
DUCK POND, CORNER OF FOURTH AND MARKET STREETS (ABOUT 1700)
The Project Gutenberg EBook of The Diatomaceae of Philadelphia and Vicinity, by Charles Sumner Boyer This eBook is for the use of anyone anywhere at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org Title: The Diatomaceae of Philadelphia and Vicinity Author: Charles Sumner Boyer Release Date: January 3, 2014 [EBook #44569] Language: English Character set encoding: ISO-8859-1 *** START OF THIS PROJECT GUTENBERG EBOOK DIATOMACEAE *** Produced by Charlene Taylor, Bryan Ness, Keith Edkins and the Online Distributed Proofreading Team at http://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)
THE DIATOMACEĈ OF
PHILADELPHIA AND VICINITY
BY
CHARLES S. BOYER, A.M., F.R.M.S.
ILLUSTRATED WITH SEVEN HUNDRED
DRAWINGS BY THE AUTHOR
PRESS OF
J. B. LIPPINCOTT COMPANY
EAST WASHINGTON SQUARE PHILADELPHIA
1916
The present contribution to the local flora is intended as an introduction to more extended research.
The study is of advantage in relation to the life history of aquatic animals, the determination of ocean currents, as proved by polar discoveries, the investigation of geological strata where other fossil forms are absent, and the analysis of water supply; and, when we consider the universal distribution of diatomaceĉ in the earth, the water and even in the air and the enormous deposits formed in past ages and still forming, we are able to realize the importance of a knowledge of these complicated forms and their function of purification.
The absence of descriptive works of reference in available form in this country, the polyglot confusion of authorities abroad and the amount of time, patience and skill required in obtaining, preparing and examining specimens, render the study one of difficulty.
The bibliography is omitted, as it is understood by those who possess the works of reference, and but few synonyms are given, having but little, except historical, value, especially when it is considered that modern investigators have no access to many of the earlier collections, when any of these exist.
So far as the marine forms are concerned, it is probable that nearly all occurring north of Florida are here included, and the fresh-water species described represent a large proportion of those found east of the Alleghanies. All of the figures are drawn to the same scale, a magnification of eight hundred diameters, from specimens in my possession, nearly all of which were found in or near Philadelphia.
If the work is of any value in inducing further investigation, I hope, in the words of Julien Deby, that "those who follow my advice will find in the study of these wonderful little organisms as much pleasure as I myself have found."
The Author.
The Delaware River rises in the Western Catskill Mountains, flows southward for about three hundred and seventy-five miles, and expands into Delaware Bay about sixty miles from the sea. Its origin is among the Devonian and Carboniferous rocks, and in its course it passes through Silurian, Triassic and Cretaceous formations, finally reaching the Cambrian and Laurentian beds. It also drains regions of the glacial drift and beds which overlie overturned Miocene strata, and are sometimes mixed with them. From the mountains, nearly four thousand feet high, to the Bay, where the depth of water is not greater than seventy-five feet, the diatomaceous flora, from Alpine cascades to the salt marshes of New Jersey, contains a larger number of species than any other equal portion of the American coast.
The city of Philadelphia, about one hundred miles from the sea, lies at the junction of the Schuylkill with the Delaware, and much of the land near the rivers, especially southward, is flat and low, composed of recent alluvial deposits. In the central districts the ground is high, the deep sub-soil being mostly a dry gravel resting upon gneiss and schist, although it is in part composed of a bluish clay which was probably laid down in the bed of the ancient river before the last period of the glacial drift. The blue clay was not all deposited at the same time, as in the lower strata many marine forms are found which do not occur in the upper layers. This is notably the case in a deposit obtained at Spreckel's Sugar Refinery and also at the east end of Walnut Street Bridge, where a layer of blue clay occurs which is overlain by glacial drift. In other parts of the city mixtures of blue clay with more recent deposits are found, including fresh-water forms from numerous creeks and rivulets which traversed what is now the city proper, and especially from the vicinity of Fourth and Market Streets, where there existed as late as the year 1700 a large pond known as the "Duck Pond" which was subject to tidal overflow from its outlet, Dock Creek. The river water at Philadelphia is not noticeably brackish, although the tide extends thirty miles above the city and, before the building of Fairmount Dam, to the Falls of the Schuylkill. At certain times, when the river is low, the influx of tide water is sufficient to produce an abundance of brackish water diatoms at Greenwich Point. The entire absence, however, at present, of many of the marine forms obtained in dredgings in the Delaware opposite the city, as at Smith's Island, now removed, and in certain well borings at Pavonia, Pensauken, Gloucester and other places in New Jersey, where the depth reached the old blue clay, indicates conditions quite different from those now prevalent. In the Bay itself comparatively few living species are found, at least in any abundance.
In the study of local forms which follows, the district included may be considered as circumscribed by the circumference of a circle having a radius of one hundred miles from Philadelphia, containing the States of New Jersey and Delaware, the southeastern part of {6}Pennsylvania, a portion of Maryland on the south and extending eastward to New York Bay and Long Island Sound as far as New Rochelle.
The greater number of fresh-water species described have been obtained from near the city along the Darby, Crum, Ridley and Brandywine Creeks and from various places in New Jersey, including the Pine Barren region of the southern part of the State. Numerous collections have been made in the Schuylkill and the various reservoirs and along the Wissahickon, "where an Alpine gorge in miniature of singular loveliness is to be found within the limits of a city." The fossil deposits are from well borings near Camden, N. J., and from excavations in various parts of the city.
There appears to be no relation between the Miocene beds of the eastern coast and the deposits here described, all of which have been formed later than the glacial period or in an interval between two such periods. Apparently no diatoms grew during the glacial era, at least in sufficient abundance to leave any perceptible traces of their existence. An examination of glacial "flour" and clays from the Catskills shows an entire absence of these forms, and I have never found them in the milky flow from the glaciers of the Alps nor in the constantly muddy streams in certain of our Western States. The opacity of the water produces the same result as the absence of light in the deep lakes of New England, where diatoms are found only on the stalks or roots of water-plants near the shore, while in shallow ponds, such as the small lake near the summit of Mt. Lafayette, the growth is abundant. Certain species will grow wherever there are moisture, light and heat, but the greater number require the presence, in small amounts, of substances produced by the decay of animal and vegetable life. An abundance of diatoms in fresh water is usually an indication of its potability, while their entire absence in shallow water may be due to an excess of bacteria.
The specimens from which the drawings are made have been collected by the author for many years; in addition to possessing an almost complete library on the subject, he has had the advantage of examining material obtained by the late Mr. Lewis Woolman and numerous slides furnished by a number of friends, including Mr. John A. Shulze, Mr. Frank J. Keeley and Mr. T. Chalkley Palmer, to whom I here take pleasure in expressing my thanks.
The difficulties of the study are well stated by Agardh in the following extract from the preface to his Systema Algarum:
"Because, indeed, in this respect, no one will wonder whether in the distinction of species and reference to synonyms we have, perchance, committed many errors. They have occurred and are bound to occur, partly from the fact that one is not permitted to see the original specimens of all authors; partly, because sometimes even the original specimens of these plants are erroneous; partly, because the figures and descriptions of authors are often lacking and imperfect....
"There is added the difficulty of the study itself of these plants, their submerged habitat, the minuteness of their structure, the rarity of their fruit, the change in the dried {7}plant, the impossibility of culture, the fallacies of microscopical vision and the chaotic condition of Algology itself to-day."
The words of Agardh, written in 1824, are almost as true to-day. The lack of authentic specimens, which we hope will be remedied in time by the collections of the Smithsonian Institute, numerous incorrectly labelled slides in amateur collections, the imperfections of figures copied and recopied, without regard to relative size or correct references, and the confusion in the attempts to harmonize different descriptions, deter the student at the outset. The remaining difficulties mentioned by Agardh add, however, to the remarkable interest these forms have always had, since no increase in optical perfection of the microscope serves to lessen the mystery of their structure and mode of growth.
The few species of diatoms first discovered were included by Lyngbye, Dillwyn, and others in the genus Conferva. In 1824, the species, increased to forty-eight, were separated by Agardh into eight genera distinguished partly by their mode of growth. But little change was made until Heiberg, in 1863, advocated the division into symmetrical and asymmetrical forms. Without entering upon a general review of the later classifications, including Pfitzer's and Petit's divisions according to the number and location of the chromatophores, or the arrangement of Prof. H. L. Smith, because of the presence or absence of a raphe, or that of Mereschkowsky into motile and immotile forms, the modification of all of these methods by Schuett is here adopted, varied in accordance with certain monographs which appear to offer advantage.
It is customary, especially among writers who are familiar with other classes of plants, to decry any classification of diatoms according to the markings of their siliceous envelopes. As, however, one of the chief distinctions of the class is the possession of a more or less siliceous and indestructible frustule, and as the cell and its contents are never seen except within the valves, their variety forms the only available method of identification. The cell contents, owing to the difficulty of observing their living condition, their continued change, their lack of distinct variation and their entire absence in fossil forms, render their consideration as a complete method of classification an impossibility. If, however, the cell contents can be brought into relation with the markings of their siliceous envelope, it will be a consummation for which the future student of these complicated forms ought to be grateful. That this result is one to be expected may be inferred from the fact that the arrangement of protoplasmic masses in the interior of the cell is coincident in some cases with markings on the valve, and the character of the endochrome is assuming a certain value in accentuating the difference between such forms as Pleurosigma and Gyrosigma, or in the resemblance between Hantzschia and Nitzschia, or between Surirella and Campylodiscus. Mereschkowsky, however, states that it is necessary to be careful in "establishing the relationship between diatoms based on the resemblance of their chromatophores," {8}and further observes that in Hantzschia amphioxys, Scoliotropis latestriata and Achnanthes brevipes, three widely separated forms, the chromatophores are essentially the same.
In one of the earliest classifications of diatoms, the individual cell received less consideration than the nature of the filament or thallus in which many species occur in the first stages of their growth. Those, however, which exist in colonies at first are, sooner or later, broken up into separate frustules, either before or at the time of their maturity or previous to conjugation, while very many species are never seen except in a free state. The union of frustules, therefore, is of secondary importance and the group must be considered as filamentous or unicellular algĉ. Their relation to other algĉ is not well determined. Among the Desmidiaceĉ, a family of the order Conjugales, of the class Chlorophyceĉ, the cells are in many forms divided by a constriction into symmetrical halves. The Conjugales are starch forming, with walls of cellulose. In the Diatomaceĉ the starch is replaced by oil globules, while the walls of cellulose are more or less filled with a deposit of silica. The Conjugales, however, reproduce by zygospores and usually contain pyrenoids, as may be seen in the parietal chromatophores of Spirogyra. In the class Heterokontĉ we have the reserve material in the form of oil, instead of starch, but there are no pyrenoids. To this class belongs the order Confervaceĉ, in which the cells are unicellular or filamentous, and to which all of the Diatomaceĉ were referred. While, therefore, Diatomaceĉ have a close affinity to the Desmidiaceĉ and to the Confervaceĉ, the determination of their origin, one from another, or from a common ancestral type, appears to be a matter of conjecture.
The cell membrane is composed of two usually equal parts, each of which consists of a valve and a girdle or zone formed of cellulose modified by silica deposited in an insoluble state from a very dilute aqueous solution. The valves are more siliceous and robust than the girdle. Both are in most species easily separable, or at least the bands of the girdle which may be more or less closely fastened to the valves have a motion over each other permitting the cell to enlarge at pleasure. The longitudinal diameter of the cell, or the distance between the centres of the two valves, will vary according to the convexity of the valve and the age of the frustule which may be often determined by the width or number of the girdle bands. These, owing to their diversity of form and arrangement, will be further described under the generic diagnoses.
The siliceous cell-wall is covered on the outside by a layer of protoplasm called the coleoderm. This layer may be quite thin and evident only when treated with fuchsin or Bismarck brown, or it may be of considerable thickness. The cell contains the cytoplasma, protoplasm, cell-sap, endochrome, pyrenoids, oil globules and nucleus, together with certain other less understood bodies.
The Cytoplasma is a thin skin of colorless plasma covering the entire inner surface of the cell. It is invisible in the living cell but is evident in plasmolysis. In long forms it is thickened at the ends and is condensed at the plasma bridge which frequently connects the two valves and divides the cell into two parts, each containing more or less protoplasm surrounding the vacuole in which are found the cell-sap and certain granules. In some forms, as Meloseira, the cytoplasma includes the entire mass of protoplasm.
The Endochrome is seen in the form of one or more bands or plates, of a yellowish or brownish color, on the inner side of the valves or connective zone, or in granules or irregular masses, more or less numerous, on the inner walls, or sometimes grouped near the centre. It consists of a mixture of chlorophyll and diatomine which differ in their relative solubility in alcohol and in their spectroscopic analyses. The color varies from green to a chocolate brown in proportion to the amount of diatomine. So far as the function of the endochrome is concerned it does not appear to differ from that of ordinary chlorophyll, absorbing, under the influence of light, the carbon, and disengaging the oxygen of the carbonic anhydride in the water. Diatoms do not live in absolutely pure or non-aërated water. The individual plates or granules of the endochrome are called chromatophores. Their number and significance will be referred to in the description of genera.
The Pyrenoids.—In the chromatophores of many species are found colorless, homogeneous bodies, strongly refractive, of various shapes, usually lenticular or fusiform, which are known as Pyrenoids (Schmitz). They are scarcely evident in the living cell, but are distinguished by the action of hĉmatoxylin and other reagents. Flat forms occur in Surirella and Pleurosigma, lens forms in Pinnularia, Stauroneis, Synedra, Fragilaria and Nitzschia, while a spherical form is found in Cymbella cuspidata. The pyrenoids are always imbedded in the chromatophore. Their growth is by division. Schmitz considers them a part of the living chromatophore, and their substance as working material which in excess has become resolved into the nature of a crystal which its form sometimes resembles. Comparisons are made between them and crystalloids found in certain monocotyledons. The pyrenoid is evidently concerned in the formation of the chromatophore, or in its division. Much of the conjecture, however, is due to the behavior of pyrenoids in other plants.
Oil Globules.—It has been established by Pfitzer that starch and sugar, as assimilation products, are replaced by oil in the cells of diatoms ("da bekannlich Staerke und Zucker bei den Bacillariaceen nicht nachzuweisen sind"). The oil drops are more or less numerous, of various sizes, and are found in the cytoplasma, the cell-sap, and sometimes the chromatophores. Mereschkowsky describes certain globules as elĉoplasts, which he divides into four kinds according to their number and position. Whether all of these are oil globules is a question not yet determined.
Other bodies, known as "Buetschli granules," or volutin, and described as "little blisters filled with a tolerably robust refractive substance," are considered by Lauterborn to be a nitrogen reserve store. They are found in the cytoplasma, or in the cell-sap, and can be fixed in picric acid and stained in methylene blue.
Note.—For a discussion of the morphology of diatoms and a valuable résumé of the investigations of Buetschli, Karsten, Lauterborn, Mereschkowsky, Mueller, Pfitzer, Schuett, and others, the student is referred to "Der Bau der Diatomzelle," by Dr. Otto Heinzerling, in "Bibliotheca Botanica," 1908.
The growth of diatoms follows the usual method of cell division as described by Sachs (Text Book of Botany, 2nd ed., p. 16): "The nucleus of a cell which is about to divide becomes broader, assuming the form of a biconcave lens, and its nucleolus breaks up into irregular granules which together with its other granular contents begin to form a nuclear disc in the equatorial plane. A delicate striation is now apparent in what is becoming the long axis of the nucleus, at right angles to the nuclear disc, and the characteristic nuclear spindle is gradually produced. The nuclear disc splits into two halves lying side by side, each of which travels to the corresponding pole of the nucleus; thus two nuclei are constituted which are connected by fibrillĉ."
The cell-wall and the chromatophore bands divide, each nucleus passes to the centre, and two new cells are formed. In the meantime, to permit of this division, the two siliceous valves separate, the girdle bands slipping over each other, and opposite the larger or enclosing valve a new valve is formed, the girdle band of which is seen later within the girdle of the mother valve. Opposite the smaller valve of the original cell and adjoining the new valve, another valve is formed which also produces a girdle within the girdle of the smaller valve. As a result of division we have, therefore, the valves of the original, or mother cell, the two new valves and four girdle bands. (Pl. 40, Figs. 18 and 19.)
In the process of division, the continual formation of new valves, enclosed in the older girdle bands, will naturally cause a reduction in the size of the frustule. While this reduction, owing to the elasticity of the girdle, does not always occur, I believe, yet, in most cases, the diameter is so reduced that a rejuvenescence of growth is required. This is caused by the production of auxospores which may appear without conjugation. In this process, the beginning of which, in certain species, may be noticed by the increase in the size of the girdle as in reduplication, the two valves separate and within is formed a more or less spherical mass about twice the size of the original frustule and which forms on its circumference two large and often shapeless valves. These valves form others which assume the appearance of the original valves, but larger, and proceed to grow in the usual way. The reduction in size of the frustule seldom proceeds further than about half the size of the type form, so that, as a general rule, it may be stated that diatoms are not often smaller than half the larger size.
The process of reproduction has been observed in many cases, but the conclusions reached are somewhat at variance with each other. The auxospore formation is simply a {11}method of rejuvenescence. When, however, the auxospores are thrown off from filamentous diatoms, it is probable that two may conjugate, their contents dividing each into two daughter cells which unite into two zygospores. The usual method is the union of two frustules, which, throwing off the old valves, coalesce into a single mass of protoplasm which produces an auxospore, sometimes called a sporangial frustule. It is stated that in some cases two frustules coalesce and produce two auxospores.
The existence of spores in diatoms is a much-disputed point. While they have never been seen, the inference that they exist is very great, as otherwise it becomes difficult to understand the sudden growth of species in localities and under conditions that seem to preclude the actual presence of the living frustule. It is a matter of common observation that, in examining collections of living forms, minute frustules or brownish globules appear to resemble larger diatoms. In gatherings of Gomphonema, when many specimens are sessile on the same object, numerous intermediate sizes, varying from minute globules to the type, are seen, yet not positively demonstrable as the same.
Conjugation, the formation of auxospores, and the actual process of cell division are seldom seen, as they occur during the night or at least in darkness. It is advisable in order to observe reduplication to obtain the material about midnight and place it in very dilute alcohol. In filamentous forms, however, the cell division is easily observed at any time in its various stages. By immersing in picric acid (saturated solution), transferring to very dilute alcohol which is gradually increased in strength, and then passing through oil of cloves and finally to the mounting medium, excellent preparations can be made. By staining with gold chloride alone the nucleus is made apparent without further treatment.
It may be assumed that diatoms originated in the sea; to deny this requires evidence of the existence of fresh-water species previous to the Miocene period which is entirely marine. In those subject to fluctuations of the waves, as pelagic diatoms, their existence appears to be contingent upon the methods by which the separate frustules can cohere. Various devices, including hooks, spiral bundles, horns and processes exuding threads of plasma, exist for holding together the frustules. When marine forms are found in quiet waters some of these devices, being no longer of any value, cease to grow, although free swimming diatoms are rare. They either occur in long chains or are stipitate or sessile. If it is further assumed that the fresh-water diatoms are found in greater abundance in later periods, the action of running streams makes necessary the provision of some means by which the species may continue to colonize. This may be recognized in the occurrence of linear forms chiefly in streams. Circular forms, such as Cyclotella which have no raphe, are found in quiet waters, such as pools or ditches, and never exist living in running streams. Those forms only would be able to live in water having a more or less swift current under one of three conditions: they must, as in Gomphonema, be adherent to surrounding objects by a stipe; or be enclosed in a gelatinous tube, as in Homœocladia; or have an independent motion powerful enough to overcome the influence of the current. It is true that many forms with a raphe have no apparent motion. In the case of Mastogloia provision is made in a gelatinous cushion in which the frustules are preserved. In Cocconeis, with a true raphe in one valve only, in Epithemia, with a partial raphe, or in certain Eunotiĉ with a trace of one, we find species evidently degenerate and parasitic. The long Synedrĉ, having only a median line, live in running streams, since they are attached at one end to other algae. Forms with a true raphe appear to be more highly developed, since they are able to seek locations favorable to growth. Given, therefore, the structure of the valve, the habitat may be inferred.
The erratic backward and forward movement of certain diatoms, especially those of the Naviculoid group, or the slow, rolling motion of Surirella, has been discussed in so many ways without definite conclusions that a brief statement will be sufficient. Osmosis, the amœboid movement of the coleoderm, the protrusion of protoplasm or protoplasmic threads through the raphe, the existence of actual organs of locomotion or cilia, and the lack of synchronism in the chemical action occurring at the ends of the cell which is sometimes divided by the plasma bridge, have been offered in explanation. The chief objection to the theory of cyclosis appears to be that the resultant motion is so greatly in excess of the rotation of protoplasm in the cell. More or less motion is observed in various kinds of free cells, but the movement of diatoms is not evident in those without either a raphe or a keel upon which and apparently by which the phenomena are produced.
Mr. T. Chalkley Palmer, in various articles in the Proceedings of the Delaware County Institute of Science, especially in Vols. 1 and 3, gives the results of exhaustive experiments. "Nothing, it would seem," he says, "could be more conclusive as to the essential sameness of the nature of motion in monads and diatoms, than the fact that both monads and diatoms require oxygen in order to perform motion, that they come to rest when oxygen becomes scarce, and that they resume their motion when oxygen is again supplied."
He also thinks "that the living substance of the cell, more or less deeply overlaid with coleoderm substance of varying consistency, and itself assuming that degree of fluidity which best meets the requirements of the situation, permeates the raphes, circulates in the keels, or in some cases protrudes quite beyond the silica, and functions as the actual propulsive agent."
Of all forms of vegetation, the Diatomaceĉ are, perhaps, the most ubiquitous. Where-ever a sufficient amount of moisture, heat and light are found, they grow. It was during the Miocene period that they first appeared, and, as marine forms, reached their greatest development, both as to size and beauty of marking, while their prevalence throughout the world in enormous quantities has been often mentioned. The Miocene beds of Richmond and Maryland continued over the Cretaceous formations of New Jersey have outcropped in certain localities within our district, but are not considered in this discussion.
The function of diatoms is not essentially different from that of other algĉ in providing food for aquatic animals, such as Salpĉ and oysters, but it is, however, in other respects that they are not only important but necessary factors in the preservation of life.
"Full nature swarms with life; one wondrous mass
Of animals, or atoms organized,
Waiting the vital breath, when parent heaven
Shall bid his spirit blow. The hoary fen,
In putrid streams, emits the living cloud
Of pestilence. Thro' subterranean cells
Where searching sunbeams scarce can find a way,
Earth animated heaves."
I am not certain if Thomson fully understood the matter, but he has remarkably described the facts. When "the vital breath" of returning spring animates the earth, the "subterranean cells" of diatoms, the "atoms organized," through the liberation of vast quantities of oxygen, immediately begin the purification of the "putrid streams." Were these streams not so purified, the accumulation of animal and vegetable débris would eventually cause an enormous bacterial growth fatal to animal life.
Unicellular or filamentous. Cells either free, sessile, united in filaments, immersed in a gelatinous envelope or in fronds composed of branching tubes; microscopic, enclosed in a more or less siliceous envelope (frustule), composed of two parts (valves), usually connected by an intervening band (zone or girdle). Cell contents include yellowish or brownish chlorophyll-like bodies which occur in one or several bands (placcochromatic), or as variously distributed granular masses (coccochromatic) lining the inner walls. Growth by ordinary cell division or by auxospores; sexual multiplication by the formation of sporangia. Valves of two kinds: (a) Those in which the markings or parts are more or less concentric (Centricĉ); (b) Those (Pennatĉ) in which the parts are more or less symmetrically divided by a line (pseudoraphe) or by a cleft (raphe).
Valves without a dividing line or cleft; markings more or less radiate; transverse section of frustule circular, polygonal, or elliptical, sometimes irregular.
Divided into four groups:
1. Discoideĉ.—Frustules (cells) discoid; valves without horns or elevations (sometimes with processes).
2. Solenoideĉ.—Frustules with numerous girdle bands.
3. Biddulphioideĉ.—Frustules box-like, i. e., with the longitudinal axis greater than in the Discoideĉ. Valves with two or more angles, elevations or horns.
4. Rutilarioideĉ.—Valves as if naviculoid, but with irregular or radial structure.
Groups 2 and 4 are not included in our description. No. 2 contains plankton genera only, while No. 4 consists of genera not yet found in this locality.
1. Coscinodisceĉ.—Valve not divided by rays or costĉ into sectors; puncta sometimes radiate; ocelli or processes absent.
2. Actinodisceĉ.—Valve with radial striĉ divided into sectors: ocelli and processes absent.
3. Eupodisceĉ.—Valve disc-shaped with mammiform processes or one or more ocelli.
(a) Meloseirinĉ.—Frustules short, in chains.
(b) Coscinodiscinĉ.—Frustules disc form, usually single, rarely in short chains.
(a) MELOSEIRINĈ
1. Meloseira.—Valve punctate, with a constriction or furrow between edge of valve and girdle.
2. Gaillonella.—Valve punctate, with a circular collar or crest near edge of valve.
3. Lysigonium.—Valve punctate, neither keeled nor constricted.
4. Hyalodiscus.—Valve punctate in the centre; border with decussating radial lines.
5. Stephanopyxis.—Border of valve with a crown of thorns; valve areolate.
6. Pyxidicula.—Valve areolate, with a border of spines.
(melos, a limb or member, and seira, a chain)
Frustules globose, ellipsoidal or cylindrical, concatenate, closely joined together. Valve either simply punctate or punctate and areolate. A constriction of the cell-wall, forming a furrow between the edge of the valve and the girdle, is more or less evident.
The genus Meloseira constituted by Agardh has been variously modified by Kuetzing, Thwaites, Wm. Smith, Van Heurck, De Toni, and others. In Systema Algarum Agardh included certain species of Conferva, of Lyngbye, Dillwyn and others, and limited his genus to frustules more or less globose (fila articulata ad genicula constricta), although in his Conspectus Criticus (p. 64), he modifies the description (fila teretia articulata, articulis diametro ĉqualibus vel longioribus) to include M. varians. As, however, Lysigonium Link, Gaillonella Bory, and other genera enlarged by Ehrenberg and Kuetzing, came to be included under Meloseira, Thwaites suggested the division of the genus into two: Orthosira, in which the frustules are not convex at the ends and Aulacosira in which no central line is apparent but with two distinct sulci. Wm. Smith adopts the genus Orthosira but rejects Aulacosira, including all forms under the former genus and Meloseira, suggesting that differences "exist in the formation of the sporangia" of the two genera. M. varians and M. crenulata appear to form auxospores or sporangial frustules in different ways, as will be noticed hereafter.
As, however, the present state of our knowledge is so limited and as much confusion would result in further changing the nomenclature, I shall adopt, for the most part, the division made by De Toni, separating Gaillonella and Lysigonium and employing the name Meloseira as emendated in Sylloge Algarum, although, as stated, it omits the species of Agardh. That a further division may be necessary is indicated by the differences existing between the Orthosira forms and the others.
ANALYSIS OF SPECIES
| Frustules cylindrical and lengthened: | |
| Valves with two distinct furrows; granules small | distans |
| Valves with coarse granules | granulata |
| Valves denticulate on the margin | crenulata |
| Valves denticulate and constricted | roeseana |
| Valves with row of large puncta on the girdle side | undulata |
| Frustules cylindrical and compressed: | |
| Valves punctate and areolate | sulcata |
The chromatophores consist of circular and compressed or irregular flat granules which lie along the wall of the cell.
MELOSEIRA DISTANS (EHR.) KUETZ.
Frustules cylindrical, slender, with two furrows, one on each side of the suture; valve in zone view with fine puncta in longitudinal rows; puncta in valve view scattered. L. 7-10 µ.
Meloseira nivalis Wm. Sm.
Coscinodiscus minor Wm. Sm.
Fresh water. Fossil in New England deposits.
Pl. 1, Figs. 8 and 9.
Note.—In all species of Meloseira, as well as Gaillonella and Lysigonium, the frustules are so closely coherent that when the filaments are broken entire frustules are less frequently found than a union of two valves of contiguous frustules.
MELOSEIRA GRANULATA (EHR.) RALFS
Frustules cylindrical, robust, 5-18 µ in diam., with large granules in longitudinal, sometimes spiral, lines, variable in size and arrangement in the same filament. Valve in valve view with scattered puncta. Variable in relative width and length, passing to M. crenulata.
Gaillonella granulata Ehr.
Orthosira punctata Wm. Sm.
Fresh water. Fossil at Coldspring, L. I.
Pl. 1, Fig. 10.
MELOSEIRA CRENULATA (EHR.) KUETZ.
Frustules cylindrical, with furrows on each side of the suture, 10-20 µ in diam.; puncta in longitudinal rows. Margins of valves denticulate at the junction of the frustules; valves with puncta scattered at the centre, radiate at the circumference.
Common in fresh water; quite variable in size.
Gaillonella crenulata Ehr.
Orthosira orichalcea Wm. Sm. in part; not Conferva orichalcea. Mertens or Gaillonella aurichalcea Ehr. and Bailey.
Pl. 1, Figs. 1 and 2.
MELOSEIRA ROESEANA RAB.
Frustules cylindrical, constricted toward each end, with coarse, longitudinal striĉ; valve convex, striĉ punctate, radiating, with several large granules at the centre. Connective zone with longitudinal rows of fine puncta. Diam. 12-45 µ.
Orthosira spinosa Grev.
Fresh water. Media, Pa. (Palmer); not common.
Pl. 1, Figs. 5 and 6.
MELOSEIRA ROESEANA VAR. EPIDENDRON (EHR.) GRUN.
Frustules denticulate at the margin; valve with coarse granules at the centre from which radiate lines of fine puncta.
Wet rocks of the Wissahickon.
Pl. 1, Figs. 3 and 4.
MELOSEIRA UNDULATA (EHR.) KUETZ.
Frustules single or in twos, usually broader than long, constricted near the margin. Valve with six to twelve internal projections forming with the outline of the constriction of the valve a polygonal figure within the circumference. Surface of the valve with radiating lines of puncta disappearing toward the centre, at which are numerous coarse puncta.
