas, they were not especially difficult to obtain with shotguns. I can see that it might be difficult for unarmed hunters to obtain the birds. The present writer (1946b:210) has recorded the extensive utilization of pigeons, rails and megapodes by Japanese troops and by their prisoners of war at Babelthuap and Koror during the latter part of the war.
During our stay at Peleliu we were unable to learn whether the pigeon was still present at Pulo Anna (Current Island), a coral island some 160 miles southeast of Peleliu. The U. S. Navy frequently dispatched a ship to the island, but we did not learn of it until our stay at Peleliu was nearly over. Dr. C. K. Dorsey, then of the U. S. Naval Epidemiology Unit at Peleliu, reported that various kinds of birds were numerous at Pulo Anna, but he did not recall seeing the pigeon. This pigeon may occur also at Fais, a raised coral island west of Yap and Ulithi in the Carolines. I know of no reports dealing with the avifauna of this phosphate island, but I examined several pictures, taken by Navy landing parties and the Military Government personnel, which show the island to be covered with extensive and luxuriant vegetation. I suspect that an intensive survey of the island will reveal several new records for birds.
Ducula oceanica teraokai (Momiyama)
Micronesian Pigeon
Globicera oceanica teraokai Momiyama, Birds Micronesia, 1922, p. 2. (Type locality, Tol, Truk Islands.)
Columba oceanica Kittlitz (part), Kupfertaf. Naturgesch. Vögel, 3, 1833, p. 25, pl. 33, fig. 1 (Lugunor); idem (part), Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 299 (Lougounor); Hartlaub (part), Archiv f. Naturgesch., 18, 1852, pp. 115, 185, (Mordlockinseln).
Carpophaga (Globicera) pacifica Gray (part), Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 41 (Mortlock's Island).
Carpophaga pacifica Finsch and Hartlaub, Fauna Centralpolynesiens, 1867, p. 146 (Lugunor).
Carpophaga oceanica Finsch, Proc. Zool. Soc. London, 1880, p. 576 (Ruk); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 330, 353 (Nukuor, Ruk); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 52 (Luganor, Ruk, Nukuor); Hartert, Novit. Zool., 7, 1900, p. 8 (Ruk).
Globicera oceanica Salvadori (part), Cat. Birds British Mus., 21, 1893, p. 176 (Ruk); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 52 (Ruk).
Globicera oceanica teraokai Kuroda, in Momiyama, Birds Micronesia, 1922, p. 55 (Ruk, ?Mortlock, ?Nukuor); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 45 (Ruk); Hand-list Japanese Birds, rev., 1932, p. 189 (Truk).
M[uscadivora] o[ceanica] oceanica Neumann (part), Verhandl. Ornith. Ges. Bayern, 25, 1922, p. 234 (Ualam = Truk).
Ducula oceanica teraokai Peters, Check-list Birds World, 3, 1937, p. 43 (Truk); Hand-list Japanese Birds, 3d ed., 1942, p. 212 (Truk); Amadon, Amer. Mus. Novit., no. 1237, 1943, p. 11 (Truk); Mayr, Birds Southwest Pacific, 1945, p. 289 (Truk).
Geographic range.—Micronesia: Caroline Islands—Truk, ?Lukunor, ?Nukuoro.
Characters.—Adult: Resembles D. o. monacha, but slightly darker on crown, nape, and mantle; back more bluish and less greenish, underparts slightly darker chestnut. Differs from D. o. townsendi by being paler and gray on crown, nape, shoulder, side of neck, and upper breast; abdomen and under tail-coverts slightly deeper chestnut. Differs from D. o. oceanica by larger size; upper parts paler; abdomen and under side of tail deeper chestnut. I agree with Amadon (1943:11) that this subspecies is only doubtfully distinct from D. o. monacha and that it might be advisable to unite these under one subspecific name.
Measurements.—Measurements are listed in table 27.
Specimens examined.—Total number, 14 (5 males, 9 females, 1 unsexed) from Caroline Islands, AMNH—Truk (Nov., Dec.).
Remarks.—The Micronesian Pigeon at Truk was observed by Kittlitz (1836:299) and later by Kubary at the islands of Lukunor and Nukuoro. Momiyama (1922:4) remarks that he did not see specimens from these two islands but concludes that they probably belong to the subspecies named from Truk. It is possible that birds at these two atolls have been exterminated, although adequate field investigations have not been made.
There is little information published concerning the natural history of this subspecies. McElroy, who visited Truk in December, 1945, did not find the bird; however, he did not visit all of the islands in the group during his stay.
Ducula oceanica townsendi (Wetmore)
Micronesian Pigeon
Globicera oceanica townsendi Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 191. (Type locality, Ponapé).
Carpophaga oceanica Finsch (part), Proc. Zool. Soc. London, 1877 (1878), p. 780 (Ponapé); Nehrkorn, Journ. f. Ornith., 1879, p. 407 (Ponapé); Finsch (part), Journ. f. Ornith., 1880, p. 292 (Ponapé); idem, 1881, pp. 113, 115 (Ponapé); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, p. 281 (Ponapé); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 52 (Ponapé); Matschie (part), Journ. f. Ornith., 1901, p. 113 (Guam, error = Ponapé).
Globicera oceanica Salvadori (part), Cat. Birds British Mus., 21, 1893, p. 176 (Ponapé).
Globicera oceanica townsendi Momiyama, Birds Micronesia, 1922, p. 6 (Ponapé); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 55 (Ponapé); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 45 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 190 (Ponapé).
Ducula oceanica townsendi Peters, Check-list Birds World, 3, 1937, p. 44 (Ponapé); Bequaert, Mushi, vol. 12, no. 2, 1939, pp. 81, 82 (Ponapé); idem, Occ. Papers Bernice P. Bishop Mus., 16, 1941, pp. 266, 290 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 212 (Ponapé); Amadon, Amer. Mus. Novit., no. 1237, 1943, p. 11 (Ponapé); Mayr, Birds Southwest Pacific, 1945, p. 289 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult: Resembles D. o. teraokai, but darker. Resembles. closely D. o. oceanica but larger and darker on crown and nape; lower parts slightly paler but chin more cream-buff in color. As Adamon (1943:11) states, there is little difference between D. o. townsendi and D. o. oceanica except in size.
Measurements.—Measurements are listed in table 27.
Specimens examined.—Total number 21 (11 males, 9 females, 1 unsexed), as follows: Caroline Islands, USNM—Ponapé, 2 (Feb. 11, 12); AMNH—Ponapé, 19 (Nov. 22, 29, Dec. 1, 2, 3).
Nesting.—Coultas (field notes) writes that the pigeon at Ponapé nests the year around, probably two or three times a year. He describes the nest as being made of loose twigs and as placed on a fork of a limb in a tall tree. One egg is laid. Coultas saw "two or three" females nesting in December.
Parasites.—Bequaert (1939:81, 82 and 1941:266, 290) found the flies (Hippoboscidae), Ornithoctona plicata and O. pusilla, on pigeons from Ponapé.
Remarks.—Coultas (field notes) writes that in 1930 several Japanese made a livelihood as professional hunters of pigeons at Ponapé. He notes, "Two or three years ago, 4 or 5 Japanese, each, averaged from 75 to 100 birds per day, which they sold to the inhabitants for 35 sen (17-1/2 cents) per bird.... Now these same hunters are fortunate if they obtain 4 or 5 Ducula each per day and are able to do so only by starting before daylight and covering great distances. Other birds are now replacing Ducula on the market." Coultas further records in his notes that the hunters used calls to attract the pigeons. In 1930, Coultas regarded the pigeon at Ponapé as a rapidly disappearing species; he found it only in small areas in remote regions of the mountains. With the shipping of supplies cut off to the Japanese garrison forces at Ponapé, as well as at Kusaie, Truk, and Yap by the effective American blockade during the latter part of the war, it is probable that the pigeons were hunted more intensively by the Japanese hunting parties than ever before. Richards obtained two specimens at Ponapé in the period from August, 1947, to January, 1948.
Ducula oceanica oceanica (Lesson and Garnot)
Micronesian Pigeon
Columba oceanica Lesson and Garnot, Dict. Sci. Nat., éd., Levrault, 40, 1826, p. 316. (Type locality, Oualan = Kusaie.)
Columba oceanica Lesson (part), Voy. "La Coquille," Zool.; Atlas, 1826, pl. 41; vol. 2, 1828, pp. 432, 708 (Oualan or Strong); idem, (part), Man. d'Ornith., 11, 1828, p. 166 (Oualan); Kittlitz (part), Kupfertaf. Naturgesch. Vögel, 3, 1833, p. 25, pl. 23, fig. 1 (Ualan); idem (part), Observ. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 284 (Ualan); Lesson, Compl. de Buffon, 2d ed., 2, Oiseaux, 1839, p. 292 (Oualan); Prévost and Knip (part), Les Pigeons, 2, 1838-43, p. 47, pl. 24 (Oualan); Bonaparte, Comptes Rendus Acad. Sci. Paris, 39, 1854, p. 1072 (Oualan); Kittlitz, Denkw. Reise russ. Amer. Micron. und Kamchat., 1, 1858, pp. 39, 49, 62 (Ualan).
Carpohaga oceanica Hartlaub (part), Archiv f. Naturgesch., 18, 1852, pp. 115, 185 (Ualan); idem, Journ. f. Ornith., 1854, p. 168 (Carolinen = Kusaie); Hartlaub and Finsch (part), Proc. Zool. Soc. London, 1872, p. 101 (Ualan); Schlegel, Mus. Pays-Bas, 6, no. 35, 1873, p. 87 (Oualan); Salvadori (part). Cronaca del R. Liceo-Ginnasio Cavour, 1878, pp. 3, 8 (Oualan); Finsch (part), Ibis, 1880, pp. 220, 331, 332 (Taluit); idem (part), Journ. f. Ornith., 1880, pp. 292, 304 (Kuschai); idem, Ibis, 1881, p. 108 (Kuschai); idem, Mitth. Ornith. Ver. Wien, 1884, p. 50 (Kuschai, Jaluit); Hartert, Katalog Vogelsamml, Senckenb., 1891, p. 190 (Ualan); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 52 (Ualan, Taluit); Matschie (part), Journ. f. Ornith., 1901, p. 113 (Ualan).
Globicera oceanica Bonaparte (part), Consp. Avium, 2, 1855, p. 31 (Oualan); idem, Comptes Rendus Acad. Sci. Paris, 43, 1856, p. 835 (Oualan); Reichenbach (part), Tauben, 1861, p. 120 (Oualan); Salvadori (part), Cat. Birds British Mus., 21, 1893, p. 176 (Kushai).
Carpophaga pacifica Finsch and Hartlaub (part), Fauna Centralpolynesiens, 1867, p. 145 (Ualan).
Carpophaga (Globicera) oceanica Gray (part), Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 41 (Oualan).
Globicera oceanica oceanica Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 191 (Kusaie); Momiyama (part), Birds Micronesia, 1922, p. 6 (Kusaie, Taluit); Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 55 (Kusaie, Taluit); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 45 (Kusaie); Takatsukasa and Yamashina, Dobutsu. Zasshi, 44, 1932, p. 221 (Jaluit, Iringlob, Kusaie); Hand-list Japanese Birds, rev., 1932, p. 190 (Kusaie, Jaluit, Elmore).
Muscadivora oceanica oceanica Neumann (part), Verhandl. Ornith. Ges. Bayern, 25, 1922, p. 234 (Kushai).
Ducula Oceanica oceanica Peters, Check-list Birds World, 3, 1937, p. 44 (Kusaie, Jaluit, Elmore); Bequaert, Mushi, 12, 1939, p. 81 (Kusaie); idem, Occ. Papers Bernice P. Bishop Mus., 16, 1941, p. 266 (Kusaie); Hand-list Japanese Birds, 3d ed., 1942, p. 212 (Kusaie, Jaluit, Elmore); Amadon, Amer. Mus. Novit., no. 1237, 1943, p. 11 (Kusaie, Jaluit, Elmore); Mayr, Birds Southwest Pacific, 1945, p. 289 (Kusaie, Jaluit, Elmore).
Geographic range.—Micronesia: Caroline Islands—Kusaie; Marshall Islands—Jaluit, Elmore.
Characters.—Adult male: A large knob-billed pigeon with breast gray, washed with buff; head and neck dark gray; feathers at base of bill and on chin buff-white; abdomen and under tail-coverts near "burnt sienna," sides grayer; mantle, back, rump, upper tail-coverts, wings and tail bronze-green edged with a dark bluish sheen; under side of wing and under side of tail brown; bill and knob black; feet blackish-red; iris reddish-brown. Adult female resembles adult male but smaller and possibly a little darker bluish-green on back, wings, and tail.
D. o. oceania resembles D. o. townsendi, but is smaller with upper parts slightly darker and abdomen and under side of tail lighter.
Measurements.—Measurements are presented in table 27.
Specimens examined.—Total number, 47 (25 males, 22 females), as follows: Caroline Islands, USNM—Kusaie, 2 (Feb. 8, 9,); AMNH—Kusaie, 45 (Jan., Feb., March).
Parasites.—Bequaert (1939:81 and 1941:266) obtained the fly (Hippoboscidae) Ornithoctona plicata from the pigeon at Kusaie.
Remarks.—The Micronesian Pigeon at Kusaie has been known since 1824, when from June 5 to June 15 of that year personnel from the corvette "La Coquille" visited the island and observed the bird. Kittlitz visited Kusaie and observed the pigeon in December, 1827, and January, 1828. Finsch (1880c and 1880d) found the bird in the [Pg 197] Marshalls at Jaluit. Takatsukasa and Yamashina (1932:221) record the bird from Elmore in the Marshalls. Coultas (field notes) writes that the pigeon was numerous at Kusaie in 1931. He remarks that they appear stupid and are easily killed by the natives, who use a call to attract them. With regard to their habits he writes, "About four o'clock in the afternoon these birds begin congregating in the high trees of the lowlands close to the salt water where they roost for the night. At daybreak they begin migrating to the high mountain sides and peaks where they spend the time feeding."
Ducula oceanica ratakensis (Takatsukasa and Yamashina)
Micronesian Pigeon
Globecera oceanica ratakensis Takatsukasa and Yamashina, Dobutsu. Zasshi, 44, 1932, p. 221. (Type locality, Aruno.)
Columba australis Chamisso, in Kotzebue's, Voy. "Rurick," 3, 1821, p. 157 (Radak).
Carpophaga oceanica Finsch, Ibis, 1880, p. 331 (Arno); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 52 (Arno); idem, Ibis, 1893, p. 211 (Marshalls).
Globicera oceanica oceanica Momiyama (part), Birds Micronesia, 1922, p. 5 (Arno); Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 55 (Arno).
Globicera oceanica ratakensis Hand-list Japanese Birds, rev., 1932, p. 190 (Arhno, Wotze); Mathews, Ibis, 1933, p. 87 (Aruno, Wozzie).
Ducala oceanica ratakensis Peters, Check-list Birds World, 3, 1937, p. 44 (Arno, Wotje); Hand-list Japanese Birds, 3d ed., 1942, p. 212 (Arhno, Wotze); Amadon, Amer. Mus. Novit., no. 1237, 1943, p. 12 (Arno, Wotje); Mayr. Birds Southwest Pacific, 1945, p. 289 (Arno, Wotje).
Geographic range.—Micronesia: Marshall Islands (Radak Chain)—Wotje, Arhno.
Characters.—Takatsukasa and Yamashina (1932:221) describe this subspecies as follows, "This form differs from all other forms of Globicera oceanica by its smaller size, more bronze-sheen on the back, more vinaceous grey on the breast and duller brown on the abdomen." On examining two specimens from Arno in the collection of the Museum of Comparative Zoölogy, Amadon (1943:12) writes that he finds no distinguishing color characters between D. o. oceanica and D. o. ratakensis. He also questions whether there is any difference in size between the two populations.
Measurements.—Measurements are listed in table 27.
Remarks.—Chamisso (1821), the naturalist on board the ship "Rurick," was the first person to write of the pigeon in the Radak Chain of the Marshall Islands. The ship visited this area in 1817. Finsch (1880b) published an account of the bird when he visited the area about 1880. Takatsukasa and Yamashina (1932:221) described this bird as new on the basis of an examination of nine skins taken at Arhno and Wotje.
Evolutionary history of Ducula oceanica in Micronesia.—The distribution and evolutionary history of Ducula oceanica have been treated by Mayr (1940) and Amadon (1943). These authors place [Pg 198] D. oceanica within a superspecies containing D. pacifica (Melanesia to Samoa and Cook Islands), D. aurorea (Society Islands), D. galeata (Marquesas Islands), and possibly other species in Papua and Malaysia. According to Mayr (1942b:fig. 7), D. pacifica is the species which is ancestral to other species of pigeons in Oceania. Apparently D. oceanica was derived from this ancestral stock and reached Micronesia via the Ellice and Gilbert islands. Records of Ducula were obtained in the Gilbert Islands in the days of exploration; Amadon (1943:11) tentatively refers these to D. o. oceanica.
The irregular distribution of D. oceanica in the islands of Micronesia and the fact that the bird exists on both "high" volcanic islands as well as on "low" coral atolls suggest that the present population may be a remnant of a once more widely distributed one. The fact that D. oceanica may be divided into several subspecies shows that a greater amount of geographic variation has occurred as compared with its probable ancestral stock, D. pacifica, which is virtually undifferentiated over most of its extensive range. The pigeon of Micronesia has a more rounded wing than that of D. pacifica, which might, as Amadon has suggested, cause the bird to be more sedentary and lend itself more readily to differentiation through geographic isolation. D. pacifica is known to fly from island to island. As shown by the measurements in table 27, the length of wing of D. oceanica differs, in the various insular populations, being longer in the west and shorter in the east. This cline has been discussed by Amadon (1943:11).
It is interesting that Ducula or some other large pigeon has not become established in the Mariana Islands. Ducula is present at Yap and Truk, which are not very distant from Guam. Another tropical pigeon, Columba vitiensis, has extended its range northward and reached the Bonin Islands; probably it arrived there via the Philippines or the Riu Kiu Islands. Thus, there are representatives of large pigeons on islands to the southeast, south, west and northwest of the Marianas, but none has become established in the Marianas themselves.
Streptopelia bitorquata dusumieri (Temminck)
Philippine Turtle Dove
Columba dusumieri Temminck, Pl. col., livr. 32, 1832, p. 188. (Type locality, Vicinity of Manila, Luzon, Philippine Islands.)
Colombe Dussumier Quoy and Gaimard, Voy. "Uranie," Zool., 1824, pp. 35, 680 (Mariannes); idem, Ann. Sci. Nat. Paris, 6, 1825, p. 148 (Mariannes).
Columba dusumieri Wagler, Syst. Avium Columba, 1827, p. 266, sp. 99 (Marianis).
Columba Dussumieri Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 305 (Guahan).
Streptopelia gaimardi Bonaparte, Consp. Avium, 2, 1854, p. 66 (Type locality, Mariannes); idem, Comptes Rendus Acad. Sci. Paris, 40, 1855, p. 18 (Mariannes); Reichenbach, Tauben, 1862, p. 76 (Mariannen).
Turtur (Streptopelia) Giamardi Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 43 (Guam).
Turtur gaimardi Gray, Hand-list Birds, 2, 1870, p. 239 (Marian).
Turtur dussumieri Schlegel, Mus. Pays-Bas, 6, no. 35, 1873, p. 120 (Mariannes); Wiglesworth, Abhandl. Und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 54 (Marianne); Salvadori, Cat. Birds British Mus., 21, 1893, p. 423 (Mariannes); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 222 (Guam); Hartert, Novit. Zool., 5, 1898, p. 60 (Guam, Saipan); Wheeler, Report Island of Guam, 1900, p. 13 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 43 (Marianas); Safford, Osprey, 1902, p. 68 (Marianas); idem, Amer. Anthro., 4, 1902, p. 711 (Guam); idem, The Plant World, 7, 1904, p. 264 (Guam); Dubois, Syn. Avium, 2, 1904, p. 760 (Marianne); Safford, Contr. U. S. Nat. Herb., 9, 1905, p. 78 (Guam); Schnee, Zeitschr. f. Naturwisch., 82, 1912, p. 466 (Marianen); Prowazek, Die deutschen Marianen, 1913, p. 101 (Marianen); Reichenow, Die Vögel, 1, 1913, p. 341 (Marianen); Cox, Island of Guam, 1917, p. 20 (Guam).
Streptopelia dussumieri Kuroda, in Momiyama, Birds Micronesia, 1922, p. 54 (Guam, Saipan); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 62 (Marianas); Hand-list Japanese Birds, rev., 1932, p. 189 (Saipan, Tinian, Rota).
Tuttur dessumieri Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam).
Streptopelia bitorquata dusumieri Peters, Check-list Birds World, 3, 1937, p. 96 (Marianne); Hand-list Japanese Birds, 3d ed., 1942, p. 211 (Saipan, Tinian, Rota); Mayr, Birds Southwest Pacific, 1945, p. 289 (Marianas); Watson, The Raven, 17, 1946, p. 41 (Guam); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 96 (Tinian); Strophlet, Auk, 1946, p. 538 (Guam); Stott, Auk, 1947, p. 526 (Saipan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 60 (Guam, Rota).
Streptopelia bitorquata Baker, Trans. 11th N. American Wildlife Conf., 1946, p. 208 (Guam); idem, Condor. 49, 1947, p. 125 (Guam).
Geographic range.—Philippine Islands, Sula Archipelago, northern Borneo. In Micronesia: Mariana Islands (introduced)—Guam, Rota, Tinian, Saipan.
Characters.—Adult: A medium-sized dove with head and nape near "light Quaker drab" with a vinous tinge; chin and upper throat whitish becoming near "vinaceous buff" on lower throat and to near "vinaceous-faun" on breast and upper abdomen; lower abdomen, vent, and under tail-coverts white; tibia grayish; neck feathers dark with grayish centers and metallic greenish-slate edges; color near "Japan rose"; back, rump, upper tail-coverts, scapulars, upper wing-coverts, and inner secondaries dark "drab"; sides, upper wing coverts, outer secondaries, and under wing-coverts lead colored; primaries blackish edged with light gray; central tail feathers like back but paler, outer feathers of tail darker with brownish tinge on edges; outermost tail feathers blackish tipped with gray and with outer webs whitish; bill dark; feet reddish; iris orange.
Measurements.—Measurements of 15 adult males from Guam, Rota and Tinian: wing, 154-162 (158); tail, 127-135 (130); culmen, 16.2-18.1 (17.2); tarsus, 24-27 (25.5); of 10 adult females from Guam and Rota: wing, 150-162 (156); tail, 120-130 (127); culmen, 16.2-19.1 (17.5); tarsus, 24-26 (25). No differences in measurements were found between populations from Guam, Rota and Tinian.
Weights.—The author (1948:61) reports the weights of five adult males as 130-167 (152) and of six adult females as 135-159 (146). These birds were taken at Guam.
Specimens examined.—Total number, 27 (16 males, 11 females), as follows: Mariana Islands, USNM—Guam, 21 (Feb. 7, May 25, 26, June 9, July 6, 7, 10, 18, 23, Aug. 2, 11, Sept. 8, Oct. 8)—Rota, 4 (Oct. 18, 22, 23, Nov. 2)—Tinian, 2 (Oct. 24, 25).
Nesting.—The NAMRU2 party found evidences of nesting by this dove at Guam in February, March, April, and June. Nests were observed on May 29 and June 28. On the latter date a nest containing one nestling and one unhatched egg was found near Mount Santa Rosa. The nest was situated approximately five feet from the ground in a low bush. Two eggs taken by Necker at Rota on October 31, 1945, are white and measure 29.6 by 23.0 and 30.1 by 23.0. Strophlet (1946:538) observed a bird carrying nest materials at Guam on November 13. Hartert (1898:60) reports on nests found at Guam in April and May. Each nest contained one egg. It is probable that this bird nests at all times of the year. The nuptial flight of these birds reminds one very much of that of the mourning dove of North America.
Remarks.—The Philippine Turtle Dove was introduced from the Philippines to Guam and other islands of the southern Marianas by the Spanish probably in the 18th Century; it was in 1771-1774 that the Philippine deer (Rusa) was introduced to Guam. Perhaps these birds were initially introduced as caged birds or possibly were liberated to offer hunting for the colonial governors. They have been a very successful introduction and are well established. At Guam (see Baker 1947b:124), this species comprised 15.5 percent of all birds seen along roadways. Although open areas appear to be preferred by this dove and although it may be on the increase owing to the clearing operations of the war effort, it appears to be equally adapted to forested areas and coconut groves. It feeds on the ground to a large extent, fitting into an ecologic niche which few other species of birds of the islands occupy. It was even observed feeding on sandy beaches and tidal flats at low tide.
In 1931, Coultas found the dove to be numerous at Guam, but thought that it was in danger of extinction at Tinian and Saipan owing to extensive hunting. Downs (1946:96) reported that in 1945 the dove was abundant at Tinian. Gleise (1945:22) estimated the population of these doves at 300 on Tinian in 1945. From the remarks of Stott (1947:526), we may assume that the population at Saipan is in no immediate danger of extinction.
A comparison of specimens from the Marianas with those from the Philippines reveals no significant difference between the two. Bonaparte described the dove in the Marianas as new, naming it Streptopelia gaimardi. The name Turtur prevostianus has been used by some authors to denote the dove in the Marianas, but this was through error as explained by Salvadori (1893:410). This name refers to a dove found on Marianne, an island of the Seychelles in the Indian Ocean.
Gallicolumba canifrons (Hartlaub and Finsch)
Palau Ground Dove
Phlegoenas canifrons Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 101. (Type locality, Pelew Islands.)
Phlegoenas canifrons Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 27, pl. 5, fig. 1 (Palau); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 57 (Pelew); Hartert; Novit. Zool., 5, 1898, p. 61 (Pelew); Matschie, Journ. f. Ornith., 1901, p. 113 (Palau); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 53 (Pelews).
Phlogoenas canifrons Sclater, Proc. Zool. Soc. London, 1877, p. 112 (Pelew); Salvadori, Ornith.] Papuasia, 3, 1882, p. 169 (Pelew); idem, Cat. Birds British Mus., 21, 1893, p. 592 (Pelew); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 227 (Palaos); Bolau. Mitteil. Naturhist. Mus. Hamburg, 1898, p. 68 (Palau); Dubois, Syn. Avium, 2, 1904, p. 772 (Pelew).
Phaps canifrons Giebel, Thes. Ornith., 3, 1877, p. 89 (Pelew).
Gallicolumba canifrons canifrons Mathews, Syst. Avium Australasianarum, 1, 1927, p. 74 (Pelew).
Gallicolumba canifrons Hand-list Japanese Birds, rev., 1932, p. 189 (Palau); Mayr, Amer. Mus. Novit., no. 828, 1936, p. 4 (Palau); Peters, Check-list Birds World, 3, 1937, p. 136 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 211 (Babelthuap); Mayr, Birds Southwest Pacific, 1945, p. 290 (Palau); Mayr, Audubon Mag., 47, 1945, p. 282 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 62 (Garakayo, Peleliu).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Peleliu, Ngabad, Angaur.
Characters.—Adult male: A small, ground dove with forehead, crown, sides of head, chin, throat, and breast ashy gray, lighter on forehead, chin, and throat, and washed with "light vinaceous-faun" on breast; occiput, nape and mantle dark "ferruginous"; rest of upper parts glossed with bronze-olive; lesser and middle wing-coverts tipped with metallic purple; wings reddish-brown with dark brown tips; under side of wing reddish-brown to brown; abdomen, vent and under tail-coverts dark grayish-brown; tail colored like back, outer feathers have a paler brown terminal band rather obscure; bill horn colored; feet red; iris brown.
Female: A female molting into adult plumage is cinnamon colored, mottled with dark brown; on the back an olive-green sheen is beginning to appear; tail brown with some greenish sheen; tips of tail edged with light brown; bill and feet light brown.
Measurements.—Measurements of six adult males are: wing, 112-119 (115); tail, 65-72 (70); exposed culmen, 15.3-16.1 (15.7); tarsus, 30.1-31.2 (30.8); of one female in postjuvenal molt: wing, 107; tail, 69; exposed culmen, 17.1; tarsus, 30.
Specimens examined.—Total number, 8 (7 males, 1 female), as follows: Palau Islands, USNM—Koror, 1 (Nov. 18)—Garakayo, 2 (Sept. 17, 19)—Peleliu, 2 (Sept. 1, Dec. 5)—Ngabad, 1 (Sept. 11); AMNH—exact locality not given, 1 (Dec. 1).
Food habits.—Stomachs of specimens taken by the NAMRU2 party at Peleliu and Garakayo contained one and one-half to two cc. of hard seeds and seed parts.
Remarks.—The Palau Ground Dove, according to Amadon (1943:19), is a member of a superspecies containing G. hoedti (Wetar), G. beccarii (New Guinea, Bismarcks, Solomons), G. sanctaecrucis (Santa Cruz, New Hebrides), and G. stairi (central Polynesia).
G. canifrons apparently came to the Palaus from either New Guinea or the region of the Bismarck Archipelago, evolving from G. beccarii or some related form. The Palau Ground Dove has a copper-colored occiput, nape, and shoulder patch, but otherwise it resembles this Melanesian species, G. beccarii. Amadon (1943:20) discusses two types of plumage of females in G. stairi; one is a male type of plumage. The lack of female specimens prevents me from determining whether this characteristic is present in G. canifrons.
Coultas (field notes) had difficulty in obtaining even one specimen of G. canifrons in the Palaus in 1931. He concluded that either the bird was practically extinct or that he just could not find it. From the experience of the NAMRU2 party in the southern Palaus in 1945, I would think that he merely did not find the bird. Although it is probably rare in comparison with some other members of the family Columbidae of these islands, we found this bird on most of the islands visited.
The NAMRU2 party arrived at Palau expecting to find the ground dove a fairly conspicuous member of the avifauna and expecting to see it sitting in trees and flying across the roads much in the same manner as did the ground dove at Guam, G. x. xanthonura. At first, we did not find the bird, but in the dense jungles a low, penetrating, and intermittent, call was heard which may be described as a moan. This was the call of the ground dove. The bird was difficult to discover because its color blended so well with the shadows and dark background of the coral rocks and forest litter. The bird was very active and moved along rapidly pecking at food particles. Also it was wary. Once the distinctive call note was recognized, it was not difficult to locate the area in which the bird was living; however, finding the bird was difficult. On one occasion I stalked a dove for at least a half an hour knowing that it was always within fifty yards of me. A bird that was flushed, flew about twenty-five feet and dropped down in open forest litter and disappeared. On the basis of specimens collected and call notes heard, I estimate that the population of the Palau Ground Dove on the islands visited in 1945 was as follows: Peleliu—a minimum of 15 (found in most forested areas which were not greatly damaged by the invasion operations); Garakayo—a minimum of 10 (the doves were found to live equally well on the steep hillsides or in flat jungle on this islet); Ngabad—5 to 10 (doves were heard in several areas on this small islet); Angaur—not estimated (one call was heard in brush near the edge of a fresh water lake).
Gallicolumba xanthonura xanthonura (Temminck)
White-throated Ground Dove
Columba xanthonura Temminck, Pl. col., livr. 32, 1823, pl. 190. (Type locality, Mariannes.)
Columba xanthonura Lesson, Compl. de Buffon, 2nd ed., 2, Oiseaux, 1838, p. 281 (Mariannes).
Columba Pampusan Quoy and Gaimard, Voy. "Uranie," Zool., 1824, pp. 121, 681, pl. 30 (Mariannes); Dumont, Dict. Sci. Nat., ed. Levrault, 40, 1826, p. 345 (Guam); Lesson, Traité d'Ornith., 1831, p. 471 (Mariannes); Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen).
Columba erythroptera Lesson, Traité d'Ornith., 1831, p. 471 (Mariannes); Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 305 (Guahan); Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen).
Columba xanthura Prévost and Knip, Les Pigeons, 2, 1838-43, p. 45, pl. 23 (Guam).
Pampusana xanthua Bonaparte, Consp. Avium, 2, 1854, p. 89 (Mariannis); idem, Comptes Rendus Acad. Sci. Paris, 40, 1855, p. 207 (Mariannes); Reichenbach, Tauben, 1861, p. 39 (Guam).
Caloenas (Pampusana) xanthura Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 45 (Guam).
Phlegoenas erythroptera Reichenbach, Tauben, 1861, p. 41 (Mariannen).
Caloenas xanthura Gray, Hand-list Birds, 2, 1870, p. 247 (Marian).
Phlegoenas yapensis Hartlaub and Finsch, 1872, p. 102 (Type locality, Uap); Gräffe, Journ. Mus. Godeffroy, 2, 1873, pp. 122, 123 (Yap); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 391 (Yap); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 57 (Yap); Hartert, Novit. Zool., 5, 1898, p. 61 (Yap); Matschie, Journ. f. Ornith., 1901, p. 113 (Yap).
Pampusana rousseaui Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 103 (Marianne).
Phaps erythroptera Giebel (part), Thes. Ornith., 3, 1877, p. 89 (Marianne).
Phaps xanthura Giebel, Thes. Ornith., 3, 1877, p. 91 (Marianne).
Phaps yapensis Giebel, Thes. Ornith., 3, 1877, p. 91 (Uap).
Phlegoenas virgo Reichenow. Journ. f. Ornith., 1885, p. 110 (Type locality, Palau-Inseln, error = Guam).
Phlogaenas erythroptera Oustalet, Le Nat., 1889, p. 261 (Mariannes).
Phlegoenas pampusan Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 55 (Marianne); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 224 (Saypan, Guam, Rota).
Phlogoenas yapensis Salvadori, Cat. Birds British Mus., 21, 1893, p. 593 (Uap); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 227 (Mackensie); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 68 (Yap); Dubois, Syn. Avium, 2. 1904, p. 772 (Uap).
Phlogoenas pampusan Salvadori, Cat. Birds British Mus., 21, 1893, p. 602 (Marianne).
Phlegoenas xanthonura Hartert, Novit. Zool., 5, 1898, p. 60 (Guam, Saipan); Wheeler, Report Island of Guam, 1900, p. 13 (Guam); Matschie, Journ. f. Ornith., 1901, p. 113 (Guam, Saipan); Safford, Amer. Anthro., 4, 1902, p. 711 (Guam); idem, Osprey, 1902, p. 68 (Mariannas); idem, The Plant World, 7, 1904, p. 264 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 78 (Guam); Cox, Island of Guam, 1917, p. 20 (Guam).
Phlogoenas xanthonura Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 42 (Marianas); Reichenow, Die Vögel, 1, 1913, p. 331 (Mariannen); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 24 (Guam).
Phlegoenas xanthonura xanthonura Kuroda, in Momiyama, Birds Micronesia, 1922, p. 54 (Guam, Rota, Saipan).
Phlegoenas xanthonura yapensis Kuroda, in Momoyama, Birds Micronesia, 1922, p. 54 (Yap).
Gallicolumba xanthonura Mathews, Syst. Avium Australasianarum, 1, 1927, p. 75 (Marianas, Mackenzie); Hand-list Japanese Birds, rev., 1932, p. 189 (Pagan, Almagan, Saipan, Tinian, Rota, Mackenzie); Mayr, Amer. Mus. Novit., no. 828, 1936, p. 4 (Marianne); Peters, Check-list Birds World, 3, 1937, p. 136 (Marianne, Yap); Hand-list Japanese Birds, 3d ed., 1942, p. 211 (Yap, Assongsong, Pagan, Almagan, Saipan, Tinian, Rota); Strophlet, Auk, 1946, p. 538 (Guam); Wharton, Ecol. Monogr., 16, 1946, p. 174 (Guam); Baker, Condor, 49, 1947, p. 125 (Guam).
Gallicolumba canifrons yapensis Mathews, Syst. Avium Australasianarum, 1, 1927, p. 74 (Yap).
Terricolumba xanthonura Yamashina, Tori, 10, 1940, p. 677 (Assongsong).
Gallicolumba xanthonura xanthonura Mayr, Birds Southwest Pacific, 1945, p. 290 (Marianas, Yap); Watson, The Raven, 17, 1946, p. 41 (Guam); Stott, Auk, 1947, p. 526 (Saipan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 61 (Guam, Rota Yap).
Gallecolumba xanthonura xanthonura Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 96 (Tinian).
Fig. 14. Geographic distribution of Gallicolumba of Micronesia and Eastern Polynesia and routes of its dispersal. (1) G. jobiensis; (2) G. x. kubaryi; (3) G. x. xanthonura; (4) G. erythroptera; (5) G. rubescens.
Geographic range.—Micronesia: Mariana Islands—Asuncion, Pagan, Almagan, Saipan, Tinian, Rota, Guam; Caroline Islands—Yap.
Characters.—Adult male: Forehead, face, chin, throat, and upper breast white, lightly washed with pale buff; crown, occiput, sides of head, and nape rusty brown to dark brown; rest of upper parts dark bronze-olive; feathers of mantle and upper wing-coverts broadly edged with metallic purple-violet; primaries, under wing-coverts and axillaries brown; tail, lower breast and rest of underparts dark brown; bill and feet dark brown.
Adult female: Resembles adult male, but smaller and with underparts colored between "ochraceous-tawny" and "cinnamon brown" instead of dark brown and white; head and neck darker and with more rufous than underparts; remainder of upper surface resembles underparts but with striking olive green sheen, especially on upper wing-coverts; primaries brown but outer webs lighter; tail rufous-brown, with a broad, black subterminal band.
The male type of plumage in the adult female is: breast light drab tinged with light brown and darkening anteriorly; crown resembles that of normal female although darker and becoming lighter and grayer on neck and nape; shoulder and wing-coverts compare favorably with that of adult male although lighter and with yellowish tinge; back bronzed olive-green as in normal female but mantle with a few purplish feathers characteristic of male; abdomen near "olive brown" with buffy-brown edges to feathers.
Immature male: Resembles adult male, but head and nape darker brown; throat and upper breast may be more brown and less white.
Immature female: Resembles adult female, but with more rufous coloring; olive-green sheen on feathers reduced in amount or absent.
Measurements.—Measurements are found in table 28.
Subspecies Number Wing Tail Culmen Tarsus G. x. xanthonura 43 males
146 102 22.0 32 (139-153) (97-111) (21.0-23.0) (31-33) 31 females
136 94 20.5 30 (131-141) (90-98) (20.0-21.5) (28-32) G. x. kubaryi 7 males
157 23.0 35 (152-160) (20.5-23.5) (33-35) 7 females
148 23.0 33 (145-151) (22.5-23.5) (32-34) There is little difference in the measurements of specimens from Guam, Rota, Tinian, Saipan, and Asuncion. No specimens from Yap were available for examination.
Weights.—The NAMRU2 party obtained weights of this ground dove from Guam as follows: seven adult males 119-154 (130); seven adult females 96-150 (118).
Specimens examined.—Total number, 96 (50 males, 38 females, 8 unsexed) as follows: Mariana Islands, USNM—Guam, 29 (Mar. 18, April 4, 17, May 20, 28, June 2, 9, 13, 14, 15, 20, 23, 24, 27, 28, July 2, 6, 10, 23, Aug. 11, 21)—Rota, 6 (Oct. 20, 22, 25, 26, Nov. 1, 2)—Tinian, 4 (Oct. 24, 26); AMNH—Guam, 40 (Jan. 17, 30, Feb. 12, 20, March 3, 5, 7, 11, 13, 23, April 13, 19, June 13, 15, July 10, 25, Aug. 4, 10, 11, 13, 15, 18, 20, 21, 22, Sept. 4, Dec. 26, 30)—Tinian, 8 (Sept. 7, 10, 11, 12, 13)—Saipan, 6 (July 13, 15, Aug. 24, Sept. 7, 8)—Asuncion, 3 (Jan. 18, Feb. 7, June).
Nesting.—The NAMRU2 party found the ground dove nesting at Guam in the winter and spring months beginning in late January. Nests were observed in tall trees, many of which were well isolated from other trees and vegetation. On February 10 a nest was discovered in a breadfruit tree near one of the NAMRU2 barracks on Oca Point. It was approximately 50 feet above the ground. On February 26 I found pieces of egg shell beneath the tree. Occasionally during the day, the male, but never the female, was observed sitting on this nest. On February 10, a dove (the male) was observed building a nest in a large banyan tree at Oca Point. Another nest was being constructed by a female on March 7. On March 17 a young female dove, just beginning to fly, was taken; another was found on April 3. Adult birds with enlarged gonads were taken in April, May, June, and July. Marche, according to Oustalet (1895:224), obtained eggs in May, 1887.
Food habits.—Stomachs of doves taken at Guam contained fruits and fruit parts. On March 9, a dove was observed feeding on the berries of the shrub known as "inkbush." This appeared to be a favorite food. Seale (1901:42) also mentions that this berry is a preferred food.
Parasites.—Wharton (1946:174) lists the chigger (Acarina), Trombicula sp., from the ground dove at Guam.
Remarks.—At Guam, the NAMRU2 party observed the ground dove to be fairly common in 1945. Along roadways, the present author (1947b:124) found that individuals of this species comprised 2.5 percent of the total population of birds observed, and the ground dove was seen on 31.2 percent of 125 road counts made. The male was much more in evidence than the female and was frequently seen flying high over the roadways and jungle areas; eighty percent of the ground doves seen while road-counts were being made were males. The female was found less frequently; it was a less conspicuous bird and was seen only occasionally in flight. Neither sex appeared to have the secretive, terrestrial habits of G. canifrons of the Palau Islands. On the basis of our observations at Guam, I would say that the name "ground dove" for the bird at Guam is not descriptive. The birds were found to spend considerable time in tall trees; the closest that I saw them to the ground was when they were feeding only three to four feet from the ground in the ink berry bushes.
The call note of this dove is much like that of the Palau Ground Dove; Seale (1901:42) describes it as follows, "These pigeons seem to prefer the deep jungle, from whence their deep low moan, like the sound of a man dying in great distress, comes with a weird uncanny effect, heightened by the gloom and darkness of the unknown forest.... This sound, which always seems to come from a long distance, is very misleading, and one is considerably surprised to find he is perhaps within a few feet of the bird." Seale writes that they were very common on Guam in 1900. In 1931, Coultas found the dove "quite common at the north end of the island." The bird apparently prefers the dense forest or second growth brushy areas, but was found also in the partly cleared areas surrounding the NAMRU2 headquarters at Oca Point in 1945. At Rota, the NAMRU2 party found the birds to be numerous in 1945. Coultas observed only a few birds on Tinian in 1931; Downs (1946:96) found only a small population at this island in 1945. The extensive cultivation and clearing activities at Tinian have removed much of [Pg 207] the habitat suitable for these, as well as other birds. At Saipan, Stott (1947:526) writes that the bird is common on "brush-covered hillsides and semi-wooded country." There is little information published regarding the status of this dove in the northern Marianas.
Gallicolumba xanthonura kubaryi (Finsch)
White-throated Ground Dove
Phlegoenas Kubaryi Finsch, Journ. f. Ornith., 1880, p. 292. (Type locality, Ruck and Ponapé.)
Phlegoenas erythroptera Bonaparte, Consp. Avium, 2, 1854, p. 89 (Carolines); Reichenbach, Tauben, 1862, p. 41 (Carolines); Finsch, Proc. Zool. Soc. London, 1877 (1878), p. 780 (Ponapé); idem, Ibis, 1881, p. 115 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 281, 353 (Ponapé, Ruk); Tristram, Cat. Birds, 1889, p. 41 (Ruk).
Phlegoenas kubaryi Reichenow and Schalow, Journ. f. Ornith., 1881, p. 75 (Ruk, Ponapé); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 55 (Ruk, Ponapé); Hartert, Novit. Zool., 7, 1900, p. 8 (Ruk, Ponapé); Matschie, Journ. f. Ornith., 1901, p. 113 (Ruck, Ponapé); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 53 (Ruk, Ponapé).
Phlogoenas erythroptera Finsch, Proc. Zool. Soc. London, 1880, p. 576 (Ponapé, Ruk); Takatsukasa and Kuroda, Tori, 1, 1915, p. 52 (Ruk).
Phlogoenas kubaryi Salvadori, Cat. Birds British Mus., 21, 1893, p. 599 (Ruk, Ponapé); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 227 (Caroline = Truk); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 68 (Ruck); Reichenow, Die Vögel, 1, 1913, p. 331 (Karolinen).
Phlegaenas kubaryi Christian, The Caroline Islands, 1899, p. 357 (Ponapé).
Gallicolumba kubaryi Mathews, Syst. Avium Australasianarum, 1, 1927, p. 74 (Caroline Is.); Hand-list Japanese Birds, rev., 1932, p. 189 (Truk, Ponapé); Peters, Check-list Birds World, 3, 1947, p. 136 (Ruk, Ponapé); Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé); Bequaert, Mushi, 12, 1939, p. 81 (Ponapé); idem, Occ. Papers Bernice P. Bishop Mus., 16, 1941, p. 266 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 211 (Truk, Ponapé).
Gallicolumba xanthonura kubaryi Mayr, Birds Southwest Pacific, 1945, p. 290 (Truk, Ponapé); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 62 (Truk).
Geographic range.—Micronesia: Caroline Islands—Truk, Ponapé.
Characters.—Adult male: Resembles adult male of G. x. xanthonura, but larger with crown, nape, and hind neck sooty-black; upper back and lesser upper wing-coverts purplish-violet, extending lower on back than in G. x. xanthonura.
Adult female: Resembles adult male, but smaller and paler with upper back glossy, bronze-green margined with purplish-violet; lower back and rump glossy, olive-green; upper tail-coverts greenish-brown; central tail feathers blackish-brown; innermost secondaries bright, glossy green tinged with bluish.
Measurements.—Measurements are presented in table 28.
Specimens examined.—Total number, 21 (9 males, 11 females, 1 unsexed), as follows: Caroline Islands, USNM—Truk, 1 (July); AMNH—Ponapé, 13 (Nov., Dec.)—Truk, 7 (Jan., Feb., May).
Nesting.—At Ponapé in November and December, Coultas obtained specimens which had enlarged gonads. He did not find the nest of this bird but writes (field notes) that the natives told him that the nest is placed in the top of the tree fern 10 or 15 feet above the ground. In contrast, the ground dove at Guam may select a nesting site considerably higher in the tree. Coultas reports that one egg is laid by C. x. kubaryi.
Food habits.—Coultas (field notes) writes that the bird feeds and lives on the ground at Ponapé. He lists food as small snails, seeds, and worms.
Parasites.—Bequaert (1939:81 and 1941:266) records the fly (Hippoboscidae), Ornithoctona plicata, from the ground dove at Ponapé.
Remarks.—Coultas (field notes) writes that in 1930 the ground dove at Ponapé was rare in the forested areas and generally found more along the sea coast and in the upland valleys. Coultas describes its call as an infrequent shrill, whistle-like call. He writes that hunting by the Japanese and natives was reducing the population of G. x. kubaryi at Ponapé in 1930. In 1945, McElroy of the NAMRU2 party found the dove at Truk on forested slopes in tall trees, and reported that its habits at Truk were similar to those of C. x. xanthonura at Guam. In 1947-1948, Richards noted (in litt.) that the dove at Ponapé was rare (he saw only one specimen). At Truk, he found the bird to be "rather common" in thickets, dry gullies, and flying over grassy slopes. He found the bird near sea level, never in country above 300 feet in altitude and not in deep forest. I offer no explanation for the conflicting reports concerning the habits of this species, unless it be that the bird is capable of varying its habits to fit particular habitats; for example, in jungle areas it may be ground-living and in open woodlands it may be tree-living.
Evolutionary history of Gallicolumba in Micronesia.—There have been two unrelated invasions of Micronesia by the genus Gallicolumba. One invasion established G. canifrons at the Palau Islands. The other established the populations of G. xanthonura in the Caroline and Mariana islands, Mayr (1936:4) points out that G. xanthonura is related to G. jobiensis (New Guinea and Northern Melanesia), G. erythroptera (Society and Tuamotu islands), and G. rubescens (Marquesas Islands). This group may be regarded as a superspecies. The adults of G. jobiensis, the male and female, resemble one another. In both, the head, neck, and auriculoloral stripes are sooty-black; the eye stripe, chin, throat, and breast are white; the abdomen is dark; and the upper parts are blackish with a coppery sheen. Immatures are rusty-brown. G. xanthonura is closely related to G. jobiensis, and they conceivably, along with G. erythroptera, might be considered conspecific. The close relationship between the G. xanthonura in Micronesia and G. erythroptera has been noted by Oustalet (1896:71). Among named kinds, G. x. kubaryi most closely resembles G. jobiensis with sooty-black coloring present on the head. The male and female of G. x. kubaryi [Pg 209] closely resemble each other, although immature type of plumage may occur in adult females as indicated by the immature plumage of a bird containing well-developed eggs taken at Ponapé by Coultas.
In G. x. xanthonura the male lacks the sooty-black head and has lost some of the coppery sheen from the middle of the back. The female has taken on the immature type of plumage, except for occasional near-male type plumage. In G. erythroptera the male has lost some of the sooty-black coloring on the forehead, anterior crown, and loral area and some of the coppery sheen in the middle of the back. The female of G. erythroptera resembles the female of G. x. xanthonura except that the throat and breast are faintly outlined by the brownish color. The head and malar stripe are also outlined in this manner. Some females have some coppery gloss on the shoulder and a few white feathers on the breast; these may be considered as in the near-male type of plumage.
The tendencies in the evolution of these insular populations of Gallicolumba include a reduction of sooty-black on the head and a reduction of coppery gloss on the back of the male and the reduction of malelike plumage in the female. G. rubescens of the Marquesas Islands is smaller and darker. It retains the coppery gloss on the back and has, in addition, a white bar on the tail and one on the wing. On the basis of color and structural characters, it is apparent that this superspecies of Gallicolumba has evolved from a center of evolution in the region of New Guinea (as shown in figure 14) with a colonization of Micronesia, from which (probably from G. x. kubaryi) an invasion of eastern Polynesia occurred establishing G. erythroptera in the Society and Tuamotu islands, although it is also possible that G. erythroptera may have reached Polynesia by way of a more direct route from Melanesia. Such a pathway of colonization as that just described is not unusual since representatives of other genera including Acrocephalus, Myzomela, and Zosterops may have followed similar paths of dispersal from Micronesia into Polynesia. Apparently a population isolated in the Marquesas has evolved the distinctive G. rubescens.
Caloenas nicobarica pelewensis Finsch
Nicobar Pigeon
Caloenas nicobarica var. pelewensis Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 159 (in reprint p. 27). (Type locality, Palau.)
Caloenas nicobarica pelewensis Mathews, Syst. Avium Australasianarum, 1, 1927, p. 77 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 188 (Palau); Peters, Check-list Birds World, 3, 1937, p. 139 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 210 (Babelthuap, Koror); Mayr, Birds Southwest Pacific, 1945, p. 291 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 62 (Garakayo).
Caloenas nicobarica Salvadori, Ornith. Papuasia, 3, 1882, p. 211 (Pelew); Wiglesworth, Abhandl. und. Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 57 (Pelew).
Caloenas pelewensis Salvadori, Cat. Birds British Mus., 21, 1893, p. 618 (Pelew); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 69 (Palau); Matschie, Journ. f. Ornith., 1901, p. 113 (Palau); Reichenow, Die Vögel, 1, 1913, p. 328 (Palauinseln); Takatsukasa and Kuroda, Tori, 1, 1915, p. 52 (Pelew).
Caloenas nicobaricus pelewensis Kuroda, in Momiyama, Birds Micronesia, 1922, p. 53 (Pelew).
Geographic range.—Micronesia: Palau Islands-Babelthuap, Koror, Garakayo.
Characters.—Adult: A large heavy-bodied pigeon with head, neck, and upper breast blackish; rest of plumage metallic bluish-green with coppery sheen; wings glossy green; tail and under tail-coverts white; feathers of hind-neck long and lanceolate; bill heavy and slightly hooked with lump at base.
Resembles C. n. nicobarica (Linnaeus), but slightly smaller and with upper parts metallic bluish-green and underparts darker and less green.
Measurements.—One adult female measures: wing, 232; tail, 82; culmen, 31; tarsus, 44; one immature female: wing, 236; tail, 89; culmen, 32; tarsus, 45.
Specimens examined.—Total number, three females from Palau Islands, AMNH—exact locality not given (undated).
Remarks.—C. nicobarica is distributed from the Nicobar Islands east through Malaysia to Melanesia as a single undifferentiated form. In the northeasternmost part of its range, in the Palau Islands, it exhibits geographic variation and is considered to be subspecifically distinct from the rest of the population. C. nicobarica appears to have no close relatives. It may represent the last remnant of some ancient group of pigeons.
The Nicobar Pigeon is rare. Coultas, who visited the islands in 1931, did not obtain the bird. The only specimens available for study are those in the collections of the American Museum of Natural History taken by Kubary in the period between 1870 and 1880. The NAMRU2 party did not obtain specimens but saw the bird on five occasions at the island of Garakayo in the middle Palaus. The writer expected the bird to be ground-living in habit, but the individuals, which I saw at Garakayo, were either perched on scrubby vegetation on high and inaccessible cliffs or were flying high overhead. In its flight overhead, the short, white tail was a particularly conspicuous mark of identification. The flight reminded me very much of that of the Black Vulture (Córagyps atrátus) of North America. No birds were found at Peleliu or Angaur, and the small population of this pigeon that remains is probably restricted to uninhabited coral islets, as Mayr (1945a:291) has already noted. Marshall (1949: 207) saw one bird on Peleliu and one on Koror in November and December, 1945. This endemic subspecies is probably on the [Pg 211] road to extinction unless governmental protection can be established and enforced.
Trichoglossus rubiginosus (Bonaparte)
Ponapé Lory
Chalcopsitta rubiginosus Bonaparte, Comptes Rendus Acad. Sci. Paris, 30, February, 1850, p. 134; Consp. Avium, 1, after April 15, 1850, p. 3. (Type locality, "ex Insulis Barabay et Guebe," error = Ponapé.)
Chalcopsitta rubiginosus Bonaparte, Proc. Zool. Soc. London, 1850, p. 26, pl. 16 ("Ins. Barabay et Guebe," error = Ponapé); Pelzeln, Reise "Novara," Vögel, 1865, pp. 99, 162 (Puynipet); Reichenow, Journ. f. Ornith., 1881, p. 162 ("Nordwestl. Polynessische subregion Carolinen" = Ponapé); Tristram, Cat. Birds, 1889, p. 73 (Ponapé); Finsch, Deut. Verein zum Schultze der Vogelwelt, 18, 1893, p. 458 (Carolinen = Ponapé); Matschie, Journ. f. Ornith., 1901, p. 112 (Ponapé).
Domicella rubiginosa Finsch, Die Papageien, 2, 1868, p. 781 (Puynipet); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 88 (Puinipet).
Lorius rubiginosus Gray, Hand-list Birds, 2, 1870, p. 153 (Puynipet); Schlegel, Mus. Pays-Bas, 3, no. 38, 1874, p. 58 (Puynipet).
Lorius rubiginosa Giebel, Thes. Ornith., 2, 1875, p. 502 (Senjawin = Ponapé).
Trichoglossus rubiginosus Finsch, Journ. Mus. Godeffroy, 12, 1876, pp. 17, 18 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 778 (Ponapé); idem, Journ. f. Ornith., 1880, p. 284 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 281 (Ponapé); Finsch, Ibis, 1881, pp. 110, 111, 114 (Ponapé); idem, Mitth. Ornith. Ver. Wien, 1884, p. 49 (Ponapé); Hartert, Kat. Vogelsamml. Senckenb., 1891, p. 161 (Puypinet); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6 1890-1891 (1891), p. 8 (Ponapé); Peters, Check-list Birds World, 3, 1937, p. 151 (Ponapé); Mayr, Proc. Sixth Pac. Sci. Congr., 4, 1941, p. 204 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 201 (Ponapé); Mayr, Birds Southwest Pacific, 1945, p. 291 (Ponapé).
Eos rubiginosa Salvadori, Ornith. Papuasia, 1, 1880, p. 267 (Puynipet); idem, Cat. Birds British Mus., 20, 1891, p. 29 (Ponapé); Christian, The Caroline Islands, 1899, p. 357 (Ponapé); Finsch, Notes Leyden Mus., 22, 1900, p. 142 (Ponapé); Dubois, Syn. Avium, 1902, p. 29 (Puinipet); Uchida, Annot. Zool. Japon., 9, 1918, pp. 484, 493 (Ponapé); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 192 (Ponapé).
Chalcopsittacus rubiginosus Finsch, Sammlung wissensch. Vorträge, 14th Ser., 1900, p. 639 (Ponapé).
Oenopsittacus rubiginosus Reichenow, Die Vögel, 1, 1913, p. 443 (Karolinen = Ponapé); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 58 (Ponapé); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 295 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 181 (Ponapé).
Eos rubiginosus Takastukasa and Kuroda, Tori, 1, 1915, p. 53 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult: A medium-sized, dark raspberry-red lory with head and nape deep purplish-red; upper back, scapulars, and upper wing-coverts raspberry-red, edged with blackish; lower back, rump, and upper tail-coverts more purplish; tail yellowish-green becoming more yellow and less green toward tip; wings black with outer webs olivaceous-green; outer edges of primaries more yellowish; lores, chin, auriculars, sides of head, and neck deep purplish-red, chin feathers faintly barred with raspberry and edged with blackish; throat, breast, abdomen, and flanks raspberry-red, feathers edged with blackish except on lower abdomen; under tail-coverts orange-red, under wing-coverts deep purple with black edges; bill of male orange, of female paler yellow; feet black; iris of male light yellowish-orange, of female grayish-white.
Immature: Resembles adult, but with narrow and more sharply pointed tail feathers.
Measurements.—Measurements are presented in table 29.
Sex No. Wing Tail Culmenybr />from
cereTarsus Adult males 18 147 105 20 16 (143-153) (100-110) (19-20) (15-17) Adult females 13 142 101 19 16 (141-146) (98-104) (18-19) (15-17) Specimens examined.—Total number, 31 (18 males, 13 females), as follows: Caroline Islands, USNM—Ponapé, 2 (Feb. 12); AMNH—Ponapé, 29 (Nov.).
Nesting.—According to Coultas (field notes) the nest is placed in the top of a coconut tree or in a hollow of a large forest tree. He says that one egg is laid, but does not record dates of nesting. Four of the birds taken by Coultas at Ponapé in November had swollen gonads.
Molt.—Specimens taken in November by Coultas were either in fresh plumage or were completing the molt when obtained.
Parasites.—Uchida (1918:484, 493) found the bird lice (Mallophaga), Psittaconirmus harrisoni and Eomenopon denticulatus, on the Ponapé Lory.
Remarks.—There is little written information concerning the habits of the Ponapé lory. Mayr (1945a:291) describes the bird as being "very noisy" and with "habits apparently similar to T. haematodus." Coultas made a number of observations on this species; some of these unpublished notes are essentially as follows: Trichoglossus is common on Ponapé. It is found everywhere on the island, preferring the coconut palms; it is noisy and quarrelsome. The parrot travels usually in small groups of two to six or eight birds, keeping up a continuous chatter all of the time. This chatter quiets down into a very pleasant-sounding crooning-tone after sunset. Trichoglossus is a continual nuisance to the hunter, inquisitive and easily attracted by the slightest noise, to which the bird responds with a frantic yapping that frightens everything within a radius of a mile. One sometimes finds a bird alone working quietly about among the low trees of the high mountain ridges. The natives' name for the bird, "se ridt," means "always hide out in rain." The bird stays under a big leaf and keeps dry during the rain. This lory is intelligent, easily tamed, and sometimes learns to repeat a few words.
