BY
R. W. SINDALL, F.C.S.
CONSULTING CHEMIST TO THE WOOD PULP AND PAPER TRADES; LECTURER
ON PAPER-MAKING FOR THE HERTFORDSHIRE COUNTY COUNCIL, THE
BUCKS COUNTY COUNCIL, THE PRINTING AND STATIONERY
TRADES AT EXETER HALL, 1903-4, THE INSTITUTE
OF PRINTERS; TECHNICAL ADVISER TO THE
GOVERNMENT OF INDIA, 1905
AUTHOR OF “PAPER TECHNOLOGY,” “THE SAMPLING OF WOOD PULP”
JOINT AUTHOR OF “THE C.B.S. UNITS, OR STANDARDS OF PAPER
TESTING,” “THE APPLICATIONS OF WOOD PULP,” ETC.
WITH ILLUSTRATIONS, AND A BIBLIOGRAPHY OF WORKS
RELATING TO CELLULOSE AND PAPER-MAKING

NEW YORK
D. VAN NOSTRAND COMPANY
23 MURRAY AND 27 WARREN STREETS
1908
Paper-making, in common with many other industries, is one in which both engineering and chemistry play important parts. Unfortunately the functions of the engineer and chemist are generally regarded as independent of one another, so that the chemist is only called in by the engineer when efforts along the lines of mechanical improvement have failed, and vice versa. It is impossible, however, to draw a hard and fast line, and the best results in the art of paper-making are only possible when the manufacturer appreciates the fact that the skill of both is essential to progress and commercial success.
In the present elementary text-book it is only proposed to give an outline of the various stages of manufacture and to indicate some of the improvements made during recent years.
The author begs to acknowledge his indebtedness to manufacturers and others who have given permission for the use of illustrations.
| PAGE | ||
| PREFACE | v | |
| LIST OF ILLUSTRATIONS | ix | |
| CHAPTER | ||
| I. | HISTORICAL NOTICE | 1 |
| II. | CELLULOSE AND PAPER-MAKING FIBRES | 20 |
| III. | THE MANUFACTURE OF PAPER FROM RAGS | 47 |
| IV. | ESPARTO AND STRAW | 72 |
| V. | WOOD PULP, AND WOOD PULP PAPERS | 95 |
| VI. | BROWN PAPERS AND BOARDS | 126 |
| VII. | SPECIAL KINDS OF PAPER | 137 |
| VIII. | CHEMICALS USED IN PAPER-MAKING | 153 |
| IX. | THE PROCESS OF “BEATING” | 175 |
| X. | THE DYEING AND COLOURING OF PAPER PULP | 199 |
| XI. | PAPER MILL MACHINERY | 214 |
| XII. | THE DETERIORATION OF PAPER | 229 |
| XIII. | BIBLIOGRAPHY | 253 |
| INDEX | 273 | |
| FIG. | PAGE | |
| 1. | SHEET OF PAPYRUS, SHOWING THE LAYERS CROSSING ONE ANOTHER | 3 |
| 2. | AN EARLY PAPER MILL (FROM “KULTURHISTORISCHEN BILDERBUCH,” A.D. 1564) | 10 |
| 3. | THE PAPER MILL OF ULMAN STROMER, A.D. 1390 (SUPPOSED TO BE THE OLDEST KNOWN DRAWING OF A PAPER MILL) | 12 |
| 4. | THE FIRST PAPER MACHINE, A.D. 1802. PLAN AND ELEVATION | 17 |
| 5. | THE IMPROVED PAPER MACHINE OF A.D. 1810 | 18 |
| 6. | A RAG SORTING HOUSE | 47 |
| 7. | A RAG DUSTER | 49 |
| 8. | A RAG CUTTER | 50 |
| 9. | INTERIOR OF PAPER MILL FOR HAND-MADE PAPER (R. BATCHELOR & SONS) | 51 |
| 10. | VIEW OF A RAG BOILER, SHOWING CONNECTIONS | 52 |
| 11. | A BREAKING AND WASHING ENGINE | 54 |
| 12. | OETTEL AND HAAS' APPARATUS FOR THE MANUFACTURE OF ELECTROLYTIC BLEACH LIQUOR | 58 |
| 13. | THE “HOLLANDER” BEATING ENGINE | 59 |
| 14. | THE HAND MOULD, SHOWING FRAME AND DECKLE | 61 |
| 15. | APPARATUS FOR SIZING PAPER IN CONTINUOUS ROLLS | 63 |
| 16. | A SUPERCALENDER | 65 |
| 17. | THE FIRST WATERMARK IN PAPER | 67 |
| 18. | COTTON | 69 |
| 19. | LINEN | 70 |
| 20. | AN ESPARTO DUSTER | 74 |
| 21. | SINCLAIR'S “VOMITING” ESPARTO BOILER | 75 |
| 22. | A PORION EVAPORATOR | 76 |
| 23. | SCOTT'S MULTIPLE EFFECT EVAPORATOR | 79 |
| 24. | A PRESSE-PÂTE FOR ESPARTO PULP | 85 |
| 25. | ESPARTO PULP | 88 |
| 26. | A CYLINDRICAL DIGESTER FOR BOILING FIBRE | 89 |
| [Pg x]27. | STRAW | 93 |
| 28. | A PAIR OF BARKERS FOR REMOVING BARK FROM LOGS OF WOOD | 98 |
| 29. | VIEW OF HORIZONTAL GRINDER (A), WITH SECTION (B) | 99 |
| 30. | A VERTICAL GRINDER FOR MAKING HOT GROUND MECHANICAL WOOD PULP | 101 |
| 31. | CENTRIFUGAL SCREEN FOR WOOD PULP | 102 |
| 32. | SECTION OF CENTRIFUGAL SCREEN FOR WOOD PULP | 103 |
| 33. | WOOD PULP DIGESTER, PARTLY IN ELEVATION, PARTLY IN SECTION | 106 |
| 34. | VIEW OF ORDINARY SULPHUR-BURNING OVENS | 108 |
| 35. | SPRUCE WOOD PULP | 114 |
| 36. | MECHANICAL WOOD PULP | 115 |
| 37. | THE SCREENS FOR REMOVING COARSE FIBRES FROM BEATEN PULP | 118 |
| 38. | THE PAPER MACHINE (WET END SHOWING WIRE) | 119 |
| 39. | PAPER MACHINE SHOWING WIRE, PRESS ROLLS, AND DRYING CYLINDERS | 123 |
| 40. | SINGLE CYLINDER OR YANKEE MACHINE | 130 |
| 41. | SECTION OF WET PRESS, OR BOARD MACHINE | 131 |
| 42. | DOUBLE CYLINDER BOARD MACHINE | 133 |
| 43. | APPARATUS FOR MAKING PARCHMENT PAPER | 138 |
| 44. | GENERAL ARRANGEMENT OF PLANT FOR MAKING “ART” PAPER | 143 |
| 45. | SECTIONAL ELEVATION OF “COATING” PLANT | 144 |
| 46. | COTTON PULP BEATEN 8 HOURS | 179 |
| 47. | COTTON PULP BEATEN 37 HOURS | 180 |
| 48. | PLAN AND SECTIONAL ELEVATION OF A “HOLLANDER” | 185 |
| 49. | BEATING ENGINE WITH FOUR BEATER ROLLS | 186 |
| 50. | UMPHERSTON BEATER | 188 |
| 51. | SECTION OF UMPHERSTON BEATING ENGINE | 189 |
| 52. | NUGENT'S BEATING ENGINE WITH PADDLES FOR CIRCULATING THE PULP | 190 |
| 53. | A “TOWER” BEATING ENGINE WITH CENTRIFUGAL PUMP FOR CIRCULATING PULP | 191 |
| 54. | WORKING PARTS OF A MODERN REFINING ENGINE | 192 |
| 55. | CONVENTIONAL DIAGRAM OF A WATER SOFTENING PLANT | 216 |
| 56. | AN “ENCLOSED” STEAM ENGINE | 220 |
| 57. | AN ELECTRICALLY DRIVEN PAPER MACHINE | 222 |
| 58. | DIAGRAM OF THE “EIBEL” PROCESS | 223 |
THE MANUFACTURE
OF PAPER
History.—The art of paper-making is undoubtedly one of the most important industries of the present day. The study of its development from the early bygone ages when men were compelled to find some means for recording important events and transactions is both interesting and instructive, so that a short summary of the known facts relating to the history of paper may well serve as an introduction to an account of the manufacture and use of this indispensable article.
Tradition.—The early races of mankind contented themselves with keeping alive the memory of great achievements by means of tradition. Valiant deeds were further commemorated by the planting of trees, the setting up of heaps of stones, and the erection of clumsy monuments.
Stone Obelisks.—The possibility of obtaining greater accuracy by carving the rude hieroglyphics of men and animals, birds and plants, soon suggested itself as an obvious improvement; and as early as B.C. 4000 the first[Pg 2] records which conveyed any meaning to later ages were faithfully inscribed, and for the most part consigned to the care of the priests.
Clay Tablets.—The ordinary transactions of daily life, the writings of literary and scientific men, and all that was worthy of note in the history of such nations as Chaldea and Assyria have come down to us also, inscribed on clay tablets, which were rendered durable by careful baking. On a tablet of clay, one of the earliest specimens of writing in existence, now preserved in the British Museum, is recorded a proposal of marriage, written about B.C. 1530, from one of the Pharaohs, asking for the hand of the daughter of a Babylonian king.
Waxed Boards.—Bone, ivory, plates of metal, lead, gold, and brass, were freely used, and at an early period wooden boards covered with wax were devised by the Romans. In fact, any material having a soft impressionable surface was speedily adopted as a medium for the permanent expression of men's fancy, so that it is not strange to find instances of documents written on such curious substances as animal skins, hides, dried intestines, and leather. The works of Homer, preserved in one of the Egyptian libraries in the days of Ptolemæus Philadelphus, were said to have been written in letters of gold on the skins of serpents.
Leaves, Bark.—The first actual advance in the direction of paper, as commonly understood, was made when the leaves and bark of trees were utilised. The latter especially came speedily into favour, and the extensive use of the inner bark (liber) made rapid headway. Manuscripts and documents written on this liber are to be found in many museums.
Papyrus.—The discovery of the wonderful properties of the Egyptian papyrus was a great step in developing the art of paper-making. The date of this discovery is very uncertain, but one of the earliest references is to be found[Pg 3] in the works of Pliny, where mention is made of the writings of Numa, who lived about B.C. 670. This celebrated plant had long been noted for its value in the manufacture of mats, cordage, and wearing apparel, but its fame rests upon its utility in quite a different direction, namely, for conveying to posterity the written records of those early days which have proved a source of unending interest to antiquaries.
The Egyptian papyrus was made from the fine layers of fibrous matter surrounding the parent stem. These layers were removed by means of a sharp tool, spread out on a board, moistened with some gummy water, and then covered with similar layers placed over them crosswise. The sheets so produced were pressed, dried, and polished with a piece of ivory or a smooth stone. Long rolls of papyrus were formed by pasting several sheets together to give what was termed a volumen.
Roman Papyri.—The Romans improved the process of manufacture, and were able to produce a variety of papers, to which they gave different names, such as Charta hieratica (holy paper, used by priests), Charta Fanniana (a superior paper made by Fannius), Charta emporetica (shop or wrapping paper), Charta Saitica (after the city of Sais), etc. The papyrus must have been used in great quantities for this purpose, since recent explorations in Eastern countries have brought to light enormous finds of papyri in a wonderful state of preservation. In 1753, when the ruins of Herculaneum were unearthed, no less than 1,800 rolls were discovered. During the last ten years huge quantities have been brought to England.
Parchment.—Parchment succeeded papyrus as an excellent writing material, being devised as a substitute for the latter by the inhabitants of Pergamus on account of the prohibited exportation of Egyptian papyrus. For many centuries parchment held a foremost place amongst the available materials serving the purpose of paper, and even to-day it is used for important legal documents. This parchment was made from the skins of sheep and goats, which were first steeped in lime pits, and then scraped. By the plentiful use of chalk and pumice stone the colour and surface of the parchment were greatly enhanced. Vellum, prepared in a similar manner from the skins of[Pg 5] calves, was also extensively employed as a writing material, and was probably the first material used for binding books. Until comparatively recent times the term “parchment” comprehended vellum, but the latter substance is much superior to that manufactured from sheep and goat skins.
Paper.—The Chinese are now generally credited with the art of making paper of the kind most familiar to us, that is from fibrous material first reduced to the condition of pulp. Materials such as strips of bark, leaves, and papyrus cannot of course be included in a definition like this, which one writer has condensed into the phrase “Paper is an aqueous deposit of vegetable fibre.”
A.D. 105.—The earliest reference to the manufacture of paper is to be found in the Chinese Encyclopædia, wherein it is stated that Ts'ai-Lun, a native of Kuei-yang, entered the service of the Emperor Ho-Ti in A.D. 75, and devoting his leisure hours to study, suggested the use of silk and ink as a substitute for the bamboo tablet and stylus. Subsequently he succeeded in making paper from bark, tow, old linen, and fish nets (A.D. 105). He was created marquis in A.D. 114 for his long years of service and his ability.
A.D. 704.—It has been commonly asserted that raw cotton, or cotton wool, was first used by the Arabs at this date for the manufacture of paper, they having learnt the art from certain Chinese prisoners captured at the occupation of Samarkand by the Arabs. The complete conquest of Samarkand does not, however, seem to have taken place until A.D. 751, and there is little doubt that this date should be accepted for the introduction of the art of paper-making among the Arabs.
Recent Researches.—Professors Wiesner and Karabacek have ascertained one or two most important and interesting facts concerning the actual manufacture of pure rag paper. In 1877 a great quantity of ancient manuscripts[Pg 6] was found at El-Faijum, in Egypt, comprising about 100,000 documents in ten languages, extending from B.C. 1400 to A.D. 1300, many of which were written on paper. The documents were closely examined in 1894 by these experts, at the request of the owner, the Archduke Rainer of Austria.
Researches of a later date resulted in the discovery of some further interesting documents which appear to establish with some degree of certainty the approximate date at which pure rag paper, that is, paper made entirely from rag, was manufactured.
Chinese documents dated A.D. 768-786, which have been reported upon by Dr. Hoernle, and others dated A.D. 781-782-787, reported upon by Dr. Stein as recently as 1901, appear to show what materials were used by the Chinese paper-makers in Western Turkestan. The manuscripts mentioned were dug out from the sand-buried site of Dandan Uilig, in Eastern Turkestan.
Professor Wiesner found that all the papers of the Rainer collection were made of linen rag, with an occasional trace of cotton, probably added accidentally. The earliest dated paper was a letter A.D. 874, but two documents, which from other reasons could be identified as belonging to A.D. 792, proved that at the end of the eighth century the Arabs understood the art of making linen paper on network moulds, and further that they added starch for the purpose of sizing and loading the paper.
Professor Karabacek advances some ingenious explanations as to the origin of the idea that raw cotton was first used for paper-making, and he suggests that the legend owes its origin to a misunderstanding of terms. In mediæval times paper was known as Charta bombycina, and sometimes as Charta Damascena, the latter from its place of origin.
Paper was also made in Bambyce, and a natural confusion[Pg 7] arose between the terms, since the word bombyx was used as a name for cotton, and the paper commonly in use suggested that material to the mind of the observer, and the name became corrupted to bombycina.
The suggestions of Professor Karabacek, together with the microscopical investigations of Dr. Wiesner, appear to show that paper made entirely from raw cotton fibre was not known.
Invention of Rag Paper.—Dr. Hoernle, in discussing this question, points out that, taking A.D. 751 as the date when the Arabs learnt the art of paper-making, and A.D. 792 as the date when paper made entirely of linen rag was produced, the date of the invention of rag paper must lie between these two dates. The documents discovered in Eastern Turkestan and bearing the dates mentioned, which papers fill up the gap between the years A.D. 751 and A.D. 792, were found to contain certain raw fibres, such as China grass, mulberry, laurel, as the main constituents, and macerated flax and hemp rags as the minor constituents.
The addition and substitution of rag evidently increased in course of time, and since the improvement thus effected soon became an obvious and established fact, the raw fibres were omitted. Hence the credit of the manufacture of pure rag paper would be given to the people of Samarkand, the date being between the years A.D. 760 and A.D. 792; and further the constitution of such paper has been shown by Dr. Wiesner to be linen, and not cotton, as commonly stated.
These researches are of such interest that we quote Professor Hoernle's translation of the summary of the principal results of Dr. Wiesner's examination of the Eastern Turkestani papers so recently discovered:—
“Taking into account the dates assigned to the papers on[Pg 8] palæographic grounds, the following conclusions may be drawn from the examination of their material:—
“(1) The oldest of the Eastern Turkestani papers, dating from the fourth and fifth centuries A.D., are made of a mixture of raw fibres of the bast of various dicotyledonous plants. From these fibres the half-stuff for the paper was made by means of a rude mechanical process.
“(2) Similar papers, made of a mixture of raw fibres, are also found belonging to the fifth, sixth, and seventh centuries. But in this period there also occur papers which are made of a mixture of rudely pounded rags and of raw fibres extracted by maceration.
“(3) In the same period papers make their appearance in which special methods are used to render them capable of being written on, viz., coating with gypsum and sizing with starch or with a gelatine extracted from lichen.
“(4) In the seventh and eighth centuries both kinds of papers are of equal frequency, those made of the raw fibre of various dicotyledonous plants and those made of a mixture of rags and raw fibres. In this period the method of extracting the raw fibre is found to improve from a rude stamping to maceration; but that of preparing the rags remains a rude stamping, and in the half-stuff thus produced from rags it is easy to distinguish the raw fibre from the crushed and broken fibre of the rags.
“(5) The old Eastern Turkestani (Chinese) paper can be distinguished from the old Arab paper, not only by the raw fibres which accompany the rag fibres, but also by the far-reaching destruction of the latter.
“(6) The previous researches of Professor Karabacek and the author had shown that the invention of rag paper was not made in Europe by Germans or Italians about the turn of the fourteenth century, but that the Arabs knew its preparation as early as the end of the eighth century.
“The present researches now further show that the beginnings of the preparation of rag paper can be traced to the Chinese in the fifth or fourth centuries, or even earlier.
“The Chinese method of preparing rag paper never progressed beyond its initial low stage. It was the Arabs who, having been initiated into the art by the Chinese, improved the method of preparing it, and carried it to that stage of perfection in which it was received from them by the civilised peoples of Europe in the mediæval ages.
“(7) The author has shown that the process of sizing the paper with starch in order to improve it was already known to the Arabs in the eighth century. In the fourteenth century the knowledge of it was lost, animal glue being substituted in the place of starch, till finally in the nineteenth century, along with the introduction of paper machines, the old process was resuscitated. But the invention of it was due to the Chinese. The oldest Eastern Turkestani paper which is sized with starch belongs to the eighth century.
“(8) The Chinese were not only the inventors of felted paper and the imitators of rag paper—though in the preparation of the latter they made use of rags only as a surrogate by the side of raw fibres—but they must also be credited with being the forerunners of the modern method of preparing ‘cellulose paper.’ For their very ancient practice of extracting the fibre from the bark and other parts of plants by means of maceration is in principle identical with the modern method of extracting ‘cellulose’ by means of certain chemical processes.”
Paper-making in Europe.—The introduction of the art into Europe seems to have taken place early in the eleventh century, when the Moors manufactured paper at Toledo. The early authorities who have studied this subject express[Pg 10-11] the opinion that the paper produced in Europe at this time was made from cotton rags and from raw cotton, but, in view of the recent researches into the composition of paper, it is difficult to say how this idea arose, unless we accept the explanation offered by Professor Karabacek. In standard encyclopædias the following statements are made as to existing early documents printed on paper made in Europe:—
| A.D. 1075. | Syriac manuscripts of early date in the British Museum. |
| A.D. 1102. | A document printed on cotton, being a deed of King Roger of Sicily, now at Vienna. |
| A.D. 1178. | A treaty of peace between the Kings of Aragon and Spain, said to be printed on linen paper, preserved at Barcelona. |
| A.D. 1223. | The “Liber Plegierum,” printed on rough cotton paper. |
One of the most interesting books on this subject is the “Historical Account of the Substances used to describe Events from the Earliest Date,” by Matthias Koops, published in 1800. This writer appears to have obtained most of his information from German authorities.
The industry of paper-making passed through Spain into Italy, France, and the Netherlands. In 1189 paper was being manufactured at Hainault, in France, and the industry rapidly spread all over the Continent. In 1390 Ulman Stromer established a mill at Nuremberg, in Germany, employing a great number of men, who were obliged to take an oath that they would not teach anyone the art of paper-making or make paper on their own account. In the sixteenth century the Dutch endeavoured to protect their industry by making the exportation of moulds for paper-making an offence punishable by death.
The bulk of the paper used in England was imported from France and Holland, and it was many years before the industry was established in England. This is not surprising in view of the protective and conservative policy of the Continental paper-makers.
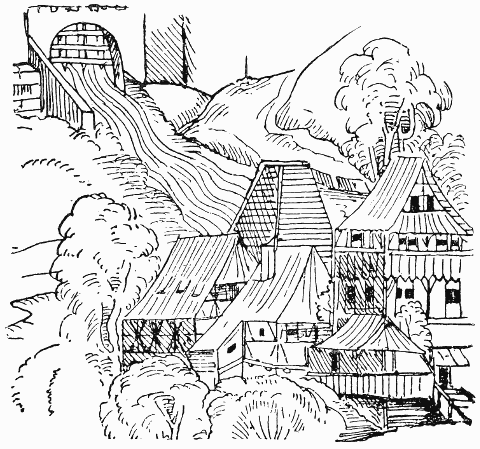
Fig. 3.—The Paper Mill of Ulman Stromer, A.D. 1390 (supposed to be the oldest known drawing of a Paper Mill).
Paper-making in England.—The actual period at which the manufacture of paper was first started in England is somewhat uncertain. The first mention of any paper-maker is found in Wynkyn de Worde's “De Proprietatibus[Pg 13] Rerum,” printed by Caxton in 1495, the reference being as follows:—
And John Tate the younger, joye mote he brok,
Which late hathe in England, doo
Make thys paper thynne,
That now in our Englyssh
Thys booke is prynted inne.
John Tate was the owner of a mill at Stevenage, Hertfordshire. In the household book of Henry VII. an entry for the year 1499 reads, “Geven in rewarde to Tate of the mylne, 6s. 8d.”
In 1588 a paper mill was erected by Sir John Spielman, a German, who obtained a licence from Queen Elizabeth “for the sole gathering for ten years of all rags, etc., necessary for the making of paper.” This paper mill was eulogised by Thomas Churchyard in a long poem of forty-four stanzas, of which we quote two:—
I prayse the man that first did paper make,
The only thing that sets all virtues forth;
It shoes new bookes, and keeps old workes awake,
Much more of price than all the world is worth:
It witnesse bears of friendship, time, and troth,
And is the tromp of vice and virtue both;
Without whose help no hap nor wealth is won,
And by whose ayde great works and deedes are done.
Six hundred men are set to worke by him
That else might starve, or seeke abroad their bread,
Who now live well, and goe full brave and trim,
And who may boast they are with paper fed.
Strange is that foode, yet stranger made the same,
For greater help, I gesse, he cannot give
Than by his help to make poore folk to live.
The industry made but little progress for some time after Spielman's death, and up till 1670 the supplies of paper were obtained almost entirely from France. The first British patent for paper-making was granted to Charles[Pg 14] Hildeyard in 1665 for “the way and art of making blue paper used by sugar bakers and others.” The trade received a great impetus on account of the presence of Huguenots who had fled to England from France in consequence of the revocation of the edict of Nantes in 1685.
In 1695 a company was formed in Scotland for the “manufacture of white and printing paper.”
Improvements in the art were slow until 1760, when Whatman, whose name has since become famous in connection with paper, commenced operations at Maidstone. Meantime the methods by which the rags were converted into paper were exceedingly slow and clumsy, so that the output of finished paper was very small.
Some interesting details as to the early manufacture of paper in England are given by Mr. Rhys Jenkins, and from his account of “Early Attempts at Paper-making in England, 1495-1788,” the following extracts have been made:—
| About | |
| 1496. | First attempts at paper-making by John Tate at Hertford. |
| 1496. | Tate's paper used by Wynkyn de Worde in “De Proprietatibus Rerum.” |
| 1557. | A paper mill in existence at Fenditton, Cambridge. |
| 1569. | A mill at Bemmarton, Wilts. |
| 1574. | Mill erected at Osterley, Middlesex, by Sir Thomas Gresham. |
| 1585. | Richard Tottyl asked for sole right to make paper for thirty-one years, which was not granted. |
| 1588. | John Spilman erected a mill at Dartford, Kent. Granted a patent for sole manufacture of paper. |
| 1588. | Churchyard's poem on the “Paper Myll built near Darthford by Master Spilman.” |
| 1612. | Robert Heyricke's mill at Cannock Chase, Staffordshire. |
| 1636. | The three or four paper mills in the neighbourhood of Hounslow and Colnbrook temporarily shut down on account of the plague, the collection of rags having been forbidden. |
| 1665. | Patent granted to Charles Hildeyard for an invention, “the way and art of making blew paper used by sugar bakers and others.” |
| [Pg 15]1675. | Approximate date of erection of mills at Wolvercote, Oxford, where the Oxford India paper is now made. |
| 1678. | Mill at Byfleet, Surrey, mentioned by Evelyn in his diary. |
| 1682. | Bladen—A patent for an engine and process whereby rags are wrought into paper. |
| 1684. | Baysmaker—A patent for “the art and mistery of making paper in whole sheets.” |
| 1684. | Jackson—A patent for “an engine, either for wind or water, which prepareth all materials whereof paper may be made.” Evidently Jackson was acquainted with the “Hollander” beating engine. |
| 1686. | A charter granted to the “White Paper Makers' Company” for the sole right of making paper exceeding 4s. a ream in value. |
| 1674. | Annual importation of paper, presumably from France, stated to be 160,000 reams, of average value of 5s. (Somers). |
| 1689. | Trade with France prohibited by royal proclamation. |
| 1696. | Price of paper very high owing to scarcity, being 11s. per ream. |
| 1712. | Duties levied on all kinds of paper, manufactured or imported. |
| 1725. | Monopoly of making paper for Bank of England notes granted to De Portal, of the Laverstoke mills, Hampshire. This paper is still made by the firm of Messrs. Portal. |
| 1739. | Galliott and Parry estimated that there were 600 paper mills in England, making 6,000 reams a day. The Commissioner of Excise reported only 278. |
| 1739. | James Whatman erected a mill at Boxley, Maidstone. |
| 1758. | Baskerville printed an edition of Virgil on so-called “woven” paper. |
Early Methods.—The most rapid development of the industry appears to have taken place in Holland. The rags used for paper-making were moistened with water and stored up in heaps until they fermented and became hot. By this means the dirt and non-fibrous matter was rendered partially soluble, so that on washing a suitable paper pulp was obtained. The washed rags were then placed in a stamping machine resembling an ordinary pestle and mortar. The mortars were constructed of stone and wood, and the stamps were kept in motion by levers which were[Pg 16] raised by projections fixed on the shaft of a waterwheel. The operation of beating thus occupied a long period, but the paper produced was of great strength.
The invention of the “Hollander,” a simple yet ingenious engine which is deservedly known by the name of the country in which it first originated, gave a tremendous impetus to the art of paper-making, as by its means the quantity of material which could be treated in twenty-four hours was greatly increased. Unfortunately the date of the invention of this important machine has not been definitely traced. The earliest mention of it seems to occur in Sturm's “Vollständige Mühlen Baukunst,” published in 1718. It was in extensive use at Saardam in 1697, so that the invention is at least some years previous to 1690.
On this point Koops says: “In Gelderland are a great many mills, but some so small that they are only able to make 400 reams of paper annually, and there are also water mills with stampers, like those in Germany. But in the province of Holland there are windmills, with cutting and grinding engines, which do more in two hours than the others do in twelve. In Saardam 1,000 persons are employed in paper-making.”
Up till the year 1799 paper was made entirely in sheets on a hand mould, but during the last few years of the eighteenth century a Frenchman, Nicholas Louis Robert, manager for M. Didot, who owned a paper mill at Essones, had been experimenting for the purpose of making paper in the form of a continuous sheet, and eventually produced some of considerable length.
The idea was taken to England by Didot's brother-in-law, Gamble, and introduced to the notice of Messrs. Fourdrinier, wholesale stationers, of London.
The first machine was naturally a very crude affair. It consisted of an endless wire cloth stretched in a horizontal position on two rollers, one of which rotated freely in a bearing attached to the frame of the machine, the other being fitted in an adjustable bearing so that the wire could be tightened up when necessary.
The beaten pulp, contained in a vat placed below the wire, was thrown up in a continual stream upon the surface of the wire, and carried forward towards the squeezing rolls. A shaking motion was imparted to the travelling wire so as to cause the fibres to felt properly. A great deal of the water fell through the meshes of the gauze, and further quantities were removed by means of the press rolls. The wet paper was then wound up on to a wooden roller, which was taken out as soon as sufficient paper had been made.
The whole process was carried on under great difficulties, but substantial improvements were soon made by the enterprising Fourdriniers, who commenced operations in Bermondsey, employing Mr. Bryan Donkin, then in the service of Messrs. Hall & Co., of Dartford, who had shown himself keenly interested in the machine. In 1803 the first “Fourdrinier,” so called, was built at Bermondsey, and erected at Two Waters Mill in Herefordshire.
In this machine the mixture of pulp and water was carried forward between two wires, and, after passing through the couch rolls, transferred to an endless felt. This arrangement proved to be faulty because the water did not escape freely enough from the wire, and a great deal of the paper was spoilt.
Donkin, however, hit upon a simple but effective device for curing this fault by altering the relative position of the two couch rolls. Instead of keeping the two rolls exactly in a vertical position one over the other, he placed them at a slight angle so that the upper one should bear gently on the web of paper carried by the wire before receiving the full pressure of the rolls, and thus remove a greater proportion of the water. In this way the paper was firmer and less liable to break when pressed between the couch rolls, an additional advantage being secured in the fact that the upper wire could be dispensed with.
The various improvements effected resulted in a machine the details of which appear in the appended diagram, the device of the inclined couch rolls being fitted about 1810.
The mixture of water and pulp flowed from a stuff chest into a small regulating box and on to the wire over a sloping board. The pulp at once formed into a wet sheet of paper, the water falling through the meshes of the wire, being caught in a bucket-shaped appliance, and conveyed back to the regulating box. The stream of pulp was confined upon the wire by means of a deckle. Further quantities of water were removed by the aid of a pair of squeezing rolls before the web passed through the couch rolls after which the paper was reeled up on a wooden spindle.
From this date the success of the machine was assured, though the inventor and his colleagues were practically ruined, an experience only too common with the early pioneers of many great and useful industrial enterprises. In fact, the firm of Messrs. Donkin were the only people to profit from the invention, for they manufactured a number of machines, as stated in the report of the Jurors of the Exhibition of 1851, and from 1803 to 1851 no less than 190 Fourdriniers were set to work.
When plants such as flax, cotton, straw, hemp, and other varieties of the vegetable kingdom are digested with a solution of caustic soda, washed, and then bleached by means of chloride of lime, a fibrous mass is obtained more or less white in colour.
This is the substance known to paper-makers as paper pulp, and the several modifications of it derived from different plants are generally known to chemists as cellulose.
Although plants differ greatly in physical structure and general appearance, yet they all contain tissue which under suitable treatment yields a definite proportion of this fibrous substance. The preparation of a small quantity of cellulose from materials like straw, rope, hemp, the stringy bark of garden shrubs, wood, and bamboo can easily be accomplished without special appliances. Soft materials, such as straw and hemp, are cut up into short pieces, hard substances like wood and bamboo are thoroughly hammered out, in order to secure a fine subdivision of the mass. The fibre so prepared is then placed in a small iron saucepan, and covered with a solution made up of ten parts of caustic soda and 100 parts of water. The material is boiled gently for eight or ten hours, the water which is lost through evaporation of steam being replaced by fresh quantities of hot water at regular intervals. When the fibrous mass breaks up readily between the fingers, it is poured into a sieve, or on a piece[Pg 21] of muslin stretched over a basin, and washed completely with hot water until clean and free from alkali. Hard pieces and portions which seem incompletely boiled are removed, and the residual fibres separated out. These fibres are placed in a weak, clear solution of ordinary bleaching powder, left for several hours, and subsequently thoroughly washed. This simple process will give a more or less white fibrous material.
The purest form of cellulose is cotton. A very slight alkaline treatment, followed by bleaching, is sufficient to remove the non-fibrous constituents of the plant, and a large yield of cellulose is obtained. For this reason the cotton fibre ranks high as an almost ideal material for paper-making, possessing the quality of durability.
Cellulose is an organic compound, containing carbon, hydrogen, and oxygen in the following proportions:—
| Carbon | 44·2 |
| Hydrogen | 6·3 |
| Oxygen | 49·5 |
| 100·0 | |
Its composition is represented by the formula C6H10O5.
The celluloses obtained from various plants are not identical either in physical structure and chemical constitution, or as to their behaviour when employed for paper-making. In fact, the well-known differences between the raw materials used for paper-making, and also between the numerous varieties of finished paper, are to be largely accounted for and explained by a careful study of the cellulose group, particularly with reference to the microscopic characteristics and the chemical composition of the individual species.
The only vegetable substance which may be regarded as[Pg 22] a simple cellulose is cotton, all others being compound celluloses of varying constitution, the nature of which cannot be appreciated without a considerable knowledge of chemistry. The classification of such plants, therefore, in a book of this description must be limited to certain distinctions having some immediate practical bearing on the question of paper manufacture.
Cotton.—Regarded as the typical simple cellulose, containing 91 per cent. of cellulose, and remarkable for its resistance to the action of caustic soda.
Linen.—The cellulose isolated from flax by treatment with alkali or caustic soda cannot readily be distinguished from cotton cellulose by chemical analysis or reactions. The difference is almost entirely a physical one.
Flax is a typical compound cellulose, to which has been given the name pecto-cellulose on account of certain properties. Other well-known plants of this class are ramie, aloe, “sunn hemp,” manila.
Esparto.—The cellulose isolated from esparto differs in composition from cotton cellulose:—
| Carbon | 41·0 |
| Hydrogen | 5·8 |
| Oxygen | 53·2 |
| 100·0 | |
It is regarded as an oxycellulose, being readily oxidised by exposure to air at 100° C. Other oxycelluloses familiar to the paper-maker are straw, sugarcane, bamboo.
Wood.—The difference between wood and the plants already mentioned is expressed by the term lignified fibre or ligno-cellulose. This term is used to indicate that the wood is a compound cellulose containing non-fibrous[Pg 23] constituents, to which has been given the name lignone. Jute is another example of this class.
These distinctions may be exemplified by reference to a simple experiment. If three papers, such as a pure rag tissue or a linen writing, an ordinary esparto printing, and a cheap newspaper containing about 80 per cent. of mechanical wood, are heated for twenty-four hours in an oven at a temperature of 105° C., the first will undergo little, if any, change in colour, while the others will be appreciably discoloured, the mechanical wood pulp paper most of all.
This change is due to the gradual oxidation of the constituents of the paper, the ligno-cellulose of the mechanical wood pulp being most readily affected by the high temperature, and the pure cellulose of the rag paper being least altered.
The process of oxidation, brought about rapidly under the conditions of the experiment described, takes place in papers of low quality exposed to air in the ordinary circumstances of daily use, but of course at an extremely slow rate. The deterioration of such paper is not, however, due to the simple oxidation of the cellulose compounds, because other factors have to be taken into account. The presence of impurities in the paper on the one hand, and of chemical vapours in the air on the other, hastens the decay of papers very considerably.
Percentage of Cellulose in Fibrous Plants.—The value of a vegetable plant for paper-making is first determined by a close examination of the physical structure of the cellulose isolated by the ordinary methods of treatment. If the fibres are weak and short, the raw material is of little value, and it is at once condemned without further investigation, but should the fibre prove suitable, then the question of the percentage of cellulose becomes important.
There are several methods employed for estimating the amount of cellulose in plants. The process giving a maximum yield is known as the chlorination method, the details of which are as follows:—About ten grammes of the air-dried fibre is dried at 100° C. in a water oven for the determination of moisture. A second ten grammes of the air-dried fibre is boiled for thirty minutes with a weak solution of pure caustic soda (ten grammes of caustic soda in 1,000 cubic centimetres of water), small quantities of distilled water being added at frequent intervals to replace water lost by evaporation. The residue is then poured on to a piece of small wire gauze, washed thoroughly, and squeezed out. The moist mass of fibre is loosened and teased out, placed in a beaker, and submitted to the action of chlorine gas for an hour. The bright yellow mass is then washed with water and immersed in a solution of sodium sulphite (twenty grammes of sodium sulphite in 1,000 cc. of water). The mixture is slowly heated, and finally boiled for eight to ten minutes, with the addition of 10 cc. of caustic soda solution. The residue is washed, immersed in dilute sodium hypochlorite solution for ten minutes, again washed, first with water containing a little sulphurous acid and then with pure distilled water. It is finally dried and weighed.
The second process for estimating cellulose is based upon the use of bromine and ammonia. About ten grammes of the air-dried fibre is placed in a well-stoppered wide-mouthed bottle with sufficient bromine water to cover it. As the reaction proceeds the red solution gradually decolourises, and further small additions of bromine are necessary. The mass is then washed, and boiled in a flask connected to a condenser with a strong solution of ammonia for about three to four hours. The fibrous residue is washed, again treated with bromine water in the cold, and subsequently[Pg 25] boiled with ammonia. The alternative treatment with bromine and ammonia is repeated until a white fibrous mass is obtained.
In practice the paper-maker is confined to two or three methods for the isolation of the fibres, viz., alkaline processes, which require the digestion of the material with caustic soda, lime, lime and carbonate of soda, chiefly applied to the boiling of rags, esparto, and similar pecto-celluloses; acid processes, in which the material is digested with sulphurous acid and sulphites. The latter methods are at present almost exclusively used for the preparation of chemical wood pulp.
Yields of Cellulose in the Paper Mill.—The object of the paper-maker is to obtain a maximum yield of cellulose residue at a minimum of cost. Usually the amount of actual bleached paper pulp obtained in the mill is less than the percentage obtained by careful quantitative analysis, for reasons easily understood.
In the first place, the raw material is digested for a stated period with a carefully measured quantity of caustic soda, for example, at a certain temperature. Now the conditions of boiling may be varied by altering one or more of these factors, the period of boiling, the strength of solution, or the steam pressure, and the paper-maker must exercise his judgment in fixing the exact relation between the varying factors so as to produce the best results.
In the second place, the mechanical devices for washing the boiled pulp and for bleaching cause slight losses of fibre, which cannot be altogether avoided when operations are conducted on a large scale. Frequently, also, a greater yield of boiled material may involve a larger quantity of bleaching powder, so that it is evident the adjustment of practical conditions requires considerable technical skill and experience.
The percentage of cellulose in the vegetable plants employed more or less in the manufacture of paper is given in the following table:—
Table Showing Percentage of Cellulose in Fibrous Plants.
| Fibre. | Cellulose, per cent. |
| Cotton | 91·0 |
| Flax | 82·0 |
| Hemp | 77·0 |
| Ramie | 76·0 |
| Manila | 64·0 |
| Jute | 64·0 |
| Wood (pine) | 57·0 |
| Bagasse | 50·0 |
| Bamboo | 48·0 |
| Esparto | 48 to 42 |
| Straw | 48 to 40 |
The Properties of Cellulose.—Cellulose is remarkably inert towards all ordinary solvents such as water, alcohol, turpentine, benzene, and similar reagents, a property which renders it extremely useful in many industries, with the result that the industrial applications of cellulose are numerous and exceedingly varied.
Solubility.—Cellulose is dissolved when brought into contact with certain metallic salts, but it behaves quite differently to ordinary organic compounds. Sugar, for example, is a crystalline body soluble in water, and can be recovered in a crystalline state by gradual evaporation of the water. Cellulose under suitable conditions can be dissolved, but it cannot be reproduced in structural form identical with the original substance.
If cellulose is gently heated in a strong aqueous solution of zinc chloride, it gradually dissolves, a thick syrupy mass being obtained, which consists of a gelatinous solution of[Pg 27] cellulose. If the mixture is diluted with cold water, a precipitate is produced consisting of cellulose hydrate intimately associated with oxide of zinc, which latter can be dissolved out by means of hydrochloric acid. The resulting product is not, however, the original substance, but a hydrated cellulose, devoid of any crystalline structure.
Cellulose is also soluble in ammoniacal solutions of cupric oxide, from which it can be precipitated by acids or by substances which act as dehydrating agents, e.g., alcohol.
Hydrolysis.—An explanation of the behaviour of cellulose towards the solvents already mentioned, and towards acid and alkali, requires a reference to its chemical composition.
The substance is a compound of carbon, hydrogen, and oxygen represented by the formula
C6H10O5
being one of a class of organic compounds known as carbohydrates, so designated because the hydrogen and oxygen are present in the proportions which exist in water.
Water = Hydrogen + Oxygen
H2 + O.
The H10O5 in the cellulose formula corresponds to 5 (H2O).
When cellulose is acted upon by acid, alkali, and certain metallic salts, it enters into combination with one or more proportions of water, forming cellulose hydrates of varying complexity. This change is usually termed hydrolysis.
With mineral acids like sulphuric and hydrochloric acids, cellulose, if boiled in weak solutions, is converted into a non-fibrous brittle substance having the composition
C12H20O10 2 H2O
to which the name hydra-cellulose has been given. Similar changes occur, but at a much slower rate, when cellulose is[Pg 28] in contact with free acids at ordinary temperatures. For this reason it is important that paper, when finished, should not be contaminated with free acid.
The nature and extent of the chemical change can be varied by altering the strength of the acid and the conditions of treatment. The manufacture of parchment paper is an example of the practical utility of the chemical reaction between cellulose and acid. A sheet of paper is dipped into a mixture of three parts of strong sulphuric acid and one part of water, when it becomes transparent. Left in the solution it dissolves, but if taken out and dipped into water in order to wash off the acid the reaction is stopped, and a tough semi-transparent piece of parchment is obtained. The cellulose is more or less hydrated, having the composition
C12H20O10 H2O,
a substance having the name amyloid.
Oxidation.—Cellulose is only oxidised to any appreciable extent by acid and alkali if treated under severe conditions. It is remarkable that the processes necessary for isolating paper pulp from plants when digested with these chemical reagents do not act upon or destroy the fibre, and this capacity for resisting oxidation has rendered cellulose extremely valuable to many of the most important industries.
The resistant power of the cellulose is, however, broken down by the use of acid and alkali in concentrated form.
Oxalic and acetic acids are obtained when cellulose is heated strongly at 250° C. with solid caustic soda.
Oxy-cellulose, a white friable powder, is produced by means of strong mineral acids. Nitric acid at 100° C. attacks the fibre very readily and produces about 30-40 per cent. of the oxidised cellulose.
The great number of compounds and derivatives, i.e., substances obtained by chemical treatment, may be judged from the following list. The substances of commercial importance are suitably distinguished from those of merely scientific interest by the printing of the names in small capitals.
Acetic Acid.—An important commercial product obtained by the destructive distillation of wood. The crude pyroligneous acid is first neutralised with chalk or lime, and the calcium acetate formed then distilled with sulphuric acid. Wood yields 5 to 10 per cent. of its weight of acetic acid according to the nature of the wood.
Acetone.—A solvent for resins, gums, camphor, gun cotton, and other cellulose products. Prepared by distilling barium or calcium acetate in iron stills, the acetate being obtained from the crude acetic acid produced by the dry distillation of wood.
Acid Cellulose.—(See Hydral-Cellulose.)
Adipo-Cellulose.—A distinct compound cellulose present in the complex cuticular tissue of plants, and separated easily by suitable solvents from the wax and oily constituents also present.
Alkali Cellulose.—When cotton pulp is intimately mixed with strong caustic soda solution, this compound is formed. It is utilised in the manufacture of Viscose.
Amyloid.—Strong sulphuric acid acts upon cellulose and converts it into a gelatinous semi-transparent substance to which the name amyloid has been given. (See Parchment Paper.)
Ballistite.—A smokeless powder composed of nearly equal parts of nitro-glycerine and nitrated cellulose, with a small quantity of diphenylamine.
Carbohydrate.—A large number of important commercial products, such as cellulose, sugars, starches, and gums, consist of the elements carbon, hydrogen, and oxygen, associated in varying proportions. The ratio of hydrogen to oxygen in these compounds is always 2:1 (H2 and O).
| Cellulose | C6H10O5. |
| Sugar | C6H12O6. |
| Dextrin n | (C6H10O5). |
To all these substances the term carbohydrate is applied.
Celloxin (Tollens).—A substance having the stated composition C8H6O6 considered to be present in oxidised derivatives of cellulose.
Celluloid.—This well-known material is made by incorporating camphor with nitro-cellulose, a plastic ivory-like substance being produced. In practice the process is as follows:—Wood pulp or wood pulp paper is saturated with a mixture of sulphuric acid (five parts) and nitric acid (two parts), which produces nitrated cellulose. The product is washed, ground, and mixed with camphor, the mastication being effected by heavy iron rollers. The mass thickens and can be removed in the form of thick sheets. These sheets are submitted to great pressure between steam-heated plates. The cake obtained is cut into sheets of any desired thickness, seasoned by prolonged storage, and afterwards worked up into boxes, combs, brush-backs, and many other domestic articles of a useful and ornamental character.
Cellulose Acetate (Cross).—If cellulose is heated with acetic anhydride at 180° C., viscous solutions of the acetates are obtained. The process yielding a definite acetate of commercial value is based upon the following reaction:—100 parts of cellulose prepared from the sulpho-carbonate are mixed with 120 parts of zinc acetate, heated and dried at 105° C. Acetic anhydride is added in small quantity, and 100 parts of acetyl chloride. At a temperature of 50° C. the mixture becomes liquid, and cellulose acetate is subsequently obtained as a white powder.
The compound can be used in the place of cellulose nitrate, and, being non-explosive, may gradually replace the latter in many industrial applications.
Cellulose-Benzoate.—When alkali cellulose is heated with benzoyl chloride and excess of caustic soda, this substance is obtained.
Cellulose Hydrate.—The substances produced by the action of acid and alkali on cellulose under certain strictly defined conditions are bodies containing cellulose united with water to form hydrates. The industrial applications of cellulose based upon this reaction are described under the special headings.
Cellulose Nitrate.—A considerable number of derivatives are obtained by bringing cellulose into contact with nitric acid. Variations in the strength of the acid, the temperature of reaction, and the time of contact determine the nature of the product. The best known nitrates are:—
Cellulose di-nitrate.
Cellulose tri-nitrate and tetra-nitrate, present chiefly in pyroxyline.
Cellulose penta-nitrate.
Cellulose hexa-nitrate, the chief constituent of gun-cotton.
Charcoal.—Not a cellulose derivative in the strict sense of the term, charcoal being a residue obtained in the dry distillation of wood.
Collodion.—A soluble nitrate of cellulose used in photography. (See Pyroxyline.)
Cordite.—A smokeless powder consisting mainly of nitro-glycerine and gun-cotton mixed with acetone. The materials are thoroughly incorporated and the resultant paste formed into threads which are dyed and then cut up into suitable lengths for cartridges.
