
In this transcription a black dotted underline indicates a hyperlink to a page, illustration or footnote; hyperlinks are also marked by aqua highlighting when the mouse pointer hovers over them. A red dashed underline indicates the presence of a hidden comment that can be revealed by hovering the mouse pointer over the underlined text. Page numbers are shown in the right margin.
The text contains some uncommon characters that will not necessarily display correctly with all viewing devices. If some of the characters look abnormal, first ensure that the device’s character encoding is set to Unicode (UTF-8). The default font might also need to be changed to a Unicode font such as Arial Unicode MS, DejaVu, Segoe UI Symbol or FreeSerif. Symbols representing male and female have been replaced by M and F in the handheld version. Subscripted and superscripted characters do not always display correctly on handheld devices.
The book contained innumerable typographical errors affecting spelling, punctuation and formatting. Most spelling errors have been corrected silently, but spelling variants that mainly reflect contemporary spellings in the original quoted sources, have been left unchanged. A list of the corrections and variations can be seen at the end of the transcription. Punctuation anomalies (errors, omissions, duplications) have mostly been corrected silently, but missing apostrophes have not been corrected because of uncertainty about intended meaning. A few missing quotation marks have been inserted within curly brackets {"} and missing words inserted by the transcriber, e.g. {sic} {and} have also been enclosed within curly brackets to differentiate them from the numerous words and phrases inserted by the authors. Redundant duplicated words have been deleted. Astute readers will probably note persisting inconsistencies involving italics, fonts, hyphenation, ellipses, accents, and ligatures (ae/æ, oe/œ) that have been left unchanged because they have no impact on interpretation of the text. The Table of Contents does not correspond accurately with headings used in the text.
Footnotes are located at the end of the book and are hyperlinked in the text. In most cases a return hyperlink will take the reader back to the appropriate place in the text, except for those footnotes that are cross-referenced multiple times. In these cases the return hyperlink will take the reader back to the location where the footnote was first referenced – it is better to make use of the browser's back-arrow to return to the correct location. An error in footnote numbering was corrected silently, and a missing footnote [120] was inserted after being identified in the original source.
Several large tables have been compressed in size to enable viewing on small screens and when necessary a key has been added to identify the column headings.

VOLUME 2
Part I
Reports of the Council
Part II
Contributions from the Laboratory
Part III
Journal Contributions: Proprietary Products
Part IV
Journal Contributions: Miscellany
PRESS OF AMERICAN MEDICAL ASSOCIATION, FIVE HUNDRED
AND THIRTY-FIVE NORTH DEARBORN STREET,—CHICAGO
1922
Copyright, 1922
by the
American Medical Association
There were nine editions of the first volume of The Propaganda for Reform in Proprietary Medicines. The ninth edition contained the most important reports of the Council on Pharmacy and Chemistry and of the Chemical Laboratory. It contained also those articles from The Journal of the American Medical Association (up to, and including, 1916) which dealt with the problems of proprietaryship in medicine and the furtherance of rational drug therapy.
The present volume contains similar material covering the period from January, 1917, to April, 1922, inclusive. Like Volume 1, this volume is divided into four parts:
Part I. The Council on Pharmacy and Chemistry: This section presents the principles and rules which govern the Council in the examination of medicaments, together with articles and reports bearing on the work of the Council, and the most important reports of the Council from 1917 to April, 1922, inclusive.
Part II. The A. M. A. Chemical Laboratory: This section, besides presenting the aims and objects of the Association’s Chemical Laboratory, also outlines some of the Laboratory’s work which is of particular interest to physicians.
Part III. Contributions from the Journal: Proprietary Products: This part contains articles on proprietary medicinal preparations and the methods by which they are exploited, which have appeared in The Journal A. M. A.
Part IV. Contributions from The Journal: Miscellany: In this section are articles dealing with matters of interest to the medical profession but not coming strictly under the classification of proprietary medicinal preparations.
A comparison of the material that has appeared in Volume 1 of The Propaganda for Reform with that which appears in this volume will reveal the changing conditions in the proprietary medicine field. Many of the reports in the first volume brought out the fact that medicinal preparations were at that time foisted on the profession with false claims of composition; reports of this character are less conspicuous in the present volume. Many of the reports in Volume 2 deal with unwarranted therapeutic claims, especially those advanced for animal organ preparations, serums, vaccines, preparations for intravenous medication, etc. The present volume will also be found of interest in its portrayal of the changed conditions in the proprietary medicine business brought about by the World War.
Special attention is directed to the index in this volume. It is, in effect, a bibliography, including references not only to articles in this book but also (1) to articles which appeared in Volume 1; (2) to articles on the same general subject in The Journal of the American Medical Association, and (3) to the articles appearing in the annual reports of the Council on Pharmacy and Chemistry and of the A. M. A. Chemical Laboratory, but not reprinted in either volume of the Propaganda for Reform in Proprietary Medicines.
From time to time The Journal of the American Medical Association has published the reports of the Council on Pharmacy and Chemistry and the Chemical Laboratory, as well as other matter on proprietary medicines. Repeated requests for some of the matter have led to the compilation of “The Propaganda for Reform in Proprietary Medicines,” which, in the present volume, attains its ninth edition.
The seventh, eighth and ninth editions have been compiled on slightly different principles from their predecessors. The therapeutic reform work of The Journal and of the Association’s Chemical Laboratory was at first confined almost entirely to the criticism and analysis of the so-called ethical proprietaries. This was right; the medical profession owed it to the public to combat the nostrum evil within its own ranks.
As the more flagrant evils of the “ethical proprietary” question were mitigated, the Association has turned the light on the more widespread and dangerous “patent medicine” evil. The articles devoted to “patent medicines” or quackery being naturally of greater interest to the general public than to the medical profession, the number of inquiries from laymen regarding various quacks and nostrums has steadily increased. It has been thought best, therefore, to publish separately all of the matter from The Journal relative to quackery and to those nostrums exploited only or chiefly to the public, and to include in the Propaganda for Reform practically none of the matter that is of direct interest primarily to laymen. In one or two instances in which the subjects were of equal interest to the profession and to the public, matter that has already appeared in “Nostrums and Quackery” is also given here; but as a general rule the contents of the ninth edition of “The Propaganda for Reform” are of strictly professional interest. Those physicians who are desirous of obtaining in convenient form the matter dealing with “patent medicines” should order the book “Nostrums and Quackery” or the various pamphlets on the same subjects that have been issued since “Nostrums and Quackery” came from the press.
The ninth edition of “Propaganda for Reform” contains a number of new articles, greatly increasing the size of the book. It also contains one novel feature which greatly enhances its value. The index includes references not only to articles in the book, but also to matter on proprietaries not accepted by the Council on Pharmacy and Chemistry which appeared in The Journal of the American Medical Association and elsewhere. This index makes of this edition of “Propaganda for Reform” a very full work of reference on proprietaries which are undeserving of recognition. It should be understood, however, that not all articles indexed are condemned; some are merely discussed and compared.
Resolved, We, Members of the House of Delegates of the American Medical Association, believe that every effort must be made to do away with the evils which result from the exploitation of the sick for the sake of gain. Earnestly believing that the continued toleration of secret, semisecret, unscientific or untruthfully advertised proprietary medicines is an evil that is inimical to medical progress and to the best interest of the public, we declare ourselves in sympathy with, endorse and by our best efforts will further, the work which has been, and is being, done by the Council on Pharmacy and Chemistry of the American Medical Association in the attempt to eliminate this evil.
| PART I: COUNCIL REPORTS | |
| PAGE | |
Foreword | 1 |
Official Rules of the Council on Pharmacy and Chemistry | 3 |
The Council on Pharmacy and Chemistry, Present and Future | 12 |
“Accepted by the Council on Pharmacy and Chemistry” | 19 |
Helping the Council | 20 |
Delays in Passing on Products | 20 |
Cooperation of the Pharmaceutical Houses | 21 |
Budwell’s Emulsion of Cod-Liver Oil, Nos. 1 and 2 | 22 |
Rheumalgine | 23 |
Gray’s Glycerine Tonic | 24 |
Tongaline and Ponca Compound | 26 |
Alfatone | 28 |
Uricsol | 30 |
Jubol | 31 |
Urodonal | 32 |
Formamint | 33 |
Hydragogin | 41 |
Filudine | 41 |
Lactopeptine and Elixir Lactopeptine | 43 |
Iodum-Miller and Iod-Izd-Oil (Miller’s) | 49 |
Elixir Iodo-Bromide of Calcium Comp. “Without Mercury” and “With Mercury” | 52 |
Lecithin Preparations Omitted from N. N. R. | 53 |
Proprietary Names for Liquid Petrolatum | 55 |
Seng | 55 |
Frosst’s Blaud Capsules | 56 |
Tyree’s Elixir of Buchu and Hyoscyamus Compound | 57 |
Hydroleine | 58 |
Curative Vaccine, Bruschettini | 58 |
Stearn’s Wine | 59 |
Protonuclein and Protonuclein Beta | 59 |
Hydropsin | 61 |
Digitalysatum | 63 |
So-Called Secretin Preparations | 64 |
Has Secretin a Therapeutic Value? | 65 |
Radio-Rem | 79 |
Olio-Phlogosis | 79 |
The Hypophosphite Fallacy | 80 |
Pulvoids Calcylates | 85 |
Sulfuryl Monal | 86 |
Mark White Goiter Serum and Mark White Iodinized Oil | 87 |
Kora-Konia | 92 |
The Therapeutic Value of the Glycerophosphates | 93 |
Hydras | 96 |
Bromin-Iodin Compound | 97 |
Ammonium Hypophosphite Omitted from N. N. R. | 98 |
Alphozone Omitted from N. N. R. | 99 |
Calcium Glycerophosphate and Sodium Glycerophosphate Omitted from N. N. R. | 99 |
Gardner’s Syrup of Ammonium Hypophosphite Omitted from N. N. R. | 100 |
Gluten Products Made by the Kellogg Food Company | 100 |
Iodo-Mangan Omitted from N. N. R. | 106 |
Liquid Albolene | 106 |
Naphey’s Medicated Uterine Wafers | 107 |
Nujol | 108 |
Pulvoids Natrium Compound | 108 |
Saloform | 110 |
Secretogen | 110 |
Iron Citrate Green | 115 |
Aspirin | 116 |
Pil. Cascara Compound-Robins | 117 |
Casta-Flora | 118 |
Firwein | 119 |
Firolyptol Plain and Firolyptol with Kreosote | 120 |
Biniodol | 121 |
| Comparative Symptoms Resulting from the Use of Several Oily Suspensions of | |
Red Mercuric Iodid (Mercury Biniodid) | 123 |
Corpora Lutea (Soluble Extract), Parke, Davis & Co. | 128 |
Wheeler’s Tissue Phosphates | 129 |
The Claimed Galactagogue Effects of Nutrolactis and Goat’s Rue Not Substantiated | 131 |
The Alleged Galactagogue Action of Galega and Nutrolactis | 131 |
The Russell Emulsion and the Russell Prepared Green Bone | 134 |
Brom-I-Phos | 136 |
Creosote-Delson and Creofos | 137 |
Triner’s American Elixir of Bitter Wine | 139 |
Trimethol | 140 |
Ferrivine, Intramine and Collosol Iodine | 144 |
Eskay’s Neuro Phosphates | 146 |
K-Y Lubricating Jelly | 147 |
Ziratol | 148 |
Gonosan | 150 |
Alcresta Ipecac | 153 |
Iodeol and Iodagol | 154 |
Capsules Bismuth Resorcinol Compound Not Admitted to N. N. R. | 157 |
Dixon’s Tubercle Bacilli Extract and Dixon’s Suspension of Dead Tubercle Bacilli | 158 |
Formosol | 158 |
Iodolene, a Solution of Iodin in Liquid Petrolatum, Inadmissible to N. N. R. | 159 |
Kalak Water | 160 |
Minson’s Soluble Iodin “Kelpidine” Not Admitted to N. N. R. | 161 |
Nutone | 162 |
Tri-Arsenole, L. O. Compound No. 1 and L. O. Compound No. 2 | 163 |
Unctol | 166 |
V-E-M (Schoonmaker Laboratories, Inc.) | 166 |
Hemo-Therapin | 168 |
Venosal | 169 |
Secretin-Beveridge and the U. S. Patent Law | 170 |
The Question of the Stability of Secretin | 171 |
Need for Patent Law Revision | 177 |
Surgodine | 180 |
Medeol Suppositories | 181 |
Guaiodine | 183 |
Several “Mixed” Vaccines Not Admitted to N. N. R. | 184 |
Ophthalmol-Lindemann | 189 |
Silvol Ineligible for N.N.R | 189 |
Katharmon | 191 |
Iodinized Emulsion (Scott) and Creosotonic (Scott) | 192 |
Campetrodin and Campetrodin No. 2 | 193 |
Carminzym | 194 |
Phillips’ Phospho-Muriate of Quinine Comp | 197 |
B. Iodine and B. Oleum Iodine | 198 |
B. Iodine Products | 199 |
| Antithyroid Preparations (Antithyroidin-Moebius and Thyreoidectin) Omitted | |
from N. N. R. | 202 |
| Cephaelin and Syrup Cephaelin-Lilly Omitted from N. N. R. and Syrup Emetic-Lilly | |
Not Accepted | 203 |
Colalin Omitted from N. N. R. | 203 |
Foral | 204 |
Granular Effervescent Bromide and Acetanilid Compound-Mulford | 206 |
Holadin and Bile Salt Mixtures | 207 |
Liquor Santaiva, S. & D., Omitted from N. N. R. | 211 |
| Maltzyme, Maltzyme with Cascara Sagrada, Maltzyme with Cod Liver Oil, Maltzyme | |
Ferrated and Maltzyme with Yerba Santa Omitted from N. N. R. | 211 |
Methaform Omitted from N. N. R. | 212 |
| Pineal Gland, Red Bone-Marrow and Thymus Gland and Their Preparations | |
Omitted from N. N. R. | 213 |
Piperazine and Lycetol Omitted from N. N. R. | 214 |
Stanolind Liquid Paraffin Omitted from N. N. R. | 214 |
Westerfield’s Digitalis Tablets | 215 |
Xeroform-Heyden and Bismuth Tribromphenate-Merck Omitted from N. N. R. | 216 |
Cream of Mustard Refused Recognition | 218 |
“Pluriglandular” Mixtures | 218 |
Cerelene Not Admitted to N. N. R. | 219 |
Collosol Cocaine Not Admitted to N. N. R. | 221 |
Cuprase Not Admitted to N. N. R. | 222 |
Collosol Preparations | 223 |
Pulvoids Calcylates Compound | 226 |
Proteogens of the Wm. S. Merrell Company | 227 |
“Arsenoven S. S.” and “Arseno-Meth-Hyd” | 231 |
Hormotone and Hormotone Without Post-Pituitary | 234 |
Formaldehyde Lozenges | 235 |
Lavoris | 237 |
Medinal | 239 |
Omission of Cotarnin Salts (Stypticin and Styptol) from N. N. R. | 240 |
Micajah’s Wafers and Micajah’s Suppositories | 241 |
Alkalithia | 242 |
Arhovin Omitted from N. N. R. | 243 |
Chloron, Chlorax and Number “3” | 244 |
Elarson Omitted from N. N. R. | 248 |
Iodiphos | 249 |
Mervenol and Armervenol Not Admitted to N. N. R. | 249 |
| Normal Phenol Serum (Cano) and Methyl-Phenol Serum (Cano) Not Accepted | |
for N. N. R. | 251 |
Soamin Omitted from N. N. R. | 253 |
Some Mixed Vaccines Not Admitted to N. N. R. | 254 |
Somnoform | 255 |
Tablets Formothalates | 256 |
Triple Arsenates with Nuclein | 256 |
“Anti-Pneumococcic Oil” and the Use of Camphor in Pneumonia | 257 |
Dial “Ciba” | 259 |
Apothesine | 260 |
Eumictine | 262 |
Platt’s Chlorides | 263 |
| Anti-Tuberculous Lymph Compound (Sweeny) and Anti-Syphilitic Compound | |
(Sweeny) | 266 |
Syrup Leptinol (Formerly Syrup Balsamea) | 268 |
Formitol Tablets, II | 271 |
Sukro-Serum and Aphlegmatol | 273 |
Supsalvs Not Admitted to N. N. R. | 274 |
| Hypodermic Solution No. 13, Iron, Arsenic and Phosphorus Compound Not | |
Accepted for N. N. R. | 275 |
Parathesin Not Admitted to N. N. R. | 276 |
Chlorlyptus | 277 |
Aquazone (Oxygen Water) | 290 |
Coagulen-Ciba Omitted from N. N. R. | 290 |
Ferric Cacodylate Omitted from New and Non-Official Remedies | 292 |
Libradol | 293 |
Helmitol Omitted from N. N. R. | 295 |
Spirocide Not Admitted to N. N. R. | 296 |
Digifolin-Ciba Not Admitted to N. N. R. | 298 |
Some of Loesser’s Intravenous Solutions | 299 |
“National Iodine Solution” Not Admitted to N. N. R. | 300 |
Mon-Arsone Not Admitted to N. N. R. | 302 |
Oxyl-Iodide Not Admitted to N. N. R. | 304 |
Quassia Compound Tablets | 306 |
Toxicide | 307 |
Pil. Mixed Treatment (Chichester) | 310 |
Atophan Omitted from N. N. R. | 313 |
Urotropin Omitted from N. N. R. | 316 |
Styptysate Not Admitted to N. N. R. | 318 |
Lipoidal Substances (Horovitz) Not Admitted to N. N. R. | 320 |
Yeast Preparations and Vitamin B Concentrates | 321 |
| PART II: CONTRIBUTIONS FROM THE A. M. A. CHEMICAL LABORATORY | |
The Chemical Laboratory of the American Medical Association | 322 |
The Work of the American Medical Association Chemical Laboratory | 322 |
Lead in “Akoz” | 328 |
Sodium Acetate in Warming Bottles | 329 |
Anti-Syphilitic Compound (Sweeny) | 330 |
“Ambrine” and Paraffin Films | 330 |
The Stability of Iodine Ointments | 337 |
Iodolene and the Solubility of Iodin in Liquid Petrolatum | 344 |
American-Made Synthetic Drugs—I | 344 |
Standardization of Commercial Bismuth Tribromphenate | 348 |
Standardization of Procain and Examination of the Market Supply | 355 |
Deterioration of Sodium Hypochlorite Solutions | 358 |
Syphilodol | 359 |
Cerelene | 362 |
Dr. De Sanctis’ Rheumatic and Gout Pills | 363 |
Iodex and Liquid Iodex | 365 |
| PART III: CONTRIBUTIONS FROM THE JOURNAL: PROPRIETARY PRODUCTS | |
Iodin in Liquid Petrolatum | 367 |
American-Made Synthetic Drugs—II | 369 |
Nostrums in Retrospect | 379 |
Bell-Ans (Pa-Pay-Ans Bell) | 380 |
Anasarcin and Anedemin | 383 |
Pepto-Mangan | 387 |
Cactina Pillets | 391 |
Ammonol and Phenalgin | 393 |
Fellows’ Syrup, and Other Preparations of the Hypophosphites | 395 |
Shotgun Nostrums | 398 |
Tyree’s Antiseptic and Aseptinol | 401 |
Neurosine and the Original Package Evil | 404 |
Anasarcin Advertising | 407 |
Antimeristem-Schmidt | 408 |
Antiphlogistine | 409 |
“Auto-Hemic Serum” | 409 |
“Autolysin” Advertising | 413 |
“Basic Cancer Research” and “Cosmopolitan Cancer Research Society” | 414 |
Seleni-Bascca | 416 |
Bell-Ans (Papayans, Bell) | 418 |
Campho-Phenique | 418 |
“Cinchophen”: Formerly “Atophan” | 419 |
“Collosols”: An Uncritical English Endorsement | 420 |
Cotton Process Ether | 421 |
Dionol | 422 |
The Eli Products of Eli H. Dunn | 424 |
Glover’s Cancer Serum | 425 |
Glyco-Thymoline and Poliomyelitis | 427 |
Glykeron: Cold Storage Testimonials | 428 |
Gray’s Glycerine Tonic: “Whose Bread I Eat His Song I Sing” | 429 |
Hagee’s Cordial of Cod Liver Oil | 429 |
Hypno-Bromic Compound | 430 |
Intravenous Compound (Loffler) | 430 |
Intravenous Specialties | 435 |
Iodex | 436 |
The William F. Koch Cancer Remedy | 437 |
The Lucas Laboratories’ Products | 440 |
“Phylacogens” | 441 |
Pineoleum Advertising Methods | 442 |
“Proteal Therapy” and Henry Smith Williams | 443 |
Proteogens | 445 |
Pulvane | 450 |
Sal Hepatica | 451 |
Salicon | 453 |
So-Called Secretin Preparations | 454 |
Succus Cineraria Maritima | 455 |
Tekarkin | 458 |
Tyree’s Antiseptic Powder Again | 462 |
Wheeler’s Tissue Phosphates | 463 |
Briefer Paragraphs | 465 |
| PART IV: CONTRIBUTIONS FROM THE JOURNAL: MISCELLANY | |
Albert Abrams, A.M., M.D., LL.D., F.R.M.S. | 472 |
Acetylsalicylic Acid, Not Aspirin | 480 |
The Allied Medical Associations of America | 486 |
“Arsenicals” | 491 |
Beer and Cancer Cures | 494 |
Biologic Therapeutics and Its Commercial Domination | 496 |
Capell’s Uroluetic Test | 497 |
Chemotherapy and Tumors | 499 |
The Direct Sales Company | 510 |
Discoveries and Discoverers | 511 |
“Drug Reform” | 513 |
Drug Therapy: The Fallibility of Textbooks | 515 |
Thomas Webster Edgar | 515 |
Glycerophosphates | 520 |
Influenza Vaccine | 520 |
Intravenous Therapy | 522 |
Iodin Fumes | 523 |
Italian Physico-Chemical Company | 524 |
What is Liquid Petrolatum? | 526 |
The Lowenthal Postgraduate Course | 527 |
Medical Society of the United States | 531 |
The National Formulary—A Review of the Fourth Edition | 535 |
Nonspecific Protein Therapy | 536 |
Willard Ealon Ogden | 538 |
“Patents” | 542 |
Pharmaceutical Barnums | 545 |
The Pharmacopeia | 546 |
Physician’s Stock in Prescription Products | 548 |
Pituitary Gland Preparations | 549 |
Proprietorship in Medicine | 550 |
Philip Rahtjen and His Discoveries | 553 |
Sodium Cacodylate in Syphilis | 555 |
Tablets: Dependability of Dosage | 556 |
Therapeutic Evidence: Its Crucial Test | 557 |
“Vaccines in Toxic Conditions” | 560 |
Vitamins: Their Distribution | 561 |
The William A. Webster Co. and the Direct Pharmaceutical Co. | 564 |
Yeast | 566 |
Briefer Paragraphs | 570 |
THE PROPAGANDA FOR REFORM IN PROPRIETARY MEDICINES
The Council on Pharmacy and Chemistry was established by the American Medical Association primarily for the purpose of gathering and disseminating such information as would protect the medical profession—and thus the public—in the prescribing of proprietary medicinal articles.
The Council consists of sixteen members, fifteen appointed for a term of five years without pay, and the sixteenth, a secretary, who is also the director of the Chemical Laboratory of the American Medical Association (see Part II).
At the present time (1921) the membership is:
C. L. Alsberg, A.M., M.D., Chief of the Bureau of Chemistry, U. S. Department of Agriculture, Washington, D. C.
C. W. Edmunds, M.D., Professor of Materia Medica and Therapeutics, University of Michigan Medical School, Ann Arbor.
R. A. Hatcher, Ph.G., M.D., Professor of Pharmacology, Cornell University Medical College, New York City.
A. W. Hewlett, M.D., Professor of Medicine, Leland Stanford Junior University School of Medicine, San Francisco.
John Howland, M.D., Professor of Pediatrics, Johns Hopkins University Medical Department, Baltimore.
Reid Hunt, M.D., Professor of Pharmacology, Medical School, Harvard University, Boston.
W. T. Longcope, A.B., M.D., New York.
G. W. McCoy, M.D., Director of the Hygienic Laboratory, U. S. Public Health Service, Washington, D. C.
Lafayette B. Mendel, Ph.D., Sc.D., Professor of Physiological Chemistry, Sheffield Scientific School, Yale University, New Haven.
F. G. Novy, Sc.D., M.D., Professor of Bacteriology, University of Michigan Medical School, Ann Arbor.
W. W. Palmer, B.S., M.D., Bard Professor of Medicine, Columbia University College of Physicians and Surgeons, New York.
W. A. Puckner, Phar.D., Secretary of the Council, Director of the Chemical Laboratory of the American Medical Association, Chicago.
L. G. Rowntree, M.D., Sc.D., Professor of Medicine, Mayo Foundation, Rochester.
G. H. Simmons, M.D., LL.D., Chairman of the Council, Editor of The Journal of the American Medical Association, Chicago.
Torald Sollmann, M.D., Professor of Pharmacology and Materia Medica, Western Reserve University School of Medicine, Cleveland.
Julius Stieglitz, Ph.D., Sc.D., Chem.D., Professor of Chemistry, University of Chicago, Vice-Chairman of the Council, Chicago.
At its first meeting in 1905, the Council began examining the proprietary and nonofficial medicinal preparations offered to physicians of the United States, and authorized the publication of a book (New and Nonofficial Remedies) containing descriptions of those preparations which were deemed worthy of the consideration of physicians. It also issued reports (Reports of the Council on Pharmacy and Chemistry) to the medical profession on those preparations which were not eligible. The Council adopted a set of rules by which to measure the eligibility of each preparation for admission to New and Nonofficial Remedies. These rules were designed primarily to protect the public—through the medical profession—against fraud, undesirable secrecy and objectionable advertising in connection with proprietary medicinal articles. The rules originally adopted have been subjected to revision from time to time to meet changing conditions. For the information of those who wish to familiarize themselves with the work of the Council the rules which are now in force (1921) follow this introduction. A summary is also to be found in the article, “The Work of the Council on Pharmacy and Chemistry, Present and Future,” page 12.
Since 1906, the Council has issued New and Nonofficial Remedies annually. In each issue are listed and described the articles that stand accepted on January 1 of the year of publication. The book describes proprietary medicinal articles on the American market that are found eligible under the rules, and also such nonproprietary, nonofficial articles as give promise of therapeutic usefulness, listing the acceptable brands. Articles of a similar character are grouped together, and each group is preceded by a general discussion for the purpose of comparison.
Since 1908, the Council has also issued an annual volume, “Reports of the Council on Pharmacy and Chemistry,” which contains reports on proprietary medicines that were found inadmissible to New and Nonofficial Remedies. The reports issued prior to 1916—and deemed of sufficient interest to physicians—were reprinted in the Propaganda for Reform in Proprietary Medicines, ninth edition (1916). The more important reports issued from 1916 to 1921, inclusive, are in this volume.
While it is the chief function of the Council to investigate and report on proprietary medicinal preparations, its work has broadened so that the Council’s work may now be characterized as a propaganda for the rational use of drugs. Thus, its Committee on Therapeutic Research encourages the investigation of questions concerning the actions of drugs. These investigations are brought together in the “Annual Reports of the Therapeutic Research Committee.” The Council also has a committee on medical teaching which has issued the publication “Useful Drugs,” a concise, but thorough and up-to-date, discussion of the more important drugs. In addition, the Council appointed a committee to prepare an “Epitome of the U. S. Pharmacopeia and National Formulary,” in which are presented those portions of the United States Pharmacopeia and the National Formulary that are of interest to physicians and in which is given a concise statement of the therapeutic usefulness of such drugs and preparations.
[May 1, 1921]
The following rules have been adopted by the Council primarily with the object of protecting the medical profession and the public against fraud, undesirable secrecy and objectionable advertising in connection with proprietary medicinal articles.
New and Nonofficial Remedies.—The book New and Nonofficial Remedies contains a description of proprietary articles which have been accepted as conforming to the rules of the Council; and of such simple nonproprietary and nonofficial substances as seem of sufficient importance to warrant their inclusion.
Mixtures.—For admission to N. N. R., proprietary pharmaceutical mixtures must comply with the rules; and, to determine such compliance, they will be investigated by the Council. The Council, however, endorses the principle that prescriptions should be written on the basis of the therapeutic effects of the individual ingredients. For this reason, it includes in this book only those mixtures that present some real advantage. There is also an appendix in which are included those proprietary articles which, so far as known to the Council, comply with the rules, but which do not possess sufficient originality to be admitted to the body of the book.
Definition of Proprietary Articles.—The term “proprietary article,” in this place, shall mean any chemical, drug or similar preparation used in the treatment of diseases, if such article is protected against free competition, as to name, product, composition or process of manufacture, by secrecy, patent, copyright, or by any other means.
Rule 1.—Composition.—No article will be accepted for inclusion in the book New and Nonofficial Remedies, or retained therein, unless its composition be furnished to the Council for publication. For simple substances, the scientific name and the chemical formula, rational or structural, if known, should be supplied. For mixtures, the amount of each active medicinal ingredient in a given quantity of the article must be stated. The general composition of the vehicle, its alcoholic percentage, and the identity of the preservatives must be furnished.
Rule 2.—Identification.—No article will be accepted or retained unless suitable tests for determining its composition are furnished to the Council. In the case of chemical compounds, these shall consist of tests for identity and purity. In the case of mixtures, description of methods for determining the amount and active strength of the potent ingredients shall be furnished, if practicable.
Rule 3.—Direct Advertising.—No article that is advertised to the public will be accepted or retained; but this rule shall not apply to: (a) disinfectants, germicides and antiseptics, provided the advertising is limited to conservative recommendations for their use as prophylactic applications to superficial cuts and abrasions of the skin and to the mucous surfaces of the mouth, pharynx and nose (but not to those of the eye, and the gastro-intestinal and genito-urinary tracts) and provided they are not advertised as curative agents (see comments to Rule 3); and (b) nonmedicinal food preparations, except when advertised in an objectionable manner.
Rule 4.—Indirect Advertising.—No article will be accepted or retained if the label, package or circular accompanying the package contains the names of diseases in the treatment of which the article is said to be indicated. The therapeutic indications and properties may be stated, provided such statements do not suggest self-medication. Dosage may be indicated. (This rule shall not apply to remedies with which self-medication is altogether improbable, to vaccines and antitoxins or to directions for administering or applying remedies when similar immediate, heroic treatment is indicated.)
Rule 5.—False Claims as to Origin.—No article will be accepted or retained concerning which the manufacturer or his agents make false or misleading statements as to source, raw material from which made, or method of collection or preparation.
Rule 6.—Unwarranted Therapeutic Claims.—No article will be accepted or retained concerning which the manufacturer or his agents make unwarranted, exaggerated or misleading statements as to the therapeutic value.
Rule 7.—Poisonous Substances.—The principal label on an article containing “poisonous” or “potent” substances must state plainly the amount of each of such ingredients in a given quantity of the product.
Rule 8.—Objectionable Names.—Proprietary names for medicinal articles will be recognized only when the Council shall deem the use of such exclusive names to be in the interest of public welfare. Names which are misleading or which suggest diseases, pathologic conditions or therapeutic indications will not be recognized (the provision against therapeutically suggestive names does not apply to serums, vaccines and antitoxins, or to foods). In the case of pharmaceutical preparations or mixtures, the name must be so framed as to indicate the most potent ingredients.
Rule 9.—Patented Products and Protected Names.—If the article is patented—either process or product, or both—the number of such patent or patents must be furnished to the Council. Furthermore, if the name of an article is registered, or the label copyrighted, the registration (trademark) number and a copy of the protected label should be furnished the Council. In case of registration in foreign countries, the name under which the article is registered should be supplied.
Rule 10.—Unscientific and Useless Articles.—No article will be accepted or retained which, because of its unscientific composition, is useless or inimical to the best interests of the public or of the medical profession.
Introduction.—The Council on Pharmacy and Chemistry was established in February, 1905, by the American Medical Association, primarily for the purpose of gathering and disseminating such information as will protect the medical profession in the prescribing of proprietary medicinal articles. In pursuance of this object, the Council examines the articles on the market as to their compliance with definite rules designed to prevent fraud, undesirable secrecy and the abuses which arise from advertising directly or indirectly to the laity. Such articles as appear to conform to the rules are accepted; and their essential features are described in the annual publication of the Council, New and Nonofficial Remedies, if they come within the scope of this book. These descriptions are based in part on investigations made by, or under, the direction of the Council, but in part also on evidence or information supplied by the manufacturer or his agents. Such interested statements are examined critically, and are admitted only if they appear to be in conformity with the evidence. It is, however, manifestly impossible for the Council to investigate the composition of every complex pharmaceutical mixture, or to check thoroughly every therapeutic claim; it can give only an unbiased judgment on the available evidence. Criticisms and corrections of the descriptions which may aid in the revision of the matter will be appreciated. The Council judges an article entirely by the facts in evidence at the time of its admission. Previous noncompliance with the rules (short of intentional fraud) does not prevent the favorable consideration of an article which is in accord with existing rules. Infringements of the rules after acceptance of an article for New and Nonofficial Remedies, or the discovery that the Council’s information was incorrect, will cause the acceptance to be reconsidered. An article is accepted for New and Nonofficial Remedies, and will continue to be included in the book, with the understanding that serious violations of the rules, after acceptance, will be followed by the omission of the article and publication of the reasons for such omission. The Council desires physicians to understand that the admission of an article does not imply a recommendation. Acceptance simply means that no conflict with the rules has been found by the Council.
Duration of Acceptance.—Unless an agreement to the contrary is made at the time of acceptance, articles admitted to New and Nonofficial Remedies will be retained for a period of three years, provided that during that period they comply with the rules and regulations which were in force at the time of their acceptance. At the end of this period all articles will be carefully reexamined for compliance with existing rules. Particular weight will be given to the question as to whether recent evidence has substantiated claims as to the therapeutic value of any preparation, this evidence to consist partly of recent statements in the literature and partly of the general esteem in which the preparation is held by clinical consultants of the Council. The reacceptance of articles after such reexamination shall be for three years unless a shorter period is specified. Any amendments to the rules, by specific requirements or by interpretation, which may be made after the acceptance of an article, shall not apply to such article until the period of acceptance has elapsed. At the end of this period the article, if it is not eligible under the amended rules, will be omitted.
The Scope of New and Nonofficial Remedies and Appendix.—To aid physicians and manufacturers in deciding what articles come within the scope of this book, or, in other words, to enable physicians to recognize whether an article which is not described in New and Nonofficial Remedies has been omitted because it does not need admission or because it has been rejected, the Council furnishes the following more detailed definitions:
Official Articles.—Articles official in the U. S. P. or N. F. do not require consideration by the Council if they are marketed under the official name and if no unestablished therapeutic claims are made for them.
These do not require consideration by the Council, since standards for them are provided in these books, and enforced under the provisions of the federal Food and Drugs Act, except that they may be mentioned for information. Consideration by the Council becomes necessary if a U. S. P. or N. F. product is offered for sale under a name other than that, or the synonyms, under which the product is described in one of these books of standards, or if the proprietors or their agents advance claims that the product possesses therapeutic properties other than those commonly accredited to it.
Modifications of U. S. P. and N. F. Products.—A pharmacopeial or National Formulary product which is marketed under the official title or synonym, but with well-founded claims that its purity, permanence, palatability or other physical properties excel the official standard, may, if no extraordinary therapeutic properties are asserted, be considered as an official article and held not to be within the scope of New and Nonofficial Remedies. When such products are marketed under the claim that they possess therapeutic properties other than those commonly accredited to the U. S. P. or N. F. products of which they are modifications, they shall be subject to the consideration of the Council.
Specifically Exempted Preparations.—Foods, in general, unless marketed with the claim that they possess therapeutic properties shall not, at the present time, be considered by the Council. Mechanical appliances, at the present time, shall not be considered by the Council. Mineral waters (natural), at the present time, shall not be considered by the Council. With these exceptions, products which in the judgment of the Council are manufactured and marketed in conformity to the principles underlying the rules of the Council may be accepted for N. N. R. Products which are manufactured and marketed in a manner which does not conform to the principles underlying the rules of the Council shall not be accepted for N. N. R. The burden of proof in establishing claims for therapeutic properties of products considered by the Council shall lie with the proprietor or, when a foreign made product, with the agent who markets the product in the United States. To avoid confusion with nonofficial substances marketed under similar names, the Council recommends that official substances be prescribed by their official titles, followed by the abbreviation “U. S. P.” or “N. F.”; thus: Tinctura Nucis Vomicae, U. S. P.; Elixir Gentianae, N. F.
Substances Described in New and Nonofficial Remedies.—In the body of the book will be described simple proprietary substances and their preparations; proprietary mixtures if they have originality or other important qualities which, in the judgment of the Council, entitle them to such place, and important, nonproprietary, unofficial articles. The Council recommends that when the latter are prescribed, they be indicated by the abbreviation, “N. N. R.,” thus insuring to the prescriber the quality of these articles laid down in the book.
Proprietary Mixtures.—A mixture will be considered as proprietary, and therefore requiring consideration by the Council for admission to the book or appendix, if it contains any proprietary article; if it is marketed under a name which is in any way protected, or if its manufacturer claims for it any unusual therapeutic qualities. Proprietary mixtures which are marketed in conformity with the rules are listed in the appendix of the book under the names of the respective manufacturers. Such proprietary mixtures are not admitted to the body of the book, save in the exceptional cases cited in the preceding paragraph.
Nonproprietary Mixtures of Official Substances.—Since the ingredients of such mixtures do not require consideration by the Council, and since the mixtures are not open to the proprietary abuses which call for the work of the Council, it is not necessary that they should be investigated by the Council. The physician must judge whether such mixtures should be directed to be prepared by the pharmacist, or whether he is justified in ordering a ready-made preparation. If he decides to use a ready-made, nonproprietary preparation, he must judge for himself whether it is marketed in accordance with the rules. It should, however, be remembered that the application of a trade name to any substance makes it proprietary.
Secrecy Objectionable.—It is not only the right but also the duty of the physician to know the essential composition of what he prescribes; the Council cannot compromise on this proposition.
Vehicles and Preservatives.—In the case of mixtures, not only the potent ingredient, but also the general character of the vehicle, the presence of alcohol, and the identity of preservatives, or of any other substance, whether added or present as an impurity, must be stated if these can under any circumstances affect the therapeutic action of the article. This, as a rule, does not mean the publication of trade secrets, such as flavors or the details of the working formula.
Trade Secrets.—Furthermore, trade secrets will not be received as confidential by the Council, since it accepts information only with the distinct understanding that this may be freely published, at its discretion.
Inspection of Factories.—The Council does not accept invitations to inspect factories; its concern is with the finished products.
On the other hand, the Council requires that the information be complete and accurate as to medicinal ingredients.
Unofficial Constituents.—Unofficial constituents of proprietary mixtures must be presented by the manufacturer in the regular way and must be acted on by the Council before the preparations containing them can be accepted.
Fraud.—When it appears that a manufacturer has made a deliberately false statement concerning a product, he is asked to furnish an explanation; and if this is not satisfactory, the product will not be accepted, even if the false statement is subsequently corrected or omitted.
Testimonials.—The foregoing paragraph applies not only to statements made to the Council, but also to statements furnished to physicians by the manufacturer or his agents, even when these statements are in the guise of testimonials.
In order to avoid errors in the case of chemical compounds, and to guard against adulterations, lack of potency or strength, and the mistaking of one chemical for another, it is necessary to have at hand suitable tests.
Tests, etc.—If these facts have appeared in the literature, or in standard textbooks, reference to them will be sufficient; but with new chemicals, especially synthetics, the manufacturer or his representatives will be required to supply such tests for publication, as will assure an intelligent opinion of these products.
Physiologic Standardization.—In cases in which chemical methods of identification are unknown or unreliable, physiologic standardization should be employed. The Council considers the phrase “physiologically standardized” or “assayed” as misleading unless the standard and method are published in sufficient detail to permit of their control by independent investigators. It is evident that when no standard is published, it is impossible to know whether the quality is high or low, and the conscientious manufacturer who sets for himself a high standard is placed on a level with the dishonest or careless one who adopts a low standard. Again, if the process of standardization is not published, it is impossible to learn, without actual trial, the relative value of one preparation as compared with that of another manufacturer, or to confirm or disprove the statements of the manufacturer as to the quality of his product.
Standardization of Disinfectants and Germicides.—No disinfectant or germicide of the phenol type will be accepted for New and Nonofficial Remedies whose phenol coefficient, determined according to the method of the Hygienic Laboratory, U. S. P. H. S., is not stated on the label of the preparation.
Lay Advertising.—The impossibility of controlling the irresponsible claims which are usually made in advertisements to the public, the well-known dangers of suggesting by descriptions of symptoms to the minds of the people that they are suffering from the many diseases described, the dangers of the unconscious and innocent formation of a drug habit, and the evils of harmful self-medication, including the dangers of the spread of many infectious and contagious diseases when hidden from the physician, and similar well-known considerations, are the reasons for discouraging, in the interest, and for the safety, of the public, this reprehensible form of exploitation. Advertising in medical journals, etc., distributed solely to physicians, does not come within the scope of this rule.
Exceptions.—In the case of subjects on which the public should be instructed, as the use of disinfectants, germicides, antiseptics and foods, advertisements to the public, if not in objectionable forms, are considered admissible. In no case shall such advertisements include recommendations for use as curative agents, nor shall the names of any diseases be mentioned in exploitation. If the preparation is sufficiently toxic to require caution in its use to prevent poisoning, this fact shall be stated on the label. On account of the deplorable results which would follow any abuse of this privilege, the conscientious cooperation of manufacturers and their agents in adhering strictly to the limitations laid down is asked; and for the same reason the acceptance of an article which is so advertised as to infringe on these limitations in any essential way (as by naming diseases or by making false and exaggerated claims) shall be summarily rescinded, and the reasons for such action may be published without notice to manufacturer or agent. A disinfectant, germicide or antiseptic will be accepted for description in New and Nonofficial Remedies, and an article of this class which has already been accepted will continue to be included in New and Nonofficial Remedies only on the explicit understanding by the manufacturer and agent that such infringements of the rule will be followed by deletion of the article and by publication of the facts as described.
Foods.—We may divide the foods into three groups. The first group contains the ordinary foods, including the well-known breakfast foods. These do not come under the supervision of the Council in any way. The second group includes a large and important class of manufactured products, such as invalid and infant foods, which in a sense stand between the first and third groups. The public has the same interest in these foods that the physician has, and usually is supplied with full information concerning them. While the primary recommendation of these articles should naturally come from the physician, it cannot be expected that their continued use should depend on repeated prescriptions. Information concerning this group of foods would come naturally and properly from a physician, and the collection and dissemination of this information may very properly be included in the work of this Council. As the products in this class are used extensively, it is not proper to limit their advertising to medical journals, but the advertising should be permitted in the lay press so long as it is conducted in a manner compatible with the rules of the Council. The third group includes medicinal foods proper, such as predigested foods. These have a relatively low food value and are characterized by a high alcohol or preservative content. They frequently contain strictly medicinal substances, or food substances for which distinct therapeutic properties are claimed. These products should be used only on the advice of the physician, and the advertisements should be restricted as in the case of ordinary medicines.
Advertisements in Foreign Countries.—The Council deals primarily, in the interest of the public and of the medical profession, with articles proposed for admission to New and Nonofficial Remedies, and, in determining the status of any article, must take into consideration any statements made regarding it or any method of advertising it employed by the manufacturer or his authorized agents or representatives, whether in this country or abroad. The Council will not regard as within its scope, however, questions concerning the marketing of articles (except the matter of direct advertising to the laity and unwarranted claims or misrepresentations) in any country which has a public body corresponding to this Council.
Matter Distributed Solely to Physicians.—It should be remembered that the sole intent of this rule is to protect the physician, so that in prescribing a proprietary medicine he shall not unconsciously advertise proprietary preparations. The rule imposes no restriction on the legitimate methods of bringing a remedy to the attention of the profession, such as advertising in medical journals, circulars and other printed matter distributed solely to physicians. The rule applies only to the package as it may reach the patient.
Naming Diseases on Labels.—The naming of diseases on the label or package is not necessary, as is shown by the very large number of proprietary products which have been successfully introduced without resorting to this expedient. This method of popularizing a proprietary remedy with the laity is most objectionable, and should not be tolerated in any form. In general, therapeutic indications should be omitted from the label and package. The Council will not insist on this point, however, when such indications are so given as not to promote self-medication, particularly in diseases which require expert diagnosis and supervision. It will be considered an infringement of the rule if an article be marketed in bottles which have the name of the article blown into the glass, or if otherwise the name or initials or other distinctive mark of the article is permanently stamped on the container, on the article itself, or is on the stoppers or seals. Articles which are marketed in any of these ways are not accepted for New and Nonofficial Remedies. Readily removable labels are not objectionable, nor is the permanent affixing of the firm’s initials or name to the trade package if such initials or name is not suggestive of the article. The Council does not countenance the use of an accepted article for advertising other articles which have not been accepted by the Council.
Source.—No false or misleading statement in regard to an article can be permitted concerning the source of material from which it is made, or the persons by whom it is made. Some glaring frauds of this nature have been perpetrated in the past, and this rule is intended to prevent such imposition.
Therapeutic Questions.—This rule insists that the claims of manufacturers or agents concerning the therapeutic properties of their products must be compatible with demonstrable facts. Manufacturers will be held responsible for all statements made or quoted in their advertising “literature” regarding their products. Recognizing the existence of honest differences of opinion on many therapeutic questions, the Council desires to be liberal in the application of this rule. It is natural that a manufacturer should be partial toward his own product, and a moderate degree of emphasis in advertising may not be objectionable. The Council, however, will not admit claims which are neither in harmony with already accepted facts nor supported by acceptable evidence. In doubtful cases the Council considers these questions with the advice and cooperation of its staff of clinical consultants.
Clinical Evidence.—To be acceptable, the clinical evidence must offer objective data with such citation of authority as will enable the Council to confirm the facts and establish the scientific value of the conclusions drawn. Clinical data are worthless when the author is not cited. The facts on which claims with regard to the value of a remedy are based must have been rendered accessible for investigation and confirmation by disinterested observers, either through publication or through the records of a hospital or other institution.
Poisons.—For the information of the pharmacist or dispenser, and to enable him to safeguard the interests of the patient and the physician, all articles containing such potent agents as the poisonous alkaloids and other organic substances and the salts of some of the metals should have the exact amount of these ingredients which is contained in the average adult dose stated on the label.
“Coined” Names.—Many of the abuses connected with proprietary medicines arise from “coined” proprietary trade names. Such names will not be recognized by the Council unless in particular instances the Council shall deem their use to be in the interest of public welfare. In every such exception the burden of proof, both for establishing and for continuing the exception, lies with those who market the product.
Proprietary (“Trade”) Names When Permitted.—In consideration of the benefits which may come from the discovery of a therapeutic agent, the Council concedes to the person or firm which, by right of discovery, controls such a product the right to name it. The Council will offer no opposition to an arbitrary name for such a new product, provided it is not misleading, therapeutically suggestive, or otherwise subversive of scientific pharmacy and therapeutics. If the discovery that a previously known substance has therapeutic value is deemed of sufficient importance, the Council may recognize a name for such a substance if the name is applied by the person who makes the discovery; or, with the consent of the discoverer or in the absence of any protest on his part, the Council may recognize a name applied by the firm which first makes such a product available to physicians. In the interest of rational drug therapy, the Council recommends that trade names be coined so as to indicate the potent element or constituent.
Scientific Names.—When the proprietary or trade name for an article is considered insufficiently descriptive of its chemical composition or pharmaceutical character, the Council may require as a condition for the acceptance of such articles that a descriptive scientific name satisfactory to the Council appear on the labels, circulars and advertisements for such an article. For all definite chemical substances it is required that the scientific name be given prominence on the labels, in circulars and advertisements.
Proprietary Names for Unoriginal Articles.—Proprietary names will not be recognized for articles which are included in the U. S. Pharmacopeia or National Formulary or for unessential modifications of such articles. Neither will proprietary names be recognized for substances or mixtures which are described in medical or pharmaceutical publications. In the marketing of unoriginal articles, the legitimate interests of the producer are fully served by identifying such products by appending the name or initials of the manufacturer or agent, or by the use of a general brand mark. No objection will be made by the Council to the use of such brand marks, provided that in no case shall such mark be used as a designation for an individual article.
For any product which, by reason of the absence or lapse of patent rights or for other reasons, is open to manufacture by more than one firm, the Council reserves the right to select a common name and to provide standards of identity, purity and strength, and then will accept such article only if it is marketed under the title adopted as the N. N. R. name or the name under which such article was introduced (to which may be appended the firm’s identifying mark).
N. N. R. to U. S. P.—When an article which has been accepted for New and Nonofficial Remedies is admitted to the U. S. Pharmacopeia or National Formulary, it will be omitted from New and Nonofficial Remedies one year after such standardization if the name of such article is used in these standards either as the main title for the product or as a synonym. If the name under which the article is described in New and Nonofficial Remedies is not used in these books of standards, the proprietary preparation will be retained provided the official name is given prominence on the labels and in the circulars and advertisements of such article. When the Council adopts a common name for an article that has been admitted under another name, it will be continued under the older name only on condition that the Council name be given prominence on the label and in the circulars and advertisements for such article.
Pharmaceutical Preparations and Mixtures.—These, with rare exceptions, are not original in composition and there is seldom any reason why they should be endowed with arbitrary names. On the contrary, it is important that the prescriber should be reminded constantly of their potent ingredients.
Therapeutically Suggestive Names.—Articles bearing therapeutically suggestive names will not be accepted for New and Nonofficial Remedies, first, because they are likely to lead physicians into prescribing names instead of remedies, and second, because they tend to encourage unwarranted self-medication by the laity. Even if the name is at first apparently meaningless to the public, its meaning will soon be understood because patients soon learn the technical names applied to their diseases and symptoms. The prohibition against therapeutically suggestive names is not applied to serums, vaccines and antitoxins, because the accepted nomenclature of the specific organisms used in their preparation makes this unavoidable and because self-medication with them is improbable.
Protection.—This information is important as a means of determining the legal status of medicinal articles and as an aid to their ready recognition in current publications.
Unscientific Compounds.—The use of articles which are unessential modifications of official or established nonproprietary articles is unscientific and serves no useful purpose. The Council will not accept products which are scientifically unsound and which, therefore, must be considered useless or inimical to the best interest of the medical profession and the public. This class includes compounds or mixtures containing an excessive number of active ingredients; those compounds or mixtures the components of which are of no probable assistance to one another, and those articles which are of no therapeutic value.
Unessential Modifications of Official Substances.—The subterfuge of obtaining proprietary rights over an official or established nonproprietary product, by introducing unessential modifications, also tends to confusion and abuses, and such articles will not be admitted by the Council. Essential and important modifications, however, will receive recognition. (The Council interprets the term “established nonproprietary product” as applying to a preparation of any formula which has been published through any recognized or reasonably accessible channel of publication, prior to its appropriation or modification by a manufacturer.) Duplicates of biologic products accepted under the name of the manufacturers will not be accepted under the names of the distributors.
Secretary, Council on Pharmacy and Chemistry
The World War marked an epoch in the existence of the Council on Pharmacy and Chemistry, as it did in all human endeavors. The information and experience which had been accumulated by the Council during its thirteen years’ existence was drawn on by our government, directly or indirectly, and it also received consideration in England, France,1 Belgium, Holland,2 Italy,3 Sweden and elsewhere. In the world wide readjustment that has begun, the efforts of the Council, past and present, will influence the plans of those who engage in the manufacture or sale of medicines, and, undoubtedly, will be the incentive to the establishment of similar bodies in other countries.
As secretary of the Council almost from the time of its organization in 1905,4 and knowing the work of its members and its collaborators, I am firmly convinced that this body has deserved the endorsement and support given it by the American medical profession. I welcome this opportunity to present an outline of the Council’s past activities and to speak of some of the problems of the future, because I feel assured that a knowledge of its endeavor to improve drug therapy will increase the profession’s confidence in the Council and add to the number of its supporters.
Organized primarily for the purpose of putting a stop to false declarations with regard to the composition of proprietary medicines, the Council’s activities have broadened until its work may be characterized as “a propaganda for the rational use of drugs.” The following are some of its activities:
1. New and Nonofficial Remedies.—This is an annual volume, issued by the Council. It describes both proprietary and nonofficial, nonproprietary drugs which are deemed worthy of consideration by the medical profession. To be admitted to this book, a preparation must comply with certain definite rules which stipulate, in effect, that its composition be declared, that no untrue or grossly exaggerated claims be made for it, and that it shall give promise of having therapeutic value.
With the exception of a few which are still under consideration, the Council has considered all proprietaries whose owners or accredited agents have requested that an examination of the products be made, and it has admitted to the book those which were found eligible. In addition, the Council has examined all of the more important or widely exploited proprietaries, even when no examination was requested, and it has admitted those of this group which were found eligible. Further, the Council has admitted to the book certain nonofficial, nonproprietary articles which seemed to give promise of therapeutic usefulness, and it has established standards for the control of their identity and purity, and listed those brands which complied with these standards.
As most proprietary medicines are of a more or less experimental nature, they are accepted for inclusion in New and Nonofficial Remedies only for a limited time—usually a period of three years. At the expiration of the period of acceptance, each preparation is reexamined and retained only if the claims made for it and the present day knowledge of its value permit this action.
Since manufacturers give information only in regard to their own products, New and Nonofficial Remedies groups together articles of a similar character, and includes in each case a general discussion of the group for the purpose of comparison, not only with each other, but also with the established or pharmacopeial drugs which members of the group are intended to supplant.
In brief, New and Nonofficial Remedies is a book in which are described preparations that have been accepted by the Council. The description includes facts that the physician should have. It is a book that should be in the hands of every physician who prescribes medicines, and who wishes to know the facts regarding the newest remedies. It is the only book in which he can find information relative to proprietary medicines that are worthy of his patronage. It will protect the physician who makes use of it against the wiles of the promoters of products not worthy of his patronage. It would certainly be of use to the physician when the detail man calls on him, for if he were being importuned to prescribe or use samples of something which he had not heretofore used and which he was unable to find in N. N. R., he might ask the detail man why. In the nature of things few physicians are sufficiently expert in chemistry and allied sciences to be able unerringly to discriminate between the true and the false as regards many preparations that he is asked to prescribe.
2. The Reports of the Council on Pharmacy and Chemistry.—A medicament may be inadmissible to New and Nonofficial Remedies for various reasons; it may be worthless or irrational, its composition may be secret or indefinite, or it may be exploited under exaggerated or unwarranted claims or in a way otherwise detrimental to the public health and scientific medicine. Of these various reasons which make an article unacceptable, the manufacturer obviously may remove all except the first, viz., worthlessness or irrationality. Consequently, a preparation which has been presented for admission is not definitely rejected until after its proprietor has been informed of the objections to his product and has failed to bring the preparation in conformity with the Council’s rules. When a preparation is found definitely inadmissible to New and Nonofficial Remedies, that is, when the proprietor cannot or will not make it acceptable, the Council prepares a report for publication. These reports are sent for publication to The Journal of the American Medical Association, and later published in the annual “Reports of the Council on Pharmacy and Chemistry.” The more important of these are also published in the book, “The Propaganda for Reform in Proprietary Medicines.”
3. Useful Drugs.—Since the domination of proprietary medicines, which was retarding medical advance and threatening therapeutic chaos, had been made possible only by the insufficient and inefficient instruction given in medical schools in subjects having to do with drugs, the Council appointed a Committee on Medical Teaching to secure the cooperation of teachers in materia medica, pharmacology and related branches. This committee has endeavored to effect an improvement in these courses of instruction. One of the results of this work was the selection of a list of drugs to serve as a basis of materia medica instruction and thus insure that medical students shall be better informed with regard to the therapeutic worth of a few well established drugs, rather than, as in the past, leaving school with a smattering of knowledge about many drugs. The outcome of these efforts is the publication of “Useful Drugs,” a concise but thorough and up-to-date discussion of the actions, uses and dosage of the more important drugs. The list of drugs presented in this book is now the basis of instruction in many schools; and many state examining boards are confining their materia medica questions to the drugs in the list.
4. Epitome of the U. S. P. and N. F.—To encourage the use of official drugs and to make available an estimate of their therapeutic value, a committee of the Council prepared an abstract of the U. S. Pharmacopeia and the National Formulary. This booklet, the “Epitome of the U. S. Pharmacopeia and National Formulary,” presents those portions of these books which are of interest to physicians, and in addition, gives a concise statement of the therapeutic usefulness of the drugs and preparations described in them.
5. Patent Law Reform.—Some of the worst abuses connected with the exploitation of proprietary medicines have been made possible by our patent and trademark laws and the method of their interpretation and enforcement. The Council, therefore, appointed a committee to study these laws and the various propositions advanced for their improvement. This committee has published, from time to time, reports on various phases of our patent and trademark laws and recently summarized these reports in an address5 sent to the commissioner of patents and the interested congressional committees. It is hoped that by means of these reports physicians will be enabled to give intelligent support to a revision of the patent and trademark laws when legislation is proposed.
6. Therapeutic Research.—Through its Committee on Therapeutic Research, and with the aid of funds provided by the Board of Trustees of the American Medical Association, the Council has encouraged the investigations of questions which might lead to a better understanding of the action of drugs. These investigations are brought together in the annual reports of the Committee on Therapeutic Research, and are an important addition to our knowledge of drug action.
In the past, the Council has in particular encouraged the investigation of the action and therapeutic value of widely used drugs regarding which our knowledge is still unsatisfactory. These investigations have included a study of the action of strychnin in cardiac disease, a comparison of the action of absorption and excretion of iodid preparations, a study of the pharmacology of the opium alkaloids, etc. Appreciating that the available knowledge of proprietary drugs is one sided in that it comes from investigations made by interested pharmaceutical concerns or from investigations made at the instigation of these firms, the Council is planning a comprehensive study of many of the synthetic drugs that have gained some vogue during recent years.
Medical research, and efficient instruction in therapeutics and related subjects, spell a diminishing influence of commercial medicine over rational therapeutics. The fact that the present shortage of German synthetics has not handicapped seriously the practice of medicine should be a lesson to American physicians for many years to come.
On the other hand, it must be remembered that the publicity given to the reports of the Council and to other contributions toward rational therapeutics by The Journal of the American Medical Association, the journals of the state organizations, and a few personally owned publications, is as nothing when compared with the persistent and wide publicity given to the propaganda of the proprietary houses. While a report setting forth the objections to a proprietary is published but once, the firm’s laudatory pronouncement goes forth again and again until the Council’s report is completely overwhelmed and forgotten. Manufacturers of proprietaries not only keep in close touch with the practicing physician by means of house organs, special “literature,” or by traveling representatives, but many of the firms, through the meritorious lines of pills, tablets, tinctures, etc., which they put out, also obtain and hold the good will and confidence of a large proportion of the medical profession.
Furthermore, some of these firms may gain the confidence of the medical profession through these high grade pharmaceuticals, and certain of their proprietaries may be of distinct therapeutic value but may fail to be acceptable for New and Nonofficial Remedies, because they do not conform to the reasonable rules of the Council. These firms do not find it profitable to force the sale of their regular nonproprietary pharmaceuticals by unwarranted claims or objectionable methods, yet they may consider it good business to market certain proprietary products by means of claims which are extravagant and without warrant, and which will lead to indiscriminate use by the profession and the public. In a word, where there is one dollar spent on behalf of rational medicine, thousands are spent for the purpose of increasing the sale of preparations which directly or indirectly are a detriment to the public health, to medicine, and to the pocketbook.
That the day of the secret nostrum of the pseudo-chemical company is not yet past is well illustrated by the recent introduction of an asserted arsphenamin preparation called “Syphilodol.” The A. M. A. Chemical Laboratory proved one form of this asserted French discovery to be essentially a pill of mercurous iodid. Another form of Syphilodol (for intravenous administration) had all the characteristics of water, and appeared devoid of any potent ingredient. Though the advertising sent out by the promoters in regard to its composition was suspiciously evasive, the Illinois Medical Journal published an advertisement of “Syphilodol,” which, possibly by a coincidence, appeared above an appeal to “Our Readers” to use wares advertised in that journal.
While such rank deceptions as “Syphilodol” are not common, there are more subtle deceptions that are even more dangerous. Types of widely exploited remedies of today comprise so-called ethical specialties composed of well known and established drugs (with “jokers” hidden away somewhere) or preparations which have a plausibly fascinating pseudo-scientific background of radiant energy, colloidal chemistry, nonspecific protein reaction, or something of the sort. The latter class of preparations in particular appeal to physicians who are striving hard to keep pace with modern science. Exposure of their fallacies requires most careful consideration on the part of the Council.
Progress toward a rational and scientific drug therapy must continue, and, therefore, it is important that the Council on Pharmacy and Chemistry should continue to make the investigation of proprietary medicines its chief work. Investigation of a proprietary medicine, however, and a report of such investigation are of value in direct ratio only to the number of physicians who read the report, endorse it and act in accordance with its conclusions. In order that you may determine to what extent those preparations which are admitted to New and Nonofficial Remedies deserve your interest and confidence, it will be worth while briefly to outline the rules which govern the Council in the admission of articles to New and Nonofficial Remedies.
Composition.—Rules 1 and 2, and in a measure 5, 7, and 9, deal with the composition of articles. Rule 1 requires that the quantitative composition of an article be furnished the Council for publication. Rule 2 requires that the manufacturer furnish methods whereby the composition of products that are definite chemicals or the potent constituents of mixtures may be determined. The Council does not require that the process of manufacture of an article be declared unless this becomes necessary in order to judge its composition. Rule 5 requires that statements with regard to the origin and source of an article shall be truthful. Rule 7 requires that for the guidance of the dispenser, the amounts of poisonous ingredients of a preparation be placed on the label. Rule 9 requires that if patent rights are claimed for a product, the Council be informed on this point.
That it is not only the right but also the duty of the physician to know the composition of what he prescribes for his patients is so generally admitted that few have attempted to market preparations of avowedly secret composition. When the Council first began its work, it was common to see chemical formulas or statements of composition published which a chemist or a pharmacist was able to pronounce at a glance as impossible.6 It was not unusual to find that the promoter published “a formula” for his preparation, rather than “the formula.”7 Today, however, a more prudent, if not more honest, course is pursued. This gives a “formula” which is correct so far as it goes, but which fails to divulge the actual composition of a preparation. When it is considered that many physicians are not any too conversant with the chemistry and pharmacy of drugs, it is not surprising that some administered the proprietary “Venarsen,” regarding the composition of which they had only the vague statement that it was “... a comparatively nontoxic organic arsenic compound, 0.6 gm. representing 247 mg. (33⁄4 grains) of metallic arsenic in chemical combination ...” in the belief that a preparation similar to that first introduced as salvarsan was being used. That “Venarsen” contained its arsenic as sodium cacodylate—a notoriously inactive state of combination—does not justify the intravenous administration of a drug of unknown composition.
While for the present it probably is not feasible to require, on the part of those who manufacture medicinal preparations, such professional training as is required of those who prescribe and those who dispense them, it certainly is not too much to require, as does Rule 2, that a manufacturer shall be able to demonstrate that his preparation has the composition claimed for it. Nor is it sufficient for him to know that the ingredients claimed as constituents were used in the manufacture. The fallacy of his method of reasoning was furnished by the physician who reported that he had personally added the required amount of mercuric iodid for a batch of “Mercol” which, nevertheless, was devoid of mercury.8 Acceptance of this rule by manufacturers will permit physicians to have a more accurate knowledge of the composition of preparations such as “Taka-Diastase”9 and “Iodeol”.10
A requirement similar to that of Rule 5 is contained in the Federal Food and Drugs Act and so no objection has been made to this rule which requires a truthful statement of the origin and source of articles. An illustration for the need of the rule was furnished by the one time popular “Vin Mariani”11 which, though very French in its makeup, was found to be largely of the “made in the United States” variety of tipple.
The issuance of a patent for a medicinal product does not prove that such a product presents a discovery or that its owner is entitled to a temporary monopoly, yet it is only fair to physicians and to other manufacturers that notice of such patent claims be given. Hence, the Council publishes in New and Nonofficial Remedies the information bearing on this point.
Lay Advertising.—Rules 3 and 4 provide against the recognition of articles that are advertised to the public directly or indirectly, exempting from this requirement preparations which the Council believes are safe to be so advertised.
It has been held with some justice that certain shotgun proprietaries are purchased by the public with as much circumspection as they are ordered by those physicians who are addicted to the prescribing of them; but even the exploiters of these mixtures have not denied that the use of medicines by the public on its own initiative is surrounded with many objections. Hence the practice of self medication should not be encouraged by prescribing or using those preparations advertised for public use.
The only objection to the rule has come from a firm which markets a brand of liquid petrolatum, the Standard Oil Company of Indiana. The Council has considered the question of exempting simple laxatives from the restrictions of Rules 3 and 4 as it has exempted antiseptics and nonmedicinal foods. The conclusion was, however, that the excessive use of a simple laxative like a liquid petrolatum, when prompted by newspaper exploitation, is likely to be detrimental to health by overuse as well as by misuse.
The indirect advertisement to the public, which Rule 4 provides against, has been the means of inducing the extensive lay use of “Antikamnia,” “Bromidia” and “Fellows’ Syrup.” Naturally Rule 4 has been bitterly opposed by most proprietary firms. Arguing that many physicians dispense their own drugs, pharmaceutical firms have insisted that every medicinal preparation should bear on its label, not only the dose of the preparation, but also a statement of the diseases in which the article is indicated. Whether manufacturers anticipated the profession’s resentment toward the claim that physicians determine the treatment and perhaps the diagnosis by means of the statements on labels, or because the Shirley amendment to the Food and Drugs Act makes the proprietor responsible for therapeutic claims on the label of a medicine, it is a fact that fewer preparations than formerly need to be refused on account of infringement on this rule. In fact, some thoroughly objectionable proprietaries make a show of being “ethical” by omitting all therapeutic discussion from the labels of their preparations.
Therapeutic Claims.—Rule 6 makes ineligible for New and Nonofficial Remedies any articles regarding which the manufacturer or his agents make unwarranted, exaggerated or misleading statements as to the therapeutic value. Recognizing the long established custom of therapeutic exaggeration, it has been most difficult to determine the degree of conservatism which might with fairness be required of a manufacturer. In view of the common acceptance of individual impressions as dependable evidence, it is often almost embarrassing to declare as incompetent the statement of some well meaning and all-too-kind-hearted doctor. However, as the pitfalls of haphazard clinical trials become better known and the physician’s mistrust of glowing accounts of marvelous cures more outspoken, the manufacturers’ claims will be more moderate.
Nomenclature.—Were it possible to enact and enforce a law which would oblige manufacturers to sell their medicinal products under properly descriptive names and which would make it illegal for a physician to prescribe it unless he understood the meaning of such properly descriptive titles, then the Council might safely disband. In that case, physicians would discontinue the use of most proprietary preparations in favor of established drugs, and successful newcomers might each year be counted on the fingers of one hand. Such a rational nomenclature is not to be thought of, at least in our generation. Rule 8 requires that the name of an article shall not be misleading, that it shall not be therapeutically suggestive, and that established drugs shall not be disguised by fanciful titles. It recognizes the right of discoverers of new drugs to name their discoveries, and interposes no objection to arbitrary names for such products so long as such names are not misleading or do not suggest the therapeutic uses of the products. As the rule provides against the recognition of coined names for established nonproprietary drugs, so it requires that mixtures of drugs shall bear names descriptive of their composition. It would be a long step forward if physicians would recognize more fully the objections to the many proprietaries which have, as their only point of originality, a non-descriptive name for an old drug or a mixture of well known drugs. It is an encouraging sign that the Federal Trade Commission, when issuing licenses for the manufacture of synthetic drugs introduced under German patents, stipulated that all manufacturers authorized to make a given drug shall use the same name for it.
Irrational Articles.—Rule 10 provides against the recognition of an article which, because of its composition, is useless or inimical to the best interests of the public and medical profession. This rule excludes medicaments which (1) are unessential modifications of established articles, or (2) are of no therapeutic value or (3) are irrational. With regard to the recognition of mixtures or compounds containing two or more active ingredients, the Council requires that the manufacturer establish the rationality of its combination. The rule has prevented the recognition of many unnecessary so-called ethical specialties. Though a mass of testimonials was often to be had for them, these contained no evidence that the mixture was superior to its potent ingredient, or that its therapeutic effect had been determined. That there is a healthy tendency to use single drugs for their definite action and to discard combinations (be they shotgun proprietaries or “mixed” vaccines) is perhaps best illustrated by the fact that at the last revision of the U. S. Pharmacopeia a considerable number of complex antiquities were omitted from that book.
Feeling confident that this meets with the endorsement of the profession, the Council is examining more critically the evidence for the value of pharmaceutical mixtures.—(From The Journal A. M. A., May 10, 1919.)
Under the caption given above, the Journal of the Missouri State Medical Association, in its July issue, speaks editorially as follows:
The Council on Pharmacy and Chemistry of the American Medical Association is a department of our national organization that has not received the plaudits and encomiums of a wildly joyous medical profession nor the grateful praises of the enthusiastic manufacturer of pharmaceuticals. The Council seems indeed to be the unloved child of the entire family of subsidiary bodies of the association. Perhaps the reason for this may be found in the character of its duties, for the Council must expose fraud, sometimes in high places, and protect the physician from being duped by avaricious persons and by persons who are themselves sometimes the victims of their own credulity. It thus happens that the sale of some proprietary article previously held in high esteem by the practitioner proves valueless, perhaps even fraudulent. The practitioner, however, may have credited much of his success in treating certain conditions to that preparation and the maker has had success in accumulating dollars from its sale and both parties emit a loud and vicious roar against the Council, because they both lose money. Nobody wants to be “protected” against making money—make it honestly, if possible, but make it—but this black sheep among the Councils of the American Medical Association insists on their making their money honestly!
Despite many obstacles thrown into its path, the Council on Pharmacy and Chemistry has serenely pursued its allotted tasks, corrected its mistakes, improved its methods, and today stands as the only medium to which the honest physician may turn for information—not misinformation—regarding proprietary articles. During the war the Council and the chemical laboratory were in close cooperation with the Surgeon-General’s Office, testing and investigating every article offered to the government for the treatment of sick soldiers. The variety and the number of fakish and fraudulent stuff offered to the Surgeon-General was a pitiable exhibit of the mental gymnastics of some people. Just now the Council and the laboratory have a new and important field before them, i. e., to protect the physicians against worthless and useless serums, vaccines and synthetics. It will be the Council’s unpleasant duty to expose the fraudulent and useless among these articles and stamp truth on those found worthy.
We seem to have wandered from the topic in our caption, but not so in reality, because the burden of our thought is to lend our influence to the spread of the motto of the Advertising Clubs of the World, namely, “Truth in Advertising.” It is our purpose to stimulate a larger degree of enthusiasm for the work of the Council on Pharmacy and Chemistry and the Chemical Laboratory, a more generous flow of inquiries concerning articles unfamiliar to the physician, and particularly to urge that the words “Accepted by the Council on Pharmacy and Chemistry of the American Medical Association” be printed on the label and on all advertising circulars of proprietary articles that have been admitted to New and Nonofficial Remedies. Then, when pamphlets and circulars are received by physicians they will read the statements of manufacturers with sympathetic understanding and with full confidence in the verity of the declarations. The importance of creating just that sort of receptivity in the mind of the prospective buyer is so well known to the astute publicity expert that it is needless for us to dwell on its advantages. Every proprietary article advertised in our journal, in The Journal of the American Medical Association, and in the other state association journals, as well as in several well-edited privately owned journals, does in effect say to the reader that the articles so advertised are accepted by the Council because only proprietary articles so accepted are accepted by us. The fact is further acknowledged when these firms are permitted to exhibit their goods at our annual sessions for again the rule is enforced that only proprietary articles which have been approved by the Council may be placed on display.
Why not complete the circle of ideas—it would not be a “vicious circle”—by printing on labels, in advertisements and circulars, the words: “Accepted by the Council on Pharmacy and Chemistry”?—(From The Journal A. M. A., Aug. 2, 1919.)
If they were built that way, the members of the Council on Pharmacy and Chemistry of the American Medical Association might become discouraged at the apparent indifference of many members of the medical profession to their efforts. There are many physicians who, while figuratively patting the Council on the back, actually do nothing to aid its efforts. On the other hand, there are men in the profession who give the Council active support instead of merely passive appreciation. The letter that follows was written by such a man to a pharmaceutical concern:
I am receiving circular advertising from you concerning —— —— solution, and I am writing to suggest that until these products have been approved by the Council on Pharmacy and Chemistry of the American Medical Association, you are wasting your postage on the practice. Aside from the fact that these products do not appeal to me personally, I feel that I am not in a position to judge the value of such products and I depend entirely on the large clinical opportunities of the Council on Pharmacy and Chemistry of the American Medical Association in addition to their laboratory facilities, in such matters as these. I may, therefore, with all due respect, suggest that ... it will pay you to eliminate my name from your mailing list.
The members of the Council on Pharmacy and Chemistry are working week in and week out without remuneration. Few appreciate how much these scientific men are doing for rational therapeutics; fewer still realize how much has been accomplished through their efforts, or how much more could be accomplished if every physician who at least believes in the work of the Council would give it his full support.—(Editorial from The Journal A. M. A., Nov. 6, 1920.)
The Council has adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
The Council frequently receives inquiries—some of them accompanied by expressions of impatience—concerning articles, reports on which appear to be delayed. It therefore seems advisable to make a statement of some of the factors which enter into this problem.
The Council fully realizes the importance of giving prompt information to the profession with regard to proprietary medicines under consideration. It therefore acts as soon as sufficient information is available to justify a definite judgment, and publishes its conclusions as soon as possible. When adequate information is available at the outset, there is no delay in the publication of the Council’s conclusions.
Unfortunately, but very naturally, there are many cases in which the information available at the time the product is submitted is not sufficient to justify the Council in coming to definite conclusions for or against the preparation. In some cases the manufacturer possesses the required information, but to obtain it from him takes time; in other cases the manufacturer does not possess the information—perhaps he did not realize the inadequacy of his evidence until the subject was brought to his attention by the Council.
Such cases might be dealt with in either one of two ways: The Council might at once reject the article because the claims for it are not supported by adequate evidence; or, the Council might suspend judgment and give the manufacturer an opportunity to supply the information.
The first method—immediate rejection—would obviously be felt by manufacturers as a hardship. To afford the fullest possible opportunity for the presentation of the case, the Council follows the second method; that is, it suspends judgment and withholds publication of a report until reasonable time has been afforded for furnishing the required information, provided the manufacturer or agent appears to be making honest and diligent efforts to supply it. The collection and compilation of such information is sometimes a lengthy process, especially when the products are of foreign manufacture.
Although it would be easier for the Council to render an immediate decision than to assist manufacturers to supply the data necessary for the formation of an authoritative judgment, the Council cannot yield to importunities for hasty action. It must rely on the medical profession to bear in mind that the character of a product under consideration by the Council has not yet been determined. The Council holds that, during this stage, a product is suitable, at most, for experimental use.—(From Reports of Council on Pharmacy and Chemistry, 1915, p. 119.)
In reply to the suggestion made last year by President Bevan that there should be closer cooperation between the large pharmaceutical houses and the Council on Pharmacy and Chemistry, the Council submitted to the Board of Trustees of the American Medical Association the statement which appears below:
“Cooperation of the Pharmaceutical Houses: At the opening meeting of the House of Delegates last year, President Arthur Dean Bevan suggested the desirability of greater cooperation between the large pharmaceutical houses and the Council on Pharmacy and Chemistry. The need of such cooperation has been recognized by the Council from the first. In no one direction has the Council made greater effort than in its endeavor to secure the fullest cooperation of the various pharmaceutical houses. The difficulty has been, and always must be, the fundamental antagonism between objectives that are largely commercial on the one hand and purely scientific on the other. Nevertheless, the Council has always believed—and has acted on the belief—that there is a possible middle ground wherein the interests of therapeutics would not be injured but would go hand in hand with a commercial development based on enlightened self-interest.
“The profits to be made by a pharmaceutical house from the sale of a staple drug—a pharmacopeial, National Formulary, or nonproprietary preparation—which enters into free competition with other drugs of the same kind, are moderate; the profits to be made from the sale of a proprietary medicine on which the manufacturer holds a monopoly are usually large—sometimes enormous. There are, broadly, two kinds of proprietary preparations advertised to physicians: One represents laborious research ending in the production of a new medicinal chemical; this product can be patented and the manufacturer can obtain a seventeen-year monopoly on its manufacture and sale. The other represents no research but comprises simple mixtures—frequently of the “shotgun” variety—of well known pharmaceuticals, or biologic products sold under trade names. As these do not represent anything new or original the manufacturer is unable to obtain a patent, but by means of the trade name he can and does obtain a perpetual monopoly. This, from a business standpoint, is more valuable than the limited monopoly granted by a patent. It is not surprising that proprietary remedies of the latter type flourish so long as physicians unthinkingly accept and prescribe them solely on the manufacturer’s valuation.
“The Council has practically the undivided support of manufacturers of medicinal chemicals; that is, of proprietaries of the first mentioned type. But pharmaceutical firms which have found it profitable to promote proprietaries of the second type—“specialties,” unscientific or ordinary mixtures of pharmaceuticals or biologic products sold under trade names—have not supported the Council.
“When the Council was organized, it was hoped and believed that all the large pharmaceutical houses would find it possible and desirable, if not actually more profitable, to shape their business methods so as to make their proprietary and other articles conform to those conservative standards on which the Council bases its rules, and thus render such articles acceptable for New and Nonofficial Remedies. It soon developed, however, that the methods of the pseudochemical companies, whose sales propaganda in the interest of unscientific nostrums with its attending damage to scientific medicine had led to the establishment of the Council, had found their lodgment in most of the pharmaceutical houses. It was a genuine disappointment to the Council to find that some of the large and old-established firms were not only unwilling to cooperate with the Council, but in many instances exhibited a definite antagonism to the Council’s work.
“The object—and duty—of the officers of pharmaceutical houses is primarily to pay dividends to their stockholders. Through skilful advertising or the persuasiveness of “detail men,” they are able to induce physicians to prescribe their controlled products, on which there are large profits, even though such products have not only not been accepted by the Council, but in many instances, have been disapproved. Is it any wonder that concerns which put out such products are indifferent or openly antagonistic to the work of the Council? The matter is largely one of business policy. When the medical profession as a unit will support the Council in its work, then such firms will find it good business policy to accede to Dr. Bevan’s suggestion—but not before.”
Evidently the problem resolves itself into this: The Council, constituted of scientific men, working without remuneration in the interest of scientific medicine and the medical profession, expects—and rightfully—the cooperation and support of the members of that profession. What is needed, therefore, is the active, sympathetic cooperation of physicians; the cooperation of pharmaceutical houses will follow as a matter of course. (J. A. M. A. 74:1235 [May 1] 1920.)
The following is the recommendation of the Reference Committee to which the Report of the Board of Trustees was referred: “A perusal of the Trustees’ Report, ‘Cooperation of the Pharmaceutical Houses’, is well worth the time of every member of the profession, and your committee would emphasize the statement of the Trustees: ‘The Council, constituted of scientific men, working without remuneration in the interest of scientific medicine and the medical profession expects—and rightfully—the cooperation and support of the members of that profession. What is needed, therefore, is the active sympathetic cooperation of physicians; the cooperation of pharmaceutical houses will follow as a matter of course.’
“Your committee would go still further and move that a vote of thanks of the House be extended to those scientific men who have devoted so much valuable time to the welfare of the Association.”
(J. A. M. A., 74:1322 [May 8] 1920; from Reports of Council on Pharmacy and Chemistry, 1920, p. 56).
W. A. Puckner, Secretary.
The Budwell Pharmacal Company, Lynchburg, Virginia, which markets these preparations, claims that “No. 1” contains cod liver oil, “Iodide of Arsenic,” “Iodide of Calcium,” and “Iodide of Manganese.” “No. 2” is said to contain in addition to the ingredients of No. 1, creosote carbonate and guaiacol.
It is known that arsenous iodid is decomposed by contact with water. It is recognized that creosote carbonate is unstable and prone to liberate creosote. Iodide of manganese not being official, the supply on the market is not controlled in any way: Tests of purity are not prescribed by the Pharmacopeia, the National Formulary, New and Nonofficial Remedies or other books of standards. Therefore doubt must be expressed as to the accuracy of the formulas as given. The Council cannot accept such statements of composition without further evidence.
“No. 1” is commended for use in
“Chronic Rheumatism, Glandular Swellings, later forms of Syphilis, convalescence from Scarlet Fever, La Grippe and Malaria, Chronic Malarial Infection, Marasmus, Joint or other suppuration of standing, diseases of skin, chorea, anaemia, neurasthenia, obstinate neuralgia, scrofulous affections in general, and diarrhea or dysentery (subacute or chronic) in childhood.”
“No. 2” is said to be
“Prepared especially for the treatment of Chronic Throat, Nasal, Bronchial and Pulmonary Diseases.”
In the advertising circular statements regarding the various ingredients of Budwell’s Emulsion are quoted from obsolete text books. These statements, for the most part, do not represent modern opinions on the subject. For instance, the circular praises the action of guaiacol as eliminated directly by the lungs, thus exerting a beneficial local effect and causing bacilli to diminish in numbers or to disappear. All of this is directly contradicted in authoritative modern publications on pharmacology, which hold that the excretion of guaiacol by the lungs is infinitesimal and its action on bacilli is nil. The Council held the preparations in conflict with its rules as follows:
1. Many of the therapeutic claims are exaggerations.
2. The method of exploitation amounts to an indirect invitation to the public to use these preparations as “consumption cures.”
3. The preparations are unscientific, they constitute a reprehensible invitation to uncritical prescribing and their use is inimical to the best interests of the profession and the public. It is difficult to imagine in what conditions such a combination would be indicated. These preparations are a remnant of the days of polypharmacy. Their use is not in keeping with present medical thought and practice.—(From The Journal A. M. A., Feb. 20, 1915.)
Rheumalgine (Eli Lilly & Co., Indianapolis) is put up both in tablet form and as a liquid. Each tablet, or teaspoonful of the liquid, is said to contain:
“Strontium salicylate from Natural Oil | 5 gr. |
Hexamethylenamin | 2 gr. |
Colchicine | 1⁄200 gr.” |
The advertising matter contains several statements regarding the individual ingredients to which objection must be made.
It is claimed (quoting from Hare) that strontium salicylate
“... is not so disagreeable to the taste as the corresponding sodium salts, and more important still, it is far less apt to disorder the stomach.”
“Taste” is a difficult subject to dispute; but in the experience of the referee, patients object more to the strontium than to the sodium salt. No evidence is submitted to prove that the strontium salt is less apt to disorder the stomach. In observations made under the direction of the referee, the nauseant and emetic doses are about the same as, or even less than, those of sodium salicylate.
Under hexamethylenamin, the recommendations are not confined to its recognized use as a urinary antiseptic; it is also said to be “unexcelled” as a “germicide,” and to prevent the formation of urate and phosphate deposits. These statements are contrary to facts.
“Rheumalgine ... may be used in all cases where the salicylates are indicated. It is superior to preparations containing sodium salicylate, in that it does not cause nausea or disturb the digestion.”
Both the preceding statements are misleading. The necessity of giving 1⁄200 grain of colchicin for each 5 grains of salicylate would certainly interfere with the use of adequate doses of the latter. The colchicin would produce digestive disturbance quite apart from the salicylate.
The mixture is described as:
“... ANTIRHEUMATIC, ANTIPYRETIC, URINARY ANTISEPTIC, AND URIC ACID ELIMINANT. Useful in Acute Articular and Chronic Rheumatism, Muscular Pains, Lumbago, Sciatica, Migraine of the Rheumatic, Gout, and in Nervous Irritability of the Gouty or Lithemic.”
The facts are: Salicylates are useful in some of these conditions, colchicin occasionally in a few, hexamethylenamin in none. The combination is conducive to uncritical prescribing. For instance, salicylates are effective in acute articular rheumatism; hexamethylenamin and colchicin are useless; salicylates are of very little use in chronic rheumatism, sciatica and nervous irritability, while hexamethylenamin and colchicin are useless in these conditions; colchicin is sometimes effective in gout, salicylates perhaps also; hexamethylenamin is not.
Attention should also be called to the high dosage of colchicin, namely, 1⁄100 to 1⁄50 of a grain of the alkaloid, every three or four hours, the dose then to be “slightly reduced,” but continued for several days; or in chronic cases, 1⁄100 to 1⁄30 grain per day, continued indefinitely. This dosage appears high, if a really active preparation is used.
Finally, the name “Rheumalgine” encourages thoughtless and unscientific prescribing. If a mixture is used at all, the prescriber should be constantly reminded of its composition.
It is therefore recommended that Rheumalgine be held in conflict with Rules 6 (unwarranted therapeutic claims), 8 (nondescriptive name) and 10 (unscientific composition).—(From The Journal A. M. A., June 26, 1915.)
The Council adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
Gray’s Glycerine Tonic Comp. (Purdue Frederick Company, New York) is a mixture said to be made according to a prescription of the late Dr. John P. Gray, superintendent of the state hospital, Utica, New York. As to the composition, the following statement is furnished by the company:
“This preparation is a combination of Glycerine, Sherry Wine, Gentian, Taraxacum and Phosphoric Acid with carminatives.”
The label declares the presence of 11 per cent. alcohol, and the dose is given at from two teaspoonfuls to a tablespoonful. A study of the ingredients will show that, aside from the alcohol, the mixture contains but one really active drug, gentian. Essentially, then, “Gray’s Glycerine Tonic” is a mixture which, in addition to the narcotic effect of the alcohol, depends on a bitter, gentian, for whatever therapeutic action it may possess.
The bitters, of which gentian is a type, were once credited with many therapeutic virtues which time has shown they do not possess. Pharmacologic research has demonstrated that their utility consists in stimulating the appetite through their action on the taste buds. On this account they were believed also to increase the secretion of the gastric juice by a psychic impression. More recently, however, even this has been questioned—by Carlson, for instance.
These facts are fully understood, presumably, by all physicians. Yet, according to the advertising circular, this “tonic,” which, for all practical purposes, is merely a simple bitter, is good for thirty-two diseases ranging from amenorrhea to whooping cough!
The conditions in which Gray’s Glycerine Tonic is asserted to be especially efficient are described on the label of the bottle and the outside wrapper, in popular terms, more or less typical of “patent medicine” exploitation, such as “catarrhal conditions,” and “stomach derangements.” Similar statements are contained in the leaflet accompanying the trade package. For instance:
“It is, therefore, an effective, reliable tonic in nervous exhaustion, general debility, impoverished conditions of the blood and nervous system, Bright’s disease, diseases of the liver, disorders of the urinary organs, etc.”
“It is an unexcelled restorative in that very common class of cases in which there is no positive organic disease, but the patient complains that he ‘does not feel well’ or ‘is out of sorts.’ ”
Here are some of the claims made in other advertising matter:
“All stages of bronchitis ... are rapidly improved by the use of Gray’s Glycerine Tonic Comp. This remedy has a direct tonic influence upon the circulation of the respiratory mucous membrane; it relieves congestion and restores tone to weakened blood vessels.”
“... improves the appetite, gives valuable aid to the digestive and absorptive processes, and reinforces cellular nutrition in ways that insure a notable gain in vitality and strength.”

Even granting that gentian may improve the appetite, how absurd it is to claim that this mixture “relieves congestion,” “restores tone to weakened blood vessels,” “gives aid to the absorptive processes,” “reinforces cellular nutrition,” or increases vitality!
Neither the composition of Gray’s Glycerine Tonic nor the clinical evidence warrants the belief that it has any therapeutic value other than that due to the psychic effect of the bitter drug gentian. Physicians who have prescribed it have done so because of the advertising. This nostrum has been kept so constantly before the eyes of medical men that they think of Gray’s Glycerine Tonic when they cannot remember the official drugs that may be indicated in the case. The moral is that liberal advertising will sell anything.
It is recommended that Gray’s Glycerine Tonic Comp. be declared not eligible for inclusion in New and Nonofficial Remedies on account of conflict with Rules 1, 6, 8 and 10.
[Editorial Note.—An old practice in hospitals—happily now practically obsolete—was to have certain stock mixtures prepared in bulk. Among these there was usually a so-called tonic mixture, used in a more or less haphazard manner when nothing in particular seemed indicated. Such a stock mixture was used in the State Hospital for the Insane at Utica, N. Y., during the many years that Dr. John P. Gray was superintendent (from the early fifties to the early eighties), although it is very doubtful whether he originated the mixture. After the death of Dr. Gray—so the story runs—one of his sons, with a partner, formed the firm of Purdue Frederick Company, and began the exploitation of the elder Dr. Gray’s name, in connection, presumably, with this stock preparation. As indicated in the Council’s report, Gray’s Glycerine Tonic Comp.—and what an absurd name!—is simply a mixture of ordinary drugs, requiring no skill whatever in compounding. If there is a physician living who cannot write a prescription offhand as good as this formula, that physician should either go back to a medical school or change his vocation. There is, and can be, no excuse for prescribing such a ready-made mixture, for every cross-roads drugstore has the ingredients and any pharmacist worthy of the name could compound it. Among the scores of nostrums that disgrace the medical profession of this country, none is more typical of all that is inimical to scientific medicine, to the medical profession and above all to the public—for, after all is said, it is the public that ultimately is humbugged.]—(From The Journal A. M. A., July 10, 1915.)
The Council, having considered “Tongaline,” “Tongaline Tablets,” “Tongaline and Lithia Tablets,” “Tongaline and Quinine Tablets” and “Ponca Compound Tablets,” found these preparations ineligible for New and Nonofficial Remedies and authorized publication of the following report.
W. A. Puckner, Secretary.
Tongaline (Mellier Drug Co., St. Louis) is a fancy name given to what is essentially a sodium salicylate mixture. The air of mystery created by the name permits the manufacturers to make claims for the product which would be ludicrous if the medical profession was fully conversant with the very ordinary character of the preparation.
Tongaline receives its name from tonga, an inert, long-discarded mixture of various barks and herbs said to be gathered and prepared by Fiji Islanders. Its constituents evidently tend to vary with the collector. The history of the introduction of this indefinite combination of simples is thus given in The Journal, May 10, 1913.
“A supply of the crude drug was carried to England by a man who had lived for a short time in the Fiji Islands and it was placed in the hands of a retail house in London. This occurred about 1879. In 1880, two English physicians of repute published laudatory articles on the therapeutic value of tonga in neuralgia and rheumatism. This created a demand for the drug which extended to the United States.”
Time showed that tonga was inert therapeutically, and authorities on pharmacology now no longer notice it. As the Council previously reported,12 the indefinite character of the mixture should, alone, be sufficient to exclude it from practical therapeutics. During the temporary popularity of tonga, the proprietary mixture Tongaline was put on the market for physicians’ use by the Mellier Drug Company, St. Louis. In this, tonga was named as the active ingredient. The commercial interests thus involved have faithfully nourished and kept alive the “tonga” myth.
In a recent advertising booklet, “The Therapeutic Properties of the Ingredients of Tongaline,” the virtues of tonga, blue cohosh, colchicum, jaborandi and salicylic acid are discussed. The label of a recently purchased bottle reads:
“Tongaline contains Tonga, Cimicifuga Racemosa, Salicylate of Sodium (the salicylic acid being made from pure natural oil) Colchicum and Pilocarpin.”
It will be noticed that Tongaline is “made from the pure, natural oil.” In fact, the statement is repeated in red ink, in large letters running across the face of the label, thus emphasizing the alleged importance of this assertion. In this connection it is only necessary to recall that it has been proved clinically, chemically and physiologically that there is absolutely no difference between the salicylic acid made from the natural oil and the synthetic.
The formula was thus commented on in the article previously quoted from The Journal:
“Tongaline ... is essentially a preparation of sodium salicylate,... The Mellier Drug Company realized the impossibility of creating any marked demand for a nostrum unless it had some real drugs in it—hence the presence of the salicylates. What the actual composition of Tongaline is, no one but the manufacturers know. At one time the following was given as the formula:
Fluid Tonga | 30 grains |
Extract of Cimicifuga Racemosa | 20 grains |
Sodium Salicylate | 10 grains |
Pilocarpin Salicylate | 1⁄100 grains |
Colchin Salicylate | 1⁄500 grains |
“These amounts refer to the quantity of drugs in each fluidram of the preparation. Whether the nostrum still has this composition we do not know, but assuming that it has, it is quite evident that sodium salicylate is the essential and active ingredient.”
The therapeutic indications given on the label of the bottle are:
“Rheumatism, Neuralgia, Grippe, Gout, Nervous Headache, Sciatica, Lumbago, Malaria, Tonsillitis, Heavy Colds, Excess of Uric Acid, and wherever the use of the Salicylates is indicated.”
In a recent booklet this semisecret salicylate mixture is recommended, not only in conditions in which salicylates are indicated, but also combined with aconite for rheumatic fever, with benzoate of soda in the treatment of “grippe,” with potassium bromid in nervous headaches, with gelsemium, glycerin and whisky for “heavy colds,” with ammonium chlorid, stramonium and cimicifuga in “rheumatic dysmenorrhea,” and even with mercury biniodid as a treatment of syphilitic eruptions!
“When administered with good judgment, Tongaline exerts a stimulating effect upon every organ of elimination; cleansing the complex sewerage system and putting it into working order. When this is done, the sluggish blood current begins to flow more freely; the lymphatic and glandular systems to give up and carry off the toxic products, so long retained ...”
Then because of a “desire to put Tongaline in a more compact and convenient form,” the same concern puts on the market Tongaline Tablets. Whether Tongaline Tablets are of the same composition, the doctor who prescribes them is not advised. In this form we have Tongaline and Lithia Tablets, and Tongaline and Quinin Tablets. Presumably those who are attracted by the word “lithia” are sufficiently uncritical to be content with the statement that:
“The addition of Lithia to Tongaline presents a most useful combination which does not rely upon its action on the kidneys alone as is the case with Lithia salts or Lithia waters as administered ...”
And the foregoing quotation, be it remembered, is for the information of the medical profession! Tongaline and Lithia Tablets, we are informed, are:
“... particularly indicated for certain diseases which are caused by deposits of urates in the joints and kidneys, and can be used with much benefit for many people who indulge in generous or intemperate habits of living.”
Tongaline and Quinine Tablets are also exploited without statement of composition. The promoters are probably justified in feeling that physicians who prescribe quinin in combination with “Tongaline” care little about the dosage.
It is unnecessary to discuss the silly claims made for Tongaline and its combinations, although it is worth while to point out that the prescribing of such nostrums by physicians is an imposition, if not a fraud, on the public.
Ponca Compound, also made by the Mellier Drug Company, St. Louis, is a “female weakness remedy” in tablet form. The name suggests that “ponca” is a medicinal substance, and, in fact, at one time, “Ext. Ponca” was named as an ingredient. The nature of “Ext. Ponca” was apparently never explained. It is now replaced in the “formula” by “senecin,” and the only information concerning the composition at present given is:
“Ponca Compound Tablets Contain Extract of Mitchella Repens, Senecin, Helonin, Caulophyllin and Viburnin.”
This “formula” is practically meaningless, not only because the amount of each ingredient is not stated, but also because “senecin,” “helonin,” “caulophyllin” and “viburnin” are in themselves variable mixtures of unknown composition.13
Presumably, “senecin,” “helonin,” “caulophyllin” and “viburnin” are extractives of some kind prepared, respectively, from senecio aureus (life root), helonias dioica (false unicorn), calophyllum thalictroides (blue cohosh) and viburnum prunifolium or opulus (black haw or cramp bark). These are, one and all, practically inert drugs. There is no reason to believe that any or all of them can have any beneficial influence in the many and varied conditions for which Ponca Compound is advertised.
The following are excerpts from the advertising matter:
“Ponca Compound is a remedy of a very beneficial character for Functional, Uterine and Ovarian troubles, which will respond to internal treatment, especially when digital examination or surgical interference is undesirable.”
“Ponca Compound is also valuable during gestation and after parturition.”
“Uterine Alterative for Leucorrhoea, Dysmenorrhoea, Amenorrhoea, Metritis, Endometritis, Menorrhagia, Metrorrhagia, Irregular Menstruation, Subinvolution, Painful Pregnancy.”
It is recommended that Tongaline and Ponca Compound and all their preparations be held in conflict with Rule 1, in view of their semisecret and indefinite composition; with Rule 6, for the grossly exaggerated therapeutic claims made for them; with Rule 8, because of their misleading names, and with Rule 10, in view of their unscientific character as irrational combinations. It is also recommended that this report be published.—(From The Journal A. M. A., July 17, 1915.)
The Council has found Alfatone ineligible for New and Nonofficial Remedies and has authorized publication of the following report.
W. A. Puckner, Secretary.
Alfalfa is good cattle feed but only nostrum exploiters have suggested its use as a medicine for human beings. While it may seem a waste of time to discuss the medicinal value of alfalfa its recent exploitation by the Norwich Pharmacal Company, Norwich, N. Y., as “a reconstructive tonic and nutrient” in the form of a mixture called “Alfatone,” calls for comment. According to the label on the preparation:
“Each fluidounce represents:
Alcohol | 15 | per cent. |
Medicago sativa (Alfalfa) | 120 | grains |
Taraxacum | 21⁄2 | grains |
Gentian | 1 | grain |
Berberine Hydrochloride | 1⁄40 | grain |
| Glycerin and Aromatics.” |
“Dose.—One to three fluidrams (4 to 12 c.c.) 4 times daily.”
Each maximum dose, therefore, should represent 45 grains of alfalfa, 1 grain of taraxacum (dandelion), 3⁄8 grain of gentian, 1⁄100 grain of berberin hydrochlorid, and 27 minims of alcohol. Since the bitter drugs are present in such small amounts that the preparation is almost devoid of bitterness, and as the medicinal value of alfalfa is practically nil, it is evident that whatever action Alfatone may have is due to the stimulant effects of the alcohol.
Some of the claims made for Alfatone are:
“A reconstructive nutritive tonic indicated in general debility, neurasthenia, convalescence, etc.”
“... a Galactagogue of merit as well.”
“... improves the appetite, aids the processes of digestion and assimilation, facilitates elimination and effects gradual but decided gains in strength, vitality and weight.”
It is suggested that:
“... in case of idiosyncrasy the addition of Tr. Nux Vomica 5 to 10 minims to the dose, unless contraindicated, will secure excellent results.”
The Norwich Pharmacal Company naively remarks:
“The dearth of medical literature on Alfalfa has lead us to present below a few of the findings of the Bureau of Plant Industry of the Department of Agriculture ... as well as those from several state experiment stations ...”
Here are the “findings”:
“... Digestible nutrients in 100 pounds of Alfalfa,... Protein, 11.0 pounds; Carbohydrates, 39.6 pounds; Ether Extract, 1.2 pounds.”
“... The high value of Alfalfa is due to the amount of protein that it contains; to the large percentage of protein that is digestible and the palatability of Alfalfa.”
“... Table showing pounds of elements removed from the soil by one ton of crop.
| Alfalfa | Wheat | |
Potash | 49.79 | 12.52 |
Phosphoric Acid | 8.27 | 9.08 |
Lime | 43.51 | 2.95 |
Nitrogen | 44.01 | 22.30” |
“... The abundance of muscle and bone producing material in Alfalfa makes this crop especially good.”
Thus estimates of the value of a farm crop and cattle fodder are made to do service as testimonials to its therapeutic merit for human beings! Has the “patent medicine” promoter ever dared to insult the intelligence of his patrons by a cruder absurdity? Yet it is not to the nontechnical and unscientific public, but to a profession presumably scientifically trained in pharmacology and therapeutics that this concern presumes to offer its fodder tincture on the basis of testimony to the agricultural value of the fodder plant.
Alfatone is a worthless alcoholic cordial. The audacity of the attempt to promote its sale by a discourse on the merits of a well-known fodder plant is the sole reason for devoting any attention to it. It is recommended that Alfatone be held ineligible for New and Nonofficial Remedies, and that this report be published.
[Editorial Note.—What a comment on American medicine that a concern can even contemplate the possibility of making a commercial success of the sale of such a silly nostrum as Alfatone! And yet, when one remembers that a proprietary in which oats constitutes one ingredient (“Pas-Avena”) for years has been advertised to physicians and presumably prescribed by them, it is not altogether inexplicable that business men should get the impression that the medical profession is “easy” enough to “fall for” anything in the line of proprietary mixtures. Perhaps we may look forward to being offered proprietaries based on other cheap and well-known fodder plants. Tincture of Timothy Hay, Blue Grass Tonic, Cornhusk Wine! Why not? The enterprising companies that may put them out can easily publish tables to show the digestible nutrients in each and indubitable testimony can be furnished to prove the excellence of any of them as stock feed. If a pitchforkful of timothy hay makes a good fattening ration for a growing steer why should not a teaspoonful of tincture of timothy hay make a “reconstructive tonic and nutrient” dose for a man? If an arm load of thistles (carduus) makes a luscious food for equus asinus why should not a pinch of thistle in alcohol and water be a good “tonic”? Great are the possibilities! They are limited only by the gullibility of the medical profession and the public. Certain it is that some proprietary manufacturers are firmly convinced that no combination can be too preposterous to be worth trying on the medical profession.]—(From The Journal A. M. A., Aug. 7, 1915.)
Below appear abstracts of the Council’s actions on articles refused recognition which were not deemed of sufficient importance to require lengthy reports.
Uricsol is marketed by the Uricsol Chemical Company, formerly of Los Angeles, now of Boston. Regarding its composition only vague statements are made. In an advertising pamphlet it is promised that the formula will be sent to physicians on request. Such a request from a physician elicited the following statement:
“URICSOL is a non-irritating, alkaline solution, containing Lithium Citrate, Acid Citric and Potassium Nitrate, together with a saline laxative in the form of Glycero Sodium Phosphate, with Vegetable Tonics added.”
The Association Laboratory has made an examination of Uricsol to determine its composition and reports as follows:
A trade package purchased in March, 1915, from a wholesale drug house was labeled:
“Uricsol Rheumatic Remedy, Uric Acid Solvent, Kidney and Liver Stimulant, Manufactured by the Uricsol Chemical Co., Los Angeles, Cal.”
This package was wrapped in a circular entitled “The Great California Remedy—Uricsol.” The preparation is a viscid, slightly turbid light brown liquid, with a faintly aromatic odor and a salty, bitter taste. The diluted solution is acid in reaction toward litmus and phenolphthalein and alkaline toward methyl orange.
Qualitative tests showed a presence of phosphate, citrate, nitrate, sodium, glycerin, and a small amount of lithium in aqueous solution. Besides these a small amount of some organic, nonalkaloidal substance was found, which from its bitter taste suggested gentian. From the qualitative tests it appeared that the phosphate was the predominating ingredient and accordingly a phosphate determination was made. The results, calculated to sodium phosphate, U. S. P., indicated the presence of 64.20 gm. per 100 c.c., held in solution by citric acid and sodium nitrate.
Uricsol evidently is a solution containing a large amount of sodium phosphate with small amounts of lithium, nitrate, citric acid and glycerin, with probably some vegetable extract.
In general Uricsol is similar to the once widely exploited proprietary “Melachol,” which has been frequently imitated. A preparation essentially identical is in the United States Pharmacopeia, under the title “Compound Solution of Sodium Phosphate.”
The Uricsol Company calls its preparation
“... the latest word in the treatment of Rheumatism and that allied group of ailments which is caused by an excess of Uric Acid.”
Hay fever, bronchial asthma and neuritis are conditions in which it is recommended. The claim is made that
“Uricsol quickly controls Vasomotor Rhinitis and eliminates such conditions from the system.” “In fact, it will correct FAULTY METABOLISM.”
To a few practitioners of an older generation the pharmacologic basis of a remedy for rheumatism was sufficiently defined by saying that it increased the solubility of uric acid or affected it in some way. This theory is obsolete; there is not, and never was, any reliable evidence on which to base the theory that rheumatism is in any way caused by uric acid. The exploitation of Uricsol as a “uric acid solvent” is merely another illustration of the way in which nostrum manufacturers play on disproved theories. Of course the claim that sodium phosphate has any particular power to control vasomotor rhinitis, hay fever, asthma, and to correct faulty metabolism is foolish.
To summarize: Uricsol is a mixture of well-known drugs, marketed with false claims as to therapeutic action, with misleading and meaningless statements as to composition and under a name which invites uncritical prescribing. Uricsol is held ineligible to inclusion in New and Nonofficial Remedies.
The following ridiculous statements are addressed, not to the laity, but to the medical profession:
DO YOU SUFFER FROM Constipation—Hemorrhoids—Enteritis—Mucous discharge—Pituita—Acidity of the stomach—Vertigo—Sick Headache—Disturbed Sleep—Insomnia—Sallow Complexion—Coated Tongue—Offensive breath—Fatigue and depression—Boils—Pimples?
“ONE of these symptoms alone shows that there is defective or insufficient function of the intestines, even if the stools are regular.
“Excrements remain too long in the intestine and set up fermentation. The harmful poisons and Ptomains which they produce are re-absorbed by the blood and poison the whole system.
“The Intestines must be cleared and re-educated with JUBOL.
“Jubolise your Intestines.”
Jubol tablets are sold in the United States by Geo. J. Wallau, Inc., New York, and are said to be prepared by J. L. Chatelain, Paris, France. The following incomplete and nonquantitative “formula” is furnished:
“... compounded chiefly [!] of Agar-Agar, Biliary Extracts and pure Extracts from all the intestinal Glands.”
It is asserted that
“The tablets are coated with a protective covering in order that they may act on the intestine only.”
The tablets contained in a regular-size trade package, obtained direct from the agent, readily separated into two halves and disintegrated within a few minutes when agitated with water. It is thus evident that, under ordinary conditions, the intestinal ferments in Jubol (if they are present, as claimed) would be destroyed during their passage through the stomach. In direct tests, however, practically no tryptic activity was demonstrated.
The composition of Jubol is not declared; grossly unwarranted and incorrect claims are made for its therapeutic actions; the name does not indicate the alleged ingredients and so much of the composition as is declared indicates an unscientific mixture. The Council decided that Jubol should be held ineligible for New and Nonofficial Remedies, and that this report should be published.
Urodonal is said to be “produced in the laboratory of J. L. Chatelain,” Paris, France. It is marketed in this country by Geo. J. Wallau, Inc., New York.
The preparation is claimed to be a chemical compound, and the advertising matter furnishes a “formula,” which consists of the formulas of lysidin, sidonal and hexamethylenamin, connected by plus signs:
That the substance is a chemical compound is highly improbable, and no evidence has been submitted to substantiate the claim. On the contrary, in the following statement the phrase “based on” is a virtual admission that the preparation is merely a mixture:
“Urodonal ... is a granular effervescent preparation based on methylglyoxalidine [Lysidine], quinate of diethylene-diamine [Sidonal] and hexamethylene-tetramine [Formin, urotropine].”
Mystery is added by the mention of undefined “special products” in the following:
“The fact of combining these two salts [lysidin and sidonal] in Urodonal, in strictly determined proportions and in the presence of special products, gives this preparation very considerable power in dissolving uric acid.”
These contradictory statements of composition conflict with Rule 1.
Urodonal is marketed in typical “patent medicine” style: the name “Urodonal” is blown in the bottle and the label contains a list of “Indications,” including rheumatism, gout and gravel (Rule 4). That this form of marketing has introduced it to the public is suggested by the following in an advertising circular:
“... Urodonal is now popular—even classic—throughout the world, where thousands of doctors and millions of patients agree in asserting that ‘Urodonal is to rheumatism what quinine is to fever.’ ”
There are also other indications that the mixture is to be exploited to the laity. For instance, the U. S. distributor sends out a portrait of Sarah Bernhardt bearing the legend:
“I am positive that URODONAL preserves youth’s freshness with clearness and strength to brain and heart. I have taken it for two years with the greatest benefit. Sarah Bernhardt.”
A circular advises this mixture
“For all who suffer from Arthritis, Rheumatism, Arterio-Sclerosis, Renal and Bilious Lithiasis, Headache, Gout, Gravel, Lumbago, Sciatic Pains, Neuralgia and all uric acid troubles.”
“In fact, Urodonal is five times more active than piperazine, and thirty-seven times more active than lithia. We are, therefore, entitled to say that no other eliminator of uric acid can be compared with it.”
“Being 37 times more active than lithia, it clears the heart valves of any sandy substances which may clog them, and checks the atheromatous degeneration of the blood vessels.”
These extracts indicate sufficiently the extravagant tone of the advertising (Rule 6): None of the ingredients are notably active in dissolving uric acid when administered by mouth. None produce any marked increase of uric acid elimination. No intelligent physician would use a uric acid solvent for “bilious lithiasis”; and their usefulness in the other conditions is open to doubt, to put it mildly.
Although the preparation is a simple mixture, the name does not indicate the components, but inclines to therapeutic suggestion (Rule 8).
Nothing is to be gained by combining several drugs which are useless, severally, for the purpose intended, as in the present case (Rule 10).
Urodonal is marketed under inconsistent statements of composition and with exaggerated therapeutic claims; the name is nondescriptive and the mixture is unscientific. The Council decided that the preparation should be declared ineligible for conflict with Rules 1, 4, 6, 8 and 10 and that this report should be published.—(From The Journal A. M. A., Aug. 14, 1915.)
The following report has been authorized for publication.
W. A. Puckner, Secretary.
Formamint is a proprietary medicine manufactured by the A. Wulfing Company (New York, London and Berlin), which is affiliated with the Bauer Chemical Company.
It has been widely advertised in Europe for several years, and is now on the American market;14 it is advertised in this country both in newspapers and medical journals.
Following is a brief review of the more important alleged investigations that have been reported from time to time in various European journals.
In “The Therapeutical Value of Foramint in Septic Affections of the Oro-Pharynx,” De Santi15 quotes Rosenberg,16 who reports the successful use of Formamint in cases of streptococcus infections, tonsillitis and acute symptoms of chronic sore throat. According to Seifert,17 Formamint is a chemical combination of formaldehyd and milk sugar. When the tablets are dissolved in the saliva, 0.01 per cent. of formaldehyd in its “status nascendi” is liberated and exercises a strong disinfectant action. Seifert states that the preparation is markedly palatable, since it contains a little citric acid to render the taste cool and refreshing. In some experiments with streptococci, pneumococci, typhoid and diphtheria bacilli, Seifert found that a solution of one tablet in 10 c.c. of water destroyed these germs in from five to ten minutes. A solution of the same strength was also added to culture tubes of broth, agar, and gelatin, with the result that no growth occurred in them, while distinct and characteristic development of the bacteria took place in control tubes. He does not state, however, how much Formamint solution was added to the mediums.
Daus18 reports successful treatment of tonsillitis, mumps and middle ear diseases. In these cases no other gargles or mouth washes were used. He states that no indication of irritant or other injurious action made its appearance even after large doses. In the same article, F. Levy reports experiments as follows: Agar plates were prepared with a culture of streptococcus from a severe case of quinsy. One half of the plate was rubbed with saliva containing Formamint in solution. (The strength of the solution used is not given.) In twenty-four hours streaks of growth had appeared on one portion of the plates while the part on which the Formamint saliva had been rubbed remained sterile. Daus also found that agar and broth cultures of streptococcus shaken with Formamint saliva remained sterile.
Rheinboldt,19 investigating the effects of Formamint and of ordinary formaldehyd on animals, concludes that formaldehyd is toxic in action while Formamint is not.

Rosenberg20 corroborates this statement. He also found that agar plates of Bacillus prodigiosus were killed by Formamint solutions in about four hours. He fails, however, to give the strength of his Formamint solutions.
Wingrave21 suggests the use of Formamint for infants! He recommends that a tablet be crushed and wrapped in “butter cloth.” The ends of the cloth are to be tied with thread, the Formamint is to be moistened, and the packet is to be held in the mouth of the baby several times each day.
Young22 published the results of some experiments by himself and Delépine on the human throat. They dissolved a tablet in the mouth and made swab cultures with the following results:
Immediately after taking the tablet | 0 colonies |
10 minutes after taking the tablet | 35 colonies |
30 minutes after taking the tablet | 150 colonies |
They found no staphylococci at any time. Other results of swabbing various parts of the throat before and after the use of Formamint, reported by these investigators, show enormous reductions in the count, claimed to be due to the action of Formamint. The count was made on agar at 37 C., but they fail to state the time elapsing between taking the Formamint and making the swab. Young also reports favorable clinical results in cases of scarlet fever, diphtheria, sore throat, and the like. It must be noted, however, that they state that the mouth and fauces must first be thoroughly cleansed by swabbing and douching before Formamint is used.
The claims made in the advertising literature of Formamint are very extravagant. Many are highly improbable. These statements will be discussed later.
The statement is made that Formamint is a new chemical compound:
“Formamint is Pentamethanallactose, 5 CHOH + C12H22O11. It is an original combination of Formaldehyde with Lactose, a definite chemical compound. The Formaldehyde molecule is locked up in it until solution in the saliva takes place, when the Formaldehyde is liberated in its nascent state and is therefore active without being irritant.”
Furthermore the makers contend that this new chemical compound is entirely harmless. For example, Daus,18 in an article on “The Disinfectant Action of Formic Aldehyde on Mucous Membranes,” declares:
“No indication of irritant or other injurious action made its appearance even after large doses. The urine remained free from albumin and sugar.”
Such statements as these are found in the advertising literature:
“Formamint tablets are absolutely harmless and innocuous, even to little children.”
“When dissolved in the saliva, Formamint Tablets liberate slowly Nascent Formaldehyde in a most active yet non-irritant form.”
They maintain that Formamint is not only absolutely harmless, but actually beneficial to the tissues. It may be used “to tone up and strengthen the tissues, prevent hoarseness, and allay irritation in singers, public speakers,” etc.
The claims urged as to its germicidal power are indeed glittering. This “new chemical compound” is claimed to liberate formaldehyd in some new and peculiar condition which, while it has a soothing and tonic effect on the cells of the human tissues, can at the same time quickly kill any form of bacterial life.
“Dissolving readily, it releases its germicidal, antiseptic qualities, which impregnate the saliva and are carried naturally and easily around the mouth and in the deepest crevices of the throat—destroying the germs where they are causing the mischief. Formamint prevents and destroys infectious germ life in a soothing grateful way.”
“In the saliva it frees a germicide, fatal to germs but harmless to the most delicate membranes. And flowing into every tiny corner of the gums, tonsils and throat, into places where no gargle ever reaches, it most effectively disinfects the throat.”
The claims as to the preventive and curative effects of the preparation cover a large portion of the category of human ailments and distresses. The following quotations indicate some of its supposed properties:
“... it is therefore self-evident that Formamint should be looked upon as a necessary part of the treatment of all forms of tonsillitis.”
“The value of Formamint is equally great in diphtheric tonsillitis, or as a prophylactic ...”
“The extraordinary success which I had with Formamint in a school epidemic of scarlet fever during May and June, 1907, was the determining factor which induced me to abandon the use of inhalations, gargles, local applications in the treatment of diseases of the throat, and to use Formamint exclusively for the future.”
“There are naturally many similar conditions in which Formamint may be used as a prophylactic, notably scarlet fever, mumps, streptococcal and staphylococcal sore throats, ‘milk outbreaks’ of sore throat, drain throats, hospital throats, and the like.”
“Formamint Tablets are indicated in Angina, Tonsillitis, Pharyngitis, Stomatitis, Gingivitis, Glossitis, ulceration, spongy or bleeding gums, Pyorrhea Alveolaris, ‘Smoker’s Sore Throat,’ Abscess or Boils, etc.”
“As a Prophylactic against Diphtheria, Scarlet Fever, Influenza, Measles, Epidemic poliomyelitis, and other pathogenic micro-organisms. To neutralize putrefaction products in and about the teeth, correct fermentative processes, deodorize and purify the breath, etc.”
“To tone up, and strengthen the tissues, prevent hoarseness and allay irritation in singers, public speakers, neutralize the effects of dust-infection or disinfect the saliva or sputum in Influenza, Tuberculosis, etc.”
One man declares that along with specific constitutional treatment he “had the best results from the use of Formamint tablets” in a case of syphilitic ulceration of the tongue.
In short, Formamint is recommended for the treatment or prevention of almost everything, from a bad breath to such grave conditions as scarlet fever, diphtheria and tuberculosis, conditions in which a delay in proper treatment—for instance, in diphtheria, a failure to administer antitoxin—may result in the death of the patient.
A series of investigations was therefore undertaken in order to discover whether the extravagant claims regarding the germicidal power of Formamint could be verified.
Two fifty-cent bottles of Wulfing’s Formamint were purchased in the open market and were kept well stoppered to prevent deterioration.
Qualitative tests showed the presence of formaldehyd and the amount was determined quantitatively by the hydrogen peroxid method as given by Sutton.23 The results were respectively, 1.99 per cent. and 2.03 per cent. of formaldehyd.

Some determinations were made of the germicidal power of Formamint in vitro, that is, under controlled laboratory conditions. A twenty-four-hour plain agar culture of Staphylococcus aureus was washed off in 10 c.c. of sterile 0.85 per cent. sodium chlorid solution. A 1:100,000 dilution of this was made in each of three flasks containing 100 c.c. of sterile saliva. Flask 1 contained 1 per cent. of Formamint, Flask 2, 5 per cent.; Flask 3, containing no Formamint, was kept as a control. At intervals samples were removed and dilutions made and plated in duplicate on standard agar. The plates were incubated twenty-four hours at 37 C., and plates containing less than 200 colonies were counted. The results are given in Table 1. After seven days there was no appreciable difference in the plates.
TABLE 1.—SHOWING TIME IN WHICH CULTURES OF STAPHYLOCOCCUS
AUREUS WERE KILLED
BY DIFFERENT AMOUNTS OF FORMAMINT
| Amount of Formamint in Saliva (Per Cent.) | Period of Standing at 37 C. (Hours) | Average Count When Plated | Count on Flask of Saliva Without Formamint |
| 1 | 3 | 32 | 3200* |
| 1 | 6 | 0 | 7000* |
| 5 | 1 | Few | 5000* |
| 5 | 2 | 0 | 4100* |
| 5 | 3 | 0 | 3200* |
| 5 | 6 | 0 | 7000* |
* The last two observations were made at the same time as on the 1 per cent. solutions.
Another test was made by adding a 1 per cent. Formamint solution to plain agar plates inoculated with B. coli. A twenty-four-hour plain agar culture of B. coli was washed off in 10 c.c. of sterile 0.85 per cent. sodium chlorid solution. A 1:1,000,000 dilution was made of this and 1 c.c. added to each plate. Varying amounts of 1 per cent. solution of Formamint were added to each plate. They were incubated seventy-two hours at 37 C. After seven days’ incubation the count was the same. The results are given in Table 2.
TABLE 2.—COUNT OF B. COLI CULTURES WITH DIFFERENT AMOUNTS OF FORMAMINT
No. c.c. of 1 per cent. Formamint | 0 | 0.1 | 0.3 | 0.5 | 0.7 | 1.0 | 1.5 | 2.0 | 3.0 |
Count | 160 | 33 | 39 | 26 | 15 | 12 | 2 | 0 | 0 |
Another experiment was made thus: One loopful of a twenty-four-hour plain agar culture of Streptococcus lacticus was mixed with a tube of North medium. One loopful from the inoculated tube was mixed with a second tube of North medium. Both tubes were poured into Petri dishes and allowed to cool. One half of each plate was well smeared with a 10 per cent. solution of Formamint in saliva. After twenty-four hours’ incubation at 37 C., only a few colonies appeared on the side to which the Formamint had been applied, while the other half was thickly covered with colonies.
This work so far corroborates that reported in the literature quoted by the manufacturers. But the fact that a compound is a germicide when brought into intimate contact with bacteria in a solution or medium in a test tube or flask does not prove that it will be effective when used in the human throat.
An attempt was made to discover whether or not the claims advanced by the manufacturers as to the perfect germicidal action of Formamint in all the nooks and crannies of the mouth and throat could be confirmed.
The first step in attacking this problem was to make comparative counts of the number of bacteria in the throat before and after the use of Formamint. The methods employed were as follows: The throat was gargled with 50 c.c. of sterile 0.85 per cent. sodium chlorid solution. In each case the same length of time, as far as possible, was used in the process. The liquid was collected in a sterile flask. The gargling in a series of experiments was begun not less than two hours after a meal. After some preliminary work the following dilutions of the 50 c.c. of salt solution were found sufficient: 1:1,000, 1:10,000 and 1:100,000. Plates were made in duplicate from each dilution and incubated seventy-two hours at 37 C. The counts were made on plates containing less than 200 colonies. Except where otherwise noted standard agar was used. The mediums were always prepared in the same way.
All the work was carried out under conditions as nearly natural as possible. The Formamint was taken according to the directions accompanying the trade package. Every opportunity was given the Formamint to penetrate all the crypts and recesses about the mouth and throat. The tablet was allowed to dissolve as slowly as possible, the time usually being five to six minutes, and saliva was thoroughly forced around the mouth before being swallowed. Plating was always done immediately after gargling so that no growth could occur in the salt solution. The results are given in Table 3. The numbers are average counts from several plates and calculated to show the number of bacteria washed out by the 50 c.c. of salt solution.
TABLE 3.—SHOWING THAT FORMAMINT DOES NOT GREATLY DECREASE THE
NUMBER
OF BACTERIA IN THE THROAT
| Conditions of Test | Time Since Preceding Test | Amount of Formamint Used | No. Found in Throat Before Use of Formamint | No. Found in Throat After Use of Formamint |
Normal | ... | 0 | 15,600,000 | ... |
Normal | 1 hour | 0 | 38,500,000 | ... |
Normal | 1 hour | 0 | 30,500,000 | ... |
Normal | ... | 0 | 12,500,000 | ... |
Normal | 1 hour | 0 | 14,500,000 | ... |
| 1 hour | 0 | 23,500,000 | ... | |
| Tablet dissolved in mouth and throat gargled one hour later | 6 days | 1 tablet | ... | 15,000,000 |
| Throat again gargled two hours after Formamint was used | 1 hour | 0 | ... | 10,050,000 |
Normal | 7 days | 0 | 62,000,000 | ... |
Normal | 1 hour | 0 | 72,500,000 | ... |
Normal | ... | ... | 61,000,000 | ... |
| Tablets were taken, one per hour, and throat gargled one hour after last tablet was taken | 2 days | 12 | ... | 39,100,000 |
| Throat was again gargled 2 hours after taking last tablet | 1 hour | 0 | ... | 59,000,000 |
Normal | 5 days | 0 | 35,000,000 | ... |
Normal | 1 hour | 0 | 62,000,000 | ... |
Normal | 1 hour | 0 | 72,000,000 | ... |
| One tablet was taken each half hour for twelve hours consecutively. Throat was gargled one hour after last tablet was taken | 4 days | 24 tablets | ... | 175,000,000 |
| Throat was again gargled two hours after last tablet was taken | 1 hour | 0 | ... | 168,750,000 |
Normal | 3 days | 0 | 129,600,000 | ... |
Normal | 1 hour | 0 | 177,000,000 | ... |
Normal | 1 hour | 0 | 147,000,000 | ... |
Normal | 3 days | 0 | 79,000,000 | ... |
| One tablet was taken immediately after preceding gargle. Throat was again gargled at end of one hour | 1 hour | 1 | ... | 83,200,000 |
| Throat was again gargled two hours after tablet was taken | 1 hour | 0 | ... | 134,750,000 |
| Normal conditions except that mouth and teeth were throughly washed with soap just before gargling | 19 days | 0 | 32,600,000 | ... |
Same as above | 1 hour | 0 | 33,125,000 | ... |
Same as above | 1 hour | 0 | 40,375,000 | ... |
| Teeth were not washed. Otherwise normal conditions | 2 days | 0 | 33,500,000 | ... |
Same as above | 1 hour | 0 | 43,330,000 | ... |
Same as above | 1 hour | 0 | 54,000,000 | ... |
Same as above | 1 hour | 0 | 50,000,000 | ... |
Same as above | 1 hour | 0 | 67,000,000 | ... |
| Mouth and teeth thoroughly washed with soap just before throat was gargled | 2 days | 0 | 5,270,000 | ... |
Same as above | 1 hour | 0 | 10,916,000 | ... |
Same as above | 1 hour | 0 | 8,275,000 | ... |
| Normal conditions, but 1 c.c. of sterile rabbit’s blood was added to each plate | 3 days | 0 | 228,750,000 | ... |
| Count from the same gargle as above. No blood used in the plates | 0 | 0 | 60,625,000 | ... |
| Normal conditions, but count was made on blood agar | 1 hour | 0 | 431,250,000 | ... |
| Count from the same gargle as above. No blood used in the plates | 0 | 0 | 59,625,000 | ... |
| Normal conditions, count was made on blood agar | 2 days | 0 | 683,300,000 | ... |
| Same gargle as above, but count was made on plain agar | 0 | 0 | 58,500,000 | ... |
| One tablet was taken just after preceding gargle. After one hour throat was again gargled. Count on blood agar | 1 hour | 1 tablet | ... | 558,300,000 |
| Same gargle as above, but count was made on plain agar | 0 | 1 tablet | ... | 55,875,000 |
Normal conditions | 2 days | 0 | 79,125,000 | ... |
| One tablet was taken just ten minutes before gargle was made | 1 hour 16 min. | 1 tablet | ... | 56,250,000 |
Normal conditions | 2 days | 0 | 46,750,000 | ... |
| One tablet was taken just ten minutes before throat was gargled | 1 hour | 1 tablet | ... | 38,500,000 |
| Teeth and mouth were thoroughly washed with soap just before gargle was made | 5 days | 0 | 47,370,000 | ... |
| Teeth washed as above and one tablet taken ten minutes before gargle was made | 1 hour | 1 tablet | ... | 21,225,000 |
Finally a determination was made of the number of streptococci in the throat before and after the use of Formamint. The throat was gargled in the manner previously described. The streptococcus count was made by the dilution method as given by Heinemann.24 Culture tubes were used instead of fermentation tubes. One per cent. dextrose broth was the medium employed. One cubic centimeter was added to each of a series of ten tubes for each dilution and the following dilutions were used: 1:10,000, 1:100,000 and 1:1,000,000.
The results given in Table 4 are the average count from a number of dilutions and are reported as the total number washed out by the 50 c.c. of salt solution.
TABLE 4.—SHOWING THAT FORMAMINT FAILS TO REDUCE THE NUMBER
OF STREPTOCOCCI IN THE THROAT
| Conditions of Test | Time Since Preceding Test | Amount of Formamint Used | No. Found in Throat Before Use of Formamint | No. Found in Throat After Use of Formamint |
Normal | ... | 0 | 1,200,000 | ... |
| One tablet was taken and throat gargled one hour later | 4 days | 1 tablet | ... | 14,750,000 |
Normal | 3 days | 0 | 9,950,000 | ... |
| One tablet was taken and throat gargled ten minutes later | 1 hour | 1 tablet | ... | 8,000,000 |
The contention that Formamint contains formaldehyd was confirmed by analysis.
The manufacturers also maintain that Formamint is a new, definite chemical compound, consisting of five molecules of formaldehyd and one molecule of lactose, and that when dissolved in the saliva the formaldehyd is liberated in some new and peculiar form, which they call nascent formaldehyd. This new kind of formaldehyd is, according to the advertising literature, especially powerful in its germicidal properties and at the same time has absolutely no irritating or harmful effects.
Thoms,25 retained as an expert by the German government, decided, after a series of chemical investigations, that Formamint was not a definite chemical compound, but that it was probably a solid solution of formaldehyd in lactose. He proved that when the process of manufacture was carried out in exactly the way called for by the Formamint patents, compounds containing a greater or less per cent. of formaldehyd could be made while the other properties remained similar to those of Formamint. The composition of the final product depended on the proportion of the components used in the process. Therefore Formamint did not form a safe means of uniform dosage.
As a result of Thoms’ work the German courts held that Formamint was not a new chemical compound. Consequently the Formamint patent (Number 189036) was annulled in Berlin, Nov. 29, 1913.
Again the contention that formaldehyd in the nascent or active condition is less poisonous and irritating than in its ordinary form is contrary to what would be expected from the behavior of such compounds. If it were liberated, as claimed, in the “nascent” condition, it would be, for that very reason, not only more active but also more harmful.
As a matter of fact, Formamint did have an irritant effect on the worker who carried out these investigations. When one tablet was taken each hour for twelve consecutive hours, marked irritation of the intestinal tract resulted. There was almost sufficient nausea to cause vomiting and uneasiness in the alimentary canal following the experiment. When the twenty-four tablets were taken the results were similar but more pronounced. This is decidedly in contradiction to the assertions of the manufacturers.
Otto Seifert,26 moreover, cites the following:
“By Effects: Only a few patients complain of an unpleasant sharp taste, burning of the tongue (Seifert, Sklarek). Among the general symptoms observed are urticaria-like exanthems (Glaser, Roters), which are accompanied by nausea, vomiting, headache, insomnia and vertigo, burning and irritability especially in the larynx (Meissner); phenomena of poisoning (Geissler); gastric disturbances (Engelmann); renal irritation (Steinhard); unsuited for diabetics (Voit).”
The contention that Formamint, when mixed directly with mediums and left in contact with bacteria, will kill the organisms was corroborated. Thus the statements and pictures in the booklet, “The Gospel of Prevention,” which is enclosed with each bottle of Formamint, showing the inhibition of growth of air bacteria on plates containing Formamint are no doubt true and authentic.
Finally, the claim that Formamint is an almost perfect throat disinfectant was by no means confirmed, as a glance at the tables will show. One hour after it is taken, even when a tablet was used each half hour for twelve hours, the number of bacteria in the throat was practically the same as when Formamint was not used. Even ten minutes after taking a tablet the number of bacteria in the throat was never greatly reduced, as is maintained by the manufacturers.
Formamint exerts no selective action in killing off the very delicate organisms which are more apt to be pathogenic. When the comparative counts were made on blood agar which would favor the growth of the delicate parasitic organisms, no reduction whatever was shown by the use of Formamint.
The number of streptococci was found to be the same, within limits of experimental error, ten minutes after taking a tablet as it was before the tablet was taken.
Therefore it seems that Formamint fails, as any such germicide would be expected to fail, to kill bacteria in the crypts and recesses of the throat, for when dissolved in the mouth it cannot reach and remain in contact with the organisms long enough to kill them before it is swallowed.
Summed up, the investigation shows:
1. That the claims made for Formamint are extravagant and misleading.
2. That the recommendations for the use of these tablets may be, in some cases, fraught with danger and are a menace, not only to the health of the individual, but also to the safety of the community.
3. That the claim that Formamint is a definite chemical compound is false.
4. That the use of Formamint may produce marked irritation of the intestinal tract.
5. That Formamint is not a throat disinfectant, as the manufacturers maintain, but its action on the bacteria of the throat is an almost negligible one and dependence on Formamint for the prevention of infection and for curing disease is not only unwise but dangerous.
6. That Formamint conflicts with the rules of the Council. False statements are made with regard to its composition (Rule 1); grossly unwarranted claims are made for its therapeutic properties (Rule 6), and therefore its exploitation to the public (Rules 3 and 4) is a public danger.
It is recommended that this report be published, to call attention not only to the falsity of the claims made for, and the danger in the use of, Formamint, but also to emphasize the utter inefficiency of all such methods of “disinfecting” the throat.—(From The Journal A. M. A., Aug. 28, 1915.)
Hydragogin (C. Bischoff & Co., New York, selling agents) is advertised as “a most powerful diuretic and cardiac tonic.” The composition given is:
“Fifteen parts of the remedy contain 0.5 parts oxysaponin, 1.5 parts tincture of digitalis, 2.5 parts tincture of strophanthus, scillipicrin and scillitoxin, the active principles of scilla maritima, and alcohol.”
It is not clear from this statement whether 15 parts of Hydragogin contain 2.5 parts of tincture of strophanthus, plus unspecified amounts of scillipicrin and scillitoxin, or 2.5 parts of a mixture, in unspecified proportions, of tincture of strophanthus, scillipicrin and scillitoxin. The activity of strophanthus, after it enters the blood stream, is about fifty times that of digitalis; hence, if the former proportion is the true one, in giving an amount of Hydragogin which ensures the full therapeutic effect of the digitalis, one would administer an almost certainly fatal amount of strophanthus. Whatever the proportion of strophanthus may be, however, the administration of a mixture of digitalis and strophanthus in fixed proportions is indefensible. At times it is advisable to follow one of these drugs with the other in the treatment of cardiac disease. The simultaneous administration of the two continuously in fixed proportions, however, is injudicious, because of the great difference between their rates of absorption and in their activity after they enter the blood stream. The action of digitalis, moreover, persists much longer than does that of strophanthus.
An advertising circular contains the following claim:
“The well-known diuretic properties of digitalis, strophanthus and squills are greatly enhanced by the addition of the oxysaponin.”
This is not true. Saponins are not synergistic with digitalis therapeutically; on the contrary, they exert a purely deleterious action on the heart when they enter the circulation.
The symptoms of cardiac disease are often difficult to distinguish from the toxic actions of the digitalis bodies. Since these bodies must often be given to the point of beginning toxic action in order to induce the full therapeutic effects, it is obvious that the administration of a mixture of digitalis, strophanthus, saponin and active principles of squill is especially liable to induce serious toxic effects which cannot be distinguished from the symptoms of the disease.
Hydragogin is a shotgun mixture of semisecret composition; it is marketed under a therapeutically suggestive name, and advertised by means of unwarranted therapeutic claims. It is therefore in conflict with Rules 1, 6, 8 and 10. The Council held Hydragogin ineligible for New and Nonofficial Remedies.—(From The Journal A. M. A., Sept. 4, 1915.)
Filudine is said to be prepared by J. L. Chatelain, Paris, and is sold in this country by Geo. J. Wallau, Inc., New York. It is offered as a remedy for “biliary insufficiency,” “hepatic insufficiency,” “intestinal dyspepsia,” “all affections of the liver (diabetes, cirrhosis, cancer, etc.),” “malaria,” “obesity” and “tuberculosis.”
No quantitative information is furnished as to the composition of the preparation and there are noteworthy discrepancies in the various statements regarding the ingredients. In one number of “Treatment,” a self-styled “Review” of medical literature (actually devoted to advertising the preparations sold by Wallau), we are told that
“This product [Filudine] is a more concentrated and potent extract of the liver, with which is combined an extract of the spleen. The liver and the spleen are so intimately interdependent, that the addition of a splenary extract to the liver extract is a signal improvement from which a synergistic action results. Thiarféine is also added, as it helps somewhat to combat the anaemia from which all diabetics suffer more or less.”
Thiarféine is said to be
“Thiomethylarsinate of Caffein, a new salt discovered by M. Chatelain.”
Another circular, which gives an imposing formula for “thiarféine” or “thiomethylarsinate of caffein,” states that
“Sulphurated methylarsinate is an arsenical preparation devoid of all toxicity on account of the intimate joining of its composing parts.”
And that
“Filudine can never be contraindicated.”
A statement of composition in a later number of “Treatment,” however, says that biliary extracts are components, in addition to the liver and spleen extracts. Moreover, thiarféine, the “new salt discovered by M. Chatelain,” is no longer “thiomethylarsinate,” but “thiocinnamate of caffein”; and a new formula is furnished for it.
We are told that
“Methyl-arsinate cannot be used in cases where fever is present....”
“M. Chatelain at first studied the action of thiomethylarsinate; clinical and physiological experimentation led him, however, to adopt thiocinnamate of caffein, of greater activity and with no contraindications.”
Nevertheless the same absence of contraindications was urged in favor of Filudine when it was said to contain the now discarded thiomethylarsinate of caffein.
The following are some of the unwarranted and even absurd claims:
“Filudine restores the liver’s functions. It is to the liver what digitalis is to the heart; it overcomes the insufficiency and stimulates the debilitated organ.”
In malaria “it is the only true specific when associated with quinine.”
“Filudine is ... the ideal medication for tuberculosis, conforming as it does with the most recent researches in the therapeusis of this affection.”
“We will not go as far as to say that Opotherapy completely restores unhealthy livers, for although the lesions of the hepatic parenchyma may be obliterated by regeneration, the lesions of the connective tissues are permanent, and may be observed at the postmortem examination. The new cells, however, do not present the same unhealthy conditions as those of the former diseased gland which they have replaced, and the liver can therefore function normally, so that the patient lives on; and he is satisfied with that.”
“Therefore, while regenerating the liver with Filudine, we cleanse it and combat its congested state with Urodonal. We cause it to produce urea from the excess of uric acid which it contains.”
“By the judicious and harmonious combination of the beneficial effects of Filudine and Urodonal, physicians not only possess the means of treating by rational methods Cirrhosis of the Liver in its various forms (which is one of the most terrible diseases which can afflict anyone) but what is still better, they can cure it.”
“The liver of a person suffering from obesity being incapable of fulfilling its functions in regard to the fatty tissues, the rational and up-to-date method of treatment is therefore to restore to the system, in the form of Filudine, the liver extracts which are lacking.”
Filudine is a mixture of semisecret composition. The therapeutic claims are manifestly unwarranted. The name is not indicative of the composition, whatever that may be, and no rational excuse is offered for the combination of liver and spleen extracts (with or without bile extracts) with “thiomethylarsinate” or “thiocinnamate” of caffein.
The Council therefore held Filudine ineligible for New and Nonofficial Remedies.—(From The Journal A. M. A., Sept. 18, 1915.)
Mixtures of pepsin and pancreatin are therapeutically irrational; the two substances are not indicated in the same conditions, nor can they act together. Under physiologic conditions, such mixtures are chemically impossible: in a liquid medium the ingredients destroy each other.
Lactopeptin is manufactured by the New York Pharmacal Association, Yonkers, N. Y. It is sold under the claim that it contains, pepsin, diastase, pancreatin, lactic acid and hydrochloric acid. This product was among the first proprietary preparations examined by the Council on Pharmacy and Chemistry. The report of the investigation was published in The Journal, March 16, 1907, p. 959. The preparation was found to be practically inert—“essentially a weak saccharated pepsin,” devoid of tryptic activity.
Six years later it was still widely advertised with the same irrational claims. A referee (A) therefore examined Lactopeptine (powdered) for the Council in 1913, and confirmed the previous findings. The referee’s report was published in The Journal, Aug. 2, 1913, p. 358.
Nearly four months after this publication, the manufacturer protested against the report, maintaining, contrary to the findings of the Council, that Lactopeptine possesses pancreatic activity and contains “loosely combined” hydrochloric acid. Referee A therefore repeated his examination, and a second referee (B), independently, examined specimens of Lactopeptine (powder) purchased on the open market for the purpose shortly before.
A few specimens examined by these two referees showed a slight tryptic activity; most of them showed none. The amount of hydrochloric acid present was insignificant.
The reports of the two referees were referred to the manufacturers, who again protested vehemently against these findings, this time on the ground that the specimens were old. The manufacturers also cited the work of three chemists to disprove the findings of the referees, and demanded that the Council reexamine Lactopeptine, making use of fresh specimens. The Council refused for the following reasons:
1. So long as the packages of Lactopeptine are not dated, the activity of specimens known to be fresh is of no practical importance. The activity of the actual market supply is the only question of interest to the profession. The only fair test is that made on specimens representative of the product sold to the ultimate consumer.
2. The evidence presented by the manufacturers did not warrant a reexamination, since the work of two of the chemists cited substantially corroborates the results obtained by the Council’s referees from the fresher specimens. The figures for tryptic activity obtained by the third chemist cited by the manufacturers could not be accepted by the Council, since it was at variance with all other known results of investigations of Lactopeptine.
3. As stated at the outset, whatever the tryptic activity of the mixture, it is therapeutically useless. A demonstration of tryptic activity in a mixture containing both pepsin and pancreatin is of merely theoretical interest.
Such activity, of course, cannot be expected, even on theoretical grounds, in liquid mixtures like Elixir Lactopeptine.
The Council therefore again declared Lactopeptine (powder and tablets) and Elixir Lactopeptine ineligible for New and Nonofficial Remedies and authorized publication of the following statement.
W. A. Puckner, Secretary
THE COUNCIL’S REPORT
Lactopeptine powder (New York Pharmacal Association, Yonkers, N. Y.) was examined by the Council in 1907. At that time it was claimed to contain
“... the five active agents of digestion—pepsin, diastase (veg. ptyalin), pancreatin, lactic acid and hydrochloric acid—combined in the proper proportion to insure the best results.”
The examination showed that the preparation was essentially “a weak saccharated pepsin,” containing but small amounts of pepsin, no hydrochloric acid, or mere traces only, and no diastase or pancreatin (The Journal, March 16, 1907).
In 1913, the product was reexamined, because the claims, as to both composition and therapeutic value, were still being made. Samples were tested both of the American product, and of a British product from John Morgan Richards & Sons, London. The original findings were confirmed and the results were published in The Journal, Aug. 2, 1913, p. 358. Nearly four months later (November 24) the New York Pharmacal Association wrote to the Council, objecting to the findings and maintaining that Lactopeptine possesses pancreatic activity and contains (“in loose chemical combination”) hydrochloric acid. In accordance with the custom of the Council, the work was sent back for review to the referee (A), whose conclusions were then tested by a second referee (B), a physiologic chemist, not a member of the Council, selected because of his special knowledge of the subject.
In December, 1913, Referee A made a large number of new tests to determine proteolytic and amylolytic power. His results show that the ferment activity of the preparation is so low as to merit no recognition in practical use. The tests also show that the amount of lactic acid or “loosely combined HCl” (or both) present is too small to have any appreciable physiologic activity and therefore to be of any therapeutic value.
Nine samples of Lactopeptine purchased in the open market in December, 1913, and January, 1914, were examined by Referee B early in 1914. His studies show absence of amylase in all samples; presence of pepsin, giving weak reactions even when compared with those of old pepsin preparations; complete absence of trypsin in seven out of nine samples, tryptic reaction being obtained in two samples, in one of which the reaction, “slight at best and of no practical import,” was obtained only after treatment for twelve hours or more.
The presence of tryptic activity in two out of the nine samples may be due to the fresher condition of these specimens, as indicated by the serial numbers. The evidence shows that it is a commercial impossibility to market mixtures of pepsin, pancreatin and lactic acid so that they can display any material tryptic activity.
It should be reaffirmed that mixtures combining peptic and pancreatic activities are not feasible, because pepsin cannot act except in the presence of acid, and pancreatin is destroyed by acid and by peptic activity. Furthermore, in conditions in which pancreatin is called for, pepsin is not, and vice versa; therefore the administration of mixtures of pepsin and pancreatin would be unjustified, even if both constituents could be expected to exert activity.
The foregoing observations apply to Lactopeptine in powder and tablet form.
While mixtures of pepsin and pancreatin are unscientific and unjustified, theoretically the two substances may coexist in a solid preparation, and the activity of such a preparation is consequently a proper subject of investigation. Theoretically as well as practically, however, pepsin and pancreatin cannot exist together in solution. The claims made for Elixir Lactopeptine and all other liquid preparations sold as mixtures of pepsin and pancreatin are therefore impossible. The Council has previously taken action (The Journal, Feb. 2, 1907, p. 434) refusing to approve for inclusion with New and Nonofficial Remedies such preparations, calling the attention of the medical profession and of manufacturers to their worthlessness, and requesting the American Pharmaceutical Association to instruct its committee on the National Formulary to omit from the next edition of that work a liquid preparation of pepsin and pancreatin recognized under the title of “elixir digestivum compositum.”
It is recommended that the Council reaffirm this previous action, and that Lactopeptine and Elixir Lactopeptine be declared ineligible for New and Nonofficial Remedies because of conflict with Rule 10 (“No article will be admitted which, because of its unscientific composition, is useless or inimical to the best interests of the public or of the medical profession”).
The foregoing was submitted, together with the findings of the two referees, to the manufacturers. They protested again, alleging that:
First.—The specimens of Lactopeptine examined by the second referee were old. The dates of manufacture corresponding to the several batch numbers are supplied by the manufacturers as follows:
2275 (Powder) | September, 1908 |
2301 (Powder) | June, 1909 |
2312 (Powder) | December, 1909 |
2348 (Powder) | October, 1911 |
2352 (Powder) | December, 1911 |
2364 (Powder) | July, 1912 |
2374 (Powder) | March, 1913 |
2383 (Powder) | October, 1913 |
1638 (Tablets) | October, 1911 |
The manufacturers assert that they do not understand how specimens of these ages could have been purchased on the open market in 1913 and 1914, inasmuch as their agents are and long have been instructed to take up from the druggist all lots of Lactopeptine which, as indicated by the batch numbers, have attained “any appreciable age.” The age of the specimens, the manufacturers declare, deprives the table in the second referee’s report of “all significance or interest.”
As previously stated, however, the specimens of Lactopeptine examined were purchased on the open market in various localities in unbroken packages, in December, 1913, and January, 1914. They thus represent stock used in filling physicians’ prescriptions or sold to the public. Neither the referees nor any one connected with the Council had any means of knowing the age of the specimens until the dates of manufacture were furnished by the New York Pharmacal Association. The first tests of the second referee were made in February, 1914, on Specimens 2374 and 2383, which were then, it would appear, about one year old and four months old, respectively. The Council has repeatedly urged that pharmaceutical substances which are subject to deterioration should be dated by the manufacturer, and a similar suggestion has been made by the Bureau of Chemistry of the U. S. Department of Agriculture concerning mixtures containing enzymes. Notwithstanding the instructions which the New York Pharmacal Association claims to have given its agents, the market supply of Lactopeptine in December, 1913, and January, 1914, was not composed of new stock, and until the manufacturers adopt the practice of dating packages, there can be no assurance that it will be fresh. In this connection, it is of interest to note that the Bureau of Chemistry of the U. S. Department of Agriculture has issued a warning that it will judge such products by the degree of their activity when they reach the consumer, i. e., as they are found on the market.
Second.—The New York Pharmacal Association cites the work of several chemists, who have examined Lactopeptine and report the presence of tryptic activity. Dr. S. R. Benedict in December, 1913, reported to the Council “distinct” tryptic activity (digestion in twelve hours by Lactopeptine of 4.2 times its weight of fibrin containing 50 per cent. moisture) in specimens examined by him. These specimens were numbered 2382, and were therefore probably manufactured in October, 1913; compare the dates furnished by the manufacturer for the specimens used by the second referee. No tests against other preparations possessing tryptic activity are reported, and Dr. Benedict expressly disclaims any opinion as to the therapeutic value of the preparation.27 Dr. P. B. Hawk, whose report was submitted by the manufacturers, found in Lactopeptine by Fermi’s method one-fifth tryptic activity of that of Merck’s pancreatin, and by Grützner’s method an activity of 18 per cent. of the pancreatin. A test for the production of tryptophan was reported positive. The New York Pharmacal Association also submitted a report from Dr. A. W. Balch, who found pepsin, rennin, trypsin, steapsin, amylopsin and lactic acid present in Lactopeptine; also an amount of combined hydrochloric acid in 1 gm. the equivalent of 1.05 c.c. tenth normal solution or 0.00383 gm. hydrochloric acid. (He reports digestion in twenty-four hours by Lactopeptine of 25 times its own weight of fibrin. “An active extract of pancreas reacted exactly like the Lactopeptine solution.”) The serial numbers of the specimens tested by Hawk and Balch are not given, but no doubt they were fresh.
The New York Pharmacal Association demanded that the referee reexamine Lactopeptine, making use of fresh specimens. The Council held that this was unnecessary, for the following reasons:
1. The previous finding of the Council, that specimens of Lactopeptine found on the open market are essentially weak saccharated pepsins, is not to be refuted by examination of fresh specimens. Even if it be assumed that all old specimens of Lactopeptine have been withdrawn from the market since the last purchase of specimens for the use of the Council’s referee, there can be no assurance that the stock will be constantly kept fresh. Unless the manufacturers date their product, physicians cannot know that their prescriptions are filled with fresh material. Nor is it reasonable to ask that the Council examine the market supply of any given proprietary at a time selected by the manufacturers.
2. Without entering into all questions of detail in the analyses, the Council is willing to accept the reports of Drs. Benedict and Hawk as representative of fresh Lactopeptine powder. It is therefore unnecessary for the Council to make further experiments along this line. The results of these two chemists in no wise contradict the conclusions of the Council’s referees, being comparable with those obtained by the referee on the fresher specimens used by them. The experiments of Drs. Hawk and Benedict show a degree of tryptic activity which, though chemically not negligible, is quite without significance practically, even if it could be assumed that the trypsin in the fresh Lactopeptine escaped destruction in the stomach. The figures for tryptic activity given by Dr. Benedict do not differ materially from those of the first referee. Those of Professor Hawk show a tryptic activity of from 18 to 20 per cent. of that of commercial pancreatin—and commercial pancreatins ordinarily are of low tryptic activity, if not inert (see Long and Muhleman: Arch. Int. Med., February, 1914, p. 314.) The reports of these chemists present no reason for changing the conclusion that “it is a commercial impossibility to market mixtures of pepsin, pancreatin and lactic acid so that they can display any material tryptic activity.”
The results which Dr. Balch obtained in a test for tryptic activity show a marked discrepancy with those obtained by Drs. Hawk and Benedict, not to mention the Council’s referees, and also with the fact that only about 11 per cent. of “pancreatin” is claimed in the published formula of Lactopeptine. The Council is unable to accept Dr. Balch’s result for trypsin or rennin as reliable. His other results are without significance and call for no special comment.
3. Even if tryptic activity were conceded to Lactopeptine, the preparation, like all preparations containing pepsin and pancreatin, would still be, as previously stated, therapeutically irrational.
The Council approved the report.
In view of the manufacturer’s reiteration of the claims for Lactopeptine powder, I have carried out further experiments to determine its proteolytic and amylolytic power.
For the proteolytic test I used fresh, well washed fibrin and examined samples of Lactopeptine powder numbered as follows:
No. 1. A part of the English product examined and reported on last spring.
No. 2. A fresh bottle obtained at a Chicago retail drug store in December, 1913.
No. 3. A fresh bottle obtained at a Chicago retail store in December, 1913.
Portions of 1 gm. each of these samples were mixed with 5 gm. fibrin, 100 mg. of sodium carbonate and 50 c.c. of water in flasks. A little toluene was added to each flask, which was then closed with a tuft of cotton and the mixtures were incubated at 40 degrees through twenty-four hours. At the end of that time there was no marked change in the quantity of the fibrin remaining in each flask, the larger part by far being undigested.
As a control I used the sample of an active commercial trypsin, of which I added 500 mg. to the same quantity of water, fibrin and sodium carbonate. This was digested in the same bath at the same time. The digestion was practically completed in less than ten minutes, only minute flakes of the fibrin remaining.
It is evident that the digestive power of the Lactopeptine must be extremely low, and only a small fraction of that exhibited by a commercially good trypsin.
In an experiment with the English sample carried out through nineteen hours as above, using 2 gm. of fibrin and 100 mg. of ferment, it was found by nitrogen tests on the filtrate that about 12.2 per cent. of the protein had been brought into solution, an amount which is practically without importance in a digestion of such duration.
To test the starch digestive power I have made a large number of experiments. In a series just completed I mixed 1 gm. portions of Samples 1 and 2 with water to make 100 c.c. volumes. Before making up to the final volumes 0.5 c.c. of normal sodium hydroxid was added to neutralize the slight acidity of the ferment as shown by phenolphthalein.
Of these mixtures 4, 6, 8 and 10 c.c. portions were mixed with 50 c.c. of 1 per cent. starch paste and incubated at 40 degrees to find the colorless end-point in the starch digestion, by the iodin test.
At the end of twenty-two hours the iodin reaction was as strong as at the beginning, indicating no appreciable starch digestion.
To the flasks in which no digestion had taken place under these conditions, 5 mg. of a pancreas ferment was added. This gave an almost immediate conversion to the colorless end-point. This ferment was a sample of Holadin which had been in the laboratory about a year. The 5 mg. completed the reaction to the colorless end-point in less than ten minutes.
In a similar test I used 2 gm. of Lactopeptine No. 3, made up to 100 c.c. with 1.2 c.c. of normal alkali. Ten and 15 c.c. portions were incubated with 50 c.c. of 1 per cent. starch paste through twenty hours at 40 degrees with no apparent result. The Holadin then added, 5 mg. being used, completed the conversion in less than ten minutes.
This shows that the medium was a proper one for the test and that the Lactopeptine must be extremely weak. No sugar tests were made because the Lactopeptine contains milk sugar to the extent of about 60 per cent.
Similar results for both protein and starch digestives have been obtained in a large number of experiments. These here quoted show that the ferment activity of the preparation is so low as to merit no recognition practically. The digestion of a few milligrams of fibrin or starch after many hours of contact, while being perhaps scientifically possible, is of no value when we come to a consideration of the use of such bodies as digestive ferments in medicine.
The amount of lactic acid or “loosely combined HCl” present in Lactopeptine is very small, since the total acid which may be titrated by sodium hydroxid and phenolphthalein is measured by 0.5 c.c. of the normal hydroxid for 1 gm. of the Lactopeptine powder, in the mean. In different samples examined the range was found to be from 0.41 c.c. to 0.6 c.c. Tests with methyl orange, methyl red and other indicators showed that the free acidity is but trifling; if the whole of this acid, as measured by phenolphthalein, were calculated to HCl, the amount would be too small to have any appreciable physiologic activity, in view of the daily dose recommended, 10 to 20 grains of the powder.
The following table gives a summary of the results of my investigations on Lactopeptine. The numbers in the extreme left-hand column are the manufacturer’s identifying marks. These, it is assumed, run serially, the higher numbers indicating fresher specimens.
TABLE SHOWING ENZYMIC POWER OF LACTOPEPTINE PREPARATIONS
| Amylase | Pepsin | Rennin | Trypsin | Lipase | |
2275 | – | + | + | – | – |
2301 | – | + | + | – | – |
2312 | – | + | + | – | – |
2348 | – | + | + | – | – |
2352 | – | + | + | – | – |
2364 | – | + | + | +(?) | – |
2374 | – | + | + | – | +(?) |
2383 | – | + | + | + | +(?) |
1638 (tablets) | – | + | + | – | – |
Pancreatin (Old) | – | .. | .. | ++ | – |
The conclusions in the foregoing summary depend on the following criteria:
Amylase: removal of starch (paste), small in proportion to begin with.
Pepsin: solution of small shreds of fresh fibrin in acid media.
Rennin: curdling of milk in moderate excess.
Trypsin: solution of small shreds of fresh fibrin in neutral and alkaline media, and tryptophan test.
Lipase: coloration of litmus-milk; exact color controls.
All tests were suitably controlled. The responses for pepsin were weak even when compared with those of old pepsin preparations.
In the table above, the interrogation points in parentheses (?) refer to results that were obtained after treatment for from twelve to twenty-four hours and indicates that the change was slight at best and of no practical import.—(From The Journal A. M. A., Oct. 23, 1915, with additions.)
The Council adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
A referee has submitted to the Council the following report of the Chemical Laboratory of the American Medical Association on Iodum-Miller and Iod-Izd-Oil (Miller’s) (Iodum-Miller Co., Kansas City, Mo.):
The unsatisfactory statements made in regard to the composition of Iodum-Miller and the far-reaching therapeutic recommendations for it induced the laboratory to make a chemical examination of the preparation. It claimed more or less directly that the preparation is entirely new and possesses novel characteristics.
It is asserted that
“Iodum-Miller is made from Soot Iodine, which is our own product. This Soot Iodine is SOLUBLE IN WATER before being combined with its base C.P. Glycerine.”
No information regarding “soot iodine” is offered and an inquiry sent to the proprietors by a physician brought only the noncommittal reply that “soot iodine”
“is made from Resublime [resublimed?] Iodine by a chemical process which renders it soluble in water before being combined with its base.”
Iodum-Miller is said to contain
“Active Free Iodine 2.2 grams per 100 c.c., 10. grains per fluid ounce, 1.7% by weight.”
“In addition to the active free iodine ... IODUM-MILLER carries a still greater per cent of Iodine in its basic combination....”
According to the label, the preparation is
“An Iodine for External and Internal use ... 45 drops equals 1 dr. by weight. Each drop equals the per cent. of iodine in 1 gr. potas. iodide.”
Iodum-Miller is a heavy, dark liquid having an odor characteristic of ether (ethyl oxid). Qualitative tests revealed the presence of glycerin, free iodin, iodid and potassium. The specific gravity at 25 degrees was 1.284. Direct titration with sodium thiosulphate solution indicated the presence of 1.68 per cent. of free iodin. A determination of the total iodin content by the Hunter method indicated 3.06 per cent. Subtraction of the amount of free iodin found from the total amount of iodin present, gives 1.38 per cent. combined iodin. Assuming this to be present as potassium iodid, as appears probable from the qualitative examination and from the quantitative determination of potassium, 1.80 per cent. potassium iodid is indicated. From this examination it is concluded that Iodum-Miller is, essentially, a solution of iodin and potassium iodid in glycerin, containing 1.68 per cent. free iodin and 1.80 per cent. potassium iodid. The examination contradicts the assumption that Iodum-Miller is either novel in principle or new. Moreover, accepting the firm’s statement that 45 drops weigh 1 dram (60 grains) the examination shows that one drop equals not “the per cent. of iodine in 1 gr. potas. iodide” but instead, the per cent. of iodin in only 1⁄20 grain potassium iodid. As the statement that “Each drop equals the per cent. of iodine in 1 gr. potas. iodide” appears on the label of the trade package, Iodum-Miller would seem to be misbranded under the federal Food and Drugs Act.
The recommended internal dosage of Iodum-Miller (from 1⁄2 to 20 drops) is equivalent to from 1⁄40 to 1 grain of potassium iodid. Its external efficacy in comparison with that of other iodin preparations may be estimated by comparing the respective free iodin contents, since the germicidal power of combined iodid is negligible. While Iodum-Miller contains 2.15 gm. free iodin in 100 c.c., tincture of iodin contains 7 gm. per 100 c.c. and compound solution of iodin (Lugol’s solution) contains 5 gm. free iodin in 100 gm.
Among the advertising literature is a circular which purports to be a “Certificate from Kansas City Testing Laboratory, by Roy Cross, Secretary.” The “certificate” attempts to prove that Iodum-Miller is vastly superior to the official tincture of iodin as a germicide, asserting that “In the process of dissolving [tincture of iodin] in water, a very large amount of the iodin is lost by precipitation....” This is not true of the tincture of iodin which is now official, though it is true of the tincture official in former editions of the Pharmacopeia. The report ignores completely the widely used aqueous solution of iodin.
Iod-Izd-Oil (Miller’s) is said to be an “Iodine Combination” made “from the same Soluble Soot Iodine as is IODUM-MILLER.” It is said to “liberate Free Soluble Iodine” when applied to the skin, mucous surfaces, etc. It is further defined as “Soluble Iodine combined with water-white Hydrocarbon Oil” and is said to liberate “Soluble Iodine 2 per cent.” While these statements suggest that Iod-Izd-Oil (Miller’s) contains the iodin-potassium iodid combination contained in Iodum-Miller, analysis indicated the oil to be a simple solution of iodin in liquid petrolatum. Quantitative determinations indicated, not 2 per cent. of iodin, as claimed, but only 0.42 per cent. and all of this was present as free iodin.
The following therapeutic claims appear on the label of a bottle of Iodum-Miller:
“EXTERNAL INDICATIONS
“Tuberculosis, Pneumonia, Pleurisy, Cough, Sore Throat, Pyorrhea, Tonsilitis, Rheumatism, Spinal Irritation, Boils, Felons or any Pain. Periostitis, Carbuncles, Fistula in Ano, Goiter, Blood Poison, Diseases of Uterus and appendages (apply full strength on cotton wrapped applicator), Gonorrhea, acute or chronic in both sexes, Orchitis, Bubo, Prostatitis, Swellings, Enlarged Glands, Etc.”
“INTERNAL INDICATIONS
“Pneumonia, Tuberculosis, Pleurisy, Typhoid Fever, Syphilis, Catarrh of Mucous surface of Alimentary Canal, Autotoxemia, Vomiting of Pregnancy, Rheumatism, Chronic Glandular and Organic Affections.”
The “certificate” from the Kansas City Testing Laboratory, mentioned above, states that Iodum-Miller was found to have a germicidal value nineteen times greater than carbolic acid—a somewhat remarkable finding in view of the fact that iodin dissolved by means of potassium iodid in alcohol or water, when tried on the typhoid bacillus has recently been found to possess only four times the germicidal value of carbolic acid in a solution of the same strength (Maben and White: Chem. and Drug., Jan. 30, 1915, p. 144). The “certificate” further states that the test “shows available iodine as found in IODUM-MILLER to have the greatest bactericidal power of any substance that we have ever tested that can be used medicinally.” There is no reason to believe that the desire to please its patrons has led the “testing laboratory” astray from the literal truth. The laboratory’s experience may be limited and the statement therefore entirely correct as far as it goes. No mention, however, is made of any tests comparing the germ-destroying power of Iodum-Miller with that of tincture of iodin, which contains 7 per cent. free iodin, unless the casual statement that “Iodum-Miller sterilized [the skin] more quickly” than tincture of iodin, be taken to imply such tests. It is not clear, however, by what means the laboratory was able to determine that there were no bacteria left alive in the skin after application of tincture of iodin and Iodum-Miller; no details are given of the methods used in arriving at this conclusion.
A circular says that Iodum-Miller
“... gives the Greatest Bactericidal and Therapeutic Action, whether used Internally, Externally, Hypodermically or Intravenously.”
In the light of the preceding report of the Chemical Laboratory of the Association, these claims require little comment. The laboratory has shown that the free iodin content (and consequently the germicidal efficiency) of Iodum-Miller is less than half that of Lugol’s solution, and less than a third of that of the official tincture of iodin. As for the advice to use Iodum-Miller internally in diseases ranging from pneumonia to syphilis and from typhoid to tuberculosis, in order to be convinced of its dangerous character, it is necessary only to recall that this treatment is equivalent to the administration of small doses of iodid—from 1⁄40 to 1 grain of potassium iodid. The mystery being removed from the composition of Iodum-Miller, the absurd extravagance of the claims made for it becomes manifest. The criticisms of the Council on the recommendations for Burnham’s Soluble Iodine (The Journal A. M. A., May 15, 1915, p. 1673) apply in almost every particular to Iodum-Miller.
Unwarranted therapeutic claims are made for Iodum-Miller; incorrect statements are made with regard to its composition and that of Iod-Izd-Oil (Miller’s); and the application of a trade name to both of these products is unjustifiable, since neither is original. It is therefore recommended that Iodum-Miller and Iod-Izd-Oil (Miller’s) be held ineligible for New and Nonofficial Remedies—(Abstracted in The Journal A. M. A., Oct. 2, 1915.)
The Tilden Company, New Lebanon, N. Y., and St. Louis, Mo., sells “Elixir Iodo-Bromide of Calcium Comp. without Mercury” and “Elixir Iodo-Bromide of Calcium Comp. with Mercury.” The latter is said to contain, in addition to the ingredients of the former, 1⁄100 grain mercuric chlorid in each fluidram. According to the label the formula of the elixir “without mercury” is:
“Formula—Salts of Iodine, Bromine, Potassium, Sodium, Calcium, Magnesium with Stillingia, Sarsaparilla, Rumex, Dulcamara, Lappa, Taraxacum, Menispermum.”
A recent circular declares that the elixir contains:
“... a number of the most powerful alteratives of the pharmacopeia such as chemically pure iodin, magnesium, potassium with sarsaparilla, stillingia, prickly ash, burdock, taraxacum, etc. ... Each fluidounce contains seventy-two grains of the combined salts.”
The same circular also alleges that each dram of the preparation contains:
“... the equivalent of one and one-half grains of the combined iodids, potassium and calcium ...”
It will be observed that, (1) the two statements quoted from the circular make no reference to bromids; (2) the statement that each dram contains “the equivalent” of 11⁄2 grains of the combined iodids, potassium and calcium, accounts for but 12 of the 72 grains of “the combined salts” per fluidounce declared in the preceding quotation; (3) the circular mentions the presence of a drug—prickly ash—not declared on the label and, finally (4) none of the “formulas” gives the quantities of all of the several constituents.
It is evident from these “formulas” that the Tilden Company continues its policy of concealment and mystification as exemplified in the cases of Hydrocyanate of Iron, Tilden (discussed in The Journal, June 19, 1909, p. 2008), Febrisol (The Journal, June 29, 1912, p. 2043) and Respirazone (The Journal, June 14, 1913, p. 1899).
In the circular just quoted (“The Conquest of Syphilis”), all hope for the syphilitic is declared to rest in mercury and iodin, and it is implied that only through Elixir Iodo-Bromide of Calcium Comp. is it possible to obtain the greatest good from these drugs.
“Were the cleansing influences of these two drugs [mercury and iodin] unavailable to the luetic patient, he, truly, would be as pitiable an object as the leper ...
“Modern Pharmacy has devised no better means of utilizing these anti-syphilitics than Elixir Iodo-Bromide of Calcium Comp. (Tilden) with or without mercury.... The Elixir, in proper dosage, acts in specific fashion and is adapted for use in all stages of the disease.
“In the early months ... Elixir Iodo-Bromide of Calcium Comp. (Tilden) with mercury is a trustworthy weapon and the physician need have no fear but that it will subjugate the disease ...
“When ... the virulent stage is passed ... Elixir Iodo-Bromide of Calcium Comp. (Tilden) without mercury may be given the patient with every assurance that medicine’s most aggressive measures are being resorted to ... From time to time, up to the very end of the time honored three years’ period of treatment, it is well to put the patient back on the bichloride, using for this purpose the form of the Elixir administered in the first stages of the disease ...
“This regime ... will indubitably antidote the virus of syphilis and eradicate from the organism its every vestige.”
While it seems incredible that any physician would jeopardize the health—even the life—of a patient by accepting this boastful magniloquence as sound therapeutic advice, still the fact that certain medical journals lend their advertising pages to advertisements for Tilden’s Elixir with the caption “The Conquest of Syphilis” makes it incumbent on the Council to record its condemnation of the employment of this unscientific, semisecret mixture.
It is recommended that Elixir Iodo-Bromide of Calcium Comp. “without mercury” and “with mercury” be held in conflict with Rule 1 (secrecy of composition), Rule 6 (unwarranted therapeutic claims) and Rule 10 (unscientific composition).—(From The Journal A. M. A., Nov. 6, 1915.)
The following report was sent to the manufacturers of the various lecithin preparations mentioned therein. As the replies of the manufacturers were obviously written from the commercial point of view and did not affect the Council’s conclusion that lecithin, when indicated, would be given more advantageously in the form of yolk of egg than in the less pure manufactured product, the Council directed that the report be published, together with extracts from the replies of the manufacturers.
W. A. Puckner, Secretary.
Commercial lecithin preparations are at best very impure substances; all are more or less altered from the original composition. Even with great care, the methods of extraction and drying always produce considerable decomposition; and in some cases the phosphorus and nitrogen contents bear but little relation to the theoretical values. (Long, J. H.: Jour. Am. Chem. Soc., xxx, 881. McLean, Hugh: Chem. Abstracts, May 20, 1915). There is not the slightest reliable evidence that commercial lecithin has any advantage over the lecithin contained in natural foods; the weight of probability is on the other side.
The doses recommended, moreover, are absurdly small; and the amount thus administered is without practical value. Why administer a few milligrams of a more or less decomposed lecithin when it is possible to give a far larger weight of a purer substance in the form of yolk of egg?
In view of these considerations the Council voted that the following proprietary products be omitted from the next edition of N. N. R.:
Glycerole of Lecithin
Lecibrin
Lecithin Solution
Lecithol
Neuro-Lecithin-Abbott
and that the general article on “Lecithin Preparations” be transferred to the annual Council Reports as a matter of record.
The report was submitted to the manufacturers. Their replies were evidently based on commercial consideration, and called for no modification in the report.
The referee recommended that the preceding report be published together with the following extracts from the replies of the manufacturers:
From Armour and Company:
“We are selling a good deal of Lecithol and it seems to be giving satisfactory results in some quarters.... We shall continue to advertise Lecithol along the lines we have employed heretofore.”
From the Abbott Laboratories:
“We can assure you of our confidence in the therapeutic value of Neuro-Lecithin. This has been attested by the reports of favorable results sent us by many physicians, as well as by the periodical literature of the last few years which contains a considerable number of very encouraging references to lecithin therapy.”
From Fairchild Bros. & Foster:
“We would like simply to say that the physician and the Council must be aware of the circumstances and the purposes which actuated us in placing lecithin at disposal, viz., the studies—research—of lecithin and the properties attributed to it and which led to inquiry for and consideration of it. The quantities proposed for medicinal use were not suggested by us; the suggestion of lecithin in small quantities as a therapeutic agent was obviously directed by those who proposed it.... The question whether lecithin, per se, has therapeutic properties in contrast to lecithin as naturally contained in food substances, is something we do not undertake to decide. The Council, on purely theoretical grounds, decides in the negative notwithstanding clinical experience—internal and hypodermic—and thus would deny lecithin the status of a new and nonofficial remedy, worthy of at least tentative progressive clinical consideration. We can only say that we offered bona fide lecithin and that we did not make the investigation of lecithin a pretext for the sale of all sorts of lecithin ‘jumbles’ with lecithin in small proportions, taking their name and making their bid on lecithin.”
Below appears the general article which has been omitted from N. N. R.:
Lecithins are fat-like bodies belonging to the group of phosphatides. They all consist of glyceryl esters containing two fatty acid radicals and the phosphoric acid radical in which one of the residual hydrogens is replaced by the choline group. The fatty acid may be palmitic, oleic or stearic and various combinations are known to exist; for example, distearyl lecithin, stearyl palmityl lecithin and so on. The commercial lecithins usually include the closely related kephalins.
On saponification the lecithins split more or less readily into choline, the fatty acids and glycerophosphoric acid, and by fusion with alkali nitrate and carbonate they yield alkali phosphate. They occur, free or in combination as lecithoproteins, most abundantly in certain animal tissues, but there are also vegetable lecithins. The lecithins of commerce are obtained usually from yolks of eggs or from calves’ or sheep’s brains.
Numerous processes have been devised for the preparation of lecithin from egg-yolk or animal tissue. From egg-yolk it may be obtained by making an alcoholic extract and precipitating by cadmium chloride. The precipitate is washed with alcohol and ether, mixed with 80 per cent. alcohol and warmed with the proper amount of ammonium carbonate to remove the cadmium. After filtering hot and concentrating the filtrate the lecithin is thrown down by cooling to a low temperature—10 C. or below. The precipitate is taken up in chloroform and reprecipitated by acetone.
From tissues it is obtained by extracting with warm alcohol and ether, concentrating the extract, precipitating with acetone and repeating the operations.
Pure lecithin is white, but the commercial preparations are yellowish-brown wax-like solids, which are not soluble in water but form milky emulsions which exhibit the myeline figures under the microscope. The solubility in cold alcohol or ether is slight, but heat aids it. Lecithins are not soluble in acetone. They are hygroscopic and the water mixtures undergo decomposition on standing. They darken on exposure to air and light.
The alcoholic solution is precipitated by platinum or cadmium chloride. It is decomposed by alkalies with the formation of choline and trimethylamine. The ash contains phosphoric acid. The different lecithins contain from 3.84 to 4.12 per cent. of phosphorus and 1.73 to 1.86 per cent. of nitrogen. The ratio of nitrogen to phosphorus should be at 1 to 2.21.
Lecithin is incompatible with alkalies; it should be kept in well-stoppered bottles and should be protected from the light.
The content of lecithin (plus kephalin) in tissues is about as follows:
| Per Cent. | ||
Egg-yolk | 8 to | 12 |
Egg-white | 0.1 to | 0.2 |
Liver | 2.0 to | 3.0 |
Kidney | 2.0 to | 3.6 |
Lung | 2.0 to | 3.0 |
Pancreas | 2.0 to | 3.0 |
Actions and Uses.—The lecithin preparations have been recommended in many pathologic conditions, especially in malnutrition and sexual debility. Moderate doses are said to bring about a marked retention of nitrogen and phosphorus, but satisfactory proof of this is lacking. It is extremely unlikely that the small doses which have been recommended in pill or tablet form or in emulsions can have any perceptible action, in view of the fact that many of our natural foods contain much greater weights of available lecithins than the medicinal doses provide. There is no good basis for the statement that the free lecithin has a greater food value or is more readily assimilated than is the substance as found in eggs or tissue. The reverse proposition is much more likely to be true, especially when it is considered that the commercial preparations are usually somewhat altered or decomposed in the process of separation.
Dosage.—Given by the mouth in the form of pills, tablets or glycero-alcoholic emulsions. The amount of actual lecithin ingested in this way is usually small because of the doubtful purity of the original preparation. Several doses, as commonly administered, would be required to furnish the amount of lecithin present in a small egg.—(From Reports of Council on Pharmacy and Chemistry, 1915, p. 122.)
The Council has accepted the following report and authorized its publication.
W. A. Puckner, Secretary.
A former report of the Council (Liquid Petrolatum or “Russian Mineral Oil,” Report Council Pharm. and Chem., The Journal, May 30, 1914, p. 1740) called attention to the large number of concerns that were placing on the market liquid petrolatum as a proprietary under coined names. Since then the number of such products has increased. The Council has been requested by several concerns to consider their products put out under proprietary brand names.
The rules of the Council affirm that “the application of ‘trade names’ to official or established nonproprietary substances tends to confusion and fosters many abuses.” In accordance with this general ruling, the Council has invariably refused to countenance proprietary names applied to liquid petrolatum. The Council holds that proprietary or coined names for this substance are detrimental to medical progress, since they are sure to foster the impression that the particular product is different from liquid petrolatum. Manufacturers have been advised that there is no objection to distinguishing their products by the addition of their firm name or the initial representing the firm name; for instance, “Liquid Petrolatum, A. B. and Co.” or “Liquid Petrolatum, Smith.” The Council also believes that such designations as “Star Liquid Petrolatum” or “Liquid Petrolatum, Anchor Brand,” may be regarded as unobjectionable, provided that the words “Liquid Petrolatum” are always used in connection with the brand designation and given equal prominence.—(From Reports of Council on Pharmacy and Chemistry, 1915, p. 127.)
The Council has adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
Seng (Sultan Drug Co., St. Louis) is called by the manufacturers:
“... a palatable preparation of Panax (Ginseng) in an aromatic vehicle.”
Regarding ginseng (Panax quinquefolia) the United States Dispensatory, nineteenth edition, page 1603, says:
“The extraordinary medicinal virtues formerly ascribed to ginseng had no other existence than in the imagination of the Chinese. It is little more than a demulcent, and in this country is not employed as a medicine.”
No discussion of ginseng is to be found in the more recently published books on pharmacology, materia medica and therapeutics, evidently because their authors agree with this estimate.
On the other hand, physicians are told through the medium of advertisements appearing in medical journals that Seng is:
“An efficient remedy in all affections in which the gastro-intestinal glands need stimulating.
“Exceptionally useful in atonic indigestion, malnutrition, convalescence from the acute diseases, and all digestive disorders characterized by deranged or depressed functions.” (Woman’s Medical Journal, July, 1914.)
According to the label, Seng is indicated in “indigestion,” “malassimilation,” “malnutrition” and “wasting diseases.” It is also stated—though the preparation is admitted to contain 18 per cent. of alcohol—that to give babies “ten to fifteen drops in water or milk during feeding” is a proper procedure and that “For Colic, Flatulency, etc., the dose for an adult or child may be repeated every half hour until relieved.”
The following are some of the exaggerated therapeutic claims made for this preparation of a worthless drug:
“As a result of its administration the gastro-intestinal secretions are augmented, the digestion of food is substantially increased, and fermentative processes are promptly overcome.”
“Seng will specifically encourage the secretion of the juices in the entire alimentary tract ...”
The formula furnished for Seng is non-quantitative and therefore meaningless. The preparation is exploited in a manner to encourage its ill-advised use by the public, and exaggerated and unwarranted therapeutic claims are made for it. The use of an inefficient or worthless drug like ginseng, moreover, is detrimental to rational therapeutics. The Council therefore voted that Seng be refused recognition for conflict with Rules 1, 4, 6 and 10.—(From Reports of Council on Pharmacy and Chemistry, 1915, p. 129.)
Frosst’s Blaud Capsules and Frosst’s Blaud, Arsenic and Strychnine Capsules were submitted to the Council by C. E. Frosst & Co., Montreal, Canada. This firm claims, on the authority of the report of a firm of analytical chemists, that:
“... of three leading Blaud preparations bought by us on the open market, the iron in Frosst’s Blaud Capsules showed the highest percentage of Ferrous carbonate.”
The Chemical Laboratory of the American Medical Association found this claim unjustified. The laboratory reported that there was no especial difference in the ferrous iron content of the various Blaud pills found on the market, and that among ten specimens examined, the total iron content was the lowest in the Frosst specimen. In view of this the Council refused recognition to Frosst’s Blaud Capsules and Frosst’s Blaud, Arsenic and Strychnine Capsules.—(From Reports of Council on Pharmacy and Chemistry, 1915, p. 164.)
Each dessertspoonful of this preparation is said to represent
Buchu Leaves | 31⁄2 | grains |
Uva Ursi | 11⁄8 | grains |
Pareira Brava | 11⁄8 | grains |
Hyoscyamus | 11⁄2 | grains |
Hops | 11⁄2 | grains |
Acetate Potash | 71⁄2 | grains |
Spirits Nitre | 5 | grains |
Alcohol5 per cent. (by volume)” | ||
The manufacturer, J. S. Tyree, Washington, D. C., offers this formula to the medical profession with the following claim:
“Approximate composition made [sic] by quantitative and qualitative analysis of the finished product.”
It is also claimed that
“An even greater advantage of Tyree’s Buchu and Hyoscyamus Compound over other drugs, lies in the fact that every constituent of the former is required to conform to a fixed standard of active principle strength; hence the results derivable from it are absolutely uniform.”
These pretentious claims of scientific accuracy look rather absurd to chemists. Many of the substances present in buchu, hops, hyoscyamus, uva ursi and pareira brava are also present in other drugs; hence it would never occur to a pharmaceutical chemist to try to ascertain the composition of such a mixture as Tyree’s Elixir by “quantitative and qualitative analysis of the finished product,” much less to determine the “active principle strength” of each ingredient, for no methods are known by which this can be done.
It is claimed that, because of the care exercised in making Tyree’s Elixir
“... the results derivable from it are absolutely uniform.”
A moment’s reflection, however, must compel any physician to attribute this statement, on the most charitable construction, to sheer ignorance. Of course, even a definite chemical principle, such as quinin, does not exert uniform clinical action, for clinical conditions vary, and accordingly the patient may or may not be cured. It is simply preposterous to claim that the clinical results obtained from such substances as hops, pareira brava, buchu and uva ursi are absolutely uniform.
A peculiarly vicious claim is that the elixir renders the mucous surfaces of the genito-urinary tract “hostile to the multiplication of the gonococci.” Since infection with the gonococcus produces the direst results, any claim which means in plain English that the remedy assists in producing a cure or in preventing infection with that organism cannot be condemned too strongly. Uva ursi, to be sure, has some slight antiseptic action but it is devoid of any curative action in gonorrhea and the minute amounts that are present in the Tyree elixir are of no more protective value against gonorrheal infection than a grain of hexamethylenamin would be.
It is further claimed that the elixir is a “specific” for “Inflammation of the Bladder, Bright’s Disease, Renal Colic, Suppurative Nephritis, Acute Cystitis, Urethritis, Catarrh of the Bladder [it would be interesting to know what distinction the manufacturer draws between ‘Inflammation of the Bladder,’ ‘Cystitis’ and ‘Catarrh of the Bladder’], Acidemia, Edema, Vesical Catarrh of Old Age, Lithemia” and that ascites and anasarca “can be reduced greatly to the satisfaction of the patient, and honor of the physician” by using a mixture of Tyree’s Elixir and infusion of digitalis. Such claims as these do not merit serious discussion, for they carry their own refutation.
It is recommended that Tyree’s Elixir of Buchu and Hyoscyamus Compound be held in conflict with Rules 5, 6 and 10 and that publication of this report be authorized.—(From Reports of Council on Pharmacy and Chemistry, 1915, p. 167.)
Hydroleine (Charles N. Crittenton Company, New York) is a cod liver oil emulsion said to contain 45 per cent. of cod liver oil, a trace of salicylic acid and 181⁄2 grains of “Pancreatin, Etc.,” per ounce. The advertising claims are based largely on the theory that cod liver oil is “that particular fat which dietetic experience and physiological chemistry have proved to be most digestible.” As a matter of fact, while the superior digestibility of cod liver oil over other oils has often been asserted, neither “dietetic experience” nor “physiological chemistry” have “proved” this by definite observations. The Crittenton Company claims that it is more readily split than other oils. This is probably not true, easy emulsification of the raw oil being often confounded with easy splitting. This latter claim, however, is offered in justification of the name “Hydroleine,” which the Crittenton Company interprets as “hydrated oil.” A circular wrapped around the bottle contains the assertion that “Cod Liver Oil has long been held in high esteem by the medical profession for the treatment of a large number of serious diseases.” This recommendation is likely to lead the public to place undue reliance on Hydroleine in the grave conditions mentioned.
The preparation is in conflict with the rules of the Council inasmuch as its name does not indicate its composition, unwarranted therapeutic claims are made for it, and the exploitation is likely to give the public unwarranted confidence in its value. The Council therefore held Hydroleine ineligible for New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1915, p. 171.)
Curative Vaccine, Bruschettini, manufactured by A. Bruschettini, Genoa, Italy, is claimed to have the properties “of acting directly on the tubercular bacillus, bringing directly into the field and determining a hyperproduction of antibacillar and antitoxic substances.” The use of the preparation is said to be indicated in “all forms of tuberculosis.”
A referee reported to the Council that he had examined the available information and believed that the use of this product had no satisfactory experimental basis. The method of preparation appears to be based more on theoretical considerations than on experimental basis.
On the recommendation of the Committee on Serums and Vaccines the Council voted that Curative Vaccine, Bruschettini, be not accepted because (1) the method used for the production of the vaccine was not satisfactorily stated; (2) the theory on which its use is based has not been satisfactorily confirmed, and (3) the value of the product is not upheld by satisfactory clinical evidence.
The Council’s findings, in accordance with its procedure, were sent to the manufacturers for comment. His reply was considered by a new referee who found that the matter presented did not warrant a revision of the Council’s conclusions. Accordingly the Council directed publication of its findings.—(From Reports of Council on Pharmacy and Chemistry, 1915, p. 176.)
Frederick Stearns & Co. market a preparation known as “Stearns’ Wine,” “Stearns’ Wine of Cod Liver Ext. with Peptonate of Iron,” and as “Vinum Ext. Morrhuae, Stearns.” The constituents are said to be “concentrated extract of fresh cod livers,” “Peptonate of Iron” and a “fine quality of prime Sherry Wine” containing 18 per cent. of alcohol.
This preparation was at one time marketed through the medical profession, but is now advertised direct to the public in typical “patent medicine” style. The label on a recently purchased bottle of Stearns’ Wine contains the following statements:
“STEARNS WINE is an ideal tonic for elderly people, for weak, pale and delicate children and convalescents.
“STEARNS WINE has for many years been successfully prescribed in the treatment of general or nervous exhaustion, anemia, malnutrition, loss of appetite, loss of sleep, faulty circulation and impoverished blood supply.”
The scope of the recommendations for the preparation is further indicated in a booklet accompanying the bottle, which begins:
“STEARNS’ WINE, What It Is and Why It Is Good for You.”
The conclusion is:
“STEARNS’ WINE is a safe medicine for the young, middle-aged and old. It is a safeguard to the family health.”
It is not necessary to discuss either these all-embracing claims as to the therapeutic efficacy of the mixture or the fallacies presented in favor of cod-liver extract and peptonate of iron. The Council reaffirms the opinion that whatever therapeutic value cod liver may have resides chiefly, if not entirely, in its fatty constituents (The Journal, Oct. 9, 1909; Reports Council Pharm. and Chem., 1909, p. 115). A confirmation of this opinion has recently been furnished by the investigations of Prof. J. P. Street (The Journal A. M. A., Feb. 20, 1915, p. 638) of several cod liver cordials, one of which (Vinol) like Stearns’ Wine, is described as a wine of cod liver extract with peptonate of iron.
Stearns’ Wine is essentially an alcoholic stimulant. It is not “a safe medicine for the young, middle-aged and old.” The unwarranted therapeutic claims and the recommendations for its indiscriminate use bring it into conflict with Rules 4 and 6. The Council voted that Stearns’ Wine be held ineligible for inclusion in N. N. R.—(From Reports of Council on Pharmacy and Chemistry, 1915, p. 177.)
The Council had adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
Protonuclein, with other products of Reed and Carnrick, was examined by the Council in 1907 and found ineligible for admission to New and Nonofficial Remedies. According to the patent specifications, it is prepared “from the thyroid and thymus glands, brain (pineal glands and pituitary body), bone-marrow, pancreas, spleen, liver, salivary glands, Brunner’s glands, Lieberkühn’s follicles and peptic glands.” These various glandular bodies, it is said, are dried at a temperature below 130 F. (preferably between 100 and 110); the fat is removed by ether, the dried glands disintegrated, the connective tissue removed by sifting and the resulting powder coated with an ether solution of benzoin and mixed with milk sugar. The dose is three to ten tablets (9 to 30 grains) daily.
Protonuclein Beta is said to be produced by the addition to Protonuclein of an equal amount of nucleoplasm and protoplasm of the spleen. The dose is from two cubes (each 5 grains) three times a day to three cubes four times a day (30 to 60 grains daily).
Special advantages over ordinary nuclein are attributed to Protonuclein, in which, it is claimed, certain unaltered cells remain that are more easily assimilated by the leukocytes than are ordinary proteins, thus leading to a multiplication of cells. In the early advertising Protonuclein was claimed to be:
“... an exact physiological product derived from the lymphoid structures of the body without the use of chemical agents.... So delicate is Protonuclein that any chemical agent is liable to disturb its cellular activity.”
After its examination of the product in 1907 (The Journal, Oct. 5, 1907, p. 1198), the Council concluded that any distinction between the action of Protonuclein and that of ordinary nuclein was purely speculative and highly improbable. “If the active ingredients are really so unstable that they are destroyed by all chemical agents, as claimed, it seems impossible that the activity would be preserved when Protonuclein is given by mouth and therefore subjected to the very profound changes of digestion.”
At that time the importance of thyroid as an ingredient had not been emphasized. The following year, however, Hunt and Seidell (The Journal A. M. A., Oct. 24, 1908) reported the results of an investigation which showed that Protonuclein was a diluted thyroid preparation, as skilfully disguised as in the antifats Rengo and Marmola. Hunt later pointed out (The Journal, Feb. 1, 1913, p. 384) that the amount of nuclein contained in a dose of Protonuclein probably would not have the slightest effect, especially when given by mouth.
The following are extracts from the Protonuclein advertising matter:
“For cancer, infectious fevers (measles, scarlet fever, typhoid and septicaemia) and as a prophylactic.”
“Protonuclein: An ideal prophylactic for all infectious Diseases.”
“A true alterative and tissue builder.”
“The value of Protonuclein depends upon its ability to increase cell power and promote tissue strength. It is therefore needed whenever the organism is below the normal standard, more especially in Anaemia, Typhoid, Neoplasms and as a Prophylactic.”
All the foregoing claims and recommendations are supposed to be based on certain alleged discoveries which the Council has previously characterized as “a tissue of vague speculations ... in direct conflict with the known facts of physiologic chemistry.” As for the third claim, Hunt and Seidell have commented on the danger of recommending thyroid, the most powerful tissue-destroying drug known, as a “tissue builder.”
Protonuclein Beta, it is said:
“... combines the reconstructive action of Protonuclein with the action of the vital principle of the spleen, making it a distinct product for use in all tubercular troubles, including phthisis, localized joint affections and scrofular conditions.”
This product, according to the manufacturers, is based on the work of a certain Dr. Bayle of Cannes, France. Dr. Bayle said that he had treated tuberculous patients with fresh ground up spleen of hogs (25–100 grams per day), mixed with fruit preserve or bouillon; in cases in which this brought on gastro-intestinal disorders, extract of the spleen pulp was administered hypodermically. Bayle reported extraordinary improvements in the physical and mental conditions of his patients even after a few days of this treatment; over 90 per cent. of his tuberculous patients, according to him, improved or were cured. This applied to all types and stages of tuberculosis in man. “With the spleen pulp treatment tuberculous glands disappear like syphilis lesions on administration of mercury and iodids.”
This “spleen specific” of Bayle lacks scientific foundation; Bayle’s own cases were not adequately controlled, and no notice has been taken of Bayle’s report by experts on tuberculosis. Hence it practically lacks both confirmation and contradiction.
The spleen is invaded by tubercle bacilli quite as frequently as are the kidneys and the liver; it has no special toxic action on these bacilli. Nor is there any reason to believe that the end products of gastric and intestinal digestion of spleen pulp, after absorption into the blood, exert such toxic action. It cannot be assumed that these end products indirectly aid the healing processes through improved metabolism, for there is no evidence that they have any specific nutritive or stimulating action after such absorption. Altogether, what we know of the physiology and pathology of the spleen does not warrant us in looking for a “specific” against tuberculosis in this organ.
If, however, the known facts did justify any hope that the spleen might furnish such a specific, manufacturers would not be warranted in exploiting or physicians in prescribing spleen products as a remedy for tuberculosis until control experiments on animals had confirmed the therapeutic value of these products. In a chronic disease like tuberculosis, no conclusions that are scientifically valid can be drawn from clinical cases until many cases have been observed for years under suitable conditions. Right here it may be said that the clinical “evidence” offered in favor of Protonuclein Beta is worthless. The observations which have been reported on this product are not such as to permit any valid final conclusions to be drawn with regard to its value.
The rational method of proving the worth of an alleged new specific such as this is by animal experimentation. So far as we know, neither Dr. Bayle nor the Reed and Carnrick company has performed any such experiments with “spleen pulp” or Protonuclein Beta; nor are we aware that any competent investigator has done so. There is, to the best of our knowledge, no scientific evidence on which to base the claims for Protonuclein Beta.
The Council reaffirms its former action with regard to Protonuclein. The objections made to Protonuclein apply with equal force to Protonuclein Beta. In view of the lack of evidence, the claims for Protonuclein Beta are unwarranted and the product is ineligible to N. N. R. on account of noncompliance with Rules 1, 6 and 8.—(From The Journal A. M. A., Jan. 1, 1916.)
The Council has adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
Hydropsin is marketed by the Ernst Bischoff Company, Inc., New York. Its composition is thus described:
“Hydropsin is the standardized dialysate of Digitalis purpurea, Betula alba, Scilla maritima, Juniperis communis, and Herniaria glabra; or, stated otherwise, it is the juice of these drugs, dialyzed and physiologically standardized.”
“Each fluid dram represents Digitalysatum 7 gtts., and 2 gtts. each of the dialysates of Betula, Herniaria, Juniper and Scilla.”
The composition of Hydropsin must be considered essentially secret since the amounts of the several constituent drugs in a given amount of “dialysate” are not disclosed. The active principle of juniper is a volatile oil which is practically insoluble in water; it is difficult to believe that the “juice” of juniper submitted to dialysis could contain any material amount of the active constituent. No information is given as to the method used whereby the several dialysates are “physiologically standardized.” It therefore remains to be proved that the manufacturer of Hydropsin possesses any method whereby the dialysates of juniper (Juniperis communis), birch (Betula alba, the common European birch) and knot weed (Herniaria glabra) are so standardized. The claim is made that:
“Herniaria has long been recognized as one of the most valuable drugs in the treatment of dropsical affections due to cardiac impairment.”
On the contrary, Herniaria belongs to that large class of drugs which have been tried, found wanting and abandoned. It is a very old remedy, and the claims made for it are an inheritance from the early herbalists, with whom it was very popular. According to King’s American Dispensatory, it was “principally employed to cure hernia (hence its name) and to increase the flow of urine. It was also said to increase the flow of bile.... Internally and externally, it was praised in snake-bites, and the powdered plant was employed to kill maggots on unhealthy sores of horses. It was reputed to ‘crush’ and expel calculi from the kidneys and bladder....”
The Ernst Bischoff Company says that:
“Betula exerts both an antiseptic and stimulating influence on the urinary passages and is particularly serviceable where a catarrhal condition of the bladder exists. When combined with other diuretics, as in Hydropsin, the drug affords highly satisfactory results in the treatment of ascites, cardiac dropsy and hydrothorax.”
Birch is another drug which has been discarded. Few textbooks on materia medica even mention it. That it can materially affect the action of such powerful drugs as squill and digitalis is exceedingly doubtful.
An unwarranted implication—that in this preparation the powerful drugs digitalis and squill have been deprived of their dangerous qualities—is the assertion:
“Dialysis, removing all resins and colloidals, results in better tolerance on part of sensitive patients, and in more rapid absorption and elimination; which, in turn, means early therapeutic effects and little or no fear of toxic accumulation.”
That removal of colloids and resins materially affects the tolerance of these drugs is improbable. To claim that because of their removal, there need be “little or no fear of toxic accumulation” is utterly without warrant. The claim that one preparation containing digitalis is less likely to produce cumulative effect than any other digitalis preparation is contradicted by a mass of evidence.
It is claimed that Hydropsin affects “favorably all forms of dropsy or Edema that are at all amenable to medical treatment.” There can be no question but that squill and digitalis, or, better, either singly, used in suitable cases, may relieve dropsical effusions; but to claim that such a complex mixture as Hydropsin can favorably affect all forms of dropsy that are amenable to medical treatment is on its face unwarranted.
The claim is made that:
“By reason of its unusual potency and relative harmlessness, Hydropsin may be employed to great advantage in all cases where it is desirable to increase the volume of urine without injury to the renal structures.”
On the basis of the claimed composition, the action of Hydropsin must be essentially that of digitalis or of digitalis and squill. Consequently, if it possesses “unusual potency,” it cannot possess “relative harmlessness,” and vice versa. Neither digitalis nor squill should be employed “in all cases” of nephritis, even if it is “desirable to increase the volume of urine.”
The composition claimed for Hydropsin brands it as an irrational mixture in which potent drugs are combined with, and more or less covered up by, others that are obsolete and inefficient. The name, instead of indicating its composition, suggests diseases in which it may be thoughtlessly and indiscriminately used. The claim that the danger of toxic or cumulative action has been removed, if accepted by physicians, tends to uncritical use with possible disastrous results. Hydropsin is ineligible for New and Nonofficial Remedies because of conflict with Rules 1, 2, 6, 8 and 10.—(From The Journal A. M. A., Jan. 8, 1916.)
The Council has adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
Digitalysatum is sold in the United States by Ernst Bischoff Company, Inc., New York. The firm claims that it is a dialysate prepared from the juice of freshly gathered digitalis, containing all the active principles, and representing the fresh plant weight for weight. It is said to be standardized physiologically and to contain 12 per cent. alcohol. Sterisol-Digitalysatum, intended for injection, appears to be the “dialysate” without alcohol, diluted with equal parts of physiologic sodium chlorid solution. The Council some years ago found both products ineligible for New and Nonofficial Remedies because of unwarranted therapeutic claims. The preparations are still being advertised to physicians under claims which imply superiority to all other digitalis preparations. For instance:
“Digitalysatum is the diuretic par excellence in cardiac insufficiency ...”
“Digitalysatum as a diuretic and cardiac stimulant is in a class by itself, being quick of action, uniform in strength, and well tolerated.”
“Digitalysatum differs from other forms of digitalis in these respects:... Digitalysatum is free from fat, resins and colloids, and is therefore well-borne by sensitive patients—the young and the feeble—and is quickly absorbed and eliminated....”
The Council has elsewhere28 expressed the conviction that tincture of digitalis produces the full therapeutic effects of digitalis; that, when properly made, the tincture is as stable as any liquid preparation of digitalis now available, and that attempts to enhance the reputation of proprietary products by exaggerating the disadvantages of the official preparation are to be deplored. No adequate evidence is offered of the claimed superiority of action of Digitalysatum.
By implication, the claim is made that Digitalysatum is superior to other digitalis preparations in respect to toxicity:
“Free from fat, resins and colloidals, it is always well borne and is quickly absorbed and eliminated. No case of toxic accumulation (faulty elimination) has ever been reported.”
That Digitalysatum is free from the dangers of toxic cumulation is highly improbable; in fact, it is inconsistent with the statement that the preparation contains all the constituents found in the fresh plant. Even if instances of cumulative action have not been reported this does not prove that such cumulative action does not occur. The tincture of digitalis has the systemic side-effects of digitalis in no greater degree than the various proprietary preparations. Attempts to create the impression that Digitalysatum possesses all the virtues of digitalis without its chief disadvantage are to be condemned as likely to lead to incautious use of the preparation.
These exaggerated claims are in the main made indirectly, but they are none the less inimical to sound therapy. The Council therefore declared Digitalysatum ineligible for New and Nonofficial Remedies and voted that this report be published.—(From The Journal A. M. A., Jan. 8, 1916.)
The Council authorized the following report for publication, and voted to endorse the work of Professor Carlson discussed therein.
W. A. Puckner, Secretary.
The Council has not accepted for inclusion in New and Nonofficial Remedies any preparations said to contain secretin or prosecretin as their active ingredient. A report giving the reasons for the rejection of one (the first of the so-called secretin preparations marketed) was published early last year;29 an article on secretin, based on work undertaken at the request of the Council on Pharmacy and Chemistry, is now published.30
Lest the appearance of this detailed study of secretin, after the rejection of so-called secretin preparations, should be interpreted (as manufacturers whose products have been rejected have endeavored to interpret such action) as a case of first condemning a preparation and then getting the facts, the Council’s methods, and their application in this case, may be briefly stated. The Council maintains that, when a manufacturer places a product on the market, the burden of proof is on that manufacturer to show that the properties of his product are in accordance with his claims for it. As stated in the introduction to N. N. R., “it is ... manifestly impossible for the Council to investigate the composition of every complex pharmaceutical mixture, or to check thoroughly every therapeutic claim; it can give only an unbiased judgment on the available evidence.” Acting on this principle, the Council examined the claims made for Secretogen, an alleged secretin product manufactured by the G. W. Carnrick Company. The conclusion was that these claims were in absolute conflict with the available evidence as to the action of secretin.
It is not necessary to review this subject again. It will suffice to state that the claims made for Secretogen rest on two fundamental propositions: (1) that deficiency of secretin (or, rather, of prosecretin) in the intestinal mucosa is a factor in gastro-intestinal diseases; (2) that secretin given by the mouth is absorbed and produces increased secretion of the pancreatic and intestinal juices and of the bile.
From an examination of the evidence available, including that submitted by the manufacturers, the Council concluded: “1. No evidence has been presented that the absence of secretin is a cause of gastro-intestinal disease. 2. There is no evidence that secretin in any form is physiologically active when administered by mouth.” That these conclusions were justified is shown again by the review given by Carlson of the literature, much of which was also reviewed in the Council’s previous report.
Since the claims of the Carnrick Company were not supported by any satisfactory evidence, no further investigation on the Council’s part was necessary to warrant rejection of the product. The Council did not undertake to determine, for instance, whether or not Secretogen and similar products actually contain secretin; the determination of this point was immaterial here, in view of the conclusiveness of the evidence that secretin given by mouth has no physiologic action.
Since firms other than the G. W. Carnrick Company are manufacturing alleged secretin preparations, and since recommendations for the use of secretin preparations in gastro-intestinal diseases have even crept into textbooks, it seemed desirable to obtain further information on certain points. The Council therefore requested Prof. A. J. Carlson of the University of Chicago to check the results of previous investigators with regard to the action of secretin administered by mouth or directly into the intestine, and, in addition, to investigate the secretin content of certain alleged secretin preparations.
Carlson and his co-workers, like all previous investigators, found that secretin given by mouth, or introduced even in enormous doses directly into the intestine, is entirely inactive. They also found that marked destruction of secretin followed contact for one minute with human gastric juice and that secretin is rapidly oxidized and rendered inert in contact with the air.
Further, they were unable to demonstrate the presence of secretin in samples of Secretogen and another supposed secretin preparation (Duodenin) bought on the open market. In the case of Secretogen there was one exception: one bottle was found which contained a little secretin, but it was necessary to administer (by intravenous injection, of course) the entire contents of the bottle (100 tablets) to obtain “a small but unmistakable secretin reaction.”
In these studies the methods employed were those by which secretin was discovered. It is only by the use of such methods that the presence or absence of secretin can be determined. Apparently the manufacturers who place so-called secretin preparations on the market do not make use of these methods, by which alone even the composition of their products can be determined.
Carlson and his collaborators conclude:
“There is as yet no reliable evidence that lack of secretin is a primary or important factor in any disease. Even should this be established, secretin therapy, to be effective, must be intravenous. Secretin has not yet been prepared in sufficiently pure state to render possible intravenous injection in man without injurious effects. And even when this is attained, the very fleeting action of secretin will in all probability render secretin therapy as futile in all the diseases in which it is theoretically indicated as epinephrin therapy is in Addison’s disease.”
In short, secretin is as ineffective taken by mouth as it would be rubbed on the skin.
The referee recommends that the work of Professor Carlson be endorsed.—(From The Journal A. M. A., Jan. 15, 1916.)
It is well established that acid chyme in the duodenum is the normal stimulus to the secretion of pancreatic juice.31 Interaction of the acid with the duodenal mucosa liberates into the blood stream a substance which, circulating through the pancreas, excites the latter to activity. This exciting substance has been termed “secretin.” It can be prepared artificially by macerating duodenojejunal mucosa in 0.4 per cent. hydrochloric acid, neutralizing the boiling mixture, and filtering. A few cubic centimeters of the filtrate injected into a vein produce invariably a powerful secretion of pancreatic juice.32 That a “chemical messenger” is at the basis of the duodenal acid reflex has been proved by even more crucial experiments—transfusion (Wertheimer,33 Enriquez and Hallion34), cross circulation (Fleig,35 Matuso36), and perfusion of the isolated pancreas (Huston37).
Prosecretin.—Secretin is soluble in water, yet a watery extract of intestinal scrapings is without action,32 even after being submitted to acid treatment.38 Starling therefore holds that secretin exists in the intestinal mucosa in an inactive form, as “prosecretin.” The content of the intestine in prosecretin decreases from the duodenum down, so that one is unable to demonstrate any prosecretin in the last 21⁄2 feet of the ileum. Prosecretin is insoluble in water, acetone, absolute alcohol or ether. Secretin, on the other hand, is readily soluble in water, normal salt solution and diluted alcohol (70 per cent.), but likewise insoluble in absolute alcohol and ether.
Preparation.—All of the more dissociated acids liberate secretin from intestinal mucosa on boiling. Their action is dependent on the degree of dissociation,39 carbonic and boric acids being inactive.40 Secretin can also be prepared with strong soaps (from 10 to 30 per cent. sodium oleate), alcohol (70 per cent.,41 0.6 per cent. sodium chlorid36). The acid and soap in the duodenum produce secretion; there is no necessary correspondence between the action of a substance in the intestine and that obtained by injection after boiling mucosa with it. The sodium chlorid, bile, maltose and glucose produce some secretion by the latter method yet none by the former.36 On the other hand, ether, chloral and oil of mustard excite secretion when in the intestine, but no secretin can be prepared from boiled mucosa by their action. The irritation of the lining cell has produced the necessary hydrolysis.38 In well-controlled experiments, Wertheimer and LePage42 found that after the introduction of acid, secretion is secreted into the lumen of the intestine. Matuso36 confirmed their results, and found this a satisfactory method for the preparation of secretin. It is said that secretin can be obtained by merely boiling the mucosa with water, but the results are inconstant.43
Action.—Secretin is an excitant not only of the pancreatic juice but also of the liver and the intestinal mucosa. The flow of bile is markedly accelerated (Henri and Portier,44 Enriquez and Hallion45), likewise that of succus entericus (Delezenne and Frouin,46 Bottazzi and Gabrielli47), and intestinal peristalsis is stimulated (Enriquez and Hallion,48 Falloise49). Injections of secretin produce a marked vasodilatation, but the secretory effect is independent of the blood pressure changes. The pancreas is not readily fatigued by secretin. Bayliss and Starling50 have obtained undiminished flow after eight hours of continuous injection. Our experience confirms this result. Also, equal doses of secretin give corresponding results at various intervals. Moreover, anesthesia does not affect the flow. Secretin is unrecoverable from the glands even after two hours of continuous injection.51 The juice obtained by secretin has been subject to many studies.52 It is of high alkalinity (about seventh normal), contains all the pancreatic ferments, and corresponds in all respects to the juice obtained in digestion from permanent pancreatic fistulas.53
Specificity.—In a maceration of the duodenojejunal mucosa, such as we have in secretin, the known substances are proteoses and peptones, acid amins, bile salts, beta-imidazolethylamin, cholin, gelatin and inorganic salts. These substances, individually and severally, together with their derivatives, are devoid of secretory action. Chemically, secretin, is then a specific entity. But like epinephrin, in its distribution, it is nonspecific. Active preparations have been made from an extraordinary variety of animals among the different classes of vertebrates (Camus,54 Bayliss and Starling,55 Chapman56). It is likewise found in the new-born and in the fetus.57 Its action, however, like its chemical composition, is markedly specific. It stimulates the flow of pancreatic juice, bile and succus entericus. Its effect on the gastric glands is negative, and on the saliva likewise.58 On the other hand, no other extracts produce pancreatic secretion. Dr. Koch, who, in collaboration with Dr. Keeton and Dr. Luckhardt, has done the most recent work on gastrin59 (a substance that most nearly resembles secretin) and has isolated an extremely active preparation, finds that gastrin injection has likewise no effect on the pancreas. Camus and Gley,60 with crude preparations, had previously obtained a similar result.
Lability.—Neutral secretin is but feebly attacked by a temperature of 100 C. If heated in an autoclave (so as to prevent oxidation), this temperature can be continued for thirty minutes without any change in its activity. Increasing the temperature increases the speed of destruction, so that at 140 C. the destructive action is marked.61 Autoclaving at 15 pounds for fifteen minutes, as an ordinary sterilization of culture mediums, produces, we found, a distinct though not serious decrease in activity. Secretin acidified to fifth-normal with hydrochloric acid loses 60 per cent. of its activity on fifteen minutes boiling. Secretin, alkalinized to fifth-normal with sodium hydroxid loses 95 per cent. of its activity in five minutes’ boiling; decreases to a trace in thirty minutes, and disappears entirely in sixty minutes. At room temperature, with fifth-normal alkalinity, 80 per cent. of secretin is destroyed in eight hours.61 The destruction probably means a secondary cleavage of the secretin molecule itself.
Secretin is oxidized readily. If left standing uncovered for a summer’s day, the preparation will be inactive.51 Even if kept in the ice-chest (no other precaution being taken), its activity is lost in a very few days. Sunlight undoubtedly hastens the oxidative process. If care is taken as to sterility, however, and the secretin is kept in the ice-chest, well stoppered and in a dark flask, it will retain its activity for several weeks.
Dixon and Hamill51 claimed that secretin disappears quantitatively on passage through a Berkefeld filter at 5 mm. pressure. Lalou,62 using higher pressure, was unable to confirm the finding, but obtained a marked decrease in activity. Our results are in accord with those of Lalou.
Analogy to Epinephrin.—The analogy of secretin to epinephrin does not generally receive enough emphasis. Both substances are nonspecific in distribution, but specific chemically, and especially physiologically, epinephrin acting on the myoneural junctions, secretin on intestinal digestion. They are both relatively simple substances of low molecular weight, and subject to rapid oxidation whereby their properties disappear. The action in both cases is very transient. They are the two examples of what Starling calls the “acute hormones,” in which it is essential that reaction take place immediately, and shall disappear as soon as the exciting cause is removed.63
Diabetes Mellitus.—Moore, Edie and Abram64 were the first to suggest a therapeutic value for secretin, having obtained favorable results with secretin administration in diabetes. They argued that the internal secretion of the pancreas may be stimulated by secretin, and that some cases of diabetes may be due to lack of this necessary excitant. Owing to the importance of the question, their announcement was followed quickly by numerous investigations by other observers. Previously, Spriggs, at the suggestion of Starling, had tried intravenous injections of secretin free from depressor substance in a diabetic patient, and had obtained negative results. Moore, Edie and Abram gave their secretin by mouth over long periods. Of the five cases cited in their first paper, two were negative. The third was that of a man, aged 25, who received daily 30 c.c. of secretin. After a latent period of three weeks, the sugar suddenly fell, and after four months the urine was sugar-free. Six months later a relapse occurred with the development of phthisis and death. The other two patients were a boy, aged 7, and a girl, aged 9, whose urine in from three to five weeks became sugar free during the secretin treatment in spite of severe diabetes. One of these patients later relapsed.65 Bainbridge and Beddard66 gave secretin a thorough trial in three cases with negative results, and are disposed to attribute the results of Moore to dieting. Dakin and Ransom67 cited one case, secretin being given for twelve weeks, with negative results; Foster,65 nine cases, all negative; Charles,68 three cases, all negative. Crofton,69 however, gave secretin a trial in one case with favorable results. Moore, Edie and Abram, in a later paper,70 report a large number of cases tried with the majority of results negative, though in some cases an improvement in the digestion, and in certain cases an increase of weight was noted.
One method of testing the basis of Moore’s theory would be by examining the prosecretin content of the intestine in diabetics. Bainbridge and Beddard found, in the paper referred to,66 that from five of the six cases of diabetics examined postmortem, little or no secretin could be prepared; but in a subsequent report of seven cases,71 they found only one in which the secretin obtained was scanty. The failure to obtain secretin in some cases they claim is probably due to the rapid postmortem degeneration of diabetic tissue. Evans,72 in Starling’s laboratory, found that in dogs made recently diabetic by total pancreatectomy, but little secretin could be obtained. Hedon and Lisbonne,73 and Pemberton and Sweet74 report, on the contrary, that the duodenum of diabetic dogs is rich in prosecretin. Bainbridge and Beddard,71 working on a diabetic cat, likewise found prosecretin to be present in normal quantity.
Digestive Disturbances.—Secretin for digestive disturbance was first used in the “acid duodenal medication” of Enriquez.75 This consisted in the giving of tartaric acid in thick keratin capsules, the acid not being liberated until the duodenum was reached, where it provoked the formation of secretin. “The secretin mechanism,” he says, “is probably capable of pathologic disturbance as would result, for example, with diminished acidity of chyme, disturbance of the normal motility of the stomach or pylorus, or diminished prosecretin in the mucosa. Such a condition would produce disturbance of the pancreatic, biliary and intestinal secretions, and interfere with intestinal movements, with a clinical syndrome of intestinal dyspepsia as a result, among the chief and most constant symptoms of which would be constipation.” “The acid duodenal medication” was submitted to wide clinical use, and very favorable results in certain obstinate cases of constipation were reported. In regard to “diminished prosecretin in the mucosa,” Wentworth76 has claimed that in infantile atrophy such is the condition, but Sweet and Pemberton77 have found that the difficulty of preparing secretin from human duodenums is such as to render Wentworth’s findings inconclusive.
Beveridge78 suggests the use of secretin in (a) pyloric stenosis, (b) pancreatic insufficiency, (c) hepatic stimulation and cirrhosis of the liver (d) to stimulate peristalsis in colonic stasis, (e) in gastro-enterostomy and short-circuiting of the intestines. He claims to have used it in over a hundred cases with “brilliant results,” and cites four typical histories. The G. W. Carnrick Company, which manufactures “Secretogen,” an alleged secretin preparation, cites a number of authorities79 as also recommending secretin for digestive disorders. Harrower, who is or was connected with the Carnrick Company, in clinical journals80 has ardently advocated the use of secretin for a large number of maladies.
Throughout its clinical use, secretin has been given by mouth; but its direct introduction into the intestine of a dog under anesthesia in even enormous quantities is without effect. This fact, first observed by Bayliss and Starling,32 was confirmed by Fleig,81 and Matuso,36 and our personal experiments have convinced us of its truth. Matuso found that ordinary secretin and that obtained from intestinal lumen gave equally negative results. Large quantities of active secretin, moreover, acidified to 0.2 per cent. hydrochloric acid, and left in the ileum for fifteen minutes, were still negative. Wertheimer and Duvillier,82 in a previous paper on this subject, had likewise found that acid solutions of secretin (which might be considered more normal for the intestine than when neutral), when introduced into the ileum gave negative or inconstant results. They conclude that it is more likely that the pancreas does not respond to such minimal stimuli, than that the secretin is not absorbed.
The destructive action of the digestive enzymes leads us to believe that it is in inactive form that secretin is absorbed. Like epinephrin, it cannot pass through the digestive tract. Bayliss and Starling state that it is destroyed by one hour’s tryptic digestion. Lalou62 worked with the action on secretin of pepsin, dog’s gastric juice, pancreatic juice, succus entericus and erepsin, and found in each case a destructive effect, even almost after mixing; and after five minutes over 75 per cent. of the activity had disappeared. Matuso36 introduced 30 c.c. of active secretin into the intestine, removed it five minutes later, and found that no activity remained.
Other methods of administration have been tried. Subcutaneous injections are practically negative (Matuso,36 Hallion83) and intrapleural injections are likewise negligible (Bayliss and Starling55).
Starling63 finds that continued intravenous injections of secretin in a healthy dog produces after a time severe symptoms of collapse, which, he believes, are due to change in the intestinal mucous membrane caused by the entry and non-neutralization of the strongly alkaline pancreatic juice.
Intestinal digestion seems little affected in achylia gastrica (Stockton,84 Ehrman and Lederer,85 Bayliss and Starling32). This may be due to other secretin stimulants as fats, or to the action of the nervous mechanisms (Meltzer86).
We have carried out in detail experiments on the digestive effect of human gastric juice on secretin. Our results in every respect confirm the findings of Lalou,62 who worked with commercial pepsin and dog’s gastric juice, but are even more striking because of the much superior quality of pure human gastric juice.
Methods.—The human gastric juice was obtained from Mr. V., the gastric fistula case of our laboratory. The chemical and digestive characters of his juice are discussed in a recent paper.87 In the different experiments, different samples of gastric juice were used. The secretin employed was always freshly prepared. Digestion was carried out in the incubator at 38 C. with the reaction of 0.4 per cent. acid, and the end of the period was marked by either boiling the mixture or (in the first two experiments) by turning the mixture alkaline. The action of the preparation, we proved, was not influenced by the method used. The dogs on which the preparations were tested were prepared for carotid blood pressure, injection into the external jugular vein, and cannula in the pancreatic duct, essentially the methods of Bayliss and Starling32 being employed. The preparations were injected at body temperature after being neutralized and filtered. Except for the addition of normal salt solution instead of gastric juice, the control injections of secretin were submitted to exactly the same treatment as the other preparations.
Results.—Our results are embodied in Table 1. We assured ourselves before beginning the series that incubation of secretin with boiled gastric juice produced no change. It is to be noted in the table that each experiment is a unit complete in itself, beginning and ending with a control injection of secretin. Special attention is called to the marked destruction that follows contact of human gastric juice with secretin for merely one minute. In Experiment 4, using 1 c.c. of human gastric juice, the action fell to 14 drops from an original secretion of 21; in Experiment 5, using 8 c.c. of gastric juice, the action fell to 6 drops from an original secretion of 20. Of interest also is the rate at which we get complete destruction of secretin. This is practically 2 hours for 2 c.c. with secretin giving originally 110 drops (Experiment 2, Fig. 1), or 30 minutes for 5 c.c. with a secretin giving originally 53 drops (Experiment 6). These results are practically parallel, though they were obtained with different samples of gastric juice and in different experiments.
TABLE 1.—THE DESTRUCTION OF SECRETIN BY HUMAN GASTRIC JUICE
| No. of Experiment | Quantity of Gastric Juice Used, C.c. | Secretion of Pancreatic Juice in Drops | |||||||
| 10 C.c. Secretin Control —Beginning Experiment | The Secretin After Incubation with Human Gastric Juice | 10 C.c. Secretin Control —End of Experiment | |||||||
| Dig. Time, Hours | Secretion Rate | Dig. Time, Hours | Secretion Rate | Dig. Time, Hours | Secretion Rate | ||||
| 1 | 2 | 28 | 6 | 0 | 4 | 0 | 2 | 0 | 16 |
| 2 | 2 | 110 | 2 | 7 | 11⁄2 | 18 | 1 | 18 | 41 |
| 3 | 2 | 40 | 1 | 7 | 3⁄4 | 7 | 1⁄4 | 8 | 31 |
| 4 | 1 | 21 | 1⁄2 | 11 | 1⁄4 | 12 | 1⁄60 | 14 | 18 |
| 5 | 8 | 20 | 1⁄2 | 1 | 1⁄4 | 3 | 1⁄60 | 6 | 18 |
| 6 | 5 | 53 | 1⁄2 | 2 | .. | .. | .. | .. | .. |
We also tried the effect of keeping the digestive time constant and varying the amount of gastric juice employed. Increasing the quantity of gastric juice used increases the quantity of secretin destroyed (Table 2).
TABLE 2.—EXPERIMENT 7*
| Preparation | Pancreatic Juice Drops |
10 c.c. secretin | 20 |
10 c.c. secretin digested with 0.5 c.c. gastric juice | 15 |
10 c.c. secretin digested with 3 c.c. gastric juice | 13 |
10 c.c. secretin digested with 10 c.c. gastric juice | 8 |
* The digestive time was kept constant at fifteen minutes. (The gastric juice used had been diluted with stomach washings.)
The reader will observe in Table 1 that the results obtained from the control injection of secretin at the beginning of the experiment is uniformly greater than that obtained after several injections of digested secretin.
In view of the established fact that equal quantities of secretin can generally be relied on to produce results,62 one might suggest that the injections of the split products of secretin have inhibited to some degree the action of the pancreas. We can submit the data in Table 3 in support of this view, showing among other things that the action of secretin is not influenced by previous injections of inert depressor substances, though it by the injection of the cleavage products of secretin. (The various injections in the experiments were made at about fifteen-minute intervals).
We have carefully analyzed the reaction in blood pressure that follows the injection of the various preparations. We find no constant effect. Digested secretin gives a fall in blood pressure that is at times less, at times equal, and at other times greater (Fig. 1) than that produced by the original preparation.
Besides the bearing that it has on the therapeutic use of secretin, this destructive action of the digestive enzymes is also of prime physiologic interest. Failure to realize it has led to misconceptions as to the intrinsic nature of secretin.
TABLE 3.—EXPERIMENTS 8 AND 9
| Preparation | Pancreatic Juice Drops |
| Experiment 8: | |
10 c.c. secretin, five injections of inert depressor substances | 29 |
10 c.c. secretin, two injections of completely digested secretin | 28 |
10 c.c. secretin, eight injections of inert depressor substances | 16 |
10 c.c. secretin | 16 |
| Experiment 9: | |
10 c.c. secretin (control, beginning of experiment) | 21 |
| 10 c.c. secretin, after thirty minutes incubation with 1 c.c. | |
boiled gastric juice | 27 |
| 10 c.c. secretin, after thirty minutes incubation with 1 c.c. | |
fresh gastric juice | 11 |
10 c.c. secretin (control, end of experiment) | 18 |
The findings of Lalou, confirmed by us, explain the anomaly that has led Delezenne88 to put forward the antisecretin theory.
It is a constant claim that so many and complex are the factors concerned in physiologic processes, that it is not unusual for clinical deductions to establish themselves in the face of a priori laboratory dicta. We considered it desirable, therefore, to test the action of secretin, orally administered, in the most direct manner, and the one freest from possible criticism. With this in view, we performed a series of experiments on normal unanesthetized dogs having permanent pancreatic fistulas.
Method.—In the operations for permanent pancreatic fistulas we followed closely the technic developed by Pawlow,89 and with excellent results. The dogs maintain themselves in splendid condition if proper care is taken. This consists in feeding them only with bread and milk, and giving sodium bicarbonate daily. The dogs were given this treatment in the evening so that experimental procedure might be carried on in the day with empty stomach under constant conditions. Freshly prepared secretin in large quantities was given by stomach tube to these dogs, and the response of the pancreas studied and compared with the response obtained from control preparations. The same preparation was generally not given on consecutive days.
TABLE 4.—DETAIL OF TYPICAL EXPERIMENTS
Dogs with pancreatic fistulas, showing that secretin given by mouth has no action on the pancreas
| Material Fed by Stomach Tube | Rate of Secretion of Pancreatic Juice in C.c. per Hr. | |||||
| Continuous Secretion Before Feeding | Continuous Secretion After Feeding | |||||
| First Hour | Second Hour | Third Hour | First Hour | Second Hour | Third Hour | |
150 c.c. active secretin, slightly acid | 6.5 | 3.6 | 3.9 | 20.0 | 6.0 | 8.0 |
150 c.c. active secretin, slightly alkaline | 13.0 | 11.0 | 5.0 | 23.0 | 26.0 | 12.0 |
150 c.c. secretin passed through Berkefeld | 7.8 | 7.5 | 7.4 | 23.0 | 13.0 | 11.0 |
150 c.c. extract of colon | 11.6 | 12.0 | 11.4 | 30.0 | 19.6 | 14.8 |
150 c.c. extract of gastric mucosa | 10.0 | 7.0 | 8.0 | 23.0 | 7.5 | 4.0 |
150 c.c. extract of muscle | 6.9 | 11.0 | 6.4 | 35.0 | 5.0 | 7.0 |
150 c.c. 0.4% HCl (diluted to 250 c.c.) | 6.0 | 8.0 | 4.0 | 33.0 | 36.0 | 17.0 |
Results.—We have data from six dogs with a total of seventy-six experiments. As shown in Table 4, the administration of secretin causes an increase in the flow of pancreatic juice, but the administration of inert substances as extracts of colon, gastric mucosa or muscle causes a like increase. The activity of the secretin may be reduced to a low value by exposure to sunlight, or filtering through a Berkefeld filter, yet the response of the pancreas is not correspondingly reduced. The secretion that occurs in the control cases, every one will admit, is but secondary to the production of gastric juice with its accompanying hydrochloric acid, that is, excited by virtue of the extractives and water in the preparations. Such, we can prove, is the only action of secretin. A mixture of gelatin, peptone and salt water, the chief incidental constituents of a secretin preparation, gives as striking results as ever obtained from secretin administration. Yet the objection may be made that the response of the pancreas that is due to the incidental constituents of secretin is maximal, and that the secretin consequently has no opportunity to display its particular potency. But, as inspection of the accompanying tables illustrate, the administration of hydrochloric acid shows that the response is by no means maximal. Let us cite a striking experiment. For three hours before the administration of hydrochloric acid, the secretion in cubic centimeters was respectively 29.4, 11.75 and 35.4 c.c.; for the three hours after, respectively 88.0, 49.0 and 40.5 c.c.

TABLE 5.—SUMMARY OF EXPERIMENTS
Dogs with pancreatic fistula, weight 14 kg. Secretin given by mouth
| No. of Experiment | Material Fed | Rate of Secretion of Pancreatic Juice in C.c. Per Hour | Increase in C.c. | |
| Three Hours Before Feeding | Three Hours After Feeding | |||
| 3 | Secretin slightly acid | 5 | 11 | 6 |
| 5 | Secretin slightly alkaline | 24 | 30 | 6 |
| 4 | Secretin passed through Berkefeld | 18 | 23 | 5 |
| 1 | Secretin exposed to sun for 4 hrs | 16 | 29 | 13 |
| 2 | Extract of colon (rabbit) | 19 | 29 | 10 |
| 3 | Extract of gastric mucosa | 14 | 23 | 9 |
| 3 | Extract of muscle | 8 | 16 | 8 |
| 2 | Mixture of gelatin, peptone and salt | 23 | 33 | 10 |
| 1 | 1 per cent. peptone solution | 6 | 8 | 2 |
| 4 | 0.2 per cent. hydrochloric acid | 13 | 37 | 24 |
| 3 | Milk and bread | 7 | 20 | 13 |
It is possible by large doses of sodium bicarbonate given shortly before the administration of a preparation so to depress the stomach that it does not respond with the usual production of hydrochloric acid. Under these conditions the administration of secretin is uniformly negative, but the administration of hydrochloric acid on the contrary still serves to increase the pancreatic secretion (Table 6).
TABLE 6.—SECRETIN IN EXPERIMENTAL “ACHYLIA GASTRICA”
| Exp. No. | Material Fed | Rate of Secretion of Pancreatic Juice in C.c. Per Hour | |||||||
| Continuous Secretion Before Feeding* | Secretion After Feeding | ||||||||
| First | Second | Third | First | Second | Third | ||||
| 1 | 150 c.c. secretin | 8.7 | 7.5 | 6.8 | 3.0 | 1.0 | 4.8 | ||
| 2 | 4.5 | 6.5 | 10.0 | 6.0 | 7.5 | 7.6 | |||
| 3 | 15.6 | 8.1 | 16.0 | 3.9 | 4.9 | 2.9 | |||
| 1 | 150 c.c. 4% HCl (diluted to 250 c.c.) | 9.8 | 7.0 | 6.0 | 65.1 | 28.0 | 7.1 | ||
| 2 | 17.4 | 18.5 | 17.0 | 34.0 | 18.0 | 20.0 | |||
* Five gm. Na HCO3 given at beginning of each first two hours.
Secretogen and Elixir Secretogen.—The Carnrick Company offers Secretogen90 for use in a large number of conditions. The following indications for the use of the preparation purport to be based on clinical tests covering a period of several years: dyspepsia, and the indigestions generally, fermentative disorders, gastric catarrh, flatulence, nausea; pancreatic insufficiency, intestinal indigestion; gastric secretory deficiencies, apepsia; constipation and hepatic torpor; intestinal stasis; diarrhea; infantile diarrhea, “summer complaint,” marasmus, inanition and malnutrition; gastric atony and dilatation; cholecystitis and gallstones; nephritis, neurasthenia, cachexia and cancer; epilepsy and high blood pressure. Testimonials are presented as to results in most of these conditions.
A quantity of “Secretogen” and “Elixir Secretogen” was bought in the open market, and the preparations were tested on suitably prepared dogs. The tablets were ground, thoroughly macerated with the solvent used (water, normal salt solution, alcohol, or 0.4 per cent. hydrochloric acid), and filtered. If hydrochloric acid was used, the pulverized tablets were boiled with it, in the manner that secretin is made from duodenal mucosa, and the preparations neutralized previous to injection. The injections were made in from 15 to 20 c.c. of the solvent. All the operations were carried on immediately before the experiment, and as rapidly as possible, so as to avoid oxidation. The Elixir Secretogen was injected directly, without dilution.
TABLE 7.—SUMMARY OF TYPICAL EXPERIMENTS SHOWING THE ABSENCE OF SECRETIN IN “SECRETOGEN”AND “ELIXIR SECRETOGEN” EXCEPT IN OCCASIONAL TESTS WHEN ADMINISTERED IN ENORMOUS DOSES
Dogs under ether anesthesia
| Exp. No. | Quantity of Secretogen and Elixir Secretogen Used* | Secretion of Pancreatic Juice in Drops, Following Intravenous Injection | ||||||
| Control 10 C.c. Secretin | Secretogen in | Elixir | Control 10 C.c. Secretin | |||||
| Distilled Water | 0.4% HCl | 70% Alcohol | 0.9% NaCl | |||||
| 1 | Secretogen, 1 tablet; Elixir, 15 c.c. | 109 | 0 | 0 | 0 | 0 | 0 | 59 |
| 1 | Secretogen, 6 tablets | ... | .. | 0 | .. | .. | .. | .. |
| 2 | Secretogen, Elixir, 15 c.c. 3 tablets; | 16 | 0 | 0 | 0 | 0 | 1(?) | 16 |
| 3 | Secretogen, 5 tablets | ... | .. | 1(?) | .. | .. | .. | .. |
| 4 | Secretogen, 25 tablets | 14 | .. | 1(?) | .. | .. | .. | 8 |
| 5 | Secretogen, 100 tablets | 110 | .. | .. | .. | 21 | .. | 67 |
| 6 | Secretogen, 100 tablets; Elixir, 125 c.c. | 19 | .. | 5 | .. | 1 | 2(?) | 8 |
| 7 | Elixir, 50 c.c. | ... | .. | .. | .. | .. | 1(?) | .. |
* One to three tablets is (according to the label) the therapeutic dose of Secretogen; 4 to 12 c.c. the dose of Elixir Secretogen.
Results.—In only one case was a slight response obtained, the others gave none. Small and large doses were equally inert (Table 7, Figs. 2, 3). The preparations, though inert, always produced a depression in blood pressure, sometimes even greater than that caused by active secretin. Among our many tests, one bottle was found, however, to be a little different from the rest (Experiment 4). Its entire content, 100 tablets, had been ground and boiled in 0.9 per cent. sodium chlorid. The extract on injection was found to have a small but unmistakable secretin reaction, equivalent to about 2 c.c. of the control secretin used. But repeated experiments were unable to duplicate this result. The “Secretogen” and “Elixir Secretogen” were all supposedly fresh preparations, the retail drug store informing us that a fresh supply was obtained from the wholesale house each week.
Secretogen, then, contains practically no secretin, and even if it did contain secretin, it can have no effect on the pancreas when taken by mouth. The indications for Secretogen, therefore, are based on false premises, and the testimonials are worthless.

