
Project Gutenberg's Scientific American Supplement, No. 288, by Various
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
Title: Scientific American Supplement, No. 288
July 9, 1881
Author: Various
Posting Date: October 10, 2012 [EBook #8391]
Release Date: June, 2005
First Posted: July 6, 2003
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC AMERICAN SUPPL., NO. 288 ***
Produced by Olaf Voss, Don Kretz, Juliet Sutherland, Charles
Franks and the Online Distributed Proofreading Team.
A Chemist of merit, Mr. A. Müntz, who has already made himself known by important labors and by analytical researches of great precision, has been led to a very curious and totally unexpected discovery, on the subject of which he has kindly given us information in detail, which we place before our readers.[1] Mr. Müntz has discovered that arable soil, waters of the ocean and streams, and the atmosphere contain traces of alcohol; and that this compound, formed by the fermentation of organic matters, is everywhere distributed throughout nature. We should add that only infinitesimal quantities are involved--reaching only the proportion of millionths--yet the fact, for all that, offers a no less powerful interest. The method of analysis which has permitted the facts to be shown is very elegant and scrupulously exact, and is worthy of being made known.
[Footnote 1: The accompanying engravings have been made from drawings of the apparatus in the laboratory of which Mr. Müntz is director, at the Agronomic Institute.]
FIG. 1.--FIRST DISTILLATORY APPARATUS.
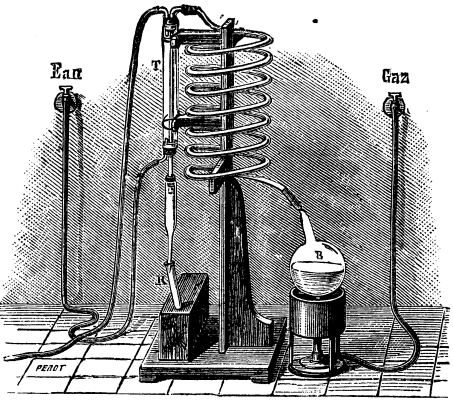
FIG. 2.--SECOND DISTILLATORY APPARATUS.
Mr. Müntz's method of procedure is as follows: He submits to distillation three or four gallons of snow, rain, or sea water in an apparatus such as shown in Fig. 1. The part which serves as a boiler, and which holds the liquid to be distilled, is a milk-can, B. The vapors given off through the action of the heat circulate through a leaden tube some thirty-three feet in length, and then traverse a tube inclosed within a refrigerating cylinder, T, which is kept constantly cold by a current of water. They are finally condensed in a glass flask, R, which forms the receiver. When 100 or 150 cubic centimeters of condensed liquid (which contains all the alcohol) are collected in the receiver, the operations are suspended. The liquid thus obtained is distilled anew in a second apparatus, which is analogous to the preceding but much smaller (Fig. 2). The liquid is heated in the flask, B, and its vapor, after traversing a glass worm, is condensed in the tube, T. The operation is suspended as soon as five or six cubic centimeters of the condensed liquid have been collected in the test-tube, R. The latter is now removed, and to its liquid contents, there is added a small quantity of iodine and carbonate of soda. The mixture is slightly heated, and soon there are seen forming, through precipitation, small crystals of iodoform. Under such circumstances, iodoform could only have been formed through the presence of an alcohol in the liquid. These analytical operations are verified by Mr. Müntz as follows: He distills in the same apparatus three to four gallons of chemically pure distilled water, and ascertains positively that under these conditions iodine and carbonate of soda give absolutely no reaction. Finally, to complete the demonstration and to ascertain the approximate quantity of alcohol contained in natural waters, he undertakes the double fractional distillation of a certain quantity of pure water to which he has previously added a one-millionth part of alcohol. Under these circumstances the iodine and carbonate of soda give a precipitate of iodoform exactly similar to that obtained by treating natural waters.

Fig. 3.--IODOFORM CRYSTALS OBTAINED
DIRECTLY (greatly magnified).

FIG. 4,--IODOFORM CRYSTALS OBTAINED WITH
RAIN WATER.
In the case of arable soil, Mr. Müntz stirs up a weighed quantity of the material to be analyzed in a certain proportion of water, distills it in the smaller of the two apparatus, and detects the alcohol by means of the same operation as before.

FIG. 5.--IODOFORM CRYSTALS OBTAINED WITH SNOW WATER.
The formation of iodoform by precipitation under the action of iodine and carbonate of soda is a very sensitive test for alcohol. Iodoform has sharply defined characters which allow of its being very easily distinguished. Its crystalline form, especially, is entirely typical, its color is pale yellowish, and, when it is examined under the microscope, it is seen to be in the form of six-pointed stars precisely like the crystalline form of snow. Mr. Müntz has not been contented to merely submit the iodoform precipitates obtained by him to microscopical examination, but has preserved the aspect of his preparations by means of micro-photography. The figures annexed show some of the most characteristic of the proofs. Fig. 1 shows crystals of iodoform obtained with pure water to which one-millionth part of alcohol had been added. Fig. 2 exhibits the form of the crystals obtained with rain water; and Fig. 3, those with water. Fig. 4 shows crystals obtained with arable soil or garden mould. The first of Mr. Müntz's experiments were made about four years ago; but since that time he has treated a great number of rain and snow waters collected both at Paris and in the country. At every distillation all the apparatus was cleansed by prolonged washing in a current of steam; and, in order to confirm each analysis, a corresponding experiment was made like the one before mentioned. More than eighty trials gave results which were exactly identical. The quantity of alcohol contained in rain, snow, and sea waters may be estimated at from one to several millionths. Cold water and melted snow seem to contain larger proportions of it than tepid waters. In the waters of the Seine it is found in appreciable quantities, and in sewage waters the proportions increase very perceptibly. Vegetable mould is quite rich in it; indeed it is quite likely that alcohol in its natural state has its origin in the soil through the fermentation of the organic matters contained therein. It is afterward disseminated throughout the atmosphere in the state of vapor and becomes combined with the aqueous vapors whenever they become condensed. The results which we have just recorded are, as far as known to us, absolutely new; they constitute a work which is entirely original, which very happily goes to complete the history of the composition of the soil and atmosphere, and which does great credit to its author.--La Nature.

FIG. 6.--IODOFORM CRYSTALS OBTAINED WITH VEGETABLE MOULD.
It appears that every article manufactured with the aid of alcohol is required on its introduction into France to pay duty on the supposed quantity of this reagent which has been used in its preparation. Certain transparent soaps of German origin are now met with, made, as is alleged, without alcohol, and the author proposes the following process for verifying this statement by ascertaining--the presence or absence of alcohol in the manufactured article: 50 grms. of soap are cut into very small pieces and placed in a phial of 200 c.c. capacity; 30 grms. sulphuric acid are then added, and the phial is stoppered and agitated till the soap is entirely dissolved. The phial is then filled up with water, and the fatty acids are allowed to collect and solidify. The subnatant liquid is drawn off, neutralized, and distilled. The first 25 c.c. are collected, filtered, and mixed, according to the process of MM. Riche and Bardy for the detection of alcohol in commercial methylenes, with ½ c.c. sulphuric acid at 18° B., then with the same volume of permanganate (15 grms. per liter), and allowed to stand for one minute. He then adds 8 drops of sodium hyposulphite at 33° B., and 1 c.c. of a solution of magenta, 1 decigrm. per liter. If any alcohol is present there appears within five minutes a distinct violet tinge. The presence of essential oils gives rise to a partial reduction of the permanganate without affecting the conversion of alcohol into aldehyd.
A simple experiment, capable of yielding results which shall be at least comparative, has long been sought after by large consumers of coal and artificial fuel abroad in order to ascertain the relative calorific power possessed by each description, as it is well known that the proportion of mineral matter and the chemical composition of coal differ widely. The determination of the ash in coal is not a highly scientific operation; hence it is not surprising that foreign merchants should have become alive to the importance of estimating its quantity. While, however, the nature and quantity of the ash can be determined without much difficulty, the determination of the chemical composition of coal entails considerable labor and skill; hence a method giving the calorific power of any fuel in an exact and reliable manner by a simple experiment is a great desideratum. This will become more obvious when one takes into consideration the many qualities and variable characters of the coals yielded by the South Wales and North of England coal fields. Bituminous coals--giving some 65 per cent, of coke--are preferred for some manufacturing purposes and in some markets. Bituminous steam coals, yielding 75 per cent, of coke, are highly prized in others. Semi-bituminous steam coals, yielding 80 to 83 per cent, of coke, are most highly valued, and find the readiest sale abroad; and anthracite steam coal (dry coals), giving from 85 to 88 per cent, of coke (using the term "coke" as equivalent to the non-volatile portion of the coal) is also exported in considerable quantity. Now the estimation of the ash of any of these varieties of coal would afford no evidence as to the class to which that coal belongs, and there is no simple test that will give the calorific power of a coal, and at the same time indicate the degree of bituminous or anthracitic character which it possesses.
In order to obtain such information it is necessary that the percentage of coke be determined together with the sulphur, ash, and water, and these form data which at once show the nature of a fuel and give some indication of its value. To ascertain the quantity of the sulphur, ash, and water with accuracy involves more skill and aptitude than can be bestowed by the non-professional public; the consequence is that experiments entailing less time and precision, like those devised by Berthier and Thompson, have been tried more or less extensively. In France and Italy, Berthier's method--slightly modified in some instances--has been long used. It is as follows:
70 grammes of oxide of lead (litharge) and 10 grammes of oxychloride of lead are employed to afford oxygen for the combustion of 1 gramme of fuel in a crucible. From the weight of the button of lead, and taking 8,080 units as the equivalent of carbon, the total heat-units of the fuel is calculated. This experiment is very imperfect and erroneous upon scientific grounds, since the hydrogen of the fuel is scarcely taken into account at all. In the first place, hydrogen consumes only one quarter as much oxygen as carbon, and, furthermore, two-ninths only of the heating power of hydrogen is used as the multiplying number, viz., 8,080, while the value of hydrogen is 34,462. In other words, one-eighteenth only of the available hydrogen present in the fuel is shown in the result obtained. Apart from this my experience of the working of Berthier's method has been by no means satisfactory. There is considerable difficulty in obtaining pure litharge, and it is almost impossible to procure a crucible which does not exert a reducing action upon the lead oxide. Some twelve months ago I went out to Italy to test a large number of cargoes of coal with Thompson's calorimeter, and since then this apparatus has superseded Berthier's process, and is likely to come into more general use. Like Berthier's method, Thompson's apparatus is not without its disadvantages, and the purpose of this paper is to set these forth, as well as to suggest a uniform method of working by means of which the great and irreconcilable differences in the results obtained by some chemists might be overcome. It has already been observed that a coal rich in hydrogen shows a low heating power by Berthier's method, and it will become evident on further reflection that the higher the percentage of carbon the greater will be the indicated calorific power. In fact a good sample of anthracite will give higher results than any other class of coal by Berthier's process. With Thompson's calorimeter the reverse is the case, as the whole of the heating power of the hydrogen is taken into account. In short, with careful working, the more bituminous a coal is the more certain is it that its full heating power shall be exerted and recorded, so far as the apparatus is capable of indicating it; for when the result obtained is multiplied by the equivalent of the latent heat of steam the product is always below the theoretical heat units calculated from the chemical composition of the coal by the acid of Favre and Silbermann's figures for carbon and hydrogen. On the other hand, when the heating power of coal low in hydrogen is determined by Thompson's calorimeter, much difficulty is experienced in burning the carbon completely; hence a low result is obtained. From a large number of experiments I have found that when a coal does not yield more than 86 per cent, of coke, it gives its full comparative heating power, but it is very questionable if equal results will be worked out if the coke exceeds the above amount although I have met with coals giving 87 per cent. of coke which were perfectly manageable, though in other cases the coal did not burn completely. It will be noted that the non-volatile residue of anthracite is never as low as 86 per cent., and this, together with the very dry steam coals and bastard anthracite (found over a not inextensive tract of the South Wales Coal field), form a series of coals, alike difficult to burn in Thompson's calorimeter. Considerable experience has shown that in no single instance was the true comparative heating power of anthracite or bastard anthracite indicated. With a view to accelerate the perfect combustion of these coals, sugar, starch, bitumen, and bituminous coals--substances rich in hydrogen--were employed, mixed in varying proportions with the anthracitic coal, but without the anticipated effect. Coke was also treated in a like manner. Without enlarging further upon these futile trials--all carefully and repeatedly verified--the results of my experiments and experience show that for coals of an anthracitic character, yielding more than 87 per cent. of coke, or for coke itself, Thompson's calorimeter is not suited as an indicator of their comparative calorific power, for the simple reason that some of the carbon is so graphitic in its nature that it will not burn perfectly when mixed with nitrate and chlorate of potash. A sample of very pure anthracite used in the experiments referred to, gave 90.4 per cent. of non-volatile residue, and only 0.84 per cent. of ash. This coal was not difficult to experiment with, as combustion started with comparative ease and proceeded quite rapidly enough, but in every instance a portion of the carbon was unconsumed, and consequently instead of about 13° of rise in temperature only 10° were recorded.
Since the calorific power of a coal is determined by the number of degrees Fahrenheit which a given quantity of water is raised in temperature by a known weight of fuel, it follows that every care should be taken that the experiment be performed under similar atmospheric conditions. The oscillation of barometric pressure does not appear to affect the working, but the temperature of the room in which the work was done, and especially that of the water, are most important considerations. It has been observed by some who have used this apparatus--and I have frequently noticed it myself--that the lower the temperature of the water is under which the fuel is burnt the higher is the result found. This has been explained on the assumption that the colder the water used, the greater is the difference between the temperature of the room and that of the water; hence it would be expedient that in all cases when such experiments are made the same difference of temperature between the air in the room and the water employed should always exist. For example, if the temperature of the room were 70°, and the water at 60°, then the same coal would give a like result with the water at 40° and the room at 50°. This has been regarded as the more evident, because the gases passing through the water escape under favorable conditions of working at the same temperature as the water, and are perfectly deprived of any heat in excess of that possessed by the water. Under these circumstances it would seem only reasonable that this assumption should be correct. It was, however, found after a large number of experiments upon the same sample of coal that this was not the case. 30 grammes of coal which raises the temperature of the water 13.4°, when the water at starting was 60° and the room at 70°, gives 13.7° rise of temperature with the water at 40° and the room at 50°. Conversely, when the water is at 70° and the room at 80°, a lower result is obtained. The explanation appears to be this: The gas which escapes from the water was not in existence in the gaseous form previous to the experiment, and the heat communicated to the gas being a definite quantity it follows that the more the gas is cooled the greater the proportion of chemical energy in the shape of heat will be utilized and recorded as calorific power.
In order, therefore, to make the experiment more simple and workable at all temperatures, a sample of coal was selected, which should be perfectly manageable and readily consumed. Appended is an analysis of the coal employed (from Ebbw Vale, Monmouthshire):
Composition per cent.
Carbon...............................88.33
Hydrogen............................. 5.08
Oxygen............................... 3.28
Nitrogen............................. 0.55
Sulphur.............................. 0.70
Ash.................................. 1.26
Water (moisture)..................... 0.80
-----
100.00
In the following experiments the standard temperature of the water was taken as 60° F., and as the coal gave 13.4° of rise of temperature, 67° F. was selected as the standard room temperature. The reason for this room temperature is obvious, for, whatever heating effect the higher temperature of the room may have upon the water in the cylinder during the time occupied by the first half of the experiment, would be compensated for by the loss sustained during the second half of the experiment, when the temperature of the water exceeded that of the room. The mean of numerous trials gave 13.4° F. rise of temperature, equal to 14.74 lb. of water per lb. of coal. When the water was at 50° and the room at 57°, the mean of several experiments gave 13.5° rise of temperature. When the water was 40° at starting and the room at 47°, 13.65° was the average rise of temperature. Trials were made at intermediate temperatures, and the results always showed that higher figures were recorded when the water was coldest. With a view of getting uniformity in the results it was thought well to make experiments, in order to find out what temperature the room should be at, so that this coal might give the same result with the water at 50°, 40°, or at intermediate temperatures. Without going much into detail, it was found that when the temperature of the room was at 40° and that of the water 40°, and the experiment was rapidly and carefully performed, 13.4° rise of temperature was given; but this result could be obtained without special effort when the room was 42° and the water 40° at starting. It is evident that the cooling effect of the air in the room upon the water cylinder is very appreciable when the water has reached 13° above that of the room. When the water was at 50° and the room at 55°, the coal gave 13.4° rise with ease and certainty, and it would not be out of place to remark here that with those coals which burn well in Thompson's calorimeter, the results of several trials are remarkably uniform when properly performed. With the water at 70° and the room at 80°, a like result was worked out. Experiments at intermediate temperatures were also carried out (see table in sequel). It is true that the whole difference of temperature we are dealing with in making these corrections is only 0.25, but 0.2 in the result, when multiplied by 537 to bring it into calories, as is done by the authorities in Italy, makes more than 100 heat units--a serious difference when 5d. per ton fine is attached to every 100 calories lower than the number guaranteed.
Taking the latent heat of steam as 537° C., and multiplying this number by 14.74, the evaporative power of the coal used in these experiments, its equivalent in calories is 7,915. From the analysis of this coal, disregarding the nitrogen and deducting an equivalent of hydrogen for the oxygen present, the total heat units given by Favre and Silbermann's figures for carbon (8,080) and hydrogen (34,462) will be 8,746. It will be seen, therefore, that the calorific power, as determined by Thompson's apparatus, gives a much lower result when multiplied by 537 than the heat units calculated from the chemical composition of the coal. When I used Thompson's apparatus in the chemical laboratory at Turin to determine the evaporative power of various cargoes of South Wales coal, it was agreed by mutual consent that the temperature of the water at starting should be 39° F. (the temperature at which the heat unit was determined). The temperature of the room was about 60°, but this varied, as the weather was somewhat severe and changeable. Under these conditions, with the water at 39° and room 60°, the coal which gives 14.74 lb. of water per lb. of coal, will give as high as 15.88 lb. of water per lb. of coal. This result multiplied by 537=8,496 calories, approaching much more nearly to the theoretic value. This method of working is still practiced abroad, but experience has shown that very widely differing results follow when working in this manner, especially if the temperature of the room is changeable, as it naturally is where ash determinations and other chemical work is proceeding simultaneously. The time the experiment lasts, taking the reading on a quickly rising thermometer and other considerations, render the experiments anything but trustworthy when 0.2 of a degree makes a difference of more than 100 calories. In the instructions supplied with Thompson's calorimeter nothing is said as to the temperature of the room in which the experiment is performed, but simply that the water shall be at 60° F. If, with the water at 60°, a room were at 50°, as it often is in winter, a good coal would give 14 lb. of water per lb. of coal as the evaporative power; but if in summer, the room were at 75° and the water at 60°, the same coal would give 15 lb. of water per lb. of coal. If further evidence were needed of the effect of temperature consideration of the experiments already referred to will show how necessary it is that some general rule shall be adopted. Considerable stress is laid (in the instructions) upon the quantity of oxygen mixture used being determined by rough experiments. This I have found leads to erroneous conclusions unless a number of experiments are tried in the calorimeter, as it often happens that the quantity which appears to be best adapted is not that which yields a trustworthy result. There are many samples of South Wales coal, 30 grains of which will require 10 parts of oxygen mixture in order to burn completely, but since a little oxygen is lost in drying and grinding, and few samples of chlorate are free from chloride, it is not safe to use less than 11 parts of oxygen mixture, but this amount is sufficient in all cases, and never need be exceeded. I have made numerous experiments with various coals (anthracite, steam, semi-bituminous, and bituminous, including a specimen of the ten yard coal of Derbyshire), and find that with 11 parts of chlorate and nitrate of potash, they are all perfectly manageable and yield the best results. It is quite clear that the excess of chlorate is decomposed in all instances, and the latent heat of the oxygen evolved, but those coals which are best to experiment with did not yield results that differed when the quantity of oxygen mixture was reduced to nearly the limit required for combustion of the coal. Under these circumstances, therefore, the constant use of 11 parts of oxygen mixture--a suitable quantity for all coals exported--would enable operators to obtain similar figures, and make the test uniform in different hands.
The following is a brief outline of the method of procedure recommended: Sample the coal until an average portion passes through a sieve having 64 meshes to the square inch. Take about 300 grains (20 grammes) of this and run through a brass wire gauze having 4,600 meshes to the square inch, taking care that the whole sample selected is thus treated. One part of nitrate of potash and 3 parts of chlorate of potash (dry) are separately ground in a mortar, and repeatedly sifted through another wire gauze sieve, having 1,000 meshes to the square inch, in order that the oxygen mixture shall not be ground to an impalpable powder, as this is very undesirable. It absorbs moisture rapidly, and interferes with the regularity of the combustion when very fine. 330 grains of the powder are weighed out (after drying), and intimately incorporated with 30 grains of coal--better with a spatula than by rubbing in a mortar--and then introduced into a copper cylinder (3½ inches long by ¾ inch wide, made from a copper tube), and pressed down in small portions by a test-tube with such firmness as is required by the nature of the coal, not tapped on the bottom, since the rougher portions of the oxygen mixture rise to the surface. As the temperature of a room is almost invariably much higher than the water supply, a little hot water is added to that placed in the glass cylinder, until the difference of temperature between the water and the room is about the mark indicated in the following table:
Room at The water should be
80° F. 70° F. 72 64 67 60 60 54 55 50 50 46 42 40
Say, for example, the room was at 57° and the water placed in the cylinder was at 46°: add a little hot water and stir with the thermometer until it assumes 52°. By the time the excess of water has been removed with a pipette until it is exactly level with the mark, and all is ready, the temperature will rise nearly 0.5°. Let the thermometer be immersed in the water at least three minutes before reading. The fuse should be placed in the mixture, and everything at hand before reading and removing the thermometer. After igniting the fuse and immersing the copper cylinder in the water, the apparatus should be kept in the best position for the gases to be evolved all around the cylinder, and the rate of combustion noted. Some coals are very unmanageable without practice, and samples of "patent fuel" are sometimes met with, containing unreasonable proportions of pitch, which require some caution in working and very close packing, inasmuch as small explosions occur during which a little of the fuel escapes combustion.
In order that the experiment shall succeed well, experience has shown that the nature of the fuse employed has much to do with it. Plaited or woven wick is not adapted, and will fail absolutely with dry coals, unless it is made very free burning. In this case not less than three-quarters of an inch in length is necessary, and the weight of such is very appreciable. I always use Oxford cotton, and thoroughly soak it in a moderately strong solution of nitrate of potash. When dry it should burn a little too fast. The cotton is rubbed between two pieces of cloth until it burns just freely enough; then four cotton strands are taken, twisted together, and cut into lengths of ¾ inch and thoroughly dried. Open out the fuse at the lower end when placing it in the mixture so as to expose as much surface as possible in order to get a quick start, but carefully avoid pressing the material, and use a wire to fill up close to the fuse. A slow start often spoils the experiment, through the upper end of the cylinder becoming nearly filled up with potassic chloride, etc.
By paying attention to such details, and following the method recommended, the apparatus yields very satisfactory results with bituminous and semi-bituminous coals.--Chemical News.
Words pass along with meanings which are simple conventionalities, marking current opinions, knowledge, fancies, and misjudgments. They attain to new accretions of import as knowledge advances or opinions change, and they are applied now to one set of ideas, now to another. Hence there is nothing truer than the saying, "definitions are never complete." The term explosion in its original introduction denoted the making of a noise; it grew to comprehend the idea of force accompanied with violent outburst; it is advancing to a stage in which it implies combustion as associated with destruction, yet somewhat distinct from the abstract idea of the resolution of any form of matter into its elementary constituents. The term, however, as yet takes in the idea of combustion as a decomposition in but a very limited degree, and it may be said to be wavering at the line between expansion and dissociation.
Strictly, in insurance, fire and explosion are different phenomena. A policy insuring against fire-loss does not insure against loss by explosion. It thereby enforces a distinction which exists, or did exist, in the popular mind; and fire, in an insurance sense, as distinct from explosion, was accurately defined by Justice McIlvaine, of the Supreme Court of Ohio (1872), in the case of the Union Insurance Company vs. Forte, i.e., an explosion was a remote cause of loss and not the proximate cause, when the fire was a burning of a gas jet which did not destroy, though the explosion caused by the burning gas-jet did destroy. Earlier than this decision, however (in 1852), Justice Cushing, of the Supreme Court of Massachusetts, in Scripture vs. Lowell Mutual Fire Insurance Company, somewhat anticipated later definition, and pronounced for the liability of the underwriter where all damage by the explosion involves the ignition and burning of the agent of explosion. That is, for example, the insurer is liable for damage caused by an explosion from gunpowder, but not for an explosion from steam. The Massachusetts Judge did not conceive any distinction as to fire-loss between the instantaneous burning of a barrel of gunpowder and the slower burning of a barrel of sulphur, and insurance fire-loss is not to be interpreted legally by thermo-dynamics nor thermo chemistry. While the legal principles are as yet unsettled, the tenor of current decisions may be summed up as follows: If explosion cause fire, and fire cause loss, it is a loss by fire as proximate cause; and if fire cause explosion, and explosion cause loss, it is a loss by fire as efficient cause. Smoke, an imperfect combustion, damages, in an insurance sense, as well as flame, which is perfect combustion; and where there is concurrence of expanding air with expanding combustion, the law settles on the basis of a common account. It's all "heat as a mode of motion."
