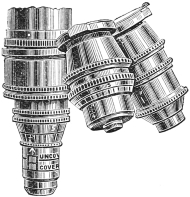
Fig. 1.—Double or Triple Nose-piece.
Apart from the following corrected
misspellings the text of this book has been preserved as in the
original:
xolol → xylol
side → slide
overstraining → overstaining
In this e-text version a black dotted underline indicates a link to a page, illustration or footnote – links are also highlighted when the mouse pointer hovers over them. Page numbers are shown in the right margin. Footnotes are located at the end of the book.
Where appropriate, illustrations and footnotes have been positioned adjacent to the relevant text.
The text contains numerous tables that might not display correctly on handheld devices and also archaic symbols that might not display at all if suitable fonts are not available.
In preparing this edition I have endeavoured to meet the requirements of students, and of practitioners who desire to keep up their histological work. Those methods are selected which have been found to work well in practice, and it has been thought better to describe a few in detail rather than give a short account of many similar methods.
I have again to express my obligation to the various instrument makers for the illustrations of microtomes, &c.; to Dr. Fearnley, of Bradford, for the description of his method for injecting blood vessels, and to Messrs. Macmillan and Co. for permission to copy figures 10 and 11.
W. S. COLMAN.
Wimpole Street, W.
Sept., 1896.
| CHAPTER I. | |
| PAGE | |
| Apparatus Required | 1 |
| CHAPTER II. | |
| Hardening Processes | 15 |
| CHAPTER III. | |
| Section Cutting | 29 |
| CHAPTER IV. | |
| Section Mounting | 55 |
| CHAPTER V. | |
| General Staining Methods | 67 |
| CHAPTER VI. | |
| Special Methods for Staining the Nerve Centres | 87 |
| CHAPTER VII. | |
| Special Methods for Staining Micro-Organisms and Blood | 103 |
| CHAPTER VIII. | |
| Injection of Blood Vessels | 120 |
| CHAPTER IX. | |
| Directions for Preparing Individual Tissues | 129 |
| Index | 153 |
Probably there is nothing more perplexing to a beginner than to decide what apparatus is required. If he consult a price list, it is difficult for him to tell which articles will be necessary, and which will be either luxuries, or required only for special investigation.
In the following account of requisites, those only will be described which it is useful to have always at hand. They will be found sufficient for ordinary work, but for special investigations a more elaborate equipment will be required.
All staining and other reagents should be made as far as possible by the worker himself, according to the directions given in later chapters. This should at any rate be done at first, as the knowledge thus gained will prove invaluable. It will also effect a great saving if articles that are used in any quantity, such as methylated spirit, distilled water, &c., are bought by the gallon, and not in small quantities.
Almost all the processes described here can be carried out without the use of a fully equipped laboratory, in fact, in an ordinary room. The only furniture required is a firm table, and a cupboard and shelves for storing reagents.
The following should also be procured:—
Jars or bottles, with well fitting stoppers or corks, to contain the tissues while being hardened. They should not hold less than two ounces. Empty drug bottles which can usually be obtained from druggists for a few pence, serve very well.
Smaller bottles should also be procured for keeping specimens in spirit after they have been hardened until one is ready to cut sections. After sections have been cut from a portion of the specimen, the rest should be preserved, in case it is wanted for further investigation. Each specimen must be labelled, with a name or a number corresponding to a reference in the note-book, and a large number of specimens may then be kept in the same jar. The best way to label them is to write the name or number on a piece of vegetable parchment in ordinary “marking ink,” and warm it until the writing is black. The little label should then be fixed to a corner of the piece of tissue with a stitch or by a fine pin, and it may be identified years afterwards. The importance of keeping tissues, sections, slides, &c., distinctly labelled cannot be too strongly impressed on the beginner. The name, date, and other particulars should be invariably written on the label at the time. At first the student will be inclined to neglect this, as he will recognize his pieces of tissue and sections so readily merely by their shape and general appearance. But as time elapses and similar specimens accumulate, he will find it most difficult or even impossible to identify one from the other.
A number of 1 oz. and 2 oz. stoppered bottles for staining reagents.
The stopper of these should be fitted with a rod. This is done by simply heating the lower end of the stopper and the upper end of a piece of glass rod of suitable length in a blow-pipe, until they are plastic, and then pressing them together.
Watch glasses.—At least a dozen watch-glasses, in which to perform the operations of staining, clarifying, &c. Those with a flat bottom should be employed as they are less easily upset than the others.
Plenty of filter papers.
Both coarse ones, for use in the manufacture of reagents, and small fine white ones (2 12 inch) for filtering the staining fluids immediately before using them, should be procured. Before using them a few drops of alcohol or distilled water should be placed in them to saturate the paper. This not only allows the fluid to pass through more rapidly, but prevents a portion of it being wasted through being absorbed by the pores of the paper.
Several needles mounted in handles.
They must be kept very bright and smooth, and care must be taken that the point does not get turned up.
A large and small funnel.
Several pipettes consisting of pieces of glass tube with an internal diameter of 18″ and about ten inches long, drawn out almost to a point at one end.
Section lifter.—This instrument is required for transferring sections from one reagent to another, or from oil of cloves, &c., to the slide. The most convenient form is Woodhead’s, made of thin sheet copper, which allows the blade to be bent at any angle to the stem. The stem or handle is about six inches long, and continuous with, and at an angle to it, a flat blade about 34 in. square with the corners rounded off. Larger ones can be obtained for mounting sections of large size, e.g., kidney, medulla oblongata, &c. The surface of the blade should be brightly polished, and kept scrupulously clean.
Ordinary dissecting forceps.
One or two scalpels.
A pair of fine scissors.
A razor or other instrument for cutting sections.
A smooth oil stone for keeping the razors and knives properly sharpened.
A spirit lamp for warming the staining fluids.
A few test tubes.
A minim measure.
Scales and small weights.
A gross of ground glass slides 3 x 1 in.
Half a gross of ground glass slides 3 x 1 12 in.
Half an ounce of thinnest coverslips, 78 in. diameter.
Quarter of an ounce of thinnest coverslips, 1 14 in. diameter.
Microscope.—This is not the place for a description of the microscope as an optical instrument, but some hints as to the selection of one may be found useful.
Showy microscopes with much brass work should be avoided, simplicity of construction being a great recommendation. The microscope should have a large heavy base, either of the horse-shoe or tripod pattern, large enough to afford a firm base when the microscope is tilted.
Mechanical stages are unnecessary and they add greatly to the expense, and very little to the utility of the instrument for ordinary histological work. Binocular arrangements also are of little use for this purpose.
The microscope should be provided with a coarse and fine adjustment, which should be most carefully tested before purchasing the instrument. They should work freely and smoothly, and the slightest turn in either direction should at once alter the focus.
There should be a reversible mirror, one side being concave and the other plane. The concave surface is the one usually employed, the plane surface being chiefly used in conjunction with the sub-stage condenser for the examination of micro-organisms. There should be an eye-piece of moderate magnifying power. Very powerful eye-pieces do not reveal additional details, but merely enlarge the image, and with it any defects that may be produced there by faults in the objective. Eye-pieces II. and IV. of most makers will be ample for most requirements.
Objectives.—These are the most important parts of the microscope, and the student will be well advised if he spends a little extra money to secure good lenses.
Most objectives and stands are now made with a universal thread, so that any objective will fit any make of stand. Many workers provide themselves with a cheap stand such as that supplied by Leitz, and then fit it with lenses by Zeiss, or other first class maker.
The most useful lenses are the 1 in. low power lens, and 15 in. or 16 in. high power, or No. 3 and No. 7 of Continental makers, or Zeiss’s A and D. A 12 in. lens will also be found very useful.
For minute work, such as bacteriology and blood investigations, higher powers will be required, 18 or 112 immersion lenses. These objectives come extremely close to the object, and very thin cover glasses must be employed. In order to avoid the refraction caused by the rays traversing the air between the coverslip and lens some immersion fluid is placed between the two. With some lenses water is employed, but usually an oil having the same refractive index as glass is used, and the one most generally employed is cedar oil (Zeiss prefers the oil from the species Juniperus virginiana). A spot of oil is placed with a rod just over the object to be examined and the objective carefully lowered by the coarse adjustment till it comes in contact with the droplet of oil. The focussing should then be managed with the fine adjustment only.
When the section has been examined the oil must be removed from the lens. For this purpose a soft silk handkerchief or a special piece of chamois leather may be employed, and used very gently. If all the oil cannot be removed, the handkerchief may be moistened with a little absolute alcohol, and the lens hastily wiped. The alcohol must not be allowed to remain in contact with the lens as it is a solvent of Canada balsam with which the lenses are often cemented in position.

Fig. 1.—Double or Triple Nose-piece.
Double or triple nose-piece (fig. 1).—This mechanical arrangement is placed on the lower end of the tube. Two or three objectives of different magnifying power are attached to it. The nose-piece rotates round a central pivot in such a way that the objectives can successively be brought accurately into position above the object on the stage. It is, therefore, a moment’s work to replace a high power objective by a low power one and vice versa. It is an extremely convenient time-saving appliance, and by its use the risk of dropping and injuring the objectives when screwing them on and off frequently is avoided. Those whose microscopes are not already fitted with this appliance can easily have one fitted on at a cost of about a sovereign.
Substage condenser.—This mechanism for concentrating light on the object is a necessity for bacteriological work. The most convenient form is Abbe’s illuminating apparatus (fig. 2).
This consists of a system of short focus lenses which collects the light received by the mirror, and throws it on the object. The amount of light received from the mirror is controlled by an “iris diaphragm,” the aperture of which can be dilated or contracted by moving a small lever at the side. It can be fitted on to most microscope stands, but it is better to get a stand in the first instance which is constructed to carry one.
The cost of a microscope varies from two guineas to two hundred. There are many excellent microscopes in the market, and of these several may be mentioned which the writer has found to work satisfactorily.
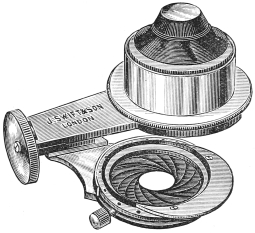
Fig. 2.—Abbe’s Illuminating Apparatus.
Of the cheaper student’s microscopes the “Star” microscope made by Messrs. R. and J. Beck, of Cornhill, E.C., will be found a safe investment. It may be obtained with coarse and fine adjustment, nose-piece, and 1 in. and 14 in. objectives, for about £5. Those who require a better instrument will find Beck’s “Pathological” microscope fitted with nose-piece, Abbe’s illuminator, &c., for £16, meet all requirements.
Leitz of Jena, supplies two good and cheap microscopes for £3 10s. and £5. They are not, however, of uniform excellence, and they should be carefully tested by some competent judge before the purchase is completed. Leitz immersion lenses are cheap, and often extremely good, but should be carefully tested beforehand, as their quality is not quite uniform. The microscopes can be obtained from Mr. A. Frazer, Teviot Place, Edinburgh.
The “Bacteriological” microscope, made by Messrs. Swift, of Tottenham Court Road, is one with which no one can be disappointed. It is sold with Abbe’s condenser, triple nose-piece, 16 in., and a 112 in. immersion objective, for just under £20. Both stand and lenses are turned out in Swift’s first-class style, and those who can afford the initial outlay will not regret it. Or the stand may be purchased, and the objectives and accessories added singly from time to time.
Among Continental makers, excellent microscopes for histological work are turned out by many makers. Zeiss’s lenses stand deservedly high in reputation, as no faulty Zeiss lens ever leaves the works, and their optical properties are nearly perfect. For this guarantee, however, the purchaser has to pay somewhat higher prices, but the money is well invested. Zeiss’s agency is at 29 Margaret Street, Regent Street, W.
Reichert, of Vienna, sells microscopes and lenses which are modelled on the lines of those of Zeiss, and though cheaper are often equal to them in excellence, but the quality is not quite uniform. His instruments can be obtained through any optician, but his agent in this country is Mr. A. Frazer, Teviot Place, Edinburgh.
Before buying a microscope the student should obtain an illustrated price list from any of the firms mentioned above, and, having selected an instrument, he should test it very carefully, or better get some experienced friend to test it for him, before deciding to purchase it. Delicate test objects such as diatoms, scales of butterfly’s wing, or a stained specimen of micro-organisms should be employed. The coarse and fine adjustments should be tried. They should work freely and smoothly and without any delay. The definition of the lens must be tested with the fine objects mentioned. The field should be quite flat, i.e., every part should be in focus at the same time, and the definition should be perfectly sharp and accurate, and the test objects without double contour. The field should be totally free from prismatic colours. If there is a halo of colour around the objects it indicates a defect in the optical properties of the objective, and another should be selected.
A microscope must always be treated with the greatest care. Jars and falls tend to slightly loosen and shift the lenses, and to permanently impair its optical properties. Dust must be most carefully excluded. This is best effected by keeping the instrument under a glass bell jar when not in use. The lenses should be wiped as little as possible, and when it is necessary, very soft chamois leather should be employed. The microscope must be kept in a dry room, or the brass work will soon tarnish and the steel parts will tend to rust.
For the satisfactory examination of tissues it is necessary that they should be “hardened” in certain fluids. The object of this is to give the specimens greater consistence, so that thin sections may be more readily obtained and more safely manipulated, and also to “fix” the tissue element as far as possible in the same relative position as in the living body. The hardening process also acts on the protoplasm of the cells, and prevents their swelling up when placed in water, and in the various staining fluids.
The fluid used must be one which will not itself injure the specimen, and which can be thoroughly removed by washing, so that it may not interfere with staining operations. The specimens should be kept while hardening in wide mouthed bottles, on the bottom of which a little cotton wool or tow has been laid. This allows the hardening fluid to come freely in contact with the under surface of the pieces of tissue, and prevents their being flattened against the hard glass bottom.
The hardening fluid requires changing occasionally. This should always be done at the end of twenty-four hours, in order to get rid of any deposit of blood, &c., that may have accumulated. Besides this, the tissue when placed in the fluid contained a good deal of water which will have diluted it and consequently an early change is desirable. Afterwards the fluid requires to be changed only as often as it becomes turbid, or any deposit occurs, usually about once a week.
While hardening, specimens should be kept in a cool place, as warmth favours changes in the cells, &c.
In manipulating the portions of organs, forceps should always be used and these with great gentleness. The specimens should never be impaled with needles, or unsightly holes, which may even be mistaken for pathological appearances, will appear when a section is examined under the microscope.
It requires some practice to know when the tissue is sufficiently hardened. The object aimed at is to make them not really hard but tough. It is almost unnecessary to add that in testing this with the fingers the utmost gentleness must be observed, or serious damage may be done to the tissue.
When the tissue is sufficiently hardened the hardening fluid must be thoroughly dissolved out. This is most quickly effected by placing the specimen in a basin into which cold water from a tap is constantly running. The tissue may then be removed (forceps always being used and never the needle) and placed in an imbedding medium as subsequently directed; or, if it is not to be cut at once, into equal parts of methylated spirit and water, in which it may be kept indefinitely, the fluid being changed if it becomes at all cloudy.
It is unnecessary for ordinary work to have
more than the following hardening fluids:—
Müller’s
fluid:—
| Potassium Bichromate | 2 14 | grms. | 3 12 | drachms. |
| Sodium Sulphate | 1 | grm. | 1 12 | drachms. |
| Water to | 100 | c.c. | 1 | pint. |
Two drachms of carbolic acid are sometimes added to each pint of the fluid but as a rule it is not necessary.
Müller’s fluid is the most generally useful of the various fluids employed, for the following reasons:—
1. It causes very little shrinking of the elements of the tissue, and hence may be employed for most delicate objects, e.g., the retina and embryos.
2. In consequence of its not making the tissues shrink, it does not squeeze the blood out of the vessels and where the organ has been congested before death, we may, by using Müller’s fluid, preserve a natural injection of the capillaries.
3. There is comparatively little danger of over-hardening the tissue and rendering it brittle.
4. Sections of organs hardened in Müller’s fluid are usually firm and easy to manipulate. They do not tend to curl up or adhere to one another as much as those hardened in spirit.
5. It readily permeates the tissues, and hence large portions of organs, or even the entire organ may be satisfactorily hardened in it.
6. It is very cheap. A gallon can be made up for about eightpence.
The fluid has however certain slight drawbacks:—
1. The hardening process is a slow one occupying four to eight weeks.
2. The fluid gives a permanent dingy colour to the tissue. This does not cause any inconvenience for microscopic purposes, but it is a disadvantage when it is intended to preserve the rest of the specimen, as a naked eye preparation. In such cases the organ should be hardened in spirit, carbolic acid, or formal.