Duodenin.—This is a preparation manufactured by Armour & Company, which purports to be “secretin plus enterokinase.” The claims for this product are similar to those for Secretogen, but somewhat less sweeping. According to the manufacturers, “Duodenin (Armour) is recommended in the treatment of intestinal disorders where an increased flow of pancreatic, hepatic and intestinal secretion is desired. It is of specific value in proteid digestion on the theory that secretin and enterokinase stimulate the pancreas and activate its secretion.”
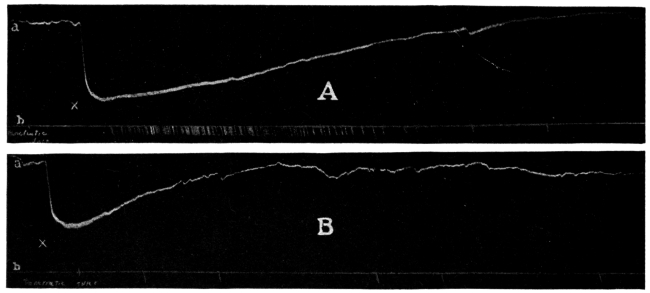
We bought a quantity of Duodenin in the open market, and carried out on this product the same series of experiments as that used in the case of Secretogen. The results were similarly negative (Table 8).
TABLE 8.—SUMMARY OF TYPICAL EXPERIMENTS SHOWING THE ABSENCE OF
SECRETIN IN “DUODENIN”
Dogs under ether anesthesia
| Exp. No. | Number Duodenin Tablets Used | Secretion of Pancreatic Juice in Drops, Following Intravenous Injection | |||||
| Control 10 C.c. Secretin | Duodenin in | Control 10 C.c. Secretin | |||||
| Distilled Water | 0.4% HCl | 70% Alcohol | 0.9% NaCl | ||||
| 1 | 3 | 29 | 0 | 0 | 0 | 1(?) | 28 |
| 1 | 6 | ... | .. | 1(?) | .. | .. | .. |
| 2 | 18 | 16 | .. | 6 | .. | .. | 16 |
| 3 | 5 | 14 | .. | 0 | 0 | 0 | 8 |
| 3 | 25 | ... | .. | 1(?) | .. | .. | .. |
| 4 | 100 | 110 | .. | 0 | .. | .. | 67 |
| 5 | 150 | 19 | .. | 0 | .. | 0 | 8 |
In regard to both Secretogen and Duodenin, we assume that the manufacturers have tried to put secretin in them, but have been unable because they have failed, in all likelihood, to check their methods by physiologic standardization. These firms do not give any details as to the procedure they employed in their manufacture of secretin. Desiccated secretin of extreme potency has been prepared by various physiologists,91 1 mg. (1⁄64 grain) of which is active when given intravenously. It is difficult to conceive that any of these methods were used in the preparation of Secretogen or Duodenin.
1. Secretin is quickly destroyed by gastric juice and by trypsin.
2. Secretin is not absorbed in active form from the alimentary tract.
3. The presence of secretin or prosecretin cannot be demonstrated in the commercial preparations “Secretogen,” “Elixir Secretogen” and “Duodenin” even when the therapeutic dose of the preparations is given intravenously. In the case of “Secretogen,” intravenous injection of 100 times the therapeutic dose reveals occasionally an insignificant trace of secretin.
It is, of course, objectionable that preparations containing no secretin should be advertised to the medical profession as containing this substance. The more important blunder, however, consists in the attempt to offer such preparations for oral administration, because even chemically pure secretin would be equally ineffective when taken by mouth. There is as yet no reliable evidence that lack of secretin is a primary or important factor in any disease. Even should this be established, secretin therapy, to be effective, must be intravenous. Secretin has not yet been prepared in sufficiently pure state to render possible intravenous injection in man without injurious effects. And even when this has been attained, the very fleeting action of secretin will in all probability render secretin therapy as futile in all the diseases in which it is theoretically indicated as epinephrin therapy is in Addison’s disease.
But there remains the alleged favorable effect from secretin therapy by mouth in various diseases in man. It is, perhaps, impertinent for laboratory men to comment on these clinical results. The ordinary “testimonials” need not be considered, but we should like to ask the serious worker who thinks he has actually obtained good results from secretin therapy how certain he is of the causal relation between the giving of secretin or alleged secretin and the abatement of the disease.
When a therapeutic measure not only lacks a positive basis in physiology and pathology but runs contrary to all the well-established experimental facts in these fundamental medical sciences, is it too much to ask that positive clinical findings be subjected to more than usual critical analysis before acceptance? “Clinical tests,” it is said, “covering a period of several years have proved that neither the condition in the stomach during digestion nor those in the intestine prevent the secretin from entering intact into the circulation.” When we meet claims such as this, should we not scrutinize the “tests” as well as the men who make them?
We are indebted to Dr. J. H. Moorehead for assistance in part of the surgical work.—(From The Journal A. M. A., Jan. 15, 1916.)
Below appear abstracts of the Council’s action on articles refused recognition which were not deemed of sufficient importance to require lengthy reports:
The Radium Therapy Company, Schieffelin & Co., selling agents, submitted to the Council radium emanation generators called “Radio-Rem Outfits,” designed to generate respectively 200, 1,000, 2,000, 5,000 and 10,000 Mache units per twenty-four hours.
Those who are well informed on the subject of radium therapy are of the opinion that the administration of small amounts of radium emanation such as generated by certain outfits is without therapeutic value. It has been stated that at the Radium Institute of London the minimum preliminary dose is 185 microcuries (500,000 Mache units), and as many as 555 microcuries (1,500,000 Mache units) are employed.
In consideration of these facts the Council voted not to accept any radium emanation generator which produces less than 2 microcuries of emanation during twenty-four hours. Accordingly, while accepting Radio-Rem Outfit No. 5, claimed to produce 10,000 Mache units (3.7 microcuries) and Radio-Rem Outfit No. 4, claimed to produce 5,000 Mache units (1.8 microcuries), the Council voted not to accept Radio-Rem Outfit No. 3, claimed to produce 2,000 Mache units (0.74 microcurie), Radio-Rem Outfit No. 2, claimed to produce 1,000 Mache units (0.37 microcurie), and Radio-Rem Outfit C, claimed to produce 200 Mache units (0.07 microcurie).
This report having been submitted to Schieffelin & Co. and their reply considered, the Council authorized publication of the report. [See also Reports of Council on Pharmacy and Chemistry, 1916, p. 631.]
Olio-Phlogosis, a liquid preparation to be applied externally by means of a cotton pad, is advertised by the Mystic Chemical Company, Kansas City, Mo., thus:
“Doctor: Don’t fail to use Olio-Phlogosis liberally for Pneumonia, Bronchitis and Pleurisy. It works quickly. Olio-Phlogosis is as far ahead of all medicated kaolin plasters as these plasters were ahead of the old-time moist and soggy poultices.”
A pamphlet advises the use of Olio-Phlogosis in
“... all cases of Inflammation and Congestion, such as Pneumonia, Bronchitis, Pleurisy, Croup, Boils, Carbuncles, Rheumatism, Swollen Glands, Peritonitis, Ovaritis, as a Surgical Dressing, Mamitis [Mastitis (?)] Vaginitis and Metritis (on cotton tampon to deplete these parts), Septic Wounds, Old Ulcers, Chilblain, Eczema, Neuralgia, Inflammation of the Eyes and Ears, Alveolar Inflammation, Burns, Scalds, Etc.”
According to the information sent to the Council by the Mystic Chemical Company, Olio-Phlogosis has the following composition per gallon:
Ol. Eucalyptus Gaultheria | drs. | 8 | |
Ol. Abies Canadensis | drs. | 8 | |
Ol. Abies Canadensis | drs. | 2 | |
Ol. Thyme (white) | drs. | 2 | |
Resublimated Iodin crystals | grs. | 32 | |
Resorcin | drs. | 1 | |
Acid Boracic C. P. | drs. | 2 | |
Quinine Bisulphate | drs. | 4 | |
Sodium Thiosulphate | drs. | 31⁄2 | |
Glycerin C. P. | q. s. ad | gal. 1 | |
A nonquantitative formula which appears on the label of a sample bottle sent to a physician enumerates the same ingredients except the sodium thiosulphate.
The A. M. A. Chemical Laboratory reports that no free iodin could be detected in the preparation.
Apparently, then, Olio-Phlogosis is essentially a skin irritant applied by means of cotton; it can be expected to be just about as effective as the old-fashioned cotton pneumonia jacket, used in conjunction with an aromatic skin irritant, such as camphorated oil or wintergreen or menthol ointment. The odor may have some psychic effect, and it is possible that some of the oily matter may be absorbed by the skin. That such small amounts, even if absorbed, can produce any considerable systemic effect, however, is highly improbable, and the advice that this preparation be relied on in pneumonia, pleurisy, peritonitis, etc., is pernicious. In the few cases of pneumonia in which heat is indicated, the plain cotton pad will usually be found sufficient. If the physician consider the addition of a skin irritant desirable, it is easy to select one from the official preparations. It will be far more rational to do so than to invoke the aid of a mystic name and a complex formula to which the patient and his family, at least, will be led to give unmerited credit.
The claims made for Olio-Phlogosis are unwarranted; its composition is complex and irrational, and the nondescriptive but therapeutically suggestive name is likely to lead to uncritical use. The Council voted that the product be refused recognition for conflict with Rules 6, 8 and 10, and that this report be published.—(From The Journal A. M. A., Aug. 19, 1916.)
The Council has adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
The introduction of hypophosphites into medicine was due to an erroneous and now discarded theory as to the cause of tuberculosis of which one Dr. J. F. Churchill of London, and later of Paris, was the promulgator and propagandist.92 This theory was that the so-called “tuberculosis diathesis” was due to a deficiency of phosphorus in the blood. Believing that the hypophosphites, while nontoxic, were capable of further oxidation in the organism, Churchill recommended them as the best means of supplying the supposedly lacking phosphorus. It is now known that tuberculosis is not due to a deficiency of phosphorus. Of more importance is the fact, now known, that little phosphorus, if any, is assimilated from the hypophosphites—far less than from phosphorus compounds of ordinary food.93 There is no justification for giving hypophosphites for the sake of their phosphorus content. For various reasons, however—partly from force of habit and partly because of the power of advertising—many physicians still prescribe hypophosphite preparations, and consequently, they are still included in the Pharmacopeia and in textbooks on materia medica and therapeutics. They are put out in the form of “specialties” and of proprietary preparations, and are lauded extravagantly by the manufacturers of the latter.
Although the overwhelming weight of evidence was against the probability that the hypophosphite preparations are of value as therapeutic agents, the Council thought it well to investigate the subject. Dr. W. McKim Marriott of Baltimore was therefore requested to review the evidence for and against the therapeutic usefulness of the hypophosphites and to conduct such experiments as seemed necessary. His report has already appeared in The Journal.94
Dr. Marriott found that nine observers (Paquelin and Joly, Vermeulen, Boddaert, Massol and Gamel, Panzer, Delaini and Berg), who endeavored to test the alleged utilization of the hypophosphites in the organism, reported that there is complete, or practically complete, elimination of hypophosphites in the urine, with little or no effect on the body. Only one experimenter (Patta) claimed that a considerable amount of ingested hypophosphite was retained in the body; however, he used a method now known to be inaccurate and made obvious errors in calculation, so that his conclusions were unwarranted.
Since the evidence was even to this extent contradictory, Marriott performed a series of experiments. The methods of this study and details of results are described in his paper, in which he also discusses the experiments of some other observers. Marriott writes:
“None of the subjects of the experiment [Marriott’s] experienced any effect whatsoever from the administration of the drug.... Almost all of the ingested hypophosphite is promptly eliminated unchanged....
“These experiments [Forbes’] demonstrate conclusively that the hypophosphites possess no specific value as a source of phosphorus for the body. This is not to be wondered at in view of the fact that 85 per cent. of the phosphorus ingested in the form of hypophosphite is excreted unchanged, and there is no proof that even the remaining 15 per cent. is available to the organism. It is doubtful if there are any conditions in which the body suffers from lack of phosphorus. Even should such conditions exist, phosphorus, in the form that it occurs in the ordinary foods, or as phosphates, is more efficient in supplying the deficit than hypophosphites that must be oxidized before utilization and which are only about 15 per cent. oxidized, if at all. For example, half a glass of milk contains more available phosphorus than three large doses of hypophosphites of 15 grains each, as great a dosage as is usually given.
“What, then, is the therapeutic value of hypophosphites? There is no reliable evidence that they exert a physiologic effect; it has not been demonstrated that they influence any pathologic process; they are not ‘foods.’ If they are of any use, that use has never been discovered.”
In view of the foregoing, it seemed to the Council advisable to examine the claims under which a few of the proprietary hypophosphite preparations are marketed. The following are representative:
No very exact information concerning the composition is furnished by the manufacturers (Fellows Medical Mfg. Co., New York). They say that the product
“... contains the chemically pure hypophosphites of iron, quinin, strychnin, calcium, manganese and potassium, agreeably blended in the form of a bland, stable syrup with a slightly alkaline reaction....
“Each fluid drachm contains the equivalent of 1-64th of a grain of pure strychnin.”
The Fellows’ Hypophosphites advertising furnishes something like a barometer of the popular status of hypophosphites. In one circular (undated, but, from certain references contained in it, presumably issued ten or fifteen years ago) we read:
“It is an indubitable fact that the hypophosphites have earned the distinction of having their therapeutic value more completely established than have any other remedial agents.... it is only by accepting the current view, which was originally advanced by Mr. Fellows, that we can satisfactorily account for the incontestable fact that the hypophosphites are of supreme importance in the treatment of a very extensive variety of affections.... the hypophosphites increase the consumption of oxygen and the elimination of carbon dioxide. In this manner, they stimulate nutrition and promote constructive metamorphosis.... It is now universally conceded that the widespread utility of the hypophosphites is due to the fact that they substantially improve metabolic processes, thus increasing the disease-resisting capacity of all the tissues.”
The circular, continuing, emphasizes the “incomparable phosphorus-contributing properties” of Fellows’ Syrup, its “extraordinary reconstructive properties” and “the magnificent results which invariably attend its employment in the treatment of anemia, chronic bronchitis, chlorosis, neurasthenia, mollities ossium, delayed union of fractures, rickets, convalescence,” etc.
A circular bearing the copyright date 1914, on the other hand, admits that:
“The theories for the favorable action of Fellows Syrup of Hypophosphites have undergone several changes.”
The same circular further maintains, however, that:
“... the fact has never been challenged that in Fellows Syrup of Hypophosphites we have one of the most efficient, most complete, most all-around tonics and roborants in the materia medica.”
No attempt is made to base this assertion on the therapeutic action of the constituents. In other words, the old theory, which formed the basis for the popularity of Fellows’ Syrup, has been thrown overboard, but no substitute is deemed necessary; the momentum already acquired is apparently regarded as sufficient to insure its continued sale.
Fellows’ Syrup of Hypophosphites is a semisecret, unscientific preparation—an affront to sound therapy—exploited by means of extravagant and misleading statements.
Little information concerning this preparation seems to be furnished at present by the manufacturers, Arthur Peter & Co., Louisville, Ky. According to an old circular, it contains, in each fluidounce,
| Grains | |
“Hypophos. Potass. | 11⁄2 |
Hypophos. Manganese | 1 |
Hypophos. Lime | 1 |
Hypophos. Iron | 11⁄2 |
Hypophos. Quinin | 6⁄16 |
Hypophos. Strichnin | 1⁄16 |
| “1⁄128 grain Strychnia to Teaspoonful.” | |
Further, according to the same circular:
“The Hypophosphites are especially useful in all diseases where there is a lack of nutrition.... They are the best of all remedies in Rachitis, non-union of fractures, Osteomalacia and Syphilitic Periostitis.”
As for Syrupus Roborans itself:
“This elegant preparation is ... the best general tonic and reconstructive known.”
The unwarranted therapeutic claims formerly made for it seem to be no longer circulated. Syrupus Roborans is an unscientific, shotgun mixture.
The Schlotterbeck & Foss Co., Portland, Maine, the manufacturers, say of their preparation:
“This solution contains 30 grains of the combined Hypophosphites of Lime and Soda to the ounce. It contains No Sugar, No Acid and it is Perfectly Neutral.”
“Indications for use.—Galactostasis, Imperfect Metabolism, Neurasthenia, Nervous Dyspepsia, Insomnia, Convalescence, Acetonuria, Cyclic Vomiting in Infants, Diabetes, Starvation, Deficiency of Lime, Mother’s Teeth during Pregnancy, Dentition of Infants, Rachitis, Furunculosis, Vomiting of Pregnancy, Obesity.”
“Migraine is often caused by conditions for which this Solution is one of the most satisfactory remedies:”
“In Insomnia due to advancing age, it will often act as a hypnotic....”
Of the hypophosphites the Schlotterbeck & Foss Company say:
“If ‘damning it with faint praise’ on the part of some of the leading medical authorities, or utterly condemning it as useless, on the part of others, would kill a medicine, the Hypophosphites would long since have disappeared as medicinal agents. Negative testimony in regard to the value of a drug does not settle anything.”
Of their own preparation they say:
“When we get the results that ought to follow the administration of Hypophosphites, we have proved that Schlotterbeck’s Solution enters the system unchanged.”
“This Solution is primarily a blood and nerve tonic and chemical food.”
Schlotterbeck’s Solution of Hypophosphites of Lime and Soda is a semisecret preparation marketed under claims that are both unwarranted and misleading.
According to the manufacturers, the Robinson-Pettet Company, Louisville, Ky., each fluidounce of this preparation contains:
“Hypophosphites Soda | 2 | gr. |
Hypophosphites Lime | 11⁄2 | gr. |
Hypophosphites Iron | 11⁄2 | gr. |
Hypophosphites Quinin | 3⁄4 | gr. |
Hypophosphites Strychnine | 1⁄16 | gr.” |
It is claimed to be
“Nutritive, Tonic Alterative. A Standard Remedy in the treatment of Pulmonary Phthisis, Bronchitis, Scrofulous Taint, General Debility, etc. Stimulates Digestion, promotes Assimilation.”
The declared composition of the preparation is unscientific, and the therapeutic claims are unwarranted.
Nelson, Baker & Co., Detroit, Mich., who market Eupeptic Hypophosphites, call this preparation:
“A superior combination containing the Hypophosphites of Potassium, Calcium, Iron and Manganese, and the bitter tonics, Quinin and Strychnin, agreeably associated with natural digestive ferments of the pancreatic secretion. It is thus a general reconstructive tonic.... The remedy is of especial value in the treatment of mental and nervous affections.... It is indicated in pulmonary tuberculosis, in all wasting diseases, in debilitated conditions generally and in all exhaustion from over work.”
On the basis of the manufacturer’s statement, Eupeptic Hypophosphites must be regarded as a semisecret, unscientific, shotgun preparation, exploited through unwarranted therapeutic claims.
So far as the recent literature and trade package are concerned, no information as to the composition of this product is furnished beyond what is conveyed in the name. The advertising for McArthur’s Syrup, like that for Fellows’ Syrup and Peters’ Syrupus Roborans, has been modified as time has passed. A few years ago it was advertised under such claims as the following:
“... Has Stood the Test during many years for unequaled efficacy in the treatment of Tuberculosis.... Indicated also as a Tonic and Tissue Builder in convalescence from Fevers, in Nervous Diseases, Rickets, Senile Debility and Bronchitis.”
“Its use is indicated in ... diseases of the chest, chronic cough, throat affections, general debility, brain exhaustion, cholera infantum and wasting diseases of children.”
At present no definite claims seem to be made for it; the manufacturers evidently find the magic name of hypophosphites sufficient to evoke the spell for which the advertisement writer’s aid was once sought. A testimonial contained in a circular which seems to be still used illustrates both the kind of aura which surrounds hypophosphites in the minds of physicians who are still living in the past, and the kind of logic which has made the reputation of this and many other equally worthless preparations.
“Just about six years ago I had a severe attack of La Grippe which almost killed me. Left me with Asthma (Catarrh) and a severe cough. Did not get out of the house for three months. Took over a dozen bottles McArthur’s Hypophos.—came out all right and since then worked hard, but last Fall took another cold, but worked on, used McArthur’s Hypophos., am using it now, am on my 12th bottle.
“I have five or six patients whom I have put on McArthur’s Hypophos., but I do not prescribe the single bottle, but wholesale no less than half dozen bottles. One patient is on his 24th bottle with orders to get another half dozen and keep it up all winter. I have given the same order to all (keep it up all winter) and I myself intend to do the same, for with its use I have lost no time—rain or shine I am doing my work. I know what it has done for me and what it is doing for my patients.”
It would be hard to find a more characteristic example of the naïve mental processes of the simple folk who in all good faith write testimonials for worthless medicines. This well-meaning practitioner (a homeopath, by the way), because he “came out all right” after an attack of grip, returns all praise to McArthur’s Hypophosphites, which he has taken “wholesale.” Not the faintest doubt of the validity of his post hoc ergo propter hoc argument seems to glimmer across his consciousness.
McArthur’s Syrup of the Hypophosphites is an irrational preparation. While its faults are fewer and less glaring than those of some other proprietaries, the circulation of such a testimonial as the one just quoted is sufficient of itself to cast suspicion on the product.
These preparations are now described in the appendix to New and Nonofficial Remedies. Borcherdt’s Malt Olive with Hypophosphites (Borcherdt Malt Extract Company, Chicago) is said to contain in each 100 c.c., 0.64 gm. each of calcium and sodium hypophosphites, with malt extract, olive oil and glycerine. Maltzyme with Hypophosphites (Malt-Diastase Company, New York) is said to contain, in each 100 c.c., 0.4 gm. each of calcium, sodium and potassium hypophosphites and 0.005 gm. each of iron and manganese hypophosphites, with maltzyme. Maltine with Hypophosphites (Maltine Company, Brooklyn, N. Y.) is said to contain in each 100 c.c., 0.64 gm. each of calcium and sodium hypophosphites and 0.42 gm. of iron hypophosphite, with maltine. Maltine with Olive Oil and Hypophosphites (Maltine Company, Brooklyn, N. Y.) is said to contain, in each 100 c.c., 0.6 gm. each of calcium and sodium hypophosphites, with maltine and olive oil. In general, no therapeutic claims are made for these mixtures so far as the hypophosphites are concerned. The addition of hypophosphites to such mixtures is irrational and, since it tends to perpetuate the hypophosphite fallacy, detrimental to sound therapeutics.
The Council endorsed the conclusions of the work of Dr. Marriott referred to above, and noted: (1) that the therapeutic use of hypophosphites (except possibly in some cases as a convenient means of administering the positive element in the salt, as ammonium in ammonium hypophosphite or calcium in calcium hypophosphite) is irrational; (2) that the merits of each hypophosphite salt submitted for consideration under the foregoing exception must be judged individually, and (3) that Fellows’ Syrup of Hypophosphites, Peters’ Syrupus Roborans, Schlotterbeck’s Solution Hypophosphites of Lime and Soda, Robinson’s Hypophosphites, the Eupeptic Hypophosphites of Nelson, Baker & Co., and McArthur’s Syrup of the Hypophosphites are ineligible for inclusion in New and Nonofficial Remedies, and that Borcherdt’s Malt Olive with Hypophosphites, Maltzyme with Hypophosphites, Maltine with Hypophosphites, and Maltine with Olive Oil and Hypophosphites be deleted from the appendix of N. N. R. Of these preparations, all are in conflict with Rule 10; Fellows’ Syrup, Schlotterbeck’s Solution, Robinson’s Hypophosphites and Nelson, Baker & Co.’s Eupeptic Hypophosphites are in conflict with Rule 6; the Fellows, Schlotterbeck, and Nelson, Baker preparations are also in conflict with Rule 1.—(From The Journal A. M. A., Sept. 2, 1916.)
Pulvoids Calcylates 5 grains was submitted by the Drug Products Company, Inc., New York, under the following claims as to composition:
| OH COO |
“Strontium Di-Salicylate 21⁄2 grs. and our especially prepared Salt of Calcium and Acid Salicylic adjusted in such nascent form, that these pulvoids upon ingestion will promptly form Calcium Neutral Di-Salicylate 21⁄2 gr.”
“A combination of Calcium and Strontium Di-Salicylate, in seemingly true chemical union.”
These statements are rather vague, possibly because they are an attempt to mystify. The product, however, may be assumed to be a mixture (not a chemical combination) of calcium salicylate and strontium salicylate. The therapeutic claims made for the preparation are:
“Superior to ordinary salicylates. Can be taken continuously and indefinitely without gastric irritation, insuring maximum efficiency.”
“Reports show surprisingly good results, even where the sodium salt fails.”
As there is no evidence to show that strontium salicylate, calcium salicylate or a mixture of the two salts has any advantage over sodium salicylate, these claims cannot be accepted. The name and the statement of composition are objectionable in that they do not reveal the identity of the drugs in “Calcylates” and in suggesting that this preparation possesses radical advantages over salicylates in other forms.
The Drug Products Company was told that the facts just mentioned rendered “Pulvoids Calcylates” ineligible for New and Nonofficial Remedies. The company in its reply objected to the Council’s conclusions, and in support of its position submitted testimonials from a number of physicians. The reply of the company embodied no facts or arguments which had not been considered by the Council’s referee, and the testimonials from physicians contained no evidence to show that the combination has any real advantage over sodium salicylate.
The Council therefore declared “Pulvoids Calcylates” ineligible for New and Nonofficial Remedies for the following reasons: Unwarranted therapeutic claims are made for the mixture (Rule 6); the name does not describe the composition (Rule 8), and the mixture is an unessential modification of an established remedy (sodium salicylate) (Rule 10).—(From The Journal A. M. A., Sept. 9, 1916.)
Sulfuryl Monal is said to be manufactured by Monal Frères, manufacturing chemists of Nancy, France. It is sold in the United States by George J. Wallau, Inc., New York City. According to the label:
| “Each Pastille | Contains: Sulfuryl (combined polysulphurets) | |
| = 0.35 centigr.” | ||
| Liberates: Nascent sulphurretted Hydrogen | ||
| = 2 cub. cent.” |
The Chemical Laboratory of the American Medical Association was requested to check the amount of available hydrogen sulphid. An original bottle of Sulfuryl Monal was used; this contained tablets having the taste of licorice extract and an odor of hydrogen sulphid. The tablets were found to liberate about 6 c.c. hydrogen sulphid to each tablet.
Among the claims made for the preparation are:
“Dissolved by the saliva, Sulfuryl Monal reaches the stomach where, under the influence of the gastric juice, it generates nascent sulphuretted hydrogen. Professor Albert Robin’s remarkable researches have proven that it is in the nascent state that drugs produce the greatest effect with the smallest dose.... Being thus eliminated by the entire respiratory tract: the lungs, bronchi and the throat, the sulphurretted hydrogen passes from the interior to the exterior, that is to say, goes right through these organs which are, as a consequence, thoroughly cleansed, antisepticized and freed of the pathogenic micro-organisms.... Then, again, part of the sulphuretted hydrogen, liberated in the stomach, is eliminated by the mouth and acts as an antiseptic and disinfectant of the mucous membranes of the throat and mouth. Hence Sulfuryl Monal is a perfect protective agent against contagious diseases.... Numerous clinical tests have demonstrated its real efficacy in diseases of the throat and of the respiratory tract: laryngitis, pharyngitis, hoarseness, granulations, tonsillitis, colds, bronchitis, pulmonary catarrh, asthma, emphysema, grippe, whooping cough, simple and infectious pneumonia, and in the first stage of pulmonary tuberculosis.”
The sulphids are practically ignored in modern textbooks. There is a rather extensive clinical literature on the subject, particularly in connection with sulphur waters; this, however, offers no good evidence for the therapeutic value of sulphids. Probably the tradition in their favor is largely due to the old popular idea that a disagreeable taste or odor is a mark of a good remedy.95
When hydrogen sulphid is introduced into the body, the small amounts that appear in the expired air are insufficient for quantitative demonstration and it is highly improbable that the amount thus excreted has any germicidal action, or that enough is excreted in the lungs to cause irritation and a reaction. The claim that Sulfuryl Monal is “a perfect protective agent against contagious diseases” is unwarranted; the recommendation for its use in “simple and infectious pneumonia, and in the first stage of pulmonary tuberculosis” is dangerous and vicious. The Council declared Sulfuryl Monal ineligible for New and Nonofficial Remedies and authorized publication of this report.
[Editorial Note..—With one exception, this product does not appear to be advertised in medical journals. We find, however, in the gallery of nostrums that grace the advertising pages of the International Journal of Surgery, that Sulfuryl Monal has its place. According to an advertisement that has been running some months in this publication, “affections of the throat and respiratory organs respond promptly” to Sulfuryl Monal whose “effects are rapid and certain” even in “incipient tuberculosis.” This preposterous pronouncement is no worse than many others appearing in the same journal, but it is bad enough to indicate how uncritical must be the physicians who support—by subscription or contribution—publications that are still debasing scientific medicine.]—(From The Journal A. M A., Sept. 16, 1916.)
The “Mark White Goiter Serum Laboratories” of Chicago asked the Council to consider its products “Mark White Goiter Serum” and “Mark White Iodinized Oil.” The “serum” was claimed to be an “antibody blood serum from a goat with thyroidosis” while the “Iodinized Oil” was said to contain “about 4 grains of iodin” to “each c.c.” The therapeutic indications for the treatment were given as:
“Simple or Exophthalmic Goiter, Hyperthyroidism-dosis, Thyrosis, Thyroidosis, Thyrotoxicosis, Dementia.”
An ampule (2 c.c.) of the “serum” is to be injected into the thyroid to be followed one week later by an ampule (2 c.c.) of the “Iodinized Oil.” Repetition of this “treatment” once or twice a month is advised.
The Council asked for more specific information as to the composition of the remedies, particularly as to the preparation and nature of the serum; it also asked for evidence of the therapeutic value of the preparations. In reply, Mark White wrote:
“All that I can say regarding the serum is that it is made from the blood of goats with thyroid affection, and it has been found that the serum from these goats has antibodies which control, or has curative effect upon thyroid affections when injected into thyroid glands of either humans or animals. As to the iodinised oil, it is only an adjunct or side treatment which is not always used or indicated, and will only be furnished to the physician for use in case in his judgment his patient needs it. We shall also advise the use of quinin ... when indicated....”
The Council was referred for further information to a paper by Rachel Watkins, M.D., published in the Illinois Medical Journal. It is to be noted, incidentally, that the letterheads used by White in his correspondence bore in one corner the notation “Rachel Watkins, M.D., Practice Limited to Goiter and Other Disorders of the Thyroid Glands,” and in the other, “Mark White, Goiter Research.”
The information regarding the composition of this goiter treatment, as furnished in Dr. Watkins’ paper, was to this effect:
“The medical treatment consists of the administration of a blood serum derived from a thyrodized goat. Formula: Iodine 0.16 grams [according to a correction by Mark White, this should read 0.26 gm.], oil 0.25 c.c., serum q. s. 1 c.c.”
This description of the treatment differs from that furnished to the Council by Mark White in that here the iodin and oil appear to be combined with the serum. Dr. Watkins’ “formula” implies that the iodin is a routine medication, thus contradicting White’s statement, which, in turn, is at variance with the statements made in submitting the treatment.

The Council does not accept any biologic product until its sale in interstate commerce has been authorized by the secretary of the treasury in accordance with the federal law regulating the sale of viruses, serums, toxins and analogous products. The sale of the Mark White Goiter Serum has not been so authorized; consequently even if the preparation complied with other rules of the Council it could not be accepted.
In addition, however, this treatment conflicts with other Council rules. The statements regarding its composition are indefinite and contradictory (Rule 1); the evidence presented to support the therapeutic claims is insufficient in itself and does not appear to have been checked by any disinterested authority (Rule 6). Moreover, the recognized variation in the morphology and pathology of the types of goiter render it impracticable to treat cases of goiter by any routine procedure.
The foregoing report was submitted to the Mark White Goiter Serum Laboratory. In reply, a letter signed “Mark White, V.M.D.,” was received, which read, in part:
“... we hope at some future time to be able to give you more detailed information, but as you possibly appreciate that we have experienced for some time a demand on the part of many physicians that we furnish to them our therapy, which necessitates us furnishing it before all the detailed work has yet been accomplished, and I trust that you will be so kind as to bear patiently with us until we are better in a position to make a complete scientific application and report to you.”
White wrote further:
“The serum and iodized oil may be mixed for immediate use, but could not be put up only separate for the use of the profession and the therapy furnished Dr. Watkins she mixed as used.”
This statement throws no light on the discrepancies in the statements with regard to the place of the iodinized oil in the treatment, namely: (a) the original statement that the oil was to be given a week after the serum; (b) White’s statement (quoted earlier in this report) that the oil “is only an adjunct or side treatment” and “is not always used or indicated”; (c) the statement in Dr. Watkins’ paper that the oil and the serum are given in combination.
The Council declared the Mark White Goiter Serum and Mark White Iodinized Oil ineligible for New and Nonofficial Remedies and authorized publication of this report.
As some of our readers will remember, on April 26, 1913, The Journal called attention to the Mark White preparation which at that time was being exploited from Denver. The Propaganda Department has in its files a number of letters sent out from the Mark White concern at various times. One mailed in May, 1911, on the embossed stationery of “The Mark White Goiter Institute,” Exchange Building, Denver, was evidently a general letter sent to physicians, calling their attention to “the most important medical discovery of the age.” “Dr. Mark White, a graduate of the University of Pennsylvania,” said the letter, had discovered “a simple and harmless remedy” that would cure goiter. “Because of the desire to preserve the secrecy of this remedy it is given only at the office here.” It was then suggested that the doctor might send those of his patients who were suffering from thyroidism to the “Mark White Goitre Institute.” If he would do so he would be “given a commission of $10, in cases of the $50 fee with the additional $5 for each $50 increase.” It closed with some casuistic arguments, presumably for the purpose of overcoming the physician’s scruples, summing up the matter with the statement:
“No right thinking man will allow a narrow and self-seeking system of ethics to stand between him and his duty to the sick and suffering.”
About 1912 the name of the concern seems to have been changed, for we have in our files a letter addressed to a layman on the stationery of the “Mark White Goitre Treatment Company.” According to this letterhead the product this concern had for sale was “Goitreine” discovered by Mark White, “President and General Manager.” Mr. White’s letter to the sufferer from goiter assured him that if he would take “Goitreine” he might “be practically sure of an immediate and permanent cure.” “Goitreine,” according to White, “has absolutely and permanently cured 90 per cent.” of all cases of goiter in which it has been used—“and the other ten showed remarkable improvement.” It was efficacious for all forms of goiter and “cannot possibly harm.”
The person who received this assurance might have had his confidence in it shaken had he seen a copy of the Denver News for May 23, 1911, in which was reported a case of collapse and death in a woman following an injection given in White’s office. The paper stated that the death certificate was signed by one W. A. Gray and gave “fatty degeneration of the heart and goiter” as the cause of death. Gray, it seems, was the licensed physician employed by Mark White to administer “Goitreine”—if that is what White happened to be calling his product at that time. For here it may be stated, parenthetically, that Mark White is not a physician; he is a veterinarian.
In February, 1913, Mark White sent a circular letter to a number of medical publications with the request that it be printed in full in the next issue, “to cover one full page of space.” The letter White wanted printed was addressed to doctors offering to “enter into a copartnership agreement” with such physicians who would be willing to treat “patients with goiter affections on a 50 per cent. commission basis.”
“You would be expected to make a cash charge to the patient for the treatment, remitting on the same day our 50 per cent. to us, when ordering the treatment, giving the treatment in no cases for less than $50.00.”
About the same time that Mark White made this “fifty-fifty” offer, he sent in an advertisement to be published in the classified column of The Journal. At that time he was told his advertisement was not acceptable; we now reprint it, however, free of charge. Here it is:
“WANTED—ONE OR MORE PHYSICIANS
in each vicinity to administer and represent
our new medical treatment for GOITER. Good
margin of profit. Write for copy of contract.
The Mark White Goitre Treatment Co.,
Denver, Colo.”
In 1914, White moved to Chicago. At least the card which we reproduce so indicates. At that time, as will be seen, “Dr. Mark White” was “personally associated” with Peter S. Clark, M.D. According to the same card Dr. F. D. Paul of Rock Island, Ill., seems to have been his “associate” for that particular locality. In this connection, it is worth noting that a Rock Island paper, in one of its issues during July, 1913, devoted a good deal of space to “Dr. Mark White” who was at that time in Rock Island “directing Dr. Frank D. Paul in the administering of the treatment.” There was nothing to indicate that this notice was an advertisement or that the editorial appearing in the same issue puffing White’s “important cure,” was paid for.

Dr. W. A. Gray, who has already been mentioned as White’s associate in Denver, seems to have been doing business in Illinois some time in 1913 and a Princeton (Ill.) paper had some uncomplimentary things to say about him. Finally in July, 1913, this item appeared in a Princeton paper.
“Dr. W. A. Gray, the goiter specialist who operated last winter at Princeton and Walnut until he became embroiled with Dr. Mark White, a Denver veterinary and originator of the cure, over a division of the spoils, has opened a goiter institute in Chicago under his own name. Advertisements of the Dr. Gray Goiter Institute appeared Sunday morning in the Chicago Examiner and other morning papers. Dr. Gray and Mark White broke off their relations after their disagreement at Walnut, and Dr. Gray slightly changed the ingredients of the goiter cure and started off on his own hook.”
One of Gray’s advertisements in Chicago newspapers made the claim that “Dr. Gray’s New Medical Treatment removes the cause of goiter in seven days.”

The Tulsa (Okla.) associate of “Dr.” White seems to have been Dr. J. H. Morgan and the Tulsa papers of June, 1914, tell of “Dr.” White’s visit to that city “for the purpose of instructing Dr. J. H. Morgan in the technique of his new medical treatment for nervous disorders and goiter.” Some months later—in December, 1915—the following little item appeared in a Tulsa paper:
“Dr. Mark White was found guilty in the county court yesterday of practicing medicine without a license and was fined $50. Doctor White is a goiter specialist.”
In September, 1915, Mr. Thomas S. Hogan, the efficient counsel for the Illinois State Board of Health, instituted action against Mark White for practicing medicine without a license. The case was tried Oct. 15, 1915, and the jury, after being out four hours, returned a verdict of “not guilty.” Attorney Hogan attributes the failure to obtain a conviction to the testimony of Dr. Rachel Watkins, who said she had a partnership arrangement with White in carrying on the medical business. It was about this time that Mark White seems to have issued some new letterheads. These bore in their upper left hand corner the device “Rachel Watkins, M. D., Practice Limited to Goiter and Other Disorders of the Thyroid Glands,” while the upper right hand corner read “Mark White, Goiter Research.”
On Dec. 9, 1915, Rachel Watkins, M. D., of Chicago, read a paper entitled “A Serum Treatment for Physiologically Defective Thyroids, With Clinical Reports” before the Stock Yards Branch of the Chicago Medical Society. The “serum treatment” discussed was Mark White’s “Goitreine” which, in the course of its checkered career, had lost its original name by the wayside. This paper appeared in the December, 1915, issue of the Illinois Medical Journal.
Probably emboldened by the ease with which a component part of the American Medical Association “fell for” a paper exploiting a “goiter cure,” Dr. Watkins requested that she be permitted to read a paper on the same subject before the Section on Pharmacology and Therapeutics at the Detroit meeting of the American Medical Association last June. The request was refused. Dr. Watkins is apparently no longer connected with White and in fact has protested against the use of her name by White in connection with his “goiter cure.”
[After the above was in type and ready for the pages of The Journal, attention was called to the Official Bulletin of the Chicago Medical Society of Sept. 16, 1916. This Bulletin contained a full page advertisement of the Mark White “goiter cure.” The advertiser referred to the preparation as having been “announced to the Chicago Medical Society” and declared it to be “an ethical therapeutic agent.” Mark White was described as “a medical research student” but no hint was given that he is a veterinarian. After again emphasizing that “this therapy is ethically proven” physicians were invited to “visit our goats when convenient” and the advertisement closed with the modest claim that “this thyroid therapy has equal curative therapeutic value in these cases as quinin in malaria.” And this sort of pseudo-scientific claptrap is presented to a presumably learned profession through its own official Bulletin—but what’s the use of commenting!]—(From The Journal A. M. A., Sept. 23, 1916.)
Kora-Konia is a “dusting powder” which at present is advertised to the medical profession through medical journals, circulars, post cards and sample packages. It is put out by the “House of Mennen,” which sells various toilet preparations such as talcum powder, shaving soap, etc. On the trade package is the statement:
“Indicated in the treatment of Acne, Dermatitis, Eczema Intertrigo; in obstinate cases of chafing, prickly heat, nettle rash, chicken pox, measles, scarlatina and irritations of the skin; as a soothing absorbent and antiseptic dusting powder and as an umbilical dressing.”
While a circular asserts that:
“Kora-konia is indicated in the treatment of acne, dermatitis, eczema and eczematous conditions of the utmost severity,... eruptive fevers,...”
What purports to be a physician’s testimonial reads:
“I used Kora-Konia in a new born case of inherited syphilis and the eruption soon cleared up.”
Germicidal powers are claimed for Kora Konia in a medical journal advertisement. In view of the various claims made and the fact that it is advertised to the medical profession, the Chemical Laboratory of the American Medical Association was asked to analyze Kora-Konia. This was done and the chemists reported as follows:
Kora-Konia is a white powder, slightly greasy to the touch. Qualitative tests showed the presence of boric acid, zinc, magnesium, a solid fatty acid and material insoluble in hydrochloric acid containing magnesium and aluminum. Starch was not found. Quantitative determinations gave the following results:
Acid-insoluble material (talc) | 48.3 per cent. |
Magnesium (Mg++) soluble in dilute acid | 1.2 per cent. |
Zinc (Zn++) | 4.5 per cent. |
Stearic acid (impure) | 39.2 per cent. |
Boric acid | 3.0 per cent. |
Carbon dioxide (CO2) | 1.5 per cent. |
From this analysis it is concluded that Kora-Konia has essentially the following composition:
Zinc stearate U. S. P. | 44 per cent. |
Talc | 48 per cent. |
Magnesium carbonate U. S. P. | 5.0 per cent. |
Boric acid | 3.0 per cent. |
Essentially this dusting powder consists of the well-known substances talc and zinc stearate in about equal proportions to which small quantities of magnesium carbonate and boric acid have been added. Inasmuch as the claim is made, by inference at least, that Kora-Konia represents original investigation carried out “with the cooperation of the medical profession” it should be stated that the preparation of commercial zinc stearate was described and recommended as a dusting and toilet powder nearly twenty-five years ago.96
There is nothing new or original in any one of these substances or in the combination. The extravagant and unwarranted claims made for this simple dusting powder are undoubtedly leading the public as well as some thoughtless physicians, to place undeserved confidence in it. In view of the small amount of boric acid present in the powder, its antiseptic powers must be slight and its germicidal powers almost nil. The Council declared Kora-Konia ineligible for New and Nonofficial Remedies and authorized publication of this report.—(From The Journal A. M. A., Sept. 30, 1916.)
The Council has adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
Glycerophosphates are the salts of glycerophosphoric acid, H2[C3H5(OH)2]PO4. This acid is produced by the interaction of glycerin and phosphoric acid. In general, only sodium glycerophosphate, Na2[C3H5(OH)2]PO4 +51⁄2H2O, and calcium glycerophosphate, Ca[C3H5(OH)2]PO4 +H2O, are used in medicine, though the glycerophosphates of lithium, potassium, manganese, magnesium, iron, quinin and strychnin are claimed as constituents of proprietary preparations. At a time when certain disorders were assumed to be due to a deficiency of phosphorus in the nerve structure in the body, glycerophosphates were introduced as “nerve foods” and “tonics” on the theory that they would be assimilated more readily than hypophosphites or ordinary phosphates. What led to this assumption was the fact that the lecithins, which form a part of the nerve structure, were known to contain the glycerophosphate radical in the molecule. The belief that inorganic phosphates cannot supply the body’s need of phosphorus is implied or expressed in most of the “literature” devoted to proprietary phosphorus preparations.
Thus, Schering and Glatz quote G. Meillière as saying that “the organism is incapable of assimilating inorganic forms of phosphorus.”
Again, when exploiters of glycerophosphates admit that the body can synthesize its phosphorus compounds from inorganic phosphates, they attempt to counterbalance the admission by contending that the use of organic compounds “spares” the system the necessity of making such synthesis. This assumption rests on the theory that the organic phosphorus compounds are absorbed and stored as such.
This theory is contradicted by evidence which has been presented97 that the organic phosphorus compounds are split up into inorganic phosphates before absorption.
The Council requested E. K. Marshall, Jr., to review the evidence for and against the therapeutic value of organic phosphorus compounds. Marshall’s study98 brings out the following points:
1. In various tissues of the animal body, enzymes have been found which hydrolyze complex organic phosphorus compounds so as to liberate the phosphorus in the form of inorganic phosphates.
2. Metabolism studies of the phosphorus balance with diets containing inorganic phosphorus compounds, as compared with diets containing organically bound phosphorus, are somewhat conflicting in their results. The balance of evidence, however, is in favor of the view that there is no difference between organically combined phosphorus and inorganic salts with respect to the phosphorus balance.
3. Experiments indicate that the organism thrives on and supplies its phosphorus needs quite as well from inorganic phosphorus compounds as from organically bound phosphorus.
Marshall concludes:
“We see that the evidence is very convincing of the view that the animal organism can synthesize its complex organic phosphorus constituents from inorganic phosphates, and that organic phosphorus is of no more value as a food than inorganic.”
In view of this report, the Council deemed it advisable to take up the consideration of certain glycerophosphate preparations on the market. As the therapeutic claims are all similar, it is not necessary to quote them extensively.
Tonols (Schering and Glatz, New York) comprise iron, lime, lithium, magnesium, manganese, potassium, quinin, sodium and strychnin “Tonols” or glycerophosphates; also Duotonol Tablets, said to contain equal parts of calcium and sodium glycerophosphates; Triotonol Tablets, each said to contain “Sodium Tonol 21⁄2 grains, Lime-Tonol 21⁄2 grains, Strychnine-Tonol 1⁄60 grain”; Quartonol Tablets, said to contain “Sodium and Lime-Tonols, each 21⁄4 grains, Quinine Tonol 1⁄2 grain, Strychnine-Tonol 1⁄200 grain”; Sextonol Tablets, said to contain “Sodium and Lime-Tonols, each 2 grains, Iron-Tonol, 1⁄2 grain, Manganese and Quinine-Tonols, each 1⁄4 grain, Strychnine-Tonol, 1⁄200 grain.”
The name “Tonols” is objectionable in that it is not only nondescriptive of the composition, but also therapeutically (and falsely) suggestive. The composition of the more elaborate Tonols is particularly unscientific; there is no justification for combining quinin, strychnin, iron, manganese, etc., in one formula.
Phosphorcin Compound, called “The Elementary Phosphorus indicated in all forms of Nervous Diseases” and the “Perfect Formula,” is said to be manufactured by the Organic Products Company, Wetzlar an der Lahn, Germany, and Greenwich, Conn. It is sold in the United States by Eimer and Amend, New York, according to whom:
| “Each two fluidrachms contain: | ||
“Acidulated Bone Phosphor O. P. Co. | 2 | grains |
“Calcium Glycerinophosphate, Merck & Co. | 11⁄2 | grains |
“Magnesium Glycerinophosphate, Merck & Co. | 11⁄2 | grains |
“Sodium Glycerinophosphate, Merck & Co. | 21⁄2 | grains |
“Lactated Pepsin | 2 | grains |
“Ignatia Extract | 1⁄20 | grain |
“C. P. Glycerin (Special Process) O. P. Co. | 50 per cent. | |
“Acidulated bone phosphor” presumably is acid phosphate of calcium. This formula is an unscientific shotgun combination.
Robinol, manufactured by John Wyeth and Brother, Philadelphia, is called a “Universal Tonic.” It is said to be:
“A preparation of the glycerophosphates of lithium, calcium, sodium, iron, manganese, quinine, with 1-16 gr. strychnine glycerophosphate in each fluidounce.”
This is a semisecret preparation, since the quantities of most of the ingredients are not given and the vehicle is not named. This complex combination, too, is unwarranted.
This is said to be prepared by the Laboratories de Pharmacologie Générale, Dr. Ph. Chapelle, Paris and New York. It is sold in this country by E. Fougera and Co., Inc., New York. It is offered in several forms, especially in that of wine, which is called the “Medicinal Wine and Tonic Par Excellence.” The alcohol is no doubt the constituent to which this preparation is indebted for such popularity as it has attained, for it is much more freely advertised than the syrup, capsules or granulated form. The usual claims are made with regard to the efficacy of calcium glycerophosphate “during convalescence, in cases of enfeebled vitality, and nervous affections associated with an excessive elimination of phosphates.”
This is manufactured by Sharp and Dohme, Baltimore. The manufacturers’ statement of composition is:
“Each fluidounce represents Nux Vomica 8 grains, Damiana 64 grains, combined with Glycerophosphates of Calcium and Sodium.”
“Alcohol 20 per cent.”
Sharp and Dohme call this mixture a “Reconstructive Nerve Stimulant, Aphrodisiac,” and claim that:
“Phosphorus in elemental form has long been prescribed under the title of Elixir Phosphorus, Nux Vomica and Damiana, but due to the rapidity of chemical change occurring in preparations containing this form of Phosphorus, much of the Physiologic action is lost. The Glycerophosphates present Phosphorus in its most available form—the form in which it exists in the brain and nervous system. They powerfully stimulate the functions of nutrition and are rapidly assimilated by the system.
“Nux Vomica is a general Nerve Tonic. Damiana exerts a stimulant effect upon the sexual appetite and function.”
The claim that the glycerophosphates may be substituted for elementary phosphorus is, at least, novel.
The elixir is an unscientific semisecret combination.
All of the preparations mentioned violate Rule 6 (unwarranted therapeutic claims). In addition, Robinol and Elixir Glycerophosphates, Nux Vomica and Damiana violate Rule 1 (secrecy of composition) in that not all the quantities of the ingredients are declared; Tonols, Phosphorcin Compound and Robinol violate Rule 8 (objectionable names). It is recommended that the Council endorse Marshall’s findings98 and declare that Tonols (Schering and Glatz), Phosphorcin Compound (Eimer and Amend), Robinol (John Wyeth and Brother), Phosphoglycerate of Lime Chapoteaut (E. Fougera and Co.), and Elixir Glycerophosphates, Nux Vomica and Damiana (Sharp and Dohme) are ineligible for New and Nonofficial Remedies.—(From The Journal A. M. A., Sept. 30, 1916.)
Hydras, sold by John Wyeth and Brother, Philadelphia, is one of the many proprietary, so-called “uterine tonics.” It is said to contain “Cramp Bark, Helonias Root, Hydrastis, Scutellaria, Dogwood and Aromatics,” but as the amounts of the several ingredients are not given the statement regarding its composition is valueless. The label declares the presence of 24 per cent. alcohol.
The name “Hydras,” taken in connection with the statement of composition, would suggest that hydrastis (golden-seal) is an important constituent. The report of the Chemical Laboratory of the American Medical Association, however, indicates that hydrastis is present in unimportant amounts:
“The hydrastin content of Hydras was determined by extraction with immiscible solvents (Pharm. Review, May, 1908, p. 132). Twenty-five c.c. was found to yield an alkaloid residue of 0.0160 gm. The preparation contains, therefore, not more than 0.064 gm. ‘hydrastin’ per 100 c.c. Inasmuch as hydrastis is required to contain about 2.5 per cent. ‘hydrastin,’ hydras contains an equivalent of not more than 2.56 gm. hydrastis (golden seal) in 100 c.c. and the stated dose of Hydras—one dessertspoonful (8 c.c.)—represents not more than 0.2 gm. or 1⁄10 of the U. S. P. average dose of hydrastis.”
The label of a recently purchased bottle of Hydras bears the following recommendations for its use:
“Indicated in treatment of Dysmenorrhea, Menorrhagia Anti-Abortive, with anodyne and tonic properties.”
“For dysmenorrhea, suppressed menses, etc., a dessertspoonful three times daily, before or after meals.”
“To relieve pain due to uterine disorders, a dessertspoonful every three hours, or increased to a tablespoonful, at the discretion of the attending physician.”
A circular wrapped around the bottle declares that Hydras is:
“A valuable preparation to the physician in the treatment of dysmenorrhea, colic, cramps, spasm, palpitation incident to pregnancy, and the various pains resulting from diseases of the female sexual organs.”
It is further claimed that:
“In the dysmenorrhea of young girls due to some mechanical difficulty, as anteflexion or of a congestive character, of suppressed menses from exposure to cold and other causes of a similar character, Hydras will prove efficient and can be administered freely without danger.”
The value of hydrastis in the treatment of the diseases and conditions mentioned is problematical at best, and the small amount present in Hydras is wholly useless. As for the other constituents, cramp bark (Viburnum opulus), helonias (false unicorn—Chamælirium luteum or Helonias dioica) and scutellaria (skullcap—Scutellaria lateriflora) are drugs which are practically ignored by most writers on materia medica and therapeutics.99 Dogwood (Cornus florida) is a mildly astringent aromatic bitter for the use of which there is no scientific evidence.100
To sum up: Of the five ingredients of Hydras (aside from alcohol and aromatics), one (hydrastis), which apparently gives the preparation its name, is present in unimportant amounts; three (cramp bark, helonias and scutellaria) are therapeutically unimportant; the fifth (dogwood) has never been shown to have any specific action on the uterus. The potent constituent, therefore, appears to be the alcohol.
But, even if every one of the several drugs said to be contained in Hydras were possessed of distinct therapeutic properties, and if each were present in known and therapeutically active amounts, still the combination in fixed proportion would be irrational. No one could foresee the joint effect of the five drugs in the several conditions for which the mixture is advertised. Hydras is evidently meant to appeal to the thoughtless and to be used at random; witness the suggestion made in the advertising that
“Owing to its palatability, it is acceptable to patients with impaired digestion, and will serve as a stomachic tonic, promoting appetite and digestion.”
A useless alcoholic nostrum “administered freely” to women and girls is as dangerous as the recommendation for such administration is reprehensible.
This preparation is semisecret. The recommendations for its use in specified diseases which appear on the label and in the advertising accompanying the bottle are sure to lead to its ill-advised use by the public. The claims made for its curative properties are exaggerated and unwarranted. The name, in view of the small content of hydrastis, is misleading. Finally, the combination of five drugs, even if individually they were of therapeutic value, is irrational. Hydras, consequently, is inadmissible to New and Nonofficial Remedies for conflict with Rules 1, 4, 6, 8 and 10, and publication of this report is authorized.
[Editorial Comment.—Products like “Hydras” are the bane of scientific medicine. The physician who prescribes them could with just as much reason prescribe any of the various alcoholic “patent medicines” of the “women’s tonic” type. In fact, his patients would be running less risk of contracting the alcohol habit if he prescribed the “patent medicines,” as these nostrums usually have less alcohol than is contained in their “ethical” prototypes—and alcohol is the only really important drug in practically all of them. Whatever one may think of reputable pharmaceutical houses who put out products of the “Hydras” type, the fault really lies with the profession which tolerates such therapeutic monstrosities.]—(From The Journal A. M. A., Oct. 7, 1916.)
“Bromin-Iodin Compound,” according to the Bromin-Iodin Chemical Company, San Diego, Calif., has the following “formula”:
Iodin | Gr. | 1 |
Bromin | Gr. | 1⁄4 |
Phosphorus | Gr. | 1⁄100 |
Thymol | Gr. | 2⁄3 |
Menthol | Gr. | 2⁄3 |
Sterilized Oil | Gr. | 1 |
The only statement regarding its method of preparation is the line “Solution in Cod Liver Oil, Norwegian.” According to the promoters, “Bromin-Iodin” is:
“A Powerful Anti-Tubercular Agent for Hypodermic Use in Pulmonary and Laryngeal Tuberculosis. Useful in other forms of Tubercular Diseases, and in Non-Tubercular Pulmonary Diseases of a Sub-Acute or Chronic Nature.”
The “formula,” in the form in which the manufacturers publish it, is either impossible or meaningless, according to the interpretation that may be given. It is impossible if it is intended to indicate the actual composition of the product because that would mean that the oil is alleged to contain free or uncombined iodin, bromin and phosphorus. Both on theoretical grounds and also in the light of the findings of the Chemical Laboratory of the American Medical Association, it is not possible that all these constituents can be present in the free state. The formula is meaningless if it is intended to convey the idea, merely, that iodin, bromin, phosphorus, thymol, menthol and sterilized oil are combined to form “Bromin-Iodin.” In the absence of any details of the method of manufacture, it is futile to attempt to pass judgment on the actual composition of the preparation.
The use of an almost identical product (said, however, to contain only 1⁄2 grain iodin to each fluidram) was described in 1908 by Dr. Ingraham of Binghamton, N. Y., in “Five Years Successful Experience with a Special Mode of Treating Pulmonary Tuberculosis.” In 1910 The Journal99 characterized the preparation as “one of the innumerable ‘treatments’ for pulmonary tuberculosis that have arisen, had their day and, more or less gracefully, retired.” If the preparation had value for the purpose for which it is recommended, its use during these twelve years should have secured its general recognition. There is no satisfactory evidence of its therapeutic efficacy. The Council refused recognition to Bromin-Iodin Comp. and, after submitting this report to the Bromin-Iodin Chemical Company, authorized its publication.—(From The Journal A. M. A., Dec. 23, 1916.)
Ammonium hypophosphite was admitted to New and Nonofficial Remedies in 1908 as a preliminary step in the consideration of a preparation containing it—“Gardner’s Syrup of Ammonium Hypophosphite”—because the Council standardizes unofficial products before considering preparations or mixtures of these.
The therapeutic use of hypophosphites being irrational (see, “The Hypophosphite Fallacy,” Report of the Council on Pharmacy and Chemistry, The Journal, Sept. 2, 1916, p. 760), the salt, ammonium hypophosphite, deserves continued recognition only on condition that this salt of ammonium is superior to other salts from which may be obtained the effect of the ammonium radical. It has been claimed that ammonium hypophosphite has a less objectionable taste than other ammonium salts used for similar purposes. This claim would merit serious consideration if in addition to being less objectionable to the taste, the effects of ammonium hypophosphite were equal to or more desirable than the official ammonium salts. There is no evidence that this condition is met by the hypophosphite salt.
Ammonium hypophosphite has long been known, yet it is not official in the Austrian, Belgian, British, French, German, Hungarian, Italian, Swedish, Swiss or United States Pharmacopeias. Neither is it mentioned in the leading textbooks on materia medica, pharmacology or therapeutics. In short it appears to be an instance of an obscure and superfluous salt selected for proprietary exploitation.
Since the continued recognition of ammonium hypophosphite would tend to perpetuate the hypophosphite fallacy, and because there is no evidence supporting its advantage as a means of securing the effect of ammonium salts the Council directed its omission from New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 51.)
The following advertisement appeared in the New Idea (September, 1916), a house organ of Frederick Stearns & Co., the proprietors of Alphozone:

In the light of our present knowledge the claim that Alphozone is a preventive of infantile paralysis is without warrant and the advice that the public depend on it for this purpose is reprehensible and dangerous. Therefore, the Council directed that Alphozone be omitted from New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 50.)
Calcium glycerophosphate and sodium glycerophosphate were accepted for New and Nonofficial Remedies chiefly in order that these products might be standardized. These mixtures now being defined in the new edition of the U. S. Pharmacopeia, this reason for including them in N. N. R. no longer exists. The report of Marshall (The Journal, Feb. 13, 1915, p. 573) which has the endorsement of the Council (The Journal, Sept. 30, 1916, p. 1033) shows that organic phosphorus compounds are split up into inorganic phosphates before absorption, that the animal organism can synthesize its complex organic phosphorus constituents from inorganic phosphates and consequently that the glycerophosphates, so far as their phosphorus value is concerned, are not superior to other phosphates. In fact, sodium and phosphate are more effectively administered as neutral or acid phosphate. It is evident that sodium glycerophosphate is a superfluous pharmaceutical preparation, particularly when the difficulty of obtaining a pure product and its high price is considered. So far as its calcium value is concerned, calcium glycerophosphate has no advantages over such calcium salts as the carbonate, phosphate, lactate, or chlorid. In view of the foregoing, the Council directed that sodium glycerophosphate and calcium glycerophosphate be omitted from New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 52.)
In recognition of the considerable revision of the therapeutic claims made by the manufacturer, Gardner’s Syrup of Ammonium Hypophosphite was retained in New and Nonofficial Remedies, 1916, and the proprietor advised of this provisional retention.
In the most recent advertising for this ammonium hypophosphite syrup the claim is made:
“Besides being an active expectorant Syrup of Ammonium Hypophosphite (Gardner) is useful as an alterative and resolvent and by virtue of its phosphorus element, which is in the form of a hypophosphite, PH2O2, has a tonic value.”
As detailed in the report of the Council “The Hypophosphite Fallacy” (The Journal, A. M. A., Sept. 2, 1916, p. 760) careful studies show that the hypophosphites are devoid of the “alterative” and “tonic” actions claimed by the manufacturer of Gardner’s Syrup of Ammonium Hypophosphite. Accordingly the Council voted to omit Gardner’s Syrup of Ammonium Hypophosphite from New and Nonofficial Remedies and authorized publication of this report.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 55.)
For over two years the Council has had under consideration certain products offered for the use of diabetics by the Kellogg Food Company of Battle Creek, Mich. These are:
Pure Gluten Biscuit.
Pure Gluten Meal.
40 per cent. Gluten Biscuit.
40 per cent. Gluten Flour.
40 per cent. Gluten Meal.
20 per cent. Gluten Meal.
The Council found these products ineligible for New and Nonofficial Remedies because the statements of composition (particularly of starch content) were insufficient and because the exploitation of the products to the laity was objectionable. June 21, 1915, the company promised to place a statement of the starch content on the package of each gluten product, to place on the gluten flour sacks a caution that diabetics use the flour only on the advice of their physicians, and to revise its advertising in accordance with the suggestions of the Council. Nothing further having been heard from the company, in April, 1916, specimens of the product were obtained, through a layman, direct from the Kellogg Food Company. These specimens, together with the advertising matter received at the same time, and also a letter of advice from the company to another layman, were sent to the Council’s referee, whose report follows. As will be seen, the referee finds that the amounts of carbohydrates contained in Pure Gluten Flour, 40 per cent. Gluten Flour and Pure Gluten Meal are greater than the amounts claimed in the company’s published analyses; that in the two first mentioned the amounts of protein are less than the amounts claimed; that exaggerated claims are made on all the labels and in the advertising literature, and that the company prescribes directly to the patient.
The following report was sent to the Kellogg Food Company for consideration. In reply the firm stated that a revision of its advertising was under consideration but would make no statement as to how soon this revision would be carried into effect. As the consideration had already consumed two years, the Council decided to give the profession the facts and authorized publication of the report. At the same time the Kellogg Food Company was advised that its products would be considered further whenever any submitted evidence warranted this.
W. A. Puckner, Secretary.
I submit herewith my report on certain foods offered by the Kellogg Food Company for the use of diabetics. I shall discuss these products from the standpoint of the claims made on the label, from the standpoint of the company toward nonmedical treatment as revealed in a letter to a layman, and lastly, on the basis of the claims made for the foods in the company’s literature.
PURE GLUTEN BISCUIT
| Referee | Company | |
Water | 8.30 | 5–10 |
Ash | 2.04 | 1–2 |
Protein (N × 5.7) | 73.87 | 75–80 |
Fiber | 0.12 | 2.4–3 |
Carbohydrates | 14.84 | 0–5 |
Fat | 0.81 | 0.25–0.70 |
Starch | 4.02 | 0–5 |
The sample analyzed does not contain the amount of protein claimed for it. It also contains more starch than one might suspect from the company’s analysis. A more conservative claim would be “starch less than 5 per cent.” The company makes the error of using the terms “starch” and “carbohydrates” as synonymous. If the maximum figures of the company’s analysis are used, the carbohydrates would amount to 5 per cent., whereas I find 14.84 per cent. The claim on the label “Guaranteed to contain less than 5 per cent. of carbohydrates” is incorrect. The next claim, “Each ounce of this gluten contains 23 grams of protein and represents 95 calories” is approximately correct, as my analysis shows 20.9 grams of protein and 103 calories.
The following remarks under “Vegetable Proteins” are in my judgment exaggerated:
“Leading authorities are now agreed that meat, fish, eggs and other animal proteins are greatly inferior to vegetable proteins in diabetes, often increasing the sugar output and the dangerous acidosis which leads to diabetic coma.... After many years of experimentation, we have succeeded in perfecting a process whereby the carbohydrates are excluded.”
In this connection, von Noorden, whom the company constantly quotes, says:
“In the slighter forms (of diabetes), the influence of meat albumins is not great and it is difficult to demonstrate the reaction of the patient to different forms of albumin. It may be necessary to add more albumin than the patient can actually take before glycosuria indication is reacted.... Once a medium amount of albumin is exceeded, say 70 to 80 grams, the glycosuria increases, no matter what the type of albumin is.”
My analysis also shows that the carbohydrates are not excluded from this food as claimed above.
40 PER CENT. GLUTEN BISCUIT
| Referee | |
Water | 8.50 |
Ash | 1.48 |
Protein (N × 5.7) | 41.15 |
Fiber | 0.08 |
Carbohydrates | 47.81 |
Fat | 0.98 |
Starch | 36.98 |
No analysis is supplied by the company, but this may be called properly a “40 per cent. Gluten Biscuit.” The company claims, however, that this is “Best for Diabetics,” which is not true.
Here, as in the case of “40 per cent. Gluten Flour,” the company’s label attributes to “Dr. Wm. Osler in ‘Practice of Medicine,’ ” the following quotation: “Of Gluten Foods, many are very unpalatable, others are frauds. A Good Gluten Flour is made by the Battle Creek Sanatarium Co., Mich.” I have no way of knowing to which gluten flour of the company Dr. Osler had reference. The “Pure Gluten Meal” might be called properly a “good gluten flour,” but this “40 per cent. Gluten Flour” is no better, and no worse, than the average gluten flour on the market. The quotation from Osler gives an entirely false impression.
40 PER CENT. GLUTEN FLOUR
| Referee | Company | |
Water | 8.62 | 5–10 |
Ash | 0.89 | 0.5–1 |
Protein (N × 5.7) | 33.63 | 40–45 |
Fiber | 0.08 | 1–3 |
Carbohydrates | 55.35 | 40–45 |
Fat | 1.43 | 0.2–0.5 |
Starch | 48.04 | ... |
My analysis shows 6.37 per cent. less protein than the company’s minimum, and 10.35 more carbohydrates than their maximum. In past years I have found the protein in this brand to range from 35.0 to 42.9 per cent. (using the factor 5.7). It is true that the manufacturer does not state what protein factor is used in his reported analysis, but as in four other brands 5.7 is used, it is fair to assume that the same factor applies to this as well. At least such should be the case, as otherwise the manufacturer’s analyses would be meaningless. Even using the factor 6.25 this later sample contains only 36.88 per cent. of protein.
The following statement, in my judgment, as applied to a food containing over 48 per cent. of starch, does not hold water: “This food is of special service in cases of Glycosuria and in the milder forms of Diabetes.” With this brand as with “40 per cent. Gluten Biscuit” the manufacturer again uses the misleading quotation from Osler.
40 PER CENT. GLUTEN MEAL
| Referee | Company | |
Water | 7.30 | 5–10 |
Ash | 1.36 | 1–2 |
Protein (N × 5.7) | 41.55 | 40–45 |
Fiber | 0.10 | 1–2 |
Carbohydrates | 48.58 | 40–45 |
Fat | 1.11 | 0.2–0.5 |
Starch | 36.59 | 40–45 |
The claimed analysis is justified by my findings. I must take exception, however, to the following statement: “Prepared with great care from a good grade of Spring Wheat, by our special process, which preserves the natural food properties of the product.” The company evidently tries to carry water on both shoulders, on the one hand claiming a reduction in the starch content, while on the other claiming the preservation of all “the natural food properties.”
20 PER CENT. GLUTEN MEAL
| Referee | Company | |
Water | 7.65 | 5–10 |
Ash | 1.22 | 1–2 |
Protein (N × 5.7) | 24.68 | 20–30 |
Fiber | 0.12 | 1–2 |
Carbohydrates | 65.41 | 65–70 |
Fat | 0.92 | 1–2 |
Starch | 51.24 | 65–70 |
The company’s analysis is confirmed. As the company claims directly that this is “Not A Diabetic Food,” any criticism of its use for that purpose is disarmed. However, again exception must be taken to the statement that “the natural food properties of the product” are preserved.
PURE GLUTEN MEAL
| Referee | Company | |
Water | 4.60 | 5–10 |
Ash | 0.96 | 1–2 |
Protein (N × 5.7) | 76.78 | 75–80 |
Fiber | 0.08 | 1–3 |
Carbohydrates | 16.77 | 0–5 |
Fat | 0.81 | 0.25–0.70 |
Starch | 6.77 | 0–5 |
The minimum claim as to protein is justified. Again the company confuses carbohydrates and starch, and the food instead of containing from 0 to 5 per cent. of “carbohydrates (starch)” actually contains 16.77 per cent. of carbohydrates, of which 6.77 per cent. is starch. Once more the statement that “the natural food properties” are preserved is untrue as applied to a wheat product deprived of most of its starch.
In justice to the company, it should be noted that on the labels of “Pure Gluten Biscuit” and “Pure Gluten Meal” appears the warning: “Every person suffering from diabetes should be under the care of an experienced physician,” and on the label of “40 per cent. Gluten Meal,” “Persons suffering from diabetes should use this food only on the advice of a physician.” On the other hand, the suggestion on the label of “Pure Gluten Meal,” “Write for a copy of Diabetic Foods and How to Use Them” is a more or less direct invitation to self-treatment. Moreover, a letter dated May 9, 1916, apparently dictated for the Kellogg Food Company by one Ruth French, in reply to an inquiry from a layman, gives direct advice with no reference whatever to a physician.
In addition to this inconsistent attitude the letter makes certain clear misstatements, as follows:
“40 per cent. Gluten Flour actually contains 40 per cent. of pure Gluten, making it a perfectly safe article of diet in all but the gravest cases of diabetes. From our Gluten Flour excellent bread, gems and puffs are made that perfectly satisfy the craving for bread with no harmful results.” This flour contains 33.63 per cent. of gluten, not 40 per cent.; it is not “a perfectly safe article of diet in all but the gravest cases of diabetes,” for if one reads the literature correctly, starch restriction is more necessary in mild than in severe cases of diabetes. Furthermore, the bread, gems and puffs made from such a flour do not “satisfy the craving for bread with no harmful results.”
In the next paragraph of the letter, undue emphasis is laid on the “objectionable properties” of flesh foods, a statement only in accord with the tenets of extreme vegetarians. I also doubt very much whether the statement is true that “under a diet of our diabetic foods the thirst to which diabetics are so often subject is usually very much relieved.”
In the next paragraph the assertion is made that “The diet indicated ... is in keeping with the ideas of the highest medical authorities.... Meat is entirely excluded from the dietary.” My reading of the literature does not show that the leading authorities take any such position. Later on reference is made to von Noorden’s claim as to the superiority of vegetable over animal proteins, which I have already discussed under “Pure Gluten Biscuit.” (Certain detached sentences of von Noorden might justify such a statement, but a reading of all he says on the subject leads to a very different conclusion.)
The whole booklet is written from the standpoint of an extreme vegetarian, and therefore is often misleading in its conclusions.
Page 5. “The researches of Ogata and others have shown that cane sugar is a less wholesome food than the natural sugars found in fruits and produced in the body by the digestion of starch, that is, fruit sugars and malt sugars.” In opposition to this I quote from von Noorden, their own authority, “Die Zuckerkrankheit und ihre Behandlung,” Berlin, 1910, page 270:
“That levulose, milk sugar and inulin are more useful than the other carbohydrates is a common opinion, but the importance of their use in practice does not correspond with the theory. In light cases the form of carbohydrates makes little difference; in severe cases the advantage from using levulose, milk sugar, etc., is only slightly greater than from using bread and flour.... Only in certain cases does it appear to me that the special form of carbohydrates possesses any particular significance.”
On page 92 of the same work von Noorden tells us that of the carbohydrates dextrose is the worst, with maltose almost as bad (in spite of the fact that Kellogg exploits his “Meltose,” the “new carbohydrate,” as of special value for diabetics). He also says that levulose increases glycosuria only about half as much as dextrose, when used occasionally, but with long use it is as bad as dextrose and starch.
Page 5. The company refers to sugar as “possibly also causing diabetes.” Sugar or any other carbohydrate may under diabetic conditions cause an increase of glucose in the urine, but I do not believe that any food or any diet can cause diabetes.
Page 7. “That the large use of meat and eggs is not only detrimental but positively dangerous in many cases of diabetes is now a well known and recognized fact.” The dietaries of well known authorities on diabetes are not in harmony with this statement.
Page 13. “It has been discovered that the complete suppression of carbohydrates from the dietary is not only unnecessary but is highly detrimental and even dangerous.” “The complete suppression of carbohydrates from the dietary” is the only means the physician has to determine the diabetic’s carbohydrate tolerance. If carbohydrate-poor foods are so “highly detrimental and even dangerous,” why does the company exploit foods like “Pure Gluten Flour” and “Pure Gluten Biscuit,” whose chief claim to excellence is their comparative freedom from carbohydrates?
Page 17. “Cream is an emulsion, and, with the exception of egg yolk, is the only form in which animal fat is found in an emulsified state.” Milk, Nature’s most wonderful emulsion, is apparently overlooked.
Page 19. “... these foods ... will be found of great value ... especially as substitutes for the breads and meats which are the most objectionable features of the ordinary diet, and which should, as far as possible, be interdicted in this class of cases.” This is simply special pleading for the Kellogg vegetarian diet.
Page 19. “Our glutens ... are all thoroughly standardized, so that in their use the physician and the patient know just the amount of starch eaten.” This standardization is largely mythical. For instance, “Pure Gluten Biscuit” claims 0 to 5 per cent. “carbohydrates (starch),” whereas I find 14.84 per cent. carbohydrates with 4.02 per cent. starch. “40 per cent. Gluten Flour” claims 40 per cent. gluten and 40 to 45 per cent. carbohydrates, whereas I find 33.63 and 55.35 per cent., respectively. “Pure Gluten Meal” claims 0 to 5 per cent. “carbohydrates (starch)” whereas I find 16.77 per cent. carbohydrates and 6.77 per cent. starch. I have a record of six analyses each of “40 per cent. Gluten Flour” and “40 per cent. Gluten Biscuit,” which show the hollowness of this claim of “standardization.” The flour showed 33.6, 35.0, 42.9, 36.8, 35.6, and 40.9 per cent. of protein, with from 40.8 to 55.4 per cent. of carbohydrates; the biscuits 32.7, 33.2, 39.5, 43.3, 33.9, and 41.2 per cent. of protein, with from 41.1 to 54.0 per cent. of carbohydrates. In fact, my experience shows that the Kellogg products are more poorly “standardized” than most of the diabetic foods on the market.
Page 20. “May be made to carry a large amount of fat in the form of butter, a most desirable thing in the treatment of diabetes,” while on page 16 the company claims that in an experiment of Minkowski on a dog, butter “passed through the body without change, none being absorbed”; these are certainly contradictory statements. The explanation is that on the one page the company is exploiting its biscuits, and on the other its nut preparations.
Page 20. Again the incorrect claim is made for “40 per cent. Gluten Flour” that “we believe this to be the only standardized gluten flour made.”
Page 21. The claim is made that flesh foods are “objectionable on account of the large amounts of ptomains and toxins which they contain.” I was not aware that fresh meats contained any ptomains whatever. On the same page the claim is again made that by the use of the Kellogg nut foods “diabetics lose their thirst,” a claim which I think is more than doubtful.
Page 22. “Nuts are a whole food, containing all the elements required for the perfect nutrition of the body.” A marked characteristic of nuts is that they are not “a whole food,” as with the exception of a few varieties, such as the chestnut, they are extremely poor in carbohydrates, which fact gives them their value in the diabetic diet.
Page 23. “With the exception of the potato, the beet and the carrot, vegetables contain little sugar or starch.” Corn, beans and peas are all vegetables which are relatively high in carbohydrates, and for this reason are specifically excluded from the diabetic’s dietary.
From the foregoing considerations I would recommend that the company’s analyses of “40 per cent. Gluten Biscuit,” “40 per cent. Gluten Meal,” and “20 per cent. Gluten Meal” be accepted as correct. Before the Council can accept any of these products, the following steps should be taken:
The company on all its labels should correct the impression that “carbohydrates” and “starch” are synonymous terms.
The labels of all the preparations examined should be changed in accordance with the criticisms given above.
In all cases in which analytic data are given, it would be preferable to state only the minimum of protein and the maximum of carbohydrates.
The booklet, “Practical Suggestions About Diet in Diabetes,” should be radically changed along the lines noted above.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 56.)
Iodo-Mangan, made by the Chemische Fabrik Helfenberg A. G., near Dresden, Germany, and sold in the United States by the Reinschild Chemical Company, New York, is a solution said to contain iron, manganese and iodin in combination with peptone. It is claimed to be a reconstructive tonic and blood-making adjuvant, with favorable action in affections of the glandular system. It was admitted to New and Nonofficial Remedies in 1907, before the Council had adopted the present Rule 10, which provides that no article shall be admitted to New and Nonofficial Remedies which, because of its unscientific composition, is useless or inimical to the best interests of the public or of the medical profession. In 1911 the Council considered the question whether or not this product was still eligible and decided in the end to retain it as probably having some merit. To determine if Iodo-Mangan was eligible for New and Nonofficial Remedies, 1917, the Reinschild Chemical Company was requested to send in the current advertising matter. As this advertising was not sent in and as apparently the product was not marketed at the present time, the Council on Pharmacy and Chemistry voted to omit Iodo-Mangan from New and Nonofficial Remedies. At the same time the Reinschild Chemical Company was informed that the preparation might be submitted for reconsideration at any time.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 64.)
As now marketed, Liquid Albolene (McKesson and Robbins, New York), is claimed to be made only from genuine Russian oil and hence to possess distinct advantages over
“... Oils purporting to be Russian, most of which are imperfectly purified and many of which are positively dangerous for continued use.”
On the other hand, a short time ago, McKesson and Robbins claimed that Liquid Albolene was then available.
“... Of as high a quality as we had supplied before the European War. Thanks to the research and scientific achievement of Our Chemists, we are now able to offer LIQUID ALBOLENE, using as a base a specially refined Domestic Oil that is in every way suitable for medicinal purposes, and having the same viscosity as Russian Oil.”
The advertising matter suggests the promiscuous, thoughtless and irrational use of Liquid Albolene and of a number of Albolene preparations by extravagant claims, such, for example, as the following:
“Albolene will never fail to bring a free, easy stool, no matter what condition may be present, from obstinate atony of the bowel to fissure, fistula, or even malignant disease, and in spite of the failure of ordinary purgatives to which the patient may have become habituated....
“Aromatic Liquid Albolene is actually the first laxative presented to the medical profession that seems to have no drawback....
“It will not have been lost upon the physician who has read the remarks on the use of Aromatic Liquid Albolene to regulate the bowels in surgical cases, that there are many instances where it would prove equally valuable during the treatment of acute diseases. In the exanthemata, in pneumonia, for example, to cite only a few of the conditions where it may be used to advantage, an absolutely reliable laxative that will not in any way weaken or distress the patient, presents obvious superiority to any of the agents heretofore in common use.”
The Council held Liquid Albolene ineligible because the product is marketed in a way to encourage its indiscriminate and irrational use by the public (Rule 4) and because unwarranted therapeutic claims are made for it (Rule 6).—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 65.)
Naphey’s Medicated Uterine Wafers were submitted to the Council by the manufacturers, Naphey & Co., some years ago and were rejected. Naphey & Co. has recently requested reconsideration of the preparation, and has submitted advertising matter, trade packages and sample packages. The label of the trade package contains the following:
“Naphey’s Wafers. For the local treatment of diseases of women, indicated in catarrhal conditions of the vagina, and of the uterine cervix. As a [sic!] adjuvant for the physician to use in carrying out treatment of disease of the uterus.”
“Zinc Sulphate, 33⁄4 gr., Sodium Sulphate, 31⁄2 gr., Sodium Borate, 4 gr., Boric Acid, 3⁄4 gr.”
“Naphey & Co., Warren, Pa., U. S. A.”
“Each box contains 25 wafers, sufficient for three months’ treatment. Price per box, 25c.”
In name, composition, and general appearance of the package, Naphey’s Medicated Uterine Wafers bear a strong resemblance to Micajah’s Medicated Uterine Wafers (The Journal, A. M. A., March 26, 1910, p. 1070). An advertising pamphlet reads:
“In every form of leucorrhea Naphey’s Medicated Uterine Wafers are indicated ...”
“What is true of leucorrhea is also true of all other functional troubles affecting the female genital canal; they are all treated best by astringents and antiseptics. And these, to be effective, must be applied in prolonged contact.”
The implication that all “functional troubles affecting the female genital canal” are best treated by astringent tablets like Naphey’s Medicated Uterine Wafers is an absurdity. The naming of disease conditions on the label, the manifestly unwarranted and exaggerated therapeutic claims, the name, which is non-descriptive of composition but suggestive of use, and the fixed formula, which cannot rationally be expected to give uniformly satisfactory results in the wide range of conditions for which the product is recommended, render Naphey’s Medicated Uterine Wafers ineligible for New and Nonofficial Remedies under Rules 4, 6, 8 and 10.
The report having been sent to Naphey & Co., the manufacturer offered, on condition that the preparation be accepted, to revise the advertising matter in minor particulars, to remove disease names from the trade package and to adopt the name Naphey’s Wafers or Naphey’s Tablets. The Council advised Naphey & Co. that the proposed names do not conform to the requirements for acceptance in New and Nonofficial Remedies because they do not indicate the composition of this pharmaceutical mixture, and moreover, that the routine use of a complex formula such as that of these tablets is irrational.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 66.)
Nujol, a liquid petrolatum (Standard Oil Company of New Jersey, Bayonne, N. J.), was submitted to the Council by the manufacturers. The Council advised the company that, before Nujol could be made eligible for New and Nonofficial Remedies, the advertising claims made for it must be revised to conform to the rules of the Council and the term “liquid petrolatum” must be used in connection with the brand designation and given equal prominence on the labels, advertisements and all circulars. The company thereupon submitted a label on which the name “Nujol” appeared in large red letters and under it in small letters the words “Liquid Petrolatum.” This did not meet the Council’s requirement with regard to the name. Moreover, Nujol continued to be advertised to the public under exaggerated and unwarranted claims.
The foregoing report was sent to the Standard Oil Company of New Jersey, which thereupon submitted revised advertising copy. This copy was decidedly less objectionable than the previous advertising but still contained exaggerated statements. The copy for use in lay journals particularly evidenced exaggeration. Observation on many occasions of a similar fact has convinced the Council of the inexpediency of admitting to New and Nonofficial Remedies any article which is advertised to the public.101
The Council held that conflict with Rules 3, 6 and 8 prevented the acceptance of Nujol and authorized the publication of this report.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 68.)
Pulvoids Natrium Compound was submitted to the Council by the Drug Products Company, Inc., New York, with the statement that each pulvoid (coated tablet, said to be made to dissolve in the intestinal tract) represents the equivalent of:
Potassium Nitrate | 21⁄2 | grs. |
Sodium Nitrite | 1⁄2 | gr. |
Sodium Bicarbonate | 2 | grs. |
Fl. Ext. Crataegus Oxycantha | 1 | min. |
Nitroglycerin | 1⁄250 | gr. |
According to the advertisements the tablets are “indicated in the treatment of high blood pressure and all forms of hypertension of the cardio-vascular system.” It is claimed that the tablets “will not irritate the kidneys.”
The Council, having submitted its objections to the manufacturer and considered the firm’s reply, held that Pulvoids Natrium Compound was inadmissible to New and Nonofficial Remedies for the following reasons:
1. The claim is made that the tablets disintegrate in the intestines; experiments conducted by the Council indicated that in most cases they would be broken up in the stomach. It was found that the tablets were visibly changed immediately after being put into gastric juice or even into distilled water; they disintegrated within from three to four hours, not only in gastric juice (obtained from a dog) at 37 C., but also in distilled water. It is quite usual for solids to remain in the stomach for more than three hours. If they make their way out of the stomach in less than that time the gastric movements must be so vigorous as further to hasten the disintegration of the tablets.
2. The rules of the Council require that the name of a pharmaceutical mixture shall indicate the potent ingredients. The name of this mixture does not indicate the presence of the nitrites, the potassium nitrate, the bicarbonate or the extract of hawthorne and the nondescriptive name is likely to lead physicians to use the tablets without fully realizing what they are giving.
3. No evidence was submitted that the tablets, as found on the market, contained the amount of sodium nitrite and nitroglycerin claimed. That is, it does not appear that the manufacturer checks the sodium nitrite and nitroglycerin content by analysis. The Council did not determine the nitrite content of the tablets. It maintains that when a manufacturer places a product on the market the burden of proof is on that manufacturer to show that the facts are in accordance with his claims for his product. Further, the examination by the Council of one or several specimens of any commercial product (particularly in the case of nitroglycerin preparations) would not be a guarantee of the constancy of its composition so long as the manufacturer does not himself control the composition by analysis. The necessity of such control of tablets containing nitroglycerin is evident from the report102 of L. F. Kebler of the U. S. Bureau of Chemistry. Dr. Kebler said:
“... nitroglycerin tablets have in a majority of cases been found deficient in the nitroglycerin content declared.”
“... these commodities are manufactured largely by rule of thumb. Little checking obtains in their manufacture and generally no analyst is employed.”
A further proof that nitroglycerin tablets are likely to be deficient in strength is contained in the convictions under the Food and Drugs Act of manufacturers who sold tablets below the declared strength, recorded from time to time (Notices of Judgments Nos. 3405, 2059, 1843, 1799).
4. There is no good evidence, experimental or clinical, to justify the simultaneous administration in fixed proportion of two vasodilators like sodium nitrite and glyceryl trinitrate (nitroglycerin). Also there is no rational excuse for combining extract of hawthorne, which is said to have a tonic effect on the heart muscle, with nitrites, which cause relaxation of the vascular system, or for the combination with these constituents of potassium nitrate or of sodium bicarbonate.
In the absence of evidence for the combination, Pulvoids Natrium Compound must be considered an irrational mixture, the use of which is a detriment to sound drug therapy and, hence, not admissible to New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 69.)
A referee submitted the following report of the American Medical Association Chemical Laboratory to the Council:
Saloform (Flexner) is advertised by the Robinson-Pettet Company of Louisville, Ky. In the advertisements for the product it is stated that:
“Saloform is a definite chemical compound the component parts of which are Hexamethylene Tetramine, Salicylic Acid and Lithia.”
“As a Uric Acid Solvent it is indicated in Rheumatism, Gout, in Phosphaturia, in Gravel, and in Renal Colic.”
“As a Genito-Urinary Antiseptic it limits suppuration anywhere along the Urinary Tract, from the Kidneys down to the orifice of Urethra.”
As, even after diligent search, no description of a compound of hexamethylenamine (hexamethylenetetramine), salicylic acid and lithia was found in chemical literature, it seemed probable that Saloform is merely a mixture of hexamethylenamine and lithium salicylate. Accordingly the separation of Saloform into its component parts by means of selected solvents was attempted. By triturating the powder with chloroform, filtering and evaporating the filtrate, a residue was obtained which gave satisfactory tests for hexamethylenamine but contained only traces of salicylic acid or lithium salicylate. The portion insoluble in chloroform was dissolved in water. The solution gave satisfactory tests for lithium salicylate but not for hexamethylenamine. From these tests it is evident that Saloform is a simple mixture of hexamethylenamine and lithium salicylate. Quantitative examination indicated that the two ingredients, hexamethylenamine and lithium salicylate, are present in approximately equal amounts.
The report of our Chemical Laboratory shows that Saloform is not a definite compound as claimed, but a simple mixture of hexamethylenamin and lithium salicylate. It is therefore in conflict with Rule 1. It is also in conflict with Rule 6, for neither hexamethylenamin, lithium, nor salicylate are therapeutically effective “uric acid solvents”; nor would any of these have any effect on “phosphaturia.”
The mixture also conflicts with Rule 10; for it is inadvisable to administer the ingredients in fixed, but unknown proportions.
It is recommended that Saloform be deemed inadmissible to N. N. R.
The Council adopted the recommendation of the referee and authorized publication of this report.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 71.)
About a year ago the Council declared Secretogen,103 a product the active ingredient of which was stated to be “pancreatic secretin” and advertised as a remedy for certain conditions of defective digestion and assimilation, to be ineligible for New and Nonofficial Remedies. The reasons for this decision were stated at the time as follows:
“1. No evidence has been presented that the absence of secretin is a cause of gastro-intestinal diseases. It is usually present, and if not present, as in achylia gastrica, there is evidently some compensating arrangement by which the pancreas is stimulated to perform its regular functions.
“2. There is no evidence that secretin in any form is physiologically active when administered by mouth.”
Since Secretogen was not the only so-called secretin preparation on the market, and since the use of secretin preparations was recommended by certain writers, notwithstanding the lack of evidence of its value, the Council caused an experimental investigation of the question to be made. This was carried out by Prof. A. J. Carlson of the University of Chicago.
No secretin was found in the commercial products examined, namely, Secretogen Tablets, Secretogen Elixir and Duodenin. Furthermore, Carlson’s results104 confirmed the Council’s previous conclusion as to the inertness of secretin administered by mouth. The Council endorsed Professor Carlson’s findings.105
The G. W. Carnrick Company has replied to the publication of this report in the letter printed below. (A portion of this letter, which consists of a communication from an unnamed correspondent of the G. W. Carnrick Company and the company’s comment thereon, has been omitted.) The Council offered to publish this if the Carnrick Company would furnish the name of the writer. This it has not done. As will be seen, the company now shifts ground, abandoning entirely the claim that Secretogen contains secretin. The Council has authorized publication of the letter (omitting the part just mentioned), together with the comment that follows.
W. A. Puckner, Secretary.
“The Council on Pharmacy and Chemistry of the American Medical Association.
“Gentlemen:—The opinion of the Council and the contribution by Professor Carlson which appeared in The Journal of the American Medical Association for Jan. 15, 1916, have been read by us with interest. The column of Current Comment dealing with ‘Tiger-Bone Therapy and Clinical Experience’ has appealed to our good nature and, under the circumstances, our sense of humor.
“Professor Carlson seems to have quite well established that the so-called secretin preparations do not contain secretin to any appreciable extent, and that they are inert in laboratory experiments on normal animals. At the same time, to do away with an apparent discrimination on the part of the management of the Council, it would have been well if Professor Carlson had included the so-called secretin preparations belonging to another well-known firm which markets such a product. This discrimination has already been referred to by us.
“Had Professor Carlson stopped at the determination of the therapeutic availability of secretin given by mouth, his work might have been accepted without comment, even if we should have thought it advisable to object to the matter published by the Council. But the professor went beyond his province entirely when, in commenting on the findings obtained by using Secretogen clinically, he said: ‘It is, perhaps, impertinent for laboratory men to comment on these clinical results.’ It is. His point was well taken and it is a profound pity that Professor Carlson did not observe his own ruling.
“In the words of a correspondent of The Journal of the American Medical Association, in discussing Professor Carlson’s criticism of Dr. Crile’s ‘Kinetic Drive,’ ‘it behooves the laboratory man to be circumspect in his criticism of clinical theories, since going beyond the bounds of well-established things weakens his position, not merely with reference to the particular subject under discussion, but with reference to clinical phenomena in general.’ Clinical results have definitely established the value of Secretogen. As the matter now stands this statement is beyond criticism.
“When Secretogen was first introduced we assumed that it depended on secretin for results produced. In this assumption we were in good company, as witnessed by the testimony of Moore, Edie and Abram when, in the course of their investigations as to the value in diabetes of a secretin-bearing extract given by mouth,106 they said: ‘In the majority of these cases ... there has been no appreciable fall in the output of sugar ... in some of these negative cases there has been noted, however, improvement in the digestion and, in certain cases, the patient’s weight has increased.’ They also state that the secretin-bearing product ‘appears to stimulate the functional activity of the duodenum.’106 They give a most significant report.107 We quote from the paper as follows:
“ ‘The patient had been under observation for six months before treatment and the sugar was not reducible by diet. Almost at once the dyspepsia from which he was suffering was relieved and his general nutrition improved to such an extent that he regained over eighteen pounds in weight, which he had previously lost, and this improvement was accompanied by complete recovery of his physical and mental energies.’106
“Inasmuch as this improvement could not have been due to the contained secretin it must have been due to some other principle contained in the extract. Our experience and that of the physicians who have used Secretogen establish the fact that Moore, Edie and Abram made no mistake when they came to the conclusion that what they termed a secretin-bearing extract stimulates the functional activity of the duodenum and improves the digestion.
“When Professor Carlson was investigating Secretogen he must have realized that he was dealing essentially with an extract of the duodenal mucosa. It is, therefore, all the more surprising, considering his extensive researches into the literature, that he should have ignored the testimony of some of his own authorities, particularly Hallion, as to the value of extracts of the duodenal mucosa in duodenal insufficiencies. The meticulous carefulness with which this evidence was avoided is hardly worthy of the best traditions of physiology, a science which has truth for its first and last aim.
“Hallion in his ‘La Pratique de l’Opothérapie’ says that the ‘aims of duodenal opotherapy are: 1, To supply deficient duodenal juice. 2, Above all to stimulate and to relieve this organ—notably to aid the production of secretin106—and so profit by the stimulating action which duodenal extract exercises on the duodenal mucosa which action we, Enriquez and myself, believe and have experimentally proved, conforms to the general principles of opotherapy. 3, By means of the production of secretin, to reinforce the biliary, pancreatic and intestinal secretions. 4, To stimulate intestinal peristalsis.
“ ‘Principal indications: Intestinal dyspepsias, intestinal autointoxications, certain forms of constipation and duodenal insufficiency.’
“At the International Congress of Medicine, Madrid, 1903, Hallion said that he felt justified in stating that duodenal opotherapy correctly carried out must be classed under the very best methods of treating dyspepsia.106 The results had been satisfactory and, in many cases, remarkable. It had been nil in a few cases but it had never been harmful in any degree. He pointed out that Marfan was the first to employ this substance clinically. Marfan had had particularly excellent results in children of 15 months to 4 years suffering with marked malnutrition, anorexia and constipation. Marfan prescribed the duodenal extract given in milk.106 Hallion further remarks that, as he is not a practitioner, he had had only one opportunity to test duodenal opotherapy clinically. The case was that of a man of 26 years with obstinate intestinal dyspepsia and severe constipation which had persisted from childhood. This patient had been treated by enemas, laxatives, diet, etc. Treatment with duodenal extract resulted in a complete cure.106 Hallion points out that the most satisfactory aspect of duodenal opotherapy is the permanent effect produced,106 which bears out his statement that these extracts have the power to aid in the restoration of function and structure of an organ.
“This has been so well established that the principle is now embodied in a law which is frequently referred to as ‘Hallion’s Law’: ‘Extracts of an organ exert on the same organ an exciting influence which lasts for a longer or shorter time. When the organ is insufficient it is conceivable that this influence augments its action and, when it is injured, that it favors its restoration.’
“In ‘La Pratique de l’Opothérapie’ Hallion points out that ‘the opotherapeutic product which corresponds to the affected organ represents in some way the stimulating and elective food for that organ, and if we supply the organ with a food which is more complete than it necessarily needs, the affected organ can exercise its elective action and take up only those substances of which it is in need.’
“Hallion’s observations on this point are beautifully borne out by the classic work of J. W. Draper, as reported in The Journal of the American Medical Association, Sept. 26, 1914. This report gives results in both laboratory and clinical experiments.
“In order to show that fed jejunal and ileac epithelium exercise some special detoxicating power, not yet understood but definitely recognizable, Draper fed a control series of dogs with intestinal obstruction, experimentally produced, on emulsified cells of liver, spleen, pancreas and muscle tissue. These animals lived a few hours longer than not-fed controls, but Draper says that it is evident that these cells had either no detoxicating action, or a very feeble one compared with intestinal epithelium. He used jejunal and ileac epithelium clinically in two instances: 1st, In a female dog which had had ‘chronic stomach trouble’ for six months. When Draper saw her she had had complete intestinal obstruction for five days, with symptoms of tachycardia, extreme nervousness and great weakness in the hind legs. Draper removed a pebble from her intestine but her condition was still grave.
“She was immediately put on small-intestine epithelium derived from two dogs of different breed. Draper says that from a long experience with duodenally obstructed dogs, he should not have expected her to recover, but the symptoms gradually subsided and she lived. The second instance in which he used the epithelium therapeutically was in the case of a man who suffered from an annular cancer of the intestine with definite symptoms of obstruction. After the operation, and realizing that the patient was in a desperate condition, he fed him an emulsion of intestinal epithelium from a dog. The pulse improved and the patient lived.
“Some of Draper’s conclusions are as follows:
“ ‘Autotoxemia in intestinal obstruction undoubtedly arises from an interference with cellular reactions of the intestinal epithelium.... When small-intestine epithelial cells of healthy animals are placed in the stomach106 of duodenally obstructed animals, such animals have lived nearly twice as long as not-fed controlled animals. This evidence is strongly opposed to the bacterial theory of origin of toxins.’
“The point to be emphasized is this: If this emulsion of intestinal epithelium had been fed to a normal dog and a normal man, what would have happened? Absolutely nothing. On the other hand, given as it was to a dog and a man in desperate need it exercised a potent effect.
“Abundant clinical testimony can be cited in support of the opinions of Moore, Edie and Abram, Hallion, Marfan and Draper as to the value of extracts of the intestinal mucosa given by mouth in pathological conditions. We have previously cited the published favorable opinions of such gastroenterologists as Anthony Bassler, Lewis Brinton, G. R. Lockwood, and R. C. Kemp, so there is no need to recapitulate their experiences with what they honestly believed to be secretin-bearing extracts, but which were essentially extracts of the duodenal mucosa.
“Supplementing the evidence of these men as to the value of these extracts we submit an excerpt from a letter from one of the best known physicians of Edinburgh:
“ ‘I can speak in very high praise of Secretogen, which I have used in both tablet form and as the elixir. There is no doubt about its value in a certain class of intractable indigestion which refuses to be benefited by any other remedy. On several occasions I have been much gratified by the definite relief obtained in this class of cases. It hits the mark also in some types of obstinate constipation—I think those cases where the trouble is wrapped up in impaired enervation of the intestine, and where stasis occurs at certain segments of the canal.’
“Hallion very pertinently points out108 that it is now accepted that opotherapy is not substitutive, but homostimulative and he remarks further that it is well to bear in mind that the so-called active substances which make the extract efficacious need not necessarily be the hormones. ‘It may be the elements of tissue structure which may come to the aid of the injured organ. The hormone should not therefore be looked on as the only active agent of opotherapy and, while its action is important, it need not necessarily be preponderant. The chemical isolation of the hormones is, of course, of interest but may not be as vital to organotherapy as we have thought.’ ...”
The G. W. Carnrick Company, which formerly claimed that Secretogen was efficacious because it contained secretin, now admits this claim to be unfounded. Notwithstanding, the manufacturers still call their product Secretogen and make for it practically the same therapeutic claims as before. They now base these claims on vague “principles of opotherapy” and on so-called “clinical testimony.” The burden of proof rests on them to show that these old claims, already discredited but put forth again on new grounds, are justified. Have they done so?
The “clinical testimony” is not convincing. So much of it as is definite enough to permit of criticism has already been dealt with. The remainder consists of mere assertions; it is not through reliance on such evidence that the Council can discharge its trust. On this side of the question there is nothing new to be said—reassertion of a refuted argument does not constitute fresh proof.
Nor is the case better on the experimental side. The statements of Hallion, Enriquez, Zuelzer and others109 as to the existence of a “peristaltic hormone” not only have failed of confirmation, but also have been positively discredited. With regard to Draper’s work, which dealt with acute intestinal obstruction, it is difficult to see what is its relevance to the present issue, particularly since Draper’s results were obtained with a product derived from the mucosa of the jejunum and ileum and not with an extract of the duodenum such as Secretogen purports to be.
The innuendo that the Council discriminates in favor of certain manufacturers, is itself a confession of weakness.
In publishing this correspondence the Council’s sole object is to put the medical profession in possession of the exact facts of the case. These may fairly be summed up as follows:
1. Secretogen was originally marketed as a preparation containing secretin. None was found in it.
2. Notwithstanding proof of this fact, the G. W. Carnrick Company retain the original name of the product, knowing that, by its association with their former erroneous assertions concerning Secretogen, this name must inevitably convey to a physician using the product the impression that he is administering secretin. In the advertising literature no hint is given that this original statement was erroneous.
3. The product called “Secretogen” has not been shown, either experimentally and by sound clinical evidence, to possess useful therapeutic properties.
Under these circumstances the Council reaffirms its decision.—(From Reports of Council on Pharmacy and Chemistry, 1916, p. 72.)
H. K. Mulford Company and E. R. Squibb and Sons submitted to the Council ampules containing solutions of iron citrate green. It thus became necessary to consider the eligibility of iron citrate green itself for admission to New and Nonofficial Remedies. As the rules of the Council provide that nonessential modifications of official or nonproprietary preparations will not be recognized, the above named firms were asked to state what advantages, if any, the so-called iron citrate green had over the official iron and ammonium citrate. In reply the H. K. Mulford Company wrote that it had come to the conclusion that iron citrate green and ampules thereof would undoubtedly be considered by the Council as a nonessential modification of an official product, adding:
“It seems to differ from the official ferric citrate so far as essentials go only in color, but custom, which is exceedingly hard to change in South America, demands that this green variety of ampules be used in place of the official product.”
In reply to a similar letter of inquiry E. R. Squibb and Sons wrote:
“Iron citrate green (iron and ammonium citrate green) differs from the U. S. P. iron and ammonium citrate in that it contains less iron and more citric acid and more ammonium citrate than does the latter. It is of course a modification of the official salt and is supplied to meet a real demand. Its reaction is quite decidedly acid and our present stock contains Fe slightly below the U. S. P. requirements for iron, assaying 15.74 per cent. instead of 16 per cent. Fe. The tests used to control its quality are those for the official product except as before indicated, it is always acid instead of neutral, as the U. S. P. requires for that salt.”
The smaller iron content (98 per cent. of the U. S. P. requirement) of the green variety referred to by E. R. Squibb and Sons is so small as to be negligible. Further, the low iron content as well as the acidity of the green salt would appear to be detriments rather than advantages. Inasmuch as no evidence has been presented to show that iron citrate green is superior in any way to the well-known iron and ammonium citrate the Council held that iron citrate green, and with it the dosage forms, was ineligible to N. N. R.
The preceding report was submitted to the Mulford Company and to E. R. Squibb and Sons for comment before publication. The former firm replied that in the present case it felt bound to supply the existing demand, the latter replied that, to give the Council its support in this matter, the sale of iron citrate green and ampules thereof would be discontinued.—(From The Journal A. M. A., Jan. 13, 1917.)
The referee’s report on Aspirin-Bayer which follows was submitted to the Council and adopted by it and, in accordance with the referee’s recommendation, was sent to the Bayer Company, Inc. The company’s reply contained nothing to warrant the continued recognition of this product by the Council. It was accordingly directed that Aspirin-Bayer be omitted from New and Nonofficial Remedies.
W. A. Puckner, Secretary.
The referee’s attention has been called to the systematic campaign of advertising aspirin to the public. He is informed that tablets have been marketed for some time in “vest-pocket” boxes, bearing the name “Aspirin” permanently affixed, which is in technical conflict with the Council’s rule against indirect advertising to the public. More recently, conspicuous advertisements have appeared in daily papers. These are technically in conflict with the rule against direct advertising to the public.
In addition to the plain technical conflicts with the Council’s rules there is a feature of the case which has not hitherto been raised and which should be fully considered: It may be remarked that the advertisements contain no therapeutic recommendation, and do not, on their face, urge the public to employ aspirin but apparently merely tell the public how it may protect itself against sophistication. In substance, they say: “If you are a user of aspirin, this is how you may obtain the genuine.” It might be said that this is not an attempt to increase the use or sale of aspirin—the ordinary object of advertising—but that the means of protection against adulteration is a “subject on which the public should be instructed.” The principle of such exceptions is stated in the comments to Rule 3 (New and Nonofficial Remedies, 1916, p. 15); and although the present case does not come under the exceptions specified under these comments, it may be urged that the exceptions need to be increased as occasion arises. The notorious adulteration of aspirin may well be urged as establishing a need for a similar exception in its use.
The general principle of protecting the public against fraud, adulteration and substitution is directly in line with the objects of the Council, and deserves commendation and support. It is obvious, however, that the means adopted for this end must be efficient, that they must not open the door to other, perhaps greater evils and that they must be used in good faith. The policy of advertising “Aspirin-Bayer” must be examined in these respects.
In the first place, the acceptance of a product by the Council implies an agreement by the manufacturers or agents that they will adhere strictly to the Council’s rules and will not depart from the letter or spirit of these rules without notice to the Council. This principle has been grossly infringed in the present case. There can be no doubt that the agents were aware that their advertisements conflicted, at least with the letter of Rule 3. Nevertheless, they did not, in any way, inform the Council of the change in policy. In this respect, at least, they have not acted in good faith.
Secondly, the wording of the advertisement implies that only the tablets stamped with “The Bayer Cross” are genuine. This is misleading, since every druggist has the right to make unstamped tablets of aspirin, fully as genuine as those stamped with the cross.
Thirdly, the cross itself cannot be considered an efficient protection; for people who imitate aspirin will not hesitate to imitate the stamp. The remedy, in either case, and as with any other drug, is the examination of trade samples, and the vigorous prosecution of those guilty of violating the law.
Fourthly, the permanent affixing of the name “Aspirin” to the vest-pocket boxes is also inefficient as a protection, and serves mainly as an advertisement.
Fifthly, whatever may have been the motives of the advertisers, and however carefully the advertisements are worded, they will inevitably tend to increase the use of aspirin by the public, and this is directly against the interests of public health. The public does not know, as physicians do, that headaches are merely symptoms of other, sometimes very serious conditions; and that they are often the signal for the need of a thorough physical examination and diagnosis. It is true that they are often also the symptoms of very minor derangements, which will right themselves spontaneously; and that, in such cases, drugs like aspirin may give relief and may do no harm. The patient, however, is not educated to distinguish one class from the other, and therefore anything that tends to promote the indiscriminate use of such remedies as aspirin is detrimental to the public health. Furthermore, aspirin itself is not always harmless. Alarming idiosyncrasies are sufficiently common that the use of the first doses, at least, should require medical supervision. With these considerations in mind, the referee is of the opinion that the direct and indirect advertising of aspirin is to be condemned.—(From The Journal A. M. A., Jan. 20, 1917.)
A circular issued by the A. H. Robins Company of Richmond, Va., contains the following statement:
“PIL. CASCARA COMPOUND-ROBINS is a rational therapeutic formula, composed of CASCARA, PODOPHYLLIN, COLOCYNTH and HYOSCYAMUS, which promotes a natural flow of secretions, which is, in turn, the physiologic stimulant of peristalsis. Thus, a normal evacuation is produced without subsequent inhibition.
“They contain no Mercury, Strychnia nor Belladonna.
“An ideal aid to any remedial agent, when a Mild, Medium or Strong alimentary stimulant is needed [sic].
“Made in two strengths, the dosage may be easily regulated so as to obtain the effects of an Anti-Dyspeptic, Aperient, Laxative or Cathartic, as desired. They never cause discomfort unless given in larger dose than needed.”
This preparation is another example of the innumerable mixtures of well-known drugs having nothing in the way of originality or of special therapeutic value to recommend them.
The advertising implies that this particular combination has a special action on the secretions of the gastro-intestinal tract; otherwise it would be hard to explain the claim that the preparation is antidyspeptic, if that means anything more than a laxative or cathartic.
The claim is made that this preparation contains no belladonna—yet it admittedly contains hyoscyamus! This manifests either ignorance on the part of the manufacturers, or an effort to impose on the medical profession. Both belladonna and hyoscyamus contain variable amounts of similar alkaloids, chiefly hyoscyamin. Hyoscyamus is feebler than belladonna in its action, as it contains less alkaloid. The qualitative differences between the two drugs, with reference to their use as laxatives, is so slight as to make the company’s claim for hyoscyamus appear either deliberately misleading or to be the result of crass ignorance. Promoting this mixture of well-known laxatives and cathartics as an “ideal aid to any remedial agent when a mild, medium or strong alimentary stimulant is needed” is a slur on the intelligence of physicians.
Pil. Cascara Compound-Robins is not acceptable for New and Nonofficial Remedies.—(From The Journal A. M. A., Jan. 27, 1917.)
Casta-Flora is one of those complex preparations which are offered to the medical profession, with plausible arguments in support of the claims made. It is put out by the Wm. S. Merrell Chemical Co., Cincinnati. Each fluidounce is said to represent:
“Castanea, fresh leaves, 40 gr.; Passiflora, fresh plant, 40 gr.; Gelsemium, green tincture, 8 minims; Inula, represented by the camphoraceous stearoptene Helenin, 20 grs.; Iodized Lime, 8 grs.; Menthol, 1-4 grs.; Aromatic Syrup Yerba Santa, 60 minims.”
It is said to be:
“A new combination of well-tried remedies of especial value in pertussis and other spasmodic coughs. It is composed of astringent, antispasmodic, sedative and expectorant agents, that control the paroxysms, relieve the irritation, promote expectoration, and give tone to mucous membranes involved.”
Still more exaggerated claims are made for the individual constituents of Casta-Flora, partly by direct statement, partly by inference. For example:
“Castanea is almost a specific in whooping cough and other spasmodic coughs.
“Passiflora is a narcotic, sedative and antispasmodic without habit-forming properties, nor does it lock up the secretions and upset digestion like opiates.
“Inula (elecampane) has been employed as a cough remedy in England for centuries. Its action is similar to guaiacol and creosote. Its active principle, helenin, is destructive of tubercle bacilli in dilutions of 1 to 10,000.
“Iodized Lime, Menthol, and Yerba Santa are too well known as expectorants and antiseptics to require more than passing mention.”
That Casta-Flora is a “new” combination may be admitted; it is improbable that exactly this combination of obsolete drugs was ever before selected for any purpose whatever, but the statement is misleading in that no new principle of therapeutics is involved. On the contrary, the combination is just what might be expected from haphazard choosing of discarded and nearly forgotten drugs. It seems incredible that a reputable firm of manufacturing pharmacists would make the positive statement that castanea is almost a specific in whooping cough. Why not say it is a specific? It would be about as true. A specific or “almost specific” for this disease would rank among great medical discoveries; but castanea is merely a slightly astringent drug neither better nor worse than scores of other astringent drugs that have been tried, found valueless and discarded.
Hardly less surprising are the statements regarding passiflora. This herb has been on the market about three quarters of a century. Not only has it never established itself in scientific medicine, but it is not even mentioned in modern standard works on therapeutics.
Of all the statements made in the circular perhaps the most remarkable, in that it is so dangerously misleading, is that regarding helenin, the active principle of elecampane. The statement that this principle (helenin) is destructive of tubercle bacilli in dilutions of 1-10,000 can only mean that it is of extraordinary value in the treatment of tuberculosis; in fact, it is definitely stated that the action of elecampane is similar to that of guaiacol and creosote.
It is obvious that any drug which would destroy the tubercle bacilli in the human lungs without exerting a toxic action on the patient would be a great contribution to medicine. But although elecampane may have been used for centuries it has proved to have little, if any, merit, and even the National Standard Dispensatory, p. 848, says: “Elecampane was formerly employed as a tonic, stimulant, diuretic, diaphoretic, expectorant, and emmenagogue, but has now largely fallen into disuse.” One looks in vain in the standard textbooks on therapeutics for a description of the uses of inula (or elecampane), and of its so-called “active principle,” helenin.
The circular to which reference has been made says, referring to the use of castanea and passiflora in the treatment of whooping cough:
“Gelsemium, when made from the fresh, green plant—as is Merrell’s—is an excellent adjuvant to the above drugs, and allays the nervous irritability so frequently present.”
H. C. Wood, Jr. (Pharmacology and Therapeutics, 1916, p. 160), says of gelsemium: “Gelsemium was originally employed as an arterial sedative and febrifuge in the malarial fevers of the South, and subsequently in sthenic fevers. It appears in some way to depress the bodily temperature, but it does not appear probable that any advantage to be derived from it will counterbalance the danger attending its employment in the large doses required. In asthma, spasmodic laryngitis, whooping cough, and nervous cough it has been recommended by Bartholow, but is little used.”
That is about as favorable a statement for the drug as is to be found in the textbooks, and it serves to illustrate how little new there is in this mixture of obsolete drugs that Merrell seeks to market as one possessing extraordinary therapeutic value.
Even though the ingredients, or certain of them, were singly useful in the treatment of those conditions for which Casta-Flora is recommended, no one could possibly foresee the effect in any given case of such a jumble of drugs, both active and inert, as is said to be represented in this preparation. The prescribing of such mixtures, the action of which cannot in any way be foreseen, is plain charlatanism.
In addition, the various drugs in Casta-Flora are present in such proportions that the dose of each of the several ingredients bears no relation to the commonly accepted dose.
Casta-Flora is not acceptable for New and Nonofficial Remedies.—(From The Journal A. M. A., Jan. 27, 1917.)
Firwein is a product of The Tilden Company, New Lebanon, N. Y. It is sold under the claim that when swallowed it has a “predilection” both for the bronchial mucosa and also for the genito-urinary organs. To quote:
“Expectorant, Sedative, Antispasmodic in the Treatment of Inflammations of the Bronchial and Genito-Urinary Mucosæ.”
“Firwein being a bland, soothing balsam possesses a wide range of adaptability and increased potency because of its healing virtues and usefulness as an expectorant, sedative and antispasmodic in bronchitis, and inflammation and catarrh of nose, throat and lungs.”
“Firwein has a special predilection for mucosæ, this being as marked in diseases of the genito-urinary system as it is in the respiratory organs. In inflammatory diseases of the genito-urinary organs, its bland, curative properties are exerted in a gratifying degree. In cystitis and uritis it is clearly indicated....”
Little information is given concerning the composition of Firwein. An old circular says:
“Firwein contains Phosphorus, Iodin and Bromin finely blended with a balsameous elixir made from the fir tree.”
From a more recent circular we quote:
“Firwein is prepared from the inside fresh green bark of the fir tree ...”
The label on the product reads:
“Firwein is pleasantly and effectively blended with salts of iodin and bromin, held in solution with 20 per cent. alcohol.”
The therapeutic claims made for Firwein and the mystery enshrouding its composition make it obvious that the product is intended to appeal to those who are either thoughtless or ignorant. This is emphasized by the suggestion that Firwein be combined with (1) cod liver oil (under the claim that it will “promote the efficiency of the oil”), with (2) whisky for the treatment of bronchorrhea of the aged, and with (3) syrup of hypophosphites for the treatment of persistent bronchitis.
As the composition of Firwein is secret, the therapeutic claims unwarranted, and its use irrational, the Council declared it inadmissible to New and Nonofficial Remedies.—(From Journal A. M. A., Feb. 17, 1917.)
Firolyptol, another product of The Tilden Company, is, we are told, composed of eucalyptol 10 drops, cottonseed oil 1⁄2 ounce and Firwein enough to make 1 ounce. As the composition of Firwein is secret, it is evident that the composition of Firolyptol is also unknown, except to the manufacturers. “Firolyptol with Kreosote” is said to contain, in addition to whatever may be the component parts of Firolyptol, 10 minims of creosote to each ounce. According to an advertisement, Firolyptol with Kreosote is “antituberculous, antistrumous” and “contains all the desired features of cod liver oil and is readily assimilated.”
The advertisements of “Firolyptol Plain” and “Firolyptol with Kreosote” seem to have for their key-note the assertion that cottonseed oil is a particularly valuable nutriment and that when combined with constituents of Firolyptol and Firolyptol with Kreosote becomes particularly valuable to the tuberculous. To quote from an advertising circular:
“Now that the reconstructive properties of cottonseed oil are better appreciated by the profession, the advantages that follow the administration of a palatable emulsion of this strengthening and fattening food product are being demonstrated in hundreds of cases where formerly reliance would have been placed in cod liver oil.... A recent writer says that pure cottonseed oil is the greatest and purest vegetable oil known to chemistry, and will do much toward revolutionizing the treatment of the GREAT WHITE PLAGUE.... If the treatment of tuberculosis could resolve itself into the administration of a fatty substance in a readily assimilated form, there would be no need for any part of FIROLYPTOL but the Cottonseed Oil.... The toxic material constantly produced in the system by the germs of tuberculosis tend to expose it more and more to the ravages of the disease, and the physiologic functions of the body suffer a constant depression. To neutralize this germ activity with a consequent production of toxins it seems most logical to employ such agents as have demonstrated their suitability for such purposes, for which reason Eucalyptol and Kreosote with Firwein are incorporated in FIROLYPTOL.”
The assertion that cottonseed oil is an especially valuable form of fat is without warrant, but even if it were true the fat is available in cheap and palatable forms in numerous other cottonseed oil products. It is unnecessary to discuss the problematic value of creosote in the treatment of tuberculosis or the value of eucalyptol (now generally abandoned), or even of the secret mixture Firwein. Food and fresh air, not drugs, constitute the fundamentals of the treatment of tuberculosis, and it is both irrational and detrimental to the interests of the tuberculous to administer various potent agents in fixed and unknown amounts with such simple articles of food as cottonseed oil. Neither of these products is acceptable for New and Nonofficial Remedies.
Editorial Note.—Firwein110 has been advertised to physicians for twenty-five or thirty years and it is a sad commentary on the intelligence of our profession that a preparation sold under such obviously false and misleading, not to say silly, claims, should still be in existence. Firwein is claimed to “prevent waste of tissue” in tuberculosis. If it had this power, it would have found its place long ago among the few great agents in drug therapy. As a matter of fact, Firwein has gained virtually no recognition outside of the “literature” of the Tilden concern. The claims made for Firwein are a peculiar mixture of studied candor—when the truth is not likely to hurt its sale—and inane vaporing—when the facts would not redound to its credit. The Tilden Company declares that “Firwein stands without a peer in its class.” But the company adds 10 drops of eucalyptol and some cottonseed oil to this peerless product and an improvement is born—“Firolyptol”! Then, to perfect the already perfectly perfected, 10 drops of creosote are added to “Firolyptol” and the profession is offered “Firolyptol with Kreosote”! In just what verbal pyrotechnics the Tilden Company might indulge, should it decide to add ten drops of something else to “Firolyptol with Kreosote,” one shudders to contemplate.
If we are accused of exhibiting undue levity in discussing a therapeutic problem, we can only answer that it is impossible to consider seriously the Charlie Chaplins of the nostrum world.—(From The Journal A. M. A., Feb. 17, 1917.)
In accordance with the usages of the Council, the report which appears below along with the reports of the clinical investigation by Drs. Cole and Keidel upon which the recommendations of the referee were based were sent to the manufacturer for comment. The reply of the manufacturer contained no evidence which justified the Council in modifying the action already taken. Publication of the report was therefore authorized.
W. A. Puckner, Secretary.
Biniodol was submitted to the Council by the manufacturer, Charles C. Yarbrough, Memphis, Tenn. The manufacturer claims the product is a solution of 1 per cent. of red mercuric iodid and 2.75 per cent. of guaiacol in bland vegetable oil. It is marketed with the implication that it is new and superior to other oil solutions of mercuric iodid. For instance:
“... it is a straight solution of this mercurial compound, as no alkaline iodide or other chemical is used to bring about the solution.” “... It is probably the first and only one-percent oil solution of straight mercury biniodide made in America....”
[The manufacturer, in a letter addressed to the secretary of the Council, explains: “By straight solution, I mean that the solution of the red mercuric iodid is effected without the aid of any alkaline iodid or other chemicals.... Biniodol was first offered early in 1912 ...”]
“Biniodol is, therefore, superior and much to be preferred to other mercurials used for like purposes. It is highly active therapeutically, producing the desired effects, usually without the inevitable disadvantages of other mercurials. It rarely causes salivation, diarrhea, or other symptoms of mercurial intolerance, even when pushed to full therapeutic effect and when given for a considerable period of time. Nor does it produce anemia.”
The Chemical Laboratory of the American Medical Association found that Biniodol contained 1 per cent. of mercuric iodid and 2.5 per cent. of guaiacol; hence the composition is essentially as claimed. It is not true, however, that Biniodol is the “first and only one-percent solution of straight mercury biniodide made in America.” As shown in The Journal A. M. A., Dec. 9, 1914, p. 2247, formulas by Lemaire and Dunning for making a “straight” solution of mercuric iodid were published in this country in 1909 and 1910, respectively. Moreover, a 1 per cent. solution of mercuric iodid in oil is on the market and is described in New and Nonofficial Remedies.
To determine whether or not Biniodol is “superior and much to be preferred to other mercurials used for like purposes,” the Council secured the cooperation of the Department of Dermatology and Syphilology of the Western Reserve University cooperating with the Cleveland City Hospital, and of the Johns Hopkins Hospital. Each received three samples, labeled respectively, 1, 2 and 3: 1 contained Biniodol; 2, a 1 per cent. solution of mercuric iodid in oil; 3, a solution made up according to the formula of Biniodol, namely, 1 per cent. of mercuric iodid and 2.5 per cent. of guaiacol in oil. All the solutions were sterile. The investigators were not informed which preparation was designated by the respective numbers, but they were asked to use the preparations when intramuscular injections of a 1 per cent. oily solution of mercuric iodid were indicated, and to note what differences, if any, were observed following the use of the different solutions regarding pain, discomfort, induration and any other evidences of effects of the medicaments.
The Cleveland investigator reports that the patients were more or less confused in their replies to inquiries and gave rather indefinite and conflicting answers. After carefully tabulating the replies, however, the following summary resulted:
1 was worse than 2 or 3 in 6 cases.
2 was worse than 1 or 3 in 5 cases.
3 was worse than 2 or 1 in 1 case.
The report from Johns Hopkins records a series of 117 injections followed by the estimated reactions recorded below:
1. Severe, 13; mild, 14; none, 4; unrecorded, 8 = 39
2. Severe, 5; mild, 15; none, 16; unrecorded, 5 = 41
3. Severe, 7; mild, 25; none, 3; unrecorded, 2 = 37
That is, when recorded in percentages:
1. (Biniodol) severe, 33.3; mild, 35.9; none, 10.3; unrecorded, 20.5.
2. (Without guaiacol) severe, 12.2; mild, 36.8; none, 39.0; unrecorded, 12.2.
3. (With guaiacol) severe, 18.9; mild, 67.5; none, 8.1; unrecorded, 5.5.
The manufacturer of Biniodol supplied the names of several physicians who have used that preparation in their practice. Correspondence with these elicited the following statements:
One had used Biniodol in forty-eight cases and states that “only a few patients complain of pain at all and then only of a general soreness in the muscle.” This physician reports a limited experience with the use of another manufacturer’s “mercury biniodide oil solution” (apparently six cases), but severe pain following the injections made it necessary to abandon that preparation.
Another of these physicians named by the manufacturer, without reference to any series of cases, reports that “Biniodol is superior to any [oily solution of mercury biniodid] that I have tried.”
A third physician has “used it [Biniodol] a few times” and is “convinced that it has no special action or virtue” over “any red mercuric iodide in oil.”
This evidence, in its most favorable estimate, shows Biniodol to be a good 1 per cent. solution of mercuric iodid in oil, but fails to justify attributing to the preparation any unique characteristics. The preparations made in the laboratory were as satisfactory, or better than the Biniodol, and the presence or absence of the guaiacol was of no consequence.
Biniodol conflicts with Rule 6, since claims of superior therapeutic efficiency made for it are not established; and with Rules 8 and 10, since it is an unessential modification of an established nonproprietary article marketed under a proprietary name.
In view of the foregoing, the referee recommends that Biniodol be not accepted for New and Nonofficial Remedies, and that this report, including the clinical investigations of Drs. Cole and Keidel, be authorized for publication.
At the request of Prof. Torald Sollmann of the Council on Pharmacy and Chemistry of the American Medical Association, we made a comparative study of several oily preparations of red mercuric iodid for intramuscular injections in syphilis.
The information, concerning the preparations submitted to the investigators, was as follows:
“It is desired to ascertain whether there is any difference between three preparations, each containing 1 per cent. of mercuric iodid, as to pain, discomfort, induration, etc. The preparations will be labeled “1,” “2” and “3.” They will be sterile.
“One of these preparations will be a plain solution in oil; another will contain, in addition, 2.5 per cent. of guaiacol; the third will be a proprietary preparation containing the guaiacol.
“It is also desirable to know how the oily solution compares with the plain watery solution; but this is of secondary importance.”
The preparations all had the same appearance. The patients were taken indiscriminately, and we attempted to keep them on the injections as long as possible, in order to compare symptoms. Owing, however, to discharge from hospital, symptoms of mercury intoxication, etc., we were unable in all cases to give a thorough trial with each preparation.
In all, eleven patients were treated and seventy-one injections given—by which time our experimental supply was exhausted.
In each case the drug was given intramuscularly in the buttocks and the patients carefully observed for subjective symptoms of pain and for objective symptoms of swelling, induration, abscess formation, etc. The details are given in Table 1.
As will be noted, in several of the cases the patients were more or less confused and gave rather indefinite and conflicting answers. In attempting to compare the results from the different drugs, by careful tabulation one finds that symptoms were more marked with the respective sample as follows:
Preparation 1 was worse than Preparation 2 or 3 in six cases.
Preparation 2 was worse than Preparation 1 in two cases.
Preparation 2 was worse than Preparation 3 in five cases.
Preparation 3 was worse than Preparations 2 or 1 in one case.
TABLE 1.—DETAILS OF INVESTIGATION BY DR. COLE*
| Case | Age | Sex† | Date | Prepara- tion | Dose, Grain | Symptoms | |
| Induration—Pain | Objective | ||||||
| 1 | 25 | ♂M | 6/11/16 | 2 | 1⁄5 | None | Still painful |
| 6/12/16 | 1 | 1⁄4 | None | None | |||
| 6/13/16 | 2 | 1⁄5 | None | Quite painful | |||
| 6/14/16 | 2 | 1⁄4 | Hurt for some time | Very tender | |||
| 6/16/16 | 2 | 1⁄5 | Hurt for some time | Very tender | |||
| 6/17/16 | 3 | 1⁄5 | Not so painful | Less tender than with Preparation 2. Can sit on area; as needle prick is only place that it hurts | |||
| 6/18/16 | 3 | 1⁄5 | Not so painful | ||||
| Discontinued (salivation) | |||||||
| 6/22/16 | 2 | 1⁄4 | Hurt, but not so long | Slight induration and slight tenderness | |||
| 6/24/16 | 2 | 1⁄4 | Hurt, but not so long | Pain “dead stinging” lasts 1 hour | |||
| 6/25/16 | 1 | 1⁄4 | Not so bad | About the same | |||
| 2 | 32 | ♂M | 6/24/16 | 2 | 1⁄4 | Some pain | No induration |
| 6/25/16 | 1 | 1⁄4 | More pain | Slight induration | |||
| 3 | .. | ♂M | 6/12/16 | 1 | 1⁄5 | No symptoms | Painful |
| 6/13/16 | 2 | 1⁄4 | No symptoms | Painful | |||
| 6/14/16 | 2 | 1⁄4 | Says the last two have hurt the more | Painful | |||
| 6/16/16 | Arseno- benzol | ||||||
| 6/17/16 | 3 | 1⁄5 | More pain than previously | Small painful area | |||
| 6/17/16 | 3 | 1⁄5 | |||||
| 6/18/16 | 3 | 1⁄5 | Not so much pain: in fact, patient says he is over it in a very short while; complained of last one | Some induration at site of injections | |||
| 6/19/16 | 3 | 1⁄5 | |||||
| 6/20/16 | 3 | 1⁄4 | |||||
| 6/21/16 | 3 | 1⁄4 | |||||
| 6/22/16 | 2 | 1⁄4 | Some pain | Considerable tenderness now after so many injections | |||
| 6/24/16 | 2 | 1⁄4 | Not so much as previously | ||||
| 6/25/16 | 1 | 1⁄4 | |||||
| 4 | 36 | ♂M | 6/22/16 | 2 | 1⁄4 | No pain | No tenderness |
| 6/24/16 | 2 | 1⁄4 | Some pain | Some tenderness | |||
| 6/25/16 | 1 | 1⁄4 | Could not sleep at night | Some tenderness; slight induration | |||
| 5 | 32 | ♂M | 6/20/16 | 3 | 20 minims | Some pain | No induration |
| 6/21/16 | 3 | 25 minims | Some pain | ||||
| 6/23/16 | 2 | 1⁄4 | Worse pain | No induration | |||
| 6/24/16 | 2 | 1⁄4 | Worse pain | ||||
| 6/25/16 | 1 | 1⁄4 | Worse than any | Slight tenderness | |||
| 6 | 20 | ♂M | 6/ 8/16 | 1 | 1⁄6 | Very little | |
| 6/10/16 | 1 | 1⁄5 | Very little | ||||
| 6/13/16 | 1 | 1⁄4 | Very little | ||||
| 6/14/16 | 2 | 1⁄4 | Bothered more than others | ||||
| 6/17/16 | 2 | 1⁄5 | Quite a little pain | Still some soreness | |||
| 6/18/16 | 2 | 1⁄5 | Quite a little pain | Still some soreness | |||
| 6/19/16 | 3 | 1⁄4 | Considerably less pain than with Preparation 2 | Very little tenderness | |||
| 6/20/16 | 3 | 1⁄4 | |||||
| 6/21/16 | 3 | 1⁄4 | |||||
| 7 | 30 | ♂M | 6/12/16 | 1 | 1⁄5 | Little pain | None |
| 6/13/16 | 2 | 1⁄4 | No pain | ||||
| 6/14/16 | 2 | 1⁄5 | Some pain | ||||
| 6/16/16 | Arseno- benzol | ||||||
| 6/17/16 | 3 | 1⁄5 | Not so much | No tenderness | |||
| 6/18/16 | 3 | 1⁄5 | Not so much | No tenderness | |||
| 6/19/16 | 3 | 1⁄5 | Very little pain | Only slight amount of induration | |||
| 6/20/16 | 3 | 1⁄4 | |||||
| 6/21/16 | 3 | 1⁄4 | |||||
| 6/22/16 | 2 | 1⁄4 | Some pain | Some little induration | |||
| 6/24/16 | 2 | 1⁄4 | Considerable pain | Some induration | |||
| 6/25/16 | 1 | 1⁄4 | “Fine” | Slight induration | |||
| 8 | 28 | ♂MM | 6/13/16 | 2 | 1⁄5 | Little pain | Little pain afterward |
| 6/15/16 | 2 | 1⁄5 | Little pain | Little pain afterward | |||
| 9 | 28 | ♀M | 6/17/16 | 2 | 1⁄5 | Some complaint of pain. Fairly severe | Very little induration |
| 6/18/16 | 2 | 1⁄5 | |||||
| 6/19/16 | 3 | 1⁄5 | Some pain; says these have hurt very much less than others | Very slight induration | |||
| 6/20/16 | 3 | 1⁄4 | |||||
| 6/21/16 | 3 | 1⁄4 | |||||
| 10 | 37 | ♂M | 6/12/16 | 1 | 1⁄5 | No symptoms | None |
| 6/13/16 | 1 | 1⁄4 | No symptoms | None | |||
| 6/14/16 | 1 | 1⁄5 | No symptoms | None | |||
| 6/15/16 | 3 | 1⁄5 | No symptoms | None | |||
| 6/16/16 | Arseno- benzol | ||||||
| 6/17/16 | 3 | 1⁄5 | “Much less pain than biniodid or grey oil” | None | |||
| 6/18/16 | 3 | 1⁄5 | No complaint | None | |||
| 6/19/16 | 3 | 1⁄5 | Says he is over it in one hour | Some induration at site of injection | |||
| 6/20/16 | 3 | 1⁄4 | |||||
| 6/21/16 | 3 | 1⁄4 | |||||
| 11 | 30 | ♀M | 6/11/16 | 1 | 20 minims | Considerable; not so much | Considerable pain and tenderness on palpation over area |
| 6/12/16 | 2 | 20 minims | |||||
| 6/13/16 | 1 | 25 minims | Not much pain | Indurated area at pt. of each. Painful | |||
| 6/14/16 | 1 | 25 minims | Not much pain | Slight induration | |||
* The diagnosis in Case 5 was primary syphilis, and in the other cases, secondary syphilis.
† In this column, ♂M indicates male, and ♀F female. In no case did Wassermann become negative.
The criticism may be raised that the number of cases and of injections is too small to permit the drawing of any just conclusions. Even should we grant it, the statistics certainly do not prove any marked superiority of any one of the preparations over the others. We wish to thank Dr. Sollmann for advising and directing us in this work, and Drs. Bailey, Bernstein, Markus and Reycraft for assistance in carrying it out.
Twenty cases were chosen at random from the syphilitic patients attending the clinic. They were given intramuscular injections of the three solutions, in amounts varying from 1 to 2 c.c., at intervals (in most instances) of two days. The injections were invariably made into the gluteal muscles, at depths of from 2 to 21⁄2 inches, and ordinary care exercised to preserve asepsis. After injection the patient was allowed to depart, and the result was recorded at the succeeding visit. The result was determined from the patient’s statement and our examination. Some patients received injections of only one solution; some were treated with first one and later with another, and one patient received all three at different times. The solutions were never mixed for a single injection, of course.
TABLE 2.—REACTIONS IN TWENTY CASES REPORTED BY DR. KEIDEL
| Preparation | Reactions | Number of Injections | |||
| Severe | Mild | None | Undetermined | ||
| 1 | 13 | 14 | 4 | 8 | 39 |
| 2 | 5 | 15 | 16 | 5 | 41 |
| 3 | 7 | 25 | 3 | 2 | 37 |
| —— | |||||
| 117 | |||||
The solutions are understood to contain a 1 per cent. solution of red mercuric iodid in oil, two of them containing in addition 2.5 per cent. of guaiacol, one of these being a proprietary preparation. The solutions are designated as Preparations 1, 2 and 3, respectively, corresponding to the numbers on the labels of the bottles in which they were originally received. The local reactions are recorded as “severe” (S), “mild” (M), “none” (O) and “Undetermined” (U). By “severe” is meant very severe pain lasting for from several hours to several days; by “mild” is meant slight pain or numbness for several hours, or less than an hour; “none” indicates that there was no local reaction, and “undetermined,” that the patient has failed to return after the last injection.
In Table 3 all the details of the investigation are recorded. Under “Local Reaction,” the letters represent the type of reaction after each injection, in the order in which they were given; when two solutions were used in the same case, the letters represent the reactions following the solution opposite which they stand. In the fifth column the plus and minus symbols indicate the Wassermann reaction; plus indicates a completely positive, and minus a completely negative reaction. When there is only one sign, it refers to the reaction at the end of treatment; when there are two, to the reaction before and after. The seventh column shows the clinical result at the end of treatment; when no note is made, it means that there was no change noted. In the eighth column are noted any objective results observed at the time of examinations of the patients.
The injections were made and the result charted by Dr. E. L. Zimmermann, of my staff, under my directions and supervision.—(Abstracted in The Journal A. M. A., Feb. 24, 1917.)
TABLE 3.—DETAILS OF INVESTIGATION BY DR. KEIDEL
| Case | No. | Prepar- ation | Local Reaction | Total Amount Solution Given, C.c. | Duration of Treatment | Effect on Wasser- mann | Type of Case | Result | General Remarks |
| 1 | 3 | 2 | OOO | 3 | 6 da. | + | Latent | ||
| 2 | 5 | 2 | MOSMS | 5.6 | 9 da. | + | Gummas | Marked improvement | |
| 3 | 7 3 | 1 2 | MMM; others U UUU | 9.5 | 3 mo. | – to + | Latent | ||
| 4 | 1 | 2 | U | 0.75 | ... | + | Latent | ||
| 5 | 4 | 1 | SSSM | 4.4 | 9 da. | – | Gummas | After 4th injection, developed diarrhea; melena | |
| 6 | 9 | 1 | OOUMSOSMU | 9.1 | 1 mo. | – | Latent | ||
| 7 | 2 | 3 | MM | 3.8 | 2 da. | + | Latent | Well tolerated | |
| 8 | 7 | 2 | OOOOMOU | 9.6 | 17 da. | + to + | Primary | Primary healed | |
| 9 | 4 | 1 | SMMU | 5.5 | 9 da. | + | Gumma | Improved | |
| 10 | 3 | 3 | MSS | 3 | 6 da. | + | Palmar syphilis; tertiary | Markedly improved | |
| 11 | 7 | 3 | MSMMMMM | 10.6 | 13 da. | + to + | Latent | ||
| 12 | 3 2 | 2 1 | MMO SM | 5.4 | 14 da. | + | Secondary (papular) | Rash disappearing | Developed toxic erythema on thighs. Cleared up on stopping HgCl2 and under local treatment |
| 13 | 10 | 3 | MMMMMMMM MMU | 12.6 | 20 da. | + to + | Secondary (lichen syph.) | Rash not improved | Small induration following injection of 1.2 c.c. |
| 14 | 6 2 | 2 1 | OOMSMM SM | 7.2 | 17 da. | + to + | Old cerebro- spinal syphilis | Responded to doses of 1 c.c. with salivation; fever after injection of 1.2 c.c. | |
| 15 | 4 | 1 | SOMS | 4.2 | 7 da. | + to + | Secondary (condylomas) | No improvement | |
| 16 | 9 2 | 3 2 | OMOMMSMSO SO | 10.4 | 12 da. | + | Secondary (pustular syph.) | Pustules dried up; head- ache and fever gone | Slight gingivitis following dose of 1.5 c.c. |
| 17 | 5 2 | 1 2 | SSMSU MS | 13.3 | 18 da. | + to + | Tertiary; aortitis | General condition im- proved | |
| 2 | 3 | MS | |||||||
| 18 | 4 | 2 | OOMM | 9.5 | 13 da. | – to + | Latent | ||
| 2 | 1 | MM | Markedly improved | ||||||
| 19 | 2 | 3 | MU | 2.5 | 5 da. | + | Gumma | ||
| 20 | 5 2 | 2 3 | MMMMO MS | 9 | 14 da. | + to + | Latent | Marked general improvement | Small induration following No. 3 |
Following inquiries, the Council took up for consideration “Corpora Lutea (Soluble Extract),” marketed by Parke, Davis & Co. in the form of ampules and proposed for hypodermic administration. The report which appears below was submitted to the Council by a committee, and was adopted by the Council. Corpora Lutea (Soluble Extract) was declared inadmissible to New and Nonofficial Remedies, and publication of the report authorized.
W. A. Puckner, Secretary.
Corpora Lutea (Soluble Extract) has not been submitted by the manufacturer. The information of the referee is based, therefore, on the claims made in the trade package, and on the statements in the price list. These show that the product is essentially secret and claims made for the actions and uses of the preparation do not make clear the essentially experimental status of the article, and are therefore misleading.
Conflict with Rule 1.—No definite statement of composition appears beyond the indefinite claim that it is an aqueous solution of “soluble Corpora Lutea Extract,” each ampule corresponding to 0.2 Gm. of desiccated gland. How these soluble products are obtained, whether they represent all the water-soluble principles, or whether some have been eliminated, are questions that are not answered. Yet such information is essential to intelligent and scientific use, for, as there is no method of standardization, the method of preparation is the only mark of identity. For instance, we do not know at this time whether proteins have anything to do with the supposed value of corpora lutea. It is, therefore, essential to know whether or not the proteins have been eliminated.
Conflict with Rule 6.—The circular in the package advises the hypodermic use of this extract, not only in functional amenorrhea and the ordinary reflex consequences of physiologic or artificial menopause, but also in:
“ ‘neurasthenic’ symptoms during menstrual life”;
“sterility, not due to pyogenic infection or mechanical obstruction”;
“repeated abortions, not due to disease or mechanical factors”;
“hyperemesis in the early months of pregnancy.”
These are not stated merely as conditions in which various enthusiasts have tried corpus luteum, but as conditions “for which it will be found serviceable.”
It is not necessary to inform the medical profession that this statement is calculated to raise expectations which cannot possibly be fulfilled. Even the manufacturers seem to realize this; at least they speak somewhat indefinitely of “suitable cases,” “good judgment,” “real indications,” etc. But they proceed to nullify this warning—if it was intended as a warning—by their illustrations of unsuitable cases, for instance, “amenorrhea due to extreme anemia, dysmenorrhea due to cervical stenosis,” etc. Finally, they sum up the case:
“Therefore, additional emphasis on the necessity for the proper selection of cases is essential in order that this useful preparation may not be unjustly discredited.”
How these cases of sterility, abortions, etc., are to be selected is not revealed. In other words, the restriction is no more than a convenient device by which every improvement is to be attributed to the medicine, and every failure to the physician.
The referee recommends that Corpora Lutea (Soluble Extract), Parke, Davis & Co., be held ineligible to N. N. R., because it is a secret preparation advertised under extravagant claims.
[Editorial Comment.—Was it not in Weir Mitchell’s “Adventures of François” that the itinerant promised to pull teeth without any pain, if the patient would hold absolutely still? And, mirabile dictu, the ones who suffered were those who had not held absolutely still!]—(From The Journal A. M. A., April 7, 1917.)
The Council held that the contribution from the A. M. A. Chemical Laboratory, “Wheeler’s Tissue Phosphates,” demonstrates that this is a semisecret, complex and irrational preparation, sold with misleading claims concerning its medicinal constituents and therapeutic properties.
The Council directed that the report be included with the Annual Council Reports and declared Wheeler’s Tissue Phosphates in conflict with Rules 1, 6, 8 and 10.
W. A. Puckner, Secretary.
“Wheeler’s Tissue Phosphates,” known also as “Compound Elixir of Phosphates and Calisaya,” is advertised as a nerve food and a nutritive tonic. The label states that it contains calcium, iron, sodium trihydrogen phosphates, alkaloids of Peruvian bark with 121⁄2 per cent. of alcohol. The preparation is sold by the T. B. Wheeler, M. D. Co., of Rouses Point, New York. According to the manufacturer, Wheeler’s Tissue Phosphates
“... is an inorganic combination of the phosphates of iron and calcium and hydrogen (phosphoric acid) together with hydrochloric acid, hydrocyanic acid, and quinine, cheerful coloring, and a delicious, cordial-like flavoring.”
“... The iron is the green, inorganic phosphate and the calcium the simple white phosphate of your early student days....”
The preparation is a red liquid, having an acid reaction, a sweet-bitter taste and the odor of wild cherry. Qualitative tests indicated the presence of calcium, iron, a phosphate, a chlorid, a sulphate, quinin or cinchona alkaloids, alcohol, sodium, cochineal coloring and invert sugar. Ammonium salts, glycerol, citrates or lactates were not found. From the quantitative values obtained the preparation may be taken to represent:
Sp. gr at 25C./25C. | 1.1087 |
Alcohol (per cent, by volume) | 11.35 |
| Gm. per 100 c.c. | |
Calcium phosphate [Ca3(PO4)2]* | 0.397 |
Iron phosphate (FePO4.4H2O)* | 0.068 |
Chlorid (as hydrochloric acid) | 0.407 |
Sodium sulphate (Na2SO4.10H2O) | 0.043 |
Quinin sulphate (U. S. P.) | 0.041 |
Sodium phosphate (Na2HPO4.12H2O) | 0.065 |
Invert sugar | 26.824 |
Water, cochineal and flavor, to make | 100 c.c. |
* It should be understood that the calcium and iron salts are held in solution by the hydrochloric acid.
The dose of Wheeler’s Tissue Phosphates recommended by the manufacturer is a tablespoonful or about 15 c.c. (1⁄2 oz.). The total calcium in a dose of the preparation is equivalent to about one-sixth of an average dose of the official calcium chlorid, and the total phosphate to each dose is equivalent to about one-fourth of a dose of the official diluted phosphoric acid. Each prescribed dose of the preparation contains about 0.01 gm. (2⁄13 grain) of iron phosphate or about one twenty-fifth of the average dose, and to obtain a Pharmacopeial dose of iron phosphate the patient would be obliged to take three-fourths of the contents of an entire bottle—or 12 ounces—of the preparation. If it be assumed that all of the chlorid present is in the form of free hydrochloric acid, each dose of the preparation contains the equivalent of about two-thirds of one Pharmacopeial dose of diluted hydrochloric acid. Each dose of the preparation contains about 0.0062 gm. (1⁄10 grain) of quinin sulphate, or about one-sixteenth of the average tonic dose. In other words, to obtain the amount of quinin sulphate given in the U. S. Pharmacopeia as the tonic dose, the patient would be required to swallow 71⁄2 fluidounces of the proprietary preparation, or the contents of nearly half a bottle. The fallacy of prescribing Wheeler’s Tissue Phosphates either for its quinin or its iron content is apparent.
Wheeler’s Tissue Phosphates is, then, a mildly bitter flavored syrup which contains nearly 12 per cent. of alcohol, small quantities each of calcium phosphate and hydrochloric acid and insignificant amounts of iron and quinin salts. In other words, essentially it is a sweetened solution of small quantities of calcium phosphate in very dilute hydrochlorid acid together with 12 per cent. of alcohol.
Bearing in mind the analysis of the preparation, how ludicrous some of the claims appear:
“Tissue Phosphates is not a hypophosphite preparation; it is not a combination of glycerophosphates or other organic salts, or so-called peptonates and manganates, all recently condemned by the best therapeutic opinion here and in Europe, as much slower and less active than the simpler salts. The iron is the green, inorganic phosphite and the calcium the simple white phosphate of your early student days. Nature takes these simple salts and builds them rapidly into lecithin, bone, and other tissue, without the delay incurred by splitting up the organic salts before she can recombine them.”
“Tissue phosphates is in fact a chemical food.”
“The formula, suggested by Professor Dusart, of Paris, combines in an easily assimilable and agreeable cordial; medium medicinal doses of Phosphorus, the Generator of Nerve Force; Calcium Phosphate, for Cell Development and Nutrition; Sodium Phosphate, a stimulant of Liver and Pancreas and Corrective of Acid Fermentation in the Alimentary Canal; Iron, generating in the Blood, Heat and Motion, Phosphoric Acid, Tonic in Sexual Debility; Alkaloids of Calisaya, Antimalarial and Antipyretic; Extract of Wild Cherry, Tonic, yet Calming Irritation and Diminishing Nervous Excitement; Ethyl Alcohol 12.5%; and Aromatics.”
Although the claim is made that the “formula” of Wheeler’s Tissue Phosphates has been “suggested by Professor Dusart,” such of Dusart’s papers as were available in this country111 failed to disclose any “formula” that was at all comparable to this product.
[Editorial Note.—The investigation verifies facts that must be obvious to every physician who has given the matter thought. “Wheeler’s Tissue Phosphates” is an unscientific, shotgun mixture whose most active and powerful drug is the alcohol it contains. That it was not years ago relegated to the realms of obsolete and discarded preparations is a commentary alike on the lack of scientific discrimination and the persuasive power of advertising. While in the past “Wheeler’s Tissue Phosphates” has been advertised extensively in medical journals, it seems that now the chief, if not the only beneficiary of the advertising appropriation for this product is the New York Medical Journal, which weekly heralds the “Delicious” and “Sustaining” qualities of “The Ideal Tonic for Fastidious Convalescents.”]—(From The Journal A. M. A., May 5, 1917.)
Specific lactagogues—drugs which stimulate the secretion of milk—are unknown to science. Yet medical publications give space to advertisements of a proprietary—“Nutrolactis”—which is said to increase the milk supply of nursing mothers. Since dependence on a preparation of this kind is likely to cause neglect of the only means of increasing a scanty milk supply of nursing mothers—care of the general health and a sufficient quantity of proper food—this proprietary and the drug “goat’s rue,” (Galega officinalis) which the proprietors hint as being the potent constituent, were subjected to a critical study to determine their possible influence on milk secretion. For this purpose the Council secured the help of A. J. Carlson, Ph.D., professor of physiology, University of Chicago. Dr. Carlson, with the aid of A. Woelfel, M.D., and Marian Lewis, Sc.M., undertook to estimate the effect of Nutrolactis and of goat’s rue on nursing dogs and goats with the intention of extending the study to nursing mothers if the animal experiments so warranted. The contribution, “The Alleged Galactagogue Action of Galega and Nutrolactis,” by Marian Lewis and A. J. Carlson from the Hull Physiological Laboratory of the University of Chicago, which appears below, shows that Nutrolactis and goat’s rue are without influence on the milk secretion in nursing animals.
The Council endorsed the work of Lewis and Carlson and held that the claimed galactagogue effects of Nutrolactis and goat’s rue are not substantiated.
W. A. Puckner, Secretary.
CHICAGO
It is well established that the food best adapted to the energy and growth requirements of the infant is normal mother’s milk. Any decrease in quantity or deterioration in quality of the maternal secretion is soon followed by a parallel impairment of growth, loss of weight, or lowered resistance to infection in the infant. The widespread occurrence of deficient milk secretion is a matter of common knowledge. The discovery of true lactagogues, or specific substances which increase the quantity and quality of the milk on being administered to nursing mothers, would therefore be of very great importance. In view of this great medical and economic interest in true lactagogues it is not surprising to find that the medical and biologic literature records discoveries of lactagogues based on hope rather than demonstration, and that spurious lactagogues are on the market.
Some of the factors known to affect milk secretion are general health, food supply, psychic state, and heredity. The mechanism of secretion and the method by which these factors affect it are imperfectly understood. In general it has been observed that milk yield improves both in quantity and in quality with improvement in general health, better food supply, and more favorable psychic state. The influence of heredity is taken advantage of by dairymen who are well acquainted with the potential milk production of the different breeds of cattle.
Among the substances which have been reported to stimulate milk secretion may be mentioned the extract of the posterior lobe of the hypophysis. But pituitary extract is not a true lactagogue, because its action is confined to the smooth musculature of the gland ducts, causing a more or less complete ejection of the milk already formed; it has no effect on the gland cells or the actual secretory process in the direction of increasing the milk yield. Extracts of thymus, corpus luteum, ovaries, uterus, placenta, fetus, and the mammary gland itself have also been reported to have a temporary stimulating effect on the quantity of milk secreted, but when these extracts are given by mouth they are apparently without specific influence on the mammary gland.
Galega, or goat’s rue (Galega officinalis), is an herb described in the National Formulary as being slightly bitter and astringent. In 1873, Gillet-Damotti,112 in a communication to the French Academy, stated that this plant when fed to cows increases the secretion of milk from 35 to 50 per cent. Other French writers have affirmed that goat’s rue is a lactagogue. In Germany, Fragner113 made a preparation called Galegal, using galega as the active principle and combining it with lactose to give it a pleasant taste and make it soluble in water, milk, coffee, and tea. This preparation was reported on favorably by Scherer,114 who asserts that he obtained positive results in fifty-four of the eighty cases in which he used it.
More recently Huët115 tested the effects of Theinhardt’s Hygiama lactogene on four lactating women. This preparation is said to be composed of hygiama,116 galega and anise. Analysis showed that it contains albumins, fat, soluble and insoluble carbohydrates, salts and water. Huët could not observe any influence from the use of this preparation, either on the quantity or on the composition of the milk secreted.
Nutrolactis117 is a commercial preparation sold by the Nutrolactis Company of New York at $1 a bottle. The label states that it contains 5 per cent. of alcohol; that it contains fluid extracts of the family of “galactagogic plants,” and that it is intended to “increase the supply of mother’s milk.” It is recommended to maintain “quality and quantity until the end of normal lactation.” Nutrolactis is also recommended for a mother debilitated by lactation. It is claimed that “Nutrolactis does not force the secretion of milk but merely assists such secretion.” Years ago Millbank118 reported good results from the use of Nutrolactis. After more than a year’s use he concluded that it was more satisfactory than any other lactagogue hitherto employed by him, which is not saying very much, as specific lactagogues are as yet unknown. Nutrolactis is still (1916) extensively advertised in various medical journals as a lactagogue.
The alleged lactagogue action of galega and Nutrolactis was tested on lactating dogs and goats. In these animals the psychic factors, or suggestion, are largely eliminated. If the results had been positive or had indicated lactagogue action, the test would have been extended to nursing women. The puppies and kids were weighed before and after nursing and a record kept of the amount of milk obtained at each nursing (the animals nursing from three to five times daily). The mothers were fed with varying doses of the drugs, and the milk yield compared with that of a control period during which no drugs were administered. An effort was made to keep the conditions of the experiments uniform throughout.
The galega was ground and mixed with the food. The Nutrolactis was mixed with food given by the stomach tube, or in some cases with a spoon. Galega was tested on two goats and Nutrolactis on one goat and nine dogs. The results are given herewith:
Goat 1: Control period, 1,600 gm., milk av. daily yield for 7 days.
Galega period (30 gm. galega mixed with oats), 860 gm., milk av. daily yield for 8 days.
Kids weaned at end of period.
Goat 2: Control period, 1,161 gm. milk av. daily yield for 9 days.
Galega period (30 gm. galega mixed with oats), 860 gm. milk av. daily yield for 8 days.
(25 gm. galega in same way), 810 gm. milk av. daily yield for 10 days.
Control period, 896 gm. milk av. daily yield for 6 days.
Goat 3: Control period, 896 gm. milk av. daily yield for 6 days.
Nutrolactis period (30 c.c. Nutrolactis mixed with oats), 658 gm. milk av. daily yield for
9 days.
Control period, 666 gm. milk av. daily yield for 5 days.
Dog 1: Control period, 176 gm. milk av. daily yield for 7 days.
Nutrolactis period (8 c.c. Nutrolactis by stomach tube), 55 gm. milk av. daily yield for
12 days.
Dog 2: Control period, 189 gm. milk av. daily yield for 6 days.
Nutrolactis period (8 c.c. Nutrolactis by stomach tube), 72 gm. milk av. daily yield for
11 days.
Dog 3: Control period, 93 gm. milk av. daily yield for 8 days.
Nutrolactis period (8 c.c. Nutrolactis on bread), 17 gm. milk av. daily yield for 5 days.
Dog 4: Control period, 28 gm. milk av. daily yield for 7 days.
Nutrolactis period (8 c.c. Nutrolactis by stomach tube), 47 gm. milk av. daily yield for
6 days.
(10 c.c. Nutrolactis by stomach tube), 43 gm. milk av. daily yield for 8 days.
Control period, 41.5 gm. milk av. daily yield for 6 days.
Nutrolactis period (10 c.c. Nutrolactis by stomach tube), 33.5 gm. milk av. daily yield for
4 days.
Dog 5: Control period, 67 gm. milk av. daily yield for 6 days.
Nutrolactis period (10 c.c. Nutrolactis on bread), 81 gm. milk av. daily yield for 6 days.
Dog 6: Control period, 40 gm. milk av. daily yield for 5 days.
Nutrolactis period (10 c.c. Nutrolactis by stomach tube), 33 gm. milk av. daily yield for
8 days.
Control period, 26 gm. milk av. daily yield for 4 days.
Dog 7: Control period, 283 gm. milk av. daily yield for 9 days.
Nutrolactis period (10 c.c. Nutrolactis by stomach tube), 155 gm. milk av. daily yield for
15 days.
(15 c.c. Nutrolactis by stomach tube), 82 gm. milk av. daily yield for 6 days.
Control period, 33 gm. milk av. daily yield for 3 days.
Dog 8: Control period, 238 gm. milk av. daily yield for 8 days.
Nutrolactis period (20 c.c. Nutrolactis on bread), 223 gm. milk av. daily yield for 4 days.
(20 c.c. Nutrolactis on bread), 46 gm. milk av. daily yield for 6 days.
Dog 9: Control period, 223 gm. milk av. daily yield for 6 days.
Nutrolactis period (10 c.c. Nutrolactis on bread), 178 gm. milk av. daily yield for 15 days.
(15 c.c. Nutrolactis on bread), 146 gm. milk av. daily yield for 5 days.
Goat 1 had already been lactating for over two months, and the yield was gradually decreasing at the time the observations were begun. The administration of galega did not check this decrease. Goat 2 should have been a very favorable subject, for the kid was about a week old at the time the observations were begun. Both galega and Nutrolactis caused a decrease in milk yield of this animal. This decrease is perhaps partly due to the animal’s distaste for the drugs and her consequent failure to eat as well as during the control periods.
Administration of Nutrolactis was accompanied by an increase in milk in only two animals, Dog 4 and Dog 5. A detailed examination of the records of these two dogs shows that in both cases there was a progressive increase in milk yield during the control period and that administration of the drug failed to accelerate this increase. On the contrary, the curve for Dog 5 takes a sudden drop immediately after the first administration of the drug.
The records of Dogs 6 and 7 show that the yield during the second control period is lower than that of the preceding periods. Although the administration of the drug in both cases was followed by a decrease in the yield, it may be urged that the drug has some lactagogue action, for its discontinuance was followed by a decrease in yield. This effect, however, is also apparent rather than real, for the data show a gradual falling off in yield during the period of administration of the drug, which decrease was not accelerated by withdrawing the drug.
Our data show that galega and Nutrolactis, when taken by mouth, and the elements of suggestion excluded, had no beneficial effect on lactation—at least in so far as the quantity of milk is concerned.—(From The Journal A. M. A., May 26, 1917.)
The following report on “The Russell Emulsion” and “The Russell Prepared Green Bone,” marketed by the Standard Emulsion Company, was submitted to the Council by a referee. The Council endorsed the report and authorized its publication.
W. A. Puckner, Secretary.
The Russell Emulsion is put up in a neat package and advertised in an attractive pamphlet, on the cover of which we are told: “Truth Always Justifies The Superlative Degree.” As what follows in the booklet and in the printed circulars certainly does not lack superlatives, this is doubtless a warning.
In addition to the pamphlet and circular advertising, the product seems to be systematically boomed by a lecture scheme in which one Dr. Hague talks before medical societies and distributes advertising matter. The lecture is succeeded by a follow-up letter scheme through which matter is sent to members of the society. Hague ostensibly discusses “lime starvation in tuberculosis,” but medical societies soon learned to estimate his work as essentially to advertise the Russell products. Last April the Medical Society of the State of Pennsylvania sent out a circular letter to its county organizations on the subject of the Russell-Hague propaganda which opens in this way:
“You have doubtless received a letter from Dr. William Grant Hague of New York, offering to address your county society on Tuberculosis. After due investigation, it is respectfully suggested that it may not be desirable to ask him to address your society....”
The statements in the pamphlet and circular published are typical of the whole method of exploitation. For example, can such claims as these be surpassed by the veriest quack?
“Science cannot improve the means employed in producing The Russell Emulsion.”
“Genius has not devised better methods than are used in manufacturing The Russell Emulsion.”
“Money cannot buy better products than are used in The Russell Emulsion.”
“Experience cannot suggest a more nutritious combination of fats than we use in The Russell Emulsion.”
The emulsion is said to be made of equal parts of beef-fat, coconut, peanut and cottonseed oils, held in suspension by albumin. The latter we are told is applied to each globule of the emulsion by an “elaborate technical process” devised by Dr. Russell. The mixture is everywhere spoken of as a “physiological” emulsion, but the word is always in quotation marks. Why it is called “physiological” is not clear, but the term may be counted on to impress the unthinking or the unscientific.
Numerous false and exaggerated statements are made about this “physiological” emulsion with reference to food value. For instance:
“The nutritional value of fats differ; the nutritional value of these fats and their increased efficiency by combination over all others have been determined by extensive clinical observation.”
And also:
“The Russell Emulsion is approximated in food value by no other emulsion or food product in existence.”
“A ‘physiological’ emulsion is a predigested food. It is absorbed with little assistance from the digestive juices, and with no waste of energy. It is, therefore, the ideal food ...”
These are sample statements found in the pamphlet and accompanying circular. A dozen or more pathologic conditions are mentioned in which this “ideal food” is specifically indicated; but we find, also, this curious statement: “Patients can rarely take this dose [speaking of the maximum dose of 2 ounces night and morning] for more than three or four weeks without showing symptoms of over-feeding.” This unguarded remark about an ingestion of 48 grams of fat daily prompts one to ask what is wrong with the “ideal predigested food.”
Russell is wedded to the idea that “lime starvation” is the main factor in tuberculosis, and insists on the importance of large amounts of fat for the “lime starved.”
“Dr. Russell was the original interpreter of the Lime Starved State and originated The Lime Starvation Treatment in Tuberculosis. He also first pointed out and emphasized the therapeutic importance of regarding the combination of lime phosphate and casein, as brought down by the rennet enzyme, as a chemical union.”
This overworked lime-starvation theory certainly lacks any tangible confirmation (see in this connection a recent paper by Halverson, Mohler and Bergeim, in The Journal, May 5, 1917), and to urge it to promote the sale of a fat preparation is preposterous. On the uninitiated the exaggerated pseudo-scientific language of the pamphlet and circular advertisement will probably make some impression. Unfortunately such things count not only with the layman who, having no technical knowledge of physiology, cannot be expected to weigh the evidence but also with those medical men who, while scientifically educated, are influenced by unscientific claims when plausibly presented. The pamphlet is a striking example of a style which is dangerous because it smacks of science.
The Russell Company sells also a mixture called “Prepared Green Bone,” said to be made by partially digesting ground chicken bones with hydrochloric acid and pepsin and adding glycerin at the end of the digestion. The product is a sticky, unappetizing looking mass, put up in little earthenware boxes and advertised as a lime food, apparently to go along with the fat emulsion. The greater value of a few glasses of milk daily is evidently overlooked.
“The Russell Emulsion” and “The Prepared Green Bone” were declared inadmissible to New and Nonofficial Remedies.
[Editorial Comment.—There are always those who are ready to exploit the unfortunate tuberculous. It is, unfortunately, a fact that many physicians accept as true, statements clothed with obscure and voluminous quasi-scientific verbiage. Such men would laugh at the bald claim that the moon is made of green cheese; when, however, one plausibly and with due solemnity, affirms that the nocturnal luminous earthly satellite is composed of an infinite aggregation of molecules of bewildering and awe-compelling complexity, built up from the recently discovered polypeptids, the whole being of a verdant tint, the person addressed looks impressed and opines that it sounds reasonable! The advertising for The Russell Emulsion and The Russell Prepared Green Bone is dangerous because it appeals to the thoughtless—layman and physician, alike.]—(From The Journal A. M. A., June 23, 1917.)
Brom-I-Phos (National Drug Company, Philadelphia) was submitted to the Council with a label bearing the following statement:
“ ‘ALCOHOL 25 PER CENT.’
COMPOSITION—Per Fluidounce
Iodin | 1 | gr. |
Bromin | 1 | gr. |
Phosphorus | 8-100 | gr. |
Aromatic Base | q. | s.” |
A request for further information in regard to the composition of Brom-I-Phos was sent to the National Drug Company. It was suggested that since the preparation cannot contain the stated amounts of free bromin, free iodin and free phosphorus, the form of combination in which these elements are present should be set forth. In reply, the firm said, first, that “Brom-I-Phos consists of Bromin, Iodin, Phosphorus, Glycerin, Wine, Water and Volatile Oils. The Iodin is rubbed up with a small percentage of Potassium Iodid and 95 per cent. Alcohol, which solution is mixed with a solution of Bromine and Spirits of Phosphorus which are combined with the base and aromatics.” The manufacturer also admitted that phosphorus reacts with bromin and iodin and that other reactions might occur, but maintained that it was “justified in assuming the greater part, if not all of these elements, are actually existent in the nascent state,” and asserted that its “printed formula complies with our working formula in point of quantities involved as well as existence of elements in an uncombined state.”
The A. M. A. Chemical Laboratory reported, on the contrary, that no free phosphorus, free bromin or free iodin could be found in Brom-I-Phos, and that no bromate or iodate could be found; bromid and iodid were present. The addition of silver nitrate to an acidulated portion, diluted with water, gave an amount of silver halid roughly agreeing with that which would be obtained had the claimed amount of bromin and iodin (together with some potassium iodid) been used in the preparation of Brom-I-Phos and in the process of manufacture become converted to bromid and iodid.
The Council declared Brom-I-Phos inadmissible to New and Nonofficial Remedies, for conflict with Rules 1, 4, 6, 8 and 10.
The statement of composition is unsatisfactory and misleading in that it suggests that the preparation contains bromin, iodin and phosphorus in the free (elementary) state. The presence of the potent elementary phosphorus is especially suggested by the small amounts of “phosphorus” declared.
The following statement on the label of the trade package constitutes an indirect advertisement to the public:
“INDICATIONS: Scrofula, Coryza, Hay Fever Necrosis, Bronchial and Throat Affections, Catarrhal Pneumonia, Glandular enlargements of the Spleen, Thyroid, and Lymphatics, Rickets and Syphilis.”
The following claims are therapeutic exaggerations:
“The Ideal Alterative”
“... indicated in all cases where an alterative is desired ...”
“The association of Bromin with Iodin in Brom-I-Phos materially enhances the product in the treatment of chronic affections of the skin, depraved conditions of the mucous membranes, tertiary syphilis, glandular enlargements, etc.”
In that it suggests that the phosphorus in Brom-I-Phos is more readily assimilated than ordinary phosphate, the following is misleading:
“The Phosphorus contained in Brom-I-Phos is readily assimilated and at once acts as a nutrient to the nervous and osseous structures of the body, stimulates metabolism and increases mental activity.”
The recommendation: “Your specification of Brom-I-Phos in the treatment of Syphilitic cases will immediately prove beneficial to the patient” is not supported by evidence. The name does not indicate that Brom-I-Phos is an alcoholic preparation with iodid as its essential constituent, but suggests that phosphorus is an important constituent, whereas the amount of phosphate or phosphite, produced by the action of iodin on elementary phosphorus (if the amount of phosphorus used in making the preparation is correctly stated) is insignificant.
The combination of bromin, iodin and phosphorus, or bromid, iodid and phosphate, is irrational because these elements are not of mutual assistance to each other in the conditions for which Brom-I-Phos is advertised.
The Council’s report was submitted to the manufacturer of Brom-I-Phos for comment; the reply contained nothing to permit a revision of the previous conclusions.
The Council declared Brom-I-Phos inadmissible to New and Nonofficial Remedies.—(From The Journal A. M. A., June 30, 1917.)
Creosote-Delson and Creofos, or Creosote with Hypophosphites, were submitted by the Delson Chemical Co., Inc., New York City. Creosote-Delson is said to be “beechwood creosote from which the irritating and caustic properties are removed by fractional distillation.” It is claimed that Creofos contains “2 grains of Creosote-Delson and 33⁄5 grains of the combined Hypophosphites in each fluidrachm of the mixture or emulsion, the lime salt predominating.” It is also claimed that “the primary object of the hypophosphites in this preparation is that of maintaining the refined creosote in a pure, unoxidized state, and that no particular claim for therapeutic action on their part is advanced.” It is explained further, however, “the addition of the lime was prompted by the belief ... that the fundamental cause of pulmonary tuberculosis is lime starvation....”
The assertions are made that Creosote-Delson is superior to the official creosote because it can be taken “abundantly and persistently without harm to or interference with stomach and kidneys” and can be “taken uninterruptedly and indefinitely,” while the dosage is “unlimited by any former knowledge of Creosote Therapy.” Creosote-Delson is not on the market except in the combination Creofos, although it is supplied on request.
Creofos is advised in the treatment of tuberculosis, whooping cough, measles, “Grippe and Colds,” bronchitis, asthma, “Intestinal Affections (Colitis, Summer Diarrhoea, etc.),” while its use is suggested for the “prevention of the spread of contagious diseases,” and for “preventing contagion in minor contagious diseases at any rate, in schools and families.”
The following advertisement has recently appeared in the New York Medical Journal and in the Therapeutic Gazette:
is the successful development of the most advanced practice in the treatment of infectious diseases. It destroys completely the causative organisms by a bactericide many times more powerful than phenol, yet absolutely harmless to animal life.
Unlike serums, its activity is not confined to any specific disease, and its use insures against sequelae (as pneumonia following grippe).
Especially valuable in the treatment of infants and patients of delicate constitution and in cases where time is of importance.
The Delson Chemical Co. was requested to supply information regarding the identity of Creosote-Delson and to support the claim that although it is “the whole drug” its dosage is “unlimited by any former knowledge of Creosote Therapy.” The reply was virtually an admission that the toxic, caustic, phenolic components of creosote were present in Creosote-Delson just as in the official creosote.
The referee of the Committee on Therapeutics in submitting his report to the Council pointed out that it is difficult to discuss the pharmacologic merits of a semisecret preparation, like Creosote-Delson, claimed to be more acceptable to the human organism than the official product it is intended to supplant, when the action of the parent drug is still questioned or disputed by eminent clinicians.
Absorption experiments have been carried out with creosote and creosote compounds, such as creosote with hypophosphites or calcium or creosote carbonate, chiefly by a study of the elimination products in the urine. But any evidence so far offered that these combinations increase absorption and lessen the irritating, caustic or toxic properties has been wholly inconclusive. The evidence offered by the Delson Chemical Co. presented no control experiments with the official creosote and did not prove that either Creosote-Delson or Creofos was less toxic than a corresponding amount of ordinary beechwood creosote.
The referee concluded that no proof had been offered that these preparations are materially superior to ordinary creosote preparations from the pharmacologic or therapeutic standpoint, and that the claims made for Creosote-Delson and Creofos are unwarranted in the light of our knowledge of the properties of creosote. The advertisement quoted above is an example of unproved and unwarranted claims.
On the recommendation of the referee, the Council declared Creosote-Delson and Creofos inadmissible to New and Nonofficial Remedies, for conflict with the rules as follows:
Creosote-Delson: The information so far available is not sufficient to define the nature, or composition, of Creosote-Delson, or to indicate in how far this product differs, if at all, from the official creosote (conflict with Rule 1). No methods are furnished for determining the identity or composition of Creosote-Delson (conflict with Rule 2). The available information does not show that Creosote-Delson has advantages over creosote (conflict with Rule 6).
Creofos: The composition of Creosote-Delson not having been furnished, the statement concerning the composition of Creofos is also unsatisfactory (conflict with Rule 1). The therapeutic claims are unsubstantiated and grossly exaggerated (conflict with Rule 6). The name is not descriptive of its composition as is required for pharmaceutical mixtures (conflict with Rule 8). There is no evidence that hypophosphites prevent decomposition of creosote (if this occurs). Hence the inclusion of hypophosphites must be considered irrational (conflict with Rule 10).
The Council’s report was sent to the Delson Chemical Co. for consideration. The firm’s reply contained nothing to warrant a revision of the report, and the Council voted that Creosote-Delson and Creofos were inadmissible to New and Nonofficial Remedies and authorized the publication of this report.—(From The Journal A. M. A., July 7, 1917.)
Triner’s American Elixir of Bitter Wine is a wine to which bitter drugs and laxatives have been added. Though evidently intended for public consumption, it is also advertised to physicians, and consequently the Council publishes this report.
Some recent advertisements read:
“It Acts Well and Is Very Palatable. These are the reasons why so many physicians recommend TRINER’S AMERICAN ELIXIR OF BITTER WINE. Free from any chemicals. Prepared from bitter herbs roots and barks of eminent medicinal value and pure natural red wine. A safe relief in auto-intoxication, constipation, weakness, etc. Price $1.00. At drug Stores. Samples gratis upon request only to physicians.”
“A Laxative Tonic. In cases of constipation and its sequelæ, autointoxication, weakness and nervousness you should try Triner’s American Elixir of Bitter Wine. This preparation consists of Cascara Sagrada, Dandelion, Gentian Root, with Licorice in Pure Red Wine as a base, with Aromatics.”
Triner’s American Elixir of Bitter Wine is put up in bottles said to hold 1 pint, 51⁄3 fluidounces. The label declares the presence of from 16 to 18 per cent. alcohol by volume, and states that “no special tax is required by the laws of the U. S. for the sale of this medicinal preparation.” The circular contains the following recommendations for its use:
“... It should be used in all cases calling for a safe evacuation of the bowels, without weakening the body or causing any pain or other discomfort; in loss of appetite, nervousness and weakness.”
“Triner’s American Elixir of Bitter Wine consists of two principal ingredients, viz., Red Wine and Medicinal Herbs.”
“Red Wine strengthens the intestines and regulates their work. It also increases the appetite, stimulates and strengthens the body.”
“Use Triner’s American Elixir of Bitter Wine always when a thorough cleaning out of the intestines is needed. Arrange the dose to suit your condition and habits.”
“In Chronic Constipation the dose of Triner’s American Elixir of Bitter Wine should be increased or taken oftener.”
“Many Female Troubles are caused or aggravated by constipation and ladies should always pay good attention to this fact.”
In addition to Triner’s Elixir of Bitter Wine, the circular—in English, Polish, Russian, Spanish and other languages—advises the use of Triner’s Angelica Bitter Tonic, Triner’s Red Pills, Triner’s Liniment and Triner’s Cough Sedative.
The composition of this “wine”—some bitter drugs, a laxative and a tannin-containing, constipating red wine—and advertising propaganda all tend to the continued use of this alcoholic stimulant and thus to the unconscious formation of a desire for alcoholic stimulation. As the medical journal advertisements may lead physicians to prescribe this secret and irrational preparation and thus unconsciously lead to alcoholism, the Council authorized publication of this report.—(From The Journal A. M. A., July 14, 1917.)
Trimethol is the trade name for a substance said to be trimethyl-methoxy-phenol of the formula C6H(CH3)3(OCH3).OH—1:2:4:5:6, originated by J. T. Ainslie Walker. It is sold as a nontoxic germicide, having a Rideal-Walker phenol-coefficient of 40, even in the intestinal canal. It is described as insoluble in water and not to be decomposed in the alimentary tract, and to be excreted unchanged in the feces.
Trimethol itself is not obtainable. Pharmaceutical preparations—Trimethol Syrup, Trimethol Capsules and Trimethol Tablets, said to contain Trimethol—are prepared by The Walker-Leeming Laboratories and sold by Thos. Leeming and Co., New York.
Trimethol preparations are advertised for use in all conditions dependent on intestinal putrefaction. The advertising claims made are very extensive and some of them give to “Trimethol” the scope of a panacea. For example:
“Physicians are constantly reporting cases where Trimethol has been especially efficient, and describing conditions (until recently not associated with intestinal infection) which have been distinctly benefited by its use. This would seem to bear out the contentions of Charcot and Metchnikoff that 90% of all human ailments have their origin in intestinal infection.
“The careful practitioner, when in doubt, will bear this in mind, now that we have a really efficient and non-toxic intestinal germicide—not a mere antiseptic.”
The Walker-Leeming Laboratories have not formally requested the Council to consider the Trimethol preparations, though in a personal letter to a member of the Council J. T. Ainslie Walker invited an investigation of his compound.
For the investigation of Trimethol and its preparation the Council secured the aid of a bacteriologist who has given much attention to the study of the intestinal flora. The Walker-Leeming Laboratories and J. T. Ainslie Walker were both asked to submit details of experimental studies and also to furnish a supply of the pure “Trimethol.” But the only data sent that had any definiteness set forth the bacterial counts made of plate cultures of stools of one patient before and after the administration of Trimethol Capsules.
The request for the pure substance was refused, on the grounds that the substance was not used in the undiluted form. The failure to furnish the chemical substance claimed as the essential constituent of the Trimethol preparations is to be deprecated if indeed it has not greater significance. At least it made it impossible for the Council’s expert to express his results in terms of absolute Trimethol of established composition. The data obtained apply only to the market preparations claimed to contain Trimethol. So far as the investigation and report go, “Trimethol” is a hypothetical substance.
Clinical or animal tests of the asserted intestinal antiseptics have hitherto given equivocal results because it is impossible, on the one hand, to predict the course of any intestinal infection, or, on the other hand, to determine what effect, if any, was produced by administration of the medicament. It therefore seemed unwise to undertake this line of investigation until the more direct laboratory bacteriologic methods had been exhausted. Consequently the investigator checked, in the first place, the phenol coefficient of one of the Trimethol preparations and then also determined its “penetrability” coefficient. Although by both methods Trimethol was found to be a germicide, the results did not indicate any remarkable potency or other properties suggesting that the drug possessed special therapeutic value. From the results obtained it appeared inadvisable to proceed further with the work until more definite evidence of the nature and of the value of the substance should be at hand. The report of the bacteriologic investigation follows:
“I have made no attempt to study the effects of internal administration of Trimethol on the intestinal flora. The methods available at the present time of enumerating the numbers of viable bacteria in the feces are probably not accurate within 100 per cent. and the precision of such determinations is equally variable. The physiologic factors involved are so complex that they would appear to make a really valuable assay a question of many months’ careful study. If it were possible to administer known amounts of Trimethol, as such, the problem might be worth while; inasmuch as the available reactive substance is not at present quantitatively assayable, this phase of the investigation barely seems practicable.
“ ‘Trimethol Syrup,’ as such, appears to be about 10 per cent. as efficient in its germicidal value as carbolic acid. If the assay, 3⁄4 m. Trimethol per drm. (as the label indicates), is correct, the substance would appear to possess germicidal merit provided enough could be administered, if it is not influenced by passage through the stomach.
“A package containing four four-ounce bottles labeled ‘Trimethol, A Non-Toxic Germicide SYRUP Representing 3⁄4 m. Trimethol per drm., Alcohol 11⁄2 per cent.’ was received at the laboratory Dec. 15, 1916. Later a smaller package containing, according to the label, 100 Trimethol tablets, each 5 gr., representing 11⁄4 m. Trimethol, was received. The tablets were apparently chocolate coated.
“Two separate series of tests were made upon the syrup. (a) Phenol coefficient, using the method outlined in Bulletin No. 82, Hygienic Laboratory, Method of Standardizing Disinfectants With and Without Organic Matter. (b) A Penetrability coefficient by the method of Kendall and Edwards, Journal of Infectious Diseases, 8, 250.
“The former method compares the viability of naked germs in a 1 per cent. carbolic acid solution as a standard, with various dilutions of the germicide to be tested. The latter measures the relative diffusibility and germicidal power of carbolic acid and various dilutions of the germicide to be tested upon Bacillus coli suspended in 1.2 per cent. agar which is molded in cylinders of one centimeter diameter after infection with the organism.
“The first method—phenol coefficient—possesses advantages and disadvantages which are well known and need no mention here. It is worthy of notice, however, that as the death rate of the bacteria increases during the progress of the test, it becomes increasingly difficult to maintain a uniform suspension of living organisms so that each loopful removed shall exactly represent the developmental potentiality of the residual organisms.
“The second method theoretically covers the possibility because all the organisms are immobilized and are exposed to the germicide in direct proportion to its diffusibility until the center of the agar mass is reached, where the residual viable bacteria are presumably located. Inasmuch as the penetrability of an intestinal mass is involved in a discussion of intestinal germicides, the propriety of utilizing this ‘penetrability coefficient’ in this connection is obvious, in spite of its patent shortcomings.
“It is unnecessary to discuss the technique—the standard broth mentioned in the Hygienic Bulletin, a temperature of 70 F., a standard 4 mm. loop and careful attention to dilutions (using distilled water) were all observed. The various dilutions of Trimethol Syrup were made with accurate volumetric pipettes, measuring flasks and distilled water was used as a diluent.
“The results of several determinations, using Trimethol Syrup from three separate bottles, were in sufficient accord to warrant the statement that a dilution of 1⁄10 of Trimethol Syrup was equivalent to a 1⁄100 dilution of carbolic acid, using Bacillus typhosus as the test organism. Both solutions—the Trimethol and phenol—killed the organism in the interval between 71⁄2 minutes and 10 minutes’ exposure. That is to say, our observations indicate that under standard conditions as defined above, a 10 per cent. solution of Trimethol Syrup is equivalent in germicidal powers, as defined by the phenol coefficient to a 1 per cent. solution of phenol. Naturally, no predictions can be drawn from these observations indicative of the value as an intestinal germicide of Trimethol itself.
“The Penetrability coefficient resulted as follows: A 5 per cent. solution of phenol killed Bacillus coli, suspended uniformly throughout a cylinder of 1.2 per cent. agar in the interval between 60 and 90 minutes. A 1 per cent. solution of phenol killed the same organisms under the same conditions in the interval between two and one half and three hours. An undiluted solution of Trimethol Syrup killed the organisms in the interval between two and one half and three hours. A 10 per cent. solution (nine volumes of distilled water to one volume of Trimethol Syrup) failed to kill the organisms in four hours. It would appear that undiluted Trimethol Syrup has the same combined penetrability and germicidal value as a 1 per cent. phenol solution.
“The Phenol coefficient: A 10 per cent. solution of Trimethol Syrup in distilled water (nine volumes of distilled water to one volume of Trimethol Syrup) possesses the same germicidal power as a 1 per cent. solution of carbolic acid. This coefficient takes no cognizance of the actual amount of Trimethol as such—it merely indicates the relative germicidal power of the Trimethol Syrup as sold.”
The preceding report shows that Trimethol Syrup has a phenol coefficient of 1⁄10, and, assuming Trimethol Syrup contains the amount of Trimethol declared, the substance Trimethol would have a phenol coefficient of 81⁄3 instead of 40, as is claimed. According to Kendall and Edwards’ method, the penetrability-germicidal value of the syrup is equal to a 1 per cent. solution of phenol.
The report of the bacteriologist was submitted to The Walker-Leeming Laboratories for comment. The following reply was received from J. T. Ainslie Walker:
(May 22, 1917) “In reply to your letter of the 15th inst., which has just been placed before me on my return to town, I have to inform you that the potent constituent of Trimethol Tablets and Trimethol Syrup is not fully available as a bactericide until it comes in contact with the pancreatic fluid.
“As you will see from the enclosed extracts from clinical reports, the therapeutic value of Trimethol has been well established.
“As regards penetrability, no claim has ever been made for Trimethol in this connection; and, as I pointed out in my original paper (American Medicine, September, 1914), when referring to the independent tests made by Dr. Frederick Sondern, ‘No attempt was made to determine the bacterial content of the solid particles, as in the opinion of the writer sterilization of the interior of these particles is not only absolutely impossible, but wholly unnecessary. The fact of the fluid contents of the canal being sterile may be taken to indicate that the exterior of all solid particles is in a like condition, and therefore harmless. It is the organisms in the fluid portions only that produce the deadly effects through the chemical substances they secrete; those in the interior of the solid portions (i. e., as evacuated) may be disregarded, as they are not available for good or evil.’
“I must confess to no little surprise on learning that your investigator is still using the Hygienic Laboratory method of determining phenol coefficients. I would respectfully suggest that you call his attention to the critical comparison of the Hygienic Laboratory and R.-W. Tests, which he will find in the enclosed reprint from the New York Medical Journal of March 11, 1916: ‘Instead of being an improvement upon the standard R.-W. Test, the so-called Hygienic Laboratory Method is so defective as to be wholly unreliable, and incapable of furnishing results of any scientific or practical value whatever.’ ”
As to the statement that the potent constituent of Trimethol Tablets and Trimethol Syrup is not fully available as a bactericide until it comes in contact with the pancreatic fluid, attention is called to a leaflet, which accompanies each bottle of Trimethol Syrup, that reads:
“Trimethol is insoluble in water, but when properly emulsified has a Rideal-Walker co-efficient of 40; that is to say, it is 40 times more efficient as a germicide than phenol (pure carbolic acid).”
The Trimethol Syrup which was used in the investigation, when mixed with water produced an almost perfectly transparent solution, which justifies the assumption that the proper physical conditions were observed and that this objection is not well founded.
As regards the relation of pancreatic fluid to bactericidal availability of Trimethol, there is little to say, other than that the published statements in the advertising accompanying the packages make no mention of this point. It would be interesting to know what, if any, relation the pancreatic fluid has to this substance, in view of the statement that it “has a Rideal-Walker coefficient of 40.”
The Trimethol “literature” does not throw light on the question, What is the germicidal value of Trimethol Syrup as compared with phenol? The only available method of determining the germicidal value of a liquid disinfectant is to make a direct comparison of the substance in question with phenol under similar conditions. Given parallel conditions, not obviously prejudicial to the substance tested in contrast to the standard solution, the results are comparable, and furnish a basis for estimating the relative germicidal power of the two substances. In the investigation, Trimethol Syrup and phenol were thus compared.
As regards the contention that the bacteria within fecal masses are harmless, this may be granted. But it must also be admitted that these intestinal masses are constantly being reformed so that buried micro-organisms do not remain in the interior. For this reason, the determination of the penetrability coefficient of a germicide is pertinent.
Regarding the respective merits of the old Rideal-Walker and the newer U. S. Hygienic Laboratory method of determining the phenol coefficient, the Rideal-Walker method was found to possess certain drawbacks, and in an attempt to overcome these the “Lancet Method” was evolved; this method in turn was improved in the U. S. Hygienic Laboratory and led to the United States Public Health Service Hygienic Laboratory method for the determination of the phenol coefficient of disinfectants (published in Hygienic Laboratory Bulletin 82). In 1913 this method was formally adopted by the Council for the valuation of disinfectants or germicides of the phenol type, and the method is now in general use for this purpose in the United States.119 In this connection Hiss and Zinsser may be quoted (Ed. 2, page 80): “The most precise method of standardizing disinfectants is that now in use in the United States Public Health Service.” Stitt, director of the United States Naval Medical Schools, in his Practical Bacteriology, Blood Work and Parasitology (Ed. 4, page 473) says: “In the United States disinfectants are rated according to the Hygienic Laboratory Phenol Coefficient.”
The Council adopted the recommendation of the Committee on Pharmacology to the effect that the claims made for Trimethol are unsupported by acceptable evidence. Accordingly, Trimethol and the pharmaceutical preparations said to contain it—Trimethol Syrup, Trimethol Capsules, and Trimethol Tablets—were held ineligible for New and Nonofficial Remedies.—(From The Journal A. M. A., Aug. 11, 1917.)
E. Fougera & Co., Inc., New York, acting as agent for The British Drug Houses, Ltd., London, advertise “Ferrivine,” “Intramine” and “Collosol Iodine” to the medical profession. A circular entitled “Ferrivine, The New Anti-Syphilitic Remedy” begins:
“FERRIVINE is the name given to ferric tri-para-amino-benzene sulphonate. This iron compound was first prepared by Mr. J. E. R. McDonagh, F. R. C. S., by whom it has been both biologically and clinically tested. It is slightly soluble in water, the solution having an acid reaction.
“INDICATIONS
“According to Mr. J. E. R. McDonagh’s researches, the phases of the Leucocytozoon syphilids are killed by the lipoid-globulin molecules of the serum, which possess a stereochemical molecular configuration homologous to those of the lipoid-globulin molecules of the parasite. The process is one of absorption, a chemico-physical reaction which is in part dependent upon the supply of active oxygen. Active oxygen is formed directly by oxidation processes and the peroxide necessary for its formation directly by reducing processes. Oxidation is increased by metals and reduction by non-metals. The non-metal which acts in the body as the normal reducing agent is sulphur, hence the discovery of Intramine (see separate pamphlet). The metal which acts in the body as the normal oxidising agent, is iron, hence the discovery of Ferrivine.”
A circular, “Intramine, a New Non-Toxic Compound for the Treatment of Protozoal and Chronic Bacterial Diseases,” expounds Mr. McDonagh’s ideas of the treatment of syphilis with Ferrivine and Intramine by means of the oxidising action of Ferrivine and the reducing action of Intramine and asserts:
“As the ultimate administration of oxidising and reducing agents will benefit almost any infection, it may be said that Intramine is indicated in all protozoal diseases, and in all chronic bacterial diseases, especially in tuberculosis, presumably in leprosy and possibly in malignant disease [cancer?]. To the administration of Intramine there are no contraindications.”
We are also told that:
“Intramine is useful injected into the urethra.... In cases of chronic urethritis and perifolliculitis ... invaluable as a local application to chronic ulcers ...”
The Intramine circular includes a “Scheme of Treatment for Syphilis” which advises, in addition to Intramine, Ferrivine or salvarsan, mercury and iodids, the use of another proprietary called “Collosol Iodine.” An inquiry addressed to Fougera & Co. in regard to the character and composition of this preparation, brought the reply that the firm had no knowledge of its identity.
This “scheme of treatment” is objectionable in that it advises the “stock” treatment of a disease which demands individualization and further in that whatever beneficial effects may result from the use of mercury and iodid is likely to be ascribed to the preparations “Intramine,” “Ferrivine” and “Collosol Iodine.”
The advertising for Ferrivine and Intramine sent out by Fougera & Co. contains no experimental or clinical data on which an estimate of their value may be based. Apparently in England, where these products were originated, little has been published regarding them.
There is, however, one report which may be accepted as a carefully controlled clinical trial. In the Lancet (June 17, 1916, p. 1214) L. W. Harrison, D.S.O., M.B., Ch.B.Glasg., and C. H. Mills, M.R.C.S., L.R.C.P.Lond., report on “The Effect of Ferrivine and Intramine on Syphilis.” After briefly reviewing the theories which form the basis of McDonagh’s proposed treatment of syphilis with his discoveries “Ferrivine” and “Intramine” the authors point out:
“... that Mr. McDonagh’s biological discoveries ... have not been publicly confirmed by any biologist of standing ...”
While:
“... eminent chemists have confessed themselves unable to understand his chemistry.”
The authors explain:
“Recognizing that this might prejudice our practical tests of Intramine and Ferrivine, we have taken particular care to guard against their influence, cross-checking our observations and submitting them to others for confirmation or otherwise.”
Harrison and Mills chose for a test three ordinary cases of secondary syphilis, cases with well marked lesions, the clinical progress of which could easily be watched and from which it was easy to obtain specimens for microscopic examination. After a detailed account of the three cases—which records grave conditions resulting from the treatment and which shows the inefficiency of the drugs—they write:
“From the above account it will be seen that the local and general reactions which follow the injection of these preparations are by no means pleasant. In the case of Intramine the pain is undiluted torture and lasts so for two or three days. One of us had previously treated four cases with Intramine and the same local reaction occurred in these. In two of them abscesses have burst outwardly, one of which is still discharging necrotic débris, ten weeks after the injection, and will take many more weeks to close. In those cases where no abscess has yet burst it is easy to feel by the gap in the muscles that considerable necrosis has occurred. None of these effects can be ascribed to sepsis, as most rigid aseptic precautions were taken. Further, particular care was taken to make the injections strictly intramuscular. The constitutional symptoms which follow immediately upon the injection of Ferrivine are distinctly alarming, and such as would cause one to hesitate before injecting this remedy into any but robust patients.”
Harrison and Mills estimate the therapeutic effects of these drugs thus:
“1. That Ferrivine entirely failed to cause S. pallida to disappear from the lesions of three well-marked cases of secondary syphilis.
“2. After the failure of Ferrivine to cause the disappearance of Spirochaeta pallida from a mucous patch a single dose of 0.3 gm. salvarsan effected this in 18 hours, and the patch, which had hitherto been uninfluenced, had healed within 48 hours.
“3. Clinically we were unable to detect any influence of either or both these compounds on syphilitic lesions, although each of them was of the variety which heals in a week or ten days under salvarsan treatment.
“4. Further syphilitic lesions appeared immediately after the treatment in one of the two cases treated with both Ferrivine and Intramine. A mucous patch appeared on one tonsil as well as further syphilitic papules from which spirochetes were obtained. The other case developed nephritis, with albumin and epithelial casts; which was not present prior to the injections.”
While from these cases the obvious conclusion was drawn that Intramine and Ferrivine “have no specific effect on early syphilis,” these authors subsequently treated a case of tertiary syphilis with the drugs. An Intramine injection caused pain for several days but did not stop the progress of the disease. Ferrivine was then administered “not without a feeling of grave responsibility” in view of their previous experiences. They state that “the reaction which resulted in this instance was the most severe” they ever experienced after an intravenous injection of any of the antisyphilitic remedies with which they had previously worked. It is stated that “for a period of some minutes there was grave doubt as to the patient’s survival.” After resuscitation the patient passed a disturbed night, and rigors which ensued lasted until the following afternoon. The author’s report that in this case also no clinical improvement occurred and that the Intramine-Ferrivine treatment was replaced by a course consisting of salvarsan, potassium iodid and mercurial inunction.
Ferrivine, Intramine and Collosol Iodine were declared inadmissible to New and Nonofficial Remedies.—(From The Journal A. M. A., Sept. 8, 1917.)
For the information of the profession the Council has prepared and authorized for publication the following report on Eskay’s Neuro Phosphates.
W. A. Puckner, Secretary.
Eskay’s Neuro Phosphates (Smith, Kline & French Co., Philadelphia) is offered to physicians under the claims that it contains alcohol, 17 per cent., and sodium glycerophosphate, 2 grains, calcium glycerophosphate, 2 grains, and strychnin glycerophosphate, 1⁄64 grain, in each dessertspoonful. It is called a “Nerve Tissue Reconstructive,” and its advertising claims are based on the discredited theories that certain disorders are due to a deficiency of phosphorus in the nerve structure of the body, and that glycerophosphates are assimilated more readily than ordinary phosphates. This assumption was based on the knowledge that the lecithins, which form a part of the nerve structure, contained the glycerophosphate radical in the molecule. In line with this, Smith, Kline & French Co. aver:
“Eskay’s Neuro Phosphates is of marked value in many acute and chronic conditions, in nervous exhaustion following mental and physical strain, neurasthenia, paralysis, anemia, tuberculosis, marasmus, debility and wasting diseases generally, and the nerve-weakness of the aged. It is particularly useful in convalescence from acute diseases and in the nervous condition following la grippe.”
In its report on “The Therapeutic Value of the Glycerophosphates” (The Journal, Sept. 30, 1916, p. 1033) the Council pointed out that the therapeutic use of the glycerophosphates was based on the assumption that the inorganic phosphates cannot supply the body’s needs of phosphorus or that the use of organic compounds “spared” the system the necessity of making such synthesis. The report presented evidence to show that the glycerophosphates are not absorbed as such, but that they are split into inorganic phosphates before absorption. The Council showed that there was convincing evidence that the animal organism synthesizes its complex organic phosphorus constituents from inorganic phosphates, and that organic phosphorus is of no more value as a food than inorganic. Despite this the Neuro Phosphates advertising makes use of the fallacious assumption regarding the action of the glycerophosphates.
Pleading for the particular mixture represented by the proprietary, it is asserted that:
“Sodium glycerophosphate is of special value in neurasthenia, Addison’s disease, phosphaturia and phthisis.”
and that calcium glycerophosphate “is employed in bone fracture, rachitis, tuberculosis and various wasting diseases.”
The phosphorus content of 1⁄64 grain of strychnin glycerophosphate is ridiculously small. Yet it is asserted that this strychnin salt is of superior value because it combines the effects of strychnin with a “food-like form of phosphorus.” Eskay’s Neuro Phosphates has an acid reaction which is capitalized, thus:
“Experiments have shown that the acid glycerophosphates are more rapidly absorbed and are more efficient than the neutral salts.”
And as a further illustration of extravagant claims:
“As a glycerophosphoric acid in the form of lecithin is normally present in spermatozoids, it is but natural that the glycerophosphates should exhibit aphrodisiac effects (as has been observed), but this result does not seem to obtain in all cases.”
Is this a clumsy attempt to exploit this “nerve phosphate” as a “lost manhood” cure?
The Council held Eskay’s Neuro Phosphates ineligible for New and Nonofficial Remedies because unwarranted therapeutic claims are made for it and because the administration of strychnin, calcium, phosphate and alcohol is not conducive to rational therapeutics, particularly when such a mixture is marketed under a name which indicates but one of its constituents.—(From The Journal A. M. A., Sept. 29, 1917.)
Because of inquiries received, the Council has authorized publication of the following report declaring K-Y Lubricating Jelly inadmissible to New and Nonofficial Remedies.
W. A. Puckner, Secretary.
K-Y Lubricating Jelly (Van Horn and Sawtell, New York), originally advertised as a lubricant for instruments and the hands, is now also recommended as a therapeutic agent. If the claims for “K-Y” were limited strictly to such effects as result from the purely mechanical properties of a lubricant, it might be held that it would not come under the purview of the Council. The preparation, however, while introduced as a lubricant, is now offered for a broader field of use, and the manufacturers make claims which are not supported by any evidence available to the Council. Evidence the following, taken from a circular that accompanies the package:
“K-Y allays smarting and burning at once through its pronounced soothing and cooling effects, and thus makes an admirable dressing for burns.”
“Many physicians make a practice of anointing the bodies of their measle and scarlet fever patients with ‘K-Y,’ in this way affording gratifying relief from itching and irritation, and effectively preventing dissemination of infectious material.”
And this from another circular:
“I had one of the most troublesome cases of pruritus vulvæ that I had ever seen. I guess I must have tried everything and the case had been referred to me by another man, who had previously tried everything, including cauterization. Well, one day I was examining her, and of course K-Y on the speculum—the irritation seemed to quiet down, and the following day she said she felt no effects from it at all. Then later on, it returned, and I couldn’t imagine what had done so much good, unless it could have been the lubricant, so I told her to buy a tube, which she did. Every once in a while she has a return of it slightly, but she just applies K-Y and clears it all up.”
The manufacturers state that they do not know why K-Y is so soothing, but suggest:
“Possibly the cooling action of the combination, and the effect of the 4% boric acid contained, are factors that enter. Be all that as it may, the fact certainly remains that oftentimes, after other local measures fail, ‘K-Y’ lubricating Jelly gives relief.”
Elsewhere it is claimed to be germicidal, and to give relief in other conditions, thus:
“Diabetic and uremic irritations, not only of the genitalia, but of other parts, have been found fully as amenable as pruritus vulvae to the soothing influence of ‘K-Y’ Lubricating Jelly, especially if the previous application is removed with water every time a new one is put on.”
The foregoing citations are obviously intended largely for the public, and make it plain that “K-Y” Jelly is not in the class of nonmedical and harmless external applications; on the contrary, these claims tend to create the impression that the spread of measles and scarlet fever can be prevented in the stage of desquamation. To place such statements in the hands of the patient supported by the tacit endorsement of a prescription is to create a false and dangerous sense of security and to lead to a failure to observe other and more important means of preventing dissemination of these diseases.
The Council held K-Y Lubricating Jelly in conflict with Rules 1, 4, 6 and 10, and authorized publication of this report.—(From The Journal A. M. A., Sept. 29, 1917.)
Ziratol (Bristol-Myers Company, New York), in compliance with the federal “insecticide law,” is declared to contain 32 per cent. water and 30 per cent. glycerin as inert constituents. Regarding its active constituents the manufacturer makes the following and meaningless statement:
“Ziratol is prepared from Phenols of the Naphthalene series and consists of a solution of such Phenols in a mixture of soap, water and glycerin.”
In response to inquiry, the A. M. A. Chemical Laboratory examined Ziratol and reported that its essential constituent appears to be alpha-napthol,120 and that it has, essentially, the following composition by weight: Alpha-napthol 18 per cent., soap 20 per cent., glycerin and water sufficient to make 100 per cent.
A Ziratol advertising circular gives a tabulated report of germicidal tests, said to have been made according to the method of the Hygienic Laboratory of the U. S. Public Health Service. When this work was done is not stated. According to these tests Ziratol possesses a phenol-coefficient of 13.66. The claim that Ziratol is ten times more efficient than carbolic acid (phenol) is evidently based on this report.
These claims of high germicidal value are contradicted by an examination made for the Council. A specimen purchased in the open market was examined independently by two operators, to determine the Hygienic Laboratory phenol-coefficient. One observer found the phenol coefficient to be 2.54. The other reported it to be 3.09. Evidently the germicidal value of Ziratol is greatly exaggerated in the advertising claims and, in fact, does not exceed that of the official compound solution of cresol (Liquor Cresolis Compositus, U. S. P.) for which a phenol-coefficient of about three has been established. (See New and Nonofficial Remedies, 1917, p. 82.) The claim that Ziratol is “the Universal Antiseptic and Germicide” is manifestly an unwarranted exaggeration.
The referee in submitting this report to the Council recommended that Ziratol be held in conflict with Rule 1 (secrecy of composition) and Rule 6 (unwarranted and exaggerated claims). After the report had been submitted, it was found that a new advertising circular, accompanying a trade package, no longer contained the claim that “Ziratol is ten times more efficient than Carbolic Acid.” The older circular made the following statement:
“1. Strong Activity.—Compared with the bactericidal action of Carbolic Acid by the method of the Hygienic Laboratory of the Marine Hospital Service, Ziratol has the Carbolic Acid Coefficient of more than TEN, that is, Ziratol is TEN times more efficient than Carbolic Acid,—a strength unapproached by any other of its class. Ziratol in dilution of 1:1400 kills the Typhoid Bacillus in 21⁄2 minutes, thus proving that it is strongly active even in very weak solutions.”
The new advertising circular reads:
“1. Strong Activity—Extensive bacteriological investigations on many pathogenic organisms, conducted in the Lederle Laboratories of New York, prove conclusively the high bactericidal value of Ziratol in extremely dilute solutions. (A copy of the complete report will be mailed upon request.)”
In response to a request, the Bristol-Myers Company sent a copy of the bacteriologic investigations of Ziratol, said to have been made by the Lederle Laboratories. The organisms employed for these tests were Staphylococcus aureus, Staphylococcus albus, Streptococcus, Green pus bacillus, B. coli, and saliva. No tests are given with the typhoid bacillus. The conclusion is reached that “in all the tests the solutions of Ziratol have several times greater killing efficiency than those of phenol.” The “coefficients” or comparative values which can be calculated from the results after exposure of 15 minutes to the disinfectants range from 2.0 to 4.0. This is in substantial accord with the referee’s findings as regards the phenol-coefficient with B. typhosus as the test object. While the new advertising circular avoids the former claim that Ziratol is ten times more efficient than carbolic acid, in germicidal value, it still makes the unwarranted claims that Ziratol is the “universal disinfectant.”
The Council declared Ziratol inadmissible to New and Nonofficial Remedies (1) because its composition is secret (Rule 1); (2) because the phenol coefficient, determined according to the method of the Hygienic Laboratory, U. S. P. H. S., is not stated on the label (Rule 2); (3) because the label and the circular accompanying the trade package advises its use by the public as a “vaginal douche” (Rule 3); and (4) because the claim that Ziratol is the “universal disinfectant” is exaggerated and unwarranted (Rule 6).
Before authorizing publication of the preceding report the Council submitted it to the Bristol-Myers Company in order to give that company the opportunity of revising its method of marketing Ziratol. In reply the company enlarged on its withdrawal (on “our own initiative”) of the claim that Ziratol had a phenol-coefficient of over ten when this claim was shown to be incorrect “by authoritative sources.” One wonders whether this is a euphemistic reference to the proceedings of the federal authorities under the Insecticide Act against the Bristol-Myers Company, just made public,121 because of the false claims made for the germicidal efficiency of Ziratol. This prosecution resulted in the seizure and condemnation of two lots of this proprietary which had passed in interstate commerce.
The Bristol-Myers Company in replying to the Council’s report made no offer to declare the exact composition of Ziratol, to state the actual phenol-coefficient, or to remove the other objections pointed out in the report of the Council. In other words, the Bristol-Myers Company has abandoned a definite but false claim of high germicidal power—a claim which subjected the firm to federal prosecution—and has substituted therefor indefinite statements which do not define the actual germicidal efficiency of Ziratol.—(From The Journal A. M. A., Oct. 6, 1917.)
The Council has adopted the following report on Gonosan and authorized its publication.
W. A. Puckner, Secretary.
Gonosan (Riedel and Company, Inc., New York City) comes in the form of capsules, each said to contain 5 minims of a mixture composed of oil of sandalwood 80 per cent., and 20 per cent. of alpha- and beta-resin of kava, isolated by a patent process. The mixture, as the name implies, is intended for the treatment of gonorrhea.
This proprietary preparation was under consideration by the Council at various times from 1905 to 1910. During this time, the Council agreed to accept the preparation if the suggestive name was changed, the therapeutic exaggerations abandoned, and the drug kava admitted to New and Nonofficial Remedies. The name was not changed, the other questions were left open, and the preparation was not accepted.
Recent and more objectionable advertising of Gonosan makes it advisable for the Council to take action and to publish a report. The tone of this advertising is reflected by the following quotation from a recent advertising circular:
“The old-established balsamic treatment of gonorrhea, for some years neglected in favor of the local injection of organic silver and other germicidal salts, has, with the increasing knowledge and attention paid to the composition and purity of the balsams, regained to a large extent the confidence formerly reposed in them.
“It may now be said that the combined treatment with local injections and internal administration of natural balsamic products completely dominates modern gonorrheal therapy.”
Any one conversant with current medical literature and practice would stamp these statements as misleading exaggerations. The balsams, oleoresins and volatile oils may have some value as minor adjuvants in the treatment of gonorrhea, but that is all. The position in this respect has not changed materially in recent years. These agents do not have a value equal to that of local treatment, as the quoted statement implies.
The claims made for Gonosan might with equal force be made for oil of santal alone. Kava kava, the other constituent, belongs to the pepper family; it had a temporary vogue some two or three decades ago, but has failed to maintain a place. It has never been recognized officially. There is no scientific evidence that it has any value either alone or as an adjuvant to sandal oil. The “clinical reports” quoted in the advertising circulars, rather curiously, nearly all date back ten years or more, viz., to a period when the attitude of the profession toward proprietary remedies was less critical than it is now. It would be interesting to know whether these authors still adhere to their opinion, or whether any of them have subsequently had experiences similar to that of a correspondent who wrote:
“Gonosan, at my hands, did not prove to be of more essential value in the treatment of gonorrhea than any other sandalwood oil preparation. The various claims made for Gonosan, that it possesses sedative and anesthetic properties, that by its continuous use the urethral discharge disappears more rapidly and that, if combined with appropriate diet and rest, it is liable to prevent complications, are, according to my experience, not corroborated by actual results.”
The only experimental work quoted in support of Gonosan, that of Pohl, is not convincing. The doses that Pohl found necessary to influence experimental purulent pleurisy makes it impossible to transfer his work to the clinic. (He found a dosage of oil of santal corresponding to an ounce per day, for man, inefficient; positive results were obtained only with 2 ounces per day.)
In order to learn the estimate placed on the therapeutic value of the “balsams,” an inquiry was sent to the authors of the papers presented to the section of Genito-Urinary Diseases at the recent meeting of the American Medical Association in New York. The inquiry read:
“Dear Doctor:—An advertising circular for Gonosan ‘Riedel’ which is now being distributed begins thus:
‘The old-established balsamic treatment of gonorrhea, for some years neglected in favor of the local injection of organic silver and other germicidal salts, has, with the increasing knowledge and attention paid to the composition and purity of the balsams, regained to a large extent the confidence formerly reposed in them.’
‘It may now be said that the combined treatment with local injections and internal administration of natural balsamic products completely dominates modern gonorrheal therapy.’
“Is the statement correct that the combined treatment with local injections and internal administration of natural balsamic products completely dominates modern gonorrheal therapy? Your reply to the above will be appreciated by the Council.”
Seventeen replies were received. They bear out the position that has been outlined. Only one writer considered the statement even approximately justified, and this in the sense that “the majority of cases receive no other treatment” than a combination of local applications and systemic medication. Another stated that, “in a general way their statement is true though a trifle too sweeping,” and then added that the field of the balsams is rather restricted. With the exception of these qualified endorsements the remaining (fifteen) replies characterized the statement as incorrect and misleading. The replies are a valuable contribution to the status of the “balsam” treatment of gonorrhea, and extracts of them are appended to this report.
It is recommended that the Council declare Gonosan inadmissible to New and Nonofficial Remedies, because the therapeutic claims are exaggerated (Rule 6); because there is no evidence that the combination of kava resin with oil of santal is superior to oil of santal alone (Rule 10); and because the therapeutically suggestive name is conducive of indiscriminate and unwarranted use of the preparation both by the profession and the public (Rules 4 and 8).
The extracts from replies received to the inquiry above referred to, follow:
Dr. B., Penn., wrote:
“In my practice I have found that local injections are very valuable in the treatment of gonorrhea, but I have never found that the internal administration of natural balsamics dominated modern gonorrheal therapy; while it is an aid, I consider the quoted statement to be very erroneous.”
Dr. F., D. C., wrote:
“While it is doubtless true that acute urethritis, gonorrheal, is now generally treated by local injections of solutions of organic silver salts, and that santal oil is often used, it is not true, as one would infer from the quotation, that the balsams are now considered more efficacious than they were formerly. So far as I know they have not lost or regained anything during the past dozen years in the way of confidence reposed in them. The indications for their use is very definite and very limited.”
Dr. B., Ga., wrote:
“... In recent years I have almost abandoned the use of balsams, etc., in the treatment of gonorrhea. Patients, who are properly treated otherwise, seem to get along as well without such drugs as with them, in fact apparently better for they have no gastric disturbance. It is important for patients to drink freely of water and when so doing the balsams are so diluted that I cannot conceive of their doing much good. Formerly my patients often lost weight during the treatment of gonorrhea; now, without balsams and with plenty of water, they usually gain in weight.”
Dr. S., Mich., wrote:
“... we believe that in a general way their statement is true though a trifle too sweeping. We do not ordinarily use the balsams in uncomplicated anterior urethritis. We do however, find indication for their administration in from sixty to seventy five per cent. of all cases of acute gonorrhea at some time during the course of the disease.”
Dr. L., Mo., wrote:
“I would say that the statement that, ‘The combined treatment with local injections and internal administration of natural balsamic products completely dominates modern gonorrheal therapy,’ is far from representing the facts. While the balsamics may occasionally have an indirect soothing effect on the mucous membranes involved, the dominant factor is local treatment, aiming at disinfection and restoration to normal of the inflamed tissues.”
Dr. R., Mich., wrote:
“Regarding your request although I am willing to reply it is difficult to do so because if I should do so in the affirmative that could apply only to certain acute cases without complication of any kind and such cases are rare. In such, however, the advertiser is not far from right—since vaccine therapy has proven absolutely worthless we must fall back on antiseptics in acute urethritis when there are no objections to such treatment ...”
Dr. K., Ill., wrote:
“I am under the impression that the internal administration of balsamics is used only when complications arise, such as acute posterior urethritis. Personally I use the balsamics very, very rarely. From my observation, however, I am led to believe that many men still use internal drugs in the treatment of gonorrhea, and during the past few years, I should say the use of hexamethylenamin has been on the increase, and the use of the balsams on the decrease. I do not believe that hexamethylenamin is of any value in the treatment of gonorrhea, and am simply citing this as my observation of the widespread use of this drug in the treatment of gonorrhea.”
Dr. T., Penn., wrote:
“... I believe that more men use salol or hexamethylenamin, or no urinary antiseptic whatsoever, than use the balsamics.”
Dr. B., Ind., wrote:
“... The only systemic treatment that is considered necessary today is rest, plenty of water and neutralize the acidity of the urine with bicarbonate of soda or some sodium salt.”
Dr. Y., Mass., wrote:
“Sandal wood oil during the acute stage of gonorrhea certainly tends to make the patient more comfortable and undoubtedly does lend some (tho I believe slight) gonococcidal action. That it plays any considerable part in actual cure I think is doubtful. The statement as quoted is true in so far as it states that local treatment plus internal medication with a balsam comprises most of the modern treatment of gonorrhea but it is grossly misleading in that it lets one draw the inference that the balsam plays a large if not the principal part.”
Dr. H., New York, wrote:
“For a period of at least three years in my hospital, dispensary and private practice, I conscientiously tried out most of the balsamics on the market (including Gonosan, which I favored for some time) both alone, and combined with local injections. As a result of this study, I have come to the conclusion that the balsamics have little, if any value in the treatment of gonorrhea. During the past few years I have relied almost entirely on local therapy, and seldom prescribed any of the balsams in my private practice, certainly in not more than 5 per cent. of the cases. My results I find are just as satisfactory, and my patients appreciate the fact that they are not loaded up with disagreeable medication. Instead of the balsamics, I am using sodium bicarbonate more and more, and feel convinced that the proper use of this drug is of more value than all of them combined.”
Dr. K., Cal., wrote:
“The statement that the combined treatment with local injections and internal administration of natural balsamic products completely eliminates modern gonorrheal therapy, would at present not be justifiable even with reference to the initial or acute stage of gonorrhea, while in the subacute and chronic forms of the disease local injections and balsams play an almost insignificant rôle as compared with various other recognized therapeutic measures.”—(From The Journal A. M. A., Oct. 13, 1917).
The Council has adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
In 1915 Alcresta Ipecac Tablets (Eli Lilly and Co.) were admitted to New and Nonofficial Remedies as a preparation of ipecac that is insoluble in the stomach but soluble in the intestines. It was supposed that this property would permit the administration of ipecac without the accompanying nausea and vomiting, and that this would be of especial advantage when using the drug in amebic dysentery. The systemic effects, of course, would be those of ipecac.
More recently, the manufacturers of Alcresta Ipecac have been advising its use in conditions which were not contemplated by the Council when the preparation was accepted for New and Nonofficial Remedies. They now claim that ipecac alkaloids have been shown to be useful in the treatment of typhoid fever, flatulence, diarrhea and constipation and that Alcresta Ipecac has these properties. Such a statement is misleading. While it is true that at one time ipecac was used promiscuously against “flatulence, diarrhea and constipation” there never has been and is not now any scientific evidence of its efficiency in such conditions except, of course, in diarrhea of the amebic type. As to the alleged usefulness of ipecac in typhoid fever: This has not even the sanction of tradition and the claim certainly should not be accepted until there is strong evidence to support it.
The advertising matter on Alcresta Ipecac also contained statements to the effect that ipecac alkaloids have a demonstrated usefulness in pyorrhea. Such an unequivocal statement is unwarranted. In spite of the enthusiastic advocacy, in the past, of ipecac alkaloids as a specific in pyorrhea alveolaris the preponderance of scientific evidence indicates that ipecac is of questionable value in this condition. Neither is there any substantial evidence to warrant the claim that ipecac alkaloids, when absorbed through the intestines, are demonstrably useful in amebic infections of the tonsils.
The reputation of the best drugs, whether unofficial or official, is bound to suffer if extravagant claims for them are permitted to go unchallenged. The referee of the Council, therefore, believed it necessary to call the attention of the manufacturers of Alcresta Ipecac Tablets to the statements made for the product and suggested that they submit evidence to substantiate the claims. This the manufacturers have refused to do. Their attitude in the matter, as well as their attitude toward the Council’s work is expressed in the following letter:
“Responding to your letter of March 10th, we beg to suggest that literature covering the different matters at issue are readily available to your referee, and all statements emanating from us are made advisedly.
“If you cannot satisfy yourselves that this preparation is a scientific product, ethically advertised, and a desirable advance in therapeutics, you can only delete it from your next issue of New and Nonofficial Remedies.”
It is to be regretted that Eli Lilly and Co. refuse either to withdraw or modify their claims or to substantiate these claims by scientific evidence. The statements as they stand are exaggerated, misleading and harmful. As such they conflict with Rule 6 of the Council and necessitate the omission of Alcresta Ipecac from New and Nonofficial Remedies. The referee recommended the adoption and publication of this report.—(From The Journal A. M. A., Oct. 20, 1917.)
Iodeol and Iodagol (formerly called Iodargol) are products of Viel and Company, Rennes, France, widely advertised in this country by David B. Levy, Incorporated, New York. The claim made for both preparations is that they depend on “colloidal iodin” for their action. They are put up in a number of forms, for instance:
“Iodeol Ampoules each containing 1 c.c. (20 centigrammes colloidal iodin in an oily vehicle).”
“Iodeol External, containing 50 per cent. colloidal iodine.”
“Iodagol Ampoules, each containing 2 c.c. (50 centigrammes colloidal iodine in an oily vehicle).”
The claim is, that, the iodin being in the colloidal state, it has the properties of elementary iodin and thus the preparations may be used in concentrations and under conditions which would make the use of free iodin impossible. The products have been extensively and extravagantly advertised for use in a wide range of conditions. Thus Iodeol has been proposed in the treatment of:
“Pulmonary Tuberculosis”
“Laryngeal Tuberculosis,”
“Glandular Tuberculosis”
“Tuberculosis of the Bones”
“Pneumonia, Broncho-pneumonia, and Congestive Conditions”
“Whooping Cough, Influenza, Asthma”
“Typhoid Fever”
“Syphilis”
“Obesity.”
Iodagol, which is for external use, has been advised in the treatment of:
“Gonorrhea and its Sequelæ”
“Cystitis”
“Tetanus”
“Wounds complicated by gaseous gangrene”
“Burns”
“Old Suppurations, ulcers, abscesses, etc.”
“Articular rheumatism”
“Abscess Alveolar”
“Pyorrhea Alveolaris”
“Stomatitis (Canker-Sores).”
Nearly two years ago the American agents requested the Council to consider Iodeol and Iodagol for admission to New and Nonofficial Remedies. The information submitted in regard to their character and composition was vague and indefinite, the pharmacologic information practically nil and the clinical data as voluminous as it was unconvincing.
On the basis of chemical, pharmacologic, bacteriologic and clinical investigation carried out under the direction of the referee and a study of the submitted evidence, the referee reported:
1. Iodeol and Iodagol do not contain the amount of iodin claimed.
2. The iodin is not present as elementary iodin, but instead the preparations behave similarly to the well-known organic iodin compounds such as iodized fats.
3. The therapeutic claims made for the preparations are exaggerated and unwarranted.
In view of his findings he recommended that Iodeol and Iodagol be declared inadmissible to New and Nonofficial Remedies for conflict with Rules 1 and 2 (misleading statements regarding composition and identification) and Rule 6 (unwarranted therapeutic claims). The Council adopted the recommendation of the referee, directing inclusion of the full report in the annual Council reports after submission to the manufacturer, and recommending publication of an abstract of this report in The Journal.
This report was brought to the attention of the American agent, David B. Levy, Inc., and through them to the French manufacturers, E. Viel and Company. The manufacturers have intimated that they will not file a reply to the report. The firm of David B. Levy, Inc., has decided to sever its connection with these products and to discontinue their sale.
W. A. Puckner, Secretary.
Iodeol and Iodagol were submitted to the Council nearly two years ago as “electro-colloidal iodine” and with the claim that they produced all the antiseptic and other effects of ordinary iodin without any of its side actions. The referee has done much work on the subject, conducted a large amount of correspondence and has contended with long delays. He feels that the consideration of these products should be brought to a conclusion and accordingly he submits this report of their consideration. The following is a summary of the report, which is appended:
I. Discrepancy in Iodin Percentage.—The examination at the Chemical Laboratory of the American Medical Association, as well as that of the referee, shows that the various samples of Iodeol and Iodagol examined contained a little less than one-half of the total iodin claimed. These facts were reported to the American agent. After a lengthy delay a reply was received which presented a double excuse: (1) that the full amount of iodin had been added, whatever had become of it later; (2) that the claims were made for “colloidal iodin” and that this is not elementary iodin in the colloidal state, but a preparation of iodin containing only 50 per cent. of real iodin. Neither explanation can be taken seriously, as they are obvious quibblings. The referee concludes that the preparations are falsely labeled as to iodin content.
II. Nature of the Iodin Compound in Iodeol and Iodagol.—In the information sent the Council, Iodeol and Iodagol were defined as “A suspension of electro-chemical colloidal iodin in a vehicle of purified oil.” Numerous inquiries have failed to elicit more specific information from the manufacturer or his agent. The statement of composition can mean only that the preparations contain free iodin (but in colloidal form) suspended in oil. No evidence to substantiate this claim has been submitted. (There is evidence that the preparations contain colloidal particles, but it does not indicate if this colloidal material is iodin, or a combination of iodin or indeed whether the colloidal component contains any iodin.) The recent statements of the agent seem to concede that what they call “electro-colloidal iodin” contains only about 50 per cent. of real iodin, in other words that it is not “colloidal iodin” at all, but a mixture or combination of iodin with some other unnamed substance. This, of course, is something very different.
Certain results reported from the American Medical Association’s Chemical Laboratory suggest that the so-called “colloidal iodin” of Iodeol may be a combination of iodin with a volatile oil. The investigations of the referee indicate that the iodin exists in a rather resistant form or combination behaving altogether differently from ordinary free iodin, and rather resembling the behavior of iodin substitution products, such as iodized fats or phenols. Briefly then the recent admissions of the agents indicate that Iodeol does not contain “colloidal iodin” in a chemical sense, and there are indications that it does contain its iodin in a rather firm (chemical) combination.
III. Chemical Properties of Iodeol.—From a study of different specimens of Iodeol, the referee concludes that fresh specimens contain no free iodin and that old ones contain small amounts as a result of decomposition. Iodeol has the solubility characteristics of fats and fat-like compounds. The examination, as a whole, shows that Iodeol contains a peculiar and rather resistant form or combination of iodin. There is nothing in the chemical data that suggests that it could act differently from ordinary iodin compounds, such as iodized fats. It would not act as ordinary iodin.
IV. Pharmacologic Data.—The pharmacologic statements which were submitted were loose and apparently meaningless or misleading. In reply to questions submitted by the referee, the manufacturer finally had some work done and submitted a report by Jean Laumonnier. The referee was unable to confirm some of this work, and as a whole it does not appear materially to elucidate the action of Iodeol. From a consideration of the submitted evidence, and as a result of his own work, the referee concludes that Iodeol does not behave like elementary iodin; it does not coagulate proteins and therefore is not irritant. It is presumably absorbed, but quite probably after chemical change; it is changed into iodid and, like organic iodids, is excreted somewhat more slowly than when inorganic iodids are administered, but the difference does not appear important.
V. Antiseptic and Bactericidal Action.—Elementary iodin is considered a fairly powerful agent in these respects. The activity is presumably due to changes in the proteins, etc., of the bacteria, analogous to the effects which produce pain, irritation and necrosis of the tissue cells. Since the latter effect is not produced by Iodeol, it seems highly improbable, if not impossible, that it should act on bacteria like elementary iodin. It is entirely unjustifiable to credit the known antibacterial qualities of ordinary iodin to “colloid” iodin. This misrepresentation is especially prominent in the circular “Notable New Therapeutic Agents,” as will be seen, for instance, from the following citations:
“Iodine has long been universally recognized as an antiseptic of extraordinary potency. Not only is it rapid and certain in its germ-destroying action, but it also possesses an attribute denied many other antiseptic agents, namely, the power to penetrate and impregnate the tissues. Other antiseptics, as is well known, act on the surface epithelium only.”
“According to Kinnaman (J. A. M. A., Aug. 26, 1905), iodine is far superior to bichloride of mercury, a two per cent. solution killing streptococcus pyogenes in two minutes. Iodine does not coagulate albumin, and is very penetrating.”
The citations imply that this “colloidal iodin” of Iodeol and Iodagol acts as an antiseptic like ordinary iodin, except that it is claimed to be more efficient by “diffusing” more readily. This is entirely unjustified and misleading. If Iodeol and Iodagol are really antiseptic, they must act by some other mechanism than that through which elementary iodin acts, and such antiseptic action would have to be demonstrated by direct observation and not assumed from the known action of free iodin.
Antiseptic and bactericidal effects are easily estimated by laboratory methods. Yet no evidence on this point appeared to have been available until the Council called for this. Laumonnier then carried out some experiments which were in turn submitted to bacteriologic control. The bacteriologist failed to obtain any results with some of the tests, and considered the other data of little value.
The claim that Iodeol and Iodagol have the antiseptic and bactericidal action of free iodin lacks proof and must be considered unwarranted and misleading in the extreme.
VI. Clinical Trials.—The manufacturers and agents of Iodeol presented many letters from physicians; but few, if any, of these gave evidence of careful, critical, controlled observations. They could not, therefore, be considered as acceptable evidence. The more important claims, letters and published papers, however, were submitted to clinical specialists collaborating with the Council, with the request that they examine these and conduct some clinical trials, if they considered it advisable. The results obtained in these preliminary trials did not appear sufficient to warrant further experimentation.
From a consideration of the evidence presented, the referee concludes that the claims made for Iodeol and Iodagol are unwarranted, exaggerated and misleading. He recommends that Iodeol and Iodagol be declared ineligible for New and Nonofficial Remedies for conflict with Rules 1 and 2 (misleading statements as to composition and identification) and with Rule 6 (unwarranted and misleading therapeutic claims). He further recommends that the Council authorize publication of the preceding summary of the consideration of Iodeol and Iodagol in The Journal and inclusion of the full report in the annual Council reports after submission to the manufacturer.—(From The Journal A. M. A., Nov. 17, 1917.)
In response to inquiries received, the Council took up the consideration of Capsules Bismuth Resorcinol Compound (The Gross Drug Company, Inc., New York City). The label, sent by the Gross Drug Company, bore the following:
Capsules
Bismuth Resorcinol Compound
Bismuth Subgallate | 2 | grs. |
Resorcinol | 1 | gr. |
Beta Naphthol | 1⁄2 | gr. |
Creosote (Beechwood) | 1 | m. |
| This combination is of acknowledged value in reducing the intestinal putrefaction and fermentation, allaying the pain and discomfort of flatulent conditions in the intestinal tract. | ||
| Dose.—One or two capsules before or after meals repeated in two hours if necessary. | ||
| The Gross Drug Company, Inc. 20 Laight Street, New York | ||
The Council held this preparation inadmissible to New and Nonofficial Remedies or the Appendix, because (1) the claim “acknowledged value in reducing the intestinal putrefaction and fermentation, allaying the pain and discomfort of flatulent conditions in the intestinal tract” is an unwarranted, exaggerated and misleading claim of therapeutic value (Rule 6); because (2) the name does not indicate the identity of the bismuth salt contained in the capsules, nor declare the presence of betanaphthol and creosote (Rule 8); and because (3) the combination of bismuth subgallate, resorcinol, betanaphthol and creosote in fixed proportions is irrational (Rule 10).—(From Reports of Council on Pharmacy and Chemistry, 1917, p. 139.)
New and Nonofficial Remedies, 1917, contains general descriptions of Dixon’s Tubercle Bacilli Extract and Dixon’s Suspension of Dead Tubercle Bacilli; the products of these manufactured by the H. M. Alexander Company being listed as dosage forms. It having become necessary to omit the preparations of the Alexander Company (see page 158) the referee recommended that the general articles of “Dixon’s Tubercle Bacilli Extract” and “Dixon’s Suspension of Dead Tubercle Bacilli” also be omitted. He reported that no other firm appears to be marketing these products and that they had not been shown to be of special value.
The Council accepted the recommendation and directed the omission as proposed. In accordance with the procedure of the Council, these have been transferred to the annual Council Reports for reference and appear below.
W. A. Puckner, Secretary.
Dixon’s Tubercle Bacilli Extract.—An extract of tubercle bacilli dissolved in normal saline solution. (See “Fluid of Dixon,” Medical News, Jan. 17, 1891.)
Dixon’s Suspension of Dead Tubercle Bacilli.—A suspension in physiologic salt solution of dead tubercle bacilli which have been defatted by prolonged treatment with alcohol and ether. (See “Possibility of Establishing Tolerance for Tubercle Bacilli,” Medical News, Oct. 19, 1889.)—(From Reports of Council on Pharmacy and Chemistry, 1917, p. 140.)
Sunshine’s Formosol (The Formosol Chemical Company, formerly the Sunshine Chemical Company, Cleveland, Ohio) is claimed to contain 18 per cent. formaldehyd in a solution of soap. It is therefore very similar to Veroform Germicide which was deleted from New and Nonofficial Remedies because of the low phenol coefficient reported by the Hygienic Laboratory of the United States Public Health Service (The Journal, Nov. 22, 1913, p. 1920.) The Council voted that in view of the Hygienic Laboratory’s finding that formaldehyd has a low germicidal value, the manufacturers of Formosol be required to produce definite evidence of the degree of germicidal value for this product.
In submitting the preparation to the Council, it was claimed that Formosol had “all properties peculiar to Formaldehyde.” This conservative tone was, however, not maintained in the form-letters submitted. These contain the following unwarranted statements:
“As the name implies, FORMOSOL is a formaldehyde preparation, which embodies all the innate antiseptic merits and eliminates all the ill features of the world’s greatest disinfectant.”
“The elimination of all the destructive elements and the incorporation of all the established therapeutic virtues of formaldehyde, have been scientifically blended in FORMOSOL.”
“Formosol is unique in the sphere of antisepsis because of its peculiar healing properties as diametrically opposed to irritation to the tissue of mucous membrane.”
“Formosol may be used for the thousand niceties of modern antisepsis, but is specific in Gynecology and Obstetrics and is indicated in Dermatology.” [Italics not in original.]
“The constant use of FORMOSOL is to develop a habit sympathetic to ethics.”
“To prescribe FORMOSOL is a great step toward Personal Hygiene, a duty of the medical fraternity to the laity.” [Italics not in original.]
The trade package recommends the use of Formosol “for cuts, wounds, ulcers, abscesses ...” This is a conflict with Rule 4. The Council held Formosol in conflict with Rules 4 and 6, and advised the manufacturers that Formosol is refused admission to New and Nonofficial Remedies until they submit evidence establishing the degree of antiseptic and germicidal efficiency, and justify the quotations listed above; or until these and any other existing conflicts with the Rules have been removed.
After submission of this report to The Formosol Chemical Company the Council authorized its publication.—(From Reports of Council on Pharmacy and Chemistry, 1917, p. 145.)
The Council was asked to consider a solution of iodin in liquid petrolatum, said to be prepared from Gulf Coast petroleum by a special process. It was to be marketed as “Iodolen” provided the Council found the preparation admissible to New and Nonofficial Remedies. The preparation was claimed to contain over 1.5 per cent. free iodin. The following claims were made:
“It is less irritating in its use on the skin, or in wounds.” “Will kill pathogenic micro-organisms present.” “Is a suitable medium for cell proliferation.” “Will penetrate a useful distance into the walls of a wound.” “Facilitates an easier, less painful and better method of dressing wounds or ulcers.”
Examination in the American Medical Association Chemical Laboratory showed a submitted sample to contain 1.32 per cent. free iodin and to emit a strong odor of hydrogen sulphid. A specimen of liquid petrolatum, said to be composed chiefly of hydrocarbons of the naphthene series, after saturation with iodin at room temperature was found to contain 1.42 per cent. free iodin. Another specimen of liquid petrolatum, said to be composed chiefly of saturated hydrocarbons, after saturation at room temperature was found to contain 1.30 per cent. free iodin.
The preparation having been shown to be an unoriginal, simple solution of iodin in liquid petrolatum, the Council declared the name “Iodolene” unacceptable (Rule 8) and the therapeutic claims made for the preparation unwarranted (Rule 6).—(From Reports of Council on Pharmacy and Chemistry, 1917, p. 148.)
The following report, submitted by a member of the Council’s committee on chemistry, was endorsed by the committee and adopted by the Council:
Kalak Water, sold by the Kalak Water Company, Inc., New York, is an artificial mineral water said to be made by adding certain salts to carbonated, distilled water and supersaturating with the gas under pressure. Such merit as it may possess by virtue of sodium bicarbonate and sodium phosphate is quite insufficient to warrant the extravagant claims made in the advertising pamphlets.
According to the analysis furnished, the water contains, in 1,000,000 parts (milligrams per liter) the following:
Sodium carbonate | 4049.0 |
Sodium phosphate | 238.5 |
Sodium chloride | 806.3 |
Calcium carbonate | 578.2 |
Magnesium carbonate | 48.9 |
Potassium chloride | 47.9 |
Among the many misleading statements found in the advertising pamphlet bearing the title “A Brief for Physiological Alkalescence” these may be quoted:
“The calcium content of Kalak is over 100% greater than ever before placed in solution in any vehicle, a fact of supreme importance when the unique alkalinizing power of the alkaline salts of this metal is considered; the ratio of calcium metabolism to its enormous waste in pregnancy, the diseases of infancy and childhood and the rapidly growing group of ‘acidoses’ make its availability in Kalak of double value.”
The first part of this statement is untrue; the last part is muddled and without much meaning. Evidently the “acidosis” fad is to be overworked as was the old “uric acid diathesis,” of unsavory memory. Again this:
“One of the most important characteristics of Kalak is the close approximation of its formula to the correlation of the contained salts as they occur in the human body, together with its freedom from salts foreign to the human economy. Another is its almost unbelievable palatability, considering its high degree of alkalinity, it being eleven times greater than any other known mineral water, artificial or natural.”
These statements are false. The salts dissolved here bear no discernible relation to the needs of the body, as disclosed by the composition of the blood or solid tissues or as shown by the character of the urinary excretion. The last statement concerning the high alkalinity is neither clear nor accurate. Then, this warning and remedy:
“It seems to be an unappreciated fact that the degree of urinary acidity, checked with the acidity of the saliva, is in direct ratio to the existing acid toxemia, and a urine acid to methyl red should be the signal for immediate and adequate alkalinizing treatment....
“Startling clinical results have been observed by physicians who have used Kalak thoughtfully and sufficiently in the more serious types of acidosis associated with diabetes, nephritis, rheumatism, gout and the acute infections. There is also evidence of its good effect in acute alcoholism and the respiratory edemas; in fact a certain few have hailed Kalak as a possible solution of the annual hay fever problem. Of perhaps supreme importance, however, is the use of Kalak throughout pregnancy as preventive medicine against the inevitable ‘toxemia of pregnancy.’ ”
Also this:
“Kalak has accomplished certain unexplainable things for the diabetic and nephritic, and if, in future years, diabetes and nephritis should prove to be constitutional diseases, based upon functionation or its lack, Kalak therapy, the embodiment of physiological alkalescence may come into its own, for if acidity retards, alkalinity must normalize functionation.”
It is not necessary to quote further. In order to insure that everyone will recognize the great need of Kalak it is advised to test the urine for acidity by means of a group of indicator solutions sent out to the physicians. Methyl red is one of these and any urine showing an acid reaction with this is said to be open to suspicion. Paranitrophenol is another of the indicators and the explanations given of the behavior of the two and the conclusions to be drawn are questionable. The methyl red solution furnished is too concentrated for proper use and perfectly normal urines from normal individuals have given a rather marked color with it. This indicator gives some color at [H+] = 1.2 × 10-6 and a strong reaction at 3 × 10-3. To condemn a urine on such a finding is entirely unwarranted.
Sodium bicarbonate is the main constituent of the water. The value of the phosphate in such a combination, with so much calcium, is problematical. In case an alkaline reaction in the intestine is reached some of it would be left as insoluble phosphate. A few grams of bicarbonate daily would have equal therapeutic value with this water. The advice based on the indications of methyl red and the urine is bad.
The committee’s report was sent to the Kalak Water Company for comment. The company promised to withdraw the advertising circular referred to in the report and disclaimed responsibility for the accuracy and value of the set of indicators which it sent out, but, on the whole, the previous advertising claims were insisted on.
In view of the absurd and false claims made for the product the Council declared Kalak Water inadmissible to N. N. R.—(From Reports of Council on Pharmacy and Chemistry, 1917, p. 148.)
Minson’s Soluble Iodin “Kelpidine” was submitted to the Council by J. J. Minson, Washington, D. C., trading as the Kelpidine Company, with the statement that in future “literature” it was to be known as Minson’s Soluble Iodin, only. The following statement of composition was furnished:
“Minson’s Soluble Iodin is somewhat of an indefinite character, chemically. Its formula is, Iodin 4 per cent., Distilled Water 6 per cent., and Absolute Alcohol q. s. 100 per cent. By a process of chilling and heating an iodid of uncertain character is produced, and because of the extreme sensitiveness of the product to chemical tests, it is hard to determine. So far as I have been able to judge, however, the result is about 3 or 31⁄2 per cent. free iodin and from 1⁄2 per cent. to 1 per cent. iodid, possibly ethyl and hydrogen iodid in combination.”
The A. M. A. Chemical Laboratory reports that the preparation is an alcoholic solution containing free iodin and iodid, probably hydrogen iodid and ethyl iodid, but that the free iodin content was only 2.69 gm. per 100 c.c.
It is claimed that the “therapeutic indications” of Minson’s Soluble Iodin are the “same as those of all iodin and iodid preparations, internally, externally, hypodermically and intravenously; excepting, however, counter irritation.” It is admitted that there are no “clinical reports” as to the hypodermic and intravenous use, but the belief is expressed “that in an emergency it is a safe remedy under proper dilution.” It is further claimed that “for all practical purposes it is nontoxic and nonirritating” and that “it has none of the undesirable features such as is the case with the iodids and the organic preparations of iodin, proprietary or otherwise.”
It was assigned for consideration to the Committee on Pharmacology, whose referee reported:
“According to the information submitted, this is a tincture of iodin; differing from the official tincture in that it is more dilute and in that hydrogen and ethyl iodid is the solvent in place of potassium iodid. It is practically immaterial for internal administration, whether the cation of the solvent iodid is hydrogen, ethyl, potassium or sodium. It would certainly be inadvisable to inject a preparation containing free iodin hypodermically. It is not ‘a safe remedy’ for intravenous injection and it would not be nonirritant. The statement that ‘it has none of the undesirable features’ of other iodin compounds is inherently impossible. Apparent freedom of any iodin preparation from undesirable effects is generally due to the use of small doses. Such claims are plainly therapeutic exaggerations and therefore in conflict with Rule 6. Even should these be removed, the preparation must be held an unessential modification of the official tincture, and therefore in conflict with Rule 10.”
The report was agreed to by the committee and adopted by the Council and Minson’s Soluble Iodin “Kelpidine” declared inadmissible to New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1917, p. 152.)
NuTone (NuTone Company, Lowell, Mass.) is a “nutritive tonic” said to have the following complex composition:
Cod Liver Oil, Pure Norwegian, 25 per cent.
Malt Extract, 91⁄3 per cent.
Beef juice,
Glycerine,
Hypophosphite Lime, Hypophosphite Soda, Chemically pure, 11⁄2 grs. each to the oz.
Fl. Ext. Nux Vomica, 3⁄64 of a minim in each teaspoonful.
It is advertised with claims that will lead thoughtless physicians and a confiding public to depend on it in cases in which fresh air, hygienic surroundings and nutritious food are of prime importance.
A sample package (the phrase “as recommended by your physician” and other statements suggest that it is expected to be given the patient by the physician and thus effectively advertise NuTone to the public) describes NuTone as an “Agreeable Concentrated Nutritive Tonic Emulsion of Malt Extract, Beef Juice and Cod Liver Oil, Combined with Nerve Tonics and Bone Nutrients.” Emphasizing the nutritive value of this “Malt Extract, Beef Juice, and Cod Liver Oil” preparation, it is advised, “As NuTone is rich in nutritive properties, it is well to begin with one-fourth teaspoonful, gradually increasing to regular dose, which is: Adults, 1 to 2 teaspoonfuls after meals and at bedtime. Children according to age.” It thus appears that adults are to take this preparation as a “nutritive” in doses which represent from 3 to 12 grains of sugar (on the assumption that malt extract may contain as much as 50 per cent. sugar) and 8 to 30 minims of cod liver oil with unstated, but probably equally small, amounts of beef juice.
A consideration of the negligible food value of NuTone as well as of the inefficiency of the other components and the claim that it is indicated in “malnutrition,” “wasting diseases” and “incipient phthisis” classes NuTone with that large group of shotgun mixtures which do harm in that dependence is placed on them in conditions in which the patient will probably be restored to health if proper medical and hygienic measures are adopted in time.
The Council declared NuTone inadmissible to New and Nonofficial Remedies because it is an irrational shotgun mixture advertised indirectly to the public with unwarranted therapeutic claims and a nondescriptive therapeutically suggestive name.—(From Reports of Council on Pharmacy and Chemistry, 1917, p. 154.)
Tri-Arsenole.—According to the advertising of the Medical Supply Company of Atlanta, Ga., “Tri-Arsenole” is “Merco-Arseno-Benzo-Chloride,” and the claim is made:
“This compound is the result of many years’ research. The toxicity has been fully tested upon animals before using clinically, the latter having proven such complete success, we take pleasure in presenting it to the public ...”
“The manufacturers of TRI-ARSENOL, before placing it upon the market, tested it biologically.”
Tri-Arsenole is “recommended and suitable for the treatment of primary, secondary, tertiary and hereditary syphilis. It has also been found very useful in pellagra and malaria.” The preparation is supplied in ampules containing varying amounts of the dry substance. It is to be dissolved in water and is to be administered intravenously. In the advertising attention is called to the yellow color of Tri-Arsenole; this, and the style of package suggest that it is a preparation similar to salvarsan.
In reply to a request sent the Medical Supply Company for the quantitative composition and chemical formula of the compound “Merco-Arseno-Benzo-Chloride” and for the details of the biologic test by which its toxicity is claimed to have been determined and evidence for its efficiency, the following statement was received:
| “Tri-Arsenole No. 1 equals to each Ampoule, | gr. |
Sodium chlorid | 41⁄2 |
Hydrarg chlor.-cor. | 1⁄4 |
Arsenous acid | 1⁄4 |
Sodium benzoate | 4 |
Hydrastin (resinoid) | 2 |
| Tri-Arsenole No. 2 equals to each Ampoule, | |
Sodium chlorid | 4 |
Hydrarg chlor.-cor. | 1⁄2 |
Arsenous acid | 1⁄2 |
Sodium benzoate | 4 |
Hydrastin (resinoid) | 2 |
| Tri-Arsenole No. 3 equals to each Ampoule, | |
Sodium chlorid | 31⁄2 |
Hydrarg chlor.-cor. | 3⁄4 |
Arsenous acid | 3⁄4 |
Sodium benzoate | 4 |
Hydrastin (resinoid) | 2 |
| Tri-Arsenole No. 4 equals to each Ampoule, | |
Sodium chlorid | 3 |
Hydrarg chlor.-cor. | 1 |
Arsenous acid | 1 |
Sodium benzoate | 4 |
Hydrastin (resinoid) | 2 |
The request for information regarding the animal experiments said to have determined the toxicity was ignored, nor were references supplied to clinical reports demonstrating the value of the product.
The Council declared Tri-Arsenole inadmissible to New and Nonofficial Remedies because of conflict with the rules as follows:
In the absence of details of the method used, the claim that the preparation has been tested biologically is in conflict with Rule 2, which requires that for preparations claimed to be physiologically standardized the method of testing must be published so as to permit of control by independent investigators.
The claims that “Merco-Arseno-Benzo-Chloride” is “the result of many years research,” that its “toxicity has been fully tested upon animals before using clinically” and that clinical use has “proven such complete success” have not been substantiated by evidence and must be held as unwarranted.
The name is in conflict with Rule 8, which requires that pharmaceutical mixtures shall bear names descriptive of their composition. Further, the name “Tri-Arsenole” by its similarity to Diarsenol, the Canadian brand of arseno-phenolamin hydrochlorid, suggests that this pharmaceutical mixture is a chemical compound similar to salvarsan. Moreover, the danger of confusion is increased by the addition of the hydrastis preparation which imparts a yellow color like that of salvarsan to the solution obtained when the colorless mercury and arsenic compounds of the mixture are dissolved. Again, the synonym “Merco-Arseno-Benzo-Chloride” conveys the false impression that Tri-Arsenole is a definite chemical compound.
The label does not declare the poisonous constituents claimed to be contained in the mixture; namely, “arsenous acid” and corrosive mercuric chlorid (Rule 7).
There is no evidence that arsenous acid (arsenic trioxid) used intravenously is efficient and safe as a spirocheticide, and the administration of this drug in conjunction with mercuric chlorid in fixed proportion is irrational and dangerous—particularly so because of the implied similarity of Tri-Arsenole to arsenphenolamin hydrochlorid (Salvarsan, Diarsenol) (Rule 10).
L. O. Compound No. 1 and L. O. Compound No. 2.—In submitting these preparations to the Council, the Medical Supply Company stated that “No. 1” was “composed of the following ingredients; chloral, camphor, menthol, iodin, and oil of gaultheria, incorporated in a fatty base. Each ounce contains fifteen grains of chloral hydrate, nine grains of resublimed iodine.” “No. 2” was said to have the same composition as “No. 1” except that the oil of gaultheria had been omitted. The Medical Supply Company was informed that the rules of the Council required declaration of the amounts of each therapeutic constituent of pharmaceutical mixtures and that, therefore, in addition to the information furnished the amounts of camphor, menthol and oil of gaultheria should be given for “No. 1” and the amount of camphor and menthol for “No. 2.” The following reply was received:
| “L. O. Compound No. 1 equals to each Tube, | |
Chloral hydrate | gr. 15 |
Camphor | gr. 22 |
Menthol | gr. 71⁄2 |
Iodin (resublime) | gr. 32⁄3 |
Oil of gaultheria | m. 3 |
Petrolatum, q. s. | oz. 1 |
| L. O. Compound No. 2, |
| The same as above formula for L. O. C. No. 1, except the oil of gaultheria which is omitted.” |
It should be noted that when the preparations were submitted each ounce of the preparation was claimed to contain 9 grains of iodin, while in the subsequent letter the company declares that they contain only 32⁄3 grains to the ounce. If it be assumed that the unit intended is the avoirdupois ounce, the preparation should contain 2.06 per cent. of iodin according to the first statement and 0.84 per cent. of iodin according to the second statement. While the dark color of the preparations suggested the presence of appreciable amounts of free iodin, the A. M. A. Chemical Laboratory reported that an examination of the specimens submitted by the Medical Supply Company showed that “No. 1” and “No. 2” each contained but 0.033 per cent. of free iodin; hence both preparations are in conflict with Rule 1.
For both preparations the labels suggest their use for the treatment of “septic wounds, burns, pustular processes of all varieties, and especially bronchial troubles.” This constitutes a conflict with Rule 4. Regarding No. 1 the advertising circular included with the trade package asserts:
“Its merits have been practically demonstrated in the following conditions. We invite your especial attention to its use in diseases of the thoracic cavity, especially Bronchitis and Pneumonia, Rheumatism, Lumbago, Migraine, Neuralgia, Orchitis, Balanitis, enlarged glands or any disturbance of the lymphatic system, anti-galactagogue, or wherever analgesic action is required.”
“No. 2” is said to be especially adapted to the needs of the surgeon, it “can be applied in any wound either aseptic or infected.” It is asserted that the usual method of preparing patients for operation may be discarded and that patients may be operated on after application of this ointment:
“... We have no other preparation to-day which serves the purpose of L. O. Compound in operative and post operative treatment.
“It is a powerful antiseptic and germicide combining anesthetic, analgesic and alterative properties.”
After attempting to discredit the approved methods of preparing the field for surgical operations, the advertising circular continues:
“Method of today: A liberal amount of L. O. Compound No. 2 is applied to the intended area of operation, massage thoroughly until absorption is complete. Patient is ready for operation ...”
Both products are in conflict with Rule 6. Further, as the names of these pharmaceutical mixtures are not descriptive of their composition, they also conflict with Rule 8.
The use of complex mixtures such as these is irrational and leads to misplaced confidence on the part of the physician; particularly when, as in this case, neither the label nor the advertising matter gives the necessary information regarding the composition of the preparations further than that, in accordance with the requirements of the Federal Food and Drugs Act, the amount of chloral is declared (Rule 10).
The Council declared L. O. Compound No. 1 and L. O. Compound No. 2 inadmissible to New and Nonofficial Remedies for conflict with Rules 1, 4, 6, 8 and 10.
The Council’s consideration of Tri-Arsenole, L. O. Compound No. 1 and L. O. Compound No. 2 was based on information received from the Medical Supply Company, the correspondence being signed “Medical Supply Co., per Dr. H. E. Pontius.” The findings having been sent to the Medical Supply Company, the following reply was received:
(June 27, 1917) “Replying to your registered letter of this A. M. relative to the Medical Supply Company’s products, will state that the party furnishing you with such information as you have in hand was misinformed. He is no longer with this company and whereabouts unknown.
Respectfully,
Medical Supply Company,
(Signed) W. B. Lingo, President.”
The Medical Supply Company then was asked to point out any statements occurring in the report, as submitted, which the company considered to be inaccurate; but no reply has been received to this request. The advertising sent out by the Medical Supply Company during the last part of August contained essentially the same statements and claims as those to which reference is made in the preceding report. A qualitative examination of Tri-Arsenole made in the A. M. A. Chemical Laboratory indicated the presence of sodium, mercury, arsenic, chlorid, benzoate and a hydrastis preparation. Quantitative determinations were not made as there was no guarantee that an analysis of the present supply would indicate the composition of that marketed later on.
In view of the statement of the president of the company, that the information submitted in the letters from the Medical Supply Company was inaccurate, Tri-Arsenole and L. O. Compound must definitely be placed with preparations, the composition of which is not divulged by their owners; hence Tri-Arsenole as well as L. O. Compound No. 1 and L. O. Compound No. 2 are in conflict with Rule 1.—(From Reports of Council on Pharmacy and Chemistry, 1917, p. 156.)
Unctol, sold by the R. R. Rogers Chemical Company, San Francisco, is a paste stated to contain approximately 40 per cent. of metallic mercury in a soap base. It is claimed that a part of the mercury is “precipitated mercury” and a part “mechanically comminuted mercury.” Unctol is sold as a substitute for mercurial ointment and is to be rubbed into the skin with the aid of water. The claim is made for Unctol that “It is more active than blue ointment because the mercury in it (40 per cent.) is more finely divided and the lathering still further subdivides the mercury particles and hence promotes absorption.”
No evidence was presented to the Council in support of the claimed superior efficacy of mercury soap paste over the official mercurial ointment. On the other hand, a consultant of the Council who has studied the absorption of mercury and mercury compounds, when applied to the skin, reported that he had used mercury preparations in which soap was the base, and that in his opinion Unctol could have no advantage over the official mercurial ointment from the standpoint of therapeutic effect. Moreover, the Council is advised that some trials with Unctol at the skin clinic of Leland Stanford University Junior School of Medicine did not confirm the claim that Unctol is more active than mercurial ointment.
The Council declared Unctol inadmissible to New and Nonofficial Remedies because: 1. The claim of superiority over mercurial ointment is not substantiated, and constitutes an unwarranted therapeutic claim (Rule 6). 2. The name does not indicate the composition of this pharmaceutical mixture (Rule 8). 3. The circular wrapped with the trade package advertises proprietary preparations not accepted by the Council (Rule 4).—(From Reports of Council on Pharmacy and Chemistry, 1917, p. 162.)
Because of inquiry received, the Schoonmaker Laboratories, Inc., New York, were requested to submit information in regard to the “V-E-M” products.
According to information received, these products have the following composition:
| V-E-M Unguentum Eucalyptol Compound | ||
Menthol | 5 | grs. |
Eucalyptol (Sander’s) | 15 | gtts. |
White Vaseline | 1 | oz. |
| V-E-M with Ichthyol | ||
Menthol | 21⁄2 | grs. |
Eucalyptol (Sander’s) | 15 | gtts. |
Ichthyol | 10 | grs. |
White Vaseline | 1 | oz. |
| V-E-M with Stearate of Zinc | ||
Menthol | 21⁄2 | grs. |
Eucalyptol (Sander’s) | 15 | gtts. |
Stearate of Zinc | 1 | drm. |
White Vaseline | 1 | oz. |
| V-E-M with Camphor | ||
Camphor | 15 | grs. |
Eucalyptol (Sander’s) | 15 | gtts. |
White Vaseline | 1 | oz. |
| V-E-M with Boric Acid | ||
Pulv. Boric Acid | 1⁄2 | drm. |
Eucalyptol (Sander’s) | 15 | gtts. |
White Vaseline | 1 | oz. |
In an advertising circular this claim is made:
“V-E-M Unguentum Eucalyptol Compound Combining, in well-balanced proportions, the cooling, soothing and healing virtues of Menthol with the antiseptic and deodorizing properties of Eucalyptol, in a base of pure, neutral white Vaseline. Furnished in five formulas as follows:
“V-E-M Unguentum Eucalyptol Compound: Menthol, Eucalyptol (Sander’s), White Vaseline.
“V-E-M with Ichthyol: Menthol, Eucalyptol (Sander’s), Ichthyol, White Vaseline.
“V-E-M with Stearate of Zinc: Menthol, Eucalyptol (Sander’s), Stearate of Zinc, White Vaseline.
“V-E-M with Camphor: Camphor, Eucalyptol (Sander’s), White Vaseline.
“V-E-M with Boric Acid: Pulv. Boric Acid, Eucalyptol (Sander’s), White Vaseline.
“For local application in the treatment of affections of the nose and throat.
“The efficacy of these combinations of remedial agents is so well established as to preclude the necessity of more than passing mention. What is obvious is that in acute coryza, in chronic and acute nasal catarrh, in dry catarrhal conditions especially, in both forms of chronic rhinitis—atrophic and hypertrophic—in the latter stages of the prevailing grippe colds, and even in hay fever, V-E-M Unguentum Eucalyptol Compound affords pronounced relief and proves a most grateful application....”
Though the identity and purity of eucalyptol are provided for by the standards of the U. S. Pharmacopeia, the claim is made that the product contained in these preparations “transcends in purity and efficiency all other brands.”
A package of V-E-M Unguentum Eucalyptol Compound, recently sent to a physician, contains the following:
“If your head is all stuffed up to-night, or you feel a cold coming on, use V-E-M just before going to bed. It will break up the cold, and you’ll wake up in the morning, with your head clear and feeling fine all over.
“If you suffer with chronic or acute catarrh, use V-E-M regularly night and morning. You’ll be agreeably surprised at the relief it will give you in a short time.
“There is nothing quicker, nothing surer to alleviate rhinitis, grippe-colds, or hay fever.
“In a word—V-E-M is the best antiseptic ointment for all diseased conditions of the nose....”
The Council declared these preparations in conflict with its rules because unwarranted therapeutic claims were made for them (Rule 6); because the public was advised to depend on them in the treatment of diseases (Rule 4), and because these combinations of ingredients, in fixed proportions, under proprietary names, are irrational (Rules 8 and 10).—(From Reports of Council on Pharmacy and Chemistry, 1917, p. 163.)
The following report on Hemo-Therapin has been adopted by the Council, and its publication authorized.
W. A. Puckner, Secretary.
According to the Hemo-Therapin Laboratories of New York City:
“Hemo-Therapin is a combination of highly refined creosols and phenols (which have been detoxicated by special processes) with salts of iron, potassium, sodium, phosphorus and calcium in minute but physiologic proportions—the solution as a whole being designed to approximately closely in various fundamental details the chemistry of the blood.”
No statement is made as to the quantities of the several ingredients, nor is any information given as to the identity of the “creosols” and “phenols,” nor the nature of the processes whereby these are “detoxicated.” It is further claimed that it is:
“... The composite character of Hemo-Therapin, the relative proportion and balance of its several ingredients, and the action of the compound as a whole, to which its potency is due.”
And it is suggested that:
“It will be apparent that the ingredients which enter into the composition of Hemo-Therapin, a remedy used intravenously exclusively, have been selected with the utmost care with the object of assuring not only maximum therapeutic potency but also absolute safety and freedom from all dangers of toxic or other unpleasant or harmful action.” [Italics in the original.]
The advertising does not explain, however, why the complex preparation should be therapeutically efficient or why the intravenous administration of this mixture should be absolutely safe and free from toxic or harmful action. Of the origin of Hemo-Therapin it is said:
“For many years Dr. E. B. Witte, a prominent physician of Trenton, N. J. [apparently owner of the Hemo-Therapin Laboratories] has devoted himself to the study of the blood. As a result of his researches, he early determined that when the blood is close to normal standards, the body is well nourished, the natural waste products are properly eliminated, an effective resistance is offered to the invasion of pathogenic bacteria, and the various functions of the body are kept normally active. But when, for one reason or another, the blood falls away from normal standards, the nutrition of the body suffers, the elimination of waste products is impaired, the resistance to germ attack is weakened, and the various functions of the body become sadly deranged and perverted. In other words, instead of the physiologic processes of the body being normally active, as soon as the blood is depreciated, they become depressed or deranged, with a loss of the physiologic harmony or equilibrium that constitutes a state of health.
“Recognizing the relation of clinical conditions to these various phenomena, Dr. Witte reached the conclusion that the correction of many aberrant or diseased conditions depended on restoring the blood to as near to its normal state as possible. He accordingly applied himself especially to investigation of the chemistry of the blood, with the object of evolving a substance in liquid form that would so closely approximate normal blood in its essential chemical characteristics that when introduced into the circulation it would bring the blood nearer to the condition in which it exists in health.”
After the usual “many years of hard painstaking labor,” Dr. Witte elaborated a “fluid meeting the foregoing conditions” and now the Hemo-Therapin Laboratories inform us that 5 to 10 c.c. of this synthetic blood administered once in one, two or three days in “acute affection” and at longer intervals in “chronic ills”—once a week is said to be usually sufficient—will restore blood to a normality and empower it to overcome most ills. While disclaiming that “Hemo-Therapin is an infallible panacea,” the medical profession is asked to believe that:
“In erysipelas, septicemia, pyemia, the acute fevers, puerperal infection, furunculosis, carbuncles, malaria, acute rheumatism, pneumonia, typhoid fever, and in various skin diseases, such as eczema, psoriasis, herpes zoster, etc., the results have been prompt and gratifying.”
It is “no less effective” in such “chronic ailments” as:
“... diabetes, chronic Bright’s disease, goiter, pulmonary tuberculosis, chronic rheumatism, the severe anemias, arterio-sclerosis [sic], various nervous disorders, locomotor ataxia, varicose and indolent ulcers....”
Evidence of the virtues of Hemo-Therapin is submitted as a series of “case reports”—unsigned—which bear a striking likeness to the testimonials of “patent medicine” almanacs. A specimen of the “case reports” is the following:
“Blood Poisoning due to Snake Bite.—Case 9.: Mrs. ——; age, 52; was bitten by a poisonous snake—a copperhead—seventeen years ago. On the anniversary of the bite the arm would swell to more than twice its normal size and there would be pain, chills and fever. After a month of this the acute symptoms would disappear and the arm would show large scaly blotches which upon being removed would disclose a thin mucous liquid. Throughout the seventeen years pain was constant, being particularly acute in midsummer around the anniversary of the bite. This patient had consulted many physicians during the seventeen years of suffering without any relief. Large doses of narcotic remedies were necessary each day to subdue the pain. Twenty-four hours after the first injection of Hemo-Therapin all pain was dissipated. After four treatments the patient was considered well and there has been no return of any of the symptoms since the last treatment six months ago.”
Hemo-Therapin is sold in ampules: 6 for $5 and 12 for $10, and a circular sent to a physician contained this typewritten note:
“Fees.—While the physician’s fee is not regulated by this company, the physicians who use Hemo-Therapin get $5.00 and $10.00 for each treatment.”—(From The Journal A. M. A., Jan. 5, 1918.)
The following report on Venosal has been adopted by the Council, and its publication authorized.
W. A. Puckner, Secretary.
“Venosal” is one of the products of the Intravenous Products Company, Denver, Colo. Its composition has been variously, and obscurely, described:
“Venosal is a sterile solution representing 1 gm. (15.4 gr.) of salicylates in combination, together with colchicum.”
“This is a product for intravenous use. The composition of which is Sodium Salicylate, 15.4 grs. (1 gm.), Iron Salicylate a minute quantity and the equivalent of approximately 2 grs. Dried Colchicum Root.”
None of these “formulas” gives the quantity of the product containing the 1 gm. of salicylate, etc., but presumably it refers to the contents of 1 ampule or 20 c.c. This inference is in accord with the analysis of the product made in the Chemical Laboratory of the American Medical Association. The analysis also brought out the fact that the amount of iron in a given ampule was 0.0008 gm. (about 1⁄80 grain). This trace of iron in the presence of salicylate gives the product a purple color.
Venosal is recommended for the treatment of “rheumatism,” meaning, the context would indicate, infectious rheumatic fever. As colchicum has no special action on this disease and as there is no apparent reason for the employment of the trace of iron present, these additions in fixed proportions are unscientific, if not absurd. According to the advertising matter:
“Venosal ... eliminates unpleasant digestive disturbances which frequently forbid the use of salicylates by mouth and, in addition, insures their full therapeutic value.”
The statement is misleading, as the cases in which the oral administration of the salicylates is contraindicated are not “frequent” but exceptional and there is no evidence to justify the implication that the “full therapeutic value” of salicylates cannot readily be attained by their oral use. Still more astonishing is the following claim:
“Venosal is a combination carrying the true salicylates (sodii) in doses much larger than given by mouth. With this preparation given intravenously, there is no nausea or disagreeable digestive after-effects, tinnitus aurium, or the accumulating effects of the drug; yet the specific action of the salicylates seems to be increased many-fold, according to reports received.”
What are the facts? By mouth sodium salicylate is given in doses of from 3 to 15 gm. in a day; whereas Venosal is advised as 1 gm., in from one to three day intervals; as a matter of elementary arithmetic it is plain that these doses of Venosal are smaller instead of being “much larger.” The absence of digestive ill effects, tinnitus, etc., is explained by the small dosage. That the specific action of the salicylates should be increased by intravenous administration is surprising when it is remembered that the drug is absorbed rapidly and completely from the intestines; in fact, the quoted statement is incredible.
The company further alleges that, on the basis of “clinical reports” it has received, it does not “hesitate to recommend this product for routine use in all streptococcic infections.” Such a therapeutic suggestion is, to put it conservatively, gross exaggeration.
The whole question of the justification of using salicylates intravenously is open to grave doubt. Since it is possible to obtain the salicylate effects promptly and certainly by oral administration, the inherent dangers of intravenous medication render its routine employment unwarranted. A further objection to Venosal, especially at this time when economy is a national policy, is the unnecessarily high expense of Venosal itself and of its administration.
The referee recommends that Venosal be declared ineligible to New and Nonofficial Remedies because of conflicts with Rule 1 (indefinite chemical composition), Rule 6 (therapeutic exaggerations) and Rule 10 (unscientific composition).—(From The Journal A. M. A., Jan. 5, 1918.)
Two years ago the Council published reports on two proprietary preparations said to contain secretin, namely, “Secretogen,” sold by the G. W. Carnrick Company (The Journal A. M. A., May 1, 1915, p. 1518), and “Duodenin,” sold by Armour and Company (The Journal A. M. A., Aug. 14, 1915, p. 639). These reports explained that there was no evidence to indicate that an insufficient amount of secretin was the cause of gastro-intestinal diseases, and further that there was no evidence that secretin in any form was physiologically active when administered by the mouth.
Subsequently, A. J. Carlson and his co-workers, at the request of the Council, studied the question of the stability of secretin and demonstrated (The Journal A. M. A., Jan. 15, 1916, pp. 178 and 208) that commercial secretin preparations contained no secretin and, further, that secretin given both by the mouth and even in enormous doses directly into the intestine is entirely inactive.
Shortly after the publication of Professor Carlson’s work the attention of the Council was called to a U. S. patent issued, May 2, 1916, to James Wallace Beveridge, “Means for and Method of Stabilizing Secretin.” In this patent Beveridge claimed to have invented “The process of producing secretin in stable form as a commercial article for therapeutic use ...” that is, a process for preparing preparations which would contain secretin when they reach the consumer and in a form resisting destruction in its passage through the stomach.
In view of the demonstrated instability of secretin, the Council asked Professor Carlson to investigate the validity of the claims of the Beveridge patent. The study on “The Question of the Stability of Secretin,” by A. J. Carlson, A. E. Kanter and I. Tumpowski, which appears below, shows that the Beveridge patent furnishes no process for the manufacture of commercially stable secretin preparations, nor any means for preventing the destruction of secretin by the gastric juice when administered orally. It further demonstrates that the preparation made by Beveridge was devoid of secretin.
The Council adopted the report of Carlson and his co-workers, and declared Secretin-Beveridge inadmissible to New and Nonofficial Remedies.
The Council directed that the report of Carlson and his collaborators be sent to the Commissioner of Patents with a protest against the granting of patents without competent and thorough investigation of the claims advanced therein.
W. A. Puckner, Secretary.
[From the Hull Physiological Laboratory of the University of Chicago]
In a letters patent, filed May 6, 1914, the patent granted May 2, 1916, James W. Beveridge, M.D., makes certain claims concerning the stability and physiologic activity of secretin prepared according to the method patented by him.
In brief, Dr. Beveridge claims that secretin prepared by digesting intestinal mucosa with a weak acid at a temperature slightly below boiling, and mixed with 0.2 per cent. to 2 per cent. blood serum, albumin or peptone (1) remains active for at least six months, (2) stimulates the pancreas when given by mouth, and (3) “may be injected intravenously in man, if desired.” The only thing in the letters patent in support of these claims is the statement: “I have found out by actual tests that the preparation maintains its stability for five or six months.”
Here are the claims in detail:
“For the source of secretin I preferably use that part of the alimentary tract of any lower animal—such as a hog or sheep—including the gastric pylorus, the duodenum and the jejunum. This part is split open and washed with a normal saline solution to clean the mucosa or mucous membrane of any detritus which may be present. The mucosa with the epithelial cells is then removed or separated from the muscular wall by scraping with a blunt knife or in any other suitable way. The scrapings or cuttings, which contain the secretin, are then macerated or broken up.”
“The macerated mass is placed in a suitable vessel and subjected to the action of an acid solution until digested. The time for the digestion of the mass will, of course, depend upon the strength and temperature of the acid solution employed. The stronger the solution and the higher the temperature, the shorter the time necessary for complete digestion. This period may vary from several minutes to several hours. In my experiments I found that the best results were obtained with hydrochloric acid solution of one-tenth to five-tenths of one per cent. in strength, although as high as eight-tenths per cent. might be used. The mixture is brought to a temperature of approximately 210 F., and it may even for a few moments exceed that temperature, but it should be kept below the boiling point, for excessive heat injures or breaks down the secretin molecule and impairs or destroys its activity. Although I prefer to use hydrochloric acid, I would have it understood that other acids—both organic or inorganic—may be employed, provided that the percentage of acidity is regulated to prevent a chemical change in the secretin, and further provided, of course, that the acid has no injurious effect on the human system.”
“After the mass has been digested in the heated solution, the decoction is decanted, and after being allowed to cool is passed through a suitable filter until the filtrate is clear. I found that by filtering the decoction from four to six times through a carbon filter, I obtained a clear colorless filtrate. This is a solution of secretin and the acid which was used, and the clearness of the solution shows that it is practically free from albumoses, gelatin and other impurities (such as cell tissues, etc.) present in the raw material under treatment.”
“To the solution of pure and active secretin prepared as above explained, there is added a suitable quantity of blood serum—say from one-fifth to two per cent. or any equivalent medium—such as albumin solution or a peptone solution—which will aid and sustain the activating power of secretin as provided by the blood. That is to say, any medium having the same power, similar quality or chemical composition that the blood-stream possesses in combining with secretin to stimulate the pancreas. The addition of such a medium to the active secretin solution increases the potency of the secretin and its degree of stability by preventing oxidation or deterioration thereof. If this strengthening or fortifying medium, as it may be properly termed, is alkaline, it performs the additional function of lowering the acidity of the secretin filtrate. It is preferable that the final product be just faintly acid. If desired, the final product may be made into an elixir by the addition of aromatics.”
“Any desired strength of secretin solution may be obtained according to the quantity of acid solution. In my experiments I used from ten to fourteen duodena to a pint of acid solution.”
“The solution of secretin prepared as above described is characterized by its ability to resist oxidation or deterioration for a sufficient period of time to render the solution available as a commercial article, and is furthermore characterized by freedom from poisonous and irritable chemical substances, whereby the secretin is chemically adapted to the human system to stimulate the pancreas to increased secretion.”
“As previously stated, the secretin prepared according to my method may be administered orally to produce the desired physiological action. Of course, if desired, the secretin might be injected intravenously, but this more or less dangerous procedure is not at all necessary, and I merely mention it here to point out that when I refer to the oral administration of my new secretin preparation, I do not mean to exclude its administration by injection.”
“As to the commercial stability of the secretin prepared according to my method, I may say that I have found by actual tests that the preparation maintains its stability for as long a period as five or six months. When I refer to my product as being “commercially stable,” I mean that it resists oxidation or deterioration for a sufficient period to render the same available as a commercial article. This period may vary from several weeks to several months, depending upon certain commercial factors well understood by the manufacturer. So, roughly speaking, I should say that secretin is commercially stable when it retains its activity from one to six months. I do not wish to be understood, however, as limiting myself to these exact figures.”
That active secretin may be extracted from macerated intestinal mucosa by weak acids below the temperature of boiling is well known. In fact, weak acids at body temperature in contact with the duodenal mucosa lead to the formation of secretin. The claims that secretin given by mouth reaches the blood and acts on the pancreas has been made for other preparations of secretin. It has also been shown that these claims are erroneous.122 Thus it would appear that the only novel element in Dr. Beveridge’s patented secretin is the addition of serum, soluble proteins or peptones. What reason is there for believing that this will render the secretin stable for months, and physiologically active when taken by mouth? We do not believe Dr. Beveridge ever injected his secretin—protein mixture—intravenously in man or animals not under anesthesia, otherwise he would not have stated: “Of course, if desired, the secretin may be injected intravenously.”
I. The Samples of Secretin Sent Us by Dr. Beveridge.—Physiological tests were made on four quart bottles of the secretin kindly sent us by Dr. Beveridge June 26, 1916. According to a letter from Dr. Beveridge of July 20, 1916, those samples of secretin were prepared June 20, that is, only six days before received by us. The material came in dark colored bottles. It was kept in the original bottles and placed in the ice box immediately on receipt. Dr. Beveridge stated the secretin “should remain active until the month of November, 1916, at least.”
Tests were made on three out of the four bottles. The fourth bottle was not opened, as we desired to learn what change it might undergo in the way of protein precipitation and bacterial decomposition. There is nothing in the Beveridge method of preparation that insures a sterile secretin unless it is passed through a Berkefeld filter. In all our crucial experiments the animals (dogs) were kept under light ether anesthesia, a cannula inserted into the pancreatic duct, the blood pressure recorded from the carotid artery and the various secretin preparations injected intravenously. When inactive secretin preparations were encountered, control tests were always made with active solutions of secretin to eliminate possible individual peculiarities of the animal. Thus when the pancreas of a dog reacts to the injection of preparation A, but not to preparation B, it is evident that absence of response to B is due to this preparation and not to the animal or to the experimental conditions.
Each of the three samples of secretin sent us by Dr. Beveridge was tested in the above manner on five dogs. The first tests were made June 27, 28 and 29, respectively, that is, within nine days of the preparation of these samples of secretin. None of the samples was active (Fig. 1), even when injected intravenously in quantities up to 50 c.c.: 40–50 c.c. of Beveridge’s secretin mixture may kill a dog by too great lowering of the blood pressure. A good secretin preparation yields a copious secretion of pancreatic juice on intravenous injection of a few cubic centimeters.
It is not difficult to prepare a secretin, by the original Bayliss or Starling method or by the Beveridge method, that retains some activity for a longer period than nine days. Hence we cannot account for the absolute inactivity of these preparations except on the assumption that they did not contain any secretin to start with; that is, faulty preparation and absence of physiologic standardization.
The sample kept intact in its original container for six months became gradually cloudy, a large mass of amorphous precipitate settled to the bottom and the odor showed bacterial decomposition. It is reprehensible, to say the least, to state concerning such a mixture: “Of course, if desired, it may be injected intravenously.” The fact that Beveridge’s secretin may be rendered clear by filtering through carbon is not sufficient evidence that it is “pure secretin,” free from bacteria and other injurious substances.
II. Beveridge Secretin Mixture Is Rapidly Rendered Inactive by Human Gastric Juice.—We prepared active secretin solutions by the Beveridge method, using 0.2 per cent. serum as the protein “stabilizer” (?). The addition of the serum does not appear to affect the activity of the fresh secretin preparation. If Beveridge’s secretin is able to act on the pancreas when given by mouth, it is obvious that it must run the gamut of gastric digestion, except in cases of complete achlorhydria. It has been repeatedly demonstrated that all other secretin preparations are rapidly destroyed by pepsin-hydrochloric acid digestion. Is Beveridge’s secretin an exception? What is there in a little serum, native albumin, or peptones to protect secretin against gastric digestion?
The pure human gastric juice used in these tests was secured from the fistula case (Mr. F. V.) that has been under observation in our laboratory for years.123
BEVERIDGE’S SECRETIN AND BAYLISS-STARLING SECRETIN PREPARED
Sept. 29, 1916
| Date of Test | Quantity of Secretin Injected, C.c. | Response of Pancreas (No. of Drops of Secretin) | |
| Bayliss-Starling Secretin | Beveridge Secretin | ||
Sept. 29 | 10 | 75 | 78 |
Oct. 2 | 10 | 61 | 61 |
Oct. 6 | 10 | 28 | 17 |
Oct. 13 | 10 | 25 | 31 |
Oct. 27 | 10 | 5 | 6 |
Nov. 3 | 10 | 7 | 6 |
Nov. 17 | 10 | 4 | 5 |
Nov. 30 | 10 | 3 | 4 |
Dec. 4 | 10 | 2 | 2 |
Dec. 20 | 10 | 0 | 0 |
Two cubic centimeters of fresh gastric juice added to 8–10 c.c. Beveridge secretin, the mixture being kept at body temperature (38 C.), renders the secretin completely inactive in from 5 to 8 minutes (Fig. 2). There is no exception to this rule, as we have repeated the test on many different secretin preparations and using different samples of human gastric juice. The secretin of Beveridge is just as vulnerable as the secretin of Bayliss and Starling to pepsin-hydrochloric acid digestion. On what kind of tests does Beveridge base his claim that his secretin mixture acts on the pancreas when given by mouth?
III. The Relative Rate of Deterioration of the Secretin Solutions Prepared According to Bayliss and Starling and According to Beveridge.—Six different preparations of the two kinds of secretin were made, kept in dark stoppered bottles in the ice box, and tested by intravenous injection in dogs under ether anesthesia from time to time until all influence on the pancreas had been lost. One typical series of these tests is given by the way of illustration. (See Table on page 126.)
It will be seen that the rate of deterioration (oxidation or decomposition) of the secretin is practically the same whether prepared according to Bayliss and Starling or according to Beveridge (Figure 3). In both preparations the rate of deterioration is most rapid the first few days after preparation. It is scarcely necessary to point out that secretin preparations not kept constantly at low temperature and in the dark, as in the above experiments, will deteriorate more rapidly.