Evolutionary history of Trichoglossus rubiginosus.—The Ponapé Lory is the only native parrot in Micronesia. It is an aberrant species and seemingly is of long residence on the island, as indicated by its differences from related forms to the southward and southwestward. The bird shows some relationships to T. ornatus (Linnaeus) of Celebes, but the plumage of T. rubiginosus lacks the brilliant red, green, and yellow of this bird. The plumage of the Ponapé Lory is also softer in texture; this is a character exhibited also by other Micronesian birds, for example, Cleptornus and Colluricincla. T. rubiginosus and T. ornatus correspond, however, in having the feathers of the breast edged with blackish. T. rubiginosus resembles also T. flavovirides of Celebes and Sula in that the edges of the feathers of the breast are dark, no markings are present on the inner web of the wing, and feathers of the upper back are edged with dark coloring. T. rubiginosus may have been derived from either of these two species; however, it shows a close relationship also to the T. haematodus group from the Papuan region. In any case, the Ponapé Lory, isolated in Micronesia, has not the multicolored plumage of its relatives and has, instead, a rather uniformly colored plumage. The presence of this parrot at only a single island in Micronesia is difficult to explain; perhaps at one time the bird was more widely distributed in Micronesia, or it may be that the population represents a single successful invasion to Ponapé. Like Aplonis pelzelni, another endemic species at Ponapé, this lory may have reached the island as a straggler, perhaps being carried north by the prevailing winds in the post-nesting season.
Cuculus canorus telephonus Heine
Common Cuckoo
Cuculus telephonus Heine, Journ. f. Ornith., 1863, p. 332. (Type locality, Japan.)
Cuculus canorus Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 100 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 12 (Palau); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 10 (Pelew); Takatsukasa and Kuroda, Tori, 1, 1915, p. 63 (Pelew).
Cuculus canorus telephonus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 57 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 181 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 201 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Breeds in northeastern Asia and Japan. Winters south to India, Malaysia, and Melanesia. In Micronesia: Palau Islands—exact locality not given.
Remarks.—The Common Cuckoo is a straggler on winter migration to the Palau Islands.
Cuculus saturatus horsfieldi Moore
Oriental Cuckoo
Cuculus horsfieldi Moore, in Moore and Horsfield, Cat. Birds Mus. Hon. East-India Co., 2, 1856-58 (1857), p. 703. (Type locality, Java.)
Cuculus striatus Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 100 (Pelew); Finsch. Journ. Mus. Godeffroy, 8, 1875, pp. 4, 12 (Palau); Takatsukasa and Kuroda, Tori, 1, 1915, p. 63 (Pelew).
Cuculus intermedius Wiglesworth. Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 10 (Pelew).
Cuculus optatus optatus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 57 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 181 (Palau).
Cuculus saturatus horsfieldi Hand-list Japanese Birds, 3d ed., 1942, p. 201 (Babelthuap, Koror); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Breeds in eastern Asia and Japan. Winters south to India, Malaysia, and Melanesia. In Micronesia: Palau Islands—Babelthuap, Koror.
Remarks.—The Oriental Cuckoo reaches the Palau Islands as a winter visitor. On November 11 and 25 of 1931, Coultas obtained four immature birds at Palau near taro swamps. The natives told him that the cuckoo visited the islands each year from December to June. On September 21 at Angaur the NAMRU2 party saw one bird which may have been this cuckoo.
Eudynamis taitensis (Sparrman)
Long-tailed New Zealand Cuckoo
Cuculus taitensis Sparrman, Mus. Carls., fasc, 2, 1787, pl. 32. (No type locality = Tahiti.)
Eudynamis tahitiensis Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap).
Eudynamis taitiensis Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 49 (Palau); idem, Journ. Mus. Godeffroy, 12, 1876, pp. 17, 20 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 778 (Ponapé); idem, Journ. f. Ornith., 1880, pp. 284, 298 (Ponapé, Kuschai, Palaos, Marshalls); idem, Ibis, 1880, pp. 331, 332 (Taluit); idem, Ibis, 1881, pp. 104, 108, 113, 114 (Kushai, Uleai, Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 281, 299, 353 (Ponapé, Mortlock, Ruk); Christian, The Caroline Islands, 1899, p. 358 (Ponapé).
Urodynamis taitensis Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 53 (Jaluit, Ponapé, Palau); Bogert, Amer. Mus. Novit., no. 933, 1937, p. 9 (Palau, Ruk, Kusaie, Ponapé, Truk, Iringlove, Wozzie, Auru, Jaluit, Ratak); Peters, Check-list Birds World, 4, 1940, p. 40 (Palaus, Carolines, Marshall); Hand-list Japanese Birds, 3d ed., 1942, p. 201 (Palau, Truk, Lukunor, Ponapé, Kusaie, Jaluit, Elmore, Aurh, Wotze).
Urodynamis taitiensis Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 11 (Pelew, Ualan, Ponapé, Luganor, Taluit); idem, Ibis, 1893, p. 212 (Marshalls); Hartert, Novit. Zool., 7, 1900, p. 7 (Ruk); Finsch, Notes Leyden Mus., 22, 1900, p. 120 (Ponapé, Palau, Kuschai, Ruk, Mortlock, Uleai, Jaluit); Takatsukasa and Kuroda, Tori, 1, 1915, p. 52 (Ruk); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 58 (Pelew, Ualan, Ponapé, Luganor, Ruk, Taluit); Hand-list Japanese Birds, rev., 1932, p. 180 (Palau, Kusaie, Ponapé, Luganor, Truk, Jaluit, Elmore, Aurh, Wotze).
Urdynamis taitiensis Finsch, Sammulung wissensch. Vorträge, 14th ser., 1900, p. 659 (Palau).
Eudynamis taitiensis Schnee, Zool. Jahrbücher, 20, 1904, p. 389 (Marshalls); Mayr, Birds Southwest Pacific, 1945, p. 302 (Micronesia).
Geographic range.—Breeds in New Zealand and adjacent islands. Winters chiefly in Polynesia, also Melanesia and Micronesia. In Micronesia: Palau Islands—exact locality unknown; Caroline Islands—Yap, Lukunor, Truk, Ponapé, Kusaie; Marshall Islands—Jaluit, Elmore, Auru, Wotze, Bikini.
Characters.—Adult: A large, long-tailed cuckoo with upper parts dark brown; top of head spotted with white; wings, upper back and tail barred with rufous; underparts pale rufous or buffy-rufous with shafts of feathers streaked with brown.
Specimens examined.—Total number, 4 (2 males, 2 females), as follows: Caroline Islands, AMNH—Truk, 1 (Jan. 7)—Kusaie, 2 (March); Marshall Islands, USNM—Bikini, 1 (May 1).
Remarks.—Bogert (1937) has summarized the information known concerning the migration of the New Zealand Long-tailed Cuckoo. Its principal winter range is in eastern and central Polynesia: Fiji, Samoa, Tonga, Union, Cook, Society, and Tuamotu islands. The bird reaches the northern extent of its range in the Marshall and Caroline islands (see map in Bogert, 1937:3-4). There are no records for the Marianas and only one record from the Palaus (taken by Peters, as recorded by Finsch, 1875:49). The bird is seemingly much more numerous as a winter visitor in the Marshall Islands than in the Caroline Islands. Coultas (field notes) writes that the cuckoo appears at Kusaie about the first of February. Bogert (1937) remarks that the cuckoo arrives at New Zealand for the breeding period in October or November and leaves for the northern wintering grounds in February or March.
Bogert (1937:11) discusses briefly the history of migration of this bird. She presents as a possible reason for the migration the fact that the cuckoo feeds principally on caterpillars and that as a consequence it moves northward to the tropics during the winter months because this food is not available at the breeding grounds in the winter months. Perhaps this cuckoo in developing its ability to fly long distances over water on migration has expanded the breadth of its range eastward into the oceanic islands, rather than westward through Malaysia and Melanesia, because it has found less competition from resident birds and from other migrants for feed and habitat. On many of the islands and atolls of the Pacific Basin, this species is the only land bird known.
Otus podarginus (Hartlaub and Finsch)
Palau Scops Owl
Noctua podargina Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 90. (Type locality, Pelew Islands.)
Noctua podargina Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 8, pl. 1, fig. 1 and 2 (Palau); Giebel, Thes. Ornith., 2, 1875, p. 720 (Pelew); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau).
Ninox podargina Sharpe, Cat. Birds British Mus., 2, 1875, p. 151 (Palau); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 51 (Palau); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 61 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 181 (Palau).
Scops podargina Sharpe, Cat. Birds British Mus., 2, 1875, p. 313 (Palau); Nehrkorn, Journ. f. Ornith., 1879, p. 394 (Palau); Wiglesworth, Abhandl. und Ber. Zool. Dresden, no. 6, 1890-1891 (1891), p. 3 (Pelew); Matschie, Journ. f. Ornith., 1901, p. 112 (Palau); Dubois, Syn. Avium, 2, 1904, p. 883 (Pelew).
P[isorhina] podargina Reichenow, Die Vögel, 1913, p. 424 (Palau).
Otus podarginus Mathews, Syst. Avium Australasianarum, 1, 1927, p. 268 (Palau); Mayr. Amer. Mus. Novit., no. 1269, 1944, p. 3 (Palau); idem, Birds Southwest Pacific, 1945, p. 291 (Palau).
Pyrroglaux podargina Yamashina, Tori, 10, 1938, p. 1 (Pelew); Peters, Check-list Birds World, 4, 1940, p. 109 (Babelthuap, Koror); Hand-list Japanese Birds, 3d ed., 1942, p. 202 (Palau).
Geographic range.—Micronesia: Palau Islands—Koror, Babelthuap, Angaur.
Characters.—Adult male: A small owl with forehead and superciliary area whitish tinged with buff and narrowly barred blackish-brown; feathers at base of upper mandible with long, blackish shafts, crown and back rufous-brown; some feathers on neck narrowly barred ochraceous and black; some scapulars with outer webs barred dark brown and white; rump and upper tail-coverts dark rufous, barred white and dark brown; tail rufous, barred indistinctly dark brown, inner webs barred white and dark brown; wings sandy rufous, outer edges of all but first primary spotted buffy-white; lores rufous, shafts white; indistinct eye ring rufous; ear-coverts whitish with rufous tips, chin white; throat white narrowly barred with wavy dark lines and tipped with rufous; breast pale rufous, feathers barred with white and black; abdomen paler rufous; under tail-coverts often barred with black and white without rufous wash; under wing-coverts white barred with dark brown; bill and feet whitish; iris brown.
Adult female: Resembles adult male, but darker brown above with fine vermiculations of blackish color; underparts may be pale or dark rufous with slight or heavy white and brown barrings and spots.
Immature: Resembles adult male, but upper parts darker brown; forehead, crown, and back barred ochraceous and black; scapulars with white shaft streaks and spots of white; underparts more heavily barred.
Measurements.—Eight males measure: wing, 155-163 (159); tail, 82-88 (84); culmen, 22.0-23.5 (23.0); tarsus, 32-35 (33); two females measure: wing, 158, 165; tail, 83, 90; culmen, 23.5, 24.0; tarsus, 33, 35.
Specimens examined.—Total number, 11 (9 males, 2 females), as follows: Palau Islands, USNM—Koror, 1 (Nov. 3); AMNH—exact locality not given, 10 (Oct., Nov., Dec.).
Remarks.—Coultas (field notes) found the Palau Scops Owl fairly common around villages on the island of Koror. He obtained specimens at night with the use of a flashlight. He writes that the bird moves about considerably remaining on one perch and calling for only approximately three minutes. The bird stays in the mangrove thickets in the daylight hours. Marshall (1949:207) also found the owl at Koror as well as at Peleliu in 1945. He observed 33 pairs on Koror (approximately one-half of the total population) and four pairs on Peleliu. The NAMRU2 party did not find the owl in the southern Palaus in 1945.
Yamashina (1938:1) gave the Palau Scops Owl the generic name, [Pg 217] Pyrroglaux. Mayr (1944b:3) has reviewed this treatment and presents evidence to show that the name Pyrroglaux should not be recognized and that the bird correctly belongs in the genus Otus. He presents a detailed discussion to show its relationship to O. spilocephalus, and that the characters possessed by O. podarginus are no more different or unusual than those found in other members of this widespread genus. It is pointed out that the reduction of the feathering is probably caused by the change in habitat—from a colder one in Asia to a warmer, tropical one in the Palaus. The bird is probably derived from O. spilocephalus of Asia and Malaysia.
Asio flammeus flammeus (Pontoppidan)
Short-eared Owl
Strix Flammea Pontoppidan, Danske, Atlas, 1, 1763, p. 617, pl. 25. (Type locality, Sweden.)
Strix stridula Quoy and Gaimard, Voy. "Uranie," Zool., 1824, pp. 680, 696 (Mariannes); idem, Ann. Sci. Nat. Paris, 6, 1825, p. 149 (Mariannes).
Otus brachyotus Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen); Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 18 (Mariannen?).
Asio accipitrinus Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 3 (Marianne); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 168 (Mariannes); Hartert, Novit. Zool., 5, 1898, p. 51 (Marianne); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 44 (Mariannes); Safford, Osprey, 1902, p. 68 (Marianas); idem, Amer. Anthro., 4, 1902, p. 711 (Guam); idem, The Plant World, 7, 1904, p. 263 (Tinian); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Tinian); Prowazek, Die deutschen Marianen, 1913, p. 88 (Marianen).
Asi accipitrimus Wheeler, Report Island of Guam, 1900, p. 12 (Guam).
Asio flammeus sandwichensis Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 61 (Marianne); Hand-list Japanese Birds (part), rev., 1932, p. 182 (Marianas).
Asio flammeus ponapensis Hand-list Japanese Birds (part), 3d ed., 1942, p. 202 (Pagan).
Asio flammeus flammeus Mayr, Birds Southwest Pacific, 1945, p. 292 (Marianas).
Geographic range.—Breeds in Europe, Asia, and North America. Winters to tropics. In Micronesia: Mariana Islands—Pagan, Tinian.
Remarks.—The Short-eared Owl was taken at Tinian by Quoy and Gaimard (1824:680, 696) and in recent years has been recorded at Pagan. The committee which prepared the Hand-list of Japanese Birds (Hachisuka et al., 1942:202) writes that the bird taken at Pagan has a short wing (288) and indicates that it belongs to A. f. ponapensis. In the present work this bird is considered to be A. f. flammeus, a migrant from Asia; possibly, however, there is an unrecorded resident population of the Short-eared Owl in the northern Marianas, which may be closely related to A. f. ponapensis of Ponapé. Owls may have at one time been resident in the southern Marianas. At Guam, for instance, owls are well known to the native peoples, and there is suitable habitat for the owl in the extensive grassland areas of the island. Perhaps an owl was resident at Guam and at other islands but has been eliminated partly by the overgrazing and burning of the grassy habitats preferred by the owl.
Asio flammeus ponapensis Mayr
Short-eared Owl
Asio flammeus ponapensis Mayr, Amer. Mus. Novit., no. 609, 1933, p. 1. (Type locality, Ponapé.)
Otus brachyotus Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 18 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 778 (Ponapé); idem, Journ. f. Ornith., 1880, p. 283 (Ponapé); idem, Mitth. Ornith. Ver. Wien, 1884, p. 47 (Ponapé); idem, Sammlung wissensch. Vorträge, 14 ser., 1900, p. 659 (Ponapé).
Asio brachyotus Finsch, Ibis, 1881, pp. 113, 114 (Ponapé).
Asio accipitrinus Ridgway, Proc. U. S. Nat. Mus., 4, 1882, p. 367 (Strong's Island = Kusaie); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 3 (Ponapé); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 169 (Ponapi).
Asio flammeus sandwichensis Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 61 (Ponapé); Hand-list Japanese Birds (part), rev., 1932, p. 182 (Ponapé).
Asio flammeus ponapensis Kelso, Oölogist, 1938, p. 183 (Kusaie); Peters, Check-list Birds World, 4, 1940, p. 170 (Ponapé); Hand-list Japanese Birds (part), 3d ed., 1942, p. 202 (Ponapé); Mayr, Birds Southwest Pacific, 1945, p. 291 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé, Kusaie?
Characters.—Adult: a large, short-eared owl, dark brown above streaked with buff and lighter below streaked with dark brown. An adult female has upper parts dark brown, outer webs of feathers buffy to give a streaked appearance; rump pale buff, feathers edged subterminally with darker brown; scapulars like head and back; wing-coverts dark brown tipped and edged with splotches of buffy to buffy-rufous; primaries and secondaries brown with large spots of pale rufous; tail brown barred with whitish buff spots, webs with dark centers; forehead whitish tinged with buff; region below and behind eye dark; chin pale with rufous tinged sides; throat and breast rufous-buff with heavy streaks of brown, becoming narrower on abdomen and under tail; under wing-coverts buffy streaked with dark brown; auxilaries buffy; feathering of tibia and tarsus pale buff; bill dark slate; feet grey-brown; iris yellow.
Resembles A. f. flammeus, but wing shorter and color darker.
Measurements.—Mayr (1933:2) lists the following measurements for two adult females: wing, 295, 307; tail, 135, 139; culmen, 17, 17.5; and tarsus, 48, 51.
Specimens examined.—Total number, 2 females, from Caroline Islands, AMNH—Ponapé (Dec.).
Nesting.—Coultas (field notes) writes that the Short-eared Owl at Ponapé builds its nest in the grass on the ground. He did not observe the nest but received reports of it from the natives.
Remarks.—The owl at Ponapé has been known since the time of Kubary. Coultas, visiting the island in 1930, was the first naturalist to record very much concerning the habits. According to him (field notes) the bird inhabits the open grasslands of Ponapé and apparently has somewhat the same habits as other members of the species. He estimated the population in 1930 as two dozen or more. He found the birds extremely secretive during the daylight hours. They were observed flying over the patches of grassland at twilight and on moonlight nights. He comments that the catlike call of this owl is heard occasionally in the night. Richards writes (in litt.) that twice [Pg 219] in late December, 1947, he saw this owl in a forested area near the summit of Jokaj Island (900 feet).
Kelso (1938:138) records the Short-eared Owl from Kusaie on the basis of a specimen taken by Gulick, which Ridgway (1882:367) thought came from the West Indies. The specimen is labeled Strong's Island, which is an old name for Kusaie. Kelso gives the measurements of this bird as: wing, 275; tail, 141; culmen from cere, 19.5, and comments that the wings are shorter than those of specimens from Asia. The skin is in the U. S. National Museum.
The Short-eared Owl at Ponapé closely resembles A. f. flammeus but is slightly smaller and darker. Apparently the owl came to Ponapé as a straggler on migration from Asia, and becoming acclimated and adapted to the grassy areas at Ponapé remained as a resident. The occurrence of A. f. flammeus in the Marianas on migration offers evidence as to how the bird originally reached Ponapé.
Caprimulgus indicus jotaka Temminck and Schlegel
Jungle Nightjar
Caprimulgus jotaka Temminck and Schlegel, in Siebold's Fauna Japonica, Aves, 1847, p. 37, pl. 12, 13. (Type locality, Japan.)
Caprimulgus indicus jotaka Hand-list Japanese Birds, rev., 1932, p. 179 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 199 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Breeds in eastern Asia and Japan. Winters south to tropics. In Micronesia: Palau Islands—exact locality unknown.
Remarks.—According to the committee who prepared the Hand-list of Japanese Birds (Hachisuka et al., 1942:199), one female was obtained by Oba in the Palaus in November, 1930. The skin was placed in the Kuroda collection. Coultas obtained a male on December 9, 1931, in the Palaus, which is in the American Museum of Natural History. The bird is apparently an occasional migrant to western Micronesia.
Caprimulgus indicus phalaena Hartlaub and Finsch
Jungle Nightjar
Caprimulgus phalaena Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 91. (Type locality, Pelew.)
Caprimulgus phalaena Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 13, pl. 2, fig. 1, 2 (Palau); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 17 (Pelew); Hartert, Cat. Birds British Mus., 16, 1892, p. 545 (Pelew); idem, Das Tierreich, no. 1, 1897, p. 51 (Palau); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 65 (Palau); Matschie, Journ. f. Ornith., 1901, p. 112 (Palau); Dubois, Syn. Avium 1, 1902, p. 124 (Pelew); Reichenow, Die Vögel, 2, 1914, p. 154 (Palau); Mathews, Syst. Avium. Australasianarum, 1, 1927, p. 396 (Pelew); Hachisuka, Birds Philippines, 2, 1934, p. 120 (Pelew).
Caprimulgus indicus phalaena Kuroda, in Momiyama, Birds Micronesia, 1922, p. 61 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 179 (Palau); Peters, Check-list Birds World, 4, 1940, p. 204 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 199 (Babelthuap, Koror); Mayr, Birds Southwest Pacific, 1945, p. 292 (Palau).
Geographic range.—Micronesia: Palau Islands—Babeltuap, Koror, Garakayo.
Characters.—Adult male: "Above grayish-brown, very finely vermiculated, more rufous on the back, with large longitudinal streaks and a few cross markings; scapulars partly with pale buff bands, mostly pale gray at the basal portion; primaries deep brown, with a white spot to the inner web of the first primary not extending to the shaft, second and third primary with fine spots to the inner web extending to the shaft and obsolete white spots to the outer web, fourth primary with a smaller and less pure white spot; chin and throat blackish brown, barred with rufous, with two white spots on the throat; breast brownish gray, vermiculated and spotted with brown and blackish; abdomen dirty ochraceous buff barred with brown, the bars wider on the lower tail-coverts; retrices rufous-brown with blackish bars, outer ones with broad white terminal spots." (Hartert, 1892:545.) Bill basally whitish with black tip; feet blackish pink; iris dark brown.
Adult female: According to Hartert (1892:545) similar to male, but with small, more or less obsolete, rufous-buff (not white) spots on the primaries; rectrices without white spots.
Immature: Resembles adult but paler and less distinctly marked.
C. i. phalaena resembles C. i. jotaka, but is paler; the male is more broadly barred and more buffy on abdomen and under side of tail; the female has paler spots on wing.
Measurements.—Measurements of four males: wing, 161-168 (165); tail, 118-129 (124); culmen, 22; tarsus, 14.0-15.1 (14.5); of four females: wing, 161-165 (163); tail, 118-127 (123); culmen, 22; tarsus, 14.5-15.6 (15.1).
Specimens examined.—Total number, 8 (4 males, 4 females), as follows: Palau Islands, USNM—Koror, 3 (Nov. 3, 20, 29); AMNH—exact locality not given, 5 (Oct., Nov., Dec.).
Remarks.—This subspecies of the Jungle Nightjar is restricted to the Palau Islands and particularly to those islands possessing damp, shady forests and mangrove swamps. In September, 1945, two birds were observed at the edge of a mangrove swamp at Garakayo at twilight by the NAMRU2 party, but neither of them was taken. Coultas (field notes) found the nightjar in mangrove swamps. He writes that they remain quiet there during the daylight hours. He took specimens both in the evening and at dawn. He considers the bird as not very common. Marshall (1949:208) obtained specimens at Koror in 1945.
Among the races of C. indicus, the coloration of C. i. phalaena resembles most closely that of C. i. jotaka; probably C. i. phalaena was derived from C. i. jotaka of Asia. Apparently this bird arrived at the Palaus by way of the Philippines. It is found only in these islands of Micronesia and maybe another one of that group of [Pg 221] species which reached the Palaus without expanding their ranges farther into Micronesia.
Collocalia inexpectata pelewensis Mayr
Edible Nest Swiftlet
Collocalia pelewensis Mayr, Amer. Mus. Novit., no. 820, 1935, p. 3. (Type locality, Palau Islands.)
Collocalia vanicorensis Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 829 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 4, 116, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, p. 89 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 15 (Palau); idem (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 24 (Palau); idem (part), Proc. Zool. Soc. London, 1880, p. 575 (Palaos); idem (part), Ibis, 1881, p. 104 (Pelew); Tristram, Cat. Birds, 1889, p. 111 (Pelew); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 18 (Pelew); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Palau).
Collocalia vanikorensis Gray, Hand-list Birds, 1, 1869, p. 66 (Pelew); Giebel, Thes. Ornith., 1, 1872, p. 737 (Pelew).
Collocalia fuciphaga Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 189 (Palaos); Reichenow, Die Vögel, 2, 1914, p. 161 (Palau).
Collocalia francica Takatsukasa and Kuroda, Tori, 1915, p. 53 (Pelew).
Collocalia fuciphaga inquieta Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 62 (Pelew).
Collocalia unicolor amelis Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 63 (Pelew).
Collocalia fuciphaga amelis Hand-list Japanese Birds, rev., 1932, p. 179 (Palau).
Collocalia (vanikorensis) pelewensis Mayr, Amer. Mus. Novit., no. 828, 1936, p. 11 (Palau).
Collocalia germani pelewensis Mayr, Amer. Mus. Novit., no. 915, 1937, p. 18 (Palau).
Collocalia inexpectata pelewensis Peters, Check-list Birds World, 4, 1940, p. 224 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 292 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 63 (Garakayo, Peleliu).
Collocalia vanikorensis pelewensis Hand-list Japanese Birds, 3d ed., 1942, p. 199 (Babelthuap, Koror).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Peleliu, Angaur.
Characters.—Adult, according to Mayr (1935:3): "Small; tarsus naked; upper parts dark fuscous-green, with a brownish tone on back; crown not much darker than back; rump pale but no distinct light gray bar across rump as in C. spodiopygia; color of the rump showing much individual variation, bases of feathers always being pale gray, but tips sometimes strongly glossy green; inner margins of wing-feathers not particularly light; feathers of chin and throat soft, with fuscous bases and rather sharply defined silvery-gray edges, but no shaft-streaks; abdomen dull gray, slightly darker than throat, inconspicuous shaft-streaks on breast and abdomen, more pronounced shaft-streaks on under tail-coverts; longest under tail-coverts fairly glossy green; white loral spot inconspicuous."
Measurements.—Measurements are listed in table 30.
Subspecies No. Wing Tail C. i. pelewensis 14 111 (109-113) 50 (47-51) C. i. bartschi 13 108 (105-108) 54 (52-57) Specimens examined.—Total number, 20 (12 males, 8 females), as follows: Palau Islands, USNM—Peleliu, 1 (Sept. 13)—Garakayo, 2 (Sept. 18)—Koror, 3 (Nov. 5, 6, 7); AMNH—exact locality not given, 14 (Oct., Dec.).
Remarks.—The NAMRU2 party found the swiftlet to be numerous on islands in the southern Palaus in 1945. The birds were observed flying in clearings and about the cliffs. Coultas writes (field notes) that they nest in caves on the smaller islands.
Collocalia inexpectata bartschi Mearns
Edible Nest Swiftlet
Collocalia bartschi Mearns, Proc. U. S. Nat. Mus., 36, 1909, p. 476. (Type locality, Guam.)
Cypselus inquietus Kittlitz (part), Obser. Zool., in Lutké., Voy. "Le Séniavine," 3, 1836, p. 304 (Guahan); idem (part), Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 26 (Guahan).
Collocalia nidifica Gray (part), Ann. Mag. Nat. Hist., (3), 17, 1866, p. 125 (Marianne); idem (part), Hand-list Birds, 1, 1869, p. 65 (Marianne).
Collocalia vanicorensis Finsch (part), Journ. Mus. Godeffroy, 12, 1876, p. 24 (Marianen); idem (part), Ibis, 1881, p. 105 (Guam); Oustalet, Le Nat., 1889, p. 260 (Mariannes); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 18 (Marianne); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Guam, Saipan).
Collocalia fuciphaga Sclater, Proc. Zool. Soc. London, 1865, p. 616 (Marianne); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 187 (Mariannes); Hartert, Novit. Zool., 5, 1898, p. 53 (Rota, Guam, Saipan); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 46 (Marianas); Safford, Osprey, 1902, p. 60 (Marianas); idem, The Plant World, 7, 1904, pp. 84, 263 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Prowazek, Die deutschen Marianen, 1913, p. 102 (Marianen); Cox, Island of Guam, 1917, p. 21 (Guam); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 25 (Guam).
Collocalia fuchphaga Wheeler, Report Island of Guam, 1900, p. 13 (Guam).
Collocalia fuciphaga fuciphaga Oberholser (part), Proc. Acad. Nat. Sci. Phila., 1906, p. 186 (Guam).
Collocalia unicolor amelis Oberholser, Proc. Acad. Nat. Sci. Phila., 1906, p. 193 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 63 (Guam).
Collocalia fuciphaga tachyptera Obersolser, Proc. U. S. Nat. Mus., 42, 1912, p. 20 (Type locality, Guam); Stresemann, Verhandl. Ornith. Gesellsch. Bayern, 12, 1914, p. 11 (Guam); Takatsukasa and Kuroda, Tori, 1, 1915, p. 63 (Marianas); Kuroda, in Momiyama, Birds Michnoseia, 1922, p. 62 (Guam, Saipan, Rota).
Collocalia unicolor bartschi Kuroda, in Momiyama, Birds Micronesia, 1922, p. 63 (Guam).
Collocalia fuciphaga bartschi Mathews, Syst. Avium Australasianarum, 1, 1927, p. 402 (Guam); Hand-list Japanese Birds, rev., 1932, p. 178 (Marianas).
Collocalia vanikorensis bartschi Mayr, Amer. Mus. Novit., no. 828, 1936, p. 11 (Marianne); Hand-list Japanese Birds, 3d ed., 1942, p. 198 (Saipan, Rota, Guam).
Collocalia germani bartschi Mayr, Amer. Mus. Novit., no. 915, 1937, p. 18 (Marianne).
Collocalia inexpectata bartschi Peters, Check-list Birds World, 4, 1940, p. 224 (Marianne); Mayr, Birds Southwest Pacific, 1945, p. 292 (Marianas); Watson, The Raven, 17, 1946, p. 41 (Guam); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 105 (Tinian); Stott, Auk, 64, 1947, p. 526 (Saipan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 63 (Guam, Rota).
Collocalia inexpectata Strophlet, Auk, 63, 1946, p. 538 (Guam); Baker, Condor, 49, 1947, p. 125 (Guam).
Geographic range.—Micronesia: Mariana Islands—Guam, Rota, Tinian, Saipan.
Characters.—Resembles C. i. pelewensis, but with wing shorter; upper parts lighter; underparts more brownish and lacking dark shaft-streaks on breast and abdomen; feathers on lores whiter basally.
Measurements.—Measurements are presented in table 30.
Weights.—The present author (1948:63) lists the weights of seven adult males as 6.4-7.3 (6.8); of three adult females as 6.8-7.6 (7.1). These birds were taken at Guam.
Specimens examined.—Total number, 48 (17 males, 19 females, 12 unsexed), as follows: Mariana Islands, USNM—Guam, 21 (Jan. 29, May 20, June 21, July 20, 29)—Rota, 1 (Oct. 27); AMNH—Guam, 18 (Jan. 22, 29, Feb. 15, July 10, Aug. 11, 12)—Saipan, 8 (Sept. 17).
Remarks.—The taxonomic relationships of the species and subspecies of the genus Collocalia are not fully known. The many different name combinations applied to the five kinds named from Micronesia are evidence of the lack of agreement among previous writers as to the correct systematic positions of the kinds. The genus is widely distributed in southeastern Asia and adjacent islands and is divisible into a number of species and subspecies. This diversity is apparently influenced by the restriction of the birds to local habitats caused, as Stresemann (1931b:83) states, by the necessity of staying by their nesting areas which are in caves. Stresemann also points out that the birds are thus dependent on "narrowly limited ecological conditions." The birds are confined to certain areas and are, therefore, isolated from other populations. Most of the volcanic islands of Micronesia have numerous caves which are suitable to the swiftlets for nesting. C. inexpectata evolved in the Malayan region and apparently spread to Micronesia via the Philippines to Palau and to the Marianas. The two subspecies of C. inexpectata in Micronesia resemble closely those to the westward but are smaller. I am following Peters (1940:224) in the treatment of these, and although some future reviser may rearrange these species and subspecies, it appears to me that the Micronesian swiftlets fall into the two natural groups (C. inexpectata and C. inquieta) now recognized, even though their parent stocks in Malaysia, in my opinion, are inadequately known.
At Guam and Rota, the NAMRU2 party found swiftlets concentrated at cliff areas, flying about in large groups. Away from the cliffs fewer were seen and singles were observed in woodland openings, over fields, and in the coconut groves. On May 18, 1945, a colony of nesting birds was found approximately two miles east of Agaña on Guam. This colony was in a coral sink-hole which was approximately 75 feet deep and 60 feet in diameter. The nests were grouped in clusters of 5 to 25 or more, on underhanging ledges, [Pg 224] sheltered from the light. The nests, which were fastened securely to the irregular ledges, were knocked down by shots from our collecting guns. Approximately 250 nests were found; no eggs were observed, the nests containing young birds. The young were in various stages of development; some were with little feather growth, others were completely feathered. Nests examined contained only one young each. The pile of guano below each cluster of nests was large; an estimate made at the time indicated that there were 10 or more tons in each pile. Guano deposits in large quantities were found also in caves at Amantes Point, Guam.
Collocalia inquieta inquieta (Kittlitz)
Carolines Swiftlet
Cypselus inquietus Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 285. (Type locality, Ualan.)
Cypselus inquietus Kittlitz (part), Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 26 (Ualan).
Collocalia ualensis Streubel, Isis, 1848, p. 368 (no type locality = Kusaie?).
Collocalia nidifica ualensis Gray, Ann. Nat. Hist., 17, 1866, p. 123 (Caroline Islands = Kusaie?).
Collocalia vanicorensis Finsch (part), Journ. Mus. Godeffroy, 12, 1876, p. 24 (Ualan); idem (part), Proc. Zool. Soc. London, 1880, p. 575 (Kuschai); idem (part), Journ. f. Ornith., 1880, pp. 285, 298 (Kuschai); idem (part), Ibis, 1881, pp. 104, 108 (Kushai); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 18 (Ualan); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Ualan).
Collocalia fuciphaga Hartert (part), Cat. Birds British Mus., 16, 1892, p. 498 (Kuschai); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 190 (Oualan).
Collocalia fuciphaga fuciphaga Oberholser (part), Proc. Acad. Nat. Sci. Phila., 1906, p. 186 (Ualan).
Collocalia fuciphaga vanikorensis Oberholser (part), Proc. U. S. Nat. Mus., 42, 1912, p. 20 (Kusaie).
Collocalia fuciphaga inquieta Stresemann, Verhandl. Ornith. Gesellsch. Bayern, 12, 1914, pp. 9, 11 (Ualan); Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 62 (Kusaie); Hand-list Japanese Birds (part), rev., 1932, p. 179 (Kusaie).
Collocalia inquieta inquieta Mayr, Amer. Mus., Novit., no. 915, 1937, p. 11 (Kusaie); Peters, Check-list Birds World, 4, 1940, p. 225 (Kusaie); Mayr, Birds Southwest Pacific, 1945, p. 292 (Kusaie).
Collocalia vanikorensis inquieta Hand-list Japanese Birds, 3d ed., 1942, p. 199 (Kusaie).
Geographic range.—Micronesia: Caroline Islands—Kusaie.
Characters.—Adult: Upper parts dark (sooty-black) with a slight greenish gloss on head and back and a more conspicuous bluish-purple gloss on the wings and tail; feathers of lores white, tipped with black; underparts smoky-gray; feet brownish; bill black; iris dark brown.
Measurements.—Measurements are presented in table 31.
Specimens examined.—Total number, 42 (21 males, 20 females, 1 unsexed), as follows: Caroline Islands, USNM—Kusaie, 1 (Feb. 8); AMNH—Kusaie, 41 (Jan., Feb., March).
Remarks.—Kittlitz obtained this swiftlet when he visited Kusaie from December 8, 1827, to January 1, 1828. In 1931, Coultas found [Pg 225] the bird common at Kusaie. The name Collocalia ualensis, published by Streubel in Isis in 1848, p. 368, is without mention of a locality, but is later used by Gray to denote the swiftlet in the Caroline Islands.
Subspecies No. Wing Collocalia i. inquieta 11 119 (116-125) Collocalia i. ponapensis 10 110 (107-114) Collocalia i. rukensis 10 (112-119.5)[C] [C] (Mayr, 1935:3).
Collocalia inquieta rukensis Kuroda
Carolines Swiftlet
Collocalia fuciphaga rukensis Kuroda, Tori, 1, 1915, pp. 58, 59, pl. 3, fig. 1. (Type locality, Ruk.)
Collocalia vanicorensis Finsch (part), Proc. Zool. London, 1880, p. 575 (Ruk); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 353 (Ruk); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 18 (Uap and Ruk); Hartert, Novit. Zool., 7, 1900, p. 11 (Ruk); Matschie, Journ. f. Ornith., 1901, p. 112 (Yap, Ruk).
Collocalia fuciphaga vanikorensis Oberholser (part), Proc. U. S. Nat. Mus., 42, 1912, p. 20 (Uala = Truk).
Collocalia fuciphaga rukensis Takatsukasa and Kuroda, Tori, 1, 1915, p. 53 (Ruk); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 62 (Ruk, Yap); Kuroda, Ibis, 1927, p. 706 (Truk); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 402 (Ruk); Hand-list Japanese Birds, rev., 1932, p. 178 (Ruk).
Collocalia fuciphaga inquieta Kuroda, in Momiyama, Birds Micronesia, 1922, p. 62 (Ruk).
Collocalia inquieta rukensis Mayr, Amer. Mus. Novit., no. 915, 1937, p. 11 (Ruk); Peters, Check-list Birds World, 4, 1940, p. 225 (Truk, Yap); Mayr, Birds Southwest Pacific, 1945, p. 292 (Yap, Truk).
Collocalia vanikorensis rukensis Hand-list Japanese Birds, 3d ed., 1942, p. 198 (Truk, Yap).
Geographic range.—Micronesia: Caroline Islands—Truk, Yap.
Characters.—Adult: Resembles C. i. inquieta but with wing shorter.
Measurements.—Measurements are given in table 31.
Specimen examined.—One unsexed bird from Caroline Islands, USNM—Truk (Feb. 16).
Remarks.—Little is known concerning this swiftlet. The bird at Yap is referred to this race; I have not seen specimens from this island. McElroy reports seeing no swiftlets at Truk in December, 1945. C. i. rukensis appears to be intermediate in size between C. i. inquieta and C. i. ponapensis. Richards writes (in litt.) that he found swiftlets common at Truk in 1948. He also noted a large swiftlike bird in "January or February," 1948, near the summit of[Pg 226] Mount Tonáchian on Moen Island. From his description, the bird may have been a large migratory swift, possibly Apus pacificus or Chaetura caudacuta, neither of which have been reported previously from Micronesia.
Collocalia inquieta ponapensis Mayr
Carolines Swiftlet
Collocalia vanikorensis ponapensis Mayr, Amer. Mus. Novit., no. 820, 1935, p. 3. (Type locality, Ponapé.)
Collocalia vanicorensis Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 23 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 778 (Ponapé); idem (part), Journ. f. Ornith., 1880, p. 285 (Ponapé); idem, Ibis, 1881, p. 115 (Ponapé); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 18 (Ponapé); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Ponapé).
Collocalia fuciphaga Hartert, Cat. Birds British Mus., 16, 1892, p. 498 (Ponapé).
Collocalia fuciphaga vanikorensis Takatsukasa and Kuroda, Tori, 1, 1915, p. 53 (Ponapé).
Collocalia fuciphaga inquieta Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 62 (Ponapé).
Collocalia vanikorensis ponapensis Mayr, Amer. Mus. Novit., no. 828, 1936, p. 12 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 198 (Ponapé).
Collocalia inquieta ponapensis Mayr, Amer. Novit., no. 915, 1937, p. 11 (Ponapé); Peters, Check-list Birds World, 4, 1940, p. 225 (Ponapé); Mayr, Birds Southwest Pacific, 1945, p. 292 (Ponapé).
Collocalia inquieta Mayr, Proc. 6th Pac. Sci. Congr., 4, 1941, p. 204 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult: According to Mayr (1936:12), "Very similar to inquieta, but much smaller; on the upper parts apparently somewhat less glossy, and not so dark, more brownish; under parts very variable, sometimes very dark (partly on account of greasing), sometimes quite silvery on the throat; very dark specimens show some greenish gloss not only on the longest under tail-coverts, but also on the entire under side, except on the throat; rump of the same color as the back; tarsus unfeathered."
Measurements.—Measurements are listed in table 31.
Specimens examined.—Total number, 37 (19 males, 18 females) from Caroline Islands, AMNH—Ponapé (Nov., Dec.).
Nesting.—Coultas obtained young birds from nests in caves in November and December.
Remarks.—I am following Mayr (1937:11) and Peters (1940:225) in this treatment of these Caroline swiftlets, even though the differences between C. inquieta and C. vanikorensis appear to be slight indeed. C. inquieta appears closest to the forms of C. vanikorensis in Northern Melanesia. The birds found in New Guinea and the Solomons are similar in size to the birds in the Carolines, while those in the Moluccas, Admiralties and Lihir are larger. Color differences are slight with the pale color of the sides of the head and underparts being variable. All of these dark-rumped birds evidently evolved in the Melanesian area.
Halcyon cinnamomina cinnamomina Swainson
Micronesian Kingfisher
Halcyon cinnamomina Swainson, Zool. Illustr., 2, 1821, text to pl. 67. (No type locality = Guam.)
Halcyon cinnamomina Hartlaub, Journ. f. Ornith., 1854, p. 167 (Marianen = Guam); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 5 (Ladrone or Marian Islands = Guam); Sharpe (part), Monogr. Alced., 1868-71, pp. xxxii, 213, pl. 80 (Guam); Gray, Hand-list Birds, 1, 1869, p. 93 (Mariannes = Guam); Oustalet, Le Nat., 1889, p. 260 (Mariannes = Guam); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 16 (Guam); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 175 (Guam); Hartert, Novit. Zool., 5, 1898, p. 53 (Guam); Matschie, Journ. f. Ornith., 1901, pp. 112, 113, 114 (Guam); Wharton, Ecol. Monogr., 16, 1946, p. 174 (Guam); Strophlet, Auk, 63, 1946, p. 538 (Guam); Baker, Condor, 49, 1947, p. 125 (Guam).
Alcedo ruficeps Dumont, Dict. Sci. Nat., 29, 1823, p. 273 (Mariannes = Guam); Pucheran, Rev. et Mag. de Zool., 1853, p. 387 (Mariannes = Guam); Hartlaub, Journ. f. Ornith., 1855, p. 423 (Mariannen = Guam).
Dacela ruficeps Lesson, Traité d'Ornith., 1831, p. 247 (Mariannes = Guam).
Halcyon cinnamomeus Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 304 (Guahan).
Dacelo cinnamomina Kittlitz, Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 131 (Guahan); Schlegel, Mus. Pays-Bas, 3, no. 17, 1863, p. 39; no. 39, 1874, p. 29 (Mariannes = Guam); Giebel, Thes. Ornith., 2, 1875, p. 3 (Mariannae = Guam).
Todiramphus cinnamominus Cassin, U. S. Expl. Exped. 1838-'42, 1858, pp. 220, 225 (Ladrone or Marianna Islands = Guam).
Sauropatis cinnamomina Cabanis, Mus. Hein., 2, 1859-'60, p. 159 (Marianen); Salvadori (part), Ornith. Papuasia, 1, 1880, p. 481 (Marianne = Guam).
Halcyon cinnamominus Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 20 (Marianen = Guam); Sharpe, Cat. Birds British Mus., 17, 1892, p. 259 (Marianne = Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 45 (Guam); Safford, Osprey, 1902, p. 69 (Guam); Dubois, Syn. Avium, 1, 1902, p. 108 (Guam); Safford, The Plant World, 7, 1904, p. 263 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Mearns, Proc. U. S. Nat. Mus., 36, 1909, p. 476 (Guam); Reichenow, Die Vögel, 2, 1914, p. 116 (Marianen = Guam); Takatsukasa and Kuroda, Tori, 1, 1915, p. 63 (Mariannes = Guam); Cox, Islands of Guam, 1917, p. 21 (Guam); Thompson, Guam and its people, 1942, p. 23 (Guam).
Halcyon rufigularis Sharpe, Cat. Birds British Mus., 17, 1892, p. 260 (No type locality = Guam).
Halcyon cinnamanea Wheeler, Report Island of Guam, 1900, p. 12 (Guam).
Halcyon cinnamonius Prowazek, Die deutschen Marianen, 1913, p. 102 (Marianen = Guam).
Souropatis cinnamominus Kuroda, in Momiyama, Birds Micronesia, 1922, p. 59 (Guam).
Hyposyma cinnamomina Mathews, Syst. Avium Australasianarum, 1, 1927, p. 384 (Marianne = Guam).
Halcyon cinnamomina cinnamomina Hand-list Japanese Birds, rev., 1932, p. 179 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 200 (Guam); Mayr, Birds Southwest Pacific, 1945, p. 293 (Guam); Peters, Check-list Birds World, 5, 1945, p. 206 (Guam); Watson, The Raven, 17, 1946, p. 41 (Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 63 (Guam).
Halcyon cinnamomius Bryan, Guam, Rec., vol. 13, no. 2, 1936, p. 25 (Guam).
Geographic range.—Micronesia: Mariana Islands—Guam.
Characters.—Adult male: Head, neck, upper back, and entire under surface near "Sanford's brown"; auriculars black with bluish wash; narrow black line extending around nape; orbital ring black; lower back, lesser wing-coverts, and scapulars deep greenish-blue; outer webs of wing feathers and tail blue; rump resembles tail but slightly lighter; under wing-coverts greenish-blue; feet dark brown; bill black, base of mandible paler; iris dark brown.
Adult female: Resembles adult male, but chin, throat, and upper breast paler; rest of underparts and under wing-coverts white; a few cinnamon-tipped feathers on tibia and at bend of wing; back and scapulars darker olive-green and less blue.
Immature: Resembles adult, but brown of crown mixed with greenish-blue; back and wing-coverts edged with pale cinnamon; chin and throat whitish; rest of underparts buffy-white in male and paler in female; feathers on breast and nape with dark edgings.
Measurements.—Measurements are listed in table 32.
Subspecies Number Wing Tail Exposed
culmenTarsus H. c. cinnamomina 31 males
102 (96-105)
77 (73-83)
37 (35-39)
15 (14-17)
25 females
102 (99-106)
79 (74-84)
38 (35-38)
15 (14-17)
H. c. pelewensis 5 males
89 (88-89)
61 (58-64)
39 (38-40)
14 (13-14)
4 females
88 (88-89)
64 (61-67)
39 (38-39)
14 (13-14)
H. c. reichenbachii 14 males
99 (95-101)
74 (72-77)
41 (39-43)
16 (16-17)
15 females
100 (96-102)
74 (71-76)
41 (39-42)
16 (15-17)
Weights.—The NAMRU2 party obtained the following weights: 11 adult males, 56-62 (59); 10 adult females, 58-76 (66).
Specimens examined.—Total number, 72 (40 males, 32 females), as follows: Mariana Islands, USNM—Guam, 38 (Feb. 14, 24, March 8, May 25, 26, 30, June 2, 3, 4, 6, 13, 14, 16, 18, 19, 28, 29, July 6, 7, 10, 18, 20, Aug. 24, 30, Nov. 19); AMNH—Guam, 34 (Jan., Feb., March, April, July, Aug., Sept., Nov., Dec.).
Nesting.—In 1945, the NAMRU2 party found the kingfisher nesting in the months of March, April, May, and July. Nests were placed in hollows of trees, usually ten or more feet above the ground. On April 3, a nest was found in a banyan tree approximately 25 feet above the ground in a hollow limb. There were two entrances to the nest cavity and both the male and female were observed to feed the young. They did not enter the hollow but placed food in the protruding beaks of the young; the parents and nestling both were exceedingly noisy throughout most of the feeding period. On July 8, McElroy found a nest containing two white eggs, partly incubated, in a cavity of a felled coconut palm at Agfayan Bay.
Molt.—Examination of specimens indicates that the time of molt is irregular or that molting may occur at any time of the year. However, there may be a peak in molting in July, August and September; many of the adult birds taken then show evidence of molting of wing and tail. This is immediately following the period of greatest nesting activity.
Food habits.—The Micronesian Kingfisher at Guam feeds on various kinds of animal life; lizards and insects are the principal items. Of three birds taken on February 14, the stomach of one contained a blue-tailed skink; one contained parts of insects and one contained parts of a gecko. I watched a kingfisher capture and swallow a skink on January 14. The bird remained motionless on its perch until the reptile approached within striking distance. Seale (1901:45) writes that the bird has a bad reputation as a chicken thief. He remarks, "I rather doubted his ability in this line until one day I actually saw him attack a brood of small chicks quite near me, and he would have undoubtedly secured one had not the mother hen rushed to the rescue."
Parasites.—Wharton (1946:174) obtained the chigger (Acarina), Trombicula sp., from the Guam Kingfisher.
Remarks.—In 1820, Quoy and Gaimard (1824:35) obtained five specimens of this kingfisher at Guam and called the bird "Martin-chasseur à têterouse." Kittlitz recorded the bird in March, 1828. Marche obtained a series of 57 skins at Guam in 1887 and 1888; these were sent to the Paris Museum. Sharpe described the female as a separate species in 1892. There is considerable variation in the coloration of adult birds, which is mostly due to fading, as suggested by Hartert (1898:52). Some individuals have the crown feathers much abraided as a result of rubbing the crown against the edge of the nest holes as the birds enter and leave them.
The kingfisher is fairly common at Guam. It is primarily a bird of the forest, preferring particularly the marginal habitats between woodlands and openings. I saw only a few birds in open country; only rarely were birds seen sitting on the telephone lines along the roads. The writer (1947b:124) found that of all the birds frequenting habitat along roadways on Guam, the kingfisher comprised only 1.2 percent. Thus, it can be said that it is not a bird of very conspicuous habits, although its noisy "rattle" may be heard in the day and at night.
Halcyon cinnamomina pelewensis Wiglesworth
Micronesian Kingfisher
Halcyon pelewensis Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 15. (Type locality, Pelew Islands.)
Halcyon reichenbachii Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 4, 118 (Pelew); Sharpe, Cat. Birds British Mus., 17, 1892, p. 261 (Pelew).
Halcyon cinnamomina Sharpe (part), Monogr. Alced., 1868-'71, pp. xxxii, 213, pl. 30 (Pelew); Tristram (part), Cat. Birds, 1889, p. 92 (Pelew).
Dacelo reichenbachii Schlegel, Mus. Pay-Bas, 3, no. 39, 1874, p. 29 (Pelew).
Halcyon reichenbachi Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 11 (Palau); Reichenow, Die Vögel, 2, 1914, p. 116 (Palau).
Halcyon cinnamominus Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 20 (Palau).
Sauropatis cinnamomina Salvadori (part), Ornith. Papuasia, 1, 1880, p. 481 (Pelew).
Halcyon pelewensis Hartert, Novit. Zool., 5, 1898, p. 53 (Pelew); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Palau); Takatsukasa and Kuroda, Tori, 1, 1915, p. 53 (Pelew); Uchida, Annot. Zool. Japan., 9, 1918, p. 483 (Palau).
Halcyon Reichenbachi var. pelewensis Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 186 (Pelew).
Halcyon cinnamominus var? pelewensis Dubois, Syn. Avium, 1, 1902, p. 108 (Pelew).
Sauropatis reichenbachii pelewensis Kuroda, in Momiyama, Birds Micronesia, 1932, p. 60 (Angaur).
Hyposyma cinnamomina pelewensis Mathews, Syst. Avium Australasianarum, 1, 1927, p. 385 (Palau).
Halcyon cinnamomina pelewensis Hand-list Japanese Birds, rev., 1932, p. 180 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 200 (Babelthuap, Koror); Mayr, Birds Southwest Pacific, 1945, p. 293 (Palau); Peters, Check-list Birds World, 5, 1945, p. 206 (Babelthuap, Koror); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, pp. 63, 64 (Peleliu, Ngabad).
Geographic range.—Micronesia: Palau Islands—Kayangel, Babelthuap, Koror, Garakayo, Ngabad, Angaur.
Characters.—Adult: Resembles adult of H. c. cinnamomina, but smaller and with underparts white; auriculars with less bluish wash; outer webs of outer tail feathers edged with white.
Immature: Resembles immature female of H. c. cinnamomina, but smaller with white underparts edged with black on throat, breast, and upper abdomen; outer webs of outer tail feathers edged with white.
Measurements.—Measurements are presented in table 32.
Specimens examined.—Total number, 17 (8 males, 8 females, 1 unsexed), as follows: Palau Islands, USNM—Babelthuap, 1 (Nov. 30)—Peleliu, 1 (Sept. 10)—Ngabad, 3 (Sept. 11); AMNH—exact locality not given, 12 (Oct., Nov., Dec.).
Food habits.—Stomachs of specimens obtained by the NAMRU2 party at Palau contained insects. One male had a large cicada in its stomach. Coultas (field notes) writes that foods of this bird consist of grubs and ants.
Parasites.—Uchida (1918:483) found the bird louse (Mallophaga), Docophorus alatoclypeatus, on this bird at Palau.
Remarks.—In 1945, the NAMRU2 party found this kingfisher in forested areas and at the edges of mangrove swamps on small islands near Peleliu. Only six birds were seen. The bird was located by listening for and determining the direction of its rasping call. After a search of the area of leafy foliage from where the call was coming, the bird would be seen sitting motionless on a near-by perch. McElroy of the NAMRU2 party saw a kingfisher with cinnamon underparts at Bulubul Island at Ulithi Atoll on August 21, 1945. It was not taken.
Halcyon cinnamomina reichenbachii (Hartlaub)
Micronesian Kingfisher
Todirhamphus Reichenbachii Hartlaub, Archiv f. Naturgesch., 18, 1852, p. 131. (Type locality, Ponapé.)
Halcyon cinnamominus Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 19 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 778 (Ponapé); idem, Journ. f. Ornith., 1880, p. 285 (Ponapé); idem, Journ. f. Ornith., 1880, p. 285 (Ponapé); idem, Mitth. Ornith. Ver. Wien, 1884, p. 47 (Ponapé).
Sauropatis cinnamomina Salvadori (part), Ornith. Papuasia, 1, 1880, p. 481 (Ponapé).
Halcyon cinnamomina Finsch, Ibis, 1881, pp. 112, 114 (Ponapé); Tristram (part), Cat. Birds, 1889, p. 92 (Ponapé).
Halcyon mediocris Sharpe, Cat. Birds British Mus., 17, 1892, p. 260 (Type locality, Ponapé); Wiglesworth, Abhandl. and Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 16 (Ponapé); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, pp. 177, 180, 181, 184, 185, 186 (Ponapi); Reichenow, Die Vögel, 2, 1914, p. 116 (Ponapé).
Halcyon reichenbachi Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 15 (Ponapé); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, pp. 176, 180, 181, 182, 183, 184, 185, 186 (Ponapi); Hartert, Novit. Zool., 5, 1898, p. 53 (Ponapé); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Ponapé); Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé).
Halcyon cinnamominus var. reichenbachi Dubois, Syn. Avium, 1, 1902, p. 108 (Ponapé).
Halcyon cinnamominus var. mediocris Dubois, Syn. Avium, 1, 1902, p. 108 (Ponapé).
Halcyon reichenbachii Takatsukasa and Kuroda, Tori, 1, 1915, p. 53 (Ponapé).
Sauropatis mediocris Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 195 (Ponapé).
Sauropatis reichenbachii reichenbachii Kuroda, in Momiyama, Birds Micronesia, 1922, p. 60 (Ponapé).
Hyposyma cinnamomina reichenbachii Mathews, Syst. Avium Australasianarum, 1, 1927, p. 384 (Ponapé).
Halycyon cinnamomina reichenbachii Hand-list Japanese Birds, rev., 1932, p. 180 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 200 (Ponapé); Mayr, Birds Southwest Pacific, 1945, p. 293 (Ponapé); Peters, Check-list Birds World, 5, 1945, p. 206 (Ponapé).
Halcyon cinnamomina reichenbachii Bequaert, Mushi, 12, 1939, p. 82 (Ponapé); idem, Occ. Papers Bernice P. Bishop Mus., 16, 1941, p. 290 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult male: Resembles adult male of H. c. cinnamomina, but with slightly smaller wing and smaller tail; slightly longer bill; top of head paler cinnamon; feathers of back tipped with cinnamon and bordered by backish; underparts white.
Adult female: Resembles adult male, but feathers forward of black nape band may be mixed white and cinnamon; back and scapulars duller and less olive.
Immature: Resembles adult, but crown streaked with greenish-black; back and scapulars darker; wing-coverts edged with cinnamon, in male chin and throat creamy, sides of throat, breast, and flanks cinnamon, and axillaries, under wing-coverts, abdomen, under tail-coverts paler cinnamon; in female chin and throat white and rest of underparts paler than in male.
Measurements.—Measurements are presented in table 32.
Specimens examined.—Total number, 49 (25 males, 24 females), as follows: Caroline Islands, USNM—Ponapé, 1 (Feb. 12); AMNH—Ponapé, 48 (Nov., Dec).
Molt.—Most of the specimens taken by Coultas in November and December are either worn or in molt.
Parasites.—Bequaert (1939:82 and 1941:290) records a fly (Hippoboscidae), Ornithoica pusilla, from the Micronesian Kingfisher at Ponapé.
Remarks.—The difference in coloration between the adults and immatures has resulted in considerable confusion concerning the taxonomy of this subspecies. According to Wiglesworth (1891a:15), the name Halcyon reichenbachii was established by Gustav Hartlaub in 1852 for a kingfisher with a white abdomen in the Dresden Museum, which had been figured by Reichenbach (Synopsis Avium, [Pg 232] Alcedineae, 1851) and called Todiramphus cinnamomina. This specimen had been mislabeled and Hartlaub and Finsch (1868a:4), noting a resemblance between this bird and specimens from the Palau Islands, used the name H. reichenbachii for the birds from the Palaus. Later, when specimens from Ponapé were taken, Hartlaub's bird was found to be identical with them; thus the name H. reichenbachii could be restricted to the bird at Ponapé, and Wiglesworth supplied the new name H. pelewensis for the population at Palau. H. mediocris was used by Sharpe to designate the cinnamon-breasted birds at Ponapé, because they were assumed to belong to a species different from the white-breasted ones. This confused situation was not clarified until additional collections were obtained by the Japanese.
Coultas (field notes) comments on the conspicuously different field characters of the two color types in this bird. In 1930, he found the bird common and usually in marginal habitat in the lowlands and at the edges of mangrove swamps.
Evolutionary history of Halcyon cinnamomina.—The three races of kingfishers belonging to the species H. cinnamomina have been derived from H. chloris. The principal distinction between the two species is the presence of the cinnamon coloring in H. cinnamomina, although within H. chloris there are some subspecies possessing traces of this coloration. The link between these two species, as pointed out to me by Mayr, appears to be H. chloris matthias Heinroth of the St. Matthias and Squally islands, which is colored like H. chloris except that on the head, especially on the occiput, there is a faint wash of color ranging from buff to ochre. This coloration of the head is a step toward the condition in the Micronesian populations of H. cinnamomina.
In H. c. pelewensis and H. c. reichenbachii, the adult birds resemble each other, although the former subspecies is slightly smaller. The immatures of H. c. reichenbachii, however, possess cinnamon coloring on the cheeks, sides of body, and breast in addition to that present on the crown and nape. The crown and nape are of this same color in the adults. In the subspecies at Guam, H. c. cinnamomina, the adult male has the immature type of plumage found in H. c. reichenbachii. The female of H. c. cinnamomina has this cinnamon coloring on the throat, but the breast, abdomen and under tail are white. The original stock from which the Micronesian birds came may have invaded the area via the Palau Islands, although Mayr (1940) is of the opinion that they reached Micronesia via Ponapé [Pg 233] (eastern Carolines) and spread to Guam and Palau. He states further (1942b:181, 182) that the presence of H. cinnamomina and H. chloris as reproductively isolated groups in the Palaus may not indicate that they are distinct species, but that they represent the overlap of terminal links of the same species, which have diverged to such an extent as to leave these terminal links reproductively isolated.
Halcyon chloris teraokai Kuroda
White-collared Kingfisher
Halcyon chloris teraokai Kuroda, Tori, 1, 1915, p. 56, pl. 3, fig. 3. (Type locality, Pelew.)
Halcyon albicilla Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 828 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 4, 118 (Pelew); Gray (part), Hand-list Birds, 1, 1869, p. 93 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 49 (Palau, Mackenzie, Matetotas); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 171 (Pelew).
Halcyon chloris Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 93 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 10 (Palau); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 14 (Pelew); Mayr, Amer. Mus. Novit., no. 469, 1931, p. 3 (Pelew).
Dacelo albicilla Giebel (part), Thes. Ornith., 2, 1875, p. 1 (Pelew).