Explosions are the resultants of elemental gases, vaporization, comminution, contact of different substances, as well as of the specifically named explosives. With new processes in manufacture, involving chemical and mechanical transformations, and other uses of new substances and new uses of old substances, explosions increase. The flour-dust of the miller, the starch-dust of the confectioner, increase in fineness and quantity, and they explode; so does the hop-dust of the brewer. In 1844, for the first time, Professors Faraday and Lyell, employed by the British government, discovered that explosion in bituminous coal mines was the quickening of the comparatively slow burning of the "fire-damp" by the almost instantaneous combustion of the fine coal-dust present in the mines. The flyings of the cotton mill do not explode, but flame passes through them with a rapidity almost instantaneous, yet not sufficient to exert the pressure which explodes; the dust of the wood planer and sawer only as yet makes sudden puffs without detonating force. Naphtha vapor and benzine vapor are getting into all places. One of the latest introductions is naphtha extracting oil from linseed, and then volatilized by steam superheated to 400° F. This combination reminds us, as to effectiveness, of the combination at the recent Kansas City fire, when cans of gunpowder and barrels of coal oil both went up together.
But it is the unsuspected causes of explosion which make the great trouble, and prominent among these is conflagration as itself the cause of explosion, and such explosion may develop gases which are non-supporters of combustion as well as those which are inflammable. You throw table salt down a blazing chimney to set free the flame-suppressing hydrochloric acid, you discharge a loaded gun up a blazing chimney to put out the fire by another agency; still the salt, with certain combinations, may be explosive, a resinous vapor may be combustive in a hydrochloric atmosphere, and gunpowder isn't harmless when thrown upon a blaze--in fact, our common fire-extinguisher, water, has its explosive incidences as liquid as well as vapor.
Gases explosive in association may be set free by the temperature of a burning building and get together. In respect to the old conundrum, "Will saltpetre explode?" Mr. A. A. Hayes, Prof. Silliman, and Dr. Hare's views were, as to the explosions in the New York fire of 1845, that in a closed building having niter in one part and shellac or other resinous material in another, the gaseous oxygen generated from the niter and the carbureted hydrogen from the resins mingling by degrees would at length constitute an explosive mixture. A brief consideration of specific explosives uniting may serve to illustrate this phase of the subject.
Though the explosion of gunpowder is the result of a chemical change whereby carbonic acid gas at high tension is evolved (due to the saltpeter and the charcoal), the effect and rapidity of action are greatly promoted by the addition of sulphur. On the contrary, dynamite, now so important, and various similar explosives, are but mixtures of nitro-glycerine with earthy substances, in order to diminish and make more manageable the development of the rending force of the base. The explosive power of any substance is the pressure it exerts on all parts of the space containing it at the instant of explosion, and is measured by comparing the heat disengaged with the volume of gas emitted, and with the rapidity of chemical action. In the case of gunpowder, the proper manipulation and division of the grains is important, because favoring rapid deflagration; but in a purely chemical explosion, each separate molecule is an explosive, and the reaction passes from the interior of one to the interior of another, suddenly driving the atoms much further apart than their naturally infinitesimal vibrations.
Purely chemical explosives like nitro-glycerine, gun-cotton, the picrites, and the fulminates, present a terrible danger from the unknown mode of the new union of atoms, and reaction of the particles within themselves, in spontaneous explosions happening in irregular manner. Some curious circumstances attend the manufacture and use of gun-cotton,[1] nitro-glycerine, and dynamite. Baron von Link, in his system of the artillery use of gun-cotton, diminishes the danger of sudden explosion by twisting the prepared cotton into cords or weaving it into cloth, thereby securing a more uniform density. Mr. Abel's mode of making gun-cotton, which explosive is now used more than any other by the British government, includes drying the damp prepared cotton upon hot plates, freely open to the air. If ignited by a flame, however, in an unconfined place, gun-cotton only burns with a strong blaze, but if confined where the temperature reaches 340° F., it explodes with terrific violence. Somewhat similar is the action of nitro-glycerine and dynamite, which simply burn if ignited in the open air, while the same substance will explode through a very slight concussion or by the application of the electric spark; a red-hot iron, also, if applied, will explode them when a flame will not. With care, nitro-glycerine can be kept many years without deterioration; and it has been heated in a sand-bath to 80° C. for a whole day without explosion or alteration. One curious experiment is deserving of mention: If a broad-headed nail be partly driven into pine wood, and then some pieces of dynamite placed on the head of the nail, the latter may be struck hard blows with a wooden mallet without exploding the dynamite so long as the nail will continue to enter the wood.
[Footnote 1: The purest gun-cotton may be regarded as a cellulose, in which three atoms of hydrogen are replaced by three molecules of peroxide of nitrogen.]
Taking gunpowder as the unit, picrate of potash (picric acid and potassium) has five times more force, gun-cotton seven and a half times, and nitro-glycerine ten times more force. There are others still more powerful, but less known and used, and some explosives are quite uncontrollable and useless.
But the particular object of these remarks is to refer to articles of merchandise non-explosive under general conditions, but so in particular circumstances, as the two fire-extinguishers, water and salt, are explosive under given conditions. The memorable fire which, in July, 1850, destroyed three hundred buildings in Philadelphia, upon Delaware avenue, Water, Front, and Vine streets, was largely extended by explosions of possibly concealed or unknown materials, the presence of the generally recognized explosives being denied by the owners of the properties.
"The germ of the first knowledge of an explosive was probably the accidental discovery, ages ago, of the deflagrating property of the natural saltpeter when in contact with incandescent charcoal."[1] Although much manipulation is deemed necessary to form the close mechanical mixture of the materials of gunpowder, it has never been proved that such intimate previous union is necessary to precede the chemical reaction causing explosion; indeed, some explosions in powder works, before the mixture of the materials, or just at its commencement, seem to point to the contrary. It is also certain that in the manufacture of gunpowder the usual nitrate of potassium (saltpeter) can be replaced by the nitrates of soda, baryta, and ammonia, also by the chloride of potassium; charcoal by sawdust, tan, resin, and starch; and though a substitute for sulphur is not easily found, the latter, or a similar substance, is not an absolute necessity in the composition of gunpowder.[2]
[Footnote 1: Encyclopædia Britannica, new edition, viii, p. 806.]
[Footnote 2: Vide Abel's Experiments in Gunpowder, as detailed in Phil. Trans. Eoy. Soc, 1874.--Vide also Bull. Soc. d'Encouragement, Nov., 1880, p. 633, Sur les Explosives.]
The generally received theory of the chemical action which makes gunpowder explosive is that it is due to the superior affinity of the oxygen of the niter (KNO3) for the carbon of the charcoal, and the production of carbonic acid gas (CO2) and carbonic oxide (CO) suddenly and in great volume. The latter extinguishes flame as well as the former, unless its own flammability is supported by the oxygen of the atmosphere until the degree of oxygenation CO2 is reached. Considering that water (H2O) is composed of two volumes of hydrogen and one of oxygen, and that under an enormously high temperature and the excessive affinity of oxygen gas for potassium or sodium (freed from nitrate union), dissociation of the water may be possible, aided by its being in the form of spray and steam, we would hesitate to deny that an explosive union of suitable crude salts could occur during the burning of a building containing them when water for extinguishment was put on. Any one who has seen the brilliance with which potassium and sodium burn upon water can easily imagine how such strong affinity of oxygen for these substances might aid in severing its union in water in their presence and under extraordinary heat. It might be safe so say that the presence of water under very high temperature may be as aidful to form an explosive among such salts as have been named, as sulphur is for the rapid combustion of gunpowder.
In the review for August, 1862 (Saltpeter Deflagrations in Burning Buildings and Vessels--Water as an Explosive Agency), it was shown that Mr. Boyden's experiments in 1861-62 proved that explosions would occur when water was put upon niter heated alone, and stronger explosion from niter, drywood, and sulphur; also explosion when melted niter was poured on water. The following points we reproduce for comparison: If common salt be heated separately to a bright heat, and water at 150° F. poured on it, an explosion will occur. Niter mixed with common salt, placed upon burning charcoal, and water added, produce a stronger explosion than salt alone. Heating caustic potash to a white heat, and adding warm or hot water, produces explosion. At a Boston fire small explosions were observed upon water touching culinary salt highly heated. Anthracite coal and niter heated in a crucible exploded when sea water was poured on them.
The production of explosion by the putting of water on nitrate of potassium and chloride of sodium arises from the union, at high temperature, of the oxygen of the water with the potash and soda. Of the three liberated gases, hydrogen only is inflammable, and the other two suffocative of flame; but together the nitrogen and chlorine are not to be undervalued, for chloride of nitrogen is ranked as the most terrible and unmanageable of all explosives. Chlorine is a great water separator, but in the present case its affinity for hydrogen would result in hydrochloric acid, a fire extinguisher.
What happens in chemical experiment may be developed on a large scale in burning grocery, drug, or drysalters' stores, when great quantities of materials, such as just mentioned, including common salt, almost always present, are heated most intensely, and then subjected to the action of water in heavy dashes, or in form of spray or steam.
Picric acid, the nature of which we have several times previously mentioned, and which explodes at 600° F. (only 28° above gunpowder), may also be an element in such explosions during fires. Its salts form, in combinations, various powerful explosives, much exceeding gunpowder in force; and they have been used to a considerable extent in Europe. Picric acid, now much employed by manufacturers and dyers for obtaining a yellow color, is always kept in store largely by drysalters and druggists, and generally by dyers, but in smaller quantity.
In a very destructive fire which occurred in Liverpool, Eng., in October, 1874, involving the loss of several "fire-proof" stores, repeated explosions of the vapor of turpentine rent ponderous brick arched vaults, and exposed to the flames stocks of cotton, etc., in the stories above. This conflagration was started by the carelessness of an employee in snuffing a tallow candle with his fingers and throwing the burning snuff into the open bung-hole of a sample barrel of turpentine, of which liquid there were many hundreds of barrels on storage in the buildings. Turpentine vapor united with chlorine gas may not produce explosion, but by spreading flames almost instantly throughout the burning buildings, such burnings have practically equaled, if not excelled, explosions, which may sometimes be fire-extinguishers. In such cases detonation may be prevented by there being ample space to receive the suddenly ignited vapor, lessening the tension of it, but carrying the flames much more rapidly than otherwise to inflammable materials at great distance.
If disastrous results have arisen from the vapor of turpentine as a fire spreader in vaults without windows, it is possible that if a quantity of hot water were suddenly converted into steam in closely confined spaces, effects of pressure might be observed, less destructive perhaps, but resembling those which other explosives might produce. If the immense temperature attained in some conflagrations be considered--sufficient to melt iron and vitrify brick--it is possible to conceive of water as being instantly converted into steam. Even a very small quantity of water thus expanded could produce most disastrous results. While such formation of steam, if it happened, would certainly extinguish most flames in direct contact, the general phenomena shown would be explosive.
A curious circumstance occurred at the Broad street (N.Y.) fire in 1845, previously mentioned. The fire extended through to Broadway, and almost to Bowling Green. A shock like a dull explosion was heard, and by many this was attributed to the effects of gunpowder and saltpeter. Several firemen were, at the moment of the shock, on the roof of the burning building, when the whole roof was suddenly raised and then let down into the street, carrying the men with it uninjured. One of the firemen described the sensation "as if the roof had been first hoisted up and then squashed down." Query: Was this like the common lifting and falling back of the loose lid of a tea-kettle containing boiling water? Was it from steam--at a low pressure perhaps--seeking vent through the roof in like manner to the raising of the kettle-lid? Without dilating on this part of the subject, we mention it as a possible cause of minor explosions--doubtless to become better known in future. It may even be that explosions happening from steam acting in close spaces may have been attributed to gunpowder, or to niter and other salts, separate, but suddenly caused to combine in chemical reaction.--American Exchange and Review.
This element, which next deserves our attention, is one of great importance and wide distribution; it occurs in nature in both the free and the combined states, and the number of compounds which it forms with other elements is very large. Unlike the previous elementary bodies we have studied, carbon is only known to us in the solid form when free, although many of its combinations are gaseous at the ordinary temperature and pressure. Carbon is known to exist in several different physical states, thus illustrating what chemists call allotropism, which means that substances of identical chemical composition sometimes possess altogether different outward and physical appearances. Thus the three states in which pure carbon exists, viz., diamond, graphite, or plumbago, and charcoal are as different as possible, and yet chemically they are all exactly the same substance. The diamond is the purest carbon, and occurs in the crystalline form known as a regular octahedron; the diamond is one of the hardest substances known, and is therefore, utilized for cutting glass; it has also a very high specific gravity, namely, 3.5, which means that it is three and a half times heavier than water, and it is far heavier than any of the other allotropic modifications of carbon. Graphite or plumbago, the second form in which carbon occurs, is widely distributed in nature, and the finer qualities are known as black lead, although no lead enters into their composition, as they are composed of carbon almost as pure as the diamond; the specific gravity of graphite is only 2.3. Charcoal, the third allotropic modification of carbon, is by far the most common, and is formed by the natural or artificial disintegration of organic matters by heat; we thus have formed wood charcoal, animal charcoal, lamp-black, and coke, all produced by artificial means, and we may also class with these coal, which is a natural product, and which contains from 85 to 95 per cent. of pure carbon.
Wood charcoal is made by heating wood in closed vessels or in large masses, when all the hydrogen, oxygen, and nitrogen are expelled in the gaseous state, and the carbon is left mixed with the mineral constituents of the wood; this form of carbon is very porous and light, and is used in a number of industrial processes.
Animal charcoal, as its name implies, is the carbonaceous residue left on heating any animal matters in a retort; and contains, in addition to the carbon, a large proportion of phosphates and other mineral salts, which, however, can be extracted by dilute acids. Animal charcoal possesses to a remarkable degree the property of removing color from solutions of animal and vegetable substances, and it is used for this purpose to a large extent by sugar refiners, who thus decolorize their dark brown sirups; in the manufacture of glucose and saccharums for brewers' use, the concentrated solutions have to be filtered through layers of animal charcoal in order that the resulting product may be freed from color. The decolorizing power of animal charcoal can be easily tested by any brewer, by causing a little dark colored wort to filter through a layer of this material; after passing through once or twice, the color will entirely disappear, or at all events be greatly reduced in intensity. Animal charcoal also absorbs gases with great avidity, and on this account it is utilized as a powerful disinfectant, for when once putrefactive gases are absorbed by it, they undergo a gradual oxidation, and are rendered innocuous, in the same way animal charcoal is a valuable agent for purifying water, for by filtering the most impure water through a bed of animal charcoal nearly the whole of the organic impurities will be completely removed.
Lamp-black is the name given to those varieties of carbon which are deposited when hydrocarbons are burned with an insufficient supply of oxygen; thus the smoke and soot emitted into our atmosphere from our furnaces and fireplaces are composed of comparatively pure carbon.
Coal is an impure form of carbon derived from the gradual oxidation and destruction of vegetable matters by natural causes; thus wood first changes into a peaty substance, and subsequently into a body called lignite, which again in its turn becomes converted into the different varieties of coal; these changes, which have resulted in the accumulation of vast beds of coal in the crust of the earth, have been going on for ages. There are very many different kinds of coal; some are rich in hydrogen, and are therefore well adapted for making illuminating gas, while others, such as anthracite, are very rich in carbon, and contain but little hydrogen; the last named variety of coal is smokeless, and is therefore largely used for drying malt.
Carbon occurs in nature also in a combined state; limestone, chalk, and marble contain 12 per cent. of this element. It is also present in the atmosphere in the form of carbonic acid, and the same compound of carbon is present in well and river waters, both in the free state and combined with lime and magnesia. All animal and vegetable organisms contain a large proportion of carbon as an essential constituent; albumen contains about 53 per cent., alcohol contains 52 per cent., starch 44 per cent., cane sugar 42 per cent., and so on. The presence of carbon in the large class of bodies known to chemists as carbohydrates, of which starch and sugar are prominent examples, can be easily demonstrated. If a little strong sulphuric acid be added to some powdered cane sugar in a glass, the mass will soon begin to darken in color and swell up, and in the course of a few minutes a mass of black porous carbon will separate, which can be purified from the acid by repeated washings; the sugar is composed of carbon, hydrogen, and oxygen, the two last-named elements being present in the exact proportion necessary to form water; the sulphuric acid having a strong affinity for water, removes the hydrogen and oxygen, and the carbon is then left in a free state.
Carbon forms two compounds with oxygen--carbon monoxide, commonly called carbonic oxide, and carbon dioxide, commonly called carbonic acid; and the last-named, being of most importance, will be studied first.
Carbon Dioxide, or Carbonic Acid, Symbol CO2.--Carbonic acid occurs, as we have already stated, in large quantities in combination with lime and magnesia, forming immense rock formations of limestone, chalk, marble, dolomite, etc.; it also issues in a gaseous state from volcanoes, and it is always present in small quantities in the atmosphere; it is found dissolved in well and river waters, and it is a product of the respiration of animals. Brewers also are well aware of the existence of this body, for it is evolved in enormous quantities during the alcoholic fermentation of saccharine fluids. When carbonaceous substances are burnt the bulk of the carbon is converted into carbonic acid, and thus our furnaces and fireplaces are continually emitting enormous quantities of carbonic acid into the atmosphere. With these different sources of supply it might reasonably be thought that carbonic acid would be gradually accumulating in our atmosphere; the breathing of animals, the eruption of volcanoes, the combustion of fuel, and the fermentation of sugar, are ever going on, and to a fast-increasing extent with the progress of civilization, and yet the proportion of carbonic acid in our atmosphere is no greater now than it was at the earliest time when exact chemical research determined its presence and quantity. A counteracting influence is always at work; nature has beautifully provided for this by causing plants to absorb carbonic acid, holding some of the carbon, and allowing the oxygen to escape again into the atmosphere to restore the equilibrium of purity. This mutual evolution and absorption of carbonic acid is continually going on; occasionally there may be either an excess or a deficiency in a particular place, but fortunately any irregularity in this respect is soon overcome, and the air retains its original composition, otherwise animal life on the face of the globe would be doomed to gradual but sure extinction.
Carbonic acid can be prepared for experimental purposes by causing dilute hydrochloric acid to act upon fragments of marble placed in a bottle with two necks, into one neck of which a funnel passing through a cork is fixed, and into the other a bent tube for conveying the gas into any suitable receiver. The evolution of carbonic acid by this method is rapid, but easily regulated, and the gas may be purified by causing it to pass through some water contained in another two-necked bottle, similar to the generator. The chemical change involved in this decomposition is expressed by the following equation:
CaCO_3 + 2HCl = CO_2 + H_2O + CaCl_2 Calcium Hydrochloric Carbonic Water. Calcium Carbonate. Acid. Acid. Chloride.
By referring to the table of combining weights given in a previous paper, it will be seen that 100 parts of calcium carbonate will yield 44 parts of carbonic acid. Instead of hydrochloric acid any other acid may be used, and in the practical manufacture of carbonic acid for aerated waters sulphuric acid is the one usually employed. Carbonic acid is colorless and inodorous, but has a peculiar sharp taste; it is half as heavy again as air, its exact specific gravity being 1529; one hundred cubic inches weigh 47.26 grains. It is uninflammable, and does not support combustion or animal respiration. Under a pressure of about 38 atmospheres, at a temperature of 32° F., carbonic acid condenses into a colorless liquid, which may also be frozen into a compact mass resembling ice, or into a white powder like snow. Carbonic acid is soluble in water, and at the ordinary pressure and temperature one volume of water will hold in solution one volume of the gas; under increased pressures, far larger quantities of the gas can be held in solution, but this is rapidly evolved as soon as the excess of pressure is removed. Upon this property the manufacture of aerated waters depends. The presence of free carbonic acid can be easily detected by causing the gas to pass over the surface of some clear lime-water. If any be present a white film of carbonate of lime will at once be formed. In testing carbonic acid in a state of combination, the gas must first be liberated by acting upon the substance with a stronger acid, and then applying the lime-water test. The presence of large quantities of carbonic acid in a gaseous mixture can be readily detected by plunging into the vessel a lighted taper, which will be immediately extinguished. This ought always to be adopted in a brewery, where many fatal accidents have happened through workmen going down into empty fermenting vats and wells without first taking this precaution.
The presence of carbon in this colorless gas can be demonstrated by causing some of it to pass over a piece of the metal potassium placed in a hard glass tube, and heated to dull redness; the potassium then eagerly combines with the oxygen, forming oxide of potassium, and the carbon is liberated and can be separated in the form of a black powder by washing the tube out with water.
Carbon Monoxide, or Carbonic Oxide. Symbol CO.--This is formed when carbon is burnt with an insufficient supply of oxygen, or when carbonic acid gas is passed over some carbon heated to redness. This gas is continually being formed in our furnaces and fire-places; at the lower part of the furnace, where the air enters, the carbon is converted into carbonic acid, which in its turn has to pass through some red-hot coals, so that before reaching the surface it is again converted into carbonic oxide; over the surface of the fire this carbonic oxide meets with a fresh supply of oxygen, and is then again converted into carbonic acid. The peculiar blue lambent flame often observed on the surface of our open fire-places is due to the combustion of carbonic oxide, which has been formed in the way we have just described. Carbonic oxide is a colorless, tasteless gas, which differs from carbonic acid by being combustible, and by not having any action on lime water.--Brewers' Guardian.
The thermometers and pyrometers usually employed are almost all based on the expansion of some fluid or other, or upon that of different metals. The first can only be constructed with glass tubes, thus rendering them fragile. The second are often wanting in exactness, because of the change that the molecules of a solid body undergo through heat, thus preventing them from returning to exactly their first position on cooling.
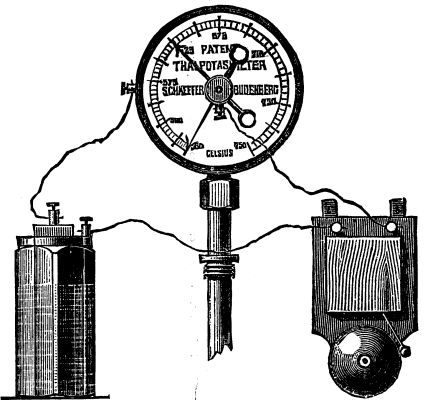
Fig. 1.--Pyrometer with Electric Indicator.
The principle of the Seyfferth pyrometer is based on the fact that the pressure of saturated vapors, that is, vapors which remain in communication with the liquid which has produced them, preserves a constant ratio with the temperature of such liquid, while, on the other hand, the temperature of the latter when shut up in a vessel will correspond exactly with that of the medium into which it is introduced.
Fig. 2.--Method of Mounting by means of a
cone on vacuum apparatus.
Fig. 3.--Mounting by means of a sleeve on vacuum apparatus.
This instrument is composed of a metallic vessel or tube which contains the liquid to be exposed to heat, and of a spring manometric apparatus communicating with the tube, and by means of which the existing temperature is shown. The dial may be provided with index needles to show minimum and maximum temperatures, as well as be connected with electric bells (Fig. 1) giving one or more signals at maximum and minimum temperatures. The vessel to contain the liquid may be of any form whatever, but it is usually made in the shape of a straight or a bent tube. The nature of the metal of which the latter is made is subordinate, not only to the maximum temperature to which the apparatus are to be exposed, but also to the nature of the liquid employed. It is of either yellow metal or iron. To prevent oxidation of the tube, when iron is employed, it is inclosed within another iron tube and the space between the two is filled in with lead. When the apparatus is exposed to a high temperature the lead melts and prevents the air from reaching the inner tube, so that no oxidation can take place.
Pyrometers filled with Ether.-These are tubular, and constructed of yellow metal, and are graduated from 35° C. to 120°. They are used for obtaining temperatures in vacuum apparatus, cooking apparatus, diffusion apparatus, saturators, etc. Figs. 2, 3, 4, and 5, show the different modes of mounting the apparatus according to the purpose for which it is designed.
Pyrometers filled with distilled water are used for ascertaining temperatures ranging from 100° to 265° C., 80° to 210° R., or 212° to 510° F.
Pyrometers filled with mercury are constructed for ascertaining temperatures from 360° to 750° C.

Fig. 4.--Mounting on horizontal pipes by
thread on the tube.
Fig. 5.--Mounting by means of a clasp
in reservoirs.
The temperature necessary for the complete carbonization of the organic substances of animal charcoal is from 430° to 500° C. In order to transmit this temperature from the cylinder to the charcoal it is indispensable that the air surrounding the cylinder be heated to 480° to 550°. If the heating of the animal black exceeds 500° the product hardens, diminishes in volume, and loses its porosity. There are two methods of ascertaining the temperature of the red-hot bone black by means of the pyrometer: First, by inserting the tube of the instrument into the black. (Fig. 6, a.) Second, by finding the temperature of the hot gases in the furnaces (Fig. 6, b.). In the first case, the plunge tube should be of sufficient length to allow its extremity to penetrate to the very bottom layer of the red-hot black. This mode of direct control of the temperature of the black is only employed for ascertaining the work accomplished by the furnace, that is to say, the ratio existing between the temperature of the hot air surrounding the cylinder and the black itself. This calculation being effected, it is useless to note the differences of temperature which arise in the spaces between the cylinders of which the furnace is composed.