Why can we hope that the addition of serum or any solution of protein will render secretin more stable? In the intact man or animal under normal conditions of digestion, secretin reaches the pancreas by way of the blood, that is, it is in solution in blood. Does that fact render the secretin stable? By no means. The reader is familiar with the fact that the response of the pancreas to a single intravenous administration of secretin is very transitory (5–15 min.). The cessation of activity is due, not to fatigue of the pancreas, as a second injection of secretin gives a prompt response of pancreatic secretion, but to the disappearance of active secretin from the blood. In fact, secretin left in the test tube or in the bottle remains active over a much longer period of time than when introduced into the blood stream.
IV. Beveridge’s Secretin Given by Mouth to the Intact Animal Has No Specific Action on the Pancreas.—Active secretin prepared according to the method of Beveridge was fed on an empty stomach to a small dog (5 kilo) with permanent fistula of one of the pancreatic ducts. On control days we gave the dog (a) equal quantities of n/10 HCl, and (b) bread and milk. The Beveridge secretin was prepared with 0.3 per cent. HCl and the addition of 0.2 per cent. serum. The results may be stated by the following summary:
GIVING BEVERIDGE’S SECRETIN BY MOUTH
| Material Fed | Number of Tests | Secretin of the Pancreas for Three Hours Following the Feeding |
150 c.c. Beveridge Secretin | 6 | 10.2 c.c. |
150 c.c. n/10 HCl | 5 | 22.7 c.c. |
Bread soaked in milk | 4 | 6.6 c.c. |
The Control experiments with pure hydrochloric acid show that the secretion of pancreatic juice following the introduction of Beveridge’s secretin into the stomach is due to the acid factor and the protein content.
The patented secretin of Beveridge is rendered inactive by gastric juice, is without effect when given by mouth, and exhibits no greater stability or keeping qualities than the secretin prepared according to Bayliss and Starling. It has no merit as a therapeutic agent. It should under no conditions be administered intravenously in man, as it contains deleterious protein split products and living bacteria.—(From The Journal A. M. A., Jan. 12, 1918.)
At the present critical time when the efficiency of this nation must be raised to the highest point, it is essential that the United States government should lead in the efforts tending to such increased efficiency. To bring this about the government must protect and stimulate science, art and industry and at the same time curb or prevent waste of the country’s resources. In this field the United States Patent Office has unlimited power for good and evil—good, in the issuance of patent grants for novel devices and substances which go to increase national efficiency; evil, in the granting of patent protection where such protection is not in the interest of national efficiency, conservation of energy and material resources.
For years the American Medical Association, in common with the national pharmaceutical bodies, has been urging amendment of the law which governs the issuance of patents on medicinal preparations and more particularly revision of the procedure under which such patents are issued. At the Chicago (1908) meeting of the American Medical Association a special committee of five was appointed by the House of Delegates to study the questions involved, and to cooperate with the Association’s committee on medical legislation in preparing and securing the enactment of a bill which would correct the abuses connected with the enforcement of our patent laws (The Journal A. M. A., June 13, 1908, p. 2003). This committee presented a comprehensive report at the Atlantic City (1909) meeting of the American Medical Association (The Journal A. M. A., June 19, 1909, p. 2063). A further report was presented at the St. Louis (1910) meeting of the American Medical Association (The Journal A. M. A., June 18, p. 2079). In 1911 (The Journal A. M. A., Nov. 25, 1911, p. 1780) the Council on Pharmacy and Chemistry of the American Medical Association issued a report which set forth the inadequacy of our patent laws as they are administered in relation to medical products particularly.
Since that time the Council has continued its study of the U. S. Patent law as it applies to medicine and has become convinced that in many instances the patent law or its enforcement is contrary to the best interest of the public, both as concerns health and prosperity. The Council feels it a duty at this time to protest against the provisions of our patent law, or the methods of its enforcement, which permit the granting of patents without thorough and scientific investigation of the claims advanced in such letters patent. As one means of improving conditions the Council urges that the U. S. Public Health Service, the Bureau of Chemistry, U. S. Department of Agriculture and other scientific departments of the United States government conversant with medicines and related subjects be consulted before the issuance of patents on medicinal preparations.
In support of the Council’s contention that the patent law procedure requires revision, the following is offered: In 1912 a U. S. Patent (No. 1,031,971) was granted on a cresol derivative, metacresyl acetate, a product described in chemical literature in 1903. When the Council inquired as to the grounds for the issuance of a patent for a substance known to science, the Patent Office replied that it was not familiar with the publication in which metacresyl acetate had been described. It seems evident that this patent would not have been issued had the application first been submitted to a government department familiar with chemical literature.
An illustration of the granting of a patent on the use of well-known chemical bodies which present no discovery or originality, is the patent issued for the use of peroxids, perborates and percarbonates as ingredients of tooth powders (U. S. Patents Nos. 760,397 and 802,099). Regarding these patents The Journal of the American Medical Association (Sept. 20, 1913, p. 978) commented:
“The patents held by McKesson and Robbins give this firm the exclusive right of manufacturing tooth powders containing peroxids, perborates and percarbonates. It is another illustration of the unfair monopolies that may be secured under our present patent laws.”
Again in 1913 U. S. Patent No. 1,081,069 was granted to a citizen of Switzerland (a country which does not grant patents on medicinal preparations) for a “composition which is intended to be used internally and which confers to the organisms immunity against the following microbial infectious illnesses: diphtheria, pneumonia, typhus, scarlet fever, influenza, septic infections, cerebral-spinal meningitis, syphilis, pest, cholera and tuberculosis; it is also effective in another kind of disease, viz., goiter.” (Italics not in original). The patent specification states that “The principal of these substances is creatinin …,” but offers no evidence whatever that this well-known chemical body has the extensive and miraculous powers claimed for it. In publishing a notice of this patent The Journal of the American Medical Association (Jan. 3, 1914, p. 54) explained:
“It appears that the inventor is dead, and that his estate took out the patent. Since this great benefactor should have been, by the use of his preparation, immune to practically all diseases, he must have died of senility, although this seems hardly to have been the case.”
and held:
“Assuredly granting patents on such claims ought to be sufficient to show the need of a change in the methods of granting patents—at least of the methods governing the issuance of patents for medicinal products.”
We submit, that had the department of the government entrusted with the enforcement of the federal Food and Drugs Act been consulted as to the claims of this patent, it would probably have advised that, if the absurd and palpably fraudulent claims set forth in this application for a patent were made on the label of a preparation of creatinin offered for sale in interstate commerce or in the District of Columbia, the vendor would be prosecuted.
In 1914 there was issued U. S. Patent No. 1,086,339. Here the “inventor” declared:
“It is the object of my invention to destroy parasitic micro-organisms, particularly on living tissue without injuring the latter, by progressively evolving sodium hydroxid contiguous to said tissue, from and in a moist mixture of calcium hydroxid, sodium carbonate, aluminum sulfate and boric acid ...”
In a word, this patent apparently was granted for the production of sodium hydroxid by a chemical reaction which had been in use for several centuries. Because the patentee had twisted the granting of this patent into a quasi-endorsement of his nostrum, the Council’s consideration of this preparation was sent the Patent Office as a protest against the present law which authorizes the granting of patents on unproved and improbable medical claims. At that time the Council was informed by the Patent Office that reforms in the issuance of patents for medicinal substances had been instituted, and that “the trouble will not be so pronounced in the future as it has been in the past.”
There was issued early in 1917 U. S. Patent No. 1,212,888 for a method of flavoring Epsom salt—yet this “discovery” is a procedure which has been practiced ever since the cathartic action of this bitter salt has been known. Not only does the patent describe a process long known to physicians and pharmacists, but it sets forth claims that the flavored cathartic salt produced by the process cures flatulency, indigestion, sick and sour stomach, colic and destroys worms. In commenting on this patent The Journal of the American Medical Association (June 23, 1917, p. 1914) was constrained to remark:
“The splendid conception of the framers of our constitution in providing a plan for promoting progress in science and useful arts by granting to inventors for a limited time the exclusive use of their inventions, in exchange for the publication of full knowledge thereof, is being debased. No branch of our government is of greater importance to the progress of the country than the patent office, provided that office is intelligently administered. When the patent office is used, however, for an extension of the nostrum business, founded on the abuse of patent and trade-mark laws, it becomes a menace to the public health. The objects of the patent law are being defeated by the practices of the patent office.”
Still further, attention is called to U. S. Patent No. 1,226,394 for a process of making hexamethylenamin tetraiodid and on the product so produced. This patent was issued after the Council had notified the Patent Office that hexamethylenamin tetraiodid had been discovered in 1888 and that a process identical in principle with that for which patent application appeared to have been made was published in 1916. On the basis of claims for which no evidence is produced this patent is issued for a well-known substance on the ground that as previously produced it contained a little free iodin or that the known processes were less economical. This patent appears to be an illustration of our patent procedure which obliged American users of acetylsalicylic acid to pay an exorbitant price because this country granted a patent which gave to the patentee, a foreigner, the exclusive right to the manufacture of the substance, whereas no such patent was issued in the patentee’s own country nor, so far as we can learn, in any other country. It forcibly illustrates the need for a revision either of our patent laws or of their methods of enforcement or both.
In further justification of the Council’s protest against the provisions of our present law, or the methods of its enforcement, which permit the granting of patents without thorough and scientific investigation of the claims advanced in such letters patent, the Council calls attention to the report, appearing above, of an investigation made by A. J. Carlson, A. E. Kanter and I. Tumpowski, “The Question of the Stability of Secretin,” which relates to U. S. Patent No. 1,181,424, issued to James Wallace Beveridge.
Whereas the regulations governing the issuance of patents demand that the processes shall be described in such detail that one versed in the sciences can confirm the claims made by the patentee, no pretense whatever of fulfilling this requirement is made in the patent specifications of this patent. The substance of the first three paragraphs of this patent has long been general knowledge. Nearly every sophomore medical student has himself performed, or seen performed such “experiments” as are therein described. The claims of novelty evidently are confined to the assertion that the preparation is able to “resist oxidation or deterioration”; that it is free from “poisonous and irritable chemical substances”; that it “may be administered orally to produce the desired physiological action.” etc., etc. Not the slightest hint is given as to how any person can substantiate these claims. As a matter of fact, the investigation of Professor Carlson and his co-workers has shown that a preparation having the properties claimed cannot be made by the process described in this patent. Any one familiar with the subject could have demonstrated readily that the applicant was withholding information concerning essential features of his process, assuming that he had any information on the subject (which he probably did not have) and would have advised against the issuance of the Beveridge patent.—(From The Journal A. M. A., Jan. 12, 1918.)
The following report submitted by a referee was adopted by the Council and authorized for publication.
W. A. Puckner, Secretary.
Surgodine (Sharp and Dohme, Baltimore, Md.), according to an advertising pamphlet, is a solution of 21⁄4 per cent. of iodin in alcohol, containing no alkaline iodid, but miscible with water in all proportions. The A. M. A. Chemical Laboratory reports that Surgodine is an alcoholic liquid (containing 91.8 per cent. alcohol by volume) containing free iodin, combined iodin and free acid, probably hydrogen iodid (hydriodic acid). Quantitative estimations gave 2.51 gm. free iodin per 100 c.c. and 1.78 gm. combined iodin (the greater part apparently was present as hydrogen iodid).
It is therefore similar to several other iodin preparations already considered by the Council. Like these, it is essentially similar to the official tincture of iodin, except that it is considerably weaker, and instead of potassium iodid it presumably contains hydrogen iodid and probably ethyl iodid to render the iodin water-soluble. Its composition, however, is secret.
There would be no objection to the use of ethyl iodid or hydrogen iodid, except perhaps the acidity of the latter, as a solvent agent rather than of potassium iodid. But neither is there any important advantage, and these preparations would have to be considered as unessential modifications of official preparations, and therefore ineligible for New and Nonofficial Remedies.
The attempt to make these modifications commercially profitable, however, seems inevitably to lead to exaggerations and misstatements. In an advertising pamphlet the following claims for Surgodine are unsupported by any evidence:
“But from the surgical viewpoint the addition of this potassium salt is most objectionable because when such solutions as the official tincture are used locally in the antiseptic treatment of open and often infected wounds the Potassium Iodide acts as an irritant to the wound and therefore produces a localized irritation which is not only objectionable from the surgical standpoint but also materially lessens the antiseptic power of the Iodine itself.”
“It has been demonstrated repeatedly that Iodine without the admixture of any alkaline iodide is much more efficient as a surgical antiseptic than any iodine solution that contains such an addition.”
“Iodine does not produce ‘iodism’ as quickly as the alkaline iodides do because it is eliminated more quickly and more perfectly than the alkaline iodides.”
The next statement intimates that iodin taken by mouth enters the intestinal tract unchanged and is there free to combine with various gases:
“Iodine in the presence of phosphorated or sulphurated gases in the gastro-intestinal tract unites with their hydrogen and thus breaks up these noxious compounds.”
This is certainly untrue at least for ordinary doses.
It is recommended that Surgodine be held inadmissible to New and Nonofficial Remedies because its composition is secret (Rule 1); because the therapeutic claims made for it are exaggerated and unwarranted (Rule 6); and because it is an unessential modification of the official tincture of iodin (Rule 10).
[Editorial Comment.—Surgodine is a good illustration of the economic waste inseparable from most proprietary medicines. A hospital pharmacist writes that whereas his hospital obtains tincture of iodin at less than 82 cents a pint, Surgodine costs $2.13 a pint. This means that while the free-iodin strength of Surgodine is only about one-third that of the official tincture, its price is between two and three times as high.]—(From The Journal A. M. A., Jan. 26, 1918)
The following report on Medeol Suppositories has been adopted by the Council, and its publication authorized.
W. A. Puckner, Secretary.
“Medeol Suppositories” (Medeol Company, Inc., New York) appear to be an imitation of “Anusol Suppositories” which, in 1907, were found to be inadmissible to New and Nonofficial Remedies. A comparison of the composition and of the claims made for the two preparations will be of interest in the present consideration of Medeol Suppositories:
| Anusol Suppositories (1909) | Medeol Suppositories (1917) | |||
Anusoli | 7.5 | Medeol | 0.25 | |
Zinc oxid | 6.0 | Zinc oxid | 0.5 | |
Balsam Peru | 1.5 | Acid. tannic | 0.15 | |
Ol. theobrom. | 19.0 | Bals. Peru | 0.16 | |
Ungt. cerat. | 2.5 | Cocoa butter and wax q. s. for 1 suppository. | ||
| for 12 suppositories. | ||||
“Anusol” was formerly said to be bismuth iodoresorcinsulphonate. The A. M. A. Chemical Laboratory published a report in 1909 showing that the suppositories contained only 1 per cent. of the iodin declared in the “formula,” and were greatly deficient in bismuth and sulphur. After the publication of the report the American agents for the product disclaimed that “Anusol” was a definite chemical compound. Today Anusol Suppositories are said to contain unstated amounts of the indefinite “bismuth oxyiodid and resorcinsulphonate.”
“Medeol” is said to be “resorcinated iodo bismuth,” but no information is vouchsafed as to the character or composition of the ingredient. The therapeutic claims made for the two preparations are similar, as the following, taken from circulars, show:
Anusol Suppositories
An innocuous, non-irritant remedy for anal, rectal and vaginal inflammatory affections, especially for Hemorrhoids!
The local medicinal treatment of hemorrhoidal and other inflammatory ano-rectal conditions has always been unsatisfactory. The usual media cannot be applied in effective concentration without producing intense inflammatory reactions; they are either ineffective or intolerable....
Anusol suppositories are absolutely free from narcotic, caustic or other injurious ingredients and may unhesitatingly be used by both sexes, at any age and under all conditions.
Medeol Suppositories
An innocuous, Non-irritant, Efficient Antiphlogistic for use in inflammatory diseases of the rectum, anus and vagina especially in Hemorrhoids.
Hitherto most of the local remedies used in these conditions have either been too irritating to be employed in sufficient concentration to be efficient or they have lacked efficiency per se....
Medeol suppositories do not contain any narcotic or any caustic or other constituent having violent action; their blandness permits of their use in either sex and at all ages.
The claims made for these preparations—as for instance “that surgical treatment ... should rarely be undertaken until Medeol Suppositories have been given a thorough trial”—are misleading in that they create the inference that the limitations in the palliative treatment of piles have been overcome. It is altogether untrue that these mixtures can be expected to “relieve the most obstinate cases,” as stated in a Medeol circular. This, from an Anusol circular, is equally misleading:
“If dietetic and other requirements are complied with, even the most obstinate chronic cases will frequently readily yield to treatment with Anusol Suppositories.”
The Council declared Medeol Suppositories inadmissible to New and Nonofficial Remedies because their composition is secret (Rules 1 and 2); because unwarranted therapeutic claims are made for these (Rule 6); because the name is objectionable (Rule 8), and because the combination is unscientific (Rule 10).
In those cases of hemorrhoids in which palliative measures may be expected to enable the patient to avoid surgical interference and afford relief from attacks, the object should be to secure cleanliness, to avoid irritation, whether it be by friction or irritating fecal matter, to reduce inflammation by astringents and, when necessary, to relieve pain by analgesics. If an antiseptic dusting powder is desired, boracic acid in impalpable powder with talc may be employed; if an astringent, finely powdered oxid of zinc may be added; if a local analgesic is necessary, a little extract of belladonna may be incorporated with petrolatum or other ointment base. The main reliance, in any event, should be to effect normal bowel movements by regulating the diet rather than by the use of purgatives; the use of warm water to insure cleanliness; the avoidance of irritation, especially that caused by friction and secretions; a mild astringent to reduce inflammation.—(From The Journal A. M. A., March 9, 1918)
The following report on Guaiodine, marketed by the Intravenous Products Company, Denver, has been adopted by the Council and its publication authorized.
W. A. Puckner, Secretary.
A referee of the Committee on Pharmacology, in submitting to the Council a report from the A. M. A. Chemical Laboratory on Guaiodine, advises that the Laboratory’s examination shows that instead of containing free “colloidal” iodin as claimed, the preparation is essentially an iodated fatty oil, containing only combined iodin. Equally misleading, in view of the Laboratory’s findings, are the implied claims that the antiseptic action of Guaiodine corresponds to that of free iodin.
Guaiodine is advertised mainly for the treatment of gonorrhea. While it may be true that the guaiacol contained in Guaiodine has some beneficial effect, especially when preceded by potassium permanganate irrigation as advised, the advertised claim that “Guaiodine acts as a specific for gonorrhea in a majority of cases” is utterly false.
The “case records” offered to establish the therapeutic value of Guaiodine are in themselves sufficient to condemn the “evidence.” The following are fair samples:
“The second boy came a day or so later with a slight discharge with the characteristic burning and itching, and with symptoms of a beginning gonorrhea, and judging from the source of the infection, it was believed to be so. Two injections of Guaiodine were given when the discharge ceased.”
“I have several cases that were completely cured in a very short time. I note this, that the first dose causes a cessation of the discharge and the second seems to increase the flow, but the color is changed. I give three doses, and then use a mild wash, and in ten days they are well. I am very pleased with this preparation and very truly believe that it is the best there is to date for the positive cure of gonorrhea.”
Guaiodine is manufactured by the Intravenous Products Company, Denver, Colorado. The “literature” which accompanies the product describes Guaiodine as:
“... an electro-chemically prepared iodin, suspended in oil, containing iodin, the same strength as the U. S. P. tincture of iodin, or 7 per cent., together with a therapeutic dose of guaiacol.”
The Intravenous Products Company claims that Guaiodine is made by an “electro-chemical process of preparing colloidal iodine,” discovered by one E. B. Page, and that by this process the tendency of iodin to produce iodism has been “overcome.” It is said to be “pre-eminently an antiseptic and germicide.” Guaiodine is a dark brown, oily liquid with a specific gravity of 0.9845 at 15.6 C. and an odor suggestive of guaiacol. Its solubilities were those of a fat. Free iodin was absent in the recently purchased specimen (traces were present in an older one). Steam distillation indicated that the product consisted of volatile and nonvolatile constituents. The volatile matter was concluded to consist, in the main, of guaiacol or some guaiacol-like body, and the nonvolatile matter to be an iodized fatty oil. Quantitative determinations indicated that Guaiodine contained about 7.25 per cent. of iodin in combination, and that it is composed approximately of 3 per cent. volatile matter and 97 per cent. nonvolatile matter. Hence Guaiodine appears to be an iodized fatty oil to which a small amount of guaiacol or some guaiacol-like substance has been added.
On the recommendation of the referee, the Council voted that Guaiodine be declared inadmissible to New and Nonofficial Remedies because of false statements as to composition and action.—(From The Journal A. M. A., April 6, 1918.)
The “mixed” vaccines which are discussed in the reports that follow were considered by the Council during the past year because inquiries had been received in regard to them.
In publishing these reports it is desirable that the attitude of the Council toward “mixed” vaccines again be stated. In view of the rapid development of bacterial therapy, the possibility for harm that attends the use of bacterial vaccines and the skepticism among experienced clinicians as to the value of vaccines representing a combination of organisms, the Council has felt that it should scrutinize the claims for such agents with exceptional care and that there should be admitted to New and Nonofficial Remedies only those vaccine mixtures for which there is acceptable evidence to indicate that the use of the particular mixtures is rational.
In considering the subject the Council has borne in mind the fact that in many institutions in which cases are studied and the results of therapeutic measures carefully observed and controlled, vaccines of any sort are practically never used—certainly here the stock mixed vaccine has no recognition. Experienced clinicians have generally come to the conclusion that mixed vaccines have no specific action and that any effect they may produce is due to a non-specific protein reaction.
As set forth in the reports, in no case was the evidence submitted by the proprietors sufficient to establish the claims made for the preparations. Hence none was accepted for New and Nonofficial Remedies.
The preparations that form the basis for the accompanying reports are only a few of the many that are being made and sold by some biological houses. Doubtless many of those not dealt with in this report are equally irrational and sold under claims equally—or probably even more—unwarranted than those with which the present report deals.
W. A. Puckner, Secretary.
In response to inquiry the Council undertook a consideration of the following “mixed{”} vaccines sold by the Abbott Laboratories:
M. Catarrhalis-Combined-Bacterin, said to contain killed Micrococcus catarrhalis, Bacillus Friedländer, Pneumococci, Streptococci, Staphylococcus aureus and Staphylococcus albus.
B. Coli-Combined-Bacterin, said to contain killed Streptococcus viridans, Streptococcus hemolyticus and Bacillus coli.
Pertussis-Combined-Bacterin, said to contain killed Bacillus pertussis, Pneumococci, Streptococci, Staphylococcus albus, Staphylococcus aureus and Micrococcus catarrhalis.
Streptococcus-Rheumaticus-Combined-Bacterin, said to contain killed “Streptococci (Rheumaticus, Viridans, etc.)” and Pneumococci.
Streptococcus-Viridans-Combined-Bacterin, said to contain killed Streptococcus viridans, Streptococcus hemolyticus, Pneumococcus and Staphylococcus albus.
The Abbott Laboratories were asked to assist in the investigation of these products and to submit evidence to establish their eligibility for admission to New and Nonofficial Remedies. The manufacturer was informed that the Council accepts “mixed” vaccines or bacterins, provided the usefulness of these products is established by acceptable clinical evidence, and references to the literature bearing on the value of the preparations were requested.
The Abbott Laboratories submitted specimens of the products, the advertising matter therefor and a considerable list of references to current literature; all of which was transmitted to the Committee on Serums and Vaccines for consideration. In due time a referee of the committee submitted the following report:
The referee has studied the literature covered by the references submitted. In general the articles are favorable to the use of vaccines, though many of these papers do not consider “mixed” vaccines; indeed, a number of the articles do not discuss treatment at all, but are devoted entirely to the consideration of etiology of the disease. Many of the papers are by those who are obviously overenthusiastic on the subject of the use of biologic preparations. One paper—not included in the references submitted by the Abbott Laboratories—records an alarming reaction following a dose of mixed vaccine; no claim is made that improvement followed.
The following comments on the submitted references are offered:
M. Catarrhalis-Combined-Bacterin.—Only four of the nine references given deal with the therapeutic use of the vaccine. The reported results in general were favorable, but sometimes in the discussion evoked by certain of the papers, views the reverse of those expressed by the author were brought forward. The enthusiasm of one writer is shown in his statement that following the use of vaccine in cases of carbuncle complicating diabetes the sugar in the urine disappeared or was reduced. One observer, who reports excellent results in nasal pharyngeal catarrh, speaks of certain vaccines as “bulk goods,” while another considers “——’s No. 7” as the proper thing. It is evident that the reports are not based on careful, scientific data, or such unscientific definition of the product employed would not be used.
B. Coli-Combined-Bacterin.—In the references cited in support of this preparation the following general statements are noted: One enthusiastic writer says, “It must be recognized that we have no satisfactory explanation of the action of vaccines, and their use at present is empirical.” One author dwelt on the superiority of autogenous vaccines but admits that occasionally stock vaccines are indicated. One vaccine therapist in concluding an article states, “It is simply impossible to practice modern urology without our modern biologic products.” Yet it is a well-known fact that many successful and capable genito-urinary surgeons avoid the use of vaccines, mixed or simple.
Pertussis-Combined-Bacterin.—These reports are uniformly favorable, but are not controlled and their value is not to be compared with a recent report from the New York City Department of Health which indicates that the vaccine is practically valueless. It is noted, further, that one of the articles cited which dealt rather fully with the treatment of pertussis did not mention vaccines.
Streptococcus-Rheumaticus-Combined-Bacterin.—The references cited in support of the preparations by the manufacturer give no support whatever for the use of mixed stock vaccines. The first reference deals with the relation of Streptococcus viridans to arthritis deformans and endocarditis and reports the following cases:
Case 1.—Vaccine case—improvement after eight months.
Case 2.—Slight improvement following use of vaccine.
Case 3.—Slight improvement following use of vaccine.
Case 4.—Marked improvement.
Case 5.—Prompt improvement.
Case 6.—Vaccine not mentioned.
Case 7.—Vaccine followed by slight improvement.
In each of the cases other methods of treatment were used. The paper shows the etiologic relation of Streptococcus viridans rather than the value of vaccines. There is no indication that stock vaccines were used, though the paper is not clear on this point. The second paper deals with the application of vaccine therapy in the treatment of arthritis. This paper is by a man who is avowedly an enthusiast on vaccine therapy. The indications are that he generally used a mixed autogenous vaccine, but the reports of cases are not always clear. This writer apparently makes no serious attempt at the classification of the joint conditions he treats. The third reference is a purely experimental study and has no bearing on the use of vaccines in treatment. The fourth article was admitted by the manufacturer to be “negative as regards evidence.” The fifth reference specifically states that “the vaccine must be autogenous.” The sixth reference deals with the experimental production of appendicitis by the use of diplococci, and has not the most remote bearing on the use of vaccines in the treatment of rheumatism.
Streptococcus-Viridans-Combined-Bacterin.—The article which bears evidence of more care than the others admits that we are not in position to state the value of vaccines in pyorrhea but the author believes they may have value supplementary to local treatment.
It is not surprising that a large number of favorable reports can be accumulated when we appreciate how promptly men report what they consider to be their successes and how commonly they leave their failures unrecorded. Bearing in mind the fact that these stock mixed vaccines, though before the profession for many years, have not been used, or continued in use, in hospitals where work is rigidly controlled and that they are used practically not at all in the large government hospital service, a candid critic must hold that there is no substantial evidence in favor of the therapeutic use of a mixed vaccine, certainly not for stock “goods” and that probably there is but a limited field for the employment of autogenous vaccines.
The referee calls attention to a shift in the advertising matter on vaccines—the tendency to recommend vaccines to be used in conjunction with drugs. A heading in the Abbott booklet reads, “The Biologics Do Not Replace Drugs”; and the paragraph speaks of serums and bacterins as “new tools, supplemental to those we already have, but not replacing them.” ... “We need them both.”
The referee recommends that the several mixed vaccines discussed in this report be not accepted on the grounds that satisfactory evidence of their value is wanting.
Having been endorsed by the Committee on Serums and Vaccines the Council adopted the report and declared M. Catarrhalis-Combined-Bacterin, B. Coli-Combined-Bacterin, Pertussis-Combined-Bacterin, Streptococcus-Rheumaticus-Combined-Bacterin and Streptococcus-Viridans-Combined-Bacterin ineligible for admission to New and Nonofficial Remedies.
Because of inquiry received, the Council requested Eli Lilly and Company to aid in determining the acceptability of the following products for New and Nonofficial Remedies: “Catarrhal Vaccine Combined,” said to contain killed cultures of the Bacillus of Friedländer, Micrococcus catarrhalis, Staphylococcus aureus and albus, Pneumococcus and Streptococcus; “Influenza Mixed Vaccine,” said to contain killed cultures of Staphylococcus albus and aureus, Streptococcus, Pneumococcus, Micrococcus catarrhalis and Bacillus influenzae.
Lilly and Company sent the circulars, etc., used in advertising these products. A circular for “Catarrhal Vaccine Combined” contained the following claim:
“Catarrhal Vaccine has been especially useful in many respiratory infections, including bronchitis, pharyngitis, rhinitis, chronic catarrh and in the mixed infections of pulmonary tuberculosis.”
A circular for “Influenza Mixed Vaccine” contained the following:
“The vaccine is useful in the treatment of influenza and ordinary colds, and in any infection in which the Bacillus influenzae is the causative agent.”
An advertising pamphlet contained the following:
“Catarrh, Acute and Chronic; Colds, Influenza.—The micro-organisms capable of producing catarrhal conditions of the nose and pharynx and most commonly isolated are B. Friedländer, M. catarrhalis, staphylococcus, pneumococcus (in infections beginning in the larynx), B. influenza and streptococcus. These organisms are found normally in the respiratory passages and acquire virulence only when resistance has been lowered through overwork, exposure to cold, etc.
“The results following the use of Catarrhal Vaccine Combined (in the non-epidemic forms) and influenza Mixed Vaccine (in the epidemic types) have been very satisfactory, due to the great vascularity of the tissues. Acute attacks are aborted altogether or shortened in duration and the danger of complications greatly minimized.”
No evidence was submitted which warrants the preceding claims nor is the Council aware of any reliable testimony to indicate that the administration of the mixture here discussed is warranted or desirable. On the recommendation of the Committee on Serums and Vaccines the Council voted that “Catarrhal Vaccine Combined-Lilly” and “Influenza Mixed Vaccine-Lilly” be not included in New and Nonofficial Remedies because satisfactory evidence of their value is wanting.
Because of inquiry received, the Council took up the consideration of “Influenza Serobacterin Mixed-Mulford,” and requested the Mulford Company to present evidence to establish the admissibility of the preparation to New and Nonofficial Remedies. The Mulford Company sent specimens of the serobacterin in question, an advertising circular and a letter by the director of its Biologic Laboratories.
According to the label on the package, the preparation is made from the following organisms: Bacillus influenzae, Staphylococcus aureus, Staphylococcus albus, Streptococcus, Pneumococcus and Micrococcus catarrhalis (group). This mixture is recommended by the manufacturer:
“For the prophylaxis and Treatment of Common Colds, Mixed Infections of the Respiratory Mucous Membranes, Acute and Chronic Catarrhal Conditions of the Nose, Throat and Respiratory Passages.”
No evidence is submitted for this recommendation except that in “colds and bronchitis and the other common infections of the upper respiratory passages ... five or six bacteria are very commonly present—two or more of them are nearly always present ...” and the letter by the director of the Mulford Biologic Laboratories expressing the belief that in his own case the use of the mixed vaccine has aborted or prevented colds.
As regards the use of this complex biologic preparation:
First, the cause of common colds is, at the present time, quite unknown. One of the most striking things is that at the beginning of a cold the organisms to be cultivated from the nasal mucous membrane are very few in number and there is no uniformity in the type of organism found. If someone of the well-known organisms (Streptococcus, Staphylococcus, Pneumococcus, Micrococcus Catarrhalis, Influenza Bacillus, etc.) were responsible, we should expect to find one of them preponderating and in overwhelming numbers. This is far from the case. After the duration of the cold for a day or two with the increased production of mucus and apparently with the infection of a mucous membrane whose powers of resistance have been greatly lowered, bacteria of all kinds are to be found in immense numbers. There is considerable reason for believing that an ultramicroscopic organism is responsible for this condition (See Foster, Journal of Infectious Diseases 21:451 [Nov.] 1917).
Second, there is no acceptable clinical evidence that vaccination with the influenza bacillus, the Streptococcus, the Pneumococcus or the Micrococcus Catarrhalis will influence the course of an infection due to one or the other of these organisms. It has been repeatedly found that a staphylococcus vaccine is of a certain degree of value when the infection with the staphylococcus is localized, but it is well known that general systemic infections with the staphylococcus are not at all benefited.
Third, the letter submitted as evidence by the Mulford Company is not convincing. The Council is not prepared to accept evidence of this sort unless it is in volume large enough to justify a definite conclusion.
Holding that there is no evidence for the value of this mixture, the Council declared “Influenza Serobacterin Mixed-Mulford” inadmissible to New and Nonofficial Remedies because its use is illogical.
Because of inquiry received the Council decided to consider this preparation and requested the manufacturer, G. H. Sherman, Detroit, Mich., to submit evidence in support of the claims made for it.
This vaccine is said to be made from killed cultures of Streptococcus, Pneumococcus, Micrococcus catarrhalis, Staphylococcus aureus, and Staphylococcus albus. In the printed matter sent out by G. H. Sherman this vaccine is recommended for hay-fever, in which it is stated that some of the symptoms are due to bacterial invasion of the respiratory mucosa; for tonsillitis, both as a remedy and as a prophylactic against rheumatic and other sequelae; for “throat infections”; for rhinitis with the claims that acute coryza can be aborted within twenty-four hours; for pneumonia in which it is advised for all stages; for laryngitis, for bronchitis, and for asthma.
No acceptable evidence was submitted as to the value of the product in the treatment of any of the foregoing conditions. In view of what is known about non-specific reactions, it seems likely that any influence which this vaccine may have on the diverse conditions enumerated by the manufacturer, is due to this, rather than to the combination of organisms used in its preparation.
On the recommendation of the Committee on Serums and Vaccines, the Council declared “Sherman’s Mixed Vaccine No. 40” ineligible to New and Nonofficial Remedies because the therapeutic claims made for it are unwarranted (Rule 6) and because the combination, in view of its complexity, is irrational and detrimental to sound therapy (Rule 10).—(From The Journal A. M. A., June 23, 1918.)
Ophthalmol-Lindemann was taken up for consideration by the Council because of inquiries received. The following report, declaring Ophthalmol inadmissible to New and Nonofficial Remedies, was adopted by the Council and its publication authorized.
W. A. Puckner, Secretary.
Ophthalmol-Lindemann (Innis, Speiden and Co., New York) is advertised as a treatment for eye diseases by “hyperemia.” The circular advertising the product is written somewhat in the style of “patent medicine” advertisements. It contains testimonials of dubious value. The principle underlying the use of Ophthalmol is that employed to a considerable extent by ophthalmologists, through the use of ethylmorphine (“dionin”), etc., viz., the production of conjunctival irritation in inflammatory eye diseases. Ophthalmol is, therefore, merely a special agent for the production of such ophthalmic irritation.
The advertising circular contains no evidence that Ophthalmol is in any respect superior to the established agents for producing conjunctival hyperemia. On the other hand, there are obvious objections to the use in the eye of a substance of unknown and apparently indefinite composition and uncertain activity. Ophthalmol is said to be an oily solution of “glandular extract of the fish Cobitis Fossilis.” Cobitis fossilis is a small fish said to be common in Germany. According to Kochs, who analyzed Ophthalmol (Arb. a. d. Pharm. Inst. d. Univ. Berl., 4:140, 1907), this fish is popularly believed to predict weather, but medical virtues are not ascribed to it. This “fishy” extract is indefinite, to say the least.
The activity of the preparation is described by the manufacturer thus: “It seems probable that the typical action of Ophthalmol is due to certain organic acids which may have formed during manufacture through the decomposition of protein bodies contained in the crude material.” The profession is not told whether this important decomposition is, or, in fact, can be controlled so as to produce a material of uniform activity.
Kochs concluded from his analysis that Ophthalmol had the properties of rancid olive oil containing about 6 to 7 per cent. mineral oil. The oil contained no nitrogen, left no ash on ignition and though traces of iodin were claimed to be present, no iodin could be found.
It is recommended that Ophthalmol be rejected first, because the use in the eye of an irritant of secret composition and uncertain activity is unscientific and against the interest of public health; second, because Ophthalmol is of secret composition (the composition claimed being practically meaningless), and, third, because no evidence has been submitted to substantiate its claimed superiority over established methods of treatment. The Council declared Ophthalmol inadmissible to New and Nonofficial Remedies.—(From The Journal A. M. A., July 6, 1918.)
The following report on Silvol (Parke, Davis & Company) was adopted by the Council and its publication authorized.
W. A. Puckner, Secretary.
The Council took up the consideration of Silvol (Parke, Davis & Company) because of inquiries received. The following report was submitted by the referee in charge of silver preparations:
Silvol (Parke, Davis & Company) is a silver-protein preparation of the Argyrol type. Like Argyrol, it is said to contain about 20 per cent. of silver. The referee finds that, like Argyrol, it is nonirritant to the nasal mucosa in a 10 per cent. solution; does not precipitate with chlorid; dissolves in water readily; a 25 per cent. solution has a high specific gravity (Silvol, 1.137 at 20 C.; Argyrol, 1.147 at 20 C.), and is not very viscid (viscosity, 1.25). A 1:1,000 solution of Silvol is clear and about 50 per cent. deeper in color than a solution of Argyrol of the same strength.
Silvol differs from Argyrol mainly in that its solutions yield a fine precipitate with egg albumin (under suitable conditions), while Argyrol is nonprecipitant; and in that Silvol solutions are not so effectively decolorized by Lloyd’s reagent.
The manufacturers did not reply to an inquiry with regard to the basis for the claims made for Silvol (see Appendix). The referee was therefore obliged to deduce these claims from the firm’s advertising matter. About the same claims are made for the local use of Silvol as are generally made for Argyrol. These may be accepted without detailed evidence in view of the similarity of the two preparations.
Its usefulness, as suggested in the advertising, when given by mouth “in the treatment of acute or chronic gastritis, gastric ulcer, or gastro-enteritis,” or the efficacy of very dilute solutions (0.2 per cent.) against dysentery, etc., is doubtful and requires substantiation by evidence. The claims that Silvol is astringent, though nonirritant and noncoagulant, that it is a “powerful germicide” or even that it is a “powerful antiseptic,” and that it may be used with advantage wherever “a silver salt is indicated,” need substantiation. There is no proof of the assertions that Silvol is “the most efficacious of silver salts”; “the most efficient antiseptic,” and “the most remarkable organic silver compound ...”
As the manufacturers have not presented any evidence for their highly improbable claims, and as they have not signified any intention of making their claims agree with substantiated facts, it is recommended that Silvol be declared inadmissible to New and Nonofficial Remedies.
The Council adopted the report of its referee and authorized its publication.
The following letter from the Secretary of the Council was sent to Parke, Davis & Company, March 20, 1917. No reply to it has been received:
The referee of the Council who is conducting an investigation of silver preparations asked me to inquire if you are willing to submit your evidence for the following claims which are made in your circulars for Silvol:
1. How it is possible for the solution to be astringent, and at the same time nonirritant and noncoagulant?
2. That intestinal irrigation with a Silvol solution containing 10 to 15 grains to the pint is sufficiently bactericidal to “be used in the abortive treatment of such infectious processes as dysentery, cholera infantum, and colitis.”
3. What evidence have you as to the degree of antiseptic and germicidal power of Silvol solutions?
4. What evidence have you as to the degree of antiseptic and germicidal power of 5 per cent. Silvol Ointment?
A reply to the above questions and any other information in regard to Silvol will receive careful consideration.—(From The Journal A. M. A., July 13, 1918.)
Following inquiries, the Council took up “Katharmon” for consideration and authorized publication of the following report.
W. A. Puckner, Secretary.
The Katharmon Chemical Company of St. Louis in advertising its Katharmon appeals especially to a profession whose members, should they live up to their ethical code, could not prescribe it.124 In 1893 (when the publication of “a formula” for proprietary preparations was thought to satisfy the requirements of scientific medicine) an advertisement in The Journal of the American Medical Association gave the following “formula” for Katharmon:
“Hydrastis Canadensis, Phytolacca Decandra, Acid Salicylous C. P. (from Oil of Wintergreen), Acid Boric C. P., Mentha Arvensis, Thymus Vulgaris, Dist. Ext. Hamamelis Virg. Conc.”
In 1907 an advertisement in the Kansas City Medical Index-Lancet declared that:
“Katharmon represents in chemical combination the active principles of Hydrastis Canadensis, Gaultheria Procumbens, Hamamelis Virginica, Phytolacca Decandra, Mentha Arvensis, Thymus Vulgaris, with two grains C. P. Boric Acid to each fluid drachm.”
Now the advertisements which appear in some medical journals state:
“KATHARMON represents in combination Hydrastis Canadensis, Thymus Vulgaris, Mentha Arvensis, Phytolacca Decandra, 101⁄2 grains Acid Borosalicylic, 24 grains Sodium Pyroborate to each fluid ounce of Pure Distilled Extract of Witch Hazel.”
A comparison of these so-called formulas shows that they have not only varied from time to time, but that in no instance was a quantitative statement with regard to all the asserted ingredients given.
The Chemical Laboratory of the A. M. A. reports: Katharmon has an alkaline reaction and therefore cannot contain boric acid, salicylic acid or “borosalicylic acid” (the latter is unknown to medical literature except as loosely applied to a simple mixture of boric and salicylic acids). The solution gives tests for sodium, borate, and salicylate and therefore probably contains sodium borate and sodium salicylate. Examined by the methods used for the determination of hydrastin in goldenseal preparations, a residue giving only a faint test for alkaloid was obtained; if present at all, hydrastis canadensis (goldenseal) is there only in very small amounts.
A circular wrapped with the trade package of Katharmon contained the following, palpably unwarranted, claims:
“Internally it is very useful in acute indigestion, Gastric Catarrh, Diarrhoea and Cholera Infantum.”
“... it has demonstrated its remarkable curative effects, not only in preventing unhealthy conditions of fresh wounds, but also in correcting the decaying of putrefactive processes peculiar to the body under certain circumstances. It has, further, a remarkable efficacy in surface inflammations, whether produced by accident or disease, and is an indispensable remedy in the affections of the mucous membranes of the nose, mouth, stomach, bowels, vagina, uterus, urethra, bladder and rectum.”
Katharmon is in conflict with Rules 1 and 4 of the Council on Pharmacy and Chemistry because of its indefinite and secret composition and the method of advertising it indirectly to the public; it is in conflict with Rules 10, 6 and 8, in that it is an irrational shotgun mixture sold under unwarranted therapeutic claims and under a name nondescriptive of its composition.—(From The Journal A. M. A., Aug. 10, 1918.)
“Iodinized Emulsion (Scott)” and “Creosotonic (Scott)” are proprietary preparations of the Dawson Pharmacal Company, Dawson Springs, Ky. The latter preparation used to be known as “Iodinized Emulsion (Scott) with Hypophosphites, Guaiacol and Creosote.” In 1907 these preparations were considered by the Council and found inadmissible to New and Nonofficial Remedies. Examination of the preparations having been again requested, the Council considered them anew because the composition and claims had been changed somewhat and because at the previous consideration no report was published.
The reports which appear below were sent to the Dawson Pharmacal Company for comment before publication. In reply the company offered to revise its claims for the preparations. The Council replied that the report sent explained that both preparations are irrational mixtures, and hence a revision of the claims would not make them eligible for New and Nonofficial Remedies. It advised that publication of the report would be withheld sixty days and that it would be revised if new information or evidence was submitted permitting such revision. After expiration of the stipulated postponement, the Dawson Pharmacal Company wrote that no new advertising matter had been prepared, but that the old circulars were not being sent out.
As these irrational preparations were still sold and advertised to the medical profession and presumably used by some physicians, the Council directed publication of its report with this explanation.
W. A. Puckner, Secretary.
The label for Iodinized Emulsion (Scott) declares:
“Each fluidram contains: Alcohol, m. 43⁄4; Rectified Ol. of Turpentine, m. 31⁄2; Iodin, gr. 1⁄8; Phenol, gr. 1⁄2; Glycerine and Elixir Lactated Pepsin with Aromatic Oils in the form of a perfect emulsion.”
A circular which gives what is asserted to be the composition of Iodinized Emulsion, declares that, among other ingredients, each fluidram contains “one and three quarters m. Tincture of Iodine.” Both the statement on the label that the preparation contains “iodin” and the one in the circular that tincture of iodin is present in the product are incorrect, for the A. M. A. Chemical Laboratory reports that no free iodin could be detected in the preparation, and that it responded to tests for iodid instead.
An advertising circular for Iodinized Emulsion (Scott) makes unwarranted claims for the therapeutic properties of the constituents. For example:
“... the great usefulness of Turpentine in diseases, especially of the Intestinal Infection, such as the Meteorism and Tympanites of Typhoid.”
And this absurdity:
“... where Turpentine, Carbolic Acid or Iodine or even Pepsin is indicated, that it will give satisfaction in each and every case.”
Iodinized Emulsion (Scott) is not a “pharmaceutical triumph”; it is an irrational mixture—a reminder of a decadent polypharmacy—sold under misleading and unwarranted claims. It is inadmissible to New and Nonofficial Remedies for conflict with Rules 1, 6, 8 and 10.
Creosotonic (Scott), advertised as a “reconstructive tonic” for the tuberculous, according to the label, contains in each fluidram:
“Alcohol, m. 21⁄2; Creosote and Guaiacol sulphonates of each, gr. 1; Compound Hypophosphites, gr. 1 (including Quinine Hypophosphites, gr. 1⁄36 and Strychnine Hypophosphites, gr. 1⁄256), with Iodinized Emulsion (Scott) m. 30.”
As in the case of Iodinized Emulsion (Scott), the advertising makes exaggerated therapeutic claims for the individual constituents of the preparation and for the heterogeneous mixture of guaiacol and creosote sulphonates, hypophosphites, quinin, strychnin, turpentine, phenol, iodin, “lactated pepsin,” etc. Thus, while it is well established that in guaiacol sulphonate and creosote sulphonate the phenolic constituent is bound so firmly that, when administered, but very little is split off in the organism, yet the advertising claims “that the system can be saturated in a shorter time and with smaller doses of creosote and guaiacol sulphonates than with any other form of these drugs” and that (on the false premise that the guaiacol and creosote from these drugs will permeate the tissues of the lungs) “they help to clear up the local infection and thus aid in returning to normal the diseased mucous membrane.”
In the advertising pamphlet, following a discussion of the effect of climate and food in the treatment of the tuberculous, we read:
“While admitting the great importance of the foregoing points, we are firmly of the opinion that proper medication is a great aid in the treatment of pulmonary tuberculosis, and, with this in view, we offer to the profession Creosotonic (Scott) believing that in it we have a superior preparation for this purpose.”
This is unwarranted. Of course suitable medication to meet special conditions is proper in the treatment of tuberculosis, but the routine administration of a complex and irrational mixture such as Creosotonic (Scott) is bound to cause inattention to the prime requisites for the proper treatment of the tuberculous—hygienic surroundings and good food.
Creosotonic (Scott) is an irrational mixture, sold under misleading and unwarranted claims. It is inadmissible to New and Nonofficial Remedies for conflict with Rules 1, 6, 8 and 10.—(From The Journal A. M. A., Aug. 24, 1918.)
The following report on Campetrodin and Campetrodin No. 2 has been adopted by the Council and its publication authorized.
W. A. Puckner, Secretary.
The following report of the A. M. A. Chemical Laboratory on “Campetrodin” and “Campetrodin No. 2,” sold by the A. H. Robins Company, Richmond, Va., was submitted to the Council by a referee of the Committee on Pharmacology:
Campetrodin and Campetrodin No. 2, Double Strength, are called “ethical medicinal specialties” by the A. H. Robins Company, Richmond, Va., which sells them. An advertisement in the Maryland Medical Journal (December, 1917) contains the following claim for composition:
“Campetrodin (Made in Two Strengths of Iodine). This preparation is an Oleaginous Solution of Iodine in Camphor.”
A booklet describing the “specialties” of the Robins Company contains the following in reference to Campetrodin: “Composition: Camphor, Iodine Element, Oleaginous Solvent.” From this it appears that the preparations are claimed to contain elementary (free) iodine in an “oleaginous solvent.” Since free iodin, as is well known, readily combines with fats, it was decided to determine the form in which the iodin was present in these preparations. The examination demonstrated that both preparations contained but a trace of free iodin. On steam distillation there was obtained from both preparations a distillate amounting to about 35 per cent. by volume which had an odor strongly suggestive of turpentine, while the residue contained the iodin and had the characteristics of an iodized fatty oil.
Quantitative determinations indicated that Campetrodin contained approximately 0.03 per cent. of free iodin and 1.3 per cent. of iodin in combination with the fatty oil. Campetrodin No. 2, Double Strength, contained approximately 0.03 per cent. free iodin and 2 per cent. of iodin in combination with the fatty oil.
Thus, contrary to the published statements, Campetrodin is not a preparation of free (elementary) iodin and Campetrodin No. 2, Double Strength, does not contain twice as much iodin as Campetrodin.
The report of the Chemical Laboratory shows that the statements made in regard to the composition of Campetrodin and Campetrodin No. 2 are incomplete in some respects and false in others. In view of the Laboratory’s findings it appears superfluous to inquire into the therapeutic claims made for the preparations: It is evident, however, that a solution containing practically no free iodin is not, as claimed by the Robins Company, “adapted for use wherever ... iodin is indicated externally....”
It is recommended that Campetrodin and Campetrodin No. 2 be declared inadmissible to New and Nonofficial Remedies because of false statements as to chemical composition and therapeutic action, constituting conflicts with Rules 1 and 6.
The Council adopted the recommendation of the referee and authorized publication of this report.—(From The Journal A. M. A., Sept. 21, 1918.)
The Council has authorized publication of the following which explains why Carminzym was not accepted for New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Carminzym is a tablet sold by Fairchild Bros. and Foster, New York. Each tablet contains, according to claims made, approximately 32 mg. of an extract of pancreas, 50 mg. sodium bicarbonate, 172 mg. prepared chalk, 1.5 mg. powdered ipecac and “aromatics q. s.” Without considering other possible conflicts with its rules, the Council held the preparation inadmissible to New and Nonofficial Remedies for conflict with Rule 10 which holds that unscientific or useless articles are not acceptable products.
The Council holds that complex mixtures of remedial agents are, from every point of view, inimical to therapeutic progress and therefore to the public welfare. Such mixtures are especially objectionable because it is impossible accurately to determine the effects which follow the simultaneous administration of a number of drugs having dissimilar actions; because the practice of prescribing such mixtures tends to discourage careful consideration of the special needs of individual patients without which there can be no rational drug therapy. On the contrary, with the use of such mixture therapeutic treatment becomes haphazard and mere guesswork.
The Council, appreciating that long established customs cannot be changed at once, has applied Rule 10 concerning the recognition of mixtures with the greatest leniency compatible with consistency. When there has been a reasonable doubt concerning the value of a mixture it has frequently directed that Rule 10 should not apply pending further clinical trial of such mixture. In no instance has subsequent experience shown that a strict interpretation of the rule would have worked hardship or injustice. The Council feels that there is no longer warrant for the admission of complex mixtures to New and Nonofficial Remedies or for the retention of any that have been admitted unless definite evidence of the therapeutic value of such combinations is available. In accordance with this decision several mixtures now described in New and Nonofficial Remedies will be omitted at the expiration of the three year period for which articles are accepted.
Reverting to the Carminzym tablet: When it is desired to obtain the effects of pancreatic extract by oral administration it must be administered with a view of preventing its destruction by the gastric fluid. With this end in view an antacid should be administered to decrease the acidity of the gastric juice. The amount of alkali may be supplied in the form of any of the official preparations, but the amount must be adjusted to the individual patient for the reason that no two successive patients are likely to have the same degree of gastric acidity.
Ipecac has a well defined though limited field of usefulness. When it is used, it should be given with a due regard to the amount needed by the patient and the frequency of the repetition of the dose. There is no reason to suppose that any two successive patients will require ipecac and extract of pancreas in a fixed proportion and with equal frequency. As a matter of fact, the amount of ipecac in Carminzym is so small that no definite therapeutic action can be assigned to it and its use in this combination is purely empirical.
In a word, the employment of mixtures of pancreatic extract, alkalis, ipecac and carminatives in fixed proportion leads to slipshod treatment and irrational therapeutics. Carminzym is an irrational mixture the use of which is detrimental to therapy.
The preceding report was sent to Fairchild Bros. and Foster for comment in accordance with the Council’s usual procedure. The following reply was received:
The long established custom of the use of mixtures of remedial agents rests upon considerations well known and generally accepted. This is equally true of combinations of drugs of similar and dissimilar properties. The drugs of these combinations, especially those of marked therapeutic action, are well known and used by themselves when indicated.
In fact, dissimilarity of action is a cause of combination, an essential of synergism.
Drugs classed as similar are by no means alike in action; laxatives, tonics, carminatives, diuretics are combined with distinct advantage, economy of dose, enhanced effect, potency not obtainable with the single drug.
Your sweeping arbitrary conclusions that complex mixtures of remedial agents are from every point of view inimical to therapeutic progress is not, it seems to us, sustained by fact and experience. There is therapeutic progress in the considerate use and observation of combinations as well as in the use of a single drug. Indeed, in the production of a synthetic chemical substance as a therapeutic agent, the combination of potent and dissimilar elements is worked out to mitigate and correct an objectionable side effect, and promote desirable action.
As for ourselves, at the very outset in our line of work we quite voluntarily declared our principles and our intentions as opposed to incompatible and therefore unstable or inert combinations of the enzymes; and against the “unnecessary multiplication of preparations”—see Fairchild’s Hand-Book of the Digestive Ferments.
Is not this after all the crux of the whole matter—does a combination contain the ingredients stated, does it possess the demonstrable properties which are to be attributed to it in consequence of this composition; and if for a certain purpose, is it well designed therefor?
Carminzym presents certain agents of well known properties, not in the least of incompatible or antagonistic action, but indeed especially suitable for the particular purpose designed; its efficacy not to be measured and judged by theory or opinion as to the efficiency of a certain dosage of a particular drug by itself. That the doses as contained are minimal and effective is distinctly advantageous.
The alkaline carbonates are in Carminzym in stated quantities; the physician adjusts the dosage to the individual patient and with obvious evidence of the efficiency of the adjustment. As we understand it, the employment of alkaline carbonates is not based on purely chemic considerations—a definite known quantity of acid of the gastric juice is to be neutralized; the whole literature and practice dealing with the alkaline carbonates show them to be accredited with a much wider field of use and repute in gastro-intestinal disorders.
The pancreatic extract in Carminzym is designed to be diffusible in the stomach, the tablet is preferable to be crushed in the mouth before swallowing, and we believe the pancreatic extract to be an effective constituent as administered in Carminzym.
You comment as follows:
“Ipecac has a well defined though limited field of usefulness. When it is used it should be given with due regard to the amount needed by the patient and the frequency of the repetition of the dose.”
This in a sense may be said of any of the most useful drugs, but not in the least special degree does it apply to ipecac, which is, on the contrary, of quite characteristic, peculiar range of therapeutic properties, useful in varying combinations and in widely varying proportions and doses according to the purpose for which it is employed.
Ipecac in well known official alkaline, carminative, laxative preparations occurs in the “average dose” in the varying quantities of 1⁄14, 1⁄10, 1⁄8, and 3⁄16 of a grain.
The ipecac in combination with the other ingredients in Carminzym is designed for a tablet which shall carry a minimal quantity whilst capable of adequate remedial action, thus admitting of increase of dosage or repetition as occasion requires. The quantity of ipecac was not taken at random, but chosen after long trial and consideration.
We believe that Carminzym possesses carminative properties in a superior degree and that, furthermore, in consequence of its composition it directly stimulates the gland secretions and thus exerts a beneficial action upon the whole digestive functions.
Carminzym is for use as occasion requires, and this is to be especially noted. Thus it is not only of direct benefit, but helpful in promoting systematic therapeutic measures and regimen.
The Council takes the ground that complex mixtures of remedial agents are so wrong that there is no longer warrant for their admission into New and Nonofficial Remedies; and that Carminzym is an irrational mixture.
We hold that certain desirable therapeutic properties may rationally be attributable to Carminzym; and that these are manifested in practice.
During the time since the description was sent and the receipt of the statement of the action of the Council, some ten months, Carminzym has proved of constantly increasing service.
The statement in the letter of Fairchild Bros. and Foster “The long established custom of the use of mixtures of remedial agents rests on considerations well known and generally accepted” might well be paraphrased to read: The one-time prevalent custom of using ill-considered combinations of remedial agents has been thoroughly discredited and is generally abandoned by progressive practitioners. Such arguments as that “laxatives, tonics, carminatives, diuretics are combined with distinct advantage” have led to the use of irrational mixtures such as the compound syrup of hypophosphites and the electuary of theriaca. The Council is confident that no one who has studied the causes and treatment of digestive disorders will find occasion to prescribe at one time all the ingredients stated to be contained in Carminzym, and certainly not in the fixed proportions present therein.
The comments in the Council’s report concerning ipecac certainly does apply to all active therapeutic agents. Ipecac was mentioned in the report because the several constituents of Carminzym were under discussion and hence it was necessary to point out the futility of the small dosage of ipecac in this mixture.
The announcement that “Carminzym has proved of constantly increasing service” is not convincing. The Council does not know of a single clinical study of the action of Carminzym under conditions which would have afforded satisfactory evidence of its therapeutic value.—(From The Journal A. M. A., Sept. 28, 1918.)
The following report on Phillips’ Phospho-Muriate of Quinine Comp. has been adopted by the Council and authorized for publication.
W. A. Puckner, Secretary.
Phillips’ Phospho-Muriate of Quinine Comp.125 is sold by the Charles H. Phillips Chemical Co., New York. According to the published formula, each fluidram contains:
Phosphoric Acid | 2 | minims | ||
| Potassium Phosphate | 21⁄4 | grains | ||
| Magnesium Phosphate | ||||
| Calcium Phosphate | ||||
| Ferric Phosphate | ||||
Quinin Muriate (equal to nearly 1⁄2 gr. Bi-Sulph.) | 1⁄4 | grain | ||
Strychnin | 1⁄120 | grain | ||
| Flavoring, Glycerin and Syrup, q. s. | ||||
Some typical claims made for the preparation are:
“With marked beneficial action upon the nervous system. To be relied on where a deficiency of the phosphates is evident.”
“... brace those tired nerves and aid that worn stomach with Phillips’ Phospho-Muriate of Quinine.”
“The maintenance of a satisfactory blood pressure level free from intervals of depression may be accomplished by the use of Phillips’ Phospho-Muriate of Quinine Compound in appropriate doses.”
“The quantities of quinin and strychnin in this preparation are so well balanced that they relieve the depression and fatigue from mental or physical exertion, without the necessity of recourse to alcoholic stimulation.”
“The other ingredients of Phillips’ Phospho-Muriate of Quinine—phosphoric acid, and the phosphates of potash, magnesia, lime, and iron—are the most rational as well as convenient means of administering these tissue remedies, and of introducing phosphorus—the vitalizing constituent of the nervous system—into the organism.”
The action of such a mixture as a whole is practically that of the sum of the actions of its constituents. The therapeutic action of strychnin and quinin are described in every text-book of therapeutics, but it is necessary to distinguish carefully between the various conditions in which these alkaloids have been used without discrimination, and those conditions in which they have been proved to be of value. While both have been widely used in a great variety of conditions, neither is of proved value in more than a distinctly limited range of diseases. The manufacturers of Phillips’ Phospho-Muriate of Quinine Comp. seem to appeal to the less discriminating who use these alkaloids without any definite conception of exactly what they seek to accomplish with them. Quinin, although used by the uncritical in a host of diseases, has a definite field of usefulness in the treatment of malaria, both prophylactic and curative, but the required dose in the treatment of malaria is many times larger than that recommended in the Phillips’ preparation. The claim that the “strychnin and quinin in this preparation are so well balanced that they produce a mild, buoyant effect, so advantageous, instead of alcoholic stimulation, to relieve depression and fatigue from mental or physical exertion” is nonsensical, if, indeed, it is not mendacious balderdash.
Calcium and potassium have important functions in the body, but any deficiency that may arise is usually attributable to an inability of the body to utilize that which is supplied, for there is seldom any deficiency of these salts in the food, and when they are needed they are best supplied as simple solutions of the salts in appropriate doses without all of the other constituents of Phillips’ Phospho-Muriate of Quinine Comp.
Phosphoric acid exerts practically the same actions as other mineral acids, hydrochloric being usually preferred for internal administration in certain forms of indigestion, aside from which they are seldom used as such.
In the more recent literature for Phillips’ Phospho-Muriate of Quinine Comp., we find the attempt to utilize the well known craze about phosphorus, which has been through so many phases, every one of which has had its day and has been discarded.
The phosphoric acid and phosphates present in Phillips’ Phospho-Muriate of Quinine are of no more value in nervous diseases than is simple sodium phosphate which does not require the addition of a host of other ingredients for its action. As a matter of fact, the phosphates of calcium and potassium present in a dose of Phillips’ Phospho-Muriate of Quinine are probably devoid of appreciable effect in practically all conditions.
To pretend that one who suffers from physical and nervous exhaustion can be materially benefited by this mixture is sheer nonsense and is unworthy of a moment’s consideration by a clinician who is called on to treat such patients.
Iron is useful in anemia, as every one knows. Iron has practically no other field of usefulness in therapeutics. When it is indicated it should be administered in a simple form, such as the pill of ferrous carbonate, for example, and not in a “shotgun” mixture that is quite as likely to do harm as good.
The claim that a satisfactory level of blood pressure can be maintained by Phillips’ Phospho-Muriate of Quinine is mentioned only to condemn as the limit of impudent therapeutic claims. It is an insult to the intelligence of any practitioner to pretend that any known agent or combination of remedial agents can maintain a uniform blood pressure in any one of innumerable conditions.
In short, Phillips’ Phospho-Muriate of Quinine Comp. is a complex and irrational mixture exploited by means of unwarranted claims. It is a survival of the old days of therapeutic chaos when impossible and fantastic chemical formulas were gravely published and as solemnly accepted without question, and also without the slightest understanding on the part of many; when the most eminent of practitioners did not hesitate to give glowing testimonials for lithia waters that contained no more lithium than ordinary river water; when no therapeutic claim was too preposterous to receive acceptance, no theory too nonsensical to justify the use of all manner of claptrap mixtures for all manner of conditions.—(From The Journal A. M. A., Oct. 19, 1918.)
The Council has authorized publication of the following report on “B. Iodine” and “B. Oleum Iodine,” together with the reply submitted by the manufacturer and a discussion thereon by the referee in charge of the preparations.
W. A. Puckner, Secretary.
Specimens of B. Iodine and B. Oleum Iodine (B. Iodine Chemical Company) and an advertising pamphlet were sent to the Council by John Bohlander, A.M., M.D., with the declaration:
“Well knowing the value of Iodin in surgical operations and dressings, prompted me for the benefit of my fellow physicians as well as myself, and for Humanity’s sake, to make Iodin my master-piece in chemistry.
“After several years of diligent work in my private laboratory I succeeded in discovering a new product of Iodin—Nitrogen, hydrate of Iodin.”
While “B. Iodine” is said to be nitrogen hydrate of iodin and “B. Oleum Iodine” a 5 per cent. solution thereof, the examination made by Prof. A. H. Clark of the University of Illinois, School of Pharmacy (working in the A. M. A. Chemical Laboratory), indicates that the first is a simple mixture of iodin and ammonium iodid, and the second a solution of iodin in liquid petrolatum. The Council adopted the report of the A. M. A. Chemical Laboratory (which appears below) and declared B. Iodine and B. Oleum Iodine inadmissible to New and Nonofficial Remedies because:
1. The composition is incorrectly declared. B. Iodine is not a newly discovered iodin compound, “Nitrogen Hydrate of Iodine,” but a mixture of iodin and ammonium iodid. B. Oleum Iodine is not a 5 per cent. solution of B. Iodine as suggested by the statement on the label and in the advertising, but a solution of iodin in liquid petrolatum containing about 0.85 per cent. of iodin.
2. Since B. Iodine is a mixture of Iodin and ammonium iodid, its solution in water will have the properties of other solutions of iodin made by the aid of iodid, such as a dilution of tincture of iodin or of compound solution of iodin (Lugol’s solution). Hence, the therapeutic claim that B. Iodine “being of a colloidal nature has the advantage of being more readily absorbed and taken up by all cellular structure, thus getting a perfect cellular medication of Iodine,” is unwarranted.
3. The names “B. Iodine” and “B. Oleum Iodine” are not descriptive of the pharmaceutical mixtures to which they are applied.
4. B. Iodine and B. Oleum Iodine are unessential modifications of established articles. B. Iodine has no advantage over tincture of iodin or compound solution of iodin. (As more convenient of transportation, the Medical Department of the U. S. Army supplies its field hospitals with a mixture of iodin and iodid ready for solution in water, either in tablet form or in powdered form in tubes.) Solutions of iodin in liquid petrolatum may be readily prepared (Reports Council Pharm. and Chem., 1917, p. 88).
[Contribution from the A. M. A. Chemical Laboratory]
“B. Iodine” products are marketed by the B. Iodine Chemical Company, Cincinnati, Ohio; John Bohlander, A.M., M.D., is said to be the discoverer. They consist of “B. Iodine,” “B. Oleum Iodine,” and “B. Aqua Iodine.” B. Iodine and B. Oleum Iodine were submitted to the Council.
In a circular submitted by the B. Iodine Chemical Company, B. Iodine is said to be “Nitrogen Hydrate of Iodin.” It is claimed that “coming in contact with water, H2O, a chemical change takes place forming Hydro Oxid of Iodin, the Nitrogen of the Nitrogen Hydrate of Iodin escaping, the balance taking up one of oxygen of the water. Its companion, the H2, escaping at the same time with the Nitrogen then combining with the remainder of the water to form the solution of Hydrogen Oxid of Iodin; so you can readily see that you really have a pure water of Iodin, nothing but the H, the O and the I.”—(From the Journal A. M. A., Feb. 1, 1919.)
According to the circular, B. Iodine is soluble in alcohol, chloroform, and ether. Also it:
“Has odor, taste, melting and boiling point, same as regular Iodin, has a great affinity for water and will respond to all the tests of Iodin. Appears in a Bluish Black Granulated mass or Powder. When heated in vaporating dish will throw off large purple volumes of Iodin leaving a slight white crystalline precipitate, which on continuous heating will entirely disappear. With careful manipulation you can get prismatic needle point like crystals, looking like spores of glass, these dissolving in water will yield pure Iodin coloring the water Iodin.
“Pharmacologic, Therapeutical and Physiological Action: Same as Iodin, being of a colloidal nature has the advantage of being more readily absorbed and taken up by all cellular structure, thus getting a perfect cellular medication of Iodin.”
A sample of B. Iodine, marked “Nitrogen Hydrate of Iodin” was submitted by the manufacturers and this sample was examined.
B. Iodine was found to be a granular powder, almost black with a purple cast. It has an odor of iodin and dissolves in water readily. It is also quite soluble in alcohol, but not entirely soluble in chloroform and ether. Ether quickly dissolves iodin from B. Iodine, leaving a residue of a white granular substance. Chloroform acts the same as ether except that the iodin is dissolved out with some difficulty. On heating B. Iodine, vapors of iodin escape. If the heating is done on a water bath, a residue of a white granular substance, subsequently identified as ammonium iodid, remains. If heated in a bunsen flame, no residue remains. These tests all indicate that iodin is held in the form of a simple mixture.
Ammonia: B. Iodine when mixed with an excess of sodium hydroxid and warmed, evolves ammonia.
Iodine: 0.1567 gm. B. Iodine dissolved in water required 5.88 c.c. tenth-normal sodium thiosulphate solution indicating 48.28 per cent. iodin. 0.3721 gm. B. Iodine required 14.18 c.c. tenth-normal sodium thiosulphate solution indicating 48.37 per cent. iodin. The average is 48.33 per cent. iodin.
Ammonium Iodide: 0.3453 gm. of the residue after heating B. Iodine on a water bath until all iodin had volatilized was dissolved in water, acidulated with phosphoric acid, and hydrogen dioxid solution added. The liberated iodin was extracted with chloroform and titrated with tenth-normal sodium thiosulphate. 23.78 c.c. were required indicating 0.3447 gm., or 99.83 per cent., ammonium iodid.
A mixture of 5 gm. iodin and 5 gm. ammonium iodid has the properties of B. Iodine mentioned above.
The conclusion is that B. Iodine is essentially a mixture of iodin and ammonium iodid in equal parts, the two substances being finely powdered and intimately mixed.
The following regarding B. Oleum Iodine is quoted from the circular submitted:
“B. OLEUM IODINE: Iodine soluble in mineral oil 5 and 10% for Nasal, Pharyngeal, Laryngeal, Bronchial, Rectal, etc., and all meucoid affections and abnormal conditions of the mucous membrane.”
A sample of B. Oleum Iodine was submitted by the manufacturer and examined. The label on the bottle states that it is 5 per cent. B. Oleum Iodine in mineral oil. This sample has the characteristics of a solution of iodin in liquid petrolatum. It is oily and has the characteristic violet color.
Ammonia: B. Oleum Iodine, since it is presumed to be a solution of B. Iodine, was examined for ammonium compounds. A small quantity was mixed with an equal volume of strong sodium hydroxid solution and heated. No ammonia was evolved. A few crystals of ammonium chlorid were added to a little of B. Oleum Iodine and treated as above. Ammonia was readily detected.
Iodine: 5.255 gm. B. Oleum Iodine was dissolved in chloroform and placed in a separator. A solution of potassium iodid was added and the iodin titrated with a tenth-normal sodium thiosulphate solution. It required 3.5 c.c. indicating 0.85 per cent. iodin.
The conclusion is that B. Oleum Iodine is a simple solution of iodin in liquid petrolatum to the extent of 0.85 per cent. and not 5 per cent. as claimed. Furthermore, it is not a solution of B. Iodine since no ammonium compound is present.
The preceding report was sent to the B. Iodine Chemical Company. The following reply was received:
Your letter of the 21st inst., received and contents noted and cannot quite agree with your report.
Reasons why: NH4I, a Nitro Hydrate Iodide; NH4I2, a Nitro Hydrate Iodate; and NH4I2I2, Per Iodide, a molecular compound, which I claim, they all being of a NH group, so what can be the objection of Nitrogen Hydrate of Iodine? Of course when your chemist, with the aid of heat, drove off all the Iodine, he naturally brought it back to a NH4I. There’s where he gets the A.M.. I claim a molecular compound.
The Oil of Iodine I sent you by mistake was a 1 per cent. and not a 5 per cent. as marked. I claim it is made from the resublimed Iodine in mineral oil and not the B. Iodine. I claim a 5 per cent. has heretofore never been accomplished, so I therefore can claim something new.
Tr. Iodine contains Alcohol and Potash as a base, the alcohol a dehydrater and Potash an escharotic, and all other soluble Iodines like the tincture have a metallic base. Mine has not. My iodine is compatible almost with all the salts, alkaloids, tannates, and even the metals. You can’t say that for the tincture or the others. Now why should mine not be superior to others?
Preparations as yet are not on the market and a few pamphlets were printed to meet with the requirements of your rulings and approval and shall be corrected if we only can agree on a proper name as you may suggest.
Yours very truly,
The B. Iodine Chemical Co.
By John Bohlander, A.M, M.D.
P.S. We are sending you under separate cover another sample of the Oil of Iodine which is a 5 per cent. solution, and allowing for deterioration will test at least four per cent.
The referee in charge of the preparations submitted the above letter to the Council with the following comments:
The principal statements in the letter are essentially erroneous or misleading: Mixtures or double salts of ammonium iodid and iodin were not discovered by Dr. Bohlander, and are nothing new. Watery solutions of iodin by means of an iodid have long been known and used in the form of Lugol’s solution.
There is no evidence that ammonium iodid is less irritating than potassium iodid. On the contrary, ammonium salts are generally more irritating than the corresponding potassium salts. B. Iodine is not compatible with alkaloids, but behaves essentially like Lugol’s solution. The A. M. A. Chemical Laboratory reports that the new sample of B. Oleum Iodine contains only 1.2 per cent. of free iodin, instead of the claimed amount. It is therefore somewhat weaker than the iodin petrolatum prepared by the A. M. A. Chemical Laboratory (Reports Council Pharm. and Chem., 1917, p. 88).
However good Dr. Bohlander’s intentions may be, the statements that he makes about his products are misleading or erroneous, and the products are ineligible for N. N. R.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 44.)
The following report explaining the omission from New and Nonofficial Remedies of antithyroid preparations (Antithyroidin-Moebius and Thyreoidectin) has been authorized for publication.
W. A. Puckner, Secretary.
New and Nonofficial Remedies, 1918, contains a discussion of “antithyroid” preparation and describes two of these: Antithyroidin-Moebius (E. Merck, Darmstadt, Germany) and Thyreoidectin (Parke, Davis & Company, Detroit, Mich.).
The referee reported that these “antithyroid preparations” evidently have not realized the expectations of their promoters, and are viewed with skepticism by practically all critical clinicians.
Consequently, notwithstanding the cautiously worded statements of claims made by the manufacturers of Thyreoidectin, the Council approved the recommendation that this preparation (Thyreoidectin) be omitted from New and Nonofficial Remedies for conflict with Rule 6 (unwarranted therapeutic claims) and Rule 10 (unscientific and useless articles) (Antithyroidin-Moebius had already been omitted because it was off the market). The Council further directed that the general article “antithyroid preparations” be also omitted.
The Council having adopted the recommendation of the referee, Thyreoidectin is omitted from N. N. R., while the general article appears below, as a matter of record:
Antithyroid preparations are obtained from the blood or milk of animals, after the removal of the thyroid glands.
The use of these preparations is based on the theory that the thyroid gland secretes products which are toxic, but which neutralize and are neutralized by, other toxic substances produced elsewhere in the body. Removal of the thyroid glands would then lead to the accumulation of these second toxic substances as evidenced by the phenomena of cachexia strumipriva and myxedema. On the other hand, the blood or milk of such animals is claimed to be capable of preventing the effects of hypersecretion of thyroid substance, such as is supposed to occur in hyperthyroidism (Basedow’s or Graves’ disease—generally called exophthalmic goiter).
These views are largely hypothetical; attempts to give to them a rational experimental basis have failed, but some clinical observers report distinctly beneficial results in the milder forms of the diseases, and in obscure nervous disorders which are supposedly connected with thyroid hypersecretion from the administration of the milk from thyroidectomized goats and also from the use of the proprietary blood preparations listed below. The value of these preparations is very doubtful. The reported improvements may only be psychical or due to associated measures, as is often seen in this disease. Other measures of treatment should not be neglected.
Improvement is said to occur in two or three weeks and to be indicated by an amelioration of the nervous symptoms, tremor, palpitation, insomnia and excitability.
The administration must be long continued. Oral and hypodermic administration are said to be equally effective, but the former is usually preferred. These preparations are not known to be toxic, even when very large doses are used.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 50.)
The Council has authorized publication of the following report, which explains the omission of cephaelin and Syrup Cephaelin-Lilly from New and Nonofficial Remedies and the non-acceptance of Syrup Emetic-Lilly.
W. A. Puckner, Secretary.
New and Nonofficial Remedies, 1918, describes cephaelin (an alkaloid obtained from ipecacuanha root) and lists Syrup Cephaelin-Lilly (containing 0.088 Gm. cephaelin hydrochlorid per 100 Cc.) as a pharmaceutical preparation of it.
The period of acceptance for Syrup Cephaelin-Lilly having expired, Eli Lilly & Company were asked to send the current advertising and labels so that the Council might determine if the acceptance of this preparation might be continued. In reply the firm wrote:
“We have changed the name Syrup Cephaeline to Syrup Emetic but the product remains the same as before. We have no circulars describing Syrup Emetic and can only send copies of the label.”
The new name “Syrup Emetic” conflicts with the rules of the Council in that it does not indicate the potent ingredient of this simple pharmaceutical preparation and in that it is therapeutically suggestive. Emetics are powerful agents, and physicians should be given every opportunity of knowing what they prescribe for the purpose.
The name being in conflict with Rule 8, the Council voted to omit Syrup Cephaelin-Lilly and not to accept Syrup Emetic-Lilly.
As the cephaelin syrup was the only preparation of cephaelin admitted to New and Nonofficial Remedies, and as the alkaloid appears to have no important therapeutic field, the Council directed that the description of cephaelin also be omitted.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 52.)
The following report explaining the omission from New and Nonofficial Remedies of Colalin has been authorized for publication.
W. A. Puckner, Secretary.
Colalin is a bile salt preparation claimed to consist essentially of hyoglycocholic and hyotaurocholic acids. It is manufactured by Rufus Crowell and Company, Somerville, Mass., and marketed by Schieffelin and Company, New York.
An examination of the current advertising by the referee of the Council in charge of bile salt preparations having revealed that claims were made for Colalin which were not in harmony with the known action of bile preparations, Schieffelin and Company were informed that in the opinion of the referee the Colalin circular matter required radical revision. In this communication the referee’s objections to the claims were set forth in detail.
No reply to this letter was received, and hence a copy of the letter was sent to Schieffelin and Company and also to Rufus Crowell and Company with the explanation that unless the statements in the Colalin advertising which the referee had questioned were substantiated by satisfactory evidence, were suitably revised, or else the advertising matter withdrawn pending revision, the referee would be obliged to recommend to the Council that Colalin be omitted from New and Nonofficial Remedies.
In reply, Schieffelin and Company wrote that they were not “engaged actively in the introduction of Colalin,” and agreed to the omission of Colalin from N. N. R.
In view of the failure to substantiate the claims objected to or an agreement to discontinue them, the Council directed that Colalin and Colalin Tablets be omitted from New and Nonofficial Remedies for conflict with Rule 6 (unwarranted therapeutic claims).
The following are the claims which the referee questioned:
“Colalin embodies the physiological function of the bile in the intestinal canal and also possesses properties of its own which are intimately connected with the function of the liver.”
The quotation implies that Colalin has properties essentially different from those of bile salts, a claim which requires substantiation.
“In the liver its action seems to be that of a general stimulant of all the hepatic functions.”
This is a claim which requires substantiation.
“By the introduction of Colalin it has therefore become possible to actually utilize the bile for therapeutic purposes.”
This is an unwarranted claim, for bile was used therapeutically before Colalin was introduced.
“As gall-stones are chiefly composed of cholesterin, experiments were made to determine whether Colalin would dissolve these concretions outside of the body. These were completely successful and were then followed by an extensive series of clinical investigations on persons suffering with cholelithiasis, which demonstrated that by the administration of Colalin in many instances gall-stones were evacuated by the natural passages and their further formation prevented without resort to surgical intervention.”
This is misleading in that the context shows that “without surgical intervention” is meant to imply a connection between the experiments showing the solvent power of Colalin and the passage of concretions.
“... Colalin not only acts as a solvent of cholesterin calculi, but prevents their further formation by removing the causes upon which their development depends.”
This conveys the impression that such solvent action is exerted in the body, that is, that such concretions in the gallbladder may be dissolved and evacuated by the use of Colalin. For this claim there is no evidence.
“To understand the value of Colalin in intestinal disorders it is necessary to bear in mind the important functions of the bile in the intestinal canal, namely, its participation in the digestion of fats, its antitoxic action, and its influence upon the peristalsis.”
“... through its antiseptic influence inhibits the production of toxins in the intestines.”
The referee believes that there is no satisfactory evidence that bile or bile salts can inhibit the production of toxins in that part of the intestine—the colon—in which they are commonly produced.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 52.)
The following report on Foral, a depilatory preparation, has been authorized for publication by the Council.
W. A. Puckner, Secretary.
Foral is sold by the Foral Products Company, Pittsburgh, Pa., as an “antiseptic depilatory” with the special claim for its use for the removal of hair prior to surgical operation or the dressing of wounds. In addition to claims made for its hair dissolving action, it is asserted that, in removing the hair from an open wound, Foral acts as “an antiseptic, which guarantees against any infection.” It is also claimed that, though hair will return after its use, “by proper use it will diminish the growth of hair and cause the hair to grow much slower, and unlike the razor, the hair will not return coarser and thicker.”
We are informed by the Foral Products Company that their preparation is used in many hospitals and that “... one and all are well pleased and a great satisfaction to do away with the old style razor ...”
Foral is stated to be made according to the following formula:
To manufacture seventy-five pounds of FORAL
Starch | 35 pounds |
Barium-Sulphide | 20 pounds |
Zinc-Oxide | 10 pounds |
Calcium-Carbonated-Precip. | 10 pounds |
Potassium-Permanganate | 10 grams |
Menthol-Crystallized | 10 grams |
Carbolic-Acid | 1⁄2 ounce |
Lilac or Citronel oil | 3 ounces |
The four above chemicals are going to a heating process before mixing or sifting.
In consideration of the preceding, the Council declared Foral inadmissible to New and Nonofficial Remedies for conflict with its rules, thus:
1. Foral is an unessential and irrational modification of an established article.
While its manufacturer states that Foral has been on the market for eighteen years, the following depilatory formula appears in a book published thirty-five years ago (A practical Treatise on Diseases of the Skin, Louis A. Duhring, Ed. 3, 1883) and is to be found in most books on dermatology:
Barium Sulphid | 2 drams |
Zinc oxid | 3 drams |
Starch | 3 drams |
Permanganates and sulphids mutually destroy each other, and therefore the addition of the small amount of potassium permanganate cannot serve any useful purpose. The amounts of phenol, menthol and “Lilac or Citronel oil” are too small to exercise any effect (other than that of a flavor) and must be considered unessential additions.
2. Foral is a pharmaceutical mixture marketed under a non-informing name.
Whereas it is in the interest of rational medicine that physicians should know the composition of the preparations which they use, the name of this pharmaceutical mixture fails to indicate that it contains the well-known and by no means always harmless barium sulphid.
3. Foral is sold under exaggerated and unwarranted claims.
In view of the small amount of phenol present and the method of using the preparation, the claim that the use of Foral which, when operating on open wounds, “guarantees against any infection,” is evidently unwarranted.
There is no evidence for the claim that the use of depilatories such as Foral retards the growth of hair or renders hair less coarse. On the contrary, the commonly prevailing opinion is that depilation, like shaving, makes the hair coarser.
To determine if “one and all” of those who had used Foral were still using the preparation, four of the testimonials, appearing in an advertising pamphlet, were investigated. The pharmacist of the hospital from which the first of these testimonials was stated to have emanated replied that the person whose name appeared in connection with it had left the hospital about ten years ago and that no depilatory preparation has been used in this hospital for some time. So far as he knew, depilatories were not now in use in the surgical wards of the hospital. In regard to the second testimonial, the pharmacist of this hospital wrote that the hospital had not bought the preparation, but that some of it had been obtained for an elderly deaconness, who had personal use for a depilatory. The physician signing the third testimonial replied that the preparation was effectual for the removal of hair from the scalp, but that “... we have gotten out of the habit of using it.” In the case of the fourth testimonial, its asserted author wrote “... if it is applied in too large a quantity or too concentrated, or permitted to remain on too long, it will vesicate. It was for this reason chiefly that I discontinued its use. It is a very bad smelling mixture and patients complain of it very bitterly.”—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 55.)
The following report explaining the omission from New and Nonofficial Remedies of Granular Effervescent Bromide and Acetanilid Compound-Mulford has been authorized for publication.
W. A. Puckner, Secretary.
The Council holds that complex mixtures of remedial agents are from every point of view inimical to therapeutic progress and therefore to the public welfare. They are especially objectionable because it is impossible accurately to determine the effects which follow the simultaneous administration of a number of drugs having dissimilar actions, and because the practice of prescribing such mixtures tends to discourage careful consideration of the special needs of the individual patients without which there can be no drug therapy. On the contrary, with the use of such mixtures, therapeutic treatment becomes haphazard and mere guesswork.
The Council, appreciating that long established customs cannot be changed at once, has applied Rule 10, concerning the recognition of mixtures, with the greatest leniency compatible with consistency. When there has been a reasonable doubt concerning the value of a mixture, it has frequently directed that Rule 10 should not apply, pending further clinical trial of such mixture.
In no instance has subsequent experience shown that a strict interpretation of the rule would have worked hardship or injustice. The Council feels that there is no longer warrant for the admission of complex mixtures to New and Nonofficial Remedies, or for the retention of any that have been admitted, unless definite evidence of the therapeutic value of such combinations is available. In accordance with this decision, several mixtures now described in New and Nonofficial Remedies will be omitted at the expiration of the three year period for which articles are accepted.
Granular Effervescent Bromide and Acetanilid Compound-Mulford is listed in the Appendix to New and Nonofficial Remedies. Each 100 Gm. of the mixture contains sodium bromide, 5 Gm., and acetanilid, 1.5 Gm. According to the label, an amount containing acetanilid, 6.5 grains, and sodium bromide, 22 grains, is to be taken at a dose, to be repeated in half an hour if necessary. For “children,” half this dose is advised.
The Council has considered the available evidence for mixtures of this sort, and has reached the conclusion that they are inimical to rational medicine and the public, and therefore in conflict with Rule 10. It holds that the use of mixtures of acetanilid and sodium bromide in fixed proportion is irrational and prone to induce their indiscriminate use by the public. Despite the perfectly frank declaration of the composition of this mixture that is made by the Mulford Company, the “directions” will be followed blindly and the preparation will be given to “children” and “repeated in half an hour, if necessary” in cases in which it would be held unwarranted to administer a dose of 3 grains of acetanilid to a child.
The period of acceptance having expired for Granular Effervescent Bromide and Acetanilid Compound-Mulford, the Council directed its omission from New and Nonofficial Remedies for conflict with Rule 10.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 58.)
Holadin and Bile Salts-Fairchild; Capsules of Bile Salts, Succinate of Soda and Phenolphthalein-Fairchild; Capsules of Holadin, Bile Salts and Phenolphthalein-Fairchild; Capsules of Holadin, Succinate of Soda and Bile Salts-Fairchild.
To explain the omission from New and Nonofficial Remedies of certain mixtures, the Council has authorized publication of the matter which appears below.
W. A. Puckner, Secretary.
The Council holds that complex mixtures of remedial agents are from every point of view inimical to therapeutic progress and therefore to the public welfare. They are especially objectionable because it is impossible accurately to determine the effects which follow the simultaneous administration of a number of drugs having dissimilar actions, and because the practice of prescribing such mixtures tends to discourage careful consideration of the special needs of individual patients without which there can be no rational drug therapy. On the contrary, with the use of such mixtures, therapeutic treatment becomes haphazard and mere guesswork.
The Council, appreciating that long established customs cannot be changed at once, has applied Rule 10 concerning the recognition of mixtures with the greatest leniency compatible with consistency. When there has been a reasonable doubt concerning the value of a mixture it has frequently directed that Rule 10 should not apply, pending further clinical trial of such mixture.
In no instance has subsequent experience shown that a strict interpretation of the rule would have worked hardship or injustice. The Council feels that there is no longer any warrant for the admission of complex mixtures to New and Nonofficial Remedies or for the retention of any that have been admitted unless definite evidence of the therapeutic value of such combinations is available. In accordance with this decision, several mixtures now described in New and Nonofficial Remedies will be omitted as soon as the three year period for which articles are accepted has expired.
The following preparations are included in New and Nonofficial Remedies, 1918:
Holadin and Bile Salts-Fairchild.—A mixture of holadin, 5 parts, with bile salts-Fairchild, 1 part, put up in 3 grain capsules.
Capsules of Bile Salts, Succinate of Soda and Phenolphthalein.—Each capsule contains bile salts-Fairchild, 0.065 Gm. (1 grain); sodium succinate exsiccated, 0.2 Gm. (3 grains), and phenolphthalein, 0.03 Gm. (1⁄2 grain).
Capsules of Holadin, Bile Salts and Phenolphthalein.—Each capsule contains holadin, 0.13 Gm. (2 grains); bile salts-Fairchild, 0.03 Gm. (1⁄2 grain), and phenolphthalein, 0.065 Gm. (1 grain).
Capsules of Holadin, Succinate of Soda and Bile Salts.—Each capsule contains holadin, 0.20 Gm. (3 grains); sodium succinate exsiccated, 0.20 Gm. (3 grains), and bile salts-Fairchild, 0.03 Gm. (1⁄2 grain).
Oxbile has long been credited with a cholagogue action, which, however, has probably been greatly overestimated. When pure bile salts were placed on the market some years ago, they and their compounds were admitted to N. N. R.
Holadin is said to represent all the constituents of the pancreas and to possess great potency in respect to the several enzymes, trypsin, amylopsin, lipase, and the milk-curdling ferment.
It is not clear when such a substance is indicated therapeutically. While it may be useful when there is a deficiency of pancreatin and gastric secretion, it should be used alone.
It is also quite possible that bile salts may have a distinct, though limited, field of usefulness when there is a deficiency of biliary secretion; but the bile salts are best administered alone, or in combination with such laxatives as may be deemed necessary by the physician while keeping in mind the fact that different patients show the widest difference in their reaction to laxatives, making combinations of these agents in fixed proportion irrational.
Phenolphthalein was popularized by nostrum makers; and while it has some therapeutic value, this has been greatly overestimated, and it should be used only in amounts deemed necessary for each patient, preferably alone.
Succinate of sodium was introduced as a saline cathartic, with the claim that it exerts an antiseptic action on the biliary passages and gallbladder. There is no satisfactory evidence to substantiate this claim.
The Council maintains a liberal attitude toward new preparations, but it feels that it is impossible to determine the value of the several constituents of such complex mixtures when used as such; it holds that these mixtures are superfluous and that the several substances of which they are composed should be used singly or at most with greater attention to the individual requirements of the patient than is possible when these fixed mixtures are prescribed.
Despite the fact that these mixtures have been in use for more than nine years, there is no satisfactory evidence that they possess any advantage over the simple laxatives or the preparations of bile or pancreatic extract. They are therefore held to be in conflict with Rule 10, and the Council has directed that they be not included in N. N. R. after Dec. 31, 1918.
Having adopted the preceding report, the Council, in accordance with its regular procedure, submitted this to Fairchild Bros. and Foster for comment.
The following reply was received:
We are entirely at variance with you in the arbitrary conclusion expressed concerning the inimical influence of mixtures on therapeutic progress, the practice of medicine and the public welfare.
If the combinations of Holadin and Bile Salts, etc., in capsules, were ever properly within the scope of New and Nonofficial Remedies, they should be retained. If, however, complex mixtures are to be held as, a priori, unworthy of consideration, the rejection of all would naturally be a logical proceeding.
We believe that the particular combination of Holadin and Bile Salts etc., have been clearly in the line of therapeutic progress—a natural evolution, improvement and development.
For many years combinations of pancreatic extract and ox gall had naturally suggested themselves.
When we realized the fact that the bile salts were quite clearly the active principles of the bile, and that they must necessarily exist in greatly varying percentages in the official inspissated or ox gall, and also because these ox gall products of pharmacy were of extremely varying density, even from that of treacle to resin—and of other objectionable character, we undertook to prepare bile salts.
These combinations are now further justified in view of physiological considerations, the simultaneous secretion of the pancreas and bile, and the state of our knowledge of the function of bile salts, and as co-ferments, promoting and supplementing the pancreas enzymes.
The question suggested as to whether the cholagogic action of ox gall (and bile salts) has been overestimated seems to us no clear purport. The bile salts are obviously employed as the means of administering and thus realizing whatever properties this secretion may have in medicine, of which the cholagogic action is by no means the only consideration.
As for phenolphthalein, which is credited with purely laxative properties, we are at a loss to see any bearing in the remark that phenolphthalein was popularized by nostrum makers. We cannot see that the physician’s or chemist’s estimate of phenolphthalein, its properties and uses, can be in the least degree influenced one way or the other by the statement that “phenolphthalein has been popularized by nostrum makers.”
The phenolphthalein and succinate of soda combinations were originally both prescribed, and we have simply placed them at the service of the physician without other exploitation of them than that designed to call attention to their use in the conditions indicated.
These combinations are offered in a form which may be administered by the mouth with the best promise of introducing the substance more directly in the intestinal tract during the digestion period or at such interval after or prior to, the digestion period, as would best, in the judgment of the physician, meet the indications.
These particular combinations are especially desirable in these “fixed forms” since they are stable and reliable resources at the command of the physicians, the enzymes retaining their stability and potency without material deterioration for many years, and they naturally possess the advantages which are obviously due to the character of the particular pancreas and bile products used in the combinations.
Furthermore, the hygroscopic and soluble organic substances in admixture cannot extemporaneously be so prepared in sealed capsules as to be readily available under the practical requirements of prescribing and dispensing. And we do not believe that those who practice medicine will be in accord with your view that the pancreas substance should necessarily be administered alone, or the bile substance alone.
It now appears that these combinations are to be dropped from New and Nonofficial Remedies in consequence of the view, so stated, that in clinical experience “for more than nine years there is no satisfactory evidence that they possess any advantage over the simple laxatives or preparations of bile or pancreatic extract.”
In reply to this we would simply make the following comment:
During these “nine years” these combinations have inevitably been put to an informing clinical trial, because of the fact that they have been employed with success in disorders of the pancreas and bile functions and often in chronic and serious cases where the clinical conditions were obvious and unmistakable.
The reports of these cases come to us from physicians widely separated and each of his own independent initiative. It would seem gratuitous, to say the least, to state that the observers are “disinterested,” since it is quite clear that there is no other interest than that of the practitioner and his patient.
It is not a case of a new drug or combinations of new remedies, but simply resources which, upon well grounded reasons, both from a theoretical and material standpoint, justify clinical trial, and with results which would seem from any ordinary human standpoint to be satisfactory clinical evidence.
As to the interpretation of competent clinical evidence by the Council, we would, in view of the circumstances and without comment, ask to embody in this text this rule:
“Clinical Evidence.”—“To be acceptable, the clinical evidence must offer objective data with such citation of authority as will enable the Council to confirm the facts and establish the scientific value of the conclusions drawn. Clinical data are worthless when the author is not cited. The facts on which claims with regard to the value of a remedy are based must have been rendered accessible for investigation and confirmation by disinterested observers, either through publication or through the records of a hospital or other institution.”
To discredit these combinations would seem to us not only unjustified, but sterile of any real advancement in medicine, or of anything in the way of helpfulness to the patient in the class of cases in which these products have been resorted to with benefit; this on no other ground really than the opinion “that they have no advantage over the simple preparations themselves.”
Naturally we shall continue to prepare these products and shall continue to take such action as we deem best to bring them to the attention of the physician, for the conduct of our business must remain in the hands of those who are personally responsible for it.
And it is now forty years since we took up this line of work and with the declared intention of devoting ourselves to the applied science of the digestive ferments and “to their development and practical application in every useful purpose in medicine.”
We have been consistently in sympathy with the fundamental purpose of the Council, which must first rest upon fact as to the character of the products offered as medicinal agents. The weight of evidence justifies the position that these particular products rationally should be, and as a matter of fact are, of important special service in the utilization of these organic secretions in medicine.
As explained in the preceding report, the Council holds that complex mixtures of remedial agents are from every point of view inimical to therapeutic progress and therefore to the public welfare. They are especially objectionable because it is impossible to determine accurately the effects which follow the simultaneous administration of a number of drugs having dissimilar actions, and because such a practice tends strongly to discourage careful consideration of the special needs of individual patients without which there can be no therapeutic progress. On the contrary, with their use, therapeutic treatment becomes haphazard and mere guesswork.
The dismissal of the holadin and bile salts mixtures does not involve the question of the usefulness of holadin or of bile salts alone; on the contrary, the possible usefulness of these preparations is admitted in the report. It is the combination of holadin, bile salts, sodium succinate and phenolphthalein to which objection is made.
The statement of Fairchild Bros. and Foster that “these combinations are now further justified in view of physiological considerations” is somewhat misleading. It is true that bile and the pancreatic secretion cooperate in intestinal digestion, but there is no evidence that in every case in which there is a deficiency of one of these secretions there is also a deficiency of the other, and it is an axiom of scientific therapeutics that no drug or remedial agent should be administered except to fill a definite want. Otherwise the practice of therapeutics becomes mere empiricism.
The properties of phenolphthalein are not in the least influenced by the manner of its introduction, as Messrs. Fairchild Bros. and Foster emphasize; but the important fact in this connection is that the popular conception of their actions is greatly influenced by the mode of introduction, and phenolphthalein has been widely advertised in a variety of conditions, so that the popular notion concerning it is not that of scientific therapeutics.
In short, the entire argument of Messrs. Fairchild Bros. and Foster concerning the exploitation of these preparations may be summed up by saying that they have been used by clinicians who believe that good results have followed their use, and that the firm will therefore continue to supply the demand. The tendency of some to use anything brought to their notice, and the readiness of manufacturers to market anything that physicians will use, presents the greatest obstacle to therapeutic progress. There was never a nostrum so irrational or worthless that honest but undiscriminating clinicians could not be found who reported wonderful results from its use.
According to Fairchild Bros. and Foster, these holadin and bile salts mixtures have been in use for some nine years. Yet the Council is not aware of any investigation of their merits that meets the requirements of scientific research.
The Council is not acquainted with a single clinical investigation of their action under conditions which afford satisfactory evidence of their therapeutic value.
It is obviously wholly insufficient for a clinician to report that the use of a mixture was followed by good results. The fallacy of such arguments was demonstrated long ago. He must make a comparison of the results obtained with the remedial agent with those obtained in as nearly similar conditions as possible except for the use of the agent. We are not aware that any such study of the mixtures in question has been made. It is in the last degree irrational to hold that because bile salts are the active constituents of bile, therefore such complex mixtures as these are necessary.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 59)
The following report explaining the omission from New and Nonofficial Remedies of Liquor Santaiva, S. & D., has been authorized for publication.
W. A. Puckner, Secretary.
So far the Council has applied Rule 10 concerning the recognition of mixtures with the greatest leniency compatible with consistency. When there has been a reasonable doubt concerning the value of a mixture, it has frequently directed that Rule 10 should not apply, pending further clinical trial of such mixture.
In no instance has subsequent experience shown that a strict interpretation of the rule would have worked hardship or injustice. The Council feels that there is no longer any warrant for the admission of complex mixtures to New and Nonofficial Remedies or for the retention of any that have been admitted, unless definite evidence of the therapeutic value of such combinations is available.
The Council being engaged in the annual revision of New and Nonofficial Remedies, the referee in charge of santal preparations reported that the three year period of acceptance had expired for Liquor Santaiva (Sharp & Dohme).
The referee held that Liquor Santaiva, S. & D., declared to be a solution of santal oil and copaiba with aromatic oils, in a mixture of alcohol and water, is plainly in conflict with the current interpretation of Rule 10, because there was no sound evidence to indicate that any useful end is gained by the simultaneous administration of santal oil and copaiba in any proportion, and that so, of course, there is no evidence of the special advantage in the fixed proportions represented by the mixture. He pointed out that the formula is essentially a survival of the discredited shotgun gonorrhea mixtures and therefore recommended that its acceptance be not continued.
The Council agreed to the recommendation of the referee and directed that Liquor Santaiva, S. & D., be omitted from New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 66)
The following report explaining the omission from New and Nonofficial Remedies of the Maltzyme preparations has been authorized for publication.
W. A. Puckner, Secretary.
In 1916, the Council voted to omit Maltzyme with Hypophosphites, and Maltzyme with Phosphate of Iron, Quinine and Strychnine. At that time the labels used on the Maltzyme preparations still in New and Nonofficial Remedies contained a list of Maltzyme combinations which included those which had been dismissed. As the Council does not permit an accepted article to be used as a means of advertising an article not accepted, it voted to continue the following preparations for a period of three years on condition that reference to the deleted articles be omitted from the labels when those then in stock had been used up: Maltzyme, Maltzyme with Cascara Sagrada, Maltzyme with Cod Liver Oil, Maltzyme Ferrated and Maltzyme with Yerba Santa. While the Maltzyme Company made no definite agreement to revise its advertising propaganda in accordance with the Council’s requirements, the Maltzyme preparations were retained in the belief that in due time the required revision of the labels would be made.
The Council being engaged in preparing the 1919 edition of New and Nonofficial Remedies, the referee in charge of malt extracts reported that the Maltzyme Company had not revised its labels in accordance with the stipulation of the Council. The referee further reported he had become convinced that the claim that Maltzyme is “rich in malt enzymes” is unwarranted and that the term “Maltzyme” (malt plus enzyme) is misleading; this because of the recognized instability of malt extracts (Jour. A. M. A., March 30, 1912, p. 954) and because the Maltzyme Company makes no definite statement with regard to the diastase (malt enzyme) content of its preparations.126 For this reason it had been the referee’s intention to propose the deletion of all Maltzyme preparations when their period of acceptance expired in 1919. As, however, the present Maltzyme preparations are in contravention with the Council’s requirements, he recommended that the acceptance of these preparations be canceled now.
The Council agreed to the recommendation of the referee and directed that Maltzyme, Maltzyme with Cascara Sagrada, Maltzyme with Cod Liver Oil, Maltzyme Ferrated, and Maltzyme with Yerba Santa be omitted from N. N. R.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 67)
The following report explaining the omission from New and Nonofficial Remedies of Methaform has been authorized for publication.
W. A. Puckner, Secretary.
Methaform is the proprietary name applied by F. Stearns & Co. to chlorbutanol.
Being engaged in the annual revision of New and Nonofficial Remedies, and the term of acceptance for Methaform having expired, a trade package was purchased to determine if the product was marketed in compliance with the rules of the Council. It was then found that a circular was wrapped with the trade package which advertised Methaform Inhalant, a preparation not accepted for New and Nonofficial Remedies.
For obvious reasons, the Council does not countenance the use of an accepted article as a means of advertising an article not accepted. Accordingly F. Stearns & Co. was advised that the Council would be obliged to withdraw the acceptance of Methaform unless the objectionable circular was omitted from the Methaform packages. Stearns & Co. did not give the requested assurance, and therefore the Council directed that Methaform be omitted from New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 68)
The following report explaining the omission from New and Nonofficial Remedies of pineal gland, red bone-marrow and thymus gland and their preparations has been authorized for publication.
W. A. Puckner, Secretary.
Pineal gland, red bone-marrow and thymus gland were admitted to New and Nonofficial Remedies when these products gave promise of having therapeutic value.
The term of acceptance for the preparations of pineal gland, red bone-marrow and thymus gland having expired, the referee in charge of animal organ preparations recommended in his report for the annual revision of N. N. R. that these products and the general articles describing them be omitted from New and Nonofficial Remedies. He held that the experimental and clinical experience with them leads to the conclusion that they are without value.
In accordance with the recommendation of the referee, the Council voted that the following preparations be omitted from New and Nonofficial Remedies: Desiccated Pineal Gland-Armour; Pineal Gland Tablets-Armour; Extract of Red Bone-Marrow-Armour; Desiccated Thymus-Armour; Thymus Tablets-Armour.
As a matter of record, the descriptive articles for pineal gland, red bone-marrow and thymus gland, which appeared in New and Nonofficial Remedies, 1918, are given below.
The functions of this gland have not yet been established but there is some pathological and some experimental evidence that there is a relation between the gland and some processes of development and growth; the nature of this relation is unknown. Adiposis is a frequent sign of disturbed pineal function, but observers are not agreed whether to interpret this as indicating hypofunction or hyperfunction, or possibly a concurrent disturbance of the pituitary. In some instances intravenous injections of pineal extract have seemed to cause a distinct fall in blood pressure. It has been inferred from observations in cases of pineal tumors in the young that the gland in young individuals furnishes a secretion which inhibits growth, particularly the development of the reproductive glands, but the results of experimental administration of pineal substance orally have led other observers to infer that the pineal secretion favors physical and possibly mental and sexual development. It has been suggested that, as all evidence points to the fact that the function of the pineal gland is one of early life, extract of adult pineal glands might be expected to be inert. Experiment has also indicated greater activity in glands obtained from young animals than in those obtained from older ones. The Council has decided to admit preparations of pineal gland to New and Nonofficial Remedies simply for experimental purposes.
Red bone-marrow consists largely (more than 90 per cent.) of fat. In new-born animals a third or more of this fat consists of lecithin. The marrow of the bones of new-born animals contains iron (up to 1 per cent. or more) in various forms of organic combination. Both lecithin and iron decrease rapidly in the first weeks after birth. The commercial preparations contain very variable amounts of these constituents.
Actions and Uses.—Red bone-marrow is supposed to stimulate the formation of red blood corpuscles; whatever action it may have in this direction is probably due largely to the iron and lecithin which it contains.
It is said to be useful in simple and pernicious anemias.
Little is known as to the functions of the thymus, but it is believed to have an important relation to growth. There also seems to be some relation between the thymus and thyroid, for the former is frequently abnormal in diseases involving the latter (hyperthyroidism).
The use of thymus is purely empirical. It has been employed in the treatment of hyperthyroidism, rickets, tuberculosis, hemophilia, and infantile marasmus and atrophy; its use in the latter conditions is said to be the most promising. It is claimed on very doubtful grounds to exert a somewhat favorable effect in certain cases of cancer.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 69)
The following report explaining the omission from New and Nonofficial Remedies of Piperazine and Lycetol has been authorized for publication.
W. A. Puckner, Secretary.
Piperazine (diethylenediamene) and Lycetol (a methyl derivative of diethylenediamene) were accepted for New and Nonofficial Remedies in 1906. Both Piperazine and Lycetol were asserted to be efficient uric acid solvents and efficacious remedies in the treatment of gout and rheumatism. These products have been retained until now because there was no investigation which definitely showed their uselessness as uric acid solvents, though their use is generally admitted to have been disappointing.
From an exhaustive and critical study of the available evidence, Hanzlik (Jour. Lab. & Clin. Med., February, 1917) concluded that scientific evidence, though limited, and clinical opinion indicate that Piperazine is valueless in gout and that there is sufficient scientific evidence to indicate the worthlessness of Lycetol.
The referee in charge of Piperazine and Lycetol recommended that these products be omitted from New and Nonofficial Remedies for the reason that they have been sufficiently tried to justify the conclusion that they are not of value. The period of acceptance having expired, the Council directed that Piperazine and Piperazine Tablets (The Bayer Company, Inc.) and Lycetol (The Bayer Company, Inc.) be omitted from New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 70.)
As explained in the report which follows, “Stanolind Liquid Paraffin” was omitted from New and Nonofficial Remedies at the request of the proprietors. Announcement of this omission was made in the preface to New and Nonofficial Remedies, 1918, but publication of the Council’s report was postponed pending actual conflict with the rules. The Council now authorizes publication of the report because a circular indirectly advertising the product to the public was found enclosed with the trade package of Stanolind Liquid Paraffin.
W. A. Puckner, Secretary.
Stanolind Liquid Paraffin was admitted to New and Nonofficial Remedies in 1916, when its method of marketing conformed to the rules of the Council. This brand of liquid petrolatum, by action of the Council, has been omitted from New and Nonofficial Remedies on request of the Standard Oil Company of Indiana, its manufacturer, who wrote to the Secretary of the Council stating that:
“In order that our facilities for the manufacture of this oil shall be constantly engaged, it will be necessary for us to find sales on a larger scale than in the past. To do this under our present advertising and marketing arrangement we feel will be impossible.”
This letter, in addition, suggested “that physicians are not prescribing Stanolind Liquid Paraffin in any considerable proportion of their orders” and “that the situation which now confronts us would not be materially helped if Stanolind was specified in all such prescriptions.” Further, the Council is asked to consider whether it “might be willing to declare this preparation as not a Council product,” on the alleged grounds that “liquid paraffin is not medicinal in its action and passes through the digestive tract in practically unaltered condition.”
The Council holds that Stanolind Liquid Paraffin is a drug, and that, therefore, its direct advertising to the public is in contravention of the Council’s rules. Constipation should be treated by dietary and hygienic means. Evacuants are only temporary measures. Liquid petrolatum is medicinal; it greatly modifies the intestinal flora; it acts as a lubricant and emollient; it modifies the absorptive powers of the intestinal mucous membrane; it is capable of influencing the digestion of fats. In short, liquid petrolatum, being a drug, its indiscriminate and excessive use should not be encouraged.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 72)
The Council has adopted the following report and authorized its publication.
W. A. Puckner, Secretary.
Westerfield’s Digitalis Tablets (The Westerfield Pharmacal Co., Dayton, Ohio) are claimed to represent a fat free tincture of digitalis and to be “enteric coated.” It is claimed that because of this coating these tablets pass the stomach unchanged and dissolve in the intestine, and that this obviates any possibility of gastric disturbance.
The circular which sets forth the asserted advantages of the tablets states that digitalis contains a fat which is an irritant to the gastric membrane. It also contains the following:
“We feel no hesitation in saying that if this remedy is given a fair trial where it is properly indicated, the result obtained will be a gratifying surprise.
“It is a common expression from physicians who have tried this remedy to say, ‘Surely I have never used Digitalis before.’ ”
If these quotations mean anything, they imply that these tablets present a distinct advance in digitalis therapy. There is no warrant for such a claim. The statement with reference to the occurrence of an oil in digitalis is partly false and partly misleading. Tincture of digitalis, which the tablets are claimed to represent, is fat free; the fixed oil that is present in the drug is not soluble in 70 per cent. alcohol, the menstruum used for the preparation of the official tincture of digitalis. Furthermore, a fairly large amount of this oil (such as is present in 100 therapeutic doses of the drug) is incapable of causing gastric disturbance. Gastric disturbance is a side action that is inseparable from slight overdosage with all true digitalis bodies and is not in any way due to local gastric action. The claim that such action is prevented by the use of enteric pills or tablets is obviously false and misleading.
The alleged “common expression from physicians who have tried this remedy” does not constitute acceptable evidence of the value of the preparation.
The Council declared Westerfield’s Digitalis Tablets inadmissible to New and Nonofficial Remedies because unwarranted therapeutic claims are made for this product.
When the preceding report was submitted to the Westerfield Pharmacal Co., a reply was received indicating that the firm did not know that progressive manufacturers had discontinued the claim that “fat free” digitalis preparations were devoid of gastric effects. It also submitted a revised circular, which, however, reiterated the claim that the tablet presented a distinct advance in digitalis therapy in that it was “fat free,” and coated to prevent disintegration in the stomach.
Since tincture of digitalis and extract of digitalis are practically devoid of fatty material, and since it is now well known that the fat does not cause gastric disturbance and that therapeutic doses of digitalis do not exert a local irritant action on the stomach, the manufacturer’s product and the claims made for it merely tend to perpetuate old errors.
The Council declared Westerfield’s Digitalis Tablets inadmissible to New and Nonofficial Remedies on the ground that this presents an unessential modification of pills of an official substance. It directed publication of its report with this explanation.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 75)
The Council has authorized publication of the following report on Bismuth Tribromphenate-Merck and Xeroform-Heyden. These two products were found not to comply with the standards for bismuth tribromphenate adopted for New and Nonofficial Remedies, and hence could not be retained. As the manufacturers of both products announce that efforts toward the production of a satisfactory product are continued, the omission of the two brands is without prejudice to their reacceptance when a satisfactory product becomes available.
W. A. Puckner, Secretary.
The referee in charge of bismuth preparations submitted the following report of the A. M. A. Chemical Laboratory which shows that Xeroform-Heyden and Bismuth Tribromphenate-Merck do not comply with the adopted standards for bismuth tribromphenate.
Some time ago a request was received from the Medical Section of the National Council of Defense for a report on a brand of bismuth tribromphenate. In accordance with this request the firm’s product was examined, and at the same time and for comparison, an examination was also made of a specimen of bismuth tribromphenate received from Merck and Company, October, 1915, and of another specimen of bismuth tribromphenate “Xeroform-Heyden” obtained from the Chicago branch of the Heyden Chemical Works in April, 1918.
The examination brought out that the bismuth tribromphenate submitted to the national Council of Defense contained a large amount of uncombined tribromphenol, while the specimen of Xeroform-Heyden contained an excessive quantity of bismuth.
When the latter finding was submitted to the Heyden Chemical Works, the firm stated: “The product had to be made in this country after importations from Europe became impossible and the first lots were not fully up to the standard ...” The firm stated that it could now furnish a product which it considered fully equal to that which was previously imported, and offered to submit “samples of the new material.”
Having been requested to do so, a specimen of Xeroform-Heyden was received from the Heyden Chemical Works, New York. This and a second specimen, purchased from a Chicago wholesale drug house, were examined. Whereas the standards for bismuth tribromphenate which had been formulated by the Laboratory and accepted by the Heyden Chemical Works required that the product should contain from 40 to 49 per cent. of bismuth and contain not more than 3.3 per cent. of uncombined tribromphenol, the specimen purchased in Chicago contained 67.7 per cent. of bismuth, while the specimen received direct from the Heyden Chemical Works contained 24 per cent. of uncombined tribromphenol. When this result was reported to the Heyden Chemical Works, the firm replied:
“It seems that we are not yet in a position to supply a product that answers a uniform standard and that we have to continue our efforts in this direction.
“We will take this matter up with you again as soon as we have been successful ...”
At the time when the preceding examination was being made, bismuth tribromphenate-Merck could not be obtained from the Chicago wholesale houses. A request sent to Merck and Company for a specimen of the market supply brought the information that the product was temporarily unavailable. Though unable to supply the product, the firm gave valuable advice for a revision of the somewhat loosely drawn tests for bismuth tribromphenate in New and Nonofficial Remedies, 1918.
Recently (November, 1918) Merck and Company sent a specimen of its product labeled “Bismuth Tribromphenate-Merck” “Merck and Company, New York, Distributors and Guarantors,” and wrote “... You will notice this sample conforms in nearly all details to the tests we submitted with our letter of June 4th. We have been able to produce better goods, but just at present unsatisfactory starting material confronts us. The sample conforms to N. N. R., 1918, but will not meet the test for uncombined tribromphenol submitted by you in your letter of September 4th ...”
Examination of the specimen demonstrated that it was soluble to a considerable extent in alcohol (the N. N. R., 1918, description provides that it should be only slightly soluble in alcohol) and according to the standards adopted for New and Nonofficial Remedies, 1919, contains 18 per cent. uncombined tribromphenol (more than five times the permitted amount).
In view of the Laboratory’s report, the referee recommended that the acceptance of Xeroform-Heyden and Bismuth Tribromphenate-Merck be withdrawn, without prejudice to their reinstatement when satisfactory products are again offered for sale. The Council adopted the recommendation of the referee, and accordingly Xeroform-Heyden and Bismuth Tribromphenate-Merck are omitted from New and Nonofficial Remedies, 1919.
When the Laboratory’s findings with regard to Xeroform-Heyden and the action of the Council deleting the article from New and Nonofficial Remedies was reported to the Heyden Chemical Works, the firm expressed regret that efforts to produce a product equal to that formerly obtained from Germany had so far not been successful and announced that it had decided to withdraw Xeroform-Heyden from the market for the present.
When Merck and Company was advised in regard to the report of the Laboratory and the Council’s action, this firm questioned the feasibility of producing a product meeting the Council’s standards and suggested that the test for free tribromphenol be revised to permit as much as 15 per cent. of this constituent. When Merck and Company was reminded that its product, submitted in 1915, essentially complied with the adopted standards and that the estimate of the therapeutic value of bismuth tribromphenate is based on a product essentially free from alcohol-soluble material, the firm replied:
“As stated in our letter of the 12th inst. we do not wish to market the chemical unless it meets all legitimate requirements of the physicians that use it. If, therefore, your standard proves to be good and it is commercially possible to make supplies conforming to it, we shall do so. We shall discontinue the article unless it is of suitable quality.”
—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 76.)
Cream of Mustard, The Cream of Mustard Co., South Norwalk, Conn., is said to be made by mixing 2 drachms of oil of mustard and 2 drachms of oil of turpentine with one pound of white petrolatum. According to the label it is “for Tonsillitis, Rheumatism, Sore Muscles, Croup, Pleurisy, Frosted Feet, Sore Throat, Neuralgia, Sprains, Bronchitis, Headache, Chilblains, Stiff Neck, Congestion, Bruises, Asthma, Lumbago, Pains and Aches, Colds in Chest.”
The Council refused recognition to Cream of Mustard:
Because it is a simple pharmaceutical mixture of well-known ingredients and has no advantage over established rubefacients which every physician knows how to prescribe and every pharmacist to compound. Incidentally, the name “Cream of Mustard” is misleading and not descriptive of the composition of this pharmaceutical of oils of mustard and turpentine.—(From Reports of Council on Pharmacy and Chemistry, 1918, p. 79)
Caps. Adreno-Spermin Comp., Caps. Antero-Pituitary Comp., Caps. Placento-Mammary Comp., Caps. Thyro-Ovarian Comp., Caps. Hepato-Splenic Comp., Caps. Pancreas Comp., and Caps. Thyroid Comp., Not Admitted to N. N. R.
After considering the evidence for the several “pluriglandular” mixtures described below, the Council declared them inadmissible to New and Nonofficial Remedies. The Council’s action was communicated to the manufacturer, Henry R. Harrower, in accordance with the usual procedure. After giving due consideration to the manufacturer’s reply the Council authorized publication of the report which appears below.
W. A. Puckner, Secretary.
With the offer “to supply you with as much literature as may be necessary and as little of the actual remedies as may be desired” if “the prospects for the inclusion of these formulas in N. N. R. are good,” Henry R. Harrower sent the Council a booklet descriptive of his preparations and labels for the following mixtures:
Caps. Adreno-Spermin Comp., each said to contain “Adrenal Gland (total) gr. 1⁄4, Thyroid Gland (U. S. P.) gr. 1⁄12, Spermin Extr. (from Gonads), Brain and Spinal Cord aa gr. 1, Calc. Glycerophosphate q. s. ad gr. 5.”
Caps. Antero-Pituitary Comp., each said to contain “Anterior Pituitary Body gr. 2, Thymus Gland gr. 1, Thyroid Gland (U. S. P.) gr. 1⁄12, Calcium-phosphorus Comp. q. s. ad gr. 5.”
Caps. Placento-Mammary Co., each said to contain “Desiccated Placenta gr. 2, Mammary Substance gr. 11⁄2, Pituitary Body (total) gr. 1⁄3, Calcium-phosphorus Comp. q. s. ad. gr. 5.”
Caps. Thyro-Ovarian Comp., each said to contain “Desic. Corpora Lutea Ovarian Substance gr. 21⁄2, Thyroid Gland (U. S. P.) gr. 1⁄12, Pituitary Gland (total) gr. 1⁄8, Calcium-phosphorus Comp. q. s. ad gr. 5.”
Caps. Hepato-Splenic Comp., each said to contain “Liver Parenchyma, Spleen Substance aa gr. 2, Powd. Bile Salts gr. 1⁄2, Adreno-Spermin Co. (No. 1) gr. 1.”
Caps. Pancreas Comp., each said to contain “Adrenal Gland, Pituitary Gland (total) aa gr. 1⁄2, Ovarian Substance gr. 1, Pancreas Substance q. s. ad gr. 5.”
Caps. Thyroid Comp., each said to contain “Desic. Thyroid Gland (U. S. P.) gr. 1⁄8, Calcium-phosphorus Comp. q. s. ad gr. 5.”
The Council declared these preparations inadmissible to New and Nonofficial Remedies, for reasons which follow:
1. Each of the mixtures contains one ingredient or more, which is neither recognized in the U. S. Pharmacopeia nor admitted to New and Nonofficial Remedies, namely: “Spermin Extract,” “Brain,” “Spinal Cord,” “Desiccated Placenta,” “Liver Parenchyma,” “Spleen Substance,” “Pancreas Substance” and “Calcium Phosphorus Comp. (Each 100 gm. represents Magnes. Phos. 1; Calc. glycerophos. 4; Potas. bicarb. 15; Sod. bicarb. 22 and Sod. chlor. q. s.).” For obvious reasons the Council does not accept a mixture containing an indefinite ingredient and hence it would be necessary as a preliminary for the consideration of any one of the mixtures that their unofficial ingredients be made eligible for New and Nonofficial Remedies by the submission of evidence that such ingredient is of uniform composition and that it is therapeutically valuable when given by mouth. There is no evidence that many of these organs have any value whatever when administered by the mouth or in any other way.
2. In the light of our knowledge the administration of gland mixtures in the host of conditions enumerated in the advertising circular is irrational and on a par with the use of the shotgun mixtures once in vogue.
Be it a pharmaceutical mixture, a “mixed” vaccine, or a “pluriglandular” product, the combination of two medicinal ingredients in a mixture must be considered contrary to rational therapy unless a good reason exists for such combination. Such mixtures are held in conflict with Rule 10 unless the manufacturer presents acceptable evidence for the value of his combination. A physician may prescribe any mixture which he considers indicated in a given case, but the marketing of mixtures of drugs in fixed proportions is in most instances irrational and a detriment to sound therapy.—(From The Journal A. M. A., Jan. 18, 1919)
The Council has authorized publication of the following report declaring Cerelene inadmissible to New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Cerelene, a paraffin preparation for the treatment of burns, was submitted to the Council by the Holliday Laboratories, with the statement that it was composed of 84 per cent. paraffin, 15 per cent. myricyl palmitate, and 1 per cent. purified elemi gum to which is added oil of eucalyptus 2 per cent. and betanaphthol 0.25 per cent. It was explained:
“Myricyl Palmitate is a purified form of Beeswax, free from all impurities, acids, etc., which is solely manufactured by this Company....”
It was also stated that on “special order” Cerelene has been made containing oil of eucalyptus and resorcin, oil of eucalyptus and picric acid, and picric acid alone. The following report on the preparation was presented to the Council by the referee to whom Cerelene had been assigned:
Cerelene is another compound wax for the treatment of burns. According to the work of Sollmann (J. A. M. A., 68:1799, 1917) it is highly improbable that compound mixtures have any advantage over simple paraffin of low melting point. Cerelene must therefore be considered as an unessential modification of paraffin, and as in conflict with Rule 10; unless definite evidence of superiority is submitted. Cerelene mixtures containing medicinal ingredients also appear unscientific since the evidence that the ingredients do not leave the wax has not been successfully contradicted. Finally, the claims made for Cerelene are rather extreme, and would need some revision before they could be accepted.
The A. M. A. Chemical Laboratory reports:
The physical properties of Cerelene are as follows:
| Melting point 50.0 C. by U. S. P. method. | |
Ductility limit | 30.5 C. |
Plasticity limit | 26.4 C. |
Not strong at | 38 C. |
Adheres moderately well; detaches with “pulling.” On heating, readily loses eucalyptol, and a small amount of resinous substance forms in the bottom of the beaker. If Cerelene is heated to 145 C. and cooled, the resulting product no longer has the properties of the original Cerelene.
It is recommended that the preceding report be sent to the Holliday Laboratories, and that unless its superiority over simple paraffins is demonstrated and the unwarranted claims abandoned, Cerelene be declared inadmissible to New and Nonofficial Remedies for conflict with Rules 6 and 10.
This report was submitted to the Holliday Laboratories with the information that it had been adopted, Oct. 3, 1917. It was also explained that before Cerelene could be accepted, the unofficial and unstandardized constituent “myricyl palmitate” would have to be considered and accepted for New and Nonofficial Remedies since, for obvious reasons, the Council does not accept a preparation which contains an unofficial and unstandardized substance not in N. N. R.
The Holliday Laboratories acknowledged receipt of the Council’s report and asked that the matter be held in abeyance until the requested evidence had been obtained. Later the Council was advised that the advertising circulars for Cerelene had been withdrawn with the exception of one giving directions for its use. Five months later, the firm stated that experiments were being made “to determine the actual strength of Cerelene in comparison with other paraffin waxes....” Nothing further has been heard from the Holliday Laboratories and no reply has been received to an inquiry made Oct. 12, 1918. The Council therefore authorizes publication of its report declaring Cerelene inadmissible to New and Nonofficial Remedies.—(From the Journal A. M. A., Feb. 15, 1919).
The report which appears below was adopted by the Council and sent to the Anglo-French Drug Co., Ltd., New York, for comment in December, 1918. No explanation has been received from the manufacturer. For the information of the profession the Council has now authorized publication of the report.
W. A. Puckner, Secretary.
“Collosol Cocaine” was submitted to the Council in October, 1918, by the Anglo-French Drug Co., Ltd., New York, under the claim that it was an “absolute colloid” and that it contained “1 per cent. cocain.” The label on the submitted specimen declares:
“Collosol Cocaine 1-100”
“... the Cocaine exists as the pure alkaloid in the Colloidal state—the condition in which it is isomorphic with the protein of the body fluids. The effect is more prolonged than that of a molecular Cocaine Solution and being non-toxic absorption presents no practical danger.”
The product was assigned to the Committee on Pharmacology for consideration. The following report was submitted and its adoption by the Council recommended by the committee:
“Collosol Cocaine” is said to be a colloidal form of cocain and is alleged to possess a remarkably low toxicity. The subjoined report of the A. M. A. Chemical Laboratory, however, shows that the preparation does not have the composition claimed for it and it is, in effect, misbranded. In fact, the English manufacturers concede that it is not an “absolute colloid” and that the declaration with regard to the percentage of cocain is incorrect.
It is recommended that, without considering other conflicts with the rules of the Council at this time, “Collosol Cocaine” be declared inadmissible to New and Nonofficial Remedies for conflict with Rule 1 which requires that the composition of an article must be correctly declared. The report of the A. M. A. Chemical Laboratory is appended.
Simpson, Hewlett and Eyre (Lancet, April 28, 1917, p. 660) reported “Collosol Cocaine” to be much less toxic than cocain. These writers, however, did not verify the statements as to the composition and in the light of subsequent chemical examination it is not to be wondered at that “Collosol Cocaine 1.0 per cent.” was much less toxic than a solution containing 1.0 per cent. of cocain hydrochlorid.
Barger, Dale and Durham report from the Department of Biochemistry and Pharmacology, Medical Research Committee (Lancet, Dec. 1, 1917, p. 825), that they examined “Collosol Cocaine” and found it to contain but 0.25 per cent. of cocain. They also found that the cocain was not present in a colloidal form. Discussing the low toxicity claimed by the manufacturers, these investigators state:
“In the samples which we examined the toxicity was, indeed, much lower than that of an ordinary 1 per cent. solution of a cocain salt; but the local anesthetic action was low to a corresponding degree, and both actions corresponded satisfactorily with the proportion of cocain chemically recoverable from the solution.”
Stroud, of the Crookes Laboratory (which manufactures the preparation), who apparently had been informed of this work in advance of publication, admits the correctness of it, and states (British Medical Journal, Nov. 24, 1918, p. 710) that “whilst the colloidal protective apparently absorbs a portion of the cocain, the remainder is found not to exhibit the attributes of a colloid,...”
The specimen of “Collosol Cocaine” submitted to the Council and labeled “Collosol Cocaine 1-100” was found to contain at most 0.4 per cent. cocain. The examination was made in accordance with the method used by Barger, Dale and Durham and calculated as cocain. This method, however, probably would not distinguish between cocain and basic decomposition products, but would include all as cocain in the amount found. The specimen of “Collosol Cocaine” examined was neutral or slightly acid, a fact which tends to confirm the conclusion of the British investigators that “Collosol Cocaine” contains cocain in noncolloidal form and precludes an increased physiologic effect due to alkalinity.
The Council adopted both the report submitted by the committee and that of the A. M. A. Laboratory and declared “Collosol Cocaine” inadmissible to New and Nonofficial Remedies.—(From The Journal A. M. A., April 12, 1919.)
The Council has authorized publication of the following report on Cuprase, sold by the Anglo-French Drug Co., Ltd. The Council’s criticisms of the advertising claims were sent to the firm, December, 1918. The firm made no reply and essentially the same claims are contained in recent advertisements.
W. A. Puckner, Secretary.
“Cuprase” is now being advertised and sold in the United States by the Anglo-French Drug Co., Ltd., the firm which also markets it in England. It is said to be “prepared in the Laboratories of F. Ducatte, 8 Place de la Medeleine, Paris.” According to an advertising circular entitled “The Medical Treatment of Cancer” “Cuprase” is “chemical colloidal copper”; in another place it is “a colloidal copper hydroxid,” which is said to be obtained chemically by the reduction of salts of copper in the presence of albumosic acid.
A box (price $8.50 less 10 per cent. discount) of “Cuprase-Doctor Gaube du Gers” was purchased recently from the Anglo-French Drug Co., Ltd. It contained eight ampules each containing approximately 6 c.c. of a brownish fluorescent liquid. No information of composition was given on the box, except the line: “Chaque ampoule contient: 0 gr. .00121 de Cuivre pur” (Each ampule contains 0.00121 gr. of pure copper). The A. M. A. Chemical Laboratory reports that the preparation does contain a small amount of copper, with some protein material and about 1 per cent. sodium chlorid.
The therapeutic claims in the advertising circular are those commonly made for cancer “cures” and are about equally convincing. The publication of such statements and quotations as the following, which appear in a pamphlet “The Medical Treatment in Cancer,” cannot be too strongly condemned in a medicament that at best has only an experimental status:
“A special preparation, Cuprase, has been introduced into therapeutics which has been remarkably successful. In the history of the therapeutics of cancer, nothing has been found which can compare with the effects produced by means of Cuprase. Clinical facts carry greater weight than theoretical deductions. It follows, from the clinical observations which I have collected, that in the large majority of cases Cuprase effects the diminution or disappearance of the pains, an improvement in the general condition, a diminution or arrest of the neoplasms, and finally in certain cases, a cure has been effected. It should be remarked that all or nearly all the observations refer to inoperable cases in which the prognosis was unfavorable at an early date. It is needless to emphasize the practical importance of a preparation capable of yielding such results, even relative, in the worst stages of a disease which has always been regarded as absolutely resisting the action of all internal remedies.”
“To sum up, Cuprase has given positive results in about 94 per cent. of the cases in which it has been employed for a sufficiently long period, and some brilliant results in about 20 per cent. of these cases. Therefore, it may be affirmed, that among the internal remedies for cancer, Cuprase is the one which has produced the most successful results, and can, under certain circumstances, compete with surgical methods, even, so far as the rapidity of their results are concerned.”
“It is indicated:
(a) apart from all operation, and as a specific and curative remedy;
(b) before an operation, in order to give tone to the patient, mobilise the tumor, destroy its toxins;
(c) after the operation, as a tonic and anti-toxic, and in order to avoid frequent relapses which are always possible.”
Essentially the same statements are made in the more recent advertisements (f. i. Urological and Cutaneous Review, Feb., 1919). Opposed to these loose statements are the results of Richard Weil (The Journal A. M. A., 1913, Sept. 27, p. 1034; ibid, 1915, April 17, p. 1283). Weil avoided pitfalls of subjective impressions and used as the essential criterion of efficiency “the demonstrable reduction in size of a tumor, of a kind not to be attributed to the natural processes of evolution of that tumor or of its associated lesions” (l. c. 1915, p. 1289).
The available evidence for Cuprase is far from meeting this criterion. That published by the manufacturers and agents presents only vague generalities, and no definite data. The evidence gathered by Weil himself permits an estimate of the value of Cuprase and it is entirely unfavorable. He states (l.c. 1915, p. 1288):
“Colloidal copper has been used in recent time for the same purpose by Gaube du Gers and by others. I have recently examined the effects of colloidal copper on malignant tumors in man, and have been unable to find that it has any therapeutic value. Furthermore, a study of the distribution of the copper in tumors obtained at operation or by necropsy from individuals so treated failed to show that the copper had been deposited therein.”
In view of the extravagant and cruelly misleading therapeutic claims, and the indefinite statements of composition, the Council voted Cuprase ineligible to N. N. R., and authorized the publication of this report.—(From The Journal A. M. A., April 12, 1919.)
The Council has adopted and authorized publication of the report which appears below declaring “Collosol Argentum,” “Collosol Arsenicum,” “Collosol Cocain,” “Collosol Cuprum,” “Collosol Ferrum,” “Collosol Hydrargyrum,” “Collosol Iodin,” “Collosol Manganese,” “Collosol Quinin” and “Collosol Sulphur” inadmissible to New and Nonofficial Remedies, because their composition is uncertain (conflict with Rule 1). In the few cases in which the therapeutic claims for these preparations were examined, the claims were found to be so improbable or exaggerated (conflict with Rules 6 and 10) as to have necessitated the rejection of these products.
W. A. Puckner, Secretary
The Anglo-French Drug Co., Ltd., London and New York, in November, 1918, requested the Council to consider the products “Collosol Argentum,” “Collosol Arsenicum,” “Collosol Cocain,” “Collosol Cuprum,” “Collosol Ferrum,” “Collosol Hydrargyrum,” “Collosol Iodin,” “Collosol Manganese,” “Collosol Quinin” and “Collosol Sulphur.” The term “Collosol” appears to be a group designation for what are claimed to be permanent colloidal solutions, marketed by the Anglo-French Drug Co., Ltd. Were this claim correct, “Collosols” should contain their active constituents in the form of microscopic or ultramicroscopic suspensions, protected against spontaneous precipitation by the presence of proteins or some similar “stabilizers.”
According to the original patent specifications for Collosols, the metals are precipitated or treated with “peptone,” which acts as the suspending or stabilizing agent. The method of using the peptone makes it doubtful, in the first place, whether the major part of the metals is present in colloidal form, or merely in the form of peptonates, i. e., as ordinary salts. Moreover, the later patents indicate that the products have been unsatisfactory; “experience having shown that some metal colloids under certain conditions not yet fully understood have the tendency to break down after a certain period” (U. S. patent No. 1,116,247). Phenol, it is claimed has a tendency to counteract this decomposition, and the patent covers the use of phenol for this purpose.
It is difficult to see how phenol could possibly have such action. In fact, it obviously does not, for a number of the samples of Collosols submitted to the Council had separated. For instance, “Collosol Hydrargyrum” was not a colloidal solution at all, but a suspension of a coarse powder. The ampules of “Collosol Ferrum” contained a considerable quantity of flocculent precipitate. If either of these preparations were injected intravenously as directed, death might result, making the physician morally if not legally liable.
The recklessness of the claims is further illustrated by the advice that these indefinite mixtures of poisonous metals can be injected in unlimited quantities. Thus, Henry Crookes stated (Chemical News, May 7, 1914, p. 218) that Collosols “contain so small a proportion of metal, viz., 1 in 2000, that even a poisonous body like arsenic can be used with impunity.” He stated that they may be applied as a lotion, intramuscular or intravenous injection, and that “one pint or more can be injected intravenously.”
In the case of “Collosol Cocain,” as was brought out in the Council’s report published in The Journal, April 12, 1919, the manufacturers have admitted that the product is not what they have claimed—and still claim—for it. The report of the A. M. A. Chemical Laboratory showed that “Collosol Cocain,” instead of containing 1 per cent. cocain as claimed, contained, in fact, at most not more than 0.4 per cent. cocain.
The report of the A. M. A. Chemical Laboratory on the Collosol products was sent by the Council to the New York office of the Anglo-French Drug Co., Ltd., in duplicate in order to facilitate reference to the London office. This was some months ago. The information which the Council requested has not yet been received, nor has the Anglo-French Drug Co., Ltd., indicated its intention of supplying such information. On the other hand, claims to which specific objection have been made, continue to appear in current advertising. Accordingly, the Council authorizes publication of this report, and declares the Collosol preparations previously named ineligible to New and Nonofficial Remedies.
In addition to the preceding the following notes of the referee on the evidence so far submitted were sent to the Anglo-French Drug Company, Ltd., for consideration:
Collosol Iodine: The leaflet which describes Collosol Iodine contains claims that are improbable, not in accord with accepted facts nor substantiated by evidence; for instance:
“This preparation contains Iodine in its most active form ...”
“The disadvantages of ‘iodism’ and nausea frequently associated with iodides never occur with Collosol Iodine.”
“In the case of Colloidal Iodine the whole of the Iodine is absorbed and enters into molecular combination with protein to form an iodo-amino acid and ... exerts a reducing action on the lipoids producing a different condition of the blood—hence the use of Iodine as an ‘alterative’.”
“Intravenously the action of Collosol Iodine is more rapid ... in cases of pyemia ... thus showing its absolute non-toxicity.”
“ ‘Per se’ Colloidal Iodine is only slightly parasitotropic and bacteriotropic but micro-organisms are very greatly influenced by its action, and not only is the effect of a subsequently administered remedy greatly increased but also the insoluble colloidal protein of serum itself is reduced to smaller particles, thus increasing its surface and adsorptive capacity and consequent germicidal power. In some cases the serum, thus aided, is enabled to throw off a milk microbial invasion. The above action can be readily demonstrated ‘in vitro’ by means of the ultramicroscope.”
“In Cancer, the intravenous injection of Collosol Iodine relieves pain, even where large dosage of morphine is ineffective.”
“In Rheumatism the ionic method of treatment with Collosol Iodine is strongly advised.”
“In Recovery from Alcoholism the internal administration of Collosol Iodine restores the normal condition of cell activity, ensuring rapid recovery.”
Collosol Hydrargyrum: This is said to be a preparation of colloidal mercury and would therefore be similar to Electromercurol (New and Nonofficial Remedies, 1919, p. 167). Colloidal mercury preparations have been used to some extent; they appear to have no decided advantage over other, noncolloidal, mercury compounds. They differ sufficiently from them, however, to justify acceptance for New and Nonofficial Remedies, providing that reasonable claims are made for them. The leaflet advertising Collosol Hydrargyrum contains statements that cannot be accepted and require thorough revision to make them acceptable. The following are instances:
“Although—especially locally—the action of mercurials is markedly antiseptic, when taken internally or injected, it has been stated by some of the best known authorities, that their action is rather to increase the natural resisting power of the body to disease, probably because of stimulation of the oxidases.”
With the soluble mercurials “considerable upset of the normal cell conditions of the tissues ensues whilst these soluble salts are being converted to a condition in which the body can make use of them.”
“The colloidal state ... is stated by some authorities in the case of mercury to be invariably precedent to absorption.... With the usual forms of mercury the danger of too great a dose per cell is considerable, but in the case of colloidal mercury, the diffusion is extremely rapid and chemical affinity low. Hence the danger to the individual leucocyte is minimized and the maximum effect obtained.”
“... absence of pain is usual in the administration of colloidal preparations and is due to their isomorphism with the colloidal lipoid and protein of the tissues and body fluids.”
“According to McDonagh,... mercury acts as an oxidizing agent and that the process of oxidation is more effective in the early stages of syphilis in producing the death of the causal organism ...”
Collosol Manganese: The circular submitted to the referee is a reprint of a paper by Sir Malcolm Morris on “The Treatment of Furunculosis and Other Deep-Seated Coccogenic Infections by Collosol Manganese.” It reports four cases of furunculosis, each of which cleared up after the intramuscular injection of a few doses of Collosol Manganese. The author seems to attribute the cure to the manganese but the evidence is not convincing. Even the author admits that, in the treatment of furunculosis in general “when at last the dismal procession ends, this often appears to be less the result of treatment than because the disease has run its natural course.” Unless much better evidence is in existence, the preparation must be considered to conflict with Rule 6, which requires therapeutic claims to be substantiated.
Collosol Argentum: The evidence submitted as to actions consists of a single reprint by Roe, which is not convincing, and this fantastic statement by Boys:
“A young girl, aged 18, came to my house with acute inflammation of one eye with an ulcer on the cornea. Two drops of Collosol Argentum were dropped in the eye at 7 p. m., and a pad placed over the eye. When she came next morning the eye was quite well; the ulcer had disappeared, and there was no inflammation.”
There is no evidence that this preparation acts as catalyzer and assists the natural resisting bodies of the tissues; or that these are “oxygen carriers.” Unless the claims are supported by better evidence, they, in the opinion of the referee, could not be accepted.
There have been submitted to the Council samples of the following metallic Collosols:
| Collosol Argentum | Collosol Ferrum |
| Collosol Arsenicum | Collosol Hydrargyrum |
| Collosol Cuprum | Collosol Manganese |
Also Collosols of Iodine and Sulphur, and finally Collosols of Cocain and Quinin. Of all the above, except sulphur, only three small ampules have been submitted. This does not admit of any chemical examination but a statement of the physical appearance may be of interest.
Collosol Arsenicum, 0.2 per cent.: Very turbid with large quantities of a lemon yellow flocculent precipitate. On shaking does not become homogeneous and rapidly separates again.
Collosol Argentum, 1-2000: The liquid has a slight opalescence. There is considerable deposit of a heavy black precipitate. Does not become homogeneous on shaking and the black substance quickly separates again.
Collosol Cuprum, 0.5 per cent.: Dark red somewhat opalescent liquid. No precipitate. May be colloidal.
Collosol Ferrum, 1-2000: Liquid clear. Large quantities of dark brown flocculent precipitate. The precipitate is not distributed evenly when the mixture is shaken and settles out quickly on standing.
Collosol Hydrargyrum, 5 per cent.: Milky liquid. Large quantities of white deposit mixed with considerable black. The deposit mixes fairly well but the greater part settles out after standing an hour or two.
Collosol Manganese, 2.5-1000: Clear reddish-brown liquid without deposit of any kind. Is not opalescent or fluorescent.
Collosol Iodin, 1-500: Very pale straw colored liquid without deposit. Has a slight opalescence.
Collosol Sulphur, 1-100: Liquid is opalescent. There is some deposit of yellow particles. A four ounce bottle was also submitted. The liquid in this bottle is milky with considerable deposit of yellow crystals like ordinary crystalline sulphur.
Collosol Cocain, 1-100: Transparent, colorless liquid with no deposit. Chemical examination showed 0.4 per cent. of what may have been cocain. This residue gave alkaloidal tests.
Collosol Quinin, 1-100: Slightly opalescent, colorless liquid, with no deposit. Gives alkaloidal reactions.—(From The Journal A. M. A., June 7, 1919.)
The Council has authorized publication of the following report, not so much because the preparation with which it deals is of any great importance, but as a protest against the large number of similar irrational complex mixtures which are still offered to physicians.
W. A. Puckner, Secretary.
Pulvoids Calcylates Compound (The Drug Products Co., Inc.) are tablets each of which is claimed to contain:
“Calcium and Strontium Disalicylate, 5 grs.; Resin Guaiac, 1⁄2 gr.; Digitalis, 1⁄4 gr.; Cochium [colchicum?] Seed, 1⁄4 gr.; Squill, 1⁄4 gr.; Cascarin, 1⁄16 gr. with aromatics.”
“Pulvoids Calcylates Compound (Sugar coated orange color)” is advertised (Medical Times, January, 1919) as being “Analgesic-Antipyretic and Diuretic,” and is included in the preparations designated by the advertiser as “Approved Remedies for LaGrippe and ‘Flu.’ ” The claim that “Their tolerance is remarkable” refers not to the physicians who tolerate such products, but to the alleged fact that Pulvoids Calcylates are tolerated remarkably well. The advertisement continues:
“May be given persistently and continuously without gastric disturbances.”
“They are uniformly efficient. More certain in effect than the ordinary Salicylates.”
It would be difficult to find an advertisement of equal length containing a greater number of misleading or directly false statements than are found in this one. The Journal (April 22, 1916, p. 1307) has called attention to the lack of justification for this absurd mixture of drugs and has discussed the preparation with especial reference to its use in acute rheumatism, in which the salicylates occupy a special field. The advertisement just quoted mentions La Grippe and “Flu” (or Influenza) as special fields of usefulness for this preparation. This, apparently, is merely an attempt to spread the sail for any breeze. Salicylates have a field of usefulness in influenza in that they often afford relief from pain. There is no reason to suppose that a mixture containing calcium and strontium salicylates—the “Calcium and Strontium Disalicylate” of Pulvoids Calcylates is probably a mixture of calcium and strontium salicylate127—has any greater salicylic effect than an equal amount of sodium salicylate. On the other hand, it is worse than useless to give colchicum, squill and digitalis for the relief of such pains.
Should cardiac dilatation develop, and digitalis medication be required it would be impossible to adjust the dose of such a mixture with special reference to the digitalis action, which alone would be indicated for that condition. No educated physician at present would think of giving resin of guaiac merely because his patient required digitalis, nor would he administer “cascarin,” whatever that may be, in fixed doses, every time he gave a dose of salicylate.
It is impossible to recognize the several effects induced by this therapeutic omneity, and the medical profession should consider it an insult to be offered mixtures such as Pulvoids Calcylates Compound.
Pulvoids Calcylates Compound is, per se, of no great importance; it is one of a type. It has been selected as one of the utterly irrational and therefore potentially dangerous mixtures, that may be found by the score or the hundred in the catalogues of practically every pharmaceutical manufacturing firm in the United States.—(From The Journal A. M. A., June 14, 1919.)
The Council has adopted and authorized publication of the statement which appears below, declaring Proteogen No. 1 (Plantex) for Cancer, Proteogen No. 2 for Rheumatism, Proteogen No. 3 for Tuberculosis, Proteogen No. 4 for Hay Fever and Bronchial Asthma, Proteogen No. 5 for Dermatoses, Proteogen No. 6 for Chlorosis, Proteogen No. 7 for Secondary Anemia, Proteogen No. 8 for Pernicious Anemia, Proteogen No. 9 for Goitre, Proteogen No. 10 for Syphilis, Proteogen No. 11 for Gonorrhea, and Proteogen No. 12 for Influenza and Pneumonia inadmissible to New and Nonofficial Remedies because their composition is secret; because the therapeutic claims made for them are unwarranted; and because the secrecy and complexity of their composition makes the use of these preparations irrational.
The Council took up the consideration of the Merrell Proteogens because of inquiries received, and on January 27 invited the Merrell Company to aid in the proposed investigation by submitting information in regard to the composition of the preparations, submitting the current advertising, and presenting evidence for the claims that were made for the preparations. While the Merrell Company agreed to submit the requested information, this had not been received at the time the report of the referee to whom the products had been assigned (Referee 1), was adopted. This report was sent to the company on April 4. In reply the Merrell Company protested against the conclusions of the report and submitted considerable material in an attempt to support the claims made for the products. This material was examined by the first referee and then transmitted to a second referee (Referee 2) for consideration. The second referee concluded that the matter submitted offered no evidence that would justify the Council in modifying the report first adopted, and hence recommended that its publication be authorized.
In accordance with this recommendation (report of Referee 2) the Council authorized the publication of the reports of both the first and second referees.
W. A. Puckner, Secretary.
“Proteogens,” according to the William S. Merrell Co., are “Polyvalent Proteins of Non-Toxic Plant Origin.” The subject of Proteogens can best be approached by recalling the history of “Autolysin,” an alleged remedy for cancer, originated by A. S. Horowitz, Ph.D. This was exploited some years ago, and was finally shown to be worthless. Proteogens are said to be prepared “under the personal supervision of the originator, Dr. A. S. Horowitz.” The composition of the different Proteogens is essentially secret. The assertion was made at one time, but is not found in the present advertising matter, that Plantex—now called “Proteogen No. 1”—is similar to Autolysin. Now the Proteogens are said to be “prepared by a special process employing various combinations of plants.” Further:
The biologic principles present are chlorophyll, chromoplast, lipoids and vitamines; these are ferments or enzymes. The vegetable acids, metalloids and metals present in all plants in colloid form act biochemically. Among the metalloids are hydrogen, carbon, manganese, oxygen, sulphur, phosphorus and chlorine; the heavy metals are iron, potassium, sodium, magnesium and copper. These biochemic principles are always present in plants as colloids.”
It is claimed by the Merrell Company that:
“Proteogens stimulate the cytogenic mechanism to higher activity; therefore, indirectly cleave the invading microorganism and eliminate their special toxins. Proteogens swing the disturbed metabolism back to normal and, by natural processes, build up effective defenses against recurrent bacterial attacks.”
Proteogen No. 1 was first introduced as “Plantex,” and at that time the Merrell Company referred to a preparation that was the result of “a series of studies” carried out by a “noted biologist” with a view of “evolving a Cancer remedy” that was “to be autolytic in character,” and announced:
“The House of Merrell always interested in the progress of plant therapy, began pharmacological experimentations to reproduce this same substance. The qualitative and quantitative analysis of the substance as used in New York having been published simplified matters. A somewhat similar remedy has now been prepared. It consists of the following substances—Menyanthes trifoliata [Buckbean], Melilotus officinalis [Yellow sweet clover], Mentha crispa [Curled mint], Brassica alba [White mustard], Anemone hepatica [Liver leaf], Viola tricolor [Pansy], Anthemis [Roman chamomile], Fructus colocynthidis [Colocynth], Lignum quassiæ [Quassia], Urtica dioica [Nettle], Radix rhei [Rhubarb root], Hedge hyssop. These substances are in approximately equal proportions with the exception of the mustard which forms 20 per cent. of the mixture, and the colocynth fruit which is 5 per cent.”
With respect also to the other Proteogens listed above, study of medical literature revealed no evidence establishing their therapeutic value; in fact, no evidence was found other than that appearing in the advertising matter of the manufacturer. The range of diseases in which Proteogens are recommended is so wide as to make obvious the lack of scientific judgment which characterizes their exploitation. A circular letter, received January, 1919, reminded the physician that about a year ago his attention had been directed to Proteogen No. 1 for cancer, that later developments enabled the firm to recommend for his consideration “a series of Proteogens (Nos. 2 to 9),” and that now “In response to an insistent demand, Dr. A. S. Horowitz has prepared two new Proteogens—No. 10 for Syphilis and No. 11 for Gonorrhea.” A postscript to this circular letter announced another preparation, “Proteogen No. 12 for Influenza and Pneumonia,” a “development out of the present influenza epidemic,” and admitted that “It has not had the clinical experimentation that precedes our introduction of a new product.”
The introduction of No. 12 was effected by means of a special bulletin which consists exclusively of clinical reports from seven physicians, all from Chicago save one, and all purporting to show most favorable results from No. 12. They describe cases which any physician with experience with influenza can duplicate without any special treatment.
It is difficult to give serious consideration to a set of alleged remedies when the only evidence is that furnished by the proponents of the alleged remedies. This is particularly true when the alleged remedy does not make a sufficient appeal to one’s sense of the rational in therapeutics to lead one to feel justified in asking a trial at the hands of careful clinical observers. Considering the grave nature of the diseases for which Proteogens are recommended, particularly cancer, tuberculosis, and pernicious anemia, the want of a rational basis for the method of treatment and the general tenor of the advertising matter, it appears safe to conclude that these agents do not represent any definite advance in therapeutics.
As the use of preparations, secret in composition, and of no established value, is contrary to rational therapy, it is recommended that the Proteogen preparations be declared in conflict with Rules 1, 6 and 10.
The report declaring the Proteogens of the William S. Merrell Company inadmissible to New and Nonofficial Remedies was adopted by the Council, but before publication it was sent to the Merrell Company for such comments as it might desire to make. In due time the reply of the firm was received. It consisted of two volumes bound in limp morocco, each stamped in gold: “Report Proteogen Therapy Requested by the American Medical Association, 1919; The Wm. S. Merrell Company.” The first volume contained 79 pages of typewritten material; the second volume contained 76 pages of typewritten material and a number of advertising booklets put out by the Wm. S. Merrell Company, exploiting the Proteogens.
Among the typewritten material was a 14-page report on “Proteogen Therapy” by its originator, A. S. Horowitz. Following this there are several pages devoted to what is termed “a short qualitative description of the ingredients of major importance in Proteogens.” Then follows a page describing the advertising of Proteogens, and the remainder of the two books is devoted to testimonials, lauding the benefit of Proteogens in diseases such as cancer, tuberculosis, rheumatism, asthma, influenza, enlarged prostate, rheumatic endocarditis, syphilis, eczema, psoriasis, diabetes, secondary anemia, gonococcic infections, etc. Finally, there are attached samples of advertising pamphlets.
The dissertation by A. S. Horowitz contains little actual information concerning these substances, but is concerned principally with discussion of foreign proteins, “antiferments,” “non-specific proteins,” “anti-virolins” and speculations on their hypothetical actions and interactions on each other and on the organs of the body and on bacteria. The report contains many questionable statements.
One finds in this report but few definite statements of facts which are known to be accurate or which could be accepted without question. The qualitative description of the proteins and their components is as vague as the previous discussion. The differentiation between the various Proteogens is extremely indefinite; that for Tuberculosis, No. 3 is described as “polyvalent, non-specific protein which rapidly attacks the acid-fast, encapsulated tubercle bacilli”; Proteogen No. 10 for syphilis is said to be a combination of “non-specific plant proteins and different chemicals which has the power to paralyze and destroy living spirochete.” It is stated that Proteogens are scientific preparations based on standard ingredients and that the standardization is more accurate than in serums, vaccines or toxins, etc. The report gives no proof of such statements.
The testimonials that are submitted are typical of “reports” that manufacturers are able to obtain from some physicians, to prove the efficacy of almost any preparation in any disease. Each consists, practically, of the opinion of the individual who has employed the Proteogens or the opinion of the patient who has been treated. Few data are given in these reports from which an impartial conclusion might be drawn. A few of the testimonials presented by the William S. Merrell Company follow. The valuelessness of such material as scientific evidence is obvious:
Rheumatism:—Proteogen No. 2.—The Doctor has one case being treated with No. 2. She has improved so rapidly she cannot express her pleasure, and will continue for some time on the treatments. She is a patient who was confined during the time she suffered from a rheumatic illness, and it seemed to affect her mental condition. This condition is clearing up also, very much to the pleasure of both patient and doctor.—November 27, 1918.
Influenza:—Proteogen No. 12.—First day, temperature 102, gave 1 c.c. Proteogen No. 12; second day, temperature 100, gave 1 c.c. Proteogen No. 12; third day, temperature 98.8, gave 1 c.c. Proteogen No. 12, and then discharged the case as recovered.—October 31, 1918.
Asthma:—Proteogen No. 4.—Splendid results obtained from a sample of Proteogen No. 4. Three ampoules affected [effected?] complete recovery.—October 9, 1918.
Cancer:—Proteogen No. 1.—Mrs. B. pronounced recovered from Cancer by Dr. O. W. A., of Catlin, after having injections of Proteogen No. 1 for some time.—October 4, 1918.
Eczema:—Proteogen No. 5.—Tried No. 5 on a patient with eczema, and with happy results. Have not done anything for him for about five months—and he is now at his business. Proteogen No. 5 also RELIEVED HIM OF CONSTIPATION AND WHAT HE CLAIMED A TRAUMATIC STRICTURE OF THE LOWER PORTION OF SIGMOID FLEXURE. He is sure pleased and recommending them to his friends. (Proteogens).—February 17, 1919.
Syphilis:—Proteogen No. 10.—I am getting such excellent results with the No. 10 Proteogen for Syphilis that I am badly in need of more, as I am treating so many cases. Please send me four dozen C. O. D.—October 9, 1918.
Enlarged Prostate:—Proteogen No. 1.—Have used Plantex in four cases, with good results in each case. One of them his father, an elderly man.—April 25, 1918.
Lobar Pneumonia:—Proteogen No. 12.—The only case I have used Proteogen No. 12, was a man who had Lobar Pneumonia of left lung following Influenza. After crisis came, patient continued to have slight rise in temperature, cough, and after using 10 doses of your Proteogen No. 12, temperature was normal, cough very much better, patient began to take on flesh and is still improving.—December 26, 1918.
Tuberculosis:—Proteogen No. 3.—The Doctor writes: The Proteogen No. 3 sent me worked wonders in my patient. The case came under my care when he was too far gone for anything to benefit him a great deal, but the Proteogen did for him more than anyone could have expected, yet he died leaving me with a few ampoules to try on the next patient.—September 20, 1918.
Gonorrheal Cystitis:—Proteogen No. 11.—My patient has taken two boxes of your Proteogen No. 11 given for gonorrheal cystitis of probably two years’ standing and at this writing I consider her almost, if not entirely, cured which I think speaks very highly of your remedy. I expect to use more of your preparations in the future.—April 12, 1919. [This testimonial, either by clerical error, or because the results were considered remarkable, was repeated elsewhere in the material submitted by the Merrell Company.]
Acute Gonorrhea:—Proteogen No. 11.—Mr. A. E. R., age 65, weight 140 pounds. First attack. Had had no previous treatment. Came to me January 2, 1919. Had discharge, all acute symptoms, burning, etc. Gave seventeen injections of Proteogen No. 11, also mild antiseptic urethral wash. Discharged on February 15, 1919, clinically cured.—April 11, 1919.
Epithelioma of Buttock.—Proteogen No. 1.—I used Proteogen No. 1 on an epithelioma of buttock some six months ago with favorable results and no return of symptoms as yet.—April 13, 1919.
It is obvious that the Proteogen preparations are in conflict with Rules 1, 6 and 10, and should not be admitted to “New and Nonofficial Remedies.” It is recommended that the previous action of the Council be allowed to stand and that publication of both reports be authorized.—(From The Journal A. M. A., July 12, 1919)
The Council authorizes publication of the following report. This report declares Arsenoven S. S. of the S. S. Products Company and Solution of Arsenic and Mercury (formerly called Arseno-Meth-Hyd) of the New York Intravenous Laboratory, inadmissible to New and Nonofficial Remedies. The Council takes this opportunity to repeat its warning against the abuses—often dangerous—to which patients are frequently subjected when “intravenous therapy” is employed.
W. A. Puckner, Secretary.
Because of inquiries received, the Council took up the consideration of Arsenoven S. S. and Arseno-Meth-Hyd (now sold as Solution of Arsenic and Mercury). The preparations having been referred to a committee for consideration, this committee reported:
“Arsenoven S. S.” is a preparation put out by the S. S. Products Company, Philadelphia. The claims are made that it is “a simplified office treatment for syphilis” and is “a combination of arsenic and mercury for office use, offering maximum efficiency, safety and convenience.” According to the company, “Arsenoven S. S.” contains Dimethylarsenin 15.4 grains, Mercury biniodid 1⁄10 grain, Sodium iodid 1⁄2 grain. With regard to the identity of “dimethylarsenin” the company claims: “This product is a compound of cacodylic acid similar to sodium cacodylate but with a more pronounced therapeutic action.” The committee recommends to the Council that “Arsenoven S. S.” be declared inadmissible to New and Nonofficial Remedies because of unwarranted therapeutic claims.
“Arseno-Meth-Hyd,” is sold by the New York Intravenous Laboratory, New York City, for the treatment of syphilis. It comes in three dosages, 2 gm., 1.5 gm., and 0.7 gm., respectively. The claim is made that “Arseno-Meth-Hyd 2 gm.” contains “2 gm. (31 grains) of Sodium Dimethylarsenate (Cacodylate), U. S. P.. and Mercury Iodid 5 mg. (1⁄12 grain)” in 5 c.c. of solution. Physicians are told:
“In primary and early secondary case administer Arseno-Meth-Hyd 2 gm. every sixth day and Mercury Oxycyanide .008 (1⁄8 grain) intravenously between each injection.”
“In Tertiary cases and those of long standing alternate with intravenous injection of Sodium Iodid 2 gm.”
The following claims are made for the alleged effectiveness and safety of the cacodylate:
“This methyl compound of arsenic has come into almost universal use for syphilis. On account of lack of toxicity an aggressive routine can be carried on. The simple technic and absence of reactions make it most desirable for the regular practitioner. This large dose gives more uniform results both as healing manifestations and negative Wassermann’s.”
“Much discussion has surrounded the use of Methyl Compounds of Arsenic and it has been demonstrated beyond doubt that Cacodylate of Soda proves an effective remedy for syphilis provided that it is properly administered.” [sic]
“The low toxicity of this Methyl compound of arsenic is remarkable. It is contraindicated only where a decided idiosyncrasy for even small doses of arsenic exists.”
These statements are essentially false and misleading. Cacodylate has not come into universal use in the treatment of syphilis, nor has its usefulness been “demonstrated beyond doubt.” On the contrary, H. N. Cole (The Journal, Dec. 30, 1916, p. 2012) has shown that doses so large as to produce renal injury were almost totally ineffective against syphilis. Obviously, “effective doses” if such exist, are not harmless. The dosage advised for Arseno-Meth-Hyd may not produce acute toxic symptoms; nevertheless smaller doses have produced nephritic phenomena. The “Arseno-Meth-Hyd” treatment includes the intravenous injection of about 1⁄4 grain of a mercury salt. Although this is less than the usual dose (about 1 grain per week), the mercury is probably more effective than the cacodylate.
The committee recommends to the Council that, because of the unwarranted therapeutic claims, “Arseno-Meth-Hyd” be held inadmissible to New and Nonofficial Remedies.
The Council adopted both reports of the committee and declared “Arsenoven S. S.” and “Solution of Arsenic and Mercury” (“Arseno-Meth-Hyd”) inadmissible to New and Nonofficial Remedies. The committee’s reports on these two products impel the Council again to call attention to the undesirable and dangerous abuses to which “Intravenous Therapy” lends itself. There is a distinct field for the intravenous administration of drugs in those cases in which immediate drug action is necessary, or when the medicament is likely to be changed if absorbed through the ordinary channels. Unless such indications exist, however, intravenous administration involves not only inconvenience and expense to the patient, but what is more important, unnecessary danger. The fact that indiscriminate intravenous administration is peculiarly profitable to certain manufacturing houses makes it all the more necessary for the medical profession to be on its guard in this matter.
In this connection it is well worth while to quote the closing paragraph from an editorial on “Intravenous Therapy” that appeared in The Journal, Nov. 11, 1916. It is as true today as when it appeared:
“Intravenous therapy will be most securely advanced if its employment is restricted to such well defined fields. [As those mentioned above.] These fields can be satisfactorily determined only by a scientific pharmacologic study of the action of these drugs when so administered in animals, as well as in man, under conditions in which the results are carefully controlled. The intravenous method is an impressive one, approaching in preparation almost to that which goes with a surgical operation. The patient is usually interested and impressed by this new, and, to him, mysterious method. There is a psychic element in his reaction to the injection which is not a factor in his reaction to the same drug when given by mouth. The intravenous injection of a complex mixture would appear to be particularly reprehensible. Little is known, as has been stated, of the results to be expected from intravenous therapy, even with simple substances. The use of complex mixtures will without doubt react against the proper use of the method.”
After the report on Arseno-Meth-Hyd had been presented to the Council, a letter was received from the New York Intravenous Laboratory announcing that the preparation “Arseno-Meth-Hyd” was now called “Solution of Arsenic and Mercury” and expressing a desire to have its products accepted for inclusion in New and Nonofficial Remedies. In view of this letter, the committee’s report on “Arseno-Meth-Hyd” and the Council’s protest against promiscuous intravenous therapy were sent the New York Intravenous Laboratory for consideration.
The reply of the New York Intravenous Laboratory contained nothing which permitted a revision of the preceding report. The change of the name of “Arseno-Meth-Hyd” to “Solution of Arsenic and Mercury” means little as the name still does not disclose the important fact that the arsenic is present as sodium cacodylate, nor does it tell the character of the mercury compound. The Council voted that “Solution of Arsenic and Mercury” and “Arsenoven S. S.” be declared inadmissible to New and Nonofficial Remedies because the therapeutic claims advanced for them are unwarranted (Rule 6) and because the names of these pharmaceutical preparations are not descriptive of their composition (Rule 8).
In filing its reply with the Council, the New York Intravenous Laboratory announced that that document would be circulated to the medical profession. This is of course the firm’s privilege. The Council notes, however, with interest, that the reply is devoted almost entirely to points which were not raised by the Council and that it fails to discuss the objections which were actually made.
The reply constantly confuses the efficiency of cacodylate in anemia and in syphilis. The Council’s report on “Arseno-Meth-Hyd” does not discuss or even touch on the question of cacodylates in anemia. It is confined to a discussion of the disappointing results obtained with cacodylates as such (i. e., without mercury) in the treatment of syphilis. This attempt on the part of the New York Intravenous Laboratory to confuse the issue and to attribute to the Council an opinion that it has never stated or held is an inexcusable misrepresentation. The company in its reply said:
“We believe that you have previously stated that a solution cacodylate of soda possesses no more action than so much water. In other words, it was inert. Now you try to show that it produces renal injury.”
The Council has never declared that cacodylates are inert. In the report it is merely stated “that doses so large as to produce renal injury were almost totally ineffective against syphilis.” Neither has the Council stated that cacodylate is “peculiarly dangerous.” In fact the absolute toxicity of cacodylates is low but Cole’s results were quoted as a caution that “effective” doses are not harmless. A great portion of the remainder of the reply is devoted to disparaging arsphenamin—a product that is not involved in this action of the Council, and one about which the physician is amply informed.
Amongst other wholly extraneous matters, the firm’s “reply” tried to resurrect the pepsin pancreatin controversy. This also has nothing to do with the efficiency or harmlessness of sodium cacodylate. In order to dispose of the matter, however, it may be pointed out that the implications are entirely misleading. The work which is quoted against the Council was undertaken by the Council itself, to clarify obscurities in the older data. The outcome of these new investigations showed the essential correctness of the deductions from the older work, namely, that pancreatin is destroyed by pepsin-hydrochloric acid. Dr. Long’s work to which the firm’s reply evidently refers, showed that under favorable conditions, namely, when protected by an excess of protein, some trypsin may escape destruction in the stomach; but it fully confirmed the original conclusion that pepsin and pancreatin mixtures as ordinarily administered are practically worthless (J. H. Long, Jour. Amer. Pharmaco. Assoc., Sept. 19, 1917).
As regards the editorial on intravenous therapy, a concession may be made the New York Intravenous Laboratory: intravenous injections are no longer quite as “impressive” as in 1916, but that does not alter the fact that they should be used only when a distinct advantage is to be gained.—(From The Journal A. M. A., Aug. 2, 1919)
“Hormotone,” of the G. W. Carnrick Company, is advertised as “A pluriglandular tonic for asthenic conditions.” “Hormotone Without Post-Pituitary” is recommended for use “in neurasthenic conditions associated with high blood pressure.” These preparations are sold in the form of tablets for oral administration. The Council declares these preparations inadmissible to New and Nonofficial Remedies because: (1) Their composition is semisecret (Rule 1); (2) the therapeutic claims are unwarranted (Rule 6); (3) they are sold under names not descriptive of their composition but suggestive of indiscriminate use as “tonics” (Rule 8); (4) in the light of our present knowledge the routine administration of polyglandular mixtures is irrational (Rule 10). In explanation of this action, the Council authorized publication of the report which appears below.
W. A. Puckner, Secretary.
Each tablet of “Hormotone” (G. W. Carnrick Co., New York City) is said to contain 1⁄10 grain of desiccated thyroid and 1⁄20 grain of entire pituitary, together with the hormones of the ovary and testes—the amounts and the form in which the latter are supposed to be present are not given. From this it will be seen that the only definite information given to the medical profession regarding the composition of Hormotone is that it is a weak thyroid and a still weaker pituitary preparation.
What results can be anticipated from one or two tablets three times daily (the recommended dose of Hormotone) each containing 1⁄10 grain of thyroid and 1⁄20 grain entire pituitary? Such doses of thyroid may, of course, have a beneficial action in a limited number of cases of myxedema and cretinism. An extract of the posterior lobe of the pituitary (Liquor Hypophysis, U. S. P., for example) will, when injected subcutaneously or intramuscularly, have a pronounced effect on the parturient uterus; its action on certain other forms of smooth muscle will be much less certain. But the oral administration (for which Hormotone is recommended) of the posterior lobe of the pituitary has not been shown to have any such effect. The use of the anterior lobe in doses of 1 to 4 grains (doses very many times larger than those recommended for the entire gland in Hormotone) is in the experimental stage and its only probable value seems to be in those cases of known gland deficiency.
As to the other alleged ingredients of Hormotone—hormones of the ovary and testes, amounts not stated: all physicians know the uncertainties attending the use of ovarian preparations and the serious question as to whether testicular extracts have any therapeutic value. Whatever may be the physicians views as to the probable therapeutic value of these organs, the first thing he desires to know is how much of the substance he is giving and from what part of the gland it is obtained.
So much for the facts; yet the physician is asked to jump from this region of solid fact into a sea of hypothesis; to believe that small amounts of the well-known drugs thyroid and pituitary, plus an unknown amount of unknown hormones of the testes and ovary are of great value in conditions that in themselves are often purely hypothetical. He is asked to believe that this combination has virtues in such conditions as “hypofunction of the adrenal system,” neurasthenia, the “fatigue syndrome,” amenorrhea, dysmenorrhea, “natural and artificial menopause,” sexual neuroses, cold extremities, cardiac asthenia, low blood pressure, infantilism, sterility, melancholic conditions, obesity, anorexia, anemia, slow metabolism, constipation, psychasthenia, lowered virility and the sexual neuroses of the unmarried, hysteria following functional exhaustion of the nerve centers, frigidity, etc., etc., especially if he guesses that the trouble is due to a “pluriglandular disturbance,” “glandular hypofunction,” an “adreno-pituitary deficiency,” suboxidation, etc.
The physician is invited to use Hormotone because, among other reasons, each alleged constituent is said to be “in physiologic sympathy and therapeutic harmony with the others,” and further, because:
“Pluriglandular therapy has the endorsement of high authorities, is both logical and effective and Hormotone is a splendid example of it. It will be seen at its best where the patient lacks snap and vim and vigor. Asthenic conditions necessarily indicate hypofunction of the adrenal system ...” etc.
“The use of gland extracts in the treatment of aplasias of the pluriglandular system has become an established therapeutic measure of miraculous potency (Bayard Holmes: The Internal Secretory Glands, Lancet-Clinic, Sept. 19, 1914).”
The G. W. Carnrick Company also advertises a “Hormotone Without Post-Pituitary,” each tablet of which is said to contain 1⁄10 grain desiccated thyroid, and to “present” “hormone bearing extracts of thyroid, anterior pituitary, ovary, and testes.” This product is just as irrational as “Hormotone.”—(From The Journal A. M. A., Aug. 16, 1919)
The Council has voted Hex-Iodin (Daggett and Miller Co., Inc., Providence, R. I.), Formitol Tablets (E. L. Patch Co., Boston), and Cin-U-Form Lozenges (McKesson and Robbins, New York City) inadmissible to New and Nonofficial Remedies, and authorized publication of the report which appears below.
W. A. Puckner, Secretary.
Some years ago, the Council published (The Journal A. M. A., Aug. 28, 1915, p. 816) a report on Formamint, a proprietary medicine widely exploited as a peculiar chemical compound of sugar of milk and formaldehyde. The formaldehyde was said to be liberated slowly by the action of the saliva, and because of this liberation of formaldehyde, Formamint was claimed to be a powerful germicide. Extravagant claims were made for its curative and prophylactic effects. The Council found that the therapeutic claims were grossly unwarranted and that its exploitation to the public was a public danger.
During the recent epidemic of influenza, a variety of tablets or lozenges were advertised, and are still being advertised, having formaldehyde, in some form or other, as the nucleus around which revolve the therapeutic claims. In some cases, the advertising clearly indicates the character of the formaldehyde compound that is claimed to be present; in others the statements are vague and indefinite or misleading.
It is hardly necessary to remind physicians that the use of tablets containing hexamethylenamin or other formaldehyde compounds can neither cure respiratory infections, nor even confer protection against such infections. To be effective, formaldehyde would need to be supplied to the entire respiratory tract continuously for some time or else in concentrations that would be distinctly irritant and damaging to the tissues. Saliva-dissolved tablets, obviously cannot reach the nasal or tracheal mucosae directly; and the application of quickly acting concentrations of formaldehyde is out of the question. This altogether aside from the fact that hexamethylenamin, the basis of some of these tablets, does not liberate formaldehyde in the mouth, and for this reason alone would be quite useless for this purpose! (See Hanzlik and Collins, Archives of Internal Medicine, November, 1913.)
An inefficient antiseptic is more than merely useless; it is a menace to public safety, in that it tends to lead to the neglect of rational and effective protective measures. It therefore seems advisable for the Council again to call the attention of physicians to the subject. Accordingly, three specimens of these products were purchased and examined in the Association’s Chemical Laboratory.
Hex-Iodin (Hexamethylenetetramine and Iodum) Lozenges are manufactured by Daggett and Miller Company, Inc., Providence, R. I. They weigh 151⁄2 grs. each, are sweetened and are flavored with mint or menthol. The package and circulars do not contain a definite statement of composition. The rather indefinite synonyms “Hexameth. and Iodine Comp.” and “Hexamethylenetetramine and Iodum” suggest that the lozenges contain hexamethylenamin and free iodin. The further statement that they “contain the combined medicinal antiseptic and prophylactic properties of Hexamethylenetetramine and Iodum” is also rather indefinite. The therapeutic action claimed for the lozenges, however, could only be produced by free iodin and by liberated formaldehyde.
It is unnecessary to discuss in detail the extravagant claims made for these lozenges. The inefficiency of hexamethylenamin has already been referred to; the limitations of iodin, free or combined, in lozenge form, need not be discussed because the examination made in the A. M. A. Chemical Laboratory showed that Hex-Iodin lozenges contained no free iodin, and only traces of combined iodin. Neither formaldehyde nor paraformaldehyde was present; hexamethylenamin was present but, the lozenges being neutral no formaldehyde is generated in contact with water or with the alkaline saliva.
Thus Hex-Iodin is shown to be worthless for the purpose for which it is advertised. Of the two important ingredients said to be present, iodin and hexamethylenamin, only traces could be found of the former while the latter, as has been shown, is incapable of exerting any effect when used as the manufacturers direct.
These tablets are prepared by the E. L. Patch Co., Boston. Each tablet weighs 131⁄2 grs. They have the odor of thymol or menthol and an acid taste and reaction. They are, according to the label:
“For the throat and mouth. Soothing, Astringent, Antiseptic. Rapidly destroys germs of infection, preventing and relieving sore throat and mouth.”
In a circular, it is stated, that one of the qualities of Formitol:
“... is the generation of formaldehyde when in contact with water or the saliva.”
“Besides generating formaldehyde, Formitol, Patch contains astringent, demulcent and soothing ingredients which render the combination unusually effective.”
A bacteriologic report is given in this circular in which it is stated that, in 21⁄2 minutes one Formitol Tablet rendered sterile a plate culture of a “characteristic throat micrococci.” The instructions are to dissolve a tablet in the mouth, slowly, once an hour or a half-tablet every half hour.
The A. M. A. Chemical Laboratory reported that Formitol Tablets contained formaldehyde (or paraformaldehyde), and ammonium compound, and some hexamethylenamin. It is probable that the formaldehyde (or paraformaldehyde) was produced by the decomposition of hexamethylenamin originally present in the tablets but decomposed by long contact with the acid.128
These tablets differ from Hex-Iodin in that they really contain active formaldehyde and, therefore, possibly produce antiseptic effect in test-tube cultures. The conditions in the mouth, however, are very different from those in the test-tube, since in the mouth the formaldehyde would be immediately “bound” or absorbed. The claimed absence of irritation indicates sufficiently the absence of efficient quantities of formaldehyde under clinical conditions.
Cin-U-Form Lozenges, manufactured by McKesson and Robbins, New York City, are marketed in bottles of 24 for 25 cents. They have a strong odor of cinnamon, weigh 151⁄2 grs. each, and are acid in taste and reaction. The label states that they contain:
“Cinnamon, Eucalyptus, Formaldehyde and Menthol—all powerful germicides against Influenzal bacilli, but not injurious to the system in this palatable form.”
A circular contains the same statement as to composition and claims further that they:
“... help to prevent the infection of Spanish Influenza, Pneumonia, Grip Colds and to guard against Sore Throat, Tonsillitis, Pharyngitis, etc.”
The A. M. A. Chemical Laboratory reported that Cin-U-Form Lozenges contained some formaldehyde (or paraformaldehyde) and no hexamethylenamin. It is obvious that the mouth and throat cannot be “disinfected” by these lozenges. They would be totally ineffective against bacteria that enter through the nose; they cannot prevent influenza, pneumonia, etc.—(From The Journal A. M. A., Oct. 4, 1919)
Lavoris was considered by the Council in 1913, and its proprietors—the Lavoris Chemical Company—were advised that the preparation was inadmissible to New and Nonofficial Remedies because of conflict with Rules 1, 4, 6, 8 and 10. No report was published at that time. As the preparation is still widely advertised to physicians, the Council has again examined Lavoris and authorized publication of the following report.
W. A. Puckner, Secretary.
In recent years Lavoris has been widely advertised as “THE IDEAL ORAL ANTISEPTIC,” particularly to the dental profession. A printed card sent out by the Lavoris Chemical Company in 1913 read: “LAVORIS, the Pyorrhea Remedy. The Original ZINC CHLORIDE Mouth Wash. One grain zinc to each ounce.” The card also gave a “formula” to the effect that each pint of Lavoris contained:
Zinc Chloride | 1.040 |
Resorcin | 0.520 |
Menthol | 0.400 |
Saccharin | 0.195 |
Formalin | 0.195 |
Ol. Cassia Zeyl | 0.780 |
Ol. Caryophyl | 0.195 |
Advertisements now appearing in medical journals repeat the older “formula” except that resorcin is omitted. The formula while seemingly frank and open is in reality indefinite and misleading in that no denomination of weight is given for the various constituents. It is uncertain, for example, if the figures in the formula are intended to represent grains, grams or percentages of the several constituents. In view of the indefinite statement of composition, a chemical examination of Lavoris was undertaken in the A. M. A. Chemical Laboratory. The report of the laboratory follows:
Zinc.—This was determined electrolytically. Fifty c.c. gave 0.026 gm. zinc and 100 c.c. gave 0.0531 gm. zinc. The average is 0.0526 gm. zinc in 100 c.c. This is equivalent to 0.1102 gm. anhydrous zinc chlorid in 100 c.c.
Chlorid.—After decolorizing some of Lavoris with chlorid-free animal charcoal, the chlorid was determined by the Volhard method. Twenty-five c.c. Lavoris required 4.328 c.c. tenth-normal silver nitrate solution equivalent to 0.01535 gm. chlorid (chloridion) or 0.0614 gm. in 100 c.c. A second 25 c.c. of Lavoris required 4.112 gm. tenth-normal silver nitrate solution equivalent to 0.01458 gm. chlorid (chloridion) or 0.05832 gm. in 100 c.c. Average is 0.05985 gm. This is equivalent to 0.1150 gm. zinc chlorid in 100 c.c. This agrees closely with the foregoing zinc determination.
Resorcin.—The method of the U. S. Pharmacopeia was used. The total bromin absorption of 25 c.c. Lavoris was 3.68 c.c. tenth-normal bromin solution. This would be equivalent to 0.00675 gm. resorcin in 25 c.c. or 0.02700 gm. in 100 c.c. In a duplicate test, 25 c.c. Lavoris required 3.8 c.c. tenth-normal bromin solution equivalent to 0.00697 gm. resorcin or 0.02788 gm. in 100 c.c. Since oil of cinnamon absorbs bromin, 50 c.c. of Lavoris was boiled until very little or no odor of the oil was noted, keeping the volume nearly constant by adding a little water from time to time, and the bromin absorption then taken. In one experiment, 0.36 c.c. of tenth-normal bromin solution was consumed, and in a duplicate no bromin was absorbed. This shows the absence of resorcin.
Residue.—On evaporating 25 c.c. Lavoris on a steam bath and subsequent drying of the residue at 100 C., 0.0455 gm. of residue was obtained. This is equivalent to 0.1820 gm. in 100 c.c.
Saccharin.—Saccharin was detected in the residue and ether-extract of the residue by its intense sweet taste when a little sodium bicarbonate was added to it.
Formaldehyd.—This could be detected by the Jarrison test. The color was not very pronounced and the quantity of formaldehyd was small.
Oil of Cinnamon.—The odor and taste of Lavoris is characteristic of cinnamon.
Menthol and Oil of Cloves.—The odor of menthol and of oil of cloves could not be detected, but no tests were made to demonstrate their presence.
The analysis thus indicates that the Lavoris of today contains no resorcin but does contain a small amount of formaldehyd, a little saccharin, and oil of cinnamon (menthol and oil of cloves could not be detected by the odor, but were not tested for). The analysis showed that the principal constituent of Lavoris is zinc chlorid, of which there is about 0.1 gm. per 100 c.c. (about 1⁄2 grain to the ounce).
The amount of zinc chlorid given in the published formula, i. e., 1.04, is meaningless because the unit of weight or measure is not given; furthermore, the analysis shows that it is inaccurate for any unit of weight that might be assumed from the published figures. Since the amount of the most active medicinal ingredient is both indefinite and inaccurate, the composition of the preparation is essentially secret. Lavoris is indirectly advertised to the public by having included in the package a circular giving a list of diseases for which the preparation was recommended. The combination of zinc chlorid, formaldehyd and oil of cinnamon (assuming the menthol and oil of cloves to be present as flavors) in a mixture is irrational and likely to lead its users to ascribe a false and exaggerated value to the preparation. The name is objectionable in that it does not indicate the composition of the potent ingredients of the mixture, but instead suggests its use as a mouth wash.
From a standpoint of public safety, the most serious objection to Lavoris, however, lies in the many unwarranted therapeutic claims and suggestions. It is generally held that zinc chlorid solutions which possess a strength of from 1 to 200 up to 1 to 500 exercise a weak antiseptic action. The strength of zinc chlorid in Lavoris is approximately 1 to 1,000. The directions for its use recommend that Lavoris should be diluted. A dilution of 1 to 4 is recommended for a variety of mouth conditions while for cystitis irrigations and as a vaginal douche, it is recommended that one tablespoonful be added to a quart of warm water or salt solution. The strength of zinc chlorid in the last suggested dilution would approximate 1 to 64,000. It is evident that no antiseptic action could be expected from such dilutions.
The recommendation that diluted Lavoris be used for the treatment of coryza, nasal catarrh, hay fever, inflamed eyes, hemorrhoids and leucorrhea is objectionable and irrational. Especially dangerous is the recommendation that members of a family exposed to diphtheria or scarlet fever should use Lavoris freely as a preventive. Such recommendations can but give a false sense of security and lead to the neglect of proved methods for preventing the spread of these diseases. Equally unwarranted is the recommendation that in gonorrhea one teaspoonful of Lavoris to eight of warm water be used with a blunt end syringe.
The use of Lavoris as recommended would not only prove valueless in many instances but might lead to serious consequences because really valuable methods of prevention or treatment might be neglected. For these reasons the preparation is in conflict with Rule 6.
The Council declared Lavoris ineligible for New and Nonofficial Remedies.—(From The Journal A. M. A., Nov. 1, 1919)
The Council has authorized publication of the following report on Medinal (Schering and Glatz, Inc.).
W. A. Puckner, Secretary.
Medinal is a proprietary name applied to barbital sodium (sodium diethylbarbiturate) the sodium salt of barbital (diethylbarbituric acid). The latter was first introduced as Veronal.
Medinal was deleted from New and Nonofficial Remedies in 1916 because the advertising issued by Schering and Glatz (who then acted as agents for Chemische Fabrik auf Actien vorm. E. Schering, the German manufacturer) contained misleading and unwarranted therapeutic claims. The Council did not publish its report because by the time the report was ready for publication the product was practically off the American market, and it was hoped that when Medinal again became available, Schering and Glatz would revise the claims and thus permit its reacceptance.
Medinal, said to be manufactured in the United States, is now marketed by Schering and Glatz, Inc. In October, 1918, the firm sent to the Council a typewritten copy of a proposed circular for Medinal. The firm was informed that this leaflet was subject to the objections that had been raised when Medinal was deleted from New and Nonofficial Remedies. In April, 1919, the firm submitted a printed circular which it was sending out. This contained numerous misleading statements, among them, these:
“Medinal removes its [Diethylbarbituric acid] one objectionable feature—insufficient solubility—and thus fulfills the three prerequisites of a truly rational hypnotic: Quick absorption, insuring prompt action, rapid and complete excretion, affording protection from cumulative toxic after effects, and the choice of rectal and subcutaneous administration.”
There is no justification for the claim that diethylbarbituric acid (barbital) has only one objectionable feature and that a minor matter of “insufficient solubility.” The Council has called the attention of Schering and Glatz, Inc., to the fact that the difference in the time of absorption between Medinal (barbital sodium) and barbital is, at the most, but one of minutes and that there is no evidence that Medinal is excreted more rapidly than barbital. Hence the claims that the danger of toxic side-actions and that cumulative after-effects are avoided in this product, are wholly unwarranted.
It is also claimed, and the claim is unsupported by satisfactory evidence, that Medinal is useful in the insomnia of tuberculosis in which condition it is said to have a double advantage owing to its favorable effects on the night-sweats. It is claimed that Medinal is used in the withdrawal treatment of morphin addiction with great success; there is no evidence that Medinal has any special usefulness in this treatment of the morphin habit. It is claimed further that success has been reported with Medinal in the treatment of whooping cough. The Council knows of no satisfactory evidence to show that Medinal is of special value in whooping cough; on the contrary, it is capable of doing a great deal of harm. The recommendations that Medinal be used for the control of labor pains and in acute neuralgic pains that resist other forms of treatment are wholly unwarranted as the value of the drug in such conditions is inherently improbable and until satisfactory evidence in support of them is forthcoming, must be deemed misleading.—(From The Journal A. M. A., Nov. 15, 1919)
The Council has authorized publication of the following report.
W. A. Puckner, Secretary.
Salts of the base cotarnin have been used as local and systemic hemostatics. The hydrochlorid was first introduced as “Stypticin,” and is now in the pharmacopeia as cotarnin hydrochlorid (Cotarninae Hydrochloridum, U. S. P.). The phthallic acid salt of cotarnin—cotarnin phthallate—was introduced as “Styptol.” Both Stypticin and Styptol were admitted to New and Nonofficial Remedies. In 1918 the Council voted to omit Stypticin because the former American agents were no longer offering it for sale. Styptol was retained and is described in N. N. R., 1919.
As was pointed out in the description (N. N. R., 1918), the evidence for the usefulness of the cotarnin salts has been contradictory and unsatisfactory; but since the available data against the efficiency were at least equally unreliable, the Council deemed it best to retain them in N. N. R. pending a thorough investigation of the subject. This was undertaken by P. J. Hanzlik, at the suggestion of the Therapeutic Research Committee of the Council.
A reliable judgment of hemostatic efficiency can be formed only on a basis of strictly controlled conditions, which can best be furnished in the laboratory. Hanzlik repeated the principal experiments published by previous investigators, and applied a number of new or improved methods. The results (published in the Journal of Pharmacology and Experimental Therapeutics 10:523, 1918; 12:71, 1919) show the following:
Direct Application to Wounds.—The widely quoted results of the gynecologist K. Abel, on the footpad of cats, were found to be quite unreliable. When the experiment is properly controlled, the results are either negative or the bleeding may be increased. Quantitative experiments on wounds of the footpad of dogs showed that cotarnin invariably increased the bleeding. Equally negative or unfavorable results were obtained with wounds to the comb of roosters, and to the liver and spleen.
Direct Action on Vessels.—The results of perfusion experiments were variable, but, in general, showed a vasodilation action instead of constriction. This holds true also of the uterine vessels. The vessels in the living animal (rabbit’s ear) were also unaffected.
Systemic Administration.—The bleeding from an irrigated wound was not modified directly by intravenous injection of cotarnin salts, but varied merely with the state of the blood pressure.
The evidence for the inefficiency of cotarnin salts as hemostatics seemed so conclusive as to warrant the Council in rescinding the acceptance of Styptol, and directing the omission of the general article on cotarnin salts and the description of Styptol from New and Nonofficial Remedies.—(From The Journal A. M. A., Nov. 22, 1919)
“Micajah’s Medicated Wafers” and “Micajah’s Suppositories,” sold by Micajah & Co., Warren, Pa., are declared inadmissible to “New and Nonofficial Remedies” because (1) their composition is essentially secret (Rule 1); (2) name of neither of these mixtures is indicative of its composition (Rule 8); (3) of exaggerated and unwarranted therapeutic claims (Rule 6), and (4) the therapeutic advice which accompanies the trade packages constitutes an indirect advertisement to the public (Rule 4).
W. A. Puckner, Secretary.
Micajah’s Medicated Wafers (formerly called “Micajah’s Medicated Uterine Wafers”) were analyzed in the A. M. A. Chemical Laboratory in 1910. They were found to consist essentially of dried (“burnt”) alum, boric acid and borax, in approximately the following proportions:
Alum, dried | 59.86 per cent. |
Borax, dried | 15.62 per cent. |
Boric acid | 5.67 per cent. |
Water of hydration | 18.85 per cent. |
There are a number of drugs that are more or less effective in the treatment of local lesions of mucous membranes and the skin. They are classed as astringents. Among these are included alum, borax and boric acid. Every physician has used them. To say that a wafer consists of alum, borax and boric acid inspires but little awe. But there is something much more mysterious and impressive in declaring that a wafer “consists of an astringent and antiseptic base, in which are incorporated certain medicaments which both locally and after absorption, contribute to the astringent, antiphlogistic, depletive, soothing and healing action of the product.” This gives the impression that some powerful and almost incomprehensible factors are at work. Yet, after all is said and done, the substances contained in Micajah’s Medicated Wafers are just the homely old alum, boric acid and borax.
In addition to “Micajah’s Medicated Wafers,” Micajah & Co. also put out “Micajah’s Suppositories for Hemorrhoids.” These have been examined in the A. M. A. Chemical Laboratory and, like the “Medicated Wafers” have been found to contain alum, boric acid and borax—and these substances practically alone—incorporated in cocoa butter. The company claims that “to these have been added Ammonii Ichthyosulphonate, Balsam of Peru, Ext. Belladonae.” The A. M. A. chemists report, however, that if extract of belladonna is present at all it is in amounts too small to be detected by the method commonly employed in the chemical examination of alkaloidal drugs. The chemists report further that while ammonium ichthyosulphonate and balsam of Peru both have a decided odor and are dark in color, the suppositories have but little color and the odor of the cocoa butter that forms their base is not covered by these drugs; obviously, therefore, if ammonium ichthyosulphonate and balsam of Peru are present at all it is in amounts utterly insufficient to exert any therapeutic effect.
It would be hard to find better examples of mischievous proprietary medicines than these two products of the Micajah Company. “Twins of Efficiency,” they are called in an advertising pamphlet. The composition is not stated. A physician using the “twins” does so absolutely in the dark. To him they are secret preparations. He is encouraged to use them in a great variety of conditions in which other drugs are much more useful. Inevitably, physicians using them will be likely to overlook, or pass over, new growths, specific infections and diseases that require radical remedial measures.
In addition to misleading and exaggerated claims, there is a reference to a report from the usual “well-known and reliable bacteriological laboratory.” The excerpts published from this report of an unnamed laboratory are sufficiently vague to incriminate no one.
From time to time it is worth while to emphasize facts regarding proprietary medicines that while obvious are sometimes forgotten. For this reason attention is directed to Micajah’s Uterine Wafers and Micajah’s Suppositories.—(From The Journal A. M. A., Nov. 29, 1919)
Alkalithia was introduced at a time when it was believed that the administration of lithium salts served to remove uric acid from the system. The product was considered by the Council in 1906, and found ineligible for New and Nonofficial Remedies. No report, however, was published at that time.
Because of inquiries received, the Council examined the current claims for Alkalithia, and authorized publication of the report which appears below.
W. A. Puckner, Secretary.
Keasbey and Mattison Company’s Effervescent Alkalithia is sold with the following statement of composition:
“Each dose or heaping teaspoonful contains 1 grain of Caffeine, 10 grains each of Bi-carbonates of soda and potash, and 5 grains of Carbonate of Lithia.”
The A. M. A. Chemical Laboratory reports that Alkalithia is an effervescent mixture which contains alkaline carbonates and bicarbonates together with caffein, free tartaric acid and free citric acid. The major portion of the alkali carbonates and bicarbonates is converted into citrates and tartrates when the preparation is dissolved in water—as is done before it is taken. An excess of alkali is present, however, as the solution has an alkaline reaction. Each “heaping teaspoonful” (which was found to be about 4.85 Gm.) contains about 0.044 Gm. of caffein (the manufacturers claim 0.0648 Gm. per heaping teaspoonful). As taken, Alkalithia, therefore, represents caffein in a solution of alkali tartrate, citrate and bicarbonate containing free carbonic acid. If it is assumed that all of the tartrate and citrate in Alkalithia is converted into carbonate in the organism, a “heaping teaspoonful” of Alkalithia would represent about 2.9 Gm. of sodium bicarbonate. This assumption is, however, not correct, for it is known that tartrates are not completely converted into carbonates in the organism.
According to the label on the bottle, this mixture of caffein and alkali salts is “a common sense remedy for the relief and treatment of conditions dependent upon perverted metabolism as manifested by neuralgic, rheumatic, cardiac and renal symptoms.” Wrapped with a trade package is a circular in which is discussed the “uric acid diathesis” as “a cause of Rheumatism in its various forms, Calculus, Gravel and Inflammation of the Bladder and Kidneys, Asthma, Hay Fever, Catarrh, Quinsy and Bronchitis, Eczema, Hives, Itching and Burning of the Skin, Palpitation of the Heart and Cold Hands and Feet, Dizziness, Mental Depression, Melancholia, Neuralgia, Chorea, Hysteria, Numbness and a great variety of purely nervous symptoms.” The arguments for the use of Alkalithia as “a safe and scientific treatment for the uric acid diathesis” found in the circular constitute an indirect appeal to the laity (conflict with Rule 4).
In the circular matter sent direct to the physicians, Keasbey and Mattison claim that in rheumatism, Alkalithia is prescribed by the medical profession more often than any other remedy. The claim is made that, “In five minutes the urine will be discolorized and analysis will show it to be loaded with urates.” The manufacturers further assert:
“You can change the character of the urinary secretion in a few minutes completely” by Alkalithia, and “In nine cases out of ten, when the doctor prescribes ‘Alkalithia’ his patient greatly improves, or gets well.”
The firm advises that “Renal Insufficiency” be determined by the old method of multiplying the ounces of urine in twenty-four hours by the last two numbers of the specific gravity, adding 10, which gives the number of grains of solids excreted in the twenty-four hours. If this is low, no matter what the cause, they advise Alkalithia, “that ideal eliminant.”
The Council declared Alkalithia inadmissible to New and Nonofficial Remedies because the claims made on the label and the circular accompanying the trade package lead the public to its detriment to depend on this preparation (Rule 4); and because the therapeutic claims are unwarranted (Rule 6).—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 65)
Arhovin is a solution of dephenylamine, thymol benzoate and ethyl benzoate, marketed by Schering and Glatz, Inc. It was omitted from New and Nonofficial Remedies because the therapeutic claims made for the preparation were unwarranted and because the firm had refused to discontinue the distribution of the advertising which contained the objectionable claims prior to Jan. 1, 1919. When the report which appears below explaining the dismissal was submitted to Schering and Glatz, Inc., the firm again promised a revision of its advertising, but refused discontinuance of the objectionable circular before Jan. 1, 1920. Since Arhovin is still marketed with unwarranted therapeutic claims, the Council has authorized publication of this report.
W. A. Puckner, Secretary.
The attention of the firm Schering and Glatz, Inc., was called to misleading statements in its booklet for Arhovin in 1915, and in 1918 the firm was informed that unless the misleading statements were withdrawn before Jan. 1, 1919, Arhovin would be omitted from New and Nonofficial Remedies.
The following quotations are taken from the circular in question, and illustrate the character of the claims to which objection was made:
“Striking also is the antiphlogistic and anesthetic effect of Arhovin on the inflamed mucosae, an effect which, as all authorities agree, is far greater than that of all other internal anti-gonorrheaics.”
“Under its influence vesical and prostatic complications, gonorrheal arthritis, endocarditis, etc., are rarely incurred.”
References to the indexes of leading textbooks, including those of Meyer and Gottlieb, Cushny, Sollman and Bastedo, fails to show that Arhovin is so much as mentioned by those authors; hence, it is obviously false to state, as is done in the first of the quotations above, that all authors agree concerning the striking effects of Arhovin.
Many of the statements are objectionable by reason of the actions implied, rather than stated directly. The following are examples:
“Arhovin in Gonorrheal Infections of the Male Genito-Urinary Organs. Anterior Urethritis. This is the class of cases in which the most favorable results from Arhovin have been reported.”
“Posterior Urethritis.
“Here also the striking effects from Arhovin medication, both in acute and chronic cases, are rapid decrease of discharge, disappearance of gonococci from the secretion, and cessation of subjective difficulties, such as strangury.”
While the firm did not agree to withdraw the objectionable advertising before Jan. 1, 1919, which made necessary the omission of Arhovin from New and Nonofficial Remedies, 1919, it did submit a proposed folder in which the most objectionable of the claims are still made.
The following statement, which was in the proposed “folder” and is included in an advertising pamphlet sent out during 1919, serves to illustrate those points:
“Its action is three-fold:
“Strong antiseptic and bactericidal effect upon the urethral and vesical mucosae, highly conducive to shortening and palliation of the acute disease course.”
No evidence has been presented that Arhovin is capable of destroying the gonococcus in the urethra, and consequently, the Council declared the recommendation for the use of Arhovin in the treatment of gonorrhea, by means of claims such as those just cited, is both misleading and dangerous.—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 66)
The report which appears below was sent to the Chlorine Products Company, Inc., May 14, 1919. In reply to an inquiry sent the Chlorine Products Company, July 8, the company wrote that it could send no reply because the medical director was still in France. However, Chloron and Chlorax are being advertised in medical journals; also essentially the same advertising as that discussed in the report was recently received by a physician from the Chlorine Products Company.
The preceding facts having been reported to the Council, publication of the report was authorized.
W. A. Puckner, Secretary.
Chloron, Chlorax and Number “3” are preparations of essentially similar composition put out by the Chlorine Products Company, Inc., New York.
Chloron, according to the label, is “A stable CHLORINE remedy for the reduction of inflammation, relief of pain and for all wounds, burns, scalds and every description of sores except cancer and lupus.” Its composition is given as:
“Free chlorine, 0.200 per cent.; calcium chloride, 0.190 per cent.; mercurous chloride, 0.030 per cent.; lithium chloride, 0.035 per cent.; calcium hydrate, 0.010 per cent.; water to 100 parts.”
The Council asked the manufacturers for further information in regard to the composition or preparation of Chloron and received this reply:
“Chlorine gas is prepared in the usual way and purified and passed into water until a saturated solution is made.
“Water to the extent of three times the volume of the chlorine solution is used to dissolve the necessary amount of calcium chloride, and the two solutions are mixed.
“The necessary amounts of Lithium and Mercurous Chloride are then intimately mixed and made into solution. This solution is then added to the above and the whole is agitated for some minutes.”
A specimen of Chloron was examined in the A. M. A. Chemical laboratory and the chemists reported:
Qualitatively the presence of the following constituents was confirmed: calcium, mercury, lithium, chlorid, free chlorin. The solution was alkaline. Of course, the declaration that Chloron contains mercurous chlorid (calomel) is obviously incorrect, as mercurous chlorid cannot exist in a solution containing active (free) chlorin, but is oxidized to mercuric chlorid (corrosive sublimate). As the solution was alkaline in reaction, it seemed unlikely that all the active chlorin was present in the free state, as declared on the label. Quantitative determination of free chlorin and of total active (“available”) chlorin gave: free chlorin, 0.036 gm. per hundred c.c.; total “available” chlorin, 0.330 gm. per hundred c.c., or 165 per cent. of the claimed amount.
A comparison of the information sent to the Council with the analytic findings leads to the conclusion that Chloron is not of reliable composition.
As evidence of the therapeutic value of Chloron, the following “case reports” were submitted:
“In a case of second degree burn involving the most of one leg from the middle of the calf down, Chloron was the only dressing used. The burn was a bad one and the patient in a rundown anaemic condition, at no time was there any appearance of pus, the surface looked clean and bright and the healing was accomplished with practically no scar whatever. The burn was kept wet with the solution by hourly applications day and night. The skin which has grown on the wound is clear, healthy and firm.
In another case of Varicose veins of long standing, the result was surprising. The patient told of two years vibrating from Hospital to Hospital and getting no real relief. Each leg had large open running sores, the only dressing used was wet compresses of this solution. The pus disappeared at once, the wound began to cicatrise from the edges and in two weeks the man was discharged from the hospital practically cured.”
“Chloron was recently tried at the —— and —— Hospital on cases presenting ulcers and other sores which did not readily yield to other methods, with good results, in fact were of an indolent type. In these cases Chloron proved very valuable.”
“I have used Chloron on a series of cases (surgical) presenting pus foci and I have found the application very beneficial and healing, the pus early disappearing. In cases of Osteomyelitis, Suppurating Arthritis, Cellulitis and Chronic Ulcers, Chloron is particularly valuable, its good effects quickly observed and the time of restoration to health shortened.”
In the first case report, there is no evidence that Chloron is more efficient in the treatment of burns than any other commonly used procedure might have been. In the case of the varicose ulcers, while there was some apparent benefit from Chloron, no credit is given to rest and the general treatment which is known to be important in the treatment of such conditions. The evidence in the other case reports is quite inconclusive. Consideration of the “case reports” leads to the conclusion that clinical evidence for the value of Chloron is lacking.
Attention should be called to the fact that the amount of active chlorin, claimed to be present in Chloron as well as the amount found by the association laboratory, is less than that considered effective by Dakin, Dunham and others; seemingly in preparing Chloron no attention has been paid to the degree of alkalinity, yet the importance of this factor is now generally recognized.
Chloron fails to comply with the requirements for surgical solution of chlorinated soda (N. N. R., 1919, p. 133), yet the manufacturers make free use of the text of Dakin and Dunham’s Handbook of Antiseptics in their advertising pamphlet. Thus:
From the Chloron pamphlet:
“This ideal antiseptic effects complete sterilization within its sphere of action without causing any damage to the cells or tissues. An important method of judging the injurious action of antiseptics is to investigate their effects on the leucocytes. From experiments in vitro by Parry Morgan and in vivo by Col. C. J. Bond with the strength of antiseptics commonly used in surgery, it has been found that Chlorine antiseptics and mercury salts have little effect on phagocytosis in comparison with other germicides.
The activity of the leucocytes from wounds which have recently been treated with CHLORON may be demonstrated experimentally.”
“In addition to its antiseptic action CHLORON is a strong oxidizing agent and deodorant and possesses to a marked degree the property of decomposing toxins. In this connection it is interesting and pertinent to note that Dean, by the regulated action of hypochlorous acid, has prepared a nontoxic dysentery vaccine and it is now a common observation that the free use of CHLORON may reduce the constitutional symptoms arising from septic processes and that they reappear on discontinuing the antiseptic treatment.”
Dakin and Dunham Handbook of Antiseptics:
“The ideal surgical antiseptic should effect complete sterilization within its sphere of action without causing any damage to animal cells. At the moment such a substance does not appear likely to be found, but on the other hand it is surprising to see how little damage may be done to animal tissues by some active antiseptics. An important method of judging of the injurious action of antiseptics is to investigate the condition of the leucocytes in wounds recently treated with the substance under consideration. In general it appears from experiments in vitro that, with the strength of antiseptics commonly used in surgery, mercury salts and hypochlorites have relatively little effect on phagocytosis as compared with phenol (Parry Morgan). It is a regular phenomenon to observe activity of the leucocytes obtained from wounds which have been recently treated with hypochlorites.
Ingenious methods for determining the influence in vivo of antiseptics on the activities of leucocytes have been worked out by Col. C. J. Bond.
“In addition to their disinfecting action, the Chlorine antiseptics are strong oxidizing agents and deodorants and moreover possess in high degree the property of decomposing toxins. By the regulated action of hypochlorous acid, Dean has prepared a nontoxic dysentery vaccine and it is a common observation that the free use of hypochlorites may reduce the constitutional symptoms arising from septic processes and that they reappear on discontinuing the antiseptic treatment.”
Chlorax is said to be “A stable CHLORINE solution for internal use,” in “Kidney Conditions,” “Diabetes,” “Acute Infections,” “Blood Dicrasias,” “Lithemias and Rheumatism,” and “Nervous Conditions.” It is claimed to have the same composition as that of Chloron with the addition of 0.016 per cent. of tincture of opium.
The A. M. A. Chemical Laboratory reported that the free chlorin in Chlorax was 0.01 gm. per hundred c.c. and the total amount of active (“available”) chlorin was 0.25 gm. per hundred c.c., or 125 per cent. of the amount claimed. The laboratory notes that though the chlorin content of Chloron and Chlorax is claimed to be the same, that of Chlorax actually is less. This is not surprising when the presence in Chlorax of reducing substances such as alcohol is borne in mind. The laboratory concludes that Chlorax is not of reliable composition.
The following is typical of the “case reports” submitted to show the value of Chlorax:
“In January last I used Chlorax on a case of Diabetes Mellitus and with excellent results.
“The patient had been suffering for about nine years and when first brought to my care Toxemia had set in, he was drowsy, irritable and unable to leave the house. I prescribed Chlorax in teaspoonful doses four times a day and am pleased to say that in one week he showed marked improvement. Soon after he was able to leave the house and attend to his business and after two months’ treatment resumed a normal diet and habits apparently without injurious effects.
“I believe that in this case Chlorax undoubtedly prolonged life.”
No mention is made of the dietary or other measures used. The wide variation in diabetes and its response to proper diet is so well known that the noncommittal statement concerning the beneficial effects of Chlorax amounts to no evidence at all in favor of the preparation.
The other “case reports” furnished by the Chlorine Products Company, Inc., which concern the treatment of gastric ulcers, acute alcoholic gastritis, tonsillitis, etc., are equally unconvincing. In fact, no satisfactory evidence for the clinical value of Chlorax has been presented.
The following from the advertising for Chlorax is unwarranted and absurd:
“Mercurous chloride (calomel) is perhaps the most widely used internal antiseptic and alterative and has established itself in the therapy of constipation, cholera, dysentery, cardiac dropsy, pleurisy, malignant fever, malaria, syphilis, worms, infectious diseases, gout and rheumatism; lithium chloride is particularly efficacious in acute and chronic parenchymatous nephritis and in various lithemic conditions; while Opium has no rival as an anodyne and can be used to stabilize and conserve the alkaline reserve of the body against the acidosing influence of infections.”
Further, on page 14 we find:
“In chills and fever malaria and other blood dicrasias, Chlorax is indicated as an internal antiseptic and it exerts a beneficial effect on the course of these diseases.”
The claims made for Chlorax are exaggerated and misleading.
According to the label, Number “3” is “A STABLE CHLORINE remedy for the purification of the blood,” with the composition:
“Free Chlorine, 0.35 per cent.; Calcium Chloride, 0.30 per cent.; Mercurous Chloride, 0.03 per cent.; Lithium Chloride, 0.04 per cent.; Calcium Hydrate, 0.01 per cent.; Opium, 0.02 per cent.; Ethyl Alcohol, 0.10 per cent.; water to 100 parts.”
It will be noticed that the composition claimed for Number “3” is essentially similar to that claimed for Chloron. It differs from Chloron in that the amounts of some of the constituents are somewhat greater, and in that, like Chlorax, it contains some tincture of opium.
The A. M. A. Chemical Laboratory reports that the free chlorin in a specimen of Number “3” was 0.024 gm. in 100 c.c. and the total active (“available”) chlorin 0.173 gm. per hundred c.c., or about 50 per cent. of the claimed amount. The examination indicates that Number “3” is of unreliable composition. The Chlorine Products Company, Inc., submitted no clinical evidence for Number “3” to which it refers as “our Syphilis remedy.” It stated that two physicians had used the preparation “with good results,” and admitted that “the company requires further evidence before pushing it.”
The Council declared “Chloron,” “Chlorax” and “Number ‘3’ ” in conflict with the rules governing admission to New and Nonofficial Remedies. All are of unreliable composition (conflict with Rule 1). The therapeutic claims made for the preparations are not substantiated by acceptable evidence and are unwarranted and misleading. Chloron is inferior as an antiseptic to the well-known surgical solution of chlorinated soda on account of its low chlorin content and uncontrolled reaction. There is no warrant for the claim that Chlorax is useful in the treatment of “Kidney Conditions,” “Diabetes,” “Acute Infections,” “Blood Dicrasias,” “Lithemias and Rheumatism,” and “Nervous Conditions,” nor is there warrant for the claim that “Number ‘3’ ” is a remedy for the purification of the blood or a “Syphilis remedy” (conflict with Rule 6).
The names of these pharmaceutical mixtures are not descriptive of their composition (conflict with Rule 8).
All three preparations are irrational. No evidence has been furnished that the lithium salt is of value in the mixtures. It is not rational to combine an active chlorin preparation and a mercury salt in one mixture, nor is there evidence that the addition of opium to the preparations proposed for internal use is of value or rational. Experimentation with Number “3” as a “Syphilis remedy” is to be severely condemned in that those on whom it is used will in the meantime be deprived of efficient medication (conflict with Rule 10).—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 70)
The Council has authorized publication of the following report announcing the omission of Elarson from New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Elarson, now sold by the Winthrop Chemical Company, Inc., was formerly sold in the United States by the Bayer Co., Inc. It was admitted to New and Nonofficial Remedies in 1914.
The circular issued by the Winthrop Chemical Co. contains several statements markedly at variance with the results of an investigation made, at the request of Fischer, by Joachimoglu (Arch. f. exper. Path. u. Pharmakol. 78:1914). The circular states that Elarson contains about 13 per cent. arsenic. Joachimoglu found from 10.8 to 11.1 per cent. to be present. The circular states further:
“The fact that Elarson represents a lipoid-like chemical combination of arsenic has an important bearing upon its absorption and utilization in the system ... there is good reason to believe that when arsenic is administered in a stable, lipoid-like combination, as in Elarson, it is more readily taken up by the cells and more completely utilized than when given in the customary manner.”
“As regards the behavior of Elarson in the system, it has been shown that its active constituent, chlorarseno-behenol, is almost completely absorbed in this form, probably as a chlor-behenolate of sodium or potassium.”
As a matter of fact, Joachimoglu found that very little arsenic was absorbed when Elarson was given to dogs and rabbits; most of it was recovered from the feces; only traces were found in the liver and kidneys and none in the blood and brain. The absence from the latter organs shows that the lipoid solubility does not obtain in the body. It is claimed in the circular that Elarson has the advantage over Fowler’s solution “in that it is free from any irritating action upon the gastro-intestinal tract”; it is stated that as many as sixty tablets have been given to dogs daily without any toxic effects. Joachimoglu, on the other hand, found powdered Elarson to be very irritating to the gastro-intestinal tract; also that the dog could not stand sixty tablets at all (gar nicht vertragen), such doses causing vomiting, diarrhea and intestinal hemorrhages; on repeated administration the symptoms became progressively more severe. Joachimoglu also found that, compared on the basis of arsenic content, Elarson, given intravenously, is from ten to twelve times as poisonous as arsenic trioxid. Elarson is recommended for the class of cases in which Fowler’s solution is used.
To sum up: None of the special claims made for Elarson—the arsenic content, ready absorbability, freedom from irritating action on the gastro-intestinal tract and its alleged better adaptation for continued administration—have been substantiated; on the contrary, they have been disproved as well as the theory of its mode of absorption proposed by Fischer and Klemperer. Furthermore, Joachimoglu found that when it actually got into the circulation (intravenous injection) in the form in which Fischer and Klemperer supposed it to be absorbed, it was from ten to twelve times as toxic as arsenic trioxid.
The Council voted to omit Elarson from New and Nonofficial Remedies because it is sold under unproved and consequently unwarranted claims and because it is an unscientific and relatively useless article. Elarson has not been shown to have advantages over Fowler’s solution; on the contrary, in some respects at least, it is inferior.—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 75.)
A report which appears below was sent Charles L. Heffner for consideration. No reply having been received, the Council authorized its publication.
W. A. Puckner, Secretary.
Iodiphos, marketed by Charles L. Heffner, Brooklyn, N. Y., is declared to contain ferric citro-iodine, 6 grains; calcium glycerophosphate, 8 grains; sodium glycerophosphate, 8 grains, and hypophosphorous acid, 2 minims in each fluidounce, and to present “the Metallic and Non-Metallic elements: Iron, Iodine, Phosphorous, Calcium and Sodium (each in separate Basic combination).”
According to the label, Iodiphos is “Alterative, Tonic, Nervine and Anti-tubercular” and is “For Treatment of BLOOD, NERVES and PULMONARY ORGANS.” An advertising circular129 asserts that “Iodiphos exerts its Physiological action rapidly in hardening of the Arteries, High Blood Pressure, Anaemia, Glandular Swelling, Neurasthenia, Hypochondria, Phthisis, Bronchitis, Asthma, Pneumonia and as an Intestinal Antiseptic and Appetizer,” and declares it to be “Indispensable as a Tonic and Restorative.”
In the advertising circular it is averred that in the production of Iodiphos “Chemistry Again Aids the Modern Physician.” Iodiphos is another instance when a decadent polypharmacy proposes haphazard medication and so obstructs the efforts of modern medicine to establish the use of single drugs to meet definite indications.
Iodiphos is inadmissible to New and Nonofficial Remedies because it is an irrational mixture of drugs sold with therapeutic claims that are unwarranted, and under a name which is not descriptive of its composition.—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 81.)
The Council has authorized publication of the report which appears below declaring Mervenol and Armervenol, marketed by the Hille Laboratories, inadmissible to New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Mervenol is stated by the proprietors—The Hille Laboratories, Chicago—to be a hydrosol (colloidal suspension) of the sulphides of mercury and copper, containing sufficient sodium chloride to make it isotonic with blood serum, and “inert proteid” and “carbohydrate” to stabilize the colloidal suspension: each cubic centimeter is stated to contain 0.005 gm. mercury, 0.0016 gm. copper, and 0.0016 gm. sulphur.
It is claimed that this preparation is of value in pneumonia, influenza, and other conditions and diseases requiring increased leukocytosis.
It is further claimed that the properties and therapeutic effects of this preparation are as follows: “Practically non-irritant; practically non-toxic; lower temperature, often crisis like; lower pulse, with better elimination; greatly accelerated recovery from Influenza; fewer Pneumonia complications; lower mortality rate in Influenza and Pneumonia; remarkable Leucocyte stimulation.” Administration by mouth and by intramuscular and intravenous injection are advocated.
In the recent influenza epidemic, it is reported that therapeutic results of some value were obtained at the Great Lakes Training Station and at Fort Sheridan. The reports of certain medical officers indicate that this preparation seemed to have some effect on the course of pneumonia and influenza, on the temperature, and on the leukocyte count. But those conducting the experiments state that it was “absolutely impossible” to fulfil ideal conditions as to controls and other observations at the time the experiments were conducted.
So far as the Council knows, no effort has been made to determine the potent constituent, or constituents, of this preparation; whether the mercury, the copper, or the protein in the mixture was responsible for the claimed benefits is an open question.
These reports were given careful consideration, but it was decided not to accept this preparation because of (1) exaggerated therapeutic claims, conflicting with Rule 6 (aside from the report of its use in influenza and pneumonia at the Great Lakes Training Station and the Post Hospital at Fort Sheridan, which reports are of work done and observations made under conditions which did not permit careful controls, no evidence has been presented to the Council supporting the therapeutic claims) and (2) being an irrational mixture, conflicting with Rule 10—a mixture containing colloidal mercury, copper and sulphur with proteins and carbohydrates in addition, it is difficult to predict the changes which occur in such mixtures on standing.
Samples of Mervenol (two 1-ounce bottles) submitted by the manufacturer, June 5, 1919, were found when opened, Aug. 18, 1919, to have undergone decomposition. A very disagreeable odor had developed, the liquid was turbid, and a large amount of precipitate had formed.
Armervenol is stated by the proprietors—The Hille Laboratories, Chicago—to be a hydrosol (colloidal suspension) of “mercury-copper-sulpharsenite” containing sufficient sodium chlorid to make it isotonic with blood serum and “inert proteid” and “carbohydrate” to stabilize the solution: each cubic centimeter is declared to contain 0.0025 gm. arsenic, 0.005 gm. mercury, 0.0016 gm. copper, and 0.0032 gm. sulphur.
The use of Armervenol as advised by the Hille Laboratories is the same as that of Mervenol, and in addition its use in syphilis is emphasized. The criticisms of this mixture are similar in every respect to those directed against Mervenol—the addition of arsenic introduces still another factor of uncertainty.
After investigating these claims, it was decided not to accept this preparation on the ground (1) that the therapeutic claims are unproved and unwarranted, conflicting with Rule 6, and (2) that the mixture is an irrational one, conflicting with Rule 10.—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 82.)
The Council has adopted the following statement declaring Normal Phenol Serum (Cano) and Methyl-Phenol Serum (Cano) ineligible to New and Nonofficial Remedies.
W. A. Puckner, Secretary.
No statement of the composition of these preparations was submitted to the Council and none appears on the labels of the trade packages. However, the advertising circular contains statements such as:
“... normal phenol serum—phenol with methyl blue dissolved in anaphylactic serum ...”
“... a combination of human or horse serum with Phenol and Methylene-blue, thereby forming a new chemico-biologic product which he termed Methyl-phenol Serum or, chemically, Chloride of Phenol Thionin Tetramethylene-Seric.”
“Methyl-phenol Serum is a chemico-biological product in which Phenol is the chief factor. Each ampule of 10 c.c. contains the therapeutic equivalent of 0.5 gm. (7.5 grains) of Phenol.”
From the foregoing it appears that both preparations contain phenol and methylthionine chloride (methylene blue) and that the second does not contain methyl phenol (cresol) as the name would indicate.
No definite evidence for the value of these preparations is brought forward and even the manufacturer is constrained to caution, “We assume no responsibility for the therapeutic action of the serum....” On the other hand there are a great many statements in the papers of Cano and his colleagues to which exception must be taken. Of these, from among many similar, the following statements are to be cited and commented on:
“Accepting that the gonorrheal infection gives systemic toxemia from absorption of the toxins ...”
It is the general opinion that in the majority of instances there is no systemic toxemia.
“The technique of intraprostatic injection, while less simple than that of the intravenous, is by no means so difficult or complicated as to place it exclusively in the category of the urologist.”
This obviously is an attempt to encourage the general use of these preparations and to minimize the necessity for careful study and special skill in their employment. It is most unwise for one to attempt intraprostatic injections unless he is specially trained in the technique of this procedure.
“This injection to be performed after the 5th or 6th intravenous injection of Methyl-phenol Serum....”
Intravenous injections have a place in sane therapy only when the medicament to be so administered is of known composition and when evidence is available which gives assurance that definite results shall follow its use. In the absence of these conditions it is manifestly unwise and even unexcusable to employ any medicament in this manner, and its repeated use is reprehensible.
“Intravenous injection of Methyl-phenol Serum alternating with intravenous injections of mercury should be given every 48 hours until infection is under control.”
This quotation further emphasizes that the treatment, as advised, carries with it a certain element of danger.
“Methylene blue prevents the phenol from exerting its usual action upon the red blood corpuscles, and ensures rapid elimination through healthy kidneys. It preserves the antiseptic power of the phenol and prevents the phenol from interfering with the chemico-biological function of the white and red blood cells. The serum component favors chemotaxis, it strengthens bodily defense, it prevents anaphylaxis even in debilitated patients, and it replaces the resistance which has been impaired by the demands that have already been made upon it.”
No evidence is submitted to substantiate these claims. It seems strange that phenol should lose its power and that this should be restored by the methylene blue.
“It has a refractory chemico-biological action, and exercises no vicious effect on the red blood corpuscles in the circulation, but, on the contrary, by its inoffensive presence, it wholly preserves all of the physiological properties of the blood.”
What “a refractory chemico-biologic action” is, is not clear, but there is no evidence that this preparation has any action which might be defined as “refractory chemico-biological,” that its presence is inoffensive or that it wholly preserves all the physiologic properties of the blood.
“The treatment of gonorrhea by Cano’s theory ... is firmly based upon chemico-biological facts and accepted authoritative theories and bears the same relation to gonorrhea that intravenous injections of arsenicals bear to syphilis.”
Quite an exaggerated and unwarranted statement. In the same way, objection is taken to the following quotations:
“Phenol administered intravenously in combination with methylene blue, to protect the red-blood globule, undergoes no change, and preserves all of its actual antiseptic effect on the gonococcus and its toxins as though employed in the test tube.”
“When thus introduced into the human body its elimination is unique, effective, antiseptic, germicidal, being completely and exclusively thrown off through the kidneys in a period varying from one-half to twelve hours without local injury or disturbance to the general economy.”
“Combinations of phenol are unstable, but they do have the advantage of mitigating direct action on the cells and globules. It is also known that ordinary phenol has a coagulant action on the albumins and an oxidizing power on the tissues, which power, if permanent, produces gangrene. By virtue of this dual action it therefore acts as a modifier; by its oxidizing power on the germ it is germicidal, and prevents the growth of the gonococcus; and by its coagulant power on the toxins it relieves paragonococcal lesions (mono- and poly-arthritides) and affections of the serous organs (endo- and pericarditis, meningitis), and some definite systemic disturbances, the pathology of which is often confused with that of other infections.”
“Lymphocytosis is often persistent in some individuals in whom the internal secretions and the processes of assimilation and disassimilation are deficient; and because of the lack of these the organic physiological ferments are insufficient for the mechanism of nutrition and the phenomena of hematopoiesis.”
Until proof is available showing that phenol, administered intravenously in the quantities employed in Cano’s Normal Phenol Serum and Cano’s Methyl-Phenol Serum, acts as a germicide and methylthionine chloride (“methylene blue”) prevents the deleterious effects of phenol on the red blood corpuscles; that repeated intravenous injections of phenol and mercury are without danger; that there is no danger of anaphylaxis; that the physiologic properties of the blood are preserved by these medicaments; and, finally, that these preparations have an effect on gonorrhea and its complications, these substances Normal Phenol Serum (Cano) and Methyl-Phenol (Cano), are inadmissible to New and Nonofficial Remedies.
The following quotations taken from the circular are admissions that these preparations are not innocuous:
“That the economy will tolerate to a surprising degree substances directly introduced through the blood stream is now well known. By the intravenous injection of 10 c.c. of methyl-phenol serum we throw into the human body a massive dose of an alien substance. The immediate effect of this injection is upon the central nervous system. The recipient usually becomes either pale or suffused, he has a ringing in his ears, has a sensation of great altitude, and occasionally has a dryness of the fauces and a metallic or a garlic taste.”
“In some patients secondary reactions occur in from one to four hours after injection. The phenomena we have observed in these secondary reactions are pronounced chill and rigor ...”
There is no doubt that considerable harm may be done by the intravenous and by the intraprostatic administration of these preparations and until there is good evidence showing the therapeutic value of the treatment, the routine use of these preparations, except perhaps at hospitals in selected and well controlled and carefully guarded cases, is to be strongly discouraged.
When the foregoing statement was sent to the Mulford Company for comment, the firm submitted a letter from Dr. Perry Townsend to the Mulford Company in which he declared that the results obtained with the Cano preparations had been satisfactory and without untoward results. In this letter, Dr. Townsend proposed that a series of injections with these preparations be carried out under the observation of members of the Council and the supervision of Dr. Cano or himself.
The report of the Council, the letter from the Mulford Company and that of Dr. Townsend were sent to a number of urologists for their opinion concerning this whole matter. It was explained that the referee held that no reason had been presented which would warrant the Council to depart from its customary procedure, namely, to require that clinical evidence be submitted in the form of published reports which permit investigation and verification by independent observers but that, before making further recommendation to the Council, he desired the opinion of urologists of recognized standing concerning the report submitted to the Mulford Company. All replies received approved the Council’s position.
The following is one of the replies received:
Your letter in regard to Normal Phenol Serum (Cano) and Methyl-Phenol Serum (Cano) received. I wish to state that I have read the correspondence between the Council and H. K. Mulford Co. and in my opinion the referee and the Council are quite correct in their attitude in the matter. In my opinion I would emphasize the following:
(1) There is absolutely nothing about the remedies directed specifically against the gonococcus and no evidence to show that any action against them is obtained. As we know there are certain states of normal serum which are highly toxic and any normal serum from another animal will produce disturbances in man when injected intravenously—particularly if repeated. The addition of substances to serum normal or otherwise is apt to and frequently does render that serum highly toxic! The substances added in the instances referred to—phenol and methylene blue are not in any way calculated to lessen the toxicity of serum. The element of danger existing in the indiscriminate use of serums intravenously is, in my opinion, increased by the addition of the substances mentioned, and it would be unwise to encourage the general use of any such remedies. Furthermore the products are condemned by the very evidence of the originators and their admissions are quite sufficient to deter anyone from using the products as they suggest.
As to the intraprostatic injections with the serums it does not at all meet my views; although the introduction of serums by this route have been frequently advocated and I have personally carried this mode out I cannot allow the impression to go out that it could be done in a routine manner—nor that no ill results could follow—for I have seen otherwise. Furthermore from theoretical standpoint serums need not be given in this way.
In consideration of the opinion expressed by the Council’s consultants the referee recommended that Normal Phenol Serum (Cano) and Methyl-Phenol Serum (Cano) be declared ineligible for New and Nonofficial Remedies because of conflict with Rule 6 (unwarranted therapeutic claims) without considering possible conflicts with other rules, and that publication of the report be authorized.—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 85.)
Soamin is the name under which the firm of Burroughs Wellcome and Company sells its brand of sodium arsanilate. The Council directed the omission of Soamin from New and Nonofficial Remedies and authorized publication of the report which appears below after the proprietors of the product had declined to withdraw or suitably revise the unwarranted therapeutic claims which it made.
W. A. Puckner, Secretary.
The proprietary brand “Soamin” of sodium arsanilate was admitted to New and Nonofficial Remedies several years ago at a time when the therapeutic value of arsanilic acid had not been definitely determined. Experience with this drug has shown that it is far more dangerous and also has a more limited field of usefulness than was at first recognized. The proprietors of the Soamin brand have continued to include in the list of conditions in which it “would seem” to be a “very effective agent” cerebrospinal meningitis and pellagra; in fact, meningitis is the first in the list of conditions mentioned, syphilis the second and pellagra third. In support of their belief in the efficacy of the remedy in meningitis, three reports, published from six to nine years ago, are quoted. In one of these it is stated that two patients “were cured”; in another report, seven of eight patients in whom the clinical, but not the microscopic, diagnosis of meningitis had been made were reported as having recovered; in the third report, fifty-six of ninety cases were reported cured; in this larger series of cases the author neglects to state the method of administration. The firm quotes but one paper (which is a very uncritical report) in regard to pellagra.
It seems to the Council that the evidence of value of sodium arsanilate in these conditions (which are now treated by more rational methods) is too slight to justify the emphasis laid on it by the firm, especially as sodium arsanilate is admittedly a dangerous agent, several cases of blindness having been reported from its use.
For these reasons it was voted to omit Soamin from New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 89.)
The Council has authorized publication of the following report.
W. A. Puckner, Secretary.
The consideration of the following “mixed” vaccines was requested by F. I. Lackenbach, San Francisco:
Special Bacterial Vaccine No. 2 (Staph-Strep. Bacterin) containing killed Staphylococcus albus, Staphylococcus aureus and streptococcus.
Special Bacterial Vaccine No. 3 (Pneumo-Staph-Strep. Bacterin) containing killed Staphylococcus albus, Staphylococcus aureus, streptococcus and pneumococcus.
Special Bacterial Vaccine No. 4 (Pneumo-Staph-Strep-Coli Bacterin) containing killed Staphylococcus albus, Staphylococcus aureus, Staphylococcus citreus, Bacillus coli, streptococcus and pneumococcus.
Special Bacterial Vaccine No. 5 (Influenza Combined Bacterin) containing killed Staphylococcus albus, Staphylococcus aureus, Bacillus Friedländer, Bacillus influenzae, Micrococcus catarrhalis, streptococcus and pneumococcus.
Special Bacterial Vaccine No. 11 (Pneumo-Strep. Bacterin) containing killed streptococcus and pneumococcus.
Special Bacterial Vaccine No. 15 (Combined Whooping Cough Bacterin) containing killed Bacillus pertussis, Staphylococcus albus, Staphylococcus aureus, Micrococcus catarrhalis, Bacillus influenzae, streptococcus and pneumococcus.
Special Bacterial Vaccine No. 16 (Mixed Gonococcus Bacterin) containing killed gonococcus, Staphylococcus albus, Staphylococcus aureus, Bacillus coli, diphtheroid bacillus, streptococcus and pneumococcus.
Mr. Lackenbach states that these bacterial mixtures were prepared for him by E. R. Squibb & Sons. Their sale in interstate commerce is permitted under the license granted to the latter firm by the U. S. Treasury Department. However, no evidence of any kind was presented to the Council proving the therapeutic efficacy of the several mixed vaccines. As a mixture of two or more kinds of organisms is accepted for New and Nonofficial Remedies only if there is satisfactory evidence that its therapeutic use is rational, the Council declared the several vaccine mixtures ineligible for New and Nonofficial Remedies (Rule 10).—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 90.)
The Council examined the available evidence for Somnoform, sold by Stratford-Cookson Company, successors to E. de Trey and Sons, and found the preparation inadmissible to New and Nonofficial Remedies. The Council authorized publication of the report which appears below.
W. A. Puckner, Secretary.
Somnoform is sold in the United States by Stratford-Cookson Company, successors to E. de Trey and Sons. According to the label on a package of Somnoform sent the Council.
“This mixture contains Chloride of Ethyl, 83 per cent.; Chloride of Methyl, 16 per cent.; Bromide of Ethyl, 1 per cent.”
Although Somnoform has been on the market for a long time, the published reports present no proof that it is superior to ethyl chlorid used alone. Moreover, the published reports and statistics do not necessarily apply to the Somnoform now sold for the reason that mixtures of varying composition have been sold as Somnoform in the past. Thus, when Somnoform was considered by the Council in 1909, it was claimed to be composed of chloride of ethyl, 60 per cent.; chloride of methyl, 35 per cent., and bromide of ethyl, 5 per cent. Federal chemists found, however, that it contained no bromide of ethyl (Notice of Judgment No. 571). It is a question, therefore, whether a given report applies to a mixture containing 5 per cent. bromide of ethyl, 1 per cent. of this substance, or none at all.
The present advertising booklet for Somnoform does not present acceptable evidence of the therapeutic value of the preparation. An ignorance concerning the elementary facts of physiology and pharmacology is evident in the second sentence: when having stated that “Somnoform is the result of several years of study and investigation by Dr. George Rolland, Dean of the Bordeau Dental School,” the pamphlet continues: “He sought an anesthetic which would enter, dwell in, and leave the body in the same manner that oxygen does....”
The claim as to the value of the 1 per cent. of ethyl bromide in the mixture is highly improbable; certainly no evidence in support of the claimed value of this constituent is available to the referee.
No evidence is submitted which proves the claim of superiority of Somnoform over similar preparations, asserted in the following:
“The peculiar manner in which the elements are combined is what makes Somnoform at once so efficient and so safe.”
The Council declared Somnoform inadmissible to New and Nonofficial Remedies because, in the absence of acceptable evidence showing its exceptional safety and value, the claims are unwarranted (Rule 6), and because the name of the mixture is not descriptive of its composition (Rule 8).—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 90.)
The Council has authorized publication of the following report which declares Tablets Formothalates (Tailby-Nason Company, Boston, Mass.) ineligible for New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Tablets Formothalates are sold by Tailby-Nason Company, Boston, Mass. On the label a formula is given: “Constituents: Acetanilid 2 gr.; phenolphthalein 1⁄2 gr. In a balanced combination with Hexamene [a name sometimes applied to hexamethylenamin] and Oil of Cinnamon. Indications: Influenza, Colds, Grippe, Headache, Neuralgia, Rheumatism.” The same formula is given in advertisements and in this advertisement it is claimed that they are “For Influenza and Grip” and if “given in the acute stage may avert a serious attack” (Boston M. & S. J., Oct. 3, 1918). The dose is given as one to two tablets at 6 p. m. and repeat at bedtimes.
The A. M. A. Chemical Laboratory reported that the tablets weigh an average of 0.4882 Gm., or 71⁄2 grains; that they have the odor and taste of cinnamon; and that they contain hexamethylenamin, are neutral and therefore give up no formaldehyde in the presence of water alone. The Laboratory further reported that they contain phenolphthalein and acetanilid. These tablets were directed to be taken internally and therefore their effect was not intended to be local.
The amount of hexamethylenamin was not determined, but in any case could not exceed 5 grains per tablet. It is evident that 4 grains of acetanilid and 10 grains of hexamethylenamin and 1 grain of phenolphthalein (in two tablets) “if given in the acute stage” of influenza would not “avert a serious attack,” as claimed in the advertisements.
The Council declared Tablets Formothalates inadmissible to New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 92.)
The Council has declared Triple Arsenates with Nuclein No. 1 and Triple Arsenates with Nuclein No. 2, tablets marketed by the Abbott Laboratories, inadmissible to New and Nonofficial Remedies because unwarranted therapeutic claims (Rule 6) are made for them and because they present an illogical combination of drugs (Rule 10). The publication of the following report has been authorized by the Council.
W. A. Puckner, Secretary.
The following claims are made for Triple Arsenates with Nuclein:
“Puts ‘pep’ and strength back into that patient recovering from Spanish Influenza, pneumonia, typhoid, or surgical operation. An extremely powerful reconstructive tonic. Try it for that ‘run down’ feeling.”
Triple Arsenates with Nuclein is said to contain “Strychnin Arsenate gr. 1⁄128, Quinin Arsenate gr. 1⁄64, Iron Arsenate gr. 1⁄64, Nuclein Solution mins. 4.” A second preparation, of double strength—Triple Arsenates with Nuclein No. 2—is also advertised. The Council voted not to accept these preparations for New and Nonofficial Remedies on the following grounds:
The quantities of quinin, iron and nuclein in the doses represented in these mixtures are negligible; thus, one tablet of Triple Arsenates with Nuclein containing 1⁄64 grain of quinin arsenate contains only about 1⁄90 grain of anhydrous quinin; the tablet containing 1⁄64 grain of iron arsenate contains 1⁄210 grain of iron; 4 minims of the nuclein solution (assuming it to be the “Nuclein Solution-Abbott”) would contain but 2⁄5 of a grain of nuclein—a substance which even in large doses is of questionable therapeutic value. The amounts of iron and nuclein contained in doses of this preparation are insignificant in comparison with the amounts present in ordinary foods. The only substances present in even small therapeutic doses are strychnin and arsenic. The effects of arsenic and strychnin are very different and there are comparatively few conditions in which they should be prescribed at the same time. Hence a preparation containing these two in fixed proportions is illogical.—(From Reports of Council on Pharmacy and Chemistry, 1919, p. 92.)
The Council has adopted and authorized publication of the report which appears below. This report declares “Anti-Pneumococcic Oil” (a solution of camphor in oil sold by Eimer and Amend, New York) ineligible for New and Nonofficial Remedies because (1) the recommendations for its use in pneumonia are not warranted by the evidence, (2) the name is not descriptive of its composition but is therapeutically suggestive, and (3) the sale of a solution of camphor in oil under a name nondescriptive of its composition is unscientific and a hindrance to therapeutic progress.
W. A. Puckner, Secretary.
The Council having decided to consider Anti-Pneumococcic Oil (Eimer and Amend, New York), the preparation was assigned to the Committee on Therapeutics for report. The report that follows was made by a member of this committee:
According to the advertising, Anti-Pneumococcic Oil is a “twenty-five per cent. solution of camphor in a thin oil” which was “originated” by August Seibert, M.D. The following directions are given for its use:
“10 c.c. (150 minims) to every 100 pounds of body weight, to be injected hypodermically every eight to twelve hours in pneumococcic pneumonia, as soon after the initial chill as possible.”
It is claimed that the prescribed dose one hour before general anesthesia begins, “safeguards against postoperative pneumonia,” and, that “animals can so be immunized against later and otherwise fatal intravenous pneumococcic infection (Boehnke, Institute for Experimental Therapy, Frankfort).” The advice is given:
“In pneumococcic meningitis, endocarditis and pleuritis, 3% of salicylic acid should be added to this oil.”
In an article by Seibert, “Camphor and Pneumococci” (Medical Record, April 20, 1912), a reprint of which is used to advertise Anti-Pneumococcic Oil, previous work (München, med. Wchnschr., No. 36, 1909) is mentioned as the starting point for the use of camphor in pneumonia. In this article, the author reports his first case, that of a young woman who entered St. Francis’ Hospital on the third day after the initial chill “with the symptoms of severe toxemia (unconscious, temperature 105.5 F., pulse 130, and respiration 40) and involvement of both lower lobes.” “Large doses of camphor,” 12 c.c. of a 20 per cent. solution, were injected hypodermically “every twelve hours, resulting in gradual improvement and recovery by the fourth day, without a crisis.” Seibert reports success in its use in twenty-one cases, but gives no case histories or protocols. He admits, however, that in four out of sixteen cases, following the first twenty-one so reported certain “limitations of this treatment were observed,” and a “sudden rise of temperature in two patients on the second and third days of treatment, respectively, proved to be due to pneumococcic nephritis, promptly subdued by appropriate doses of urotropin, while the camphor injections were continued and resulting in speedy recovery.” He further admits that empyema occurs, and states: “This proves that the camphor brought into the blood cannot prevent the as yet living organisms, constantly entering the blood current from the affected alveoli, from colonizing in the renal and pleural tissue.”
He reports, among thirty-seven patients treated in this manner, one death, that of a man 68 years old, weighing 200 pounds, with a fatty heart. Heart failure was the real cause of death. Seibert also reports some very incomplete experimental work; Dr. Hensel, assistant and pathologist of the German Hospital, found that “1⁄10,000 part of camphor added to the usual culture media inhibited the growth of pneumococci, while the controls all thrived”; Dr. J. C. Welch, pathologist of the Lying-in Hospital, found that rabbits infected with lethal doses of pneumococcus cultures intravenously were saved by large doses of camphorated oil; fragmentary protocols are given. The assistant pathologist of St. Francis’ Hospital carried on the experimental work, adding salicylic acid to the camphor. No blood cultures are reported. The conclusion reached by Dr. Seibert is that salicylic acid up to 3 per cent., added to the camphorated oil, is effective in preventing pleural infection. In the article by Dr. Seibert, there appear most sketchy reports of cases, recovery being reported without crisis in from three to nine days.
The referee has made a careful search of the literature, with the following results: Boehnke (Berl. klin. Wchnschr. 50:818, 1913), using white mice, failed to confirm the experiments reported in Seibert’s paper, unless camphorated oil were given before the pneumococci, and even then, he felt that the results were too irregular to be of great significance. When given with anti-pneumococcic serum, however, he felt that there was some benefit to be seen by the administration of camphor; his protocols, however, are not detailed. There is no report of blood cultures, etc.
Another worker, H. Leo (Deutsch. med. Wchnschr. 39:690, 1913), reported that camphor water given intravenously prolonged the lives of thirty-eight rabbits inoculated with pneumococci. Here again there were no adequate protocols and very little evidence of careful experimental work appears.
In the literature of the past ten years, there appear sketchy clinical articles on the value of huge doses of camphor in pneumonia. Markevitch (Russk. Vrach, June 27, 1914; abstr., The Journal, Dec. 5, 1914, p. 2081) treated 226 cases of pneumonia with 5 c.c. of camphorated oil hypodermically four times daily, at the same time giving digitalis (amount not stated), with a mortality of 6.6 per cent., whereas, in 322 cases untreated, there was a mortality of 13.3 per cent. He reports 133 grave cases; sixty-six received no camphor; 48 per cent. died. Of sixty-seven treated with camphor, only 22 per cent. died. He reports temperature falling by lysis when camphor is used, and comments on the symptomatic improvement following its use. With the great variation in the clinical course of pneumonia, the above figures, though suggestive, certainly need further support before the routine use of camphor as recommended by Seibert can be sanctioned.
Later articles found on the subject refer to it in a very cursory way, giving no protocols and no cases, and giving the referee the feeling that the conclusions were very impressionistic.
After a careful search of the literature, the referee concludes that: Huge doses of camphor, to 250 grains in twenty-four hours, may be given to man without serious results. No satisfactory evidence, however, appears that camphor has a specific germicidal action on pneumococci (similar to that of ethylhydrocuprein). The clinical evidence, as found in the literature, is certainly of very little value. It appears that the sale of a simple solution of camphor in oil under the guise of “Anti-Pneumococcic Oil” is to be deplored (a 20 per cent. solution of camphor in cottonseed oil is official in the U. S. Pharmacopeia as camphor liniment). It is recommended that the preparation be held inadmissible to New and Nonofficial Remedies because exaggerated therapeutic claims are advanced for it, and because the name is not descriptive of the composition, but is, instead, therapeutically suggestive.—(From The Journal A. M. A., Jan. 3, 1920.)
Dial “Ciba” has not been accepted for “New and Nonofficial Remedies” because, as the report which follows shows, unwarranted claims are made for the product. It is a definite new chemical compound which might be made eligible for N. N. R. if misleading therapeutic claims were eliminated. The Council directed that Dial “Ciba” be included with Articles Described but Not Accepted, so that physicians might be informed with regard to its character and properties.
W. A. Puckner, Secretary.
Dial “Ciba” is a hypnotic manufactured by the Society of Chemical Industry of Basle, Switzerland, and is sold in the United States by A. Klipstein and Company, Inc., New York. Chemically, Dial “Ciba” is diallylbarbituric acid and is, therefore, closely related to diethylbarbituric acid or barbital (“veronal”).
The claims made for Dial “Ciba” are (1), that the “allyl” group in its molecule makes it more readily decomposed by oxidizing agents than barbital, which contains the “ethyl” group; (2) that because of this ease of oxidation, it is more readily decomposed in the body and more rapidly and completely eliminated, and (3) that because of its alleged rapid elimination, it is devoid of the after effects of barbital and other hypnotics.
The Council took up the substance in February, 1918, and referred the matter to the referee in charge of barbital preparations. The referee considered unwarranted the claim that Dial “Ciba” did not have the after-effects of other hypnotics due to its alleged total decomposition in the body. The American agents, A. Klipstein and Company, were informed of the referee’s objections. Their attention was also called to the fact that, notwithstanding the claimed absence of after-effects in one part of the advertising, other parts of the same advertising admitted certain post-hypnotic effects of the product. It was pointed out also that while it was claimed in one of the advertising circulars that lowering of the blood pressure is never observed after administration of Dial “Ciba,” yet two of the authors quoted in the same circular definitely stated that a lowering of the blood pressure followed even small doses of the drug and these authors warn against this very danger in certain conditions.
A year later, a circular letter sent out by A. Klipstein and Company reiterated the claim that the asserted decomposition of Dial “Ciba” in the body prevents after-effects, the drug being still contrasted with barbital (“veronal”). In view of the reiteration of this highly improbable claim, the referee undertook to study the comparative action of Dial “Ciba” as compared with other hypnotics. It was found that the actions of Dial “Ciba” are not distinguishable, qualitatively, from those of barbital, there being no perceptible difference in the after-effects or in the nature of the side actions. In toxic doses, both caused profound depression with the temperature falling to that of the room (or about one degree above), the respiration being extraordinarily slow and shallow as one would expect with lowering of the temperature. There were also the same evidences of nausea that are so frequently seen after toxic doses of the various hypnotics of this group. In view of these results, the Council declared that it is unwarranted to claim freedom from after-effects for Dial “Ciba.”
The Council held that the following statement is unwarranted:
“The therapeutic field for Dial ‘Ciba,’ as shown by tests on rabbits, is just as broad as the field for Diethylbarbituric Acid.”
Tests on rabbits do not and cannot show the breadth of the therapeutic field for a hypnotic. The Council also declared the following statement improbable, and contrary to the evidence obtained by the referee:
“In dogs, the increase of dosage beyond the therapeutic dose to the point of death is decidedly in favor of Dial ‘Ciba,’ which required a larger dose [than diethylbarbituric acid] to produce death.”
The referee’s experiments on cats show that Dial “Ciba” is several times as toxic as hydrated chloral, and more than twice as toxic as diethylbarbituric acid (barbital).
Since the circular to which objection was made in 1918 was still being sent out in December, 1919, the Council held Dial “Ciba” inadmissible to N. N. R. and voted that report of its action in the matter be authorized for publication. The Council further directed that Dial “Ciba” be included with Articles Described but Not Accepted.—(From The Journal A. M. A., Jan. 24, 1920.)
Apothesine is a synthetic drug for producing local anesthesia, made by Parke, Davis & Company. In the fall of 1917 the Council wrote to Parke, Davis & Company offering its aid in establishing the identity, purity and therapeutic efficiency of this synthetic local anesthetic with the ultimate object of accepting the product for inclusion in New and Nonofficial Remedies should the facts warrant such acceptance. The Council’s letter was never acknowledged. After Apothesine was put on the market the Council desired to accept it for inclusion in New and Nonofficial Remedies but, unfortunately, was unable to do so because some of the claims made for the product were not justified by acceptable evidence. The manufacturers were notified of the Council’s desire to admit this product to N. N. R. and the wish was expressed that the company would either so modify its claims as to make the product acceptable under the Council’s rules or else would submit evidence to the Council in proof of the claims made and thus permit the Council to revise its conclusions. Parke, Davis & Company were, apparently, either unwilling or unable to submit evidence that would sustain their claims; neither did they offer to modify the claims themselves. The product, therefore, is ineligible to inclusion in New and Nonofficial Remedies; it will, however, be listed in the “Described But Not Accepted” department of New and Nonofficial Remedies. The report on Apothesine that follows has been authorized for publication.
W. A. Puckner, Secretary.
Apothesine, “the hydrochlorid of diethyl-amino-propyl-cinnamate,” is an efficient local anesthetic. It belongs to the procain rather than to the cocain type, that is, it belongs to that type which, while effective for injection anesthesia (especially when combined with epinephrin) is relatively inefficient when applied to mucous membranes. Apothesine may also be used for spinal anesthesia. Its absolute toxicity is less than that of cocain (as 20 is to 15, see table below) but about twice that of procain (as 20 is to 40, see table below). It is non-irritant, is easily soluble and makes a stable solution so that it may readily be sterilized.
The Council took exception to certain claims made by Parke, Davis & Company for their product on the ground that these claims were not supported by acceptable scientific evidence. One of the claims was that Apothesine is applicable in any case in which any other local anesthetic is used. This statement, made in many advertisements, is distinctly misleading as used. When applied to mucous membranes Apothesine is far inferior to cocain and to some other local anesthetics, yet the claim obviously suggests that Apothesine is an efficient substitute for any local anesthetic.
The manufacturers claimed, too, that Apothesine is as potent as cocain. The claim would lead the physician to think that Apothesine had the same anesthetic potency as cocain in solution of equal strength. This statement, so far as it refers to the drug when applied to mucous membranes, is not in accord with the facts and is true for injection anesthesia only when stronger solutions are used. The only support for the claim of equal efficiency appears to be the experiments with intracutaneous injections made by H. C. Hamilton130 in Parke, Davis & Company’s laboratory. These differed considerably from the results of Sollmann.131 A further series of experiments were made by Sollmann to compare still further the diverse results previously reported by him and Hamilton. The latest series, while showing considerable variations in the susceptibility of different skin areas, especially toward Apothesine, demonstrated in every case that the efficiency of Apothesine is unmistakably lower than that of cocain, being at best one half. The series also showed that the potency of Apothesine was never greater than procain and averaged considerably below it.
Another claim made for Apothesine which the Council holds is not supported by evidence is that of superior safety. This claim is made on the basis of hypodermic injections in guinea-pigs carried out in the laboratory of Parke, Davis & Company. Such experiments prove little because of the fact—well known to laboratory workers—that the use of rodents in toxicity tests made by injecting a drug into the subcutaneous tissues does not give a reliable index of the relative toxicity of such a drug for man. This is due partly to the peculiar resistance of rodents to poisons and partly to the great importance of the rate of absorption. The organism destroys most local anesthetics so rapidly that the rate of absorption is more important than the absolute dose. The absorption from hypodermic injections into guinea-pigs differs, of course, from that in clinical accidents, especially where the drug has been applied to mucous membranes. One cannot, therefore, reliably estimate the degree of clinical danger on animals.
It has been shown that when toxicity tests of local anesthetics are made on cats these animals seem to respond to the drugs in a manner more closely approximating humans and it is a suggestive fact that the more toxic of local anesthetics, as shown by tests on cats, have been found the most dangerous in clinical use. The absolute toxicity of Apothesine has been measured by Eggleston and Hatcher132 by the intravenous injection in cats. The fatal doses, in terms of milligrams per kilogram ranged as follows:
Alypin, Holocain | 10 |
Beta Eucain | 12.5 |
Cocain | 15 |
Apothesine | 20 |
Tropacocain | 20–25 |
Stovain | 25–30 |
Nirvanin | 30–35 |
Procain | 40–45 |
The absolute toxicity of Apothesine is, therefore, only a little lower than that of cocain, and is twice as great as that of procain. The clinical dangers cannot be predicted by either method, since clinical accidents depend, in most instances, on idiosyncrasies, or the technic of application.—(From The Journal A. M. A., Jan. 24, 1920.)
The Council has adopted and authorized publication of the report which appears below. This report declares “Eumictine” ineligible for New and Nonofficial Remedies because (1) it conflicts with Rule 10 in that it is unscientific, (2) it conflicts with Rule 6 in that it is sold under unwarranted therapeutic claims, (3) it conflicts with Rule 4 against indirect advertising to the public in that the name “Eumictine” is blown in the bottle for the obvious purpose of bringing the product to the attention of the public when it is prescribed in the original package, and (4) because the name is therapeutically suggestive and not in any way descriptive of its composition.
W. A. Puckner, Secretary.
Eumictine is a preparation from the laboratory of Maurice Le Prince, Paris, France, and is marketed in this country by George J. Wallau, Inc., New York. It is claimed that the product is “a balsamo-antiseptic preparation composed of Santalol, Salol, and Hexamethylene-Tetramine, in the form of gluten-coated capsules.” Nowhere in the advertising are the amounts of the ingredients given. According to the American agent, however, “each capsule is supposed to contain 20 centigrams of Santalol, 5 centigrams of Salol, 5 centigrams of Hexamethylene-Tetramine.”
Eumictine is advised “in treating genito-urinary diseases (urethritis, cystitis, prostatitis, pyelitis, etc.).” It is claimed to be “both an antiphlogistic modifying agent, a well-tolerated diuretic” which “may be administered for long periods without ill effects.”
The Council declares Eumictine ineligible for New and Nonofficial Remedies because it is exploited in conflict with the following rules:
It is unscientific (Rule 10). Eumictine is composed of hexamethylenamin, salol and sanalol in fixed proportions. Hexamethylenamin may serve a useful purpose in some forms of infection of the urinary tract, but neither it nor salol is of any considerable value in gonorrhea. It is now known that the balsamic preparations, formerly so widely used, do not have the curative effects in gonorrhea and associated conditions that used to be ascribed to them. To combine three substances, none of which has any distinct therapeutic value in the conditions for which Eumictine is proposed, does not enhance their value. There is nothing original in the combination used in Eumictine, or in the manner of dispensing it.
It is sold under unwarranted therapeutic claims (Rule 6). These claims are made not only for the components of Eumictine but for the combination itself. Though santalol has certain advantages over the somewhat variable oil of santal and other balsamic resins, it is not true that santalol “does not cause congestion of the renal epithelium” or that it does not “produce exanthema as do copaiba, cubebs, and the ordinary santal oil.” It is not true that salol is “devoid of toxicity.” Neither is it correct to say that salol “asepticizes and disinfects the bladder, the prostate and the urethra.” The claim that hexamethylenamin “is of value when any acute symptoms or tendency to inflammation subsist” is not justified. The claim that hexamethylenamin “renders soluble the uric acid and urates” is also without foundation. The following paragraph is characteristic of the claims made for Eumictine:
“Anti-gonorrhoic by its Santalol, diuretic, urolytic and analgetic by its hexamethylenetetramin (Urotropin) antiseptic and antipyretic by its Salol, Eumictine represents a real therapeutic advance in the scientific treatment of diseases of the urinary passages.”
Instead of being “a real therapeutic advance” in the treatment of diseases of the urinary passages, Eumictine presents one of the complex combinations that have long retarded the scientific treatment of these diseases. Eumictine also conflicts with Rules 4 and 8 of the Council.—(From The Journal A. M. A. Feb. 21, 1920.)
The Council has authorized publication of the following report on “Platt’s Chlorides.” It also declares the preparation inadmissible to New and Nonofficial Remedies because its composition is uncertain and indefinite and because the claims made for it are exaggerated and misleading.
W. A. Puckner, Secretary
“Platt’s Chlorides,” marketed by Henry B. Platt, New York, is sold as a disinfectant and germicide. Only incomplete and contradictory statements have been made in regard to its composition. Many years ago (about 1899) the composition of Platt’s Chlorides was given as “The Chlorids of Zn 40 per cent., Pb 20, Ca 15, Al 15, Mg 5, K 5.” The statement that the preparation contained 20 per cent. of lead chlorid is interesting, in view of the fact that lead chlorid is soluble in water at ordinary temperatures to the extent of less than 1 per cent. In a booklet, also issued a number of years ago, the following “Formula of Platt’s Chlorides” was given:
“A saturated solution of Metallic Chlorids combined in the following proportions:
“Sol. Zinc Chlorid | 40 | per cent. |
“Sol. Aluminum Chlorid | 15 | per cent. |
“Sol. Lead Chlorid | 20 | per cent. |
“Sol. Calcium Chlorid | 15 | per cent. |
“Sol. Magnesium Chlorid | 5 | per cent. |
“Sol. Potassium Chlorid | 5 | per cent.” |
The label on a bottle purchased in 1911, describes Platt’s Chlorides as:
“A Highly Concentrated Solution of the Chlorids of Aluminum, Calcium, Lead, Zinc, etc.”
The label of a bottle purchased in 1919 reads:
“Contains Inert Material: Water 84.0%. Sodium Chlorid 4.8%. Calcium Chlorid 0.3%.”
This statement is obviously made to meet the requirements of the federal Insecticide Act. This law requires either that the identity and the amounts of potent ingredients in disinfecting preparations be declared or else that the percentage of the inert ingredients of such preparations be given. The omission from the label of all statements with regard to the potent ingredients of the preparation and the absence of such a statement in recent advertising matter suggests either that the older statements about its composition were false or else that the composition has been changed.
Tscheppe published (Pharmaceutische Rundschau 8:109, 1890) an analysis of Platt’s Chlorides which has been quoted in other publications as indicating the composition of the preparation. He reported that he found each quart of the preparation to contain aluminum sulphate 6 ounces, zinc chlorid 11⁄3 ounces, sodium chlorid 2 ounces, calcium chlorid 3 ounces.
Some years ago (about 1911) the company made the following statement relative to the germicidal power (phenol co-efficient) of Platt’s Chlorides:
“... for some time the carbolic acid co-efficiency of our output has been from 2.5 to 4.3, the average being about 3; namely about three times stronger than pure carbolic acid.”
In 1912, the U. S. Public Health and Marine Hospital Service reported (Bulletin 82, Public Health and Marine Hospital Service, p. 69) that the phenol coefficient of a sample of Platt’s Chlorides was so low that it could not be determined and also that the sample was found to contain some mercuric chlorid. In 1913, the North Dakota Agricultural Experiment Station reported (Bulletin, July, 1913, p. 292), that Platt’s Chlorides contained principally zinc chlorid, also some aluminum chlorid, calcium chlorid, and traces of mercuric chlorid. The phenol coefficient, determined by the Hygienic Laboratory method, was found to be 0.05.
The preceding suggests that the composition of Platt’s Chlorides had been changed (without notice to the consumer) and that it had been fortified by the addition of mercuric chlorid. Years ago part of the advertising of this product was a testimonial from a health official which declared that, for disinfection, “bichlorid of mercury is useless in disinfecting sputum or discharges from the bowels, being rendered inert by the albumin present” and it lauded Platt’s Chlorides as devoid of such drawbacks.
To determine the present composition of Platt’s Chlorides and to compare it with that sold formerly, the A. M. A. Chemical Laboratory has made an analysis of a specimen purchased in 1919 and also of one purchased in 1911 and since kept unopened in the files of the Council on Pharmacy and Chemistry. The following table contains the results of these analyses (all quantities given are Gm. per 100 c.c.):
| 1911 Specimen | 1919 Specimen | ||
Color | Colorless | Straw Color | |
Odor | None | None | |
Specific Gravity at 25 Cc. | 1.1229 | 1.1313 | |
Total Solids (residue at 100 Cc.) | 16.49 | 18.33 | |
Chlorid (Cl-) | 7.60 | 10.74 | |
Sulphate (SO4- -) | 1.11 | .16 | |
Aluminum (Al+++) | .22 | .90 | |
Calcium (Ca++) | .19 | .13 | |
Zinc (Zn++) | 5.11 | 3.93 | |
Lead (Pb++) | .046 | Traces | |
Mercury (Hg++) | ....... | .0086 | |
Sodium (Na+) | 1.01 | 1.39 |
These quantities transposed to hypothetical combinations would indicate that Platt’s Chlorides has the following composition:
| 1911 Specimen | 1919 Specimen | ||
Aluminum Sulphate | 1.32 | .18 | |
Aluminum Chlorid | .07 | 4.29 | |
Calcium Chlorid | .54 | .37 | |
Zinc Chlorid | 10.66 | 8.19 | |
Lead Chlorid | .06 | Traces | |
Mercury Chlorid | ....... | .0116 | |
Sodium Chlorid | 2.57 | 4.81 | |
Hydrogen Chlorid | .43 | None |
In the past, the advertising has suggested, more or less directly, that, as chlorinated lime (bleaching powder) may be made to give off chlorin gas which disinfects, so the air in a room may be disinfected by evaporating Platt’s Chlorides. Thus the label of the 1911 specimen contains the following:
“For Store Rooms, Refrigerators, and Closets, keep a sponge saturated with the pure liquid in a saucer on an upper shelf.”
On the label of the 1919 specimen, the statement reads:
“Refrigerators and Storerooms—As a disinfectant wash regularly with one part Chlorides to eight of water. As a deodorant, keep in an open vessel a sponge or cloth saturated with the Chlorides full strength.”
That the owner of Platt’s Chlorides really believes that the vapors of the preparation have disinfecting properties is seen from a letter over the name of Henry B. Platt printed in the New York Tribune in 1916. This read, in part:
“... by keeping in a dish or saucer on radiators Platt’s Chlorides diluted one-half, the hot solution will evaporate and purify the air, thus destroying the grip germ which is the cause of all the trouble.”
From the analysis of Platt’s Chlorides, it is evident that when the preparation is evaporated, water vapor only escapes.133 Whatever disinfecting or germicidal action the preparation may possess is exercised only when the solution is brought in direct contact with the substance to be disinfected.
The aluminum and zinc salts present may be useful as deodorants but they are not effective as germicides. The presence of mercuric chlorid in a concentration of 1 to 10,000 is hardly to be considered as materially increasing the efficiency. The directions recommend the use of a mixture of 1 part of Platt’s Chlorides to 10 parts of water for rinsing the hands, and a mixture of 1 part to 4 parts of water for the disinfection of discharges. It is further stated that 1 quart makes 2 gallons sufficiently strong for general use. It is evident that such dilutions decrease considerably the feeble germicidal action of the original fluid.—(From The Journal A. M. A., March 27, 1920.)
The Council has authorized publication of the reports which appear below, declaring Anti-Tuberculous Lymph Compound (Sweeny) and Anti-Syphilitic Compound (Sweeny) ineligible for New and Nonofficial Remedies.
W. A. Puckner, Secretary.
“Anti-Tuberculous Lymph Compound (Sweeny)” is put out by the National Laboratories of Pittsburgh, Dr. Gilliford B. Sweeny, “Medical Director.” Sweeny has claimed at different times that he became interested in the subject of von Behring’s efforts to immunize cattle to tuberculosis at a time when he was an assistant in von Behring’s laboratory. He claims to have conceived the idea while there of transferring bovine immunity to tuberculosis to the human subject and later to have evolved his “treatment” at the Pasteur Institute in Paris.
Just how Anti-Tuberculous Lymph Compound is made today is not stated—at least so far as one is able to learn from recent advertising. Some years ago Sweeny declared that his “Anti-Tubercular Lymph” (as it was then called) was derived from a bullock which had been immunized to tuberculosis. Then:
“The immunized animal having been slaughtered, the contents of the lymph reservoirs are carefully collected and an aqueous extract is made from the grey cerebral substance, spinal cord and the lymph glands. It is then filtered under high pressure and de-albuminized by succussion. To this, the lymph, together with a definite proportion (50 per cent.), of the naturally phosphorized brain fats is added, with a small amount of chloride of gold (about 1-60 gr. to the dose), the latter as a preservative.”
It is a fair assumption that however the preparation may have been made originally, it is not now made in such a manner as to bring it under the federal laws governing the preparation of serums and similar preparations. The claims made for Anti-Tuberculous Lymph Compound are of the usual uncritical and unscientific type. Mainly, of course, they are of the testimonial class. The physician is told that the preparation has been carefully tested by men whose judgment is worthy of consideration; that the verdict has been altogether favorable to the “Compound.” Thus:
“... the remedy was submitted to a selected body of skilled physicians, recognized for their skill and care in making therapeutic observations. These men represented widely varying conditions, climatic and otherwise. Those who said ten years ago that Anti-Tuberculous Lymph Compound has a specific immunizing influence upon the tuberculosis patient, find the same to be true today.”
Careful reading of the matter just quoted will reveal its ambiguity and inherent lack of frankness. The inference conveyed is that the “selected body of skilled physicians” have unqualifiedly endorsed Anti-Tuberculous Lymph Compound (Sweeny)—but it does not say so!
It is the history of all such preparations, introduced to the medical profession with the usual blare of trumpets, that a certain number of favorable testimonials can be obtained. It is also the history of such products that one has but to wait a few years and the physicians who had written most enthusiastically regarding the preparation—in the first flush of their optimism following its use and the perusal of the manufacturers’ literature—will acknowledge that they were mistaken in their original estimate and are no longer using the agent. In this connection an investigation of some of the old testimonials for Anti-Tuberculous Lymph Compound by the Propaganda department of The Journal is instructive.
In a somewhat elaborate booklet published in 1907 by Sweeny, an Indiana physician was said to have reported favorable results following the administration of the “lymph.” A letter written to this physician in October, 1919, asking for his present opinion on the product brought this reply, in part:
“... it being twelve years since using the serum and no reference or repeated orders since should surely suffice as evidence of my lack of faith in the serum....”
An Illinois physician was reported in the same booklet to have described a case of a young man with an active tuberculosis, who was given injections of the “lymph” in February, 1907. The patient, it was claimed, showed immediate improvement and the Sweeny booklet (published in August, 1907) stated that “improvement in this case continued and terminated in complete recovery.” A letter written to the physician in October, 1919, brought out the fact that the young man in question, after receiving “Anti-Tuberculous Lymph Compound” and other treatment was removed “on a stretcher” “to New Mexico, where he remained for three or four years” and recovered. The doctor adds:
“I do not think that the Anti-Tuberculous Lymph had anything to do with the man’s recovery, although I realize the difficulty of definitely analyzing just what did effect the cure. I did since that time use that preparation in several other cases without beneficial results so that I gave it up a good many years ago adding it to that large heap of pharmaceutical material ‘weighed and found wanting.’ ”
A physician in Texas also reported in the 1907 booklet as having had very satisfactory results with the Anti-Tuberculous Lymph Compound in one case of pulmonary tuberculosis was written to in October, 1919. He replied:
“I will state that subsequent use of this compound did not bear out the apparent good results from its use in the first case or two.”
In a “Bulletin” issued by the Sweeny concern in 1912, a Pennsylvania physician was quoted as having treated three cases with Anti-Tuberculin Lymph Compound with resultant cures. This physician was written to in October, 1919, and he replied:
“I have no knowledge of the use of my name by any Pittsburgh concern and know nothing of a lymph of the name of Sweeny; neither do I recollect ever curing three cases of tuberculosis with any lymph.”
The same “Bulletin” quoted the alleged statement by a Delaware physician to the effect that he believed Anti-Tuberculous Lymph Compound to be the most successful treatment of tuberculosis extant. This in 1912. To an inquiry sent in October, 1919, this physician briefly replied:
“Am not using it now.”
The result of the Propaganda department’s questionnaire was what might have been expected. Every physician who answered the inquiry regarding his previous and present opinions of Anti-Tuberculous Lymph Compound (Sweeny) declared, in effect, that he had long since ceased to have faith in its value or efficacy.
According to claims made in Sweeny literature, “Anti-Tuberculous Lymph Compound exercises its immunizing power through a specific action upon the blood cells.” The statement that “it destroys the tuberculosis germ when this is present in the system of the patient” is untrue. The facts are, no serum or lymph has thus far been proved to have any value in the treatment of tuberculosis even when fortified by “a small proportion of chloride of gold and soda” as one circular tells us the “lymph” is. In spite of research by competent investigators, we are still without any aid in the form of a serum in the treatment of tuberculosis.
Anti-Tuberculous Lymph Compound (Sweeny) is one of those preparations that need no elaborate laboratory tests, nor even exact therapeutic research, to convince any clear-thinking person that it is patently and obviously worthless. One would hesitate before asking any reputable clinician to test a preparation of this sort. It is a constant source of surprise that some physicians allow themselves to be persuaded by advertising literature that is obviously uncritical and unscientific, to use preparations which have no more reasonable foundation than this one.
The Council declares Anti-Tuberculous Lymph Compound (Sweeny) not acceptable for New and Nonofficial Remedies.
This preparation also is made by or under the direction of the same Dr. Gilliford B. Sweeny whose researches (?) led to the production and evolution of the Anti-Tuberculous Lymph Compound (Sweeny). According to the data at hand, this preparation is made by suspending benzoate of mercury in lymph from the bullock. Case reports are given of alleged cures of syphilis after two months of treatment; indeed, the circular exploiting the agent makes the statement that it is seldom necessary to continue the treatment beyond two months, which, if one chose to be credulous, would indicate extraordinary power for the mercury.
Mercury of course has a proper place in the treatment of syphilis, but that any physician could be induced to place his trust in this preparation is almost unthinkable though testimonials—which the “National Laboratories” claim to have received from physicians—are published. They all stamp the writers as not only gullible but also incompetent. The tenor of the claims is on a par with those made for the Anti-Tuberculous Lymph Compound; they do not justify the time required for detailed consideration.
The Council declares Anti-Syphilitic Lymph Compound (Sweeny) not acceptable for N. N. R.—(From The Journal A. M. A., April 3, 1920.)
The Council has authorized publication of the following report on “Syrup Leptinol” (formerly “Syrup Balsamea”). The product is inadmissible to “New and Nonofficial Remedies,” first, because the manufacturers fail to give the profession information regarding either the amount of the potent ingredient or the method of determining its identity and uniformity; second, because of the unwarranted recommendation for its use in such infectious diseases as pneumonia and epidemic influenza and for lack of satisfactory supporting evidence of its alleged therapeutic efficacy in other diseases and, third, because the recommendations for its use appearing on and in the trade package constitute an indirect advertisement to the public.
W. A. Puckner, Secretary.
Syrup Leptinol is sold by the Balsamea Co. of San Francisco. It was first introduced as Syrup Balsamea. In recent advertising, Syrup Leptinol is also referred to simply as “Leptinol.”
According to the statements of the Balsamea Co., Syrup Leptinol is prepared from the root of a species of Leptotaenia (a plant belonging to the parsnip family) which grows in Nevada and which has heretofore not been used in medicine. The manufacturer states that the botanists who have been consulted have been unable to agree on the botanical classification of the plant. The dried root of this unclassified species of Leptotaenia is extracted with alcohol and from the extract so obtained the syrup is made, but no information has been furnished to show how the alcohol-soluble material is incorporated in the syrup. Further, the manufacturer has not announced tests whereby the identity and uniformity of the finished preparation may be determined.
A booklet contains the following:
“The species of Leptotaenia from which Leptinol is produced was first used in medicine by Dr. E. T. Krebs, who, after thorough laboratory investigation and clinical application over a period of several months, which resulted in the perfecting of Leptinol, prescribed the preparation for Influenza during the epidemic of that disease in 1918 with remarkably good results. Since this first use, Leptinol has been exhaustively tested by clinicians in private practice and in hospitals in the treatment of Pneumonia, Influenza Bronchitis, etc., and has been universally endorsed.”
In a circular letter it is asserted that the use of “Leptinol” during the “influenza epidemic” of 1918–1919 “demonstrated its almost specific action in respiratory affections”; that “during this epidemic it proved to be five times as efficacious as any other treatment in pneumonia ...”; and that “it is now as firmly fixed in the mind of many doctors for respiratory diseases as quinine is for malaria and the salicylates for rheumatism.”
In the booklet it is further stated that the therapeutic action of the preparation is primarily that of a “stimulating expectorant” and secondarily as a “sedative expectorant”; that “its antiseptic action in the respiratory tract is prompt”; that it “is an effectual cardiac tonic where the tone of the heart muscle is impaired by fever”; that “in acute pulmonary conditions it effectively improves the respiratory action and allays cerebral irritation due to fever and toxins”; that it acts “as a vital stimulant and nerve sedative”; that “it stimulates the excretion of acid by the skin and in fever it has a strongly diaphoretic and antipyretic action without depressing the circulation or the central nervous system”; that it is “mildly diuretic” and “slightly augments the biliary flow” and that “it increases the gastric and intestinal secretions and allays intestinal fermentation.”
No evidence has been presented to the Council which shows that Syrup Leptinol has the actions ascribed to it. The reports of clinical trial are little more than chance observations and lack all control. This applies also to the following, stated to be a quotation from the report of the Tonopah Mines Hospital Association:
“In the spring of 1919 a recurrence of the Influenza epidemic of the previous winter was experienced. During the first period of this second epidemic, prior to April 15th, there were treated one hundred sixteen cases of Influenza, fourteen of which developed Influenzal Pneumonia, with six deaths. The Pneumonia was of the very virulent type which prevails in this high altitude.... After April 15th, when the clinical use of Leptinol was inaugurated, three hundred and sixty-eight cases of Influenza were treated and not a single case developed Pneumonia. Twenty-two cases of Influenzal Pneumonia were received and treated with Leptinol, with a consequent one hundred per cent. recovery....
“In the cases where Leptinol was used the treatment was the same as had been previously followed, as to diet, fresh air, etc., but the medication was confined to Leptinol. Syrup Leptinol was started immediately in one-dram doses at one-hour intervals, in cases with high temperatures, and this was continued until temperature and pulse subsided. It was then used in one-dram doses at three-hour intervals as recovery progressed. On admission to the hospital, calomel in 1⁄4 grain doses, was given at fifteen minute intervals for eight doses. The last calomel was followed in six hours by 1⁄2 ounce Magnesium Sulphate in saturated solution. The second day 1⁄10 grain of calomel was given at one-hour intervals for ten doses....”
Medical journals are replete with reports of remarkable results obtained with the most varied forms of treatment instituted at the time that the “influenza epidemic” had been reached. In these cases it is more than probable that the lessened virulence of the causative factor of the disease, the gradually established resistance of those stricken with it in the latter period and the improved management resulting from experience deserve the credit for the successful outcome of the treatment, rather than the particular form of medication employed.
The report of the Tonopah Mines Hospital Association directly implies that Syrup Leptinol prevents the development of pneumonia in practically all cases of influenza in which it would develop and that it entirely abolishes the mortality of that disease. However, it is well known that innumerable remedies have been recommended as specifics in the treatment of pneumonia on the basis of the treatment of a limited number of cases which recovered, and that eventually these asserted specifics have been discarded as of little value. In the present instance, the recovery of twenty-two cases in succession afford prima facie evidence that those cases were not the virulent type of pneumonia in which the death rate is very high under any methods of treatment. While no effort appears to have been made to determine the nature of the infecting organism, the records show fairly conclusively that they belonged to those causing the milder type of pneumonia.
The Council finds Syrup Leptinol (formerly Syrup Balsamea) inadmissible to New and Nonofficial Remedies because: (1) the information in regard to composition does not state the amount of potent ingredient, nor permit the determination of its identity and uniformity; (2) the recommendation for its use in such infectious diseases as pneumonia and epidemic influenza is unwarranted and its claimed therapeutic efficacy in other diseases is without satisfactory supporting evidence; and (3) the recommendations for its use which appear on the label and the circular wrapped with the trade package constitute an indirect advertisement to the public.
The Council accepts the explanation of the manufacturer that he has been unable to obtain a satisfactory classification of the plant from which Syrup Leptinol is made. It would be undesirable to exclude from therapeutic use a valuable drug simply because its botanical character has not been determined or because an exhaustive chemical examination had so far not been made. However, in the absence of such information the manufacturer should give full information with regard to the preparation or standardization of his remedy and the therapeutic claims made for it should be accompanied by indisputable, thoroughly controlled clinical evidence. In the case of Syrup Leptinol, there is no satisfactory evidence available showing that the preparation has any value in the treatment of epidemic influenza, pneumonia, whooping cough, etc. While it is probable that a balsamic syrup, such as Syrup Leptinol, has palliative properties in coughs, such action does not at all justify the claim that it is useful in the contagious diseases for which it is proposed. The Council cannot recognize a syrup presenting an unknown plant in uncertain proportions which is recommended in a variety of dangerous contagious diseases in which it ultimately may be harmful, even though in early stages of these diseases it may serve to allay some of the milder symptoms.
Concerning the composition of the plant from which Syrup Leptinol is prepared, the Balsamea Company states that it contains “Alkaloids, acids, glucosides, volatile and fixed oils, gums and resins.” This information is valueless, since no information is given concerning the character, amounts or pharmacologic action of the ingredients. Further, it is unreliable as far as the presence of alkaloids is concerned since the A. M. A. Chemical Laboratory has been unable to find any alkaloids in the specimen of the crude drug furnished by the manufacturers.
In accordance with its regular procedure, the Council submitted the preceding statement to the manufacturer.
In reply the Balsamea Company stated that it is more than ever of the belief that Syrup Leptinol is deserving of recognition by the Council, basing this opinion on further clinical experience with it in the treatment of influenza.
The manufacturer stated that the use of the words “Leptinol” and “Syrup Leptinol” interchangeably was due to an oversight and promised to limit the use of the word “Leptinol” to an alcoholic extract of the plant.
Concerning the method of preparation of this alcoholic extract and the amount used in the preparation of Syrup Leptinol the Balsamea Company replied as follows:
“The alcoholic extract of the Leptotaenia, which we have termed ‘Leptinol’ is a preparation of definite and uniform strength, as determined by two methods: (a) the gravity test using the U. S. Hydrometer Scale for spirits, by which Leptinol registers 52 degrees at 60 degrees F., and (b) by gentle evaporation of the alcohol content and the measuring of the active constituents, which measures twenty-five per cent. by weight.
“The alcoholic extract ‘Leptinol’ is glycerinated in a machine, using one part of the alcoholic concentration to four parts of glycerin. This is then added to eleven parts of a heavy syrup, containing 71⁄2 pounds of sugar to the gallon of syrup, and thoroughly mixed in an agitating machine. Leptinol is the sole active ingredient of Syrup Leptinol. Syrup Leptinol is a preparation of uniform strength. It is far more uniform in strength than most of the syrups of the U. S. P. made from fluid extracts which are made from crude drugs which are not uniform in strength.”
This claim cannot be allowed as meeting the conflict with Rule 1. It is well known that plants vary in their composition at different times of the year; under different conditions of cultivation and growth; and under other conditions; hence the claim that alcoholic extracts of equal specific gravity insure uniformity of composition in active principles must be considered entirely illogical, especially since the exact nature of the active principles, if any be present, is unknown. If these are known their nature should be stated and tests for their identity be given. If they are unknown it is manifestly misleading to state that the preparation is of uniform strength.
It is evident that the Council cannot approve of the use of a preparation of unknown composition without satisfactory evidence of its value, especially when it is recommended in a variety of serious infectious diseases such as influenza and pneumonia. The mere fact that a small number of patients who have received the drug recover is no evidence of its curative value, and until carefully controlled clinical tests of the preparation are made, it is not entitled to the consideration of physicians.—(From The Journal A. M. A., June 5, 1920.)
The Council has authorized publication of the following supplementary report on Formitol Tablets.
W. A. Puckner, Secretary.
In the Council report (The Journal A. M. A., Oct. 4, 1919, p. 1077) on the ineffectiveness of lozenges claimed either to contain formaldehyd or to liberate formaldehyd in the mouth, the composition of Formitol Tablets of the E. L. Patch Co. was briefly discussed in the following terms:
“The A. M. A. Chemical Laboratory reported that Formitol Tablets contained formaldehyd (or paraformaldehyd), an ammonium compound, and some hexamethylenamin. It is probable that the formaldehyd (or paraformaldehyd) was produced by the decomposition of hexamethylenamin originally present in the tablets but decomposed by long contact with the acid.”
At the time this report was published, the label and the advertising matter contained but vague and indefinite statements with regard to the composition of Formitol Tablets. In the October, 1919, issue of Patchwork, the house organ of the E. L. Patch Co., it was denied that these tablets contain hexamethylenamin since none had ever been used in their manufacture. It was also claimed that the company had a “printed sheet giving the formula of these tablets.”
The Council advised the E. L. Patch Co. that it desires to publish only facts about the products which it examines and that if the report on Formitol Tablets was inaccurate in any way the Council would want to correct any error it might have unintentionally made. As the Formitol advertising in the files of the Council contained no information as to the composition of the tablets, the firm was also requested to send the printed sheet giving the “formula.”
When this printed “formula” came it was found to be a sheet used by the E. L. Patch Co. for the purpose of giving its salesmen information regarding Formitol tablets, to be passed on to the physician. This printed sheet conveyed the information that Formitol Tablets contain ammonium chlorid, benzoic acid, citric acid, guaiac, hyoscyamus, menthol, paraformaldehyd and tannic acid, but it gave no information in regard to the amount of any of the ingredients except that it declared that each tablet represents the equivalent of 10 minims of a 1 per cent. formaldehyd solution.
Because of the nonquantitative, and, therefore meaningless printed “formula” and because, also, of its complexity, it was thought desirable to make a more complete analysis of Formitol Tablets. Experience has shown that frequently the real formula of a thing is quite different from the alleged formula published by the manufacturer. The details of the laboratory’s later analysis will appear in the Annual Reports of the Chemical Laboratory or may be had on request.
The result of the laboratory’s additional experimental work, especially when taken in connection with investigations made elsewhere on the interaction of formaldehyd and ammonium chlorid justifies the conclusion that Formitol Tablets do contain some hexamethylenamin, even though the amount may be very small. As the E. L. Patch Co. declare that no hexamethylenamin is put into Formitol Tablets the conclusion drawn in the Council’s original report to the effect that the formaldehyd probably was formed by the decomposition of hexamethylenamin was evidently an error. The hexamethylenamin present is doubtless produced by the action of the paraformaldehyd on the ammonium chlorid present.
The analysis also showed that more than 78 per cent. of the weight of Formitol Tablets was made up of sugars and about 16.5 per cent. was starch and other material, some of which was talcum or similar material. This means that about 94 per cent. of the total weight of the tablets is sugar and starch, neither of which is mentioned in the printed “formula.” The significance of this is apparent when it is considered that there are eight ingredients listed in the “formula” for which therapeutic effects are claimed. Since a tablet weighs about 13.5 grains, the combined weight of all the claimed active ingredients is less than 1 grain per tablet!
The amount of ammonium chlorid found, as indicated by the total nitrogen, was not more than 1.0 per cent. or about 1⁄8 grain per tablet. The amount of benzoic acid found was 0.34 per cent. or 1⁄25 grain per tablet. Yet these two drugs are said to exert their peculiar expectorant action. (The U. S. P. lozenge of ammonium chlorid contains 11⁄2 grains ammonium chlorid or twelve times the amount of this drug in a Formitol Tablet.)
The tannic acid contained in the tablets could not be determined with accuracy but it was much less than 1 per cent. (or 1⁄8 grain per tablet) yet it is said to add valuable astringent qualities to Formitol Tablets! (The U. S. P. lozenge of tannic acid contains 1 grain of tannic acid.)
The quantity of guaiac (as resin) is but a fraction of 1 per cent. Yet it is said to impart to Formitol Tablets “stimulant resolvent” properties and it is intimated that there is sufficient to be of value in “cases of abscess of the throat and inflammation of the tissues.”
The total acidity indicates the presence of about 2 per cent. of citric acid or 1⁄4 grain per tablet. Yet this amount is said to be “antiseptic” and “aids in the general results.”
While the presence of the drug hyoscyamus (henbane) was not positively identified by microscopic examination, alkaloids were present.
The manufacturers claim that the tablets contain menthol yet only a suggestion of menthol could be obtained from the odor. However, the odor of methyl salicylate—a constituent not declared in the “formula”—predominated throughout the operations of analysis.
Formitol Tablets furnish a good illustration of some well established but often ignored truths:
1. “Formulas” that are nonquantitative are valueless or worse than valueless.
2. The fact that a manufacturer puts certain drugs in a mixture, is no proof that these drugs are there when the mixture reaches the patient. The physician must be assured that they are there when he prescribes them.
3. Complex mixtures should be avoided. It is absurd to expect, as is claimed in the case of Formitol Tablets, anodyne, antiseptic, astringent, expectorant, and resolvent action all at the same time.—(From The Journal A. M. A., June 19, 1920.)
Two years ago, American newspapers contained accounts of an alleged cure for pulmonary tuberculosis “discovered” by Prof. Domenico Lo Monaco of Rome, Italy. At that time no reference to the “cure” could be found in medical journals which had come from Italy and other European countries (The Journal A. M. A., July 13, 1918, p. 142). Later, reports were published of experiments carried out in Italy, according to which the intramuscular injection of solutions of sugar (saccharose—cane sugar) diminished pulmonary secretion and was of considerable value in the treatment of tuberculosis (The Journal A. M. A., Sept. 28, 1918, p. 1083). On the whole the reports of the trial of what has been called the Italian Sugar Cure for Consumption have been unfavorable. At a meeting in Paris in October, 1918, Drs. Louis Rénon and Mignot reported that they had found that the disease in guinea-pigs was not modified by the treatment and with humans the results were also negative (Paris Letter, The Journal A. M. A., Nov. 23, 1918, p. 1760).
In view of the exploitation of this treatment in the United States by the Anglo-French Drug Co., which offers “Sukro-Serum,” and by G. Giambalvo & Co., which sells “Aphlegmatol,” and because of inquiries received, the Council has authorized publication of the statement which follows.
W. A. Puckner, Secretary.
A circular issued by the Anglo-French Drug Co., describes “Sukro-Serum” as a “Sterilized Solution of lacto-gluco-saccharose.” By reading the circular to the end, however, one learns that “Sukro-Serum” is not a “serum” in the ordinary sense but apparently it is a solution of ordinary sugar (sucrose). “Sukro-Serum is a sterilized, specially prepared solution of Saccharose.”
Sukro-Serum has been advertised (N. Y. Med. Jour., Sept. 6, 1919) as an “INTRAMUSCULAR INJECTION FOR TUBERCULOSIS” “... ready for use in cases of Pulmonary and general Tuberculosis” with the assertion that “It is quite certain that in the near future Sukro-Serum will be largely used and its value fully recognized.” The circular received from the Anglo-French Drug Co. contains quotations from an article by Professor Lo Monaco in the British Medical Journal (Aug. 24, 1918) setting forth the merits of intramuscular injections of sucrose in tuberculosis. It is recommended that “Néocaine-Surrénine” (which the Anglo-French Drug Co. supplies) be used for the control of pain when Sukro-Serum is injected.
The circular enclosed with a package of “Aphlegmatol,” purchased from G. Giambalvo & Co., contained the following with reference to the composition of this preparation:
“A solution of Hydrats of Carbon After the formula of Prof. D. Lo Monaco, Director of the Institut of Physiological Chemistry of the University of Rome. Contents: Sucrose (C12H22O11) Glucose and Galactose (C6H12O6).”
The package contained ampules of thin, fragile, brown colored glass, containing approximately 21⁄2 c.c. of light, clear, amber colored, thick, sticky fluid, having a distinct caramel odor. Reaction pH = 5.0. A reducing substance (probably glucose) amounting to 7.4 per cent. was found by using Benedict’s method for estimating glucose quantitatively; after hydrolysis with hydrochloric acid, 55.5 per cent. glucose was found. There was no reaction for albumin. No attempt was made to identify the sugars, as it seemed probable that in the preparation caramel had been produced.
The circular which accompanied the package of Aphlegmatol contained the following information (spelling and composition as in original) about its use and effects:
To be emploied where a large bronchial secretion is present in the respiratory branches disease. The secretion will diminish and, in non complicated cases, it will completely disappear.
Fever, cough, hemottisis, night perspiration, vomiting and difficulty of breathing are, in the meantime, diminuished.
Aphlegmatol acts also as a riconstituent, being itself a nurrishing composition, improves the digestive function of the body and increases the arterial pressure.
5 c.c. (2 Phials) of Aphlegmatol per day must be injected intramuscularly in the Gluteus.
If the patient wishes two injections may be made, one at the right immediately followed by a second one at the left.
The cure must not be interrupted untill sometime after expectoration has disappeared, which result may be obtained only after fifty or sixty days, in the meantime the patient must be controlled by his home physician, especialy when thermal elevation of the body takes place.
Improvement will be manifested on or about the tenth day of the first injection.
In the advertising circular, which is apparently intended for general distribution, much the same information is given as in the sheet enclosed with the ampules, except that in the directions we find: “If the injections are painful—especially in cases where patients are very emaciated—physicians are advised to inject together with Aphlegmatol, as an anesthetic, a vial with 1 c.c. solution of Stovain at 3%.” The advertising for Aphlegmatol contains many misspelled words and appears to be the work of those ignorant of the English language.
Tuberculosis is a widespread disease and a majority of the uninformed are only too willing and ready to try such a “cure.” The preparations appear to be nothing more than concentrated solutions of sugar. It is probable that a small amount of the cane sugar might be inverted to glucose and fructose, but experiments have shown that cane sugar subcutaneously administered in the small amounts used in this instance is largely excreted in the urine unchanged. Less is known about galactose, but the evidence available would indicate that galactose is largely excreted in the urine unchanged when given subcutaneously. Glucose would be absorbed as such, and in the amounts under consideration, used by the system much the same as when given by mouth.—(From The Journal A. M. A., Aug. 21, 1920.)
The Council has authorized publication of the following report declaring Supsalvs (Anglo-French Drug Company) inadmissible to New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Supsalvs are advertised by the Anglo-French Drug Company as “stable suppositories of ‘606’ (of French manufacture)” with the claim that by the rectal administration of these suppositories the effects of arsphenamine may be obtained. The asserted efficacy of Supsalvs medication is based in part on the claim that for these suppositories an excipient was found which mixes with the cocoa butter base “to form an assimilable emulsion.”
“The active principle and the vehicle being bound to one another, the mucous membrane is able to absorb both simultaneously and progressively in the form of an organic emulsion.”
As no information was furnished the Council by the Anglo-French Drug Company on the origin or quality of the arsphenamine used in the preparation of Supsalvs or the character of the vehicle which was “bound” to the arsphenamine in such a way as to permit the absorption of this combination in the form of an “organic emulsion,” the firm was requested to furnish: (1) Evidence that the arsphenamine used in Supsalvs complies with the N. N. R. standards and that deterioration of it does not occur in the preparation of the suppositories or on keeping. (2) The identity of the ingredients composing the suppository.
The Anglo-French Drug Company did not supply the requested evidence and consequently the Council judged the preparation on the basis of the information received from the company, and that contained in the available advertising and circulars. It found Supsalvs inadmissible to New and Nonofficial Remedies, first because the quality of the medicament contained in the suppositories has not been established, and second because the claimed efficacy of this preparation as a means of securing the effects of arsphenamine lacks substantiating proof.
During the past few years some French physicians have reported favorably on the intrarectal administration of arsphenamine. Boyd and Joseph at Panama published (The Journal, Aug. 17, 1918, p. 521) an enthusiastic report on intrarectal injection of arsphenamine but did not refer to its use in the form of suppositories. In a comprehensive report, on the “Treatment of Syphilis” (Quarterly Journal of Medicine, July, 1917) L. W. Harrison stated that arsphenamine (Salvarsan) in the shape of an enema is definitely less effective than intravenously and that “Neisser and the vast majority of workers can see no value in the rectal method.” Schamberg and Hirschler (A Safe and Efficient Intensive Method of Treating Syphilis, Therapeutic Gazette, November, 1919, p. 761) have given a rather thorough trial of this method; the results were most disappointing: “A certain or rather uncertain amount of arsphenamine is absorbed into the blood, but the quantity is obviously too small to be at all comparable in its effect with the intravenous administration. Our conclusions are that the rectal administration of arsphenamine or neoarsphenamine is an extremely feeble method of administering these drugs.”
The report of the Special Committee on the Manufacture, Biological History and Clinical Administration of Salvarsan and Other Substances of the British National Health Insurance Medical Research Committee contains the following: “The rectal method of administration, either in the form of solution or as suppositories, has been advocated by a few observers mainly for cases in which there is difficulty in the adoption of the intravenous method. The experiments made by Mills at Rochester Row show that three enemata of ‘606’ (0.6 Gm. in each) on successive days failed to produce any effect on the spironemes in the lesions. The general opinion of experienced workers is that the rectal method is ineffective, and in this view the Committee concur.”—(From The Journal A. M. A., Oct. 30, 1920.)
The Council has authorized publication of the following report.
W. A. Puckner, Secretary.
Hypodermic Solution No. 13, Iron, Arsenic and Phosphorus Compound (Burdick-Abel Laboratory) is said to contain in each c.c.:
Ferrous citrate | 0.06 | Gm. |
Sodium cacodylate | 0.06 | Gm. |
Sodium glycerophosphate | 0.1 | Gm. |
Chloretone | 0.005 | Gm. |
The preparation is advertised as “the old reliable hematinic” which is “indicated in all forms of anemia, where both red and white cells are low.” It is for hypodermic or intramuscular administration. The product is inadmissible to New and Nonofficial Remedies because:
1. It does not contain ferrous citrate as claimed. Instead the iron is in the ferric condition, apparently in the form of the unofficial and unstandardized “iron citrate green” for which there is no evidence of superiority over the official iron and ammonium citrate.134
2. Its name gives no information on the form in which the iron, the arsenic and the phosphorus occur therein. The term “arsenic” does not indicate whether the mild cacodylate or the potent arsenous oxid is being administered nor does the term “phosphorus” tell the physician that he is administering the practically inert sodium glycerophosphate.135—(From The Journal A. M. A., Nov. 13, 1920.)
The Council has authorized publication of the following report.
W. A. Puckner, Secretary.
The local anesthetic ethyl paraminobenzoate was first introduced as “Anesthesin” or “Anæsthesin.” Ethyl paraminobenzoate is not patented in the United States and it may be manufactured, therefore, by any firm which chooses to do so. In order that a common name by which to designate the drug might be available, the Council coined the name “Benzocaine,” as being short and easily remembered, but yet suggestive of its composition and character (“benzo” to indicate its derivation from benzoic acid and “caine” to indicate its cocaine-like properties). As the term “anesthesin” had become a common name for the drug, the Council recognized this as a synonym for benzocaine.
One of the accepted brands for benzocaine is “Anesthesin,” manufactured by the H. A. Metz Laboratories, Inc. (see New and Nonofficial Remedies, 1920, p. 33). However, on April 19, 1920, the Metz Laboratories requested that its product be recognized under the designation of “Parathesin.” As the use of one substance under several names causes confusion and retards rational therapeutics, the Council’s rules provide against the recognition of proprietary names for nonproprietary, established drugs. In view of this and because the legitimate interests of the manufacturer may be safeguarded by appending his name or initials to the common name, benzocaine or anesthesin, the Council voted not to recognize the designation “Parathesin.”—(From The Journal A. M. A., Nov. 13, 1920.)
The condensed report on Chlorlyptus which follows and also a complete detailed report was sent to the proprietor, Jan. 9, 1920. In reply he requested that publication be postponed pending the submission of further clinical evidence. As after nine months this evidence had not been received the Council has authorized publication of its report.
W. A. Puckner, Secretary.
Chlorlyptus is manufactured by Chas. A. Weeks, trading as the Weeks Chemical Company, Philadelphia. It is prepared by chlorinating eucalyptus oil until it has bound 30 per cent. of chlorin, the chlorin being in relatively stable combination. It is claimed that Chlorlyptus is a new “chlorinated antiseptic,” highly efficient as a wound antiseptic and at the same time nonirritant and nontoxic. Chlorlyptus is offered for use in the treatment of local infections of all types, as well as of burns, and also as an antiseptic in the alimentary and genito-urinary tracts.
The claims were based largely on reports of investigations made by Philip B. Hawk and his collaborators. These reports the referee of the committee in charge of Chlorlyptus considered incomplete and unconvincing. Being advised of this Mr. Weeks caused further investigations to be made. Some of the information was checked and extended by the A. M. A. Chemical Laboratory and by the referee.
The laboratory side of the investigation may now be considered as complete. The results show that Chlorlyptus is a feeble antiseptic of the aromatic oil type, considerably weaker than eucalyptus oil, both as to therapeutic and toxic qualities. The chlorin contained in it is bound too firmly to have any action; in fact, the chlorination appears to have accomplished nothing more than a considerable destruction or weakening of the eucalyptus oil. As far as the referee can judge, this object could have been accomplished just as effectively by diluting ordinary eucalyptus oil with some indifferent solvent.
The manufacturer of Chlorlyptus contends that if the experimental findings are against his product, it should be judged by the clinical data. The clinical evidence, however, is not decisive. It shows that wounds healed and infections were prevented or successfully combated in cases in which Chlorlyptus was used in combination with good surgery, but it does not show how much of the result was due to the surgery and how much, if any, to the use of Chlorlyptus. Even if it were granted as probable that the Chlorlyptus contributed to the favorable outcome, it would still be a question whether it equals other established antiseptics, or whether it possesses any material advantages over diluted eucalyptus oil. Until these points are established the clinical reports cannot offset the unfavorable results of the laboratory investigation.
The manufacturer has endeavored to obtain more convincing clinical reports, but the lack of success in this direction during the past nine months gives little encouragement that acceptable clinical evidence will be available within a reasonable time.
Believing that the information which has been obtained should be made available to the profession, the Council authorized publication of this statement and also of the detailed report. The Council voted not to accept Chlorlyptus for New and Nonofficial Remedies because of the unfavorable results of the laboratory investigation, but with the agreement that the product would receive further consideration should more convincing clinical data become available.
Chlorlyptus is prepared by chlorinating eucalyptus oil until it has bound 30 per cent. of chlorin. “Chlorlyptol” is prepared in an analogous manner from eucalyptol. There has been some confusion as to the composition; but the principal constituent is now stated to be “a dichloride of eucalyptus oil,” to which the formula C10H16OCl2 has been assigned. It differs from the “chlorinated eucalyptus oil,” as ordinarily used for making dichloramin-T solutions, and which contains only 2⁄3 per cent. of chlorin.
The chlorin content of chlorlyptus is almost entirely firmly bound, and therefore not “available,” in contrast to the group of so-called chlorinated antiseptics (i. e., the hypochlorite and chloramin type). For instance, it does not directly liberate iodin from iodid. It contains a very small quantity of free hydrochloric acid, or perhaps some acid esters, and liberates a little more on prolonged contact with water; but the total quantity liberated under reasonable conditions is very small. According to Hawk’s data, they correspond only to 1⁄8 per cent. HCl even after standing with water overnight and to only 1⁄5 per cent. of HCl after two weeks. The referee has shown that this quantity of acid has no therapeutic significance.
The “bound” chlorin of chlorlyptus, being chemically inactive, would have no more practical significance than the bound chlorin in common salt. The “ozone” said to be used during the preparation, to expel the HCl, has also practically disappeared, to judge by the slowness with which iodin is liberated from potassium iodid.
Some constituents of chlorlyptus hydrolyze slowly and to a slight degree with the liberation of a trace of free hydrochloric acid. According to the data of Hawk’s report, the free acidity, in term of HCl, is 1⁄12 per cent. On standing with water over night, this increases to 1⁄8 per cent.
On this basis, Hawk proposed a theory that the claimed antiseptic effects of chlorlyptus are due to the continuous liberation of hydrochloric acid.
Experiments by the referee show this to be untenable. The traces of acid are neutralized and absorbed by the tissues so rapidly that an acid reaction is not maintained. These experiments are described in the appendix.
They were submitted to the manufacturers, who in the name of Mr. Weeks (May 9, 1919) concede this conclusion and state that “there is no doubt that the referee’s statements as to action in mouth, contact with living tissue and improbability that the acidity is effectively antiseptic is correct, and I am willing to accept the referee’s statement as conclusive in this respect.”
Mr. Weeks submitted a statement by Hawk to the effect that chlorlyptus has a phenol coefficient of 2.6, determined by the standard Hygienic Laboratory procedure.
He also quotes Rockefeller War Hospital that chlorlyptus kills Staphylococcus aureus in concentra of 1 dram: 1 gallon (about 1:1,000), but not in more dilute solutions.
More recently, he presented a more comprehensive report by Rivas, which is reproduced in the appendix. The essential results are tabulated herewith. This tabulation shows that chlorlyptus fails to kill the organisms after an hour’s exposure of the following concentrations:
Typhoid in bouillon, 10 per cent. of chlorlyptus.
Staphylococci in pus, 5 per cent. of chlorlyptus.
Staphylococci in serum, 1 per cent. of chlorlyptus.
It seems to the referee that a substance that is ineffective with an hour’s exposure to these concentrations is not at all likely to kill or check bacteria under clinical conditions. In other words, it is not an antiseptic in the ordinary sense.
The referee is not impressed by the superior power attributed by Rivas to chlorlyptus in the presence of pus. Inefficiency of 10 per cent. for one-half hour or of 5 per cent. for two hours seems a failure rather than a success. The referee also notes the absence of any data as to the relative efficiency of chlorlyptus against staphylococci in pus and in bouillon. The data on serum indicate that chlorlyptus is much weaker than phenol and show that it is less effective in the presence of pus than in other mediums.
The referee fails to grasp the bearing of the oil experiments on any clinical condition. Moreover, the inconstant results mentioned by Rivas suggest the possibility that the incorporation of the bacteria in oil may have prevented their effective distribution in the culture medium. If any significance is to be attached to these experiments, they should be checked by controls, without antiseptics.
SUMMARY OF RIVAS’ IN VITRO EXPERIMENTS
| Minimal Germicidal Concentrations | Maximal Not Germicidal Concentrations | |
| Typhoid Bacilli in Bouillon: | ||
| Chlorlyptus (Exp. 3) | 10%, 2 to 4 hours | 10% for 1 hour |
| 5% for 2 hours | ||
| Eucalyptus oil (Exp. 1) | 5% within 5 minutes | No data |
| Phenol (Exp. 5) | 1% within 10 min. | No data |
| Streptococci and Staphylococci in Olive Oil: | ||
| Chlorlyptus (Exps. 7 and 8) | 1%, almost at once, sometimes | No data |
| Eucalyptus oil | No data | No data |
| Phenol (Exps. 9 and 10) | 1%, almost at once, | No data |
| Staphylococci in Pus: | ||
| Chlorlyptus (Exp. 11) | 10% for 1 hour | 10% for 1⁄2 hour |
| 5% for 2 hours | ||
| Eucalyptus oil | No data | No data |
| Phenol | No data | No data |
| Staphylococci in Human Blood Serum: | ||
| Chlorlyptus (Exp. 12) | 5% in 1 hour | 1% in 1 hour |
| Eucalyptus oil | No data | No data |
| Phenol | 5% almost at once | 1% in 1 hour |
Dr. Rivas reports two series of experiments, in each of which three guinea-pigs received staphylococcus suspensions in the peritoneum. One guinea-pig in each series was left untreated; the others received injections of chlorlyptus into the peritoneum at various intervals.
The following results were obtained:
| Chlorlyptus | Results | |
| Exp. 19, No. 1 | None | Survived |
| Exp. 20, No. 1 | None | Died |
| Exp. 19, No. 2 | At once | Died |
| Exp. 19, No. 3 | After 24 hours | Survived |
| Exp. 20, No. 2 | After 18 hours | Died |
| Exp. 20, No. 3 | After 24 hours | Died |
This shows mortalities of:
1 in 2, i. e., 50 per cent., without chlorlyptus.
3 in 4, i. e., 75 per cent., with chlorlyptus.
It is doubtful whether so small a series of experiments on so variable a phenomenon as is infection should receive any serious consideration. So far as they go, they would indicate that chlorlyptus is useless or worse.
The referee determined the acute toxicity of chlorlyptus by hypodermic injection of oily solutions into white rats. Comparative experiments were made with ordinary eucalyptus oil. The details are given in the appendix. The end-results may be summarized as follows:
| Survived | Chlorlyptus | Eucalyptus Oil |
| 1.56 c.c. | ||
| 3.75 c.c. | ||
| 5.00 c.c. | ||
| 6.25 c.c. | 1.25 c.c. | |
| 8.65 c.c. | 2.5 c.c. (3 days) | |
| Died (in days) | 12.5 c.c. (1 day) | 3.75 c.c. (3 days) |
| 12.5 c.c. (1 day) | 5.00 c.c. (3 days) | |
| 18.75 c.c. (1 day) | 6.25 c.c. (11⁄2 days) | |
| M. F. D. | 8.75 to 12.5 c.c. per kg. | 1.25 to 2.5 c.c. per kg. |
Fatality.—The doses are calculated for cubic centimeters of the undiluted drugs per kilogram of rat.
Dr. Rivas reports a series of toxicity experiments on guinea-pigs. Assuming a uniform weight of 400 gm. per animal, his results (details in appendix) may be summarized as:
| Minimal Fatal Dose C.c. per Kg. | Maximal Survived Dose C.c. per Kg. | |
Chlorlyptus, peritoneal (Exp. 14) | 7.5 c.c. | 5.0 c.c. |
Chlorlyptus, pleural (Exp. 15) | 5.0 c.c. | 2.5 c.c. |
Eucalyptus oil, peritoneal (Exp. 16) | 2.5 c.c. | No Data |
Eucalyptus oil, pleural (Exp. 16) | 1.25 c.c. | No Data |
Dichloramin-T, peritoneal (Exp. 16) | 1.25 c.c. | No Data |
The comparative toxicity in the various series is therefore approximately as follows:
| Chlorlyptus | : | Eucalyptus | |
Referee, rats, hypodermic | 1⁄5 | : | 1 |
Rivas guinea-pig, peritoneal | 1⁄3 | : | 1 |
Rivas guinea-pig, pleural | 1⁄4 | : | 1 |
Evidently, the toxicity of chlorlyptus is about one-fourth of that of eucalyptus oil. The difference is considerable, but not fundamental. Moreover, the symptoms of chlorlyptus resemble the characteristics of eucalyptus oil.
According to the tabulation of Barker and Rowntree,136 the mean fatal dose of eucalyptus oil for man, in the twenty-nine clinical cases reported in the literature, is about 20 c.c. If the toxicity ratio of the two substances were the same as for the rat experiments (a rather hazardous assumption), the fatal dose of chlorlyptus for man would be about 80 c.c.
Rivas’s Experiment 14 shows that chlorlyptus gives very definite irritation, apparently similar to that produced in Experiment 16 by eucalyptus oil in one-fourth the dose.
Incidentally, the referee may add from personal experience that the “chlorlyptus oil, 5 per cent. Cl” is markedly irritating in the nostrils, although marked “non-irritating” on the label.
According to the label, “Chlorlyptus” is a “Synthatized Chlorinated Oil of Eucalyptos, with Acid Reaction, containing approximately 30 per cent. Chlorine and possesses excellent Germicidal Properties, when made under our special process.” It is manufactured by the Weeks Chemical Company, Philadelphia, Pa. This product was submitted to the Council on Pharmacy and Chemistry by the manufacturers, and in turn the Laboratory was asked to examine it with the idea of comparing it with the nonproprietary brands of “chlorinated eucalyptol” (used as a solvent for dichloramine-T; see New and Nonofficial Remedies, 1919, p. 70). In the submission, certain tests were described, most of which were followed. Among the statements given under the chemical properties of chlorlyptus are:
“On distillation, chlorlyptus begins to boil at about 100 C. The temperature rises as the distillation continues, accompanied by the decomposition of the chlorlyptus and the evolution of hydrochloric acid and chlorine.”
“When brought into contact with water, chlorlyptus undergoes a process of hydrolysis ...”
Notwithstanding the foregoing the statement is made on the label that chlorlyptus “is a Stable Compound, not affected by heat, light or water.”
The following comparisons of chlorlyptus, chlorinated eucalyptol-Abbott and chlorinated eucalyptol-Squibb were made:
Chlorlyptus is a viscous, dark brown liquid, with an acrid odor and having a specific gravity of 1.2098. Chlorinated eucalyptol-Abbott is a mobile, light yellow liquid, with a eucalyptus odor, having a specific gravity of 0.9317. Chlorinated eucalyptol-Squibb is a mobile, colorless liquid, and its specific gravity is 0.9303.
An alcoholic solution of silver nitrate added to an alcoholic solution of chlorlyptus yields a heavy precipitate of silver chloride. In the case of the Abbott chlorinated eucalyptol a slight turbidity is caused by this test; the Squibb product shows no reaction.
A 10 per cent. solution of potassium iodide is overlaid with an equal volume of chlorlyptus. Iodine is slowly liberated, being noticeable in one-half hour. With chlorinated eucalyptol-Abbott, a trace of free iodine is discernible after four hours, while with chlorinated eucalyptol-Squibb there is no free iodine present. When the respective products are shaken with an alcoholic solution of potassium iodide, no iodine is immediately liberated, thus showing the absence of “active chlorine” (difference from the hypochlorite derivatives).
When chlorlyptus is dissolved in concentrated sulphuric acid, some blackening occurs and the odor of hydrogen chloride is very noticeable. Both the Abbott and Squibb brands of chlorinated eucalyptol give a reddish mixture, with no perceptible evolution of hydrogen chloride, and still retain the characteristic eucalyptol odor.
On heating, chlorlyptus decomposes and begins to boil at from 103 to 105 C. Then a higher fraction comes over at 178 C. The distillate has a sharp odor, is acid, and frees very little iodine from potassium iodide. Chlorinated eucalyptol-Abbott does not seem to decompose. Some gaseous substance is given off at 80 C, but the liquid distills at 173 C. The distillate has no acid odor, is neutral, and liberates no iodine from potassium iodide. (In both cases the distillation was not carried to completion, approximately only about half of the volume being distilled over.)
PRELIMINARY TESTS ON CHLORLYPTUS AND CHLORINATED EUCALYPTOL
| Chlorlyptus | Chlorinated Eucalyptol-Abbot | Chlorinated Eucalyptol-Squibb | |
| Odor | Acrid | Like eucalyptus | Like eucalyptus |
| Density and color | Dark brown; viscous, heavier than water | Light yellow; mobile; lighter than water | Colorless; mobile; lighter than water |
| AgNO3 added to alcoholic solution | Heavy ppt. | Slight turbidity | Clear |
| Equal parts with KI solution | Gives free iodin slowly, noticeable in 1⁄2 hour | Gives free iodin in 4 hours; not much | No free iodin in 4 hours |
| Equal parts with 10% KI, 10% KIO3 solution | Much iodin immediately | Small amount of free iodin in few numbers; does not noticeably increase | No free iodin in 3 hours |
| Equal parts with conc. H2SO4 | Some blackening; odor of HCl | Reddish mixture; no HCl; eucalyptol odor | Same |
| Alcohol KI | No iodin liberated | Same | Same as Abbott product |
| Heating | Decomposes and boils at 103–105 C.; then higher fraction comes over at 178 C.; distillate has sharp odor, is acid, but frees very little I2 from KI; distillation not completed | Apparently does not decompose; some gas given off when T=80; the liquid distilled at 173 C.; the distillate did not have much odor; no HCl gas detected; no I2 from KI; distillate was neutral (distillation not completed) |
The addition of chlorlyptus to a mixture of 10 per cent. potassium iodide, 10 per cent. potassium iodate solution, brings about the liberation of iodine, increasing perceptibly on standing. This shows that the hydrogen chloride is gradually split off, and in time will cause a solution having a considerable degree of acidity. When this test is carried out on chlorinated eucalyptol-Abbott, a small amount of iodine is liberated in a few minutes but does not increase, showing a slight initial acidity without further hydrolysis. Chlorinated eucalyptol-Squibb yields no free iodine after standing three hours.
When the chlorine content of chlorlyptus is determined according to the method of Carius, the amount is found to be 29.6 per cent. (The manufacturers give a method of determining chlorine by Hunter’s fusion method. It is believed that in this method hydrogen chloride may be lost, and this opinion is substantiated by the firm’s statement, “Chlorlyptus analyzed in this manner shows approximately 25 per cent. of chlorine.”) The chlorine content of chlorinated eucalyptol-Abbott is found to be 0.67 per cent., and that of the Squibb brand to be 0.62 per cent. (about one-fiftieth as much as in chlorlyptus).
To sum up: Chlorlyptus differs from chlorinated eucalyptol in odor, color, density, in reaction to silver nitrate, potassium iodide, sulphuric acid and the aqueous solution of potassium iodate and potassium iodide. The distillation of the two products occurs differently. Chlorlyptus contains nearly 30 per cent. of chlorine, which is approximately fifty times as much as in chlorinated eucalyptol. Thus it appears to have considerable chlorine in the negative form (Cl-) which may be relatively easily split off as hydrogen chloride.
This “chlorinated ozonized eucalyptus oil” is distinctly acid to litmus paper. It is claimed that further quantities of acid are liberated on contact with water. This is credited with producing a continuous acid reaction on the surface of tissues to which the oil may be applied and this in turn is stated to be antiseptic or germicidal.
This theoretical speculation does not take into account the large quantity of reserve alkali in the body by which it combats attempts to alter its normal reaction. It is therefore not convincing, unless it is supported by direct evidence.
In the absence of such data on the part of the promoters of the preparation, experiments were made to determine whether the oil preserves its acid reaction in contact with mucous and serous membranes. The answers were clearly in the negative.
In the mouth, the reaction becomes neutral within ten or fifteen minutes; in the pleura and peritoneum within half an hour, and probably in much shorter periods.
More detailed data follow:
Experiment.—Chlorlyptus and to less extent Chlorlyptus Oil, are acid to litmus. They are applied:
(a) Drop to litmus paper and this to gums.
(b) Several drops directly to tongue.
(c) Same to gums.
The reaction to litmus paper is tried from time to time.
Results.—(a) Applied to gums on litmus paper:
Chlorlyptus: Red color becomes gradually feebler and does not spread on
the paper.
Chlorlyptus Oil: Turns blue in a few minutes.
(b) Dropped on tongue:
Chlorlyptus: Acid taste at once. Does not increase, but on contrary, becomes
less.
Litmus applied after ten minutes: not acid.
Litmus applied after five minutes: distinctly acid.
(c) Dropped on inside of cheek:
Chlorlyptus, 1⁄3 c.c.: After six minutes, litmus very red.
After ten minutes, faintly red.
After fifteen minutes, blue.
Chlorlyptus Oil, 1 c.c.
After three minutes, faintly red.
After eight minutes, neutral.
Conclusions.—On contact with living tissues, the acid of chlorlyptus is rapidly neutralized and absorbed.
The surface is neutral within ten or fifteen minutes.
It is therefore very improbable that the acidity is effectively antiseptic.
A comparison of chlorlyptus with dilute acetic acid shows that the chlorlyptus does not maintain the acidity even as well as 1 per cent. acetic acid.
| Acetic Acid | Chlorlyptus |
| Tongue, a drop of 5 per cent.; still slightly acid to litmus after ten minutes; taste almost gone in two minutes | Neutral between five and ten minutes |
| Gums, a few drops between cheeks and gums: Five per cent. still strongly acid in twelve minutes; distinctly acid in seventeen minutes. One per cent. still strongly acid in twenty-one minutes | Neutral between ten and fifteen minutes |
CHLORLYPTUS: REACTION (LITMUS PAPER) ON CONTACT WITH TISSUE
| Serial No. | Animal | When Injected | Quantity, C.c. | Time of Death | Blue Litmus | Symptoms or Toxicity |
| 1 | Rat | Pleura | 1 | 1⁄2 hour | Remains blue | None; killed; pleura not congested; lung spec. = 21; slight congestion |
| 2 | Rat | Pleura | Less than 1 | 1 hour | Remains blue | Negative |
| 3 | Rat | Pleura | 1 | 23 min. | Remains blue | Almost at once bad gasping respiration and died in 23 m.; heart distend.; possibly injection penetrated lung |
| Peritoneum | 1 | 23 min. | Turns red | |||
| 4 | Rabbit | Pleura | 1 | ........ | ........ | Died overnight |
| 5 | Dog | Pleura | 1 | 1⁄4 hour | Remains blue 20 m. p. m. | |
| Peritoneum | 1 | 1⁄4 hour | Remains blue 20 m. p. m. | |||
| 6 | Dog | Pleura | 1 | 3 min. | Remains blue 45 m. p. m. | |
| Peritoneum | 1 | 3 min. | Remains blue 45 m. p. m. | |||
| 7 | Dog | Pleura | 1 | 20 min. | Remains blue 20 m. p. m. | |
| Peritoneum | 1 | 20 min. | Remains blue 20 m. p. m. |
In these experiments, 1 c.c. of chlorlyptus was injected into the pleura or peritoneum. After a stated time, the animal was killed, and the reaction of the pleural or peritoneal surface was tested with blue litmus paper. The results are shown in the table.
White rats were injected hypodermically with chlorlyptus or with eucalyptus oil, diluted with olive oil in the ratio of 1:4. The larger doses were divided between two or more sites of injection.
Hypodermic injections in white rats. Drugs diluted with 3 parts of olive oil. Doses are given as cubic centimeters of pure drug per kilogram of rat.
Experiment 1.—1.25 c.c.; injected VII.9.19: Active; walks about. No depression at any time. VII.10.19. Appears normal.
Experiment 2.—2.5 c.c.; injected VI.30.19: Quiet—not very depressed, reflexes good (six
hours).
VII.1.19—Active—reflexes good, eats moderately.
VII.2.19—Animal acts normal—eats moderately, reflexes good; active (a.m.). Later in
day, depressed.
VII.4.19—Died during night of VII.3.19.
Experiment 3.—3.75 c.c.; injected VI.24.19: Quiet; depressed; pain reflex diminished.
Animal lay on ventral surface, not supported by legs. Will get on to feet very sluggishly if
turned on side (twenty-four hours). Does not eat.
VI.26.19—Depressed slightly; pain reflex present.
VI.27.19—Fairly active; eats a little.
VI.28.19—Depressed.
Died during night of VI.29.19 (three days).
Experiment 4.—5 c.c.; injected VI.24.19: Quiet; markedly depressed (one hour). Does
not get on feet when turned on side; ataxia well marked. Slight watery secretion in eyes.
Reflexes diminished. Does not eat (twenty-four hours).
VI.26.19—Heart slowed and arrhythmic. Animal lies on side. Unable to walk; markedly
depressed.
VI.27.19—Lies on side; does not eat. Died during night of VI.27.19 (three days).
Experiment 5.—6.25 c.c.; injected VI.24.19: Quiet; very markedly depressed. Heart and
respiration greatly slowed. Lies on side; tears in eyes; does not eat (twenty-four hours).
VI.25.19—Temperature subnormal; cold to touch; tail stiffened and straight.
Died during night of VI.25.19 (one and one-half days).
Postmortem: Lungs congested. Liver pale in color. Spleen very dark red. Kidneys
normal. Other organs normal.
Experiment 1.—1.56 c.c.; injected VI.24.19: Rather restless for an hour. Active during next four hours and following twenty-four. Eats well, reflexes good. Acts normal on VII.1.19 and since VI.26.19.
Experiment 2.—3.75 c.c.; injected VI.24.19: More quiet; active during next twenty-four hours. Reflex all right. Eats well; normal VII.1.19, since VI.26.19.
Experiment 3.—5 c.c.; injected VI.24.19: Quiet; defecation in four hours. Rather quiet for six hours. Eats well. Reflexes good; normal VII.1.19, since VI.26.19.
Experiment 4.—6.25 c.c.; injected VI.24.19: Quiet and breathing labored in four hours; active after twenty-four hours. Eats well. Somewhat depressed on VI.26.19; pain reflex present. On VI.26.19, eats well and fairly active. Active and eats, VI.27.19. Appears normal, VII.1.19.
Experiment 5.—8.75 c.c.; injected VI.30.19: Rather quiet during next two hours. Morning of VII.1.19, lies on stomach; quiet; does not eat very much. Pain reflexes good. VII.2.19, still depressed; does not eat. Appears normal, VII.3.19.
Experiment 6.—12.5 c.c.; injected VI.25.19: Quiet, but reflexes good; more quiet and
depressed after several hours. Some loss of oil from wound. Died night of VI.25.19 (one
day). Tail stiff. Temperature low.
Postmortem: Lungs markedly congested. Spleen and liver dark red. One kidney congested.
Other viscera normal.
Experiment 7.—12.5 c.c.; injected VII.9.19: Quiet for one-half hour; 1.5 hours twitching of muscles of whole body, lies on side, ataxia present. Died night of VII.9.19 (one day).
Experiment 8.—18.75 c.c.; injected VI.25.19: Quiet; reflexes good (three hours). Some
loss of oil. Depressed and turns on side (six hours). Died night of VI.25.19 (one day).
Postmortem: Lungs congested. Spleen and liver very dark red. Right kidney much
darker red. Viscera normal.
The following are the results of experiments conducted by me, during the past four months, on the germicidal action of chlorlyptus (chlorinated oil of eucalyptus, principal constituent C10H17OCl2) in vitro and in vivo, and comparison also with carbolic acid, oil of eucalyptus and dichloramine in test for irritation and toxicity.
Germicidal Action.—Based on the results obtained, chlorlyptus when used in a 5 per cent. paraffin oil solution was found to be a mild germicidal against typhoid B, streptococcus and staphylococcus when these organisms were suspended in ordinary bouillon culture or sterile salt solutions.
The germicidal action was found stronger when these micro-organisms were suspended in a sterile oily or lipoid substance, such as olive oil. The results of these experiments were not constant, owing probably to the imperfect suspension of the bacteria. Thus, while in some of the experiments chlorlyptus in 1 per cent. oil solution destroyed these micro-organisms, in other cases the same strength solution failed to give same result in same time.
The increased germicidal action of chlorlyptus on bacterial suspensions in olive oil may be accounted for by the fact that chlorlyptus is soluble in olive oil and not an admixture, as in the case of paraffin oil.
Chlorlyptus is not a coagulant, as are germicides of the phenol or hypochlorite types, and the germicidal action is therefore not strictly comparable.
The germicidal action of chlorlyptus oil solution, on pathogenic bacteria, on streptococcus and staphylococcus, suspended in pus, was found to be stronger than when these micro-organisms were suspended in ordinary bouillon culture or sterile salt solution. In one of the experiments, similar results were obtained when these micro-organisms were suspended in olive oil, chlorlyptus showing marked germicidal action.
Irritation and Toxicity.—The irritating action was found to be relatively mild in tests on laboratory animals. Thus, from 0.5 to 1 c.c. of chlorlyptus in paraffin oil 5 per cent. solution, injected into peritoneal or pleural cavities of guinea-pigs weighing 400 gm. was found to be without any appreciable disturbance in the health of the animal, and in some cases the injection of as much as 2 c.c. did not kill the animal.
Therapeutic Action.—Guinea-pigs were inoculated with purulent material containing streptococcus, staphylococcus and B. coli in peritoneal and pleural cavities respectively, and after six hours 1 c.c. of chlorlyptus 5 per cent. in paraffin oil solution was injected. Other infected animals were similarly treated twenty-four hours after inoculation, and another series forty-eight hours after inoculation. In some of these cases the animals died from shock but in a clearly defined series in which the injection of 1 c.c. of the chlorlyptus solution was made in the peritoneum of the guinea-pigs twenty-four hours after the inoculation, the animals lived. The control animal, inoculated with the purulent material and not treated with chlorlyptus oil solution, died.
In consideration that the injection of chlorlyptus oil solution [sic, referee] were made [? referee] in the peritoneal cavity this substance is apt to affect the vital organs in the abdominal cavity. It is my belief that in case of wall abscess of chronic inflammation, by limiting the action of chlorlyptus to the infected area, preventing at the same time the infection of the vital organs, chlorlyptus, because of its non-irritating quality, can be used effectively as an antiseptic.
1. Chlorlyptus is a mild and relatively nonirritating antiseptic of marked action on pus and suppuration.
2. When bacteria were suspended in olive oil or in pus, chlorlyptus showed marked germicidal action.
3. Chlorlyptus can be injected into the peritoneum or the pleural cavities of guinea-pigs in the proportion of 1 c.c. per 400 gm. of body weight without detriment to the animal.
4. Chlorlyptus in 5 per cent. oil solution (taking Clause 3 as comparison) can perhaps be injected in man as an antiseptic agent when there is a walled-in abscess in the peritoneum or pleural cavity where there is drainage, in the proportion of 0.5 to 1 c.c. per pound of body weight with good result.
Experiment 1.—The germicidal action of eucalyptus oil.—Typhoid bacillus was destroyed in less than five minutes when exposed to the action of a 5 per cent. suspension of oil of eucalyptus. The exposure for four hours in a 5 per cent. suspension of chlorlyptus in paraffin oil was without effect on typhoid bacillus. It requires an exposure of two to four hours in a 10 per cent. suspension of chlorlyptus in paraffin oil to destroy typhoid bacillus.
Experiment 2.—Bacilliary action of chlorlyptus on the growth of pathogenic bacteria.—Typhoid
and anthrax bacilli were selected for the experiment. Two series of five tubes each
were made. The culture medium used was nutrient bouillon. Chlorlyptus was added in the
following proportions: Tube 1, 1:10; Tube 2, 1:100; Tube 3, 1:1,000; Tube 4, 1:10,000, and
Tube 5, 1:100,000. One series was inoculated with typhoid bacillus. All tubes were incubated
for three days at 37 C.
Chlorlyptus inhibited the growth of typhoid bacillus when added to the bouillon in the proportions
of 1:10. The growth of anthrax bacillus was inhibited by chlorlyptus when it was
added in the proportions of 1:10, 1:100 and 1:1,000, as shown in the accompanying table.
[The table was not submitted.—Ed.] In one instance the growth was markedly inhibited by
chlorlyptus when added in the proportion of 1:10,000.
Experiment 3.—Germicidal action of chlorlyptus on typhoid bacillus.—Bouillon cultures of
typhoid bacillus forty-eight hours old, and a suspension of forty-eight-hour agar cultures of
typhoid bacillus in sterile salt solution were used for the experiment. Chlorlyptus was added
in the proportion of 1:1,000; 1:1,500; 1:100; 2 per cent.; 3 per cent.; 4 per cent.; 5 per
cent. and 10 per cent., respectively.
Inoculations were made in trypsinized peptone bouillon after the addition of chlorlyptus
at different intervals, namely: at once, after five minutes, after ten minutes, after fifteen
minutes, after thirty minutes, after one hour and after two hours, and tubes incubated at
37 C. for forty-eight hours.
Result: Growth was shown in all tubes except those in which chlorlyptus was added in the
proportion of 10 per cent. and after the action of the antiseptic for two hours or longer.
Experiment 4.—Inhibitory action of chlorlyptus in the growth of typhoid bacillus.—Chlorlyptus
was added to sterile bouillon in the proportion of 1:100, 1:1,000, 1:10,000 and
1:100,000, and incubated for forty-eight hours at 37 C. to eliminate any possible contamination
of the bouillon during the manipulations. All tubes were found sterile and inoculated with
typhoid bacillus.
Result: All tubes were found sterile again after being inoculated with typhoid bacillus and
incubated at 37 C. for forty-eight hours, which shows chlorlyptus inhibited and the growth of
typhoid bacillus in bouillon when this antiseptic was added in the proportions of 1:100 to
1:100,000.
Remarks: In another experiment made, chlorlyptus showed a weaker inhibitory action on
the growth of typhoid bacillus.
Experiment 5.—Germicidal action of carbolic acid.—The technic was the same as that outlined
in Experiment 1. except that carbolic acid was used instead of chlorlyptus.
Result: Carbolic acid showed a distinct germicidal action on typhoid bacillus in the proportions
of 1 per cent. in ten minutes.
Experiment 6.—Action of nitrogen gas on the growth of typhoid bacillus in bouillon and
nutrient agar when chlorlyptus was added to this culture medium.—Chlorlyptus was added to
the bouillon in the proportions of 1:100, 1:1,000, 1:10,000 and 1:100,000, as outlined in
Experiment 2; also to agar kept melted at 45 C. Tubes were inoculated with typhoid bacillus;
plates were made of the inoculated agar tubes; all plates and tubes were incubated at 37 C.
for forty-eight hours in an atmosphere of nitrogen gas.
Duplicate experiments were made with cultures of typhoid bacillus as above in bouillon and
agar plates containing the same amount of chlorlyptus and incubated at 37 C. in ordinary
atmosphere as control.
Result: Nitrogen gas did not show any appreciable increase of the germicidal action of
typhoid bacillus when grown in medium containing chlorlyptus. Growth was about the same
in cultures supplied with nitrogen gas as in those growing in ordinary atmosphere.
Experiment 7.—Germicidal action of chlorlyptus on pyogenic bacteria suspended in an oily
medium.—Experiment with streptococcus: Cultures of streptococcus in blood agar three days
old were suspended in olive oil (sterile), and chlorlyptus was added in the proportions of
1, 5 and 10 per cent. and inoculated in trypsinized bouillon at different intervals, namely: at
once, after five minutes, after ten minutes, after fifteen minutes, after thirty minutes, and after
one hour. Tubes were incubated at 37 C. for forty-eight hours.
Result: All tubes remained sterile. The germicidal action of chlorlyptus on streptococcus
suspended in oil was almost at once and with certainty after five minutes when added in the
proportion of 1, 5 and 10 per cent.
Experiment 8.—Germicidal action of chlorlyptus on staphylococcus, suspended in sterile
olive oil.—The technic employed was the same as in Experiment 5, except that a culture of
staphylococcus was used.
Result: All tubes remained sterile. The germicidal action of chlorlyptus was almost at
once in the proportions of 1, 5 and 10 per cent.
Remarks: By repeating this experiment the result showed some variations. The discrepancy
was probably due to an imperfect suspension of the micro-organism in the oil.
Experiment 9.—Germicidal action of carbolic acid on streptococcus suspended in olive oil.—The
technic employed was the same as in Experiment 5, except that carbolic acid was used
instead of chlorlyptus.
Result: The germicidal action of carbolic acid of streptococcus suspended in olive oil was
almost at once in the proportions of 1, 5 and 10.
Experiment 10.—Germicidal action of chlorlyptus on staphylococcus.—The technic employed
was the same as in Experiment 6 except that the carbolic acid was used instead of chlorlyptus.
Result: The germicidal action of carbolic acid on staphylococcus suspended in olive oil
was almost at once, in proportions of 1, 5 and 10 per cent.
Experiment 11.—Germicidal action of chlorlyptus on pyogenic bacteria suspended in pus.—Chlorlyptus
was added to sterile pus in the proportions of 1, 5 and 10 per cent., and then
inoculated with staphylococcus and cultures were made in bouillon at once, after five minutes,
after ten minutes, after fifteen minutes, after thirty minutes, after one hour and after two
hours, respectively, and tubes incubated for forty-eight hours at 37 C.
Result: Growth was shown in all tubes except those inoculated from tubes in which
chlorlyptus was added in the proportions of 10 per cent. after one hour.
Experiment 12.—Germicidal action of chlorlyptus on streptococcus suspended in sterile
human blood serum.—Staphylococcus culture in agar forty-eight hours old was suspended in
sterile human blood serum, and to the suspension chlorlyptus 5 per cent. in paraffin oil was
added in the proportions of 1, 5 and 10 per cent. Inoculations were made at intervals, at
once, after five minutes, after ten minutes, after fifteen minutes and after one hour in trypsinized
bouillon. Tubes were incubated at 37 C. for forty-eight hours.
Result: Chlorlyptus showed inhibitory action on the growth of staphylococcus in the
strength of 10 per cent., but did not produce complete sterilization. Similar results were
shown with the 5 per cent., and in the 1 per cent. chlorlyptus did not show any inhibitory
action at all.
Experiment 13.—Germicidal action of carbolic acid on staphylococcus suspended in human
blood serum (sterile).—The technic employed was the same as in Experiment 10 except that
carbolic acid was used instead of chlorlyptus.
Result: Carbolic acid produced a complete sterilization in the strength of 10 per cent.
almost at once, and with certainty after five minutes. Similar results were produced with the
5 per cent. The 1 per cent. carbolic acid did not show any appreciable germicidal action on
staphylococcus.
Experiment 14.—Toxic and irritant action of chlorlyptus.—Six normal guinea-pigs were
used for the experiment. Guinea-Pig 1 was injected peritoneally with 1 c.c. of chlorlyptus,
Guinea-Pig 2 with 2 c.c. of chlorlyptus, Guinea-Pig 3 with 3 c.c. of chlorlyptus, Guinea-Pig 4
with 4 c.c. and Guinea-Pig 5 with 5 c.c. 5 per cent. respectively. Guinea-Pig 6 was used as a
control and not injected.
Result: Guinea-Pigs 1 and 2 did not show any appreciable disturbance. Guinea-Pig 3 was
sick for four days, after which it gradually recovered but it became sick again after one week
and died ten days after the injection. Guinea-Pig 4 died over night. Guinea-Pig 5 died six
hours after injection. Guinea-Pig 5 was injected at 11:30 with 5 c.c. chlorlyptus. Ten minutes
after the injection it was lying relaxed, respiration and heart normal, conjunctive reflex
present. One hour after the injection the animal seemed to present symptoms resembling those
of narcosis: respiration and heart were normal. After four hours there was no change in the
condition of the guinea-pig except that the respiration was irregular. Five and a half hours
after it showed prostration with irregular respiration and heart action. Six hours after
injection the animal was dead.
Autopsy: The peritoneum showed a congestion and a fibrinous exudation, amount of liquid
increased, some part of which was probably chlorlyptus unabsorbed. Spleen about normal,
liver congested, kidney about normal, suprarenal glands about normal, lungs normal, pleural
cavity obtained no exudation, heart soft, flabby and congested.
Experiment 15.—Toxic and irritant action of chlorlyptus when injected into the pleural
cavity.—Six normal guinea-pigs used for the experiment. Chlorlyptus was injected in the
pleural cavity as follows: Guinea-Pig 1, 0.5 c.c.; Guinea-Pig 2, 1 c.c.; Guinea-Pig 3, 2 c.c.;
Guinea-Pig 4, 3 c.c., and Guinea-Pig 5, 4 c.c. Guinea-Pig 6 was used as a control.
Result: Guinea-Pigs 1 and 2 recovered about four hours after injection. Guinea-Pig 3
died three days after and Guinea-Pigs 4 and 5 four and two hours after, respectively.
Conclusions: Guinea-pigs weighing on the average of 400 gm. may be injected peritoneally
with one or two c.c. or intrapleurally with 0.5 to 1 c.c. of chlorlyptus without having fatal
results from the injection.
Experiment 16.—Toxic and irritant action of eucalyptus oil.—Three normal guinea-pigs
were used for the experiment. Guinea-Pig 1 was injected with 1 c.c. of oil of eucalyptus in
the peritoneum, and Guinea-Pig 2 with 0.5 c.c. in the pleural cavity. Guinea-Pig 3 was used
as a control.
Result: Guinea-Pig 1 died about three hours after injection, and Guinea-Pig 2 about two
hours after the injection.
Autopsy: Both guinea-pigs showed marked congestion and a moderate degree of exudate
in the peritoneum.
Experiment 17.—Toxic and virulent action of eucalyptus.—Three normal guinea-pigs were
selected for the experiment, as in Experiment 16. The injection was made in the pleural
cavity. Guinea-Pig 1 was injected with 0.5 c.c. and Guinea-Pig 2 with 1 c.c. of eucalyptus oil.
Result: Guinea-Pig 1 died the following day, and Guinea-Pig 2 one hour after the injection.
Experiment 18.—Toxic and irritant action of dichloramin-T, 0.5 per cent. in chlorcozane.—One
guinea-pig was used for each experiment. Guinea-Pig 1 was injected with 0.5 c.c. and
Guinea-Pig 2 with 1 c.c. of dichloramin-T peritoneally.
Result: Both animals became restless immediately after the injection, and died twelve hours
after of acute hemorrhagic peritonitis.
Experiment 19.—Effect of chlorlyptus on staphylococcus suspended in salt solution and
one of that solution injected into the peritoneum of the guinea-pig.—Three guinea-pigs were
used for the experiment. Guinea-Pig 1 was injected with 0.5 c.c. of staphylococcus suspension
as control. Guinea-Pig 2 was given the same, and immediately after received 1 c.c. of
chlorlyptus. Guinea-Pig 3 was injected with the same amount, and chlorlyptus was injected
twenty-four hours after injection.
Results: Guinea-Pig 1 was sick and weak with loss of appetite for some days, but gradually
recovered. Guinea-Pig 2 died over night.
Autopsy: There was a large amount of exudate in the peritoneal cavity, irritation of the
intestine, and other signs of acute inflammation. A moderate degree of congestion; spleen
not enlarged; liver showed cloudy swelling and fibrinous exudate; lungs and heart about
normal except for a moderate degree of congestion but no exudate. Guinea-Pig 3 was sick
for some days, but recovered gradually one week after.
Experiment 20.—Effect of chlorlyptus in vivo on staphylococcus.—The experiment was
conducted in the same way as in Experiment 17, but 2 c.c. were used instead of 1 c.c.
Result: Guinea-Pig 1 was injected with 2 c.c. staphylococcus suspension and died over
night. Autopsy showed that the animal died of acute peritonitis. The peritoneum showed
some fibrinous exudate and mesenteric vessels. Guinea-Pig 2 was injected with 2 c.c. of
staphylococcus, and eighteen hours after was injected with 1 c.c. of chlorlyptus. The animal
died two weeks after injection. Guinea-Pig 3 was injected with 2 c.c. staphylococcus suspension,
and twenty-four hours after with 1 c.c. of chlorlyptus. The Guinea-Pig died ten days
after. Autopsy revealed bronchopneumonia of the left lung and acute miliary abscess in the
liver.
—(From The Journal A. M. A., Nov. 27, 1920, with additions.)
Aquazone is stated by the Aquazone Laboratories, Inc., Los Angeles, California, to be a supersaturated solution of oxygen in water, carrying approximately five and one-half times as much dissolved oxygen as ordinary water. In an advertising booklet, it is suggested that Aquazone is of value in the treatment of influenza, pneumonia, typhoid, Bright’s disease and kindred disorders. It was also stated therein that in the treatment of fevers it lowers the temperature, and that the administration of three bottles of Aquazone (representing 0.033 gm.—11⁄2 grain—of oxygen) is of value for “preventive and tonic purposes.”
The evidence which the Aquazone Laboratories submitted did not show that the effects were other than those which might be obtained from the administration of ordinary potable water. The Council declared Aquazone inadmissible to New and Nonofficial Remedies, because the therapeutic claims made for it were unwarranted, and because its use is irrational for the reason that oxygen given by stomach in this way is of little or no value.—(Abstracted from Reports of Council on Pharmacy and Chemistry, 1920, p. 50.)
The Council has authorized publication of the following report announcing the deletion of Coagulen-Ciba from New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Coagulen-Ciba, a product of the Society of Chemical Industry, Basle, Switzerland, was admitted to New and Nonofficial Remedies in 1915. It is stated to be an extract prepared from blood platelets and to contain thromboplastic substances (cytozym, thrombokinase, thrombozym) mixed with lactose. Extensive clinical reports appeared to justify its acceptance for New and Nonofficial Remedies with Fibrin Ferments and Thromboplastic substances.
In 1918, Dr. Arthur D. Hirschfelder reported to the Council that of a number of specimens of Coagulen-Ciba examined by him, failed to accelerate the coagulation time of blood.
In view of Dr. Hirschfelder’s findings, the Therapeutic Research Committee of the Council invited Dr. P. J. Hanzlik to undertake an exhaustive investigation of thromboplastic substances, the Council, in the meantime temporarily retaining Coagulen in New and Nonofficial Remedies until the investigation was completed.
The following report on the eligibility of Coagulen-Ciba was made to the Council by Dr. Hanzlik:
Object: To test the claims of thromboplastic and hemostatic activities.
Claims: Coagulen is alleged to be a “physiological styptic prepared from the natural coagulants of animal food contained in the blood platelets. It has the characteristics of a lipoid.” (If cephalin is meant it is difficult to understand why platelets should be selected in preference to other abundantly supplied organs such as brains).
“Coagulen is indicated in all cases of external and internal hemorrhage due to a deficiency of the coagulating power of the blood: epistaxis, hemophilia, hemorrhage from gastric or duodenal ulcer, melaena neonatorum, hemorrhage from the gums, the lungs, the bladder, the uterus, hemorrhage during or after operations (turbinectomy, tonsillectomy). It has also been used as a prophylactic before operations, likely to produce severe hemorrhage.”
“In cases of true hemophilia one application of 5 grains of coagulen usually suffices to control the hemorrhage.” “In gastric and intestinal hemorrhage the internal administration of coagulen will be found effective.” “In bonegrafting, plastic surgery, dentistry and nose and throat surgery the application of a 10 per cent. solution of Coagulen will be found to be of valuable assistance in controlling hemorrhage and oozing.”
“It is a non-toxic and non-irritating powder to which a certain amount of sugar has been added, with a view to ensuring its prompt solution in water or physiological sodium chloride solution.”
Description: “Coagulen is a yellowish granular powder with but slight odor, a sweet taste and is readily soluble in water or a normal salt solution.” The dry Coagulen obtained corresponds to the description claimed. Old specimens show the presence of dark brown particles. Coagulen is marketed in 3 forms: (1) as dry powder containing lactose, which, it is claimed, facilitates solution in water; (2) as 3 per cent. sterile solution in ampoules;137 (3) tablets.
Methods of Study: The alleged thromboplastic activity was tested by the method of Howell and a modification of this method by Fenger as described in “New and Nonofficial Remedies.” In the Howell method dog or cat blood is used, while beef blood at body temperature is used in Fenger’s method. In other respects the methods are essentially the same. Briefly these consist of noting the acceleration of coagulation time in a mixture of equal parts of serum and the thromboplastic agent to which about an equal part of oxalate plasma is added. Under these conditions cephalin causes clotting in about 1 minute or even less as compared with 20 to 30 minutes or more of the control.
The effects were compared with freshly prepared cephalin and other thromboplastic agents, using saline (0.9 per cent. NaCl) as control. The effect of different concentrations was also studied.
The literature of the manufacturers claims that Coagulen is harmless. This was tested by making intravenous and subcutaneous injections into guinea-pigs, using saline and cephalin as controls.
Bloods of 4 different species were used, namely, cat, dog, beef and human. Dog’s peptonized blood and plasma were also tried.
The 15 different tests that were made in vitro were carried out with 3 different samples of fresh dry Coagulen (from manufacturer), 2 old samples (one from Council on Pharmacy and Chemistry and one of our own), 3 fresh specimens of sterile solution in ampoules (from manufacturer), one old specimen and 4 small ampoules (Council on Pharmacy and Chemistry).
The tablets were not tested since these are made from dry Coagulen and the results would hardly be expected to show anything different.
Results: The results obtained may be briefly summarized as follows: (1) 0.1 per cent. to 5 per cent. Coagulen did not accelerate the coagulation time of blood and oxalate plasmas in the majority of tests any more than the controls of saline, while 0.1 per cent. cephalin was found to shorten the coagulation time from 1⁄3 to 1⁄2.
(2) There was no difference between the behavior of old and fresh specimens.
(3) No acceleration of coagulation in vitro was observed even with the highest concentrations tried, namely 25 and 50 per cent.
(4) Irrigations made with fresh dry coagulen in solution and sterile solution in ampoules on superficial bleeding from the foot-pads of 3 normal and peptonized dogs and local application to hemorrhages from dissected femoral arteries and bone and liver wounds of 3 dogs showed that coagulen was no more active than normal saline.
Toxicity: Subcutaneous and intravenous injections of different doses of Coagulen solutions (fresh ampoules) and dry Coagulen in solution in 8 guinea-pigs produced definite anaphylactoid symptoms with injury to the circulatory and respiratory systems as indicated by cardiac dilatation, abdominal congestion and pulmonary hemorrhages, congestion, distention and sometimes thrombi. On the other hand, the control animals injected with saline and cephalin remained practically unharmed.
Conclusions: The results obtained justify the following conclusions:
(1) Coagulen is entirely inactive as a thromboplastic and hemostatic agent.
(2) Coagulen is distinctly injurious when injected systemically.
(3) The claims of hemostatic efficiency and harmlessness for Coagulen by the manufacturer
appear exaggerated and unjustified.
Recommendations: Because of its uncertain composition, the possible dangers when injected systemically, and its inactivity as a thromboplastic and hemostatic agent when tested by several different methods, Coagulen merits no recognition as a therapeutic agent for inclusion in New and Nonofficial Remedies.
The detail evidences used as the basis of this brief report concerning Coagulen will be published shortly in the Journal of Pharmacology,138 together with the results with other thromboplastic agents.
The preceding report was sent to the American agent for the Society of Chemical Industry, Sept. 8, 1919.
In reply the American agent, Ciba Co., Inc., on March 22, 1920, sent the Council “some additional clinical reports on the use of Coagulen-Ciba in the treatment of Hemorrhages supporting our claims of the merits of Coagulen-Ciba.”
The material submitted by the Ciba Co., contains no objective evidence for or against the efficiency of Coagulen-Ciba but merely opinions. As a rule these opinions are favorable though conditional and hedging and quite unconvincing. Nothing was submitted to offset or challenge the findings of Dr. Hanzlik’s report.
Since the evidence indicates that Coagulen-Ciba has little, if any, efficacy as a hemostatic, the Council directed its omission from New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1920, p. 53.)
The Council has authorized publication of the report which appears below, explaining the omission of ferric cacodylate from New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Iron cacodylate, the ferric salt of cacodylic acid, was admitted to New and Nonofficial Remedies in 1917. It is required to contain from 39.7 to 44.9 per cent. of arsenic (As).
The following statement of the action, uses and dosage of iron cacodylate appears in the 1920 edition of New and Nonofficial Remedies:
“Actions and Uses.—Ferric Cacodylate has the properties of iron salts and of arsenic. Its use has been proposed in conditions in which the effects of iron and the mild arsenic action of cacodylates is desired.
“Dosage.—from 0.015 to 0.1 Gm. (1⁄4 to 11⁄2 grains).”
The period for which the iron cacodylate preparations now in New and Nonofficial Remedies were accepted coming to an end with the close of 1920, the Council decided to determine if sufficient evidence for the value of ferric cacodylate has accumulated to warrant its continued recognition. The following is the report of the referee of the Committee on Therapeutics to whom the matter was assigned:
“As far as the Referee knows, the only claim that Iron Cacodylate has as a therapeutic agent is that it forms a convenient method for the administration of Iron and Cacodylate (while there is no reason why a drug should not be given by mouth, usually intramuscularly, and apparently it has recently been given intravenously). The effects to be expected from its use are those of iron and arsenic.
“Granted that iron and arsenic are valuable therapeutic agents, Iron Cacodylate is not a satisfactory preparation in which to administer these drugs for the following reasons:
“1. It would appear that Cacodylates are not the best form in which to administer arsenic. Cacodylates in therapeutic doses exert but a feeble action. Small quantities may be reduced to cacodyl (CH2)4As2, and varying amounts to inorganic arsenic. The amount transformed to arsenic is apparently unknown and probably varies in different individuals. On these grounds alone the use of the cacodylates where an arsenic effect is desired seems dubious.
“2. The amounts of iron and cacodylates contained in the doses recommended are small when compared with the usual doses of either iron or cacodylate. The amount of iron in the Iron Cacodylate preparations is small, about .0036 gram per dose, while the preparations admitted to ‘Useful Drugs’ contain much larger amounts per dose recommended. The list follows:
Massa Ferri Carbonates | Fe per dose | .042 gm. |
Pilulae Ferri Carbonates | " | .058 gm. |
Tinctura Ferri Chloride | " | .022 gm. |
Ferri et Ammonii Citrae | " | .042 gm. |
“The approximate amount of arsenic in Iron Cacodylate in the commonly recommended doses varies from .012 gm. to 0.024 gm., while the amount of arsenic in Sodium Cacodylate in the recommended doses varies between .021 and .35 gms. It would seem that a much more rational method of administration of these two drugs would be separately, in which case a better control over the dosage is possible.
“3. The Referee has been unable to secure reliable clinical evidence that Iron Cacodylate is a serviceable preparation. A search of the available literature for the past fifteen years has been made, also Drs. Edsall, Longcope, Stengel, Hoover, Phillips and Miller have been consulted. These physicians know nothing of its use.
“4. In view of the above, it appears to the Referee that Iron Cacodylate is an irrational and useless method of the administration of iron and arsenic.”
The Council adopted the report of the referee and directed that iron cacodylate be omitted from the 1921 edition of New and Nonofficial Remedies.—(From Reports of Council on Pharmacy and Chemistry, 1920, p. 62.)
The Council has authorized for publication the following report which explains why Libradol was found ineligible for New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Libradol is manufactured by Lloyd Bros., Cincinnati. According to a circular (a “readily removable” label) which accompanies the trade package, its “uses” are: “In colds, croup and acute bronchitis. In local congestions; in lung trouble, in acute inflammations of this or any other organ, especially if pain or soreness be present. In lumbago, sciatica, or in rheumatic pains of the joints or muscles. Applied to the forehead, it induces sleep.”
Libradol is offered in two forms, “Libradol Mild” for infants and supersensitive persons which is said to be “destitute of drug energy” and Libradol “Regular” which is “highly medicated,” the “constituents” being “DRACONTIUM, SANGUINARIA, CEPHAELIS, MELALEUCA, LOBELIA, LAURUS, CAPSICUM, TOBACCO.”
According to a circular, “The sanitary plasma Libradol” is a “homogeneous, highly medicated, and exceedingly potent compound, in plastic form,” which “carries the energies of its drug constituents and the high antiseptic qualities of Laurus Camphora and Melaleuca.” It is stated: “The Drug Influence of Libradol is necessarily different from that of any known single member of the Materia Medica. But yet, no mystery either in medicine or of pharmacy is claimed as a part of its composition or process of manufacture. It is a thing peculiar to itself, the result of the study of the drugs from which it is derived and compounded. These drugs may be studied at leisure by whoever cares to do so....”
The following information bearing on the composition of Libradol was furnished by Lloyd Brothers in response to a request from the Council to aid in the consideration of the preparation:
“ ‘Compound Lobelia Powder’ has been, since 1852, official in the American Dispensatory, in the first edition of which (1852) its formula is given, as follows:
“ ‘Take of Lobelia, in powder, twelve ounces; Bloodroot and Skunk Cabbage, in powder, of each, six ounces; Ipecacuanha, eight ounces; Capsicus, in powder, two ounces. Mix them.’
“This preparation came increasingly into demand with the Eclectic profession, the principal use for which it was first employed (as an emetic), being finally displaced by its local application in bronchial pneumonia troubles, when sprinkled on a greased cloth and applied to the chest.”
“In 1898, Dr. Finley Ellingwood petitioned Lloyd Brothers to make for him, in plasma form, ready for application, a compound carrying the ingredients of the old ‘Compound Lobelia Powder,’ strengthened by the addition of Melaleuca leucadendron, Laurus camphora and Nicotiana tabacum. Experiments not very encouraging in a pharmaceutical sense were made, and it was not until repeated requests had been made that a product was at last satisfactorily prepared and forwarded to Dr. Ellingwood (1900), with no thought other than that of serving him personally in his practice. This product he used and commended to his professional friends, and under his commendation it came into professional demand.”
An examination of the information submitted by Lloyd Brothers showed Libradol to be in conflict with the principles and rules that govern in the acceptance of articles for New and Nonofficial Remedies as follows:
Composition (Rule 1).—The information which has been received gives little idea of the actual composition of the preparation; for example, the statement that Libradol “carries the energies of its drug constituents and the high antiseptic qualities of Laurus Camphora and Melaleuca” gives no indication as to the part or parts of the Laurus Camphora or Melaleuca employed. If the statement is correct, that Libradol “is a homogeneous, highly medicated, and exceedingly potent compound,” it is essential that the several potent ingredients be stated clearly and not merely hinted at by their qualities. Other conflicts with Rule 1 might be enumerated, but the foregoing citations state the direct conflict; and this has not been removed, although an inquiry was sent to Lloyd Brothers for a statement of the amount of each potent ingredient in a given quantity of Libradol.
Indirect Advertising (Rule 4).—The recommendation for the use of Libradol in the treatment of colds, bronchitis, lumbago, sciatica and rheumatic pains, which accompanies the trade package, is prone to lead the public to depend on it in cases where definite treatment is imperative.
Unwarranted Therapeutic Claims (Rule 6).—Libradol is recommended in a great variety of conditions and is especially claimed not only to relieve pain, but to remove the cause of pain. This is explained as follows: “In the study of the physiological action of many drugs, it was found that the constituent remedies in this combination exercised a most salutary influence, not only upon the sensibility of the nerves involved, but upon the capillary circulation within the diseased area, the muscular structures therein included, and, subsequently, upon the course of the advancement of the congestive and inflammatory processes, and upon secretion, exudation, adhesion, induration, hypertrophy, suppuration and excretion.”
Granting, for the sake of argument, that carefully controlled experimental clinical evidence were available to substantiate this statement with reference to a single case of pain, the statement would be misleading when considered as a general explanation of the preparation’s relieving pain by removing the cause of pain when taken in connection with the conditions for which it is recommended and in which pain is even a minor symptom. Still, if pain were relieved in these cases by removing the cause, the patient would be cured of the conditions which give rise to the pain, and these include: “Acute pain in the chest;... acute inflammation in the chest;... persistent local pain;...” (This might be interpreted as including tuberculosis; pneumonia; cancer, and appendicitis.) “lumbago; sciatica; articular rheumatism” (gonorrheal infections?).
Name (Rule 8).—The name, derived from Dolar and Liber, suggests the claimed action of the preparation (the relief of pain) rather than the drugs said to be presented by it.
Irrational Composition (Rule 10).—It is quite possible that Libradol will relieve pain in certain instances and that the drug constituents present in Libradol “Regular” make this more effective than “Libradol Mild” which is “destitute of drug energy”; this, however, is no justification for the use by physicians of a cataplasm containing or made from skunk cabbage, bloodroot, ipecac, melaleuca (oil of cajeput?), lobelia, laurus comphora (camphor?), capsicum and tobacco. The combination is thoroughly irrational and a reminder of a past century. Further, the Council knows of no evidence to support the following claims:
“As a stimulant Capsicum has the power of neutralizing depressant remedies like Lobelia and Tobacco.”
“Our association of its desirable constituents with those of Lobelia, in connection with the modifying influence of Capsicum, Melaleuca, and Laurus Camphora, permits a more free use in Libradol than would be possible were it to be employed alone.”
“Capsicum, Melaleuca, and Laurus Camphora in Libradol tend to counteract the excessive relaxative and depressant effects of Lobelia.”
“The great value of Melaleuca in Libradol is its quality of modifying and controlling the action of the associated energetic constituents of the drugs Tobacco and Lobelia, which reduce congestion and inflammation, but which would, unsupported, be too depressant.”
Libradol is inadmissible to New and Nonofficial Remedies because its composition is complex, irrational and semi-secret, and because its name and the unwarranted therapeutic recommendations made for it will lead to its ill-advised use.—(From Reports of Council on Pharmacy and Chemistry, 1920, p. 65.)
Helmitol is hexamethylenamin methylencitrate. It was introduced with the claim that it was superior to hexamethylenamin—which acts in acid fluids only—in that it is equally efficient whether the urine is alkaline or acid.
In 1918 The Bayer Company, which then marketed the product in the United States, was notified that the Council questioned the claims made for Helmitol and desired evidence to substantiate them. In 1919 the same notification was sent the Winthrop Chemical Company, which in the meantime had secured control of the product. Pending the submission of the evidence, the Council continued the acceptance of Helmitol for New and Nonofficial Remedies with the statement that the actions and uses of hexamethylenamin anhydromethylencitrate were those of hexamethylenamin.
W. A. Puckner, Secretary.
The following report on Helmitol was made by the referee in charge of hexamethylenamin compounds and preparations, adopted by the Council and sent the Winthrop Chemical Company:
“Helmitol is a compound of anhydromethylencitric acid and hexamethylenamin. It was introduced with the claim that it would be antiseptic even in alkaline urine. The Council did not entirely trust the evidence, but continued to list Helmitol in N. N. R., merely as a salt of hexamethylenamin, until satisfactory data should become available. These have now been furnished by Hanzlik (Journal of Urology 4:145) who has shown that:
“1. The alkalinity required to split off formaldehyd from anhydromethylencitric acid is greater than exists in the urine, even in advanced ammoniacal fermentation.
“2. Even if any formaldehyd were liberated in ammoniacal fermentation, it would at once become inactive by combining with ammonia.
“3. Urine after the administration of anhydromethylencitric acid actually putrefies readily.
“4. Less than 5 per cent. of the anhydromethylencitric radical reaches the urine, the remainder being destroyed in the body.
“The only reason for the existence of Helmitol was this claim of antiseptic action in alkaline and putrefying urines. Since this has been disproved, there remains no reason for retaining Helmitol in N. N. R.; on the contrary, its retention would only tend to continue the fallacy on which it is based.
“It is, therefore, recommended that Helmitol be no longer listed with New and Nonofficial Remedies, and that this report be published, after the usual submission to the manufacturers.”
In accordance with the recommendation of the report, the Council has directed the omission of Helmitol from New and Nonofficial Remedies and has authorized the publication of this report.—(From The Journal A. M. A., Jan. 22, 1921.)
The Council has authorized publication of the following report.
W. A. Puckner, Secretary.
“Spirocide” (The Spirocide Corporation of New York) is advertised as a new and successful treatment of syphilis by fumigation and inhalation. According to the information presented to the Council, Spirocide is a mechanical mixture of metallic mercury 25 per cent., copper sulphate 25 per cent., cypress cones 20 per cent., henna 20 per cent., nut gall 5 per cent., and dried pomegranate 5 per cent. It is supplied in the form of greenish-gray tablets weighing about 10 gm. each, and containing, therefore, about 2.5 gm. (about 38 grains) of mercury. It is sold in packages of six tablets.
The following directions for its use are contained in a pamphlet recently distributed:
“Spirocide is administered by means of fumigation and inhalation. The patient is disrobed to the waist and placed in a light chair, preferably with arms. A pastil or tablet of Spirocide is placed on a small plate, or open receptacle, after being ignited by holding in a gas or alcohol flame for a minute or so until it begins to smoulder. The plate with the burning Spirocide is then placed on the floor between the patient’s feet or just under the chair. A small shelf or platform between the lower rounds of the chair is an excellent location for the plate containing the burning mass. When all is in position a sheet should be thrown over the patient and arranged to enclose the whole. The patient should breathe naturally and inhale the vapor, which will rise and fill the canopy surrounding him. The treatment will require 15 to 30 minutes, or until the Spirocide is burned up. The patient may complain at first of a slight choking sensation, and there may be some tendency to cough. This can be removed by raising the sheet long enough to let in a little clear air. The eyes should be closed or lightly bandaged to avoid smarting.”
Experiments conducted in the A. M. A. Chemical Laboratory show that Spirocide, when ignited, burns slowly with consequent volatilization of mercury. The several organic constituents serve as fuel and the copper sulphate possibly acts as a regulator of the combustion. During the burning process the cypress cones, henna, etc., are consumed but most, if not all, the copper remains behind, the mercury only being vaporized. It is asserted in the advertising pamphlet that Spirocide is indicated in all stages of syphilis, primary, secondary and tertiary, and in all its complications or sequelae. In these varying conditions one tablet daily or every other day is recommended until six treatments have been taken, though it is stated that “occasionally, depending on the severity or the duration of the disease, it may be wise to give nine treatments, the last three at intervals of two, three or more days.”
Some of the results which it is claimed are obtained with Spirocide are:
“At the completion of this course of treatment with Spirocide, all signs or evidences of syphilis are removed, and in ten days to three months all Wassermann tests prove negative. Any further treatments than the original course of fumigations are rarely needed. Wassermann’s will be found uniformly negative after a period which, according to the patient, may vary from ten days to three months. These results have been obtained in cases in which Salvarsan and kindred preparations have been employed without the slightest benefit.”
In a letter to the Council the “scientific observer” of the Spirocide Corporation declared:
“We do not claim that the vaporization method is new. We do claim, however, that this combination of mercury produces more rapid volatilization, certain absorption and undoubted effect than any form of mercury administered by any method known to science without the usual danger. That this is so we are willing to prove by comparison with other methods both by ourselves and many observers scattered over the United States....”
To determine the validity of the claims made for Spirocide, the Corporation was asked to present the evidence which it offered. In reply, the corporation’s “scientific observer,” Dr. J. Lewengood, submitted 83 case reports from a number of different observers, including those from military hospitals and a state institution, and also a reprint of an article published by him in the New York Medical Journal, Feb. 21, 1920, wherein were reported eight cases which received “Spirocide Treatment.” In no case were controls with other methods of mercury administration carried out.
This material the Council sent to two recognized syphilographers for an opinion. One of the consultants reported that of the 83 cases, 20 dealt with patients who had also received arsphenamin medication and, therefore, these 20 cases could not be considered as evidence concerning the value of Spirocide. As to the remaining cases, he found on the whole that the history and data furnished were far from sufficient to warrant the claims made. In many of the cases emphasis was laid on the Wassermann test, as though this test were the only thing to be considered in a case of syphilis. He pointed out that in one case the reaction changed from negative to strongly positive after six treatments and that in several cases the phenomena reported cannot be explained by anything else than a desire to get a negative blood test. For example, one case had Spirocide treatment and a Wassermann, 1 plus, 55 days after; the author then reports that 19 days later the reaction had become negative and, therefore, the change must be due to Spirocide. In several of the cases reported it is even questionable if the patients were syphilitic. The consultant concluded that the evidence submitted by the Spirocide Corporation failed to prove the claims made for Spirocide. He pointed out on the other hand that patients readily become salivated from the use of Spirocide, often after 8 or 10 treatments.
The second consultant replied that in his opinion the claim that Spirocide produces more “undoubted effect than any form of mercury administered by any method known to science without the usual danger,” was not substantiated. He believed that it was not as effective as some other methods, that the dosage is not as exact, and, therefore, it is not as free from danger when the drug is pushed.
The Council’s two consultants were also asked whether or not, in their opinion, the administration of mercury by inhalation is a method which the Council should endorse to the extent of recognizing a preparation based on this principle. This inquiry was also sent to the members of the editorial board of the Archives of Dermatology and Syphilology. Five replies were received. One advised a thorough study of the different methods of administering mercury by inhalation. The other four were opposed to such recognition on the ground that as the dosage is not exact the effects, therefore, are not certain.
In consideration of the opinions expressed by its consultants, the Council declared Spirocide inadmissible to New and Nonofficial Remedies because (1) the claims made for it are unproved and unwarranted, (2) the routine use of an inexact method for the administration of mercury is detrimental to sound therapy and (3) the name is not descriptive of its composition, thus failing to remind the physician who uses these pastils that he is administering metallic mercury.—(From The Journal A. M. A., Jan. 22, 1921.)
The Council has authorized the publication of the following report, declaring Digifolin-Ciba inadmissible to New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Digifolin-Ciba is a product of the Society of Chemical Industry of Basle, Switzerland. It is marketed in the United States by the Ciba Company, 91 Barclay Street, New York City. It is claimed that Digifolin-Ciba is “a preparation of digitalis leaves that has been freed from the useless and harmful principles such as Digitonin (saponin), coloring and inert matter, etc., but does contain all the really valuable, therapeutically active constituents of the leaves, namely: digitoxin and digitalein in their natural proportions.” There is no evidence that digifolin contains all of the glucosides of digitalis as they exist in the leaf, and it is extremely improbable that this is the case because one cannot remove saponin without altering the other active principles of digitalis.
The Ciba Company sends out the following pamphlets relating to Digifolin:
“ ‘Concerning Digifolin-Ciba, A New Preparation of Digitalis,’ by C. Hartung, M.D., Ph.D. Extracts from the work ‘Ueber Digifolin, Ein Neues Digitalis-Praeparat’ in the Munich Medical Weekly, No. 36, page 1944, 1912.”
“ ‘Digitoxin Contents of Digifolin-Ciba,’ by C. Hartung, M.D., Ph.D., Basle, Switzerland. Reprints from the Pharmaceutical Post, 1913. No. 34, page 357. No. 40, page 431.”
“ ‘Pharmacological Tests of Digitalis,’ by M. J. Chevalier, Chef Des Travaux Pratiques de Pharmacologie et Matiere Medicale, Faculte De Medecine De Paris. Report Presented to the Societe de Therapeutique at Their Meeting, May 28, 1913.”
In the reprint “Concerning Digifolin, ‘Ciba.’ ” Hartung lays stress on the presence of harmful and inert substances present in the leaf and galenical preparations with the direct or implied statement that digifolin has an advantage in that these are absent from it. This is misleading. It is true that Boehm whom Hartung cites, found saponin to be irritating, but Boehm states that it required 100 mg. per kilogram of body weight to induce vomiting after its oral administration. Furthermore, saponin is present in traces only in infusion of digitalis, so that the therapeutic dose contains a wholly negligible amount of it.
The following occurs in “Pharmacological Tests of Digitalis,” by M. J. Chevalier:
“Hartung’s Digifolin merits our attention, especially because it seems to possess all the pharmacodynamic properties of galenic preparations of digitalis without showing any of their disadvantages.”
This claim scarcely needs comment, since it is well established that the chief “disadvantages” of digitalis are inherent in the principles which produce the desired effects of digitalis and may be avoided to a large extent by a carefully regulated dosage of any digitalis preparation. In short, the advertising for Digifolin asserts that this digitalis preparation has all the advantages of digitalis itself, but none of its disadvantages. This claim has been refuted so frequently that manufacturers must be aware that it is untenable. Further the claims now made for Digifolin are essentially those made nearly four years ago at which time the attention of the American agent was called to their unwarranted character.
The Council declared Digifolin-Ciba inadmissible to New and Nonofficial Remedies because the therapeutic claims advanced for it are misleading and unwarranted.—(From The Journal A. M. A., April 2, 1921.)
The Council has authorized the publication of the following report on “Loeser’s Intravenous Solution of Hexamethylenamin,” “Loeser’s Intravenous Solution of Hexamethylenamin and Sodium Iodid,” “Loeser’s Intravenous Solution of Sodium Salicylate,” “Loeser’s Intravenous Solution of Salicylate and Iodid,” “Loeser’s Intravenous Solution of Sodium Iodid” and “Loeser’s Intravenous Solution of Mercury Bichlorid,” put out by the New York Intravenous Laboratory, Inc.
W. A. Puckner, Secretary.
The intravenous solutions of “Hexamethylenamin,” “Hexamethylenamin and Sodium Iodid,” “Sodium Salicylate,” “Sodium Salicylate and Sodium Iodid,” “Sodium Iodid” and “Mercuric Chlorid” marketed by the New York Intravenous Laboratory, Inc., are solutions of official substances sold under their official names. They would, therefore, be outside the scope of the Council, were it not that special and general therapeutic claims are made for them. Such special claims, for instance, are contained in an advertisement in the Illinois Medical Journal for Oct. 20, 1920, which gives, under the various drugs, a list of diseases in which the drugs are said to be “indicated.” The Council is unable to agree with some of these recommendations. The fundamental objection, however, is the general claim of superiority and safety of the intravenous method.
The intravenous solutions named above would naturally have little sale if such special claims were not made for them. While the claims may not be made directly, they are carried by such display phrases as “For the progressive physician seeking improved clinical results” and “A safe practical office technique.”
The Council continues to hold that intravenous medication, generally, is not as safe as oral medication even with relatively harmless substances (a fact again illustrated by the results of Hanzlik and Karsner, 1920, Journal Pharmacology and Experimental Therapeutics, 14, 379), and that it does not give “improved clinical results” except under rather narrowly confined circumstances—namely, if the drug undergoes decomposition in the alimentary tract, if it is not absorbed, if it causes serious direct local reaction or if time is an urgent element. Each intravenous preparation for which advantage over oral administration is claimed, directly or by implication, must be examined from these points of view.
The Council has recognized intravenous preparations which satisfied these requirements. It is evident, however, that hexamethylenamin, sodium iodid and sodium salicylate do not. When given orally they do not undergo material decomposition in the digestive tract, they are rapidly absorbed, they cause no direct local reaction, and in the conditions in which they are used the hour or so which is required for absorption is immaterial, especially as they are used continuously for some time. Mercuric chlorid does indeed produce some local irritation, but there is as yet no convincing evidence that its intravenous injection causes less injury than oral administration. More experience under controlled conditions is needed before the intravenous use of mercuric chlorid can be approved. Especially objectionable are the fixed proportion mixtures of sodium iodid with sodium salicylate and with hexamethylenamin. The dosage of all three drugs has to be adapted to individual conditions. This is impossible when giving them in fixed proportions.
The Council voted not to accept “Loeser’s Intravenous Solution of Hexamethylenamin,” “Loeser’s Intravenous Solution of Hexamethylenamin and Sodium Iodid,” “Loeser’s Intravenous Solution of Sodium Salicylate,” “Loeser’s Intravenous Solution of Salicylate and Iodid,” “Loeser’s Intravenous Solution Sodium Iodid” and “Loeser’s Intravenous Solution of Mercury Bichlorid” for New and Nonofficial Remedies because they are sold under misleading claims regarding their alleged safety and efficiency. In view of this fundamental objection the individual claims for each preparation were not passed on.—(From The Journal A. M. A., April 16, 1921.)
The Council has authorized publication of the following report.
W. A. Puckner, Secretary.
“National Iodine Solution” is a proprietary sold by the National Drug Co., Philadelphia, Pa. From inquiries received by the Council on Pharmacy and Chemistry it is evident that the product is extensively brought to the attention of physicians by means of circulars. The name implies that it is a solution of iodin and the inference is given that it has the advantages of iodin without the disadvantages.
In view of the foregoing, the Council took up the investigation of “National Iodine Solution,” and in turn asked the A. M. A. Chemical Laboratory to analyze it. The chemist’s report follows:
According to the label of National Iodine Solution, “each fluidounce represents three grains Proteo-albuminoid compound of iodin (National)”; also an alcohol declaration of 7 per cent. is made. Otherwise no information is given as to the composition either of the “solution” or of “Proteo-albuminoid compound of Iodine.”
Each bottle contained about 115 c.c. (nearly 4 ounces) of a yellowish solution, acid in reaction, having an odor resembling witch hazel; its specific gravity at 25 C. was 0.9860. Qualitative tests indicated the presence of zinc, alcohol, sulphate, an iodin compound (the solution gave tests which indicated a very small amount of free iodin; most of the iodin was in the form of ordinary iodid), a small amount of vegetable extractives, and traces of aluminum and potassium. If any protein was present, it was in amounts too small to be identified, though a small amount of a nitrogenous compound was present. The amount of solids in “National Iodine Solution” was equivalent to 0.72 per cent, and the amount of ash, to 0.2 per cent. Quantitative estimations yielded the following:
Alcohol (by volume) | 7.0 | per cent. |
Zinc (Zn++) | 0.096 | per cent. |
Iodin (free and combined) | 0.029 | per cent. |
Sulphate (SO4- -) | 0.146 | per cent. |
Protein (N × 6.36) | 0.012 | per cent. |
The above findings indicate that each 100 c.c. contains about 7 c.c. of alcohol, 0.5 gram of zinc sulphate U. S. P. (ZnSO4+7H2O.), 0.03 gram of iodin, 0.01 gram of protein (calculated as such from nitrogen times the factor 6.36) and some hamamelis water. Expressed in equivalent apothecary terms, each fluidounce contains essentially:
Zinc sulphate | 21⁄3 | grains |
Iodin (free and combined) | 1⁄8 | grain |
Protein | 1⁄25 | grain |
Alcohol | 34 | minims |
This amount of alcohol is equivalent to about 31⁄2 fluidrams of witch hazel water. Although the label states that each fluidounce contains three grains of “proteo-albuminoid compound of iodine,” yet the sum of the protein (calculated from nitrogen content) and iodin components is equivalent to less than 1⁄5 grain.
“National Iodine Solution” appears to be very similar to “Gonocol” (The National Drug Co., Philadelphia, Pa.), which was analyzed by the Bureau of Chemistry of the U. S. Department of Agriculture. The bureau stated that “it [Gonocol] consisted essentially of an aqueous solution of zinc sulphate, hamamelis water, a small amount of alcohol, 0.38 grain of iodin, and 0.36 grain of protein per fluidounce.”
It is evident that “National Iodine Solution” is not a solution of free elementary iodin as the name suggests; instead it appears to be a solution of zinc sulphate in witch hazel water containing less than 0.03 per cent. of combined iodin and not more than a trace of free iodin. “National Iodine Solution” is one more to be added to that already long list of proprietaries which makes capital of the high esteem in which physicians hold iodin.
An advertising circular sent to physicians begins:
“Dear Doctor: We beg to suggest a line of treatment while using National Iodine Solution which our many years of experience has proven to us to give the best and quickest results in the treatment of inflammation of the urethral tract ...”
In it are given directions for the treatment of “acute gonorrhea, male,” “anterior urethritis,” “anterior-posterior urethritis,” “ardor urinæ and chordee,” etc., by means of National Iodine Solution and other proprietaries of the National Drug Company’s make. In fact the solution is claimed to be “Indicated in All Conditions of Urethra Accompanied by a Discharge.”
The therapeutic claims made for “National Iodine Solution” are unwarranted. Such a solution is not indicated in all conditions of the urethra accompanied by discharge. The advice contained in the circular is equivalent to mail-order treatment of gonorrhea.
It is of interest to note that the claims for an identical or a similar solution prepared by the National Drug Company as a treatment for gonorrhea and intended for use by the laity, has been adjudged misbranded by the federal authorities (Notice of Judgment No. 8150, issued Jan. 25, 1921) in that it misled and deceived the purchaser or purchasers thereof in the statements regarding the therapeutic or curative effects of the article, which falsely and fraudulently represent it to be indicated in all conditions of the urethra accompanied with a discharge, “whereas in truth and in fact it was not.”
The Council would emphasize that if physicians give heed to advertising such as that sent out by the National Drug Company for this preparation the medical profession cannot with good grace protest against the routine treatment of venereal diseases by quacks and “patent medicine” venders.—(From The Journal A. M. A., June 4, 1921.)
The Council has authorized publication of the following report.
W. A. Puckner, Secretary.
Mon-Arsone is offered by the Harmer Laboratories Company as “a new and non-toxic arsenical for the treatment of syphilis.” In the advertisements for Mon-Arsone it has been claimed that with this drug “the toxic, corrosive and uncertain reactions attending the use of arsphenamine have been entirely eliminated” and that “it has a therapeutic value equal to arsphenamine, but extensive case reports fail to record the slightest toxic reaction following its use.”
According to the manufacturers, Mons-Arsone is disodiumethylarsonate, the sodium salt of ethylarsonic acid, derived from arsenic acid by replacement of one hydroxyl group by the ethyl group—AsO(CH2CH3)(OH)2. Mons-Arsone is related to sodium cacodylate, which is the sodium salt of dimethyl-arsenic acid—AsO(CH3)2OH—derived from arsenic acid by replacement of two hydroxyl groups by two methyl groups. Ethylarsonic acid and its potassium salt were described by La Coste139 more than thirty-five years ago, and the use of the sodium salt of methylarsonic acid was proposed in France some years ago. The Harmer Laboratories Company claims originality for Mons-Arsone in that it was the first to prepare the sodium salt of ethylarsonic acid and to propose its therapeutic use.
It was reported several years ago by Castelli140 that sodium cacodylate and the sodium salt of methyl arsenic acid were devoid of effect on experimental trypanosomiasis and spirochete infections. Careful clinical observations in this country by H. J. Nichols141 and H. N. Cole142 have demonstrated the inefficacy of sodium cacodylate in the treatment of human syphilis.
Animal experiments carried out in the U. S. Hygienic Laboratory by Voegtlin and Smith143 show that Mon-Arsone is devoid of any practical trypanocidal action. Thus the “therapeutic ratio” (the ratio of the minimal effective dose to the lethal dose) was about 1, that is, it was effective therapeutically only in approximately fatal doses; the therapeutic ratio for arsphenamine in similar conditions was 17, and that of neoarsphenamine, 28.
The findings that sodium dimethylarsenate (sodium cacodylate), sodium methylarsenate, and sodium ethylarsenate are devoid of any practical trypanocidal action and the conclusion that sodium cacodylate is inefficient in the treatment of human syphilis does not prove that Mon-Arsone is without effect on the disease. These findings, however, certainly demand convincing therapeutic evidence to warrant the recommendation for the use of the drug in the treatment of syphilis—particularly because the drug is proposed as a substitute for arsphenamine, the value of which is established.
When the Council first took up the consideration of Mon-Arsone, the only evidence for the claim that it “has a therapeutic value at least equal to that of arsphenamine” consisted, with one exception, of reports from those who had experimented with the drug for the Harmer Laboratories Company, including a report by B. L. Wright, L. A. Kennell, and L. M. Hussey,144 the latter of the Harmer Laboratories Company. These reports appeared to show that the administration of Mon-Arsone caused less reaction than arsphenamine, and that the immediate effects, judged by clinical symptoms and the response to the Wassermann test, appeared to be good. These trials extended over too short a period of time to permit judgment as to the permanence of the results. A report by an independent observer seemed to indicate that Mon-Arsone does not have the sterilizing action on syphilitic lesions which it is usually believed arsphenamine exercises.
After examining the available evidence, the Council advised the Harmer Laboratories Company that the claim that Mon-Arsone has a therapeutic value equal to arsphenamine appeared unwarranted; that, in the opinion of the Council, Mon-Arsone should not be used except under conditions that justify the experimental trial of an unproved drug, and should not be used in a routine way until the permanence of its effects has been established; and consequently any advertising propaganda for the drug by the Harmer Laboratories Company was to be deprecated.
In its reply the Harmer Laboratories Company admitted that its advertising claim, that Mon-Arsone was at least equal to arsphenamine therapeutically, had been based on reports on fifty cases and on additional reports that were beginning to come in at that time. The Harmer Laboratories Company submitted a list of hospitals and physicians using Mon-Arsone. A letter of inquiry sent by the Council to those who, according to the names in the list supplied by the Harmer Laboratories Company, had used Mon-Arsone, brought seven replies.
The clinical evidence contained in these replies was to the effect that Mon-Arsone had been used in the various types of syphilis and that there was a certain beneficial effect, both clinically and as shown by the Wassermann reaction. In certain instances the Wassermann reaction changed from a four plus to a negative reaction. The reports showed that the efficiency of Mon-Arsone as compared with that of arsphenamine preparations has not been adequately studied. One physician who has used Mon-Arsone extensively reports that in many of the cases treated there seemed to be nearly as good results from the use of Mon-Arsone as is frequently obtained in the use of arsphenamine. He reports, however, that it was necessary in eleven out of one hundred cases to change from Mon-Arsone to neoarsphenamine.
In view of the fact that there is definite lack of evidence to show that Mon-Arsone is the equal of arsphenamine therapeutically, and because of the reports that in some cases it is inferior, Mon-Arsone should not be used in the treatment of syphilis generally until its therapeutic status has been more rigidly investigated and conclusive evidence of its superiority to arsphenamine preparations obtained.
The Council voted not to admit Mon-Arsone to New and Nonofficial Remedies and reaffirmed its conclusion that the claim that Mon-Arsone has a therapeutic value equal to that of arsphenamine is premature and unwarranted; that Mon-Arsone should not be used except under conditions that justify the experimental trial of an unproved drug; and that the advertising propaganda for the drug by the Harmer Laboratories Company is to be deprecated.
When the preceding report was sent to the Harmer Laboratories Company, the firm submitted a reply in which it was stated:
1. That in certain instances patients improved under Mon-Arsone who, previously, had not improved under arsphenamine, and that this should be taken to offset the report of the one hundred cases in which the use of Mon-Arsone had to be abandoned in 11 per cent. of the cases.
2. That the Harmer Laboratories Company has abandoned the claim that Mon-Arsone is therapeutically equal to arsphenamine and that it now furnishes the drug to such men as care to use it simply on the basis of its special and useful characteristics.
The Council heartily endorses the recent warning against the use of untried medicaments which was issued by the U. S. Public Health Service.145
Since the Council’s report was prepared a report on the effects of Mon-Arsone on experimental syphilis has been published by Nichols,146 from the Division of Laboratories, Army Medical School, which concludes:
1. Disodium-ethylarsinate, or mon-arsone, tested on rabbits infected with syphilis shows no spirocheticidal power. The tissues are fatally poisoned as soon as or before the spirochetes are affected.
“2. For its practical use in syphilis there is no such germicidal basis as exists in case of the arsphenamine group.”—(From The Journal A. M. A., June 18, 1921.)
“Oxyl-Iodide” (Eli Lilly and Co.) is said to be the hydroiodid of cinchophen and the claim is made that it exerts the effects of cinchophen and of iodid. Because of inquiries which have been received the Council decided to determine the eligibility of “Oxyl-Iodide” for New and Nonofficial Remedies. Dr. P. J. Hanzlik—formerly Associate Professor of Pharmacology at Western Reserve University School of Medicine, now Professor of Pharmacology at Leland Stanford Junior University Medical School—who has made a study of the action of salicylates and cinchophen, was asked to report on the therapeutic value and the rationality of “Oxyl-Iodide.” This he consented to do and his report appears below.
After considering Doctor Hanzlik’s report, the Council declared “Oxyl-Iodide” inadmissible to New and Nonofficial Remedies because it is an irrational combination, marketed under claims that are unproved and consequently unwarranted.
W. A. Puckner, Secretary.
“Oxyl-Iodide,” marketed by Eli Lilly & Co., is claimed to be the hydroiodid of phenylcinchoninic acid, containing 33 per cent. of iodin and 67 per cent. of phenylcinchoninic acid (cinchophen). Its solubility resembles that of cinchophen, being low in water and acid mediums, and higher in the presence of alkalis. Whether “oxyl-iodide” is decomposed into its constituents in the presence of alkalis does not appear to have been determined. However, if this were the case, the intestine, after administration of “oxyl-iodide,” would contain cinchophen and sodium iodid in the same forms as if these agents were administered individually so that nothing would be gained by administering “oxyl-iodide.” Being, like cinchophen, practically insoluble in acid mediums, “oxyl-iodide” would have no advantage over the latter so far as gastric irritation is concerned.
The dosage advised is from one to three tablets containing 3 grains (0.2 gm.) each of “oxyl-iodide.” The total dosage would depend on the condition to be treated. In rheumatic fever, which requires a full therapeutic or so-called, “toxic” dose of cinchophen, about 12 to 13 gm. would be administered intensively. Since each tablet of “oxyl-iodide” contains 0.13 gm. of cinchophen, the total number of tablets of “oxyl-iodide” required would be 100, or two and one-half bottles of forty tablets each. At the same time the patient would receive 6.6 gm. of iodin (as iodid). This might be distinctly objectionable because of the production of the disagreeable symptoms of iodism in some persons, and indicates that the fixed proportion of the iodin constituent would be objectionable.
Even a smaller dosage, such as 5 gm. of cinchophen, which gives partial relief in rheumatism and similar conditions, would still require a patient to take a full bottle, or forty tablets, of “oxyl-iodide,” and at the same time about 2.7 gm. of iodin would have to be ingested.
Furthermore, rheumatic fever, the arthritides, gout and related conditions in which cinchophen is indicated do not require iodid. Therefore, “oxyl-iodide” would not be the remedy of choice in these conditions, and its use would be irrational and illogical.
No data on the pharmacologic actions of “oxyl-iodide” are presented in the manufacturer’s literature. Presumably, the compound would exhibit the actions of its individual components, i. e., cinchophen and iodin (as iodid), though probably less efficiently, owing to its low solubility. This is also indicated by the following statements of the manufacturer: “The analgesic action of ‘oxyl-iodide’ is gradual. A word of caution is necessary to those who may expect immediate relief from pain.” Therefore, why use “oxyl-iodide” in place of more dependable analgesics, such as salicylate or cinchophen. The following statements appear far-fetched: “There is a stimulation of the endocrines which is perhaps more marked in the thyroid gland, although it is probably shared by the pituitary and other glands which function in a chain-like control.... There is stimulation of cells with increased flow of secretion, visibly demonstrated by the nasal mucous membrane after ‘oxyl-iodide’ has been taken for some time. The general action on mucous membranes favors elimination of toxins and waste products.”
It is probable that “oxyl-iodide” acts as a uric acid eliminant, though there is no reason to suppose that it is more effective than cinchophen alone. No data are given for this in the manufacturer’s literature.
Successful use of “oxyl-iodide” is claimed in brachial and sciatic neuritis, lumbago, muscular rheumatism, arthritis deformans, chronic arthritis (“... in some instances were apparently cured”), subacute bronchitis, circumflex neuritis, traumatic orchitis, eczema and rheumatism. However, a careful reading of the protocols of seven cases, representing these conditions, gives an unfavorable impression as to the real contribution to the recovery by, or value received from, “oxyl-iodide.” Summarized, the opinions as quoted by the manufacturers in support of their claims for “oxyl-iodide” are briefly as follows:
Case 1. “Of course, the case is not complete yet, but I am looking for continued betterment.”
Case 2. “For two weeks past her improvement has been marvelous.”
Case 3. “The joints are still enlarged and we do not hope to clear them entirely....”
Case 4. “Undoubtedly, removal of the kidney had much to do with improvement.”
Case 5. “I think I have gotten very good results.”
Case 6. “Some apparent benefit.”
Case 7. “She is practically free from pain, and the muscle and joint stiffness is now slight.”
These inconclusive opinions certainly do not agree with the favorable impression which other portions of the manufacturer’s literature create. If the factor of natural recovery in the conditions represented by these seven cases is given due weight, little, if anything, is left to the credit of “oxyl-iodide.” Such clinical evidence as is supplied by the manufacturer indicates that the therapeutic efficiency of “oxyl-iodide” is doubtful, and not an improvement over either cinchophen or iodid.
Iodism cannot be avoided by the use of “oxyl-iodide,” for the manufacturer’s literature states that “the dosage of ‘oxyl-iodide’ may be pushed to iodism as manifested by skin symptoms.... To avoid iodism there should be an occasional interruption of treatment.” “Oxyl-iodide,” therefore, has no advantage over ordinary sodium iodid to avoid iodism. Usually, the conditions which require cinchophen do not require the simultaneous administration of iodids, and vice versa. If administration of iodid and cinchophen together should be indicated or desirable, these can be given separately with the added advantage that the iodid can be easily reduced or withdrawn in case iodism supervenes, and the cinchophen could be continued if necessary. Since conditions do not arise frequently enough to warrant the use of iodid and cinchophen together, the existence of such a product as “oxyl-iodide” is unwarranted.
Finally, the manufacturer himself recognizes that phenylcinchoninic acid (cinchophen) can take the place of “oxyl-iodide.” Under “dosage,” the circular states: “A few patients may be idiosyncratic to the iodides and find they cannot take ‘oxyl-iodide.’ For the latter chloroxyl, the hydrochloride of phenylcinchoninic acid, is recommended.” The action of the hydrochlorid of phenylcinchoninic acid does not differ, of course, from that of cinchophen. The difficulties of assigning a clear-cut, definite, therapeutic rôle to “oxyl-iodide” in order to justify its existence, alongside well-known and tried remedies are self-evident.
“Oxyl-iodide” is pharmacologically and therapeutically an illogical, irrational and unjustified substitute for cinchophen and iodids. The conditions which require the administration of cinchophen do not as a rule require the administration of iodid and vice versa. If it is desirable to secure the effects of iodid and cinchophen together, these can be more conveniently and advantageously administered as separate agents, permitting in that way a better control of their actions. This cannot be accomplished with “oxyl-iodide,” in which the proportion of iodid and cinchophen are fixed. Symptoms of iodism cannot be avoided by the administration of “oxyl-iodide.” The objective evidences for its actions and uses are totally lacking; and the clinical opinions concerning its therapeutic benefits in different disease conditions are inconclusive and hedging, and, if anything, contradictory to the favorable impressions which the language of the advertising matter is likely to create.—(From The Journal A. M. A., July 2, 1921.)
The Council has authorized publication of the following report, declaring that Quassia Compound Tablets (Flint, Eaton and Company) are inadmissible to New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Quassia Compound Tablets, marketed by Flint, Eaton and Company, Decatur, Ill., according to the label on a trade package submitted to the Council, contain in each tablet:
Quassia | 3⁄4 | grain | Aloin | 1⁄4 | grain | |
Chionanthus | 1 | grain | Ipecac | 1⁄16 | grain | |
Wahoo | 3⁄4 | grain | Podophyllin | 1⁄4 | grain | |
Nux Vomica | 1⁄2 | grain | Gingerine | q. s. | ||
Cascara | 1⁄3 | grain |
In the advertising the “Cascara” of the label is replaced by the indefinite term “Cascarin” and the “Gingerine q. s.” by “Carminative Antigripe q. s.” Flint, Eaton and Company informed the Council that “Carminative Antigripe is C. P. Sodium Sulphite of which each tablet contains 1⁄4 grain.” The tablets were treated with dilute hydrochloric acid and the odor of sulphur dioxid became apparent. This shows that the company’s statement to the Council, that the tablets contain a sulphite, is correct and the formula on the label is incorrect.
In the advertising for this preparation we read:
“A careful study of this formula [which formula? That on the label or that in the general advertising?—Council] will reveal the outstanding fact that, while there are several drugs employed, each ingredient is there for a purpose and all do splendid teamwork. If your patient is constipated because the stomach is not sufficiently energetic, the Quassia stimulates that organ to an increased secretion of digestive fluids and sets it to working normally. If the liver be sluggish, the Chionanthus and Wahoo prompt it to increased activity. Chionanthus has no superior for producing a sustained healthy hepatic condition. Should the bowels be slow and uncertain, the small doses of Aloin, Cascarin and Podophyllin stimulate to free peristaltic action, while the Nux Vomica sets the nervous system right. We use an effective Antigripe so that there is no griping.”
It is absurd to suppose that a complex mixture of drugs in fixed proportions can have the actions claimed for Quassia Compound Tablets. As regards the claim that “Chionanthus has no superior for producing a sustained healthy hepatic condition,” it was brought out in a report of the Council on “Some Unimportant Drugs” (Reports of Council on Pharmacy and Chemistry, 1912, p. 36) that the “claims for this remedy [Chionanthus] are not supported by experimental evidence and the clinical reports of its use fail to show indications of discriminating critical observation. It is not noticed by most pharmacologic authorities.”
Of Wahoo (Euonymus N. F.) the “Epitome of the U. S. P. and N. F.” says: “Actions and Uses.—Obsolete cathartic; toxic digitalis effects. Caption: the uncertain absorption of this drug makes its use inadvisable.”
Quassia Compound Tablets (Flint, Eaton and Company) are inadmissible to New and Nonofficial Remedies because (1) they contain drugs of unproved value; (2) their composition is needlessly complex, and, therefore irrational; (3) unwarranted therapeutic claims are made for them; (4) the name is misleading and not descriptive of their composition, and (5) the statement of their composition is indefinite and incorrect.—(From The Journal A. M. A., July 9, 1921.)
The Council has authorized the publication of the following report:
W. A. Puckner, Secretary.
Toxicide (Toxicide Laboratories, Chicago) is alleged to be a remedy which “increases systemic resistance,” is “used for immunizing against septic infections” and “is indicated in any case of septic infection, capable of inducing inflammation and pus formation, regardless of location or kind of tissue involved.” The following statements bearing on the composition of the preparation are furnished by the manufacturers:
“Toxicide contains Lachesis 12X, Tarantula 6X, Psorinum (special) 15X, Silicia 6X and Excipient q. s. (the excipient is sweet milk).
“These remedies are combined in the sweet milk and put through a process of development, which produces the curative agent which we call ‘Toxicide’ ...
“Put up in tablet form, sugar coated and colored red.”
No information is given as to the proportions, either relative or actual, of the ingredients. Neither is any information given regarding the “process of development” to which the mixture is subjected, nor the amount of the finished mixture which is contained in Toxicide tablets.
The Toxicide Laboratories present the following “theory”:
“In combining these remedies and processing with milk, we develop a latent immunizing active principle, which usually controls the most virulently, active, septic infections promptly.”