Halcyon sanctus Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 50 (Palau); Sharpe, Cat. Birds British Mus., 17, 1892, p. 267 (Pelew).
Dacelo albicilla Giebel (part), Thes. Ornith., 2, 1875, p. 1 (Pelew).
Sauropatis chloris Salvadori, Ornith. Papuasia, 1, 1880, p. 470 (Pelew).
Halcyon chloris teraokai Uchida, Annot. Zool. Japon., 9, 1918, p. 482 (Palau); Kuroda, Ibis, 1927, p. 707 (Pelew); Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 484 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 180 (Palau); Bequaert, Mushi, 2, 1939, p. 82 (Palau); idem, Occ. Papers Bernice P. Bishop Mus., 16, 1941, p. 290 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 201 (Babelthuap, Koror, Angaur); Mayr, Birds Southwest Pacific, 1945, p. 293 (Palau); Peters, Check-list Birds World, 5, 1945, p. 209 (Babelthuap, Koror, Angaur); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 64 (Peleliu, Garakayo).
Sauropatis chloris teraokai Oberholser, Proc. U. S. Nat. Mus., 55, 1919, p. 357 (Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 59 (Angaur); Mathews, Syst. Avium Australasianarum, 1, 1927, p. 381 (Palau).
Geographic range.—Micronesia: Palau Islands—Kayangel, Babelthuap, Koror, Garakayo, Peleliu, Angaur.
Characters.—Adult male: Dorsal surface bluish, head slightly darker, back and scapulars more greenish, rump lighter blue; outer webs of feathers of wing and of tail dark blue, entire first primary blue, inner webs of other primaries black; collar and underparts white; ariculars black with bluish wash, the black extending around neck above white band; spot on upper lores and narrow line above eye white; orbital ring and lower part of lores black; under wing-coverts white; under tail black; feet black; bill black, mandible with whitish base; iris dark brown.
Adult female: Resembles adult male, but crown and back more green and less blue; auriculars with greenish-blue wash.
Immature: Resembles adult, but feathers of forehead edged with buff; spot on lores and underparts buffy margined with dusky.
H. c. teraokai resembles closely H. c. chloris (Boddaert), but more greenish and less bluish, especially on tail.
Measurements.—Measurements are listed in table 33. Adult males and females have similar measurements and are treated together.
Subspecies No. Wing Tail Exposed
culmenTarsus H. c. teraokai 17
113 (110-116)
76 (72-81)
45 (41-52)
14 (13-16)
H. c. orii 9
111 (109-116)
80 (78-83)
44 (42-45)
16 (15-16)
H. c. albicilla 17
116 (109-119)
81 (78-84)
46 (42-49)
16 (14-17)
H. c. owstoni 3
115 (114-116)
81 (80-82)
44 (42-45)
17 (16-17)
Specimens examined.—Total number, 53 (25 males, 28 females), as follows: Palau Islands, USNM—Garakayo, 3 (Sept. 20)—Peleliu, 14 (Aug. 27, 29, 30, 31, Sept. 1, 5, 6, Nov. 7); AMNH—exact locality not given, 36 (Oct., Nov., Dec.).
Food habits.—Unlike H. cinnamomina, H. chloris obtains much of its food by fishing in inland waters or in tidal flats and lagoons. It does, however, obtain terrestrial foods also. Stomachs of birds taken by the NAMRU2 party at Palau contained insects, fish, crab, and shrimp. One stomach contained 3 cc. of fragments of crab, another 2 cc. of shrimp and other crustacea, and another 2 cc. of grasshoppers. Marshall (1949:210) records the house mouse as a food of this bird.
Parasites.—Uchida (1918:483) records the bird louse (Mallophaga), Docophorus alatoclypeatus, from this bird at Palau. Bequaert (1939:82 and 1941:290) lists the fly (Hippoboscidae), Ornithoica pusilla, from H. c. teraokai.
Remarks.—The White-collared Kingfisher at Palau is a showy and conspicuous bird. It cannot be classed as a forest bird but seems to prefer openings and marginal woodlands. Its range does not overlap that of the secretive and inconspicuous H. cinnamomina pelewensis, which prefers the denser forests. In 1945, the NAMRU2 party found H. c. teraokai to be numerous in the cleared battle areas at Peleliu and Angaur. A favorite perch of this bird was the telephone lines, from which a number of our specimens were shot. Usually the bird was observed singly; occasionally two birds were found together. A pair was seen in copulation on August 29. The call of this bird, a loud and harsh rattle, is noticeably different from the low rasping note of H. c. pelewensis. Coultas found H. c. teraokai to be numerous in 1931. He comments (field notes) that the bird frequents salt water areas, especially the mangrove swamps. He noted the bird fishing at the outer reef.
Halcyon chloris orii Takatsukasa and Yamashina
White-collared Kingfisher
Halcyon chloris orii Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 484. (Type locality, Rota.)
Halcyon albicillus Sharpe (part), Cat. Birds British Mus., 17, 1892, p. 249 (Marianne = Rota).
Halcyon albicilla Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 169 (Rota); Hartert (part), Novit. Zool., 5, 1898, p. 53 (Rota).
Sauropatis albicillus Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 58 (Rota).
Halcyon chloris orii] Hand-list Japanese Birds, rev., 1932, p. 180 (Rota); Hand-list Japanese Birds, 3d ed., 1942, p. 200 (Rota, Saipan as straggler); Mayr, Birds Southwest Pacific, 1945, p. 293 (Rota); Peters, Check-list Birds World, 5, 1945, p. 210 (Rota); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 64 (Rota).
Geographic range.—Micronesia: Mariana Islands—Rota.
Characters.—Adult: Resembles H. c. teraokai, but loral spot larger and more buffy; occiput lightly streaked with white and white line above eye; top of head and back more oily green and less blue, darker in female.
Immature: Resembles adult, but underparts and loral spot buffy with dusky edges; feathers of forehead tipped with buff; remainder of upper parts slightly darker.
Measurements.—Measurements are listed in table 33.
Weights.—The author (1948:64) lists the weights of two adult females as 84 and 85.
Specimens examined.—Total number, 11 (4 males, 6 females, 1 unsexed), from Mariana Islands, USNM—Rota (Oct. 18, 19, 22, 26, Nov. 2).
Molt.—The 11 specimens taken by the NAMRU2 party at Rota in October and November are in molt.
Remarks.—The kingfisher at Rota was taken by Marche in June and July, 1888, and reported by Oustalet (1895:169). It was taken later by the Japanese and described by Takatsukasa and Yamashina as a new subspecies. Apparently, no other specimens were taken until the NAMRU party visited Rota in October and November, 1945, and obtained 11 skins. The bird is conspicuous and common at Rota.
The color characters of white feathers intermingled with the bluish coloring of the crown and the occiput and the large, whitish loral spot place this subspecies as intermediate between H. c. teraokai and the two subspecies known from the more northern Marianas.
Halcyon chloris albicilla (Dumont)
White-headed Kingfisher
Alcedo albicilla Dumont, Dict. Sci. Nat., éd. Levrault, 29, 1823, p. 273. (Type locality, Marianne = Tinian.)
Alcedo albicilla Pucheran, Rev. et Mag. Zool., 1853, p. 388 (Marianne = Tinian); Hartlaub, Journ. f. Ornith., 1855, p. 423 (Mariannen = Tinian); Cassin, U. S. Expl. Exped. 1838-'42, 1858, p. 225 (Mariannes = Tinian).
Todiramphus albicilla Reichenbach, Syn. Avium, Alcedineae, 1851, p. 30 (Mariannen = Tinian).
Halcyon albicilla Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen = Tinian); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 5 (Ladrone or Marian Islands = Tinian); Gray (part), Hand-list Birds, 1, 1869, p. 93 (Mariannes = Tinian); Oustalet, Le Nat., 1889, p. 260, (Saypan); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 14 (Marianne = Tinian); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 169 (Saypan); Hartert (part), Novit. Zool., 5, 1898, p. 52 (Saipan); Matschie, Journ. f. Ornith., 1901, pp. 112, 113, 114 (Saipan); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 45 (Saipan).
Dacelo albicilla Giebel (part), Thes. Ornith., 2, 1875, p. 1 (Marianne = Tinian).
Sauropatis albicilla Salvadori, Ornith. Papuasia, 1, 1880, p. 470 (Marianne = Tinian).
Halcyon albicillus Sharpe (part), Cat. Birds British Mus., 17, 1892, p. 249 (Marianne = Saipan).
Halcyon saurophagus Schnee, Zeitschr. f. Naturwisch., 82, 1912, p. 463 (Saipan).
Sauropatis albicillus Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 58 (Saipan).
Leucalcyon albicilla albicilla Mathews (part), Syst. Avium Australasianarum, 1, 1927, p. 376 (Saipan).
Halcyon chloris albicilla, Hand-list Japanese Birds, rev., 1932, p. 180 (Saipan, Tinian); Hand-list Japanese Birds, 3d ed., 1942, p. 200 (Saipan, Tinian, Yap?); Mayr, Birds Southwest Pacific, 1945, p. 293 (Saipan, Tinian); Peters, Check-list Birds World, 5, 1945, p. 210 (Saipan, Tinian); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 97 (Tinian); Stott, Auk, 64, 1947, p. 526 (Saipan).
Geographic range.—Micronesia: Mariana Islands—Saipan, Tinian.
Characters.—Adult: Resembles H. c. teraokai, but slightly larger; pileum white; white collar broad; black band on nape narrow and faint in some individuals; back and scapulars more oily green and less blue.
Immature: Resembles adult, but pileum pale buff streaked with bluish-green; back and scapulars darker; upper wing-coverts edged with white; breast feathers edged with dusky black.
Measurements.—Measurements are listed in table 33.
Specimens examined.—Total number, 20 (12 males, 8 females), as follows: Mariana Islands, USNM—Saipan, 1 (Sept. 27)—Tinian, 4 (Oct. 18, 23, 26); AMNH—Saipan, 11 (July 8, 9, 11, 13, 15, 17, August 5, 21, 26)—Tinian, 4 (Sept. 7, 8, 10).
Nesting.—Hartert (1898:42) records an egg found in a hole of a tree at Saipan on July 31, 1895. He writes that the egg "is only slightly glossy, very thin, pure white, but soiled all over with deep brown spots, evidently from the decaying wood in the nest hole. It measures 33:25 mm."
Molt.—Most of the birds taken in July, August, September, and October are in molt.
Remarks.—Quoy and Gaimard, who visited the Marianas while on the expedition in the "Uranie," obtained this kingfisher at Tinian. Additional material was taken by Marche in 1887 at Saipan and by Owston's Japanese collectors in 1895. In 1932, Coultas (field notes) found the bird to be common on both Tinian and Saipan, especially in open country. At Saipan, Stott (1947:526) found the birds as singles or in pairs on wooded hillsides. At Tinian, Gleise (1945:220) estimated the population in 1945 as 150.
The completely white head in H. c. albicilla closely resembles that in H. s. saurophaga Gould of Melanesia. These two species resemble [Pg 237] each other in several other respects. H. saurophaga is smaller than H. chloris with black or greenish blue on the anterior part of the ear-coverts and the color of the back, wings, and tail is more greenish. The presence of both H. saurophaga and H. chloris on the same islands in Melanesia is an indication that the two groups are specifically distinct.
Halcyon chloris owstoni Rothschild
White-collared Kingfisher
Halcyon owstoni Rothschild, Bull. British Ornith. Club, 15, 1904, p. 6. (Type locality, Asuncion.)
Halcyon albicillus Sharpe (part), Cat. Birds British Mus., 17, 1892, p. 249 (Marianne = Pagan, Agrigan).
Halcyon albicilla Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, pp. 169, 170 (Pagan, Agrigan); Hartert, Novit. Zool., 5, 1898, p. 52 (Pagan, Agrigan).
Sauropatis chloris owstoni Kuroda, in Momiyama, Birds Micronesia, 1922, p. 59 (Asuncion).
Leucalcyon albicilla owstoni Mathews, Syst. Avium Australasianarum, 1, 1927, p. 376 (Asuncion).
Halcyon chloris owstoni Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 484 (Asuncion); Hand-list Japanese Birds, rev., 1932, p. 180 (Asuncion); Hand-list Japanese Birds, 3d ed., 1942, p. 200 (Assongsong, Pagan, Almagan); Mayr, Birds Southwest Pacific, 1945, p. 293 (Almagan, Pagan, Agrigan, Asuncion); Peters, Checklist Birds World, 5, 1945, p. 209 (Asuncion, Pagan, Alamagan); Borror, Auk, 64, 1947, p. 417 (Agrigan).
Geographic range.—Micronesia: Mariana Islands—Asuncion, Agrigan, Pagan, Almagan.
Characters.—Adult: Resembles H. c. albicilla, but hind part of crown blue-green and black collar broader.
Immature: Resembles adult, but forehead buffy and edges of feathering on anterior crown, upper wing-coverts, and tips of secondaries brownish.
Measurements.—Measurements are listed in table 33.
Specimens examined.—Total number, 4 (2 males, 1 female, 1 unsexed), as follows: Mariana Islands, AMNH—Asuncion, 4 (Jan., July).
Remarks.—Marche obtained specimens of this bird at Pagan in November, 1887, and at Agrigan in December, 1888, and in February, 1889. Owston's Japanese collectors obtained birds at Asuncion in 1904, which were named as new by Rothschild. Apparently he used an immature specimen in preparing the diagnosis of his new subspecies. Borror (1947:417) visited Agrigan in 1945 and obtained specimens of this kingfisher. He reports that the bird is a "common and abundant species and probably nests on the island."
Evolutionary history of Halcyon chloris in Micronesia.—Halcyon chloris is distributed from eastern Africa at the Red Sea eastward through southern Asia to Malaysia, Australia and the Pacific islands. Peters (1945:207-213) recognized 47 subspecies within this species.
In its colonization of Micronesia, H. chloris apparently arrived first at the Palaus probably from the Philippines or the Moluccas. [Pg 238] Whether H. cinnamomina was established at Palau prior to the arrival of H. chloris is unknown. H. chloris teraokai dominates most of the available habitats at Palau, although it has differentiated but little from subspecies to the west and southwest of Palau. Among named kinds it most closely resembles H. c. chloris (Boddaert) of the Moluccas, Lesser Sundas and adjacent areas in color and structure. The species did not succeed in establishing itself in the Carolines or at Guam, but did so in the Marianas at Rota and northward. In comparison with other subspecies of H. chloris those in the Marianas are characterized by a slight increase in size and a replacement of the bluish-green coloring of the head either partly or wholly by white. It is noteworthy that on the islands of Tinian and Saipan, which occupy a geographically intermediate position in the Mariana chain, the bird has an almost completely white head, whereas the birds on islands to the north and south have only partly white heads.
The geographic ranges of H. chloris and H. cinnamomina in Micronesia overlap only at Palau as shown by Mayr (1942b:181). Even here each is restricted to a different habitat. Possibly the present ranges resulted from competition between each group, and both may have had more extensive ranges in Micronesia in the past. Another possibility is that the original stock of H. chloris arrived in Micronesia via the Palaus and that of H. cinnamomina via Ponapé (eastern Carolinas), and that the resulting successful colonizations were a matter of chance. If this were the case the present day ranges may represent the total amount of dispersal that has taken place. The absence of kingfishers from Kusaie, Yap, Truk and other apparently suitable islands favors this possibility.
Eurystomus orientalis connectens Stresemann
Dollar Bird
Eurystomus orientalis connectens Stresemann, Novit. Zool., 20, 1913, p. 302. (Type locality, Moa.)
Eurystomus orientalis connectens Yamashina, Tori, 10, 1940, p. 675 (Babelthuap); Hand-list Japanese Birds, 3d ed., 1942, p. 199 (Babelthuap).
Eurystomus orientalis pacificus Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Celebes and adjacent islands, Lesser Sunda Islands from Lombock to Damar, Southeastern Islands. In Micronesia: Palau Islands—Babelthuap.
Remarks.—Yamashina (1940:675) records an adult male taken at Babelthuap in 1938. He assigns it to E. o. connectens, comparing it with a series of 15 specimens of this race from Celebes, Halmahera and Batchian. Mayr (1045a:302) refers this visitor to Palau to E. o. pacificus (Latham); this form is migratory and may fly [Pg 239] north from Australia to the Melanesian area between breeding seasons.
Hirundo rustica gutturalis Scopoli
Eastern Barn Swallow
Hirundo gutturalis Scopoli, Del. Flor. et Faune, Insubr., 2, 1786, p. 96. (Type locality, "in Nova Guinea," error = Panay, Philippine Islands.)
Hirundo rustica Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 112 (Yap); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 391 (Yap).
Hirundo rustica gutturalis Hand-list Japanese Birds, rev., 1932, p. 178 (Koror); Hand-list Japanese Birds, 3d ed., 1942, p. 198 (Koror); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau); Baker, Smithson. Mus. Coll., vol. 107, no. 15, 1948, p. 65 (Guam, Angaur, Ngesebus).
Geographic range.—Breeds in northeastern Asia, winters south to Australia and Pacific islands. In Micronesia: Mariana Islands—Guam, Tinian; Palau Islands—Babelthuap, Koror, Ngesebus, Peleliu, Angaur; Caroline Islands—Yap.
Specimens examined.—Total number, 13 (9 males, 3 females, 1 unsexed), as follows: Mariana Islands, USNM—Tinian, 10 (Oct. 23, 25); Palau Islands, USNM—Babelthuap, 1 (Nov. 27)—Angaur, 1 (Sept. 21); AMNH—exact locality not given, 1 (Oct. 26).
Remarks.—This swallow is a winter migrant to western Micronesia from Asia. In the Palau Islands in September, 1945, the NAMRU2 party saw the swallow at Ngesebus and Angaur in small flocks. At Guam, the NAMRU2 party saw one bird on October 7 and four birds flying near Agaña River on October 11. Strophlet (1946:535) saw one bird on October 28, 1945, and six birds on November 16 at Guam. Marshall (1949:221) found swallows at Tinian, Saipan and Palau from October to February. He found only immature birds.
Edolisoma tenuirostre monachum (Hartlaub and Finsch)
Cicada Bird
Campephaga monacha Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 99. (Type locality, Pelew Islands.)
Volvocivora monacha Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 19, pl. 3, fig. 2-3 (Palau); idem, Journ. Mus. Godeffroy, 12, 1876, p. 28 (Palau); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau).
Lalage monacha Sharpe, Cat. Birds British Mus., 4, 1879, p. 105 (Pelew); Tristram, Cat. Birds, 1889, p. 186 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 25 (Pelew); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 53 (Palau); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Palau); Dubois, Syn. Avium., 1, 1902, p. 303 (Pelew); Reichenow, Die Vögel, 2, 1914, p. 276 (Palau); Takatsukasa and Kuroda, Tori, 1, 1915, p. 54 (Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 68 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 175 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 194 (Babelthuap, Koror).
Edolisoma monacha Mathews, Syst. Avium Australasianarum, 2, 1930, p. 541 (Pelew).
Edolisoma tenuirostre monacha Stresemann, Ornith. Monatsber., 47, 1939, p. 126 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 294 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 65 (Peleliu).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Peleliu.
Characters.—Adult male: Forehead, crown, nape, back, and underparts near "Tyrian blue"; auriculars darker than back; lores and chin black; throat black washed with blue gray; wing feathers black, margined with pale blue; black tail tipped with whitish, and basal part of middle two rectrices colored like back; under wing dark except for whitish inner margins of secondaries; bill and feet black; iris dark brown.
Adult female: Resembles adult male, but forehead and under eye pale buff; superciliary stripe darker buff; crown, nape, and sides of neck dark slate-blue; mantle brown, feathers with buffy centers; back brown washed with burnt brown; feathers of rump and upper tail-coverts with terminal black bar edged with buff; wing and tail brownish-black, primaries margined with buff, innermost three secondaries and upper wing-coverts broadly edged with lighter buff, tail tipped with buff, more broadly so on outermost tail feathers, two outermost tail feathers with outer edge buff; two central tail feathers basally dark ochre; ear-coverts buff, tinged with black; chin, throat, and under wing-coverts deep buff; breast, abdomen, and flanks buff, feathers with subterminal blackish bar; under tail buff.
Immature: Resembles adult female, but crown, nape, and sides of neck brown; back faintly mottled with buff; tail feathers and primary wing-coverts tipped with white; younger birds may have upper parts margined with pale buff.
Measurements.—Measurements are listed in table 34.
Specimens examined.—Total number, 23 (13 males, 10 females), as follows: Palau Islands, USNM—Koror, 4 (Nov. 6, 14, 26, Dec. 5)—Peleliu, 2 (Aug. 29, 30); AMNH—exact locality not given, 17 (Oct., Nov., Dec.).
Subspecies No. Wing Tail Exposed
culmenTarsus E. t. monachum 10 98 80 21.0 23.0 96-103 76-83 20.0-22.5 22.5-24.0 E. t. insperatum 35 109 86 23.0 24.0 107-112 82-91 22.0-24.0 23.0-25.0 Molt.—Molt in this bird appears to take place in the period from August to December. Most of the specimens taken in August, October, November and December were in molt. None was taken in other months.
Food habits.—This bird feeds principally on insects. A female taken on August 29 had in its stomach about one and a half cc. of parts of grasshopper. Marshall (1949:212) records both animal and vegetable matter in the stomach of this bird.
Remarks.—The Cicada Bird at Palau inhabits the jungles, especially the marginal areas between the thick jungle and the more open woodlands. In 1945, the NAMRU2 party observed only two birds, both of which were obtained. These were found at Peleliu in a small area of undisturbed woodland at the edge of a mangrove swamp. [Pg 241] Each bird was perched approximately 25 feet above the ground on the outer branches of a densely foliated tree. The bird is thought not to be so rare as our records indicate; probably its secretive habits conceal it from man except as he makes special search for it. Coultas (field notes) describes the bird as one of the true forest. He found it shy and retiring and possessing a very weak voice.
It may be noted that Delacour (1946:2) does not accept the genus Edolisoma but places birds which are currently assigned to it in the genus Coracina.
Edolisoma tenuirostre nesiotis (Hartlaub and Finsch)
Cicada Bird
Campephaga nesiotis Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 98. (Type locality, Uap.)
Campehaga nesiotis Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 391 (Yap).
Volvocivora nesiotis Finsch, Journ. Mus. Godeffroy, 12, 1876, p. 28 (Yap).
Edoliisoma nesiotis Sharpe, Cat. Birds British Mus., 4, 1879, p. 56 (Yap); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 25 (Uap); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 53 (Yap); Matschie, Journ. f. Ornith., 1901, p. 112 (Yap); Dubois, Syn. Avium, 1, 1902, p. 299 (Uap); Reichenow, Die Vögel, 2, 1914, p. 274 (Karolinen = Yap); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 68 (Mackenzie, Yap).
Edolisoma nesiotis Mathews, Syst. Avium Australasianarum, 2, 1930, p. 542 (Mackenzie group); Hand-list Japanese Birds, rev., 1932, p. 174 (Yap); Hand-list Japanese Birds, 3d ed., 1942, p. 194 (Yap).
Edolisoma tenuirostre nesiotis Stresemann, Ornith. Monatsber., 49, 1939, p. 126 (Yap); Mayr, Birds Southwest Pacific, 1945, p. 294 (Yap).
Geographic range.—Micronesia: Caroline Islands—Yap.
Characters.—Adult male: Resembles adult male of E. t. monachum. Adult female: Resembles adult female of E. t. monachum, but wings and upper parts less buffy and more rufous; eye-stripe rufous; breast barred on sides only.
Remarks.—No specimen of the Cicada Bird from Yap has been examined by me. For a long time this bird was thought to be a species distinct from any other member of this genus, but Stresemann (1939:126) arranged it as a subspecies of Edolisoma tenuirostre. The type specimen is an immature, and the adult is unknown. The presence of rufous coloring shows a relationship with E. t. insperatum of Ponapé, but Mayr, who has examined the type of E. t. nesiotis in the Hamburg Museum, and has obligingly showed me his notes on the bird, says that it has a greater resemblance to the Cicada Bird at Palau especially because of the amount of barring on the underparts. The true status of this bird, as well as that of other members of the avifauna of Yap, will be incompletely known until such time as good collections are available from this island group.
Edolisoma tenuirostre insperatum (Finsch)
Cicada Bird
Volvocivora inseperata Finsch, Proc. Zool. Soc. London, 1875, (1876), p. 644. (Type locality, Ponapé.)
Volvocivora insperata Finsch, Journ. Mus. Godeffroy, 12, 1876, pp. 17, 27 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 779 (Ponapé); idem, Ibis, 1881, pp. 110, 112, 115 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 281 (Ponapé).
Volvozivora insperata Finsch, Journ. f. Ornith., 1880, p. 289 (Ponapé).
Lalage insperata Sharpe, Cat. Birds British Mus., 4, 1879, p. 108 (Ponapé); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 25 (Ponapé); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 53 (Ponapé); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Ponapé); Reichenow, Die Vögel, 2, 1914, p. 276 (Karolinen = Ponapé); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 68 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 174 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 194 (Ponapé).
Lisomada insperata Mathews, Novit. Zool., 24, 1928, p. 372 (new generic name); idem, Syst. Avium Australasianarum, 2, 1930, p. 545 (Ponapé).
Edolisoma tenuirostre insperata Stresemann, Ornith. Monatsber., 47, 1939, p. 126 (Ponapé); Mayr, Birds Southwest Pacific, 1945, p. 294 (Ponapé).
Edolisoma tenuirostre Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult male: Resembles adult male of E. t. monachum, but larger; upper parts more grayish-blue; wings with outer edges bluish-gray and inner webbings grayish-white; central tail feathers with subterminal, roundish, black spots; two outermost tail feathers black tipped with broad, pale bluish-gray coloring; lores more bluish-gray and less black; ear-coverts pale bluish-gray; chin, throat, breast, abdomen, flanks, under wing, and under tail-coverts grayish-blue; bill and feet black; iris dark brown.
Adult female: Resembles adult female of E. t. monachum, but larger; forehead slate-gray; crown brownish-gray, browner on nape; back chocolate-brown; rump rufous; upper tail-coverts more cinnamon; wing and tail brownish-black, outer margins of primaries edged with buff; outer margins of secondaries and upper wing-coverts except primary wing-coverts edged with rufous; central tail feathers like back but tipped with buff, other tail feathers more broadly tipped with buff; lores grayish-black; malar stripe to auriculars darker and more brownish-black with lighter shafts; underparts rufous, under wing paler and more buffy.
Immature: Resembles adult female, but forehead grayish tinged with ochre; crown and neck brown becoming slightly more reddish on back and more burnt reddish-brown on rump; tail edged and tipped with buff; primaries tipped with whitish, secondaries broadly edged with buff, primary wing-coverts tipped with buffy-white; lores blackish; ear-coverts rufous with lighter shafts; tail feathers pointed while in adult more rounded. Younger birds resemble older ones, but plumage except wings and tail may be spotted or barred with buff and black with whitish margins.
Measurements.—Measurements are listed in table 34.
Specimens examined.—Total number, 46 (23 males, 23 females), from Caroline Islands, AMNH—Ponapé (Nov., Dec.).
Nesting.—Coultas (field notes) writes that the nest is cup-shaped, made of grasses and strands of hair fern, and placed at low elevations in small trees and bushes. He was told that two eggs are laid. He comments that the nesting season had just been completed in November and December (the time of his visit to Ponapé), because he noted juveniles being attended and fed by the adults.
Molt.—Most of the specimens taken by Coultas in November and December are in fresh plumage or in the final stages of molt, indicating that the molt was initiated possibly in September and would be completed possibly in January. This time of molt appears to be approximately one month later than the time of molt of E. t. monachum of Palau. Probably the bird at the Palau Islands breeds slightly earlier in the year than the subspecies on Ponapé.
Examination of the large series of birds taken by Coultas at Ponapé shows the presence of three types of plumages. The writer has not made a thorough diagnosis of these plumages, but suspects that the phenomenon obtained here is the same as was found by Mayr (1933e) in his study of Neolalage banksiana (Gray), which is a related bird. Immatures of E. t. insperatum seemingly present two plumages, which, if Mayr's arrangement is followed, may be interpreted as a more primitive or "retarded" type in one case, with less striking plumage, barred with black and buff, and a more advanced or "progressive" type in the other case, with plumage of the latter resembling more the adult type, especially the adult female. It was not ascertained whether any of these specimens represented adult birds in "retarded" plumage.
Remarks.—The Cicada Bird at Ponapé resembles in habits its related subspecies at Palau. Coultas (field notes) writes that it is a forest bird, with retiring habits. He observed the birds in small groups, and describes their musical call notes as "to-to-wee, to-to-wee" repeated several times.
Evolutionary history of Edolisoma tenuirostre in Micronesia.—Mayr (in Stresemann, 1939:126) first pointed out the close relationship between the cicada birds of Micronesia and Edolisoma tenuirostre of the Solomon Islands. Up to that time the Micronesian birds were considered to belong to the genus Lalage. The cicada birds probably invaded Micronesia along two independent routes from a dispersal center in the Papuan area. The form at Palau, E. t. monachum, resembles closely several of the subspecies to the south and southwest, particularly those in the New Guinea area. Aside from the smaller size of the Palau form there are differences in coloration between this bird and those of Melanesia. In the adult female and the juvenile there are differences in the amount of barring on the underparts and in the shade of color on the upper parts. In the adult male there are differences in the marginal coloring of the primaries and secondaries. E. t. nesiotis may have arrived at Yap from Palau. Little is known concerning the taxonomic position of this bird. On the basis of the information available, it appears closer to the Palau bird than the Ponapé bird in color; however, in size it probably more closely approaches the latter subspecies.
The Ponapé Cicada Bird, E. t. insperatum, appears to represent a colonization distinct from that which established the populations at Yap and Palau. This conclusion is based on the fact that the adult female of E. t. insperatum has distinctive reddish coloring and lacks the barring on the underparts, and that it may have been derived from an ancestral stock, which was reddish and not barred, such as E. t. remotum of the New Ireland area. The three subspecies in Micronesia may represent remnants of a single colonization, since additional material from Yap may prove that this island population has characters intermediate between those of the other subspecies of Micronesia.
Dicrurus macrocercus harterti S. Baker
Black Drongo
Dicrurus ater harterti S. Baker, Novit. Zool., 26, 1918, p. 299. (Type locality, Formosa.)
Dicrurus macrocercus Baker, Trans. 11th N. Amer. Wildlife Conf., 1946, p. 211 (Rota).
Dicrurus macrocercus harterti Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 65 (Rota).
Geographic range.—Formosa. In Micronesia: Mariana Islands—Rota (introduced).
Specimens examined.—Total number, 7 (4 males, 3 females), from Mariana Islands, USNM—Rota (Oct. 18, 19, Nov. 2).
Remarks.—This drongo was introduced from Formosa to Rota by the Japanese South Seas Development Company (Nanyo Kohatsu Kabushiki Kaisha) apparently in 1935. An illustrated booklet, printed by this organization and seen by members of the NAMRU2 party at the Rota Civil Government headquarters, showed pictures of the captive birds before release and indicated that they had been brought to Rota for the purpose of controlling destructive insects. Dr. Charles Vaurie has examined these birds and compared them with a series of drongos from Formosa in the collection of the American Museum of Natural History.
The drongo appears well adapted at Rota, where it prefers cultivated areas and the bombed village sites to thick woodlands. Birds were found in small flocks often perched in large shade trees in village areas. Weights of two immature males are 53 and 61 grams. One adult male measures: wing, 144, tail, 153, culmen, 26, tarsus, 22.
Corvus kubaryi Reichenow
Marianas Crow
Corvus Kubaryi Reichenow, Journ. f. Ornith., 1885, p. 110. (Type locality, Palau, error = Guam.)
Corvus solitarius Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 305 (Guahan); Bonaparte, Comptes Rendus Acad. Sci. Paris, 37, 1853, p. 830 (Mariannes); Kittlitz, Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 143 (Guahan); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 216 (Guam and Rota).
Corvus spec. Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen); Gray, Hand-list Birds, 2, 1870, p. 12 (Marianne).
Corvus kubaryi Hartert, Novit. Zool., 5, 1898, p. 59 (Guam, Rota); Wheeler, Report Island of Guam, 1900, p. 13 (Guam); Matschie, Journ. f. Ornith., 1901, p. 112 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1901, p. 55 (Guam); Safford, Osprey, 1902, p. 69 (Guam); idem, The Plant World, 7, 1904, pp. 3, 264 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Prowazek, Die deutschen Marianen, 1913, pp. 87, 102 (Marianen); Reichenow, Die Vögel, 2, 1914, p. 306 (Palau); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Marianne); Cox, Island of Guam, 1917, p. 21 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 69 (Guam, Rota); Meinertzhagen, Novit. Zool., 33, 1926, p. 73 (Guam); Hand-list Japanese Birds, rev., 1932, p. 169 (Guam, Rota); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 25 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 187 (Guam, Rota); Mayr, Birds Southwest Pacific, 1945, p. 298 (Guam, Rota); Watson, The Raven, 17, 1946, p. 41 (Guam); Wharton, Ecol. Monogr., 16, 1946, p. 174 (Guam); Strophlet, Auk, 1946, p. 540 (Guam); Baker, Ecol. Monogr., 16, 1946, p. 408 (Guam); idem, Condor, 49, 1947, p. 125 (Guam); idem, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 66 (Guam, Rota).
Corone phillipina Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 46 (Marianne).
Corone kubaryi Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 46 (Pelew, error = Guam).
Geographic range.—Micronesia: Mariana Islands—Guam, Rota.
Characters.—Adult: A small, black crow with a slight greenish-black gloss on head; back, wings, and tail with bluish-black gloss; underparts with dull, greenish-black gloss; bases of feathers light grayish, more nearly white on neck, producing a somewhat ragged appearance; nasal bristles short but extending over nostrils and base of culmen; bill and feet black; iris dark brown. Female smaller.
Immature: Resembles adult, but feathers with less gloss; wings and tail browner.
Measurements.—Measurements of Corvus kubaryi are listed in table 35.
Weights.—The NAMRU2 party obtained weights of the Marianas Crow as follows: from Guam, 5 males, 231-270 (256), 11 females, 205-260 (242); from Rota, 1 male, 256; 1 female, 260 grams.
Location Number
and
sexWing Tail Full
culmenTarsus Guam 9 males
236 (229-244)
165 (158-170)
55 (51-57)
51 (49-52)
19 females
227 (222-241)
151 (143-166)
50 (47-54)
50 (46-54)
Rota 3 males
235 (233-236)
167 (166-169)
54 (53-56)
50 (49-51)
Specimens examined.—Total number, 49 (20 males, 27 females, 2 unsexed), as follows: Mariana Islands, USNM—Guam, 26 (May 25, 29, June 4, 7, 8, 9, 18, 28, 29, July 10, 12, 18, Sept. 5, 11)—Rota, 4 (Oct. 22, 25, 29); AMNH—Guam, 19 (Jan., Feb., March, Aug., Sept., Dec.).
Nesting.—In the spring of 1945, the NAMRU2 party obtained records of nesting activities by crows. One nest was observed on March 8 in a banyan tree. Specimens collected from May to September were not in breeding condition, and it is thought that the nesting period is concentrated in the winter and spring months. Watson (1946:41) reports finding a young crow being fed on May 8 by an adult.
Molt.—The Marianas Crow molts in the period from May to August or September. Most of the birds taken by the NAMRU2 party in this period were in the process of molt. Skins obtained at Rota in late October also exhibit signs of molt. Specimens taken in December, January and February are in fresh or slightly worn plumage. The crow presents an exceedingly shabby appearance in molt, because the grayish and whitish basal parts of the feathers are exposed.
Food habits.—The crow is an omnivorous feeder. Stomachs examined contained both plant and animal food. Both Seale (1901:55) and Safford (1905:79) comment on the damage which the crow does to the corn crop at Guam. Seale remarks that the crow has a reputation for plundering nests of other birds. The NAMRU2 party saw crows being chased by starlings on several occasions.
Parasites.—Wharton (1946:174) obtained the chigger (Acarina), Trombicula sp., from the crow at Guam.
Remarks.—The Marianas Crow is confined to the forested areas and to the coconut plantations at Guam. The birds were seen as singles or in small flocks, often along the roadways. In a count of the number of birds seen along the roadways of Guam, the author (1947:124) found crows to constitute 2.4 per cent of the total population of birds counted and observed the crow on 21.6 per cent of the 125 roadway counts made. Coultas (field notes) noted the birds at the northern part of Guam. The NAMRU2 party found the birds distributed in most parts of the island but usually they were infrequent near areas where large numbers of service personnel were stationed. The birds were often noisy when flying in small flocks or in pairs; Seale (1901:55) also notes this. When observed in jungle areas, the birds were generally quiet, feeding and perching in dense foliage. At Rota, the NAMRU2 party found the bird to be fairly numerous and with habits resembling those of the crow at Guam. No differences in color or structure could be found between the specimens of crows obtained at the two islands.
Kittlitz (1836:305) was the first person to write an account of the crow at Guam. He called it Corvus solitarius and remarked that he later found the same species in the Philippines. Wiglesworth (1891a:46) also considered the crow at Guam to resemble one found in the Philippines and called it Corone phillipina. Later Reichenow named the bird Corvus kubaryi with the type locality as the Palau Islands. This locality proved to be erroneous and the bird was judged to be from Guam by Hartert (1898:59), who did not use the name C. solitarius because it was a nomen nudum, and recognized C. kubaryi as the correct name.
Evolutionary history of Corvus kubaryi.—Meinertzhagen (1926:59) writes that "Environmental influences seem to be mainly, if not entirely, responsible for geographic differences in the genus Corvus." Such may be the case in C. kubaryi, which is a small, dull-colored crow with a relatively unmodified bill. In structure, it has little resemblance to other crows found in the Pacific area. Kittlitz was the first to note a resemblance between the bird at Guam and one in the Philippines. Oustalet (1896:70) wrote that the bird at Guam is related to crows of the Moluccas and New Guinea. Although not closely related to the Hawaiian Crow, C. tropicus, both have little gloss on their feathers, a character which is common to many of the insular populations of crows. Mayr (1943:46) is of the opinion that the Hawaiian bird was derived from a North American ancestor, although Bryan (1941:187) suggests that it is related to C. macrorhynchus of southeastern Asia and remarks that the Hawaiian Crow, "has some relation to the Guam Crow." In looking for the ancestral stock of C. kubaryi, the several species of crows which occur to the north, west and south of the Marianas have been examined. In size and general structure, C. kubaryi appears to be closest to the C. enca group, and not as closely related to the C. macrorhynchus group. The small size, the shape of the culmen, the lack of pointed feathers on the breast, and the presence of white on the basal parts of the feathers of the nape are characters which C. kubaryi has in common with C. enca. Nasal bristles cover the frontal base of the culmen in C. kubaryi; this character is found also in C. enca florensis. C. kubaryi differs from the C. enca group by lacking the purple sheen on the upper parts; this sheen is conspicuous in the latter species. C. kubaryi appears to have little in common with C. meeki of the Solomons and C. orru of the Moluccas and New Guinea area. There is apparently no close relation between the Marianas Crow and the crow which reaches the Bonins. The latter crow, according to the Hand-list of Japanese Birds (Hachisuka et al., 1932:1), is called C. coronoides hondoensis Momiyama and is apparently now extinct in the Bonins.
In summary, it may be said that C. kubaryi is an isolated and modified species of crow, which probably has been living at Guam and Rota for a considerable length of time. Whether it once lived on other islands in Micronesia is unknown, but it is entirely possible that the present population may represent a remnant of one which formerly had a more extensive distribution. The characters which show its distinctness from possible ancestral species include its [Pg 248] small size, its slender bill, and its dull coloration. It is thought to have been derived from the C. enca group, C. e. pusillus of the Philippines or C. e. celebensis of the Celebean area.
Luscinia calliope calliope (Pallas)
Siberian Rubythroat
Motacilla Calliope Pallas, Reise durch versch. Prov. russ. Reichs, 3, 1776, pp. 261, 325, 697. (Type locality, Yenesei.)
Luscinia calliope calliope Hand-list Japanese Birds, rev., 1932, p. 178 (Koror); Hand-list Japanese Birds, 3d ed., 1942, p. 197 (Koror); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Breeds in northeastern Asia. Winters south to Malaysia. In Micronesia: Palau Islands—Koror.
Remarks.—The Siberian Rubythroat is considered to be a casual winter visitor to the Palau Islands.
Monticola solitaria philippensis (Müller)
Chinese Blue Rock Thrush
Turdus philippensis Müller, Natursystem Supplements- und Register-Band, 1776, p. 145. (Type locality, Philippine Islands, ex Buffon.)
Monticola philippensis philippensis Hand-list Japanese Birds, rev., 1932, p. 177 (Koror); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Monticola solitarius philippensis Hand-list Japanese Birds, 3d ed., 1942, p. 197 (Koror).
Geographic range.—Breeds in northeastern Asia and Japan. Winters south to Malaysia. In Micronesia: Palau Islands—Koror.
Remarks.—The Chinese Blue Rock Thrush is apparently an infrequent winter visitor to the Palau Islands.
Turdus obscurus obscurus Gmelin
Dusky Thrush
Turdus obscuras Gmelin, Syst. Nat., 1, 1789, p. 816. (Type locality, Lake Baikal.)
Turdus obscuras Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 96 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 22 (Palau); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 66 (Pelew).
Merula obscura Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 39 (Pelew).
Turdus obscuras obscuras Hand-list Japanese Birds, rev., 1932, p. 177 (Koror); Hand-list Japanese Birds, 3d ed., 1942, p. 197 (Koror); Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Breeds in northeastern Asia. Winters south to Malaysia. In Micronesia: Palau Islands—Koror.
Remarks.—The Dusky Thrush is considered to be a casual winter visitor to the Palau Islands. It was first taken there by Captain Heinsohn, according to Hartlaub and Finsch (1872:96).
Psamathia annae Hartlaub and Finsch
Palau Bush-warbler
Psamathia annae Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 5, pl. 2. (Type locality, Pelew Islands.)
Psamathia annae Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 116, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 89, 94 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 5, 22 (Palau); Nehrkorn, Journ. f. Ornith., 1879, pp. 399, 404 (Palau); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Sharpe, Cat. Birds British Mus., 7, 1883, p. 101 (Pelew); Tristram, Cat. Birds, 1889, p. 155 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 40 (Pelew); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 57 (Palau); Matschie, Journ. f. Ornith., 1901, p. 112 (Palau); Reichenow, Die Vögel, 2, 1914, p. 536 (Palau); Takatsukasa and Kuroda, Tori, 1, 1915, p. 54 (Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 67 (Pelew); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 629 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 177 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 196 (Babelthuap, Koror, Peleliu); Delacour, Ibis, 84, 1942, p. 514 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 294 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 197 (Peleliu, Ngabad).
Calamodyta annae Gray, Hand-list Birds, 1, 1869, p. 208 (Pelew).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Peleliu, Ngabad.
Characters.—Adult: A medium-sized warbler with a rather long bill and tail; upper parts near "buff olive," slightly lighter on head; lores olive-gray to olive-green; supraloral stripe and orbital ring pale yellow-buff; auriculars yellow-brown; underparts lighter and more olive-yellow than back, especially in midsection; chin paler; sides, tibia and under tail-coverts darker and more olivaceus; wings and tail dark brown with outer edges olive; under wing-coverts light yellow; axillaries more whitish; upper mandible horn-colored, darker at base; lower mandible yellowish, darker at base; legs and feet light yellowish-brown; iris grayish-brown. Adult female resembles adult male but is slightly smaller. Immature: Resembles adult but forehead and crown slightly lighter and more yellowish; back and rump more brownish.
Measurements.—Measurements are listed in table 36.
Sex No. Wing Tail Exposed
culmenTarsus Adult males 7
74 64 21.0 28.5 (72-77) (62-68) (19.5-22.5) (27.0-30.0) Adult females 11
69 58 21.0 26.5 (65-74) (55-61) (19.5-22.0) (25.0-29.0) Specimens examined.—Total number, 23 (9 males, 14 females), as follows: Palau Islands, USNM—Koror, 5 (Nov. 7, 9, 11, 18, 19)—Peleliu, 4 (Aug. 29, 30, Sept. 4, Dec. 5)—Ngabad, 1 (Sept. 11); AMNH—exact locality not given, 13 (Nov., Dec.).
Nesting.—Nehrkorn (1879:399, 404) records the egg of Psamathia from Palau. The NAMRU2 party obtained no evidence of nesting of this bird in August and September, 1945. In 1931, Coultas secured birds in November and December, which had enlarged gonads. Marshall (1949:219) records breeding in November and December.
Molt.—Most of the skins taken from August to December have worn or molting feathers. Apparently there is a high point in the molting process in autumn and early winter.
Food habits.—Stomachs obtained from birds taken by the NAMRU2 party in August and September contained parts of insects and small seeds. One stomach contained about one-half cc. of parts of insects. Coultas (field notes) found the bird scratching "on the ground for seeds as well as working in the low trees and bushes." Marshall (1949:212) records insects and snails as food items.
Remarks.—Psamathia has the habit of a typical bushwarbler, occurring in jungle undergrowth and along woodland margins. In 1945, specimens were obtained by the NAMRU2 party in the scrub vegetation which was growing over the devastated battle areas of Peleliu. The bird was not common in this habitat, nor was it very numerous on the smaller offshore islands. Coultas (field notes) found the bird to be rather tame and frequently to live close to human habitation. Its call, as noted by Coultas, is a loud whistle that breaks off into a beautiful song. The bird is quick in its movements; one seen by the writer at Ngabad was constantly moving about in low, second-growth vegetation and was making a low, whistling call. The resemblance of Psamathia to Rukia palauensis is noteworthy. These two unrelated birds live together in jungle areas, although Psamathia is perhaps confined more to the forested undergrowth and is more solitary in its habits. Aside from its longer legs and bill, Psamathia closely resembles Rukia in shape and coloration. They appear to have developed along somewhat similar evolutionary lines with regard to structure, color and ecologic requirements.
The Palau Warbler was first discovered by Captain Tetens and described as belonging to a new genus by Hartlaub and Finsch (1868a:5). In the original description the authors remark that, "The generic position of this new form is in the Calamoherpe group; the feet are the same as in Calamoherpe; but the beak is weaker and slenderer, and the wings are very different. Calamoherpe has the first quill quite spurious, the third is the longest, and the second and sixth are subequal. In Calamoherpe there are twelve tail-feathers; in Psamathia I can find only ten. Tatare is a very different form, with a scutellated tarsi, a very different structure of the plumage, a much more elongated beak, and a twelve-feathered tail. Tatare syrinx is a typical Calamoherpe. In the structure of the wing of [Pg 251] Psamathia, there seems to be a great resemblance to the genus Arundinax of Blyth, a form with which it is not in my power to compare." The genera Calamoherpe and Tatare are now included in Acrocephalus; the describers were comparing the Palau Warbler with the reed-warblers of Micronesia and Polynesia.
Sharpe (1883:93) writes that the Palau Bush-warblers are "Aberrant reed warblers, and should, in my opinion, be placed in future classifications of the Cichlomorphae near the genera Cettia and Acrocephalus, from which they are separated by their larger first primary only. Through Megalurus and Sphenoeacus they approach the grass-warblers and Cisticolae especially."
Mayr (1941b:203) cites Psamathia as an example of "restricted endemism" and points out that the nearest relative occurs in the Philippines. Delacour (1942:514), in a discussion of the bush-warblers of the genera Cettia, Bradypterus and related forms, says, "Psamathia annae, from Palau Islands, is related to Cettia, differing mainly in its much longer bill and legs."
Psamathia is a specialized bush-warbler and has followed a pattern of evolution which characterizes some of the other island birds in that the bill and legs are long and the wing is rather short and rounded. Psamathia resembles many of the bush-warblers, as well as the reed-warblers {Acrocephalus); in general, body coloring being paler below and darker above. It differs from Acrocephalus by having a longer tenth primary, smaller second and third primaries, only ten tail feathers, a more rounded wing, differently shaped nostrils, and by much softer plumage (the latter character is found also in Collurcincla tenebrosus and Cleptornis marchei of Micronesia). Rather than being related to the reed-warblers, as was supposed by Hartlaub and Finsch, Psamathia seems closest to Cettia, especially to Cettia (Horeites) diphone seebohmi of the Philippine Islands. Psamathia has a longer bill than this bird, but the general appearance and structure of the feet, tail, wing, body and bill are the same.
Thryothorus luscinius Quoy and Gaimard, Voy. "l'Astrolabe," Zool., 1, 1830, p. 202, pl. 5, fig. 2. (Type locality, Marian Is. = Guam.)
Sylvia syrinx Kittlitz (part), Obser. Zool., in Lutké, Voy. "Le Seniavine," 3, 1836, p. 306 (Guahan); idem (part), Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 141 (Guaham).
Tatare luscinia Gray, Genera Birds, 3, 1849, App. 8 (Marian Is. = Guam); Hartlaub, Journ. f. Ornith., 1854, p. 167 (Mariannen = Guam); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 14 (Ladrone or Marian Is. = Guam); Finsch, Journ. Mus. Godeffroy, 12, 1876, p. 31 (Guaham); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 41 (Guam); Büttikofer, Notes Leyden Mus., 14, 1892, p. 16 (Guam); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 209 (Guam).
Tatare luscinius Bonaparte, Consp. Avium, 1, 1850, p. 224 (Guam); idem, Comptes Rendus Acad. Sci. Paris, 41, 1855, p. 1111 (Mariannes = Guam); Gray, Hand-list Birds, 1, 1869, p. 194 (Ladrone = Guam).
Hybristes [luscinia] Reichenbach, Syst. Avium, 1850, pl. 57, fig. 7 (no locality = Guam).
Acrocephalus orientalis Pelzeln, Reise, "Novara," Vögel, 1865, p. 64 (Guaham).
Tatares luscinius Giebel, Thes. Ornith., 3, 1877, p. 599 (Marianae).
Acrocephalus mariannae Tristram, Ibis, 1883, p. 45 (Type locality, Guam).
Tatare mariannae Sharpe, Cat. Birds British Mus., 7, 1883, p. 528 (Marianne = Guam); Oustalet, Le Nat., 1889, p. 260 (Mariannes = Guam).
Acrocephalus luscinia Hartert, Novit. Zool., 5, 1898, p. 57 (Guam, Saipan); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 53 (Guam, Saipan); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Guam, Saipan); Safford, Osprey, 1902, p. 69 (Guam); Dubois, Syn. Avium, 1, 1902, p. 369 (Marianne); Safford, Amer. Anthro., 4, 1902, p. 711 (Guam); idem, The Plant World, 7, 1904, p. 264 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, pp. 30, 79 (Guam); Reichenow, Die Vögel, 2, 1914, p. 545 (Marianen); Cox, Island of Guam, 1917, p. 21 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 67 (Guam, Saipan); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 25 (Guam); Thompson, Guam and its people, 1942, p. 23 (Guam); Strophlet, Auk, 1946, p. 539 (Guam).
Conopoderas luscinia Mathews, Syst. Avium Australasianarum, 2, 1930, p. 594 (Marianas); Hand-list Japanese Birds, rev., 1932, p. 177 (Marianas).
Conopoderas luscinia hivae Yamashina, Bull. Biogeogr. Soc. Japan, 12, 1942, p. 81 (Type locality, Saipan); Hand-list Japanese Birds, 3d ed., 1942, p. 196 (Almagan, Saipan).
Conopoderas luscinia luscinia Hand-list Japanese Birds, 3d ed., 1942, p. 197 (Guam).
Acrocephalus luscinia luscinia Mayr (part), Birds Southwest Pacific, 1945, p. 294 (Guam, Saipan, Almagan); Stott, Auk, 1947, p. 526 (Saipan); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 67 (Guam).
Geographic range.—Micronesia: Mariana Islands—Guam, Saipan, Almagan.
Character.—Adult: A rather large warbler with long, curved bill; upper parts near "Saccardo olive"; feathers of head grayer because of darker shafts; rump paler and browner; lores dark; supraloral stripe light buffy-yellow; auriculars, cheeks, and sides of neck slightly darker; chin, throat, breast, and abdomen pale buffy-yellow; tibia darker and more olivaceous-brown; under tail-coverts pale yellow-buff; wing and tail feathers brown, edged with ochraceous; under wing grayish, inner edges lighter; axillaries pinkish-white; upper mandible dark horn colored; lower mandible lighter yellow; feet light gray; iris brown. Female resembles male but is slightly smaller.
| Subspecies | No. | Sex | Wing | Tail | Exposed culmen |
Tarsus |
| A. l. luscinia |
11 |
males | 84 | 83 | 36.0 | 30.5 |
| (81-86) | (80-86) | (35.5-39.0) | (30.0-31.0) | |||
|
1 |
female | 78 | 73 | 37.0 | 28.5 | |
| A. l. syrinx |
31 |
males | 78 | 71 | 26.5 | 26.5 |
| (76-80) | (68-75) | (25.0-27.0) | (25.0-29.0) | |||
|
12 |
females | 75 | 68 | 25.5 | 26.0 | |
| (74-78) | (65-70) | (24.0-27.0) | (24.0-26.0) |
Immature: Resembles adult, but upper parts duller and more brown and less olive; underparts less yellow; wing and tail feathers lighter brown.
Measurements.—Measurements are listed in table 37.
Weights.—The weights of three adult males obtained at Guam by the NAMRU2 party are 30, 30, and 31 grams. An adult female from Guam weighed 27 grams.
Specimens examined.—Total number, 12 (11 males, 1 female), as follows: Mariana Islands, USNM—Guam, 6 (June 2, 13, July 2, 18)—Saipan, 6 (Sept. 27, 30).
Nesting.—Oustalet (1895:209) writes that Marche found nests at Guam in June, 1887. The NAMRU2 party obtained two males with enlarged gonads in June, 1945.
Molt.—Specimens taken in June, July, and September are either in worn plumage or in molt. Birds in worn plumage become a faded straw-brown above. Oustalet apparently interpreted this coloring of the worn plumage as a seasonal coloration.
Food habits.—Seale (1901:53) reports that four stomachs which he examined contained insects and larvae. Marshall (1949:21) lists as food items: lizards, snails, spiders, and insects.
Remarks.—The Nightingale Reed-warbler at Guam is restricted to cane thickets and adjacent areas in and near fresh and brackish water marshes. In 1945, the NAMRU2 party found the bird fairly numerous in some of these habitats. Seale (1901:53) writes, "This bird is now quite scarce on the island of Guam. It lives exclusively among the reedy swamps, and those swamps are now being drained to make room for the Chinaman's rice paddies." Mayr (1945a:295) also notes the rarity of the species. As a result of the late war, the cultivation of rice was reduced and the reed-warbler probably has been able to increase in some of the now fallow areas. The most extensive range of this bird at Guam is found in the Agaña Swamp, where there is a large area consisting of thick cane. Here, and in the other large cane patches, the chief hazard to the bird population appears to be fire. In dry periods, the entire habitat might be easily destroyed by fire. The birds are extremely shy; their melodious songs may be heard in the reeds, but their active movements in the thick cane are difficult to observe. While hunting for these birds along the edges of Agaña Swamp on June 2, the writer observed, or located the calls of, at least six or seven individuals but could only get within shooting range of three birds. Within the cane thickets, these birds feed and move about near the ground or the surface of the water. Rarely do they perch in a conspicuous manner in the upper parts of the cover. Their color patterns blend perfectly with the coloration of the dry cane stalks. Perhaps failure to find many of the birds because of their secretive habits has caused many [Pg 254] observers to assume that the bird is near extinction. Nevertheless, it is my contention that the bird, being restricted to these limited areas, has never been very abundant at Guam. The absence of natural enemies, especially snakes, may be one of the principal reasons why they have been able to survive.
Reed-warblers were not found by the NAMRU2 party at Rota in 1945, nor have they been reported from Tinian. Yamashina in 1942 described the populations at Saipan and Almagan as distinct. I have not seen this description, but on the basis of examinations of specimens from Saipan, I can see no recognizable differences between these and birds from Guam.
Sylvia syrinx Kittlitz, Mém. Acad. Imp. Sci. St. Petersbourg, 2, 1835, p. 6, pl. 8. (Type locality, Lugunor and Ulcei = Woleai.)
Sylvia syrinx Kittlitz (part), Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 297 (Lougounor); idem, Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 92 (Ualan, Lugunor, Ulea).
Eparnetes Reichenbach, Syst. Avium, 1850, pl. 57 (no locality = Carolines); Bonaparte, Comptes Rendus Acad. Sci. Paris, 41, 1855, p. 1111 (Carolines).
Tatare syrinx Hartlaub, Archiv f. Naturgesch., 18, 1852, p. 131 (Ualan, Lugunor); Pucheran, Voy. Pôle Sud, 3, 1853, p. 92 (Hogoleu = Truk); Hartlaub, Journ. f. Ornith., 1854, pp. 164, 168 (Hogoleu); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 14 (Ualan); Sharpe, Cat. Birds British Mus., 7, 1883, p. 527 (Carolines); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 41 (Ruk, Ualan, Luganor, Uleei, Nukuor, Ponapé); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 210 (Ruk, Ponapi, Mortlock, Kusaie, Uleei, Nukuor).
Acrocephalus orientalis Pelzeln, Reise "Novara," Vögel, 1865, pp. 63, 162 (Puynipet, Lugunor, Ulcei).
Calamodyta syrinx Gray, Hand-list Birds, 1, 1869, p. 208 (Ualan); Giebel, Thes. Ornith., 1, 1872, p. 529 (Carolin.).
Calamoherpe syrinx Finsch, Journ. Mus. Godeffroy, 12, 1876, p. 17 (Ponapé, Lugunor, Ruck, Ualan, Uleei); idem, Proc. Zool. Soc. London, 1877 (1878), p. 778 (Ponapé); idem, Journ. f. Ornith, 1880, pp. 287, 297 (Ponapé, Ruck, Mortlocks, Kuschai); idem, Proc. Zool. Soc. London, 1880, p. 575 (Ruk, Ponapé); idem, Ibis, 1881, pp. 108, 112, 115, 247 (Kuschai, Ruck, Ponapé, Mortlocks); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 298, 330, 353 (Ponapé, Mortlocks, Nukor, Ruk); Finsch, Ibis, 1883, p. 143 (Ruck); idem, Mitth. Ornith. Ver. Wien, 1884, p. 49 (Ponapé); idem, Sammlung wissensch. Vorträge, 14 ser., 1900, p. 659 (Carolinen).
Acrocephalus syrinx Seebohm, Cat. Birds British Mus., 5, 1881, p. 100 (Ponapé); Tristram, Ibis, 1883, p. 44 (Ponapé, Ruk, Mortlock, Lugunor, Uleei); idem, Cat. Birds, 1889, p. 152 (Ponape, Ruk); Nehrkorn, Kat. Eiers., 1899, p. 33 (Ponapé, Ruk); Hartert (part), Novit. Zool., 5, 1898, p. 58 (Carolines); idem, Novit. Zool., 7, 1900, p. 3 (Ruk); Seale (part), Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 53 (Ponapé); Matschie, Journ. f. Ornith., 1900, pp. 112, 113 (Ruk, Ponapé, Ualan); Dubois, Syn. Avium, 1, 1902, p. 369 (Ponapé); Reichenow, Die Vögel, 2, 1914, p. 545 (Ponapé); Takatsukasa and Kuroda, Tori, 1, 1915, p. 54 (Ponapé, Ruk); Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé).
Conopoderas syrinx Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 214 (Ponapé, Truk); Takatsukasa and Yamashina, Dobotsu. Zasshi, 43, 1931, p. 485 (Caroline Is.); Yamashina, Tori, 7, 1932, p. 405 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 177 (Carolines).
Acrocephalus stentoreus syrinx Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 67 (Ruk, Ualan, Lugunor, Wolea, Nukuoro, Ponapé).
Conopoderas luscinia syrinx, Hand-list Japanese Birds, 3d ed., 1942, p. 197 (Wolea, Lamotrek, Truk, Lukunor, Nukuoro, Ponapé, Kusaie).
Acrocephalus luscinia syrinx Mayr, Birds Southwest Pacific, 1945, p. 294 (Carolines); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 67 (Truk).