Cuto-Cellulose.—Synonymous with adipo-cellulose.
Dextron.—A compound prepared from the waste liquors of the bisulphite process used for the manufacture of wood pulp. Resembles dextrin in its physical properties.
Dextrose.—A carbohydrate which can be obtained by the action of mineral acids on cellulose. Commercial dextrose, or glucose, is prepared by the conversion of starch with sulphuric acid. The starch is mixed with dilute acid at a fixed temperature, and the starch milk obtained poured gradually into a vessel containing dilute acid, which is maintained at boiling point. The conversion is complete and rapid.
Explosives.—The production of the several cellulose nitrates has given rise to a great number of highly explosive substances.
Blasting Gelatine.—A mixture of nitro-glycerine with cellulose nitrates.
Amberite, Ballistite, Cordite, and other smokeless powders, consisting of nitro-glycerine and cellulose nitrates in about equal proportions.
Sporting powders made by mixing nitro-cellulose with barium nitrate, camphor nitro-benzene, such as indurite, plastomenite, etc.
Glucose.—(See Dextrose.)
Gun-cotton.—An explosive prepared by the action of nitric acid on cotton. Selected cotton waste suitably opened up is immersed in a mixture of three parts of nitric acid by weight (1·50 sp. gr.) and one part of sulphuric acid by weight (1·85 sp. gr.) and submitted to a number of processes by which the nitration is properly effected so as to produce a nitro-cellulose of uniform composition. The material is washed, reduced to pulp, and moulded into various forms.
Hemi-Cellulose.—The constituents of plant tissues are extremely varied in character. Many plants contain substances which resemble true cellulose, but differing from it in being easily converted by hydrolysis, and by the action of dilute acids, into carbohydrates. Plants which contain a large proportion of such constituents are termed hemi-celluloses. In some cases certain crystallisable sugars can be obtained by hydrolysis under suitable conditions.
Hydral-Cellulose (Bumcke).—A compound of merely scientific interest, resulting from the treatment of cellulose with hydrogen peroxide. When acted upon by alkali it is decomposed into cellulose and acid cellulose, the latter a derivative of unstable composition.
Hydro-Cellulose.—This product, a white, non-structureless, friable powder, is obtained by treating cellulose with hydrochloric or sulphuric acid of moderate strength. The substance itself has no commercial value, but the reaction is useful in separating cotton from animal fabrics. If a woollen cloth containing cotton is soaked in dilute sulphuric acid, washed, and dried at a gentle heat, the cotton is acted upon, and can be beaten out of the fabric, the wool resisting the acid treatment.
Lignin.—The complex mixture of substances which is associated with cellulose in wood, jute, and other[Pg 34] ligno-celluloses. The conversion of wood into chemical pulp effects the removal of this material more or less completely. The well-known “phloroglucine” test for mechanical wood in papers is based upon the presence of lignin in the wood.
Ligno-Cellulose.—Wood and jute are typical bodies consisting of cellulose and complex non-cellulose, generally described as lignin, associated together in the plant tissue. The chemistry of the non-cellulose portion of wood is a matter still under investigation, its importance from a commercial point of view being obvious from the fact that the removal of the lignin during the conversion of the wood into wood-cellulose results in a loss of 50 per cent. of the weight of wood.
Lustra-Cellulose.—Synonymous with and suggested as a more appropriate name for the material usually described as artificial silk.
Mercerised Cotton.—When cotton is immersed in strong solutions of caustic soda a remarkable change sets in. The physical structure of the fibre is entirely altered from the long flattened tube having a large central canal to a shorter cylindrical tube in which the canal almost disappears. Hydration of the cellulose takes place, and these changes are taken advantage of in the production of mercerised cloth (so named from the discoverer of the reaction, Mercer). Cotton goods, particularly those made of long stapled cotton, when mercerised, exhibit a beautiful lustre, and some magnificent crêpon effects are obtained by the process.
Methoxyl.—A constituent of the complex compound known as ligno-cellulose, which is present in wood and similar fibres. The amount of methoxyl in lignified tissue can be accurately determined, and it has been suggested that the proportion of methoxyl found in a cheap[Pg 35] printing paper could be used as a measure of mechanical wood pulp present.
Muco-Cellulose.—This term is applied to certain compound celluloses present chiefly in mucilages, gums, and in seaweeds (Algæ). The natural substances are all of commercial importance—Iceland moss, Carragheen, Algin, etc.
Naphtha.—One of the products of the dry distillation of wood, usually described as wood-naphtha, or wood spirit.
Nitro-Cellulose.—The treatment of cellulose with nitric acid gives a number of nitro-celluloses according to the conditions of the process. (See Cellulose Nitrates.)
Oxalic Acid.—A substance of great commercial importance prepared by heating the sawdust of soft wood, such as pine, fir, and poplar, with strong solutions of mixed caustic soda and potash to dryness. The wood yields after six hours a greyish mass containing about 20 per cent. of the acid, which is separated out by water and then crystallised.
It is used for bleaching, and as a discharge in calico printing and dyeing.
Oxy-Cellulose.—A white friable powder produced by treating cellulose with nitric acid at 100° C. The oxidation of cellulose is brought about by several reagents such as chromic acid, hypochlorites of lime and soda, chlorine, and permanganates. The extent to which cloth has been damaged by overbleaching may be determined by a simple test with methylene blue solution, which is readily absorbed by oxy-cellulose present in such fabrics.
Parchment.—A tough paper prepared by the action of sulphuric acid on unsized paper. (See page 137.)
Pectins.—(See Pecto-Cellulose.)
Pecto-Cellulose.—A generic term applied to many important fibrous materials, such as flax, straw, esparto, bamboo, phormium, ramie, &c., which on alkaline treatment yield cellulose for paper-making, and a non-fibrous soluble residue of complex composition. These soluble derivatives are known as pectin (C32H48O32), pectic acid (C32H44O30), and metapectic acid (C32H28O36). Although the soluble constituents of the pecto-celluloses amount to 50 per cent. by weight in most cases, no process for the recovery of the product in a commercial form has yet been devised. (See description of Soda recovery, page 78.)
Pyroxyline.—A substance prepared by nitrating cotton. The cotton is immersed in a mixture of nitric and sulphuric acids of carefully regulated strength, and subsequently washed free of the acid. Three volumes of nitric acid (sp. gr. 1·429) are diluted with two volumes of water and nine volumes of strong sulphuric acid (sp. gr. 1·839) added. To the solution when cool the cotton is added in small quantities at a time. The resultant pyroxyline is soluble in a mixture of equal quantities of alcohol and ether, and in the soluble form is utilised as collodion for photography.
Silk, Artificial.—A remarkable substance made from wood or cotton cellulose, closely resembling silk in appearance and physical properties.
Nitrated cellulose is dissolved in a mixture of equal parts of alcohol and ether.
The solution is forced through five capillary tubes under high pressure, and the filament so obtained solidifying at once is wound together with other similar filaments upon suitable bobbins. Various modifications of this general process are in use, such as the solidification of the solution into threads by [Pg 37]passing it into water; the application of solvents less inflammable than ether and alcohol; the use of other forms of dissolved cellulose such as those prepared by means of zinc chloride, ammoniacal copper oxide, or acetic anhydride. In all cases the yarn or thread is submitted to further chemical treatment for the removal of nitric acid and to render the material non-explosive and less inflammable. The finished product is soft and supple, can be easily bleached and dyed, and is capable of acquiring a high lustre.
Smokeless Powders.—(See Explosives.)
Sulpho-Carbonate.—(See Viscose.)
Sulphate Cellulose.—Chemical wood pulp prepared by the sulphate process. (See page 107.)
Sulphite Cellulose.—Chemical wood pulp prepared by the sulphite process. (See page 107.)
Viscose.—A soluble sulpho-carbonate of cellulose, prepared by treating cellulose with a 15 per cent. solution of caustic soda, and shaking the product with carbon bisulphide in a closed vessel. The mixture forms a yellowish mass soluble in water, giving a viscous solution which has some remarkable and valuable properties.
This viscose, on standing, coagulates to a hard mass which can be turned and polished.
If spread on glass and coagulated by heat, films are obtained from which the alkaline by-products can be washed out. These films are transparent, colourless, very tough and hard.
Vulcanised Fibre.—Fibre or pulp treated with zinc chloride in acid solution, or otherwise, for the manufacture of hard boards. (See page 139.)
Willesden Goods.—Paper, fibre, and textiles when treated[Pg 38] with cuprammonium oxide are partially gelatinised on the surface and rendered waterproof. (See page 139.)
Wood Spirit.—(See Naphtha.)
Xylonite.—(See Celluloid.)
Although the vegetable world has been explored from time to time for new supplies of cellulose, and some plants have been found serviceable in certain directions, yet the number of fibres in actual use is very limited.
The following table indicates the principal sources of the material required for paper-making:—
| Fibre. | Source of the Fibre. | Application of the Fibre. |
| Linen | Rags, textile waste. | High class writings and printings. |
| Cotton | Rags, textile waste. | High class writings and printings. |
| Esparto | Natural grass. | Writings and printings. |
| Straw | Straw from various cereals—wheat, barley, oats, etc. | Printings, box and card boards. |
| Wood | Mechanically ground wood. | Cheap papers, boxboards, middles, tickets and cards, writings and printings. |
| „ | Chemically prepared wood. | Writings and printings. |
| Flax | Threads, waste from spinning mills. | Wrappings, boards, cable papers. |
| Hemp | Spinning refuse, old rope, sailcloth, etc. | Wrappings, boards, cable papers, strong writings. |
| Jute | Waste, old gunny bags. | Wrappings, boxboard, cards. |
| Bamboo | Natural stems. | Writings and printings (not in Europe, and only limited quantities elsewhere). |
| Ramie | Bast fibres of the plant; textile refuse. | Rarely used, except in special cases. |
| Bagasse | Sugar-cane refuse. | Common papers (chiefly experimental results). |
| Manila Hemp | Textile and rope refuse. | Wrappings, cable papers. |
Exploiting New Fibres.—The exploitation of any new paper-making fibre requires attention to certain important details, which may be fairly considered in the following order:—
(1) Supply.—The supply of material must be plentiful and obtainable in large quantities. Too often this question is entirely neglected by those who bring new fibres to the notice of paper-makers, probably because they do not realise that enormous quantities of material are necessary to supply even a very small section of the paper trade, the fact being that few plants yield more than half their weight of paper-making fibre.
(2) Suitability.—The fibre should be properly examined as to its chemical and physical properties in a laboratory equipped with appliances for its conversion into bleached paper pulp on a small scale. The examination of the fibre would include tests as to the amount of pulp which can be obtained from one ton of raw material, the approximate cost of treatment, and details as to the value of the fibre for paper-making.
(3) Cost of Raw Material.—If the supply of material seems to be sufficient, and the paper pulp obtained possesses suitable qualities, then it is necessary to get accurate information as to the cost of the fibre delivered to some given spot at or near the place of collection.
The exploitation of any new fibre for paper-making purposes will involve a recognition of the fact that the raw material must be converted into pulp at or near the place where the material is most abundant.
The only interesting exception to this is the case of esparto fibre, which is imported into England in large amount, but this is only possible because esparto possesses most valuable paper-making qualities, and is obtained in countries close to England, where large quantities are[Pg 40] consumed. It is doubtful whether other fibres could be utilised in the same way.
(4) The Cost of Manufacture at or near the place of collection requires to be carefully worked out, due consideration being given to the actual cost of chemicals on the spot, cost of labour, and the conditions under which the maintenance of machinery can be efficiently looked after.
(5) Carriage and Freight Charges are the last, but by no means the least, items of importance. It is not too much to say that the whole success of the exploitation of new paper-making fibre hangs entirely upon this item, the majority of many fibres which have been brought to the notice of the trade being suitable, but impracticable, solely on account of these and similar commercial considerations.
In the pages of the trade press for the last few years the following fibres have been noticed:—
(1) Flax Pulp.—This material was to be obtained from flax straw. Attempts were made on a commercial scale to produce quantities of flax fibre, but so far the efforts made have not been very successful.
(2) Ramie Fibre.—This material has been exploited over and over again, chiefly for textile trades, its application as a paper-making material being limited to small quantities used for special purposes such as bank notes. The fibre is too valuable, except for textile industries, and can only come into the paper trade as a waste material from such sources.
(3) Tobacco Fibre has been before the trade for some years, the idea being to utilise tobacco stems and other tobacco waste for the manufacture of paper suitable for use as wrappers for cigars, cigarettes, and similar purposes.
(4) Agave Fibre.—This name is given to a large and important genus of fibre-yielding plants found chiefly in Central America. It is also found in India, and in 1878 an[Pg 41] experiment was made for the manufacture of paper at a mill near Bombay, but this did not give any satisfactory results, probably on account of the primitive methods used in treatment.
(5) Bagasse.—The waste material from sugar-cane has been looked upon for many years as a desirable fibre, much time and labour having been given to the utilisation of this material. In spite of these efforts bagasse still remains an almost useless and unworkable material. This is partly due to inferiority of the pulp and partly due to difficulties connected with its treatment. Probably cultivation of the plant for the sake of its fibre instead of the sugar might give better results.
(6) Peat.—The attempts made to utilise peat for paper-making are probably fresh in the minds of those paper-makers interested in the production of wrappers and boxboards. The nature of peat, however, is such as to exclude the hope of making any useful article. The material has been exploited by companies in Austria, Ireland, and Canada on a fairly large scale, with but a limited amount of success.
(7) Cotton-seed Hulls.—Many patents have been taken out for the chemical treatment of cotton-seed waste and having for their object the removal of the particles of seed hulls, so as to obtain a pure cotton pulp. The scheme sounds attractive, but there are so many conditions which have to be taken account of that the commercial success of any undertaking based on the use of cotton-seed hulls is very questionable. The fact is that the hulls have a market value quite apart from the possibility of their application to paper-making, and this initial cost would prevent paper-makers from buying the material owing to the large quantity necessary for the manufacture of one ton of pure pulp.
(8) Apocynum.—This plant is said to be utilised to some extent by the Russian Government in the manufacture of bank notes, the plant being cultivated at Poltava. This is an instance of the particular application of a fibrous material in limited quantities, a proposition which is always feasible in the case of special requirements.
(9) Cornstalk.—This fibre has been chiefly exploited in America, experts having been attracted by the enormous quantities of cornstalk available in the several wheat-producing States. The manufacture of paper pulp from this material on a large scale has yet to be established.
(10) Japanese Paper Fibres.—In Eastern countries a great number of fibrous plants are utilised in small quantities for the manufacture of special papers. It is obvious that in these Eastern countries the employment of fibres which are not cultivated in large bulk is readily possible when the question of price obtained for the paper and the cost of production are considered. Of such fibres may be mentioned the Mitsumata and Kodzu, easy of cultivation and giving a good yield of material per acre of ground. The waxed papers used for stencils in duplicating work on the typewriter are made from these fibres. The paper Mulberry is also a well-known fibre; while a third species particularly valuable for thin papers is the Gampi.
(11) Antaimoto Fibre.—The bark of this shrub is utilised in Madagascar in very small quantities for local purposes and possesses little interest for paper-makers.
(12) Refuse Hempstalk.—The suggestion of the use of this material comes from Italy, the hempstalk having been experimented with at San Cesario Mill. This also is a fibre of a local interest only. The percentage of cellulose is very high, being over 50 per cent.
(13) Papyrus.—The revival of this celebrated material is of comparatively recent date. It should be noted that the[Pg 43] manufacture of papyrus as carried out by the Egyptians, by smoothing out layers of bark in order to utilise them as sheets of paper, and the present day proposals which involve the production of paper pulp from papyrus, are two entirely different propositions, and the success of the old Egyptian method cannot be referred to as any assurance of success for the production of paper from papyrus along modern lines. The exploitation of this fibre must follow the lines of modern research and commercial investigation, and its value, if any, could then be established.
(14) Pousolsia.—This is a fibre of the same family as hemp and ramie. The value of this material is at present unknown, but the ultimate fibre appears to possess a most extraordinary length. Very little information is available at present as to its value for paper-making.
(15) Bamboo.—This material has been before the paper trade for many years, having first been exploited seriously by Mr. Thomas Routledge in 1875. Since that date a good deal of work has been done in connection with the fibre, but not until recently has the investigation been made of a sufficiently extensive character to enable paper-makers to form some conclusions as to the best methods of obtaining a reliable paper pulp. The researches of the writer in India go to prove that with any fibre it is necessary to take into account all the factors likely to affect the final cost of the paper pulp delivered to any given paper mill.
The figures given in a report recently published, “The Manufacture of Paper and Paper Pulp in Burma,” show the necessity of thorough investigation into all the points likely to affect the final results, viz., the price at which the paper pulp can be sold in England, assuming that the fibre in question is suitable for the manufacture of paper.
Examination of Fibres.—The exact chemical analysis of[Pg 44] a new fibre is necessary in order to establish completely its value for textile and paper-making purposes, but the investigation of the suitability of the fibre for paper-making may be simplified by simple reduction of the raw material with caustic soda. The following process is sufficient for all practical purposes:—
Condition of Sample.—A record should be made of the general appearance of the sample, its condition and the amount available for the investigation. Any information available as to the source of supply and the growth of the plant should also be noted.
Preparation of Sample.—The material is cut up into small pieces. The most convenient appliance for this purpose is a mitre cutter as used by picture-frame makers. If the sample is a piece of wood, sections one inch thick cut across the grain of the wood are most suitable, as they can be readily cut up into thin flakes by this machine.
Moisture in Sample.—A small average sample should be dried at 100° C. for the determination of moisture.
Treatment with Caustic Soda.—About two hundred grams of the raw material is closely packed into a small digester or autoclave and covered with a solution of caustic soda having a specific gravity of 1·050. A perforated lead disc should be placed above the sample in the digester to prevent any of it from floating above the level of the solution. The material should be digested for five or six hours at a pressure of 50 lbs. The conditions of treatment here given will need to be varied according to the nature of the fibre. Some materials can be readily converted into pulp with weaker liquor and at a lower pressure, while others will require prolonged treatment. These conditions must be varied according to judgment or according to the effects produced by the conditions already set out.
Unbleached Pulp.—The contents of the digester are[Pg 45] emptied out into an ordinary circular sieve provided with a fine copper wire bottom, having a mesh of about sixteen to the inch. The sieve is immersed in water and the contents partially washed with hot water. The partially washed material is squeezed out by hand and tied up in a strong cloth and then kneaded thoroughly by hand in a basin of water which is repeatedly renewed until the fibre is thoroughly washed. The process of kneading at the same time reduces the fibre to the condition of pulp. The water is carefully squeezed out of the pulp by hand, and the moist pulp is then divided into two equal parts, the first of which is made up into sheets of any convenient size, care being taken that none of the fibre is lost. These sheets are then dried in the air and preserved as samples of unbleached pulp, a record being made of the weight produced.
Bleached Pulp.—The second portion of the moist pulp is mixed with a solution of bleach, the strength of which has been accurately determined by the usual methods. The amount of bleach added should be about 20 per cent. of the weight of air-dry fibre present in the moist sample of pulp. The pulp should be bleached at a temperature not exceeding 38° C., and when the colour has reached a maximum the amount of bleach remaining in solution is ascertained by titration with standard arsenic solution. In this way the amount of bleaching powder required to bleach the pulp is determined. The product is then made up into sheets of pulp which are dried by exposure to air and subsequently weighed.
Yield of Pulp.—The percentage yield of finished pulp obtained from the raw material is determined from the figures arrived at in the experiment described, and the weight of raw material necessary to produce one ton of bleached pulp is readily calculated.
Examination of Bleached Fibre.—The fibre should be[Pg 46] carefully examined under the microscope and a record made of general microscopic features, especially with reference to the length and diameter of the fibres, and the proportion of cellular matter present, if any.
Sample of Paper.—It is only in the case of short-fibred material similar to esparto and straw that sheets of paper capable of giving comparative results as to strength can be made. The figures obtained with fibrous materials of this kind are only comparative, because it is possible in practice to make a much stronger sheet of paper when the material is beaten properly under normal conditions.
A similar investigation should be made by submitting the fibre to treatment with bisulphite of lime, that is to say, if the fibre lends itself to such a process. A lead-lined digester is necessary, and the solution employed is bisulphite of lime prepared according to the directions given on page 160.
The preparation of sulphite pulp requires more attention than the manufacture of soda pulp. It is most important that the digester should be absolutely tight in order to prevent the escape of any free sulphurous acid gas, and the contents of the digester must be heated slowly until the maximum pressure has been reached.
The word rag is used to designate a very wide range of raw material suitable for conversion into paper. In the case of high-class hand-made writing papers only the best qualities are employed, such as new linen and cotton[Pg 48] cuttings from factories, or well-sorted rags of domestic origin. The usual classification adopted by merchants who supply the paper mills is somewhat as follows:—
New white linen cuttings (from textile factories).
New white cotton cuttings (from textile factories).
Fine whites (domestic rags).
Outshots (a quality between fines and seconds).
Seconds (a grade inferior to fines).
Thirds (inferior and dirty well-worn rags).
Coloured prints (of all grades and colours).
Fustians and canvas.
Manila and hemp rope.
Baggy, gunny, and jute.
The total amount of rag used in England for paper-making is not known. The only figures available refer to rags imported; and these cannot be regarded as a measure of consumption, which could only be arrived at by first ascertaining the quantity of home rags used. The imports of rag at stated periods are given in the appended table:—
Rags Imported into England.
| — | 1872. | 1882. | 1892. | 1902. | 1905. |
| Weight (tons) | 22,254 | 21,200 | 23,032 | 18,692 | 23,681 |
| Value | £373,035 | £303,349 | £214,065 | £173,732 | £224,232 |
Sorting and Cutting.—All rags on arrival at the mill are carefully sorted. This process is conducted entirely by women, who sort and cut up the rags at special tables provided with cutting knives curved in shape similar to a scythe. These are fixed at an angle in the centre of the table, with the back towards and in front of each work-woman. The top of the table is made of thick coarse wire[Pg 49] so that some of the dirt and foreign impurities may fall through. All buttons, hooks and eyes, pins, leather, pieces of rubber, and other articles are carefully removed, while seams and hems are also opened out. The rags are cut into slips 3-5 inches long and then recut crosswise, and thrown into suitable baskets or receptacles standing round the table, by which means the sorting operation is effectually carried out. The care and attention given to the sorting is an important item in the manufacture of papers of uniform quality, and in the best mills the sorting is carried out to such an extent that twenty or twenty-five grades are obtained.
Dusting.—The rags are next passed through a machine which removes dirt. This is a hollow cylindrical or conical[Pg 50] drum having an external covering of coarse wire cloth, which rotates inside a wooden box. The shaft is provided with projecting spikes, so that the rags are violently agitated in their passage through the machine. The dirt and other impurities fall through the wire on to the floor of the room, while the clean rags are discharged from the lower end of the drum. The loss in weight varies according to the condition of the rags. With good materials the loss[Pg 51] may only be 1-2 per cent., while with dirty common rags the loss during cleaning and dusting may amount to 10 per cent.
Boiling.—The further purification of the rags is effected by a chemical treatment, viz., boiling at a high temperature with alkaline substances, which process removes fatty, glutinous, and starchy matter from the material.
For this purpose a spherical digester is used, generally 7-9 feet diameter, and capable of holding 2-2½ tons of rag. The boiler or digester is filled with dusted rags, and the requisite amount of alkaline solution added. The manhole is then closed, and steam admitted through the hollow[Pg 52] trunnions until the pressure reaches 20 or 30 lbs., at which pressure the boiling is continued for three to six hours according to requirements, the digester rotating slowly the whole time in order that the rags may be evenly and thoroughly boiled.
The liquor employed for boiling is a solution of caustic soda, carbonate of soda, or milk of lime. In the case of caustic soda the amount required varies from 5 to 10 per cent. of the weight of rag. Caustic soda is preferable to lime, because it acts upon the grease and other fatty[Pg 53] matters, forming a soluble compound which is freely removed in the subsequent process of washing. Many paper-makers, however, use milk of lime, carefully strained through fine cloth, almost exclusively. Considerable experience and skill are necessary in this operation in order to avoid injury to the fibre not only as regards its strength, but also its colour.
Washing.—When the rags have been sufficiently boiled, the steam is turned off and the pressure allowed to fall. This can be effected quickly by blowing off from a valve fixed at the bottom of the boiler opposite to the manhole. The cover is removed from the boiler and the boiler slowly rotated in order that the contents may be discharged into a tank placed below. The “black liquor,” as it is called, is then drained away from the rags, which are immediately subjected to a preliminary washing. The process of washing must be carried out in a thorough manner in order to remove all soluble compounds, which if left would cause an unnecessary waste of bleach in the subsequent stages of purification. There are many schemes employed for washing, most of them being devised with the idea of using a minimum quantity of water.
The most general practice, in the absence of special machinery, is the preliminary treatment in the tank below the digester, followed by a more complete washing process in a machine known as a breaking engine.
This apparatus is a shallow oval-shaped vessel with circular ends, divided lengthwise by a partition called a mid-feather, which, however, does not extend the full length of the apparatus. In one of the two channels into which the vessel is thus divided a heavy roll is fitted, which is provided with a number of steel knives. On the floor of this channel there is fixed a “bed-plate,” also provided with projecting knives which are parallel with the knives[Pg 54] in the roll. The distance between the knives in the roll and those in the “bed-plate” may be altered as required by means of an adjusting screw. In the other channel of the breaking engine there is fitted a “drum-washer,” which serves for the removal of the dirty water from the machine. This drum is divided into sections by means of partitions which reach from the centre to the circumference. The surface of the “drum-washer” consists of a fine brass wire cloth supported by a coarser material placed underneath.
The breaking engine is half filled with clean water, and the rags are thrown into the engine until it is suitably filled. The rotation of the heavy roll causes the mixture of rags and water to circulate round the vessel, the floor of which is so constructed that the pulp is drawn between the roll and “bed-plate” and discharged over the “backfall,” which is that portion of the sloping floor behind the “bed-plate.”
The “drum-washer” rotates with its surface in contact with the mixture in the engine, so that the dirty water passes through the wire cloth and is caught in the curved sections or buckets inside the drum and discharged into a trough adjacent to the centre, and thereby conveyed away from the engine. Clean water is allowed to run into the vessel at one end while the dirty water is discharged by means of the “drum-washer.” At the same time the rags are broken up by means of the knives on the roll, so that when the rags are sufficiently washed, a process which usually occupies four hours, they are also partially disintegrated.
Bleaching.—The clean disintegrated rag is next bleached by means of ordinary bleaching powder solution. Bleaching powder is a substance prepared by the action of chlorine gas on dry slaked lime, resulting in the formation of a compound which has the property of bleaching or “whitening” vegetable matters. The clear solution obtained by treating the powder with water is utilised by the paper-maker for bleaching the rag pulp.
Various methods are used for this purpose. Sometimes the requisite volume of clear bleach liquor is added to the pulp in the breaker, and the material kept in constant circulation until the operation has been completed. In other cases the broken pulp is transferred to a “potcher,” which is a vessel similar in shape to the breaker, but merely provided with paddles for keeping the pulp in circulation, and bleached by the addition of chloride of lime solution.
Another method frequently adopted is to discharge the pulp from the breaker, immediately after the addition of the bleach, into brick or cement tanks, allowing the bleaching action to proceed spontaneously without prolonged agitation.
In some instances the process is hastened by adding dilute sulphuric acid to the pulp after the bleach liquor has[Pg 56] been run in, or by heating the mixture with steam. For high-class papers such devices as this are seldom resorted to, as experience shows that the colour of pulp bleached by drastic methods does not maintain a high standard.
The pulp is then thoroughly washed in order to remove every trace of residual bleach, and also the soluble compounds which have been formed during the operation. Very large quantities of water, clear and free from suspended dirt, are necessary. In some mills any excess of bleach is neutralised by the use of an “antichlor” such as sodium hyposulphite, or sodium sulphite, but the best results are undoubtedly obtained when the quantity of chemicals used is kept at a minimum.
If the pulp is bleached in a breaker or potcher, the washing is effected by the aid of the drum-washer. With pulp treated in steeping tanks, fresh water is allowed to percolate or drain slowly through the mass.
The substitution of a sodium hypochlorite solution for the ordinary calcium hypochlorite solution obtained from common bleaching powder has been the aim of specialists for many years. As early as 1851 a patent was taken out by Charles Watt for decomposing chlorides of the alkali metals and the formation of hypochlorites. It was not until 1886 that a practical method was devised for producing an electrolysed solution of salt, but in that year Hermite introduced a continuous process in which an electrolysed solution having a strength of three grammes chlorine per litre was passed continuously into the potcher.
Many patents for the electrolysis of salt have been taken out during the last twenty years, of which the Bird-Hargreave process is in operation in England, the Rhodin process in America, the Siemens and Halske in Norway,[Pg 57] and the Oettel and Haas apparatus in Germany. The figures relating to the latter apparatus may be mentioned as typical of the present condition of electrolytic bleaching. The apparatus consists of a narrow rectangular trough divided into a number of chambers through which a solution of brine flows at a constant and steady rate. The electric current is passed through the solution by suitable electrodes, the temperature being kept down by means of a cooling coil. The cost of producing the bleach liquor as given by the inventors of the apparatus from the results of actual working are shown in the following table:—
Table giving Analysis of Cost for Producing Bleach Liquor.
| Capacity of tank | 750 litres = 166 gallons. |
| Strength or density of brine | 1·5 Baumé, or 23 Twaddell. |
| 286 lbs. of common salt required for 166 gallons. | |
| Hours worked | 2 | 4 | 6 | 8 | 10 | 12 |
| Grammes of chlorine per litre produced | 4·35 | 7·38 | 9·9 | 12·42 | 14·31 | 16·20 |
| Temperature C. of brine during operation | 20 | 21 | 20 | 21 | 20 | 20 |
| Ampères of 110 volts | 55 | 50 | 46 | 52 | 47 | 43 |
| Power in h.p. hours | 16 | 31 | 45 | 61 | 75 | 89 |
| Cost of the h.p. at ·22d. per h.p. hour | 3½d. | 6¾d. | 10d. | 1½d. | 4½d. | 7½d. |
| Cost of salt | 1s. 6d. | 1s. 6d. | 1s. 6d. | 1s. 6d. | 1s. 6d. | 1s. 6d. |
| Total cost | 1s. 9½d. | 2s. 0¾d. | 2s. 4d. | 2s. 7½d. | 2s. 10½d. | 3s. 1½d. |
| Total chlorine obtained in kilos. | 3·262 | 5·535 | 7·425 | 9·315 | 10·732 | 12·150 |
| Cost of chlorine per kilo. | 6·6d. | 4½d. | 3¾d. | 3·4d. | 3·2d. | 3d. |
| Salt used per kilo chlorine | 35 | 20 | 15 | 12 | 10 | 9 |
The above costs have been estimated on prices as follows:—
| Coal | 10s. per ton. |
| Salt | 12s. per ton. |
After 12 hours the 166 gallons (750 litres) are converted into electrolytic bleach liquor containing 26¾ lbs. of active chlorine (12·15 kilos.).
Beating.—Although the rags are reduced by the breaking engine to a condition of fibrous lint, called “half-stuff,” they are not fit for conversion into paper. They have to be beaten in special machinery until a complete separation of the single fibres has been effected, and this process is rightly regarded by many paper-makers as the most important stage of manufacture.
The beating engine is similar in construction to the breaking engine, but there are certain essential differences in arrangement and manipulation. There is usually no drum-washer; the roll contains a large number of knives which are fixed in clumps or sets of three round the circumference; the lowering of the roll upon the bed-plate is carefully watched and controlled, and the desired effects[Pg 59] are only obtained by strict attention to the condition of the pulp during the whole process.
The beater is first partially filled with water, and the drained half-stuff added gradually until the “furnish,” a convenient term applied to the contents of the engine, has the proper consistency, which varies according to the nature and quality of paper required.
The mass is circulated steadily round the engine by the action of the beater roll, which is lowered from time to time until the distance between the knives on the roll and those on the bed-plate has been set to the desired adjustment. This lowering of the roll and its proper adjustment call for the greatest care.
Influence of the Beating.—The importance of this operation can easily be judged from one or two specific examples.[Pg 60] In the case of rag papers the two extremes of variation are represented by the ordinary blotting paper on the one hand and a hard strong writing paper known as a loan on the other. Now the great difference in these papers may be traced to the careful selection of the rag and the treatment in the beater as the two primary causes of the final results.
For blotting papers it is essential that the rags should be old and tender. In the beating operation subsequent to the usual boiling and bleaching processes the half-stuff is beaten quickly with sharp knives, the roll being lowered soon after the engine is filled, so that the beating is finished in about one to one and a half hours.
For the strong writing paper new strong rags are selected. In the beating process the knives used are dull, the roll is lowered slowly and cautiously, and the beating goes on for eight to ten hours.
The effect of such difference in treatment is easily seen by examination of the fibres of the papers under the microscope. In the first case the fibres appear short with clean cut ends, the shape little distorted, the structure well defined, bearing a strong resemblance to the unbeaten material. In the case of the well-beaten paper the ends of the individual fibres appear to be drawn or frayed out, the fibres do not possess the sharp well-defined outline characteristic of blotting paper; they are partly split up into fibrillæ which lie together in a confused mass.
In the blotting paper these effects are produced because the knives being sharp cut up the material quickly, and in the writing paper because the dull “tackle” tends to draw out the fibres and tear them up lengthwise.
The practical result is a spongy, soft, and bulky blotting and a hard, strong, heavy writing paper. Of course the great difference between a blotting and a writing paper is[Pg 61] not all due to this one operation, but is obtained by a series of operations, of which one of the most important is, however, the beating.
Colouring the Paper.—The pulp is brought to any desired tint by the addition of mineral pigments or aniline dyes to the contents of the engine. The latter soluble dyes, however, are seldom used for high-class rag papers. Prussian blue, ultramarine, and smalts are chiefly used for this purpose, giving toned blue, azure, and blue laid papers.
Making the Paper.—The beaten pulp, when duly prepared, is run from the engine into store tanks known as stuff chests, ready for the actual manufacture. The pulp properly diluted with water is strained through special screens to remove any insufficiently beaten material and any impurities present, after which it is run off into the vat, a square-shaped vessel built of wood or stone.
The apparatus used in forming the sheets is called a hand mould. The mould is a rectangular frame of mahogany upon which is stretched tightly a fine wire cloth, the surface of the latter being kept flat by a coarser wire cloth fixed underneath, supplemented by wedge-shaped pieces of wood. A second frame called the deckle fits on to the mould in such a manner as to form a shallow tray, the bottom of which is the fine wire cloth.
The vatman takes up the mould with both hands and dips it into the vat full of pulp in a slanting position, drawing it through the stuff towards him in a peculiar manner and lifting it out from the vat with a definite quantity of[Pg 62] the mixture in the frame. As the water drains away from the pulp, through the wire cloth, he imparts a shaking motion to the mould in order to cause the fibres to “felt” properly, this felting or interlacing of the fibres being an essential feature in the manufacture of a good sheet of paper. When the water has drained away sufficiently from the pulp, the vatman removes the deckle from the mould and passes the latter over to the coucher, who takes the mould, reverses it, and presses the contents, which may now be described as a wet sheet of paper, down on to a damp piece of felt, by which means the paper is transferred to the felt. He returns the mould to the vatman, who meanwhile has made another sheet with a duplicate mould, and then, having laid a second felt upon the wet sheet of paper, he proceeds to transfer the next sheet of paper to the second felt. This process is continued until a pile is formed consisting of wet sheets of paper alternated with pieces of felt.
The pile is at once submitted to great pressure in the hydraulic press, and the excess water slowly forced out, while at the same time the sheets are compressed and thus “closed up,” as it is termed. When all the excess water has been removed as far as possible, the pile is taken away and the sheets of damp paper taken out, the felts being placed in one pile ready for further use, and the sheets of paper in a second ready for the next process.
The papers are put back into the press without felts between the sheets and left for some time. In most cases the sheets are turned round or mixed in with the sheets of another pile, before pressing. In this way any unevenness or irregularity in the sheets is counteracted and a more uniform result obtained.
When these changes are repeated several times the paper acquires an even texture and becomes firm and hard.
Drying the Paper.—The sheets are hung up in the loft,[Pg 63] as the drying room is called, upon poles or ropes. The moisture gradually evaporates, and the paper is thus dried by exposure to air. In winter it is necessary to warm the air in the loft, as the air is then saturated with moisture. In lofts of limited capacity the air is heated in order to hasten the process, but the best paper is allowed to dry naturally, as by this means the shrinkage is gradual and a maximum strength is attained.
Sizing the Paper.—The dried paper as it leaves the loft is termed Waterleaf because, being unsized, it readily absorbs water, and therefore before it can be used it must be sized. For this purpose it is dipped into a solution of gelatine, an operation described as tub-sizing or animal-sizing, the former term being used on account of the tub in which the size is kept, and the latter on account of the fact that the gelatine is made from animal matter such as hides, cartilage, hoofs, and other refuse.
Animal Size.—This is prepared from hide pieces, skins,[Pg 64] and the like by a simple process, which, however, requires a good deal of care in order to obtain the best results. The material is first thoroughly washed in plenty of clean water, and then heated with a definite quantity of water in a steam jacketed copper pan. The pieces slowly dissolve until a solution of gelatine is produced, and after the dirt and impurities have settled to the bottom of the pan the clear liquid is drawn off into store vessels. There are many details of a technical character to be attended to in the manufacture of good gelatine, and as the process is expensive, considerable attention is demanded at this stage in the completion of a sheet of paper.
The dry sheets of paper are sized by the simple expedient of dipping, or by the passage of the paper through a long trough. In the first case the workman takes up a number of sheets and dips the bunch into a vat of size at the proper temperature, about 100° Fahrenheit. He then allows the surplus size to drain off, and the sheets are submitted to a slight pressure in order to remove the excess of gelatine that will not drain off.
In the second case a different method is adopted in that the sheets of paper are carried by travelling felts through a bath of heated size, the excess gelatine being removed by the action of rubber or wooden rollers through which the papers are passed before leaving the apparatus. The papers are quickly and evenly sized by this method, which is now most generally used.
Glazing.—When the sheets of paper are quite dry they are ready for glazing, a process which turns the dull rough surface of the sized sheet into a highly polished smooth surface fit for use. The sheets are placed singly between copper or zinc plates, and a pile of these passed several times through heavy iron rollers, great pressure being applied to the latter during the operation.
The amount of polish imparted by this plate-glazing process, as it is termed, can be varied considerably. With a light pressure and few rollings, the sheet of paper can be turned out having a fairly smooth surface, and without a conspicuously shiny appearance. By employing a great pressure and repeated rolling a much higher surface is attainable. If the plates are hot a still higher finish is possible. Machine-made rag papers are glazed usually by means of the supercalender, which is a stack of alternate steel and paper rolls placed one above the other in a vertical position. The reel of paper passes between these rolls and becomes highly surfaced.
This operation effects many changes in the paper, besides imparting a good finish. The thickness of the sheet is reduced by about 40 per cent., the fibres being compressed much closer together. The tensile strength of the paper is also materially increased, and in every way the paper is improved. Moderation is essential in this as in everything, because excess of glazing weakens a paper, rendering it brittle and liable to crack when folded.
Laid and Wove Papers.—When certain papers are held up to the light and carefully examined it will be noticed that they appear to contain delicate transparent lines running parallel with one another at equal distances of about an inch, and that these are intersected by similar transparent lines running at right angles, which are much closer together. Such papers are known as Laid Papers, and the peculiar formation of the transparent lines is due to the construction of the mould used in the making. The wire surface of this mould consists of a number of somewhat stout wires placed about one inch apart, interwoven with finer wires running across and at right angles, which are threaded much closer together. When the mould is dipped into the vat and withdrawn, the water drains away[Pg 67] from the under surface of the wire, and the moist pulp settles down on the upper surface; but since the coarser wires project a little from the finer threads, the paper is slightly thinner along those wires, though to an almost infinitesimal extent, with the result that on drying the sheet appears to contain transparent lines.
Wove papers are so called from the nature of the mould used. The surface of the mould in this case consists of fine wires equally distributed, being woven in such a manner that the wires are equidistant from one another, as in ordinary wire gauze. A wove paper, on being examined in the light, simply shows a number of small diamond-shaped spaces, which in the majority of instances are difficult to detect.
The Watermark.—The transparent device observed in many papers when held up to the light is known as the watermark, a term probably derived from the conditions existing at the time the sheet of paper is made on the mould. The effect is produced by means of a raised design sewn or soldered to the surface of the mould, the design being fashioned out of fine wire.
When a mould thus fitted with the design is dipped into a vat of pulp and lifted out, the water falls through the wire, and the pulp sinks down on to the surface of the mould, forming a replica, so to speak, of the design, which is easily seen when the dry paper is held up to the light, because the paper is thinner just at those points where the wires forming the design come into contact with the wet pulp.
Some of the watermarks are very elaborate and interesting. A familiar illustration of a beautiful design of this[Pg 68] description is to be found in the Bank of England notes. As a general rule the ordinary watermark consists of a mere trade term such as “Vellum,” “Zenobia,” or of the name of the manufacturer, such as “J. Whatman,” “R. Batchelor,” and so on. In the earlier days of paper-making many highly interesting designs were used, and some of these are still extant. In fact many of the names by which certain standard sizes of paper are known owe their origin to the watermarks employed.
The earliest known watermark bears the date A.D. 1301, being in the form of a globe and cross, as shown. Of equal interest are those designs from which certain papers are called foolscap, crown, pott, post, royal, columbier, and so on. The watermarks are now little used, but the terms are still retained, as indicating the size of the sheet.
The cotton fibre is about 30 mm. long, with an average diameter of ·025 mm. of tube-like shape, and having a prominent central canal. There are no cross markings on the cell walls, and the ends of the fibre are rounded off into a somewhat blunt point. It exhibits a marked tendency to twist itself, especially if dry, and this peculiarity is readily observed with the raw material.
The process of paper-making alters the characteristic structure of the fibre very greatly. The ends of the fibre are seldom to be seen; the curious twist is less prominent, and the fibres are torn and destroyed. The effect of the beating process, for example, on cotton is easily to be noticed by comparing the fibres of a blotting paper under the microscope with the fibres of a bank or loan paper.
The distortions produced by prolonged beating renders[Pg 69] the determination of the exact percentage of cotton in a rag paper rather difficult, but the features to be looked for are the absence of pores, cross markings, the existence of a central canal, striations produced in many cases on the cell walls parallel to the length of the fibre. The structural features are more readily observed when the fibres are stained with a suitable reagent. (See page 71.)
The linen fibre has an average length of 27 mm. with a diameter of ·02 mm. The raw flax is very different from raw[Pg 70] cotton and is easily distinguished. The fibre is slender in shape, having thickened knots at regular intervals throughout its length, the general appearance of which may be compared to a stick of bamboo. The central canal of the fibre is extremely narrow, running like a small thread through the length of the fibre. The cell walls are further marked by numerous pores, which appear as small dark lines running from side to side, but not meeting in the centre.
In the treatment necessary for making paper these characteristics are largely destroyed, and while it is quite easy to ascertain that a paper is of linen, or of cotton, or that a paper is mainly cotton with a small percentage of linen, yet there are conditions under which it is difficult to determine the exact percentage of cotton or linen in a rag paper. If, for example, a paper contains nearly equal quantities of cotton and linen, the exact proportions cannot be determined closer than 10 per cent., especially in well-beaten papers.
Preparation.—Dissolve 2·1 grams potassium iodide and 0·1 grams iodine in 5 c.c. of water. Mix this solution with a solution containing 20 grams of dry zinc chloride in 10 c.c. of water. Allow the mixture to stand; pour off the clear liquid into suitable bottles.
Cotton, linen, hemp.—Wine red.
Esparto, straw and wood cellulose.—Bluish violet.
Mechanical wood, unbleached jute.—Yellow.
Manila hemp.—Blue, bluish grey to yellow.
The value of Esparto for the manufacture of high-class printing and medium quality writing paper is well known. This material has qualities which cannot readily be obtained from other fibres, such as rag and wood pulp. It is chiefly used in papers required for lithographic printing, books, and art illustration, since it gives a sheet having a good surface and one which is soft and flexible.
The grass is obtained from Spain, Morocco, Algeria, Tunis, and Tripoli, in which countries it grows wild, requiring very little cultivation. The condition of the crop is improved by proper treatment, and in districts where the grass is cut for export as a paper-making material attention is given to cultivation.
The plant grows to a height of three or four feet, and when mature the long blades of grass curl up into the form of a cylinder resembling a piece of wire. The leaf consists of two parts, the stalk and a sheath, which are easily separated when harvested. The grass is pulled up by hand and stacked into heaps in order that it may be dried by the heat of the sun, after which process it is carefully picked over for the removal of all extraneous matter and impurities. It is then graded, the best sorts being kept for weaving, and the remainder being sold for paper-making. It is packed up into large bales of about 4 cwt. capacity, compressed into small bulk by powerful presses, and shipped to England.
Esparto Pulp.—The first process in the manufacture of the paper is cleaning. The bundles of grass are opened up, shaken out, and put through a willowing machine. This consists of a hollow conical drum, the outer surface of which is a coarse wire cloth. Inside the drum is fitted a shaft provided with wooden teeth, and as the grass passes through it is tossed about and the dust removed. The clean grass is conveyed by travelling belts to the digester house. For the production of a high-class paper the grass is often examined by girls, who stand on either side of the travelling conveyer and take out any coarse root ends and foreign material not removed by the willowing machine.
Boiling.—The object of submitting esparto to chemical treatment is to obtain a pure paper-making fibre known as cellulose. The composition of this raw material is shown by the following analysis:—
Spanish Esparto.
| Cellulose | 48·25 | |
| Water | 9·38 | |
| Aqueous extract | 10·19 | |
| Pectous matter | 26·39 | |
| Fatty matter | 2·07 | |
| Ash | 3·72 | |
| 100·0 | ||
| Yield of dry cellulose obtained in actual practice from good raw material | 45 to 48 % | |
By boiling the esparto with caustic soda under pressure for a stated time, the non-fibrous constituents are removed, leaving the cellulose in a more or less pure form according to the severity of the chemical treatment.
In practice the grass is packed tightly into upright stationary digesters and a definite quantity of caustic soda solution added, the amount of chemical used being equal to 15-18 per cent. of the weight of grass packed into the digester. The form of digester almost universally employed is that known as the Sinclair's “vomiting” boiler, which is constructed so that a continuous circulation of the liquid is maintained by means of what are called “vomit” pipes. These are fitted to the sides of the digester in such a manner that the caustic soda solution circulates from the bottom of the digester, up through the “vomit” pipes, and is discharged downwards upon the contents of the boiler through a perforated plate fixed in the upper part of the digester. The requisite quantity of caustic soda solution is placed in the digester, and steam admitted into[Pg 75] the bottom of the vessel while the grass is being thrown in. In this way a much larger weight of grass can be boiled at one operation, since the bulk is greatly reduced when the grass has become thoroughly soft and wet.
When the boiler is loaded the inlet is closed up and steam[Pg 76] turned on to the full pressure of about 40 or 50 lbs., this being maintained for a period of about four hours. The non-fibrous constituents of the esparto are gradually dissolved out by the caustic soda, and when the operation is completed the black liquor is run off from the digester into large store tanks, and the esparto grass which remains in the digester is then completely washed until the soda is almost entirely washed out.