There is no evidence, however, that any effort has been made to demonstrate the presence of a “latent immunizing active principle” by scientific methods of modern immunology. The following claims for the use of Toxicide appear on the label:
“Acne, boils, carbuncles, furuncles and abscesses of the most virulent types usually begin to show improvement within 4 to 12 hours after beginning administration.
“In badly infected wounds, Toxicide will check the further destruction of live tissue and should always be given for a few days before and after operations on pus cases.
“For gunshot wounds and other conditions difficult to sterilize or drain, Toxicide is the ideal remedy.
“For abscesses existing or threatened in any obscure location, the middle ear, the mastoid, the frontal or any accessory sinuses, Toxicide is of inestimable value.
“If administered early, in fractures, compound or simple, or for laceration and other injuries, inflammation, swelling, soreness and destruction of tissue will be greatly mitigated.”
In support of these claims there are offered letters from physicians who have used Toxicide with good results. None of these testimonials present evidence that the reported effects were due to Toxicide. The asserted—and highly improbable—action of Toxicide could be determined only by an extensive series of carefully controlled clinical trials—and such evidence is entirely lacking. In fact, the claims appear to have no better basis than the coincidence which is stated to have led to the discovery of the “remedy”; namely, that a boil on the neck disappeared shortly after the administration of Toxicide!
The Council finds Toxicide inadmissible to New and Nonofficial Remedies because (1) the identity and amount of the potent constituent or constituents have not been furnished; (2) the preparation is advertised indirectly to the public; (3) the name “Toxicide” is therapeutically suggestive, and (4) the therapeutic claims, being unsubstantiated by evidence, are unwarranted.

[Editorial Comment.—It seems rather preposterous that a scientific body, such as the Council on Pharmacy and Chemistry, should have to waste its time in investigating and reporting on such an obviously unscientific product as “Toxicide.” So long, however, as there are physicians who will take preparations of this sort seriously, the Council feels that it is its duty to report on such products. The problem, in fact, was well stated in a letter addressed to the editor some months ago by the secretary of a county medical society who had just received a visit from a representative of the Toxicide Laboratories and who sent to The Journal some of the advertising matter that he had received from the same source. This physician wrote:
“I do not wish to trouble you with this kind of material, usually deposited safely in my waste paper basket, but the enclosed was handed to me today by a ‘bird’ who is calling on all the doctors and making strong statements. When he claimed that ‘Toxicide’ is being used in the Presbyterian Hospital, Chicago, and that the Council on Pharmacy and Chemistry is considering it seriously, etc., etc., I wish to know whether I am missing any real good thing. If it has any real virtue, I would like to know about it, but if it has not, it seems to me that something ought to be done to head him off as some doctors are sure to fall for some of it.”
The Toxicide Laboratories is, apparently, merely a trade name used by the alleged originator of “Toxicide,” J. F. Ruckel, M.D. According to our records, Ruckel was born in 1860 and was graduated by the Chicago Homeopathic Medical College in 1886. He claims to have originated Toxicide about twenty years ago and to have prescribed it “in over 3,000 cases.” In addition to Toxicide, the Toxicide Laboratories also put out “Dianasiac for Nymphomania and Satyriasis” and “Somnosine for Insomnia.”]—(From The Journal A. M. A., Oct. 8, 1921.)
The Council has authorized publication of the following report:
W. A. Puckner, Secretary.
“Pil. Mixed Treatment (Chichester)” is a proprietary preparation of the Hillside Chemical Co., Newburgh, N. Y. It is sold in the form of pills, each said to contain 1⁄20 grain of mercuric iodid and 5 grains of potassium iodid.
In 1907 the Council examined the therapeutic claims advanced for this preparation and found that they were unwarranted, exaggerated and misleading. It found, also, many misleading statements in regard to the product itself. Furthermore, the A. M. A. Chemical Laboratory found the pills to be “short weight” in potassium iodid content.
At the time that the Council examined Pil. Mixed Treatment (Chichester), a dermatologist of recognized standing, to whom the “literature” for this product had been submitted for an opinion, made the following report:
“Assuming that this pill contains what is claimed for it, one-twentieth (1⁄20) of a grain of biniodid of mercury and five (5) grains of potassium iodid, it presents neither an original nor a very useful formula.
“The literature furnished by the company abounds in suggestions that the mixture, as they prepare it, represents some unusual potency which is not possessed by the ordinary mixture of these same drugs in the same proportion. These suggestions may of course be dismissed without consideration. There is nothing mysterious in a mixture of potassium iodid and biniodid of mercury and this formula is no more entitled to special consideration than any other pill or tablet of the same composition prepared by any reputable pharmaceutical firm.
“The formula of this pill, however, does not represent a good combination. It is offered for use both during the active secondary period of syphilis and for tertiary lesions. The pill does not contain enough mercury to be an efficient remedy for secondary syphilis and not enough potassium iodid to be satisfactory in the treatment of tertiary lesions. It is neither fish, flesh, fowl, nor good red herring. A patient with secondary syphilis should not be dosed all the time with potassium iodid and for the treatment of tertiary lesions he should have a very much larger quantity of potassium iodid than can be given in these pills without giving toxic doses of mercury.
“The statement that this pill ‘does not impair the appetite nor disturb digestion and is well borne by patients who cannot tolerate iodids otherwise administered’ is a bald claim which cannot be justified by experience. The most unsatisfactory way of administering potassium iodid is in solid form. A patient who can stand potassium iodid in pill form, as it is furnished in this preparation, can stand it in any form in which it is ever administered.
“In short this preparation is neither agreeable nor efficient. The greatest objection to it is its inefficiency, for it is offered as an adequate preparation for the treatment of syphilis in all of its stages, whereas it is neither satisfactory for the treatment of secondary syphilis nor of tertiary lesions.”
During the fourteen years which have elapsed since the Council’s first examination of Pil. Mixed Treatment (Chichester), arsphenamin has been added to the syphilographer’s armamentarium and much has been learned about syphilis and its treatment. While there exist differences of opinion as to the exact value of arsphenamin in the treatment of syphilis and there are even some who desist from the use of arsenic compounds of any kind, no syphilographer of standing countenances the routine treatment of syphilis with a fixed combination of mercuric iodid and potassium iodid. The use of Pil. Mixed Treatment (Chichester) is on a par with the use of certain “blood purifiers” which were advocated at a time when the treatment of syphilis was a baffling problem.
The present advertising, which reads as if it had been written in the heyday of proprietary license, is, in effect, an invitation to treat syphilis in its various stages and manifestations with Pil. Mixed Treatment (Chichester). If heeded by those who read the advertising of the Hillside Chemical Co., it will result in much harm to the public and the profession. For this reason, the present report of the Council is published as a protest against any advertising propaganda advocating the routine treatment of a disease which requires that each case be studied carefully so that prompt and efficient measures may be applied to the various manifestations of the disease.
The following advertisement appeared recently in several medical journals:
“Medicine is an Exact Science—on Paper Only!” Every general practitioner of medicine is called upon to treat Syphilis occasionally. He cannot depend upon the use of arsenicals alone. In most cases, “mixed treatment” the giving of mercury and iodides is required to get satisfactory results. Pil. Mixed Treatment (Chichester) accurately and successfully meets the indications and assures definite action. Important advantages:
Ready solubility of mercury in combination with Potassium Iodide.
Avoidance of gastric, buccal or intestinal disturbance.
Easy administration, can be taken at any time, anywhere.
Economical, both drugs in one combination.
Accurate adjustment of dosage to each individual case.
Full physiological action—assured by purity of content.
Secrecy—patient or friends do not know nature of medicine. Pil Mixed Treatment (Chichester) has been time tested and trial proven. It needs no introduction to the thousands of physicians who prescribe or dispense it.
While the advertisement does not directly so advise, yet it is a subtle invitation to the general practitioner to use Pil. Mixed Treatment (Chichester) and thus save himself and his patient the time and inconvenience which the rational treatment of syphilis imposes. A circular “The Treatment of Syphilis Simplified and Improved” begins:
“No therapeutic fact is more conspicuously and decisively established than that a radical cure of syphilis can be effected by the continuous administration, from the period of development, of a proper combination of mercury with iodine.”
Continuing, it is admitted that mercury is the most efficacious drug in the primary and secondary stages of syphilis and iodin in the tertiary stage, but it is asserted that:
“... it is now granted by all syphilologists that the antiluetic action of these drugs is immeasurably augmented by properly combining them, and that the best results are obtained when they are conjunctively administered throughout the entire course of the disease.”
Arguing along the same lines, this circular continues:
“... it was not until mercury and iodine in the form of Pil. Mixed Treatment (Chichester) was evolved that the marked advantages of the combined employment of these drugs in the various stages of syphilis became a scientific certainty.”
Further we are asked to believe that:
“Because of the greatly increased potency of mercury and iodine when combined, as in Pil. Mixed Treatment (Chichester), the foremost syphilologists are now agreed that the employment of these drugs in such form should be enjoined as soon as the disease develops, and should be thus continued until a cure has been effected; in other words, Pil. Mixed Treatment (Chichester) should be made the sole antisyphilitic medication throughout all stages of the disease.”
The circular illustrates the extent to which our knowledge of drugs may be distorted and misrepresented and the public health jeopardized in the exploitation of a proprietary medicine.

In its advertising, the Hillside Chemical Co. claims that Pil. Mixed Treatment (Chichester) both as to formula and method of preparation “in the incapsulated powder form” was “brought to the notice of the profession by Dr. W. R. Chichester of New York, an eminent Syphilographer and recognized authority in the therapeutics of Syphilis.” It is claimed that this pill “is perfectly soluble, tasteless, nonirritant, and therefore well adapted to a sensitive stomach.” It is claimed that the pill “is always preferable to one extemporaneously prepared, which, even if identical in composition, often gives negative results.”
An examination made in the chemical laboratory of the association to determine if the product now marketed contains the claimed amount of potassium iodid indicated that this was the case. The chemist who made this examination commented as follows on the claim that in this pill, potassium iodid is rendered tasteless, that the pill is “perfectly soluble” and that extemporaneous pills of “identical ... composition often give negative results.”
“That the potassium iodid has been rendered tasteless is false, naturally; the pills when placed in the mouth, after removal of the coating, have the characteristic taste of alkali iodids. The claim that the pills are entirely soluble is incorrect; they contain a large amount of insoluble material, probably kaolin. The assertion that an extemporaneous compound prescription even if identical in composition with the Chichester pill is often inert, is absurd and a reprehensible attack by suggestion of the ideal that the physician shall write his prescription to meet the individual needs of his patient and that the pharmacist shall compound the prescriptions of the physician as they are required. It should also be pointed out that while much is said about the potassium iodid in the Chichester pill being in powdered form, the pill mass is solid and very slowly soluble and the claim of being in powdered form is, if immaterial, also incorrect.”
As to the asserted standing of the alleged discoverer of the formula for Pil. Mixed Treatment: Dr. William R. Chichester appears to have lived and practiced in New York since 1886 or longer, but the claim that he is an “eminent syphilographer” seems to have originated with the exploiters of “Pil. Mixed Treatment.” Search failed to show the name of W. R. Chichester among authors of textbooks of syphilis or any other branch of medicine or among authors of contemporary literature in the Index Medicus from 1907 down to the present; nor did a search of the catalogue to the Surgeon-General’s Library reveal W. R. Chichester as ever having published anything on syphilis or any other subject.
Pil. Mixed Treatment (Chichester) is sold under therapeutic claims which are unwarranted and misleading. The preparation well illustrates the abuses which are connected with the exploitation as proprietaries of established drugs or mixtures of established drugs.—(From The Journal A. M. A., Oct. 22, 1921.)
The Council has authorized publication of the following report explaining why Atophan has been omitted from New and Nonofficial Remedies. Schering and Glatz, Inc., the firm which markets this brand of cinchophen in the United States, has refused to place either the U. S. Pharmacopeial name, “Phenylcinchoninic Acid (Acidum Phenylcinchoninicum)” or the N. N. R. name, “Cinchophen,” on the label and in the advertising matter so as to make the identity of the product clear to physicians. Furthermore, the product is sold under therapeutic claims which the Council holds to be exaggerated and unwarranted.
W. A. Puckner, Secretary.
The substance, 2-phenyl-quinolin-4-carboxylic acid, was described by Doebner and Gieseke in 1887 (Ann. d. Chem. [Liebig’s] 242:291). The therapeutic properties of this compound were described by Nicolaier and Dohrn in 1908 (Deutsch. Arch. f. klin. Med. 93:331). Subsequently the product was placed on the market and extensively advertised by the Chemische Fabrik auf Actien (vorm. E. Schering), Berlin, Germany. This firm also took out a patent in the United States on its production and in 1911 secured a U. S. trademark on the name “Atophan.” In 1912 Atophan was passed on by the Council and admitted to New and Nonofficial Remedies.
When the government of the United States took charge of German-owned patents during the World War, the Federal Trade Commission, and later the Chemical Foundation, Inc., issued licenses to American firms whereby these were authorized to manufacture the compound. In the meantime, Schering and Glatz, Inc., who had been the U. S. representatives for the Chemische Fabrik auf Actien, also undertook to supply the drug, but did not obtain a license from the boards in charge of German patents. Also, this firm secured, in 1919, a trademark of the word “Atophan,” apparently after the German-owned trademark had been canceled.
The drug “Atophan” was admitted to the U. S. Pharmacopeia as “Phenylcinchoninic Acid (Acidum Phenylcinchoninicum).” As this name proved too cumbersome, the Council on Pharmacy and Chemistry coined the abbreviated name “Cinchophen” for it, and this name is now used by all the firms which are marketing the product in the United States, with the exception of Schering and Glatz, Inc., who use the term “Atophan,” first owned by the Chemische Fabrik auf Actien.
Because of the confusion which is bound to arise from giving various names to one drug, the Council selects a common name and provides standards of identity, purity and strength for any drug which, by reason of the absence or lapse of patent rights or for other reason, is open to manufacture by more than one firm. The Council, then, will accept such article only if it is marketed under the title adopted for New and Nonofficial Remedies. The rules provide, however, that when the Council adopts a common name for an article that has been admitted under another name, such article will be retained in New and Nonofficial Remedies under the older name if the Council name is given prominence on the label and in the circulars and advertisements, in order to avoid confusion. Accordingly, when the period of acceptance for Atophan in New and Nonofficial Remedies was about to expire, Schering and Glatz were notified that Atophan could be retained in that publication only on condition that the name, “Cinchophen,” or else the pharmacopeial name, “Phenylcinchoninic Acid (Acidum Phenylcinchoninicum)” be placed on the label and used in the circulars and advertising.
At the time that the Council asked Schering and Glatz to adopt cinchophen or phenylcinchoninic acid as a synonym for Atophan, the firm was also requested to omit from future advertising a number of therapeutic claims to which the Council was obliged to take exception. Schering and Glatz refused the first request and made no definite promise with regard to the second. The Council, therefore, directed the omission of Atophan from New and Nonofficial Remedies, 1921.
The advertising to which the Council took exception does not appear to be distributed at present. A pamphlet has been sent out, however, which is equally objectionable. It contains unwarranted therapeutic claims and suggests that Atophan be used in conditions in which it is not indicated. For instance:
“No longer the vague, hypothetical, ‘test-tube demonstrated’ principle of uric acid elimination by solution, but a definite, scientifically and clinically established, physiologic stimulation of the uric acid excretion. Performed innocuously and controllable to a nicety by dosage and by urine and blood tests.”
The “innocuousness” of Atophan has not been proved; on the other hand there is evidence that it is not innocuous, as the recent investigations of Hanzlik and Scott and their collaborators (Cinchophen, Neocinchophen and Novaspirin in Rheumatic Fever, J. A. M. A. 76:1728 [June 18] 1921) show that it may injure the kidney.
The circular also contains the following:
“No longer, hit and miss relief of pain at the expense of the heart, the intestines, the kidneys and the nervous system, but the promptest and most reliable analgesic, anti-inflammatory and decongestive action so far known, with notable freedom from heart depressant, renal irritant, constipating and cumulative toxic by-effects. No contraindications, except chronic nephritis and the presence of kidney concretions.”
This is misleading. The drug depresses the circulation, injures the kidney and produces symptoms of salicylism or “toxicity.” It is not the promptest and most reliable analgesic; morphin is superior and salicylate is just as efficient. The phrase “decongestive action” is vague. Treatment of pulmonary congestion from phosgene, and congestion of the conjunctiva in mustard oil chemosis of cats, with large doses of Atophan was ineffective; in fact, it proved distinctly harmful. This was shown by such workers as Laqueur and Magnus, and Heubner and Gildemeister (Ztschr. f. d. ges. exper. Med. 13:200, 1921). It is incorrect to ascribe “decongestive” or “anticongestive” action in the true sense to Atophan (cinchophen). The principal assets of the salicylate-cinchophen class of drugs in the treatment of rheumatism and gout are their analgesic and antipyretic qualities.
The claim is made:
“In Rheumatic and Gouty Disorders, whether of the well-known muscular and arthritic type, or their Eye, Ear, Nose and Throat manifestations.”
The suggestion that Atophan is indicated in “their Eye, Ear, Nose and Throat manifestations” is a vague generalization without definite meaning, but nevertheless calculated to impress physicians and promote the sale of Atophan for common and minor ailments. Rhinitis and sore throat are, of course, self-limited conditions which require chiefly good habits, personal and general hygiene as prophylactic measures, and simple hot baths with rest, instead of medication, for symptomatic relief. When it comes to ear and eye conditions, Atophan certainly would do no good in otitis media, panophthalmitis, choroiditis, retinitis, etc.
The administration of Atophan is proposed “in Migrains, Hemicrania, Eyestrain, etc., often vaguely grouped as ‘Headaches.’ ” Eyestrain and headaches are vague symptoms often arising from numerous causes that require no medication, but rather good habits, hygiene and similar corrective measures. There is always the possibility of habituation from the use of drugs for such common and vague symptoms, resulting eventually in more harm than good to the patient.
The use of Atophan is proposed “In Influenza (Grippe) for the ready alleviation of the respiratory congestion, pain and stiffness of limbs and back.” Probably the entire claim is without warrant, since influenza is a self-limited disease. Atophan might relieve pain in the joints, reduce the fever, etc., but at the same time it would tend to impair the functional efficiency of the heart, which may be impaired already by the disease. Cardiac failure is one of the causes of death in influenza. The recommendation for “alleviating respiratory congestion” is certainly without warrant, since in actual trial in pulmonary congestion by Magnus et al., Atophan was found to be deleterious and not beneficial. Phosgenized cats are probably as good a test object for the alleged decongestive action of Atophan as anything could be, since, according to Underhill and Ringer (J. A. M. A. 75:1531, 1920) the pathological physiology of the circulation and respiration in phosgene poisoning and influenza are nearly identical.
Further, Atophan is recommended “In Pyorrhea Alveolaris as a systemic support to local and specific measures.” Atophan is not indicated here. Pyorrhea requires local medication, if anything at all. It could exert no local beneficial effects in this condition; indeed, the employment of Atophan might lead to irritation. Good dental treatment is more essential than medication.
Finally, Schering and Glatz advise Atophan “In Eczema, Pruritus and similar irritant and itching Skin Diseases with lowered blood alkalinity.” The assumption that blood alkalinity is lowered in irritant and itching disease is unsupported by evidence in medical literature and the recommendation is incorrect and misleading. Neither does Atophan alter the reaction of the blood. Amelioration in these capricious conditions occurs without medication so that any relief that might be obtained could not be attributed to Atophan. The entire paragraph is misleading and will undoubtedly tend to extend the use of Atophan in conditions for which it is not suited.—(From Reports of Council on Pharmacy and Chemistry, 1921, p. 8.)
Urotropin is a proprietary name applied to the substance which is known in chemical literature as hexamethylenetetramin and which is designated hexamethylenamine in the U. S. Pharmacopeia. The Council has authorized publication of the following report explaining that Urotropin was omitted from New and Nonofficial Remedies because Schering & Glatz, Inc. (the firm that markets this brand of hexamethylenamin in the United States), refused to place the U. S. Pharmacopeia name Hexamethylenamine (hexamethylenamina) on the label and in its advertising so as to make clear to physicians the identity of the product, and, furthermore, because it was sold under therapeutic claims which the Council held unwarranted.
W. A. Puckner, Secretary.
This substance which is generally referred to in chemical literature as hexamethylenetetramin, the cyclic condensation product of formaldehyd and ammonia, appears to have been described first in 1860 (Butlerow: Ann. d. Chem. 115:322, 1860). Subsequently, numerous references to the preparation, properties and constitution of the substance appeared in chemical literature.
Hexamethylenetetramin is said to have been first used for therapeutic purposes by G. Bardet, who, in 1894, reported to the Société de Thérapeutique that he believed this substance to be a uric acid solvent. At about the same period, A. Nicolaier, who gave Bardet credit for suggesting the use of hexamethylenetetramin as a uric acid solvent, announced the discovery of its antiseptic action (Centralbl. f. d. med. Wissensch. 32:897, 1894; Deutsche med. Wchnschr. 21:541, 1895). Shortly thereafter as a result of Nicolaier’s publication, the Chemische Fabrik auf Aktien vorm. E. Schering, Berlin, Germany, began to offer the product to the medical profession under the trademarked and nondescriptive name “Urotropine.” In the United States, it was marketed by Schering and Glatz, who then were acting as American agents for the Schering works of Germany.
It soon became evident that hexamethylenetetramin was a valuable drug. As the substance was introduced at a time when new “synthetic” drugs were rapidly appearing and when unlimited and uncritical confidence was placed in them, and before the medical profession became skeptical of the claims advanced by manufacturers for their respective “discoveries,” it was not long before this new drug was placed on the market by many firms, each applying its own name and often keeping the chemical character of it in the background. Some of the names which were thus applied to hexamethylenamin were Cystogen, Aminoform, Formin, Uritone, Urisol, {and} Cystamine.
In 1907 the late Prof. J. O. Schlotterbeck, then a member of the Council, protested against the confusion caused by the marketing of a given drug under different names. He stated that it was not uncommon for a physician to prescribe two or more of these identical substances in the same mixture, expecting to get the combined action of different urinary antiseptics; also, that patients had been treated first with hexamethylenamin under one name and later by the same substance under another name (The Journal, Jan. 19, 1907, p. 241).
Hexamethylenetetramin was admitted to the eighth revision of the U. S. Pharmacopeia. In part because of this official recognition and standardization and in part because the extravagant reports of its virtues had been largely discounted, physicians have in general prescribed the drug by its pharmacopeial name, with one notable exception: Urotropin. One reason for this is that Urotropin was the first proprietary brand of hexamethylenetetramin introduced, a second reason is that through the extensive and persistent advertising of the proprietary name under which the substance was introduced, it has become firmly fixed in the minds of many physicians. The other is that the product was claimed to be of greater purity than the product sold under the pharmacopeial or other name although no evidence confirmatory of this claim has ever been published. On the other hand, Daniel Base, as long ago as 1907, found that hexamethylenamin sold under its pharmacopeial name is just as pure as when sold under proprietary names. When, in 1907, urotropin was admitted to New and Nonofficial Remedies, the published description showed that it was manufactured by the Chemische Fabrik auf Aktien vorm. E. Schering, Berlin, and that Schering and Glatz were the United States agents. In 1919, the description was revised to show that Schering and Glatz were no longer selling the German product.
As it is the general practice to omit articles that are admitted to the U. S. Pharmacopeia for the reason that their quality is guaranteed under the federal Food and Drugs Act and because pharmacopeial nonproprietary articles are rarely advertised with claims that require the Council’s control, yet, in the case of Urotropin, it was retained because it was sold under a name not recognized in the pharmacopeia and because special (proprietary) claims were made for it.
The period for which Urotropin stood “Accepted” expired with the close of 1921. To determine its continued eligibility for New and Nonofficial Remedies, the Council examined the labels and circular matter sent by Schering and Glatz for the purpose and also a booklet “Urotropin,” subsequently sent by the firm to physicians.
It was found that the pamphlet contained a number of unwarranted statements. Particularly objectionable are the claims made for the use of Urotropin as an antiseptic in body fluids that are alkaline, such as the cerebrospinal fluid, bile, aqueous humor of the eye, saliva, the excretions caused by middle ear infection and other excretions of the nasal, bronchial, laryngeal and mucous membranes. The lack of efficacy of hexamethylenamin in alkaline secretions is generally admitted and the clinical references to the use of hexamethylenamin in the pamphlet are obsolete. In the introduction to the pamphlet, Schering and Glatz state that they are well acquainted with the scientific research work discrediting the efficiency of hexamethylenamin in nonacid mediums, but that they feel that the accumulated evidence for its efficacy in such conditions should not be “brushed aside.” However, the pamphlet is not made up of quotations, but of unqualified statements. With one exception, all references to the antiseptic properties of the drug in alkaline mediums are previous to 1913, that is, before the importance of reaction of the medium was fully appreciated. To quote these earlier articles without regard to the later work, which in most eyes discredited them, constitutes in effect an exploitation of this brand of hexamethylenamin under unwarranted therapeutic claims.
In consideration of the confusion which arises from the application of different names to an identical article, the rules of the Council provide that when an article which has been accepted for New and Nonofficial Remedies is admitted to the U. S. Pharmacopeia under another name, it will be retained, provided the official name is given prominence on the label and in the advertising of such article. Neither the label nor the advertising for Urotropin gives prominence to the pharmacopeial name as a synonym nor indeed does it bring out the fact that Urotropin is a brand of hexamethylenamine, U. S. P. Schering and Glatz, Inc., was advised that Urotropin could be retained in New and Nonofficial Remedies only on condition that the objections to the therapeutic recommendations were removed and on agreement that the U. S. P. name appear on the labels and circular matter. The firm did not offer to make the product eligible for continued recognition; accordingly the Council directed the omission of Urotropin because of conflict with Rule 6 (Unwarranted Therapeutic Claims) and with Rule 8 (Objectionable Names).—(From Reports of Council on Pharmacy and Chemistry, 1921, p 71.)
The Council has authorized publication of the following report, declaring Styptysate (Ernst Hischoff Co., Inc.) inadmissible to New and Nonofficial Remedies.
W. A. Puckner, Secretary.
Styptysate, according to the advertisement of Ernst Bischoff Co., Inc., New York, is “obtained by dialysis from Bursa Pastoris (Sheppard’s [sic!] Purse).” It is claimed to be “The Remedy For Hemorrhages,” to be “Superior to Ergot and Hydrastis,” “of particular advantage in Menorrhagia and Metrorrhagia” and to have been “found of great value in vesical hemorrhages and hemorrhages from mucous membranes in general.” The Styptysate label bears the synonym “Dialysate Herba Bursa Pastoris”; the statement that it contains “alcohol 11 per cent.” and that it is “made in Germany.” No other statement of the composition or strength of “Styptysate” is furnished nor is the name of the German manufacturer disclosed.
In an advertising circular entitled “Styptysate, a New Reliable Hemostatic,” it is declared that in recent years the plant, Shepherd’s Purse (Capsella bursa pastoris), “has been submitted to clinical tests in the form of a concentrated dialysate, known as Styptysate, by Loewy, Oppenheim, Krummacher and others, and that their reports coincide in regard to Styptysate as a hemostatic par excellence, particularly in uterine hemorrhages, even in cases where ergot and hydrastis had failed to produce satisfactory results.” The circular also reprints some “short clinical reports” without reference to their authorship; one ascribed to Krummacher and two ascribed to “B.H.M., Kansas City, Mo.,” and the following references: “A. Krummacher, M.D., Monthly Review for Obstetrics and Gynecology, Berlin, Vol. XLIX, 4, and Vol. LII.” “H. Oppenheim, M.D., Medical Clinic, Berlin, 1920, 35.”
Shepherd’s Purse is a weed common in the United States and in Europe. Like most other herbs, it has some reputation as a folk medicine. It is used by eclectics and homeopaths, being included in the Homeopathic Pharmacopeia of the United States. Shepherd’s Purse receives no consideration at the hands of the authors of standard works on materia medica, pharmacology or therapeutics.
From an examination of recent German medical publications, it appears that the use of Shepherd’s Purse was proposed as a substitute for ergot and hydrastis, when the latter drugs became scarce in Germany. These publications, in the main, emanate from those in the employ of pharmaceutical firms and deal with proprietary preparations or they are written by physicians who used these proprietary preparations at the solicitation of the manufacturers. For this reason the reported results must be accepted with reserve.
One of the proprietary preparations discussed in the German publications is Styptysate, manufactured by Isalfabrik Johannes Buerger, Wernigerode. It is said to be produced by submitting the juice of fresh Shepherd’s Purse to dialysis and preserving the dialysate by the addition of alcohol. There is no statement as to the drug strength or the chemical or biological standards, if any, used in its manufacture; hence, the preparation is essentially a secret one. As first produced, the preparation seems to have been fortified by the addition of cotarnin: the dose was then given as ten to fifteen drops. Later, as the cost of cotarnin went up, this drug was omitted, and the drug strength increased; the dose of the new preparation is given as twenty-five to thirty drops. Just what relation, if any, the Styptysate of Ernst Bischoff Co., Inc., bears to that of the Isalfabrik Johannes Buerger, Wernigerode, cannot be determined from the Bischoff advertising. If it has any relationship the announcement that no narcotic order is required when ordering Styptysate would indicate that the new preparation is supplied; the old one with its addition of cotarnin would require a narcotic order. On the other hand, the recommended dose of the cotarnin-free preparation is twenty-five to thirty-drops, whereas the product sold by Bischoff and Co. is to be given in doses of ten to fifteen drops—that is, in the amount proposed for the cotarnin-fortified product.
What justification is there for the claim that Styptysate has been submitted to clinical tests by Loewy, Oppenheim and Krummacher and found to be a hemostatic par excellence and efficient even where ergot had failed to give satisfactory results? Loewy (Zentralblatt für Gynäcologie 42:920, 1921) made some pharmacologic tests on guinea-pigs with the cotarnin-containing preparation, but reported no clinical trials. Hans Oppenheim (Medizinische Klinik, Aug. 29, 1920, p. 906) reported that he was agreeably surprised at the excellent results (vorzueglichem Erfolg) obtained with the drug but he did not assert that it is superior to ergot.
Krummacher reported on thirteen cases of profuse menstruation in which the patients were treated with Styptysate, using for a part, the preparation containing cotarnin and for the other a preparation without cotarnin. He reported as good results with the cotarnin-free preparation in larger dosage, as with the cotarnin-containing preparation in smaller dosage. Krummacher did not compare Styptysate with ergot. Some of Krummacher’s cases are quoted, with some typographical errors, in the Bischoff circular.
On the assumption that the product discussed in German publications is the Styptysate marketed in the United States, the best that can be said for it is, that during a shortage of ergot it was used in place of that established drug. There is no evidence to warrant the use of this indefinite proprietary in place of the biologically standardized fluidextract of ergot or other standardized ergot preparations.
Styptysate (Ernst Bischoff and Co., Inc.) is inadmissible to New and Nonofficial Remedies because its composition is semisecret and indefinite and there is no evidence that its uniformity and strength is controlled (Rules 1 and 2); further, it is inadmissible because the therapeutic claims advanced for it are exaggerated and unwarranted (Rule 6) and because there is no evidence that it possesses any advantage over established drugs such as the biologically standardized fluidextract of ergot or the definite ergot preparations admitted to New and Nonofficial Remedies.—(From The Journal A. M. A., Feb. 11, 1922.)
The Council has authorized publication of the following report declaring Lipoidal Substances (Horovitz) inadmissible to New and Nonofficial Remedies because its composition is essentially secret and because the curative claims made for it are unsubstantiated and, therefore, unwarranted.
W. A. Puckner, Secretary.
In the advertising of the Horovitz Biochemic Laboratories Co. (A. S. Horovitz, president) we read:
“Horovitz proves by careful paralleled investigations of normal and of pathological tissues, both in addiction disease and in other diseases, that in patients suffering from narcotic addiction disease there is an inactivity of the lymph-glands due to the use of the drug and that the system is not supplied with the necessary fats.” “Horovitz further found that the lipoidal content of the cerebro-spinal system varies in strict accordance with the pathological processes introduced by infection or by alkaloids. Furthermore, he has found that the lipoids of various other organs, as well as those of the nervous system, may be extracted and consumed by the administration of narcotic alkaloids.”
It is further stated in the advertising that:
“After a long and very careful research investigation, Dr. Horovitz worked out a method of rational treatment for narcotic addiction disease which involves the restoration of the lipoids, which have been lost through the action of the drug, and of the toxins, by means of a combination of lipoidal substance from various plant lipoids in the form of a sterile solution. This preparation not only replaces the lipoids lost by the tissues, but also protects the nerve tissues, from attacks by the toxins elaborated during the use of narcotics, and, this by detoxicating the tissues, brings about permanent freedom from the craving of narcotics, instead of the temporary relief afforded by other methods of treatment.”
The “combination of lipoidal substance of various plant lipoids” which was worked out by Horovitz, the Horovitz Biochemic Laboratories offer as “Lipoidal Substances.” This preparation is supplied in ampoules said to contain 1 c.c. of solution. The treatment with “Lipoidal Substances” consists, first, in the complete withdrawal of the narcotic; second, in free catharsis; and third, in the intramuscular injection of the preparation. The initial dose is given as 8 to 12 minims repeated with increase of 3 to 4 minims every three hours during the first day. On the second, third and fourth day 16 minims is to be given twice a day and “from the fifth day until the medication is stopped (usually 28 to 35 days) it will be necessary usually to give but 1 injection of 16 minims each day.”
In a request for the admission of its preparation to New and Nonofficial Remedies, the Horovitz Biochemic Laboratories Co. stated:
“The composition of Lipoidal Substance is (a) Lipoids of plant origin, (b) Vitamines (water soluble) of plant origin, (c) Non-specific plant proteins, (d) Preservatives—None.”
While the communication abounded in generalities, it gave neither the identity nor character of the lipoids, of the vitamins nor of the nonspecific protein, nor their quantities or methods for their control. The firm presented no evidence that the injection of “Lipoidal Substances” produced any effect other than by suggestion. Also, while a long list of references to publications bearing on lipoids was submitted (many of which had no bearing on the subject under consideration) there was no reference to the work of Horovitz quoted in the firm’s advertising.
After examining the information which had been submitted, the Council requested the manufacturer to supply:
1. Information as to the character (identity) of the several ingredients contained in the preparation that it marketed, the amount of each ingredient so far as known and the method used for their control.
2. Evidence that the administration of “Lipoidal Substances” is of value in the treatment of drug addiction.
3. Evidence for the claims that the “researches” of Horovitz have proved that “in patients suffering from narcotic addiction disease there is an inactivity of the lymph-glands ... and the system is not supplied with the necessary fats” and that “lipoidal content of the cerebro-spinal system varies in strict accordance with the pathological processes introduced by infection or by alkaloids” and that “the lipoids of various other organs as well as those of the nervous system, may be extracted and consumed by the administration of narcotic alkaloids.”
The Horovitz Biochemic Laboratories replied that the requested information would be supplied in about two weeks. At the expiration of three months the promised information and evidence had not been received; neither had any reports to show the value of the treatment come to the attention of the Council. The Council, accordingly, declared “Lipoidal Substances” (Horovitz Biochemic Laboratories) inadmissible to New and Nonofficial Remedies because the composition is essentially secret and because the curative claims are unsubstantiated and unwarranted.—(From The Journal A. M. A., Feb. 25, 1922.)
The Council has adopted the following principles as a guide in the consideration of yeast preparations and vitamin B concentrates for New and Nonofficial Remedies.
W. A. Puckner, Secretary.
1. The claim that deficiency of vitamin B and diseases resulting therefrom are common conditions in the United States is not at this time supported by adequate acceptable evidence.
2. The claim that yeast preparations or extracts are, in principle or in general, essentially more effective or more practical or more available means of administering vitamins than the commonly available vitamin-containing foods is not at this time supported by adequate acceptable evidence. (Any claims for superiority made for such products proposed for inclusion in New and Nonofficial Remedies must be presented in detail and passed on specially by the Council.)
3. The claim that therapy with yeast or yeast preparations has as yet more than an experimental status is not at this time supported by adequate acceptable evidence.
Preparations for which such claims are made, directly or by implication or in one-sided quotations, in advertisements or letters or by salesmen, cannot be admitted to or retained in New and Nonofficial Remedies.—(From The Journal A. M. A., April 15, 1922.)
The Chemical Laboratory of the American Medical Association was established in 1906 to assist the Council on Pharmacy and Chemistry in the investigation of proprietary remedies.
In accordance with the principle of its foundation, the Laboratory examines and checks the claims made for the composition and chemical properties of the products under examination by the Council, and when these are admitted to New and Nonofficial Remedies, it insures the establishment of tests and standards whereby the identity and purity of these products may be controlled. In addition, the Laboratory supplies information, secured by reference to chemical and pharmaceutical literature or by actual analytic work, in regard to proprietary and unofficial medicines, either for publication in The Journal of the American Medical Association or through direct correspondence.
Those portions of the Laboratory’s activities which are of special interest to physicians and which were not included in the Reports of the Council on Pharmacy and Chemistry were included in the Propaganda for Reform in Proprietary Medicines, ninth edition (1916), so far as they had been published up to the time when the edition was issued; those made during the last five years are included in Part II of this volume.
For a detailed report of the Laboratory’s work, the reader is referred to the article that follows on “The Work of the American Medical Association Chemical Laboratory.” Those who are interested in the analysis of drugs are referred to the Reports of the Chemical Laboratory issued annually for the details of the analyses which have been made by the Laboratory.
The American Medical Association Chemical Laboratory was established nearly ten years ago—in fall of 1906. The reason for its existence was primarily the fact that the Council on Pharmacy and Chemistry found it difficult to secure from outside sources such help as it needed in checking up the composition and properties of proprietary medicines under investigation. Medical schools and similar institutions were found ready to lend their assistance in pharmacologic and medical investigations; but the chemical investigation required the establishment of a laboratory under the control of the American Medical Association.
As years have passed, the scope of the laboratory has been extended: Its services have been requisitioned by The Journal in various ways. Thus, when requested, the laboratory reviews and verifies the chemical data contained in editorials and original contributions. The laboratory is often called on for information as to the character and composition of quack treatments and so-called “patent medicines.” Through the columns of The Journal and through direct correspondence, the laboratory responds to requests of physicians with information regarding the composition of medicines which they prescribe or in which they are interested. The laboratory attempts to be to the members of the American Medical Association what the prescription pharmacist is, or should be, to the prescribing physician—a storehouse of chemical and pharmaceutical information. In the belief that an insufficient familiarity with the chemistry and pharmacy of drugs constitutes the chief reason for the extensive use of unscientific, worthless or fraudulent proprietary remedies, this service is rendered by the laboratory as a contribution to the cause of rational therapy.
Since the efficiency of the American Medical Association Chemical Laboratory will increase as its activities are better known, the following more detailed statement of its work is offered:
As stated in the rules of the Council on Pharmacy and Chemistry, it is “manifestly impossible for the Council to investigate the composition of every complex pharmaceutical mixture ...”; “it can only give an unbiased judgment on the available evidence.” In line with this, the laboratory does not undertake to prove the composition of constitution of all new synthetics, nor does it attempt to determine the individual composition of proprietary mixtures. It checks all claims that seem doubtful, however, and uses its best endeavors to secure correction of misstatements with regard to proprietary remedies and improvement in the quality of these products. Further, it reexamines, when this seems desirable, the products which have been admitted by the Council to New and Nonofficial Remedies, and thus determines, from time to time, their dependability. The fact that no product admitted to New and Nonofficial Remedies has later been shown to be untrue to its claimed composition is, it is believed, an indication that in this respect the laboratory has succeeded in performing the work for which it was primarily created.
In this connection the question may be asked, Are many proprietary medicines exploited to the medical profession with false claims in regard to their composition? Also it may be asked, Has the number of proprietaries marketed with false statements of composition decreased since the Council and the laboratory began their work? Answering the latter question first: There is no doubt that today fewer proprietary medicines are being sold with false claims as to composition than there were ten years ago. When the Council began its work, medical journal advertising teemed with statements regarding the composition of medicines which any chemist familiar with medicine would not hesitate at sight to brand as untrue. Today such manifestly false claims are rare. Coming to the former question: Many false statements regarding the identity and composition of remedies have been made in ignorance. This is not surprising when it is remembered that the most ignorant may and do engage in the manufacture of medicine. Besides ignorance, however, an accommodating conscience on the part of the manufacturer and a failure on the part of the medical profession to appreciate the danger which lies in the use of medicines of unknown composition unquestionably have greatly encouraged the marketing of falsely declared medicines. A glaring illustration of the ignorance of manufacturers—for it is hard to believe that any business concern would deliberately court prosecution by the federal authorities through false statements on labels—is the fact that nearly thirty years ago A. B. Lyons published a report147 pointing out that the proprietary Iodia was falsely declared as to composition and that in 1914 when the Council examined this preparation such incorrect declaration appeared on the label.148 That many physicians do not recognize the danger to their patients and their reputation in the use of medicines, the composition of which they do not know, is illustrated by the fact, disclosed by inquiries sent to the laboratory, that physicians were found willing to employ an arsenical preparation (Venarsen), advertised for intravenous use, although its promoters vouchsafed no information in regard to the nature of the arsenic compound contained therein.
The purpose of the federal Food and Drugs Act is to secure the prosecution and punishment of all who sell medicines which are adulterated or misrepresented as to composition. As a matter of fact, the wording of the law relating to the adulteration and misbranding of drugs is such that the federal authorities have been able to do little more than to require that the drugs for which standards are provided in the Pharmacopeia shall when sold comply with those standards. Similarly, those states which attempt to improve the quality of drugs sold within their borders—few states do efficient work along these lines—limit their work to the enforcement of the Pharmacopeial standards. This leaves the vast number of unofficial drugs and medicaments beyond the control of federal or state authorities. While most of these drugs are relatively unimportant, and while the amounts of them which are used are not great individually, the total consumption of them is large. With a view of furnishing to physicians standards for drugs of this sort the Council has described in New and Nonofficial Remedies not only distinctly proprietary drugs, but also some of the unofficial drugs which are apparently of therapeutic value and used to a considerable extent. Aiding the Council in this line of endeavor, the laboratory has attempted to establish standards for these little used drugs, and New and Nonofficial Remedies, 1916, provides standards for such unofficial and non-proprietary drugs as quinin and urea hydrochlorid quinin, tannate, sodium acid phosphate, and sodium perborate. An example of work which furnished much needed standards for an unofficial article is the investigation of zinc permanganate by W. S. Hilpert.149 Reference to the published reports of the laboratory will give an idea of the amount of work such standardization entails. A reference to the new U. S. Pharmacopeia, when this comes from the press, will show that a considerable number of unofficial articles described in New and Nonofficial Remedies have been admitted to the Pharmacopeia along with the standards worked out in this laboratory.
While in a way the work done in connection with these less important drugs has attracted little attention from the medical profession, it has had an effect on pharmaceutical manufacturers. In the past, pharmaceutical houses, ever anxious to market something new, on the slightest provocation have placed on the market, in the form of pills, powder, elixir, ampule, etc., every drug for which some sort of medical recommendation could be found. In marketing these dosage forms, the manufacturer has too often been little concerned about the quality of the drugs used.150 Just at present, for instance, some interest is being shown in iron cacodylate; but while manufacturers appear to be most ready to take advantage of this interest by offering the drug in the form of ampules, etc., they have given little help toward the establishment of standards for this arsenic compound. Manufacturers are ever ready to sell drugs of all sorts, but in view of the small demand they cannot or will not safeguard the identity and purity of such drugs. A further illustration of the unreliability of unofficial drugs is the recent report by Levy and Rowntree151 showing not only that the various dosage forms of emetin hydrochlorid obtained from different manufacturers varied from manufacturer to manufacturer, but also that the product of the same manufacturer was variable and that the supply furnished by one pharmaceutical firm was so toxic as to make its use dangerous.
In the preface to the first annual report of the chemical laboratory it was stated that the laboratory “occasionally takes up the examination of ‘patent medicines’ ...” At that time it was felt that the widespread use by the medical profession of irrational and even secret medicines made it necessary to devote the laboratory’s attention to the correction of this evil. As the years have passed on, these conditions have been remedied to some extent, at least so far as chemical analysis can correct them. On the other hand, public opinion has been aroused to the many evils connected with the exploitation of “patent medicines,” and has more and more insistently demanded that the medical profession aid in the correction of this evil. Accordingly, the laboratory has paid much attention to the analysis of “patent medicines” during the last few years. As the chief asset of “patent medicines” is the element of secrecy which surrounds their composition, it is hoped that the laboratory’s analysis of such widely used “patent medicines” as Nature’s Creation,152 Mayr’s Wonderful Stomach Remedy,153 Sanatogen,154 Eckman’s Alterative,155 Tonsiline,156 and Bromo-Quinin157 has been worth the labor. In addition, the work of this laboratory has been published, including not only the results of its analyses, but also the methods which are used. In view of the dearth of published reports regarding the methods used in the analysis of “patent medicines,” it is hoped that this feature of the laboratory’s work has been of aid to chemists engaged in similar work.
The laboratory’s activities along these lines have done much to discount the claim of proprietary manufacturers that chemical analysis is unable to determine the character of “patent medicines.” The recent Wine of Cardui trial has brought it out prominently that chemical analysis can determine the presence of potent constituents, and that “patent medicines” which fail to reveal such potent ingredients to the analyst may safely be put down as worthless. The demonstration that the essential composition of medicinal preparations may be determined by chemical analysis should also prove an effective answer to the manufacturers in their protest against the requirement, now being urged for enactment into law in various states, that the medicinal ingredients of their wares must be declared on the label. Manufacturers have held that this would lay them open to competition with imitations and substitutions. The possibility of chemical identification proves, however, that secrecy of composition, though it prevents consumers from knowing the character of a “patent medicine,” will not be a hindrance to the imitator and substitutor.
In the past, much of the experimental work in medicine has seriously suffered in that the identity of the material used in such investigations was not established. In view of this the laboratory has watched the contributions submitted to The Journal, and whenever necessary and feasible has urged the authors to identify their material before publication of the findings. For instance, a number of staining agents—so-called “anilin dyes”—have been found to possess therapeutic action. Since the identity of many of these staining agents is today essentially secret, the laboratory has urged through The Journal that those who experiment with these substances make an effort to determine their identity whenever possible and to give preference to those the chemical identity of which is known. The need for such identification has been discussed in the reports of the laboratory.158 The amount of work involved in the chemical identification of drugs used for experimental work is illustrated in a contribution entitled “An Examination of Several Commercial Specimens of Opium Alkaloids or Their Salts.”159 by L. E. Warren, in which was determined the identity of the various opium products used in an investigation by D. I. Macht, carried out under a grant of the Therapeutic Research Committee.
In the past much of the information in regard to the composition and properties of medicines which has appeared in pharmaceutical journals has not become available to medicine. In many cases medical journals could not afford to publish such data because this would have been contrary to the interest of their advertisers, and hence the publications regarding the irrational character of Lactopeptine, of Bromidia, etc., which appeared in the pharmaceutical journals did not become a matter of common medical knowledge. Through the laboratory an attempt has been made to keep the medical profession informed in regard to pharmaceutical literature. The laboratory has a good working pharmaceutical and chemical library, and subscribes to the important American and foreign pharmaceutical and chemical publications. The discussion of new remedies, such as medinal and sodium veronal,160 salvarsan, atoxyl and arsacetin,161 and neosalvarsan162 soon after their introduction, illustrates the work of the laboratory along these lines.
The laboratory naturally is in thorough sympathy with the present day efforts toward a more rational use of drugs, as exemplified in the Council’s publication “Useful Drugs.” Two recent contributions of the laboratory may be cited as a further support of the movement for limiting prescribing to the more widely used drugs. In line with the general tendency of manufacturers to put out all sorts of modifications and asserted improvements over official substances, there have been placed on the market a number of preparations said to represent some improvement over the pharmacopeial Blaud pills. The report, “The Quality of Commercial Blaud’s Pills,”163 by L. E. Warren, shows that the ordinary pharmacopeial Blaud pill is in every way the equal of the semiproprietary preparations claimed to be improvements. Further, the examination of the various brands of sodium and theobromin salicylate as compared with the preparation diuretin by P. N. Leech164 shows that the former preparations, sold at 35 cents per ounce at the time the examination was made, are fully the equal of the proprietary Diuretin, which then cost the druggist $1.75 per ounce.
It is generally admitted that the proprietary medicine business, particularly the exploitation of complex mixtures, attained the extensive vogue which it has or had because instruction in medical schools was deficient in materia medica, pharmacy and chemistry. As a result of lack of knowledge along these lines, the young graduate after some trial became fearful of formulating his own prescriptions, and in time became dependent on pharmaceutical firms which provided him with medicines ready to dispense. That physicians have been insufficiently trained in regard to the pharmacy and chemistry of drugs has often been emphasized in pharmaceutical journals where prescriptions containing incompatible drugs are reported and where even plans are brought forward whereby the pharmaceutical profession may aid in remedying this difficulty.
During my pharmaceutical experience I was often sorely vexed as to what to do when prescriptions contained drugs which on mixing would undergo decomposition which the physician surely did not anticipate. I remember well a prescription directing that potassium permanganate be made into pills with extract of gentian and other things, and how, the physician having spurned the suggestion to modify the prescription so as to avoid decomposition of the permanganate, I was obliged to select a mortar, gently triturate the drugs until a conflagration was started, and to finish the prescription after the combustion had subsided. However, in my pharmaceutical experience I generally found the physician most ready to receive suggestions from the pharmacist which would prevent incompatibilities, improve the palatability and appearance of his prescriptions, and protect the patient from unnecessary expense.
Similarly it has been my experience since the establishment of the Association’s laboratory that physicians are anxious to receive information in regard to the materia medica, pharmacy and chemistry of drugs. As the druggist earns the respect and support of the physician when he makes available to him the pharmaceutical knowledge and experience which he has, so this laboratory has aimed to gain the endorsement of the American Medical Association membership by furnishing to physicians information in regard to the composition, chemistry and pharmacy of drugs through replies in the Query and Minor Notes Department of The Journal as well as through direct correspondence. It has been most gratifying to the laboratory that The Journal receives an increasing number of inquiries both as regards the chemical and pharmaceutical questions involved in the writing of prescriptions and as regards the composition of secret and semisecret proprietaries (often because they are prescribed by the inquirer’s colleague) and “patent medicines” (which are taken by his patient). The laboratory has tried its best to answer the many inquiries received. Many of the questions which come in can be answered by a pharmacist or chemist without hesitation. Others, particularly as to the composition of medicines, the laboratory has been able to answer by reference to its library and its extensive card index. Still others have required experimentation and chemical analysis.
While, as stated a moment ago, the laboratory has encouraged the sending of inquiries and has earnestly striven to furnish the information asked for, it is obvious that the amount of chemical work which can be done is limited. The small size of the laboratory force, consisting of three chemists engaged in actual analytical work, makes it necessary to select for investigation those problems which shall be of general interest to the medical profession. As the American Medical Association is national in its scope, the laboratory has held that it can do analytical work only when such work will be of general interest to physicians and of value both to the medical profession and the public. In view of this it has refrained from undertaking analyses which would benefit only the physician making the inquiry and possibly his patient. The laboratory further has not felt justified in undertaking work of merely local interest; instead it has used its endeavors to secure the investigation of such local problems by municipal or state authorities.—(From The Journal A. M. A., Nov. 25, 1916.)
Akoz is a mineral product sold by the Natura Company of San Francisco, and said to possess most remarkable medicinal properties.
A circular issued by the Natura Company begins thus:
“While scientists have been striving through the centuries to compound remedies for man’s various ills, Nature, greatest chemist of them all, has been working wonders in her crucibles and has achieved results far beyond man’s greatest expectation.”
“Nature’s chief handicap has been the difficulty of placing her gifts in the hands of those whom she would benefit. By accident or fate, as you will, one of Nature’s greatest medicinal products has just been discovered. It is the mineral given the name of Akoz by John D. Mackenzie, president and manager of the Natura Company of San Francisco, which is now giving this rare remedy of Nature to the public.”
The circular then describes how the power of the “rare remedy” to cure rheumatism is claimed to have been discovered and asserts that:
“Akoz was subjected to every known scientific test before being presented to the public. It was practically determined that the ore contained a new element having radium-like qualities but containing nothing poisonous or harmful.”
“After the curative virtues of Akoz for rheumatism, stomach trouble, eczema, catarrh, piles, ulcers and numerous other ailments had been fully established in chemical laboratory, hospital clinic, and the private practice of physicians in various parts of the world, Mr. Mackenzie effected the organization of the Natura Company.”
This product, put up in the form of “Akoz Medicinal Mineral Water, Akoz Ointment, Akoz Powder and Akoz Suppositories,” was submitted to the Council on Pharmacy and Chemistry for consideration some years ago with the claims that “Akoz” itself consists essentially of zinc sulphid, barium sulphate and aluminum oxid. The submitted analysis did not declare the presence of lead or of uranium though “special tests” for the latter had been “run.” Without checking the claimed composition, the Council at that time refused recognition to Akoz because there was no evidence submitted for the very extravagant and altogether improbable therapeutic claims.
After the Council had concluded the consideration of Akoz a letter was received from a California physician stating that according to an analysis submitted to him Akoz contained 0.34 per cent. of lead in the form of lead sulphate. The correspondent held that, while the lead sulphate did not pass into solution, persons drinking the supernatant liquid from Akoz (the “medicinal mineral water” is made by adding Akoz to ordinary water) might inadvertently swallow some of the powder. He was inclined to believe that this might account for a case of lead poisoning which had been observed in a patient who had been taking Akoz.
Inasmuch as it has been demonstrated by Carlson and Woelfel (Carlson, A. J., and Woelfel, A.: Solubility of Lead Sulphate and Basic Lead Carbonate in Human Gastric Juice.... In Hygiene of the Painter’s Trade by Alice Hamilton, Bull. of U. S. Bureau of Labor Statistics No. 120, May 13, 1913, pp. 22–32) that even small quantities of lead sulphate when taken into the system for a long time, have produced lead poisoning, the laboratory deemed it important that the products be examined for lead.
A specimen of “Akoz Powder” submitted to the Council by the Natura Company and contained in a sifter-top can was taken for analysis. The contents of the can were thoroughly mixed. To determine the presence of lead some of the powder was extracted with ammonium acetate solution.
Qualitative tests showed the presence of lead and sulphate in the ammonium acetate solution.
The presence of lead was demonstrated by the black precipitate with hydrogen sulphid, the yellow precipitate with potassium chromate and the typical yellowish crystalline precipitate with potassium iodin.
The presence of sulphates in the ammonium acetate solution was shown by the formation of a precipitate with barium chlorid solution and acetic acid.
Two 2 gm. samples (A and B) were taken for the quantitative determination of lead. Each was treated repeatedly with a saturated solution of ammonium acetate until the filtered ammonium acetate solution gave no appreciable precipitate with potassium chromate solution. The ammonium acetate extractions from each specimen were combined and treated with hydrogen sulphid, the precipitated lead sulphid filtered off and washed, and ignited with sulphuric acid at a low heat. The crucible with the residue of lead sulphate was cooled and weighed.
A yielded 0.0469 gm., or 2.34 per cent., lead sulphate.
B yielded 0.0440 gm., or 2.20 per cent., lead sulphate.
While the laboratory has no evidence to show that the amount of lead-sulphate thus found to be present is likely to prove harmful, the following cautionary letter was sent to the Natura Company:
“According to information which you sent to the Council on Pharmacy and Chemistry your product “Akoz” does not contain lead. In view of reports received ascribing symptoms, resulting from the internal use of Akoz, to chronic lead poisoning, an examination of a specimen of Akoz Powder, which you sent to the Council, was made. This examination indicates the presence in Akoz Powder of about 2.2 per cent. lead sulphate. In view of the disastrous results likely to follow the internal use of products containing even small amounts of lead, the above is submitted to you for your consideration.”
No reply to the foregoing was received from the Natura Company.—(From Reports A. M. A. Chemical Laboratory, 1916, p. 103.)
Recently the laboratory’s attention was called to the “ThermoR Waterless Hot Bottle,” manufactured by the Royal Thermophor Sales Co., New York. The following claims appear in one of the advertising pamphlets:
“There is moist heat.” “Rubber hot-water (? ? ?) naturally give a moist heat.” It (ThermoR) gives a dry heat.
“The ‘THERMOR’ Bottle is not a hot-water bottle—it acts on a principle that is entirely different and new.”
“... gives you first, last and all the time a fixed degree of dry usable heat—a heat that holds steadily at 125 degrees for fully twelve hours—you will easily see why it is that ‘THERMOR’ relieves and cures where hot-water bottles fail.”
The bottle was nickel plated, 83⁄8 inches in diameter and 11⁄2 inches thick, and in appearance resembled an exaggerated closed Ingersoll watch.
The bottle is not flexible and weighs 31⁄2 pounds. The contents consisted essentially of sodium acetate. This salt melts when heated. When it cools the temperature inside the bottle is relatively constant, as it will remain at the “freezing point” until all of the sodium acetate has solidified. The duration of the time that it remains warm when well wrapped is simply in inverse proportion to the conductivity of the surrounding environment. When two ordinary towels were carefully arranged about it, the air between the bottle and the wrappings was maintained at a temperature of 40–50 C. (104–122 F.) for a period of eight hours.
The company’s implication that the heat given out by the Thermor bottle differs from that given out by an ordinary hot-water bottle is an absurdity. The use of sodium acetate in the preparation of warming bottles has been in practice many years, and is not “a principle that is entirely different and new.” Furthermore, the therapeutic claims are extravagant.—(From Reports A. M. A. Chemical Laboratory, 1916, p. 105.)
A specimen of Anti-Syphilitic Compound (Sweeny), sold by The National Laboratories of Pittsburgh, was received from a physician. The package (1 ounce size) has been opened by the sender and about three fourths of the contents removed.
From the rather indefinite statements in the literature of the manufacturer it is gathered that the preparation is claimed to be a “sterile, oily emulsion” which contains 1⁄20 grain of mercuric benzoate in each 5 minims, together with some sodium chlorid. According to information furnished by the Laboratory’s correspondent, the price asked for the preparation is $15 an ounce.
The quantity of the preparation received was too small to permit a complete examination, but, from the tests which it was possible to make, the preparation appears to be an aqueous solution containing some suspended matter and small quantities of mercuric benzoate and a chlorid, presumably sodium chlorid. There was no evidence of the presence of an “oily emulsion.” Quantitative tests indicated the presence of a mercuric salt, equivalent to about 0.2783 gm. of crystallized mercuric benzoate per 100 c.c. This corresponds to about 0.00086 gm. in each 5 minims, or about 26.5 per cent. of the amount claimed.—(From Reports A. M. A. Chemical Laboratory, 1916, p. 106.)
In the last year or so, the hot-wax or paraffin treatment of burns has been widely discussed both in medical and lay periodicals. Although the treatment is simply a modification of the well-known use of oil and ointments, it has received unusual attention, owing to the widespread sensationalism following the exploitation in France of a secret and therefore mysterious mixture, “Ambrine,” the formula of Dr. Barthe de Sandfort. Owing to this publicity, it seemed desirable to investigate the chemical composition, and to compare its physical properties with other waxlike substances.
“Ambrine” is promoted as a dressing for burns, frostbites, neuritis, varicose ulcers, phlebitis, neuralgia, rheumatism, sciatica, gout, etc. It is a smoky-appearing substance, resembling paraffin in consistency and without odor. For application, “Ambrine” is melted and applied to the wound either with a brush or with a specially devised atomizer. It cools quickly, and leaves a solid, protecting film.