Geographic range.—Micronesia: Caroline Islands—Woleai, Lamotrek, Truk, Lukunor, Nukuoro, Ponapé, Kusaie.
Characters.—Adult: Resembles A. l. luscinia, but smaller; with shorter, straighter bill; head and neck more reddish-brown; back, rump, wing, and tail edged with cinnamon; flight feathers faintly tipped with white.
Immature: Resembles adult, but lighter and more rufous in color; wings and rump paler, wings edged with rufous buff.
Measurements.—Measurements are listed in table 37.
Specimens examined.—Total number, 62 (35 males, 20 females, 7 unsexed), as follows: Caroline Islands, USNM—Ponapé, 1 (Feb. 12)—Truk, 4 (Feb. 16, Mar. 15); AMNH—Ponapé, 35 (Nov., Dec.)—Truk, 22 (Feb., March, May, June, Nov.).
Nesting.—Birds nest in reedy swamps and scrub vegetation in the Caroline Islands, although Finsch (1881b:115), recording a field note by Kubary, states that nests were found in trees at Mortlock Atoll (= Lukunor). Yamashina (1932a:405) reports the collecting of seven sets of eggs at Ponapé in July and August, 1931. The sets consisted of one or two eggs each. McElroy of the NAMRU2 party obtained specimens with enlarged gonads at Truk in December and noted that birds were carrying nest materials to cane swamps. Of the birds secured by Coultas in November and December at Ponapé, only a small number had enlarged gonads. He also found nests containing no eggs in low bushes at Ponapé. Hartert (1900:3) reports that at Truk Owston's Japanese collectors obtained "many nests" from the end of May to the beginning of July. These nests contained one or two eggs and were found 7 to 20 feet above the ground in breadfruit, coconut and ivory-nut palm trees. Hartert writes, "The eggs are white, covered with darker and lighter brown patches, and underlying ashy grey or lavender-grey spots. These spots are generally thicker near the broad end, sometimes forming a loose ring, and they are sometimes equally spread over the whole surface." He lists measurements of 48 eggs.
Molt.—Of the specimens examined by me, those taken in the spring and summer are in fresh or worn plumage; those taken in fall and winter are in molt, with a few skins exhibiting worn or fresh plumage in the latter period. Apparently the peak in the molting process occurs from September to December.
Food habits.—The reed-warbler is an insect feeder. Coultas, in his observations of the bird at Ponapé, relates that he was able to locate the warbler by listening for the "snapping of the mandibles as the bird is catching food."
Remarks.—From the observations of Kittlitz, Kubary, Coultas, McElroy, and others, it is apparent that the Nightingale Reed-warbler in the Caroline Islands is restricted to the lower elevations of the islands. Whereas the reed-warbler at Guam seems closely associated with cane swamps and adjacent vegetation, the bird in the Carolines may range more extensively into brush lands, forest margin [Pg 256] and grass lands. Coultas (field notes) notes that the reed-warbler at Ponapé is a "common bird of the small bush and grasslands. One is attracted by its warbler-like song. The bird spends hours perched on a stem of a bush caroling the time of day. When feeding, one finds it on the ground or working away quietly among the bushes. Acrocephalus is a friendly bird who does not become frightened easily. He responds to man-made calls."
The Nightingale Reed-warbler is found on many of the islands in the Caroline Chain, including both the "high" volcanic islands (Ponapé and Truk) and the "low" coral islands (Lukunor and Nukunor). Although the bird has been recorded at Kusaie by Kittlitz and Finsch, it was not taken there by Coultas in 1931. Reed-warblers are unknown at Yap, Ulithi, Fais or at other islands of the extreme western Carolines, or in the Palau Archipelago.
They are unrecorded also in the Marshall Islands, but at Nauru in the Gilbert Islands, to the southeast, an isolated population of this bird occurs and has been named A. l. rehsei (Finsch).
Conopoderas yamashinae Takatsukasa, Dobutsu. Zasshi, 43, 1931, p. 485. (Type locality, Pagan.)
Tatare syrinx Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 210 (Pagan).
Acrocephalus syrinx Hartert (part), Novit. Zool., 5, 1898, p. 58 (Pagan); Seale (part), Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 53 (Pagan).
Acrocephalus stentoreus syrinx Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 67 (Pagan).
Conopoderas yamashinae, Hand-list Japanese Birds, rev., 1932, p. 177 (Pagan).
Conopoderas luscinia yamashinae Hand-list Japanese Birds, 3d ed., 1942, p. 196 (Pagan).
Acrocephalus luscinia yamashinae Mayr, Birds Southwest Pacific, 1945, p. 294 (Pagan).
Geographic range.—Micronesia: Mariana Islands—Pagan.
Characters.—Resembles A. l. syrinx, but duller and more brownish and less olive-rufous on back, rump and tail; bill shorter and more curved.
Takatsukasa (1931:485) gives the following description: "Upperparts dark olive brown, paler on the lower rump; remiges and rectrices dark olive-brown, margined with brown. Superciliary stripe distinct and buff; chin, throat, breast and abdomen pale brown; ear-coverts, sides of neck, sides of breast and flanks dusty greyish brown, belly and under tail-coverts pale buff. Bill clove brown, legs grey, and iris Van Dyke brown." He continues, "It differs from Conopoderas syrinx of Caroline Islands by its colouration and the shape of the bill, namely in the new form the culmen is more curved and more stout, and the tail is less roundish and nearly square."
Measurements.—Takatsukasa and Yamashina (1931b:485) lists the following measurements: 13 adult males—wing, 75-80; tail, 65-70; culmen, 20-22; 6 adult females—wing, 73-77; tail, 60-65; culmen, 20-22.
Mayr examined seven specimens from Pagan in the Paris Museum. His measurements are: five males—wing, 76-79; tail, 66-69; bill from nostril, 14-14.5; two females—wing, 75, 77; tail, 66, 67; bill from nostril, 14.5, 15.
Remarks.—No specimens have been examined by me. Oustalet (1895:210) was the first to note the difference between the reed-warblers from Pagan and those from Guam and Saipan (A. l. luscinia). He regarded those from Pagan as similar to the population in the Carolines, calling them Tatare syrinx. Hartert, Seale, and Momiyama followed Oustalet in this regard, and it was not until 1931 that the population at Pagan was recognized as distinct, when further collections were made by the Japanese.
Conopoderas luscinia nijoi Yamashina, Tori, 10, 1940, p. 674. (Type locality, Agiguan.)
Conopoderas luscinia nijoi Hand-list Japanese Birds, 3d ed., 1942, p. 196 (Agiguan).
Acrocephalus luscinia luscinia Mayr (part), Birds Southwest Pacific, 1945, p. 294 (Agiguan).
Geographic range.—Micronesia: Mariana Islands—Agiguan.
Characters.—Adult: Resembles A. l. luscinia, but with shorter bill. Yamashina (1940:674) describes the birds as, "upper parts much less rusty in colour and the flanks and bellies are darker and more brownish than those of the specimens from Almagan and Saipan."
Measurements.—Yamashina (1940:674) gives the measurements of five adult birds from Agiguan as: exposed culmen 27-29, bill from nostril 17.0-20.0; as compared with 27 adult birds from Almagan and Saipan as: exposed culmen 30-34, bill from nostril 21.2-24.5.
Remarks.—No specimens have been examined by me. The island of Agiguan is a very small one lying offshore from Tinian and not far from Saipan, where A. l. luscinia occurs. A. l. nijoi is given tentative recognition, on the basis of the measurements of the five adult specimens given by Yamashina. These indicate that the population has a distinctly shorter bill.
Evolutionary history of Acrocephalus luscinia.—The species of Acrocephalus in Micronesia and Polynesia have received several taxonomic treatments. In regard to the Micronesian forms, Quoy and Gaimard called the population at Guam Thryothorus while Kittlitz called the population in the Carolines, Sylvia. Evidently to emphasize the distinctness of these two birds, Reichenbach in 1850 renamed the bird in the Marianas as Hybristes and the bird in the Carolines as Eparnetes. The birds were later placed in the genus, Tatare, by Hartlaub, Gray, Sharpe and other workers. Gray also used the name, Calamodyta, for the bird in the Carolines. [Pg 258] The generic term, Calamoherpe, was employed also by a number of workers for the Caroline population. Sharpe (1883:525) placed the reed-warblers in the family Timelidae and retained the name, Tatare, for the Micronesian and Polynesian forms. In distinguishing Acrocephalus from Tatare he has the following to say of Acrocephalus: "besides having a much shorter bill, possesses a very much more pointed wing, the distance between the primaries and the secondaries being much more than the length of the hind toe and claw; whereas in Tatare the wing is much more obtuse, and the distance between the primaries and the secondaries is less than the length of the hind toe and claw." More recent authors have followed Sharpe using the generic name, Conopoderas (= Tatare, old name preoccupied). However, Tristram (1883:38-46) regarded the separation of these oceanic forms from Acrocephalus as a taxonomic error. He said that this is "one of the very few links (the others being the solitary Hirundo tahitica and the Merulae) between the avifauna of Oceania and our own; and it has a much wider range east and west than either of the other links, extending from the Carolines in the east to the Marquesas in the west." Mayr has pointed out (orally to the writer) that the separation of the Oceanic reed-warblers from Acrocephalus is an unnatural one, although it is perfectly true that the extreme members (A. caffra and A. l. luscinia) have a very long bill, but forms with shorter bills like A. l. syrinx point to the close affinity between the continental species and these insular birds. This has also been noted by Hartert (1898:58). Mayr (in litt.) comments that "There is no difference between Acrocephalus and Conopoderas in regard to the wing formula, provided that we compare the Polynesian species with the tropical forms of Acrocephalus (such as toxopei and cervinus). The character mentioned by Sharpe is very artificial and merely indicates the difference in the wing between a migrant of the temperate zone and a resident of the tropics. There is no denying that some of the warblers of eastern Polynesia are no longer reed-warblers but have become dwellers of trees and bushes. However, this same tendency prevails among some of the unquestionable species of Acrocephalus (scirpaceus and palustris) and at any rate a slight change in habits is not sufficient for generic separation." Earlier, Mayr (1942b:169) used Conopoderas as one of the several genera that is based on "morphologically distinct geographic forms." The degree of modification that has occurred in these oceanic reed-warblers, would, if the birds were in a continental area, undoubtedly be considered [Pg 259] worthy of specific or even generic rank by some authors; however, as Mayr (1942b:162) points out, "the majority of well-isolated subspecies have all the characters of good species and are indeed considered to be such by the more conservative systematists." Owing to their differentiation, the Micronesian and Polynesian reed-warblers might not be considered by some ornithologists as belonging to a single superspecies; however, all evidence seems to point to the origin of this group by a single invasion from Asia."
Tristram (1883:41) was the first worker to recognize the relationship of the Micronesian and Polynesian reed-warblers to the continental forms, when he placed them within the genus Acrocephalus. Rothschild (1893:2) further stated, "Tatare cannot be separated generically from Acrocephalus." In discussing the status of the Hawaiian species, A. familiaris, Hartert (1898:58) also follows this treatment. Bryan (1941:187) also comments on the relationship of the "miller" birds of Laysan and Nihoa to species at Guam, Christmas and other islands of the Pacific.
The reed-warblers of Polynesia and Micronesia represent an ancient invasion from Asia. The continental form, Acrocephalus arundinaceous, is apparently closest to the ancestral stock of these oceanic birds. This species resembles the oceanic populations in size, general coloring, shape of bill, and wing and tail structure. Some of the continental races of this species have a shorter first primary which is similar to that in the oceanic forms. How rapid the spread was of the reed warbler through the large insular area that it now occupies is unknown. A. syrinx of Micronesia has a shortened wing and some populations have a long bill. Species in Polynesia have stronger wings than the one in Micronesia, but have become differentiated in other ways, as, for example, by the long bill of A. caffra and the small size of A. aequinoctialis. In addition, call notes have become varied, as noted by Chapin (in Mayr, 1942b:54). Also certain of the reed-warblers have become bush and tree-living birds. The Hawaiian birds are reduced in size and have become tree-living in a manner similar to that of other Polynesian species. These modifications of the reed-warblers of the Oceanic area appear, according to Murphy and Mathews (1929), to indicate their long-time residence in the islands, as compared with subspecies of A. arundinaceous that are found in Melanesia. The latter birds, which are not ancestral to the Polynesian birds, resemble closely their Asiatic ancestors and have also retained their swamp-living habits. This would seem to indicate that the birds in Melanesia may be of more [Pg 260] recent occurrence. Stresemann (1939b:324) presents a map of the distribution of A. arundinaceous in southeastern Asia and adjacent islands. The original stock came from a point in China, north of Indochina, spreading to the Philippines and to Celebes, from where it reached the Solomons and New Guinea via the Lesser Sundas and Australia.
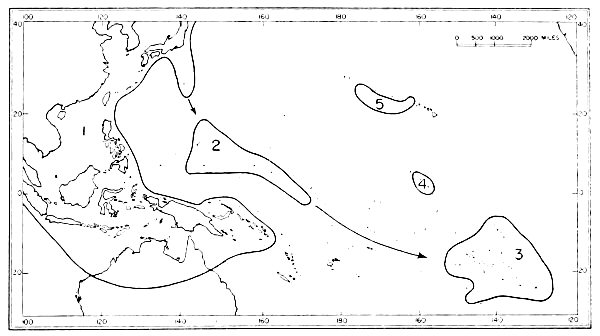 Fig.
15. Geographic distribution of Acrocephalus in the Pacific area and routes of its dispersal. (1) A. arundinaceus; (2) A. luscinia; (3) ranges of A. atypha,
A. caffra, and A. vaughani; (4) A. aequinoctialis; (5) A. familiaris.
Fig.
15. Geographic distribution of Acrocephalus in the Pacific area and routes of its dispersal. (1) A. arundinaceus; (2) A. luscinia; (3) ranges of A. atypha,
A. caffra, and A. vaughani; (4) A. aequinoctialis; (5) A. familiaris.The path of invasion of Oceania by the reed-warbler is pictured in figure 15. Probably the birds became established in Micronesia by an invasion from the Bonins, where A. arundinaceus orientalis is known to occur today. From the Marianas and Carolines, the birds spread to Polynesia; A. l. rehsei of the Gilbert Islands (Nauru) might well be a connecting link. Possibly, the Hawaiian birds came as a separate invasion via the Volcano and Bonin islands or through the Micronesia Chain, or through the Line and Christmas islands from the south. It seems evident, however, that owing to their geographic proximity and comparative structural similarity, the species in Hawaii is closest to A. luscinia of Micronesia. The absence of reed-warblers from the western Carolines and Palaus seems to reduce the possibility of an invasion from the Philippine region. However, reed-warblers are absent from the Marshall and the northern Gilbert islands, where there is undoubtedly suitable habitat for their occurrence. Possibly these islands were once occupied by the birds but they were eliminated by natural causes or by man and his land uses.
Rhipidura Uraniae Oustalet, Bull. Soc. Philom. Paris, (7), 5, 1881, p. 76. (Type locality, Mariannes = Guam.)
Rhipidura pectoralis Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 17 (Ladrone or Marian Is. = Guam).
Rhipidura uraniae Reichenow and Schlow, Journ. f. Ornith., 1884, p. 398 (Mariannes = Guam); Hartert, Novit. Zool., 5, 1898, p. 53 (Guam); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 20 (Marianne = Guam); Büttikofer, Notes Leyden Mus., 15, 1893, p. 76 (Guam); Wheeler, Report Island of Guam, 1900, p. 13 (Guam); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 48 (Guam); Safford, Osprey, 1902, p. 69 (Guam); Dubois, Syn. Avium, 1, 1902, p. 277 (Guam); Safford, The Plant World, 7, 1904, p. 263 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Mearns, Proc. U. S. Nat. Mus., 36, 1909, p. 477 (Guam); Schnee, Zeitschr. f. Naturwisch., 82, 1910, p. 464 (Marianen = Guam); Reichenow, Die Vögel, 2, 1914, p. 267 (Marianen = Guam); Cox, Island of Guam, 1917, p. 21 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 65 (Guam); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 25 (Guam).
Rhipidura atrigularis Reichenow, Journ. f. Ornith., 1885, p. 110 (Type locality, Palau, error = Guam); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 21 (Pelew, error = Guam); Takatsukasa and Kuroda, Tori, 1, 1915, p. 63 (Marianne = Guam).
Rhipidura versicolor Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 190 (Guam).
Rhipidura rufifrons uraniae Mathews, Syst. Avium Australasianarum, 2, 1930, p. 490 (Marianne = Guam); Hand-list Japanese Birds, rev., 1932, p. 176 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 195 (Guam); Mayr, Birds Southwest Pacific, 1945, p. 295 (Guam); Watson, The Raven, 17, 1946, p. 42 (Guam); Mayr and Moynihan, Amer. Mus. Novit., no. 1321, 1946, pp. 3, 9 (Guam); Baker, Proc. Biol. Soc. Washington, 59, 1946, p. 77 (Guam); idem, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 67 (Guam).
Rhipidura rufifrons Wharton, Ecol. Monogr., 16, 1948, p. 174 (Guam); Strophlet, Auk, 1946, p. 339 (Guam).
Geographic range.—Micronesia: Mariana Islands—Guam.
Characters.—Adult: Forehead and anterior crown near "cinnamon-buff"; lores and orbital ring black, auriculars more brownish than lores; malar stripe white; a few feathers in posterior malar region tipped with "citrine drab"; anterior part of chin white; posterior part of chin, throat, and upper breast black; feathers on breast edged with white; lower breast, abdomen, sides, flanks, tibia, vent, and under tail-coverts near "royal brown," becoming lighter on breast and more rufous on under tail-coverts; sides of neck and back near "Dresden brown," becoming grayer on neck and crown where feathers have darker shafts; rump and upper tail-coverts near "orange rufous"; basal half of tail slightly lighter than rump; terminal part of tail black, tipped with white; wings dark edged with coloring like back; under wing grayish with axillaries tipped with buffy-white; bill black with base of upper mandible lighter; feet dark brown; iris dark brown.
Immature: Resembles adult, but head, neck, scapulars, and secondaries edged with rufous; feathers of chin and throat edged with whitish. Younger birds may have less rufous on head but feathers of body more rufous with creamy edges.
Measurements.—Measurements are listed in table 38.
| Subspecies | Number and sex |
Wing | Tail | Exposed culmen |
Tarsus |
| R. r. uraniae |
11 males |
66 | 78 | 13.6 | 16.6 |
| (64-69) | (75-82) | (13.1-14.5) | (15.6-17.2) | ||
|
6 females |
65 | 76 | 12.3 | 16.8 | |
| (61-68) | (73-81) | (11.6-12.5) | (16.1-17.6) | ||
| R. r. saipanensis |
7 males |
68 | 81 | 13.3 | 17.3 |
| (68-69) | (80-83) | (13.0-13.5) | (16.2-18.4) | ||
|
6 females |
64 | 76 | 12.7 | 17.9 | |
| (62-66) | (72-81) | (12.4-13.4) | (17.2-18.1) | ||
| R. r. mariae |
2 males |
65, 67 | 82, 82 | 12.1, 12.4 | 17.1, 17.2 |
| R. r. kubaryi |
14 males |
77 | 88 | 14.4 | 20.0 |
| (75-79) | (82-95) | (13.6-15.0) | (19.0-21.0) | ||
|
10 females |
72 | 87 | 14.5 | 20.0 | |
| (69-75) | (83-90) | (14.0-15.0) | (20.0-20.5) |
Weights.—The NAMRU2 party recorded the weights of nine males as 9.0-10.0 (9.0); of three females as 7.2-9.6 (8.8) grams.
Specimens examined.—Total number, 41 (19 males, 14 females, 8 unsexed), as follows: Mariana Islands, USNM—Guam, 17 (May 29, 30, June 6, 14, 18, July 12, 20); AMNH—Guam, 24 (Jan., Feb., March, Aug., Sept., Dec.).
Nesting.—Hartert (1898:54) recorded nests taken at Guam in February and March.
Molt.—On the basis of specimens examined, it is apparent that molt begins in August or September and continues through the months of the fall.
Parasites.—Wharton (1946:174) obtained the chigger (Acarina), Trombicula sp., from this bird at Guam.
Remarks.—The Rufous-fronted Fantail at Guam is a bird of the forest and forest scrub. It prefers the areas where leafy undergrowth is present. It moves rapidly about continually fluttering its wings and spreading its long fanlike tail. The birds are usually observed in pairs. On January 21, 1945, E. W. Coleman of the NAMRU2 party killed a fantail but before he could retrieve it, a large toad (Bufo marinus) seized the fallen bird and carried it into a hole in the ground.
Rhipidura saipanensis Hartert, Novit. Zool., 5, 1898, p. 54. (Type locality, Saipan).
Rhipidura versicolor Oustalet, Le Nat., 1889, p. 260 (Mariannes = Saipan); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 21 (Marianne = Saipan); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 190 (Saipan).
Rhipidura saipanensis Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Saipan); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 48 (Saipan); Dubois, Syn. Avium, 1, 1902, p. 277 (Saipan); Takatsukasa and Kuroda, Tori, 1, 1915, p. 63 (Marianne = Saipan).
Rhipidura rufifrons saipanensis Kuroda, in Momiyama, Birds Micronesia, 1922, p. 65 (Saipan); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 490 (Saipan); Hand-list Japanese Birds (part), rev., 1932, p. 176 (Saipan, Tinian); Hand-list Japanese Birds (part), 3d ed., 1942, p. 195 (Saipan, Tinian); Mayr (part), Birds Southwest Pacific, 1945, p. 295 (Saipan, Tinian); Mayr and Moynihan (part), Amer. Mus. Novit., no. 1321, 1946, p. 3 (Saipan, Tinian); Baker, Proc. Biol. Soc. Washington, 59, 1946, p. 77 (Saipan, Tinian); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 98 (Tinian).
Rhipidura lepida saipanensis Stott, Auk, 64, 1946, p. 527 (Saipan).
Geographic range.—Micronesia: Mariana Islands—Saipan, Tinian.
Characters.—Adult: Resembles adult of R. r. uraniae, but forehead and anterior crown more rufous; posterior crown and nape lighter; rump and upper tail-coverts lighter and richer in color; white malar stripe broader; chin with white feathering more extensive, covering edge of upper throat.
Measurements.—Measurements are listed in table 38.
Specimens examined.—Total number, 16 (9 males, 6 females, 1 unsexed), as follows: Mariana Islands, USNM—Saipan, 1 (Dec. 15)—Tinian, 3 (Oct. 16, 23); AMNH—Saipan, 6 (July, Aug.)—Tinian, 6 (Sept.).
Molt.—Molt begins in July and extends through the autumn. Most of the specimens examined, that were taken in this period, are in molt.
Food habits.—Stott (1947:527) writes that the fantail forages for insects in the undergrowth and also while on the wing captures flying insects. Downs (1946:99) made similar observations concerning this bird at Tinian.
Remarks.—In studying the collection of fantails obtained by Marche at Guam and Saipan, Oustalet (1895:191) reached the conclusion that the birds from these two islands were the same as the bird from Yap, which he called R. versicolor. He thought that the white-throated birds were in breeding plumage, and that the black-throated birds (from Guam) were in autumn and winter dress. This error was corrected by Hartert (1898:53).
Downs (1946:98-100) has published some interesting observations concerning the fantail at Tinian. He describes feeding behavior and the song which he says is "a beautiful rolling whistle, starting rather shrilly, then rolling on. Something like a meadow-lark and song sparrow combined." Gleise (1945:220) estimated the population of fantails at Tinian to be "40-50" in 1945. In 1931, Coultas found the bird at Tinian but not at Saipan. Stott (1947:527) observed the bird at Saipan "in forested areas and vine-draped crevices in the lava above Magicienne Bay."
Rhipidura rufifrons mariae R. H. Baker, Proc. Biol. Soc. Washington, 59, 1946, p. 7. (Type locality, Rota.)
Rhipidura rufifrons saipanensis Takatsukasa and Yamashina, Dobutsu. Zasshi, 44, 1932, p. 222 (Rota); Hand-list Japanese Birds (part), rev., 1932, p. 176 (Rota); Hand-list Japanese Birds (part), 3d ed., 1942, p. 195 (Rota); Mayr (part), Birds Southwest Pacific, 1945, p. 295 (Rota); Mayr and Moynihan (part), Amer. Mus. Novit., no. 1321, 1946, p. 3 (Rota).
Rhipidura rufifrons mariae Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 68 (Rota).
Geographic range.—Micronesia: Mariana Islands—Rota.
Characters.—Adult: Resembles adult of R. r. saipanensis, but with richer brown coloring on the breast and abdomen; darker above, especially the forehead, rump, and basal part of tail; chin with small mount of white; malar line of white thinner.
Measurements.—Measurements are listed in table 38.
Weights.—Baker (1946:78) records the weights of two adult males from Rota as 8.3 and 9.0 grams.
Specimens examined.—Total number, 2 males, from Mariana Islands, USNM—Rota (Oct. 22).
Remarks.—Takatsukasa and Yamashina (1932:222) published the first account of the fantail from Rota although Coultas (field notes) obtained a report of its presence at Rota in 1931. The NAMRU2 party obtained the two specimens studied, and reported that the birds were numerous in the forested areas of Rota in 1945.
Rhipidura versicolor Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 96. (Type locality, Uap.)
Rhipidura versicolor Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Sharpe, Cat. Birds British Mus., 4, 1879, p. 320 (Yap); Nehrkorn, Journ. f. Ornith., 1879, p. 402 (Yap); Oustalet, Bull. Soc. Philom. Paris, (7), 5, 1881, p. 76 (Uap); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 391 (Yap); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 21 (Uap); Büttikofer, Notes Leyden Mus., 15, 1893, p. 78 (Uap); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 191 (Yap); Hartert, Novit. Zool., 5, 1898, p. 54 (Yap); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 54 (Yap); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Yap); Dubois, Syn. Avium, 1, 1902, p. 277 (Yap); Reichenow, Die Vögel, 2, 1914, p. 267 (Yap); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Mackenzie = Yap); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 66 (Yap); Hand-list Japanese Birds, rev., 1932, p. 176 (Yap).
Rhipidura rufifrons versicolor Mathews, Syst. Avium Australasianarum, 2, 1930, p. 489 (Uap); Hand-list Japanese Birds, 3d ed., 1942, p. 196 (Yap); Mayr, Birds Southwest Pacific, 1945, p. 295 (Yap); Mayr and Moynihan, Amer. Mus. Novit., no. 1321, 1946, p. 3 (Yap).
Geographic range.—Micronesia: Caroline Islands—Yap.
Characters.—Adult: Resembles R. r. uraniae, but chin and upper throat white; upper parts darker; abdomen whitish.
The description of the adult given by Hartlaub and Finsch (1872:96) is "Upper parts a rich brown with a slight reddish tinge; forehead bright rufous; upper and under tail-coverts rufous; throat white, margined underneath by an irregular jugular band of pure black; pectoral plumes black, broadly margined with yellowish white; middle of abdomen whitish, sides of a paler olive-brown under wing-coverts whitish; wing-feathers blackish brown; tail feathers brownish black, all largely tipped with white, the four middle ones rufous at the base, the white terminal spots becoming smaller towards the middle; beak fuscous, the under mandible paler except at tip; feet fuscous."
Hartert (1898:54) writes that R. r. saipanensis differs from the bird at Yap "in having the bases of all rectrices rufous, the rump and upper tail-coverts rufous. The sides of the abdomen are not olive-brown, but rufous."
Remarks.—No specimens of the Rufous-fronted Fantail of Yap have been seen by me. On the basis of published descriptions and comments, it appears that the bird is subspecifically distinct from the forms in the Marianas but shows close relationships to them. R. r. versicolor has the chin and throat white; R. r. saipanensis has the chin and part of the throat white and a heavy, white line in the malar region; R. r. mariae has the chin and only a small amount of the throat white and a thinner, white malar stripe; R. r. uraniae has only a small amount of white present on the chin and a very thin, white line in the malar region.
Rhipidura kubaryi Finsch, Proc. Zool. Soc. London, 1875 (1876), p. 644. (Type locality, Ponapé.)
Rhipidura kubaryi Finsch, Journ. Mus. Godeffroy, 12. 1876, pp. 17, 29, pl. 2, fig. 2 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 779 (Ponapé); Nehrkorn, Journ. f. Ornith., 1879, p. 403 (Ponapé); Finsch, Journ. f. Ornith., 1880, p. 289 (Ponapé); idem, Ibis, 1881, pp. 110, 112, 115 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 281 (Ponapé); Tristram, Cat. Birds, 1889, p. 198 (Ponapé); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 20 (Ponapé); Büttikofer, Notes Leyden Mus., 15, 1893, p. 76 (Ponapé); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Ponapé); Dubois, Syn. Avium, 1, 1902, p. 277 (Ponapé); Takatsukasa and Kuroda, Tori, 1, 1915, pp. 54, 64 (Ponapé); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zool., 63, 1919, p. 204 (Ponapé); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 65 (Ponapé); Yamashina, Tori, 7, 1932, p. 403 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 176 (Ponapé); Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 196 (Ponapé).
Rhipidura kubarii Sharpe, Cat. Birds British Mus., 4, 1879, p. 314 (Ponapé); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 55 (Ponapé).
Rhipdura rufifrons kubaryi Mayr, Birds Southwest Pacific, 1945, p. 295 (Ponapé); Mayr and Moynihan, Amer. Mus. Novit., no. 1321, 1946, pp. 3, 6, 9, 11, 12, 15, 16 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult: Upper parts smoky olivaceous-brown, less smoky on rump and upper tail-coverts; anterior forehead and supraloral region narrowly edged with white; lores and orbital ring black; auriculars brown; feathers of chin and malar region tipped with white; rest of chin and throat black, lower feathers of throat edged with white; abdomen dark olivaceous-brown with whitish mid-portion anteriorily; sides and under tail-coverts ashy, the latter broadly tipped with white; wings and tail dark, tail tipped with white and outer rectrices more broadly so; axillaries and under wing-coverts gray, broadly tipped with white; bill and feet black, mandible basally whitish; iris dark brown.
R. r. kubaryi resembles R. r. uraniae, but larger; lacking rufous coloring; smaller and shorter, white malar stripe; white on chin reduced.
Measurements.—Measurements are listed in table 38.
Specimens examined.—Total number, 40 (24 males, 15 females, 1 unsexed), as follows: Caroline Islands, USNM—Ponapé, 1 (Feb. 12); AMNH—Ponapé, 39 (Nov., Dec.).
Nesting.—Yamashina (1932a:403) records nests containing one or two eggs taken at Ponapé in 1931 on the following dates: July 11, August 2, 14, 19, 22, 30. Coultas (field notes) obtained reports that the eggs are two in number and laid in a cup-shaped nest of grass and fern, which is placed near the ground.
Molt.—Many of the specimens examined that were taken in November and December are in fresh or slightly worn plumage. Only a few are molting. Apparently molt occurs earlier, perhaps beginning in August and continuing until October or November.
Remarks.—Coultas obtained a large series of these birds at Ponapé in 1931. He writes (field notes) that the fantail is a common bird and is found in forest and brush lands. This bird has a nervous behavior similar to that of other fantails and is constantly "wagging its long tail." Coultas describes it as an aggressive bird, chasing honey-eaters and white-eyes.
Rhipidura lepida Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 6. (Type locality, Pelew Islands.)
Rhipidura lepida Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 117, 118 (Pelew Islands); Gray, Hand-list Birds, 1, 1869, p. 331 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 97 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 21, pl. 4, fig. 2-3 (Palau); Sharpe, Cat. Birds British Mus., 4, 1879, p. 322 (Pelew); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Tristram, Cat. Birds, 1889, p. 198 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 21 (Pelew); Büttikofer, Notes Leyden Mus., 15, 1893, p. 81 (Pelew); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 55 (Palau); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Palau); Dubois, Syn. Avium, 1, 1902, p. 278 (Pelew); Reichenow, Die Vögel, 2, 1914, p. 267 (Palau); Takatsukasa and Kuroda, Tori, 1, 1915, p. 54 (Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 66 (Pelew); Mathews, Syst. Avium Australasianarum], 2, 1930, p. 484 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 176 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 196 (Babelthuap, Koror, Peliliu); Mayr and Moynihan, Amer. Mus. Novit., no. 1321, 1946, pp. 3, 5, 8, 10, 12, 19 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 68 (Peleliu).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Peleliu, Ngabad.
Characters.—Adult: Upper parts near "cinnamon-rufous," slightly lighter on the upper wing-coverts, scapulars, edges of inner secondaries, and rump; lores blackish; orbital ring and auriculars dark brown; chin, upper throat, and malar region white; lower throat and upper breast black with ashy-gray sides; lower breast whitish; rest of underparts like back but slightly paler; wings dark; tail black with tips rufous, inner rectrices with rufous tips narrower than on tail; under wing-coverts and axillaries broadly edged with rufous; bill blackish, lower mandible whitish at base; feet brownish; iris dark brown. Female slightly smaller.
Immature: Resembles adult, but head and neck brown; throat coloring dingy; patch on breast blackish cinnamon. Younger individuals may be more tinged with rufous above and below.
Measurements.—Measurements are listed in table 39.
| Number and Sex | Wing | Tail | Culmen | Tarsus |
| 7 adult males | 80 | 89 | 15.5 | 23.3 |
| (77-83) | (85-94) | (14.5-16.0) | (23.0-24.0) | |
| 7 adult females | 77 | 86 | 15.5 | 22.5 |
| (76-79) | (83-88) | (14.5-15.0) | (21.7-23.0) |
Specimens examined.—Total number, 18 (9 males, 9 females), as follows: Palau Islands, USNM—Koror, 2 (Nov. 6, 18)—Babelthuap, 1 (Nov. 27)—Peleliu, 4 (Aug. 29, 30, 31); AMNH—exact locality not given, 11 (Nov., Dec.).
Molt.—Some of the birds taken in August are in molt. Specimens taken in November and December are mostly in fresh plumage. Apparently this bird molts in late summer and early fall.
Remarks.—In 1945 the NAMRU2 party found the Palau Fantail in small numbers at Peleliu, Garakayo and Ngabad. At Peleliu the birds were noted as singles or in pairs in brushy undergrowth in forested areas. The birds were observed also in the second growth vegetation in the battle areas. Coultas (field notes) found the bird to be rare and restricted to the true forest, when he visited the Palau Islands in 1931. The fantail is one of the most attractive birds found in the jungles of the Palau Islands. Its bright rufous coloring is conspicuously displayed by the rapid movements of the wings and tail as the bird moves and feeds in the undergrowth. The population is apparently not large, and the individual or pair of birds probably ranges in a relatively large home territory.
Evolutionary History of Rhipidura in Micronesia.—The evolutionary history of Rhipidura in Micronesia has been studied considerably more than that of some of the other genera in the area. Oustalet (1896:70) notes a close relation between Rhipidura of the Marianas and R. rufifrons of Australia. Mayr (1941b:202, 203) regards the genus Rhipidura as typical of the Polynesian area and remarks that speciation within this genus has proceeded at a relatively rapid rate. Mayr and Moynihan (1946) have devoted a 21-page paper to a thorough discussion of the R. rufifrons group, [Pg 268] based on the extensive collections at the American Museum of Natural History. They remark that no other genera are closely related to Rhipidura and that evolution has proceeded further in R. rufifrons than in any other species of the genus. These authors regard the Papuan area, probably New Guinea, as the original home of this group. From their study they point out that many of the subspecies of R. rufifrons of the Papuan area, especially those of the Louisiades and the Solomons, appear to be the least specialized of the species, and that this lack of specialization in these subspecies indicates that the ancestral stock of the species R. rufifrons acquired its specificity somewhere in that area. With regard to the kinds of Rhipidura in Micronesia, Mayr and Moynihan (1946:fig. 2) have logically found three separate colonizations within the area: one represented today by R. lepida at Palau; one of subspecies of R. rufifrons at Yap and in the Marianas; and one by R. r. kubaryi at Ponapé.
R. lepida, according to Mayr and Moynihan (1946), is a result of an early colonization by Rhipidura. It is related to R. dedemi, R. superflua, and R. teijsmanni, which are mostly monotypic or have only two or three subspecies within the species. These three species are found in the region including Celebes and the Moluccas. R. lepida apparently invaded the Palau Islands from Celebes or an adjacent area and, among named species, most closely resembles R. teijsmanni. Both of these species have a white chin and throat, black breast patch, and rufous abdomen. R. lepida has become differentiated chiefly by the presence of a rufous head and back, a more distinct breast band, and proportionately different amounts of rufous and black coloration of the tail feathers.
Mayr and Moynihan (1946:6) give as the chief characters of R. rufifrons the following: "a rufous forehead, a grayish brown head and upper back, a well-defined rufous rump, a white chin and throat, a black breast band with scaling at its lower edge, and a dark brown tail with a distinct rufous base and a white tip." The Micronesian subspecies of R. rufifrons at Yap and in the Marianas display these characters. Of the four subspecies found in the area including Yap and the Marianas, R. r. versicolor, R. r. saipanensis, R. r. mariae and R. r. uraniae, the two first named most closely approach the ancestral stock, which may have been R. r. commoda Hartert of the northern Solomons or some near relative in Melanesia. The amount of white on the chin and throat and on the malar stripe, in R. r. versicolor and R. r. saipanensis is probably [Pg 269] nearer that which obtained in the ancestor. At Rota, R. r. mariae, exhibits less white on the throat and a thinner, white malar stripe, while at Guam, R. r. uraniae possesses only a small amount of white on the chin and only a very thin line of white in the malar region. This variation in coloration suggests that the birds may have originally become established at Yap, Saipan and Tinian and later, birds from Saipan and Tinian spread to Rota and lastly to Guam.
R. r. kubaryi of Ponapé, although considered as a subspecies of R. rufifrons by most workers, has lost the rufous coloring found in most members of the species. Mayr and Moynihan (1946:6) point to its evolution through subspecies in the Santa Cruz Islands, where in R. r. agilis Mayr the rufous of the lower back is restricted to the upper tail-coverts, and in R. r. melanolaema Sharpe and R. r. utupuae Mayr the rufous is absent. In the latter two subspecies, as well as in R. r. kubaryi, the forehead is white instead of rufous.
The invasion of Micronesia by Rhipidura has undoubtedly been the result of abnormally long flights by a relatively weak flyer. The fact that Rhipidura has succeeded in establishing itself at only a few of the seemingly suitable islands in Micronesia may indicate that the possibilities for chance migration and resulting colonization are small, but that new colonization may be expected in the future.
It is my opinion that the populations of Rhipidura, as I have observed them in Micronesia, are small because each individual or pair of birds is dependent on a relatively large area of woodland to satisfy its habitat requirements, especially for food. This suggestion needs to be tested by observation made in the field. In comparison with the insect fauna of New Guinea or some other large island, that of Micronesia is indeed small in number of kinds. Hesse, Allee and Schmidt (1937:524) explain the absence of insectivorous animals such as "swallows, swifts, flycatchers, and insectivorous bats" in island communities on the basis of the small number of flying insects in these communities. Probably Rhipidura is able to forage for sedentary insect life as well as for the flying forms.
Muscicapa Rugensis Hombron and Jacquinot, Ann. Sci. Nat. Paris, (2), 16, 1841, p. 312. (Type locality, Roug = Truk.)
Monarcha rugensis Hartlaub, Archiv. f. Naturgesch., 18, 1852, p. 133 (Gruppe Roug. = Truk); idem, Journ. f. Ornith., 1854, p. 168 (Carolinen = Truk); idem, Proc. Zool. Soc. London, 1867 (1868), p. 829 (Hogoleu = Truk); Gray, Hand-list Birds, 1, 1869, p. 321 (Caroline = Truk); Giebel, Thes. Ornith., 2, 1875, p. 614 (Carolinae = Truk); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 353 (Ruk).
Colluricincla rugensis Pucheran, Voy. Pôle Sud, Zool., 3, 1853, p. 62 (Ruk); Hartlaub, Journ. f. Ornith., 1854, p. 162 (Roug = Truk).
Metabolus rugensis Bonaparte, Comptes Rendus Acad. Sci. Paris, 38, 1854, p. 650 (no locality = Truk); Sharpe, Cat. Birds British Mus., 4, 1879, p. 238 (Ruk); Finsch, Proc. Zool. Soc. London, 1880, p. 575 (Ruk); Tristram, Cat. Birds, 1889, p. 197 (Ruk); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 19 (Ruk); Nehrkorn, Kat. Eiers., 1899, p. 26 (Ruk); Hartert, Novit. Zool., 7, 1900, p. 4 (Ruk); Matschie, Journ. f. Ornith., 1901, p. 112 (Ruk); Reichenow, Die Vögel, 2, 1914, p. 262 (Karolinen = Truk); Takatsukasa and Kuroda, Tori, 1, 1915, p. 54 (Ruk); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zool., 63, 1919, p. 203 (Truk); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 63 (Ruk); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 470 (Ruk); Yamashina, Tori, 7, 1932, p. 404 (Truk); Hand-list Japanese Birds, rev., 1932, p. 178 (Truk); Hand-list Japanese Birds, 3d ed., 1942, p. 197 (Truk); Mayr, Birds Southwest Pacific, 1945, p. 295 (Truk).
Monarcha (Metabolus) rugensis Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 19 (Caroline Islands).
Geographic range.—Micronesia: Caroline Islands—Truk.
Characters.—Adult male: White feathers (with dark bases) throughout except for black ones on forehead, lores, chin, and throat; chin and throat with steel-blue gloss; tips of second to fifth or seventh primaries black, black coloring extending along inner webs; shafts of primaries and basal half of tail feathers black; bill and feet black.
Adult female: Resembles adult male, but generally sooty-black, darker above; under tail-coverts and sometimes rump splotched with white; white coloring may also be present on tips of secondaries, on chin, and on tail.
Immature: Resembles adult, but bright cinnamon on upper parts and on wings and tail; inner webs of primaries grayish or dark brown, shafts of primaries lighter on basal half; lores, chin and throat white or washed with creamy-buff; breast and abdomen whitish, washed with cinnamon, sides darker rufous, under wing-coverts, axillaries, and under tail-coverts usually light rufous although somewhat variable in color; basal part of bill yellow, tip of bill horn colored. Immatures may be observed in all stages of color change toward the adult condition.
Measurements.—Measurements are listed in table 40.
| Number and Sex | Wing | Tail | Culmen | Tarsus |
| 8 males | 103 | 91 | 27 | 26 |
| (98-105) | (88-93) | (26-28) | (25-27) | |
| 6 females | 100 | 87 | 27 | 26 |
| (97-101) | (86-89) | (26-28) | (25-27) |
Specimens examined.—Total number, 27 (14 males, 13 females), as follows: Caroline Islands, USNM—Truk, 2 (Feb. 16, not dated); AMNH—Truk, 25 (Jan. 29, Feb. 1, 8, 10, 11, May 6, 9, June 11, 13, 14, 15, Oct. 11, 31, Nov. 2, 11, Dec. 3, 12, 17, 20).
Nesting.—Yamashina (1932a:404) reports on the taking of a nest containing one egg at Natsushima, Truk Atoll, in May, 1931. According to Hartert (1900:5) Owston's collectors obtained nests on June 1, 4, and 12. Two were in breadfruit trees about twenty feet above the ground. Each nest contained one egg. Hartert writes, "The eggs are cream-coloured, speckled with brownish red, more frequently and often very thickly on the large end, and with some deeper lying pale purplish grey patches, and one has some very fine black lines on the large end."
Molt.—A study of adult specimens obtained at various times of the year indicates that Metabolus normally molts in the period from about October through January.
Mayr (1933e:1-10) has studied the variation of immature and adult plumages in Neolalage banksiana (Gray) and other birds pointing out the occurrence of "retarded" and "progressive" plumages. Bogert has followed this work in interpreting the condition of the plumages in Metabolus, and through the kindness of Ernst Mayr I have examined Bogert's unpublished manuscript on the series of Metabolus at the American Museum of Natural History, from which the following account of the plumage is taken.
In the series of skins, there are specimens of non-molting, immature males with "normal" plumage (that is to say, plumage with upper parts cinnamon-colored and lower parts whitish and darker buff) taken in October and in February. There are also specimens of non-molting, immature females with "normal" plumage taken in November and in May. These immatures are in fresh or slightly worn plumages. In addition, there is one non-molting, male specimen (November) which has some white on the crown and throat, some black on the lores and chin, but because the black feathers are fresh, the specimen is considered to be a "transition" bird and may be either a "retarded" adult or a "progressive" immature male. One non-molting female (October) shows some sooty-black mottling on the chin and throat and a few black feathers on the crown; this is apparently a "progressive" immature because the lower mandible has a yellow basal part, characteristic of the immature. Another female (June) shows black feathers on the crown, nape, chin, throat, and breast; this bird is in the process of molting with the black feathers representing new growth and is an immature assuming the adult condition—in "progressive" plumage. One non-molting male (January) has an intermingling of white feathers in the cinnamon coloring of the head and body, black on the forehead, chin and throat, primaries black with cinnamon edges, and bill similar in color to that of the adult; it is considered to be an adult with "retarded" plumage. Two molting males (December) resemble adults except for cinnamon coloring on shoulders, back, primaries, retrices and a slight cinnamon wash on breast feathers; these may be "retarded" adults. One molting female (June) has mixed cinnamon and sooty-black feathering; this may also be a "retarded" adult. Another molting female (December) with more sooty-black feathering and less cinnamon feathering is also considered to be a "retarded" adult. In fully adult birds there is considerable individual variation, especially in the males where the amount of black on the throat, the extent of the black on the terminal part of the primaries, and the extent of the black on the basal part of the tail feathers is variable. Scattered white feathers may be present on adult females.
Remarks.—Hombron and Jacquinot first obtained the Truk Monarch, but it was not until the time of Kubary and of the Japanese [Pg 272] collectors of Owston that very much was learned concerning the bird. In 1945, McElroy of the NAMRU2 party reported that he found no birds at the several islands of Truk that he visited in December. Some of the Japanese residents of the islands told McElroy that they did not know the bird. Evidently, its numbers are low or it has been eliminated, at least on the islands then populated by the Japanese.
Metabolus belongs to a group of flycatchers including the genera Pomarea, Mayrornis, Neolalage, Monarcha, and Clytorhynchus. The different plumages of the adults and the immatures are not unusual in this group of genera, this feature being observed in many of the flycatchers of Oceania. Mayr (1933c:1) points out some of the relationships between Metabolus and some of these other genera; he comments that all of them have rather thin bills, in contrast to those of other flycatchers.
Metabolus became established at Truk probably as the result of an independent colonization. It is a well differentiated genus showing little resemblance to Monarcha godeffroyi of Yap. In looking over the genera found in the Pacific area, it appears that Metabolus is closest to Clytorhynchus of the Melanesian region, especially to Clytorhynchus hamlini Mayr, which is resident at Rennell in the Solomon Islands. The bills of these two birds are similar, both being long and thin, with a pronounced hook. In coloration there is some resemblance; C. hamlini has the blackish forehead and chin like the male Metabolus and also the burnt-orange underparts. In C. hamlini, however, the sexes are similar, Metabolus also resembles C. nigrogularis. Like Metabolus, the immatures of this latter species are different in color from the adults.
Monarcha godeffroyi Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 829, pl. 38. (Type locality, Yap.)
Monarcha godeffroyi Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 50 (Yap); Sharpe, Cat. Birds British Mus., 4, 1879, p. 432 (Yap); Nehrkorn, Journ. f. Ornith., 1879, p. 403 (Yap); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 56 (Yap); Dubois, Syn. Avium, 1, 1902, p. 289 (Yap); Reichenow, Die Vögel, 2, 1914, p. 261 (Yap); Mayr, Birds Southwest Pacific, 1945, p. 295 (Yap).
Monarcha godeffroyi Gray, Hand-list Birds, 1, 1869, p. 321 (Yap); Hand-list Japanese Birds, rev., 1932, p. 175 (Yap); Hand-list Japanese Birds, 3d ed., 1942, p. 194 (Yap).
Monarches godeffroyi Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 97 (Yap); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 391 (Yap); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 63 (Yap).
Pomarea godeffroyi Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 19 (Yap); Matschie, Journ. f. Ornith., 1901, p. 112 (Yap).
Monarcharses geoffroyii Mathews, Bull. British Ornith. Club, 45, 1925, p. 94 (new generic name); idem, Syst. Avium Australasianarum, 2, 1930, p. 514 (Yap).
Monarcharses godeffroyi Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 486 (Yap?).
Geographic range.—Micronesia: Caroline Islands—Yap.
Characters.—Adult male: according to Sharpe (1879:432). "General colour above white, from the hind neck to the rump and including scapulars; wings black, the quills browner; upper tail-coverts and tail black; head all around black, including the lower throat; sides of neck and rest of under surface, from the foreneck downwards, pure white; thighs and under tail-coverts black; under wing-coverts black, quills ashy blackish below; white along the inner edge of the primaries; 'bill entirely blue; feet whitish blue; iris black' (Kubary M. S.)."
Adult female: "Entirely black, excepting the hind neck and upper mantle, sides of neck, lower throat, and fore neck, which are pure white" (Sharpe, 1879:432).
Immature: "Above brown, the head and hind neck ashy grey, the scapulars rufescent at the tips, the rump rufous, becoming paler and more fulvous on the upper tail-coverts; wing-coverts dusky brown, broadly edged externally with rufous-buff, becoming fulvous on the median and greater coverts; quills dark brown, externally edged with rufous, the primaries narrowly, the secondaries more broadly, the innermost of the latter edged and tipped with buff; tail-feathers ashy brown, narrowly edged with ochraceous brown and tipped with white, more broadly on the outer feathers; lores and a broad eyebrow rufous-buff; ear-coverts rather deeper rufous, shading on to the sides of the throat; under surface of body light cinnamon-rufous inclining to rufous on the throat and under tail-coverts; under wing-coverts light cinnamon, like the breast; quills light brown below, whitish along the inner web; 'bill horn-colour, the point brown, under mandible paler, feet dirty white, iris black' (Kubary M. S.)." (Sharpe, 1879:433).
Remarks.—No specimens of this species have been seen by me. Most taxonomists have regarded this bird as a member of the genus Monarcha, although Mathews did propose the name Monarcharses for this bird. On the basis of descriptions and pictures (especially plate 38 in Hartlaub, 1868:828) the bird appears to be related to the monarch flycatchers of the Melanesian area. It may be closest to Monarcha menckei from the Bismarcks, M. manadensis of the New Guinea region, M. barbatus from the Solomons or to M. leucurus from Buru. The drab color of the immatures and the black and white color of the adults are characteristics of the Yap Monarch which are shared with some of the other species of Monarcha. The connection between M. godeffroyi and Metabolus rugensis of Truk is not known, but they evidently represent separate colonizations. M. takatsukasae of Tinian appears to be an offshoot of M. godeffroyi of [Pg 274] Yap, in which the black and white plumage has been suppressed (or never developed). As indicated by the published descriptions, the immature of M. godeffroyi shows a close resemblance to the adult of M. takatsukasae. The latter also shows relationships to immature specimens of M. leucotis and to M. guttula of Melanesia.
The relationship of the two species of Monarcha in Micronesia to the Hawaiian Flycatcher, Chasiempsis sandwichensis is not known. It is apparent that this Hawaiian form was derived from some ancestor from Melanesia, which arrived in the Hawaiian Islands by way of either Polynesia or Micronesia. Mayr (1943:45) has already pointed out that Chasiempsis is "related to the Monarcha group (Pomarea, Mayrornis, etc.)."
Monarcharses takatsukasae Yamashina, in Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 485. (Type locality, Tinian.)
Monarcha takatsukasae Yamashina, Tori, 7, 1932, p. 400 (Tinian); Hand-list Japanese Birds, rev., 1932, p. 175 (Tinian); Hand-list Japanese Birds, 3d ed., 1942, p. 195 (Tinian); Mayr, Birds Southwest Pacific, 1945, p. 296 (Tinian); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 100 (Tinian).
Geographic range.—Micronesia: Mariana Islands—Tinian.
Characters.—Adult male: Forehead, lores, eyering, auriculars, and underparts rufous, chin paler; under tail-coverts white; crown and nape dark slate-gray; back reddish-brown; rump white; wing and tail dark brown, outer edges of first three primaries white, tail with white tips, more broadly tipped on outer tail feathers; outer edges of scapulars and secondaries buffy but tips more whitish, forming two wing bars; under wing-coverts whitish; bill slate-blue, tip pearl; feet dark slate; iris dark brown.
Adult female: Resembles adult male, but slightly smaller and crown more brownish.
Immature: Resembles adult, but base of bill lighter and underparts paler.
According to the original description by Yamashina, M. takatsukasae resembles closely the immature M. godeffroyi of Yap in coloration; however, the Tinian Monarch has a shorter wing.
Measurements.—Measurements are listed in table 41.
| Number and Sex | Wing | Tail | Full Culmen | Tarsus |
| 6 males | 70 | 68 | 18.0 | 22.0 |
| (67-72) | (65-70) | (17.5-19.0) | (21.0-23.0) | |
| 10 females | 67 | 67 | 17.2 | 22.5 |
| (65-68) | (64-69) | (17.0-19.0) | (21.7-23.0) |
Specimens examined.—Total number, 20 (10 males, 10 females), as follows: Mariana Islands, USNM—Tinian, 10 (Oct.); AMNH—Tinian, 9 (Sept.); KMNH—Tinian, 1 (Sept.).
Nesting.—Yamashina (1932a:400, 401) records two nests of the Tinian Monarch. He writes of one nest containing two eggs taken at Churo, Tinian, on January 29, 1932, that was "hung on a fork of an upward pointing branch of a road side tree commonly called 'Oba' 1.5 m. high from the ground in a forest.... The ground color of the egg shells is white. The spots are pale reddish-brown and distributed all round the surface like small dots, being concentrated especially round the larger end." Another nest containing three eggs was found on January 29, 1932. Yamashina writes that the eggs measure 20.5 x 15, 21 x 15, and 18 x 15 mm. In describing these nests Yamashina notes, "The shape of the two nests mentioned above is like a deep cup. The outer layer of them is made chiefly of dead leaves, fibers, cotton, wools and moss, and the inner layer of fine stems and fibers only."
Downs (1946:101) writes that a nest found near Lake Hagoi at Tinian on August 31, 1945, "was about three feet from the ground carefully woven into the framework of a triangular crotch.... It was composed exteriorly of small leaves, scattered white feathers, and heavy grass; interiorly of grasses only." In the nest he found a young bird which "was black-skinned, with ugly white quills and a few short dark feathers on its tail and wings. The back feathers were rusty brown as were the tufted head feathers." Marshall (1949:219) assumes that this bird breeds all year.
Molt.—Birds taken by Coultas in September are in fresh plumage.
Remarks.—The Tinian Monarch is known only from Tinian, where it was described in 1931 by Yamashina. Downs (1946:100-103) presents a detailed account of this bird as he saw it in 1945. He found it living in brushy woodlands where other birds, including Rhipidura rufifrons, were observed. From his description, the actions and food-catching behaviors of this bird must be much like those of Rhipidura. Gleise (1945:220) estimated the population of these birds to be 40 to 50 in 1945.
Myiagra erythrops Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 6. (Type locality, Pelew Islands.)
Myiagra erythrops Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 117, 118 (Pelew Islands); idem, Proc. Zool. Soc. London, 1872, pp. 89, 97 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 20 (Palau); Giebel, Thes. Ornith., 2, 1875, p. 658 (Pelew); Nehrkorn, Journ. f. Ornith., 1879, pp. 399, 403 (Palau); Sharpe, Cat. Birds British Mus., 4, 1879, p. 383 (Pelew); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 23 (Pelew); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 195 (Palaos); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 55 (Palau); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Palau); Dubois, Syn. Avium, 1, 1902, p. 283 (Pelew); Reichenow, Die Vögel, 2, 1914, p. 260 (Palau); Takatsukasa and Kuroda, Tori, 1, 1915, p. 54 (Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 64 (Pelew); Yamashina, Tori, 10, 1940, p. 674 (Palau); Handlist Japanese Birds, 3d ed., 1942, p. 195 (Babelthuap, Koror); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 69 (Peleliu, Ngabad, Garakayo).
Submyiagra erythrops Mathews, Syst. Avium Australasianarum, 2, 1930, p. 504 (Palau); Hand-list Japanese Birds, rev., 1932, p. 176 (Palau).
Myiagra oceanica erythrops Mayr, Birds Southwest Pacific, 1945, p. 296 (Palau).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Peleliu, Ngabad.
Characters.—Adult male: Crown, occiput, nape, and auriculars dark "slate-blue"; forehead, lores and orbital ring dark "cinnamon-rufous"; black and upper wing-coverts olive-brown; rump more like crown; underparts near "cinnamon," paler on middle of abdomen, sides, and under tail-coverts; wings and tail dark brown, edged with white; secondaries edged with brownish; under wing-coverts whitish with dusky bases; bill and feet black.
Adult female: Resembles adult male, but slightly smaller and paler in color.
Immature: Resembles adult, but head and rump browner; forehead, lores, and orbital ring sandy in some individuals, more rufous in others; underparts usually paler than in adult; bill basally lighter.
Measurements.—Measurements are listed in table 42.
| Subspecies | Number and sex |
Wing | Tail | Exposed culmen |
Tarsus |
| M. o. erythrops |
14 males |
69 | 53 | 16.4 | 19.5 |
| (68-71) | (51-56) | (16.0-17.3) | (18.5-20) | ||
|
11 females |
66 | 51 | 16.1 | 19.5 | |
| (64-68) | (48-53) | (15.5-17.0) | (18.5-20) | ||
| M. o. freycineti |
25 males |
70 | 60 | 16.3 | 19.5 |
| (67-73) | (57-64) | (15.8-17.0) | (18.5-20) | ||
|
16 females |
67 | 57 | 16.0 | 19.0 | |
| (65-70) | (55-62) | (15.5-17.0) | (18.0-19) | ||
| M. o. oceanica |
11 males |
81 | 68 | 20.1 | 20.0 |
| (78-83) | (65-71) | (19.5-20.5) | (19.5-21) | ||
|
10 females |
79 | 66 | 20.0 | 20.0 | |
| (77-81) | (65-68) | (20.0-20.5) | (19-20.5) | ||
| M. o. pluto |
14 males |
82 | 74 | 17.5 | 19.0 |
| (79-83) | (71-77) | (17.5-18.0) | (18.5-20) | ||
|
14 females |
80 | 73 | 17.5 | 19.0 | |
| (78-84) | (69-77) | (17.0-18.0) | (18.5-20) |
Specimens examined.—Total number, 33 (17 males, 15 females, 1 unsexed), as follows: Palau Islands, USNM—Babelthuap, 1 (Nov. 27)—Koror, 4 (Nov. 6, 19, 26)—Garakayo, 1 (Sept. 18)—Peleliu, 2 (Aug. 30)—Ngabad, 2 (Sept. 11); AMNH—exact locality not given, 23 (Oct., Nov., Dec.).
Molt.—Molt apparently takes place in fall and early winter. Of the specimens examined, there is little evidence of molt in those obtained in August and September while there is considerably more evidence of molt in those taken in November and December.
Food habits.—A bird taken by the writer on September 17, 1945, at Garakayo had approximately one-half cc. of insect parts in its stomach.
Remarks.—The Micronesian Broadbill at Palau is a friendly little bird and easily called-up to within a few yards of a person by imitating its note. It was seen by the NAMRU2 party in 1945 as singles and in pairs in the dense underbrush of the undisturbed forested areas. The bird was seen at only one woodland area at Peleliu (Southeastern Peninsula), but it was observed more frequently on the smaller islands of Ngabad and Garakayo. Coultas (field notes) also notes that in 1931 this bird was found more frequently on the smaller islands. Myiagra was found to be much less conspicuous at Palau than Rhipidura lepida. Myiagra appears to be less active, more solitary in its habits, and possibly more restricted in the territory that it covers in feeding than Rhipidura.
Myiagra freycineti Oustalet, Bull. Soc. Philom. Paris, (7), 5, 1881, p. 73. (Type locality, Mariannes = Guam.)