The position that the pyrometer should occupy is subordinate to the construction of the furnace. Fig. 6 shows the type which is most employed.
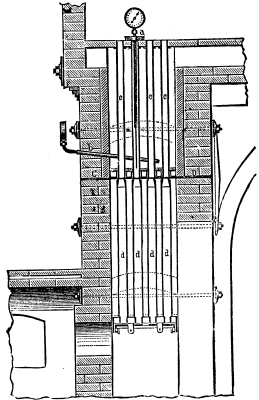
Fig. 6.--The Pyrometer mounted on a bone-black furnace.
In a furnace with lateral fire-place, cc are the heating cylinders, and dd the cooling cylinders. C D is the plate on which are mounted vertically the former, and from which are suspended the latter, b shows the pyrometer, the length of which must be such that the manometric apparatus shall stand out one or two inches from the external surface of the wall, while its tube, traversing the wall, shall reach the very last row of heating cylinders.
That the apparatus may form a permanent regulator for the stoker it is well to adapt to it an arrangement permitting of a graphic control of the work accomplished and signaling by means of an electric bell when the temperature of the gases in the furnace descends below 480° C. or rises above 550° C.
The operation of heating brick furnaces is generally performed according to empirical methods, the temperature having to vary much according to the products that it is desired to obtain. It is necessary, however, for a like product to maintain as uniform a temperature as possible. These observations are particularly applicable to continuous furnaces such as annular brick furnaces, etc., in which a uniformity of temperature in the different chambers is of vital importance to perfect the baking. In these furnaces the tube of the pyrometer is inserted through one of the apertures at the top, as shown in Fig. 7. The dial is graduated up to 750°, which is more than sufficient, since the temperature of the upper part of a compartment fully exposed to the heat rarely exceeds 670° to 680° C.
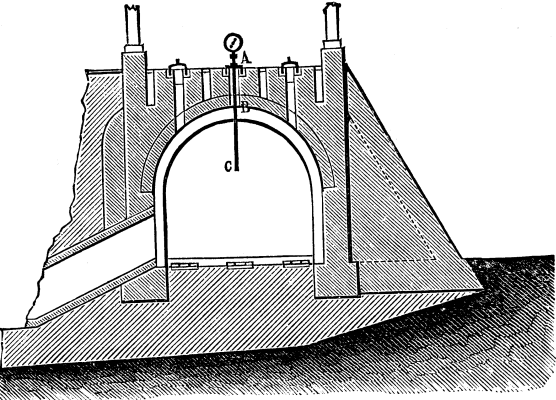
Fig. 7.--The Pyrometer mounted on a brick furnace.
Potash soaps are generally superior to soda soaps for most purposes, but more especially in washing wool and woolen goods. The difference between the use of a potash and a soda soap for these purposes is very marked. Potash lubricates the fiber of the wool, renders it soft and silky, and to a certain extent bleaches it; soda, on the other hand, has a tendency to turn wool a yellow color, and renders the fiber hard and brittle. It cannot be too strongly insisted upon, therefore, that nothing but a potash soap (or some form of potash in preference to soda if an alkali alone is employed) should be used in washing wool in any form--either manufactured or unmanufactured. This is fully borne out by nature, who invariably assimilates the most appropriate substances. Wool when growing in its natural state is lubricated and protected by a sticky substance called "grease" or "suinte;" this consists to the extent of nearly half its weight of carbonate of potash, hardly a trace of soda being present. It is very evident, therefore, that potash must be more suitable for washing wool than soda, as the teaching of nature is always correct.
There are certain prejudices against the use of potash soap, which have, to a great extent, prevented its more extensive use. Many consumers of soap fancy that because a potash soap is soft it necessarily must contain more water than a soda soap; this, however, is quite an erroneous notion. A potash soap is soft, because it is the nature of all potash soaps to be so, just in the same way that on the other hand all soda soaps are hard. As an actual fact a good potash soap contains less water than many quite hard soda soaps that are now in the market. Another reason is that soapmakers have had every interest in using soda in preference to potash--particularly when latterly soda has been so cheap.
Potash not only is a more expensive alkali, but its combining equivalent is greatly against it as compared with soda; that is to say, that thirty-one parts of actual or anhydrous soda will saponify as much tallow or oil as forty-seven parts of anhydrous potash. It will be evident, therefore, that the use of potash instead of soda is decidedly more advantageous to the soapboiler, and more particularly in the present age, when the demand is for cheap articles, often quite without regard to the quality or purpose for which they are to be used. As far as consumers are concerned, this has been a mistake. Potash soap, though it may cost more, is in most cases actually the most economical. Soap is never used in exact chemical equivalents, but an excess is always taken. Potash soap is much more soluble than a soda soap; it therefore penetrates the fiber, and consequently removes dirt and grease much more quickly. Notwithstanding, also, that its chemical combining equivalent is greater than that of soda, it is, nevertheless, the strongest base, and always combines with any substance in preference to soda. For these reasons--probably combined also with the fact that in the whole realm of the animal and vegetable kingdoms, to which all textile fabrics belong, potash is more naturally assimilated than soda--a smaller quantity of potash soap will do more practical work than a larger quantity of soda soap.
There are other reasons why potash soaps have not been used; originally soft soap was made either with fish oil or olive oil. Fish oil is objectionable, as the strong smell imparted to the soap renders it unfit for many finishing purposes. Nothing can be better than olive oil soap, but it is a costly article, and only can be used for finer purposes. There are now, however, many of the seed oils that are much cheaper. Linseed, rape seed, and cotton seed all produce a good soap. Cotton seed oil is particularly suitable for the purpose; the manufacture of this oil during the last few years has been brought to great perfection, and the cost is now much less than that of tallow or of any other seed oil. It is now difficult to distinguish a well refined cotton seed oil from olive oil; it is therefore in every way suitable for making soft soap. One of the chief causes, however, why potash soap has not been more generally made is that a convenient form of potash has been unobtainable. For many years the only source of potash was from the ashes of burnt trees. These ashes are collected, mixed with lime, lixiviated, and the resulting lye boiled down. The result is a very impure form of potash, also of a very variable composition, depending upon the trees used for the purpose. Canada has been the principal source of supply of this form of potash; hence the commercial name of Montreal potashes. The classification of "firsts," "seconds," and "thirds" is from the inspection at the warehouse there; this, however, is exceedingly superficial, the ashes being simply tested for their alkaline strength, with no discrimination between potash and soda, which is a difficult and delicate chemical test. Soda being now far cheaper than potash, and also the alkaline equivalent, as previously explained, being greatly in favor of soda, there has been every inducement to "enterprising" producers of ashes to adulterate them with soda, which, in many cases, has been largely done. Another source of potash has been beetroot ashes, very similar to wood ashes, and also German carbonate of potash, which latter about corresponds to a common soda ash, as compared with caustic soda; with these articles, a tedious boiling process, very similar to the old process for the production of hard soap, had to be adopted, the ashes, or carbonate of potash, previously being dissolved and causticized with lime by the soap maker. The production of a first-class soft soap was also a very difficult operation, as the impurities and soda contained varied considerably, often causing the "boil" to go wrong and give considerable trouble to the soapboiler.
During the last two years, however, caustic potash has been introduced, that manufactured by the Greenbank Alkali Co., of St. Helens, being very nearly pure. With this article there is no difficulty in producing a pure potash soap, either for wool scouring, fulling, or sizing, by a cold process very similar to that described for the production of hard soda soap with pure powdered caustic soda.
The following directions will produce an excellent soap for wool scouring: Fifty pounds of Greenbank pure caustic potash are put into eight gallons of soft water; the potash dissolves immediately, heating the water. This lye is allowed to cool, and then slowly added, with continual mixing, to 20 gallons of cotton seed oil, mixed with 20 pounds of melted tallow, the whole being brought to a temperature of about 90° F. After stirring for some minutes, so as to completely combine the lye and oil, the mixture is left for two days in a warm place, when a slow and gradual saponification of the mass takes place. If when examined the oil and lye are then found not completely combined, the stiff soap is again stirred and left two days, when the saponification will be found complete, the result being the formation of about 330 pounds of very stiff potash soap, each pound being equal to about two pounds of the ordinary "fig" soap sold. The requisite quantity is thrown into the scouring vat with about five per cent of its weight of refined pearl ash to increase the alkali present, the weight depending somewhat upon the kind of wool washed on purpose for which the soap is required. If the wool is very dirty or greasy, rather a stronger soap is sometimes advisable. This can easily be attained by reducing the quantity of oil used to 18 gallons.
The advantages to be gained by the wool scourer or other consumer making his own potash soap are that a pure, uniform article can always be thus produced at a less cost than that at which the soap can be bought. Potash soap, like soda soap now sold, is much adulterated, in addition to all the impurities originally contained in the potash used, and which, unlike soda soap, cannot be separated by any salting process. Many other adulterations are added to increase the weight and cheapen the cost. Silicate of potash, resin, and potato flour are all more or less employed for this purpose, to the gain of the soap maker and at the expense of the consumer.
The production of potash soap for fulling and sizing, and the most suitable oils and tallow for the production of the various qualities required for these purposes, must be reserved for the next issue.--Textile Manufacturer.
We have, on a previous occasion, described the process of "maceration" or "enfleurage," that is, the impregnation of purified fat with the aroma of certain scented flowers which do not yield any essential oil in paying quantities. At present we wish to describe an apparatus which is used in several large establishments in Europe for obtaining such products on the large scale and within as short a time as possible. The drawing gives the idea of the general arrangement of the parts rather than the actual appearance of a working apparatus, for the latter will have to vary according to the conveniences and interior arrangements of the factory.[1]
[Footnote 1: Our illustration has been taken from C. Hofmann, "Chemisch-technisches Universal-Receptbuch," 8vo, Berlin, 1879, p. 207.]
A series of frames with wire-sieve bottoms are charged with a layer of fat in form of fine curly threads, obtained by pressing or rubbing the fat through a finely-perforated sieve. The frames are then placed one on top of the other, and to make the connection between them air-tight, pressed together in a screw press. A reservoir, E, is charged with a suitable quantity of the flowers, etc., and tightly closed with the cover, after which the bellows are set into motion by any power most convenient. Scented air is thereby drawn from the reservoir, E, through the pipe, G B, toward the stack of frames containing the finely divided fat, which latter absorbs the aroma, while the nearly deodorized air is sent back to the reservoir by the pipe, D, to be freshly charged and again sent on its circuit. This apparatus is said to facilitate the turning out of nearly twenty times the amount of pomade for the same number of frames and the same time, as the old process of "enfleurage." It might be called the "ensoufflage" process.--New Remedies.
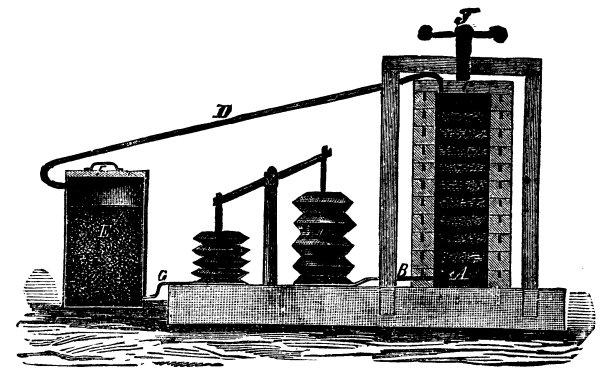
"ENSOUFFLAGE" APPARATUS FOR PERFUMES.
At a recent meeting of the London Chemical Society, Mr. W. Jago read a paper "On the Organic Matter in Sea-water." On p. 133 of the "Sixth Report of the Rivers Commission," it is stated that the proportion of organic elements in sea-water varies between such wide limits in different samples as to suggest that much of the organic matter consists of living organisms, so minute and gelatinous as to pass readily through the best filters. At the suggestion of Dr. Frankland, the author has investigated this subject. The water was collected in mid-channel between Newhaven and Dieppe by the engineers of the London, Brighton, and South Coast Railway in stoppered glass carboys. The author has used the combustion method, the albuminoid ammonia, and in some cases the oxygen process of Prof. Tidy. To determine how the various methods of water-analysis were effected by a change of the organic matter from organic compounds in solution to organisms in suspension, some experiments were made with hay-infusion. The results confirm those of Kingzett (Chem. Soc. Journ., 1880, 15). the oxygen required first rising and then diminishing. The author concludes that the organic matter of sea-water is much more capable of resisting oxidizing agents than that present in ordinary fresh waters, and that the organic matter in sea-water is probably organized and alive.
W. M. Hamlet, in a paper before the London Chemical Society, said: Flasks similar to those of Pasteur ("Etudes sur la Biere," p. 81), holding about ¼ liter, were used. The liquids employed were Pasteur's fluid with sugar, beef-tea, hay infusion, urine, brewers' wort, and extract of meat. Each flask was about half filled, and boiled for ten minutes, whereby all previously existing life was destroyed. The flask was then allowed to cool, the entering air being filtered through a plug of glass wool or asbestos. The flask was then inoculated with a small quantity of previously cultivated hay solution or Pasteur's fluid. Hydrogen, oxygen, carbonic oxide, marsh-gas, nitrogen, and sulphureted hydrogen, were without effect on the bacteria. Chlorine and hydric peroxide (about 7 per cent, of a 5 vol. solution) were fatal to bacteria. The action of various salts and organic acids in 5 per cent, solution was tried. Many, including potash, soda, potassic bisulphite, sodic hyposulphite, potassic chlorate, potassic permanganate, oxalic acid, acetic acid, glycerin, laudanum, and alcohol, were without effect on the bacterial life. Others--the alums, ferrous sulphate, ferric chloride, magnesic and aluminic chlorides, bleaching powder, camphor, salicylic acid, chloroform, creosote, and carbolic acid--decidedly arrested the development of bacteria. The author has made a more extended examination of the action of chloroform, especially as regards the statement of Müntz, that bacteria cannot exist in the presence of 2½ per cent, of chloroform, which substance is therefore useful in distinguishing physiological from chemical ferments. The author concludes that amounts of chloroform, phenol, and creosote, varying from ¼ to 3 per cent., do not destroy bacteria, although their functional activity is decidedly arrested while in contact with these reagents. To use the author's words, bacteria may be pickled in creosote and carbolic acid without being deprived of their vitality. The author concludes that the substances which destroy bacteria are those which are capable of exerting an immediate and powerful oxidizing action, and that it is active oxygen, whether from the action of chlorine, ozone, or peroxide of hydrogen, which must be regarded as the greatest known enemy to bacteria.
Mr. Hamlet, in replying to some remarks of Messrs. Kingzett and Williams, said that in all cases the solution which he had used had been completely sterilized by exposure to a temperature of 105° for ten minutes. The India-rubber tubing he had used was steamed. Carbolic acid solution must contain at least 5 per cent, of carbolic acid to be fatal to bacteria. He was quite aware of the importance of distinguishing between the action of the substances on various kinds of bacteria, and was quite prepared to admit that a treatment which would be fatal to one kind of bacterium might not injure another.
[Footnote: Read before the American Chemical Society, June 3,1881.]
Noticing the recent advertisements in the city regarding the "Baby Elephant," it occurred to me that perhaps no analysis of the milk of this species of the mammalia had been recorded. This I found corroborated, for though the milk of many animals had been subjected to analysis, no opportunity had ever presented itself to obtain elephants' milk.
Through the courtesy of Jas. A. Bailey I was enabled to procure samples of the milk on several occasions.
On March 10, 1880, the elephant Hebe gave birth to the female calf America. Hebe is now twenty eight years old, and the father of the calf, Mandrie, thirty-two. Since the birth of the "Baby," the mother has been in excellent health, except during about ten days, when she suffered from a slight indisposition, which soon left her.
When born the calf weighed 213½ lbs., and in April, 1881, weighed 900 lbs. A very fair year's growth on a milk diet. At the time I procured the samples both mother and calf were in fine health.
To obtain the milk was a matter of some difficulty. The calf was constantly sucking, nursing two or three times an hour, morning, noon, and night. The milk could be drawn from either of the two teats, but only in small quantity. The mother gave the fluid freely enough, apparently, to her infant, but sparingly to inquisitive man, so the ruse had to be resorted to of milking one teat while the calf was at the other.
When I first examined the specimens they seemed watery, but to my surprise, on allowing the milk to stand, I could not help wondering at the large percentage of cream.
The following represents approximately the daily diet of the mother:
Three pecks of oats, one bucket bran mash, five or six loaves of bread, half a bushel of roots (potatoes, etc.), fifty to seventy-five pounds of hay, and forty gallons of water.
Elephants eat continually, little at a time, to be sure, but are constantly picking. This habit is also observable in the way the calf nurses. The first specimen of milk was procured on the morning of April 5, the second on the 9th, and the third on the 10th.
The last exceeded the others in quantity, and is therefore the fairest of the three. It took several milkings to get even these, for the calf would begin to nurse, then stop, and when she stopped the flow of milk did also.
I was assured by Mr. Cross and the keeper, Mr. Copeland, that the milk I obtained had all the appearances of that drawn at various times since the birth of the calf. Mr. Cross, when in Boston, compared the milk with that from an Alderney cow, and found the volume of cream greater.
I endeavored to have the calf kept away from the mother for some hours, but could not, since she is allowed her freedom, as she worries under restraint, and besides, has never been taken from the mother. The calf picked at oats and hay, but was dependent on the mother for nourishment.
It would have been a matter of great satisfaction to me had I been able to obtain a larger quantity of the milk, or to have gained even an approximate knowledge of the daily yield, but was obliged to content myself with what I could get. By comparing several samples, however, a just conclusion regarding the quality was found. The analyses of the samples gave the following results:
No. I. II. III. April 5, April 9, April 10, Morning. Noon. Morning.
Quantity, 19 cc. 36 cc. 72 cc. Cream, 52-4, vol.% 58 62 Reaction, Neutral. Slightly alkaline. Slightly acid. Sp.gr., ---- ---- 1023.7
In 100 parts by weight. Water............67.567 69.286 66.697 Solids...........32.433 30.714 33.303 Fat..............17.546 19.095 22.070 Solids not fat...14.887 11.619 11.233 Casein...........14.236 3.694 3.212 Sugar............14.236 7.267 7.392 Ash.............. 0.651 0.658 0.629
Ten grammes were taken for analysis, and in No. III. duplicates were made.
It is evident from these analyses that the milk approaches the composition of cream, yet it did not have the consistency of ordinary cream--as cream even rose upon it. Under the microscope the globules presented a very perfect outline, and were beautifully even in size and very transparent.
The cream rose quickly, leaving a layer of bluish tinge below. The milk was pleasant in flavor and odor, and very superior in these respects to that of many animals such as goats or camels, and in quality equal to that of cows. Nor did the milk emit any rank odor on heating.
When ten grammes were evaporated to dryness, the last portions of water were hard to remove, as the residue fairly floated with oil. Only by long-continued application of heat, and in analysis III. over sulphuric acid in vacuo, could a constant weight be obtained.
I would have used sand in the drying, or Baumhauer's method of fat extraction, but for the small quantity of milk at my disposal and from fear of loss of fat in the latter case.
The fat in III. was determined by extracting the dried residue and also with 20 c. c. of milk by adding alkali and shaking with ether, removing and evaporating the ether and weighing the fat.
As is shown in the table the sp. gr. is very low, though the solids and solids not fat are great. The ash, casein, and sugar are in about the usual proportion. The weight of casein, it is true, is but half that of the sugar. The milk indeed shows an unusually great preponderance of the non-nitrogenized elements, and this seems to correspond with the wants of the animal, since fatty tissues are greatly developed in elephants. According to Mr. Cross, who has had large experience with these animals, they are fatter in the wild state than in bondage. These specimens must appear as exceptional; they may be considered by some as "strippings;" but as against such a view we have the recurrence in each sample of the same characteristics in the milk and a near correspondence in the composition. As may be seen from the subjoined analyses, given by v. Gorup Besanez,[1] the milk belongs to the class of which woman's and mare's milk are members, especially as regards the proportion of the non-nitrogenized to the nitrogenized elements.
[Footnote 1: "Lehrhuch der Physiologischen Chemie," pp. 423 and 424.]
Constituents. Woman. Cow. Goat. Ewe. Ass. Mare.
Water. 86.271 84.28 86.85 83.30 89.01 90.45 Solids. 13.729 15.72 13.52 16.60 10.99 9.55 Fat. 5.370 5.47 4.34 6.05 1.85 1.31 Casein. \ 3.57 2.53 \ \ \ 2.950 5.73 3.57 2.53 Albumen. / 0.78 1.26 / / / Milk Sugar. 5.136 4.34 3.78 3.96 \ 5.42 5.05 Ash. 0.223 0.63 0.65 0.68 / 0.29
Constituents. Buffalo. Camel. Sow. Hippo- Elephant. potamus.
Water. 80.640 86.34 81.80 90.43 66.697 Solids. 19.360 13.66 18.20 9.57 33.308 Fat. 8.450 2.90 6.00 4.51 22.070 Casein. \ \ \ 4.40 \ 4.247 3.67 5.30 3.212 Albumen. / / / / Milk Sugar. 4.518 5.78 6.07 [1] 7.392 Ash. 0.845 0.66 0.83 0.11 0.629
[Footnote 1: Milk Sugar included.]
It may be remarked that though approaching the composition of cream it still differs enough to require it to be considered milk.
Perhaps if a larger quantity of the milk could be collected, it would have a more watery character, and approximate more nearly to other milks in that respect. However this may be the quality of the fat deserves some attention.
The fat has a light yellow color, resembling olive oil, is very pleasant in odor and taste, is liquid at common temperatures, but solidifies at 18° C. or 64° F.
The cow must yield a considerable quantity of milk, since the growth of the calf has been constant, and at the time these samples were milked the mother gave as freely to her babe as she ever had since its birth. The calf having gained seven to eight hundred pounds on a milk diet in one year, it is presumable that it had no lack of nourishment.
In size the "Baby" compared equally with other elephants in the same menagerie, who were known to be four and five years old.
From whatever standpoint, therefore, we view the lacteal product of these four-footed giants, we are fully warranted in ascribing to it not only extreme richness, but also great delicacy of flavor.
Rice contains much more starch, but on the other hand, much less albuminous matter and ash, than maize and barley. The compositions of different kinds of dried rice do not vary very much, but as the amount of moisture in the raw grain ranges from 5 to 15 per cent., no brewer ought to buy rice without having first of all inquired with the assistance of a chemist as to the percentage of water present in the sample.
Another point requiring attention is that of taking notice of the acidity, which also varies a good deal for different sorts of rice. In comparing the nutritive values of the three kinds of grain before us, Pillitz obtained the following numbers:
Barley. Maize. Rice.
-------------- ------------- ------------------
Air Dried at Air Dried at Air Dried at With
Dry. 100° C. Dry. 100° C. Dry. 100° C. Husk.
Moisture. 13.88 --- 13.89 --- 12.51 --- 12.00
Starch. 54.07 62.65 62.69 73.27 74.88 85.41 74.50
Dextrin and
sugar. 5.66 6.67 3.57 4.14 1.12 1.26 ---
Total albumen
matter. 14.00 16.28 10.63 12.35 9.19 10.40 7.80
Mineral matter. 2.33 2.70 1.48 1.71 0.84 0.94 2.30
Fatty matter. 2.30 2.68 4.36 5.03 0.78 0.88 0.30
Cellulose
matter. 7.76 9.02 3.38 4.50 0.68 1.11 3.10
-----------------------------------------------------------
100.00 100.00 100.00 100.00 100.00 100.00 100.00
On looking over this table, we notice that rice contains by about 20 per cent, more starch than barley, and by about 10 to 12 per cent, more than maize.
But on the other hand, barley and maize are richer in albuminous matter and in ash. The extractive matter, i. e., the part which is soluble in cold water, is also much greater in barley and maize than in rice. The extractive matter is for barley 8.7 per cent., for maize 6.3 per cent., while rice contains only 2.1 per cent., and it consists in each case of dextrin, sugar, the soluble part of the ash, and of some nitrogenous matter (soluble albumen).
The amount of woody fiber or cellulose is considerable for rice with its husk, but only slight for samples without husk. The seat of the mineral matter of the grain of rice is mainly in the husk, and as this ash is very valuable as nourishment for the yeast plant, it is an open question whether it would not be preferable to use for brewing purposes rice with its husk. The comparatively largest amount of fat is contained in maize; and as such oil is not desirable for brewing purposes, different recommendations have been advanced for freeing the grain from it. In the following table some of the mineral constituents of the three kinds of grain are compared with each other. These data refer to 100 parts of ash, and are taken from analysis given by Dr. Emil Wolf.
100 parts of
Potash Lime Magnesia Phosphoric Silica grain contain
acid ash.
Barley. 21.9 2.5 8.3 32.8 27.2 2.55 p. ct.
Rice with
husk. 18.4 5.1 8.6 47.2 0.6 7.84 "
Rice without
husk. 23.3 2.9 13.4 51.0 3.0 0.39 "
Maize. 27.0 2.7 14.6 44.7 2.2 1.42 "
The excessive amount of ash in rice with its husk is very remarkable, and as this mineral matter consists to a great extent of phosphoric acid and potash, the larger part of it is soluble in water. Consequently on using rice with its husk for brewing purposes, the yeast will be provided with a considerable amount of nutritive substance.