Meloseira gowenii A. Schmidt.
Blue clay of Philadelphia, especially common at Twelfth and Market Sts.
Pl. 1, Figs. 15, 16, 17.
MELOSEIRA SULCATA KUETZ.
Frustules quite robust, with diam. several times the length, deeply furrowed at the margin, areolate and punctate. Valve with radiating striĉ disappearing toward the centre, and with a double row of cells near the margin, the outer one having the appearance of a crown of teeth.
Gaillonella sulcata Ehr.
Paralia sulcata (Ehr.) Cleve.
Paralia marina Heib.
Marine and brackish. Common in all parts of the world, and fossil in the Miocene. The Philadelphia form is the var. genuina Grun.
Pl. 1, Figs. 11 and 12.
In a gathering from Media of Meloseira crenulata (Palmer leg.), occasional filaments are noticed with much longer and narrower frustules which become enlarged in the middle and are seen to contain inner frustules in the process of still further division, as shown in Fig. 2, Pl. 38.
Meloseira dickei Thwaites shows internal box-like cells placed one within the other, which were supposed by Thwaites to be a method of reproduction. Wm. Smith doubts this, but is unable to offer any explanation. In the present form the mode of reduplication is that usually found in filamentous forms, but in this case the presence of perfect frustules enclosing others in the process of still further division has been heretofore unfamiliar to me. The swelling in the middle appears to indicate that not all filamentous diatoms are reduced in size by subdivision. In outline the valve is like that of a "truncated cone," as described by Petit in referring to Gaillonella granulata var. bambusina Petit (Diat. Nouv. et Rares, Jour. de Micrographie, 1890).
(named after Gaillon, a botanist of Dieppe)
Frustules ellipsoidal, united in long filaments, usually found in pairs; each valve is furnished with a circular collar or crest extending at right angles to the convex edge. Valve hyaline at the centre from near which radiate lines of fine puncta, 18-20 in 10 µ.
Note.—The original names of both Meloseira and Gaillonella are retained, as there is no good reason for contracting the Greek diphthong in the first, and the second is the correct spelling.
GAILLONELLA NUMMULOIDES (DILLW.) BORY
Frustules as in the generic diagnosis. Diam. 30 µ.
Conferva nummuloides Dillwyn (Brit. Confervĉ, p. 45, Sup. Pl. B).
Meloseira nummuloides Ag.
Heiberg and O'Meara assign this species to Lysigonium moniliforme (Muell.) Link, which is not keeled. While Dillwyn's and Lyngbye's figures do not show the keel, it is probable from their descriptions that the angular outline produced by the keel was noticed.
Marine or brackish. Coast of New Jersey; Hudson River (Bail.).
Pl. 1, Figs. 13 and 14.
Gaillonella moniliformis of Bailey is this form, as he describes it as having "two minute projections of the delicate transverse ridges seen near the ends of the two globules belonging to a joint." (Amer. Jour. Science, 1842, p. 89, Pl. 2, Fig. 3.)
(luo, to loose, and gonu, a joint)
Frustules globose, concatenate; valve simply punctate.
LYSIGONIUM MONILIFORME (MUELL.) LINK
Frustules usually in twos, not keeled; valve with puncta in longitudinal lines, the puncta of the enveloping zone larger and in transverse rows. L. 25-40 µ (De Toni).
Conferva moniliformis Mueller (1783).
Conferva nummuloides Eng. Bot. pl., 2287, not Dillwyn.
Meloseira borreri Grev.
Lysigonium nummuloides (Lyngb., Kuetz.) O'Meara = Gaillonella nummuloides (Dillw.) Bory. See O'Meara, p. 248.
Marine and brackish. Long Island Sound and coast of New Jersey.
Pl. 1, Fig. 7.
LYSIGONIUM VARIANS (AG.) DE TONI
Frustules cylindrical, in long filaments, slightly constricted on each side of the suture; puncta in oblique rows in zone view. Valves 15-35 µ in diam. (De Toni), sub-plane, with fine puncta in lines radiating from the centre. Under medium magnification the frustules appear smooth. Very variable in size.
Meloseira varians Ag.
Fresh water. Common in ditches and springs.
Pl. 1, Figs. 18 and 19.
(hyalos, transparent, and discus, a disc)
Frustules spheroidal; valve with a flattened, irregularly punctate umbilicus from which proceed radiating or decussating lines of fine puncta.
ANALYSIS OF SPECIES
| Valves divided into sectors | stelliger |
| Valves not divided but interrupted by short dark lines at intervals | radiatus |
| Valves with very fine puncta | scoticus |
HYALODISCUS STELLIGER BAIL.
Valve with puncta in oblique decussating rows which, by reason of the difference in obliquity, form numerous sectors. Umbilicus irregular, with scattered, coarse puncta. Margin wide, striated.
Podosira maculata Wm. Sm.
Blue clay. Not common.
Pl. 1, Fig. 22.
HYALODISCUS RADIATUS VAR. ARCTICA GRUN.
Valve with radiating puncta from a rather small umbilicus, the rays interspersed with short, dark lines, having the appearance of spines, at irregular intervals. Margin broad, striated.
Pyxidicula radiata O'Meara.
The Philadelphia form corresponds exactly to Grunow's variety which has closer puncta than the type form.
Blue clay. Rather rare.
Pl. 1, Fig. 21.
HYALODISCUS SCOTICUS (KUETZ.) GRUN.
Valve small, with puncta about 24 in 10 µ, appearing hyaline.
De Toni remarks that it resembles a small form of H. subtilis which occurs north and south of our limits and is yet likely to be recorded.
Cyclotella scotica Kuetz.
Podosira hormoides Wm. Sm.
Blue clay. Not rare.
Pl. 1, Fig. 20.
Endochrome in the form of four flaps or patches bound together about a common pyrenoid. In H. subtilis numerous rod-shaped chromatophores lie in a row and are not bound in the centre (Mereschkowsky).
(stephanos, a crown, and pyxis, a kind of vase or box)
Frustules ellipsoidal, concatenate; valves tumid, of unequal convexity, coarsely areolate, the cells in rows parallel to the longitudinal axis, not radiate, with stray spines or teeth placed concentrically more or less near the margin.
According to Karsten the chromatophores are round or angular discs which lie near the connective zone.
STEPHANOPYXIS TURRIS (GREV.) RALFS
Valve cylindrical, with a crown of stout spines less than the diameter of the valve near the margin. Cells hexagonal, about 2 in 10 µ, sometimes punctate. The valve having the greater convexity has the larger spines, though usually less of them.
Creswellia turris Grev. (Gregory, Diat. of the Clyde, T. R. S. E., vol. 21, part 4, p. 66.)
Stephanopyxis appendiculata Ehr.?
Creswellia is incorrectly based, as stated by Ralfs, on the concatenation of the valves which was not noticed by Ehrenberg in the fossil forms. It had been suggested by Kuetzing in Systema Algarum (p. 126).
Blue clay. Port Penn and Smith's Island.
Pl. 2, Figs. 1 and 2.
STEPHANOPYXIS CORONA (EHR.) GRUN.
Valve larger than in turris, sub-globose, coarsely areolate cells, 4-5 in 10 µ. One valve furnished with a crown of teeth shaped like the letter T and united at the top into a ring above the margin of the valve; the other valve with long spines more or less concentrically arranged.
Blue clay. Not common. Fossil in the Nottingham deposit.
Pl. 2, Fig. 3.
Note.—The diatomaceous deposit, so often called "Bermuda" or "Bermuda tripoli," especially by foreign writers, is in reality the Miocene stratum extending for miles along the Patuxent River near the village of Nottingham, Md. The author is perfectly familiar with the location, having made large collections there. The mistake in the name is due to the fact that Prof. Bailey received material from Mr. Tuomey marked "Bermuda Hundred," which is located near Petersburg, Va. Attempts have been made to find material there and while there is an earth containing Miocene diatoms at Petersburg, it does not exactly correspond to the material sent to Ehrenberg by Bailey, who was in doubt as to the locality. The Bermuda Islands are of coral formation and have no deposits of diatomaceous earth.
(dim. of pyxis, a box)
Frustules globular, solitary or in short fasciĉ. Valve more or less hemispherical, areolate, destitute of spines.
PYXIDICULA CRUCIATA EHR.
Valve hemispherical, with large, hexagonal cells. An inner stratum is finely punctate.
Blue clay. Walnut St. Bridge. Rare.
Pl. 38, Fig. 8.
This form is not usually described as having punctate areolĉ, but it does not apparently differ from other forms of Pyxidicula of Ehrenberg as described by Kuetzing (Species Algarum, pp. 21-23), including P. areolata. In fact, it differs from Stephanopyxis, which is also sometimes punctate, only in the absence of spines. In fossil deposits the absence of an easily detached stratum is not significant. The difference, except in size, between it and P. mediterranea Grun. (V. H. S., Pl. 95, Figs. 15 and 16), I am unable to determine.
Although many species of Meloseira are fresh-water, the habitat of the group Meloseirinĉ is, in general, marine. It more nearly coincides in structure and development with other algĉ not diatomaceous, the siliceous envelope constituting its most distinctive feature. As we proceed in the classification, the structure both of the frustule and contents becomes more complicated.
(b) COSCINODISCINĈ
1. Cyclotella.—Valve with two concentric divisions of different structure, one a wide border and the other a central surface.
2. Coscinodiscus.—Valve areolate or punctate, with a narrow border of the same structure.
(cyclos, a circle)
Frustules single or geminate, cylindrical, short, in zone view rectangular or with undulating sides. Valve usually with smooth or punctate striĉ, centre sometimes bullose, smooth, or with granules scattered or radiating.
Chromatophores numerous along the valves (Pfitzer).
CYCLOTELLA STRIATA (KUETZ.) GRUN.
Valve 30-80 µ in diam., with coarse striĉ, 7-12 in 10 µ, centre coarsely punctate and bullose.
Coscinodiscus striatus Kuetz.
Cyclotella dallasiana Wm. Sm.
Common in the blue clay.
Pl. 2, Fig. 9.
CYCLOTELLA MENEGHINIANA KUETZ.
Frustule in zone view rectangular, undulated; valve, 10-20 µ in diam., marginal striĉ robust and transversely punctate, centre radiately punctate.
Cyclotella kuetzingiana Wm. Sm. (not Thwaites).
Crum Creek.
Pl. 2, Fig. 8.
CYCLOTELLA MENEGHINIANA, VAR. STELLIGERA CL. AND GRUN.
Differs from the type in the coarse radiating lines at the centre.
Broomall Lake, Media.
Pl. 2, Fig. 4.
CYCLOTELLA MENEGHINIANA, VAR. STELLULIFERA CL. AND GRUN.
As in type but with the central rays granulate.
Broomall Lake, Media.
Pl. 2, Fig. 12.
CYCLOTELLA STYLORUM (BR.?) V. H.
Margin striated, the alternate striĉ thickened near the border, producing an appearance of subquadrate cells. Centre faintly granulate, the outer border of which is encircled by 10-12 puncta, each of which is surrounded by a small hyaline space.
Blue clay. Rare.
Van Heurck gives this form doubtfully as a variety of striata, while De Toni makes it synonymous with it. Van Heurck's figure is not that of Brightwell, but as the specimen above described is, I believe, exactly the same as Van Heurck's, I retain his name.
Pl. 2, Fig. 10.
CYCLOTELLA COMTA (EHR.) KUETZ.
Valve with marginal striĉ well marked, each third or fourth costa more robust than the others. Central part finely striated, the striĉ punctate, radiating.
Fresh water.
Pl. 2, Fig. 7.
The form here figured is probably the variety radiosa Grun. and is from a New England specimen. It is quite likely to occur in this locality.
CYCLOTELLA OPERCULATA (AG.) KUETZ.
Frustules in zone view undulated. Angles rounded. Marginal costĉ alternating with minute spines; centre nearly smooth, depressed, convex or flexuose.
Fresh water.
Pl. 2, Figs. 5 and 6.
The figure is drawn from a specimen from Boston, Mass., H. L. Smith Type Slide No. 107, marked equivalent to C. minutula Wm. Sm.
CYCLOTELLA ANTIQUA WM. SM.
Marginal costĉ alternating with thick puncta; centre finely granulate with subtriangular elevations. Frustules in zone view rectangular.
Blue clay.
Pl. 2, Fig. 11.
The form corresponds to the original specimens of Wm. Smith in the deposit of Stavenger, Norway.
The genus Cyclotella comprises about seventy specific names, many of which may be referred to other genera, while some of Ehrenberg's are incapable of verification on account of the small size of the figures and the lack of sufficient description. About half of the forms are marine. The fresh-water species are usually found living in more or less stagnant water or in pools contaminated with drainage, being an exception to the general rule that diatoms are more abundant in water free from deleterious matter.
(coscinon, a sieve, and discus)
Frustules solitary, cylindrical, compressed; valve circular or elliptical; surface flat or sometimes convex near the border; markings more or less angular, radiating, sometimes fasciculate; border usually well defined. Central space, if present, hyaline, sometimes surrounded with a rosette of large cells.
Chromatophores round, angular or irregular discs usually without pyrenoids (Karsten).
Rattray's classification is here followed, so far as it refers to our species.
Excentrici.—Valves circular; central space absent; markings angular, in oblique, decussating rows.
Lineati.—Central space absent; markings angular, oblique decussating rows straight.
Fasciculati.—Markings fasciculate, or sometimes only near the border.
Radiati.—Markings rounded or angular, more or less radiate.
Elaborati.—Valves elliptical, markings rounded.
EXCENTRICI
COSCINODISCUS EXCENTRICUS EHR.
Valve with a hyaline excentric space from which proceed, usually in six directions, rows of polygonal markings decreasing toward the narrow, coarsely striated border, the rows appearing convex toward the centre. Apiculi at unequal distances apart. Quite variable in size.
Common in the blue clay and along the coast.
Pl. 2, Figs. 14 and 20.
Fig. 20 is probably var. perpusilla Grun. (Diat. Fr. Jos. L., Pl. 4 (D), Fig. 7).
LINEATI
COSCINODISCUS LINEATUS EHR.
Valve circular, markings hexagonal, cells in parallel rows. Border narrow, cellular.
Blue clay and Atlantic coast. Not common.
Pl. 3, Fig. 8.
FASCICULATI
COSCINODISCUS NITIDUS GREG.
Valve flat, markings rounded, distant, radiate, decreasing toward the border which is coarsely striate. Quite variable in size and in the distance between the markings.
Blue clay and Atlantic coast. Common.
Pl. 2, Fig. 18.
COSCINODISCUS NITIDULUS GRUN.
Valve usually not quite circular; markings smaller than in nitidus and fasciculate near the border.
Blue clay.
Pl. 2, Fig. 19.
Various intermediate forms between nitidus and nitidulus occur.
COSCINODISCUS SUBTILIS EHR.
Markings polygonal, irregular at the centre, but forming numerous fasciculi radiating {22}toward the border, the rows parallel to the central row of each fasciculus. Border narrow with fine striĉ; apiculi often present between the fasciculi.
Blue clay and along the coast. Very common in the water supply of Philadelphia and Camden, where the diameter seldom exceeds 40 µ and the markings on the semi-radius are 10 in 10 µ.
Pl. 2, Fig. 17.
COSCINODISCUS DENARIUS SCHMIDT
Markings larger than in C. subtilis, equal, forming usually ten fasciculi, each beginning near the semi-radius and containing ten parallel rows of granules.
Common in the blue clay and sparingly along the coast.
Pl. 2, Fig. 13.
Forms are found intermediate between C. subtilis and C. denarius, as shown in Fig. 15.
COSCINODISCUS POLYACANTHUS GRUN.
Markings angular, 10 in 10 µ, decreasing toward the border, fasciculate. Apiculi large, twelve or more, usually inserted at the middle of each fasciculus, and extending into the interior of the cell. The apiculi in outline resemble the heads of horse-shoe nails, and are seen with difficulty except when the valve is examined from the inner side. Border narrow, striated. Diam. 70 µ.
Pensauken, N. J., artesian well.
Pl. 38, Fig. 5.
Rattray's description of C. polyacanthus var. intermedia Grun., from Cape Wankarema, Siberia, gives the diam. as 60 µ, and there are about 7 markings by actual count in 10 µ in Grunow's figure (Diat. Fr. Jos. Land, Pl. 3 (C), Fig. 25). The apiculi are more numerous, but there appears to be little doubt of the general similarity. The Philadelphia form is abundant in the Pensauken well deposit at a depth of 33 ft. The apiculi become quite distinct in slides stained with silver nitrate by Mr. F. J. Keeley; they are distinct from small apiculi sometimes evident between the fasciculi. The specimens in the Pensauken deposit are mingled with other forms which cannot be distinguished from C. subtilis. Whether the two are identical, I am unable to determine. Rattray (Rev. Cos., p. 47) refers to H. L. Smith's Type Slide No. 100, from rice-field mud, Savannah, Ga., as C. subtilis. In Smith's slide, in my possession, a number of the forms show faint outlines of the large apiculi and are otherwise exactly like C. polyacanthus.
RADIATI
COSCINODISCUS VELATUS EHR.
Markings angular, decreasing slightly toward the coarsely striated border, covered with fine puncta.
Blue clay.
Pl. 3, Fig. 2.
COSCINODISCUS MARGINATUS EHR.
Markings rounded, large, decreasing toward the broad border, which is coarsely marked with distant striĉ. The cells are punctate.
Common in the blue clay.
Pl. 3, Fig. 9.
In the fossil forms the puncta are not evident, hence the species is usually described as not punctate.
COSCINODISCUS RADIATUS EHR.
Markings polygonal, slightly decreasing toward the border where they are much smaller; border well marked, striate. Quite variable in size.
Common in the blue clay and along the coast.
Pl. 3, Fig. 11. Fig. 1 is probably a smaller form.
COSCINODISCUS SUBAULACODISCOIDALIS RATTR.
Markings small, decreasing toward the border in somewhat fasciculate rows. About one-third the distance from the border are five (Rattray finds six) well-marked apiculi somewhat resembling those of Aulacodiscus. Border narrow, hyaline.
Rare in the lower stratum of the blue clay.
Pl. 3, Fig. 4.
COSCINODISCUS ARGUS EHR.
Markings angular with central dots, increasing from the centre toward the border, where they are smaller.
Blue clay.
Pl. 3, Fig. 7 (a small form).
COSCINODISCUS BIANGULATUS SCHMIDT
Central space and rosette absent, markings large, angular, not punctate, with large central papillĉ, decreasing toward the border. Border wide, coarsely marked with rows of granules, and with two indentations on the inner side distant from each other about two-thirds of the diameter.
Blue clay.
Pl. 3, Fig. 3.
Distinguished from Coscinodiscus asteromphalus var. omphalantha Grun., which also has two constrictions, by the absence of punctate markings.
COSCINODISCUS ASTEROMPHALUS EHR.
Central space small, surrounded by a rosette of large polygonal cells from which radiate hexagonal cells, increasing about half way toward the border and then slightly decreasing. Cells punctate.
Blue clay.
Pl. 2, Fig. 16; Pl. 40, Fig. 12.
COSCINODISCUS ASTEROMPHALUS VAR. OMPHALANTHA (EHR.) GRUN.
Central space absent, rosette evident. Markings 2½ in 10 µ, somewhat smaller near the rosette and decreasing near the border, which is constricted in two places, as in C. biangulatus.
Blue clay.
Pl. 38, Fig. 10.
COSCINODISCUS OCULUS-IRIDIS EHR.
Central space and rosette distinct; markings polygonal, not punctate, with large papillĉ, smaller near the rosette, increasing toward the semi-radius, and then decreasing to the striated border which is comparatively narrow.
Blue clay and Atlantic coast.
Pl. 3, Fig. 10.
ELABORATI
COSCINODISCUS LEWISIANUS GREV.
Valves elliptical, major axis a little more than twice the minor. From a point, usually near one side, radiate rows of granules in lines nearly parallel to the major axis. Border broad, with distinct striĉ.
Great Sedge Island, N. J. (artesian well), and in outcrops later than the Miocene, where it is usually found.
Pl. 3, Fig. 5.
ACTINOPTYCHINĈ
Valves divided into sectors alternately elevated and depressed.
(1) Actinoptychus.—Sectors plane.
(2) Polymyxus.—Sectors convex.
(actis, a ray, and ptyx, a fold)
Frustule cylindrical, less in length than the diameter, in zone view undulated. Valve divided into six or more sectors alternately raised and depressed, areolate and punctate, varying in the alternate divisions. The areolation is confined to the outer layer of the valve while the punctation is usually on an inner valve often found detached. Processes on the border, three or more. Umbilicus circular or angular, hyaline.
ANALYSIS OF SPECIES
| Sectors, six | undulatus |
| Sectors, eight or more, cellular | heliopelta |
| Sectors, fourteen, punctate | vulgaris |
ACTINOPTYCHUS UNDULATUS (KUETZ.) RALFS
Valve areolate and punctate in quincunx, divided into six equal sectors, alternately elevated or depressed, their areolations appearing different. Margin well defined. Umbilicus smooth, hexagonal. Processes three, sometimes six, inserted within the margin of each alternate division. Very variable in size and appearance.
This is the Actinocyclus of Bailey, figured and described in Amer. Jour. Science, 1842, p. 93, Pl. 2, Fig. 11, but not named. Kuetzing describes and names it and refers to Bailey.
Actinoptychus omphalopelta Ehr.
Actinoptychus cellulosa Ehr., H. L. Smith Sp. Typ., 384.
Quite common in marine and brackish water and in the blue clay.
Pl. 4, Figs. 1, 2, 4 and 6.
ACTINOPTYCHUS VULGARIS VAR. INTERRUPTA N. VAR.
Valve with fourteen sectors, the alternate ones divided by a smooth lanceolate space for about one-half the radius, forming with the smooth, circular umbilicus a seven pointed star. The sectors thus divided have coarser puncta in quincunx than the other sectors, ending in a smooth area near the margin, and also larger black puncta scattered from the centre to the semi-radius.
Near A. vulgaris var. neogradensis Pant.
Blue clay. Not common.
Pl. 4, Fig. 5.
ACTINOPTYCHUS HELIOPELTA GRUN. VAR.?
Valve circular, sectors, eight, umbilicus circular, without rays; border wide, cellular, with distinct rays. Inserted at a distance within the inner edge of the border are large processes, one on each of four alternate sectors, and two on each of the others. The sectors are cellulate and punctate.
Near A. heliopelta var. versicolor Brun., which, however, in the specimen in my collection from Atlantic City (artesian well), has a greater number of processes and they are situated on the edge of the border.
Outcrop at Buckshutem, N. J. Rare.
Pl. 4, Fig. 3.
It has been quite well determined, I think, that the typical forms of A. heliopelta occur at the base of the Miocene. At Rock Hall, Md., on the eastern shore of Chesapeake Bay, at a depth of from 21 to 130 ft., and at Wildwood, N. J., at a depth of from 78 to 179 ft., diatomaceous beds occur considered by Mr. Lewis Woolman (Geol. Surv. of N. J., 1898, pp. 116-121) "as synchronous in age," the former being deposited in the Delaware River Delta and the latter in the Chesapeake in post-miocene times. In each of these beds a small form of A. heliopelta is rarely found. The material at Buckshutem is post-miocene, and the form here figured shows a marked variation from the Miocene species and a gradual approach toward A. undulatus.
Valve circular, usually divided into fourteen sectors which are on the same plane at the centre, but the alternate ones are elevated into mammillated projections terminated by small processes on the margin. Zone view rectangular with undulations subconical, terminated by the processes.
POLYMYXUS CORONALIS L. W. BAIL.
Central space hyaline, rounded or slightly stellate, from which radiate rows of fine puncta in quincunx, shown in the figure only on the alternate elevations, the depressed interspaces being out of focus. The mammillĉ are stated by Bailey to vary from six to ten.
Very rare in the blue clay (Walnut St. Bridge). Occurs also in the Wildwood deposit (Bull. Torrey Bot. Club, 1895, p. 261).
Pl. 4, Fig. 7, and Pl. 5, Fig. 2.
Aulacodiscinĉ.—Valves with mammiform elevations near the border surmounted by nipple-like processes.
AULACODISCUS—THE ONLY GENUS AS ABOVE
Eupodiscinĉ.—Valves with ocelli.
(1) Actinocyclus.—Valve with one small ocellus; striĉ radial.
(2) Eupodiscus.—Valve with one or more ocelli; striĉ not radial.
(3) Auliscus.—Valve with large, elevated ocelli. Central area hyaline. Markings granular and costate.
(4) Pseudauliscus.—Valve with radiating granules. No central space.
(aulax, a furrow, and discus)
Valve usually circular, plane or with an elevated zone, frequently inflated beneath the processes; central space irregular or rounded, sometimes absent; markings granular, radial, sometimes in a reticulum.
The genus comprises more than one hundred species most of which are fossil, and is represented in this locality by a single form, A. argus, included by Rattray in his section "Retiformes," distinguished by the presence of a reticulum.
AULACODISCUS ARGUS (EHR.) SCHMIDT
Frustule in zone view elliptical. Valve circular, 125-190 µ in diam., closely covered with two kinds of markings, one, a mesh of large, radiating, angular cells, the outer plate, and the other, radiating rows of circular granules with hyaline spaces intervening and closer near the border, forming the inner plate which can occasionally be seen detached. Central space absent. The walls of the angular cells are crossed with fine lines and are probably composed of granules compressed so closely as to produce partial opacity, the depth of which depends in a measure not only on the superposition of the two plates, but on the relative closeness and thickness of the cell-walls. In a fully-developed specimen the effect is to produce more or less triangular cells containing three or four granules. In some cases the opacity is so great as to render detail invisible.
In the figure the valve is supposed to be divided into three sectors, illustrating at "a" the lower plate, at "c" the combination of the upper and lower plates, and in the other sector the cellular mesh of the upper plate. Processes, usually three, quite robust and inserted at from one-fourth to one-fifth the length of the radius from the border which is striated on the inner side. A form with four processes is found in the lower blue clay.
Tripodiscus argus Ehr.
Eupodiscus argus (Ehr.) Wm. Sm.
Not uncommon in the blue clay.
Pl. 4, Fig. 8.
(actis, a ray, and cyclos)
Valve circular or elliptical; surface flat at the centre, sloping toward the border. Central space usually evident, rounded or irregular. Markings rounded, granular, punctiform, in radial, or nearly radial, rows, sometimes fasciculate. A nodule, more or less evident, is found near the border which is usually striate.
Chromatophores round discs or granules.
ANALYSIS OF SPECIES
| Valve circular, rows radial, hyaline lines at the border | barkleyi |
| Valve circular, rows fasciculate | moniliformis |
| Valve elliptical | ellipticus |
The nodule is generally supposed to be a thickening of the cell-wall, and, in the opinion of Rattray, a projection outward, but "whether there may not be at the same time a slight inward protuberance is difficult to determine," though, as a rule, he seems to "think there is not."
ACTINOCYCLUS BARKLEYI VAR. AGGREGATA RATTR.
Surface flat from centre to semi-radius. Central space irregular, sometimes with a few scattered granules. Markings round with central dots distinct, about 7 at the centre, decreasing in straight radial rows to 12 in 10 µ at the border, where they form moniliform striĉ. Border narrow with striĉ about 16 in 10 µ. Hyaline interspaces at the origin of the shorter rows, but not at equal intervals. At the border, linear hyaline spaces occur at somewhat irregular intervals between the moniliform striĉ owing to the termination of certain radial rows before they reach the circumference. Nodule small, from one-seventh to one-fourth the radius from the border.
According to Rattray the distinction between A. ralfsii and A. barkleyi is partly in the absence of the zone arrangement of the hyaline spaces in the latter, and to the slight differences in the number of granules. The variety aggregata differs from the type form of barkleyi mainly in the distance of the nodule from the border. I have specimens from the blue clay material at Walnut St. Bridge, and from Smith's Island, in which the distance from the border in one case is, as stated above, quite different from that in the other. In specimens from Morris Cove, Conn., the locality referred to by Rattray, variations occur.
Blue clay.
Pl. 6, Fig. 1.
In the figure the subulate hyaline spaces at the border are, in some instances, wider than usual.
ACTINOCYCLUS MONILIFORMIS RALFS
Surface flat, from centre to about five-sixths of the radius. Central space rounded, with one or more granules. Markings, 8 in 10 µ, round, in radial rows, fasciculate, the oblique transverse rows irregular, very slightly decreasing until near the edge of the flattened zone, and then suddenly decreasing and appearing as decussating lines oblique to the border. Apiculi distinct, interfasciculate within the border. Nodule quite evident, surrounded by a rather wide irregular hyaline space on the margin of the flattened zone in the middle of the fasciculus. Border wide, with striĉ about 20 in 10 µ.
Blue clay. Port Penn. Not common.
Pl. 6, Fig. 2.
Equivalent to Actinocyclus ehrenbergii, H. L. S. Type Slide 10.
In a valve from Port Penn, Delaware Bay, two nodules occur nearly opposite each other.
ACTINOCYCLUS ELLIPTICUS VAR. DELAWARENSIS N. VAR.
Valve rhombic-elliptical. Markings somewhat angular, 6 in 10 µ at the centre where they are sub-concentric, thence decreasing in lines radiating more or less toward the border, where they suddenly become punctiform, striĉ about 20 in 10 µ. Border equal to one-fifth the radius. A nodule is found on the inner side of the border. Apiculi apparently absent.
The markings are larger than in the Richmond forms which are associated by Rattray with Actinocyclus ellipticus Grun. The form corresponds closely to Witt's Cestodiscus ovalis var.? (Witt, Polierschief. von Archangelsk-Kurojedowo, Pl. 8, Fig. 2), except as to the border. It does not answer to Van Heurck's figure or any other.
Blue clay. Very rare.
Pl. 3, Fig. 6.
(eu, well, pous, a foot, and discus)
Valve circular, 45-117 µ in diam. (De Toni). Central space absent, surface plane with angular cells. At the border short, circular processes or ocelli.
EUPODISCUS RADIATUS BAIL.