Myiagra freycineti Reichenow and Schalow, Journ. f. Ornith., 1884, p. 395 (Mariannes = Guam); Oustalet, Le Nat., 1889, p. 260 (Mariannes = Guam); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 24 (Marianne = Guam); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 194 (Guam); Hartert, Novit. Zool., 5, 1898, p. 54 (Guam); Wheeler, Report Island of Guam, 1900, p. 13 (Guam); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 50 (Guam); Safford, Osprey, 1902, p. 69 (Guam); idem, Amer. Anthro., 4, 1902, p. 711 (Guam); idem, The Plant World, 7, 1904, p. 263 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Reichenow, Die Vögel, 2, 1914, p. 260 (Marianen); Cox, Island of Guam, 1917, p. 21 (Guam); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 65 (Guam); Bryan, Guam Rec., vol. 13, no. 2, 1936, p. 25 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 195 (Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 68 (Guam).
Submyiagra freycineti Mathews, Syst. Avium Australasianarum, 2, 1930, p. 504 (Guam); Hand-list Japanese Birds, rev., 1932, p. 176 (Guam).
Myiagra oceanica freycineti Mayr, Birds Southwest Pacific, 1945, p. 296 (Guam).
Myiagra oceanica Strophlet, Auk, 1946, p. 539. (Guam).
Geographic range.—Micronesia: Mariana Islands—Guam.
Characters.—Adult male: A small flycatcher with head and neck near "dark delft blue" with a metallic luster; lores and anterior forehead ashy-gray, more bluish and darker on auriculars and sides of neck than on lores; back and upper wing-coverts near "green-blue slate" but darker and with metallic luster less apparent than on head; rump grayer than back; chin and throat white; breast light "cinnamon," fading to pale buff and white on abdomen, sides, and under tail-coverts; tibia smoky-gray, tips of feathers paler; wings dark brown edged with light bluish-gray; tail bluish-slate, especially middle rectrices, tips of tail feathers edged with white; bill and feet black; iris dark brown.
Adult female: Resembles adult female of M. o. erythrops, but crown and neck near "deep Payne's gray," auriculars grayer than neck; anterior forehead and lores buffy and tinged with cinnamon; back browner than lores with upper wing-coverts and scapulars edged with slightly lighter brown; rump resembles crown but grayer; underparts paler than those of M. o. erythrops, especially chin and throat; tibia more brownish.
Immature male: Resembles adult male, but back more brown and less blue-green, lacking luster; anterior forehead more rufous; scapulars, upper wingcoverts, and wings edged with light brown; underparts variable but generally more buffy than those of adult.
Immature female: Resembles adult female, but more brown and less blue on head and back; underparts more buffy; base of bill paler.
Measurements.—Measurements are listed in table 42.
Weights.—The author (1948:68) records the weights of five adult males as 10.5-12.5 (11.9), and those of two adult females as 11.4 and 12.0 grams.
Specimens examined.—Total number, 64 (33 males, 22 females, 9 unsexed), as follows: Mariana Islands, USNM—Guam, 26 (Jan. 21, March 16, May 21, 29, 30, June 1, 3, 14, 24, 26, July 10, 12, 13, 20, 23, Aug. 30); AMNH—Guam, 38 (Jan., Feb., March, July, Aug.).
Nesting.—The writer (1948:68) records a nest containing one egg found by Muennink at Guam near Mt. Santa Rosa on May 7, 1945. The nest was in a bamboo stump approximately six feet from the ground. The egg hatched on about May 21. Seale (1901:50) reports on a nest and egg taken in the period from May to July. The NAMRU2 party obtained a female on March 15 with an enlarged gonad. Strophlet (1946:539) observed a pair of broadbills building a nest on September 20, 1945; it was completed on October 4 and was approximately seven feet above the ground. Hartert (1898:33) reports on a nest taken at Guam on February 14, 1895.
Molt.—As shown by the specimens examined, molt begins in June or July.
Food habits.—The stomach of a bird obtained on January 21, 1945, contained one unidentified bug (Hemiptera) and several parts of other insects.
Remarks.—The Micronesia Broadbill at Guam is not a common bird, and like its relative Rhipidura rufifrons is an inhabitant of forested areas, especially those containing brushy undercover. It is an active bird, although less conspicuous than Rhipidura. The birds were found as singles or in pairs. The pair of birds which had a nest at the west base of Mount Santa Rosa in May, 1945, allowed the observers to approach closely to them. The birds are easily attracted by squeaking sounds. There is considerable variation in the amount of cinnamon coloring on the breasts of adult birds.
The Micronesian Broadbill at Guam was first discovered by Quoy and Gaimard, who called it "Moucherolle à gorge rouge." Kittlitz (1836:304) evidently records two species of flycatchers from Guam, which he calls Muscicapa. I judge these birds to be Myiagra and Rhipidura. It was not until 1881 that Oustalet recognized this bird [Pg 279] to be new. The first large series of specimens was obtained by Marche for the Paris Museum and was reported on by Oustalet (1895:194). Marche collected 12 skins in August and September, 1887, and 4 additional skins in February, 1889.
Myiagra oceanica Pucheran, Voy. Pôle Sud, Zool., 3, 1853, p. 77. (Type locality, Hogoleu = Truk.)
Myiagra oceanica Hartlaub, Journ. f. Ornith., 1854, p. 168 (Carolinen = Truk); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 18 (Hogoleu = Truk); Finsch and Hartlaub, Fauna Centralpolynesiens, 1867, p. 94 (Hogoleu = Truk); Gray, Hand-list Birds, 1, 1869, p. 328 (Caroline Is. = Truk); Pelzeln, Journ. f. Ornith., 1875, p. 51 (Hogoleu = Truk); Sharpe, Cat. Birds British Mus., 4, 1879, p. 383 (Hogoleu = Truk); Nehrkorn, Journ. f. Ornith., 1879, p. 403 (Ruk); Finsch, Proc. Zool. Soc. London, 1880, p. 575 (Ruk); Oustalet, Bull. Soc. Philom. Paris, (7), 5, 1881, p. 73 (Carolines = Truk); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 353 (Ruk); Reichenow and Schalow, Journ. f. Ornith., 1884, p. 395 (Carolines = Truk); Tristram, Cat. Birds, 1889, p. 200 (Ruk); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 23 (Ruk); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 196 (Hogoleu = Truk); Nehrkorn, Kat. Eiers., 1899, p. 30 (Ruk); Hartert, Novit. Zool., 7, 1900, p. 5 (Ruk); Matschie, Journ. f. Ornith., 1901, pp. 111, 112, 113 (Ruck); Dubois, Syn. Avium, 1, 1902, p. 283 (Hogoleu = Truk); Reichenow, Die Vögel, 2, 1914, p. 260 (Karolinen = Truk); Takatsukasa and Kuroda, Tori, 1, 1915, p. 54 (Ruk); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 204 (Truk); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 64 (Ruk); Hand-list Japanese Birds, 3d ed., 1942, p. 195 (Truk); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 68 (Truk).
Myiagra albiventris Finsch and Hartlaub, Fauna Centralpolynesiens, 1867, p. 93 (Hoguleu = Truk); Giebel, Thes. Ornith., 2, 1875, p. 658 (Carolinae = Truk).
Submyiagra oceanica Mathews, Syst. Avium Australasianarum, 2, 1930, p. 505 (Ruk); Hand-list Japanese Birds, rev., 1932, p. 175 (Truk).
Myiagra oceanica oceanica Mayr, Birds Southwest Pacific, 1945, p. 296 (Truk).
Geographic range.—Micronesia: Caroline Islands—Truk.
Characters.—Adult male: Resembles M. o. freycineti, but larger with crown and nape less green and with less metallic luster; lores and anterior forehead darker gray; chin, throat, and sides of neck more buffy-cinnamon; back, rump, upper wing-coverts, and scapulars less blue and more ashy gray; tibia, wings, and tail more brownish.
Adult female: Resembles adult male, but smaller with less blue and more gray on crown; lores and anterior forehead lighter.
Immature: Resembles adult, but crown and nape grayish, slate-blue; under-parts paler.
Measurements.—Measurements are listed in table 42.
Specimens examined.—Total number, 23 (12 males, 10 females, 1 unsexed), as follows: Caroline Islands, USNM—Truk, 2 (Feb. 16); AMNH—Truk, 21 (Feb., June, Nov., Dec.).
Nesting.—Hartert (1900:5) reports the taking of several nests in the period from March to July by Owston's Japanese collectors. One nest contained two eggs, the other nests contained one.
Remarks.—The broadbill at Truk was first taken by Hombron and Jacquinot, who called it "Platyrhynque océanien." Later, Kubary obtained material which was studied by Finsch (1880e:575). [Pg 280] In December, 1945, McElroy of the NAMRU2 party examined two adults with enlarged gonads. Specimens obtained by him at Truk were lost in shipment to the United States. In coloration this subspecies is closest to M. o. freycineti; in size it is closest to M. o. pluto.
Myiagra pluto Finsch, Proc. Zool. Soc. London, 1875 (1876), p. 644. (Type locality, Ponapé.)
Myiagra pluto Finsch, Journ. Mus. Godeffroy, 12, 1876, pp. 17, 19 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 779 (Ponapé); Sharpe, Cat. Birds British Mus., 4, 1879, p. 380 (Ponapé); Nehrkorn, Journ. f. Ornith. 1879, p. 404 (Ponapé); Finsch, Journ. f. Ornith., 1880, p. 288 (Ponapé); idem, Proc. Zool. Soc. London, 1880, p. 576 (Ponapé); idem, Ibis, 1881, pp. 110, 112, 115 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 280 (Ponapé); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 23 (Ponapé); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 195 (Ponapi); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 55 (Ponapé); Nehrkorn, Kat. Eiers., 1899, p. 26 (Ponapé); Christian, The Caroline Islands, 1899, p. 358 (Ponapé); Matschie, Journ. f. Ornith., 1901, pp. 111, 112, 113 (Ponapé); Dubois, Syn. Avium, 1, 1902, p. 283 (Ponapi); Reichenow, Die Vögel, 2, 1914, p. 260 (Ponapé); Takatsukasa and Kuroda, Tori, 1, 1915, p. 54 (Ponapé); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 204 (Ponapé); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 64 (Ponapé); Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 195 (Ponapé).
Submyiagra pluto Mathews, Syst. Avium Australasianarum, 2, 1930, p. 505 (Ponapé); Yamashina, Tori, 1, 1932, p. 401 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 176 (Ponapé).
Myiagra oceanica pluto Mayr, Birds Southwest Pacific, 1945, p. 296 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult male: A dark, bluish-gray broadbill with head, ear-coverts, and nape dark, metallic, steel-blue; back and rump darker and more slate-blue than head; upper tail-coverts blackish; tail black edged with greenish gloss; wings dark brown, scapulars and secondaries with outer edges tinged with metallic bluish-gray; lores black; chin, throat, and upper breast dark with light metallic-blue wash; lower breast and abdomen slate-gray; under wing-coverts brownish-black; bill black; feet bluish-black; iris dark brown. Female resembles male, but slightly smaller and somewhat duller. Immature duller.
Measurements.—Measurements are listed in table 42.
Specimens examined.—Total number, 42 (23 males, 19 females), as follows: Caroline Islands, USNM—Ponapé, 3 (Feb. 11); AMNH—Ponapé, 39 (Nov., Dec.).
Nesting.—Yamashina (1932a:401) records nests and eggs of the Ponapé broadbill. The nests were at heights of between .9 and 2.2 meters above the ground. Nests, each containing a single egg, were obtained on July 21, 25, and August 6. The eggs measure 19.5 by 16, 20.5 by 15.7, 20.5 by 16, and 20.2 by 16. Coultas (field notes) describes the nest as a cup-shaped structure, made of fine grasses and ferns, and placed in small trees and bushes at low elevations. Of specimens taken by Coultas in November and December, 1931, approximately fifty percent of the males had enlarged gonads. According to his specimen labels, none of the females was in breeding condition.
Molt.—Of the large series of broadbills taken by Coultas, approximately twenty percent of those taken in November were in molt whereas only approximately ten percent of those taken in December were in molt. Specimens taken in February were not in molt. It is evident that molting takes place in the fall, possibly from August to December.
Remarks.—The coloration of the Micronesian Broadbill at Ponapé is in marked contrast to that of other representatives of Myiagra in Micronesia, being dark, bluish-gray in color. Probably the bird has taken on melanistic characters, which is not unusual in birds which have become isolated; examples of this condition may be observed in Rhipidura, Terpsiphone and other genera.
Coultas (field notes) writes that the bird is "Common everywhere on the island except in the grasslands. Two birds are working together usually, darting around in the low trees, among the branches or on the ground. The birds are playful, friendly and inquisitive. I should not call them noisy as one or more will sit for many minutes watching the intruder without making a peep. Their call, "Que Que," is a spasmodic outburst that might be repeated many times or just once. The male, presumably, erects the long crown feathers when calling. Perhaps both male and female do this, I can't say. The bird flutters on the wing and displays the feathers as does Rhipidura. When sitting, the bird often erects the crest and fluffs the tail and feathers."
Evolutionary History of Myiagra oceanica.—According to Mayr (1933d:1) Myiagra "is easily recognizable by its broad bill and the color pattern which is similar in all species." The range of the genus Myiagra extends from Australia and Tasmania westward to Timor, northward to the Moluccas, and Micronesia, and eastward to Polynesia. Myiagra oceanica is restricted to Micronesia and consists of four subspecies, which until recently have been considered as four separate species. Unlike many of the species of this genus, M. oceanica shows comparatively little sexual dimorphism. The male of M. oceanica has metallic coloring on the head and the upper back and often has rich, rufous coloring on the breast. The female is less brilliant in coloring, lacking the sheen. The four subspecies vary from each other in size, color and even, to some extent, in basal breadth of the bill. M. oceanica resembles several broadbills, including M. galeata of the Moluccas, M. rubecula of Australia, M. vanikorensis of Fiji, and M. ruficollis of Australia and the Lesser Sundas; however, in my opinion, it has probably been derived from M. galeata of the Moluccan area or from a closely related species. In Micronesia, M. o. oceanica and M. o. freycineti appear to resemble closely this parent stock, whereas M. o. erythrops [Pg 282] and M. o. pluto are more differentiated but are considered to have been derived from this same colonization. M. o. pluto bears some resemblance to M. atra of the Papuan area, particularly in the dark coloring; this is probably only a parallel evolution, since they have little else in common. M. vanikorensis of the Fiji area is close to M. oceanica in color and structure; the two species, I suspect, have been derived from a common source rather than from each other. Study of the evolutionary history of the entire genus is necessary before we can understand fully the derivation of the Micronesian and Polynesian species. It seems safe to say that the center of dispersal has been in the Australian region; the lack of diversity of this genus within the Papuan area is at present unexplained.
Muscicapa narcissina Temminck, Pl. Col., 3, 1835, pl. 577, fig. 1. (Type locality, Japan.)
Muscicapa narcissina narcissina Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Breeds in eastern Asia and Japan. Winters south to Malaysia. In Micronesia: Palau Islands—exact locality unknown.
Remarks.—Mayr (1945a:302) records the Narcissus Flycatcher as a migrant visitor to the Palau Islands on the basis of two specimens in the Turloff collection, formerly in the Zoölogical Museum in Hamburg.
Hemichelidon griseisticta Swinhoe, Ibis, 1861, p. 330. (Type locality, Amoy.)
Hemichelidon griseisticta Hand-list Japanese Birds, rev., 1932, p. 175 (Koror); Hand-list Japanese Birds, 3d ed., 1942, p. 194 (Koror).
Muscicapa griseisticta Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau); Marshall, Condor, vol. 51, 1949, p. 221 (Palau).
Geographic range.—Breeds in northwestern Asia and Japan. Winters south to Malaysia. In Micronesia: Palau Islands—Koror.
Remarks.—The Chinese Gray-spotted Flycatcher is a casual winter visitor to the Palaus. Marshall (1949:221) took two specimens at Palau on November, 1945.
Rectes tenebrosus Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 6. (Type locality, Pelew Islands.)
Rectes tenebrosus Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 118 (Pelew Islands); idem, Proc. Zool. Soc. London, 1872, pp. 89, 99 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 18, pl. 3, fig. 1 (Palau); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 27 (Pelew).
Colluricincla tenebrosa Gray, Hand-list Birds, 1, 1869, p. 386 (Pelew); Dubois, Syn. Avium, 1, 1902, p. 496 (Pelew); Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 5 (Palau); idem, Birds Southwest Pacific, 1945, p. 297 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 69 (Peleliu, Ngabad, Garakayo).
Pinarolestes tenebrosus Sharpe, Cat. Birds British Mus., 3, 1877, p. 298 (Pelew); Matschie, Journ. f. Ornith., 1901, p. 112 (Palau); Reichenow, Die Vögel, 2, 1914, p. 296 (Palau); Takasukasa and Kuroda, Tori, 1, 1915, p. 54 (Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 69 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 174 (Palau).
Myiolestes tenebrosus Tristram, Cat. Birds, 1899, p. 188 (Pelew).
Caleya tenebrosus Mathews, Syst. Avium Australasianarum, 2, 1930, p. 649 (Pelew).
Malacolestes tenebrosus Mayr, Amer. Mus. Novit., no. 590, 1933, p. 5 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 193 (Babelthuap, Koror, Peliliu).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Peleliu, Ngabad.
Characters.—Adult: Upper parts between "snuff brown" and "bister," head blacker; chin, throat, and upper breast like upper parts but darker; lower breast and abdomen lighter and more buffy, sides darker; feathers of underparts with darker shafts producing a streaked appearance; underside of wing and under tail-coverts light-colored; bill dark brown; feet lighter brown; iris yellowish. Female smaller.
Immature: Resembles adult, but head and neck lighter; ear-coverts, sides of neck, throat, upper breast darker; lower breast and abdomen paler.
Measurements.—Measurements are listed in table 43.
| Number and Sex | Wing | Tail | Full Culmen | Tarsus |
| 20 males | 104 | 76 | 23.5 | 31 |
| (100-107) | (73-79) | (22.5-24.5) | (29-31) | |
| 9 females | 97 | 73 | 23.0 | 30 |
| (94-101) | (71-76) | (22.0-24.0) | (30-31) |
Specimens examined.—Total number, 32 (21 males, 11 females), as follows: Palau Islands, USNM—Koror, 6 (Nov. 5, 18)—Garakayo, 3 (Sept. 18)—Peleliu, 5 (Aug. 29, 30, Sept. 1, 6)—Ngabad, 2 (Sept. 11); AMNH—exact locality not given, 16 (Oct. 8, 13, 26, Nov. 11, 13, 17, 19, 21, 23, Dec. 9).
Molt.—The molting process in this species seemingly takes place from August until December. Most of the birds taken by the NAMRU2 party in August and September were in molt. Molting specimens were obtained by Coultas in October, November and December.
Food habits.—The Palau Morning Bird feeds on plant and animal materials. Stomachs obtained by the NAMRU2 party contained green plant material, seeds, insect parts, and grit. The bird feeds principally on the ground or in low bushes.
Remarks.—The Palau Morning Bird is a thrushlike bird which spends its time on or near the ground in areas where ground cover is thick. In 1945, the NAMRU2 party found the bird in the thick [Pg 284] matting of vines which had covered over the battle-cleared areas. I did not find the bird at elevations of more than three to four feet above the ground. When flushed, it would flutter a short distance and disappear into the brush. It has a sweet song and may be considered as one of the finest singers in Micronesia. It heralds the break of day with its melodious carol, and its name is derived from its calling early in the morning. I heard the bird only infrequently in the hot part of the day, although it would sing when the skies were overcast. Its song could be heard also as evening approached. The bird is moderately common, and evidently is more abundant on the smaller islands than on Peleliu. Its occurrence on the smaller islands was noted also by Coultas.
The taxonomic status of the Palau Morning Bird has been one of uncertainty as shown by the fact that the bird has been treated under six generic names since its discovery by Captain Tetens. Mayr (1933a:5) erected a new genus, Malacolestes, for the morning bird pointing to its differences from "Rhectes (= Pitohui) and Pinarolestes (= Myiolestes)." Later, he (1944b:5) disregards this name and places the bird in the genus Colluricincla stating that its special characters "are due to isolation." This treatment is followed here. The Palau Morning Bird is the most northern representative of a group of birds which have their center of dispersal in the New Guinea and Australian area. As Mayr has pointed out, C. tenebrosus appears closest to the C. megarhynchus group of New Guinea. These species have bills of similar shape, coloration which is darker above and lighter below, soft feathers on underparts, and streaked appearance of throat and breast. The resemblances between C. tenebrosus and C. megarhynchus might be such as to indicate that these are merely subspecifically distinct from each other.
Artamus pelewensis Finsch, Journ. Mus. Godeffroy, 12, 1876, p. 41. (Type locality, Palau.)
Artamus leucorhynchus Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 116, 118 (Pelew); idem, Proc. Zool. Soc. London, 1872, pp. 89, 99 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 18 (Palau); Walden, Ibis, 1876, p. 188 (Pelew).
Artamus pelewensis Finsch, Proc. Zool. Soc. London, 1877 (1878), p. 739 (Pelew); Tweeddale, Ibis, 1878, p. 385 (Pelew); Sharpe, Cat. Birds British Mus., 13, 1890, p. 9 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 26 (Pelew); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 62 (Palau); Matschie, Journ. f. Ornith., 1901, p. 112 (Palau); Dubois, Syn. Avium, 1, 1902, p. 533 (Pelew); Reichenow, Die Vögel, 2, 1914, p. 346 (Pelew).
Artamus leucorhynchus pelewensis Stresemann, Novit. Zool., 20, 1913, p. 293 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 193 (Babelthuap, Koror); Mayr, Birds Southwest Pacific, 1945, p. 297 (Palau).
Artamus melanoleucus pelewensis Kuroda, in Momiyama, Birds Micronesia, 1922, p. 69 (Pelew); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 635 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 174 (Palau).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Angaur.
Characters.—Adult: Upper surface black, except for back which is slightly brownish and for rump which is white; underparts white, except for chin, throat and upper breast which are black; wings with grayish tips; bend of wing black; bill milky blue, nostril and tip black; feet black; iris dark brown.
Immature: Resembles adult, but black feathers with brownish tinges; primaries tipped with white.
Measurements.—Measurements are listed in table 44.
| Number and Sex | Wing | Tail | Culmen | Tarsus |
| 5 males | 134 | 68 | 25 | 16.5 |
| (132-136)) | (66-69) | (24-26) | ||
| 4 females | 134 | 68 | 24 | 16.5 |
| (132-136) | (67-69) | (16.5-17.0) |
Specimens examined.—Total number, 12 (7 males, 5 females), from Palau Islands, AMNH—exact locality not given (March, Nov., Dec.).
Remarks.—Little is known concerning the habits and distribution of the white-breasted Wood-Swallow at Palau. Coultas obtained a series of eight birds in 1931; he writes (field notes) that his native hunter took every bird that he saw. The natives told him that they did not know the nest of the bird. Coultas concluded that the bird was not common. He commented that it may be found perched in the top of a tree on a dead branch or "even displaying in the air." The NAMRU2 party found no evidence of this bird in the southern Palaus in 1945. The specimens obtained by Coultas in November and December, 1931, were in molt and had small gonads.
This wood-swallow is the only Micronesian representative of Artamus leucorhynchus. Like several other species of birds it has become established only at the Palau Islands, and has either been unsuccessful in colonizing other parts of Micronesia or has not had the opportunity to do so. This bird had been compared with specimens representing ten subspecies of A. leucorhynchus in Melanesia and Malaysia. A. l. pelewensis differs from these subspecies examined by its blacker appearance, with only a faint brownish wash on the back, and by its shorter, first primary. The curvature of the upper mandible of the bird in the Palaus is similar to that of P. l. leucorhynchus of the Philippines; the mandible is less curved than [Pg 286] that of P. l. celebensis of Celebes; the mandible is slightly thicker than that of P. l. leucopygialis of the New Guinea and Australian region. In length of wing P. l. pelewensis resembles closely P. l. leucorhynchus; P. l. celebensis has a longer wing and P. l. leucopygialis has a shorter one. Stresemann (1913:293) points to a close relationship between P. l. pelewensis and P. l. musschenbreeki of Tenimber and Babber islands and P. l. melaleucus of New Caledonia; Mayr (1945a:284) says the bird in the Palaus came from the Papuan area. Probably P. l. pelewensis has reached the Palau Islands from the New Guinea area by way of the Philippines.
Lamproth[ornis] opaca Kittlitz, Kupfertaf. Naturgesch. Vögel, 2, 1833, p. 11, pl. 15, fig. 2. (Type locality, Ualan = Kusaie.)
Turdus colombinus Lesson (part), Traité d'Ornith., 1832, p. 406 (Carolines = Kusaie?).
Lamproth[ornis] opaca Kittlitz, Mém. Acad. Imp. Sci. St. Petersbourg, 2, 1935, p. 7 (Ualan); idem (part), Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 285, 297 (Ualan); Pelzeln, Reise "Novara," Vögel, 1865, p. 68 (Ualan).
Lamprotornis columbinus Bonaparte (part), Consp. Avium, 1, 1850, p. 417 (Carolinen = Kusaie?).
Lamprotornis columbina Hartlaub, Archiv f. Naturgesch., 18, 1852, p. 133 (Ualan); idem (part), Journ. f. Ornith., 1854, p. 168 (Carolinen = Kusaie?); Kittlitz, Denkw. Reise russ. Amer. Micron. und Kamchat., 1, 1858, p. 376 (Ualan).
Calornis opaca Gray (part), Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 26 (Oualau = Kusaie); Tristram, Cat. Birds, 1889, p. 255 (Kusaie); Hartert, Kat. Vogelsamml. Senckenb., 1891, p. 75 (Ualan).
Calornis kittlitzi Finsch and Hartlaub (part), Fauna Central polynesiens, 1867, p. 109 (Ualan, Puynipet, Marianen; type locality, by subsequent restriction, Ualan = Kusaie); Finsch (part), Journ. Mus. Godeffroy, 8, 1875, p. 23 (Ualan).
Calornis kittlitzii Hartlaub, Proc. Zool. Soc. London, 1867 (1868), p. 830 (Ualan).
Amadina Kittlitzi Gray, Hand-list Birds, 2, 1870, p. 58 (Ualan).
Calornis pacifica Sharpe, Ibis, 1876, p. 47 (Caroline Is. = Kusaie?); Finsch (part), Mitth. Ornith. Ver. Wien, 1884, p. 49 (Kuschai).
Calornis pacificus Finsch (part), Journ. Mus. Godeffroy, 12, 1876, p. 32 (Ualan); idem (part), Journ. f. Ornith., 1880, pp. 289, 301 (Kuschai); idem, (part), Proc. Zool. Soc. London, 1880, p. 576 (Kuschai); idem, (part), Ibis, 1881, pp. 103, 104, 108, 111 (Kuschai).
Aplonis kittlitzi Sharpe (part), Cat. Birds British Mus., 13, 1890, p. 136 (Kuschai); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 44 (Ualan); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 216 (Oualan); Hartert (part), Novit. Zool., 5, 1898, p. 59 (Ualan); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Ualan); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Kusaie).
Lamprocorax kittlitzi Dubois (part), Syn. Avium, 1, 1902, p. 542 (Kuschai).
Aplonis opaca Oberholser, Bull. U. S. Nat. Mus., 98, 1917, p. 59 (Ualan); Wetmore (part), in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 219 (Kusaie).
Aplonis kittlitzi kittlitzi Momiyama (part), Tori, 2, 1920, p. 1 (Kusaie).
Aplonis opaca opaca Momiyama (part), Birds Micronesia, 1922, pp. 6, 12 (Kusaie); Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 70 (Kusaie); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 847 (Kusaie); Takatsukasa and Yamashina, Tori, 7, 1931, p. 109 (Kusaie); Hand-list Japanese Birds, rev., 1932, p. 170 (Kusaie).
Aplornis opaca opaca, Hand-list Japanese Birds, 3d ed., 1942, p. 188 (Kusaie).
Aplonis opacus opacus Mayr, Birds Southwest Pacific, 1945, p. 298 (Kusaie).
Geographic range.—Micronesia: Caroline Islands—Kusaie.
Characters.—Adult: Feathers black with dusky appearance caused by lighter bases; edges of feathers with slight amount of steel-green gloss; underparts slightly duller than upper parts; bill black, with maxilla rather strongly curved; feet black, iris yellow. Females slightly smaller.
Immature: Resembles adult, but upper parts more brown and less black; underparts dusky with edges of feathers tinged with smoky yellow producing a streaked appearance; base of bill horn-colored.
Measurements.—Measurements are listed in table 45.
Specimens examined.—Total number, 30 (18 males, 12 females), as follows: Caroline Islands, USNM—Kusaie, 5 (Feb. 8); AMNH—Kusaie, 25 (Jan., Feb., March).
| Subspecies | Number and sex |
Wing | Tail | Full Culmen |
Depth of culmen at nostril |
| A. o. opacus |
15 males |
124 | 80 | 24 | 9.5 |
| 121-125 | 76-85 | 24-26 | 9.0-10.0 | ||
|
12 females |
119 | 77 | 24 | 9.0 | |
| 115-125 | 72-82 | 23-26 | 8.5-9.0 | ||
| A. o. ponapensis |
17 males |
133 | 87 | 27 | 9.5 |
| 130-138 | 85-91 | 26-29 | 9.0-10.0 | ||
|
11 females |
126 | 83 | 27 | 9.0 | |
| 122-127 | 81-85 | 26-28 | 8.5-9.0 | ||
| A. o. angus |
16 males |
129 | 88 | 28 | 9.5 |
| 125-131 | 84-92 | 27-29 | 8.0-9.0 | ||
|
7 females |
124 | 85 | 27 | 8.5 | |
| 121-129 | 83-88 | 25-28 | 8.0-9.0 | ||
| A. o. orii |
11 males |
128 | 86 | 27 | 7.5 |
| 124-131 | 83-90 | 25-28 | 7.5-8.5 | ||
|
7 females |
124 | 79 | 26 | 7.5 | |
| 121-126 | 77-82 | 25-27 | 7.5-8.0 | ||
| A. o. guami |
41 males |
128 | 86 | 27 | 9.5 |
| 120-136 | 81-92 | 24-29 | 8.5-10.5 | ||
|
32 females |
121 | 84 | 26 | 9.5 | |
| 117-126 | 78-89 | 24-30 | 8.5-10.5 |
Remarks.—The Micronesian Starling at Kusaie was first taken by Kittlitz (1833:11), who named it in the following manner: "Turdus columbinus Gm. L. oder Lamproth. opaca Lichtenstein." The bird was later given the name of Calornis kittlitzi by Finsch and Hartlaub (1867:109). Oberholser (1917:59) has shown that the specific name opaca is applicable, since the manuscript name [Pg 288] Lamprothornis opaca of Lichtenstein is made available by Kittlitz's published description and figure, and since it is the earliest name used. Mathews (1938:342) reports that the name Aplornis appeared a few days before the name Aplonis. I have been unable to check his source of information.
The Micronesia Starling is one of the most abundant birds at Kusaie. Coultas (field notes) observed the bird in all parts of the island, when he visited there in 1931. He found the bird in flocks of two to six or more and noted that birds in immature plumage seemed to outnumber the birds in adult plumage approximately five to one. This subspecies is characterized by the presence of only a slight amount of gloss on the black feathers of the adult.
Aplonis opaca ponapensis Takatsukasa and Yamashina, Tori, 7, 1931, p. 109. (Type locality, Ponapé.)
Calornis columbina Pelzeln, Reise "Novara," Vögel, 1865, pp. 88, 162 (Puynipet).
Calornis kittlitzi Finsch and Hartlaub (part), Fauna Centralpolynesiens, 1867, p. 109 (Puynipet); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, p. 298 (Ponapé).
Calornis opaca Gray (part), Hand-list Birds, 2, 1870, p. 27 (Seniavin = Ponapé).
Calornis pacificus Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 32 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 779 (Ponapé); idem (part), Journ. f. Ornith., 1880, p. 289 (Ponapé); idem, (part), Proc. Zool. Soc. London, 1880, p. 576 (Ponapé).
Calornis pacifica Finsch, Ibis, 1881, p. 115 (Ponapé); idem, (part), Mitth. Ornith. Ver. Wien. 1884, p. 49 (Ponapé).
Aplonis kittlitzi Sharpe (part), Cat. Birds British Mus., 13, 1890, p. 136 (Ponapé); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 44 (Ponapé); Bolau (part), Mitteil. Naturhist. Mus. Hamburg, 1898, p. 62 (Ponapé); Nehrkorn, Kat. Eiers., 1899, p. 122 (Ponapé); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Ponapé); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 55 (Ponapé).
Lamprocorax kittlitzi Dubois (part), Syn. Avium, 1, 1902, p. 542 (Ponapé).
Aplonis opaca Wetmore (part), in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 219 (Ponapé); Mayr. Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé).
Aplonis kittlitzi kittlitzi Momiyama (part), Tori, 2, 1920, p. 1 (Ponapé).
Aplonis opaca opaca Momiyama (part), Birds Micronesia, 1922, p. 12 (Ponapé); Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 70 (Ponapé).
Aplonis opaca ponapensis Yamashina, Tori, 7, 1932, p. 394 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 170 (Ponapé).
Aplonis opaca ponapensis Hand-list Japanese Birds, 3d ed., 1942, p. 188 (Ponapé).
Aplonis opacus ponapensis Mayr, Birds Southwest Pacific, 1945, p. 297 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult: Resembles A. o. opacus, but larger with a longer bill and richer green luster on the back and breast.
Immature: Resembles immature of A. o. opacus, but underparts more brightly streaked but still dingy in appearance.
Measurements.—Measurements are listed in table 45.
Specimens examined.—Total number, 47 (31 males, 16 females), as follows: Caroline Islands, USNM—Ponapé, 1 (Feb. 11); AMNH—Ponapé, 46 (Nov., Dec.).
Nesting.—Yamashina (1932a:394) reports the taking of an egg on August 2, 1931, and two eggs on August 30, 1931, at Ponapé. Coultas (field notes) writes that the nests of these birds are hidden in the tops of the tree-ferns and in holes in the trees. The natives told him that the starling lays two eggs.
Molt.—Most of the adult specimens taken by Coultas in November and December, 1931, are in molting plumage.
Remarks.—Coultas (field notes) writes that the starling is a common bird at Ponapé. He found it in flocks of from two to 12 or more birds. As at Kusaie he noted more birds in the immature plumage than in the adult plumage at Ponapé. The starling occurs in large numbers even though the people of the island hunt this bird persistently for part of their food supply.
The Micronesian Starling at Palau has the longest wing of any of the subspecies of Aplonis opacus. It most closely resembles A. o. opacus; both of these subspecies have only a faint amount of bronzy-green luster of the feathers, and the immatures have dingy yellow streaks on the abdomen.
Aplonis opaca anga Momiyama, Birds Micronesia, 1922, p. 6. (Type locality, Toroas, Ruk Island.)
Lamproth[ornis] opaca Kittlitz (part), Observ. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 297 (Lougounor = Lukunor).
Lamprotornis columbinus Bonaparte (part), Consp. Avium, 1, 1850, p. 417 (Carolinen = Lukunor?).
Lamprotornis columbina Hartlaub (part), Journ. f. Ornith., 1854, p. 168 (Carolinen = Lukunor?).
Calornis kittlitzi Hartlaub and Finsch (part), Proc. Zool. Soc. London, 1872, pp. 89, 100 (Mackenzie = Ulithi?); Finsch (part), Journ. Mus. Godeffroy, 8, 1875, p. 23 (Mackenzie = Ulithi?); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, pp. 298, 330, 353 (Mortlock, Nukuor, Ruk).
Calornis pacificus Finsch (part), Journ. Mus. Godeffroy, 8, 1875, p. 23 (Mackenzie = Ulithi?); idem (part), Journ. f. Ornith., 1880, p. 290 (Ruck, Mortlocks); idem (part), Proc. Zool. Soc. London, 1880, p. 576 (Ruk); idem (part), Ibis, 1881, p. 111 (Ruk).
Calornis pacifica Finsch (part), Mitth. Ornith. Ver. Wien, 1884, p. 49 (Rukgruppe).
Aplonis kittlitzi Sharpe (part), Cat. Birds British Mus., 13, 1890, p. 136 (Ruk, Lugunor); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 44 (Ruk or Luganor, Nukuor); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 216 (Ruk, Nukuor, Luganor); Hartert (part), Novit. Zool., 5, 1898, p. 59 (Ruk, Luganor); idem, Novit. Zool., 7, 1900, p. 6 (Ruk); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Ruck); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 55 (Ruk).
Lamprocorax kittlitzi Dubois (part), Syn. Avium, 1, 1902, p. 542 (Ruk, Luganor).
Aplonis opaca Wetmore (part), in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 219 (Truk).
Aplonis kittlitzi kittlitzi Momiyama (part), Tori, 2, 1920, p. 1 (Truk, Wolea).
Aplonis opaca anga Kuroda, in Momiyama, Birds Micronesia, 1922, p. 71 (?Luganor or Ruk, ?Nukuor, Wolea or Oleai); Takatsukasa and Yamashina, Tori, 32, 1930, p. 109 (Ruk); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 847 (Ruk); Hand-list Japanese Birds, rev., 1932, p. 170 (Uluthi, Feys, Wolea, Ifalik, Faraulep, Lamotrek, Truk, Nukuoro).
Aplornis apaca anga Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 458 (Truk?); Hand-list Japanese Birds, 3d ed., 1942, p. 188 (Uluthi, Feys, Wolea, Ifalik, Faraulep, Lamotrek, Truk, Nukuoro).
Aplonis opacus angus Mayr, Birds Southwest Pacific, 1945, p. 297 (Truk and western Carolines); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, pp. 70, 71 (Ulithi Truk).
Geographic range.—Micronesia: Caroline Islands—Ulithi, Fais, Wolea, Ifalik, Faraulep, Lamotrek, Truk, Nukuoro, Lukunor.
Characters.—Adult: Resembles A. o. opacus, but larger and with bill less deep and feathers with distinct greenish luster both on the upper parts and the lower parts. Female smaller.
Immature: Resembles immature of A. o. opacus, but underparts streaked with brighter, buffy-yellow coloring.
Measurements.—Measurements are listed in table 45.
Specimens examined.—Total number, 38 (24 males, 14 females), as follows: Caroline Islands, USNM—Ulithi, 27 (Aug. 15, 16, 19, 20, 21, 22)—Truk, 2 (Feb. 16, Dec. 13); AMNH—Truk, 9 (Jan. 29, Feb. 1, 28, June 14, Oct. 9, 14).
Nesting.—Hartert (1900:6) reports that at Truk nests of the starling were obtained by Owston's Japanese collectors from May to July and one in March. Nests contained from one to three eggs each.
Molt.—Adult birds taken by the NAMRU2 party at Ulithi in August are in molting plumage.
Food habits.—The stomachs of starlings obtained in August at Ulithi contained pieces of fruit and seeds. Twelve stomachs contained between one and three cc. of these foods. Papaya and small berries were the foods most frequently observed in the stomachs.
Remarks.—The Micronesian Starling of the central and western Carolines is one of the few land birds which lives on both the "high" islands and the "low" coral islands in Micronesia. It is found on several of the coral atolls in the Carolines. In the Hand-list of Japanese Birds (Hachisuka et al., 1932:170), the birds at Ulithi and Fais are placed in the subspecies A. o. angus, although these islands are only a short distance from Yap, at which place another subspecies, A. o. kurodai, occurs. Specimens from Yap are not available for comparison. Specimens from Ulithi and from Truk closely resemble one another.
The NAMRU2 party found the starling to be numerous at Truk and at Ulithi in 1945. At both places the natives make use of the birds as food. At Truk, McElroy found a larger number of birds in immature plumage than that of birds in adult plumage. Similar observations have been made at several other islands in Micronesia.
At Ulithi, the NAMRU2 party found the starling at all islands in [Pg 291] the atoll visited in 1945. The bird was more numerous at the islands of Potangeras and Mangejang, and less numerous at the island of Losiep; the former two islands were occupied—at the time of the visit in 1945—by service personnel and the vegetation was disturbed, whereas Losiep was uninhabited and rarely visited by people. I attribute the smaller population of starlings at Losiep to the fact that on this island the large monitor lizard, Varanus indicus, was numerous while at Potangeras and Mangejang it was apparently entirely absent. These large lizards depend principally on the birds, rodents, and insects for their food supply. At Potangeras the rat Rattus exulans was exceedingly numerous, while at Losiep no sign of rodents was found nor were any taken in traps set during the daytime.
Aplonis kittlitzi kurodai Momiyama, Tori, 2, 1920, p. 1. (Type locality, Yap.)
Calornis kittlitzi Hartlaub and Finsch (part), Proc. Zool. Soc. London, 1872, p. 100 (Uap); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 123 (Yap); Finsch (part), Journ. Mus. Godeffroy, 8, 1875, pp. 5, 24 (Yap); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, p. 298 (Yap).
Calornis pacificus Finsch (part), Journ. Mus. Godeffroy, 12, 1876, p. 32 (Yap).
Aplonis kittlitzi Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 44 (Yap); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 216 (Yap); Hartert (part), Novit. Zool., 5, 1898, p. 58 (Yap); Bolau (part), Mitteil. Naturhist. Mus. Hamburg, 1898, p. 62 (Yap); Matschie (part), Journ. f. Ornith., 49, 1901, p. 112 (Yap); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 64 (Yap).
Aplonis opaca kurodai Momiyama, Birds Micronesia, 1922, p. 11 (Yap); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 71 (Yap); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 848 (Yap); Hand-list Japanese Birds, rev., 1932, p. 170 (Yap).
Aplonis opaca kurodai Takatsukasa and Yamashina, Dobutsu, Zasshi, 43, 1931, p. 458 (Yap?); Hand-list Japanese Birds, 3d ed., 1942, p. 188 (Yap).
Aplonis opacus kurodai Mayr, Birds Southwest Pacific, 1945, p. 297 (Yap); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 71 (Yap).
Geographic range.—Micronesia: Caroline Islands—Yap.
Characters.—Adult: According to Momiyama (1922:11), "Similar to A. o. anga from Ruk group, but the bill thicker (9-10.5 mm.; that of the latter 8.5-9.5 mm.) and much longer (24-27.5 mm.; that of the latter 21.5-25 mm.) and the wing also longer in average (119.5-130 mm. instead of 116.5-129.5 mm.). It differs from typical opaca by the edge of feathers of both body sides very distinctly tinged with a bronzy-green lustre, by the bill being longer and thicker (in typical opaca exposed culmen 21.5-24.5 mm., depth of bill 9-9.5 mm.)."
of body somewhat deeper in colour and the edge of feathers distinctly tinged with lustrous bronzy-green. It differs from the same stage of A. o. anga by the under-parts being without pale-yellowish area." Momiyama (1922:11).
Young: "Similar to the young of typical bird, but differs from it by the mantle being very faintly tinged with bronzy-green and by the under-parts being somewhat tinged with brown. In the same stage of the typical form, the under-parts are much more greyish-ashy in colour." Momiyama (1922:11).
Remarks.—No specimens have been examined. Momiyama (1920:1) regarded the birds at Yap and at Saipan as A. o. kurodai. Later (1922:10) he separated the birds at Saipan as A. o. harterti, remarking that the birds from Saipan differ "from A. o. kurodai Momiyama from Yap islands, by the green lustre on both sides of body being less distinct and showing tendency to a purplish lustre, by the bill being decidedly shorter, and by the same thickness."
Price (1936a:19) describes a method by which starlings and other birds are captured by the natives of Yap. The natives make slashes in the trunk of a breadfruit tree and allow the exuding juice to harden. This material is then chewed until soft and adhesive. It is then placed on a stick which has been secured directly under a papaya fruit. When the birds alight on this perch, they become stuck and are captured.
Aplornis opaca orii Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 458. (Type locality, Coror, Pelew Islands.)
Calornis kittlitzii Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 7, 117, 118 (Pelew).
Calornis opaca Gray (part), Hand-list Birds, 2, 1870, p. 27 (Pelew).
Calornis kittlitzi Hartlaub and Finsch (part), Proc. Zool. Soc. London, 1872, p. 89 (Pelew); Finsch (part), Journ. Mus. Godeffroy, 8, 1875, pp. 5, 23 (Palau); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, p. 298 (Palau).
Calornis kittlitzi Kubary, Journ. Mus. Godeffroy, 4, 1873, p. 225 (Palau-Inseln).
Calornis pacificus Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 32 (Palau); idem (part), Journ. f. Ornith., 1880, p. 289 (Palau); idem (part), Proc. Zool. Soc. London, 1880, p. 576 (Palau); idem (part). Ibis, 1881, p. 111 (Pelew).
Aplonis kittlitzi Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 44 (Pelew); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 212 (Palaos); Hartert (part), Novit. Zool., 5, 1898, p. 58 (Pelew); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Palau); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 55 (Pelew).
Aplonis opaca subsp nov.? Momiyama, Birds Micronesia, 1922, p. 13 (Pelew); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 72 (Pelew).
Aplornis opaca orii Hand-list Japanese Birds, 3d ed., 1942, p. 188 (Babelthuap, Koror, Peliliu, Anguar).
Aplonis opaca orii Hand-list Japanese Birds, rev., 1932, p. 169 (Palau); Yamashina, Tori, 10, 1940, p. 673 (Palau).
Aplonis opacus orii Mayr, Birds Southwest Pacific, 1945, p. 297 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 71 (Peleliu, Ngesebus, Garakayo).
Geographic range.—Micronesia: Palau Islands—Kayangel, Babelthuap, Koror, Garakayo, Ngesebus, Peleliu, Ngabad, Angaur.
Characters.—Adult: Resembles adult of A. o. opacus, but slightly larger with bill longer and shallower, and feathers with distinct greenish gloss both on the upper parts and the lower parts. Resembles A. o. angus in the amount of greenish gloss on feathers, but bill shallower. Depth of bill of A. o. opacus measures, on the average, 9.5 for males and 9.0 for females; of A. o. angus 8.5 for both males and females; of A. o. orii 7.5 for both males and females.
Immature: Resembles immature of A. o. angus, but streaking on underparts duller.
Measurements.—Measurements are listed in table 45.
Specimens examined.—Total number, 40 (21 males, 19 females), as follows: Palau Islands, USNM—Koror, 3 (Nov. 6)—Garakayo, 2 (Sept. 19)—Ngesebus, 1 (Sept. 20)—Peleliu, 7 (Aug. 28, 29, 30, 31, Sept. 5); AMNH—exact locality not given, 27 (Oct., Nov., Dec.).
Molt.—Many of the specimens taken in August and September show evidence of molt; most of the specimens taken in October, November and December are not in molt.
Remarks.—The amount of greenish gloss on the feathers of A. o. orii and A. o. angus appears to be the same, but the streaked underparts of the immature of A. o. orii are duller than those of the immature of A. o. angus. The shallower bill in the Palau starling is caused by the lower edge of the mandible being generally straighter than that in A. o. angus and A. o. opacus. In comparing A. o. orii with A. o. kurodai, Takatsukasa and Yamashina (1931a:458) state that "the greenish gloss is less pronounced and of a duller shade than that of A. o. kurodai Momiyama."
The starling is probably the most abundant land bird in the Palaus. It was found as singles or in small flocks at all islands visited by the NAMRU2 party in 1945. As at the other islands of Micronesia, the starling at Palau is noisy and conspicuous. It is a most inquisitive bird, often following the collector through the woodlands. Apparently the starling prefers the open woodlands and marginal areas to the thicker jungles; as a result of clearing operations during the war, the bird probably has increased. The starling is primarily a vegetarian; I found no animal matter in stomachs examined at Palau or at Ulithi or Guam. At Palau, as at other islands, more of the starlings seen were in immature plumage than in adult plumage. Coultas (field notes) found the birds to be abundant at Koror and highly prized as food by the natives and Japanese. He writes, "It is surprising what a fine wholesome meal certain people can get out of handful of rice and a starling's breast."
Aplonis opaca guami Momiyama, Birds Micronesia, 1922, p. 9. (Type locality, Guam).
Turdus columbinus Lesson (part), Traité d'Ornith., 1831, p. 406 (Mariannes = Guam).
Lamproth[ornis] opaca Kittlitz (part), Kupfertaf. Naturgesch. Vögel, 2, 1833, p. 11, pl. 15, fig. 2 (Marianen = Guam); idem (part), Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, pp. 298, 304 (Guahan).
Lamprotornis columbinus Bonaparte (part), Consp. Avium, 1, 1850, p. 417 (Mariann. =Guam).
Lamprotornis columbina Hartlaub (part), Journ. f. Ornith., 1854, p. 167 (Mariannen =Guam); Kittlitz, Denkw. Reise russ. Amer. Micron, und Kamchat., 1, 1858, pp. 367, 376 (Guaham).
Calornis opaca Gray (part), Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 26 (Ladrone or Marian Is.); idem, (part), Hand-list Birds, 2, 1870, p. 27 (Ladrone = Guam?).
Calornis kittlitzi Finsch and Hartlaub (part), Fauna Centralpolynesiens, 1867, p. 109 (Marianen = Guam?); Oustalet, Le. Nat., 1889, p. 261 (Mariannes).
Calornis columbina Giebel (part), Thes. Ornith., 2, 1875, p. 427 (Marianae = Guam?).
Calornis pacificus Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 32 Marianne).
Aplonis kittlitzi Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 44 (Marianne; Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 212 (Guam, Saypan); Hartert (part), Novit. Zool., 5, 1898, p. 58 (Guam, Saipan); Wheeler, Report Island of Guam, 1900, p. 13 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 54 (Marianas); Matschie, Journ. f. Ornith., 1901, p. 112 (Guam); Safford, Osprey, 1902, p. 69 (Guam); idem, The Plant World, 7, 1904, p. 264 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Mearns, Proc. U. S. Nat. Mus., 36, 1909, p. 477 (Guam); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 64 (Marianas); Cox, Island of Guam, 1917, p. 21 (Guam); Bryan, Guam Rec, vol. 13, no. 2, 1936, p. 25 (Guam).
Aplonis opaca Wetmore (part), in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 219 (Guam).
Aplonis kittlitzi kurodai Momiyama, Tori, 2, 1920, p. (Saipan).
Aplonis opaca guami Kuroda, in Momiyama, Birds Micronesia, 1922, p. 71 (Guam); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 847 (Guam); Yamashina, Tori, 7, 1932, p. 394 (Saipan, Rota); Hand-list Japanese Birds, rev., 1932, p. 169 (Guam, Rota, Tinian, Saipan).
Aplonis opaca harterti Momiyama (part), Birds Micronesia, 1922, p. 10 (Type locality, Saipan); Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 71 (Saipan); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 847 (Saipan).
Aplornis opaca harterti Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 487 (Saipan).
Aplornis opaca guami Takatsukasa and Yamashina, Dobutsu. Zasshi, 44, 1932, p. 221 (Tinian, Rota); Hand-list Japanese Birds, 3d ed., 1942, p. 188 (Saipan, Tinian, Rota, Guam).
Aplonis opacus guami Mayr, Birds Southwest Pacific, 1945, p. 297 (Guam, Rota, Tinian, Saipan); Watson, The Raven, 17, 1946, p. 41 (Guam); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 103 (Tinian); Stott, Auk, 1947, p. 527 (Saipan, Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 69 (Guam, Rota, Tinian, Saipan).
Aplonis opacus Wharton, Ecol. Monogr., 16, 1946, p. 174 (Guam); Strophlet, Auk, 1946, p. 540 (Guam); Baker, Condor, 49, 1947, p. 125 (Guam).
Geographic range.—Micronesia: Mariana Islands—Guam, Rota, Tinian, Saipan.
Characters.—Adult: Resembles closely A. o. angus in the amount of greenish gloss present on the body feathers, but with slightly shorter and deeper bill.
Immature: Resembles the immature of A. o. angus but streaks on underparts brighter and less-dingy yellow.
Measurements.—Measurements are listed in table 45. The writer (1948:69) has given average measurements for the length of wing of adult males from Guam as 127, from Rota as 122, from Tinian as 131, and from Saipan as 131; for depth of bill of adult males from Guam as 9.0, from Rota as 9.0, from Tinian as 9.5, and from Saipan as 10.0.
Weights.—The NAMRU2 party obtained weights of six adult males from Guam as 84-96 (87); of eight adult females from Guam as 78-108 (86); of two juvenal males from Guam as 88 and 90; of five juvenal females from Guam as 77-87 (80); of two adult males from Rota as 70 and 83; and of five juvenal males from Rota as 64-80 (76).
Specimens examined.—Total number, 95 (55 males, 37 females, 3 unsexed), as follows: Mariana Islands, USNM—Guam, 44 (Jan. 21, 22, Feb. 5, March 8, 13, April 12, May 18, 22, 24, 27, 29, 30, June 3, 4, 6, 14, 16, 18, July 6, 7, 14, 20, Aug. 24, Oct. 8, Nov. 19, 23)—Rota, 12 (Oct. 18, 19, 26, 27, Nov. 2)—Tinian, 4 (Oct. 12, 18); AMNH—Guam, 16 (Jan. 23, 24, 29, March 3, 12, 13, 24, May, Aug. 12, Nov. 23, 28, Dec. 26)—Tinian, 15 (Sept. 7, 8, 10, 11, 12)—Saipan, 4 (July 9, 17, Aug. 26, Sept. 2).
Nesting.—The NAMRU2 party found evidence of nesting by starlings at Guam as early as January 28, in 1945. On this date a bird was seen to carry food into a hollow tree at Oca Point. Signs of nesting activities were observed in the months that followed, the last record being obtained on June 11. Starlings nest in cavities in trees, in holes in rocky cliffs, and probably in the tops of coconut palms. On June 2 a nest was found by Muennink in a cavity of a banyan tree at Oca Point, Guam. The nest was approximately 12 feet from the ground and consisted of a flattened mass of green foliage at the bottom of the cavity. Two eggs found in the nest have been described by the author (1948:69) as "Niagara green" with scattered, irregular spots of color, near "russet," "Mars brown" and "pallid purple-drab," most abundant near the large ends. Measurements are 32.1 by 22.1 and 32.0 by 22.4.
Yamashina (1932a:394) records two eggs taken at Saipan on April 14, 1931; two eggs taken at Rota on March 10, 1931; and one egg taken at Rota on March 11, 1931. Seale (1901:54) writes that the starling nests in a hole in the dead trunk of the coconut palm and may lay three or four eggs. Hartert (1898:59) reports that two eggs were taken at Guam on March 11.
Food habits.—Probably the chief food of the starling at Guam is the fruit and seeds of the papaya. This plant grows in most parts of the island, especially in the lowlands where land uses have disturbed the climax vegetation. Many of the garden plots lay fallow during the war and were allowed to grow up in thick stands of papaya. As a fruit began to ripen, the starlings would peck out one side of a ripe fruit, feeding on the tissues and the seeds. It was seldom that a fully ripe papaya fruit was found that had not been at least partly eaten by the starlings. Apparently the birds do not feed on the fruit before it is fully ripened. Seeds of other types of vegetation were also eaten by the birds.
Parasites.—Wharton (1946:174) records the chigger (Acarina), Trombicula sp., from the starling at Guam.
Remarks.—According to Oustalet (1895:212), the starling was taken in the Marianas by the expedition in the "Uranie" in 1820 and by the expedition in the "Astrolabe" in 1829. Kittlitz, who visited Guam from March 1-20, 1828, also recorded the starling. It was not until 1922, however, that the starling in the Marianas was recognized as subspecifically distinct from the birds in the Carolines and Palaus. [Pg 296] The Japanese ornithologists named the bird at Guam as A. o. guami and the bird at Saipan as A. o. harterti, but later regarded these as a single subspecies A. o. guami. Momiyama (1920:2) had, previously to the naming of the new forms in the Marianas, considered the bird at Saipan as belonging to the same subspecies as that found at Yap. Among named kinds, A. o. guami found at Guam, Rota, Tinian, and Saipan appears to be most closely related to A. o. angus. These two subspecies differ in that the streaking of the underparts in the immatures is brighter in A. o. guami and duller in A. o. angus. The bird at Saipan has a longer wing and a deeper bill than the bird at Guam; however, birds at Tinian show intermediate measurements.
At Guam, the starling is the most numerous land bird. The writer (1947b:124), in counting birds along the roadways of Guam, recorded the starling on all of the 125 counts and found the birds to include more than one-half (57.3 percent) of all the birds seen. Starlings may have increased during the years of the war, with the disruption of normal agricultural activities allowing the growth of papaya and other food plants in fallow areas; however, the use of the birds as food by the islanders probably increased during the war.
As at other islands in Micronesia, the numbers of birds in immature plumage at Guam seemingly exceeds the number of birds in adult plumage. Animals which may prey on the starling at Guam include the feral house cat, Rattus mindanensis, Corvus kubaryi, and the large lizard Varanus indicus. The starling spends little time on the ground; it feeds principally in the trees, which might limit the amount of damage done to it by the feral house cats which are numerous on the island. The rat, R. mindanensis, is a semi-arboreal animal and may feed on eggs and young birds in nest cavities of trees or on cliffs. The crow, C. kubaryi, has a reputation for stealing chicken eggs from poultry yards and may prey on the eggs and young of the starling. The monitor lizard, V. indicus, is known to prey on the starling, as well as on the domestic chickens at farm houses. On January 31, 1945, one of these large lizards was seen descending a tree after robbing a nest of a starling; one of the starling's eggs was seen in the mouth of the lizard. The noise and commotion set up by the parent birds and by other starlings, which had been attracted to the area, did not appear to perturb the uninvited guest.
Downs (1946:103) writes that the starling at Tinian is less common than the white-eye, Zosterops conspicillata saypani. Gleize (1945:220) estimated the population of starlings on Tinian at 200. [Pg 297] Coultas (field notes) found the starling abundant at Tinian in 1931, but he did not find the bird at Saipan. According to Stott (1947:527), the starling was abundant at Guam but "appeared to be common only locally on Saipan." He saw large flocks at the Marpi Point and Kingman Point areas on Saipan but found the bird less numerous elsewhere on the island. At Rota, the NAMRU2 party found the birds to be numerous and widely distributed over the island in 1945.
At Guam, the present writer observed behavior of the starling on January 31, 1945, which may have been a courtship ceremony. Two adults were perched on a palm frond approximately 20 feet above the ground. The bird which was perched more distally on the frond opened its tail fan-fashion, spread its wings and at irregular intervals picked up in its beak a part of the frond and then released it. As this behavior was taking place, the birds would call in a sweet ascending song, which reminded me very much of the song of the redwing blackbird of North America. This was indeed a contrast to the usual squawking notes of this subspecies.
Aplornis opaca aenea Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 487. (Type locality, Pagan.)
Aplonis kittlitzi Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 212 (Pagan, Agrigan).
Aplonis opaca harterti Momiyama (part), Birds Micronesia, 1922, p. 11 (Pagan, Agrigan); Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 71 (Pagan, Agrigan).
Aplornis opaca aenea Hand-list Japanese Birds, 3d ed., 1942, p. 187 (Asongsong = Asuncion, Agrigan, Pagan, Almagan).
Aplornis opaca aenea Takatsukasa and Yamashina, Dobutsu. Zasshi, 44, 1932, p. 221 (Pagan, Almagan).
Aplonis opaca aenea Hand-list Japanese Birds, rev., 1932, p. 169 (Agrigan, Pagan, Almagan); Yamashina, Tori, 10, 1940, p. 673 (Asongsong).
Aplonis opacus aeneus Mayr, Birds Southwest Pacific, 1945, p. 297 (Agrigan, Pagan, Almagan); Borror, Auk, 64, 1947, p. 417 (Agrihan).
Geographic range.—Micronesia: Mariana Islands—Alamagan, Pagan, Agrihan, Asuncion.
Characters.—Adult: According to Takatsukasa and Yamashina (1931:487), A. o. aeneus resembles A. o. orii of Palau, but has a bronze rather than green luster. A. o. aeneus resembles A. o. opacus, but has a smaller bill.
Remarks.—No specimens of this subspecies have been examined by me. Little information is available regarding the occurrence of this subspecies in the northern Marianas. Oustalet (1895:212) writes that Marche collected four specimens at Pagan and three at Agrihan. Borror (1947:417) writes that in 1945, it was a "common and [Pg 298] abundant species" at Agrihan. He obtained one specimen between July 27 and August 14 and comments that it had a grasshopper in its stomach.