The conditions for boiling and bleaching esparto are varied by the paper-maker as circumstances require. A maximum yield of fibre is obtained when the least possible quantity of caustic soda is used, but a larger percentage of bleaching powder may be necessary to ensure a well bleached pulp. The use of an excess of caustic soda is[Pg 77] probably the general practice for several reasons, amongst which may be noted the advisability of guarding against irregularities in the quality of the esparto, and consequent insufficient boiling, as well as the advantage of having some free caustic in the spent liquors to prevent the furring up of the tubes of the evaporating apparatus in the soda recovery department.
The following experiments, given by a contributor to the Paper Trade Review some years ago, are interesting as showing the effect of varying proportions of caustic soda used per unit of grass:—
Experiments re Yield of Air-dry Bleached Pulp from Oran Esparto.
Air-dry Pulp containing 10 per cent. water.
| No. of Experiment. | Esparto. | Soda Liquor. | Conditions of Boiling. | Weight of Air-dry Pulp. Grams. | Dry Pulp on Dry Esparto. Per cent. | Bleaching Powder. Per cent. | |||
| Wt. taken. Grams. | Volume, C.C. | Per cent. Na2O. | Time. Hours. | Temp. °C. | Pressure. Lbs. | ||||
| 1 | 200 | 800 | 1·58 | 3 | 142 | 55 | 87·30 | 43·65 | 29·5 |
| 2 | 200 | 800 | 2·13 | 3 | 142 | 55 | 80·67 | 40·33 | 18·5 |
| 3 | 200 | 800 | 2·69 | 3 | 142 | 55 | 72·00 | 36·00 | 10·5 |
Practical Data calculated from Experiments.
| No. of Experiment. | Boiling. | Weight of Esparto to give 1 ton Pulp. Cwts. | 60 per cent. Caustic Soda required to Digest Esparto. Cwts. | Bleaching Powder required to Bleach 1 ton Air-dry Pulp. Cwts. | For One Ton of Esparto used. | ||
| Time. Hours. | Pressure. Lbs. | 60 per cent. Caustic. Lbs. | Bleaching Powder. Lbs. | ||||
| 1 | 3 | 55 | 45·8 | 4·30 | 5·26 | 210 | 260 |
| 2 | 3 | 55 | 49·5 | 6·27 | 3·39 | 282 | 156 |
| 3 | 3 | 55 | 55·5 | 8·90 | 1·96 | 358 | 79 |
Recovery of Spent Liquor.—As it is possible to recover 75 to 80 per cent. of the soda originally used in digesting the esparto, the washing of the boiled grass is conducted on scientific principles in order to ensure a maximum recovery of soda at a minimum cost.
The recovery is effected by evaporating down the black liquor, together with the washing waters, to a thick syrupy mass, which can be burnt. The organic and resinous constituents of the esparto which have been dissolved out by the caustic soda, forming the soluble soda compounds, ignite readily, and during combustion the organic soda compounds are converted more or less completely into crude carbonate of soda.
It is obvious, then, that the cost of recovery depends mainly on the quantity of weak washing water which has to be evaporated. Consequently methods are devised by means of which the grass is thoroughly washed with as little water as possible, and some of the methods are very ingenious.
The spent liquors and washing waters are evaporated to a small bulk in a vacuum multiple effect apparatus, and the thick liquid mass obtained by evaporation is burnt either in a rotary furnace or on an ordinary hearth. Every precaution is taken to effect this operation with a minimum quantity of coal. The burning off of this mass results in the formation of a black substance which is taken away from the furnace and allowed to char or slowly burn until the impure white soda ash, or carbonate of soda, is obtained.
Two systems of recovery are in general use, which deserve a brief notice:—
Direct Evaporation.—The liquors may be evaporated to a small bulk ready for incineration by treatment in long shallow pans or furnaces, the heat necessary for the process[Pg 80] being obtained mainly from the combustion of the thick concentrated liquor. The most familiar type of this form of apparatus is the Porion evaporator.
The combustion of the concentrated liquor is started by a coal furnace at one end of the apparatus. The thick viscous mass catches fire and burns with a fierce flame, and the heat is utilised in evaporating the weaker liquors which flow continuously through shallow brick troughs, the surface of which is freely exposed to the heat and flames from the hearth where the organic soda compounds produced in the boiling of esparto are being incinerated and converted into soda ash.
Under suitable conditions this evaporator is most economical in its results. It can be erected cheaply, and when all the heat is fully used in every possible direction it can be worked at a low cost compared with the more modern multiple effect evaporators.
Vacuum Multiple Effect Evaporation.—Advantage is taken of the fact that water boils at a lower temperature in a vacuum than at the ordinary pressure of the atmosphere. There are many forms of apparatus based on this principle, amongst which the most recent is Scott's evaporator. The black liquor from the boilers is pumped through tubes heated externally by high-pressure steam. The liquor is passed into a chamber in which a slight vacuum is maintained, so that immediately on entering, the liquor parts with a good deal of water in the shape of steam. The steam liberated is utilised in producing further evaporation of the partially concentrated liquor, and this operation is repeated several times until the concentration is effected to the desired point.
In most cases the actual incineration of the thick liquor is carried out in a rotary furnace when such an apparatus as this is used.
Evaporation Table.
Showing the volume of liquor obtained by evaporating 1,000 gallons of weak black lye of density d to a higher density D.
| Lower Density d (at 100° F.). | Higher Density D (Twaddell) at 100° F. | ||||||||
| 20. | 25. | 30. | 35. | 40. | 45. | 50. | 55. | 60. | |
| 2 | 100 | 80 | 66·6 | 57·1 | 50 | 44·4 | 40 | 36·3 | 33·3 |
| 3 | 150 | 120 | 100 | 85·7 | 75 | 66·6 | 60 | 54·5 | 50 |
| 4 | 200 | 160 | 133·3 | 114·3 | 100 | 88·8 | 80 | 72·7 | 66·6 |
| 5 | 250 | 200 | 166·6 | 143 | 125 | 111·0 | 100 | 90·9 | 83·3 |
| 6 | 300 | 240 | 200 | 171·4 | 150 | 133·3 | 120 | 109 | 100 |
| 7 | 350 | 280 | 233·3 | 200 | 175 | 155·5 | 140 | 127 | 116·6 |
| 8 | 400 | 320 | 266·6 | 228·6 | 200 | 177·6 | 160 | 145·5 | 133·3 |
| 9 | 450 | 360 | 300 | 257 | 225 | 200 | 180 | 163·5 | 150 |
| 10 | 500 | 400 | 333·3 | 286 | 250 | 222 | 200 | 181·8 | 166·6 |
Example:—1,000 gallons of weak liquor at a density of 7° Twaddell are reduced to a volume of 200 gallons having a density of 35° Twaddell, or to a volume of 140 gallons with a density of 50° Twaddell, by evaporation.
Preparation of Caustic Soda.—The crude soda ash recovered from previous boiling operations is dissolved in large lixiviating tanks and extracted with hot water. The clear solution obtained after all impurities have been allowed to settle is pumped up into the causticising tanks, where it is converted into caustic soda, the loss due to the amount of soda not recovered being made up by the addition of ordinary soda ash. The causticising pans are large circular iron vessels usually 9 feet diameter and 8 or 9 feet deep, into which a known volume of the recovered carbonate of soda solution is placed.
A weighed quantity of ordinary quicklime is then put into a perforated iron cage which is fixed inside the causticising pan at such a level that the whole of the lime is immersed in the solution. The liquor is kept in[Pg 82] constant circulation by means of an agitator and heated to boiling point, with the result that the chemical reaction sets in, the carbonate of soda being converted into caustic soda and the lime being thrown out as chalk. When the operation is completed, the steam is turned off and the chalk allowed to settle. The clear liquor is carefully strained off and pumped up into store tanks from which the required quantities are drawn off into the digesters as circumstances demand.
Washing.—The grass which has been partially washed in the digester is dug out by the workmen and discharged through a manhole fitted on one side of the digester near the bottom. It is then conveyed in any convenient manner to the breaking engine, in which the grass is more completely washed. This important machine has already been described on page 53. The floor of the vessel slopes slightly upward towards the front of the roll and falls suddenly behind the roll, in order to promote a circulation of the contents of the engine round and round the vessel.
A definite weight of boiled grass is thrown into the engine together with a large quantity of fresh water. The circulation of the roll draws the mixture of pulp and water between the knives, breaking it up and at the same time discharging it behind the beater roll, and producing a continuous circulation of the mixture in the two sections of the vessels.
The dirty water is continuously removed from the vessel by means of a “drum-washer.” This is a large hollow drum, the outer surface of which consists of a fine wire cloth, the interior of the washer being fitted with specially curved scoops. The drum-washer is lowered until it is half immersed in the mixture of pulp and water, and as it rotates the dirty water finds its way through the wire cloth, being caught up by the internal scoops and discharged through a[Pg 83] pipe to a drain outside the breaking engine. At the same time fresh water is run into the vessel at one end, and the continuous washing of the pulp thus effected.
Bleaching.—The clean boiled grass is bleached by means of a solution of chloride of lime.
There are several methods used for this purpose, each of which has special advantages of its own, though this is largely a question of local conditions:—
(A) The pulp can be bleached in the washing engine directly the grass has been sufficiently cleaned. In this case the flow of fresh water is stopped and as much water as possible removed by means of the drum-washer. The drum-washer is then raised out of the pulp and a known volume of bleaching powder solution corresponding to a definite weight of dry powder is added to the contents of the breaking engine. The amount used depends on the quantity of dry grass in the breaking engine, the usual proportion being 8 to 10 per cent. on the calculated air-dry weight of raw grass. As the stuff circulates round the engine the colour gradually changes from dark yellow to white.
The process is sometimes hastened by blowing a small quantity of steam into the mixture and thereby raising its temperature. Considerable care must be exercised in using heat, because pulp bleached quickly by this means is liable to lose colour at the later stages of manufacture.
When the pulp has been bleached to the required extent, the drum-washer is again lowered into contact with the bleached pulp, and the latter is thoroughly washed so as to be quite free from traces of bleach and other soluble impurities.
(B) Esparto is often bleached in a “Tower” bleaching engine which consists of a tall cylindrical vessel of 9 feet diameter, and 15 or 16 feet deep, at the bottom of which is fixed a small centrifugal pump.
The boiled grass together with sufficient water and clear bleaching powder solution is placed in the engine; the centrifugal pump draws the mixture from the bottom of the vessel and discharges it, by means of a large external pipe, direct into the top of the vessel, where, as it falls, it comes into contact with a circular baffle-plate, which distributes the pulp evenly over the surface of the mixture in the vessel. A continuous and rapid circulation is thus maintained, and the process is said to be very effective. The bleached pulp is subsequently washed free from any traces of bleach.
(C) Esparto is frequently bleached by the “steeping” process. In this case the pulp is washed in the breaking engine, mixed with the required quantity of bleach, and at once discharged through the outlet pipes of the engine into large brick tanks, where the bleach is allowed to act quietly upon the boiled grass. This method produces a pulp of good colour and is economical.
Whichever process of bleaching is adopted, it is necessary to remove all the by-products formed during the process, as these soluble by-products if left in the mixture produce a lowering of colour.
The presence of small traces of bleaching powder solution can be detected by the use of starch and potassium iodide test papers. If a handful of the pulp after bleaching, when squeezed out, does not turn the test paper violet or blue, then the absence of any free bleach is taken for granted. The slightest trace of bleach will turn such test papers blue or violet according to the amount present. This is the test usually applied by the men in charge of the bleaching operations.
Making Sheets of Esparto Pulp.—For convenience in handling, it is usual to work up the washed and bleached pulp into the form of moist sheets. This is effected on a[Pg 86] machine known as a “presse-pâte,” an apparatus which closely resembles the wet end of a paper machine. It consists of a set of flat strainers or screens, a horizontal wire similar to the paper machine wire, provided with deckles, the usual couch rolls, and press rolls.
The pulp diluted with water is passed through the screens and on to the horizontal wire, where it is formed into a moist sheet, the water draining away from the wire, and also being removed by vacuum pumps. The thick sheet of pulp is carried through the couch rolls and press rolls, being finally wound up on a wooden roller at the end of the machine. In this moist condition it is ready for use in the mill.
Dry Esparto Pulp.—When the bleached pulp is intended for export a more elaborate machine is used—to all intents and purposes a paper-making machine—by means of which the continuous sheet of moist pulp is dried and cut up into smaller sheets of suitable size. These dried sheets are packed up in bales containing 2 cwt. or 4 cwt. of dried pulp, then wrapped in hessian and bound with iron wires.
Other Methods.—Since the yield of esparto pulp from the raw material is less than 50 per cent. and it requires 45 cwt. of grass to make one ton of finished pulp, methods have been devised for treating the grass in the green state in the districts where it is grown, but so far nothing has been done on a large scale.
The isolation of the cellulose by alkaline treatment in the cold has been suggested, but the method never passed beyond the experimental stage. This process was indeed first mentioned by Trabut, who many years ago considered that the removal of non-fibrous constituents from fresh grass could be readily accomplished by the less drastic treatment of the esparto with alkaline carbonates of soda and potash at ordinary temperatures.
The production of esparto pulp by bacteriological fermentation is an idea of later date. According to the inventor, the grass is crushed mechanically by means of rollers and then immersed in sea water inoculated with special bacillus obtained from esparto, and gradually resolved into cellulose and soluble by-products by fermentation which is complete in about eleven days. The commercial value of this idea has not yet been demonstrated.
The pulp of esparto when examined under the microscope is easily recognised, first by the characteristic appearance of the long slender cylindrical-shaped fibres, and secondly by the numerous cells always present. These cells consist of cuticular vessels with serrated edges, and also of small pear-shaped seed hairs, the shape of which is a ready means of identifying esparto. An examination of the transverse section of the raw material indicates the source of these pear-shaped vessels.
Test for Esparto in Papers.—Paper containing esparto fibre may be tested by means of a weak solution of aniline sulphate. The suspected paper is gently heated in the test reagent, and if esparto is present the paper turns a rose-red or pink colour, the depth of colour being a measure of the amount of esparto. Most of the modern book papers are prepared from chemical wood pulp and esparto mixed in varying proportions, and while this test can be used as a means of detecting a small or a large proportion of esparto, a microscopical examination is required for a more accurate estimation.
The proportions used by the paper-maker depend upon the weighing out of the wood pulp and esparto more or less accurately, while the microscopical test is based upon the relative proportions as represented by the volume of fibres[Pg 88] of each class on the glass slip placed under the microscope. Since the wood pulp consists of a number of broad flat ribbon-like fibres, and the esparto of small cylindrical fibres, considerable practice is necessary in making a proper analysis of the two constituents in paper.
The use of straw for the manufacture of paper was first brought prominently into notice about the year 1800 by[Pg 89] Matthias Koops, who published a book printed on paper made from straw, but it was not until 1860 that this material was used in any large quantity.
Straw is now converted into a bleached paper pulp for news and printings, and is also utilised for the manufacture of straw boards.
The production of a white paper pulp from straw is carried out in a manner similar to that used in the case of esparto fibre, viz., by digestion with caustic soda under pressure and subsequent bleaching. As the straw contains considerable quantities of siliceous matter, the chemical treatment necessary to reduce the material to paper pulp is more severe, a stronger solution of caustic soda being used,[Pg 90] and the process of digestion being carried out at a higher temperature.
For the best quality of straw cellulose, the material is cut up into small pieces by machines which resemble an ordinary chaff-cutter, and the knots taken out by a separating machine. In most cases, however, the whole straw is simply cut up into small lengths of about one to two inches long, and placed at once in the digester. When the straw is contaminated with foreign weeds, sand, husks, and similar substances, as is usually the case, it is carefully hand-picked by girls, who remove these impurities, which tend to produce particles of unbleached matter in the finished pulp. The expense of this preliminary cleaning process is more than compensated for by the enhanced value of the bleached straw pulp.
Digesting.—The cut straw is boiled in rotary cylindrical or spherical vessels, stationary upright boilers of the vomiting type being seldom employed because the circulation of the caustic soda liquor does not take place freely with straw packed in the latter.
As the material is very bulky, some of the liquor is first put into the boiler and the steam admitted while the straw is being thrown in. By this means the straw is softened and reduced in bulk, so that a larger quantity can be added before the digester is quite full. The full amount of caustic soda is then made up by further additions of liquor, and the contents of the digester heated by high-pressure steam for four to six hours.
The conditions of treatment are shown by the following trial:—
| Amount of straw | 5,600 lbs. |
| Caustic soda, 20 per cent. | 1,120 lbs. |
The caustic soda was added in the form of a liquor,[Pg 91] having a volume of 2,012 gallons and a specific gravity of 1·055.
| Time of boiling | 5 hours. |
| Pressure | 60 lbs. |
Washing.—The boiled straw is discharged into large tanks placed below the digester and washed with hot water, the smallest possible quantity being used consistent with complete washing in order to prevent the accumulation of large volumes of weak lye. The spent liquor and washing waters are drained off into store tanks and evaporated in a multiple effect apparatus by the same process as that used for esparto pulp. The last washings are usually run away because the percentage of soda in them is too small to pay for the cost of recovery.
The final washing of the straw pulp is completed by the use of a breaking engine or potcher. As straw pulp contains a large proportion of cellular matter which cannot be regarded as true fibres, there is always a danger of considerable loss in yield if the use of the breaking engine is extensively adopted, because the short cells escape through the meshes of the drum-washer. The washing is most economically effected in the tanks if a good yield of pulp is required.
Separating out Knots.—The broken pulp from the breaking engines is diluted with large quantities of water and pumped over sand traps in order to remove knots and weeds which have resisted the action of the caustic soda. These traps consist of long shallow trays, perhaps sixty to eighty yards long, one yard wide, and nine inches deep, containing boards which stretch from side to side, sloping at an angle, and nailed to the bottom of the trays. The dilute pulp flows through the trays, leaving the heavy particles, knots, and foreign matter behind the sloping[Pg 92] boards, and finally passes over the strainers, which retain any large coarse pieces still remaining.
Making Sheets of Pulp.—The mixture from the strainers contains a large excess of water which has to be removed before the pulp can be bleached. For this purpose a wet press machine (see page 103) or a presse-pâte (see page 85) is employed, and the wet sheets of pulp are then ready for bleaching.
Bleaching.—The process by which the pulp is bleached is exactly similar to that used for treating esparto.
From 1870 to 1890 large quantities of straw were used for the manufacture of newspaper in conjunction with esparto and wood pulp, but the price of the material was gradually advanced so that it could not be used with advantage, especially as the production of wood pulp gave a material which was much cheaper, and which could be utilised at once without chemical treatment.
In the manufacture of newspaper the tendency during recent years has been to make the paper mill operations as mechanical as possible and to dispense with the preliminary operations which are essential for the manufacture of half-stuff, the chemical processes being left in the hands of the pulp manufacturers.
The manufacture of straw cellulose is now practically confined to Germany, but small quantities of the bleached straw cellulose are imported because the pulp imparts certain qualities to paper which improve it, notably in making cheap printing papers harder and more opaque.
The paper pulp obtained from straw consists of a mixture of short fibres together with a large proportion of oval-shaped cells. The fibres are short and somewhat resemble esparto, but the presence of the smaller cells is a sure[Pg 93] indication of the straw pulp. The fibres themselves closely resemble the fibres of esparto, but as a rule the latter are long slender fibres, while the straw fibre is very often bent and twisted or slightly kinked.
The only method of distinguishing between straw and esparto is by examination with the microscope. There is no chemical reagent known which will produce a colour reaction on a paper containing straw that will serve to distinguish it from a paper containing esparto. If such papers are gently[Pg 94] heated in a weak solution of aniline sulphate a pink colour is slowly developed, the intensity of which is to some extent a measure of the amount of straw or esparto present.
Straw and esparto are usually described in text-books under one heading, partly because the fibres possess strong resemblances in physical and chemical constitution, and partly because the methods of manufacture are identical. At the same time the qualities of the two pulps are so different that they cannot be used indiscriminately, the one for the other. Straw cellulose cannot be utilised in the place of esparto, particularly for light bulky papers. Hence in magazine and book papers containing a fibre which gives a pink coloration with aniline sulphate it is fairly safe to assume that esparto pulp is present.
Wood is converted into pulp suitable for the manufacture of paper by methods which produce two distinct varieties. The first is mechanical wood pulp, so called because it is made by a purely mechanical process. The second is termed chemical wood pulp from the fact that the material is submitted to chemical treatment.
Ground Wood and Cellulose.—The two varieties of pulp are sometimes distinguished by the use of the terms ground wood and cellulose. In the former case the description implies a product consisting of pulp obtained by grinding wood into a fibrous condition, while in the second the word suggests a purified chemical product freed from the resinous and non-fibrous constituents found in wood. This is, in fact, the essential difference, for mechanical wood pulp consists of fibres which have been torn away from wood by means of a grindstone; it differs but slightly in chemical composition from the original raw material and contains most of the complex substances natural to wood. Chemical wood pulp, on the other hand, consists of fibre isolated from wood in such a manner that the complex non-fibrous substances are more or less entirely removed. The difference between these two pulps is shown in the following approximate analysis of spruce wood, and of the pulp derived from it. The composition of the mechanical pulp is practically identical with that of the wood itself.
Composition of Spruce Wood, and of Chemical Wood Pulp (Spruce).
| — | Wood (Spruce). | Chemical Wood Pulp. |
| Cellulose | 53·0 | 88·0 |
| Resin | 1·5 | 0·5 |
| Aqueous Extract | 2·5 | 0·5 |
| Water | 12·0 | 8·0 |
| Lignin | 30·5 | 2·5 |
| Ash | 0·5 | 0·5 |
| 100·0 | 100·0 | |
The use of mechanical wood pulp is generally confined to the manufacture of news, common printings and packing papers, cardboards, and boxboards. It possesses very little strength, quickly discolours when exposed to light and air, and gradually loses its fibrous character. The chemical wood pulp is a strong fibre, from which high-class papers can be manufactured, the colour and strength of which leave little to be desired.
Species of Wood.—The woods most commonly used for the manufacture of wood pulp belong to the order Coniferæ, or cone-bearing trees. In Europe the spruce and silver fir are the chief species, while in America spruce, balsam, pine, and fir are employed. The harder woods, such as hemlock, beech, larch and others, are not converted into pulp by the mechanical process.
Timber Operations.—The trees are cut down in the early part of winter by gangs of men specially trained to the work. The organisation of a lumber camp when the operations are of an extensive character is very complete and carefully arranged, every detail being attended to in order to get out the wood as cheaply and expeditiously as possible. The[Pg 97] branches and small tops are removed from the trees when they are fallen, and the trunks cut into logs of 12, 14, or 16 feet in length, and afterwards piled up on the banks of the nearest river, or on the ice, ready for the breaking up of the winter.
As soon as the ice breaks up and the rivers become navigable the logs are floated down to their destination, in some cases hundreds of miles from the scene of operations. Where rivers are not available the timber is brought out by horses or bullocks, or by means of a light railway.
Log Cutting.—As the timber arrives at the mill it is carefully measured, both as to its diameter and length, in order that a record may be kept of the quantity used. Some of the logs are piled up in the storeyard for use in the winter, and the remainder converted into pulp day by day. The logs are first cut into short pieces about 2 feet long by means of a powerful circular saw, the arrangements for this work being devised so as to keep down the cost of labour as much as possible. All waste pieces are thrown aside to be utilised as fuel.
Barking.—The bark on the logs is removed in one or two ways. Much of it is knocked off during the transfer from the forest to the mill, but even then the wood requires to be cleaned. In Norway and Sweden the wood is treated in a tumbler or a barker, while in America and Canada the use of the tumbler is practically unknown.
The barker consists of a heavy iron disc fitted with knives, usually three in number, which project from the surface of the disc about half or three-quarters of an inch. The barker rotates in a vertical position, and the short pieces of wood are brought one by one into contact with the disc in such a manner that the bark is shaved off by the knives. The machine is provided with conveniences for[Pg 98] pressing the wood against the disc and for turning the logs as they are barked.
The machine is encased in a strong cast-iron cover, and all the bark shaved off is carried away by the strong current of air set up by the rapid motion of the disc, and subsequently burnt.
The tumbler system is quite different. In this case the short pieces are thrown into a large circular drum with hot water, and the bark taken off by the friction of the pieces as the drum rotates. The loss of material is of course less[Pg 99] in this process, but the wood is not cleaned quite so effectively.
The wood at this stage can be used either for the manufacture of mechanical or chemical pulp. As a general rule the pieces are taken indiscriminately for either process, but sometimes the wood is sorted out, the clean stuff free from knots and blemishes being reserved for high quality chemical pulp.
Grinding.—The main feature of the grinding process is the attrition of the wood when held against the surface of a rapidly revolving grindstone, the fibres as they are rubbed off being instantly carried away from the stone by a current of water. A complete description of the machines used and the modifications of the process practised by manufacturers is impossible in this book, but the following points will be sufficient.
The machine consists of a large grindstone about 54 inches in diameter, and 27 inches thick. It rotates in a vertical or in a horizontal position at a high speed. The stone revolves inside a casing which is provided with a number of pockets, so called, into which the pieces of wood are thrown at regular intervals, as fast as the wood is ground by the friction of the stone.
A continual stream of water playing upon the surface of the stone washes away the pulp into a tank or pit below the machine.
The quality of the pulp may be varied by the conditions under which it is made. By limiting the proportion of water so that the wood remains in contact with the stone for a longer time the temperature of the mass in the pockets rises. Such hot ground pulp, as it is termed, is tough and strong.
When the fibres are washed away from the stone as fast as they are produced the temperature does not rise, and cold ground pulp is made, which is not characterised by the somewhat leathery feel of the pulp made at the higher temperature.
The surface of the stone plays an important part also. If the stone is smooth the wood is rubbed away slowly, but if the surface has been roughened and grooved by means of a special tool the fibres are torn away quickly. In the first case the pulp comes from the stone in a finely-ground state[Pg 101] and in a uniform condition, while in the second the pulp is coarse and chippy.
The output of the machine is, however, much increased by the use of sharp stones and by the application of considerable pressure to the blocks of wood.
Screening.—The mixture of water and pulp leaving the grinder falls into a tank below the stone, all large chips being retained by means of a perforated plate. The finer pulp, still too coarse for use, is then pumped to the screens, which serve to remove all chippy and coarse fibres and[Pg 102] produce a uniform material. The shaking sieve consists of a shallow tray, the bottom of which is a brass plate or series of plates perforated with small holes or slits. The pulp flows on to the tray, which is kept in a state of violent agitation, the fine pulp passing through the holes and the coarser pieces working down to the lower edge of the tray into a trough which carries them away. The flat screen is somewhat different in construction, but the principle of separation is the same. It consists of brass perforated plates forming the bottom of a shallow cast-iron tray, continually agitated by means of cams fixed to the under surface of the trays.
The centrifugal screen is a cage made of finely perforated[Pg 103] brass sheeting which revolves at a very high rate of speed inside a circular cast-iron vessel. The pulp flows into the interior of the cage, the fine fibres being forced through the screen by the centrifugal action of the machine, and the coarse material is retained.
Wet Pressing.—The pulp leaving the screens is mixed with such a large quantity of water that it is necessary to concentrate it. This is effected by means of the wet press machine (Fig. 41). The pulp and water are pumped into a wooden box in which revolves a large hollow drum, the surface of this drum consisting of a fine wire cloth of about 60 or 70 mesh. The drum is not entirely immersed in the mixture, so that as it rotates the pulp forms a skin or thin sheet on the surface, and the water passes away through the wire into the interior of the[Pg 104] hollow drum. The drum carries the thin sheet out of the box and above the level of the mixture until it comes into contact with an endless blanket or felt, which is pressed against that part of the drum not immersed in the liquid.
By this means the thin sheet is transferred to the felt and carried between squeezing rolls to the finishing rolls. The felt, carrying on its upper surface the thin sheet of pulp, passes between two rolls, usually 16 to 20 inches in diameter, the upper being made of wood and the lower one of cast iron. The pulp adheres to the upper drum and the felt passes round the lower drum back to the box containing the mixture of pulp and water; the thin sheet is continuously wound on the upper roll until a certain thickness is reached.
When this occurs the attendant removes the thick sheet by a dexterous movement of a sharp stick across the face of the roll. The wet pulp at this stage consists of 30 per cent. air-dry pulp and 70 per cent. of water.
Hydraulic Pressing.—The sheets taken from the wet press machine are folded into a convenient shape and piled up, coarse pieces of sacking being placed between the sheets. At stated intervals the piles are submitted to pressure in hydraulic presses in order to remove further quantities of water, which slowly drains away through the sacking. In this way a mass of pulp in the form of thick folded sheets containing 50 per cent. of dry wood pulp is produced.
The pieces of sacking are taken out and the sheets put up in bales of any required weight, usually 2 cwt. or 4 cwt.
Most vegetable fibres are converted into pulp by alkaline processes, that is by digesting the raw material with caustic soda and similar alkaline substances. Wood may be treated[Pg 105] in two ways, one of which is the ordinary soda process, and the other an acid treatment requiring the use of sulphurous acid.
Preparation of the Wood.—The logs of wood are cut up and barked exactly as in the case of mechanical pulp. The short two-foot pieces are then cut up into small flakes about one inch square and half an inch thick by means of a machine known as a chipper. This is similar in construction to a barker, consisting of a heavy iron disc rotating at a high speed inside a stout cover. The disc revolves in a vertical position, and three projecting knives slice up the logs into flakes. For this purpose the disc is provided with three slots which radiate from the centre towards the circumference for about 12 inches. The knives can be adjusted so that they stand up through the slots and above the surface of the disc to any required distance.
In order to ensure uniformity in the size of the chips, the practice is frequently adopted of sifting the wood leaving the chipper. The sieve is a large skeleton drum, the outer surface of which is made of a coarse wire cloth capable of passing all pieces of the size mentioned. Larger chips and pieces are retained in the drum as it revolves in a horizontal position and only fall out on reaching the extreme end of the machine.
The Digesters.—The object of boiling the wood under pressure with chemicals is to dissociate the valuable fibrous portion of the plant from the resinous and non-fibrous portion. In this process the wood loses half its weight, the yield of pulp being about 50 per cent., and the remainder is dissolved out by the chemical solution. The conditions of treatment are extremely varied in character, the quality of the pulp produced varying in proportion.
The digesters are either spherical, cylindrical, or egg-shaped, being constructed to revolve at a slow rate of speed,[Pg 106] or fixed permanently in an upright position. Spherical boilers are usually 9 or 10 feet in diameter, the cylindrical digesters being 40 or 50 feet high and 12 or 15 feet diameter, the larger ones being capable of taking 20 tons of wood for each operation.
For the alkaline process the interior of the digester does not require any special treatment, but with the acid process the internal portion of the boiler is carefully lined with a thick layer of acid-resisting brick and cement.
The contents of the digester are heated by means of high-pressure steam, which is blown direct into the mass or passed through a coil lying at the bottom of the vessel. In the former case the steam is condensed by the liquor, the volume of which is consequently increased, while in the latter case[Pg 107] the condensed steam is drawn off continuously from the pipes. Each system has its own particular advantages.
Different Kinds of Chemical Wood Pulp.—According to the method of treatment so the quality of the pulp varies. The chemicals used, the system of boiling, the temperature of digestion, the strength of the solutions, the duration of the cooking period, and, last but not least, the species of wood, are all determining factors in the value of the ultimate product.
Soda Pulp.—This is prepared by digesting wood with caustic soda in revolving boilers for eight or ten hours at a pressure of 60 to 80 lbs.
Sulphate Pulp.—Prepared by digesting the wood with a mixture of caustic soda, sulphide of soda, and sulphate of soda.
Sulphite Pulp.—The process most generally adopted for the manufacture of wood pulp is the treatment of the material in brick-lined digesters with bisulphite of lime for eight to nine hours at a pressure of 80 lbs.
Mitscherlich Pulp.—This is sulphite pulp prepared by digesting the wood at a much lower temperature and for a longer period than the ordinary sulphite. The steam is not blown direct into the mass of wood, and the pressure seldom exceeds 45 or 50 lbs., the time of boiling occupying 45 to 50 hours. So called from the name of the inventor.
Sulphite Wood Pulp.—This name is given to pulp prepared by digesting wood with solutions containing sulphurous acid, or salts of sulphurous acid. The acid is produced by burning sulphur or certain ores containing sulphur, such as copper or iron pyrites, in special ovens. The most modern form of oven consists of a cylindrical cast-iron drum revolving slowly in a horizontal position on suitable bearings. The sulphur is thrown at intervals, or fed automatically, into the oven, the amount of air being[Pg 108] carefully regulated to avoid the formation of sulphuric acid in the later stages of preparation. The sulphur is also burnt in stationary ovens which consist of flat shallow closed trays.
The hot sulphurous acid gas passes through pipes and is cooled, after which it is brought into contact with water and lime for the production of the bisulphite of lime. This is accomplished by one of two methods as follows.
Tower System.—The cool gas is drawn into high towers usually built of wood, 7 or 8 feet diameter, which are filled with masses of limestone. From tanks at the top of each tower a carefully regulated quantity of water flows down upon the limestone and absorbs the ascending column[Pg 109] of gas, this being drawn into the tower from the bottom. The limestone is simultaneously dissolved, and the liquid which flows out from the pipes at the bottom of the tower consists of lime dissolved in sulphurous acid, together with a certain proportion of free sulphurous acid. This is generally known as a solution of bisulphite of lime.
Tank System.—The somewhat costly tower system has in many cases been superseded by the use of a number of huge wooden vats, 10 to 12 feet diameter and 8 to 10 feet high. These tanks are filled with water and a known quantity of slaked lime. The gas is forced into the tanks by pressure or drawn through by suction, and the conversion of the milk of lime into bisulphite of lime proceeds automatically. In order to ensure complete absorption the gas passes through the tanks in series, so that the spent gases leaving the vats do not contain any appreciable amount of sulphurous acid.
In order to obtain pulp of uniform quality it is necessary that the liquor should be of constant composition. The formula differs in the various mills according to the conditions which are found most suitable.
Sulphite Digesters.—The almost universal form of boiler employed in cooking wood by the sulphite process is a tall cylindrical vessel of about 50 feet in height, and 14 to 15 feet internal diameter, lined with acid-resisting brick.
This form of digester is capable of holding 20 tons of wood at one charge, yielding 10 tons of finished pulp.
The chipped wood is discharged into the digesters from huge bins erected just above the openings to the digesters, so that the latter can be filled without any delay and the requisite quantity of sulphite liquor added.
The manhole or cover is at once put on, securely fastened, and steam turned on gradually until the pressure reaches 70 or 80 lbs., at which pressure the cooking is steadily[Pg 110] maintained. The progress of the operation is watched and samples of the liquor drawn from the boiler at intervals to be tested, so that the boiling may be stopped when the results of the testing show the wood is sufficiently cooked.
There is no special difficulty in this operation, provided the necessary conditions are observed. It is important that the wood should be dry, and that the proportion of sulphite liquor per ton of dry wood should be constant. If the wood happens to be wet, due allowance must be made for the excess water and a somewhat stronger liquor used in order to compensate for this. Other precautions of a similar character are observed in order to minimise the danger of an insufficiently cooked pulp.
Washing.—When the pulp has been boiled, a process which generally occupies seven or eight hours, the steam is shut off and the contents of the boiler blown out into large vats known as blow-out tanks, the pressure of steam remaining in the digester being sufficient to empty the softened pulp in a few minutes. Much of the spent sulphite liquor, now containing the dissolved resinous and non-fibrous portions of the original wood, drains away from the mass in the tank, and then copious supplies of clean water are added in order to wash out the residual liquors which it is essential to remove.
Numerous other devices are employed to ensure the complete washing of the boiled pulp.
Screening.—The production of a high-class pulp necessitates proper screening to eliminate coarse pieces of unboiled wood and the knots, the latter not being softened completely. The methods adopted vary according to requirements.
For uniform clean pulp that can be bleached easily the material from the blow-out tanks is, after washing, mixed with large quantities of water and run through sand traps,[Pg 111] which consist of long shallow wide boxes provided with slanting baffle-boards to retain knots and large pieces of unsoftened wood, the pulp thus partially screened being subsequently treated in the proper screening apparatus.
Sometimes the washed pulp is sent direct to the screens and the well-boiled fibres sorted out by a system of graded screens, which separate the completely isolated fibres from the bulk and retain the larger pieces, these being broken down in a suitable engine and put back on the screens.
The machinery employed for screening chemical pulp is identical with that used for the treatment of mechanical wood pulp.
Finishing.—The ordinary sulphite pulp is worked up into the form of dry sheets for the market and not sent out in a wet state as the mechanical wood. There are several practical disadvantages in preparing the latter in a dry condition which do not, however, occur with chemical pulp.
Hence the pulp after being screened is not pressed but submitted to a different process. From the screens the mixture of pulp and water, the latter being present in large quantity, is pumped into a concentrator, or slusher, as it is termed, by means of which some of the water is taken out.
The slusher consists of a wooden box divided into two compartments by a vertical partition. In the larger compartment a hollow drum covered with a fine wire cloth revolves, the construction and purpose of which are precisely the same as that of the wet press machine used for mechanical pulp.
As the drum revolves the pulp adheres to the outer surface, while the water passes through the wire cloth. The drum is not completely immersed in the mixture, so that the skin of pulp is brought out of the water by the rotation of the drum. When this takes place the contact of a wooden or felt covered roll which revolves on the top of[Pg 112] the drum causes the pulp to be transferred from the drum to the roll. The wet pulp is continuously scraped off by an iron bar or doctor, as it is called, resting on the surface of the roll, and it finally drops into the second compartment of the slusher in a more concentrated form ready for the drying machine.
Drying.—The mass of wet pulp from the slusher is conveyed into a circular reservoir or stuff chest, which serves to supply the machine used for converting the pulp into dry sheets.
The machine is to all intents and purposes a Fourdrinier paper machine, and the process is similar to that used for the manufacture of paper. The pulp flows in a continuous stream on to a horizontal endless wire, which carries it forward as a thin layer; the water drains through the meshes of the wire, further quantities being removed by suction boxes, which draw away the water by virtue of the vacuum produced by special pumps. The wet sheet then passes between the couch rolls which compress the pulp, squeezing out more water, and then through press rolls, which finally give a firm adherent sheet of pulp containing 70 per cent. of water. The sheet is dried by passing over a number of steam heated cylinders, which cause all the moisture to evaporate from the pulp. At the end of the machine the dry pulp is cut up into sheets of any convenient size, and packed up in bales of two or four cwts.
Mitscherlich Sulphite Pulp.—This term is applied to sulphite wood prepared by submitting the chipped wood to a comparatively low pressure for a long period. The wood is placed in the stationary upright form of digester with the requisite amount of liquor, and the heating produced by the passage of steam through a leaden coil lying at the bottom of the digester, so that the steam does not condense in the liquor but in the coil, from which it is[Pg 113] drawn off. The pressure seldom exceeds 45 lbs. but the duration of the cooking is thirty-six to forty-eight hours. The boiler is not emptied under pressure, but the pulp is discharged from the digester after the pressure has been lowered, and the manhole taken off. The contents are usually shovelled out by the workmen.
The pulp is carefully washed, screened and made up into wet sheets on the ordinary wet press machine. This pulp is never dried on the Fourdrinier like the common sulphite, as its special qualities can only be preserved by the treatment described. This pulp is particularly suitable for parchment papers, grease proofs and transparent papers.
Soda Wood Pulp.—The chipped wood is boiled in stationary or revolving digesters for eight or nine hours at a pressure of 70 or 80 lbs. A solution of caustic soda is employed, about 16 to 20 per cent. of the weight of the wood being added to the contents of the digester. Live steam is blown direct into the mass, and after the operation the spent liquor is carefully kept for subsequent treatment. The pulp is washed in such a manner that the amount of water actually used is kept down to the smallest possible volume consistent with a complete removal of soluble matters. This is done in order that the spent liquors may be treated for the recovery of the soda.
Recovery of Spent Liquors.—When wood is cooked by the soda and sulphate processes the solutions containing the dissolved organic matter from the wood can be evaporated, and the original chemical recovered. In the case of soda pulp the method of treatment is as follows: the spent liquors and the washings are evaporated by means of a multiple effect vacuum apparatus to a thick syrup. The concentrated liquor produced is then burnt in special furnaces, all the organic matter being consumed, leaving a black mass which consists mainly of carbonate of soda.[Pg 114] The mass is washed with water to remove the carbonate which is afterwards converted into caustic soda by being boiled with lime.
The spent liquors from the sulphite process have no value, for they cannot be recovered by this method. At present the whole of the sulphur used and the organic matter dissolved from the wood is lost. This means the loss of about 250 to 350 lbs. of sulphur and nearly 50 per cent. of the weight of wood for every ton of pulp produced.
Mechanical and chemical pulps are readily distinguished under the microscope. The former consists of fibres of irregular shape and size, mixed with a large proportion of structureless particles, all bearing evidence of having been torn apart and separated by mechanical methods. The chemical pulp, on the other hand, consists of fibres isolated by a process which preserves them in perfect condition and[Pg 116] form. The pulp from the various woods can be differentiated by minute details in fibre structure, some of the woods being determined from the presence of characteristic cells.
The use of aniline sulphate can also be resorted to, and for microscopic work the most useful reagent is a mixture of zinc chloride and iodine. This produces an intense yellow colour with mechanical pulp and a bluish colour with sulphite and other chemical wood pulps.
The newspapers of the present day are made almost exclusively of wood pulp. The use of the latter material for paper-making has steadily increased from the date of its introduction about A.D. 1870, when wood pulp was imported into England in considerable quantities.
News and cheap printings consist of mechanical and chemical wood pulps mixed in varying proportions determined chiefly by the price paid for the finished paper. In some cases the proportion of mechanical wood pulp is as much as 85 per cent., though the average composition of a cheap wood paper is represented by the following proportions: Mechanical pulp, 70 per cent.; sulphite pulp, 20 per cent.; loading, 10 per cent.
Some idea of the enormous quantity of material used for the daily press may be judged from one or two examples. A certain popular weekly newspaper having a circulation of one and a quarter million copies per week requires every week 137 tons of paper produced from 170 tons of wood. A popular halfpenny newspaper boasting a circulation of about one-half million copies per day consumes 185 tons of paper manufactured from 230 tons of wood, every week.
It is easy also from these facts to estimate the amount of timber which must be cut down to supply the demand for newspapers and cheap printings.
The manufacture of news calls for considerable skill and able management, owing to the keen competition amongst the paper mills devoted to this class of paper. The process as carried on in England is as follows:—
The mechanical pulp, reaching the mill in the form of thick sheets suitably packed up into bales, is first broken up again into moist pulp. Various machines are used for this, such as Wurster's kneading engine, Cornett's breaker, or some similar contrivance. An old potcher, such as is used for the breaking and washing of rags, makes a good pulp disintegrator. The broken pulp is discharged into beating engines in any suitable or convenient manner and the right proportion of chemical wood pulp added in the form of dry sheets. The beating process only occupies thirty to forty minutes in the case of the common news, a marked contrast to the eight or nine hours required by rags. China clay is added to the contents of the beater, ten to twelve per cent. being the general practice. This is followed by a measured quantity of rosin size, and after thorough incorporation the size is precipitated upon the fibres by means of alum.
In the commoner qualities of these papers the materials are added in the dry state, but for finer grades of newspaper the china clay is mixed with water, and carefully drained through a fine sieve before use. The alum cake is also dissolved and treated in a similar manner in order to keep out dirt and coarse particles likely to produce holes in the paper.
The paper machine used for the manufacture of cheap printings is constructed to produce as much as 100 to 180 tons of finished paper per week, every detail being arranged for a large output at a very high speed. In the modern machine it is possible to produce paper at the rate of 450 to 550 feet per minute, the width of the sheet being from 120 to 160 inches.
Careful attention is paid to economy of every kind with regard to the power required for driving the machine, the amount of steam consumed in drying the paper, recovery of excess of fibre and china clay which escapes from the machine wire, and similar details of a mechanical order.
The beaten pulp, after being sized and coloured, is discharged into huge circular brick tanks, or stuff chests, two of which are found with each paper machine. The supply of pulp and water for the machine is taken from one stuff chest while the second is being filled up from the[Pg 120] beating engines, in order to secure a mixture of constant composition.
The pulp is pumped from the stuff chest into a small regulating box placed above the machine wire, and this box is kept full of beaten pulp so that the supply of pulp and water to the machine is perfectly constant. The pulp, diluted with the proper quantity of back-water, is carefully strained through rotary screens and allowed to flow through a distributing box on to the machine wire, where it rapidly forms a sheet of paper.
The excess of water, together with a certain proportion of fine fibre and china clay, falls through the wire, and is caught below in a shallow box, called the save-all. This back-water, as it is called, is used over again for diluting the beaten pulp to the right consistency, as already described.
The whole of the water obtained in this way is not all utilised in the regulating box, and any surplus is pumped up continually into large store tanks and used in the beating engines for breaking down the dry pulp.
In many cases, where a large quantity of water is used on the machine, special methods have to be adopted for the recovery of all the fibre and clay, which would otherwise be lost, and there are many ingenious systems in use whereby this saving is effected.
The most usual practice is to allow the excess of water, which contains from 8 to 15 lbs. of suspended matter per thousand gallons, to flow through a series of brick tanks at a slow rate of speed. The clay and fibre settle to the bottom of the tanks, and the water passes away from the last tank almost clear and free from fibre and loading.
The drying of the moist paper leaving the press rolls of the machine is effected in the usual manner by means of drying cylinders. On account of the great increase of[Pg 121] speed at which the paper is produced, the number of drying cylinders has also been increased, and at the present time a machine of this description is provided with 28 or 32 cylinders, the object being to dry the paper economically.
The presence of mechanical wood pulp in paper is detected by means of several reagents, which produce a definite colour when applied to a sheet of paper containing mechanical wood. The depth of colour obtained indicates approximately the percentage present, but considerable practice and experience is necessary to interpret the colour exactly. A more reliable method of estimating the percentage of mechanical wood in a paper is by microscopic examination.
The reagents which can be used are—
(1) Nitric Acid.—This produces a brown stain on the paper, but it is not a desirable reagent for ordinary office purposes.
(2) Aniline Sulphate.—A solution of this is prepared by dissolving 5 parts of aniline sulphate in 100 parts of distilled water. When applied to the surface of news a yellow coloration is produced, more or less intense according to the amount of mechanical wood present. It can only be used with white papers, or papers very slightly toned.
(3) Phloroglucine.—This sensitive reagent, which gives a rose-pink colour when brushed on to the surface of the paper, is prepared by dissolving 4 grammes of phloroglucine in 100 c.c. of rectified spirits, and adding to the mixture 50 c.c. of pure concentrated hydrochloric acid.
There are several other aniline compounds which give colour reactions of a similar character, but they are not often used. The phloroglucine reagent fails as a test for[Pg 122] mechanical wood in papers which have been dyed with certain aniline colours, for example, metanil yellow. Paper which has been coloured with this dye will, when moistened with the phloroglucine reagent, give an intense pink colour, even if no mechanical wood is present. This is due to the fact that the dye itself is acted upon by the hydrochloric acid in the test reagent. The same colour is produced on the paper with hydrochloric acid per se.