Valve with radiating hexagonal cells, sometimes slightly curved toward the large ocelli inserted near the border which are hyaline at the centre. Border wide, coarsely striate.
The number of ocelli heretofore recorded is four. Specimens with five processes are found in the artesian well at St. Augustine, Fla., and in material at Twelfth and Brandywine Sts. Mr. Hugo Bilgram has discovered valves with three and six ocelli.
Not common in the blue clay, but abundant along the southern coast of the Atlantic states and the Gulf of Mexico.
Not Eupodiscus radiatus Wm. Sm, which is Biddulphia smithii (Ralfs) V. H.
Pl. 5, Fig. 3.
(aulax, a furrow, referring to the grooves in certain species, according to De Toni, but preferably from auliscos, a small reed, referring to the processes?)
Frustule cylindrical; zone with longitudinal rows of fine puncta. Valve circular or elliptical, plane except near the processes; central area hyaline, usually circular. Markings of two kinds, granules radiating or scattered and radiating, costate lines, prominent or indistinct. Processes, two or three, large, short, cylindrical, with hyaline surface, near the ends of the major axis in a line oblique to it.
Auliscus is divided by Rattray into fourteen sections, defined chiefly by the character and arrangement of the markings. About eighty species are described, but as many of the forms are fossil, occuring in the Miocene of California, Oamaru and elsewhere, and as so few species are found in this locality, I shall refer but briefly to this division.
| Striolati.—No transverse median areas, striĉ inconspicuous | punctatus |
| Lineolati.—Markings distinct, pruinose, interrupted | pruinosus |
| Costati.—Transverse median areas usually distinct, markings continuous, costate | sculptus cĉlatus |
AULISCUS PUNCTATUS BAIL.
Valve broadly elliptical, or suborbicular, covered with delicate interrupted striĉ radiating in sinuous lines to the circumference, more evident on the transverse median area; puncta 3 in 10 µ, grouped into a rounded area on each side of the median line, elsewhere scattered. Central space rounded, processes two, large, suborbicular.
Port Penn, Delaware River. Rare.
Pl. 5, Fig. 6.
AULISCUS PRUINOSUS BAIL.
Valve elliptical, with distinct, interrupted, pruinose, irregular markings diverging in curved lines toward the circumference in the median part and converging toward the processes, interspersed with numerous darker markings having the appearance of apiculi. Central space nearly circular, sometimes with several granules. Processes large near the ends of the major axis and not oblique to it, or scarcely so, the edges with a crenulate border.
Blue clay. Rather rare.
Pl. 5, Fig. 8.
AULISCUS SCULPTUS (WM. SM.) RALFS
Valve elliptical or subcircular, median areas distinct, rounded, circumscribed by coarse distant costĉ radiating near the border where they are more evident, and converging toward the processes. Central space rounded, sometimes indefinite. Processes, two, circular.
Typical specimens show wide, coarse, distant costĉ, but, in some cases, the median areas are indistinctly outlined.
Blue clay.
Pl. 5, Fig. 5.
AULISCUS CĈLATUS BAIL.
Valve elliptical or subcircular, with radiating costĉ, more evident around the median areas and at the border, converging toward the processes, with intermediate punctate radiating lines. Central space rounded or irregular. Processes circular.
A. sculptus has coarser costĉ and the interspaces are hyaline, or apparently so, while in A. cĉlatus the punctate striĉ between the costĉ are more evident.
Blue clay. Not uncommon.
Pl. 5, Fig. 4.
Fig. 7 is a small, indefinite form intermediate between A. sculptus and A. cĉlatus. The numerous variations in this genus make it difficult to satisfactorily differentiate the species. The size of the four above described varies from 40 to 150 µ.
Valve circular or subcircular, nearly flat or depressed at the centre. Central space not evident. Processes circular, with narrow border, near the circumference. Border narrow, striated. Markings granular, radiating, sometimes interspersed with striĉ and apiculi.
Differs from Auliscus chiefly in the absence of a central space and costĉ.
PSEUDAULISCUS RADIATUS (BAIL.) RATTR.
Valve circular, or nearly so, flat. Central area with scattered granules radiating and increasing in size outward in diverging rows toward the border which is coarsely striated. Processes, two, circular. Two small apiculi are inserted at about one-fifth the radius from the border near the ends of the minor axis.
Blue clay. Rare.
Pl. 5, Fig. 9.
The apiculi are not always figured. They appear in a number of specimens from the Miocene of Maryland, Atlantic City, Harvey Cedars and Newbern.
PSEUDAULISCUS SPINOSUS (CHRISTIAN) RATTR.
Valve subcircular or slightly quadrangular, depressed at the centre and rising to an elevated zone near the border, the two zones separated by a distinct line. The inner zone indistinctly reticulate with fine puncta radiating from the centre and apiculi at intervals. The outer zone with smaller apiculi surrounding the inner zone and with intermingled rows of fine puncta and interrupted diverging striĉ. Near each end of the minor axis is a rather long, robust spine inserted at one-fourth the radius from the border which is narrow and striated. Processes circular, close to the circumference.
Auliscus spinosus Christian.
Blue clay. Rare.
Pl. 5, Fig. 10.
The genus is named by Schmidt, described by Leuduger-Fortmorel and emendated by Rattray.
(a) Triceratiinĉ.—Frustule cylindrical or prismatic, with three or more sides.
(b) Biddulphiinĉ.—Frustule cylindroid; valve with ends elevated into round processes or long horns.
(c) Anauleĉ.—Valve elliptical, lunate or triangular, with internal septa.
(d) Euodieĉ.—Frustule cuneate in zone view; valve lunate.
(a) TRICERATIINĈ
(1) Ditylum.—Frustule imperfectly siliceous. Zone with numerous divisions. Valve with central spine.
(2) Trinacria.—Processes with sharp spines.
(dis, two, and tyle, a swelling, referring to the outline of the frustule)
Frustule quadrangular, convex at the ends. Valve triangular, with undulating sides, the angles ending in a sharp point surmounted by a bristle. Surface of valve convex at centre from which projects a long stout spine.
DITYLUM INTRICATUM (WEST) GRUN.
Valve with the angles separated from the central part by lines imitating septa. Surface with radiating lines of fine puncta.
Blue clay. Rare.
Pl. 6, Fig. 4.
Detached valves only have been found in the blue clay. The form is regarded as but slightly siliceous and, therefore, the zone or girdle not being found in the fossil deposits, I am unable to illustrate it from material in the vicinity. On Plate 38, Figs. 6 and 7, I have sketched the zone and valve views of specimens found recently at Vera Cruz and labelled by H. L. Smith Triceratum intricatum West. I can find no difference between the recent and fossil forms of the valves. The zone is covered with fine puncta in quincunx, not visible under ordinary illumination.
The form as figured in Plate 6 corresponds to the figure of Lithodesmium undulatum Ehr. in Van Heurck, and West, in describing the Triceratium undulatum Wm. Sm. (figured as T. striolatum), thought that his T. intricatum was distinct from Ehrenberg's form on the ground that the latter came from the "Bermuda" (Nottingham) earth and must be strongly siliceous. Lithodesmium is characterized by the envelopment of the frustules by a cellular membrane which does not appear, evidently, in Ditylum. D. brightwellii is distinguished by its crown of spines on the margin; otherwise it closely resembles D. intricatum.
(treis, three, and acra, a point)
Valve triangular, angles elevated into spines. Cells at the margin large.
TRINACRIA PILEOLUS (EHR.) GRUN.
Valve with concave sides. Surface concave with unequal punctiform and scattered markings with central dots. Cells at the margin large, rounded. At the angles, which vary in elevation, a few puncta are seen.
Triceratium pileolus Ehr.
Blue clay. Rare.
Pl. 6, Fig. 9.
(b) BIDDULPHIINĈ
(a genus, constituted from Conferva biddulphiana of the English Botany, named after a Miss Biddulph)
Frustule prismatic or subcylindrical, concatenate, filamentous, or in zig-zag, or, as usually found, free. Zone well developed. Valve triangular, polygonal, elliptic or subcircular, convex, more or less elevated at the angles into processes or horns. Markings cellular or punctate. Chromatophores, small plates of various forms.
KEY TO THE SPECIES
| Valves costate | biddulphiana |
| Valves not costate: | |
| Markings cellular, angles elevated into horns | favus |
| Markings cellular, angles not elevated | antediluviana |
| Markings punctate, angles with subconical processes and long spines | granulata |
| spines short | rhombus |
| spines minute | smithii |
| processes truncate, valve elliptical | turgida |
| processes truncate, valve orbicular | lĉvis |
| processes absent, valve divided by irregular lines | alternans |
| not so divided | reticulum |
BIDDULPHIA BIDDULPHIANA (SMITH)
Frustule quadrangular with convex ends and rounded angles. Valve elliptical with undulated sides, divided by septa into three or more sections. Processes large, rounded, globular or subconical. Zone varying in width. Surface with rounded reticulations in longitudinal and transverse rows, except at the centre where they are concentric and smaller.
Conferva biddulphiana Smith (English Botany, 1807, Pl. 1762, upper figures).
Diatoma biddulphianum Ag.
Biddulphia pulchella Gray.
Blue clay. Hoboken Tunnel. Along the coast.
Pl. 7, Figs. 1, 2, 3, and 4.
Quite variable in size and number of septate divisions. Fig. 3 is an unusual form with narrow zone, having but one row of large reticulations, evidently a young frustule.
BIDDULPHIA FAVUS (EHR.) V. H.
Frustule quadrangular, elevated at the angles into subconical processes oblique to the longitudinal axis. Valve triangular or quadrangular, plane, of two layers, the outer layer composed of large hexagonal cells in rows parallel to the sides, the inner of small puncta radiating from the centre. Zone punctate in quincunx, never found open.
Triceratium favus Ehr.
Blue clay. Common along the coast.
The quadrangular form occurs only southward.
Pl. 6, Fig. 6. At "a" a cell showing the lower punctate layer. Pl. 40, Fig. 16, a transverse section of a portion of the valve showing the cellular structure and the punctated lower stratum.
BIDDULPHIA ANTEDILUVIANA (EHR.) V. H.
Frustules quadrangular, sometimes united in zig-zag chains. Valve quadrangular with more or less concave sides, sometimes cruciform. Surface with angular cells arranged in concentric and radiating lines increasing toward the circumference. At each angle is a large, rounded process, which, as well as the secondary layer, scarcely visible, is finely punctate.
Amphitetras antediluviana Ehr.
Amphitetras tessellata Shad.
Blue clay. Rare.
Pl. 6, Fig. 3.
A cruciform variety occurs at Pensauken, N. J., artesian well (Coll. F. J. Keeley).
BIDDULPHIA GRANULATA ROPER
Valve elliptical-lanceolate, convex, with diagonal rows of puncta 12 in 10 µ and sometimes with small scattered spurs. Processes inflated at the base, obtuse at the ends, which are curved outward toward alternate sides. Near each process and on opposite sides of the longitudinal axis is placed a stout spine bent or curved inward near the middle. Connective zone with diagonal rows of puncta smaller than those on the valve.
Pavonia, N. J., artesian well. Fossil in the Pleistocene. Along the coast. Not common.
Pl. 7, Fig. 6.
BIDDULPHIA RHOMBUS (EHR.) WM. SM.
Valve rhomboidal, sometimes triangular, with subconical processes. Surface convex with hexagonal reticulations, 7-9 in 10 µ, irregular at the centre and radiating to the circumference. Minute spurs are scattered over the surface, and on each side are usually two or three short spines.
Common along the coast and fossil in the Miocene and later deposits.
Pl. 7, Fig. 5 (somewhat inclined, as usually seen).
BIDDULPHIA SMITHII (RALFS) V. H.
Valve orbicular, convex, with reticulations 5 in 10 µ radiating from the centre and decreasing toward the margin and processes which are truncate. A short spine is found on each side half way between the processes. Zone narrow with fine puncta 12 in 10 µ in longitudinal rows.
Cerataulus smithii Ralfs.
Eupodiscus radiatus Wm. Sm.
Blue clay. Along the coast southward.
Pl. 7, Fig. 8.
BIDDULPHIA TURGIDA (EHR.) WM. SM.
Valve elliptical or orbicular, surface convex. Processes very large, cylindrical, placed obliquely and inclined by the torsion of the frustule. Between the processes are two stout spines, one on each side, frequently forked at the ends. Puncta fine, irregular at the centre and radiating toward the circumference.
Cerataulus turgidus Ehr.
Blue clay. Along the coast. Quite variable in size.
Pl. 7, Fig. 7.
BIDDULPHIA LĈVIS EHR.
Valve suborbicular or triangular, with short, truncate processes. Surface with fine puncta about 13 in 10 µ radiating in straight or curved lines toward the circumference and with fine spurs at intervals. Nearer one process than the other, and about half way between centre and circumference, are two small spines, one on each side. Quite variable in size.
Blue clay. Common along the coast.
Pl. 7, Fig. 9.
Fig. 10 (magnification about 260 diameters only) illustrates sporangial frustules discovered by Mr. T. Chalkley Palmer at Reedy Island, Delaware River. In frustules having a cylindrical form, the endochrome lines the cell-walls in the form of granules which become congregated toward the centre in the sporangia.
BIDDULPHIA ALTERNANS (BAIL.) V. H.
Valve triangular or, rarely, quadrangular, with sides straight or slightly concave, usually unequal. Angles obtuse, separated from the centre by costate lines. Surface with puncta of irregular shape, large at the centre, with smaller puncta interspersed. In many valves several lines appearing like costĉ extend inward from the border in various directions. Angles with small puncta in transverse and longitudinal rows.
Triceratium alternans Bail.
Blue clay. Along the coast.
Pl. 6, Fig. 7 and probably Fig. 8.
BIDDULPHIA RETICULUM (EHR.)
Frustule quadrangular. Valve triangular with straight or concave sides and rounded angles. Surface convex at the centre and angles. Markings of unequal size, mostly larger at the centre, scattered; at the angles, small puncta in longitudinal rows.
Triceratium sculptum Shad.
Triceratium punctatum Br.
Triceratium obtusum Br.
For explanation of the synonymy see "Biddulphoid Forms of N. A. Diat.," Proc. Acad. Nat. Sci., 1900, p. 724.
Blue clay. Along the coast.
Pl. 6, Fig. 5.
(c) ANAULEĈ
(eu, well, noton, a back, and gramma)
Frustule quadrangular. Valve elliptical or lunate divided by septa which constrict the margin. Surface flat with punctate markings.
EUNOTOGRAMMA LĈVE GRUN.
Valve lunate with obtuse ends. Septa, from four to eleven or more. Surface with puncta in transverse and longitudinal rows, sometimes indistinct and scattered.
Shark River. Rare. More common southward. Fossil at Buckshutem, N. J.
Pl. 7, Fig. 11, and Pl. 10, Fig. 15.
I am unable to distinguish between E. lĉve and E. debile, as intermediate forms occur.
(terpsinoos, gladdening?)
Frustules quadrangular, adnate in filaments, usually free. Valve elliptical or triangular, with undulating sides divided by septa into three or more sections.
TERPSINOË AMERICANA (BAIL.) RALFS
Valve lobed at each end or angle. Central space rounded, hyaline. Surface with fine puncta in radiating lines.
Blue clay. Not common.
Pl. 6, Fig. 10.
TERPSINOË NOVĈ-CĈSAREĈ BOYER
Valve triangular, with concave sides and broad angles equally three-lobed, separated from the central part by septa. Central space small or absent. Puncta delicate, radiating or scattered. L. of side 62 µ.
Pleistocene clay at Buckshutem, N. J. Fossil at Wildwood, N. J.
T. americana, forma trigona Pant.? (Le Diatomiste, Vol. 2, p. 207.)
Pl. 6, Fig. 11.
(d) EUODIEĈ
(derivation uncertain; apparently from euodia, fragrant, probably a euphemism)
Frustule in zone view cuneate. Valve semi-lunate, coscinodiscoid.
EUODIA GIBBA BAIL.
Valve with rounded markings, larger and scattered at the centre, radiating at the circumference and in indefinite straight rows at the semi-radius.
Delaware Bay (Mann).
Pl. 5, Fig. 1.
I have not seen this in the Philadelphia material. The figure is drawn from a specimen from the Gulf Stream, S. Atlantic.
Valve zygomorphous. Structure pinnate, not concentric. Valve divided either by a true raphe or cleft or by a linear space or line imitating a raphe.
Divided into three Groups:
1. Fragilarioideĉ.—Valves without a raphe; usually with a pseudoraphe or median line.
2. Naviculoideĉ.—Either one or both valves with a true raphe.
3. Surirelloideĉ.—Valves in which the raphe is concealed near the margin on one or both sides of each valve in a more or less elevated keel or wing.
(a) Tabellarieĉ.—Valve symmetrical with respect to both the longitudinal and transverse axes; septate, not cuneate.
(b) Meridioneĉ.—Valve symmetrical with respect to the longitudinal axis, asymmetrical to the transverse axis, cuneate, finely striated.
(c) Fragilarieĉ.—Valve of varied shape, not cuneate; costate or with transverse rows of puncta.
Frustule in zone view rectangular, in valve view linear or linear-elliptical, sometimes constricted in the middle, symmetrical to both axes, not cuneate; with two or more septa or annuli.
Chromatophores numerous, granular.
Rhabdonema.—Frustules with numerous septate partitions having one or several foramina. Transverse costĉ or rows of coarse puncta.
Tabellaria.—Frustules with two to six nearly straight septa. Transverse striĉ subtly punctate.
Grammatophora.—Frustules with two sinuate perforate curved septa. Transverse striĉ subtly punctate.
Striatella.—Frustules with alternate partitions, septate or partly so.
Attheya.—Frustules not septate but with numerous annuli.
(rhabdos, a rod, and nema, a thread)
Frustules quadrangular, concatenate, composed of numerous septate partitions with transverse costĉ or rows of puncta. Valves elliptical, with a pseudoraphe and transverse apparent costĉ and punctate lines; the partitions with one or several foramina.
Chromatophores in rosettes of various kinds (Karsten); usually parallel to the septa.
RHABDONEMA ARCUATUM (LYNG.) KUETZ.
Valve hyaline at the ends, with transverse rows of puncta producing the appearance of costĉ between the rows; pseudoraphe distinct; foramen single.
Diatoma arcuatum Lyngbye.
Common along the coast.
Pl. 8, Figs. 1, 2, and 3; Pl. 40, Fig. 10.
According to T. H. Buffham (Jour. Quek. M. C., Series 2, Vol. 2, p. 131), the frustules are of two kinds, those in which the length and breadth are the same and those which are much lengthened, with a wide hyaline girdle frequently in the middle. At the time of fructification the smaller frustules are attached to a larger one which produces a sporangium at the end of the girdle from which the other end of the frustule has disappeared, or, if the two halves of the frustule remain, two sporangia are formed.
RHABDONEMA MINUTUM KUETZ.
Frustules small; valve not smooth at the ends, elliptical or lanceolate-elliptical, with transverse rows of puncta; pseudoraphe distinct. Foramen single, alternating above and below in adjoining partitions.
Common in the blue clay and along the coast.
Pl. 8, Fig. 7 and Pl. 38, Fig. 11.
RHABDONEMA ADRIATICUM KUETZ.
Valve linear-lanceolate, with smooth angles; rows of puncta transverse, the intervals appearing as costĉ, as in arcuatum. Foramina, three.
Blue clay in the Pensauken and Pavonia deposits and along the coast.
Pl. 8, Figs. 4, 5 and 6.
(tabella, a tablet)
Frustules quadrangular, adnate in filaments, frequently found in zig-zag chains, united by a gelatinous isthmus, at length separating. Valve linear, inflated in the middle and at the ends; striĉ transverse.
Chromatophores numerous, small, along the zones.
TABELLARIA FENESTRATA (LYNG.) KUETZ.
Valve elongated; pseudoraphe narrow; transverse striĉ faint. In the zone view a straight septum is shown at each end of a valve.
Common, especially in the cedar swamps and ponds of the Pine Barren region, N. J.
Pl. 8, Figs. 11 and 12.
TABELLARIA FLOCCULOSA (ROTH) KUETZ.
Valve linear, with median inflation larger than the terminal; pseudoraphe rather broad in the middle; transverse striĉ subtly punctate. In zone view the frustules are quadrangular, or nearly so, with about six sometimes curved septa at one end alternating with those on the other end.
Conferva flocculosa Roth.
Common especially in the Pine Barrens of New Jersey.
Pl. 8, Figs. 8, 9 and 10.
(from gramma, a letter, and phoreo, I bear)
Frustules quadrangular, adnate, in zig-zag, united by an isthmus, or, usually, found free; divided by two sinuate and perforate curved septa. Valve linear or oblong, sometimes with sinuate sides, and with a pseudoraphe and transverse punctate lines.
Chromatophores granular.
GRAMMATOPHORA MARINA (LYNG.) KUETZ.
Valve linear-elliptical, with smooth apices. Septum with a wide undulation near its origin, thence straight and incrassate at the end. Striĉ in quincunx, 18-21 in 10 µ.
Diatoma marinum Lyngbye.
Blue clay. Along the coast.
Pl. 8, Figs. 17 and 18.
GRAMMATOPHORA MARINA VAR. SUBTILISSIMA (BAIL.) V. H.
Valve linear, slightly constricted near the smooth apices. Septum undulated near its origin and then straight, incrassate at the end. Puncta in quincunx very subtle, 34-36 in 10 µ.
Grammatophora subtilissima Bail.
Grammatophora oceanica var. subtilissima (Bail.) V. H., according to De Toni. G. marina and G. oceanica are united by some authors; the latter has more subtle striĉ.
Along the coast.
Pl. 8, Figs. 13 and 14.
GRAMMATOPHORA SERPENTINA RALFS
Valve linear-elliptical, long, measuring to 150 µ (De Toni); smooth at the apices. Septum with numerous undulations and hooked at the apex. Puncta in quincunx, 17 in 10 µ.
Along the coast.
Pl. 8, Fig. 21.
GRAMMATOPHORA ANGULOSA VAR. HAMULIFERA (KUETZ.) GRUN.
Frustule nearly quadrate; valve with rounded but not smooth apices. Septum bent into a sharp angle near its origin and ending in a broad hook. Puncta in transverse rows, 14 in 10 µ.
Along the coast.
Pl. 8, Figs. 15 and 16.
GRAMMATOPHORA ISLANDICA EHR.
Frustule oblong; valve elliptical-lanceolate. Septum robust with several undulations and hooked at the end. Pseudoraphe distinct; transverse rows of puncta, 10 in 10 µ.
Reported by Kuetzing in the Atlantic Ocean and by Kain at Belmar, N. J. I have not found it on our coast and I believe, in some cases, it has been confused with G. angulosa var. hamulifera. The figure is drawn from an Iceland form in H. L. Smith T. S., 186.
Pl. 8, Figs. 19 and 20.
(dim. of stria, referring to the lines on the frustule)
Frustules tabulate, adnate in short, stipitate filaments, scarcely siliceous, divided into partitions, septate or partly so at alternate ends.
STRIATELLA UNIPUNCTATA (LYNG.) AG.
Frustules with numerous bent septa extending the entire length. Valve lanceolate, somewhat unsymmetrical, subtly punctate, with pseudoraphe quite distinct.
"The specific name is derived from the appearance of the endochrome which in the living specimen is invariably collected in a central mass with slender threads radiating in all directions toward the cell-wall" (Wm. Sm.). Pyrenoids cuneate, in the centre of the endochrome, numerous.
Long Island Sound and along the coast.
Pl. 8, Figs. 22 and 23.
STRIATELLA INTERRUPTA (EHR.) HEIB.
Frustules quadrangular, with robust alternate septa extending to the middle. Puncta in quincunx, 22 in 10 µ.
Tessella interrupta Ehr.
Very rare along the coast.
Pl. 8, Fig. 24. (From a form found at Stonington, Conn.)
(named after Thomas Atthey)
Frustules quadrangular, tabulate, with numerous annuli. Valve elliptical-lanceolate, with a pseudoraphe and a central punctum. Extending from each end is a strong spine half as long as the valve.
ATTHEYA DECORA WEST
The only species. Diagnosis of the genus. The valves are imperfectly siliceous, scarcely visible in balsam.
Very local. Abundant at Shark River, N. J.
Pl. 8, Fig. 25.
Valve symmetrical in zone and valve view along the sagittal line, but asymmetrical to the transverse axis, cuneate. In zone view sometimes with wedge-shaped septa. Valve finely striated, without central and usually without terminal nodules; a pseudoraphe present.
Licmophora.—Frustules cuneate in stipitate fan-shaped fascicles.
Meridion.—Frustules cuneate in spiral fascicles.
(licmos, a fan, and phoreo, I bear)
Frustules wedge-shaped, joined together into fan-shaped, stipitate fascicles. Valve cuneate, rounded at both ends, septate. Chromatophores granular, round or oval in our species.
ANALYSIS OF SPECIES
(In accordance, so far as it relates to our species, with the classification of C. Mereschkowsky, Diagnoses of New Licmophorĉ, Nuova Notarisia, 1901.)
| Placatĉ—valve narrow, striĉ very fine, septa superficial | flabellata |
| Dubiĉ—valve bacilliform, septa shallow, frustule with thick walls | ovulum |
| Paradoxĉ—valve with lower end produced, striĉ fine, pseudoraphe distinct, septa deep | paradoxa gracilis tincta baileyi ? |
| Lyngbyeĉ—valve narrow, attenuated at both ends, distinct, septa deep | lyngbyei |
| Peristriatĉ—valve broad, pseudoraphe wide, striĉ robust | ehrenbergii |
LICMOPHORA FLABELLATA (CARM.) AG.
Frustule elongate, narrow; valve narrow, lanceolate-cuneate, enlarged at the base; striĉ very fine, 30 in 10 µ.
Echinella flabellata Carm.
Licmophora splendida Wm. Sm.
Common along the coast.
Pl. 9, Figs. 1 and 2.
LICMOPHORA OVULUM MER.
Valve ovate, attenuated to the rounded inferior apex; pseudoraphe indistinct, striĉ fine, 24 in 10 µ. Zone view broad, cuneate, angles rounded, inferior apex broad; frustule robust, septa superficial, straight. (Mereschkowsky, in part.)
Atlantic City. Common.
Pl. 9, Figs. 8 and 9.
LICMOPHORA PARADOXA (LYNG.) AG.
Frustule broad, with rounded angles; septa curved; valve ovate, inferior apex produced. Pseudoraphe distinct; striĉ varying from 25 below to 30 above in 10 µ.
Echinella paradoxa Lyng.
Rhipidophora paradoxa Kuetz.
Along the coast.
Pl. 9, Figs. 6 and 7.
LICMOPHORA GRACILIS (EHR.) GRUN.
Frustule cuneate, narrow, with sinuate margin; valve clavate, linear at the base; striĉ, 20 to 22 in 10 µ.
New Rochelle. Along the coast.
Pl. 9, Fig. 11.
LICMOPHORA GRACILIS VAR. ELONGATA (KUETZ.) DE TONI
As in the type, but more graceful and with deeper septa.
Rhipidophora elongata Kuetz.
Along the coast. Not common.
Pl. 9, Figs. 12 and 13.
LICMOPHORA TINCTA (AG.) GRUN.
Frustules cuneate, narrow, usually found in twos. Valve clavate, hyaline, rather broad at the base; septa moderately deep; pseudoraphe indistinct; striĉ, 27 at the base, 30 in the middle and 33 at the apex in 10 µ.
Gomphonema tinctum Ag.
Along the coast. Abundant from about the middle of July to the middle of August.
Pl. 9, Figs. 14 and 15.
LICMOPHORA BAILEYI (EDW.) GRUN.
Frustule broadly cuneate or with convex margins, rarely almost orbicular; valve spatulate or ovate with slender, produced base; septa very deep; pseudoraphe distinct; striĉ 20 in 10 µ.
Podosphenia baileyi (Edw.) Lewis.
Long Island Sound and upper coast of New Jersey.
This form is placed in a doubtful position by Mereschkowsky. As it corresponds more closely to the Paradoxĉ, it is placed here provisionally. The girdle face and apex of the valve are round, the pseudoraphe is distinct and the septa deep, but the stipe is short.
Pl. 9, Fig. 10 and Pl. 38, Figs. 3 and 4.
LICMOPHORA LYNGBYEI (KUETZ.) GRUN.
Frustule cuneate, slightly rounded at the angles. Valve oblanceolate; pseudoraphe distinct; septa deep; striĉ, 12 in 10 µ below, and 16 in 10 µ above.
Podosphenia lyngbyei Kuetz.
Along the coast.
Pl. 9, Figs. 3 and 4.
LICMOPHORA EHRENBERGII (KUETZ.) GRUN.
Frustule cuneate, broad. Valve obovate-lanceolate; pseudoraphe wide; striĉ coarse, 8 in 10 µ, moniliform.
Podosphenia ehrenbergii Kuetz.
Along the coast.
Pl. 9, Fig. 5.
(merizo, I divide)
Frustules in zone view cuneate, adnate in circular or spiral fasciĉ, at length becoming free. Valve symmetrical with respect to the longitudinal axis, more or less cuneate; costĉ and striĉ transverse.
Chromatophores numerous, small, elongated, in irregular rows on the zone (Pfitzer).
MERIDION CIRCULARE (GREV.) AG.
Transverse costĉ coarse, variable in number and distance apart, sometimes interrupted or indistinct; striĉ interstitial, 16 in 10 µ.
In springs and small streams of pure water.
Echinella circularis Grev.
Meridion constrictum Ralfs, sometimes given as a variety of M. circulare, differs only in the constriction below the apex. The two kinds of frustules are usually found growing together and as the variation is often extremely slight they are here included under the earlier name.
Pl. 10, Figs. 1, 2 and 3.
Fig. 1 represents the constricted form which is the more common. Fig. 3 is a sporangial form.
The sporangial frustules vary in shape and size, some being long and slender, others clavate, but they are all more or less tumid in the middle, with costĉ more indefinite than in perfect valves. All gradations occur, one end becoming shorter until the valve has the shape of the variety known as constrictum. It would seem, therefore, that the non-constricted form is a passage from the sporangial to the smaller or adult form, or is of no specific importance. All forms are found living together. The adult frustules are the smaller ones; it is from them that the sporangia are produced.