Evolutionary history of Aplonis opacus.—Aplonis opacus is known from the Mariana, Palau, and Caroline islands in Micronesia. It consists of several subspecies, which have relatively few distinguishing characteristics. No starlings are known in the Marshall and Gilbert islands, although atolls occur in these island-chains that offer a habitat approximately the same as those in the western Carolines now occupied by A. o. angus.
In regard to parental stock, Sharpe (1876:47) considered A. opacus as "nothing but a slightly more metallic race of C. mysolensis, with a still stouter bill." The species with which Sharpe compared A. opacus is known from Mysol, Buru, and Ceram. Oustalet (1896:70) thought that the Aplonis in Micronesia belonged to a group of starlings whose members are scattered through the Pacific islands including Cook, Samoa, Tonga, Fiji, New Britain, New Guinea, Banta, Mysol, Salwatti, and Timor. Mayr (1941b:204) is of the opinion that Aplonis in Micronesia was derived from central Polynesia. Amadon (1943:8), in his study of the genera of starlings, places A. opacus within a superspecies containing A. cinerascens, A. tabuensis, A. fuscus, and possibly A. feadensis and A. cantoroides. All of these are blackish birds with greenish gloss with immatures having the underparts streaked. In comparing A. opacus with these mentioned species and with other species of Aplonis, I find that A. opacus more closely resembles A. feadensis and A. cantoroides than any others. Although there are differences in size of the bill, wing, and tail, these structures are proportionally the same. The streaked underparts of the immatures of A. cantoroides are much like that of the immatures of A. opacus, whereas the immatures of A. feadensis are only faintly streaked with whitish below. The eye of A. cantoroides is red, and that of A. opacus is more nearly yellow. The ancestral stock from which A. opacus developed in Micronesia seemingly reached the area from Melanesia. In Micronesia the birds dispersed to various groups of islands from some point in the Caroline Islands. The birds are absent from the Marshall Islands. Perhaps the birds never reached the Marshall Islands or they may have been present in former times and disappeared since then.
Aplonis pelzelni Finsch, Proc. Zool. Soc. London, 1875 (1876), p. 644. (Type locality, Ponapé.)
Aplonis pelzelni Finsch, Journ. Mus. Godeffroy, 12, 1876, pp. 17, 32, pl. 2, fig. 3 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 779 (Ponapé); idem, Journ, f. Ornith., 1880, p. 290 (Ponapé); idem, Ibis, 1881, pp. 110, 112, 115 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 281 (Ponapé); Sharpe, Cat. Birds British Mus., 13, 1890, p. 136 (Ponapé); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 43 (Ponapé); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 215 (Ponapé); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 62 (Ponapé); Matschie, Journ. f. Ornith., 1901, pp. 111, 112 (Ponapé); Dubois, Syn. Avium, 1, 1902, p. 542 (Ponapé); Reichenow, Die Vögel, 2, 1914, p. 355 (Ponapé); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Ponapé); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 70 (Ponapé); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 849 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 170 (Ponapé); Bequaert, Mushi, 12, 1939, p. 82 (Ponapé); Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, pp. 204, 213 (Ponapé); Bequaert, Occ. Papers Bernice P. Bishop Mus., 16, 1941, p. 290 (Ponapé); Mayr. Birds Southwest Pacific, 1945, p. 298 (Ponapé).
Aplornis pelzelni Hand-List Japanese Birds, 3d ed., 1942, p. 189 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult: A small, dark starling with upper parts sooty-brown, darker on head with forehead and lores blackish; wings, rump, upper tail-coverts, and tail lighter and more brownish than head; underparts paler and washed with olive-brown; bill and feet black; iris brown.
Immature: Resembles adult, but lighter brown, especially the underparts.
A. pelzelni differs from A. opacus by having no gloss on the feathers, smaller size, more slender bill, and a brown iris.
Measurements.—Measurements are listed in table 46.
Specimens examined.—Total number, 59 (32 males, 24 females, 3 unsexed), from Caroline Islands, AMNH—Ponapé (Dec).
Nesting.—Coultas (field notes) obtained reports that the Ponapé Mountain Starling nests in cavities in trees and lays two eggs.
| Number and Sex | Wing | Tail | Exposed Culmen |
Depth of bill at nostril |
Tarsus |
|
10 adult males |
103 | 65 | 20.0 | 6.5 | 27 |
| 101-105 | 63-67 | 19.0-21.0 | 6.0-7.0 | 26-28 | |
|
10 adult females |
99 | 61 | 19.5 | 6.0 | 27 |
| 97-102 | 57-64 | 19.5-20.5 | 6.0-6.5 | 26-27 |
Parasites.—Bequaert (1939:82 and 1941:290) records the fly (Hippoboscidae), Ornithoica pusilla, from A. pelzelni.
Remarks.—Coultas (field notes) writes that "the Mountain Starling is a bird of the true mountain forest.... I did not record it below 1,400 feet. Natives tell me that the Mountain Starling formerly covered the whole of the island and that now some individuals can be found on the low atoll of Ant, to the westward of Ponapé. Unfortunately, I was not permitted to visit either Ant or Pakin." Coultas notes also that the birds are quiet and usually travel in pairs. They are easily attracted by squeaking the lips against the hand or by the cries of a wounded bird. Many of these starlings were taken in fruit trees. Coultas describes the call of A. pelzelni as "weaker and finer" than that of A. opacus. These two species may be found together, according to Coultas, but A. opacus is apparently the more aggressive and often drives A. pelzelni away. Richards (in litt.) found this bird to be "very rare" while on his visit to Ponapé in 1947-1948. He observed two individuals on January 15, 1948, at an elevation of approximately 600 or 700 feet. A male was taken.
Evolutionary history of Aplonis pelzelni.—The Ponapé Mountain Starling is a distinctive bird which evidently represents an ancient and single colonization of Micronesia. It lacks the green gloss which is found on many of the other starlings of the Pacific region. It has a brown iris, and the immatures lack the streaked underparts which are characteristic of A. opacus and other species. The structure of its wing resembles that of A. opacus, but the primaries are more rounded. It is apparently better adapted to forested uplands, whereas A. opacus and its relatives, A. cantoroides and A. feadensis, appear to prefer lowland forests and coconut plantations. In habits and habitat preference, A. pelzelni seems to resemble A. santovestris, which is restricted to mountain environment on Espiritu Santo in the New Hebrides. The describers of this starling, Harrisson and Marshall (1937:149), write that "Aplonis santovestris apparently most closely resembles A. pelzelni from Ponapé, especially in bill and tarsus." According to the description, A. santovestris is approximately the size of A. pelzelni with brownish coloring, crown dark brown, lower back and rump dark rufous, wing and tail blackish-brown, underparts rufous-brown, and iris grayish-green. These two birds are separated geographically and apparently exhibit evidences of parallel development. Possibly they came from a common ancestral stock. Mayr (1941b:204) writes that A. pelzelni belongs with the starlings of the Polynesian area. I have compared A. pelzelni with other starlings of the Southwest Pacific, including A. [Pg 301] feadensis, A. cantoroides, and A. zealandicus, but see no close resemblances.
Lamprothornis corvina Kittlitz, Kupfertaf. Naturgesch. Vögel, 2, 1833, p. 12, pl. 15, fig. 3. (Type locality, Ualan = Kusaie.)
Lamprothornis corvina, Kittlitz, Mem. Acad. Imp. Sci. St. Peterbourg, 2, 1835, p. 7, pl. 9 (Ualan); idem, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 285 (Ualan).
Lamprotornis corvina Bonaparte, Consp. Avium, 1, 1850, p. 417 (Ualan); Hartlaub, Archiv. f. Naturgesch., 18, 1852, p. 133 (Ualan); Kittlitz, Denkw. Reise russ. Amer. Micron. und Kamchat., 2, 1858, pp. 25, 43, 59, 103 (Ualan); Finsch, Ibis, 1881, p. 104 (Kuschai).
Lamprocorax corvinus Hartlaub, Journ. f. Ornith., 1854, p. 168 (Carolinen = Kusaie); Sclater, Ibis, 1859, p. 327 (Caroline = Kusaie); Dubois, Syn. Avium, 1, 1902, p. 543 (Kuschai).
Calornis (Lamprocorax?) corvina Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 25 (Oualan).
Sturnoides corvina Finsch and Hartlaub, Fauna Centralpolynesiens, 1867, p. 108 (Ualan); Finsch, Journ. f. Ornith., 1880, pp. 297, 302 (Kuschai).
Calornis corvina Gray, Hand-list Birds, 2, 1870, p. 27 (Caroline = Kusaie); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 100 (Ualan); Giebel, Thes. Ornith., 2, 1875, p. 427 (Caroline = Kusaie); Sharpe, Cat. Birds British Mus., 13, 1890, p. 137 (Kuschai); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 46 (Ualan or Kushai); Matschie, Journ. f. Ornith., 1901, p. 112 (Ualan); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Kusaie).
Sturnoides corvinus Finsch, Ibis, 1881, pp. 107, 108 (Kushai).
Kittlitzia corvina Hartert, Kat. Vogelsamml. Senckenb., 1891, p. 75 (Ualan); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 72 (Kusaie); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 853 (Kusaie); Hand-list Japanese Birds, rev., 1932, p. 169 (Kusaie); Hand-list Japanese Birds, 3d ed., 1942, p. 187 (Kusaie).
Aplonis corvina Reichenow, Die Vögel, 2, 1914, p. 356 (Ualan); Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 213 (Kusaie).
Aplonis corvinus Mayr, Birds Southwest Pacific, 1945, p. 298 (Kusaie).
Geographic range.—Micronesia: Caroline Islands—Kusaie, probably extinct for many years.
Characters.—According to Sharpe (1890:137), "Shining black; each feather with a glossy margin, varying from steel-green to purplish red; bill and feet black (Kittlitz)."
Remarks.—Kittlitz obtained two specimens of a unique starling at Kusaie when he visited the island in December and January, 1827-'28. He named the birds as new and deposited the specimens in the museum in St. Petersburg. The bird has not been found at Kusaie since that time. Sharpe (1890:137-138, footnote) writes "This species I have never seen, and Dr. Finsch did not meet with it during his visit to Kuschai. He writes to me:—'It no doubt exists on Kuschai, just as it did when Kittlitz visited the island. Nobody has reached the mountains in the interior since Kittlitz's time; and it is strictly a mountain bird.'" Coultas spent considerable time searching the higher areas of Kusaie for the bird in 1931.
The Kusaie Mountain Starling apparently represents an early [Pg 302] invasion of Micronesia, independent of that of any other starling in the area and perhaps the earliest of the three colonizations by starlings in Micronesia. The drawing of the bird as pictured by Kittlitz (1833:pl. 14, fig. 3) shows the long bill to be one of its distinctive characters. This suggests relationship to A. atrifuscus of Samoa, as noted by Mayr (1942a:6). A. atrifuscus is larger than A. opacus with a longer bill and gloss on some of the feathering of the body; it looks a good deal like the drawing of A. corvinus by Kittlitz. A. corvinus may also have some relation to A. magnus of Biak, although this species has a longer tail and a shorter bill. A. corvinus probably has undergone an evolutionary development which parallels that of A. atrifuscus and possibly other species in the Polynesian and Melanesian areas. The ancestral stock from which A. corvinus was derived may have been close to A. grandis, which is found in the Solomon area. A. grandis is a forest bird, somewhat solitary in habits.
[Motacilla] philippensis Forster, Ind. Zool., 1781, p. 41. (Type locality, Philippines.)
Sturnus philippensis Mayr, Birds Southwest Pacific, 1945, p. 302 (Palau).
Geographic range.—Breeds in Japan. Winters to the Philippine Islands. In Micronesia: Palau Islands—exact locality unknown.
Remarks.—Mayr (1945a:302) records this starling as a migrant visitor to the Palau Islands. Coultas obtained an immature female of this species at Palau on October 13, 1931.
Sturnus cineraceus Temminck, Pl. Col. 2, 1832, pl. 556. (Type locality, Japan.)
Spodiopsar cineracea Kishida, Lansania, 1, 1929, p. 17 (Saipan); Hand-list Japanese Birds, 3d ed., 1942, p. 187 (Saipan).
Geographic range.—Breeds in eastern Asia and Japan. Winters in southern China and Philippines. In Micronesia: Mariana Islands—Saipan.
Remarks.—The Ashy Starling has been reported from Saipan by Kishida. It probably is a casual winter migrant.
Ptilotis Marchei Oustalet, Le Nat., 1889, p. 260. (Type locality, Saypan.)
Cleptornis marchei Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 35 (Saypan); Hartert, Novit. Zool., 5, 1898, p. 56 (Saipan); Matschie, Journ. f. Ornith., 1901, p. 112 (Saipan); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 60 (Saipan); Dubois, Syn. Avium, 1, 1902, p. 722 (Marianne = Saipan); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Marianne = Saipan); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 75 (Saipan); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 788 (Saipan); Hand-list Japanese Birds, rev., 1932, p. 171 (Saipan); Hand-list Japanese Birds, 3d ed., 1942, p. 190 (Saipan); Mayr, Birds Southwest Pacific, 1945, p. 298 (Saipan); Stott, Auk, 64, 1947, p. 527 (Saipan).
Ptilotis (Cleptornis) marchei Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 202 (Saypan).
Geographic range.—Micronesia: Mariana Islands—Saipan.
Characters.—Adult: A small honey-eater with head, rump, and underparts near "light cadmium" becoming lighter on the chin and darker on the nape; back near "orange-citrine"; wings and tail feathers brown with outer edges colored like back and inner edges whitish; orbital ring pale yellow; breast, belly, sides, and under tail- and upper tail-coverts near "raw sienna"; under wing-coverts pale yellow; axillaries yellow; bill and feet light yellow-brown, maxilla darker; iris chestnut-brown. Immature has lighter bill.
Measurements.—Measurements are listed in table 47.
| Number and Sex | Wing | Tail | Full Culmen | Tarsus |
| 7 adult males | 79 | 64 | 19.5 | 26 |
| (77-80) | (61-66) | (19.0-20.0) | (25-27) | |
| 5 adult females | 73 | 58 | 18.0 | 24 |
| (72-75) | (56-59) | (17.5-18.5) | (23-25) |
Specimens examined.—Total number, 17 (9 males, 8 females), as follows: Mariana Islands, USNM—Saipan, 4 (July 11, Dec. 15); AMNH—Saipan, 13 (July 8, Aug. 1, 10, 13, 14, 21, 30, Sept. 3, 7, 9, 15).
Nesting.—Hartert (1898:56) reports that one nest of the Golden Honey-eater was found on July 7. It was hung from a fork of a branch, "like the nest of a golden Oriole." He writes that four other nests were obtained in late August. Hartert describes the egg as "pale blue without gloss, spotted over and over with rufous, more so on the thicker end, and measures about 20:15 mm."
Molt.—Specimens taken in July, August, and September are molting.
Remarks.—Oustalet (1895:202) writes that Marche obtained 25 specimens of the Golden Honey-eater at Saipan in May, June, and July, 1887. Little is known regarding its habits; Moran (1946:262) writes that the bird "reminds one of the prothonotary warbler, with a long, curved, black bill." Stott (1947:527) writes that "it appears to be restricted to a single habitat, that of dense forest." He found the bird in forest on the north shore of Magicienne Bay. Coultas obtained only one specimen on his visit to Saipan in 1931. Marshall (1949:216) records some interesting observations of this bird made in 1945. He notes (op. cit. p. 219) that the bird breeds in January, February and April.
Not only is it remarkable that the Golden Honey-eater has become established on a single island in a rather closely associated chain of islands, but it is also difficult to determine from where the bird came. It seemingly has no close relatives in the Micronesian area. Oustalet (1895:202) points out that one has to go to New Guinea, Moluccas, Australia, Fiji, Samoa, and Tonga in order to find related forms. In looking through the large collections of Meliphagidae in the American Museum of Natural History, I found only a few genera to which the Saipan Golden Honey-eater seems to be closely related. Timeliopsis of New Guinea has some resemblances to Cleptornis, although the coloration is different. Timeliopsis has a similar bill, but has a longer tail and longer wing; the shortness of the wing in Cleptornis is not unusual since other insular forms also exhibit this characteristic.
Perhaps Cleptornis is closer to the genus Meliphaga of New Guinea and Australia, which has become differentiated into a number of diverse species and subspecies. Cleptornis compares rather favorably with M. pencillata carteri of Australia, but differs by the softness of its feathers and the shorter wing and shorter tail. It shows also some affinities with M. flava of Australia, particularly in shape of bill; the coloration of the feathers is light olive-green in M. flava. The bird at Saipan seemingly has no relationships with the Hawaiian honey-eaters.
Cinnyris rubrater Lesson, Dict. Sci. Nat., éd. Levrault, 50, 1827, p. 30. (Type locality, Oualan = Kusaie.)
Cinnyris rubrater Lesson (part), Voy. "La Coquille," Zool., 2, 1828, pp. 433, 678 (Oualan); idem (part), Man. d'Ornith., 2, 1828, p. 55 (Oualan); idem (part), Traité d'Ornith., 1831, p. 299 (Oualan); Kittlitz (part), Kupfertaf. Naturgesch. Vögel, 1832, p. 6, pl. 8, fig. 1 (Ualan); idem (part), Denkw. Reise russ. Amer. Micron. und Kamchat., 1, 1858, pp. 364, 381; 2, 1858, pp. 39, 49 (Ualan).
Certhia Cardinalis Kittlitz, Mém. Acad. Imp. Sci. St. Pétersbourg, 2, 1835, p. 4 (Ualan).
Cinnyris cardinalis Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 285 (Ualan).
Myzomela sanguinolenta Bonaparte, Consp. Avium, 1, 1850, p. 394 (no loc. = Kusaie?).
Myzomela rubrater Hartlaub (part), Archiv. f. Naturgesch., 18, 1852, pp. 109, 131 (Ualan); Finsch and Hartlaub, Fauna Centralpolynesiens, 1867, p. 57 (Ualan).
Myzomela rubratra Hartlaub (part), Journ. f. Ornith., 1854, p. 168 (Carolinen = Kusaie); idem (part), Proc. Zool. Soc. London, 1867 (1868), p. 829 (Carolines = Kusaie); Hartlaub and Finsch (part), Proc. Zool. Soc. London, 1872, p. 95 (Ualan); Giebel (part), Thes. Ornith., 2, 1875, p. 681 (Carolinae = Kusaie); Finsch (part), Journ. Mus. Godeffroy, 12, 1876, p. 26 (Ualan); Forbes (part), Proc. Zool. Soc. London, 1879, p. 271 (Ualan); Finsch (part), Journ. f. Ornith., 1880, pp. 285, 298 (Kuschai); idem (part), Ibis, 1881, pp. 103, 108, 111 (Kuschai); idem (part), Mitth. Ornith. Ver. Wien, 1884, p. 48 (Ualan); Hartert, Kat. Vogelsamml. Senckenb., 1891, p. 31 (Ualan); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 31 (Ualan); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, pp. 201, 202 (Kushai); Hartert (part), Novit, Zool., 5, 1898, p. 56 (Ualan); Dubois (part), Syn. Avium, 1, 1902, p. 716 (Carolines = Kusaie).
Certhia sanguinolenta Kittlitz, Denkw. Reise russ. Amer. Micron, und Kamchat., 1, 1858, p. 364 (Ualan).
Myzomela major Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 11 (Oualan?).
Myzomela rubrata Matschie (part), Journ. f. Ornith., 1901, p. 112 (Ualan).
Myzomela rubratra rubratra Wetmore, Proc. Biol. Soc. Washington, 30, 1917, p. 117 (Kusaie); Wetmore (part), in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 219 (Kusaie); Momiyama, Birds Micronesia, 1922, pp. 15, 20, 21, 22, (Kusaie); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 72 (Kusaie); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 743 (Oualan); Hand-list Japanese Birds (part), rev., 1932, p. 172 (Kusaie); Hand-list Japanese Birds (part), 3d ed., 1942, p. 191 (Kusaie).
Myzomela cardinalis rubratra Mayr, Birds Southwest Pacific, 1945, p. 299 (Kusaie).
Geographic range.—Micronesia: Caroline Islands—Kusaie.
Characters.—Adult male: Head (except lores), neck back, rump, upper tail-coverts, chin, throat, breast, and upper abdomen black with feathers tipped with coloring between "scarlet" and "scarlet-red"; rest of feathering black; bill long and curved and black; feet black; iris dark brown.
| Subspecies | Number and sex |
Wing | Tail | Full Culmen |
Tarsus |
| M. c. rubratra |
21 adult males |
79 | 55 | 19.5 | 22 |
| (76-81) | (53-56) | (18.5-20.5) | (21-22) | ||
|
20 adult females |
71 | 49 | 18.5 | 20 | |
| (69-74) | (45-51) | (17.5-19.5) | (19-21) | ||
| M. c. dichromata |
24 adult males |
78 | 53 | 21.5 | 22 |
| (76-80) | (51-56) | (20.0-23.0) | (21-23) | ||
|
22 adult females |
69 | 47 | 19.0 | 20 | |
| (66-72) | (45-49) | (17.5-20.5) | (19-21) | ||
| M. c. major |
9 adult males |
77 | 55 | 20.0 | 22 |
| (75-78) | (54-59) | (19.5-20.5) | (21-22) | ||
|
2 adult females |
70 | 50 | 19.0, 20.5 | 21.5 | |
| M. c. saffordi |
47 adult males |
73 | 55 | 20.0 | 22 |
| (69-77) | (51-56) | (19.0-20.5) | (21-24) | ||
|
14 adult females |
65 | 49 | 18.5 | 21 | |
| (63-71) | (46-51) | (17.5-19.5) | (19-21) | ||
| M. c. kurodai |
2 adult males |
74, 75 | 52 | 20.0, 20.5 | 20, 21 |
| M. c. kobayashii |
17 adult males |
74 | 54 | 20.5 | 21 |
| (71-76) | (51-57) | (19.0-22.0) | (20-22) | ||
|
8 adult females |
67 | 48 | 18.0 | 20 | |
| (65-68) | (45-50) | (17.5-19.0) | (19-21) |
Adult female: Resembles adult male, but smaller; red coloring duller; wings and tail more brownish and less blackish; abdomen and under tailcoverts dark gray.
Immature: Resembles adult, but duller and less blackish and more grayish with less red coloring on feathers and an olivaceous-brown tinge to plumage.
Measurements.—Measurements are listed in table 48.
Specimens examined.—Total number, 62 (35 males, 27 females), as follows: Caroline Islands, USNM—Kusaie, 3 (Feb. 9); AMNH—Kusaie, 59 (Jan., Feb., March).
Nesting.—Finsch records the taking of eggs of the honey-eater at Kusaie on February 26 and March 10, 1880.
Molt.—Evidence of molt was observed in a few specimens taken in January and in larger number of birds taken in March. In addition, some skins obtained in March showed fresh plumage. Although there is little evidence available, I suppose that nesting activities of M. r. rubratra at Kusaie occur in the winter months of December, January, February, and March, and that molt begins in January, especially in the males, and possibly reaches a peak in March.
Remarks.—M. r. rubratra was first described by Lesson, who referred to it under the name Cinnyris rubrater. The bird was found by Lesson at Kusaie, when he visited the island in June, 1924, as a member of the expedition from the ship "La Coquille." In his description he also stated that the bird was found in the Philippines by Dussumier. The report of the bird's occurrence in the Philippines proved to be erroneous, as was pointed out by Wetmore (in Townsend and Wetmore, 1919:220). Oustalet (1895:200) contended that Lesson's description was based on the specimens taken by Quoy and Gaimard in the Marianas; he stated that none of the birds which Lesson mentions from Kusaie was preserved. Bonaparte also considered Cinnyris rubrater to be from the Marianas, and he gave the name Myzomela major to the honey-eater of the Caroline Islands (apparently including Kusaie) on the basis of specimens taken by Hombron and Jacquinot at Truk. Wetmore (in Townsend and Wetmore, 1919:220) settles the argument and assigns Lesson's name rubratra to the honey-eater at Kusaie; apparently this treatment is the correct one inasmuch as Lesson used his own field notes and records of the occurrence of this honey-eater at Kusaie in preparing his description, even if the actual specimens were not preserved. This arrangement makes Bonaparte's name major available for the population at Truk and makes Wetmore's name saffordi available for the population in the Marianas. The placing of the honey-eaters of Micronesia within the species Myzomela cardinalis by Mayr (1932:19) is, I think, justified.
Little information is available concerning the habits of the honeyeater at Kusaie. In 1931, Coultas (field notes) regarded the bird as [Pg 307] common in the lowlands, especially in the coconut groves. He did not find the bird at high elevations on the island.
Myzomela rubratra dichromata Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 220. (Type locality, Ponapé.)
Myzomela rubratra Pelzeln, Reise "Novara," Vögel, 1865, pp. 55, 162 (Puynipet = Ponapé); Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 26 (Ponapé); idem, Proc. Zool. Soc. London, 1877 (1878), p. 778 (Ponapé); Forbes (part), Proc. Zool., Soc. London, 1879, p. 271 (Ponapé); Finsch (part), Journ. f. Ornith., 1880, p. 285 (Ponapé); idem (part), Proc. Zool. Soc. London, 1880, p. 575 (Ponapé); idem (part), Ibis, 1881, pp. 111, 115 (Ponapé); idem (part), Mitth. Ornith. Ver. Wien, 1884, p. 48 (Ponapé); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 31 (Ponapé); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 202 (Ponapi).
Myzomela rubrata Nehrkorn (part), Journ. f. Ornith., 1879, p. 397 (Ponapé); Christian, The Caroline Islands, 1899, p. 358 (Ponapé); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Ponapé); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 55 Ponapé); Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé).
Myzomela chermesina Gadow, Cat. Birds British Mus., 9, 1884, p. 137 (Ponapé); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Ponapé).
Myzomela rubratra dichromata Momiyama, Birds Micronesia, 1922, pp. 15, 20, 21, 22 (Ponapé); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 73 (Ponapé); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 743 (Ponapé).
Myzomela rubratra rubratra Yamashina, Tori, 7, 1932, p. 395 (Ponapé); Hand-list Japanese Birds (part), rev., 1932, p. 172 (Ponapé); Hand-list Japanese Birds (part), 3d ed., 1942, p. 191 (Ponapé).
Myzomela cardinalis dichromata Mayr, Birds Southwest Pacific, 1945, p. 299 Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult male: Resembles adult males of M. c. rubratra, but with more extensive black markings on lores and below eye; tips of feathers lighter "scarlet."
Adult female: Resembles adult female of M. c. rubratra, but duller and with red coloring much reduced; head, neck, shoulder, ear-coverts, and sides of neck sooty brownish-gray; rest of upper parts dark brownish-gray with plumage of middle of back, rump, and upper tail-coverts tipped with scarlet; wings and tail dark brown with outer edges olivaceous-gray; chin and throat reddish; breast light brownish-gray, may be washed with reddish; axillaries, abdomen, and under tail-coverts grayish.
Immature male: Resembles adult male, but scarlet coloring less brilliant and thinner on forehead, middle of back, rump, upper tail-coverts, and underparts and absent, or nearly absent, on crown and neck.
Immature female: Resembles adult female, but scarlet coloring thinner and present only on underparts, back, rump, and upper tail-coverts; abdomen and under tail-coverts washed with buff.
Measurements.—Measurements are listed in table 48.
Specimens examined.—Total number, 52 (26 males, 24 females, 2 unsexed), as follows: Caroline Islands, USNM—Ponapé, 3 (Feb. 11, 12); AMNH—Ponapé, 49 (Nov., Dec.).
Nesting.—Yamashina (1932a:395) records a large collection of eggs of the honey-eater, taken at Ponapé in 1931. Of 13 sets of eggs listed, 10 include two eggs per set and 3 include one egg per set. These were obtained from July 20 to September 2. Coultas (field notes) found one nest with young in a tree-fern in the period of November and December, 1930. The nest was cup-shaped and made of fern and fine grasses and lined with lichens. Coultas writes that only the female feeds the young. He suspects that the honey-eater nests at all times of the year.
Molt.—Most of the birds taken by Coultas in November and December are in molting plumage.
Remarks.—The Cardinal Honey-eater at Ponapé is, according to Coultas, found in most habitats of the island. He found it to be an aggressive bird, often chasing the white-eye Zosterops cinerea. The committee (Hachisuka et al.) which prepared the Hand-list of Japanese Birds in both the revised edition (1932) and the third edition (1942) does not recognize the Ponapé honey-eater as separable from the bird at Kusaie. I see no reason for this action and find the bird at Ponapé to be a well-marked subspecies.
Myzomela major Bonaparte, Comptes Rendus Acad. Sci. Paris, 38, 1854, p. 264. (Type locality, "ex Ins. Carolinis ab Homb. et Jacq." = Truk.)
Myzomela major Gray, Hand-list Birds, 1, 1869, p. 153 (Caroline = Truk); Giebel, Thes. Ornith., 1875, p. 681 (Carolinae = Truk?); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Ruk); Kuroda, Dobutsu, Zasshi, 27, 1915, p. 28 (Ruk); idem, Dobutsu Zasshi, 28, 1916, p. 71 (Ruk).
Myzomela rubratra Finsch (part), Proc. Zool. Soc. London, 1880, p. 575 (Ruk); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 253 (Ruk); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 31 (Ruk); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 202 (Ruk); Hartert (part), Novit. Zool., 5, 1898, p. 56 (Ruk); idem (part), Novit. Zool., 7, 1900, p. 2 (Ruk); Dubois (part), Syn. Avium, 1, 1902, p. 714 (Carolines = Truk?).
Myzomela rubrata Matschie (part), Journ. f. Ornith., 1901, p. 112 (Ruck); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 55 (Ruk).
Myzomela rubratra rubrata Wetmore (part), in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 221 (Uala).
Myzomela rubrata wetmorei Momiyama, Birds Micronesia, 1922, p. 15 (Type locality, Ruk); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 73 (Ruk); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 743 (Ruk); Hand-list Japanese Birds, rev., 1932, p. 172 (Truk); Hand-list Japanese Birds, 3d ed., 1942, p. 190 (Truk).
Myzomela cardinalis major Mayr, Birds Southwest Pacific, 1945, p. 299 (Truk); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 72 (Truk).
Geographic range.—Micronesia: Caroline Islands—Truk.
Characters.—Adult male: Resembles adult male of M. c. rubrata, but tips of plumage lighter "scarlet."
Adult female: Resembles adult female of M. c. rubrata, but underparts more heavily tipped with scarlet; abdomen and under tail-coverts black; tail slightly darker. Differs from M. c. dichromata by presence of scarlet tips on feathers of head.
Immature male: Resembles adult female, but scarlet coloring of tips of feathers of head and neck narrower.
Immature female: Resembles immature female of M. c. rubrata, but upper parts grayer; underparts darker.
Measurements.—Measurements are listed in table 48.
Specimens examined.—Total number, 19 (13 males, 6 females), as follows: Caroline Islands, USNM—Truk, 2 (Feb. 16, Dec. 13); AMNH—Truk, 17 (Feb., March, Nov., Dec.).
Nesting.—Concerning the honey-eater at Truk, Hartert (1900:2) writes "many nests were found from end of May to July, and one in March." McElroy examined three males in December, which had swollen testes. As seems to be the case with other races of this species, the Cardinal Honey-eater at Truk may nest at all times of the year.
Molt.—Specimens examined that were taken in November, December and February are in fresh or in molting plumages.
Remarks.—Bonaparte described his Myzomela major as "Similis praecedenti, sed major et percoccinea." He compares it here with Myzomela rubrata, which he considered as a resident of the Mariana Islands. According to Oustalet (1895:202) Hombron and Jacquinot obtained one specimen of the honey-eater at Truk in 1841. This subspecies, as well as most of the others of M. cardinalis in Micronesia, is best distinguished by the characteristics of the female. The male of the different subspecies shows much less geographic variation.
Myzomela rubratra saffordi Wetmore, Proc. Biol. Soc. Washington, 30, 1917, p. 117. (Type locality, Guam.)
Cinnyris rubrater Lesson (part), Dict. Sci. Nat., éd. Levrault, 50, 1827, p. 30 (Mariannes); idem (part), Voy. "La Coquille," Zool., 2, 1828, p. 678 (Mariannes); idem (part), Man. d'Ornith., 2, 1828, p. 55 (Mariannes); idem (part), Traité d'Ornith., 1831, p. 299 (Mariannes); Kittlitz (part), Kupfertaf. Naturgesch. Vögel, 1, 1832. p. 6, pl. 8, fig. 1 (Guaham); idem (part), Denkw. Reise russ. Amer. Micron. und Kamchat., 1, 1858, pp. 364, 381; 2, 1858, pp. 39, 49 (Guaham).
Certhia cardinalis Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 304 (Guaham).
Myzomela rubrater Hartlaub (part), Archiv f. Naturgesch., 18, 1852, p. 109 (Mariannen); Finsch and Hartlaub (part), Fauna Centralpolynesiens, 1867, p. 57 (Guaham).
Myzomela rubratra Bonaparte, Comptes Rendus Acad. Sci. Paris, 38, 1854, p. 263 (Mariannes); Hartlaub (part), Journ. f. Ornith., 1854, p. 167 (Mariannen); Gray (part), Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 11 (Guam); idem (part), Handlist Birds, 1, 1869, p. 154 (Marian); Finsch (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 26 (Marianen); Forbes (part), Proc. Zool. Soc. London, 1879, p. 270 (Marianis); Giebel (part), Thes. Ornith., 2, 1875, p. 681 (Marinae); Finsch (part), Mitth. Ornith. Ver. Wien, 1884, p. 48 (Guam); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 31 (Marianne); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 197 (Guam, Rota, Saypan, Pagan, Agrigan); Hartert (part), Novit. Zool., 5, 1898, p. 55 (Guam, Saipan, Pagan, Agrigan); idem (part), Novit. Zool., 7, 1900, p. 2 (Guam); Wheeler, Report Island of Guam, 1900, p. 13 (Guam); Seale (part), Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 55 (Marianae); Safford, The Plant World, 7, 1904, p. 263 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Mearns, Proc. U. S. Nat. Mus., 36, 1909, p. 477 (Guam); Reichenow (part), Die Vögel, 2, 1914, p. 482 (Marianen); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 64 (Marianas); Cox, Island of Guam, 1917, p. 21 (Guam).
Myzomela rubrata Oustalet, Le Nat., 1889, p. 260 (Mariannes); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Guam, Saipan); Safford, Osprey, 1902, p. 69 (Guam); Prowazek, Die deutschen Marianen, 1913, p. 101 (Saipan).
Myzomela rubratra saffordi Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 221 (Guam, Saipan); Momiyama, Birds Micronesia, 1922, pp. 17, 20, 21, 22 (Guam, Rota, Saipan, Pagan, Agrigan); Kuroda in Momiyama, Birds Micronesia, 1922, p. 74 (Guam, Rota, Saipan, Pagan, Agrigan); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 744 (Guam); Yamashina, Tori, 7, 1932, p. 395 (Marianas?); Hand-list Japanese Birds, rev., 1932, p. 171 (Marianas); Bryan, Guam Rec., vol. 13, no. 2. 1936, p. 25 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 190 (Guam, Rota, Agiguan, Tinian, Saipan, Almagan, Pagan, Agrigan, Assongsong).
Myzomela rubrata saffordi Yamashina, Tori, 19, 1940, p. 673 (Assongsong, Agiguan).
Myzomela cardinalis saffordi Mayr, Birds Southwest Pacific, 1945, p. 299 (Marianas); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 103 (Tinian); Borror, Auk, 1947, p. 417 (Agrihan); Stott, Auk, 1947, p. 527 (Saipan, Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 72 (Guam, Rota).
Myzomela cardinalis Watson, The Raven, 17, 1946, p. 41 (Guam); Strophlet, Auk, 1946, p. 540 (Guam); Baker, Condor, 49, 1947, p. 125 (Guam).
Geographic range.—Micronesia: Mariana Islands—Guam, Rota, Tinian, Agiguan, Saipan, Almagan, Pagan, Agrihan, Asuncion.
Characters.—Adult male: Resembles M. c. rubratra, but smaller with red coloring lighter and more orange; edges of wing and tail feathers olivaceous. Differs from adult males of M. c. dichromata and M. c. major by smaller size and presence of olivaceous edgings on wing and tail feathers.
Adult female: Resembles adult female of M. c. rubratra, but smaller and paler with upper parts dark olivaceous-gray, sparsely mottled with scarlet; outer edges of wing and tail feathers greenish-olive; abdomen and under tail-coverts buffy-gray. Differs from M. c. dichromata by smaller size and presence of scarlet tips of feathers on top of head. Differs from M. c. major by smaller size and presence of broad olivaceous edges on tail feathers.
Immature male: Resembles adult male, but red coloring less brilliant, upper parts, lower breast, and abdomen more narrowly edged with the red coloring; plumage of breast, abdomen, and under tail-coverts buffy-gray, lighter in very young birds.
| Island | No. | Wing | Tail | Full culmen | Tarsus |
| Guam |
0_535 |
72 | 54 | 20.0 | 22 |
| (69-75) | (51-56) | (19.5-20.5) | (21-23) | ||
| Rota |
1 |
73 | 20.0 | 22 | |
| Tinian |
5 |
73 | 53 | 19.5 | 22 |
| (71-74) | (52-55) | (19.0-20.0) | (21-24) | ||
| Saipan |
4 |
74 | 54 | 19.5 | 22 |
| (72-76) | (53-55) | (19.0-20.5) | (22-23) | ||
| Agrihan |
1 |
77 | 55 | 20.0 | 22 |
Immature female: Resembles adult female, but paler with upper parts darker brown; underparts pale buffy-brown; outer edges of wing and tail. feathers greenish-olive, more extensive than in adult.
Measurements.—Measurements of the subspecies of M. cardinalis in Micronesia are listed in table 48. Measurements of male specimens of M. c. saffordi from various islands in the Marianas are listed in table 49.
Weights.—The author (1948:72) records weights of M. c. saffordi from Guam as: 17 adult males, 12.7-18.0 (15.0), and 5 adult females, 10.4-15.0 (12.7).
Specimens examined.—Total number, 80 (61 males, 17 females, 2 unsexed), as follows: Mariana Islands, USNM—Guam, 43 (Jan. 22, May 26, 30, June 2, 3, 5, 7, 8, 9, 13, 18, 19, 25, 28, July 6, 10, 12, 17, 19, 20, 21, Sept., Nov. 20, 21)—Rota, 2 (Oct. 10)—Tinian, 3 (Oct. 23, 25)—Saipan 2 (Sept. 27, 30); AMNH—Guam, 23 (Jan. 22, 23, Feb. 5, 7, 9, 16, March 8, 10, 11, 13, 23, June 28, July 8, 21, Aug. 22, Nov. 25, Dec. 4, 11)—Tinian, 2 (Sept. 7, 14)—Saipan, 3 (July 8, Aug. 5, 22)—Asuncion, 1 (June)—Agrihan, 1 (June).
Nesting.—Seale (1901:55) obtained nests and eggs in the period from May to July at Guam. He found the nests 8 to 15 feet above the ground. Strophlet (1946:540) observed a pair of honey-eaters with two young on October 9 at Guam. In 1945 at Guam the NAMRU2 party obtained individuals with enlarged gonads on January 22, June 2, 5, July 21 and 23, and found evidence of nesting on June 16. Hartert (1898:56) writes that Owston's Japanese collectors obtained nests in January, February, and March. Each nest contained two eggs; they were placed four to eight feet from the ground. Probably the Cardinal Honey-eater in the Marianas nests at most times of the year.
Molt.—Specimens, with molting plumage, have been examined that were taken at most times of the year. I suspect that this bird molts at irregular intervals.
Food habits.—The honey-eater feeds partly on insect life and partly on nectar and juices from flowers. At Guam, the honey-eater was frequently found at flowers of the ink berry bush, where evidently both nectar and insects were obtained. The birds were attracted also to the coconut palms, especially when the reproductive parts of the palms were developing.
Remarks.—The Cardinal Honey-eater is one of the most conspicuous land birds in the Mariana Islands. Its scarlet plumage and characteristic fluttering flight cause it to stand out against its habitat of forest, scrub, and garden. At Guam, the author (1947b:124) found the honey-eater on 37.6 percent of the 125 roadside birds counts made in 1945. The species included 3.9 percent of all of the birds observed on these counts. Seale (1901:55) and Strophlet (1946:540) also commented on its abundance at Guam; however, in 1931, Coultas (field notes) wrote that the bird was rare; he obtained only one skin at Guam. At Rota, the NAMRU2 party found the honey-eater to be abundant. Coultas obtained only a few birds at Tinian and Saipan in 1931. In 1945, Downs (1946:103) saw only a single pair at Tinian; Gleise (1945:220) estimated the population at Tinian to be 12 in 1945. At Agrihan, Borror (1947:417) reported that the honey-eater was a common bird in 1945.
Table 49 lists the measurements of males of M. c. saffordi from several islands in the Marianas. Measurements of birds from Guam, Rota, Tinian, and Saipan are fairly similar, although the birds at Saipan seem to have a slightly longer wing than those at Guam. A single skin from Agrigan has larger measurements than those of birds obtained in the southern Marianas. Whether the birds in the northern Marianas are separable because of larger size can only be ascertained by the studying of more material from that region.
Mayr (1945a:102) writes that males of M. cardinalis seem to outnumber the females by approximately four to one. On the basis of collections and field observations, the males were found to outnumber the females in the Micronesian islands; although the ratio may not be so great as four to one. At Guam, the NAMRU2 party obtained 21 males and 8 females. Although these birds are often seen as pairs (male and female), single males are frequently observed. The females do not appear to have more secretive habits than the males.
Myzomela rubratra kurodai Momiyama, Birds Micronesia, 1922, p. 17. (Type locality, Yap.)
Myzomela rubratra Hartlaub and Finsch (part), Proc. Soc. London, 1872, pp. 89, 94 (Uap); Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 122 (Yap); Finsch (part), Journ. Mus. Godeffroy, 8, 1875, p. 4 (Yap); Forbes (part), Proc. Zool. Soc. London, 1879, p. 271 (Yap); Wiglesworth (part), Abhandl. und. Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 31 (Uap); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 202 (Yap); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 64 (Yap); Kuroda, Dobutsu. Zasshi, 27, 1915, pp. 331, 332 (Yap).
Myzomela rubrata Matschie (part), Journ. f. Ornith., 1901, p. 112 (Yap).
Myzomela rubrata kurodai Kuroda, in Momiyama, Birds Micronesia, 1922, p. 74 (Yap); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 743 (Yap); Hand-list Japanese Birds (part), rev., 1932, p. 172 (Yap); Hand-list Japanese Birds (part), 3d ed., 1942, p. 190 (Yap).
Myzomela cardinalis kurodai Mayr, Birds Southwest Pacific, 1945, p. 299 (Yap).
Geographic range.—Micronesia: Caroline Islands—Yap.
Characters.—Adult male: According to Momiyama (1922:17), M. c. kurodai is "Similar to M. r. saffordi Wetmore from Southern Marianne islands, but the tarsus is decidedly shorter, not exceeding 21 mm. (more than 21 mm. in M. r. saffordi), and the colour of plumage is not so much tinged with vermillion. It differs from M. r. rubratra, M. r. dichromata, and M. r. wetmorei by the body measuring much shorter, and by the scarlet colour of plumage being less pronounced. The length of bill in M. r. wetmorei and kurodai is nearly the same."
Adult female: According to Momiyama (1922:17), "Upper-parts of body dark olivaceous brown; under-parts, including chin, throat and fore neck like upper-parts, but somewhat paler; breast and abdomen yellowish ashy-white; head, lower back, rump, upper tail-coverts, chin, throat as well as lower breast tinged with scarlet (the red colour more distinct on lower back but less so on lower breast); pale olive margin to the outer web of flight-feathers."
Measurements.—Measurements are listed in table 48.
Specimens examined.—Total number, 2 males, from Caroline Islands, AMNH—Yap (Sept.).
Remarks.—This subspecies is tentatively recognized as distinct from M. c. kobayashii of Palau. No female has been examined, and the two males seen and the description by Momiyama indicate that the population at Yap closely resembles the one at Palau. The Hand-list of Japanese Birds (Hachisuka et al., 1932:172) places the birds from Yap and Palau in the same subspecies.
Myzomela rubratra kobayashii Momiyama, Birds Micronesia, 1922, p. 19. (Type locality, Pelew Islands.)
Cinnyris rubrater Lesson (part), Dict. Sci. Nat., éd., Levrault, 50, 1827, p. 30 (Pelew); idem (part), Voy. "La Coquille," Zool., 1, 1828, p. 678 (Pelew); idem (part), Man. d'Ornith., 2, 1828, p. 55 (Pelew).
Myzomela rubratra Gray (part), Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 11 (Pelew); Hartlaub (part), Proc. Zool. Soc. London, 1867 (1868), p. 829 (Pelew); Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 5, 116, 118 (Pelew); Gray (part), Hand-list Birds, 1, 1869, p. 154 (Pelew); Hartlaub and Finsch (part), Proc. Zool. Soc. London, 1872, pp. 89, 94 (Pelew); Finsch (part), Journ. Mus. Godeffroy, 8, 1875, pp. 4, 16 (Palau); idem (part), Journ. Mus. Godeffroy, 12, 1876, pp. 17, 26 (Palau); Forbes (part), Proc. Zool. Soc. London, 1879, p. 270 (Pelew); Finsch (part), Mitth. Ornith. Ver. Wien, 1884, p. 48 (Palau); Gadow, Cat. Birds British Mus., 9, 1884, p. 129 (Pelew); Tristram, Cat. Birds, 1889, p. 206 (Pelew); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 31 (Pelew); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 202 (Palaos); Nehrkorn, Kat. Eiers., 1899, p. 79 (Palau-inseln); Seale (part), Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 57 (Pelew); Reichenow (part), Die Vögel, 2, 1914, p. 482 (Palau); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 64 (Pelew).
Myzomela rubratra Nehrkorn (part), Journ. f. Ornith., 1879, p. 397 (Palau); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Palau); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 55 (Pelew); Kuroda, Dobutsu. Zasshi, 28, 1916, p. 71 (Pelew).
Myzomela rubratra kobayshii Kuroda, in Momiyama, Birds Micronesia, 1922, p. 74 (Pelew); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 722 (Pelew).
Myzomela rubratra kurodai Hand-list Japanese Birds (part), rev., 1932, p. 172 (Palau); Hand-list Japanese Birds (part), 3d ed., 1942, p. 190 (Babelthuap, Koror, Peleliu).
Myzomela rubratra kurodai Yamashina, Tori, 10, 1940, p. 674 (Palau).
Myzomela cardinalis kobayashii Mayr, Birds Southwest Pacific, 1945, p. 299 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 72 (Peleliu).
Geographic range.—Micronesia: Palau Islands;—Babelthuap, Koror, Garakayo, Peleliu, Angaur.
Characters.—Adult male: Resembles M. c. rubratra, but smaller and with red coloring darker, near "scarlet-red"; margins of wing feathers olivaceous. Differs from adult males of other subspecies of M. cardinalis by red coloring of feathers being darker.
Adult female: Resembles adult female of M. c. dichromata but red coloring darker, top of head only partly red; abdomen, under tail-coverts, and axillaries buff-gray; outer edges of wing and tail feathers light olive. Differs from adult females of other subspecies of M. cardinalis by having top of head only partly red.
Immature male: Resembles adult male, but red coloring lighter and thinly distributed; wings and tail brownish-olive; abdomen and under tail-coverts grayish.
Immature female: Resembles adult female, but red coloring paler and underparts more buffy and less grayish.
Measurements.—Measurements are listed in table 48.
Specimens examined.—Total number, 42 (28 males, 11 females, 3 unsexed), as follows: Palau Islands, USNM—Koror, 4 (Nov.)—Peleliu, 11 (Aug. 29, 30, 31, Sept. 1, 5); AMNH—exact locality not given, 27 (Oct., Nov., Dec.).
Molt.—Many of the specimens taken from late August to December are in molt. Of the adult males obtained during this period almost a half had enlarged testes.
Food habits.—Stomachs of specimens obtained by the NAMRU2 party in August and September, 1945, contained vegetable matter, seeds and small insects.
Remarks.—Honey-eaters were found by the NAMRU2 party in open woodlands, in coconut groves and about human habitations. They were not seen in dense jungle areas, and appeared to prefer the plantation areas.
The Cardinal Honey-eater at Palau is distinguished from other subspecies of M. cardinalis in Micronesia by its deeper red coloring. In size, it closely resembles the bird at Yap and in the Marianas.
Evolutionary history of Myzomela cardinalis in Micronesia.—The genus Myzomela is found in Australia, northward to Timor, Tenimber, Moluccas, Celebes, Melanesia, Polynesia and Micronesia. The range of the species M. cardinalis includes the islands from the eastern Solomons, New Hebrides, and Loyalty Islands east to central Polynesia and north to Micronesia. It appears likely that M. cardinalis was derived, probably along with M. nigrita, M. lafargei and others, from an ancestral stock in the Melanesian area. Within the species M. cardinalis there is one group of subspecies which exhibits a marked degree of sexual dimorphism, with the males having a much greater amount of red coloration than the females. These subspecies occur in the southern part of the range of the species (Loyalty, Santa Cruz, New Hebrides, and Samoa islands). A second group of subspecies exhibit a lesser amount of sexual dimorphism, the females possessing more of the red coloration and resembling the males more closely. This second group includes subspecies which occur in the more northern part of the range of the species (Solomons, Micronesia, and Rotuma islands). The [Pg 315] males of the various subspecies of M. cardinalis vary one from another considerably less than do the females.
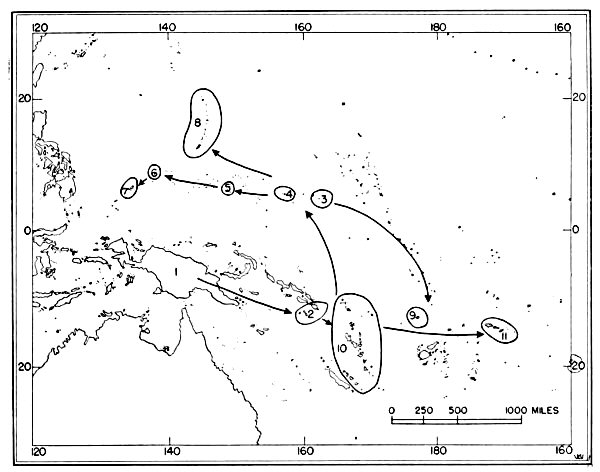 Fig.
16. Geographic distribution of Myzomela cardinalis and routes of its dispersal. (1) Probable center of dispersal of Myzomela; (2) ranges of M. c. sanfordi and
M. c. pulcherrima in the Solomon Islands; (3) M. c. rubratra; (4) M. c. dichromata; (5) M. c. major; (6) M. c. kurodai; (7) M. c. kobayashii; (8) M.
c. saffordi; (9) M. c. chermesina; (10) range of M. cardinalis in the Santa Cruz, New Hebrides, Banks and Loyalty islands; (11) M. c. nigriventris.
Fig.
16. Geographic distribution of Myzomela cardinalis and routes of its dispersal. (1) Probable center of dispersal of Myzomela; (2) ranges of M. c. sanfordi and
M. c. pulcherrima in the Solomon Islands; (3) M. c. rubratra; (4) M. c. dichromata; (5) M. c. major; (6) M. c. kurodai; (7) M. c. kobayashii; (8) M.
c. saffordi; (9) M. c. chermesina; (10) range of M. cardinalis in the Santa Cruz, New Hebrides, Banks and Loyalty islands; (11) M. c. nigriventris.Figure 16 shows the probable routes of colonization used by M. cardinalis to attain its present distribution in the Pacific islands. The subspecies in the eastern Solomon Islands (M. c. pulcherrima Ramsey and M. c. sanfordi Mayr) may be representative of the first colonization by the supposed ancestral stock. From a focal point in this area, M. cardinalis has dispersed by what may be considered as two routes. One route evidently was to the south as far as the Loyalty Islands with a side branch extending to the Samoan Islands where M. c. nigriventris Peale occurs. The second route extended north to the islands of Micronesia. The Caroline Islands were seemingly inhabited initially, with invasions of the Palaus made via Yap, and of the Marianas via Kusaie or Ponapé (as indicated by the comparison of specimens). Mayr (in conversation) has pointed out the close relationship between the subspecies in Micronesia and M. c. [Pg 316] chermesina Gray of Rotuma Island. This subspecies at Rotuma, which is located between Santa Cruz and Samoa, resembles closely M. c. dichromata of Ponapé, especially in the case of the female. It is evident that the honey-eater arrived at Rotuma from Micronesia, rather than from the Solomon and Santa Cruz area to the west.
Dicaeum conspicillatum Kittlitz, Kupfertaf. Naturgesch. Vögel, 2, 1833, p. 15, pl. 19, fig. 1. (Type locality, Guaham.)
Dicaeum conspicillatum Kittlitz, Mém. Acad. Imp. Sci. St. Pétersbourg, 2, 1835, p. 3, pl. 4 (Guaham); idem, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 305 (Guaham).
Zosterops conspicillatum Bonaparte, Consp. Avium, 1, 1850, p. 398 (Mariann. = Guam).
Zosterops conspicillata Reichenbach, Syn. Avium, 1852, p. 92 (Guaham); Hartlaub, Journ. f. Ornith., 1854, p. 187 (Mariannen = Guam); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 16 (Guam); Hartlaub, Journ. f. Ornith., 1865, pp. 5, 17 (Guaham); Gray, Hand-list Birds, 1, 1869, p. 163 (Ladrone = Guam); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 95 (Guaham); Giebel, Thes. Ornith., 3, 1877, p. 775 (Ladrone = Guam); Gadow, Cat. Birds British Mus., 9, 1884, p. 187 (Guam); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 37 (Guam); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 205 (Guam); Hartert (part), Novit. Zool., 5, 1898, p. 57 (Guam); Hartert, Novit. Zool., 7, 1900, p. 3 (Guam); Matschie (part), Journ. f. Ornith., 1901, pp. 112, 113 (Guam); Seale, Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 58 (Guam); Finsch (part), Das Tierreich, no. 15, 1901, p. 37 (Guam); Safford, Osprey, 1902, p. 69 (Guam); Dubois, Syn. Avium, 1, 1902, p. 711 (Guam); Safford, The Plant World, 7, 1904, p. 264 (Guam); idem, Contr. U. S. Nat. Herb., 9, 1905, p. 79 (Guam); Takatsukasa and Kuroda (part), Tori, 1, 1901, p. 64 (Marianne = Guam); Cox, Island of Guam, 1917, p. 21 (Guam); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 706 (Guam); Bryan, Guam. Rec., vol. 13, no. 2, 1936, p. 25 (Guam); Strophlet, Auk, 1948, p. 540 (Guam).
Zosterops conspicillatus Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 76 (Guam).
Zosterops conspicillata conspicillata Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 227 (Guam); Hand-list Japanese Birds, rev., 1932, p. 173 (Guam); Hand-list Japanese Birds, 3d ed., 1942, p. 192 (Guam); Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Guam); idem, Birds Southwest Pacific, 1945, p. 299 (Guam); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, pp. 72, 73 (Guam).
Geographic range.—Micronesia: Mariana Islands—Guam.
Characters.—Adult: A small white-eye with upper parts near "serpentine green," becoming slightly lighter on the rump; orbital ring broad and white; fronto-loral band light yellowish-white; auriculars grayish-green; chin and throat yellowish-white; breast and abdomen dingy yellow; wing and tail feathers dark brown with greenish-yellow edges; upper mandible horn colored, lower mandible lighter yellow; legs and feet dark olive-gray; iris light umber. Adult female may be lighter on underparts.
Immature: Resembles adult, but underparts paler yellow and upper mandible light yellowish-brown.
Measurements.—Measurements of Z. c. conspicillata are listed in table 50. Males and females have measurements which are nearly equal.
| Subspecies | No. | Wing | Tail | Full culmen | Tarsus |
| Z. c. conspicillata |
43 |
56 | 41 | 13.5 | 19 |
| (52-59) | (37-43) | (13.0-14.5) | (18-20) | ||
| Z. c. saypani |
29 |
52 | 38 | 12.5 | 18 |
| (50-55) | (35-40) | (12.0-13.5) | (17-19) | ||
| Z. c. rotensis |
3 |
53 | 42 | 13.0 | 18 |
| (51-55) | (42-43) | (13.0-13.5) | (18-19) | ||
| Z. c. semperi |
28 |
55 | 38 | 12.5 | 18 |
| (54-57) | (36-41) | (12.0-13.5) | (17-19) | ||
| Z. c. owstoni |
22 |
55 | 36 | 12.5 | 19 |
| (52-57) | (34-38) | (12.0-13.0) | (18-20) | ||
| Z. c. takatsukasai |
16 |
54 | 36 | 13.0 | 19 |
| (53-55) | (34-39) | (13.0-14.0) | (19-20) |
Weights.—The author (1948:73) records the weights of 11 adult males as 9.5-14.0 (10.5), of 3 adult females as 8.0-10.0 (9.3).
Specimens examined.—Total number, 61 (33 males, 17 females, 11 unsexed), as follows: Mariana Islands, USNM—Guam, 27 (May 24, 29, 30, June 2, 3, 25, 28, July 12, 18, 19, 20, 23, 26, Sept., Oct. 8); AMNH—Guam, 34 (Jan., March, July, Aug., Sept., Nov., Dec.).
Nesting.—Seale (1901:58) reports the taking of one nestling and three nests with eggs of the bridled white-eye at Guam in the period from May to July. The NAMRU2 party obtained little evidence of nesting in late May to July. Three males taken in the period of June and July had enlarged gonads. Hartert (1898:57) records several nests taken in February and March at Guam. He writes, "The nest is a fairly deep cup, placed in the fork of a branch, woven together of fine grasses and roots, and on the outside ornamented with cobwebs, wool and cottonwood, varying in width from 8 to 5 cm. The clutches consist of 2 or 3 eggs. The eggs are pale blue, like all Zosterops eggs. They measure 18:13, 17:13.2, 17:12.2, 15.5:12:5, 17:13.5, and between these measurements." Coultas obtained specimens with enlarged gonads in August. According to Oustalet (1895:207), Marche found nests and young in May or June.
Remarks.—Kittlitz obtained the Bridled White-eye at Guam, when he visited the island, in March, 1828. He found the birds common and they reminded him of titmice. Marche obtained a series of 21 skins at Guam in August and September, 1887, and in February and March, 1888. Seale (1901:58) observed the birds in flocks of 10 to 20 in roadside bushes and in waste areas. He mentions that their principal foods are insects. The NAMRU2 party found the birds to be restricted to certain areas on Guam, where they were found in small flocks moving about in low trees. They [Pg 318] were taken at only five localities, two of these being at the northern end of the island in vegetation along the high, coastal cliffs. The other localities were in the central part of the island in low trees in the uplands. Strophlet (1946:540) found them in grasslands on the foothills. Arvey (field notes) saw a flock of 12 white-eyes at Mount Tenjo in July, 1946.
The white-eye is a very active bird, always moving rapidly through the vegetation or flying across open areas to disappear into scrub foliage. As they move about they make a twittering sound, which is considered to be a flocking call.
Zosterops conspicillata Saypani Dubois, Syn. Avium, 1, 1902, p. 711. (Type locality, Saypan.)
Zosterops conspicillata Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 205 (Saypan); Hartert (part), Novit. Zool., 5, 1898, p. 57 (Saipan); Finsch (part), Das Tierreich, no. 15, 1901, p. 37 (Saipan); Matschie (part), Journ. f. Ornith., 1901, pp. 112, 113 (Saipan); Prowazek, Die deutschen Marianen, 1913, p. 101 (Saipan); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 64 (Marianne = Saipan).
Zosterops conspicillata var. saypani Snouckaert, Alauda, (2), 3, 1931, p. 22 (Saypan).
Zosterops conspicillatus Kuroda (part), in Momiyama, Birds Micronesia, 1922; p. 76 (Saipan).
Zosterops saipani Mathews, Syst. Avium Australasianarum, 2, 1930, p. 706 (Saipan).