There is little difficulty in distinguishing between the colour arising from the presence of such a dye, because the effect is instantaneous, whereas the coloration due to mechanical wood develops gradually. Moreover, the reaction due to the presence of metanil yellow gives a perfectly even coloured surface, whereas with mechanical wood pulp the fibres appear to be more deeply stained than the body of the paper.
Output of a Paper Machine.—The quantity of paper which can be produced on the paper machine is readily calculated from the following data:—
| Speed of machine in feet per minute | F |
| Nett deckle width in inches | D |
| Width of sheet of paper in inches | W |
| Length of sheet of paper in inches | L |
| Number of sheets in ream | S |
| Weight of paper per ream | R |
The general formula for the output of paper per hour is
| Output in lbs. per hour = | 720 × F × D × R | . |
| S × L × W |
When the number of sheets in the ream is 480, this formula simplifies to
| Output in lbs. per hour = | 1½ × R × F × D | . |
| L × W |
The term “nett deckle width” applies to the width of[Pg 124] the trimmed finished paper at the end of the machine. The formula takes no account of the allowance required for trimming edges. In most cases the deckle width of the machine is arranged so that the paper is cut into strips of equal width when leaving the calenders, e.g., a deckle of 80 inches will give 4 sheets, each 20 inches wide.
The method by which the general formula is obtained may be explained by an example.
What is the output of a machine having a speed of 100 feet per minute, with an 80-inch deckle, producing a sheet of paper 20 inches by 30 inches, weighing 30 lbs. per ream of 480 sheets?
The machine produces every minute a sheet of paper 100 feet long and 80 inches wide.
Hence output per minute in square inches
| = 12 × 100 × 80. |
Output per hour in square inches
| = 60 × 12 × 100 × 80. |
Now each (20 × 30 × 480) square inches is area of one ream.
Output of paper per hour in reams
| = | 60 × 12 × 100 × 80 | . |
| 480 × 30 × 20 |
Output of paper per hour in lbs.
| = | 720 × 100 × 80 × 30 |
| 480 × 30 × 20 | |
| = | 600 lbs. |
The general formula may be applied for the purpose of calculating the speed at which the machine must be driven.
Example.—A machine with 75-inch deckle is required to produce 6 cwts. per hour of a paper 25 inches by 18 inches[Pg 125] (500 sheets), weighing 19 lbs. to the ream. At what speed is the machine to be driven?
Output in lbs. per hour
| = | 720 × F × D × R |
| S × L × W | |
| 672 = | 720 × F × 75 × 19 |
| 500 × 18 × 25 | |
| F = | 148 feet per minute. |
Common Browns.—The raw material used in the manufacture of common brown papers is chiefly jute and waste fibres of every description, such as waste cuttings from boxboard factories, old papers, wood pulp refuse, and other substances of a like nature. The jute, in the form of sacking or old gunny bags, and the hemp refuse, in the shape of old rope and string, are subjected to a slight chemical treatment just sufficient to isolate the fibres to a condition in which it is possible to work them up into paper. The bagging and string are cut up in a rag chopper and boiled in revolving boilers with lime or caustic soda for several hours at a pressure of 20-30 lbs., the lime being used when it is desired to manufacture a harsh paper, and the caustic soda being employed for the production of paper having a softer feel. The pulp is not always washed very completely after the process of digestion, as is the case with white papers, and it is often possible to extract from brown papers of this class a considerable proportion of the alkaline matter which has not been thoroughly removed from the boiled pulp. The presence of this alkaline residue does not affect the quality of ordinary brown paper, but is frequently a serious defect in the case of middles or straw boards, which are afterwards utilised for boxes and covered with coloured papers. The colour of the paper pasted on to such incompletely washed boards is frequently spoilt by the action of the alkali when moistened with the paste[Pg 127] used, many aniline dyes being susceptible to the small proportion of alkali present.
The stronger materials, such as jute or old rope and string, are either used by themselves or blended with inferior raw material according to the quality of the paper being made. The jute and hemp fibres are generally beaten by themselves in the engine before the other materials are added. The pulp is mixed with the required amount of loading, while the sizing and colouring operations are carried out in the usual way.
The common brown papers are known by a variety of trade names which at one time indicated the nature of the fibrous constituent, but at the present day the name is no guide or indication of the material used for the manufacture of the paper. The common heavy brown used for wrapping sugar and sundry groceries made in heavy grey and blue shades is a coarse paper made from cheap materials and containing a large proportion of mineral matter. It is usually supplied under the trade name of royal.
A somewhat lighter and stronger wrapping paper of a white or buff colour, used for wrapping groceries, tea, and cotton goods, is that known as casings, a name probably derived from the application of this paper originally to the lining of cases.
Manila papers so called were originally made from rope, but the term is now applied to papers which may be made entirely of wood pulp.
Rope browns are common papers made of fairly strong material of a miscellaneous character, this name having been derived from the fact that rope and similar fibre were at one time used exclusively.
Wood Pulp Wrappers.—Most of the papers of the present day are made from wood pulp, this material giving a thin, light, tough paper, which is pleasant to handle and forms a[Pg 128] great contrast to the dense, opaque, heavily loaded, and inartistic specimens produced some years ago. Paper of this kind, though apparently more expensive than common browns, is really more economical in use. The paper is not only stronger, but it is possible to obtain a larger number of sheets for a given weight. The great advantage in the improvement of brown papers dates from the introduction of the now well-known kraft papers, which are of comparatively recent origin.
Kraft Paper.—The term Kraft, meaning “strength,” is applied to a remarkably strong cellulose paper prepared from spruce and other coniferous woods by the soda treatment, the special feature of the process being an incomplete digestion of the wood.
The wood previously chipped into pieces 1 inch to 1½ inches in length, is boiled with caustic soda, the digestion being stopped before the wood pulp has been quite softened, and while the pulp is still too hard to be broken up into isolated fibres by simple agitation in water. The pulp after thorough washing is disintegrated by means of an edge-runner, or some form of breaking engine, the first mentioned probably giving the most satisfactory results, and converted into paper by the usual methods.
The wood can also be reduced by the sulphate process, in which case the chipped wood is boiled in a liquor to which about 25 per cent. of spent lye from a previous cooking is added.
The best results are obtained by attention to the cooking process to ensure an under-cooked pulp, by careful isolation of the fibres in a kollergang, or edge-runner, which machine is capable of separating the fibres without shortening them, and by proper manipulation on the paper machine.
The paper produced under favourable conditions in this direction is wonderfully tough and strong and may be[Pg 129] quoted as the most recent example of the fact that the latent possibilities of wood pulp have by no means been exhausted or even thoroughly investigated.
Imitation Kraft Paper.—If wood is boiled in water at high temperatures the fibre is softened and much of the resinous matter is removed. Such wood, if ground in the same way and by the same methods as ordinary mechanical wood pulp, is readily disintegrated, and a long-fibred pulp may be obtained. The process of boiling short 2 feet logs of wood in a digester under a pressure of 20-50 lbs. has long been known. The wood after boiling is partly washed and then worked up into pulp by the usual mechanical process. The wood is easily ground and yields pulp containing long fibres which in their physical properties closely resemble those of pure wood cellulose, but the original constituents of the wood are present almost unchanged, just as in mechanical pulp. The product obtained by grinding is a very tough flexible material of a brownish yellow colour, and the paper is known as Nature brown. It is chiefly used for the preparation of tough packing papers, for the covers of cheap pocket-books, and other miscellaneous purposes. When this brown mechanical wood pulp paper is glazed on both sides it is then known as ochre glazed, the word ochre referring to the colour. When made up into light weight papers it is sold as imitation kraft paper.
A great variety of wrapping papers are now made from wood pulp, such as sealings, sulphite browns, manilas, sulphite caps, but the distinctions between these papers relate chiefly to the amount of finish, the colour and size of the sheet. The methods of manufacture only differ in small details as indicated by these distinctions.
Fine Wrappings.—The papers used for packing small goods such as silver ware and other delicate articles are[Pg 130] generally tissues, the better qualities of which are made from rag, and the cheaper qualities from wood pulp. These papers are known as tissue, crêpe, crinkled tissue, manila tissue, and by a variety of trade terms.
Many of the fine wrappings of the tissue class and the somewhat heavier papers known as M. G. Caps are manufactured on the single cylinder machine, which produces a paper having a highly polished surface on one side and a rough unglazed surface on the other side.
In the single cylinder machine the beaten pulp passes[Pg 131] from the stuff-chest on to the wire of the ordinary Fourdrinier machine and through the press rolls, but instead of being dried over a number of cylinders the paper is led over one single cylinder of very large diameter which is heated internally with steam. The paper is usually pressed against the surface of the cylinder by means of a heavy felt, which is, however, sometimes omitted. The side of the paper coming into contact with the cylinder becomes highly polished, the surface in contact with the felt remaining in an unfinished rough condition. This paper is said to be machine glazed and is known as an M. G. paper.
Boards.—Cards, millboards, middles, boxboards, carriage panels, and similar paper products are manufactured either on a single board machine, by means of which single sheets of any required thickness can be obtained, or on a[Pg 132] continuous board machine, which is capable of producing cards and plain or duplex boards of moderate thickness.
The raw material used consists, as in the case of browns and wrappers, of every conceivable fibrous substance mixed with mineral matter and then suitably coloured. The preliminary processes for the treatment of the pulp are exactly the same as those employed in the case of brown papers up to the point at which the beating has been effected.
The beaten pulp, diluted with large quantities of water, is pumped continuously into a large wooden vat of rectangular shape. Inside this vat revolves slowly a hollow cylindrical drum, the circumference of which is covered with wire gauze of fine mesh. The drum is not completely immersed in the mixture of pulp and water, so that as it revolves the water passes through the wire, while the pulp adheres to the surface. The water flows regularly into the interior of the drum and runs away through pipes fitted at each side of the vat near the axis of the drum, and the pulp is brought up out of the water until it comes into contact with a travelling felt. The thin moist sheet of pulp adheres to this felt, passes through squeezing rolls which remove part of the water, and is finally carried between two wooden or iron rollers of large diameter. The pulp adheres to, and is wound up on the upper roller, the felt being carried back by the lower roller to the vat. When the sheet on the upper roller has attained the desired thickness, it is immediately cut off and transferred to a pile of similar sheets, a piece of coarse sacking or canvas being interposed between every wet board. The dimensions of the full-sized board are determined by the diameter of the upper roller and its length. A roll 74 inches wide and 14 inches diameter will give a board 74 inches by 44 inches.
As soon as a sufficient number of wet boards has been obtained they are submitted to pressure in order to remove the excess of water and at the same time compress the material into dense heavy boards. The pieces of sacking are then taken out and the boards dried by exposure to air at the ordinary temperature or in a heated chamber.
The dried boards are finished off by glazing rolls. These rolls compress the boards still further and impart a polished surface. The amount of “finish” may be varied by the pressure, number of rollings, temperature of the rolls, and by damping the surface of the dry boards just before they are glazed. The boards are cut to standard sizes before or after glazing.
Duplex Boards.—If the single board machine is fitted with two vats instead of one, it is possible to manufacture a board with different coloured surfaces. A board coloured red on one side and white on the other is manufactured by having one vat full of pulp coloured red and the second vat full of white pulp. The thin moist sheets from the two vats are brought together and passed through the glazing rolls, which cause the moist sheets to adhere closely to one another, the double sheet of pulp so formed being wound up on the rollers at the end of the machine. The board is then dried, glazed, and finished in the usual way.
The same principle is occasionally adopted on the Fourdrinier machine for duplex wrappers. Thus a common brown pulp is worked up in conjunction with a dyed pulp to produce a brown paper having one surface of good paper suitably coloured. The brown pulp flows on to the wire of the paper machine, and after it has been deprived of part of the water at the suction boxes, a thin stream of coloured pulp, diluted to a proper consistency, flows from a shallow trough, placed across and above the wire, on to the wet brown web of paper in such a manner as to completely cover it as a thin even sheet of coloured pulp. The adhesion of the latter to the surface of the brown paper is practically perfect, and the weight of the couch and press rolls ensures uniform felting of the fibres.
Middles.—This term is applied to a thin or thick cardboard made of common material, the colour and appearance of which is of little importance for inferior goods. Boards of this kind are covered subsequently with papers of all colours and qualities, and the origin of the word “middle” is easily seen. The manufacture of a board consisting of two outside papers of good material and a middle produced from common stuff is effected by the continuous boxboard machine, unless the board is too[Pg 135] thick to be passed over drying cylinders, calendered, and reeled, in which case the boards are produced on an ordinary wet machine and the paper pasted on the surface of the dry board.
The term is, however, now also applied to a common paper made of mechanical wood pulp with perhaps a little chemical pulp, used for tram tickets, cheap advertising circulars, common calendar cards, and similar purposes, to which no outer surface of a special character is added.
This machine differs from the single board machine in that the finished board can be produced from the pulp at one operation. It is used principally for cards and boards of moderate thickness which can be wound up in the form of a reel at the end of the machine.
The mixture of pulp and water is pumped into two or more vats and formed into a number of thin sheets, which are all brought together between squeezing rolls and passed through heavy press rolls which compress the several layers into a compact mass. The thick sheet obtained is dried over steam-heated cylinders which are placed at the end of the press rolls, and calendered. The whole process, indeed, resembles that of ordinary paper-making, the main difference being the method of producing the wet sheet or card.
Some machines are constructed with six or seven vats and forty to fifty drying cylinders, and are capable of turning out a large quantity of finished material.
The board can be made of uniform quality and texture throughout, or be finished off with high-grade paper on one or both sides. In the latter case the constituents of the “middle” part are waste papers and raw material of inferior quality, the outer surface of wood pulp, white or[Pg 136] coloured according to circumstances. The variety of papers and boards which can be produced is due to the fact that the several vats of pulp are independent of one another and can be filled with any kind of paper stock. The combined sheets forming the ultimate board are dried on the ordinary cylinders, calendered, and reeled up at the end of the machine.
There are many varieties of paper products obtained by submitting finished paper to a number of special processes. Of these only a few of the more important will be described.
These products can be divided approximately into three classes:—
(1) Papers coated on one side or both sides with various substances, such as “art,” photographic papers, etc.
(2) Papers impregnated with chemicals, such as blue print, medicated, and cheque papers.
(3) Paper pulp converted into modified products by chemical treatment, such as vulcanised board, viscoid, etc.
Of the first class, the coated papers used for art and chromo illustrations are the most important.
Of the second class, the blue prints and papers impregnated with chemicals, chiefly employed for the production of engineers' drawings, may be regarded as typical.
In the third class, vegetable parchment and vulcanised board are the most familiar.
Parchment Paper.—This is produced by the action of sulphuric acid upon ordinary paper, the most suitable for this purpose being made from unsized cotton rag, free from such additions as mechanical wood pulp. The presence of the latter substance should be avoided, as it is liable to char or burn, so that in the finished product it[Pg 138] shows itself in the form of small holes. The process depends upon the power of sulphuric acid to change the surface of the paper into a gelatinous mass, which has been shown to consist of a substance called amyloid.
The best parchment is made from pure cellulose such as rag or chemical wood pulp. The quality of the parchment depends upon attention to the strength of the acid, the temperature of the acid bath, the period of immersion, the complete removal of the acid, and the careful drying of the wet parchment.
The acid is employed at a strength of 1·71 specific gravity, being prepared by diluting the commercial concentrated acid in a leaden vessel, with a sufficient quantity of water.
The parchment is generally prepared by passing a continuous sheet of paper through a bath of acid of the proper strength at a speed which ensures the correct period of immersion. As the treated paper leaves the bath it passes through squeezing rolls which remove the excess of acid, and the paper is then led through a series of tanks containing fresh water, the last traces of acid being neutralised by small additions of ammonia, or some alkali, to the last washing tank. The wet parchment is then passed through suitable rollers and carefully dried over cylinders heated internally by steam. The paper is kept perfectly stretched as it dries, because it shrinks[Pg 139] enormously, and would otherwise become cockled and uneven.
Thick sheets of parchment paper are frequently made by passing three sheets of paper through the acid bath and bringing them together between the rollers before washing. The sheets unite when pressed together; the remainder of the process being the same as that employed for single sheets.
The parchment exhibits remarkable differences to the original paper, the strength being increased three or four times, the density about 30 per cent., the latter being shown by the shrinkage, which amounts to at least 30 per cent.
Vulcanised Paper.—Zinc chloride has the property of parchmentising paper in a manner similar to sulphuric acid. The product obtained when this reagent is used is generally termed vulcanised fibre. The paper is passed as a continuous sheet into a bath of strong zinc chloride, having a density of 160-170 Twaddell, which causes the cellulose to swell up and partly gelatinise. A very large excess of strong zinc chloride is necessary, and the process is only rendered commercially possible by careful recovery of the zinc from the washing waters, which are submitted to chemical treatment.
The vulcanised product is subsequently treated with nitric acid or with a mixture of nitric and sulphuric acids to render them waterproof. Dextrin is frequently employed to retard the chemical action to permit of the necessary manipulation of the material before it is finally washed. The complete removal of the excess of zinc and acid is a necessary feature of the whole operation.
Willesden Paper.—When paper is passed through an ammoniacal solution of copper oxide, a superficial gelatinisation of the surface takes place, so that the paper when washed and dried is impregnated with copper oxide, which[Pg 140] helps to preserve it, and it becomes waterproof. Such material is well known as Willesden paper.
Blue Print or Cyanotype Papers.—This name is usually given to the process by means of which blue prints of engineers' and architects' plans can be reproduced. It was discovered in 1842 by Sir John Herschel. It is a useful method of reproducing drawings, and incidentally is of great value to the amateur photographer because of the facility with which it can be applied for getting proofs from negatives quickly and easily without special baths and chemicals. The process is based upon the reduction of a ferric salt to the ferrous condition by light, and the formation of Prussian blue by the action of potassium ferricyanide. The negative cyanotype gives white lines on a blue ground. Various formulæ are in common use.
| — | Herschel. | Clark. | Watt. | Rockwood. |
| Solution 1. | ||||
| Potassium ferricyanide | 16 | 27 | 48 | 10 |
| Water | 100 | 100 | 100 | 100 |
| Ammonia | — | 2·3 | — | — |
| Saturated solution of oxalic acid | — | 20 | — | — |
| Solution 2. | ||||
| Ammonia-citrate of iron | 20 | 30 | 50 | 30 |
| Water | 100 | 100 | 100 | 100 |
| Boric acid | — | — | 0·5 | — |
| Dextrin | — | — | — | 5 |
Equal parts of the two prepared solutions are mixed when required and spread evenly over well-sized paper. The paper is hung up, dried, and preserved in a dark dry place.
The positive cyanotype gives blue lines on a white ground, being the reverse of the ordinary blue print. That is, no image is formed where the light acts, and the reaction is[Pg 141] the formation of blue due to the union of a ferrous salt with ferrocyanide of potassium.
Pizzighelli in 1881 gave the following formula:—
| — | Solution 1. | Solution 2. | Solution 3. | Solution 4. |
| Water | 100 | 100 | 100 | 100 |
| Gum arabic | 20 | — | — | — |
| Ammonia-citrate of iron | — | 50 | — | — |
| Ferric chloride | — | — | 50 | — |
| Potassium ferrocyanide | — | — | — | 20 |
Mix the first three solutions in the following order in the proportions stated:—
| Solution 1. | 20 | parts. |
| Solution 2. | 8 | „ |
| Solution 3. | 5 | „ |
As soon as the solution, which at first gets thick and cloudy, is clear and thin, it is spread over the surface of well-sized paper, which is then dried in a warm room.
The print, which appears yellow on a dark yellow ground, is treated with the developer (solution 4) by means of a brush dipped in the solution. When the image is deep blue in colour, the print is washed in water and then placed in dilute hydrochloric acid (1 part of acid to 10 parts of water) till the ground is quite white. A final washing with water is then necessary.
Waterhouse gives the following formula:—
| — | Solution 1. | Solution 2. | Solution 3. | Solution 4. |
| Water | 650 | 150 | — | 100 |
| Gum arabic | 170 | — | — | — |
| Tartaric acid | — | 40 | — | — |
| Ferric chloride solution 45° Baumé | — | — | 150 | — |
| Ferrocyanide of potassium | — | — | — | 20 |
Solutions 1 and 2 are mixed and No. 3 added gradually with constant stirring. The mixture is left twenty-four hours, and diluted with water to a specific gravity of 1·100.
The paper is coated with the solution and used as already directed, being developed in ferrocyanide of potassium solution and washed with water, treated with weak hydrochloric acid, and then finally cleaned from all traces of acid.
Black Lines on a White Ground.—This modification of the ordinary blue print is arrived at with the following formula:—
| Water | 96·0 | parts. |
| Gelatine | 1·5 | „ |
| Perchloride of iron (in syrupy condition) | 6·0 | „ |
| Tartaric acid | 6·0 | „ |
| Sulphate of iron | 1·5 | „ |
The paper is coated with the solution. After printing, the image is developed with a solution containing
| Gallic acid | 1 | part. |
| Alcohol | 10 | parts. |
| Water | 50 | „ |
A final washing of the print with water completes the operation.
This term should properly include all the varieties of special papers which are coated with extraneous matter for particular purposes, such as art, chromo, tinfoil, gilt, emery, carbon, photographic, marble, and sand papers. In practice however, the term is almost entirely limited to “art” papers used for illustration work and half-tone printing.
An “art” paper, using the definition given above, consists of an ordinary sheet of paper, one or both sides of[Pg 143] which have been coated by the application of a mixture of a mineral matter, such as china clay or satin white, and some adhesive, like casein or glue. The object of the coating is to impart to the paper a perfectly smooth surface, rendered necessary because of the conditions under which the printing of the illustrations is carried out.
The machine used for coating the paper consists of a large hollow drum about 40 inches diameter and 48 inches wide. The paper is brought over upon the drum in a continuous sheet, and the coating mixture applied to the surface by means of a revolving brush or an endless felt which rotates in a copper trough containing a coating mixture which is usually maintained at a temperature of 120° Fahr.
The amount of material put on to the surface of the[Pg 144] paper is varied by altering the proportion of water in the trough. As the wet coated paper is drawn over the drum it comes into contact with a number of flat brushes which move from side to side and brush the coating well into the paper.
The last two or three brushes on the drum are made of very fine bristles, so that when the coated paper leaves the machine the surface is perfectly even and free from brush marks. The wet paper is then drawn up an inclined ladder by an ingenious device, which causes the paper to fall into festoons or loops, and these are carried bodily forward by means of travelling chains. The process, somewhat difficult to describe, is more easily understood by a study of the illustrations given.
The paper is dried by a current of warm air which can be obtained by means of steam pipes placed below the festoons or with a special air blower. The dry paper is then led through guide rolls and wound up in the form of a reel.
The paper at this stage has a dull coated surface, which[Pg 145] is somewhat rough and unfinished, and a high polish is imparted to it by a machine known as a supercalender.
The supercalender consists of a number of alternate steel and cotton or paper rolls placed vertically in a stack one above the other. When the coated paper is led through this machine the friction of the alternate steel and cotton rolls produces a high finish on its surface.
An art paper coated on both sides is manufactured by passing the paper through the coating machine twice. Machines have been devised for coating both sides of the paper at one operation, but these are not in very general use.
Tinted art papers are prepared in the same manner, the desired colour being obtained by the addition of pigments or aniline dyes to the mixture in the trough containing the coating materials. When the two sides of such tinted papers are coloured differently, they are often described as duplex coated papers.
Imitation Art Papers are prepared by quite a different process, although they have the appearance, more or less, of the coated paper. They are merely esparto papers very heavily loaded, containing frequently as much as 25 to 30 per cent. of mineral matter prepared as follows:—
Bleached esparto half-stuff is beaten together with any suitable proportion of chemical wood pulp in an ordinary beating engine, and a large quantity of china clay is added at the same time. The beating is carried out under conditions which favour the retention of as much china clay as the pulp will hold while being converted into paper on the Fourdrinier machine.
After the paper passes over the drying cylinders of the machine it is passed through the calenders in the usual way, but the surface of the paper is damped by means of a fine water spray just before it enters the calender rolls.[Pg 146] The result is that a “water-finish,” so called, is imparted to the paper, and a close imitation of the genuine art paper is obtained, the effect of this peculiar treatment being to compress the fibres and bring the clay up, as it were, to the surface.
A paper containing such a large proportion of mineral matter intimately mixed with the fibre is naturally very weak. It easily tears, and if moistened with water goes all to pieces. At the same time it is a cheap substitute for high-class art paper, being suitable for circulars, temporary catalogues, and similar printed matter.
In an “art” paper the nature of the fibrous constituents is too often regarded as a matter of secondary importance, because in the process of printing the ink does not come into contact at all with the paper, and an impression is produced merely on a layer of clay which is bound together by the glue.
The illustrations are not absolutely permanent, and it is perfectly easy to remove the whole of the impression and the coating itself by immersing a sheet of the paper in warm water and rubbing the surface gently with the fingers, or with a camel-hair brush.
In fact the amount of coating matter which has been brushed on to a paper can be determined approximately by weighing a piece of the coated paper, removing the mineral matter and glue from both sides as indicated, allowing the paper to dry again, and then re-weighing, the loss in weight representing the amount of coating.
It is not surprising to find that the true paper is merely regarded as a convenient means of producing, so to speak, a smooth surface of clay, and an examination of the material between the two clay surfaces often reveals a paper of very low quality.
There are one or two empirical methods for testing the[Pg 147] condition of coating on an art paper. If the coating is firm and adherent, then on pressing the moistened thumb on to the surface none of the coating matter is removed, but in a badly-made art paper some of the coating adheres to the thumb.
Another method is to crumple a sheet of paper between the fingers, and if any of the coating comes away easily the paper is considered of poor quality.
The complete examination of an art paper, apart from the practical test of printing, involves the determination of the amount of coating matter added to the paper, the proportion of glue in the coating, and the usual analysis of the paper itself.
This term may be applied to wrappings specially treated with substances which render the paper air and water proof. They are principally used for preserving food, or such articles as tobacco, which require to be kept slightly moist.
Waxed Paper.—The paper in the form of a continuous sheet is passed through a bath of melted wax at a high temperature, any excess being removed by squeezing rolls through which the hot waxed paper is passed. The paper is led over skeleton drums and thoroughly cooled before being cut into sheets.
Butter Paper.—Ordinary parchment paper is generally used, but for special purposes a solution containing albumen and saltpetre is utilised for impregnating paper.
Hardware Paper.—Needles and silver goods are frequently wrapped in paper impregnated or mixed with substances which are supposed to prevent deleterious fumes from coming into contact with them. The use of black papers[Pg 148] heavily loaded with pigment, sized with glue and an excess of alum, is commonly resorted to. For silver ware, paper dipped in a solution of caustic soda containing zinc oxide is used. A recent patent suggests the impregnation of paper with heavy hydrocarbon oils, which being slightly volatile cover the goods, such as needles, with a thin film.
Paraffin Paper.—Large quantities of this paper are consumed for packing food and other articles which need protection from air and moisture.
The paper is either passed through a bath of paraffin or passed over a roller which rotates in a trough of paraffin.
If the paper is to be coated on both sides it is passed through the bath containing the paraffin in a melted condition, the excess of which is scraped from the paper as it leaves the bath. The paper is cooled by exposure to air, and when the paraffin has solidified upon the sheet the paper is wound up on a roller at the end of the machine.
If the paper is to be coated on one side only it is passed over a heated roller which revolves in a bath of melted paraffin, the other operations of drying and finishing being the same as in the case of a paper coated on both sides.
Tinfoil Papers, required for packing tea, coffee, and similar foodstuffs, are prepared by coating cheap paper with a solution of gum and finely powdered tin. The manufacture of the fine powder is accomplished by melting tin at a low temperature and shaking it continually as it cools down, whereby a mixture of fine powder and large particles is produced, the latter being separated out by agitation of water.
Tin in a fine state of division can also be obtained by a[Pg 149] chemical process. Granulated tin is dissolved in strong hydrochloric acid, the solution diluted with water, and a stick of zinc introduced into the solution. The tin is gradually precipitated.
The dried powder is coated on to the paper with gum, and when the paper is dry the necessary degree of brilliancy produced by suitable calendering.
Transfer Papers.—A number of important operations require the use of what are known as transfer papers, so that a design written or printed upon a specially prepared surface can be transferred to another surface from which duplicate copies may be obtained. The principle upon which all such operations are based is the coating of suitable paper with starch, flour, and gum, singly or mixed, so as to give a surface firm enough to take the design, but which readily breaks up when the printed side is pressed against the wood, stone, or metal object intended to receive the design.
Thus a paper may first be dusted over with dry starch, or coated with starch paste and then dried. A layer of dextrine may then be put over the starch coating, and the design printed upon the dextrine surface. When the paper is turned face downward on a sticky metal plate the design adheres to the metal, and the paper is easily pulled off, owing to the dry starch layer between it and the dextrine being non-adhesive.
This principle is utilised in producing designs upon tins used for packing, metal advertisement plates, domestic articles of every kind, stoneware and earthenware goods.
It is further applied in the preparation of lithographic stones required for printing.
Each class of work demands paper of a suitable character, but the principle of an easily detached surface-coating is the[Pg 150] same for all. The main difficulty experienced is the liability of paper to stretch when damped, and various methods are devised to obviate this, either by employing paper which stretches very little when damp, or by making the paper partially waterproof before use.
Papier-mâché.—This name indicates a preparation of paper or paper pulp mixed with various mineral substances firmly cemented together by animal or vegetable adhesives.
The paper pulp used for high-class goods consists of pure wood cellulose, while for the commoner qualities mechanical wood pulp, waste papers, and any similar fibrous material are employed.
The mineral substances used are china clay, chalk, gypsum, barytes, ochre, sienna, and other mineral pigments.
The adhesive materials are glue, casein, gum, starch, paste, dextrine, Iceland moss, or wax.
For experimental purposes, small quantities of papier-mâché may be prepared in the following manner:—
When old newspapers or brown papers are used as the fibrous basis of the papier-mâché, they are first torn up into small pieces, moistened with hot water, tied up in a small cloth bag or sack, which must only be half filled, and then immersed in a basin of warm water and thoroughly kneaded by hand, so that the paper is gradually reduced to the condition of pulp. If the kneading process is carried out thoroughly the paper is entirely reduced to pulp. The excess of water can be removed by pressure and the preparation of the final mixture completed by the incorporation of clay, pigment, and adhesive.
In the preparation of papier-mâché for goods on a large scale a beating engine is used in order to break up the old paper or wood pulp into a fibrous condition.
The following formulæ can be used for making papier-mâché:—
| (1) | (2) | (3) | (4) | ||||
| Pulp | 22 | Pulp | 22 | Pulp | 12 | Pulp | 33 |
| Clay | 37 | Chalk | 30 | Rosin size | 22 | Starch | 9 |
| Casein | 37 | Glue | 4 | Flour | 11 | Clay | 9 |
| Water | 4 | Water | 44 | China clay | 11 | Water | 49 |
| Water | 44 | ||||||
| 100 | 100 | 100 | 100 | ||||
Plaster Moulds.—Plaster of Paris or gypsum is the main article used for moulds and pattern. The preparation of gypsum for casting is made as follows:—The gypsum is gradually worked up into a creamy paste with water, the mixing being done quickly yet thoroughly.
The pattern of which it is desired to form a mould must be coated with oil. Around the pattern placed on a table a wall of wood or pasteboard is fixed, so that a basin will be formed of suitable depth, preventing the gypsum from flowing away. Patterns of figures or of curved articles have to be made in two or more parts. For that purpose the pattern is usually cut into two pieces. Two moulds are now readily obtainable by first oiling the pattern and by pouring the gypsum in a thin state gradually over the surface, to avoid the forming of air bubbles.
The rapid drying of the soaked gypsum is sometimes inconvenient, but the addition of a saturated solution of borax in water to the gypsum mixture can be resorted to as a check.
Various means are employed for hardening and strengthening the plaster cast, such as the addition of coarse paper fibres, shreds of canvas, iron filings, or wire.
Colouring.—Usually a cheap water colour only is required; a light coating of a cheap varnish may be sufficient. In other cases a water colour serving as a filler for smoothing the surface may receive a finish of one or more coats of resinous solutions in alcohol or of copal varnish. Many goods are coated with asphaltum or Japan varnish and dried in cold or hot air.
Some of the articles may be decorated with scrolls or arabesques in oil colours or enamels, or the lines may be covered with bronze powder, or with metal, gold, or aluminium leaf.
Varnishing.—The following varnish recipes are suitable:—
| (1) | (2) | (3) | (4) | ||||
| Shellac | 20 | Shellac | 10 | Shellac | 6 | Sandarac | 15 |
| Alcohol | 70 | Rosin | 10 | Sandarac | 3 | Mastic | 5 |
| Lamp black | 10 | Alcohol | 60 | Mastic | 18 | Turpentine | 5 |
| Lamp black | 20 | Alcohol | 73 | Alcohol | 75 | ||
| 100 | 100 | 100 | 100 | ||||
The manufacture of paper is a highly technical industry, which requires a practical knowledge of mechanical engineering, as well as an intimate acquaintance with the many important chemical problems connected with the art.
The following brief description of the various chemicals used in the manufacture of paper is divided into certain classes, based upon the order of the operations through which the raw material passes before its final conversion into paper:—
(1) The alkaline processes used for treating raw fibre: soda ash; caustic soda; lime; recovered ash.
(2) The conversion of wood into sulphite pulp: sulphur; limestone.
(3) The operation of bleaching: bleaching powder; antichlors; acids.
(4) The sizing and loading of paper: casein; gelatine; rosin size; alum; starch; silicate of soda; pigments and soluble dyes; mordants.
Mineral substances for loading: clay, blanc fixe, etc.
Carbonate of Soda.—This substance, also known under the trade names of alkali and soda ash, is used in the paper mill for the manufacture of caustic soda. It is purchased by the paper-maker from the chemical works, and used together with the recovered ash (see page 78) for the production of caustic soda solution, which is required in the treatment of raw fibres.
It is also used for the preparation of rosin size (see “Rosin Size”) and in softening hard waters for steam-raising purposes.
Sodium Carbonate Table.
Showing percentage by weight and pounds per 100 gallons in solutions of various densities.
| Twaddell. | Percentage by Weight. | 100 gallons contain pounds of | |||
| Na2O. | Na2CO3. | Na2O. | Na2CO3. | 48 per cent. Ash. | |
| 1 | 0·28 | 0·47 | 2·76 | 4·72 | 5·74 |
| 2 | 0·56 | 0·95 | 5·61 | 9·60 | 11·68 |
| 3 | 0·84 | 1·42 | 8·42 | 14·41 | 17·56 |
| 4 | 1·11 | 1·90 | 11·34 | 19·38 | 23·64 |
| 5 | 1·39 | 2·38 | 14·26 | 24·40 | 29·73 |
| 6 | 1·67 | 2·85 | 17·10 | 29·36 | 35·77 |
| 7 | 1·95 | 3·33 | 20·16 | 34·46 | 42·00 |
| 8 | 2·22 | 3·80 | 23·12 | 39·52 | 48·15 |
| 9 | 2·50 | 4·28 | 26·17 | 44·72 | 54·50 |
| 10 | 2·78 | 4·76 | 29·71 | 50·00 | 60·90 |
| 11 | 3·06 | 5·23 | 32·27 | 55·18 | 67·22 |
| 12 | 3·34 | 5·71 | 35·36 | 60·50 | 73·72 |
| 13 | 3·61 | 6·17 | 38·43 | 65·72 | 80·07 |
| 14 | 3·88 | 6·64 | 41·57 | 71·06 | 86·58 |
| 15 | 4·16 | 7·10 | 44·65 | 76·33 | 93·03 |
| 16 | 4·42 | 7·57 | 47·80 | 81·77 | 99·61 |
| 17 | 4·70 | 8·04 | 51·02 | 87·24 | 106·31 |
| 18 | 4·97 | 8·51 | 54·25 | 92·74 | 113·10 |
| 19 | 5·24 | 8·97 | 57·45 | 98·26 | 119·70 |
| 20 | 5·52 | 9·43 | 60·67 | 103·70 | 126·42 |
| 21 | 5·79 | 9·90 | 63·98 | 109·40 | 133·45 |
| 22 | 6·06 | 10·37 | 67·32 | 115·10 | 140·12 |
| 23 | 6·33 | 10·83 | 70·63 | 120·81 | 147·10 |
| 24 | 6·61 | 11·30 | 74·00 | 126·62 | 154·20 |
| 25 | 6·88 | 11·76 | 77·38 | 132·30 | 161·12 |
| 26 | 7·15 | 12·23 | 80·83 | 138·20 | 168·51 |
| 27 | 7·42 | 12·70 | 84·31 | 144·12 | 175·70 |
| 28 | 7·70 | 13·16 | 87·67 | 150·20 | 182·70 |
| 29 | 7·97 | 13·63 | 91·28 | 156·15 | 190·14 |
| 30 | 8·24 | 14·09 | 94·77 | 162·00 | 197·40 |
Analysis.—The value of soda ash, carbonate of soda, and recovered ash depends on the amount of available alkali [Pg 155](Na2O) present.
A weighed quantity (15·5 grammes conveniently) is dissolved in a measured volume of distilled water (500 c.c.), and titrated with standard normal hydrochloric acid, methyl orange indicator being used.
Caustic Soda.—Raw vegetable fibres may be reduced to the condition of paper pulp by treatment with caustic soda. In practice this process is largely resorted to for the manufacture of pulp from esparto, straw, and wood, the spent caustic soda being recovered and used again.
The paper-maker prepares the caustic required for digesting the raw material from recovered ash and carbonate of soda.
A convenient volume of clear liquor obtained by lixiviating the recovered ash is boiled with lime in suitable causticising pans, the reaction being represented as follows:—
| Na2CO3 | + | CaO | + | H2O | = | 2 NaOH | + | CaCO3. |
| Soda ash | + | Lime | + | Water | = | Caustic soda | + | Chalk. |
According to this equation, 100 lbs. of soda ash require 53 lbs. of quicklime, but a slight excess is generally added, 58 or 60 lbs. being the usual amount actually employed. Several precautions should be observed in the process of causticising.
(1) The liquor from the recovered soda should be bright and clear, indicating complete incineration of the ash.
(2) The liquor is best causticised at a density between 1·050 and 1·100 (10-20, Twaddell). With stronger solutions the reaction is complicated and the yield of caustic soda reduced. Lunge has shown that if the density of the solution is 1·025 the proportion of soda causticised is 99·5 per cent., whereas at a density of 1·150 it is only 94·5 per cent. In the latter case the caustic soda formed acts upon the chalk produced and is reconverted into carbonate.
(3) The large quantities of chalk residue resulting from the reaction must be thoroughly and carefully washed. The economy of the whole process depends in no small measure upon this seemingly small detail.
Caustic Soda Tables.
Showing quantity of liquor obtained from 1 cwt. of caustic soda and the amount of caustic soda in 100 gallons of liquor (adapted from Lunge and others).
| Twaddell. | Gallons obtained per hundredweight of Caustic. | Twaddell. | Pounds of Caustic Soda per 100 gallons Liquor. | |||
| 60 per cent. Caustic. | 77 per cent. Caustic Pure. | 60 per cent. Caustic. | 77 per cent. Caustic Pure. | |||
| 1 | 1,777 | 2,358 | 1 | 6·3 | 4·75 | |
| 2 | 896 | 1,179 | 2 | 12·5 | 9·5 | |
| 3 | 596 | 767 | 3 | 18·8 | 14·6 | |
| 4 | 448 | 574 | 4 | 25·0 | 19·5 | |
| 5 | 359 | 457 | 5 | 31·2 | 24·5 | |
| 6 | 298 | 384 | 6 | 37·6 | 29·2 | |
| 7 | 256 | 330 | 7 | 43·8 | 34·0 | |
| 8 | 223 | 287 | 8 | 50·1 | 39·0 | |
| 9 | 199 | 256 | 9 | 56·2 | 43·7 | |
| 10 | 178 | 229 | 10 | 62·9 | 48·9 | |
| 11 | 162 | 208 | 11 | 69·1 | 53·7 | |
| 12 | 148 | 190 | 12 | 75·7 | 58·7 | |
| 13 | 136 | 176 | 13 | 82·1 | 63·7 | |
| 14 | 126 | 166 | 14 | 88·5 | 67·5 | |
| 15 | 117·5 | 152 | 15 | 95·0 | 73·5 | |
| 16 | 110 | 141·5 | 16 | 101·5 | 79·0 | |
| 17 | 103·5 | 135 | 17 | 107·8 | 83·0 | |
| 18 | 98 | 125·5 | 18 | 114·4 | 89·0 | |
| 19 | 92·8 | 119·5 | 19 | 120·8 | 93·8 | |
| 20 | 88 | 114 | 20 | 127·2 | 98·0 | |
| 25 | 70 | 90·3 | 25 | 159·5 | 124·0 | |
| 30 | 56·5 | 73 | 30 | 197·3 | 153·0 | |
| 35 | 48 | 61·5 | 35 | 234·9 | 182·2 | |
| 40 | 41 | 53 | 40 | 272·6 | 211·6 | |
| 45 | 35·3 | 45·5 | 45 | 317·4 | 246·3 | |
| 50 | 31 | 40 | 50 | 362·1 | 281·0 | |
Dilution Table for Strong Liquors.
Showing number of gallons of water required to reduce the density of 100 gallons of liquor from a higher density, D, to a lower density, d. (See page 163).
| Higher Density, D (Twaddell). | Lower Density, d. | ||||||||||
| 14. | 13. | 12. | 11. | 10. | 9. | 8. | 7. | 6. | 5. | 4. | |
| 42 | 200 | 223 | 250 | 281·8 | 320 | 367 | 425 | 500 | 600 | 740 | 950 |
| 40 | 185 | 207 | 233·3 | 263·6 | 300 | 344·4 | 400 | 471·4 | 566·6 | 700 | 900 |
| 38 | 171 | 192 | 216·6 | 245·5 | 280 | 322·2 | 375 | 442·8 | 533·3 | 660 | 850 |
| 36 | 157 | 177 | 200 | 227·3 | 260 | 300 | 350 | 414·3 | 500 | 620 | 800 |
| 34 | 143 | 161·5 | 183·3 | 209·1 | 240 | 277·7 | 325 | 385·7 | 466·6 | 580 | 750 |
| 32 | 128·6 | 146 | 166·6 | 191 | 220 | 255·5 | 300 | 357·1 | 433·3 | 540 | 700 |
| 30 | 114·3 | 130·6 | 150 | 172·8 | 200 | 233·3 | 275 | 328·5 | 400 | 500 | 650 |
| 28 | 100 | 115·3 | 133·3 | 154·6 | 180 | 211·1 | 250 | 300 | 366·6 | 460 | 600 |
| 26 | 85·7 | 100 | 116·6 | 136·4 | 160 | 188·8 | 225 | 271·4 | 333·3 | 420 | 550 |
| 24 | 71·4 | 84·6 | 100 | 118·2 | 140 | 166·6 | 200 | 243 | 300 | 380 | 500 |
| 22 | 57·1 | 69·2 | 83·3 | 100 | 120 | 144·4 | 175 | 214·4 | 266·6 | 340 | 450 |
| 20 | 43 | 53·6 | 66·6 | 81·8 | 100 | 122·2 | 150 | 185·7 | 233·3 | 300 | 400 |
| 18 | 28·6 | 38·4 | 50 | 63·7 | 80 | 100 | 125 | 157 | 200 | 260 | 350 |
| 16 | 14·3 | 23 | 33·3 | 45·5 | 60 | 77·7 | 100 | 128·5 | 166·6 | 220 | 300 |
Lime and Limestone.—Carbonate of soda and recovered ash are converted into caustic soda by means of lime. About sixty parts of lime are necessary for the conversion of 100 parts of carbonate of soda. Large quantities of insoluble carbonate of lime are produced in this operation, and great care is necessary to prevent a loss of caustic soda which occurs if the residue is not thoroughly washed. In some cases the residual chalk is drained by vacuum filters in order to remove all traces of soluble alkali. Processes have been devised for calcining the residue so as to convert the carbonate into caustic lime to be used over again, but no economical and practical method has yet been found. The treatment of the residual chalk with sulphuric acid for the production of calcium sulphate appears feasible, but the substance obtained is very impure, and therefore has little commercial value.
Limestone is required in considerable quantity for the preparation of sulphite of lime for the manufacture of wood pulp.
Recovered Ash.—The black liquor obtained during the process of the boiling of straw, esparto, and other paper-making fibres contains a large proportion of non-fibrous organic constituents derived from the fibres, the quantity of which may be gauged from the fact that these fibres generally lose 50 per cent. of their weight when being boiled. The black liquor on evaporation yields a thick resinous mass, which is converted into carbonate of soda when burnt.
Advantage is taken of this fact to carry out a process of incineration on a large scale, so that heat derived from the burning off of the resinous mass is utilised for evaporation of weaker liquors. The ash is drawn from special furnaces, put aside, and allowed to char quietly, so that the carbonaceous matter is more or less completely burnt away. The ash in this form contains about 40 per cent. of soda, its composition being determined by the nature of the fibre which has been treated. In the case of straw, the amount of silicate is considerable, as shown by the following typical analysis:—
| Sodium carbonate | 70·2 |
| Sodium hydrate | 2·3 |
| Sodium sulphate | 4·1 |
| Sodium chloride | 7·5 |
| Silica | 7·5 |
| Oxides of iron and alumina | 0·75 |
| Unburnt carbon, etc. | 7·65 |
| 100·00 | |
At the present time there is no process in general use for the recovery of the liquors used in the treatment of wood[Pg 159] by the sulphite process. Many schemes have been proposed, the most promising of which is that of Drewsen.
Sulphur and Sulphites.—The pale yellow brittle substance known as sulphur is too familiar to require any detailed description. It unites with oxygen in various proportions, and these in contact with water form the various sulphur acids known to commerce. Sulphur burned with a limited quantity of air forms sulphurous acid gas, and this substance is the chief product of oxidation, which by further treatment can be converted into sulphites.
In the manufacture of the sulphur compounds required in the preparation of wood pulp, the furnace for burning the sulphur consists of a flat-bottomed cast iron retort which is very shallow, and provided with a curved top, to which a pipe is fixed, so that the sulphurous acid may be conveyed away from the furnace. In the most recent form of sulphur oven a small conical-shaped revolving furnace is employed, which produces a satisfactory gas of constant composition very economically.
Bisulphite of Lime.—This compound is obtained when the sulphurous acid gas is brought into contact with moistened limestone. In the manufacture of bisulphite of lime on a large scale the sulphurous acid gas is drawn or pumped up tall circular towers filled with blocks of limestone, kept moistened by a carefully regulated stream of water flowing from the top of the tower.
In another system known as the acid tank process, the gas is forced into large circular vats containing milk of lime.
In either case a solution is prepared containing bisulphite of lime, together with a certain proportion of free sulphurous acid, the object of the pulp manufacturer being to obtain a solution containing as large a proportion of free sulphurous acid as possible. The composition of a solution will vary[Pg 160] on this account, and the following may be quoted as being an example of such a liquor:—
| Free sulphurous acid | 3·23 | per | cent. |
| Combined sulphurous acid | 0·77 | „ | „ |
| 4·00 | „ | „ | |
For experimental purposes the bisulphite of lime solution may be prepared by passing sulphurous acid gas into a mixture of water and sulphite of lime. The latter compound is insoluble in water, but gradually dissolves when the gas is absorbed. A known weight of sulphite of lime is added to a measured volume of water, and the sulphurous acid gas discharged into the mixture from a siphon of compressed sulphurous acid. The amount of gas absorbed is determined by weighing the siphon before and after use, the loss of weight representing the gas discharged.