In conclusion it need hardly be mentioned that the use of rice with its husk would also be of considerable pecuniary advantage. There is very little oil in the husk of rice, as shown above by analysis, and it is not likely that the flavor of the brew would suffer by it.--London Brewers' Journal.
Nothing is in more general use than petroleum, and but few things are known less about by the majority of persons. It is hydra-headed. It appears in many forms and under many names. "Burning fluid" is a popular name with many unscrupulous dealers in the cheap and nasty. "Burning fluid" is usually another name for naphtha, or something worse. Gasoline, naphtha, benzine, kerosene, paraffine, and many other dangerous fluids which make the fireman's vocation necessary are all the product of petroleum. These oils are produced by the distillation or refining of crude petroleum, and inasmuch as the public, especially firemen, are daily brought into contact with them it is proper that they should know something of their properties. Refining as commonly practiced involves three successive operations. The apparatus employed consists of an iron still connected with a coil or worm of wrought-iron pipe, which is submerged in a tank of water for the purpose of cooling it. The end of this pipe is fixed with a movable spout, which can be transferred or switched from one to another of half a dozen pipes which come around close to it, but which lead into different tanks containing different grades of the distillate. When the still has been filled with crude oil the fire is lighted beneath it, and soon the oil begins to boil. The first products of distillation are gases which, at ordinary temperatures, pass through the coil without being condensed, and escape. When the vapors begin to condense in the worm the oil trickles from the end of the coil into the pipe leading to the appropriate receiving tank.
The first oil obtained is known as gasoline, used in portable gas machines for making illuminating gas. Then, in turn, come naphthas of a greater or less gravity, benzine, high test water white burning oil, such as Pratt's Astral common burning oil or kerosene, and paraffine oils. When the oil has been distilled it is by no means fit for use, having a dirty color and most offensive smell; it is then refined. For this purpose it is pumped into a large vat or agitator, which holds from two hundred and fifty to one thousand barrels. There is then added to the oil about two per cent, of its volume of the strongest sulphuric acid. The whole mixture is then agitated by means of air pumps, which bring as much as possible every particle of oil in contact with the acid. The acid has no affinity for the oil, but it has for the tarry substance in it which discolors it, and, after the agitation, the acid with the tar settles to the bottom of the agitator, and the mixture is drawn off into a lead-lined tank. After the removal of the acid and tar, the clear oil is agitated with either caustic soda or ammonia and water. The alkali neutralizes the acid remaining in the oil, and the water removes the alkali, when the process of refining is finished. A few refiners improve the quality of their refined oil by redistilling it after treating it with acid and alkali. All distillates of petroleum have to be treated with acid and alkali to refine them. There is one thing peculiar about the distillates of petroleum, and that is that the run which follows naphtha, which is called "the middle run oil," is the highest test oil that is made, running as high as 150 and 160 degrees flash, while the common oil which follows, viz., from 45 down to 33 degrees Baume, will range at only about 100 flash, or 115 and 120 degrees burning lest.
An oil that will stand 100 flash will stand 110 burning test every time. Kerosene oil, at ordinary temperature, should extinguish a match as readily as water. When heated it should not evolve an inflammable vapor below 110 degrees, or, better, 120 degrees Fahrenheit, and should not take fire below 125 to 140 degrees Fahrenheit. As the temperature in a burning lamp rarely exceeds 100 degrees Fahrenheit, such an oil would be safe. It would produce no vapors to mix with the air in the lamp and make an explosive mixture; and, if the lamp should be overturned, or broken, the oil would not be liable to take fire. The crude naphtha sells at from three to five cents per gallon, while the refined petroleum or kerosene sells at from fifteen to twenty cents. As great competition exists among the refiners, there is a strong inducement to turn the heavier portions of the naphtha into the kerosene tank, so as to get for it the price of kerosene. In this way the inflammable naphtha or benzine is sometimes mixed with the kerosene, rendering the whole highly dangerous. Dr. D. B. White, President of the Board of Health of New Orleans, found that experimenting on oil which flashed at 113 degrees Fahrenheit, an addition of one per cent. of naphtha caused it to flash at 103 degrees; two per cent. brought the flashing point down to 92 degrees, five per cent. to 83 degrees, ten per cent. to 59 degrees, and twenty per cent. of naphtha added brought the flashing point down to 40 degrees Fahrenheit. After the addition of twenty per cent. of naphtha the oil burned at 50 degrees Fahrenheit. There are two distinct tests for oil, the flashing test and the burning test. The flashing test determines the flashing point of the oil, or the lowest temperature at which it gives off an inflammable vapor. This is the most important test, as it is the inflammable vapor, evolved at atmospheric temperatures, that causes most accidents. Moreover, an oil which has a high flashing test is sure to have a high burning test, while the reverse is not true. The burning test fixes the burning point of the oil, or the lowest temperature at which it takes fire. The burning point of an oil is from ten to fifty degrees Fahrenheit higher than the flashing point. The two points are quite independent of each other; the flashing point depends upon the amount of the most volatile constituents present, such as naphtha, etc., while the burning point depends upon the general character of the whole oil. One per cent. of naphtha will lower the flashing point of an oil ten degrees without materially affecting the burning test. The burning test does not determine the real safety of the oil, that is, the absence of naphtha. The flashing test should, therefore, be the only test, and the higher the flashing point the safer the oil.
In regard to the danger of using the lighter petroleum oils, the following, under the head of "Naphtha and Benzine under False Names," is taken from Prof. C. F. Chandler's article on "Petroleum" in Johnson's Cyclopedia. He says: "Processes have been patented, and venders have sold rights throughout the country, for patented and secret processes for rendering gasoline, naphtha, and benzine non-explosive. Thus treated, these explosive oils, just as explosive as before the treatment, are sold throughout the country under trade names. These processes are not only totally ineffective, but they are ridiculous. Roots, gums, barks, and salts are turned indiscriminately into the benzine, to leave it just as explosive as before. No wonder we have kerosene accidents, with agents scattered through the country selling county rights and teaching retail dealers how to make these murderous 'non-explosive' oils. The experiments these venders make to deceive their dupes are very convincing. None of the petroleum products are explosive per se, nor are their vapors explosive under all circumstances when mixed with air. A certain ratio of air to vapor is necessary to make an explosive mixture. Equal volumes of vapor and air will not explode; three parts of air and one of vapor gives a vigorous puff when ignited in a vessel; five volumes of air to one of vapor gives a loud report. The maximum degree of violence results from the explosion of eight or nine parts of air mixed with vapor. It requires considerable skill to make at will an explosive mixture with air and naphtha, and it is consequently very easy for the vender not to make one. In most cases the proportion of vapor is too great, and on bringing a flame in contact with the mixture it burns quietly. The vender, to make his oil appear non-explosive, unscrews the wick-tube and applies a match, when the vapor in the lamp quietly takes fire and burns without explosion. Or he pours some of the 'safety oil' into a saucer and lights it. There is no explosion, and ignorant persons, biased by the saving of a few cents per gallon, purchase the most dangerous oils in the market. It is not possible to make gasoline, naphtha, or benzine safe by any addition that can be made to it. Nor is any oil safe that can be set on fire at the ordinary temperature of the air. Nothing but the most stringent laws, making it a State prison offense to mix naphtha and illuminating oil, or to sell any product of petroleum as an illuminating oil or fluid to be used in lamps, or to be burned, except in air gas machines, that will evolve an inflammable vapor below 100 degrees, or better, 120 degrees Fahrenheit, will be effectual in remedying the evil. In case of an accident from the sale of oil below the standard, the seller should be compelled to pay all damages to property, and, if a life is sacrificed, should be punished for manslaughter. It should be made extremely hazardous to sell such oils." Prof Chandler is professor of analytical chemistry, School of Mines, Columbia College.
There is no substance on earth, or under the earth, which will chemically combine with naphtha, or that will destroy its peculiar volatile and explosive properties. The manufacturers of petroleum products have exhausted the whole resources of chemistry to make this product available as a safe burning oil, and their inability to do so proclaims the fact that it cannot be done. Chemistry has shown that naphtha, and, in fact, the other products of petroleum, will not part with their hydrogen or change the nature of their compounds, except by decomposition from a union with oxygen, that is, by combustion. These humbugs, who deceive people for their own gains, may put camphor, salt, alum, potatoes, etc., into naphtha, and call it by whatever fancy name they please. The camphor is dissolved, the salt partially; potatoes have no effect whatever. The camphor may disguise the smell of the naphtha, and sometimes myrhane or burnt almonds may be used for the same purpose. But, no matter what is used, the liability to explosion is not lessened in any degree. The stuff is always dangerous and always will be. There is not much danger in the use of kerosene, if it is of the standard required by law in several of the States. At the same time petroleum is dangerous under certain conditions. Where oil is heated it is more or less inflammable, and, in fact, inflammability is only a question of temperature of the oil, after all. Burning oils should be kept in a moderately cool place, and always with care. Of course, if a lighted lamp is dropped and broken, the oil is liable to take fire, though the lamp may be put out in the fall, or the light drowned by the oil, or the oil not take fire at all. This will be the effect if the oil is cool and of high flash test. When a lamp is lighted, and remains burning for some time, it should never be turned down and set aside. The theory is, that while lighting, a certain supply of gas is created from the oil, and that when the wick is turned down that supply still continues to flow out, and not being consumed, forms an inflammable gas in the chimney, which will explode when a sufficient quantity of air is mixed with it in the presence of light, which may happen if a person blows down the chimney; but a lamp should never be extinguished in that way. A good, high test kerosene oil can be made with ordinary care as safe as sperm oil, though, of course, it is not so safe as a matter of fact. We are sure to hear of it when an accident happens, but we never hear of the reckless use of kerosene where an accident does not occur, and yet there are few things so generally carelessly handled as burning oils.--Fireman's Journal
All portions of this petroleum contain saturated carbides of the formula CnH2n, which the authors name paraffenes. At a bright red heat they yield benzinic carbides, CnH2n-6, naphthalin and a little anthracen. At dull redness the products are along with unaltered paraffenes, products which unite energetically with bromine, and which are converted into resinous polymers of ordinary sulphuric acid. It is difficult to isolate, by means of fractional distillation, definite products with constant boiling points.
[Footnote: From the Archiv der Pharmacie.]
This oil, on account of its fragrance, which is described by most observers as extremely pleasant, has attained to some importance, so that it appears to me not superfluous to submit the following remarks upon it and the plant from which it is derived.
The tree, of which the flowers yield the oil known under the name "Ilang-ilang" or "Alanguilan," is the Cananga odorata, Hook. fil. et Thomp.,[1] of the order Unonaceæ, for which reason it is called also in many price lists "Oleum Anonæ," or "Oleum Unonæ" It is not known to me whether the tree can be identified in the old Indian and Chinese literature.[2] In the west it was first named by Ray as "Arbor Saguisan," the name by which it was called at that time at Luçon[3] Rump[4] gave a detailed description of the "Bonga Cananga," as the Malays designate the tree ("Tsjampa" among the Javanese); Rumph's figure, however is defective. Further, Lamarck[5] has short notices of it under "Canang odorant, Uvaria odorata." According to Roxburgh,[6] the plant was in 1797 brought from Sumatra to the Botanical Gardens in Calcutta. Dunal devoted to the Ucaria odorata, or, properly, Unona odorata, as he himself corrected it, a somewhat more thorough description in his "Monographic de la Famille des Anonacees,"[7] which principally repeats Rumph's statements.
[Footnote 1: "Flora Indica," i (1855), 130.]
[Footnote 2: "No mention of any plant or flowers, which might be identified with Cananga, can be traced in any Sanskrit works."--Dr. Charles Rice, New Remedies, April, 1881, page 98.]
[Footnote 3: Ray. "Historia Plantarum, Supplementum," tomi i et ii "Hist. Stirpium Insulæ Luzonensis et Philippinarum" a Georgio Josepho Canello; London, 1704, 83]
[Footnote 4: "Herbarium Amboinense, Amboinsch Kruidboek," ii. (Amsterdam, 1750), cap. xix, fol. 195 and tab. 65.]
[Footnote 5: "Encyclopédie méthodique. Botanique," i (1783), 595.]
[Footnote 6: "Flora Indica," ii. (Serampore, 1832), 661.]
[Footnote 7: Paris, 1817, p. 108, 105.]
Lastly, we owe a very handsome figure of the Cananga odorata to the magnificent "Flora Javæ," of Blume;[1] a copy of this, which in the original is beautifully colored, is appended to the present notice. That this figure is correct I venture to assume after having seen numerous specimens in Geneva, with De Candolle, as well as in the Delessert herbarium. The unjustifiable name Unona odoratissima, which incorrectly enough has passed into many writings, originated with Blanco,[2] who in his description of the powerful fragrance of the flowers, which in a closed sleeping room produces headache, was induced to use the superlative "odoratissima." Baillon[3] designated as Canangium the section of the genus Uvaria, from which he would not separate the Ilang-ilang tree.
[Footnote 1: Vol. i. (Brussels, 1829), fol. 29, tab ix et xiv. B.]
[Footnote 2: "Flora de Filipinas," Manila, 1845, 325. Unona odoratissima, Alang-ilan. The latter name, according to Sonnerat, is stated by the Lamarck to be of Chinese origin; Herr Reymann derives it from the Tagal language.]
[Footnote 3: "Dictionnaire de Botanique."]
CANAGA ODORATA
The notice of Maximowicz,[1] "Ueber den Ursprung des Parfums Ylang-Ylang," contains only a confirmation of the derivation of the perfume from Cananga.
[Footnote 1: Just's "Botanischer Jahresbericht," 1875, 973.]
Cananga odorata is a tree attaining to a height of 60 feet, with few but abundantly ramified branches. The shortly petioled long acuminate leaves, arranged in two rows, attain a length of 18 centimeters and a breadth of 7 centimeters; the leaf is rather coriaceous, and slightly downy only along the nerves on the under side. The handsome and imposing looking flowers of the Cananga odorata occur to the number of four on short peduncles. The lobes of the tripartite leathery calyx are finally bent back. The six lanceolate petals spread out very nearly flat, and grow to a length of 7 centimeters and a breadth of about 12 millimeters; they are longitudinally veined, of a greenish color, and dark brown when dried. The somewhat bell-shaped elegantly drooping flowers impart quite a handsome appearance, although the floral beauty of other closely allied plants is far more striking. The filaments of the Cananga are very numerous; the somewhat elevated receptacle has a shallow depression at the summit. The green berry-like fruit is formed of from fifteen to twenty tolerably long stalked separate carpels which inclose three to eight seeds arranged in two rows. The umbel-like peduncles are situated in the axils of the leaves or spring from the nodes of leafless branches. The flesh of the fruit is sweetish and aromatic. The flowers possess a most exquisite perfume, frequently compared with hyacinth, narcissus, and cloves.
Cananga odorata, according to Hooker and Thomson or Bentham and Hooker,[1] is the only species of this genus; the plants formerly classed together with it under the names Unona or Uvaria, among which some equally possess odorous flowers, are now distributed between those two genera, which are tolerably rich in species. From Uvaria the Cananga differs in its valvate petals, and from Unona in the arrangement of the seeds in two rows.
[Footnote 1: "Genera Plantarum," i, (1864), 24.]
Cananga odorata is distributed throughout all Southern Asia, mostly, however, as a cultivated plant. In the primitive forest the tree is much higher, but the flowers are, according to Blume, almost odorless. In habit the Cananga resembles the Michelia champaca, L.,[1] of the family Magnoliaceæ, an Indian tree extraordinarily prized on account of the very pleasant perfume of its yellow flowers, and which was already highly celebrated in ancient times in India. Among the admired fragrant flowers which are the most prized by the in this respect pampered Javanese, the "Tjempaka" (Michelia champaca) and the "Kenangga wangi" (Cananga odorata)[2] stand in the first rank.
[Footnote 1: A beautiful figure of this also is given in Blume's "Flora Javæ," iii., Magnoliaceæ, tab. I.]
[Footnote 2: Junghuhn, Java, Leipsic, 1852, 166.]
It is not known to me whether the oil of cananga was prepared in former times. It appears to have first reached Europe about 1864; in Paris and London its choice perfume found full recognition.[1] The quantities, evidently only very small, that were first imported from the Indian Archipelago were followed immediately by somewhat larger consignments from Manila, where German pharmacists occupied themselves with the distillation of the oil.[2]
[Footnote 1: Jahresbericht d. Pharmacie, by Wiggers and Husemann, 1867, 422.]
[Footnote 2: Jahresbericht, 1868, 166.]
Oscar Reymann and Adolf Ronsch, of Manila, exhibited the ilang-ilang oil in Paris in 1878; the former also showed the Cananga flowers. The oil of the flowers of the before-mentioned Michelia champaca, which stood next to it, competes with the cananga oil, or ilang-ilang oil, in respect to fragrance.[1] How far the latter has found acceptance is difficult to determine; a lowering of the price which it has undergone indicates probably a somewhat larger demand. At present it may be obtained in Germany for about 600 marks (£30) the kilogramme.[2] Since the Cananga tree can be so very easily cultivated in all warm countries, and probably everywhere bears flowers endowed with the same pleasant perfume, it must be possible for the oil to be produced far more cheaply, notwithstanding that the yield is always small.[3] It may be questioned whether the tree might not, for instance, succeed in Algeria, where already so many exotic perfumery plants are found.
[Footnote 1: Archiv der Pharmacie, ccxiv. (1879), 18.]
[Footnote 2: According to information kindly supplied by Herr Reymann, in Paris, Nice, and Grasse, annually about 200 kilogrammes are used; in London about 50 kilogrammes, and equally as much in Germany (Leipsic, Berlin, Frankfort).]
[Footnote 3: 25 grammes of oil from 5 kilogrammes of flowers, according to Reymann.]
According to Guibourt,[1] the "macassar oil," much prized in Europe for at least some decades as a hair oil, is a cocoa nut oil digested with the flowers of Cananga odorata and Michelia champaca, and colored yellow by means of turmeric. In India unguents of this kind have always been in use.
[Footnote 1: Histoire Naturelle des Drogues Simples, iii. (1850), 675.]
The name "Cananga" is met with in Germany as occurring in former times. An "Oleum destillatum Canangæ" is mentioned by the Leipsic apothecary, Joh. Heinr. Linck[1] among "some new exotics" in the "Sammlung von Naturund Medicin- wie, auch hierzu gehorigen Kunst- und Literatur Geschichten, so sich Anno 1719 in Schlesien und andern Ländern begeben" (Leipsic und Budissin, 1719). As, however, the fruit of the same tree sent together with this cananga oil is described by Linck as uncommonly bitter, he cannot probably here refer to the present Cananga odorata, the fruit-pulp of which is expressly described by Humph and by Blume as sweetish. Further an "Oleum Canangæ, Camel-straw oil," occurs in 1765 in the tax of Bremen and Verden.[2] It may remain undetermined whether this oil actually came from "camel-straw," the beautiful grass Andropogon laniger.
[Footnote 1: Compare Flückiger, "Pharmakognosic," 2d edit, 1881, p. 152.]
[Footnote 2: Flückiger, "Documente zur Geschichte der Pharmacie," Halle (1876), p 93.]
From a chemical point of view cananga oil has become interesting because of the information given by Gal,[1] that it contains benzoic acid, no doubt in the form of a compound ether. So far as I, at the moment, remember the literature of the essential oils, this occurrence of benzoic acid in plants stands alone,[2] although in itself it is not surprising, and probably the same compound will yet be frequently detected in the vegetable kingdom. As it was convenient to test the above statement by an examination I induced Herr Adolf Convert, a pharmaceutical student from Frankfort-On-Main, to undertake an investigation of ilang-ilang oil in that direction. The oil did not change litmus paper moistened with alcohol. A small portion distilled at 170° C.; but the thermometer rose gradually to 290°, and at a still higher temperature decomposition commenced. That the portions passing over below 290° had a strong acid reaction already indicated the presence of ethers. Herr Convert boiled 10 grammes of the oil with 20 grammes of alcohol and 1 gramme of potash during one day in a retort provided with a return condenser. Finally the alcohol was separated by distillation, the residue supersaturated with dilute sulphuric acid, and together with much water submitted to distillation until the distillate had scarcely an acid reaction. The liquid that had passed over was neutralized with barium carbonate, and the filtrate concentrated, when it yielded crystals, which were recognized as nearly pure acetate. The acid residue, which contained the potassium sulphate, was shaken with ether; after the evaporation of the ether there remained a crystalline mass having an acid reaction which was colored violet with ferric chloride. This reaction, which probably may be ascribed to the account of a phenol, was absent after the recrystallization of the crystalline mass from boiling water. The aqueous solution of the purified crystalline scales then gave with ferric chloride only a small flesh-colored precipitate. The crystals melted at 120° C. In order to demonstrate the presence of benzoic acid Herr Convert boiled the crystals with water and silver oxide and dried the scales that separated from the cooling filtrate over sulphuric acid. 0.0312 gramme gave upon combustion 0.0147 gramme of silver, or 47.1 per cent. The benzoate of silver contains 46.6 per cent, of metal; the crystals prepared from the acid of ilang-ilang oil were, therefore, benzoate of silver. For the separation of the alcoholic constituent, which is present in the form of an apparently not very considerable quantity of benzoic ether, far more ilang-ilang oil would be required than was at command.
[Footnote 1: Comptes Rendus, lxxvi. (1873), 1428, and abstracted in the Pharmaceutical Journal [3], iv., p. 28; also in Jahresbericht, 1873, p. 431.]
[Footnote 2: Overlooking Peru balsam and Tolu balsam.]
Besides the benzoic ether and, probably, a phenol, mentioned above, there may be recognized in ilang-ilang oil an aldehyde or ketone, inasmuch as upon shaking it with bisulphite of sodium I observed the formation of a very small quantity of crystals. That Gal did not obtain the like result must at present remain unexplained. Like the benzoic acid the acetic acid is, no doubt, present in cananga oil in the form of ether.
The following letter has been received by the editors of the Repertoire de Pharmacie: For some months past, a good deal has been heard about a product of our island that had quite fallen into disuse, and which no one cared to gather, so much had the demand fallen off because a substitute for it had been found in Europe; I mean Chian turpentine.
As this product is destined to take a certain part in the treatment of cancer, according to some English physicians, permit me, sir, to give your readers a few interesting details, obtained on the spot, concerning the turpentine tree and its product.
The turpentine tree (Pistacia terebinthus L.) has existed in our island for many centuries, judging from the enormous dimensions of some of these trees, compared, too, with their slow rate of growth. The trunks of some measure from 4 to 5 meters in circumference, and their heights vary from 15 to 20 meters. On my own land there is an enormous tree, by far the largest on the island, the circumference of its trunk being 6 meters. Many of these great trees have been used in the construction of mills, presses, etc., on account of the hardness of their wood. It is in the vicinity of the town and in three or four neighboring villages that these trees are found. To-day, at a careful estimate, there may be 1,500 trees capable of yielding 2,000 kilos of turpentine, mixed with at least 30 per cent of foreign matter. There are no appliances for refining the product here, except the sieves through which it is passed to remove the pebbles and bits of wood which are found in it.
It is gathered from incisions made in the tree in June. Axes are used for this purpose, and the incision must be through the whole thickness of the bark. Through these outlets the turpentine falls to the foot of the tree, and mixes with the earth there. On its first appearance the turpentine is of a sirupy consistence, and is quite transparent; gradually it becomes more opaque, and of a yellowish-white color. It is at this period also that it gives off its characteristic odor most abundantly.
It is, however, not the product "turpentine" that is most esteemed by the natives, but the fruit of the tree, a kind of drupe disposed in clusters. The fruit is improved by the incisions made in the tree for the escape of the turpentine, otherwise the resin, having no other outlet, would impregnate the former, hinder its complete development, and render it useless for the purposes for which it is cultivated. One circumstance worth noting is that, as soon as the fruit commences to ripen, the flow of turpentine completely ceases. This is toward August; the fruit is then green; it is gathered, dried in the sun, bruised, and a fine yellowish-green oil is drawn from it, which is soluble in ether. This oil is used for alimentary purposes, but rarely for illumination since the introduction of petroleum. It is mostly used in making sweet cakes, and often as a substitute for butter, in all cases where the latter is employed. I use it daily myself without perceiving any difference.
I may here be permitted to correct a slight mistake that has crept into several standard botanical works. It is therein stated that the inhabitants of this country extract from the fruit of the lentisc (Pistacia lentiscus L., a well-known shrub growing on this island, from which Chian mastic is obtained), an alimentary and illuminating oil. This fruit has never been gathered for its oil within the memory of man. The lentisc has probably been thus mistaken for the turpentine tree.
For the last twenty years the gathering of turpentine has been almost abandoned, although the incisions in the trees have been regularly made, but the value was so small that proprietors did not care to collect it, and left it to run to waste. There were but a few pharmacists of Smyrna and the neighboring islands who took a small quantity for making medicinal plasters. An utterly insignificant quantity found its way into Europe. How is it then that, after so many years, it was found in Europe? The problem is easily explained--the greater part came from Venice. This is indubitable, and, lately, an English chemist, Mr. W. Martindale, in a communication to the Chemical Society of London, expressed doubts as to the authenticity of the turpentine used in the treatment of cancer. If turpentine can really somewhat relieve this disease, and if this treatment is generally accepted in Europe, I much fear you will only obtain substitutions of very inferior quality to the turpentine produced in our island.