Meridion intermedium H. L. Smith (Amer. Quart. Mic. Jour., Vol. 1, p. 12) is characterized by less evident costĉ and is more delicate in general appearance. Some forms are capitate and others are not. Prof. Smith compares the M. intermedium with Peronia erinacea Bréb. and Arnott which he has named M. erinaceum, hitherto found only in Europe, and points out the relation of the two forms to Licmophora. An examination of the H. L. S. type slides of the two diatoms proves that Peronia has very delicate costĉ and a distinct pseudoraphe not noticeable in Meridion. On the slide of Peronia are frustules exactly similar to certain of the sporangial variations of M. circulare.
The fan-like arrangement of Licmophora, the marine form, and the circular chains of Meridion, the fresh-water genus, are similar. Both are stipitate at the beginning of their growth.
Divided into three sections:
Diatominĉ.—Valve circular, elliptical to linear, quadrate or cruciform, with transverse costĉ; without raphe, a pseudoraphe sometimes wanting.
Fragilariinĉ.—Valve elongate, with small central and terminal elevations, without costĉ but with transverse punctate striĉ; without genuine central nodule.
Eunotiinĉ.—Valve lunate; a raphe sometimes partially formed with terminal nodules near the edges.
DIATOMINĈ
Diatoma.—Frustules in filaments. Valve linear or elliptical, costate.
Plagiogramma.—Frustules in fasciĉ or free. Valve costate.
Opephora.—Valve costate, with an inner punctate stratum.
(diatemno, I cut in two)
Frustules oblong or quadrate, adnate in filaments, attached by alternate angles and finally separating. Valve linear or elliptical, with transverse costĉ and rows of puncta and a pseudoraphe.
Chromatophores large granules without definite arrangement. (See Pl. 40, Fig. 11.)
DIATOMA VULGARE BORY.
Valve elliptical-lanceolate, with apices sometimes rostrate or capitate; pseudoraphe narrow; costĉ, 5 in 10 µ.
Common everywhere in pure fresh water and extremely variable.
Pl. 10, Figs. 9 and 10.
Var. elongatum (Ag.) = var. ehrenbergii (Kuetz.)—elliptical-lanceolate, constricted near the apex.
Var. grande (Wm. Sm.) Grun.—linear, elongated, constricted near the apices.
Pl. 10, Fig. 4.
Both of these varieties, with numerous intermediate forms, are abundant near Newtown Square. Varieties of Grunow, known as breve, ovate-lanceolate; productum, ovate-lanceolate with produced apices; capitulatum, lanceolate with capitate extremities, are mingled together in the same gathering.
DIATOMA ANCEPS (EHR.) KIRCHN.
Valve linear with rostrate apices; costĉ robust; striĉ delicate, 20 in 10 µ. Zone view quadrangular.
Common in fresh water.
Pl. 10, Figs. 5 and 6. Fig. 11, Pl. 40, shows frustules containing the nuclei and chromatophores.
DIATOMA HIEMALE (LYNG.) HEIB.
Valve ovate-lanceolate to lanceolate; apices obtuse, not produced. Costĉ not numerous, robust; striĉ moniliform. Zone view quadrate, the costĉ as septa deeply dividing the valve into convex elevations.
Common in springs.
Pl. 10, Figs. 7 and 8.
In all species of Diatoma a punctum, or pore, is observed, usually at alternate ends of the two valves, by means of which a communication exists between adjoining frustules and causes them to adhere in zig-zag chains when partially separated.
(plagios, on the side, and gramma, a letter)
Frustules quadrangular, adnate in fasciĉ, or free. Valve linear, elliptical, or elliptical-lanceolate, divided by two or more median and two terminal costĉ or with a central and two terminal hyaline spaces.
| Valve with two median and two terminal costĉ: | |
| Linear, pseudoraphe distinct | pygmĉum |
| Linear, with striĉ at the ends | wallichianum |
| Ovate-lanceolate | obesum |
| Valve without costĉ but with central and terminal nodules: pseudoraphe absent | tessellatum |
PLAGIOGRAMMA PYGMĈUM GREV.
Valve linear-elliptical; pseudoraphe distinct; rows of granules transverse, usually six in each compartment, moniliform, three on each side.
Blue clay. Not common.
Pl. 10, Fig. 13.
PLAGIOGRAMMA WALLICHIANUM GREV.
Valve linear, rounded at the ends; pseudoraphe absent; transverse rows of granules, six or seven in each compartment, and two or three rows of smaller granules at each end.
Blue clay. Not common.
Pl. 10, Fig. 14.
PLAGIOGRAMMA OBESUM GREV.
Valve rhombic-lanceolate, the costĉ scarcely visible; pseudoraphe rather wide; rows of granules, about seven in each compartment, slightly radiating.
Blue clay. Not common.
Pl. 10, Fig. 12.
PLAGIOGRAMMA TESSELLATUM GREV.
Valve elliptical-lanceolate; central space transversely elliptical to the major axis, half the diameter of the valve; terminal spaces more or less circular or ovate. Granular markings large, quadrangular, in transverse rows. Pseudoraphe not distinct. As the central space does not reach the margin, it is a question whether this form is a Plagiogramma or a new genus.
Blue clay. Rare.
Pl. 10, Fig. 11.
(ope, an opening, and phoreo)
Frustule rectangular. Valve cuneiform, linear or elliptical-lanceolate, with broad, transverse striĉ and a well-defined pseudoraphe or median area.
The genus "portant des stries en forme de boutonnières," as Petit remarks, is quite near Fragilaria, under which the species here described were originally included. (See Schmidt's Atlas, Pl. 298, where numerous forms of F. pinnata are figured.)
OPEPHORA SCHWARTZII (GRUN.) PETIT
Valve obovate-lanceolate or nearly linear with rounded apices; striĉ transverse, broad, 3 or 4 in 10 µ; median area lanceolate.
An inner stratum, with puncta in transverse rows, is apparent.
Blue clay. Not uncommon. Variable in size.
Pl. 10, Figs. 16 and 19.
OPEPHORA PACIFICA (GRUN.) PETIT
Valve linear, oblong, with rounded apices. Median area linear, narrow; striĉ punctate.
Blue clay.
Pl. 10, Fig. 18.
Petit (Diat. Cap Horn) in his diagnosis states that the valves are cuneiform, but they are not always so.
OPEPHORA PINNATA VAR. LANCEOLATA N. VAR.
Valve lanceolate; costĉ slightly radiate, punctate; median area broad, lanceolate.
Differs from O. pinnata in outline, radiation of the costĉ and median area.
Blue clay. Rare.
Pl. 10, Fig. 17.
FRAGILARIINĈ
Fragilaria.—Frustules in fasciĉ. Valve with transverse striĉ. Pseudoraphe indistinct.
Rhaphoneis.—Striĉ radiate; pseudoraphe distinct.
Dimerogramma.—Pseudoraphe broad.
Trachysphenia.—Valve cuneiform.
Synedra.—Valve elongate.
Asterionella.—Frustules in star-shaped clusters.
(fragilis, because of the fasciĉ easily breaking up)
Frustules rectangular, adnate in fasciĉ, soon breaking up. Valve lanceolate, oblong or elliptical in general outline, with convex or sinuate margins; without costĉ; pseudoraphe narrow or indistinct; striĉ transverse. Chromatophores vary according to species. In some they consist of four bands on the valves; in others they are granular (Mereschkowsky).
Brun divides the genus into two sections, Fragilaria proper and Staurosira. The former, with an indistinct pseudoraphe, includes the species virescens, arctica, undata and linearis, while the latter, with distinct pseudoraphe, includes capucina, harrisonii, construens and parasitica.
FRAGILARIA VIRESCENS RALFS
Frustules in long fasciĉ. Valve elliptical-lanceolate, obtuse at the apices; pseudoraphe indistinct; striĉ, 17 in 10 µ, punctate.
Very common in springs and pure streams. The fasciĉ are often a foot or more in length.
Pl. 10, Figs. 20 and 21.
FRAGILARIA ARCTICA GRUN.
Valve oblong or elliptical, 10 µ in length; striĉ subtle, with coarse, short striĉ at intervals on the margin and evident in zone view.
Marine. Common at Cape May, N. J.
Pl. 10, Figs. 22 and 23.
FRAGILARIA UNDATA WM. SM.
Valve in general outline linear-elliptical, with extremities produced; striĉ subtle; pseudoraphe distinct.
Fresh water.
Pl. 10, Figs. 24, 25, 27, 28 and 29.
FRAGILARIA LINEARIS CSTR.
Valve linear, with rounded apices; striĉ subtle; pseudoraphe indistinct.
Marine. Cape May.
Pl. 10, Fig. 37. Fig. 36 is an indeterminate form occasionally found in the blue clay.
FRAGILARIA CAPUCINA VAR. MESOLEPTA RAB.
Valve linear, constricted at the hyaline middle; apices slightly produced; striĉ, 17 in 10 µ. Quite variable in size.
Schuylkill River. Morrisville (Keeley).
Pl. 10, Fig. 34.
FRAGILARIA HARRISONII (WM. SM.) GRUN.
Frustules rectangular, solitary or in twos. Valve cruciform; pseudoraphe narrow, lanceolate; striĉ robust, radiating in the middle, composed of confluent puncta, larger at the circumference.
Blue clay.
Pl. 10, Fig. 31.
FRAGILARIA CONSTRUENS (EHR.) GRUN.
Valve in general outline lanceolate, with produced apices; pseudoraphe lanceolate, distinct or broad; striĉ subtle, 15 in 10 µ. L. of valve, 10-45 µ.
Staurosira construens Ehr.
Odontidium tabellaria Wm. Sm.
Blue clay.
Pl. 10, Fig. 30.
FRAGILARIA PARASITICA (WM. SM.)
Frustules solitary or in twos. Valve lanceolate, sometimes constricted in the middle; pseudoraphe wide, lanceolate; striĉ subtle. Parasitic on other diatoms.
Odontidium parasiticum Wm. Sm.
Not common. Media (Palmer).
In the constricted form it is known as F. construens var. binodis (Ehr.) Grun.
Pl. 10, Fig. 35.
An examination of the synonymy of the species of Fragilaria will convince the student of the difficulty of determining the correct name even in well-known forms. If all of the species of Fragilaria proper have granular chromatophores, and all of Staurosira are placcochromatic, a satisfactory division can be made, but so long as these facts are not known in all species, and as authors have repeatedly confused the two divisions, the nomenclature will be uncertain. F. harrisonii is probably in any case to be separated from the others. De Toni includes it under its original name of Odontidium, which genus he places near to Diatoma. The number of species in our locality is too limited to render further discussion of any value.
(rhaphis, a needle)
Frustule in zone view linear. Valve lanceolate or elliptical-lanceolate; pseudoraphe distinct; striĉ radiating, moniliform.
RHAPHONEIS AMPHICEROS EHR.
Valve lanceolate, broad, with apices produced; striĉ in curved lines, moniliform, the large granules in longitudinal lines.
Blue clay.
Pl. 10, Fig. 38.
RHAPHONEIS AMPHICEROS VAR. RHOMBICA GRUN.
Valve as in type form but shorter, with larger and more remote granules.
Blue clay.
Pl. 10, Figs. 39 and 40.
RHAPHONEIS BELGICA VAR. INTERMEDIA GRUN.
Valve lanceolate, rostrate; granules in longitudinal and nearly transverse, not radiating, lines.
Absecon, N. J.
Pl. 10, Fig. 41.
(dis, two, meros, a part, gramma, a letter)
Frustules quadrangular, inflated at the angles, in fasciĉ. Valve ovate or lanceolate; striĉ moniliform, transverse or slightly radiate; median area or pseudoraphe broad, lanceolate.
DIMEROGRAMMA MARINUM (GREG.) RALFS
Valve lanceolate or linear and inflated in the middle; striĉ moniliform, transverse or slightly radiate; median area linear or lanceolate, sometimes not reaching the smooth extremities; striĉ, 8 in 10 µ.
Pl. 12, Figs. 9 and 10.
Fig. 9 differs in its lanceolate outline, in having four puncta on each side in a row, and in the striĉ which are radiate.
DIMEROGRAMMA SURIRELLA (EHR.) GRUN.
Valve elliptical-lanceolate, with rounded apices; striĉ moniliform, radiate; pseudoraphe narrow, lanceolate.
Blue clay.
Pl. 12, Fig. 11.
DIMEROGRAMMA MINUS (GREG.) RALFS
Valve rhombic-lanceolate; striĉ punctate, radiate; pseudoraphe lanceolate; apices smooth.
Blue clay. Along the coast.
Pl. 12, Figs. 12, 13, 14.
(trachys, rough, and sphen, a wedge)
Frustules rectangular. Valve cuneiform with coarse puncta in transverse and longitudinal lines; pseudoraphe narrow, linear. One species only.
TRACHYSPHENIA AUSTRALIS PETIT
Characters of the genus. Valve small; puncta, 6 in 10 µ. Allied to Dimerogramma.
Shark River, N. J. Rare.
Pl. 12, Fig. 15.
(synedrion, a sitting together)
Frustules adnate in small stipitate clusters or free. Valve elongate, linear or linear-lanceolate; pseudoraphe distinct; costĉ absent.
The genus Synedra has few distinctive characters. As Brun remarks (Diat. des Alpes et du Jura, p. 122), the dilatation of the extremities and the pseudo-nodule are of little value in classification, as the intermediate forms are so numerous. Fragilaria occurs in very long ribbons or fasciĉ, Synedra in short fasciĉ or radiating clusters. Fragilaria is seldom longer than three or four times the width, while Synedra is nearly always so. The former has fine, often subtle, markings and narrow pseudoraphe, while the latter has coarser punctate striĉ and a more distinct pseudoraphe.
Chromatophores usually consist of two bands, one on each of the valves. Karsten states that in the marine forms the chromatophores are oval or polygonal discs, each of which usually encloses a pyrenoid.
SYNEDRA ULNA (NITZSCH) EHR.
Frustules solitary or in twos. Valve 150-250 µ in length, linear or linear-lanceolate, with rostrate apices; striĉ, 9 in 10 µ.
Common in rivers and streams.
Pl. 11, Figs. 4, 7 and 11 (?).
Frequently interrupted in the middle. The distinction made by Wm. Smith as to the presence or absence of the central blank space is probably not necessary, as both forms are found which are otherwise identical.
Fig. 5 represents the formation of a sporangial frustule which differs from the usual form in its inflated ends prolonged into rostrate apices. Figs. 1 and 6 are sporangial frustules.
SYNEDRA BICEPS (KUETZ.) SCHMIDT
Valve sublanceolate, inflated at the ends, apices rounded; central space not always distinct; pseudoraphe narrow; striĉ radiate at the ends.
This is not Kuetzing's species, if the descriptions and figures are accepted, nor is it H. L. Smith's Type No. 545, which is S. ulna var. danica, nor is it S. biceps Wm. Smith, but it is exactly Schmidt's form (Atlas, Pl. 303, Figs. 10-15).
Schuylkill River.
Pl. 11, Fig. 3.
SYNEDRA DANICA KUETZ.
Valve lanceolate, suddenly constricted at the rounded apices; central space frequently absent.
Very common in streams.
Pl. 11, Fig. 2.
The figure represents an unusually large form. It differs from S. ulna only in its apices.
SYNEDRA CAPITATA EHR.
Valve long, linear, dilated into triangular acute apices; pseudoraphe distinct; striĉ radiate at the ends.
Blue clay.
Pl. 11, Fig. 8.
SYNEDRA ACUS KUETZ.
Valve very narrow, lanceolate, acicular, with obtuse apices.
Common in the Schuylkill River.
Pl. 11, Figs. 9 and 18.
SYNEDRA GOULARDI BRÉB.
Valve constricted in the middle; apices sub-acute, sometimes slightly rostrate or capitate; central space evident.
Neshaminy Creek (Palmer). Blue clay. Crum Creek.
Pl. 11, Figs. 12 and 13.
SYNEDRA PULCHELLA (RALFS) KUETZ.
Valve lanceolate, tapering to the sub-acute, rostrate or slightly capitate apices; dilated at the central hyaline space; pseudoraphe distinct. Very variable in size.
Crum Creek. Schuylkill River. Rather common.
Pl. 11, Figs. 14, 15, 16.
SYNEDRA PULCHELLA VAR. ABNORMIS MACCHIATI?
Valve as in type form, except that one end is curved like a beak, as in S. hamata Wm. Sm., which it resembles.
Not uncommon in the Schuylkill River.
Pl. 11, Fig. 17.
SYNEDRA OXYRHYNCHUS VAR. UNDULATA GRUN.
Valve linear-lanceolate with produced rostrate apices, asymmetrical, sigmoid; pseudoraphe narrow; pseudo-nodule large.
Common in fresh water.
Pl. 12, Fig. 1.
SYNEDRA PULCHELLA VAR. FLEXELLA N. VAR.
Frustule slightly attenuated at the ends, truncate, somewhat tumid in the middle and flexed. Valve lanceolate, with obtuse or subcapitate apices and with two almost imperceptible constrictions at the middle producing a tumid appearance; pseudoraphe distinct; pseudo-nodule absent. L. 56 µ; striĉ, 14-16 in 10 µ.
Some valves are bent and incised on one side. The outline of the valve is that of pulchella.
Common at Newtown Square.
Pl. 12, Fig. 2.
SYNEDRA RADIANS KUETZ.
Frustules linear, in small fasciĉ. Valve 34 µ in length, linear, with apices rostrate, obtuse, sometimes slightly capitate; pseudoraphe distinct; striĉ about 20 in 10 µ.
Fresh water.
Pl. 10, Figs. 32 and 33.
There is difficulty in recognizing S. radians K. as described and figured by different authors. On Plate 12, Fig. 8, I have drawn a specimen from H. L. Smith's Type Slide No. 574, labelled S. radians Kuetz., not Wm. Smith, which, however, corresponds closely to Smith's figure (Brit. Diat. 1, Pl. 11, Fig. 89). De Toni gives S. radians Kuetz. as equivalent to S. tenera Wm. Sm. Van Heurck's figure of S. radians, and also the figure of ulna var., said to be synonymous with H. L. Smith's S. radians, which does not correspond to the specimens on Smith's slide in my possession, are confusing. In Van Heurck's Synopsis the striĉ are said to be 16 or 17, while De Toni describes them as subtle and from 17 to 24 in 10 µ. The length is quite variable.
Several species of Synedra resemble S. radians in the mode of growth, as they are adnate at first, in short bands, the frustules being sessile on other plants or objects, attached at the terminal nodules which, although scarcely visible in most forms, are probably present in all. The frustules are not closely connected at the free end, and soon become entirely detached.
In Diatoma and Fragilaria, we find a punctum or pore at one end of a valve, but not in line with the pseudoraphe; in Synedra, a minute pore is usually found in the position of the terminal nodule and, in some species, indications of a central nodule are observed; the median line is wider but there is no raphe. In the fresh-water Synedrĉ, many of which are among the longest of diatoms, living in running streams, the terminal nodules are much more indistinct, while the marine forms have distinct terminal nodules, are not, as a rule, found in bands, and assume a more naviculoid outline.
SYNEDRA VAUCHERIĈ VAR. PARVULA (KUETZ.) RAB.
Valve lanceolate, with produced or rostrate apices; pseudo-nodule wide, excentric. L. 17 µ.
Crum Creek.
Pl. 12, Fig. 5.
Fig. 6 represents a variety with coarser striĉ from the Schuylkill River. Both are easily mistaken for Fragilaria intermedia.
SYNEDRA FULGENS (GREV.) WM. SM.
Frustules geminate or flabellate on a stipe. Valve slightly inflated in the middle and at the apices; pseudoraphe narrow; striĉ finely punctate, radiate at the ends.
Marine. Atlantic City.
Pl. 11, Fig. 10.
SYNEDRA AFFINIS KUETZ.
Valve lanceolate; striĉ marginal, leaving a broad lanceolate pseudoraphe.
Common along the coast.
Pl. 12, Fig. 3.
SYNEDRA AFFINIS VAR. PARVA (KUETZ.) V. H.
Valve lanceolate, slender; striĉ marginal, shorter than in the type.
Synedra gracilis Kuetz.
Common along the coast.
Pl. 12, Fig. 7.
SYNEDRA AFFINIS VAR. TABULATA (AG.) V. H.
Valve linear-lanceolate; striĉ, 11 in 10 µ, very short.
Not common. New Rochelle.
Pl. 12, Fig. 4.
(dim. of aster, a star)
Frustules linear, slightly inflated at the ends, arranged in star-shaped clusters which soon break up. Valve linear, unequally inflated at the ends.
ASTERIONELLA FORMOSA HASS.
Valve clavate at the ends; striĉ transverse, 17 in 10 µ, pseudoraphe very narrow or indistinct; an ovoid, hyaline area at each end.
Newark, N. J. Broomall's Lake, Media (Palmer).
Pl. 12, Figs. 19, 20, 21.
ASTERIONELLA INFLATA HEIB.
Valve linear, capitate at each end and tumid in the middle; striĉ distinctly punctate; pseudoraphe indistinct, or not apparent. L. 30 µ.
Fresh water. May's Landing, N. J.
Pl. 12, Fig. 22.
EUNOTIINĈ
Eunotia.—Frustules either free, in fasciĉ or epiphytic. Valves arcuate.
Actinella.—Frustules, solitary or in small clusters, cuneate. Valve inflated at one end.
(eu, well, and noton, a back, referring to the strong, ridged dorsum)
Frustules free, in fasciĉ or epiphytic. Valve arcuate, without costĉ, transversely striated; pseudoraphe absent; pseudo-nodules at each end.
Chromatophores laminate along the concave zone and the valves.
Very many species of Eunotia have been created to differentiate size and number of crenĉ or undulations. An examination of certain fossil deposits of New England, as well as a gathering from the blue clay of Philadelphia, will show forms which vary infinitely. E. major and E. gracilis are scarcely distinguishable because of the intermediate variations. The striĉ in all forms are punctate, but the puncta are frequently confluent.
ANALYSIS OF SPECIES
Eunotia is divided into two sections, Himantidium and Eunotia proper. In Himantidium, the frustules are in fasciĉ, either short or long. Among those with short fasciĉ are major, gracilis, and nymanniana; those with long fasciĉ are pectinalis, solierolii and veneris. Eunotia proper includes frustules, free or epiphytic, in which the valves are not dentate on the dorsal margin, such as lunaris, hemicyclus, biceps and prĉrupta; and those in which the valves are dentate or crenate on the dorsum, such as monodon, triodon, diadema and others.
The resemblance between Eunotia and Epithemia is noticeable. In both, the epiphytic character of the valve is seen in the shape of the frustule which is arched, and, in the free forms, is adherent at the ends only. In Epithemia, the median is more evident than the terminal nodules. In Eunotia, there is no median nodule, but the end nodules, in some species, are quite evident, and a tendency is shown to produce a very short raphe. The arrangement of puncta in valve view is similar in both genera.
Section 1. Himantidium
EUNOTIA MAJOR (WM. SM.) RAB.
Valve arcuate, linear, subcapitate, recurved. Striĉ punctate, 12 in 10 µ L. 90-190 µ.
Common in fresh water.
Pl. 13, Figs. 1 and 2.
EUNOTIA GRACILIS (EHR.) RAB.
Valve with sides parallel; apices slightly capitate and revolute; striĉ, 10 in 10 µ. The striĉ on the connective membrane more delicate than in E. major. Intermediate forms occur.
Common in fresh water.
Pl. 13, Fig. 3. Fig. 4 is indeterminate.
EUNOTIA NYMANNIANA GRUN.
Valve small, curved, with parallel dorsal and ventral margins; apices truncate and recurved into dorsal elevations; striĉ delicate.
Blue clay. Not common.
Pl. 13, Fig. 32.
EUNOTIA PECTINALIS (KUETZ.)
Valve linear, arcuate, apices slightly rostrate; striĉ distinctly punctate with puncta in longitudinal rows nearer together at the ends.
Himantidium pectinale Kuetz.
Common in fresh water.
Pl. 13, Figs. 6 and 7.
The fasciĉ are associated in large masses, sometimes an inch or more in diameter, and late in August are found a foot or more in length, of a beautiful chocolate color. Exceedingly abundant in the cedar-swamp streams of the Pine Barren regions of New Jersey. In winter, the dead frustules form a parchment-like coating upon the twigs, dead leaves, and other débris on the borders of streams.
This species can scarcely be referred to Dillwyn's Conferva pectinalis, as, in his description, quoting Mueller, he says that "the filaments are of a dirty green color; seldom exceeding half an inch in length." Dillwyn's form is probably Fragilaria virescens, which equals Fragilaria pectinalis Ehr., while Kuetzing's species is Fragilaria pectinalis Ralfs. It is not impossible to confuse Fragilaria virescens and Eunotia pectinalis when the zone only is seen under a low power and their mode of growth is similar.
EUNOTIA PECTINALIS VAR. UNDULATA RALFS
Valve as in type form, but with undulate margins.
Common in the cedar swamps of New Jersey.
Pl. 13, Figs. 8 and 10.
EUNOTIA PECTINALIS VAR. SOLIEROLII (KUETZ.)
Valve as in type, but with internal divisions as though in the process of reduplication.
Not common. Moorestown, N. J. (Palmer).
Pl. 13, Fig. 9.
EUNOTIA PECTINALIS VAR. VENTRICOSA GRUN.
As in type, but with the valves tumid in the middle.
May's Landing, N. J.
Pl. 13, Fig. 12.
Fig. 11 is a form found in the blue clay. It differs in the coarser puncta from the var. ventricosa. In outline it resembles Eunotia arcus Wm. Sm., which is Ceratoneis arcus (Ehr.) Kuetz., but the central nodule is not present as in the latter form, which connects Eunotia and Cymbella. It may be a form of E. luna Ehr. (A. S., Atlas, Pl. 286, Figs. 33 and 34.)
EUNOTIA VENERIS KUETZ.
Valve with convex dorsal and straight ventral margins, more or less constricted near the sub-acute apices. Striĉ subtle, punctate.
Eunotia incisa Greg.
May's Landing, N. J. Blue clay, Pavonia, N. J.
Pl. 13, Figs. 30 and 31.
Eunotia (proper)
EUNOTIA LUNARIS (EHR.) GRUN.
Frustules sessile, solitary or in clusters. Valve arcuate, narrow, attenuated toward the apices, which are sometimes slightly rostrate or rostrate-capitate; transverse striĉ, 14 in 10 µ, punctate.
Very common in ditches, especially in the spring. Variable in length.
Pl. 12, Figs. 24 and 25.
EUNOTIA HEMICYCLUS (EHR.) RALFS
Valve semicircular, with obtuse apices; striĉ transverse, punctate; terminal nodules minute and indistinct.
Hammonton Pond, N. J. Rare.
Pl. 12, Fig. 23.
The genus Pseudo-Eunotia was created by Grunow for forms like Eunotia, but without terminal nodules. As, however, in E. lunaris and E. hemicyclus nodules are evident, although not so large as in many species, I include these two forms as heretofore under Eunotia.
EUNOTIA BICEPS EHR.
Valve linear, slightly arcuate, narrow, with rounded apices somewhat revolute; striĉ, 16 in 10 µ.
May's Landing, N. J.
Pl. 13, Fig. 27.
EUNOTIA PRĈRUPTA EHR.
Valve convex on dorsal side, apices dilated and truncate; striĉ distant at centre.
Common in the blue clay.
Pl. 13, Fig. 5.
EUNOTIA PRĈRUPTA VAR. BIDENS GRUN.
Valve with two undulations; otherwise as in type.
Eunotia bigibba Greg.
With the type.
Pl. 13, Fig. 19.
EUNOTIA ROBUSTA RALFS
Valve arcuate, with several or numerous dorsal ridges or crenĉ which decrease in relative size in proportion to their number. Striĉ radiate, variable in distance apart, and in size of puncta.
Ralfs included under this one name the following species named by Ehrenberg: E. diodon (2 crenĉ); E. triodon (3); E. tetraodon (4); E. pentodon (5); E. diadema (6); E. heptodon (7); E. octodon (8); E. enneadon (9); E. decadon (10); E. hendecadon (11); E. duodecadon (12); E. serra (13); E. prioritis (14); all more than 20, E. polyodon. E. scalaris, with from 15 to 17 crenĉ, and E. icosodon with 20, may be added.
It is probable that all of these forms occur at May's Landing, N. J. The forms with more than eight crenĉ are comparatively rare. In the blue clay those with from four to six are most common.
Pl. 13, Figs. 13, 14, 15, 16, 17, 21, 24, 25.
EUNOTIA BACTRIANA EHR.
Valve linear, apices revolute, acute, dentate on the dorsal margin, with one acute crena near each end.
Tom's River, N. J. Rare.
Pl. 13, Fig. 18.
EUNOTIA BIDENTULA WM. SM.
Valve with straight ventral margin, and with two undulations on the dorsum; apices large, rounded.
May's Landing, N. J. Rare.
Pl. 13, Fig. 20 (not Schumann's form, which has angular crenĉ).
EUNOTIA FORMICA EHR. VAR.?
Valve turgid in the middle and at the apices which are unilaterally truncate.
Pensauken, N. J. (artesian well).
Pl. 13, Fig. 26 (not a typical form).
The following are forms which appear to be indeterminate, or, in any case, are scarcely worthy of distinction by specific names, as might be said of others of the innumerable variations of this genus:
Fig. 23, Pl. 13, probably a form of prĉrupta. Newtown Square.
Fig. 28, Pl. 13, from the blue clay.
Fig. 29, Pl. 13, an asymmetrical form, apparently abnormal, but not rare at May's Landing, N. J.
Fig. 17, Pl. 38. Valve convex on the dorsal side, incised on the ventral; striĉ about 15 in 10 µ, closer at the ends; L. 30 µ. Schuylkill River.
Fig. 18, Pl. 38. Valve arcuate, asymmetrical, broader at one end; terminal nodules large; striĉ, 10 in 10 µ; L. 47 µ. Gloucester, N. J., artesian well.