The following figures may be quoted as an example:—
| Quantities | used. | |
| Calcium sulphite | 536 | grammes. |
| Water | 7100 | c.c. |
| Gas absorbed | 534 | grammes. |
| Density of solution | 18° | Twaddell. |
The composition of the solution prepared is—
| Combined sulphurous acid | 3·50 |
| Free sulphurous acid | 6·54 |
| Lime | 3·06 |
| Water | 86·90 |
| 100·00 | |
Analysis.—The examination of sulphite liquors for free and combined sulphurous acid is made by means of standard iodine solution and normal caustic soda solution.
A known volume of the sulphite liquor is first titrated with standard iodine solution, the number of cubic centimetres required being a measure of the total sulphurous acid.
Each cubic centimetre standard iodine solution = ·0032 grammes SO2. The titrated liquor is then treated with standard caustic soda in quantity sufficient to exactly neutralise the acid. The volume of caustic soda solution used minus the number of cubic centimetres of iodine first added is a measure of the free sulphurous acid.
Bleaching Powder.—This substance is prepared on a large scale by allowing chlorine gas to act upon dry slaked lime. The lime absorbs nearly one-half its weight of chlorine and forms a dry white powder, having a very pungent odour. The best bleaching powder contains about 37 per cent. of what is termed “available chlorine.” The substance, on being treated with water, gives a greenish-coloured solution known as bleach liquor, and when raw paper-making material, after having been digested with caustic soda, is treated with this solution, it is gradually bleached to a white colour. The composition of the powder may be represented approximately as follows:—
| Available chlorine (combined with lime) | 36·00 |
| Chlorine in the form of chloride | 0·32 |
| Chlorine in the form of chlorate | 0·26 |
| Lime | 44·66 |
| Magnesia | 0·43 |
| Silica, iron oxides, etc. | 1·33 |
| Insoluble matter | 17·00 |
| 100·00 | |
Since the amount of bleach used for wood pulps varies from 8 per cent. to 25 per cent. of powder on the dry wood pulp, the cost of bleaching in some cases is considerable. The economy of the process depends in some measure[Pg 162] upon the care exercised in the purchase of bleaching powder of standard quality, the storage of same in a dark, cool place, and the efficient treatment or exhaustion of the powder when the bleach liquor is prepared.
The powder is usually agitated for about an hour with water sufficient to produce a liquor of 13°-15° Twaddell. The undissolved powder is allowed to settle and the clear solution siphoned off, after which the sediment is washed once or twice to remove all the soluble matter completely.
Bleach Liquor Table.
Showing for bleaching powder solutions of known density the quantity of powder necessary to produce 100 gallons of liquor and the number of gallons obtained from 1 cwt. of powder (adapted from Lunge and Beichofen).
| Twaddell. | Available Chlorine Pounds per 100 gallons. | Number of Gallons obtained from 112 lbs. of Powder. | Pounds of Powder per 100 gallons of Liquor. | ||
| 34 per cent. Powder. | 35 per cent. Powder. | 34 per cent. Powder. | 35 per cent. Powder. | ||
| 0·25 | 0·70 | 5,464 | 5,600 | 2·05 | 2·00 |
| 0·50 | 1·40 | 2,725 | 2,800 | 4·11 | 4·00 |
| 1 | 2·71 | 1,405 | 1,445 | 7·97 | 7·74 |
| 2 | 5·58 | 681 | 702 | 16·41 | 15·94 |
| 3 | 8·48 | 448 | 462 | 24·95 | 24·23 |
| 4 | 11·41 | 334 | 340 | 33·55 | 32·60 |
| 5 | 14·47 | 264 | 270 | 42·58 | 41·34 |
| 6 | 17·36 | 219·5 | 225 | 51·06 | 49·60 |
| 7 | 20·44 | 186 | 191 | 60·11 | 58·40 |
| 8 | 23·75 | 160 | 165 | 69·85 | 67·85 |
| 9 | 26·62 | 141 | 147 | 78·30 | 76·57 |
| 10 | 29·60 | 129 | 132·5 | 87·06 | 84·54 |
| 11 | 32·68 | 116·5 | 120 | 96·11 | 93·37 |
| 12 | 35·81 | 106·5 | 109·5 | 105·32 | 102·31 |
| 13 | 39·10 | 98 | 100 | 115·00 | 111·70 |
| 14 | 42·31 | 90 | 92·5 | 124·45 | 120·90 |
| 15 | 45·70 | 84 | 86 | 134·41 | 130·56 |
| 16 | 48·96 | 78 | 80 | 143·80 | 139·71 |
| 17 | 52·27 | 73·5 | 75 | 153·53 | 149·34 |
| 18 | 55·18 | 69 | 71 | 162·30 | 157·65 |
| 19 | 58·40 | 65·5 | 67 | 171·00 | 166·86 |
| 20 | 61·50 | 61·5 | 64 | 180·88 | 175·71 |
The best method for extracting powder is to agitate the material with water for a short period, and to stop the mixing process directly the maximum density has been obtained, which usually takes place in 15 minutes. Prolonged agitating prevents the powder from settling readily.
The maximum quantities of liquor which can be obtained from bleaching powder are shown on page 162. The following table is useful as showing the amount of water required for diluting strong liquors, the figures being applicable to any solution independent of the nature of the dissolved substance.
Dilution Table for Weak Liquors.
Showing number of gallons of water required to reduce the density of 100 gallons of liquor from a higher density, D, to a lower density, d. (See page 157.)
| Higher Density, D (Twaddell). | Lower Density, d. | |||||||||||
| 12. | 11. | 10. | 9. | 8. | 7. | 6. | 5. | 4. | 3. | 2. | 1. | |
| 16 | 33·3 | 45·4 | 60 | 77·7 | 100 | 128·5 | 166·6 | 220 | 300 | 433·3 | 700 | 1,500 |
| 15 | 25·0 | 36·4 | 50 | 66·6 | 87·5 | 114·3 | 150 | 200 | 275 | 400 | 650 | 1,400 |
| 14 | 16·6 | 27·3 | 40 | 55·5 | 75 | 100 | 133·3 | 180 | 250 | 366·6 | 600 | 1,300 |
| 13 | 8·3 | 18·2 | 30 | 44·4 | 62·5 | 85·7 | 116·6 | 160 | 225 | 333·3 | 550 | 1,200 |
| 12 | 9·1 | 20 | 33·3 | 50 | 71·4 | 100 | 140 | 200 | 300 | 500 | 1,100 | |
| 11 | 10 | 22·2 | 37·5 | 57·1 | 83·3 | 120 | 175 | 266·6 | 450 | 1,000 | ||
| 10 | 11·1 | 25 | 42·8 | 66·6 | 100 | 150 | 233·3 | 400 | 900 | |||
| 9 | 12·5 | 28·5 | 50 | 80 | 125 | 200 | 350 | 800 | ||||
| 8 | 14·2 | 33·3 | 60 | 100 | 166·6 | 300 | 700 | |||||
| 7 | 16·6 | 40 | 75 | 133·3 | 250 | 600 | ||||||
| 6 | 20 | 50 | 100 | 200 | 500 | |||||||
| 5 | 25 | 66·6 | 150 | 400 | ||||||||
| 4 | 33·3 | 100 | 300 | |||||||||
Antichlors.—The residues of chlorine which may be left in pulp after bleaching are frequently neutralised by the use of substances termed antichlors, which react with the calcium hypochlorite, converting it into chlorides.
The sodium hyposulphite is the most frequently used[Pg 164] antichlor, the reaction between this and hypochlorite resulting in the formation of calcium sulphate and sodium chloride; 100 lbs. of commercial bleaching powder will require 30 lbs. of crystallised sodium hyposulphite.
The sulphites of soda and lime also act as antichlors, reducing the hypochlorite of calcium into sulphate of lime or soda. The chief advantage of the use of sulphites is to be found in the fact that the substances obtained by the reaction are neutral.
The best practice in bleaching is to avoid the necessity for using any forms of antichlors by careful regulation of the bleaching process. It has already been suggested in previous references to bleaching that the desired results are obtained when the pulp and bleach are left in contact with one another in tanks or drainers until the bleach is completely exhausted, the residual salts in solution being removed by thorough washing.
Gelatine.—For animal-sized or tub-sized papers gelatine is used. It can be prepared by the paper-maker from hide clippings, sheep skins, bone, etc., or can be purchased ready made.
Beadle gives the following interesting details as to the amount of gelatine which can be obtained from wet hide pieces:—
Weight of Wet Hide Pieces, 2,128 lbs.
| Draught. | Gallons. | Per cent. Gelatine in Solution. | Weight of Gelatine. Lbs. |
| 1 | 126·48 | 6·775 | 85·64 |
| 2 | 128·96 | 6·052 | 78·04 |
| 3 and 4 mixed | 135·20 | 9·446 | 127·63 |
| Total | 390·64 | 291·31 | |
Percentage of gelatine on weight of wet skins = 13·69.
A similar trial on the same class of wet hide pieces gave a yield of 13·23 per cent.
Two trials, of a somewhat different class of wet hide pieces, gave respectively 13·11 and 12·8 per cent.
The temperature of the draught water should be approximately as follows:—
| Draught. | At Beginning. | At End. |
| 1 | 120° F. | 150° F. |
| 2 | 130° F. | 160° F. |
| 3 and 4 | 140° F. | 180° F. |
In the final draught it is often necessary to use live steam at the finish, but this should be avoided if possible.
The water contained in wet hide pieces varies from 77 to 90 per cent. in the different pieces, but in the bulk the average may be taken at 85 per cent.
Casein.—Casein is the nitrogenous principle of milk, and belongs to the class of proteids which are definite compounds of oxygen, hydrogen, carbon, and nitrogen, forming the basis of the most important constituents of all animal fibres, albumen, casein, and gluten. A very pure form of casein is cheese made from skimmed milk. Casein belongs to that class of albumens which are soluble in water, e.g., egg albumen, blood albumen or serum, and lactalbumen, or milk albumen; these are mostly precipitated from solution by saturation with sodium chloride (common salt) or magnesium sulphate; but they are all coagulated by heat.
By the action of rennet on milk the proteid or albumen principle is converted into a curd (casein). This curd, when freed from fats, is insoluble in water, but is soluble in dilute acids, or alkalies, or alkaline carbonates, from[Pg 166] which substances, however, it is reprecipitated by acidulation. Instead of the above method, casein may be precipitated from milk by saturation with sulphate of magnesia, and washing the precipitate with a solution of that salt until the washings contain no albumen, and then redissolving the prepared casein by adding water. The salt still adhering to the precipitate enables it to dissolve. On a large scale the casein is usually prepared by treating the milk with acid.
Casein is readily dissolved by alkalies and alkaline carbonates, borax, boracic acid solution, caustic soda, and bicarbonate of soda.
Starch.—This substance is used in many classes of paper for improving the surface and finish. It is added to the pulp in the beating engine in the dry form as powder, or in the form of starch paste, produced by boiling the starch in water.
The viscosity of the starch paste is somewhat increased by the addition of a small quantity of alkali, but due care must be exercised in boiling, which should only be carried out sufficiently to cause the starch granules to burst, as any excessive boiling causes the starch paste to lose some of its viscosity.
The presence of starch in paper is detected by the blue coloration produced when the paper is dipped into a weak solution of iodine. The determination of the exact percentage of starch in a paper is a matter of some difficulty.
Silicate of Soda.—The precipitation of gelatinous silica upon the pulp in the beating engine is generally regarded as favourable to the production of a sheet of paper having what is known as a harder finish. The precipitation is effected by adding a solution of silicate of soda to the beating engine, with the subsequent addition of sufficient sulphate of alumina to react with the silicate of soda.
Analysis of Commercial Alums.
(Griffin and Little.)
| — | (1) | (2) | (3) | (4) |
| Insoluble in water | 0·05 | 10·61 | 0·11 | 0·56 |
| Alumina (Al2O3) | 15·47 | 14·96 | 11·64 | 16·58 |
| Iron protoxide (FeO) | 0·02 | 0·13 | 0·06 | — |
| Iron sesquioxide (Fe2O3) | 0·00 | 1·08 | 1·17 | 0·04 |
| Zinc oxide (ZnO) | — | — | — | — |
| Soda (Na2O) | 1·72 | 0·57 | 4·75 | 0·56 |
| Magnesia (MgO) | — | — | 0·45 | — |
| Sulphuric acid (SO3) combined | 37·26 | 37·36 | 35·98 | 39·17 |
| Sulphuric acid (SO3) free | — | 1·08 | 5·13 | — |
| Water by difference | 45·48 | 34·21 | 40·71 | 43·09 |
| 100·00 | 100·00 | 100·00 | 100·00 | |
| Sizing test (parts of dry neutral rosin size precipitated by one part of the alum) | 3·32 | 3·47 | 3·19 | 3·71 |
Table showing Value of Solutions of Aluminium Sulphate.
| Twaddell. | Pounds per 100 gallons. | ||
| Al2O3. | SO3. | Sulphate of Alumina containing 15 per cent. Al2O3. | |
| 1 | 1·4 | 3·3 | 9·0 |
| 2 | 2·8 | 6·5 | 19·0 |
| 3 | 4·2 | 9·8 | 28·0 |
| 4 | 5·6 | 13·0 | 37·0 |
| 5 | 7·0 | 16·3 | 47·0 |
| 6 | 8·4 | 19·6 | 56·0 |
| 7 | 9·8 | 22·8 | 65·0 |
| 8 | 11·2 | 26·1 | 75·0 |
| 9 | 12·6 | 29·4 | 84·0 |
| 10 | 14·0 | 32·6 | 93·0 |
| 11 | 15·4 | 35·9 | 103·0 |
| 12 | 16·8 | 39·1 | 112·0 |
| 14 | 20·3 | 47·3 | 135·0 |
| 16 | 23·1 | 53·8 | 155·0 |
| 18 | 26·2 | 60·3 | 172·0 |
| 20 | 29·4 | 68·5 | 196·0 |
| 25 | 37·1 | 86·5 | 247·0 |
| 30 | 44·8 | 104·4 | 299·0 |
| 35 | 53·2 | 124·0 | 355·0 |
| 40 | 60·9 | 142·0 | 405·0 |
| 45 | 68·6 | 159·9 | 456·0 |
| 50 | 77·7 | 181·0 | 578·0 |
| 55 | 86·1 | 200·6 | 575·0 |
| 60 | 95·2 | 221·8 | 635·0 |
Alum.—Alum is one of the most important substances required in the manufacture of paper, its chief function relating to the sizing of paper. Various forms are utilised for this purpose, the purest being sulphate of alumina, required for high grade papers, and the cheaper form known as alum cake, for news and common printing.
The alum is manufactured on a large scale by heating china clay or bauxite with sulphuric acid. This reaction gives sulphate of alumina together with silica. If the mass is heated to dryness, it is sold under the name of alum cake. If the mass is extracted with hot water and the insoluble silica filtered off, the solution can be evaporated down for the production of sulphate of alumina, which is sold in the form of large cakes or in the form of crystals.
By careful selection of raw material a sulphate of alumina can be prepared almost entirely free from iron. The presence of the latter is undesirable, since on exposure to air the sulphate of iron produced during the manufacture of the alum is slowly oxidised and turns brown. Ultimately this affects the colour of the finished paper.
Alum is added to solutions of animal size or gelatine in order to thicken the solution and render it more viscous. It also acts as a preservative, and is used for regulating the absorption of the gelatine by the paper, the penetration effects being materially varied by the extent to which the alum is utilised.
In the process of engine sizing, a term applied to the application of rosin size on account of the fact that the process is completed in the beating engine, alum plays an important part. The mere addition of the prepared rosin soap to the mixture of pulp and water in the beating engine does not size the paper, but the alum precipitates the rosin from its solution, producing a complex mixture said to consist of resinate of alumina and free rosin particles, and[Pg 169] subsequently the heat of the paper machine drying cylinders renders the paper more or less impermeable to moisture.
The appearance and tone of paper, more particularly of coloured papers, are brightened by the use of an excess of alum over and above that necessary to precipitate the rosin soap.
Rosin Size.—This substance is used chiefly for the sizing of news and cheap printing papers, and is also employed together with gelatine for the commoner writing papers. It is prepared by boiling rosin with carbonate of soda under various conditions.
Rosin, sometimes called colophony, is obtained from the sap of certain firs and pine trees. This on distillation yields spirits of turpentine, leaving behind as a residue the mixture of substances to which is given the name rosin. It behaves as an acid, and therefore will combine with certain alkaline oxides, producing soluble resinates.
The nature of the rosin soap used in the paper mill varies according to the conditions under which the size is prepared. If a large proportion of rosin is used, then the size obtained consists of a mixture of resinate of soda together with free rosin dissolved in the solution. If the proportion of rosin is small compared with the amount of carbonate of soda, the composition of the final mixture is quite different. The difference in treatment results in the formation of—
(A) Neutral Size, prepared by boiling a known weight of rosin with sufficient alkali to combine with it and form a neutral resinate of soda. Theoretically this may be obtained by using 630 parts of rosin to 100 parts of soda ash. It is doubtful how far the reaction is completed so as to produce an exactly neutral solution containing only resinate of soda.
(B) Acid Size.—When the proportion of rosin is largely increased the soda becomes converted into the alkaline resinate, and the excess of rosin is gradually dissolved in the resinate formed.
The practical operations necessary for the preparation of the size are comparatively simple. In the case of size containing relatively small percentages of free rosin, the boiling is conducted in open vessels, but for the manufacture of rosin size containing large proportions of free rosin boiling under pressure in closed vessels must be resorted to.
With the open pan process a steam jacketed pan is used, and the required quantity of alkali, dissolved in water, is placed therein and heated to boiling point. The rosin well powdered is added in small quantities from time to time, this being effected cautiously in order that the carbonic acid gas set free during the process may readily escape. The rosin is generally completely saponified after four or five hours' boiling. It is then passed through strainers into store tanks, from which it is drawn into the beating engines as required.
In the case of rosin boiled under pressure a cylindrical vessel provided with a manhole at the top is used. The correct amounts of alkali and water are put into the digester, and also the rosin in a powdered form, the digester being fitted with a perforated plate placed about two feet above the bottom of the vessel in order to prevent the rosin forming into a hard mass at the bottom of the digester.
It is possible in this way to manufacture a thick size containing 30 or 40 per cent. of free rosin and a comparatively small proportion of water. Many paper mill firms prefer to purchase such size ready made.
The most recent modification of the ordinary rosin size is a compound prepared by treating rosin with silicate of soda. This alkali dissolves rosin readily, and the soap[Pg 171] obtained when suitably diluted with water decomposes in the beating engine on the addition of aluminium sulphate, with the precipitation of a gelatinous silica which assists in hardening the paper.
Bacon has patented a process in which powdered rosin is melted down with dry crystalline silicate of soda. The resultant product is ground to a fine powder, which is then ready for use. It dissolves easily in water, and when decomposed with the proper proportion of alum gives a gelatinous viscous mass said to have excellent sizing properties.
The advantages of a dry powdered rosin size readily soluble in water are obvious.
Loading.—The term “loading” is applied to the various substances which are employed for the purpose, as it is commonly supposed, of making paper heavy. But china clay and similar materials are not added simply in order to give weight to the paper, since they serve to produce opacity and to improve the surface of papers which could not be satisfactorily made unless such materials were used.
Examination of Paper for Loading.—If a piece of paper is crumpled up, placed in a small crucible, and then ignited until all the carbonaceous matter has been burnt off, a residue is left in the crucible which may be white or coloured. This is usually termed the ash of the paper. The amount of ash present is determined by taking a weighed quantity of paper and weighing the residue obtained. Special appliances can be obtained for making rapid determinations of the ash in paper, but for occasional analyses they are not required.
China Clay.—This is the best known and most commonly used loading. The purest form of this material is kaolin, a natural substance formed by the gradual decomposition[Pg 172] of felspathic rocks arising from exposure to the long-continued action of air and water. The clay occurs in great abundance in Dorset, Cornwall, and Devon, the southern counties in England, where the most famous deposits are found.
The natural mineral is levigated with water, and the mixture allowed to flow through a series of settling ponds, so that the clay gradually settles in the form of a fine deposit. The clay is dried and packed in bags. Its value is controlled largely by the purity of its colour and its freedom from grit and sand. It is essentially a silicate of alumina, having the approximate composition—
| Silica (SiO2) | 43·00 |
| Alumina (Al2O3) | 35·00 |
| Combined water | 10·00 |
| Moisture and impurities | 12·00 |
| 100·00 | |
The specific gravity of the dry substance is 2·50.
It is utilised as a loading in all kinds of paper, and forms also the main ingredient in the coating found on ordinary art and chromo papers.
Ash containing China Clay.—In news, cheap printings, and common art papers the ash almost invariably contains china clay. This substance is insoluble in dilute acids, but is acted upon by concentrated sulphuric acid when digested for some time. A simple test for the presence of china clay in ash is the blue coloration which is obtained when the ash after being ignited is gradually heated with a few drops of solution of cobalt nitrate. China clay can be decomposed by fusion with carbonate of soda in a crucible. By this means silicate of alumina is decomposed, and the alumina goes into solution, the silica remaining as an[Pg 173] insoluble residue. The filtered solution is boiled with an excess of ammonia which gives a gelatinous precipitate of aluminium hydrate.
Sulphate of Lime.—This compound is valued chiefly for its brilliancy of colour, being used in high-class papers. It is slightly soluble in water, to the extent of about 23 lbs. in 1,000 gallons, and this fact must be taken into account when the material is added to the pulp in the beating engine.
It occurs naturally in a variety of forms, such as gypsum, alabaster, selenite, the first of which when finely powdered is sold to the paper-maker as gypsum, powdered plaster, and under other fancy names.
It can be prepared artificially by adding sulphuric acid to solutions of calcium salts; and the precipitated product so obtained is sold as terra alba, pearl hardening, satinite, mineral white, etc.
The tests for sulphate of lime in paper ash are based upon the following reactions:—
Calcium sulphate is soluble in dilute hydrochloric acid. The addition of a few drops of barium chloride to the solution produces a dense heavy precipitate, indicating the sulphate. A small quantity of ammonium oxalate solution added to another portion of the dissolved calcium salt previously neutralised with ammonia produces a precipitate and indicates calcium.
A microscopic test of paper for the presence of sulphate of lime is based upon the slight solubility of the salt in water. The paper is boiled with some distilled water. The water is evaporated to a small bulk and transferred to a glass slip, and the gradual formation of characteristic sulphate of lime crystals can be seen by means of the microscope as the water cools down.
French Chalk.—This material is prepared by grinding[Pg 174] talc into a fine powder, and possesses a good colour and a somewhat soapy feel. It is a silicate of magnesia, having the approximate composition—
| Silica (SiO2) | 62·00 |
| Magnesia (MgO) | 33·00 |
| Water | 4·30 |
| Traces of oxides, etc. | 0·70 |
| 100·00 | |
Other silicates of magnesia used for paper-making are agalite and asbestine, the latter being a finely ground asbestos.
The composition of asbestos is approximately—
| — | Italian. | Canadian. |
| Lime and magnesia | 38·0 | 33·0 |
| Silica | 42·0 | 41·0 |
| Oxides of iron and alumina | 5·0 | 12·0 |
| Total water | 13·0 | 12·0 |
| Traces of soda, etc | 2·0 | 3·0 |
| 100·00 | 100·00 | |
Introduction.—The process of beating has for its object the complete breaking down of the bleached pulp to the condition of single fibres, and the further reduction of the fibres, when necessary, into smaller pieces. The disintegration of the material is essential for the production of a close even sheet of paper, and the amount of beating required varies greatly according to the nature of the raw material, and the class of paper to be produced.
The textile trade, on the other hand, depends on a raw material composed of strong fibres, or of filaments characterised by great length, and any processes of treatment which tend to reduce the length of such fibres are carefully avoided, and it is therefore obvious that fibres which are of no value for textile purposes can be appropriated for paper-making.
Condition of Fibres.—The great differences in the physical characteristics and structure of the fibres employed for paper-making suggest that the possible variations in the final product obtained by beating are very numerous. This is a well-known fact, and it is further to be noted that this mechanical operation brings about not merely alterations of a physical order, but introduces some interesting and important chemical changes.
Of the better-known materials linen, with an average fibre length of 28 mm., the structure of which lends itself to considerable alteration by beating, is in marked contrast[Pg 176] to esparto, the fibre length of which is only 1·5 mm. If the process of beating a linen rag merely resulted in the cutting of all the fibres of 28 mm. long into short fragments of 1·5 mm., there would be nothing remarkable in it, but the changes which occur in reducing the long linen fibre to 1·5 or 2·0 mm. are of a far more important character than this.
Early Methods.—In the early days of paper-making the disintegration of the half-stuff was effected by a true “beating” process, the rags being subjected to the action of heavy stampers, which broke up the mass of tangled fibre into a uniform pulp. The fibres for the most part retained their maximum length in this operation, which was exceedingly slow and tedious, though at the same time giving a sheet of paper of remarkable strength.
The nearest imitation of these old-time rag papers is to be seen in the well-known Japanese papers, which are extraordinarily strong. Some of these the writer has examined in order to determine the length of the fibre. The sheets when held up to the light appear “cloudy” and “wild” owing to the presence of the long fibres, which have only been separated or teased out by the primitive methods of beating used, and not completely disintegrated.
Conditions of Beating.—About A.D. 1700 there began a great epoch in the history of paper-making. With the invention of the Hollander engine about A.D. 1670, the process of disintegration was greatly hastened, because it was possible to reduce the half-stuff much more readily. The substitution of the idea of plain “beating” by a principle which combined the gradual isolation of the individual fibres with a splitting up of those fibres lengthwise and crosswise was not only an advantage in point of economy of time and cost, but also a material advance in the possibilities of greater variations in the finished paper.
The conditions of the process of beating carried out with a Hollander permit of considerable alteration, so that these changes in the fibre are not surprising when properly understood. In fact, it is now conceded that a close study of the theory and practice of beating is likely to bring about still more remarkable improvements in this important department of the paper-maker's work. The quality and character of the paper made may be varied with—
(1) The origin of the raw material, e.g., rags, esparto, or wood;
(2) The condition of the material, e.g., old or new rags, green or mature esparto, mechanical or chemical wood pulp;
(3) The time occupied in beating, e.g., four hours for an ordinary rag printing and twelve hours for a rag parchment;
(4) The state of the beater knives, e.g., sharp tackle for blottings and dull tackle for cartridge papers;
(5) The speed of the beater roll, also its weight;
(6) The rate at which the beater roll is lowered on to the bedplate;
(7) The temperature of the contents of the engine.
The Beater Roll.—If the beater roll is fitted with sharp knives, and this is put down close to the bedplate quickly, the fibres are cut up short, and they do not assimilate the water. If the roll is fitted with dull knives, or “tackle,” as it is sometimes called, and it is lowered gradually, the fibres are drawn and bruised out without being greatly shortened. In this condition the stuff becomes very “wet,” or “greasy,” as it is termed. The cellulose enters into association with water when beaten for many hours, and the pulp in the beating engine changes into a curious greasy-like mass of a semi-transparent character. Rag pulp beaten for a long time produces a hard, translucent, dense sheet of paper. Flax thread beaten 48 to 60 hours is used in practice[Pg 178] for the manufacture of gramophone horns and similar purposes.
Soft porous papers like blottings, filtering papers, heavy chromos, litho papers, antiques, light printings, are made from pulps which are beaten quickly with the roll put down close to the bedplate soon after the stuff has been filled in.
With strong, dense, hard papers, such as parchments, banks, greaseproofs and the like, the pulp is beaten slowly and the roll lowered gradually.
The nature of the pulp and the time occupied in beating are also important factors in producing these different papers, three to four hours being ample for an ordinary wood pulp printing, whereas a wood pulp parchment requires seven to eight hours.
Beating Pulps Separately.—The use of esparto and wood pulp in conjunction with one another, or blended with rag, has introduced new problems into the question of beating. Perhaps the most important of these is the advisability of beating the pulps separately and eventually passing them through a mixer of some kind before discharging into a stuff chest. The necessity for differentiating the amount of beating is already partly recognised when very dissimilar pulps, such as strong rag and esparto, are blended, but the whole subject ought to be carefully studied by the paper-maker and investigated on its merits from the standpoint of “beating effects,” apart from questions of cost and expediency. The former fully understood and exhaustively examined by practical tests would of course only be developed if proved to be advantageous.
The field of research in this direction has not yet been seriously explored. With the enormous consumption of wood pulps of varying quality made from many different species of wood by several processes, there is ample room for interesting and profitable enquiry, particularly as the[Pg 179] types of beating engine are so numerous. The effects produced by the Hollander, the refiner, the edge runner, the stone beater roll, and other mechanisms, are all of varying kinds.
The importance of a knowledge of the precise effects produced by the beating of pulp cannot be emphasised too much, and any contributions to the subject along the lines of special research will be welcomed by all students of cellulose.
Some experiments were conducted by the writer in 1906 with cotton rags, in order to determine the results obtained[Pg 180] by beating the pulp for a prolonged period under exact and specific conditions.
The cotton rags, of good quality, were boiled with caustic soda in the usual way for six or seven hours, at a pressure of 15 to 20 lbs., washed and partially broken down in the rag breaker, and finally bleached, made into half-stuff, and then transferred to a Hollander beating engine.
The particular conditions specified for the beating operation were that the beaterman should manipulate the pulp according to his usual routine for the manufacture of the paper which he was accustomed to make from these rags. In this case the routine process meant beating for eight hours, by which time the pulp was ready for the paper machine. In the ordinary course the pulp would be[Pg 181] discharged into the stuff chest, and converted into a strong, thin, bank paper.
During the prolonged beating the pulp became very soft and “greasy,” and when made up into sheets the paper as it dried exhibited remarkable differences in shrinkage, the dry sheets obtained from pulp beaten thirty-seven hours being much smaller than those obtained from pulp beaten only four or six hours. The actual shrinkage is shown in the following table:—
| Hours. | Area of Sheet. Sq. mm. | Loss of Area. Sq. mm. | Relative Areas. Deckle 100 | Shrinkage per cent. |
| 0 | 26,384·0 | — | 100·0 | — |
| 4 | 26,076·0 | 308·0 | 98·9 | 1·1 |
| 6 | 25,520·1 | 863·9 | 96·7 | 3·3 |
| 8 | 25,160·0 | 1,224·0 | 95·4 | 4·6 |
| 10 | 24,794·8 | 1,589·2 | 93·9 | 6·1 |
| 13 | 24,467·4 | 1,916·6 | 92·8 | 7·2 |
| 15 | 24,215·2 | 2,168·8 | 91·8 | 8·2 |
| 17 | 24,024·0 | 2,360·0 | 90·9 | 9·1 |
| 19 | 23,616·2 | 2,767·8 | 89·6 | 10·4 |
| 21 | 23,616·0 | 2,768·0 | 89·6 | 10·4 |
| 23 | 23,535·7 | 2,848·3 | 89·3 | 10·7 |
| 25 | 23,329·9 | 3,054·1 | 88·5 | 11·5 |
| 27 | 22,920·5 | 3,463·5 | 86·9 | 13·1 |
| 29 | 22,831·2 | 3,552·8 | 86·5 | 13·5 |
| 31 | 22,492·9 | 3,891·1 | 85·3 | 14·7 |
| 33 | 21,917·2 | 4,466·8 | 83·1 | 16·9 |
| 35 | 21,226·1 | 5,157·9 | 80·5 | 19·5 |
| 37 | 20,778·8 | 5,605·2 | 78·8 | 21·2 |
If these results are plotted in the form of a curve the relation between the period of beating and the shrinkage in area is clearly shown. For the first twenty hours the shrinkage is proportional to the period of beating, after which the curve assumes an irregular shape, showing a tendency for shrinkage to proceed at a faster rate.
Weight and Substance of the Paper.—The shrinkage of the paper after prolonged beating indicates a closer and denser sheet, so that for papers of equal thickness the weight per unit area was much greater in the case of the pulp beaten for the full period. The results obtained are very interesting, and the following summary for a few of the readings obtained will serve to show the alteration effected.
| Hours. | Weight of 20,000 sq. mm. Grams. | Thickness of Sheet. mm. | Grams per sq. metre. | Lbs. per ream 480 sheets, 20" × 30". |
| Class A 8-10 hrs. | 1·875 | ·183 | 93·75 | 38·23 |
| Class B 19-21 hrs. | 2·043 | ·189 | 102·15 | 41·65 |
| Class C 33-35 hrs. | 2·203 | ·189 | 110·15 | 44·93 |
Sizing and Glazing Effects.—The behaviour of the waterleaf paper after sizing and glazing gave some interesting results. In the first place, the effect of the altered density of the paper is strikingly shown by the amount of the size absorbed. Certain selected sheets were passed through a solution of ordinary gelatine in the usual way, and subsequently dried. The amount of gelatine absorbed differs in a remarkable degree, as shown in table.
Tensile Strength of the Paper.—It is interesting to note that the tensile strength of the waterleaf papers appears to remain fairly constant throughout the whole period of beating. But this uniformity is greatly altered by the operations of sizing and glazing.
Percentage of Air-dry Gelatine absorbed by the Waterleaf Sheets.
| Hours. | Percentage of Size absorbed. | Mean. | ||
| 1st Trial. | 2nd Trial. | 3rd Trial. | ||
| 8 | 5·5 | 6·0 | 6·2 | 5·9 |
| 10 | 5·4 | 6·8 | 6·5 | 6·2 |
| 19 | 3·8 | 5·0 | 4·5 | 4·4 |
| 21 | 4·8 | 3·9 | 4·6 | 4·4 |
| 33 | 2·7 | 1·7 | 2·4 | 2·3 |
| 35 | 2·4 | 1·9 | 1·7 | 2·0 |
These results are rather remarkable. The prolonged beating does not seem to have affected the tensile strength of the waterleaf, and the practical loss of strength which actually occurs in the more completely finished paper does not manifest itself until after the sizing process. The importance of the gelatine as a factor in the ultimate strength is thus clearly and strikingly demonstrated.
Tests for Strength on Original Waterleaf Paper.
| Hours. | Mean result of Readings. Lbs. | Mean Strength of the Paper. Lbs. |
| 8 | a 14·1 | 12·1 |
| b 10·1 | ||
| 10 | a 15·4 | 13·2 |
| b 10·9 | ||
| 19 | a 16·5 | 14·0 |
| b 11·4 | ||
| 21 | a 15·2 | 14·0 |
| b 12·8 | ||
| 33 | a 13·4 | 12·4 |
| b 11·4 | ||
| 35 | a 14·5 | 13·6 |
| b 12·7 | ||
Tests for Strength on Papers, Sized only.
| Hours. | Mean result of Readings. Lbs. | Mean Strength of the Paper. Lbs. |
| 8 | a 22·7 | 20·0 |
| b 17·3 | ||
| 10 | a 28·5 | 23·2 |
| b 18·0 | ||
| 19 | a 22·5 | 21·0 |
| b 19·5 | ||
| 21 | a 26·0 | 21·7 |
| b 17·5 | ||
| 33 | a 15·0 | 15·0 |
| b 15·0 | ||
| 35 | a 14·2 | 15·3 |
| b 16·5 | ||
Tests for Strength on Paper Sized and Glazed.
| Hours. | Mean result of Readings. Lbs. | Mean Strength of the Paper. Lbs. |
| 8 | a 25·8 | 23·6 |
| b 21·4 | ||
| 10 | a 28·4 | 23·6 |
| b 18·9 | ||
| 19 | a 27·0 | 22·9 |
| b 18·9 | ||
| 21 | a 24·9 | 22·7 |
| b 20·6 | ||
| 33 | a 16·1 | 15·2 |
| b 14·4 | ||
| 35 | a 17·5 | 16·2 |
| b 15·0 | ||
It may also be noticed that the strength of the finished paper after twenty hours' beating, as in class B, is equal to that of the paper after nine hours' beating, as in class A. This is curious, especially in view of the fact that the percentage of gelatine in the papers of class B. is only 4·4 per cent. as against 6·0 per cent. in class A.
The relation of the percentage of gelatine to the period of beating thus becomes a matter of interest, and well worth investigation. The figures are suggestive of further experimental research along definite lines.
Developments in Beating Engines.—Since the introduction of the Hollander beating engine, about A.D. 1670, other types of beater almost too numerous to mention have been devised to supersede it, but the fact remains that the principle of the original Hollander and its general design are still adhered to in the engines used by paper-makers for high-class work.
The alterations and improvements which have taken[Pg 186] place during the last fifty years relate chiefly to the modifications naturally arising from the introduction of fibres not requiring such drastic treatment as rags.
The machines now in use for reducing half-stuff to beaten pulp ready for the paper machine may be classified as follows:—
(1) Beaters of the Hollander type, in which the circulation of the pulp in the engine and the actual beating process are both effected by the beater roll.
(2) Beaters of the circulator type, in which the movement of the pulp is maintained by a special contrivance, and the beater roll used only for beating.
(3) Beaters of the stone roll type in which the roll and bedplate are either or both composed of stone, granite, or similar non-metallic substance.
(4) Refiners, containing conical shaped beater rolls working in a conical shell fitted with stationary knives.
The Hollander.—This beating engine in its simplest form consists of an oval shaped trough, divided into two channels by a “midfeather,” which does not, however, reach completely from one end to the other.
In one of the channels the bed of the trough slopes up slightly to the place where the “bedplate” is fixed. The bedplate consists of a number of stout metal bars or knives firmly fastened into an iron frame, which lies across this[Pg 187] channel. The beater roll, a heavy cast-iron roll provided with projecting knives or blades arranged in clumps of three around the circumference, and supported on bearings at each side of the engine, revolves above the bedplate with the knives adjusted to any required distance from it, the raising or lowering of the beater roll for this purpose being effected by the use of adjustable bearings.
The bed of the trough behind the beater roll rises sharply up from the bedplate and then falls away suddenly, as shown in the diagram, forming the “backfall.”
When the engine is in operation the mixture of water and pulp is drawn between the knives and circulated round the trough. The material is disintegrated into fibres of the required condition, discharged over the backfall, and kept in a state of continual circulation, and the beating maintained until the stuff has been sufficiently treated.
The dimensions of the engine vary according to the capacity, which is usually expressed in terms of the amount of dry pulp the beater will hold, and the following figures may be taken as giving the average sizes:—
| — | 2 cwt. Engine. | 5 cwt. Engine. |
| Length | 11 ft. 0 in. | 16 ft. 0 in. |
| Width | 5 ft. 6 in. | 8 ft. 0 in. |
| Depth (average) | 2 ft. 3 in. | 2 ft. 9 in. |
| Diameter of roll | 3 ft. 6 in. | 3 ft. 6 in. |
Sundry modifications in the form and arrangement of the beater have been tried from time to time. In 1869 Granville patented the substitution of a second beater roll in place of the stationary bedplate for the purpose of hastening the operation. Repeated attempts have been made to construct a beating engine with two or more rolls, but it is evident that such a device could hardly succeed, since it would be[Pg 188] impossible to ensure proper adjustment of the rolls, and in that case one roll might be doing all the work.
The first machine of this type was patented in 1872 by Salt. Similar beaters were devised by Forbes in 1880, Macfarlane in 1886, Pickles in 1894, who proposed to use three rolls, and Partington in 1901. Hoffman describes a beating engine which was working in America containing four rolls, as shown in the diagram.
The Umpherston.—A notable modification of the Hollander, having an arrangement by which the two channels of the engines are placed under one another, and one which is largely used for fibres, is the Umpherston. Several engines differing in detail, but embodying the same principle, have been built in imitation of this one.
Bedplates of large working surface were first tried in England by Cooke and Hibbert, in 1878, but in practice it has been found that no serious deviations from the narrow type of plate are of much value. As a matter of fact it is held by some paper-makers that one or two knives would be sufficient if they could be relied on to keep true and in proper adjustment.
The Circulating Type of Beater.—The addition of some device for keeping the pulp in circulation apart from the action of the roll has received considerable attention. The early experiments in this direction with the Hollander led ultimately to the construction of the engine of the circulator type mentioned in class 2.
Thus, in 1872, Nugent patented a special paddle to be used in the Hollander, by which the pulp in the trough of the beater was impelled towards the roll. Many other plans were tried for this purpose, and details can be seen in the List of Patents (see page 192).
The introduction of the beaters with special means of circulating the pulp was found to be of the greatest service in the treatment of stuff like esparto and wood pulp, since these materials did not require the drastic measures necessary with rag pulp. In 1890 several engines of this class were being adopted, amongst which may be mentioned Hemmer's, Reed's and Taylor's. The pulp discharged from the beater roll was drawn through an independent pipe or channel by means of an Archimedean screw, or a centrifugal pump.
Stone Beater Rolls.—The substitution of stone for metal in the roll and bedplate of the engine brings about some[Pg 190] remarkable changes in the nature of the beaten stuff. The fibre is submitted to the action of rough surfaces rather than that due to the contact of sharp edges, with the result that the disintegration is much more rapid, and produces a “wet” working pulp suitable for imitation parchments and similar papers. The latest materials used for this purpose are basalt lava stone in Germany, and carborundum in America.
Care is necessary in the manipulation of these beaters to prevent fracture of the stone parts. In the Wagg Jordan engine this danger is materially reduced by the construction of the working parts.
Refiners.—In these engines the beater roll is a conical shaped drum carrying the knives, which revolve inside a conical shell completely lined with fixed knives. The fibres are thus cut up to the desired length, but before discharge from the engine they pass between two circular discs,[Pg 191] one stationary and the other revolving in a vertical position. The effect of the discs is to tear or bruise the fibres rather than to cut them.
The refiner is best employed to clear or brush out the mass of pulp after a certain amount of preliminary treatment in the beater, as the refiner cannot produce the effects obtained by actual beating as in the Hollander.
Power Consumption.—The long treatment required to thoroughly pulp a strong material demands a great amount of power. Engines differ considerably in their power consumption, and comparisons are frequently made in terms of the power required to beat a given weight of pulp. But this is not always a true criterion of efficient work. Some types of beater are suitable for producing certain[Pg 192] results, and the mere substitution of a beater consuming less power is worse than useless unless it can be shown that the same effects are being obtained. The efficiency of the Hollander for the beating of rag pulp, in spite of the high power consumption, is a case in point.
With this factor properly considered, the power required for beating becomes an interesting study. Many detailed experiments have been published from time to time, the most recent being those described by Beadle.
1855. Park (1170).—A small steam engine was attached to the shaft of the beater roll, so that it could be driven direct.
1856. Kingsland (2828).—A form of refiner in which the pulp was beaten by a vertical disc rotating in an enclosed case.
1860. Jordan (792).—A machine devised for mixing size with pulp, made like a conical refining engine, the rubbing surface being provided with teeth or cutters.
1860. Jordan (2019).—An engine of the refiner type, constructed with a conical drum rotating in a conical casing. The knives at the larger end of the drum are placed closer together than those on the smaller end.
1863. Park (1138).—Two beaters placed side by side are driven by one steam engine placed between them, the operations being so timed that one rag engine is used for breaking while the other is finishing.
1864. Ibotson (2913).—The pulp is passed continuously from one engine roll to another, or from one part of a beater roll to another part of the same roll through slotted plates.
1866. Roeckner (140).—A beating engine of the refiner type with conical drum and casing.
1866. Berham (3299).—A beating engine of the conical type with the beater roll rotating vertically instead of horizontally.
1867. Crompton (482).—Device for raising the bars in the beater roll as the edge of the plate wears away.
1867. Wood (914).—Modification in the form of the beater bars (of little importance).
1867. Edge (3673).—The knives of the beater roll distributed at equal distances apart all round the roll, alternated with strips of wood.
1869. Granville (1041).—Substitution of a second beater roll for the stationary bed-plate, the knives being set spirally round the roller.
1869. Newell (2905).—Weight of the beater roll counter[Pg 194]poised to allow of the exact regulation of the pressure on the stuff in the beating engine.
1870. Rose (997).—An intercepting plate fixed to the cover of the beating engine which causes that part of the stuff which was usually carried right round by the roll to fall back behind the backfall.
1870. Bentley and Jackson (1633).—A beater roll having the same width as the engine, and provided with a cover fitted with a pipe which conducted the material back to the front of the roll.
1871. Patton (1336).—Bottom of beating engine curved in order to prevent the stuff settling or accumulating at any portion of the machine.
1872. Salt (1901).—A beating engine of usual type, but having two beater rolls and two drum washers, one pair in each of the two channels.
1873. Gould (769).—A curious engine with horizontal shaft having a circular disc at the lower end, fitted with knives on the under-surface, which are in contact with fixed knives lying at the bottom of the vessel. The circulation of the pulp is effected by the centrifugal force generated.
1873. Martin (3751).—A beating engine with two rolls in the same trough, the first roll working in conjunction with a smooth surfaced beating roll, the other being in contact with a bedplate of the usual type, the object of the first roll being to partially disintegrate the material without danger of choking.
1874. Johnstone (3708).—A pulping engine in which the rubbing action of two grindstones one upon the other is utilised as a means of beating.
1876. Gardner (307).—A beating engine in which the beater roll is conical in shape, working vertically in contact with the bottom of the beating engine, which is also conical in shape, the engine itself being circular.
1878. Cooke and Hibbert (4068).—The bedplate constructed in the form of a circular segment with a much larger face than usual, and capable of adjustment, the beater roll itself being fixed in the bearings.
1880. Forbes (692).—A long oval shaped beating engine divided into three channels instead of two. In the two outer channels are placed beater rolls and drum washers. The stuff discharged over the backfalls from the two beating engines flows down the central channel and is circulated by a special paddle constructed in such a manner as to deliver the pulp in two equal streams into the outer channels to each of the beater rolls.
1880. Umpherston (1150).—An engine constructed with a passage below the backfall so that the stuff circulates in a trough underneath the beater roll, the object being to ensure more effective treatment and to save floor space.
1883. Aitchison (5381).—A beating engine of usual form, but with the beater roll made conical in shape with the larger circumference outwards, and the bedplate placed on an incline parallel with the knives on the beater roll.
1884. Mayfield (2028).—The backfall of the beating engine is of entirely different construction to the ordinary machine, for the purpose of improving the circulation.
1884. Hoyt (11177).—An engine resembling the Umpherston, but with a larger roll, the diameter of which is equal to the full depth of the engine, the backfall being in a line with the axis of the beater roll.
1885. Jordan (7156).—Additions to the Jordan engine for admitting water and steam to the engine as required.
1885. Korschilgen (9433).—The beater roll made of stone or of metal with a stone casing furnished with ribs or knives placed close together.
1886. Hibbert (4237).—A beating engine fitted with an ordinary beater roll, and having in addition a heavy disc[Pg 196] rotating vertically, the disc being fitted with knives on one surface which rotate in contact with knives fixed on a stationary disc.
1886. Kron (9885).—A device for securing better circulation of the pulp, the stuff leaving the beater roll being divided into two streams which are brought together again in front of the roll.