This year the Chians have been surprised by an extensive demand for this product, from London in the first place, and secondly from Vienna, and the proprietors, although but poorly provided at the moment, sent away nearly 600 kilos Paris has not yet made any demand. Yours, etc.,
DR. STIEPOWICH.
Chio, Turkey.
In previous notes I have established, first, that the galvanic depositions experience a change of volume, from which there results a pressure exercised on the mould which receives them; second, that the Peltier phenomenon is produced at the surface of contact of an electrode and of an electrolyte. Fresh observations have caused me to believe that the two phenomena are connected, and that the first is a consequence of the second. The Peltier effect can clearly be proved when the electrolysis is not interfered with by energetic secondary actions, and particularly with the sulphate and nitrate of copper, the sulphate and chloride of zinc, and the sulphate and chloride of cadmium. For any one of these salts it is possible to determine a value, I, of the intensity of the current which produces the metallic deposit such that, for all the higher intensities the electrode becomes heated, and such that it becomes cold for less intensities. I will designate this intensity, I, under the name of neutral point of temperatures.
The new fact which I have observed is, that in the electrolysis of the same salts it is always possible to lower the intensity of the current below a limit, I', such that the compression produced by the deposit changes its direction, that is to say, instead of contracting the metal dilates in solidifying. This change, although unquestionable, is sufficiently difficult to produce with sulphate of copper. It is necessary to employ as a negative electrode a thermometer sensitive to 1/200 of a degree, and to take most careful precautions to avoid accidental deformations of the deposit; but the phenomenon can be observed very easily with nitrate of copper, the sulphate of zinc, and the chloride of cadmium. There is, therefore, a neutral point of compression in the same cases where there is a neutral point of temperatures. With the salts of iron, nickel, etc., for which the neutral point of temperatures cannot be arrived at, there is also no neutral point of compression; and the negative electrode always becomes heated, and the deposit obtained is always a compressing deposit.
I have determined, by the help of observations made with ten different current strengths, the constants of the formulæ which I have explained elsewhere, and which gives the apparent excess, y, of the thermometer electrode compressed by the metallic deposit in terms of the time, t, during which the metal was depositing:
A t
(1) y = -------
B + t
The constant, A, is proportional to the variation of volume of the unit of volume of the metal. The values of A, without being exactly regular, are sufficiently well represented within practical limits by the formula:
(2) A = - a'i + b'i²,
of the same form as the expression E:
E = - ai + bi²,
of the heating of the thermometer electrode. Further, every cause which affects the coefficients, a or b, also affects in the same way a' and b': such causes being the greater or less dilution of the solution, the nature of the salt, etc. It is, therefore, impossible not to be struck by the direct relation of the thermic and mechanical phenomena of which the negative electrode is the origin. The following is the explanation which I offer: The thermometer indicates the mean temperature of the liquid just outside it; this temperature is not necessarily that of the metal which incloses it. The current, propagated almost exclusively by the molecules of the decomposed salt, does not act directly to cause a variation in the temperature of the dissolving molecules; these change heat with the molecules of the electrolyte, which should be in general hotter than those when a heating is noticed and colder when a cooling is observed. Suppose it is found, in the first case, that the metal, at the moment when it is deposited, is hotter than the liquid, and, consequently, than the thermometer; it becomes colder immediately after the deposit, and consequently contracts; the deposit is compressed. The reverse is the case when the metal is colder than the liquid; the deposit then dilates. If this hypothesis is correct, the excess, T, of the temperature of the metal over the liquid which surrounds the thermometer should be proportional to the contraction, A, represented by the formula (2), and the neutral point, I', of the contraction corresponds to the case where the temperature of the metal is precisely equal to that of the liquid.
It might be expected, perhaps, from the foregoing, that I' = I; this would take place if the excess of temperature of the metal, measured by the contraction, were rigorously proportional to the heating of the liquid, for then the two quantities would be null at the same time. Careful experiment proves that this is not the case. The sulphate of copper gives compressing deposits on a thermometer which is undoubtedly cooling; chloride of zinc of a density 200 can give expanding deposits on a thermometer which is heating. There is, therefore, no proportionality; but it must be remarked that the temperature of the metal which is deposited does not depend only on the quantities of heat disengaged in an interval of molecular thickness which is infinitely small compared with the thickness of the layer, of which the variations of temperature are registered by the thermometer. There is nothing surprising, therefore, that the two variations of temperature, according exactly with one another, do not follow identically the same laws.--Comptes Rendus.
The analyses of rice soils was undertaken at the instance of the Revenue Settlement Survey, who wanted to know if the chemical composition of the soil corresponded in any way to the valuation as fixed from other evidence. It was found that the amount of phosphoric acid in the soil in any one district corresponded pretty well with the Settlement Officers' valuation, but on comparing two districts it was found that the district which was poorer in phosphoric acid gave crops equal to the richer one. On inquiry it was found that in the former the rice is grown in nurseries and then planted out by hand, whereas in the latter, where the holdings are much larger, the grain is sown broadcast. The practice of planting out the young crops enables the cultivator to get a harvest 20 per cent. better than he would otherwise do, and hence the poorer land equals the richer.
The deductions drawn from this investigation are, first, that, climate and situation being equal, the value of soil depends on the phosphoric acid in it; and, second, that the planting-out system is far superior to the broadcast system of cultivation for rice.
Results of two analyses of soils from Syriam, near Rangoon, are appended:
_Soluble in Hydrochloric Acid_.
I. II.
Virgin Soil.
Organic matter 4.590 8.5?8
Oxide of iron and alumina 8.939 7.179
Magnesia 0.469 0.677
Lime trace. 0.131
Potash 0.138 0.187
Soda 0.136 0.337
Phosphoric acid 0.100 0.108
Sulphuric acid 0.025 0.117
Silica ---- 0.005
-------- ---------
14.397 17.249
_Soluble in Sulphuric Acid_.
Alumina 17.460 15.684
Magnesia 0.459 0.446
Lime 0.286 trace.
Potash 0.616 1.250
Soda 0.317 0.285
--------- ---------
19.138 17.665
_Residue_.
Silica, soluble 11.675 \
69.546
" insoluble 49.477 /
Alumina 3.062 4.178
Lime 0.700 0.134
Magnesia 0.212 trace.
Potash 0.276 1.180
Soda 0.503 1.048
-------- ---------
100.000 100.000
These are alluvial soils from the Delta of the Irrawaddy.
A large number of scientific and other gentlemen interested in mechanical refrigeration lately visited the works of Messrs. J. & E. Hall, of Dartford, to inspect the working of one of their improved horizontal dry air refrigerators!
The machine, which is illustrated below, is designed to deliver about 10,000 cubic feet of cold air per hour, when running at the rate of 100 revolutions per minute, and is capable of reducing the temperature of the air from 90 deg. above, to about 50 deg. below zero, Fah., with an initial temperature of cooling water of 90 deg. to 95 deg. Fah. It can, however, be run at as high a speed as 140 revolutions per minute. The air is compressed in a water-jacketed, double-acting compression cylinder, to about 55 lb. per square inch --more or less according to the temperature of the cooling water--the inlet valve being worked from a cam on the crank shaft, to insure a full cylinder of air at each stroke, and the outlet valves being self acting, specially constructed to avoid noise in working and breakages, which have given rise to so much annoyance in other cold air machines. The compressed air, still at a high temperature, is then passed through a series of tubular coolers, where it parts with a great deal of its heat, and is reduced to within 4 deg. or 5 deg. of the initial temperature of the cooling water. Here also a considerable portion of the moisture, which, when fresh air is being used, must of necessity enter the compression cylinder, is condensed and deposited as water.
COMPRESSION CYLINDER. SCALE 1/60
After being cooled, the compressed air is then admitted to the expansion cylinder, but as it still contains a large quantity of water in solution, which, if expansion was carried immediately to atmospheric pressure, would, from the extreme cold, be converted into snow and ice, with a positive certainty of causing great trouble in the valves and passages. It is got rid of by a process invented by Mr. Lightfoot, which is at the same time extremely simple and beautiful in action, and efficient. Instead of reducing the compressed air at once to atmospheric pressure, it is at first only partially expanded to such an extent that the temperature is lowered to about 35 deg. to 40 deg. Fah., with the result that very nearly the whole of the contained aqueous vapor is condensed into water. The partially expanded air which now contains the water as a thick mist is then admitted into a vessel containing a number of grids, through which it passes, parting all the while with its moisture, which gradually collects at the bottom and is blown off. The surface area of the grids is so arranged that by the time the air has passed through them it is quite free from moisture, with the exception of the very trifling amount which it can hold in solution at about 35 deg. Fah., and 30 lb. pressure. The expansion is then continued to atmospheric pressure and the cooled air containing only a trace of snow is then discharged ready for use into a meat chamber or elsewhere. In small machines the double expansion is carried out in one cylinder containing a piston with a trunk, the annulus forming the first expansion and the whole piston area the second, but in larger machines two cylinders of different sizes are used, just as in an ordinary compound engine. To compensate for the varying temperature of the cooling water the cut-off valve to the first or primary expansion is made adjustable; and this can either be regulated as occasion requires by hand, or else automatically. The temperature in the depositors being kept constant under all variations in cooling water, there is the same abstraction of moisture in the tropics as in colder climates, and the cold air finally discharged from the machine is also kept at a uniform temperature.
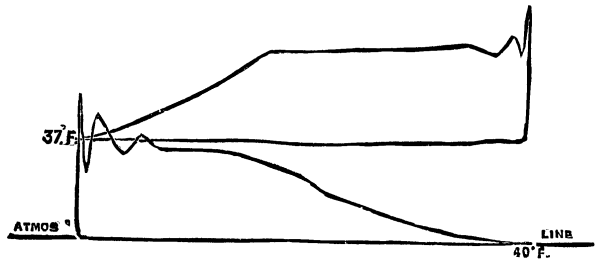
Expansion Cylinder. Scale 1/60.92° F.
temperature of entering
air. Cooling water
entering
in at 86° F.
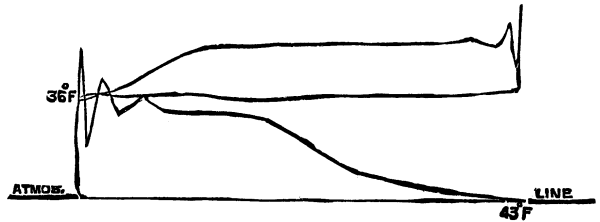
Expansion Cylinder. Scale 1/60.
68° F. temperature of entering air. Cooling water entering
in at 65° F. 125 revs. per minute, or 312 ft.
per minute per piston speed.
The diagrams are reduced from the originals, taken from the compression cylinder when running at the speed of 125 revolutions per minute, and also from the expansion cylinder, the first when the cooling water was entering the coolers at 86 deg. Fah., and the latter when this temperature was reduced to 65 deg. Fah. In all cases the compressed air is cooled down to within from 3 deg. to 5 deg. of the initial temperature of the cooling water, thus showing the great efficiency of the cooling apparatus. The machine has been run experimentally at Dartford, under conditions perhaps more trying than can possibly occur, even in the tropics, the air entering the compression cylinder being artificially heated up to 85 deg. and being supersaturated at that temperature by a jet of steam laid on for the purpose. In this case no more snow was formed than when dealing with aircontaining a very much less proportion of moisture. The vapor was condensed previous to final expansion and abstracted as water in the drying apparatus. The machine was exhibited at work in connection with a cold chamber which was kept at a temperature of about 10 deg. Fah., besides which several hundredweight of ice were made in the few days during which the experiments lasted. This machine is in all respects an improvement on the machine which we have already illustrated. In that machine Messrs. Hall were trammeled by being compelled to work to the plans of others. In the present case the machine has been designed by Mr. Lightfoot, and appears to leave little to be desired. It is a new thing that a cold air machine may be run at any speed from 32 to 120 revolutions per minute. In its action it is perfectly steady, and the cold air chamber is kept entirely clear of snow. The dimensions of the machine are also eminently favorable to its use on board ship.-The Engineer.
DRY AIR REFRIGERATING MACHINE
The rotary or steam wheel, the invention of J.E. Thomas, of Carlinville, Ill., shown in the annexed figure, consists of a wheel with an iron rim inclosed within a casing or jacket from which nothing protrudes except the axle which carries the driving pulley, and the grooved distributing disk. Within this jacket, which need not necessarily be steam-tight, there is a movable piece, K, which, pressing against the rim, renders steam-tight the channel in which the pistons move when driven by the steam. At the extremities of this channel there are plates which are kept pressed against the wheel by means of spiral springs, thus rendering the channel perfectly tight.
The steam enters the closed space (which forms one-fourth of the circumference) through the slide-valve, S, presses against the pistons, d, and causes the wheel to revolve in the direction of the arrows. The slide-valve is closed by the action of the external distributing mechanism, the piston passes beyond the steam-outlet, A, and a new piston then comes in play. Altogether, there are six of these pistons, each one working in an aperture in the rim, and kept pressed outwardly by means of a spiral spring. The steam acts constantly on the same lever arm and meets with no counter-pressure. The other defects, likewise, of the ordinary steam engines in use are obviated to such an extent that the effective power of the steam-wheel is 50 per cent, greater than that of other and more complicated machines--at least this is the experience of the inventor.
IMPROVED STEAM-WHEEL.
To the inner ends of the pistons there are attached rods which pass through the rim of the wheel (where they are provided with stuffing-boxes) and abut against spiral springs. These rods are, in addition, connected with levers, h, which are pivoted on the spokes of the wheel, and whose other extremities carry rods, 2. These latter run through guides on the external face of the rim of the wheel and engage by means of friction-rollers, in an undulating groove formed in the inner surface of the jacket. When a piston arrives in front of the upper extremity of the steam channel, the friction roller at that moment enters one of the depressions in the groove, and thus lifts up the piston and allows it to pass freely beyond the plate which closes the channel.
You have assembled in convention for the first time outside the limits of the United States, and I congratulate you on the selection of this beautiful city, in which and its immediate neighborhood there are so many interesting engineering works, constructed with the skill and solidity characteristic of the British school of engineering. Nine of our members are Canadian engineers, which must be the excuse of the other members for invading foreign territory.
The society was organized November 3, 1852, and actively maintained up to March 2, 1855. Eleven only of the present members date from this period. October 2, 1867, the society was reorganized on a wider basis, and from that time to the present it has been constantly increasing in interest and usefulness.
The membership of the society is now as follows:
Honorary members........ 11
Corresponding members... 3
Members................. 491
Associates.............. 21
Juniors................. 57
Fellows................. 53
----
Total................... 636
During the last year we have lost six members by death and five by resignation, and fifty-six new members have been elected and qualified.
The most interesting event to the society since the last convention has been the purchase of a house in the City of New York, as a permanent home, at a cost of $30,000. This has been accomplished, so far, without taxing the resources of the society, the required payments having been met by subscription. The sum of $11,900 had been subscribed to the building fund up to the 25th ult., by seventy members and twenty-nine friends of the society who are not members. The subscription is still open, and it is expected that large additions will be made to it by members and their friends to enable the society to make the remaining payments without embarrassment.
Meetings of the society are held twice in each month during ten months in the year, for the reading and discussion of papers and other purposes. The new house affords much better accommodations for these purposes than we have ever had before, and also for the library, which now contains 8,850 books and pamphlets, and is constantly increasing. A catalogue of the library is being prepared. Part I., embracing railroads and the transactions of scientific societies, has been printed and furnished to members.
Water power in many of the States is abundant and contributes largely to their prosperity. Its proper development calls for the services of the civil engineer, and as it is the branch of the profession with which I am most familiar, I propose to offer a few remarks on the subject.
The earliest applications were to grist and saw mills; carding and fulling mills soon followed; these were essential to the comfort of the early settlers who relied on home industries for shelter, food, and clothing, but with the progress of the country came other requirements.
The earliest application of water power to general manufacturing purposes appears to have been at Paterson, New Jersey, where "The Society for Establishing Useful Manufactures" was formed in the year 1791. The Passaic River at this point furnishes, when at a minimum, about eleven hundred horse power continuously night and day.
The water power at Lowell, Massachusetts, was begun to be improved for general manufacturing purposes in 1822. The Merrimack River at this point has a fall of thirty-five feet, and furnishes, at a minimum, about ten thousand horse power during the usual working hours.
At Cohoes, in the State of New York, the Mohawk River has a fall of about one hundred and five feet, which was brought into use systematically very soon after that at Lowell, and could furnish about fourteen thousand horse power during the usual working hours, but the works are so arranged that part of the power is not available at present.
At Manchester, New Hampshire, the present works were commenced in 1835. The Merrimack River at this point has a fall of about fifty-two feet, and furnishes, at a minimum, about ten thousand horse power during the usual working hours.
At Lawrence, Massachusetts, the Essex Co. built a dam across the Merrimack River, commencing in 1845, and making a fall of about twenty-eight feet, and a minimum power, during the usual working hours, of about ten thousand horse power.
At Holyoke, Massachusetts, the Hadley Falls Co. commenced their works about 1845, for developing the power of the Connecticut River at that point, where there is a fall of about fifty feet, and at a minimum, about seventeen thousand horse power during the usual working hours.
At Lewiston, Maine, the fall in the Androscoggin River is about fifty feet; its systematic development was commenced about 1845, and with the improvement of the large natural reservoirs at the head waters of the river, now in progress, it is expected that a minimum power, during the usual working hours, of about eleven thousand horse power will be obtained.
At Birmingham, Connecticut, the Housatonic Water Co. have developed the water power of the Housatonic River by a dam, giving twenty-two feet fall, furnishing at a minimum about one thousand horse power during the usual working hours.
The Dundee Water and Land Co., about 1858, developed the power of the Passaic River, at Passaic, New Jersey, where there is a fall of about twenty-two feet, giving a minimum power, during the usual working hours, of about nine hundred horse power.
The Turners Falls Co., in 1866, commenced the development of the power of the Connecticut River at Turners Falls, Massachusetts, by building a dam on the middle fall, which is about thirty-five feet, and furnishes a minimum power, during the usual working hours, of about ten thousand horse power.
I have named the above water powers as being developed in a systematic manner from their inception, and of which I have been able to obtain some data. In the usual process of developing a large water power, a company is formed, who acquire the title to the property, embracing the land necessary for the site of the town, to accommodate the population which is sure to gather around an improved water power. The dam and canals or races are constructed, and mill sites, with accompanying rights to the use of the water, are granted, usually by perpetual leases subject to annual rents. This method of developing water power is distinctly an American idea, and the only instance where it has been attempted abroad, that I know of, is at Bellegarde in France, where there is a fall in the Rhone of about thirty-three feet. Within the last few years works have been constructed for its development, furnishing a large amount of power, but from the great outlay incurred in acquiring the titles to the property, and other difficulties, it has not been a financial success.
The water powers I have named are but a small fraction of the whole amount existing in the United States and the adjoining Dominion of Canada. There is Niagara, with its two or three millions of horse power; the St. Lawrence, with its succession of falls from Lake Ontario to Montreal; the Falls of St. Antony, at Minneapolis; and many other falls, with large volumes of water, on the upper Mississippi and its branches. It would be a long story to name even the large water powers, and the smaller ones are almost innumerable. In the State of Maine a survey of the water power has recently been made, the result, as stated in the official report, being "between one and two millions of horse power," part of which will probably not be available. There is an elevated region in the northern part of the South Atlantic States, exceeding in area one hundred thousand square miles, in which there is a vast amount of water power, and being near the cotton fields, with a fine climate, free from malaria, its only needs are railways, capital, and population, to become a great manufacturing section.
The design and construction of the works for developing a large water power, together with the necessary arrangements for utilizing it and providing for its subdivision among the parties entitled to it according to their respective rights, affords an extensive field for civil engineers; and in view of the vast amount of it yet undeveloped, but which, with the increase of population and the constantly increasing demand for mechanical power as a substitute for hand labor, must come into use, the field must continue to enlarge for a long time to come.
There are many cases in which the power of a waterfall can be made available by means of compressed air more conveniently than by the ordinary motors. The fall may be too small to be utilized by the ordinary motors; the site where the power is wanted may be too distant from the waterfall; or it may be desired to distribute the power in small amounts at distant points.[1] A method of compressing air by means of a fall of water has been devised by Mr. Joseph P. Frizell, C.E., of St. Paul, Minnesota, which, from the extreme simplicity of the apparatus, promises to find useful applications. The principle on which it operates is, by carrying the air in small bubbles in a current of water down a vertical shaft, to the depth giving the desired compression, then through a horizontal passage in which the bubbles rise into a reservoir near the top of this passage, the water passing on and rising in another vertical or inclined passage, at the top of which it is discharged, of course, at a lower level than it entered the first shaft.
[Footnote 1: Journal of the Franklin Institute for September, 1877.]
The formation at waterfalls is usually rock, which would enable the passages and the reservoir for collecting the compressed air to be formed by simple excavations, with no other apparatus than that required to charge the descending column of water with the bubbles of air, which can be done by throwing the water into violent commotion at its entrance, and a pipe and valve for the delivery of the air from the reservoir.
The transfer of power by electricity is one of the problems now engaging the attention of electricians, and it is now done in Europe in a small way. Sir William Thomson stated in evidence before an English parliamentary committee, two years ago, that he looked "forward to the Falls of Niagara being extensively used for the production of light and mechanical power over a large area of North America," and that a copper wire half an inch in diameter would transmit twenty-one thousand horse power from Niagara to Montreal, Boston, New York, or Philadelphia. His statements appear to have been based on theoretical considerations; but there is no longer any doubt as to the possibility of transferring power in this manner--its practicability for industrial purposes must be determined by trial. Dr. Paget Higgs, a distinguished English electrician, is now experimenting on it in the City of New York.
Great improvements in reaction water wheels have been made in the United States within the last forty years. In the year 1844, the late Uriah Atherton Boyden, a civil engineer of Massachusetts, commenced the design and construction of Fourneyron turbines, in which he introduced various improvements and a general perfection of form and workmanship, which enabled a larger percentage of the theoretical power of the water to be utilized than had been previously attained. The great results obtained by Boyden with water wheels made in his perfect manner, and, in some instances, almost regardless of cost, undoubtedly stimulated others to attempt to approximate to these results at less cost; and there are now many forms of wheel of low cost giving fully double the power, with the same consumption of water, that was obtained from most of the older forms of wheels of the same class.
A frequent inconvenience in the use of water power in cold climates is that peculiar form of ice called anchor or ground ice. It adheres to stones, gravel, wood, and other substances forming the beds of streams, the channels of conduits, and orifices through which water is drawn, sometimes raising the level of water courses many feet by its accumulation on the bed, and entirely closing small orifices through which water is drawn for industrial purposes. I have been for many years in a position to observe its effects and the conditions under which it is formed.
The essential conditions are, that the temperature of the water is at its freezing point, and that of the air below that point; the surface of the water must be exposed to the air, and there must be a current in the water.
The ice is formed in small needles on the surface, which would remain there and form a sheet if the surface was not too much agitated, except for a current or movement in the body of water sufficient to maintain it in a constant state of intermixture. Even when flowing in a regular channel there is a continued interchange of position of the different parts of a stream; the retardation of the bed causes variations in the velocity, which produce whirls and eddies and a general instability in the movement of the water in different parts of the section--the result being that the water at the bottom soon finds its way to the surface, and the reverse. I found by experiments on straight canals in earth and masonry that colored water discharged at the bottom reached the surface at distances varying from ten to thirty times the depth.[1] In natural water courses, in which the beds are always more or less irregular, the disturbance would be much greater. The result is that the water at the surface of a running stream does not remain there, and when it leaves the surface it carries with it the needles of ice, the specific gravity of which differs but little from that of the water, which, combined with their small size, allows them to be carried by the currents of water in any direction. The converse effect takes place in muddy streams. The mud is apparently held in suspension, but is only prevented from subsiding by the constant intermixture of the different parts of the stream; when the current ceases the mud sinks to the bottom, the earthy particles composing it, being heavier than water, would sink in still water in times inversely proportional to their size and specific gravity. This, I think, is a satisfactory explanation of the manner in which the ice formed at the surface finds its way to the bottom; its adherence to the bottom, I think, is explained by the phenomenon of regelation, first observed by Faraday; he found that when the wetted surfaces of two pieces of ice were pressed together they froze together, and that this took place under water even when above the freezing point. Professor James D. Forbes found that the same thing occurred by mere contact without pressure, and that ice would become attached to other substances in a similar manner. Regelation was observed by these philosophers in carefully arranged experiments with prepared surfaces fitting together accurately, and kept in contact sufficiently long to allow the freezing together to take place. In nature these favorable conditions would seldom occur in the masses of ice commonly observed, but we must admit, on the evidence of the recorded experiments, that, under particular circumstances, pieces of ice will freeze together or adhere to other substances in situations where there can be no abstraction of heat.
[Footnote 1: Paper clx., in the Transactions of the Society, 1878, vol. vii., pages 109-168.]