It is said that de Sandfort “stumbled on this treatment by accident.”165 Being a sufferer from rheumatism, he had been benefited by hot mud baths; on returning home he sought a substitute, and finally made a mixture of paraffin, oil of amber and amber resin. This was applied hot, serving as a firm poultice. “Years later, he went on service to a railway in China and was in Yunnan at the time of the incendiary insurrection, and many badly burned Chinese were brought in for treatment. Remembering that Ambroise Paré treated such cases with hot oil, he tried the effect of covering the burn with his melted ambrine, which at once glazes over, forming a coat impervious to the air, and his patients ceased to suffer.”166
“Ambrine” has been sold in America under two names: “Hyperthermine,” as exploited to physicians, and “Thermozine,” as advertised to the public. Physical comparison alone shows that Ambrine as now sold differs from “Hyperthermine” of a few years ago; the probable reason is that “Ambrine” has changed its formula. This is borne out by Matas,167 who states that de Sandfort “admitted that Ambrine was a compound of paraffin, oil of sesame and resins, but was not at liberty to divulge its exact composition, as the formula and manufacture of this substance was now the property of a private corporation, which was exploiting it as a proprietary and secret remedy.” The later formula differs from the original.
Besides the foregoing paraffin preparations, two others have recently been placed on the American market, “Parresine” (nonsecret) and “Mulene” (secret).
“Ambrine” comes in rectangular cakes, about 11⁄2 inches wide, 6 inches long and 1⁄2 inch thick. It is moderately soft, but somewhat brittle at ordinary room temperature. A black substance is present, which evidently settles out during the compounding, as in one side of the cake these particles can be clearly discerned by holding it up to the light; in the other side there are no suspended particles. When melted, the solution is not clear, and a sediment forms. The melting point (U. S. P. method; see later) is 48.4 C. The plasticity and ductility168 are 27 and 30.5, respectively. It is pliable and strong at body temperature. The saponification number and acid number are both very low, but a fatty oil is present. Tests indicated oil of sesame. Ninety-eight per cent. of “Ambrine” is soluble in ether; this soluble portion may be treated with low-boiling ligroin (petroleum ether), out of which, on standing, a black asphalt-like substance separates. Of the ether-insoluble substance, 65 per cent. is soluble in chloroform. The remaining insoluble substance contains a small amount of silica and vegetable fiber. The paraffin obtained from “Ambrine” melted at 48.6 C. As a result of various experiments, it appears that the composition of “Ambrine” is essentially as follows:
Paraffin (M. P. 48.6 C.) | 97.0 per cent. |
Fatty oil (sesame?) | 1.5 per cent. |
Asphalt-like body | 0.5 per cent. |
Coloring matter, and undetermined | 1.0 per cent. |
| —— | |
| 100.0 |
A cursory examination of “Mulene,” manufactured by the Mulene Company, Pittsburgh, was also made. This appears to contain paraffin, beeswax, a fat-soluble red dye and considerable rosin. When heated carefully in a beaker, the rosin “sticks” to the bottom, and does not go into solution readily.169
“Paresine,”170 according to the manufactures, is a mixture composed of paraffin, 94 to 96 per cent.; gum elemi, 0.20 to 0.25 per cent.; Japan wax, 0.40 to 0.50 per cent.; asphalt, 0.20 to 0.25 per cent., and eucalyptol, 2 per cent., the whole being colored with alkannin and gentian violet.171
In a recent article, Sollmann172 presented various suggestions for the compounding of paraffin films. Some of the formulas were promising and others were not, but all were simple. He did not try to imitate “Ambrine.” Lieut.-Col. A. J. Hull173 of the Royal Army Medical Corps, after experimenting with different combinations, concluded that a mixture of “1 part resorcin, 2 parts eucalyptus oil, 5 parts olive oil, 25 parts soft paraffin [petrolatum]174 and 67 parts hard paraffin” served the purpose as well as “Ambrine.” The following formula, which might be called Asphalt-Paraffin No. 21, much more closely resembles “Ambrine,” and it seems to have certain advantages, due to the use of a more suitable grade of paraffin:
Paraffin175 (M. P. by U. S. P. method 47.2 C.) | 97.5 | gm. | |
Asphalt | from 3 | to 5 drops | |
Olive oil | 1.5 | c.c. | |

About 10 c.c. of “asphalt varnish” (B. Asphaltum)176 is placed in a beaker and heated on the steam bath for one-half hour. From 3 to 5 drops, delivered from a 1 c.c. pipet, are then placed in a casserole, and 1.5 c.c. of olive oil added. The mixture is heated and stirred for a few minutes until perfect solution is effected. To this is then added, with stirring, the paraffin, which has been previously melted. When it is cooled, a brown solid is obtained.177 The physical factors of this paraffin mixture are, melting point 45.4 C. (U. S. P. method); plasticity, 28.5; ductility, 29; it is very pliable and strong at 38 C., and adheres exceedingly well to the skin, although it detaches easily. This mixture, which is easy to prepare, is inexpensive, the cost of the materials being approximately 10 cents a pound.
Both Hull and Sollmann noticed that tarlike substances and melted paraffin do not mix well. This is noticeable in “Ambrine,” which cannot be called an “elegant” preparation. The difficulty may be overcome by first mixing hot olive oil and asphalt; the asphalt will then go into solution. It is interesting to note that the suggested formula (as well as others which were also prepared) is not as plastic as the paraffin itself.178 This is also true of “Ambrine.” On the other hand, the melting point of the paraffin is higher. The important point, however, in compounding all paraffin preparations, is to select a proper grade of paraffin as elaborated below.

The name “paraffin” generally applies to a colorless and tasteless waxlike substance that is solid at ordinary temperature. It is composed of saturated hydrocarbons, that is, they are unable to take up any more hydrogen, and thereby are quite stable; the hydrocarbons in paraffin have the general formula of CnH2n+2, ranging as high as C24H50 to C27H56. Paraffin may be found in crude form in coal, from which source the first paraffin candles were made. It may be produced from the distillation of brown coal, as in Germany, or from bituminous shale. In America, it is obtained chiefly from the distillation of crude petroleum, being in the residue after the distillation of such products as naphtha (gasoline), kerosene and the lubricating oils. The residue is treated by one of a number of processes causing the unpurified solid paraffin to be made available. The crude paraffin is either sold as such, or is refined. Paraffin or “paraffin waxes”179 are designated in the trade by their melting points (which in the “American standard” is expressed in Fahrenheit degrees), and as to their state of refinement as “crude,” “semirefined” and “fully refined” paraffin. There are certain chemical and physical differences so that two refined waxes having the same melting point would not have the same plasticity. The higher melting point varieties of paraffin are hard and tough at room temperature: when melted, paraffin expands and forms a thin mobile liquid.

The significant requirements of paraffin for surgical dressings are that it should be solid at body temperature, at the same time having flexibility and adhesiveness, together with a certain amount of strength. A number of brands of paraffin are sold in the United States, so that it seemed advisable to examine some of them and compare them with certain paraffin-film preparations. They were tested as to their melting points, plasticity, ductility, strength of film, etc.
Melting Point Determination.—The melting point was determined by the method of the U. S. Pharmacopeia IX, p. 596. The melting point as obtained by this method is lower than the melting point used by manufacturers of paraffin (after conversion to Fahrenheit).
Pliability and Ductility, Limit Temperature.180—A little of the melted wax was poured from a teaspoon on the surface of the water at about 40 C., in a tin pan (bread mold). This formed a fairly thin film. The temperature of the water was then lowered by the addition of cold water. At each temperature the pliability and ductility were tested thus:
Pliability Test.—The film, immersed in water, was doubled on itself, note being taken whether or not it broke.
Ductility Test.—The film was pulled under water, note being taken whether it stretched on being pulled and broke with a ragged fracture; or whether it broke sharp without stretching. It is desirable that the pliability and ductility be preserved at as low a temperature as possible.
Cotton Films, Adhesives and Detachability.180—The melted wax was applied as it would be for burns; namely, a thin layer was painted on the inner surface of the forearm with a camel’s hair brush,181 a transverse strip about an inch wide being made. This was covered with a very thin layer of absorbent cotton, and over this another layer of melted wax was painted. As soon as this had cooled a little, it was covered by a few layers of bandage and left on for at least an hour. At the end of that time, the bandage was removed. The cotton film should be found at the place at which it was applied, showing that it is sufficiently adherent. It should detach without “pulling” the skin.

The results of these tests are given in the accompanying table. It can be seen that nearly all the paraffins examined have properties which would make them useful, the notable exceptions being Nos. 8, 15 and 16. The more satisfactory products would be those having a melting point about 47 C., ductility of 30 or below, and plasticity of 28 or below. The paraffin described in the U. S. Pharmacopeia is not so satisfactory, the required melting point being between 50 and 57 C.
The use of paraffin bandages has been suggested by Fisher182 and Sollmann.183 In such cases, it may very likely be that a paraffin of higher melting point would be more satisfactory, owing to its greater resistance and tougher fiber.
1. “Ambrine” is essentially paraffin in which a small amount of fatty and asphalt-like body is incorporated; like most secret mixtures, its composition varies.
2. A simple formula for a paraffin film, similar in chemical composition but superior in physical properties to “Ambrine,” is that described as Formula 21. The superiority is due to using a grade of paraffin that is better adapted to the purpose. The cost of materials is about 10 cents a pound.
3. The properties of the paraffin used for a surgical dressing are important. A number of different grades have been examined, in order to determine the ones that appear most promising. Paraffins Nos. 3, 4, 10, 11 and 25 are the best in the table, and surpass “Ambrine” itself.
4. It is exceedingly probable that further experience will show that for most purposes simple paraffin will serve just as well as the mixtures—if, indeed, not better.
(Reprinted from the Annual Report of the Chemical Laboratory of The American Medical Association, Vol. 10 (1917), p. 32)
Since the foregoing was published, two other products—“Cerelene” and “Stanolind Surgical Wax”—were submitted to the Council on Pharmacy and Chemistry for investigation as to their acceptability for inclusion in New and Nonofficial Remedies. In this connection the Laboratory was requested to examine them.
“Cerelene” is manufactured by the Holliday Laboratories, Pittsburgh. According to the manufacturers, “Cerelene” is a compound composed of 84 per cent. paraffin, 15 per cent. myricyl palmitate and 1 per cent. elemi gum. As ordinarily marketed, “Cerelene” contains the following materials: To the beeswax is added Oil of Eucalyptus, U. S. P., 2 per cent., and Betanaphthol, U. S. P., 0.25 per cent. The manufacturer further states that the myricyl palmitate is a purified form of beeswax, free from all impurities, acids, etc., which is solely manufactured by this company and for which patents are pending. The properties described for “Cerelene” were as follows:
When cold, Cerelene is a solid wax-like cake of a fine yellow brown color. On exposure to air for long periods, the amber color darkens to some extent. It is entirely free from solids, odorless and tasteless; does not separate or change when melted repeatedly, and cannot in the melted state be separated by fractional crystallization. It is entirely neutral to indicators being perfectly free from both acids and bases.
| Tests: | Melting Point, U. S. P. method, 126 F. |
| Density, U. S. P. method, 0.907. | |
| Iodin value, 0.5. | |
| Saponification number, 0.9. |
“Stanolind Surgical Wax” is manufactured by the Standard Oil Company of Indiana. In the submission of the product to the Council on Pharmacy and Chemistry, it was stated that the product was a specially prepared paraffin “free from dirt or other deleterious matter.... It has been steamed and resteamed to drive out any free oil and repeatedly filtered.”
The examination of the foregoing products yielded the figures described in Table “B.”—(From The Journal A. M. A., May 19, 1917.)
In general, the literature on the keeping qualities of iodine ointment, and on the stability of iodine if mixed with ointment bases, is confusing. The recorded evidence is often contradictory. The attention of the writer was brought to this condition by studies of several proprietary preparations, Iodex,184 Iod-Izd-Oil,185 Iocamfen, and Iocamfen Ointment.186
Iodex was sold under the claim that it is
“... an embodiment of vaporized iodine, in an organic base, reduced and standardized at 5 per cent. by incorporation with a refined petroleum product.”
The exact composition of Iodex is a trade secret. Analysis showed that it contains petrolatum-like substances and combined iodine, the latter probably in combination with oleic acid. Tests for free iodine were made in five specimens of Iodex. In one of these no free iodine was present; in the others the merest traces were found.
Two years ago a preparation called “Iod-Izd-Oil” was examined. This was claimed to contain 2 per cent. of free iodine in liquid petrolatum. At the time of the examination the age of the preparation was not known, but it had been obtained just prior to the analysis, and was thought not to be very old. The analysis showed that it contained but about 0.43 per cent. of iodine, all of which was in a free state. The fact that all of the iodine present was in the free state appeared to indicate that iodine is relatively stable in liquid petrolatum solutions.
Iocamfen is a liquid composed of iodine, camphor and phenol. It was claimed to contain 10 per cent. of free iodine. Analysis showed that it contained 9.3 per cent. of total iodine (of which 7.5 per cent. was present in an uncombined state), 66.1 per cent. of camphor and 19.7 per cent. of phenol. After storing for several months a second assay of Iocamfen showed no appreciable loss in iodine content. This would indicate that iodine is relatively stable in presence of phenol and camphor, although immediately after mixing there is some loss of free iodine. The Iocamfen Ointment was supposed to contain 50 per cent. of Iocamfen (equivalent to 5 per cent. of free iodine) in a lard-wax-cacaobutter base. The analysis showed that the ointment contained but 0.4 per cent. of free iodine, the balance being in combination. From the results of the examination, and from correspondence with the manufacturers (Schering and Glatz), it became evident that the only plausible explanation for the loss of free iodine in the preparation of Iocamfen Ointment from Iocamfen lay in the combination of the free iodine with the ingredients of the ointment base. It seems likely that the free iodine originally present in Iocamfen for the most part had gradually gone into combination with the fatty substances after the ointment had been prepared.
The literature was then examined to determine the consensus of opinion concerning the stability of iodine in iodine ointment. In the older literature the belief that iodine ointment is unstable appears to be quite general. Such statements as the following are typical:
The ointment should be prepared only when wanted for use, for it undergoes change if kept, losing its deep, orange-brown color, and becoming pale upon its surface.187
It is better to prepare it only as it is required for use.188
This ointment must not be dispensed unless it has recently been prepared.189
In 1909 Lythgoe,190 of the Massachusetts Board of Health laboratory, reported an examination of four samples of iodine ointment. Three were found to be pure, the fourth was low in iodine. Experiments showed that iodine ointment deteriorates rapidly; consequently, no further collections of samples were made.
In 1912 Pullen191 reported that he had prepared two specimens of iodine ointment according to the British Pharmacopeia, one being from new lard and the other from a specimen of lard at least 2 years old. Assays for free iodine were carried out immediately after the preparations were made, and at intervals afterward up to four months. The following values were found:
| Sample I | Sample II | |
| Ointment from new lard, per cent. | Ointment from old lard, per cent. | |
Iodine introduced | 4.0 | 4.0 |
Iodine found immediately after making | 3.95 | 3.38 |
Iodine found after twenty-four hours | 3.30 | 3.15 |
Iodine found on the third day | 3.18 | 2.62 |
Iodine found on the seventh day | 3.15 | 2.46 |
Iodine found on the fourteenth day | 3.00 | 2.45 |
Iodine found after one month | 3.00 | 2.39 |
Iodine found after two months | 2.90 | 2.31 |
Iodine found after four months | 2.92 | 2.26 |
Pullen found that the loss in free iodine could be accounted for by the iodine which had gone into combination with the fats of the ointment base.
Pullen also found that if the potassium iodide and glycerin were omitted in the preparation of the ointment, the loss in free iodine was very rapid, the preparation containing practically no free iodine (only 1⁄20) after a few hours. He concludes that the use of potassium iodide and glycerin is necessary for the preservation of the ointment. He obtained specimens of iodine ointment in drug stores, and assayed them for free iodine. It is to be presumed that the ages of the several specimens were not known. The results are found in the following table:
Specimen No. 1 | 2.74 per cent. |
Specimen No. 2 | 2.85 per cent. |
Specimen No. 3 | 2.62 per cent. |
Specimen No. 4 | 2.48 per cent. |
Specimen No. 5 | 2.53 per cent. |
Specimen No. 6 | 2.79 per cent. |
Fried192 prepared iodine ointment according to the U. S. P. VIII formula, and assayed it at intervals. His results are tabulated herewith:
| Per cent. | |
Iodine introduced | 4.00 |
Iodine found immediately after making | 3.89 |
Iodine found one hour after making | 3.51 |
Iodine found one day after making | 3.48 |
Iodine found five days after making | 3.06 |
Iodine found ten days after making | 2.84 |
Iodine found thirty days after making | 2.81 |
Iodine found ninety days after making | 2.81 |
Iodine found eight months after making | 2.81 |
Iodine ointment has been official in the U. S. Pharmacopeia since 1870. Briefly, the method now used for making the preparation is as follows:
Four gm. of iodine, 4 gm. of potassium iodide and 12 gm. of glycerin are weighed into a tared mortar and the mixture triturated until the iodine and potassium iodide are dissolved and a dark, reddish-brown, syrupy liquid is produced. Eighty gm. of benzoinated lard are then added in small portions and with trituration after each addition. The mass is then triturated until of uniform consistence.193
PARAFFINS AND PARAFFIN PREPARATIONS—TABLE A
| Formula | Substance | Melting Point, U. S. P. | Ductility Limit | Plasticity Limit | (a) Adhesiveness and Detachability (b) Strength of Film at 38 C. |
| 1 | “Parowax,” Stand. Oil Co. of Ind. | 50.8 | 32.5 | 29.0 | (a) Adheres and detaches well; rather hard (b) Pliable and strong |
| 3 | “Paraffin 118–120 F.,” Stand. Oil Co. of Ind. | 46.8 | 28.5 | 24.5 | (a) Does not adhere well; detaches easily (b) Pliable but not strong |
| 4 | “Paraffin 120–122 F.,” Stand. Oil Co. of Ind. | 47.2 | 29.0 | 24.5 | (a) Adheres well; detaches well (b) Pliable and fairly strong |
| 5 | “Paraffin 123–125 F.,” Stand. Oil Co. of Ind. | 48.8 | 31.5 | 28.5 | Same as 4 |
| 6 | “Paraffin 128–130 F.,” Stand. Oil Co. of Ind. | 52.0 | 33.0 | 30.0 | (a) Adheres well; detaches not so easily (b) Pliable and strong |
| 7 | “Texwax,” Texas Co., Port Arthur, Texas | 51.2 | 32.5 | 29.8 | Same as 6 |
| 8 | “Paraffin Wax 122–124 F.,” Warren Refining Co., Warren, Pa. | 50.6 | 36.0 | 34–35 | (a) Unsatisfactory; does not adhere (b) Only slightly pliable; too tough |
| 9 | “Paraffin No. 910,” Waverly Oil Works, Pittsburgh | 47.0 | 30.5 | 26–27 | (a) Adheres well; detaches well (b) Pliable and strong |
| 10 | “Paraffin No. 920,” Waverly Oil Works, Pittsburgh | 44.4 | 27.5 | 25.0 | (a) Adheres well; detaches well (b) Pliable and fairly strong |
| 11 | “Hard Paraffin,” Rob’t Stevenson & Co., Chicago | 48.0 | 28.5 | 24.5–25.5 | (a) Adheres well; detaches well (b) Pliable and strong |
| 12 | “Paraffin,” Island Petroleum Co., Chicago | 47.2 | 33.0 | 32.5 | Not quite as good as 11 |
| 13 | “Paraffin 122 F.,” Gulf Refining Co., Pittsburgh | 46.8 | 30.5 | 27.5–28 | (a) Does not adhere so well; detaches well (b) Very pliable |
| 14 | “Paraffin 125 F.,” Gulf Refining Co., Pittsburgh | 50.0 | 32.0 | 31.0 | About as 13 |
| 15 | “Paraffin 132 F.,” Gulf Refining Co., Pittsburgh | 54.8 | 35.5 | 34.0 | (a) Does not adhere well (b) Not very pliable, but strong |
| 16 | “Paraffin No. 301,” National Refining Co., Cleveland | 50.2 | 33.0 | 32–32.5 | (a) Does not adhere well (b) Not very pliable |
| 18 | Paraffin recovered from “Ambrine” | 48.6 | 30.5 | 28–28.5 | (a) Adheres well; detaches well (b) Pliable but not strong |
| 19 | “Hyperthermine” | 49.4 | 33.5 | 30.5–31 | (a) Does not adhere well; detaches well (b) Very pliable and strong |
| 20 | “Ambrine” | 48.4 | 30.5 | 27.0 | (a) Adheres well; detaches well (b) Very pliable and strong |
| 21 | Paraffin 120–122 F. (see 3), 97.5; olive oil, 1.5; asphalt, 4 drops | 45.4 | 29.0 | 28.5 | (a) Adheres excellently; detaches well (b) Very pliable and strong |
| 22 | “Parowax” (see 1), 97.5; olive oil, 1.5; asphalt, 4 drops | 49.2 | 32.0 | 30.5 | (a) Adheres well; detaches well (b) Pliable and strong |
| 23 | “Mulene” | 51.0 | 36.0 | 28.0 | (a) Adheres but detaches with difficulty (b) Pliable but not strong |
| 24 | “Parresine,” Abbott Laboratories, Chicago | 46.0 | 29.5 | 26.0 | (a) Adheres well; detaches easily (b) Pliable and fairly strong |
| 25 | “Paraffin 118–121 F.,” The Atlantic Refining Co., Philadelphia | 45.8 | 26.4 | 23.2 | (a) Adheres well; detaches easily (b) Pliable and fairly strong |
| TABLE B | |||||
| 26 | “Cerelene,” Holliday Lab.,* Pittsburgh | 50.0 | 30.5 | 26.5 | (a) Adheres well; detaches with pulling (b) Not strong at 38 C. |
| 27 | “Stanolind” Surgical Wax,† Standard Oil Co. of Ind. | 47.0 | 28.8 | 25.0 | (a) Adheres well; detaches easily (b) Fairly strong at 38 C. |
* On being heated, it readily loses eucalyptol, and a small amount of resinous substance forms in the bottom of the beaker. If “Cerelene” is heated to 145 C. and cooled, the resulting product no longer has the properties of the original “Cerelene.”
† Accepted by the Council on Pharmacy and Chemistry for inclusion in New and Nonofficial Remedies.
Iodine ointment is officialized also in several foreign pharmacopeias, although the iodine strength of the several preparations is not uniform. The formula in the British Pharmacopeia is exactly like that in the U. S. Pharmacopeia except that pure lard is directed to be used instead of benzoinated lard. Some of the foreign pharmacopeias also specify that the preparation must be freshly prepared when wanted. In the earlier editions the U. S. Pharmacopeia directed the ointment to be prepared by using water as the solvent for the potassium iodide. In the U. S. Pharmacopeia VIII the formula was changed so as to employ glycerin, and that solvent is now official. Water is still prescribed as the potassium iodide solvent by the Pharmacopeias of the Netherlands and of France.
From the examination of the literature it seems probable that iodine ointments which contain petrolatum products only as the ointment bases are apt to be relatively stable, so far as the content of free iodine is concerned. On the other hand, ointments the bases of which contain fats of the unsaturated fatty acid series, such as oleic acid, do not satisfactorily preserve the iodine in the free state. In the latter class it seems likely that the iodine enters into combination with the unsaturated fatty acids. Accordingly, on theoretical grounds, an ointment base composed of pure stearin (if such substance were available) but softened by an admixture of liquid petrolatum would preserve the iodine satisfactorily. Cocoanut oil (iodine No. 8) ought to be suitable also if mixed with hard paraffin.
Since the literature was not sufficiently concordant to warrant positive conclusions concerning the stability of ointments containing free iodine, it seemed worth while to conduct experiments with preparations of known origin. Accordingly, a number of preparations containing free iodine were made under varying conditions and each was assayed for its free iodine content immediately after its manufacture and from time to time later.
Leaf lard of the best quality obtainable was purchased from a butcher. This was rendered in an open dish on the steam bath. The preparation was of a fine color, and uniform consistence and had a faint but not unpleasant odor. Two specimens of lard were furnished by the research department of Armour and Company. An effort was made to procure specimens of lard having iodine absorption numbers as far apart as possible, i. e., one with a low and the other with a high iodine value. This was done in order to determine whether the keeping qualities of the ointments prepared from the two would be alike.
One of the specimens (a) was described as
“Natural lard; iodine value, 57.1. Leaf lard used exclusively for butterine and benzoinated lard.”
The other specimen was described as
“Prime steam lard. Good, commercial grade of lard for general use; iodine value, 69.0.”
The iodine absorption numbers of the three specimens were determined by the U. S. P. process to be as follows:
Laboratory rendered specimen | 57.1 |
Armour specimen (a) | 57.65 |
Armour specimen (b) | 67.55 |
Each specimen was benzoinated according to the process described in the U. S. P. IX and 100 gm. of iodine ointment were prepared from each according to the U. S. P. process. Another specimen was made from benzoinated lard and iodine only194 without the addition of either glycerin or potassium iodide. This was made to contain 4 per cent. of iodine.
Immediately after preparation each of these iodine ointments was assayed for free iodine, and each was reassayed at intervals later. The method for the determination of iodine in the ointment was that employed in this laboratory for the determination of iodine in Iocamfen Ointment.195 It is essentially the same as was employed by Pullen for the determination of uncombined iodine in iodine ointment.196 As carried out in this laboratory for iodine ointment it is as follows:
From 5 to 8 gm. of the ointment were weighed in a small porcelain capsule, the capsule and contents placed in a 16 oz. salt mouth bottle together with 20 c.c. of chloroform, 10 c.c. of potassium iodide solution and 40 c.c. of water. Tenth-normal sodium thiosulphate was slowly added with agitation until the pink color of the chloroform layer had nearly disappeared. A little soluble starch was then added and the titration continued until a blue color in the aqueous layer could no longer be obtained by repeated shaking.
The findings for the several assays are tabulated herewith:
| Age at time of assay | U. S. P. Ointment from laboratory rendered lard | U. S. P. Ointment from commercial lard Grade I | U. S. P. Ointment from commercial lard Grade II | Ointment from lard and iodine only (laboratory rendered lard) |
| (% I) | (% I) | (% I) | (% I) | |
Freshly made | 3.32 | 3.26 | 3.30 | 0.32 |
After 3 days | 3.25 | ...... | ...... | 0.23 |
After 7 days | 2.99 | 3.17 | 3.15 | ...... |
After 3 weeks | 3.01 | 3.19 | 3.07 | ...... |
After 7 weeks | 3.12* | 3.10 | 3.02 | ...... |
After 3 months | 2.98 | 2.88 | 2.88 | ...... |
* This slight rise in iodine content followed by a fall could not be accounted for. The specimen was believed to have been very thoroughly mixed at the time of manufacture.
That the fatty constituents of the ointment contained iodine after the preparation had been made for some time was demonstrated. Some of the material was examined as follows:
A portion of the ointment which had been made for nearly three months was shaken in a separator with chloroform and a dilute mixture of potassium iodide and sodium thiosulphate solutions. After all of the free iodine had been removed the chloroformic solution of the fats was washed several times with a very dilute solution of sodium thiosulphate. The chloroformic solution was filtered, evaporated and the residue dried over sulphuric acid.197
The separated fat was then tested for iodine by Kendall’s method.198 It was found to contain iodine in considerable amounts, but quantitative determinations were not made.
The Pharmacopeia of the Netherlands directs that iodine ointment shall contain 3 per cent. of potassium iodide and 2 per cent. of iodine instead of equal proportions (4 per cent. of each) as prescribed by the U. S. Pharmacopeia. Likewise the French Pharmacopeia directs that 10 per cent. of potassium iodide and only 2 per cent. of iodine shall be used. Both of these pharmacopeias use water instead of glycerin as the solvent. Loose combinations of iodine and potassium iodide, such as are represented by the compound having the formula KI3, have been described. The quantity of potassium iodide prescribed by the U. S. Pharmacopeia for the preparation of iodine ointment is not sufficient to form such a compound as KI3 with all of the iodine directed to be used. Since some of the pharmacopeias use larger proportions of potassium iodide (more than sufficient to form the compound, KI3), it seemed worth while to determine whether an ointment containing a greater proportion of potassium iodide than that required by the U. S. Pharmacopeia would be more stable than the official article. Accordingly a specimen was prepared to contain 4 per cent. of iodine, 8 per cent. of potassium iodide (twice the U. S. P. requirement), 12 per cent. of glycerin and 76 per cent. of lard. This was assayed for its free iodine content immediately after preparation, and found to contain 3.68 per cent. Nine days later it contained 3.70 per cent. Another specimen of the same iodine strength prepared from grade No. 2 of commercial lard assayed 3.69 per cent. at the initial assay, and seven days later 3.40 per cent. From these experiments it seems likely that the free iodine content of the U. S. Pharmacopeia iodine ointment could be raised somewhat by increasing the proportion of potassium iodide.
The results of these studies confirm the findings of Pullen and of Fried in all essential particulars. It appears that during the process of manufacture of iodine ointment about 20 per cent. of the free iodine goes into combination with the fatty constituents of the ointment. On standing for a month approximately an additional 5 per cent. goes into combination, after which there is practically no loss in free iodine content. In other words iodine ointment which is a month old is a relatively stable preparation. It appears to make no noticeable difference upon the rate and amount of iodine absorption whether the lard from which the ointment is made has a high or a low iodine absorption value. The composition of iodine ointment, which has been made sufficiently long to have reached equilibrium, is approximately as follows:
Free iodine | 3 | per cent. |
Iodine combined with fat | 1 | per cent. |
Potassium iodide | 4 | per cent. |
Benzoinated lard (containing iodine) | 80 | per cent. |
The U. S. Pharmacopeia requirement that iodine ointment shall be freshly prepared when wanted appears to be unnecessary. Probably most pharmaceutical manufacturers are aware of this, for many of them include the preparation in their trade lists. The presence of an iodide appears to be necessary, to prevent practically all of the iodine from entering into combination with the fat.199—(From the American Journal of Pharmacy, August, 1917.)
The Council on Pharmacy and Chemistry was asked to examine a preparation submitted with the statement that it was “iodin crystals incorporated in a petroleum product.” The name “Iodolene” was proposed by the promoters, providing the product was found eligible for New and Nonofficial Remedies.
Iodolene was stated to have been prepared by treating a liquid petrolatum, obtained from Gulf Coast petroleum, with an excess of iodin; the mixture was subsequently “placed in an oven for three hours.” The claim was made that this method of procedure produced a preparation containing more iodin than market specimens which had been examined, namely: “over 1.50 per cent. free iodine.”
Two specimens of the product were submitted, one stated to have been unfiltered, and the other filtered. Both of the specimens emitted a strong odor of hydrogen sulphide upon removing the stopper from the respective containers.
Iodin Content of Iodolene.—The iodin content of the filtered specimen was determined thus: A weighed amount—3 to 5 gm.—was transferred to a separator by means of 20 c.c. of ligroin, used in portions. Twenty c.c. of 10 per cent. potassium iodid solution was added and the free iodin titrated with tenth-normal sodium thiosulphate solution (with agitation), the end point being the absence of a yellow color in the aqueous layer. The amount of free iodin was found to be 1.32 per cent.
The Solubility of Iodin in Liquid Petrolatum.—To determine the solubility of iodin in Liquid Petrolatum, 200 c.c. of Liquid Petrolatum-Squibb (said to be composed of hydrocarbons of the naphthene series) and 200 c.c. of Stanolind Liquid Paraffin (said to be composed chiefly of marsh gas hydrocarbons) were each treated with 5 gm. of iodin crystals. The two mixtures were maintained for a week at a temperature somewhat above that of the room and agitated occasionally. Each was then cooled to room temperature (about 22 C.), agitated for a day and then filtered. The amount of iodin in the preparation made with Liquid Petrolatum-Squibb was found to be 1.42 per cent. The iodin content of the preparation made with Stanolind Liquid Paraffin was 1.30 per cent.
In view of these findings the prospective manufacturer was advised that the Council cannot countenance a proprietary name for an unofficial, simple solution of iodin in liquid petrolatum.—(From Reports A. M. A. Chemical Laboratory, 1917, p. 87.)
At the request of the Council on Pharmacy and Chemistry, the A. M. A. Chemical Laboratory has undertaken examinations of American-made synthetic drugs. The most extensively used synthetic is acetylsalicylic acid and hence an investigation of this product was deemed expedient.
For seventeen years acetylsalicylic acid was protected by a United States Patent (the proprietors were not given a patent in other countries) and sold under the name “Aspirin.” In February, 1917, the patent expired, and since then a number of firms have engaged in the manufacture of acetylsalicylic acid, selling it either as such or as aspirin, modified, of course, by a distinctive firm designation. During this period the former manufacturers (The Bayer Co., New York, in past years called Farbenfabriken of Elberfeld Co., New York) have been extensively advertising, both to physicians and the public, the alleged superior qualities of their product. The chemical examination, therefore, was concerned chiefly with tests of purity, and the comparison of the American brands with the formerly patented product.
In European countries, acetylsalicylic acid200 is described in the various pharmacopeias as a condensation product of acetic anhydride or acetyl chloride with salicylic acid (o-hydroxybenzoic acid). Generally the test of identification is hydrolysis of acetylsalicylic acid and qualitative tests for acetic acid and salicylic acid. For purposes of purity the requirements are essentially that the specimen should have a certain melting point, should show absence of salicylic acid by means of ferric chloride (the manipulations for the tests are variously described) and leave no appreciable ash. The two tests of purity most generally employed, however, are the melting point and the reaction with ferric chloride.
The melting point of acetylsalicylic acid has been given at various temperatures from 118 to 137 C.201; the British Pharmacopeia describes the melting point at 133 to 135 C.; the German Pharmacopeia “about 135 C.;” the French Pharmacopeia at 135 C.; New and Nonofficial Remedies, 1917, 134 to 136 C. The Bayer Company, in the patent trial at Chicago a number of years ago, gave among the “four infallible tests” a melting point of “about 135 C.” Several men have carefully determined the melting point in recent years. Emery and Wright202 in 1912 found that “Aspirin, Bayer” melted at 130.5 to 131 C. In France, François203 has determined the melting point of pure acetylsalicylic acid, which, according to his method, is 132 C. When various samples of acetylsalicylic acid were examined in this laboratory, it was found that the melting point of none was as high as that described in New and Nonofficial Remedies or the British, French, or German pharmacopeias when taken according to the general method of the U. S. Pharmacopeia, Vol. 9, p. 596. On critical observation, it may be seen that the melting point of acetylsalicylic acid is preceded and accompanied by decomposition. If the sample in the melting tube is heated from the original room temperature of the bath to 120 C., the temperature of melting will be lower than if the bath is first heated to 120 C. and the melting-point tube then placed in the bath.204 Thus the melting point of acetylsalicylic acid, like so many organic compounds which decompose and do not melt sharply, is unsatisfactory and cannot be taken as an “infallible test” of purity, especially when determined by different operators who do not give their method in detail. After making a large number of melting-point determinations of acetylsalicylic acid, alone and in parallel with other operators, it was decided to use the method described in the U. S. Pharmacopeia modified by first heating the bath to 120 C. before attaching the melting-point tube to the thermometer.
The melting point of purified acetylsalicylic acid was found to be 131.5 to 132.5 C. (corr.).205 With the exception of one specimen, which was obviously impure, the various specimens examined melted between 128 and 133 C. as may be seen in the accompanying table. It would appear that this range of melting points would be more acceptable and reliable than the melting points described in various standards.
It is generally conceded that the presence of salicylic acid in amounts more than traces is deleterious. Furthermore, the amount of salicylic acid is a good index of the purity of the acetylsalicylic acid, because the test is so delicate that, under favorable conditions, mere traces may be determined and, as a rule, the better the product, the less the amount of free salicylic acid.
The tests appearing in various pharmacopeias for salicylic acid as an impurity in acetylsalicylic acid do not give concordant results, different workers interpreting the results differently, nor are they detailed in such a manner as to yield maximum delicacy.
After experimentation, it was decided to establish a “limit” test of approximately 0.1 per cent. free salicylic acid, when carried out according to the following method:
0.1 gm. of the substance was placed in a dry colorimeter tube and 1 c.c. of alcohol,206 previously distilled over NaOH, was added. After the acetylsalicylic acid had dissolved, 48 c.c. of water and 1 c.c. of fresh 0.1 per cent. ferric chloride (FeCl3.6H2O) solution were added. At the same time a control was run by treating 1 c.c. of a “standard” salicylate solution the same as above.207 If within two minutes the color given by acetylsalicylic acid is not more intense than the color given by the “standard,” the presence of not more than 0.1 per cent. free salicylic acid is proved.208
The solutions used were prepared as follows:
Redistilled alcohol was treated with a small amount of sodium hydroxide for twenty-four hours, then again distilled.
The color standard was made by dissolving 0.116 gm. of dried sodium salicylate in water, adding 1 minim of glacial acetic acid, and making up to 1,000 c.c. Each c.c. represents 0.1 mg. of salicylic acid.209
The ferric chloride solution was made by diluting 1 c.c. ferric chloride (FeCl3.6H2O) test solution U. S. P. with 99 c.c. of water. The diluted solution must be freshly prepared each day.
With one exception, all of the commercial specimens examined responded satisfactorily to the above test showing less than 1 part salicylic acid in 1,000 parts acetylsalicylic acid. The individual results are given in the accompanying table.
Melting Point and Salicylic Acid Determinations
| Brand | Melting Point Corrected | Free Salicylic Acid Colorimetrically |
Acetylsalicylic acid, P. W. R.¹ | 130.0–131.0° | Colored, but showing less than 0.1 per cent. |
Acetylsalicylic acid, Millikin² | 130.0–131.0° | No color |
| Acetylsalicylic acid, Millikin² | ||
5-grain capsules | 129.0–130.0° | No color |
| Acetylsalicylic acid, Millikin,¹ | ||
5-grain capsules³ | 128.0–129.0° (a) | Colored, but showing less than 0.1 per cent. (a) |
| 125.5–126.5° (b) | Considerably more than 0.1 per cent. (b) | |
Acetylsalicylic acid, Squibb² | 131.0–132.0° | No color |
Acetylsalicylic acid (Aspirin),¹ Monsanto | 131.0–132.0° | No color |
Acetylsalicylic acid, M. C. W.¹ | 130.5–131.5° | Colored, but showing less than 0.1 per cent. |
Acetylsalicylic acid, M. C. W.¹ | 131.5–132.5° | Colored, but showing less than 0.1 per cent. |
Acetylsalicylic acid, M. C. W.¹ | 131.0–132.0° | Colored, but showing less than 0.1 per cent. |
Aspirin, Bayer¹ (before patent expired) | 131.5–132.5° | No color |
Aspirin, Bayer¹ ⁴ (after patent expired) | 128.5–129.5° | Colored, but showing less than 0.1 per cent. |
Aspirin, Bayer¹ ⁴ (after patent expired) | 129.5–130.5° | Colored, but showing less than 0.1 per cent. |
Aspirin, Lehn and Fink² | 130.5–131.5° | 0.1 per cent. |
Aspirin, Lehn and Fink² | 130.5–131.5° | Colored, but showing less than 0.1 per cent. |
Aspirin, Lehn and Fink¹ | 131.0–132.0° | Colored, but showing less than 0.1 per cent. |
1: Obtained on the open market.
2: Obtained from manufacturer.
3: One-third of the capsules (a) contained a white powder; two-thirds of the capsules (b)
contained a pink powder having strong odor of acetic acid and not complying with the tests.
4: Not described in “New and Nonofficial Remedies, 1917”; the other products are.
New and Nonofficial Remedies, 1917, requires that acetylsalicylic acid shall form a clear solution with warm sodium carbonate solution; that sulfates, chlorides and heavy metals shall be absent; that 0.5 gm. shall leave no weighable ash. All the brands reported in this paper complied with these requirements.
So far there has been no satisfactory quantitative estimation of acetylsalicylic acid. True, various methods have been proposed, but they are objectionable. It was thought that hydrolysis of acetylsalicylic acid and then titrating the solution by comparing the color formed by ferric chloride with that of a standard control might yield interesting results, providing that the conditions were alike. For this purpose 1 gm. of acetylsalicylic acid was dissolved in 10 c.c. of alcohol and diluted to 1,000 c.c. The solution was then heated at 98 to 100 C. for two hours, allowing the alcohol to evaporate, then allowed to stand at room temperature (22 C.) for twenty-two hours. After adding water sufficient to make 1,000 c.c., it was compared colorimetrically for salicylic acid strength. The amount of hydrolysis varied so with different samples under the same conditions, that it was realized that an approximate assay by this method was unreliable. If the assay were made under more exact conditions, quantitative comparisons might be possible. In one experiment, after sixty days the hydrolysis of the acetylsalicylic acid was 61 per cent., which is in rough agreement with the work of Tsaklatos and Horsh.210
Apart from the proposed revision of the standards for the melting point and limit of salicylic acid in acetylsalicylic acid, the examination shows that there is no appreciable difference between the various brands of acetylsalicylic acid examined, all of them with one exception (acetylsalicylic acid, Millikin, 5-grain capsules, purchased on the open market) complying with the tests described in this paper. The Journal of the American Medical Association, in past years, has protested repeatedly against the monopoly given to the Bayer Company for their “Aspirin,” contending that acetylsalicylic acid (aspirin) was not new, and that “Aspirin, Bayer” was simply a good brand of acetylsalicylic acid which could be bought in foreign countries at much lower prices than here. Although the patent in the United States has expired, “Aspirin, Bayer” is still being retailed at higher prices than other products which are now enjoying the privilege of American manufacture.
Mr. Paul Bakewell,211 in an opinion answering the warning circular of the Bayer Co. in reference to the use of the word “aspirin” by firms other than Bayer, argues very ably that acetylsalicylic acid, before the patent was granted, meant the impure substance which was not used therapeutically, while “aspirin” was designated as the improved product (a new article of manufacture, the particular acetylsalicylic acid made under the Hoffman patent) and “is the substance now known in pharmacy as aspirin” (statement made by an officer of the Farbenfabriken of Elberfeld Co. in U. S. Circuit Court, 1909). The products reported in this paper are (with the one exception) the same as described in the Hoffman patent, and, in the sense of Mr. Bakewell’s argument, are “aspirin.” However, it would seem better if the name acetylsalicylic acid, instead of aspirin, were used, especially by physicians in their prescriptions because (1) it is a generic, scientific name; (2) “Aspirin, Bayer” is sold at higher prices than other products, whereas chemically equivalent products sold under the descriptive name may be purchased at a lower price. Finally, the manufacture of acetylsalicylic acid in this country is another example of the fact that American chemists can produce the drug synthetics, and at the same time make products as good as, if not better than, those of German origin.
I express my appreciation to Dr. W. A. Puckner for his kind interest.—(From the Journal of Industrial and Engineering Chemistry, April, 1918.)
This work was begun in view of a request received by the Council on Pharmacy and Chemistry from the Medical Section of the Council of National Defense for a report on the quality of bismuth tribromphenate, offered to the government by a certain firm.
In submitting a specimen of its product, “Bismuth Tribromphenolate,” the firm claimed that “it is of high character, matching exactly the German product formerly imported into this country,” and expressed the belief that it would be found to conform to the standards for this preparation in New and Nonofficial Remedies. Later a second specimen was received from the same company, with the request that this be substituted for that first received. It was explained that the first had been taken from an experimental lot, and that the second, taken from the regular factory output, was identical with the first except that it was free from odor because of the more thorough washing to which it had been subjected. Accordingly, the examination which is reported below refers to the second specimen only.
New and Nonofficial Remedies, 1918, defines bismuth tribromphenate as basis bismuth tribromphenate having the formula Bi(C6H2Br3O)2OH.Bi2O3, and it is required to yield not less than 49.5 per cent. of bismuth oxid (the chemical formula requires 46.2 per cent. bismuth, or 51.6 per cent. bismuth oxid, Bi2O3, and 49.2 per cent. tribromphenate, C6H2Br3.OH). It describes it as a “fine, yellow, nearly odorless and tasteless powder, neutral in reaction,” and “only slightly soluble in water, alcohol, chloroform, liquid petrolatum and vegetable oils.” It is required to yield tribromphenol (to which a melting point of 95 C. is assigned) when decomposed by alkali and the alkali tribromphenate decomposed by acid, the separated tribromphenol purified and dried.
As the New and Nonofficial Remedies description appeared loosely drawn—it had been based on information furnished for the product Xeroform when this, because of patent protection, was the only bismuth tribromphenate on the market—it was decided to include in the examination also specimens of the two brands of Bismuth Tribromphenate included in New and Nonofficial Remedies, namely, Bismuth Tribromphenate-Merck (Merck and Company) and Xeroform-Heyden (The Heyden Chemical Works). The Merck specimen had been received by the Council from Merck and Company in 1915, while the Heyden preparation was obtained direct from the firm’s Chicago branch in April, 1918. At this time Bismuth Tribromphenate-Merck could not be obtained from the Chicago wholesale houses.
All three specimens were nearly odorless. Two of the specimens (the Research Council Specimen and Merck products) were of a lemon-yellow color, while the Heyden preparation was of a grayish color.
Four methods for the determination of the bismuth content of the specimens were tried:
(A). Direct Ignition to Bismuth Oxid.—This method was abandoned because of the tendency to ignite suddenly during the incineration and the consequent loss of material.
(B). The Method of the Japanese Pharmacopeia, Third Revised Edition, Translated by the Pharmaceutical Society of Japan.—The method consists in treatment of the product with nitric acid, evaporation and subsequent heating to bismuth oxid. This method also was abandoned because of tendency toward sudden ignition with loss of material.
(C). The Method of Kollo (Apotheker Zeitung, 1910, p. 99).—The method consists in decomposition of the product by heating on water bath with normal sodium hydroxid solution, with formation of soluble sodium tribromphenate and insoluble bismuth hydroxid. The bismuth hydroxid is collected on a filter, washed with hot water until a few drops of the filtrate no longer turn litmus paper blue, dried and heated to constant weight and weighed as bismuth oxid.
(D). A. M. A. Method (Reports A. M. A. Chem. Lab., 1911, p. 18).—This method consists in dissolving the product in hot, strong hydrochloric acid, diluting, filtering and precipitating by saturation with hydrogen sulphid. The bismuth sulphid obtained is dissolved in nitric acid and from the solution obtained the bismuth is precipitated by addition of an excess of ammonium hydroxid and ammonium carbonate. The precipitate is collected and converted to bismuth oxid by heat.
The following tabulation shows the results obtained by Methods “C” and “D”:
TABLE 1.—BISMUTH CONTENT OF BISMUTH TRIBROMPHENATE
| Method | Gm. of Salt | Gm. of Bi2O3 | Per Cent. of Bi2O3 | |
No. 1 Research Council Spec | C | 2.1312 | 1.1754 | 55.1 |
No. 1 Research Council Spec | D | 0.5151 | 0.2772 | 50.03 |
No. 2 (Merck & Company) | C | 2.0287 | 1.2543 | 61.8 |
No. 2 (Merck & Company) | D | 0.5064 | 0.2634 | 52.01 |
No. 3 (Heyden Chem. Works) | C | 2.0472 | 1.6020 | 78.2 |
No. 3 (Heyden Chem. Works) | D | 0.5227 | 0.3546 | 67.8 |
It is seen from the tabulation that the results obtained by the Kollo method (Method C) are higher than those by the sulphid method (Method D) and that duplicate determinations show a rather wide variation. The results by the sulphid method are somewhat lower than those by the Kollo method, but duplicates agree fairly well. In view of the fact that the Kollo method will give excessive results if impurities such as talcum, etc., are present and in consideration of the satisfactory results obtained in previous work with the sulphid method, the figures obtained by this method are taken as indicative of the bismuth content of the specimens examined. Calculating the per cent. of bismuth oxid obtained to bismuth (Bi), the following values are obtained:
Bismuth Tribromphenolate, Research Council Specimen: Bismuth, 44.8 per cent.
Bismuth Tribromphenate-Merck, Merck & Co.: Bismuth, 46.6 per cent.
Xeroform, Heyden Chemical Works: Bismuth, 60.7 per cent.
The content of tribromphenate radical, C6H2Br3O-, was determined by the method of Kollo (Apotheker Zeitung, 1910, p. 99). It consists in titrating the filtrate of the bismuth oxid determination of Kollo, described under “C” (bismuth determinations), with normal hydrochloric acid, using phenolphthalein as an indicator. The cubic centimeters of normal alkali consumed multiplied by the theoretical factor 0.331 gives the weight of tribromphenol (combined and free) contained in the specimen.
The following results were obtained:
TABLE 2.—DETERMINATION OF TOTAL TRIBROMPHENOL IN BISMUTH TRIBROMPHENATE
| Gm. of Salt Taken | Gm. Tribromphenol Calculated from Theoretical Factor | Per Cent. of Total Tribromphenol | |
No. 1 (Research Council Spec.) | 1.7817 | 1.0592 | 59.44 |
No. 2 (Merck & Co.) | 0.9743 | 0.5627 | 57.75 |
No. 3 (Heyden Chem. Works) | 2.0440 | 0.4303 | 21.04 |
The definite chemical formula given in New and Nonofficial Remedies for bismuth tribromphenate and the statement that it is “only slightly soluble in ... alcohol ...” requires the absence of uncombined tribromphenol, but no method for its detection or determination is provided.
In the U. S. Patent 516,358 (expired March 13, 1911), issued to Bruno Richard Seifert, assignor to Dr. F. Von Heyden, for “Phenol Bismuth Compound” the freedom from uncombined tribromphenol was provided for by the direction to wash with alcohol the product obtained.
In the Swiss Pharmacopeia the permissible content of uncombined tribromphenol is limited thus:
“If 0.5 gm. be shaken with 5 c.c. of alcohol and 1 c.c. of the filtrate be diluted with 15 c.c. of water, neither a turbidity nor a flocculent precipitate should appear....”
When this test was applied to the three specimens under examination, the Merck and Heyden specimens complied, while the Research Council specimen did not comply, with this requirement.
Method 1.—About 1 gm. of bismuth tribromphenate was placed in a flask, 20 c.c. of 95 per cent. alcohol added and shaken for fifteen minutes, after which it was filtered by suction through a Gooch filter into an Erlenmeyer flask. The flask was rinsed with 10 c.c. of alcohol and finally the filter was washed with 10 c.c. of alcohol, 25 c.c. of tenth-normal sodium hydroxid solution were added to the alcoholic filtrate (which was nearly but not perfectly clear) containing the tribromphenol, and the residual alkali titrated with tenth-normal hydrochloric acid.
The number of cubic centimeters of tenth-normal alkali consumed multiplied by 0.331 gave the weight of tribromphenol (Table 3).
TABLE 3.—DETERMINATION OF FREE TRIBROMPHENOL
| Gm. of Salt Taken | Gm. Tribromphenol Calculated from Theoretical Factor | Per Cent. Free Tribromphenol | |
Research Council Spec. | 2.3351 | 0.3806 | 16.31 |
Merck & Co. | 0.7980 | 0.0364 | 4.56 |
Heyden Chemical Works | 1.9460 | 0.0132 | 0.68 |
Method 2.—About 2 gm. of bismuth tribromphenate were placed in a glass stoppered Erlenmeyer flask, 100 c.c. of alcohol were measured in and shaken during one-half hour and allowed to stand over night. Fifty c.c. of the supernatant liquid were then removed by means of a pipet, a slight excess of tenth-normal sodium hydroxid added and the residual alkali titrated with tenth-normal HCl.
Table 4 gives results obtained.
TABLE 4.—PER CENT. OF TRIBROMPHENOL BY METHOD 2
| Gm. of Salt Taken | Gm. Tribromphenol Calculated from Theoretical Factor | Per Cent. Free Tribromphenol | |
Research Council Spec. | 2.0712 | 0.3905 | 18.85 |
Merck & Co. | 1.9417 | 0.0760 | 3.92 |
Heyden Chemical Works | 2.0440 | 0.0198 | 0.97 |
Table 5 gives a comparison of the results obtained by the two methods.
TABLE 5.—COMPARISON OF RESULTS BY METHODS 1 AND 2
| Method 1 | Method 2 | |
Research Council Spec. | 16.31 | 18.85 |
Merck & Co. | 4.56 | 3.92 |
Heyden Chemical Works | 0.68 | 0.97 |
The results obtained in Method 1 (the percolation method) apparently are reliable and, as the method is the more simple, may be given preference.
The amount of tribromphenol existing in the specimen in combination was calculated by subtracting from the per cent. of total tribromphenol determined, the per cent. of free tribromphenol found by Method 1.
The figures obtained are given in Table 6.
TABLE 6.—THE TRIBROMPHENATE CONTENT OF BISMUTH TRIBROMPHENATE
| Per Cent. of Combined Tribromphenol | |
Research Council Specimen | 43.13 |
Merck & Co. | 53.19 |
Heyden Chemical Works | 20.36 |
From the foregoing the specimens examined contain the percentages shown in Table 7 of bismuth (Bi), combined tribromphenate and free tribromphenol.
TABLE 7.—PERCENTAGES OF BISMUTH AND OF COMBINED TRIBROMPHENATE AND FREE TRIBROMPHENOL
| Per Cent. Bismuth | Per Cent. Combined Tribromphenate | Per Cent. Free Tribromphenol | |
Research Council Specimen | 44.8 | 43.13 | 16.31 |
Merck & Co. | 46.6 | 53.19 | 4.56 |
Heyden Chemical Works | 60.7 | 20.36 | 0.68 |
This examination shows:
1. The Bismuth Tribromphenolate submitted to the Council of National Defense, does not correspond to the description of bismuth tribromphenate in New and Nonofficial Remedies.
2. As now supplied, Xeroform-Heyden does not meet the requirements for bismuth tribromphenate, nor does its composition correspond to that of the product formerly supplied.
3. The description in New and Nonofficial Remedies of bismuth tribromphenate should provide an upper, as well as a lower, limit for the bismuth content; it should provide tests for the absence of adulterants, and also set a limit of permissible uncombined tribromphenol.
The results of this examination with reference to the Research Council specimen having been submitted to the Council on Pharmacy and Chemistry, this body advised the Medical Section of the Council of National Defense as follows:
1. The specimen of “Bismuth Tribromphenolate” sent to the Council of National Defense complies with the New and Nonofficial Remedies description for bismuth tribromphenate, except that it contains considerable amounts (approximately 16 per cent.) of alcohol-soluble, uncombined tribromphenol.
The results of the examination of the three specimens were sent to the Heyden Chemical Works and to Merck and Co. (in each case disclosing the identity of the particular firm’s product), asking aid in the standardization of the product. After Merck and Co. had submitted valuable advice for a revision of the somewhat loosely drawn standards for bismuth tribromphenate in N. N. R., 1918, the inquiry whether the following proposed revision of the description of bismuth tribromphenate in New and Nonofficial Remedies was acceptable, was submitted to both firms:
BISMUTH TRIBROMPHENATE.—Bismuthi Tribromphenas.—Bismuth Tribromphenol.—Xeroform.—A basic bismuth tribromphenate of variable composition.
An amorphous, yellow, nearly odorless and tasteless powder, neutral to moistened litmus paper.
It is only slightly soluble in water, alcohol, chloroform, liquid petrolatum and vegetable oils. Alkalies and strong acids decompose it. It is stable at temperatures below 120 C.
When about 1 gm. of the salt is boiled with 10 c.c. of sodium hydroxide test solution, the liquid filtered, and the filtrate acidulated with sulphuric acid, the white curdy precipitate produced, when washed and dried, melts at 90 to 95 C. (tribromphenol). The contents of the filter dissolve completely in dilute hydrochloric acid (insoluble inert material).
Boil 1 gm. of bismuth tribromphenate with 20 c.c. of a mixture of equal parts of acetic acid and distilled water, cool the solution and filter. Free the filtrate from bismuth by the addition of hydrogen sulphide, boil the mixture and again filter. The latter filtrate leaves not more than 0.005 gm. of residue on evaporation and gentle ignition (alkalies and alkali earths).
Shake for one minute in a separatory funnel, 2 gm. of bismuth tribromphenate, 20 c.c. of ether, and 20 c.c. of a mixture of equal volumes of hydrochloric acid and distilled water. Draw off the aqueous portion and concentrate to about 4 c.c.; pour it into 100 c.c. distilled water, filter, evaporate the filtrate on the water bath to 30 c.c., again filter and divide this filtrate into portions of 5 c.c. each. Mix one portion with an equal volume of dilute sulphuric acid; it does not become cloudy (lead). Treat another portion with a slight excess of ammonia water; the supernatant liquid does not exhibit a bluish tint (copper). Another portion is not immediately affected by barium nitrate test solution (sulphate).
Heat gently a mixture of about 0.2 gm. of bismuth tribromphenate with 5 c.c. of potassium hydroxide test solution and about 0.2 gm. of aluminum wire; the vapors evolved do not turn red litmus blue (nitrates).
Shake 1 gm. of bismuth tribromphenate frequently during fifteen minutes with 30 c.c. of alcohol (95 per cent.), filter and rinse flask with two separate 10 c.c. portions of alcohol, allowing the washings to run through filter. To the combined filtrate and washings add 20 c.c. of tenth-normal sodium hydroxide and a few drops of phenolphthalein solution and determine the excess of alkali with tenth-normal hydrochloric acid. Not more than 1 c.c. of tenth-normal sodium hydroxide should have been consumed by the alcoholic liquid (free tribromphenol).
Add 2 c.c. of nitric acid to 2 gm. of bismuth tribromphenate in a porcelain crucible, carefully evaporate to dryness on a sand bath and incinerate. Dissolve the residue in 5 c.c. of concentrated hydrochloric acid and add to the solution 10 c.c. of a saturated solution of stannous chloride in concentrated hydrochloric acid. The mixture should not darken on standing thirty minutes (arsenic).
Mix 0.5 gm. of the salt with 10 c.c. of a mixture of equal parts of hydrochloric acid, U. S. P., and distilled water. No effervescence should occur (carbonate).
To about 0.5 gm. of bismuth tribromphenate, accurately weighed, add 20 c.c. of hydrochloric acid and digest on water bath. Add 150 c.c. of distilled water and filter. Rinse the beaker with 30 c.c. of distilled water and allow the washings to run through the filter. Saturate the combined filtrate and washings with hydrogen sulphide, filter off the bismuth sulphide, wash and dissolve in hot dilute nitric acid. Add a slight excess of ammonia water followed by 2 c.c. of ammonium carbonate test solution. Allow to stand thirty minutes, filter off the precipitated bismuth hydroxide and heat to constant weight at dull red heat. The residue of bismuth oxide (Bi2O3) should not be less than 45 per cent. nor more than 55 per cent. of the original weight of bismuth tribromphenate taken, corresponding to not less than 40 per cent. nor more than 49 per cent. of bismuth.
The Heyden Chemical Works accepted the proposed monograph. Regarding the Laboratory’s findings, the firm stated that “the product had to be made in this country after importations from Europe became impossible and the first lots were not fully up to the standard.” Later the firm stated that it could furnish a product which it considered equal to that which was previously imported and offered to submit “samples of the new material.”
Merck and Co. acknowledged the receipt of the monograph but made no statement as to its acceptance or suggestions for its revision. As the new monograph was accepted by the Heyden Chemical Works and as Merck and Co. offered no objections, it was adopted for N. N. R., 1919, by the Council on Pharmacy and Chemistry.
In November, 1918, Merck and Co. sent a specimen labeled “Bismuth Tribromphenate-Merck,” “Merck and Co., New York, Distributors and Guarantors” and wrote: “You will notice this sample conforms in nearly all details to the tests submitted with our letter of June 4. We have been able to produce better goods, but just at present unsatisfactory starting material confronts us.”
Examination of the specimen demonstrated that it was soluble to a considerable extent in alcohol (the N. N. R., 1918, description provides that it should be only slightly soluble in alcohol) and, according to the standards adopted for New and Nonofficial Remedies, 1919, contained 18 per cent. of uncombined tribromphenol (more than five times the permitted amount).
In December, 1918, Merck and Co. submitted another specimen and said: “We believe this is a better grade than we have been able to make in the recent past. It seems to meet all the tests for N. N. R., 1919, with two exceptions: these are (a) solubility in alcohol, and (b) the test for uncombined tribromphenol.{”}
When the two recent samples of bismuth tribromphenate-Merck and two samples of Xeroform-Heyden were examined according to the new monograph the results given in Table 8 were obtained.
TABLE 8.—EXAMINATION OF TRIBROMPHENATE AND XEROFORM
| 1. BISMUTH. | |||
| Brand and Date Received | Weight Taken, Gm. | Weight of Bi2O3 Obtained, Gm. | Per Cent. of Bismuth, Gm. |
Xeroform-Heyden (from mfr.) July, 1918 | 0.6754 | 0.3565 | 47.2 |
Xeroform-Heyden (open market) July, 1918 | 0.8259 | 0.6156 | 66.7 |
Bismuth tribromphenate-Merck Nov., 1918 | 0.4882 | 0.2512 | 46.1 |
Bismuth tribromphenate-Merck Dec., 1918 | 0.8869 | 0.4495 | 45.5 |
| 2. UNCOMBINED TRIBROMPHENOL. | |||
| Brand and Date Received | Weight Taken, Gm. | No. C.c. of Tenth-Normal NaOH Con- sumed, C.c. | Per Cent. of Free Tribromphenol |
Xeroform-Heyden (from mfr.) July, 1918 | 1 | 7.4 | 24.5 |
Xeroform-Heyden (open market) July, 1918 | 1 | 0.7 | 2.3 |
Bismuth Tribromphenate-Merck Nov., 1918 | 1 | 5.7 | 18.8 |
Bismuth Tribromphenate-Merck Dec., 1918 | 1 | 5 | 16.5 |
In view of the laboratory’s report the referee of the Council on Pharmacy and Chemistry in charge of bismuth tribromphenate recommended that the acceptance of Xeroform-Heyden and bismuth tribromphenate-Merck be withdrawn, but that this should be without prejudice to their reinstatement when satisfactory products are again offered for sale. The Council adopted the recommendation of the referee and accordingly Xeroform-Heyden and bismuth tribromphenate-Merck are omitted from New and Nonofficial Remedies, 1919.
When the laboratory’s findings with regard to Xeroform-Heyden and the action of the Council deleting the article from New and Nonofficial Remedies was reported to the Heyden Chemical Works, the firm expressed regret that efforts to produce a product equal to that formerly obtained from Germany had so far not been successful and announced that it had decided to withdraw Xeroform-Heyden from the market for the present. When Merck and Co. was advised in regard to the report of the laboratory and Council’s action, this firm questioned the feasibility of producing a product meeting the Council’s standards and suggested that the test for free tribromphenol be revised to permit as much as 15 per cent. of this constituent. When Merck and Co. was reminded that its product submitted in 1915 essentially complied with the adopted standards (an old sample of Xeroform-Heyden was also found to comply) and that the estimate of the therapeutic value of bismuth tribromphenate is based on a product essentially devoid of free tribromphenol, the firm replied:
“As stated in our letter of the 12th inst., we do not wish to market the chemical unless it meets all legitimate requirements of the physicians that use it. If, therefore, your standard proves to be good and it is commercially possible to make supplies conforming to it, we shall do so. We shall discontinue the article unless it is of suitable quality.”—(From Reports A. M. A. Chemical Laboratory, 1918, p. 93.)
Procain, which chemically is the mono-hydrochlorid of para-amino-benzoyldiethyl-amino-ethanol, is the nonproprietary name selected by the Federal Trade Commission as the official designation for the drug previously known under the proprietary name “novocaine.” Before the war procain was obtainable in this country only through the Farbwerke Hoechst Co., the American representative of the German establishment, Farbwerke, vorm, Meister, Lucius and Bruening, under the name “novocaine.” This monopoly on “novocaine” was exercised by virtue of United States patent No. 812554, which was issued to Alfred Einhorn, Munich, Germany, assignor to Farbwerke, vorm, Meister, Lucius and Bruening, Hoechst a. M., in 1906. With the outbreak of hostilities, Congress passed the Trading with the Enemy Act, and under this, the Federal Trade Commission took charge of the novocain patent with a view of securing the production of this product in the United States. To ensure an adequate supply of the drug, the Federal Trade Commission on recommendation of the Committee on Synthetic Drugs of the National Research Council, in addition to issuing a license to the Farbwerke Hoechst Company (which license was later transferred to the H. A. Metz Laboratories) granted authority to the Abbott Laboratories and the Rector Chemical Company to manufacture it under the U. S. patent after specimens submitted by these firms had been found satisfactory in the Association’s laboratory and at the Cornell Pharmacologic Laboratory.
When the first specimen of American made procain was sent to the American Medical Association Chemical Laboratory it was necessary to work out adequate standards. The standards were formulated on the basis of the novocain monograph in the German Pharmacopeia, 1910, Ed. 5, p. 363, Remedia “Hoechst,” p. 242, and New and Nonofficial Remedies, 1918, p. 32, and the work carried out in this laboratory.
The following description has been adopted for New and Nonofficial Remedies, 1919, and all specimens of procain were subjected to these tests:
Procain occurs in small colorless and odorless crystals, or a crystalline powder which if placed on the tongue produces a transient sense of numbness.
It melts at 153–155 C.212
One gm. of procain is soluble in 0.7 c.c. of water and in 20 c.c. of alcohol U. S. P. (95 per cent.) at 20 C. From the aqueous solution, which is neutral, alkali hydroxids and carbonates precipitate the free base in the form of a colorless oil, which soon congeals to a crystalline mass, but solutions of sodium bicarbonate are miscible with solutions of procain without producing precipitations or turbidity.
Dissolve 1 gm. of procain in water. Separate portions of the solution yield a white precipitate with potassium mercuric iodid solution, a white precipitate with mercuric chlorid test solution, a brown precipitate with iodin test solution and a yellow precipitate with picric acid test solution. Acidify a portion with dilute nitric acid. A white curdy precipitate is thrown down on the addition of silver nitrate test solution.
Dissolve about 0.1 gm. of procain in 5 c.c. of water, add 2 drops of dilute hydrochloric acid and 2 drops of sodium nitrite solution (10 per cent.) and mix with a solution of 0.2 gm. of betanaphthol in 10 c.c. of sodium hydroxid solution (10 per cent.). A scarlet red precipitate is thrown down.
To a solution of about 0.1 gm. of procain in 5 c.c. of water add 3 drops of dilute sulphuric acid and mix with 5 drops of potassium permanganate test solution. The violet color of the latter disappears immediately (distinction from cocain).
Dissolve about 0.1 gm. procain in 1 c.c. sulphuric acid U. S. P. The solution is colorless (organic impurities).
Dissolve 0.1 gm. of the salt in 10 c.c. of water and saturate with hydrogen sulphid. No coloration or precipitation occurs (salts of the heavy metals).
Incinerate about 0.5 gm. of procain accurately weighed. Not more than 0.1 per cent. of residue remains.
To obtain specimens representing the market supply, orders for the three brands of procain were placed with pharmaceutical firms in New York, Baltimore and San Francisco. The Baltimore and San Francisco firms supplied specimens of procain-novocain brand and procain-Rector brand but reported that the Abbott brand was not procurable. The New York correspondent was able to supply procain-Rector only. As the entire output of the Abbott Laboratories was stated to go to the government, specimens of this product were obtained through the surgeon-general of the army from the general purchasing office, Medical Dept., U. S. Army. The following specimens were obtained and examined:
1. Procain-Abbott, 6 specimens: The first specimen bore no serial number but the five later specimens were designated respectively, No. 89999, No. 89998, No. 89997, No. 89996, and No. 810995, representing batches from which shipments are to be made on contracts placed by the general purchasing office, Medical Department, U. S. Army, with the Abbott Laboratories of Chicago.
2. Procain-novocain brand, 4 specimens: These were designated respectively, A56, A57, A63, and A67. The first two specimens were labeled “Manufactured by the Farbwerke-Hoechst Co. at the H. A. Metz Laboratories.” The third specimen (not in original container) was labeled “H. A. Metz Laboratories” and the fourth was marked “Manufactured by the H. A. Metz Laboratories.”
3. Procain-Rector, 3 specimens: Each bore the statement “Manufactured by the Rector Chemical Company” but had no “lot number.”
From this examination it appears that all the specimens of procain received complied satisfactorily with all tests of identity and purity with the following exceptions: (1) One specimen of procain-Abbott had a melting point slightly below the permitted range; however, the last five specimens had the required melting point. (2) Five specimens of procain-Abbott and the last three specimens of procain-Rector were not entirely colorless, but had a yellow or light brown tinge.
The toxicity experiments, which were carried out by Dr. R. A. Hatcher of the Cornell Pharmacologic Laboratory, were reported as being satisfactory.
When the Council on Pharmacy and Chemistry referred the matter of the discolored specimens of procain to the Rector Chemical Company for explanation, the firm wrote that for a short time for some unexplainable reason its procain had been slightly yellowish in color, but that every batch had been carefully tested and found to answer all chemical requirements. The firm stated that the product which it had sent out for some time past had been white and yielded a colorless solution.
To a like inquiry from the Council the Abbott Laboratories replied that the five samples which were found discolored were products manufactured by the Rector Chemical Company and represented goods which it had purchased to assist in filling delayed orders, because the firm had found itself unable to keep pace with the demand on account of delay in securing needed apparatus. The firm submitted protocols to show that the procain made by it, by Rector and by Metz were of equal toxicity.
In the accompanying table the results of the examination are given. For comparison the findings for the specimens examined previously are included.
| Brand | Date Received | Color | Melting Point, C. | Ash % |
| Procain (Abbott), from Committee on Synthetic Drugs | 12/21/17 | White | 154–155 | None |
| Procain (Abbott), submitted to Council P. and C | 1/29/18 | White | 153.5–154.5 | None |
| Procain (Abbott), Gen. Pur. Off. U. S. Army | 8/31/18 | White | 152.5–153.5 | None |
| Procain (Abbott), Gen. Pur. Off. U. S. Army, No. 89999 | 9/30/18 | Slight brownish tint | 153.5–154.5 | None |
| Procain (Abbott), Gen. Pur. Off. U. S. Army, No. 89998 | 9/30/18 | Slight brownish tint | 153–154.5 | 0.005 |
| Procain (Abbott), Gen. Pur. Off. U. S. Army, No. 89997 | 10/ 8/18 | Slight brownish tint | 153–154 | None |
| Procain (Abbott), Gen. Pur. Off. U. S. Army, No. 89996 | 11/ 4/18 | Slight brownish tint | 153.5–154.5 | None |
| Procain (Abbott), Gen. Pur. Off. U. S. Army, No. 810995 | 11/ 4/18 | Slight brownish tint | 153.5–154.5 | None |
| Procain (Farbwerke Hoechst Co.), submitted to Council | 10/24/17 | White | 153–154 | None |
| Procain (Farbwerke Hoechst Co.), submitted to Council | 12/10/17 | White | 153–154.5 | None |
| Procain (Farbwerke Hoechst Co.), submitted to Council, market spec. “A56” | 8/ 9/18 | White | 153.5–154.5 | None |
| Procain (Farbwerke Hoechst Co.), submitted to Council, market spec. “A57” | 9/ 9/18 | White | 153.5–154.5 | None |
| Procain (H. A. Metz Lab.), market spec. “A63” | 8/23/18 | White | 153–154 | None |
| Procain (H. A. Metz Lab.), market spec. “A67” | 9/23/18 | White | 153–154 | 0.018 |
| Procain (Rector), from Com. on Synthetic Drugs | 12/18/17 | White | 153–154.5 | None |
| Procain (Rector), market spec. | 8/20/18 | Slight brownish tint | 153–155 | None |
| Procain (Rector), market spec. | 8/23/18 | Slight brownish tint | 153–155 | None |
| Procain (Rector), market spec. | 8/23/18 | Slight brownish tint | 153–154.5 | None |
So far as the evidence goes, there was nothing to indicate that the yellowish or brownish colored specimens of procain were seriously impure. On the contrary, the compliance with the chemical and toxicologic tests indicated that the color was due to an insignificant trace of some colored substance produced in the manufacturing process. In view of this, the Council considered the use of the discolored product to be justified in the present emergency, although it urged that the future supply of procain should be free from color and also comply to the tests of purity. It made this request in the interest of the medical and dental professions, which use the drug, and also in a desire that in the manufacture of synthetic drugs, the United States should occupy a high place.—(From The Journal A. M. A., Jan. 11, 1919, with additions.)
The following note on two hypochlorite solutions is published as a slight addition to the inconclusive available information concerning the rate of deterioration of solutions containing sodium hypochlorite:
Hyclorite.—This is a solution of chlorinated soda, 100 gm. of which is said to contain sodium hypochlorite 4.05 gm., sodium chlorid 3.20 gm., calcium hydroxid 0.25 gm., inert ingredients 0.92 gm. It is declared to contain, when placed on the market, not less than 3.85 per cent. of available chlorin, and to deteriorate at the rate of about 12 per cent. per year. In order that the available chlorin content at the time of use may be judged, the date of bottling is stamped on each package. The solution is prepared by decomposing chlorinated lime suspended in water with sodium carbonate and adding to the solution obtained a freshly prepared solution of electrolyzed sodium chlorid. The composition and keeping qualities of hyclorite were reported on by this laboratory (Ann. Rep. Chem. Lab., A. M. A. 9:123, 1916). Hyclorite is fully described in New and Nonofficial Remedies, 1918, p. 153.
To further check the keeping qualities of hyclorite, a specimen received from the manufacturer in June, 1918, and said to have been bottled in April, 1918, was examined in September, 1918. It was found to contain 3.6 per cent, “available chlorin” (equivalent to 3.79 gm. sodium hypochlorite in 100 gm.). This indicated a loss of 6.2 per cent. during five months (equivalent to 14.9 per cent. per year) on the assumption that it contained the amount of “available chlorin” declared on the label.
Concentrated Solution Sodium Hypochlorite-Mulford.—This is described as a 5 per cent, aqueous solution of sodium hypochlorite containing free chlorin equivalent to from 0.2 to 1 per cent. sodium hypochlorite. It is prepared by treating a solution of sodium carbonate and sodium bicarbonate with chlorinated lime. The solution is filtered and standardized by determining the “available chlorin” and adjusting it to contain the equivalent of 5 per cent. of sodium hypochlorite.
It is proposed for use in the irrigation treatment of infected wounds after dilution with nine times its volume of water and the addition of a determined amount (stated on the label of each bottle) of boric acid to render it neutral to phenolphthalein. The manufacturer has found that development of a red color (due to formation of permanganate from the manganese contained in the chlorinated lime) is indicative of deterioration, and therefore warns against any solution which has become pink.
A specimen of concentrated solution of sodium hypochlorite-Mulford was sent the Council on Pharmacy and Chemistry in June, 1917, with a view of having the product admitted to New and Nonofficial Remedies. At that time it was found to contain 4.18 per cent. “available chlorin” (equivalent to 4.4 gm. sodium hypochlorite in 100 gm.). Another specimen received at the same time and kept unopened in a dark place, was examined in September, 1918, and was found to contain 2.88 per cent. available chlorin (equivalent to 3 gm. sodium hypochlorite per 100 gm.). On the assumption that the second specimen contained, at the time of its receipt, the amount of “available chlorin” found in the first, this second specimen lost 31 per cent. of its “available chlorin” during fifteen months.
At the time the specimens were received from the Mulford Company, the firm reported experiments which were under way to determine the keeping qualities of the solution. These experiments indicated marked deterioration of the specimens, which had become red from permanganate formation, and also that one specimen, which had not become red, had lost 5 per cent. of its available chlorin in one month. The Mulford Company explained that when sufficient data had been accumulated, a decision would be made either as to placing a time limit on the solution or making a claim as to the rate of deterioration. When the extreme deterioration found by this laboratory was reported to the Mulford Company, the firm replied that this was a much greater loss than the average deterioration found in its chemical laboratory, namely, an average of 10 or 12 per cent. per year. It advised that because of the instability of concentrated solution of sodium hypochlorite, its manufacture had been discontinued.—(From Reports A. M. A. Chemical Laboratory, 1918, p. 81.)
The shortage of arsphenamin (salvarsan) has made the sale of substitutes a profitable business. In many of these substitutes the earmarks of dishonesty have been obvious, so that detection of their falsity was relatively simple. In the case of “Syphilodol” marketed by the French Medicinal Company, Inc., New York, the deception has been practiced more skilfully. In the circular announcing their preparations, we read:
“It seems fitting at this time, when the American physicians are doing so much for France, that there should be a reciprocation in some way.
“Attempting to enhance somewhat this mutual interchange, we are presenting some of those scientific products, which have been so successfully used in France, ——”
“The effect of SYPHILODOL is very similar to salvarsan and neosalvarsan, but it has the advantage of being more lasting in its results and more pleasing in the manner of its preparations, in that it is put up in the form of tablets, and, also, in hermetically closed glass syringes or ampules, so that it may be administered either by the mouth, intravenously or intramuscularly, at the discretion of the physician. Patients averse to the use of the hypodermic needle may be treated expeditiously by the use of the tablet form of the medicine.”
In addition to Syphilodol, the French Medicinal Co. also sells “Vichi Fruti,” a combination of salts, “Urodol,” an “alkaline salt of the famous European Springs which is noted for breaking up and dissolving uric acid rapidly” and “Syloiodol,” “French Preventive,” which is described as “a solution of iodol incorporated into bougie.”
“Syphilodol,” we are told, is “a synthetic chemical product of silver, arsenic and antimony, scientifically prepared after the formula of the late Dr. Alfred Fournier of Paris.” (Italics ours—Ed.). It is also claimed that “Prof. Metchnikoff and other noted French scientists have made exhaustive tests of syphilodol and found it superior to the other products, in the treatment of syphilis.” In the advertisements, Fournier and Metchnikoff are the only names given of alleged endorsers; both of these men are dead and cannot protest. True, Fournier did considerable work on a legitimate synthetic of antimony, silver and arsenic having a general chemical constitution similar to arsphenamin, but so far as we are aware, there has been no publication by these men on “Syphilodol.” It would seem that the valuable work and high reputation of Fournier and Metchnikoff are being capitalized by the French Medicinal Company in their endeavor to foist a nostrum on the medical profession of this country.
“Syphilodol” comes in two forms—ampules and tablets. An order for two 0.4 ampules brought an elaborate case, much like those used to hold the popular style safety razors. The ampule itself was a “classy” affair evidently made by a glass expert; the hypodermic needle was enclosed in a novel sealed glass device. The price of each ampule is $3. No such fancy garnishments came with the tablets, although they are listed at $4.50 for twenty-five—18 cents a tablet! In the “Syphilodol” advertising it is emphasized that both the tablets and ampules are to be administered. For example:
“Syphilodol is dispensed in the form of tablets and also hermetically closed glass syringes or ampules so that it may be used either by the mouth, intravenously or intramuscularly at the discretion of the physician. An advantage of the tablets is that they can and should be given during the interim between the injections.”
Several samples of “Syphilodol” were sent to the American Medical Association Chemical Laboratory by readers of The Journal. An original bottle of tablets was ordered direct from the French Medicinal Company. The bottle contained 25 yellow tablets, having an average weight of 0.276 gm. (41⁄4 grains). After being powdered, “Syphilodol” was found to be only partially soluble in water (the excipient is soluble) and to be neutral in reaction. These findings contradict the claims on the circular accompanying the bottle to the effect that “Syphilodol is a yellow powder, soluble in water, and has an acid reaction.” Qualitative tests indicated the presence of mercury, sucrose (cane sugar), iodid, calcium, sulphate, fatty material, a trace of silver, a trace of arsenic and a very minute trace of antimony; a red dye was also present. Both qualitative and quantitative data showed that the mercury was present in the form of mercurous iodid (yellow iodid of mercury—hydrargyri iodidum flavum). Quantitative estimations yielded the following:
Silver (Ag+) | 0.001 | per cent. |
Mercury (Hg+) | 11.1 | per cent. |
Iodid (I-) | 7.8 | per cent. |
Sucrose (cane sugar) | 72.0 | per cent. |
Ash (calcium sulphate) | 2.5 | per cent. |
Ether-soluble material (fatty material—petrolatum) | 3.5 | per cent. |
Thus each tablet of “Syphilodol” contains approximately, 3⁄4 grain of mercurous iodid. An ampule of “Syphilodol,” labeled 0.4 gram, contained approximately 1.5 c.c. of a liquid which after evaporation on a water-bath left a residue weighing 0.8 mg., or 1⁄80 grain. A second ampule held about 2 c.c. of liquid, which contained a trace of arsenic (less than 0.00001 gm., or 1⁄6000 grain); a very small amount of mercury was indicated but not definitely established. The liquid had the physical characteristics of water.
Accompanying “Syphilodol” advertising sent to physicians is a circular letter inviting the doctor to become a member in the “United States Bacteriological and Research Institute.” The “institute” seems to be a means of suggesting that the physician have bacteriologic, pathologic and serologic examinations made on behalf of his patients. In view of the fact that it is to the commercial interest of the French Medicinal Company to have as many users of “Syphilodol” as possible, it would be interesting to know what proportion of the Wassermann tests are reported negative.
Shorn of its mystery, Syphilodol the “synthetic chemical product of silver, arsenic and antimony” is essentially mercurous iodid—yellow iodid of mercury.
In France there has been on the market for some time a synthetic compound of silver, arsenic and antimony having the general structure of arsphenamin. Structurally, the formula as given by Bonard, Danyss and Tournier is (C12H12N2As2) 2AgBrSbO (H2SO4)2— dioxy═ diamino arsenobensolstibicosilver sulphate. As the advertising matter for “Syphilodol” referred to the synthetic compound of silver, antimony and arsenic, and also to its use in syphilis by Fournier, the above compound was first suspected. However, the general characteristics of syphilodol tablets, such as partial solubility in water, but not soluble in sodium hydroxid, sodium bicarbonate or acids, threw doubt on the hypothesis. When a small amount of the powdered tablets was treated with water, a yellow residue could be filtered off; the filtrate was pink, opalescent, which on standing gave a clear pink solution, and a small yellow precipitate. The residue, when allowed to remain in sulphuric acid solution (20 per cent.) over night became red; on boiling, the red precipitate with sulphuric acid, the precipitate volatilized and could be condensed in a watch glass. Adding a pinch of manganese dioxid to the hot sulphuric acid mixture caused an evolution of iodin fumes. A small amount of powdered syphilodol tablets was placed in the sunlight; they turned from yellow to black. All these reactions are typical of mercurous iodid—yellow iodid of mercury.
I. Mercury.—Two methods were used to determine the mercury: (a) 1.4535 gm. of powdered syphilodol was treated with 10 c.c. of a 50 per cent. sodium sulphid solution. The solution was then transferred with washings (about 20 c.c.) to a cathode cup, previously weighed with its contained mercury. The mercury compound was electrolyzed by a current of about 8 volts and 3 amperes, using a rotating anode. The solution (and some sulphur suspension) was removed by siphon, pouring in water until the amperage of the current was close to zero (U. S. P., IX, p. 587). The increased weight in mercury was 0.1612 gm.
II. To serve as a check on the foregoing method, mercury was also determined in the following method, which also allowed systematic tests for silver, antimony and arsenic. (b) 1.1023 gm. of the sample was placed in an Erlenmeyer flask, 50 c.c. of water, 50 c.c. of sodium hydroxid solution (10 per cent.) and 20 c.c. of formaldehyd solution, U. S. P., added. The solution was boiled for ten minutes and maintained at temperature of steam bath for two hours. (This reduces the mercury salt to mercury and any silver salt to silver; antimony would probably be likewise reduced.) The precipitated mercury was transferred by water, and concentrated nitric acid added. (The nitric acid solution is boiled to oxidize all mercurous nitrate to mercuric nitrate.) A small white precipitate was obtained at this point which seemed to be insoluble in aqua regia (calcium sulphate). The filtrate from this precipitate, which was washed well, was tested with one or two drops of dilute hydrochloric acid and a faint precipitate formed; this was filtered off through extra fine filter paper and washed repeatedly. The paper and precipitate was heated with potassium cyanid solution over night, filtered and the filtrate electrolyzed in a platinum dish. The increase in weight of the dish was 0.00018 gm., or 0.001 per cent. Into the platinum dish some nitric acid was poured, then diluted, and a drop of hydrochloric acid added. A turbidity was produced which cleared on the addition of excess of ammonium hydroxid solution (silver). The filtrate from the nitric acid treatment was electrolyzed, this time in a platinum dish, and the liquid carefully removed, washed carefully with redistilled alcohol and ether. The mercury, which could be seen easily by the naked eye, weighed 0.1200 gm., equivalent to 10.89 per cent. of mercury.
III. Arsenic and Antimony.—About 3 gm. of the powdered specimen was digested with sulphuric acid in a Kjeldahl flask. One-half portion (which was evaporated almost to dryness and treated with 5 c.c. of concentrated hydrochloric acid) was submitted to treatment with hydrogen sulphid, diluted, and saturated with hydrogen sulphid. The precipitate was treated in the usual manner of the group separation with warm ammonium sulphid solution. The filtrate from this treatment was acidulated with hydrochloric acid, the precipitate removed, and treated with concentrated hydrochloric acid. The substance insoluble in hydrochloric acid was treated with more concentrated hydrochloric acid and a crystal of potassium chlorate. The solution was tested after the Gutzeit method of the Pharmacopeia IX, for arsenic. A very small amount was indicated. The hydrogen sulphid test was not indicative. The solution which might contain the antimony was tested with hydrogen sulphid. In one case only was a slight orange coloration produced. No antimony was deposited on platinum foil in the presence of granulated zinc. These tests were run in triplicate.
Iodid.—Iodid was determined by the Carius method (a) 0.7412 gm. yielded 0.1112 gm. silver iodid, equivalent to 8.09 per cent.; (b) 0.5319 gm. yielded 0.0751 gm., equivalent to 7.80 per cent. The iodid and mercury were in proportions comparable to mercurous iodid.
Ash.—(a) 0.9159 gm. when ignited to constant weight yielded 0.0232 gm., equivalent to 2.52 per cent. ash; (b) 1.3008 gm. treated with water and the residue filtered on a Gooch filter and ignited. The ash of the residue was 2.51 per cent. (the mercurous iodid volatilized). The ash was calcium sulphate.
Sucrose.—1.3008 gm. of the sample was treated with water and filtered by suction through a Gooch crucible. The filtrate and washing were carefully transferred to 500 c.c. volumetric flask, and allowed to stand one week; 50 c.c. portions were used to determine sugar according to the Daufresne-O’Sullivan method. The weights of cupric oxid averaged 210 mg., or 72 per cent.
Ether Soluble Material.—1.6998 gm. of the powdered specimen was extracted with ether and the ether extract evaporated to dryness. The residue weighed 0.0600 gm., equivalent to 3.53 per cent.
Water.—The liquid from one ampule was distilled over very carefully. The freezing point of the liquid was +0.1 C., and it was neutral to methyl orange and phenolphthalein.
Arsenic.—The contents of one ampule was placed in a small florence flask, 20 c.c. of concentrated sulphuric acid added and heated to 70 C.; 0.5 gm. of potassium permanganate was added in small amounts. The procedure was then carried on as described by Engelhardt and Winters in J. Am. Pharm. Assn., 1915, p. 1469. To the mixture from 5 to 10 c.c. of hydrogen peroxid solution were added drop by drop until the color had disappeared. The liquid was diluted with 20 c.c. of water, boiled fifteen minutes, diluted again and boiled fifteen minutes, then cooled and made up to exactly 100 c.c. A blank was also run alongside. Five c.c. of this solution was then tested quantitatively for arsenic according to the U. S. P. IX method, using all precautions. Comparisons of stains showed less than 0.00001 gm. of arsenic (As).—(From The Journal A. M. A., May 18, 1918.)
Cerelene, a paraffin preparation for the treatment of burns, was submitted to the Council by the Holliday Laboratories, with the statement that it was composed of 84 per cent. paraffin, 15 per cent. myricyl palmitate, and 1 per cent. purified elemi gum to which is added oil of eucalyptus 2 per cent. and betanaphthol 0.25 per cent. It was explained:
“Myricyl Palmitate is a purified form of Beeswax, free from all impurities, acids, etc., which is solely manufactured by this Company....”
It was also stated that on “special order” Cerelene has been made containing oil of eucalyptus and resorcin, oil of eucalyptus and picric acid, and picric acid alone. The following report on the preparation was presented to the Council by the referee to whom Cerelene had been assigned:
Cerelene is another compound wax for the treatment of burns. According to the work of Sollmann (J. A. M. A. 68:1799, 1917) it is highly improbable that compound mixtures have any advantage over simple paraffin of low melting point. Cerelene must therefore be considered as an unessential modification of paraffin, and as in conflict with Rule 10, unless definite evidence of superiority be submitted. Cerelene mixtures containing medicinal ingredients also appear unscientific since the evidence that the ingredients do not leave the wax has not been successfully contradicted. Finally, the claims made for Cerelene are rather extreme, and would need some revision before they could be accepted.
The A. M. A. Chemical Laboratory reports:
The physical properties of Cerelene are as follows:
Melting point by U. S. P. method | 50.0 C. |
Ductility limit | 30.5 C. |
Plasticity limit | 26.4 C. |
Not strong at | 38.0 C. |
Adheres moderately well; detaches with “pulling.” On heating, readily loses eucalyptol, and a small amount of resinous substance forms in the bottom of the beaker. If Cerelene be heated to 145 C. and cooled, the resulting product no longer has the properties of the original Cerelene.
After two years’ delay on the part of the manufacturer, the Council authorized publication declaring Cerelene inadmissible for New and Nonofficial Remedies because its superiority over single paraffins had not been demonstrated and the unwarranted claims had not been abandoned.—(Abstracted from The Journal A. M. A., Feb. 15, 1919.)
Dr. DeSanctis’ Rheumatic and Gout Pills are sold by Edward Cleaver, 13 Clerkenwell Road, London, England. The American agents are E. Fougera and Co., Inc., New York. The package is a round pill box and contains twelve pills and a circular, which directs that one pill be taken every eight hours until relieved. In the package there is also a circular advertising Dr. DeSanctis’ Gout and Rheumatic Paint, with directions for its use. On the cover of a box, which contained six of the retail packages, is the statement that these pills have been in general use for nearly 100 years, and that their sale has been built up without advertising.
DeSanctis’ pills are round, uncoated, and have a light brown color. There was some variation in the color of different lots, one lot in particular being gray rather than brown. A little arrowroot starch was found in each box, this evidently having been used as a dusting powder. The pills were very hard, rather brittle, but quite difficult to powder. The pills were not readily disintegrated by water or diluted acids, even when warmed, but when warmed with a dilute sodium hydroxid solution they readily disintegrated.
Ten pills weighed 3.213 gm., an average of 0.3213 gm., or 5 grains. The arrowroot starch used as a dusting powder was removed as completely as possible by rolling the pills in a cloth. Several dozen pills were then powdered and the powder thus obtained used for the analysis.
A microscopic examination of the powder showed powdered colchicum seed in abundance and also traces of arrowroot starch, no doubt from that used as the dusting powder.
Since colchicum seed was so abundant, the powder was assayed by the U. S. Pharmacopeial method for colchicum seed (U. S. P. IX, p. 120), slightly modified so that less of the powdered pills than directed there could be used. In one assay 3.75 gm. gave 0.0204 gm. of colchicin or 0.54 per cent. In a duplicate, 5 gm. gave 0.0234 gm. of colchicin or 0.47 per cent.; average 0.5 per cent.
The alkaloid obtained had the characteristic appearance and odor of colchicin when separated from the seed under these conditions. The solution in water and acid was yellow; the aqueous solution was intensely bitter, and the yellow color intensified with acids. The dry residue became intensely yellow with concentrated sulphuric acid; with nitric acid it became violet turning to yellow, and with concentrated sulphuric acid and potassium nitrate it gave a yellowish green color, turning to violet and finally to a wine color. All these reactions are typical of colchicin.
From 1 gm. of the powdered pills there was obtained 0.0425 gm. of ash, or 4.25 per cent.
When the powdered pills were extracted with chloroform in a Soxhlet apparatus, a very uniform quantity of extract was obtained. From 5 gm. there was obtained, in one case, 0.581 gm.; in another, 0.5755 gm., and in a third, 0.588 gm., the average being 0.5815 gm. or 11.63 per cent.
On still further extracting with alcohol, a small amount of extractive was obtained, the amount depending on the length of time the extraction was continued.
On extracting with hot water the residue left after exhaustion with chloroform and with alcohol, a further extract was obtained. In one case, it amounted to 0.4763 gm. or 9.53 per cent., and in another case it amounted to 0.470 gm., or 9.40 per cent.; average 9.47 per cent.
In attempting to dry the pills or the above-mentioned chloroformic extract at 100 C., a crystalline sublimate was obtained which had the odor of benzoic acid. The crystals were acid, their neutral solution gave a flesh-colored precipitate with ferric chlorid, and they melted at 120–121 C. This crystalline substance appeared to be benzoic acid.
The quantity of benzoic acid in this extract was determined by heating it to about 140 C. A current of air was drawn through the flask and the sublimed benzoic acid collected in a cooled tube. The benzoic acid was washed out of the tube with neutral alcohol, and the solution was titrated with tenth normal potassium hydroxid. In one case, 11.25 c.c. of tenth-normal alkali was used, indicating 0.1373 gm, of benzoic acid; in another, 12.27 c.c., indicating 0.1498 gm. of benzoic acid; average 0.1436 gm., or 2.87 per cent. In a third case the temperature reached 250 C., and there was some decomposition of the fat in the flask and some colored material distilled over. For this sublimate 15.54 c.c. of tenth-normal alkali were required.
After evaporating the alcohol and acidulating the solutions obtained in the previous experiments, the benzoic acid was extracted with chloroform. In the first case, 0.1383 gm. was obtained; in the second, 0.1541 gm.; average 0.1462 gm., or 2.92 per cent. of benzoic acid.
When the original chloroformic extract was heated until all of the benzoic acid had been driven off, the residue had the appearance of a semisolid fat. It compared quite closely in color, odor, etc., with the fatty material obtained by extracting colchicum seed with chloroform, although the odor was more suggestive of oleic or stearic acid. It was distinctly acid, which is also true of the fatty material obtained from a sample of colchicum seed.
The extract obtained with hot water was light yellow; gummy, at first, but dried to a glass-like brittle mass. It had a slight burned-sugar odor and taste, and was neutral in reaction. It was strongly dextrogyrate and at once reduced Fehling’s solution as well as alkaline silver nitrate solution. On boiling with potassium hydroxid solution, it turned deep red. It also gave the Molisch carbohydrate reaction, and the ozazone test in seventeen minutes as described in Mulliken (Identification of Pure Organic Compounds, Ed. 1, 1905, p. 26). These are all characteristic reactions of lactose or milk sugar.
From this examination we conclude that DeSanctis’ pills contain powdered colchicum seed, benzoic acid, and sugar of milk. There is also present fatty material which resembles the fat of colchicum seed, but may be, in part, added fatty acid. The percentage of colchicin found (0.50) is about that of a good quality of colchicum seed, the U. S. Pharmacopeial standard being not less than 0.45 per cent. Since the pills contain material other than colchicum seed, this assay would indicate a colchicum seed of high alkaloidal content, or the possible reinforcement of the pills with colchicum extract or colchicin.
The amount of benzoic acid, 2.92 per cent., or about 1⁄7 grain per pill, is insignificant from a therapeutic standpoint, since an average dose is 0.5 gm., or 8 grains. Fatty acids, and the fatty matter from colchicum seed are inert, at least in the quantities found here. The only office which fatty acids might perform, would be to give the pills an enteric quality, preventing their absorption until they reach the intestine. The sugar of milk, about 10 per cent., or 1⁄2 grain per pill, no doubt is simply an excipient.
DeSanctis’ pills are therefore essentially 5 grain doses of powdered colchicum seed, of which the average dose is 0.2 gm., or 3 grains (U. S. P. IX, p. 120).
The Journal in presenting the facts contained in the above report made the following comments:
“Here then, we have sold for self-medication an extremely poisonous drug, with no warning of the risk the public runs in using it. While the directions call for “one pill every eight hours until relieved,” it is notorious that the public takes the attitude toward “patent medicines” that, if a little is good, more is better, and the average user of remedies for self-treatment is likely, unless there is some warning, to use his own discretion as to the amount taken.
“The individual dose is above that of the average recommended in the United States Pharmacopeia. Colchicum or its alkaloids—or for that matter, any drug as toxic as colchicum—have no place in preparations of the home-remedy type. In the case of all “patent medicines,” public interest demands that the full quantitative formula of the therapeutically active ingredients should be given on the label, for when the public prescribes for itself, it has a right to know what it is taking. Unfortunately, public interest clashes with vested interests and, as usual, vested interests get the better of it. In the case of such dangerous preparations as DeSanctis’ pills, if their sale is to be permitted at all, not only should the names and quantities of all therapeutically active ingredients in the mixture be given, but the law should require that the word Poison be plainly printed on the label.”—(Abstracted from The Journal A. M. A., July 19, 1919.)
The A. M. A. Chemical Laboratory examined Iodex in 1915.213 The claims made, at that time, by the exploiters, Menley & James, were shown to be contrary to facts in that Iodex contained only traces of free iodin while they claimed “5 per cent. Therapeutically Free Iodin.” Even the total quantity of iodin was shown to be only about one half of the 5 per cent. claimed to be present as free iodin.
An examination of the advertising matter sent out by Menley & James in 1919 showed that substantially the same claims were being made as in 1915. This at once suggested the inquiry: Since the claims are the same as previously made, have the manufacturers altered the composition to conform to the claims? The answer is found in the results of the analysis of two samples purchased in the open market early in 1919.
This analysis shows conclusively that Iodex is essentially the same as in 1915, that is, that it contains no free iodin and only about three fifths of the total amount of iodin claimed.
It would seem that Iodex (Ung. Iodi., M. & J.) is in obvious conflict with Section 7 of the Food and Drugs Act. While it is sold under a name recognized by the U. S. Pharmacopeia, namely, Ung. Iodi., it does not conform to the standards of the U. S. Pharmacopeia for that product. Iodin ointment U. S. P. is made with 4 per cent. of free iodin, 4 per cent. of potassium iodid, 12 per cent. of glycerin, and a benzoinated lard base. It should then contain approximately 7 per cent. of total iodin. It has been shown by Warren214 that about 75 per cent. of the iodin in the U. S. P. ointment remains in the free state even after months of standing. Ung. Iodi., U. S. P., then, should contain about 3 per cent. of free iodin. Iodex contains no free iodin, or but traces, and no potassium iodid. Furthermore, the Iodex label declares the presence of 5 per cent. of “therapeutically free” iodin. As a matter of fact, the amount of iodin is variable, the highest amount found being 3.5 per cent. and samples containing as low as 2.63 per cent. have been examined.
It would seem further that Iodex is misbranded under the Sherley amendment in that it is said that it “may be used externally with advantage in all cases where the action of iodin is desired.” Since it contains no iodin as such this cannot possibly be true. It is also stated in a circular accompanying the trade package that “Thirty minutes after inunction iodin can be found in the urine.” This statement has also been shown to be untrue.—(Annual Reports A. M. A. Chem. Lab., 1915, p. 89.)
Iodex.—This is a rather soft ointment, almost black but with a decided greenish cast in thin layers. It is soluble in chloroform but is only partly saponified and dissolved by alcoholic potassium hydroxid. Iodex has a distinct odor like oleic acid.
Free Iodin.—When examined by the method previously used215 only minute traces of free iodin were found.
Total Iodin.—The methods employed were as follows: 1. Iodex was saponified by boiling for from two to three hours with alcoholic potassium hydroxid. The alcohol was then evaporated and the iodin determined by the method described in the U. S. Pharmacopeia for thymol iodid.
2. The same as Method 1, except that after ignition of the saponified mixture the halogen was determined by weighing as silver iodid.
3. The Carius method.
It should be noted that Methods 2 and 3 determine chlorin and bromin should any be present with the iodin.
When 5 gm. of Sample 1 was assayed by Method 1, it required 73.56 c.c. of tenth-normal sodium thiosulphate, equivalent to 3.11 per cent. of iodin. In a duplicate, 2.7565 gm. of Iodex required 38 c.c. of tenth-normal sodium thiosulphate, equivalent to 2.92 per cent. of iodin; average of the two, 3.02 per cent. of iodin.
A weight of 2.5800 gm. of Sample 1, assayed by Method 2, gave 0.1582 gm. of silver halid, equivalent to 0.0855 gm. of iodin, or 3.31 per cent.
A weight of 0.588 gm. of Sample 2, assayed by the Carius method, gave 0.0388 gm. of silver halid, indicating 0.02096 gm. of iodin, or 3.52 per cent. In a duplicate, 0.5342 gm. gave 0.0338 gm. of silver halid, indicating 0.01826 gm. of iodin, or 3.42 per cent.; average, 3.49 per cent. of iodin.
Liquid Iodex.—This is sold by Menley & James, Ltd., the firm selling Iodex Ointment. According to a circular in a trade package “the valuable properties of Free Iodine are available in Liquid ‘Iodex’ in a state of greatly enhanced activity; but the irritating, corrosive and hardening drawbacks of ordinary solutions of the drug are absent.” The label on a bottle reads as follows: “Liquid ‘Iodex’ (Liq. Iodi. M. & J.). A nonirritant preparation of iodine (21⁄2%) ... This product contains Free Iodine....”
The sample of Liquid Iodex purchased on the open market was found to be a reddish liquid with an odor like oleic acid. It dissolved completely in chloroform.
Free Iodin.—A weight of 6.2936 gm. was dissolved in chloroform and the solution shaken with 25 c.c. of a solution of potassium iodid. The iodin which passed into the potassium iodid solution was titrated with tenth-normal sodium thiosulphate, 0.81 c.c. being required. This indicates 0.01022 gm. of iodin, or 0.16 per cent.
Total Iodin.—Total iodin was determined by Method 1 as given above under Iodex. A weight of 4.466 gm. required 32.93 c.c. of tenth-normal sodium thiosulphate, equivalent to 0.06964 gm. of iodin, or 1.55 per cent. In a duplicate, 5 gm. of material required 33.3 c.c. of tenth-normal sodium thiosulphate, equivalent to 0.7043 gm. of iodin, or 1.41 per cent.; average, 1.48 per cent. of iodin.
Liquid Iodex, then, contains but little (0.16 per cent.) free iodin and only about three fifths of the total iodin claimed.
I. G. O. is an iodin ointment. It is said to be made by Dr. H. S. Lambdin, Peru, Kansas. In a circular distributed by the manufacturer, it is stated that “I. G. O. is a saturated solution of Iodine Gas in petrolatum at 130 degrees with oil of eucalyptus. The heat of the body liberates the iodine and it is absorbed as free iodine.”
A sample of I. G. O., received from a physician, was examined. It was found to be a black ointment, green in thin layers, with a slight odor like crude petroleum. By the methods used for the examination of Iodex, I. G. O. was found to contain 0.59 per cent. of free iodin.—(From Reports A. M. A. Chemical Laboratory, 1919, p. 104.)
Of all the things used in medicine nothing seems to have attracted the attention of all classes of users as has iodin. Perhaps more romantic schemes for the cure of all the ills which afflict mankind have centered in iodin therapy than in any other one drug. Iodin is being used in every conceivable way from crystals to colloid; in vapor; combined as iodid, iodate and the like; organic, inorganic, simple and complex; internal, external and by injection, and yet there seems to be no end to the ingenious schemes for its exploitation.
One of these schemes, and one so simple that it seems at first sight to be hardly worth serious consideration, is that of a solution of iodin in liquid petrolatum. Solutions of this kind have frequently been offered to physicians and the laity. The thing of particular interest is the claim made as to the percentage of free iodin. Five per cent. is frequently claimed. Examination of some of these products in the chemical laboratory of the A. M. A.216 revealed the fact that they did not contain the claimed amount of free iodin. These questions at once arose: Was the low free iodin content due to intentional fraud, the result of carelessness, or of ignorance? Was it impossible to prepare a solution containing 5 per cent., or did the iodin slowly combine with the oil and disappear?
Several years ago the A. M. A. Chemical Laboratory217 conducted some experiments on the solubility of iodin in liquid petrolatum, which indicated that a saturated solution would contain about 1.4 per cent. These experiments did not show conclusively that no iodin was absorbed by the petrolatum during the process of solution. For this reason, further experiments were conducted with the view of determining both the solubility in and the extent to which iodin is absorbed (disappears as free iodin), if at all, by liquid petrolatums of various kinds. Theoretically such hydrocarbons should not absorb iodin. The results of these experiments are here given.
A sample of iodin was prepared by sublimation from a mixture with potassium iodid. This sample when dried over sulphuric acid assayed 99.98 per cent. of iodin. Portions of this sample were used in all of the subsequent experiments. To prepare solutions of definite concentrations, in all cases expressed as percentage by weight, an accurately weighed quantity of iodin was placed in a glass-stoppered bottle and an accurately weighed quantity of liquid petrolatum added. The mixture was subjected to treatment as indicated in the various experiments and from the weights of iodin and petrolatum used the percentage of iodin was calculated.
The method of assay employed was as follows: A weighed quantity of the iodin solution was transferred to a bottle or flask by means of several small amounts of chloroform, about 50 c.c. in all. To this was added about 25 c.c. of potassium iodid solution. The mixture was then titrated with tenth-normal sodium thiosulphate until on thorough shaking no iodin passed into the aqueous layer.
To 2.1248 gm. of iodin was added 199.3 gm. of liquid petrolatum. The mixture was shaken frequently each day and after forty days there seemed to be still a few particles of iodin undissolved. The supernatant solution was assayed, however, and found to contain 1.038 per cent. of iodin. The iodin added was 1.055 per cent. Six months later 1.025 per cent. of iodin was found.
To 5.1832 gm. of iodin was added 199.5 gm. of liquid petrolatum. The mixture was heated to 100 C. for four hours with frequent shaking. The iodin was in perfect solution. The per cent. of iodin would then be 4.95. Upon cooling, iodin in abundance crystallized out. After standing a few hours, with frequent shaking, the iodin in solution was determined. This was found to be 1.425 per cent.
These two experiments indicate: First, that the previous findings of the A. M. A. Chemical Laboratory are correct in that only about 1.4 per cent. of free iodin is retained in solution in liquid petrolatum at room temperature. Second, that the quantity of iodin absorbed by liquid petrolatum at room temperature, in seven months at least, is practically none. Third, that iodin dissolves rather slowly in liquid petrolatum at room temperature.
In the experiments, the results of which are tabulated below, the iodin and liquid petrolatum were heated at 100 C. for about four hours, shaking frequently to hasten solution. After cooling, the specimens were assayed and were again assayed at intervals as indicated in the table.
| Kind of Liquid Petrolatum Used | Date of Manufacture and First Assay | Weight | Per Cent. Iodin Used | Per Cent. Iodin Found | Per Cent. Iodin Nov. 17, 1918 | Per Cent. Iodin† Nov. 19, 1919 | |
| Iodin | Petrolatum | ||||||
| Stanolind | 10/17/18 | 2.089 | 188.4 | 1.096 | 1.085 | 1.068 | 1.067 |
| Squibb | 10/14/18 | 1.9569 | 186.78 | 1.0306 | 1.0232 | 1.013 | 1.009 |
| Unknown, bulk* | 10/28/18 | 1.9497 | 158.2 | 1.225 | 1.133 | 1.075 | 1.095 |
| Parke, Davis & Co | 10/24/18 | 2.0869 | 167.43 | 1.241 | 1.2488 | 1.191 | 1.180 |
* Considerable dark sediment formed in this sample during the heating process.
† It should be pointed out here that while every sample showed some absorption, the amount, with the exception of the unknown bulk, is so small that it might even be accounted for on the basis of “experimental error.” Every ordinary precaution was taken to insure accuracy, but since about 15 gm. of the solution was used for each determination, it is seen that an error of 0.3 c.c. in the titration would indicate a greater absorption of iodin than that noted.
Conclusions: These experiments show: A solution of iodin in liquid petrolatum is saturated when it contains about 1.4 per cent. of iodin. The amount of iodin absorbed (disappearing as free iodin) by liquid petrolatum, when in contact at room temperature for as long as seven months, or in contact at 100 C. for four hours, or both, is relatively insignificant. Also all the absorption seems to take place during the heating and in the first month of contact.—(From Reports A. M. A. Chemical Laboratory, 1919, p. 21.)
Before European hostilities, the United States was so dependent on Germany for synthetic drugs that the dependence was considered a necessity; this was strikingly manifested by the precipitous rise in prices immediately after the embargo was declared against Germany. Since then the shortage of German-made synthetics has caused two important results: 1. The physician can do without most of the German drugs, because the prewar demand had been stimulated artificially. 2. Those few synthetics, which were in great need, are being rapidly replaced by the American-made drugs.218 In connection with the second result, the Chemical Laboratory of the American Medical Association has endeavored to contribute its services.
In September, 1917, it was announced219 that the A. M. A. Chemical Laboratory would make studies of American-made synthetics. Just prior to this announcement, the National Research Council established a committee on synthetic drugs220 “to facilitate the manufacture of synthetic drugs in this country and thus to relieve shortage and reduce the exorbitant prices which have resulted from the war.”221 Also during this time Congress was considering the “trading with enemy” act, first known as the Adamson bill—the purpose of which was to confer authority on the President to license American firms to use U. S. patents owned by German subjects. The act became law, September 28; the Federal Trade Commission was designated by the President to carry out the provisions of the law as it referred to enemy-owned patents. As a result of a conference, Oct. 30, 1917,222 with various agencies, the Federal Trade Commission decided to consider licenses for manufacturers of synthetic drugs, after recommendations had been made by the Committee on Synthetic Drugs of the National Research Council; this committee in turn invoked the aid of the A. M. A. Chemical Laboratory in testing the manufacturer’s products. The essence of the laboratory’s work up to July 1, 1919, is reported in this paper.
“Partly in order to help insure to licensees a market for their products after the war, in larger part inspired by the idea of encouraging the establishment of a permanent American industry in these important articles, the [Federal Trade] Commission wisely decided that American houses should be put on the same footing as competing foreign houses for after-the-war competition, by imposing on all licensees the obligation to use new official names for the articles, names which after the war will be open to all competitors, domestic and foreign.”223
The new American names are:
Arsphenamin224 (contracted from the scientific name arsenphenolamin) for
salvarsan, arsenobenzol, diarsanol, arsaminol.
Barbital (contracted from the scientific name diethyl-barbituric acid) for
veronal.
Barbital-sodium (the sodium salt of barbital) for “veronal-sodium” and
“medinal.”
Cinchophen for atophan or phenylcinchoninic acid (the U. S. P. IX name).
Procain for novocain hydrochlorid (from “pro” and “(co)caine”).
Procain nitrate for novocain nitrate.
In testing chemically the products which had been submitted to the Federal Trade Commission, the aims were that the product should conform to a high degree of purity; at the same time the candidate for license should not be inflicted with undue hardships in making the product, such as an unnecessarily high degree of purity. It was insisted that the purity of the drugs should be equal to, if not greater than, that of the respective former German-made products, in order to uphold the name and reputation of the American manufacturers in the after-the-war competition. Consequently, in the chemical work the American product was always examined parallel with the German-made product, authentic samples of the latter of which the laboratory had in its possession. Whenever possible, the tests described in books of standards were carried out.
Barbital was introduced into medicine under the proprietary name “veronal,” and was manufactured in Germany by Friedr. Bayer & Co., Leverkusen, and E. Merck & Co., Darmstadt, Germany. Barbital is described in New and Nonofficial Remedies, 1919,225 as diethylbarbituric acid (diethylmalonyl urea) having the formula:
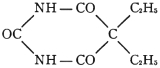
It is official in the British Pharmacopeia under the name “barbitone,” and in the German Pharmacopeia as “acidum diethylbarbituricum.” Barbital “may be prepared by the interaction of esters of diethylmalonic acid with urea in the presence of metallic alcoholates.... It is also obtained by condensation of diethylcyanacetic ester with urea by means of sodium alcoholate.” Barbital is used in medicine chiefly as a hypnotic.
The different brands of barbital which were submitted to the laboratory were subjected to the tests given in the books referred to above.226 The products were:
1. Barbital (Abbott) Sample A, to Federal Trade Commission.
2. Barbital (Abbott) Sample B, to Federal Trade Commission.
3. Barbital (Abbott) Sample C, to Red Cross.
4. Barbital (Antoine Chiris), to Federal Trade Commission.
5. Barbital (V. Halter), to Federal Trade Commission.
6. Barbital (Rector Chemical Company) to Federal Trade Commission.
7. Diethylbarbituric acid (Merck), to Council.
8. “Veronal,” manufactured by Farb. vorm Fried. Bayer & Co., Germany.
All responded satisfactorily to the tests. In Table 1 are given the respective melting points and percentages of ash found. (The melting point of a mixture of the sample with the original “veronal” was always taken.)
TABLE 1.—MELTING POINT
| Ash | Ash | ||||
1 | 188.5–189.0 | 0.01 | 5 | 188.0–188.5 | 0.01 |
2 | 188.5–189.0 | 0.01 | 6 | 188.0–188.5 | 0.01 |
3 | 188.0–188.5 | 0.01 | 7 | 188.0–188.5 | 0.01 |
4 | 188.0–188.5 | 0.04 | 8 | 188.0–188.5 | 0.02 |
Barbital does not seem to form an insoluble salt with chlorplatinic acid; nor an ether-insoluble hydrochlorid or oxalate; nor an insoluble barium salt. It does not respond to many urea tests, and is not affected by urease as would be expected in light of the extensive investigations made on this enzyme by Van Slyke and Cullen.
As barbital is also sold in the form of tablets or mixtures, a reliable method for its quantitative determination in the presence of other substances is needed. Some experiments in this direction were made, but the press of other work did not permit their continuation. When time permits, this work will be resumed.
At the time of writing this article, licenses for manufacture had been granted by the Federal Trade Commission to the Abbott Laboratories, to Antoine Chiris Company, and to the Rector Chemical Company.
Barbital sodium, formerly sold under the proprietary names “veronal-sodium” and “medinal,” is, as the former name suggests, the sodium salt of diethylbarbituric acid. Its therapeutic advantages are stated to be that more rapid results are obtained because of its increased solubility over barbital alone.227 Barbital sodium should yield, according to theory, 11.19 per cent. of sodium and 89.31 per cent. of diethylbarbituric acid. A number of years ago, when “veronal-sodium” and “medinal” were being introduced, Puckner and Hilpert228 found that these products yielded results corresponding closely to the theoretical amounts of sodium and diethylbarbituric acid. A recent examination of veronal-sodium, Merck, made for the Council on Pharmacy and Chemistry, showed it to be of the same composition as that previously reported.
| CCONNaCONHCO └─────────┘ |
It is possible that in preparing the sodium salt of diethylbarbituric acid, the ring opens up, forming a compound not so easily affected by dilute mineral acids.
TABLE 2.—EXTRACTION OF A SAMPLE OF BARBITAL-SODIUM
| Length of Time | Diethylbarbituric Acid per Cent. | |
| a. | Immediately | 75.5 |
| a1. | 3⁄4 hour | 82.0 |
| b. | Immediately | 82.0 |
| c. | 11⁄2 hours | 80.5 |
| d. | 4 hours | 82.82 |
| e. | 4 hours | 83.56 |
| f. | 4 hours | 83.41 |
| g. | 451⁄2 hours | 84.89 |
| h. | 451⁄2 hours | 84.73 |
| Theory | ....... | 89.31 |
| Veronal-Sodium | (Puckner and Hilpert) | 89.01 (average) |
| Medinal | (Puckner and Hilpert) | 88.95 (average) |
Phenetidyl-acetphenetidin hydrochlorid was introduced in the United States under the name of “holocain hydrochloride” by Farbwerke, vorm Meister Lucius and Bruening, Hoechst a. M. Germany; the product apparently had not been patented in this country, although it was protected in Germany under patents No. 78868 and 80568. New and Nonofficial Remedies, 1918, describes “holocain hydrochlorid” as ethenyl-paradiethoxy-diphenyl-amidin hydrochlorid CH3:(NC6H4OC2H5)(NHC6H4OC2H5)HCl. It is used as a local anesthetic for the eye.
The standards, such as had been described, were meager and unsatisfactory. Hence when the first specimen of American-made phenetidyl-acetphenetidin was sent to the A. M. A. Chemical Laboratory through the agency of the Federal Trade Commission and the Committee on Synthetic Drugs, it was necessary for the laboratory to work out adequate standards.231 As a result of the chemical work, a rather comprehensive monograph was drawn up, which was published in the 1918 Laboratory Reports. A summary of the products examined, with some of the chemical data, is given in Table 3. It will be seen that one specimen had a deficiency of about 2 per cent. of free base.
TABLE 3.—DATA ON PHENETIDYL-ACETPHENETIDIN HYDROCHLORID
| Manufacturer | Appearance | Moisture | Melting Point | Phosphorus Compounds | Phenetidin* | Indol Reaction | Ash | Per Cent. Base by Weight | Per Cent. Base by Titration | Melting Point of Base | Per Cent. Platinum in Platinum Salt + |
| John T. Milliken Co. | White crystalline powder | 5.13 | 191.5 to 192 | Absent | Negative | Positive | 0.00 | 89.16 | 89.16 | 116 to 117 | 19.02 |
| Synthetic Products Co. | White crystalline powder | 2.90 | 192 to 192.5 | Absent | Negative | Positive | 0.13 | 87.49 | 87.26 | 116 to 117 | 19.3 |
| H. A. Metz Laboratories, Inc. | White crystalline powder | 4.99 | 192 to 192.5 | Absent | Negative | Positive | 0.00 | 89.14 | 88.55 | 117 | 19.34 |
| Farbwerke-Hoechst Co. (German specimen) | Slightly pink crystal | 5.09 | 190 to 191 | Absent | Negative | Positive | 0.16 | 89.65 | 89.64 | 116 to 117 | 19.00 |
* The phenetidin test is not very sensitive.
The melting point of the free base is given by a number of writers at 121 C. Although Kennert232 stated it to be 117 C. and not 121 C., his findings seemingly went unheeded. It will be noted that our work shows the melting point to be in accord with that announced by Kennert.
The Federal Trade Commission has not issued any licenses for the manufacture of “holocain hydrochlorid.” The John T. Milliken Company has withdrawn its application. The H. A. Metz Laboratories (Successor to Farbwerke Hoechst Company, New York) are making the product in this country.
Cinchophen (phenylcinchoninic acid) was introduced in the United States as a medicine under the proprietary name “atophan,” by Schering and Glatz, New York City, who before the war were the American agents for the German manufacturers “Chemische Fabric auf Actien von E. Schering, Berlin.” Phenylcinchoninic acid (2 phenyl-quinolin-4 carboxylic acid) was first described by Doebner and Gieseke233 in 1887, who prepared it by warming together pyro-racemic acid, benzaldehyd and anilin in alcoholic solution; it has the structural formula:
The chief use of phenylcinchoninic acid is as an antiuric acid agent, especially indicated in gout.
In 1913, the German house of Schering was made the assignee of patent 1045759 granted by the United States government234 for the manufacture of phenylcinchoninic acid: at about the same time the product was admitted to the U. S. Pharmacopeia IX, under very loosely constructed standards.
Some time after the beginning of the European war the proprietary “atophan” became scarce in America. In 1917, however, Schering and Glatz, New York, placed American-made atophan on the market and submitted it to the Council on Pharmacy and Chemistry. Later, other firms began to manufacture the product and also submitted specimens. During the time it was investigating these products, the Federal Trade Commission decided that a license was needed to manufacture phenylcinchoninic acid under the patent just referred to, so that altogether the laboratory had a number of specimens to examine.
In making the examinations for the Council, the laboratory was practically confined, by virtue of the Food and Drugs Law, to limit its requirements of purity to those of the Pharmacopeia. Practically, the only tests were melting point, ash and solubility. According to the U. S. Pharmacopeia the melting point is “about 210.” In New and Nonofficial Remedies, 1918, it was explained that atophan “complies with the standards for phenylcinchoninic acid, U. S. P., but melts between 208 and 212 C.” The U. S. Pharmacopeia requires that no weighable ash remains on incinerating about 0.5 gm. of phenylcinchoninic acid. Considerable variations, especially in melting points, were found, as can be seen from Table 4.
TABLE 4.—MELTING POINTS AND ASH
| Product No. | Manufacturer | Melting Point, C. | Ash, % |
| 1 | Abbott Laboratories, Chicago | 208.5–210.5 | 0.05 |
| 2 | Abbott Laboratories, Chicago | 212 –213 | 0.05 |
| 1 | Calco Chem. Co., Bound Brook | 209 –210.5 | 0.07 |
| 1 | Morgenstern, New York | 204.5–207.5 | 2.8 |
| 2 | Morgenstern, New York | 208.5–211.5 | None |
| 1 | Schering and Glatz, New York | 206 –208 | None |
| 2 | Schering and Glatz, New York | 209 –211 | None |
| 3 | Schering and Glatz, New York | 208.5–210 | 0.17 |
| 4a | Schering and Glatz, New York (1) | 208.5–210 | 0.2 |
| 4b | Schering and Glatz, New York (2) | 208.5–209.5 | 0.3 |
| 4c | Schering and Glatz, New York (3) | 208.5–210 | 0.025 |
| 1 | Wm. H. Sweet and Co., Columbus | 204 –208 | None |
| 2 | Wm. H. Sweet and Co., Columbus | 209.5–211.5 | 0.04 |
| 1 | German specimen from Schering and Glatz | 210 –212 | None |
By referring to this table on melting points and ash content it will be noted that the production of a better grade of products resulted after the respective firms had submitted samples to the A. M. A. Chemical Laboratory for criticism, and from a chemical standpoint, the last products examined were found to be as satisfactory as the German-made “atophan.”
Solubility of Cinchophen (Phenylcinchoninic Acid).—As methods of determining impurities, or estimating the degree of purity of phenylcinchoninic acid were not described in the U. S. Pharmacopeia, it was decided to try extraction methods.235 This in turn led to the question of solubilities. The U. S. Pharmacopeia gives the solubility of phenylcinchoninic acid only in general terms; hence it was deemed advisable to determine its solubilities and describe them in more definite terms. The sample of phenylcinchoninic acid employed to determine the solubility was obtained by repeated recrystallization from alcohol of a commercial specimen. Solubilities were determined in water; 95.0 per cent. alcohol; 48.5 per cent. alcohol;236 chloroform and ethyl acetate.237 Complete saturation of the solvent was attained according to the U. S. P. IX method (p. 599). The bath was maintained at a temperature of 25 C., with a range of ± 0.2 degrees. The solution was analyzed by the method of Seidell.238 The data obtained for the solubility of phenylcinchoninic acid are given in Table 5.
TABLE 5.—SOLUBILITY OF CINCOPHEN
| Solvent | Gm. per Hun- dred Gm. of Sat. Solution | Solubility, Parts by Weight | |
Distilled water | 0.0160 | 1 in | 6,216.0 |
95 per cent. ethyl alcohol | 0.8343 | 1 in | 119.0 |
Dilute ethyl alcohol | 0.0875 | 1 in | 1,142.6 |
Chloroform | 0.1075 | 1 in | 929.7 |
Ethyl acetate | 1.4151 | 1 in | 70.6 |
The Abbott Laboratories, Chicago, have been licensed by the Federal Trade Commission to manufacture cinchophen. Other firms, however, have decided to manufacture it without the formality of obtaining a license, evidently considering the German-obtained patent not to be valid.239
Procain was introduced in medicine under the proprietary name “novocain,” and before the war was obtainable in this country only through the Farbwerke Hoechst Company, the American representative of the German establishment, Farbwerke vorm Meister Lucius Bruening, Hoechst a. M. Chemically it is the mono-hydrochlorid of para-amino-benzoyl-diethyl-amino-ethanol, having the structural formula:
NH2—![]() COO-CH2-CH2—N(C2H5)2.HCl
COO-CH2-CH2—N(C2H5)2.HCl
It is prepared according to U. S. patent No. 812554 (issued to Alfred Einhorn, Munich, Germany) by treating para-nitro-benzoylchlorid with ethylene chlorhydrin and diethylamin with subsequent reduction of the nitro groups, the resulting product being purified by recrystallization.
Procain is employed largely in infiltration anesthesia. It is less toxic than cocain, but its anesthetic action is not sustained. This drawback is overcome by the simultaneous injection of epinephrin, and for this reason procain is often compounded with epinephrin in tablets, thus obviating the necessity of separate solutions.
When the first specimens of the American-made product were submitted through the channels of the Federal Trade Commission, it was necessary to compile a monograph.240 This was prepared from descriptions in the available literature, mostly from tests described in New and Nonofficial Remedies, 1918, and the German Pharmacopeia V.
The submitted products were found satisfactory chemically. The toxicity determinations made by Dr. R. A. Hatcher, with the assistance of Dr. Carey Eggleston241 indicated that none of the specimens are to be considered dangerous when used in ordinary dosage for normal individuals. Therefore the Federal Trade Commission, on recommendation of the Committee on Synthetic Drugs of the National Research Council (aided by the A. M. A. Chemical Laboratory), issued licenses for the manufacture of procain to the Farbwerke-Hoechst Company (which license was later transferred to the H. A. Metz Laboratories), to the Abbott Laboratories, to the Calco Chemical Company and to the Rector Chemical Company.
Subsequently the products of the licensed firms were submitted to the Council on Pharmacy and Chemistry, which in turn invoked the aid of the A. M. A. Chemical Laboratory and the Cornell University Pharmacologic Laboratory. Later the Council asked the laboratory to examine the market supply. Altogether, therefore, a number of products were examined which were found to respond satisfactorily to the tests outlined (Table 6).
TABLE 6
| Brand | Date Received | Color | Melting Point, C.* | Ash, % |
| Procain (Abbott), from Committee | ||||
on Synthetic Drugs | 12/21/17 | White | 154–155 | None |
| Procain (Abbott), submitted to | ||||
Council P. and C. | 1/29/18 | White | 153.5–154.5 | None |
| Procain (Abbott), Gen. Pur. Off. | ||||
U. S. Army | 8/31/18 | White | 152.5–153.5 | None |
| Procain (Abbott), Gen. Pur. Off. | ||||
U. S. Army, No. 89999 | 9/30/18 | Slight brownish tint | 153–154.5 | None |
| Procain (Abbott), Gen. Pur. Off. | ||||
U. S. Army, No. 89998 | 9/30/18 | Slight brownish tint | 153–154.5 | 0.005 |
| Procain (Abbott), Gen. Pur. Off. | ||||
U. S. Army, No. 89997 | 10/ 8/18 | Slight brownish tint | 153–154 | None |
| Procain (Abbott), Gen. Pur. Off. | ||||
U. S. Army, No. 89996 | 11/ 4/18 | Slight brownish tint | 153.5–154.5 | None |
| Procain (Abbott), Gen. Pur. Off. | ||||
U. S. Army, No. 810995 | 11/ 4/18 | Slight brownish tint | 153.5–154.5 | None |
| Procain (Calco), from Committee | ||||
on Synthetic Drugs | 2/ 7/18 | White | 153.5–154.5 | None |
| Procain (Farbwerke-Hoechst Co.), | ||||
submitted to Council | 10/24/18 | White | 153–154 | None |
| Procain (Farbwerke-Hoechst Co.), | ||||
submitted to Council | 12/10/17 | White | 153–154.5 | None |
| Procain (Farbwerke-Hoechst Co.), | ||||
submitted to Council, market spec. “A 56” | 8/ 9/18 | White | 153.5–154.5 | None |
| Procain (Farbwerke-Hoechst Co.), | ||||
submitted to Council, market spec. “A 57” | 9/ 9/18 | White | 153.5–154.5 | None |
| Procain (H. A. Metz Lab.), | ||||
market spec. “A 63” | 8/23/18 | White | 153–154 | None |
| Procain (H. A. Metz Lab.), | ||||
market spec. “A 57” | 9/23/18 | White | 153–154 | None |
| Procain (Rector), from | ||||
Committee on Synthetic Drugs | 12/18/17 | White | 153–154.5 | None |
| Procain (Rector), from | ||||
Committee on Synthetic Drugs | 5/ 2/18 | White | 152.5–153 | None |
Procain (Rector), market spec | 8/20/18 | Slight brownish tint | 153–155 | None |
Procain (Rector), market spec | 8/23/18 | Slight brownish tint | 153–155 | None |
Procain (Rector), market spec | 8/23/18 | Slight brownish tint | 153–154.5 | None |
* U. S. Patent 812,554—the novocain patent—declares that the salt melts at 156 C. Evidently based on this, both the German Pharmacopeia and past editions of New and Nonofficial Remedies give this melting point. Two specimens of German-made novocain obtained from our files, stated to be manufactured by Farbwerke-Hoechst vorm. Meister, Lucius and Bruening, Hoechst a. M., were found to melt, respectively, between 154 and 155 C. and between 153.5 and 154.5 C. when the melting point was determined according to the direction of the U. S. Pharmacopeia, ninth revision. The various specimens examined at that time melted between 153 and 155 C., and it was decided to permit this range.
An examination of some American-made procain-suprarenin tablets was also made. The procain was determined by liberation of the alkaloid with ammonia water, extraction with chloroform, evaporation of the chloroform, dissolving the alkaloid in one hundredth normal sulphuric acid solution and titrating excess acid with one hundredth normal sodium hydroxid solution. The epinephrin was determined according to the method employed by Seidell,242 with slight modifications. The tablets contained the claimed amounts of ingredients.
Before the war, the American physician was literally bombarded with new and wonderful (?) coal-tar synthetics, most of which were originated in Germany. In fact, it seemed that if a by-product in the manufacture of dyes could not be used for a dye per se, then a place might be found for it in the ever increasing lists of medicaments. By clever advertising and propaganda among physicians, an artificial stimulation for coal-tar drugs was created which evidently yielded lucrative financial returns. As a result of the war, it is interesting to observe that of all the synthetic drugs imported into this country from Germany and on which the American patents were controlled by the Germans (up to the time of our entrance into the war), the demand was really sufficient enough to warrant the commercial manufacture of only four of them by American firms. Of course, a larger number of nonpatented drugs, also imported from Germany, are now being made in sufficient quantities in this country; many of the drugs in this class were never patented or are the ones which have survived after the patent had expired, such as acetanilid, acetphenetidin, and acetylsalicylic acid.
In view of the agitation to found an institute for cooperative research as an aid to the American drug industry under the auspices of the American Chemical Society, it will be well for the medical profession to be on its guard against too enthusiastic propaganda on the part of those engaged in the laudable enterprise of promoting American chemical industry. Unless it is, it may be inflicted in the future, as in the past, with a large number of drugs that are either useless, harmful or unessential modifications of well-known pharmaceuticals. It will be well also for the chemists—those engaged in this enterprise—to be sure that the product is of therapeutic value before asking its use as a medicine. The American medical profession has learned that relatively few of the many German synthetics were really valuable or decided improvements over established drugs. If American chemists desire to retain their prestige with the medical profession, they should earnestly endeavor to see that the advantages derived from the war and from such an institute as proposed are not abused in the worthy desire to popularize chemistry both educationally and commercially. They should realize that physicians are in no receptive mood for a flood of synthetics, even though “American-made.”
On the other hand, the constructive possibilities of chemistry in the service of medicine should serve as a stimulus for American research. Notwithstanding all the pharmaceutical shrubbery which Germany sent to us, still it did contain some synthetics that were worth while. As therapeutics has been benefited by these organic chemicals, it is logical to reason by analogy that there remain other synthetics to be discovered which will occupy places of equal distinction in the modern materia medica. For example, vaccines are of undoubted merit in the field of immunology, but their action is, in the end, chemical; as soon as chemical technic is refined by medicochemical research, it is quite possible that a definite chemical agent (synthetic) will supersede the indefinite bacterial vaccine. Obviously the American chemist has the opportunity of showing his resourcefulness in aiding the public health of America and the world. In this connection, a cooperative institute devoted to purely scientific drug research, and governed in such a manner as to inspire confidence in its humanitarianism and unbiased judgment, should serve a most commendable purpose. The hopes of American men of science are for a monumental research institution—cooperative with all the allied professions—and, as the Chicago Chemical Bulletin stated, “Stripped of all professional or commercial pettishness and not dominated by any one group of scientists.”243
As for the results of the work so far, they can be summed up in two sentences.
1. American chemists are producing synthetic drugs formerly controlled by Germany, and thus have declared their independence of German chemicals.
2. Judging from the evidence at hand, we can feel assured that the quality of American synthetics will be second to none.—(From The Journal A. M. A., Sept. 6, 1919.)
[Foreword.—It is more than twelve years since the Council on Pharmacy and Chemistry of the American Medical Association was created. Since then there have been but few issues of The Journal that have not called the attention of the medical profession to the debasing influence on scientific medicine of unscientific or worthless proprietary mixtures advertised to physicians for their use in prescribing. The Council on Pharmacy and Chemistry has investigated and shown many of these preparations to be fraudulent in one way or another, and these reports have been published in The Journal. In spite of this, these preparations have been advertised continuously to physicians, through medical journals and otherwise, and prescribed by a large number of physicians. One reason for this is that there are many physicians who have never seen these reports—who were not in active practice at the time, or who were not reading The Journal. We probably have among our readers at the present time 35,000 or 40,000 physicians who were not among the readers of The Journal twelve years ago. It is desirable, then, that we should take up, in more or less detail, several of the more widely advertised products that have been the subjects of previous reports. It has been repeatedly stated in The Journal that many of the proprietary mixtures—the so-called ethical proprietaries advertised to physicians—were no better and no worse than “patent medicines” advertised to the public.
Every physician who has the welfare of medicine at heart should put these questions squarely to himself if he has not already taken a firm stand on this whole problem: What is my attitude toward the work of the Council? Are its reports worthy of acceptance? Am I upholding the Council in its efforts to place therapeutics on a rational basis, not by blind faith alone, but by an honestly critical attitude toward it? Am I following the path of indolence by taking the advice of nostrum makers without any serious effort to determine whether they are true or false? In a word, am I really practicing medicine, or am I an unpaid agent and a dupe of nostrum makers? There are other revolutions than political. The public can be wronged just as certainly by the abuse of its confidence in clinicians as by the usurpers of political power, and when the public is thoroughly aroused the heavy hand of retribution is not likely to be too discriminating. That the sins of clinicians are standing out plain for any one who wishes to read is becoming more and more evident. There is but one short and ugly word that properly characterizes the physician who accepts a fee for prescribing that about which he has no more knowledge than has the one for whom he prescribes it. Are you with the nostrum makers or with decent medicine?
The article below is the first of a series written for The Journal by one who is thoroughly conversant with the work of the Council on Pharmacy and Chemistry and can speak authoritatively on questions dealing with the action of drugs and the treatment of diseases. We believe that these articles will prove of interest and profit and that they will help physicians to answer the questions just propounded.]
Bell-ans, for years advertised only in medical journals under the name “Pa-pay-ans (Bell),” is now advertised in newspapers as a remedy that “Absolutely Removes Indigestion.” As it is still being advertised to physicians, we propose to analyze the claims made for it with as much care as would be exercised in the discussion of the newest discovery in medicine, because we believe that it is desirable to show the trend of exploitation of a certain type of preparation in the medical press.
In the New York Medical Journal the following advertisement recently appeared on the front cover:
Acute Indigestion
Yesterday a great soldier and today the head of a big trust succumbed to an attack of Acute Indigestion; and every day we hear from some physician of some case he has saved with BELL-ANS by giving SIX (6) tablets dissolved in a glass of hot water and repeating if necessary. Can any doctor who reads this fail to provide himself with the free supply of BELL-ANS which we gladly send for his emergency case?

Typical of Bell-ans advertisements as appearing in medical journals.
A recently purchased package of Bell-ans contained a circular in which it was stated that Bell-ans removes flatulence, vertigo, weakness and other symptoms of indigestion quickly and pleasantly; that it aids the digestion of food and tends to restore the digestive tract to a normal condition; that it relieves vomiting in pregnancy, alcoholism, seasickness and cholera morbus, besides being pleasant, harmless and effective for colic, sour stomach, feverishness, and wakefulness of infants and children. The circular contained paragraphs purporting to be taken from various medical journals, including the New York Medical Journal, Wisconsin Medical Recorder, the Lancet Clinic, International Journal of Surgery, and Massachusetts Medical Journal. No exact references were given to permit verification or to determine whether or not the quotations were from “reading notices” (advertisements) or from the scientific part of the journals in question. To quote one of the statements given:
“The results from the use of Bell-ans (Pa-Pay-ans Bell) in the treatment of indigestion are so prompt and so generally good—and the evidence of this fact is accumulating so rapidly and from such reliable sources—that we venture to suggest to our readers who have not tried this remedy that they prescribe one original sealed package of Bell-ans (Pa-pay-ans Bell) and that they carefully note the results from its use.
“We suggest an original sealed package because the preparation is widely and badly imitated, and unless such a package is specified an imitation of little value may be substituted and the experiment be thus rendered useless.”
It is possible that Bell-ans has been imitated, but it is not true that it is widely imitated, for no such imitation has ever been called to our attention, and we strongly suspect that the main reason for desiring that an original package be dispensed is that the patient may see for himself the name BELL-ANS plainly blown in the glass.
The circular in question states that there is no derangement of the digestive organs on which the proper dose of Bell-ans will not act quickly and pleasantly! These are samples of the claims made for Bell-ans. Let us inquire into the nature of the conditions for which the preparation is recommended and the treatment advised by well known clinicians.
The subject of indigestion is discussed by Robert Hutchison and Robert Saundby under the general title of dyspepsia in the “Index of Treatment by Various Writers,” Edition 6, 1912, pp. 260–265. Hutchison says: “In the first place it must be remembered that in many patients who complain of ‘indigestion’ the seat of the trouble is not in the stomach at all.”

The general principles to be observed in the treatment of functional dyspepsia, as given by Hutchison, are: (1) to remove the cause; (2) to adapt the diet to the impaired functional power of the stomach; (3) to administer such drugs as are calculated to stimulate or correct the particular function or functions which happen to be impaired, or disordered. Proper diet, proper mastication of food, hygiene of the mouth, and constipation are enumerated as deserving attention. Careful attention to securing a proper diet is essential. The choice of drugs depends, of course, on the conditions that give rise to indigestion, and he calls attention to the necessity of avoiding all routine treatment and compiling one’s prescription with an eye to the special disorder or disorders of function, whether secretory, motor or sensory, believed to be present. Hutchison gives the following typical prescriptions to illustrate the use of drugs in the different disorders of function:
FOR HYPERSECRETION (HYPERCHLORHYDRIA, ACID DYSPEPSIA, ETC.)
Sodium bromid | 10 | grains |
Bismuth subcarbonate | 15 | grains |
Chloroform water | 1⁄2 | ounce |
This mixture to be taken before meals.
Sodium bicarbonate.
Bismuth subcarbonate.
Heavy magnesium carbonate, of each equal parts.
A small teaspoonful of the powder to be taken mixed with a little water or milk about two
hours after meals.
FOR DEFICIENT SECRETION (HYPOCHYLIA, ACHYLIA, GASTRITIS, ETC.)
Sodium bicarbonate | 10 | grains |
Tincture of nux vomica | 10 | minims |
Spirit of chloroform | 8 | minims |
Compound infusion of gentian | 1⁄2 | ounce |
This mixture to be taken before meals.
Dilute hydrochloric acid and glycerin, of each 15 minims with enough water to make half
an ounce, to be taken about twenty minutes after meals.
FOR DEFECTIVE MOTILITY (ATONIC DYSPEPSIA, GASTROPTOSIS, ETC.)
Hutchison recommends the use of 10 minims of tincture of nux vomica in an aromatic vehicle, such as infusion of quassia and compound tincture of cardamom; but another aromatic bitter, such as the compound tincture of gentian, will serve quite as well, of course. This is to be taken before each meal, and for the flatulence that often accompanies this trouble he gives menthol, aromatic spirit of ammonia and spirit of chloroform, as may be needed.
FOR ACID DYSPEPSIA
Robert Saundby recommends the following to be used before each meal for the relief of acid dyspepsia: sodium bicarbonate, bismuth subcarbonate, magnesium carbonate, of each 10 grains; mucilage of tragacanth 15 minims, and enough peppermint water to make an ounce.
These are only a few of the conditions that are discussed by Hutchison and Saundby, but they serve to show that the treatment of indigestion by a single prescription or combination is wholly irrational.
Bell-ans, both under its present name and under its older name, “Pa-pay-ans (Bell),” has always been alleged by its manufacturers to contain papain or to be a preparation of the digestive juice from the fruit of Carica papaya (papaw) with other substances. Various chemists have attempted to find papain present and to determine the digestive power of the tablets, but without success. For this reason The Journal suggested that the change of name from “Pa-pay-ans (Bell)” to “Bell-ans” was probably not made entirely for euphonious reasons, as alleged, especially when one considers that the name of a nostrum is its most valuable asset. It is much more likely that as analyses indicated there was not and probably never had been any papain present in the product, the name was changed for fear that some day the misleading term “Pa-pay-ans” might bring the preparation in conflict with the federal Food and Drugs Act.
Pa-pay-ans (Bell) was examined for the Council on Pharmacy and Chemistry in 1909 and the tablets were found to consist of charcoal, sodium bicarbonate, ginger, saccharin and oil of gaultheria. No digestive ferment could be detected in the tablets. Sodium bicarbonate is antacid and serves to dissolve mucus; ginger, if in sufficient amount, causes the expulsion of flatus, and charcoal, while an absorbent in the dry state, is probably useless for any therapeutic purpose whatever after it becomes saturated with gastric juice. Bell-ans, then, has all of the virtues, which are few, and all of the limitations, which are many, of a tablet of sodium bicarbonate and ginger. Its value in the treatment of acute indigestion would be limited to the value of a tablet of such a composition. It is absurd to suppose that it could have the slightest value in the far more serious conditions attended with intestinal indigestion, with the toxemia and autointoxication to which they give rise.
Bell-ans is now advertised directly to the public—but it is no less valuable on that account. True, it is a “patent medicine” in the commonly accepted sense of the term, but it is no more a “patent medicine” today than it was fifteen years ago when it reached the public, not through the direct medium of the newspapers but the more indirect route of the medical journals and undiscriminating physicians. It is true that, in view of the serious nature of many conditions which are loosely spoken of by the public as “indigestion,” its present method of exploitation is likely to make it just that much more dangerous because of the larger publicity that will be given. The point to be borne in mind is that Bell-ans is now in fact what it has always been in essence, a “patent medicine.”

Typical of Bell-ans advertisements as appearing in newspapers.
Again we ask the question: How do you wish to be classified, Doctor—among those who follow blindly the lead of a firm of nostrum makers, or among the intelligent members of the profession who study their cases carefully and prescribe intelligently? The manufacturers of Bell-ans claim that 100,000 American physicians now prescribe Bell-ans, and that 600,000,000 of the tablets are sold annually. If this is even approximately true it is a serious reflection on the medical profession, for the Council examined Bell-ans and reported its findings nearly eight years ago (J. A. M. A. 53:569 [Aug. 14] 1909), and the statements made in that report are as incontrovertible today as they were then.—(From The Journal A. M. A., Nov. 24, 1917.)
“Anasarcin” and “Anedemin” are the twin nostrums of cardiac pseudotherapy. They are dubbed “twin nostrums” not so much because of any similarity in their formulas, that being a minor consideration in the average nostrum, but because of the close similarity in their methods of exploitation, the therapeutic claims made for them, and the time and place of their birth.
It may be remembered that they both claim Winchester, Tenn., as their birthplace, and they appeared on the market at about the same time; furthermore, a comparison of the claims formerly made for both of them indicated that one mind conceived the main idea that lies back of their exploitation. While Anasarcin is especially dealt with in this article, much of the discussion applies with equal force to Anedemin.
Cardiac disease, with its resultant renal involvement, is frequently encountered; and running, as it does, a chronic course, it offers an almost ideal field of exploitation for the typical nostrum vender. By a typical nostrum vender we mean one whose knowledge of his product is far below that of his appreciation of a certain element of human character. On this element rests the whole secret of the nostrum vender’s success. It is variously termed credulity, gullibility and childlike simplicity, but it is that which often causes even the most conscientious clinician to turn aside from the use of the best known and most dependable drugs at his command, in the face of disappointment and failure, and employ some vaunted mixture which, in his saner moments, he scorns to use.
Anedemin is said to consist of a “Scientific Combination of three of the more recently investigated members of the Digitalis Series, with Sambucus”; that is, of apocynum, strophanthus and squill with elder. It is difficult to know just what idea the statement that it is a “scientific combination” of these drugs is intended to convey, for it is unscientific to mix three drugs of this group for use in fixed proportion in a wide range of conditions, if indeed, there is ever any indication for their use.
The great disadvantages of strophanthus and apocynum pertain to the extreme uncertainty of their absorption from the gastro-intestinal tract. Strophanthus is occasionally absorbed promptly, sometimes so slowly that the therapeutic effects are not induced until an amount equal to several times that which would prove fatal if all of it were absorbed into the circulation has been administered, and, unfortunately, one cannot control the absorption which may continue until a fatal effect is induced. This is true to an even greater degree of apocynum, and it was due to the recognition of this fact that apocynum was not admitted to the U. S. Pharmacopeia IX, the committee on dosage having agreed that no safe and effective dose could be given.
In 1907 the Council on Pharmacy and Chemistry examined the literature used in the exploitation of Anasarcin and Anedemin and published its report. Anasarcin tablets, it was pointed out, were said to contain the active principles of Oxydendron arboreum (sour wood), Sambucus canadensis (elder) and Urginea scilla (squill), and the following claims were made for the nostrum:
“Does what dropsy medicaments have hitherto failed to accomplish.”
“Superior to digitalis, strophanthus, scoparius, squills, acetate of potash and the hydragogue cathartics all put together.”
“The only known relief and permanent cure of dropsies.”
“Unrivaled heart tonic.”
“The most powerful agent known.”
“Safe in administration.”
“Non-toxic as ordinarily administered.”
“Will nauseate some persons,” but “the reaction from the temporary depression is prompt.”
“In Bright’s disease, both the interstitial and parenchymatous forms of nephritis, acute or chronic, no remedy ... to equal it in efficacy.”
“Without increasing the debility of the patient or interfering with nutrition by producing loss of appetite ...”
“This treatment is to be continued without cessation until all symptoms of dropsy have disappeared.”
A comparison of the earlier claims with those now being made (see advertisement reproduced from the New York Medical Journal) illustrates one of the results of the work of the Council. Today the nostrum exploiter avoids the cruder forms of obvious misstatement, but continues to make, by inference, claims that are equally misleading. It will be observed in this case that a more cautious pen worded the later advertisement, but there is still evident the intent to convince the reader that Anasarcin is superior to the official drugs in the treatment of cardiovascular diseases. The facts are that Anasarcin is at best a dangerous remedy in the hands of the average clinician in the treatment of such conditions, and its use is at all times to be condemned.
No competent investigator has ever investigated the pharmacology of sour wood (Oxydendron arboreum), and it appears to have no therapeutic value other than that due to a slight acidulousness. Elder (Sambucus canadensis) contains a trace of a volatile oil as its most important constituent, according to the British Pharmaceutical Codex of 1911 (p. 908), but it is difficult to explain why a trace of volatile oil should be considered important. Elder may be dismissed without further consideration in connection with Anasarcin tablets.
This leaves only squill among the constituents of Anasarcin for consideration. Sollmann (Manual of Pharmacology, 1917, p. 409) in discussing the advantages claimed for squill over other drugs of the digitalis group, says: “Dixon, 1906, points out that any superiority is outweighed by its disadvantages: uncertain absorption; strong gastro-intestinal irritation.” Squill was formerly used as an expectorant and diuretic, the activity having been attributed to two amorphous glucosids, scillipicrin and scillitoxin, but Ewins, 1911, found these to be impure mixtures. A later investigator claimed to have isolated two glucosidal agents from squill, but similar claims have often been made only to be disproved later, and we know of no confirmation of the claims regarding the isolation of any pure principles from squill having any true typical digitalis action.
The statement quoted from Sollmann is accepted by practically all pharmacologists, and we may say with certainty that squill is decidedly inferior to digitalis in the treatment of cardiovascular, and cardiorenal diseases, and certainly no active principles of squill were known to the scientific world at a time that the remarkable claims were first made for Anasarcin by an obscure pharmacist of Winchester, Tenn. Indeed, if Anasarcin were all that it was claimed to be, its discovery would have made Winchester as famous as a certain Wisconsin city was said to have been made by a popular beverage.
It has been abundantly demonstrated, and it is now almost universally accepted among well informed pharmacologists and clinicians, that all digitalis principles exert the same kind of action on the heart after they enter the circulation in effective doses, though they differ to an extraordinary degree in the intensity of their action and in their undesired sideactions, such as nausea and vomiting. When the use of Anasarcin (squill) is followed by immediate improvement after digitalis has failed, it merely shows that the dosage of digitalis was insufficient or that it was discontinued and the squill mixture was substituted before the full therapeutic effects of the digitalis developed.
If the administration of a sufficient dose of digitalis is not followed by improvement in the circulation, it shows that the heart is incapable of responding to such treatment and the further use of any of the drugs of this group is distinctly contraindicated. This is confirmed by the experience of nearly every competent observer of digitalis therapy, and numerous fatalities have resulted from the failure to appreciate this fact and further administer some other member of the group, such as strophanthus or squill.
It is now well known that the cardiac effects of toxic doses of squill, and other members of the group, resemble closely those of cardiac disease, and it is often impossible to determine whether the behavior of the heart in a given case is attributable to insufficient dosage, to excessive dosage, or to the progress of the cardiac disease itself. If this occurs when one uses the best known members of the group, it is certain that it occurs even more frequently when others that are less understood are employed. In the light of this knowledge of the dangers attending the incautious use of any member of the digitalis group, and more especially the use of impure principles, such as are commonly obtained from squill, it is impossible to condemn sufficiently the recommendation that the use of Anasarcin should be continued without cessation until all symptoms of dropsy have disappeared.
Digitalis bodies are not suited for the treatment of all cardiac disturbances, and it is, of course, self-evident that a time must come in the treatment of chronic cardiac disease when the heart is incapable of responding to any form of treatment with improvement. But, unfortunately, it never loses its response to toxic doses, and to push the administration of any drug or mixture containing any drug of the entire digitalis group—and especially those, like squill, in which the side actions are most prominent—beyond the point of tolerance is to court certain disaster.
While it is quite certain that many lives have been sacrificed to the failure to understand this phase of cardiac therapy, it is equally certain that many lives have been sacrificed because of insufficient dosage, and one can steer a safe course between these dangers only by using the best known preparation available; and in the present state of our knowledge it is indisputable that digitalis and the tincture of digitalis are best suited for the treatment of cardiac disease except in those few cases in which intramuscular or intravenous administration must be employed temporarily for immediate effect.

The secret of prescribing successfully for the relief of dropsy in cardiac disease consists in understanding the effects of digitalis on the heart, in administering it until these effects indicate that the desired object has been obtained, and stopping, or interrupting, the administration at that point until the effects begin to wear off. Cumulation, so called, is a positive advantage in such cases. It merely means that the desired therapeutic effects once induced persist for a time, and that further medication is unnecessary during such persistence of action. Eggleston has recently shown (Arch. Int. Med. 16:1 [July] 1915; abstr., J. A. M. A. 44:459 [July 31] 1915) that the full therapeutic effects of digitalis can be induced in suitable cases within a few hours even with oral administration.
We are not aware of a single publication in which a careful, detailed clinical study of Anasarcin has been reported. The claims made for Anasarcin, past and present, indicate either a deliberate purpose to mislead or crass ignorance of the rudiments of pharmacology and therapeutics. The exploiters of the nostrum claim that thousands of physicians have found Anasarcin tablets of unsurpassed remedial value in the treatment of disorders of the circulatory system and of ascitic conditions.244 It must be admitted that too many physicians have prescribed Anasarcin, otherwise the manufacturers would not have continued to spend thousands of dollars in advertising it in medical journals during a period of more than ten years.
Doctor, this article is meant to be a candid discussion with you, whether you use Anasarcin or not, because every clinician is vitally interested in the customs that obtain in the practice of medicine, and we wish to put a hypothetic question to you. Answer it, at least to yourself, in exactly the spirit in which it is put. Suppose that you prescribe Anasarcin for a patient who is critically ill with cardiac disease. He dies. Are you willing to tell the relatives frankly just what you used and the nature of the evidence on which you based your choice of this nostrum? Let the supposition be carried further and say that the case was hopeless, and agree that digitalis and all other drugs would have been equally ineffective. Granting all this, would your explanation satisfy? Would you in all candor dare to offer such an explanation? Try it as a hypothetic case before you are forced to apply it.—(From The Journal A. M. A., Dec. 8, 1917.)
It would be interesting, and even instructive, to know how many educated physicians, if any, are now prescribing Pepto-Mangan (Gude): interesting as indicating the number who have neglected to avail themselves of the work of the Council on Pharmacy and Chemistry, especially the earlier work; instructive in that it would show how many are still prescribing by the rule of thumb, and who are taking their therapeutic instructions from purely commercial sources instead of striving to learn how to choose those drugs that are most effective in the treatment of disease.
It has been pointed out many times in the pages of The Journal that many nostrums are advertised first to physicians, and that after physicians have served as the unpaid agents of the manufacturers in introducing the preparations, their exploitation is then commonly continued by means of advertisements in the public press. This plan has been followed successfully in so many cases that we have now come to look on it as the regular course. It is in keeping with this rule that we find Pepto-Mangan now advertised in the public press, the physicians having served the manufacturer’s purpose.
It will be recalled that many years ago the theory was held that hydrogen sulphid (sulphureted hydrogen) interfered with the absorption of the iron of the food, and that the administration of medicinal iron prevented this interference by neutralizing the hydrogen sulphid (sulphureted hydrogen). It was only a short step to argue that manganese might replace the medicinal iron in combining with the hydrogen sulphid, permitting the food iron to be absorbed, and it was held that only food iron could be utilized in the formation of hemoglobin.
It is hardly necessary to remind the reader that this theory rests on numerous fallacies. There is no hydrogen sulphid worth mentioning in the small intestine where iron is absorbed; food iron cannot be utilized directly in the formation of hemoglobin but must be broken into simple forms for absorption; and, further, inorganic iron, such as ferrous carbonate, serves the purpose admirably when iron is indicated. With the acceptance of these well established facts, all possible excuse for the therapeutic employment of Pepto-Mangan in place of iron vanished; but as plain and simple as this fact is, the unnecessary and expensive Pepto-Mangan continues to be prescribed by physicians who will not take the slight trouble to investigate the claims for this nostrum.
There is not merely a difference of opinion between the exploiters and the Council, but there has been also actual misrepresentation in the exploitation of this nostrum to physicians. This has been shown on more than one occasion. About twelve years ago, the M. J. Breitenbach Company, the proprietors of Pepto-Mangan, claimed that the report of the commission that had been appointed for the investigation of anemia in Porto Rico “would alone suffice to establish Pepto-Mangan at once as the foremost hematinic known.” Examination of the report showed that the commission made no such claims; on the contrary the commission protested against this misrepresentation (J. A. M. A. 45:1099 [Oct. 7] 1905).

From the New York Medical Journal.
Undaunted by this exposure of their methods, the Breitenbach Company later sent out a statement of results purporting to have been obtained by one Mateo M. Gillen, in the treatment of infantile anemia on Randall’s Island in New York City. At the instance of The Journal the hospital records in these cases were examined, and it was found that the pretended report was little more than a tissue of falsehood (J. A. M. A. 48:1197 [April 6] 1907).
About two years ago the Council reported that while the statements just referred to were no longer made, they had never been definitely admitted by the Breitenbach Company to be erroneous, and that Pepto-Mangan was then being exploited to the public indirectly. (Council Reports, 1914, p. 121.)
We reproduce an advertisement that has been appearing weekly in the New York Medical Journal for several months. One can only suppose that this advertisement was intended to mislead physicians, and it would be an insult to the intelligence of the average reader to attempt any detailed discussion of it, but enough has been said to show how misleading the statements are. One should note particularly the advice—old as the nostrum business itself—contained in the advertisement, to prescribe an original bottle. The reason for such advice is simple. Experience has shown that when original bottles are dispensed patients soon learn to buy the nostrum without consulting the physician, for they shrewdly suspect that he knows no more about the preparation than they, and that he gets his information from precisely the same sources that are available to them. They are obviously right. In truth, the physician who prescribes Pepto-Mangan as a hematinic shows ignorance of the most rudimentary facts of iron therapy, and the intelligent patient soon perceives his limitations.

A newspaper advertisement of Pepto-Mangan.
The investigation of the problems of iron therapy and its utilization in the formation of hemoglobin forms one of the most brilliant chapters in pharmacologic research, and there is no better established fact in therapeutics than that any organic or inorganic preparation of iron that does not irritate the stomach may be employed effectively when the administration of iron is indicated. “Useful Drugs” contains a list of iron preparations that are suitable for all conditions which call for iron, and the clinician may rest assured that he will never have occasion to go outside that list to prescribe any substitute.
As a matter of fact, it seems probable that the very number of available iron preparations has served to cause confusion, thus affording an opportunity for the nostrum maker to introduce his superfluous compounds. It may be difficult at times to select the preparation of iron best suited to the individual patient; and it is this difficulty that has led the clinician to listen to the seductive claims made for the various pretended substitutes for iron. One should approach the question of choosing the proper form of iron for therapeutic use with the recognition of the fact that there is no such thing as a substitute for iron in the formation of hemoglobin, that there are no ideal forms of iron other than those found in the foodstuffs. Further, the clinician cannot avoid the disadvantages inherent in all forms of iron that he can prescribe, and he must therefore seek that which seems best suited for the individual patient.
Bunge estimated the amounts of iron present in various foods; and a table based on this, and other data, is given in “Pharmacology of Useful Drugs” (published by the American Medical Association). Ordinary foods in an ample diet contain enough iron to supply the normal daily loss, which amounts to only a few milligrams, but many persons who have poor appetites take an insufficient amount of iron in their food and become anemic. In such cases the additional iron required can be supplied best by adding spinach, eggs, apples, or other iron-rich food to the dietary.
William Hunter discusses the subject of anemia and its treatment at considerable length in the “Index of Treatment,” Ed. 6, pp. 17–37, and gives many prescriptions containing iron for use under different conditions; and while it is unnecessary to reproduce all of these here, a few may be given in order to suggest suitable methods of prescribing iron when it cannot be given in sufficient amounts in the food.
In chlorosis Hunter advises that that form of iron which experience has shown to be least disturbing to the patient’s stomach should be used, and he suggests separate stomachic mixtures to be used simultaneously, not mixed with the iron itself. When constipation exists—and this is a very common accompaniment of chlorosis—he gives the following aperient iron combination:
| Gm. or c.c. | ||||
| ℞ | Ferrous sulphate | │25 | gr. iv | |
Magnesium sulphate | 4 | │ | Ʒ i | |
Aromatic sulphuric acid | │5 | ♏ vii | ||
Tincture of ginger | │7 | ♏ x | ||
Compound infusion of gentian (B. P.) q. s., ad | 30 | │ | ℥ i | |
This, constituting a single dose, is to be taken twice daily—at 11 a. m. and 6 p. m. A little compound tincture of gentian and water may be used in place of the compound infusion of the British Pharmacopeia. He modifies this somewhat as occasion demands by using sodium sulphate and adding sodium bicarbonate (which converts the sulphate of iron into ferrous carbonate) and adds 10 minims of spirit of chloroform to act as a stomachic.
Hunter also suggests the use of pills of aloes and iron in place of the mixture described above, and when constipation has been corrected, the aloes may be omitted and the pill of ferrous carbonate alone may be used for the iron. Hunter’s comment regarding this pill is, “very satisfactory.”
The same form of iron is available in the compound iron mixture, formerly official, which Hunter says is exceedingly good. In this country the compound solution of iron and ammonium acetate, Basham’s mixture, so called, has long enjoyed a wide reputation as causing very little disturbance of the stomach, and the homely tincture of ferric chlorid is probably useful in a large majority of cases in which the stomach is not especially irritable.
We may say with assurance that one of the forms suggested here will suffice for practically every case in which it is necessary to reinforce the amount of iron available in the food by some pharmaceutical preparation. If these do not satisfy your requirements, consult a really competent pharmacist and enlist his aid in devising a mixture especially suited to your individual patient.—(From the Journal A. M. A., Dec. 29, 1917.)
This preparation may be considered briefly in view of the recent discussion in this series of articles of the pharmacology of the digitalis group and the principles of treatment in cardiovascular disease. The manufacturers maintain that cactina is wholly unlike digitalis, and that is the truth, as we shall show; but since they claim that it is useful in certain conditions of the heart in which digitalis is commonly employed by well informed clinicians, it is necessary to consider its cardiac actions—or its lack of them! It is difficult to determine just what action cactina is supposed to exert on the heart. For example, one advertisement contains the following:
“Cactina Pillets. A gentle cardiac tonic that supports and sustains the heart through its capacity to improve cardiac nutrition.”
Just how the cardiac nutrition is to be improved without an improved coronary circulation is not explained. It would be interesting to know in what other way this is to be accomplished, and how an improved coronary circulation can be induced without acting on the heart or vessels. But that is what digitalis does, and you should remember that cactina is so very different from digitalis! Then again:
“Cactina Pillets. A remedy that steadies and strengthens the heart by imparting tone to the heart muscle.”
That is a pretty direct statement, but digitalis imparts tone; and we must not forget that “cactina” is wholly unlike digitalis, and we are told that “cactina” is:
“Invaluable in all functional cardiac disorders such as tachycardia, palpitation, arrhythmia, and whenever the heart’s action needs regulating or support.”
If these are merely functional disorders of the heart, it is highly desirable to know what are the symptoms of really serious cardiac disease! Since the manufacturers give us no information concerning the mode of action of “cactina” we will turn to the literature of disinterested observers. If one attempts to discover the origin of “cactina,” he will probably meet with disappointment, for various bibliographies fail to mention the name of Sultan, who is said to have isolated “cactina” from Cactus grandiflorus. It seems that Sultan worked with Cactus, or some other plant, when a student of pharmacy, and it is to be remembered that Cactina Pillets are manufactured by the Sultan Drug Company.
It is doubtful whether Sultan actually worked with genuine Cactus grandiflorus; and, in fact, there is good reason for thinking that he did not, for all subsequent workers who have taken pains to secure genuine Cactus grandiflorus have failed to detect the presence of any active principle, except possible traces that are of no therapeutic importance whatever.
The Council on Pharmacy and Chemistry examined the literature relating to cactus and certain proprietary preparations, including Cactina Pillets, alleged to be made from cactus, and has reported the results of its investigation (J. A. M. A. 54:888 [March 12] 1910) and we will quote from that report.
“The therapeutic value of this plant has been variously estimated by different observers. Experimental evidence as to its action is scanty and no complete chemical examination has ever been made.
“Reputable men have testified that some of the plants of the cactus family contain very active principles, but so far experiments seem to prove that Cactus grandiflorus has neither the action of digitalis nor that of strychnin. The principal contributions, clinical and experimental, for and against the drug are set out below.”
The report then proceeds to analyze the work of O. H. Myers, R. A. Hatcher, Boinet and Boy-Teissier, Sayre, Gordon Sharp, S. A. Matthews, P. W. Williams, Aulde and Ellingwood, and comes to conclusions that are set forth as follows, in brief:
1. It is uncertain what part of the plant contains the active principle, if any such principle exists.
2. Part of the experimental and clinical work has been published under proprietary auspices.
3. The value of clinical evidence when unsupported by animal experimentation is much diminished by the tendency of enthusiastic and untrained observers to attribute to the drug given the effect really due to general remedial measures, psychic suggestion and so forth.
In other words, the literature does not afford a report of a single piece of careful painstaking work the results of which lend support to the claims made for Cactina Pillets as stated above, for it is obvious that if Cactus grandiflorus contains no active principle, no active principle can be extracted from it. Some time after the report of the Council was published, Hatcher and Bailey secured genuine Cactus grandiflorus directly from a competent botanist, Dr. C. A. Purpus, of Vera Cruz, Mexico, and studied it experimentally. They reported (J. A. M. A. 56:26 [Jan. 7] 1911) in part as follows:
“We have been unable to obtain any evidence that the true Mexican Cactus grandiflorus possesses any pharmacologic action whatever; but, on the contrary, it appears to be a singularly inert substance when administered either by the mouth or by the vein.”
When colossal doses of Cactus grandiflorus are given by the vein, they sometimes—but not always—appear to exert an extremely feeble action on the heart; but this action is so slight that its nature could not be determined. Even the most colossal doses of Cactus grandiflorus administered by the mouth to cats, dogs and frogs exert no perceptible effect.
Sollmann thus satirizes the absurd claims made by the exploiters of proprietary forms of cactus: “Should the heart be too slow, cactus quickens it; if the heart is too fast, cactus slows it; should the heart be too weak, cactus strengthens it; if the heart is too strong, cactus weakens it; does the heart wobble, cactus steadies it; if the heart is normal, cactus does not meddle with it” (J. A. M. A. 51:52 [July 4] 1908).
Will physicians continue to accept the statements of an interested nostrum vender—who submits not a shred of evidence to support his claims, but who has a financial interest in convincing them—even when his statements are diametrically opposed to all the evidence that the Council on Pharmacy and Chemistry has been able to secure?—(From The Journal A. M. A., Jan. 19, 1918.)
At the time that synthetic chemical drugs were coming into fame and when every manufacturer who launched a new headache mixture claimed to have achieved another triumph in synthetic chemistry, Ammonol and Phenalgin were born. Of course, these twins of analgesic pseudotherapy were claimed to be synthetics and were duly christened with “formulas.” They were among the first of the nostrums examined for the Council on Pharmacy and Chemistry, and the false claims made for them were exposed.
The analyses made for the Council showed that Ammonol and Phenalgin were simple mixtures, having the following composition:
| ACETANILID | SODIUM BICARBONATE | AMMONIUM CARBONATE | |
| Ammonol | 50 | 25 | 20 |
| Phenalgin | 57 | 20 | 10 |
The reports of the Council on, and numerous references to, these two nostrums may be found in The Journal of various dates.245 The reports will prove interesting to those who are not familiar with, or have forgotten, the methods of nostrum exploiters at the time the Council was formed. Following the Council’s exposure of the false claims made by the manufacturers of Phenalgin, the Medical Record published an advertisement of that nostrum in which an attempt was made to discredit the Council’s report. The editor of the Medical Record was requested by the Council to publish the facts in the case but he refused to do so.
Long after the death of Dr. Cyrus Edson, the claim was made that Phenalgin was made under his direction and that it was his “discovery.” As a matter of fact, Dr. Edson had favored the use of Ammonol at one time, and when the Council exposed the false claims then being made for Phenalgin, The Journal charged that a fraud was being perpetrated on the medical profession. Despite the exposure of the methods used in exploiting Ammonol and Phenalgin, one finds just as glaringly false statements made in the advertisements of Phenalgin today as were made in its unsavory past. This would seem to indicate either that physicians have short memories or that they are strangely indifferent to the welfare of their patients, to their own reputations and to the good name of medicine.
The New York Medical Journal of Dec. 22, 1917, contained an advertisement of Phenalgin—it has been running for months—from which the following is quoted:
“For the relief of PAIN the ‘logical supplanter of opium and other habit-forming drugs’ is Phenalgin. No matter how severe or where located pain is promptly and satisfactorily controlled by this effective anodyne—and without disturbing the digestion, suppressing the secretions, causing constipation or inducing a drug habit.
“This is why Phenalgin has superseded opium and its derivatives for relieving Headaches, Rheumatism, Gout, La Grippe, Lumbago, Neuralgia, Disorders of the Female, Dysmenorrhea, and Painful Conditions generally. To thousands of physicians Phenalgin ‘is the one dependable analgesic—the logical supplanter of opium.’ ”
If we are to suppose that the composition of Phenalgin is today essentially the same as when it was examined, the claims just quoted are obviously false for, of course, such a mixture must have the properties of acetanilid with all of its drawbacks and limitations. We may contrast the statements made in the advertisement just quoted with those made in Bulletin 126 of the Bureau of Chemistry of the U. S. Department of Agriculture. This bulletin on “The Harmful Effects of Acetanilid, Antipyrin and Phenacetin” summarizes the replies received from 400 physicians to whom a questionnaire had been sent. The information thus gained was tabulated and the figures that follow are from these tables. There were reported no fewer than 614 cases of poisoning by acetanilid with 16 deaths and 112 cases of its habitual use. The larger number of cases of poisoning followed the administration of the drug, by physicians, in doses larger than those now regarded as fairly safe. This large number reported by only 400 physicians indicated an excessively large number in the whole country. Since the questionnaire was sent to nearly a thousand physicians, of whom about 500 failed to reply, it may be assumed that had it been sent to the entire 130,000 physicians in the country, at least 75,000 cases of poisoning would have been reported.
Prior to the passage of the federal Food and Drugs Act (the “Pure Food Law”) many nostrum makers had declared that their preparations contained no acetanilid. When that law went into effect, some of these manufacturers triumphantly pointed to the fact that they were still able to make the same claim without conflicting with the requirements of the law. This was accomplished in fact by changing the formula and substituting acetphenetidin (phenacetin) for the acetanilid. While acetphenetidin is somewhat less toxic than acetanilid, bulk for bulk, the toxicity and therapeutic activity of the two drugs are nearly proportional.
The claim made by many proprietary medicine manufacturers that they are “strictly ethical” because they advertise only to physicians is mere verbal camouflage. There may be no more certain way of insuring the continued use of a nostrum by the public than to have it prescribed by physicians; and none know this better than the makers of nostrums. A proprietary individuality is obtained by giving some special form to the tablets and package or a special coloring to the capsules (“Specify ‘Phenalgin Pink Top Capsules’ ”) so as to indicate the identity of the products in such a way that the patient may in the future procure them without the advice or warning of the physician. When a proprietary preparation with the name or initials stamped on it or attached to it is prescribed, the patient immediately is aware of the fact, and his respect for the physician’s intelligence and wisdom is naturally lessened.
The physician should never place such dangerous drugs as acetanilid and acetphenetidin, or ready made mixtures of them, in the hands of the patient in such a way that they can be employed without his supervision or control. He should never prescribe more than is needed at the time and should not form the habit of using fixed doses or combinations of drugs without a special reference to the particular needs of the individual.
Certain forms of headache yield more readily to a mixture of caffein and acetanilid or caffein and acetphenetidin than to either acetanilid or acetphenetidin alone. When the physician wishes to prescribe such a mixture he may combine 1 grain of caffein or 2 grains of citrated caffein with 3 grains of acetanilid or 4 grains of acetphenetidin in a powder or capsule. Under supervision such a dose may be repeated at intervals of from two to four hours if necessary to control pain. It is necessary to remember, however, that when small doses fail to give relief, increase in the dose is useless. This fact is especially important, and disregard or ignorance of it has been responsible for many cases of poisoning. Further, it should be remembered that while it was taught for many years that the admixture of caffein with acetanilid lessened the effect of the latter drug on the heart, Hale has shown that this is not the case and such mixtures must be used with special caution.—(From The Journal A. M. A., Feb. 2, 1918.)
We hope that it is clear to those who have read the several articles of this series that their purpose is to present evidence that will enable the reader to form a correct estimate of the literature employed in the exploitation of various nostrums. The distinction between mere assertion—however plausible, and from however eminent an authority—and evidence should again be emphasized. Satisfactory evidence rests on careful observation by those who are capable of accurately determining to what extent any changes that may be observed are due to the therapeutic agent employed and not mere accompaniments of such treatment.
When the Council on Pharmacy and Chemistry was organized in 1905, the greater part of the literature of the nostrums was so palpably misleading, the statements often so ludicrously false, that it was only necessary to call attention to this fact to have those claims collapse. As a result of the Council’s work, the exploiters of worthless nostrums have developed a greater degree of shrewdness in avoiding the easily exploded falsehoods. This has made it increasingly difficult to point out the exact statements on which many of the false claims now rest, even though the exploitation as a whole is as inherently dishonest as before. If a nostrum is worthless, any exploitation must be false and misleading in effect, even though not one single false direct statement is made.
A platitude may be given an appearance of importance if uttered in an impressive manner, and it may be employed to suggest far more than it categorically affirms. These two facts are appreciated by many nostrum exploiters and we find that they have adopted the impressive manner to secure attention, and the platitude to suggest far more than they could defend in direct statement. Thus we have the “lie with circumstance.”
A full page advertisement, which has been appearing regularly for about a year and which must represent a good deal of money, is used to give an appearance of importance to a few words which, if printed in ordinary type, would either pass wholly unnoticed or would lead one to assume that something essential to the full meaning had been omitted. The statement, in full reads:
“Fellows’ Syrup differs from other preparations of the hypophosphites. Leading clinicians in all parts of the world have long recognized this important fact. Have you? To insure results, prescribe the genuine ℞ Syr. Hypophos. Comp. Fellows’. Reject cheap and inefficient substitutes. Reject preparations ‘just as good.’ ”
The only direct statement contained in the advertisement is to the effect that many clinicians have observed that Fellows’ syrup and other preparations of the hypophosphites are not alike. In truth, Fellows’ is not like the better preparations of this type, since after standing it contains a muddy looking deposit that any pharmaceutical tyro would be ashamed of. Technically, then, the statement is true, but it is hardly credible that the manufacturer is paying for an entire page in a medical journal to make this statement without any attempt to suggest something else.
The advertising pages of six medical journals were examined in the order in which they chanced to come to hand. In five of these, the entire advertisement of Fellows’ syrup was in the words just quoted; not a single word more. In one there was the further statement:
“Not a new-born prodigy or an untried experiment, but a remedy whose usefulness has been fully demonstrated during half a century of clinical application.”
These advertisements show that the exploiters of Fellows’ Syrup are spending a great deal of money to induce physicians to prescribe the preparation, and it is equally evident that they wish to convey the impression that the preparation has some therapeutic value. Since we find nothing directly false, in the first mentioned advertisement at least, we must take the evident intent for consideration and determine what therapeutic value, if any, this preparation has, and whether it is advisable for physicians to employ it in any case.
The preparation, according to the statement just cited, has been in use for fifty years. As the exploiter of any preparation cites the most convincing evidence in his possession in support of his views, this claim may be assumed to be the strongest available, and if this evidence fails we must reject the contention as not proved. Here we face a dilemma, for examination of the literature used in the exploitation of Fellows’ Syrup fails to disclose any evidence of the kind that we have described as satisfactory; and we are, therefore, forced to conclude that none has ever been found. By this it is not to be implied that no reputable physician has ever reported favorably concerning the therapeutic effects of this preparation. It is quite possible that an extensive literature of that sort might be found if one examined the older medical journals. But the day has passed when every improvement that follows the administration of a preparation is blindly attributed to the drug in question. Clinical research today is far more exacting.
We will assume that the reader who has investigated the question with an open mind will have come to the decision that the contention that Fellows’ syrup is of especial therapeutic value is not proved. We might rest with that assumption and ask the clinician whether he is prepared to use a nostrum that has been before the medical profession for half a century without any satisfactory evidence having been gained that it possesses therapeutic value. We might ask him whether he would be willing to tell his patients that he was prescribing such a nostrum for them in the face of the absence of any such evidence of its value.
But we prefer to go even further and show him that not only is there an entire absence of any evidence of its therapeutic value so far as we have been able to learn, but in addition there is an abundance of evidence that the hypophosphites are devoid of any such therapeutic effect as they were formerly reputed to have, and that, in fact, they are, so far as any effect based on their phosphorus content is concerned, singularly inert.
While we have thus far taken the Fellows’ preparation as the subject of the discussion, we may take a broader view and examine the subject of the hypophosphites in general, and the substitutes containing phosphorus that have been introduced from time to time. It hardly needs to be said that if the hypophosphites are without therapeutic value, it is impossible to give them value by combining them in a muddy-looking, ill-made preparation such as Fellows’ Syrup. Such evidence was submitted to the medical profession in a report of the Council on Pharmacy and Chemistry (J. A. M. A. 67:760 [Sept. 2] 1916); and we would strongly advise any one who is disposed to act on the suggestion contained in the advertisements of Fellows’, and other hypophosphite preparations, to read that report in full and to think the matter over before prescribing one of these nostrums. Quoting briefly from the report in question:
“Although the overwhelming weight of evidence was against the probability that the hypophosphite preparations are of value as therapeutic agents, the Council thought it well to investigate the subject. Dr. W. McKim Marriott of Baltimore was therefore requested to review the evidence for and against the therapeutic usefulness of the hypophosphites and to conduct such experiments as seemed necessary.”
The Council was not content to rest on the mere absence of evidence for the value of these preparations or any one of them, but sought to obtain evidence that would fulfil the conditions mentioned above, and in pursuance of this plan it secured the cooperation of a trained investigator, one who would work under the best of conditions for learning the truth. The results of Dr. Marriott’s investigation were published in The Journal, Feb. 12, 1916, p. 486, and should be read by everyone who has any interest in the problem. Lest some of our readers may fail to refer to the original of Marriott’s paper, we will quote briefly from it:
“None of the subjects of the experiment experienced any effect whatsoever from the administration of the drug ... Almost all of the ingested hypophosphite is eliminated unchanged....
“These experiments (Forbes) demonstrate conclusively that the hypophosphites possess no specific value as a source of phosphorus for the body.... It is doubtful if there are any conditions in which the body suffers from lack of phosphorus. Even should such conditions exist, phosphorus, in the form that it occurs in the ordinary foods, or as phosphates, is more efficient in supplying the deficit than hypophosphites that must be oxidized before utilization and which are only about 15 per cent. oxidized if at all. For example, half a glass of milk contains more available phosphorus than three large doses of hypophosphites of 15 grains each, as great a dosage as is usually given.
“What then, is the therapeutic value of hypophosphites? There is no reliable evidence that they exert a physiologic effect; it has not been demonstrated that they influence any pathologic process; they are not ‘foods.’ If they are of any use, that use has never been discovered.”
The case seems to stand about like this: A nostrum maker spends thousands of dollars to tell physicians that his cloudy preparation is not like other preparations, and physicians are expected to accept that as convincing evidence that they should prescribe and their patients, perforce, take it. This too, in spite of the evidence gained by careful scientific investigators that the hypophosphites in fairly large doses contain less available phosphorus than half a glass of milk, and that there is no evidence available that they exert any therapeutic effects at all.
Should we take the meaningless statement of a nostrum maker, who does not submit evidence of any therapeutic value of his preparation—unless one can call certain careless habits of prescribing evidence—and assume the responsibility of prescribing a nostrum that according to all scientific evidence available is useless, and of no more effect than a few teaspoonfuls of milk, so far as its hypophosphite content is concerned? It may be argued that it possesses some value because of its bitter nature. We will not deny that it is bitter; so is strychnin, so is quinin, so are scores of simple drugs, but what physician would care to admit to his patients that he did not know how to prescribe a simple bitter, such as nearly every layman can select for himself, without recourse to a preparation such as Fellows’ Syrup?
We have felt that it is not wholly satisfactory to discourage the use of a given nostrum without making an effort to assist the physician in choosing wisely in the treatment of the condition for which the nostrum is claimed to be useful. In the present instance, however, we fear that would prove a task beyond our powers, for the hypophosphites have been used in such a variety of conditions that the discussion would have to include nearly the whole materia medica if we were to follow our usual procedure.—(From the Journal A. M. A. Feb. 16, 1918.)
Formerly it was customary to prescribe mixtures of many drugs on the assumption that if one of the ingredients missed the mark another might be expected to hit it, just as a poor marksman is more likely to hit a target at short range with a blunderbuss than with a high powered rifle. Increased precision in every branch of science has become the outstanding feature of civilization. The soldier today must shoot straight with a rifle that sends a single ball. There is none of the disposition to rely on chance as when the blunderbuss was used. A capable physician directs his drug straight at the seat of the trouble, and we now have many drugs that can be depended on to exert definite actions. The complex mixture is just as preposterous in modern therapeutics as the blunderbuss would be on a modern battlefield.
Every drug exerts undesired side actions, and it is the aim of the modern physician to try to select the one which will have a maximum of therapeutic with a minimum of undesired actions. When a complex mixture is employed, it is obvious that only the best is utilized, whereas all the undesired side actions come into play. We do not pretend that even the best studied drug has not much to be learned about it; but the nostrum maker who exploits a complex mixture either knows practically nothing of the side actions that it will exert, or, if he knows, he conceals that knowledge. He knows that massive doses of hydrated chloral combined with various narcotics can be relied on to cause unconsciousness in nearly all cases, but he prefers to speak of this as a hypnotic action. This is plain gambling with human life. When the patient dies, it is difficult to prove that death was caused by the mixture alone.
The Council on Pharmacy and Chemistry has expended a great deal of time and energy in combating the “shotgun” nostrum evil. It is easy to understand the disadvantages of such mixtures but it is not so easy to demonstrate the misleading character of the claims made, with an entire disregard of the truth, for these mixtures. No one believes that a pot of gold lies at the end of the rainbow, but no one has actually gone there to see for himself.
There are many types of “shotgun” nostrum. Some are dangerous, as in the case of “Bromidia”; some are preposterous, therapeutic monstrosities which excite the contempt of educated physicians, as in the case of “Tongaline”; some are merely useless mixtures of well known drugs, sold under grotesquely exaggerated claims, as in the case of “Peacock’s Bromides.”
Various formulas have been given for Bromidia. The manufacturers appear to be more cautious under those circumstances in which falsehood might lead them into collision with the federal authorities, than when giving reign to fancy and considering only the best means of winning the favor of the physician. It is said to consist of hydrated chloral, potassium bromid, Indian cannabis, and hyoscyamus. It is impossible to determine from the published formulas just how much hydrated chloral and potassium bromid it contains, but is probable that there are about 15 grains of each of these two drugs to the fluidram, and variable amounts of Indian cannabis and a small amount of either extract or tincture of hyoscyamus.
This much is certain: Bromidia is a distinctly dangerous mixture for indiscriminate use. The claim of the manufacturers, implied, rather than directly stated, that it is superior to an extemporaneously prepared mixture of those drugs is especially reprehensible because it tends to create the impression that the nostrum is safer in effective doses, conducing to a false sense of security on the part of those who are deluded into prescribing it in larger doses than they would a mixture of the same drugs prepared extemporaneously.
A report of the Council on Pharmacy and Chemistry published in The Journal, May 16, 1914, p. 1573, mentions three instances in which death is reported to have followed the use of Bromidia. The manufacturers of Bromidia have no magic power to render hydrated chloral harmless, while it retains its hypnotic action. It depresses the central nervous system, and it is nothing less than monstrous for any one to pretend to rob this drug of its dangerous properties while it retains its hypnotic effects. If the patient requires a hypnotic, the physician should choose that one which his judgment and experience dictate as the best for that particular patient. If he needs hydrated chloral, the physician should prescribe exactly as much as he believes the patient needs. If the effect is slightly greater or slightly less than anticipated, no harm is done and the physician has gained experience that will be valuable in future prescribing. If Bromidia is prescribed and unexpected effects are induced, it is impossible to know whether these were due to the hydrated chloral or to one of the other narcotics or to a synergistic action; and there is nothing to guide in the further use of the nostrum, for mixtures of narcotics commonly have much less uniformity of action than a single drug.
The irritant action of hydrated chloral on the stomach can be avoided by the use of bland fluids or dilute solutions. The following serves as an example of the way in which it may be prescribed conveniently:
| Gm. or c.c. | |||
Hydrated chloral | 2 | │6 | gr. xl |
Syrup of orange peel | │ | ||
Water of each | 30 | │ | fl ℥ i |
A tablespoonful (15.0 c.c.) of this mixture, containing 10 grains (0.65 gm.) of hydrated chloral, will often induce sleep in the absence of severe pain or serious disturbance, and seldom does this dose have to be repeated more than once in such simple cases. Hydrated chloral is often used in somewhat smaller doses in combination with potassium bromid, which may be prescribed in a mixture such as the following:
| Gm. or c.c. | |||
Hydrated chloral | 1 | │3 | gr. xx |
Potassium bromid | 3 | │9 | gr. lx |
Syrup of orange peel | │ | ||
Water of each | 30 | │ | fl ℥ i |
In producing sleep when severe pain is absent this is as effective as the preceding, in similar doses. The use of repeated doses of hydrated chloral in such a mixture as this, or in the form of Bromidia or other nostrum when sleeplessness is due to severe pain is highly dangerous. It should be remembered that while hydrated chloral is an effective hypnotic in case of simple sleeplessness, it is not actively analgesic except in distinctly dangerous doses. Bromidia in repeated doses will induce sleep even in the presence of pain, of course; but any active narcotic does that, and it is correspondingly dangerous. Small doses of morphin given alone are preferable when sleeplessness is due to severe pain.
“Tongaline” is an example of the type of “shotgun” nostrum that would be merely ludicrous if we could look on anything that degrades therapeutics so lightly. A report was made to the Council on Pharmacy and Chemistry, and published in The Journal, July 17, 1915, p. 269, and in this report it is stated that Tongaline is said to consist of tonga, cimicifuga racemosa, sodium salicylate, colchicum, and pilocarpin. Whether the formula was cut short just there because the office boy ran out of breath at that point, or because the discoverers of this wonderful combination had not heard of the eminently potent substances that the witches added to their cauldron, we can leave to the reader’s imagination, for it is manifestly impossible to present an orderly discussion of the pharmacology and therapeutics of such a preposterous jumble of drugs.
“Peacock’s Bromides” belongs to a slightly different class. It is said to consist of the bromids of sodium, potassium, ammonium, calcium and lithium. In the absence of a logical explanation of the pretended superiority of this mixture over one that is made extemporaneously, the exploiters seem to have been driven to the necessity of pretending that its freedom from contaminating chlorids explains its claimed advantages over mixtures of the official or commercial bromids. The truth is that the chlorids are used as antidotes in bromid poisoning.
Disregard the claims made for Peacock’s Bromides, and ask yourself the question whether you have ever actually seen any ill results following the use of the official bromids that you could reasonably attribute to contaminating chlorids. Furthermore, carefully consider the relative advantage of a single bromid (say the bromid of potassium, or bromid of sodium, if you prefer it), with the opportunity of observing its effects and adjusting the dose in accordance with the results of your experience, and a mixture such as Peacock’s Bromides, the composition of which you do not know, and which the manufacturer can alter to suit his own convenience.
While it is true that the therapeutic art will not degenerate in its entirety merely because some physicians continue to use the most fraudulent and worthless nostrums, yet, on the other hand, to the extent that a physician continues to be guided by the false teachings of nostrum venders who have no therapeutic training, he is plunged into therapeutic chaos.—(From The Journal A. M. A., March 2, 1918.)
It may seem paradoxic to say that recent progress in the medical sciences has made therapeutic chaos possible, but it is true nevertheless. Revolutions are sometimes slow and orderly, sometimes sudden and attended with confusion. The revolutionary changes in the medical sciences have been so numerous and so rapid that the general practitioner has been unable to keep pace with them, and in the resulting confusion the nostrum maker has seen his opportunity for exploiting his useless, dangerous or unscientific preparations. The greater the confusion, the greater his opportunity; and it is no exaggeration to say that he has been the most potent factor in maintaining the chaos of therapeutics.
The majority of our readers would probably say that the existing scientific medical literature insures the permanence of established beliefs, but every one who has delved into the literature has found instances of truths that had been established and forgotten—buried under the ever-increasing avalanche of contributions to that literature.

Typical half-page Tyree advertisement appearing in medical journals.
Rapid advances are still being made in the medical sciences and unless constant vigilance is exercised therapeutics will return to the chaotic condition from which it has so recently emerged. It was in recognition of these facts—the danger of this return to chaos, and the difficulty, in fact, impossibility, of any individual’s keeping pace with all of the medical sciences—that the American Medical Association secured the cooperation of men in various branches of medicine in the Council on Pharmacy and Chemistry, in order that it may place the results of therapeutic progress before the readers of The Journal in an impartial manner.
Are you profiting by this work, or are you still depending on your unaided efforts to distinguish the false teachings of the nostrum venders from that of scientific medicine? Are you prescribing “Antikamnia” and “Ammonol” or a simple member of the group, such as acetanilid or phenacetin? Are you depending on “Tyree’s Antiseptic,” so called, or are you using an antiseptic about which there is no mystery, for which no false claims are made, and one which is really effective? In short, are you using drugs of unquestioned value, such as are described in “Useful Drugs,” or are you taking your therapeutic instructions from nostrum makers’ circulars?
Perhaps you have been led to believe that the Council on Pharmacy and Chemistry is composed of “theorists” and that the nostrums represent the work of “practical men.” Every one should strive to be practical, of course, and it is worth while to inquire whether scientific experimenters, who so largely mold medical literature, should be termed theorists, or practical men. A practical man practices that which is useful in the treatment of the sick, and he must determine who is capable of furnishing him with a better materia medica. A perusal of medical literature will convince any unbiased mind that medical science progresses only by means of experiment, hence experimenters must be considered the really practical men while those who cling to outworn theories are really the “theorists.”

Typical Aseptinol advertisement.
When Lister introduced antiseptic methods into surgery he inaugurated a veritable revolution, which afforded the nostrum makers opportunities for reaping rich harvests through the exploitation—under extraordinary claims—of cheap mixtures of little, or no, value. There is no lack of antiseptics of extraordinary activity in the test tube that are practically harmless to man, and it would seem natural to suppose that such antiseptics could be used to control the development of bacteria in such diseases as typhoid fever, but, unfortunately, such hopes have not been fulfilled. Ehrlich experimented with many phenol derivatives that showed decided antiseptic activity in the test tube, in the hope that he might find some that could be used to combat such common diseases as diphtheria and typhoid fever, but while many of these are of low toxicity for man, he was unable to find even one that could be used effectively in the treatment of any of these diseases. His discovery of arsphenamin (“salvarsan”) resulted from quite another type of investigation.
Many practitioners lose sight of the essential difference between antiseptics and disinfectants and employ antiseptics in cases in which only a disinfectant action would be of value. An antiseptic does not destroy bacteria, it merely inhibits their growth; and when it is diluted too much, it loses its effects and the bacteria may begin to multiply as though no antiseptic had been used. This is especially true after the use of weak antiseptics in the mouth. These are soon diluted or removed by the saliva, and the bacteria continue to multiply with only a momentary interruption at best; hence to advise the use of an oral antiseptic as an effective means of treating diphtheria is little short of criminal.
“Tyree’s Antiseptic Powder” was submitted to the Council nearly twelve years ago. The label on the package stated:
“This preparation is a scientific combination of borate of sodium, alumen, carbolic acid, glycerin and the crystallized principles of thyme, eucalyptus, gaultheria and mentha in the form of a powder.”

A leaflet issued several years ago by the Aseptinol Manufacturing Company states that “Pulv. Aseptinol Comp.” combines in an elegant form boric acid, the salts of aluminum, crystallized phenol, and the active crystalline principles of thymus, mentha and gaultheria.
A comparison of these formulas would justify the designation of the two preparations as twins, but even one twin may have a wart where the other lacks it. The formula of Pulv. Aseptinol Comp. given in the leaflet also includes Hydrastis canadensis, but we believe that a wart should be quite as much of an addition to the anatomy of man as the hydrastis is to this already preposterous formula. Similar as the formulas of these two nostrums were said to be, the general methods of exploiting them were even more similar. A partial list of the diseases for which each has been recommended by its exploiters shows the similarity of methods pursued:
| Tyree’s was Said to be Useful in the Treatment of: | Pulv. Aseptinol Comp. was Said to be Useful in the Treatment of: |
| Leucorrhea | Leucorrhea |
| Gonorrhea | Gonorrhea |
| Vaginitis | Vaginal inflammation |
| Pruritus | Pruritus |
| Ulcerated conditions of the mucous membrane | Ulceration of vagina or cervix |
| Scrofulous ulcers | Chronic ulcers |
| Syphilitic ulcers | Prophylactic against specific disease |
| Disinfecting offensive cavities | Cleansing pus cavities |
| Deodorant | Deodorant |
| Profuse and offensive perspiration | Checks abnormal secretion |
We stated that the formula furnished by Tyree was that given above, but the Council was never able to learn when Tyree actually employed the formula except for advertising purposes; and analysis of the powder showed that Tyree’s Antiseptic Powder was essentially a mixture of boric acid and zinc sulphate, with insignificant amounts of odorous principles.
A remarkable fact brought out in the course of the consideration of the preparation by the Council was that Tyree admitted that he had changed the formula without having published the new one. The Council then showed that a specimen of the “antiseptic” that had been kept in a retail drug store for several years was essentially similar to that sold at the later date. Thus it would seem that Mr. Tyree had been making his powder by one formula and publishing an entirely different one for years before the Council published the facts in the case.
If Tyree found it necessary to change the formula of his powder—if indeed, he ever used the published formula—why did the Aseptinol Manufacturing Company adopt it, or one so closely resembling it?
It is obvious that both of these twin nostrums are utterly unfit for treating the various conditions for which they are or have been recommended; and in view of the misrepresentation in one case, it is difficult to understand why it should be taken as the model for the other. Do physicians believe that a simple mixture of boric acid and zinc sulphate, or a mixture such as that given in the formula of “Aseptinol” powder, is in any way superior to a prescription such as any physician could write?
There is a far more important question to consider than the relative merits of such nostrums and a prescription of the physician’s own devising. That question is whether the medical profession is going to help perpetuate the chaotic conditions that the use of such nostrums fosters or to assist in therapeutic progress by maintaining its independence of such false teachers, and seeking to aid in the establishment of a rational use of drugs and remedial measures.—(From The Journal A. M. A., March 30, 1918.)
We called attention recently to the skill which the nostrum vender displays in avoiding the particular thorn that pricks him, and his development of the art of impressively saying, “Nothing in General,” as exemplified in the advertisements of Fellows’ Syrup. Nostrum sellers are more canny than original; and when once an idea finds lodgment with one of them, it is made to serve many masters. Formerly exploiters of either vicious or worthless nostrums were wont to boast that their preparations were exploited in a “strictly ethical manner.” Recent perusal of as choice a lot of advertisements as can be found in the most degraded of medical journals failed to disclose this claim in a single instance, although the claim that a preparation is “advertised only to physicians” is still common.
The advertisement of “Neurosine,” which we reproduce, was the first one which came to our attention when we searched through some medical journals for one that would illustrate a discussion of the “original package” evil. This is the only reason for selecting Neurosine rather than another. Such half page advertisements and others of similar size in various medical journals cost a good deal of money and they presuppose that the Dios Chemical Company is interested in having original bottles of Neurosine dispensed every time that nostrum is prescribed.
Why should the firm have any such deep interest in seeing that an original bottle gets to the patient? Why should it be necessary to do anything more than see that the genuine mixture reaches the patient? Does it seem within the bounds of reason that substitution is so commonly practiced by pharmacists that this firm must go to large expense to prevent the substitution of spurious mixtures for its product? Is dishonesty the rule among pharmacists? Common sense rejects the plea as placing too great a strain on one’s credulity. Obviously, then, the advertisement does not tell the whole truth, though it does indeed tell exactly what the nostrum maker wishes to have done, that is, to have only original bottles dispensed when physicians prescribe that nostrum. The fact we have; the reason is not far to seek.