1886. Horne (10237).—A long rectangular vessel with a large beater roll at one end, contrived so as to force the pulp leaving the beater roll to pass down a partition separating it from the pulp going towards the beater roll.
1886. Macfarlane (11084).—An engine fitted with two beater rolls which rotate in opposite directions, the stuff being mixed between them.
1887. Nacke (746).—A centrifugal circulating wheel rotating horizontally in the centre of the beating engine is used in combination with a parallel cutting disc.
1887. Marshall (1808).—A conical refiner having in addition at its large end a pair of grinding discs fitted with knives and rotating vertically.
1887. Voith (6174).—An alteration to the covers of the beater rolls which prevent stuff from being carried round the cylinder, and cause it to pass over the backfall freely.
1890. Hemmer (17483).—A beating engine provided with a separate return channel for the pulp, the circulation through the channel being effected by a small centrifugal pump.
1890. A. E. Reed (19107).—A beating engine in which the pulp discharged over the backfall is delivered to the front of the beater roll by a screw propeller.
1891. Karger (11564).—A beater similar to the Umpherston, but provided with a circulating roll fitted with radial projections which delivers the stuff to the front of the beater roll.
1892. Taylor (7397).—A beating engine in which the beater roll operates in a closed chamber above the vat full of pulp, the stuff being continually circulated by a centrifugal pump which draws the stock from the bottom of the vat and delivers it to the beater roll.
1892. Annandale (9173).—A conical-shaped beating engine with the beater roll rotating in a vertical position; the larger end of the cone being downwards.
1892. Umpherston (15766).—An addition to the beating engine arranged so that two fixed bedplates are used instead of one.
1892. Miller (15947).—A machine in which two fixed bedplates are used, one below the beater roll and one above, the engine being fitted with suitable baffle plates to ensure proper circulation.
1893. Pearson and Bertram (11956).—A special form of refining engine in which the pulp is subjected to the action of discs rotating vertically, the knives being arranged radially on the disc.
1893. Caldwell (15332).—A rotary beating engine in which the beating surfaces admit of accurate adjustment.
1894. Cornett (945).—An outlet is fixed to the beater roll casing close to the discharge from the bedplate, so that the roll is not impeded by the weight of the pulp, which is subsequently pumped to the front of the beater roll.
1894. Shand and Bertram (4136).—A beating engine similar to the Umpherston beater in which the beater roll is raised up out of the pulp and the circulation effected by means of a worm which delivers the pulp to the front of the beater roll.
1894. Pickles (20255).—A beating engine somewhat similar to an Umpherston, but fitted with three beater rolls and bedplates.
1894. Hibbert (25040).—A beating engine in which the[Pg 198] pulp is beaten between two discs rotating vertically, the pulp being brought between the discs through the hollow shaft of one of the discs.
1895. Brown (1615).—An engine in which the beater roll and bedplate both revolve, but in opposite directions, and at different speeds in order to draw out the fibres.
1895. Schmidt (24730).—A device by means of which the pulp discharged from the beater roll is diverted into supplementary channels on either side which come together again in front of the beater roll.
1900. Hadfield (2468).—An adjustable baffle board passing through the cover of the beater roll which prevents the pulp being carried round by the roll, more or less.
1900. Masson and Scott (5367).—An improved form of Taylor beating engine in which the chest of the engine is vertical instead of horizontal.
1901. Partington (24654).—A continuous elliptical trough provided with two beater rolls.
1902. Picard (19635).—Improvements in the form of the propellers used for circulating the material.
1902. Pope and Mullen (22089).—Improvements in propellers for circulating the pulp.
1903. Annandale (26012).—A new form of beating engine somewhat on the principle of a steam turbine.
1905. Bertram (1727).—A beater similar to Masson's tower beater, but in which a pair of reciprocating wheels fitted with projecting knives are used instead of a centrifugal pump.
1907. Wagg's Jordan Engine (6788).—A conical refiner fitted with specially arranged metal or stone knives.
Nearly all papers, even those commonly regarded as white, are dyed with some proportion of colouring matter. With the ordinary writing and printing papers the process is usually confined to the addition of small quantities of pigments or soluble colours sufficient to tone the pulp and correct the yellow tint which the raw material possesses even after bleaching. In the case of cover papers, tissues, and similar coloured papers, the process is one of dyeing as it is generally understood.
The colouring matters which have been employed by the paper-maker are—
(A) Added to the pulp in the form of mineral in a finely divided state.
Yellow.—This colour is obtained by the use of ochres, which are natural earth colours of varying shades, from bright yellow to brown.
Red.—Ordinary red lead.
Various oxides of iron, such as Indian red, Venetian red, red ochre, rouge.
Blue.—Smalts—An expensive pigment prepared by grinding cobalt glass.
Ultramarine—A substance of complex composition prepared by heating a mixture of china clay, carbonate of soda, sulphate of soda, sulphur, [Pg 200]charcoal, and sometimes quartz, rosin and infusorial earth.
Prussian Blue—A compound prepared by adding potassium ferrocyanide to a solution of ferrous sulphate.
Brown.—Natural earth colours, such as sienna, umber, Vandyke brown.
Black.—Lamp-black, bone-black, Frankfort black.
(B) Produced by the reaction of soluble salts upon one another when added to the pulp in the beating engine.
Yellow.—Chrome Yellow—The paper pulp is first impregnated with acetate of lead, and potassium or sodium bichromate added. This precipitates the chromate of lead as a yellow pigment.
Chrome Orange—The addition of caustic alkali to the bichromate solution converts the chrome yellow into an orange.
Blue.—Prussian Blue—The paper pulp impregnated with iron salts is treated with potassium ferrocyanide. The blue colour is at once obtained.
Brown.—Iron Buff—A light yellow-brown colour due to the precipitation of ferrous sulphate by means of an alkali.
Bronze.—Manganese chloride followed by caustic soda.
(A) Natural Dyes. These colouring matters are now seldom used.
Yellow and Brown.—The vegetable extracts, such as fustic, quercitron, cutch, turmeric, have practically all been replaced by aniline colours.
Red.—Madder (Turkey red), Brazilwood, cochineal (a dye obtained from dried cochineal insects). Safflower.
Black.—Logwood, used in conjunction with an iron salt. Cutch, used with an iron salt.
(B) Coal Tar Dyes. The dyeing and colouring of paper pulp by means of the artificial organic substances has become a matter of daily routine, the expensive natural dyes and the ordinary pigments having been almost completely superseded. The numerous colouring matters available may be classified either by reference to their chemical constitution or simply on general lines, having regard to certain broad distinctions.
If the latter classification is taken, then the dyes familiar to the paper-maker may be divided into—
(a) Acid dyes, so called because the full effect of the colouring matter is best obtained in a bath showing an acid reaction.
(b) Basic dyes, so called because the colour is best developed in an alkaline solution, without any excess of mordant.
(c) Substantive dyes, which do not require the use of a mordant, as the colour is fixed by the fibre without such reagents.
Some of the most frequently used colouring matters are shown in the accompanying table on page 202.
The distinction between acid and basic dye-stuffs is largely due to certain characteristics possessed by many of them. Thus magenta, which is the salt of the base known as Rosaniline, belonging to the basic colouring matters, a group of dyes which do not possess the fastness of colour peculiar to acid dyes, has a limited application. But by treatment with sulphuric acid magenta is converted into an acid magenta, and this dye has wider application than the basic salt. Similarly the basic dye called aniline blue is insoluble in water, and therefore has only a limited use, but by treatment with sulphuric acid it is converted into alkali blue,[Pg 202] soluble blue and so on, which dissolve readily in water and are good fast colours. The acid dyes generally have a weaker colouring power than the basic dyes, but they produce very even shades.
| Colour. | Acid. | Basic. | Substantive. |
| Yellow | Metanil yellow. | Auramine. | Cotton yellow. |
| and | Paper yellow. | Chrysoidine. | Chrysophenine. |
| Orange. | Orange II. | ||
| Naphthol yellow S. | |||
| Quinoline yellow. | |||
| Red. | Fast red A. | Rhodamine. | Congo red. |
| Cotton scarlet. | Paper scarlet. | Benzopurpurin. | |
| Erythrine. | Safranine. | Oxamine red. | |
| Ponceau. | Magenta. | ||
| Blue | Water blue 1 N. | Methylene blue. | Azo blue. |
| and | Fast blue. | Victoria blue. | |
| Violet. | Acid violet. | New blue. | |
| Indoine blue. | |||
| Methyl violet. | |||
| Crystal violet. | |||
| Brown | Naphthylamine brown. | Bismarck brown. | |
| Vesuvine. | |||
| Black | Nigrosine. | Coal Black B. | |
| Brilliant black B. | |||
| Green | Diamond green. | ||
| Malachite green. | |||
The difference in the composition of the basic and acid dyes is taken advantage of in the dyeing of paper pulp to secure a complete distribution of the colouring matter upon the pulp, with the result that the intensity of colour is increased, its fastness strengthened, and the process of dyeing generally rendered more economical. This is effected by the judicious addition of a suitable acid dye to the pulp already coloured with the basic dye.
The direct colouring matters have but a very limited application for paper dyeing owing to their sensitiveness to acids and alkalies.
In the colouring of paper pulp, attention is given to many important details, such as:—
Fading of Colour.—Some loss of colour almost invariably occurs even with dyes generally looked upon as fast to light. The shade or tint of the paper is affected not only by exposure to light, but by contact of the coloured paper with common boards on which it is often pasted. The alkalinity of straw boards, for example, is frequently one source of serious alteration of colour, and the acidity of badly made pastes and adhesives another.
In all such cases, the dyes must be carefully selected in order to obtain a coloured paper which will show a minimum alteration in tint by exposure to light or by contact with chemical substances. This is particularly necessary in coloured wrapping paper used for soap, tea, cotton yarn, and similar goods.
Unevenness of Colour.—The different affinity of the various paper-making fibres for dyes is apt to produce an uneven colour in the finished paper. This is very noticeable in mixtures of chemical wood pulp or cellulose and mechanical wood pulp. The ligno-cellulose of the latter has a great affinity for basic dyes, and if the required amount of dye is added to a beater containing the mixed pulps in an insufficiently diluted form, the mechanical wood pulp becomes more deeply coloured than the cellulose. If the former is a finely ground pulp, the effect is not very noticeable, but if it is coarse, containing a large number of coarse fibres, then the paper appears mottled. The defect is still further aggravated when the paper is calendered, especially if calendered in a damp condition. In that case the strongly coloured fibres of mechanical wood are very prominent.
When dyes have been carelessly dissolved and added to the beating engine without being properly strained,[Pg 204] unevenness of colour may often be traced to the presence of undissolved particles of dye.
Irregular Colour of the two Sides.—Many papers exhibit a marked difference in the colour of the two sides. When heavy pigments are employed as the colouring medium, the under side of the sheet, that is, the side of the paper in contact with the machine wire, is often darker than the top side. The suction of the vacuum boxes is the main cause of this defect, though the amount of water flowing on to the wire, the “shake” of the wire, and the extent to which the paper is sized are all contributory causes. By careful regulation of these varying conditions the trouble is considerably minimised.
The under surface of the paper is not invariably darker than the top surface. With pigments of less specific gravity the reverse is found to be the case. This is probably to be explained by the fact that some of the colouring matter from the under side is drawn away from the paper by the suction boxes, and the pigment on the top side is not drawn away to any serious extent, because the layer of pulp below it acts as a filter and promotes a retention of colour on the top side.
It is interesting to notice that this irregularity sometimes occurs with soluble dyes, as for example in the case of auramine. The decomposition of this dye when heated to the temperature of boiling water is well known, and the contact of a damp sheet of paper coloured by auramine with the surfaces of steam-heated cylinders at a high temperature brings about a partial decomposition of the dye on one side of the paper. Generally speaking, acid dyes are more sensitive to heat than basic dyes.
The presence of china clay in a coloured paper is also an explanation of this irregular appearance of the two sides. China clay readily forms an insoluble lake with basic[Pg 205] dyes, and when the suction boxes on the machine are worked with a high vacuum the paper is apt to be more deeply coloured one side than another.
The Machine Backwater.—Economy in the use of dyes to avoid a loss of the colouring matter in the “backwater,” or waste water from the paper machine, is only obtained by careful attention to details of manufacture on the one hand and by a knowledge of the chemistry of dyeing on the other. The loss is partly avoided by regulating the amount of water used on the machine, so that very little actually goes to waste, and further reduced by ensuring as complete a precipitation of the soluble dye as possible.
The acid dyes generally do not give a colourless backwater, and all pulps require to be heavily sized when acid dyes are used.
The basic dyes are more readily precipitated than the acid dyes, particularly if a suitable mordant is used, even with heavily coloured papers. The addition of an acid dye to pulp first coloured with a basic dye is frequently resorted to as a means of more complete precipitation.
Dyeing to Sample.—The matching of colours has been greatly simplified through the publication of pattern books by the firms who manufacture dyes, in which books full details as to the composition of the paper, the proportion of colour and the conditions for maximum effects are fully set out. The precise results obtained by treating paper pulp with definite proportions of a certain dye, or a mixture of several dyes, is determined by experimental trials. A definite quantity of moist partially beaten and sized pulp, containing a known weight of air-dry fibre, is mixed with a suitable volume of water at a temperature of 80° to 90° F. and the dye-stuff added from a burette in the form of a 1 per cent. solution. If preferred a measured volume of a 1 per cent. solution of the dye can be placed in a mortar,[Pg 206] and the moist pulp, previously squeezed out by hand, added gradually and well triturated with the pestle.
The dyed mixture is then suitably diluted with water, made up into small sheets of paper on a hand mould or a siphon mould, and dried.
The effect of small additions of colour to the contents of a beating engine is frequently examined in a rough and ready way by the beaterman, who pours a small quantity of the diluted pulp on the edge of the machine wire while the machine is running. This gives a little rough sheet of paper very quickly.
The comparison of the colour of a beaterfull of pulp with the sample paper which it is desired to match is also effected by reducing a portion of the paper to the condition of pulp, so that a handful of the latter can be compared with a quantity of pulp from the engine. This is not always a reliable process, especially with papers coloured by dyes which are sensitive to the heat of the paper machine drying cylinders.
Detection of Colours in Papers.—The examination of coloured papers for the purpose of determining what dyes have been employed is a difficult task. With white papers which have been merely toned the proportion of dye is exceedingly small, and a large bulk of paper has to be treated with suitable solvents in order to obtain an extract containing sufficient dye for investigation.
With coloured papers dyed by means of pigments, the colour of the ash left on ignition is some guide to the substance used, a red ash indicating iron oxide, a yellow ash chromate of lead, and so on.
With papers dyed by means of coal tar colours the nature of the colouring matter may be determined by the methods of analysis employed for the examination of textile fibres.
The following hints given by Kollmann will be found useful:—
Tear up small about 100 grammes of paper, and boil it in alcohol, in a flask or a reflux condenser. This must be done before the stripping with water, so as to extract the size which would otherwise protect the dye from the water. Of course the alcohol treatment is omitted with unsized paper. The paper is now boiled with from three to five lots of water, taking each time only just enough to cover the paper. This is done in the same flask after pouring off any alcohol that may have been used, and also with the reflux condenser. The watery extract is mixed with the alcohol extract (if any). Three cases may occur:—(1) The dye is entirely stripped, or very nearly so. (2) The dye is partly stripped, what remains on the fibres showing the same colour as at first or not. (3) The dye is not stripped. To make sure of this the solution is filtered, as the presence in it of minute fragments of fibre deceive the eye as to the stripping action. In the first two cases the mixed solutions are evaporated down to one half on the water bath, filtered, evaporated further, and then precipitated by saturating it with common salt. The dye is thrown out at once, or after a time. It may precipitate slowly without any salt. The precipitated dye is filtered off and dried. To see whether it is a single dye or a mixture, make a not too dark solution of a little of it in water, and hang up a strip of filter paper so that it is partly immersed in the solution. If the latter contains more than one dye they will usually be absorbed to different heights, so that the strip will show bands of different colours crossing it. If it is found that there is only one dye, dissolve some of it in as little water as possible, and mix it with “tannin-reagent,” which is made by dissolving equal weights of tannin and sodium acetate in ten times the weight of either of water. If there is a[Pg 208] precipitate there is a basic dye, if not, an acid dye. In the former case mix the strong solution of the dye with concentrated hydrochloric acid and zinc dust, and boil till the colour is destroyed. Then neutralise exactly with caustic soda, filter, and put a drop of the filtrate on to white filter paper. If the original colour soon reappears on drying, we draw the following conclusions:—
(a) The colour is red; the dye is an oxazine, thiazine, azine, or acridine dye, e.g., safranine. (b) It is orange or yellow; the dye is as in (a), e.g., phosphine. (c) It is green; the dye is as in (a), e.g., azine green. (d) It is blue; the dye is as in (a), e.g., Nile blue, new blue, fast blue, or methylene blue. (e) It is violet; the dye is as in (a), e.g., mauveine. If the original colour does not reappear on drying, but does so if padded with a 1 per cent. solution of chromic acid, we draw the following conclusions:—
(a) The colour is red; the dye is rhodamine or fuchsine, or one of their allies. (b) It is green; the dye is malachite green, brilliant green, or one of their allies. (c) It is blue; the dye is night blue, Victoria blue, or one of their allies. (d) It is violet; the dye is methyl violet, crystal violet, or one of their allies.
If the original colour does not reappear even with chromic acid, it was in most cases a yellow or a brown, referable to auramine, chrysoidine, Bismarck brown, thioflavine, or one of their allies.
If the tannin reagent produces no precipitate, reduce with hydrochloric acid and zinc, or ammonia and zinc, and neutralise and filter as in the case of a basic dye. The solution when dropped on to white filter paper may be bleached (a), may have become a brownish red (b), may have been imperfectly and slowly bleached (c), or may have undergone no change (d).
(a) If the colour quickly returns the dye is azurine,[Pg 209] indigo-carmine, nigrosine, or one of their allies. If it returns only on padding with a 1 per cent. solution of chromic acid, warming, and holding over ammonia, some of the dye is dissolved in water mixed with concentrated hydrochloric acid, and shaken up with ether. If the ether takes up the dye, we have aurine, eosine, erythrine, phloxine, erythrosine, or one of their allies. If it does not, we have acid fuchsine, acid green, fast green, water blue, patent blue, or one of their allies. If the colour never returns, heat some of the dye on platinum foil. If it deflagrates with coloured fumes, the dye is aurantia, naphthol yellow S., brilliant yellow, or one of their allies. If it does not deflagrate, or very slightly, dissolve a little of the dye in one hundred times its weight of water, and dye a cotton skein in it at the boil for about fifteen minutes. Then rinse and soap the skein vigorously. If the dyeing is fast with this treatment we have a substantive cotton yellow or thiazine red; if it is not, we have an ordinary azo dye. (b) The dye is an oxyketone, such as alizarine. (c) The dye is thiazol yellow, or one of its allies. (d) The dye is thioflavine S., quinoline yellow, or one of their allies.
If the dye is not stripped by alcohol and water, it is either inorganic or an adjective dye, such as logwood black, cutch, fustic, etc.; and we proceed according to the colour as follows:—
If it is red or brown, the dyed fibre is dried and divided into two parts. One is boiled with bleaching powder. If it is bleached entirely or to a large extent, the dye is cutch. If the bleach has no action, incinerate some of the dyed fibre in an iron crucible and heat the ash on charcoal before the blowpipe. If a globule of lead is formed, we have saturn red. The second portion is boiled with concentrated hydrochloric acid. If there is no action, we have Cologne[Pg 210] umber; if there is partial action, we have real umber; if the dye dissolves completely to a yellow solution, we have an ochre; if the solution is colourless instead of yellow, and chlorine is evolved during solution, we have manganese brown.
If the colour is yellow or orange, boil with concentrated hydrochloric acid. If we get a green solution and a white residue, we infer chrome yellow or orange. If we get a yellow solution, we boil it with a drop or two of nitric acid and then add some ammonium sulphocyanide. A red colour shows an ochre or Sienna earth.
If the colour is green, boil with caustic soda lye. If the fibre turns brown, we have chrome green. If no change takes place, boil with concentrated hydrochloric acid. A yellow solution shows green earth; a red colour logwood plus fustic.
If the colour is blue or violet, boil with caustic soda lye. If the fibre turns brown, we have Prussian blue. If no change takes place, boil with concentrated hydrochloric acid. A yellow solution shows smalts. If the colour is destroyed, and the smell of rotten eggs is developed, we have ultramarine.
If the colour is black, warm with concentrated hydrochloric acid containing a little tin salt. If the black is unchanged, we have a black pigment. If we get a pink to deep red solution we have logwood black.
By means of the tests above detailed at length the group to which the dye belongs is discovered, and often the actual dye itself. Once the group is known it is generally easy, by means of the special reactions given in many books, e.g., in Schultz and Julius's “Tabellarische Übersicht,” to identify the particular dye.
When one has to deal with a single dye and simply desires to determine its group, the following table, due to[Pg 211] J. Herzfeld, will suffice. Originally intended for textiles, it will serve, with some modifications here made in it, for the rapid testing of paper.
Boil the paper with a mixture of alcohol and sulphate of alumina. If no dye is extracted or a fluorescent solution is formed, we have an inorganic pigment, or eosine, phloxine, rhodamine, safranine, or one of their allies. Add bleaching powder solution, and heat. If the paper is bleached, add concentrated hydrochloric acid. A violet colour shows safranine or an analogue. If there is no colour, but the fluorescence disappears, we have eosine, phloxine, rhodamine, or one of their allies. If the paper is not bleached test for inorganic colouring matters. Cutch brown is partly but not entirely bleached.
If the alumina solution gives a red or yellow solution without fluorescence, add to it concentrated sodium bisulphite. If bleaching takes place, heat a piece of the paper with dilute spirit. A red extract shows sandal wood, fuchsine, etc. If there is little or no extract, we have acid fuchsine or one of its allies. If the bisulphite causes no bleaching, boil a piece of the paper with very dilute hydrochloric acid. If the colour is unchanged, heat another piece of the paper with dilute acetate of lead. If no change takes place, we have an azo dye. If the colour turns to a dark brownish red, we have cochineal or the like. If the boiling with very dilute hydrochloric acid darkens the colour we have a substantive cotton dye.
Heat some of the paper with a not too dilute solution of tin salt in hydrochloric acid. If the colour is unchanged,[Pg 212] with a colourless or yellow solution, boil some more paper with milk of lime. A change to reddish or brown shows turmeric or a congener. Absence of change shows phosphine, quinoline yellow, or a natural dye-stuff. If the acid tin solution turns the paper red, and then quickly bleaches it to a pale yellow, we have fast yellow, orange IV., metanil yellow, brilliant yellow, or the like. If the tin turns the paper greyish, heat another portion with ammonium sulphide. A blackening shows a lead or iron yellow. If there is no change, we have naphthol yellow, auramine, azoflavine, orange II., chrysoidine, or one of their allies.
Heat a sample of the paper in dilute spirit. If the spirit acquires no colour, warm for a short time with dilute sulphuric acid. If both paper and solution become brownish red, we have logwood plus fustic. If this fails, boil with concentrated hydrochloric acid. A yellow solution shows green earth. If this fails, boil with concentrated caustic soda. Browning shows chrome green. If the spirit becomes blue, it is a case of paper which has been topped with blue on a yellow, brown, or green ground. The solution and the insoluble part are separately tested. The case is probably one of an aniline blue dyed over a mineral pigment. If the spirit becomes green, heat with dilute hydrochloric acid. If the fibre is completely or nearly bleached, and the acid turns yellow, the dye is brilliant green, malachite green, or one of their allies.
Heat some of the paper with dilute spirit. If the alcohol remains colourless, we have Prussian blue or ultramarine. If it becomes blue or violet, shake some of the paper with[Pg 213] concentrated sulphuric acid. A dirty olive green shows methylene blue, and a brownish colour shows spirit blue, water blue, Victoria blue, methyl violet, etc. If the spirit turns yellow, and the colour of the paper changes, we have wood blue or wood violet.
In the case of common printings and writings, which form the great bulk of the paper made, the possibility of one mill competing against another, apart from the important factor of the cost of freight, coal, and labour, is almost entirely determined by the economy resulting from the introduction of modern machinery.
The equipment of an up-to-date paper mill, therefore, comprises all the latest devices for the efficient handling of large quantities of raw material, the economical production of steam, and the minimum consumption of coal, matters which are of course common to most industrial operations, together with the special machinery peculiar to the manufacture of paper.
The amount of material to be handled may be seen from the table on page 215, which gives the approximate quantities for the weekly output of a common news and a good printing paper.
Economy in Coal Consumption.—The reduction to a minimum of the amount of coal required for a ton of paper has been brought about by the use of appliances for the better and more regular combustion of the coal, such as mechanical stokers, forced and induced draught, the introduction of methods for utilising waste heat in flue gases by economisers, and the waste heat in exhaust steam and condensed water by feed-water heaters, the adoption of machines for securing the whole energy of the live steam[Pg 215] by means of superheaters, adequate insulation of steam mains and pipes, high pressure boilers, and engines of most recent design.
The firing of steam boilers is now conducted on scientific principles, the coal being submitted regularly to proper analysis for calorific value, the evaporative power of the boilers being determined at intervals by adequate trials, the condition of the waste flue gases being automatically
Table showing the Materials required for News and Printings.
| — | Common News. | Good Printings. | ||
| Weekly output of paper, say | 600 | tons | 250 | tons |
| Mechanical wood pulp, moist, 50 per cent. dry | 800 | „ | Nil. | |
| Chemical wood pulp, dry | 200 | „ | 150 | tons |
| Esparto | Nil. | 200 | „ | |
| Soda ash | Nil. | 16 | „ | |
| Coal | 600 | tons | 800 | „ |
| Lime | Nil. | 45 | „ | |
| China clay | 60 | tons | 25 | „ |
| Bleach | Nil. | 30 | „ | |
| Alum, rosin, and chemicals | 20 | tons | 20 | „ |
| Water, per ton paper | 8,000 | gallons | 40,000 | gallons |
The Sarco Combustion Recorder.—This instrument is a device which automatically records the percentage of carbonic acid gas in the waste gases from boiler furnaces. The flue gases are analysed at frequent and regular intervals, and the results of the analysis can be seen on a chart immediately, so that it is possible to determine the effect of an alteration in the firing of the boilers within two minutes of its taking place. The apparatus is rather complicated, but the principle upon which it is based is simple.
Measured quantities of the flue gases are drawn into graduated glass tubes and brought into contact with strong caustic soda solution, which absorbs all the carbonic acid gas. The remaining gases not absorbed by the caustic soda are automatically measured and the percentage of carbonic acid gas registered on the chart.
The use of suitable boiler feed-water is also an important factor in modern steam-raising plant. The hot condensed water from the paper machine drying cylinders, and exhaust steam from the engines and steam-pipes, is returned to the stoke-hole to be utilised in heating up the cold water which has been previously softened by chemical treatment.
Fig. 55.—Conventional Diagram of a Water Softening Plant.
A. Water supply.
B. Regulating tank.
C. Lime mixer.
D. Soda tank.
E. Settling tank and filter.
F. Outlet for softened water.
Water Softening.—The water softeners available on the market are numerous, and as each possesses special advantages of its own, it would be almost invidious to select any one for particular notice.
They are based upon the principle of mixing chemicals with the water to be treated, so as to precipitate the matters in solution and give a boiler feed-water free from carbonates and sulphates of lime and magnesia. The chemicals are[Pg 217] added in the form of solutions of carefully regulated strength to the water, which flow in a continuous stream into a tank. The flow of the water and chemical reagent is adjusted by previous analysis.
The various machines differ in details of construction, and in the methods by which the mixing of the water and reagents is effected. The object to be achieved is the complete precipitation of the dissolved salts and the production of a clear water, free from sediment, in an apparatus that will treat a maximum quantity of water at a cheap rate per 1,000 gallons.
The process needs proper attention. The addition of reagents in wrong proportions will do more harm than good, and possibly result in hardening the water instead of softening it. The following may be quoted as an example:—
| Composition of Water. | Before Treatment. | After Treatment. | Change. | |
| Calcium carbonate | 13·863 | 38·920 | 25·057 | gain |
| Calcium oxide (lime) | 0·0 | 14·300 | 14·300 | „ |
| Calcium silicate | 2·062 | 3·591 | 1·529 | „ |
| Calcium sulphate | 1·625 | 2·121 | 0·496 | „ |
| Magnesia | 0·0 | 0·266 | 0·266 | „ |
| Ferric oxide, etc. | 0·447 | 0·987 | 0·540 | „ |
| Scale forming mineral | 17·997 | 60·185 | 42·188 | gain |
| Calcium chloride | 1·331 | 2·114 | 0·783 | gain |
| Magnesium chloride | 0·672 | 0·0 | 0·672 | loss |
| Sodium chloride | 0·478 | 0·476 | 0·003 | „ |
| Soluble salts | 2·482 | 2·590 | 0·108 | gain |
| Total mineral matter | 20·479 | 62·776 | 42·297 | gain |
| Carbonic acid gas | 9·71 | 0·0 | 9·71 | loss |
| Oxygen gas | 0·66 | 0·66 | 0·0 | „ |
Treatment required: 1·8 lbs. of lime, 0·2 lbs. soda ash per 1,000 gallons. Apparently 5·5 lbs. of lime were being used and no soda (Stromeyer).
Superheated Steam.—The effective application of the energy of the high pressure steam is probably one of the most important problems in paper mill economy. The use of superheated steam is being extended in every direction, and, in addition to the advantages obtained in the steam engine itself, its wider possibilities for the boiling of esparto, wood, and fibres generally have been noted. The following case may be quoted as the result of a trial at a paper mill, showing for stated conditions the advantages of superheated steam:—
| — | Superheated Steam. | Ordinary Steam. |
| Duration of test hours | 26 | 34 |
| Coal consumed (lbs.)— | ||
| Per hour | 610·5 | 661·5 |
| Per 1 h.-p. hour | 1·83 | 2·08 |
| Water evaporated (lbs.)— | ||
| Per hour | 4,832 | 5,679 |
| Per 1 h.-p. hour | 14·55 | 17·8 |
| From and at 212° F. | 8·7 | 8·94 |
| Steam, temperature F. | 464 | 334 |
| Pressure | 90·3 | 90·8 |
| Steam engine— | ||
| 1 h.-p. total | 331·5 | 323·2 |
| Temperature F. | 381·8 | 333·8 |
| Coal used per 1 h.-p.— | ||
| Per hour at boiler | 1·83 | 2·08 |
This appears to show a saving of 12 per cent.
Gas Producers.—The substitution of gas for steam in the paper mill has not yet proved a success. The fact that heat is required for the drying cylinders of a paper machine, and that the heat is most cheaply and readily obtained in the form of exhaust steam from the engines driving the paper machine, militates considerably against economies which might otherwise be possible. The difficulties of heating[Pg 219] such cylinders, or rather of properly controlling and regulating the temperature by any other means than steam, may easily be surmised.
Gas engines of over 200 h.-p. seem to give considerable trouble at present, but no doubt in course of time the required improvements will be effected.
It is generally supposed that gas producers can only be economical when utilised for the production of gas on a large scale, and for distribution to engines of smaller capacity than the main steam engine required in a paper mill. The peculiar conditions of the manufacture of paper do not appear to be favourable to the adoption of the gas producer system in its present form.
Motive Power.—The paper-maker has taken advantage of every modern improvement in steam engines for the purpose of reducing the cost of motive power. Amongst other alterations in this direction the use of a high speed enclosed engine and the employment of the modern steam turbine may be noted.
In the enclosed engine the working parts are boxed in by a casing fitted with oil-tight doors. The cranks and connecting rods splash into the oil, which is thus thrown about in all directions, so as to ensure sufficient lubrication. Another feature of this engine is the variable speed, and it is possible to run the paper machine at speeds varying from 100 to 500 ft. per minute without the use of change wheels.
Electrical Driving.—The application of electricity for motive power has made steady advances in the paper mill. At first it was limited to the driving of machinery in which variations of speed or load were not required to any large extent, but of recent years beating engines, calenders, and paper machines have all been fitted with electrical drives.
The following details relate to the installation at the Linwood Paper Mills:—
The installation consists of 250-K.W. steam dynamos. The engines are Willan's high speed triple expansion, working with a boiler pressure of 250 lbs. per square inch at the stop valve, the steam being superheated to give a temperature of 500° Fahr. at the engine. By means of jet condensers a vacuum of 25 to 25½ inches is obtained on the engines. The two boilers are of the Babcock type, and have 3,580 square feet of heating surface each. The furnaces have chain grate stokers, and the boilers are arranged with their own superheaters. The motor equipment consists of eight 80, two 50, and ten 25 B.H.P. motors.
Six of the 80 B.H.P. drive the beating engines, and it has been found that the motors readily respond to an overload of 50 per cent. without beating or other trouble. To remedy the excessive and sudden variation a belt drive was adopted. An 80 motor drives the pulp refining engine. The two paper-making machines have each two motors, one a 25 and a 50 and the other two 25 B.H.P. motors. The speed can be regulated with exactitude. The auxiliary plant of the paper-making machine, pumps, agitators, etc., is worked from lines of shafting driven by motors.
Calender motors are of the variable speed type, being designed to run from 100 revolutions per minute to 600 revolutions per minute. Variations from 300 to 600 revolutions per minute can be regulated by the shunts, the loss being negligible. Several of the motors are geared up to the various machines, as is the case with the calender.
As regards cost, the capital outlay on the 500-K.W. generating plant, including engines, dynamos, boilers, condensers, steam pipes, filters, etc., and all engine room accessories, was £9,500.
In addition to the above, the plant also contains a Parson's[Pg 223] steam turbine of 1,000 K.W., driving two continuous current dynamos.
The Eibel Patent.—One of the most important improvements in connection with the manufacture of newspaper is the Eibel process, designed to increase the speed of the machine and to reduce the amount of suction at the vacuum box. In the ordinary machine the wire has usually been arranged to move in a horizontal plane. In some machines means have been provided for adjusting the breast-roll end of the wire to different elevations to provide for dealing with different grades of stock, but the wire has never hitherto been so inclined as to cause the paper stock to travel at a speed, under the action of gravity, to equal or approximate the speed of the wire. In all previous methods of working, the wire has for a considerable portion of its length, starting from the breast-roll, drawn the stock along in consequence of the wire moving much faster than the stock, and the stock has waved, or rippled, badly near the breast-roll end of the wire. This has gradually diminished until an equilibrium has been established and an even surface obtained, but not until the waving or rippling has ceased at some considerable distance from the breast-roll have the fibres become laid uniformly, and the machines have therefore necessarily been run slowly to give ample time for the water to escape and for the fibres to lie down so as to make them a uniform sheet. In many cases the breast-roll has[Pg 224] been raised 14 or 15 inches, and the stock rushes, as it were, downhill.
As, during the formation of the paper, the stock and the wire practically do not move relatively to each other, there is no drag of the stock upon the wire; consequently there is a more rapid and uniform drainage of the water from the stock, the full influence of the “shake” is made effective to secure uniformity in the distribution and interlocking of the fibres, and the regularity of the formation of the paper is not disturbed by waves or currents, which would otherwise be caused by pull of the wire upon the stock.
This ingenious device is now working successfully in many paper mills.
Machinery.—In setting out the plant necessary for a paper mill which is designed to produce a given quantity of finished paper, the manufacturer takes into consideration the class of paper to be made and the raw material to be employed. The following schedule has been prepared on such a basis:—
Paper.
High-class printings made of wood pulp and esparto, used alone or blended in varying proportions as required. Quantity, 250 tons weekly.
Raw Material.
Esparto; chemical wood pulp.
Quantity: esparto, about 200 tons; wood pulp, 150 to 160.
China clay and usual chemicals.
In the estimation of materials required for the production of about 250 tons of paper, it is assumed that the 200 tons of esparto fibre will yield 90 tons bleached esparto fibre, and[Pg 225] that the mechanical losses which take place during manufacture are counterbalanced by the weight of china clay added to the pulp. These conditions naturally vary in different mills, but such variations do not affect the schedule of machinery.
Unloading Sheds.
2 steam or electric cranes for handling fibre, clay, alum, bleach, rosin, coal, and finished paper.
1 3-ton weighbridge.
1 5-cwt. platform scales.
Steam Plant.
6 8-ft. by 30-ft. Lancashire boilers.
Fuel economiser.
Feed-water pump and tank.
Water softening apparatus.
1 500-h.-p. main steam engine, for fibre departments and beater floor.
Chemical Department.
Hoist for clay, alum, bleach, lime, &c.
4 causticising pans, 9 ft. diameter, 9 ft. deep.
2 storage tanks.
2 chalk sludge filter presses.
2 clay-mixing vats, 6 ft. diameter, 6 ft. deep.
1 starch mixer, 6 ft. diameter, 6 ft. deep.
1 size boiler, 8 ft. diameter, 8 ft. deep.
3 size storage tanks, 1,000 gallons each.
3 bleach-mixing vats.
3 bleach liquor settling tanks.
2 clear bleach liquor storage tanks.
1 alum dissolving tank.
Recovery Department:—
Soda.
1 multiple effect evaporating plant.
1 rotary furnace.[Pg 226] 4,2]
4 lixiviating tanks, 2,000 gallons each.
2 storage tanks for clear liquor from lixiviating tanks, 20,000 gallons capacity.
Fibre.
2 tanks for receiving machine backwater.
2 Fullner's stuff catchers, or some other system of treating backwater.
2 filter presses.
Esparto Department.
1 esparto duster.
Travelling conveyer for cleaned esparto.
6 Sinclair vomiting boilers, each of 3 tons capacity.
2 measuring tanks for caustic liquor.
4 washing engines, 15 cwt. capacity.
6 Tower bleaching engines.
1 presse-pâte.
10 galvanised iron trucks.
Wood Pulp Department.
4 pulp disintegrators and pumps.
4 Tower bleaching engines.
4 washing tanks or drainers.
6 galvanised iron trucks.
Beater Floor.
8 1,200-lbs. beating engines.
2 Marshall refiners.
6 galvanised iron trucks.
Paper Machine Room.
2 paper machines, 106 in. wide, with stuff chests, strainers, and engines complete.
1 paper machine, 120 in. wide, with stuff chests, strainers, and engines complete.
Patent dampers for each machine.
Calendering Room.
2 110-in. supercalenders. 4,2]
2 100-in. supercalenders.
2 6-reel cutters.
1 200-h.-p. main steam engine.
Finishing Room.
Sorting tables.
Packing press.
Weighing machine.
Repairs Department.
Usual repair outfit, such as lathes, planing machine, drilling tools, etc.
Blacksmith's shop outfit.
Carpenter's shop outfit.
Calender roll grinder.
Water Supply.
Main storage tank, 50,000 gallons capacity.
Water pumps.
Piping and connections to various departments.
Bell's patent filters (if necessary).
Recent complaints about the quality of paper and the rapid decay of manuscripts and papers have resulted in arousing some interest in the subject of the durability of paper used for books and legal documents, and in the equally important question of the ink employed. The Society of Arts and the Library Association in England and the Imperial Paper Testing Institute in Germany have already appointed special committees of inquiry, and from this it is evident that the subject is one of urgent importance.
It is sometimes argued that the lack of durability is due to the want of care on the part of manufacturers in preserving the knowledge of paper-making as handed down by the early pioneers, but such an argument is superficial and utterly erroneous. The quality of paper, in common with the quality of many other articles of commerce, has suffered because the demand for a really good high-class material is so small. The general public has become accustomed to ask for something cheap, and since the reduction in price is only rendered possible by the use of cheap raw material and less expensive methods of manufacture, the paper of the present day, with certain exceptions, is inferior to that of fifty years ago.
The causes which favour the deterioration of paper are best understood by an inquiry into the nature of the fibres and other materials used and the methods of manufacture employed.
The Fibres Used.—Cotton and linen rags stand preeminent amongst vegetable fibres as being the most suitable for the production of high-class paper capable of withstanding the ravages of time. This arises from the fact that cotton and linen require the least amount of chemical treatment to convert them into paper pulp, since they are almost pure cellulose, cotton containing 98·7 per cent. of air-dry cellulose, and flax 90·6 per cent. The processes through which the raw cotton and flax are passed for the manufacture of textile goods are of the simplest character, and the rags themselves can be converted into paper without chemical treatment if necessary. As a matter of fact certain papers, such as the O. W. S. and other drawing papers, are manufactured from rags without the aid of caustic soda, bleach, or chemicals. The rags are carefully selected, boiled for a long time in plain water, broken up and beaten into pulp, and made up into sheets by purely mechanical methods.
The liability of papers to decay, in respect of the fibrous composition, is almost in direct proportion to the severity of the chemical treatment necessary to convert the raw material into cellulose, and the extent of the deviation of the fibre from pure cellulose is a measure of the degradation which is to be expected. The behaviour of the fibres towards caustic soda or any similar hydrolytic agent serves to distinguish the fibres of maximum durability from those of lesser resistance. It may be noted that in the former the raw materials, viz., cotton, linen, hemp, ramie, etc., contain a high percentage of pure cellulose, while in the latter the percentage of cellulose is very much lower, such fibres as esparto, straw, wood, bamboo, etc., giving only 40-50 per cent. of cellulose. The two extremes are represented by pure cotton rag and mechanical wood pulp. Other things being equal, the decay which may[Pg 230] take place in papers containing the fibre only, without the admixture of size or chemicals, may be considered as one of oxidation, which takes place slowly in cotton, and much more rapidly with mechanical wood pulp. Experimental evidence of this oxidation is afforded when thin sheets of paper made from these materials are exposed to a temperature of 100° to 110° C. in an air oven. The cotton paper is but little affected, while the mechanical wood pulp paper soon falls to pieces.
The order of durability of various papers in relation to the fibrous constituents may be expressed thus: (1) rag cellulose; (2) chemical wood cellulose; (3) esparto, straw, and bamboo celluloses; (4) mechanical wood pulp. The rate and extent of oxidation is approximately shown by the effect of heat as described. The differences between the celluloses are also shown by heating strips of various papers in a weak solution of aniline sulphate, which has no effect on wood or rag cellulose, dyes esparto and straw a pinkish colour, and imparts a strong yellow colour to mechanical wood pulp and jute.
Physical Qualities.—The permanence of a paper depends not only upon the purity of the fibrous constituents and the freedom from chemicals likely to bring about deterioration, but also upon the general physical properties of the paper itself. Other things being equal, the more resistant a paper is to rough usage the longer will it last. The reason why rag papers are so permanent is that not only is the chemical condition of the cellulose of the highest order, but the physical structure of the fibre is such that the strength of the finished paper is also a maximum.
The methods of manufacture may be modified to almost any extent, giving on the one hand a paper of extraordinary toughness, or on the other hand a paper which falls to pieces after a very short time. Thus a strong bank-note[Pg 231] paper may be crumpled up between the fingers three or four hundred times without tearing, while an imitation art paper is broken up when crumpled three or four times.
A thorough study of the physical qualities of a paper is therefore necessary to an appreciation of the conditions for durability. The physical structure of the fibre, the modifications produced in it by beating, the effect of drying, sizing, and glazing upon the strength and elasticity of the finished paper, are some of the factors which need to be considered.
Strength.—The strength of a paper as measured by the tensile strain required to fracture a strip of given width, and the percentage of elongation which the paper undergoes when submitted to tension, are properties of the utmost importance. The elasticity, that is, the amount of stretch under tension, has not received the attention from paper-makers that it deserves. If two papers of equal tensile strength differ in elasticity, it may be taken for granted that the paper showing a greater percentage of elongation under tension is the better of the two.
The strength of a paper, as already indicated, is greatly influenced by the conditions of manufacture. This has been explained in the chapter devoted to the subject of beating, and other examples are briefly given in the following paragraphs.
Bulk.—The manufacture during recent years of light bulky papers for book production has accentuated the problem in a marked degree, and the factor of bulk as one of the causes of deterioration is therefore a comparatively new one. It is interesting to notice that the rapid destruction of such books by frequent use is in no way related to the chemical purity of the cellulose of which it is composed, or to the influence of any chemical substance associated with the fibre. It is purely a mechanical question, to be explained by reference to the process of manufacture.
This paper is made from esparto entirely, or from a mixture of esparto and wood pulp. The pulp is beaten quickly, and for as short a time as possible, little or no china clay being added, and only a very small percentage of rosin size. The wet sheet of paper is submitted to very light pressure at the press rolls, and the bulky nature is preserved by omitting the ordinary methods of calendering.
The paper thus produced consists of fibres which are but little felted together. The physical condition and structure of the paper are readily noticeable to the eye, and when these peculiarities are reduced to numerical terms the effect of the conditions of manufacture is strikingly displayed.
The effect of this special treatment is best seen by contrasting the bulky esparto featherweight paper with the normal magazine paper made from esparto. In the latter case a smoother, heavier, stronger sheet of paper is made from identically the same raw material. But the pulp is beaten for a longer period, while mineral matter and size are added in suitable proportions. The press rolls and calenders are used to the fullest extent.
The difference between these two papers, both consisting, as they do, of pure esparto with a small proportion of ash may be emphasised by comparing the analysis by weight with analysis by volume. The two papers in question when analysed by weight proved to have the following composition:—
| — | Parts by Weight. | |
| Featherweight. | Ordinary. | |
| Esparto fibre | 96·0 | 95·4 |
| Ash, etc | 4·0 | 4·6 |
| —— | —— | |
| 100·0 | 100·0 | |
But if the papers are compared in terms of the composition by volume, it will be found that the featherweight contains a large amount of air space.
| — | Composition by Volume. | |
| Featherweight. | Ordinary. | |
| Esparto fibre | 28·0 | 65·5 |
| Ash, etc | 0·7 | 1·8 |
| Air space | 71·3 | 32·7 |
| ——- | —— | |
| 100·0 | 100·0 | |
In other words, the conditions of manufacture for the bulky paper are such that the fibres are as far apart from one another as possible, and the cohesion of fibre to fibre is reduced to a minimum.
While paper of this description is agreeable to the printer, and probably to the general reading public, yet its strength and physical qualities, from the point of view of resistance to wear and tear, are of the lowest order. It is very difficult to rebind books made from it, which is not altogether to be wondered at, seeing that the bookbinder's stitches can hardly be expected to hold together sheets containing 60 to 70 per cent. of air space.
This concrete case emphasises the necessity for including in a schedule of standards of quality a classification of papers according to strength and bulk.
Surface.—The introduction of new methods of printing has brought about some changes in the process of glazing and finishing paper which are not altogether favourable to the manufacture of a sheet having maximum qualities of strength and elasticity, two conditions which are essential[Pg 234] to permanence. In other words, the very high finish and surface imparted to paper by plate-glazing, supercalendering, water finish, and other devices of a similar character is carried to excess.
All papers are improved in strength by glazing up to a certain point, but over-glazing crushes the paper, renders it brittle and liable to crack. Unfortunately, the maximum strength of a paper is generally reached before the maximum of finish, with the result that the former is frequently sacrificed to the latter. The usual result of glazing is found in an increase of 8 to 10 per cent. in the tensile strength, but a diminution of elasticity to the extent of 8 to 10 per cent. With supercalendered magazine papers, the high surface is imparted for the sake of the illustrations which are produced by methods requiring it. The addition of considerable quantities of clay or mineral substances improves the finish, so that the question of the relation of glazing to strength, surface, and loading is one which affects the subject of deterioration of paper very materially. With writing paper the false standard of an “attractive” appearance is almost universally accepted by the public as the basis of purchase without any reference to actual quality.