When a piece of ice of considerable size comes in contact under water with ice or other substance, it would usually touch in an area very small in proportion to its mass, and other forces acting upon it, and tending to move it, would usually exceed the freezing force, and regelation would not take place. In the minute needles formed at the surface of the water the tendency to adhere would be much the same as in larger masses touching at points only, while the external forces acting upon them would be extremely small in proportion, and regelation would often occur, and of the immense number of the needles of ice formed at the surface enough would adhere to produce the effect which we observe and call anchor ice. The adherence of the ice to the bed of the stream or other objects is always downstream from the place where they are formed; in large streams it is frequently many miles below; a large part of them do not become fixed, but as they come in contact with each other, regelate and form spongy masses, often of considerable size, which drift along with the current, and are often troublesome impediments to the use of water power.
Water powers supplied directly from ponds or rivers, or canals frozen over for along distance immediately above the places from which the water is drawn, are not usually troubled with anchor ice, which, as I have stated, requires open water, upstream, for its formation.
This drawing has been admitted into the Exhibition of the Royal Academy this year. The cottages are of red brick, tiled roof, white woodwork, as usual, rough-cast in the gables; but they are not built yet. Design of Arthur Cawston.--Building News.
SUGGESTIONS IN ARCHITECTURE.--A PAIR OF ENGLISH
COTTAGES.--BY A.
CAWSTON.
Within the past five years, scientific men have surpassed previous efforts in close measurement and refined analysis. By means of instruments of exceeding delicacy, processes in nature hitherto unknown, are made palpable to sense. Heat is found in ice, light in seeming darkness, and sound in apparent silence. It seems that physicists and chemists have almost if not quite reached the ultimate atoms of matter. The mechanism must be sensitive, as such properties of matter as heat, light, electricity, magnetism, and actinism, are to be handled, caused to vanish and reappear, analyzed and measured. With such instruments nature is scrutinized, revealing new properties, strange motions, vibrations, and undulations. Throughout the visible universe, the faintest pulsations of atoms are detected, and countless millions of infinitely small waves, bearing light, heat, and sound, are discovered and their lengths determined. Refined spectroscopic analysis of light is now made so that when any material burns, no matter what its distance, its spectrum tells what substance is burning. When any luminous body appears, it can be told whether it is approaching or receding, or whether it shines by its own or reflected light; whence it is seen that rays falling on earth from a flight of a hundred years, are as sounding lines dropped in the appalling depths of space. We wish to describe a few of these intricate instruments, and mention several far-reaching discoveries made by their use; beginning with mechanism for the manipulation of light. Optics is based on the accidental discovery that a piece of glass of certain shape will draw light to a focus, forming an image of any object at that point. The next step was in learning that this image can be viewed with a microscope, and magnified; thus came the telescope revealing unheard of suns and galaxies. The first telescopes colored everything looked at, but by a hundred years of mathematical research, the proper curvature of objectives formed of two glasses was discovered, so that now we have perfect instruments. Great results followed; one can now peer into the profound solitudes of space, bringing to view millions of stars, requiring light 5,000 years to traverse their awful distance, and behold suns wheeling around suns, and thousands of nebulæ, or agglomerations of stars so distant as to send us confused light, appearing like faint gauze like structures in measureless voids. The modern telescope has astonishing power, thus: When Mr. Clark finished the great twenty-six-inch equatorial, now at Washington, he tested its seeing properties. A photographic calligraph, whose letters were so fine as to require a microscope to see them, was placed at a distance of three hundred feet. Mr. Clark turned the great eye upon the invisible thing and read the writing with ease. But a greater feat than this was accomplished by the same instrument-- the discovery of the two little moons of Mars, by Prof. Asaph Hall, in 1877. They are so small as to be incapable of measurement by ordinary means, but with an ingenious photometer devised by Prof. Pickering of Harvard College, he determined the outer satellite to be six and the inner seven miles in diameter. The discovery of these minute bodies seems past belief, and will appear more so, when it is told that the task is equal to that of viewing a luminous ball two inches in diameter suspended above Boston, by the telescope situated in the city of New York. (Newcomb and Holden's Astronomy, p. 338.)
Phobos, the nearest moon, is only 4,000 miles from the surface of Mars, and is obliged to move with such great velocity to prevent falling, that it actually makes a circuit about its primary in only seven hours and thirty-eight minutes. But Mars turns on its axis in twenty-four hours and thirty-seven minutes, so the moon goes round three times, while Mars does once, hence it rises in the west and sets in the east, making one day of Mars equal three of its months. This moon changes every two hours, passing all phases in a single martial night; is anomalous in the solar system, and tends to subvert that theory of cosmic evolution wherein a rotating gaseous sun cast off concentric rings, afterward becoming planets. Astronomers were not satisfied with the telescope; true, they beheld the phenomena of the solar system; planets rotating on axes, and satellites revolving about them. They saw sunspots, faculæ, and solar upheaval; watched eclipses, transits, and the alternations of summer and winter on Mars, and detected the laws of gravity and motion in the system to which the earth belongs. They then devised the micrometer. This is a complex mechanism placed in the focus of a telescope, and by its use any object, providing it shows a disk, no matter what its distance, can be measured. It consists of spider webs set within a graduated metallic circle, the webs movable by screws, and the whole instrument capable of rotating about the collimation axis of the telescope. The screw head is a circle ruled to degrees and minutes, and turns in front of a fixed vernier in the field of a reading microscope. One turn of the screw moves the web a certain number of seconds; then as there are 360° in a circle, one-three-hundred-and-sixtieth of a turn moves the web one-three-hundred and-sixtieth of the amount, and so on. Thus, when two stars are seen in the field, one web is moved by the screw until the fixed line and the movable one are parallel, each bisecting a star. By reading with the microscope the number of degrees turned, the distance apart of the stars becomes known; the distance being learned, position is then sought; the observance of which led to one of the greatest discoveries ever made by man. The permanent line of the micrometer is placed in the line joining the north and south poles of the heavens, and brought across one of the stars; the movable web is then rotated until it bisects the other, and then the angle between the webs is recorded. Double stars are thus measured, first in distance, and second, their position. After this, if any movement of the stars takes place, the tell tale micrometer at once detects it.
In 1780, Sir Wm. Herschel measured double stars and made catalogues with distances and positions. Within twenty years, he startled intellectual man with the statement that many of the fixed stars actually move--one great sun revolving around another, and both rotating about their common center of gravity. If we look at a double star with a small telescope, it looks just like any other; using a little larger glass, it changes appearance and looks elongated; with a still better telescope, they become distinctly separated and appear as two beautiful stars whose elements are measured and carefully recorded, in order to see if they move. Herschel detected the motion of fifty of these systems, and revolutionized modern astronomy. Astronomers soared away from the little solar system, and began a minute search throughout the whole sidereal heavens. Herschel's catalogue contained four hundred double suns, only fifty of which were known to be in revolution. Since then, enormous advance has been made. The micrometer has been improved into an instrument of great delicacy, and the number of doubles has swelled to ten thousand; six hundred and fifty of them being known to be binary, or revolving on orbits--Prof. S. W. Burnham, the distinguished young astronomer of the Dearborn Observatory, Chicago, having discovered eight hundred within the last eight years. This discovery implies stupendous motion; every fixed star is a sun like our own, and we can imagine these wheeling orbs to be surrounded by cool planets, the abode of life, as well as ours. If the orbit of a binary system lies edgewise toward us, then one star will hide the other each revolution, moving across it and appearing on the other side. Several instances of this motion are known; the distant suns having made more than a complete circuit since discovery, the shortest periodic time known being twenty-five years.
Wonderful as was this achievement of the micrometer, one not less surprising awaited its delicate measurement. If one walks in a long street lighted with gas, the lights ahead will appear to separate, and those in the rear approach. The little spider lines have detected just such a movement in the heavens. The stars in Hercules are all the time growing wider apart, while those in Argus, in exactly the opposite part of the Universe, are steadily drawing nearer together. This demonstrates that our sun with his stately retinue of planets, satellites, comets, and meteorites, all move in grand march toward the constellation Hercules. The entire universe is in motion. But these revelations of the micrometer are tame compared with its final achievement, the discovery of parallax.
This means difference of direction, and the parallax of a star is the difference of its direction when viewed at intervals of six months. Astronomers observe a star to-day with a powerful telescope and micrometer; and in six months again measure the same star. But meanwhile the earth has moved 183,000,000 miles to the east, so that if the star has changed place, this enormous journey caused it, and the change equals a line 91,400,000 miles long as viewed from the star. For years many such observations were made; but behold the star was always in the same place; the whole distance of the sun having dwindled down to the diameter of a pin point in comparison with the awful chasm separating us from the stars. Finally micrometers were made that measured lines requiring 100,000 to make an inch; and a new series of observations begun, crowning the labors of a century with success. Finite man actually told the distance of the starry hosts and gauged the universe.
When the parallax of any object is found, its distance is at once known, for the parallax is an arc of a circle whose radius is the distance. By an important theorem in geometry it is learned, that when anything subtends an angle of one second its distance is 206,265 times its own diameter. The greatest parallax of any star is that of Alpha Centauri--nine-tenths of a second; hence it is more than 206,265 times 91,400,000 miles--the distance of the sun--away, or twenty thousand billions of miles. This is the distance of the nearest fixed star, and is used as a standard of reference in describing greater depths of space. This is not all the micrometer enables man to know, When the distance separating the earth from two celestial bodies that revolve is learned, the distance between the two orbs becomes known. Then the period of revolution is learned from observation, and having the distance and time, then their velocity can be determined. The distance and velocity being given, then the combined weights of both suns can be calculated, since by the laws of gravity and motion it is known how much weight is required to produce so much motion in so much time, at so much distance, and thus man weighs the stars. If the density of these bodies could be ascertained, their diameters and volumes would be known, and the size of the fixed stars would have been measured. Density can never be exactly learned; but strange to say, photometers measure the quantity of light that any bright body emits; hence the stars cannot have specific gravity very far different from that of the sun, since they send similar light, and in quantity obeying the law wherein light varies inversely as the squares of distance. Therefore, knowing the weight and having close approximation to density, the sizes of the stars are nearly calculated. The conclusion is now made that all suns within the visible universe are neither very many times larger nor smaller than our own. (Newcomb and Holden's Astronomy, p. 454.)
Another result followed the use of the micrometer: the detection of the proper motion of the stars. For several thousand years the stars have been called "fixed," but the fine rulings of the filar micrometer tell a different story. There are catalogues of several hundred moving stars, whose motion is from one-half second to eight seconds annually. The binary star, Sixty-one Cygni, the nearest north of the equator, moves eight seconds every year, a displacement equal in three hundred and sixty years to the apparent diameter of the moon. The fixed stars have no general motion toward any point, but move in all directions.
Thus the micrometer revealed to man the magnitude and general structure, together with the motions and revolutions of the sidereal heavens. Above all, it demonstrated that gravity extends throughout the universe. Still the longings of men were not appeased; they brought to view invisible suns sunk in space, and told their weight, yet the thirst for knowledge was not quenched. Men wished to know what all the suns are made of, whether of substances like those composing the earth, or of kinds of matter entirely different. Then was devised the spectroscope, and with it men audaciously questioned nature in her most secluded recesses. The basis of spectroscopy is the prism, which separates sunlight into seven colors and projects a band of light called a spectrum. This was known for three hundred years, and not much thought of it until Fraunhofer viewed it with a telescope, and was surprised to find it filled with hundreds of black lines invisible to the unaided eye. Could it be possible that there are portions of the solar surface that fail to send out light? Such is the fact, and then began a twenty years' search to learn the cause. The lines in the solar spectrum were unexplained until finally metals were vaporized in the intense heat of the electric arc and the light passed through a spectroscope, when behold the spectra of metals were filled with bright lines in the same places as were the dark lines in the spectrum of the sun. Another step: if when metals are volatilized in the arc, rays of light from the sun are passed through the vapor and allowed to enter the spectroscope, a great change is wrought; a reversal takes place, and the original black bands reappear. A new law of nature was discovered, thus: "Vapors of all elements absorb the same rays of light which they emit when incandescent." Every element makes a different spectrum with lines in different places and of different widths. These have been memorized by chemists, so that when an expert having a spectroscope sees anything burn he can tell what it is as well as read a printed page. Men have learned the alphabet of the universe, and can read in all things radiating light, the constituent elements. The black lines in the solar spectrum are there because in the atmosphere of the sun exist vapors of metals, and the light from the liquid metals below is unable to pass through and reach the earth, being absorbed kind for kind. Gaseous iron sifts out all rays emitted from melted iron, and so do the vapors of all other elements in the sun, radiating light in unison with their own. Sodium, iron, calcium, hydrogen, magnesium, and many other substances are now known to be incandescent in the sun and stars; and the results of the developments of the spectroscope may be summed up in the generalization that all bodies in the universe are composed of the same substance the earth is.
The sun is subject to terrific hurricanes and cyclones, as well as explosions, casting up jets to the height of 200,000 miles. In the early days of spectroscopy these protuberances could only be seen at a time of a total solar ellipse, and astronomers made long journeys to distant parts of the earth to be in line of totality. Now all is changed. Images of the sun are thrown into the observatory by an ingenious instrument run by clockwork, and called a heliostat. This is set on the sun at such an angle as to throw the solar image into the objective of the telescope placed horizontally in a darkened observatory, and the pendulum ball set in motion, when it will follow the sun without moving its image, all day if desired. At the eye end of the telescope is attached the spectroscope and the micrometer, and the whole set of instruments so adjusted that just the edge of the sun is seen, making a half spectrum. The other half of the spectroscope projects above the solar limb, and is dark, so if an explosion throws up liquid jets, or flames of hydrogen, the astronomer at once sees them and with the micrometer measures their height before they have time to fall. And the spectrum at once tells what the jets are composed of, whether hydrogen, gaseous iron, calcium, or anything else. Prof. C. A. Young saw a jet of hydrogen ascend a distance of 200,000 miles, measured its height, noted its spectrum and timed its ascent by a chronometer all at once, and was astonished to find the velocity one hundred and sixty miles per second--eight times faster than the earth flies on its orbit. By these improvements solar hurricanes, whirlpools, and explosions can be seen from any physical observatory on clear days.
The slit of the spectroscope can be moved anywhere on the disk of the sun; so that if the observer sees a tornado begin, he moves the slit along with it, measures the length of its tract and velocity. With the telescope, micrometer, heliostat, and spectroscope came desire for more complex instruments, resulting in the invention of the photoheliograph, invoking the aid of photography to make permanent the results of these exciting researches. This mechanism consists of an excessively sensitive plate, adjusted in the solar focus of the telespectroscope. In front of the plate in the camera is a screen attached to a spring, and held closed by a cord. The eye is applied to the spectroscopic end of the complex arrangement to watch the development of solar hurricanes.
Finally an appalling outburst occurs; the flames leap higher and higher, torn into a thousand shreds, presenting a scene that language is powerless to describe. When the display is at the height of its magnificence, the astronomer cuts the cord; the slide makes an exposure of one-three thousandth part of a second, and an accurate photograph is taken. The storm all in rapid motion is petrified on the plate; everything is distinct, all the surging billows of fire, boilings, and turbulence are rendered motionless with the velocity of lightning.
At Meudon, in France, M. Janssen takes these instantaneous photographs of the sun, thirty inches in diameter, and afterward enlarges them to ten feet; showing scenes of fiery desolation that appalls the human imagination. (See address of Vice President Langley, A. A. A. S., Proceedings Saratoga Meeting, p. 56.) This huge photograph can be viewed in detail with a small telescope and micrometer, and the crests of solar waves measured. Many of these billows of fire are in dimensions every way equal in size to the State of Illinois. Binary stars are photographed so that in time to come they can be retaken, when if they have moved, the precise amount can be measured.
Another instrument is the telepolariscope, to be attached to a telescope. It tells whether any luminous body sends us its own, or reflected light. Only one comet bright enough to be examined has appeared since its perfection. This was Coggia's, and was found to reflect solar from the tail, and to radiate its own light from the nucleus.
Still another intricate instrument is in use, the thermograph, that utilizes the heat rays from the sun, instead of the light. It takes pictures by heat; in other words, it sees in the dark; brings invisible things to the eye of man, and is used in astronomical and physical researches wherein undulations and radiations are concerned. And now comes the magnetometer, to measure the amount of magnetism that reaches the earth from the sun. It points to zero when the magnetic forces of the earth are in equilibrium, but let a magnetic storm occur anywhere in the world and the pointer will move by invisible power. It detects a close relation between the magnetism of the earth and sun. The needle is deflected every time a solar disturbance takes place. At Kew, England, an astronomer was viewing the sun with a telescope and observed a tongue of flame dart across a spot whose diameter was thirty-three thousand seven hundred miles. The magnetometer was violently agitated at once, showing that whatever magnetism may be, its influence traversed the distance of the sun with a velocity greater than that of light.
Not less remarkable is the new instrument, the thermal balance, devised by Prof. S. P. Langley, Pittsburgh. It will measure the one-fifty-thousandth part of a degree of heat, and consists of strips of platinum one-thirty-second of an inch wide and one-fourth of an inch long; and so thin that it requires fifty to equal the thickness of tissue paper, placed in the circuit of electricity running to a galvanometer. "When mounted in a reflected telescope it will record the heat from the body of a man or other animal in an adjoining field, and can do so at great distances. It will do this equally well at night, and may be said, in a certain sense, to give the power of seeing in the dark." (Science, issue of Jan. 8,1881, p. 12.) It is expected to reveal great facts concerning the heat of the stars.
Indeed, the thermopile in the hands of Lockyer has already made palpable the heat of the fixed stars. He placed the little detective in the focus of a telescope and turned it on Arcturus. "The result was this, that the heat received from Arcturus, when at an altitude of 55°, was found to be just equal to that received from a cube of boiling water, three inches across each side, at the distance of four hundred yards; and the heat from Vega is equal to that from the same cube at six hundred yards." (Lockyer's Star Gazing, p. 385.) Thus that inscrutable mode of force heat traverses the depths of space, reaches the earth, and turns the delicate balance of the thermopile. Another discovery was made with the spectroscope; thus, if a boat moves up a river, it will meet more waves than will strike it if going down stream. Light is the undulation of waves; hence if the spectroscope is set on a star that is approaching the earth, more waves will enter than if set on a receding star, which fact is known by displacement of lines in the spectroscope from normal positions. It is found that many fixed stars are approaching, while others are moving away from the solar system.
We cannot note the researches of Edison, Lockyer, or Tyndall, nor of Crookes, who has seemingly reached the molecules whence the universe is composed.
The modern observatory is a labyrinth of sensitive instruments; and when any disturbance takes place in nature, in heat, light, magnetism, or like modes of force, the apparatus note and record them.
Men are by no means satisfied. Insatiable thirst to know more is developing into a fever of unrest; they are wandering beyond the limits of the known, every day a little farther. They survey space, and interrogate the infinite; measure the atom of hydrogen and weigh suns. Man takes no rest, and neither will he until he shall have found his own place in the chain of nature.--Kansas Review.
Prof. J. Perry lately delivered a lecture on this subject at the Society of Arts, London, which contains in an epitomized form the salient points of the hopes and fears of the more sanguine spirits of the electrical world. Prof. Perry is one of the two professors who have been dubbed the "Japanese Twins," and whose insatiate love of work induced one of our most celebrated men of science to say that they caused the center of experimental research to tend toward Tokyo instead of London. Professors Ayrton and Perry have for some time been again resident in England, but it is evident that they did not leave any of their energy in Japan, for those who know them intimately, know that they are pursuing numerous original investigations, and that so soon as one is finished, another is commenced. It would have been difficult then to have found an abler exponent of the future of electricity.
Prof. Perry, after referring to what might have been said of the great things physical science has done for humanity, plunged into his subject. The work to be done was vast, and the workers altogether out of proportion to the task.
The methods of measurement of electricity are not generally understood. Perhaps when electricity is supplied to every house in the city at a certain price per horse power, and is used by private individuals for many different purposes, this ignorance will disappear. Electrical energy is obtained in various ways, but the generators get heated; and one great object of inventors is to obtain from machines as much as possible electrical energy of the energy in the first place supplied to such machine. The lecturer called particular attention to the difference between electricity and electrical energy, and attempted to drive home the fundamental conceptions of electrical science by the analogies derivable from hydraulics. A miller speaks not only of quantity of water, but also of head of water. The statement then of quantity of electricity is insufficient, except we know the electrical property analogous to head of water, and which is termed electrical potential. A small quantity of electricity of high potential is similar to a small quantity of water at high level. The analogies between water and electricity were collected in the form of a table shown on a wall sheet as follows:
We Want to Use Water. We Want to Use Electricity.
1. Steam pump burns coal, 1. Generator burns zinc, or and lifts water to a higher uses mechanical power, and level. lifts electricity to a higher level or potential.
2. Energy available is 2. Energy available is amount of water lifted x amount of electricity x difference difference of level. of potential.
3. If we let all the water 3. If we let all the electricity flow away through channel flow through a wire from one to lower level without doing screw of our generator to the work, its energy is all other without doing work, all converted into heat because the electrical energy is of frictional resistance of converted into heat because of pipe or channel. resistance of wire.
4. If we let water work a 4. If we let our electricity hoist as well as flow through work a machine as well as channels, less water flows flow through wires, less flows than before, less power is than before, less power is wasted in friction. wasted through the resistance of the wire.
5. However long and narrow 5. However long and thin may be the channels, the wires may be, electricity water maybe brought from may be brought from any distance distance, however great, however great, to give to give out almost all its out almost all its original original energy to a hoist. energy to a machine. This requires This requires a great head a great difference of and small quantity of water. potentials and a small current.
The difference between potential and electro-motive force was explained thus: "difference of potential" is analogous with "difference of pressure" or "head" of water, howsoever produced; whereas electromotive force is analogous with the difference of pressure before and behind a slowly moving piston of the pump employed by an unfortunate miller to produce his water supply. Electricians have very definite ideas upon the subject they are working at, and especial attention is paid to the measurements on which their work depends. Examples of these measurements were shown by the following tables on wall sheets:
ELECTRICAL MAGNITUDES (SOME RATHER APPROXIMATE).
Resistance of One yard of copper wire, one-eighth of an inch diameter...............................0.002 ohms. One mile ordinary iron telegraph wire, .........10 to 20 " Some of our selenium cells ............. 40 to 1,000,000 " A good telegraph insulator ........... 4,000,000,000,000 "
Electro-motive force of A pair of copper-iron junctions at a difference of temperature of 1 deg. Fah......... =0.0000 volt. Contact of zinc and copper ..................... =0.75 " One Daniell's cell ............................. =1.1 " Mr. Latimer Clark's standard cell .............. =1.45 " One of Dr. De la Hue's batteries ...... =11,000 " Lightning flashes probably many millions of volts.
Current measured by us in some experiments:
Using electrometer....... = almost infinitely small currents. Using delicate galvanometer =0.00,000,000,040 weber. Current received from Atlantic cable, when 25 words per minute are being sent ................ = 0.000,001 weber Current in ordinary land telegraph lines ......................... = 0.003 weber Current from dynamo machine.... = 5 to 100 weber
In any circuit, current in webers = electro-motive force in volts / resistance in ohms.
In the whole of a circuit=current in webers x electro-motive force in volts / 746. In any part of circuit=current in webers x difference of potential at the two ends of the part of the circuit in question / 746. Or, =square of current in webers x resistance of the part in ohms / 746.
If there are a number of generators of electricity in a circuit, whose electromotive forces in volts are E1, E2, etc., and if there are also opposing electro-motive forces. F1, F2, etc., volts, and if C is the current in webers, R the whole resistance of the current in ohms, P the total horse-power taken at the generators, Q the total horse-power converted into some other form of energy, and given out at the places where there are opposing electro-motive forces, H the total horse-power wasted in heat, because of resistance, then:
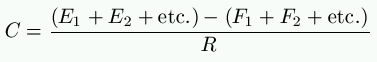
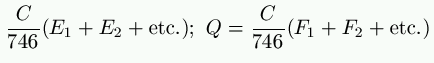
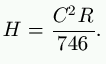
The lifting power of an electro-magnet of given volume is proportional to the heat generated against resistance in the wire of the magnet.
The future of many electrical appliances depends on how general is the public comprehension of the lessons taught by these wall sheets. If a few capitalists in London would only spend a few days in learning thoroughly what these mean, electrical appliances of a very distant future would date from a few months hence.