Müller’s fluid can be used for almost any tissue. It is especially useful for those which contain a large quantity of fluid, or of blood, and is essential for nerve tissues which it is intended to stain by Pal’s method (p. 89).
To harden a specimen in it at least twenty times the bulk of fluid must be employed.
The fluid must be changed on the third day, and afterwards about every week as may be required.
Methylated spirit is a very useful hardening agent. It hardens in one to three weeks according to the size of the tissue and the quantity of spirit used. Its disadvantages are:—
1. It is more apt to overharden than Müller’s fluid.
2. It causes a great deal of shrinking of the tissue and thus squeezes much of the blood out of the vessels.
It is most useful in hardening tissues containing much epithelium, e.g., kidney, epithelioma, &c.
Spirit is also frequently employed to complete the hardening by Müller’s fluid and to preserve tissues after they have been hardened.
About ten or fifteen times the bulk of spirit should be used for one of the tissues. The fluid should be changed on the third day and afterwards as required.
Müller’s fluid and spirit.—This is a useful combination for many purposes. It is made thus:—Müller’s fluid, three parts; methylated spirit, one part.
The fluid must be allowed to cool after mixing before being used, and if necessary filtered. It will harden specimens satisfactorily in three weeks.
Müller’s fluid and formal.—Is an extremely useful mixture made by adding one part of formal to nine of Müller’s fluid. It hardens in a much shorter time than Müller’s fluid and causes very little shrinkage.
Absolute alcohol.—Used as a hardening agent where the tissues are to be examined for micro-organisms, and for specimens to be stained by Nissl’s method (p. 101). A cheaper and equally effective hardening medium is made by dehydrating methylated spirit by adding one ounce of fused carbonate of potassium to each pint of methylated spirit, and decanting.
Small pieces must be used. The depths of the block should not exceed 38 inch. The fluid should be changed on the third day. Hardening will be completed in about ten days or even earlier.
Osmic acid.—For rapidity of action, and for rapid fixing of all the tissue elements in their natural position osmic acid is one of the best hardening reagents we possess.
Its disadvantages as a hardening agent are:—
1. Its expense.
2. Its irritating and corrosive vapour.
3. The fact that only small pieces of tissue can be hardened in it, since the external surface is very rapidly hardened and thus the fluid is prevented from penetrating into the centre of the lump.
It is most frequently used as a hardening agent for very delicate structures, such as the retina, or embryos, or for fresh sections of brain (p. 95).
The acid itself may be procured in sealed tubes, each containing one gramme. These should be broken in a bottle under sufficient distilled water to make a one per cent. solution. The bottle containing it should be covered with brown paper to exclude the light. For hardening purposes small pieces of the tissue, not much larger than a pea, should be placed in the acid, the one per cent. solution being diluted with five to ten volumes of distilled water. The tissues may be left in this for from three to five days. They must then be thoroughly washed in distilled water and may afterwards be preserved in methylated spirit.
Both the hardening and the subsequent washing must be carried on in the dark.
Osmic acid is also a most valuable staining reagent (see p. 81).
Carbolic acid (5 per cent.).—May be used to harden almost any tissue, but is particularly useful for hardening nervous tissues such as brain or spinal cord which are afterwards to be preserved as museum specimens. It does not discharge the colour of a specimen so rapidly as spirit.
Three or four times the bulk of fluid should be used. It requires changing at the end of twenty-four hours, and again at the end of the first week.
Saturated aqueous solution of corrosive sublimate is one of the most convenient hardening reagents for small pieces of delicate tissue, e.g., embryos. It hardens them in a few days. When they are sufficiently hardened the mercurial salt should be removed by washing first in methylated spirit for a few hours and then in running water.
Formal.—An aqueous solution containing about 35 per cent. of formaldehyde. It is a rapid hardening agent, causes very little shrinkage of the tissues, and does not discharge the colour of the specimens as much as alcohol. For hardening formal should be used as a two to five per cent. solution in distilled water. It may also be used as a ten per cent. solution for mounting museum preparations, but there is some tendency for a cloudy deposit to form on the glass after a time. It is the most suitable hardening agent at our disposal for eyes. It rapidly fixes the tissue elements, but does not cause much contraction. It may also be used for hardening the brain and spinal cord. Large quantities of fluid must be used for the latter purpose and it must be frequently changed. As soon as they are sufficiently hardened they should be transferred to methylated spirit.
Marchi’s fluid.—This consists of:—
| Müller’s fluid | 2 | parts. |
| Osmic acid solution (one per cent.) | 1 | part. |
It is used for hardening specimens as a preliminary to Golgi’s method for staining nerve cells (p. 97), and also to complete the hardening of sections of spinal cord, &c., before employing Schäfer’s modification of the Weigert Pal hæmatoxyline method (p. 91).
It is also used as a stain for recently degenerated nerve tracts and fibres, especially after experimental lesions.
The fluid has little penetrating power, and therefore tissues must be cut into small pieces, about 38 inch cube. It is not necessary to place them in this fluid at once on removal from the body, but the preliminary hardening must be in Müller’s fluid and not in alcohol, &c.
1. Alcohol.
2. Flemming’s solution (modified by Friedmann):—
| Osmic acid (one per cent.) | 3 | c.c. | (♏xxx.). |
| Glacial acetic acid | 2 | c.c. | (♏xx.). |
| Chromic acid (one per cent.) | 42 | c.c. | (℥j.) |
Small pieces should be hardened in this fluid for twelve to twenty-four hours, and then washed and transferred to alcohol for some days before staining.
3. Nitric acid.—A ten per cent. solution in distilled water. It hardens the tissue in three to four hours, and should be followed by 70 per cent. alcohol, the hardening being completed in absolute alcohol.
In using any of these methods it is necessary that the tissue be removed from the body during life or immediately after death. They are employed for revealing the changes in the cells and their nuclei in rapidly growing or inflamed tissue, for studying karyokinesis in cancer cells, and investigating the appearance of nerve cells and gland cells at rest, when actively employed and when fatigued; and they are also most useful in preparing specimens of the “parasitic bodies” which have been described in many cancer cells.
Used in the preparation of bone, tooth, osseous tumours, &c. The two best fluids for general use are:—
Chromic and nitric fluid.—This is made as follows:—
| Chromic acid | 1 | gramme | 45 | grains. |
| Nitric acid | 2 | grammes | 1 12 | drachms. |
| Water | 200 | c.c. | 1 | pint. |
If the bone is not very compact the fluid may be used diluted with an equal quantity of water. A large quantity of fluid should be used, and like all decalcifying fluids, it should be frequently changed.
As soon as the specimen is sufficiently flexible, it should be thoroughly washed in running water for some hours, and then transferred to spirit until it is convenient to cut sections.
Von Ebner’s solution:—
| Hydrochloric acid | 1 | gramme | 1 12 | drachms. |
| Common salt | 10 | grammes | 2 | ounces. |
| Water to | 100 | c.c. | 1 | pint. |
It is a very useful decalcifying agent, but causes the fibrous elements to swell up rather more than chromic and nitric fluid. A large quantity of the fluid must be used, and it should be changed daily. It must be very thoroughly washed out in running water when the decalcification is completed.
Bleaching solution (eau de Javelle).
| (1) | “Chloride of lime” (bleaching powder) | 20 | 12 | oz. |
| Water | 100 | 2 12 | oz. |
Shake up well.
| (2) | Carbonate of potassium | 20 | 12 | oz. |
| Water | 100 | 2 12 | oz. |
Mix the two solutions. Allow them to stand for an hour and filter.
It is used chiefly for clearing vegetable sections but may also be used for sections containing a large quantity of pigment. It is particularly useful in decolourizing sections of “madura foot” due to the presence of a black fungus.
Embedding of sections.—Before sections are made the tissues require to be embedded in some fluid, which will permeate their interstices, and is capable of being rendered firm so as to support the most delicate parts when the knife passes through the tissue.
The most generally useful substances are:—
(1) gum, (2) celloidin, (3) paraffin or wax.
Gum.—Picked colourless gum arabic 2 parts, cold water 3 parts.
Leave with frequent stirring until dissolved. Add ten drops of carbolic acid to each ounce of the mucilage.
Specimens are thoroughly freed from all trace of the hardening fluid by washing in water, and the tissue is then placed in the gum solution for at least twelve hours, or if enough carbolic acid be added, it may be left there for an indefinite time.
When frozen, gum forms a firm non-crystalline mass, which supports the tissue on all sides. It must not be frozen too deeply, or it becomes hard and rather brittle and is apt to injure the razor. If this have occurred the surface can be softened sufficiently by breathing gently on it.
After cutting in gum, the sections are gently removed from the knife into distilled water by a soft camel’s hair brush, and left there for an hour or two, until the medium is entirely dissolved out. They may then be stained and mounted, or they may be put away in spirit for an indefinite time, and then stained and mounted.
Celloidin is for many purposes almost an ideal embedding medium. (1) It has great penetrating power; (2) it can be made of an admirable consistence for cutting purposes; (3) after sections are made it allows them to be very freely manipulated without fear of injuring them: (4) and being perfectly transparent and homogeneous in thin sections, it does not require to be removed from a section before mounting. It is insoluble in water, and in weak spirit; slightly soluble in alcohol of more than 90 per cent. strength, and very readily soluble in ether, or in a mixture of alcohol and ether. The last solvent is the one commonly employed.
The embedding solution is made thus:—
Take some pure celloidin (“Schering’s,” sold in boxes containing an ounce of shavings, is very good) and pour on it about eight times its volume of a mixture of equal parts of absolute alcohol and ether. Allow this to stand all night until the celloidin is dissolved. The solution should be made about the consistence of ordinary mucilage.
It is also convenient to have a thinner solution made by using double the proportion of alcohol and ether. Both solutions should be kept in wide mouthed stoppered bottles, and the stopper should be well greased with vaseline as an additional obstacle to the evaporation of the volatile ether.
Before embedding a specimen it is necessary to dehydrate it thoroughly for twelve to twenty-four hours in absolute alcohol. It should then be placed in a mixture containing alcohol and ether for an hour or two, and afterwards transferred to the thin solution of celloidin for twenty-four hours, and then to the thick solution for the same period. The celloidin penetrates slowly and in the case of nerve tissues and other delicate structures it is wise to give the full allowance of time for the different steps. When the tissue has been thoroughly permeated by the celloidin, it is gently removed from the celloidin and placed in position on a piece of cork of suitable size for clamping in the holder of the microtome. Celloidin is painted round the object so that it is supported on every side. It is then left exposed to the air until the surface has become firm, when the cork is placed, with the tissue downwards, in methylated spirit. The cork floats but the tissue and celloidin remain submerged. At the end of twenty-four hours the celloidin will have become semi-opaque and opalescent, and of the same consistence as hard boiled white of egg. When it is impossible to wait so long, rapid hardening of the celloidin may be secured by immersing it in methylated chloroform in place of spirit, but the slower method gives more uniformly satisfactory results.
Pieces of tissue embedded in celloidin may also be cut on a freezing microtome. After the celloidin has become firm by immersion in methylated spirit, the tissue with the celloidin round it may be cut off the cork, washed in water to remove the alcohol, and then soaked for an hour or two in gum, placed on the plate of an ether spray microtome, frozen and cut in the usual way.
Subsequent staining operations are conducted in the same way as for sections cut by hand or in gum. As celloidin is only slightly stained by hæmatoxylin, alum carmine, borax carmine, &c., it is not necessary to remove it from the sections, but it exhibits so intense a staining reaction with aniline dyes that it is necessary to remove it by treatment with alcohol and ether either before or after the staining operation.
The sections after staining may be mounted in Farrant’s solution (p. 59), or in Canada balsam (p. 61). If the latter medium is employed, the section should be clarified, after dehydration in alcohol, by means of oil of bergamot, or oil of origanum, instead of oil of cloves, as the latter dissolves out the celloidin and causes the section to break up.
Celloidin is most useful for cutting sections of the coats of the eye, of the internal ear, and of bone marrow. It should always be used for the Weigert-Pal hæmatoxyline method of staining the nervous centres, as it protects the section from being injured by the transference from one fluid to another which is repeatedly required during the process. The stain is completely discharged from the celloidin by the decolourising solution used (p. 90).
Paraffin.—Paraffin is a very convenient embedding medium for delicate structures, as very thin sections can be obtained and the paraffin need not be removed from the section until the latter is safely on the slide. It is unsuitable for large sections. Staining operations are not easily carried out after cutting in paraffin, and it is better to stain the blocks of tissue in bulk before embedding. The best stains for penetrating are borax carmine (p. 75), alum carmine (p. 76), and Kleinenberg’s hæmatoxyline (p. 70). The tissue must be left in them for four to ten days.
Various kinds of paraffin are employed. It is usual to keep two kinds, one “soft,” melting at 110° F., and another “hard,” melting at 140° F. A mixture of two parts of the hard and one of the soft will be found most generally useful. In winter a large proportion of the soft variety and in hot weather a larger proportion of the hard may be required. A paraffin mass which is always available has been suggested recently by Dr. F. E. Batten, who employs an ordinary white candle, composed of paraffin and wax. If the mass is found to be too hard, it can easily be made of a suitable consistence by adding a little paraffin with a low melting point.
To prepare a piece of tissue for embedding in paraffin, it should be stained, washed in distilled water, and as much moisture as possible removed by blotting paper. The block is then dehydrated, first in methylated spirit for several hours, finally in absolute alcohol. It is taken carefully by means of forceps from the alcohol and placed in xylol for an hour or two according to size. Superfluous xylol is removed from the surface, and the tissue placed in the melted paraffin. This will set round the cold tissue at once, but soon melts again and must be kept at a temperature just above melting point for one to four hours, according to size. The tissue is then transferred to a mould (which can be easily made of paper), about half an inch cube, and melted paraffin poured round it until the mould is full. The mould may be made by folding a piece of paper to form a box about half an inch cube, or a small pill box may be used. Another convenient method is to place two L-shaped pieces of lead in contact with each other so as to enclose a space of suitable size as in the diagram (fig. 3). The tissue is now hermetically sealed, and can be kept indefinitely if it is not convenient to cut it at the time. To prepare it for cutting, all superfluous paraffin is trimmed away with a warm knife, and the block is fixed on a piece of wood, cut so as to suit the clamp of the microtome, by melting the lower end of the paraffin block with a hot needle or wire and pressing it down on the wood.
When sections are cut they may be transferred singly to the slide (which should be lightly smeared beforehand with a saturated solution of celloidin in oil of cloves), or they may be cut so that the back of one section of the paraffin block adheres to the front of the next, and in this way a continuous delicate ribbon of serial sections is obtained. The ribbon is broken up into lengths of about two and a half inches and transferred to the slide, on which several ribbons may be placed side by side, and so a large number of sections kept in the order in which they are cut. A mark should be made on the slide to indicate where the series begins, and each slide should be numbered, so that the exact position of each section in the series can be recognised at once.
Before mounting, the paraffin must be removed from the sections. This is easily done on the slide in the case of single sections and of ribbons. If the sections are curled, a little warmth will make them unbend and lie flat. The slide is warmed over a spirit lamp until the paraffin just melts. The sections will keep their places owing to the celloidin beneath. Xylol is then allowed to flow over the slide from a pipette, until the paraffin has been completely dissolved, which can be ascertained by glancing at the sections under the low power of the microscope. The slide is placed in an almost vertical position to let the xylol drain off, excess is wiped off from the edge of the slide with blotting paper, a drop of Canada balsam solution (p. 61) is run on the slide, and a cover-glass of suitable size is applied.
Microtomes.—After a large amount of practice, persons with a fair amount of manual dexterity may acquire sufficient skill to be able to cut very satisfactory sections of specimens embedded in paraffin, &c., by hand. In the Pathological Laboratory of a large German University, until quite recently the use of a microtome was prohibited by the Professor, who is himself a most distinguished histologist. The amount of time expended before one acquires the necessary skill, and the cheapness and great convenience of the modern microtome have combined to throw hand cutting into the background, and some form of microtome is now almost universally adopted.
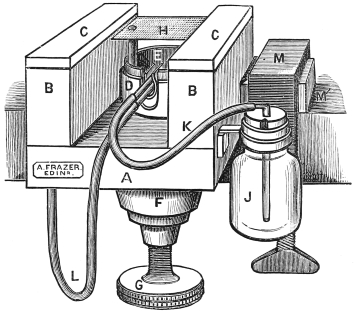
Fig. 4.—Cathcart’s Ether Spray Microtome.