Mineral Substances.—China clay, sulphate of lime, agalite and other inert mineral substances are important factors in lowering the quality of paper, not so much in promoting the actual deterioration of paper by any chemical reaction with the fibres, as in making the paper less capable of resistance to the influence of atmospheric conditions and ordinary usage. Clay in small, well-defined quantities serves a useful purpose, if added to some papers, because it favours the production of a smooth surface, but when the combination of mineral substances is carried to an extreme, then the result from the point of view of permanence is disastrous. This is well recognised by all[Pg 235] paper-makers, and in Germany the limits of the amount of clay or loading in high-grade paper have been rigidly fixed. In the case of imitation art paper, which contains 25 to 30 per cent. of its weight of clay, the strength and resistance of the sheet is reduced to a minimum. The paper falls to pieces if slightly damped, the felting power of the fibres being rendered of no effect owing to the weakening influence of excessive mineral matter. This paper is used chiefly for catalogues, programmes, circulars, and printed matter of a temporary and evanescent character, and so long as it is confined to such objects it serves a useful purpose, being cheap, and suitable for the production of illustrations by means of the half-tone process; but its lasting qualities are of the lowest order. The addition of 10 per cent. of any mineral substance must be regarded as the maximum allowance for papers intended for permanent and frequent use.
Coating Material.—The ingenious method for producing an absolutely even surface on paper by the use of a mixture of clay or other mineral substance and an adhesive like glue or casein brushed on to the surface of the paper, is responsible for many of the complaints about the papers of the present day.
The sole merit of this substance is the facility with which half-tone process blocks can be utilised for the purpose of picture production. Beyond this, nothing can be said. The paper is brittle, susceptible to the least suspicion of dampness, with a high polish which in artificial light produces fatigue of the reader's eye very quickly, heavy to handle, and liable to fall to pieces when bound up in book form.
As the fibrous material is completely covered by mineral substances, it is frequently considered of secondary importance, with the result that the “value” of the paper is[Pg 236] judged entirely by the surface coating, with little regard to the nature of the body paper. In such cases, with an inferior body paper, the pages of a book very quickly discolour, and the letterpress becomes blurred.
Analysis of a Typical Art Paper.
| — | Per Cent. by Weight. | — | Volume Composition per Cent. |
| Fibre | 77·5 | Fibre | 68·3 |
| Ash, etc. | 22·5 | Ash | 12·0 |
| Air space | 19·7 | ||
| —— | —— | ||
| 100·0 | 100·0 | ||
Rosin.—The presence of an excess of rosin is a well-known factor in the disintegration of the paper, even when the fibrous composition is of the highest order. The decomposition is largely due to the action of light, many experiments having been made by Herzberg and others to determine the nature of the reactions taking place. One of the chief alterations is the change brought about in the ink-resisting qualities of the paper.
The actual character of the chemical reactions as far as the effect on the fibre is concerned is not accurately known. The degradation of a hard-sized rosin paper by exposure to strong sunlight, for example, is probably due to the alteration in the rosin size, and not to any material change in the cellulose. It is hardly conceivable that in a pure rag paper sized with rosin and yielding readily to ink penetration, after about one year's exposure to light, the cellulose itself had undergone any chemical changes capable of detection.
Gelatine.—Papers properly sized with gelatine are preferable to those sized with rosin for the majority of books and documents preserved under normal circumstances. But the nature of a tub-sized paper may be, and often is, greatly altered by unusual climatic conditions. In hot, damp countries papers are quickly ruined, and high-class drawing papers sized with gelatine often rendered useless. The change is scarcely visible on the clean paper, and is only observed when the paper is used for water-colour work, the colour appearing blotchy in various parts of the sheet where the gelatine has been decomposed by the united action of heat and damp.
The artist is frequently compelled in such cases to put a layer of heavy white colour on the sheet of paper before proceeding to paint the picture.
The storage of books under favourable conditions has a great deal to do with the permanence of the paper, and the degradation of a paper in relation to the tub-sizing qualities is much hastened by the presence of moisture in the air.
Starch.—The same is true of starch, which is largely employed as a binding or sizing material in paper. The degradation of gelatine, starch, and similar nitrogenous substances is due to the action of organisms, and the following experiments, suggested by Cross, are interesting in this connection.
If strips of paper are put into stoppered bottles with a small quantity of warm water and kept at a temperature of about 80° F., fungus growths will be noticed on some of them after the lapse of fourteen days. Rag papers sized with gelatine will show micro-organisms of all kinds. A pure cellulose paper, like filter paper, will not produce any such effects. The result in the first case is due to the nitrogenous substance, viz., the gelatine used in sizing,[Pg 238] since the two papers are identical as far as the cellulose fibres are concerned. High-class wood pulp papers, unless sized with gelatine, would not show similar results. The action of the organisms upon the nitrogenous material by a process of hydrolysis is in the direction of the production of soluble compounds allied to the starch sugars capable of being assimilated by organisms.
The cellulose of esparto and straw are readily attacked, and it is on this account that the tissues of the various straws are digested more or less when eaten by animals. It is for this reason that the celluloses from straw and esparto are inferior to the cotton cellulose in producing a paper likely to be permanent.
Chemical Residues.—The necessity for manufacturing a pure cellulose half-stuff is fully recognised by paper-makers. This was not the case in the early days of the manufacture of wood pulp, for it is a matter of common experience that many of the books printed on wood pulp paper between 1870 and 1880 are in a hopeless condition, and it is quite easy to find books and periodicals of that date the pages of which crumble to dust when handled. This serious defect has been proved to be due to the presence of traces of chemicals used in manufacture which have not been thoroughly removed from the pulp.
The precautions necessary in bleaching pulp by means of chloride of lime, in order to prevent (1) any action between the fibre and the calcium hypochlorite, (2) the presence of residual chlorine or soluble compounds derived from it, and (3) the presence of by-products arising from the use of an antichlor, are also well known to paper-makers. The subject has been closely studied by chemists, who have shown that the deterioration of many modern papers may be ascribed to carelessness in bleaching.
The questions relating to the chemical residues of paper[Pg 239] can only be adequately dealt with by a discussion of actual cases which arise from time to time. There are certain conditions in manufacture, common to all papers, which may give rise to the presence of chemical residues, of which two have already been mentioned.
The acidity of papers is frequently quoted as an instance. It is true that the presence of free acid in a paper is most undesirable, as it seriously attacks the cellulose, converting it into an oxidised form. This in course of time renders the paper so brittle as to destroy its fibrous character.
The change is brought about by the acid, which itself suffers no material alteration, so that the process of deterioration is continued almost indefinitely until the cellulose is completely oxidised. Most papers, however, show an acid reaction when tested with litmus, the usual reagent employed by those not familiar with the proper methods of testing paper. All papers which have been treated with an excess of alum for sizing purposes would show an acid reaction with litmus without necessarily containing any free acid.
The presence of iron is undesirable, particularly in photographic papers, and since cellulose has a remarkable affinity for iron, the conditions of manufacture which tend to leave iron in the pulp have to be taken into consideration. The presence of minute quantities of iron in the form of impurities must not be confused with the presence of iron in large quantities derived from the toning and colouring of paper by means of iron salts.
The fading of colour is frequently observed when coloured papers are tested on boxboards, particularly those made of straw. This fading may often be traced to the presence of alkali in the straw board which has not been completely removed in the process of manufacture.
The blurring of letterpress is a defect which often occurs with printing papers made of chemical wood pulp. The oil[Pg 240] in the ink seems to separate out on either side of the letter, producing a discoloration. In such cases the paper itself frequently exhibits an unpleasant smell.
These defects are usually determined by the presence of traces of sulphur compounds in the paper resulting from incomplete washing of the pulp in manufacture. The presence of sulphur compounds sometimes associates itself with papers which have been coloured by means of ultramarine, which in presence of alum is slightly decomposed by the heat of the drying cylinders.
Some knowledge of the effect of chemical residues in paper is important, not only in regard to the deterioration which takes place in the fibre itself, but also in relation to the fading of the ink which is used. The subject of the ink has received much attention from chemists on account of the serious difficulties which have been experienced by State departments in various countries.
The United States Department of Agriculture have devised certain methods for ascertaining the suitability of stamping ink used by the Government and suggest the qualities desirable in such an ink. The ink, first of all, must produce an indelible cancellation; that is, it must be relatively indelible as compared with the ink used for printing the postage stamps. The post-mark made with the ink must dry quickly in order that the mail matter may be handled immediately without any blurring or smearing of the post-mark.
Both this property and the property of the indelibility involve the question of the rate at which the ink penetrates or is absorbed by the fibre of the paper. A satisfactory ink does not harden or form a crust on the ink-pad on exposure to air. There must be no deposition of solid matter on the bottom of the vessel in which the ink is stored, and the pigments on which the indelibility of the ink depends, if[Pg 241] insoluble, must not settle out in such a way as to make it possible to pour off from the top of the container a portion of the ink which contains little or none of the insoluble pigment or pigments.
Colour.—If the subject of deterioration of paper is to be considered in its broadest sense as including changes of any kind, the fading of colour must be taken into account. The use of aniline dyes which are not fast to light results in a loss of colour in paper just as with textiles, and the fading may be regarded as a function of the dye and not as arising from its combination with the paper.
The gradual fading of some dyes, however, and of many water-colour pigments may be traced to the presence of residual chemicals in the paper and to the presence of moisture in an atmosphere impregnated with gaseous or suspended impurities. In fact the latter is a greater enemy to permanence of colour than light, since it has been proved by experiment that most colours do not fade when exposed to light in a vacuum. The oxygen of the air in combination with the moisture present is the principal agent in bringing about such changes. The dulling of bronze, or imitation gold leaf, on cover papers is a practical illustration of this, though this can hardly be quoted as an instance of actual deterioration of the paper.
The maintenance of the original colour can only be assured by the careful selection of pure fibrous material, the use of fast dyes, and the preservation of the book or painting from the conditions which favour the fading as described above. For common papers such precautions become impossible, but for water-colour drawings and valuable papers they are essential.
The demand for an abnormally white paper is indirectly the cause of deterioration in colour, but in this case the ultimate effect is not a fading but a discoloration of white[Pg 242] to a more or less distinct yellow or brown colour, due to changes in the fibre which may often be traced to excessive bleaching. In this case the fading of colour is directly due to deterioration of the paper itself, and may occur in celluloses of the best type. With lower-grade papers containing mechanical wood pulp the degradation of colour and fibre is inevitable.
Air and Moisture.—The exact effects produced on paper freely exposed, or in books as ordinarily stored, depend upon the condition of the atmosphere. Pure air has little or no action upon paper, cellulose being a remarkably inert substance, and even in impure mechanical wood pulp, if merely exposed to pure dry air, the signs of decay would be delayed considerably. The combined action of air and moisture is of a more vigorous character in promoting oxidation changes in the fibres, or a dissociation of the sizing and other chemical ingredients of the paper. The presence of moisture is, indeed, absolutely essential for the reaction of some substances upon one another, and it is easy to show that certain chemical compounds can be left in ultimate contact, if absolutely dry, for a lengthened period without reacting, but the addition of a little moisture at once produces chemical union. This may be shown by a simple experiment.
Thus a piece of coloured paper which may be bleached immediately if suspended in an atmosphere of ordinary chlorine gas will remain unbleached for several hours if first thoroughly dried in an oven and exposed to dry gas.
In the case of books and papers, these conditions which promote slow disintegration are aggravated by the presence of impurities in the air, such as the vapours of burning gas, the traces of acidity in the atmosphere of large manufacturing towns, the excessive dampness and perhaps heat of a climate favouring the growth of organisms. All these[Pg 243] factors are of varying degrees in different places, so that the deterioration of papers does not proceed in the same measure and at the same rate everywhere.
Moisture.—It may not be out of place to discuss some important relations between moisture and the physical qualities of a sheet of paper. A paper in its normal condition always contains a certain proportion of water as one of its ingredients, and the presence of this moisture has much to do with the strength, elasticity, and use of the paper, the absence of moisture giving rise to defects and troubles in the use of the paper which to a certain extent lower its commercial value and deteriorate it, though not perhaps in the sense of permanent degradation of quality.
One trouble frequently experienced by stationers and others is that known as wavy edges. The edges of a stack containing sheets of paper piled upon one another frequently twist and curl, producing what are known as wavy edges. This arises from the fact that the paper when manufactured was deficient in natural moisture, and that when stacked it has gradually absorbed moisture, which is taken up first by the edges exposed to the air. This causes unequal expansion of the fibres with the production of the so-called wavy edges. The only remedy in such cases is the free exposure of the sheets before printing, so that the moisture is absorbed equally all over the sheet. The cracked edges of envelopes may be explained by reference to the same conditions. The paper is worked up into envelopes in an over-dry condition, and the fibres, being somewhat brittle, readily break apart from one another. If the paper is kept in stock for some time before use this defect can be very largely remedied.
With supercalendered papers it is only possible to obtain the best results by allowing the paper to stand for several days after making before it is glazed.
It is evident from these few examples that many of the troubles experienced by printers are due to the fact that orders for paper are frequently accompanied by an instruction for immediate delivery, under which circumstances it is impossible to obtain the best results. The expansion of papers used for lithography, and the bad register frequently seen in colour work, may be explained by reference to the behaviour of the individual fibres towards moisture. The expansion is usually greater in one direction of the paper than in the direction at right angles to it, and this is due to the fact that fibres have a greater ratio of expansion in the diameter than in the length.
The behaviour of papers when damped is a peculiarity well known to paper-makers and printers. For certain purposes it is desirable that paper should not show any material alteration when damped, since any expansion of the sheet is liable to throw the printing out of “register.” The liability of papers to such stretch or expansion is largely minimised by careful manipulation of the pulp during the process of beating, and also by a proper regulation of the web of paper as it passes from the wet end of the paper machine over the drying cylinders to the calenders. The paper which fulfils the necessary qualifications as to a minimum stretch is prepared from pulp which has not been beaten for too long a period, so that the pulp obtained is fairly light and bulky. By this means the expansion of the fibres takes place in the sheet itself without making any material alteration in its size. That is to say, as the sheet of paper is fairly open, there is sufficient room for expansion, which thus takes place with the least alteration of the total area of the sheet. The paper which is allowed to shrink on the machine during the process of drying, without undue tension, usually exhibits a minimum amount of expansion subsequently in printing.
It is important to notice that the expansion of paper is different for the two directions, that is for the machine and cross directions.
This arises from the fact that in the machine-made paper the greater proportion of the fibres point in the direction of the machine while the paper is being made. In consequence of this the expansion of the paper is greatest in what is known as the cross direction of the paper, that is, in the direction at right angles to the flow of the pulp along the machine wire.
This is to be explained by reference to the behaviour of fibres when damped or brought into contact with an excess of water. The question of the exact changes in the dimensions of a fibre due to absorption of water has been dealt with in an interesting manner by Hohnel. He points out that the well-known peculiarity of the shrinkage of ropes which have been lying in the water can be explained by an examination of the behaviour of the single fibres. He relates in detail the experiment which can be carried out for the exact observation of the fibres when in contact with water. A dry fibre when soaked in water appears to become 20 to 30 per cent. greater in diameter, whereas in length it is usually only increased by one-tenth per cent.
The method adopted by Hohnel was to place a fibre of convenient length on a glass slip down the centre of which was a fine narrow groove capable of holding water, so that the fibre could be wetted. Over the fibre was a cover glass with a small scale marked on it. The loose end of the fibres passed over a small roller and was stretched by a light weight. The movements of the fibre were measured by means of an eye-piece micrometer.
In this way it is possible to determine alterations in length to within 0·005 per cent., and this variation can be directly seen under the microscope.
Hohnel observes in his account of the experiments that[Pg 246] all fibres become thicker when wetted, that vegetable fibres are more susceptible than animal fibres.
Animal fibres expand about 10 to 14 per cent. in diameter, but vegetable fibres as much as 20 per cent., as shown in the following table:—
| Animal Fibre. | Per Cent. | Vegetable Fibre. | Per Cent. |
| Human hair | 10·67 | New Zealand flax | 20·0 |
| Angora wool | 10·2 | Aloe hemp | 25·8 |
| Alpaca wool | 13·7 | Hemp | 22·7 |
| Tussah silk | 11·0 | Cotton | 27·5 |
The reverse is the case when the expansion of the fibres in regard to length is considered, since animal fibres expand 0·50 to 1·00 per cent. of their length, and vegetable fibres only 0·05 to 0·10 per cent.
The maximum amount of expansion in the case of the vegetable fibres is obtained by gently breathing upon them rather than by the use of an excess of water.
These figures are important as explaining many of the peculiar characteristics of vegetable and animal fibres. Advantage is taken of the greater expansion of the latter in the manufacture of instruments for the measurement of moisture, such as the hair hygrometer, in which the elongation of a stretched hair registers the variation in the moisture of the atmosphere.
Quality of Book Papers.—The Committee of the Society of Arts in dealing with the evidence as to the permanence of finished papers suggest the following classification as indicating the desired standards of quality:—
A. Cotton, flax, and hemp.
B. Wood celluloses, (a) sulphite process, and (b) soda and sulphate process.
C. Esparto and straw celluloses.
D. Mechanical wood pulp.
The Committee find little fault with the Principles which govern the trade in the manufacture of high-class papers, and limit the result of their investigation to the suggestion of a normal standard of quality for book papers required in documents of importance according to the following schedule:—
Fibres.—Not less than 70 per cent. of fibres of Class A.
Sizing.—Not more than 2 per cent. rosin, and finished with the normal acidity of pure alum.
Loading.—Not more than 10 per cent. total mineral matter (ash).
With regard to written documents, it must be evident that the proper materials are those of Class A, and that the paper should be pure, sized with gelatine and not with rosin. All imitations of high-class writing papers, which are in fact merely disguised printing papers, should be carefully avoided.
These recommendations are good as far as they go, but in order to establish the proper standards of quality some specifications must be laid down with regard to the strength of the paper and its physical properties, together with a reference to the use for which the paper is intended. The physical condition of the paper itself apart from the nature of the fibre has much to do with its resistance to wear and tear, and this is easily proved by comparing modern book papers made from esparto with book papers of an earlier date made from the same material.
The only official schedule of requirements in relation to public documents is that issued by the Stationery Office.
The details set out relate chiefly to questions of weight and strength, the limits being expressed in definite form and not allowing much margin for variation in respect of strength[Pg 248] or fibrous constituents. Mechanical wood pulp is excluded in all papers except common material as stated in the schedule. The papers required for stock are divided into twelve classes. In each class the trade names of various sized papers are given, the size of the sheet and the weight of the ream, and, where required, any special characteristics are set out. The schedule is as follows:—
Class 1. Hand-made or Mould-made.
General Specification.—Hand-made or mould-made. Animal tub-sized. (“Hand-made” or “Mould-made” to be marked on the wrapper.)
Where special water-marking is required mould will be supplied by the Stationery Office for those papers made by hand.
Class 2. Writings, Air-dried.
General Specification.—Plate rolled. Machine made. Animal tub-sized. Air-dried. (Must bear ink after erasure.)
Note.—The mean breaking strain and mean stretch required are given for each paper. The figures represent the mean of the results obtained for both directions of the sheet, and are calculated on a strip of paper five-eighths of an inch wide and having a free length of seven inches between the clips.
Class 3. Writings, Ordinary.
General Specification.—Rolled. Machine-made. Animal tub-sized.
Class 4. Writings, Coloured.
Specification.—Highly rolled. Machine-made. Animal tub-sized.
Class 5. Blotting Papers.
Specification.—All rag. Machine-made. Free from loading.
Class 6. Printing and Lithographic Papers.
General Specification.—Rolled. Machine-made. Engine-sized. Loading not to exceed 15 per cent.
Class 7. Coloured Printings.
General Specification.—Rolled. Machine-made. Engine-sized.
Class 8. Copying and Tissue Papers.
Specification.—Machine-made. Free from loading. (Copying papers are required to give three good copies.)
Class 9. Brown Papers, Air-dried.
Specification.—Air-dried. Machine-made.
Note.—The mean breaking strain and mean stretch required are given for each paper. The figures represent the mean of the results obtained for both directions of the sheet, and are calculated on a strip of paper two inches wide and having a free length of seven inches between the clips.
In the case of papers indicating a larger breaking strain than the minimum required, a proportional increase in the stretch must also be shown.
Class 10. Brown Paper, Cylinder-dried.
General Specification.—Machine-made.
Note.—The mean breaking strain required is given for each paper. The figures represent the mean of the results obtained for both directions of the sheet, and are calculated on a strip of paper two inches wide and having a free length of seven inches between the clips.
Class 11. Smallhands.
General Specification.—Machine-made. Engine-sized.
Class 12. Buff Papers.
Specification.—Highly finished both sides. Machine-made. Hard engine-sized.
Mechanical wood pulp must not be used in the manufacture of any papers, with the exception of engine-sized coloured printings, and buff papers, where an addition up to 25 per cent. will be allowed.
All animal tub-sized papers are required to be as far as possible free from earthy matter; and, except where specially stated, the amount of loading added to other papers must not exceed 6 per cent.
When sulphite or soda pulps are used, either separately or conjointly, in the manufacture of printing papers, the quantity of neither material shall separately exceed 50 per cent.
The most complete specification as to the requirements for standard papers is that published by the Paper Testing Institute in Germany, and used as the basis of most contracts, at least for public and official documents.
Standards of Quality in Germany.—The classification of papers according to the raw materials used and the nature of the finished paper is very complete. The classification is made under three headings: (A) Raw Material; (B) Strength; (C) Uses.
(A) Classification according to Material.
(1) Paper made from rags only (linen, hemp, and cotton).
(2) Paper made from rags with a maximum of 25 per cent. of cellulose from wood, straw, esparto, manila, etc., but free from mechanical wood pulp.
(3) Paper made from any fibrous material, but free from mechanical wood pulp.
(4) Paper of any fibrous material.
(B) Classification according to Strength.
| Class | 1. | 2. | 3. | 4. | 5. | 6. |
| Mean tearing length in metres | 6,000 | 5,000 | 4,000 | 3,000 | 2,000 | 1,000 |
| Elasticity per cent. | 4 | 3·5 | 3 | 2·5 | 2 | 1·5 |
| Resistance to folding (Schoppers' method, number of foldings) | 190 | 190 | 80 | 40 | 20 | 3 |
The tests for tearing length, resistance to folding, elasticity, etc., are made in air showing relative humidity of 65 per cent. The calculations for tearing length are made on strips of paper dried at 100° C.
(C) Classification according to Use.
| Class. | Uses. | Fibre. Class. | Strength. Class. | Size of Sheets. Cm. | Weight of | |
| 1,000 Sheets. Kg. | 1 Sq. Metre. Grms. | |||||
| 1 | Writing papers for important documents | 1 | 1 | 33 × 42 | 15 | — |
| Paper for State documents | 1 | 1 | 26·5 × 42 | 12 | — | |
| 2 | Paper for registers, account books, and ledgers— | |||||
| (a) First quality | 1 | 2 | 33 × 42 | 14 | — | |
| (b) Second quality | 1 | 3 | 33 × 42 | 13 | — | |
| 3 | Documents intended to be preserved longer than ten years— | |||||
| (a) Foolscap paper | 2 | 3 | 33 × 42 | 13 | — | |
| Letter paper (quarto size) | 2 | 3 | 26·5 × 42 | 10·4 | — | |
| [Pg 252] | Letter paper (octavo size) | 2 | 3 | 26·5 × 21 | 5·2 | — |
| Duplicating paper | 2 | 3 | 33 × 42 | 7 | — | |
| (b) Official writing paper | 2 | 4 | 33 × 42 | 13 | — | |
| 4 | Paper for documents of lesser importance— | |||||
| (a) Foolscap paper | 3 | — | 33 × 42 | 12 | — | |
| Letter paper (quarto size) | 3 | — | 26·5 × 42 | 9·6 | — | |
| Letter paper (octavo size) | 3 | — | 26·5 × 21 | 4·8 | — | |
| (b) Official writing paper | 3 | 4 | 33 × 42 | 12 | — | |
| 5 | Envelopes and wrappers— | |||||
| (a) First quality | — | 3 | — | — | — | |
| (b) Second quality | — | 5 | — | — | — | |
| 6 | Writing paper of medium quality | — | 5-6 | — | — | — |
| 7 | Covers for documents— | |||||
| (a) That required for frequent use | 1 | Tearing | 36 × 47 | 81·2 | 480 | |
| length 2,500 Elasticity 3·5% | ||||||
| (b) For other purposes | 3 | Tearing | 36 × 47 | 42·3 | 250 | |
| length 2,500 Elasticity 2·5% | ||||||
| 8 | Printing paper— | |||||
| (a) For important printed matter | 1 | 4 | — | — | — | |
| (b) For less important printed matter | 3 | 4 | — | — | — | |
| (c) For common use | — | 5-6 | — | — | — | |
ANALYSIS, TECHNOLOGY, ETC.
Abel, Dr. E. Hypochlorite und electrische Bleiche. Halle, 1905.
Arabol Manufacturing Co.—Theory and Practice of the Sizing of Paper. New York, 8o, 1895.
Behrens, H.—Anleitung zur mikrochemischen Analyse der wichtigsten Verbindungen. Heft 2. Die wichtigsten Faserstoffe. Hamburg und Leipzig, 1896.
Beveridge, J.—Paper-makers' Pocket Book. London, sm. 8o, 1901.
Bourdillat, E. Die Entfärbung und das Bleichen der Hadern. Weimar, 1867.
Corput, E. Van den.—De la fabrication du papier au point de vue de la technologie chimique. 2e éd. Paris, 8o, 1861.
Cross, C. F. and Bevan, E. J.—A Text-book of Paper-making. London, sm. 8o, 1888.
Ditto, 2nd edition. 1900.
Ditto, 3rd edition. 1907.
Cross and Bevan.—Manuel de la fabrication du papier. Traduit de la 2e édition Anglaise. Par L. Desmarest. 1902.
Cross, Bevan, Beadle and Sindall.—The C.B.S. Units: a Book on Paper Testing. 1904.
Deterioration of Paper.—Society of Arts Report. 1898.
Engelhardt, B. Hypochlorite und electrische Bleiche (Technisch-Constructiver Teil). Halle, 1904.
Engels, J. A.—Ueber Papier und einige andere Gegenstände der Technologie und Industrie. Duisburg, sm. 8o, 1808.
Engländer.—Technologie der Papierfabrikation. Lehrbuch für Spezialkurse an Handelsfachschulen u. fachlich. Fortbildungsschulen sowie Lehrbehelf zum Selbststudium. 1906.
Erfurt, J. Färben des Papierstoffs. Mit 145 Proben in Stoffgefärbten Papiere, 2te Aufl. Berlin, 8o, 1900.
Erfurt, J. The Dyeing of Paper Pulp; from the 2nd German edition, by J. Hübner. London, 8o, 1901.
Finkener.—Ueber die quantitative Bestimmung des Holzschliffes in Papier nach Goddefroy und Coulon. 1892.
Flatters.—Microscopical Research. 1906.
Griffin, R. B. and Little, A. D.—The Chemistry of Paper-making, with Principles of General Chemistry. New York, 8o, 1894.
Hassak.—Wandtafeln für Warenkunde u. Mikroskopie. 1904.
Haywood, J. K.—Arsenic in Papers and Fabrics. 1904. (U.S.A. Department of Agriculture.)
Herzberg, W.—Mikrosk. Untersuchung des Papiers. 1887.
Herzberg, W.—Papierprüfung. Leitf. bei d. Unters. v. Papier. 1888.
Ditto, 2nd edition. 1902.
Ditto, 3rd edition. 1907.
Herzberg, W.—Paper Testing as carried out in the Government Laboratory at Charlottenburg. From the German, by P. N. Evans, London, 8o, 1892.
Herzberg, W.—Mitteilungen aus den Königl. technischen Versuchsanstalten zu Berlin. 1887, et seq.
Höhnel, F. v.—Die Mikroskopie der technisch verwendeten Faserstoffe. 1905.
Hölbling, V.—Die Fabrikation der Bleichmaterialien. Berlin.
Hoyer, E.—Le papier; étude sur sa composition, analyses et essais. De l'Allemand. Paris, 8o, 1884.
Hoyer-Kraft.—Die Spinnerei, Weberei und Papierfabrikation, 4 Aufl. 1904.
Jagenberg, F.—Die thierische Leimung für endloses Papier. Berlin, 8o, 1878.
Johannsen.—Mitteilungen über Mikrophotographie von Faserstoffen im durchfallenden und auffallenden Licht. 1906.
Klemm, P.—Papier Industrie Kalender. 1898, et seq.
Lauboeck.—Über die Saugfähigkeit der Löschpapiere. Mitteilungen des k.k. Technologischen Gewerbe-Museums. Wien, 1897.
Leach, C. E.—On the Shrinkage of Paper (excerpt). Newcastle, 8o, 1884.
Martens, A.—Mitteilungen aus den Königl. Technischen Versuchsanstalten (jährlich). Erscheinen seit 1883. Die Jahrgänge[Pg 255] 1884 bis 1903 enthalten aus der Abteilung für Papierprüfung die im Jahrgang 1905, dieses Kalenders verzeichneten Arbeiten.
Martens, A.—Apparaten zur Untersuchung der Festigkeitseigenschaften von Papier. Königl. Techn. Versuchsanstalten. Mitteilungen. Ergänzungsheft. No. 3. 8o, 1887.
Martens, A.—Ueber Druckpapier der Gegenwart. Königl. Techn. Versuchsanstalten. Mittheilungen. Ergänzungsheft. No. 4. 8o, 1887.
Martens, A.—Untersuchung Japanischer Papiere. Königl. Techn. Versuchsanstalten. Mittheilungen. Ergänzungsheft. No. 4. 8o, 1888.
Martens und Guth.—Das königliche Materialprüfungsamt der technischen Hochschule Berlin auf dem Gelände der Domäne Dahlem beim Bahnhof Gross-Lichterfelde West. Berlin, 1904.
Melnikoff, N.—Prüfung von Papier und Pappe nebst Adressbuch der russischen Papierfabriken. Petersburg, 1906.
Müller, L.—Die Fabrikation d. Papiers in Sonderheit d. a. d. Maschine gefertigten. 2 Aufl. 1855.
Müller und A. Haussner.—Die Herstellung u. Prüfung des Papiers. 1905.
Müller, A.—Qualitative und quantitative Bestimmung des Holzschliffes im Papier. 1887.
Muth. Die Leimung der Papierfaser im Holländer und die Anfertigung fester Papiere. 1890.
Naylor, W.—Trades Waste. London, 1902.
Normalpapier.—Sammlung der Vorschriften für amtliche Papier- und Tintenprüfung. Berlin, 1892.
Piette, L.—Traité de la coloration des pâtes à papier. Précédé d'un aperçu sur l'état actuel de la fabrication du papier. Avec échantillons de papiers colorés. Paris, 8o, 1863.
Rejtö, A.—Anleitung für Private zur Durchführung der Papierprüfung. Budapest, 1893.
Rossel.—Papiere und Papierprüfung mit Berücksichtigung der in der Schweiz verwendeten Schreib- und Druckpapiere. Biel, 1895.
Schumann, Dr. G.—Welche Ursachen bedingen die Papierqualität. Biberach, 1901.
Sindall, R. W.—Paper Technology. London, 1906.
Stevens, H. P.—The Paper Mill Chemist. London, 1907.
Wiesner, J.—Mikroskopische Untersuchung des Papiers mit besonderer Berücksichtigung der ältesten orientalischen und europäischen Papiere. Wien, 1887.
Wiesner, J.—Mikroskopische Untersuchung alter ostturkestanischer[Pg 256] und anderer asiatischer Papiere nebst histologischen Beiträgen zur mikroskopischen Papieruntersuchung. Wien, 1902.
Winkler, O.—Die Trockengehalts-Bestimmung d. Papierstoffe. 1902.
Winkler, O., und Karstens, H.—Papieruntersuchung. 1903.
Wurster.—Le collage et la nature du papier. Paris, 1901.
Wurster, Dr. C.—Die neuen Reagentien auf Holzschliff und verholzte Pflanzenteile zur Bestimmung des Holzschliffs im Papier. Berlin.
Zirm, A.—Der Papierfärber. Tilsit, 1904.
CELLULOSE, ETC.
Beadle, C.—Viscose and Viscoid. Franklin Institute reprint. 1896.
Bersch, J.—Cellulose, Celluloseprodukte u. Kautschuksurrogate. 1903.
Bockmann, F.—Das Celluloid, sein Rohmaterial, Fabrikation, Eigenschaften u. technische Verwendung. 1880. 2te Aufl. 1894.
Bornemann, Gr.—Ueber Cellulose and neuere Umwandlungsprodukte derselben. Biberach, 1901.
Bottler, M.—Die vegetabilischen Faserstoffe—Hartleben's chemisch-technische Bibliothek. 1900.
Butschli, O.—Untersuchgn. an Gerinnungsschaumen, Sphärokystallen u. d. Struktur v. Cellulose. 1894.
Cross, C. F., and Bevan, E. J.—Cellulose. London, 1885.
2nd edition. 1895.
Cross and Bevan.—Researches on Cellulose. 1895-1900.
Ditto, 1900-1905.
Margosches, Dr. B.—Die Viskose, ihre Herstellung, Eigenschaften und Anwendung. Leipzig, 1906.
Schlesinger.—Künstliche Seide (Zellstoff-Seide). Mechanisch-technologische Untersuchung der aus nitriertem Zellstoffs hergestellten Seide. 1895.
FIBRES, ETC.
Andés, L. E.—Die Verarbeitung des Strohes. Wien, 1898.
Bagshaw.—Photomicrography. Elementary.
Bengal Government.—Jute in Bengal, and on Indian Fibres available for the Manufacture of Paper. Report by H. Kerr. Calcutta, fol., 1874.
Bleekrode, S.—Grondstoffen voor Papierbereiding, bijzonder in Neerlandsch-Indië (excerpt). 8o, 1859.
Bottler, M.—Die animalischen Faserstoffe. 1901.
Carter.—Spinning of Fibres. 1904.
Cobbett.—A Treatise on Cobbett's Corn. 1828. (Printed on paper made of corn husks.)
Christy.—Commercial Plants and Drugs. 1882.
Cross and Bevan.—Report on Indian Fibres. 1887.
Cross, C. F.—Report on Miscellaneous Fibres. 1886.
Cross, C. F.—Bast Fibres. Manchester, 1880.
Dalen, G.—Jute. Manila, Adansonia. 1902.
Dépierre, J.—Traité des apprêts et spécialement des tissus de coton, blancs, teints et imprimés.
Dodge, C. R.—Leaf Fibres of the United States. 1903.
Garçon, Jules.—Bibliographie de la technologie chimique des fibres textiles. Paris, 1893.
Gelder Zonen, van.—Een woord over nieuwe Grondstoffen voor Papier, met monsters van ded proeven, etc. Amsterdam, sm. 4o, 1866.
Georgievics, G. v.—Lehrbuch der chemischen Technologie der Gespinnstfasern. 1895-98.
Georgievics, G. v.—Lehrbuch d. chemischen Technologie d. Gespinnstfasern. 2te Tle. 1898-1902.
Georgievics, G. v.—Technology of Textile Fibres; from the German. 1902.
Grothe, H.—Die Technologie der Gespinnstfasern. 1876-82.
Goodale.—Physiological Botany. 1890.
Hammarsten, O.—Untersuchungen über d. Faserstoffgewinnung, 1875.
Hannan, W. I.—Textile Fibres of Commerce. 1902.
Hoyer, E. von.—Die Verarbeitung der Faserstoffe. (Spinnerei, Papierfabrikation.) 3te Aufl. 1900.
Johnstone.—Esparto. (Society of Arts Lecture.) 1870.
Kew Bulletin.—Vegetable Fibres. 1901.
Lecomte, H.—Les textiles végétaux; leur examen microchimique. Paris, 1891.
Liotard.—Materials in India suitable for Paper-making. Calcutta, 1880.
Morris, Dr.—Commercial Fibres. (Cantor Lectures.) 1895.
Müller, Hugo.—Pflanzenfaser. Leipzig, 1873.
Payen, A.—Succédanés des chiffons. Paris Universal Exhibition. Rapports du Jury International, Classe 7, sect. ii. 8o, 1867.
Pfuhl, E.—Papierstoffgarne, ihre Herstellung, Eigenschaften u. Verwendbarkeit. 1904.
Posselt, E. A.—The Structure of Fibres, Yarns, and Fabrics, being a Practical Treatise for the use of all persons employed in the Manufacture of Textile Fibres. 2 vols., 1902.
Rostaing and Others.—Précis historique, descriptif, analytique et photomicrographique, des végétaux propres à la fabrication de la cellulose et du papier. Paris, 8o, 1900.
Routledge, T.—Bamboo considered as a Paper-making Material, with Remarks upon its Cultivation and Treatment. London, 8o, 1875.
Routledge, T.—Bamboo and its Treatment. 1879.
Silbermann, H.—Fortschritte auf dem Gebiete der chemischen Technologie d. Gespinnstfasern, 1885-1900. 2te Tle., 1902-03.
Trabut.—Étude sur l'alfa. 1889.
Urbain, V.—Les succédanés du chiffon en papeterie. Paris, 16o, 1897.
Vétillart.—Études sur les Fibres Végétales. Paris, 1876.
Wieck, F. G.—Bilder aus Gewerbskunst (aus Tomlinson's “Objects in Art Manufacture”), i. Papier. Leipzig, sm. 8o, 1855.
Witt, O. N.—Chemische Technologie der Gespinnstfasern, ihre Geschichte, Gewinnung, Verarbeitg. u. Veredlung. 1888-1902.
Zetzsche.—Die Wichtigsten Faserstoffe der europäischen Industrie. Anleitung zur Erkennung und Unterscheidung. 1905.
Zimmermann, A.—Morphologie und Physiologie der Pflanzenzelle.
HISTORICAL.
Blanchet, Augustin.—Essai sur l'histoire du papier et de sa fabrication. Paris, 1900.
Breitkoff, J. G. J.—Ursprung der Spielkarten, die Einführung des Leinenpapieres, etc., in Europa. (Completed by J. G. F. Roch.) Leipzig, 2 vols., 4o, 1784-1801.
Briquet, C. M.—Bemerkungen über das Sammeln von Wasserzeichen oder Papiermarken, überreicht bei der Ausstellung der alten Papiermarkerkunst zu Paris. 1900.
Briquet, C. M.—Papiers et filigranes des archives de Gênes 1154-1700. Geneva, 1888.
Briquet, C. M.—Geschichte der Papierzeichen von ihrem Erscheinen gegen 1282 bis 1600. Mit Beigabe von 15500. 1906.
Butler Paper Co.—The Story of Paper-making. Chicago, sm. 8o, 1901.
Collett, C. D.—History of Taxes on Knowledge. London, 1899.
Congress.—International congress de fabricants de papier et carton, Antwerp. Comptes rendu des séances. Bruxelles, 8o, 1894.
Dropisch, B.—Die Papiermaschine, ihre geschichtliche Entwicklung u. Construction. 1878.
Egger, E.—Le papier dans l'antiquité et dans les temps modernes. Paris, 16o, 1866.
Evans, L.—The Firm of John Dickinson & Co., with an Appendix on Ancient Paper-making. London, sm. 8o, 1896.
Gamble, J.—Collection of Documents (Specifications, Official Reports, etc.) respecting the Claims of L. Robert as the Original Inventor, and of J. Gamble as the First Introducer of the French Paper-machine. Fol., 1801—58.
Hoernle, A. F.—Who was the Inventor of Rag Paper? 1903.
Hössle, F. von.—Geschichte der alten Papiermühlen in ehemaligen Stift Kempten und in der Reichsstadt Kempten. 1901, 4o, 1900.
Hunter, J.—Specimen of Marks used by Early Manufacturers of Paper (Excerpt). London, 4o, 1858.
Imberdis, J.—Le papier ou l'art de fabriquer de papier. Traduction au Français de (papyrus sive ars conficiendæ papyri, 1693), par A. Blanchet. Avec le texte latin. 1899.
Jackson, J. B.—An Essay on the Invention of Engraving and Printing in Chiaro Oscuro, as practised by Durer, etc., and its Application to the Making of Paperhangings. London, sm. 4o, 1754.
Jansen, H.—Essai sur l'origine de la gravure, etc. Paris, 2 vols., 8o, 1808.
Jenkins, R.—Paper-making in England, 1495, etc., from the Library Association Record, September, 1900-April, 1902. London, 8o.
Karabacek, J.—Das arabische Papier. Wien, 1887.
Kent & Co.—Paper and Paper-making Chronology. London, 8o, 1875.
Kirchner, E.—Die Papiere des XIV. Jahrhunderts im Stadtarchive zu Frankfurt a. M. 1893.
Kirchner, E.—Das Papier. Die Geschichte d. Papierindustrie; die Rohstofflehre d. Papierindustrie. 3 Bde., 1897-99.
Kirchner, E.—Das Papier. Historisch-technologische Skizzen. Jahresbericht der Techn. Lehranstalten in Chemnitz. 1903.
Klein, A.—Entwicklung und Aufgaben der Papierindustrie. Biberach, 1906.
Klemm, P.—Papier-warenzeichen ... vom 1 Okt., 1894, bis Ende 1902, für Klasse 27, umfassend Papier, etc., eingetragenen Wort- und Bildzeichen. Leipzig, sm. 8o, 1903.
Koops, M.—Historical Account of Paper, and of Substances used prior to its Invention (printed on paper made from straw and wood). London, 8o, 1800.
Ditto, 2nd edition, 1801.
Lacroix, A.—Historique de la papeterie d'Angoulême suivi d'observations sur le commerce de chiffons en France. Paris, 8o, 1863.
Lalande, J. J. Le F. de.—L'art de faire le papier. Acad. Roy. des sciences. Description des Arts et Métiers, vol. 1. Fol., 1761.
Lettre sur les découvertes de M. Didot aîné dans les arts de ... la papeterie (l'invention du papier-vélin). Paris, 12o, 1783.
Leuchs, J. C.—Beschreibung der in den letzten acht Jahren in der Papierfabrikation gemachten Verbesserungen. Nachtrag. Nürnberg, 8o, 1828.
Marabini.—Bayrische Papiergeschichte. 1 Teil. Die Papiermühlen im Gebiete der weiland freien Reichsstadt Nürnberg. Nürnberg, 1894.
Maurel, F.—Le papier japonais. Histoire et fabrication d'après les documents Anglais et indigènes (excerpt). Paris, 4o, 1871.
Meerman, G., and others.—Epistolæ, etc., de chartæ vulgaris lineæ origine. Ed. J. Van Vassen, Hagæ Com. Sm. 8o, 1767.
Midoux, E., and Matton, A.—Étude sur les filigranes des papiers employés en France aux 14e et 15e siècles. Paris, 8o, 1868.
Millar, O.—Papier-Industrie. Schweizerische Landesausstellung, 1883. Berichte, Gruppe 8, 1884.
Murray, J.—Practical Remarks on Modern Paper, etc., with an Introductory Account of its Former Substitute. Edinburgh, 8o, 1829.
Parlatore, P.—Mémoire sur le papyrus des Anciens et sur le papyrus de Sicile, Acad. des Sciences. Paris. Mèm. par divers Savans.... 2e Serie, Tome 12. 4o, 1854.
Peignot, E. G.—Essai sur l'histoire du parchemin et du vélin. Paris, 8o, 1812.
Penig.—(Patentpapierfabrik zu Penig.) Ein Beitrag z. Geschichte d. Papiers, 1897.
Robert, N. L.—Le centenaire de la machine a papier continu. Son[Pg 261] invention par N. L. Robert en 1799. Biographie de l'inventeur, par J. Breville. Historique des divers perfectionnements ... par Didot Saint-Leger, 1800-1818. Paris, 8o, 1901.
Robertson.—Fifty Years' Experience in Paper-making. Leith.
Schaeffer, J. C.—Proefnemingen en Monster-Bladen om Papier te maaken zonder Lumpen of met een gering Byvoegzel derzelven. Uit het Hoogduits vertaald. Deel 1-2. Amsterdam, 2 vols., sm. 4o, 1770.
Schaeffer, J. C.—Sämtliche Papierversuche, 2te Aufl. Nebst 81 Mustern und 13 Kupfertafeln. Regensburg, 6 vols. in one, sm. 4o, 1772.
Schaeffer, J. C.—Erweis in Musterbogen dass die neuen Papierarten ... sich allerdings auch zu Tapeten übermahlen und gebrauchen lassen. Regensburg, fol.
Smith, J. E. A.—History of Paper, Genesis and Revelations. Holyoke, Mass., U.S.A., 1882.
Sotheby, S. L.—The Typography of the 15th Century ... Exemplified in a Collection of Facsimiles from 100 Works, with their Watermarks. London, fol. 1845.
Sotheby, S. L.—Principia Typographica. An Attempt to Elucidate the Paper Marks of the Period. London, 3 vols., fol. 1858.
Spechthausen.—Hundert Jahre der Papierfabrik Spechthausen. Festschrift, z. 1887.
Spicer, A.—The Paper Trade. London, 1907.
Stoppelaar, J. H. de.—Het Papier in de Nederlanden gedurende de middeleeuwen, inzonderheid in Zeeland. Middelburg, 8o, 1869.
Tomlinson, C.—Illustrations of the Useful Arts. No. 3, Paper. London, 32o, 1859.
Villette, C. Marquis de.—Œuvres, with Specimens of Paper. Londres, 16o, 1786.
Willkomm, M.—Über den Lotos und Papyros der alten Ägypter und die Papiererzeugung in Altertume. Prag, 1892.
PAPER MANUFACTURE.
Archer, T. C.—The Manufacture of Paper. Bevan. British Manufacturing Industries, viii. Sm. 8o, 1876.
Arnot.—Technology of the Paper Trade (Cantor Lecture, Society of Arts). London, 1877.
Barse, J.—Études comparées sur l'industrie Française, ii. La[Pg 262] fabrication et le commerce du papier en 1860 et en 1864. Paris, l. 8o, 1864.
Beadle, C.—Paper Manufacture—Lectures. 1901.
Beadle, C.—Chapters on Paper-making. Vol. 1. London, 1904.
Vol. 2, Answers to Technological Questions. 1906.
Vol. 3, Practical Points in Paper Manufacture. 1907.
Vol. 4, Ditto. 1907.
Beaumont, F.—Report on Apparatus and Processes used in Paper-making, etc. Paris Universal Exhibition, 1867. British Commercial Reports, Vol. 4. 8o, 1867.
Bennett, J. B.—Paper-making Processes and Machinery, with Illustrations of Paper-making Machinery constructed by Bertrams, Ltd. Edinburgh, 8o, 1892.
Bertrams, Ltd.—Specimens of Paper. Edinburgh, obl. 16o, 1892.
Blanchet, A.—Fabrication du papier. Rapports, Paris Universal Exhibition, 1900.
Brown, H. T.—The Manufacture of Paper from Wood in the United States. 1886.
Burot.—Note sur la fabrication du papier de paille. Paris, 8o, 1883.