When the pharmacist puts up an ordinary, nonproprietary prescription, the patient gets no clue from the package as to the nature of the prescription employed. But when an original bottle of Neurosine is dispensed, even though the pharmacist puts his own prescription label on it, the patient sees the difference at once and knows just why the usual prescription bottle was not employed. He also knows that he can get the medicine with its original wrapper or label by merely showing the bottle to the druggist, for the words “Neurosine” and “Dios Chemical Co.” are blown in the glass. Here, then, may be a plausible reason for desiring that only original bottles be dispensed.
You may ask, “What difference does it make if the patient does learn the name of the nostrum, he must go to his physician for advice concerning its use?” Having learned the name of the remedy that has been prescribed for sleeplessness, let us say, he proceeds to use it whenever he imagines that he needs it; and that need, real or imaginary, has a way of increasing in frequency. As a result, the patient takes far more Neurosine than the physician would think of permitting if the matter had not passed entirely beyond his control.
Not only has the patient acquired a dangerous habit of self-prescribing, but he takes especial delight in recommending his favorite remedy to friends whose symptoms, real and imaginary, seem to resemble his own. This offers him an opportunity to prescribe with an air of authority. It was prescribed for him by Dr. Blank, and it gave relief, ergo it may be depended on to give relief to others! Thus is the basis laid for its general use by the laity, when this process is multiplied sufficiently. The statement is susceptible of easy proof by any one who cares to investigate the matter for himself. There is probably no physician worthy of the name who will attempt to deny that the promiscuous use of hypnotics and narcotics is dangerous, and certainly no careful physician will deliberately place a narcotic in the hands of patients to be used freely and without control.
Since we have selected Neurosine at random, so far as this particular discussion is concerned, it is worth while to inquire into its composition, the claims that have been made for it and the evidence, if any exists, for or against its therapeutic value. Even the most active of hypnotics are worse than useless if they are inferior to other readily available hypnotics, or if they have undesired side-actions that outweigh any advantages that they might otherwise have.
The Council on Pharmacy and Chemistry investigated the literature relating to Neurosine and published its report in The Journal, Jan. 9, 1915, p. 165. According to this report the manufacturers of Neurosine claimed that each fluidounce contained:
Bromid of potassium, C. P. | 40 | grains |
Bromid of sodium, C. P. | 40 | grains |
Bromid of ammonium, C. P. | 40 | grains |
Bromid of zinc | 1 | grain |
Extract lupulin | 32 | grains |
Cascara sagrada, fl. ex. | 40 | minims |
Extract henbane | 0.075 | grain |
Extract belladonna | 0.075 | grain |
Extract cannabis indica | 0.60 | grain |
Oil bitter almonds | 0.60 | grain |
| Aromatic elixir |
This chemical blunderbuss was recommended for use in insomnia, hysteria, neurasthenia, migraine, neuralgia, delirium tremens, epilepsy and many other conditions. Also it was called an ideal calmative for children suffering from chorea, the exploiters claiming that “All authorities recommend the bromids, hyoscyamus and cannabis indica in this disease.” Oliver T. Osborne, professor of therapeutics in Yale Medical School, does not mention one of these three drugs in his discussion of the Medicinal Treatment of Chorea, in the Handbook of Therapy, though he quotes several authorities in this article. Indeed, he does not mention one of the ten drugs included in the above formula of Neurosine in connection with the treatment of this disease. It is a curious fact that Osborne gives the greatest prominence to the use of that drug which is claimed to be wanting in the formula of Neurosine, namely, hydrated chloral.
Perhaps you may have seen temporary relief follow the administration of Neurosine in chorea, and may argue that theorizing is of little value in the face of personal experience. We shall not deny that some may have had that experience, for Osborne calls attention to the fact that the success of any medicinal treatment must be judged in the light of the fact that chorea is self-limited, and the intensity of the symptoms will abate in from two to four weeks. In view of this, we would hardly dispute the claim that one may administer narcotics, such as those contained in Neurosine, and the symptoms of chorea may abate in spite of such mistreatment. In all the years that Neurosine has been exploited to physicians with such remarkable claims, we have never seen a report of a careful clinical study in which the product has been used under the conditions which scientific investigation demands. Would you prescribe any nonproprietary preparations which had never been studied clinically, if a horse-shoer or grocer’s boy told you it would cure epilepsy or malaria?
According to an editorial note appended to the report of the Council on Neurosine, the Dios Chemical Company consisted at that time (1915) of J. H. Chambers, his wife and two sons. It appeared that Chambers never claimed to have any special knowledge of chemistry, pharmacy or medicine, yet we find that he arrogated to himself or to his employees the right to offer therapeutic advice to the medical profession, and even to direct them as to how they should prescribe a given mixture.
We sometimes fail to see the forest because of the trees. It may help us to obtain a better perspective, in a problem that concerns us intimately, by resorting to a hypothetic case, if a close analogy is maintained. In order that we may see ourselves as others see us in such a situation, let us consider the following imaginary case: You become involved in a lawsuit in which an effort is made to deprive you of your property and your liberty. You seek what you had reason to believe was competent legal advice; but, nevertheless, you lose your case and find yourself deprived of your property and your liberty. Now let us suppose further that you discover, when too late to permit you to correct your mistake, that your legal adviser (we can hardly call such a man a lawyer) had been acting all along under the guidance of a plumber who made no pretense of knowing anything about law. How would you feel regarding that pretended lawyer? Would you feel that you had been treated fairly? Would you feel disposed to speak with all charity of him, to recommend him to those in need of legal advice?
You would probably feel toward such a lawyer as patients must feel toward physicians who prescribe proprietary nostrums based on information and advice offered by those who, though without any special knowledge of chemistry, pharmacy or medicine, will be benefited financially if their information and advice are accepted and acted on.—(From The Journal A. M. A., April 27, 1918.)
To the Editor:—As an old Fellow of the A. M. A. I beg to present the following facts to you, and to ask if anything can be done by you to expose the methods of these people: A concern calling itself “The Anasarcin Chem. Co.” of Winchester, Tenn., has caused to be sent to physicians a chart on the subject of “Diagnostics of Renal Diseases.” This chart contains eighteen plates, which were all taken without knowledge or permission of either myself or my publishers, William Wood & Co., from the third edition of my book on “Urinary Analysis and Diagnosis.” The plates are partly composite plates, but mostly portions of plates, exactly reproduced from my book. I at once caused my publishers to write to the Anasarcin Company; and a few days ago I received a letter from a Dr. H. Elliott Bates of 118 East Twenty-Eighth Street, New York, whose letterhead says, “Medical Advertising.” In this letter the writer says that it was he who suggested the sending of such a chart, and admits that all the plates were taken from my book. In this letter he offers to have a letter sent to every physician of the country “in which it is explicitly stated that the cuts on the chart were taken from your book, and that complete information regarding the matters treated on the chart can be found in your book.” In other words he offers to advertise my book free of cost to me, so that I should take no further steps in the matter. I consider this entire matter an outrage, and thought it best to write to you for advice, since my publishers seem to think that in spite of the violation of the copyright nothing can be done.
Besides the cuts, some of the text on the chart is bodily taken from my book, while some of the other text, not taken from my book, but apparently compiled from different articles, is in part entirely wrong, so much so that I must be ashamed of its being associated with any of my own work.
By giving this letter your early consideration, and advising me what you think it best for me to do, you would greatly oblige
Louis Heitzman, M.D., New York.
[Comment.—Readers of The Journal are, of course, familiar with the articles246 that have been published on “Anasarcin,” the “dropsy cure”! Knowing the standard of ethics that the Anasarcin concern adopts in the exploitation of its ridiculous squill mixture, our readers will not be surprised at the standard of commercial ethics which would justify the appropriation of copyrighted scientific material for nostrum advertising purposes. The statement of Dr. Heitzmann’s publishers that “in spite of a violation of copyright nothing can be done” is, of course, incorrect. Something can be done by those who hold the copyright.—Ed.]—(From The Journal A. M. A., Oct. 18, 1919.)
Some, possibly many, of our readers have received a letter from Cologne, Germany, from the “Bakteriologisch-Chemisches Laboratorium Wolfgang Schmidt.” The letter contains a circular directing the attention of American physicians to “Antimeristem-Schmidt.” It also contains some advertising leaflets. One physician in sending this material to The Journal writes:
“A copy of the enclosed circulars has been sent to many of the physicians in this city, and probably elsewhere. Perhaps it has already been called to your attention. Let us be as liberal as possible with our recent enemies. The sooner the old channels of scientific communication are re-opened, the better. But let us not allow such blatant commercialism from a foreign country to go unprotested, any more than we should if it were from our own.”
It should be noted in passing that the envelop in which the Wolfgang Schmidt letter came has on its face a rubber-stamped impress to the effect: “Concerns Cancer Treatment.” The circular letter declares that by means of Antimeristem-Schmidt “either a cure or improvement has been effected in numerous inoperable cases” of malignant tumors. American physicians are asked “to employ the preparation when occasion arises” and are assured that “every medical man in city or country will be able to carry out treatment without preliminary knowledge.” With the letter are two leaflets discussing the use and administration of the product; one contained what was called a “Synopsis of some of the more recent publications regarding the employment of Antimeristem-Schmidt in inoperable malignant tumors.” The “recent” publications comprised three articles published in 1910 and one published in 1912!
Antimeristem-Schmidt was rather widely exploited some six or seven years ago. As was explained in The Journal, March 8, 1913, p. 766, it is a preparation claimed to be useful in the treatment of inoperable cancer and as a supplementary treatment after operations for cancer. The treatment is founded on a theory advanced by one O. Schmidt that the cause of cancer is found in a fungus, Mucor racemosus, which, Schmidt at first asserted, carried a protozoon which he regarded as the real cause of the disease. The vaccine is said to be prepared from cultures from this fungus. While Schmidt claims that he has been able to produce cancer by means of the organism, scientific research has not verified his claims. Extensive clinical trials have shown the treatment to be without effect. The Journal also advised its readers on April 19, 1913, that no license for the sale of Antimeristem-Schmidt had been granted by the Treasury Department and, therefore, its importation into this country was prohibited. Neither the therapeutic nor the legal status of the product has been changed since then.—(From The Journal A.M.A., Dec. 6, 1919.)
To the Editor:—Last September, my chief, Dr. J. S. Millard, received a letter from the Denver Chemical Mfg. Co., manufacturers of “Antiphlogistine.” This letter purported to quote many large commercial concerns as testifying to the value of Antiphlogistine. Recently, I doubted the veracity of these claims and wrote to some of those quoted. I quote from the original letter of the Antiphlogistine company:
“The surgeon to the electric light and electric railroad company in New Orleans says that Antiphlogistine is the finest thing he has ever used in burns, especially flash and brush burns.
“The physician to the New York Edison Co. makes a similar statement. He says that the application gives speedy relief and the burns heal quickly without scars.”
I wrote to Dr. John Woodman, the physician to the New York Edison Co., who replied in part as follows:
“The Denver Chemical Manufacturing Company have no authority to quote me.... I gave Antiphlogistine a thorough trial, and found it had a very limited use, and I cannot recommend it for burns....”
Again, the Antiphlogistine letter said:
“It may be of interest to you to know that at the emergency hospital of the Ford Automobile Co. in Detroit, Antiphlogistine is carried in stock and is used extensively by the three physicians in burns, bruises, infected wounds, sprains and other traumatic conditions which are constantly arising in such a plant....”
I wrote to Dr. Mead who replied as follows:
“In answer to your letter of January 25th, will state that no Antiphlogistine has been purchased or used in this hospital for years past, and I cannot imagine why the representative of the Denver Chemical Company should make such a statement as attributed to him....”
He adds that “Antiphlogistine has never been used” in his department “on an open wound, abrasion or burn.” Is there not some way that such exploitation of our large companies can be prevented?
A. G. Gould, M.D., Akron, Ohio.
Plant Physician, the Goodyear Tire & Rubber Co.-(From the Journal
A.M.A., Feb. 23, 1918.)
The following letters are typical of many that have been received asking for information regarding Dr. L. D. Rogers and his “Auto-Hemic Serum.” This from a physician in New York state:
“Can you give me any information in reference to Dr. Rogers of Chicago, Ill., who has an Auto-Hemic Institute?”
And this from Kansas:
“Just received a letter from a Dr. L. D. Rogers, 2812 North Clark St., who is anxious to sell me a course in ‘Auto-Hemic Therapy.’ Would you kindly inform me what he has to sell? He did not tell me what it consisted of; am inclined to believe it is a rank fake. Kindly let me know what The Journal thinks about it. Just what is it? In the letter they claim that it is practically a panacea for every blood disease.”
This from Maine:
“What is Auto-Hemic Therapy? I have a handsome red and yellow circular from the Ideal Life Extension Press, 2812 North Clark St., Chicago, soliciting subscriptions to their publication, offering as a bonus this book, ‘Auto-Hemic Therapy’ by L. D. Rogers, A.M., M.D., LL.D., Chicago, and membership in the American Medical Union.”
In order better to appreciate the probable scientific status of “Auto-Hemic Serum,” it is well briefly to sketch some of the previous activities of its discoverer, Dr. L. D. Rogers. For many years Rogers was the head and chief owner of the National Medical University of Chicago, a low-grade school of the “sun-down” variety. The “university” is now out of existence and for some time before it went out of existence was not recognized either by the board of health of the state in which it operated or by the boards of the majority of the other states in the Union. The report of the Carnegie Foundation on medical education had this to say about the laboratory facilities of Rogers’ school:
“The school occupies a badly lighted building, containing nothing that can be dignified by the name of equipment. There has been no dissecting thus far (October to the middle of April, 1909), anatomy being didactically taught. Persistent inquiry for the ‘dissecting-room’ was, however, finally rewarded by the sight of a dirty, unused, and almost inaccessible room containing a putrid corpse, several of the members of which had been hacked off. There is a large room called the chemical laboratory, its equipment ‘locked up,’ the tables spotless. ‘About ten’ oil-immersion microscopes are claimed—also ‘locked up in the storeroom.’ There is not even a pretense of anything else. Classes in session were all taking dictation.”
Dr. Rogers is, or was, if he is not still, “Permanent Secretary” of the “National Association of Panpathic Physicians”—whatever that is. In fact, one of Dr. Rogers’ specialties seems to be the founding of quasimedical organizations—organizations, apparently, which may prove useful in the promulgation of such projects as he may, at the time, be interested in. A few years ago, Rogers was exploiting a “cancer serum” and, presto, the “American Cancer Research Society” came into being, L. D. Rogers, president. Soon thereafter certain members of the profession were circularized urging them to purchase shares in the “Cancer Research Laboratory and Hospital,” par value $10. Apparently, the profession did not invest.
A few years ago, also, L. D. Rogers’ name appeared on the “Faculty” list of the “American Post-Graduate School,” a concern which granted—on the mail-order plan—a long line of sonorous degrees and an equally complete line of ornate diplomas.
Then, in 1915, there appeared in the classified columns of certain newspapers the following advertisement:
TUBERCULOSIS—New Japanese treatment;
to prove merits and give discovery quick
publicity will send 10 days’ treatment free.
DR. ROGERS, 546 Surf St., Chicago.
So far as we have been able to learn, Rogers, for some unexplained reason, did not call into existence out of the vastly deep a “Japanese-American Tuberculosis Research Society.” This consumption cure apparently died of inanition.
Then came the “Auto-Hemic Serum” with its inevitable sequel, the “National Society of Auto-Hemic Practitioners.” Another adjunct to the serum exploitation is the North American Journal of Homeopathy, the official organ of the “Auto-Hemic Practitioners” and of the “American Medical Union” and possibly of some other “societies”—but not representative of homeopathy!
What is this new therapy? According to a very lurid poster, it is described as “The Missing Link in Medicine”—possibly referring to the ease with which one may make monkeys of certain physicians. More specifically, although still vaguely, we learn:
“It consists in giving the patient a solution made by attenuating, hemolizing, incubating and potenizing a few drops of his or her own blood, and administering it according to a refined technic developed by the author.”
Elsewhere it is said to consist:
“... in taking five drops (or some multiple of five) of blood from a vein and putting it into nineteen times as much sterilized, distilled water, and incubating it at fever temperature for twenty-four hours, and then making further dilutions according to the needs of the case, as can be determined only by a physician skilled in its use.”
Neither of these statements, of course, describes the “refined technic” of those “skilled in its use,” but those who are interested can, by sending Dr. L. D. Rogers, “One Hundred Dollars cash-in-advance” get a mail-order course in this new marvel.
But if it is rather expensive to learn just how to use “Auto-Hemic Serum,” it does not cost so much to learn what the “serum” will do. Rogers has written a book on the subject, “Auto-Hemic Therapy,” which is used as a premium for subscriptions to the North American Journal of Homeopathy, price $5.00 per year, payable in advance. In the book Dr. Rogers modestly assures his readers that he considers his discovery more important than that of Alexis Carrel, winner of a Nobel Prize.
One of the chief virtues claimed for this serum is that of developing in the patient who takes it an unbounded energy that, apparently, makes him want to work himself to death. In some sensational articles that have appeared in Sunday editions of newspapers on Rogers’ serum, the stuff has been described as “Lazy Serum.” One of the first cases described in the Rogers book is that of a young waiter, “a good-for-nothing lazy fellow who would not work and would not pay for medical services” and who was turned over to Dr. Rogers’ free clinic. He was given the serum on Thursday and was told to report Saturday. He did not return until Monday, his excuse being that “he worked all day Saturday until midnight and all day Sunday and felt as if he could work all day and all night without rest.” The “case report” ends:
“... finally remarking, ‘I feel like a bird’ he flew out of the classroom and we never saw him again.”
The next case described is that of a servant girl who had not worked for a year; within a week after taking the “Auto-Hemic Serum,” “she voluntarily beat carpets till she blistered her hands.” Then there was the rooming house keeper who had spent more than half of each day in bed. After an “Auto-Hemic” injection she “discharged her maid and janitor ... and did all the work of her twelve room house herself, beating rugs, firing furnace and carrying out ashes besides doing some of the laundry.” “Case No. 7176” is interesting: A man, generally considered the laziest person in his community and with a habit of “drinking thirty whiskies a day,” took “Auto-Hemic Serum.” He stopped drinking, shaved himself and changed from “a “bum” to that of a sober, clean, wholesome, bright and honest workman.” Then there was the case of the “lady physician” who “took the serum one evening and the next morning reported that she had the ‘giggles’ all day”; also she became “more magnetic.” More remarkable still was the case of the young woman clerk in a retail store who, after taking the serum, “astonished her employer by volunteering to work overtime.” In the chapter dealing with “Ills Peculiar to Women” Dr. Rogers details the moving story of a man to whom the “serum” was given and who reported that “about the third twenty-four hours after taking it his bowels moved forty times”—nevertheless, “he felt no exhaustion.”
In all phases of human activity the serum seems to work wonders. “The cases are numerous in which the frigidity of both sexes have [sic] melted after Auto-Hemic treatment.” A young married woman with a morbid dislike for her husband took the serum and within a week “became normal.” The discoverer suggests that in some cases there is no doubt that this serum “would prevent divorce.” A 40 year old woman who could not endure to wear any waists but white or black was able, it seems, after taking the serum to tolerate a veritable Jacob’s coat.
Is, then, “Auto-Hemic Serum” good for everything? Let Dr. Rogers answer:
“Briefly stated, without any great exaggeration, this new modified serum treatment is good for anything that is the matter with you, provided the cause is not organic, mechanical or bacterial.”
One infers that in the inorganic, mental, spiritual and nonbacterial spheres the stuff is supreme. But it has its limitations. For instance, Dr. Rogers states that he once had “a very troublesome cough which lasted several weeks, but did not yield to this serum.” Reaching the conclusion that some other treatment was necessary “he had the bones of his neck ‘adjusted’ and got immediate relief.”
The serum “cannot be made up by the barrel and sold at wholesale or retail”:
“If it could be bottled and stored and sold at retail like a patent medicine, the demand for it as a complexion beautifier alone would net the proprietor millions. More than one person a few days after taking the treatment has been wrongly accused of painting.”
Should any of The Journal readers decide to take the $100 mail-order course in “Auto-Hemic Therapy” he should realize that even after he has done so there are certain restrictions in the practice of this “therapy.” In no case must he administer “a course of Auto-Hemic Treatment” for “less than $100, paid in advance.” The only exceptions to this rule are “cases of absolute charity, expectant mothers and to persons positively unable to pay that amount.” Furthermore, Dr. Rogers says that for the reputation of his method, as well as for the good of all concerned, “I insist that the entire fee be paid in advance and that the course extend over a period of one year whether the patient needs few or many treatments.”
For those who do not wish to take the mail-order course, Rogers offers to prepare individual specimens of the “serum” from blood that is sent to him by the physician. The cost of this “serum” is $5.00, “in advance,” of course.
Still emphasizing the commercial side, “Auto-Hemic Therapy” is especially recommended to “the general practitioner growing old and the physician who is ambitious to build up a creditable and lucrative practice” because “the health of four people out of five (old or young, whether they consider themselves sick or well) taken at random can be improved by this method of treatment”! An Ohio physician was said to have doubled his $3,000 practice in two years after starting the “Auto-Hemic” method. A Virginia physician is alleged to have “increased his income $10,000 a year.” A Pennsylvania physician urged by Rogers to send $150.00 for the mail-order course, was assured that this “is merely a nominal amount, as most of the doctors have been able to get this amount back the first month.”
But enough. The story, were it not for the tragic element that forms the background, would be amusing. But it is tragic!—(From The Journal A.M.A., Feb. 14, 1920.)
To the Editor:—Enclosed is a little booklet I received today from the Goodhue Publishing Co., of New York, exploiting the Horowitz-Beebe cure-all for cancer, which, were it not for certain obvious serious features, would make humorous reading.
What psychologic explanation can be made of the fact that there are always sufficient numbers of suckers to make such pseudoscientific adventures profitable?
H. C. Dodge, M.D., steamboat Springs, Colo.
To the Editor:—In my professional life I have been flooded with the usual number of insults to intelligence both by mail and by the softspoken detail man. As a result, I have no doubt, of the active propaganda for reform carried on by The Journal, these insults have lost a certain quality of “rawness” and become much more cleverly done.
One of these has just been perpetrated on the profession which will probably hold the championship pennant for 1916, although I admit that it is early in the year to begin prophecy. A very modestly bound, well printed volume comes to my desk with the compliments of the publishers. At the end of the volume is a group of highly ethical advertisements of other books of the author. So far, so good. The last four pages, however, contain the advertisement of a forthcoming book on the “autolysin” treatment of inoperable cancer. Perhaps we might forgive this were it not for the following paragraph: “This book tells how the general practitioner ... may take an active hand in fighting the malady. The weapons he requires are an ordinary hypodermic syringe and some ampules of Autolysin. The syringe he already possesses. Autolysin he may secure, if he is a legally qualified practitioner, by writing,” etc. Incidentally, the book is advertised to the Intelligent Layman.
Isn’t it beautiful? Too bad the lamented F. F. F. with his mock turtles or those prominent eugenists of scopolamin-morphin fame could not take a lesson in advertising. It was not very long ago that we were invited to come East and learn how to use “autolysin,” or else pay the rather heavy fee for an imported tutor. Now all we need is a “gun” and some of the “dope.” All this is interesting in view of the recent article on the failure of “autolysin” in mouse tumor. It is a foregone conclusion that a lot of “autolysin” will be used, so cancer patients, who have been told that they have cancer, will get better through suggestion, and a lot of enthusiastic reports will pour in from medical brethren who have never studied psychology. Then the thing will slump and we shall all be ready for the next fad.
Nevertheless, each one of these things furnishes us with a text for another sermon on ethics of medical advertising, so I suppose they do not live in vain.
J. W. Force, M.D., Berkeley, Calif.
Assistant Professor of Epidemiology, University of California.
[Comment.—With each of the foregoing communications is a circular letter from the Goodhue Company, advertising Dr. Henry Smith Williams’ book on “The Autolysin Treatment of Cancer.” With this circular is a booklet entitled “Notes on the Treatment of Inoperable Cancer with the New Remedy Autolysin (Horowitz-Beebe) Issued by the Autolysin Laboratory.” Similar circular letters and pamphlets have been sent to The Journal from various parts of the country. The Goodhue Company, publishers, therefore are apparently killing two birds with one stone—advertising the book as well as “Autolysin.”
The Journal has been informed that Henry Smith Williams in some of his magazine articles uses the pen name “Stoddard Goodhue,” and that Henry Smith Williams is a part owner of the Goodhue Publishing Company.
Articles on “Autolysin” will be found in The Journal, Nov. 6, 1915, pp. 1641, 1647 and 1662. The article on “Action of ‘Autolysin’ on Mouse Tumors,” by Dr. Francis Carter Wood, appeared in The Journal, Jan. 8, 1916, p. 94.— Ed.]—(Correspondence in The Journal A.M.A., Jan. 29, 1916.)
Medical journals, and some other technical publications, have received recently what purport to be items of news value sent out by the “Medical News Bureau,” 77 Seventh Ave., Brooklyn, New York. The “manager” of this alleged bureau is given as D. E. Woolley. These “news items” are undated but are marked: “(For immediate release)” One of these starts with the statement, attributed to Mme. Curie, that cancer can be cured by radium and then continues:
“Cancer can be cured by the use of selenium and tellurium, more plentiful and less costly elements,” says F. W. Humphreys of Brooklyn, an American born student of chemistry and science who has devoted years to the study of the cause of cancer and the discovery of methods for relief....
“For the purpose of further developing methods of control and treatment of disease by the use of selenium and tellurium discovered by a number of local scientists, chemists and physicians, the Basic Cancer Research has been organized and an efficient laboratory established at 847 Union Street, Brooklyn....”
“Through the education of the people and special instruction to physicians it is hoped it may soon be possible to gain control of and eradicate the disease which now appears so great a menace. Mr. F. W. Humphrey, one of the organizers of the new institution, estimates that within ten years, or perhaps less time, cancer will no longer be considered a fatal disease.”
Evidently the joker here is the “Basic Cancer Research” of 847 Union Street, Brooklyn!
Newspapers are approached from a different angle. They receive free publicity matter on stationery reading “Cosmopolitan Cancer Research Society” (D. E. Woolley, secretary), 847 Union St., Brooklyn, N. Y. With this matter is a letter from Woolley addressed to the editor of the paper to which the stuff is sent and asking:
“In the interest of suffering humanity will you please give space to the enclosed?
“No object of greater importance has ever been presented for your helpful consideration. Thousands are dying whom you can help save.”
According to the “news item” that accompanies this letter the “Cosmopolitan Cancer Research Society” has been founded for the purpose of “investigating and developing methods” by which cancer “may be successfully combated and eventually eradicated.” It states further that the “society” will “disseminate information concerning symptoms, diagnosis, treatment and methods of prevention” of cancer. Furthermore, the membership of the society “includes physicians, scientists and chemists of prominence, laymen of means, and the sympathetically inclined from all walks of life.” Nor is this all!
“Doctor Frederick Klein the eminent authority on urinology and the chemistry of cancer, has evolved a new colorimetric test which is a most wonderful and valuable discovery in the diagnosis of cancer and various other diseases. This test will be particularly valuable in all life extension work because it determines, even in children the possibility of predisposition toward any particular disease, whether tuberculosis, cancer, diabetes or any of the diseases which in later life may become fatal. It determines also the vitality of the subject enabling the physician to accurately determine the condition of any of the vital organs.”
We learn in closing that memberships in the “society” are “graduated from $1.00 upwards according to the ability and disposition of those who may be interested.”
Located at 77 Seventh Avenue, from which the press agent material of the “Medical News Bureau” is sent, is the “Basic Chemical Corporation of America.” According to such information as we have been able to get, the president of this concern is F. W. Humphreys, the “student of chemistry and science who has devoted years to the study of the cause of cancer and the discovery of methods of relief.” We are informed that Mr. Humphreys was for a while in the employ of a “chemical company” of Philadelphia, and has been in the photographic line down in Virginia and later was connected with a real estate concern in Brooklyn. Another officer of the Basic Chemical Corporation is said to have been in the grocery line in a small village in Missouri, selling out and later coming to Brooklyn and entering the insurance business. Still another officer, it seems, was in the fish business. In addition to these three officers, there are two directors, one of whom is in the fancy grocery line, and the other is a local practicing physician whose name we find in the Propaganda department’s testimonial file under Sanmetto and Arsenauro.
The Dr. Frederick Klein, who is described as the “eminent authority on urinology and the chemistry of cancer,” is not a physician but claims a Ph.D. from Munich, Bavaria. Klein claims to have developed certain urinary tests. One of these, according to him, “indicates the body Vitality with great accuracy,” another proves the presence of cancer, a third is the “syphilis test” and a fourth is the “pregnancy test.” And these are not all!
Those who read the reports of the Council on Pharmacy and Chemistry may remember that Frederick Klein is the gentleman who made “Sulfo-Selene,” which the Council, in refusing it recognition, described as a “mixture containing a selenium compound of undetermined composition produced by reduction of nitro-selenous acid with sulphurous acid, mixed with bile salts and diluents.” Sulfo-Selene was widely exploited in the newspapers in 1916 as a remedy for cancer, and Klein got a good deal of publicity at that time.
Just what product the Basic Chemical Corporation of America is putting, or is about to put, on the market we do not know. From the rather vague talk about selenium and Frederick Klein’s marvelous diagnostic discoveries, it might be inferred that “Sulfo-Selene” was to be resurrected. Be that as it may, it seems fairly obvious that the material being sent out by D. E. Woolley—whether as “Manager” of the “Medical News Bureau” or as “Secretary” of the “Cosmopolitan Cancer Research Society”—is advertising matter in the guise of news.
In this connection it is worth noting that the American Newspaper Publishers’ Association, in a special bulletin issued in 1909, published a very complete list of press agents and the interests these agents represented. This list contains the name D. E. Woolley, who then was sending out press notices for the National Association of Piano Dealers of America. Is this the gentleman who is now acting as press agent for the Basic Chemical Corporation of America? If it is, it may be that the slump in the piano trade has caused Mr. Woolley to turn from musical instruments to cancer cures.—(From The Journal A. M. A., Sept. 3, 1921.)
In the issue of September 3 The Journal called attention to a campaign of free publicity that was being instituted by a Brooklyn concern that, apparently, had for sale an alleged remedy for cancer. The press agent material was of two kinds—for medical journals and for newspapers. That which went to the medical journals was sent out on the stationery of the “Medical News Bureau,” 77 Seventh Ave., Brooklyn. The “manager” of the bureau was given as D. E. Woolley. The items sent out to medical journals stated that the “Basic Cancer Research” had been organized to develop a treatment of cancer by the use of selenium and tellurium.
The material received by newspapers was sent out by the “Cosmopolitan Cancer Research Society,” 847 Union St., Brooklyn (the same address as the “Basic Cancer Research”). The “Secretary” of the “Cosmopolitan Cancer Research” was D. E. Woolley!
The name of one “Dr. Frederick Klein” loomed large in the matter sent out by the “Cosmopolitan Cancer Research Society.” Klein, we were told, is “the eminent authority on urinology and the chemistry of cancer.” The Journal called attention to the fact that Frederick Klein’s name was not unknown in the Propaganda files, as he was the gentleman who manufactured “Sulfo-Selene,” a product that was widely heralded in the newspapers in 1916 as a remedy for cancer. It was also brought out that Klein, who is not a physician, claims to have evolved certain remarkable urinary diagnostic tests whereby the presence of cancer, syphilis, etc., may be determined.
More than a month after the publication of The Journal’s article, a letter was received (October 8) from Frederick Klein. To quote literally from part of the letter:
“In the above Journal dated Sept. 3th, Vol. 77, on page 805, regarding the ‘Cosmopolitan Cancer Research Society’ you have amongst others, mentioned my name Dr. Frederick Klein.
“I wish to inform you that I have given my legal adviser the order to write a note to the above Cosmopolitan Cancer Research Society, 847 Union St., Brooklyn, forbidden them to the effect that my name should not be used by above society in any form or writing in any of their transactions, this has been done some time ago to prevent unethical conceptions concerning myself.”
Shortly after the article of September 3 another item appeared in the newspapers throughout the country to the effect that the Cancer Research Society was offering a “$100,000 Cancer Prize” for a “medicinal cure for cancer.” Many of the newspapers of the country seemed to bite on this piece of free publicity. This was in the first week of October. In the third week of the same month a Brooklyn paper announced that 3,000 people had submitted formulas for curing cancer to the Cosmopolitan Cancer Research Society. The article containing this announcement gave interesting descriptions of some of the “cures” submitted and closed with the statement that the Cosmopolitan Cancer Research Society was establishing “clinics” in various cities. It ended with the statement:
“All treatments are confidential. In this respect the society had the cooperation of the Brooklyn Bureau of Charities. It also has the cooperation of the American Medical Association.”
The closing sentence is, of course, unequivocally false.
At the time of The Journal’s article the name of the particular preparation which the Basic Chemical Corporation of America was putting out was unknown. Shortly after the article appeared it was learned that the product was on the market as “Seleni-Bascca.” A physician, himself a sufferer from carcinoma, after reading the article of September 3, sent The Journal some correspondence he had received from the Cosmopolitan Cancer Research Society regarding the alleged cure. One piece was a letter signed “F. W. Humphrey, Acting Director; Dictated by Dr. George D. Barney,” which read in part:
“Our claim is a very simple one indeed, namely that the use of a proper preparation of Selenium (Seleni-Bascca) restores the Sulphur metabolism to normal; we claim that cancer cannot exist in any form, when the Sulphur metabolism is normal, the results from the proper use of Seleni-Bascca in cases of Carcinoma are quick and lasting, the Medical Profession can hardly realize that in this modest treatment a remedy for the Dreaded Carcinoma has been discovered.
“Seleni-Bascca in its colloidal form is quickly taken up by the blood stream, reaches the finest tissues and almost immediately resists the further growth of the disease. The research work has been going on since 1901, under the direction of Dr. Frederick Klein, in connection with Medical Men who have proved to their own satisfaction that Seleni-Bascca should be used as a treatment in every case of malignancy.”
Seleni-Bascca comes in small vials containing fifty tablets. Each vial bears a label reading:
“SELENIBASCCA. A mixture of Colloidal Selenium in tablet form. Recommended in the internal treatment of Carcinoma and some other cases of faulty metabolism.”
Some of the preparation was turned over to the A. M. A. Chemical Laboratory with the request that the tablets be examined to determine whether or not they contained, as claimed, selenium in colloidal form. The laboratory report follows:
“An original vial of ‘Seleni-Bascca’ (Basic Chemical Corporation of America) was examined in the A. M. A. Chemical Laboratory to determine whether or not the substance contained colloidal selenium. The bottle contained 50 tablets weighing approximately 0.1 gm. (about 11⁄2 gr.) each. The major portion of the tablet was soluble in hot water. Qualitative tests indicated the presence of chlorid, sulphate, small amount of nitrate, potassium, sodium, starch, talc and selenium. Tellurium was not found to be present. The ash was equivalent to 5.5 per cent.; over one-half of the ash consisted of a talc-like substance. The amount of selenium present in the specimen examined was only about 1.3 per cent.
“In the literature sent out by The Basic Chemical Corporation, ‘Dr. Frederick Klein’ is mentioned as chemist. Several years ago, the Council on Pharmacy and Chemistry investigated ‘Sulfo-Selene,’ a cancer remedy, with which the same ‘Dr. Klein’ was connected. The alleged composition of ‘Sulfo-Selene,’ as given to the Council, was:
“Selenium | .25 |
“Sulphur (partially in colloidal and partially in crystalloid state) | .10 |
“Potassium carbonate | .10 |
“Nitrogen | .05 |
“Bile Salts | .50 |
| “To which is added an inert base or vehicle; as sugar of milk or amylum.” |
“It was claimed that ‘Sulfo-Selene’ was prepared by reducing nitro-selenious acid with sulphurous acid, neutralizing with potassium bicarbonate and then adding bile salts. Assuming that the composition claimed for ‘Sulfo-Selene’ was correct the analysis of ‘Seleni-Bascca’ shows that the two products resemble each other. The tests, however, failed to reveal in ‘Seleni-Bascca’ the presence of the bile salts claimed to have been present in ‘Sulfo-Selene.’ ”
“The product is not colloidal as claimed as the selenium can be removed by ordinary filtration.”—(From The Journal A. M. A., Nov. 19, 1921.)
To the Editor:—My attention has been called to the fact that there appears in a recent issue of The Journal of the American Medical Association a statement that the Cosmopolitan Cancer Research Society, located at 847 Union Street, Brooklyn, has the cooperation of the Brooklyn Bureau of Charities. In reply may I say that the Bureau of Charities has no connection, understanding, or relationship whatever, with the Cosmopolitan Cancer Research Society, and has never sent a patient to them.
T. J. Riley, Brooklyn.
Secretary, Brooklyn Bureau of Charities.—Correspondence from The Journal
A. M. A., Dec. 24, 1921.
“Why avoid draughts? Sit by an open window if you want to! Just take a few drops of Sneeze-o before you go into the draught and after you come out of it, and you’ll never catch cold.
“Don’t be afraid of contagion. Kiss your Uncle Ebenezer, even if he’s dying of tuberculosis! Just fortify yourself with a sip of Lungicide before you go to his bedside, and another when you come away, and you’ll be taking no risk.
“Are you going to sit there and let the other folks eat up all the good things just because you are afraid to pitch in, when 2 or 3 Bell-Ans taken before and after the meal would enable you to enjoy your share of all that’s coming without a bit of discomfort or distress? Bell-Ans has restored the pleasures of the table to thousands who say: ‘I can now eat anything and plenty of it, too.’ ”
“The first two blurbs are The Ad-Visor’s. The third is a bona fide advertisement of Bell-Ans, aimed to catch the holiday trade. They are all patterned after the same style and the first two are no more lacking in logic than the last. Overeat—deliberately court indigestion—invite gout—don’t be a gourmet, be a gourmand—be an anti-Hoover and eat a lot of food, whether you need it or not; than take Bell-Ans. If it doesn’t ‘absolutely remove indigestion,’ your druggist will give you back your money! Could anything be fairer than that?
“Such copy as this is not limited in its evil effects to the misguided individual who eats lobster and ice cream at midnight and trusts to Bell-Ans to atone for his indiscretion. The most serious effect of such reckless advice is the example which the advertising sets to other advertisers.”
The comments just quoted are from the Ad-Visor department of the New York Tribune of Feb. 7, 1918. They are respectfully referred to the New York Medical Journal, the International Journal of Surgery and the Woman’s Medical Journal—three presumably scientific publications that through their advertising pages urge physicians to prescribe Bell-ans.—(From The Journal A.M.A., Feb. 23, 1918.)
The secretary of the Harvard University Medical School received from the Campho-Phenique Company of St. Louis a letter that, presumably, has been sent to most of the medical colleges of the country. It read:
“We wish to supply the senior class of all Medical Colleges with physicians’ samples of Campho-Phenique Liquid and Campho-Phenique Powder, and Ointment for 1918.
“We will thank you very kindly if you will send us a communication stating the number of students in your graduating class, and if possible, we would like the name of each and every student, that we may send him personally a sample of Campho-Phenique. In this way, we are sure the party receives the sample.”
Presumably, the Campho-Phenique concern believes in following the old advice: Catch ’em young! In this connection, it may be well briefly to call to the attention of fourth-year medical students the results of the investigation of the Council on Pharmacy and Chemistry of Campho-Phenique. The Council’s findings on Campho-Phenique Liquid were to the effect that the preparation, which was exploited under a false “formula,” was, essentially, a solution of camphor and phenol in liquid petrolatum, substances well known in medicine and none of which under its own name has been credited with possessing any superlative virtues. The Council’s verdict on Campho-Phenique Powder was that “for all practical purposes it is essentially a camphorated talcum powder” containing, apparently, sufficient camphor and phenol to give the talcum powder an odor. It was further brought out in the Council’s report that the Campho-Phenique Company was in effect one of the numerous trade names adopted by one James F. Ballard of St. Louis. Mr. Ballard seems to market a number of “patent medicines,” most of them sold direct to the public, but some, as in the case of Campho-Phenique, exploited to the public via the medical profession. “Herbine,” a “marvelous preparation” that “puts the liver in healthy condition”; “Ballard’s Snow Liniment” that when applied to wounds performs “a perfect cure that leaves no scar”; “Dr. T. L. Stephens’ Chemical Eye Salve” which “acts quickly in all cases” and cures “failing vision,” are some of the numerous “patent medicines” made and sold by Ballard. “Collins Ague Remedy,” “Swaim’s Panacea,” “Swayne’s Panacea” and “Renne’s Pain Killing Oil” are four more of Mr. Ballard’s products, for each of which he has pleaded guilty in the federal courts to making false and fraudulent claims knowingly and wantonly.
If medical colleges of the better class were turning out graduates today who could be caught by free samples of such nostrums as Campho-Phenique, then, indeed, would the outlook for the future of scientific medicine be a gloomy one. But they are not. The young man or woman who goes out today from a reputable medical college is imbued with the scientific spirit, has developed habits of straight thinking and will not, we believe, be so uncritical as to accept at their face value claims made for nostrums of the Campho-Phenique type.—(From The Journal A. M. A., Feb. 9, 1918.)
It will be remembered that the Federal Trade Commission adopted the names arsphenamin and neoarsphenamin for the drugs first introduced as “salvarsan” and “neosalvarsan,” respectively; the terms barbital and barbital sodium for the substances first introduced as “veronal” and “veronal sodium,” and the word procain as the name for the compound first marketed as “novocain.” In issuing licenses for the use of the patents on these drugs, the commission stipulated that the drugs should be sold under the new American title unless the firm desired to use a new trade designation, in which case the titles chosen by the commission should be given equal prominence. The Council on Pharmacy and Chemistry has cooperated with the Federal Trade Commission and has adopted the new names as the descriptive names which appear in New and Nonofficial Remedies. The Chemical Foundation, Inc., which has purchased some 4,500 German-owned patents, many of them for synthetic drugs, proposes to continue the wise policy of the Federal Trade Commission by requiring that those who receive licenses for the use of patents for synthetic drugs must use a common designation for each drug selected by the foundation. “Cinchophen” has been selected as the designation for the substance introduced as “atophan” (also described in the U. S. Pharmacopeia under “phenylcinchoninic acid”). In consideration of this action on the part of the Chemical Foundation, and also because physicians found it difficult to use the pharmacopeia name “phenylcinchoninic acid,” the Council on Pharmacy and Chemistry has recognized the contracted term “cinchophen” as a name for the drug introduced as “atophan.” It is hoped that physicians will support this simplified and nonproprietary nomenclature in the same spirit with which they adopted the terms “arsphenamin,” “barbital” and “procain.”—(Editorial from The Journal A.M.A., Aug. 9, 1919.)
Under the auspices of the British Association for the Advancement of Science, there has just appeared a report on the present status of colloid chemistry.247 The work has been recognized as sufficiently important to receive the endorsement of the government Department of Scientific and Industrial Research. Of particular interest to physicians is the chapter on “Administration of Colloids in Disease” written by Alfred B. Searle, “consulting chemist, Sheffield.” After a somewhat academic generalization of colloidal drugs, the “thesis” is devoted largely to the “Collosols”—proprietary preparations made by the Crookes Laboratories. The “scientific” evidence presented by Searle for colloids in medicine reads as if the advertising literature of the Crookes concern had been considered ample source of information. Thus: “Colloidal Manganese,” besides having been “used with remarkable and surprising results in the treatment of coccogenic skin diseases,... gives excellent results [in impetigo, chronic seborrheic eczema and acute folliculitis] when employed in conjunction with intramine”! The grave danger of the intramine therapy has been known for more than two years, both here and abroad,248 in fact, one author stated that in cases of intramine injections, “the pain is undiluted torture.” In a style as bombastic and verbose as the usual house-organ write-up, the report recklessly details all sorts of conditions in which so-called colloids—and particularly the “Collosol” brand—have been recommended, but derogatory findings are conspicuous by their omission. Even Sir Malcolm Morris is quoted as lending his name (and title) to the endorsement of “Collosols.”
In the United States the medical profession has created a means whereby physicians need not be misled by such “high” authorities as evidently has been the case with our English confrères. Once more the value of the Council on Pharmacy and Chemistry is strikingly manifested. What are the facts about “Collosols”? The Council has reported that a number of the “Collosol” preparations were not colloids at all, and “if ... injected intravenously as directed, death might result, making the physician morally if not legally liable”;249 that in the cases in which the therapeutic claims were examined, the claims were found to be either exceedingly improbable or exaggerated; furthermore, that the A. M. A. Chemical Laboratory found “Collosol Cocaine,” on analysis, to contain only 40 per cent. of the claimed amount of cocain.250
Such are the findings which have been presented to the American physician. But the British physician is now being made the object of an intensive advertising campaign for “Collosols,” based in part on an uncritical, pseudogovernmental endorsement. Just so long as the English profession will not protect itself by creating a competent board to examine and judge proprietary medicines and to control methods of exploitation, just so long will such extravagant and even cruelly misleading claims continue to impede scientific progress in therapeutics.—(Editorial from The Journal A. M. A., Oct. 18, 1919.)
To the Editor:—Has anything been published on the efficacy of “Collosol Manganese” in malaria? I recently read the Council’s report which indicated the fakishness of the “Crooke’s Collosols,” but I also was told that the War Office of England had requested a study to be made of colloidal manganese in malaria.
J. B., Columbus, Ohio.
Answer.—Stephens, Yorke, Blacklock, Macfie, Cooper and Carter report in the Annals of Tropical Medicine and Parasitology (Feb. 28, 1919, p. 345) the results of their investigation for the English government and conclude: “Collosol Manganese in the doses used is of no value in the treatment of simple tertian malaria.”—(Query in The Journal A. M. A., May 3, 1919.)
To the Editor:—Please let me know what information you have about the enclosed clipping?
E. W. Carpenter, M.D., Greenville, S. C.
To the Editor:—“Cotton Process Ether,” manufactured by the Du Pont Co., has been given considerable notoriety in the lay press. A letter of inquiry addressed to the firm elicits the information that “Cotton Process Ether is a very highly refined Di-ethyl Ether charged with Ethylene Gas.” ... What is your opinion of the “Cotton Process Ether”? Has the Council on Pharmacy and Chemistry investigated this product?
John L. Atlee, M.D., Lancaster, Pa.
To the Editor:—I have been waiting for some reference to the new anesthetic referred to in the enclosed clipping, but if any has been made in the medical press I have failed to notice it. If there is anything of interest in connection with this item, and it is not too much trouble, I will thank you to put me in touch with the situation.
Holman Taylor, M.D., Fort Worth, Tex.
Answer.—About January 20, the “News Service” of the “E. I. Du Pont De Nemours and Co., Inc.,” circularized the press of the country with what it was pleased to term a “good ‘filler’ ”; this particular piece of press agent work dealt with “The New Du Pont Ether.” To quote one paragraph from the “News Item”:
The new anesthetic, which is a highly refined di-ethyl ether, modified by the addition of gases, has the following characteristics: (1) the property of inducing and maintaining anesthesia with practical freedom from postoperative nausea, and (2) the property of inducing and maintaining analgesia (conscious insensibility to pain) as distinguished from anesthesia (insensibility to pain plus narcosis).
The Du Pont Ether and the claims made for it are seemingly based on the work of one man, “James H. Cotton, M.A., M.D., Toronto, Canada,” who published an article on “Cotton Process Ether and Ether Analgesia,” in the American Journal of Surgery for April, 1919. However, Cotton did not give the composition of the “new” ether nor, so far as we are aware, has his work been corroborated. In view of the inquiries received, the Secretary of the Council on Pharmacy and Chemistry asked the Du Pont Chemical Works for the composition of the new ether. From the firm’s reply we quote one paragraph:
“... The procedures of manufacture, and the exact composition of our ether, we regard as confidential information which we are entitled to retain unless a condition were to arise in which we were unable alone to satisfy the demand for this type of ether.”
It has been recognized—and incorporated in the “Principles of Medical Ethics”—that the use of a therapeutic agent of unknown composition is unscientific and contrary to the best interests of the medical profession and the public; but it is many times more serious for a physician to employ a secret or semisecret substance as an anesthetic. A physician using such a semisecret substance would have little defense if the patient should die.—(Query in The Journal A. M. A., Feb. 21, 1920.)
In the Query and Minor Notes department of The Journal of February 21, some inquiries from physicians relative to “Cotton Process Ether” were answered. In referring to the composition of this product it was stated that the secretary of the Council on Pharmacy and Chemistry had asked the manufacturers, the Du Pont Chemical Works, for information on this point and one paragraph from the firm’s reply was quoted. Another paragraph from the same letter was omitted; and to this omission the manufacturers took exception, expressing the opinion that by it The Journal led its readers to infer that the concern had “refused to furnish any information whatever” regarding the composition of the ether. The following paragraph, italicized as in the original letter, is the one in question:
“Cotton Process Ether contains no components which do not occur in other anesthesia ethers. Its peculiar properties result from the thorough methods taken to exclude harmful impurities, such as aldehydes, peroxides, traces of acids, carbon monoxide, sulphur compounds, etc., and to include carefully regulated quantities of only such of the usual components as we have found to give distinctly beneficial properties to the ether. We are willing to state that in this class we consider properly prepared ethylene of greatest importance, but we have not announced which of the beneficial components of anesthesia ether we include in our ether, or the amount of such components.”
As the quotation shows, the paragraph is informative in a negative rather than in a positive way in that it states what Cotton Process Ether is not rather than what it is. Since that time, however, the manufacturers have notified The Journal that they have definitely decided to present Cotton Process Ether to the Council on Pharmacy and Chemistry for consideration and that in preparing the data required by the Council will define Cotton Process Ether as follows:
“An improved anesthesia ether consisting of highly refined diethyl oxid (C2H5)2O, plus approximately two volumes of ethylene (C2H4), 1⁄2 volume of carbon dioxide (CO2) and 1 per cent. by weight of ethyl alcohol.”—(From The Journal A. M. A., May 22, 1920.)
“Dionol” is advertised to physicians by the Dionol Company of Detroit. If one takes the word of the manufacturers, the therapeutic possibilities of Dionol are apparently limited only by the blue sky. Even the company admits that “the unprecedented range of action” of this marvel “may come as a surprise.” A glance over the published “case reports” confirms the inference. From “Bed Sores,” “Bubo,” “Catarrh” and “Circumcision” through “Croup,” “Deafness,” “Dysmenorrhea” and “Eczema,” including “Endometritis,” “Erysipelas,” “Gastritis” and “Hemorrhoids,” not omitting “Osteomyelitis,” “Otitis Media,” “Pneumonia” and “Ptomaine Poisoning,” down through the pathologic alphabet to “Quinsy,” “Sciatica,” “Spinal Curvature,” “Varicose Veins,” and “Whooping Cough” one concludes that here at last is a catholicon indeed.
What is Dionol? First it should be said that the preparation comes in two forms: as an ointment and as an emulsion. The ointment, so declare the manufacturers, “is always required”; the emulsion may be used “as an auxiliary treatment.” The Dionol “literature” when stripped of the verbal camouflage with which it abounds may be said to propound the following theories and propositions: First, that the nerves of the body are electric conductors insulated from the surrounding tissues by the nerve sheaths; second, that inflammation breaks down the insulation with the resultant escape of the current and an interference with the normal metabolic action of the cells; third, that Dionol, when applied to the body, penetrates the tissues, “coating the cells and with them the nerve sheaths with a nonconducting layer which is sufficient to insulate the nerve sheaths and stop the leak.”
So much for the theory on which the alleged action of Dionol is based. Dionol itself is a sort of glorified petrolatum. Not, of course, that the manufacturers describe it in any such crude and understandable language. According to the company, Dionol is “composed of pure hydrocarbons, especially selected with regard to specific gravity, viscosity and other necessary physical properties” which has been “perfectly deionized by our special scientific process under the Baines Method.” It appears, from further reading, that ordinary petrolatum will not “turn the trick”; presumably because it does not overcome the human short circuits which the Dionol Company declare are always present in inflammation. When, however, the petrolatum has been subjected to the “Baines Method” it achieves, it seems, an esoteric value that puts to shame its plebeian origin.
The whole thing is very simple. To those physicians that like this sort of thing this preparation should make a strong appeal.—(From The Journal A. M. A., Jan. 26, 1918.)
An Indiana physician sends us in a batch of leaflets detailing the marvels of “Dionol” and thus comments:
“I received the enclosed in the mail today and I am puzzled, perplexed and astounded. I had formed the opinion that the profession was getting better; that it was more scholarly than formerly when the two course school was still in existence and any one could matriculate; that it was no longer possible for a ‘patent medicine’ manufacturer to palm off his wares on us. After reading this stuff and realizing that such methods must be remunerative, I am deeply humiliated. Is it possible that educated physicians respond to this kind of advertising? Or has some one perpetrated a joke on me? If the profession can be thus successfully exploited one can no longer wonder at the following which every new ‘ic’ and ‘ism’ acquires.”
It is a pity that the medical profession generally does not react to the Dionol and similar advertising as does our correspondent. As the concern continues to do business, the presumption is that at least some physicians are using Dionol. As was pointed out in The Journal of Jan. 26, 1918, Dionol seems to be a glorified and esoteric form of petrolatum. The exploitation of Dionol is based on the following theory: (1) The brain is a generator of neuro-electricity; (2) the nerves are the conductors of this electricity; (3) the nerve sheaths are the insulator; (4) wherever there is local inflammation the nerves are short circuited, due to a breaking down of the insulation resistance of the nerve sheath; (5) this results in “an escape of neuro-electricity;” (6) Dionol coats the nerve sheaths with a nonconducting layer and this restores the insulation and “stops the leak.”
Whether this ingenious theory was invented to lend an air of verisimilitude to an otherwise bald and unconvincing tale and give a “reason for being” for Dionol or whether Dionol was first invented and it became necessary to evolve a theory that would give some plausibility to the claims made for this etheralized petrolatum, we are unable to say. In any case the theory and the product are exploited together.
Among the material sent in by a correspondent are some “Dionol Case Reports.” Neither the names nor the addresses of the physicians making these reports are given, but the company states that they may be had “on request.” One special “report” is featured under the heading “Infected Wound. Striking Results After United States and French Government Army Surgeons Failed” is signed “Dr. W.” It is dated July 19, 1919. A few months ago the Dionol Company was sending out this same testimonial with the full name and address of the “doctor” giving it. Investigation showed that the “doctor” in question was an osteopath whose specialties, according to his advertisement in his local newspaper, are “Catarrhal Deafness and Hay Fever, Acute and Chronic Diseases”! In this connection it is worth noting that investigation of some of the earlier testimonials sent out by the Dionol concern and alleged to have been given by “doctors” showed that the gentlemen in question were “drugless healers.”
As a “true indication of the value which the medical profession is placing on Dionol” the Dionol Company has published the names of some physicians who, it is alleged, have used the preparation.—(From The Journal A. M. A., Feb. 7, 1920.)
Physicians are receiving some miscellaneous advertising matter from a concern that seems to operate under various names such as “E. H. Dunn & Co.,” “Eli H. Dunn,” “Eli Laboratory,” etc. The concern is located at 3820 Main St., Kansas City, Mo. One Journal reader, who is evidently not greatly impressed by this material, forwards the stuff to us with the laconic request: “Will you please give me your opinion on this junk?”
The “junk” referred to comprised, in part, an advertising leaflet on “Eli 606 Capsules,” another leaflet on “Eli Vaginal Capsules,” still another on “Eli ‘Vim’ Restorative;” then there was reference to the inevitable nostrum for intravenous use: “Ampoules Eli Venhydrarsen.” A four-page leaflet, headed in large and very black letters “Confidential Guide to Live Wire Physicians Only,” expressed its key-note in the opening paragraph:
“How to make MONEY as well as REPUTATION in the treatment of all CHRONIC AILMENTS and all types, forms and sequella of VENEREAL diseases.”
The “Eli ‘Vim’ Restorative” is said to be a “tonic aphrodisiac.” The “action” of the product is to “Arouse Sexual Ardor and Desire. Influx blood supply to the genital organs.” A postscript to the “Guide” urges physicians:
“If you do not already use Intravenous Serums, by all means get an outfit, if for no other reason than to meet the popular DEMAND.”
A “Special Note” in the “Confidential Guide” advises physicians who “have to deal with Hysteria” to “write the Author of this Guide, who will explain by personal letter a method of cooperation by which such Convulsions may be At Once and forever stopped.... There will be $100 for You from every case treated.” One physician wrote to the “Author of this Guide”—Eli H. Dunn, M.D.—asking for further information on this treatment for hysteria. He received in reply two letters both signed Eli H. Dunn; one was to be shown to the patient, the other was for the doctor’s own information. The letter for the patient to see described the marvelous effects of “Dunn’s Intravenous and Restorative Treatment” in hysteria and recommended it “with the utmost confidence in every case able to pay you the fee commensurate with the service you render.” Then followed these two paragraphs:
“The cost of the treatment when administered by yourself is $300 CASH WITH ORDERS which includes one complete outfit and technique for administering.
“Should you call me personally in consultation an additional fee of $150 per diem covering the time I am away from my Kansas City office; fees to be collected and held until I arrive.”
The letter that was intended only for the doctor’s eye declared:
“You are to have $100 of the fee and $50 of the per diem.”
It explained that the “complete outfit” referred to in the “patient’s letter” would “consist in part of a tube of intravenous medication” and doses of “Restorative Capsules” and “Eli 606 Capsules.”
Eli H. Dunn seems to have had a somewhat varied and spectacular career. After being graduated in 1885 he apparently started practice in Orion, Ill. During the nineties he was practicing at Elma, Iowa, and about 1900 he seems to have moved to Kansas City, Mo. During 1906 and 1908, he also had an additional office at Denver, Col. About this time he was exploiting “Dunn’s Uterine Evacuant” which was “a strictly legitimate” product which could “be injected within the uterus with perfect safety and immediate effect.” This stuff was advertised both from the Kansas City and the Denver offices. The “Personal Column” of a Kansas City paper in 1910 carried the message to “Ladies” that “Dr. Dunn” was a “Regular physician for women only,” Dunn’s violation of the postal laws in 1911 and of the federal Food and Drugs Act in 1912 need not be gone into at this time.
The Journal would feel like apologizing for devoting space to such a preposterous scheme were it not for the fact that physicians, being human, sometimes “fall for” preposterous schemes. Some, we know, have nibbled at Dunn’s bait; others may do so. The gross commercialism that permeates the advertising matter sent out by Dunn again emphasizes the fact that the fad for intravenous medication offers an attractive field for those who would exploit our profession.—(From The Journal A. M. A., Nov. 22, 1919.)
Scores of letters have reached—and are reaching—The Journal office similar in effect to the following:
“I am enclosing ‘literature’ received from the ‘T. J. Glover Research Laboratory.’ Though purporting to come from Toronto, where the $25.00 are to be sent, if you please, the envelope bears the New York postmark.”
The above is from New Jersey while the two following are from Michigan and Illinois, respectively:
“Have you any information in regard to this party and his treatment for cancer? This is the first I have heard of any such work having been done. One wonders if it is presented in good faith or if the money god has overcome the gentleman’s scientific spirit.”
“Is this just one more of them? Why a roan horse? Some people might want serum from a nice bay or calico cow pony.”
The literature referred to comes in an envelop bearing the name of “T. J. Glover, Research Laboratory, Toronto, Canada,” but mailed, apparently, from New York City. The enclosures are a single sheet circular signed Thomas Joseph Glover and entitled “Etiology of Cancer,” a “Directions” slip and a card quoting prices. In the circular Dr. Glover states that he has prepared a serum from immunized horses, “between ages of seven and nine years, of the roan type,” and has injected this intramuscularly “into patients in the advanced stages of cancer and noticed that it has a specific action on every known type of cancer.” Further:
“Up to the present time I have apparently cured cancer of the face, eye, nose, lip, mouth, tongue, stomach, bowel, bladder, breast and uterus.”
In addition to the circular, was a small leaflet giving directions for the injection of the serum and also a card bearing Dr. Glover’s name and Toronto address and reading:
This is to advise you that Dr. T. J. Glover’s Serum for the treatment of cancer can now be had by application to office at above address.
PRICE FIVE DOLLARS PER TREATMENT. FIVE TREAT-
MENTS
MINIMUM NUMBER SENT AT ONE TIME.
Send money by Post-Office Money Order or Certified Cheque.
DIRECTIONS FOR TREATMENT WITH EACH ORDER.
This advertising material, which is evidently being widely circulated in the United States, would indicate that the Glover Research Laboratory had received a permit from the United States Public Health Service licensing the interstate sale of this serum in the United States. No such license has been issued.
The Journal briefly reported in the department of Medical News, Oct. 30, 1920, that the Academy of Medicine of Toronto had appointed a committee to investigate the claims made for the Glover “cancer serum.” In the meantime, the most charitable thing that can be said is that the “treatment” is in the experimental stage and the reported results have not been corroborated by independent investigators.—(From The Journal A.M.A., Jan. 1, 1921.)
The method of exploitation of the alleged cancer serum being put out by Dr. T. J. Glover of Toronto, Canada, was briefly discussed in this department of The Journal for January 1. At that time it was pointed out that the medical profession of the United States was being widely circularized by Dr. Glover and that, while the letters purported to come from Toronto, they were, in fact, mailed from New York City. Since this article appeared the circularization seems to have continued undiminished and physicians in various parts of the United States have sent in the Glover advertising material. Oddly enough, the matter now sent out, while identical in every respect with that dealt with in the previous article, bears a different return address on the back of the envelop. The envelops are the same; but the legend “T. J. Glover Research Laboratory, 538 Jarvis St., Toronto, Canada,” has been crudely crossed out and there has been substituted by means of a rubber stamp the legend “MRS. STEWART, 309 W. 54th St., New York.” Still later letters have been modified to the extent that the letters “RS” of “MRS.” have been cut out of the stamp and it now reads “M . STEWART.”
There has now come to hand a report just published by a special committee appointed by the council of the Academy of Medicine, Toronto, to investigate the Glover Serum. The report of this committee may be summed up by one of its closing paragraphs, which reads:
“The data which your committee has been able to obtain have not convinced it that the results of treatment obtained by the use of Dr. Glover’s serum are better than those obtained by similar methods introduced by others, and which have ultimately disappointed the hopes entertained of them.”
The committee’s report deals with the claims that Dr. Glover has made for his serum, both experimental and clinical. It seems that Dr. Glover has claimed that, experimentally, he had (1) cultured cancer cells and from these cells had isolated and cultured an organism which he declared was confined to, and present in, every type of cancer; (2) produced cancer in a number of animals by inoculation with these cells and organisms; (3) obtained a serum—from a horse that had been injected with cultures of these cells and organisms—which, when injected into experimental animals rendered them immune to inoculation, and (4) produced improvement or cure in cases of human cancer by the injection of his serum. The committee reported that it was unable to obtain any evidence to substantiate Dr. Glover’s claims on the experimental aspect of the question as Dr. Glover had refused to permit representatives of the committee to visit his laboratory; had refused the request of the committee to be allowed to examine his cultures and experimental material; had not acceded to the request of the committee that he demonstrate his ability to culture cancer cells and organisms and to produce cancer by inoculation or to immunize animals against it.
The committee attempted also to collect information which would enable it to pass on the clinical claims made by Dr. Glover, first, as to whether he has succeeded in producing cures, either regularly or occasionally, in cases definitely established as cancer and, second, to enable the committee to decide whether his serum in cases definitely established as cancer produces improvement beyond that which occasionally occurs spontaneously or under palliative measures. On both of these points, the committee reported that it found no evidence to warrant the hope that a specific cure for cancer has been discovered by Dr. Glover or that the serum had produced a cure in any case definitely established as cancer.
It should be understood, that the committee’s investigations and findings were completed before the present advertising campaign of the Glover serum was initiated.—(From The Journal A.M.A, Feb. 5, 1921.)
One characteristic of the “patent medicine” business is that it trades on fear. Should an epidemic occur the market is flooded with new nostrums purporting to cure or prevent the disease in question, while the manufacturers of older “patent medicines” revamp their advertising so as to make it appear that their preparations are all that stand between the scourge and the public. One has but to remember “Peruna’s” exploitation of the yellow fever epidemic in New Orleans some years ago and the way in which the exploiters of “Pond’s Extract” played on the fears of the public at the time of the former meningitis epidemic in New York City.
At present the public is much exercised over the epidemic of infantile paralysis. Anticipating that the nostrum fraternity would attempt to reap a golden harvest from the public distress, the federal officials issued a bulletin of warning on the subject. Naturally, the bulletin was addressed to the lay public, the government assuming that physicians knew enough to avoid being misled by any such advertising campaigns. Apparently, the assumption is too broad. At any rate, the manufacturers of “Glyco-Thymoline” are circularizing physicians, one of whom writes as follows:
To the Editor:—I am enclosing circular letter that I received this morning which seems to me almost a crime. I do not suppose that there is any way to prevent anything of this sort, but it is certainly a shame to attempt to deceive people in this way. As I recollect, Glyco-Thymoline is almost inert, practically no more efficient than Dobell’s Solution.
E. Fletcher Ingals, M.D., Chicago.
The circular letter referred to was on the stationery of Kress & Owen Company, manufacturers of Glyco-Thymoline. It read:
Dear Doctor:—Regarding Infantile Paralysis, it is conceded that the source of infection is through the Nose, Mouth and Throat.
Taking this measure to be correct, we believe that there is no safer prophylactic measure than the use of Glyco-Thymoline, with three parts of water, as a mouth, tooth and nasal wash, by means of the K. & O. Nasal Douche and the toothbrush.
Glyco-Thymoline tends to promote exosmosis, and prevents the absorption of the germ or toxic matter.
We would be glad to send you samples of both Glyco-Thymoline and the Douche should you so desire.
With best wishes, we beg to remain,
Yours very truly,
Kress & Owen Company.
Glyco-Thymoline has been discussed in these pages. A report of the Council on Pharmacy and Chemistry pointed out that this “patent medicine” is simply a weak antiseptic, so feeble that even in full strength it does not kill Staphylococcus aureus in four hours and is of little, if any, greater therapeutic value than sterile salt solution. Yet, Glyco-Thymoline has been recommended by its manufacturers, either directly or inferentially, for such diseases as diphtheria, ophthalmia neonatorum and consumption. Today its manufacturers put it forward as one of the safest prophylactic measures against infantile paralysis and have the effrontery to make this suggestion, not to the uninstructed public but to the medical profession. Presumably, as a business organization, the concern believes it will convince a sufficient number of physicians of the therapeutic efficacy of its product to pay for the cost of this advertising campaign. If it appraises the situation correctly there need no longer be any wonder expressed that in the recent suit against The Journal, “patent medicine” makers were able to enlist the help of medical men.—(From The Journal A. M. A., Sept. 16, 1916.)
The law which limits the length of time that food products may be kept in cold storage could with advantage have its scope extended to include “patent medicine” testimonials. Physicians recently received through the mails—at a time when the mails were frightfully congested with Christmas business—a sixteen page pamphlet sent out in a plain envelop as first class matter. The caption of the pamphlet reads: “Cough and Its Treatment in Pulmonary and Laryngeal Tuberculosis: By Henry Levien, M.D., While Medical Director and Physician-in-Charge of the Liberty Sanitarium, Liberty, N. Y. From the Buffalo Medical Journal.” The pamphlet is devoted to the alleged virtues of that dangerous and widely advertised nostrum, “Glyco-Heroin (Smith),” whose more recent and less descriptive name is now “Glykeron.” Physicians might assume, and doubtless will assume, from the pamphlet that this reprint represents a recent pronouncement on the subject with which it deals. The facts are that the “Liberty Sanitarium” has, apparently, been out of existence for at least fifteen years, while the article itself originally appeared more than eighteen years ago—September, 1901. One of many physicians who sent in the copies received called attention to the fact that he had left the address to which the pamphlet was directed, more than six years ago. Even at that, the mailing lists of the concern that sells this heroin-containing nostrum are more than twelve years ahead of its “clinical reports.”—(Editorial from The Journal A. M. A., Jan. 17, 1920.)
Last September the United States Department of Agriculture issued a press bulletin describing the work of the Bureau of Chemistry in prosecuting the venders or manufacturers of fraudulently exploited “patent medicines.” At the end of the bulletin was a tabulated list of “other preparations against which the government’s charge that they were falsely or fraudulently labeled was sustained by the federal courts.” Tucked away in the list was a product often euphemistically described as an “ethical proprietary” but none the less essentially a “patent medicine”—“Gray’s Glycerine Tonic.” The editor of the Atlanta Journal of Medicine, apparently not having read the bulletin with any great degree of care, published it verbatim. Thus it was that the Atlanta Journal-Record of Medicine for September, 1915, presented the interesting sight of a half-page advertisement of “Gray’s Glycerine Tonic” in the same issue that contained the government’s article classifying “Gray’s Glycerine Tonic” among the false and fraudulent products! What happened? In the very next issue the Atlanta Journal-Record of Medicine apologized thus editorially:
“In our September issue, Gray’s Glycerine Tonic Comp. was inadvertently included in a list that seemed to be under the ban of the Government and very likely an injustice has been done the Purdue Frederick Company which we desire to undo as far as possible.”
Did the editor mean by “inadvertently included,” that he would have omitted “Gray’s Glycerine Tonic” from the government’s list had he noticed it in time? If so, on what grounds? It is a fact that “Gray’s Glycerine Tonic” was one of the “Fifty Falsely Labeled Medicines”; it is also a fact that it is one of the products that government officials and the federal courts have declared to be sold under claims that are “false, fraudulent and misleading.” If “Gray’s Glycerine Tonic” was fraudulently exploited—and the government and the courts have so declared it—why is it necessary for the editor of a medical journal to apologize to his subscribers for having told them so?—(Editorial from The Journal A. M. A., Jan. 1, 1916.)
“Under the deceptive heading ‘Making Cod Liver Oil Palatable,’ the Charlotte Medical Journal in its December issue prints a boost for ‘Cord. Ext. Ol. Morrhuae Comp. (Hagee),’ or, as it is generally known to the drug trade, ‘Hagee’s Cordial of Cod Liver Oil.’
“The boost intimates that this is a preparation in which cod liver oil has in some way been rendered palatable, and then goes on to say that this is a cod liver oil product which has not suffered the least loss of those essential elements which make the crude oil such a high-class reconstructive.
“At first sight one might question whether a cod liver oil product which contains absolutely no cod liver oil had not suffered the loss of essential elements. But a closer reading discloses a significant qualification, namely, the phrase, ‘those elements which make the crude oil such a high-class reconstructive.’
“The boost is misleading from beginning to end. The manufacturers have not succeeded in this preparation in ‘making cod liver oil palatable,’ nor does their preparation in any way possess the virtues of cod liver oil. These claims have again and again been refuted, but they continue to be published—at a price but rarely in reputable medical journals.”
The above is quoted from the Weekly Bulletin of the Department of Health of the City of New York. The Bulletin is issued for the enlightenment of the public.—(From The Journal A. M. A., Jan. 8, 1916.)
A physician in Vermont writes:
“This is simply a word of inquiry—and of possible warning to other practitioners—regarding a preparation known as Hypno-Bromic Compound manufactured by H. K. Wampole & Co. This compound is dispensed by druggists without prescription and contains in each ounce:
“Cannabis indica | 1 | gr. |
“Morphin | 1⁄4 | gr. |
“Potassium bromid | 48 | gr. |
“Hyoscyamus | 1 | gr. |
“Chloral hydrate | 96 | gr. |
“I have at the present time three young women who are addicts to this preparation as the result of thoughtless prescriptions from physicians. This mixture evades the working of the Harrison Act and may be dispensed freely at the discretion of the druggist and, as a result, these three cases of mine have been able, by visiting at the various drug stores in town, to keep an ample supply on hand at all times.”
“Hypno-Bromic Compound” is more than an unscientific mixture; it is a dangerous product and should not be sold indiscriminately over the drug counter. Before the Harrison Narcotic Law went into effect, “Hypno-Bromic Compound” contained half a grain of morphin sulphate to the ounce instead of its present one-fourth grain. Physicians remember that Section 6 of the Harrison law contains a joker—put over by the “patent medicine” interests—that exempts proprietary remedies containing one-fourth grain of morphin or less to the ounce from the restrictions of that act. While it is illegal for a physician to write a prescription which contains morphin, no matter how small the amount, unless he conforms in all ways to the requirements of the Harrison Narcotic Law, “patent medicine” concerns can sell indiscriminately nostrums containing morphin up to this amount and the public can buy them without let or hindrance. No reputable druggist would sell a layman over 700 grains of chloral hydrate or 2 grains of morphin or 8 grains of extract of cannabis indica, without a prescription, yet, the druggist may hand over 8 ounce bottles of Hypno-Bromic Compound which contain 768 grains of choral hydrate, 2 grains of morphin sulphate, 8 grains of extract of cannabis indica, 8 grains of hyoscyamus and 384 grains of potassium bromid! Physicians who prescribe such products as Hypno-Bromic Compound and druggists who indiscriminately sell such stuff are disgracing two honorable professions.—(From The Journal A. M. A., Feb. 7, 1920.)
For some time past inquiries have been received regarding Charles Lyman Loffler, his Post-graduate Course in Intravenous Therapy and especially relative to “Intravenous Compound (Loffler).” For instance, a physician writes:
“Can you tell me anything about the Physicians Drug Syndicate.... They are pushing the sale of Thymozene and offering One Hundred Dollars’ worth of stock fully paid and non-assessable, free to those sending in their order, and also a copy of Dr. Loffler’s Lectures on the Blood.”
And from another physician:
“What do you know of Charles Loffler, M.D., and his Intravenous Compound? A few evenings ago a man who appeared to be about 40 years old came to my office and tried to interest me in the above-mentioned article; he claimed to be Dr. Charles Loffler of Chicago. With him was a young lady whom he introduced as Miss B——. Miss B—— said that she had been with Dr. X—— [a physician of high standing in Los Angeles] for two months and that he was using the Intravenous Compound; also quoted other physicians.... His whole layout looks quackish, and were it not for the fact that he showed me a letter that appeared to be from Dr. X——, I should not have given him a second thought.”
And this also:
“Charles Loffler, M.D., or his agent was traveling around inducing one M.D. in each town to take up his methods of blood examination and treatment and with a little advertising of blood examinations free the doctor selected gets quite a run of patronage.”
Another physician writes:
“My attention has been called by another physician to Loffler’s Intra-Venous Compound. May I trouble you to give me any information that you may have with regard to its composition and its value as a therapeutic agent?”
C. L. Loffler does business from Rooms 1101–1102, Venetian Bldg., Chicago, the location of the “Intravenous Chemical Co.,” the “Physicians Drug Syndicate” and the “Ma-Oze Chemical Co.” Of these, more later. The Journal has in its files a large amount of material regarding Loffler. A brief résumé of that part of the material dealing with Loffler’s professional activities will be given for the purpose of allowing physicians to evaluate the scientific status of Loffler’s “Lectures,” “Post-Graduate Courses,” his therapeutic “discoveries” and his products.
It seems that Loffler was reared in Yankton, S. D. In 1898–1899, Loffler was a senior student at John Creighton Medical College, but, for reasons that need not be gone into here, he was never graduated. He received a diploma from Barnes Medical College in 1900, and in the same year was licensed to practice in South Dakota. In 1902 he was at Le Mars, Iowa; in 1904 his name appears in the medical directory, under Sioux Falls, S. D., as “Specialist in Chronic Troubles.”
Charles L. Loffler’s “specialty” is “Intravenous Medication.” In 1912 and 1913, as the Intravenous Company of Colorado Springs, he was sending out a booklet entitled “Consumption.” This described the alleged marvelous results to be obtained in the treatment of tuberculosis by the use of “Intravenous Compound”; there was also a side line, “The Loffler Internal Bath Plate.” At that time the administration of “Intravenous Compound” was recommended intravenously, hypodermically, by rectum, by mouth and even by insufflation. When the stuff was to be given by rectum, the recommendation was made: “First wash out the bowels with a preliminary injection of two or three quarts of warm water, using for this purpose the Loffler Internal Bath.”
In 1913 Loffler sought a larger field for his peculiar talents and left Colorado Springs. After a short stay in Denver he is next found in Minneapolis, where he was also “engaged in the practice of intravenous therapy” and, incidentally, seems to have been an organizer and manager of a common law concern known as the Automatic Thrasher Co.
In 1919 we find Loffler in Chicago as president of the “Physicians Drug Syndicate.” This concern—another common law organization—had for its vice president one A. E. Erling, M.D., and for its secretary and treasurer, Arthur C. Hanson. Erling was discussed251 in an article that appeared in The Journal, July 5, 1919, on the egregious “Allied Medical Association of America” of which organization C. L. Loffler was “President” in 1918.
Hanson, the secretary and treasurer of the Physicians Drug Syndicate, is said to have hailed originally from Minot, N. D., where he was in the drug business. His name appears in the Propaganda files as the manager of the Ma-Oze Chemical Co. of Minneapolis, which, in October, 1919, was advertising in a daily paper of that city:
“Protect yourself against influenza. Don’t let the germs get a foothold in your system. Kill them with Ma-Oze Antiseptic Powder. Use it as a gargle. It is ... sure death to all kinds of disease germs.”
In a preliminary statement sent out by Hanson in the early part of 1919 it seems that the Physicians Drug Syndicate was conceived “primarily to supply physicians with a product to be used in Leucorrhea and personal cleanliness of women.” This product, apparently, was the Ma-Oze of influenza fame in Minneapolis. It was to be put out, however, under the name of “Thymozene,” which, “packed in 4 ounce unlabeled carton for dispensing,” would “show nearly 100 per cent. profit to the organization over the profit which you make if you dispense your own drug.”
In October, 1919, the Physicians Drug Syndicate was circularizing physicians in Iowa trying to get them to send in $6 for “1 Dozen Thymozene 4 oz.” For this $6 the doctors were to get, in addition to the marvelous Thymozene, the following rights, privileges and emoluments:
1. A free Post-Graduate Course in Intravenous Therapy by Dr. Charles Loffler.
2. A gift of $100 worth of stock in the Physicians Drug Syndicate.
3. A copy of Dr. Loffler’s Lectures on Blood.
4. The privilege of purchasing future supplies of Thymozene “at wholesale prices less discount of 331⁄3 per cent.”
The letter making these offers mentioned incidentally:
“Besides our product Thymozene we have been forced to add a Uterine Wafer to be used in connection with hot Thymozene douches in Leucorrhea. These wafers are simply miracle workers.”
In addition to this circular letter there was a membership blank leaflet detailing the marvels of “Thymozene.” There was another leaflet headed in very large, black type “Influenza” and recommending “Ma-Oze Antiseptic Powder” or “Thymozene” for this condition. Still another leaflet accompanying it lauded “Intravenous Compound (Loffler)” and reprinted laudatory puffs of this preparation that were credited to H. H. Witherstine, M.D., Rochester, Minn., Joseph B. Klinehans, M.D., Chicago, and the “Loring Park Sanatorium” of Minneapolis.
In addition to the Intravenous Compound (Loffler) there is, of course, certain “apparatus for the giving of the treatment” which the Intravenous Chemical Co. supplies. The “compound” must be given just so, and the Intravenous Chemical Co. “reserves the right to refuse to supply any physician with Intravenous Compound (Loffler) who, either through lack of proper apparatus or proper care in preparation of solution, or for any reason, uses it in such a manner that will cast discredit upon it.”
The complete apparatus, including 2 ounces of Intravenous Compound (Loffler), sells for $24. What is Intravenous Compound? Apparently, nobody knows except Charles L. Loffler, who asks physicians to inject—and we regret to say some are injecting—this nostrum of unknown composition into the veins of their patients. To a physician who had raised the point of secrecy Loffler wrote in part:
“I am sure that you will agree with me that it is far better to place this treatment in the hands of competent physicians, such as Dr. Witherstine, and many more whose names I will gladly send you, and to protect the honest and competent doctor who investigates and takes up the work, than to publish the formula and give to the unscrupulous a chance to try to make the product and no doubt to claim to cure disease that is beyond hope. The formula is not kept secret for profit ... but is so kept upon the advice of a number of good men who have the interest of the doctor at heart.... I am willing and anxious to place the product and the results in thousands of cases before the A. M. A. on the one condition that the formula shall be kept secret for the benefit of the reputable physician.”
In another letter written more recently to a physician who called attention to the secrecy of the nostrum, Loffler wrote:
“The Intravenous Compound contains approximately 58 per cent. oxygen, 12 per cent. chlorine, 16 per cent. potassium, 9 per cent. sodium and 5 per cent. boron. I have no hesitancy in giving it, and it was due to an incompetent man in this office that this was not given fully in the booklet. He made the changes without my consent and has caused me to answer many inquiries by physicians.”
A seeming frankness is a trick as old as nostrum exploitation itself. Loffler’s “formula” is meaningless. A quack who was putting out a mixture of 1 part baking soda and 2 parts common salt might with equal frankness say that his marvelous combination contained approximately 35.4 per cent. sodium, 4.8 per cent. carbon, 19 per cent. oxygen, 40.4 per cent. chlorin, and 0.4 per cent. hydrogen.
In order that the profession might know more about this product a specimen was turned over to the A. M. A. Chemical Laboratory for analysis. Here is what the chemists report:
“One original 2 ounce bottle of ‘Intravenous Compound (Loffler) for Intravenous Use’ was submitted to the Association’s Chemical Laboratory for examination. According to the label, the product is sold by the ‘Intravenous Chemical Co., Chicago.’ The bottle contained a white granular substance, which appeared as if the ingredients had been fused together. The product responded to tests for sodium, potassium, chlorate, borate and nitrate. As this same set of chemical radicals was found by Puckner and Hilpert (J. A. M. A., May 22, 1908, p. 1706) to be present in ‘Oxychlorin’ and ‘Zyme-oid,’ a quantitative comparison of ‘Intravenous Compound (Loffler)’ was made.
“The analysis indicated that all three products are essentially the same:
| Oxychlorin, Per Cent. | Zyme-Oid, Per Cent. | Intravenous Compound, Per Cent. | |
Potassium (K+) | 12.26 | 13.50 | 13.79 |
Sodium (Na+) | 8.20 | 9.84 | 9.82 |
Boric acid anhydride (B2O3) | 18.63 | 13.42 | 15.20 |
Chlorate (ClO3-) | 25.52 | 27.53 | 26.44 |
Nitrate (NO3-) | 21.70 | 24.22 | 23.75 |
Water calculated | 13.29 | 10.42 | 11.72 |
“Assuming that the chlorate in ‘Intravenous Compound (Loffler)’ is present as potassium chlorate and the nitrate is present as sodium nitrate, the figures obtained by the analysis correspond to a mixture approximately as follows:
Potassium chlorate (KClO3) | 38.6 | per cent. |
Sodium nitrate (NaNO3) | 32.6 | per cent. |
Potassium borate (K2B4O7) | 4.9 | per cent. |
Sodium borate (Na2B4O7) | 4.0 | per cent. |
Boric acid | 21.1 | per cent. |
“From the results of the examination it is concluded that this preparation is a mixture of alkali chlorate and nitrate and boric acid, probably produced by fusing together the constituents. It is practically the same mixture as Oxychlorine and Zyme-oid as analyzed nearly fourteen years ago in the A. M. A. Chemical Laboratory.”
Throughout the advertising of “Intravenous Compound (Loffler)” the physician is reminded of the financial returns that the product offers.
“... The financial return will prove as interesting to yourself as results are to the patients.”
“And lastly but not less interesting, the financial returns are commensurate with results.”
“... the instruction given me in the use of your Intravenous Compound and the opportunity presented adds four to five hundred dollars per month to my bank account.”
“... will not only give you more positive results than have ever obtained in chronic and progressive diseases but a very remunerative business.”
“Intravenous Compound (Loffler) is supplied in granular form, 2 ounces to a bottle, at $2 per bottle. An ounce will average fifteen treatments and treatments are at from $3 to $5 each, according to the ability of the patient to pay.”
A physician whose name the Intravenous Chemical Company had given as a user of Intravenous Compound (Loffler) was written to by another physician who was interested in the matter and he was asked frankly for his opinion. He replied in part:
“The treatment makes a profound impression on the recipient and is usually followed by a marked improvement mentally, and I have not been keen enough to draw the line of just how far the physical or material improvement went and when the psychical began.
“For the office ‘specialist’ of the advertising type this would be a boon, but I am not entirely satisfied that its use completely justifies its claims.”
Intravenous Compound (Loffler) stands revealed as a nostrum of secret composition which physicians are asked to inject into the veins of their patients. It must be purchased in connection with some supplementary material, “a complete set of apparatus,” sold by the same concern. Its successful administration is said to depend on following a technic detailed either in a booklet sent out by Loffler or given by Loffler in a “Post-graduate Course” which costs physicians $50 unless they have purchased six dollars’ worth of another nostrum, “Thymozene.”
The intravenous administration of drugs is impressive. To the patient the technic is mysterious and its psychic effect striking. Its dangers—infection, air embolism, intravascular clotting, sudden death—are matters of record. Every conservative physician will admit that there is no excuse for the intravenous administration of even those drugs that are well known and whose effects have been carefully studied, except when distinct advantages are to be secured. As The Journal has stated before, “Little is known of the results to be expected from intravenous therapy even with simple substances.”
What, then, can be said of the physician who subjects his patients to the intravenous injection—“at from $3 to $5 each, according to the ability of the patient to pay”—of a preparation of whose composition he is as ignorant as he must be of its effects? Intravenous Compound (Loffler) has been on the market ten years; it is unmentioned in the literature of scientific medicine. The name of its exploiter, while not unknown in the twilight zone of professionalism as the exploiter of a nostrum, as a “Specialist” in “Chronic Troubles” and “Intravenous Therapy,” as well as in other capacities even less savory, is equally unknown to scientific medicine.—(From The Journal A. M. A., Nov. 12, 1921.)
To the Editor:—There is a salesman here in Salt Lake City making extravagant claims about the medicines advertised in the enclosed pamphlet. Would you kindly advise me as to your opinion of it?
W. C. Schulte, M.D., Salt Lake City.
To the Editor:—I am interested in knowing the attitude of the Council on Pharmacy and Chemistry regarding the products of the Intravenous Products Company of America, 121 Madison Avenue, New York City. If the Council has already reported, please refer me to the appropriate number of The Journal. If it has not, please give me any information available.
H. B. Gessner, M.D., New Orleans.
Answer.—The Intravenous Products Company of America has not requested the Council on Pharmacy and Chemistry to examine any of its intravenous specialties, nor have they been discussed in The Journal or examined in the American Medical Association Chemical Laboratory. The firm’s list of specialties bears a striking resemblance to those of other “intravenous specialty” firms. Endoarsan, like Venarsen of the Intravenous Products Company of Denver, is stated to contain a cacodylate (dimethylarsenate) along with mercury and iodid. Venarsen was reported on unfavorably by the Council (The Journal, May 22, 1915, p. 1780), the inferior efficacy of sodium cacodylate was discussed (The Journal, March 25, 1916, p. 978) and the worthlessness of sodium cacodylate as a spirocheticide confirmed by H. N. Cole (The Journal, Dec. 30, 1916, p. 2012), William G. Ward (The Journal, Feb. 3, 1917, p. 390), and R. L. Sutton (The Journal, Feb. 17, 1917, p. 566). Endosal, like Venosal of the Intravenous Products Company of Denver, is said to contain salicylate and a colchicum preparation (the latter is also said to contain iodids). Venosal was found unacceptable for New and Nonofficial Remedies by the Council on Pharmacy and Chemistry. Like other “intravenous” firms, this company advertises the intravenous administration of drugs such as sodium iodid and hexamethylenamin. The objections to and the dangers of indiscriminate administration of drugs intravenously was recently emphasized in a report of the Council on Pharmacy and Chemistry “Some of Loeser’s Intravenous Solutions” (The Journal, April 16, 1921, p. 1120).—(Query from The Journal A. M. A., Dec. 10, 1921.)
At fairly frequent intervals physicians receive through the mail free samples of “Iodex,” a black ointment sent out in small, circular aluminum boxes. Iodex is sold by Menley and James, Ltd., New York City, under the claim that it is a preparation of free iodin,252 minus the objectionable features that go with free iodin. The preparation was examined in the A. M. A. Chemical Laboratory in 1915, and found practically devoid of free iodin. The laboratory also reported that when 1 or 2 grams of Iodex was rubbed on the skin of the forearm on several subjects and the urine collected and tested for iodin, the results were negative. This disproved the claim that “thirty minutes after inunction [with Iodex] iodine can be found in the urine.”
The findings of the laboratory, which were summed up in a report (The Journal, June 19, 1915) of the Council on Pharmacy and Chemistry on Iodex, were essentially as follows:
1. The composition is incorrectly stated; the actual iodin content is only about half of that claimed.
2. The action of Iodex is not essentially that of free iodin, although that is the impression conveyed by the advertising.
3. The assertion that iodin may be found in the urine shortly after Iodex has been rubbed on the skin has been experimentally disproved.
At the time the laboratory reported its findings, it pointed out the obvious contradiction in the claim that Iodex is not only an “effective free iodine application without drawbacks” but also a means of “really efficient external iodine therapy without stain or irritation.” It is impossible to have free iodin present in sufficient quantities to be therapeutically efficient and not get skin stains and irritation.
In a recent issue of the house organ, Pharmacal Advance, there was a large display advertisement of Iodex under the heading: “For prophylaxis and to ‘Double Cross’ Disease,” with the claims:
“Free Iodine.”
“Rub Through Skin.”
“Does Not Irritate nor Stain.”
On other pages of the same issue these claims appeared:
“There is no therapeutic virtue in Iodex which is not inherent—though often latent—in Free Iodine; and there is no virtue in Free Iodine which is not available in Iodex.”
“In Iodex all the beneficent properties of Iodine are emphasized and all its disadvantages are eliminated—in a word, Iodex is Pure Free Iodine presented therapeutically active and efficient, ready for use in all conditions, with all the well-known powers of Free Iodine, but without the sequelæ of unpleasant effects, as irritation, corrosion, desquamation, staining, etc., which defeat the ends of treatment when ordinary preparations of Iodine are used. The fact that Free Iodine in the form of Iodex can now be used in rectal and vaginal treatment, without irritation, speaks volumes for its penetrability and bland action.”
These quotations are sufficient to show that the manufacturers of Iodex still persist in their claim that the product contains free iodin. In view of this, the A. M. A. Chemical Laboratory has again examined Iodex, having recently purchased specimens on the open market. It reports that Iodex gives no test for free iodin, or at most, but minute traces.
An interesting side-light on the methods of Menley and James is also brought out in the issue of Pharmacal Advance just quoted. Under a “department” misnamed “Book Reviews” the following appears:
“The Actions of Drugs.—Torald Sollmann, M.D. Published by W. B. Saunders Co., Philadelphia. This is a book of lectures designed for students in pharmacy and deals with the subject in plain and simple language. The author in his introduction has brought out the fact that over-counter prescribing is baneful both to the public and to the pharmacist himself. Among some of the interesting points brought out that Pharmacol Advance has always maintained, namely, that ‘Potassium iodid is not absorbed efficiently by the skin; hence the ointment of potassium iodid is unscientific.’
“We would especially call attention to Ungt. Iodi U. S. P., containing Potassium Iodid, used as a solvent for its iodin content. Accepting Sollmann’s statement, it is to be assumed that Ungt. Iodi U. S. P. has not 100 per cent. efficiency.”
Garbling statements from scientific works for the purpose of puffing proprietaries is not unusual in nostrum exploitation. The facts are that the statement in Sollmann’s book, introduced in the Menley and James house organ under the guise of a book review, appeared in a discussion of iodin compounds. In this the author points out that to obtain systemic iodid effects, it is irrational to apply iodin preparations externally. So far as the free iodin content of the official ointment of iodin is concerned, L. E. Warren (Reports of the A. M. A. Chemical Laboratory, 1917) has shown that even after more than six months this ointment still contains about 75 per cent. of the free iodin originally added. The official ointment (Unguentum Iodi, U. S. P.), therefore, so far as its free iodin content is concerned, is far superior to Iodex, which contains no iodin in its free state.—(From The Journal A. M. A., May 3, 1919.)
A number of inquiries have been received of which those that follow are typical. This from a Philadelphia physician:
“Would you give me any information you have about one so-called ‘Dr. W. S. Koch,’ Detroit, Michigan? This man is said to claim to have in his possession a cure for cancer, the nature of which I do not know. I know, however, that he obtained a very large fee not very long ago in treating a case, but without success ...”
While a Chicago physician writes:
“I have at hand a pamphlet from Wm. F. Koch, M.D., Ph.D., of Detroit, Mich., which is supposed to be a reprint from the Medical Record of Oct. 30, 1920, entitled ‘A New and Successful Diagnosis and Treatment of Cancer.’ Will you kindly advise me what you know about this man’s work on this subject and how much stock I can put in the claims he makes in this article?”
And this from a physician in Seattle, received a few days ago:
“Has your office any knowledge of the cancer cure devised by Dr. William F. Koch, Ph.D., M.D., of Detroit? He published an article on it in the Medical Record, Oct. 30, 1920.... I enclose copy of letter received by one of our patients from his ‘western representative’ which reads like pure quackery. I do not find Dr. Koch’s name in either the A. M. A. or Polk’s medical directories.”
The letter referred to in the last inquiry as coming from Dr. Koch’s “western representative” was addressed to a woman who had written to Dr. Koch with reference to his alleged cancer cure. The letter, dated Jan. 19, 1921, was signed “Chas. L. Tisdale, 1898 Geary Street, San Francisco.” It read:
“Dear Madam:—Your letter of January 10th written to Dr. Koch of Detroit in reference to his cancer cure has been sent to me by Dr. Koch. I am the western representative of Dr. Koch and am giving the treatments with his remedy. I am now treating 14 cases here with some most wonderful results. The amount of the remedy that Dr. Koch can supply me with is limited and it is a very expensive substance. None of it can be sent to Seattle or any other place for I have only enough to treat the cases that are constantly presenting themselves here. If you could come to San Francisco and have the money to pay a reasonable fee, say enough to pay for the remedy, I would be very glad to do everything I can for you.
“The results that have already shown in many of these cases warrant me in believing that almost any case of cancer can be cured if the treatment is persisted in.”
According to our records, Dr. William F. Koch of Detroit was born in 1885. Some years ago he graduated in chemistry and for some time held the position of professor of physiology and physiologic chemistry at the Detroit College of Medicine and Surgery. In 1918, Dr. Koch received his degree in medicine from this same college. Less than a year after his graduation, Dr. Koch declared that he had “developed a real specific cure for cancer.” In the Detroit Medical Journal for July, 1919, there appeared a brief article by William F. Koch, entitled “A New and Successful Treatment and Diagnosis of Cancer.” A more extensive article bearing the same title was published in the New York Medical Journal of Oct. 30, 1920.
As a result of the publicity that was given the Koch treatment, the Wayne County (Detroit) Medical Society appointed a committee to investigate the treatment. Its first report appeared in the Bulletin of the society for Dec. 22, 1919. Briefly, this report said that the Board of Health of Detroit had placed at the disposal of the committee twelve beds in a local hospital with the necessary special nurses and everything else required free of charge. The committee sent certain patients to the hospital; and there were also some other patients recommended by different physicians as proper cases for treatment. There were nine altogether. After going over the cases carefully, the committee found some in which the diagnosis was doubtful. There were five cases, however, of undoubted cancer, a positive diagnosis having been made from specimens and microscopic examination. The management and treatment of these patients were turned over to Dr. Koch.
Dr. Koch seems to have raised certain objections and to have made certain criticisms. He also insisted that he ought to have some representative on the committee. The committee offered to put on any and all he would name. He failed to name any. The committee reported further that Dr. Koch was very negligent in his treatment of the patients and finally, on November 26, the committee met with Koch and went over all the cases with him. At that time he gave the patients injections and promised to attend to the treatment regularly in the future. According to the report, he saw the patients only once more (three days later) and then did not come near them again. As the patients became disgusted with the neglect, some of them left and the committee sent the rest home and closed its connections with the investigation of the subject.
In the same issue of the Bulletin of the county society in which this committee’s report was published, the editor of the Bulletin stated that from all sections of the country inquiries were coming relative to the treatment and “from long distances patients are coming to Detroit to be ‘cured’ of cancer.” The editor further stated: “It is reported that Dr. Koch is treating many patients, promising much and charging well.” To this Dr. Koch retorted that only about 30 per cent. of his patients had “contributed.” The rest were treated free.
The Wayne County Medical Society Bulletin for Jan. 5, 1920, was devoted almost exclusively to another discussion of Dr. Koch’s “cancer cure.” It was there stated that a second committee had been appointed to gather what information could be obtained from outside sources relative to cases treated by Dr. Koch. This committee reported that of fifty-six cases of which it was able to obtain data, only three of the patients showed clinical improvement; twenty-one of the patients were dead. Three more patients treated both by the Koch injections and by operation were reported as clinically improved. The condition of eighteen of the patients was reported as stationary, or unimproved. In eleven of the cases, the results were unknown but the surgeons reported unfavorably.
The committee reported further that Dr. Koch’s records were incomplete and that he had submitted no proof that his injections have any particular merit and the committee concluded that the study “is entirely experimental and improperly supervised.”
Evidently, the most that can be said of Dr. Koch’s alleged “cure” for cancer is that the claims made for it have not been supported by independent investigators.—(From The Journal A. M. A., Feb. 12, 1921.)
Last week some space was given to the alleged cure for cancer put out by Dr. William F. Koch of Detroit. Incidentally, it should be mentioned that Dr. Koch’s article of Oct. 30, 1920, to which reference was made, appeared not in the New York Medical Journal, as stated, but in the New York Medical Record.
The following correspondence throws additional light on the subject:
To the Editor:—To the number of inquiries which you have received regarding the alleged cure of cancer by Dr. Koch, permit me to add the following personal experience. On July 1, 1920, I was asked to examine an ex-patient of mine whom I had not seen professionally for many years. Her husband frankly told me that for several months his wife had been treated by Dr. W. F. Koch for inoperable carcinoma of the pelvic organs, that he wished Dr. Koch to retain charge of the treatment but hoped I would give my opinion regarding certain nervous manifestations in the patient which were causing him (her husband) much concern.
At the same time, he showed me a letter written by Dr. Koch purporting to explain the symptoms and offering suggestions regarding treatment. I called on the patient and found her in the last stages of generalized carcinomatosis. Simple palpation of the abdomen revealed multiple nodules involving both lower and upper abdominal quadrants. I did not feel justified in making a pelvic examination but noted a profuse foul-smelling discharge on the vulvar pad. My prognosis did not meet with the deluded husband’s approval. The patient died within a week and a necropsy confirmed the clinical picture of carcinomatosis. Enclosed is Dr. Koch’s letter; the patient’s name should, of course, be omitted if you see fit to publish this note.
George de Tarnowsky, M.D., Chicago.
The letter from Dr. Koch which Dr. de Tarnowsky enclosed with his own, follows. We have, of course, deleted the name of the patient.
Dear Doctor: Mrs. —— has absorbed and is still absorbing some killed tumor tissue. She has absorbed some three pounds, I judge. The results of the absorption are intoxication quite general (nervous, muscular, perhaps nephritic). The myocardium at present shows no signs of poisoning but the skeletal muscles and nerve do. The important toxin liberated by the killed tissue is methyl cyanimide which combines ammonia (NH2){sic} from the amino acids, and thus becomes methyl guanidine. This latter has produced in my patients an intoxication varying in similarity to: idiopathic tetany in children, chorea in children, eclampsia in women, and has even been so severe as tetanus in some of the muscle spasms; a toxic albuminuria has resulted in some of my cases.
All of my cases have cleaned up so far. Of course, I cannot predict in any individual case, except that when the absorption has been completed and the toxin all eliminated, everything should return to normal, unless the toxin has destroyed tissue beyond physiological repair. My suggestions as to treatment would be elimination, saving the kidneys as much as possible, by whatever methods you find best and necessary.
At present I am treating symptomatically thus—atropin as a guanidine antidote, arsenic as a chorea coupled antidote as a prevention to the production of guanidine from the cyanimide, the use of dilute hydrochloric acid has proven successful to me. Even a urine boiling solid—albumen has cleared up in one case in three days just by taking large quantities of 1⁄2 per cent HCl. I am explaining the factors I have contended with in these cases, but do not want to influence your plan of treatment when your judgment finds me insufficient.
Sincerely,
Wm. F. Koch.
I shall have a publication out very soon on the treatment of these tetanics and eclampsia with HCl.
It is worth noting that this letter of Dr. Koch’s was written June 28, just three days before Dr. de Tarnowsky saw Mrs. —— and less than a week before she died of generalized carcinoma.
Not the least important element in the story which these two letters tell is the optimism engendered in the husband of the poor cancer patient by the widely vaunted treatment of Koch. And herein lies one of the most pernicious features connected with the exploitation of alleged cures for cancer, tuberculosis, etc. All such remedies, whether fraudulent both in their inception and exploitation or those which while equally worthless are at least honestly put forward and are based on a certain amount of scientific investigation, produce a profound and marked temporary change in the patient’s condition. It is this that tends to warp the judgment not only of the unscientific layman, but also of the physician. The psychic element in cancer has been well described by Weil:
“It is, indeed, very remarkable that a patient who has been consigned to death as a victim of a hopeless malady, should regain his spirits and his appetite, when he is again confronted with the hope of a cure, and of the eradication of his disease? It is a phenomenon well known to every student of the disease that a large proportion of cases responds in just this manner to any treatment which is offered them. Osler has described a case of cancer of the stomach in which the mere visit to a consultant of sanguine temperament, though poor judgment, whose assurance of the patient that there was no possibility of cancer, resulted in the disappearance of all the symptoms and a gain of 18 pounds in weight. It is this psychic influence, which has occasionally deluded the honest student of cancer cure, and which has also so generously played into the hands of the dishonest.”—(From The Journal A. M. A., Feb. 19, 1921.)
The Journal has received several inquiries about the products put out by the Lucas Laboratories, Incorporated, of New York City. A typical inquiry is that received from Dr. F. A. Jewett of Brooklyn, who writes:
“The enclosed circular is sent out to the medical profession by Dr. William Lucas, 287 W. 70th St., New York. What do you know of this man and his methods?”
William H. Lucas was graduated by the Medical College of Ohio in 1895 and was licensed in 1897. He is not a member of his local medical society. The products put out by the Lucas Laboratories are for intravenous use, and their method of exploitation indicates that the concern is less interested in the science of therapeutics than it is in taking commercial advantage of the present fad for intravenous medication. The Journal has protested editorially against the unnecessary use of the intravenous administration of drugs, and the abuse of this method of drug giving prompted the Council on Pharmacy and Chemistry recently to emphasize the danger of indiscriminate intravenous medication.
The products of the Lucas Laboratories, Inc., have not been examined either by the A. M. A. Chemical Laboratory or by the Council on Pharmacy and Chemistry. The composition of these products is essentially secret, which in itself should be sufficient to deter physicians from using them. Of course, in accordance with all the tenents of orthodox nostrum exploitation, “formulas” are furnished. Even the crude hieroglyphics that used to be palmed off on the medical profession by nostrum exploiters under the guise of “graphic formulas” are outdone by the Lucas Laboratories in publishing the alleged formulas of its preparations. If we, as physicians, knew more chemistry, the Lucas Laboratories would not find it profitable to publish such ineffable nonsense as that which characterizes their “literature.” For instance:
“ ‘Luvein’ Arsans (Plain)” is said to be: “Di hypo sodio calcio phosphite hydroxy arseno mercuric iodid.” The first part of this “formula” might stand for sodium and calcium hypophosphite. The remainder is meaningless except that it suggests (but does not insure) the presence of arsenic and mercury iodide.
“ ‘Luvein’ Arsans, Nos. 1, 2 and 3.”—“Meta hydroxy iodide sodio arsano mercuric dimethyl benzo sodio arsenate, ai oxy sodio tartaria sulpho disheuyl hydrazin.” Who can venture even a conjecture as to the possible significance of this?
“ ‘Luvein’ Creosophite.”—“Ammonio hydroxy calcio sodio hypo-phosphite arsenous pentoxy iodide.” While the name suggests creosote, the “formula” gives no hint of this. It might refer to hypophosphites of ammonium, calcium and sodium with iodide of arsenic. Whether arsenous (trivalent arsenic) or arsenic (pentavalent arsenic) iodide or both are intended, is a question.
“ ‘Luvein’ Hexacol.”—“Hexa methylenepyro catechin mono methyl amino ether glycerite.” By moving these syllables around like the old “fifteen puzzle” they can be arranged to represent hexamethylenamin and monomethyl-ether of pyrocatechin, or guaiacol, having the “glycerite” left over.
It is futile to discuss the therapeutic claims made for the various preparations put out by the Lucas Laboratories. One might as profitably discuss the therapeutic claims made for “Peruna” or “Paine’s Celery Compound” for the exploitation of the latter products is on just as high a scientific plane as the exploitation of the “Luvein” nostrums. The proposition offered to physicians by the Lucas Laboratories, Inc., is an insult to the intelligence of the medical profession. Not that the products themselves are necessarily any worse or any better than many offered for intravenous use; the selling methods are more crude, that is all.
The facts are, we have entered a new cycle of nostrum development. The unscientific mixtures for oral administration that characterized so large and disreputable a part of the proprietary medicine business of the past two or three decades are giving way to equally unscientific mixtures for intravenous use. The dangers of the older nostrums are accentuated in the newer by the added element of risk that is inseparable from intravenous therapy. Add to this the temptation to the physician in the way of more substantial fees which, legitimately enough, may be charged when intravenous administration is called for, and the menace of the new style nostrum becomes evident. The Journal can only reiterate the warning that intravenous therapy should be employed only when most positively indicated. Further, because of the danger that is inseparable from this method of drug administration, physicians should be doubly careful to see that products employed for intravenous use come from firms of unquestioned scientific standing.—(From The Journal A. M. A., Sept. 20, 1919.)
A physician in Florida writes:
“I am enclosing a copy of a circular letter just received from Parke, Davis & Company, and will call your attention to a marked paragraph in this letter on which I would like to have an expression of your opinion.”
The circular letter which the doctor forwards is devoted to singing the praises of “Pneumonia Phylacogen.” It opens with the statement: “Influenza, we learn, has appeared in your section.” The paragraph marked by our correspondent reads:
“Pneumonia Phylacogen has been found to be a dependable means of preventing and treating pneumonic complications of Influenza. In one large city it became a routine measure to give all persons affected with Influenza an injection of Pneumonia Phylacogen as a prophylactic of pneumonia. The results were remarkable. Not only did the cases improve rapidly, but in a great majority of them the pneumonia did not occur.”
The “Phylacogens” were repeatedly discussed in The Journal during 1913 and 1914 when these products were being pushed with much vigor by the manufacturers. We know of no evidence that calls for a revision of the statements then made regarding them. The injection of phylacogens is simply the administration of a mixture of the filtered products of several bacterial species. The results which follow represent the reaction of the bacterial protein—a reaction for good or evil. There is no scientific evidence to show that they possess any specific prophylactic virtue. To recommend their use in cases of influenza, as a prophylactic against pneumonia, is unwarranted, and the physician who acts on the advice of the manufacturer must assume the responsibility for the results. In case of mishap he cannot fall back on the manufacturer; he will find no scientific evidence to support him.—(From The Journal A. M. A., Nov. 15, 1919.)
To the Editor:—Enclosed is a postal card which a physician in Oklahoma has sent me together with thirty-six cents in stamps. The envelop was addressed to me at the address of the Pineoleum Company. The postoffice corrected the address and sent it to me. It is evident, therefore, that the physician in Oklahoma thought I was sending these postals as an employee of the Pineoleum Company, or, at least, was endorsing their products.

Kindly do me the favor to publish this letter in The Journal as a protest against the dishonesty of this method of advertising. What is quoted from an article that I wrote appeared originally in the New York State Journal of Medicine and was abstracted in The Journal of the American Medical Association of August 2, 1919. The obvious inference to be drawn from this postal is that I referred to the products of the Pineoleum Company in that article. I did not have the products of the Pineoleum Company in my mind. I never have used their products and never prescribed them.
This form of advertising is done with intent to deceive and did deceive the doctor in Oklahoma. It was therefore a successful falsehood, its success depending on the false use of the name of the President of the American Medical Association to bolster up the sale of the product.
I resent the use of my name in connection with the quack advertising of nostrum venders. The low, vulpine cunning of the method used is on the same level as the deceit and dishonesty which use this form of advertising to the injury of my name and reputation. As President of the American Medical Association I must insist that you protect me by publishing this letter in The Journal, giving it as widespread publicity as possible.
Alexander Lambert.
[Comment.—“Pineoleum” is a “patent medicine” advertised in the cheapest and most effective way—by the aid of the easy going and complacent physician. In 1906 Pineoleum was being marketed by the Winslow Laboratory of New York City, which also put out three or four other nostrums—“Morumalt,” “Egeriol,” “Digestylin,” and “Ford’s Nucleo-Peptone.” Pineoleum was advertised to the public then as it is advertised now, via the medical profession. Physicians are circularized and are offered a petty graft in the form of a cheap nebulizer and a sample bottle of Pineoleum. Some time ago the company seems to have developed a scheme whereby physicians could make money “dispensing Pineoleum nebulizer outfits at more than 140 per cent. profit.” The Pineoleum concern for years has also polluted the stream at its source by attempting to get the secretary of the senior class of every medical school to distribute its free nebulizer outfits to members of the class and receive therefor 5 cents for each outfit distributed! The life history of Pineoleum is that of the typical nostrum. Epidemics, of course, are utilized as opportunities for pushing the product. In 1911 a card was sent out featuring “A Special LaGrippe Offer”; in 1916 the profession was circularized recommending Pineoleum as “The Ideal Prophylactic” in infantile paralysis; during the past year influenza has again been the selling point.
The case described by Dr. Lambert is not the first example of the misuse of names and statements of physicians. Last December the Pineoleum concern was sending out an advertising card in which Dr. McCoy of the United States Public Health Service was quoted as recommending Pineoleum as the “bulwark of prevention” and “battery of relief” in influenza. Of course, Dr. McCoy never said anything of the sort. A protest against this particular falsehood resulted in another card being sent out several months later by the Pineoleum people purporting to explain and apologize for the misquotations and putting the blame on the printer. The “apology” ended with a postscript (in larger and bolder face type than the body of the card) that urged physicians to “secure our liberal introductory advertising proposition on improved oil nebulizer outfits.” From the standpoint of publicity for Pineoleum, the “explanation and apology” was doubtless as good an advertisement as the original card of misrepresentation.—Ed.]—(From The Journal A. M. A., Nov. 1, 1919.)
To the Editor:—Will you please advise as to the success and safeness in using the Proteal treatment for tuberculosis by Henry Smith Williams, M.D., LL.D., 104 East 40th Street, New York?
C. P. Burchard, Alamogordo, N. M.
To the Editor:—Kindly send me any available information on “The Proteal Treatment for Cancer.” An article by Dr. Henry Smith Williams, 120 West 32 Street, New York City, in April Hearst’s has caused relatives to request its use in a case of carcinoma of the liver under my care.
M. M. Reppard, Middlebourne, W. Va.
To the Editor:—I am enclosing a leaflet, mailed to me on request, by Dr. Henry Smith Williams of New York City, who published a series of articles during the last year in Hearst’s Magazine on “Proteal Therapy.” If you have investigated this man and his proteal treatment, I should like to know the result of your findings. I am a consumptive and am, therefore, particularly interested in its alleged benefactions for the treatment of tuberculosis.
Michael A. Long, Glen Lake Sanitarium, Hopkins, Minn.
To the Editor:—What information can you give me regarding Henry Smith Williams, M.D., LL.D., 104 East Fortieth Street, New York, and the therapeutic value of the “Proteal Therapy” that he has originated?
M. D. Baker, M.D., San Jose, Calif.
The above letters are selected from many received on the subject. Henry Smith Williams is better known in the journalistic world than in the field of scientific medicine. He was graduated by the Chicago Medical College in 1884. In the thirteen issues of medical directories of the United States that have been published during the past thirty years Dr. Williams’ name does not appear—except for the issues of 1890 and 1893—until the 1914 edition. So far as we have been able to find, Dr. Williams had not until 1915 contributed any articles to medical journals. The catalog of the Surgeon General’s Library contains no reference to any articles of Dr. Williams except those that have appeared in popular magazines. The volumes of the Index Medicus from 1907 until 1914, inclusive, also contain no references to any articles by him in medical journals. The Journal‘s author index to current medical literature from 1900 to 1914, inclusive, fails to record any articles by Dr. Williams in medical journals. Dr. Williams’ articles, however, in popular magazines have been voluminous and numerous. Sometimes his articles have been under his own name and sometimes under the nom de plume, “Stoddard Goodhue, M.D.” Under the latter name the Cosmopolitan published articles on “Adding Years to Your Life,” “Battle of the Microbes,” “Do You Choose Your Children?” and “What is the Matter With Your Brain?” Under his own name articles have appeared in popular magazines on such subjects as “Burbank’s Way with Flowers,” “Every Woman Her Own Burbank,” “Why Not Live Forever?” “Science of Breeding Kings,” “New Cancer Treatment” and “New Hope for Rheumatism Sufferers.” In addition, Dr. Williams has published books on such subjects as “History of the Art of Writing,” “Historians’ History of the World,” “Story of Nineteenth Century Science,” “Luther Burbank,” “Twilight Sleep” and others. The Goodhue Company of New York City, which publishes some of Dr. Williams’ books has, we understand, for its president, Dr. Henry Smith Williams, for its vice president, Dr. Williams’ wife, and for its secretary-treasurer, Dr. Williams’ daughter.
Readers of The Journal will remember the publicity given in 1915 and 1916 to an alleged treatment for cancer, sometimes called the “Horowitz-Beebe Autolysin Treatment.” The method was heralded widely both in a certain portion of the medical press and in popular magazines and newspapers. A popular article by Henry Smith Williams on “The New Cancer Treatment” appeared in the Illustrated World for October, 1915, with pictures of Dr. Horowitz, Dr. Beebe, etc. A month or two later, physicians received, gratis, from the Goodhue Company a neatly bound little book on “Alcohol Hygiene and Legislation,” by E. H. Williams, M.D. (brother of Henry Smith Williams). Enclosed with it was a letter from the Goodhue Company asking physicians to accept the book. The body of the letter was devoted to calling the attention of physicians to an “important work” by Dr. Henry Smith Williams on “The Autolysin Treatment of Cancer” that the Goodhue Company was publishing. With the letter, there was a small advertising pamphlet “Issued by the Autolysin Laboratory” and advertising that product. In addition, the last thirteen pages of the book on “Alcohol Hygiene” contained advertisements of the Goodhue Company’s publications with particular emphasis (four pages of it) on the “Autolysin Treatment of Cancer,” by Henry Smith Williams.
In May, 1917, physicians in the West received a letter from the “Ellison-White Chautauqua System” informing them that Dr. Henry Smith Williams was to lecture at “your Chautauqua” and reminding them that “he has recently issued two volumes, ‘The Autolysin Treatment of Cancer’ which he believes will be his greatest contribution to medical science.” The present “Proteal” treatment appears to be a modification of the “Autolysin” treatment. Dr. Williams, in attempting to justify the use of his “Proteal” in tuberculosis, cancer, rheumatism, etc., takes advantage of certain investigations bearing on the nonspecific reactions resulting from the parental injection of foreign proteins. So far as we can discover, there is no scientific evidence to indicate that the “Proteal” treatment expounded by Williams is of value in the treatment of cancer, tuberculosis or the other numerous diseases for which the “Proteals” are recommended.
It is a question whether such articles as those on “The Proteal Treatment of Cancer,” “New Hope for Rheumatism Sufferers,” etc., published in popular magazines or newspapers serve any useful public purpose. May they not, on the contrary, by raising false hopes, cause much mental suffering and do scientific medicine great harm?—(From The Journal A. M. A., July 6, 1918.)
A report of the Council on Pharmacy and Chemistry that appears elsewhere253 in this book deals with another attempt to foist on our profession a series of essentially secret preparations whose therapeutic value has not been scientifically demonstrated. Grotesquely extravagant claims are advanced as to the therapeutic potency and range of action of substances of whose nature and effects we have no trustworthy information. Physicians are advised to use—and many undoubtedly are using—these alleged remedies in the treatment of diseases in which delay in the proper kind of treatment may be of the greatest danger to the patient. As stated, there is available no reliable information regarding the effects of these substances when they are introduced in the human body. They may have no effect whatever, or they may produce more or less direct injury; in either case, there is the chance that damage, even irreparable to the patient, may result because rational treatment is withheld.
If we accept the statement that the preparations are largely vegetable proteins, it is a fair inference that, under certain conditions, they may cause a febrile reaction of the same general nature as that caused by other foreign proteins when injected into the body. We know that such reactions are not without danger and that the treatment of certain infections by induced reactions to foreign proteins is strictly an experimental procedure to be undertaken only under very special conditions. There is, therefore, no known valid reason why a physician should assume the responsibility for using these alleged remedies in the treatment of his patients; there is a very obvious reason why he should not—the therapeutic instructions of “the House of Merrell, always interested in the progress of plant therapy” to the contrary notwithstanding. It is the old story of exploiting physicians through commercial pseudoscience; of trading on the credulity of the profession to the detriment of the public. As Osler254 recently protested so vigorously:
Some time ago a pamphlet came from X and Company, characterized by brazen therapeutic impudence, and indicating a supreme indifference to anything that could be called intelligence on the part of the recipients. That these firms [manufacturing pharmacists] have the audacity to issue such trash indicates the state of thraldom in which they regard us. And I would protest against the usurpation on the part of these men of our function as teachers. Why, for example, should Y and Company write as if they were directors of large genito-urinary clinics instead of manufacturing pharmacists? It is none of their business what is the best treatment for gonorrhea—by what possibility could they ever know it, and why should their literature pretend to the combined wisdom of Neisser and Guyon? What right have Z and Company to send on a card directions for the treatment of anemia and dyspepsia, about which subjects they know as much as an unborn babe, and, if they stick to their legitimate business, about the same opportunity of getting information? For years the profession has been exploited in this way, until the evil has become unbearable, and we need as active a crusade against the pseudoscience in the profession as has been waged of late against the use of quack medicines by the public. We have been altogether too submissive, and have gradually allowed those who should be our willing helpers to dictate terms and to play the rôle of masters. Far too large a section of the treatment of disease is today controlled by the big manufacturing pharmacists, who have enslaved us in a plausible pseudoscience.
What shall the profession do to protect itself against this humiliation—to throw off the credulity that extols pseudoscience and makes commercialized empiricism financially profitable? Osler says the remedy is obvious: “Give our students a firsthand acquaintance with disease, and give them a thorough practical knowledge of the great drugs, and we will send out independent, clear-headed, cautious practitioners who will do their own thinking and be no longer at the mercy of the meretricious literature, which has sapped our independence.” Excellent! But must humanity wait a generation? Why not stop this evil at once? The American Medical Association has provided the means whereby this can be done, if physicians will only make use of it—the Council on Pharmacy and Chemistry.—(Editorial from The Journal A. M. A., July 12, 1919.)
To the Editor:—I note in the issue of The Journal for July 12, a statement regarding the so-called “Proteogens” manufactured by the Wm. S. Merrell Company of Cincinnati.
My attention has been called to the fact that salesmen of this company have been exhibiting a letter purporting to show that this department has endorsed their products in the treatment of venereal diseases. The letter in question was written by a physician employed in one of the clinics conducted jointly by this department and the U. S. Public Health Service, and the stationery of the department was used without authority. The physician in question has made numerous efforts to recall the letter, but the Merrell people profess an inability to control its use.
I need not add that this department has not endorsed and will not endorse these products, and has no evidence that they are of any value whatsoever.
Allen W. Freeman, M.D., Commissioner of Health,
State of Ohio, State Department of Health.
—(Correspondence in The Journal A. M. A., July 26, 1919.)
To the Editor:—Allow us to direct your attention to several misstatements which appear in the letter signed, “Allen W. Freeman, M.D., Commissioner of Health, State of Ohio,” published in The Journal of the American Medical Association for July 26.
1. Salesmen of this company have not been exhibiting a “letter purporting to show that this department has endorsed their products in the treatment of venereal diseases,” as stated by Dr. Freeman.
2. The author of the letter has not “made numerous efforts to recall the letter, but the Merrell people profess an inability to control its use,” as stated by Dr. Freeman.
A physician employed in one of the clinics used our Proteogens Nos. 10 and 11 extensively and is still using them to a large extent in his private practice. He is a man of standing in the community in which he practices and is also a professor in one of the leading medical colleges in the state.
The letter in question cites the case of a man who had been under treatment for three years with 606, 914 and most of the other treatments in general use, and on August 31, a year ago, still gave a Wassermann test plus 4. He was given Proteogen No. 10, and by the middle of December the Wassermann was negative and the man was discharged as cured.
While this letter was written on the stationery of the Bureau of Venereal Diseases of the Department of Health, State of Ohio, it was written in the first person, and made no pretension in any way to being official nor was any such pretense made or authorized by the Merrell Company.
The author of the letter has not made “numerous efforts to recall the letter,” nor has the Merrell Company “professed an inability to control its use.”
The physician did ask that the letter be returned to him, and his request was complied with promptly.
[Then follows the full text of the letter in question. As its contents have no bearing on the question under discussion, it is omitted.—Ed.]
In over ninety-one years of honorable service as manufacturers of medicinal preparations, the Wm. S. Merrell Company has never endeavored to advance its interests through misrepresentation.
The Wm. S. Merrell Company,
Chas. G. Merrell, Pres.
[The letter above was submitted to Dr. Allen W. Freeman, Commissioner of Health of the State of Ohio. Dr. Freeman’s comments appear below.—Ed.]
To the Editor:—The plain issue of veracity raised in the communication of the Merrell Company must be settled on the evidence, which is unfortunately too voluminous to be published in full in The Journal. Copies of the correspondence in the case have been furnished the editor, and the originals are on file in the office of the state department of health in Columbus.
1. Whether or not the photographic reproduction of a letter written on the letter head of this department, and the distribution of copies to salesmen for display to physicians, was a conscious effort on the part of the firm in question to create the impression that the letter was an official one is perhaps a matter of inference. That it did create such an impression is evidenced by the letters of inquiry received from physicians who saw it.
2. The statement that the Merrell Company refused to return the letter is perhaps erroneous. They did apparently return the original letter but not the photographic copies which had been distributed to their salesmen. On May 22 the firm wrote as follows:
“A number of physicians who are in cooperation with both state and national bureaus of venereal diseases have been using our Proteogens with marked success and there are doubtless many letters carried by our salesmen—reports from some of these physicians.”
This was interpreted to mean that the firm had no method of knowing what letters were carried by their salesmen and was not responsible for them. Whether or not this interpretation is correct is again, perhaps, a matter of opinion.
The purpose of the original communication was to make plain to those of the profession who have already seen or might subsequently see the letter referred to that the communication was the expression of an individual and not of the Department.
A. W. Freeman, Commissioner.
—(Correspondence in The Journal A. M. A., Sept. 6, 1919.)
Our readers will remember the recent correspondence published in The Journal of July 26 and September 6, by Dr. A. W. Freeman, Commissioner of Health of the State of Ohio and the Wm. S. Merrell Co. The letters dealt with the use that had been made by the Wm. S. Merrell Co. of a letter, written on the official stationery of the Bureau of Venereal Diseases of the State Department of Health of Ohio, puffing one of the company’s proprietary remedies—Proteogen No. 10.
Dr. Freeman wrote to The Journal calling the attention of the profession to the use of this letter and explaining that the letter was merely the expression of opinion of an individual, and not an expression from the State Department of Health. The Wm. S. Merrell Co. took exception to certain inferences made in Dr. Freeman’s letter and in the course of a letter to The Journal regarding this, incorporated the contents of the testimonial letter. The Journal, in publishing the Merrell letter, omitted this testimonial on the ground that the contents of the letter had no bearing on the question under discussion.
We have now received a letter from the company protesting against this omission. The Journal, therefore, takes this opportunity of briefly restating such facts as it has been able to get regarding the entire matter and publishing the letter. The facts are as follows:
1. In February of this year a Cincinnati physician, Dr. C. J. Broeman, wrote to Dr. A. S. Horovitz relative to alleged results with Proteogen No. 10. The letter was written—without authority—on the official stationery of the Bureau of Venereal Diseases of the State Department of Health of Ohio.
2. The Wm. S. Merrell Co. had linen mounted photographs made of Dr. Broeman’s letter and distributed them to their Proteogen detail men. Accompanying these photographic copies was a communication to these detail men describing the photographed letter as one written by:
“... a Cincinnati physician who is now Acting Assistant Surgeon, U. S. Public Health Service, cooperating with the Bureau of Venereal Diseases of the Department of Health of the State of Ohio.”
3. The right hand top corner of the official stationery, as can be seen by the reproduction, bore the name of “James D. Bauman, Deputy Commissioner.” Dr. Broeman’s signature was rather illegible and could easily be mistaken, by those not knowing the handwriting of either man, for the signature of Deputy Commissioner Bauman. In at least one instance it was so mistaken, and the physician who was misled wrote to the Director of the Bureau asking whether the testimonial for Proteogen No. 10 which had been shown him by the Merrell detail man was really an official communication.
4. On May 15, 1919, Commissioner of Health Freeman wrote to the Merrell Co. stating that he had been informed that one of the Merrell representatives was using as an advertisement a letter bearing the letterhead of the Bureau of Venereal Diseases of the State Department of Health and what purported to be a report signed by “Mr. Bauman, Deputy Commissioner.”
5. On May 19, the Wm. S. Merrell Co. wrote Dr. Freeman that he was certainly mistaken in regard to the use of any “report signed by Mr. Bauman.” Dr. Freeman then sent to the company the letter he had received from the physician who had mistaken Broeman’s letter for an official letter by Bauman. Although it would seem that this letter and Commissioner Freeman’s protest should have made plain to the Wm. S. Merrell Co., the fact that the letter, incorrectly referred to as Mr. Bauman’s, was in reality Dr. Broeman’s, the company remained silent regarding its use of the Broeman letter and, on May 22, merely reiterated that there had been “no letter circulated by this company containing a testimonial of your Mr. Bauman.” On May 28 (six days later) the Merrell company sent to its Proteogen detail men another general letter, “for personal use of agents,” in which it again called their attention to the “photographic copy mounted on linen” of Dr. Broeman’s letter. This communication to the detail men also declared that it “has been suggested that the further use of Dr. Broeman’s letter might antagonize the State Department of Health” and, therefore the detail men were told to “discontinue using the photographic copy in question” and to return the photographs to the head office.

Here, briefly are the bald facts in the case. The essential point at issue is whether these photographic copies of Dr. Broeman’s letter would or would not be likely—whether or not they were so intended—to mislead physicians into believing that the endorsement was an official one by the State Board of Health rather than an individual one. One can but wonder why, if, as the Merrell company so vehemently asserts, there was no intention of misleading physicians on this point, the company should have gone to the trouble and expense of photographing the entire letter, including the letterhead, rather than making typewritten or mimeographed copies of the contents of the letter.—(From The Journal A. M. A., Sept. 27, 1919.)
To the Editor:—In the September 27 issue of The Journal my name was mentioned in connection with the Merrell Chemical Company’s “Proteogens” in the treatment of syphilis. The Merrell Chemical Company promised not to use my name at any time in connection with their “Proteogens” injection and they know that the use of my name has been distinctly against my wishes. I feel that in justice to myself, as well as the public, I should report the result of my experiments with their “Proteogens” in private practice.
In explanation I might say that I began the use of their “Proteogens” in April, 1918, and I feel that I now have enough data to give a complete report. I might say that all my results have been practically nil; particularly is this true in my cases of syphilis, which all had a four plus Wassermann reaction when I discontinued using this form of treatment.
Very truly yours,
C. J. Broeman, M.D., Cincinnati.
—(Correspondence in The Journal A. M. A., Oct. 11, 1919.)
In a twelve-page pamphlet, sent out by the Pulvane Laboratories, Inc., of Des Moines, Iowa, and purporting to deal with “The Therapy of Pulvane, an advanced method for the treatment of Respiratory Diseases,” we are told that Pulvane “was developed in a United States Army General Hospital by officers of the Medical Department.”
Pulvane “originally was intended only for its germicidal action upon tubercle bacilli in the lung,” but it is now also recommended for asthma, hay fever, bronchitis, rhinitis, laryngitis and “other affections of the air passages.” Of the alleged action of Pulvane on tuberculosis we read:
“It destroys the spores of the bacilli as well as the germs themselves. It prevents infection of new areas by aspiration, gravity or surface contact.
“In cases where sputum is positive it is a very noteworthy fact that shortly after treatment is begun, the bacilli begin to disappear, gradually diminish in number, and finally the sputum becomes negative.”
Pulvane is administered, by inhalation, at the offices of the Pulvane Laboratories, Inc. Its “discoverer” chanced on a method of “introducing into solution and volatizing a certain germicide, extremely rare in its usage because of its resistance heretofore to attempts to bend it to scientific will.” This “rare” medicament is alpha naphthol! But since the discovery of this volatizing method “three other ingredients of high therapeutic value have been added.” What are these other ingredients?
“They would be named were it not that Pulvane requires special technique in its preparation and administration. Our medical directors do not consider it advisable to identify them here because of the possibility of incompetent hands attempting their use. The medical directors, however, will be glad to name every ingredient of Pulvane for any reputable member of the profession. Pulvane Laboratories reserve only the method of compounding.”
Presumably, therefore, if physicians desire to know what Pulvane is, the Pulvane Laboratories, Inc., “will be glad to name every ingredient of Pulvane.” It is worth noting that nothing is said about quantities. It is also worth remembering that “Peruna” and some other “patient medicines” have for years printed on the label the names of the alleged ingredients. How much longer is the medical profession going to be fooled with the trick of nostrum exploiters pretending a frankness that means nothing?
From a recent issue of a Des Moines newspaper we learn that the Pulvane Laboratories are about to establish a sanatorium where the Pulvane treatment can be given. This announcement is said to be made by John P. Mosher, the alleged discoverer of Pulvane. Mosher is not a physician. The newspaper article states, further, that Mosher’s experiments were tried out “under the observation of Major Sharpe,” commander at Fort Des Moines. It appears also that an ex-newspaper reporter is connected with the Pulvane Laboratories. The value of having a good publicity man is obviously recognized. There also seems to be connected with the concern a Dr. Harry P. Hall. We find in the records reference to one Harry P. Hall who was graduated by the Medical Department of Drake University of Des Moines, Iowa, in 1894, and was licensed in Iowa in 1896. Our records indicate that he has not been in practice for some years. We also find in our files some newspaper clippings regarding a Dr. Harry P. Hall who, in 1914, pleaded guilty to a charge of using the mails to defraud and was fined in the federal courts. Whether there is any connection between these two names, we do not know.
Reverting to the claims made by the Pulvane Laboratories that Pulvane was “developed in a United States Army General Hospital by officers of the Medical Department” the following statement has recently been received by The Journal from Surgeon-General Ireland of the United States Army:
“It has been brought to my attention that a concern in Des Moines, Iowa, known as the Pulvane Laboratories, has issued a pamphlet in which statements are made which would naturally lead medical men to believe that the experiments, etc., referred to therein were made with the approval of and more or less under the direction of the Medical Department of the Army. I wish to say that this is not so; that the Medical Department had nothing whatever to do with the matter and that it thoroughly disapproves of the methods used by the promoters of this concern.—(From The Journal A. M. A., March 11, 1922.)
Sal Hepatica is a saline laxative sold by the Bristol-Myers Company of New York. Little information is given, or, apparently, ever has been given, concerning the composition of this product. Many years ago the stock medical journal advertisement contained this statement:
“Composition.—Sal Hepatica contains all of the Tonic, Alterative and Laxative Salts of the celebrated ‘Bitter Waters’ of Europe, especially those of Bohemia, as determined by actual chemical analysis of these waters, and fortified by the addition of Lithium and Sodium Phosphates.”255
Sal Hepatica no longer “contains all the tonic, alterative and laxative salts ...,” etc., for the label on a package recently purchased reads:
“Sal Hepatica is an effervescent saline combination possessing medicinal properties similar to the natural ‘Bitter Waters’ of Europe, and fortified by the addition of Sodium Phosphate.”
In 1909, the Druggists Circular published an analysis of Sal Hepatica which showed that the preparation contained only 0.04 per cent. of lithium phosphate. By referring to the two quotations just given it will be noticed that today the manufacturers make no claim that their preparation is fortified with any salt of lithium. A circular accompanying recent trade packages states:
“Sal Hepatica is composed solely of harmless salts, being absolutely free from Acetanilid, Phenacetin, Caffein, Calomel, opium or coal tar derivatives.”
Since neither the names nor the amounts of the “harmless salts” are mentioned, the composition of Sal Hepatica is secret. It is a trick of the nostrum exploiter, old but ever popular, to mention numerous drugs which his preparation does not contain; it helps to distract attention from the fact that he does not tell what the preparation does contain!
In the old-time medical journal advertisements, one reads, “Sal Hepatica is the most powerful solvent of Uric Acid known.” (The same advertisement as it appeared in those days in The Journal shows that claim toned down to, “Sal Hepatica is a powerful solvent of Uric Acid.”) In those easy going days, the Bristol-Myers Company declared that “diabetes is treated with decided advantage by means of Sal Hepatica ... it ... possesses the property of arresting the secretion of sugar in the liver.” In the old days, too, Sal Hepatica was recommended in the treatment of cirrhosis of the liver, Bright’s disease, gravel, phthisis, etc.
The present advertising circular recommends Sal Hepatica as an eliminant, laxative or cathartic in gout, autointoxication, “Bilious Attacks,” rheumatism, acute indigestion, catarrhal conditions of the stomach, pyorrhea, headache, dizziness, heart burn, “Summer Complaints,” “Derangements of the Stomach and Liver,” skin diseases, colic, alcoholic excesses, and as a “preventive of Seasickness.”
In 1914 the Council on Pharmacy and Chemistry published256 a report on Sal Hepatica declaring it secret in composition and sold under exaggerated and unwarranted claims.
In view of the inquiries which The Journal continues to receive it seemed worth while to make a chemical examination of the present-day product. Accordingly specimens were purchased and analyzed in the A. M. A. Chemical Laboratory. The report that follows was submitted by the chemists:
“Sal Hepatica is a white, granular, odorless powder. It effervesces on the addition of water in which it eventually dissolves. The aqueous solution, after boiling to remove carbon dioxid, has an acid reaction to litmus.
“Since a great many medicinal substances are sold in effervescent form, and since practically no information is given by the manufacturer concerning the composition of Sal Hepatica, it became necessary to test for a considerable number of therapeutic agents. The absence of acetanilid, acetphenetidin, alkaloids, ammonium salts, benzoates, caffein, citrates, heavy metals, hexamethylenamin, magnesium, potassium, salicylates and sugars was demonstrated by appropriate tests. The presence of a carbonate (probably in the form of a bicarbonate), a phosphate, a sulphate, a chlorid, tartaric acid, sodium and traces of lithium was shown by qualitative tests.
“Quantitative analysis indicated that the composition of the specimens examined was essentially as follows:
Sodium phosphate, anhydrous | 4.4 | per cent. |
Sodium sulphate, anhydrous | 26.5 | per cent. |
Sodium tartrate, anhydrous | 12.7 | per cent. |
Sodium bicarbonate | 19.5 | per cent. |
Tartaric Acid, free | 20.8 | per cent. |
Sodium chlorid | 8.9 | per cent. |
Lithium phosphate | trace | |
Water of hydration (by difference) | 7.2 | per cent. |
“From the results of the analysis, it appears probable that the composition of the mixture before ‘granulation’ was approximately as follows:
Sodium phosphate | 4 | per cent. |
Sodium sulphate | 25 | per cent. |
Sodium bicarbonate | 30 | per cent. |
Tartaric Acid | 30 | per cent. |
Sodium chlorid | 8 | per cent. |
Lithium phosphate | trace | |
Water of hydration (by difference) | 3 | per cent. |
“Sal Hepatica, therefore, is essentially an effervescing mixture of dried sodium sulphate (Glauber’s salt) and sodium tartrate with a little dried sodium phosphate and table salt added. It is similar to the effervescent artificial Carlsbad Salt described in the National Formulary.
“In 1909 the Druggists Circular published the following analysis of Sal Hepatica:
Sodium phosphate | 29.80 | parts |
Sodium sulphate (Glauber’s salt) | 26.27 | parts |
Sodium bicarbonate (baking soda) | 18.00 | parts |
Sodium chlorid (salt) | 13.05 | parts |
Lithium phosphate | 0.04 | parts |
Citric and tartaric acids (to make 100) | 12.84 | parts |
“A comparison of the recent analysis with the earlier one would seem to indicate that considerable changes have been made in the formula since the first examination. The proportions of sodium phosphate have been greatly reduced, while the sodium bicarbonate and tartaric acid have been increased and the citric acid entirely eliminated.”
Sal Hepatica, then, is a simple effervescent saline laxative, essentially secret in composition and sold under claims that would be laughed at were the full formula of the product a matter of public knowledge.—(From The Journal A. M. A., Oct. 29, 1921.)
“Salicon” is marketed by the K. A. Hughes Company, Boston, as “an improved aspirin.” In a circular sent out to the public a little over a year ago the following claims were made for it:
“We rendered aspirin absolutely harmless and yet retained all its virtues as a medicine.”
“It positively will not depress the heart nor upset the stomach no matter how large amounts of it are taken.”
“... the Massachusetts state medical authorities ... adopted its use at all the state camps for fighting the Spanish influenza....”
The first two statements quoted above are obviously false. The third statement might have been true although it seemed unlikely. A letter was, therefore, written to the Department of Public Health of the Commonwealth of Massachusetts and the claim of the K. A. Hughes Company relative to the adoption of Salicon in all the state camps by the “state medical authorities” was brought to their attention. The reply of the department on this point was emphatic:
“The State Department of Health of Massachusetts did not endorse the use of Salicon for any purpose.”
Some Salicon was purchased on the open market and submitted to the A. M. A. Chemical Laboratory for analysis. Here is the chemists’ report.
“One original bottle of ‘Salicon’ (K. A. Hughes Company, Boston) was submitted by the Propaganda department of The Journal to the Association’s Chemical Laboratory for examination. The bottle contained 100 white tablets having an average weight of 0.407 gram (6.3 grains), each. The amount of ash was 20.9 per cent. Qualitative tests indicated the presence of magnesium, carbonate, starch, acetylsalicylic acid and a trace of calcium; a very small amount of a petrolatum-like substance was present. Alkaloids and drugs used for a laxative effect were not found. The amount of acetylsalicylic acid extracted by chloroform was 50.7 per cent. The amount of magnesium present as magnesium oxid was 14.3 per cent. The amount of magnesium oxid derived from magnesium carbonate U. S. P. is variable; but calculating on the lowest limit, 14.3 per cent. of magnesium oxid is equivalent to at least 35.5 per cent. of magnesium carbonate. This figure agreed closely with that obtained from the U. S. P., method of assay. The acetylsalicylic acid was not combined with the magnesium. From the above, it may be stated that each tablet consisted essentially of a mixture of 3.2 grains of acetylsalicylic acid (aspirin), 2.2 grains of magnesium carbonate and some starch. Although labeled 5 grains, each tablet did not contain 5 grains of the most active ingredient, acetylsalicylic acid.”
The same old story. An ordinary mixture of well known drugs put on the market as a new discovery and foisted on the public under false and misleading claims.—(Correspondence in The Journal A. M. A., Feb. 5, 1921.)
In China the administration of powdered tiger-bone is—or was—a favorite form of treatment in cases of supposed cardiac weakness. The theory is, presumably, that the cardiac strength of the tiger would be a good thing for the patient to acquire. Since many patients have recovered after taking tiger-bone, and no one has proved that they might not have died had they failed to take it, “clinical experience” stands back of the treatment; and where is the skeptic so rash as to challenge that? The Chinese physician believes in his tiger-bone therapy, and, with the best interests of his patient at heart, insists on obtaining absolutely true and authentic tiger-bone. Not satisfied with the assertions of the dealers, the conscientious Chinese physician subjects his tiger-bone to a kind of physiologic standardization. He offers the bone in question to a dog! If it is an ox-bone—a frequent form of substitution—the dog will seize and eagerly gnaw it, whereas, according to all the teachings of Chinese pharmacognosy, if it is a tiger-bone the dog will depart hurriedly with his tail between his legs. Very foolish? Yes! But before we smile superciliously at the Chinese medical man, let us turn to the report of the Council on Pharmacy and Chemistry on “So-Called Secretin Preparations.”257 After reading this report let us put to ourselves, squarely and honestly, the question: Has the attitude toward secretin therapy, of a certain portion of those who represent Western modern medicine, really been much more scientific than that of the Chinese medical profession toward tiger-bone therapy? On the basis of a hypothesis scarcely less crude and unsubstantiated than that which assumes that tiger-bone is of value in heart disease, it has been assumed that secretin is of value in gastro-intestinal diseases. On the ground of “clinical evidence” scarcely more critical than that exhibited by our confrères in the antipodes, it has been asserted that alleged secretin preparations actually are efficious. Indeed, in one respect the methods of the Chinese physician appear more scientific than those of his Western brethren. To the best of his ability, the Oriental at least makes sure that he is administering genuine tiger-bone; he does not rely on the unverified word of his dealer alone. The American physician has not been making the least effort to ascertain whether his supposed secretin preparations are truly such; and, as a matter of fact, scientific investigation seems to indicate that some of these products contained no secretin at all! Whatever one may think of the validity of his test, the Chinese physician does his best according to his lights. As to “clinical experience,” Dr. Jacobi has well said that some people make the same mistake a hundred times and call it “experience.”—(Editorial from The Journal A. M. A., Jan. 15, 1916.)
The Walker Pharmacal Company of St. Louis was, we understand, if it is not still, one of the subsidiary concerns of the Luyties Homeopathic Pharmacy Company. It has for years sold a nostrum, “Succus Cineraria Maritima,” under the claim that by simply dropping this stuff into the eye, twice daily, cataract and other opacities of the eye will be cured. For instance:
“... the only remedy for the relief of cataract and other opacities of vision, which stands before the medical fraternity on a firm foundation of accomplished results....”
“... possesses a specific power in removing the obstruction to vision.”
“In this class of cases [cataract] physicians can place reliance on Succus Cineraria Maritima (Walker) which does not require the services of a specialist but is simply dropped into the eye with an ordinary medicine dropper twice daily....”
“... has been used with success in cataract, both lenticular and capsular, pterygium and opacities of the cornea, softening the opaque deposits, causing dissolution, and by its stimulating properties, hastening absorption.”
Succus Cineraria Maritima is advertised to the medical profession in true “patent medicine” style by means of testimonials from doctors, obscure and deceased. The preparation is valueless for the purposes for which it is sold and “has about as much effect on the dissolution or dispersal of opacities due to organic changes in the lens as pouring the same down the back of the patient’s neck!” More than five years ago the Council on Pharmacy and Chemistry reported on the worthlessness of the drug, Cineraria maritima, and, at the same time, The Journal pointed out that the drug would have been forgotten long ago had it not been for the prodigal use of printers’ ink by the Walker Pharmacal Company in advertising its Succus Cineraria Maritima.
These facts are given for the purpose of refreshing the memory of our readers and are but incidental to the object of this article. In due time the federal authorities proceeded against the Walker Pharmacal Company charging that Succus Cineraria Maritima was misbranded under the federal Food and Drugs Act. The government chemists reported that analysis “showed that the product was essentially an aqueous solution of glycerin, boric acid and vegetable drug extractives carrying tannin-like bodies.” The direct and inferential claims made in the advertising matter accompanying the trade package were quoted by the federal authorities, who pointed out that the Walker Pharmacal Company was selling the nostrum under claims that would create in the minds of the purchasers the belief that Succus Cineraria Maritima was a remedy for cataract and other opacities of the eye causing impaired vision and that it was a cure for senile cataract, trachoma, secondary opacities, etc. These claims the government charged were “false and fraudulent in that the same were applied to the article knowingly, and in reckless and wanton disregard of their truth or falsity,” because “in truth and in fact it was not, in whole or in part, composed of, and did not contain, such ingredients and medicinal agents” as would produce the therapeutic effects claimed.

These charges put the matter flatly up to the Walker Pharmacal Company. This company has for years been telling physicians that their stuff could and would do just what the federal authorities insisted it can not and will not do. Did the Walker Pharmacal Company attempt to defend its claims? Did it demonstrate that Succus Cineraria Maritima would cure cataract? Did it produce evidence of the numerous cases of recovery from blindness or partial blindness which must have been available if the preparation had the powers claimed for it? No! The Walker Pharmacal Company in February, 1916, pleaded guilty—and was fined a paltry $10 and costs.
This, however, is not the end of the story. The company was prosecuted because it had published the false and fraudulent claims in the trade package, thus bringing the claims within the purview of the federal Food and Drugs Act. Had the Walker Pharmacal Company confined its false statements to medical journal advertisements, to the circular letters sent to physicians or to any other advertising matter not part of the trade package, it could have snapped its fingers at the Food and Drugs Act.
It was in February, 1916, that the Walker Pharmacal Company pleaded guilty to the charge of making false and fraudulent claims for Succus Cineraria Maritima. In October, 1916, they were still sending out circular letters to physicians urging the use of Succus Cineraria Maritima in the treatment of cataract and enclosing the usual booklet of testimonials claiming cures for cataract and other opacities of the lens and cornea!

Can one conceive a better illustration of the inadequacy of the Food and Drugs Act? The dishonest exploiter of proprietary medicines cares little that the law requires him to keep within certain bounds of truthfulness in the advertising that accompanies the trade package. It isn’t the claims in the trade packages that sell the product; it’s the advertising in medical journals, in circular letters, etc. Yet, the Food and Drugs Act offers no check or curb on false statements or fraudulent claims made for proprietary or “patent medicines” in any other place than the trade packages.
A few weeks ago The Journal called attention to a flagrant case of fraud; and at that time it said, “It is justifiable to assume that when any man, whatever his business, admits in court that he has made fraudulent claims and then continues to make the same claims through channels that are not controlled by penal enactment, that man’s standard of business ethics is such that the public needs protection against it. There are many such men in the ‘patent medicine’ world. The only way in which the public may properly be protected against being defrauded in such cases is for the federal Food and Drugs Act to have its scope extended to cover all advertising of the products coming under the purview of the act.”—(From The Journal A. M. A., March 17, 1917.)
From various parts of the country The Journal has received a sixteen page pamphlet, Therapeutic Leaves. The publication, which has a saffron colored cover, is said to be published by the National Bio-Chemical Laboratory, Mount Vernon, N. Y. The National Bio-Chemical Laboratory seems to be a style used by Dr. Edward Percy Robinson. The “editorial offices” of Therapeutic Leaves are given as “501 Knox Bldg., 5th Ave. at 40th St., New York,” which is a roundabout way of describing 452 Fifth Ave., the office address of Edward Percy Robinson. The first number (February, 1921) of Therapeutic Leaves gives the names of the “editors” as “E. P. Robinson, M.D., and W. A. Jenner, B.A.” In addition, there is “Assistant Editor, F. J. Geiger,” and “Gen’l Manager, Beverly K. Robinson.” The first and second numbers of Therapeutic Leaves (February and March, 1921) are practically identical, being evidently printed from the same plates. Therapeutic Leaves purports to be a periodical published as “a medium for the dissemination of knowledge, pertaining to therapeusis.” Actually, it is an advertising medium dealing with the products of the National Bio-Chemical Laboratory: “Osmo-Calcic Solution,” “Tekarkin” and “Osmotic Mangano-Potassic Solution.”
These preparations are said to be the “formulas” of Dr. Edward Percy Robinson who lives in Mt. Vernon, N. Y., and has an office at 452 Fifth Ave., New York City. They are used by Dr. Robinson in the treatment of cancer. At an earlier stage they seem to have been known under different names: “Tekarkin” was first “Hypotonic Sal-Cella” and then “Neoanabolin-X;” “Osmo-Calcic Solution” was “Osmotonic Calcic” while “Osmotic Mangano-Potassic Solution” was “Osmotonic Drops.” The three solutions are put up in one package containing 4 c.c. (about 65 minims) of “Tekarkin” and 1 ounce each of the other preparations. The package sells for $10.00. “Remittance with order ... We have no agents.”

Most of the material in Therapeutic Leaves is a rehash of four papers published by Edward Percy Robinson in the New York Medical Record of various dates between September, 1917, and July, 1920. In these Robinson advances the theory that cancer is caused by an excess of sodium chlorid (table salt) in the blood and tissues and that it can be cured by administering a solution of potassium nitrate. Such a treatment sounds ideally simple. One might assume that all that was necessary was to make up a solution of potassium nitrate and inject it. One might further wonder how it would be possible to commercialize such a “treatment.” “Homemade solutions,” says Dr. Robinson, “are apt to be disappointing.” Their use is likely to cause “considerable swelling at the site of an injection, accompanied with tenderness and some heat.” Moreover, “a wide hyperemic area with red blotches has been observed in a number of instances.” In order to avoid “accidents of this sort” which would “bring discredit upon an excellent agent,” Dr. Robinson, “after considerable experimental work” has obtained “a solution of this chemical which would meet the ideal requirements.” This is available under the name “Tekarkin.” Dilute potassium nitrate solution sold under the name “Tekarkin” sells for $67 an ounce. The physician can make his own solution, of the purest and highest grade potassium nitrate on the market, at an expense, for the chemical, not exceeding 5 cents an ounce.
Therapeutic Leaves also contains the usual number of those “clinical reports” which bulk so large in the literature of “cures” for cancer. Then there is a full page advertisement of a side-line of the National Bio-Chemical Laboratory: “Vitamines (Compressed) Tekarkin Brand;” “They have a meaty taste.”
The medical profession, naturally, is interested in knowing more about the physician who admits that he has discovered the cause and cure of cancer. According to our records, Edward Percy Robinson was born in 1871 and was graduated in 1897 by Bellevue Hospital Medical College. He was licensed in New York State the same year and has practiced in New York City continuously since that time. He is not, and apparently never has been, a member of his local medical society.

In 1914 Robinson was specializing in “facial contouring.” One piece of advertising purports to be the reprint of an interview with “Dr. E. P. Robinson, Specialist, as he sat in his office at 116 West 39th Street, having questions fired at him by the reporter.” Thus Dr. Robinson:
“There are physicians everywhere who abandon the general, or family, practice of medicine, to devote their life to some specialty. My specialty is the improvement of the facial features and the beautifying of the shoulders, neck and arms. I round out hollow cheeks, build up the neck, eradicate wrinkles, make irregular noses perfect and remove defects by a process which is my own secret. I claim no superhuman power or ability; I have simply bent my whole professional study and energy to the one line of remodeling—so to speak—the human features, and I employ only scientific methods and aids in my operations.”
In another piece of advertising, a little booklet bearing Edward Percy Robinson’s name, we find the following:
“This is what I accomplish....
“Remove all wrinkles and traces of age from the forehead, or about the eyes and mouth. Lift sag from cheeks and chin.
“Round out hollow cheeks.
“Remove depressions and defects from the chin.
“Build up the neck and shoulders.
“Build up and enlarge the bust.
“Round out and give symmetry to unshapely arms and remove the lines of age from the hands.
“Correct many of the defects not mentioned here, but which may be possessed by exceptional cases.”
Still another advertising leaflet purports to be a reprint of an “editorial” from the Mercantile and Financial Times of March 11, 1914. It is a pretentious puff of Robinson, telling about his “scientific attainments” and his marvelous secret preparations used in “Youthifying the Face.” The Mercantile and Financial Times is an utterly discredited sheet run for the purpose of selling what appear to be editorial comments. Such “editorial” puffs are paid for through the purchase of a certain number of copies of the paper by the party who desires the publicity. The Associated Advertising Clubs of the World exposed this publication in a special bulletin issued in June, 1919, and described it as an “example of publications that serve as convenient tools of fake promoters.” In 1911 the Mercantile and Financial Times published an “editorial” endorsement of the consumption cure “Nature’s Creation.” It has done the same for a fakish device known as the “Ideal Sight Restorer.” It published a puff on the “Oxypathor,” a swindle so preposterous that the exploitation of this “gaspipe” fake was debarred from the U. S. mails and its exploiter was sent to the federal penitentiary.

We also find in our files a testimonial signed E. P. Robinson, M.D., 1402 Broadway (Edward Percy Robinson’s address in 1912), extolling the virtues of a foolish piece of quackery, the obesity cure “Get Slim.” This nostrum was exposed in The Journal some years ago and was also exposed by Dr. Wiley in Good Housekeeping. The “Get Slim” concern sued Good Housekeeping for libel but a jury decided that Good Housekeeping had told the truth. In the “Get Slim” testimonial Robinson is quoted as saying that he is “acquainted with the ingredients entering into its manufacture” and he describes it, as did the “Get Slim” concern, as “a purely vegetable combination.” The fact is the Association’s chemists found this “purely vegetable combination” to consist of sugar and tartaric acid, each colored pink, and baking soda.
And this is the gentleman who claims to have discovered the cause of, and offers for sale a cure for, one of the most baffling scourges known to modern medicine—cancer. Except for the articles that have been published during the past three years in the Medical Record, we are unable to find anywhere in representative medical literature anything to indicate that Edward Percy Robinson can lay any claim to special knowledge of, or skill in the treatment of, cancer. What we do find are advertisements describing Edward Percy Robinson’s alleged abilities as a “face beautifier,” puffs from utterly uncritical or discredited sources and a testimonial to the value of a preposterous “fat cure” fake.
With the best brains of the world at work on the problem of cancer, it is reasonable to assume that any man who has found out even a little more than has previously been discovered or is able to accomplish even a little better results than the average in the treatment of this dreaded disease, would be well known to scientific medicine.
After this article was in type physicians began sending in No. 3 (April, 1921) of Therapeutic Leaves. This is still another reprint of Nos. 1 and 2, with minor changes. In the first two, Tekarkin is described as “a solution of potassium nitrate of special strength;” in No. 3 it becomes “a special solution containing potassium nitrate.” In Nos. 1 and 2, Robinson described an alleged case of “Cancer of the Rectum Treated with Tekarkin.” In No. 3 this becomes “Medicinal Treatment Cures Cancer of the Rectum.” In No. 3 the names of the editors, assistant editor and general manager are eliminated.

The inside back cover of No. 3 contains an advertisement of Tekarkin, in which physicians are warned that “Cancer of the Lung May Present Diagnostic Signs of Tuberculosis.” It contains the further startling information that the particular micro-organism responsible for pulmonary tuberculosis is the Klebs-Loeffler bacillus! Thus:
“The Klebs-Loeffler bacillus may find a suitable habitat in a malignant area of lung tissue and thrive therein. The presence of the bacillus does not necessarily exclude the presence of cancer. A chronic cough with blood-streaked sputum may be the result of tuberculosis and cancer.”—(From The Journal A. M. A., May 28, 1921.)
“I am fond of the retail drug business and follow it every day of my life. I know and observe to the fullest extent its ethical and commercial requirements.” This from a circular letter recently received by physicians, and signed J. S. Tyree, who asks that he be forgiven for writing you personally, but there are several reasons why he thinks the circumstances warrant it. All of which is preliminary to calling attention to an enclosure, which accompanies the circular letter, and is described as a “short memorandum” submitted for “your consideration.”
The “memorandum” is a four-page leaflet of which three pages are devoted to “Tyree’s Antiseptic Powder.” One of these three pages is a reproduction of a letter on the stationery of the Surgeon General’s Office of the War Department, and signed “W. M. Gray, M.D., Microscopist, Army Medical Museum; Pathologist to Providence Hospital.” The letter describes a series of “bacteriological and comparative tests” made by Dr. Gray with Tyree’s Antiseptic Powder. The entire second page of the circular is given over to the results of these bacteriologic tests which compare various strengths of Tyree’s Antiseptic Powder with “mercuric bichlorid,” phenol and formaldehyde.
The physicians who received this advertising material in April, 1919, might easily overlook the fact that Dr. Gray has been dead several years, that the letter which is reproduced is dated Jan. 3, 1890, and that the bacteriologic tests were made in 1889—thirty years ago!
The Council on Pharmacy and Chemistry in 1906258 published the results of an analysis of Tyree’s Antiseptic Powder which showed that although the stuff was advertised as a mixture of borax and alum, it was in fact essentially a mixture of zinc sulphate and boric acid. The publication of the Council’s report in 1906, showing the falsity of the formula, brought out the admission that the composition had recently been changed. Certain it is, however, that for at least a decade past, the Tyree product has been a zinc sulphate-boric acid preparation. Yet, according to the manufacturer’s own statement, Tyree’s Antiseptic Powder in 1889, when Dr. Gray made his bacteriologic tests, was an entirely different substance from the present mixture.
Here then we have a manufacturer publishing in 1919, in behalf of a certain product, tests that were made in 1889 with a product of different composition, although of the same name! Is this observing “to the fullest extent” the “ethical and commercial requirements” of the “retail drug business”?
There is no scientific excuse for such a mixture as Tyree’s Antiseptic Powder. If, however, physicians feel that they must use an irrational conglomeration such as this, why not prescribe Pulvis Antisepticus, N. F.? Like the Tyree product, this, too, is essentially a mixture of zinc sulphate and boric acid, with minute amounts of phenol, eucalyptol, menthol and thymol, to say nothing of a dash of salicylic acid. This official article has at least the virtue of constancy of strength, composition and purity assured under the federal Food and Drugs Act.—(From The Journal A. M. A., May 17, 1919.)
“The Commissioner of Health directs me to call to your attention the enclosed advertisement issued by T. B. Wheeler, M.D., Company, Montreal, Canada, in which the name of the Association’s Journal is being used.”
Accompanying this brief note to The Journal from the secretary of Dr. Haven Emerson, Commissioner of the Department of Health of the City of New York, was a four page leaflet devoted to the exploitation of “Wheeler’s Tissue Phosphates.” The trend of the circular is to lead the average reader to infer that The Journal of the American Medical Association has endorsed Wheeler’s Tissue Phosphates. For example, in describing the preparation one reads:
“It embodies ... the best recent scientific opinion concerning the treatment of the disease (tuberculosis) as stated ... by the official Journal A. M. A.”
Elsewhere in the circular The Journal’s criticisms of the hypophosphites and the glycerophosphates (proprietary preparations which are competitors of the Wheeler product) are quoted and twisted into a tribute to the ingredients of Wheeler’s Tissue Phosphates. Garbling quotations, distorting statements, separating phrases from their contexts and omitting qualifying clauses, all for the purpose of making out a case for some proprietary remedy is a trick as old as quackery itself. That it should be used in advertising Wheeler’s Tissue Phosphates is entirely fitting. Obviously, the T. B. Wheeler, M.D., Company esteems the opinion of The Journal on pharmacologic matters. This being the case, it should, in the interest of truth and scientific accuracy, publish in its advertising circulars just what The Journal has said about Wheeler’s Tissue Phosphates. It could not do this better than by quoting from a recent editorial note which commented on a report of the Chemical Laboratory on this preparation. Here is part of the The Journal’s comment:
“ ‘Wheeler’s Tissue Phosphates’ is an unscientific shotgun mixture whose most active and powerful drug is the alcohol it contains. That it was not years ago relegated to the realms of obsolete and discarded preparations is a commentary alike on the lack of scientific discrimination and on the power of advertising.”
Here we have “Wheeler’s Tissue Phosphates” stripped of the verbal camouflage with which its exploiters have invested it.—(Editorial from The Journal A. M. A., Sept. 22, 1917.)
To the Editor:—What is the composition of Alcresta Lotion?
L. T. A. Hotten, M.D., Paris, Idaho.
According to a circular in our files, “Alcresta Dental Lotion-Libby” contains “Emetin, the active amebicidal principle of Ipecac, together with Benzoic Acid, Thymol, Eucalyptol and Aromatics.” The theory that emetin is an active amebicide against pyorrhea alveolaris has been exploded. In this connection, it is interesting to note that the firm does not list the product in the latest catalogue in our files.—(Query from The Journal A. M. A., Oct. 29, 1921.)
To the Editor:—What is the composition of calcidin tablets (Abbott) and what is their value?
J. S.
Answer.—Calcidin is claimed to be a mixture of iodin, lime and starch. In contact with water, the iodin and lime react to form calcium iodid and calcium iodate. By the acid of the gastric juice, the calcium iodid and calcium iodate are decomposed with liberation of free iodin. The administration of calcidin tablets amounts to giving free (elementary) iodin. In the past, the advertising for calcidin has contained the unwarranted claim more or less directly that it was the most effective and only noninjurious preparation of iodin for internal use, and that it possesses all of the valuable properties of the iodin with all of the objectionable effects left out. So far as we know, the effects produced by the administration of free iodin do not differ from those produced by the administration of iodids and, therefore, calcidin has no advantage over the iodids, such as sodium iodid.—(Query in The Journal A. M. A., Sept. 25, 1920.)
To the Editor:—Do you have any literature or information relative to the Di-Crotalin treatment for epilepsy? I will be very grateful if you can furnish information as to method of preparation, rationale of the treatment, etc.
R. C. Decker, Captain, M. R. C.,
U. S. Soldiers’ Home, Washington, D. C.
Answer.—Di-Crotalin is a rattlesnake venom preparation sold by the Swan-Myers Company of Indianapolis as a “Treatment for Epilepsy, Chorea, Bronchial Asthma, Chronic or Hereditary Nervous Headache, Nervous Prostration Incident to Change of Life, Hysteria-Mania, Insomnia, Neurasthenia, etc.” Dr. Thomas J. Mays of Philadelphia advocated the use of rattlesnake venom for tuberculosis. Later his former assistant, Dr. R. H. Spangler, used the same material in the treatment of epilepsy. That any measure of success sufficient to justify the adoption of the rattlesnake venom or crotalin treatment for epilepsy has resulted is not to be concluded from the available reports. Still less evidence is there for the use of rattlesnake venom in the list of conditions for which the Swan-Myers Company has recommended its preparation. There are a number of good reasons why a cautious physician will shun the administration of this treatment and advise against it. J. F. Anderson, working in the hygienic laboratory of the United States Public Health Service, reported a death from the crotalin treatment in consequence of infection, and reports that the market supply of crotalin solution and crotalin tablets is highly contaminated. He also found both crotalin and crotalin solution to vary in activity. The use of rattlesnake venom was discussed in The Journal, March 15, 1913, p. 850.—(Query in The Journal A. M. A., Aug. 17, 1918.)
To the Editor:—What is “Estevin,” or something like that? It is said to be good in hay-fever.
Constant Reader.
Answer.—The product called “Estivin” is sold by Schieffelin and Company, New York. A request for a statement of the composition of this preparation sent to Schieffelin and Company by the Council on Pharmacy and Chemistry brought the indefinite and, therefore, meaningless statement that “ ‘Estivin’ is an extract of Rosa Gallica containing no alcoholic or foreign ingredients.”—(Query from The Journal A. M. A., Nov. 12, 1921.)
To the Editor:—Can you inform me how iron arsenite can be prepared for subcutaneous injection? A commercial firm furnishes physicians with ampules of arsenite of iron. Is this really arsenite of iron?
S. H. Kempner, M.D., New York.
Answer.—Ferric arsenite (iron arsenite) is in itself relatively insoluble in water, but may be treated with ammonium citrate, the resulting product thus being soluble; the latter substance was at one time described in New and Nonofficial Remedies as “Ferric Arsenite, Soluble” and is sometimes sold as a solution in ampule form. In 1912, the Council on Pharmacy and Chemistry deleted “Ferric Arsenite, Soluble” from New and Nonofficial Remedies because “one cannot, in administering Ferric Arsenite, Soluble, give a useful dose of iron without giving too much arsenic; and, vice versa, one cannot give a safe dose of arsenic without giving too little iron.” The Council, therefore, held the preparation to be irrational and unscientific.—(Query in The Journal A. M. A., Feb. 19, 1921.)
To the Editor:—1. What is the composition of “K-Y Lubricating Jelly”? 2. Can you furnish a formula for a simple nongreasy lubricating jelly?
S. T.
Answer.—1. The composition of “K-Y Lubricating Jelly” has not been divulged. Examination of the advertising matter reveals only meaningless statements, such as: “This is a judicious combination of vegetable products ... Combined in well balanced proportions with non-irritating antiseptics...,” “It incorporates a sufficient quantity of mild antiseptics (of the Thymol class) ...” and “... contains NO formaldehyde.”
2. Probably a simple tragacanth jelly, which can be made cheaply, will produce the same effects as those of the proprietary preparation. The following formula was published by Mr. J. K. Thum, apothecary at the German Hospital, Philadelphia (Druggists Circular, September, 1915, p. 586):
LUBRICATING JELLY
Tragacanth, whole | 3 | gm. |
Glycerin | 25 | c.c. |
Phenol | 1.5 | gm. |
Distilled water, a sufficient quantity to make 300 c.c. The tragacanth is broken in small pieces, and put into a wide-mouthed bottle; the other ingredients are added and the bottle frequently shaken.
In regard to this formula, Mr. Thum writes:
It has been used in our gynecologic department for years.... For the last six years we have been dispensing it in collapsible tubes throughout the hospital for general work.—(Correspondence in The Journal A. M. A., May 12, 1917.)
To the Editor:—Collier’s has a special article this week on “Nikalgin.” Have you any information on this subject? It sounds like nostrum stuff.
P. R. Minahan, M.D., Fond du Lac, Wis.
Answer.—“Nikalgin” is said to be the “invention” of Gordon Edwards, an engineer. Large claims for its anesthetic and antiseptic virtues have been made. While no very definite information seems to be forthcoming regarding the preparation, it has been said to be “composed of quinin, hydrochloric acid and urea.” This would indicate that “Nikalgin” may be nothing more wonderful than the well known local anesthetic, quinin and urea hydrochlorid, the Quininae et Ureae Hydrochloridum of the U. S. Pharmacopeia, or a modification of it.—(Query in The Journal A. M. A., Sept. 22, 1917.)
To the Editor:—A short time ago I received a sample of “Pertussin” and used some in an obstinate case of bronchitis with excellent results. I have since received a catalog from a pharmaceutical firm, which advertises syrup of thyme. I have searched for a formula to make my own syrup of thyme, but have not been able to find one. Will you publish one?
E. F. Benner, M.D., Salfordville, Pa.
Answer.—The subjoined formula yields a product very similar to “Pertussin” in taste, flavor, composition, and probably in activity as well:
Fluidextract of thyme | 15 c.c. | |
Glycerin | 15 c.c. | |
Syrup | to make | 100 c.c. |
The original German preparation contained 1.5 gm. of sodium bromid in each hundred cubic centimeters, and this might be added to the foregoing formula with advantage, so far as action is concerned. However, a sample of “Pertussin” purchased in the open market in the United States failed to respond to tests for bromids.
As fluidextract of thyme is not official, this formula is presented as furnishing an acceptable preparation:
Thyme, in No. 60 powder | 100 gm. |
Moisten with a mixture of:
Water | 25 | c.c. |
Alcohol | 15 | c.c. |
Glycerin | 10 | c.c. |
After standing five hours, pack in a percolator. Exhaust with a menstruum of alcohol, 1 volume, and water, 3 volumes. Reserve the first 85 c.c. of percolate. Concentrate the weak percolate to a soft extract and dissolve in the reserved portion. Make up to 100 c.c. by addition of a mixture of alcohol, 1 volume, and water, 3 volumes.
Other aromatic expectorants, such as terebene, terpin hydrate or creosote, might be expected to have similar but greater effect in chronic bronchitis.—(Query in The Journal, A. M. A., March 27, 1920.)
To the Editor:—Could you tell me why quinin and urea hydrochlorid has not become more popular for local anesthesia? Is it less efficacious or more toxic than other preparations? If it is useful, can you name some trustworthy firm or brand? Please omit my name in answering.
L. F. C., M.D., Mexico.
Answer.—Quinin and urea hydrochlorid “has the actions of quinin. When injected hypodermically it exerts an anesthetic action much more prolonged than that of cocain” (Useful Drugs, Ed. 4, 1920, p. 127). It has been pointed out editorially in The Journal (Feb. 14, 1920, p. 462) that quinin has been regarded for more than half a century by toxicologists as a protoplasmic poison capable of destroying various forms of animal and vegetable cells, and hence it need not be surprising that tissue necrosis may be produced by strong solutions of the quinin salts. That this deleterious reaction actually does occur and has militated against the general use of quinin and urea hydrochlorid is confirmed by the report of the Committee on the Advantages and Disadvantages of Local Anesthesia in Nose and Throat Work (The Journal, July 31, 1920, p. 315). To quote:
The only local anesthetic that produces edema and sloughing is quinin and urea hydrochlorid. So many statements were found in the literature that this anesthetic has been abandoned in other fields of medicine because of edema and sloughing, that writers who had presented favorable reports in nose and throat operations were communicated with by your committee. One writer who had recorded 390 cases of tonsillectomies extolling this anesthetic, which he had used for four years and is still so recorded, now states that he has not used it in two years, although no publication has been made retracting his former endorsement. Still another writer, who stated that quinin-urea came nearest the ideal local anesthetic, now states that he has ceased using it. Your committee finds that as far as nose and throat operations are concerned, this drug has practically gone into “innocuous desuetude.”
The anesthesia produced by this drug at the time of operation is good and the recovery of the patient might often be enhanced by its use if it did not have the serious drawback. The product is official in the U. S. Pharmacopeia, and may be obtained from any reputable pharmaceutical house.—(Query in The Journal A. M. A., Aug. 21, 1920.)
To the Editor:—My mail is frequently cluttered with pseudo-scientific data from various manufacturers of proprietary remedies which contain as much real scientific information as the Police Gazette. I am enclosing a sample page of such a periodical. The article has been so cleverly worded in the first paragraph, as to impress the unthinking with the idea that sodium cacodylate is superior to arsphenamin, when we know in reality that sodium cacodylate has been proved practically worthless in syphilis (vide “Venarsen”). One case is reported, in which twenty injections of sodium cacodylate were administered intravenously, from October 23 to December 14. On December 18, a Wassermann test proved negative, it had been strongly positive on October 20, but during the same interval from October 23 to December 14, the patient had been taking by mouth “Ricord pills” each containing half a grain of yellow iodid of mercury; granted that he had taken these pills regularly, during all that time, it might well be that the Wassermann would be sharply influenced by them. Again, a negative Wassermann in the midst of treatment proves little; it might be positive again in a few days. The article stimulates the further use of a product of known worthlessness in the treatment of syphilis. How any one can use sodium cacodylate in preference to arsphenamin in syphilis is beyond me. If I mistake not, the Propaganda Department has not taken up the matter of these various pamphlets of the drug companies, such as the Doctor’s Factotum, Therapeutic Notes, etc., lauding to the skies such articles as “Seng,” “Cactina Pillets,” etc., ad nauseam. The saddest part of the whole thing is that it must bring returns from the unthinking, otherwise they would soon disappear, which would be a great relief for the scrubwomen who empty our waste baskets.
Paul E. Bechet, M.D., New York.
[Comment.—The “sample page” sent by Dr. Bechet is from the March-April, 1918, number of Parke, Davis & Company’s Therapeutic Notes. It contains an “Original Communication” on “The Treatment of Syphilis with Sodium Cacodylate, by Adolph Lappner, M.D., Detroit, Mich.” The “article,” while nominally devoted to the praise of sodium cacodylate, is virtually a puff for “Ricord Pills.” a Parke, Davis & Co. product.]—(Correspondence in The Journal A. M. A., July 13, 1918.)
To the Editor:—I am very anxious to know whether tin or stannous oxid (SnO) has or has had any place among useful drugs. I have seen such a prescription given in the treatment of mucous colitis, and would be very glad to learn what its use may be.
Carlos Manuel Garcia, M.D., Havana, Cuba.
Answer.—Recently, on the assumption that tin workers are less troubled with boils than the average person, two French investigators proposed the use of tin compounds in the treatment of staphylococcic infections. Based on their work, a proprietary preparation—Stannoxyl—has been placed on the market which is claimed to be “composed of stannous oxide and specially purified metallic tin.” Absurd claims are made for the product: for instance, “... We have no hesitation in offering STANNOXYL—in Tablets or Cachets—as the only true specific for diseases of Staphylococcus origin.” The available evidence is unconvincing and in no way warrants such exaggerated statements.—(Query in The Journal A. M. A., March 6, 1920.)
To the Editor:—I was much interested in your answer to a query about Stannoxyl (The Journal, March 6, p. 629). I submit the following experience as a confirmatory note:
While serving with the Royal Army Medical Corps in Egypt, I for some time had charge of the medical division of a hospital in which most of the skin diseases occurring among soldiers in the district were treated. The most common conditions were boils and septic sores, chiefly due to staphylococcal infection, though several of the latter cases were diphtheritic. The treatment adopted was that in ordinary use, namely, incision and evacuation of pus, application of antiseptic dressings, and in most cases employment of the specific vaccine. It was possible to judge of the efficacy of any variations of treatment, as there were always plenty of cases undergoing the usual treatment with which the results could be controlled.
An available supply of Stannoxyl, a proprietary remedy consisting of a mixture of metallic tin and tin oxid, enabled me to give it a fair trial in full doses in eight cases of boils of average severity, in which culture revealed the infecting organism to be Staphylococcus aureus. The boils were treated locally as usual, but no vaccine was given. No improvement could be demonstrated in these cases that could not be shown in other cases similarly treated with the omission of Stannoxyl; in fact, three of the treated cases were much longer in clearing up than the untreated controls. Eight cases do not constitute a very large series from which to draw conclusions; but if the preparation were as good as the descriptive literature would lead one to believe, one should have expected an evident result in at least one of these cases.
It has been stated that Stannoxyl does not inhibit the growth of staphylococci, but only renders the growth less virulent. It is known that a certain amount of tin may be absorbed from the intestine, and Salant, Rieger and Treuhardt have shown that in certain cases tin may be retained for some time in the skin; but it is questionable whether, when preparations of tin are given by mouth, any reaches the staphylococci in the boils, or at any rate enough materially to influence their growth or virulence.
In the cases treated by me, the results did not at all suggest that Stannoxyl was a “specific for diseases of staphylococcal origin.”
J. W. C. Gunn, M.A., M.B., Ch.B., Cape Town.
Professor of Pharmacology, University of Cape Town.
—(Correspondence in The Journal A. M. A., Nov. 20, 1920.)
To the Council on Pharmacy and Chemistry:—If you have not already done so, will you kindly examine and report on “Syphilodol” advertised in the enclosed pamphlet?
B. C. Pedersen, M.D., New York.
To the Editor:—Have you any information concerning the enclosed half page advertisement [of Syphilodol] from the Urologic and Cutaneous Review?
Edward S. Newell, M.D., Pelham, N. Y.
To the Editor:—I am enclosing an advertisement for a substance called “Syphilodol” manufactured in New York. Am not familiar with this article nor have I seen it advertised in the higher class journals. Can you tell me whether The Journal’s department of New and Nonofficial Remedies has passed on this article or whether you have any data concerning it? It looks a trifle fishy to me.
Louis Leroy, M.D., Memphis, Tenn.
To the Editor:—I am sending the enclosed correspondence [Syphilodol letters] to you as it looks as if it might have some interesting features from the point of view of your nostrum department.
Isadore Dyer, New Orleans, La.
Answer.—These are but some of the inquiries that have been received on this subject and it is encouraging to note the scientifically critical attitude of physicians toward new therapeutic agents. According to the French Medicinal Company, Inc., which markets this product, “Syphilodol is a synthetic chemical product of silver, arsenic and antimony....” Nowhere in the advertising matter is there any more definite statement as to the composition of this new “synthetic” than that just quoted. The product is now under examination in the Association’s laboratory and when this is completed a more detailed report will doubtless be forthcoming. At present the work has progressed sufficiently to show that Syphilodol tablets contain considerable quantities of mercury! Although the advertising leaflets claim that the preparation is “the formula of the late Dr. Alfred Fournier of Paris” and had been exhaustively tested by Metchnikoff, who is alleged to have found it superior to salvarsan and neosalvarsan, yet, strange to say, a careful search of French medical journals fails to show any reports on Syphilodol. Verb. sap.—(Query in The Journal A. M. A., Feb. 23, 1918.)
To the Editor:—Kindly inform me regarding thialion, manufactured by the Vass Chemical Company, Danbury, Conn. Please omit my name and address in answering in The Journal.
H. C. W.
Answer.—Thialion is an heirloom of the days when lithium salts were supported to be nature’s antidote for all kinds of ailments, supposedly due to excess of uric acid. It was advertised as a uric acid eliminant and therefore good for all kinds of diseases. The Council on Pharmacy and Chemistry published a report on thialion in The Journal, Nov. 3, 1906. At that time thialion was advertised by the Vass Chemical Company as a “laxative salt of lithia” with the chemical formula “3Li2O.NaO.SO3.7HO,” and an elaborate structural formula was also furnished. The Council reported that the product was not a definite chemical compound, but a mixture consisting chiefly of sodium sulphate, sodium citrate and small amounts of lithia. In recent advertisements, thialion is referred to as “A Non-Effervescing Lithiated Laxative Salt,” “a non-hygroscopic, non-deliquescent, granular salt of lithia,” etc., but the chemical formula does not appear, nor is any definite statement of composition furnished. According to this advertisement, the “indications” for thialion are: “gout, rheumatism, uric acid diathesis, constipation, acute and chronic, sluggish liver, Bright’s disease, albuminuria of pregnancy, asthma, incontinence of urine, gravel, cystitis, chronic lead poisoning, headache, neuralgia, neurasthenia and lumbago, Hay fever, etc.”—(Query in The Journal A. M. A., Dec. 6, 1919.)
Venarsen—To the Editor:—The following is a copy of a letter sent to the Intravenous Products Company, which needs no explanation:
June 8, 1917.
The Intravenous Products Co., Denver, Colo.
Gentlemen:—In reply to your circular letter under date of June 3, may I say that after using a great quantity of Venarsen both in clinical and private cases, I can see no more effect upon these cases than if so much water had been administered.
This is also the report of Don R. Black, pathologist for Bell Memorial Hospital, University of Kansas. In our experiments all bloods were tested before and after each administration of this product.
William A. Wilson, M.D., Kansas City, Mo.
(Correspondence in The Journal A. M. A., July 7, 1917.)
For some time The Journal has received inquiries of which the following recent examples are typical. This from an Ohio physician:
“Please give me some information concerning Dr. Abrams and his diagnostic and therapeutic devices known as reflexaphore and oscilloclast. If this is published please withhold my name.”
A physician in Massachusetts writes:
“Can you give me any information concerning Dr. (?) San Francisco, California, who reports himself able to diagnose syphilis from a drop of blood sent him on a blotting paper? He has caused a patient of mine a great deal of needless worry.”
And from Rhode Island a physician facetiously inquires:
“I am interested to know of the ‘Reactions of Abrams.’ Have you any information that you can give me in regard to this matter? They apparently do wonderful things in the West.”
While a New York physician acknowledges his failure to keep up with the times thus:
“To-day I had occasion to see a patient who mentioned having an Abrams test for gonorrheal infection of the prostate. He also stated he wished to have Abrams’ treatment for the same condition. Could you enlighten me as to what these are? I thought I had kept myself up to date as to all new tests and treatments in my line; but evidently I have been delinquent.”
According to our records, Albert Abrams, A.M., M.D., LL.D., F.R.M.S., was born in San Francisco in 1864. He was graduated in medicine by the University of Heidelberg, Germany, in 1882. Dr. Abrams is a member of his local medical society and through that holds fellowship in the American Medical Association. Dr. Abrams has written voluminously. In 1910, his book on “Spondylotherapy” (“Physio-Therapy of the Spine”) was reviewed in The Journal. “Spondylotherapy” is a neologic creation of Dr. Abrams. According to its disciples, it concerns itself “only with the excitation of the functional centers of the spinal cord” and has been called “the science of evoking the reflexes of the body both to diagnose and to cure disease.” In bringing its review of Abrams’ book on “Spondylotherapy” to a close, The Journal said:
“... one wonders whether this is an attempt to explain osteopathy and chiropractic to the understanding of the regular practitioner, or to exploit the very ingenious percussion devices of the author, or whether it is really true that medical men really know practically nothing about the cure of disease through treatment of the spine. Let us hope that it is the latter and that a careful study of this unique volume may open new avenues of therapy heretofore undreamed of.”
While the review was obviously critical, yet in advertising the book, the publisher picked out part of the closing sentence, omitted the context, and quoted The Journal as having said:
“Let us hope that a careful study of this unique volume may open new avenues of therapy heretofore undreamed of.”
When this matter was brought to the attention of Dr. Abrams, he replied, “I fail to see any real difference in the two quotations” and “only one ... with an astigmatic mentality” could “see any incongruity between the context and the concluding sentence.” Yet, in this same letter which attempted to justify the garbling of a quotation so as to make a critical review appear a laudatory one, Dr. Abrams declared that the review in question was “conceived and executed in a malicious spirit.”
Between 1912 and 1914 Dr. Abrams gave “clinical courses” on “Spondylotherapy” in various parts of the country—price $50. These “courses” were widely advertised by an Ohio concern that seems to make a specialty not only of handling the advertising campaigns of those members of the medical profession who have unusual or bizarre methods to exploit, but also of acting as an agent for the sale of such devices and publications as may be necessary to the proper practice of the particular brand of therapy that is being exploited. At the time this concern was featuring Abrams’ course it called attention to the alleged fact that “no class were [sic!] so busy as those employing mechanical treatment such as Osteopathy, Chiropractic, Mechanotherapy, etc.”
Says Dr. Abrams:
“Despite the fury of tongue or the truculence of the pen, the osteopath and chiropractor are inspiring the confidence of the community with their systems. Right or wrong in their theory, they are, in vulgar parlance, ‘delivering the goods.’ Spondylotherapy was a product of necessity—the translation of an ignored field of medicine from a chaotic to a scientific basis.”
Possibly the following testimonial published by Dr. Abrams as typical of many received, and credited to “Dr. Henry Stacy Dodge, Richmond, Va.,” may explain the field that “Spondylotherapy” is to cover. Incidentally “Dr.” Dodge is listed in the Richmond telephone directory as a chiropractor:
“I have been in practice for fifteen years in Chiropractic and ten years an Osteopath and I wish to say that during the last three years I have received more genuine and sincere satisfaction from the application of Spondylotherapy than all other methods combined. My success in gastrology alone is worth many times the cost of the information.”
More recently, Dr. Abrams has advertised that he gives a “course” in Spondylotherapy in San Francisco, beginning on the first of each month. The course last four weeks. “The honorarium for this course is $200.00.”
In 1912 an organization was created devoted to this new therapeutic method: the “American Association for the Study of Spondylotherapy.” Later Dr. Abrams was made Honorary President. Whether the organization is still viable we do not know.
In addition to “Spondylotherapy,” Dr. Abrams has also evolved what he calls the “Electronic Reactions of Abrams.” These are said to make possible long-distance diagnoses, it being necessary only to send a few drops of blood taken from the patient and allowed to dry on a slide. There are, it seems, certain instruments and devices used in the performance of these diagnostic feats. By means of the “Electronic Reactions” Dr. Abrams (while admitting the protective factor of vaccination against smallpox) has discovered that practically all the vaccines obtained from reliable firms yield the reaction (“electronic tests”) of congenital syphilis, and that many of them also yield the reaction of tuberculosis and of streptococci and staphylococci. Further, “from the cicatrices of all vaccinated persons, one can always elicit a reaction of congenital syphilis and in early scars a tuberculous reaction.” Dr. Abrams also declares that exposing vaccine virus for ten minutes to blue light will destroy the syphilitic, streptococcic and staphylococci reactions and exposing it for the same period to yellow light will destroy the tuberculous reaction.
One of Dr. Abrams’ disciples—Sir James Barr—frequently quoted with evident satisfaction, declares that from a fresh sample of blood spread over four square inches of white blotting paper, “Dr. Abrams can diagnose the sex, race and disease of the patient.” However, there are certain precautions that must be taken: The patient should face West, “the blood should be taken in a subdued light and there should be no strong red or yellow coloring material in the room.”
In various places Dr. Abrams has asseverated that “if splancho-diagnosis is approached with a prejudiced mind, it is better not to attempt it, for there are ‘none so blind as those that will not see.’ ”
Dr. Abrams founded and edits Physico-Clinical Medicine. It is published by “Physico-Clinical Co.” at 2135 Sacramento St., San Francisco—the address, according to the telephone directory, of Dr. Abrams’ residence. It is a quarterly “Devoted to the Study of the Electronic Reactions of Abrams and the Visceral reflexes of Abrams, in the Diagnosis, Treatment and Pathology of Disease.” Single copies, one dollar; by the year, two dollars. The publication is, apparently, not entered as second class matter, in fact, presumably, it could not be, as it seems obviously to be an advertising affair. Each issue contains material dealing with “Spondylotherapy,” “Splanchno-Diagnosis,” “Electronic Reactions” and other discoveries and theories of Dr. Abrams. In it also is published a list of “Some recent visitors at Dr. Abrams’ laboratory,” the names and addresses of the “Lessees of Oscilloclast” (about which more later), testimonials for Dr. Abrams, etc.
Of course, it carries advertisements of Dr. Abrams’ “Physico-Clinical Laboratory” (also at 2135 Sacramento St.) and his “Practical Courses in Spondylotherapy and Electronic Diagnosis and Treatment” ($200 in advance). Some of the devices of Dr. Abrams are also advertised. “No apparatus sold on credit. Terms cash.” Among these are:
“Dr. Abrams’ Electrodes for Electronic Diagnosis | $ 6 | .00 |
“Biodynamometer | 36 | .00 |
“Dr. Abrams’ Reflex Set | 36 | .00 |
“Dr. Abrams’ Electro-Concusser | 120 | .00” |
But what seems to be the outstanding piece of apparatus, devised or invented by Dr. Abrams, the pièce de resistance, as it were, of physicoclinical diagnosis and treatment, is the “Oscilloclast.”
This device is not for sale. It can be had only on lease. The first payment is $200 or $250, according to whether it is wired for alternating or direct current. Then there is a monthly payment of $5. Dr. Abrams publishes a list of more than 130 men who have leased one or more “Oscilloclasts.” Sir James Barr’s name heads the list. According to Dr. Abrams, the “Oscilloclast” owes its conception to the therapeutic principles he advocates. These, in part, are:
“1. Physiologic phenomena are manifestations of electronic energy.
“2. Pathologic phenomena are manifestations of perturbed electronic energy.
“3. The energy in health and disease has an invariable and definite rate of vibration (determinable by the electronic reactions).
“4. Specific drugs possess a like vibratory rate as the diseases for which they are effective.
“These like vibratory rates (hemovibrations) of drugs owe their efficiency to their inherent radioactivity. Thus, an obsolete drug like gamboge painted on the chest in incipient tuberculosis will effect a symptomatic cure within a few weeks. Gamboge possesses the same vibratory rate as tuberculosis. Our conception that drug action is dependent on direct cellular contact is thus demolished ...
“5. All forms of energy whether derived from heat, electricity or magnetism may be made to yield different rates of vibration and these rates corresponding to the diseases are utilized for their destruction.”
If one accepts one of Dr. Abrams’ theories, the possibilities of such a piece of machinery as the “Oscilloclast” would seem to loom large, not only in therapeutics, but also in economics. All one needs to do, according to Dr. Abrams, is to ascertain “the vibration rate of a drug” and then to substitute the same vibration as produced by the “Oscilloclast.” Thus, if one substitutes the “vibratory rate of atropin” for the drug itself “the mouth dries or the subject feels as if it were puckered.” Conversely, if you switch the “Oscilloclast” to the pilocarpin vibratory rate, there is a copious flow of saliva.
What some of the lessees of the oscilloclast are accomplishing (if we are to believe the clinical reports published in Physico-Clinical Medicine) may be gathered from the following quotations:
“Woman, Age 52.—Diagnosis of acquired syphilis made by one of our most eminent clinicians. (?) Abrams test showed tuberculosis of the apex of the right lung. No syphilis. Fourteen treatments with the Oscilloclast at 5. Patient gained fourteen pounds in three weeks. Now in perfect health.”
“Mechanic, Age 22.—Acute acquired syphilis. General eruption, throat, mouth symptoms and chancre. Thirteen treatments with the Oscilloclast at 3, and splenic sterilization only. Complete abatement of all symptoms.”
“Woman, Age 42.—Strep infection of the second upper cuspid tooth of three years’ standing. Well developed sinus. Regular discharge of pus. Eight treatments with the Oscilloclast at 2. Clinically cured.”
“Cancer of the pylorus and pylorectomy executed at the Mayo Clinic. Later, vomiting, severe pains, loss in weight, etc. After the third treatment [with “Oscilloclast”] pains ceased and, after 14 treatments, she was well and continued so when I last saw her.”
“Cancer of uterus. Inoperable. Severe uterine hemorrhages. Electrode of Oscilloclast to cervix and hemorrhage ceased after second treatment. After 14 treatments the patient declared she was well. Another case of the same character was followed by equally good results.”
It also seems to be a great business-getter, as the following testimonials published by Dr. Abrams show:
“The Oscilloclast has doubled my business.”—S. King, M.D. (Pa.).
“I am doing good work with the Oscilloclast in T. B. and when I get more room I shall want another machine.”—H. Michener (Kas.).
“We are swamped with work and our three cord Oscilloclast is working to full capacity. We are still astonishing the incredulous and keeping busy. We must have another Oscilloclast at once for there are so many here who demand treatment.”—W. P. Myers, M.D. (Cal.).
More recently, Dr. Abrams has extended his observations and experiments, using what apparently is a modification of the old fashioned pith ball suspended by a silk thread from a rubber rod with which we all experimented during our high school days. This device Dr. Abrams has called the “Electrobioscope.” It is for sale by the Physico-Clinical Co. The “Electrobioscope,” in addition to doing many other things, has demonstrated (to Dr. Abrams) the “sexuality of numbers and sounds.” Thus, if the pith ball is charged negatively and the numbers 1 to 9 are marked on a narrow board and the vowels and consonants are marked on another board, it will be found—still according to Dr. Abrams—that even numbers repel the pith ball while odd numbers attract it. Vowels repel and consonants attract. “A female hair repels and a male hair attracts.” From these data Dr. Abrams deduces that “even numbers and vowels are female and odd numbers and consonants are male.”
The value of music as a therapeutic agent is briefly touched on by Dr. Abrams and we are told that the overture of “Tannhäuser” will increase the pulse rate whereas “Meditation” diminishes blood pressure and pulse rate. “In dogs, music augments elimination of carbonic acid and increases the consumption of oxygen.” Love, says Dr. Abrams, “is dependent upon matter in vibration and the passional component has a wave metric index of 14 in both sexes.” In referring to legendary lore, Dr. Abrams apparently assigns a scientific basis for the belief among the bucolic that carrying around a potato has therapeutic virtue. Thus:
“A cut potato (carried on the person) prevents elicitation of the stomach reflex when the negative pole of a bar-magnet is presented to the stomach region whereas the positive pole will evoke dulness.”
It seems also that the “rheumatic rings” of iron “when worn yield a neutral energy which prevents the elicitation of the stomach reflex by either pole of a bar-magnet.” We learn, too, that the divining rod “no longer belongs to occultism but is entitled to consideration as a scientific fact.”
Dr. Abrams also has investigated methods whereby the sex of the fetus may be diagnosed. In the human these investigations have, apparently, been so limited as to permit only tentative conclusions. In the case of eggs of the domestic fowl, Dr. Abrams reports that with four eggs that yielded negative polarity, the result of incubation was four hens. Of five eggs yielding a positive polarity only two hatched, one was a hen and one a rooster, giving an “error in observation.” Three eggs tested yielded neutral polarity and “as predicted the eggs were sterile.” In case of an egg yielding a negative (female) polarity “an attempt was made to reverse the sex by painting one end of the egg with a yellow coloring material.” The result was a rooster.
Much more might be written about what one of our correspondents calls the wonderful things they are doing in the West, but space forbids. “Neither the fury of tongue,” says Dr. Abrams in the preface to his book, New Concepts in Diagnosis and Treatment, “nor the truculence of pen can discredit the author’s observations which are capable of analyzation and demonstration.” If there is any scientific foundation for the marvels that Dr. Abrams so picturesquely features, the scientific world has not yet found it out!—(From The Journal A. M. A., March 25, 1922.)
In this department of The Journal for March 25 appeared an article on Dr. Albert Abrams and some of his discoveries. We have now received a letter from a physician, who asks that his name be not published, reading:
“To the Editor:—I notice in your article on Albert Abrams the statement that he was born in 1864 and received his degree of M.D. in Heidelberg 1882; if these data are furnished by himself and not a typographical error—though I find the same data in the American Medical Directory for 1916—then it is high time that some board of censors should make a careful examination of his credentials.
“Anybody, like myself, who is acquainted with medical matters in Germany knows that it is preposterous to assume that anybody could obtain the degree of M.D. in any German university at the age of 18 years.
M. D., Leipzig.”
Eighteen is rather young to receive an M.D. degree from Heidelberg! By again going over the various sources of information available the following data were collected: In Polk’s Medical Directory for 1886 Dr. Abrams’ name appears as a graduate of the University of Heidelberg, 1882, and of Cooper Medical College in 1883. The records we have from these two institutions confirm these dates. The year of Dr. Abrams’ birth seems less clear. In the early part of 1902 the American Medical Association sent Dr. Abrams a blank for him to fill out for a permanent record. This was returned in due course and, according to it, Dr. Abrams was born in San Francisco Dec. 8, 1863. This same date appears in various editions of “Who’s Who in America.” A blank sent by the A. M. A. Directory Department to Dr. Abrams in 1908 asking for a personal biographical report was returned Aug. 20, 1908; it gave Dr. Abrams’ date of birth as Dec. 8, 1864. A similar blank sent in the earlier part of 1909 was returned giving the same birth date. We learn, however, that an affidavit executed in 1917 states that Albert Abrams was born in San Francisco Dec. 8, 1862.
Just how long Dr. Abrams attended Heidelberg University before he was granted the M.D. degree, we do not know. Apparently, at that time the standards for admission to that institution were not especially severe and the length of time one would have to attend before being admitted to an examination seems to have depended on the educational credentials that the matriculant offered. What credentials Dr. Abrams submitted, we do not know. Assuming that the earliest date (1862) represents Dr. Abrams’ date of birth, he could have been but twenty years old when he received his M.D. from Heidelberg. This indicates a precocity that might have forecast Dr. Abrams’ later achievements.
Throughout the records of Dr. Abrams’ educational credentials there appears the statement that he also graduated from the “University of Portland” in 1892, receiving the degree of A.M. From references available we have been unable to find any record of a “University of Portland.”—(From The Journal A. M. A., April 8, 1922.)
A somewhat voluminous letter has been received from Mr. Upton Sinclair, which is a defense of Dr. Albert Abrams of San Francisco. We publish Mr. Sinclair’s letter because we believe it is written in honesty and sincerity—and because The Journal readers will enjoy it! It is worth mentioning in this connection that Mr. Sinclair in his latest book devotes a few pages to a eulogy of Dr. Abrams and his methods. This material has not only been reproduced by Dr. Abrams in his “house organ” Physico-Clinical Medicine but is reprinted in leaflet form and is being distributed by some of the individuals who are exploiting the Abrams methods. Such reprints have been sent to this office by both laymen and physicians.
To the Editor.—A few weeks ago you published an article dealing with the discoveries or claims of Dr. Albert Abrams of San Francisco. I happen to be attending Dr. Abrams’ clinic at the time and have discussed this article with him at some length. Dr. Abrams follows the policy of ignoring attacks on his work, taking the view that in the long run, the man who cures disease makes his way in the world in spite of all opposition. However, it is easy to see that he has been deeply hurt by this attack on his reputation, and as one of his friends and most ardent admirers I am taking the liberty of addressing a letter to you.
I do not know if the rules of your publication permit intervention in medical affairs by a mere layman. Permit me to introduce myself as a layman who for some twenty years tried faithfully to be cured of various diseases by many doctors of the best reputation in many parts of the world, and failed; and who, therefore, was compelled, as a matter of self-protection, to look into the question of health for himself. I have read so many different kinds of books on health and made so many experiments of my own that nowadays when I meet with a group of physicians I find that before long they come to accept me as one of themselves. You may not go that far, but at least you may be so generous as to allow me to tell you a little of what I have seen during the time I have spent in the clinic of Dr. Albert Abrams.
I observe that in the course of your two page article dealing with this subject, you nowhere have anything to charge against Dr. Abrams, nor do you show that you have investigated his work. You consider that all you have to do is to quote Dr. Abrams’ own words as to what he can do, and that these words refute themselves. [Italics our.—Ed.]. Also you quote Dr. Abrams’ schedules of prices, and imply that his motives are mercenary. I will take up these two questions one at a time.
First, as to what Dr. Abrams can do: I have been here and have seen him do all that he claims to do. Therefore, you will understand that this portion of your argument does not produce much impression on me. I merely say to you, why do you not come and see, or why do you not send some reliable representative to see—before you take it for granted that Abrams is a knave or a lunatic? This man is not merely a colleague of yours; he is a fellow of the Royal Medical Society of Great Britain [We know of no such society.—Ed.] and surely he was entitled to a little elementary courtesy from you. Why did you not at least write to him and permit him to put before you a little of his evidence on the genuineness of his work? You admit that he is a graduate of the Universities of Heidelberg and Stanford; [Dr. Abrams is not a graduate of “Stanford.”—Ed.] you admit that he was graduated from Heidelberg at the age of twenty. It happens that this was the youngest and remains the youngest age at which any man has taken a Doctor’s Degree at that University in a hundred years. If you had inquired further you might have learned that ten years ago Abrams was one of the most respected physicians in San Francisco. What has he done since to forfeit the honors of a lifetime? All that he has done is to shut himself up in his laboratory and make the most revolutionary discoveries of this or any other age; and now when he emerges and offers this work to the world, you can think of nothing to do but jeer at him.
I spent two weeks in his clinic; then I took six months to write to his physicians all over the country, and to experiment with his cures on a great number of my friends. [Italics again ours.—Ed.] Now I am spending another two weeks in his clinic, and I venture to stake whatever reputation I have, or hope to have in this world, upon the statement that Albert Abrams has discovered the great secret of the diagnosis and cure of all the major disease. [Again we must italicize.—Ed.] He has proven by diagnosing with the taps of his own sensitive finger tips over 15,000 people, and my investigation convinces me that he has cured over 95 per cent. of these who have taken his treatments. Moreover, he has taught his method to 200 or 300 other physicians, and some 80 per cent. of these have submitted to me answers to a questionnaire in which they claim thousands of cures.
You may say, perhaps, that I am not competent to judge of cures. For the sake of argument, I will grant that; but I assert that I am competent to judge of physicians, for I have tested several score of them, and if I ever knew a devoted scientist and a great humanitarian, it is Albert Abrams. In his clinic I have met perhaps a hundred physicians, and I venture to assert that a number of these are men both of integrity and capacity, and when I asked them why they came, I got invariably one answer: “Because I sent him blood specimens and I found that invariably he sent me a correct diagnosis.” Not once, but at least two score times, I have seen Albert Abrams take a blood specimen brought to him, without even the name of the patient, and heard him diagnose cancer or sarcoma, and from the blood specimen locate the growth PRECISELY TO AN INCH. [Italics fail one here!—Ed.] Then I have seen the patient, an entire stranger to Abrams, brought into the clinic and examined, not merely by Abrams, but by a score of other physicians, and the growth found precisely at the spot indicated. (This was done twice between the time when this letter was dictated and the time when it was transcribed.) Three times, yesterday, I saw a diagnosis made of syphilis and the patient brought in, and all the standard reactions demonstrated. I have seen, not once, but hundreds of times, tubercular lesions diagnosed and located from the blood specimen and the patient brought in and the condition demonstrated by percussion. All these things are going on day after day. They are being done in other clinics in several score of cities, and you may have the addresses for the asking. Why do you not ask? [We have some such addresses in the Propaganda files.—Ed.]
I take up the second criticism, that Albert Abrams is mercenary. He charges $200.00 for the clinical course, which may last as long as the physician wishes. It seems to me that that price is to be judged somewhat in relation to what he has to teach. He maintains a large establishment; he has need of many assistants, and expensive apparatus for his research work. He charges for the use of his oscilloclast a deposit of $250.00, and a rental of $5.00 per month. The former item covers the cost of manufacturing the machine, and the second item must be compared with the fact that a great number of physicians who are using this instruments are today enjoying incomes of from $1,000.00 to $2,000.00 per week. [Once more, italics!—Ed.]
A few weeks ago I visited a physician who told me he had treated thirty-two patients that day with his one instrument, and that his income was over $1,300.00 for that week, and I could name several who have given similar accounts. It may be, of course, that you will say they should not charge so much. The average charge is about $200.00 for a guaranteed cure of such diseases as syphilis, tuberculosis, cancer and sarcoma. [Italics our again.—Ed.]. Do you know anyone who will guarantee to cure a cancer or sarcoma at any price? [No!—Ed.]
I am sure you will agree with me that it would be possible to find physicians who would be willing to put up many hundreds of dollars to guarantee that neither cancer nor sarcoma can at the present time be cured except by operation. And I can recall many cases in my lifetime when I paid hundreds and even thousands of dollars to be cured of diseases by the medical profession, and I am unable to recall a single case where I was ever cured of anything. [Still this need not be an indictment of scientific medicine.—Ed.]
Finally, as regards to the subject of mercenary motives, permit me to state that I have in my possession a letter from Dr. Abrams stating that what he desires is to have established an institute for the purpose of making his work known to the world, and that if such an institute is established he is prepared to give up all his other work and devote all his time, without compensation, to the institute. Furthermore, he is willing to furnish his instruments without charge to any medical institution which requests them. Within the last few days, on account of the enormous number of blood specimens brought into his clinic, Dr. Abrams has signed in my presence, and is prepared to issue a statement to the effect that his charge for examining blood specimens is to be raised from $10.00 to $25.00 and all checks are to be made payable to a Trust Fund which is to be immediately established, for the purpose of founding the institution above referred to. I do not see how the medical profession can ask for more than this; but if you do, I should be pleased to receive your suggestions and transmit them to my friend.
Now, this failure to recognize a great medical discovery is an old story. It was the experience of Harvey [We knew poor Harvey would be dragged into this.—Ed.], of Jenner and of Lister. But the world moves on, and men’s brains should improve, and it should be possible to shorten the time of persecution which the great pioneers of science have to suffer. I put to you this simple proposition: Send a reliable man of science to the clinic of Albert Abrams, and let him stay there as long as he pleases and see all that he wishes to see, and then send you a report, and if it indicates that you have blundered in your condemnation, be honest and say so, and save your profession from another black mark against its name.
Upton Sinclair, Pasadena, Cal.
A testimonial is of value to the extent that the person giving it is an authority on the subject on which he testifies. When Mr. Sinclair testifies on socialism we may listen respectfully, believing him competent to express an opinion; but when Mr. Sinclair gives a testimonial on certain bizarre methods of interpreting difficult and obscure problems in medicine, he leaves us cold.
Mr. Sinclair says that he has spent time in Dr. Abrams’ clinic and is wonderfully impressed with Dr. Abrams’ achievements. So is the small boy impressed with the marvelous facility with which the magician extracts the white rabbit from the silk hat. Mr. Sinclair is convinced “that Albert Abrams has discovered the great secret of the diagnosis and cure of all the major diseases.” The small boy is equally convinced that the prestidigitator has solved the mystery of producing snow white bunnies from airy nothings.
Great store seems to be placed by Mr. Sinclair on the favorable reports that he obtained from those who are relieving the public—of from $1,000 to $2,000 a week—by the Abrams methods of diagnosis and treatment. What kind of evidence did he expect to get from such obviously ex parte sources? Mr. Sinclair’s naïveté may be childlike, but it is not scientific. While the significance of the statement may not be apparent to Mr. Sinclair, it is a fact that when the names of the one hundred or more lessees of the Abrams “Oscilloclast” were checked up it was found that a number of these individuals were already in the Propaganda files in some other connection. That these disciples of Abrams, who are “enjoying incomes of from $1,000 to $2,000 a week,” should speak favorably of the Abrams method was inevitable!
Some years ago Upton Sinclair wrote a book on his (at that time) panacea for human ailments. It was the “Fasting Cure.” At that time he told of individual acquaintances suffering from various ailments: one was “dying of kidney trouble”; another was “in the hospital from nervous breakdown”; still another had “only a year to live,” while a fourth was “a nervous wreck, craving for death.” Of these Mr. Sinclair said at the time: “And there is not one of these people whom I could not cure if I had him alone for a couple of weeks.”
At that time Mr. Sinclair was greatly perturbed at the attitude of the medical profession toward his dictum that “the fast is Nature’s remedy for all diseases.” There was just one physician “who was really interested.” This man lived “in an out of the way town in Arkansas” and asked Sinclair to “let him print several thousand copies of the article in the form of a pamphlet to be distributed among his patients.” As Mr. Sinclair said at the time, “one single mind among all the 140,000 [physicians], open to a new truth!” And this “open mind,” that of a man who was practicing in a small town in Arkansas and needed “several thousand copies” of the Sinclair article to distribute to his patients!
After his “fasting cure” experience, Mr. Sinclair had the “raw food” fad—also abandoned in due time. In one of his recent books (“The Brass Check”) he refers to his outgrown fads in the following words: “I ... was willing to try anything in the hope of solving the health problem, which I have since realized is insolvable—there being no diet or system of any sort which will permit a man to overwork with impunity.” He states further in this same connection:
“I look back in retrospect and have not a little fun over my ‘monkey diet’ days.”
Who shall say that ten years hence Mr. Sinclair may not be able to look back, good humoredly, in retrospect, to another time when he was “monkeying” with a subject that was beyond his ken?—(From The Journal A. M. A., April 29, 1922.)
The Council on Pharmacy and Chemistry publishes a report in this issue giving its reasons for deleting “Aspirin-Bayer” from New and Nonofficial Remedies. In order that a standard may be provided, the drug acetylsalicylic is retained259 in N. N. R. under its scientific name, acetylsalicylic acid, aspirin appearing as a synonym. The attempt on the part of the Bayer Company to perpetuate the monopoly it has had for seventeen years in the United States was briefly discussed editorially in The Journal, Aug. 12, 1916. We quoted from Printers’ Ink, a magazine devoted to advertising, in part as follows:
“The manufacturers of aspirin are about to launch an extensive advertising campaign to clinch the market as far as possible before the expiration of their patent rights next year.... The purpose of the campaign is to identify the product with the trademark of the Bayer Company and to this extent hamper competition after the expiration of the patent.”
It is worth while reminding physicians of the privileges the Bayer Company has enjoyed for so many years, owing largely to our inequitable and crude patent laws, or to their construction. First, it should be remembered that practically no other country in the world, not even the original home of the preparation, would grant a patent on either acetylsalicylic acid, the product, or on the process for making that product. The United States granted both! As a result, for seventeen years it has been impossible in this country for anybody except the Bayer Company to manufacture or sell acetylsalicylic acid, either under its chemical name or under any other name. Neither was it possible for individuals, hospitals or any other institutions to import it, legally, for their own use.
Needless to say, the American people have been made to pay exorbitantly for the monopoly our patent office granted this firm. Three or four years ago The Journal, through the American consuls, obtained information regarding the price at which acetylsalicylic acid was sold in foreign countries. At that time, acetylsalicylic acid, as “aspirin,” was costing American druggists—and of course the American public had to pay still more for it—43 cents an ounce. Just across the border in Canada it sold for one-third the price asked here. In some of the foreign countries, acetylsalicylic acid under its scientific name could be purchased by the druggists of those countries at from one-sixth to less than one-tenth the price that it cost American druggists. Here are some of the figures:
Austria-Hungary | 4 cents an ounce | Holland | 4 cents an ounce |
British Isles | 6 cents an ounce | Norway | 4 cents an ounce |
Denmark | 4 cents an ounce | Sweden | 4 cents an ounce |
France | 4 cents an ounce | United States | 43 cents an ounce |
Germany | 4 cents an ounce |
Not content with the iron-bound monopoly which it had been granted through our patent laws, the company attempted further to clinch its exclusive rights by giving the preparation a fancy name, “aspirin,” and getting a trademark on this name. The patent on acetylsalicylic acid expires next month (February, 1917). After its expiration the product, and its method of manufacture, become common property. American manufacturers will now be able to do what manufacturers in other countries, other than the patentees, have long been doing—make and sell acetylsalicylic acid.260
Unfortunately, it is extremely improbable that any American manufacturer will market acetylsalicylic acid under the name aspirin, although we believe they would have a legal right to do so. The courts have held in related instances that when a patented article has been known during the life of the patent under a trademarked name, with the expiration of the patent the name as well as the product becomes common property. The classical “Singer Sewing Machine” decision and the lanolin case are in point. The Bayer Company, through a widespread newspaper advertising campaign, seems to be attempting to perpetuate its seventeen-year monopoly by leading the public to believe that there can be only one brand of genuine acetylsalicylic acid on the market—that made by the Bayer Company.
The firm will, of course, continue to manufacture and advertise the product under the name “Aspirin-Bayer,” and will probably charge high prices for it, as was the case with phenacetin (acetphenetidin). In any event, physicians hereafter should do what for a long time we have been advising should be done, namely, prescribe the compound under its scientific name, acetylsalicylic acid. They should do this if for no other reason than that they would be using the name which carries with it a reminder of the composition of the preparation. Of course, for those who have been writing “aspirin” it will be rather difficult to write “acetylsalicylic acid,” just as a quarter of a century ago it was difficult for the physician of that day who had been using the copyright name “antifebrin” to write “acet-anilid,” a name which nowadays is easy, even for laymen.—(Editorial from The Journal A. M. A., Jan. 20, 1917.)
Under the caption “What’s in a Name?” the current (April) issue of the Journal of Industrial and Engineering Chemistry has an editorial dealing with the nomenclatures—common and proprietary—of acetylsalicylic acid. The editorial was prompted by an article by Dr. Leech printed in the same issue. Replying to its own question:
“The answer to this question so far as it applies to acetylsalicylic acid (popularly known as aspirin) is the difference between eighty-eight cents, the price the druggist must pay for every one hundred tablets of Bayer aspirin, and forty cents, the cost of an equally pure American product. Naturally, this difference in cost is passed on to the individual consumer.
“That no scientific justification exists for this difference in cost is clearly shown in the contribution by Dr. Paul Nicholas Leech, of the Chemical Laboratory of the American Medical Association, page 288 of this issue.
“On the other hand, the excess profit fully warrants the extensive and shrewdly-worded advertising campaign now in progress, a campaign which must eventually fail, because in the first place, it is contrary to the prevailing spirit of modern advertising, the motive of which is constructive rather than destructive, and, in the second place, it appeals merely to the temporary ignorance of the public at large, and has no basis in fact.
“We have been informed that the Custodian of Alien Enemy Property has taken charge of the stock interests of alien enemies in the company conducting this propaganda. Surely the Custodian will not care, even in a trustee capacity, to continue as a participant in a misleading campaign whose sole purpose is the perpetuation of a monopoly hitherto enjoyed under full patent protection.”
The article to which the editorial refers is a somewhat technical one giving the findings of an examination made, at the request of the Council on Pharmacy and Chemistry, in the Chemical Laboratory of the American Medical Association by Paul Nicholas Leech, Ph.D., of various American brands of acetylsalicylic acid (aspirin). The result of the investigation may be summed up briefly in the statement that there are on the American market, made by American firms, several brands of acetylsalicylic acid that are just as good as, if not better than, the Bayer product.
The Journal has called attention to the misleading propaganda on the part of the Bayer Company (Farbenfabriken vorm. Friedr. Bayer & Co.), in its attempt to perpetuate the monopoly granted under our inequitable patent laws. This is done by conveying the inference that the only pure acetylsalicylic acid on the market is that known as “Aspirin-Bayer.” Physicians should again be reminded of the facts in the case of aspirin: Practically no other country in the world, and certainly not Germany, the original home of aspirin, would grant a patent either on acetylsalicylic acid, itself, or the process for making it. The United States granted both! As a result no one in this country except the Bayer Company could for seventeen years manufacture or sell acetylsalicylic acid either under its chemical name or under any other name. Nor was it permissible for hospitals or individuals to import it. While the monopoly held, the American people were compelled to pay from six to ten times as much for acetylsalicylic acid as were the people of Great Britain, France, Germany, Austria-Hungary, Denmark, Holland, Norway or Sweden. At a time when American druggists were compelled to pay 43 cents an ounce for acetylsalicylic acid as aspirin, just across the border in Canada it sold for about one-third the price.
About a year ago, the Council on Pharmacy and Chemistry announced that “Aspirin-Bayer” had been deleted from New and Nonofficial Remedies while the scientific term acetylsalicylic acid was retained along with standards to insure its quality. The necessity for a standard becomes evident when it is remembered that acetylsalicylic acid is not yet an official drug, and its purity, therefore, is not subject to the control of the federal Food and Drugs Act. It is worth while at this time to remind physicians that several brands of acetylsalicylic acid (aspirin) have been found to comply with the standards set by the Council on Pharmacy and Chemistry and have been admitted to New and Nonofficial Remedies.261 These, of course, are thereby subject to the control of the federal law to conform to the standard to which they profess.
Leech’s report gives still greater weight to the suggestion that has been made for some time, viz., that physicians should describe acetylsalicylic acid under its scientific name rather than its proprietary name, even though, in the opinion of The Journal, the proprietary name, aspirin, has become common property since the expiration of the acetylsalicylic acid patent. Every consideration of public interest, of patriotism and of ordinary common sense should prompt physicians to specify acetylsalicylic acid in writing prescriptions.—(Editorial from The Journal A. M. A., April 13, 1918.)
The Journal has received two letters, one from a physician who had written to the New York Tribune protesting against an advertisement of “Aspirin (Bayer)” that appeared in the rotogravure supplement of a Sunday edition and the other the New York Tribune’s answer to the protest. The two letters make an editorial in themselves. Here is the letter of the physician—Dr. Edwin H. Shepard of Syracuse, N. Y.—which was addressed to the editor of The Journal:
“When a great daily newspaper takes a stand for honest advertising it seems worthy that acknowledgement should be made. On April 14 the illustrated Sunday supplement of the New York Tribune, together with many of the other papers of the country, published a duplicate of the enclosed advertisement of ‘Aspirin.’ Your own instructive editorial on ‘Acetylsalicylic Acid, or What’s in a Name?’ had appeared in the copy of The Journal of the day preceding.
“Believing in the sincerity of the Tribune in its effort for honest advertising, I sent them a copy of your editorial together with the page of advertisement, also calling attention to the statements in the advertisement which seemed questionable. Among the questionable matters in the advertisement were the statements, ‘The one genuine Aspirin,’ ‘No other is genuine,’ ‘That which is genuine possesses qualities of excellence never found in imitations,’ ‘For your protection ... every package and tablet is marked with the Bayer cross,’ ‘Your guarantee of purity,’ and ‘Refuse substitutes as they may prove ineffective and harmful.’
“The Tribune was requested to investigate into the standing of the Bayer company and its product. A few days later the enclosed letter was received from the paper’s Bureau of Investigations.”
And here is the New York Tribune’s answer, signed by R. R. Baer, assistant director of that paper’s Bureau of Investigations:
“We have your letter of April 14th, which was acknowledged on the 22nd, in re Aspirin. For your information: Our rotogravure supplement is printed a number of days in advance of the Sunday paper. When these copies which have already been printed are used, no further Aspirin copy will appear. This means a loss of some four pages.”
How many of the numerous medical journals that are still carrying the “Aspirin (Bayer)” advertising would make such a financial sacrifice for mere principle?—(From The Journal A. M. A., May 25, 1918.)
“Aspirin” as a trademark will no longer exist if the recommendation of the Examiner of Interferences of the United States Patent Office is upheld, as it probably would be, should the matter be taken to the courts. The opinion of the Patent Office was the result of a petition by the United Drug Company in the case of that company against the Bayer Company, or, as it was called at the time the suit was brought, the Farbenfabriken of Elberfeld Company. The stand taken by the Patent Office is directly in line with that that has been held in this and other cases by The Journal, which has for years insisted that it was against public policy to permit patentees to extend the seventeen-year monopoly, which the patent laws grant, to a perpetual monopoly by the simple device of obtaining a trademark for the name of the thing patented. It is a fundamental principle in law that “no one can have a monopoly in the name of anything.” This, of course, has been recognized and admitted even by those manufacturers who have attempted to invoke the trademark laws to obtain an unwarranted advantage.
The manufacturers of aspirin have held that the chemical name “monoaceticacidester of salicylic acid” was the true name of the patented article, and was the only name which became public property when the patent right expired. The Patent Office points out, however, that for years the only name that the public ever saw on the brand of monoaceticacidester of salicylic acid made by the holders of the patent on this product was “Aspirin.” The Examiner of Interferences in his decision points out that, previous to 1915, the Bayer Company sold no tablets to the retail-purchasing public, but marketed its product as a powder; further, that it did sell vast quantities of the powder to tablet-makers, who sold “Aspirin Tablets,” and that the consuming public knew the product only by the name “Aspirin.” This name, then, had a significance to the purchaser, similar to that of the word “quinin” on a package of quinin tablets, or the word “calomel” on a package of calomel tablets. As the Patent Office says: “In other words, the prima facie significance of this word ‘Aspirin’ to such purchasers was that of a name”—and as a name it is “necessarily incapable of exclusive use by any one.”
The Patent Office’s decision also brings out the fact that, until the owners of the aspirin patent commenced making tablets themselves, the aspirin tablets on the market were not uniform, and that this lack of uniformity was a fraud on the public which the owners of the aspirin patent should have prevented. The concern did prevent it when it began to make the tablets itself, but maintained in its contention against the United Drug Company that it was unable to control the matter previously—a contention to which the Patent Office gives short shrift. It is further pointed out that the Bayer Company evidently recognized the weakness of its contention by the emphasis it placed through its advertising on the “Bayer Cross.”
When the Bayer Company began manufacturing its own aspirin tablets, it made a pretense of complying with the letter of the law, while violating its spirit, by placing on the label under the word “Aspirin,” the statement that “the monoaceticacidester of salicylic acid in these tablets is the reliable Bayer manufacture.” Says the Patent Office: “With regard to the expression ‘monoaceticacidester of salicylic acid,’ a mere inspection of it is sufficient to apprise any one of its inherent unsuitability for use as a name by the lay purchasing public.” This attempt on the part of the company to “beat the devil around a stump” tended, in the opinion of the Patent Office decision, “to show that the respondent was familiar with the methods of some modern traders to meet the trend of the law.” And, discussing such methods, the Examiner of Interferences says: “A very popular one is for a trader to seemingly bend to the necessity of the situation by placing on the label a notation which in theory, but not in practice, may be used by the public to identify the article after the monopoly has expired. To the examiner this practice seems to be merely a manifestation of that keen commercial instinct which endeavors to keep just ahead of the law. This instinct is fairly common in traders, and is clearly disclosed in trademark infringement cases.”
Summed up, the decision is to the effect that, as in any case prior to 1915, the public had been driven to look on the word “Aspirin” as the name of a thing, and as the Bayer Company had not used the word as a “trademark” within the meaning of the law, the Patent Office recommends that the registration of “Aspirin” as a trademark be canceled. If no appeal is taken from this decision, or in case an appeal is taken, should the opinion be sustained, the attempt on the part of the patentees of aspirin to get a perpetual monopoly on their product through the trademark laws will have been definitely defeated.—(Editorial from The Journal A. M. A., Jan. 11, 1919.)
A correspondent, who asks that his name be not published, writes:
“Your editorial on ‘Aspirin or Acetylsalicylic Acid—An Important Court Decision’ is timely. Too often, I fear, physicians forget ‘what’s in a name.’ I have been told that the Sterling Products Co., the present owners of the Aspirin-Bayer rights, are manufacturers of other ‘patent medicines.’ Are they interested in the Winthrop Chemical Company, which firm seems to be using the much vaunted ‘Bayer Cross’ on the labels of the products formerly imported from Germany by ‘The Bayer Company’? If you answer this in The Journal, kindly omit my name.”
The recent history of Bayer & Co., is somewhat as follows: Shortly after the United States entered the war, the Alien Property Custodian took over the property of Bayer & Co. (Inc.) and its subsidiary, the Synthetic Patents Co. In his report to congress the Custodian said:
“The stock of Bayer & Co. (Inc.) and of Synthetic Patents Co. was sold by me at public auction, the successful bidder being the Sterling Products Co., a West Virginia corporation dealing in proprietary medicines. This company had previously agreed to dispose of the dye plant and patents, in case it secured the property, to Grasselli Chemical Co., one of the largest makers of heavy chemicals in the country. The price paid was $5,310,000, plus back taxes and other obligations of many hundred thousands more.”
After the Sterling Products Company had acquired the pharmaceutical end of the business, the Winthrop Chemical Co. was incorporated in the state of New York. This concern seemingly secured control of all the Bayer pharmaceutical specialties except “Aspirin.” The Bayer Co., it was announced, had been merged with the Sterling Products Co., and “Aspirin-Bayer” added to the latter firm’s list of “patent medicines”: Cascarets, Danderine, Pape’s Diapepsin, California Syrup of Figs, Neuralgine and Dodson’s Livertone. The business is apparently a paying one financially as witness the following excerpt from a recent announcement in a drug journal:
“Stockholders of the Sterling Products Co., Inc., of Wheeling, manufacturers of Neuralgine, Cascarets, Bayer’s Aspirin, and other well known products, and the largest proprietary medicine organization in the world, at their annual meeting received a report of Manager W. E. Weiss, which showed that the company did a $10,000,000 business in 1920. The total profits were $2,100,000, while a total of $1,080,000 was paid out in dividends.
Just what relationship exists between the Winthrop Chemical Co., and the Sterling Products Co., we do not know. As our correspondent points out, the “Bayer Cross” is used on the label of the Winthrop products.
The advertising campaign of “Aspirin, Bayer” since it entered the “patent medicine” field has been typical of that field. By half truths and inferential falsehoods the public has been led to believe that the only genuine aspirin on the market is that put out under the Bayer name. The facts are, of course, that the aspirin of any reputable firm is just as good as the aspirin put out by the makers of Livertone, Danderine and Cascarets.
There is one point, however, that is of vital importance to the medical profession; The decision recently rendered in the United States District Court of Southern New York makes it obligatory for druggists, when filling a physician’s prescription calling for “aspirin,” to dispense the Bayer product. When the public buys aspirin on its own responsibility—without specifying any particular brand—the druggist may give the purchaser any make of acetylsalicylic acid he sees fit. To repeat what was said in The Journal’s comment on this decision: “Unless a physician wishes to cater to the concern owning the Bayer rights and to aid in perpetuating what was a monopoly for seventeen years, he should be careful to prescribe the drug under the term ‘acetylsalicylic acid,’ The court now places the responsibility directly on the medical profession. Avoid ‘aspirin’—write ‘acetylsalicylic acid.’—(From The Journal A. M. A., June 11, 1921.)
It was once said, in the days when diploma mills flourished, that it seemed easier to start a “university” than it was to open a grog shop. A study of quasimedical organizations convinces one that it is easier to found a “medical society” than it is to establish a peanut stand. Most reputable practitioners of medicine who care to affiliate themselves with medical organizations are members of the American Medical Association, its component societies, or similar scientific bodies. It is not surprising then, that those who live and move in the twilight zone of professionalism, from visionaries riding bizarre medical hobbies to those who have special interests to exploit, should create and make use of hybrid medical organizations. Such organizations multiply as rapidly as rabbits. They flourish for a while, obtain more or less newspaper and other publicity—usually more, because of the sensational methods of those controlling them—then, having served the purpose of those who brought them into being, they lapse into innocuous desuetude.
The official accouchement of the Allied Medical Associations of America occurred, according to that organization’s report, May 18, 1918. On the official stationery of the Allied Medical Associations of America in use in May, 1919, we find the names of the “Officers,” “Censors,” etc. These constitute, presumably, the more prominent members of this organization. We give briefly, some data regarding some of these so that a rational perspective may be obtained:
L. M. Ottofy, M.D., St. Louis, Mo.—Dr. Ottofy seems to have been the chief organizer, if not, indeed, the founder. He has been its “Secretary-Treasurer” since its inception; he is also “editor” of its journal. Ottofy, according to our records, was born in 1865 at Budapest, Hungary, and was graduated in 1888 by the Homeopathic Medical College of Missouri. In Polk’s Medical Directories for 1914 and 1917, Ottofy has those extended notices which any physician can obtain who cares to pay for them. According to these notices, Ottofy is, or has been, affiliated with the following “societies”:
President of the International Cancer Research Society.
Ex-President of the St. Louis Society of Medical Research.
Second Vice President of the Missouri Institute of Homeopathy.
General Secretary of the American Association of Progressive Medicine.
Chairman of the Board of Censors of the Missouri Institute of Homeopathy.
Member of the American Institute of Homeopathy.
Member of the Southern Homeopathic Association.
Member of the American Association of Orificial Surgeons.
Member of the Southern Homeopathic Medical Society.
Member of the Kansas City Society of Medical Research.
Honorary member of the Chicago Society of Medical Research.
In December, 1911, numerous newspaper clippings show that Dr. Ottofy was obtaining much publicity relative to his antivaccination activities. At that time the papers reported that Ottofy was suing the St. Louis Board of Education for $25,000 damages, because the board would not admit to the schools of the city a child he had “internally” vaccinated. In November, 1913, the St. Louis Republic reported that Ottofy had claimed to have discovered a serum for the cure of cancer, and quoted Ottofy as claiming “a record of 72 per cent. of cures” in “selected cases.” In February, 1914, the newspapers reported that Ottofy was making a trip east “on the trail of radium for use in his practice in the cure of cancer” and quoted him as stating, “I have learned on good authority that there is radium in Missouri, and just where I refuse to divulge at this time.” In January, 1915, the St. Louis Republic reported that Ottofy, at a meeting of the “St. Louis Society of Medical Research,” had announced that he had perfected a serum treatment for cancer, which “is curing patients who have been pronounced incurable by so-called ‘cancer experts.’ ” In January, 1916, the St. Louis Star reported that Ottofy had sought an injunction against the Board of Education of St. Louis to restrain it from using its funds for “the maintenance of a Board of Hygiene.” In July, 1916, St. Louis papers recorded that Ottofy, who was then running for coroner, had been cited to appear before the prosecuting attorney to explain a charge of passing out, at a political meeting, a card alleged to have borne an indecent drawing of President Wilson. The prosecuting attorney was said to have instructed Ottofy to bring the plates from which the cards were printed to his office. Two days later the papers stated that Ottofy had sent the cards and plates by messenger to the prosecuting attorney’s office.
N. La Doit Johnson, M.D., Chicago.—Dr. Johnson’s name appears as the “First Vice-President” of the Allied Medical Associations of America. A few years ago, Dr. Johnson’s name also appeared as the “Dean of the Faculty” of the “American Post Graduate School.” This “school” was a mail-order concern which, according to the “Annual Announcement,” would grant diplomas and confer degrees as follows: “Master of Surgery,” “Bachelor of Medicine,” “Bachelor of Science,” “Master of Electro-Therapy,” “Doctor of Osteopathy,” “Doctor of Psychology,” “Master of Massage,” etc.
H. M. Goehring, D.O., M.D., Pittsburgh, Pa.—The “Second Vice-President,” according to the letterheads of the “Association” carries the letters D.O., M.D., after his name. So far as our records show, and they are most complete and based on official data, H. M. Goehring is an osteopath, but not a doctor of medicine.
A. E. Erling, M.D., Milwaukee, Wis.—A. E. Erling, according to the stationery, is “Chairman” of “Censors.” Our records fail to show that Erling ever graduated in medicine. The Health Department of Milwaukee, however, says that Erling, when interviewed, claimed to have “a diploma from the German Medical College of Chicago, but refused to show or present the same.” The American Medical Directory has this item:
German Medical College, Chicago. Chartered Dec. 28, 1891, by Johann Malok. Fraudulent. Extinct.
A few years ago Erling was in La Crosse, Wis.; and in 1908 a circular letter bearing his name and picture was sent out from which the following extracts are taken. Capitalization as in the original:
“Dear Friend:—Permit me to call your attention to the fact that Dr. A. E. Erling, the eminent specialist, after many years of travel, practice and medical research, has given up his extensive road practice and severed his connection with the several medical institutes which have heretofore occupied considerable of his attention ... Dr. Erling’s success in the treatment of all CHRONIC DISEASES is truly remarkable. Nervousness, all BLOOD DISEASES, RHEUMATISM, DISEASES PECULIAR TO WOMEN, CATARRH, DEAFNESS, CHRONIC CONSTIPATION ... APPENDICITIS ... PILES, STOMACH TROUBLES, PARTIAL PARALYSIS, etc., give way as if by magic under his skillful method of treatment ... Understand please, that Dr. Erling DOES NOT ACCEPT A CASE FOR TREATMENT unless he can PROMISE A SPEEDY AND POSITIVELY PERMANENT CURE.”
The Journal also has in its files advertisements (vintage of 1915), from some Wisconsin country newspapers, which notify the afflicted that “Drs. Erling and Karass, the expert German Specialists,” could be seen in their offices in the “Schlegel Hotel,” the “Schlitz Hotel,” etc., as the case might be. Whether one of these “German Specialists” was Dr. Arnold E. Erling, The Journal does not know. Official medical records fail to show, at least, that there is any other Erling in the state of Wisconsin.
W. W. Fritz, M.D., Philadelphia.—Another of the “Censors.” This presumably is W. Wallace Fritz, M.D., D.D.S., N.D., D.O., D.C., who was the “Dean” of the “American College of Neuropathy,” and “Professor of Neuropathy” at the same institution. According to newspaper reports published when the “dean” of the American College of Neuropathy was called into court to testify regarding the “school,” Fritz admitted that when he became dean of this “college,” the “college” had three students and thirty “Faculty Members”! Fritz, it should be mentioned in passing, is a member of the Philadelphia County Medical Society and by virtue of this membership he has qualified as a Fellow of the American Medical Association! Recently Fritz’s name appeared in connection with the formation of a new organization, founded, it appears, for the laudable purpose of fighting the “Medical Trust.” Fritz, according to the newspaper reports, is treasurer of this new organization, which has adopted the inspiring title, “Constitutional Liberty League of America” and seems to be a later edition of the mushroom “National League for Medical Freedom.” Quoting from the newspaper report:
“Dr. W. Wallace Fritz, a member of the American Medical Association, created a profound impression when he said that all health laws were written by agents of, or members of, the American Medical Association, and that this organization was at once the most powerful and the most baneful of all the American Trusts. Dr. Fritz then went on to say: ‘Most of the drugs administered are worthless. Most of the doctors who prescribe them are incompetent, but both the injurious drug and the ignorant prescriber are protected, in and out of court, by the American Medical Association, which trust is now raising a vast fund with which to drive all drugless healers out of the profession. Medicine is the camouflage used to conceal the most alert, the most rapacious and the least patriotic of all the trusts milking the American people. The tyranny of the Medical Trust is unbelievable. It is also un-American.’ ”
The Philadelphia Sunday Transcript of May 4, 1919, had a five column article under the name of W. Wallace Fritz. It is a most vituperative affair, and reeks with fire and brimstone. It is directed chiefly against the American Medical Association, and physicians are dubbed “Prescription Writing Drug Peddlers Who Prosper Through Monopolistic Laws Rather than by the Practice of an Exact Science.” In the course of this diatribe we read:
“The members of the American Medical Association are manifesting an unwarranted interest in the dear people, who, in their assumption, need quinin and mercurial guardian; who under this class legislation confines us to this monopoly of the big and little pill, is trying by hook and crook to shut out the natural and rational methods of cure which are driving the drug monopoly from the face of the earth. Diagnosis and consultation consist in four or five medical doctors, whose faces denote death, sitting around a sick man and guessing what ails him. After that has been performed they guess at what will cure him, and that is generally a sure sign the undertaker will follow.”
C. O. Linder, M.D., Spokane, Wash.—This gentleman (another “Censor”), seems to be an osteopath, who some years ago was “Assistant Secretary” of the “Washington’s Physicians’ Association,” founded apparently by rebels within the osteopathic ranks who denounced the Washington Osteopathic Association as a “professional trust”! Linder apparently claims graduation in 1905 from the “Thompsonian Medical College” of Allentown, Pa. The following item from the American Medical Directory regarding this school is of interest:
“Thompsonian Medical College, Allentown. Organized in 1904. Fraudulent. No evidence to show classes were ever held.”
A. H. Flower, M.D., Boston.—Still another “Censor.” Flower, according to the notice that appears in Polk’s Directory for 1917, claims graduation in 1888 from the “American Health College” of Cincinnati, and in 1894 from the “American Health University” of Chicago. Quoting again from the American Medical Directory, here is what we find regarding the former “college”:
“American Health College, Cincinnati. Organized in 1874 and re-organized in 1876. Conducted by a Dr. Campbell who originated and copyrighted the so-called ‘Vitapathic System,’ Fraudulent. Extinct about 1888.”
We have no record of an “American Health University” of Chicago, although there was an “Illinois Health University” of Chicago, one of the numerous diploma-mill swindles operated by Armstrong. It was declared fraudulent by the federal authorities and its charter was revoked in 1897. Flower, according to the notice in Polk’s Directory, is:
Ex-President Maine Eclectic Society.
Ex-President New England Eclectic Medical Association.
Member National Eclectic Medical Association.
Member American Progressive Medical Society.
Member Massachusetts Eclectic Medical Society.
Z. L. Baldwin, M.D., Kalamazoo, Mich.—Possibly the data just given concerning some of those whose names appeared on the organization’s stationery are more than sufficient for the average physician to get a perspective of the Allied Medical Associations of America. Still, it is worth mentioning that in a letter recently sent out by Ignatz Mayer, extending an invitation to the annual convention of the Allied Medical Associations of America, Mayer took the opportunity of incorporating in his letter a letter which one of the members of the “association” had been sending out, urging individuals to join. The member in question was Dr. Z. L. Baldwin of Kalamazoo, Mich. Dr. Baldwin, as some of our readers may remember, is the gentleman who, a few years ago, was exploiting an “Intravenous Treatment” for the cure of tuberculosis. According to the claims made at that time:
“... for the first time in the history of medicine, we have a successful treatment for tuberculosis.
“... we are able to kill the germs of the disease in the body, thoroughly ridding it of all tubercular infection, destroying the germ and its poisons likewise.”
This was a few years ago. Whether Dr. Baldwin is still specializing in consumption we do not know; apparently not, as we notice that at the first meeting of the Allied Medical Associations, Baldwin’s name was on the program for the “Cure of Goiter by Adjustment of Lenses.”
George Starr White, M.D., F.S.Sc., Lond., Los Angeles, Calif.—A letter received by a physician a few days before the recent convention of the Allied Medical Associations, held out as an inducement to be present the fact that “Geo. S. White will show you how to diagnose disease by means of dif. colored lights and the reaction of the body to the magnetic meridian.” Dr. George Starr White was the “Second Vice-President” of the Allied Medical Associations in 1918. White, according to our records, was graduated in 1908 when he was forty-two years old, by the New York Homeopathic Medical College and Hospital. He was licensed in New York in 1908, in California, Connecticut and Nevada in 1913, and in Michigan in 1916. He seems to have been one of the proponents of “spondylotherapy,” “zonetherapy,” etc., and in 1915 it was announced that he would give one week courses in “Spondylotherapy” in Chicago, Kansas City and Denver, respectively. In his advertisement he emphasized that he was a Fellow of the American Medical Association, which, while true at the time, is no longer true, as on Feb. 4, 1916, he was expelled from membership in the Los Angeles County Medical Association. In May, 1915, White was arrested in Chicago and fined $100 and costs for practicing medicine without a license. Dr. White’s specialty seems to be what is ponderously known as “Bio-Dynamo-Chromatic Diagnosis.” This has been described by one of its enthusiastic adherents as “Diagnosis by Sympathetic Vagal-Reflex.” To obtain the “Sympathetic Vagal-Reflex” it seems the patient must face east or west and have his bare abdomen percussed until a dull area is located. The patient is then faced north or south and again percussed. Then, it seems, different colored lights are thrown on the patient, the location of the areas of dullness being determined meanwhile. A combination of ruby and blue lights “will cause a reflex in cases of gonorrhea,” a “green light will cause a reflex in cases of liver or gallbladder trouble,” while the color for carcinoma is orange red! During the height of the influenza epidemic last winter, White seems to have put on the market “Valens Essential Oil Tablets” which were for “Gripping the Flu out of Influenza,” and were also said greatly to benefit or cure incipient tuberculosis, hay-fever, asthma, and “catar.” The letters “F.S.Sc., Lond.” after Dr. White’s name look well, sound well, and have an air of erudition and mystery that is well worth what they cost. They mean “Fellow of the Incorporated Society of Science, Letters and Arts of London, Ltd.” The “Fellowship” costs one guinea. Not a few “patent medicine” exploiters in the United States carry these mystic letters after their names. The society in question was a seriocomic concern that was exposed by London Truth some years ago and was also dealt with in The Journal of May 29, 1909, in connection with the “Aicsol Consumption Cure” exposé.
So much for the Allied Medical Associations of America. At their recent meeting in New York City they got much newspaper publicity because of their action on the prohibition question. According to the newspaper reports, the organization adopted a resolution declaring that “properly brewed lager beer is absolutely essential in the treatment of certain cases.” They were further reported as endorsing the manufacture of light wines and of beer containing not to exceed 2.75 per cent. alcohol. As a piece of publicity work this resolution was all that its sponsors could expect. The Journal office was flooded with telegrams and letters from physicians, temperance workers, congressmen, church organizations, and others, asking, in effect, What is the Allied Medical Associations of America? This is our apology for giving the amount of space necessary to a proper understanding of this organization. Today the rocket of the Allied Medical Associations of America is blazing a more or less erratic course across the sky of publicity. The stick will be down anon! Any resolution or expression of opinion by this organization, or others of its type, when dealing with the broader problems of public health, is wholly without scientific significance, whether such resolutions are good, bad or indifferent.—(From The Journal A. M. A., July 5, 1919.)
The September issue of the Archives of Dermatology and Syphilology presents a number of significant features regarding the use of arsphenamin and related compounds that are at present being widely employed in the treatment of syphilis. To one who studies these statements of laboratory and clinical investigators in the special field involved there must come the conviction that many therapeutic perplexities still remain at the end of nearly a decade of trial for the types of compounds which Ehrlich introduced. It is well for the practitioner to realize this, especially when expert workers still make an appeal for conservative interpretations. Stokes forcefully summarized the situation when he stated at the New Orleans session of the American Medical Association:
Too short a time has elapsed since the discovery of these drugs, and too little is as yet known about the ultimate problems of the pathology, immunology and parasitology of syphilis, to justify the announcing of new infallibilities. The necropsy pathologist of the next fifty years may well, like Warthin, upset our most plausible generalizations of today. Seasoned tradition and conservatism are still the wisest guides in our interpretation of clinical cure. Arsphenamin has made it apparently possible and even probable, but only to the inexperienced has cure been made absolute and inevitable.
It is recognized that the exact composition of arsphenamin in its available form is not fully determined. As has been emphasized again, the quantitative determination of arsenic alone in arsphenamin is insufficient to estimate its purity; in fact, the interstate sale of arsphenamin is controlled by toxicity tests on guinea-pigs made by the Hygienic Laboratory of the United States Public Health Service. Consequently, practical medicine must be on its guard to employ a product which is carefully controlled by such toxicity tests as well as by other criteria. It will not do to charge untoward results offhand solely to idiosyncrasy of the patient, faulty administration or other errors in technic. The drug itself still has inherent dangers. It should be borne in mind also that neo-arsphenamin behaves differently in the animal organism from arsphenamin, and should not be regarded simply as arsphenamin in a convenient form for administration.
It is gratifying to learn from a government expert that after the long struggle to produce satisfactory products, arsphenamin preparations made in the United States are generally less toxic than those of foreign manufacture. Neo-arsphenamin preparations made in the United States compare favorably, and in certain instances are decidedly less toxic than most of the foreign products. Timely presentations of the faults and dangers as well as the undisputed advantages of current therapy in the management of syphilis should be welcomed.—(Editorial from The Journal A. M. A., Oct. 9, 1920.)
The Public Health Service some time ago262 warned against the use in syphilis of new arsenicals which are not related to arsphenamin; it was stated that a number of such were being sold with unwarranted claims as to their value. At least three such arsenicals have in recent years been the subject of some exploitation for use in this disease: sodium cacodylate, the sodium salt of methyl arsenic acid (“Arrhenal”) and the sodium salt of ethyl arsenic acid (“Mon-Arsone”).263 As regards the first two, Castelli showed several years ago that neither has any action on experimental trypanosomiasis and spirochete infections; careful clinical observations in this country have confirmed the inefficacy of sodium cacodylate in human syphilis.264 Voegtlin and Smith265 of the Hygienic Laboratory have now shown in animal experiments that ethyl arsenic acid (“Mon-Arsone”) is devoid of any practical trypanocidal action. Thus the “therapeutic ratio” (the ratio of the minimal effective dose to the lethal dose) was about 1, that is, it was effective therapeutically only in approximately fatal doses, the therapeutic ratio for arsphenamin in similar conditions was 17, and that of neo-arsphenamin, 28. In fact, the conditions with ethyl arsenic acid were no more favorable than were those with arsenous acid (the active constituent of solution of potassium arsenite), although it was far less poisonous. The validity of such experiments in determining the probable value of drugs in human syphilis cannot be questioned:266 it was by such experiments that Ehrlich and his co-workers found two or three of six hundred and six arsenic preparations studied to be of value, and of the next three hundred or more studied only one (neo-arsphenamin) worthy of trial in human medicine. The time has passed when a high arsenic content of a compound and a low toxicity, and a number of cases of apparent clinical improvement, can be assumed to indicate that a drug has any real value in the treatment of syphilis. Many organic compounds of arsenic as well as other drugs may cause temporary or apparent improvement in syphilis, but to date only those related to arsphenamin have proved of real value and comparatively safe. Others which had some real value proved to have dangerous side effects; readers will recall the history of arsanilic acid (“Atoxyl” or “Soamin”) and its acetyl derivative (“Arsacetin”).—(Editorial from The Journal A. M. A., Feb. 26, 1921.)
The Journal has already commented on the difficulty in securing salvarsan, on the moral and ethical question as to whether or not it is justifiable for one person to control the output of a drug necessary to public health. This week we publish an account of the action of the St. Louis and Chicago medical societies, which are calling on the medical profession to appeal to their senators and congressmen to abrogate this patent. The Journal believes that this patent should be abrogated, not alone because the patentees have not supplied the demand, not alone because they have dictated to the medical profession who should have the drug and how much a physician might have, not alone because of the war with Germany, not alone because of the special needs of the government at this time for the control of venereal diseases, not alone because, as some claim, the patent at Washington does not correctly describe the product, but also because the people who are supplying this product are charging prices that are exorbitant compared to the price at which others in this country can supply it. The fact is that the salvarsan one can obtain today costs $4.50 per ampule of 0.6 gram, whereas the same dose of arsenobenzol—a preparation identical with, if not better than, salvarsan—costs $2.00 at retail, and as Dr. Schamberg says: “If we are permitted to continue marketing the same drug after the war, we can sell it at $1.00 or less per tube.” To abrogate this patent would be doing an injury to no one. Certainly the patentees of salvarsan have already reaped their harvest—and a pretty rich one. The supply of salvarsan at a reasonable price in proportion to its actual cost of production is in the interest of the health of the entire population of the country, whereas to let matters rest as they are, is to the benefit of one man. While we are emphasizing here the cost, there is after all a greater question, and that is the supply necessary to help control the ravages of one of the most serious diseases which afflict humanity today. It is the duty of Congress to abrogate the patent on this preparation and, incidentally, on all medicinal preparations of importance.—(Editorial from The Journal A. M. A., April 21, 1917.)
The Adamson Bill, known as the “trading with the enemy act,” has recently been passed by the House of Representatives, is now before the Senate, and will doubtless be enacted into a law. One of its clauses confers authority on the Federal Trade Commission to grant licenses to citizens of this country to operate patents owned by enemy aliens. Physicians are interested in the bill primarily because it includes the salvarsan situation. The manner in which salvarsan has been supplied in this country has been so arbitrary and the prices charged so tremendously above the actual cost, that we should not be satisfied unless the monopoly is ended so that the drug can be supplied at least at a fairly moderate figure, and the old methods eliminated. It is to be hoped, therefore, that the Federal Trade Commission will not grant exclusive control—that is, exclusive license—to any one person or firm. To do so would simply perpetuate the old monopoly and the old conditions. England has adopted a law, which, in principle, is similar to the Adamson Bill, and there several concerns have been licensed to manufacture the product. The same should be done here. The Dermatologic Research Laboratories of Philadelphia announce that they can supply arsenobenzol at $1.50 a tube, and that there is immediately available a supply sufficient for any demand that may be made. The same laboratories have announced also that in a few months they will be able to supply hospitals for $1.00 a tube. Considerable responsibility rests on the Federal Trade Commission in this matter, for it is not only a question of monopoly, but also a question of scientific qualifications and ability to make the product on the part of some who may make application. Undoubtedly the commission will secure the cooperation of the United States Public Health Service, under whose supervision these drugs should be manufactured no matter who shall be licensed to make the product.—(Editorial from The Journal A. M. A., July 21, 1917.)
No, this is not a new chemical; it is simply the name adopted by the Federal Trade Commission for the hydrochlorid of 3-diamino-4-dihydroxy-1-arsenobenzene—in other words, salvarsan. As our readers already have been informed three firms have been licensed to manufacture and sell arsphenamin; but, while each manufacturer may have his own trade name on the label, the official name must be the prominent one on all packages. Hence, physicians should at once make it a point to learn and use the name “arsphenamin” in place of salvarsan. At first sight, arsphenamin looks formidable. In reality, it is just as easy to familiarize oneself with the word “arsphenamin” as it was to learn to use the terms “salvarsan,” “arsenobenzol” or any other of the trade names.—(Editorial from The Journal A. M. A., Jan. 19, 1918.)
Our readers may remember that an article appeared in this department of The Journal for July 6, 1918, under the title “Henry Smith Williams and ‘Proteal Therapy.’ ” “Proteal Therapy” is a treatment exploited by Henry Smith Williams, M.D., of New York, for use in tuberculosis, cancer, rheumatism, etc. It is apparently a modification of the “Autolysin” cancer “cure” which Williams had previously puffed in Heart’s Magazine.
The Journal’s article pointed out that Henry Smith Williams, although entitled to write “M.D.” after his name, is essentially a journalist. He has written voluminously for some years in lay publications on various subjects, both under his own name and under his nom de plume, “Stoddard Goodhue, M.D.” In addition, Williams runs a publishing concern called the Goodhue Company, which issues a number of books, many of them being reprints of Williams’ own articles.
Closely associated with Henry Smith Williams is his brother, Edward Huntington Williams, who also is a prolific writer. The Journal’s previous article called attention to the fact that there had been sent broadcast to physicians a neat little cloth-bound book, entitled, “Alcohol, Hygiene and Legislation.” This book, which evidently cost somebody a good deal of money to distribute gratis, was published by the Goodhue Company, and was written by Edward Huntington Williams. Enclosed with the book was an advertising leaflet on the “Autolysin” cancer cure and also a letter from the Goodhue Company, asking physicians to accept it “with our compliments and the compliments of the author.” The letter was chiefly devoted to calling attention to Henry Smith Williams’ “new book, the Autolysin Treatment of Cancer.” The last thirteen pages of the book “Alcohol, Hygiene and Legislation” contained advertisements of the Goodhue Company’s publications, particular emphasis being placed on the “Autolysin Treatment of Cancer,” by Henry Smith Williams.
So much by way of retrospect. Now comes information that may throw an interesting side-light on the matter just presented. There is at present being conducted by a committee of the United States Senate, an investigation relative to the purchase of a Washington (D. C.) newspaper with money alleged to have been furnished by those interested in the brewing industry.
At the opening hearing before the Senate Committee, Tuesday, November 19, the secretary of the United States Brewers’ Association, after admitting that brewers’ propaganda had been published in the International Monthly, edited by Viereck (of the Fatherland), also declared that the Publication Committee of the brewers’ association employed writers to “write up certain subjects” relating to the brewers’ trade. One of the writers mentioned in this connection was, according to the newspaper reports, “Dr. Edward H. Williams, author of articles published in medical and other journals.”
With this fact before us, it seemed worth while to go through the book that had been distributed so lavishly to physicians with the compliments of the Goodhue Company and Dr. Edward Huntington Williams, in the exploitation of “Autolysin,” and Henry Smith Williams’ book on the subject.
The first chapter of “Alcohol, Hygiene and Legislation” consists of a reprint of an article from the New York Medical Journal of May 8, 1915. The article is a skilful presentation of the case for the defenders of the lighter alcoholic beverages, especially beer. This chapter and all succeeding chapters of the book attempt to discredit prohibitory legislation, and argue that prohibition drives the public to the use of the more ardent alcoholic beverages, while preventing the use of the milder beverages, such as beer, which one is led to infer is not particularly harmful. Throughout the book, also, the state of Kansas is held up as an example of the harm done by prohibition, and the theme is developed that insanity and the use of cocain and other habit-forming drugs follows in the wake of prohibition. The following extracts are from Chapter I:
The evil effects of beer and wine, for example, are greatly less than those produced by spirituous liquors.... [Italics ours.—Ed.]
If our theory of immunity is correct we should expect to find that the older beverages, such as beer and wine, which have been used for thousands of years, are less productive of alcoholic insanity, for example, than the spirituous liquors which are recent innovations. In point of fact we find this to be the case: the spirituous liquors are almost wholly responsible for all forms of alcoholic insanity. [Italics ours.—Ed.]
Chapter II is a reprint of an article that appeared in Everybody’s Magazine, August 1914, and deals with “Legislation from a Medical Viewpoint.” It is to the effect that drug addiction and insanity, together with special forms of mental disease directly attributable to alcoholism, seem to flourish best in prohibition territory.
Chapter III deals with “The Peace and War Footing of Alcohol,” and is a reprint from the Medical Record, Aug. 7, 1915. It, too, sings the praises of the “lighter beverages,” while deprecating the use of “ardent spirits.” For instance:
An overwhelmingly large proportion of persons who develop alcoholic psychoses in America are drinkers of whisky, or some corresponding ardent spirit, whereas this condition is seldom seen in beer and wine drinkers. [Italics ours.—Ed.]
Thus we find the highest percentage of alcohol psychoses among the whisky drinkers who come from western Europe, while the wine and beer drinking races of central and southern Europe show a distinctly lower percentage, in some instances only about one-fourth as many per thousand. [Italics ours.—Ed.]
Chapter IV deals with “Some Aspects of Liquor Legislation.” Like Chapter II it is an indictment of prohibition, and the United States Census Bureau’s reports are called on to sustain this thesis. Quotations, too, are made from the writings of Henry Smith Williams further to prove the point. “Dry” Kansas and “wet” Nebraska are frequently compared, to the detriment of the former. One who accepts the statements in this chapter will get the impression that Kansas has more lawlessness, illiteracy, pauperism, and insanity than Nebraska.
Chapter V deals with “The Problem of Legislation.” It is based on the premise that “prohibition does not prevent the consumption of liquor,” but on the contrary, “prohibitive legislation induces the consumption of the most harmful form of liquors.” Stated in another way, it is equivalent to charging that prohibition is hard on the brewers, but beneficial to the distillers. In fact, E. H. Williams, in another book (“The Question of Alcohol”—Goodhue Co.) which also champions the case for the milder alcoholics, quotes Henry Smith Williams as saying, relative to prohibitory legislation: “In general, it would appear that, if our legislators of recent years had been in league with the distiller, they could not have served his purpose better.”
Whether or not Edward H. Williams’ or Henry Smith Williams’ conception of the alcohol problem is good, bad or indifferent, need not at this time concern us. The medical profession, however, has a right to ask two questions: First, Is the Dr. Edward Huntington Williams who wrote “Alcohol, Hygiene and Legislation” the “Dr. Edward H. Williams” who was employed by the brewers to write propaganda favorable to the brewing interests? Second, Was the cloth-bound book, “Alcohol, Hygiene and Legislation,” which was distributed by the Williams brothers, paid for, wholly or in part, by the United States Brewers’ Association?
For those who wish to read Dr. Edward Huntington Williams’ opinion on the alcohol question, the following bibliography may be of service:
“Liquor Legislation and Insanity”: Medical Record 84:791, 1913.
“The Liquor Question in Medicine”: Medical Record 85:612, 1914.
“Inebriety as a Medical Problem”: Post-Graduate 29:603, 1914.
“The Problem of Inebriety”: N. Y. Medical Journal 101:940, 1915.
“Aspects of Inebriety in Alcohol”: British Journal of Inebriety 13:9, 1915–1916.
“The Peace and War Footing of Alcohol”: Medical Record 88:226, 1915.
“Alcohol and Therapeutics”: Medical Record 92:666, 1917.—(From The Journal
A. M. A., Nov. 30, 1918.)
The danger of commercialized therapeutics has been enormously increased by the introduction of biologic products. These substances offer a rich field for the commercially minded, first, because of the remarkable results which seem to have followed the use of certain products of this type; second, because the field is new and the mode of action of these substances not readily understood and, third—and most important—because, by the very nature of the problems involved, few physicians are well informed concerning them. The influenza epidemic of last year was widespread and fatal in character. It stimulated earnest research in methods of prevention and cure. We were all in a frame of mind to grasp at any straw. Here and there some worker would cry “Eureka”—only to be disappointed when his product was actually put to the test. However, there were more than enough manufacturers ready to place any product on the market with specious claims that could not be positively denied. Vaccines, serums, proteins—all were advanced with such glowing statements as to their properties that only those physicians who kept their feet firmly on solid ground could resist the appeal. Now we have had another epidemic—mild, it is true—but the memories of last year make the average physician ready to accept anything which promises hope, and the manufacturers “make hay while the sun shines.” Physicians have been and are being deluged with literature on the prophylaxis and treatment of influenza. So far as we know, few publications have contained any word of warning on these matters. One exception has just come to notice: the Medico-Military Review, a semimonthly mimeographed publication sent to medical officers of the Army by the Surgeon General’s Office. This says:
You Are Reminded that so far a comprehensive analysis of results obtained by the use of monovalent and polyvalent vaccines in the prevention of influenza has not demonstrated their value. Much carefully controlled experimental work is now being carried out on this subject both in civil institutions and in the Army, and any worthwhile advances will be reported in the Review from time to time. If a prospective vaccine is developed, it will be prepared at the Army Medical School for general distribution and all medical officers will be duly notified. The general use of the present commercial polyvalent protective against influenza is not considered desirable. Numerous telegrams and other requisitions are being received for influenza vaccine. In view of the fact that no prophylactic influenza vaccine is available, such requisitions should be discontinued.—(Editorial from The Journal A. M. A., Feb. 14, 1920.)
U. S. Marine Hospital, Chicago.
To the Editor:—A member of the consultant staff of this hospital recently referred to us a “Doctor” H. F. Matthews, who was supposed to give demonstrations of a new test for syphilis—“Capell’s ‘Uroluetic’ Test.” The test was to be made of the urine of the patient. The above mentioned consultant was under the impression that the said “Doctor” Matthews was a graduate physician.
“Doctor” Matthews came to the hospital according to the appointment made by the consultant, and proceeded to give his demonstration. Several of the junior officers and interns were present to witness it. He was asked questions in an attempt to determine the scientific status of the test which he was demonstrating. His answers were always vague and indefinite and not clothed in scientific words.
We became suspicious of him, and he was asked if he was a graduate physician. He admitted that he was not. He was further asked if he had studied chemistry and bacteriology; he stated that he had in 1888. Inquiry was made as to where; he replied that it was at the University of Illinois. He was further asked if he was familiar with the Wassermann reaction. He stated that he was not.
This man is going around representing himself as a physician who has a new test which he claims is superior to, and more delicate than, the Wassermann test; yet he knows nothing whatever of the technic of the Wassermann reaction.
In one case, we gave him the same specimen of urine in four different containers. He read a different degree of reaction for each of them. In other words, in a specimen from the same patient, his four different tests showed, respectively, a +, a ++, a +++ and a ++++ reaction.
It occurred to me that it might be well to inform you of this man’s methods, as he told us that he had been to a good many institutions, and I am sure he will soon start a plan to systematically force his pseudoscientific test on credulous physicians everywhere.
J. O. Cobb, M.D., Senior Surgeon in Charge.
The Propaganda Department has in its files a business card reading: “Capell’s Laboratories, Room 1510 Masonic Temple, Chicago. Dr. H. F. Matthews, Special Representative.” Capell’s Laboratory has its headquarters in Omaha, and is apparently conducted by Dr. W. L. Capell, who, for many years, seems to have been more or less interested in proprietary medicines. Some years ago he was connected with a concern known as “Acneine Pharmacal Company,” which, apparently, was dissolved some time in 1910; and soon thereafter a new company was organized known as the LeRoy Drug Company. In 1917 W. L. Capell was connected with the Capell, Cameron Co., Inc., of Lincoln, Neb., which was selling “Capell’s Uroluetic Test”, “Capell’s Treatment for Syphilis” and other remedies. The “Treatment for Syphilis” was said to be
“Painless, Pleasant, Harmless, Efficacious, and requires usually from 30 to 90 days only to eradicate the disease.”
The name of the treatment is “Mercarodin”—earlier it was called “Camit”—and it is now being sold from “Capell’s Laboratory,” Omaha. In addition, Capell’s Laboratory sells Acneine, which apparently is the same product that was sold in 1906 and 1907 under the name of “Sambu-Co” by the Holtman-Stringer Co. of Omaha and later was put out by the Acneine Pharmacal Company of Omaha.
While Capell’s Laboratory sells proprietary remedies, it is the “Uroluetic Test” which the concern now seems to be featuring. The claims made for this are:
“This test requires no expert knowledge, is inexpensive, and can be made in a few minutes, and is so plain that it cannot be mistaken.”
The idea of being able to determine the absence or presence of syphilis by a simple color test of the urine is a fascinating one. The present reliable diagnostic tests are, as Capell’s Laboratory so plausibly emphasizes, somewhat involved, and call for rather delicate technic. But there are no short-cuts to knowledge.
A physician who ordered Capell’s Uroluetic Test some weeks ago received with the bill the letter that follows: It is given not so much for what it says, as for how it says it. It is copied verbatim et literatim:
“Your letter received, and we have mailed you as per your letter 1 Doz. of Capell’s ‘Uroluetic’ Tests. In close find statement and instructions, for same.
“The ‘Uroluetic’ Test is meeting a far greater approval from the Medical profession than we had expected, while we do not claime that it is perfect, yet we have only received one unfavorable report, and we daily feel incuraged in its efficacy.
“You know Doctor that there are two dangerous elements in this world, one is the extreme pessimist and the other is the extreme optimist. The immoral Lincoln said, ‘That there was nothing that was wholly good or wholly evil,’ and we presume that this is equally true of the ‘Uroluetic’ test. But we want the truth no matter what it is.”
“Capell’s Uroluetic Test” would be “important if true.” Unfortunately, its scientific value to the sufferer is negligible compared with its economic value to the exploiter. It is not so much a test for lues in the patient as of credulity in the doctor.—(From The Journal A. M. A., Aug. 23, 1919.)
To the Editor:—Will you kindly inform me whether the test in the enclosed “literature” is what it is represented to be?
Charles M. Thomas, M.D., Sunbury, Pa.
Answer.—The “literature” referred to by Dr. Thomas dealt with the “Uri-Na Test” sold by the Standard Appliance Company of Philadelphia. There seems to be a strong family resemblance between this alleged test and that known as “Capell’s Uroluetic Test,” which was discussed in the Propaganda Department of The Journal, Aug. 23, 1919. Of that The Journal said: “Unfortunately, its scientific value to the sufferer is negligible compared with its economic value to the exploiter. It is not so much a test for lues in the patient as of credulity in the doctor.” The same may be said of the “Uri-Na Test.” The facts are, there is no method at present known by which the absence or presence of syphilis may be determined by a simple color test of the urine.—(Query in The Journal A. M. A., Nov. 22, 1919.)
Within the last three years a number of reports have appeared in the medical press which bear on the treatment of malignant growths in human beings by chemical preparations. The most persuasive and the most insistent claims have been made in connection with the colloidal solutions of certain metalloids and metals, notably selenium, vanadium and copper. At the same time a number of drug houses, both in this country and abroad, have placed on the market proprietary preparations of these substances in various forms, for which the claim is made that they produce striking therapeutic effects and sometimes even cures in malignant neoplasms.
The impulse toward the use and production of this type of preparation is directly traceable to a series of scientific experiments on the tumors of animals, which date back no farther than the year 1911. In that year Wassermann and his co-workers267 published a report on the treatment of rat tumors by means of the intravenous injection of selenium compounds. This paper received wide notoriety through its enthusiastic diffusion by the lay press. Shortly afterward Neuberg and his co-workers268 published their observations upon the therapeutic effects of certain metallic compounds. The clinical application of the encouraging results obtained by these authors in animal tumors followed rapidly, and up to the present time a number of papers have appeared in which the claim is made that human tumors also may be favorably influenced through the constitutional use of substances similar to those used by Wassermann or Neuberg. In some cases, use has been made of colloidal solutions of the heavy metals, such as copper; in others, selenium compounds have been used, while in a third set of observations the therapeutic agent represents an attempt to combine the virtues of these two types of therapy by employing selenium in colloidal form. As an example of the first class, may be cited the cuprase of Gaube du Gers;269 of the second, the seleniovanadic ointment of Roemer and the sulpho-selene of Walker; of the third, seleniol and electro-selenium.
Inasmuch as this new type of cancer therapy derives its origin, its justification and its support, in very large measure, from the laboratory results obtained in animals, it is a matter of considerable importance to examine those results with care, in order to determine whether they furnish a satisfactory basis for human therapy, and whether they justify the hopes to which they have given rise.
It is safe to assert that the application of chemotherapy to the treatment of tumors practically dates from the publications of Wassermann. He stated the principle that a rational therapy of tumors must be based on constitutional treatment. It appears evident that local treatment can have only local effects. The lymphatic extensions of tumorous growths, and the often unsuspected metastases in distant organs must of necessity escape the effects of purely local treatment. Hence, Wassermann reached the conclusion that all treatment of cancer which was to be effective, and not merely palliative, must be carried to all parts of the body by means of the blood stream. He therefore introduced the use of intravenous injections in the experimental therapy of rat and mouse tumors. An accidental observation led him to believe that selenium was a substance possessing a high degree of affinity for tumor cells.
In order to insure the penetration of the tumor in the live animal by this substance, however, he considered it essential to combine it with some other highly diffusible substance. This type of substance, which was to act as a carrier of the selenium, he described under the name “cytotrochin,” from the Greek word τροχιά {trochia}, meaning road. For this purpose he selected eosin. The eosin and the selenium were then combined by a method and in a form the details of which have never been published. All that we know of this preparation is contained in the statement that it is very difficult to produce, and that it is extremely unstable and difficult to keep. Mice can be given amounts of from 2 to 3 mg. of this substance in solution. Wassermann experimented with mice inoculated with transplanted tumors of the types of carcinoma and sarcoma. After from three to five intravenous injections of the drug, he noted that the tumors become softer and fluctuate. After still further injections the fluid mass undergoes absorption, and the tumor gives the impression of an empty sac. If it is possible to carry the injections up to the number of ten or twelve, recovery ensues. In such cured animals there remain only the unabsorbed portions of the fibrous capsule. Recurrences were not observed in the cured animals. Wassermann further stated that two spontaneous tumors in mice which had been treated by this method presented favorable results.
Wassermann’s original presentation gave few experimental details, and has not been followed by the promised scientific report. From his article it is impossible to determine what proportion of his animals were cured and what proportion failed to survive the treatment. From a later paper by Keysser270 we learn that by far the larger portion of the animals perished during the treatment in the stage of softening, so that a cure was accomplished in from only 3 to 5 per cent. of the animals. This is a point of great importance, inasmuch as it furnishes an indication of the highly dangerous character of this mode of treatment. Fatal results are attributed by Keysser to the absorption of toxic products from the tumor. This contention, however, is supported by no observations, and it is certainly equally fair to assume that death results from the toxic effects of the compound. A microscopic study of tumors taken from animals undergoing treatment was made by Hansemann. He found that the death of the cells was the result of nuclear destruction.
Within a very few months after Wassermann’s publication, Neuberg and Caspari268 published a paper which was the first of a series of studies on the therapeutic effects of the heavy metals on the animal tumors. They used zinc, platinum, tin, selenium, copper, silver and cobalt in the form of certain complex organic compounds, the composition of which is not revealed. Owing to the fact that intravenous injections of these compounds produced a specific effect on the tumors, they are described as “tumoraffin” substances. Immediately after the intravenous injection of these preparations, there followed a marked hyperemia of the tumor, whereas the remainder of the mouse’s body appeared markedly anemic. The hyperemia was often attended by hemorrhage into the tumor. This first stage was succeeded by liquefaction and absorption followed by recovery in favorable cases. The authors failed to state in what proportion of their experiments the animals died, and in what proportion recovery ensued.
The second paper on this subject is by Neuberg, Caspari and Löhe,268 in which further details are vouchsafed. They state that with the compounds used by them the toxic and the therapeutic doses approximate very closely, from which it follows that the treatment, as with the Wassermann method, results in a very high mortality. Smaller doses produce no therapeutic effect; on the contrary, they seem to act as a stimulus to the tumor, accelerating the normal rate of growth. Spontaneous tumors show similar effects, but no cures are recorded. Only in tumors in which autolysis is active intra vitam does the method exert any effect. Consequently the benign primary tumors of animals, such as fibromas, are not influenced by it.
Neuberg and Caspari are to a great extent responsible for the colloidal theory of treatment in tumors. Accepting the observations of Petri and others that the autolytic ferments in tumors are quantitatively greater and qualitatively different from those present in the normal tissues of the body, they venture the assumption that the process of recovery in the experimental tumors of animals is due to the self-digestion of the tumor by these ferments. Ascoli and Izar271 had shown that such ferments are materially stimulated by the presence of metals, and more especially of metals in colloidal form. This contention is apparently in harmony with the well-established fact that certain colloidal metals of themselves are capable of acting under certain circumstances as ferments. Neuberg and Caspari were at first of the belief that the compounds produced by them circulate in colloidal form. Subsequently they stated that these compounds were crystalline substances, but they assumed, under the influence of the theoretical consideration mentioned above, that these substances are broken up in the tumor and there undergo transformation into the colloid state.
In connection with the preceding observations there are certain other experimental results which require mention. Izar271 succeeded in curing rat tumors by means of injection of colloidal sulphur. C. Lewin272 cured subcutaneous mouse tumors with various preparations of gold. Werner and Szécsi273 produced similar results through a combination of selenium-vanadium with cholin-borate; in these experiments the selenium-vanadium was supposed to be present in colloidal form.
Within a comparatively brief period of time, therefore, it fell to the lot of a number of observers, using strikingly different substances, to produce therapeutic effects amounting in a certain percentage of cases even to cure in the experimental tumors of the lower animals. The various procedures have little in common. Both metals and nonmetallic substances have been employed either in colloidal form or in combination with organic radicals. In some instances a diffusible carrier is combined with the basic substances; in others not. All of the preparations appear to possess a high degree of toxicity, although adequate data on this very essential feature are almost invariably withheld.
Wassermann’s results with eosin-selenium were soon critically examined by other observers. Uhlenhuth274 and Contamin275 were unable to confirm his observations, but their negative results are attributed by Keysser to the fact that they were not in possession of the proper formula for the preparation of the eosin-selenium compounds as used by Wassermann. Apolant,276 however, in Ehrlich’s name confirmed Wassermann’s findings.
The most important critique of eosin-selenium has been contributed by the subsequent investigations of one of Wassermann’s original collaborators, F. Keysser.277 Keysser’s publication contains a large number of very careful observations on the various forms of eosin supplied by the German manufacturers, as well as on other matters which cannot here be considered in detail. He finally reached the conclusion that the eosin furnished by the manufacturing house of Sandoz was the most effective for his purposes, inasmuch as it combined the lowest grade of toxicity with the highest capacity for discoloring the tissues. The selenium he used in the form of selenio-vanadium furnished by Clin of Paris, which was the identical preparation used by Werner and Szécsi in combination with borcholin. The maximum dose of this selenio-vanadium is 0.06 c.c. for each gram of mouse. Eosin, 0.01 gm., dissolved in 0.5 c.c. of physiologic salt solution, is mixed with 0.5 c.c. of the selenio-vanadium. This mixture is then used for intravenous injections. The results produced by the injection of this mixture are to all intents and purposes similar to those obtained by Wassermann, except that Keysser induced cure in a larger proportion of animals, namely, from 6 to 8 per cent. It is evident from his careful description of his experiments that the treatment is extremely toxic to the animals. The therapeutic dose is considerably greater than one-half the toxic dose. This accounts for the fact that an extremely large proportion of the animals perish during the course of the treatment. The tumors failed to be influenced unless the dose given fell very little short of the fatal amount. Moreover, in order to accomplish a complete cure, at least eight to ten injections must be given, and in some instances not less than fourteen.
Keysser’s most important conclusions, however, were obtained by following an altogether different line of procedure. It has been pointed out by Carl Lewin272 that the therapeutic results obtained from subcutaneous mouse tumors, however encouraging, could not be logically applied to the treatment of human cancers. The subcutaneous transplanted tumors, as is well known, are as a rule limited by a distinct capsule and show no tendency to infiltrative growth. In this particular they present a most striking difference when compared with human tumors. On the other hand, the metastases of mouse tumors in the internal organs present an infiltrative mode of growth and thus approximate very much more closely to the human type of tumors. Keysser, therefore, determined to test the therapeutic effectiveness of his compounds on tumors implanted in various organs. He developed a technic which enabled him to implant tumors in the liver, the spleen, the kidneys and other parts of the mouse by means of injection through special needles, often without the assistance of a cutting operation.
The tumors so implanted grew rapidly, and within from two to three weeks reached the size of cherry pits. The growth was characteristically infiltrative. Animals with these tumors were then submitted to intravenous injection of the therapeutic agents in exactly the same fashion as the animals carrying subcutaneous tumors. The results, however, were absolutely different. Whereas the subcutaneous tumors invariably showed a much more intense discoloration than the other tissues of the mouse, this feature was entirely lacking in the case of the internal tumors. Softening and liquefaction, which almost invariably follows on the third or fourth injection in the case of subcutaneous tumors, and which is the first symptom of cure, never presented itself in the case of the internal tumors. Their consistency throughout the treatment was indistinguishable from that of the tumors of control animals. The treatment, in fact, appeared to exercise not the slightest influence on internal tumors. There was neither cessation nor retardation in growth, but the tumors continued their normal rate of destructive increase with the production of metastases, leading eventually to the death of the animal either during the course of the treatment or shortly thereafter. Microscopic changes, such as had been observed by Hansemann in the case of subcutaneous tumors, were entirely lacking. No matter in what organ the tumors were implanted, these results remained the same. No matter what type of tumor was employed, whether carcinoma, adenocarcinoma or sarcoma, the therapeutic outcome was regularly and consistently nil.
These results induced Keysser to determine whether or not eosin-selenium could actually be shown to exercise a deleterious effect on cancer cells outside the body. In order to do this he made a suspension of mouse tumor cells in salt solution and mixed this with the eosin-selenium-vanadium, using the latter in amounts equivalent to three times the fatal dose for a mouse. After the mixture had stood from one to three hours, it was injected either subcutaneously or intravenously into mice in order to test the vitality of the cells. In every instance the injections resulted in the production of tumors which could be in no way distinguished from the tumors produced by untreated cancer cells. In other words, the therapeutic preparation had absolutely no effect on the tumor cells.
In the same way Keysser carried out experiments along the lines inaugurated by Neuberg, using a combination of glycocoll and copper. He also tested the combination of borcholin with selenium-vanadium used by Werner and Szécsi. He was able to confirm the fact that both of these substances produced an unmistakable therapeutic effect on subcutaneous tumors. On the other hand, they were absolutely without influence on the internal tumors. In this respect, therefore, they were entirely comparable with the eosin-selenium compound. The theoretical basis constructed by Neuberg, which rests on the assumption that the metallic compounds stimulate autolytic processes in the tumors, was also subjected by Keysser to destructive criticism.
Finally, Keysser showed that none of these therapeutic agents were effective even in the case of subcutaneous tumors, unless the latter had reached at least the size of cherry pits. If a therapeutic injection were made immediately after inoculation of the tumors, no effect was observed. The tumors grew exactly as in the control animals, and the injected animals died in about the same period of time as they.
All of these facts, which taken together constitute a very remarkable and convincing piece of scientific investigation, permit of but one conclusion. It is quite clearly established that none of the preparations of which the therapeutic effectiveness has hitherto been proclaimed exercise any direct influence on the life or development of the tumor. Under certain very definite and restricted conditions, however, they do appear to produce certain changes in the tumors, and in a small proportion even cures. These results, however, are obtained only in the case of tumors which are subcutaneous in location and not smaller than a cherry pit in size. Keysser’s interpretation of the striking differences between tumors is of interest in this connection. He believes that the constant palpation and examination of the subcutaneous tumors, which is prompted by interest in the experiment, produces circulatory changes with hyperemia and hemorrhage. These circulatory changes are responsible for the increased tendency of the injected substances to lodge in the tumors, thereby possibly increasing the tendency to autolysis which the circulatory changes have inaugurated. It is, of course, questionable whether this explanation can be regarded as final. In a series of experiments which I performed many years ago, I was able to show that sodium iodid when injected intravenously accumulates in tumors in larger amounts than in any other tissue of the body in rats. A similar observation has been recorded by Wells, De Witt and Corper.278 In the same way I found that various dyes, such as Congo red, when injected intravenously, could be demonstrated in tumors long after the rest of the body had recovered its normal color; the liver alone vied with the tumors in this respect. The dyestuff was invariably sharply localized in the necrotic portions of the tumor. The conclusion seemed obvious that, owing to circulatory conditions or possibly even to chemical conditions, the dye was retained longest in the necrotic parts of the tumor. This effect was unquestionably not due to handling, inasmuch as the animals in my experiments were not palpated from the time of injection until death.
I have, however, had an even more striking demonstration of the same fact. I have given intravenous injections of dyes to patients suffering with various forms of internal tumors, as, for example, cancer of the breast, in the hope of favorably influencing the growths. At operation, the picture presented by the tumor is striking in the extreme. It presents areas of various size which are intensely discolored by the dye. These areas, both to the naked eye and under the microscope, are the necrotic parts of the tumor. The actively growing areas of tumor tissue and all the normal tissues of the organ present their normal color. All of these observations lead to the conclusion that the necrotic areas in tumors either possess a higher affinity for sodium iodid and for the dyes than do the normal tissues, or that these substances are more slowly absorbed from the necrotic areas owing to the circulatory deficiency. Whichever of these explanations is accepted, it is quite reasonable to believe that necrotic areas might well undergo liquefaction under the influence of the various substances which have been used for therapeutic injection. Such a result is, of course, without direct effect on the growth or vitality of the living part of the tumor. This fact is quite clearly evidenced by the experimental data, which show that the internal portions of the tumor might undergo liquefaction and yet the tumors were not cured. Indeed, Löhe, who made microscopic examinations of the tumors treated by Caspari and Neuberg, states particularly, with reference to a tumor which had been subjected to treatment, that “the central portion of the tumor showed softening, while the external margin was composed of actively growing cells.” The central portions of implanted tumors are, of course, those which first undergo spontaneous necrosis.
It still remains to explain the small percentage of cures achieved by Wassermann and by Keysser. It does not appear to me that this problem presents any insuperable difficulties. The fact must be emphasized that practically 95 per cent. of the animals die under the treatment, which sufficiently indicates the toxic effects of the agent used. We must remember that transplanted tumors are under all circumstances at a certain disadvantage as compared with the normal tissues of the body. After all, they are implanted on a foreign soil. Their blood supply is impoverished and imperfect. They have a natural tendency to undergo necrosis, and in many cases spontaneous retrogression. It is not strange, therefore, that they should prove in slight degree more susceptible to toxic effects than are the normal tissues of the body.
If we remember that the various therapeutic agents introduced in all probability reach a somewhat higher degree of concentration in the necrotic areas of the tumor than in the normal tissues of the body, an assumption which is entirely in accord with the facts as observed in the case of sodium iodid and of various dyes, we may be quite prepared to believe that this factor is sufficient to induce the destruction of the marginal healthy and living cells of the tumor. The fact that small subcutaneous tumors were found by Keysser to be entirely refractory to the treatment is entirely in accord with this assumption, in view of the fact that tumors of this size present practically no central necrosis. The same explanation holds of the observation previously cited from Caspari that the primary spontaneous tumors of animals do not yield to the treatment. Indeed, he himself states that the treatment is effective only in tumors in which autolysis takes place during life. The word autolysis, however, in this connection is a misnomer and represents a gratuitous assumption; as an actual fact, one is entitled to say only that such tumors undergo central necrosis, in all probably owing to defective circulatory supply. The process is exactly similar to the coagulation necrosis described in the case of tubercles by Weigert. If autolysis occurs, it is only secondary to the preceding necrosis.
This explanation, however, is confronted by the fact that the internal tumors produced by Keysser showed no tendency to effect a localization of the dyes, and correspondingly no tendency to be affected by the therapeutic agents. One might be permitted to inquire whether these internal tumors had undergone any necrosis. Keysser unfortunately makes no mention of this matter. It is certainly true that the infiltrative mode of growth of the internal tumors, which is entirely different from that of the subcutaneous implantations, is associated with a much better blood supply and a lessened tendency to undergo necrosis. That such tumors can undergo necrosis, however, is evidenced by certain illustrations given by Carl Lewin in his paper on internal tumors. But such changes usually occur only in advanced stages. To judge from his plates, Keysser worked with relatively small tumors, an assumption which is rendered even more likely by the fact that his injections were undertaken in a fairly early stage of their growth. In this connection I may quote certain experiments of my own on internal tumors.279 The implantations made in my experiments were produced by intravenous injections of a tumor suspension into the jugular vein of rats. Such injections resulted almost invariably in the production of a large number of tumors in the lungs, which, as is well shown in the figures accompanying the original article, differed very markedly in size. The smaller of these tumors are composed throughout of actively growing cells, while the large tumors present an area of central necrosis exactly as do the subcutaneous tumors. If such an animal be given an intravenous injection of a dye such as Congo red, it will be found that the larger tumors present an area of central discoloration corresponding to the area of previous necrosis, while the smaller tumors, like normal tissues, are not colored. Thus, it is clear that the internal tumors implanted in animals are subject to the same laws concerning the distribution of dyes and, of course, other substances as are the subcutaneous tumors. As I have stated previously, an exactly analogous observation has been made in a human breast tumor. In the absence of any contradictory evidence, therefore, I think that it is perfectly justifiable to assume that Keysser failed to achieve a result in the internal growths simply owing to the fact that those growths presented practically no areas of necrosis at the time of his injection.
Another theoretical question which bears closely on the recent therapeutic investigations in human beings concerns the rôle of colloids, as such, in the procedure. It is quite clear from what has already been said that all experiments with animal tumors have been largely influenced by the belief that metals in the colloidal form exercise a peculiar and characteristic influence on the destruction of tumors. Even when the therapeutic agents have been introduced in crystalline form, as by Neuberg and Caspari, the authors find themselves compelled to assume that the metals are reduced to colloidal form within the tumors. For the latter assumption there is absolutely no evidence; it is due simply to the influence of the colloidal theory. If one critically examines the data on which this theory is based, one is forced to the conclusion that it has practically no established claim to validity. If we grant that colloidal metals have been shown to stimulate autolysis in the test tube, the same fact must be admitted of metals in noncolloidal solution. The experiments, however, are very far from establishing either of these facts satisfactorily. But even were this the case, it is an unjustifiable inference that living tumor cells would be influenced in anything like the same manner as are the dead cells observed in test tube experiments. As an actual fact, we know from the work of Evans and Schulemann that only the “scavenger cells” of the body take up foreign colloids, and to this class the tumor cells do not belong. Moreover, the form in which metals are introduced into the circulation is not necessarily or even probably the form in which they act on the tissues. Colloidal solutions of the metals are certainly subject to precipitation and other changes on entering the blood. This fact I have shown experimentally in a previous study on colloidal copper.280 In the same way it is probable, as has been pointed out by Wells, that metals when introduced in crystalloid form may rapidly be altered so that they are carried throughout the body in colloidal form. All of these considerations indicate how unjustifiable is the assumption that colloidal metals exercise a peculiar action on growing tumors. It is hardly surprising that their empiric use has failed to measure up to expectations based on so slim a foundation of fact.
Clinicians have not been slow in following the lead suggested by the therapeutic experiments in animals. It is perfectly proper that this should be the case. In dealing with a disease of the character of cancer, in many instances entirely beyond our power to influence, no one can question the advisability of trying any and every agent which holds out the slightest promise. Unfortunately, a closer analysis of the animal experiments fails to vindicate even that degree of faith. When one considers the facts which have been analyzed in the preceding discussion, it would appear not only futile but actually dangerous to attempt to benefit cancer sufferers by means of any of the agencies which have been employed in animal experimentation. Nevertheless, the fact remains that a variety of preparations have been used in the human clinic. The various types of preparations may be satisfactorily grouped under four classes, namely:
1. The crystalline salts of selenium.
2. Selenium in colloidal solution.
3. Other metals in colloidal solution.
4. Compounds of metals with organic radicals.
These substances have been administered by injection or by the mouth. In the case of injection, the injections have been made either into the subcutaneous tissues, intramuscularly, or intravenously, or finally, directly into the tumors. Before passing to a further consideration of this subject in detail, it may be well to recall the fact that in the experimental tumors of animals, no matter what preparation has been used, it has been possible to accomplish therapeutic effects only by the use of relatively enormous doses of the medicament, of doses, in fact, which were scarcely lower than the lethal dose. Certain experimenters have noted that smaller doses actually stimulated the growth of the tumors. In the second place, it has almost invariably been found necessary to administer the treatment intravenously, inasmuch as the other modes of administration failed of therapeutic effect. It is quite apparent that a procedure in human beings in any degree analogous to that pursued in animals is entirely impossible. The doses used, with one notable exception to be subsequently mentioned, have invariably been relatively small. Hence it is apparent at the outset that at least one fundamental condition of success in the treatment of animal tumors has been necessarily excluded in the clinical application.
The salt used by Wassermann is not stated in his original publication. Wolff281 speaks of it as a sodium salt, whereas Keysser says that it was a combination with potassium cyanid. In only one instance, as far as I am aware, has the sodium salt been used therapeutically in human beings. Delbet282 states that he employed this salt intravenously in one case, and that its use was shortly followed by death. Unquestionably the salts of selenium are very much too toxic to be used in this way.
The majority of those who have worked with selenium have used it in colloidal form, either preparing it themselves or employing one of the preparations put on the market by the pharmaceutic firms. Of the latter the best known are the electro-selenium of Clin, and the Seleniol of Couturieux. Of those who have made use of selenium in these forms may be mentioned Cade and Girard,283 Bougeaut and Galliot,284 Blumenthal,285 Thiroloix and Lancien,286 Delbet, Laurent and Bohec,287 and most extensively of all, M. Touche.288 All of these authors have described cases of malignant new growths of the most varied character which were treated by these preparations.
The results obtained are fairly concordant. The intravenous injection of the preparation produces but slight disturbance. There is leukocytosis, a moderate rise of temperature, and not infrequently a chill. Otherwise the substance seems to possess no toxicity. The effects produced on the tumors have almost invariably been described as encouraging. Touche, who treated twenty-seven cases in this way and has described each case in detail, states that under the treatment the surface of the tumors, if ulcerated, became cleaner and healthier; the tumors became softer; the rate of growth was arrested, and there was relief of pain and of the accompanying functional disturbances; often, too, there was a gain in weight and an improvement in general well-being.
Touche concludes his article with the statement that “it is certain that the effect is not curative but it is actually palliative.” Delbet, on the other hand, states that he has seen no beneficial effects from the use of colloidal selenium injected intravenously. In the discussion on Delbet’s paper, Ledoux-Lebard states that he has observed nothing from selenium further than the temporary improvement which is shown by almost all cancer cases on the application of any new therapeutic measure. In one or two instances the claim is made in the literature of an actual cure of malignant growth through the use of selenium. Such, for example, is the case described by Blumenthal. From the clinical description this might have been a cancer of the tongue, and was judged to have been such in view of the negative Wassermann reaction. No microscopic examination was made. Arsphenamin was given. The patient recovered. It is clear that instances of this type cannot be accepted as beyond criticism, and it is safe to say that nothing more convincing in the way of actual cure is offered in the rather voluminous literature on the use of selenium.
Numerous compounds of selenium, some of them claiming to circulate in colloidal form, have been described, and have been put on the market for use in malignant disease. Such are Walker’s sulpho-selene, and selenio-vanadium, which has been prepared in the form of an ointment by Schering and Glatz. These preparations lay claim to the same palliative effects which have been previously described for colloidal selenium.
Of the other metals in colloidal form, chiefly silver and copper have come into use. Colloidal silver was first recommended for malignant growths by Vogel. It is obtainable on the market in proprietary form under the name of fulmargin, and also as electrargol. Recently Rohdenburg289 has made a careful study of the effects of colloidal silver in experimental and in human tumors, and finds that they have no value. Colloidal copper has been used in recent times for the same purpose by Gaube du Gers and by others. I have recently examined the effects of colloidal copper on malignant tumors in man, and have been unable to find that it has any therapeutic value. Furthermore, a study of the distribution of the copper in tumors obtained at operation or by necropsy from individuals so treated failed to show that the copper had been deposited therein.
Finally, preparations similar to those used by Werner and by Caspari in animals have also been used in human beings. In these cases also the authors have been able to record palliative effects on the tumor, but in no instance cures.
We have seen that it has been quite impossible to duplicate in human beings the therapeutic technic employed in animal experiments. We have seen further that the use of a modified technic in animal experimentation has never been productive of favorable results even at the hands of enthusiastic adherents. In striking contrast to these conclusions are the observations made in human therapeutics. For every type of preparation described in the preceding paragraphs, the claim has been made practically without exception that it exercises a markedly beneficial effect on malignant diseases in the human being. Not only are the subjective symptoms alleviated, but also the tumors appear to become cleaner and softer; the rate of growth is retarded; necrosis and metastasis are prevented, and inoperable tumors become operable. How are we to interpret these observations? How are we to explain the fact that they are the almost invariable accompaniment of the most diverse methods of treatment? I have already quoted the statement of Ledoux-Lebard that every therapeutic novelty appears to exercise a favorable effect on cancer cases. The same fact has been observed in a variety of other diseases, such as locomotor ataxia.
In order to arrive at a safe and reliable estimate as to the value of any new or experimental procedure in cases of cancer, it seems advisable to accept certain definite therapeutic criteria by which the cases are to be judged. In the absence of such a method, alterations in symptoms which are actually of no real value or importance receive undue emphasis. The natural course of the disease is associated with such fluctuations that a sanguine therapeutist can gain some encouragement from even the most hopeless cases. Hence it follows that every mode of treatment has found adherents. The market is flooded with cancer drugs, and cancer charlatans flourish in the most highly educated communities. Unfortunately, even well trained, honest and reputable physicians have fallen victims to this fallacy, and have lent their names to the support of modes of treatment which in reality produce no determinable effect on the natural evolution of the disease. It was the desire to combat this unfortunate tendency which led me some time ago to attempt to establish a reliable set of criteria of therapeutic effects in cancer. These were embodied in an article280 which appeared two years ago, and I may be here permitted to quote them in extenso:
In determining the effects of any given mode of treatment on a tumor, a variety of criteria may be relied on. Circulatory changes in the tumor, the relief of pain and the restoration of a secondarily impaired function are certain of the criteria on which stress has been laid by the majority of observers in the past. Important as are these criteria in determining the progress of purely inflammatory processes, it is unquestionable that their value in judging of the effects of therapeutic methods when applied to malignant disease is open to criticism. It is a curious and interesting fact that almost every therapeutic claim made in recent years in connection with cancer has included among its virtues the relief of pain. This is true of vaccination with cancer tissue, of Hodenpyl’s method and of many others. In view of this very general effect, not much stress can be laid on this symptom, and it is probably fair to assume that in the great majority of these cases the result is in no small measure psychic. The improvement of function is also largely a subjective phenomenon, and as such requires most careful criticism. Osler relates that he has known a patient with gastric cancer to be relieved of digestive disturbances and to gain 18 pounds in weight as the result simply of the visit of a sanguine consultant who denied the presence of a tumor. Improvement in the ability to chew food, to articulate words or to move a limb are phenomena familiar to those who attempt to treat cases of cancer. The victims of this disease seem to be in a very high degree “suggestible” and impressionable and respond nobly to every therapeutic effort.
Circulatory changes in tumors offer an interesting group of clinical symptoms. The observation has often been made, especially in ulcerated new growths, that treatment is associated with swelling, peripheral hyperemia, and an altered character of the discharge. In spite of the fact that there is no reasonable relationship between this congeries of symptoms and the actual cure of the tumor, they generally receive considerable emphasis and are cited as an indication of the specific local action of the agent employed. It is also true, however, that the growth may continue to advance in spite of their presence. It is of some importance to inquire into the mechanism which produces these circulatory changes and into their clinical interpretation. It is a well known fact that many drugs, when introduced into the body either by the mouth or through the skin, are excreted not only by the normal channels of elimination, such as the kidney or the intestine, but also from such ulcerated surfaces as may be present on the body. This is easily shown to be true, for example, of certain of the anilin dyes, which, when introduced by way of the veins, produce an intense discoloration of the dressings over ulcers. It is likewise true of certain of the metals, such as arsenic. In order to understand the series of events previously enumerated it is therefore only necessary to assume that the therapeutic agent is excreted from the ulcerated surface of tumors. If an irritant, it will tend to produce hyperemia of the margins of the ulcer, and an increase of the secretions. If an astringent, however, it may produce just the opposite of these effects. Such a result, however striking, is purely accidental, and has no necessary bearing on the growth or destruction of the tumor itself. It constitutes a symptom on which no reliance should be placed.
Excluding from consideration all of these secondary factors, we may conclude that the observation of the size of the tumor itself is the sole criterion on which we can place reliance in judging of the effect of therapeutic measures. This implies, in the first place, that a tumor must be accessible to fairly accurate measurement. Tumors of the uterus, for example, and intra-abdominal growths will only exceptionally fall into this class. In the second place, indirect evidence of a decrease in the size of tumors, such as is afforded by the increased permeability of obstructed passages, as in the case of tumors of the esophagus, pylorus or intestine, must be accepted only with great reserve. Remissions in the obstructive symptoms characteristic of such tumors are a frequent feature of the normal evolution of the clinical history of such growths. The relief of obstruction, however, may be due either to necrosis of the obstructing portions of the tumor, while the remainder continues to grow progressively, or to a relief of the accompanying muscular spasm. Finally, evidence of decrease afforded by the roentgenogram is not sufficiently exact in most cases to afford ground for so important a conclusion as that at present in question.
Not only must there be unquestionable evidence, however, of the diminution in size of the tumor, but this diminution must be of a kind not ordinarily attributable to the natural evolution of the tumor.... It is safe to say that multiple tumors offer enormous difficulties in the matter of interpreting therapeutic results. At present we have in the wards of the hospital a patient with multiple metastatic carcinomas of the skin. For several months we have at intervals made accurate measurements of certain of these tumors and have found that some have undergone retrogression, others have entirely disappeared, while still others have continued to grow steadily. In the case which afforded the ascitic fluid used in Hodenpyl’s experiments, many of the lymphatic metastases underwent complete retrogression, while the metastatic process in the liver, as was demonstrated at necropsy, increased progressively, and ultimately almost destroyed that organ. Thus, in multiple carcinosis, the retrogression of individual nodules is no indication that therapeutic intervention has produced an improvement.
I shall not delay to emphasize those variations in the size of solid tumors which accompany hemorrhage and its absorption, edematous swelling, necrosis in the depths, and other familiar factors which clinically simulate, or induce, the softening and the reduction that are so often attributed to therapeutic interference. But it is important to draw attention to a similar feature in that type of superficial epithelioma known as rodent ulcer. These new growths not infrequently advance at one point of the periphery, while they recede at another, and thus cicatrization and contracture may simulate a partial recovery. This effect is due in part to alterations not in the growth itself, but in the accompanying ulcerative process. The secretions from the growths, especially if confined under dressings, may have eroded and destroyed the surrounding skin, and it is tempting to interpret a recession of the associated ulcerative disease as an indication of a favorable effect on the new growth. It is unquestionably this aspect of rodent ulcers which plays so generously into the hands of the numerous venders of nostrums for this disease.
In brief, the demonstrable reduction in size of a tumor, of a kind not to be attributed to the natural processes of evolution of that tumor or of its associated lesions, is the one essential feature of effective therapeutic intervention.
When the various methods of treatment which have been discussed in this paper are judged by the standard advocated above, it is apparent that none of them can lay claim to therapeutic effectiveness. The modifications of the disease attributed to them are modifications which occur spontaneously in a very large proportion of cases as a result of the natural evolution of the disease process. This is a fact which cannot be too strongly emphasized. Owing unfortunately to the hopeless character of cancer, men are not prone to study with care all the lesser changes which the disease and the patient present under ordinary conditions; but when a “cure” is under investigation, the patient and his medical attendant note every apparent improvement with painstaking attention and enthusiasm. As a result, some evidence of improvement in treatment is entered on the books.—(From The Journal A. M. A., April 17, 1915.)
During the past four or five years, The Journal has had inquiries similar in effect to this, just received from Dr. E. P. Jewett of Gardner, Mass.:
“Will you kindly inform me regarding a drug manufacturing company by the name of the Direct Sales Company, Buffalo, New York? Are their products standard and reliable so far as you know?”
The Direct Sales Company, Inc., Buffalo, has, according to its letterhead, the following officers:
Geo. J. Dotterweich, President and Treasurer,
C. K. Dotterweich, Vice-President,
Louis B. Seufert, Secretary.
This concern circularizes physicians and emphasizes that it sells “Only by Mail.” It also features a “profit sharing rebate” scheme, whereby purchasers receive a coupon representing 10 per cent. of the invoice value of each purchase. After $100 worth of merchandise has been purchased the $10 worth of coupons when “presented for redemption at one time” will be “honored as cash”—presumably on the purchase of additional goods.
The Direct Sales Company catalogues have for some years, carried a guaranty, which reads, in part:
“We absolutely guarantee all preparations to be in exact accordance with the National Pure Food and Drugs Act, June 30, 1906.
“We also absolutely guarantee all preparations bearing our label to be equal, if not superior, to any on the market.”
In one of the Quarterly Bulletins of the State Board of Health of New Hampshire, issued last year, this paragraph appeared:
“The Direct Sales Company, Inc., Buffalo, N.Y., is a pharmaceutical concern which until recently has done business direct with New Hampshire physicians. In two or three instances complaints have been received by this department that the preparations sold seemed to be lacking in potency. Some time ago a physician sent us a specimen of codein sulphate tablets, one-fourth grain, concerning which he was suspicious, admission being made that the price paid was very much less than current quotations. The amount of codein sulphate actually found per tablet proved to be but one-sixteenth grain. Later on, having subsequently received a new lot from this source, the same physician sent us a second sample, the composition of which was found to be practically identical with the first. Acting under the federal law, 500 lot packages of the following preparations were next purchased of the company direct, the analytical results indicating serious deficiency in every case, as follows:
“Tablets salicylic acid, 5 grains ... 1.72 grains found.
“Tablets acetylsalicylic acid, 5 grains ... 2.31 grains found.
“Tablets acetanilid, 3 grains ... 1.88 grains found.
“Tablets codein sulphate, 1⁄4 grain ... 1⁄15 grain found.
“Tablets nux and pepsin No. 2, claiming pepsin 1 grain, extract nux vomica, 1⁄10 grain,
found to have a gross average weight per tablet of only 1.17 grains, 0.54 grains of which
was represented by sugar and other medicinally inert material.
“Tablets Infant’s Anodyne (Waugh) showed serious discrepancy from formula.”
The Bulletin added the statement that, as the company could not be reached under the New Hampshire laws, the federal authorities were appealed to. The result of this appeal appeared in Chemical Supplement 54, issued Aug. 21, 1918, by the Bureau of Chemistry of the U. S. Department of Agriculture. This government bulletin contained Notice of Judgment No. 6193, which describes cases of adulteration and misbranding of some of the drugs put out by the Direct Sales Company. Briefly, it may be said that some 2 grain acetanilid tablets sold by this concern were found by the government chemists to contain, roughly, about 12⁄3 (1.61) grains; some 1⁄4 grain calomel tablets were found to contain only about 1⁄6 (0.163) grain; some 1 grain quinin sulphate tablets were found to have only about 2⁄3 (0.63) grain; some 21⁄2 grain salol tablets contained only about 11⁄3 (1.39) grain; some 5 grain sodium salicylate tablets contained less than half that amount (2.32 grain). In addition, the federal chemists found that some Elixir of Iron pyrophosphate Quinin and Strychnin (Elix. Ferr. Pyrophos. Quin. et Strych. N. F.) fell considerably below the standard of strength laid down by the National Formulary by having less than one-eighth the amount of quinin sulphate which the official standard calls for, and only about one-fifth the amount of sugar, saccharine, which is not a normal ingredient of the official preparation, having been substituted for part of the sugar. The chemists found, too, that some hydriodic acid sold by the same concern, instead of containing, as the label declared and as the United States Pharmacopeia requires, 1 per cent. of absolute hydriodic acid, contained less than one half of 1 per cent. The Direct Sales Company pleaded guilty in this case and was fined $700.—(From The Journal A. M. A., Sept. 27, 1919.)
In spite of the wonderful achievements of modern science, it seems impossible to get the public to think in scientific terms. This is doubtless due to a fundamental weakness in our educational system. The tendency still is to think in terms of the eighteenth century rather than of the twentieth. Many times The Journal has been chided, even by its friends, for failing to take seriously preposterous claims made for alleged discoveries in medicine by well-meaning but self-deluded enthusiasts or by shrewd and conscienceless charlatans. Far too often the attitude is that any alleged discovery in medicine, no matter how bizarre or how humanly improbable, should be taken up in all seriousness and subjected to the tests of modern laboratory methods. It was only a few years ago that a quack of unsavory antecedents brought forth an alleged cure for consumption—a disease that for years has been the subject of study by the best brains in the world—and a medical college spent thousands of dollars “investigating” the “cure,” thereby giving it a standing that it would never have received otherwise and incidentally obtaining for the school an amount of publicity that may or may not have been desired. As The Journal said at the time, it would have been just as pertinent for a body of astronomers to determine by scientific methods whether or not the moon is really made of green cheese.
The point we would make is that the strides made by modern science have practically eliminated the possibility of men without training or special knowledge evolving any epoch-making discovery. In this connection an editorial in the Scientific American of recent date, dealing with the mechanical sciences rather than the medical, is well worth quoting in part. The editorial discussed the “Garabed” incident. “Garabed,” as Our readers know, was a name given to a device which one Garabed T. K. Giragossian claimed to have developed and which, so far as could be learned from the generalities in which Mr. Giragossian indulged, would take energy out of the cosmos and transfer it directly into mechanical motion. Mr. Giragossian would give no details regarding his “engine,” but was so able to hypnotize Congress into a belief that he had something worth looking into that it passed a joint resolution calling for the appointment of five scientists to pass on the claims for Garabed. The investigation proved, as might have been expected, that the thing was unsound in principle and nonoperative as a device.
The methods by which Garabed was brought before the public savored strongly of those used by quacks in the medical world, the one difference being that Giragossian was apparently perfectly sincere and unequivocally honest. The point that we bring out, however, and which, as we have said before, was so well expressed by the Scientific American, is the utter futility of wasting the time of scientific men on alleged inventions or discoveries by men without training who substitute secrecy and glittering generalities for facts and accomplishments. Quoting the Scientific American:
Scientific discovery, once an open field for all comers, is today becoming more and more a matter calling for the most intensive special qualifications. As the body of human knowledge broadens and deepens, it becomes increasingly difficult to make any material addition to it. Any one undertaking such a task must of necessity bring to it a long and careful training, acquired either in the refined atmosphere of the laboratory, or in the rougher school of close contact with the operation of apparatus constructed by those who have already qualified. In particular, he must possess a carefully developed power of making accurate observations and drawing correct conclusions. It is rather the habit to point to men like Edison and Maxim in refutation of these necessities; but they are not to be so refuted. These men are examples, raised to the nth power, of the great inventor who has qualified in the University of Hard Knocks and Long Experience.
On these grounds, when a man comes before us in the self-assigned rôle of a great inventor, it is incumbent upon him to answer, not necessarily the bald question “Who are you?” but certainly the more searching one, “What are your qualifications to undertake this work?” Only by his answer can we decide whether he possesses a competence deserving of attention, or is but a dilettante playing with fire. Yet this obligation was one which Mr. Giragossian, far from meeting, did not even appear to comprehend. To every effort to ascertain his qualifications he replied in the same terms, that he was an honest man, and could prove it by letters from his technically nondescript collection of friends and sponsors. The very fact that more than personal integrity is necessary in a man who would unravel the secrets of the creation of energy appears to have escaped his comprehension.
The fundamentals thus stated apply with equal force to the sphere of medical discovery. At the time when medicine was pure empiricism it was not only possible but also probable that the medicinal value of certain products or combinations of products might be stumbled on by those untrained and unskilled. That time has passed. Today, while it is not impossible, it is so improbable that there is no justification in taking up the time of scientific men in investigating alleged discoveries by men who are utterly lacking in the fundamental qualifications needed for the study of the complex problems of human pathology.—(Editorial from The Journal A. M. A., Aug. 10, 1918.)
The matter which follows appeared originally as an editorial in the Cleveland Medical Journal, November, 1915. It expresses, we believe, the attitude of the thinking physician toward the subject discussed:
Physicians have come to the realization that drugs are as a two-edged sword—under proper conditions, striking against the disease; otherwise, against the patient’s health. The first condition for their proper use is adequate knowledge of their composition and purity; of their actions and malefactions; their field and limitations. Slowly and painstakingly—sometimes painfully—this scientific knowledge has been gathered, is still being gathered, by chemists and pharmacists, pharmacologists and clinicians, with increasing thoroughness, care and discrimination.
Where wisdom fears to venture, unwisdom and cupidity find ample room. The wise physician knows that there are ills that drugs cannot cure; that drugs generally only aid or relieve; and that to obtain even this aid efficiently and safely, the existing scientific knowledge is none too great. Not so the unwise. He who sees in disease only a name, to him a name is a sufficient cure. Let there be a mixture with a convenient and suggestive name and a pleasant taste, a compendious index of diseases and symptoms—and a lively imagination—and the cure is accomplished. Few things could be easier, and few more false. It is not surprising that the “man on the street” should fall into these errors; it is sad that any physician should be misled by the sophistry of interested drug vendors.
Physicians have the moral obligation to instruct the public in matters of health. Preaching before practice is of little avail. It behooves the medical profession to make at least a reasonable effort to clean its own house before it passes the broom to the public. Realizing this responsibility, the American Medical Association some years ago established its Council on Pharmacy and Chemistry. This Council is strictly an educational agency—it collects and disseminates knowledge about drugs, especially those drugs that are advertised to physicians and that are not described in the legal pharmacopeias. Physicians are thus put in a position to discriminate. Many have done so; others will, a few may never see the light on this earth.
Journals can no longer claim that they mislead their readers in good faith. Some—the Cleveland Medical Journal among the first—have frankly acknowledged their obligations, and sacrificed a lucrative income from advertisements; others are still occupied in compounding the matter with their conscience. Manufacturers are in a similar position. Those who are on the side of scientific progress—or to put it materially, those who realize that honesty is the best policy—are taking the opportunity to separate themselves from the dishonest and ignorant.
Altogether, the medical profession may safely advise the public on the subject of drugs without laying itself open to the charge that it preaches what it is unwilling to practice.
Meantime, the public itself has had a somewhat similar awakening. The progress of the profession has necessarily spread more or less to the laymen. All sorts of educational agencies have been working to convince the public that individual and national health is too precious an asset to be entrusted to any quack who may spell his praise in printer’s ink. Legislators have passed food, drug, and antinarcotic laws which have aroused interest and discussion. Even the “drugless cults” have somewhat offset their harm by causing the public to reflect that drugging is not a panacea for all ills. All this has not been without effect. The public is in a receptive mood; it is not convinced, but wishes to learn. Legislators are prepared to follow public opinion. The purveyors of patent medicines are watching events.
What, under these conditions, should be the attitude of the medical profession? Plainly, it should continue to be what it always has been: to stand aggressively for the protection of the public health, without any compromise. In doing so, it is true, physicians will expose themselves to the imputation of selfish motives. Selfishly commercial minds cannot or will not understand the unselfish ideals of a profession—that is their loss. Physicians, however, must be careful not to give a semblance of reason to the charge; for that would diminish the effect of their attitude. They must confine themselves to informing the public of the facts; and to guarding the health of the public at large, and of their own patients in particular.
No one, in a free country, can force a diseased individual to seek effective treatment or prevent him from using an ineffective treatment, unless his disease imperils the health of others. At that point, and not before, the government can and should take personal measures. However, it is a well recognized function of the government to protect individuals against their own ignorance. It does this when it forces the child to go to school; it does this when it places the swindler in jail.
On exactly the same principle, the government has the right and the duty to protect the uninformed public against the flagrant evil of the patent medicine traffic—and the patent medicine traffic as now carried on is a flagrant evil and series of evils. The government should protect the public against advertisements that are framed to suggest or create imaginary ailments, with their attendant miseries; it should protect the public against being deluded by false promises of cure; against the specious relief that merely hides the disease and blinds the patient to its dangers; against drugs that may and do work positive harm; against the veil of mystery that makes these abuses possible.
The individual layman cannot protect himself against these dangers, and has a right to expect that the government will prohibit the indiscriminate sale of any medicine that may be harmful to him. He has a right to expect, when the government permits the sale of a patent medicine, that the medicine will do him no harm; just as he has a right to expect that any physician whom the government permits to practice, should be competent.
These are some of the reasons why physicians oppose patent medicines as they are now exploited; and for these reasons, physicians should take an absolutely uncompromising attitude, and use every opportunity to educate the public. The patent medicine interests naturally try to obscure the issue. By the art in which they are so skilful, they aim to suggest to the public that physicians are opposed to patent medicines, in order to drive patients to their offices. They “forget” to mention that physicians have never conducted a “campaign” against really efficient preventive public-health measures, no matter how many prospective patients were involved. No physician has ever refused to give diphtheria antitoxin because this would diminish the number of his visits. A short memory is a very convenient asset for self-interested persons. It is not so convenient for the public—but it is all too frequent. Physicians must, therefore, make it plain that their stand is not against patent medicines, but for the protection of the health of the public.—(From The Journal A. M. A., March 4, 1916.)
Until very recently, we were compelled to acknowledge that little, if any, progress was being made in internal medicine so far as drug therapy was concerned. Everybody knows of the progress made in other branches—in bacteriology, in pathology, in biologic chemistry, in surgery, in etiology and in application of technical methods to diagnosis. Recently, however, pharmacologic research and the application of scientific methods in the study of the physiologic action of drugs are resulting in definite, positive progress. An important lesson, incidentally learned through this scientific investigation, is the fallibility of the drug therapy described in textbooks. The explanation is, of course, that many of these textbooks are mere compilations containing false statements, unproved theories, and unverified clinical evidence representing the guesswork of ancient uncritical observers. Many drugs have been, and still are, vaunted in textbooks as valuable in a variety of conditions, whereas scientific investigation and controlled clinical observation have proved them to be totally worthless; others are proving to be of value in an extremely limited number of conditions. The sooner writers of textbooks realize this fact and enter into the spirit of the new era, the better for the public and for scientific medicine.—(Editorial from The Journal A. M. A., May 27, 1916.)
During the last two or three years The Journal has received inquiries regarding one Thomas Webster Edgar, M.D., of New York City, first, relative to his alleged treatment for diabetes and more recently about his “monkey gland” treatment for sex stimulation. Here is one from a physician in Washington:
“Have you any knowledge of the efficacy of a serum made from the pancreas of rabbits for the relief or cure of diabetes? It is made by Dr. T. W. Edgar of 766 West End Ave., New York City.”
And this from a layman in Pennsylvania:
“Last year there was published in the New York Herald an account of the new treatment for diabetes in which a serum was injected in the veins and as a result it was claimed that over sixty-five per cent. of the treatments made were successful. The account further stated that they proposed to establish some sort of a sanitarium in New York City used especially for the treatment. The writer having mislaid the account, wrote the New York Herald as to the doctor who had charge of it and in return was given the name and address. Dr. Edgar in a letter under date of last year stated that the cost of the treatment was $300.00, payable beginning of the treatment, and he gave very little information as to the success of it, with the exception that if the treatment did not give the desired effect after the end of three months, it would be continued without any further cost. The writer wrote and asked him the names of one or two of the patients who had been cured, because it seemed rather unusual that if the treatment were a success, it was necessary for a patient to pay the cost of the treatment in advance. To that letter I have never received a reply.”
While a physician from Illinois writes:
“I am enclosing a clipping from a Chicago paper relative to Dr. Thomas Webster Edgar of New York and his operation for transplanting the glands of ring-tailed monkey. I note that he is a member of the New York County Medical Society! What is there to this? I have seen no mention of these wonders in The Journal.”
Thomas Webster Edgar was born in 1889. The records show that he was graduated in medicine by the University and Bellevue Hospital Medical College in 1913, and was licensed to practice medicine in the State of New York the same year. In March, 1919, an article by T. Webster Edgar appeared in the New York Medical Journal on “Diabetes Mellitus.” In this Edgar gave a theory of the cause of diabetes mellitus and stated that he had “treated successfully, twenty cases of definite diabetes.” In the article he spoke positively of the successful results he had obtained by the “intramuscular injections of my diabetic serum.” No information was given regarding this serum except that he mentioned vaguely that it was “prepared from normal blood after the animal is exercised to the point of fatigue.”