A, B. Wooden frame and supports. C. Glass runners. G. Screw for raising the zinc plate H. J. Ether bottle. L. Tube from air bellows.
Of these there are a very large number in the market, each having special advantages, and often special drawbacks. A few of the more generally useful only will be described. We have microtomes for cutting in gum frozen by ether spray or ice, and those intended for cutting in paraffin or celloidin.
Cathcart’s ether spray microtome (fig. 4).—This, or its more recent modifications (see later), is perhaps the most useful and economical microtome for the purposes of the student. Its prime cost is low, it is small and portable as well as being clean and inexpensive to work with.
It consists of an oak frame which can be firmly clamped on to a table. On this frame are two narrow parallel supports about two inches high, which are covered by strips of plate glass, and serve as smooth rests along which the razor may glide in making sections. Between them is a brass well and in this a zinc plate firmly fixed in the horizontal position, which is almost at the level of the glass runners. It is capable of being raised or lowered through about 38 inch by means of a screw with a very fine and accurate thread. This screw is turned by a large milled wheel beneath the microtome. Just beneath the zinc plate are two small tubes, one connected with an india-rubber bellows, the other with a bottle at the side which contains ether. As the air issues from the first tube, it passes over the open end of the second, and thus draws the ether out and makes it play on the zinc plate, and at the same time causes it rapidly to evaporate, and so reduces the temperature of the zinc plate.
In cutting sections with this microtome the tissue is taken out of the gum and placed on the zinc plate. The bellows are then worked until the gum on the zinc plate is completely frozen. The plate should be lowered by means of the screw until the surface of the piece of tissue is on a level with the glass runners. These and the razor should then be wetted with water. The razor being held firmly in the hand is pushed along the glass runners in a rather oblique direction. The plate should then be raised by turning the screw below through a very small arc, another section taken off and so on. Sections are carefully removed from the razor to a vessel of water by means of a soft wet camel’s hair brush. The needle should never be used for this purpose.
If the specimen is very delicate, and likely to be spoiled by being curled up on the knife, the latter should be kept cold by frequently dipping it in a vessel containing lumps of ice in water. The gum will then remain frozen after cutting, and support the tissue better. Each section should be at once transferred to a glass slide from the knife, washing it off with a stream of ice water from a pipette.
The knife that is used may be an ordinary razor, with the edge ground straight. It requires to be held steadily with both hands. As this is rather inconvenient, Dr. Sheridan Délépine suggested the employment of an ordinary plane iron such as is used in a carpenter’s plane. This only requires one hand, and the other can be kept on the head of the screw beneath to raise the plate at once after each stroke of the knife. Its disadvantages are that it is rather heavy for prolonged working, and that it is less easy to “set” than a razor.
A. Frazer has recently introduced a valuable improvement in the Cathcart microtome (fig. 5).
In this the brass frame carrying the zinc plate and ether spray tubes is surrounded by a brass cylinder, in which it fits accurately, and is pushed up as desired by turning the screw beneath the instrument. This brass frame and with it the zinc plate, &c., can be easily drawn altogether out of the outer tube, and replaced by a second brass well, which exactly fits its place and can be raised by the screw as desired. In this is a small toothed clamp which can be screwed up so as to hold a piece of wood carrying a piece of tissue embedded in paraffin. Sections can also be cut in celloidin with this instrument, but as oblique strokes with the knife cannot be made, it is impossible to get very thin sections. The combined microtome can be obtained for a guinea from Frazer, 22 Teviot Row, Edinburgh.
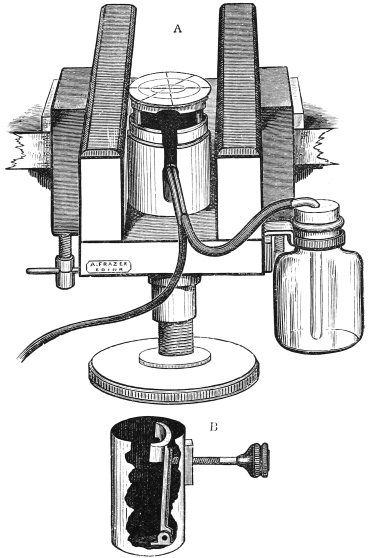
Fig. 5.—Frazer’s Modification of Cathcart’s Microtome.
A. Microtome arranged for ether spray. B. Cylinder with clamp for holding object embedded in celloidin, &c. to replace ether spray apparatus.
There is another modification which is more generally useful, and at the same time more expensive than the original model. In this, instead of glass runners to support the knife, there is a flat glass plate about eight inches square sufficiently large to allow of “Swift’s plough” (fig. 6) being used for the purpose of cutting sections. This instrument consists of a triangular brass frame, supported on three legs, each of which is a screw, tipped with ivory. There is one screw in front and two behind. Beneath the plate, and held in position by the posterior screws in front, and a little clamp behind, is a razor with the edge directed forwards. The edge can be raised or depressed by turning the anterior screw, on which the frame is supported. Before sections are cut the edge of the razor should be brought down to the level of the tissue, taking care that all the legs are equal in length. The plough should then be firmly grasped with both hands, (the index finger of one hand being left free to turn the anterior screw) and pushed rather obliquely through the tissue. The edge of the razor is then slightly lowered by turning the screw through a very small angle, and another section made, and so on. With a little practice very thin uniform sections may be made with great rapidity.
Another useful ether spray microtome is that made by Jung of Heidelburg. The knife swings round a pivot, and there is an ingenious ratchet arrangement which works synchronously with each swing of the knife, to raise the tissue automatically the requisite distance for the next section to be made. The exact thickness of the sections can be graduated with great nicety by a simple contrivance. The instrument can be obtained in this country for about £2. It works satisfactorily, but, with practice, the student will get equally good results with the cheaper “Cathcart.”
Williams’ ice freezing microtome (fig. 6).
This consists of a round mahogany water-tight box provided with an exit tube below, and covered with a strong plate glass lid. Firmly fixed in the centre of the floor of the box is a stout brass pillar surmounted by a brass disc which fits into a hole in the centre of the glass lid, so that its surface is on a level with that of the lid.
To use it, the box is filled with alternate layers of pounded ice and salt; the lid is then put on and fixed by means of a lateral screw. The tissue to be frozen is gently removed from the gum and placed on the brass disc and plenty of gum painted round it. It should then be covered with a tin cap for a few minutes until frozen. Sections are made with a Swift’s Plough (p. 44).
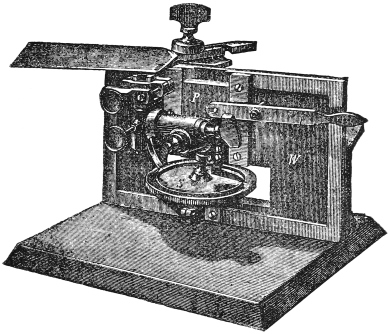
Fig. 7.—Schanze Microtome (see text).
Schanze microtome (fig. 7) is the pattern used in the Leipsic laboratories. It consists of a heavy iron frame with a large base. The knife is carried in a clamp which slides along the full length of the instrument, gliding upon two smooth plates of iron which are arranged at an angle to one another. The knife must be moved very steadily and gently, as when using a long blade vibrations are easily set up which prevent good sections being obtained. The surfaces of contact must be kept scrupulously free from dust, and lubricated with equal parts of olive oil and castor oil. There are several object holders, which can be removed and interchanged, one connected with an ether spray apparatus, another suitable for holding an object embedded in paraffin, and a third for grasping an object embedded in celloidin. When celloidin is employed, a specially long knife must be used, and it must be fixed very obliquely in the clamp. The object holder is raised by a fine screw worked by a large brass toothed wheel. There is a ratchet arrangement, by which the object may be raised automatically any desired distance, after each stroke of the knife. It gives most satisfactory results with celloidin and paraffin. (Messrs. R. and J. Beck are the agents). Its cost is about £5.
Becker’s microtome is made on exactly the same principles as the Schanze. The modifications are that the carrier glides on glass plates instead of iron ones, and that instead of the whole surface of the carrier being in contact with the plates, there are a few smooth ivory buttons only. Friction is thus reduced to a minimum, and very uniform sections can be obtained. The price is the same as that of the Schanze.
Frazer has introduced a “student’s sliding microtome” on the same principle as the Schanze which costs about £3.
The Cambridge Rocking microtome.—This instrument, as made by the Cambridge Scientific Instrument Company, or the slightly modified form made by Messrs. Swift (fig. 8), is the best instrument for cutting sections of small objects embedded in paraffin. Ribbons of serial sections can be obtained from it with greater ease and certainty than with other microtomes. This microtome differs from those which have been previously described in that the knife is fixed, while the object is moveable. The microtome consists of an oblong heavy metal stand. A long bar is arranged so that it rides in see-saw fashion on two strong vertical pillars arising from the frame. One end of this bar is hollow, and receives the piece of wood carrying the tissue embedded in paraffin, which is firmly clamped in position. This end is depressed by means of a strong spiral spring. In order to raise it there is an arrangement by which the other end of the bar is depressed by a cord which revolves round a pulley. When the handle is turned, the tissue is raised, and when the cord is relaxed, the spring pulls the tissue firmly and steadily down. The razor, which must have a straight edge, is fixed firmly by screws, with its edge upwards at the end of the microtome. The object is then adjusted so that in its descent a thin slice is taken off by the razor. There is an ingenious arrangement by which the depression of the bar to raise the section pushes it a little further in the direction of the razor. The distance can be graduated from 1500 to 13000 inch. The actual working of the machine is therefore very simple. The position of the block containing the tissue to be cut having been adjusted so that the razor just cuts it, the free end is depressed by means of the pulley. This also pushes the section a little beyond the razor. The strong spring then draws the tissue steadily past the edge of the razor, and a thin section is left on the blade. This may be at once transferred to a slide, or if the paraffin be of the proper consistence, another cut may be made, when the two sections should adhere by their edges, and so by repeating the movement a continuous ribbon may be obtained. If there is difficulty in obtaining a good ribbon, it will usually be got over by taking a little soft paraffin and attaching it by means of a hot needle to the lower end of the paraffin block. The cost of the instrument is about £5.
Fresh sections.—Although these are not so satisfactory as hardened specimens for accurate histological work, it is often very useful to make them both in the post-mortem room where an immediate opinion of the nature of the tumour or diseased organ is desired, and also in the operating theatre. With a little practice sections may be cut, stained, and mounted, within ten minutes of the removal of the specimen from the body. In this way important information may be afforded to the operating surgeon, and in not a few cases it has caused the proposed treatment to be entirely altered. Thus, in one case, a supposed chronic periostitis was shown to be a sarcoma, and the limb was amputated. In another, a supposed sarcoma of the thigh was found to be a gumma, when a portion was removed and microscopically examined.
A portion of the specimen should be placed without any preparation on the zinc plate of the freezing microtome, and some gum painted round it. It is then frozen. The serum in the tissues is not in sufficient mass to injure the knife when it is frozen. The knife should be wetted with, and sections transferred to, either pericardial serum, or 34 per cent. solution (70 grains to the pint), of common salt, neither of which causes the cells to swell up as plain water does. They should be carefully floated out on a glass slide, an operation which requires much more patience than in the case of hardened sections, as fresh sections are less coherent and also more sticky, so that the edges tend to curl up on the knife, &c. They should then be examined, one unstained, simply mounted in salt solution; another stained with picrocarmine and examined in the saline solution; and a third stained in picrocarmine, mounted in Farrant’s solution, and preserved. The last usually gives the best results, the picrocarmine staining becoming quite brilliant after a week. The glycerine, however, is apt to make the sections shrink a good deal, and the weight of the cover-glass tends to break up the unhardened section.
1. By flotation.
In this method the section whether stained or unstained is placed in a bowl of water, or normal salt solution (p. 53). A clean slide is then introduced into the water at an angle of about 60°, a little more than half of its length being submerged. The section is then brought up by the needle and floated as far as possible into position on the slide. One corner is then fixed by the needle, and on gently withdrawing the slide the section should lie flat. If any folds are left no attempt should be made to smoothen them out with a needle, but the slide should be re-immersed until the folded part of the section is under water. It should then be gently withdrawn, when the fold will disappear. This manœuvre must be repeated in different directions until the section lies quite smoothly on the slide. Stained and unstained sections are floated out in this way before being mounted in Farrant’s medium, and unstained sections previous to staining in picrocarmine.
2. By transference with a section lifter.
This method is employed in mounting in Canada balsam in order to transfer the section from the clarifying agent (p. 63) to the slide. The lifter is polished, and insinuated under the section. The section being held in position by the needle is now raised from the fluid, excess of which is removed by holding the section in position with a mounted needle, and tilting the lifter so as to allow it to drain off.
Removal of air bubbles from sections.—When sections contain many air bubbles, the best plan is to leave them in methylated spirit for a time. The bubbles then coalesce and escape from the section.
For delicate structures and for fresh sections the transference to spirit, and the subsequent flying out of the section when returned to water are risky, and the best method of treating these is to put the vessel containing them under the receiver of an air pump, if one is available, and slightly exhausting the air.
The most frequent cause of air bubbles in mounted specimens, however, is the employment of cover-glasses which have not been thoroughly cleansed. Proper cleansing is best effected by placing the covers when bought in a shallow wide mouthed stoppered bottle containing strong nitric acid, and leaving them in this fluid for twenty-four hours. The acid should then be drained off and water run through the vessel from a tap, until the washings no longer give an acid reaction with litmus paper. The water should then be drained off, and the glasses covered with absolute alcohol. They can be removed one by one and rapidly dried as required. With cover-glasses properly cleansed in this manner, not only will air bubbles be avoided, but the covers will be dried much more easily with the cloth, and fewer will be broken in the process.
Another very frequent cause is the transference of air bubbles with the mounting medium on the glass rod. This occurs especially if the rod be fused to the stopper. The proper bottles to use, both for Farrant’s medium and balsam are “balsam bottles” which have no stopper, but the mouth is closed by a glass cap which fits accurately (fig. 9). A short glass rod is attached to the cap, and is used to transfer the medium to the slide.
Treatment of folded sections.—The folding may be due:—
(1.) To the section having creased through being cut with a knife whose surface was not perfectly smooth. This is best remedied by placing the section in methylated spirit for a minute, and then transferring it to a bowl of clean water, when the section will rapidly rise to the top, and spread itself out flat on the surface of the water, in consequence of the alcohol rapidly diffusing out at the edges into the surrounding water.
(2) To the section containing a large amount of fat, as in those of the skin and subcutaneous tissue. The fat may be removed from the fat cells without materially altering the appearance of the section. This is done by dehydrating the section in alcohol, and then transferring to a watch glass containing ether or chloroform to extract the fat. The tissue should be washed free from ether in the alcohol and then transferred to the bowl of water, and allowed to float out. This process does not interfere with subsequent staining operations.
Farrant’s solution:—
| Gum Arabic (picked, colourless) | | equal parts. |
| Glycerine | ||
| Water |
In making this solution the best gum arabic must be used, and only the clearest pieces of this. “Powdered gum acacia” should be avoided, as though it looks white it often yields a brown mucilage, and besides is frequently adulterated with starch, &c.
The glycerine and water should be mixed and the gum arabic added. The mixture should be allowed to stand for some weeks, with frequent stirring until the whole of the gum is dissolved. Then allow it to stand for a week or two longer in order that the dirt may subside, and the bubbles rise to the top. The scum should be removed and the clear fluid decanted from the sediment into a “Balsam bottle” (p. 58) containing a few drops of a saturated solution of arseniate of sodium and a small lump of camphor.
If properly made it is an extremely useful mounting reagent. It does not clarify the tissues too much, and in consequence of its containing gum it dries at the edges and cements the cover-glass more or less firmly in a week or two. If this is not the case the medium contains too much glycerine and more gum must be added to compensate for this. This drying at the edge prevents any further evaporation while the glycerine keeps the section permanently moist.
The camphor and arseniate of sodium prevent the formation of fungi. Sections preserve their original appearance in this medium for many years. After a long time they are apt to become a little cloudy and granular.
Unstained sections should always be mounted in Farrant’s medium, as the Canada balsam process renders them quite transparent. It is suitable for almost any tissue stained or unstained, but sections of the nervous centres require to be mounted in Canada balsam, owing to the opacity of myelin when mounted in glycerine.
Canada balsam solution:—The medium is made thus:—
The ordinary Canada balsam which is of a treacly consistence is heated gently in a water bath for some hours, to drive off turpentine and other volatile oils. It is then allowed to cool to a yellow vitreous mass. Take of
| Dried Canada balsam | | equal parts. |
| Xylol |
Leave till dissolved, stirring occasionally.