Campredon, E.—Le Papier. Étude monographique sur la papeterie Française et en particulier sur la papeterie Charentaise. i. Historical; ii. Descriptive of Modern Paper-making; iii. Co-operative Paper-making. Paris, 8o, 1901.
Charpentier, P.—Le Papier. Fremy, E. Encycl. Chim., Tome X. 8o (83), 1890.
Clapperton, G.—Practical Paper-making. London, sm. 8o, 1894.
Ditto, 2nd edition, 1907.
Coney, E.—Paper-making Machinery and Fibres. Philadelphia International Exhibition, 1876. U.S.A. Centennial Commission Reports and Awards, Group xiii. 8o, 1876.
Dalheim, C. F.—Taschenbuch f. d. prakt. Papierfabrikanten. 3te Aufl. 1896.
Dammer, O.—Papierfabrikation.
Davis, C. T.—The Manufacture of Paper. Philadelphia, 8o, 1886.
Doumerc and others.—Matériel et procédés de la papeterie, etc. Paris Univ. Exhibition, 1867. Rapports du Jury International, Classe 59. 8o, 1867.
Doyle, P.—Paper-making in India, being Notes of a Visit to the [Pg 263]Lucknow Paper Mill. Lucknow, 8o, 1885.
Dropisch, B.—Handb. d. Papierfabrikation. 3e Aufl. 31 Taf. in fol. 1881.
Dunbar, J.—The Practical Papermaker. Leith, 12o, 1881.
Hartmann, C.—Handb. d. Papierfabrikation. Taf. 1842.
Hassak, K.—Die Erzeugung des Papieres.
Hausner, A.—Der Holländer. Eine kritische Betrachtung seiner Arbeitsweise mit Bezug auf die Einzelabmessungen seiner Teile und die verarbeiteten Fasern. 1901.
Herring, R.—Paper and Paper-making, Ancient and Modern. London, 8o, 1854.
Ditto, 2nd edition, 1855.
Ditto, 3rd edition, 1863.
Hofmann, C.—A Practical Treatise on the Manufacture of Paper in All its Branches. Philadelphia, 4o, 1873.
Hofmann, C.—Praktisches Handbuch d. Papierfabrikation. 1873.
Hoffmann, Th.—Papierprägung. Berlin.
Hoyer, E.—Das Papier, seine Beschaffenheit und deren Prüfung. München, 1882.
Hoyer, E.—Über die Entstehung und Bedeutung der Papiernormalien, sowie deren Einfluss auf die Fabrikation des Papieres. München, 1888.
Hoyer, E.—Die Fabrikation des Papiers. 1900.
Hoyer, E.—Die Fabrikation des Papiers, nebst Gewinnung d. Fasern. 1887.
Hübner, J.—Paper Manufacture (Cantor Lectures to the Society of Arts). 1903.
Jagenberg, F.—Das Holländergeschirr. Remscheid. 1894.
Kirchner-Strohbach.—Holländer-Theorie. Biberach. 1904.
Klemm, Dr. P.—Über Papier. Klimsch's Graphische Bibliothek Bd. 3 (Farbe und Papier im Druckgewerbe). 2 Teil. Frankfurt a. M. 1900.
Korschilgen und Selleger.—Technik und Praxis der Papierfabrikation. Berlin, 1906.
Kraft, M.—Grundriss der mechanischen Technologie. Abt. ii. Spinnerei, Weberei, und Papierfabrikation. 2te Aufl. Wiesbaden, 8o, 1895.
Lenormand, L. S.—Manuel du fabricant de papier. Paris, 2 vols., 18o, 1833.
Ditto, 2nd edition. 1834.
Lenormand, L. S.—Nouveau manuel complet du ... fabricant de papiers peints. Nouv. ed. par Vergnand. Paris, 18o, 1854.
Lenormand, L. S.—Handbuch der gesammten Papierfabrikation, 2te Aufl., von C. Hartmann. Weimar, 2 vols., 12o, 1862.
Merz.—Behandlung der Papiermaschine.
Meynier, H.—Papier und Papier-Fabrikate. Paris Univ. Exhibition, 1867. Austrian Comm. Berichte. Heft 8. 8o, 1867.
Mierzinski, St.—Handbuch d. Papierfabrikation. 3 Bde. 1886.
Müller, F. A. L.—Die Fabrikation des Papiers, in Sonderheit der auf der Maschinen gefertigten, etc. 3te Aufl. Berlin, 8o, 1862.
Müller, Dr. L.—Die Fabrikation des Papiers. Berlin, 1877.
Olmer, Georges.—Du papier mécanique.
Onfroy.—L'art du papier et le papier d'Arches. 1907.
Paper-Making.—Paper-making, by the Editor of the Paper Mills Directory, London. 2nd edition. 8o, 1876.
Paper-Maker.—The Paper-makers' Handbook and Guide to Paper-making, by a Practical Paper-maker. London, sm. 8o, 1878.
Paper-Manufacture.—Essays by a Society of Gentlemen. No. vi., pp. 21-27. 1717.
Parkinson, R.—Treatise on Paper, with Outline of Manufacture. 1886.
Ditto, 2nd edition, 1896.
Payen, A., and others.—La fabrication du papier et du carton. 3e ed. Paris, 8o, 1881.
Payen, A., and Vigreux, L.—La papeterie. Études sur l'Exposition de 1867. Vol. 8. 8o, 1867.
Pfau, F.—Der junge Papierhändler. Berlin, 1902.
Piette, L.—Manuel ... de papeterie et les succédanés (des chiffons). Paris, 2 vols., 8o, 1861.
Planche, G.—De l'industrie de la papeterie. Paris, 8o, 1853.
Planche, G.—Der Papierfabrikation. Bearbeitet von C. Hartmann. Weimar, 12o, 1853.
Planche, G.—Bericht über die Reinigung der Stoffe zur Papierfabrikation. Uebersetzt und vervollständigt durch eine chronologische Skizze der Papier-Erzeugung und der Verbesserungen an den Maschinen zur Reinigung des Papier-Stoffs von A. Rudel. Leipzig, 8o, 1862.
Prouteaux, A.—Practical Guide for the Manufacture of Paper and (Paper) Boards. With a chapter on Wood Paper in the U.S. by H. T. [Pg 265]Brown. Philadelphia, 8o, 1866.
Prouteaux, A.—Guide de la fabrication du papier et du carton. Paris, 12o, 1864.
Raab, R.—Die Schreibmaterialen und die gesamte Papierindustrie. Hamburg, 1888.
Reed, A. E.—Paper Manufacture. Society for the Promotion of Scientific Industry. Artisans' Reports upon the Vienna Exhibition. 8o, 1873.
Richardson, W. H.—The Industrial Resources of the Tyne ... [Paper]. 1864.
Schubert, M.—Traité pratique de la fabrication de la cellulose. Trad. p. E. Bibas. Toile. 1893.
Schubert, M.—Die Praxis der Papierfabrikation mit besond. Berücksichtigung der Stoffmischungen und deren Calculationen. 1897.
Schubert, M.—Die Papierverarbeitung. 2 Bde. 1900-1901.
Bd. I. Die Kartonnagen-Industrie.
Bd. II. Die Buntpapierfabrikation.
Sindall, R. W.—The Manufacture of Paper Pulp in Burma. Government Press. Rangoon, 1907.
Sindall, R. W.—The Manufacture of Paper. 1908. Constable & Co. London.
Twerdy, E.—Papier industrie. Berichte. Wien, 1873.
Vachon, M.—Les arts et les industries du papier. France, 1871-1894.
Valenta, E.—Das Papier, seine Herstellung, Eigenschaften, Prüfung. 1904.
Wanderley, G.—Die Papierfabrikation und Papierfabrikanlage. Leipzig, 1876.
Watt, A.—The Art of Paper-making, with the Recovery of Soda from Waste Liquors. London, sm. 8o, 1890.
Weber, R.—Papier-Industrie. Vienna Universal Exhibition, 1873.
Wehrs, G. F.—Vom Papier, den vor der Erfindung desselben üblich gewesenen Schreibmassen und sonstigen Schreibmaterialien. Halle, 8o, 1789.
Winkler, O.—Der Papierkenner. 1887.
PAPER, SPECIAL KINDS.
Andés, L. E.—Papier-Spezialitäten, praktische Anleitung zur Herstellung. 1896.
Andés, L. E.—Treatment of Paper for Special Purposes. Translated from German. 1907.
Andés, L. E.—Die Fabrikation der Papiermaché und Papierstoff-Waren. Leipzig, 1900.
Andés, L. E.—Blattmetalle, Bronzen und Metallpapiere, deren Herstellung und Anwendung. Wien, sm. 8o, 1902.
Boeck, J. P.—Die Marmorirkunst für Buchbindereien, Buntpapierfabriken. Wien, sm. 8o, 1880.
Briquet, M.—De quelques industries nouvelles dont le papier est la base. Genève, 1885.
Exner, W. F.—Tapeten- und Buntpapier-Industrie. Paris Univ. Exhibition, 1867. Austrian Comm. Berichte. Heft 8. 1867.
Exner, W. F.—Tapeten- und Buntpapier. Vienna Universal Exhibition, 1873. Officieller Ausstellungs-Bericht. Heft 53. 8o, 1873.
Fichtenberg.—Nouveau manuel complet du fabricant de papiers de fantaisie, papiers marbrés, etc. Paris, 18o, 1852.
Herring, R.—Guide to Varieties and Value of Paper. 1860.
Hofmann, A. W.—Report on Vegetable Parchment (Gaine's Patent, No. 2834 of 1853). London, 8o, 1858.
Kaeppelin, D.—Fabrication des papiers peints. Lacroix E., Études sur l'exposition de 1867. Vol. 1. 8o, 1867.
Kaeppelin, D.—Fabrication des papiers peints. 1881.
Lindsey, G.—Pens and Papiermaché. Bevan, G. P., Brit. Manufacturing Industries (iii.). 12o, 1876.
Morton, G. H.—The History of Paper-hangings, with Review of other Modes of Mural Decoration. Liverpool, 8o, 1875.
Sanborn, K.—Old Time Wall Papers. 1905.
Schmidt, C. H.—Die Benutzung des Papiermaché. Weimar, 12o, 1847.
Schmidt, C. H.—Die Papier-Tapetenfabrikation. 3te Aufl. Weimar, 12o, 1856.
Schmidt, C. H.—The Book of Papiermaché and Japanning. London, 1850.
Seeman, Th.—Die Tapete, ihre aesthetische Bedeutung u. Techn. Darstellung, sowie kurze Beschreibung der Buntpapierfabrik. 1882.
Silcox.—Manufacture of Paper Barrels. Vienna Exhibition, 1873. U.S.A. Reports, ii.
Smee, A.—Report on Vegetable Parchment (Gaine's Patent, No. 2834 [Pg 267]of 1853). London, 8o, 1858.
Thon, C. F. G.—Der Fabrikant bunter Papiere, 3te Aufl. Weimar, 12o, 1844.
Weichelt, A.—Buntpapier-Fabrikation. Berlin, 8o, 1903.
Whiting Paper Co.—How Paper is Made. Holyoke, Mass., 32o, 1893.
Winzer, A.—Die Bereitung und Benutzung der Papiermaché und ähnlicher Kompositionen, 3te Aufl. Weimar, 12o, 1884.
Ditto, 4th edition, 1907.
Woolnough, C. W.—The Whole Art of Marbling, as applied to Paper, Book Edges, etc. London, 8o, 1881.
Wyatt, Sir M. D.—Report on Paper-hangings. Paris Univ. Exhibition, 1867. Brit. Comm. Report, Vol. II. 8o, 1867.
STATISTICS AND VARIOUS.
Akesson.—Lexikon der Papier-Industrie. Deutsch-Englisch-Französisch, 2te Aufl. 1905.
Archer, T. C.—British Manufacturing Industries. Vol. 15. Industrial Statistics. London.
Barth, E.—Arbeitsregeln für Fabriken mit besonderer Berücksichtigung von Papierfabriken. Karlsruhe, 1897.
Baudisch, J.—Einige ins Papierfach schlagende Berechnungen. Biberach, 1893.
Dyson.—Mosely Commission Report. Manchester, 1903.
Ermel.—Rapport sur le matériel et les procédés de la papeterie, etc. Paris Univ. Exhibition, 1878. Rapports. Classe 60. 8o, 1881.
Foreign Office, No. 4 (1871).—Reports on the Manufacture of Paper in Japan. London, fol., 1871.
Geyer, A.—Registry of Water-marks and Trade-marks. Compiled from the American Paper Trade (2nd edition). New York, 1898.
Ditto, 5th edition, 1903.
Gratiot, A.—Description de la papeterie d'Essonnes, London International Exhibition of 1851, Prospectuses of Exhibitors. Vol. 2. 8o, 1851.
Krawany, F.—Warte der Papier-Halbstoff- und Pappenfabriken Oesterreich-Ungarns. 1905.
Landgraf, J.—Papier-Holzschliff und seine Zollpolitische Würdigung. Mannheim.
Lockwood & Co.—American Dictionary of Printing and Bookmaking. New York, 1895.
Ludwig, G.—Trockengehalts-Tabellen. Pirna, 1897.
MacNaughton, J.—Factory Book-keeping for Paper Mills. 1900.
Mahrlen.—Papierfabrikation, im Königr. Württemberg (im Jahre 1860). Stuttgart, 8o, 1861.
Marr, D.—Kosten der Betriebskräfte bei 1-24 stündiger Arbeitszeit täglich und unter Berücksichtigung des Aufwandes für die Heizung. München u. Berlin.
Melnikoff, N.—Lehrbuch der Papier-Holzschliff, Zellstoff und Pappenfabrikation. Petersburg, 1905.
Melnikoff, N.—Kleines Handbuch Papierfabrikation. Petersburg, 1906.
Melnikoff, N.—Geschichte, Statistik u. Literatur der Papierindustrie nebst russischen Wasserzeichen. Petersburg, 1906.
Munsell, J.—Chronology of Paper-making. Albany, 8o, 1857.
Ditto, 4th edition, 1870.
Munsell, J.—Chronology of the Origin and Progress of Paper and Paper-making. Albany, 1876.
Munsell, J.—Observations Illustrative of the Operation of the Duties on Paper. London, 8o, 1836.
Munsell, J.—Matériel et procédés de la papeterie, etc., 1889. Rapports du Jury. Classe 58. 8o, 1889.
Paris Univ. Exhibition.—Papiers peints, 1889. Rapports du Jury. Classe 21. 8o, 1891.
Passerat, A. L.—Barème complet pour papeteries. Paris.
Patents.—Patent Abridgments. Class 96. Patent Office Abstracts on Paper-making. From 1855 to date.
Roulhac.—Papeterie. Paris Univ. Exhibition, 1867. Rapports du Jury. Classe 7, sect. 1. 8o, 1868.
Sampson, J. T.—Paper-staining. Mansion House Committee. Artisans' Reports, Paris Exhibition. 8o, 1889.
Treasury.—Report of the Excise Commission. 1835.
Vogel, K.—Papierindustrie, etc., Auf der Weltausstellung in Chicago. Chicago Exhibition, 1893. Austrian Central Committee. Officieller Bericht. Heft iv. 8o, 1894.
Voigt, G.—Papiergewichtstabellen. Merseburg, 1894.
Ward, Sir W.—Report on German Paper-making Industry. Parliamentary Paper, 1905.
Water-marks.—Water-marks and Trade-marks Registry (2nd ed.). [Pg 269]New York, 16o, 1898.
WOOD PULP AND PULP WOOD.
British and Colonial Printer.—History of Wood Pulp. Vol. 8. 1882.
Dunbar.—Wood Pulp and Wood Pulp Papers.
Fittica, Dr. F.—Geschichte der Sulfitzellstoff-Fabrikation. Leipzig, 1901.
Fittica, Dr. F.—Forestry and Forest Products. [Edinburgh Forestry Exhibition. 1884.]
Gottstein.—Holzzellstoff in seiner Anwendung für die Papier- und Textil-Industrie und die bei seiner Herstellung entstehenden Abwässer. 1904.
Griffin, M. L.—Sulphite Processes. American Society C. E. 417. 1889.
Harper, W.—Utilisation of Wood Waste by Distillation. U.S.A., 1907.
Harpf, A.—Die Erzeugung von Holzschliff und Zellstoff. Wien, 1901.
Harpf, A.—Flüssiges Schwefeldioxyd. Stuttgart, 1901.
Hubbard.—Utilisation of Wood Waste. 1902.
Johnson, G.—Wood Pulp of Canada. 1902-08. Yearly.
Michaelis, O. E.—Lime Sulphite Fibre Manufacture in the United States. With Remarks on the Chemistry of the Processes, by M. L. Griffin (excerpt). New York, 8o, 1889.
Phillips, S. C.—Uses of Wood Pulp. 1904.
Rosenheim, G. M.—Die Holzcellulose. Berlin, 1878.
Schubert, M.—Die Holzstoff oder Holzschliff-Fabrikation. 1898.
Schubert, M.—Die Cellulosefabrikation (Zellstofffabrikation). Praktisches Handbuch für Papier- u. Cellulosetechniker. 1906.
Sindall, R. W.—The Sampling of Wood Pulp. London, 8o, 1901.
Veitch, L. P.—Chemical Methods for Utilising Wood. U.S.A. Department of Agriculture. 1907.
Veitch, L. P.—Wood Pulp, Uses of. U.S.A. Consular Reports, vol. xix.
Banks and Crate.—Pulpwood Problems. Letters to the Globe, Toronto, Canada. 1907.
Gamble, J.—Indian Timbers.
Graves.—The Woodsman's Handbook. U.S.A.
Pinchott, G.—Forestry Primer. U.S.A., 1900.
Pinchott, G.—The Adirondack Spruce. U.S.A.
Rattray, J., and Mill, H. R.—Forestry and Forestry Products. Edinburgh, 1885.
Schlich.—Forestry Manual.
Some more or less interesting articles on “Paper” will be found in the following encyclopædias, etc.:—
DATE.
1738. Chambers's Encyclopædia.
1757. Barrow. Dictionary of Arts.
1759. New. Universal History of Arts.
1770. Royal Dictionary of Arts.
1788. Howard. A Royal Encyclopædia.
1806. Gregory. A Dictionary of Arts and Sciences.
1807. Encyclopædia Perthensis.
1809. Nicholson. The British Encyclopædia.
1813. Martin. Circle of the Mechanical Arts.
1813. Pantologia.
1819. Rees' Cyclopædia.
1821. Encyclopædia Londoniensis.
1827. Jamieson's Dictionary.
1828. Oxford Encyclopædia.
1829. The London Encyclopædia.
1830. Edinburgh Encyclopædia.
1833. Phillip's Dictionary of Arts.
1835. Partington. British Cyclopædia.
1836. Archæologia, vol. xxvi.
1836. Barlow. Encyclopædia of Arts.
1840. The Penny Encyclopædia.
1845. Encyclopædia Metropolitana.
1848. Useful Arts of Great Britain. S.P.C.K
1851. Knight's Cyclopædia of Industry.
1855. Appleton's Dictionary of Mechanics.
1860. Hebert. Mechanic's Encyclopædia.
1861. Knight's English Cyclopædia.
1861. New American Cyclopædia.
1866. Tomlinson's Dictionary of Arts.
1871. Yeats. The Technical History of Commerce.
1874. Clarke's Practical Magazine.
[Pg 271]1875. Ure's Dictionary of Arts.
1875. Globe Cyclopædia.
1876. American Mechanical Dictionary.
1877. Johnson's Universal Cyclopædia.
1880. Wylde. Industries of the World.
1882. Spon's Encyclopædia of Manufactures.
1886. Encyclopædia Britannica.
1889. Chambers's Encyclopædia.
1889. Blaikie. Modern Cyclopædia.
1890. Popular Encyclopædia.
1892. Spon's Workshop Receipts.
1903. Gilman. International Encyclopædia.
1904. Encyclopædia Americana.
1904. Tweney's Technological Dictionary.
Newspapers.
England.
Papermaker and British Paper Trade Journal. S. C. Phillips, London.
Papermakers' Circular. Dean & Son, London.
Papermakers' Monthly Journal. Marchant, Singer & Co., London.
Paper Box and Bag Maker. S. C. Phillips, London.
Papermaking. London.
The Paper and Printing Trades' Journal. London.
World's Paper Trade Review. W. J. Stonhill, London.
Canada.
Pulp and Paper Magazine. Biggar-Wilson, Ltd., Toronto.
United States of America.
American Bookmaker. Howard Lockwood & Co., New York.
The Paper Trade. Chicago.
The Stationer. Howard Lockwood & Co., New York.
Paper Mill and Wood Pulp News. L. D. Post & Co., New York.
Paper Trade Journal. Howard Lockwood & Co., New York.
The Paper World. C. W. Bryan & Co., Holyoke, Mass.
France.
Bulletin Journal des Fabricants de Papier. Paris.
Journal des Papetiers. M. Edmond Rousset, Paris.
Le Moniteur de la Papeterie Française. Paris.
La Papeterie. Paris.
La Revue de la Papeterie Française et Étrangère. M. Edmond Rousset, Paris.
Le Papier. H. Everling, Paris.
Germany.
Centralblatt für die Österreichisch-Ungarische Papierindustrie. Adolf Hladufka, Wien.
Der Papierfabrikant. Otto Elsner, Berlin.
Der Papier-Markt. Carl Dobler, Frankfurt a. Main.
Deutsche Papier- und Schreibwarenzeitung. S. Richter, Berlin.
Die Postkarte. Gustav Fahrig, Leipzig.
Export-Journal. G. Hedeler, Leipzig.
Holzstoff-Zeitung. Camillo Drache, Dresden.
Papierhändler Zeitung für Österreich-Ungarn. Wien.
Papier-Industrie. Berlin.
Papier- und Schreibwaren-Zeitung. Wien.
Papier-Zeitung. C. Hofmann, Berlin.
Schweizer Graphischer Central-Anzeiger. H. Keller, Luzern.
Wochenblatt für Papierfabrikation. Guntter-Staib Biberach (Württ).
Wochenschrift für den Papier- und Schreibwarenhandel. Dr. H. Hirschberg, Berlin.
ANALYSIS, TECHNOLOGY.
Beadle and Stevens.—Blotting paper, nature of absorbency. 1905.
Winkler.—Estimation of Moisture in Wood-pulp. 1902. Translated by Dr. H. P. Stevens.
Hauptversammlung.—Published annually by the Verein der Zellstoff- und Papier-Chemiker. Berlin, 1907 et.
FIBRES, etc.
Dodge, C. R.—Catalogue of useful Fibre-plants of the World. Report No. 9. Dept. of Agriculture. U.S.A., 1897.
Duchesne, E. A.—Répertoire des plantes utiles et des plantes vénéneuses du globe, etc. Bruxelles, 1846.
Gabalde, B.—Essai sur le bananier et ses applications à la fabrication de papier. 1843.
Montessus de Ballore.—Alfa et papier d'Alfa. 1908.
Pecheux.—Les textiles, les tissus, le papier. 6 pp. Paris, 1907.
Renouard.—Études sur les fibres textiles. Paris.
Renouard.—Les fibres textiles de l'Algérie. Paris.
Riviere, Auguste et Charles.—“Les Bambous.” Société d'Acclimatation. Paris.
Richmond, G. F.—Philippine Fibres and Fibrous Substances. Manila, Bureau of Printing, 1906.
HISTORICAL.
Briquet, C. M.—Recherches sur les premiers Papiers employés du Xe au XIVe siècle. pp. 77. Paris, 1886.
Briquet, C. M.—De la valeur des Filigranes du Papier comme moyen de déterminer l'âge de documents. pp. 13. Genève, 1892.
Briquet, C. M.—La Légende paléographique du Papier de Coton. pp. 18. Genève, 1884.
Briquet, C. M.—Lettre sur les Papiers usités en Sicile à l'occasion de deux manuscrits en papier dit le coton. 16 pp. Palermo, 1892.
Desmarest, N.—Art de la Papeterie. Paris, 1879.
Delon, C.—Histoire d'un livre. Paris, 1879.
Didot, A. F.—Le centenaire de la Machine à Papier continu. pp. 79. Paris, 1900.
Dickinson, J.—Dickinson's Paper Mills. Calcutta, 1884.
Girard, A.—Le Papier. Ses ancêtres. Son histoire. Lille, 1892.
Julien, S.—Description des procédés chinois pour la fabrication du papier. Traduit de l'ouvrage chinois par Thien-Kong-Kha-We. 1840.
Kay, J.—Paper, its history. pp. 100. London, 1893.
Lempertz, H.—Beiträge zur Geschichte des Leinen-Papiers. Köln, 1891.
PAPER MANUFACTURE.
Bory, P.—Les Métamorphoses d'un Chiffon. Abbeville, 1897.
Chabrol, L.—La Réglementation du Travail dans l'industrie du papier. pp. 168. Paris, 1901.
Demuth, F.—Die Papier-Fabrikation. 1903.
Demuth, F.—Die Störungen im deutschen Wirtschaftsleben 1900. Leipzig, 1903.
Limoge.—Cercles d'Études commerciales, Le Papier. pp. 140. Limoge, 1892.
PAPER, SPECIAL KINDS.
Spalding and Hodge.—Printing papers; a handbook. London, 1905.
STATISTICS, etc.
Beadle, C.—Development of Water-marking. London, 1906 (Society of Arts).
Dumercy.—Bibliographie de la Papeterie. pp. 28. Bruxelles, 1888.
Bruce, H.—Gladstone and Paper Duties. Edinburgh, 1885.
Ellis, J. B.—Hints for the Paper Warehouse. Leeds, 1887.
Webster, J.—Synopsis of Sizes of Paper. Southport, 1889.
Whitson, W.—The Concise Paper Calculator. Edinburgh, 1903.
WOOD PULP, etc.
Dropisch, B.—Holzstoff und Holzcellulose. Weimar, 1879.
Acid dyes, 201
in papers, 239
size, 170
Agave, 40
Alum, 167, 168
Aniline dyes, 201
sulphate, 121
Animal size, 63, 164
Antichlors, 163
Art paper, 142
imitation, 145
testing, 147
Asbestos, 174
Ash in paper, 171
Backwater, 120, 205
Bagasse, 41
Bamboo, 43
Barker, 97
Beating engines, 186
patents, 192
power consumed, 191
Beating, conditions of, 197
early methods of, 176
experiments in, 179
process of, 58, 175
Bibliography, 253
Bisulphite of lime, 159
Bleaching, 57, 83
powder, 161
Blue prints, 140
Board machine, 132, 135
Boards, manufacture of, 131
duplex, 132, 134
Book papers, quality of, 246
Books, decay of, 237
Brown papers, 127
Carbonic acid recorder, 215
Casein, 165, 235
Caustic soda, 81, 155
Cellulose, 21
derivatives of, 29
hydrolysis of, 27, 229
oxidation of, 28
percentage of, in plants, 23
properties of, 26
Chemical residues in paper, 238
wood pulp, 104
Chemicals, 153
China clay, 117, 150, 171, 204, 234
Coal consumption, 214
Coated paper, 142
Cold ground pulp, 100
Colophony, 169
Colour of paper, fading of, 203, 241
matching, 205
unevenness of, 203
Colouring of paper pulp, 199
analysis of, 206
[Pg 274]
Cotton, 22, 69
Cyanotype papers, 140
Cylinder machine, 131
Density of paper, 181
Deterioration of paper, 228, 246
Digesters, 52, 89, 109
Dilution tables, 157, 163
Duplex boards, 134
Dyeing of paper, 199
Eibel patent, 223
Electrical power, 219
Electrolytic bleaching, 57
Engine sizing, 117, 167
Esparto, 72
bleaching of, 83
composition of, 73
test for, in papers, 87
yield of, 77
Evaporation apparatus, 76, 79
tables, 81
Featherweight papers, 232
Fibres for paper-making, 38
examination of, 43
reagents for staining, 71
Flax, 40
Fourdrinier machine, early, 16
French chalk, 173
Gas producer, 218
Gelatine, 63, 164, 237
Glue, 137, 142, 235
Grinders, 100
History of paper, 1
Hoernle, 7
Hollander, 16, 59, 176, 185
Hot ground pulp, 100
Imitation art paper, 145, 235
Kraft paper, 129
parchment, 137
Improvements in paper-making, 214
Iron in paper, 229
Kraft papers, 128
Laid papers, 66
Lime, 52, 157
bisulphite, 159
sulphate, 173
Linen fibre, 70
Loading, 171
M. G. caps, 130
Machinery, 214, 224
Manila paper, 127
Mechanical pulp, 95
detection of, 121
Metanil yellow, 122
Middles, 134
Mitscherlich pulp, 107
Moisture, influence of, 243
Multiple effect evaporation, 79
Neutral size, 169
Newspaper, 116, 215
Output of a paper machine, 122
Paper, art, 142
ash in, 171
brown, 127
bulk of, 231
chemical residues in, 238
clay in, 234
colour of, 199, 241
colour in, analysis of, 207
deterioration of, 229
fibres for, 38
history of, 1, 5
iron in, 239
[Pg 275]
permanence of, 230
rags used for, 47
sizing of, 63
special kinds of, 137
standards of quality, 246
strength, of, 184, 231
surface of, 233
volume composition of, 233
Paper machine, early, 16
output of, 122
Papier-maché, 150
Papyrus, 2, 42
Paraffin paper, 148
Parchment, 4
paper, 137
Peat, 41
Phloroglucine, 121
Pigments, 199
Porion evaporator, 76
Presse-pâte, 86
Prussian blue, 200
Rag paper, manufacture of, 47
origin of, 5
Rags, bleaching, 55
boiling, 51
classification, 48
sorting, 48
Ramie, 40
Records, early, 1
Recovered ash, 158
Recovery processes, 78, 113
Refiners, 90
Rope browns, 127
Rosin size, 117, 169, 236
Screens, 102
Sealings, 129
Shrinkage of paper, 181
Sizing of paper, 63, 117, 167
Society of Arts, 246
Soda, 153
Soda pulp, 107, 113
recovery, 78
silicate of, 166, 171
Softening of water, 216
Spent liquors, 78, 113
Staining reagents for fibres, 71
Standards of quality, 246, 248, 250
Starch, 166, 237
Stationery Office, 248
Stone beater rolls, 189
Straw, 88
Sulphate pulp, 107
Sulphite pulp, 107
Sulphites, 159, 163
Supercalender, 65
Superheated steam, 218
Tinfoil paper, 148
Transfer paper, 149
Ultramarine, 199
Volume composition of paper, 233
Vulcanised fibre, 139
Water softening, 216
Watermarks, 67
Wavy edges, 243
Waxed paper, 147
Wet press machine, 103
Wiesner, 6
Willesden paper, 139
Wood, 22
pulp, 95
chemical, 104
mechanical, 95
soda, 107, 113
sulphite, 107
Wove papers, 66
Wrappers, 127
BRADBURY, AGNEW, & CO. LD., PRINTERS, LONDON AND TONBRIDGE.
VAN NOSTRAND'S
“Westminster” Series
Bound in uniform style. Fully Illustrated. Price $2·00 net each.
The Volumes in the “Westminster” Series have been designed to meet the growing demand for books on practical subjects; to bring within the ken of the non-technical reader an accurate knowledge of manufacturing processes and the practical application of modern science to industries. Each volume is written by an expert to the end that practical readers and all who are engaged in the numerous allied branches of the engineering and technical trades may have reliable works of reference. The series provides for a class not hitherto reached in published works. The volumes can be easily read by the general public, and make excellent handbooks at a moderate price for the student.
The series is well suited to public libraries and will be found valuable for libraries in engineering shops and factories.
D. VAN NOSTRAND COMPANY
Publishers and Booksellers
23, Murray and 27, Warren Streets, New York.
Coal. By James Tonge, M.I.M.E., F.G.S., etc. (Lecturer on Mining at Victoria University, Manchester). With 46 Illustrations, many of them showing the Fossils found in the Coal Measures.
List of Contents: History. Occurrence. Mode of Formation of Coal Seams. Fossils of the Coal Measures. Botany of the Coal-Measure Plants. Coalfields of the British Isles. Foreign Coalfields. The Classification of Coals. The Valuation of Coal. Foreign Coals and their Values. Uses of Coal. The Production of Heat from Coal. Waste of Coal. The Preparation of Coal for the Market. Coaling Stations of the World. Index.
This book on a momentous subject is provided for the general reader who wishes accurate knowledge of Coal, its origin, position and extent, and its economical utilization and application.
Iron and Steel. By J. H. Stansbie, B.Sc. (Lond.), F.I.C. With 86 Illustrations.
List of Contents: Introductory. Iron Ores. Combustible and other materials used in Iron and Steel Manufacture. Primitive Methods of Iron and Steel Production. Pig Iron and its Manufacture. The Refining of Pig Iron in Small Charges. Crucible and Weld Steel. The Bessemer Process. The Open Hearth Process. Mechanical Treatment of Iron and Steel. Physical and Mechanical Properties of Iron and Steel. Iron and Steel under the Microscope. Heat Treatment of Iron and Steel. Electric Smelting. Special Steels. Index.
The aim of this book is to give a comprehensive view of the modern aspects of iron and steel, together with a sufficient account of its history to enable the reader to follow its march of progress. The methods of producing varieties of the metal suitable to the requirements of the engineer, foundryman and mechanician are described so that the worker may learn the history of the material he is handling.
Natural Sources of Power. By Robert S. Ball, B.Sc., A.M.Inst.C.E. With 104 Diagrams and Illustrations.
Contents: Preface. Units with Metric Equivalents and Abbreviations. Length and Distance. Surface and Area. Volumes. Weights or Measures. Pressures. Linear Velocities, Angular Velocities. Acceleration. Energy. Power. Introductory Water Power and Methods of Measuring. Application of Water Power to the Propulsion of Machinery. The Hydraulic Turbine. Various Types of Turbine. Construction of Water Power Plants. Water Power Installations. The Regulation of Turbines. Wind Pressure, Velocity, and Methods of Measuring. The Application of Wind Power to Industry. The Modern Windmill. Constructional Details. Power of Modern Windmills. Appendices A, B, C. Index.
Two departments of Engineering and their applications to industry form the subject of this volume: the “natural” sources of water(3) and wind power which supply mechanical energy without any intermediate stage of transformation. Most people will be surprised at the extent to which these natural power producers are used. The widespread application of water power is generally known, but it is interesting to learn that the demand for windmills was never so great as it is to-day, and there are signs of abnormal expansion in the direction of their useful application in the great agricultural countries of the world. Though primarily of importance to the engineer, this work will be of great interest to every manufacturer who in economizing his means of power production can take the natural forces that lie to his hand and harness them in his service. The author is the son of Sir Robert Ball, the eminent mathematician and astronomer.
Liquid and Gaseous Fuels, and the Part they play in Modern Power Production. By Professor Vivian B. Lewes, F.I.C., F.C.S., Prof. of Chemistry, Royal Naval College, Greenwich. With 54 Illustrations.
List of Contents: Lavoisier's Discovery of the Nature of Combustion, etc. The Cycle of Animal and Vegetable Life. Method of determining Calorific Value. The Discovery of Petroleum in America. Oil Lamps, etc. The History of Coal Gas. Calorific Value of Coal Gas and its Constituents. The History of Water Gas. Incomplete Combustion. Comparison of the Thermal Values of our Fuels, etc. Appendix. Bibliography. Index.
The subject of this book has, during the last decade, assumed such importance that it is hoped this account of the history and development of the use of various forms of combustible liquids and gases for the generation of energy may do some service in its advancement.
Electric Power and Traction. By F. H. Davies, A.M.I.E.E. With 66 Illustrations.
List of Contents: Introduction. The Generation and Distribution of Power. The Electric Motor. The Application of Electric Power. Electric Power in Collieries. Electric Power in Engineering Workshops. Electric Power in Textile Factories. Electric Power in the Printing Trade. Electric Power at Sea. Electric Power on Canals. Electric Traction. The Overhead System and Track Work. The Conduit System. The Surface Contact System. Car Building and Equipment. Electric Railways. Glossary. Index.
The majority of the allied trades that cluster round the business of electrical engineering are connected in some way or other with its power and traction branches. To members of such trades and callings, to whom some knowledge of applied electrical engineering is desirable if not strictly essential, the book is particularly intended to appeal. It deals almost entirely with practical matters, and enters to some extent into those commercial considerations which in the long run must overrule all others.
Town Gas and its Uses for the Production of Light, Heat, and Motive Power. By W. H. Y. Webber, C.E. With 71 Illustrations.
List of Contents: The Nature and Properties of Town Gas. The History and Manufacture of Town Gas. The By-Products of Coal Gas Manufacture. Gas Lights and Lighting. Practical Gas Lighting. The Cost of Gas Lighting. Heating and Warming by Gas. Cooking by Gas. The Healthfulness and Safety of Gas in all its uses. Town Gas for Power Generation, including Private Electricity Supply. The Legal Relations of Gas Suppliers, Consumers, and the Public. Index.
The “country,” as opposed to the “town,” has been defined as “the parts beyond the gas lamps.” This book provides accurate knowledge regarding the manufacture and supply of town gas and its uses for domestic and industrial purposes. Few people realize the extent to which this great industry can be utilized. The author has produced a volume which will instruct and interest the generally well informed but not technically instructed reader.
Electro-Metallurgy. By J. B. C. Kershaw, F.I.C. With 61 Illustrations.
Contents: Introduction and Historical Survey. Aluminium. Production. Details of Processes and Works. Costs. Utilization. Future of the Metal. Bullion and Gold. Silver Refining Process. Gold Refining Processes. Gold Extraction Processes. Calcium Carbide and Acetylene Gas. The Carbide Furnace and Process. Production. Utilization. Carborundum. Details of Manufacture. Properties and Uses. Copper. Copper Refining. Descriptions of Refineries. Costs. Properties and Utilization. The Elmore and similar Processes. Electrolytic Extraction Processes. Electro-Metallurgical Concentration Processes. Ferro-alloys. Descriptions of Works. Utilization. Glass and Quartz Glass. Graphite. Details of Process. Utilization. Iron and Steel. Descriptions of Furnaces and Processes. Yields and Costs. Comparative Costs. Lead. The Salom Process. The Betts Refining Process. The Betts Reduction Process. White Lead Processes. Miscellaneous Products. Calcium. Carbon Bisulphide. Carbon Tetra-Chloride. Diamantine. Magnesium. Phosphorus. Silicon and its Compounds. Nickel. Wet Processes. Dry Processes. Sodium. Descriptions of Cells and Processes. Tin. Alkaline Processes for Tin Stripping. Acid Processes for Tin Stripping. Salt Processes for Tin Stripping. Zinc. Wet Processes. Dry Processes. Electro-Thermal Processes. Electro-Galvanizing. Glossary. Name Index.
The subject of this volume, the branch of metallurgy which deals with the extraction and refining of metals by aid of electricity, is becoming of great importance. The author gives a brief and clear account of the industrial developments of electro-metallurgy, in language that can be understood by those whose acquaintance with either(5) chemical or electrical science may be but slight. It is a thoroughly practical work descriptive of apparatus and processes, and commends itself to all practical men engaged, in metallurgical operations, as well as to business men, financiers, and investors.
Radio-Telegraphy. By C. C. F. Monckton, M.I.E.E. With 173 Diagrams and Illustrations.
Contents: Preface. Electric Phenomena. Electric Vibrations. Electro-Magnetic Waves. Modified Hertz Waves used in Radio-Telegraphy. Apparatus used for Charging the Oscillator. The Electric Oscillator: Methods of Arrangement, Practical Details. The Receiver: Methods of Arrangement, The Detecting Apparatus, and other details. Measurements in Radio-Telegraphy. The Experimental Station at Elmers End: Lodge-Muirhead System. Radio-Telegraph Station at Nauen: Telefunken System. Station at Lyngby: Poulsen System. The Lodge-Muirhead System, the Marconi System, Telefunken System, and Poulsen System. Portable Stations. Radio-Telephony. Appendices: The Morse Alphabet. Electrical Units used in this Book. International Control of Radio-Telegraphy. Index.
The startling discovery twelve years ago of what is popularly known as Wireless Telegraphy has received many no less startling additions since then. The official name now given to this branch of electrical practice is Radio-Telegraphy. The subject has now reached a thoroughly practicable stage, and this book presents it in clear, concise form. The various services for which Radio-Telegraphy is or may be used are indicated by the author. Every stage of the subject is illustrated by diagrams or photographs of apparatus, so that, while an elementary knowledge of electricity is presupposed, the bearings of the subject can be grasped by every reader. No subject is fraught with so many possibilities of development for the future relationships of the peoples of the world.
India-Rubber and its Manufacture, with Chapters on Gutta-Percha and Balata. By H. L. Terry, F.I.C., Assoc.Inst.M.M. With Illustrations.
List of Contents: Preface. Introduction: Historical and General. Raw Rubber. Botanical Origin. Tapping the Trees. Coagulation. Principal Raw Rubbers of Commerce. Pseudo-Rubbers. Congo Rubber. General Considerations. Chemical and Physical Properties. Vulcanization. India-rubber Plantations. India-rubber Substitutes. Reclaimed Rubber. Washing and Drying of Raw Rubber. Compounding of Rubber. Rubber Solvents and their Recovery. Rubber Solution. Fine Cut Sheet and Articles made therefrom. Elastic Thread. Mechanical Rubber Goods. Sundry Rubber Articles. India-rubber Proofed Textures. Tyres. India-rubber Boots and Shoes. Rubber for Insulated Wires. Vulcanite Contracts for India-rubber Goods. (6)The Testing of Rubber Goods. Gutta-Percha. Balata. Bibliography. Index.
Tells all about a material which has grown immensely in commercial importance in recent years. It has been expressly written for the general reader and for the technologist in other branches of industry.
Glass Manufacture. By Walter Rosenhain, Superintendent of the Department of Metallurgy in the National Physical Laboratory, late Scientific Adviser in the Glass Works of Messrs. Chance Bros. and Co. With Illustrations.
Contents: Preface. Definitions. Physical and Chemical Qualities. Mechanical, Thermal, and Electrical Properties. Transparency and Colour. Raw materials of manufacture. Crucibles and Furnaces for Fusion. Process of Fusion. Processes used in Working of Glass. Bottle. Blown and Pressed. Rolled or Plate. Sheet and Crown. Coloured. Optical Glass: Nature and Properties, Manufacture. Miscellaneous Products. Appendix. Bibliography of Glass Manufacture. Index.
This volume is for users of glass, and makes no claim to be an adequate guide or help to those engaged in glass manufacture itself. For this reason the account of manufacturing processes has been kept as non-technical as possible. In describing each process the object in view has been to give an insight into the rationale of each step, so far as it is known or understood, from the point of view of principles and methods rather than as mere rule of thumb description of manufacturing manipulations. The processes described are, with the exception of those described as obsolete, to the author's definite knowledge, in commercial use at the present time.
Precious Stones. By W. Goodchild, M.B., B.Ch. With 42 Illustrations. With a Chapter on Artificial Stones. By Robert Dykes.
List of Contents: Introductory and Historical. Genesis of Precious Stones. Physical Properties. The Cutting and Polishing of Gems. Imitation Gems and the Artificial Production of Precious Stones. The Diamond. Fluor Spar and the Forms of Silica. Corundum, including Ruby and Sapphire. Spinel and Chrysoberyl. The Carbonates and the Felspars. The Pyroxene and Amphibole Groups. Beryl, Cordierite, Lapis Lazuli and the Garnets. Olivine, Topaz, Tourmaline and other Silicates. Phosphates, Sulphates, and Carbon Compounds.
An admirable guide to a fascinating subject.
Patents, Designs and Trade Marks: The Law and Commercial Usage. By Kenneth R. Swan, B.A. (Oxon.), of the Inner Temple, Barrister-at-Law.
Contents: Table of Cases Cited—Part I.—Letters Patent. Introduction. General. Historical. I., II., III. Invention, Novelty, Subject Matter, and Utility the Essentials of Patentable Invention. IV. Specification. V. Construction of Specification. VI. Who May Apply for a Patent. VII. Application and Grant. VIII. Opposition. IX. Patent Rights. Legal Value. Commercial Value. X. Amendment. XI. Infringement of Patent. XII. Action for Infringement. XIII. Action to Restrain Threats. XIV. Negotiation of Patents by Sale and Licence. XV. Limitations on Patent Right. XVI. Revocation. XVII. Prolongation. XVIII. Miscellaneous. XIX. Foreign Patents. XX. Foreign Patent Laws: United States of America. Germany. France. Table of Cost, etc., of Foreign Patents. Appendix A.—1. Table of Forms and Fees. 2. Cost of Obtaining a British Patent. 3. Convention Countries. Part II.—Copyright in Design. Introduction. I. Registrable Designs. II. Registration. III. Marking. IV. Infringement. Appendix B.—1. Table of Forms and Fees. 2. Classification of Goods. Part III.—Trade Marks. Introduction. I. Meaning of Trade Mark. II. Qualification for Registration. III. Restrictions on Registration. IV. Registration. V. Effect of Registration. VI. Miscellaneous. Appendix C.—Table of Forms and Fees. Indices. 1. Patents. 2. Designs. 3. Trade Marks.
This is the first book on the subject since the New Patents Act. Its aim is not only to present the existing law accurately and as fully as possible, but also to cast it in a form readily comprehensible to the layman unfamiliar with legal phraseology. It will be of value to those engaged in trades and industries where a knowledge of the patenting of inventions and the registration of trade marks is important. Full information is given regarding patents in foreign countries.
The Book; Its History and Development. By Cyril Davenport, V.D., F.S.A. With 7 Plates and 126 Figures in the text.
List of Contents: Early Records. Rolls, Books and Book bindings. Paper. Printing. Illustrations. Miscellanea. Leathers. The Ornamentation of Leather Bookbindings without Gold. The Ornamentation of Leather Bookbindings with Gold, Bibliography. Index.
The romance of the Book and its development from the rude inscriptions on stone to the magnificent de Luxe tomes of to-day have never been so excellently discoursed upon as in this volume. The history of the Book is the history of the preservation of human thought. This work should be in the possession of every book lover.
Van Nostrand's “Westminster” Series
LIST OF NEW AND FORTHCOMING VOLUMES.
Timber. By J. R. Baterden, A.M.I.C.E.
Steam Engines. By J. T. Rossiter, M.I.E.E., A.M.I.M.E.
Electric Lamps. By Maurice Solomon, A.C.G.I., A.M.I.E.E.
The Railway Locomotive. By Vaughan Pendred, M.I.Mech.E.
Leather. By H. Garner Bennett.
Pumps and Pumping Machinery. By James W. Rossiter, A.M.I.M.E.
Workshop Practice. By Professor G. F. Charnock, A.M.I.C.E., M.I.M.E.
Textiles and their Manufacture. By Aldred Barker, M.Sc.
Gold and Precious Metals. By Thomas K. Rose, D.Sc., of the Royal Mint.
Photography. By Alfred Watkins, Past President of the Photographic Convention.
Commercial Paints and Painting. By A. S. Jennings, Hon. Consulting Examiner, City and Guilds of London Institute.
Ornamental Window Glass Work. By A. L. Duthie.
Brewing and Distilling. By James Grant, F.C.S.
Wood Pulp and Its Applications. By C. F. Cross, E. J. Bevan and R. W. Sindall.
The Manufacture of Paper. By R. W. Sindall.
D. VAN NOSTRAND COMPANY
Publishers and Booksellers
23, MURRAY AND 27, WARREN STREETS, NEW YORK.