Numerous variations of the above species are illustrated in Schmidt (Atlas, Pls. 285-291).
(dim. of actin, a ray)
Frustules solitary, or in small clusters, sub-cuneate or nearly linear. Valve arcuate, rounded at one end and suddenly widened at the other into a cup-shaped or lychnoid inflation.
ACTINELLA PUNCTATA LEWIS
Valve with fine, transverse striĉ; on the margin, puncta at intervals; terminal nodules distinct.
May's Landing, N. J.
Pl. 12, Figs. 16, 17, 18.
Fig. 17, from Tom's River, N. J., is an approach toward A. brasiliensis Grun.
Fig. 18 represents the frustules geminate, a frequent occurrence.
In discussing the Naviculoid group, the general divisions of Cleve are here followed, and all diatoms having a true raphe are included. I have added the genus Epithemia and also Rhopalodia, partly because they contain a raphe of a certain kind and partly because they resemble the markings of certain of the genus Hantzschia in the following group, although in other respects there is probably no similarity.
The difficulty of combining the numerous genera into groups which are naturally affiliated is avoided in the following arrangement based on superficial similarities, and is intended merely as an artificial key. To unite all forms having a raphe and which are symmetrical with valves similar and not sigmoid, under the one genus Navicula, as has been the custom previous to the publication of Cleve's monograph, would result in associating species differing in so many respects in relation to structure of the valve and cell contents that it seems advisable to retain the new genera, especially as the original genus is likely to be still further reduced when more is known of the structure and life history of the group.
KEY TO THE GENERA
| Valves dissimilar. Achnantheĉ | |
| symmetrical | Cocconeis |
| asymmetrical | |
| to the longitudinal axis | Anorthoneis |
| to the transverse axis | Rhoicosphenia |
| in zone view | Achnanthes |
| Valves similar and asymmetrical | |
| asymmetrical to the longitudinal axis | |
| valves parallel | Cymbella |
| valves not parallel | Amphora |
| valves keeled, twisted (sometimes symmetrical) | Amphiprora |
| valves keeled | Tropidoneis |
| valves reniform and keeled | Auricula |
| median line sigmoid at the ends | Scoliotropis |
| asymmetrical to the transverse axis | |
| striĉ punctate and costate | Gomphoneis |
| striĉ punctate | Gomphonema |
| Valves similar, symmetrical and sigmoid | |
| striĉ oblique | Pleurosigma |
| striĉ at right angles | Gyrosigma |
| Valves similar, symmetrical, not sigmoid | |
| striĉ punctate, nodules elongated | Frustulia |
| striĉ subtly punctate, central nodule forked | Amphipleura |
| striĉ punctate and reticulate, in two strata | Dictyoneis |
| striĉ punctate and alveolate, in three strata | Trachyneis |
| striĉ punctate, in two strata | Brèbissonia |
| striĉ interrupted by blank lines | Anomœoneis |
| {56}
striĉ crossed by longitudinal lines |
Caloneis |
| striĉ oblique, median fissures in opposite directions | Neidium |
| striĉ punctate and costate, median line with horns | Diploneis |
| striĉ punctate; valves separated by septate plates | Mastogloia |
| striĉ punctate, central area dilated into a stauros | Stauroneis |
| striĉ punctate, area without stauros or horns | Navicula |
| striĉ costate, not punctate | Pinnularia |
Frustules stipitate, free or parasitic. Valves cuneate, elliptical or suborbicular, dissimilar, bent along the transverse or the longitudinal axes, the lower valve with a true raphe and central and terminal nodules, the upper valve with a pseudoraphe or median line.
Rhoicosphenia.—Stipitate; valves with transverse puncta, bent along the transverse axis, cuneate, with diaphragms at the ends.
Anorthoneis.—Free; puncta radiate; valves bent slightly along the transverse axis, suborbicular.
Cocconeis.—Parasitic; valves elliptical, usually bent along the longitudinal axis; striĉ punctate, transverse and longitudinal.
Achnanthes.—Stipitate; valves lanceolate or elliptical, bent along the transverse axis; striĉ transverse, punctate; costĉ sometimes present.
(rhoicos, curved, and sphen, a wedge)
Frustule in zone view curved; valves cuneate, dissimilar, the upper with a pseudoraphe, the lower with a raphe.
Chromatophore a single plate along both valves, and one of the inner walls of the zone. Conjugation as in Gomphonema, with which it is generally associated in classification.
RHOICOSPHENIA CURVATA (KUETZ.) GRUN.
Valve clavate, with rounded apex and base; lower valve with raphe, a narrow axial area and slightly radiate, punctate striĉ; the upper valve with a narrow pseudoraphe and parallel striĉ; a short diaphragm at the ends of each valve. Length usually from 15 to 25 µ, but frequently of twice the size.
Common in Crum Creek.
Pl. 19, Figs. 25, 26, 27.
(anorthos, not straight)
Valves dissimilar, the upper valve with an excentric axial area, the lower with an excentric raphe.
ANORTHONEIS EXCENTRICA (DONK.) GRUN.
Valves orbicular, with radiating, punctate striĉ, closer at the circumference, producing the appearance of a border. Axial area not reaching the ends. Frustules occur free on the sands of the sea-shore. L. 25 to 50 µ.
Belmar, N. J.
Pl. 16, Figs. 30 and 31.
(coccos, a berry)
Valves elliptical, dissimilar, the upper valve with a pseudoraphe and the lower with a genuine raphe and nodules, usually with a rim or annulus. Frustules epiphytic.
Cocconeis is generally considered as a degenerated form of Mastogloia, as indicated by the "obsoletely loculiferous rim." The frustules are usually bent along the longitudinal axis, probably because of the attachment to the curved stems of water-plants.
The cell contents of only a few species are known. In C. pediculus, a single chromatophore occurs on the inside of the upper valve. In conjugation, two cells open and secrete a gelatinous mass from which an auxospore is formed.
Cleve separates the forms having a loculiferous rim (Cocconeis) from those without a rim (Eucocconeis). As the rim is easily detachable, the distinction is often made with difficulty.
COCCONEIS SCUTELLUM EHR.
Valves elliptical, the upper with a linear or lanceolate pseudoraphe and coarse puncta in transverse and radiating lines; the lower valve with much finer puncta in radiating lines, a lanceolate axial area and, sometimes, a loculiferous rim.
Along the coast. Common, but extremely variable.
Pl. 16, Fig. 21 (upper valve). Fig. 18, var. ?
COCCONEIS SCUTELLUM VAR. ORNATA GRUN.
Upper valve with linear axial area, and transverse and radiating punctate lines which end at the border in a double row of finer puncta; lower valve with much finer puncta, a lanceolate axial area and a loculiferous rim.
Atlantic City. Common.
Pl. 16, Figs. 27 and 28.
The forms along the coast vary infinitely both in size and appearance. The var. ornata is very abundant along the entire coast. In any gathering, valves are found with or without the rim which is frequently seen detached. The upper valve is sometimes without the double row of puncta. Fig. 21 represents an upper valve more coarsely punctate than usually occurs. Very many intermediate forms might be noticed.
COCCONEIS PEDICULUS EHR.
Valves rhombic-elliptical, very convex, somewhat asymmetrical; the upper valve with a linear pseudoraphe, sometimes widened near the ends, and slightly radiating, finely punctate striĉ; lower valve with narrow, axial area and finely punctate, radiating striĉ.
Not uncommon in fresh water. Abundant in a ditch at Paoli, Pa.
Pl. 16, Figs. 23 and 24.
COCCONEIS PLACENTULA EHR.
Valve elliptical; upper valve with a linear or lanceolate axial area, and punctate striĉ in transverse and radiating rows, the puncta at equal distances; the lower valve with a lanceolate axial area, radiating rows of puncta, and a wide border of finely punctate, radiating striĉ, separated from the central part of the valve by a narrow hyaline zone.
Common in salt, brackish and fresh water.
Pl. 16, Figs. 19 and 20.
COCCONEIS PLACENTULA VAR. LINEATA (EHR.) V. H.
As in the type, except that the upper valve has the puncta arranged in zig-zag, giving the appearance of sinuous, longitudinal lines.
Common along the coast.
Pl. 16, Fig. 29.
C. pediculus and C. placentula are the only species I have found in fresh water. Cleve states that the former occurs also in brackish water.
The following are among the species placed by Cleve in a new genus, Eucocconeis, distinguished by the absence of a loculiferous rim.
COCCONEIS DIRUPTA GREG.
Valves elliptical, the lower with fine puncta in slightly radiating lines, a narrow axial area and a central area dilated into a lanceolate, stauriform space; the terminal fissures turned in opposite directions; the upper valve similar to the lower valve except in the absence of raphe and nodules.
Along the coast. New Rochelle.
Pl. 16, Fig. 22 (lower valve).
COCCONEIS PELLUCIDA GRUN.
Valves elliptical, the upper with broad axial area on each side of which are fine, longitudinal rows of short striĉ; the lower valve with more numerous longitudinal rows, a marginal line and indistinct raphe; the terminal fissures small and turned in opposite directions.
New Rochelle.
Pl. 16, Figs. 25 and 26.
In the var. minor Grun. the median line of the lower valve is sometimes slightly sigmoid.
(achne, froth or down, and anthos, a flower)
Frustules stipitate, solitary or in short fasciĉ, flexed. Valves elliptical or lanceolate, naviculoid, dissimilar, the lower with a raphe and median and terminal nodules, and the upper with a pseudoraphe or median space.
The genus has no apparent affinity with any other.
ACHNANTHES LONGIPES AG.
Valves linear-elliptical, obtuse at the apex, sometimes slightly constricted in the middle. Connective zone with transverse, subtly punctate striĉ, interrupted by longitudinal lines. Central nodule of lower valve dilated into a stauros reaching the margin. Valves costate, the costĉ alternating with double rows of fine puncta.
Along the coast, in estuaries.
Pl. 16, Figs. 1 and 2.
A. longipes is the only species in our locality considered by Cleve as belonging to the genus; the other forms, distinguished by the absence of costĉ, are included in the genus Achnanthidium of Kuetzing.
In A. longipes, the chromatophores consist of scattered, rounded granules, while in Achnanthidium the chromatophore is a single plate along the upper valve, or a double one {59}along the connective zone. It is necessary, therefore, to distinguish between A. longipes and the following group, but, because of the long continued union of all of the stipitate forms having the general appearance of a true Achnanthes, I shall continue to describe the local species under the generally accepted name.
ACHNANTHES BREVIPES AG.
Valves without costĉ; striĉ moniliform; upper valve with excentric pseudoraphe or median line; otherwise as in A. longipes.
Along the coast, in estuaries.
Pl. 16, Fig. 3.
ACHNANTHES SUBSESSILIS KUETZ.
Valves linear-elliptical, rounded at the ends; upper valve with excentric pseudoraphe; striĉ moniliform, puncta smaller than in A. brevipes.
Along the coast, in estuaries.
Pl. 16, Figs. 4, 5, 6.
The three species described above are named from the length of the stipe, but this varies considerably and is not of special significance.
ACHNANTHES INFLATA (KUETZ.) GRUN.
Valves more or less inflated in the middle, usually with the stauros of the lower valve asymmetrical and wider than in A. subsessilis, with which it agrees in size and markings.
Gloucester, N. J. (artesian well).
Pl. 16, Figs. 7 and 8.
ACHNANTHES COARCTATA (BRÉB.) GRUN.
Valves lanceolate, oblong, broad at the ends and constricted in the middle. Stauros wide; pseudoraphe of the upper valve excentric; striĉ slightly radiate on the lower valve; puncta small.
Blue clay.
Pl. 16, Fig. 9.
ACHNANTHES LANCEOLATA (BRÉB.) GRUN.
Valves more or less elliptical; striĉ radiating, 12 in 10 µ, punctate; on the lower valve a horse-shoe shaped hyaline space on one side of the centre; on the upper valve an irregular stauros, not reaching the margin. L. 8-20 µ.
In springs. Abundant at Newtown Square.
Pl. 16, Figs. 10, 11, 12.
ACHNANTHES EXIGUA GRUN.
Valves oblong-lanceolate, with rostrate ends, sometimes slightly constricted in the middle. Stauros rather wide; striĉ punctate, radiating, 22 in 10 µ. L. 10-12 µ.
Stauroneis exilis Kuetz. (not Achnanthes exilis Kuetz.)
Frequently found in aquaria where I have kept it growing continuously for years.
Pl. 16, Figs. 14 and 15.
ACHNANTHES LINEARIS FORMA CURTA H. L. SMITH
Frustules solitary or geminate. Valves linear-elliptical, or elliptical-lanceolate. Lower valve without distinct axial area; upper valve with axial area widened in the middle; striĉ slightly radiate (?). L. 7 µ. One of the smallest of diatoms.
This form I found in a pure gathering covering the sides of a greenhouse tank at Elm, N. J. It was sent to Prof. H. L. Smith, who determined it as forma curta of A. linearis.
Pl. 16, Figs. 16 and 17.
ACHNANTHES DANICA (FLOEGEL) GRUN.
Valves rhombic-lanceolate, with subacute ends. Striĉ, 25 in 10 µ, radiate. Lower valve with stauros widened toward the margin, and cleft into three divisions.
Pavonia, N. J. (artesian well).
Pl. 16, Fig. 13.
I have seen the lower valve only. Cleve states that the upper valve is costate with "alternating fine lineolĉ twice as close as the costĉ."
(cymbe, a boat)
Frustules free, stipitate or enclosed in tubes. Valve boat-shaped; median line asymmetrical, straight or curved.
Chromatophore single, covering the entire interior of the frustule, except the ventral part of the zone and the median lines. Its longitudinal axis is on the dorsal part of the zone. A pyrenoid lies in a fold of the chromatophore on the dorsal part.
The genus includes the former genera of Cocconema, characterized by stipitate forms, and Encyonema in which the frustules are frequently enclosed in gelatinous tubes.
Section 1.—Cymbella Proper. Frustules free or sometimes stipitate
CYMBELLA HETEROPLEURA (EHR.) KUETZ.
Valve nearly symmetrical, lanceolate, with rostrate, produced apices; median line nearly straight; axial area linear, widened in the middle; striĉ radiate, punctate.
Blue clay.
Pl. 18, Fig. 10.
CYMBELLA CUSPIDATA KUETZ.
Valve broad, elliptical, with rostrate, somewhat acute, apices and nearly straight, ventral margin; median line straight, axial area linear, widened in the middle; striĉ radiate, punctate.
Blue clay.
Pl. 18, Fig. 17.
CYMBELLA NAVICULIFORMIS AUERSWALD
Valve linear-elliptical, with abruptly produced apices; ventral margin straight; median line almost straight; axial area narrow, central area large, rounded; striĉ distant in the middle, closer at the ends.
Fresh water.
Pl. 18, Fig. 6.
CYMBELLA EHRENBERGII KUETZ.
Valve lanceolate, with ventral margin nearly straight and apices sub-rostrate; median line straight, excentric; axial area narrow; central area widened in the middle; striĉ coarsely punctate.
Fresh water.
Pl. 18, Fig. 9.
CYMBELLA AFFINIS KUETZ.
Valve about three times as long as broad, strongly convex on the dorsal side and straight on the ventral; apices sub-rostrate; striĉ punctate; axial area narrow, not widened in the middle; median line curved; a small or indistinct punctum on the ventral side of the median line (not shown in the figure).
Common in ponds. Abundant in East Park Reservoir.
Pl. 18, Fig. 18.
CYMBELLA EXCISA (KUETZ.) DE TONI
Valve as in affinis, but with tumid and excised ventral margin; a punctum is found on the ventral side (not shown in the figure).
According to Cleve this is a variety of C. affinis.
Common in ponds.
Pl. 18, Figs. 15, 19?
CYMBELLA PARVA (WM. SM.) CL.
Valve semi-lanceolate, with produced apices; ventral margin slightly tumid; axial area narrow; striĉ coarsely but obscurely punctate.
C. affinis and C. parva are quite variable, the latter differing by its lanceolate form and the absence of a punctum, which, however, is sometimes difficult to recognize. In a gathering of C. parva, it is quite possible to find numerous abnormal forms which appear to be sporangial, so that specific distinctions are difficult if based on occasional specimens.
Common in ponds.
Pl. 38, Fig. 14.
CYMBELLA AMPHICEPHALA NĈGELI
Valve unequally elliptical, with broad, rostrate apices; axial area narrow; median line straight; central area small, rounded; striĉ, 12 in 10 µ on the dorsal, closer on the ventral, side and at the ends.
Kirkwood Pond, N. J.
Pl. 18, Fig. 16.
CYMBELLA SINUATA GREG.
Valve linear-elliptical, gibbous on the ventral side; axial area indistinct; central area widened on the ventral side nearly to the margin.
Crum Creek.
Pl. 18, Fig. 13.
Section 2.—Cocconema. Frustules stipitate
CYMBELLA ASPERA (EHR.) CL.
Valve large, cymbiform, arcuate on the dorsal, slightly gibbous on the ventral side; axial area linear, broad, slightly widened in the middle; no row of puncta on the ventral side. The puncta form curved longitudinal lines and the innermost row on the ventral side appears sometimes distant from the others, but not as in C. cistula.
Cocconema asperum Ehr.
Cymbella gastroides Kuetz.
Not Cymbella gastroides H. L. Smith, Type No. 118, which is C. mexicana A. S., having a punctum in the middle of the central nodule; in outline it is like C. gastroides var. minor Kuetz.
Blue clay.
Pl. 18, Fig. 1 (an unusual form, but it resembles Grunow's. (Diat. Franz Jos. Land, Pl. 1, Fig. 7.)
CYMBELLA CYMBIFORMIS (KUETZ.) BRÉB.
Valve cymbiform, slightly gibbous on the ventral margin; apices broad, somewhat truncate; a punctum occurs on the ventral side of the median line; striĉ, 8 in 10 µ, closely punctate.
Kirkwood Pond, N. J.
Pl. 18, Fig. 2.
CYMBELLA CISTULA (HEMPR.) KIRCHN.
Valve cymbiform, with gibbous ventral margin and truncate apices; a distinct row of several puncta occurs below the median line in typical forms.
Blue clay.
Pl. 18, Fig. 3.
CYMBELLA LANCEOLATA (EHR.) KIRCHN.
Valve cymbiform, with gibbous ventral margin; apices truncate; axial area very narrow, scarcely widened in the middle; striĉ with fine close puncta.
Kirkwood Pond, N. J.
Pl. 18, Fig. 4.
CYMBELLA MEXICANA (EHR.) A. S.
Valve broad, with gibbous ventral margin and sub-rostrate, truncate apices; median line with reflexed terminal fissures; striĉ with coarse puncta; a large punctum occurs in the centre of the central area.
Blue clay.
Pl. 18, Fig. 5.
CYMBELLA TUMIDA (BRÉB.) V. H.
Valve cymbiform, with gibbous ventral margin and abruptly rostrate ends; median line arcuate; axial area narrow; central area large, orbicular; below the central nodule is a punctum; striĉ punctate.
Crum Creek.
Pl. 18, Fig. 7.
Section 3.—Encyonema. Frustules in tubes
CYMBELLA VENTRICOSA KUETZ.
Valve lunate, with straight or slightly gibbous ventral margin; axial area indistinct; median line straight or nearly so; striĉ punctate.
Very common, but extremely variable. The ventral margin is sometimes straight and sometimes quite gibbous.
Pl. 18, Figs. 14, 22; Pl. 38, Fig. 16; Pl. 40, Fig. 8.
C. ventricosa is considered by some authors to be equivalent to C. affinis var. semicircularis Lagerst., Encyonema prostratum (Berk.) Ralfs, E. cĉspitosum Kuetz. and E. auerswaldii Rab. H. L. Smith's Type Slide of C. ventricosa Ag. is said to equal C. affinis Kuetz., but the specimens appear to me to be equivalent to C. ventricosa Kuetz. Cleve unites many forms, including E. cĉspitosum, under C. ventricosa.
CYMBELLA PROSTRATA (BERK.) CL.
Valve semi-elliptical, obtuse at the apices, which are sometimes prolonged and turned downwards; median line straight, terminal nodules distant from the ends; axial area narrow, central area rounded; striĉ in radiating, slightly curved lines, indistinctly punctate.
Common in fresh water; occasional in brackish.
Pl. 18, Fig. 21 (represents a frequent variation).
CYMBELLA PHILADELPHICA N. SP.
Valve semi-elliptical-lanceolate, with rounded apices; ventral margin strongly gibbous; terminal nodules distant from the ends; axial area broad, central area widened on the dorsal side; striĉ radiate, not curved nor of unequal length, indistinctly punctate, 10 in 10 µ on the dorsal, 8 in 10 µ on the ventral side. L. 86 µ.
This form approaches Encyonema prostratum (Berk.) Ralfs, Schmidt's Atlas, Pl. 71, Fig. 7, but differs in the striĉ and the axial and central areas.
Blue clay of Philadelphia. Rare.
Pl. 18, Fig. 8.
CYMBELLA TRIANGULUM (EHR.) CL.
Valve semi-elliptical, with acute ends; median line straight; ventral side half the width of the dorsal, with straight, slightly convex or concave margin; striĉ radiate, coarsely punctate.
Glœonema triangulum Ehr.
Baker's Run, Willistown, Pa.
Pl. 18, Fig. 24.
CYMBELLA TURGIDA (GREG.) CL.
Valve semi-elliptical, with acute ends; ventral margin gibbous; ventral side half the width of the dorsal; median line straight; terminal fissures turned downwards; axial area broad; striĉ radiate, coarsely punctate.
Baker's Run, Willistown, Pa.
Pl. 18, Fig. 23.
CYMBELLA TURGIDA (GREG.) CL. VAR. ?
Valve lunate, with gibbous ventral margin; median line straight; terminal fissures turned downwards near the ends; axial area lanceolate, striĉ radiate on the dorsal side, 8 in 10 µ, punctate, 9 on the ventral side, closer at the ends where they are convergent. L. 65 µ. Not a typical form.
Willistown, Pa.
Pl. 18, Fig. 12.
CYMBELLA RHOMBOIDEA N. SP.
Valve rhomboidal, with acute ends; dorsal part one and a half times the width of the ventral; median line nearly straight, with terminal fissures turned downwards near the ends; axial area broad, not widened in the middle, except slightly on the ventral side; striĉ {64}radiate, distant in the middle of the dorsal side where they are 7 in 10 µ, coarsely punctate, the puncta in longitudinal lines, 9 in 10 µ on the ventral side, closer at the ends. L. 69 µ.
Baker's Run, Willistown, Pa.
Pl. 18, Fig. 11.
CYMBELLA GRACILIS (RAB.) CL.
Valve semi-lanceolate, with acute ends; median line nearly straight, with terminal fissures turned downwards, distant from the ends; axial area linear; ventral margin straight or slightly gibbous in the middle.
Hammonton Pond, N. J.
Pl. 18, Fig. 20.
CYMBELLA LACUSTRIS (AG.) CL.
Valve elliptical-lanceolate, with obtuse ends, nearly symmetrical; median line straight, terminal fissures distant from the ends; striĉ radiate in the middle, convergent at the ends, coarsely lineate.
Belmar, N. J.
Pl. 18, Fig. 25.
(amphora, a jar)
Valves asymmetrical along the longitudinal axis, as in Cymbella, but with the plane passing through the dorsal and ventral sides of one valve at an angle with that of the other. As Cleve states, Cymbella and Amphora are forms of Navicula "with both valves similar and asymmetrical along the longitudinal axis," and the difference between Cymbella and Amphora is in the "degree of asymmetry." If, following H. L. Smith's diagrams (Lens, Vol. 2, 1873, p. 66), we assume that the usual form of the valve in Navicula is elliptical or lanceolate, and the zone view is rectangular, we have in Cymbella an arcuate median line and a more or less reniform valve, while the zone view remains rectangular with the valves parallel. Now, if the valves are asymmetrical along the longitudinal axis, and one side of one valve is separated from the corresponding side of the opposite valve by a wider connective zone than is the case on the other side, the transverse section of the frustule will appear cuneate, as in Amphora, and the connective zone will be wider on one side than the other. When, therefore, we examine an entire frustule as it is usually seen, we shall find the two raphes of the valves in focus at the same time on the ventral side, and, by changing the focus, the convex sides of the same valves are seen, the dorsal view with, usually, a wider connective zone. As an illustration, compare Figs. 5 and 6, on Plate 15, Fig. 6 being the ventral, and Fig. 5 the dorsal view.
As Amphorĉ are epiphytic or parasitic, they are considered, as Cleve remarks, like Achnanthes and Cocconeis, as "degenerated forms."
Chromatophores usually single, lying on the ventral connective zone. Mereschkowsky describes nine forms.
Cleve divides the genus into a number of groups as follows:
Amphora proper.—Connective zone not complex; valves with longitudinal lines on the dorsal side; coarsely punctate or costate.
Diplamphora.—Zone complex; otherwise as in Amphora.
Halamphora.—Longitudinal lines absent; frustule elongate, with protracted ends.
Oxyamphora.—Zone complex; longitudinal lines absent; frustule elliptical; valve lunate, with or without a central stauros; striĉ punctate.
Amblyamphora.—Zone complex; frustule rectangular; valve lunate; striĉ punctate; axial and central areas indistinct.
Psammamphora.—Zone not complex; frustule rectangular; central nodule frequently dilated to a stauros; no axial or central area.
Cymbamphora.—Valve semi-lanceolate; median line straight, approximate to the ventral margin.
Amphora
AMPHORA ROBUSTA GREG.
Frustule elliptical, truncate; valve lunate, with straight ventral margin; median line biarcuate; ventral side with coarse, radiate striĉ, 6 in 10 µ, on both sides of the median line.
Along the coast.
Pl. 15, Fig. 1.
AMPHORA PROTEUS GREG.
Frustule elliptical, truncate; valve lunate, with straight ventral margin; median line biarcuate; no central area. Striĉ on the dorsal side not interrupted, 9 in 10 µ. Ventral side striate toward the ends.
Differs from A. robusta chiefly in size and coarseness of puncta. Extremely variable in size.
Common along the coast.
Pl. 15, Figs. 5, 6, and 19.
AMPHORA OVALIS (BRÉB.) KUETZ.
Frustule elliptical, truncate; valve lunate; median line biarcuate; striĉ on dorsal side 10-16 in 10 µ.
Var. libyca (Ehr.) Cl.—Central area distinct on the dorsal side.
Var. pediculus (Kuetz.) Cl.—Central area and nodule quite distinct. Striĉ finer than in var. libyca.
Common in ponds. Quite variable.
Pl. 15, Fig. 7.
AMPHORA GIGANTEA VAR. FUSCA A. S.
Frustule elliptical; valve lunate, with straight ventral margin. Axial area absent on the dorsal side; dorsal striĉ, 10 in 10 µ, punctate. Ventral part hyaline except at the ends, which are obliquely striated, with short, punctate lines. L. 70-120 µ.
Absecon, N. J.
Pl. 38, Fig. 1.
Diplamphora
AMPHORA CRASSA GREG.
Valve linear-elliptical, with obtuse, incurved ends. Median line biarcuate. Axial and central areas indistinct on the dorsal side; striĉ coarsely punctate, interrupted by a longitudinal line on the dorsal side.
Along the coast.
Pl. 15, Fig. 3.
AMPHORA AREOLATA GRUN.
Valve with straight ventral margin; median line straight, approximate to the ventral margin; axial area indistinct; several longitudinal lines crossed by apparent costĉ which alternate with rows of fine puncta.
Blue clay. Rare.
Pl. 15, Fig. 11.
Halamphora
AMPHORA COFFĈIFORMIS (AG.) KUETZ.
Frustule lanceolate, truncate; zone with numerous divisions. Valve arcuate on the dorsal and nearly straight on the ventral side; ends protracted or slightly capitate.
A. aponina Kuetz.
A. salina Wm. Sm.
Along the coast.
Pl. 15, Figs. 8 and 18.
Oxyamphora
AMPHORA LINEOLATA EHR.
Frustule membranaceous, elliptical, truncate, with broad ends. Zone with numerous divisions. Dorsal part striated transversely; ventral side with longitudinal lines.
A. plicata Greg.
A. hyalina H. L. Smith, Type No. 64.
Along the coast.
Pl. 15, Figs. 9 and 10.
AMPHORA OSTREARIA BRÉB.
Frustule oblong, with rounded angles. Zone with five or more divisions transversely striated. Central area narrow, biarcuate; central nodule dilated to a stauros. Valve narrow, with arcuate dorsal and straight ventral margin, acute at the ends. Striĉ transverse, finely punctate.
A. vitrĉa Cl.; A. porcellus Kitton; A. quadrata Bréb.; A. elegans Greg. Appearance varies according to the position of the valve.
Along the coast.
Pl. 15, Figs. 12 and 21.
AMPHORA LĈVIS GREG.
Frustule oblong, hyaline and membranaceous. Valve linear or slightly arcuate, with ventral margin tumid in the middle; ends obtuse; central nodule dilated to a stauros; median line very narrow, biarcuate, coinciding with the dorsal margin at the ends; striĉ transverse, punctate.
Blue clay.
Pl. 15, Fig. 13.
AMPHORA ACUTA GREG.
Valve lunate, with acute ends; ventral margin straight; ventral side very narrow. Central nodule dilated to a stauros; striĉ transverse, punctate.
Along the coast.
Pl. 15, Fig. 20.
Amblyamphora
AMPHORA OBTUSA GREG.
Frustule rectangular. Valve linear, obliquely rounded at the ends, with arcuate dorsal, and straight ventral, margin; median line biarcuate; striĉ, 18-20 in 10 µ.
Along the coast. Common.
Pl. 15, Fig. 4.
Psammamphora
AMPHORA ARENARIA DONK.
Frustule hyaline, rectangular, slightly tumid in the middle, with rounded angles. Valve linear with broad ventral side and straight or sinuate ventral margin. Striĉ, 24-27 in 10 µ (Cleve).
Common along the coast.
Pl. 15, Fig. 17.