Zosterops conspicillata saipani Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 227 (Saipan); Hand-list Japanese Birds, rev., 1932, p. 173 (Saipan, Tinian); Hand-list Japanese Birds, 3d ed., 1942, p. 192 (Saipan, Tinian); Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Tinian, Saipan); idem, Birds Southwest Pacific, 1945, p. 299 (Saipan, Tinian); Downs, Trans. Kansas Acad. Sci., 49, 1946, p. 104 (Tinian); Stott, Auk, 64, 1947, p. 527 (Saipan); Baker, Smithson, Misc. Coll., vol. 107, no. 15, 1948, p. 73 (Saipan, Tinian).
Zosterops conspicillatus saipani Yamashina, Tori, 7, 1932, p. 398 (Tinian).
Geographic range.—Micronesia: Mariana Islands—Tinian, Saipan.
Characters.—Adult: Resembles Z. c. conspicillata, but slightly smaller with fronto-loral band more greenish yellow; auriculars olivaceous; orbital ring narrower; upper parts brighter olive; underparts pale yellowish-white; bill darker. Birds from Saipan resemble closely birds from Tinian, but upper parts may be slightly brighter and underparts slightly more yellowish; iris chestnut.
Measurements.—Measurements are listed in table 50. Twenty-three birds from Tinian measure: wing, 51 (50-53); tail, 38 (35-41); full culmen, 12.0 (12.0-13.0); tarsus, 18 (17-18); six birds from Saipan measure: wing, 54 (52-55); tail, 37 (35-39); full culmen, 13.0 (13.0-15.0); tarsus, 18 (17-19). Birds from Saipan are slightly larger than birds from Tinian.
Specimens examined.—Total number, 33 (18 males, 13 females, 2 unsexed), as follows: Mariana Islands, USNM—7 (Oct. 7, 8, 9, 10, 23); AMNH—26 (July, Aug., Sept.).
Nesting.—Yamashina (1932a:398) records the taking of three nests of the Bridled White-eye at Tinian on January 8, 1932. The nests contained one, two, and three eggs, respectively. The color of the eggs is uniformly pale blue; the nests were situated two to four meters from the ground. Oustalet (1895:207) writes that Marche obtained records of nesting at Saipan in the period from May to July. Of 18 birds taken by Coultas at Tinian in September, 1931, one-half of them had enlarged gonads.
Molt.—Specimens examined that were taken in July, August, September, and October have molting plumage.
Remarks.—Marche obtained the first skins of this white-eye at Saipan; he got 23 specimens in May, June, and July, 1887. The population at Saipan was initially considered similar to that at Guam; it was later given subspecific separation by Dubois. The birds at Tinian exhibit some differences from the birds at Saipan, and it is possible that these two populations should be regarded as subspecifically distinct from one another.
In 1931, Coultas (field notes) found this white-eye common at Saipan and Tinian. He writes "The little fellow has adjusted himself to the gardens and shrubs in the villages. He is a seed eater and makes himself at home now around human habitation. I have seen him climbing over potted plants on the window ledges of dwellings. His cheerful little sibilation uttered continuously while at work or while on the wing makes him friends wherever he goes. He is no longer a bird of the forest as he has none here to go to." Several observers in the late war have published notes on this white-eye. Stott (1947:527) writes that he was reminded of the bush-tit (Psaltriparus) when he observed the behavior of this white-eye; Moran (1946:262) writes that it is "Similar in size and behavior to our vireos." Gleise (1945:220) estimated the population of white-eyes at Tinian at 500 plus in 1945. Downs (1946:104-105) found the birds to be abundant at Tinian; he found them in small flocks in low brush or trees and at edges of open fields as well as elsewhere. He saw a white-eye eating "a large green fuzzy caterpillar."
Zosterops semperi rotensis Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 486. (Type locality, Rota.)
Zosterops semperi Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 207 (Rota); Hartert (part), Novit. Zool., 5, 1898, p. 57 (Rota); Finsch (part), Das Tierreich, no. 15, 1901, p. 30 (Rota); Seale (part), Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 58 (Rota); Dubois (part), Syn. Avium, 1, 1902, p. 710 (Rota); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 64 (Marianne = Rota).
Zosterops semperi semperi Momiyama (part), Birds Micronesia, 1922, p. 23 (Rota); Kuroda, (part) in Momiyama, Birds Micronesia, 1922, p. 75 (Rota).
Zosterops semperi rotensis Snouckaert, Alauda. (2), 4, 1932, p. 459 (Rota); Yamashina, Tori, 7, 1932, p. 399 (Rota); Hand-list Japanese Birds, rev., 1932, p. 173 (Rota).
Zosterops conspicillata rotensis Hand-list Japanese Birds, 3d ed., 1942, p. 193 (Rota); Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Rota); idem, Birds Southwest Pacific, 1945, p. 299 (Rota); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 73 (Rota).
Geographic range.—Micronesia: Mariana Islands—Rota.
Characters.—Adult: Upper parts and sides of neck between "warbler green" and "pyrite yellow" becoming lighter on the rump; auriculars light yellowish-green; orbital ring white; fronto-loral band narrowly tinged with yellow; underparts dingy yellow; wing and tail feathers dark with light greenish-yellow edges; upper mandible light brown; lower mandible light yellowish-brown; feet light brown.
Resembles Z. c. conspicillata, but brighter greenish-yellow above; chin and throat yellow like rest of underparts; fronto-loral band tinged with bright yellow; auriculars resemble closely the upper parts in color; narrow orbital ring.
Measurements.—Measurements are listed in table 50.
Specimens examined.—Total number, 5 (3 males, 1 female, 1 unsexed), from Mariana Islands, USNM—Rota (Oct. 18, 20, 22).
Nesting.—Yamashina (1932a:399) records the taking of one nest containing two eggs at Rota on March 7, 1931.
Molt.—Specimens taken in October were in molt.
Remarks.—Oustalet (1895:207) reported on two specimens of white-eye taken at Rota by Marche. He considered them as being similar to the birds at Palau. The birds at Rota were named as a separate subspecies by Takatsukasa and Yamashina in 1931. The NAMRU2 party found the birds to be numerous at Rota in October, 1945.
Zosterops semperi Hartlaub, in Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 117. (Type locality, Pelew Islands.)
Zosterops semperi Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 95 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 16, pl. 4, fig. 1 (Palau); Giebel, Thes. Ornith., 3, 1877, p. 777 (Pelew); Nehrkorn, Journ. f. Ornith., 1879, p. 396 (Palau); Finsch (part), Journ. f. Ornith., 1880, p. 286 (Palau); idem (part), Ibis, 1881, p. 111 (Pelew); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Finsch (part), Mitth. Ornith. Ver. Wien, 1884, p. 48 (Palau); Gadow (part), Cat. Birds British Mus., 9, 1884, p. 183 (Pelew); Tristram, Cat. Birds, 1889, p. 212 (Pelew); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 37 (Pelew); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 208 (Palaos); Hartert (part), Novit. Zool., 5, 1898, p. 57 (Pelew); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Palau); Finsch (part), Das Tierreich, no. 15, 1901, p. 30 (Palau); Seale (part), Occ. Papers Bernice P. Bishop Mus., 1, 1901, p. 58 (Pelew); Dubois (part), Syn. Avium, 1, 1902, p. 710 (Palau); Takatsukasa and Kuroda (part), Tori, 1, 1915, pp. 55, 64 (Pelew).
Zosterops semperi semperi Hartert, Novit. Zool., 7, 1900, p. 2 (Pelew); Momiyama (part), Birds Micronesia, 1922, pp. 22, 23 (Pelew); Kuroda (part), in Momiyama, Birds Micronesia, 1922, p. 75 (Pelew); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 705 (Pelew); Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 486 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 174 (Palau).
Zosterops conspicillata semperi Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 227 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 193 (Babelthuap, Koror, Peliliu); Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Palau); Mayr, Birds Southwest Pacific, 1945, p. 299 (Palau); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1945, p. 73 (Garakayo).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Peleliu.
Characters.—Adult: Resembles adult of Z. c. rotensis, but fronto-loral band lighter yellow, and coloring is usually not continuous above; auriculars paler; breast and abdomen paler yellow; maxilla and feet darker; mandible whitish. Resembles adult of Z. c. conspicillata, but brighter greenish-yellow above; coloring of chin and throat like that of rest of underparts; auriculars colored like back; fronto-loral band narrowly tinged with bright yellow and not completely connected above; orbital ring narrow; iris grayish-white.
Measurements.—Measurements are listed in table 50.
Specimens examined.—Total number, 30 (15 males, 14 females, 1 unsexed), as follows: Palau Islands, USNM—Babelthuap, 2 (Nov. 27)—Koror, 4 (Nov. 14, 19)—Garakayo, 4 (Sept. 18, 19); AMNH—exact locality not given, 20 (Oct., Nov., Dec.).
Molt.—All birds examined (taken in September, October, and November) are in molting plumage.
Food habits.—At Garakayo, birds were observed in small flocks feeding in low trees. Two stomachs examined, which were from individuals of these flocks, contained very small seeds.
Remarks.—Oustalet (1895:207) first pointed out the relationship between the Bridled White-eye at Palau and the one at Rota. Hartert (1898:57) thought that the occurrence of the same kind of bird at Palau and at Rota was "very peculiar." It was not until 1931 that Takatsukasa and Yamashina separated the two populations by name.
Coultas (field notes) found the Bridled White-eye to be uncommon in the Palaus in 1931. He observed them in the tops of trees, noting that they were wary and easily frightened away by the shooting of a gun. Coultas writes that he found the birds to be numerous at Peleliu; in 1945, the NAMRU2 party did not find the birds at that island. The only locality where they were found to occur was on the small island of Garakayo where the writer shot four Bridled White-eyes on September 18 and 19. He found two or three small flocks in low trees near the summit of a hill on the island. Approximately 25 birds were in this area.
Zosterops semperi owstoni Hartert, Novit., Zool., 7, 1900, p. 2. (Type locality, Ruk.)
Zosterops semperi semperi Finsch (part), Journ. f. Ornith., 1880, p. 287 (Ruck); idem (part), Proc. Zool. Soc. London, 1880, p. 575 (Ruk); idem (part), Ibis, 1881, p. 110 (Ruk); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, p. 353 (Ruk); Gadow (part), Cat. Birds British Mus., 9, 1884, p. 183 (Central Carolines=Truk); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 37 (Ruk); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 208 (Ruk); Hartert (part), Novit. Zool., 5, 1898, p. 57 (Ruk); Nehrkorn, Kat. Eiers, 1899, p. 80 (Ruk).
Zosterops semperi owstoni Dubois, Syn. Avium, 1, 1902, p. 710 (Ruk); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 223 (Truk); Momiyama, Birds Micronesia, 1922, p. 24 (Ruk); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 75 (Ruk); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 705 (Ruk); Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 496 (Ruk); Yamashina, Tori, 7, 1932, p. 400 (Truk); Hand-list Japanese Birds, rev., 1932, p. 174 (Truk).
Zosterops owstoni Finsch, Das Tierreich, no. 15, 1901, p. 31 (Ruk); Matschie (part), Journ. f. Ornith., 1901, pp. 112, 113 (Ruck); Reichenow, Die Vögel, 2, 1914, p. 470 (Karolinen = Truk); Takatsukasa and Kuroda, Tori, 1, 1915, pp. 55, 64 (Ruk).
Zosterops conspicillata owstoni Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 277 (Truk); Hand-list Japanese Birds, 3d ed., 1942, p. 193 (Truk); Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Truk); idem, Birds Southwest Pacific, 1945, p. 299 (Truk); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, pp. 73, 74 (Truk).
Geographic range.—Micronesia: Caroline Islands—Truk.
Characters.—Adult: Resembles adult of Z. c. semperi, but upper parts darker olive and less yellowish-green; fronto-loral band deeper yellow; auriculars slightly darker; black line on lores and under eye more distinct; underparts deeper yellow; abdomen with greenish tinges. Resembles adult of Z. c. rotensis, but upper parts duller, more green and less yellow; fronto-loral band lighter and less distinct, coloring near that of Z. c. semperi; auriculars darker green; underparts slightly darker, more olive-green and less yellow.
Measurements.—Measurements are listed in table 50.
Specimens examined.—Total number, 23 (12 males, 10 females, 1 unsexed), as follows: Caroline Islands, USNM—Truk, 3 (Feb. 16); AMNH—Truk, 20 (Feb., March, May, Nov.).
Nesting.—Yamashina (1932a:400) records the taking of a nest with one egg at Truk in May. Hartert (1900:2) records nests containing single eggs taken at Truk from May to July. Nests were found in bushes and trees four to eight feet above the ground. The eggs are pale blue. He gives measurements of seven eggs.
Remarks.—Kubary obtained the first specimens of the Bridled White-eye at Truk. Hartert described the population as a new subspecies using material taken by Owston's collectors. The bird was named in honor of Alan Owston. McElroy of the NAMRU2 party visited Truk in December, 1945. He found this white-eye in the mountainous areas at Moen and Udot islands.
Zosterops semperi takatsukasai Momiyama, Birds Micronesia, 1922, p. 22. (Type locality, Ponapé.)
Zosterops semperi (part), Finsch, Journ. f. Ornith., 1880, p. 286 (Ponapé); idem (part), Proc. Zool. Soc. London, 1880, p. 575 (Ponapé); idem (part), Ibis, 1881, p. 115 (Ponapé); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, p. 281 (Ponapé); Finsch (part), Mitth. Ornith. Ver. Wien, 1884, p. 48 (Ponapé); Gadow (part), Cat. Birds British Mus., 9, 1884, p. 183 (Central Carolines, Ponapé); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 37 (Ponapé); Oustalet (part), Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 208 (Ponapé); Finsch, Das Tierreich, no. 15, 1901, p. 30 (Ponapé); Dubois (part), Syn. Avium, 1, 1902, p. 710 (Ponapé); Takatsukasa and Kuroda (part), Tori, 1, 1915, pp. 55, 64 (Ponapé).
Zosterops owstoni Matschie (part), Journ. f. Ornith., 1901, pp. 112, 113 (Ponapé).
Zosterops semperi takatsukasai Kuroda, in Momiyama, Birds Micronesia, 1922, p. 76 (Ponapé); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 705 (Ponapé); Snouchaert, Alauda, (2), 3, 1931, p. 22 (Ponapé); Takatsukasa and Yamashina, Tori, 7, 1932, p. 400 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 174 (Ponapé).
Zosterops conspicillata takatsukasai Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 227 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 193 (Ponapé); Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Ponapé); idem, Birds Southwest Pacific, 1945, p. 299 (Ponapé); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 73 (Ponapé).
Zosterops conspicillata Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult: Resembles adult of Z. c. semperi, but slightly smaller with fronto-loral area more sulfur-yellow; underparts brighter, especially the coloring of the abdomen and under tail-coverts; iris light chestnut.
Measurements.—Measurements are listed in table 50.
Specimens examined.—Total number, 20 (10 males, 9 females, 1 unsexed) from Caroline Islands, AMNH—Ponapé (Nov., Dec.).
Nesting.—Yamashina (1932a:400) records nests and eggs of Z. c. takatsukasai. The nests, each containing a single egg, were taken on July 10 and 20, 1931. Coultas (field notes) writes that the nest consists of a small, cup-shaped structure of grasses and hair. The natives told him that two eggs were laid. In birds taken by Coultas in November the gonads were beginning to enlarge; specimens taken in December had swollen gonads. From the evidence at hand, it would appear that the Bridled White-eye at Ponapé breeds at two periods of the year, the winter and the summer.
Molt.—Specimens examined, which were taken by Coultas in November and December, are in fresh plumage.
Remarks.—In 1931, Coultas (field notes) found this white-eye to be rare at Ponapé. He obtained almost every one that he saw to get his series of 20 specimens. He found the birds usually in pairs around yellow-flowering bushy trees. A specimen taken by Richards had "small insects" in its stomach.
Zosterops hypolais Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 95. (Type locality, Uap.)
Zosterops hypolais Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 122 (Yap); Giebel, Thes. Ornith., 3, 1877, p. 776 (Carolinae=Yap); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 391 (Yap); Gadow. Cat. Birds British Mus., 9, 1884, p. 186 (Uap); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 37 (Uap); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 208 (Uap); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 60 (Yap); Finsch, Das Tierreich, no. 15, 1901, p. 24 (Yap); Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Yap); Dubois, Syn. Avium, 1, 1902, p. 708 (Uap); Reichenow, Die Vögel, 2, 1914, p. 469 (Karolinen=Yap); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Mackenzie=Yap); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 76 (Yap); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 700 (Yap); Hand-list Japanese Birds, 3d ed., 1942, p. 192 (Yap).
Zosterops conspicillata hypolais Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 227 (Yap); Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Yap); idem, Birds Southwest Pacific, 1945, p. 299 (Yap).
Zosterops hypolais Hand-list Japanese Birds, rev., 1932, p. 173 (Yap).
Geographic range.—Micronesia: Caroline Islands—Yap.
Characters.—According to Hartlaub and Finsch (1872:95), "Upper parts of a pale greyish green, throat and under tail-coverts a pure but very pale whitish-yellow; breast and abdomen of a mixed pale grey and pale yellow; wing- and tail-feathers pale blackish, margined with greenish colour of the back; under wing-coverts and inner margins of remiges white; eye-ring indistinct; beak fuscous, the under mandible paler, except at the tip; feet plumbeous."
Remarks.—No specimen has been examined by me. I am following Stresemann (1931:227) in placing the Bridled White-eye at Yap as a subspecies of Z. conspicillata. This is one arrangement; the committee who prepared the Hand-list of Japanese Birds (1942:192) treat this bird as a separate species. The Japanese probably have more specimens of this bird than anyone else and may be in a better position to judge its taxonomic status. Specimens of this white-eye were taken by Fisher in 1946 at Yap. His report (soon to be published) may throw additional light on the degree of distinctness of Z. c. hypolais. On the basis of published descriptions it is evident that Z. c. hypolais has a few characters in common with other members of the species.
Evolutionary history of Zosterops conspicillata.—The small olive-green and yellow white-eyes of Micronesia have been considered as belonging to several species by authors in the past. As late as 1930, Mathews (1930; 700, 706) placed them in four species. Stresemann (1931a: 227) put them all in the species Z. conspicillata, an arrangement which is being followed in this report. It is evident, however, that these subspecies of Z. conspicillata can be associated into three groups. The author (1948:73) states that Z. c. conspicillata and Z. c. saypani have pale chins and throats, light fronto-loral bands, blackish coloring at the bend of the wings and broad, white orbital rings. Another group, Z. c. rotensis, Z. c. semperi, Z. c. owstoni, and Z. c. takatsukasai, have bright yellow chins and throats, matching the rest of the underparts, obscure fronto-loral bands, which are narrowly tinged with yellow, yellowish coloring at the bend of the wings, and narrow, white orbital rings. Z. c. hypolais apparently falls into a third group by itself, as indicated by the published descriptions. There is apparently some variation in the color of the eyes of these subspecies; they may be either whitish or chestnut in color. The data are insufficient to determine the significance of this color character.
Z. conspicillata is restricted to Micronesia and appears to have little close relationship to other species of the genus. Z. conspicillata [Pg 325] shows little affinity to white-eyes to the north and northwest of Micronesia belonging to the species Z. japonica, of which representatives are found in the Bonin and Volcano islands. Z. conspicillata shows greater affinity to species found to the west and to the south of Micronesia.
It may have colonized Micronesia from the south or southeast (Polynesia), even though the species is absent at Kusaie; however, Z. conspicillata shows more relationships to species now living to the westward and the southwestward, and it probably invaded Micronesia from some place in that direction. Z. conspicillata differs from species found in Melanesia and Malaysia chiefly in color of the forehead, lores, fronto-loral band, crown, nape, breast, abdomen, orbital ring, and bill. Also there are differences in the breadth of the orbital ring.
Z. conspicillata shows evidence of relationships with Z. nigrorum of the Philippines and Z. montanus of the Philippines and other parts of Malaysia. Z. nigrorum resembles Z. c. semperi of Palau in size, but is brighter yellow-green above with a darker and less curved bill and brighter underparts. The fronto-loral band and the lores are colored the same in Z. nigrorum and Z. c. semperi. Z. montanus resembles Z. conspicillata especially in size and in shape of the bill. Z. lutea intermedia of the Makassar area shows some affinity to Z. conspicillata, although the bill is heavier. The Micronesia species also bears a close resemblance to Z. griseotincta of the Papuan region. This is especially true of Z. c. takatsukasai at Ponapé; however, Z. griseotincta has a heavier and larger bill. Z. lateralis from southern Melanesia and Australia is not very different from Z. conspicillata aside from its grayish and brownish coloring.
Z. conspicillata probably was derived from an ancestral stock which came to Micronesia from the Philippine or Moluccan area, rather than directly from Melanesia. Z. conspicillata seemingly shows the closest resemblance to Z. nigrorum or to some of its relatives in the Australo-Moluccan area. The subspecies at Palau, Z. c. semperi, appears to be the connecting link. Whether the form at Yap represents an independent colonization is not known; such might also be true in the case of the subspecies at Guam and at Saipan and Tinian. If these are considered as separate colonizations, then the populations can be regarded as separate species. Mayr, (in conversation) has pointed out the affinity of the white-eye at Samoa, Z. samoensis, with Z. conspicillata and suggests that Z. samoensis is derived from the Micronesian species.
Drepanis cinerea Kittlitz, Kupfertaf. Naturgesch. Vögel, 1, 1832, p. 6, pl. 8, fig. 2. (Type locality, Ualan = Kusaie.)
Drepanis cinerea Kittlitz, Mém. Acad. Imp. Sci., St. Pétersbourg, 2, 1835, p. 4, pl. 5 (Ualan); idem, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 285 (Ualan); Reichenbach, Syn. Avium, 1853, p. 242 (Ualan); Kittlitz, Denkw. Reise, russ. Amer. Micron. und Kamchat., 1, 1858, p. 367 (Ualan).
Zosterops cinerea Hartlaub, Archiv f. Naturgesch., 18, 1852, p. 131 (Ualan); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 16 (Oualan); idem, Hand-list Birds, 1, 1869, p. 163 (Caroline = Kusaie); Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 96 (Ualan); Finsch, Journ. Mus. Godeffroy, 12, 1876, p. 27 (Ualan); idem, Ibis, 1881, pp. 107, 108 (Kuschai); Gadow, Cat. Birds British Mus., 9, 1884, p. 198 (Kushai); Tristram, Cat. Birds, 1889, p. 210 (Kuschai); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 36 (Ualan); Hartert, Kat. Vogelsamml., Senckenb., 1891, p. 31 (Ualan); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 208 (Oualan); Finsch, Das Tierreich, no. 15, 1901, p. 45 (Kusaie); Dubois, Syn. Avium, 1, 1902, p. 713 (Kusaie); Takatsukasa and Kuroda, Tori, 1, 1915, pp. 55, 64 (Kusaie); Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 224 (Kusaie); Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 230 (Kusaie); Hand-list Japanese Birds, rev., 1932, p. 173 (Kusaie); Hand-list Japanese Birds, 3d ed., 1942, p. 192 (Kusaie).
Dicaeum cinereum Hartlaub, Journ. f. Ornith., 1854, p. 168 (Carolinen = Kusaie).
Zosterops cinereus Finsch, Journ. Mus. Godeffroy, 8, 1875, p. 17 (Ualan); idem, Journ. f. Ornith., 1880, pp. 286, 297, 300 (Kuschai); idem, Mitth. Ornith. Ver. Wien, 1884, p. 48 (Kuschai).
Zosterops Kittlitzi Finsch, Journ. f. Ornith., 1880, p. 300 (Type locality, Kusaie); Reichenow and Schalow, Journ. f. Ornith., 1881, p. 94 (Kusaie?).
Tephras cinereus Matschie, Journ. f. Ornith., 1901, pp. 111, 112, 113 (Ualan).
Tephras cinerea Kuroda, in Momiyama, Birds Micronesia, 1922, p. 77 (Kusaie); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 712 (Oualan).
Zosterops cinerea cinerea Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Kusaie?); idem, Birds Southwest Pacific, 1945, p. 300 (Kusaie).
Geographic range.—Micronesia: Caroline Islands—Kusaie.
Characters.—Adult: A small, dusky white-eye with upper parts smoky olivaceous-gray; lores dingy white; auriculars brownish; no white orbital ring; wing and tail feathers dark brownish-gray with paler greenish-gray outer edges; underparts pale ashy-gray, chin lighter, flanks darker; bill black; feet light brown; iris brown.
Measurements.—Measurements of Z. cinerea are listed in table 51. Males and females have approximately equal measurements.
| Subspecies | No. | Wing | Tail | Culmen | Tarsus |
| Z. c. cinerea |
47 |
63 | 37 | 15.0 | 20 |
| (60-65) | (35-39) | (14.0-16.5) | (19-20) | ||
| Z. c. ponapensis |
38 |
59 | 38 | 13.5 | 20 |
| (57-61) | (36-40) | (13.0-14.5) | (18-21) | ||
| Z. c. finschii |
30 |
65 | 43 | 17.5 | 21 |
| (63-67) | (40-46) | (16.0-18.5) | (20-23) |
Specimens examined.—Total number, 50 (33 males, 17 females), as follows: Caroline Islands, USNM—Kusaie, 1 (Feb. 9); AMNH—Kusaie, 49 (Jan., Feb., March).
Nesting.—Coultas found that approximately one-half of the males which he obtained in March, 1931, had swollen gonads.
Molt.—Many of the birds obtained in January and February were molting, and many of those obtained in March were in fresh plumage.
Remarks.—Coultas obtained a large series of these birds at Kusaie in 1931, where he found them to be common.
Zosterops ponapensis Finsch, Proc. Zool. Soc. London, 1875 (1876), p. 643. (Type locality, Ponapé.)
Zosterops ponapensis Finsch, Journ. Mus. Godeffroy, 12, 1876, pp. 17, 27, pl. 2, fig. 1 (Ponapé); idem., Proc. Zool. Soc. London, 1877 (1878), p. 778 (Ponapé); Nehrkorn, Journ. Mus. Godeffroy, 1879, p. 396 (Ponapé?); Finsch, Journ. f. Ornith., 1880, pp. 286, 300 (Ponapé); idem, Ibis. 1881, pp. 110, 111, 115 (Ponapé); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 281 (Ponapé); Finsch, Mitth. Ornith. Ver. Wien, 1884, p. 48 (Ponapé); Gadow, Cat. Birds British Mus., 9, 1884, p. 198 (Ponapé); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 36 (Ponapé); Bolau, Mitteil. Naturhist. Mus. Hamburg. 1898, p. 60 (Ponapé); Nehrkorn, Kat. Eiers., 1899, p. 80 (Ponapé); Finsch, Das Tierreich, no. 15, 1901, p. 46 (Ponapé); Dubois, Syn. Avium, 1, 1902, p. 713 (Ponapé); Reichenow, Die Vögel, 2, 1914, p. 470 (Ponapé); Takatsukasa and Kuroda, Tori, 1, 1915, pp. 55, 65 (Ponapé); Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 230 (Ponapè); Yamashina, Tori, 7, 1932, p. 397 (Ponapé); Hand-list Japanese Birds, rev., 1932, p. 173 (Ponapé); Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 192 (Ponapé).
Tephras ponapensis Matschie, Journ. f. Ornith., 1901, pp. 111, 112, 113 (Ponapé); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 77 (Ponapé); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 712 (Ponapé).
Zosterops ponapenensis Wetmore, in Townsend and Wetmore, Bull. Mus. Comp. Zoöl., 63, 1919, p. 224 (Ponapé).
Zosterops cinerea ponapensis Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Ponapé?); idem, Birds Southwest Pacific, 1945, p. 300 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult: Resembles adult of Z. c. cinerea, but smaller with upper parts umber-brown, forehead pale gray; underparts mostly pale gray, sides of breast and abdomen brownish-buff; under tail-coverts pale buffy-gray.
Measurements.—Measurements are listed in table 51.
Specimens examined.—Total number, 47 (28 males, 17 females, 2 unsexed), as follows: Caroline Islands, USNM—Ponapé, 1 (Feb. 11); AMNH—Ponapé, 46 (Nov., Dec.).
Nesting.—Yamashina (1931a:397-398) describes two nests of Z. c. ponapensis, each containing one egg. These were taken at Ponapé on August 4 and 11, 1931. The nests were located 2.5 meters from the ground. The eggs are light blue and pale greenish-blue in color; one measures 18.5 by 13.5. He writes, "The nest consists of two layers, the inner and the outer. The outer layer is made of fine roots, fibers, leaves and petals, interwoven with a large quantity of cotton-wool, and the inner layer is made of fibers of fine roots only." Coultas found that a large number of birds taken in November had enlarged gonads, especially the males; in December, fewer birds with swollen gonads were obtained.
Remarks.—Coultas found this white-eye to be common at Ponapé, when he visited that island in November and December, 1930. He observed the birds in flocks and found them noisy and quarrelsome. They feed in bushes and small trees on seeds and insects. Richards obtained "small large-seeded blackish berries" from the stomach of a female from Ponapé. He found the birds to frequent low altitudes in and about native gardens.
Tephras finschii Hartlaub, in Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, p. 6, pl. 3. (Type locality, Pelew Islands.)
Tephras finschii Hartlaub and Finsch, Proc. Zool. Soc. London, 1868, pp. 117, 118 (Pelew Islands).
Zosterops finschii Gray, Hand-list Birds, 1, 1869, p. 164 (Pelew); Gadow, Cat. Birds British Mus., 9, 1884, p. 197 (Pelew); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 60 (Palau).
Zosterops finschi Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, pp. 89, 96 (Pelew); Finsch, Journ. Mus. Godeffroy, 8, 1875, pp. 4, 17 (Palau); idem, Journ. Mus. Godeffroy, 12, 1876, p. 27 (Palau); Giebel, Thes. Ornith., 3, 1877, p. 775 (Pelew); Finsch, Journ. f. Ornith., 1880, p. 300 (Pelew?); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 407 (Palau); Tristram, Cat. Birds, 1889, p. 211 (Pelew); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 36 (Pelew); Finsch, Das Tierreich, no. 15, 1901, p. 45 (Palau); Dubois, Syn. Avium, 1, 1902, p. 713 (Pelew); Reichenow, Die Vögel, 2, 1914, p. 470 (Palau); Takatsukasa and Kuroda, Tori, 1, 1915, pp. 55, 64 (Pelew); Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 230 (Palau); Hand-list Japanese Birds, rev., 1932, p. 173 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 192 (Babelthuap, Koror).
Tephras finschi Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Palau); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 77 (Pelew); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 712 (Pelew).
Zosterops cinerea finschi Mayr, Birds Southwest Pacific, 1945, p. 300 (Palau).
Zosterops cinerea finschii Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 74 (Peleliu, Garakayo).
Geographic range.—Micronesia: Palau Islands—Babelthuap, Koror, Garakayo, Peleliu, Ngabad.
Characters.—Adult: Resembles adult of Z. c. cinerea, but upper parts mostly browner; wing and tail feathers browner; head blacker; rump lighter than back; auriculars grayish-brown; lores dark; sides of head and neck brownish; underparts mostly dark; chin and throat smoky gray; breast and abdomen more brown less gray; sides, flanks and under tail-coverts brown. Resembles adult of Z. c. ponapensis, but larger with underparts more buffy; upper parts darker.
Measurements.—Measurements are listed in table 51.
Specimens examined.—Total number, 37 (15 males, 19 females, 3 unsexed), as follows: Palau Islands, USNM—Babelthuap, 1 (Nov. 27)—Koror, 3 (Nov. 4, 5)—Garakayo, 6 (Sept. 18)—Peleliu, 5 (Aug. 27, Sept. 10); AMNH—exact locality not given, 22 (Oct., Nov., Dec.).
Molt.—Many of the specimens of Z. c. finschii taken in the period from August to December show evidences of molt. Some of the birds taken in November and in December appear to be in fresh plumage. All three subspecies of Z. cinerea evidently undergo a period of molt in the late summer and fall.
Remarks.—The Micronesian Dusky White-eye of Palau was found on several of the islands of the southern Palaus by the NAMRU2 party in 1945. The bird was observed in flocks of five or more individuals moving rapidly through the foliage of trees and shrubs. It was not found in the dense, undisturbed jungle areas, but rather in second growth vegetation and along the margins of woodlands. At Peleliu, birds were noted in trees and shrubs along the roadways; at Garakayo, birds were seen in low trees near the summits of hills. At Garakayo, Z. cinerea and Z. conspicillata were found in the same areas near the tops of the hills. Both species appeared to be feeding on seeds of the same trees (unidentified but resembling the hibiscus). Z. cinerea was more numerous than Z. conspicillata and appeared (from observations made on September 18, 1945) to be the dominant species and was seen to chase the smaller Z. conspicillata away. Coultas (field notes) found Z. cinerea "fairly common" in 1931 at Palau.
Evolutionary history of Zosterops cinerea.—The dusky white-eyes of Micronesia were considered as separate species until 1944, when Mayr (1944b:7) treated them as conspecific, stating that the bird at Ponapé has characters intermediate between those at Kusaie and Palau. Earlier, Hartert (1900:3) suggested a close association between Z. cinerea and the species at Truk (now Rukia ruki). Mayr concludes that Z. cinerea and R. ruki are not closely related, and points out that the absence of a white orbital ring in Z. cinerea does not necessarily mean that the bird should be considered as belonging to a genus other than Zosterops.
The pathway of colonization and the ancestral stock of Z. cinerea are not certainly known. Among the white-eyes of the Polynesian, Melanesian and Malayan areas, there are few kinds which Z. cinerea resembles closely. Mayr (1941b:204) writes that the Z. cinerea at Ponapé was derived from either Polynesia or Papua. I find little in common between Z. cinerea and the species in these areas, and in my opinion Z. cinerea is closest to Z. atriceps of the Moluccas. Z. atriceps has plumage which is part grayish and part brownish. Its underparts resemble those of Z. c. cinerea but are paler gray; crown, neck, and shoulder much like that of Z. c. ponapensis and Z. c. finschii; and bill resembling that of Z. c. cinerea. Z. atriceps differs by having olive-green coloring on back and wings and yellowish coloring on under side of tail. Thus, it is possible that Z. cinerea invaded Micronesia from the Moluccan region, reaching either Palau or Ponapé initially.
Cleptornis palauensis Reichenow, Journ. f. Ornith., 1915, p. 125. (Type locality, Babeldzuap = Babelthuap, Palauinseln.)
Megazosterops palauensis Stresemann, Ornith. Monatsber., 38, 1930, p. 159 (Baobeltaob); Snouckaert, Alauda (2), 3, 1931, p. 26 (Palau); Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 235 (Baobel Taob = Babelthuap); Mathews, Ibis, 1931, p. 48 (Palau); Hand-list Japanese Birds, rev., 1932, p. 172 (Palau); Yamashina, Tori, 10, 1940, p. 674 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 191 (Babelthuap, Peleliu).
Rukia palauensis Mayr, Amer. Novit., no. 1269, 1944, p. 7 (Palau); idem, Birds Southwest Pacific, 1945, pp. 294, 300 (Peliliu); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, pp. 67, 74 (Peleliu).
Geographic range.—Micronesia: Palau Islands-Babelthuap, Peleliu.
Characters.—Adult: A large white-eye with upper parts near "Saccardo's olive" (some individuals darker brown), head and neck more olivacious, rump browner; auriculars blackish with pale yellow streaks; narrow supra-orbital stripe pale olive; orbital ring indistinct; underparts near "olive lake," chin lighter, under tail-coverts light yellowish-brown; wing and tail feathers dark brown, except for tawny outer edges and whitish inner edges; maxilla horn-color; mandible yellowish to tawny; feet tawny; iris grayish-brown.
Measurements.—Measurements of Rukia are listed in table 52. Measurements of males and females are comparable within the same species.
| Species | No. | Wing | Tail | Culmen | Tarsus |
| R. palauensis |
19 |
80 | 54 | 21.5 | 25 |
| (76-84) | (51-57) | (20.0-22.5) | (24-26) | ||
| R. ruki |
8 |
81 | 52 | 21.5 | 23 |
| (76-85) | (51-52) | (20.0-23.0) | (22-24) | ||
| R. sanfordi |
18 |
70 | 44 | 23.0 | 21 |
| (67-71) | (41-47) | (22.0-24.0) | (20-22) |
Specimens examined.—Total number, 21 (12 males, 9 females), as follows: Palau Islands, USNM—Peleliu, 11 (Aug. 27, 29, 30, Sept. 4, 5, 6, 7, Dec. 4, 5); AMNH—Peleliu?, 10 (Dec.).
Molt.—Specimens taken in August and September are in worn plumage, a few individuals show evidence of molt. Specimens taken in December are in fresh plumage, although two or three individuals are in the final stages of molt. This places the period of molt as September, October, and November. Nesting evidently occurs in the summer; one male taken on August 27, 1945, had enlarged gonads.
Remarks.—The Palau Greater White-eye was described under the generic name Cleptornis by Reichenow. This generic allocation was not followed by subsequent authors; Stresemann proposed the [Pg 331] generic name Megazosterops in 1930, and Mayr (1944b:7) placed this white-eye in the genus Rukia along with other large white-eyes from Micronesia. In employing this name, Mayr writes, "The generic names Rukia (for ruki) and Kubaryum (for oleaginea) were published simultaneously in the same publication. As first reviser I select the name Rukia, which not only is shorter but is also based on a species which I have been able to examine."
R. palauensis is recorded from Babelthuap and Peleliu of the Palau Islands. In 1931, Coultas found the birds only at the island of Peleliu, where he obtained nine specimens from a flock. In 1940, Yamashina (1940:674) writes that it is a very rare species at Palau. Marshall (1949:219) found the bird at Peleliu but at no other islands visited. In 1945, the NAMRU2 party obtained eight specimens at Peleliu from two localities on the eastern side of the island in jungle areas relatively undisturbed by war activities. The birds were fairly common in the brush and vines of the jungle undergrowth at these two areas. There were no flocks seen; usually singles or pairs were noted. The bird bears a striking resemblance to Psamathia annae, which lives in the same environment and has a somewhat similar coloration, shape and posture. These two birds probably have undergone a parallel development. Competition between the two was not noted. Psamathia is evidently less restricted in its distribution.
R. palauensis has a restricted distribution in the Palau Islands, as indicated by the observations of Coultas, the Japanese and the NAMRU2 party. The disturbance resulting from the war activities has undoubtedly influenced the population and restricted further the preferred habitat of this white-eye, especially at Peleliu.
Zosterops oleaginea Hartlaub and Finsch, Proc. Zool. Soc. London, 1872, p. 95. (Type locality, Uap.)
Zosterops oleaginea Gräffe, Journ. Mus. Godeffroy, 2, 1873, p. 122 (Yap); Gadow, Cat. Birds British Mus., 9, 1884, p. 187 (Yap); Finsch, Das Tierreich, no. 15, 1901, p. 24 (Yap); Dubois, Syn. Avium, 1, 1902, p. 708 (Uap); Reichenow, Die Vögel, 2, 1914, p. 469 (Karolinen=Yap); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Mackenzie); Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 230 (Yap); Hand-list Japanese Birds, rev., 1932, p. 173 (Yap); Hand-list Japanese Birds, 3d ed., 1942, p. 192 (Yap).
Zosterops oleaginea Giebel, Thes. Ornith., 3, 1877, p. 777 (Mackenzie); Schmeltz and Krause, Ethnogr. Abth. Mus. Godeffroy, 1881, p. 391 (Yap); Wiglesworth, Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 37 (Uap); Oustalet, Nouv. Arch. Mus. Hist. Nat. Paris, (3), 7, 1895, p. 208 (Uap); Bolau, Mitteil. Naturhist. Mus. Hamburg, 1898, p. 60 (Yap).
Tephras oleaginea Matschie, Journ. f. Ornith., 1901, pp. 112, 113 (Yap).
Kubaryum oleaginus Momiyama, Birds Micronesia, 1922, p. 1 (Yap); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 77 (Yap).
Kubaryum oleagineum Mathews, Syst. Avium Australasianarum, 2, 1930, p. 712 (Yap).
Rukia oleaginea Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Yap); idem, Birds Southwest Pacific, 1945, p. 300 (Yap).
Geographic range.—Micronesia: Caroline Islands—Yap.
Characters.—According to Hartlaub and Finsch (1872:95), "General colour a deep oil-green, with a decided fulvous hue; underparts a little paler, and a little more yellowish; eye-ring satin-white; ears blackish; upper and under tail coverts with a slight rufous tinge; wing- and tail-feathers blackish, with oil-green margins; under wing-coverts whitish-grey; beak fulvous, under mandible, except at the tip, yellowish; feet pale, probably yellow; iris reddish white."
Remarks.—No specimens of R. oleaginea have been examined by me, and I am following Mayr (1944b:7) in including it with the other large white-eyes of Micronesia in the genus Rukia.
Tephras ruki Hartert, Bull. British Ornith. Club, 7, 1897, p. 5. (Type locality, Ruk.)
Tephras ruki Hartert, Ibis, 1898, p. 144 (Ruk); idem, Novit. Zool., 7, 1900, p. 3 (Ruk); Matschie, Journ. f. Ornith., 1901, pp. 111, 112, 113 (Ruck); Mathews, Syst. Avium Australasianarum, 2, 1930, p. 712 (Ruk).
Zosterops ruki Finsch, Das Tierreich, no. 15, 1901, p. 46 (Ruk); Dubois, Syn. Avium, 1, 1902, p. 713 (Ruk); Reichenow, Die Vögel, 2, 1914, p. 470 (Ruk); Takatsukasa and Kuroda, Tori, 1, 1915, p. 64 (Ruk); Stresemann, Mitt. Zool. Mus. Berlin, 17, 1931, p. 230 (Truk); Hand-list Japanese Birds, rev., 1932, pp. 172 (Truk); Hand-list Japanese Birds, 3d ed., 1942, p. 191 (Truk).
Rukia ruki Momiyama, Birds Micronesia, 1922, p. 2 (Ruk); Kuroda, in Momiyama, Birds Micronesia, 1922, p. 78 (Ruk); Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Truk); idem, Birds Southwest Pacific, 1945, p. 301 (Truk).
Geographic range.—Micronesia: Caroline Islands—Truk.
Characters.—According to Hartert (1897:5), "Entirely sepia-brown, the inner webs of the remiges and under wing-coverts lighter, inclining to whitish; the primaries darker, the outer webs bordered with the same colour as the back. Bill black; iris red; tarsi and feet orange-rufous; claws mouse-brown." R. ruki may be distinguished from other species of Rukia by its dark olive-brown coloring.
Measurements.—Measurements are listed in table 52.
Specimens examined.—Total number, 7 (4 males, 2 females, 1 unsexed), from Caroline Islands, AMNH—Truk (Nov., Dec.).
Remarks.—This white-eye was first obtained by Owston's collectors in 1895 at Truk. Hartert (1900:3) writes, "It is most peculiar that the late J. Kubary, who was an excellent collector, and who spent more than fourteen months on Ruk, did not obtain this bird. It is probably not numerous, and occurs only on a certain secluded spot not visited by Kubary." In like manner, R. palauensis [Pg 333] was not described from Palau until 1915, although several collectors had visited the island at previous times. Hartert included the Truk Greater White-eye in the genus Tephras of Hartlaub. Later, Momiyama (1922:2) made this bird the type for his new genus Rukia, in which Mayr has placed all of the large white-eyes of Micronesia.
Rhamphozosterops sanfordi Mayr, Ornith. Monatsber., 39, 1931 [mailing date, Nov. 4, 1931, ex Mayr, 1944b:8], p. 182. (Type locality, Ponapé.)
Cinnyrorhyncha longirostra Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931 [printed date, Oct. 15, 1931, but mailing date for extra-Japanese recipients, Nov. 23, 1931, ex Mayr, 1944b:8], p. 599. (Type locality, Ponapé); Hand-list Japanese Birds, rev., 1932, p. 172 (Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 191 (Ponapé).
Cinnyrorhyncha longirostris Mathews, Ibis, 1933, p. 94 (Ponapé).
Rhamphozosterops sanfordi Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé).
Rukia sanfordi Mayr, Amer. Mus. Novit., no. 1269, 1944, p. 7 (Ponapé); idem, Birds Southwest Pacific, 1945, p. 301 (Ponapé).
Geographic range.—Micronesia: Caroline Islands—Ponapé.
Characters.—Adult: upper parts buffy-olive, head greenish, rump and upper tail-coverts buffy-brown; wing and tail feathers dark brown, outer edges yellowish-olive; underparts grayish-buff, chin and throat faintly washed with greenish-yellow; under tail-coverts darker; bill long, curved and brownish-black, base of mandible paler; feet yellowish; iris chestnut. R. sanfordi is distinguished from other species of Rukia by its smaller size, its paler coloration and its longer and more curved bill.
Measurements.—Measurements are listed in table 52.
Specimens examined.—Total number, 18 (12 males, 6 females), from Caroline Islands, AMNH—Ponapé (Nov., Dec.).
Remarks.—Coultas obtained this white-eye at Ponapé in 1931; he writes (field notes) that it is "a very rare bird on Ponapé. I found them at one tree, a sort of a gum-tree, at about 2,000 feet, where they were collecting from the flowers of the tree. I was attracted by their deep-throated sibilation that is uttered while feeding. They were not in the least disturbed by the noise of the gun and remained long enough for me to collect a substantial series. One old man, who lives not far from the tree, was the only one I could find who knew the bird." Six males and one female taken in December had swollen gonads. Richards found this bird to be rare at Ponapé in 1947-1948. He writes (field notes) that the bird was seen twice (he obtained one male), once in deep forest at about 700 feet and once at the summit of Jokaj at 900 feet. He observed a group of three birds "wildly and loudly chasing one another from tree to tree." The male obtained had yellowish sap adhering to its bill.
The Ponapé Greater White-eye has an appearance very much like that of some of the honey-eaters. Takatsukasa and Yamashina (1931c:599) write, "General appearance very much like either Cinnyris or Myzomela, but it differs from them by its very small first primary, which is far shorter than the primary coverts, and also the smooth cutting edge of the bill, though the bill is similarly shaped as to that of Cinnyris. These characteristics show that this bird belongs to Zosteropidae but not Nectarinidae or Meliphagidae."
Mayr and the Japanese workers, Takatsukasa and Yamashina, published descriptions of this white-eye at Ponapé almost simultaneously. Mayr (1944b:8) contends that his name, Rhamphozosterops sanfordi, is valid because the mailing date of the journal (Ornithologische Monatsberichte) in which R. sanfordi was proposed was November 4, 1931, while his investigations show that the earliest mailing date to European and American ornithologists and libraries of the issue of Dobutsugaku Zasshi in which the name Cinnyrorhyncha longirostra, proposed by Takatsukasa and Yamashina, appeared was November 23, 1931. Mayr (1944b:8) points out that Japanese friends of the authors of the name C. longirostra assert that they saw copies of the description [inferentially printed copies] prior to November 23, 1931. These Japanese, as far as is known, have not claimed that they saw copies before November 4, 1931, and Mayr's conclusion that his name, R. sanfordi, has priority is here accepted. If the name C. longirostra Takatsukasa and Yamashina appeared in printed form and if copies, in requisite number, were distributed to specialists or libraries in Japan, or anywhere else, on or before November 3, 1931, the name C. longirostris has priority over R. sanfordi.
Evolutionary history of Rukia in Micronesia.—There is little known concerning the status of the large white-eyes of Micronesia. Most of them were not found by the earlier collectors and are at present reported to be rare or restricted in their distribution. Little is known concerning the food preferences and nesting activities of the birds and also whether they are actually in danger of extermination or whether their populations are normally as low as have been reported. Originally described under four different generic names, they are now considered as belonging in a single genus, Rukia.
I have compared specimens of Rukia with those of other members of the family Zosteropidae found in the Pacific area. Rukia is apparently not closely related to Z. conspicillata and Z. cinerea of Micronesia but has been derived from a different source or sources. [Pg 335] The author has compared Rukia with the genera Zosterops, Woodfordia, Hypocryptadius, Apoia, Chlorocharis, Pseudozosterops, and Tephrozosterops. Results of these comparisons indicate that large and well-differentiated white-eyes are found on a number of the islands of Oceania. These white-eyes include Woodfordia, Rukia, Zosterops inornata, Z. albogularis, Z. tenuirostris, and Z. strenua. These birds are all large, have large bills (either longer or stouter or both), large and long tarsi, and often short and rounded wings. Rukia apparently has undergone a differentiation which parallels that which has taken place in these other white-eyes, but there is no evidence of a close relationship between these birds and Rukia. There are some resemblances between Rukia and Woodfordia superciliosa of Rennell Island; W. superciliosa is the same size and has a bill somewhat similar to that of R. ruki and a coloration not very different from that of R. sanfordi. R. ruki and R. sanfordi may have been derived originally from a common ancestral stock in Melanesia, with subsequent isolation on small islands for considerable time where differentiation took place. Rukia also shows some resemblance to the genus Apoia, especially to A. pinaiae of Ceram. There is also a possibility that the large white-eyes of Micronesia are merely highly modified species of the genus Zosterops; this has been suggested by Mayr (1944b:7). It is my opinion that Rukia is a valid genus and is as much different from the genus Zosterops (or more so) than other recognized genera of large white-eyes (Woodfordia and Apoia). There is also the strong possibility that the large white-eyes of Micronesia have been derived from more than one source (and are falsely united in one genus); however, it is my feeling that they represent a single colonization, which successfully established itself at four islands and evolved into four divergent species. Possibly R. oleaginea is the least specialized and is closest to the ancestral stock; however, this supposition is based on study of the original description and on a colored plate of the bird in a paper by Kuroda (1922b:pl. 7, fig. 4).
In summary, it seems that the large Micronesian white-eyes of the genus Rukia came originally from Melanesia. Possibly they came from Malaysia. Probably the birds have been derived from a single ancestral stock, that became established at four islands of Micronesia and became differentiated along diverse lines, so much so that some ornithologists have considered them as belonging to separate endemic genera.
Fringilla trichroa Kittlitz, Mém. Acad. Imp. Sci. St. Pétersbourg, 2, 1835, p. 8, pl. 10. (Type locality, Ualan = Kusaie.)
Fringilla trichroa Kittlitz, Obser. Zool., in Lutké, Voy. "Le Séniavine," 3, 1836, p. 285 (Ualan); idem, Denk. Reise russ. Amer. Micron. und Kamchat., 2, 1858, p. 38 (Ualan).
Estrelda trichroa Gray, Genera Birds, 2, 1849, p. 369 (Kusaie?); Gray, Cat. Birds Trop. Is. Pacific Ocean, 1859, p. 27 (Oualan).
Erythrura trichroa Bonaparte, Consp. Avium, 1, 1850, p. 457 (Ualan); Hartlaub, Archiv f. Naturgesch., 18, 1852, p. 133 (Carolinen = Kusaie); idem, Journ. f. Ornith., 1854, p. 168 (Carolinen = Kusaie); Gray, Hand-list Birds, 2, 1870, p. 58 (Ualan); Giebel, Thes. Ornith., 2, 1875, p. 118 (Carolinen = Ualan); Finsch, Journ. Mus. Godeffroy, 12, 1876, p. 36 (Ualan); idem (part), Journ. f. Ornith., 1880, pp. 290, 297, 302 (Kusaie); idem (part), Ibis, 1881, pp. 104, 108 (Kuschai); Salvadori (part), Ornith. Papuasia, 2, 1881, p. 442 (Carolinis = Kusaie?); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, p. 281 (Kusaie); Sclater (part), Ibis, 1881, p. 545 (Ualan); Sharpe (part), Cat. Birds British Mus., 13, 1890, p. 385 (Carolines = Kusaie); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 42 (Ualan); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Ualan); Dubois (part), Syn. Avium, 1, 1902, pp. 583 (Carolines = Kusaie); Takatsukasa and Kuroda (part), Tori, 1, 1915, p. 64 (Kusaie).
Erythrura kittlitzi Bonaparte, Consp. Avium, 1, 1850, p. 457 (ex Bonaparte MSS.) (Type locality, Ualan); Gray, Hand-list Birds, 2, 1870, p. 58 (Caroline Islands = Kusaie).
Erythrura trichros trichros Hartert (part), Novit. Zool., 7, 1900, p. 6 (Kusaie); Kuroda (part), in Momiyama, Birds Micronesia, 1922, pp. 27, 29, 78 (Kusaie); Mayr (part), Amer. Mus. Novit., no. 489, 1931, p. 4 (Kusaie); Takatsukasa and Yamashina, Tori, 7, 1931, p. 110 (Kusaie); Hand-list Japanese Birds, rev., 1932, p. 170 (Kusaie); Hand-list Japanese Birds, 3d ed., 1942, p. 189 (Kusaie); Mayr, Birds Southwest Pacific, 1945, p. 302 (Kusaie).
Chloromunia trichroa trichroa Mathews (part), Syst. Avium Australasianarum, 2, 1930, p. 840 (Ualan).
Geographic range.—Micronesia: Caroline Islands—Kusaie.
Characters.—Adult: A small finch with thick, stout bill; head, neck, back, and scapulars between "parrot green" and "grass green"; forehead, orbital area, auriculars, and malar area bluish; sides of neck green tinged with yellowish; edge of forehead and lores blackish; wing-coverts and outer margins of wings yellowish-green; underparts like back but paler green; rump, upper tail-coverts and outer edges of tail feathers near "Pompeian red"; wing and tail feathers mostly brownish; bend of wing greenish; under wing-coverts brownish; axillaries buffy tinged with greenish; bill black; feet light yellowish-brown; iris brown. Adult female duller than male.
Immature: Resembles adult, but lacks bluish coloring on sides of head and on forehead; underparts washed with buffy brown; rump and tail duller carmine.
Measurements.—Measurements are listed in table 53.
Specimens examined.—Total number, 14 (12 males, 2 females), from Caroline Islands, AMNH—Kusaie (Feb., March, April).
Molt.—Specimens taken in February and March have mostly new feathers, molt having been almost completed when obtained.
Remarks.—Kittlitz was the first person to describe the Blue-faced Parrot-finch; he found it at Kusaie when he visited the island in the winter of 1827-28. Later, it was found to have an extensive range in [Pg 337] Micronesia, Melanesia, northern Australia, Celebes, and the Moluccas. This small finch may be kept as a pet in a cage by native peoples, but as far as I know there is no evidence that the bird has been introduced to island areas as a result of this practice.
| Subspecies | No. | Wing | Tail | Culmen | Tarsus |
| E. t. trichroa |
6 |
58 | 46 | 13.0 | 17 |
| (57-59) | (43-48) | (12.5-13.5) | (16-17) | ||
| E. t. clara |
29 |
59 | 45 | 13.5 | 17 |
| (57-62) | (41-50) | (13.0-14.5) | (17-18) | ||
| E. t. pelewensis* |
1 |
61.5 | 51 | 13.5 | 18 |
* Kuroda (1922:28).
Coultas observed the finch at Kusaie in 1931; he wrote (field notes) that it is a common bird but difficult to obtain. He found it in most parts of the island and at all elevations; the bird appeared to prefer dense underbrush of the jungle or marginal vegetation. He found no evidence of breeding activity in February, March or April.
Erythrura trichroa clara Takatsukasa and Yamashina, Tori, 7, 1931, p. 110. (Type locality, Ruk Island.)
Erythrura trichroa Finsch (part), Journ. f. Ornith., 1880, p. 290 (Ponapé, Hügeln = Truk); idem, Proc. Zool. Soc. London, 1880, p. 576 (Ruk); idem (part), Ibis, 1881, pp. 104, 110, 112, 115 (Ponapé); Schmeltz and Krause (part), Ethnogr. Abth. Mus. Godeffroy, 1881, p. 353 (Ruk); Salvadori (part), Ornith. Papuasia, 2, 1881, p. 442 (Ponapé); Sclater (part), Ibis, 1881, p. 545 (Ponapé, Ruk); Sharpe (part), Cat. Birds British Mus., 13, 1890, p. 385 (Carolines = Truk, Ponapé); Wiglesworth (part), Abhandl. und Ber. Zool. Mus. Dresden, no. 6, 1890-1891 (1891), p. 42 (Ponapé, Ruk); Nehrkorn, Kat. Eiers, 1899, p. 122 (Ruk); Matschie (part), Journ. f. Ornith., 1901, p. 112 (Ruk, Ponapé); Dubois (part), Syn. Avium, 1, 1902, p. 583 (Carolines = Ponapé); Takatsukasa and Kuroda (part), Tori, 1, 1915, pp. 55, 64 (Ponapé); Mayr, Proc. 6th Pacific Sci. Congr., 4, 1941, p. 204 (Ponapé).
Erythrura trichroa trichroa Hartert (part), Novit. Zool., 7, 1900, p. 6 (Ruk, Ponapé); Kuroda (part), in Momiyama, Birds Micronesia, 1922, pp. 27, 28, 29, 78 (Ponapé, Ruk); Mayr (part), Amer. Mus., Novit., no. 489, 1931, p. 4 (Ponapé, Ruk).
Chloromunia trichroa Mathews, Birds Australia, 12, 1925, p. 208 (Ruk).
Chloromunia trichroa trichroa Mathews (part), Syst. Avium Australasianarum, 2, 1930, p. 840 (Carolines = Truk, Ponapé).
Erythrura trichroa clara Hand-list Japanese Birds, rev., 1932, p. 170 (Truk, Ponapé); Hand-list Japanese Birds, 3d ed., 1942, p. 189 (Truk, Ponapé); Mayr, Birds Southwest Pacific, 1945, p. 302 (Truk, Ponapé); Baker, Smithson. Misc. Coll., vol. 107, no. 15, 1948, p. 74 (Truk).
Lobospingus trichroa clara Mathews, Ibis, 1933, p. 96 (Ruk, Ponapé).
Geographic range.—Micronesia: Caroline Islands—Truk, Ponapé, Lukunor?
Characters.—Adult: Resembles adult of E. t. trichroa, but slightly larger with underparts more yellowish and less greenish; blue on head slightly paler; sides of neck tinged more strongly with yellowish. Birds from Ponapé are slightly paler than those from Truk.
Measurements.—Measurements are listed in table 53. Birds from Ponapé and Truk differ but little in measurements.
Specimens examined.—Total number, 39 (22 males, 16 females, 1 unsexed), as follows: Caroline Islands, USNM—Truk, 2 (May 5, Dec.); AMNH—Truk, 15 (March, June, Nov.)—Ponapé, 22 (Dec.).
Molt.—Birds taken in March and June are not in molt. Some of the specimens obtained in November and December are in molt.
Remarks.—The differences between E. t. trichroa at Kusaie and E. t. clara at Ponapé and Truk are slight. Takatsukasa and Yamashina (1931d:110) separate E. t. clara from E. t. trichroa of Kusaie on the basis of a paler blue coloring on head, body more yellowish green and sides of neck more distinctly golden-yellow.
Coultas obtained specimens at Ponapé in 1930 and reports (field notes) that the bird occurs in the extensive grassland areas of the island but that the numbers are small. He estimates the population to be less than 100 individuals. He learned that the Japanese had trapped them for shipment to Japan as caged birds. Coultas writes that the finch at Ponapé "is very shy and flies readily when he is disturbed. As soon as a call of alarm is uttered the whole flock flies up from the ground and heads for the true forest where they will hide. They will also work along in the grass, and make a getaway. The bird has a little hissing sybilation that it utters when on the wing." He found the bird in flocks of 3 to 20; immatures were frequently found alone.
McElroy of the NAMRU2 party obtained a female at Moen Island in the Truk Atoll in December, 1945. He found small flocks of these birds in dense vegetation along streams.
Erythrura trichroa pelewensis Kuroda, in Momiyama, Birds Micronesia, 1922, p. 27. (Type locality, Pelew Islands).
Erythrura trichroa pelewensis Kuroda, Ibis, 1927, p. 692 (Pelew); Mayr. Amer. Mus. Novit., no. 489, 1931, p. 4 (Pelew); Hand-list Japanese Birds, rev., 1932, p. 171 (Palau); Hand-list Japanese Birds, 3d ed., 1942, p. 189 (Babelthuap); Mayr, Birds Southwest Pacific, 1945, p. 301 (Palau).