Unless the solution be perfectly clear, it must be filtered through a very thin paper, previously wetted with xylol. If the medium be too thick more xylol should be added, if too thin, the xylol should be allowed to evaporate until the medium is of the consistence of a thin syrup.
If the medium is made too thin much annoyance will be caused by its evaporating at the edge of the cover-glass, leaving an air-space, which will increase daily until the section is left quite dry. This should be remedied by putting another drop of balsam at the edge of the coverslip and allowing it to run in and displace the air. A ring of cement should be put on as early as possible afterwards.
The bottle in which the balsam is preserved must be very carefully dried before being filled and then rinsed out with absolute alcohol, and afterwards with xylol. Turpentine or benzol are often used instead of xylol in the preparation of the medium, and in the same proportion, but the latter is less apt to dissolve out the aniline colours from the sections.
To mount sections in Canada balsam they must be transferred first to a watch glass containing absolute alcohol or an alcoholic solution of some staining reagent, e.g., eosine (p. 72) and left in it, no attempt being made to spread it out, until it is perfectly dehydrated, i.e., in about two minutes. It should then be transferred to the clarifying oil on a mounted needle, or on a section lifter, which must be perfectly dry as any spot of moisture that gets on to the section will resist the clarifying action of the oil, and will cause unsightly opaque areas when the section is mounted. Even breathing on the section on its way to the clarifying agent will prevent uniformity of clearing. Should white spots appear in the section while in the oil it must be taken out with as little oil as possible, and again dehydrated in absolute alcohol.
The process of clarifying must be performed in some medium in which Canada balsam is readily soluble, and which is also readily miscible with alcohol. Those most frequently employed are oil of cloves, xylol, oil of bergamot, oil of cedar, and origanum oil. The first named has always been much used because of its agreeable odour, its cheapness, and the ease with which it can be obtained. But it has the disadvantage of dissolving out many important staining reagents, especially eosine and the various aniline colours. In addition as it dissolves celloidin, sections cut in this medium tend to fall to pieces when transferred to oil of cloves, and one of the other oils (which have no solvent action on celloidin) should always be employed with celloidin sections. Oil of bergamot is the most generally useful, but rather expensive. Where there are special reasons for employing other dehydrating agents, they will be indicated in the special directions for particular staining methods in Chapters VI. and VII.
As soon as the section is plunged into the oil, the alcohol rapidly diffuses out, so that the edges of the section fly out with it, and the section floats quite flat on the surface of the oil. When it is completely clarified (in about a minute), as shown by its sinking in the oil, it should be transferred to the slide by the section lifter, and the oil drained off. Excess of oil may be removed by pressing gently on the section with a flat piece of filter paper folded several times. If carefully performed this manœuvre will not injure the section, but it requires practice.
If the tissue is very delicate, and likely to be injured by changing from one vessel to another, or if it is larger than the section lifter will conveniently carry, it should be floated out on a glass slide, and, as much water as possible having been removed by blotting paper, should be dehydrated by adding a little alcohol from a pipette once or twice. Most of the alcohol should then be removed by tilting the slide, and before the remainder has evaporated, some oil of cloves or bergamot should be added from another pipette. The section will float on the oil at first, but the latter will gradually come through and appear on the top of the section. When this occurs the clarification is complete, and the oil may be run off by tilting the slide and the section mounted in Canada balsam.
Cementing of cover-glasses.—The cover-glasses may be cemented down to prevent their shifting and spoiling the specimen. If the cover-glass be circular, a Shadbolt’s turntable should be used. It consists simply of a horizontal heavy brass disc, rotating easily on a pivot. There are a number of circles traced on the disc concentrically. The slide is then fixed on the disc by means of the clips, so that the circumference of the cover-glass corresponds to one of the circles. The disc is then rotated and the cement applied to the edge of the cover-glass with a brush.
Many materials are employed. The most suitable are:—(1) Canada balsam, which is almost colourless and transparent and looks very neat. (2) Gold size. (3) Marine glue.
When these are dry a finished appearance may be given to the slide by laying on a ring of zinc white. This is made as follows:—
| Oxide of zinc | 12 drachm. | |
| Benzole | | half an ounce of each. |
| Gum dammar | ||
Preservation of sections.—They should be kept flat, and preserved from both light and dust. Very useful cardboard trays are now sold by almost all dealers in boxes made to contain twenty-four dozen slides for about eight shillings, or suitable cabinets may be constructed by a carpenter.
Much information may be obtained from unstained sections, and in most cases one section should be examined unstained, but the specimens mounted in this way are so transparent that it is difficult to study the details of the tissue. They are therefore usually prepared by treating them with some staining reagent, not merely to render them less transparent, but also to “differentiate” the elements of the section, by staining one part more deeply than another, or of a different colour. Thus hæmatoxyline stains the nuclei and rapidly growing parts of the tissue, leaving the formed material, as a rule, much more lightly tinted. Methyl violet again stains healthy tissues blue, and parts affected with waxy degeneration a red-violet colour. By combining stains also much differentiation of the tissue elements may be obtained. Sections should be stained with several reagents, as their effect on individual specimens varies a good deal.
The following are the most useful stains for general purposes:—
Logwood.—This or its purified principle hæmatoxyline is the most useful general stain. The hæmatoxyline itself is preferable, giving more constant results, and less diffuse staining.
For general staining purposes the following
formula will be found to give excellent results:—
Hæmatoxyline.
Schuchardt’s formula.—
| (a) | Hæmatoxyline | 3 | grms. | 30 | grs. |
| Absolute alcohol | 16 | c.c. | 2 12 | drms. | |
| (b) | Pure alum | 3 | grms. | 30 | grs. |
| Distilled water | 100 | c.c. | 2 | ozs. |
Add (a) to (b) drop by drop and with constant agitation. Keep for some days exposed to diffuse daylight until its colour is so deep that it will not transmit the light. It should then be filtered, and a crystal of thymol added. It will not give very satisfactory staining reactions at first, and should be allowed to ripen at least a month or six weeks before using. It improves as a dye with every month that it is kept. Whenever hæmatoxyline has been made up with alum as in the above formula, an abundant reddish-brown precipitate forms after some time. This in no way interferes with the activity of the solution, but it must always be filtered before being used.
Barrett’s formula.—Introduced by Dr. W. H. Barrett, of Belfast. It gives almost as good results as the above. It is made from ordinary English extract of logwood, and is considerably cheaper.
The extract should be dried, and finely powdered, and then extracted with absolute alcohol for several days.
| Powdered extract of logwood | 2 | grms. | 1 12 | drms. |
| Absolute alcohol | 10 | c.c. | 1 | oz. |
Filter and add slowly to
| Benzoate of sodium | 1 | grm. | 36 | grs. |
| Alum | 1 | grm. | 36 | grs. |
| Distilled water | 100 | c.c. | 10 | ozs. |
The strength of the solution will vary with different samples of logwood and must be estimated by trial. This solution is comparatively cheap and is useful for class purposes.
Ehrlich’s hæmatoxyline.—This very useful nuclear stain is made as follows:—
| (a) | Hæmatoxyline | 2 | grms. | 9 | grs. |
| Absolute alcohol | 100 | c.c. | 2 | ozs. | |
| (b) | Glycerine | 100 | c.c. | 2 | ozs. |
| Distilled water | 100 | c.c. | 2 | ozs. | |
| Alum | 120 | grms. | 2 12 | ozs. | |
| Glacial acetic acid | 5 | c.c. | 24 | mins. |
Add (a) slowly to (b) with constant agitation.
Allow to ripen in sunlight for two months before using. It may be employed as a rapid stain undiluted but far better results are obtained by using a weak solution, a few drops to a watch-glass full of distilled water, and staining slowly for from half an hour to two hours. The solution improves by keeping. If after a time the staining becomes diffuse it is an indication that the acetic acid has evaporated, and a few drops more should be added.
Kleinenberg’s hæmatoxyline.—This formula differs from the previous one in being an alcoholic solution. The calcium chloride is added because it “sets up diffusion currents between the alcohol in the material to be stained and the alcoholic staining solution, so enabling the latter to penetrate more rapidly” (Squire). It is much used in staining embryonic specimens in bulk before embedding in paraffin, and was strongly recommended for that purpose by Foster and Maitland Balfour.
Various formulæ have been given from time to time. That advised by Squire (Methods and Formulæ, p. 25) can be accurately made up without much difficulty.
| (a) | Crystallised calcium chloride | 20 | grs. | 12 | oz. |
| Distilled water | 10 | c.c. | 2 | drms. | |
| (b) | Alum | 3 | grms. | 32 | grs. |
| Distilled water | 16 | c.c. | 170 | mins. |
Mix and add
| Rectified spirit | 240 | c.c. | 8 | ozs. |
Allow it to stand and any excess of calcium sulphate, &c., to separate. Filter and add
| Hæmatoxyline | 2 12 | grms. | 25 | grs. |
A little thymol should be added as a preservative.
In making up these solutions care must be taken that only distilled water is used, and that all the vessels employed have been previously rinsed out with it, otherwise precipitation of the hæmatoxyline will occur.
Should sections be overstained in hæmatoxyline, this may be remedied by washing it in a half per cent. solution of acetic acid, until sufficient of the stain is discharged, but the staining is more diffuse than if the happy mean had been hit in the first instance.
Hæmatoxyline stains the nuclei of the cells a beautiful violet colour, and also tints, more or less lightly, the cell protoplasm and the fibrous elements. It also stains the axis cylinders of nerves, and is much used in special staining of the nerve centres as will be described later, (pp. 88–91).
The stain is permanent. Sections may be mounted either in Farrant’s solution, or in Canada balsam, the latter being preferable.
Eosine.—Much more satisfactory results are obtained from the commercial eosine (an amorphous orange powder used in dyeing and in the manufacture of red ink), than from the pure crystalline form.
It may be used as an aqueous solution ( 130 per cent.) or as a solution in absolute alcohol ( 115 per cent.). Sections stained in the former should be rapidly passed through a one per cent. solution of acetic acid in order to “fix” the stain, and then washed in distilled water.
It is a very transparent stain, and the most delicate details of a section stained with it are perfectly visible.
It stains the nucleus but slightly, while it stains the cell protoplasm and fibrous tissues and especially muscular tissues a beautiful rose colour.
It will be seen, therefore, that it stains those parts which are left unstained by hæmatoxyline, and vice versâ. This complementary action is applied in the following method.
Double staining with eosine and hæmatoxyline.—Sections having been stained in hæmatoxyline in the ordinary way, are washed in distilled water, and dehydrated in a solution (about 1 in 1500) of eosine in absolute alcohol. They should remain in this for about two minutes, and then be passed through oil of cloves and mounted in Canada balsam in the ordinary way.
This method gives extremely useful and beautiful results with almost all tissues, and is superior to picrocarmine for differentiating the tissue elements. Thus, the nuclei are stained violet, the cell protoplasm a much paler and warmer violet, the fibrous tissues pink, and red blood corpuscles orange or brick red.
The alcoholic solution of eosine is also used as a contrast stain after staining for micro-organisms with blue or violet dyes.
Carmine.—It is made as follows:—
| Carmine (best) | 2 | 1 | drachm. |
| Strong ammonia | 2 | 1 | drachm. |
| Distilled water | 100 | 6 | ounces. |
Rub the carmine with a little water in a mortar, add the ammonia, when the liquid will turn black. Gradually add the rest of the water, rubbing it up all the time. It should be bottled, allowed to stand for a few days, and then filtered, and a piece of camphor put in the bottle.
Lithium carmine resembles closely ammonia carmine in its staining effects. It is usually a matter of individual preference which is employed.
| Carmine | 2 12 | grms. | 10 12 | grs. |
| Saturated aqueous solution of lithium carbonate | 100 | c.c. | 2 | ozs. |
Dissolve and filter.
Sections may be sufficiently stained in either of these fluids in from three to five minutes, but more satisfactory results are to be obtained by diluting with twenty times the bulk of distilled water, and leaving sections to stain for twenty-four hours.
After staining in carmine the sections must be passed through a half per cent. solution of acetic acid, in order to fix the carmine in the tissues, as otherwise the water will dissolve the stain out.
Borax carmine—
| (a) | Borax | 4 | grms. | 3 | drachms. |
| Carmine | 2 | grms. | 1 12 | drachms. | |
| Distilled water | 100 | c.c. | 5 | ounces. |
Dissolve with the aid of heat and add slowly to (b).
| (b) | Alcohol | 70 | c.c. | 3 12 | ounces. |
| Distilled water | 30 | c.c. | 1 12 | ounce. |
Allow to stand for a fortnight. Filter, and add a lump of camphor.
To use it, place sections, or the tissue in bulk, in it for from four to twenty-four hours, according to size, and then transfer to alcohol (seventy per cent.) containing a drop to the ounce of hydrochloric acid, for twenty-four hours, and then wash thoroughly in water. The tissue may then be placed in gum if it is to be frozen, dehydrated in alcohol if paraffin or celloidin is to be employed.
Its advantage is that it is very diffusible, and so can be used to stain tissues in bulk. It takes a considerable time to stain sufficiently deeply, but there is little fear of overstaining.
It stains nerve-cells and axis cylinders brightly, and also the connective tissue, bringing a sclerosed patch out very prominently.
Alum carmine:—
| Alum five per cent. solution in distilled water | 100 | c.c. | 1 | oz. |
| Pure carmine | 1 | grm. | 4 12 | grs. |
Boil for twenty minutes. Filter. Add a few drops of carbolic acid.
In using this reagent it should be filtered into a watch glass, and the sections placed in it for at least an hour. There is no fear of overstaining, and they may be left all night. After they have been stained they must be thoroughly washed in water to remove the alum, otherwise numerous crystals of it will be seen in the field when the section is mounted. Sections may be mounted in Farrant’s solution or in Canada balsam. The staining effect improves very much after the section has been kept a few days.
If desired its staining action may be complemented by dehydrating it in an alcoholic solution, either of eosine (1 in 1500) or of picric acid, and then clearing up in oil of cloves, and mounting in Canada balsam.
By itself it gives a stain very like that of hæmatoxyline, only warmer. It picks out the nuclei and axis cylinders of nerves, stains cell protoplasm slightly, and the fibrous elements scarcely at all.
It may be used for the same purposes as hæmatoxyline. The colour is less attractive, and not so deep as that of the latter, but as it does not overstain sections, even when left in it for a week, it is a very convenient stain for general purposes.
It is particularly useful as a contrast stain for sections of brain and spinal cord, after the Weigert-Pal hæmatoxylin process (p. 88).
Ammonia-picrocarmine was formerly very largely used as a staining reagent. Its place has now to a large extent been taken by lithio-picrocarmine.
In its preparation the best carmine must be used.
It is made as follows:—
| Carmine | 1 | part. |
| Liq. ammon. fort. | 3 | parts. |
| Distilled water | 3 | parts. |
Dissolve with gentle heat, and add
| Cold saturated aqueous solution of picric acid | 200 | parts. |
Bring the mixture to the boiling point, and then place in a shallow vessel, covered with a glass plate, and leave it in full sunlight for a month or more. Filter, bottle, and add six drops of carbolic acid to each ounce of the mixture. It will keep indefinitely and improves with age. It requires filtering from time to time, as a gelatinous crimson mud tends to deposit from the solution.
Lithio-picrocarmine.—Prepared as follows:—
| Carmine | 2·5 | grms. | 10 | grs. |
| Saturated solution lithium carbonate | 100 | c.c. | 1 | oz. |
Dissolve, and add
| Saturated solution picric acid | 250 | c.c. | 2 12 | oz. |
Add a few drops of carbolic acid to each ounce.
It should stand a day or two in sunlight and then be filtered. It improves by keeping.
It should be kept in a stoppered bottle with a glass rod fused into the stopper.
When sections are to be stained they are to be floated out on a clean glass slide as described on page 55. The slide should then be tilted to allow the water to drain off, and superfluous moisture round the section removed by a soft rag, or blotting paper. A drop or two of the stain should then be transferred to the slide, which should be left lying quite flat for about ten minutes. Unless the room is very warm it is advisable to heat the slide very gently over a spirit lamp, as this causes the tissues to stain more brightly and more rapidly.