A number of experiments were shown, in some of which electrical energy was converted into heat, in others into sound, in others into work. At this part of the lecture reference was made to the work of Prof. Ayrton and his pupils at Cowper street (City and Guilds of London Institute Classes). They measure (1) the gas consumed by the engine, (2) the horse-power given to the dynamo machine, (3) the current in the circuit in webers, and (4) the resistance of the circuit. Thus exact calculations can now be made as to the horse power expended in any part of the circuit, and the light given out in any given period by an electric lamp. The dynamometers used in these measurements were described, but at present, in some cases, the description given is for various reasons incomplete, so that we shall take a future opportunity of writing of these instruments. To measure the light a photometer, constructed by Profs. Ayrton and Perry, is used, which obviates the necessity of large rooms, and enables the operator to give the intensity in a very short period of time. A number of measurements of the illuminating power of an electric lamp were rapidly made during the lecture with this photometer. By means of a small dynamo machine, driven by an electric current generated in the Adelphi arches, a ventilator, a sewing machine, a lathe, etc., were driven; in the latter a piece of wood was turned. "What," said the lecturer, "do these examples show you?" "They show that if I have a steam-engine in my back yard I can transmit power to various machines in my house, but if you measured the power given to these machines you would find it to be less than half of what the engine driving the outside electrical machine gives out. Further, when we wanted to think of heating of buildings and the boiling of water, it was all very well to speak of the conversion of electrical energy into heat, but now we find that not only do the two electrical machines get heated and give out heat, but heat is given out by our connecting wires. We have then to consider our most important question. Electrical energy can be transmitted to a distance, and even to many thousands of miles, but can it be transformed at the distant place into mechanical or any other required form of energy, nearly equal in amount to what was supplied? Unfortunately, I must say that hitherto the practical answer made to us by existing machines is, 'No;' there is always a great waste due to the heat spoken of above. But, fortunately, we have faith in the measurements, of which I have already spoken, in the facts given us by Joule's experiments and formulated in ways we can understand. And these facts tell us that in electric machines of the future, and in their connecting wires, there will be little heating, and therefore little loss. We shall, I believe, at no distant date, have great central stations, possibly situated at the bottom of coal-pits where enormous steam engines will drive enormous electric machines. We shall have wires laid along every street, tapped into every house, as gas-pipes are at present; we shall have the quantity of electricity used in each house registered, as gas is at present, and it will be passed through little electric machines to drive machinery, to produce ventilation, to replace stoves and fires, to work apple-parers and mangles and barbers' brushes, among other things, as well as to give everybody an electric light."
It is possible, as Prof. Ayrton first showed in his Sheffield lecture, that electrical energy can be transmitted through long distances by means of small wires, and that the opinion that wires of enormous thickness would be required is erroneous. The desideratum required was good insulation. He also showed that, instead of a limiting efficiency of 50 per cent., the only thing preventing our receiving the whole of our power was the mechanical friction which occurs in the machines. He showed, in fact, how to get rid of electrical friction. A machine at Niagara receives mechanical power, and generates electricity. Call this the generator. Let there be Wires to another electric machine in New York, which will receive electricity, and give out mechanical work. Now this machine, which may be called the motor, produces a back electromotive force, and the mechanical power given out is proportional to the back electromotive force multiplied into the current. The current, which is, of course, the same at Niagara as at New York, is proportional to the difference of the two electromotive forces, and the heat wasted is proportional to the square of the current. You see, from the last table, that we have the simple proportion: power utilized is to power wasted, as the back electromotive force of the motor is to the difference between electromotive forces of generator and motor. This reason is very shortly and yet very exactly given as follows:
Let electromotive force of generator be E; of motor F. Let total resistance of circuit be R. Then if we call P the horse-power received by the generator at Niagara, Q, the horse-power given out by motor at New York, that is, utilized; H, the horse-power wasted as heat in machines and circuit; C, the current flowing through the circuit:
C=(E-F) / R
P=E(E-F) / (746 R)
Q=F(E-F) / (746 R)
H=(E-F)_2 / (746 R)
Q:H::F:E-F
The water analogy was again called into play in the shape of a model for the better demonstration of the problem. The defects in existing electric machines and the means of increasing the E.M.F. were discussed, the conclusions pointing to the future use of very large machines and very high velocities. The future of telephonic communication received a passing remark, and attention called to the future of electric railways. The small experiments of Siemens have determined the ultimate success of this kind of railway. Their introduction is merely a question of time and capital. The first cost of electric railways would be smaller than that of steam railways; the working expenses would also be reduced. The rails would be lighter, the rolling stock lighter, the bridges and viaducts less costly, and in the underground railways the atmosphere would not be vitiated.
"About two years ago, it struck Professor Ayrton and myself, when thinking how very faint musical sounds are heard distinctly from the telephone, in spite of loud noises in the neighborhood, that there was an application of this principle of recurrent effects of far more practical importance than any other, namely, in the use of musical notes for coast warnings in thick weather. You will say that fog bells and horns are an old story, and that they have not been particularly successful, since in some states of the weather they are audible, in others not.
"Now, it seems to be forgotten by everybody that there is a medium of communicating with a distant ship, namely, the water, which is not at all influenced by changes in the weather. At some twenty or thirty feet below the surface there is exceedingly little disturbance of the water, although there may be large waves at the surface. Suppose a large water-siren like this--experiment shown--is working at as great a depth as is available, off a dangerous coast, the sound it gives out is transmitted so as to be heard at exceedingly great distances by an ear pressed against a strip of wood or metal dipping into the water. If the strip is connected with a much larger wooden or metallic surface in the water the sound is heard much more distinctly. Now, the sides of a ship form a very large collecting surface, and at the distance of several miles from such a water siren as might be constructed, we feel quite sure that, above the noise of engines and flapping sails, above the far more troublesome noise of waves striking the ship's side, the musical note of the distant siren would be heard, giving warning of a dangerous neighborhood. In considering this problem, you must remember that Messrs. Colladon and Sturn heard distinctly the sound of a bell struck underwater at the distance of nearly nine miles, the sound being communicated by the water of Lake Geneva."
The next portion of the lecture discussed the great value of a rapid recurrence of effects, the obtaining of sound by means of a rapid intermission of light rays on selenium joined up in an electric circuit being instanced as an example. Then recent experiments on the refractive power of ebonite were detailed--the rough results tending to give greater weight to Clerk-Maxwell's electro-magnetic theory of light. The index of refraction of ebonite was found by Profs. Ayrton and Perry to be roughly 1.7. Clerk-Maxwell's theory requires that the square of this number should be equal to the electric specific inductive capacity of the substance. For ebonite this electric constant varies from 2.2 to 3.5 for different specimens, the mean of which is almost exactly equal to the square of 1.7.
The author discusses the question whether, according to the experiments of Crookes, the assumption of an especial fourth state of aggregation is necessary, or whether the facts may be satisfactorily explained without such hypothesis? He shows that the latter alternative is possible with the aid of a mechanical theory of electricity. If the radiant matter produced in the vacuum is a phenomenon sui generis, produced by the action of electricity and heat upon the molecules of gas remaining in the receiver, it is, in the first place, doubtful to apply to it the conception of an aggregate condition. The author considers it impossible to form a clear understanding of the phenomena in accordance with the theory of Crookes, or to find in the facts any evidence of the existence of radiant matter. An explanation of the latter phenomenon is thus given: Particles become separated from the surface of the substance of the negative pole, they are repelled, and they move away from the pole with a speed resulting from the antagonistic forces in a parallel and rectilinear direction, preserving their speed and their initial path so long as they do not meet with obstacles which influence their movement. At a certain density of the gases present in the exhausted space, these particles, in consequence of the impact of gaseous molecules more or less opposed to their direction of movement, lose their velocity after traveling a short distance and soon come to rest. The more dilute the gas the smaller is the number of the impacts of the gaseous molecules encountering the molecules of the poles, and at a certain degree of dilution the repelled polar particles will be able to traverse the space open to them without any essential alteration in their speed, the small number of the existing gaseous molecules being no longer able to retard the molecules of the polar no their journey through the apparatus. The luminous phenomena of the Geissler tubes the author supposes to be produced by the intense blows which the gaseous molecules receive from the polar molecules flying rapidly through the apparatus. The intensity of the luminous phenomena will naturally decrease with the number of the photophorous particles occupying the space. Accordingly in the experiments of Crookes, on continued rarefaction of the gas, a condition was reached where a display of light is no longer perceptible, or can be made visible merely by the aid of fluorescent bodies. A condition may also appear, as is shown by Crookes' experiment, with the metallic plate intercalated as negative pole in the middle of. a Geissler tube, with the positive poles at the ends. In this case the gaseous molecules are, so to speak, driven away by the polar particles endowed with an equal initial velocity, till at a certain distance from the pole the mass of the gaseous molecules and their speed become so great that a luminous display begins. In an analogous manner the author explains the phenomena of phosphorescence which Crookes' elicits by the action of his radiant matter. In like manner the thermic and the mechanical effects are most simply explained, according to the expression selected by Crookes himself, as the results of a "continued molecular bombardment." The attraction of the so called radiant matter, regarded as a stream of metallic particles by the magnet, will not appear surprising.
Mr. W. H. Preece writes to the Journal of Arts as follows:
At the South Kensington Museum, very careful observations have been made on the relative cost of the two systems, i. e., gas and electricity. The court lighted is that known as the "Lord President's" (or the Loan) Court. It is 138 feet long by 114 feet wide, and has an average height of about 42 feet. It is divided down the middle lengthwise by a central gallery. There are cloisters all around it on the ground floor, and the walls above are decorated in such a way that they do not assist in the reflection or diffusion of the light. The absence of a ceiling--the court being sky-lighted--is to some extent compensated for by drawing the blinds under the sky-lights.
The experiments commenced about twelve months ago, with eight lamps only on one side of the court. The system was that of Brush. The dynamo machine was driven by an eight horse-power Otto gas engine, supplied by Messrs. Crossley. The comparison with the gas was so much in favor of electricity, and the success of the experiment so encouraging, that it was determined to light up the whole court.
The gas engine, which was not powerful enough, was replaced by a 14-horse power "semi-portable" steam engine, by Ransomes & Co., of Ipswich--an engine of sufficient power to drive double the required number of lights. The dynamo machine is a No. 7 Brush. There are sixteen lamps in all--eight on each side of the court. The machine has given no trouble whatever, and it has, as yet, shown no signs of wear. The lamps were not all good, and it was found that they required careful adjustment, but when once they were got to go right they continued to do so, and have, up to the present, shown no signs of deterioration, although the time during which they have been in operation is nine months.
The first outlay has been as follows:
Engine and fixing, including shafting and
belting................................ £420
Dynamo machine......................... 400
Lamps, apparatus, and conducting wire . 384
------
£1,204
The cost of working has been, from June 22, to December 31, during which period the lights were going on 87 nights for a total time of 359 hours:
£ s. d.
Carbons............................... 18 9 0
Oil, etc.............................. 4 11 6
Coal.................................. 11 14 0
Wages................................. 34 7 6
----------
£69 2 0
being at the rate of 3s. 10d. per hour of light.
Now, the consumption of gas in the court would have been 4,800 cubic feet per hour, which, at 3s. 4d. per 1,000 cubic feet, would amount to 16s. per hour, thus showing a saving of working expenses of 12s. 2d. per hour, or, since the museum is lit up for 700 hours every year, a total saving at the rate of £426 per annum.
In estimating the cost as applied to this court, only half the cost of the engine should be taken, for a second dynamo machine has lately been added to light up some of the picture galleries, and the "Life" room of the Art School. The capital outlay should, therefore, be £994. In making a fair estimate of the annual cost, we should also allow something for percentage on capital, and something for wear and tear. Take--
£ s.
5 per cent, on the capital............................. 49 10
5 per cent, for wear and tear of electrical apparatus.. 39 0
5 per cent, for depreciation of engines, etc........... 21 0
-------
Total.......... £109 10
leaving a handsome balance to the good of £316 10s. as against gas. The results of the working, both practically and financially, have proved to be, at South Kensington, a decided success.
I am indebted to Colonel Festing, R.E., who has charge of the lighting, for these details.
The same comparison cannot be made at the British Museum, for no gas was used in the reading-room before the introduction of the electric light, but the cost of lighting has proved to be 5s. 6d. per hour--at least one-third of that which would be required for gas. The system in use at the Museum is Siemens', the engine being by Wallis and Steevens, of Basingstoke.
"An excellent example of economic electric lighting, is that of Messrs. Henry Tate & Sons, sugar refinery, Silvertown. A small Tangye engine, placed under the supervision of the driver of a large engine of the works, drives an 'A' size 'Gramme' machine, which feeds a 'Crompton' 'E' lamp. This is hung at a height of about 12 feet from the ground in a single story shed, about 80 feet long, and 50 feet wide, and having an open trussed roof. The light, placed about midway, lengthways, has a flat canvas frame, forming a sort of ceiling directly over it, to help to diffuse the illumination. The whole of the shed is well lit; and a large quantity of light also penetrates into an adjoining one of similar dimensions, and separated by a row of columns. The light is used regularly all through the night, and has been so all through the winter. Messrs. Tate speak highly of its efficiency. To ascertain the exact cost of the light, as well as of the gas illumination which it replaced, a gas-meter was placed to measure the consumption of the gas through the jets affected; and also the carbons consumed by the electric illumination were noted. A series of careful experiments showed that during a winter's night of 14 hours' duration the illumination by electricity cost 1s. 9d., while that by gas was 3s. 6d., or 1½d. per hour against 3d. per hour. To this must be added the greatly increased illumination, four to five times, given by the electric light, to the benefit of the work; while this last illuminant also allowed, during the process of manufacture of the sugar, the delicate gradations of tint to be detected; and so to avoid those mistakes, sometimes costly ones, liable to arise through the yellow tinge of gas illumination. This alone would add much to the above-named economy, arising from the use of electric illumination in sugar works."
I am indebted for these facts to Mr. J. N. Shoolbred, under whose supervision the arrangements were made.
Some excellent experience has been gained at the shipbuilding docks in Barrow-in-Furness, where the Brush system has been applied to illuminate several large sheds covering the punching and shearing machinery, bending blocks, furnaces, and other branches of this gigantic business. In one shed, which was formerly lighted by large blast-lamps, in which torch oil was burnt, costing about 5d. per gallon, and involving an expenditure of £8 9s. per week, the electric light has been adopted at an expenditure of £4 14s. per week.
The erecting shop, 450 feet by 150 feet, formerly dimly lit by gas at a cost of £22 per week, is now efficiently lit by electricity at half the cost.
I am indebted for these facts to Mr. Humphreys, the manager of the works.
The Post office authorities have contracted with Mr. M. E. Crompton, to light up the Post-office at Glasgow for the same price as they have hitherto paid for gas, and there is no doubt that in many instances this arrangement will leave a handsome profit to the Electric Light Company. They are about to try the Brockie system in the telegraph galleries, and the Brush system in the newspaper sorting rooms of the General Post-office in St. Martin's-le-Grand.
[Footnote: From the Philosophical Magazine for December, 1880.]
Any portion of non-conducting space disturbed by electricity is called an electric field. At every point of this field, if a small electrified body were placed there, there would be a certain resultant force experienced by it dependent upon the distribution of electricity producing the field. When we know the strength and direction of this resultant force, we know all the properties of the field, and we can express them numerically or delineate them graphically, Faraday (Exp. Res., § 3122 et seq.) showed how the distribution of the forces in any electric field can be graphically depicted by drawing lines (which he called lines of force) whose direction at every point coincides with the direction of the resultant force at that point; and Clerk-Maxwell (Camb. Phil. Trans., 1857) showed how the magnitude of the forces can be indicated by the way in which the lines of force are drawn. The magnitude of the resultant force at any point of the field is a function of the potential at that point; and this potential is measured by the work done in producing the field. The potential at any point is, in fact, measured by the work done in moving a unit of electricity from the point to an infinite distance. Indeed the resultant force at any point is directly proportional to the rate of fall of potential per unit length along the line of force passing through that point. If there be no fall of potential there can be no resultant force; hence if we take any surface in the field such that the potential is the same at every point of the surface, we have what is called an equipotential surface. The difference of potential between any two points is called an electromotive force. The lines of force are necessarily perpendicular to the surface. When the lines of force and the equipotential surfaces are straight, parallel, and equidistant, we have a uniform field. The intensity of the field is shown by the number of lines passing through unit area, and the rate of variation of potential by the number of equipotential surfaces cutting unit length of each line of force. Hence the distances separating the equipotential surfaces are a measure of the electromotive force present. Thus an electric field can be mapped or plotted out so that its properties can be indicated graphically.
Fig. 1
The air in an electric field is in a state of tension or strain; and this strain increases along the lines of force with the electromotive force producing it until a limit is reached, when a rent or split occurs in the air along the line of least resistance--which is disruptive discharge, or lightning.
Fig. 2
Since the resistance which the air or any other dielectric opposes to this breaking strain is thus limited, there must be a certain rate of fall of potential per unit length which corresponds to this resistance. It follows, therefore, that the number of equipotential surfaces per unit length can represent this limit, or rather the stress which leads to disruptive discharge. Hence we can represent this limit by a length. We can produce disruptive discharge either by approaching the electrified surfaces producing the electric field near to each other, or by increasing the quantity of electricity present upon them; for in each case we should increase the electromotive force and close up, as it were, the equipotential surfaces beyond the limit of resistance. Of course this limit of resistance varies with every dielectric; but we are now dealing only with air at ordinary pressures. It appears from the experiments of Drs. Warren De La Rue and Hugo Muller that the electromotive force determining disruptive discharge in air is about 40,000 volts per centimeter, except for very thin layers of air.
Fig. 3
If we take into consideration a flat portion of the earth's surface, A B (fig. 1), and assume a highly charged thunder-cloud, C D, floating at some finite distance above it, they would, together with the air, form an electrified system. There would be an electric field; and if we take a small portion of this system, it would be uniform. The lines, a b, a' b'...would be lines of force; and cd, c' d', c" d' ...would be equipotential planes. If the cloud gradually approached the earth's surface (Fig. 2), the field would become more intense, the equipotential surfaces would gradually close up, the tension of the air would increase until at last the limit of resistance of the air, e f, would be reached; disruptive discharge would take place, with its attendant thunder and lightning. We can let the line, e f, represent the limit of resistance of the air if the field be drawn to scale; and we can thus trace the conditions that determine disruptive discharge.
Fig. 4
If the earth-surface be not flat, but have a hill or a building, as H or L, upon it, then the lines of force and the equipotential planes will be distorted, as shown in Fig. 3. If the hill or building be so high as to make the distance H h or L l equal to e f (Fig. 2), then we shall again have disruptive discharge.
If instead of a hill or building we erect a solid rod of metal, G H, then the field will be distorted as shown in Fig. 4. Now, it is quite evident that whatever be the relative distance of the cloud and earth, or whatever be the motion of the cloud, there must be a space, g g', along which the lines of force must be longer than a' a or H H'; and hence there must be a circle described around G as a center which is less subject to disruptive discharge than the space outside the circle; and hence this area may be said to be protected by the rod, G H. The same reasoning applies to each equipotential plane; and as each circle diminishes in radius as we ascend, it follows that the rod virtually protects a cone of space whose height is the rod, and whose base is the circle described by the radius, G a. It is important to find out what this radius is.
Fig. 5
Let us assume that a thunder-cloud is approaching the rod, A B (Fig. 5), from above, and that it has reached a point, D', where the distance. D' B, is equal to the perpendicular height, D' C'. It is evident that, if the potential at D be increased until the striking-distance be attained, the line of discharge will be along D' C or D' B, and that the length, A C', is under protection. Now the nearer the point D' is to D the shorter will be the length A C' under protection; but the minimum length will be A C, since the cloud would never descend lower than the perpendicular distance D C.
Supposing, however, that the cloud had actually descended to D when the discharge took place. Then the latter would strike to the nearest point; and any point within the circumference of the portion of the circle, B C (whose radius is D B), would be at a less distance from D than either the point B or the point C.
Hence a lightning-rod protects a conic space whose height is the length of the rod, whose base is a circle having its radius equal to the height of the rod, and whose side is the quadrant of a circle whose radius is equal to the height of the rod.
I have carefully examined every record of accident that was available, and I have not yet found one case where damage was inflicted inside this cone when the building was properly protected. There are many cases where the pinnacles of the same turret of a church have been struck where one has had a rod attached to it; but it is clear that the other pinnacles were outside the cone; and therefore, for protection, each pinnacle should have had its own rod. It is evident also that every prominent point of a building should have its rod, and that the higher the rod the greater is the space protected.
Hantzel has communicated to the Saxon Royal Society of Science some interesting observations on the production of electricity by light in colored fluor-spar. The centers of the fluor-spar cubes become negatively electric by the action of light. The electric tension diminishes toward the edges and angles, and frequently positive polarity is produced there. With very sensitive crystals a short exposure to daylight is sufficient; by a long exposure to light the electric current increases. The direct rays of the sun act much more powerfully than diffused daylight, and the electric carbon light is more powerful even than sunlight. The photo-electric action of light belongs principally to the "chemically active" rays; this is shown by the fact that the production of electricity is extremely small behind a glass colored with cuprous oxide, and behind a film of a solution of quinine sulphate; while it is not appreciably diminished by a film of a solution of alum. The photo-electric excitability of fluor-spar crystals is increased by a moderate heat (80° to 100° C.).
The January and February numbers of the Elektrotechnische Zeitschrift contain a number of articles on this interesting subject by several eminent electricians. Professor Foerster, director of the observatory in Berlin, points out the great importance of the careful study of earth currents, first observed at Greenwich, and now being investigated by a committee appointed by the German Government. He further points out, according to Professor Wykander, of Lund, in Sweden, that a close connection exists between earth currents, the protuberances of the sun, and the aurora borealis, and that the nearly regular periodical reappearance of protuberances in intervals of eleven years coincides with similar periods of excessive magnetic earth currents and the appearance of the aurora borealis. The remarkable disturbing influences on telegraph wires and cables of the aurora borealis observed from the 11th to 14th of August, 1880, have been carefully recorded by Herr Geh. Postnath Ludwig in Berlin, and a map of Europe compiled, showing the places affected, with the extent to which telegraph wires and cables were influenced and disturbed. Although the aurora was but faintly visible in England and Germany, and in Russia only as far as 35° north, disturbing influences were reported from all parts of Europe, the Mediterranean, and Africa, and even Japan and the east coast of Asia. As far south as Zanzibar, Mozambique, and Natal disturbances were also noticed. They were in Europe most intense on the morning of August 12, when they lasted the whole day, and increased again in intensity toward eight o'clock in the evening, while they suddenly ceased everywhere almost simultaneously. Scientific and careful observations were only taken at a few places, but the existence of earth currents in frequently changing direction and varying intensity, was noticed everywhere. Long lines of wires were more affected than short ones, and although some lines--for instance the Berlin-Hamburg in an east-west direction--were not at all influenced, no general law was noticed according to which certain directions were freed from the disturbing influence. While, for instance, the Red Sea cable was not noticeably affected, the land line to Bombay, forming a continuation of this cable, was materially disturbed. The Marseilles-Algiers cable, so seriously influenced in 1871, showed no signs at all, but as may be expected, the north of Europe suffered more than the south, and in Nystad, Finland, the galvanometer indicated an intensity of current equal to that of 200 Leclanché cells.
Since thunderstorms are generally local, it is only natural that their effect upon telegraph cables should also be confined to one locality. Numerous careful observations, carried out over considerable periods of time, show that the disturbing influences of thunderstorms on telegraph lines are of less duration and more varying in direction and intensity than those of the aurora borealis. Long lines suffer less than short lines; telegraph wires above ground are more easily and more intensely affected than underground cables. It is, however, possible, that this is mainly due to the fact that in the districts where strict records were kept, in the German Empire, most of the long lines are underground cables, while most of the short local lines are overground wires. The results of the disturbances varied; in Hughes's apparatus the armatures were thrown off, lines in operation indicated wrong signs, dots became dashes, and the spaces were either multiplied in size or number, according to the direction of the earth currents induced by the thunderstorms. Since these observations extended over nearly 2,000 cases, some conclusions might fairly be drawn from them. For the purpose of a more complete knowledge on this subject, Dr. Wykander recommends a series of regular observations on earth currents to be carried out at different stations, well distributed over the whole surface of the globe, these observations to be made between six and eight A.M., and at the same time in the evening. Special arrangements to be made at various stations to record exceptionally intense disturbances during the phenomena of the aurora borealis, notice to be taken of time, direction, intensity, and all further particulars. Since this question appears to bear a considerable amount of influence on underground cables, it is one that deserves serious attention before earth cables are more generally introduced; there can, however, be little doubt that they are not nearly so much exposed as overhead wires to disturbing influences of other kinds, such as snow, rain, wind, etc., while they certainly do suffer, though perhaps in a less degree, by electrical disturbances.--Engineering.
[Footnote: A communication to the Sheffield Photographic Society in the British Journal of Photography.]
It is quite possible that in the remarks I propose making this evening in connection with the photographic art I may mention topics and some details which are familiar to many present; but as chemistry and optical and physical phenomena enter largely into the theory and practice of photography, the field is so extensive there is always something interesting and suggestive even in the rudiments, especially to those who are commencing their studies. Although this paper may be considered an introductory one, I do not wish to load it with any historical account, or describe the early methods of producing a light picture, but shall at once take for my subject, "The Photographic Image: What It Is," and under this heading I must restrict myself to the collodion and silver or wet process, leaving gelatine dry plates, collodio-chloride, platinum, carbontype, and the numerous other types which are springing up in all directions for future consideration.
Now, in an ordinary pencil, pen and ink, or sepia sketch we have a deposit of a dark, non-reflecting substance, which gives the outline of a figure on a lighter background. The different gradations of shade are acquired by a more or less deposit of lead, ink, or sepia. In photography--at least in the ordinary silver process--the image is formed by a deposition of metallic silver or organic oxide in a minute state of division, either on glass, paper, or other suitable material. This is brought about by the action of light and certain reagents. Light has long been recognized as a motive power comparable with heat or electricity. Its action upon the skin, fading of colors, and effect on the growth of vegetable and animal organisms are well known; and, although the exact molecular change in many instances is not clearly understood, yet certain salts of silver, iron, the alkaline bichromates, and some organic materials--as bitumen and gelatine--have been pretty well worked out.