Chloromunia trichroa pelewensis Mathews, Syst. Avium Australasianarum, 2, 1930, p. 840 (Pelew).
Geographic range.—Micronesia: Palau Islands—Babelthuap.
Characters.—Kuroda (1922a:27) describes the bird as follows, "Resembles E. trichroa (Kittlitz) from Carolines (the type from Kusaie), but distinguishable from it by the bill being much thicker and stouter, by the chin being tinged with blue, by the under-parts being paler throughout and somewhat tinged with bluish, by the rump and upper tail-coverts being bright crimson instead of dull crimson, by the central tail-feathers brownish red instead of dull crimson, by the distinct shafts of central tail-feathers and by longer wing and tail."
Measurements.—The measurements by Kuroda of a single specimen are listed in table 53.
Remarks.—Only one specimen of this subspecies is known. The NAMRU2 party did not obtain any record of it in the southern Palaus in 1945. If still present in the islands, it may be confined to the higher forested areas of Babelthuap.
Evolutionary history of Erythrura trichroa in Micronesia.—The Blue-faced Parrot-finch has been recorded from Kusaie, Ponapé, Truk and Palau, which are all "high" islands of southern Micronesia. This bird belongs to a species which occurs in Melanesia, northern Australia, Celebes, and the Moluccas. Stresemann (1940:40) points out the interesting observation that this species ranges only east of Wallace's Line. Mayr (1931c:1-10) has reviewed the parrot-finches of the genus Erythrura and places E. trichroa in the subgenus Erythrura, noting that E. t. cyaneifrons from Banks and the New Hebrides is similar to the subspecies found in Micronesia. As a group the subspecies of E. trichroa are very similar, but the populations in Micronesia appear closest to subspecies from the Solomons, Admiralty Islands and possibly to E. t. modesta from the Moluccas, which appears to indicate that Micronesia was invaded from the south or from the southwest via the Moluccas. Whether the little known subspecies at Palau represents an independent invader from the Moluccas is uncertain.
Munia (Donacola) hunsteini minor Yamashina, in Takatsukasa and Yamashina, Dobutsu. Zasshi, 43, 1931, p. 600. (Type locality, Ponapé.)
Lonchura hunsteini minor Hand-list Japanese Birds, rev., 1932, p. 171 (Ponapé, Truk); Hand-list Japanese Birds, 3d ed., 1942, p. 190 (Ponapé, Truk).
Donacola hunsteini minor Mathews, Ibis, 1933, p. 95 (Ponapé).
Lonchura nigerrima minor Mayr, Birds Southwest Pacific, 1945, p. 301 (Ponapé, ?Truk).
Geographic range.—Micronesia: Caroline Islands—Ponapé, Truk?
Characters.—Yamashina in Takatsukasa and Yamashina (1931c:600) characterizes this subspecies as similar to M. hunsteini from New Ireland, but smaller; the wing of the adult of the bird from Ponapé is from 46 to 49 mm, instead of 50-51 mm. as in the New Ireland bird. Moreover the crown and nape are white instead of pearl gray.
Remarks.—Little is known concerning this subspecies named by [Pg 340] Yamashina at Ponapé. No specimens have been seen by me. Richards obtained one male at Ponapé in 1947-1948. He found the birds in large flocks.
Munia cabanisi Sharpe, Cat. Birds British Mus., 13, 1890, p. 353. (Type locality, Luzon.)
Munia punctulata cabanisi Kuroda, in Momiyama, Birds Micronesia, 1922, p. 78 (Yap).
Lonchura punctulata cabanisi Yamashina, Tori, 7, 1932, p. 395 (Yap); Hand-list Japanese Birds, rev., 1932, p. 171 (Yap); Hand-list Japanese Birds, 3d ed., 1942, p. 189 (Yap).
Geographic range.—Philippine Islands and Micronesia. In Micronesia: Palau Islands; Caroline Islands—Yap.
Characters.—A small finch with upper parts light grayish-brown, feathers with white shafts producing a streaked appearance; lores, anterior part of auriculars, malar region, and feathers of chin and throat chocolate-brown with faint white shafts; breast and sides mottled white and dark brown, middle of abdomen and under tail-coverts pale buffy-white, wings brown with lighter edges, under wing dark with lighter coverts; upper tail-coverts and middle tail feathers dark olive, outer tail feathers colored like wings; bill heavy and black; feet dark brown.
Remarks.—The Philippine Nutmeg Mannikin is a resident on the island of Yap. Yamashina (1932a:395) records a nest containing one egg taken there on May 15, 1932. Marshall (1949:221) records this bird at Palau on November 6 and December 2, 1945. Whether this bird was introduced to Yap and Palau by man or whether it reached there by independent invasion is unknown.
The avifauna of Micronesia consists of 206 kinds of birds belonging to 37 families and 91 genera. Of these, 30 kinds are sea birds, 29 kinds are migratory shore birds, and 146 kinds are land and freshwater birds. Of the 30 sea birds, 18 kinds are resident; of the 147 land and fresh-water birds, 104 kinds are resident and 6 kinds have been introduced by man. There are no resident shore birds in Micronesia. The following conclusions can be drawn from this study:
1. The islands of Micronesia are oceanic islands and were seemingly formed independently of any present day continental land mass. Terrestrial organisms have reached these islands by "over-water dispersal." The avifauna of Micronesia has been received from the following sources: Polynesia, Melanesia, the Moluccas, Celebes, Phillipines, and Palearctica (see figure 8).
2. Oceanic birds are among the oldest forms of bird life inhabiting [Pg 341] Micronesia. The presence of elevated islands containing phosphate, resulting from the deposition of guano by oceanic birds, is some indication of the length of time during which these birds have been present. In number of individuals, the oceanic birds inhabiting the inshore zone are more numerous than those inhabiting the offshore and pelagic zones, although twelve of the eighteen resident kinds of oceanic birds prefer the offshore and pelagic zones. Most of the species of oceanic birds resident in Micronesia are circumtropical in distribution; no residents are known in Micronesia which have been derived from Palearctica or the North Pacific. Micronesia has no endemic oceanic birds.
3. On the migratory flights, shore birds reach Micronesia along three distinct flyways, which in this report are named the Asiatic-Palauan Flyway, the Japanese-Marianan Flyway, and the Nearctic-Hawaiian Flyway (see figure 7). The shore birds began to utilize the Pacific islands as wintering grounds by gradually spreading from the Eastern Hemisphere rather than from the Western Hemisphere.
4. More than half (52 percent) of the land birds and fresh-water birds in Micronesia were derived directly from ancestral stocks in Melanesia. The areas of the Moluccas and of Celebes (Malaysia) supplied 21 percent of the birds; the Philippines, 10 percent; Polynesia, 9 percent; and Palearctica, 8 percent. Results of this study show that there may have been only 46 actual colonizations of Micronesia by birds from other areas, and that many of the large number of endemics present have been the result of secondary colonizations within the islands of Micronesia. It is concluded that Micronesia, except for the Marshall Islands, has a much closer affinity to Melanesia than to any other area as regards avifauna. The Marshall Islands may be regarded as a part of the Polynesian Subregion from the viewpoint of avian zoogeography.
5. Endemism in the land birds and fresh-water birds of Micronesia is extreme. Of 104 native, resident birds, 97 (93.5 percent) have become differentiated and can be separated taxonomically from related forms. In Micronesia, there are 5 endemic genera, 31 endemic species, and 76 endemic subspecies. The families containing the greatest number of endemic forms are Muscicapidae (14), Zosteropidae (14), Columbidae (13), and Sturnidae (9).
6. It is concluded that some of the more important factors controlling the dispersal of the bird life to Micronesia are the direction and the intensity of the winds, the small size of the islands, the isolation of the islands (especially those "high" islands), and the insular [Pg 342] climates, which appear to favor colonists from tropical homes rather than those from Palearctic homes.
7. The factors most important in the process of differentiation of birds in the islands of Micronesia are isolation, paucity in numbers of individuals, freedom from predation, absence (and presence) of interspecific and intraspecific strife, and nutrition. The importance of the "dilution" factor is discussed, and the possibility of cross-breeding between different kinds of birds is considered. It is concluded that genetic change altering the phenotypic expression of avian characteristics is no more apt to occur in insular populations than in continental populations, but such changes have a greater chance of being perpetuated in insular populations.
Amadon, D. 1942. Notes on some non-passerine genera, 1. Amer. Mus. no. 1175:1-11, 1 fig. 1943. Notes on some non-passerine genera, 3. Amer. Mus. Novit., no. 1237:1-22. Austin, O. L., Jr. 1948a. Japanese ornithology and mammalogy during World War II. Natural Resources Section, General Headquarters, Supreme Commander Allied Powers. Report No. 102:1-47. 1948b. The birds of Korea. Bull. Mus. Comp. Zool., 101, no. 1:1-301, 1 pl.
Baker, R. H. 1946a. A new race of Rhipidura rufifrons from Rota Island, Mariana Islands. Proc. Biol. Soc. Washington, 55:77-78. 1946b. Some effects of the war on the wildlife of Micronesia. Trans. 11th N. Amer. Wildlife Conf.: 205-213, 3 figs. 1946c. A study of rodent populations on Guam, Mariana Islands. Ecol. Monogr., 16:393-408, 11 figs. 1947a. Observations on the birds of the North Atlantic. Auk, 64:245-259, 5 figs. 1947b. Size of bird populations at Guam, Mariana Islands. Condor, 49:124-125. 1948. Report on collections of birds made by United States Naval Medical Research Unit No. 2 in the Pacific war area. Smithsonian Misc. Coll., 107, no. 13:ii + 74 pp., 6 pls. Bequaert, J. C. 1939. Hippoboscidae of the Caroline Islands (including the Palau Group). Mushi, 12:81-82. 1941. The Hippoboscidae of Oceania (Diptera). Occ. Papers Bernice P. Bishop Mus., 16:247-292, 5 figs. Berlioz, M. J. 1929. Catalogue systematique des type de la collection d'oiseaux du muséum. Bull. Mus. Hist. Nat., Paris, 2nd Ser., 1:58-69. Bogert, C. 1937. The distribution and the migration of the long-tailed cuckoo (Urodynamis taitensis Sparrman). Amer. Mus. Novit., no. 933:1-12, 1 map. Bolau, H. 1898. Die typen der Vogelsammlung des Naturhistorischen Museums zu Hamburg. Mitteil. Naturhist. Mus. Hamburg, 15:47-71. Bonaparte, C. R. 1850-'57. Conspectus generum avium. 1850, 1:1-543; 1855-57, 2:1-232. Borror, D. J. 1947. Birds of Agrihan, Auk, 64:415-417. Brigham, W. T. 1900. An index to the islands of the Pacific Ocean. Mem. Bernice P. Bishop Mus., 1, no. 2:1-170, maps. [Pg 344] Bryan, E. H., Jr. 1936. Birds of Guam. Guam Recorder [newspaper]. 13, no. 2:14-15, 24-25; no. 3:10; no. 4:14, 30; no. 5:15-16, 30-31; no. 6:14-15, 34; no. 7:18-20, 34-35. 1941. A summary of the Hawaiian birds. Proc. 6th Pacific Sci. Cong., 1939, 4:185-189. Bryan, E. H., Jr., and Greenway, J. C., Jr. 1944. Contribution to the ornithology of the Hawaiian Islands. Bull. Mus. Comp. Zoöl., 94:80-142. Bryan, W. A. 1903. A monograph of Marcus Island. Occ. Papers Bernice P. Bishop Mus., 2:77-139. Büttikofer, J. 1893. A review of the genus Rhipidura. Notes Leyden Mus., 15:65-98. Buxton, P. A. 1938. The formation of species among insects in Samoa and other oceanic islands. Proc. Zool. Soc. London: 264-267.
Cabanis, J. 1859-'60. Museum Heineanum. Verzeichniss der Ornithologoschen Sammlung. pt. 2:1-175. Cassin, J. 1858. United States Exploring Expedition during the years 1838, 1839, 1840, 1841, 1842 under the command of Charles Wiles, U. S. N. Mammalogy and ornithology. Philadelphia, viii + 466 pp. Chamisso, A. von 1821. Remarks and opinions of the naturalist of the expedition, in Kotzebue's Voyage "Rurick," London, 2:351-433; 3:1-318. Christian, F. W. 1899. The Caroline Islands, travels in the sea of little islands. Charles Scribner's Sons, New York: xiii + 412 pp., illus. 1926. The megapode bird and the story it tells. Journ. Polynesian Soc., 35:260. Churchill, W. 1911. The peopling of Yap. Bull. Amer. Geogr. Soc., 43:510-518. Cox, L. M. 1917. The island of Guam. U.S. Govt. Print. Office, Washington, 95 pp., illus., map. Cram, E. B. 1927. Bird parasites of the nematode suborders Strongylata, Ascaridata and Spirurata. Bull. U.S. Nat. Mus., 140:xvii + 465 pp. Crampton, H. E. 1925. Studies on the variation, distribution and evolution of the genus Partula. The species of the Mariana Islands, Guam and Saipan. Publ. Carnegie Inst., Washington, 228A:vi + 116 pp., 14 pls.
Darlington, P. J. 1938. The origin of the fauna of the Greater Antilles, with discussion of dispersal of animals over water and through the air. Quart. Rev. Biol., 13:274-300, 5 figs. [Pg 345] Delacour, J. 1942-'43. The bush-warblers of the genera Cettia and Bradypterus, with notes on allied genera and species. Ibis, 84 (1942):509-519; 85 (1943):27-40. 1943. A revision of the subfamily Estrildinae of the family Ploceidae. Zoologica, 28:69-86. 1946. Notes of the taxonomy of the birds of Malaysia. Zoologica, 31:1-8. 1947. Birds of Malaysia. The Macmillan Co., New York, xvi + 382 pp., 84 figs. Delacour, J., and Mayr, E. 1945a. The family Anatidae. Wilson Bull., 57:3-55, 24 figs. 1945b. Notes on the taxonomy of the birds of the Philippines. Zoologica, 30:105-117. 1946. Birds of the Philippines. New York, xv + 309 pp., 69 figs., 1 map. Desmarest, A. G. 1826. Columba oceanica. Dict. Sci. Nat., éd. Levrault, Paris, 40:316. Dobzhansky, T. G. 1941. Genetics and the origin of species. Columbia Univ. Press, New York, 2d ed., rev., ix + 466 pp., 24 figs. Downs, T. 1946. Birds on Tinian in the Marianas. Trans. Kansas Acad. Sci., 49:87-106, 3 figs., 1 map. Dubois, A. 1902-'04. Synopsis avium. Nouveau manuel d'ornithologie. Bruxelles, 1 (1899-1902):xv + 1-729, pls. i-xii; 2 (1902-1904):ix + 730-1399, pls. xii-xvi.
Eliott, D. G. 1878a. The genus Porphyrio and its species. Stray Feathers, 7:6-25. 1878b. On the fruit-pigeons of the genus Ptilopus. Proc. Zool. Soc. London: 500-575.
Finsch, O. 1867-'68. Die Papageien. Leiden, 1 (1867):xii + 561 pp.; 2 (1868):vii + 996 pp. 1870. Zur Ornithologie der Tonga-Inseln. Journ. f. Ornith., 18:119-140. 1852. Zur Ornithologie der Samoa-Inseln. Journ. f. Ornith., 20:30-58. 1875. Zur Ornithologie der Südsee-Inseln. 1. Die Vögel der Palau-Gruppe. Journ. Mus. Godeffroy, 8:133-183 (in reprint: 1-51), 5 pls. 1876a. Characters of six new Polynesian birds in the Museum Godeffroy at Hamburg. Proc. Zool. Soc. London for 1875:643-644. 1876b. Zur Ornithologie der Südsee-Inseln. II. Ueber neue und weniger bekannte Vögel von Viti-, Samoa- und Carolinen-Inseln. Journ. Mus. Godeffroy, 12:1-41. 1876c. Über den Schwalbenwürger (Artamus) der Palau Inseln. Journ. Mus. Godeffroy, 12:41-42. 1878. On the birds of the island of Ponapé, eastern Carolines. Proc. Zool. Soc. London for 1877:777-782. 1880a. Beobachtungen über die Vögel der Inseln Ponapé (Carolinen). Journ. f. Ornith., 28:283-295. [Pg 346] 1880b. Beobachtungen über die Vögel der Inseln Kuschai (Carolinen). Journ. f. Ornith., 28:296-310. 1880c. Ornithological letters from the Pacific, no. II. Taluit (Bonham) Island. Ibis, 4th Ser., 4:218-220. 1880d. Ornithological letters from the Pacific, no. III. Taluit (Bonham) Island. Ibis, 4th Ser., 4:329-333. 1880e. List of the birds of the islands of Ruk in the central Carolines. Proc. Zool. Soc. London: 574-577. 1881a. On two species of pigeons from the Caroline Islands. Proc. Zool. Soc. London for 1880: 577-578. 1881b. Ornithological letters from the Pacific. No. V. Kushai; no. VI. Ponapé. Ibis, 4th Ser., 5:102-115. 1881c. Ornithological letters from the Pacific, no. VII. Nawodo (Pleasant Island). Ibis, 4th Ser., 5:245-249. 1883. On a new reed-warbler from Nawodo or Pleasant Island, in the western Pacific. Ibis, 5th Ser., 1:142-144. 1884. Über Vögel der Südsee. Auf Grund eigener Beobachtungen und Sammlungen. Mitt. Ornith. Ver. Wien, V. Micronesia: 46-56. 1893a. Ueber die Monasaralle von Kuschai [Kittlitzia Monasa (Kittlitz) und die bisher mit ihr verwechselten Arten]. Mitth. Ornith. Ver. Wien, "Die Schwalbe," 17:1-5. 1893b. Einiges über Südsee-Rallen. Anzeigeblatt Ornith. Monats. des Deut. Ver. z. Schutze der Vogelwelt, 18:457-463. 1900a. Zur Catalogisirung der ornithologischen Abtheilung. Notes Leyden Mus., 22:75-125. 1900b. Meine Beobachtungen über Fregattvögel (Fregata aquila L., F. ariel Gould). Anzeigeblatt Ornith. Monats. des Deut. Ver. z. Schutze der Vogelwelt, 25:446-452. 1901. Das Tierreich. no. 15. Zosteropidae. Berlin, xiv + 54 pp., 31 figs. Finsch, O., and Hartlaub, G. 1867. Beitrag zur Fauna Centralpolynesiens. Ornithologie der Viti-, Samoa- und Tonga-Inseln. Halle, xl + 290 pp., 14 pl. Fisher, H. I. 1946. The type localities of Puffinus pacificus cuneatus Salvin and Pterodroma leucoptera hypoleuca (Salvin). Auk, 63:587-588. 1947. Utinomi's Bibliographica Micronesica: Chordate Sections. Pacific Science, 1, no. 3:129-150. Forbes, W. A. 1879. A synopsis of the Meliphagine genus Myzomela, with descriptions of two new species. Proc. Zool. Soc. London: 256-279.
Gadow, H. 1884. Catalogue of the Passeriformes or perching birds in the collections of the British Museum. Cinnyrimorphae. 9:xii + 310 pp., 7 pls. Gaimard, P. J. 1823. Memoire sur un nouveau genre de Gallinaces, establi sous le nom de Megapode. Bull. Gen. et Univ. des Annonces et des Nouvelles Sci., 2:450-451. [Pg 347] Giebel, C. G. 1872-'77. Thesaurus ornithologiae. Reportorium der gesammten ornithologischen Literatur und Nomenclatur sämmtlicher Gattungen und Arten der Vögel nebst Synonymem und geographischer Verbreitung. F. A. Brockhaus, Leipzig, 1 (1872):vii + 868 pp.; 2 (1875):vi + 787 pp.; 3 (1877):vi + 861 pp. Gleize, D. A. 1945. Birds of Tinian. Bull. Mass. Audubon Soc., 29:220. Gleize, D. A., and Genelly, D. 1945. in With the colors. Bull. Mass. Audubon Soc., 29:221-222. Godman, F. 1907-1910. A monograph of the Petrels. Witherby, London, lv + 381 pp., pls. Gräffe, E. 1873. Die Carolineninsel Yap oder Guap. Journ. Mus. Godeffroy, 2:84-130 (in reprint 1-58). Gray, G. R. 1859. Catalogue of the birds of the tropical islands of the Pacific Ocean in the collection of the British Museum. Taylor and Francis, London: 1-72. 1866. A synopsis of the species of the genus Collocalia, with descriptions of new species. Ann. and Mag. Nat. Hist., 3d ser., 17:118-128. Gray, J. E. 1866. Naturalization of Zosterops dorsalis in New Zealand. Ann. and Mag. Nat. Hist., 3d ser., 17:237. Gulick, A. 1932. Biological peculiarities of oceanic islands. Quat. Rev. Biol., 7:405-427.
Hachisuka, M. U. 1931-'35. The birds of the Philippine Islands. Witherby, London, 1 (1931-'32):xx + 439 pp.; 2 (1934-'35):xxxi + 469 pp. 1939a. New races of a rail and a fruit pigeon from Micronesia and Palawan. Bull. British Ornith. Club, 59:151-153. 1939b. The red jungle fowl from the Pacific islands. Tori, 10:596-601.
Hachisuka, M. U., Kuroda, N., Takatsukasa, N., Uchida, S., and Yamashina, Y. 1932. A hand-list of the Japanese birds, revised. The Ornith. Soc. of Japan, Tokyo, iv + 211 pp. 1942. A hand-list of Japanese Birds, third revised edition. The Ornith. Soc. of Japan, Tokyo, vii + 238 pp. Harrison, T. H., and Marshall, A. J. 1937. Aplonis santovestris, sp. nov. Bull. British Ornith. Club, 57:148-150. Hartert, E. 1891. Katalog der Vogelsammlung in Museum der Senckenbergischen Naturforschenden Gesselschaft in Frankfurt am Main. Frankfurt am Main, xxii + 259 pp. 1892. in Salvin and Hartert, Catalogue of the Picariae in the collection of the British Museum. Coraciae. 16:434-654, pls. 10-14. 1897. On a new species of Tephras (Zosteropidae) from the Caroline Islands. Bull. British Ornith. Club, 7:5. [Pg 348] 1898. On the birds of the Marianne Islands. Novit. Zool., 5:51-69. 1900. The birds of Ruk in the central Carolines. Novit. Zool., 7:1-11. 1903-'22. Die Vögel der paläarktischen Fauna. Berlin, 1 (1910):xlix + 1-832, figs. 1-134; 2 (1912-21):xxiv + 833-1764, figs. 135-256; 3 (1921-22): xii + 1765-2328, figs. 257-268. 1917. On some Rallidae. Novit. Zool. 24:265-274. 1926. Types of birds in the Tring Museum. B. Types in the general collection, VII. Tubinares. Novit. Zool., 33:344-357. 1927. Types of birds in the Tring Museum. B. Types in the general collection, VIII. Columbae-Struthionidae. Novit. Zool., 34:1-38. 1930. List of the birds collected by Ernst Mayr. Novit. Zool., 36:27-128. Hartlaub, G. 1852. R. Titian Peale's Vögel der "United States Exploring Expedition." Archiv f. Naturg., 18:93-138. 1854. Zur Ornithologie Oceanien's. Journ. f. Ornith., 2:160-170. 1865. Monographischer Versuch über die Gattung Zosterops. Journ. f. Ornith., 13:1-30. 1868. On a collection of birds from some less-known localities in the western Pacific. Proc. Zool. Soc. London for 1867:828-832, pl. 38. 1892. Vier selten Rallen. Abh. Nat. Ver. Bremen, 12:389-402. Hartlaub, G., and Finsch, O 1868a. On a collection of birds from the Pelew Islands. Proc. Zool. Soc. London: 4-9, pls. 2-3. 1868b. Additional notes on the ornithology of the Pelew Islands. Proc. Zool. Soc. London: 116-118. 1872. On a fourth collection of birds from the Pelew and Mackenzie islands. Proc. Zool. Soc. London: 87-114. Hedley, C. 1899. A zoogeographic scheme for the mid-Pacific. Proc. Linnean Soc. New South Wales, 24:391-417. Hesse, R., Allee, N. C., and Schmidt, K. P. 1937. Ecological Animal Geography. An authorized, rewritten edition based on Tiergeographie auf oekologischer Grundlage by R. Hesse, prepared by N. C. Alee and K. P. Schmidt. John Wiley & Sons, New York, xiv + 597 pp., 135 figs. Hobbs, W. H. 1945. Fortress islands of the Pacific. J. W. Edwards, Ann Arbor, xiii + 186 pp., 107 figs. Hombron, J. B., and Jacquinot, C. H. 1841. Description de plusieurs oiseaux nouveaux ou peu connus, provenant de l'expedition autour du monde faite sur les corvettes l'Astrolabe et La Zélée. Ann. Sci. Nat., Paris, 2d ser., 16:312-320. Huxley, J. S. 1938. Species formation and geographical isolation. Proc. Zool. Soc. London: 253-264.
Kelso, L. 1938. An addition to the range of the short-eared owl. Oölogist, 60:138. Kirby, H., Jr. 1925. The Birds of Fanning Island, Central Pacific Ocean. Condor, 27:185-196, figs. 47-54. [Pg 349] Kittlitz, F. H. von 1832-'33. Kupfertafeln zur Naturgeschichte der Vögel. Frankfurt am Main, parts 1-3:1-28, 36 pls. 1835. Über einige noch unbeschriebene Vögel von der Insel Luzon, den Carolinen und den Marianen. Mém. Acad. Imp. Sci. St. Pétersbourg, 2:1-9, pls. 1-10. 1836. Observations zoologiques faites pendant l'expedition de la corvette Le Séniavine, par F. H. de Kittlitz, in Lutké, Voyage "Le Séniavine," 3:237-330. 1858. Denkwürdigkeiten einer Reise nach dem russischen Amerika, nach Micronesien und durch Kamtschatka. Gotha, 1:383 pp.; 2:463 pp. Krieger, H. W. 1943. Island peoples of the western Pacific. Micronesia and Melanesia. Smithson. Inst. Publ. 3737 (War Background Ser. No. 16):iv + 104 pp., 21 pls., 2 maps. Kuroda, N. 1922a. Descriptions of two new forms of birds from Pelew Islands, Micronesia. in Momiyama, Birds of Micronesia, pt. 1:25-30. 1922b. A list of the birds of Micronesia Group, exclusive of Magalhaes, Gilbert and Ellice islands. in Momiyama, Birds of Micronesia, pt. 1:31-78. 1925. A contribution to the knowledge of the avifauna of the Riu Kiu Islands and vicinity. publ. by the author, Tokyo, vi + 293 pp., illus. 1932. A revision of the types of birds described by Japanese authors during the years 1923 to 1931. Novit. Zool., 37:384-405. 1941-'42. A study of the Marianas Duck, Anas oustaleti. Tori, pt. 1, 11:99-119, 6 pls; pt. 2, 11:443-448, 3 photos. [In Japanese.]
Lack, D. L. 1947. Darwin's finches. Cambridge Univ. Press, Cambridge, x + 209 pp., 8 pls., 27 figs. Lesson, R. P. 1828. Manuel d'ornithologie, ou description des genres et des principales espèces d'oiseaux. Paris, 1:iv + 421 pp.; 2:448 pp. 1829. Voyage autour du monde, éxecuté par ordre du Roi, sur la corvette de la Majesté La Coquille. pendant les années 1822, 1823, 1824, et 1825. Zoologie. Paris, 1:iv + 1-360; 2:361-743; Atlas:157 col. pls. 1830-'31. Traité d'ornithologie, ou description des oiseaux réunis dans les principales collection de France. Paris, xxxii + 659 pp., 119 col. pls. Linsley, L. N. 1935. Curious things about Guam: the mountain chicken. Guam Recorder [newspaper], 12:249-250. Lister, J. J. 1911. The distribution of the avian genus Megapodius in the Pacific islands. Proc. Zool. Soc. London: 749-759, 1 fig. Lutké, F. 1835-'36. Voyage autour de monde, exécuté par ordre de sa majesté l'empereur Nicolas 1, sur la corvette Le Séniaville, dans les années 1826, 1827, 1828, et 1829. Partie historique avec un atlas, lithographié d'aprés les dessins originaux d'Alexandre Postels et du Baron Kittlitz. Didot, Paris, 1 (1835):xxiv + 410 pp.; 2 (1835):387 pp.; 3 (1836):xiv + 352 pp. [Pg 350] McAtee, W. L. (editor) 1945. The ring-necked pheasant and its management in North America. Amer. Wildlife Inst., Washington: xi + 320 pp., 31 pls., 12 figs.
Marche, A. 1891. Rapport général sur une mission aux îles Mariannes. Nov. Arch. Missions Sci. et Litt., 1:241-280. Marshall, J. T., Jr. 1949. The endemic avifauna of Saipan, Tinian, Guam and Palau. Condor, 51:200-221, figs. 35-40. Mathews, G. M. 1925. Monarcharses, gen. nov. Bull. British Ornith. Club, 45:95. 1926. On some changes in names. Nycticorax caledonicus pelewensis subsp. nov. Bull. Brit. Ornith. Club, 46:60. 1927-30. Systema Avium Australasianarum. British Ornith. Union, pt. 1 (1927):1-426; pt. 2 (1930):427-1048. 1931. Additions and corrections to the 'Systema Avium Australasianarum'. Ibis, 13th ser., 1:44-57. 1932. Additions and corrections to the 'Systema Avium Australasianarum'. pt. II. Ibis, 13th ser., 2:132-161. 1933. Additions and corrections to the 'Systema Avium Australasianarum'. pt. III. Ibis, 13th ser., 3:87-97. 1934. A check-list of the order Procellariiformes. Novit. Zool., 39:151-206. 1938. Aplornis versus Aplonis. Ibis, 14th ser., 2:342. Matschie, P. 1901. Bemerkungen zur Zoogeographie des westlichen Mikronensiens. Journ. f. Ornith., 49:109-114. Mayr, E. 1931a. Notes on Halcyon chloris and some of its subspecies. Amer. Mus. Novit., no. 469:1-10. 1931b. A systematic list of the birds of Rennell Islands with descriptions of new species and subspecies. Amer. Mus. Novit., no. 486:1-29. 1931c. The parrot finches (genus Erythrura). Amer. Mus. Novit., no. 489:1-10. 1931d. Notes on fantails of the genus Rhipidura. Amer. Mus. Novit., no. 502:1-21. 1931e. Rhamphozosterops nov. gen. Ornith. Monatsber., 39:182. 1932. Notes on Meliphagidae from Polynesia and the Solomon Islands. Amer. Mus. Novit., no. 516:1-30. 1933a. Rhamphozosterops versus Cinnyrhyncha. Ibis, 13th ser., 3:389-390. 1933b. Three new genera from Polynesia and Melanesia. Amer. Mus. Novit., no. 590:1-6, 1 fig. 1933c. Two new birds from Micronesia. Amer. Mus. Novit., no. 609:1-4. 1933d. Notes on Polynesian flycatchers and a revision of the genus Clythorhynchus Elliott. Amer. Mus. Novit., no. 628:1-21. 1933e. Notes on the genera Myiagra and Mayrornis. Amer. Mus. Novit., no. 651:1-20. 1933f. Notes on the variation of immature and adult plumages in birds and a physiological explanation of abnormal plumages. Amer. Mus. Novit., no. 666:1-10. [Pg 351] 1933g. Die Vogelwelt Polynesiens. Mitt. Zool., Mus. Berlin, 19:306-323. 1935. Descriptions of twenty-five new species and subspecies. Amer. Mus. Novit., no. 820:1-6. 1936. Descriptions of twenty-five species and subspecies. Amer. Mus. Novit., no. 828:1-19. 1937. Notes on New Guinea birds I. Amer. Mus. Novit., no. 915:1-19. 1938. Notes on New Guinea birds IV. Amer. Mus. Novit., no. 1006:1-16. 1940. Speciation phenomena in birds. Amer. Nat., 74:249-278, 7 figs. 1941a. Borders and subdivisions of the Polynesian Region based on our knowledge of the distribution of the birds. Proc. 6th Pacific Sci. Cong. 1939, 4:191-195. 1941b. The origin and the history of the bird fauna of Polynesia. Proc. 6th Pacific Sci. Cong., 1939, 4:197-216. 1942a. Notes on the Polynesian species of Aplonis. Amer. Mus. Novit., no. 1166:1-6. 1942b. Systematics and the origin of species from the viewpoint of a zoologist. Columbia Univ. Press, New York, xiv + 334 pp., 29 figs. 1943. The zoogeographical position of the Hawaiian Islands. Condor, 45:45-48. 1944a. The birds of Timor and Sumba. Bull. Amer. Mus. Nat. Hist., 83:127-194, 4 figs. 1944b. Notes on some genera from the Southwest Pacific. Amer. Mus. Novit., no. 1269:1-8. 1945a. Birds of the Southwest Pacific. The Macmillan Co., New York, xix + 316 pp., 3 pls., 16 figs., 1 map. 1945b. Bird habitats in the Southwest Pacific. Audubon Mag., 47:207-221, 5 figs. 1945c. Bird conservation problems in the Southwest Pacific. Audubon Mag., 47:279-282, 2 figs. Mayr, E., and Amadon, D. 1941. Geographical variation in Demigretta sacra (Gmelin). Amer. Mus. Novit., no. 1144:1-11, 1 map. Mayr, E., and Moynihan, M. 1946. Evolution in the Rhipidura rufifrons group. Amer. Mus. Novit., no. 1321:1-21, 6 figs. Mearns, E. A. 1909. A list of birds collected by Dr. Paul Bartsch in the Philippine Islands, Borneo, Guam, and Midway Island, with descriptions of three new forms. Proc. U. S. Nat. Mus., 36:463-478. Meinertzhagen, R. 1926. Introduction to a review of the genus Corvus. Novit. Zool., 33:57-121, 12 pls. Merrill, E. D. 1945. Plant life of the Pacific world. Fighting Forces Ed., The Infantry Journal, Washington, xviii + 298 pp., 256 figs. 1947. A botanical bibliography of the islands of the Pacific. Contr. U. S. Nat. Herb., 30, pt. 1:i-v, 1-322. [Pg 352] Momiyama, T. T. 1920. Description of a new subspecies of Aplonis from the islands of the western Micronesia. Tori, 2:1-3. 1922. Birds of Micronesia. Descriptions of two new genera and nine new subspecies of birds from Micronesia. The Ornith. Soc. Japan, pt. 1:1-24. 1930. On the birds of Bonin and Iwô Islands. Annot. Ornith. Orientalis. 2:1-186. Moran, J. V. 1946. Birds of Saipan and Tinian. Bull. Mass. Audubon Soc., 30:261-262. Murphy, R. C. 1927. On certain forms of Puffinus assimilis and its allies. Amer. Mus. Novit., no. 276:1-15, 1 fig. 1929. On Pterodroma cooki and its allies. Amer. Mus. Novit., no. 370:1-17, 1 fig. 1936. Oceanic birds of South America. Amer. Mus. Nat. Hist., New York, 1:i-xxii + 1-640, 5 col. pls. (unnumbered), numbered pls. 1-38, figs. 1-61; 2:641-1245, 10 col. pls. (unnumbered), numbered pls. 39-42, figs. 62-80. 1938. The need of insular exploration as illustrated by birds. Science, 88:533-539, 2 figs. Murphy, R. C., and Mathews, G. M. 1929. Birds collected during the Whitney South Sea Expedition. VI. Amer. Mus. Novit., no. 350:1-21, 1 fig. Nehrkorn, A. 1879. Mittheilungen über Nester und Eier des Museums Godeffroy zu Hamburg. Journ. f. Ornith., 27:393-410. 1899. Katalog der Eiersammlung. Braunschweig, vii + 256 pp. Nelson, E. W. 1921. The importance of systematic exploration of the land fauna of islands in the Pacific. Proc. 1st Pan-Pacific Sci. Conf., Honolulu, 1920:265-276. Neumann, O. 1922. Neue Formen aus dem papuanischen und polynesischen Inselreich. Verh. Ornith. Ges. Bayern, 25:234-238.
Oberholser, H. C. 1906. A monograph of the genus Collocalia. Proc. Acad. Nat. Sci. Phila., 58:177-212. 1912. A revision of the forms of the edible-nest swiftlet, Collocalia fuciphaga (Thumberg). Proc. U. S. Nat. Mus., 42:11-20. 1915. A synopsis of the races of the crested tern, Thalasseus bergii (Lichtenstein). Proc. U. S. Nat. Mus., 49:515-526, pl. 66. 1917. The birds of the Anamba Islands. Bull. U. S. Nat. Mus., 98:v + 75 pp., 2 pls. 1919. A revision of the subspecies of the white-collared kingfisher, Sauropatis chloris (Boddaert). Proc. U. S. Nat. Mus., 55:351-395. Ogilvie-Grant, W. R. 1893. Catalogue of the game birds in the collection of the British Museum. 22:xvi + 585, 8 pls. [Pg 353] 1898. In Sharpe and Ogilvie-Grant, Catalogue of the Plataleae, Herodiones. Steganopodes, Pygopodes, Alcae, and Impennes in the collection of the British Museum. 26:329-653, pls. 5-8. Oliver, W. R. B. 1930. New Zealand birds. Fine Arts Ltd., Wellington, viii + 541 pp., illus. Oustalet, M. E. 1881a. Observations sur divers oiseaux de l'Asie et de la Nouvelle-Guinée. Bull. Soc. Philom. de Paris, ser. 7, 5:71-80. 1881b. Monographie des oiseaux de la familie des Mégapodiidés. III. Description des genres et speces. Ann. Sci. Nat., Zool., ser. 6, 9:1-182. 1889. Sur la faune ornithologique des Iles Mariannes. Le Naturaliste, ser. 2, 3:260-261. 1891. Note sur las mégapode de la Pérouse. Ann Sci. Nat. Zool., Paris, 17th ser., 11:196. 1895-'96. Les mammifères et les oiseaux des Iles Mariannes. Nouv. Arch. Mus. Hist. Nat. Paris, ser. 3, 7 (1895):141-228, 7 pls.; 8 (1896):24-74.
Pelzeln, A. von 1865. Reise der östereichischen Fregatte Novara um die Erde in de Jahren 1857, 1858, 1859, unter den Befehlen des Commodure B. von Wüllerstorf-Urbair, 1, pt. 2, Vögel. Vienna, iv + 176 pp., 6 pls. Peters, J. L. 1930. Notes on some night herons. Proc. Boston Soc. Nat. Hist., 39:266-277. 1931-'45. Check-list of the birds of the World. Harvard Univ. Press, Cambridge, 1 (1931):xviii + 345 pp.; 2 (1934):xvii + 401 pp.; 3 (1937):xiii + 311 pp.; 4 (1940):xii + 291; 5 (1945):xi + 306. Peters, J. L., and Griscom, L. 1928. A new rail and a new dove from Micronesia. Proc. New England Zoöl. Club, 10:99-106. Phillips, J. C. 1923. A natural history of the ducks. Houghton Mifflin Co., Boston and New York, 2:xii + 409 pp., 26 pls., 38 maps. Price, W. 1936a. The South Sea adventure. The Hokuseido Press, Tokyo, xiv + 313, illus. 1936b. Mysterious Micronesia, Yap, Map and other islands under the Japanese Mandate, etc. Nat. Geogr. Mag., 69:481-510. Prowazek, S. von 1913. Die deutschen Marianen. Leipzig, 125 pp. Pucheran, J. 1853. Voyage au Pôle Sud et dans l'Océanie, sur les corvettes l'Astrolabe et la Zélée, exécuté par ordre du Roi, pendant les années 1837-40. Paris. vol. III, Zoologie, Mammiferes et Oiseaux: 1-166.
Quinn, J. 1946. Pheasants flown to Guam. Calif. Fish and Game, 32:32-33. [Pg 354] Quoy, J. R. C., and Gaimard, P. J. 1824-'26. Voyage autour du monde. Entepres par ordre du Roi. Exécuté sur les corvettes de S. M. l'Uranie et la Physicienne, pendant les années 1817, 1818, 1819, et 1820. Par M. Louis de Freycinet, Capitaine de Vaisseau. Paris, Zoologie: 712 pp., Atlas: 96 pls. 1825. Notice sur les mammifères et les oiseaux des îles Timor, Rawak, Boni, Vaigiou, Guam, Rota et Tinian. Ann. Sci. Nat. Zool., 6:138-150. 1830-'35. Voyage de decouvertes de la corvette l'Astrolabe, exécuté par ordre du Roi, pendant les années 1826, 1827, 1828, et 1829 sous le commandement de M. J. Dumont D'Urville. Paris, Zoologie:xlx + 268 pp.
Reichenow, A. 1885. Bericht über die Januar-Sitzung. Journ. f. Ornith., 33:108-110. 1899. Aufzeichnung. (Vogelzug auf der Marschall Inseln.) Ornith. Monatsber., 7:41. 1901. Eine auffallende Vogelzustrasse vom nordwestlichen Nordamerika nach Polynesien. Ornith. Monatsber., 9:17-18. 1902. Vögel von Nauru. Journ. f. Ornith., 50:254-257. 1913-'14. Die Vögel. Handbuch der systematischen Ornithologie. Stuttgart, 1 (1913):viii + 529 pp.; 2 (1914):viii + 628 pp. 1915. Neue Arten. Journ. f. Ornith., 63:124-129. Reichenow, A., and Schalow, H. 1881. Compendium der neu beschriebenen Gattungen und Arten. Journ. f. Ornith., 29:70-102. Reid, C. F. 1939. Bibliography of the island of Guam. The H. W. Wilson Co., New York, 109 pp. Rensch, B. 1938. Some problems of geographical variation and species-formation. Proc. Linnean Soc. London: 275-285. Ridgway, R. 1882. Description of a new owl from Porto Rico. Proc. U. S. Nat. Mus., 4:366-371. 1912. Color standards and color nomenclature. Published by the author, Washington, D. C.: iii + 43 pp., 53 pls. 1919. The Birds of North and Middle America. Bull. U. S. Nat. Mus., 50, pt. VIII: xvi + 852 pp., 34 pls Ripley, S. D. 1944. The bird fauna of the West Sumatra Islands. Bull. Mus. Comp. Zoöl., 94:305-430, 2 pls. 1948. First record of Anhingidae in Micronesia, Auk, 65:454-455. Ripley, S. D., and Birckhead, H. 1942. On the fruit-pigeons of the Ptilinopus purpuratus group. Amer. Mus. Novit., no. 1192:1-14. Rothschild, W. 1893-1900. The avifauna of Laysan and the neighbouring islands: with a complete history to date of the birds of the Hawaiian possessions. London: 34 + 320 + 21 pp., 83 col. pls. [Pg 355] 1904. New species of kingfishers from Bougainville Island, Solomon Archipelago and Asuncion Island, N. Mariannes. Bull. British Ornith. Club, 15:5-7. Rutland, J. 1896. Traces of civilization: An inquiry into the history of the Pacific. Trans. and Proc. New Zealand Inst., 29:1-51.
Safford, W. E. 1902. Birds of the Marianne Islands and their vernacular names. Osprey, 6:39-42 and 65-70. 1902-'04. Extracts from the note-book of a naturalist on the island of Guam. I-XXV. The Plant World, 5 (1902):161-168, 193-198; 6 (1903):25-32, 49-54, 73-78, 97-103, 123-130, 147-153, 173-179, 205-211, 232-237, 257-262, 278-284; 7 (1904):1-8, 25-31, 53-60, 81-87, 113-118, 141-146, 163-169, 189-195, 213-220, 237-245, 261-268, 286-298. 1903. Guam and its people. Amer. Anthro., (n. s.), 4:707-729, pls. 27-30. 1905. The useful plants of the island of Guam. Contr. U. S. Nat. Herb., 9:416 pp., 69 pls., 1 map. Salvadori, T. 1879. Di alcune specie del genere Porphyrio Brisson. Atti della Reale Accad. delle Sci. di Torino. 14:1165-1170. 1880-'89. Ornithologia della Papuasia e delle Molucche. Turin, 1 (1880):xi + 573 pp.; 2 (1881):xvi + 705 pp.; 3 (1882):xv + 595 pp.; suppl. (1889):244 pp 1894. Anas oustaleti, n. sp. Bull. British Ornith. Club, 20:1. 1895. Catalogue of the Chenomorphe, Crypturi and Ratitae in the collection of the British Museum. 27:xv + 636 pp., 19 pls. Salvin, O. 1888. Critical notes on the Procellariidae. Ibis, 3d ser., 6:351-360. 1896. In Saunders and Salvin, Catalogue of the Gaviae and Tubinares in the British Museum. 25:340-475, 8 pls. Saunders, H. and Salvin, O. 1896. Catalogue of the Gaviae and Tubinares in the collection of the British Museum. 25:1-339, 1 pl. Schmeltz, J. D. E., and Krause, R. 1881. Ein Beitrag Zur Kunde der Südsee-Völker. Die Ethnographische-Anthropologische Abth. Mus. Godeffroy in Hamburg. L. Friederichsen & Co., Hamburg; xxxii + 687 pp., 46 pls., 1 map. Schnee, P. von, and Krause, R. 1901. Eine auffallende Vogelzustrasse vom nordwestlichen Nordamerika nach Polynesien. Ornith. Monatsber., 9:131-132. 1904. Die Landfauna der Marshall-Inseln. Zool. Jahrbüch., 20:387-412. 1912. Einiges über die höhere Tierwelt der Marianen. Zeits. Naturwiss., Leipzig, 82:458-470. Seale, A. 1901. Report of a mission to Guam. Occ. Papers Bernice P. Bishop Mus., 1:17-128. Seebohm, H. 1881. Catalogue of the Passeriformes, or perching birds, in the collection of the British Museum. Cichlomorphae, part II. Turdidae. 5:xvi + 426 pp., 18 pls. [Pg 356] 1891. On the birds of the Volcano Islands. Ibis, 6th ser., 3:109-194. Semper, K. 1881. Animal life as affected by the natural causes of existence. D. Appleton & Co., New York, xvi + 472 pp., illus. Sharpe, R. B. 1876. Contributions to the ornithology of Borneo. Pt. I. Ibis, pp. 29-52. 1879. Catalogue of the Passeriformes, or perching birds, in the collection of the British Museum. Cichlomorphae, part I. 4:xvi + 494 pp., 14 pls. 1881. Catalogue of the Passeriformes, or perching birds, in the collection of the British Museum. Cichlomorphae, part III. Timeliidae. 6:xiii + 420 pp., 18 pls. 1883. Catalogue of the Passeriformes, or perching birds, in the collection of the British Museum. Cichlomorphae, part IV. Timeliidae. 7:xvi + 698 pp., 15 pls. 1890. Catalogue of the Passeriformes, or perching birds, in the collection of the British Museum. Sturniformes. 13:xvi + 701 pp., 15 pls. 1892. Aphanolimnas, n. gen. Bull. British Ornith. Club, 1:19-20. 1894. Catalogue of the Fulicariae and Alectorides in the collection of the British Museum. 23:xiii + 353 pp., 9 pls. 1896. Catalogue of the Liminolae in the collection of the British Museum. 24:xii + 794 pp., 7 pls. 1898. in Sharpe and Ogilvie-Grant, Catalogue of the Plataleae, Herodiones, Steganopodes, Pygopodes, Alcae, and Impennes in the collection of the British Museum. 26:1-328, pls. 1-4. Sharpe, R. B., and Ogilvie-Grant, W. R 1892. Catalogue of the Picariae in the collection of the British Museum. Coraciae (cont.) and Halcyones. 17:1-346 pp., pls. 1-12. Stearns, H. T. 1946. An integration of coral-reef hypotheses. Amer. Journ. Sci., 244:245-262 Stickney, E. H. 1943. Northern shore birds in the Pacific. Amer. Mus. Novit., no. 1248:1-9, 1 fig. Strophlet, J. J. 1946. Birds of Guam. Auk, 63:534-540. Stott, K., Jr. 1947. Notes on Saipan birds. Auk, 64:523-527. Stresemann, E. 1913. Ornithologische Miszellen aus dem indo-australischen Gebiet. Novit. Zool., 20:289-324. 1930. Megazosterops novum genus Zosteropidarum. Ornith. Monatsber., 38:159. 1931a. Die Zosteropiden der indo-australischen Region. Mitt. Zool. Mus. Berlin, 17:201-238. 1931b. Notes on the swiftlets and distribution of some swiftlets (Collocalia) of Malaysia and adjacent subregions. Bull. Raffles Mus., 6:83-191. 1939a. "Heterogynie" im Rassenkreis Edolisoma morio. Ornith. Monatsber., 49:124-126. [Pg 357] 1939-41. Die Vögel von Celebes. Journ. f. Ornith, (1939b):299-425; (1940):1-135, 389-487; (1941):1-102. Swarth, H. S. 1931. The avifauna of the Galápagos Islands. Occ. Papers Calif. Acad. Sci., 18:1-299, map. 1934. The bird fauna of the Galápagos Islands in relation to species formation. Biol. Rev., 9:213-234.
Takatsukasa, N. 1932-38. The birds of Nippon. 1, pts. 1-7 (Aug. 15, 1932; Nov. 10, 1938) incompleted serial. Takatsukasa, S., and Kuroda, N. 1915a. A list of birds collected in the North Pacific islands by the Ornithological Society of Japan. Tori, 1:49-55. 1915b. A table of birds known at present from the various islands and island-groups of western Pacific, formerly belonging to Germany but now occupied by Japan. Tori, 1:60-64. Takatsukasa, S., and Yamashina, Y. 1931a. On Aplornis opaca orii subsp. nov. from the Palau Islands. Dobutsu. Zasshi, 43:458-459. 1931b. Some new birds from the Palau and Mariana Islands. Dobutsu. Zasshi, 43:484-487. 1931c. On two new birds from the Caroline Islands. Dobutsu. Zasshi, 43:599-600. 1931d. On two new forms of the Micronesian birds. Tori, 7:109-110. 1932. Second report on the birds of the South Sea. Dobutsu. Zasshi, 44:221-226.. Townsend, C. H., and Wetmore, A. 1919. Reports on the scientific results of the expedition to the tropical Pacific in charge of Alexander Agassiz, on the US Fish Commission steamer "Albatross" from August, 1899, to March, 1900, Commander Jefferson F. Moser, USN, commanding. XXI. The birds. Bull. Mus. Comp. Zoöl., 63:151-225. Tristram, H. B. 1883. On the position of the Acrocephaline genus Tatare, with descriptions of the two new species of the genus Acrocephalus. Ibis, 5th ser., 1:38-46, pls. 1-2. 1889. Catalogue of a collection of birds belonging to H. B. Tristram. Durham, xvi + 278 pp.
Uchida, S. 1918. Mallophaga from birds of the Ponapé I. (Carolines) and the Palau Is. (Micronesia). Annot. Zool. Japon., 9:480-493. United States Navy Department 1943. Military Government Handbook. Marshall Islands. Office Chief Naval Operations, U. S. Navy Dept., Washington, OPNAV 50E—1:113 pp., 1 map. 1944a. Civil Affairs Handbook. East Caroline Islands (published as a restricted publication, February 21, 1944; unclassified May 2, 1945). Office Chief Naval Operations, U. S. Navy Dept., Washington, OPNAV 50E—5:213 pp., 43 figs., 1 map. [Pg 358] 1944b. Civil Affairs Handbook. West Caroline Islands. Office Chief Naval Operations, U. S. Navy Dept., Washington, OPNAV 50E-7:222 pp., 40 figs., 1 map. 1944c. Civil Affairs Handbook. Mandated Marianas Islands. Office Chief Naval Operations, U. S. Navy Dept., Washington, OPNAV 50E-8:205 pp., 26 figs., 1 map.
Wallace, A. R. 1876. The geographical distribution of animals. Harper and Brothers, New York, 1:xxi + 503 pp., 13 pls., 5 maps. 1881. Island life. Harper and Brothers, New York:xvi + 522 pp., illus., 3 maps. Watson, R. J. 1946. Bird notes from Guam. The Raven, 17:40-42. Wheeler, J. 1900. Report on the island of Guam. Adjutant-General's Office, War Dept., no. 28:1-51. Wetmore, A. 1917. A new honey-eater from the Marianne Islands. Proc. Biol. Soc. Washington, 30:117-118. 1940. A systematic classification for the birds of the world. Smithsonian Misc. Coll., 99, no. 7:1-11. Wharton, G. W. 1946. Observations on Ascoschongastia indica (Hirst 1915). Ecol. Monogr., 16:151-184, 24 figs. Wharton, G. W., and Hardcastle, A. B. 1946. The genus Neoschöngastia (Acarinida: Trombiculidae) in the western Pacific area. Journ. Parasit., 32:286-322. Wiglesworth, L. W. 1891a. Aves Polynesiae. A catalogue of the birds of the Polynesian Subregion. Abhandl. und Ber. Zool. Mus. Dresden, no. 6 (1890-1891): vi + 92 pp. 1891b. On the Polynesian members of the genus Ptilopus. Ibis, 6th ser., 3:566-584. 1893. Remarks on the birds of the Gilbert Islands. Ibis, 6th ser., 5:210-215. Wilson, H. 1788. An account of the Pelew Islands, situated in the western part of the Pacific Ocean, composed from the journals and communications of Captain Henry Wilson and some of his officers, who, in August, 1783, were there shipwrecked, in the Antelope, a packet belonging to the honourable East India Company, compiled by George Keate. 2d ed., London:xxvii + 378 pp., illus. Wright, S. 1931. Evolution in Mendelian populations. Genetics, 16:97-159, 21 figs. Wynne-Edwards, V. C. 1935. On the habits and distribution of birds on the North Atlantic. Proc. Boston Soc. Nat. Hist., 40:233-346, pls. 3-5. [Pg 359]
Yamada, Y. 1926. The phyto-geographical relation between the Chlorophyceas of the Mariannes, Carolines and Marshall Islands and those of the Malay Archipelago, Australia and Japan. Proc. 3d Pan-Pacific Congr., Tokyo, 1:964-966. Yamashina, Y. 1932a. On a collection of birds' eggs from Micronesia. Tori, 7:393-413. 1932b. On the distribution of the birds in Micronesia. Bull. Biogeogr. Soc. Japan, 3:139-148, pls. 8-12 1938. A new genus of the owl. Tori, 10:1-2. 1940. Some additions to the "List of the birds of Micronesia." Tori, 10:673-679. 1942. A new subspecies of Conopoderas luscinia from the Mariana Islands. Bull. Biogeogr. Soc. Japan, 12:81-83, 1 fig. 1948. Notes on the Marianas Mallard. Pacific Science, 2:121-124.
Transmitted July 28, 1949.
22-8131
UNIVERSITY OF KANSAS PUBLICATIONS
The University of Kansas Publications, Museum of Natural History, are offered in exchange for the publications of learned societies and institutions, universities and libraries. For exchanges and information, address the Exchange Desk, University of Kansas Library, Lawrence, Kansas, U. S. A.
Museum of Natural History.— E. Raymond Hall, Chairman, Editorial Committee.
This series contains contributions from the Museum of Natural History. Cited as Univ. Kans. Publ., Mus. Nat. Hist.
| Vol. 1. | 1. | The pocket gophers (genus Thomomys) of Utah. By Stephen D. Durrant. Pp. 1-82, 1 figure in text. August 15, 1946. |
| 2. | The systematic status of Eumeces pluvialis Cope, and noteworthy records of other amphibians and reptiles from Kansas and Oklahoma. By Hobart M. Smith. Pp. 85-89. August 15, 1946. | |
| 3. | The tadpoles of Bufo cognatus Say. By Hobart M. Smith. Pp. 93-96, 1 figure in text. August 15, 1946. | |
| 4. | Hybridization between two species of garter snakes. By Hobart M. Smith. Pp. 97-100. August 15, 1946. | |
| 5. | Selected records of reptiles and amphibians from Kansas. By John Breukelman and Hobart M. Smith. Pp. 101-112. August 15, 1946. | |
| 6. | Kyphosis and other variations in soft-shelled turtles. By Hobart M. Smith. Pp. 117-124. July 7, 1947. | |
| 7. | Natural history of the prairie vole (Mammalian genus Microtus). By E. W. Jameson, Jr. Pp. 125-151, 4 figures in text. October 6, 1947. | |
| 8. | The postnatal development of two broods of great horned owls (Bubo virginianus). By Donald F. Hoffmeister and Henry W. Setzer. Pp. 157-173, 5 figures in text. October 6, 1947. | |
| 9. | Additions to the list of the birds of Louisiana. By George H. Lowery, Jr. Pp. 177-192. November 7, 1947. | |
| 10. | A check-list of the birds of Idaho. By M. Dale Arvey. Pp. 193-216. November 29, 1947. | |
| 11. | Subspeciation in pocket gophers of Kansas. By Bernardo Villa-R. and E. Raymond Hall. Pp. 217-236, 2 figures in text. November 29, 1947. | |
| 12. | A new bat (Genus Myotis) from Mexico. By Walter W. Dalquest and E. Raymond Hall. Pp. 237-244, 6 figures in text. December 10, 1947. | |
| 13. | Tadarida femorosacca (Merriam) in Tamaulipas, Mexico. By Walter W. Dalquest and E. Raymond Hall. Pp. 245-248, 1 figure in text. December 10, 1947. | |
| 14. | A new pocket gopher (Thomomys) and a new spiny pocket mouse (Liomys) from Michoacán, Mexico. By E. Raymond Hall and Bernardo Villa-R. Pp. 249-256, 6 figures in text. July 26, 1948. | |
| 15. | A new hylid frog from eastern Mexico. By Edward H. Taylor. Pp. 257-264, 1 figure in text. August 16, 1948. | |
| (Continued on inside of back cover.) | ||
| (Continued from inside of front cover.) | ||
| Vol. 1. | 1. | The pocket gophers (genus Thomomys) of Utah. By Stephen D. Durrant. Pp. 1-82, 1 figure in text. August 15, 1946. |
| 16. | A new extinct emydid turtle from the Lower Pliocene of Oklahoma. By Edwin C. Galbreath. Pp. 265-280, 1 plate. August 16, 1948. | |
| 17. | Pliocene and Pleistocene records of fossil turtles from western Kansas and Oklahoma. By Edwin C. Galbreath. Pp. 281-284, 1 figure in text. August 16, 1948. | |
| 18. | A new species of heteromyid rodent from the Middle Oligocene of northeastern Colorado with remarks on the skull. By Edwin C. Galbreath. Pp. 285-300, 2 plates. August 16, 1948. | |
| 19. | Speciation in the Brazilian spiny rats (Genus Proechimys, Family Echimyidae). By João Moojen. Pp. 301-406, 140 figures in text. December 10, 1948. | |
| 20. | Three new beavers from Utah. By Stephen D. Durrant and Harold S. Crane. Pp. 407-417, 7 figures in text. December 24, 1948. | |
| 21. | Two new meadow mice from Michoacán Mexico. By E. Raymond Hall. Pp. 423-427, 6 figures in text. December 24, 1948. | |
| 22. | An annotated check list of the mammals of Michoacán, Mexico. By E. Raymond Hall and Bernardo Villa-R. Pp. 431-472, 5 figures in text. December 27, 1949. | |
| 23. | Subspeciation in the kangaroo rat, Dipodomys ordii. By Henry W. Setzer. Pp. 473-573, 27 figures in text. December 27, 1949. | |
| 24. | Geographic range of the hooded skunk, Mephitis macroura, with description of a new subspecies from Mexico. By E. Raymond Hall and Walter W. Dalquest, Pp. 575-580, 1 figure in text. January 20, 1950. | |
| 25. | Pipistrellus cinnamomeus Miller 1902 referred to the genus Myotis. By E. Raymond Hall and Walter W. Dalquest. Pp. 581-590, 5 figures in text. January 20, 1950. | |
| 26. | A synopsis of the American bats of the genus Pipistrellus. By E. Raymond Hall and Walter W. Dalquest. Pp. 591-602, 1 figure in text. January 20, 1950. | |
| Index, Pp. 605-638. | ||
| Vol. 2. | (Complete) Mammals of Washington. By Walter W. Dalquest. Pp. 1-444, 140 figures in text. April 9, 1948. | |
| Vol. 3. | 1. | The avifauna of Micronesia, its origin, evolution, and distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June 12, 1951. |