The distinction between A. obtusa and A. arenaria is not always evident if the valves alone are seen. The former has a complex zone, the latter a simple zone, and the valve has finer striĉ. Cleve's descriptions and references in regard to these two forms do not agree with the descriptions and figures of H. L. Smith, or with the figures of Schmidt. The valves of most Amphorĉ are capable of assuming various outlines according to their position.
AMPHORA OCELLATA VAR. CINGULATA CLEVE
Frustule rectangular. Valve linear, with dorsal margin arcuate and the ventral margin straight. Central nodule with a stauros on the dorsal side.
Squan River, N. J.
Pl. 15, Figs. 14 and 15.
Cymbamphora
AMPHORA ANGUSTA VAR. EULENSTEINII GRUN.
Valve lanceolate, acute at the ends. Median line straight, approximate to the margin. Axial area widened on the dorsal side, indistinct on the ventral; striĉ punctate.
A. eulensteinii A. S.
Common along the coast.
Pl. 15, Fig. 16.
On Pl. 40, Figs. 21, 22, and 23, I have attempted, imitating H. L. Smith's figures (Lens, l.c.), to illustrate the difference in the transverse sections of Navicula, Cymbella and Amphora.
Fig. 21 represents the transverse section of a convex Navicula, in which the valves ecg and fdh are parallel, and the median nodules c and d are central.
Fig. 22 is a transverse section of Cymbella in which the valves are nearly parallel and the median nodules are excentric. The girdles on one side, ea and af, are narrower than gb and bh on the other side.
Fig. 23 is a transverse section of an Amphora in which the valves appear in zone view with the median nodules of both valves on the same side. The girdles on the ventral side, ea and af, are narrower than gb and bh on the dorsal side. The girdles on the dorsal side are seldom as broad as gb and bh, the valve extending over a great part of the dorsal side to g′ and h′.
(amphi, on both ends, and prora, a prow)
Frustule twisted in the longitudinal axis, constricted in the middle; zone complex, with numerous divisions crossed by fine striĉ. Valve lanceolate, acute. The raphe confined within a sigmoid keel or extension of the valve; the central and terminal nodules indistinct. Striĉ transverse, punctate, with coarser striĉ at the junction of the keel and lower part of the valve.
Chromatophores single, with indented border except in A. pulchra, in which there are two chromatophores with entire borders.
AMPHIPRORA ALATA KUETZ.
Frustule with a row of puncta at the junction line. Valve linear, acute at the ends. Median line sigmoid. Striĉ lineate on the lower part of the valve, punctate on the keel.
Along the coast. Not common.
Pl. 14, Fig. 3.
AMPHIPRORA PULCHRA BAIL.
Frustule with sigmoid connective zone. Valve very convex, with sinuate keel and junction lines evident. In zone view and in valve view, one half of the frustule, owing to the elevation of the keel, is wider than the other half. Striĉ punctate, coarser on the keel.
Not uncommon along the coast.
Pl. 14, Figs. 1 and 2.
AMPHIPRORA CONSPICUA GREV.
Valve linear or elliptical, with acute ends. Median line sigmoid, but the junction lines not evident. Striĉ lineate, with coarser lines near the middle.
Not common. Port Penn, Delaware River.
Pl. 14, Fig. 4.
AMPHIPRORA ORNATA BAIL.
Frustule membranaceous, constricted in the middle, with well-marked folds extending from the junction line in both directions. Valve lanceolate, constricted in the middle and with protracted ends. Keel undulate on the edge.
A beautiful, transparent and delicate form, the only fresh-water species in our locality.
Delaware Water Gap, Pa.
Pl. 14, Figs. 6 and 7.
AMPHIPRORA PALUDOSA WM. SM.
Frustule membranaceous, constricted, with truncate ends. Valve linear, with acute ends. Striĉ scarcely visible.
Cape May (Cleve).
Pl. 14, Fig. 5.
(tropis, a keel)
Frustule oblong, constricted in the middle; keel not sigmoid. Axial area not evident. Striĉ very fine, punctate, in longitudinal lines.
TROPIDONEIS LEPIDOPTERA (GREG.) CLEVE
Valve with straight, median excentric line. Keel unilateral, projecting above the median line in zone view; central area small. Transverse striĉ finely punctate. As usually seen, the valve is inclined. According to Karsten there are two chromatophores on the connective zone, each divided into four parts, each of which contains a large oval pyrenoid.
Amphiprora lepidoptera Greg.
Along the coast.
Pl. 14, Figs. 8 and 9.
(auricula, the ear, the shape of the valve)
Frustule globose. Valve reniform or cymbiform, elevated into a keel which is not sigmoid. Median line biarcuate. Differs from Amphiprora in not having a sigmoid keel.
AURICULA MUCRONATA (H. L. SMITH) PERAGALLO
In zone view, the median line deeply bisects the longitudinal axis, ending in a mucronate central nodule. Connective zone complex. Valve very complex, with ventral margin nearly straight and raphe excentric. Central nodule near the margin, terminal nodules small. Striĉ, 35-40 in 10 µ (Cleve). Chromatophore single, on the ventral part.
Amphora mucronata H. L. Smith.
Amphora (?) insecta Grun.
Auricula insecta (Grun.) Cleve.
"A rare and very curious pelagic species" (Peragallo, Diat. Villefranche).
Prof. H. L. Smith included this form in his first century of "Species Typicĉ Diatomacearum," which was issued prior to 1876, the date of publication, in Schmidt's Atlas, of Amphora insecta Grun.
Atlantic City, N. J. Rare.
Pl. 15, Fig. 2.
(scolios, twisted, and tropis, a keel)
Frustule linear, oblong. Median line sigmoid near the ends. Valve with transverse costĉ alternating with two intermediate rows of puncta in oblique lines.
SCOLIOTROPIS LATESTRIATA VAR. AMPHORA CLEVE
Valve asymmetrical, with the median line curved. Frustule sub-acute at the ends. Median lines not on the same side of each valve of the frustule.
Abundant at Cape May, N. J. Not common elsewhere.
Pl. 14, Figs. 10 and 11.
(gomphos, a peg, and neis (naus))
Valve elongated, asymmetrical to the transverse axis; axial area narrow; central area rounded, stigmatic; striĉ radiating, costĉ alternating with double rows of fine puncta. An indistinct, longitudinal line near the border.
Chromatophores and conjugation have not been determined.
GOMPHONEIS HERCULANEUM (EHR.) CL.
Valve clavate, with rounded apex; costĉ, 13 in 10 µ, alternating with double rows of fine puncta, 22 in 10 µ, in oblique rows; axial area narrow, central area rounded, with one stigma.
Gomphonema capitatum Ehr var. herculaneum Ehr., H. L. S., Type Slide No. 177.
Common in the blue clay.
Pl. 19, Fig. 2.
Pl. 38, Fig. 15, zone view of young frustule.
GOMPHONEIS MAMILLA (EHR.) CL.
Valve lanceolate, with rounded apex and base; striĉ costate, 10 in 10 µ, alternating with double rows of fine puncta; axial area linear, sometimes oblique, central area small, with one or more stigmas.
Blue clay. Rare.
Pl. 19, Fig. 1.
In one frustule I noticed one valve with one stigma and the other with four stigmas.
The difference between G. mamilla and G. elegans is not very great. In the latter the central area is larger and the longitudinal lines not so near to the margin. The stigmas form a circlet. There appears to be a coincidence in the relation of Gomphoneis to Gomphonema, and that of the true Achnanthes to the group described by Cleve under Achnanthidium. In Gomphoneis and Achnanthes the striation is both costate and punctate while in Gomphonema and Achnanthidium the striation is punctate only.
(gomphos, a peg, and nema, a filament)
Valve elongated, asymmetrical with respect to the transverse axis; striĉ transverse, usually radiate, punctate.
Chromatophore band single, the middle lying on one zone.
In conjugation, according to Thwaites and Pfitzer, from two mother cells, which do not form a positive union, two auxospores are developed parallel to the original frustules. In Plate 19, Fig. 19, I have drawn a representation of the auxospore formation as I have frequently observed it in a gathering sent me by Mr. T. C. Palmer, containing G. angustatum, a common species in this locality. The sagittal plane of the valve of the auxospore is at right angles to the plane of the valve of the mother cell. Two valves of one of the mother cells are seen separated, one on each side of the auxospore which is nearly twice the length of the original frustules. The two valves of the other mother cell are not shown as they are not usually found closely united. In the figure one valve alone of the auxospore is seen, the opposite valve not being in focus. The valves of the auxospore are usually more or less arcuate, as in Cymbella, to which the genus is closely allied.
Grunow divides Gomphonema into two groups, Asymmetricĉ and Symmetricĉ, according to the presence or absence of stigmas. Cleve suggests Stigmaticĉ and Astigmaticĉ as more suitable in order to agree with the Cymbellĉ. The Stigmaticĉ are found chiefly in fresh water, sometimes in brackish. All of the marine forms belong to the Astigmaticĉ, which, however, include some common fresh-water forms. Many species of Gomphonema are stipitate, some occur in gelatinous masses, and others are free.
GOMPHONEMA MONTANUM SCHUM.
Valve slightly biconstricted, with obtuse apex and basis, somewhat cuneate; axial area linear, widened in the middle unilaterally; stigma, one; striĉ about 11 in 10 µ, more distant in the middle, punctate.
Gomphonema subclavatum var. montana (Schum.) Cl.
Pavonia, N. J., artesian well. Rare.
Pl. 19, Fig. 3.
GOMPHONEMA GEMINATUM LYNG.
Valve biconstricted, with large, rounded, sub-truncate apex and broad, sub-truncate basis; striĉ, 9 in 10 µ, radiate in the middle, alternately longer and shorter, transverse at the basis and near the apex where they again radiate, coarsely punctate, puncta, 12 in 10 µ. Axial area linear; central area rounded, with several large stigmas in a longitudinal row; terminal fissures hook-shaped.
Blue clay.
Pl. 19, Fig. 4.
GOMPHONEMA LANCEOLATUM VAR. INSIGNIS (GREG.) CL.
Valve lanceolate; axial area narrow; central area unilateral with one stigma; striĉ with coarse and distant puncta.
Common and variable.
Gomphonema insigne Greg.
Pl. 19, Figs. 6 and 12.
Fig. 12 shows a unilateral central area. Fig. 6 is more clavate in outline with small central area. In both forms the coarse puncta are in distinct longitudinal lines in the middle.
GOMPHONEMA ACUMINATUM VAR. TURRIS (EHR.) CL.?
Valve clavate, with cuneate, acute apex; axial area distinct; central area unilateral with one stigma.
Blue clay.
Pl. 19, Fig. 11.
GOMPHONEMA ACUMINATUM VAR. TURRIS (EHR.) CL.
Valve clavate, with cuneate apiculate apex and narrow basis; axial area narrow, with a unilateral central space; stigma opposite the short striĉ; striĉ more radiate in the upper part, distant in the middle.
Smith's Island, Delaware River.
Pl. 19, Fig. 5.
GOMPHONEMA ACUMINATUM VAR. CORONATA (EHR.) CL.
Valve twice constricted, with broad, cuneate apex; striĉ radiate in the middle, convergent near the apex and radiate at the apex. Variable in size and outline.
Blue clay. Fresh water. Common.
Pl. 19, Fig. 7.
GOMPHONEMA ACUMINATUM VAR. TRIGONOCEPHALA (EHR.) CL.
Valve broad, with cuneate apex; axial area narrow; central area unilateral with one stigma.
Pavonia, N. J., artesian well.
Pl. 19, Fig. 20.
GOMPHONEMA CONSTRICTUM EHR.
Valve clavate, constricted beneath the abruptly rounded apex, gibbous in the middle, striĉ alternately longer and shorter; axial area narrow, central area unilateral, with one stigma.
Common in fresh water.
Pl. 19, Fig. 8.
GOMPHONEMA SPHĈROPHORUM EHR.
Valve clavate, with capitate or rostrate-capitate apex and narrow basis; axial area very narrow; central area small, unilateral, with one stigma.
Common in fresh water.
Pl. 19, Figs. 9 and 10. Fig. 10 appears to be a transitional form having a more distinct axial area and rostrate apex.
GOMPHONEMA AUGUR EHR.
Valve broadly clavate, truncate and apiculate at the apex; basis sub-acute; axial area distinct; central area small, unilateral with one stigma; striĉ with distant puncta.
Blue clay. Willistown, Pa.
Pl. 19, Fig. 21.
GOMPHONEMA INTRICATUM KUETZ.
Valve narrow, lanceolate, slightly gibbous in the middle; axial area distinct; central area transverse with one stigma; striĉ parallel. Quite variable.
Common in fresh water.
Pl. 19, Fig. 14.
GOMPHONEMA ANGUSTATUM KUETZ.
Valve lanceolate, with sub-rostrate apex and basis; axial area indistinct; central area unilateral, with one small stigma; striĉ slightly radiate, indistinctly punctate.
Very common in fresh water.
Pl. 19, Figs. 18 and 19.
Fig. 19, as stated above, represents the formation of an auxospore.
GOMPHONEMA ĈQUALE GREG.
Valve linear-lanceolate, nearly symmetrical, with capitate apex and basis; axial area narrow; central area unilateral, with one stigma; striĉ radiate in the middle, slightly convergent at the ends.
Gomphonema intricatum var. ĉquale (Greg.) Cl.
Blue clay. Not common.
Pl. 19, Fig. 15.
GOMPHONEMA SARCOPHAGUS GREG.
Valve linear, irregular in outline, with rounded apex and basis; axial area distinct; central area small, unilateral, with one stigma; striĉ irregular with coarse, distinct puncta.
Occasional in fresh water.
Pl. 19, Fig. 16.
GOMPHONEMA CAPITATUM EHR.
Valve clavate, broad at the sub-truncate apex and slightly constricted, or with parallel margins; axial area linear, central area stellate, with one stigma; striĉ in the middle alternately longer and shorter.
Blue clay.
Pl. 19, Fig. 22.
GOMPHONEMA PARVULUM VAR. MICROPUS (KUETZ.) CL.
Valve clavate, with rounded apex and basis; axial area indistinct; central area unilateral, with a small stigma; striĉ distant in the middle.
Common.
Pl. 19, Fig. 17.
GOMPHONEMA VENTRICOSUM GREG.
Valve clavate, with broad apex and produced, rounded basis; axial area narrow, widened in the middle; stigma one; striĉ distant in the middle, finely punctate.
Blue clay.
Pl. 19, Fig. 13.
GOMPHONEMA OLIVACEUM LYNG.
Valve clavate, with broad apex and narrow basis; axial area very narrow; central area irregular, without stigma; striĉ radiate, finely punctate.
Very common.
Pl. 19, Fig. 23.
GOMPHONEMA BRASILIENSE VAR. DEMERARĈ GRUN.?
Valve lanceolate, with sub-cuneate apex and narrowed basis; axial area lanceolate, broad; no stigma; median fissures remote; striĉ parallel, 12 in 10 µ, punctate, the puncta obsolescent, small or interrupted.
Willistown, Pa. Rare.
Pl. 19, Fig. 24.
(pleura, a side, and sigma, the letter s)
Valve lanceolate, sigmoid; axial area very narrow, central area small; striĉ punctate, in transverse and oblique lines.
Cleve divides the forms usually known as Pleurosigma into two genera, Pleurosigma and Gyrosigma. Pleurosigma includes all forms having oblique rows of puncta, while Gyrosigma includes all having longitudinal rows. Both have transverse striĉ. The former consists entirely of marine species, while in the latter the species are found in fresh, brackish and salt water.
The endochrome in Pleurosigma, according to Mueller, consists of two bands which differ in the median part of each valve. Mereschkowsky says that the endochrome is so divided as to form four bands, two on each valve, that their position is different in different species, and that they are not the same on valves of the same frustule.
Cleve prefers to classify the species of Pleurosigma and Gyrosigma in accordance with the outline of the valve and the flexure of the median line. I shall, however, retain the method used by Peragallo and Grunow and arrange the forms according to the striation.
(1) Oblique Striĉ about 90 Degrees, More Distinct Than the Transverse
PLEUROSIGMA FORMOSUM WM. SM.
Valve elongated, slender, gently sigmoid, acute at the ends; oblique striĉ crossing each other at about 90 degrees; 10-16 in 10 µ; transverse striĉ, 14-20 in 10 µ (Cleve).
Along the coast.
Pl. 22, Fig. 5.
PLEUROSIGMA OBSCURUM WM. SM.
Valve linear, not sigmoid, or scarcely so; ends obtuse, subconical; raphe sigmoid, near the margin at the extremities; transverse and oblique striĉ equidistant, 28 in 10 µ (Wm. Sm.).
Abundant at Greenwich Point, Philadelphia.
Pl. 22, Fig. 4.
(2) Oblique Striĉ Closer At The Ends
PLEUROSIGMA NAVICULACEUM BRÉB.
Valve lanceolate, slightly sigmoid at the extremities; raphe strongly sigmoid near the margin at the ends; central nodule large, rounded; oblique striĉ, 13-14 in the middle, closer at the ends; transverse striĉ, 18-20 in 10 µ (Peragallo).
Long Island Sound.
Pl. 22, Fig. 6.
PLEUROSIGMA VIRGINIACUM H. L. SMITH
Valve slightly sigmoid, with acute ends; raphe more sigmoid than the valve, excentric near the ends; oblique striĉ in different directions at the centre, 13 in 10 µ, closer and less distinct at the ends; central nodule small but prominent because of its thickness, producing by diffraction an apparently wide area (somewhat exaggerated in the figure). L. 95 µ, usually larger.
P. affine var. fossilis Grun. (Peragallo).
P. normanii var. fossilis Grun. (Cleve).
Common in the blue clay.
Pl. 22, Fig. 8.
(3) Oblique Striĉ 60 Degrees
PLEUROSIGMA ANGULATUM (QUEKETT) CL.
Valve rhomboidal, with sub-rostrate or produced ends; central nodule rhomboidal; raphe central; transverse and oblique striĉ at an angle of 60 degrees, equidistant, 18-22 in 10 µ.
Navicula angulata Quekett.
Along the coast.
Pl. 22, Fig. 3.
PLEUROSIGMA STRIGOSUM WM. SM.
Valve lanceolate, with sub-acute, somewhat revolute, apices; oblique striĉ at an angle of about 60 degrees, otherwise as in angulatum.
Along the coast. Not common.
Pl. 22, Fig. 1.
PLEUROSIGMA ĈSTUARII BRÉB.
Valve lanceolate, with produced apices; raphe less sigmoid than the valve and excentric; oblique striĉ, 19-21 in 10 µ, at an angle of about 60 degrees.
Along the coast. Common.
Pl. 22, Fig. 7.
(4) Oblique Striĉ 60 Degrees, The Transverse More Distant
PLEUROSIGMA RIGIDUM WM. SM.
Valve nearly straight or slightly sigmoid, with obtuse ends; raphe central, excentric near the ends; oblique striĉ, 17-21, transverse, 16-19 in 10 µ. (Peragallo).
New Rochelle, N. Y.
Pl. 22, Fig. 2 (very near the var. gigantea Grun.)
(gyros, curved, and sigma)
Valve lanceolate, sigmoid; axial area very narrow, central area small; striĉ punctate, in transverse and longitudinal rows.
Chromatophores two, in long and narrow bands, perforated, differing from those of Pleurosigma. The elĉoplasts are also arranged differently in the two genera. (Mereschkowsky, Études sur l'Endochrome des Diatomées, Imperial Academy of Petrograd, 1901, Vol. 11, No. 6, p. 18 et seq.)
The arrangement is according to Peragallo.
(1) Longitudinal Striĉ More Distant Than The Transverse
GYROSIGMA HIPPOCAMPUS (EHR.)
Valve lanceolate, sigmoid, with obtuse ends; raphe nearly central; transverse striĉ 15-17, longitudinal, 10-12 in 10 µ.
Navicula hippocampus Ehr.
Pleurosigma hippocampus (Ehr.) Wm. Sm.
Gyrosigma attenuatum (Kuetz.) Cl.
Long Island Sound.
Pl. 23, Fig. 3.
(2) Longitudinal and Transverse Striĉ Nearly Equal
GYROSIGMA BALTICUM (EHR.) CL.
Valve with margins parallel nearly to the extremities, which are suddenly unilaterally sub-conical and obtuse; raphe sigmoid; transverse and longitudinal striĉ nearly equally distant, 15 in 10 µ (Per.). L. 200-360 µ.
Navicula baltica Ehr.
Pleurosigma balticum (Ehr.) Wm. Sm.
Common along the coast.
Pl. 23, Fig. 2.
GYROSIGMA PARKERI VAR. STAURONEIOIDES GRUN.
Valve lanceolate, slightly sigmoid, ends produced into beaks with sub-acute apices; raphe straight in the middle part; central nodule elliptical; transverse striĉ, 21, and longitudinal, 24 in 10 µ (Per.).
An apparent stauros, variable in width, extends to the margin and, in consequence, the median transverse striĉ are more evident. L. 75 µ.
Schuylkill River. Rather rare.
Pl. 23, Fig. 7.
GYROSIGMA SIMILE (GRUN.)
Valve slightly sigmoid, broad, with obtuse ends; raphe sigmoid, nearly central; transverse striĉ, 15, longitudinal, 16-17 in 10 µ (Per.).
Pleurosigma simile Grun.
Gyrosigma balticum var. similis (Grun.) Cl.
Shark River, N. J.
Pl. 23, Fig. 4.
(3) Transverse Striĉ More Distant
GYROSIGMA ACUMINATUM (KUETZ.) CL.
Valve sigmoid, tapering to the sub-acute ends; raphe central; transverse and longitudinal striĉ nearly equally distant, 17 or 18 in 10 µ (Per.).
Frustulia acuminata Kuetz.
Port Penn, Delaware River.
Pl. 23, Fig. 5.
GYROSIGMA STRIGILIS (WM. SM.) CL.
Valve sigmoid, with obtuse ends; raphe doubly sigmoid; axial area rather wide; transverse striĉ, 13, and longitudinal, about 16 in 10 µ.
Long Island Sound. Not common.
Pl. 23, Fig. 1.
GYROSIGMA KUETZINGII (GRUN.) CL.
Valve sigmoid, lanceolate, with sub-acute ends; raphe central, the central nodule elliptical; transverse striĉ, 21-23, and longitudinal, 25-26 in 10 µ.
Pleurosigma spencerii var. acutiuscula Grun.
Pleurosigma spencerii var. kuetzingii Grun.
Common in fresh water.
Pl. 38, Fig. 12.
GYROSIGMA SCALPROIDES (RAB.) CL.
Valve slightly sigmoid, with obtuse ends; raphe nearly straight; central nodule elliptical; transverse striĉ, 22, slightly radiate and more distant in the middle; longitudinal striĉ, 29 in 10 µ. L. 60 µ.
Common in streams.
Pl. 38, Fig. 9.
In Pl. 23, Fig. 6 represents a form more sigmoid.
GYROSIGMA SPENCERII VAR. NODIFERA GRUN.
Valve sigmoid, with obtuse ends; raphe central; central nodule obliquely elongated; transverse striĉ, 17-18 in 10 µ, curved in the middle of the valve, longitudinal striĉ, 22 in 10 µ. L. 150 µ.
Blue clay.
Pl. 23, Fig. 8.
GYROSIGMA PROLONGATUM (WM. SM.) CL.
Valve narrow, lanceolate, produced into beaks, curved in a contrary direction; raphe central; transverse striĉ, 20-21 in 10 µ, longitudinal closer. L. 140 µ.
Along the coast, northward.
Pl. 38, Fig. 13.
I have not seen any specimens south of New England, but they will probably occur.
(4) Striĉ Alike, Extremities Produced
GYROSIGMA FASCIOLA (EHR.) CL.
Valve lanceolate, attenuated into curved beaks turned in opposite directions; raphe central, straight, except at the beaks; transverse striĉ, 22, longitudinal, 24 in 10 µ (Per.).
New York Bay.
Pl. 23, Fig. 9.
(frustulum, a small piece)
Valves naviculoid, similar, usually free but sometimes enclosed in gelatinous tubes or embedded in mucus. Median line between two thickened ribs. Central and terminal nodules frequently elongated. Surface of valve with fine puncta in longitudinal and transverse lines appearing hyaline under medium powers.
Chromatophores, two, extending along the girdle. They differ from those of Navicula in being separated from the wall in the middle by a hemispherical mass of protoplasm. According to Pfitzer, each chromatophore is divided in the middle, allowing a connection between the hemispherical mass and the central plasma mass. Schmitz states that the chromatophore is thickened in the middle and contains a pyrenoid.
In conjugation, two frustules form two cylindrical bodies which later become conical and from which are formed the sporangial valves twice the usual size.
FRUSTULIA LEWISIANA (GREV.) DE TONI
Valve elliptical or linear, with rounded ends; terminal nodules elongated, at a distance from the ends; striĉ, 24 in 10 µ.
Port Penn, Delaware River. Along the coast.
Pl. 17, Fig. 1.
FRUSTULIA RHOMBOIDES (EHR.) DE TONI
Valve lanceolate or rhombic-lanceolate, rounded at the ends; central and terminal nodules short; striĉ, 20 in 10 µ, sometimes coarser.
Common in fresh water.
Pl. 17, Fig. 2.
FRUSTULIA RHOMBOIDES VAR. AMPHIPLEUROIDES GRUN.
Valve rhombic-lanceolate; central and terminal nodules elongated; median line somewhat excentric.
Blue clay.
Pl. 17, Fig. 3.
FRUSTULIA RHOMBOIDES VAR. SAXONICA (RAB.) DE TONI
Valve smaller than in rhomboides, with somewhat produced ends, closer median ribs and rounded central nodule.
Fresh water.
Pl. 17, Fig. 6.
FRUSTULIA VULGARIS (THWAITES) DE TONI
Valve elliptical-lanceolate, with rounded or sometimes sub-rostrate ends; central and terminal nodules slightly elongated; striĉ delicate, closer at the ends. Frustules at first in gelatinous tubes.
Colletonema vulgaris Thwaites.
Fresh water.
Pl. 17, Fig. 4.
FRUSTULIA INTERPOSITA (LEWIS) DE TONI
Valve linear-elliptical, rounded at the ends; terminal nodules short.
Navicula interposita Lewis.
Along the coast. Port Penn, Delaware River.
Pl. 17, Fig. 5.
(amphi, on both sides, pleura, a side)
Frustules free, in gelatinous masses or in tubes. Valve linear-lanceolate; central nodule narrow, extending half the length of the valve or more, then forking toward the ends. Terminal nodules prolonged, as in Frustulia, into a "porte-crayon-shaped" figure.
Chromatophores two, very short.
AMPHIPLEURA PELLUCIDA KUETZ.
Frustules free or in mucous masses. Valve fusiform; forks about one-fourth the length of the valve; striĉ transverse, punctate, 36-40 in 10 µ (J. J. Woodward).
Occasional in the Delaware River.
Pl. 17, Fig. 9.
AMPHIPLEURA RUTILANS (TRENTEPOHL) CL.
Frustules enclosed in gelatinous tubes. Valve linear-lanceolate, obtuse at the ends; forks about one-third the length of the valve; striĉ, 28 in 10 µ.
Conferva rutilans Trentepohl.
Schizonema dillwynii Wm. Sm.
Abundant at Belmar, N. J.
Pl. 17, Fig. 10.
Fig. 11 represents a portion of the gelatinous tube containing frustules.
(dictyon, a net)
Frustules oblong. Valve lanceolate, constricted in the middle (in our species); an outer layer finely punctate and an inner layer of reticulations; the margin of the valve divided into large, quadrate cells.
The genus Dictyoneis includes species at one time ascribed to Mastogloia and Navicula. The structure, however, is not like that of either, as the loculi are attached to the valve and are not separable as in Mastogloia, and the cell-wall is not like that of any Navicula.
Cleve remarks that Dictyoneis is found in warm waters. Lewis found one specimen at Black Rock Harbor, L. I., and one in the Delaware River blue clay. The specimens here described I found living on the New Jersey coast.
DICTYONEIS MARGINATA VAR. TYPICA CLEVE
Valve panduriform, with cuneate lobes; axial area narrow, linear, scarcely, or not at all, widened in the middle; terminal fissures in contrary directions; outer stratum finely punctate, about 25 in 10 µ, in parallel striĉ; inner stratum coarsely reticulated. Four and one-fourth times longer than broad; marginal cells, 5 in 10 µ, smaller or obsolescent in the middle of the valve; cells of the valve in irregular transverse rows, 10-12 in 10 µ. L. 93 µ.
Navicula marginata Lewis.
Absecon, N. J.
Pl. 20, Fig. 3.
DICTYONEIS MARGINATA VAR. COMMUTATA CLEVE
Valve four and one-half times longer than broad; cells of the valve in irregular, transverse rows about 11 in 10 µ; marginal cells nearly equal, 6 in 10 µ. L. 125 µ.
Absecon, N. J.
Pl. 20, Fig. 2.
DICTYONEIS MARGINATA VAR. MAXIMA N. VAR.
Valve with cuneate segments; marginal cells, 4 in 10 µ; cells of the valve, 5 in 10 µ, obsolescent in the middle and smaller; transverse striĉ, 25 in 10 µ.
Atlantic Coast. Rare.
Pl. 20, Fig. 1 (from a specimen found at Colon).
(trachys, rough, and neis (naus), named from the chief species)
Valve more or less linear or linear-lanceolate. It appears to be composed of three strata, one an interior, coarsely dotted, an exterior of fine puncta in longitudinal striĉ, scarcely visible, and a median of transverse anastomosing costĉ forming irregular alveoli.
Chromatophores, two or four bands on the zone (Mereschkowsky).
TRACHYNEIS ASPERA VAR. INTERMEDIA GRUN.
Valve linear-elliptic; axial area a stauros widened outward and unilateral. Striĉ of the median layer of radiating rows of oblong alveoli.
Along the coast. Not common.
Pl. 17, Fig. 15.
The type form and its numerous varieties are quite ubiquitous. Very large specimens occur in the Antarctic regions, especially in material from Ross Island, S. Victoria Land (Shackleton Ant. Exp.).