The excess of the picrocarmine should be allowed to run off the slide, and the latter wiped. Some of the stain should, however, be left on the section, as its effects go on increasing, and are often not fully seen until a few weeks have elapsed. They should be mounted in Farrant’s medium. As a rule those mounted in Canada balsam do not give such good results. Should there be special reasons for using this medium, as in mounting spinal cord sections, &c., they should be dehydrated after staining in picrocarmine in an alcoholic solution of picric acid (one part of a saturated alcoholic solution to five of alcohol), before clarifying in oil of cloves, as otherwise the alcohol will dissolve out the picric acid, and much of the differential staining effect will be lost. The nuclei should be stained a bright crimson, the protoplasm of the cells yellow, or a dull pink, the fibrous elements a bright pink, red corpuscles green, and all dead material, e.g., caseous matter, bright yellow. It also stains nerve-cells, and the axis cylinders of nerve fibres very brightly. It is, however, a rather uncertain dye. The results are most brilliant in the case of fresh sections.
Osmic acid is invaluable for staining fatty particles in the cells.
For ordinary use the one per cent. stock solution (p. 21) should be diluted with ten times its bulk of distilled water, and sections stained in it all night in a dark cupboard, or the watch glass containing them may be placed inside a small box.
The sections must be washed thoroughly in plenty of water. If desired they may be stained subsequently in picrocarmine or methyl violet if waxy degeneration also be present. Sections should be mounted in Farrant’s solution, as Canada balsam usually gives disappointing results.
It demonstrates the most minute fatty particles in degenerating cells, &c., staining them black. It may be employed to demonstrate the globules of fat blocking up the vessels in fat embolism.
It stains the myelin sheaths of nerves black, and will be again referred to when speaking of methods of staining the spinal cord.
Nitrate of silver is employed for staining the intercellular cement of epithelial cells. It stains this substance a deep black, while the rest of the tissue takes on a brown colour. It is used as a half per cent. solution in distilled water, and kept in a stoppered bottle carefully covered up with brown paper. To use it take some epithelial tissue, e.g., the omentum from a recently killed animal, or a section of some epithelial tumour, immediately after excision. Wash thoroughly in distilled water to remove all chlorides, and then place in a watch glass containing the silver solution. Keep this in the dark for half an hour and then wash thoroughly in plenty of water. The section should be mounted in glycerine or Farrant’s medium and kept from the light or it will become too darkly stained.
Chloride of gold is employed to demonstrate the peripheral terminations of nerves. It can only be employed within the first half hour after the tissue has been removed from the living body. The pieces of tissue must be small and may be stained in bulk, sections being subsequently made.
A half per cent. solution in distilled water is employed. The tissue is transferred to this on its removal from the body, until it becomes lemon coloured. It is then exposed in a one per cent. solution of acetic acid to a strong light until it assumes a purplish tinge, which takes from two hours to two days. Sections should be mounted in Farrant’s medium. It stains the cells of the tissue, and nerve cells reddish purple, and nerve fibrils, especially the terminal ones, rather more violet. This is very well seen in the cornea.
It is useful sometimes for clinical purposes to excise a portion of muscular tissue and examine the nerve endings by this method. Unfortunately the stain is somewhat uncertain in its action. Better results are obtained by Sihler’s chloral hæmatoxyline method (p. 92).
Methyl violet.—A very satisfactory solution may be obtained ready made in the “telegraphen tinte,” prepared by Leonhardi, of Dresden, as recommended by Woodhead. It may also be used as a one per cent. solution in distilled water, a few drops of carbolic acid being added to prevent the growth of fungi.
It is a very useful selective stain. It gives two reactions, red violet, and blue violet. Thus it stains the matrix of hyaline cartilage blue violet, but the cells red violet. It has also a most important pathological application, as it picks out any parts which have undergone “waxy” or “lardaceous” degeneration, staining them red violet, but the rest of the section blue violet.
About ten drops of a one per cent. solution should be filtered into a watchglassful of water, and the sections stained for about five minutes. They must then be passed through a half per cent. solution of acetic acid and washed thoroughly for some time in a large quantity of water till no more colour comes away.
If these steps are not taken with care, the dye will diffuse out after the section has been mounted, blurring all details and spoiling the appearance of the section.
Sections may be mounted in Farrant’s solution (to which a spot of formic acid may be added): if mounted in Canada balsam the sections must be overstained as both the alcohol and oil of cloves rapidly dissolve out the dye.
Safranine.—Employed as a freshly made saturated solution in aniline oil water warmed to 60° C. (140° F.). Filter into a watch glass. Stain for not more than a minute. Dehydrate in alcohol which will remove much of the stain, clarify in oil of cloves or origanum oil. Mount in balsam.
Another method is to stain for about ten minutes, and then leave for a minute in Gram’s iodide solution. The sections are then washed in alcohol, dehydrated, clarified in oil of cloves, and mounted in balsam. By these methods the stain is withdrawn except from certain elements, e.g., those undergoing colloid or calcareous degeneration.
A quarter per cent. watery solution is sometimes employed as it stains nucleoli and actively dividing nuclei very brightly, while the rest of the cell is stained faintly. It may be employed to study karyokinesis in the cells of a rapidly growing cancer.
Ehrlich-Biondi stain.—This stain has been much employed for staining specimens of blood, for studying karyokinesis, and for investigations on the supposed parasitic bodies found in cancer cells.
It is prepared by mixing saturated aqueous solutions of the following aniline dyes, slowly and with constant agitation:—
| Solution of Orange G | 100 | parts |
| " Rubin S | 20 | " |
| " Methyl Green OO | 50 | " |
finally add
| Distilled water | 70 | " |
Filter from the copious precipitate which forms. The solution must be made up frequently as it does not keep well.
Sections may be stained rapidly for half an hour or an hour, but better results are obtained by diluting the fluid with twenty volumes of water, and staining all night. Sections should be washed in water and then passed rapidly through absolute alcohol and xylol and mounted in Canada balsam.
1. For staining nerve fibres.
Three methods (two of which are modifications of the first) are employed far more often than any others. By these methods the myelin coating is stained. Tissues must have been hardened previously for many weeks in Müller’s fluid, or some other bichromate solution. They are then overstained in a solution of hæmatoxyline, and the section treated with a suitable bleaching reagent, when the colour is discharged from all the tissue elements except the nerve fibres. This method displays not merely the nerve fibres in the white matter, but also the fine network in the grey matter of the brain and spinal cord. Degenerated fibres are left unstained and so degenerated tracts shew up as unstained spots on a dark background. The sections may be subsequently stained with alum carmine or eosine to shew the cells and neuroglia.
Weigert’s method.—The piece of cord to be cut after prolonged hardening in Müller’s fluid is transferred without washing to absolute alcohol and dehydrated preparatory to embedding in celloidin (p. 30). When sections are cut they are transferred at once to Weigert’s hæmatoxyline solution:—
| Hæmatoxyline | 1 | 4 | grains. |
| Alcohol | 10 | 45 | minims. |
| Distilled water | 100 | 1 | ounce. |
They are stained in this for twenty-four hours or longer, until they are quite black.
The staining will take place much more rapidly if the fluid be kept at 100° F. in the incubator. After staining they are transferred to Weigert’s differentiating solution:—
| Borax | 2 | 160 | grs. |
| Potassium ferricyanide | 2·5 | 200 | grs. |
| Distilled water | 100 | 1 | pint. |
They are left in this solution for several hours, until the ground work becomes nearly decolourised.
Sections will sometimes stain more satisfactorily if they are treated, according to Weigert’s original directions, for a few hours with a half saturated solution of acetate of copper. If the hardening in Müller’s fluid has been sufficiently prolonged, this step is usually superfluous.
Pal’s modification of Weigert’s method.—By this method quicker and more complete decolouration of the neuroglia, nerve cells, &c., is obtained. Sections are prepared in exactly the same way as in Weigert’s method and then transferred to Weigert’s hæmatoxyline. Pal recommends that this solution be diluted to half the strength and a few drops of a saturated solution of lithium carbonate added. The writer finds the results equally good if the ordinary Weigert solution be employed.
When the sections have been thoroughly stained they are washed in distilled water and placed in a three-quarter per cent. solution of permanganate of potassium. The time required in this solution depends on the time the specimen has been in Müller’s fluid. It should not be less than half a minute, and in very thoroughly hardened specimens, five minutes may be allowed with advantage. In this solution the sections will become of an opaque brown colour. They are washed in distilled water, and transferred to:—
Pal’s differentiating solution.
| Potassium sulphite | 1 | grm. | 40 | gr. |
| Oxalic acid | 1 | grm. | 40 | gr. |
| Distilled water | 200 | cc. | 1 | pint. |
They are kept in this for one to five minutes, according to the depth of staining, until the white and grey matter are clearly defined and the brown colour is completely discharged. If the brown stain does not readily clear up, the section should be returned to the permanganate solution for about half a minute, and again treated with “Pal’s solution.” This manœuvre may be repeated several times. As soon as the sections are thoroughly differentiated they are transferred one by one to a large vessel of water and thoroughly washed. The blue stain of the hæmatoxyline becomes brighter during the washing process. The sections may be mounted at once, but more beautiful results will be obtained if they are stained in alum carmine for 24 hours. They should then be washed, dehydrated in alcohol, clarified in oil of bergamot, and mounted in Canada balsam.
The very prolonged hardening in Müller’s fluid which is a necessary preliminary for this method led to the introduction of:—
Schäfer’s modification of Pal’s method.—In this method hardening in Müller’s fluid for three or four weeks is sufficient. The sections are made exactly as in the previous method, and transferred to Marchi’s fluid (p. 24) for six hours. They are washed and stained all night in the following:—
| Hæmatoxylin | 1 | 4 | grs. |
| Alcohol | 10 | 45 | min. |
| Acetic acid (2 per cent. aqueous solution) | 100 | 1 | oz. |
The subsequent processes of differentiation, bleaching, &c., are exactly the same as in Pal’s method.
Osmic acid.—Employed with fresh and also with hardened specimens to demonstrate the medullary sheath. Much the best results are obtained with the former. The nerves, or small pieces of the central nervous system are placed in half to one per cent. solution of osmic acid as soon as possible after death and kept in the dark for about a week. The tissue must be very thoroughly washed in running water to remove all traces of osmic acid, and then stained for a couple of days in borax carmine to demonstrate the nuclei and axis cylinders. Sections may be made in gum, or the tissue may be teased with needles and then mounted in Farrant. Embedding in celloidin, and mounting in balsam are inadvisable, because the ether tends to dissolve out myelin, and the clarifying oil to render it too transparent.
2. Intra-muscular ramifications of nerves:—
Sihler’s chloral hæmatoxyline method.—This method reveals the intra-muscular nerve-endings, and also brings into prominence the curious “muscle spindles” which Sherrington has shown to be connected with the posterior nerve roots, and which are believed by some to be the end organs subserving muscular sense.
A piece of muscle is taken as soon as possible after death, or from an amputated limb, and slices cut about one-tenth of an inch thick with the freezing microtome. Transfer for twenty-four hours to the following solution:—
| Acetic acid | 1 | part. |
| Glycerine | 1 | " |
| One per cent. aqueous solution of chloral hydrate | 6 | parts. |
The tissues swell up in this fluid and become translucent and gelatinous in appearance. They are now placed in pure glycerine until saturated as shown by their sinking to the bottom of the dish. This usually takes several days. They may now be stained in the following solution:—
| Ehrlich’s hæmatoxyline (p. 70) | 1 | part. |
| Glycerine | 1 | " |
| One per cent. aqueous solution of chloral hydrate | 6 | parts. |
They may be left in this from three days to a week with little fear of overstaining. Portions may then be teased with needles, and mounted in glycerine, or the stained tissue may be pressed out into a sufficiently thin layer by squeezing it forcibly between two glass slides.
Motor and sensory nerve endings.—These are best stained by the chloride of gold method (p. 82).
Specimens must be taken from the body immediately after death. The method is therefore useless for the post-mortem room, but may be used for tissues removed by operation. Small pieces of tissue must be employed and must be stained in bulk, sections being made subsequently.
For motor nerve endings the muscle of a frog or human muscle from a limb just amputated may be taken. Specimens should be prepared after staining by teasing in preference to making sections. Mount in Farrant.
Sensory nerve endings may be conveniently studied in the cornea of a recently killed frog or rabbit, or in a freshly extirpated human eye. Tactile end organs may be studied in the lip or finger tips, taste buds in the papilla foliata of the rabbit’s tongue, and Pacini’s corpuscles are well seen in the mesentery of a thin cat.
3. Staining nerve cells.
Bevan Lewis’s aniline blue-black method:—This method is the best for demonstrating the wealth of nerve cells in the fresh cerebral cortex. The solution of aniline blue-black should be of the strength of 1 in 400, about a grain to the ounce. A piece of the cerebral cortex with pia mater attached, should be removed as soon as possible after death by parallel cuts about 18 inch apart, and perpendicular to the surface of the convolution, placed on the plate of the freezing microtome and just frozen—not too hard or the tissue will be brittle and will also injure the edge of the razor. As soon as a good section is obtained the razor should be plunged into a large bowl of cold water to detach the section, which is at once floated on a glass slide, and osmic acid solution, 14 per cent. allowed to flow over it from a pipette. This will fix the tissue elements in about two minutes. The section is again floated off into the bowl of water and thoroughly washed to free it from the osmic acid. It is then stained either on the slide, or in a watch-glass, with the aniline blue-black solution for an hour in the cold, or half-an-hour if the solution is slightly warmed. The dye is thoroughly washed away with distilled water, excess of moisture wiped off the slide with blotting paper, and the section allowed to dry under a glass bell jar. It is not practicable to dehydrate by means of alcohol as it would cause sudden shrinking of the tissues. When the section is dry a drop of Canada balsam is applied and it is covered with alcohol in the usual way. The nerve cells and their processes are stained a deep slate colour, as are the nuclei of the connective tissue cells, while the ground work of the neuroglia is faintly stained and of a neutral grey tint. This method gives beautiful results both with normal and morbid specimens.
Various other aniline dyes, indulin, methylene blue, gentian violet, have been employed in the same way, but none of them give such good or uniform results as aniline blue-black.
Hardened specimens may also be stained with aniline blue-black, but the results are not to be compared with those obtained by the fresh method. The stain is usually diffuse, but this can be improved by placing the sections for 12 –2 minutes in a 2 per cent. solution of chloral hydrate in distilled water after staining.
Golgi’s metallic stains for nerve cells.
Golgi introduced the methods of producing a metallic deposit of mercury or silver in the nerve cell, revealing both the cell and its processes. This method has been very fruitful in discoveries, especially in the hands of Ramon y Cajal, Köllicher, Van Gehucten and others. It gives best results with embryonic tissues. To ensure good results it is important that the tissue be removed immediately after death. Sections of brains removed some hours after death usually give disappointing results.
There are several methods now in vogue, all slight modifications of Golgi’s original methods.
Silver nitrate method.—Small pieces not more than a quarter of an inch cube, are transferred straight from the body to a large quantity of Marchi’s fluid (p. 24) and kept in it for about a week, or longer in the case of adult specimens. On removal from Marchi’s solution the tissue should be washed for a few seconds in distilled water, and then placed in a large quantity of a 34 per cent. solution of nitrate of silver solution in distilled water for at least a week. The lump of tissue becomes of a brick red colour owing to a coating of silver chromate. On removal from the silver solution the tissue should be washed in methylated spirit for a few minutes and the incrustation of silver chromate brushed off. Sections may be cut in gum and celloidin; or they may be fixed on a cork with celloidin or spirit varnish and cut without embedding: very thin sections are not required. Dehydrate in alcohol, clear in xylol, and mount in balsam. Goodall advises a mixture of pyridine and xylol for clearing, and mounts in strong xylol-dammar solution, without a cover-glass.
Very careful attention to details and much practice are required before uniformly good results can be obtained. The results are extremely beautiful and well repay the labour expended on them. The cells and their processes appear black on a yellowish ground.
A method has been employed for deepening the colour of the stain, but the writer has no experience of it. Kallus (Zeitsch. f. Wiss. Mikr., 1893, 477) dilutes an ordinary hydrokinone developing solution (prepared as for developing an ordinary photographic plate) with about ten times its volume of distilled water. Just before using a third part of absolute alcohol is added. Sections which have been through the silver process when placed in it become grey or black in a few minutes, and, after washing in methylated spirit, are transferred to a 20 per cent. aqueous solution of hyposulphite of soda for a couple of minutes and then washed very thoroughly in distilled water for twenty-four hours. They are then dehydrated and mounted in balsam.
Buckley’s modification of the silver method.—Described in Brain, Winter number, 1895.