It is a remarkable and well-known fact that the chloride, iodide, and bromide of silver--called "sensitive salts" in photography--are not susceptible (at least only slowly) to change when exposed to the yellow, orange, and red rays. The longer wave lengths of the spectrum, as you know, form, with violet, indigo, blue, and green, white light. The diagram on the wall shows this dispersion and separation of the primitive colors. These--the yellow, orange, and red-- are called technically "non actinic" rays, and the others in their order become more actinic until the ultra violet is reached. The action of white light, or rays, excluding yellow, orange, and red, has the effect of converting silver chloride into a sub-chloride; it drives off one equivalent of chlorine. Thus, silver chloride, Ag2Cl2=Ag2Cl+Cl. When water is present the water is decomposed. Hydrochloric acid, HCl, hypochlorous acid, HClO is formed.
The iodide of silver in like manner is changed into a sub-iodide; but with water hydriodic acid is formed unless an iodine absorbent be present--then into hypoiodic acid. The silver bromide undergoes a similar change. When with light alone, a sub-bromide, Ag2Br2=Ag2Br+Br, and with water hypobromous acid. It is important to bear this in mind, as one or other, and frequently both iodide and bromide of silver, is the sensitive salt requisite or used in producing the invisible image.
The theory regarding these sensitive salts of silver is that, being very unstable, i. e., ready to undergo a molecular change, the undulations produced in the ether, which pervades all space, and the potential action or moving power of light is sufficient to disturb their normal chemical composition; it liberates some of the chlorine, iodine, or bromine, as the case may be. This action, of course, applies to light from any source--the sun, electricity, or the brighter hydrocarbons, also flame from gas or candle, whether it comes direct as rays of white light or is reflected from an object and conducted through a lens as a distinct image upon the screen of a camera.
I have no time to speak on the subject of lenses, only just to mention that they are, or ought to be, achromatic, so as to transmit white light and of perfect definition, and the amount of light passed through should be as much as possible consistent with a sharp image--at least when rapid exposure is attempted.
I shall touch very lightly on the manipulative part of photography, as that would be unnecessary; but a brief account of the chemicals in use is essential to a right appreciation of the theory of developing the image. In the first place, our object is to get a film of some suitable material coated with a thin layer of a sensitive salt of silver--say a bromo-iodide. By mixing certain proportions of ammonium iodide and cadmium bromide, or an iodide and bromide of cadmium with collodion--which is pyroxyline, a kind of gun-cotton dissolved in ether and alcohol--a plate of glass is coated, and before being perfectly dry is immersed in the nitrate of silver bath. The silver nitrate solution, adhering and entering to a slight extent the surface of the collodion, becomes converted by an ordinary chemical action of affinity into silver iodide and bromide.
The ammonium and cadmium play a secondary part in the process, and are not absolutely necessary in forming the image. The plate is now extremely sensitive to light. When we have entered it into the dark slide and camera, and then exposed to light, the change I mentioned has taken place. The film is transformed into different quantities of sub-iodide and sub-bromide of silver, according to brilliancy of light. In addition, there is on the plate an amount of unchanged silver nitrate which becomes useful in the second stage, or development. The image is not seen as yet, being latent, and requiring the well-known developing solution of sulphate of iron, acetic acid, alcohol, and water. Practically we all recognize the effect of a nicely-balanced wave of developer worked round a plate. The high lights are first to appear as a darker color, till the details of shadow come out; when this is reached the developer is washed off. The chemical action is briefly thus, and it can be shown by solutions without a photographic plate, as in a test tube: Pour into this glass a solution of silver nitrate, AgNO, and add a solution of ferrous sulphate, FeSO4. The ferrous sulphate combines with the nitric acid, forming two new salts--ferric nitrate and ferric sulphate. The silver is deposited. Any other substance which will remove oxygen from silver nitrate without combining with the silver would do the same, and metallic silver would be thrown down. The formula, as shown on the diagram, explains the interchange.
When the developer is poured over the plate it attacks first the free silver nitrate, and causes it to deposit extremely fine particles of metallic silver. The question arises: How is it these particles arrange themselves to form an image? This is explained by the physical movement known as molecular attraction or affinity. These particles are attracted first to the portions of the plate where there is most sub-iodide and sub-bromide. In the shady parts less silver is deposited. When the image is once started it follows that particles of silver produced by the iron developer will cause more to fall down on the face of those already present, and the image is, of course, built up if the silver nitrate be all consumed on the plate. The developer then becomes useless or injurious. The presence of acetic acid checks the reduction of the silver, and the alcohol facilitates the flow when the bath becomes charged with ether and spirit.
The molecular attraction just mentioned is made plainer by reference to the simple lead tree experiment. We have here in this bottle a piece of zinc rod introduced into a solution of acetate of lead. A chemical change has taken place. The zinc has abstracted the acetic acid and the lead is deposited on the zinc, and will continue to be so until the solution is exhausted. The irregularities of surface and arborescent appearance are well shown. If the change were rapidly conducted the lead particles would from their weight sink directly to the bottom instead of aggregating together like ordinary crystals. I have constructed a diagram of colored card, which will perhaps more clearly demonstrate the relation of the different constituents. The lower portion (Fig. a) represents a section of the glass plate or support, the collodion film (Fig. b) having upon its surface a thin layer of bromo-iodine silver (Fig. c), which, when exposed to a well-lighted image, as in a camera, changes into different gradations of sub-bromide and sub-iodide, as indicated by irregular, dark masses in the film. The dotted marks immediately above these are intended for the silver deposit (Fig. d)--clusters of granules, more abundant in the well lighted and less in the shaded parts of the picture, corresponding to the amount of sub-bromide and iodide beneath.

SECTION OF SENSITIVE PLATE AFTER EXPOSURE AND DURING
DEVELOPMENT.
d Silver deposit--Image, c Sub-bromide and sub-chloride
(gradations of), b Collodion film--Substratum, a Section
of glass plate--Support.
The next point to consider is that of intensification--a process seldom required in positive pictures, and would not be needed so often in negatives if there was enough free silver nitrate on the plate during development. The object, as we all know, in a wet-plate negative is to get good printing density without destruction of half-tone. It is a rule, I believe, in an over-exposed picture to intensify after fixing the image, and in an under-exposed picture to intensify before fixing. Whichever is done the intention is similar, namely, to intercept in a greater degree the light passing through a negative, so as to make a whiter and cleaner print. The usual intensifier--and, I suppose, there is no better--is pyrogallic acid, citric acid, water, and a few drops of silver nitrate solution. Pyrogallic is the most active agent, and might be used alone with water; but for special reasons it is not desirable. As a chemical it has a great affinity for oxygen, and will precipitate silver from a solution containing, for instance, nitrate of silver. It also combines with the metal, forming a pyrogallate--a dark brown, very non-actinic material. The use of a few drops of AgNO3 solution is very evident. A deposit is added to the image already formed. Citric acid is the retarder in this case. Alcohol is unnecessary, as the film is well washed with water before the intensifier is used, consequently it flows readily over the plate.
As regards fixing, or, more properly, clearing the image: it is the simple act of dissolving out or from the film all free nitrate, chloride, iodide, or bromide. Cyanide of potassium does not attack the metallic deposit unless very strong. It has then a tendency to reduce the detail in the shadows.
THOMAS H. MORTON, M.D.
[Footnote: A communication to the Photographic Society of Ireland.]
Few of those who work with gelatine dry plates seem to be aware of the great beauty of the transparencies for lantern or other uses which can be made from them by ferrous oxalate development with the greatest ease and certainty.
I think this a very great pity, for I hold the opinion that the lantern furnishes the most enjoyable and, in some cases, the most perfect of all means of showing good photographic pictures. Many prints from excellent negatives which may be passed over in an album without provoking a remark will, if printed as transparencies and thrown on the screen, call forth expressions of the warmest admiration; and justly so, for no paper print can do that full justice to a really good negative which a transparency does. This difference is more conspicuous in these days of dry gelatine plates and handy photographic apparatus, when many of our most interesting negatives are taken on quarter or 5 x 4 plates the small size of which frequently involves a crowding of detail, much of which will be invisible in a paper print, but which, when unraveled or opened out, as it were, by means of the lantern, enhances the beauty of the pictures immensely.
When I last had the pleasure of bringing this subject before the members of our society, it may be remembered that I demonstrated the ease and simplicity with which those beautiful results maybe obtained, by printing in an ordinary printing frame by the light of my petroleum developing lamp, raising one of its panes of ruby glass for the purpose for five seconds, and then developing by ferrous oxalate until I got the amount of intensity requisite. On that evening, in the course of a very just criticism by one of our members, Mr. J. V. Robinson, he pointed out what was undoubtedly a defect, viz., a slightly opalescent veiling of the high lights, which should range from absolutely bare glass in the highest points. He showed that, in consequence of this veiling, the light was sensibly diminished all over the picture. This veiling of the high lights was a serious disadvantage in another important particular, inasmuch as it lessened the contrast between the lights and shadows of the picture, thereby robbing it of some of its charm and deteriorating its quality.
Since that evening I have endeavored, by a series of experiments, to find out some means by which this opalescence might be got rid of in the most convenient manner. Cementing the transparency to a piece of plain, clear glass with Canada balsam, as suggested by Mr. Woodworth, I found in practice to be open to two formidable objections. One of these was that Canada balsam used in this manner is a sticky, unpleasant substance to meddle with, and takes a long time--nearly a month--to harden when confined between plates in this manner. The other objection was of extreme importance, namely, that, in consequence of commercial gelatine plates not being prepared on perfectly flat glasses in all cases, I found that, after squeezing out the superfluous balsam and the air bubbles that might have formed from between the two plates, they are liable to separate at the places where the transparency is not flat, causing air bubbles to creep in from the edges, as you may see from these examples. I, therefore, have discarded this method, although it had the effect desired when successfully done.
I have hit, however, upon another way of utilizing Canada balsam, which, while retaining all the good qualities of the former method, is not subject to any of its disadvantages. This consists in diluting the balsam with an equal bulk of turpentine, and using it as a varnish, pouring it on like collodion, flowing it toward each corner, and pouring it off into the bottle from the last corner, avoiding crapy lines by slowly tilting the plate, as in varnishing. If the plate be warmed previously, the varnish flows more freely and leaves a thinner coating of balsam behind on the transparency. When the plate has ceased to drip, place it in a plate drainer, with the corner you poured from lowest, and leave it where dust cannot get at it for four or five days, when it will be found sufficiently hard to be put into a plate box. The transparency may be finished at any time afterward by putting a clean glass of the same size along with it, placing one of the blank paper masks sold for the purpose--either circular or cushion-shaped to suit the subject--between the plates, and pasting narrow strips of thin black paper over the edges to bind them together. This method is very successful, as you may see from the examples. It renders the high lights perfectly clear, and leaves a film like glass over all the parts of the transparency where the varnish has flowed.
In order to avoid the risk of dust involved in this process, I tried other means of arriving at similar results and with success, for the plates I now submit to you have been simply rubbed or polished, as I may say, with a mixture of one part of Canada balsam to three parts of turpentine, using either a small tuft of French wadding or a small piece of soft rag for the purpose, continuing the rubbing until the plate is polished nearly dry. This method is particularly successful, rendering the clear parts of the sky like bare glass. I have here a plate which is heavily veiled--almost fogged, in fact--one half of which I have treated in this way, showing that the half so treated is beautifully clear, while the other half is so veiled as to be apparently useless.
I have tried to still further simplify this necessary clearing of those plates, and find that soaking tor twelve hours in a saturated solution of alum, after washing the hypo out of the plate, is successful in a large number of cases; and where it is successful there is no further trouble with the transparency, except to mount it after it becomes dry. Where it is not entirely successful I put the plate into a solution of citric acid, four ounces to a pint of water, for about one minute, and have in nearly all cases succeeded in getting a beautifully-clear plate. The picture must not be left long in the citric acid solution, or it will float off; neither do I like using citric acid until after trying the alum, for a similar reason.
I may mention that I recommend a short exposure in the printing-frame and slow development, in order to get sufficient intensity. Of course the exposure is always made to a gas or petroleum light. I also still prefer the old method of making the ferrous oxalate solution, pouring it back into the bottle each time after using, and using it for two or three months, keeping the bottle full from a stock bottle, and occasionally putting a little dry ferrous oxalate into the bottle and shaking it up, allowing it to settle before using next time. By treating it in this way it retains its power fairly well for a long time; and as it becomes less active I give a little longer exposure, balancing one against the other. Making the ferrous oxalate solution from two saturated solutions of iron sulphate and potassium oxalate has not succeeded so well with me for transparencies. The tone of the picture is not so black as when developed by the old method; and I do not like gray transparencies for the lantern. I also recommend very slow gelatine plates, about twice as sensitive as wet collodion--not more, if I can help it.
I have demonstrated, I hope to your satisfaction, the possibility of producing lantern slides from commercial gelatine plates of a most beautiful quality--ranging from clear glass to deep black, and giving charming gradation of tones, showing on the screen a film as structureless as albumen slides, without the great trouble involved in making them. You must not accept the slides put before you this evening as the best that can be done with gelatine. Far from it; they are only the work of an amateur with very little leisure now to devote to their manufacture, and are merely the result of a series of experiments which, so far as they have gone, I now place before you.--Thomas Mayne, T. C., in British Journal of Photography.
[Footnote: Read at a meeting of the Physical Society, Feb. 26.]
By C.V. BOYS.
All the integrating machines hitherto made, of which I can find any record, may be classed under two heads, one of which, Ainslee's machine, is the sole representative, depending on the revolution of a disk which partly rolls and partly slides on the paper, and the other comprising all the remaining machines depending on the varying diameters of the parts of a rolling system. Now, none of these machines do their work by the method of the mathematician, but in their own way. My machine, however, is an exact mechanical translation of the mathematical method of integrating y dx, and thus forms a third type of instrument.
The mathematical rule may be described in words as follows: Required the area between a curve, the axis of x and two ordinates; it is necessary to draw a new curve, such that its steepness, as measured by the tangent of the inclination, may be proportional to the ordinate of the given curve for the same value of x, then the ascent made by the new curve in passing from one ordinate to the other is a measure of the area required.
The figure shows a plan and side elevation of a model of the instrument, made merely to test the idea, and the arrangement of the details is not altogether convenient. The frame-work is a kind of T square, carrying a fixed center, B, which moves along the axis of x of the given curve, a rod passing always through B carries a pointer, A, which is constrained to move in the vertical line, ee, of the T square, A then may be made to follow any given curve. The distance of B from the edge, ee, is constant; call it K, therefore, the inclination of the rod, AB, is such that its tangent is equal to the ordinate of the given curve divided by K; that is, the tangent of the inclination is proportional to the ordinate; therefore, as the instrument is moved over the paper, AB has always the inclination of the desired curve.
The part of the instrument that draws the curve is a three-wheeled cart of lead, whose front wheel, F, is mounted, not as a caster, but like the steering wheel of a bicycle. When such a cart is moved, the front wheel, F, can only move in the direction of its own plane, whatever be the position of the cart; if, therefore, the cart is so moved that F is in the line, ee, and at the same time has its plane parallel to the rod, AB, then F must necessarily describe the required curve, and if it is made to pass over a sheet of black tracing paper, the required curve will be drawn. The upper end of the T square is raised above the paper, and forms a bridge, under which the cart travels. There is a longitudinal slot in this bridge in which lies a horizontal wheel, carried by that part of the cart corresponding to the head of a bicycle. By this means the horizontal motion communicated to the front wheel of the cart by the bridge, is equal to that of the pointer, A; at the same time the cart is free to move vertically.
The mechanism employed to keep the plane of the front wheel of the cart parallel to AB is made clear by the figure. Three equal wheels at the ends of two jointed arms are connected by an open band, as shown. Now, in an arrangement of this kind, however the arms or the wheels are turned, lines on the wheels, if ever parallel, will always be so. If, therefore, the wheel at one end is so supported that its rotation is equal to that of AB, while the wheel at the other end is carried by the fork which supports F, then the plane of F, if ever parallel to AB, will always be so. Therefore, when A is made to trace any given curve, F will draw a curve whose ascent is (1/K) f y dx, and this, multiplied by K, is the area required.
AN INTEGRATING MACHINE.
Not only does the machine integrate y dx, but if the plane of the front wheel of the cart is set at right angles instead of parallel to AB, then the cart finds the integral of dx / y, and thus solves problems, such, for instance, as the time occupied by a body in moving along a path when the law of the velocity is known.
Some modifications of the machine already described will enable it to integrate squares, cubes, or products of functions, or the reciprocals of any of these.
Of the various curves exhibited which have been drawn by the machine, the following are of special physical interest.
Given the inclined straight line y = cx, the machine draws the parabola y = cx² / 2. This is the path of a projectile, as the space fallen is as the area of the triangle between the inclined line, the axis of x, and the traveling ordinate.
Given the curve representing attraction y = 1 / x² the machine draws the hyperbola y = 1 / x the curve representing potential, as the work done in bringing a unit from an infinite distance to a point is measured by the area between the curve of attraction, the axis of x, and the ordinate at that point.
Given the logarithmic curve y = ex, the machine draws an identical curve. The vertical distance between these two curves, therefore, is constant; if, then, the head of the cart and the pointer, A, are connected by a link, this is the only curve they can draw. This motion is very interesting, for the cart pulls the pointer and the pointer directs the cart, and between they calculate a table of Naperian logarithms.
Given a wave-line, the machine draws another wave-line a quarter of a wave-length behind the first in point of time. If the first line represents the varying strengths of an induced electrical current, the second shows the nature of the primary that would produce such a current.
Given any closed curve, the machine will find its area. It thus answers the same purpose as Ainslee's polar planimeter, and though not so handy, is free from the defect due to the sliding of the integrating wheel on the paper.
The rules connected with maxima and minima and points of inflexion are illustrated by the machine, for the cart cannot be made to describe a maximum or a minimum unless the pointer, A, crosses the axis of x, or a point of inflexion unless A passes a maximum or minimum.
[Footnote: A paper read before the Philosophical Society of Washington. D. C., June 11, 1881.]
In August, 1880, I directed attention to the fact that thin disks or diaphragms of various materials become sonorous when exposed to the action of an intermittent beam of sunlight, and I stated my belief that the sounds were due to molecular disturbances produced in the substance composing the diaphragm.[1] Shortly afterwards Lord Raleigh undertook a mathematical investigation of the subject and came to the conclusion that the audible effects were caused by the bending of the plates under unequal heating.[2] This explanation has recently been called in question by Mr. Preece,[3] who has expressed the opinion that although vibrations may be produced in the disks by the action of the intermittent beam, such vibrations are not the cause of the sonorous effects observed. According to him the aerial disturbances that produce the sound arise spontaneously in the air itself by sudden expansion due to heat communicated from the diaphragm--every increase of heat giving rise to a fresh pulse of air. Mr. Preece was led to discard the theoretical explanation of Lord Raleigh on account of the failure of experiments undertaken to test the theory.
[Footnote 1: Amer. Asso. for Advancement of Science, August 27, 1880.]
[Footnote 2: Nature, vol. xxiii., p. 274.]
[Footnote 3: Roy. Soc., Mar. 10, 1881.]
Fig. 1. A B, Carbon Supports. C, Diaphragm.
He was thus forced, by the supposed insufficiency of the explanation, to seek in some other direction the cause of the phenomenon observed, and as a consequence he adopted the ingenious hypothesis alluded to above. But the experiments which had proved unsuccessful in the hands of Mr. Preece were perfectly successful when repeated in America under better conditions of experiment, and the supposed necessity for another hypothesis at once vanished. I have shown in a recent paper read before the National Academy of Science,[1] that audible sounds result from the expansion and contraction of the material exposed to the beam, and that a real to-and-fro vibration of the diaphragm occurs capable of producing sonorous effects. It has occurred to me that Mr. Preece's failure to detect, with a delicate microphone, the sonorous vibrations that were so easily observed in our experiments, might be explained upon the supposition that he had employed the ordinary form of Hughes's microphone shown in Fig. 1, and that the vibrating area was confined to the central portion of the disk. Under such circumstances it might easily happen that both the supports (a b) of the microphone might touch portions of the diaphragm which were practically at rest. It would of course be interesting to ascertain whether any such localization of the vibration as that supposed really occurred, and I have great pleasure in showing to you tonight the apparatus by means of which this point has been investigated (see Fig. 2).
[Footnote 1: April 21, 1881.]
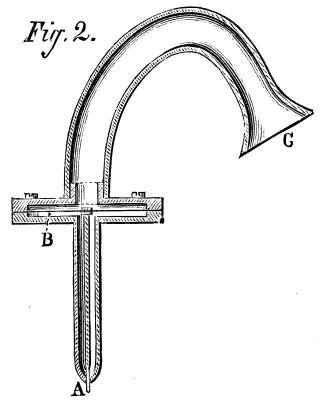
Fig. 2. A, Stiff wire. B, Diaphragm. C, Hearing
tube.
D, Perforated handle.
The instrument is a modification of the form of microphone devised in 1872 by the late Sir Charles Wheatstone, and it consists essentially of a stiff wire, A, one end of which is rigidly attached to the center of a metallic diaphragm, B. In Wheatstone's original arrangement the diaphragm was placed directly against the ear, and the free extremity of the wire was rested against some sounding body--like a watch. In the present arrangement the diaphragm is clamped at the circumference like a telephone diaphragm, and the sounds are conveyed to the ear through a rubber hearing tube, c. The wire passes through the perforated handle, D, and is exposed only at the extremity. When the point, A, was rested against the center of a diaphragm upon which was focused an intermittent beam of sunlight, a clear musical tone was perceived by applying the ear to the hearing tube, c. The surface of the diaphragm was then explored with the point of the microphone, and sounds were obtained in all parts of the illuminated area and in the corresponding area on the other side of the diaphragm. Outside of this area on both sides of the diaphragm the sounds became weaker and weaker, until, at a certain distance from the center, they could no longer be perceived.
At the point where we would naturally place the supports of a Hughes microphone (see Fig. 1) no sound was observed. We were also unable to detect any audible effects when thepoint of the microphone was rested against the support to which the diaphragm was attached. The negative results obtained in Europe by Mr. Preece may, therefore, be reconciled with the positive results obtained in America by Mr. Tainter and myself. A still more curious demonstration of localization of vibration occurred in the case of a large metallic mass. An intermittent beam of sunlight was focused upon a brass weight (1 kilogramme), and the surface of the weight was then explored with the microphone shown in Fig. 2. A feeble but distinct sound was heard upon touching the surface within the illuminated area and for a short distance outside, but not in other parts.
In this experiment, as in the case of the thin diaphragm, absolute contact between the point of the microphone and the surface explored was necessary in order to obtain audible effects. Now I do not mean to deny that sound waves may be originated in the manner suggested by Mr. Preece, but I think that our experiments have demonstrated that the kind of action described by Lord Raleigh actually occurs, and that it is sufficient to account for the audible effects observed.
A catalogue, containing brief notices of many important scientific papers heretofore published in the SUPPLEMENT, may be had gratis at this office.
Terms of Subscription, $5 a Year.
Sent by mail, postage prepaid, to subscribers in any part of the United States or Canada. Six dollars a year, sent, prepaid, to any foreign country.
All the back numbers of THE SUPPLEMENT, from the commencement, January 1, 1876, can be had. Price, 10 cents each.
All the back volumes of THE SUPPLEMENT can likewise be supplied. Two volumes are issued yearly. Price of each volume, $2.50, stitched in paper, or $3.50, bound in stiff covers.
COMBINED RATES--One copy of SCIENTIFIC AMERICAN and one copy of SCIENTIFIC AMERICAN SUPPLEMENT, one year, postpaid, $7.00.
A liberal discount to booksellers, news agents, and canvassers.
MUNN & CO., Publishers,
37 Park Row, New York, N. Y.
In connection with the Scientific American, Messrs. MUNN & Co. are Solicitors of American and Foreign Patents, have had 35 years' experience, and now have the largest establishment in the world. Patents are obtained on the best terms.
A special notice is made in the Scientific American of all Inventions patented through this Agency, with the name and residence of the Patentee. By the immense circulation thus given, public attention is directed to the merits of the new patent, and sales or introduction often easily effected.
Any person who has made a new discovery or invention can ascertain, free of charge, whether a patent can probably be obtained, by writing to MUNN & Co.
We also send free our Hand Book about the Patent Laws, Patents, Caveats. Trade Marks, their costs, and how procured, with hints for procuring advances on inventions. Address
MUNN & CO., 37 Park Row, New York.
Branch Office, cor. F and 7th Sts., Washington, D. C.
End of Project Gutenberg's Scientific American Supplement, No. 288, by Various
*** END OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC AMERICAN SUPPL., NO. 288 ***
***** This file should be named 8391-h.htm or 8391-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/8/3/9/8391/
Produced by Olaf Voss, Don Kretz, Juliet Sutherland, Charles
Franks and the Online Distributed Proofreading Team.
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation information page at www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at 809
North 1500 West, Salt Lake City, UT 84116, (801) 596-1887. Email
contact links and up to date contact information can be found at the
Foundation's web site and official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For forty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.