(named after Alphonse de Brébisson, the distinguished French naturalist)
Frustules stipitate; valve lanceolate; striĉ transverse in the middle, radiate at the ends. Median area narrow, central nodule elongated, terminal fissures at a distance from the ends. Valve with an outer finely punctate stratum.
At one end of one valve in each frustule is found a conspicuous punctum, the plasma pore of Otto Mueller, through which the frustule is connected with the gelatinous stipe, analogous to the pore in Diatoma connecting the zig-zag frustules.
Chromatophore single, lying on one girdle and passing over to each valve.
BRÉBISSONIA BŒCKII (KUETZ.) GRUN.
Valve lanceolate, with sub-acute apices; striĉ, 3-4 in 10 µ, not reaching the median line.
Blue clay. Very rare. Common in brackish water at Chestertown, Md. (T. C. Palmer)
Pl. 17, Fig. 7.
BRÉBISSONIA PALMERII, N. SP.
Valve rhombic-lanceolate, with cuneate ends and produced apices. Central nodule more elongate and terminal fissures further from the ends than in B. bœckii.
Pavonia, N. J. (artesian well, depth of 40 ft.). Rare.
Pl. 17, Fig. 8.
I take pleasure in naming this species after Mr. T. Chalkley Palmer, of Media, Pa., the author of numerous papers on the Diatomaceĉ.
Lewis partly describes a similar form, which he does not name, as a species of Navicula found in the blue clay at Kaighn's Point, N. J. (Lewis, "New and Intermediate Forms," etc., p. 15, Pl. 1, Fig. 8.)
(anomoios, unlike, and neis (naus), a boat)
Valve lanceolate, axial area narrow, central area widened; transverse striĉ punctate, the puncta in longitudinal rows or interrupted by blank lines.
A single chromatophore lies along one of the girdle sides and extends over the valves, each of the two parts being deeply notched or slit at the ends. According to Schmitz there are two pyrenoids, but Heinzerling thinks there is but one.
Cleve considers this genus not well founded, as it is based upon the cell contents of but one species, the structure of the other species not being known. As the forms here described are easily recognized by the interrupted puncta, the genus is, at least, convenient.
ANOMŒONEIS SPHĈROPHORA (KUETZ.) CL.
Valve elliptic-lanceolate, ends rostrate-capitate. Axial area narrow, central area rounded, larger on one side of the median line than the other. Striĉ very slightly radiate, 16 in 10 µ, punctate, the puncta interrupted by longitudinal blank lines.
Pfitzer states that the central plasma mass is unequal on the two sides.
Navicula sphĉrophora Kuetz.
Fresh and brackish water. Not common.
Pl. 40, Fig. 2.
ANOMŒONEIS SERIANS (BRÉB.) CL.
Valve lanceolate, acute; axial area lanceolate; striĉ, 24 in 10 µ; puncta elongate.
Not common in this locality, but abundant northwards; fossil in the peat deposits of New England.
May's Landing, N. J.
Pl. 17, Fig. 12.
Forma minor—Valve rhombic-lanceolate, smaller than the type.
May's Landing, N. J.
Pl. 17, Fig. 13.
ANOMŒONEIS FOLLIS (EHR.) CL.
Valve rhomboid, tumid in the middle and obtuse at the produced ends. Central area lanceolate; striĉ radiate in the middle, transverse at the ends.
Navicula follis Ehr.
Navicula trochus Kuetz.
Reported by Lewis as very rare in the blue clay of the Delaware River. I have not seen it in this locality. The figure is drawn from a specimen in the W. Bridgewater, Mass., deposit.
Pl. 17, Fig. 14.
(calos, beautiful)
Valve convex, linear or lanceolate in general outline, with transverse, smooth or finely punctate striĉ crossed by one or more longitudinal lines.
Endochrome of two chromatophores lying one on each valve, entire in some species and deeply cleft in others.
CALONEIS LIBER (WM. SM.) CL.
Valve linear, with parallel margins and rounded ends; axial area narrow, central area orbicular; striĉ transverse in the middle, slightly divergent at the ends, 16 in 10 µ; terminal fissures slightly curved in the same direction; longitudinal line median. L. 82 µ.
Atlantic coast, chiefly southward.
Pl. 40, Fig. 1.
CALONEIS SILICULA (EHR.) CL.
Valve linear, gibbous in the middle, with broad sub-cuneate ends; axial area narrow, central area rounded; longitudinal line marginal; striĉ parallel or nearly so, 16 to 18 in 10 µ.
Navicula silicula Ehr.
Navicula limosa Donk.
Blue clay.
Pl. 21, Fig. 3 (var. genuina Cl.).
CALONEIS SILICULA VAR. INFLATA (GRUN.) CL.
Valve gibbous in the middle, with rounded ends; central area elliptical.
Schuylkill River.
Pl. 21, Fig. 4.
C. silicula may be recognized by its yellow color when dry. Its varieties are extremely numerous.
CALONEIS TRINODIS (LEWIS)
Valve divided into three segments of equal width; ends cuneate and usually produced; axial area elliptical with a lunate marking on each side; striĉ radiate in the middle, elsewhere parallel, about 20 in 10 µ, finely punctate; longitudinal line marginal, scarcely visible; the striĉ become fainter toward the axial area.
Occasional in streams and in the blue clay. Abundant in a water-trough at Ashbourne, Pa.
Pl. 21, Fig. 8.
I have retained Lewis' name as specific. Lewis, wrongly, I think, ascribes his species to Navicula trinodis Wm. Sm., which is not figured by Smith, but is illustrated by Van Heurck (Syn. Pl. 14, Fig. 31a), and is named by Cleve Navicula contenta var. biceps Arnott. {82}De Toni includes Lewis' name under Rhoiconeis trinodis (Wm. Sm.) Grun. Rhoiconeis is achnanthiform, with frustules arcuate, and the species is named by Cleve Achnanthes trinodis (Arnott). Caloneis schumanniana (Grun.) Cl., to which as a variety Cleve unites Lewis' form, appears to resemble it only in the lunate marks.
Fig. 9 represents a single specimen found in the Pavonia deposit and which I believe to be an abnormal form of C. trinodis, differing only in the degree of inflation and in the larger central area.
Navicula trinodis var. inflata Schultze, from Staten Island, is the same form figured by Lewis, who states that certain specimens have produced apices.
CALONEIS PERMAGNA (BAIL.) CL.
Valve lanceolate, with produced apices; median line nearly straight; axial area lanceolate, irregular or slightly unilateral, about half the width of the valve; striĉ, 9 in 10 µ, radiate and indistinctly punctate; longitudinal lines double. L. 100-200 µ.
Pinnularia permagna Bail.
Common in brackish water.
Pl. 21, Fig. 1.
CALONEIS PERMAGNA VAR. LEWISIANA N. VAR.
Valve lanceolate, with undulating sides and sub-cuneate apices; axial area less than one-third the width of the valve; striĉ radiate, 12 in 10 µ, indistinctly punctate; longitudinal lines double, closer together than in the type. L. 140 µ.
Lewis illustrates this variety in "New and Rare Species," Pl. 2, Fig. 11, and states that it is probably Navicula esox Kuetzing. This is an error, as Kuetzing's species is Pinnularia esox Ehr., a form near P. major.
Rather common in the Delaware River.
Pl. 21, Fig. 2.
CALONEIS FORMOSA (GREG.) CL.
Valve lanceolate, with sub-cuneate apices; axial area one-fourth to one-fifth the width of the valve, somewhat unilateral, dilated in the middle; striĉ, 12-14 in 10 µ radiate, punctate; longitudinal lines double, distinct. Variable in size and outline.
Abundant along the shores of the Delaware River.
Pl. 21, Fig. 18.
CALONEIS BREVIS VAR. VEXANS (GRUN.) CL.
Valve elliptical-lanceolate; apices obtuse; median fissures distant; axial area narrow; central area large, orbicular; longitudinal lines close together, median.
Shark River, N. J.
Pl. 21, Fig. 5.
CALONEIS WARDII CL.
Valve linear, ends cuneate; axial area linear; central area dilated to a stauros reaching the margin; striĉ parallel, radiate at the ends, 18 in 10 µ; longitudinal lines marginal.
Not uncommon in the Delaware River.
Pl. 21, Figs. 6 and 7.
CALONEIS POWELLII (LEWIS) CL.
Valve linear, with cuneate ends; axial area linear; central area large, quadrate, united to the wide longitudinal lines; striĉ parallel, smooth, 8 in 10 µ.
Long Island (Lewis); Smith's Island, Delaware River.
Pl. 21, Fig. 10.
(neidion, dim. of naus, a boat)
Valve linear or lanceolate; median fissures turned in opposite directions, terminal fissures appearing bifurcate (?); striĉ transverse, usually oblique, finely punctate, crossed by one or several longitudinal blank lines.
Chromatophores, two, lying on the girdle side, in cell division each forming a partially divided pair. A large pyrenoid is said to be found in the middle of each chromatophore, but Mereschkowsky states that the pyrenoids are absent, but that in N. affine four elĉoplasts are always seen in the centre of the frustule.
A genus easily recognized by the peculiar terminal and median fissures and by the yellowish or brownish color of the valves when dry, darker than in Caloneis.
NEIDIUM AFFINE (EHR.) PFITZER
Valve linear, with protracted, sub-rostrate or capitate ends.
Navicula affinis Ehr.
NEIDIUM AFFINE VAR. GENUINA FORMA MAXIMA CL.
Striĉ, 14 in 10 µ, punctate, oblique in the middle, convergent at the ends; puncta, 15 in 10 µ. L. 238 µ.
Pensauken, N. J. (artesian well).
Pl. 21, Fig. 11.
Var. genuina forma minor Cl.—L. 26 µ; striĉ, 24 in 10 µ.
Brandywine Creek.
Pl. 21, Fig. 12.
NEIDIUM AFFINE VAR. AMPHIRHYNCUS (EHR.) CL.
Valve linear, with protracted capitate ends; striĉ transverse, interrupted by several longitudinal lines.
Willistown, Pa.
Pl. 21, Fig. 13.
NEIDIUM AMPHIGOMPHUS (EHR.) PFITZER
Valve with parallel margins and cuneate ends; striĉ transverse, interrupted by several longitudinal lines; central area widened transversely.
Navicula amphigomphus Ehr.
Wissahickon.
Pl. 21, Fig. 14.
NEIDIUM PRODUCTUM (WM. SM.) CL.
Valve linear, elongate, with capitate apices; striĉ slightly oblique; longitudinal lines marginal; axial area very narrow, central area small.
Navicula producta Wm. Sm.
Newtown Square.
Pl. 21, Fig. 16.
NEIDIUM IRIDIS (EHR.) CL.
Valve linear or lanceolate-elliptical, with sub-cuneate or rounded ends; striĉ oblique, about 18 in 10 µ; central area orbicular.
Navicula iridis Ehr.
Navicula firma Kuetz.
Willistown, Pa.; Middletown, Delaware Co., Pa. (Palmer).
Pl. 21, Fig. 17.
The form here figured is probably the variety ampliata (Ehr.) Cl. with less acute apices and more elliptical outline. The species occurs in many variations, the larger being found northward, especially in the peat deposits of New England.
NEIDIUM HITCHCOCKII (EHR.) CL.
Valve linear, with triundulate margin and cuneate ends; striĉ transverse, oblique.
Navicula hitchcockii Ehr.
Pavonia, N. J. (artesian well); Kirkwood Pond, N. J.
Pl. 21, Fig. 15.
(diplos, double)
Valve elliptical or panduriform; median line enclosed in strongly siliceous horns corresponding to the lyre-shaped areas of Navicula lyra but never punctate; central nodule, quadrate; valve costate, or striate, or both; between the horns and the outer part are thinner spaces or sulci, and, in some species, outside of the sulci are narrow spaces known as lunulĉ.
Chromatophores, two, upon the girdle or the valves. Pyrenoids have been found in one species only, D. interrupta.
DIPLONEIS ELLIPTICA (KUETZ.) CL.
Valve elliptical; central nodule large; sulci narrow, curved, close to the horns; striĉ punctate, in rows radiating more and more toward the ends. Variable in size and in the coarseness of puncta which are from 10 to 13 in 10 µ (Cleve).
Cleve describes D. ovalis Hilse as having the central nodule rounded, but otherwise about the same as D. elliptica, and as equivalent to Navicula ovalis A. Schmidt (Atlas, Pl. 7, Figs. 33 to 36).
Very common in fresh water and occasional in brackish.
Pl. 20, Fig. 14.
DIPLONEIS SMITHII (BRÉB.) CL.
Valve elliptical; central nodule not broad; furrows evenly curved on the outer edge, crossed by costĉ and double oblique rows of alveoli. Variable in size and in the curvature of the furrows.
Cleve forms a new species, D. major, of the large form figured by Schmidt (Atlas, Pl. 7, Figs. 18, 19, 21 and 22), stating that the structure is much coarser and the form is larger with broad furrows. In the specimen here figured the size is median and the furrows are as in D. major.
Marine and brackish. Common.
Pl. 20, Fig. 17.
DIPLONEIS CRABRO VAR. PANDURA (BRÉB.) CL.
Valve constricted, segments tongue-shaped; central nodule small; horns narrow, nearly parallel, with a row of large puncta; costĉ, 4 in 10 µ, convergent in the middle, radiating at the ends, alternating with a double row of puncta, 11 in 10 µ.
Pavonia, N. J. (artesian well).
Pl. 20, Fig. 4.
DIPLONEIS CRABRO VAR. EXPLETA (A. S.) CL.
Valve slightly constricted, segments tongue-shaped; costĉ robust, 5 or 6 in 10 µ, alternating with double rows of rather coarse puncta. L. 56 µ.
Port Penn, Delaware River.
Pl. 20, Fig. 15.
DIPLONEIS CRABRO VAR. PANDURELLA CL.?
Valve constricted, the lobes elliptical; central nodule large, with horns parallel in the middle, convergent at the ends; furrows wide, with faint costĉ; no lunula; costĉ parallel in the middle, radiate at the ends, 9 in 10 µ, alternating with very fine double rows of puncta (not shown in the figure). L. 65 µ.
Blue clay.
Pl. 20, Fig. 13.
DIPLONEIS CRABRO VAR.?
Valve constricted, segments elliptical; costĉ, 8 in 10 µ, converging in the middle, radiating at the ends; horns narrow; furrows wide, costate; lunulĉ indistinct. L. 75 µ.
Resembles var. pandurella except in the convergence of the costĉ and in the lunula.
Squan River. Marine.
Pl. 20, Fig. 9.
DIPLONEIS FUSCA VAR. DELICATA (A. S.) CL.
Valve elliptical; furrows broad, crossed with rows of faint costĉ and alveoli; costĉ, 6 or 7 in 10 µ; alveoli, 10 in 10 µ, in short, irregular, longitudinal rows. L. 84 µ.
Port Penn, Delaware River.
Pl. 20, Fig. 11.
DIPLONEIS GRUENDLERI (A. S.) CL.
Valve constricted, segments tongue-shaped, often unequal; horns broad, divergent in the middle; furrows narrow; costĉ transverse, crossed by from 3 to 7 longitudinal costĉ, interrupted in the middle at the border.
Blue clay.
Pl. 20, Figs. 7 and 8.
DIPLONEIS PUELLA (SCHUM.) CL.
Valve elliptical, sometimes orbicular; furrows very narrow; striĉ, 20 in 10 µ, indistinct. L. 15 µ.
Diploneis elliptica var. minutissima Grun.
Shark River, N. J. Brackish.
Pl. 20, Fig. 12.
DIPLONEIS EXCENTRICA, N. SP.
Valve elliptical; central nodule quadrate; furrows of the same width throughout, nearly parallel; costĉ radiating toward the ends, 10 in 10 µ, indistinct on the furrows, alternating with alveoli, 7 in 10 µ, in irregular, longitudinal lines. One side of the valve is one and a half times the width of the other. L. 49 µ.
I can find neither description nor figure of any species to which I can ascribe this form. It approaches D. elliptica. The alveoli are quite distinct and distant from each other.
Brackish water. Very abundant in a gathering from Squan River, N. J.
Pl. 20, Fig. 10.
DIPLONEIS OCULATA (BRÉB.) CL.
Valve elliptical; striĉ radiate at the ends, about 20 in 10 µ, coarsely punctate. L. 23 µ.
Fresh water.
Pl. 26, Fig. 7.
The figure is drawn from Brébisson's original material in H. L. Smith's Type Slide No. 299.
Navicula oculata Bréb.
Reported from New Jersey. I have not seen this species in this locality. Navicula oculata, referred to by Kain as occurring in Shark River, is not this form.
DIPLONEIS GEMMATA (GREV.) CL.
Valve oblong-linear, with cuneate ends and parallel or slightly concave sides; central nodule large; horns parallel; furrows about one-third the width of the valve. Costĉ about 5 in 10 µ, alternating with double rows of fine puncta; short costĉ occur along the borders of the horns.
Port Penn, Delaware River.
Pl. 20, Fig. 16.
DIPLONEIS CAMPYLODISCUS (GRUN.) CL.
Valve suborbicular; central nodule quadrate; horns divergent; costĉ, 6 in 10 µ, alternating with double rows of alveoli; furrows broad, costate near the horns.
Differs from Cleve's description in having 6, instead of 4, costĉ in 10 µ.
Pensauken, N. J. (artesian well). Rare.
Pl. 20, Fig. 6.
(mastos, a breast, and gloios, gelatinous, referring to the "mamillate cushion" in which the frustules are often immersed)
Frustule rectangular. Valves similar, naviculoid. Central and axial areas usually narrow or indistinct; striĉ punctate, parallel in the middle. On each side, between the valve and the zone, is a septate plate.
ANALYSIS OF SPECIES
| Striĉ interrupted by a hyaline furrow on each side of the median line | kinsmanii |
| Striĉ not interrupted: | |
| Loculi, five, or less | exigua |
| Loculi, more than five, equal, ending at distance from the ends | smithii |
| Loculi, ending near the ends, distinct | lanceolata |
| Loculi, ending near the ends, indistinct | elegans |
| Loculi, very numerous | apiculata |
| Loculi, unequal | angulata |
Karsten states that there are two chromatophores, each of which extends from the middle of one valve to the end and down the middle of the other valve. Mereschkowsky says, however, that there are four plates or chromatophores, sometimes on the valve, sometimes on the zone, according to the species, and that two long pyrenoids unite the two opposite chromatophores.
MASTOGLOIA KINSMANII LEWIS
Valve lanceolate-elliptical, with sub-rostrate ends; loculi more numerous than in M. angulata but less than in M. apiculata, the middle ones larger. Median line with a sulcus on each side; central area quadrate.
Mastogloia braunii Grun. (According to Cleve).
Atlantic City.
Pl. 17, Fig. 16.
MASTOGLOIA EXIGUA LEWIS
Valve elliptical- or linear-lanceolate; loculi, 2-5, usually 3, larger in the middle and rounded; central space small; striĉ, 20-24 in 10 µ.
Along the coast.
Pl. 17, Fig. 24.
MASTOGLOIA SMITHII THWAITES
Valve lanceolate, sub-rostrate; loculi forming a wide band ending at a distance from the ends; striĉ transverse, with puncta forming longitudinal rows; central area rounded or transversely elliptical.
Along the coast.
Pl. 17, Fig. 19.
MASTOGLOIA LANCEOLATA THWAITES
Valve lanceolate, with sub-rostrate apices; loculi very numerous; median and central areas indistinct; striĉ, 19 in 10 µ, punctate, convergent at the ends.
Along the coast.
Pl. 17, Fig. 18.
MASTOGLOIA ELEGANS LEWIS
Valve lanceolate, acute; loculi indistinct or rudimentary, extending to the ends; central area apparently quadrate, sometimes indistinct; puncta distinct, 15 in 10 µ, in transverse and longitudinal rows.
Along the coast. Common.
Pl. 17, Fig. 20.
MASTOGLOIA APICULATA WM. SM.
Valve elliptical-lanceolate, sometimes with slightly produced apices; median line between two ribs; central space very small; loculi numerous; puncta in slightly radiating rows and in longitudinal lines.
Along the coast.
Pl. 17, Figs. 21, 22, 23.
MASTOGLOIA ANGULATA LEWIS
Valve elliptical, with produced apices; loculi usually less than 12, unequal, the larger in the middle; striĉ, 12 in 10 µ, puncta in decussating rows. "Differs from apiculata in its more broadly elliptical shape, the smaller number of its loculi and the angular character of its striation" (Lewis).
Considered by Cleve as synonymous with M. apiculata Grun., not Wm. Smith, and by De Toni as synonymous with M. apiculata Wm. Sm. In any case, M. angulata Lewis is not the same as M. apiculata Wm. Sm., the loculi of which are equal.
Atlantic City. H. L. Smith T. S. No. 211.
Pl. 17, Fig. 17.
(stauros, a cross, and neis (naus), a boat)
Frustules free, sometimes geminate; valve as in Navicula but with a stauros. Cell contents as in Navicula. Mereschkowsky, however, says that the chromatophores always contain more pyrenoids than are found in Navicula. Heinzerling gives the number as two to four in each chromatophore.
Cleve includes under Naviculĉ Microstigmaticĉ all species of Stauroneis, Pleurostauron, Schizostauron, certain Schizonemĉ and Naviculĉ. As a matter of convenience, and because I have already included certain Schizonemĉ and Scoliopleura under Navicula, and because of the small number of species in our locality, I have arranged them under the three divisions of Cleve as follows:
Stauroneis.—Forms having a true stauros, without diaphragms.
Pleurostauron.—Forms like Stauroneis but with diaphragms at the ends.
Schizostauron.—Forms having a bifid stauros.
STAURONEIS PHŒNICENTERON EHR.
Valve lanceolate, obtuse; striĉ radiate, 18 in 10 µ, distinctly punctate. L. usually 125 µ but sometimes 200 µ.
Common in fresh water.
Pl. 27, Fig. 1.
STAURONEIS ANCEPS EHR.
Valve lanceolate, with rostrate or capitate ends; stauros in some cases does not reach the margin. The varieties are very numerous.
Var. gracilis (Ehr.) Cl.—Valve lanceolate, striĉ very fine; margin of stauros striated. L. 100 µ. Cape May, N. J. Pl. 27, Fig. 5.
Var. amphicephala (Kuetz.) Cl.—Valve capitate at the ends; striĉ, 24 in 10 µ. L. 47 µ. Fresh water. Pl. 27, Fig. 7.
Var. ?—Valve with produced ends; striĉ, 30 or more in 10 µ. L. 104 µ. Willistown, Pa. Pl. 27, Fig. 4.
Var. ?—Valve with produced ends; striĉ, about 28 in 10 µ, punctate. L. 47 µ. Newtown Square. Pl. 27, Fig. 8.
Var. ?—Valve with produced ends; striĉ, 22 in 10 µ, showing a tendency to form longitudinal rows of puncta as in Stauroneis stodderi Greenleaf, but the rows are not so evident. L. 60 µ. Pavonia, N. J., artesian well. Pl. 27, Fig. 9.
STAURONEIS FRICKEI VAR. ANGUSTA N. VAR.
Valve lanceolate, gradually tapering to the obtuse ends; terminal fissures prominent, forking at a distance of 7 µ from the ends. Frustules frequently geminate. L. 173 µ.
Newtown Square. Rare.
Pl. 26, Fig. 18.
Near Stauroneis frickei A. S. (Atlas, Pl. 242, Fig. 16), except that the stauros is narrow at the margin.
STAURONEIS SALINA WM. SM.
Valve lanceolate, obtuse; stauros narrow, with short, scattered striĉ at the margin, 18 in 10 µ, punctate. L. 65 µ.
Along the coast. Common.
Pl. 27, Fig. 6.
STAURONEIS LEGUMEN EHR.
Valve elliptical-lanceolate, inflated in the middle, with produced sub-capitate or rostrate ends separated by diaphragms. Stauros wide, striated at the margins; axial area very narrow; striĉ radiate, about 26 (?) in 10 µ, punctate. L. 28 µ.
Pavonia, N. J., artesian well.
Pl. 39, Fig. 15.
In Cleve's description and Van Heurck's figure, the median inflation is "not larger than the others." In the present form the median inflation is wider.
STAURONEIS ACUTA WM. SM.
Valve rhombic-lanceolate, obtuse; a diaphragm at each end; stauros widened outwards; striĉ, 15 or 16 in 10 µ, punctate. L. 130 µ.
Blue clay.
Pl. 27, Fig. 2.
STAURONEIS AMERICANA A. S.
Valve elliptical-lanceolate, obtuse; striĉ, 14 in 10 µ. L. 119 µ.
Pavonia, N. J., artesian well. Rare.
The only specimen found is asymmetrical with respect to the transverse axis.
On Plate 40, Fig. 4, is illustrated an abnormal form of Stauroneis, apparently near S. acuta, having an elongated central nodule and radiating, curved and coarsely punctate striĉ. Blue clay.
STAURONEIS SMITHII GRUN.
Valve lanceolate, inflated in the middle and at the ends, which have diaphragms and are produced into rostrate apices; stauros reaching the margin; striĉ parallel, about 25 in 10 µ (28 to 30, Cleve), distinctly punctate.
Not uncommon in meadow pools near Newtown Square.
Pl. 27, Fig. 11.
STAURONEIS CRUCICULA (GRUN.) CL.
Valve lanceolate, with obtuse, produced ends; stauros bifid; striĉ, 24 in 10 µ, oblique, parallel to the branches of the stauros, closer at the ends, punctate. L. 32 µ.
Newtown Square. East Park Reservoir. Rare.
Pl. 27, Fig. 10.
(dim. of navis, a boat)
Valve linear to elliptical; ends acute, rounded, rostrate, capitate or truncate; axial area usually distinct; central area distinct, rounded or rarely extended into a transverse fascia; striĉ transverse or radiate, punctate; central area not dilated into a transverse stauros nor into horns.
The endochrome in the greater number of species consists of two chromatophores extending along the zone and sometimes partly over the valves. Sometimes, however, as in N. hennedyi, N. lyra and N. humerosa, the bands are on the valves. Certain species have four bands, others eight, and in one the endochrome is granular. (Mereschkowsky, l. c., p. 9 et seq.) Pyrenoids are usually absent. On account of the diversity of the chromatophores, Mereschkowsky considers the genus not homogeneous. The difficulty of arranging groups according to the cell contents, however, is so great that, for the present, the species must be described by the usual characteristics of the valves and divided as follows, according to Cleve, to the extent of employing the classification of all Naviculoid forms as applicable, especially to the species of Navicula. Van Heurck's analysis includes Pinnularia, Trachyneis, Diploneis, Caloneis, Neidium and Anomœoneis, which are here separated, while N. lyra and N. hennedyi are placed in different groups, although they are closely related. In other respects Cleve's divisions correspond, to some extent, to those of Van Heurck.
The genus Navicula at one time included the following: Dictyoneis, Pleurosigma, Gyrosigma, Caloneis, Neidium, Diploneis, Frustulia, Trachyneis, Anomœoneis, Pinnularia and Stauroneis, and few forms with a raphe escaped. For this reason the diagnosis of the present genus is somewhat limited. Pleurosigma and Gyrosigma differ from Navicula in their outline, Dictyoneis in the double stratification, Caloneis in the marginal lines, Neidium in the median and terminal fissures, Diploneis in the horns, Frustulia in the terminal nodules, Trachyneis in the stratification of the valve, Anomœoneis in the longitudinal arrangement of the puncta, Pinnularia in the smooth costĉ and Stauroneis in the stauros.
As the object of the present work is to aid the student of local forms in the identification of species by the briefest methods, the further discussion of the reasons for classification will be left for his gratification in referring to the authorities on the subject.
Punctatĉ Cleve
Valve elliptical to lanceolate; central nodule not stauroid or continued into lyriform spaces; striĉ distinctly or coarsely punctate, in radiate rows.
NAVICULA MACULATA (BAIL.) CL.
Valve lanceolate-elliptical, with produced or sub-rostrate ends; axial area narrow, wider near the ends and dilated to a rounded, transverse central area; striĉ radiate, 6 in 10 µ, puncta, 7 in 10 µ, in irregular, longitudinal rows. L. 90 to 120 µ (Cl.).
Stauroneis maculata Bail.
Navicula fischeri A. S.
Blue clay. Along the coast, especially southward.
Pl. 24, Fig. 1.
NAVICULA LATISSIMA GREG.
Valve oblong-elliptical or elliptical-lanceolate, with sub-cuneate ends; axial area lanceolate, widened in the middle to an orbicular space; striĉ radiate, 7 in 10 µ, puncta, 11 in 10 µ, the median striĉ alternating with short striĉ along the sides. L. 50-150 µ (Cl).
Blue clay. Pavonia, N. J., artesian well.
Pl. 24, Fig. 3.
NAVICULA LATISSIMA VAR. ELONGATA (PANT.) CL.
Valve elliptical-lanceolate, with rounded ends; striĉ and puncta closer than in the type form; axial area narrow, widened in the middle; terminal fissures hook-shaped, turned in different directions.
Navicula humerosa var. elongata Pant.
Fossil at Buckshutem, N. J.
Pl. 24, Fig. 5.
NAVICULA FUCHSII PANT.
Valve elliptical, with slightly produced apices; axial area wide, lanceolate; central area orbicular; striĉ alternately longer and shorter in the middle, 10-12 in 10 µ; puncta on the border of the axial area larger, elongated; median fissures incrassate.
Navicula humerosa var. fuchsii (Pant.) Cl.
Navicula (latissima var.?) fuchsii Pant.
Port Penn, Delaware River.
Pl. 24, Fig. 6.
NAVICULA HUMEROSA BRÉB.
Valve lanceolate-elliptical or oblong-elliptical, with sub-cuneate or sub-rostrate ends; axial area narrow, lanceolate; central area rounded, somewhat transverse; terminal fissures hook-shaped, in the same direction; central pores incrassate; striĉ, 11 in 10 µ, the middle alternately longer and shorter, closer at the ends. L. 60-86 µ. Variable in size, outline and fineness of striation.
N. monilifera Cleve (N. granulata Bréb.) differs in having coarser striĉ.
Blue clay. Along the coast.
Pl. 25, Fig. 5.