The method is applicable to specimens that have been hardened in Müller’s fluid. Thin slices are cut in the usual way, and then immersed in
| Bichromate of potassium, 3 per cent. solution | 5 | parts |
| Osmic acid, 1 per cent. solution | 1 | part |
for three to five days. Excess of bichromate is removed from the sections by blotting paper, and they are transferred to the freshly prepared staining mixture:—
| Phospho-molybdic acid (10 per cent.) | 1 | minim | 2 | drops. |
| Nitrate of silver (1 per cent.) | 1 | ounce | 60 | c.c. |
which must not be filtered. Stain for several days.
The sections should be cut at once after removal from the staining solution. It is claimed that the minute details of structure of the cell processes are better shewn by this method.
Corrosive sublimate method.—This method is similar in its mode of action to the last, mercury being deposited in the cell instead of silver. It is rather less certain and requires more practice. It seldom stains uniformly. One cell will be found exquisitely stained while those in its vicinity are unaffected.
Small pieces of cortex are hardened for several weeks in Müller’s fluid, or other bichromate solution, and are then transferred direct to a one-half per cent. aqueous solution of corrosive sublimate, in which they should be left from three to six weeks. Shorter periods will only give disappointing and inconstant results. Sections should be cut, if possible, in gum. They may be mounted in Farrant, or dehydrated and mounted in balsam. Tal has proposed to render the effect sharper by transforming the deposit of mercury into mercuric sulphide, by treating the sections with a solution of sulphide of sodium, which he prepares by saturating a ten per cent. solution of caustic soda with sulphuretted hydrogen and then adding an equal quantity of fresh soda solution. They are stained in this for a few minutes and then thoroughly washed.
By this method the pyramidal cells and their delicate processes appear as black opaque objects on a light ground. The neuroglia cells with their fine delicate processes are often also beautifully stained.
Nissl’s aniline method.—This method is complementary to Golgi’s method. The latter impregnates the cell rendering it opaque and shewing its form with great definiteness.
Nissl’s method stains the protoplasm without greatly reducing its transparency and allows us to study details of cell structure. Small portions of tissue, removed as soon as possible after death, are hardened in alcohol. Sections are then cut, preferably in gum, as celloidin is inconvenient owing to its staining so deeply with aniline dyes.
Sections are transferred from alcohol to a one-half per cent. aqueous solution of methylene blue, which is heated in a watch glass till it steams freely, but short of the boiling point. Stain for about a quarter of an hour and allow to cool. Transfer the sections to a mixture containing one part of aniline oil and ten of absolute alcohol, and move them about till no more colour comes away. Transfer the section to a slide with a section lifter, drain, and dry well by pressing folded filter paper carefully on the section. Allow some origanum oil to flow over the section and remove excess of this by pressure with blotting paper. Moisten with benzine,1 and add a drop of colophonium resin dissolved in benzine. The slide is warmed cautiously till the benzine is driven off and the colophonium liquefied by heat alone, and then the cover-glass is applied.
Magenta and other aniline dyes may also be employed in a similar manner.
It is impossible, within the limits of this work, to attempt any adequate description of the modern methods of bacteriological investigation. Some of these are very lengthy and complicated, and require much skill and practice before good results can be relied on. But those who do not desire to make a special study of bacteriology may often require to examine for the presence of organisms in sections, or in various excretions, and it is hoped that they may find the following short description of special methods sufficient for their purpose. For more elaborate work they must consult one of the many excellent textbooks on the subject.
The student should provide himself with the following dyes in powder:—
Methylene blue.
Gentian violet.
Methyl violet.
Fuchsine.
Bismarck brown.
The following solutions of these dyes are used:—
1. Saturated alcoholic solutions which may be kept in stoppered bottles.
2. One per cent. aqueous solutions. These must be freshly made each time of using.
In filtering either alcoholic or aqueous solutions it is well to moisten the filter paper beforehand with alcohol or water as the case may be.
The following special solutions will also be wanted:—
Löffler’s methylene blue.—In this solution a weak solution of caustic potash is employed as a mordant:—
| Saturated alcoholic solution of methylene blue | 3 | volumes. |
| Caustic potash, aqueous solution 1 : 10,000 | 10 | volumes. |
Filter.
This solution is perhaps the most generally useful stain. It colours most bacilli and micrococci, and while rapid in its action rarely overstains. It must be made up fresh on each occasion. It is the best counterstain after staining tubercle bacilli, &c., with fuchsine.
Ziehl’s carbol-fuchsine.
| Carbolic acid (5 per cent. aqueous solution) | 100 | volumes. |
| Fuchsine (saturated alcoholic solution) | 11 | volumes. |
The solution must be filtered immediately before being used.
Gram’s iodine solution.—Sections are placed in this solution after being stained with aniline dyes. The iodine in some way fixes the dye in the organisms, so that they are not decolourised along with the rest of the tissues.
It is made thus:—
| Iodine | 1 | grm. | 1 12 | grains. |
| Iodide of potassium | 2 | grms. | 3 | grains. |
| Distilled water | 300 | c.c. | 1 | ounce. |
Ten per cent. aqueous solutions of nitric and sulphuric acids should be prepared and may be kept indefinitely.
The following are the general methods of employing these reagents for the purpose of staining organisms in sections. Special methods are required for special organisms, but one or two only can be given.
Weigert’s method.—The sections must be placed in a freshly made one per cent. aqueous solution of methyl violet, gentian violet, fuchsine, &c. The solution may be kept at the temperature of the body in an incubator. The organisms will often stain more readily if the section be passed through a 1 in 2000 solution of corrosive sublimate before putting it into the staining fluid. After staining the section is washed in distilled water and then in methylated spirit until it appears almost decolourised. Some prefer to decolourise the tissues by washing in a half per cent. solution of acetic acid instead of methylated spirit. Practice is required before the correct time for decolourising is accurately estimated. The beginner should float a section rapidly on the slide now and then, put on a cover-glass and examine it under a low power to see if the decoloration has been carried far enough. A contrast stain may then be used, such as picrocarmine, after which the section may be mounted in Farrant’s medium: or a weak solution of another aniline colour may be used as a counter stain, after which the section is clarified in xylol, and mounted in balsam dissolved in xylol.
Gram’s method.—Place some aniline oil in a test tube and add ten times its volume of distilled water. Close the end with the thumb and shake very thoroughly. Filter ninety drops into another clean test tube, and add ten drops of a saturated solution of gentian violet or some similar dye. Filter the mixture into a watch glass. Stain sections in it for from three minutes to half-an-hour according to the temperature,—the shorter time for the incubator at 100°, the longer when the sections are stained at the ordinary temperature of the room. Wash in distilled water, and transfer to Gram’s iodine solution until they become black, usually in a few minutes. They are then decolourised in absolute alcohol. This often takes some time. It may be hastened, as Crookshank suggests, by placing the section in clove oil, returning to alcohol, and so on.
Ehrlich’s modification of Gram’s method. The contrast stain is here used first.
Stain the section (e.g., that of a mitral valve in a case of ulcerative endocarditis), in an alcoholic solution of eosine (1 in 1500). Transfer to a solution of some aniline dye, such as gentian violet, dissolved in aniline oil water, exactly as in Gram’s method. The section floats on the surface and spreads out, owing to the alcohol diffusing out. Stain for about twenty minutes. Wash the section in water, and float out (p. 55) on a glass slide. Allow the water to drain off and add Gram’s iodine slowly from a pipette so as not to disarrange the section. When the section has become quite black pour off the Gram’s solution. Remove all superfluous fluid from the slide with blotting paper, and dry the section by carefully and firmly pressing on it a folded piece of blotting paper. If this is done with care the section need not be injured in the least. Decoloration is effected on the slide with aniline oil, instead of alcohol as in the preceding method. The slide is rocked about so that the colour may be evenly discharged by the aniline. When no more colour comes away, the aniline oil is poured off, the section clarified in xylol, and mounted in Canada balsam.
As soon as the section is decolourised it may be treated with a contrast stain, the most suitable being alcoholic solutions of eosine or Bismarck brown if a blue stain has been employed, or methylene blue if fuchsine has been the first stain used.
The following will be found the most useful stains and contrast stains:—
| Stains. | Contrast Stains. | ||
| Gentian violet. Methyl violet. Methylene blue. | | Picrocarmine. Eosine. Bismarck brown. Safranine. | |
| Magenta. Fuchsine. | Methylene blue. | ||
| And vice versâ. | |||
Much practice is required in using either of the methods before one can judge accurately how long to leave sections in the staining reagents or decolourising agents, and the beginner must not be discouraged if at first he is unable to obtain good results although he follows the book directions most minutely.
Ehrlich method for tubercle bacilli.—Sections are stained for six to twenty-four hours in a one per cent. solution of gentian violet, methyl violet, methyl blue or fuchsine. They will stain more rapidly if the staining fluid be kept in an incubator at the body temperature. They should be removed from the staining fluid, and washed in distilled water, and then transferred (preferably on a glass section lifter) to a ten per cent. solution of nitric acid in distilled water until they are nearly decolourised. They should then be very thoroughly washed in distilled water. They may then be treated with some suitable contrast stain and mounted in Canada balsam.
Neelsen’s stain for tubercle bacilli.—Sections are placed in Ziehl’s carbol-fuchsine solution (p. 103) which should be warmed for ten minutes to half-an-hour. They are then decolourised in a solution of sulphuric acid. Twenty-five per cent. is the strength originally recommended, but a ten per cent. solution does equally well and injures the section less. They are then very thoroughly washed in a large quantity of water, and afterwards may be treated with a contrast stain.
Gibbes’ double stain for tubercle bacilli.—
| (1) | Rosaniline hydrochlorate | 2 | grms. | 25 | grs. |
| Methyl blue | 1 | grm. | 12·5 | grs. |
Triturate in a glass mortar,
| (2) | Aniline oil | 3 | c.c. | 37·5 | grs. |
| Rectified spirit | 15 | c.c. | 3 12 | drms. |
Dissolve and add slowly to (1).
| (3) | Lastly add slowly to the mixture | ||||
| Distilled water | 15 | c.c. | 3 12 | drms. | |
Some of the solution is filtered into a watch glass and warmed. The sections are placed in it and left for some hours. They are then washed in methylated spirit till they are sufficiently decolourised, and then rapidly passed through absolute alcohol and oil of cloves and mounted in balsam and xylol. It is a very useful stain for examining the sputum for tubercle bacilli.
In order to stain fluids, such as blood, pus, or sputum, for organisms, a very thin layer should be obtained by placing a little of the fluid between two clean cover-glasses and pressing them together. They are then separated and allowed to dry. The film is fixed by holding the cover-glass in a pair of forceps, and passing it slowly through the flame of a spirit lamp two or three times. Films of pus should be ‘cleared’ after fixing by placing them in a twenty per cent. solution of acetic acid for three minutes.
For clinical purposes it is often necessary to examine urine, fæces or vomited matter for bacilli. Films are prepared in the usual way and allowed to evaporate slowly, and then fixed by passing through the flame, and then washed in distilled water before staining. In the case of vomited matter and fæces this is usually done without difficulty. In the case of urine however it is often difficult to get the urine to evaporate completely. A syrupy layer remains, and if more heat be applied it decomposes and chars, and the products cause precipitation of aniline during subsequent staining processes. This may be partly avoided by gently washing the film in distilled water before staining.
Another plan is to mix the urinary deposit with a little gelatine free from organisms, such as that in unused culture tubes. The gelatin is liquefied by heat, and mixed with the deposit. Films are made from this mixture, and allowed to set, and then thoroughly washed in distilled water. The film is then dried thoroughly, and the cover-glass laid flat with the film uppermost, and a few drops of the staining fluid filtered on to it. After it has been stained sufficiently the stain is drained off, and the slip gently washed. The film may then be stained with some contrast stain in exactly the same way as sections, again washed, dried between folds of blotting paper, and mounted in balsam.
It is sometimes difficult to tell which is the side of the cover-glass which bears the film. This is readily done by holding the glass obliquely so that light from a window is reflected from its surface. The side which is coated appears dull; while the other is smooth and bright.
In all these methods blood is obtained by pricking the skin of one of the fingers, or the lobule of the ear, preferably the latter. The skin must previously be washed with soap and water or ether, to remove any grease or epithelial scales. The puncture should be made firmly so that blood may escape freely. The finger or ear must not be squeezed. Specimens must be made rapidly before red corpuscles have run into rouleaux. The slides and coverslips employed must be scrupulously clean, or it is impossible to get really good films. They should be cleaned with nitric acid and alcohol according to the directions on page 57.
Fresh specimens should be examined. The coverslip is made just to touch the drop of blood at one edge, so as to transfer a small quantity only, and is at once lowered on to the slide with the aid of a mounted needle. If slide and coverslip be perfectly clean the blood will spread out into a thin film, the corpuscles lying quite flat. If there be any delay, or if the cover-glass be not quite clean the red corpuscles will run into masses and the specimen will be useless for minute examination. Another specimen may be mixed with a little of Ferrier’s solution (p. 129) before mounting. Permanent coverslip films may also be prepared.
Here again the use of absolutely clean coverslips is essential, and the blood must be taken immediately it escapes from the puncture. A little blood is taken on a cover-glass which is held horizontally. Another cover-glass is lowered on to this and by its weight and by capillary attraction, the drop of blood quickly becomes transformed into a thin film. The two covers are separated as soon as the film is formed by rapidly sliding them off one another. This manœuvre requires a little practice and dexterity. The movement of the slips must be in an exactly parallel direction otherwise the coating left will be uneven, just as when two pieces of bread and butter are pulled apart. Even with practice it is difficult to get more than one good film, the lower being usually best. There are four ways of fixing the film.
1. Exposure to osmic acid vapour.
The film while still moist is held over the mouth of a bottle containing at least one per cent. solution of osmic acid. In a minute or two the fixation will be complete, and the film becomes of a dirty brown colour. It is then left exposed to the air to get rid of all traces of osmic acid, and may afterwards be stained as described below.
2. Treatment with saturated aqueous solution of corrosive sublimate (Muir’s method).
The cover-glass on which the film has been spread, is floated before the latter has time to dry, film downwards on a saturated solution of corrosive sublimate in a watch glass for half an hour. The cover-glass is placed in distilled water and then in alcohol to remove excess of corrosive sublimate, and then stained. A little care is required when washing the film to prevent it sliding bodily off the cover-glass.
3. By drying and passing rapidly through the flame of a Bunsen burner, exactly as in preparing specimens of sputum, &c. (p. 111). This method is handy for ordinary clinical purposes.
4. By keeping the coverslips at a temperature of about 200° F. (Ehrlich’s method).
Ehrlich uses for this purpose a strip of copper about two inches wide and a foot long which is supported on a retort stand in a horizontal position. One end is heated by a Bunsen’s burner beneath. The point in the copper strip at which the temperature is at boiling point is readily ascertained by dropping a little water on. The point at which a drop of water assumes the spherical state indicates a temperature there of 212° F. The coverslips are placed an inch or two further than this point, and kept there at a temperature of about 200° F. for some hours.
Fresh blood may be stained by mixing with Ferrier’s fuchsine solution:—
| Fuchsine | 1 | grm. |
| Distilled water | 150 | c.c. |
Dissolve and add
| Alcohol (80 per cent.) | 50 | c.c. |
| Neutral glycerine | 200 | c.c. |
A spot of this solution is mixed with the blood on a slide by means of a mounted needle, and covered with a clean cover-glass. The red corpuscles are slightly stained, while the nuclei of the white corpuscles are stained a bright crimson, and the “blood plates” a deep pink colour.
Stained preparations may also be obtained by using Toison’s fluid, which serves also for diluting the blood in order to determine the exact number of red and white corpuscles present by means of Gowers’ or the Thoma-Zeiss hæmocytometer. It is prepared thus:—
| Glycerine | 30 | c.c. | 1 | oz. |
| Sodium sulphate | 8 | grms. | 2 | drms. |
| Sodium chloride | 1 | grm. | 15 | grs. |
| Methyl violet | ·25 | grm. | 4 | grs. |
| Distilled water | 160 | c.c. | 5 | oz. |
It stains the nuclei and blood plates, but does not alter the shape of the red cells. It requires to be made up fresh occasionally as torulæ are apt to form and multiply in it.
Dried films may be stained with hæmatoxyline, picrocarmine, or any of the general stains. The nuclei of the leucocytes may be stained rapidly in a couple of minutes in a one per cent. solution of methyl violet, washing in water, drying between blotting paper and mounting in balsam. The best method for general purposes is to stain with a saturated aqueous solution of methyl blue for half an hour or longer. Wash in water, and then stain for ten minutes in a half saturated aqueous solution of eosine. In this way the eosinophile granules of the leucocytes and the red corpuscles, are stained by the eosine, while the nuclei of the leucocytes are stained by the methyl blue.