A few days after the appearance of this article in the New York Medical Journal, newspaper articles appeared regarding a cure for diabetes perfected by “Dr. Thomas Webster Edgar, 766 West End Avenue, New York City.” According to these reports, Edgar said:
“I tried the blood of rabbits and found what I wanted. In obtaining the blood I first put the rabbit upon a treadmill and keep it there until it reaches a stage of fatigue. Then I draw the blood, and after heating it to 60 degrees centigrade separate the corpuscles from the serum. When the serum has been treated after the method I have discovered, I inject it immediately subcutaneously.
“I have attained success in 65 per cent. of my cases and I have had 100 cases. I do not say that the cure is infallible, but I am now certain that it will work in most cases, particularly when the patient observes the rules laid down and undergoes faithful treatment.”
In April, 1919, a physician in Kansas wrote to Edgar at the request of a diabetic patient asking for information about the “serum.” Edgar replied that it would be impossible to send the physician any of the serum for administration unless the “patient is willing to pay me for the cost of same, which will be approximately the sum of $25.” He stated further that, in a few months’ time, he hoped to be able to manufacture the serum in larger quantities which would “more than cut the expense in half.”
In the same month a layman in Chicago who read the newspaper story wrote to Edgar and asked for details regarding terms and the arrangements that would have to be made to take the “treatment.” Edgar replied that he expected to be in Chicago in a few weeks’ time and would see the man in consultation with his regular physician, that he would administer the first injection and give instructions to the physician as to subsequent injections. Edgar added:
“My custom is to have all fees paid in advance and my charge is $200.00 by certified cheque or money-order.”
A layman in one of the smaller cities of New York wrote to Edgar in May, 1919, and received a reply from Edgar’s secretary stating that the treatment extends “over a period of three months, cost $150.” He was also told that the serum could be sent to his physician for administration “for the sum of $25 prepaid by money-order.” The letter closed with the statement that Edgar “has been very successful with the serum.”

A layman in South Carolina who wrote to Edgar in June, 1919, was told that the treatment as administered by Edgar “extends over a period of two months; fee $300” and that if he wanted the serum administered by his own physician the cost would be “$50 prepaid.”
In May, 1920, Edgar had another article on diabetes, also in the New York Medical Journal. In this, too, he refers to his serum in the following words:
“In conclusion I may state that I have been able to produce some rather startling results by the use of my serum, which is prepared from the blood of rabbits after they have undergone a series of maneuvres capable of activating the various internal secretory glands to increased action. The serum contains the internal secretions in hormone form.”
Gradually the newspaper publicity on Edgar’s diabetic “serum” died down. Then, in November, 1920, there appeared—again in the New York Medical Journal—an article by Edgar on “Sterility, Sex Stimulation and Endocrines.” Edgar there stated that he wished to place himself “on record as being interested in sex stimulation” and that he wanted to notify the profession that he had another serum which he was using “with success in the treatment of this condition.” Thus:
“... I feel entitled to state that I have a distinctly beneficial serum for the alleviation of presenile and senile deficiency; and that my product is capable of producing a new lease of life in those whose functions have been reduced to a minimum.”
How long Edgar has been featuring his “serum” for “sex stimulation” it is difficult to determine, but during the last year the newspapers have carried sporadic reports of alleged remarkable results produced by “Dr. Thomas Webster Edgar of 766 West End Ave., New York,” through the transplantation of the “interstitial gland” taken from “a special species of orangoutang.” A layman who wrote Edgar some months ago regarding this “gland implantation” received a letter from Edgar’s secretary stating that the treatment “has been most successful in all cases” and assuring him that “the experimental stage had been passed, and the operation is advised in all cases presenting symptoms of presenility or age.” A week later the same man received a letter written by Edgar himself in which he reiterated the claim that all of the operations had been successful. Edgar added that he was now treating all cases “by operation instead of the serum,” and that “the fee for operation is $500, inclusive of the sanitarium,” the patients remaining in the “sanitarium” “for from two to three days.” A month or two later the prospective patient received another letter signed, “Thomas Webster Edgar, M.D.,” assuring him that “the effect is permanent, and does not wear off. No ill effects can possibly result.”
Commencing, Oct. 1, 1921, a series of sensational articles appeared regarding one of Edgar’s alleged monkey gland implantations performed on an individual described as “one time lawyer and then a writer.” These articles purport to be written partly by one of the newspaper staff, partly by the man undergoing the “operation” and at least one by Thomas Webster Edgar. The material is played up in the style typical of yellow journalism. In addition to repeated pictures of the individual who is being operated on, there also are given pictures of Thomas Webster Edgar and one of his “ring-tailed monkeys.” Doubtless the “story” has sold many newspapers. Its sensational character, the element of mystery and above all its sex slant will appeal to that large class of newspaper readers that hunger for stuff of this sort. Doubtless, too, it has proved a large advertising asset for Thomas Webster Edgar.
The statement that appears in the series to the effect that Edgar “is a member of the County Medical Society of New York” is incorrect. Edgar is not a member.
The further newspaper claim that Edgar is “an authority on glandular transplantation” should also be accepted with reservations. “Authorities” are created with ease in the pages of newspapers. Edgar may possibly be termed an authority in a newspaper or, shall we say, Pickwickian sense.—(From The Journal A. M. A., Oct. 15, 1921.)
The Journal recently published in this department some inquiries regarding Thomas Webster Edgar, M.D., of New York City, relative to some alleged serums that Dr. Edgar had developed for diabetes and sex stimulation, respectively, and relative also to the newspaper publicity given Dr. Edgar in connection with the alleged transplantation of glands from “ring-tailed monkeys.”
We are in receipt of a letter signed, “Thomas Webster Edgar, M.D.,” and reading as follows. It is given verbatim et literatim:
“Gentlemen:—I have read with great interest your editorial regarding the publicity given my work in metabolism, and gland implantation.
“Your pseudo, expose, and distinctly libelous insinuations are unjust, and they lead me to believe that you are going to be called to account at a very early date.
“My profession is the practice of medicine, and the policy of my practice is not controlled by the editorial department of the journal. I am progressive, and a firm believer that legitiment medicine and surgery can not be practiced if the physician be governed by a set of medical clerks, who disdainfully boast that they control, and govern the healing art through out the breadth of the land, with a sceptre that is biased and steeped in the unadulterated commercialism of a certain medical clique.
“Aside from the fact that I am an associate editor on a medical publication, it is disgraceful, as well as unjust that you have written such an editorial with out first investigating the therapeutic value of my serum, and implantation operation.
“The psychology of your editorial, only reflects on your editorial department, and will tend to belittle some of the greatest surgeons in the country.
“It may be to your advantage to know, that this very afternoon, I was on the program with the following men.
“Dr. Lewis Gregory Cole—New York
“Dr. Charles H. Mayo—Rochester, Minn.
“Dr. John B. Deaver—Philadelphia
“Dr. Charles Peck—New York.
“My paper was entitled—Senility, its etiology and treatment by gland implantation. I am sure the above mentioned gentlemen are thoroughly ashamed of your actions in the matter, as well as thoroughly disgusted with the baby like attitude you have displayed. You have no sense of fair play, and if it is with in my power to undue the wrong which you have wrought me, I shall endeavor to vindicate myself in the eyes of the clear thinking members of the profession.
“I sincerely trust you will publish this communication, in order that my brethren shall understand and appreciate that your thrust has not gone unnoticed.
“It is my hope that the various medical societies through out the country, will call upon me to read a paper on my work, so that I may be able to offer substantial evidence to the fact that you have done me an injustice.
“Very truly yours,
TWE/AEL[Signed] “Thomas Webster Edgar, M.D.”
Dr. Edgar’s statement that he had been on the program with Drs. Cole, Mayo, Deaver and Peck was sufficiently startling to prompt further investigation. It was found that the program in question was that of the annual meeting of the New York and New England Association of Railway Surgeons. It was further found that Edgar’s name did appear on some of the printed programs but not on others. It was rather naturally assumed that the name had been put on the program before the officers of this organization had seen the crude publicity to which The Journal recently called attention. It was found, however, that after several hundred programs had been printed about 150 more were needed and “in the meantime, Dr. Edgar had come into the limelight” in his ring-tailed monkey gland transplantation rôle and “was invited to read a paper on the subject.” While he accepted this invitation the secretary of the organization tells us that Edgar did not read his paper but, when the paper was called, declined, saying it was time for him to be in his office!
As for the rest of Dr. Edgar’s communication, The Journal appreciates that courtesy is due “an associate editor on a medical publication”—referring doubtless to the Western Medical Times. Dr. Edgar’s pronouncement that “legitimate medicine and surgery can not be practiced if the physician be governed by a set of medical clerks” seems reasonable—if cryptic. But it is when he charges that these “clerks” govern the healing art “with a sceptre that is biased and steeped in the unadulterated commercialism of a certain medical clique,” that he really shines. Whatever opinion one may hold of Dr. Edgar’s ability to compound serums, surely no one can question his skill as a mixer of metaphors. His reference to “sceptres” deserves to be embalmed in every textbook on rhetoric with the classic of the Hibernian statesman who passionately declared: “I smell a rat! I see it floating in the air! But, mark you, Sir, I shall nip it in the bud!”—(From The Journal A. M. A., Dec. 3, 1921.)
Physicians who prescribe on definite principles must often be puzzled by the number and variety of glycerophosphates on the market. All available evidence indicates that, as sources of phosphorus to the animal organism, the glycerophosphates possess no advantages over the ordinary inorganic phosphates.290 The glycerophosphates are split up in the intestine, and liberate inorganic phosphates. In this form they are absorbed and utilized, if they are utilized at all. There is no evidence that glycerophosphates have any pharmacologic action to warrant the belief that they are of use as therapeutic agents. The theory that organic phosphorus compounds are more readily assimilable than inorganic compounds and hence a better means of introducing phosphorus into the system is still kept alive in the promotion of certain proprietary mixtures, in spite of the scientific evidence that the organism can assimilate phosphorus quite as readily from inorganic as from organic phosphorus compounds.291 The glycerophosphates will continue to be manufactured until physicians refuse to prescribe them. A chemist in the “research laboratory” of a well known manufacturing firm has recently given a rather interesting reason for the use of glycerophosphates—from the manufacturers’ point of view. He is quoted as saying: “On account of the instability of phosphorus in elixir of phosphorus, nux vomica and damiana we have quite recently replaced the phosphorus by glycerophosphates. Such a preparation is apparently equally as effective, for we continue to have a great demand for it.” This is doubtless a sufficient reason for the substitution from the manufacturers’ point of view; but how about the patient, who, after all, is the one to be considered? Is it not a matter of considerable importance to the patient whether he receives phosphorus, one of the most powerful drugs known, or the inert glycerophosphates? The chemist’s statement seems to imply that it is not. It may be of interest to recall that a member of the firm whose chemist gives this “reason” for the use of glycerophosphates, in a recent address, was rather severe in his condemnation of institutions of learning, hospitals, etc., for their lack of cooperation with manufacturers: he said that “they should welcome an opportunity to let any manufacturer try out or test his products in their clinics, laboratories, etc.” A test as to whether there is a difference between the action of glycerophosphates and ordinary poisonous yellow phosphorus, especially when the former are mixed with extracts of nux vomica and damiana, would not be likely to appeal to many hospitals and laboratories as a very promising field of research at this day since, as has been stated, the scientific evidence at present available does not furnish any warrant for the therapeutic use of glycerophosphates.—(Editorial from The Journal A. M. A., April 15, 1916.)
With the appearance of the epidemic of influenza, reports began to appear, chiefly in newspapers, as to new serums, vaccines, drugs and other methods for checking and even for curing the disease. A few samples of such as have come to The Journal appear in our Tonics and Sedatives Department this week. In Massachusetts, Commissioner E. R. Kelly appointed two committees to investigate the value of influenza vaccines as a preventive agent and as a treatment of the disease. The first committee, a special board for scientific investigation, consisting of Dr. M. J. Rosenau, chairman, and Frederick P. Gay and George W. McCoy, was appointed to consider the evidence available on the prophylactic and therapeutic use of vaccines against influenza. This committee presented the following conclusions:
1. The evidence at hand affords no trustworthy basis for regarding prophylactic vaccination against influenza as of value in preventing the spread of the disease, or of reducing its severity. The evidence from the present epidemic, though meager, suggests that the incidence of the disease among the vaccinated is smaller than among the nonvaccinated. The board, therefore, concludes that further experimental evidence should be collected.
2. The evidence at hand convinces the board that the vaccines we have considered have no specific value in the treatment of influenza.
3. There is evidence that no unfavorable results have followed the use of the vaccines.
The second committee, known as the Special Board of Statistical Investigation, consisted of Dr. George C. Whipple, chairman, William H. Davis and F. C. Crum. This committee reported:
1. The weight of such statistical evidence as we have been able to accumulate indicates that the use of the influenza vaccine which we have investigated is without therapeutic benefit. Exceptional cases where apparent benefit has resulted from the use of the vaccine can be matched by other cases where similar recoveries have been made without vaccination.
2. The statistical evidence, as far as it goes, indicates a probability that the use of this influenza vaccine has some prophylactic value.
3. There is also some evidence to the effect that other methods of protection, such as open-air treatment and the use of proper masks, are effective in protecting exposed attendants, and the use of vaccine should not be taken as an excuse for omitting such safeguards.
As a result, the following recommendations were made:
That the state encourage the distribution of influenza vaccine intended for prophylactic use, but in such manner as will secure scientific evidence of the possible value of the agent. The use of such vaccine is to be regarded as experimental.
That the state shall neither furnish nor endorse any vaccine at present in use for the treatment of influenza.
These reports are conservative, and offer to other health commissioners and their communities a reliable guide as to procedures that should be adopted before subjecting or trying out on the public any method of prevention or treatment that may be offered. These matters are the domain of medical science, and medical scientists of recognized ability should be called on to make the decision.—(Editorial from The Journal A. M. A., Oct. 19, 1918.)
With respect to serums and vaccines in influenza, there are certain simple facts and considerations that physicians will do well to keep in mind at this times. The main point to keep always in sight is that unfortunately we as yet have no specific serum or other specific means for the cure of influenza, and no specific vaccine or vaccines for its prevention. Such is the fact, all claims and propagandist statements in the newspapers and elsewhere to the contrary notwithstanding. This being the case, efforts at treatment and prevention by serums and vaccines, now hurriedly undertaken, are simply experiments in a new field, and the true value of the results cannot be predicted by any one. Indeed, the exact results can be determined if at all only after a time, in most cases probably not until the epidemic is past and all the returns fully canvassed. Consequently, the physician must keep his head level and not allow himself to be led into making more promises than the facts warrant. This warning applies especially to health officers in their public relations.
As to serum treatment, the only noteworthy new method so far is the injection in severe cases of influenzal pneumonia of the serum of patients who have recovered from such pneumonia.292 The principle of this method is rational; analogous procedures have given seemingly good results in scarlet fever and other diseases; and the results reported in influenzal pneumonia appear promising. Further trial of this treatment under proper conditions consequently seems to be warranted. It should be borne in mind, however, that McGuire and Redden292 made their observations in the declining phase of the epidemic when the organism or organisms concerned appeared to be losing virulence. For this and other reasons, the expectations as to what may be accomplished by this method must be kept within reasonable bounds. Influenza is a self-limited disease with variable complications and of variable severity in different places, thus offering great difficulties in the way of evaluation of different methods of treatment.
At least two kinds of vaccine are in use in the hope that they may have preventive effects. One consists solely of killed influenza bacilli; it being extensively used in the East. We have as yet no decisive figures as to its effects, but there is an impression that it may have some value. The other vaccine is a mixed vaccine of the more important bacteria in the respiratory tract in influenza, principally pneumonococci, streptococci and influenza bacilli. It appears that vaccines of this nature are in extensive use, but we have no evidence that any benefit will be derived from them. To say that thousands have been vaccinated with apparently good results means nothing at all, simply because we are still in the midst of the outbreak, in many places even in the earlier stages. How slender the basis of this anti-influenzal vaccination when it is considered that the real nature of influenza is still unknown! In any event, it will require many carefully elaborated and controlled observations before anything definite may be learned in regard to the effect of these vaccines, and it is probably safe to say that nothing on which to rely in the future can be learned from the indiscriminate vaccination now going on. There is, therefore, no basis on which promise of protection from vaccines may be made. They may be harmless, and they may or may not be of preventive value.—(Editorial from The Journal A. M. A., Oct. 26, 1918.)
The intravenous administration of drugs is a new departure in therapy, but one which is rapidly increasing in use. Among its reputed advantages are that it is the quickest means of obtaining the effects of a drug, the effects are obtained with a certainty not obtained by other methods, and they are so marked that they cannot fail to impress the observer. These advantages in many cases are apparent rather than real; but even were they real advantages, they should not blind us to the various and serious dangers which this method involves. The technic, although not difficult, must be thoroughly mastered, or undue pain, infection, air embolism, or even death may result. Such accidents, however, are ordinarily easily avoided, and should be considered quite inexcusable. More serious is the fact that the drugs given intravenously reach the system, and especially the heart, in a different manner and concentration from that to which physicians are accustomed with ordinary methods of administration. Pharmacologists have long practiced intravenous administration, when studying acute effects of drugs, and they have observed that frequently the immediate result of such injections is a prompt fall of blood pressure, not obtained when the same drugs are given by mouth or even hypodermically. This fall in blood pressure is commonly attributed to irritation of the endocardium. It is usually of short duration, but is certainly undesirable and sometimes may have serious results.
It has also been observed that several drugs, for instance, quinin and potassium, depress the cardiac muscle when given intravenously much more than when given in other ways. Furthermore, any substance which tends to precipitate proteins must be injected slowly and with extreme caution, or it will produce intravascular clotting and sudden death. Deaths have resulted not only from a lack of knowledge of the technic of intravenous therapy, but also from a lack of knowledge of drugs which may be so administered. Sudden death has been reported following the injection of an iron preparation containing peptone, and also following intravenous injection of ether. Intravenous injections, while sometimes superior to the slower methods, are distinctly inferior when a continuous, rather than a sudden, action is desired. Drugs leave the blood system with great rapidity, and therefore their action on the circulation will cease promptly unless they are continuously supplied. It would be undesirable to inject intravenously such drugs as iodids, nitrites, iron or salicylates.
With these dangers and disadvantages in mind, it seems unwise to resort to promiscuous intravenous medication until the effects of this method have been studied in detail for the drugs employed, and unless there are distinct advantages to be secured. This is the case when an immediate action is necessary in emergencies, as in the use of strophanthin for cardiac collapse, quinin in pernicious malaria, etc., or if the drug would be destroyed in the stomach or tissues as in the case of salvarsan, or when the drug is not adequately absorbed by any other channel, as in the case of epinephrin.
Intravenous therapy will be most securely advanced if its employment is restricted to such well defined fields. These fields can be satisfactorily determined only by a scientific pharmacologic study of the action of these drugs when so administered in animals, as well as in man, under conditions in which the results are carefully controlled. The intravenous method is an impressive one, approaching in preparation almost to that which goes with a surgical operation. The patient is usually interested and impressed by this new, and to him, mysterious method. There is a psychic element in his reaction to the injection which is not a factor in his reaction to the same drug when given by mouth. The intravenous injection of a complex mixture would appear to be particularly reprehensible. Little is known, as has been stated, of the results to be expected from intravenous therapy, even with simple substances. The use of complex mixtures will without doubt react against the proper use of the method.—(Editorial from The Journal A. M. A., Nov. 11, 1916.)
One of the important factors connected with therapeutics as a science is the method of administration of medicinal substances. Drugs may be given by mouth, by hypodermic or intravenous injection, by inhalation, by inunction or, less frequently, by the use of other entrances into the body. In choosing a method, the physical characters of the substance to be administered and the immediate effects of the substance on the body tissues with which it may come in contact must be especially taken into consideration.
These factors apply particularly in the case of substances like iodin, arsenic, mercury or the biologic products in which the mode of administration radically modifies the action. For some time, manufacturers have urged substitutes for tincture of iodin, claiming that their substitutes were free from the undesirable properties of the tincture, and, at the same time, possessed special virtues which the tincture could not possess. More recently, attention has been directed to the administration of iodin in the form of vapor. The diffusing and penetrating powers of gases have particularly attracted the attention of therapeutists, since by this method drugs may be applied to rather inaccessible portions of the body, such as the lining of the lungs, the throat and the mucous membranes of the genito-urinary tract. Furthermore, it has been asserted that iodin in the form of fumes has increased combining powers, and is thus far more potent in effect than iodin administered by any other route. There do not seem to have been any adequate scientific investigations of the subject, however, until the recently published results of Luckhardt and his collaborators293 at the University of Chicago. In their experiments, both on man and on animals, accurately determined quantities of iodin were vaporized in a special device, and the fumes applied to the skin. At the same time, the tincture was applied to the skin of other persons as a control. Iodin was also applied to the skin of dogs with hyperplastic thyroid glands; and the effects on the gland, before and after administration, studied. Dogs were also used to determine whether iodin fumes were absorbed from the lungs. As a result of these investigations, which are reported in great detail, it was found that iodin, when deposited on the skin in the form of fumes, is absorbed. More iodin was recovered from the urine, following the application of the tincture, than was recovered following the use of the fumes. This result is explained by the authors on the ground that probably more iodin was actually applied, and that the iodin so deposited was held in combination with the protein during the process of coagulation of the latter by the alcohol of the tincture, leading to a state of continuous absorption. It is probable, furthermore, that the iodin deposited on the skin in the form of fumes is revaporized to some extent by the heat of the body.
Most important were the effects of iodin administered intratracheally in the forms of fumes. Iodin given in this way seems to be rapidly and completely absorbed; but it was found that the administration of the fumes of iodin by inhalation through the respiratory passages, even in small quantities, is fraught with great danger. Such administration induces dyspnea; and when it is given in large quantities, acute and fatal pulmonary edema ensues within twenty-four hours. When respiratory disorders are present at the time of administration, the fatal edema supervenes very quickly. Thus far, no device designed to deliver fumes controls the dosage.
It is interesting to consider, as do the authors, the fact that the fumes of iodin have the same effect as those of two other halogens, bromin and chlorin. The results of these experiments with iodin fumes on the dog, as shown by necropsy findings, are practically identical with those reported by military surgeons as found in soldiers gassed with chlorin during the war.
The results of these researches are additional evidence as to how scientific research may confirm or deny conclusions based on empiric therapeutic observations. The work may well serve as a model for similar experiments, now being made, on the therapeutic use, intravenously, of such substances as nonspecific proteins or organic preparations of toxic drugs. The patient should at least have the chance that is afford him by preliminary experiments, scientifically performed on animals in the research laboratory.—(Editorial from The Journal A.M.A., May 29, 1920.)
Many and various are the letters received by The Journal asking for information about an alleged scientific organization in Italy styled l’Académie Physico-Chimique Italienne. This Italian Physico-Chemical Academy is operated from Palermo, Italy. Here is the scheme: Dr. John Doe, an American physician receives an imposing-looking letter bearing the Palermo, Sicily, postmark and addressed to “Monsieur le Docteur John Doe, Médecin.” On opening the letter “Monsieur le Docteur” finds that the “Council” of l’Académie Physico-Chimique Italienne has nominated him “Honorary Member of this Academy” and furthermore has bestowed on him “a First Class Medal for technical work and scientific merit.” All this, “in consideration of your many dignities and great learning.” Dr. Doe is told that as soon as he will write an acceptance of this honor “in conformity with Section 19 and 22 of the Constitution” he will be sent “the Medal, Diploma and all the other documents relating to the title accorded.” The joker in the scheme lies in the necessity for Dr. John Doe “conforming” with “Section 19 and 22 of the Constitution.” Here are the sections:

“Sec. 19.—The entrance fee to cover office and postal expenses, including postage of diploma is 5 Dollars, and is payable once at the admission to the Academy by special bulletin filled up, stamped and signed.”
“Sec. 22.—Those to whom medals are awarded and who wish to possess them must pay for their coinage 10 Dollars as the Academy does not, at present, possess the necessary funds for this purpose....”
In short the whole thing means that if Dr. Doe is willing to send $15 in good American money he will receive in due time from the academy a “diploma” and a gilt (not gold) medal.
About four years ago when the “Academy” seemed to be making a particularly heavy bid for American dollars the member of The Journal staff in charge of the Propaganda Department wrote to the “Academy,” on his personal stationery, asking about the cost of membership in the “Academy” and asking also for a copy of the “prospectus.” And that was all. In reply he received a letter stating that “in consideration of” his “many dignities and great learning” he had been nominated “an officer of this academy” and had been awarded “la médaille de première classe” for humanitarian work and scientific merit. In order to obtain these tokens of the “Academy’s” regard it would be necessary to inform the “Academy” of acceptance “in conformity with Section 19 and 22 ...” As the Propaganda Department did not consider the diploma and gilt medal worth $15 even as exhibit for its museum of fakes, the “Form of Acceptance” was not filled in and returned “in accordance with Section 19 and 22.”

The leading spirits in the operation of this diploma and medal mill are D. and G. Bandiera, who, so far as we can learn, are neither physicians nor pharmacists nor have any scientific standing. The “Academy” has been referred to at various times294 by The Journal.—(From The Journal A. M. A., Feb. 26, 1916.)
The use of liquid petrolatum in chronic constipation, which has recently become the vogue, has naturally been commercialized; as a result, also naturally, claims of superiority of one brand over another have been made. Some of these claims may have been well founded; others certainly are not. Some have claimed superiority for those products made from Russian oil over those made from American oils. As naphthene hydrocarbons predominate in Russian crude petroleums, and paraffin hydrocarbons in many or most American crude petroleums, it was assumed that the petrolatums derived from these sources differed from one another in like manner. Both the naphthenes and the paraffins are chemically inert; but some unexplained therapeutic superiority has been assumed to reside in the naphthenes. Consequently, it has been urged that the American liquid petrolatums should not be used internally. So far these claims and counterclaims have been based on much theory and little fact. The Journal publishes this week a contribution by Benjamin T. Brooks, Senior Fellow in charge of petroleum investigations at Mellon Institute, Pittsburgh. Brooks calls attention to the fact that Marcusson, in 1913, pointed out that most of the so-called “mineral oils” used for therapeutic purposes contain no paraffin hydrocarbons whatever; that they consist solely of naphthenes and polynaphthenes. Brooks confirms this statement so far as American liquid petrolatums are concerned. He states that many American petroleums, such as most of those from the Gulf region, are like the Russian in containing no paraffin; and that, in the case of those petroleums that do contain it, the customary refinery method of removing paraffin is sufficient to produce true naphthene and polynaphthene petrolatums. “The claim that only Russian oils belong in this class,” he says, “has no basis in fact and has been advanced presumably for business reasons.” The name “paraffin oil” applied to these liquid petrolatums, then, is a misnomer. The new name, “white naphthene oils,” suggested by Brooks, seems superfluous, however, since the pharmacopeial title, “liquid petrolatum,” is subject to no such objection.—(Editorial from The Journal A. M. A., Jan. 1, 1916.)
During the past year The Journal has received letters from physicians in various cities asking for information regarding the “Post Graduate Course of Lectures and Clinics on Nervous and Mental Diseases” which was going to be given in their respective cities by Dr. Albert A. Lowenthal of Chicago. The following inquiries are typical:
“To the Editor.—Please note the enclosed letter from the American Organotherapy Company which appears to be conducted by Lowenthal. The proposition of conducting these clinics impresses me as a piece of colossal gall which is amazing even in these days. Do you know anything about this matter?”
“To the Editor.—Who the dickens is Albert A. Lowenthal, M.D.? Note the circular enclosed. I have blue circled the remarks he evidently thinks will attract.”
In May, 1919, Chicago physicians received a form letter, signed, and on the stationery of, Albert A. Lowenthal, notifying them that Dr. Lowenthal was about to “give a Post Graduate Course of Lectures and Clinics on nervous and mental diseases” in the “Banquet Hall, Morrison Hotel.” Enclosed was a “Programme and Reservation Card” and a self-addressed envelop for physicians to notify Dr. Lowenthal that they would be present. In addition to showing physicians “how to make diagnoses accurately,” Dr. Lowenthal offered to “explain fully how to scientifically check the Christian Scientists and increase your earning power!” And all for nothing!
At later dates similar letters were received by physicians in other cities, on the stationery of the “American Organotherapy Company, Room 902, 31 North State St., Chicago.” Dr. Lowenthal, whose Chicago office is Room 901, 31 North State St., is, apparently, president, treasurer and practical owner of this company. Enclosed with each of these letters—which offered the same inducements, free—was an envelop addressed to Albert A. Lowenthal in care of the hotel at which Dr. Lowenthal would stay while in that city. There was also a “Programme and Reservation Card” as in the case of the letters sent to Chicago physicians.
According to our records, Dr. Albert A. Lowenthal was born in Chicago in 1874 and was graduated by the College of Physicians and Surgeons, Chicago, in 1895, receiving his license the same year.
In a leaflet issued some time ago by Albert A. Lowenthal, M.D., “for the sole purpose of enlightening Prospective Patients in regard to the therapeutic value of the Organo Therapy Treatment for Nervous Diseases,” we learn that Dr. Lowenthal is, or was:
“Professor Nervous and Mental Diseases, Chicago Hospital College of Medicine.”
“Formerly Professor Nervous and Mental Diseases, Dearborn Medical College, Jenner
Medical College.”
“Adjunct Professor on Neurology and Psychiatry, University of Illinois College of
Medicine.”
“Formerly Physician Illinois Eastern Hospital for the Insane.”
“Formerly Supt., Riverview Hospital for Nervous Diseases, Kankakee, Ill.”
“Formerly on Advisory and Associated Attending Staff Cook County Hospital.”
In Polk’s Medical Directory for 1904, Dr. Albert A. Lowenthal’s name appeared, under Chicago, at 910–912 Chicago Opera House Building. He was described as “Superintendent of Lowenthal’s Sanitarium.” In the same issue of the directory, there was a display advertisement of the Lowenthal Sanitarium, which, while located at Kankakee, Ill., had its “main offices” at 912 Chicago Opera House Bldg., Chicago. The advertisement was headed “GOAT LYMPH TREATMENT,” and read in part:
“Goat Lymph has revolutionized medicine, and has been adopted by the scientific medical world as the only therapeutical agent that will absolutely bring about positive results in chronic conditions, such as Neurasthenia, Nervous Collapse, Paralysis, Locomotor Ataxia, Brain Fag, Oncoming Insanity, Chronic Stomach Disorders, in fact such diseases needing cell stimulation.”
It mentioned further that Dr. Albert Lowenthal “introduced Goat Lymph to the medical world as a curative agent.”
A few years ago a Chicago concern, known as the “American Animal Therapy Co.,” put out such products as “Lymphoid Compound (Lowenthal),” “Ova Mammoid (Lowenthal),” “Prostoid (Lowenthal),” etc. The American Animal Therapy Co. had for its manager James M. Rainey. Rainey also operated the “Rainey Medicine Co.,” a mail-order “patent medicine” concern that sold “Vitaline,” a “general debility cure.” The Rainey “Vitaline” quackery was exposed in The Journal, Oct. 1, 1910, and the matter appears in “Nostrums and Quackery.”
When the American Animal Therapy Company was operating from 84 Adams St., Chicago, it claimed to have a hospital and laboratory at Kankakee. At the same time letters were being sent out on the stationery of “The Lymph Hospital,” signed Albert A. Lowenthal, M.D. Although this “hospital” was at Kankakee, Ill., the address on the stationery was 84 Adams St., Chicago, and its telephone number was that of the American Animal Therapy Company. According to the stationery, the “Medical Department” of the Lymph Hospital was “under the personal direction of Dr. Albert A. Lowenthal, who introduced the Lymph Compound and Lymphoid Compound to the Scientific Medical World as a curative agent in Chronic Nervous conditions.” A layman received a letter from the “Lymph Hospital” urging him to take “Lymphoid Compound.” Later he received a “follow-up” letter, from which the following extracts are made. Capitals used as in the original:
“Do you know that the doctors of this country are using the Lymphoid Compound Exclusively in all cases, where the nervous system is greatly involved, with the most Marvelous Results. Isn’t that Sufficient Proof as to the merit of the remedy?”
“... Nobody can tell you there is something just as good, because THERE IS NOTHING JUST AS GOOD AS THE LYMPH—in fact IT IS THE ONLY THING THAT CAN BE DEPENDED UPON.”
“... Our Dr. Lowenthal gives his personal attention to all cases at the Hospital and devotes a portion of his time advising by mail those persons under treatment who are unable to come to the Hospital. He is a man of WORLD WIDE REPUTATION IN TREATING NERVOUS DISEASES—HIS ADVICE ON CASES LIKE YOURS IS WORTH EVERYTHING TO YOU.”
“Think this over and if you do, you will write an order today for the Lymphoid Compound. The home treatment costs $9.50 for thirty three days—think of that. You have our physician’s advice and care free of charge—could anybody ofter more to you?”
In 1908 Dr. Lowenthal appeared as a witness for Edward R. Hibbard, who was being prosecuted by the federal authorities. Hibbard operated a “men’s specialist” office in Chicago; it had two entrances and a different name for each entrance—the “Boston Medical Institute” and the “Bellevue Medical Institute.” Hibbard was found guilty of fraud in the operation of this concern and was fined $1,500. The transcript of the testimony in the Hibbard case records that Dr. Albert A. Lowenthal, when on the stand, claimed to “have treated as many nerve patients as any nerve specialist in Chicago.” He further declared, according to the transcript, that physicians who make a specialty of nervous diseases “mature in about ten years” and that after that time most of them become nervous wrecks or insane. This was in 1908. In this connection it is worth noting that in letters sent out by Lowenthal in May, 1919, he claimed:
“In the past twenty-five years I have limited my work to neurological and psychological cases....”
In 1908 also, Dr. Lowenthal was sending out letters to Illinois physicians in his capacity as secretary of the “Physicians’ League of Illinois.” The “league” issued a “report on candidates for governor and members of legislature,” giving the names of the various political candidates for office whom “the members of the league can safely support.” There were no “membership” fees and a physician who wrote asking “who foots the bills” received no reply.
In 1915 Albert A. Lowenthal, whose “valuable discoveries in the domains of Organo Therapy, Neurology and Pediatrics, have given him an international reputation as a Neurologist, Alienist and Climatological Expert of high standing,” was “Medical Superintendent” of the “National Sanitarium Information Bureau.” This purported to represent the “Leading Sanitariums and Health Resorts in the U. S.” The “Bureau” expected to make its “profit from the 10 per cent. honorarium received on every referred patient.” The “Business Manager” of this concern was one Hubert Miller, M.D. The following advertisement appeared in the classified department of the St. Louis Post Dispatch in 1915:

A layman who wrote in answer to this advertisement received a letter from Dr. Lowenthal in which he said that it was his intention to take about thirty patients south with him for four months—cost of trip $500, which includes medical treatment, board, etc. Dr. Lowenthal stated further:
“I have treated probably more cases of Locomotor Ataxia and Paralysis than any Physician in United States and can honestly state that with Organo Therapy Treatment your walk can be improved and pains controlled.”
In March, 1919, Dr. Lowenthal paid a visit to Spokane, Wash., and Portland, Ore. A Portland paper heralded his coming and printed a picture of “Dr. A. A. Lowenthal, World famous alienist.” The paper described Dr. Lowenthal as “the alienist consulted in the Harry Thaw case” and the one “who treated John Alexander Dowie of Zion City fame and Pope Leo XIII.” The fulsome puffery that Dr. Lowenthal got while in Spokane drew criticism from one or two members of the local medical profession, who wrote to the newspapers protesting. One of the physicians who thus wrote declared that Lowenthal’s “coming was announced in a circular sent through the Owl Drug Company which is agent for the sale of products of an organo-therapy company.”
Apparently, it was after Dr. Lowenthal’s return from the Pacific Coast that he commenced to announce his “Post-Graduate Course of Lectures and Clinics” to the physicians of Chicago, Denver, St. Louis, Columbus, etc.—and, incidentally, to bring to the attention of the medical world the alleged virtues of the products of the American Organo-Therapy Company.—(From The Journal A. M. A., July 3, 1920.)
The “Medical Society of the United States” has for its “Honorary President” one A. H. Ohmann-Dumesnil, A.M., M.D., M.E., Sc.D., Ph.D., and for its “Secretary and Treasurer” one Emory Lanphear, M.D., C.M., Ph.D., LL.D. As originally planned, the “society” seems to have been based on the idea of organizing the “fee-splitters.” In May, 1916, the birth of the organization was announced to the medical profession through a letter signed Emory Lanphear, written on the stationery of the “Medical Society of the United States.” Even in its embryonic state the society had A. H. Ohmann-Dumesnil, A.M., M.D., M.E., for its president, and Emory Lanphear, M.D., Ph.D., LL.D., as its treasurer. The letter read in part:
“We—the majority of the medical profession—who believe in division of fees (i. e., that the surgeon should not ‘hog’ the whole of a patient’s money and leave nothing for the family doctor), are no longer welcome in the A. M. A. We are therefore organizing the Medical Society of the United States, which will not be conducted for the benefit of a few selfish egotists. We would like to have you with us.
“It costs only $1.00 to join. This covers dues for 1916, and includes expense for the beautiful certificate of membership (suitable for framing), which you will receive on admission. Fill enclosed blank and return to me with $1.00.”
But presumably the idea of organizing on a basis of “fee splitting” did not make a hit, so the lure was changed. Today physicians are approached with the plea that the “Medical Society of the United States” will make the medical world free for democracy; it is, we are assured, a “Society of Protest Against the Autocracy of the A. M. A.,” and a “Society of Medical Democracy.”
Membership costs “only $1.00 ... including the cost of a beautiful certificate of membership.” No penalties or punishments are involved for belonging to other societies, and:
“Joining our body need not affect your membership in any other society—even the A. M. A., if you wish to belong to it, and be ‘bossed’ by the ‘Simmons Gang’.”
The dollar for the “beautiful certificate” and membership is solicited by means of circular letters signed “Emory Lanphear,” coming from 3447 Pine St., St. Louis, Mo., the address of what has been variously called the “American Polyclinic,” the “American Hospital,” and later, the “German Hospital.” The “Surgeon-in-Charge” of the “German Hospital” is Emory Lanphear, M.D., C.M., Ph.D., LL.D. When running under the name of the “American Hospital,” Lanphear solicited operative work on a “division of fees” basis, which, the general practitioner was told, meant that “you are to have 40 per cent. of all fees received from your patients sent to our staff for operation or treatment.”

With the change in name from “American Hospital,” to “German Hospital,” Lanphear appealed for a “portion of your operative work on a basis of pure reciprocity.” This “pure reciprocity” seems to have been a still more liberal distribution of the patient’s money, for from a 40 per cent. basis it was raised to an even fifty-fifty. Said Lanphear, in a letter sent out a few months ago:
“I wish also to inform you in spite of the despicable opposition of the hypocritical gang in charge of the A. M. A., and the no less contemptible action of the St. Louis Medical Society, I am going to remain in St. Louis and continue to do surgical work upon a ‘division of fee’ basis. To be more explicit, if you bring me a case for operation I shall allow you one half of the fee for your time, trouble, responsibility and help in the management of the case.”
Before leaving the interesting professional personality of Lanphear, and carefully avoiding any details of a personal nature, we may remind our readers that as long ago as 1908 Lanphear was the “Dean” of the “Hippocratean College of Medicine,” with A. H. Ohmann-Dumesnil, A.M., M.D., M.E., Sc.D., “Vice-Dean.” At that time Lanphear sent out letters to physicians proposing the organization of a “Post Graduate Faculty” on the following basis:
“Those who hold full professorships shall purchase stock in the corporation to the amount of $1,000.00; those who become lecturers or instructors shall pay in the sum of $500.00; those who are to be merely clinical assistants will buy ten shares of stock, $100.00.”
The “Hippocratean College” was a “sundown” affair; it never graduated a student, and expired in 1910.
But to come back to the “Society of Medical Democracy”: The “Medical Society of the United States” seems to have been born in 1916. Its parents, so far as is apparent, seem to have been Lanphear and Ohmann-Dumesnil. The latter, it may be remembered, used to be the editor and proprietor of the St. Louis Medical and Surgical Journal, a publication so obviously venal, that its value to the nostrum makers, whose interests it espoused, must have been small. Advertising pages, “original articles” and “editorials”—all were used to puff nostrums of the crudest type. It was Ohmann-Dumesnil and his journal that came to the defense of the “patent medicine” interests when they were so hard hit by Mr. Adam’s “Great American Fraud” series. In commenting on this phase of “patent medicine” activities, Collier’s, in January, 1907, said:
“Headache powders came in for a considerable share of attention in the patent medicine articles. There was much talk of libels among the headache powder makers, but they decided upon the safer methods of hiring a meretricious medical publication, the St. Louis Medical and Surgical Journal, to print an article in which the Collier’s statements were branded as lies, and the Collier’s editors and writers as liars and libelers. This article the Proprietary Association of America circulated in pamphlet form. The journal which printed it died a natural death a few weeks later. Its editor, one A. H. Ohmann-Dumesnil, has just appeared in the public prints in an unsavory connection with a corrupt lobbying project in St. Louis.”
Some of the nostrums that Ohmann-Dumesnil has recommended are: “Sanmetto,” “Gonosan,” “Cactina Pillets,” “Pepto-Mangan,” “Satyria,” “Campho-Phenique,” “Tongaline,” “Germiletum,” “Narkogen,” “Nosophen,” “Mercauro,” “Arsenauro,” and “Hydrozone.” Many of these testimonials were, of course, used by the manufacturers in their advertising “literature.”
At the time that the Medical Society of the United States was being organized—in 1916—there was published what purported to be a preliminary program of its first meeting. The meeting was held in St. Louis, and the program, while containing the names of men with special fads or interests to exploit, also contained the names of some men of standing. It appeared, however, on investigation, that at least some of the latter had but a hazy conception of the use to which their names were being put, and protested vigorously on learning the facts, repudiating the organization.

Now, in 1918, another drive is on for membership; letters signed “Emory Lanphear” are being sent to various selected groups of physicians. For example, the Eclectics are being coaxed by a letter which commences:
“We want every reputable Eclectic practitioner in this country to join our society of protest against the iniquities of the A. M. A.”
An identical letter has been addressed to Homeopaths, the words “Homeopathic practitioner” being substituted for “Eclectic practitioners.” In all of the letters the “beautiful certificate of membership” is emphasized, and the trivial cost—“only $1.00 a year”—is referred to, while the plea: “surely you are willing to help to that amount to ‘down’ the ‘gang’ in charge of the A. M. A.,” is featured. Another group of letters has gone out to the graduates of the Barnes Medical College. This commences:
“Most graduates of ‘Old Barnes’ have joined our society of protest against the iniquities of the A. M. A. Why should you also not come in? It costs only $1.00 to become a member, including the cost of a beautiful certificate of membership.”
Still another group appeal is based on sex; thus Lanphear:
“We want every reputable ‘lady physician’ in this country to join our society of protest against the iniquities of the A. M. A.”
And yet another:
“You formerly belonged to the Tri-State Medical Society, of which I was Treasurer for 20 years. It is now dead. I wish you would join our new society which has superseded Tri-State in this territory.”
With these various letters is enclosed a “preliminary program” of the 1918 meeting which is to be held October 8 and 9 in Chicago. As might be expected, many of the names on the program are characteristic of the organization and an interesting “story” might be made from the material in The Journal’s files on the individuals. Such names are of men, who, professionally speaking, range from faddists, who ride grotesque and bizarre medical hobbies, to those who with special interests to exploit and unable to use reputable medical organizations for that purpose, take refuge in such hybrid conglomerations as the Medical Society of the United States. Not that the program contains the names of crude quacks, or obvious medical swindlers. It is representative, rather, of that twilight zone of professionalism, the penumbra, in whose uncertain light it is difficult to distinguish between the unbalanced visionary, with a fad, and the more sinister near-quack, with a “scheme.”—(From The Journal A. M. A., Oct. 5, 1918.)
The fourth edition of the National Formulary appears simultaneously with the U. S. Pharmacopeia IX, and is to become official at the same time (September 1). The principles which determine its scope, as frankly set forth in the preface, are apparently the same as those applied, though more faint-heartedly, in the compilation of the Pharmacopeia. A statement in the preface of the new National Formulary runs:
“The scope of the present National Formulary is the same as in previous issues, and is based on medical usage rather than on therapeutic ideals. The committee consists entirely of pharmacists, or of men with a pharmaceutical training, and it cannot presume either to judge therapeutic practice or to follow any particular school of therapeutic practice. The question of the addition or deletion of any formula was judged on the basis of its use by physicians and its pharmaceutical soundness. The considerable use by physicians of any preparation was considered sufficient warrant for the inclusion of its formula in the book, and a negligible or diminishing use as justifying its exclusion.”
Part I of the volume contains formulas, good, bad and indifferent, including the equivalents of a large number of shotgun proprietaries. Part II contains descriptions of drugs. This is a new feature. The purpose is to provide standards for those drugs not described in the Pharmacopeia but used in N. F. preparations. Many of these drugs were described in the U. S. Pharmacopeia VIII, but have not been included in the ninth revision. Practically all are either worthless or superfluous. Part III contains descriptions of special tests and reagents.
Among the therapeutically useful formulas are those for aromatic castor oil, emulsion of castor oil, sprays or nebulae, solution of aluminum acetate, solution of aluminum subacetate and wine of antimony. The two last named are also included in “Useful Drugs.” Several formulas for new classes of preparations which may or may not be found superior to old forms are paste pencils for the application of medicaments to limited areas of the skin, mulls, which are ointments spread like plasters, and fluidglycerates, which are fluidextracts in which glycerin takes the place of alcohol. It should be noted also that, as a result of criticism, the alcohol content of some preparations has been reduced.
As a whole, the present edition of the National Formulary, like its predecessors, is “pharmaceutically useful but not a therapeutic necessity.” To say that it is not a therapeutic necessity is to state the matter mildly, since most of the formulas and almost all of the drugs described have been discarded long since by rational therapeutists. So long as there are physicians who prescribe therapeutic monstrosities, however, the druggist should have the aid that is furnished by this book in compounding them. From the pharmacist’s point of view, therefore, the book is a valuable one. Physicians who have a scientific training in the pharmacology of drugs will not want it; others will be better off without the temptations offered by its many irrational formulas.—(Book Review in The Journal A. M. A., Sept. 2, 1916.)
The treatment by nonspecific methods in a series of cases of influenzal pneumonia has been the subject of two recent papers.295 These methods are a development of the work of Ichikawa, Kraus, Lüdke, Jobling and Petersen, and others on the treatment of typhoid fever and of Miller and Lusk’s work on arthritis. In the original work in this field it was recognized that there were certain inherent dangers in the method and that wide application would be permissible only with the greatest caution and under careful control.
When vaccines and other toxic protein substances are injected intravenously a train of reactions takes place that includes: (a) a primary leukopenia, followed by a leukocytosis; (b) a primary lessening of the coagulability of the blood, followed after some interval by a reduction of the coagulation time; (c) a pronounced lymphagogue effect, the flow of lymph from the thoracic duct being increased threefold; (d) a hyperperistalsis of the intestinal tract, and (e) a marked splanchnic engorgement with a resulting lowering of the systemic blood pressure. The alteration of the coagulability of the blood, together with the vascular engorgement of the splanchnic area and the coincident increase in motility of the intestinal tract that follow the therapeutic injection, all tend to increase the possibility of intestinal hemorrhage. Protein therapy is therefore not a safe procedure in this particular disease. That we are able to terminate a certain number of cases of typhoid fever by crisis by means of such injections is of very great interest from a theoretical point of view.
In the treatment of arthritis, the results seem much more satisfactory. The work of Miller and Lusk296 has been confirmed by a number of observers, among them Culver, Cecil, Snyder, Cowie and Calhoun; and there seems little doubt that we may be able to give prompt relief and even permanent freedom from symptoms in a considerable percentage of cases of acute and subacute arthritis, especially those classed as of rheumatic origin—and this with practically no risk to the patient.
As with other new therapeutic measures, there is still some uncertainty as to the proper dosage, which is a matter of considerable importance, in order to arrive at a just estimate of the relative advantage or danger in the treatment. Typhoid vaccines have been extensively used because they are readily procured and give a prompt and sharp reaction. However, they have the disadvantage of inexactitude in the bacterial count, as well as being of varying degrees of toxicity, the latter factor depending not only on the use of different strains of bacteria in their preparation but on the age of the vaccine. Synder,297 as well as other workers, is of the opinion that the primary dose should be small—from five to ten million organisms—and that the dose of typhoid bacilli injected should never exceed two hundred and fifty million. While a sharp reaction on the part of the patient is apparently a desideratum, a sufficient response can usually be elicited with a relatively small dose. There is no object in subjecting the patient to the risk of the profound depression that follows occasionally in the wake of large doses. Indeed, the only serious results so far ascribed as due to this form of therapy have followed very large doses or the use of relatively large doses in moribund patients; or such unreasonable procedures as the intravenous injection of milk. It is true that milk injections were recommended by some of the German investigators, but they were always used intramuscularly.
In the treatment of pneumonia, Roberts and Cary298 have employed a vaccine made up of 100 million of each of the following organisms per cubic centimeter: influenza bacilli, pneumococci, staphylococci and streptococci. Of this vaccine they injected, intravenously, first 0.5 c.c., later 1 c.c. In the series of 200 patients so treated there was no evidence of injury to the patients in any way. The mortality in this series was 9.5 per cent.; in a series of eighty-six patients not treated with vaccine, the mortality was 31.2 per cent. In the untreated series, 20 per cent. recovered by crisis; in the treated, 36 per cent. so recovered. Before any reliance is placed on such statistics they should be analyzed and compared carefully according to age periods, as the death rate may vary at different ages. Cowie and Beaven298 used typhoid vaccine in the treatment of their patients, and they consider the vaccine shock as indicated only in the early stages of pneumonia.
Before applying the treatment to such diseases as pneumonia it would seem that prudence would demand a thorough familiarity with the range of the reaction and the degree of toxicity of the preparation it is intended to use by first employing it in some arthritic cases. In pneumonia we must ever keep before us the vital factor of cardiac impairment; and certainly we must not undertake any measure that may depress the function of the heart. In arthritis this danger is largely a negligible one; and, with proper precaution, nonspecific therapy is not only without risk but indeed frequently followed by gratifying clinical improvement. Only in the light of experience gained in the manner indicated would it seem permissible for us to attempt to extend this form of therapy to more acute infections.—(Editorial from The Journal A. M. A., May 17, 1919.)
Within the past few weeks a number of inquiries have reached The Journal from physicians in Ohio, Indiana and Pennsylvania. Those that follow are typical:
“I am in receipt of literature from H. L. Roberts, 1126 Masonic Temple, Chicago, advertising clinic in Cleveland by Dr. Willard E. Ogden who claims to be a member of the Chicago Medical Society and the A. M. A. What can you say of this man and his methods?”
“I am enclosing a folder received a short time ago. I would be glad to know if Dr. Ogden is a member of the A. M. A. as he claims to be.”
“The enclosed folder has been sent to many doctors in Indiana. The purpose is plain. The attached post card on this one was returned to him for further literature.”

In each case the correspondents send in a four-page folder bearing the title “Proctology, A Clinic. Who? Where? Why?” Three of the four pages purport to answer the interrogations given on the title page. Under “Who?” we read:
DR. WILLARD E. OGDEN
Chicago, Ill.
SPECIALIST IN PROCTOLOGY
Member Chicago Medical Society and A. M. A.
Author of “Improved Method of Treating Rectal Diseases”
Formerly associated with Drs. Burleson & Burleson
Grand Rapids, Mich.
Under the question “Where?” there appears the statement that “Dr. Ogden Will Hold a Clinic for The Treatment of Rectal Diseases” and the name of the city and the dates of the “clinic” are inserted with a typewriter.
Under “Why?” we read:
“Dr. Ogden does not use the usual surgical methods. His many years of experience in the treatment of Rectal Diseases (during which time he has been associated with the leading Proctologists of America) have enabled him to develop a system of office treatment which is not taught by any other practitioner.
“Tear off, sign and mail attached postcard and I will send you a booklet giving you full particulars as to the course.
Yours truly,
H. L. Roberts, Business Manager.
“Eighty-three per cent. of the people have some Rectal trouble. THIS IS THE DAY OF SPECIALISTS. Why not fit yourself to specialize in Proctology?”
The fourth page is a post card addressed to “H. L. Roberts, Room 1126, Masonic Temple, Chicago.” On the reverse side there is a printed statement which the recipient is expected to sign to the effect that he is interested in “Dr. Ogden’s Clinic” and wishes to have “full particulars of the course.”
A visit to Room 1126, Masonic Temple, failed to disclose the name of H. L. Roberts, either on the door (or doors, for there are two rooms having this number) or on the office building directory board. In fact, Rooms 1126 seem to contain a somewhat miscellaneous assortment. The signs, either on the door or on the directory board, show that there is a public stenographer (who operates a “Mailing Service,” and does “Addressing, Mailing, Multigraphing, Mimeographing”), a bookstore, a chocolate company, a publishing company, a lumber company, and one or two other concerns; but the name of “H. L. Roberts” does not appear. Incidentally, no “H. L. Roberts” is to be found listed in the Chicago telephone directory.
A few yards away from Rooms 1126 and on the same floor there appears the name, “Dr. Willard E. Ogden” on Room 1102.
According to our records, Willard Ealon Ogden was born in 1866. Before taking up the study of medicine he seems to have been a preacher. In 1899 he was graduated by the Saginaw Valley Medical College, Saginaw, Mich. He was licensed in Michigan in 1900, in Illinois and Indiana in 1913, and in Wisconsin in 1921. From 1900 until 1904 he practiced in Lyons, Mich.; from 1906 until 1911 he was at Ionia, Mich.
In 1911, he was in Grand Rapids, Mich., and was associated with Burleson and Burleson, an advertising pile cure concern. From some of the voluminous Burleson advertising on file, we learn that they “cure all diseases of the rectum (except cancer);” and claim to have “the most successful method ever discovered,” and to have cured “many desperate cases that have been given up to die.” Furthermore, they “guarantee to cure in every case or make no charge.”
On Jan. 1, 1914, Ogden was sending out a card to physicians in which he stated that he had removed from Grand Rapids, Mich., and LaPorte, Ind., to 36 W. Randolph St., Chicago, and that he would limit himself “exclusively to the treatment of diseases of the rectum.” Later, Ogden was sending out an advertising booklet filled with testimonials.
In 1914, Ogden was carrying display advertisements in Chicago papers reading, in part, in large back-faced type: “Piles Cured Absolutely Without Knife, Anesthetics, Pain or Loss of Time.... Cure Guaranteed or Money Refunded.”
In March, 1918, he became a member of the Chicago Medical Society and qualified for Fellowship in the American Medical Association, August, 1918.
In 1921, Ogden had a copyrighted mail-order course on the “Treatment of Rectal Diseases by Improved Method.” This “course” consisted of thirty-eight pages of foolscap printed on one side in imitation typewriting. The material abounded in typographical errors. Among the proprietary products recommended in this “course” as “essential” to those taking it, was “Mecca Ointment.” This nostrum, made by a Chicago concern, was declared misbranded in 1916 because of false and fraudulent claims made knowingly, recklessly and wantonly. The “course” was divided into ten parts, and with it, apparently, came ten consecutively numbered sealed envelops, and the purchaser was instructed to open these envelops, one at a time, as he completed the corresponding part in the “course.” He would there find questions which were to be answered and returned to Ogden. This, according to the description, was to enable Ogden to determine whether it was necessary to “enter more into detail upon that particular subject,” which, he stated, he would gladly do if necessary.

Furthermore, the purchaser had the privilege of asking questions of Ogden relative to symptoms, diagnosis and treatment for a period of six months after the purchase of the “course.” Although, in Ogden’s opinion, “you should have the subject well understood long before that time.”
The charge for this course and “services as outlined” was $200, but in order to show his confidence in the ability of those who purchased it, Ogden was willing to take $100 down and the other $100 paid in “five per cent. of monies received from CURED patients” until the balance was paid.
Reverting to the present “post-graduate course” and “clinic”: Those who send in the postal card to “H. L. Roberts” receive a form-letter, signed “H. L. Roberts” in facsimile handwriting, stating that information was enclosed “regarding THE OGDEN METHODS” and stating that Dr. Ogden would be in Indianapolis or Cleveland or Pittsburgh, as the case might be, on a certain date and that the fee for the “clinic” would be $100. With this letter is an eight-page pamphlet entitled “Some Facts Concerning the Ogden Method of Treating Rectal Diseases.” The first page is headed in black-faced type: “About References and Endorsements.” It then states that the “usual references and endorsements are omitted from this booklet.” Further:
“As to Dr. Willard E. Ogden: The professional and social standing of Dr. Ogden is such that he does not need to offer any.
“As to ‘THE OGDEN METHOD’ and its value to you in your professional work: What others say or think has little if any weight. You are your own man. You do your own thinking. You decide for yourself—Do you not?”

The booklet gives an outline of the “Course of Instruction,” which is almost identical, word for word, with the outline given in the letter advertising the mail-order course previously referred to.
The booklet further states that “THE OGDEN METHOD has entirely eliminated the use of cautery, the ligature or any injections, in the treatment of hemorrhoids,” but that “the use of the electric current has proved to be the very correct method in such cases, as will be demonstrated at the clinic.” The booklet reiterates the statement that Ogden’s association with the Burleson and Burleson concern at Grand Rapids makes him “eminently well qualified to instruct members of the medical profession in this important branch of the medical science!”
In addition to this booklet there is a four-page advertising leaflet illustrating and describing the “Ogden Rectal Cabinet” and also the “Ogden Rectal Table and Stool.” There is also a little postcard—addressed, of course, to “H. L. Roberts”—for the physician to fill in stating that “you may enroll me as intending to attend Dr. Ogden’s Clinic in Proctology, to be held at——.” Should the recipient not fill in and mail this enrolment card he gets another form letter calling attention to the fact that the enrolment card has not been received and stating further that “available hotel facilities make it necessary to limit our enrolment to twenty students.”
Careful search fails to disclose that Dr. Willard Ealon Ogden has ever distinguished himself in the practice of the specialty in which he now wishes to instruct physicians. Equally careful search fails to show that Dr. Ogden has ever published a paper either on any proctologic subjects or on any other phase of medicine or surgery. Neither does there seem to be any evidence for the claim that Dr. Ogden “has been associated with the leading Proctologists of America.”—(From The Journal A. M. A., Feb. 4, 1922.)
The inequity of our patent laws, or possibly it would be more correct to say, of the interpretation of our patent laws, has been commented on many times in The Journal. The Journal also has had occasion to call attention to patents that have been issued for obviously unscientific and quackish devices and preparations. The cases of the preposterous gas-pipe fake “Oxydonor” and the creatinin mixture for the alleged conferring of immunity against diphtheria, pneumonia, scarlet fever, syphilis, tuberculosis, etc., are cases in point.
In a patent issued the early part of this year for the “discovery” of a method of flavoring Epsom salt, the patent office has, in fatuity, piled Pelion on Ossa. The “inventor” declares that his invention relates to a pharmaceutical preparation and a special method of treatment of the medicinal agents whereby said agent will be rendered much more efficient in character. He further avows that the “prime object” of his “invention” is to “disguise the normal taste and impart an agreeable odor or smell to salts commonly employed as a cathartic.” Parenthetically it may be said that probably not a day passes that some physician in the United States does not do substantially the same thing when writing a prescription. The “inventor” further claims that the object of his “invention” is to utilize the salts as a vehicle to carry an antiseptic and anesthetic agent whereby the salts when administered as a cathartic “will also act beneficially on the entire digestive tract” and “whereby cramped and spasmodic conditions are at once relieved with a resulting cure of flatulency, indigestion, sick and sour stomach, colic and the destruction of worms, etc.”
Such claims are so absurd that the only excuse for commenting on them is the effect they have on the public mind. The layman reading the specifications of this patent would naturally conclude that an invention of great importance had been made—of such importance as to warrant the government in rewarding its inventor by granting him a seventeen-year monopoly on the sale of his invention.
The law requires that, to be patentable, inventions shall be new and useful and shall show a higher degree of skill in their inception than is naturally to be expected from those who are skilled in the arts to which the inventions belong. It has been decided again and again that physicians’ prescriptions are not patentable because it is assumed that an educated physician will utilize his knowledge of pharmacy in devising proper compounds of medicines to meet the indications of disease. When a physician prescribes a dose of Epsom salt to be taken in one of the official aromatic waters, he does not produce or create a new invention by so doing. Of course, in one sense every prescription is an invention—an invention to meet the conditions presented by the patient—but such inventions are not patentable, because they represent the ordinary skill of a physician in carrying on his vocation.
If the patent office goes on granting patents for such “inventions” as flavored Epsom salt, and it should be found financially profitable to secure such patents and place the products on the market, it will only be a matter of time before the materia medica will be so restricted that a physician will be unable to write a prescription without infringing on somebody’s patent.
The splendid conception of the framers of our constitution in providing a plan for promoting progress in science and useful arts by granting to inventors for a limited time the exclusive use of their inventions, in exchange for the publication of full knowledge thereof, is being debased. No branch of our government is of greater importance to the progress of the country than the patent office, provided that office is intelligently administered. When the patent office is used, however, for an extention of the nostrum business, founded on the abuse of patent and trade-mark laws, it becomes a menace to the public health. The objects of the patent law are being defeated by the practices of the patent office.—(Editorial from The Journal A. M. A., June 23, 1917.)
In this issue we publish two reports of the Council on Pharmacy and Chemistry which illustrate the weaknesses of the present working of the United States patent laws. In the first report the Council presents an investigation of a recently granted patent, and shows that the patent was issued on the mere claims of the applicant and without the presentation of any evidence for such claims. The second report—“Need for Patent Law Revision”—is an appeal to the Patent Office for a more enlightened administration of the patent law, and it presents a few illustrations of the unfair protection which has been granted by the Patent Office. The protest of the Council appears at an opportune time. In Science299 the “Patent Office Society,” an association of employees of the U. S. Patent Office, announces that a committee has been created on request of the National Research Council to make a study of the U. S. Patent Office and its service to science and arts. It states that this committee will meet in Washington shortly to consider the adequacy of the present Patent Office equipment and the simplification of procedure as well as responsiveness to present national and international requirements. The committee also hopes to coordinate, in the interest of an improved public service, the endeavors of the various national societies, manufacturing interests, patent bar associations and all others aiming at genuine patent reform. Unquestionably, there is a growing conviction that in the case of medicines the monopoly given by the patent laws, if granted at all, should be granted with greater consideration of the public welfare. Too often the United States Patent Law has been used to obtain an unfair monopoly on a medicament or to abet quackery. There is no question that one of two things is needed: either a radical change in the patent law itself or the application of more brains in its administration.—(Editorial from The Journal A. M. A., Jan. 12, 1918.)
The patent on aspirin300 (acetylsalicylic acid), controlled by the Bayer Company, American representative of the Farbenfabriken of Elberfeld Company, will expire next year (1917). The Journal has previously stated that the grant of this patent was regrettable and worked injustice to American citizens. It is unnecessary again to go into the grounds for this statement; neither in the Farbenfabriken’s home country, Germany, nor in any other country except in the United States, has a patent been granted for this product. Owing to their monopoly, the manufacturers have been able to exact a much higher price for acetylsalicylic acid, or aspirin, in this country, than elsewhere. Naturally, the Bayer Company, the American agents, view with disfavor the prospect of being compelled to share this rich field with competitors. The foregoing furnishes the answer to inquiries which have reached us from all over the country with regard to the campaign of publicity which the Bayer Company has inaugurated in the lay press. A presumably authentic and apparently candid exposition of the methods used and the motives behind the aspirin advertising is furnished in Printers’ Ink:301
“The manufacturers of aspirin are about to launch an extensive advertising campaign to clinch the market as far as possible before the expiration of their patent rights next year.... The purpose of the campaign is to identify the product with the trademark of the Bayer Company and to this extent hamper competition after the expiration of the patent.”
The business of the Bayer Company, the article goes on to say, has been hurt by the sale of worthless or even harmful imitations put on the market by irresponsible and unauthorized persons when the present war stopped importations from Germany.
“The public knew aspirin, but did not know who made it [italics ours].... When the Bayer Company, Inc., took over the manufacture of aspirin in this country, the first steps were taken to identify the product with the firm who made it.... Of course, there are good reasons why the makers were loth to advertise the product or to exploit their trademark. As every one knows, the advertising of a medical proposition is an extremely ticklish subject.... It is easy to make a misstep. Aspirin is one of those proprietary drugs that are extensively prescribed by physicians. If anything were done that might possibly associate this drug with the patent medicines that are in disfavor with the profession, the valuable influence and cooperation of thousands of doctors might be lost. It is believed that this knotty phase of the question is being answered in the present advertising.... Since nothing is mentioned about ‘medicine,’ ‘cures’ or ‘ailments,’ it is anticipated that there will be but little objection to the copy. All that the advertising attempts to do is to link up the name ‘Bayer’ with aspirin.... The nearest the copy gets to medical talk is in this sentence in very small type at the bottom of the advertisement, ‘The trademark “Aspirin” (Reg. U. S. Patent Office) is a guarantee that the monoacetic acid ester of salicylic acid in these tablets is of the reliable Bayer manufacture.’ ”
From this it appears that, not content with seventeen years of monopoly, the aspirin people are attempting to retain a hold on the market in perpetuo by associating the name of the company with the trade name “aspirin.” There can be no better time than the present, therefore, for the medical profession to substitute, for the nondescriptive name “aspirin,” the descriptive and correct name acetylsalicylic acid.—(Editorial from The Journal A. M. A., Aug. 12, 1916.)
In the past, therapeutic agents and apparatus have been controlled by patents and trademarks for profit. If there have been exceptions, they have been rare. The Principles of Medical Ethics of the American Medical Association contain this statement: “It is unprofessional to receive remuneration from patents for surgical instruments or medicines.” This does not mean that the patenting is wrong in itself; there are occasions when it is wise, if not necessary, to obtain a patent in the interest of the public, and, in the case of surgical instruments and medicines, of the medical profession. In certain instances it is absolutely necessary that the article produced shall maintain a definite standard of quality and purity—and, it may be added, shall be sold at a reasonable price. Enterprising pharmaceutical manufacturers have usually been ready to appropriate the results of scientific research by investigators or therapeutic measures suggested by practicing physicians. Not infrequently, in such instances, the desire for financial gain has caused the marketing of such products with extravagant, if not false, claims as to their value. Yet the patent laws may be used so as to protect and benefit the public and the medical profession. In research laboratories, work is being carried on resulting in the production of new therapeutic agents. It is important that these agents shall be so controlled that they may be made available without subordination to commercial interests. It has become practically necessary, therefore, for research workers to protect their products in the interest of the public welfare and scientific medicine. It has not been an easy matter to decide how best to bring about the desired results. This question has been before the Board of Trustees of the American Medical Association; and, in 1914, the House of Delegates passed a resolution authorizing the board to accept at its discretion patents for medical and surgical instruments and appliances; as trustees, for the benefit of the profession and the public, provided that neither the Association nor the patentee should receive remuneration from these patents. The Rockefeller Institute for Medical Research has solved the problem in a similar manner. In connection with the report of the discovery of several new arsenic compounds, Jacobs and Heidelberger,302 working in the Rockefeller Institute, say:
It may be appropriate to mention here that this substance and related compounds, described in the present and following papers of the series, are covered by U. S. Patents Nos. 1280119-27. Patents have also been applied for in foreign countries. All discoveries made at the Rockefeller Institute are made freely available to the public, in accordance with the philanthropic purposes of the institution. In order to insure purity of product and protection against exploitation, it has been deemed necessary in certain instances to protect the discoveries by patents. It is the purpose of the institute to permit any drugs which may prove of practical therapeutic value to be manufactured under license by suitable chemical firms and under conditions of production which will insure the biological qualities of the drugs and their marketing at reasonable prices. Other than through the issuance of license, the Rockefeller Institute does not participate in any way in the commercial preparation or sale of the manufactured chemicals; and it receives no royalties or other pecuniary benefits from the licenses it issues.
Here we have medicine at its best. The altruism of pure science operating for the benefit of the general public: scientific therapeutics freed from commercial domination.—(Editorial from The Journal A. M. A., Oct. 18, 1919.)
Does the public love to be humbugged? We doubt it. That we, whether sage or fool, are humbugged is undeniable. We are humbugged just to the extent that we are ignorant. There lies one of the most powerful factors operating to the advantage of the “patent medicine” maker and the quack. The layman’s ignorance of the possibilities and limitations of drugs is wide and deep. Hence the ease with which he is fooled on this subject. A seeming frankness in advertising being the order of the day, the nostrum maker makes a pretense of telling what is in his stuff without disclosing any facts that will tend to lift the veil of mystery and thus destroy his greatest asset. So the exploiter of nostrums to the medical profession, realizing that at least a pretense must be made of giving the composition of medicaments offered to the physician, declares that his clay poultice has for its base “anhydrous and levigated argillaceous mineral.” This sounds much more imposing than dry and finely powdered clay, and satisfies by its very sonorousness. Now comes a product exploited chiefly to members of the dental profession but also, it seems, to physicians. Tablets, “activated tablets,” if you please! They are “an anodyne, analgesic febrifuge sedative, exorcising [sic!] antineuralgic and antirheumatic action.” And their composition? Simply “an activated, balanced combination of the mono-acetyl-derivative of para-amidophenetol together with a feebly basic substance in the alkaloidal state from the Thea-Sinensis.” As clear as the Missouri River! Some day some dentist or physician is going to investigate and find that this awe-inspiring, polysyllabic example of exuberant verbosity means nothing more mysterious than our old friends acetphenetidin (phenacetin) and caffein. In the meantime, the exploiters may smile softly and murmur, “Barnum was right!”—(Editorial from The Journal A. M. A., Jan. 1, 1921.)
The ninth revision of the United States Pharmacopeia became official this week, Sept. 1, 1916. It is more fully reviewed elsewhere;303 here we desire merely to call attention to two points; what the book is and what it is not. It is a book of standards for drugs; it is not a book of standard remedies. The Committee of Revision of the Pharmacopeia included physicians and pharmacists (retail, wholesale and manufacturing), but the pharmacists were in the majority and in control. The majority of the representatives of the medical profession on this committee would have preferred to see the bulk of the Pharmacopeia reduced and its value as a work of reference enhanced by the rejection of therapeutically worthless drugs. The representatives of commercial interests, on the other hand, argued that it was necessary for the Pharmacopeia to provide standards for drugs in more or less general use, whether worthless or otherwise. The force of this argument is somewhat impaired by the fact that the National Formulary, which has also been made a book of legal standards, now includes individual drugs as well as combinations; the new edition of the Formulary, in fact, contains a large number of drugs which had been dropped from the U. S. Pharmacopeia VIII. The principle of making use the sole criterion for admission to the Pharmacopeia, however, on the whole carried the day. It has not been strictly observed; good results from the efforts of the medical contingent are to be observed here and there, as in the deletion of elixir of the phosphates of iron, quinin and strychnin and of emulsion of cod liver oil with hypophosphites. That these instances were not expressions of policy on the part of the Committee on Revision, but merely deviations from policy, may be seen by a glance at the contents of the new Pharmacopeia. These include substances which have been shown to be inert, like the hypophosphites (calcium, potassium and sodium hypophosphites), complex and obsolete mixtures, like the compound syrup of sarsaparilla, and drugs which have been tried and found wanting, like saw palmetto berries. Even substances seldom used by the medical profession, but chiefly or altogether by the public, like sassafras, hops and peppermint (the herb), are standardized and made official. It seems difficult to discover any principle by which the sphere of the Pharmacopeia may be definitely marked off from that of the National Formulary. There is one great advantage in specifying U. S. P. drugs and preparations: Physicians who do so invoke legal standards of purity and identity. The only way to be sure of obtaining substances of therapeutic efficiency, however, is to exercise discrimination. The Pharmacopeia is no guide. Being prepared mainly by pharmacists to meet the needs of pharmacists, the Pharmacopeia of course contains much matter of little interest to physicians and entirely foreign to scientific medicine.—(Editorial from The Journal A. M. A., Sept. 2, 1916.)
The ninth revision of the United States Pharmacopeia, which has been in the hands of the Committee of Revision for more than six years, has just appeared. As was to be expected, the desire of medical men on the Committee of Revision to have therapeutic value made a requirement for admission to the Pharmacopeia has not been fully realized; it remains a book of standards for therapeutically good, bad and indifferent remedies. Among the drugs of little or no therapeutic importance or value are musk, arnica, eriodictyon, quassia, pumpkin seed, saw palmetto berries, sarsaparilla and couch grass. Many superfluous drugs and preparations are included. For instance, of the nine forms of quinin described (quinin alkaloid, bisulphate, dihydrochlorid, hydrobromid, hydrochlorid, salicylate, sulphate and tannate, and quinin and urea hydrochlorid), at least four might well have been eliminated. Two insoluble forms (the alkaloid and the tannate), two soluble forms (the hydrochlorid and quinin and urea hydrochlorid), and a moderately soluble form (the sulphate) are all that could reasonably be demanded by even the most extreme partisans of the doctrine of “pharmaceutic necessity.” Further, the use of quinin salicylate for its salicylic acid content and of quinin hydrobromid for its bromid content is unscientific. The inclusion of these salts in the Pharmacopeia is regrettable.
Those interested in the promotion of rational therapy will also regret the inclusion of a number of fluidextracts of violently toxic drugs, such as aconite and gelsemium (dose 1⁄2 minim each), belladonna root, digitalis, nux vomica and ipecac (dose 1 minim each), and lobelia (dose 21⁄2 minims). The more diluted forms, the tinctures, of these drugs are preferable. The inclusion of such fluidextracts in the Pharmacopeia is playing into the hands of certain pharmaceutical manufacturers, who recommend the tincture be prepared from fluidextracts—an unscientific procedure.
The efforts of the medical members of the committee, however, have not been entirely fruitless. Of the articles described in the U. S. Pharmacopeia VIII, 243 have been deleted; sixty-seven new articles have been added. The loss of 167 titles may be set down as a gain. Moreover, most of the new substances give promise of therapeutic usefulness. Thirty-six are taken over from New and Nonofficial Remedies; nineteen are substances which are in the edition of Useful Drugs now in the press. It cannot be said, however; that all of the additions have been judiciously selected. It is an infelicitous time to add calcium and sodium glycerophosphate just when grave doubts of their therapeutic efficiency are being felt. The addition of the extracts of aconite, hydrastis and viburnum prunifolium is likewise unfortunate. All are superfluous preparations, the first because a drug so powerful that an average dose of the extract is only 10 mg. or 1⁄6 grain is better given in the form of tincture; the second because hydrastis is a drug of uncertain value, already represented by three preparations, and the third because viburnum prunifolium has been discarded and discredited by the best therapeutic authorities. It must be accounted clear gain, on the other hand, that the deletions include many inert, obsolete or superfluous substances like bismuth citrate, kaolin cataplasm, pipsissewa, coca leaves, ladyslipper, wahoo, cotton root bark, compound acetanilid powder and compound syrup of hypophosphites, not to mention nine salts of iron and thirty-eight fluidextracts of various drugs. Wines, unmedicated and medicated, whisky and brandy are also among the articles dropped.
A number of new features are introduced, such as microscopic standards for powdered drugs, standard abbreviations for titles, the use of the term “mil” instead of “cubic centimeter,” and a chapter each on sterilization, diagnostic reagents, biologic assays, electrolytic determination of metals and the determination of alcohol, the melting point, the boiling point and the congealing point.
The chemical nomenclature is substantially the same as that adopted in the previous revision; so is the nomenclature of drugs. The addition of official abbreviations for the Latin titles of drugs will doubtless be found a useful feature.
Less commendable is the change from the familiar “Cc.” to “mil.” The term “cubic centimeter” is so thoroughly established and so widely used, wherever the metric system is employed, that it cannot be expected that it will be universally displaced by the word “mil.” The latter is therefore only a superfluous synonym, and as such out of harmony with the simplicity of the metric system. Perhaps it may even be taken for the abbreviation of “millimeter,” “milligram” or other words derived from “mille,” which would be equally entitled to the same abbreviation.—(Book Review in The Journal A. M. A., Sept. 2, 1916.)
The letter that follows comes from a physician who feels that he has a grievance regarding a company in which he holds stock:
“In 1914, I bought some stock of the —— —— Company, and in 1917 bought some more stock in the same company. I notice that the company advertises in The Journal of the American Medical Association, and I believe it does this not so much to acquaint the medical profession with its product, as to acquaint physicians with its name in order that its stock salesmen can keep on unloading more stock to members of the medical profession.
“The company gets the doctors’ money through the sale of stocks, it gets its product on the market with the doctors’ assistance and through their influence, and it looks to me as if the doctors were getting very little in return, as the dividend checks have been few and far between since I have known anything of the company.
“It is not my idea to criticize the product; but I do believe and feel that the stockholders are entitled to a square deal from a company which in turn is expecting so much from them, and again I feel that the publishers of The Journal should be made aware of these conditions so that they do not either consciously or unconsciously foster a concern that is depriving the physician of his hard-earned money.
“If this letter is unfair, I am willing to be shown otherwise. Kindly publish it in The Journal, omitting my name and address.”
The company to which our correspondent refers put out a proprietary product prescribed by physicians and used by the public. Some years ago the company in question advertised its product in The Journal until its stock-selling scheme was brought to the attention of The Journal; the advertisements were then rejected. Some years later, on evidence that the company had discontinued its stock-selling methods to physicians, its product was again admitted to the advertising pages of The Journal. Our correspondent says that he believes that the physicians who hold stock in this company “are entitled to a square deal.” What about the public? Is it getting a square deal when physicians are financially interested in the products that they may be called on to prescribe? Is the average layman’s confidence in the medical profession likely to be enhanced when he learns that the physician to whom he went for treatment has a financial interest in the therapeutic agent which was prescribed? Our correspondent’s complaint against the company seems to be, not that the company sold stock to physicians, but that “the dividend checks have been few and far between,” the assumption being that had the dividends come regularly, there would have been no complaint. It cannot be too often emphasized that it is against public interest and scientific medicine for physicians to be financially interested in the sale of products which they may be called on to prescribe for the sick. It is perfectly true that there are many physicians who would not consciously permit financial considerations to warp their judgment; but it is not humanly possible to remain unbiased in cases of this sort. It is conceivable that a judge on the bench might make every effort to dispense impartial justice in a suit in which one of the parties was a firm in which he, the judge, had financial interest. Nevertheless, it would be obviously improper for such a judge to try a case of this kind. Yet, in this supposititious case the only harm that could result would be of a financial nature. In the case of the physician, the harm is not to the public’s purse but to the public’s health.—(From The Journal A. M. A., Dec. 11, 1920.)
The importance of the standardization of preparations of the posterior, or infundibular, lobe of the pituitary gland (the liquor hypophysis of the new United States Pharmacopeia, pituitary solution, pituitary extract, etc.) is exemplified by a recent publication of Roth.304 As is well known, the active constituent or constituents of this gland have not been isolated, and there is no chemical method of determining the activity and therapeutic value of various preparations. There are, however, certain physiologic methods by which the activity of such preparations may be determined with a considerable degree of exactness. The last revision of the Pharmacopeia, recognizing that the best attested field of usefulness for such preparations is in obstetrics, adopted as a test their activity on the uterus of the guinea-pig; the details of the method adopted by the Pharmacopeia are those described by Roth.305 Roth now reports on the activity of seven samples of commercial infundibular extracts, the products of five American manufacturing pharmacists. Four of these samples were found to be of Pharmacopeia strength; the other three were much weaker. Of the latter, one had but one tenth, another but one fifth and the third but one fourth of the required activity. Those preparations which had been accepted by the Council on Pharmacy and Chemistry for inclusion in New and Non-official Remedies corresponded to the Pharmacopeia requirements. Roth also compared the activity of these seven preparations on the blood pressure, another method by which it has been proposed to standardize infundibular extracts. The four preparations which were equally active on the uterus were found to be equally active on the blood pressure; the other three were much weaker. Roth points out, however, that the results of the two methods are not necessarily parallel; in one instance, for example, two samples caused equal rises of blood pressure, but one was twice as active as the other on the uterus. Hence it is evident that the blood pressure test is not a satisfactory method for determining the activity of a preparation on the uterus, and vice versa.
The subject of pituitary standardization, or perhaps it may be said the application of the present method is, however, in need of further study. Thus the statement has recently been made306 that commercial preparations are on the market which have from three to five times the activity of the Pharmacopeia standard; this was not the case, however, with the preparations examined by Roth. It is probable that some have used for comparison a weaker standard than that proposed by the Pharmacopeia; this, of course, would lead to the conclusion that the commercial preparations were stronger than the Pharmacopeia standard. Roth suggests that the employment of standards of unequal activity by the various supply houses could easily be eliminated by having a central laboratory distribute material for use as a standard. It will be recalled that before the United States Public Health Service established and began the distribution of standards for diphtheria and tetanus antitoxins, the commercial preparations of these varied even more in activity than do those of the pituitary extracts at present.
It is unnecessary to emphasize the importance of this subject; this is sufficiently evident to those who have followed the recent clinical literature on the use of pituitary extracts in obstetrics. These preparations are used in times of emergency; a weak preparation is valueless, whereas overdosage, either from too strong a preparation or from too free use of a preparation of the official strength, is often followed by disaster to the mother or child or both. Roth cites a number of cases of ruptured uterus and other injuries resulting from their use.—(Editorial from The Journal A. M. A., May 5, 1917.)
To the Editor:—I am enclosing a reprint of my article on the “Present Status of Pituitary Extract in Labor,” which appeared in The Journal, June 2, 1917, p. 1601, and also the September issue of Parke, Davis and Company’s Therapeutic Notes, on page 89 of which they quote this article, that you may compare the two. The Therapeutic Notes article is ostensibly a copy, but as a matter of fact, it gives it only in part, which seems to me to be a gross misrepresentation, and one which I do not think should go unnoticed.
Joseph J. Mundell, M.D., Anacostia, D. C.
[Comment.—Therapeutic Notes is one of the house organs of Parke, Davis and Company. A part of each issue is usually devoted to “excerpts” from current literature. The Therapeutic Notes may be judged from the manner of “excerpting” the article of Dr. Mundell. Naturally the interest of Parke, Davis and Company is in those sections of the article which may be expected to promote the use of Parke, Davis and Company’s proprietary preparation of pituitary extract—pituitrin. The following passages from the article of Dr. Mundell were not among those “excerpted” in Therapeutic Notes:
“Used here in properly selected cases, after due consideration by one who has good obstetric judgment, its results are usually happy, and it is a boon to the tired mother and her attendants.”
“To step beyond these narrow confines of indications is indeed entering on dangerous territory. Especially is this true as regards the life of the baby. It is recommended in small doses by some good authorities and is frequently used in cases of slight contraction at the brim with sometimes very good results if the birth occurs within a few minutes, but frequently with disastrous results to the baby if delivery is delayed. In such cases, forceps are urgently indicated. Its use in such cases is risky beyond question. Pituitary extract is recommended in cases of postpartum hemorrhage, but ergot is undoubtedly to be preferred.”
“All means should be exhausted to arrive at a definite diagnosis, and the dangers of its use should be fully appreciated and due consideration given before its administration in any case, for such a powerful drug, used indiscriminately, will surely produce sad results to mother or child or both.”
“During the past two years a number of untoward effects and consequences of severe character have arisen. As far as the maternal accidents and complications are concerned, I firmly believe that were the slogan of the hour “safety first” borne in mind, a number of them could have been prevented, for beyond question this drug has been greatly abused, as it has been given in too large doses, in cases in which its use was strongly contraindicated, and often, I am sorry to say, for no reason other than the accoucheur’s expediency. Its use has been reckless and careless. The many reports of its rapid and safe action have been one of the greatest dangers. DeLee says, ‘It provides for the physician and his brother gynecologist a lot of chronic sufferers, often incurable, even after mutilating operations.’ ”
“An analysis of the detailed reports of all these cases of ruptured uterus with one or two exceptions reveals the fact that pituitary extract was abused, being given to patients who should not have had it. To my mind, to give a dose of pituitary extract to a woman who has a contracted pelvis, mild or severe, when the head has not passed through the pelvis, is criminal and, if the obstetrician is not aware of the contraction, he is still little short of being a criminal.”
In the latter part of his article in The Journal, Dr. Mundell analyzes the reports of twelve cases of rupture of the uterus, thirty-four cases of fetal deaths, and forty-one cases of asphyxia pallida in which “resuscitation was effected only after prolonged and vigorous efforts.” These also were not excerpted.—Ed.]—(Correspondence in The Journal A. M. A., Nov. 24, 1917.)
To the Editor:—The article in The Journal, November 24, page 1818, on proprietorship in medicine does us a gross injustice, and in reply thereto we beg leave to submit the following:
For reasons which every publisher (yourself included) understands, it is not practicable for us to reproduce in full, in the columns of Therapeutic Notes, all the clinical papers to which we wish to direct the attention of our readers. But that the article of Dr. Mundell was not garbled to make capital for Parke, Davis & Co. is quite apparent on comparison of the omitted portions with a previous paper by the same author, reprinted in the January (1917) issue of the Notes, and herewith submitted together with clippings from other issues of the Notes which prove that we have not hesitated to present to our readers the dangers incidental to the misuse of Pituitrin as well as the advantages of its proper use.
Therapeutic Notes, in quoting other journals, puts into its readers’ hands the means of investigating the fairness of its quotations. It is a house organ—true enough; but the organ of a house which has always appealed to the honor as well as to the progressiveness of the medical profession. Its publishers could not afford to resort to deception in advertising their products, through this or any other medium.
The profession is indebted to Parke, Davis & Co. for Pituitrin (among other medicaments), and it is to the profession that the manufacturers look for the ultimate verdict. The contraindications are quite as important as the indications, and, as the excerpts submitted show, we have taken account of these, not only in forming our own estimate, but in presenting the evidence to the readers of Therapeutic Notes.
We cite these facts that you may give us a square deal in an early issue of The Journal if so disposed.
Parke, Davis & Co., Detroit.
[Comment.—The Journal has no desire to discuss Parke, Davis and Company’s motives in omitting certain parts of Dr. Mundell’s paper. What The Journal did was to publish those parts of Dr. Mundell’s paper on the “Present Status of Pituitary Extract in Labor” that Parke, Davis and Company left out of their circular. That it is not practicable, as Parke, Davis and Company points out, for the manufacturers of proprietary products to reproduce in full all clinical papers dealing with such products is obvious. It is not so obvious why such concerns in abstracting or quoting papers of this kind should delete those parts that are unfavorable to the products dealt with rather than those that are favorable. Curiously, however, whenever an author is quoted only in part those parts are almost invariably those favorable to the product.—Ed.]—(Correspondence in The Journal A. M. A., Dec. 8, 1917.)
To the Editor:—The following experiences seem to add one more to the many reasons offered to explain why proprietaries and ready-made preparations flourish at the expense of the official drugs and preparations: A few days ago I prescribed Troches of Ammonium Chloride, U. S. P., for a patient of exceptional perseverance. The next day he had not yet secured the troches and told me that he had submitted the prescription to seven pharmacies, including the largest, and three of the best known and admittedly the best equipped in New York. All told him that these troches were “not being made any more,” and that they were therefore unable to supply him. He thereupon communicated with one of the largest wholesale manufacturing pharmaceutical houses in America and received precisely the same answer. I then took the matter up with a first class pharmacist whom I knew and induced him to prepare this difficult (!) troche, for which the U. S. Pharmacopeia gives the following directions: “Rub the powders together until they are thoroughly mixed; then form a mass with syrup of tolu and divide ...”
Seven pharmacists declined to fill a prescription for an official preparation because they could not buy the preparation from a wholesaler, and it required some persuasion to get the eighth to make the preparation. But even worse, several of the pharmacists offered my patient some ready-made troche more or less resembling the official, or offered compressed tablets of ammonium chlorid.
That this is not an isolated example of what often poses as pharmacy is shown by the fact that I have found it extremely difficult to find a pharmacist who would extemporaneously coat pills with gelatin. Most want the physician to alter his prescription so that one of the ready-made gelatin coated pills can be dispensed, if a gelatin coating is necessary. Some gelatin, hot water, a large cork, and a few domestic sewing needles are all that is required for very satisfactory coating of pills with gelatin, yet few pharmacists seem willing to perform this simple procedure.
Two other illustrations, not so recent, have come to me from a colleague. A few years ago he was unable to obtain from either of two pharmacists an emulsion of cod liver oil without the hypophosphites because, as both said, “It does not come without hypophosphites.” On another occasion four of the best drug stores in Boston were asked for the Compound Laxative Pill, U. S. P., then official in the Pharmacopeia. In every case he was told that he must have meant the compound cathartic pill, which in no way resembles the pill he sought.
With this attitude on the part of the men supposed to be serving the public and the medical profession by the practice of pharmacy, is it any wonder that it is difficult to induce the medical profession to prescribe official preparations or combinations of official drugs in place of ready-made commercial substitutes largely drawn from among the proprietaries or specialties? Real pharmacy by real pharmacists is a necessity if we are to succeed in combating the proprietary evil.
Cary Eggleston, M.D., New York.
—(Correspondence in The Journal A. M. A., Aug. 21, 1920.)
Recent newspaper reports regarding the alleged “discovery of the Germ of Pernicious Anemia” and the development of “an antitoxin and serum” by Dr. Philip Rahtjen of Pasadena, California, have brought inquiries of which the two that follow are typical. This from a physician in Indiana:
“Please let me know about the supposed recent discovery of Dr. Philip Rahtjen concerning pernicious anemia. The information I have is from a newspaper clipping of October 21, Pasadena, California. Kindly omit my name.”
A New York physician writes:
“If you could send me any information as to the enclosed I would appreciate it. The article impresses one as absolutely inconclusive. However, I promised the patient I would investigate the matter.”
The enclosures referred to consisted of a reprint and a letter from “Ph. Rahtjen, M.A., Ph.D.,” Pasadena, Calif., both of which had been sent to a layman who had written to Rahtjen. The reprint was a translation of a brief article by Rahtjen “On the Etiology of Idiopathic Anemia,” translated from the Centralblatt für Bakteriologie Parasitenkunde und Infektionskrankheiten. Rahtjen’s letter to the layman read:
“Your inquiry relative to my isolation and classification of the Germ of Anemia received.
“I herein enclose my paper published in August in the Central Magazine of Bacteriology.
“I have succeeded in immunizing goats against the Germ therein described. Five thousand injections of the Serum have been given. Three hundred cases diagnosed as Anemia and Chlorosis were treated under observation. Six cases of Pernicious Anemia were observed under treatment. All responded favorably.
“The Serum is at your disposal from my laboratory here for the use of your physician. The price is five dollars for twelve ampoules each containing 1 ccm., the amount of one injection.
“The treatment consists of intramuscular injection every second day accompanied with a nitrogenous free diet, preferably milk diet. Your attending physician should very easily give them.”
Just what Rahtjen’s serum is we do not know. Nor have we been able to find any information on the subject in any available medical literature. In fact, a rather careful search of American medical literature for some years past fails to reveal any article by Rahtjen on any subject.
Philip Rahtjen is not a physician. In the Propaganda files is a circular issued in 1917 by the “Rahtjen Tuberculosis Sanatorium” of San Francisco, Calif. This exploits “The Rahtjen Cure for Tuberculosis” and tells of “The Discovery of Dr. Philip Rahtjen.” The circular states that:
“Dr Rahtjen studied in Heidelberg, Berlin, Munich, Marburg, and Rostock, Germany, from which latter school in 1904, he graduated in chemical pathology as Doctor of Philosophy. He became assistant professor of pathology at the Imperial Biological Station at Heligoland, and was later appointed assistant to Dr. Piorkowsky, head of the Deutsche Schutz und Heilserum Gesellschaft.”
The same circular summarizes the potentialities of “Rahtjen’s Cure for Tuberculosis” thus:
“The remedy seems to cure tuberculosis in all its forms with equal celerity and certainty. The evidences indicate that it does not matter how far the disease has progressed, if there be tissue of the attacked organ remaining sufficient to sustain life, the disease can be wholly eradicated and the patient restored to health. This is indicated alike in tuberculosis of the lungs, of the throat, of the bladder, of the kidneys.”
The booklet stated further that patients might be treated at one of two places: at the offices of the sanatorium in the city of San Francisco, or at the sanatorium itself near Glenwood. The cost of treatment at the sanatorium was to be $1,000, which would entitle “the patient to residence and attention there for four months.” According to the leaflet, “This is regarded as a period sufficient to restore the patient to health whatever be the stage of his disease; provided only, as we remark, that he has enough left of the infected organ to sustain life with the T. B. expelled.”
“At the end of four months the patient is sent to his home, not alone relieved of his disease, but in a highly vigorous state of health.”
All this, as stated previously, was in 1917. And yet people are still dying of tuberculosis!
In March, 1920, Rahtjen (so the newspapers have it) was offering a “New-Life Fluid.” According to a San Francisco paper, Dr. Philip Rahtjen “announces the discovery that by the injection of secretions from the ductless glands the human body may be reinvigorated.” The paper described the discovery “as a long step forward in the fight to counteract old age” and stated that a syndicate was being formed by Rahtjen and others to “produce the extract in such quantity that it may be available for every one.” The newspaper article showed the learned doctor in a laboratory apron in the characteristic pose of the newspaper “scientist” pouring something from a beaker into a test tube—and gazing intently at the camera while doing it! This was in March, 1920; yet people still grow old.
Within the last month the Los Angeles Examiner has heralded some more wonderful accomplishments of Rahtjen. According to this paper Rahtjen has:
1. Isolated the “germ of pernicious anemia.”
2. Found the “serum” for the cure of this disease.
3. Discovered the secret of human virility.
4. Evolved a fluid “from the glands of selected bulls and cows” which will “restore ‘pep’ for worn-out human bodies! Give added weight, clearer eyes, brighter minds, quicker bodies and a generally ‘firmer grip’ on oneself!”
This “amazing discovery” was, according to the Los Angeles paper, the culmination of “five years of continuous study” and had only just been revealed by Rahtjen.
“Dr. Rahtjen has for years been working silently in a bio-chemical laboratory in Pasadena, surrounded by microscopes, scales, test-tubes, acids, alkalis, reagents and all the accompanying stage settings that spell bio-chemical science.”
All of these wonders might still have been a closed book to the public had not “friends” of Dr. Rahtjen brought the matter to the attention of the Examiner.
“Dr. Rahtjen yesterday, with the usual reserve of the ethical scientist, was disinclined to talk of his work until publication of it in a scientific journal.”
Fortunately for a palpitating public, the Los Angeles Examiner “was able to learn the essence of his study” and pass the information on. It seems from this newspaper report that Rahtjen first made his extracts from the glands of goats and sheep but these extracts “were found to be too strong.” As a result “Dr. Rahtjen is now using the glands of specially selected Mexican bulls and cows.” The male patients who are “weak, uninterested in life, unable to concentrate in thought” are given the extract of bull; the female patients who are in a similarly deplorable condition receive an “injection of the cow gland extract.”
We have not yet learned whether the Los Angeles Examiner has deprecated Dr. Rahtjen’s use of Mexican bovines. Remembering the attitude of the Hearst papers toward all things Mexican, one may look for the suggestion that Mr. Rahtjen use 100 per cent. American bull.—(From The Journal A. M. A., Nov. 26, 1921.)
To the Editor:—I was much interested in the study of this subject by Dr. H. N. Cole (The Journal, Dec. 30, 1916, p. 2012.)
In 1913 I treated a series of cases of syphilis with sodium cacodylate; but, not getting the desired results, I discontinued its use. In 1915, I became interested again because of the writings of Dr. J. B. Murphy, and applied it in three cases in which the patients had initial lesions:
Case 1.—J. M., man aged 21, single, shoeworker, came to me with an initial lesion of the penis to the right of the frenum. I began intramuscular injections of sodium cacodylate, 5 grains, in ampules made by Parke, Davis & Co., every day for ten days. Then I halted for ten days and repeated ten more injections. The sore on the penis entirely disappeared about the ninth day. There was a slight, faintly macular eruption of the forearms and abdomen, which soon disappeared. There was no alopecia. When he returned, after the last series of ten injections, there were mucous patches in the throat and some involvement of the left tonsil. I put the patient on mixed treatment, which cleared his throat. He had, at end of twenty doses of 5 grains of sodium cacodylate each, a positive Wassermann reaction. After mercury and potassium iodid for two months there was a positive Wassermann reaction. To date, after three salvarsan treatments intravenously there have been two negatives.
Case 2.—F. S., man, aged 28, married, machinist, had an initial lesion on the penis. Treatment with sixty injections of 5 grains of sodium cacodylate gave results as follows: The initial sore on the penis disappeared in ten injections; there were severe mucous patches of the mouth; the tonsils were badly infected. There was a positive Wassermann reaction. There were syphilids of both arms and shins; marked papular eruption; malaise, and a slight trace of albumin in the urine. I placed the patient on mercurials and at last give him three salvarsan injections three weeks apart. The result was a negative Wassermann reaction, the skin was clear and the patient felt fine.
Case 3.—D. C., woman, aged 21, single, seamstress, had an initial lesion on the left side of the cervix, and a macular eruption on the face, neck and shoulders, and also, though faint, on the forearms. Thirty injections of sodium cacodylate of 5 grains each were given. The initial lesion disappeared in one week. Mucous patches of the mouth appeared and persisted. The Wassermann reaction was positive. I gave mercurials and potassium iodid for seven months, and salvarsan once. The Wassermann reaction is now negative.
My conclusion after two trials of the use of sodium cacodylate in small or large doses is that it has no effect toward curing the condition; in fact, the throat symptoms were seemingly increased in severity by its use. It has no effect on syphilids of the forearms and shins, and if anything makes them worse.
It improves the appetite, as one would expect. It has some effect on the kidneys, as noted in Case 2; it has some effect in healing the initial lesion, as noted in all three of this series; why, I do not know.
I am entirely satisfied that it has no beneficial effect on syphilitics and have discontinued its use entirely in my practice.
I am glad to have read Cole’s excellent article, as it shows me that I was correct in my decision not to use it again, as it was worthless.
William G. Ward, M.D., Lynn, Mass.
To the Editor:—Dr. William G. Ward’s letter (The Journal, Feb. 3, 1917, p. 390), and the recent admirable article by Dr. Harold N. Cole (The Journal, Dec. 30, 1916, p. 2012) recall to mind Dr. J. B. Murphy’s clinical note on the use of sodium cacodylate in the treatment of syphilis (The Journal, Sept. 24, 1910, p. 1113), and the experimental work of Cap. H. J. Nichols, U. S. Army (The Journal, Feb. 18, 1911, p. 492). The results of Nichols’ work conclusively proved, at least from a laboratory standpoint, that this drug was of very little value as a spirocheticide in combating syphilis. Prior to the publication of Dr. Murphy’s letter I had employed sodium cacodylate extensively as a remedy in psoriasis, and I still continue to use it in selected cases of the disease.
Adopting Dr. Murphy’s suggestion, I gave the agent an extensive trial in syphilis in all stages of the disease. The results were extremely disappointing, from both clinical and serologic points of view. More recently, during the scarcity of salvarsan, I gave the drug a second trial, employing it in large dosage in the hope that the previous failure had been due to the employment of insufficient amounts. The results were not tabulated, but, judging roughly from my experience in a score of cases, its therapeutic value as an antisyphilitic was nil. A few of the patients underwent a temporary improvement, probably owing to the tonic effect of the drug, but in every instance the serologic findings were unaffected.
R. L. Sutton, M.D., Kansas City, Mo.
—(Correspondence in The Journal A. M. A., Feb. 3, 1917.)
The tablet form of administering medicines is popular among many physicians because of its convenient availability and dosage. There is no doubt about the convenience of tablets, but the accuracy of the dosage content is not always to be depended on. One reason for this is that the demand for palatable and convenient “medicaments has led manufacturers to attempt to produce in tablet form mixtures which, from the nature of the case, are not suited to that method of compounding.” In a series of painstaking experiments307 on bismuth, opium and phenol tablets, conducted a number of years ago in the A. M. A. Chemical Laboratory, it was shown that no tablets on the market then contained the amount of phenol the label indicated, the variation being from 12.3 to 112.5 per cent. Similarly, the laboratory found that in the case of several different brands of Aromatic Digestive Tablets,308 the amount of hydrochloric acid present in these absurd combinations was true to label in only one half of the specimens, notwithstanding the fact that the amounts claimed to be present were ridiculously small; in two specimens, there was no hydrochloric acid whatever present, while a third contained only a trace. These examples illustrated clearly the very evident unwisdom of attempting the pharmaceutically impossible merely for the sake of convenience or pharmaceutical “elegance.”
Another reason for doubting the accuracy of dosage, irrespective of the characteristics of the drugs composing the tablets, has been the manifest lack of care in their manufacture. In 1914, Kebler309 reported the results of a far-reaching investigation of tablet compounding in which he pointed out that tablets on the market were not as uniform or accurate as was generally believed, the variations being “unexpectedly large in numbers and amount.” During the past year, the Connecticut Agricultural Experiment Station310 undertook the examination of tablets—proprietary and nonproprietary—taken from the stock of dispensing physicians. The variations found in weights of the tablets were strikingly similar to those reported by Kebler.
VARIATION IN WEIGHTS OF TABLETS
| Variation | Kebler Per Cent. | Connecticut Per Cent. |
Less than 10 per cent. | 43 | 44 |
More than 10 per cent. | 57 | 56 |
More than 12 per cent. | 44 | 35 |
More than 15 per cent. | 28 | 26 |
More than 20 per cent. | 9 | 10 |
The determinations of the composition of the tablets when compared with that claimed for them showed wide variation—from 54 per cent. above to 70.5 per cent. below; in almost two thirds of the tablets examined, the variation amounted to more than 10 per cent.; in three fifths of the tablets, the variation was more than 15 per cent.; in one fourth, more than 20 per cent., and in one twentieth, more than 50 per cent.; only in one eighth of the tablets was the variation less than 5 per cent.
The Connecticut investigators substantiate once again the work previously reported, namely, that there are a number of firms who are either incompetent or careless. For tablets of simple composition, a variation from the declaration of 10 per cent. should be amply sufficient to compensate for the errors of careful manufacture. It may be added that the best tablets originate generally from firms having competent chemical control.—(From The Journal A. M. A., July 27, 1918.)
According to the good old truism, the last and crucial proof of the pudding is in the eating thereof; and so, the last and crucial test of a therapeutic agent is its consumption by a patient. There is, however, one essential difference: When the pudding is eaten, with a sense of satisfaction, we know that it was a good, or at least an eatable, pudding.
If the patient improves after taking a remedy, we do not yet know that he improved on account of the remedy. The post hoc type of reasoning or logic is not respectable; but it is all too apt to creep in unawares, unless one takes great precautions indeed.
Clinical evidence needs especially to be on its guard against this pitfall, for the conditions of disease never remain constant; nor is it possible to foresee with certainty the direction which they are going to take. It is just this point which makes the clinical evidence so much more difficult to interpret than laboratory evidence, in which the conditions can be more or less exactly controlled, and any changes foreseen. It is on this account, also, that clinical experiments must be surrounded with extra painstaking precautions.
In brief, while the “proof” of a remedy is on the patient, that is not the whole story, but merely an introduction. The real problem is to establish the causative connection between the remedy and the events. The imperfect realization of this has blocked therapeutic advance, has disgusted critical men to the point of therapeutic nihilism, and has fertilized the ground for the commercial exploitation of drugs that are of doubtful value or worse.
This has been impressed on me particularly by my service on the Council on Pharmacy and Chemistry. In the course of its work of passing on the claims advanced for commercial remedies, this Council is forced to inquire critically into the basis of the claims of manufacturers.
It is interesting to note the qualitative differences in the evidence for the various kinds of claims: The chemical data are usually presented in such a form that it is possible to tell at a glance whether or not they are based on demonstrated facts, which could usually be verified or refuted without special difficulty. The deductions are usually such as can be legitimately drawn from the data, or else they are obviously absurd. All this agrees with the relatively exact status of chemical science.
In passing to data and deductions from animal experiments, a distinct change is noticeable: Not only are the data less reliable, and less worthy of confidence, but they are more often stated in a less straightforward manner. The presentation of the data often shows evidence of manipulation of the results, so as to make them most favorable to a preconceived conclusion that would recommend the drug. This is not always intentional, but is partly due to the less exact nature of animal experimentation, which leaves a wider play to the arbitrary interpretation of the reporter. A certain amount of this is unavoidable. No serious objection can be raised, provided the experimenter presents all the essential data, and discusses fairly all of the interpretations that would apply to them.
On the whole, it is usually possible to form a fairly definite estimate of the value of experimental data.
When one comes to the clinical evidence, an entirely different atmosphere obtains. When the Council demands evidence of the usefulness of a remedy, the manufacturers generally respond with every sign of enthusiasm. They may have ready a series of articles already published, or they instruct their agents to bring in letters from physicians. The last method seems to meet the most cordial response, judging from the deluge of letters and opinions that floods the Council.
The quality of the published papers is a fair reflection of the deficiencies of what is still the common type of clinical evidence. A little thought suffices to show that the greater part cannot be taken as serious evidence at all. Some of the data are merely impressions—usually the latest impressions of an impressionable enthusiast—the type of man who does not consider it necessary to present evidence for his own opinions; the type of man who does not even realize that scientific conclusions must be based on objective phenomena.
Some of the papers masquerade as “clinical reports,” sometimes with a splendid disregard for all details that could enable one to judge of their value and bearing, sometimes with the most tedious presentation of all sorts of routine observations that have no relation to the problem.
The majority of reports obtained by the agents belong to these classes, notwithstanding the fact that they are often written for the special use of the Council, and therefore with the realization that they are likely to be subjected to a thorough examination, and therefore presumably representing the best type of work of which the reporter is capable. So, at least, one would suppose.
It is also possible, however, that some of these reports are written merely out of thoughtlessness, or perhaps often to get rid of an importunate agent. This is illustrated by the following correspondence, taken literally from the files of the Council.
A letter from a prominent physician “A,” endorsing a certain preparation “D,” having been submitted to the Council, the secretary was directed to write to Dr. A as follows:
Dear Dr. A:—The B Company of C has requested the Council on Pharmacy and Chemistry to admit its preparation D to New and Nonofficial Remedies. As part evidence for the value of the preparation, the company submitted a letter from you which contains the following:
So far as my experience has thus far gone, they are certainly superior to a number of other iodine compounds now on the market, and I should judge that they ought to take a superior place in therapy involving the use of iodine.
The referee of the Council in charge of D writes that he was interested by your letter and asks that I inquire: As compared with sodium or potassium iodid, what would you say are the differences between, and real advantages of, D and the alkaline iodids? Did you make any comparative experiments and keep a record of them? If so, the referee would like to receive an account of your trials. In what direction could D be expected to occupy a superior place in iodin therapy?
I hope that you can give the information asked by the referee and thus aid the Council in arriving at a correct estimate regarding the value of D.
The following reply was received from the physician in response to the foregoing:
Dear Professor Puckner:—In reply to yours of January 19, I did not proceed far enough in the investigation of D to draw conclusions of any particular value for the purpose of the Council on Pharmacy and Chemistry; and I so stated in my letter to the proprietors of that remedy.
Answers to the questions you put in your letter require an amount of investigation of the remedy far beyond anything I undertook. As a matter of fact, I returned about five sixths of the capsules sent me, because of lack of time and opportunity to carry out the extensive clinical experiments that I plainly saw would be required to give an opinion at all worth while. I believe you had better not consider me in the matter at all.
The report was furnished by a physician for whom I have a high personal regard. I introduce it here, not so much in a spirit of criticism, but as a justification of the opinion that I have formed of clinical evidence obtained by manufacturers through their clinical adjutors.
When commercial firms claim to base their conclusions on clinical reports, the profession has a right to expect that these reports should be submitted to competent and independent review. When such reports are kept secret, it is impossible for any one to decide what proportion of them are trustworthy, and what proportion thoughtless, incompetent or accommodating. However, if this were done it is quite possible that such firms would find much more difficulty in obtaining the reports. Those who collaborate should realize frankly that under present conditions they are collaborating, not so much in determining the scientific value, but rather in establishing the commercial value of the article.
Often the best type of clinical reports—those in which the observations are directed to the significant events and not to mere side lines, and in which the significant events are correctly and adequately reported—generally lack one important essential, namely, an adequate control of the natural course of the disease.
Since this cannot be controlled directly, it must be compensated indirectly. For this purpose, there are available two methods:
The first is the statistical method, in which alternate patients receive or do not receive the treatment. This method can usually only be of value when a very large series of patients is available. Even then, its value is limited or doubtful, because it cannot take sufficient account of the individuality of cases.
The second method consists in the attempt to distinguish unknown preparations by their effects—the method that might be called the “comparative method” or the “blind test.”
In this, the patient, or a series of patients, is given the preparation which is to be tested, and another preparation which is inactive, and the observer aims to distinguish the two preparations by their effects on the patient. Surely if the drug has any actions at all it will be possible to select correctly in a decided majority of the administrations.
The same principle can be applied in distinguishing the superiority of one preparation over another. In this case, the two preparations would be given alternately to different patients, and the observer would try to distinguish them by their effects. Here again, if one drug is really superior or otherwise different from another, to a practically important extent, the observer will surely be able to make the distinction.
This method is really the only one that avoids the pitfalls of clinical observation; it is the only method that makes the results purely objective, really independent of the bias of the observer and the patient. It is the only method, therefore, which determines whether it was really the pudding that was eaten and not some other dessert.
In principle this method does not usually offer any very great difficulties. It is, of course, necessary that the two preparations to be compared shall resemble each other so closely or shall be flavored, etc., so that they cannot be distinguished by their physical properties. This is usually not a very difficult matter. The method does not jeopardize the interests of the patient, for it is understood that no drug would be tested in this way unless there is some reason to believe that it has a value. When the patient’s condition is such as to demand treatment, then he would be receiving either the standard drug or the drug which the experimenter believes may be superior to the standard.
The final and crucial test of a remedy is on the patient; but the test must be framed so as to make it really crucial. Most clinical therapeutic evidence falls far short of this. The “blind test” is urged to meet the deficiencies.—(From The Journal A. M. A., July 21, 1917.)
Under the title “Vaccines in Toxic Conditions,” what purports to be a scientific contribution appears in the original department of the official organ of a state medical society.311 The apparent purpose of the article is to overcome any hesitancy on the part of practitioners to use vaccines in toxic infectious conditions for fear that they might thereby cause harm. Such a thesis is interesting and might be important—if true. Two outstanding facts, however, give pause. First, the theory promulgated is contrary to the experience of those who have studied the subject; second, the man who writes the article is in the business of making and selling vaccines! The former fact is a matter of fairly general knowledge among the better informed members of the medical profession; the latter fact is nowhere made evident in the article, which the reader might infer came from a disinterested investigator in the realms of immunology.
The article purports to prove that the special investigations carried on by its author show that there is no basis for the well-grounded fear that vaccines might be harmful to a patient suffering from toxic infectious conditions. Thus:
From a closer study of these infective processes we find that this toxic condition is due to the rapid multiplication of the infecting organisms with the incidental production of ferments which the germs secrete to digest the food on which they live. These toxic ferments have a distinct destructive tendency on tissue cells, without any marked influence in stimulating tissue cells for antibody production. The crying need, however, in these extensive acute infections is rapid antibody formation to neutralize these germ-produced poisons and to eliminate the germs.
Now vaccines, we are informed, are not toxic and so stimulate the production of antibodies. In other words, the same organism that in the body is toxic and without marked antigenic properties becomes nontoxic and actively antigenic when converted into a vaccine. The details of the experiments of the “closer study” made by the author of this paper (and the manufacturer of vaccines) which give such definite and convincing results are not published. Possibly the article is a preliminary contribution, and future issues of the same publication will carry further articles on the same subject. The follow-up system is well recognized in the advertising world. At all events, this “closer study” has convinced the author of the article that:
... even in extreme toxic conditions, in acute infections, bacterial vaccines may be employed without the least fear of doing any harm. In fact, we find that in extreme acute infections, bacterial vaccines not only give the best clinical results, but they may also be given in larger doses at shorter intervals with less reactions than in minor or chronic infections and the earlier they are given the better the results.
Here again no details are given; there are no comparative results of the careful study of a series of cases. The sum and substance of this remarkable contribution to a scientific publication is to the effect (1) that the organism that in the body is toxic becomes nontoxic when introduced in vaccine form; (2) that the organism that in the body is but little antigenic becomes when introduced in vaccine form actively antigenic, and (3) that “in extreme acute infections” when the body is affected profoundly by the infectious agent and its product, the oftener and the more one injects of these very materials, the better the results!
And this astounding plea for the use of vaccines in conditions in which vaccines are generally held to be contraindicated, or even injurious, is made by one whose business is the manufacture of vaccines and selling them to the medical profession!—(Editorial from The Journal A. M. A., Oct. 23, 1920.)
Our knowledge of the accessory food factors, commonly spoken of as vitamins, is so recent, comparatively speaking, and the exact nature of these factors still so enveloped in mystery, that it was inevitable that the public’s lack of knowledge on the subject should be capitalized. It is not surprising that there are on the market a number of preparations of the “patent medicine” type that are being sold under the claim that they are rich in vitamins—although the exploiters of these fail to explain which, if any, of the three accessory food factors their products contain. The renaissance of yeast as a therapeutic agent has given an opportunity to the manufacturers of this product of unduly stressing the fact that yeast is particularly rich in the antineuritic vitamin (water-soluble B). Because milk and certain milk products are rich in the fat-soluble A factor, the dairy interests would apparently have the public believe that this particular vitamin is to be obtained only from their products. Thus, a journal devoted to the dairy interests recently claimed that those who want vitamins must get them in their milk, butter, cheese and other milk products. The truth is, the accessory food factors are so well distributed throughout the dietary of modern man that, generally speaking, the individual who uses ordinary judgment in selecting his food is in no danger of suffering from a deficiency of any of these three factors. It would be well if every physician might read the excellent monograph on the present state of knowledge concerning accessory food factors written by a committee appointed jointly by the Lister Institute and Medical Research Committee. In this report the distribution of the vitamins in our common foodstuffs is thus briefly summarized: “... broadly speaking it is safe to say that the individual always finds sufficient supply of vitamins in his food so long as that food is reasonably varied and has received no artificial or accidental separation into parts, and so long as no destructive influence has been applied to it.” At the end of the committee’s report is a table (reproduced on page 562) which shows the distribution of the three accessory factors in the commoner foodstuffs.—(Editorial from The Journal A. M. A., Aug. 13, 1921.).
Distribution of Three Accessory Factors in Commoner Foodstuffs
| Classes of Foodstuff | Fat-Soluble A or Anti- rachitic Factor | Water-Soluble B or Antineuritic (Antiberiberi) Factor | Antiscor- butic Factor |
| Fats and Oils: | |||
Butter | +++ | ||
Cream | ++ | ||
Cod-liver oil | +++ | ||
Mutton fat | ++ | ||
Beef fat or suet | ++ | ||
Peanut oil | + | ||
Fish oil, whale oil, etc. | ++ | ||
Margarin prepared from animal fat | Value in proportion to amount of animal fat contained | ||
Nut butters | + | ||
| Meat, Fish, etc.: | |||
Lean meat (beef, mutton, etc.) | + | + | + |
Liver | ++ | ++ | + |
Kidneys | ++ | + | |
Heart | ++ | + | |
Brain | + | ++ | |
Sweetbreads | + | ++ | |
Fish, white | very slight, if any | ||
Fish, fat (salmon, herring, etc.) | ++ | very slight, if any | |
Fish roe | + | ++ | |
Canned meats | ? | very slight | |
| Milk, Cheese, etc.: | |||
Milk, cow’s whole, raw | ++ | + | + |
Milk, skim, raw | + | + | |
Milk, dried whole | less than ++ | + | less than + |
Milk, boiled, whole | undetermined | + | less than + |
Milk, condensed, sweetened | + | + | less than + |
Cheese, whole milk | + | ||
| Eggs: | |||
Fresh | ++ | +++ | ? |
Dried | ++ | +++ | ? |
| Cereals, Pulses, etc.: | |||
Wheat, maize, rice, whole grain | + | + | |
Wheat germ | ++ | +++ | |
Wheat, maize, bran | ++ | ||
Linseed, millet | ++ | ++ | |
Dried peas, lentils, etc. | ++ | ||
Soy beans, haricot beans | + | ++ | |
Germinated pulses or cereals | + | ++ | ++ |
| Vegetables and Fruits: | |||
Cabbage, fresh (raw) | ++ | + | +++ |
Cabbage, fresh (cooked) | + | + | |
Cabbage, dried | + | + | very slight |
Cabbage, canned | very slight | ||
Swede (rutabaga) raw expressed juice | + | +++ | |
Lettuce | ++ | + | |
Spinach (dried) | ++ | + | |
Carrots, fresh raw | + | + | + |
Carrots, dried | very slight | + | |
Beetroot, raw, expressed juice | less than + | ||
Potatoes, raw | + | + | |
Potatoes, cooked | + | ||
Beans, fresh, scarlet runners, raw | ++ | ||
Onions, cooked | + at least | ||
Lemon juice, fresh | +++ | ||
Lemon juice, preserved | ++ | ||
Lime juice, fresh | ++ | ||
Lime juice, preserved | very slight | ||
Orange juice, fresh | +++ | ||
Raspberries | ++ | ||
Apples | + | ||
Bananas | + | + | very slight |
Tomatoes (canned) | ++ | ||
Nuts | + | ++ | |
| Miscellaneous: | |||
Yeast, dried | +++ | ||
Yeast, extract and autolyzed | ? | +++ | |
Malt extract | + in some specimens |
Commenting on the trend of medical research concerning vitamins, the latest report of the British Medical Research Council says:
The present situation is a curious one, upon which posterity will probably look back with great interest. We still have almost no knowledge of the nature of these elusive food substances or of their mode of action, but we have gained empiric knowledge already of the greatest practical value for the prevention of scurvy and of other grave diseases and for the promotion of health and beauty in the population.
This statement, it will be noted, emphasizes the foundation on which rests our present use of vitamins. From time to time The Journal has commented on our lack of actual knowledge of these mysterious substances, emphasizing particularly the generally accepted fact that the taking of a well-balanced diet results in providing the individual with such vitamins as are necessary to his growth and nutrition. Last week appeared a brief report of a meeting of the Chicago Medical Society devoted to this subject, and it was gratifying to have the conservative view which The Journal has emphasized substantiated by many of those who took part in the discussion. Moreover, the British Medical Journal, in its leading editorial for February 11, reiterates that an abundant supply of vitamins exists in all fresh vegetables, and that a considerable quantity occurs in milk and meat, provided the latter substances are obtained from animals fed on fresh foods. “A normal adult,” it says, “living on an ordinary diet containing a reasonable proportion of fresh vegetables is, therefore, certain of obtaining a plentiful supply of vitamins.” Of all the mass of evidence which has accumulated relative to these substances, this fact is the point of greatest importance. It is, however, very unfortunately, the one point which those commercially inclined are unwilling to recognize.—(Editorial from The Journal A. M. A., March 11, 1922.)
Thus the British Medical Journal in its current issue:
In spite of the fact that ordinary fresh foods are the simplest, cheapest and richest sources of vitamins, the public apparently demands to be supplied with vitamins in the form of medicinal products.
The public “demands” vitamins in pill form! Why? For the same reason that the public, lay or medical, demands many things today that it does not need—because the whole trend of modern advertising is toward creating demands, rather than supplying needs. Vitamin concentrates are being “demanded” by the public because shrewd and forward-looking “patent medicine” exploiters are using all the subtle arts of modern advertising to convince the public that it is in serious danger of vitamin starvation, and that the only hope lies in buying these alleged concentrates to make up a hypothetical deficiency. It seems inconceivable that a rational man would pay a tremendously high price for certain food factors which are already present in his ordinary diet. But he will; and advertising is the reason. Advertising campaigns such as these of the vitamins constitute a vicious circle; an artificial demand is created and then the manufacturer excuses his business on the ground that he is merely supplying a demand! As our British contemporary says, “ordinary fresh foods are the simplest, cheapest and richest sources of vitamins.”—(Editorial from The Journal A. M. A., March 18, 1922.)
The following letter from a Detroit physician was received a few days ago.
To the Editor:—I have just received a letter from the Direct Pharmaceutical Co. of St. Louis, Mo., quoting prices on drugs which are not more than one half what the leading manufacturers are quoting on the same drugs. I have received previous literature from this company but have not done business with them. I would be unwilling to prescribe their drugs unless I were satisfied that they are what is claimed for them. I would be glad to receive any information regarding this firm that may be available.
The Journal has also received some letters from physicians regarding the William A. Webster Co. of Memphis, Tenn., relative to a letter the concern was sending physicians in the form of a testimonial (reproduced in miniature on this page) and alleged to be from Dr. F. W. P. Butler of Columbia, S. C. Typical letters on the Webster advertising follow:
To the Editor:—Is there not some way through which the dignity of the medical profession can be protected from the circulation of such idiotic drivel as the enclosures display?
To the Editor:—I am sending you an example of the sort of “evidence” which some so-called ethical pharmaceutical houses expect physicians to take for scientific proof. It is pathetic that there are some in our profession who “fall for” such rot. I trust you will continue your campaign for honest and intelligent medicine.
The “evidence” to which one of the correspondents refers and which another characterizes as “idiotic drivel” is reproduced on the following page in miniature. It is a testimonial for William A. Webster Company’s “Ferritonic-Woods.”