Kanthack and Drysdale recommend that the film should first be stained with a half per cent. solution of eosine in 50 per cent. alcohol, then washed, dried and fixed in the flame, and stained for a short time in Löffler’s solution of methylene blue (p. 104).
These films may be stained for micro-organisms in the way described for cover-glass preparations (p. 112).
Injection of blood vessels may be performed on small animals, or on individual human organs after removal from the body. The object is to fill the vessels with a coloured fluid which will solidify afterwards. It is possible in the same organ to inject the arteries with a red medium, the veins blue, and secretory ducts, such as bile ducts, yellow or blue.
The most convenient basis for an injection mass is gelatine, as its solutions liquefy at a temperature of about 100° F., and solidify a little below that point, and when solidified cut readily, and do not tend to become brittle. The various masses are prepared as follows:—
Red injection mass (Woodhead’s formula) consists of gelatine softened by mixture with water and coloured by carmine.
| (1) | Carmine | 4 | grms. |
| Liq. ammoniæ B.P. | 8 | grms. | |
| Distilled water | 150 | c.c. |
Dissolve the carmine in the ammonia in a mortar. Pour on the water. Mix thoroughly and filter.
| (2) | Gelatine | 10 | grms. |
| Distilled water | 50 | c.c. |
Allow it to stand in the cold water until the water is absorbed and the gelatine has become soft.
Warm (1) almost to boiling point over a Bunsen burner, and add the gelatine slowly. Stir thoroughly and add a ten per cent. solution of acetic acid until the solution becomes slightly acid. This will be shewn by the mass assuming a darker and duller colour. A little salicylic acid may be added to preserve it.
Blue injection mass.—To the gelatine mass (2) prepared as above, and liquefied by heat, add instead of carmine
| Soluble Prussian blue | 5 | grms. |
| Distilled water | 60 | c.c. |
Every trace of alkali must be kept away from the mass during and after the preparation. Sections of injected organs should be mounted in Farrant’s solution slightly acidulated with formic or acetic acid. With every care, however, the blue colour is apt to fade in the course of time.
Green injection mass. Robin’s formula (modified).
| (1) | Arseniate of soda (sat. sol.) | 80 | c.c. |
| Glycerine | 50 | " | |
| (2) | Sulphate of copper (sat. sol.) | 40 | " |
| Glycerine | 50 | " |
Mix and add one part to three parts of the gelatine mass made as for the red and blue injections.
Method of injection.—In injecting the vessels of tissues it is necessary that the organ or the entire animal, as the case may be, shall be kept during injection at a temperature well above that at which the gelatine mass will melt, otherwise the gelatine will “set” in the arteries and will never reach the capillaries. This warming is effected by immersing the animal in a water bath. The liquefied gelatine is forced into the artery by a syringe or by air pressure. It is essential that the pressure be uniform and steady. This is so much more easily managed with air pressure that this method is strongly recommended to the beginner. But, whatever method be adopted, perfect results can only be obtained with certainty after long practice. Sometimes too high pressure will be employed and the vessels give way, at others the injection may not reach the capillaries at all. The most scrupulous attention to details is essential.
By far the most effective apparatus for injecting is the modification of Ludwig’s constant pressure apparatus devised by Fearnley.2 Although the apparatus appears complicated, the various parts are easily obtained and it would be easy to improvise a substitute for the water bath.
The apparatus which is shewn in figures 10 and 11 consists of a bath deep enough to contain the animal, and a vessel containing the injection fluid. The bath is kept at a temperature of about 110° by an ordinary Bunsen burner. A large Wolff’s bottle (20–40 oz.) with three necks, is fitted with three india-rubber stoppers perforated by glass tubes. Through the central stopper a glass tube connected by a rubber tube, with an ordinary Higginson’s syringe, passes almost to the bottom of the bottle. From one of the other necks a rubber tube passes to an ordinary mercurial manometer, while from the third a tube passes to the flask containing the liquefied injection mass, which is immersed in the water bath. This flask is also firmly stoppered, and should be about half filled with injection material. The delivery tube from the large Wolff’s bottle should only just come through the cork. Another glass tube passes down almost to the bottom of the flask, and is connected by a rubber tube with the cannula inserted into the artery. It will be evident from figure 11 that when water is pumped by the Higginson’s syringe into the Wolff’s bottle the pressure there will be raised (as indicated by the manometer). This increase of pressure will equally affect the air inside the bottle containing the injection fluid, and the fluid will be forced out along the tube and through the cannula into the artery.
Before using the apparatus a clamp should be placed on the exit tube of the vessel containing the injection fluid, and the pressure should be raised to see that the apparatus is everywhere air-tight. Any leaks should be sealed before the actual injection is commenced.
If an isolated organ is to be injected, a cannula of glass or brass should first be inserted into the artery and securely tied in position. The organs, if cold, must be soaked in water at 120° F. for about half an hour and then transferred to a water bath.
In the case of injecting an entire animal, such as a rabbit, rat, or guinea pig, the injection is best made a few minutes after death. The animal may be chloroformed, and then bled to death by opening a large vein. As soon as death has occurred incise the skin over the thorax in the middle line. Cut through the costal cartilages to the right of the sternum, and through the junction of the manubrium and body of the sternum. These incisions being for most part through non-vascular parts will not lead to escape of fluid during injection. The sternum being forcibly raised towards the left, the pericardium will be exposed and must be carefully divided. An incision must be made into the left ventricle, and a cannula passed up into the aorta and firmly secured by a ligature passed round the aorta with the assistance of forceps or an aneurism needle. Any blood is cleaned away and the animal is then placed in the water bath for about ten minutes. The tube from the bottle containing the injection fluid is then filled by gentle pressure on the syringe, and clamped when full. Its end is then placed on the cannula and secured there by a ligature. The pressure should be raised by squeezing the syringe until the manometer registers one inch. The clamp should then be removed and the injection commenced. The pressure should be raised very gently and constantly by working the syringe, and the condition of the gums, lips, and eyes of the animal observed. The gums will soon shew a pink tinge. The best indications are obtained by watching the effect on the small vessels of the sclerotic. When these are completely filled, which will be in about five to ten minutes according to the rate at which the air pressure has been increased, the injection may be stopped. This result will be obtained, under good conditions, before the manometer indicates a pressure of five inches. The aorta should now be ligatured, and the animal placed in cold water frequently renewed until it is thoroughly cooled. The organs may then be removed and placed in methylated spirit and hardened. Sections are afterwards cut and mounted in the usual way.
Normal histology.—It cannot be too strongly impressed on the beginner that a thorough mastery of the normal appearances of tissues and organs is absolutely necessary before attempting to make an accurate study of morbid changes in them. He should not be satisfied with examining one specimen of an organ but as many as he conveniently can, in order to be fully acquainted with the many deviations from normal which may exist without actual disease. He should therefore obtain several animals, such as small dogs, cats, rabbits, frogs, &c., and remove their organs with all care, and harden them in the various appropriate fluids. He should also obtain specimens of normal human organs from the post-mortem room. Many normal tissues (skin, muscle, tendon, bone, &c.), can also be prepared from a limb amputated for an accident to a healthy patient. By preparing specimens in this way he will not only become the possessor of a set of slides illustrating normal histology, but will find also that he has acquired that proficiency in hardening and staining the specimens which practice alone can give.
The following account of the method of preparing different tissues is merely intended to indicate the lines on which the beginner should proceed. After some practice he will be quite able to select the modes of hardening and staining which special circumstances or cases may seem to demand.
The first part of these directions will refer to the preparation of normal tissues, the second part to morbid histology.
Blood.—For special methods of examination see Chapter VII.
Blood crystals—Hæmoglobin crystals, obtained from the blood of an animal, or enough may be collected at any operation. A little water or a little ether is added to the blood which is allowed to stand for half-an-hour after which a drop is allowed to evaporate slowly on a clean slide.
Hæmatin crystals.—The student should make himself thoroughly familiar with these, as their presence affords positive proof of the existence of blood colouring matter in a stain.
To obtain them a drop of blood should be allowed to dry on a slide. The dried blood is scraped into a little heap with a small piece of clean glass, and a drop of glacial acetic acid added. As it evaporates minute reddish-brown acicular crystals will appear.
Hæmatoidin crystals.—Obtained from the site of a bruise, or an old hæmorrhage, e.g., a cerebral apoplexy or a hæmatocele.
Simple squamous epithelium.—(Endothelium). Carefully strip off the lining of the parietal pericardium or parietal pleura, of a recently killed animal, or spread out its omentum on a piece of cork, and (1) stain the intercellular cement with nitrate of silver (p. 82) so as to reveal the outlines of the cells. (2) Stain other specimens with hæmatoxyline or alum carmine to reveal the nuclei.
Stratified squamous epithelium.—Specimens from skin of various parts, finger, groin, lip, tongue should be prepared. Harden in Müller’s fluid.
Transitional epithelium.—Occurs in the pelvis of the kidney, ureter and bladder. It is very readily detached, especially if not hardened immediately after death. Remove as early as possible. If the bladder is taken it should be cut open and pinned out as flat as possible. Harden in osmic acid, or Müller’s fluid and spirit. Embed preferably in celloidin.
Simple columnar epithelium.—Occurs in many parts. It may be studied in the salivary ducts, the intestine, kidney, &c., of any mammal.
Goblet-cells.—Seen abundantly among the columnar cells of the intestinal glands, and in the mucous glands of the mouth and of the cervix uteri.
Stratified columnar epithelium.—Occurs only in the urethra. Harden the penis of a cat in Müller’s fluid, and cut transverse sections.
Ciliated epithelium.—Harden the trachea of a recently killed cat in osmic acid or Müller’s fluid. Beautiful specimens may also be obtained from an ordinary nasal polypus, which should be put into hardening fluid immediately after removal.
Stain all sections of epithelium in picrocarmine, and in eosine and hæmatoxyline.
Ordinary areolar tissue.—Difficult to obtain free from fat. It may be studied in the subcutaneous tissue of the section of the cat’s penis already made. A fragment of the tissue should also be removed and carefully teased in a drop of picrocarmine. Areolar tissue may also be studied in sections of skin, and in the capsules of the different internal organs.
Elastic tissue.—May also be studied in most sections of skin. If the ligamentum nuchæ of a large quadruped (horse, bullock), &c., is available it yields the best specimens, or the human ligamenta subflava may be examined. Pin a piece out on a piece of wood or wax. Harden in Müller’s fluid. Stain in picrocarmine. Both sections and teased specimens should be prepared.
Tendon.—Readily obtained from an amputated limb. Harden in Müller’s fluid. Make transverse and longitudinal sections. Stain with eosine and hæmatoxyline.
A preparation should also be made by teasing a little of the fresh tendon in normal salt solution, and staining with picrocarmine.
Retiform or lymphadenoid tissue.—Seen in lymphatic glands and in the lymphoid follicles scattered along the sub-mucous coat of the alimentary canal.
Prepare sections in the ordinary way. Stain in eosine and hæmatoxyline or in picrocarmine.
Some sections should also be prepared by pencilling (i.e., dabbing with a camel’s hair brush) or by shaking sections up in a test tube with water or normal salt solution. By this means the leucocytes are removed, and the structure of the adenoid tissue itself becomes more evident.
Fat.—Best studied in sections of skin and subcutaneous tissue, or in the mesentery of the cat. One specimen should be stained with osmic acid and picrocarmine and mounted in Farrant’s medium, and another in eosine and hæmatoxyline and mounted in Canada balsam.
Pigment cells.—Branched cells are best studied in the living foot of the frog, where amœboid movements may be seen in them when the light falling on the retina is made to vary in intensity. Permanent preparations are most conveniently made from the pallium of the common snail. The shell is removed, and the pallium snipped out with the scissors. It is then pinned out flat, hardened for a day in methylated spirit, and mounted unstained in Farrant’s medium. They are also well seen in sections of the choroid coat of the eye.
Hyaline cartilage.—Specimens may be obtained from any joint, from the costal cartilages of young animals, or from the thyroid cartilage and tracheal rings. It may be hardened in spirit. Stain with picrocarmine, eosine and hæmatoxyline, and with methyl violet.
Elastic cartilage.—Prepared from the epiglottis, or from the cartilages of the ear, e.g., of a cat. Harden in spirit. Stain in picrocarmine or in dilute fuchsin.
White fibro-cartilage.—Obtained from intervertebral disc. Prepare and stain as for hyaline cartilage.
Bone:—
Unsoftened Bone.—Cut as thin a section as possible with a fine saw. Rub the section with the hand on a dry oil stone until it is as thin as possible. Then cement it by Canada balsam (liquefied by warming) to a piece of plate glass and continue the rubbing process with this, examining it now and then with the low power to see if it is thin enough. As soon as it is thin enough it is washed off the slide with methylated spirit, and washed to get rid of the fine bone dust. It should then be transferred to turpentine and may be mounted in balsam.
Softened bone.—Specimens may be obtained from an amputated limb or from the femur of a cat.
Specimens should be decalcified in chromic and nitric fluid, and the hardening completed in spirit. In studying the process of ossification, e.g., in the head of the humerus of a kitten, it is best to embed the specimen in celloidin before cutting sections, as the trabeculæ of bone are very delicate, and easily detached.
Very beautiful double staining effects may be obtained with either picrocarmine, or eosine and hæmatoxyline, and with eosine and methyl violet.
Bone marrow.—To obtain good sections of red bone marrow, take a piece of the clavicle or a rib, or of one of the carpal or tarsal bones. Decalcify in chromic and nitric fluid. Embed in celloidin. Stain with eosine and logwood, eosine and alum carmine, or alum carmine and picric acid. Mount in Canada balsam. The various cells present in bone marrow may also be studied by squeezing some fresh marrow from a rib, and making a cover-glass film, and preparing in exactly the same way as is directed in the case of blood films on page 116.
Tooth.—Best cut in situ from the jaw of a cat. Decalcify in chromic and nitric fluid, and cut both vertical and transverse sections. Stain in picrocarmine, or eosine and hæmatoxyline.
Developing tooth.—Extremely good specimens may be obtained from the jaw of a newly-born kitten or puppy. Sections can easily be made shewing a milk tooth and a developing permanent tooth by its side.
The enamel is dissolved by decalcifying fluids. To study it a specimen of unsoftened tooth should be made, according to the directions given for bone.
Striped muscle.—Should be studied in various animals.
The leg of an insect such as a cockroach may be hardened in osmic acid. One leg should be hardened in a straight position so as to fix the fibrils in the fully extended position, another should be bent up so as to get specimens of relaxed fibrils.
Portions of muscle should be removed, and teased on a glass slide in some staining fluid such as picrocarmine, a tenth per cent. solution of eosine or quarter per cent. of safranine.
Sections of amphibian and mammalian muscle should be prepared to show their differences in structure. The most convenient part to select is the tongue, as a view of the fibres is obtained both in longitudinal and transverse sections. Sections should be stained in eosine and hæmatoxyline which gives a beautiful effect. For special stains for intra-muscular nerve endings see page 92.
Heart muscle.—A portion should be teased fresh in picrocarmine or eosine, another portion hardened in Müller’s fluid, and sections made and stained with eosine and hæmatoxyline.
Unstriped muscle may be obtained by teasing a fresh portion of the muscular coat of the small intestine of an animal, or by sections of the hardened intestine, bladder or uterus. Stain in picrocarmine or preferably eosine and hæmatoxyline.
Nerves.—The special methods for staining nerve tissues are detailed in Chapter VI. The student must remember that the ordinary staining methods are also applicable to nervous tissues.
Nerve terminations:—
Meissner’s corpuscles.—Take the tip of an index finger immediately after amputation. Place part of it at once in chloride of gold solution, and the rest in Müller’s fluid until it is hardened.
Sections stained with chloride of gold should be mounted in Farrant’s medium. The other sections may be stained in picrocarmine or eosine and hæmatoxyline.
Pacini’s corpuscles.—May be dissected out on the smaller branches of the digital nerves, or may be found in the mesentery of the cat. The latter should be spread out on wood, hardened in Müller’s fluid, stained in hæmatoxyline, and mounted in balsam.
Other forms of tactile corpuscles may be studied in the tongues of frogs, ducks, or geese. A network of nervous fibrils should be studied in the cornea. Take the cornea of a newly killed frog or cat and stain with chloride of gold (p. 82).
The end plates in which the nerves terminate in muscle may be studied by placing specimens of living muscle of some cold blooded animal into chloride of gold solution, and staining rather deeply.
Arteries.—Take a piece of the aorta, a piece of some medium artery, as the renal or radial, and harden in Müller’s fluid. Stain in picrocarmine and always in eosine and hæmatoxyline. Arterioles are best studied in sections of the various organs. Thus they are seen in each Malpighian body of the spleen, in the boundary zone of the kidney, and so on. A longitudinal surface view can also be obtained by staining and examining the pia mater.
Veins.—Remove, harden, and stain in the same way.
Capillaries.—May be very well seen in the foot of the frog.
Stun a frog by striking its head, or by chloroforming it. Fix it on a piece of card with a V shaped notch at one end. Tie one of the hind feet by means of threads attached to its toes so that the web of the foot is gently stretched over the V. The foot can then be readily examined under a 12 inch objective. The foot must be brushed from time to time with normal salt solution to keep it moist. The movement of blood in the capillaries, &c., can then be studied for an hour or two. After death the mesentery should be spread out on a piece of wood, and hardened for a few days in Müller’s fluid.
Stain with eosine and hæmatoxyline.
Lymphatics.—Commencement of lymphatics in serous membrane. Stain a piece of cat’s omentum in nitrate of silver (p. 82) for some minutes. After washing keep in glycerine for about a week and then stain in hæmatoxyline and mount in Farrant’s medium.
Lymphatic glands.—The lymphatic glands of the neck of the cat may be used. Harden in Müller’s fluid. Stain in picrocarmine, eosine and hæmatoxyline.
Skin and sweat glands.—Sections should be made from pieces taken (a) from the sole, (b) from the skin of the body, (c) from the axilla of an adult to study the pigment. Harden in Müller’s fluid. Stain in picrocarmine or eosine and hæmatoxyline.
Hairs and sebaceous glands.—Take a portion of the scalp, or of the skin of a puppy. Harden in Müller’s fluid. Stain in eosine and hæmatoxyline, and mount others unstained.
Hairs from various parts of the body should also be soaked for some hours in liq. potassæ and mounted unstained in Farrant’s medium. They may be bleached subsequently by treatment with eau de Javelle (p. 27).
Brain and spinal cord.—Must be removed from the body with extreme care, all stretching or squeezing being avoided. Harden slowly in Müller’s fluid to which a fourth of its bulk of water may be added.
The best staining reagents to employ are eosine and hæmatoxyline, alum carmine or borax carmine, aniline blue-black, &c. Staining methods, see Chapter VI.
Eye.—Harden the eye of a recently killed bullock, cat, or other animal in formal (p. 23), puncturing the sclerotic in places to allow the hardening fluid to penetrate. In about a week make a horizontal section through the eye. The anterior half (the lens having been removed) may be satisfactorily cut in gum. Sections of the crystalline lens are not very satisfactory. The best way to get specimens of the fibres is to tease a piece of the fresh lens of a fish (e.g., a cod) in a 140 per cent. aqueous solution of eosine. Wash the eosine off the slide with 12 per cent. acetic acid, and mount in Farrant’s solution.
The posterior half of the eye should be embedded in celloidin, as otherwise it is extremely difficult to get sections of the retina in its proper relation to the other coats.
Mount some specimens unstained. Stain others with the ordinary stains.
Internal ear.—Decalcifying the temporal bone of a cat, dog, guinea pig, &c., in chromic and nitric fluid. As soon as the bone is decalcified complete the hardening of the soft parts in methylated spirit, embed in celloidin, and cut sections in the longitudinal axis of the cochlea. Owing to the extreme hardness of the bone in adults it will be found best to use the petrous bone of newly born animals.
The semi-circular canals will be most readily studied in the temporal bone of fishes, or of birds, e.g., the common fowl. They also must be cut in celloidin, and stained in the ordinary way.
Nose and olfactory epithelium.—It is difficult to obtain specimens from the human subject, but very satisfactory preparations may be made from the dog, or more conveniently in a new born puppy where the bones are still cartilaginous. Harden the latter in Müller’s fluid, decalcify adult specimens in chromic and nitric fluid. Specimens of ciliated epithelium, &c., will be obtained from the lower part, and of the special olfactory epithelium from the upper part. Stain in eosine and hæmatoxyline.
Lungs.—Carefully remove the lungs of a cat without injuring the bronchi or trachea. Introduce a cannula into the trachea and gently inflate the trachea with air. Ligature the trachea and place the lung in Müller’s fluid, a weight being attached to keep the organ submerged. Harden for about six weeks, and then make sections of the various parts.
To demonstrate the endothelium of the alveoli, inject instead of air, nitrate of silver. Allow it to remain in for half an hour, then remove it by washing, and harden in Müller’s fluid.
Beautiful casts of the alveoli, &c., may be obtained by placing a cat’s or human lung under the receiver of an air-pump, and when the air is completely exhausted, injecting fusible metal into the bronchus. The lung tissue is then removed by corrosion or by maceration. Portions of the casts should be removed, fixed in a glass cell with a spot of Canada balsam, and examined by reflected light.
Thyroid gland.—Best obtained from a young subject either human or an animal.
Harden in Müller’s fluid. Stain in picrocarmine or eosine and hæmatoxyline. Also stain sections in safranine, which stains the colloid material, and also picks out any colloid formation in the cells themselves.
Thymus.—Remove from a fœtus or a very young animal, and prepare in the usual way.
Tongue.—That of the cat or rabbit serves very well.
Ordinary transverse sections should be made, and also sections through the circumvallate papillæ in order to study the “taste buds.”
Salivary glands.—Those of a cat or dog do very well.
Sections should be made from each of the three glands.
Stomach.—That of the cat or dog should be studied. The organ must be removed immediately after death before any post-mortem digestion of the coats has occurred. The stomach should be opened, washed gently and pinned out flat, with as little stretching as possible on a piece of wood, and hardened in Müller’s fluid.
Sections should be made (a) longitudinally through the cardiac end to show the transition from the œsophageal to the gastric mucous membrane, (b) from a portion of the greater curvature, (c) longitudinally through the pyloric valve.
Eosine and hæmatoxyline form the best stain for the alimentary canal.
Intestine.—Prepare in the same way as the stomach. Make sections from (a) the upper part of the duodenum to show Brunner’s glands, (b) the ileum, (c) a Peyer’s patch, (d) the vermiform appendix, (e) the colon.
Liver.—Make an injection of one specimen with carmine and gelatin (p. 120). Harden in methylated spirit. Others should be hardened in Müller’s fluid and stained in the usual way.
Kidney, supra-renal, and pancreas.—Same preparation as for liver.
Spleen.—Harden in Müller’s fluid.
Mount one section unstained. Shake another up with water in a test tube to shew the structure of the pulp. Stain others in eosine and hæmatoxyline.
Bladder.—Must be removed and pinned out immediately after death, as otherwise the epithelium will be macerated off. Consequently it must be taken from an animal, as a cat. Harden in osmic acid. Cut in celloidin as the coats are very apt to become detached.
Penis and testis.—Readily obtained from dog, cat, or rat.
Stain with eosine and hæmatoxyline.
Uterus, ovaries, and Fallopian tubes.—May be obtained from the post-mortem room or from the lower animals. Harden in Müller’s fluid, and make sections from the cervix, the body of the uterus, the Fallopian tube, and the ovary.
Stain with eosine and hæmatoxyline.
Embryological specimens.—For systematic work special manuals should be consulted.
Specimens should be hardened in osmic acid or in Müller’s fluid, and cut in celloidin, or paraffin.
Cloudy swelling.—Specimens are obtained from organs of subjects who have died in the early stage of some fever. They should be always hardened in Müller’s fluid, as the appearances alter if the tissue is kept in spirit for any length of time.
Fatty degeneration.—Prepare from patients who have died of exhausting diseases, phosphorus poisoning, &c.
Stain in osmic acid. Mount in Farrant’s medium and keep in the dark.
Mucoid degeneration.—Study in goblet cells of normal intestine or of ovarian cysts. There are no satisfactory selective stains for mucin.
Colloid degeneration.—Occurs in the thyroid gland, in the tubules of the kidney in many diseases, and the prostate of the old.
Stain in safranine.
Waxy or lardaceous degeneration.—Best studied in liver, spleen, or kidneys. It should be searched for in persons who have died from a long illness, accompanied by suppuration, e.g., phthisis or bone disease. Mount one section unstained, stain another in methyl violet, a third in a weak solution of iodine, and examine the latter at once both by transmitted and reflected light. The iodine stain is not permanent. Another section should be stained in osmic acid, followed by methyl violet, as waxy and fatty degeneration frequently co-exist.
Hyaline degeneration.—Seen in the arterioles of the spleen in some cases of typhoid and diphtheria. The ordinary staining methods must be used.
Calcareous degeneration.—Occurs after fatty degeneration in gummata and in atheromatous arteries. It also occurs in the matrix of the costal cartilages after middle life. Mount one section unstained and examine if possible with the polariscope. Stain others in safranine.
Pigmentary degeneration.—May be studied in brown atrophy of heart, nutmeg liver, &c. It is also seen well in spinal and cerebral nerve cells of the aged. Harden in Müller’s fluid and mount sections unstained.
It will be unnecessary to recapitulate the methods for hardening the various diseased organs as the directions for the normal organs hold good. If the presence of micro-organisms be suspected, harden in methylated spirit or absolute alcohol, but as a rule both for diseased organs and tumours Müller’s fluid will be found the most satisfactory reagent for general use.
It sometimes happens, however, that it is inconvenient to wait several weeks, until the Müller’s fluid has hardened the specimen sufficiently, before making sections. In this case the best plan is to make fresh sections, or else to cut a slice about one-eighth of an inch thick, and harden for about three days in plenty of methylated spirit, or in formal (p. 23).
Tumours.—Müller’s fluid should be employed, unless a more rapid agent is required.
Methylated spirit may be used in the case of epithelioma, adenoma, &c., but for sarcoma, myxoma, tumours containing cysts or much blood, Müller’s fluid yields by far the best results.
Methods in Microscopical Anatomy—Whitman.
Practical Pathology—Woodhead.
Textbook of Bacteriology—Crookshank.
Manual for Physiological Laboratory—Harris and Power.
Practical Histology—Fearnley.
Practical Pathology and Histology—Gibbes.
Journal of Microscopical Society.
Methods and Formulæ—Squire.
The Human Brain—Goodall.
Practical Bacteriology—Kanthack and Drysdale.
Methods of Microscopical Research—Cole.
Abbe’s condenser, 11
Absolute alcohol, 21
Acetate of copper, 89
Air bubbles, removal of, 56
Alum carmine, 76
hæmatoxyline, 68, 70
Amyloid degeneration, 149
Aniline blue-black, 94
oil, 102, 108
oil water, 107
Apparatus required, 1
Areolar tissue, 133
Bacteria, stains for, 103
Balsam bottle, 58
Barrett’s logwood solution, 69
Bevan Lewis’s method, 94
Bichromate of potassium, 17
Bismarck brown, 104
Bleaching solution, 27
Blood crystals, 130
Blood, methods of examining, 113
Blood-vessels, injection of, 120
Blue injection mass, 121
Bone marrow, 136
Bone, sections of, 136
Borax carmine, 75
Brain, methods of staining, 94
Buckley’s modification of Golgi’s method, 99
Calcareous degeneration, 149
Canada balsam solution, 61
Carbolic acid, 23
Carmine, 74
injection mass, 120
Cathcart microtome, 39
Cathcart-Frazer microtome, 42
Cedar oil, 8, 63
Celloidin, 30
Cementing cover-glasses, 65
Chloral hæmatoxyline, 92
Chloride of gold, 82, 94
Chromic and nitric decalcifying fluid, 26
Ciliated epithelium, 132
Circulation in frog’s foot, 141
Clarifying sections, 63
Clearing agents, 63
Cloudy swelling, 148
Clove oil, 63
Colloid degeneration, 149
Columnar epithelium, 132
Corrosive sublimate hardening, 23
staining, 100
Cover-glasses, cleansing of, 57
Cover-glass preparations, 111
Decalcifying solutions, 26
Dehydration, 63
Eau de Javelle, 27
Ebner’s solution, 27
Ehrlich-Biondi fluid, 85
Ehrlich’s hæmatoxyline, 70
method of fixing blood-films, 116
method for tubercle bacilli, 110
Ehrlich-Gram method for staining bacteria, 108
Elastic cartilage, 135
tissue, 133
Embedding methods, 29
Endothelium, 131
Eosine, 72
Eosine and hæmatoxyline, 73
Epithelial cement, 131
Ether spray microtome, 39
Fæces, staining for bacilli, 112
Farrant’s solution, 59
Fat, removal from sections, 59
staining of, 134
Fatty degeneration, 148
Fearnley’s injection apparatus, 123
Ferrier’s fuchsine solution, 117
Flemming’s solution, 25
Flotation of sections, 55
Folded sections, treatment of, 58
Formal, 23
Fresh sections, 52
Fuchsine, 104
Gentian violet, 104
Gibbes’ stain for tubercle bacilli, 111
Gold chloride, 82, 94
Golgi’s silver method, 96
sublimate method, 99
Gram’s iodide solution, 105
method for staining bacteria, 107
Green injection mass, 122
Gum, 29
Hæmatin crystals, 131
Hæmatoidin, 131
Hæmatoxyline, Ehrlich’s, 70
Kleinenberg’s, 70
Schuchardt’s, 68
Sihler’s, 92
Weigert’s, 88
Hæmoglobin crystals, 130
Hardening processes, 15
Hyaline cartilage, 135
degeneration, 149
Ice freezing microtome, 46
Immersion lenses, 8
Injection of blood-vessels, 120
pulmonary alveoli, 145
Internal ear, 143
Intestines, 146
Iodine solution, 105
Jung’s ether spray microtome, 45
Kleinenberg’s hæmatoxyline, 70
Lardaceous degeneration, 149
Lithio-picrocarmine, 79
Lithium carmine, 74
Liver, 147
Löffler’s methyl blue, 104
Logwood, 68
Lymphoid tissue, 134
Marchi’s fluid, 24
Marrow, 136
Methyl blue, 101, 104
violet, 83
Methylated spirit, 19
Micro-organisms, stains for, 103
Microscope, 6
Microtome, Becker, 49
Cambridge rocking, 49
Cathcart, 39
Cathcart-Frazer, 42
Jung, 45
Schanze, 47
Swift’s, 49
Williams’, 46
Mould for paraffin embedding, 36
Mounting methods, 55
Mucoid degeneration, 148
Muscle, 138
Muir’s method of hardening films, 116
Müller’s fluid, 17
and formal, 20
and spirit, 20
Neelsen’s stain for tubercle bacilli, 110
Nerve cells, stains for, 94
endings, 139
fibres, stains for, 87
Nissl’s aniline method, 101
Nitrate of silver, 82
Nitric acid as hardening agent, 25
decalcifying agent, 26
decolourising agent, 110
Normal salt solution, 53
Nose piece, 9
Objectives, 7
Oil of bergamot, 63
cedar, 8, 63
cloves, 63
origanum, 63
Osmic acid as hardening agent, 21
staining reagent, 81
Pal’s method, 86
solution, 90
Paraffin, 34
Picrocarmine, 78
Pigment cells, 134
Pigmentary degeneration, 150
Plane iron microtome knife, 42
Rapid hardening, 150
Retina, 143
Safranine, 85
Salivary glands, 146
Schäfer-Pal method, 91
Schanze microtome, 47
Schuchardt’s hæmatoxyline, 68
Sihler’s chloral hæmatoxyline, 92
Silver nitrate, stain for nerve cells, 96
epithelial cement, 131
Skin, 142
Spinal cord, 86, 142
Spleen, 147
Sputum, staining of, 111
Squamous epithelium, 131
Staining methods, 67 seq.
Stomach, 146
Striped muscle, 138
Sulphuric acid, 105
Sweat glands, 142
Tendon, 133
Testing a microscope, 13
Thymus gland, 145
Thyroid gland, 145
Toison’s fluid, 118
Tooth, sections of, 137
Transitional epithelium, 132
Tubercle bacillus, stains for, 110
Tumours, hardening of, 150
Unstriped muscle, 139
Urine, examination for bacilli, 112
Uterus, 148
Von Ebner’s decalcifying solution, 27
Waxy degeneration, 149
Weigert’s hæmatoxyline method, 88
method for staining bacteria, 106
Williams’ ice freezing microtome, 46
Woodhead’s injection mass, 120
Xylol, 61
Ziehl’s carbol-fuchsine, 105
1 The student will bear in mind the danger of working with benzine near a naked light.
2 “Practical Histology,” (Macmillan & Co.).