Our readers may wonder why we are discussing in one article the William A. Webster Company of Memphis, Tenn., and the Direct Pharmaceutical Co. of St. Louis. The reason is that the Direct Pharmaceutical Co. of St. Louis is apparently merely a sales agency for the William A. Webster Company of Memphis. It appears that orders sent in to the Direct Pharmaceutical Co. go to Memphis to be filled.
The following information regarding some of the products that have in the past been put out by the William A. Webster Company should be of interest to the profession. In government bulletins issued by the Department of Agriculture in October, 1913, there were reported some cases of adulteration and misbranding on the part of the William A. Webster Co., of Memphis, Tenn. A “Pure Concentrated Extract of Lemon” shipped by this concern was found by the federal chemists to be colored with a coal-tar dye “whereby inferiority was concealed,” and while purporting to be a concentrated lemon extract, “in fact, it was not a concentrated lemon extract.” Some “Pure Concentrated Extract of Banana” was found to have mixed with it an imitation banana flavor and an artificial color so as to “injuriously affect its quality and strength” and so that “its inferiority was concealed.” “Pure Concentrated Extract of Pineapple” was found to have had mixed with it “an imitation extract of pineapple artificially colored.” “Pure Concentrated Extract of Strawberry” had been mixed with “an imitation strawberry extract artificially colored.” The same bulletins described the case of the government against a shipment of “Syrup Iron Iodide” made by the Webster concern in which the amount of iron iodid was less than half that claimed on the label. In each of the cases just described, the company pleaded guilty and was fined.
In a similar bulletin issued August, 1914, there were recorded several more cases of adulteration and misbranding charged against the William A. Webster Company. Some “Wine Coca Leaves” was held adulterated in that the amount of alcohol present was wrongly declared on the label; it was held misbranded in that while it contained cocain, the label failed to bear any statement regarding the quantity of proportion of this drug. Tablets of “Acetanilid and Sodium Bromid Compound” were found deficient in strength. “Anti-Vomit Tablets,” “Aspirin Tablets,” “Bismuth and Calomel Tablets,” “Quinin Laxative Tablets,” “Salol Tablets,” “Sodium Salicylate Tablets,” “Neuralgic Tablets,” “Diarrhea Calomel Pills” and “Morphin Sulphate Hypodermic Tablets” were also misbranded in that the amount of certain ingredients found in them failed to tally with the amount declared on the label. In all of these cases also the William A. Webster Company pleaded guilty and was fined.
In a government bulletin issued in June, 1917, the same company was charged with adulterating and misbranding a quantity of Aspirin tablets which, instead of containing 5 grains as labeled, contained only a fraction over 1 grain. In this case, too, the company pleaded guilty and was fined. The table that follows briefly summarizes some of the cases just referred to:
| Amount Claimed | Amount Found | |||
| “Syrup Iron Iodid, U. S. P.” | ||||
Ferrous Iodid | 10% | 4.6% | ||
| “Acetanilid and Sodium Bromid Tablets” | ||||
Acetanilid | 3.50 | gr. | 2.94 | gr. |
| “Anti-Vomit Tablets” | ||||
Bismuth subnitrate | 2.50 | gr. | 1.76 | gr. |
Cerium Oxalate | 2.50 | gr. | 1.78 | gr. |
Cocain Hydrochl. | 0.0833 | gr. | 0.0637 | gr. |
“Aspirin Tablets” | 5.0 | gr. | 3.82 | gr. |
| “Bismuth and Calomel Comp. Tablets” | ||||
Bismuth subnitrate | 0.1 | gr. | 0.22 | gr. |
Calomel | 0.1 | gr. | 0.22 | gr. |
| “Quinin Laxative Tablets” | ||||
Acetanilid | 2.0 | gr. | 1.69 | gr. |
“Salol Tablets” | 2.50 | gr. | 2.05 | gr. |
“Sodium Calicylate Tablets” | 5.0 | gr. | 3.88 | gr. |
| “Neuralgic Pills” | ||||
Morphin sulphate | 0.05 | gr. | 0.015 | gr. |
| “Diarrhea Calomel Pills” | ||||
Morphin sulphate | 0.062 | gr. | 0.05 | gr. |
“Morphin Sulphate Hypodermic Tablets” | 0.25 | gr. | 0.21 | gr. |
“Aspirin Tablets” | 5.00 | gr. | 1.13 | gr. |
—(From The Journal A. M. A., Oct. 18, 1919.)
From time to time yeast has attained a transitory popularity as a therapeutic agent. Its use in this way in practical medicine has been based essentially on empiric considerations. Yeast is rich in nucleic acid, but this has not found any special applications. The fatlike substances obtainable from yeast have been recommended in certain alimentary conditions, without finding any widespread acceptance.
More recently yeast has acquired interest from somewhat different angles. In these days of food shortage and enforced conservation, it has come to be realized that the minute yeast cells are endowed with a remarkable capacity of synthesizing one of the most valued nutrients, namely, protein. This substance can be built up out of the simplest forms of nitrogenous compounds by yeasts, in contrast with the incapacity of the higher organisms to construct protein out of anything less complex than the ready made aminoacids. It is reported that in Europe yeast has actually been grown on a large scale in solutions of sugar, salt and simple nitrogenous compounds for the sake of securing the much desired proteins. The utilization of yeast protein for cattle feeding is a current practice abroad; and the satisfactory digestibility and availability of the same product by the human organism has repeatedly been announced since the beginning of the war. In this country the yeast which is produced as a by-product of the brewing industry is for the most part discarded as waste; in the distilleries it becomes a part of the distillers’ grains that are extensively employed as feeds in the dairy industry.
Still newer is the indication that yeast is comparatively rich in at least one of the as yet unidentified accessory factors in nutrition now popularly spoken of as vitamins. Hopkins of the University of Cambridge, England, first directed attention to this unique property of yeast. It has been verified by Funk and Macallum, and recently Osborne and Mendel have given substantial evidence of the potency of yeast to render a diet not otherwise capable of inducing maintenance effective in nutrition.
Yeast has been used, like extracts of rice polishings, to cure the experimental polyneuritis induced in birds by a diet of polished rice. From the experiments of Osborne and Mendel it appears that less than 2 per cent. of dried brewers’ yeast suffices to induce small experimental animals to grow on artificial food mixtures on which alone they fail to thrive. How the use of yeast as an adjuvant to otherwise inadequate food mixtures exerts its beneficial effect is not yet made clear. Satisfactory growth in these cases is promoted by liberal eating. Anything which renders food more palatable may stimulate one to eat more liberally of it. This can scarcely be the explanation of the potency of the yeast as it is effective even when fed apart from the rest of the food. It may have a favorable effect on the metabolism and thus improve the general condition so that more food is consumed. Small quantities of milk and extracts of many of the common plant foods, such as the cereal grains, have been found to act in the same way. There seems to be little doubt, therefore, that yeast also contains something comparable with the so-called water-soluble vitamins of the diet. A specific need for yeast can scarcely be predicated on this fact, however; for any well selected human dietary containing the usual variety of animal and vegetable foods is not likely to be devoid of the widely distributed water-soluble type of vitamin. We mention this to check premature enthusiasm for a new vitamin.—(Editorial from The Journal A. M. A., Sept. 8, 1917.)
To the Editor:—Is there available information concerning the medicinal use of yeast? How is it taken? I should like to know whether the use of it would cause any digestive disturbance, and whether the flesh gained is normal and permanent.
S. E. L., Bridgeport, Conn.
Answer.—Yeast is one of those remedies that have undergone alternating cycles of use and of disuse; just at present it appears again to be in its ascendency. No doubt, the reason for these cycles has been excessive praise and uncritical use, followed by disappointment and consequent discard.
Hawk and his associates (Hawk, P. B.; Knowles, F. C.; Rehfuss, M. E., and Clarke, J. A.: The Use of Bakers’ Yeast in Diseases of the Skin and of the Gastro-Intestinal Tract, The Journal, Oct. 13, 1917, p. 1243) have recently called renewed attention to its laxative qualities. When from one-half to one cake of yeast was given three times daily before meals, it produced regular bowel movements in a number of patients suffering from constipation. That this result is not due to any vital processes in the yeast is shown by the fact that yeast killed by boiling water was employed with success. It is suggested that such yeast might be preferred for patients troubled with flatulence. Aside from the tendency of living yeast to produce diarrhea, and the possibility that it may aggravate flatulence, no digestive disturbance has been charged against it. Aaron, in his “Diseases of the Digestive Organs,” speaks favorably of its use in atonic constipation.
The much debated question whether yeast may serve as a food can be answered in the affirmative in view of such work as that of the Germans on “Nährhefe”—yeast food (Schottelius, Deutsch. med. Wchnschr., July 8, 1915, p. 817) and Boruttau (ibid., July 29, 1915, p. 924) and of Hawk and his associates. There is no reason to assume that weight gained under its use would be more readily lost than weight gained from any other food. However, in view of its laxative action, the average individual can ingest only from 1 to 2 gm. of nitrogen a day in this form. This obviously greatly limits its value as a food. Owing to its high nuclein content, it is contraindicated in gout.
As a source of water soluble growth promoting as well as antineuritic vitamin, yeast has become thoroughly established as the result of the recent works of numerous investigators. However, as such common foods as milk, rice, wheat, oats and beans also contain such vitamin, there is little likelihood of its proving of therapeutic value on that account. In other words, yeast and other vitamin containing foods have specific growth promoting qualities only when the stunting is due to lack of vitamin. A minute amount of this substance suffices to produce maximal results. More is of no use. Hess (Proc. Soc. Exper. Biol. & Med. 13:145, 1916) found yeast of no value in infantile scurvy.
The most important question in connection with yeast therapy is to what extent it is endowed with “antibiotic” power, that is, to what degree it is capable of inhibiting the growth of other organisms. That this frequently occurs in cultures in vitro is shown by the fact that yeast contamination may practically eradicate the growth of certain other organisms. That, on the other hand, this is not true for all forms of bacterial life is shown by the fact that there is definite symbiosis between yeast and lactic acid bacilli (Northrup: Soc. Tech. Bull. 15, Mich. Agr. Expa. Sta., 1912).
That its “antiseptic power is, on the whole insignificant” has been shown by Palier (Diet. & Hyg. Gaz., March, 1906), who found commercial yeasts commonly contaminated with numerous bacteria, the most frequent being Bacillus coli-communis or one of its congeners. An antagonistic action by yeast is claimed against Staphylococcus pyogenes, and on the strength of this, Buchholtz (Ueber Acne und eine neue erfolgreiche Behandlung derselben, Berl. klin. Wchnschr., Feb. 2, 1914, p. 215) employed it locally in the treatment of acne and obtained a positive but temporary effect. He believes that the effect is improved by the combination of yeast with an equal quantity of boric acid. He employed this as a dusting powder applied freely to the skin once daily, after the application of a thin layer of a boric acid salve (boric acid powder from 40 to 50, glycerin and water, of each 100) to make it stick better. In cases in which the nose was markedly involved, he also used this as a snuff. Yeast poultices have been employed with asserted great benefit in the treatment of wound infection of all kinds (Kempf, E. J.: Ind. M. J., September, 1904, p. 97).
The use in leukorrhea was recommended by Hippocrates Abraham (Mon. Geb. Sym., 1910) and many others report favorable results from yeast in the treatment of gonorrheal vaginitis. In various gastro-intestinal infections, yeast has been lauded by many, among others, Thiercelin and Chevrey. It has been given by mouth, but most especially in high rectal enemas.
Still more from a theoretical standpoint is the reassertion of the curative value of the oral administration of yeast in various cutaneous disorders. Thus Hawk and his collaborators report cure or improvement in all of seventeen cases of acne vulgaris and eight cases of acne rosacea. They also report seventeen cases of furunculosis, in all but one of which there was cure or improvement from yeast treatment. They are unable to decide whether the result is due to the laxative action, the production of leukocytosis, or to other influences.
Yeast is probably best taken incorporated in food. Hawk and his associates found that yeast may well be incorporated in wheat biscuits, and that in this way a yeast-wheat combination of most agreeable flavor was produced: that, in fact, the biscuits with the yeast tasted better than those without it. They found by tests that in bread making as much as 20 per cent. of the flour might be replaced by dry yeast, and that thereby a loaf would be produced that was excellent in every way and possessed of an attractive flavor. The dry yeast was prepared by desiccating compressed yeast at 105 C. in a current of air, and then milling it to produce a flour of the approximate fineness of ordinary wheat flour. They also found that yeast may be added to meat preparations, such as Hamburger steak, to the extent of 2.5 per cent., yielding a preparation of very satisfactory taste.—(Query in The Journal A. M. A., Aug. 23, 1919.)
A laborer went to a Brooklyn physician for treatment and was given three prescriptions. One of the prescriptions, according to the Food and Drug Bulletin of the Department of Health, City of New York, called for “Laxol,” the word being written on a piece of blank paper without directions. The drug clerk misread the prescription and dispensed an “original” bottle of “Lysol” which bore the usual poison label with skull and cross bones. The man drank the entire 3 ounces of Lysol and died half an hour later. The case is now in the hands of the District Attorney, the drug clerk being held under $10,000 bail. “Laxol,” as our readers know, is castor oil sweetened with saccharin and flavored with peppermint. There is no excuse for prescribing the product. The official Aromatic Castor Oil (Ol. Ricin. Arom.) of the National Formulary would answer every purpose served by the proprietary preparation.—(Editorial from The Journal A. M. A., May 29, 1920.)
Modern business has become so complex that it is no longer possible for those engaged in trade to know, offhand, the financial responsibility of their prospective customers. The commercial agency is a natural development; it aims to supply the technical (financial) information which the conservative business man needs but is otherwise unable to get. When John Doe & Co. contemplates entering into business arrangements with Henry Roe & Son to a degree that involves financial obligations, it looks up Roe in the rating book of Dun or Bradstreet and probably calls for a special commercial report on the concern. These facts are so elemental and obvious as to be trite. The complexity of modern medicine, especially in the pharmacologic field, has made it a physical impossibility for physicians to know the scientific status of scores of pharmaceutical products put out under proprietary or brand names. It was recognition of this fact that brought about the creation by the American Medical Association of the Council on Pharmacy and Chemistry. This body of experts, serving without remuneration and reporting without fear or favor on the newcomers to the pharmaceutical world, places at the disposal of physicians unbiased information, free alike from prejudice or prepossession. As the commercial agency reports on the commercial probity of individuals and firms, so the Council on Pharmacy and Chemistry reports on what might be called the scientific probity of proprietary and unofficial pharmaceutical products. The commercial agency issues, at no small expense to its customers, rating books; the council on Pharmacy and Chemistry issues, at a nominal price, “New and Nonofficial Remedies.” The commercial agency, for a substantial fee, will furnish reports on business concerns; the Council on Pharmacy and Chemistry will, without any expense to the profession, furnish reports on proprietary products used for the relief or cure of human ailments. The careful business man avails himself of the services of the commercial agency; there are financial interests at stake. The conscientious physician will avail himself of the services of the Council; there are, it may well be, lives at stake.—(Editorial from The Journal A. M. A., April 24, 1920.)
Last week The Journal published a letter received by a physician in Milwaukee from a firm of lawyers representing the Farbwerke-Hoechst Company. In this communication the physician was threatened with suit if he published further unfavorable reports regarding that firm’s preparation. Quoting from the attorney’s letter:
“Mr. Metz directs us to inform you that the publication of this article and the statements therein were seriously damaging to the Farbwerke-Hoechst Company, and directs us to say further to you that he and the corporation will hold you personally responsible for any repetition, oral or written, of the same or of similar statements to the same effect.”
We were under the impression that the time had passed when a proprietary medicine manufacturer would presume to threaten a physician for making an honest report of his results with any therapeutic agent. Such condition did exist once, before the Council on Pharmacy and Chemistry undertook its work. Now comes Mr. Metz to revive this relic of an historic but infamous period in the history of American proprietary medicine manufacturing. Even Mr. Vanderbilt regretted that he ever said “The public be d——.” One of the elementary principles in the practice of medicine is that the individual physician shall let others know his results, whether good or bad, in any line of treatment. It is by such interchange of knowledge and experience that progress in medicine is possible. Yet Mr. Metz would interfere with the diffusion of such knowledge and experience when it applies to proprietary medicines. His legal threat against Dr. Sargent is “terrorism” applied to the medical profession.—(Editorial from The Journal A. M. A., June 8, 1918.)
Few but ignorant darkies have any great faith in the therapeutic efficacy of the left hind foot of a rabbit caught in the churchyard in the dark of the moon. In the light of modern therapeutics one is tempted to believe, however, that had some one person or firm an exclusive proprietary right to this particular brand of rabbits’ feet, there would be many intelligent people—and not all of them laymen—ready to swear by rabbits’ foot therapy. In medical journals (whose advertising pages set forth the virtues of the pedal extremities of Lepus sylvaticus) many solemnly scientific articles would probably appear relating the success that the writers had had with this form of therapy in the treatment of some distressing stubborn conditions that had failed to respond to all previous efforts. Is it ubiquity that has saved the homely cotton-tail from being a therapeutic hero?—(Editorial from The Journal A. M. A., Sept. 29, 1917.)
Many hundreds, possibly thousands, of inquiries are received each year by The Journal from physicians asking for information on, or an opinion of certain proprietary remedies. In many instances the preparations in question are essentially secret in composition, although advertised to the profession under a fair-seeming exterior of apparent frankness. There are on the market today—and used by members of the American Medical Association—dozens, yes scores, of widely advertised proprietaries that are, to all intents and purposes, secret. The physicians who prescribe them do not know and cannot know what they are giving their patients. On this point Section 6 of Chapter II of the Principles of Medical Ethics of the American Medical Association says:
“... it is ... unethical to prescribe or dispense secret medicines or other secret remedial agents, or manufacture or promote their use in any way.”
The inherent and basic reasonableness of the various requirements of the Principles of Medical Ethics needs no exposition or defense. A large number of proprietary remedies which at present degrade medicine would be wiped out of existence or, at any rate, go over to the “patent medicine” class, where they belong, if physicians would live up to Section 6, Chapter II, of the Principles.—(Editorial from The Journal A. M. A., Sept. 27, 1919.)
To the Editor:—I am curious to learn the value of the violet ray in the treatment of disease. The violet ray seems to be much in evidence in Canada at the present time in various towns. It is well advertised, not in the same way as a “patent medicine” would be, but as a genuine form of treatment. The enclosed booklet gives a brief outline of what the agents for the “Sterling Violet Ray Generator” claim it will do. The reason I am troubling you about the matter is that I feel if there is anything in it as is claimed, it should be better known. It also seems that if this treatment is not capable of doing what is claimed for it, it is a rather serious thing for a person who may defer calling a physician.
J. A. G.
Answer.—The “Sterling Violet Ray Generator” is a small high frequency apparatus with some vacuum and possibly other electrodes. There is a violet color in these vacuum electrodes when they are energized. The apparatus is not one for producing violet or ultraviolet rays in the scientific meaning of those words. The apparatus certainly will not do the things claimed for it in the booklet which includes the treatment of practically every ailment known to mankind.—(Correspondence in The Journal A. M. A., April 14, 1917.)
Sodium salicylate is a valuable drug. It is official and cheap; it is the compound generally relied on when salicylate effects are desired. And there is no mystery about it. With the other salicylates, mystery begins. For this reason, such studies as that of Blankenhorn on strontium salicylate are of special value. Blankenhorn shows that strontium salicylate possesses no advantages over sodium salicylate, as regards either therapeutic efficacy or freedom from undesirable by-effects. He calls attention to the fact that “the salicyl content of strontium salicylate is about four-fifths that of sodium salicylate based on the amount of available anion.” The question naturally arise whether this smaller salicylate content may not contribute to the notion that strontium salicylate is less likely to cause salicylism. The impression as to the greater freedom of this salt from undesirable by-effects may have arisen in part also from the fact that the more expensive preparations are more likely to be given in small doses than is the cheaper sodium salicylate. As Blankenhorn suggests, when once such a tradition gains currency, it will be “lugged along” from one textbook to another, with little or no attempt at critical examination.—(Editorial from The Journal A. M. A., Jan. 29, 1916.)
To the Editor:—I am chief surgeon for a large steel industry in Canton, and desire to do all in my power to prevent the threatened recurrence of influenza. What is the status of the various vaccines as a preventive or prophylactic measure? Would you advise their use as a preventive measure, to immunize the workers in the industries?
M.D., Canton, Ohio.
Answer.—The status of vaccine therapy as a prophylactic for influenza may be ascertained from the two articles appearing in The Journal, Aug. 9, 1919: that of E. C. Rosenow and B. F. Sturdivant entitled “Studies in Influenza and Pneumonia: Further Results of Prophylactic Inoculations,” and that of G. W. McCoy, director, Hygienic Laboratory, U. S. Public Health Service, on “Status of Prophylactic Vaccination Against Influenza.” In brief, the conclusion of Rosenow and Sturdivant is: “It appears from all of the facts at hand that by the use of a properly prepared vaccine it is possible to rob influenza of some of its terrors.” On the other hand, McCoy states: “The general impression gained from uncontrolled use of vaccines is that they are of value in the prevention of influenza; but, in every case in which vaccines have been tried under perfectly controlled conditions, they have failed to influence in a definite manner either the morbidity or the mortality.” To make a conservative statement: The use of vaccine as a prophylactic in influenza is an experiment.—(Query in The Journal A. M. A., Sept. 27, 1919.)
To the Editor:—Has there been work done of sufficient extent to be of value in justifying use of mixed “shotgun” vaccines to abort or immunize “common colds,” that is, rhinitis, pharyngitis, acute bronchitis, coryza, etc.?
Charles E. Bennett, M.D., Aneta, N. D.
Answer.—We know of no investigation which demonstrates that the use of the commercial mixed vaccines are of value in the prevention or treatment of “common colds” or of similar affections. The Council on Pharmacy and Chemistry accepts for New and Nonofficial Remedies mixed vaccines only on condition that their usefulness has been established by acceptable clinical evidence; so far it has not admitted any of the “influenza” or “catarrhal” mixed vaccines.—(Correspondence in The Journal A. M. A., Nov. 10, 1917.)
To the Editor:—Please advise me of the latest and best vaccine for common colds.
L. J. Smith, M.D., Wilson, N. C.
Health Officer, City and County Health Department.
Answer.—There is no scientific evidence that common colds can be prevented by the use of vaccines, despite the glowing recommendations of vaccine makers and the patter of the detail man. Colds characterized by catarrhal inflammation of the mucous membranes of the nose and throat are caused by various organisms, including a number of the commoner cocci and the bacillus of Pfeiffer. They are contagious, and spread rapidly from one person to another by the transfer of the bacteria concerned, so that small epidemics of colds are continually occurring in homes and communities. The organism concerned in one small epidemic may be different from that in another, and it is impossible to anticipate what organism is about to invade the household or community. The inoculation of mixed vaccines in the hope of providing against a number of possible invaders fails to produce immunity sufficient to prevent the infection of mucous membranes. Where completely controlled experiments have been made with large numbers of persons, colds have occurred among the inoculated in as large proportion as among the uninoculated. During the war, some evidence was obtained which indicated that preventive inoculation of troops with a vaccine containing large numbers of pneumococci reduced the incidence and mortality of pneumonia. In the case of superficial infection of the nasal and pharyngeal mucous membranes with diverse etiology, less can be expected, and practical results indicate that this skepticism based on theoretical considerations is well founded.—(Query in The Journal A. M. A., Nov. 13, 1920.)
[See Closing Paragraph to Preface, Page IV.]
| A | B | C | D | E | F | G | H | I | J | K | L | M |
| N | O | P | Q | R | S | T | U | V | W | X | Y | Z |
| APAGE |
| Abbott Laboratories— |
B. Coli-Combined-Bacterin185 |
Barbital371 |
Calcidin Tablets465 |
M. Catarrhalis-Combined-Bacterin184 |
Neuro-Lecithin53 |
Pertussis-Combined-Bacterin185 |
Procain-Abbott356 |
Triple Arsenates with Nuclein256 |
Abrams, Albert472 |
Abrams, Albert, defense of, by Upton Sinclair477 |
Acetanilid and Sodium Bromid Tablets566 |
Acetanilid Compound, Mulford206 |
| Acet-Phenetidin Compound, P.-M. Co., Tablets (Pitman-Moore Co.), Reports Council Pharm. & Chem., 1918, p. 73. |
| Acetylsalicylic Acid: See also Aspirin. |
Acetylsalicylic Acid, American-made, examination of344 |
Acetylsalicylic Acid (Aspirin) Monsanto347 |
Acetylsalicylic Acid, M. C. W347 |
Acetylsalicylic Acid, Millikin347 |
Acetylsalicylic Acid, not Aspirin480 |
Acetylsalicylic Acid, P. W. R347 |
Acetylsalicylic Acid, Squibb347 |
Acetylsalicylic Acid—“What’s in a Name.”482 |
Adreno-Spermin Comp., Caps219 |
Advertising by means of “original” articles in medical journals560 |
Advertising principles—lay and medical483 |
| Agar-lac (E. Fougera & Co., Inc.), The Journal, Nov. 14, 1914, p. 1777; Reports Council Pharm. & Chem., 1914, p. 124; Propaganda, ed. 9, p. 10. |
| Agmel (Maguey Products Co.), The Journal, Oct. 12, 1912, p. 1392. |
Akoz, lead in328 |
Akoz Medicinal Mineral Water328 |
Akoz Ointment328 |
Akoz Powder328 |
Akoz Suppositories328 |
Albolene, Liquid106 |
| Albolene, Liquid (McKesson & Robbins), The Journal, July 26, 1913, p. 296; Reports Council Pharm. & Chem., 1916, p. 65. |
| Alborum (The Whitehouse Chemical Co., Inc.), The Journal, Dec. 12, 1914, p. 2148; Reports Council Pharm. & Chem., 1914, p. 129. |
Alcohol, Hygiene and Legislation495 |
| Alcresta Dental Lotion-Lilly (Eli Lilly & Co.), The Journal, Oct. 29, 1921, p. 1441. |
Alcresta Ipecac153 |
| Alcresta Ipecac (Eli Lilly & Co.), The Journal, Oct. 20, 1917, p. 1373; Reports Council Pharm. & Chem., 1917, p. 62. |
Alcresta Lotion465 |
| Aletrin, The Journal, Nov. 13, 1909, p. 1655; Reports Council Pharm. & Chem., 1909, p. 135. |
| Aletris Compound, Elixir (Parke, Davis & Co.; Ray Chemical Co.); Reports Council Pharm. & Chem., 1912, p. 46. |
| Aletris Cordial (Rio Chemical Co.), The Journal, Oct. 17, 1914, p. 1411; Feb. 13, 1915, p. 606; Reports Council Pharm. & Chem., 1914, p. 99; Propaganda, ed. 9, p. 43. |
| Alexander, H. M., Company— Dixon’s Tubercle Bacilli Extract and Dixon’s Suspension |
of Dead Tubercle Bacilli158 |
Alfatone28 |
| Alfatone (Norwich Pharmacal Company), The Journal, Aug. 7, 1915, p. 548; Reports Council Pharm. & Chem., 1915, p. 62. |
| Alkaline Digestine (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 44. |
Alkalithia242 |
| Alkalithia (Keasbey and Mattison Company), Reports Council Pharm. & Chem., 1919, p. 65. |
| Alkalol (Alkalol Co.), The Journal, Nov. 6, 1915, p. 1665; Reports Chem. Lab., 1915, p. 110. |
| Alleotone (B. F. Copeland), The Journal, Feb. 1, 1908, p. 379; Propaganda, ed. 9, p. 264. |
Allied Medical Associations of America486 |
Alphozone99 |
| Alphozone (Frederick Stearns & Co.), Reports Council Pharm. & Chem., 1916, p. 50. |
Ambrine, analysis of332 |
“Ambrine” and Paraffin Films330 |
| Ambrine, The Journal, April 7, 1917, p. 1057; May 19, 1917, p. 1497; Reports Chem. Lab., 1917, p. 20. |
American Animal Therapy Co528 |
American Elixir of Bitter Wine, Triner’s139 |
American Medical Association Chemical Laboratory, foreword322 |
American Medical Association Chemical Laboratory, work of322 |
| American Medical Association Council on Pharmacy and Chemistry, delays in |
passing on products20 |
American Medical Association Council on Pharmacy and Chemistry, foreword1 |
| American Medical Association Council on Pharmacy and Chemistry offers services similar to |
Dun and Bradstreet570 |
American Medical Association Council on Pharmacy and Chemistry, official rules of3 |
American Medical Association Council on Pharmacy and Chemistry, present and future12 |
| American Medical Association Council on Pharmacy and Chemistry endorsed by The |
Journal of the Missouri State Medical Association19 |
American Organotherapy Company527 |
Ammonium Chloride, Troches of, druggists refuse to supply552 |
Ammonium Hypophosphite98 |
Ammonium Hypophosphite, Gardner’s Syrup of100 |
| Ammonium Hypophosphite, Reports Council Pharm. & Chem., 1916, p. 51. |
Ammonol393 |
| Ammonol (Ammonol Chemical Co.), The Journal, June 3, 1905, p. 1791; Feb. 2, 1918, p. 337; Reports Council Pharm. & Chem., 1905, 1908, p. 7; Propaganda, ed. 9, p. 9. |
| Amolin Deodorant Powder (Amolin Chemical Co.), The Journal, Feb. 22, 1908, p. 626; Reports Chem. Lab., 1909, p. 63. |
| Anadol (Wheeler Chemical Works), The Journal, May 21, 1910, p. 1704; Propaganda, ed. 9, p. 245. |
| Analutos and Analutos Tablets (Royal Pharmaceutical Works, Meppel, Holland), The Journal, Feb. 20, 1915, p. 684; Reports Council Pharm. & Chem., 1915, p. 135; Reports Chem. Lab., 1915, p. 131. |
Anasarcin383 |
Anasarcin advertising407 |
| Anasarcin (Anasarcin Chemical Co.), The Journal, May 4, 1907, p. 1535; Dec. 8, 1917, p. 1992; Reports Council Pharm. & Chem., 1905–8, p. 54; Propaganda, ed. 9, p. 11. |
| Anderson System for Treatment of Alcoholism (The Anderson Laboratories), Reports Council Pharm. & Chem., 1916, p. 51. |
Anedemin383 |
| Anedemin (Anedemin Chemical Co.), The Journal, May 4, 1907, p. 1535; Dec. 8, 1917, p. 1992; Reports Council Pharm. & Chem., 1905–8, p. 54; Propaganda, ed. 9, p. 11. |
Anesthesin276 |
| Angier’s Emulsion (Angier Chemical Co.), The Journal, Sept. 12, 1914, p. 962; Reports Council Pharm. & Chem., 1914, p. 48; Reports Chem. Lab., 1914, p. 55; Propaganda, ed. 9, p. 169. |
| Anglo-French Drug Co., Ltd.— |
Collosol Cocaine221 |
Collosol preparations223 |
Cuprase222 |
Sukro-Serum273 |
Supsalvs274 |
| Anistamina (M. Olivetti), Reports Council Pharm. & Chem., 1915, p. 162. |
Antero-Pituitary Comp., Caps219 |
| Antidiabeticum, Bauer (Sanin-Gesellschaft), The Journal, July 30, 1910, p. 418; Propaganda, ed. 9, p. 267. |
| Antidysenteric Serum (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Antikamnia (Antikamnia Chemical Co.), The Journal, June 3, 1905, p. 1791; Feb. 8, 1908, p. 467; Reports Council Pharm. & Chem., 1905–8, p. 7; Reports Chem. Lab., to 1909, p. 60; Propaganda, ed. 9, pp. 9, 268, 307. |
| Antikamnia and Quinin (Antikamnia Chemical Co.), The Journal, July 1, 1905, p. 55. |
| Anti-Malta Fever Serum (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1917, p. 136. |
Antimeristem-Schmidt408 |
| Antimeristem-Schmidt (Laboratorium W. Schmidt), The Journal, March 8, 1913, p. 766; Dec. 6, 1919, p. 1787. |
Antiphlogistine409 |
| Antiphlogistine (Denver Chemical Mfg. Co.), The Journal, June 1, 1907, p. 1875; Feb. 23, 1918, p. 557. |
| Anti-Pneumococcic Oil (Eimer and Amend), The Journal, Jan. 3, 1920, p. 46; Reports Council Pharm. & Chem., 1919, p. 52. |
“Anti-Pneumococcic Oil” and the use of camphor in pneumonia257 |
| Antipneumococcus Serum (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Antiseptic Powder, Maignen (Maignen Institute), The Journal, Nov. 14, 1914, p. 1778; Reports Council Pharm. & Chem., 1914, p. 57; Propaganda, ed. 9, p. 19. |
Antiseptic Powder, Tyree’s462 |
| Antiseptic Powder, Tyree’s (J. S. Tyree), The Journal, Oct. 20, 1906, p. 1316; Aug. 24, 1912, p. 666; March 30, 1918, p. 949; Reports Council Pharm. & Chem., 1905–8, p. 22; Propaganda, ed. 9, pp. 21, 404; May 17, 1919, p. 1482. |
| Antiseptic Tablets Clover (Sharp & Dohme), The Journal, Aug. 26, 1911, p. 755. |
Antiseptic, Tyree’s401 |
| Antistaphylococcus Serum (Burroughs Wellcome & Co.), Reports Council Pharm. & Chem., 1917, p. 137. |
| Antistreptococcus Serum, Aronson’s (Schering & Glatz, Inc.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Antistreptococcus Serum “Hoechst” (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Anti-Syphilitic Compound Sweeny (National Laboratories of Pittsburgh), The Journal, April 3, 1920, p. 965; Reports Council Pharm. & Chem., 1920, p. 12. |
| Antithermoline (G. W. Carnrick Co.), The Journal, Nov. 1, 1913, p. 1649. |
Antithyroid Preparations (Antithyroidin-Moebius and Thyreoidectin)202 |
| Antithyroid Preparations, Reports Council Pharm. & Chem., 1918, p. 50. |
| Antithyroidin-Moebius (Merck & Co.), Reports Council Pharm. & Chem., 1918, p. 50. |
Antithyroidin-Moebius and Thyreoidectin, Antithyroid Preparations202 |
Anti-Tuberculous Lymph Compound (Sweeny)266 |
| Anti-Tuberculous Lymph Compound, Sweeny (National Laboratories of Pittsburgh), The Journal, April 3, 1920, p. 965; Reports Council Pharm. & Chem., 1920, p. 12. |
Anti-Vomit Tablets566 |
Anusol Suppositories182 |
| Anusol Suppositories (Schering & Glatz, Inc.), The Journal, Oct. 2, 1909, p. 1112; Oct. 11, 1913, p. 1392; Jan. 31, 1914, p. 395; March 9, 1918, p. 719; Reports Chem. Lab., 1909, p. 32; Propaganda, ed. 9, pp. 227, 280, 281. |
| Apergols (H. K. Wampole Co., Inc.), The Journal, Dec. 12, 1914, p. 2149; Reports Council Pharm. & Chem., 1914, p. 64; Propaganda, ed. 9, p. 26. |
Aphlegmatol273 |
| Aphlegmatol (G. Giambalvo & Co.), The Journal, Aug. 21, 1920, p. 556; Reports Council Pharm. & Chem., 1920, p. 23. |
Apothesine260 |
Aquazone (Oxygen Water)290 |
| Aquazone (Oxygen Water) (Aquazone Laboratories, Inc.), Reports Council Pharm. & Chem., 1920, p. 50. |
| Arbor Vitae, Reports Council Pharm. & Chem., 1912, p. 38. |
Arhovin243 |
| Arhovin (Schering & Glatz, Inc.), Reports Council Pharm. & Chem., 1919, p. 66. |
Armervenol249 |
| Armervenol (Hille Laboratories), Reports Council Pharm. & Chem., 1919, p. 82. |
| Armour and Company— |
Duodenin76 |
Lecithol53 |
Pineal Gland Preparations213 |
Red Bone-Marrow Preparations213 |
Thymus Gland Preparations214 |
“Arrhenal”492 |
| Arrhenal (E. Fougera & Co.) The Journal, Feb. 26, 1921, p. 595. |
Arsenates, Triple, with Nuclein256 |
Arsenic and Mercury, Solution of231 |
| Arsenic and Mercury, Solution of (New York Intravenous Laboratory), The Journal, Aug. 2, 1919, p. 353; Reports Council Pharm. & Chem., 1919, p. 26. |
Arsenic, Iron and Phosphorus Compound, Hypodermic Solution No. 13275 |
“Arsenicals”491 |
Arsenicals, pharmacology of492 |
| Arseno-Meth. Hyd.: See Arsenic and Mercury, Solution of. |
“Arsenoven, S. S.”231 |
| Arsenoven, S. S. (S. S. Products Co.), The Journal, Aug. 2, 1919, p. 353; Reports Council Pharm. & Chem., 1919, p. 26. |
| Aseptikons (Chinosol Co.), The Journal, Nov. 14, 1914, p. 1778; Reports Council Pharm. & Chem., 1914, p. 124; Propaganda, ed. 9, p. 26. |
Aseptinol401 |
| Aseptinol (Aseptinol Mfg. Co.), The Journal, March 30, 1918, p. 949. |
| Aspirin: See also Acetylsalicylic Acid. |
| Aspirin (The Bayer Co., Inc.), The Journal, Jan. 20, 1917; April 13, 1918, p. 1097; June 12, 1920, p. 1664; May 14, 1921, p. 1356; June 11, 1921, p. 1697; Reports Council Pharm. & Chem., 1916, p. 43. |
Aspirin—a common name484 |
Aspirin, Acetylsalicylic Acid not Aspirin480 |
Aspirin advertisements483 |
“Aspirin Bayer” and the Sterling Products Company485 |
Aspirin, Lehn and Fink347 |
Aspirin Tablets566 |
| Aspiro-Lithine (McKesson & Robbins), The Journal, May 28, 1910, p. 1803; Propaganda, ed. 9, p. 281. |
Atlanta Journal-Record of Medicine and Gray’s Glycerine Tonic429 |
Atophan (Phenylcinchoninic Acid, U.S.P.), Cinchophen373 |
“Auto-Hemic Serum”409 |
| Auto-Hemic Serum (L. D. Rogers), The Journal, Feb. 14, 1920, p. 477. |
| Autolysin (Autolysin Laboratory), The Journal, July 24, 1915, p. 336; Nov. 6, 1915, pp. 1647, 1662. |
“Autolysin” advertising413 |
| B |
B. Coli-Combined-Bacterin185 |
| B. Coli-Combined-Bacterin (The Abbott Laboratories), The Journal, June 22, 1918, p. 1967; Reports Council Pharm. & Chem., 1918, p. 11. |
B. Iodine198 |
| B. Iodine (B. Iodine Chemical Co.), The Journal, Feb. 1, 1919, p. 365; Reports Council Pharm. & Chem., 1918, p. 44. |
B. Iodine Product199 |
B. Oleum Iodine198 |
| B. Oleum Iodine (B. Iodine Chemical Co.), The Journal, Feb. 1, 1919, p. 365; Reports Council Pharm. & Chem., 1918, p. 44. |
| Baby Taeniafuge-Grape (Grape Capsule Co.), Reports Council Pharm. & Chem., 1915, p. 174. |
| Bacilli Emulsion, Bovine (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Bacilli Emulsion, Koch’s (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Bacillicide (Prophytol Products Co.), The Journal, Nov. 14, 1914, p. 1778; Reports Council Pharm. & Chem., 1914, p. 125. |
| Bacillus Acidophilus Cultures, The Journal, Dec. 20, 1919, p. 1895; Reports Council Pharm. & Chem., 1919, p. 51. |
| Bacillus Vaccine, Friedländer. See Friedländer Bacillus Vaccine. |
Bacterial Vaccine, Special, Nos. 2, 3, 4, 5, 11, 15 and 16254 |
| Bakurol (Sharp & Dohme), The Journal, July 10, 1915, p. 175. |
Baldwin, Z. L., and the Allied Medical Associations of America490 |
Balsamea Co.—Syrup Leptinol (formerly Syrup Balsamea)268 |
| Baneberry, Reports Council Pharm. & Chem., 1912, p. 38. |
| Baptisin, The Journal, Nov. 13, 1909, p. 1655; Reports Council Pharm. & Chem., 1909, p. 135. |
Barbital (Abbott)371 |
Barbital (Antoine Chiris)371 |
Barbital (Halter)371 |
Barbital (Rector Chemical Company)371 |
Barbital (Veronal)370 |
Barbital Sodium (Medinal or Veronal-Sodium)371 |
“Basic Cancer Research”414 |
| Baume Analgesique, Bengué (Thos. Leeming & Co.), The Journal, Dec. 14, 1912, p. 2173; Propaganda, ed. 9, p. 267. |
| Bayer Company, Inc.— |
Helmitol295 |
Lycetol214 |
Piperazine214 |
| Bee, Honey, Reports Council Pharm. & Chem., 1912, p. 38. |
| Beef Extract (Swift & Co.), The Journal, Jan. 23, 1909, p. 311; Propaganda, ed. 9, p. 471. |
| Beef Extract, Coin Special (G. H. Hammond & Co.), The Journal, Jan. 23, 1909, p. 311; Propaganda, ed. 9, p. 472. |
| Beef Extract, Concentrated Fluid (Armour & Co.), The Journal, Jan. 23, 1909, p. 311; Propaganda, ed. 9, p. 471. |
| Beef Extract, Fluid (Cibilis Co.), The Journal, Jan. 23, 1909, p. 311; Propaganda, ed. 9, p. 472. |
| Beef Extract, Fluid, “Rex” (Cudahy Packing Co.), The Journal, Jan. 23, 1909, p. 311; Propaganda, ed. 9, p. 471. |
| Beef, Extract of, Premier (Libby, McNeil & Libby), The Journal, Jan. 23, 1909, p. 311; Propaganda, ed. 9, p. 471. |
| Beef Juice, Wyeth’s (John Wyeth & Bro.), The Journal, Nov. 20, 1909, p. 1754; Reports Council Pharm. & Chem., 1909, p. 137; Propaganda, ed. 9, p. 123. |
Beer and cancer cures494 |
| Bell-Ans (Bell & Co.), The Journal, Aug. 24, 1909, p. 569; May 9, 1914, p. 1492; Nov. 24, 1917, p. 1815; Feb. 23, 1918, p. 557; Reports Council Pharm. & Chem., 1909, p. 108; Propaganda, ed. 9, pp. 151, 282. |
| Benetol (Northern Chemical Assn.), The Journal, April 15, 1911, p. 1128; Reports Chem. Lab., 1911, p. 82. |
| Betul-ol (E. Fougera & Co., Inc.), The Journal, Dec. 12, 1914, p. 2148; Reports Council Pharm. & Chem., 1914, p. 62; Reports Chem. Lab., 1914, p. 74; Propaganda, ed. 9, p. 27. |
Beveridge, James Wallace—Secretin-Beveridge170 |
Bile Salt and Holadin Mixtures207 |
Bile Salts, Holadin and Phenolphthalein, Capsules of208 |
Bile Salts, Succinate of Soda and Holadin, Capsules of208 |
Bile Salts, Succinate of Soda and Phenolphthalein, Capsules of208 |
| Bile Salts, Succinate of Soda and Phenolphthalein, Capsules of, Fairchild (Fairchild Bros. & Foster), Reports Council Pharm. & Chem., 1918, p. 59. |
Biniodol121 |
| Biniodol (Charles C. Yarbrough), The Journal, Feb. 24, 1917, p. 650; Reports Council Pharm. & Chem., 1917, p. 10; Reports Chem. Lab., 1916, p. 108. |
Biologic therapeutics and its commercial domination496 |
| Biosol (Vito Chemical Laboratories), The Journal, March 8, 1913, p. 767; Propaganda, ed. 9, p. 284. |
Bischoff, C., & Co.—Hydragogin41 |
| Bischoff, Ernst, Company, Inc.— |
Digitalysatum63 |
Hydropsin61 |
Styptysate318 |
| Bismon (Kalle Color and Chemical Co.), Reports Council Pharm. & Chem., 1921, p. 12. |
Bismuth and Calomel Comp. Tablets566 |
| Bismuth and Iron Citrate Soluble (Wellcome Brand) (Burroughs Wellcome & Co.), Reports Council Pharm. & Chem., 1920, p. 51. |
| Bismuth and Lithium Citrate Soluble (Wellcome Brand) (Burroughs Wellcome & Co.), Reports Council Pharm. & Chem., 1920, p. 51. |
| Bismuth Iodo-Resorcin Sulphonate, The Journal, Feb. 11, 1911, p. 441; Reports Chem. Lab., 1911, p. 14. |
| Bismuth, Opium and Phenol Tablets, The Journal, July 25, 1908, p. 330; Dec. 17, 1910, p. 2169; May 6, 1911, p. 1344; Reports Chem. Lab., to 1909, p. 28; 1910, p. 85; 1911, p. 22. |
| Bismuth, Opium and Phenol Tablets (Hance Bros. & White; Wm. S. Merrell Chemical Co.; Sharp & Dohme; F. Stearns & Co.; Truax, Greene & Co.; H. K. Wampole & Co., Inc.; Wm. R. Warner & Co.), The Journal, July 25, 1908, p. 330; Dec. 17, 1910, p. 2169; May 6, 1911, p. 1344; Reports Chem. Lab., to 1909, p. 28; 1910, p. 85; 1911, p. 22. |
Bismuth, Resorcinol Compound, Capsules157 |
| Bismuth Resorcinol Compound, Capsules (Gross Drug Co., Inc.), Reports Council Pharm. & Chem., 1917, p. 139. |
Bismuth Tribromphenate, standardization of348 |
Bismuth Tribromphenolate, report to Council of National Defense352 |
| Bi-Taride Tablets (Germicidal Products Corporation), The Journal, Sept. 16, 1916, p. 895; Reports Council Pharm. & Chem., 1916, p. 21. |
| Bitter Bark, Reports Council Pharm. & Chem., 1912, p. 39. |
Bitter Wine, Triner’s American Elixir of139 |
| Bladder Wrack, Reports Council Pharm. & Chem., 1912, p. 39. |
| Blandine Laxative, Mulford (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1914, p. 136. |
Blaud, Arsenic and Strychnine Capsules, Frosst’s56 |
| Blaud, Arsenic and Strychnine Capsules, Frosst’s (C. E. Frosst & Co.), Reports Council Pharm. & Chem., 1915, p. 164. |
Blaud Capsules, Frosst’s56 |
| Blaud Capsules, Frosst’s (C. E. Frosst & Co.), Reports Council Pharm. & Chem., 1915, p. 164. |
| Blaud’s Pills, The Journal, April 17, 1915, p. 1344; Reports Chem. Lab., 1915, p. 7. |
| Blood Tonic, Alterative (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 47. |
| Blue Cohosh, The Journal, Sept. 11, 1915, p. 972. |
Borcherdt’s Malt Olive with Hypophosphites84 |
| Borolyptol (Palisade Mfg. Co.), The Journal, Nov. 15, 1913, p. 1812. |
| Borotetramine (Takamine Laboratories), The Journal, Feb. 19, 1921, p. 538; Reports Council Pharm. & Chem., 1921, p. 13. |
| Bovinine (The Bovinine Co.), The Journal, Nov. 20, 1909, p. 1754; Reports Council Pharm. & Chem., 1909, p. 137; 1914, p. 105; Propaganda, ed. 9, p. 123. |
Breitenbach, M. J., Company—Pepto-Mangan387 |
| Bristol-Myers Company— |
Sal Hepatica451 |
Ziratol148 |
| Brobor: See Episan. |
Broeman, C. J.—Final report on Proteogens450 |
| Bromide and Acetanilid Compound-Mulford, Granular Effervescent (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1918, p. 58. |
Bromide, Granular Effervescent and Acetanilid Compound-Mulford206 |
Bromides, Peacock’s400 |
| Bromides, Peacock’s (Peacock Chemical Co.), The Journal, April 3, 1915, p. 1177; March 2, 1918, p. 643; Reports Council Pharm. & Chem., 1915, p. 24; Propaganda, ed. 9, p. 28. |
| Bromides with Cypripedium Compound (Truax, Greene & Co.), Reports Council Pharm. & Chem., 1912, p. 43. |
Bromidia399 |
| Bromidia (Battle & Co.), The Journal, May 16, 1914, p. 1573; March 2, 1918, p. 642; Reports Council Pharm. & Chem., 1914, p. 15; Propaganda, ed. 9, p. 31. |
Bromin-Iodin Compound97 |
| Bromin-Iodin Compound (Bromin-Iodin Chemical Co.), The Journal, June 4, 1910, p. 1884; Dec. 23, 1916, p. 1956; Reports Council Pharm. & Chem., 1916, p. 40; Propaganda, ed. 9, p. 285. |
Brom-I-Phos136 |
| Brom-I-Phos (The National Drug Co.), The Journal, June 30, 1917, p. 2001; Reports Council Pharm. & Chem., 1917, p. 32; Reports Chem. Lab., 1917, p. 43. |
| Bromo-Mangan (Reinschild Chemical Co.), Reports Council Pharm. & Chem., 1915, p. 165. |
| Broom Corn, Reports Council Pharm. & Chem., 1912, p. 39. |
| Bruschettini Curative Vaccine: See Curative Vaccine, Bruschettini. |
Buchu and Hyoscyamus Compound, Tyree’s Elixir of57 |
| Buchu and Hyoscyamus Compound, Elixir of, Tyree’s (J. S. Tyree), Reports Council Pharm. & Chem., 1915, p. 167. |
| Buchu and Pareira Compound, Elixir (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Buchu, Juniper and Acetate Potassium, Elixir (Pitman-Moore Co.), Reports Council Pharm. & Chem., 1915, p. 167. |
Budwell’s Emulsion of Cod-Liver Oil, Nos. 1 and 222 |
| Burdick-Abel Laboratory—Hypodermic Solution No. 13, Iron, Arsenic and Phosphorus |
Compound276 |
Burroughs Wellcome and Company—Soamin253 |
| C |
Cacodylate, Ferric292 |
| Cactin, now Cactoid (The Abbott Laboratories), The Journal, Sept. 21, 1907, p. 1021; March 21, 1908, p. 956; April 4, 1908, p. 1140; March 12, 1910, p. 888; Aug. 6, 1910, p. 455; Reports Council Pharm. & Chem., 1910, p. 41; Propaganda, ed. 9, p. 37. |
| Cactina (Sultan Drug Co.), The Journal, Sept. 21, 1907, p. 1021; March 21, 1908, p. 956; April 4, 1908, p. 1140; March 12, 1910, p. 888; Aug. 6, 1910, p. 455; Jan. 19, 1918, p. 185; Reports Council Pharm. & Chem., 1910, p. 41; Propaganda, ed. 9, p. 37. |
Cactina Pillets391 |
| Cactus Compound Pills (Heart Tonic), The Journal, April 29, 1916, p. 1387. |
| Cactus Grandiflorus, The Journal, Sept. 21, 1907, p. 1021; March 12, 1910, p. 888; Jan. 7, 1911, p. 26; Reports Council Pharm. & Chem., 1910, p. 40; Propaganda, ed. 9, p. 36. |
| Calcidin Abbott (The Abbott Laboratories), The Journal, Sept. 7, 1907, p. 865; Reports Chem. Lab., to 1909, p. 7. |
Calcidin Tablets (Abbott)465 |
| Calcidin Tablets (The Abbott Laboratories), The Journal, Sept. 25, 1920, p. 892. |
Calcium Comp., Elixir Iodo-Bromide of, “Without Mercury” and “With Mercury”52 |
Calcium Glycerophosphate and Sodium Glycerophosphate99 |
| Calcium Glycerophosphate, Reports Council Pharm. & Chem., 1916, p. 52. |
| Calcylates Compound, Elixir, The Journal, April 22, 1916, p. 1307. |
Calcylates Compound, Pulvoids226 |
| Calcylates Compounds, Pulvoids (The Drug Products Co.), The Journal, June 14, 1919, p. 1784; Reports Council Pharm. & Chem., 1919, p. 19. |
Calcylates, Pulvoids85 |
| Calmine (The Abbott Laboratories), The Journal, Jan. 14, 1911, p. 137; Propaganda, ed. 9, p. 286. |
Calomel and Bismuth Comp. Tablets566 |
Campetrodin and Campetrodin No. 2193 |
| Campetrodin and Campetrodin No. 2 (A. H. Robins Company), The Journal, Sept. 21, 1918, p. 993; Reports Council Pharm. & Chem., 1918, p. 27. |
| Camphenol (Johnson & Johnson), The Journal, Nov. 5, 1910, p. 1662; Reports Chem. Lab., 1910, p. 112; Propaganda, ed. 9, p. 287. |
Campho-Phenique418 |
| Campho-Phenique (Campho-Phenique Co.), The Journal, April 20, 1907, p. 1365; Feb. 9, 1918, p. 408; Reports Council Pharm. & Chem., 1905–8, p. 51; Propaganda, ed. 9, p. 40. |
| Campho-Phenique Powder (Campho-Phenique Co.), The Journal, April 20, 1907, p. 1365; Reports Council Pharm. & Chem., 1905–8, p. 51; Propaganda, ed. 9, p. 40. |
Camphor in pneumonia257 |
Cancer cures and beer494 |
Cancer, Edward Percy Robinson’s “Cure” for (Tekarkin)458 |
| Cancer Remedy, Koch’s (Wm. F. Koch), The Journal, Feb. 12, 1921, p. 466; Feb. 19, 1921, p. 537. |
Cancer Remedy, William F. Koch’s437 |
Cancer Research, Basic, and Cosmopolitan Cancer Research Society414 |
Cancer Serum, Glover’s425 |
| Cancer Serum, Glover’s (T. J. Glover), The Journal, Jan. 1, 1921, p. 52; Feb. 5, 1921, p. 396. |
| Cannabis Compound, Syrup (Pitman-Moore Co.), Reports Council Pharm. & Chem., 1915, p. 168. |
Cano’s Methyl-Phenol Serum251 |
Cano’s Normal Phenol Serum251 |
| Capell’s Uroluetic Test: See Uroluetic Test, Capell’s. |
| Caps. Adreno-Spermin Comp. (Henry R. Harrower), The Journal, Jan. 18, 1919, p. 213; Reports Council Pharm. & Chem., 1918, p. 42. |
| Caps. Antero-Pituitary Comp. (Henry R. Harrower), The Journal, Jan. 18, 1919, p. 213; Reports Council Pharm. & Chem., 1918, p. 42. |
| Caps. Folio Digitalis (Upsher Smith), Reports Council Pharm. & Chem., 1920, p. 52. |
| Caps. Hepato-Splenic Comp. (Henry R. Harrower), The Journal, Jan. 18, 1919, p. 213; Reports Council Pharm. & Chem., 1918, p. 42. |
| Caps. Pancreas Comp. (Henry R. Harrower), The Journal, Jan. 18, 1919, p. 213; Reports Council Pharm. & Chem., 1918, p. 42. |
| Caps. Placento-Mammary Comp. (Henry R. Harrower), The Journal, Jan. 18, 1919, p. 213; Reports Council Pharm. & Chem., 1918, p. 42. |
| Caps. Thyroid Comp. (Henry R. Harrower), The Journal, Jan. 18, 1919, p. 213; Reports Council Pharm. & Chem., 1918, p. 42. |
| Caps. Thyro-Ovarian Comp. (Henry R. Harrower), The Journal, Jan. 18, 1919, p. 213; Reports Council Pharm. & Chem., 1918, p. 42. |
Capsules Bismuth Resorcinol Compound157 |
| Captol (Mühlens & Kropff), The Journal, Sept. 10, 1910, p. 959; Reports Chem. Lab., 1910, p. 70. |
Carminzym194 |
| Carminzym (Fairchild Bros. & Foster), The Journal, Sept. 28, 1918, p. 1081; Reports Council Pharm. & Chem., 1918, p. 28. |
| Carnick, G. W., Co.— |
Elixir Secretogen75 |
Hormotone and Hormotone without Post-Pituitary234 |
Secretin-Beveridge170 |
Secretogen, 75110 |
| Carnine (E. Fougera & Co., Inc.), The Journal, Nov. 20, 1909, p. 1754; Reports Council Pharm. & Chem., 1909, p. 137; Propaganda, ed. 9, p. 123. |
| Caroid (Mead Johnson & Co.), Reports Council Pharm. & Chem., 1914, p. 109. |
| Caroid, Essence of (Mead Johnson & Co.), Reports Council Pharm. & Chem., 1914, p. 109. |
| Carpanutrine (John Wyeth & Bro.), The Journal, May 11, 1907, p. 1612; Reports Council Pharm. & Chem., 1905–8, opp. p. 64; Propaganda, ed. 9, p. 133. |
| Casca-Aletris (Pullen-Richardson Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
Cascara, Compound-Robins, Pil.117 |
Cascara Sagrada, with Maltzyme211 |
| Cascarans, Bell (Bell & Co.), The Journal, Aug. 14, 1909, p. 569; Reports Council Pharm. & Chem., 1909, p. 111; Propaganda, ed. 9, p. 154. |
Casta-Flora118 |
| Castaflora (The Wm. S. Merrell Chemical Co.), The Journal, Jan. 27, 1917, p. 303; Reports Council Pharm. & Chem., 1916, p. 45. |
| Castrox (Purdue Newberry Co.), The Journal, Dec. 23, 1916, p. 1956; Reports Council Pharm. & Chem., 1916, p. 41. |
Catarrhal Vaccine Combined-Lilly187 |
| Catarrhal Vaccine Combined-Lilly (Eli Lilly & Co.), The Journal, June 22, 1918, p. 1967; Reports Council Pharm. & Chem., 1918, p. 11. |
| Caviblen (A. Grimme), Reports Council Pharm. & Chem., 1915, p. 176. |
| Cedron Seed, Reports Council Pharm. & Chem., 1912, p. 40. |
| Celerina (Rio Chemical Co.), The Journal Oct. 17, 1914, p. 1411; Feb. 13, 1915, p. 606; Reports Council Pharm. & Chem., 1912, p. 40; 1914, p. 99; Propaganda, ed. 9, p. 43. |
| Celery, Reports Council Pharm. & Chem., 1912, p. 40. |
| Celery and Guarana Compound, Elixir (Ray Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Celery and Guarana, Elixir (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Celery Compound, Elixir (Nelson, Baker & Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Celery Compound, Elixir (Ray Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Celery Compound, Elixir (Smith, Kline & French Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Celery Compound, Elixir (F. Stearns & Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Celery, Elixir Guarana and (Hance Bros. & White), Reports Council Pharm. & Chem., 1912, p. 40. |
| Cellasin (Mead Johnson & Co.), The Journal, Sept. 12, 1908, p. 931; Oct. 30, 1909, p. 1406; Reports Council Pharm. & Chem., 1905–8, p. 198; 1909, p. 118. |
Cephaelin203 |
| Cephaelin, Reports Council Pharm. & Chem., 1918, p. 52. |
| Cerelene (Holliday Laboratories), The Journal, Feb. 15, 1919, p. 513; Reports Council Pharm. & Chem., 1918, p. 48. |
| Chapoteaut’s Wine: See Wine, Chapoteaut’s. |
| Chemical Laboratory of the American Medical Association: See American Medical Association. |
Chemotherapy and tumors499 |
Chichester, Pil. Mixed Treatment310 |
| Chiodrastis (H. K. Wampole & Co., Inc.), Reports Council Pharm. & Chem., 1912, p. 42. |
| Chionacea (Nelson, Baker & Co.), Reports Council Pharm. & Chem., 1912, p. 42; The Journal, June 14, 1919, p. 1787. |
| Chionanthus Compound, Elixir (Ray Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 42. |
| Chionanthus (Special), Elixir (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 42. |
| Chionia (Peacock Chemical Co.), The Journal, April 3, 1915, p. 1177; Reports Council Pharm. & Chem., 1912, p. 42; 1915, p. 24; Propaganda, ed. 9, p. 28. |
| Chlorax (Chlorine Products Company, Inc.), Reports Council Pharm. & Chem., 1919, p. 70. |
Chlorides, Platt’s263 |
Chlorinated Eucalyptol, compared with Chlorlyptus281 |
“Chlorinated Soda” Solutions, deterioration of358 |
Chlorine Products Company, Inc.—Chloron, Chlorax and Number “3”244 |
Chlorlyptus277 |
| Chlorlyptus (Weeks Chemical Co.), The Journal, Nov. 27, 1920, p. 1512; Reports Chem. Lab., 1920, p. 75; Reports Council Pharm. & Chem., 1920, p. 28. |
Chlorlyptus, comparison of, with chlorinated eucalyptol281 |
Chlorlyptus, persistence of acid reaction of, in body283 |
Chlorlyptus, report of Dr. D. Rivas on286 |
Chlorlyptus toxicity experiments285 |
Chloron245 |
| Chloron (Chlorine Products Company, Inc.), Reports Council Pharm. & Chem., 1919. p. 70. |
| Chologen (Leonard A. Seltzer), The Journal, Feb. 1, 1913, p. 383; Propaganda, ed. 9, p. 288. |
| Chologestin (F. H. Strong Co.), The Journal, Dec. 11, 1915, p. 2108. |
| Chromiac Tablets (Maltbie Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 44. |
| Ciba Company— |
Coagulen-Ciba290 |
Digiolin-Ciba298 |
“Cinchophen:” formerly “Atophan”419 |
| Cineraria Maritima, The Journal, Nov. 11, 1911, p. 1630; Reports Council Pharm. & Chem., 1911, p. 48; Propaganda, ed. 9, p. 49. |
Cin-U-Form Lozenges237 |
| Cin-U-Form Lozenges (McKesson and Robbins), The Journal, Oct. 4, 1919, p. 1077; Reports Council Pharm. & Chem., 1919, p. 35. |
| Citarin (The Bayer Company, Inc.), The Journal, Feb. 20, 1915, p. 685; Reports Council Pharm. & Chem., 1914, p. 135. |
| Citrocoli (Cellarius Co.), The Journal, Jan. 21, 1911, p. 210; Reports Council Pharm. & Chem., 1911, p. 7; Propaganda, ed. 9, p. 85. |
Cleveland Medical Journal, “drug reform” as it appears to513 |
| Clover Compound, Syrup Red (Nelson, Baker & Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
Coagulen-Ciba290 |
| Coagulen-Ciba (Society of Chemical Industry, Basle, Switzerland), Reports Council Pharm. & Chem., 1920, p. 53. |
| Cod-Liver, Extract of, Wampole’s Perfected Tasteless Preparation of (H. K. Wampole & Co., Inc.), The Journal, April 5, 1913, p. 1093; April 10, 1915, p. 1262; Reports Council Pharm. & Chem., 1915, p. 140; Propaganda, ed. 9, p. 52. |
Cod-Liver Oil, Budwell’s Emulsion of, Nos. 1 and 222 |
| Cod-Liver Oil, Budwell’s Emulsion of, Nos. 1 and 2 (Budwell Pharmacal Co.), The Journal, Feb. 20, 1915, p. 684; Reports Council Pharm. & Chem., 1915, p. 135. |
| Cod-Liver Oil Compound, Hagee’s Cordial of the Extract of (Katharmon Chemical Co.), The Journal, Oct. 13, 1906, p. 1208; April 10, 1915, p. 1262; Reports Council Pharm. & Chem., 1915, p. 138; Propaganda, ed. 9, pp. 51, 289. |
| Cod-Liver Oil Compound, Waterbury’s Metabolized (Waterbury Chemical Co.), The Journal, Oct. 9, 1909, p. 1201; Reports Council Pharm. & Chem., 1909, p. 115; Propaganda, ed. 9, p. 291. See also Compound, Waterbury’s. |
Cod-Liver Oil, Hagee’s Cordial of429 |
Cod-Liver Oil with Maltzyme211 |
| Cohosh, Blue, Reports Council Pharm. & Chem., 1912, p. 40. |
Colalin203 |
| Colalin (Schieffelin & Co.), Reports Council Pharm. & Chem., 1918, p. 52. |
| Colchi-Methyl Capsules (H. K. Wampole & Co., Inc.), Reports Council Pharm. & Chem., 1915, p. 169. |
| Colchi-Sal (E. Fougera & Co., Inc.), The Journal, March 20, 1915, p. 1016; Reports Council Pharm. & Chem., 1915, p. 136; Propaganda, ed. 9, p. 58. |
“Colds,” Vaccines for573 |
| Colloid Solution Materials for Intravenous Transfusion, Hogan’s (E. R. Squibb & Sons), Reports Council Pharm. & Chem., 1917, p. 147. |
| Colloidine (Boracol Chemical Co.), The Journal, March 11, 1916, p. 831; Reports Council Pharm. & Chem., 1916, p. 7; Reports Chem. Lab., 1915, p. 127. |
| Collosol Cocain (Anglo-French Drug Co., Ltd.), The Journal, April 12, 1919, p. 1094; Reports Council Pharm. & Chem., 1919, p. 8. |
| Collosol Iodine (E. Fougera & Co., Inc.), The Journal, Sept. 8, 1917, p. 841; Reports Council Pharm. & Chem., 1917, p. 49. |
Collosol Preparations223 |
| Collosol Preparations (Collosol Argentum, Collosol Arsenicum, Collosol Cuprum, Collosol Ferrum, Collosol Hydrargyrum, Collosol Iodin, Collosol Manganese, Collosol Quinin, and Collosol Sulphur) (Anglo-French Drug Co., Ltd.), The Journal, June 7, 1919, p. 1694; Reports Council Pharm. & Chem., 1919, p. 14. |
“Collosols:” an uncritical English endorsement420 |
| Collyrium, Wyeth (John Wyeth & Bro.), The Journal, May 17, 1913, p. 1557; Propaganda, ed. 9, p. 292. |
| Compound Elixir of Phosphates and Calisaya: See Tissue Phosphates, Wheeler’s. |
| Compound, Waterbury’s (Waterbury Chemical Co.), The Journal, March 20, 1915, p. 1016; Reports Council Pharm. & Chem., 1915, p. 138; Propaganda, ed. 9, p. 57. |
Concentrated Solution Sodium Hypochlorite-Mulford358 |
| Condurango, Reports Council Pharm. & Chem., 1911, p. 54. |
| Cooperation of the Pharmaceutical Houses, Reports Council Pharm. & Chem., 1920, p. 56. |
| Copper Phenolsulphonate (The Abbott Laboratories), Reports Council Pharm. & Chem., 1916, p. 54. |
Cordial of Cod Liver Oil, Hagee’s429 |
Corpora Lutea (Soluble Extract) Parke, Davis & Co128 |
| Corpora Lutea (Soluble Extract) (Parke, Davis & Co.), The Journal, April 7, 1917, p. 1056; Reports Council Pharm. & Chem., 1917, p. 20. |
| Corydalis Compound, Elixir (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 47. |
“Cosmopolitan Cancer Research Society” and Basic Cancer Research414 |
Cotarnin Salts (Stypticin and Styptol)240 |
| Cotarnin Salts, Reports Council Pharm. & Chem., 1919, p. 39. |
| Coto, Reports Council Pharm. & Chem., 1913, p. 39. |
| Cotoin, Reports Council Pharm. & Chem., 1913, p. 39. |
Cotton Process Ether421 |
| Council on Pharmacy and Chemistry: See American Medical Association. |
Cream of Mustard218 |
| Cream of Mustard (The Cream of Mustard Co., South Norwalk, Conn.), Reports Council Pharm. & Chem., 1918, p. 79. |
| Cream of Sulphur, O’Grady’s Medicated Mineral (John H. O’Grady, Minneapolis), Reports Council Pharm. & Chem., 1918, p. 81. |
| Creo Ferrum (The Gross Drug Co., Inc.), Reports Council Pharm. & Chem., 1921, p. 16. |
Creofos137 |
| Creofos (Delson Chemical Co.), The Journal, July 7, 1917, p. 58; Reports Council Pharm. & Chem., 1917, p. 34. |
Creosote-Delson137 |
| Creosote-Delson (Delson Chemical Co.), The Journal, July 7, 1917, p. 58; Reports Council Pharm. & Chem., 1917, p. 34. |
| Creosotonic (Scott) (Dawson Pharmacal Co.), The Journal, Aug. 24, 1918, p. 680; Reports Council Pharm. & Chem., 1918, p. 25. |
Crittenton Company, Charles N.—Hydroleine58 |
Crookes Laboratories—Collosols420 |
| Croustils, Simple No. 1 (Oaten Bread) (LaPorte & Gauthier), Reports Council Pharm. & Chem., 1921, p. 17. |
| Croustils, No. 2 (Dechloridised and Lactosed) (LaPorte & Gauthier), Reports Council Pharm. & Chem., 1921, p. 17. |
| Croustils, No. 3 (Glutinized) (LaPorte & Gauthier), Reports Council Pharm. & Chem., 1921, p. 17. |
| Cu-Co-Ba Tarrant (The Tarrant Co.), The Journal, Sept. 25, 1920, p. 891. |
Cuprase222 |
| Cuprase (Anglo-French Drug Co., Ltd.), The Journal, April 12, 1919, p. 1095; Reports Council Pharm. & Chem., 1919, p. 10. |
| Curare, The Journal, Jan. 15, 1910, p. 219; Reports Council Pharm. & Chem., 1910, p. 7. |
| Curarin, The Journal, Jan. 15, 1910, p. 219; Reports Council Pharm. & Chem., 1910, p. 7. |
Curative Vaccine, Bruschettini58 |
| Curative Vaccine, Bruschettini (A. Bruschettini), Reports Council Pharm. & Chem., 1915, p. 176. |
| Cypridol Capsules (E. Fougera & Co., Inc.), The Journal, Dec. 19, 1914, p. 2247; Reports Council Pharm. & Chem., 1914, p. 77; Propaganda, ed. 9, p. 59. |
| Cystogen (Cystogen Chemical Co.), The Journal, Dec. 12, 1914, p. 2148; Reports Council Pharm. & Chem., 1914, p. 66; Propaganda, ed. 9, p. 60. |
| Cystogen Aperient (Cystogen Chemical Co.), The Journal, Dec. 12, 1914, p. 2148; Reports Council Pharm. & Chem., 1914, p. 66; Propaganda, ed. 9, p. 60. |
| Cystogen Lithia (Cystogen Chemical Co.), The Journal, Dec. 12, 1914, p. 2148; Reports Pharm. & Chem., 1914, p. 66; Propaganda, ed. 9, p. 60. |
| Cysto-Sedative (Strong, Cobb & Co.), The Journal, Dec. 12, 1914, p. 2148; Reports Council Pharm. & Chem., 1914, p. 130; Propaganda, ed. 9, p. 61. |
| D |
Daggett and Miller Company, Inc.—Hex-Iodin236 |
| Damiana, Allen’s Compound Extract of (Allen-Pfeiffer Chemical Co.), The Journal, July 19, 1913, p. 211. |
| Darpin (Rio Chemical Co.), The Journal, Feb. 13, 1915, p. 606; Reports Council Pharm. & Chem., 1914, p. 99; Propaganda, ed. 9, p. 43. |
Dawson Pharmacal Company—Iodonized Emulsion (Scott) and Creosotonic (Scott)192 |
Delson Chemical Co., Inc.—Creosote-Delson and Creofos137 |
Denver Chemical Mfg. Co.—Antiphlogistine409 |
De Sanctis’ Rheumatic and Gout Pills363 |
Desiccated Pineal Gland—Armour213 |
Desiccated Thymus—Armour213 |
| Diabetic Biscuit (Jireh Diabetic Food Co.), The Journal, March 22, 1913, p. 922. |
| Diabetic Flour, Jireh (Jireh Diabetic Food Co.), The Journal, March 22, 1913, p. 922. |
| Diabetic Food, Jireh (Jireh Diabetic Food Co.), The Journal, Dec. 14, 1912, p. 2174. |
Dial “Ciba”259 |
| Dianol I, Dianol II, and Dianol III (Kalle & Co.), Reports Council Pharm. & Chem., 1913, p. 34; Reports Chem. Lab., 1913, p. 75. |
Diarrhea Calomel Pills566 |
| Diastos, Liquor (H. K. Mulford Co.), The Journal, Feb. 9, 1907, p. 533. |
| Diatussin (E. Bischoff & Co.), The Journal, May 17, 1913, p. 1557; Propaganda, ed. 9, p. 293. |
| Di-Crotalin (Swan-Myers Co.), The Journal, Aug. 17, 1918, p. 592. |
Di-Crotalin treatment of epilepsy465 |
Diethylbarbituric Acid (Merck)371 |
| Digalen (Hoffmann-LaRoche Chemical Works), The Journal, Sept. 5, 1914, p. 881; Reports Council Pharm. & Chem., 1914, p. 33; Propaganda, ed. 9, p. 68. |
| Digestive Tablets, Aromatic (Fraser Tablet Co.; Wm. S. Merrill Chemical Co.; H. K. Mulford Co.; Parke, Davis & Co.; Sharp & Dohme), The Journal, Aug. 20, 1910, p. 710; Reports Chem. Lab., 1910, p. 67; Propaganda, ed. 9, p. 232. |
| Digestive Tonic (Truax, Greene & Co.), Reports Council Pharm. & Chem., 1912, p. 44. |
Digifolin-Ciba298 |
| Digifolin-Ciba (Ciba Company, Inc.), The Journal, April 2, 1921, p. 952; Reports Council Pharm. & Chem., 1921, p. 20. |
Digitalis Tablets, Westerfield’s215 |
| Digitalis Tablets, Westerfield’s (Westerfield Pharmacal Co.), Reports Council Pharm. & Chem., 1918, p. 75. |
| Digitalone (Parke, Davis & Co.), The Journal, June 12, 1909, p. 1938; Dec. 7, 1912, p. 2074; Jan. 11, 1913, p. 143. |
Digitalysatum63 |
| Digitalysatum (E. Bischoff & Co.), The Journal, Feb. 15, 1913, p. 499; Jan. 8, 1916, p. 135; Reports Council Pharm. & Chem., 1915, p. 93. |
Dionol422 |
| Dionol (Dionol Company), The Journal, Jan. 26, 1918, p. 257; Feb. 7, 1920, p. 410. |
| Dioradin (Dioradin Co.), The Journal, Oct. 26, 1912, p. 1556; Reports Council Pharm. & Chem., 1912, p. 23; 1913, p. 37; Propaganda, ed. 9, p. 73. |
Dios Chemical Company—Neurosine404 |
| Dioscorea Compound, Elixir (H. K. Mulford Co.; Parke, Davis & Co.; Ray Chemical Co.; F. Stearns & Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Dioviburnia (Dios Chemical Co.), The Journal, Aug. 31, 1912, p. 735; Jan. 9, 1915, p. 166; Reports Council Pharm. & Chem., 1912, p. 46; 1914, p. 86; Propaganda, ed. 9, pp. 139, 410. |
| Diphtheria Antitoxin (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Diphtheria Antitoxin, Concentrated (National Vaccine and Antitoxin Institute), Reports Council Pharm. & Chem., 1921, p. 23. |
| Diphtheria Bacillus Vaccine, Reports Council Pharm. & Chem., 1918, p. 54. |
Direct Pharmaceutical Co. and William A. Webster Co.564 |
Direct Sales Company510 |
Discoveries and Discoverers511 |
| Diuretin (Knoll & Co.), The Journal, April 4, 1914, p. 1108; Reports Chem. Lab., 1914, p. 7; Propaganda, ed. 9, p. 251. |
| Diurol (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1912, p. 45. |
Dixon’s Suspension of Dead Tubercle Bacilli158 |
| Dixon’s Suspension of Dead Tubercle Bacilli, Reports Council Pharm. & Chem., 1917, p. 140. |
Dixon’s Tubercle Bacilli Extract158 |
| Dixon’s Tubercle Bacilli Extract; Reports Council Pharm. & Chem., 1917, p. 140. |
| Dogwood, Flowering, Reports Council Pharm. & Chem., 1912, p. 41. |
Dosage, dependability of tablet dosage556 |
Dotterweich, G. J., and C. K., Officers of Direct Sales Company510 |
| Drug Products Co., Inc.— |
Pulvoids Calcylates85 |
Pulvoids Calcylates Compound226 |
Pulvoids Natrium Compound108 |
“Drug Reform” as it appears to the Cleveland Medical Journal513 |
Drug Therapy: the fallibility of textbooks515 |
Drugs, crucial test of therapeutic evidence557 |
Dunn, Eli H., Eli products of424 |
Duodenin76 |
| Duodenin, Armour (Armour & Co.), The Journal, Aug. 14, 1915, p. 639; Jan. 15, 1916, pp. 178, 208; Reports Council Pharm. & Chem., 1915, pp. 96, 99, 151; 1916, p. 72. |
Duotonol Tablets94 |
| Duotonol Tablets (Schering & Glatz, Inc.), The Journal, Sept. 30, 1916, p. 1033; Reports Council Pharm. & Chem., 1916, p. 34. |
Du Pont De Nemours, E. I. and Co., Inc.—Cotton Process Ether421 |
| Dysentery Bacterin-Mulford (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1917, p. 141. |
| Dyspepsia Compound, Elixir (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1912, p. 44. |
| Dyspepsia, Elixir Atonic, Phenolated (Wm. S. Merrell Chemical Co.), The Journal, Feb. 9, 1907, p. 533. |
| E |
| Echinacea, The Journal, Nov. 27, 1909, p. 1836; Reports Council Pharm. & Chem., 1909, p. 144; Propaganda, ed. 9, p. 79. |
| Echitone (Strong, Cobb & Co.), The Journal, Jan. 2, 1915, p. 71; July 17, 1920, p. 193; Reports Council Pharm. & Chem., 1914, p. 80; Propaganda, ed. 9, p. 81. |
| Echthol (Battle & Co.), The Journal, March 13, 1909, p. 904; Jan. 2, 1915, p. 71; Reports Council Pharm. & Chem., 1909, p. 144; 1912, p. 38; 1914, p. 80; Propaganda, p. 81. |
| Echtisia (Wm. S. Merrell Chemical Co.), The Journal, Jan. 2, 1915, p. 71; Reports Council Pharm. & Chem., 1914, p. 80; Propaganda, ed. 9, p. 81. |
| Edema Improved, Tablet (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 41. |
| Edema Tablet (Parke, Davis & Co.; Smith, Kline & French Co.), Reports Council Pharm. & Chem., 1912, p. 41. |
Edgar, Thomas Webster515 |
| Edgar, Thomas Webster, Journal receives a letter denouncing “Medical Clerks” and |
“Biased Sceptres”518 |
Edward Percy Robinson’s “Cure” for Cancer458 |
| Eimer and Amend— |
“Anti-Pneumococcic Oil”257 |
Phosphorcin Compound94 |
Elarson248 |
| Elarson (Winthrop Chemical Company, Inc.), Reports Council Pharm. & Chem., 1919, p. 75. |
| Elder, Reports Council Pharm. & Chem., 1912, p. 41. |
Electrobioscope472 |
| Electrargol (E. Fougera & Co.), Reports Council Pharm. & Chem., 1920, p. 58. |
Electronic Reactions472 |
Eli products of Eli H. Dunn424 |
Eli 606 Capsules, 324425 |
Eli Vaginal Capsules424 |
Eli Vim Restorative424 |
Elixir Glycerophosphates, Nux Vomica and Damiana95 |
| Elixir Glycerophosphates, Nux Vomica and Damiana (Sharp & Dohme), The Journal, Sept. 30, 1916, p. 1034; Reports Council Pharm. & Chem., 1916, p. 35. |
Elixir Iodo-Bromide of Calcium Comp. “Without Mercury” and “With Mercury”52 |
Elixir Lactopeptine43 |
| Elixir Novo-Hexamine (Upsher Smith), Reports Council Pharm. & Chem., 1917, p. 142; Reports Chem. Lab., 1917, p. 70. |
Elixir of Bitter Wine, Triner’s American139 |
| Elixir of Bitter Wine, Triner’s American (Jos. Triner), The Journal, July 14, 1917, p. 139; Reports Council Pharm. & Chem., p. 36. |
Elixir of Buchu and Hyoscyamus Compound, Tyree’s57 |
Elixir Secretogen75 |
| Empyroform (Schering & Glatz, Inc.), Reports Council Pharm. & Chem., 1918, p. 55. |
| Emulsio Minerolein (T. R. D. Barse Co.), Reports Council Pharm. & Chem., 1915, p. 169. |
| Emulsio Phen-Oleum (T. R. D. Barse Co.), Reports Council Pharm. & Chem., 1915, p. 169. |
Endoarsan435 |
Endosal435 |
| Endotin (Morgenstern & Co.), Reports Council Pharm. & Chem., 1914, p. 136. |
| Enesol (E. Fougera & Co., Inc.), The Journal, July 26, 1913, p. 293. |
| Enteronol (Enteronol Co.), The Journal, March 21, 1908, p. 977; Reports Chem. Lab., 1909, p. 64; Propaganda, ed. 9, p. 294. |
Epilepsy, Di-Crotalin treatment465 |
| Episan (Gaynor-Bagstad Co.) The Journal, Sept. 25, 1915, p. 1130; Reports Council Pharm. & Chem., 1915, p. 164. |
Epsom Salt Flavoring a “discovery”179 |
| Ergoapiol (Martin H. Smith Co.), The Journal, Dec. 12, 1914, p. 2149; Reports Council Pharm. & Chem., 1914, p. 64; Propaganda, ed. 9, p. 82. |
| Ergone (Parke, Davis & Co.), The Journal, Oct. 7, 1911, p. 1211; Oct. 14, 1911, p. 1302. |
| Ergotole (Sharp & Dohme), The Journal, Oct. 7, 1911, p. 1211; Oct. 14, 1911, p. 1302. |
Erling, A. E., and the Allied Medical Associations of America488 |
Erling, A. E., and the Physicians’ Drug Syndicate432 |
| Erpiol, Dr. Schrader (Wm. S. Merrell Chemical Co.), The Journal, June 3, 1911, p. 1670; Reports Council Pharm. & Chem., 1911, p. 18; Propaganda, ed. 9, p. 83. |
Eskay’s Neuro Phosphates146 |
Estivin466 |
| Estivin (Schieffelin and Company), The Journal, Nov. 12, 1921, p. 1595. |
| Ether, Anesthesia (Cotton Process) (Du Pont Chemical Works), The Journal, Feb. 21, 1920, p. 544; May 22, 1920, p. 1474. |
Ether, Cotton Process421 |
Ethics, secret remedies and the principles of571 |
Eucalyptol, Chlorinated, comparison of Chlorlyptus with281 |
Eucalyptus, toxicity experiments285 |
| Euca-Mul (The Edward G. Bintz Co.), The Journal, Oct. 29, 1921, p. 1438. |
Eumictine262 |
| Eumictine (Geo. J. Wallau, Inc.), The Journal, Feb. 21, 1920, p. 542; Reports Council Pharm. & Chem., 1920, p. 7. |
| Eunatrol (C. Bischoff & Co.), The Journal, Feb. 22, 1908, p. 627. |
Eupeptic Hypophosphites83 |
| Eupeptic Hypophosphites (Nelson, Baker & Co.), The Journal, Sept. 2, 1916, p. 761; Reports Council Pharm. & Chem., 1916, p. 15. |
| Eusoma (Eusoma Pharmaceutical Co.), Reports Council Pharm. & Chem., 1912, p. 38. |
| Expurgo Anti-Diabetes (Expurgo Mfg. Co.), The Journal, Jan. 24, 1914, p. 312; Reports Chem. Lab., 1914, p. 27; Propaganda, ed. 9, p. 299. |
| Expurgo Lapis (Expurgo Mfg. Co.), The Journal, Nov. 8, 1913, p. 1733. |
Extract of Red Bone Marrow (Armour)213 |
| F |
| Fairchild Bros. & Foster— |
Carminzym194 |
Holadin and Bile Salt Mixtures207 |
Lecithin53 |
| False Unicorn, The Journal, Nov. 27, 1909, p. 1836; Reports Council Pharm. & Chem., 1909, p. 146; Propaganda, ed. 9, p. 84. |
| Farbwerke-Hoechst Co.— |
Threatens physician with suit for publishing unfavorable report of its product, 570571 |
| Febrisol (The Tilden Co.), The Journal, June 29, 1912, p. 2043. |
| Febri-Tone (Arthur Peter & Co.), The Journal, Feb. 1, 1908, p. 379. |
Fellows’ Syrup and other preparations of Hypophosphites395 |
Fellows’ Syrup of Hypophosphites82 |
| Fermenlactyl (Anglo-American Pharmacal Co., Ltd.), The Journal, Jan. 30, 1909, pp. 372, 397. |
| Ferric Arsenite, Soluble, Reports Council Pharm. & Chem., 1912, p. 30. |
Ferric Cacodylate292 |
| Ferric Cacodylate, Reports Council Pharm. & Chem., 1920, p. 62. |
| Ferritonic-Woods (William A. Webster Company), The Journal, Oct. 18, 1919, p. 1231. |
Ferrivine144 |
| Ferrivine (E. Fougera & Co., Inc.), The Journal, Sept. 8, 1917, p. 841; Reports Council Pharm. & Chem., 1917, p. 49. |
| Figwort, Reports Council Pharm. & Chem., 1912, p. 42. |
Filudine41 |
| Filudine (Geo. J. Wallau, Inc.), The Journal, Sept. 18, 1915, p. 1045; Reports Council Pharm. & Chem., 1915, p. 156. |
Firolyptol Plain120 |
| Firolyptol Plain (The Tilden Co.). The Journal, Feb. 17, 1917, p. 564; Reports Council Pharm. & Chem., 1917, p. 8. |
Firolyptol with Kreosote120 |
| Firolyptol with Kreosote (The Tilden Co.), The Journal, Feb. 17, 1917, p. 564; Reports Council Pharm. & Chem., 1917, p. 49. |
Firwein119 |
| Firwein (The Tilden Co.), The Journal, Feb. 17, 1917, p. 564; Reports Council Pharm. & Chem., 1917, p. 7. |
Flint, Eaton and Company—Quassia Compound Tablets306 |
Flower, A. H., and the Allied Medical Associations of America489 |
Foral204 |
| Foral (Foral Products Co.), Reports Council Pharm. & Chem., 1918, p. 55. |
Formaldehyde Lozenges235 |
| Formaldehyde Lozenges, The Journal, Oct. 4, 1919, p. 1077; Reports Council Pharm. & Chem., 1919, p. 32. |
Formamint33 |
| Formamint (A. Wulfing & Co.), The Journal, Jan. 27, 1912, p. 295; Feb. 24, 1912, p. 572; Aug. 28, 1915, p. 816; Reports Council Pharm. & Chem., 1915, p. 64; Propaganda, ed. 9, p. 303. |
| Formic Acid, Reports Council Pharm. & Chem., 1921, p. 25. |
| Formicin (Kalle Color Chemical Co.), Reports Council Pharm. & Chem., 1919, p. 76. |
| Formidin (Parke, Davis & Co.), The Journal, Sept. 5, 1908, p. 818; Reports Council Pharm. & Chem., 1905–8, pp. 164, 192; 1912, p. 48. |
| Formitol Tablets (E. L. Patch Co.), The Journal, Oct. 4, 1919, p. 1077; June 19, 1920, p. 1730; Reports Council Pharm. & Chem., 1919, p. 34; 1920, p. 20; Reports Chem. Lab., 1920, p. 40. |
Formosol158 |
| Formosol, Sunshine’s (The Formosol Chemical Co.), Reports Council Pharm. & Chem., 1917, p. 145. |
Formothalates, Tablets256 |
| Formothalates, Tablets (Tailby Nason Company), Reports Council Pharm. & Chem., 1919, p. 92. |
| Formurol (Cellarius Co.), The Journal, Jan. 21, 1911, p. 210; Reports Council Pharm. & Chem., 1911, p. 7; Propaganda, ed. 9, p. 85. |
| Fortossan (A. Klipstein & Co.), The Journal, Jan. 30, 1915, p. 456; Reports Council Pharm. & Chem., 1915, p. 131; Propaganda, ed. 9, p. 178. |
| Fougera E., and Co., Inc.— |
Collosol Iodine144 |
Dr. De Sanctis’ Rheumatic and Gout Pills363 |
Ferrivine144 |
Intramine144 |
Phosphoglycerate of Lime (Chapoteaut)95 |
| Freeman, Allen W., protests regarding advertising methods used by |
| Friedländer Bacillus Vaccine, Reports Council Pharm. & Chem., 1919, p. 78. |
| Fringe Tree, Reports Council Pharm. & Chem., 1912, p. 42. |
Fritz, W. W., and the Allied Medical Associations of America488 |
| Frosst’s Blaud Capsules: See Blaud Capsules. |
| Frutosen (The Frutosen Drug Co.), Reports Council Pharm. & Chem., 1921, p. 26. |
| G |
| G. G. Phenoleum Disinfectant (G. G. Phenoleum Co., Inc.), The Journal, Jan. 30, 1915, p. 456; Reports Council Pharm. & Chem., 1915, p. 131. |
Galactagogue action of Galega and Nutrolactis131 |
Galactagogue, alleged Galactagogue effects of Nutrolactis and Goat’s Rue not substantiated131 |
| Galactagogue (Eli Lilly & Co.), Reports Council Pharm. & Chem., 1912, p. 43. |
Galega and Nutrolactis, alleged Galactagogue action of131 |
Gardner’s Syrup of Ammonium Hypophosphite100 |
| Gastrogen Tablets (Bristol-Myers Co.), The Journal, Dec. 12, 1914, p. 2149; Reports Council Pharm. & Chem., 1914, p. 131; Propaganda, ed. 9, p. 87. |
| Gelesmine Hydrochlorid, Reports Council Pharm. & Chem., 1911, p. 57. |
| Gelseminine, Reports Council Pharm. & Chem., 1911, p. 57. |
| Genitone (Wm. S. Merrell Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 44. |
| Genoform (C. Bischoff & Co.), The Journal, Feb. 26, 1916, p. 676. |
| Germaseptic Lubricant “Bing” (Chas. M. Griswold, St. Petersburg, Fla.), Reports Council Pharm. & Chem., 1918, p. 79. |
| Germiletum (Dios Chemical Co.), The Journal, Jan. 9, 1915, p. 165; Reports Council Pharm & Chem., 1914, p. 86; Reports Chem. Lab., 1910, p. 11; Propaganda, ed. 9, p. 139. |
Giambalvo, G., & Co.—Aphlegmatol273 |
| Ginseng, The Journal, Oct. 24, 1914, p. 1486; Reports Council Pharm. & Chem., 1912, p. 42. |
| Ginseng Compound, Elixir (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1912, p. 42. |
| Ginseng Compound (Special), Elixir (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 42. |
| Glidine (Menley & James), The Journal, June 28, 1913, p. 2037. |
| Globeol (Geo. J. Wallau, Inc.), The Journal, Sept. 18, 1915, p. 1046; Reports Council Pharm. & Chem., 1915, p. 157. |
Glover’s Cancer Serum425 |
| Gluten Biscuit, Pure (Kellogg Food Company), Reports Council Pharm. & Chem., 1916, pp. 56, 57. |
| Gluten Biscuit, 40 per cent. (Kellogg Food Company), Reports Council Pharm. & Chem., 1916, pp. 56, 58. |
| Gluten Flour, 40 per cent. (Kellogg Food Company), Reports Council Pharm. & Chem., 1916, pp. 56, 59. |
| Gluten Meal, Pure (Kellogg Food Company), Reports Council Pharm. & Chem., 1916, pp. 56, 60. |
| Gluten Meal, 20 per cent. (Kellogg Food Company), Reports Council Pharm. & Chem., 1916, pp. 56, 60. |
| Gluten Meal, 40 per cent. (Kellogg Food Company), Reports Council Pharm. & Chem., 1916, pp. 56, 59. |
Gluten products made by the Kellogg Food Company100 |
| Glutol-Schleich (Schering J. Glatz, Inc.), Reports Council Pharm. & Chem., 1915, p. 170. |
| Glycerinated Vaccine Virus (National Vaccine and Antitoxin Institute), Reports Council Pharm. & Chem.. 1921, p. 23. |
| Glycerine Tonic, Gray’s (Purdue Frederick Co.), The Journal, July 10, 1915, p. 189; Reports Council Pharm. & Chem., 1915, p. 56. |
| Glycero-Lecithin, Pill (Westerfield Pharmacal Co.), Reports Council Pharm. & Chem., 1915, p. 170. |
Glycerole of Lecithin53 |
| Glycerole of Lecithin (Fairchild Bros, and Foster), Reports Council Pharm. & Chem., 1915, p. 122. |
| Glycerophosphate Comp. No. 1, Mulford, Ampuls (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1916, p. 49. |
| Glycerophosphate Comp, Ampuls, 1 Cc. Squibb (E. R. Squibb & Sons), The Journal, Feb. 3, 1917; Reports Council Pharm. & Chem., 1916, p. 48. |
Glycerophosphate, Sodium99 |
Glycerophosphates520 |
| Glycerophosphates, The Journal, Sept. 30, 1916, p. 1033; Reports Council Pharm. & Chem., 1916, p. 32. |
Glycerophosphate, Calcium99 |
Glycerophosphates, Elixir, Nux Vomica and Damiana95 |
Glycerophosphates, Schering’s (Tonols)94 |
Glycerophosphates, therapeutic value of93 |
| Glycerosal (Röhm & Haas), Reports Council Pharm. & Chem., 1918, p. 57. |
| Glyco-Heroin, Smith (Martin H. Smith & Co.), The Journal, June 6, 1914, p. 1826; Reports Council Pharm. & Chem., 1914, p. 29; Propaganda, ed. 9, p. 88. |
| Glyco-Thymoline (Kress & Owen Co.), The Journal, March 14, 1914, p. 868; Oct. 10, 1914, p. 1312; Sept. 16, 1916, p. 895; Reports Council Pharm. & Chem., 1914, p. 54; Propaganda, ed. 9, p. 92. |
Glyco-Thymoline and poliomyelitis427 |
| Glycozone (Drevet Mfg. Co.), The Journal, June 5, 1909, p. 1851; Reports Council Pharm. & Chem., 1909, p. 103; Propaganda, ed. 9, p. 95. |
Glykeron: cold storage testimonials428 |
Goat Lymph Treatment528 |
Goat’s Rue, claimed Galactagogue effects of, not substantiated131 |
| Goat’s Rue, The Journal, May 26, 1917, p. 1570; Reports Council Pharm. & Chem., 1912, p. 42; 1917, p. 24. |
Goehring, H. M., and the Allied Medical Associations of America488 |
Goiter Serum, Mark White87 |
| Goiter Serum, Mark White (Mark White Serum Laboratories), The Journal, Sept. 23, 1916, p. 967; Reports Council Pharm. & Chem., 1916, p. 23. |
Goitreine88 |
| Gomenol (Charles R. Bard), The Journal, April 4, 1914, p. 1110; Propaganda, ed. 9, p. 304. |
| Gonococcic Vaccine (National Vaccine and Antitoxin Institute), Reports Council Pharm. & Chem., 1921, p. 53. |
| Gonococcide (Cox Chemical Co.), The Journal, Aug. 24, 1907, p. 708. |
Gonococcus Bacterin, Mixed, Special Bacterial Vaccine No. 16254 |
Gonosan150 |
| Gonosan (Riedel & Co., Inc.), The Journal, Oct. 13, 1917, p. 1287; Reports Council Pharm. & Chem., 1917, p. 57. |
| Gossypin, The Journal, June 3, 1911, p. 1670; Reports Council Pharm. & Chem., 1911, p. 19; Propaganda, ed. 9, p. 84. |
Gout and Rheumatic Pills, Dr. De Sanctis’363 |
Granular Effervescent Bromide and Acetanilid Compound-Mulford206 |
| Granular Effervescent Sodium Phosphate Compound (Squibb) (E. R. Squibb & Sons), Reports Council Pharm. & Chem., 1920, p. 63. |
Green Bone, Russell Prepared134 |
Gross Drug Company, Inc.—Capsules Bismuth Resorcinol Compound157 |
| Guiaialin (Organic Chemical Mfg. Co.), The Journal, Sept. 5, 1908, p. 818; May 8, 1909, p. 1511; Reports Council Pharm. & Chem., 1905–8, p. 166; 1909, p. 76. |
Guaiodine183 |
| Guaiodine (Intravenous Products Co.), The Journal, April 6, 1918, p. 1026; Reports Council Pharm. & Chem., 1918, p. 9. |
Gude’s Pepto-Mangan387 |
| H |
| H-M-C.: See Hyoscin-Morphin-Cactin. |
Hagee’s Cordial of Cod Liver Oil429 |
| Hair Cap Moss, Reports Council Pharm. & Chem., 1912, p. 43. |
Hanson, A. C., and the Physicians’ Drug Syndicate432 |
Harmer Laboratories Company—Mon-Arsone302 |
| Harrower, Henry R.—“Pluriglandular” Mixtures: Caps. Adreno-Spermin Comp., Caps. |
| Antero-Pituitary Comp., Caps. Placento-Mammary Comp., Caps. Thyro-Ovarian Comp., |
Caps. Hepato-Splenic Comp., Caps. Pancreas Comp., and Caps. Thyroid Comp218 |
| Havens’ Wonderful Discovery (E. C. Havens), The Journal, March 22, 1919, p. 883; Reports Council Pharm. & Chem., 1919, p. 7. |
| Hay Fever Fall Pollen Extract-Mulford (H. K. Mulford Co.), Reports Council Pharm. Chem., 1921, p. 45. |
| Hay Fever Spring Pollen Extract-Mulford (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1921, p. 45. |
| Hectine (Geo. J. Wallau, Inc.), The Journal, Aug 8, 1914, p. 502, Propaganda, ed. 9, p. 308. |
Heffner, Charles L.—Iodiphos249 |
| Hélénin and Globules of “Hélénin De Korab” (De Korab Bojemski), Reports Council Pharm. & Chem., 1919, p. 78. |
Helmitol295 |
| Helmitol (Winthrop Chemical Co.), The Journal, Jan. 22, 1921, p. 260; Reports Council Pharm. & Chem., 1920, p. 49. |
| Helonias Compound, Cordial, Elixir (Ray Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 41. |
| Helonias Compound, Elixir (Hance Bros. & White; H. K. Mulford Co.; Parke, Davis & Co.; Smith, Kline & French Co.), Reports Council Pharm. & Chem., 1912, p. 41. |
| Helonias Compound, Fluidextract (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 41. |
| Hemaboloids (Palisade Manufacturing Co.), Reports Council Pharm. & Chem., 1919, p. 80. |
| Hemaboloids Arseniated with Strychnia (The Palisade Mfg. Co.), The Journal, Dec. 27, 1913, p. 2306. |
| Hemo (Thompson’s Malted Food Co.), The Journal, Oct. 24, 1914, p. 1494; Propaganda, ed. 9, p. 319. |
Hemo-Therapin168 |
| Hemo-Therapin (Hemo-Therapin Laboratories), The Journal, Jan. 5, 1918, p. 48; Reports Council Pharm. & Chem., 1917, p. 116. |
| Hepatico Tablets (David Laboratories, Inc.), The Journal, Oct. 20, 1917, p. 1734; Reports Council Pharm. & Chem., 1917, p. 64. |
Hepato-Splenic Comp., Caps219 |
| Hexa-Co-Sal-In (Hexa-Co-Sal-In Co.), The Journal, Oct. 2, 1915, p. 1203; Reports Council Pharm. & Chem., 1915, p. 159; Reports Chem. Lab., 1915, p. 107. |
| Hexalet (Riedel & Co.), Reports Council Pharm. & Chem., 1921, p. 27. |
| Hex-a-lith (Smith-Dorsey Co.), The Journal, Feb. 14, 1914, p. 555. |
Hexamethylenamin, commercial history of316 |
| Hexamethylenamin Methylencitrate: See Helmitol. |
Hex-Iodin236 |
| Hex-Iodin (Daggett and Miller Co., Inc.), The Journal, Oct. 4, 1919, p. 1077; Reports Council Pharm. & Chem., 1919, p. 33. |
Hille Laboratories—Mervenol and Armervenol249 |
Hillside Chemical Co.—Pil. Mixed Treatment (Chichester)310 |
| Hogan’s Colloid Solution Materials for Intravenous Transfusion.—See Colloid Solution Materials for Intravenous Transfusion, Hogan’s. |
Holadin and Bile Salts—Fairchild207 |
| Holadin and Bile Salts, Fairchild (Fairchild Bros, and Foster), Reports Council Pharm. & Chem., 1918, p. 59. |
Holadin, Bile Salts and Phenolphthalein, Capsules of208 |
| Holadin, Bile Salts and Phenolphthalein, Capsules of, Fairchild (Fairchild Bros, and Foster), Reports Council Pharm. & Chem., 1918, p. 59. |
Holadin, Succinate of Soda and Bile Salts, Capsules of208 |
| Holadin, Succinate of Soda and Bile Salts, Capsules of, Fairchild (Fairchild Bros. & Foster), Reports Council Pharm. & Chem., 1918, p. 59. |
Holocain Hydrochlorid372 |
Hormotone234 |
| Hormotone (G. W. Carnrick Co.), The Journal, Aug. 16, 1919, p. 549; Reports Council Pharm. & Chem., 1919, p. 30. |
Hormotone without Postpituitary235 |
| Hormotone Without Postpituitary (G. W. Carnrick Co.), The Journal, Aug. 16, 1919, p. 549; Reports Council Pharm. & Chem., 1919, p. 30. |
Horovitz Biochemic Laboratories Co—Lipoidal Substances320 |
Horowitz-Beebe—“Autolysin”413 |
| Horse Nettle, Reports Council Pharm. & Chem., 1912, p. 43. |
Hughes, K. A., Company—Salicon453 |
Hyclorite358 |
Hydragogin41 |
| Hydragogin (C. Bischoff & Co.), The Journal, Jan. 27, 1906, p. 288; Sept. 4, 1915, p. 894; Reports Council Pharm. & Chem., 1915, p. 154. |
| Hydrangea and Lithia, Elixir (Hance Bros. & White), Reports Council Pharm. & Chem., 1912, p. 45. |
| Hydrangea, Lithiated (Lambert Pharmacal Co.), Reports Council Pharm. & Chem., 1912, p. 42. |
Hydras96 |
| Hydras (John Wyeth and Bro.), The Journal, Oct. 7, 1916, p. 1107; Reports Council Pharm. & Chem., 1916, p. 36; Reports Chem. Lab., 1916, p. 29. |
| Hydrastis and Cramp Bark Compound, Elixir (Parke, Davis & Co.), The Journal, Aug. 31, 1912, p. 735; Reports Council Pharm. & Chem., 1912, p. 44; Propaganda, ed. 9, p. 410. |
| Hydrastis and Viburnum Compound, Elixir of (Smith, Kline & French Co.), The Journal, Aug., 31, 1912, p. 735; Propaganda, ed. 9, p. 410. |
| Hydrocyanate of Iron, Tilden (The Tilden Co.), The Journal, June 19, 1909, p. 2008; Reports Chem. Lab., 1909, p. 27; Propaganda, ed. 9, p. 235. |
Hydroleine58 |
| Hydroleine (Charles N. Crittenton Co.), Reports Council Pharm. & Chem., 1915, p. 171. |
| Hydron (Wm. S. Merrell Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 44. |
| Hydronaphthol (Seabury & Johnson), The Journal, Sept. 3, 1910, p. 878; Reports Chem. Lab., 1910, p. 108; Propaganda, ed. 9, p. 308. |
Hydropsin61 |
| Hydropsin (E. Bischoff & Co., Inc.), The Journal, Jan. 8, 1916, p. 135; Reports Council Pharm. & Chem., 1915, p. 94. |
| Hydrozone (Charles Marchand), The Journal, Sept. 23, 1905, p. 936; Propaganda, ed. 9, p. 309. |
| Hymosa (Walker Pharmacal Co.), The Journal, June 11, 1910, p. 1955; Reports Chem. Lab., 1910, p. 51; Reports Council Pharm. & Chem., 1912, p. 44; Propaganda, ed. 9, p. 238. |
| Hyoscin-Morphine-Cactin, now Hyoscin-Morphin-Cactoid (The Abbott Laboratories), The Journal, Dec. 21, 1907, p. 2103. |
Hyoscyamus Compound, Tyree’s Elixir of Buchu and Hyoscyamus Compound57 |
| Hyperol (Purdue Frederick Co.), The Journal, April 18, 1914, p. 1271; Reports Council Pharm. & Chem., 1914, p. 12; Propaganda, ed. 9, p. 100. |
Hyperthermine331 |
| Hyperthermine (Pasteur Chemical Co.), The Journal, May 19, 1917, p. 1497. |
Hypno-Bromic Compound430 |
Hypodermic Solution No. 13, Iron, Arsenic, and Phosphorus Compound275 |
| Hypodermic Solution No. 13, Iron, Arsenic and Phosphorus Compound (Burdick-Abel Laboratory), The Journal, Nov. 13, 1920, p. 1358; Reports Council Pharm. & Chem., 1920, p. 27. |
| Hypophosphite, The Journal, Sept. 2, 1916, p. 760; Reports Council Pharm. & Chem., 1916, p. 11. |
Hypophosphite, Ammonium98 |
Hypophosphite, Ammonium, Gardner’s Syrup of100 |
Hypophosphite Fallacy80 |
Hypophosphites, Borcherdt’s Malt Olive with84 |
Hypophosphites Comp. (Lime and Soda)—McArthur’s Syrup of84 |
Hypophosphites, Eupeptic83 |
Hypophosphites, Fellows’ Syrup and other Preparations of395 |
Hypophosphites, Fellows’ Syrup of82 |
Hypophosphites, inertness of397 |
Hypophosphites, Maltine with Olive Oil and84 |
Hypophosphites, Maltzyme with84 |
| Hypophosphites of Lime and Soda, Schlotterbeck’s Solution (Liq. Hypophosphitum, |
Schlotterbeck’s)83 |
Hypophosphites, Preparations of395 |
Hypophosphites, Robinson’s83 |
| Hypophosphites, Robinson’s (Robinson-Pettet Company), The Journal, Sept. 2, 1916, p. 761; Reports Council Pharm. & Chem., 1916, p. 15. |
Hypophosphites, Syrup, Comp. with Quinin, Strychinin and Manganese, Syrupus Roborans82 |
| Hypoquinidol (R. W. Gardner), The Journal, Jan. 10, 1914, p. 148; Propaganda, ed. 9, p. 310. |
| I |
I. G. O.367 |
| Ichthynate (Mallinckrodt Chemical Works), Reports Chem. Lab., 1912, p. 110. |
| Ichthytar (Szel Import & Export Co.), The Journal, March 10, 1917, p. 796; Reports Council Pharm. & Chem., 1917, p. 18. |
Influenza Combined Bacterin (Special Bacterial Vaccine No. 5)254 |
Influenza Mixed Vaccine-Lilly187 |
| Influenza Mixed Vaccine-Lilly (Eli Lilly & Co.), The Journal, June 22, 1918, p. 1967; Reports Council Pharm. & Chem., 1918, p. 11. |
| Influenza Prophylactic-Lederle (Lederle Antitoxin Laboratories), Reports Council Pharm. & Chem., 1919, p. 81. |
Influenza Serobacterin Mixed-Mulford187 |
| Influenza Serobacterin Mixed-Mulford (H. K. Mulford Co.), The Journal, June 22, 1918, p. 1967; Reports Council Pharm. & Chem., 1918, p. 11. |
Influenza, Serums and Vaccines521 |
Influenza, vaccine as a prophylactic in572 |
Influenza Vaccines520 |
| Ingluvin (Wm. R. Warner & Co.), The Journal, July 11, 1908, p. 142; Reports Council Pharm. & Chem., 1905–8, p. 116; Propaganda, ed. 9, p. 101. |
Innis, Speiden & Co.—Ophthalmol-Lindemann189 |
| Interol (Van Horn & Sawtell), The Journal, July 10, 1915, p. 175. |
| Intestinal Antiseptic W-A (The Abbott Laboratories), The Journal, Dec. 19, 1914, p. 2247; Reports Council Pharm. & Chem., 1914, p. 78; Propaganda, ed. 9, p. 103. |
Intramine144 |
| Intramine (E. Fougera & Co., Inc.), The Journal, Sept. 8, 1917, p. 841; Reports Council Pharm. & Chem., 1917, p. 49. |
| Intravenin P-H (Intravenin Products Co.), Reports Council Pharm. & Chem., 1915, p. 120. |
Intravenous Compound (Loffler)430 |
| Intravenous Compound (Loffler) (Charles Lyman Loffler), The Journal, Nov. 12, 1921, p. 1591. |
| Intravenous Products Company of America— |
Endoarsan435 |
Endosal435 |
Venosal435 |
| Intravenous Products Company of Denver— |
Guaiodine183 |
Intravenous Specialties435 |
Venarsen471 |
Venosal169 |
| Intravenous Solution, Bannerman’s (William Bannerman), The Journal, May 31, 1913, p. 1724; Jan. 2, 1915, p. 70; Reports Council Pharm. & Chem., 1914, p. 131; Propaganda, ed. 9, p. 105. |
Intravenous Solution of Hexamethylenamin, Loeser’s299 |
| Intravenous Solution of Hexamethylenamin, Loeser’s (New York Intravenous Laboratory, Inc.), The Journal, April 16, 1921, p. 1120; Reports Council Pharm. & Chem., 1921, p. 43. |
Intravenous Solution of Hexamethylenamin and Sodium Iodid, Loeser’s299 |
| Intravenous Solution of Hexamethylenamin and Sodium Iodid, Loeser’s (New York Intravenous Laboratory, Inc.), The Journal, April 16, 1921, p. 1120; Reports Council Pharm. & Chem., 1921, p. 43. |
Intravenous Solution of Sodium Iodid, Loeser’s299 |
Intravenous Solution of Mercury Bichlorid, Loeser’s299 |
| Intravenous Solution of Mercury Bichlorid, Loeser’s (New York Intravenous Laboratory, Inc.), The Journal, April 16, 1921, p. 1120; Reports Council Pharm. & Chem., 1921, p. 43. |
Intravenous Solution of Salicylate and Iodid, Loeser’s299 |
| Intravenous Solution of Salicylate and Iodid, Loeser’s (New York Intravenous Laboratory, Inc.), The Journal, April 16, 1921, p. 1120; Reports Council Pharm. & Chem., 1921, p. 43. |
| Intravenous Solution of Sodium Iodid and Sodium Salicylate, Loeser’s (New York Intravenous Laboratory, Inc.), The Journal, April 16, 1921; page 1120; Reports Council Pharm. & Chem., 1921, p. 43. |
Intravenous Solution Sodium Salicylate, Loeser’s299 |
Intravenous Solutions, Loeser’s299 |
Intravenous Specialties435 |
Intravenous Therapy522 |
Iocamfen338 |
Iocamfen Ointment338 |
Iodagol154 |
| Iodagol (David B. Levy, Inc.), The Journal, Nov. 17, 1917, p. 1725; Reports Council Pharm. & Chem., 1917, pp. 65–116; Reports Chem. Lab., 1917, p. 80. |
| Iodalia (Geo. J. Wallau, Inc.), The Journal, Dec. 12, 1914, p. 2149; Reports Council Pharm. & Chem., 1914, p. 69; Reports Chem. Lab., 1914, p. 75; Propaganda, ed. 9, p. 106. |
Iodeol154 |
| Iodeol (David B. Levy, Inc.), The Journal, Nov. 17, 1917, p. 1725; Reports Council Pharm. & Chem., 1917, p. 65–116; Reports Chem. Lab., 1917, p. 80. |
| Iodex (Menley & James), The Journal, Nov. 30, 1912, p. 1992; June 19, 1915, p. 2085; Reports Council Pharm. & Chem., 1915, p. 144; Reports Chem. Lab., 1915, p. 89; 1919, p. 104; Propaganda, ed. 9, p. 107; The Journal, May 3, 1919, p. 1315. |
Iodex, Liquid365 |
| Iodex, Liquid (Menley and James), Reports Chem. Lab., 1919, p. 104. |
| Iodia (Battle & Co.), The Journal, Nov. 21, 1914, p. 1871; Reports Council Pharm. & Chem., 1914, p. 60; Reports Chem. Lab., 1914, p. 58; Propaganda, ed. 9, p. 108. |
| Iodin, Burnham’s Soluble (Burnham Soluble Iodin Co.), The Journal, March 28, 1908, p. 1055; May 15, 1915, p. 1673; Reports Council Pharm. & Chem., 1915, p. 50; Reports Chem. Lab., to 1909, p. 30; Propaganda, ed. 9, pp. 110, 233. |
Iodin Fumes523 |
Iodin in liquid petrolatum367 |
| Iodin Petrogen (John Wyeth & Bro.), The Journal, Nov. 30, 1912, p. 1992. |
| Iodin Tablets, Burnham’s Soluble (Burnham Soluble Iodin Co.), The Journal, March 28, 1908, p. 1055; Reports Chem. Lab., to 1909, p. 32; Propaganda, ed. 9, p. 233. |
Iodine, B. Iodine198 |
Iodine, B. Iodine Products199 |
Iodine, B. Oleum Iodine198 |
Iodine ointments, stability of337 |
Iodine Solution, National300 |
Iodinized Emulsion (Scott)192 |
| Iodinized Emulsion (Scott) (Dawson Pharmacal Co.), The Journal, Aug. 24, 1918, p. 680; Reports Council Pharm. & Chem., 1918, p. 25. |
Iodinized Oil, Mark White87 |
| Iodinized Oil, Mark White (Mark White Laboratories), The Journal, Sept. 23, 1916, p. 967; Reports Council Pharm. & Chem., 1916, p. 23. |
| Iodinol (Toledo Pharmacal Co.), The Journal, Aug. 20, 1921, p. 637. |
Iodiphos249 |
| Iodiphos (Charles L. Heffner), Reports Council Pharm. & Chem., 1919, p. 81. |
| Iodival (Knoll & Co.), The Journal, March 4, 1911, p. 685. |
Iod-Izd-Oil338 |
Iod-Izd-Oil (Miller’s)49 |
| Iod-Izd-Oil (Miller’s) (Iodum Miller Co.), The Journal, Oct. 2, 1915, p. 1202; Reports Council Pharm. & Chem., 1915, p. 76; Reports Chem. Lab., 1915, p. 106. |
Iodo-Bromide of Calcium Comp. “Without Mercury” and “With Mercury,” Elixir52 |
| Iodo-Bromide of Calcium Comp., “Without Mercury” and “With Mercury,” Elixir (The Tilden Co.), The Journal, Nov. 6, 1915, p. 1662; Reports Council Pharm. & Chem., 1915, p. 160. |
Iodolene and solubility of iodin in liquid petrolatum344 |
Iodolene, a solution of iodin in liquid petrolatum159 |
Iodo-Mangan106 |
| Iodo-Mangan (Reinschild Chemical Co.), Reports Council Pharm. & Chem., 1916, p. 64. |
| Iodomuth (Organic Chemical Mfg. Co.), The Journal, Sept. 5, 1908, p. 818; May 8, 1909, p. 1511; Reports Council Pharm. & Chem., 1905–8, p. 166; 1909, p. 75. |
| Iodonucleoid (Dinet & Delfosse Pharm. Co.), The Journal, July 22, 1911, p. 309; Reports Chem. Lab., 1911, p. 92; Propaganda, ed. 9, p. 310. |
| Iodotone (Eimer & Amend), The Journal, Dec. 12, 1914, p. 2149; Reports Council Pharm. & Chem., 1914, p. 72; Propaganda, ed. 9, p. 113. |
| Iodovasogen (Lehn & Fink), The Journal, Feb. 13, 1909, p. 575; Propaganda, ed. 9, p. 408. |
Iodum-Miller49 |
| Iodum-Miller (Iodum-Miller Co.), The Journal, Oct. 2, 1915, p. 1202; Reports Council Pharm. & Chem., 1915, p. 76; Reports Chem. Lab., 1915, p. 102. |
| Iosaline (Iosaline Co.), The Journal, March 15, 1913, p. 849; Reports Council Pharm. & Chem., 1913, p. 13; Propaganda, ed. 9, p. 113. |
Ipecac, Alcresta153 |
| Iridium Medicinal (Platinum Company of America), The Journal, April 23, 1910, p. 1389; Propaganda, ed. 9, p. 312. |
Iron, Arsenic, and Phosphorus Compound, Hypodermic Solution No. 13275 |
Iron Arsenite466 |
Iron Citrate Green115 |
| Iron Citrate Green, The Journal, Jan. 13, 1917, p. 135; Reports Council Pharm. & Chem., 1916, p. 42. |
| Iron Solution for Intravenous Therapy—Perkins and Ross (Perkins & Ross), The Journal, Nov. 14, 1914, p. 1778; Reports Council Pharm. & Chem., 1914, p. 125. |
| Iron Tropon (Tropon Works), The Journal, April 23, 1910, p. 1389; Propaganda, ed. 9, p. 313. |
| Isopral (The Bayer Company, Inc.), The Journal, Aug. 8, 1908, p. 487; Reports Council Pharm. & Chem., 1905–8, p. 119. |
Italian Physico-Chemical Company524 |
| Ittiolo (Guiseppi W. Guidi), Reports Council Pharm. & Chem., 1920, p. 64. |
| J |
| Jaroma (Jaroma Co.), The Journal, Sept. 2, 1911, p. 835; Reports Chem. Lab., 1911, p. 103. |
Johnson, N. La Doit, and the Allied Medical Associations of America488 |
Jubol31 |
| Jubol (Geo. J. Wallau, Inc.), The Journal, Aug. 14, 1915, p. 639; Reports Council Pharm. & Chem., 1915, p. 152. |
| Juglandin, The Journal, Nov. 13, 1909, p. 1655; Reports Council Pharm. & Chem., 1909, p. 135. |
| K |
| Kal Pheno Tooth Paste (Kal Pheno Chemical Co.), Reports Council Pharm. & Chem., 1921, p. 41. |
| Kal Pheno Tooth Powder (Kal Pheno Chemical Co.), Reports Council Pharm. & Chem., 1921, p. 41. |
Kalak Water160 |
| Kalak Water (Kalak Water Co., Inc.), Reports Council Pharm. & Chem., 1917, p. 148. |
Katharmon191 |
| Katharmon (Katharmon Chemical Co.), The Journal, Aug. 10, 1918, p. 487; Reports Council Pharm. & Chem., 1918, p. 23. |
Keasbey and Mattison Company—Alkalithia242 |
| Kefilac (Kefilac Co.), The Journal, Jan. 30, 1909, pp. 372, 397. |
Kellogg Food Company—Gluten products100 |
| Kelpidine: See Minson’s Soluble Iodin. |
| Keratin, Reports Council Pharm. & Chem., 1911, p. 58. |
| Kinazyme (G. W. Carnrick Co.), The Journal, Nov. 1, 1913, p. 1649. |
Klein, Frederick415 |
Klipstein, A., and Company, Inc.—Dial “Ciba”259 |
Koch, William F., Cancer Remedy437 |
| Kola Compound, Elixir (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Kola Compound, Elixir (Ray Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Kolynos (Kolynos Co.), The Journal, Nov. 15, 1913, p. 1812. |
Kora-Konia92 |
| Kora-Konia (Gerhard Mennen Chemical Co.), The Journal, Sept. 30, 1916, p. 1034; Reports Council Pharm. & Chem., 1916, p. 31; Reports Chem. Lab., 1916, p. 26. |
| Koyol (The Koyol Co.), Reports Council Pharm. & Chem., 1815, p. 172. |
Kress & Owen Company—Glyco-Thymoline427 |
| Kutnow’s Powder (Kutnow Bros.), The Journal, Nov. 9, 1907, p. 1619; Propaganda, ed. 9, p. 314. |
| K-Y Lubricating Jelly (Van Horn & Sawtell), The Journal, May 12, 1917, p. 1430; Sept. 29, p. 1102; Reports Council Pharm. & Chem., 1917, p. 53. |
| L |
| Labordine (Labordine Pharmacal Co.), The Journal, March 30, 1907, p. 1121; Reports Council Pharm. & Chem., 1905–8, p. 45; 1912, p. 40; Propaganda, ed. 9, p. 115. |
| Lackenbach, F. I.— |
Special Bacterial Vaccine, Nos. 2, 3, 4, 5, 11, 15, 16254 |
| Lacteol (Dr. Boucar, Paris), The Journal, Dec. 21, 1916, p. 1959. |
| Lactobacilline (The Franco-American Ferment Co.), The Journal, April 17, 1915, p. 1346; Sept. 18, 1915, p. 1049; Reports Council Pharm. & Chem., 1915, p. 143; Propaganda, ed. 9, p. 120. |
| Lactone (Parke, Davis & Co.), The Journal, Jan. 30, 1909, pp. 372, 397. |
| Lactopeptine (New York Pharmacal Association), The Journal, March 16, 1907, p. 959; Aug. 2, 1913, p. 358; Oct. 23, 1915, pp. 1466, 1467, 1477; Reports Council Pharm. & Chem., 1905–8, p. 43; 1913, p. 21; 1915, p. 79; Propaganda, ed. 9, p. 121. |
Lactopeptine and Elixir Lactopeptine43 |
| Lactopeptine, Elixir (New York Pharmacal Association), The Journal, Feb. 9, 1907, p. 533; Oct. 23, 1915, pp. 1466, 1467, 1477; Reports Council Pharm. & Chem., 1915, p. 79. |
| Lactucarium, Aubergier’s Syrup of (E. Fougera & Co., Inc.), The Journal, Nov. 9, 1912, p. 1732; Feb. 15, 1913, p. 538; Propaganda, ed. 9, p. 399. |
Lambdin’s I. G. O.367 |
Lambert, Alexander—protest regarding Pineoleum advertising methods443 |
Lanphear, Emory—and the Medical Society of the United States531 |
Lavoris237 |
| Lavoris (Lavoris Chemical Company), The Journal, Nov. 1, 1919, p. 1380; Reports Council Pharm. & Chem., 1919, p. 35. |
| Laxaphen (Parke, Davis & Co.), The Journal, April 30, 1910, p. 1458, Propaganda, ed. 9, p. 344. |
| Laxine (Columbus Pharmacal Co.), The Journal, April 30, 1910, p. 1458; Propaganda, ed. 9, p. 344. |
Laxol and Lysol, the short and catchy proprietary name570 |
| Laxothalen Tablets (Pitman-Moore Co.), The Journal, April 30, 1910, p. 1458; Propaganda, ed. 9, p. 344. |
Lecibrin53 |
| Lecibrin (Fairchild Bros. and Foster), Reports Council Pharm. & Chem., 1915, p. 122. |
Lecithin, Glycerole of53 |
| Lecithin, Reports Council Pharm. & Chem., 1915, p. 122. |
Lecithin Solution53 |
| Lecithin Solution (Fairchild Bros. & Foster), Reports Council Pharm. & Chem., 1915, p. 122. |
| Lecithine, Gare’s Granular (Gare Pharmacal Co.), Reports Council Pharm. & Chem., 1916, p. 56. |
Lecithol53 |
| Lecithol (Armour & Co.), Reports Council Pharm. & Chem., 1915, p. 122. |
Leeming, Thos., and Co—Trimethol140 |
Lehn and Fink—Aspirin347 |
Leptinol, Syrup (formerly Syrup Balsamea)268 |
| Lettuce Calmative (Nelson, Baker & Co.), Reports Council Pharm. & Chem., 1912, p. 43. |
| Lettuce, Wild, Reports Council Pharm. & Chem., 1912, p. 43. |
| Leucocytic Extract, Archibald’s (The Western Laboratories), Reports Council Pharm. & Chem., 1916, p. 65. |
Levy, David B., Inc.—Iodeol and Iodagol154 |
Libby’s Alcresta Lotion465 |
Libradol293 |
| Libradol (Lloyd Bros.), Reports Council Pharm. & Chem., 190, p. 65. |
| Lilly, Eli, and Co.— |
Alcresta Ipecac153 |
Catarrhal Vaccine Combined-Lilly187 |
Cephaelin203 |
Influenza Mixed Vaccine-Lilly187 |
Oxyl-Iodide304 |
Rheumalgine23 |
Syrup Cephaelin-Lilly203 |
Syrup Emetic-Lilly203 |
Linder, C. O., and the Allied Medical Associations of America489 |
Lipoidal Substances (Horovitz)320 |
Liquid Albolene106 |
Liquid Iodex365 |
| Liquid Peptone (Eli Lilly & Co.; Stevenson & Jester Co.), The Journal, May 11, 1907, p. 1612; Reports Council Pharm. & Chem., 1905–8, opp. p. 64; Propaganda, ed. 9, p. 133. |
| Liquid Peptones with Creosote (Eli Lilly & Co.), The Journal, May 11, 1907, p. 1612; Reports Council Pharm. & Chem., 1905–8, opp. p. 64; Propaganda, ed. 9, p. 133. |
Liquid Petrolatum526 |
Liquid Petrolatum, Iodolene and the solubility of Iodin in344 |
Liquid Petrolatum, proprietary names for55 |
Liquor Santaiva, S. & D.211 |
| Liquor Santaiva, S. & D. (Sharp & Dohme), Reports Council Pharm. & Chem., 1918, p. 66. |
| Lithia and Hydrangea, Elixir (Parke, Davis & Co.; Ray Chemical Co.; Smith, Kline & French Co.), Reports Council Pharm. & Chem., 1912, p. 45. |
| Lithiated Sorghum Compound (Sharp & Dohme), Reports Council Pharm. & Chem., 1912, p. 39. |
| Liver Leaf, Reports Council Pharm. & Chem., 1912, p. 48. |
Lloyd Bros.—Libradol293 |
L. O. Compound No. 1 and L. O. Compound No. 2, Tri-Arsenole163 |
| L. O. Compound No. 1 and No. 2 (Medical Supply Co.), Reports Council Pharm. & Chem., 1917, p. 156. |
Loeser’s Intravenous Solutions299 |
Loeser’s Intravenous Solution of Hexamethylenamin299 |
Loeser’s Intravenous Solution of Mercury Bichloride299 |
Loeser’s Intravenous Solution of Salicylate and Iodid299 |
Loeser’s Intravenous Solution of Sodium Iodid299 |
Loeser’s Intravenous Solution of Sodium Salicylate299 |
Loffler’s Intravenous Compound430 |
Lowenthal Postgraduate Course527 |
Lucas Laboratories’ Products440 |
| Lucas Laboratories’ Products (Lucas Laboratories, Inc.), The Journal, Sept. 20, 1919, p. 927. |
Luvein Arsans, Nos. 1, 2 and 3441 |
Luvein Arsans (Plain)440 |
| Luvein Arsans (Plain) (Lucas Laboratories, Inc.), The Journal, Sept. 20, 1919, p. 927. |
| Luvein Arsans, Nos. 1, 2 and 3 (Lucas Laboratories, Inc.), The Journal, Sept. 20, 1919, p. 927. |
Luvein Creosophite441 |
| Luvein Creosophite (Lucas Laboratories, Inc.), The Journal, Sept. 20, 1919, p. 927. |
Luvein Hexacol411 |
| Luvein Hexacol (Lucas Laboratories, Inc.), The Journal, Sept. 20, 1919, p. 927. |
Luytie’s Homeopathic Pharmacy—Succus Cineraria Maritima455 |
Lycetol214 |
| Lycetol (The Bayer Co., Inc.), Reports Council Pharm. & Chem., 1918, p. 70. |
| Lymph Compound R-H (New Animal Therapy Co.), The Journal, Dec. 14, 1912, p. 2176; Propaganda, ed. 9, p. 317. |
Lymphoid Compound (Lowenthal)528 |
| Lysoform (Lysoform Gesellschaft), The Journal, Nov. 21, 1914, p. 1870; Reports Council Pharm. & Chem., 1914, p. 126. |
| Lysoform, Crude (Lysoform Gesellschaft), The Journal, Nov. 21, 1914, p. 1870; Reports Council Pharm. & Chem., 1914, p. 126. |
| Lysol (Lehn & Fink), The Journal, Dec. 14, 1912, p. 2173; Reports Council Pharm. & Chem., 1912, p. 53; Propaganda, ed. 9, p. 318. |
Lysol and Laxol—the short and catchy proprietary name570 |
| M |
McArthur’s Syrup of Hypophosphites Comp. (Lime and Soda)84 |
| McKesson and Robbins— |
Cin-U-Form Lozenges237 |
Liquid Albolene106 |
| Maizavena (Wm. S. Merrell Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 44. |
| Maizo-Lithium (Henry Pharmacal Co.), The Journal, Feb. 6, 1915, p. 528; Reports Council Pharm. & Chem., 1915, p. 9; Reports Chem. Lab., 1914, p. 65; Propaganda, ed. 9, p. 198. |
| Malt Extract with Alteratives, Borcherdt’s (Borcherdt Malt Extract Co.), Reports Council Pharm. & Chem., 1918, p. 51. |
| Malt Extract with Pepsin and Pancreatin (Wm. S. Merrell Chemical Co.; Parke, Davis & Co.), The Journal, Feb. 9, 1907, p. 533. |
| Malt Extract with Yerba Santa, Borcherdt’s (Borcherdt Malt Extract Co.), Reports Council Pharm. & Chem., 1917, p. 138. |
Malt Olive, Borcherdt’s, with Hypophosphites84 |
| Malt Peptonates with Arsenic, Borcherdt’s (Borcherdt Malt Extract Co.), Reports Council Pharm. & Chem., 1917, p. 138. |
Malt-Diastase Company—Maltzyme with Hypophosphites84 |
Maltine with Olive Oil and Hypophosphites84 |
Maltzyme211 |
| Maltzyme (Maltzyme Company), Reports Council Pharm. & Chem., 1918, p. 67. |
Maltzyme Ferrated211 |
| Maltzyme Ferrated (Maltzyme Company), Reports Council Pharm. & Chem., 1918, p. 67. |
Maltzyme with Cascara Sagrada211 |
| Maltzyme with Cascara Sagrada (Maltzyme Company), Reports Council Pharm. & Chem., 1918, p. 67. |
Maltzyme with Cod Liver Oil211 |
| Maltzyme with Cod Liver Oil (Maltzyme Company), Reports Council Pharm. & Chem., 1918, p. 67. |
Maltzyme with Hypophosphites84 |
Maltzyme with Yerba Santa211 |
| Maltzyme with Yerba Santa (Maltzyme Company), Reports Council Pharm. & Chem., 1918, p. 67. |
| Mammary Gland, Reports Council Pharm. & Chem., 1921, p. 44. |
| Manaca, Reports Council Pharm. & Chem., 1912, p. 43. |
| Manaca and Salicylates Compound, Elixir (Hance Bros. & White; Wm. S. Merrell Chemical Co.; H. K. Mulford Co.; Nelson, Baker & Co.; Parke, Davis & Co.; Ray Chemical Co.; Smith, Kline & French Co.; Sharp & Dohme; F. Stearns & Co.; Truax, Greene & Co.), Reports Council Pharm. & Chem., 1912, p. 44. |
| Manaca and Salicylates Compound, Elixir (Sharp & Dohme), Reports Council Pharm. & Chem., 1912, p. 44. |
| Manacaline (Pullen-Richardson Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 44. |
| Manola (Manola Co.), The Journal, April 2, 1910, p. 1154; Reports Chem. Lab., 1910, p. 105; Propaganda, ed. 9, p. 323. |
| Marienbad Tablets (J. Sicke), The Journal, July 18, 1908, p. 237. |
Mark White Goiter Serum87 |
Mark White Iodinized Oil87 |
| Meat Extract, “Rex” Brand (Cudahy Packing Co.), The Journal, Jan. 23, 1909, p. 311; Propaganda, ed. 9, p. 472. |
| Meat Juice, Valentine’s (Valentine’s Meat Juice Co.), The Journal, Nov. 20, 1909, p. 1754; May 2, 1914, p. 1419; Reports Council Pharm. & Chem., 1909, p. 137; 1914, p. 14; Propaganda, ed. 9, pp. 123, 129, 472. |
Medeol Suppositories181 |
| Medeol Suppositories (Medeol Company, Inc.), The Journal, March 9, 1918, p. 719; Reports Council Pharm. & Chem., 1918, p. 7. |
Medical Society of the United States531 |
Medical Supply Company—Tri-Arsenole, L. O. Compound No. 1 and L. O. Compound No. 2163 |
| Med-O-Lin (Waverly Oil Works Co.), The Journal, July 10, 1915, p. 175; Reports Council Pharm. & Chem., 1915, p. 172. |
| Mellier Drug Company— |
Ponca Compound26 |
Tongaline26 |
Tongaline Tablets27 |
| Meningococcus Serum “Hoechst” (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
Mennen, House of—Kora-Konia92 |
| Merck and Company— |
Antithyroidin-Moebius202 |
Barbital-Sodium (Medinal or Veronal-Sodium)371 |
Diethylbarbituric Acid371 |
| Mercol, Howell’s (H. B. Howell & Co., Ltd.), The Journal, Jan. 16, 1909, p. 225; May 15, 1909, p. 1595; Propaganda, ed. 9, p. 326. |
| Mercuric Iodid, Red, (Mercury Biniodid) comparative symptoms resulting from use of |
several oily suspensions of123 |
| Mercury Biniodid, comparative symptoms resulting from use of several oily suspensions |
of red mercuric iodid123 |
| Mercury Sozoiodolate, The Journal, Feb. 13, 1909, p. 573; Reports Chem. Lab., 1909, p. 19. |
| Mercury Sozoiodolate Solution, The Journal, Feb. 13, 1909, p. 573; Reports Chem. Lab., 1909, p. 19. |
| Merrell, W. S., Chemical Co.— |
Casta-Flora118 |
Proteogens, 227445 |
Mervenol249 |
| Mervenol (Hille Laboratories), Reports Council Pharm. & Chem., 1919, p. 82. |
Methaform212 |
| Methaform (F. Stearns & Co.), Reports Council Pharm. & Chem., 1918, p. 68. |
Methyl-Phenol Serum (Cano)251 |
| Methyl-Phenol Serum (Cano) (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1919, p. 85. |
| Methyl-Santal (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1915, p. 173. |
| Metz, H. A., Laboratories, Inc.— |
Anesthesin276 |
Parathesin276 |
Phenetidyl-Acetphenetidin Hydrochlorid (Holocain Hydrochlorid)372 |
Procain-Novocain brand356 |
Micajah’s Suppositories241 |
| Micajah’s Suppositories (Micajah & Co.), The Journal, Nov. 29, 1919, p. 1715; Reports Council Pharm. & Chem., 1919, p. 49. |
Micajah’s Wafers241 |
| Micajah’s Wafers (Micajah & Co.), The Journal, Nov. 29, 1919, p. 1715; Reports Council Pharm. & Chem., 1919, p. 49. |
Micrococcus Catarrhalis-Combined-Bacterin184 |
| Micrococcus Catarrhalis-Combined-Bacterin (The Abbott Laboratories), The Journal, June 22, 1918, p. 1967; Reports Council Pharm. & Chem., 1918, p. 11. |
| Micrococcus Neoformans Vaccine, Reports Council Pharm. & Chem., 1917, p. 152. |
| Migrainin (Farbwerke-Hoechst Co.), The Journal, June 5, 1909, p. 1851; Reports Council Pharm. & Chem., 1909, p. 105; Propaganda, ed. 9, p. 135. |
Miller’s Iod-Izd-Oil49 |
Millikin’s Acetylsalicylic Acid347 |
Minson’s Soluble Iodin “Kelpidine”161 |
| Minson’s Soluble Iodin “Kelpidine” (J. J. Minson), Reports Council Pharm. & Chem., 1917, p. 152. |
| Mist. Helonin Comp. (Schlotterbeck & Foss), The Journal, Dec. 18, 1915, p. 2186. |
| Mitchella Compound (Dr. J. H. Dye Medical Institute), Reports Council Pharm. & Chem., 1912, p. 46. |
Mixed Vaccine No. 40, Sherman’s188 |
| Mixed Vaccine No. 40, Sherman’s (G. H. Sherman), The Journal, June 22, 1918, p. 1967; Reports Council Pharm. & Chem., 1918, p. 11. |
| Mon-Arsone (The Hammer Laboratories Co.), The Journal, Feb. 26, 1921, p. 595; June 18, 1921, p. 1781; Reports Chem. Labs., 1920, p. 67; Reports Council Pharm. & Chem., 1921, p. 47. |
Monsanto’s Acetylsalicylic Acid (Aspirin)347 |
Morphin Sulphate Hypodermic Tablets566 |
| Morphine Meconate, Reports Council Pharm. & Chem., 1919, p. 84. |
| Mother’s Cordial (Eli Lilly & Co.), The Journal, Aug. 31, 1912, p. 735; Reports Council Pharm. & Chem., 1912, p. 46; Propaganda, ed. 9, p. 410. |
| Mother’s Cordial (Ray Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Motherwort, Reports Council Pharm. & Chem., 1912, p. 44. |
| Mucol Powder (Mucol Co., Inc.), The Journal, Nov. 15, 1913, p. 1812; Reports Council Pharm. & Chem., 1913, p. 43; Propaganda, ed. 9, p. 329. |
Mulene332 |
| Mulene (Mulene Co.), The Journal, May 19, 1917, p. 1497. |
| Mulford, H. K., Company— |
Acetanilid Compound—Mulford206 |
Concentrated Solution Sodium Hypochlorite358 |
Granular Effervescent Bromide206 |
Influenza Serobacterin Mixed—Mulford187 |
Iron Citrate Green115 |
Mustard, Cream of218 |
| Muthol (Demuth’s Laboratories), The Journal, July 10, 1915, p. 175. |
Mystic Chemical Company—Olio-Phlogosis79 |
| N |
Naphey’s Medicated Uterine Wafers107 |
| Narkine (The Tilden Co.), The Journal, Oct. 24, 1908 p. 1439; Propaganda, ed. 9, p. 329. |
National Bio-Chemical Laboratory (Therapeutic Leaves)458 |
| National Drug Company— |
Brom-I-Phos136 |
“National Iodine Solution”300 |
National Formulary—a review of the fourth edition535 |
“National Iodine Solution”300 |
| National Iodine Solution (National Drug Co.), The Journal, June 4, 1921, p. 1592; Reports Council Pharm. & Chem., 1921, p. 50. |
| National Laboratories of Pittsburgh— |
Anti-Tuberculous Lymph Compound (Sweeny)266 |
National Medical University410 |
| Natrium Compound, Pulvoids. See Pulvoids Natrium Compound. |
Natura Company—“Akoz”328 |
| Nazol (Nazol Antiseptic Co.), Reports Council Pharm. & Chem., 1918, p. 81. |
| Neisser Serobacterin Mixed (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1916, p. 67. |
Nelson, Baker & Co.—Eupeptic Hypophosphites83 |
| Nephritin (Reed & Carnrick Co.), The Journal, Oct. 5, 1907, p. 1198; Reports Council Pharm. & Chem., 1905–8, p. 79. |
| Nephroson (Wm. S. Merrell Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 39. |
| Nerve Vitalizer, Wheeler’s (J. W. Brant Co., Ltd.), The Journal, April 11, 1908, p. 1206; Reports Chem. Lab., to 1909, p. 66; Propaganda, ed. 9, p. 411. |
Neuralgic Pills566 |
| Neurilla (Dad Chemical Co.), The Journal, March 27, 1915, p. 1093; Reports Council Pharm. & Chem., 1915, p. 20; Propaganda, ed. 9, p. 136. |
| Neurocaine (Schieffelin & Co.), Reports Council Pharm. & Chem., 1915, p. 173. |
Neuro-Lecithin53 |
| Neuro-Lecithin-Abbott (The Abbott Laboratories), Reports Council Pharm. & Chem., 1915, p. 122. |
Neuro Phosphates, Eskay’s146 |
| Neuro Phosphates, Eskay’s (Smith, Kline & French Co.), The Journal, Sept., 29, 1917, p. 1102; Reports Council Pharm. & Chem., 1917, p. 52. |
| Neurosine (Dios Chemical Co.), The Journal, Jan. 9, 1915, p. 165; April 27, 1918, p. 1251; Reports Council Pharm. & Chem., 1914, p. 86; Propaganda, ed. 9, p. 139. |
Neurosine and the original package evil404 |
| New York Intravenous Laboratory— |
Arseno-Meth-Hyd231 |
Loeser’s Intravenous Solutions299 |
New York Pharmacal Association—Lactopeptine and Elixir Lactopeptine43 |
“Nikalgin”467 |
| Noitol (Wheeler Chemical Works), The Journal, May 21, 1910, p. 1704; Reports Chem. Lab., 1910, p. 45; Propaganda, ed. 9, p. 245. |
| Normal Horse Serum, Sterile (National Vaccine and Antitoxin Institute), Reports Council Pharm. & Chem., 1921, p. 53. |
Normal Phenol Serum (Cano)251 |
| Normal Phenol Serum (Cano) (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1919, p. 85. |
| Noron (The Heron Company), Reports Council Pharm. & Chem., 1921, p. 53. |
Norwich Pharmacal Company—Alfatone28 |
| Nose-Ions (Nose-Ions Company), The Journal, Dec. 4, 1915, p. 2026; Reports Chem. Lab., 1915, p. 123. |
Nostrums in retrospect379 |
Nostrums, Shotgun398 |
| Nourry Wine (E. Fougera & Co., Inc.), The Journal, Dec. 12, 1914, p. 2150; Reports Council Pharm. & Chem., 1914, p. 74; Propaganda, ed. 9, p. 115. |
Novocain, Procain375 |
Novocaine355 |
| Nuclein, Reports Council Pharm. & Chem., 1921, p. 54. |
Nuclein, Triple Arsenates with256 |
| Nucleinic Acid, Reports Council Pharm. & Chem., 1921, p. 54. |
Nujol108 |
| Nujol (Standard Oil Co. of New Jersey), The Journal, July 10, 1915, p. 175; Reports Council Pharm. & Chem., 1916, p. 68. |
Number “3”, 244247 |
| Number “3” (Chlorine Products Company, Inc.), Reports Council Pharm. & Chem., 1919, p. 70. |
Nutone162 |
| NuTone (NuTone Company), Reports Council Pharm. & Chem., 1917, p. 154. |
| Nutrient Wine of Beef Peptone (Armour & Co.), The Journal, May 11, 1907, p. 1612; Reports Council Pharm. & Chem., 1905–8, opp. p. 64; Propaganda, ed. 9, p. 133. |
| Nutritive Liquid Peptone (Parke, Davis & Co.), The Journal, May 11, 1907, p. 1612; Reports Council Pharm. & Chem.,1905–8, opp. p. 64; Propaganda, ed. 9, p. 133. |
Nutrolactis131 |
| Nutrolactis (The Nutrolactis Co.), The Journal, May 26, 1917, p. 1570; Reports Council Pharm. & Chem., 1917, p. 24. |
| Nuxated Iron (Dae Health Laboratories), The Journal, Oct. 21, 1916, p. 1244; Oct. 28, 1916, p. 1309; Nov. 4, 1916, p. 1376; Feb. 24, 1917, p. 642; Reports Chem. Lab., 1916, p. 29. |
| O |
| Oats, Reports Council Pharm. & Chem., 1912, p. 44. |
Ogden, Willard Ealon, and his cure for piles538 |
Ohio State Department of Health protests use of name in advertisements of Proteogens446 |
Ohmann-Dumesnil, A. H.531 |
Oleum Iodine, B. oleum iodine198 |
Olio-Phlogosis79 |
| Olio-Phlogosis (Mystic Chemical Company), The Journal, Aug. 19, 1916, p. 631; Reports Council Pharm. & Chem., 1916, p. 10. |
Olive Oil and Hypophosphites, Maltine with84 |
Ophthalmol-Lindemann189 |
| Ophthalmol-Lindemann (Innis, Speiden & Co.), The Journal, July 6, 1918, p. 59; Reports Council Pharm. & Chem., 1918, p. 21. |
| Orchitic Fluid Tablets (New Animal Therapy Co.), The Journal, Dec. 14, 1912, p. 2176; Propaganda, ed. 9, p. 317. |
| Orsudan (Burroughs Wellcome & Co.), The Journal, April 16, 1910, p. 1323. |
Oscilloclast472 |
| Osmium Tetroxide, Reports Council Pharm. & Chem., 1921, p. 55. |
Ottofy, L. M., and The Allied Medical Associations of America487 |
Ova Mammoid (Lowenthal)528 |
| Oxychlorine (Oxychlorine Co.), The Journal, July 6, 1907, p. 54; Reports Council Pharm. & Chem., 1905–8, p. 68; Propaganda, ed. 9, p. 147. |
| Oxydendron Compound, Fluidextract (Nelson, Baker & Co.), Reports Council Pharm. & Chem., 1912, p. 43. |
Oxygen Water, Aquazone290 |
Oxyl-Iodide304 |
| Oxyl-Iodide (Eli Lilly and Co.), The Journal, July 2, 1921, p. 57; Reports Council Pharm. & Chem., 1921, p. 56. |
| Oxyntin (Fairchild Bros. and Foster), Reports Council Pharm. & Chem., 1915, p. 174. |
| P |
| Palmetto Compound (Wm. S. Merrell Chem. Co.), Reports Council Pharm. & Chem., 1912, p. 44. |
| Palpebrine (Dios Chemical Co.), The Journal, Jan. 9, 1915, p. 167; Reports Council Pharm. & Chem., 1914, p. 86; Propaganda, ed. 9, p. 139. |
| Pam-ala (Pam-Ala Co.), The Journal, Feb. 28, 1914, p. 715; Propaganda, ed. 9, p. 149. |
Pancreas Comp., Caps219 |
| Pancreopepsin, Liquid (Wm. R. Warner & Co.), The Journal, Feb. 9, 1907, p. 533. |
| Pan-peptic Elixir (Sharp & Dohme), The Journal, Feb. 9, 1907, p. 533. |
| Papain, The Journal, Feb. 9, 1907, p. 522. |
| Pa-pay-ans, Bell (Bell & Co.): See Bell-ans. |
| Papine (Battle & Co.), The Journal, April 29, 1911, p. 1278; Reports Chem. Lab., 1911, p. 82; Propaganda, ed. 9, p. 330. |
| Para Coto, Reports Council Pharm. & Chem., 1913, p. 39. |
| Paracotoin, Reports Council Pharm. & Chem., 1913, p. 39. |
Paraffin Films330 |
Paraffin Films, formula for333 |
Paraffin, Stanolind Liquid214 |
Paraffins and paraffin preparations, examination of334 |
Parathesin276 |
| Parathesin (H. A. Metz Laboratories, Inc.), The Journal, Nov. 13, 1920, p. 1358; Reports Council Pharm. & Chem., 1920, p. 27. |
| Parke, Davis & Company— |
Apothesine260 |
Corpora Lutea (Soluble Extract)128 |
Phylacogens441 |
Pituitary Extract550 |
Pituitrin550 |
Ricord Pills468 |
Silvol189 |
Therapeutic Notes550 |
Thyreoidectin202 |
Parresine332 |
| Pasadyne (John B. Daniel), The Journal, March 8, 1913, p. 766; Propaganda, ed. 9, p. 332. |
| Pas-Avena (Pas-Avena Chemical Company), The Journal, March 7, 1908, p. 783; Reports Chem. Lab., to 1909, p. 69; Propaganda, ed. 9, p. 333. |
| Pasiflora, The Journal, March 19, 1910, p. 983; Reports Council Pharm. & Chem., 1912, p. 38; Propaganda, ed. 9, p. 156. |
| Pasiflora Incarnata, Daniel’s Concentrated Tincture of (John B. Daniel), The Journal, March 19, 1910, p. 983; Reports Council Pharm. & Chem., 1910, p. 44; 1912, p. 38; Propaganda, ed. 9, p. 156. |
Pasteur Chemical Company—Thermozine334 |
Patent-Law Revision, need for177 |
Patent Laws and Patent Office Practice542 |
Patent Laws, archaic543 |
Patent medicine, physicians not against patent medicine but for protection of public513 |
Patenting therapeutic agents544 |
Patents perpetuated by trade names544 |
| Pautauberge’s Solution (Geo. J. Wallau, Inc.), The Journal, March 7, 1910, p. 1560. |
Peacock’s Bromides400 |
| Pepsin and Pancreatin Compound, Elixir (Eli Lilly & Co.), The Journal, Feb. 6, 1907, p. 533. |
| Pepsin and Pancreatin Compound, Tablets (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Pepsin and Pancreatin, Elixir (Eli Lilly & Co.; Sharp & Dohme; Smith, Kline & French Co.; F. Stearns & Co.; Wm. R. Warner & Co.), The Journal, Feb. 9, 1907, p. 533. |
| Pepsin and Pancreatin with Caffein, Elixir (Eli Lilly & Co.; Parke, Davis & Co.), The Journal, Feb. 9, 1907, p. 533. |
| Pepsin, Bismuth and Pancreatin, Elixir (Parke, Davis & Co.; Sharp & Dohme; Smith, Kline & French Co.; F. Stearns & Co.), The Journal, Feb. 9, 1907, p. 533. |
| Pepsin, Bismuth, Strychnin and Pancreatin, Elixir (Parke, Davis & Co.), The Journal, Feb. 9, 1907, p. 533. |
| Pepsin, Elixir Lactated (H. K. Mulford Co.; Parke, Davis & Co.; F. Stearns & Co.), The Journal, Feb. 9, 1907, p. 533. |
| Pepsin, Pancreatin and Bismuth, Elixir (Eli Lilly & Co.), The Journal, Feb. 9, 1907, p. 533. |
| Pepsin, Pancreatin, Bismuth and Strychnin, Elixir (Eli Lilly & Co.), The Journal, Feb. 9, 1907, p. 533. |
| Pepsin, Strychnin, Bismuth and Pancreatin, Elixir (Sharp & Dohme), The Journal, Feb. 9, 1907, p. 533. |
| Peptenzyme, Elixir (Reed & Carnrick Co.), The Journal, Feb. 9, 1907, p. 533; Oct. 5, 1907, p. 1198; Reports Council Pharm. & Chem., 1905–8, p. 79. |
| Peptenzyme Powder (Reed & Carnrick Co.), The Journal, Oct. 5, 1907, p. 1198; Reports Council Pharm. & Chem., 1905–8, p. 79. |
| Peptic Digestant (Columbus Pharmacal Co.), The Journal, Feb. 9, 1907, p. 533. |
| Peptic Essence Comp., Peters’ (Arthur Peters & Co.), The Journal, Feb. 9, 1907, p. 533. |
Pepto-Mangan387 |
| Pepto-Mangan (M. J. Breitenbach Co.), The Journal, Sept. 23, 1905, p. 934; April 6, 1907, p. 1197; Dec. 29, 1917, p. 2202; Reports Council Pharm. & Chem., 1914, p. 121; Propaganda, ed. 9, p. 159. |
| Peptone, Reports Council Pharm. & Chem., 1913, p. 41. |
| Peptonic Elixir (Wm. S. Merrell Chemical Co.), The Journal, May 11, 1907, p. 1612; Reports Council Pharm. & Chem., 1905–8, opp. p. 64; Propaganda, ed. 9, p. 133. |
| Perfection Liquid Food (Perfection Liquid Food Co.), Reports Council Pharm. & Chem., 1913, p. 44; Reports Chem. Lab., 1913, p. 80. |
Pertussin467 |
| Pertussin (Lehn & Fink), The Journal, March 8, 1913, p. 766; Propaganda, ed. 9, p. 334. |
Pertussis-Combined-Bacterin185 |
| Pertussis-Combined-Bacterin (The Abbott Laboratories), The Journal, June 22, 1918, p. 1967; Reports Council Pharm. & Chem., 1918, p. 11. |
| Peter, Arthur, & Co.—Syrupus Roborans (Syrup Hypophosphites Comp. with Quinin, |
Strychnin and Manganese)82 |
Petrolatum, Liquid526 |
Petrolatum, Liquid, Iodin in367 |
Petrolatum, Liquid, proprietary names for55 |
Petrolatum, liquid, solubility of Iodin in344 |
Pharmaceutical Barnums545 |
Pharmacopeia, the Ninth Decennial Revision546 |
| Phecolates (F. Waldo Whitney), The Journal, Nov. 21, 1914, p. 1870; Reports Council Pharm. & Chem., 1914, p. 127; Propaganda, ed. 9, p. 174. |
| Phecolax (F. Waldo Whitney), The Journal, Nov. 21, 1914, p. 1870; Reports Council Pharm. & Chem., 1914, p. 127; Propaganda, ed. 9, p. 174. |
| Phecotones (F. Waldo Whitney), The Journal, Nov. 21, 1914, p. 1870; Reports Council Pharm. & Chem., 1914, p. 127; Propaganda, ed. 9, p. 174. |
| Phecozymes (F. Waldo Whitney), The Journal, Nov. 21, 1914, p. 1870; Reports Council Pharm. & Chem., 1914, p. 127; Propaganda, ed. 9, p. 174. |
| Phenalein (Pax Chemical Co.), The Journal, April 30, 1910, p. 1458; Propaganda, ed. 9, p. 344. |
Phenalgin393 |
| Phenalgin (Etna Chemical Co.), The Journal, June 3, 1905, p. 1791; Jan. 27, 1912, p. 293; Feb. 8, 1918, p. 337; Reports Council Pharm. & Chem., 1905–8, p. 8; Propaganda, ed. 9, pp. 10, 335. |
Phenetidyl-Acetphenetidin Hydrochlorid (Holocain Hydrochlorid)372 |
| Pheno-Bromate (Pheno-Bromate Co.), The Journal, July 14, 1906, p. 125; April 18, 1908, p. 1282; Propaganda, ed. 9, p. 343. |
Phenol, Methyl-Phenol Serum (Cano)251 |
Phenol Serum, Normal (Cano)251 |
| Phenol Sodique (Hance Bros. & White), The Journal, Nov. 9, 1907, p. 1617; Reports Council Pharm. & Chem., 1905–8, p. 99; Propaganda, ed. 9, p. 175. |
| Phenolax Wafers (Upjohn Co.), The Journal, April 30, 1910, p. 1458; Propaganda, ed. 9, p. 344. |
| Phenolphthalein Laxative (El Zernac Co.), The Journal, April 30, 1910, p. 1458; Propaganda, ed. 9, p. 344. |
Phenylcinchoninic Acid, U. S. P.373 |
Phillips’ Phospho-Muriate of Quinine Comp.197 |
Phosphoglycerate of Lime (Chapoteaut)95 |
| Phosphoglycerate of Lime (Chapoteaut) (E. Fougera and Co., Inc.), The Journal, Sept. 30, 1916, p. 1034; Reports Council Pharm. & Chem., 1916, p. 35. |
Phospho-Muriate of Quinine Comp., Phillips’197 |
| Phospho-Muriate of Quinine Comp.-Phillips (Charles H. Phillips Chemical Co.), The Journal, Oct. 19, 1918, p. 1335; Reports Council Pharm. & Chem., 1918, p. 32. |
Phosphorcin Compound94 |
| Phosphorcin Compound (Organic Products Company), The Journal, Sept. 30, 1916, p. 1033; Reports Council Pharm. & Chem., 1916, p. 34. |
| Phosphorus, Amorphous, The Journal, March 7, 1914, p. 793; March 28, 1914, p. 1033; Propaganda, ed. 9, p. 478. |
| Phosphorus, Amorphous, Pill, S. & D. (Sharp & Dohme), The Journal, March 7, 1914, p. 793; March 28, 1914, p. 1033; Propaganda, ed. 9, 478. |
Phosphorus Compound, Iron and Arsenic, Hypodermic Solution No. 13275 |
| Phosphorus Tonic, Compound, Dowd’s (The Richardson Company), The Journal, Dec. 20, 1913, p. 2258; Propaganda, ed. 9, p. 476. |
| Phospho-Vanadiol (Vanadium Chemical Co.), The Journal, Jan. 18, 1913, p. 225; Reports Council Pharm. & Chem., 1913, p. 7; Propaganda, ed. 9, p. 209. |
Phylacogens441 |
| Phylacogens (Parke, Davis & Co.), The Journal, Feb. 1, 1913, p. 384; Feb. 22, 1913, pp. 602, 615; March 15, 1913, p. 849; Aug. 29, 1914, p. 785; Nov. 15, 1919, p. 1542; Propaganda, ed. 9, p. 346. |
Physicians’ Drug Syndicate—Thymozene432 |
Physician’s League of Illinois529 |
Physician’s stock in prescription products548 |
| Phytin (A. Klipstein & Co.), The Journal, Jan. 30, 1915, p. 456; Reports Council Pharm. & Chem., 1915, p. 131; Propaganda, ed. 9, p. 178. |
Pil. Cascara Compound-Robins117 |
| Pil. Cascara Comp.-Robins (A. H. Robins Co.), The Journal, Jan. 27, 1917, p. 303; Reports Council Pharm. & Chem., 1916, p. 47. |
Pil. Mixed Treatment (Chichester)310 |
| Pil. Mixed Treatment (Chichester) (Hillside Chemical Co.), The Journal, Oct. 22, 1921, p. 1355; Reports Council Pharm. & Chem., 1921, p. 60. |
| Pineal Gland-Armour, Desiccated (Armour & Co.), Reports Council Pharm. & Chem., 1918, p. 69. |
Pineal Gland, Desiccated, Armour213 |
| Pineal Gland, Reports Council Pharm. & Chem., 1918, p. 69. |
Pineal Gland Tablets—Armour213 |
Pineoleum advertising methods442 |
| Pineoleum (The Pineoleum Company), The Journal, Nov. 1, 1919, p. 1380. |
| Pinus Canadensis, Kennedy’s, Dark: See Darpin. |
Piperazine214 |
| Piperazine (The Bayer Co., Inc.), Reports Council Pharm. & Chem., 1918, p. 70. |
| Piperazine Water (Lehn & Fink), The Journal, Feb. 29, 1908, p. 704. |
Pituitary Extract in labor550 |
Pituitary Gland Preparations549 |
Pituitrin550 |
| Pix Cresol (Pix Cresol Chemical Co.), The Journal, June 10, 1911, p. 1738; Reports Chem. Lab., 1911, p. 37; Propaganda, ed. 9, p. 247. |
Placento-Mammary Comp., Caps219 |
Plantex, Proteogen No. 1 (Plantex) for cancer227 |
| Plasmon (Plasmon Milk Products Co.), Reports Chem. Lab., 1914, p. 88. |
Platt’s Chlorides263 |
| Platt’s Chlorides (Henry B. Platt), The Journal, March 27, 1920, p. 903; Reports Chem. Lab., 1920, p. 28; Reports Council Pharm. & Chem., 1920, p. 8. |
| Plurasav, Young’s (The Plurasav Co., Columbus, Ohio), Reports Council Pharm. & Chem., 1918, p. 82. |
“Pluriglandular” mixtures218 |
| Pluto Spring Water, Concentrated (French Lick Springs Hotel Co.), The Journal, March 29, 1913, p. 1013. |
Pneumonia, camphor in257 |
Pneumo-Staph-Strep. Bacterin, Special Bacterial Vaccine No. 3254 |
Pneumo-Staph-Strep-Coli Bacterin, Special Bacterial Vaccine No. 4254 |
Pneumo-Strep. Bacterin, Special Bacterial Vaccine No. 11254 |
| Pneumo-Strep-Serum (H. K. Mulford Co.), The Journal, Jan. 31, 1920, p. 342. |
| Pollantin, Fall (Fritzsche Bros.), Reports Council Pharm. & Chem., 1916, p. 69. |
| Pollantin Powder, Fall (Fritzsche Bros.), Reports Council Pharm. & Chem., 1916, p. 69. |
| Pollen Antigen (Lederle Antitoxin Laboratories), Reports Council Pharm. & Chem., 1918, p. 65. |
| Pollen Antigen-Lederle (Fall Type) (Lederle Antitoxin Laboratories), Reports Council Pharm. & Chem., 1921, p. 45. |
| Pollen Antigen-Lederle (Spring Type) (Lederle Antitoxin Laboratories), Reports Council Pharm. & Chem., 1921, p. 45. |
Ponca Compound28 |
| Ponca Compound (Mellier Drug Co.), The Journal, July 17, 1915, p. 269; Reports Council Pharm. & Chem., 1912, p. 46; 1914, p. 58. |
| Poslam (Emergency Laboratories), The Journal, May 22, 1909, p. 1678; Reports Chem. Lab., 1909, p. 25. |
| Potassium Iodo-Resorcin Sulphonate, The Journal, Feb. 11, 1911, p. 441; Reports Chem. Lab., 1911, p. 21. |
Prepared Green Bone, Russell134 |
Prescription products, physician’s stock in548 |
| Probilin Pills (Schering & Glatz, Inc.), The Journal, Aug. 24, 1907, p. 702; Nov. 2, 1907, p. 1541; Reports Council Pharm. & Chem., 1905–8, p. 70; Propaganda, ed. 9, p. 344. |
Procain (Calco)377 |
Procain (Metz)377 |
Procain (Novocain)375 |
Procain-Novocain brand356 |
Procain, standardization of, and examination of market supply355 |
Proprietaries, why they flourish552 |
Proprietary medicine, secret remedies and the principles of ethics571 |
Prostoid (Lowenthal)528 |
Proteal Therapy494 |
“Proteal Therapy” and Henry Smith Williams443 |
| Proteals (Henry Smith Williams), The Journal, July 6, 1918, p. 58. |
Protein Therapy, Nonspecific536 |
Proteogen No. 1 (Plantex) for cancer227 |
Proteogen No. 2 for rheumatism227 |
Proteogen No. 3 for tuberculosis227 |
Proteogen No. 4 for hay fever and bronchial asthma227 |
Proteogen No. 5 for dermatoses227 |
Proteogen No. 6 for chlorosis227 |
Proteogen No. 7 for secondary anemia227 |
Proteogen No. 8 for pernicious anemia227 |
Proteogen No. 9 for goitre227 |
Proteogen No. 10 for syphilis227 |
Proteogen No. 11 for gonorrhea227 |
Proteogen No. 12 for influenza and pneumonia227 |
| Proteogens (Wm. S. Merrell Co.), The Journal, July 12, 1919, pp. 109 and 128; July 26, 1919, p. 288; Sept. 6, 1919, p. 781; Sept. 27, 1919, p. 1000; Oct. 11, 1919, p. 1152; Reports Council Pharm. & Chem., 1919, p. 20. |
Proteogens, alleged endorsement repudiated446 |
Proteogens, Dr. Broeman’s final report on450 |
Protonuclein59 |
| Protonuclein (Reed & Carnrick Co.), The Journal, Oct. 5, 1907, p. 1198; Oct. 24, 1908, p. 1388; Jan. 1, 1916, p. 48; Reports Council Pharm. & chem., 1905–8, p. 79; Reports Council Pharm. & Chem., 1915, p. 90; Propaganda, ed. 9, p. 348. |
Protonuclein Beta59 |
| Protonuclein Beta (Reed & Carnrick Co.), The Journal, Jan. 1, 1916, p. 48; Reports Council Pharm. & Chem., 1915, p. 90. |
| Prunoids (Sultan Drug Co.), The Journal, April 30, 1910, p. 1458; Jan. 2, 1915, p. 71; Reports Council Pharm. & Chem., 1914, p. 133; Reports Chem. Lab., 1914, p. 63; Propaganda, ed. 9, pp. 178, 344. |
| Psora (Pix Cresol Co.), Reports Council Pharm. & Chem., 1912, p. 39. |
Pulvane450 |
Pulvoids, Calcylates85 |
| Pulvoids, Calcylates (Drug Products Company), The Journal, Sept. 9, 1916, p. 827; Reports Council Pharm. & Chem., 1916, p. 18. |
Pulvoids Calcylates Compound226 |
| Pulvoids Calcylates Compound (The Drug Products Co., Inc.), The Journal, June 14, 1919, p. 1784; Reports Council Pharm. & Chem., 1919, p. 19. |
Pulvoids Natrium Compound108 |
| Pulvoids Natrium Compound (Drug Products Company, Inc.), Reports Council Pharm. & Chem., 1916, p. 69. |
Purdue Frederick Company—Gray’s Glycerine Tonic24 |
| Purgen (Lehn & Fink), The Journal, Jan. 5, 1907, p. 64; Sept. 14, 1907, p. 954; Propaganda, ed. 9, p. 349. |
| Pyo-atoxin (H. O. Hurley), The Journal, Feb. 14, 1914, p. 552; Reports Chem. Lab., 1914, p. 32; Propaganda, ed. 9, p. 350. |
| Pyocyaneus Bacillus Vaccine, The Journal, May 18, 1918, p. 1486; Reports Council Pharm. & Chem., 1918, p. 11. |
| Pyxol (Barrett Mfg. Co.), The Journal, Feb. 23, 1918, p. 559. |
| Q |
Quartonol Tablets94 |
| Quartonol Tablets (Schering and Glatz, Inc.), The Journal, Sept. 30, 1916, p. 1033; Reports Council Pharm. & Chem., 1916, p. 34. |
Quassia Compound Tablets306 |
| Quassia Compound Tablets (Flint, Eaton and Company), The Journal, July 9, 1921, p. 141; Reports Council Pharm. & Chem., 1921, p. 64. |
| Queen of the Meadow, Reports Council Pharm. & Chem., 1912, p. 45. |
| Quina LaRoche (E. Fougera & Co., Inc.), The Journal, March 21, 1908, p. 978. |
Quinin and Urea Hydrochlorid467 |
| Quinin Arsenate, The Journal, July 16, 1910, p. 325; Reports Council Pharm. & Chem., 1910, p. 73. |
| Quinin Glycerophosphate, Reports Chem. Lab., 1912, p. 107. |
Quinin Laxative Tablets566 |
Quinine Comp., Phillips’ Phospho-Muriate of197 |
| R |
Rabbit-Foot Therapy571 |
| Radelium and Radelium Generator (Radio-Active Water Company), Reports Council Pharm. & Chem., 1915, p. 128. |
Radio-Rem79 |
| Radio-Rem Outfit No. 2, No. 3 (Schieffelin & Co.), The Journal, Aug. 19, 1916, p. 631; Reports Council Pharm. & Chem., 1916, p. 9. |
| Radio-Rem Outfit C (Schieffelin & Co.), The Journal, Aug. 19, 1916, p. 631; Reports Council Pharm. & Chem., 1916, p. 9. |
| Radium Solution for Intravenous Use, Standard (Radium Chemical Co.), The Journal, June 26, 1915, p. 2156; Reports Council Pharm. & Chem., 1915, p. 147. |
Rahtjen, Philip, and his discoveries553 |
Rainey, James M.528 |
| Rattlesnake-Venom, The Journal, March 15, 1913, p. 850; March 29, 1913, p. 1001; June 7, 1913, p. 1811. |
| Rector Chemical Company— |
Barbital371 |
Procain-Rector, 356377 |
Red Bone-Marrow—Armour, Extract of213 |
| Red Bone-Marrow, Reports Council Pharm. & Chem., 1918, p. 69. |
| Red Bone-Marrow—Armour, Extract of (Armour & Co.), Reports Council Pharm. & Chem., 1918, p. 69. |
| Red Mercuric Iodid (Mercury Biniodid) comparative symptoms resulting from use of |
several oily suspensions of123 |
| Redintol (Johnson & Johnson), The Journal, July 28, 1917, p. 306. |
Reed and Carnrick—Protonuclein and Protonuclein Beta59 |
Reinschild Chemical Company—Iodo-Mangan106 |
| Resinol (Resinol Chemical Co.), The Journal, Nov. 6, 1909, p. 1578; Propaganda, ed. 9, p. 352. |
| Resor-Bisnol (Resor-Bisnol Chemical Co.), The Journal, June 1, 1912, p. 1706; Reports Chem. Lab., 1912, p. 85; Propaganda, ed. 9, p. 353. |
| Respirazone (The Tilden Company), The Journal, June 14, 1913, p. 1899. |
Restorative Capsules425 |
Rheumalgine23 |
| Rheumalgine (Eli Lilly & Co.), The Journal, June 26, 1915, p. 2156; Reports Council Pharm. & Chem., 1915, p. 148. |
Rheumatic and Gout Pills, De Sanctis’363 |
| Rheumatic Bacterin (Mixed), (No. 47), Swan’s (Swan-Myers Co.), The Journal, Nov. 6, 1915, p. 1662; Reports Council Pharm. & Chem., 1915, p. 160. |
| Rheume Olum (Rheumeolum Chemical Co.), The Journal, March 17, 1917, p. 865; Reports Council Pharm. & Chem., 1917, p. 19. |
| Ricinol-Grape Tape-Worm Remedy (Grape Capsule Co.), Reports Council Pharm. & Chem., 1915, p. 174. |
Ricord Pills and house organ therapeutics.468 |
Riedel and Company, Inc.—Gonosan150 |
Robinol95 |
| Robinol (John Wyeth & Bro.), The Journal, July 6, 1914, p. 49; Sept. 30, 1916, p. 1034; Reports Council Pharm. & Chem., 1916, p. 34; Propaganda, ed. 9, p. 353. |
| Robins, A. H., Company— |
Campetrodin and Campetrodin No. 2193 |
Pil, Cascara Compound—Robins117 |
| Robinson-Pettet Company— |
Robinson’s Hypophosphites83 |
Saloform110 |
Robinson’s “Cure” for Cancer—Tekarkin458 |
Robinson’s Hypophosphites83 |
Roborans, Syrupus, (Syrup Hypophosphites Comp. with Quinin, Strychnin and Manganese)82 |
Rogers, L. D.—Auto-Hemic Serum409 |
Rogers, R. R., Chemical Company—Unctol166 |
Royal Thermophor Sales Co.—Thermor Waterless Hot Bottle329 |
Russell Emulsion134 |
| Russell Emulsion (The Standard Emulsion Co.), The Journal, June 23, 1917, p. 1931; Reports Council Pharm. & Chem., 1917, p. 29. |
Russell Prepared Green Bone134 |
| Russell Prepared Green Bone (The Standard Emulsion Co.), The Journal, June 23, 1917, p. 1931; Reports Council Pharm. & Chem., 1917, p. 29. |
| S |
| Sal-Codeia, Bell (Bell & Co.), The Journal, Nov. 4, 1905, p. 1422; Propaganda, ed. 9, p. 357. |
Sal Hepatica451 |
| Sal Hepatica (Bristol-Myers Co.), The Journal, March 26, 1910, p. 1071; Feb. 7, 1914, p. 427; April 12, 1919, p. 1078; Oct. 29, 1921, p. 1438; Reports Council Pharm. & Chem., 1914, p. 7; Propaganda, ed. 9, p. 179. |
| Sal Hyl (New York Salesthyl Corporation), The Journal, Feb. 20, 1915, p. 684; Reports Council Pharm. & Chem., 1915, p. 134. |
| Salacetin (Bell & Co.), The Journal, June 3, 1905, p. 1791; Reports Council Pharm. & Chem., 1905–8, p. 8; Propaganda, ed. 9, p. 152. |
| Salen (A. Klipstein & Co.), Reports Council Pharm. & Chem., 1917, p. 155. |
| Salenal (A. Klipstein & Co.), Reports Council Pharm. & Chem., 1917, p. 155. |
| Salesthyl (New York Salesthyl Corporation), The Journal, Feb. 20, 1915, p. 684; Reports Council Pharm. & Chem., 1915, p. 134. |
Salicon453 |
| Salicylic Acid, “Natural,” The Journal, Sept. 20, 1913, p. 979; Reports Council Pharm. & Chem., 1913, p. 23. |
| Saliodin (Saliodin Chemical Co.), The Journal, Oct. 26, 1907, p. 1453; Reports Council Pharm. & Chem., 1905–8, p. 95; Propaganda, ed. 9, p. 249. |
| Salit (Heyden Chemical Works), The Journal, June 5, 1909, p. 1852; Reports Council Pharm. & Chem., 1909, p. 106. |
Saloform110 |
| Saloform (Robinson-Pettet Company), Reports Council Pharm. & Chem., 1916, p. 71; Reports Chem. Lab., 1916, p. 36. |
Salol Tablets566 |
Salvarsan: abrogate the patent493 |
| Sanatogen (Bauer Chemical Co.), The Journal, April 20, 1912, p. 1216; Dec. 6, 1913, p. 2085; March 28, 1914, p. 1035; Sept. 26, 1914, p. 1127; Reports Chem. Lab., 1912, p. 71; Propaganda, ed. 9, pp. 358, 378, 385. |
de Sandfort’s Ambrine330 |
| Sanmetto (The Od Chemical Co.), The Journal, July 8, 1905, p. 116; March 13, 1915, p. 926; Reports Council Pharm. & Chem., 1915, p. 17; Propaganda, ed. 9, p. 182. |
| Sanol (Expurgo Mfg. Co.),—See Expurgo Anti-Diabetes. |
Santaiva, S. & D. Liquor211 |
| Santal Midy Capsules (E. Fougera & Co.), The Journal, Oct. 9, 1920, p. 1016. |
| Saphanol Aromatic (Saphanol Products Co.), Reports Council Pharm. & Chem., 1921, p. 66. |
| Saphanol Concentrate (Saphanol Products Co.), Reports Council Pharm. & Chem., 1921, p. 66. |
| Schering and Glatz, Inc.— |
Arhovin243 |
Atophan313 |
Cinchophen (Phenylcinchoninic Acid, U. S. P.; Atophan)373 |
Medinal239 |
Tonols (Schering’s Glycerophosphates)94 |
Urotropin316 |
| Schieffelin and Company— |
Colalin203 |
Estivin466 |
Radio-Rem79 |
| Schlotterbeck’s Solution Hypophosphites of Lime and Soda (Liq. Hypophosphitum, |
Schlotterbeck’s)83 |
Schmidt’s Antimeristem408 |
Schoonmaker Laboratories, Inc.—V-E-M166 |
| Scopolamin-Morphin Mixtures, The Journal, Feb. 5, 1910, p. 446; Feb. 12, 1910, p. 516; June 7, 1913, p. 1814; Reports Council Pharm. & Chem., 1910, p. 11. |
Scott Iodinized Emulsion192 |
| Scullcap Compound, Fluidextract (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 43. |
| Secretin-Beveridge (James Wallace Beveridge), The Journal, Jan. 12, 1918, p. 116; Reports Council Pharm. & Chem., 1917, p. 120. |
Secretin-Beveridge and the U. S. Patent Law170 |
Secretin-Beveridge, question of stability of171 |
Secretin, commercial preparations of75 |
Secretin, has it a therapeutic value?65 |
| Secretogen Elixir (G. W. Carnrick Co.), The Journal, Nov. 1, 1913, p. 1649; May 1, 1915, p. 1518; Sept. 9, 1916, p. 828; Reports Council Pharm. & Chem., 1915, p. 46; 1916, p. 72; Propaganda, ed. 9, p. 185. |
| Secretogen Tablets (G. W. Carnrick Co.), The Journal, Nov. 1, 1913, p. 1649; May 1, 1915, p. 1518; Jan. 15, 1916, pp. 178, 208; Sept. 9, 1916, p. 828; Reports Council Pharm. & Chem., 1915, pp. 46, 96, 99; 1916, p. 72; Propaganda, ed. 9, p. 185. |
| Sedobrol “Roche” (Hoffmann-LaRoche Chemical Works), The Journal, Jan. 2, 1915, p. 71; Reports Council Pharm. & Chem., 1914, p. 134. |
Seleni-Bascca416 |
| Seleni-Bascca (Cosmopolitan Cancer Research Society), The Journal, Nov. 19, 1921, p. 1672. |
Selenium in cancer506 |
| Semprolin (W. Browning & Co.), The Journal, July 10, 1915, p. 175. |
Seng55 |
| Seng (Sultan Drug Co.), Reports Council Pharm. & Chem., 1912, p. 42; 1915, p. 129. |
| Serum Vaccine, Bruschettini (R. G. Berlingieri), The Journal, Nov. 21, 1914, p. 1870; Reports Council Pharm. & Chem., 1914, p. 127. |
Serums521 |
Seufert, L. B., Secretary, Direct Sales Company510 |
| Seven-Bark, Reports Council Pharm. & Chem., 1912, p. 45. |
| Sevetol (John Wyeth & Bro.), The Journal, July 6, 1914, p. 49; Propaganda, ed. 9, p. 353. |
Sextonol Tablets94 |
| Sextonol Tablets (Schering & Glatz, Inc.), The Journal, Sept. 30, 1916, p. 1033; Reports Council Pharm. & Chem., 1916, p. 34. |
Sharp and Dohme—Elixir Glycerophosphates, Nux Vomica and Damiana95 |
Liquor Santaiva211 |
Surgodine180 |
Sherman, G. H., on vaccines in toxic conditions560 |
| Sherman’s Mixed Vaccine No. 40. See Mixed Vaccine No. 40, Sherman’s. |
Shotgun Nostrums398 |
Silvol189 |
Sinclair, Upton, defense of Abrams by477 |
| Sinkina (Metropolitan Pharmacal Co.), The Journal, Sept. 27, 1913, p. 1056; Reports Council Pharm. & Chem., 1913, p. 24; Propaganda, ed. 9, p. 188. |
| Sirolin (Sirolin Co.), The Journal, June 21, 1913, p. 1974. |
Smith, Kline & French Co.—Eskay’s Neuro Phosphates46 |
Soamin253 |
| Soamin Tabloid (Burroughs Wellcome & Co.), Reports Council Pharm. & Chem., 1919, p. 89. |
Sodium Acetate in warming bottles329 |
Sodium Cacodylate in syphilis555 |
| Sodium Carbonate and Sodium Chloride Mixture for making Fisher’s Hypertonic Alkaline Solution (E. R. Squibb & Sons), Reports Council Pharm. & Chem., 1917, p. 147. |
Sodium Glycerophosphate99 |
| Sodium Glycerophosphate, Reports Council Pharm. & Chem., 1916, p. 52. |
Sodium Hypochlorite-Mulford, Concentrated Solution358 |
Sodium Hypochlorite Solutions (“Chlorinated Soda” Solutions), Deterioration of358 |
| Sodium Salicylate, “Natural,” The Journal, Sept. 20, 1913, p. 979; Reports Council Pharm. & Chem., 1913, p. 23. |
Sodium Salicylate, Strontium Salicylate not superior to572 |
Sodium Salicylate Tablets566 |
| Sofos (General Chemical Co.), Reports Council Pharm. & Chem., 1921, p. 67. |
Solution Hypophosphites of Lime and Soda (Liq. Hypophosphitum, Schlotterbeck’s)83 |
| Solution Hypophosphites of Lime and Soda, Schlotterbeck’s (Liq. Hypophosphitum, Schlotterbeck’s), (Schlotterbeck and Foss Co.), The Journal, Sept. 2, 1916, p. 761; Reports Council Pharm. & Chem., 1916, p. 14. |
Somnoform255 |
| Somnoform (Stratford-Cookson Co.), Reports Council Pharm. & Chem., 1919, p. 90. |
| Somnos (H. K. Mulford Co.), The Journal, Sept. 15, 1906, pp. 863, 872; Sept. 29, 1906, p. 1033; Reports Council Pharm. & Chem., 1905–8, p. 12; Propaganda, ed. 9, p. 193. |
| Sourwood, Reports Council Pharm. & Chem., 1912, p. 45. |
| Sourwood Compound, Elixir (Eli Lilly & Co.), Reports Council Pharm. & Chem., 1912, p. 45. |
| Sourwood Compound, Elixir (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 39. |
| Sourwood Compound (Special), Elixir (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 45. |
Special Bacterial vaccine Nos. 2, 3, 4, 5, 11, 15, and 16254 |
| Spermin, Poehl (Prof. Dr. v. Poehl & Soehne), The Journal, April 15, 1911, p. 1132; Propaganda, ed. 9, p. 395. |
Spirocide296 |
| Spirocide (The Spirocide Corp.), The Journal, Jan. 22, 1921, p. 259; July 30, 1921, p. 394; Reports Chem. Lab., 1920, p. 58; Reports Council Pharm. & Chem., 1920, p. 46. |
Spondylotherapy472 |
| Squaw-Vine, Reports Council Pharm. & Chem., 1912, p. 45. |
| Squaw-Vine and Black-Haw Compound, Elixir (Eli Lilly & Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Squaw-Vine Compound, Syrup (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Squibb, E. R., and Sons— |
Acetylsalicylic Acid347 |
Iron Citrate Green115 |
S. S. Products Company—Arsenoven S. S.231 |
Standard Appliance Company—Uri-Na Test498 |
Standard Emulsion Company—Russell Emulsion and Russell Prepared Green Bone134 |
| Standard Oil Company of Indiana— |
Stanolind Liquid Paraffin214 |
Stanolind Surgical Wax337 |
Standard Oil Company of New Jersey—Nujol108 |
Stannoxyl469 |
| Stannoxyl (E. Fougera & Co.), The Journal, March 6, 1920, p. 692. |
Stanolind Liquid Paraffin214 |
| Stanolind Liquid Paraffin (Standard Oil Company of Ind.), Reports Council Pharm. & Chem., 1918, p. 72. |
Stanolind Surgical Wax337 |
Staph-Strep. Bacterin, Vaccine No. 2, Special Bacterial254 |
| Stearns, Frederick & Co.— |
Alphozone99 |
Methaform212 |
Stearns’ Wine59 |
Stearns’ Wine59 |
Sterling Products Company and “Aspirin Bayer”485 |
“Sterling Violet Ray Generator”572 |
| Stillingia Compound, Elixir (Hance Bros. & White; Ray Chemical Co.; Smith, Kline & French Co.), Reports Council Pharm. & Chem., 1912, p. 47. |
| Stillingia Compound, Fluidextract (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1912, p. 42. |
| Stillingia Compound, Syrup (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 42. |
Stock, Physician’s, in Prescription Products548 |
| Stone Root, Reports Council Pharm. & Chem., 1912, p. 46. |
Stratford-Cookson Company—Somnoform255 |
| Streptococcus-Rheumaticus-Combined-Bacterin (The Abbott Laboratories), The Journal, June 22, 1918, p. 1967; Reports Council Pharm. & Chem., 1918, p. 11. |
| Streptococcus-Viridans-Combined-Bacterin (The Abbott Laboratories), The Journal, June 22, 1918, p. 1967; Reports Council Pharm. & Chem., 1918, p. 11. |
Strontium Salicylate not superior to Sodium Salicylate572 |
| Strychnin Arsenate, The Journal, Sept. 24, 1910, p. 1128; Reports Council Pharm. & Chem., 1910, p. 74. |
Stypticin240 |
| Stypticin, The Journal, Nov. 22, 1919, p. 1628; Reports Council Pharm. & Chem., 1919, p. 48. |
Styptol240 |
| Styptol (E. Bilhuber), The Journal, Nov. 22. 1919, p. 1628; Reports Council Pharm. & Chem., 1919, p. 48. |
Styptysate318 |
Succinate of Soda, Bile Salts and Phenolphthalein, Capsules of208 |
Succinate of Soda, Holadin and Bile Salts, Capsules of208 |
| Succinolac (Succinolac Company), Reports Council Pharm. & Chem., 1916, p. 79. |
| Succus Alterans (Eli Lilly & Co.), The Journal, June 26, 1909, p. 2115; Reports Council Pharm. & Chem., 1909, p. 107; Propaganda, ed. 9, p. 195. |
Succus Cineraria Maritima455 |
| Succus Cineraria Maritima, Walker (Walker Pharmacal Co.), The Journal, Nov. 11, 1911, p. 1630; March 17, 1917, p. 864; Reports Council Pharm. & Chem., 1911, p. 48; Propaganda, ed. 9, p. 50. |
Sukro-Serum273 |
| Sukro-Serum (Anglo-French Drug Co.), The Journal, Aug. 21, 1920, p. 556; Reports Council Pharm. & Chem., 1920, p. 23. |
Sulfo-Selene417 |
Sulfuryl Monal86 |
| Sulfuryl Monal (Geo. J. Wallau, Inc.), The Journal, Sept. 16, 1916, p. 895; Reports Council Pharm. & Chem., 1916, p. 22; Reports Chem. Lab., 1916, p. 23. |
| Sulpho-Lythin (Laine Chemical Co.), The Journal, Dec. 8, 1906, p. 1930; Reports Council Pharm. & Chem., 1905–8, p. 34; Propaganda, ed. 9, p. 196. |
| Sulpho-Selene (C. H. Walker), The Journal, April 17, 1915, p. 1283; Dec. 16, 1915, p. 1864; Reports Council Pharm. & Chem., 1915, p. 28; 1916, p. 80. |
| Sultan Drug Co.— |
Cactina Pillets391 |
Seng55 |
Supsalvs274 |
| Supsalvs (Anglo-French Drug Co.). The Journal, Oct. 30, 1920, p. 1219; Reports Council Pharm. & Chem., 1920, p. 25. |
Surgodine180 |
| Surgodine (Sharp & Dohme), The Journal, Jan. 26, 1918, p. 257; Reports Council Pharm. & Chem., 1917, p. 134; Reports Chem. Lab., 1917, p. 59. |
Swan-Myers Company—Di-Crotalin465 |
Sweeny, Anti-Tuberculous Lymph Compound266 |
Synthetic drug situation376 |
Syphilis, sodium cacodylate in555 |
| Syphilodol (French Medicinal Company, Inc.), The Journal, May 18, 1918, p. 1485. |
Syrup Balsamea268 |
Syrup Cephaelin-Lilly203 |
| Syrup Cocillana Compound (Parke, Davis & Co.), The Journal, March 18, 1911, p. 834; Feb. 15, 1913, p. 537; Reports Council Pharm. & Chem., 1912, p. 43; Propaganda, ed. 9, p. 396. |
Syrup Emetic-Lilly203 |
| Syrup Emetic-Lilly (Eli Lilly & Co.). Reports Council Pharm. & Chem., 1918, p. 52. |
Syrup, Fellows’, of Hypophosphites82 |
Syrup, Gardner’s, of Ammonium Hypophosphite100 |
Syrup of Ammonium Hypophosphite, Gardner’s100 |
| Syrup of Ammonium Hypophosphite, Gardner’s (R. W. Gardner), Reports Council Pharm. & Chem., 1916, p. 55. |
| Syrup of Hydriodic Acid, Gardner’s (R. W. Gardner), The Journal, Nov. 14, 1908, p. 1712; Reports Council Pharm. & Chem., 1905–8, p. 200; Propaganda, ed. 9, p. 97. |
Syrup of Hypophosphites Comp. (Lime and Soda) McArthur’s84 |
| Syrup of the Hypophosphites Comp. (Lime and Soda), McArthur’s (McArthur Hypophosphite Co.), The Journal, Sept. 2, 1916, p. 761; Reports Council Pharm. & Chem., 1916, p. 16. |
Syrup of Hypophosphites, Fellow’s82 |
| Syrup of Hypophosphites, Fellows’ (Fellows Medical Mfg. Co.), The Journal, Sept. 2, 1916, p. 760; Feb. 16, 1918, p. 478; Reports Council Pharm. & Chem., 1916, p. 13. |
Syrup Iron Iodid, U. S. P.566 |
| Syrup Leptinol (Balsamea Co.), The Journal, June 5, 1920, p. 1591; Reports Council Pharm. & Chem., 1920, p. 15. |
Syrup Leptinol (formerly Syrup Balsamea)268 |
| Syrup of Malt Williams’ (American Malt Extract Co.), The Journal, Sept. 4, 1915, p. 895; Reports Council Pharm. & Chem., 1915, p. 155. |
Syrup of Thyme467 |
Syrupus Roborans (Syrup Hypophosphites Comp. with Quinin, Strychnin and Manganese)82 |
| Syrupus Roborans (Syrup Hypophosphites Comp. with Quinin, Strychnin and Manganese) (Arthur Peter & Co.), The Journal, Sept. 2, 1916, p. 760; Reports Council Pharm. & Chem., 1916, p. 14. |
| T |
Tablets: dependability of dosage556 |
Tablets Formothalates256 |
| Tablets Formothalates (Tailby-Nason Company), Reports Council Pharm. & Chem., 1919, p. 92. |
| Tablogestin (F. H. Strong Co.), The Journal, Dec. 11, 1915, p. 2108. |
Tailby-Nason Company—Tablets Formothalates256 |
| Taka-Diastase (Parke, Davis & Co.), The Journal, July 11, 1908, p. 140; July 6, 1912, p. 50; Reports Council Pharm. & Chem., 1905–8, p. 110; 1912, p. 14; Propaganda, ed. 9, p. 62. |
| Taka-Diastase, Liquid (Parke, Davis & Co.), The Journal, July 11, 1908, p. 140; July 6, 1912, p. 50; Reports Council Pharm. & Chem., 1905–8, p. 110; 1912, p. 14; Propaganda, ed. 9, p. 62. |
| Tannyl (Charles Goslar), Reports Chem. Lab., 1912, p. 108. |
| Tartarlithine (Tartarlithine Co., McKesson & Robbins), The Journal, April 13, 1907, p. 1284; Propaganda, ed. 9, p. 401. |
| Taurocol Compound Tablets (Paul Plessner Company), The Journal, April 24, 1915, p. 1441; Reports Council Pharm. & Chem., 1915, p. 143; Propaganda, ed. 9, p. 198. |
| Taurocol Tablets (Paul Plessner Company), The Journal, April 24, 1915, p. 1441; Reports Council Pharm. & Chem., 1915, p. 143; Propaganda, ed. 9, p. 198. |
Tekarkin458 |
| Tekarkin (National Bio-Chemical Laboratory), The Journal, May 28, 1921, p. 1514; Nov. 19, 1921, p. 1675. |
| Terpin Hydrate with Codein Sulphate, Elixir, The Journal, April 15, 1916, p. 1199. |
Textbooks, fallibility of515 |
| Thalosen (The Abbott Laboratories), The Journal, April 30, 1910, p. 1458; Propaganda, ed. 9, p. 344. |
Therapeutic evidence: its crucial test557 |
Therapeutic Leaves458 |
Therapeutic Notes550 |
Therapeutic Notes, Ricord Pills written up in468 |
Thermor Waterless Hot Bottle329 |
| Thermozine (Pasteur Chemical Co.), The Journal, May 19, 1917, p. 1497. |
Thialion470 |
| Thialion (Vass Chemical Co.), The Journal, Nov. 3, 1906, p. 1500; Reports Council Pharm. & Chem., 1905–8, p. 26; Propaganda, ed. 9, p. 205. |
| Thioform (Otto Hann & Bro), Reports Chem. Lab., to 1909, p. 79. |
| Thiosinamine and Thiosinamine Compounds, Reports Council Pharm. & Chem., 1920, p. 68. |
| Thoxos (John Wyeth & Bro.), The Journal, March 21, 1914, p. 949; Reports Chem. Lab., 1914, p. 41; Propaganda, ed. 9, p. 402. |
| Three Chlorides (Henry), (Henry Pharmacal Co.), The Journal, Feb. 6, 1915, p. 528; Reports Council Pharm. & Chem., 1915, p. 9; Reports Chem. Lab., 1914, p. 65; Propaganda, ed. 9, p. 198. |
Thyme, Syrup of467 |
Thymozene432 |
Thymus, Desiccated, Armour213 |
| Thymus Gland, Reports Council Pharm. & Chem., 1918, p. 69. |
| Thymus-Gland-Armour, Desiccated (Armour & Co.), Reports Council Pharm. & Chem., 1918, p. 69. |
Thymus Tablets—Armour213 |
Thyreoidectin202 |
| Thyreoidectin (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1918, p. 50. |
Thyroid Comp., Caps219 |
Thyroid Preparations (Antithyroidin-Moebius and Thyreoidectin)202 |
Thyro-Ovarian Comp., Caps219 |
| Tilden Company— |
Elixir Iodo-Bromide of Calcium Comp. “Without Mercury” and “With Mercury”52 |
Firolyptol Plain and Firolyptol with Kreosote120 |
Firwein119 |
| Tincture of Digitalis (Upsher Smith), Reports Council Pharm. & Chem., 1920, p. 52. |
| Tissue Phosphates, Wheeler’s (T. B. Wheeler, M.D., Company), The Journal, May 5, 1917, p. 1336; Sept. 22, 1917, p. 1010; Reports Council Pharm. & Chem., 1917, p. 21; Reports Chem. Lab., 1917, p. 13. |
| Tonga, The Journal, May 10, 1913, p. 1477; Reports Council Pharm. & Chem., 1912, p. 46. |
| Tonga and Salicylates, Elixir (Sharp & Dohme), The Journal, May 10, 1913, p. 1478; Reports Council Pharm. & Chem., 1912, p. 47. |
| Tonga Compound, Elixir (Hance Bros. & White; Eli Lilly & Co.; Nelson Baker & Co.; Ray Chemical Co.; F. Stearns & Co.), The Journal, May 10, 1913, p. 1478; Reports Council Pharm. & Chem., 1912, p. 47. |
| Tonga Compound, Elixir (Wm. S. Merrell Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Tonga Compound, Elixir (Parke, Davis & Co.; Wm. R. Warner & Co.), The Journal, May 10, 1913, p. 1478. |
| Tonga Compound (Special), Elixir (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 47. |
| Tonga Salicylates, Elixir (Wm. S. Merrell Chemical Co.), The Journal, May 10, 1913, p. 1478. |
| Tongaline (Mellier Drug Co.), The Journal, May 10, 1913, p. 1477; July 17, 1915, p. 269; March 2, 1918, p. 643; Reports Council Pharm. & Chem., 1912, p. 47; 1915, p. 58. |
| Tongaline and Quinine Tablets (Mellier Drug Co.), The Journal, July 17, 1915, p. 269. |
Tongaline Tablets27 |
| Tonga-Salicyl (H. K. Wampole & Co.), The Journal, May 10, 1913, p. 1478; Reports Council Pharm. & Chem., 1912, p. 47. |
| Tonic Beef, S. & D. (Sharp & Dohme), The Journal, May 11, 1907, p. 1612; Reports Council Pharm. & Chem., 1905–8, opp. p. 64; Propaganda, ed. 9, p. 133. |
Tonols (Schering’s Glycerophosphates)94 |
| Tonols (Schering’s Glycerophosphates), (Schering & Glatz, Inc.), The Journal, Sept. 30, 1916, p. 1033; Reports Council Pharm. & Chem., 1916, p. 34. |
Toxicide307 |
| Toxicide (Toxicide Laboratories, Chicago), The Journal, Oct. 8, 1921, p. 1197; Reports Council Pharm. & Chem., 1921, p. 68. |
| Toxinol (Hughes Chemical Co.), The Journal, Dec. 2, 1916, p. 1687; Reports Council Pharm. & Chem., 1912, p. 39; 1916, p. 38. |
Trade Names, Patents perpetuated by544 |
| Tri-Arsenole (Medical Supply Co.), Reports Council Pharm. & Chem., 1917, p. 156. |
Tri-Arsenole, L. O. Compound No. 1 and L. O. Compound No. 2163 |
| Trifolium Compound, Extract (Wm. S. Merrell Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 39. |
| Trifolium Compound, Fluidextract (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Trifolium Compound, Syrup (Hance Bros. & White; Eli Lilly & Co.; H. K. Mulford Co.; Parke, Davis & Co.; Ray Chemical Co.; F. Stearns & Co.), Reports Council Pharm. & Chem., 1912, p. 39, 40. |
| Trifolium Compound with Cascara Syrup (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 40. |
| Tri-Iodides (Henry) (Henry Pharmacal Co.), The Journal, Feb. 6, 1915, p. 528; Reports Council Pharm. & Chem., 1915, p. 9; Reports Chem. Lab., 1914, p. 65; Propaganda, ed. 9, p. 198. |
| Trilene Tablets, Reports Council Pharm. & Chem., 1912, p. 39. |
Trimethol140 |
| Trimethol (Thos. Leeming & Co.), The Journal, Aug. 11, 1917, p. 485; Reports Council Pharm. & Chem., 1917, p. 43. |
Triner’s American Elixir of Bitter Wine139 |
Triotonol Tablets94 |
| Triotonol Tablets (Schering & Glatz, Inc.), The Journal, Sept. 30, 1916, p. 1033; Reports Council Pharm. & Chem., 1916, p. 34. |
Triple Arsenates with Nuclein256 |
| Triple Arsenates with Nuclein (Abbott Laboratories), Reports Council Pharm. & Chem., 1919, p. 92. |
Troches of Ammonium Chloride, druggists refuse to supply552 |
| Trophenine (Reed & Carnrick Co.), The Journal, Oct. 5, 1907, p. 1198; Reports Council Pharm. & Chem., 1905–8, p. 79. |
| Trypsogen (G. W. Carnrick Co.), The Journal, Nov. 1, 1913, p. 1649; Propaganda, ed. 9, p. 403. |
Tubercle Bacilli, Dixon’s Suspension of Dead158 |
| Tubercle Bacilli Emulsion, Polygeneous (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
Tubercle Bacilli Extract, Dixon’s158 |
| Tubercle Bacilli, Triturated (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tubercle Germs, Dried Dead (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tubercle Vaccine, Sherman’s Non-Virulent (G. H. Sherman) The Journal, Nov. 21, 1914, p. 1870; Reports Council Pharm. & Chem., 1914, p. 128. |
| Tuberculin, Bovine (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculin, Bovine, T. R. (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculin, “Koch” New (T. R.) (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculin “Koch” (Old) (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculin Old, Bovine (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculin-Rosenbach (Kalle Color & Chemical Co.), Reports Council Pharm. & Chem., 1917, p. 161. |
| Tuberculin Test Plate, Keller’s (A. H. Keller), The Journal, Dec. 19, 1914, p. 2250. |
| Tuberculin T. O. A. (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculin, Vacuum (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculin, Vacuum Bovine (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculoids (Columbus Pharmacal Co.), The Journal, Feb. 22, 1908, p. 704. |
| Tuberculosis-Diagnostic “Hoechst” (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculosis-Diagnostic “Hoechst” Dry in Tubes (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculosis-Diagnostic “Hoechst” (0.1 per cent.) Solution (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 146. |
| Tuberculosis Serum Vaccine “Hoechst” (Farbwerke-Hoechst Co.), Reports Council Pharm. & Chem., 1917, p. 161. |
| Tubo-Arg (Tubo Pharmacal Co.), Reports Council Pharm. & Chem., 1915, p. 175. |
Tumors and Chemotherapy499 |
| Turkey Corn, Reports Council Pharm. & Chem., 1912, p. 47. |
Tyree’s Antiseptic401 |
Tyree’s Antiseptic Powder462 |
Tyree’s Elixir of Buchu and Hyoscyamus Compound57 |
| U |
| Ulax Salt (F. H. Strong Co.), Reports Council Pharm. & Chem., 1915, p. 175. |
Unctol166 |
| Unctol (R. R. Rogers Chemical Co.), Reports Council Pharm. & Chem., 1917, p. 162. |
| Unguentine (Norwich Pharmacal Co.), The Journal, March 27, 1909, p. 1047; July 17, 1915, p. 272; Reports Chem. Lab., 1909, p. 21; Propaganda, ed. 9, p. 254. |
| Unguentum Selenio Vanadic (v. Roemer) (A. von Roemer), The Journal, Nov. 21, 1914, p. 1870; Reports Council Pharm. & Chem., 1914, p. 129; Propaganda, ed. 9, p. 207. |
| Unicorn Root, The Journal, Jan. 22, 1910, p. 304; Reports Council Pharm. & Chem., 1910, p. 10; Propaganda, ed. 9. p. 208. |
| Uranoblen (A. Grimme), Reports Council Pharm. & Chem., 1915, p. 176. |
| Urasol (Organic Chemical Mfg. Co.), The Journal, Sept. 5, 1908, p. 818; May 8, 1909, p. 1511; Reports Council Pharm. & Chem., 1905–8, p. 166; 1909, p. 64. |
Urea, Hydrochlorid and Quinin467 |
| Uricedin (Fischer Chemical Importing Co.), The Journal, Nov. 23, 1907, p. 1788; Reports Chem. Lab., 1909, p. 20; Propaganda, ed. 9, p. 256. |
Uricsol30 |
| Uricsol (Uricsol Chemical Co.), The Journal, Aug. 14, 1915, p. 638; Reports Council Pharm. & Chem., 1915, p. 149; Reports Chem. Lab., 1915, p. 96. |
“Uri-Na Test”498 |
| Uriseptin (Gardner-Barada Chemical Co.), The Journal, Aug. 29, 1908, p. 773; Reports Chem. Lab., to 1909, p. 40; Propaganda, ed. 9, p. 256. |
Urodonal32 |
| Urodonal (Geo. J. Wallau, Inc.), The Journal, Aug. 14, 1915, p. 639; Reports Council Pharm. & Chem., 1915, p. 153. |
Uroluetic Test, Capell’s497 |
| Uroluetic Test, Capell’s (Capell’s Laboratory), The Journal, Aug. 23, 1919, p. 626. |
| Uron (Uron Chemical Co.), The Journal, Nov. 3, 1906, p. 1500; Reports Council Pharm. & Chem., 1905–8, p. 26. |
Urotropin316 |
Urotropin a brand of hexamethylenamin, U. S. P.318 |
Urotropin marketed under unwarranted therapeutic claims317 |
| Uterine Sedative, Elixir (Eli Lilly & Co.), The Journal, Aug. 31, 1912, p. 735; Reports Council Pharm. & Chem., 1912, p. 44; Propaganda, ed. 9, p. 410. |
| Uterine Tonic, Buckley (The Abbott Laboratories), Reports Council Pharm. & Chem., 1912, p. 41. |
| Uterine Tonic, Girard (Girard Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 41. |
| Uterine Tonic (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Uterine Tonic, Elixir (F. Stearns & Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Uterine Tonic (Tablet), (Maltbie Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 41. |
| Uterine Wafers, Micajah’s (Micajah & Co.), The Journal, March 26, 1910, p. 1070; Sept. 25, 1915, p. 1128; Reports Council Pharm. & Chem., 1916, p. 66; Reports Chem. Lab., 1910, p. 18; 1915, p. 100; Propaganda, ed. 9, p. 240. |
Uterine Wafers, Naphey’s Medicated107 |
| Utero Tonic (Nelson, Baker & Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Utros (H. K. Mulford Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| V |
Vaccine as a prophylactic in influenza572 |
Vaccine, B. Coli-Combined-Bacterin185 |
Vaccine, Catarrhal Vaccine Combined-Lilly187 |
Vaccine, Curative Vaccine, Bruschettini58 |
| Vaccine, Friedmann’s (Standard Distributing Co.). Reports Council Pharm. & Chem., 1914, p. 136. |
Vaccine-Lilly, Influenza Mixed187 |
Vaccine, M. Catarrhalis-Combined-Bacterin184 |
| Vaccine, Non-Virulent Tubercle Bacillus (G. H. Sherman).—See Tubercle Vaccine, Sherman’s Non-Virulent. |
Vaccine, No. 2 (Staph-Strep. Bacterin), Special Bacterial254 |
Vaccine No. 3 (Pneumo-Staph-Strep. Bacterin), Special Bacterial254 |
Vaccine No. 4 (Pneumo-Staph-Strep-Coli Bacterin), Special Bacterial254 |
Vaccine No. 5 (Influenza Combined Bacterin), Special Bacterial254 |
Vaccine No. 11 (Pneumo-Strep. Bacterin), Special Bacterial254 |
Vaccine No. 15 (Combined Whooping Cough Bacterin), Special Bacterial254 |
Vaccine No. 16 (Mixed Gonococcus Bacterin), Special Bacterial254 |
Vaccine, No. 40, Sherman’s Mixed188 |
Vaccine, Pertussis-Combined-Bacterin185 |
Vaccine, Streptococcus-Rheumaticus-Combined-Bacterin185 |
Vaccines-Abbott, Mixed184 |
Vaccines and Serums521 |
Vaccines for “Colds”573 |
“Vaccines” in toxic conditions560 |
Vaccines, Influenza520 |
| Vaccines, Mixed, The Journal, June 22, 1918, p. 1967; Reports Council Pharm. & Chem., 1918, p. 11. See also: B. Coli-Combined-Bacterin; Catarrhal Vaccine-Combined-Lilly; Influenza Mixed Vaccine-Lilly; Influenza Serobacterin Mixed-Mulford; M. Catarrhalis-Combined Bacterin; Mixed Vaccine No. 40, Sherman’s; Pertussis-Combined-Bacterin; Streptococcus-Rheumaticus-Combined-Bacterin; Streptococcus-Viridans-Combined-Bacterin. |
Vaccines, Several “Mixed”184 |
Van Horn and Sawtell—K-Y Lubricating Jelly147 |
| Vanadic Acid, The Journal, May 9, 1908, p. 1548; July 24, 1909, p. 309. |
| Vanadiol (Vanadium Chemical Co.), The Journal, Jan. 18, 1913, p. 225; Reports Council Pharm. & Chem., 1913, p. 7; Propaganda, ed. 9, p. 209. |
| Vanadioseptol (Vanadium Chemical Co.), The Journal, Jan. 18, 1913, p. 225; Reports Council Pharm. & Chem., 1913, p. 7; Propaganda, ed. 9, p. 209. |
| Vanadium, The Journal, June 3, 1911, p. 1648. |
| Vanadium Solution for Intravenous and Hypodermic Use (Vanadium Chemical Co.), The Journal, Jan. 18, 1913, p. 225; Reports Council Pharm. & Chem., 1913, p. 7; Propaganda ed. 9, p. 209. |
| Vanadoforms (Vanadium Chemical Co.), The Journal, Jan. 18, 1913, p. 225; Reports Council Pharm. & Chem., 1913, p. 7; Propaganda, ed. 9, p. 209. |
| Vapo-Cresolene (Vapo-Cresolene Co.), The Journal, April 4, 1908, p. 1135; Reports Chem. Lab., to 1909, p. 65; Propaganda, ed. 9, p. 408. |
| Vasogen (Lehn & Fink), The Journal, Feb. 13, 1909, p. 575; Propaganda, ed. 9, p. 408. |
Vass Chemical Company—Thialion470 |
V-E-M (Schoonmaker Laboratories, Inc.)166 |
V-E-M Unguentum Eucalyptol Compound167 |
| V-E-M Unguentum Eucalyptol Compound (Schoonmaker Laboratories, Inc.), Reports Council Pharm. & Chem., 1917, p. 163. |
V-E-M with Boric Acid167 |
| V-E-M with Boric Acid (Schoonmaker Laboratories, Inc.), Reports Council Pharm. & Chem., 1917, p. 163. |
V-E-M with Camphor167 |
| V-E-M with Camphor (Schoonmaker Laboratories, Inc.), Reports Council Pharm. & Chem., 1917, p. 163. |
V-E-M with Ichthyol167 |
| V-E-M with Ichthyol (Schoonmaker Laboratories, Inc.), Reports Council Pharm. & Chem., 1917, p. 163. |
V-E-M with Stearate of Zinc167 |
| V-E-M with Stearate of Zinc (Schoonmaker Laboratories, Inc.), Reports Council Pharm. & Chem., 1917, p. 163. |
Venarsen471 |
| Venarsen (Intravenous Products Co.), The Journal, May 22, 1915, p. 1780; March 25, 1916, p. 978; Reports Council Pharm. & Chem., 1915, p. 52; Reports Chem. Lab., 1915, p. 82; Propaganda, ed. 9, p. 212. |
| Vencalxodine (Intravenous Products Co.), The Journal, Feb. 16, 1918, p. 481. |
| Venodine (Intravenous Products Co.). The Journal, June 26, 1915, p. 2155; March 25, 1916, p. 278; Reports Council Pharm. & Chem., 1915, p. 145; Propaganda, ed. 9, p. 214. |
| Venomer (Intravenous Products Co.), The Journal, March 25, 1916, p. 978. |
| Venosal (Intravenous Products Co.), The Journal, Jan. 5, 1918, p. 48; Reports Council Pharm. & Chem., 1917, p. 118; Reports Chem. Lab., 1917, p. 57. |
| Veracolate (Marcy Co.), The Journal, April 30, 1910, p. 1458; Aug. 1, 1914, p. 420; April 24, 1915, p. 1440; Reports Council Pharm. & Chem., 1915, p. 141; Propaganda, ed. 9, pp. 216, 344. |
| Veracolate with Iron, Quinine and Strychnine (Marcy Co.), The Journal, April 24, 1915, p. 1440; Reports Council Pharm. & Chem., 1915, p. 141. |
| Veracolate with Pepsin and Pancreatin (Marcy Co.), The Journal, April 24, 1915, p. 1440; Reports Council Pharm. & Chem., 1915, p. 141. |
| Veroform Germicide (Veroform Hygienic Co.), The Journal, Nov. 22, 1913, p. 1920; Reports Council Pharm. & Chem., 1913, p. 29. |
Veronal371 |
Veronal (Barbital)370 |
Veronal-Sodium371 |
| Viburn-Ovaro (Ray Chemical Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Viburnum Compound, Hayden’s (New York Pharmacal Co.), The Journal, Aug. 31, 1912, p. 735; Jan. 23, 1915, p. 359; Reports Council Pharm. & Chem., 1914, p. 95; Propaganda, ed. 9, pp. 218, 409. |
| Viburnum Compound, Elixir (Nelson, Baker & Co.), The Journal, Aug. 31, 1912, p. 735; Propaganda, ed. 9, p. 410. |
| Viburnum Compound (Tablets), (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 41. |
| Viburnum Compound (Tablet) (Uterine Tonic), (Parke, Davis & Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Viburnum Compound (Tablet) (Smith, Kline & French Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Viburnum Sedative (Fraser Tablet Co.), Reports Council Pharm. & Chem., 1912, p. 46. |
| Viburnumal (Louisville Pharmacal Works), The Journal, Aug. 31, 1912, p. 735; Reports Council Pharm. & Chem., 1912, p. 44; Propaganda, ed. 9, p. 410. |
| Vibutero (F. Stearns & Co.), The Journal, Aug. 31, 1912, p. 735; Reports Council Pharm. & Chem., 1912, p. 46; Propaganda, ed. 9, p. 410. |
| Vigoral (Armour & Co.), The Journal, Jan. 23, 1909, p. 311; Propaganda, ed. 9, p. 472. |
| Vin Mariani (Mariani & Co.), The Journal, Nov. 24, 1906, p. 1751; Reports Council Pharm. & Chem., 1905–8, p. 29; Propaganda, ed. 9, p. 221. |
Vinum Extract Morrhuae, Stearns59 |
Violet Ray Generator, Sterling572 |
| Virol (Etna Chemical Co.), The Journal, Feb. 20, 1915, p. 683; Reports Council Pharm. & Chem., 1915, p. 132; Propaganda, ed. 9, p. 225. |
Vitamin B Concentrates and Yeast Preparations321 |
Vitamins: their distribution561 |
| Vitaphos (The Grain Chemical Company, Inc.), Reports Council Pharm. & Chem., 1921, p. 74. |
| W |
Walker Pharmacal Company—Succus Cineraria Maritima455 |
| Wallau, George J., Inc.— |
Eumictine262 |
Filudine41 |
Jubol31 |
Sulfuryl Monal86 |
Urodonal32 |
Wampole, H. K., & Co.—Hypno-Bromic Compound430 |
Warming bottles, Sodium Acetate in329 |
| Water, Buffalo Lithia Springs (Buffalo Lithia Springs Water Co.), The Journal, Sept. 12, 1908, p. 931. |
| Water Eryngo, Reports Council Pharm. & Chem., 1912, p. 47. |
Webster Co., William A. and the Direct Pharmaceutical Co.564 |
Weeks Chemical Company—Chlorlyptus277 |
Westerfield’s Digitalis Tablets215 |
White, G. S., and the Allied Medical Associations of America490 |
| White, Mark, Goiter Serum Laboratories—Mark White Goiter Serum and Mark White |
Iodinized Oil87 |
| White Sulphur Salts (White Sulphur Springs, Inc.), The Journal, Nov. 21, 1914, p. 1870; Reports Council Pharm. & Chem., 1914. p. 128. |
| Whiteruss (Lubric Oil Co.), The Journal, July 10, 1915, p. 175. |
Whooping Cough Bacterin, Combined, Special Bacterial Vaccine No. 15254 |
| Wild Indigo, The Journal, Jan. 22, 1910, p. 304; Reports Council Pharm. & Chem., 1910, p. 19; Propaganda, ed. 9, p. 208. |
| Wild Yam, The Journal, Jan. 22, 1910, p. 304; Reports Council Pharm. & Chem., 1910, p. 10; Propaganda, ed. 9, p. 208. |
William F. Koch Cancer Remedy437 |
| Wine, Chapoteaut’s (E. Fougera & Co., Inc.), The Journal, Dec. 19, 1914, p. 2247; Reports Council Pharm. & Chem., 1914, p. 77; Propaganda, ed. 9, p. 60. |
| Wine, Stearns’ (F. Stearns & Co.), Reports Council Pharm. & Chem., 1915, p. 177. |
Winslow Laboratory—Pineoleum advertising methods442 |
| Winthrop Chemical Company, Inc.— |
Elarson248 |
Helmitol295 |
Woolley, D. E., Manager of Cosmopolitan Cancer Research Society414 |
| Worlds Wonder Remedy (W. W. Remedy Co., Superior, Wis.), Reports Council Pharm. & Chem., 1918, p. 82. |
Wulfing, A., Company—Formamint33 |
| Wyeth, John, and Brother— |
Hydras96 |
Robinol95 |
| X |
| Xanol (Wm. S. Merrell Chemical Co.), Reports Council Pharm. & Chem., 1911, p. 64. |
Xeroform352 |
| Y |
Yarbrough, Charles C.—Biniodol121 |
Yeast566 |
Yeast Preparations and Vitamin B Concentrates321 |
Yerba Santa, Maltzyme with211 |
| Yogurt (Yogurt Co.), The Journal, Jan. 30, 1909, pp. 372, 397. |
| Yohimbin Spiegel (Lehn & Fink), The Journal, March 30, 1907, p. 1127. |
| Z |
| Zemacol (Norwich Pharmacal Co.), The Journal, May 14, 1910, p. 1626; Reports Chem. Lab., 1910, p. 42; Propaganda, ed. 9, p. 259. |
Ziratol148 |
| Ziratol (Bristol-Myers Co.), The Journal, Oct. 6, 1917, p. 1191; Reports Council Pharm. & Chem., 1917, p. 55; Reports Chem. Lab., 1917, p. 51. |
| Zyme-oid (Oxychlorin Chemical Co.), The Journal, May 23, 1908, p. 1706; Reports Chem. Lab., to 1909, p. 34; Propaganda, ed. 9, p. 261. |
| Zymotoid, Arnold’s (Zymotoid Co.), The Journal, April 6, 1912, p. 1030; Reports Chem. Lab., 1912, p. 68; Propaganda, ed. 9, p. 412. |
[A] Read before the Chicago Medical Society, March 26, 1919.
1 “New and Nonofficial Remedies” in France, Foreign News, J. A. M. A. 71:1331 (Oct. 19) 1918; 70:1783 (June 8) 1918.
2 Nederl. Tjdschr. v. Geneesk. Oct. 5, 1918, p. 1201.
3 An Italian View of the Proprietary Evil, Foreign News, J. A. M. A. 71:840 (Sept. 7) 1918; The Council on Pharmacy and Chemistry and the Patriotic Medical League in Italy, ibid. 71:918 (Sept. 14) 1918.
4 Although the Council on Pharmacy and Chemistry was established in 1905, it is likely that only a small percentage of physicians know just what the Council is, or have any conception as to its personnel and its ability to judge the available evidence for proprietary medicaments. The personnel has changed from time to time since 1905. At present its membership is: C. L. Alsberg, A.M., M.D., chief of the Bureau of Chemistry, U. S. Department of Agriculture, Washington, D. C.; R. A. Hatcher, Ph.G., M.D., professor of pharmacology, Cornell University Medical College, New York City; A. W. Hewlett, M.D., professor of medicine, Leland Stanford Junior University Medical School, San Francisco; John Howland, M.D., professor of pediatrics, Johns Hopkins University Department of Medicine, Baltimore; Reid Hunt, M.D., professor of pharmacology, Harvard University Medical School, Boston; Henry Kraemer, Ph.D., professor of pharmacognosy, University of Michigan College of Pharmacy, Ann Arbor, Mich.; W. T. Longcope, A.B., M.D., Bard Professor of the Practice of Medicine, College of Physicians and Surgeons of Columbia University, New York City; G. W. McCoy, M.D., director of the Hygienic Laboratory, United States Public Health Service, Washington, D. C.; Lafayette B. Mendel, Ph.D., Sc.D., professor of physiologic chemistry, Sheffield Scientific School, Yale University, New Haven, Conn.; F. G. Novy, M.D., Sc.D., professor of bacteriology, University of Michigan, Ann Arbor, Mich.; W. W. Palmer, B.S., M.D., associate professor of medicine, College of Physicians and Surgeons of Columbia University, New York City; L. G. Rowntree, M.D., professor of medicine, University of Minnesota Medical School, Minneapolis; Torald Sollmann, M.D., professor of pharmacology and materia medica, Medical Department, Western Reserve University, Cleveland; Julius Stieglitz, Ph.D., Sc.D., Chem.D., vice chairman of the Council, professor of chemistry, University of Chicago, Chicago; G. H. Simmons, M.D., LL.D., chairman of the Council, editor of The Journal of the American Medical Association, Chicago, and W. A. Puckner, Phar.D., secretary of the Council, director of the Chemical Laboratory of the American Medical Association, Chicago.
5 Need for Patent Law Revision, A. M. A. Council on Pharmacy and Chemistry Reports, 1917, p. 130.
6 Puckner, W. A.: The Abuse of Chemical Formulas, Reports A. M. A. Chemical Laboratory 3:7, 1910.
7 The Formula for Glyco-Thymoline, J. A. M. A. 52:147 (Jan. 9) 1909.
8 Hunt, Reid, and Seidell, Atherton: Howell’s Mercol, J. A. M. A. 52:225 (Jan. 16) 1909. Howell’s Mercol Again: Another Analysis Fails to Reveal the Presence of Mercury, J. A. M. A. 52:1595 (May 15) 1909.
9 Taka-Diastase and Liquid Taka-Diastase: Report of the Council on Pharmacy and Chemistry, J. A. M. A. 59:50 (July 6) 1912.
10 Iodeol and Iodagol: Report of the Council on Pharmacy and Chemistry, J. A. M. A. 69:1725 (Nov. 17) 1917.
11 Vin Mariani: Official Report of Council on Pharmacy and Chemistry—With Comments, J. A. M. A. 47:1751 (Nov. 24) 1906.
12 Reports of the Council on Pharm. and Chem., 1912, p. 40.
13 See Report of the Council on Pharmacy and Chemistry on “Resinoids and Concentrations,” J. A. M. A., Nov. 13, 1909, p. 1655.
14 The Journal A. M. A., Feb. 24, 1912, p. 572.
15 De Santi: Medical Magazine 16:141, 1907.
16 Rosenberg: Lancet, London 2:1871, 1905.
17 Seifert: Pharmakol. u. therap. Rundschau, 1904, No. 14; quoted by De Santi (Note 2).
18 Daus: Med. Klin. 2:4110, 1906.
19 Rheinboldt: Deutsch. med. Wchnschr. 32:587, 1906.
20 Rosenberg: Therap. d. Gegen. 7:55, 1905.
21 Wingrave: Lancet, London 2:1067, 1906.
22 Young: Lancet, London 1:975, 1908.
23 Sutton: Volumetric Analysis, Edition 10, p. 390.
24 Heinemann: Laboratory Guide in Bacteriology, p. 86.
25 Thoms: Arb. a. d. Pharm. Inst. d. Universität, Berlin 11:210, 1914.
26 Seifert, Otto: Die Nebenwirkungen der modernen Arzneimittel, 1915.
27 Dr. Benedict’s personal communication to a member of the Council is as follows:
“In the report of the Council upon Lactopeptine which you sent to me, I find the following statement: ‘Careful examination failed to show the presence of either diastase or pancreatin.’ In this connection I will cite to you the following experiment carried out by myself: A package containing a 1-ounce bottle of Lactopeptine (powder) with seal unbroken was purchased in the open market and opened in this laboratory. The label bore the special Number 6 2382. Two hundred milligrams of this product was dissolved in 50 c.c. of a 0.25 per cent. solution of sodium carbonate in water. This solution was divided into two portions of 25 c.c. each. One of these portions was boiled at once, and after cooling was added to 1 gm. of moist fibrin contained in a flask. The other portion (unboiled) was also added to 1 gm. of moist fibrin contained in a flask. Both flasks (after addition of 5 c.c. of toluene to each) were stoppered and placed in an incubator at 37 degrees, and left there for twelve hours. Examination of the two flasks at the end of this period showed that the one to which the unboiled solution of Lactopeptine [powder] had been added contained much less solid protein than did the other. Although this fact was obvious to the naked eye, the exact extent of digestion in the two flasks was determined by heating both to boiling, acidifying with acetic acid, diluting to definite volume, filtering and determining the nitrogen in the filtrate by Kjeldahl’s method. Subtracting the trace of nitrogen contained in the filtrate of the control flask, the results showed that 42 per cent. of the original fibrin present had been dissolved by the unboiled Lactopeptine solution. This can be ascribed only to tryptic activity under the conditions of this experiment. Furthermore, this is not simply a ‘trace’ of activity, but is at least sufficiently marked to warrant a statement that this sample showed a distinct tryptic activity. Inasmuch as I have obtained exactly similar results with two other samples of Lactopeptine (powder) (these being the only ones I have examined), I am inclined to question the correctness of the Council’s statement regarding the absence of trypsin from this preparation. [As noted above, a fresh preparation was used.—Ed.]
“May I again add that I am making no statement regarding therapeutic value of preparation, and that I have no opinion upon that matter one way or the other? My work was undertaken solely out of interest to see whether trypsin could exist in the powder (which gives a markedly acid solution when dissolved in water). The Elixir Lactopeptine could theoretically show no tryptic activity, nor have I found any trace of such activity in one sample of the Elixir examined.
“In making use of any of the contents of my letters kindly include the statement that my work upon Lactopeptine was done without remuneration of any kind, and was done only for the scientific interest attached to the question.”
28 Report on Proprietary Digitalis Preparations, J. A. M. A., Dec. 4, 1915, p. 2024.
29 Secretogen, J. A. M. A., May 1, 1915, p. 1518.
30 Carlson, A. J.; Lebensohn, J. E., and Pearlmann, S. J.: Has Secretin a Therapeutic Value? J. A. M. A. 66:178 (Jan. 15) 1916.
[B] From the Hull Physiological Laboratory of the University of Chicago.
[C] This investigation was undertaken at the request of the Council on Pharmacy and Chemistry. The following report, having been submitted to the Council, received its endorsement (see preceding report of the Council on Pharmacy and Chemistry, “So-Called Secretin Preparations”).
31 Pawlow: The Work of the Digestive Glands, 1912.
32 Bayliss and Starling: Jour. Physiol. 28:325, 1902.
33 Wertheimer: Compt. rend. Soc. de biol. 54:475, 1902.
34 Enriquez and Hallion: Compt. rend. Soc. de biol. 55:233, 363, 1903.
35 Fleig: Arch. internat. de Physiol., 10:206, 1910.
36 Matuso: Jour. Physiol. 45:477, 1913.
37 Huston: Ann. et bull. Soc. roy. de sc. méd. et nat. 70:178, 1912.
38 Starling: Lancet, London 2:433, 1905.
39 Frouin and Lalou: Compt. rend. Soc. de biol., 71:189, 1911.
40 Camus: Compt. rend. Soc. de biol., 1902, 54:442, 1902.
41 Fleig: Jour. de physiol. et de path. gén. 6:32, 50, 1904.
42 Wertheimer and LePage: Jour. de physiol. et de path. gén. 4:1061, 1070, 1902.
43 Stepp: Jour. Physiol. 43:441, 1912.
44 Henri and Portier: Compt. rend. Soc. de biol. 54:620, 1902.
45 Enriquez and Hallion: Presse méd. 1:105, 1903.
46 Delezenne and Frouin: Compt. rend. Soc. de biol. 56:319, 1904.
47 Bottazzi and Gabrielli: Arch. internat. de physiol. 111:156, 1905.
48 Enriquez and Hallion: Bull. gén. de thér. 162:202, 1911.
49 Falloise: Bull. de l’Acad. roy. de Belgique 5:945, 1902.
50 Bayliss and Starling (Note 2). Matuso (Note 6). Arch. internat. de physiol. 10:335, 1911.
51 Dixon and Hamill: Jour. Physiol., 1909, xxxv, 314.
52 Bayliss and Starling: Jour. Physiol., 1904, xxx, 61. Bierry: Compt. rend. Soc. de biol., 1907, lxii, 433. Bierry and Terroine: Compt. rend. Acad. de sc., 1905, cxli, 146. Lalou: Comp. rend. Acad. de sc., 1910, xxix, 824. Morel: Compt. rend. Soc. de biol., 1909, lxvii, 36. Strassano and Billoro: Compt. rend. Soc. de biol., 1902, liv, 937.
53 Bayliss and Starling (Note 23).
54 Camus: Jour. de physiol. et de path. gén. 4:998, 1902.
55 Bayliss and Starling: Jour. Physiol. 29:174, 1903.
56 Chapman: Proc. Linnaean Soc., New South Wales 1:92, 1905.
57 Camus: Compt. rend. Soc. de biol. 61:59, 1906. Hallion and Lequex: Compt. rend. Soc. de biol. 61:33, 1906.
58 Derouaux: Arch. internat. de physiol. 3:44, 1905. Lambert and Myer: Compt. rend. Soc. de biol. 54:1044, 1902. Starling: Lancet, London 2:501, 1905.
59 Keeton and Koch: Am. Jour. Physiol. 37:481, 1915.
60 Camus and Gley: Compt. rend. Soc. de biol. 54:648, 1902.
61 Lalou (Note 21). May: Jour. Physiol. 30:400, 1904.
62 Launoy: Arch. internat. de Physiol. 3:62, 1906. Morel and Terroine: Compt. rend. Soc. de biol. 67:36, 1909. Zunz: Arch. internat. de physiol. 8:181, 1909. Lalou: Jour. de physiol. 14:465, 1912.
63 Starling: Proc. Roy. Soc. Med., 8, No. 4, 1914, Therap. and Pharm. Section, p. 29.
64 Moore, Edie and Abram: Biochem. Jour., 1:28, 1906.
65 Foster: Jour. Biol. Chem., 2:297, 1906.
66 Bainbridge and Beddard: Biochem. Jour., 1:429, 1906.
67 Dakin and Ransom: Jour. Biol. Chem., 2:305, 1906.
68 Charles: Med. Press and Cir., 133:578, 1906.
69 Crofton: Lancet, London, 176:607, 1909.
70 Moore, Edie and Abram: Biochem Jour., 3:82, 1908.
71 Bainbridge and Beddard: Biochem. Jour. 3:82, 1908.
72 Evan: Jour. Physiol. 44:461, 1912.
73 Hedon: Compt. rend. Soc. de biol. 74:375, 1913.
74 Pemberton, Ralph, and Sweet, J. E.: Further Studies on the Influence of the Ductless Glands on the Pancreas, Arch. Int. Med., May, 1910, p. 466.
75 Enriquez: Bull. du Lab. de biol. Appliq. 2, No. 2-No. 8, 1904.
76 Wentworth, A. H.: The Cause of Infantile Atrophy, J. A. M. A., July 20, 1907, p. 204.
77 Sweet, J. E., and Pemberton, Ralph: Experimental Observations on Secretin, Arch. Int. Med., February, 1908, p. 231.
78 Beveridge: Am. Med. 20:255, 1914.
79 Lockwood, G. R.: Diseases of Stomach, 1913, Chapter on Achylia. Bassler, Anthony: Am. Jour. Gastro-Enter., 1914; Kemp, R. C.: Diseases of Stomach, Intestine and Pancreas, 1912. Reed, Boardman: Am. Jour. Gastro-Enter., October, 1912. Ewald (Therapie der Gegenwart, 1915, p. 5) reports favorable results with Secretogen in one of thirteen cases.
80 Harrower: Pediatrics 25:430, 1913; New York M. J. 118:315, 1913; Arch. f. Verdauungskr. 20:577, 1914.
81 Flieg: Arch. gén. de méd. 191:1482, 1903.
82 Wertheimer and Duvillier: Compt. rend. Soc. de biol. 68:535, 1910.
83 Hallion: Presse méd. 20:433, 1912.
84 Stockton: In Osier and McCrae’s Modern Medicine 3:19, 1914.
85 Ehrman and Lederer: Deutsch. med. Wchnschr. 35:879, 1909.
86 Meltzer, S. J.: The Factors of Safety in Animal Structure and Animal Economy, J. A. M. A., Feb. 23, 1907, p. 655.
87 Carlson: Am. Jour. Physiol. 38:248, 1915.
88 Delezenne and Pozerski: Jour. de Physiol., 14:540, 1912.
89 Pawlow: Ergeb. de Physiol., O., p. 266, 1902.
90 Secretogen, Report of the Council on Pharmacy and Chemistry, J. A. M. A., May 1, p. 1518, 1915.
91 Stepp (Note 13). Dale and Laidlow: Jour. Physiol. 44:11, 1912. Launoy and Ochslin: Comp. rend. Soc. de biol., 74:338, 1913.
92 Churchill, J. F.: De la cause immédiate et du traitement spécifique de la phthisie pulmonaire et des maladies tuberculeuses, Paris, 1858.
93 The Hypophosphite Fallacy, J. A. M. A., April 25, 1914, p. 1346.
94 Marriott, W. McKim: The Therapeutic Value of the Hypophosphites, J. A. M. A., Feb. 12, 1916, p. 486.
95 Liquid Sulphur—Sulphume, J. A. M. A., Dec. 2, 1911, p. 1853.
96 Proc. Am. Pharm. A. 40:488, 1892.
97 McCollum and Hart, Grosser and Husler, Plimmer, and Bayliss and Plimmer, quoted by Marshall (Note 2).
98 Marshall, E. K.: The Therapeutic Value of Organic Phosphorus Compounds, J. A. M. A., Feb. 13, 1915, p. 573.
99 See reports of the Council, J. A. M. A., Jan. 9, 1915, p. 165; Jan. 23, 1915, p. 359; Nov. 27, 1919, p. 1836; March 27, 1915, p. 1093.
100 See Reports Council Pharm. and Chem., 1912, p. 36.
101 Since publication of this report the Council on Pharmacy and Chemistry has revised its rule against recognition of articles advertised to the public so that this shall not apply (a) to disinfectants, germicides and antiseptics, provided the advertising be limited to conservative recommendations for their use as prophylactic applications to superficial cuts and abrasions of the skin and to the mucous surfaces of the mouth, pharynx and nose, and provided they are not advertised as curative agents, and (b) to non-medicinal food preparations, except when advertised in an objectionable manner.
102 J. A. M. A., Nov. 2, 1912, p. 1604.
103 J. A. M. A., May 1, 1915, p. 1518.
104 Carlson. A. J.; Lebensohn, J. E., and Pearlman, S. J.; Has Secretin a Therapeutic Value? J. A. M. A., Jan. 15, 1916, p. 178. Reports Council on Pharm. and Chem., 1915, p. 98.
105 So-Called Secretin Preparations, J. A. M. A., Jan 15, 1916, p. 208; Reports Council on Pharm. and Chem., 1915, p. 96.
106 All italics are ours. G. W. Carnrick Company.
107 Bio-Chem. Jour. 1:28, 1906.
108 Presse Médicale, 1912, p. 433.
109 Cf. interal. Schagindweit, E.: Experimentelle Versuche mit Hormonal, Arch. Internat. de Pharmacod., 1913, p. 77.
110 Three other Tilden products have been the subject of deserved and unfavorable comment in the J. A. M. A: “Narkine” in the issue of Oct. 24, 1908, “Hydrocyanate of Iron-Tilden” in the issue of June 19, 1909, and “Febrisol,” in the issue of June 29, 1912. The first two articles are reprinted in the latest (9th) edition of “The Propaganda for Reform.”
[D] From the Department of Dermatology and Syphilology of the Western Reserve University and of the Cleveland City Hospital.
111 Dusart, L.: Recherches expérimentales sur le rôle physiologique et thérapeutique du phosphate de chaux, Paris, 1870; Quel est l’acide du suc gastrique? Lille, 1874, unbound, 8 pages; Notice sur l’emploi et les proprietés du lacto-phosphate de chaux, Clichy, 1868, unbound, 8 pages. Dusart and Blache: Recherches sur l’assimilation du phosphate de chaux, Paris, 1868, unbound, 15 pages.
[E] From the Hull Physiological Laboratory of the University of Chicago.
[F] This investigation was begun in 1915 by Drs. A. Woelfel and A. J. Carlson.
112 Gillet-Damotti: Comp. rend. Acad. d. Sc., July 7, 1873.
113 Fragner: Wien. med. Wchnschr. 60:1033–1036, 1910.
114 Scherer: Wien. med. Wchnschr. 60:1033–1036, 1910.
115 Huët: Nederlandsch Tijdschr. v. Geneesk. 1:1353–1370, 1914.
116 Hygiama is said to be a food consisting of condensed milk, with (fatless) cocoa and cereals added to it (Encyclopedia and Dictionary of Medicine and Surgery, 1907).
117 The North Dakota Agricultural Experiment Station has recently published (Bulletin 22, 1915, p. 386) a complete chemical analysis of Nutrolactis. It contains only 0.60 per cent. solids (including strychnin and emodin). It has a bitter taste. The alcohol content was 3.5 per cent. The report concludes: “a little strychnin, a little alcohol, and a little laxative is about all there is to cause an increase in the milk secretion.”
118 Millbank: New York M. J. 50:544, 1889.
119 Those who are interested in the relative merits of the Rideal-Walker, the Lancet and the Hygienic Laboratory methods for the valuation of disinfectants, should read the following: Method of Standardizing Disinfectants with and without Organic Matter, J. A. M. A., Aug. 24, 1912, p. 667; Standardization of disinfectants, Report of the Council on Pharmacy and Chemistry, J. A. M. A., April 26, 1913, p. 1316; Standardizing Disinfectants, J. A. M. A., Sept. 30, 1916, p. 883.
120 Alpha-napthol was also found to be the basis of the nostrum Benetol. See The Journal, April 15, 1911, p. 1128.
121 U. S. Dept. of Agric., Insecticide and Fungicide Board, Service and Regulatory Announcements, No. 16, issued Aug. 8, 1917. No. 244, Misbranding of “Ziratol.” U. S. v. 100 bottles, more or less, of “Ziratol”; consent decree of condemnation and forfeiture; product ordered released on bond, p. 248. No. 256, Misbranding of “Ziratol.” U. S. v. 936 bottles and 6 jugs of Ziratol, consent decree of condemnation and forfeiture; product ordered released on bond, p. 260.
122 Carlson, Lebensohn and Pearlman, The Journal, Jan. 15, 1916, p. 178.
123 Carlson: The Control of Hunger in Health and Disease, Chicago, 1916.
124 “... it is equally unethical to prescribe or dispense secret medicines or other secret remedial agents,...” Sec. 6, Art. I, Chapter II, Principles of Medical Ethics.
125 The evolution of “Phillips’ Phospho-Muriate of Quinine Comp.” from “Phillips’ Wheat Phosphates” may be interesting. Every one knows that therapeutics tends to fashions, and “Phillips’ Wheat Phosphates” appears to have had its inception as the result of the observation that super-refined white flour contains less phosphates than the corresponding amount of wheat. It was assumed that such flour must be deficient in an essential constituent, and the Wheat Phosphates preparation was apparently designed to fill the want. It was exploited for the relief of numerous conditions that were supposed, without satisfactory evidence, to result from this deficiency. When iron, quinin and strychnin mixtures became the vogue a quarter of a century ago, it was only natural to ride on the wave of popularity and the already widely advertised “Wheat Phosphates” was further enhanced—commercially—by the addition of the iron, quinin and strychnin, the amount of alkaloid added being practically negligible. Those who are not familiar with the various phases of the phosphorus, phosphoric acid, lactophosphate, lecithin, nuclein and glycerophosphate propaganda are referred to a report of the Council on Pharmacy and Chemistry in The Journal A. M. A., Sept. 30, 1916, p. 1033.
126 Manufacturers are warned by the Department of Agriculture, through the Bureau of Chemistry, that combinations claiming to contain digestive enzymes must be active when sold. If preparations tend to deteriorate in a short time, each lot should be dated and not sold after the period when they become inactive. While every manufacturer must be considered innocent until proved guilty, and ignorant until proved knowing, it is a matter of knowledge that manufacturers have marketed their various digestive mixtures with full appreciation of their worthlessness.—(Jour. A. M. A., Dec. 19, 1914, p. 2234.)
127 See report, The Journal, Sept. 9, 1916, p. 827.
128 The E. L. Patch Company declares that “no hexamethylenamine has ever been used in the manufacture of Formitol tablets,” and that ammonium chloride and paraformaldehyde are among the ingredients used in the manufacture of these tablets. The hexamethylenamine present in the tablets, therefore, must have been produced by interaction of the paraformaldehyde and ammonium chloride. This does not alter the laboratory findings regarding the composition of the tablets, namely, that they “contain formaldehyde (or paraformaldehyde), an ammonium compound and some hexamethylenamine.”
129 After publication of the foregoing report had been authorized by the Council, a letter was received from Charles L. Heffner, advising that the distribution of the circulars has been discontinued.
130 The Comparative Values of Some Local Anesthetics by H. C. Hamilton, Detroit, Mich., from the Research Laboratory of Parke, Davis & Co., J. Lab. & Clin. M. 4:60 (Nov.) 1918.
131 Comparative Efficiency of Local Anesthetics, V, by T. Sollmann, from the Pharmacological Laboratory of the School of Medicine, Western Reserve University, J. Pharmacol. & Exper. Therap. 11:69 (Feb.) 1918.
132 A Further Contribution to the Pharmacology of the Local Anesthetics by Eggleston and Hatcher, from the Department of Pharmacology, Cornell University Medical College, New York City, J. Pharmacol. & Exper. Therap. 13:433 (Aug.) 1919.
133 It is well known that when a solution of mercuric chlorid in water is evaporated, mercuric chlorid passes off with the water vapors, but under any condition the amount is but a fraction of the whole. As in Platt’s Chlorides other metallic chlorids are present, the formation of complex mercuric compounds which is bound to have occurred, should retard or prevent the volatilization of mercuric chlorid. That this actually occurs was confirmed by the following experiment: When 1 gm. mercuric chlorid was dissolved in 1 liter of water and the solution distilled, the distillate contained a very small amount of mercury. Then the experiment was repeated after adding sodium chlorid to the solution to simulate the conditions in Platt’s Chlorides. In this case no mercury was found in the distillate. Even were all the mercury in a bottle of Platt’s Chlorides volatilized in a room 10 by 12 by 9 feet, this would be equivalent to only about 1⁄500 grain mercuric chlorid per cubic foot.
134 Iron Citrate Green, The Journal A. M. A., Jan. 12, 1917, p. 135; Reports Council Pharm. and Chem., 1916, p. 42.
135 Glycerophosphates, The Journal A. M. A., Sept. 30, 1916, p. 1033; Reports Council Pharm. and Chem., 1916, p. 32. Sodium glycerophosphates. Reports Council Pharm. and Chem., 1916, p. 52.
136 Barker and Rowntree (Bull. Johns Hopkins Hospital 29:215, 221 [Oct.] 1918) obtained the following results with eucalyptus oil:
Cat, hypodermic; survived 3 c.c. per kg.; killed by 5.5 c.c. per kg.
Cat, intraperitoneal; killed by 5 c.c. per kg.
Dog, hypodermic; survived 1.3 c.c. per kg.
They quote from Browning that the following doses, c.c. per kilogram, are not fatal: frogs, 0.5; rabbits, 1 to 5; guinea-pigs, 1.
137 An ampoule labeled as follows: “Coagulen-Ciba, 20 c.c. in sterile solution ready for use. To be shaken. Importé de Suisse. Op. No. 968” was found to measure only 15 c.c. Another ampoule with the same label and Op. No. 9641 contained considerable sediment.
138 Since the report was sent to the manufacturers, the results have been published. Hanzlik, P. J., and Weidenthal, C. M., Plasma and Blood Clotting Efficiency of Thromboplastic Agents in Vitro and their Stability, J. Pharmacol. and Exper. Therap. 14:157 (October) 1919; Hanzlik, P. J., Karsner, H. T., and Fetterman, J., Anaphylactoid Conditions, J. Pharmacol. and Exper. Therap. 14:189 (Oct.) 1919; Hanzlik, P. J., Karsner, H. T., and Fetterman, F., Anaphylactoid Phenomena from Thromboplastic Agents, J. Pharmacol. and Exper. Therap. 14:229 (Nov.) 1919.
139 La Coste: Annalen der Chemie (Liebig’s) 208:34.
140 Castelli, G.: Arch. f. Schiffs- u. Tropen-Hyg. 16:605, 1912.
141 Nichols, H. J.: Salvarsan and Sodium Cacodylate, J. A. M. A. 56:492 (Feb. 18) 1911.
142 Cole, H. N.: A Study of Sodium Cacodylate in the Treatment of Syphilis, J. A. M. A. 67:2012 (Dec. 30) 1916.
143 Voegtlin, Carl, and Smith, H. W.: J. Pharmacol. and Exper. Therap. 16:449, 1921.
144 Wright, B. L.; Kennell, L. A., and Hussey, L. M.: M. Rec. 97:607 (April 10) 1920.
145 J. A. M. A. June 12, 1920, p. 1654.
146 Nichols, H. J.: The Spirocheticidal Value of Disodium Ethyl Arsenate (Mon-Arsone), J. A. M. A. 76:1335 (May 14) 1921.
[G] Read before the Section on Pharmacology and Therapeutics at the Sixty-Seventh Annual Session of the American Medical Association, Detroit, June, 1916.
147 Lyons, A. B.: Detroit Lancet, 1882, 6, 157.
148 The Journal A. M. A., Nov. 21, 1914, p. 1871.
149 Zinc permanganate, J. A. M. A., Feb. 6, 1909, p. 488; Reports Chem. Lab. 2:15, 1909.
150 The Unreliability of Unimportant Medicaments, The Journal A. M. A., Sept. 28, 1912, p. 1156.
151 Levy, R. L., and Rowntree, L. G.: On the Toxicity of Various Commercial Preparations of Emetin Hydrochlorid, Arch. Int. Med., March, 1916, p. 420.
152 The Journal A. M. A., March 5, 1910, p. 806.
153 The Journal A. M. A., Aug. 19, 1911, p. 671.
154 The Journal A. M. A., April 20, 1912, p. 1216.
155 The Journal A. M. A., April 27, 1912, p. 1298.
156 The Journal A. M. A., April 4, 1914, p. 1109.
157 The Journal A. M. A., Nov. 27, 1915, p. 1932.
158 Reports A. M. A. Chemical Laboratory, 1912, v, 102.
159 Am. Jour. Pharm., 1915, 87, 439.
160 The Journal A. M. A., Jan. 23, 1909, p. 311.
161 The Journal A. M. A., Dec. 31, 1910, pp. 2303 and 2314.
162 The Journal A. M. A., Oct. 5, 1912, p. 1295.
163 The Journal A. M. A., April 17, 1915, p. 1344.
164 The Journal A. M. A., April 4, 1914, p. 1108.
[H] Contribution from the Chemical Laboratory of the American Medical Association.
165 The Outlook, Jan. 17, 1917, p. 100.
166 Med. Rec., New York, Jan. 27, 1917, p. 160.
167 Matas, Rudolph: Burns Treated with Paraffin Mixtures, New Orleans Med. and Surg. Jour., April, 1917, p. 681.
168 These determinations will be described later.
169 When the sample was first obtained, this feature was not observed.
170 Made by the Abbott Laboratories, Chicago, and accepted by the Council on Pharmacy and Chemistry for New and Nonofficial Remedies, The Journal, May 12, 1917, p. 1406.
171 No chemical examination was made.
172 Sollmann, Torald: Suggested Formulas for Paraffin Films, The Journal A. M. A., April 7, 1917, p. 1037.
173 Hull, A. J.: The Treatment of Burns by Paraffin, Brit Med. Jour., Jan. 13, 1917, p. 37; The Treatment of Burns by Paraffin, Therapeutics, The Journal A. M. A., Feb. 3, 1917, p. 373.
174 The “soft paraffin” of the British Pharmacopeia resembles petrolatum, U. S. P., Queries and Minor Notes, The Journal A. M. A., April 28, 1917, p. 1281.
175 The paraffin used in this formula was supplied by the Standard Oil Company of Indiana; the melting point given by the manufacturers is from 120 to 122 F., which, according to the American Standard of taking melting points, gives higher results than the method described in the pharmacopeia.
176 The “Asphalt Varnish” used was obtained from Remien & Kuhnert Company, Chicago.
177 While needless, a color resembling “Ambrine” may be obtained by the addition of coloring agents.
178 In a personal communication Dr. Sollmann expressed the opinion that the synthetic preparation is inferior to the paraffin used in the formula, basing the view on the greater plasticity of the paraffin. For practical purposes, the paraffin will most probably serve as well as the mixture, especially when it is held in place by bandages, but I believe that the mixture is more adhesive.
179 Paraffin is sometimes spoken of as “white wax.” This is unfortunate, as “white wax” is an official name for “White Beeswax, U. S. P.” The term “white wax” is also often applied to “Chinese wax,” which is formed from an insect living on the tree Ligustrum lucidum.
180 I am indebted to Dr. Torald Sollmann for these methods.
181 When painting a surface with a paraffin film, I found that the temperature of the paraffin should not be too close to the melting point, but several degrees above; otherwise it does not “set” well.
182 Fisher, H. E.: Nonadhering Surgical Gauze, The Journal A. M. A., March 25, 1916, p. 939.
183 Sollmann, Torald: Paraffin-Covered Bandages, The Journal A. M. A., April 21, 1917, p. 1178.
184 Rep. Chem. Lab., A. M. A., 1915, 8, 89.
185 Rep. Chem. Lab., A. M. A., 1915, 8, 106.
186 Rep. Chem. Lab., A. M. A., 1916, 9, 118.
187 U. S. Disp., ed. 19, p. 1315.
188 Am. Disp., ed. 2, p. 2022.
189 U. S. Pharmacopeia, IX, p. 481.
190 Rep. Mass. Bd. Health, 1909, 41, 477.
191 Pharm. Jour., 1912, 89, 610.
192 Pharm. Jour., 1912, 89, 610.
193 The time required to complete the process after the initial portion of lard has been added should be about twenty minutes.
194 In order to facilitate the incorporation of the iodine with the fatty base the iodine was first powdered by trituration with alcohol and drying the powder in the air.
195 Rep. Chem. Lab., A. M. A., 1916, 9, 118.
196 Pharm. Jour., 1912, 89, 610.
197 The resultant fatty residue was of a brownish-green color. It no longer had either the taste, color or odor of lard. It was noted that the fats, after removal by this method from the freshly prepared ointment, were nearly white. As the ointment aged the fat became successively darker in color.
198 The method depends upon the conversion of all of the iodine compounds into iodate by fusion with sodium hydroxide and oxidation with potassium nitrate. The melt is dissolved in water, a little sodium bisulphite added, the solution cooled and neutralized with phosphoric acid, using methyl orange as indicator. An excess of bromine water is added, and the mixture boiled to expel carbon dioxid and bromine. A little sodium salicylate is added, the solution cooled, an excess of potassium iodid added, and the liberated iodine titrated with tenth-normal sodium thiosulphate in the usual way. One sixth of the iodine found is obtained from the material assayed, the balance being furnished by the potassium iodide added.—Jour. Biochem., 1914, 19, 251.
199 In order to determine whether the iodine which is in combination with fat is absorbed through the skin, a few experiments were carried out. The dark-colored iodine-containing fat (obtained from the ointment and washed free from potassium iodide by the method described above) was rubbed thoroughly into the skin of the forearm. It was allowed to remain for four hours, after which the limb was scoured with soap suds. Beginning at the time of the application the urine was collected for forty-eight hours. This was evaporated to small bulk and the residue tested for iodine by Kendall’s method. Small amounts of iodine were found. These findings were taken to indicate that the iodine-containing fat is absorbed to some extent by the skin. It is generally believed that potassium iodide is not absorbed by the unbroken skin. Therefore it seems reasonable to suppose that the principal iodine effects obtainable from iodine ointment are those due to the free iodine contained in the preparation, supplemented to a slight extent by the iodine which is contained in the fatty ointment base.—Jour. Biochem., 1914, 19, 251.
200 Unfortunately, the nondescriptive name “aspirin” has been used extensively in European literature and has even got into European pharmacopeias, instead of the scientific name “acetylsalicylic acid.”
201 For reference to older literature see Beilstein, II, 1496 (889).
202 “The Melting Temperature of Aspirin and Salicylic Acid Mixtures,” Proc. Assoc. Off. Agr. Chem., 1912; Bureau of Chemistry, Department of Agriculture, Bull. 162.
203 “Assay of Aspirin,” J. Pharm. Chem., 15 (117), No. 7, 213.
204 Similar observations were made by Emery and Wright, who state: “An accurate determination of the melting temperature in this way (the rate of heating was such as to give a rise in temperature of about 1° per minute) is rendered difficult by the fact that ‘aspirin’ decomposes on heating, as evidenced in the depression of the melting temperature of the pure substance of about 1° for every five minutes’ heating just below its melting temperature.”
205 Isolated crystals attached to the walls of the melting-point tube, apart from the bulk acetylsalicylic acid, melted at a lower temperature.
206 An excess of alcohol destroys or lessens the color when only a very minute amount of salicylic acid is present.
207 The control should be made each time as standing in the air changes its tinctorial power.
208 The presence of pure acetylsalicylic acid does not seem to affect the iron (Fe+++) salicylic acid coloration. The small amount of acetic acid was added to the sodium salicylate control solution (1) to stimulate an acidity approximating the acidity of the acetylsalicylic acid, and (2) since acetylsalicylic acid gives by hydrolysis both acetic acid and salicylic acid, it was thought advisable to add acetic acid to the standard. If there is any free acetic acid in a sample of acetylsalicylic acid containing salicylic acid (which I believe is generally the case when salicylic acid is present) then it would modify the color given by the same amount of salicylic acid alone. For this reason it was thought to be more comparable to have the standard contain a slight amount of acetic acid.
209 This standard is somewhat similar to the one proposed by T. W. Thoburn and Paul J. Hanzlik, J. Biol. Chem., 23, 175.
210 Apoth. Ztg., 1915, p. 247; Bull. soc. chem., 17 (1915), 401. “Studies of the decomposition of aspirin determined by titrametric methods and by conductivity measurements indicate that the reaction is exceedingly complex,” T. and H. Chem. Abs., 10, 591.
211 “In the Matter of Aspirin. Answer to the warning circular of the Bayer Co. of June 1, 1917,” by Mr. Paul Bakewell, Monsanto Chemical Works.
212 U. S. patent number 812,554—the novocain patent—declares that the salt melts at 156 C. Evidently based on this, the German Pharmacopoeia Remedia “Hoechst” and past editions of New and Nonofficial Remedies give this melting point. Two specimens of German made novocain obtained from our files, stated to be manufactured by Farbwerke-Hoechst vorm. Meister, Lucius and Bruening, Hoechst a.M. were found to melt, respectively, between 154 and 155 C. and between 153.5 and 154.5 C. when the melting point was determined according to the directions of the U. S. Pharmacopoeia, 9th revision. The various specimens examined at that time melted between 153 and 155 C. and it was decided to permit this range.
213 Annual Reports of the Chem. Lab. of the A. M. A., 1915, p. 89.
214 Warren, L. E.: Iodin Ointment, Am. J. Pharm., August, 1917, p. 339.
215 Ibid., p. 90.
216 Reports A. M. A. Chemical Laboratory, 1915, p. 106; Ibid., 1917, p. 87.
217 Ibid., 1917, p. 87.
[I] From the Chemical Laboratory of the American Medical Association.
[J] The first article of this series dealt with the purity of acetylsalicylic acid. Leech, P. N.: Examination of American-Made Acetylsalicylic Acid, J. Indust. & Engin. Chem., April, 1918, p. 288. “What’s in a Name?” ibid., p. 255. Acetylsalicylic Acid, or “What’s in a Name?” Editorial, J. A. M. A. 70:1097 (April 13) 1918.
218 Stieglitz, Julius: Synthetic Drugs II, J. A. M. A. 70:688 (March 9) 1918. Leech, P. N.: The Vindication of the American Chemist; Synthetic Drugs, Chicago Chem. Bull. January, 1918, p. 230.
219 The Quality of American-Made Synthetics, J. A. M. A. 69:1018 (Sept. 22) 1917.
220 This committee is composed of Julius Stieglitz, chairman, professor of chemistry, University of Chicago; W. A. Puckner, secretary of the Council on Pharmacy and Chemistry, American Medical Association, and Moses Gomberg, professor of chemistry, University of Michigan.
221 Stieglitz, Julius: Shortage of Synthetic Remedies, J. A. M. A. 69:400 (Aug. 4) 1917.
222 Foreign Patents to Be Open to American Manufacturers, J. A. M. A. 69:1550 (Nov. 3) 1917.
223 For an interesting discussion, see Stieglitz, Julius: Synthetic Drugs, J. A. M. A. 70: 536 (Feb. 23); 688 (March 9); 923 (March 30) 1918. Bracken, L. L.: Federal Trade Commission Requests Use of Official Names, ibid. 70:558 (Feb. 23) 1918.
224 The testing and standardizing of arsphenamin is being done by the Hygienic Laboratory, U. S. Public Health Service. For chemical tests see reprint 472, Public Health Reports. For a review of the patent literature see article by H. F. Lewis, J. Indust. Engin. Chem., Feb. 1, 1919, p. 141.
225 New and Nonofficial Remedies, 1919, published by The Council on Pharmacy and Chemistry of the American Medical Association, p. 82.
226 The pharmaceutic monograph on barbital has been omitted. It was published in the 1918 edition of the Annual Reports of the Chemical Laboratory of the American Medical Association.
227 New and Nonofficial Remedies, 1918, p. 96.
228 Puckner, W. A., and Hilpert, W. S.: Veronal-Sodium and Medinal, J. A. M. A. 52:311 (Jan. 23) 1909; Rep. A. M. A. Chemical Lab., 2:13.
229 Since this was written, the Council on Pharmacy and Chemistry has also accepted “Barbital-Sodium Abbott.”
230 No short, scientific name has been given for this substance although several are under consideration.
231 Certain chemical tests are described by E. H. Rankin, Indian J. M. Res. 4:237, 1916; also Chem. Abst. 10:524. Other references are Schmidt: Pharmazeutische Chemie 2:990, Beilstein II, (403). Arends, G.: Neue Arzneimittel und pharmazeutische Spezialitäten, Ed. 4, 1913, p. 271.
232 Kennert: Chem. Zentralbl. 2:556, 1897.
233 Doebner and Gieseke: Ann. d. Chem. (Liebigs) 240:291, 1887.
234 The validity of this patent is to be doubted.
235 Attempts were made to make salts of phenylcinchoninic acid with metals such as copper, mercury, barium and calcium, and also the chloroplatinic acid or periodid addition products. Reliable quantitative results could not be obtained.
236 This corresponds to “diluted alcohol, U. S. P.”
237 The ethyl acetate was Merck’s product (redistilled), stated to contain 81.6 per cent. of ethyl acetate, 10 per cent. alcohol and alcohol derivatives.
238 Seidell, A.: Bull. 67, Hyg. Lab., U. S. P. H. S., p. 11.
239 Very recently the Chemical Foundation, Inc., has undertaken to grant licenses for cinchophen. The Calco Chemical Company has obtained one.
240 The monograph appears in New and Nonofficial Remedies, 1919.
241 The report of these and subsequent toxicity experiments on procain appeared in the report of the Council on Pharmacy and Chemistry, J. A. M. A. 72:136 (Jan. 11) 1919.
242 Seidell: J. Biol. Chem. 14:19, 1913.
243 Proposed Institute for Drug Research, editorial Chicago Chem. Bull., April, 1919, p. 67.
[K] See also ????.
244 Former estimates of the number of physicians who prescribed Anasarcin appear to have been too high, possibly based on the ratio obtaining in Winchester, Tenn. Inquiry at five fairly busy drug stores in a large eastern city showed that in no instance was the pharmacist even acquainted with the name. One pretended to be, and manifested pity for the inquirer’s ignorance in supposing that it could be imported during the war! He was obviously merely less honest than the others, who frankly admitted they had never heard of it.
245 J. A. M. A. 44:1791 (June 3) 1905; ibid. 44:1997 (June 24) 1905; ibid. 45:935 (Sept. 23) 1905; ibid. 46:134 (Jan. 13) 1906; ibid. 46:290 (Jan. 27) 1906; ibid. 58:280 (Jan. 27) 1912.
[L] See index for other articles on Anasarcin.
246 J. A. M. A. 46:288 (Jan. 27) 1906; ibid. 48:1535 (May 4) 1907; ibid. 48:1614 (May 11) 1907, and ibid. 49:1992 (Dec. 8) 1917.
[M] See index for additional article on Bell-Ans.
247 British Association for the Advancement of Science. Second Report on Colloid Chemistry. Published for the Department of Scientific and Industrial Research by His Majesty’s Stationery Office.
248 Ferrivine, Intramine and Collosol Iodine, J.A.M.A. 69:841 (Sept. 8) 1917.
249 Collosol Preparations, J.A.M.A. 72:1694 (June 7) 1919.
250 Collosol Cocaine Not Admitted to N. N. R., J. A. M. A. 72:1094 (April 12) 1919.
[N] Glyco-Heroin
251 Here is what The Journal published on Erling:
A. E. Erling according to the stationery, is “Chairman” of “Censors.” Our records fail to show that Erling ever graduated in medicine. The Health Department of Milwaukee, however, says that Erling, when interviewed, claimed to have “a diploma from the German Medical College of Chicago, but refused to show or present the same.” The American Medical Directory has this item:
German Medical College, Chicago. Chartered Dec. 28, 1891, by Johann Malok. Fraudulent. Extinct.
A few years ago Erling was in La Crosse, Wis., and in 1908 a circular letter bearing his name and picture was sent out, from which the following extracts are taken. Capitalization as in the original:
“Dear Friend:—Permit me to call your attention to the fact that Dr. A. E. Erling, the eminent specialist, after many years of travel, practice and medical research, has given up his extensive road practice and severed his connection with the several medical institutes which have heretofore occupied considerable of his attention.... Dr. Erling’s success in the treatment of all CHRONIC DISEASES IS truly remarkable. NERVOUSNESS, all BLOOD DISEASES, RHEUMATISM, DISEASES PECULIAR TO WOMEN; CATARRH, DEAFNESS, CHRONIC CONSTIPATION ... APPENDICITIS ... PILES, STOMACH TROUBLES, PARTIAL PARALYSIS, etc., give way as if by magic under his skilful method of treatment.... Understand, please, that Dr. Erling DOES NOT ACCEPT A CASE FOR TREATMENT unless he can PROMISE A SPEEDY AND POSITIVELY PERMANENT CURE.”
The Journal also has in its files advertisements (vintage of 1915), from some Wisconsin country newspapers; which notify the afflicted that “Drs. Erling and Karass, the expert German Specialists,” could be seen in their offices in the “Schlegel Hotel,” the “Schlitz Hotel,” etc., as the case might be. Whether one of these “German Specialists” was Dr. Arnold E. Erling, The Journal does not know. Official medical records fail to show, at least, that there is any other Erling in the state of Wisconsin.
252 “Free” or elementary iodin (such as the tincture of iodin) is used externally for its local irritant and antiseptic effects. “Combined iodin” (e. g., iodid of potassium), does not produce these effects; and when preparations containing iodin in combined form are used, it is with the expectation of obtaining the systemic (“alterative”) effects such as are produced by iodids.
[O] This matter was largely reprinted in the Propaganda for Reform, eighth and ninth editions.
[P] See index for additional articles on proteogens.
253 Page 227.
254 Advance pages, the Oxford Medicine, 1919, Vol. 1, Part. 3, p. 245.
255 Some of the Sal Hepatica advertising has claimed that it “is a saline combination with the addition of Sodium Phosphate and Lithia Citrate!”
256 J. A. M. A., Feb. 7, 1914, p. 472.
257 Page 64.
258 At this time Tyree’s Antiseptic Powder was an “ethical proprietary”—heaven save the mark!—and advertised only to physicians. Later, as The Journal has shown, it entered the “patent medicine” field as “ideal for douche” and the “best preventative known.” The articles on this nostrum are reprinted in the ninth edition of “The Propaganda for Reform.”
259 See index for additional article.
260 The Bayer people may try to convey the impression that “Aspirin” is pure and reliable whereas other brands are not. Since acetylsalicylic acid is a definite chemical compound, there is no more likelihood of this being sophisticated than there is of quinin being adulterated. Furthermore, the Council in accepting acetylsalicylic acid for New and Nonofficial Remedies has provided standards of purity which will insure a uniform product. The brand of one firm—Powers-Weightman-Rosengarten Co., of Philadelphia—has been accepted by the Council on Pharmacy and Chemistry for inclusion in New and Nonofficial Remedies, 1917.
261 The following brands of acetylsalicylic acid conform to the standards of the Council and are in New and Nonofficial Remedies:
“Aspirin—L. and F.”: Lehn & Fink, New York.
“Acetylsalicylic Acid—Squibb”: E. R. Squibb & Sons, New York.
“Acetylsalicylic Acid—Merck”: Merck & Co., New York.
“Acetylsalicylic Acid—Milliken”: John T. Milliken & Co., St. Louis.
“Acetylsalicylic Acid—M. C. W.”: Mallinckrodt Chemical Works, St. Louis.
“Acetylsalicylic Acid—Monsanto”: Monsanto Chemical Works, St. Louis.
“Acetylsalicylic Acid—P. W. R.”: Powers-Weightman-Rosengarten Company, Philadelphia.
262 Warning Against Untried Medicaments, J. A. M. A. 74:1654 (June 12) 1920.
263 Wright, B. L.; Kennell, L. A., and Hussey, L. M.: Med. Rec. 97:607 (April 10) 1920.
264 Nichols, H. J.: Salvarsan and Sodium Cacodylate, J. A. M. A. 56:492 (Feb. 18) 1911.
265 Voegtlin, Carl, and Smith, H. W.: J. Pharmacol. & Exper. Therap. 16:449, 1921.
266 Compare Schamberg, J. F.; Kolmer, J. A., and Raiziss, G. W.: Am. J. M. Sc. 150:25
(July) 1920.
[Q] From the Cancer Research Service of the General Memorial Hospital, New York.
[R] This critical discussion of the status of chemotherapy in tumors was prepared at the request of the Council on Pharmacy and Chemistry of the American Medical Association.
267 Wassermann, Keysser and Wassermann: Deutsch. med. Wchnschr. 37:2389, 1911. Wassermann and Hansemann: Berl. klin. Wchnschr. 49:4, 1912.
268 Neuberg and Caspari: Deutsch. med. Wchnschr. 38:375, 1912. Neuberg, Caspari and Löhe: Berl. klin. Wchnschr. 49:1405, 1912.
269 Gers, Gaube du: La cuprase et le cancer, Paris, 1913.
270 Keysser: Wien. klin. Wchnschr. 26:1664, 1913.
271 Izar: Ztschr. f. Immunitätsforsch., 1913. Izar and Basile: Berl. klin. Wchnschr., 1913, p. 1312.
272 Lewin, Carl: Berl. klin. Wchnschr., 1913, p. 147; Berl. klin. Wchnschr., 1913, p. 541.
273 Werner and Szécsi: Ztschr. f. Chemotherap., 1913, Orig., i, 358. Szécsi: Ibid., ref., 1913, ii, 1060.
274 Uhlenhuth, Dold and Bindseil: Ref., München. med. Wchnschr., 1912, p. 1782.
275 Contamin, Detoeuf and Thomos: Bull. de l’assn. franç. pour l’étude du cancer, vi, 62.
276 Apolant, H.: VI Tag. der freien Vereinigung für Mikrobiologie., Berlin, 1912. Ref. München. med. Wchnschr., 1912, p. 659.
277 Keysser, F.: Ztschr. f. Chemotherap., 1914, Orig., ii, 188.
278 Wells, H. G., De Witt, and Corper: Ztschr. f. Chemotherap., 1914, Orig., ii, 110.
279 J. M. Research, 1913, p. 497.
280 Weil, Richard: The Effects of Colloidal Copper with an Analysis of the Therapeutic Criteria in Human Cancer, J. A. M. A. 61:1034 (Sept. 27) 1913.
281 Wolff: Die Lehre von der Krebs Krankheit 3:1913.
282 Delbet, P.: Bull. de l’Assn. franç. pour l’étude du cancer 5:121, 1912; ibid. 6:85, 1913.
283 Cade and Girard: Bull. Soc. méd. d. hôp. de Lyon 11:397, 1912.
284 Bougeaut and Galliot: Clinique, Paris 7:501, 1912.
285 Blumenthal, A.: Jour. méd. de Bruxelles, 1912, 17:325; Presse méd. belge 65:919, 1913.
286 Thiroloix and Lancien, A: Bull. et mém. Soc. méd. d. hôp. de Paris 33:197, 1912.
287 Laurent, M., and Bohec, J.: Med. Press and Circular 94:461, 1912.
288 Touche, M.: Bull. et mém. Soc. méd. d. hôp. de Paris 35:451, 1913.
289 Rohdenburg, H.: J. M. Research 26:331, 1915.
290 Organic Phosphorus Compounds, Editorial, J. A. M. A. 40:1958 (June 21) 1913. Marshall, E. K.: The Therapeutic Value of Organic Phosphorus Compounds, J. A. M. A. 44:573 (Feb. 13) 1915.
291 Marshall, E. K.: The Therapeutic Value of Organic Phosphorus Compounds, J. A. M. A. 44:573 (Feb. 13) 1915.
292 McGuire, L. W., and Redden, W. R.: Treatment of Influenza Pneumonia by the Use of Convalescent Human Serum: Preliminary Report, J. A. M. A. 71:1311 (Oct. 19) 1918.
293 Luckhardt, A. B.; Koch, F. C.; Schroeder, W. F.; and Weiland, A. H.: The Physiological Action of Fumes of Iodin, J. Pharmacol & Exper. Therap. 15:1 (March) 1920.
294 J. A. M. A. 48:2196 (June 29) 1907; Editorial 57:1373, Berlin letter, p. 1380 (Oct. 21) 1911; 58:1455 (May 11) 1912; 60:770 (March 8) 1913; 60:1480 (May 10) 1913; 61:1737 (Nov. 8) 1913.
295 Roberts, Dudley, and Cary, E. G.: Bacterial Protein Injections in Influenzal Pneumonia, J. A. M. A. 72:922 (March 29) 1919. Cowie, D. M., and Beaven, P. W.: Nonspecific Protein Therapy in Influenzal Pneumonia, J. A. M. A. 72:1117 (April 19) 1919.
296 Miller, J. L., and Lusk, F. B.: The Treatment of Arthritis by the Intravenous Injection of Foreign Protein, J. A. M. A. 66:1756 (June 3) 1916; The Use of Foreign Protein in the Treatment of Arthritis, ibid. 67:2010 (Dec. 30) 1916.
297 Snyder, R. G.: A Clinical Report of Nonspecific Protein Therapy in the Treatment of Arthritis, Arch. Int. Med. 22:224 (Aug.) 1918.
298 Report of International Health Board, Social Medicine, Medical Economics and Miscellany, J. A. M. A. 72:751 (March 8) 1919.
299 Dec. 28, 1917, p. 629.
300 Granted Feb. 27, 1900.
301 Printers’ Ink, June 29, 1916, p. 189; July 13, 1916, p. 100.
302 Jacobs, W. A., and Heidelberger, M.: Aromatic Arsenic Compounds, II, The Amides and Alkyl Amides of N—Arylglycine Arsonic Acids, J. Am. Chem. Soc. 41:1587 (Oct.) 1919.
303 J. A. M. A. 67:764 (Sept. 2) 1916.
304 Roth, G. B.: Pituitary Standardization, Bull 109, Hyg. Lab., U. S. P. H. S., 1917.
305 Roth, G. B.: Bull 100, Hyg. Lab., U. S. P. H. S.
306 Pittenger, P. S., and Vanderkleed, C. E.: Jour. Am. Pharm. Assn. 6:131, 1917.
307 Puckner, W. A., and Clark, A. H.: Examination of Tablets of Bismuth, Opium and Phenol, The Journal A. M. A., July 25, 1908, p. 330. Puckner, W. A., and Hilpert, W. S.: Tablets of Bismuth, Opium and Phenol, Dec. 17, 1910, p. 2169, May 6, 1911, p. 1344. Unreliable Pharmaceutical Products, editorial, May 6, 1911, p. 1335.
308 Puckner, W. A., and Warren, L. E.: Aromatic Digestive Tablets, The Journal A. M. A., Aug. 20, 1910, p. 710.
309 Kebler, L. F.: The Tablet Industry, Jour. Am. Pharm. Assn., 1914, 3, 820, 937, 1062.
310 Bull. 200, Connecticut Agricultural Station, Food and Drug Products, 1917, p. 161.
[S] Read before the Section on Pharmacology and Therapeutics at the Sixty-Eighth Annual Session of the American Medical Association, New York, June, 1917.
[T] This article clearly states the difficulties experienced by the Council in estimating the merits of a proprietary medicinal product and clearly defines the method which has been found to be practical in judging of the therapeutic value of such preparations. The Council has approved this discussion of the subject and has directed that the paper be published in the annual Council reports.
W. A. Puckner, Secretary.
311 Sherman, G. H.: Vaccines in Toxic Conditions, Illinois M.J. 38: 314 (Oct.) 1920.
Return to transcriber’s notes
Spelling variations:
amenorrhea/amenorrhoea
ampoule/ampule/ampul
anaemia/anemia
analgetic/analgesic
any one/anyone
arsenphenolamin/arseno-phenolamin
arsphenamin/arsphenamine
bromid/bromide
cerebral-spinal/cerebro-spinal/cerebrospinal
chlorid/chloride
cocain/cocaine
Cod-Liver Oil/Cod Liver Oil
diethylenediamene/diethylene-diamine
dioxid/dioxide
drams/drachms
dulness/dullness
envelop/envelope
every one/everyone
extention/extension
formaldehyd/formaldehyde
golden-seal/goldenseal
gonorrhoic/gonorrheaic
Grip/Grippe
guaranty/guarantee
Heitzman/Heitzmann
hexamethylenetetramin/hexamethylenetetramine/hexamethylene-tetramine/hexamenthylenetetramin
iodid/iodide
iodin/iodine
melena/melaena
oxid/oxide
Parresine/Paresine
pharmacopeia/pharmacopoeia
post-graduate/postgraduate
sanatarium/sanitarium/sanatorium
selenio-vanadium/selenium-vanadium
sequelæ/sequelae/sequella
sulphurated/sulphureted/sulphuretted/sulphurretted
technique/technic
vitamines/vitamins
Spelling corrections:
accompanished —> accomplished
accurtely —> accurately
acethphenetidin —> acetphenetidin
advertiseing —> advertising
advertisments —> advertisements
aluminium —> aluminum
Amercan —> American
amples —> ampules
an ypure —> any pure
and —> an
and —> on
Anelgesic —> Analgesic
anitseptic —> antiseptic
anodoyne —> anodyne
anohter —> another
aortis —> aortitis
artifically —> artificially
asosciated —> associated
Assimiliation —> Assimilation
at —> as
atttention —> attention
authenic —> authentic
author —> authors
Bacilus —> Bacillus
baillus —> bacillus
Barbitol —> Barbital
beeen —> been
benefical —> beneficial
betanaphtol —> betanaphthol
betwen —> between
bibliograpy —> bibliography
Binghampton —> Binghamton
binodide —> biniodide
Botazzi —> Bottazzi
carbonic —> carbolic
carodylate —> cacodylate
carrer —> carrier
carthartic —> cathartic
characteristc —> characteristic
Chautaqua —> Chautauqua
chefly —> chiefly
Chemisty —> Chemistry
Chlorilyptus —> Chlorlyptus
Choron —> Chloron
cities —> cites
claims —> claim
clipping —> clippings
clorless —> colorless
coeffiecient —> coefficient
Collosal —> Collosol
compained —> complained
compaint —> complaint
compatable —> compatible
Compond —> Compound
compostion —> composition
Concernng —> Concerning
conditons —> conditions
conection —> connection
coneentrated —> concentrated
confréres —> confrères
connenction —> connection
constitutent(s) —> constituent(s)
Contro —> Control
convicition —> conviction
corespondence —> correspondence
corpuscules —> corpuscles
crystaline —> crystalline
davantages —> advantages
deparment —> department
destroyes —> destroys
develope —> develop
diehylbarbituric —> diethylbarbituric
Diethylbarbiutric —> Diethylbarbituric
digtalis —> digitalis
dirctory —> directory
distate —> distaste
distils —> distills
does —> doses
dulness —> dullness
dystentery —> dysentery
Ehlrich —> Ehrlich
Electobioscope —> Electrobioscope
emipric —> empiric
employe —> employee
entiled —> entitled
eperiments —> experiments
esesntially —> essentially
essentally —> essentially
eucalytpus —> eucalyptus
examation —> examination
extravant —> extravagant
exurberant —> exuberant
Farbkerke-Hoechst —> Farbwerke-Hoechst
Farbwercke-Hoechst —> Farbwerke-Hoechst
Farbwerke-Hochest —> Farbwerke-Hoechst
Farbwerke-Hoechest —> Farbwerke-Hoechst
Farkwerke —> Farbwerke
Ferbwerke —> Farbwerke
finsh —> finish
Firwin —> Firwein
fitrate —> filtrate
flgrant —> flagrant
fluorish —> flourish
FOM —> FROM
form —> from
fradulent —> fraudulent
Fritsche —> Fritzsche
gactric —> gastric
galactogogic —> galactagogic
galactogogue —> galactagogue
Galatagogue —> Galactagogue
Giesecke —> Gieseke
Glyecrin —> Glycerin
Goering —> Goehring
gonoccoccus —> gonococcus
gualtheria —> gaultheria
guiacol —> guaiacol
habtual —> habitual
Hallon —> Hallion
Hcl —> HCl
he —> be
Heath —> Health
hexamenthylenamin —> hexamethylenamin
Hexamenthylenetetramin —> Hexamethylenetetramin
Hexamethylentetramine —> Hexamethylenetetramine
Higienic —> Hygienic
hundrance —> hundrance
Hussy —> Hussey
hydiodic —> hydriodic
hydochloric —> hydrochloric
hyerplastic —> hyerplastic
hyocyamus —> hyocyamus
hyoscymus —> hyoscymus
idodin —> iodin
idosyncrasy —> idiosyncrasy
inections —> injections
ingorance —> ignorance
insommia —> insomnia
intractions —> interactions
intramuscuarly —> intramuscularly
itme —> time
jubject —> subject
lechithin —> lecithin
liklihood —> likelihood
lisited —> listed
LYPMH —> LYMPH
Maganese —> Manganese
Magnesim —> Magnesium
manufacturer —> manufacturers
Mazazine —> Magazine
mecurial —> mercurial
mecuric —> mercuric
medicince —> medicine
Metorrhagia —> Metrorrhagia
Meyers —> Myers
minimum —> minim
mixutre —> mixture
monoceticacidester —> monoaceticacidester
monoply —> monopoly
Naphtol —> Naphthol
napthal —> napthol
Napthol —> Naphthol
neuralga —> neuralgia
nirtic —> nitric
Nutrolactic —> Nutrolactis
O. H. —> A. H.
odide —> iodide
Ophtalmol —> Ophthalmol
or —> of
orginal —> original
pag —> page
pamphet —> pamphlet
parffin —> paraffin
parminobenzoate —> paraminobenzoate
particuarly —> particularly
pasticity —> plasticity
patent —> patient
pharmaceptical —> pharmaceutical
Pharmacopia —> Pharmacopeia
pharmactists —> pharmactists
pharmceutical —> pharmaceutical
phoshite —> phosphite
Phram. —> Pharm.
physicial —> physical
Pineolum —> Pineoleum
Pinoleum —> Pineoleum
Pitsburgh —> Pittsburgh
pituiary —> pituitary
Plurig andular —> Pluriglandular
Pneumocci —> Pneumococci
Pneumococccus —> Pneumococcus
pnuemonia —> pneumonia
popularily —> popularly
populary —> popularly
postive —> positive
Power —> Powder
precipiate —> precipitate
prefare —> preface
preparatons —> preparations
prescibed —> prescribed
prevous —> previous
prinicple —> principle
procedues —> procedures
profesion —> profession
proper ties —> properties
proponderant —> preponderant
Protier —> Portier
Protogen —> Proteogen
puporting —> purporting
pyrogenic —> pyogenic
qestions —> questions
quantiatively —> quantitatively
Querry —> Query
Quine —> Quinine
radicle —> radical
Rathjen —> Rahtjen
recomendation —> recommendation
recommmend —> recommend
recommmended —> recommended
refree —> referee
rememebered —> remembered
Reparts —> Reports
ressistance —> resistance
restrospect —> retrospect
reults —> results
Rhatjen —> Rahtjen
saccarine —> saccharine
same —> some
santorium —> sanatorium
scientfic —> scientific
seleninum —> selenium
seleno-vanadium —> selenio-vanadium
series —> serious
Shering —> Schering
simiplicity —> simplicity
specifially —> specifically
Staphylococus —> Staphylococcus
submited —> submitted
submittted —> submitted
substaneous —> subcutaneous
supernatent —> supernatant
suport —> support
syphilus —> syphilis
Syracue —> Syracuse
teasponful —> teaspoonful
tehnic —> technic
tetraoidid —> tetraiodid
that —> than
thearapeutic —> therapeutic
thiomethylarsniate —> thiomethylarsinate
Thyroidectin —> Thyroidectin
tisssues —> tissues
tisues —> tissues
to —> too
travenous —> intravenous
treament —> treatment
tremely —> extremely
troat —> throat
tubercuclosis —> tuberculosis
unbeliveable —> unbelievable
vasty —> vastly
vially —> vitally
Wulflng —> Wulfing
240:2'1, 1887. —> 240:291, 1887.
Return to transcriber’s notes