NAVICULA PUSILLA WM. SM.
Valve ovate-elliptical, with rostrate or sub-rostrate ends; axial area narrow; central area elliptical; striĉ radiate, 10-12 in 10 µ in the middle where they are longer and shorter alternately, closer at the ends; median fissures somewhat incrassate, terminal in the same direction. L. 47 µ.
Smith's Island, Delaware River.
Pl. 25, Figs. 4, 6?
Cleve gives the striĉ as 13-18 in the typical form, and 11-13 in varieties. In the form here figured the striation is as stated by De Toni, but is about 19 at the ends.
Fig. 6 appears to be a small form of N. pusilla, near lanceolata Grun., at least according to the figure in "Arctic Diatoms," but not Gregory's figure. It occurs rarely in fresh water at Newtown Square. It may be a small form of N. punctulata and, if so, is probably accidental, as the material is entirely fresh-water.
NAVICULA PUSILLA VAR. SUBCAPITATA N. VAR.
Valve elliptical with rostrate-capitate and truncate ends; striĉ about 12 in 10 µ in the middle where they are unequal; axial area narrow, slightly widened in the middle; central pores incrassate, terminal fissures in the same direction. Differs from type in outline and centre.
Pavonia, N. J., artesian well. Rare.
Pl. 25, Fig. 8.
NAVICULA DELAWARENSIS GRUN.
Valve elliptical-lanceolate, with sub-rostrate ends; axial area narrow, lanceolate, widened in the middle; striĉ about 10 in 10 µ; in the middle, much closer at the ends; puncta in the middle, 9 in 10 µ, closer and much smaller at the ends. L. 58-95 µ.
Cleve (Le Diatomiste, Vol. 2, p. 14) states that this form is very near N. pusilla but is much larger. Specimens from Smith's Island measure 58-65 µ, from Wildwood, 95 µ in length.
Pl. 25, Fig. 3.
NAVICULA PUNCTULATA WM. SM.
Valve elliptical-lanceolate, with sub-rostrate ends; axial area narrow, central area rounded; striĉ, 11 in 10 µ, closer at the ends, a few shorter in the middle; puncta, 10 in 10 µ. L. 54 µ.
Navicula marina Ralfs.
Port Penn, Delaware River (brackish water).
Pl. 25, Fig. 9.
"Although this species is described as marine in the Synopsis of Prof. Smith, I have never found it in purely marine localities" (Donkin).
NAVICULA PUNCTATA VAR. ASYMMETRICA LAGERSTEDT
Valve lanceolate, with rostrate ends; axial area narrow, central area transverse, irregular; striĉ radiate, punctate, 12 in 10 µ. L. 36 µ.
Navicula amphibola Cleve.
Blue clay.
Pl. 27, Fig. 15.
NAVICULA BRASILIENSIS VAR. BICUNEATA CL., FORMA CONSTRICTA
Valve oblong-elliptical, slightly constricted, with cuneate-rostrate ends; axial area narrow; central area dilated transversely and unilaterally; striĉ, 9 in 10 µ; puncta closer at the border and in irregular longitudinal rows in the middle; terminal fissures small, hook-shaped, turned in the same direction. L. 93 µ.
Corresponds closely to Cleve's variety except in the constriction.
Blue clay.
Pl. 25, Fig. 2.
NAVICULA LACUSTRIS GREG.
Valve lanceolate, sub-acute; axial area narrow; central area orbicular; striĉ radiate, 14 in 10 µ, punctate, the median puncta sometimes more distant than the others.
Blue clay. Rare.
Pl. 27, Fig. 12.
Lyratĉ Cl.
Valve elliptical or elliptical-lanceolate; striĉ punctate, transverse; axial area narrow or indistinct; central area expanded on each side into lyre-shaped or horn-like blank spaces.
NAVICULA PRĈTEXTA EHR.
Valve elliptical; lateral areas not regular, with scattered puncta; striĉ radiate, 5 or 6 in 10 µ; puncta, 7 or 8 in 10 µ; along the axial area, a single or double row of puncta; at {93}the middle of the border, on each side, two striĉ approach each other closely with a short stria between them; terminal fissures small, in the same direction. L. 120 µ.
Port Penn, Delaware River.
Pl. 24, Fig. 2.
While variable in size and striation, approaching N. hennedyi, this species, as here figured, is found in the Miocene and later deposits and is extant in most parts of the world.
NAVICULA IRRORATA GREV.
Valve oblong-elliptical, with cuneate-rostrate ends; striĉ, 7 or 8 in 10 µ, puncta, 7 in 10 µ; axial area bordered by puncta in unequal, transverse rows. L. 84 µ.
Blue clay. Rare.
Pl. 24, Fig. 4.
NAVICULA HENNEDYI WM. SM.
Valve elliptical; areas semilanceolate; striĉ about 11 in 10 µ, sometimes longer and shorter on the margin; short rows of transverse striĉ along the axial area.
Blue clay.
Pl. 25, Fig. 12.
Var. circumsecta Grun.—As in the type but with the lateral areas faintly striate or punctate.
Var. manca A. S.—Valve lanceolate-elliptical, the lateral areas narrow and convergent toward the ends; short rows of transverse striĉ along the axial area; striĉ, 9 in 10 µ; central pores incrassate.
Blue clay.
Pl. 25, Fig. 11.
NAVICULA LYRA EHR.
Valve elliptical, with rounded, sub-rostrate or sub-cuneate ends; lateral areas narrow; striĉ, 6 to 14 in 10 µ (Cl.), punctate. L. 50-180 µ.
Var. ehrenbergii Cl.—Lateral areas constricted in the middle, divergent at the ends. Cleve refers to Schmidt, Atlas, Pl. 2, Fig. 25, which is not divergent at the ends.
Along the coast.
Pl. 25, Fig. 10.
A narrower form occurs which has the areas divergent.
Var. ?—Valve elliptical, lateral areas narrow, convergent at the ends with short rows of punctate striĉ; marginal striĉ, 10 in 10 µ, punctate. L. 60 µ.
Squan River, N. J.
Pl. 20, Fig. 5.
Var. dilatata A. S.—Valve elliptical, rostrate; lateral areas convergent in the middle and nearly parallel or convergent at the ends.
Blue clay.
Pl. 25, Fig. 13.
N. lyra is exceedingly variable in outline, fineness of striation and in the lateral areas. Intermediate forms occur approaching N. hennedyi and N. spectabilis. In N. hennedyi the lateral areas are broad, semilanceolate, not narrowed in the middle. In N. spectabilis the lateral areas are broad and narrowed in the middle. In N. lyra the lateral areas are narrow and either constricted or not in the middle. In many forms in {94}these three species the lateral areas are more or less striated or punctate. Cleve does not consider this a distinction of any importance, although certain varieties are founded upon it. All three species are very common in the blue clay and along the coast, but their varieties are too numerous to describe or figure.
NAVICULA SPECTABILIS VAR. EMARGINATA CL.
Valve elliptical; lateral areas broad, narrowed in the middle, delicately striated; marginal striĉ, 10 in 10 µ. L. 70 µ.
Blue clay.
Pl. 25, Fig. 7.
NAVICULA PYGMĈA KUETZ.
Valve elliptical, appearing hyaline; axial and central areas faint; lateral areas convergent in the middle; striĉ indistinct, about 25 in 10 µ. L. 23 µ.
Brandywine Creek (Palmer).
Pl. 27, Fig. 23.
Decussatĉ Cl.
Valve elliptical or lanceolate; axial area narrow; central area small; striĉ punctate, in transverse and oblique, curved rows.
NAVICULA PLACENTA EHR.
Valve elliptical, with short, rostrate-capitate ends; axial area narrow; central area elliptical; striĉ in two directions, the transverse about 22 (to 27, Cl.) in 10 µ, the oblique striĉ crossing in both directions in curved lines appearing "coarser than the transverse" (Lewis).
A very peculiar species which, as Cleve remarks, seems not to be allied to any other. L. about 35 µ, quite constant in size. It is reported from Finland, Scotland, Hungary and New Zealand. Dr. Lewis found it in the Delaware River. It is occasional in the Schuylkill River and the blue clay, and very abundant on Marchantia and mosses on the wet rocks of the upper Wissahickon (F. J. Keeley).
Pl. 27, Fig. 17.
Lineolatĉ Cl.
Valve more or less lanceolate; axial area narrow or indistinct; striĉ radiate or parallel, lineate, that is, with the puncta closer than the striĉ.
NAVICULA RADIOSA KUETZ.
Valve lanceolate with sub-rostrate apices; axial area indistinct; central area small; striĉ radiate in the middle, from 6 to 8 in 10 µ, and convergent at the ends, about 12 in 10 µ. L. 47 µ.
Very common in fresh water.
Pl. 26, Fig. 17; Pl. 40, Fig. 9.
NAVICULA PEREGRINA EHR.
Valve lanceolate, obtuse; axial area narrow; central area large, rounded or slightly irregular; striĉ coarse in the middle, 5 in 10 µ, radiate; convergent at the ends, 7 or 8 in 10 µ.
Abundant in brackish water. Delaware River.
Pl. 26, Fig. 20.
NAVICULA CYPRINUS (WM. SM.)
Valve lanceolate, slightly gibbous in the middle, sub-cuneate at the ends; axial area narrow; central area small; striĉ radiate in the middle, 10 in 10 µ, with shorter, transverse striĉ intermediate; transverse at the extreme ends. L. 82 µ.
Navicula digito-radiata var. cyprinus (Ehr. ?) Wm. Sm. Whether the form here figured is Ehrenberg's or not, it is the species known as Pinnularia cyprinus Ehr. of Wm. Smith.
Common in Shark River, N. J.
Pl. 26, Fig. 21.
NAVICULA REINHARDTII GRUN.
Valve elliptical or elliptical-lanceolate, with broad, rounded ends; axial area narrow, widened at the ends to the width of the valve; central area widened transversely to an irregular, quadrate space; striĉ coarse, 8 in 10 µ, distinctly lineate, alternately longer and shorter in the middle, radiate, nearly transverse at the ends. L. 59 µ.
Blue clay. Rare.
Pl. 26, Fig. 22.
NAVICULA LANCEOLATA VAR. ARENARIA (DONK.) CL.
Valve lanceolate; axial area very narrow or indistinct; central area small, rounded; striĉ radiate, 11 in 10 µ in the middle, closer at the ends. L. 47-54 µ.
Navicula arenaria Donk.
Shark River, N. J.
Pl. 26, Fig. 23.
NAVICULA SALINARUM GRUN.
Valve elliptical-lanceolate with produced sub-capitate or rostrate ends; striĉ radiate in the middle, longer and shorter; transverse at the ends, lineate. L. 32 µ.
Atlantic City, N. J.
Pl. 26, Fig. 24.
NAVICULA VIRIDULA VAR. ROSTELLATA KUETZ.
Valve lanceolate with rostrate ends; axial area very narrow, central area orbicular; striĉ radiate in the middle, about 12 in 10 µ, convergent at the ends and closer. L. 43 µ.
Common in fresh water.
Pl. 26, Fig. 16.
NAVICULA GRACILIS VAR. SCHIZONEMOIDES (EHR.) V. H.
Valve lanceolate, obtuse; axial area widened in the middle; striĉ radiate in the middle, about 12 in 10 µ, transverse or slightly convergent at the ends. L. 45-60 µ. Occurs in gelatinous tubes; usually found free.
Colletonema neglectum Thwaites.
Fresh water.
Pl. 26, Fig. 19.
NAVICULA RAMOSISSIMA (AG.) CL.
Valve lanceolate, sub-acute; axial area very narrow; central area scarcely widened; striĉ, 12 in 10 µ, parallel throughout. L. 45 µ.
Micromega ramosissimum Ag.
Schizonema smithii Kuetz. (not Ag.).
East River, N. Y.
Pl. 26, Fig. 14.
NAVICULA ANGLICA RALFS
Valve elliptical, with sub-capitate or rostrate ends; axial area narrow, central area small; striĉ radiate, 12-13 in 10 µ, distinctly punctate. L. 26 µ.
Fresh water.
Pl. 26, Fig. 26.
NAVICULA GASTRUM EHR.
Valve elliptical, with rostrate ends; axial area narrow, central area transverse or irregular; striĉ radiate, 9 in 10 µ in the middle. L. 26 µ.
The form here figured approaches N. anglica.
Kirkwood Pond, N. J.
Pl. 26, Fig. 25.
NAVICULA DICEPHALA WM. SM.
Valve linear, with rostrate or rostrate-capitate ends; axial area narrow, central area rectangular, transverse; striĉ radiate, 12 in 10 µ. L. 32 µ.
Fresh water.
Pl. 27, Fig. 16.
NAVICULA HUMILIS DONK.
Valve elliptical, with broad, rostrate ends; axial area narrow; central area small; striĉ radiate and distant in the middle, convergent at the ends, coarse, appearing costate, averaging 9 in 10 µ. L. 19 µ. As Donkin states, the striĉ are "very conspicuous."
Navicula hungarica var. capitata (Ehr.) Cl.
Navicula globiceps Lagerstedt, according to Cleve.
Willistown, Pa.
Pl. 27, Fig. 24.
NAVICULA PINNATA PANT. ?
Valve lanceolate, obtuse; axial area narrow, widened in the middle; striĉ coarse, 7 in 10 µ in the middle, radiate, 10 in 10 µ at the ends and transverse, indistinctly lineate. L. 40 µ.
Near Navicula ardua Mann (Diat. Albatross Voy., Cont. U. S. Nat. Herbarium Vol. 10, Part 5, p. 336, Pl. 53, Fig. 2) which, however, is said to have "strictly unbeaded costĉ."
Pavonia, N. J., artesian well.
Pl. 27, Fig. 20.
NAVICULA PENNATA A. S.
Valve lanceolate, acute; axial area narrow; central area quadrate, transverse; striĉ radiate, coarse, 5 in 10 µ, lineate. L. 68-95 µ (Cleve).
Pavonia, N. J., artesian well.
Pl. 27, Fig. 22.
NAVICULA INFLEXA GREG.
Valve slightly elliptical-lanceolate, sub-acute, smooth at the ends; axial area narrow, widened in the middle; striĉ radiate, 11 in 10 µ, lineate. Frustule in zone view constricted in the middle. L. 28-45 µ.
Common along the coast.
Pl. 27, Figs. 18 and 19.
NAVICULA OBLONGA KUETZ.
Valve linear-lanceolate, with broad, rounded ends; margin sometimes undulate; axial area narrow; central area large, orbicular; striĉ in the middle distant, radiate, convergent at the ends and curved or sharply bent, 7 in 10 µ, lineate. L. 70-200 µ (Cleve).
Blue clay. Occasional in fresh water.
Pl. 27, Fig. 21.
NAVICULA HASTA PANT.
Valve lanceolate, gently tapering to the obtuse, produced ends; axial area lanceolate, widened to an orbicular space in the middle; striĉ radiate, the median coarse and quite distant, 5 in 10 µ, becoming closer at the ends where they are 12 in 10 µ, lineate. The distance between the median striĉ gives the appearance of a stauros.
Occasional in the blue clay.
Pl. 27, Fig. 13.
NAVICULA HASTA VAR. PUNCTATA N. VAR.
Valve as in type but with striĉ in the middle distinctly punctate and reaching the median line.
Greenwich Point, Philadelphia.
Pl. 27, Fig. 14.
NAVICULA RHYNCOCEPHALA KUETZ.
Valve lanceolate, with produced ends; axial area indistinct; central area small, rounded; striĉ radiate in the middle, convergent at the ends, 10-11 in 10 µ, punctate. L. 42 µ.
Fresh water. Common.
Pl. 31, Fig. 8.
NAVICULA CRYPTOCEPHALA KUETZ.
Valve lanceolate, with rostrate ends; axial area indistinct; central area small; striĉ, 16 in 10 µ, lineate, radiate in the middle, convergent at the ends. L. 28 µ.
Common in fresh water.
Intermediate forms occur between N. rhyncocephala and N. cryptocephala.
Pl. 31, Fig. 9.
NAVICULA LONGA (GREG.) RALFS
Valve slender, rhombic, elongated, with acute ends; axial area indistinct; central area small; striĉ, 6 or 7 in 10 µ, radiate in the middle, elsewhere transverse; central pores closely approximate. L. 120 µ.
New Rochelle, N. Y.
Pl. 31, Fig. 10.
Cleve refers this form to N. directa var. remota Grun. Some specimens are found in this locality showing the "generally twisted" median line mentioned by Gregory.
Mesoleiĉ Cl.
Valve linear or elliptical; axial area narrow; central area quadrate; striĉ radiate, finely punctate.
NAVICULA MUTICA KUETZ.
Valve ovate, elliptical or lanceolate; axial area narrow; central area dilated into a stauros not reaching the margin; striĉ about 20 in 10 µ, more distant in the middle, radiate, punctate. A punctum occurs on one side of the central nodule.
Reported from New Jersey in fresh water. I have not found it. The figure is from a specimen from another locality.
Pl. 26, Fig. 6.
NAVICULA MINIMA GRUN.
Valve broadly elliptical, 13-15 µ in length; axial area narrow; central area small but with a quadrate pseudo-stauros which is striated; striĉ, about 28 in 10 µ, radiate.
Agrees closely with N. saugeri var. Grun. in V. H. Synopsis, Pl. 14, Fig. 16, said to be intermediate between N. minima and N. atomoides Grun. N. minima var. atomoides Grun. is smaller.
Common in water-troughs.
Pl. 26, Fig. 13.
NAVICULA PUPULA VAR. BACILLARIOIDES GRUN.
Valve linear, with rounded ends; axial area linear, expanding on both sides near the ends of the valve, forming a transverse lunate space; central area small, apparently expanded into a stauros, which, however, is striated; striĉ, 18 in 10 µ, at the middle, closer at the ends, punctate. L. 54 µ.
Pavonia, N. J., artesian well.
Pl. 26, Fig. 9.
Bacillares Cl.
Valve linear or linear-elliptical, with broad ends; axial area narrow, the median line enclosed in siliceous ribs; striĉ finely punctate, more distant in the middle.
NAVICULA BACILLUM EHR.
Valve linear, with rounded ends; axial area enclosed in siliceous ribs and slightly expanded on each side at the ends; terminal nodules incrassate; central area small, elliptical; striĉ, 15 in 10 µ in the middle, transverse, distinctly punctate, closer at the ends L. 47 µ.
Fresh water.
Pl. 26, Fig. 10.
Cleve describes the form as having slightly radiate striĉ in the middle. There is considerable difference in the descriptions of Cleve, Donkin, Grunow and Van Heurck, as also in all of the figures.
NAVICULA AMERICANA EHR.
Valve oblong-linear, with rounded ends, sometimes slightly constricted; axial area about one-half the width of the valve, dilated in the middle; striĉ parallel in the middle, radiate at the ends, 15-16 in 10 µ. A punctum is usually found in the central nodule. L. 55-154 µ.
Blue clay. Occasional in fresh water.
Pl. 26, Fig. 8.
Decipientes Cl.
Valve lanceolate, with obtuse ends; axial area narrow; central area orbicular; striĉ radiate in the middle and more distant.
NAVICULA SEMEN EHR.
Valve elliptic-lanceolate, with sub-rostrate, truncate apices; axial area narrow, {99}sinuous; central area orbicular; terminal fissures small, hook-shaped; striĉ robust, 7 or 8 in the middle, closer at the ends, indistinctly punctate or lineolate.
Blue clay. Not common.
Pl. 26, Fig. 11.
Cleve states that this form belongs to the post-glacial deposits and is found living only in the Hartz Mountains.
NAVICULA INTEGRA WM. SM.
Valve lanceolate with triundulate margins and rostrate-apiculate ends; striĉ radiate, more distant in the middle, 20-23 in µ, punctate; axial area very narrow, central area rounded or elliptical. L. 33-43 µ.
Pavonia, N. J., artesian well. Common in Chester River, Md.
Pl. 26, Fig. 5.
Microstigmaticĉ CL.
Valve lanceolate; axial area narrow; central area small, rounded; striĉ finely punctate, nearly parallel. (Includes here only the division Libellus.)
NAVICULA TUMIDA (BRÉB.) CL.
Valve lanceolate, with rounded ends; axial area narrow, central area elliptical; raphe slightly sigmoid; striĉ, 13 in 10 µ, finely punctate, a few shorter in the middle.
Scoliopleura tumida (Bréb.) V. H.
Cape May, N. J.
Pl. 25, Fig. 1.
NAVICULA GREVILLEI (AG.) CL.
Frustules in gelatinous tubes, rectangular; zone with numerous longitudinal divisions. Valve elliptical-lanceolate, obtuse; axial area narrow, central area small; striĉ lineate, about 18 in 10 µ in the middle where they are slightly radiate and more evident, closer near the ends and transverse; median line with terminal pores distant from the ends. L. 60 µ.
Schizonema grevillei Ag.
East River, N. Y.
Pl. 31, Figs. 3 and 4.
NAVICULA LIBELLUS GREG.
Valve rhombic-elliptical, obtuse at the ends; axial area narrow, central rounded, small; striĉ punctate, slightly radiate, about 19 in 10 µ; terminal fissures close to the ends, indistinct. L. 60 µ.
Cleve describes this form as having acute ends, while Gregory states that it is "more obtuse and broader than N. rhombica." Gregory's Figure 101 apparently shows the ends acute, but he says that the valve view is "rhombic or elliptic-lanceolate, broad, with obtuse ends" (Diat. of the Clyde, p. 57, Pl. 6).
Hackensack Swamp, N. J.
Pl. 31, Fig. 5.
Orthostichĉ Cl.
Valve lanceolate or elongated; axial area narrow; central area sometimes apparently dilated into a stauros; striĉ punctate, the puncta in transverse and longitudinal rows.
NAVICULA CUSPIDATA KUETZ.
Valve rhombic-lanceolate, with acute ends; axial area linear, narrow, not widened in the middle; striĉ transverse, 14-19 in 10 µ (Cl.). L. 70-150 µ.
Blue clay. Not uncommon in fresh water.
Pl. 26, Figs. 1 and 2.
Fig. 2 represents an inner valve or stratum, with strong costĉ variable in size, formerly known as Surirella craticula Ehr.
N. cuspidata var. ambigua (Ehr.) Cl.—Valve elliptical-lanceolate, with rostrate ends, smaller than the type and with finer striĉ.
Crum Creek.
Pl. 26, Fig. 3.
NAVICULA SPICULA (HICKIE) CL.
Valve narrow, lanceolate with acute ends; axial area narrow, central area dilated into a stauros reaching the margin; transverse striĉ, 25-29 in 10 µ, longitudinal closer. L. 50-130 (Cl.).
Sometimes confused with N. crucigera.
Stauroneis spicula Hickie.
Newark, N. J.
Pl. 26, Fig. 4.
NAVICULA CRUCIGERA (WM. SM.) CL.
Valve lanceolate, narrow, with acute apices; central nodule a stauros reaching the margin but crossed by two or three coarser striĉ; transverse striĉ, 12 in 10 µ, punctate, the puncta about 25 in 10 µ. L. 80-100 µ (Cl.). Frustules in gelatinous tubes or free.
Schizonema cruciger Wm. Sm.
Pl. 26, Fig. 15.
Reported as occurring in New York Bay, but I have not seen it. The figure is from a specimen from another locality.
Minusculĉ Cl.
Valve lanceolate or elliptical, chiefly distinguished by the small size; axial area indistinct; central area small; striĉ radiate, very finely punctate.
NAVICULA ATOMUS NĈGELI
Valve elliptical, 6-8 µ in length; striĉ radiate, 26-30 µ, closer near the ends; axial area linear, scarcely widened in the middle.
Water-troughs and ditches. Probably common, but frequently not noticed because of its minuteness. A mounting medium of the highest refractive index, such as realgar, is required to resolve the striĉ. In the figure the striĉ are drawn a little coarser than they appear in most specimens.
Pl. 26, Fig. 12.
Lĉvistriatĉ Cl.
Valve lanceolate, axial area distinct; central area orbicular; striĉ coarse, indistinctly punctate, approaching the costĉ of Pinnularia.
NAVICULA YARRENSIS GRUN.
Valve elliptical-lanceolate, with rounded ends; axial area lanceolate, widened in the middle; striĉ, 5 in 10 µ. L. 97 µ.
Cape May, N. J. Common.
Pl. 25, Fig. 14.
Fig. 15, a smaller form, 65 µ in length; striĉ, 6 in 10 µ.
Fig. 16, 54 µ in length; striĉ, 8 in 10 µ (near var. valida Pant.).
NAVICULA ELEGANS WM. SM.
Valve elliptical-lanceolate, with produced ends; axial area very narrow, central area large, orbicular; striĉ strongly divergent in the middle, slightly, if at all, convergent at the ends, curved toward the margin, indistinctly lineate, 9 in 10 µ. L. 95 µ.
Blue clay. Not rare.
Pl. 31, Fig. 1.
Navicula elegans var. cuspidata Cl.—Valve as in type form but smaller and with rostrate apices; striĉ, 10 in 10 µ. L. 82 µ.
Belmar, N. J.
Pl. 31, Fig. 2.
Cleve remarks that the type form is acute and the striĉ 9, while the var. cuspidata has 12 striĉ in 10 µ. In Fig. 1, Pl. 31, is represented a valve having 9 striĉ in 10 µ, but not acute, while Fig. 2, with but slight variation in striĉ, is more cuspidate. It is probable there are intermediate variations.
NAVICULA PALPEBRALIS BRÉB.
Valve elliptical-lanceolate, with acute apiculate ends; axial area broad, lanceolate; striĉ radiate, lineate, about 11 in 10 µ. L. 60 µ.
Along the coast.
Pl. 31, Figs. 6 and 7.
On Plate 40, Fig. 5, is represented an abnormal form of Navicula in which the central pores are in a line transverse to the longitudinal axis and each raphe is curved in a line which almost returns to the centre. The puncta are in curved lines radiating from the rounded hyaline centre.
Pavonia, N. J., artesian well.
Weissflog has described valves of Navicula somewhat similar in punctation.
(pinnula, a small feather)
Valve linear or nearly so, with rounded ends; axial area broad; central and terminal areas large; costĉ smooth, transverse or radiating, usually convergent at the ends.
The costĉ are channels on the inside of the valve, closed, except in the middle where elliptical foramina, opening into the interior of the valve, give rise through their terminal margins to the two longitudinal lines on each side of the valve. The raphe begins as a groove in the side of the conical central nodule and continues as a cleft at right angles to the plane of the surface of the valve, in which case the raphe forms a single line; if the raphe is inclined to the valve surface, then two lines appear in projection, the upper and lower edges of the cleft. In some forms the surface of the edge of the raphe on one side is folded or grooved for a considerable distance, and the opposite edge is elevated into a ridge or {102}tongue fitting into the groove. In such cases it is possible, in projection, to see the upper or outer edges of the raphe, the lower edges and the edges of the tongue and groove, thus showing four lines; sometimes, when the tongue and groove do not meet, six lines. The so-called inner channel is the part of the raphe on the inside of the tongue, and the so-called exterior channel is the part of the raphe on the outside of the tongue. If, in addition to this formation of the raphe, the plane of cleavage changes toward the terminal nodules, the lines will cross each other and, when two are superimposed, disappear altogether. For the careful examination of the raphe it is necessary to employ large forms, and it is advisable to use nitrate of silver which remains in the raphe, and, as in slides mounted by Mr. F. J. Keeley, shows in a beautiful manner the entire outline of raphe and fissures. The terminal fissures owe their separation to the different directions taken by the two edges of the raphe on each side, one edge bending in a wide curve toward the end of the valve, showing two lines, the upper and lower edges of one side of the raphe when inclined to the plane of the surface, and the other edge of the raphe turning suddenly in an opposite direction and ending abruptly in a curve, giving rise to the appearance, by diffraction, of a punctum.
Pl. 40, Figs. 13, 14 and 15.
Endochrome consists of two chromatophores lying on the zones.
Pinnularia is usually divided into the Majores, or larger, and the Minores, or smaller forms, the latter being further divided according to their striĉ. The following classification is chiefly that of Cleve.
Majores.—Valve large, linear with parallel or slightly radiate striĉ and broad axial area.
Gracillimĉ.—Valve small, striĉ parallel or nearly so; axial area very narrow.
Capitatĉ.—Valve with capitate or rostrate ends; striĉ radiate.
Divergentes.—Striĉ strongly radiate.
Brevistriatĉ.—Striĉ short.
Distantes.—Striĉ distant.
Tabellariĉ.—Striĉ radiate in the middle, strongly convergent at the ends.
Marinĉ.—Marine forms.
Majores
PINNULARIA MAJOR (KUETZ.) WM. SM.
Valve linear, usually slightly gibbous in the middle and at the ends; raphe oblique; axial area less than one-third the width of valve, convergent at the ends; striĉ, 7 or 8 in 10 µ, radiate in the middle, convergent at the ends, crossed by a narrow band. L. ? to 300 µ.
Blue clay. Fresh water. Abundant at Middletown, Delaware Co. (T. C. Palmer).
Pl. 28, Fig. 4.
Fig. 9, Pl. 29, is one of a number of smaller forms which are difficult to determine, approaching P. viridis.
PINNULARIA MAJOR VAR. PULCHELLA N. VAR.
Valve strongly gibbous in the middle and gradually widened to the rounded ends; axial area broad, less than one-third the width of the valve, widened unilaterally in the middle; striĉ, 7 in 10 µ, crossed by a band nearly as wide as the length of the costĉ and scarcely distinct. L. 273 µ.
The central nodule is scarcely evident, probably because it is not so thick as in other forms. The outline is near to that of N. mesogongyla and certain forms of N. nobilis, differing from the latter in the median line, striĉ and band which is wider than that of P. latevittata var. domingensis Cl.
Hammonton Pond, N. J.
Pl. 28, Fig. 2.
A very beautiful form which I cannot find described or figured. It does not appear to be N. major var. turgidula Cl., which has a narrow band. In the fossil deposit from Hopkinton, N. H., valves occur similar in outline but smaller.
PINNULARIA NOBILIS EHR.
Valve slightly gibbous in the middle and at the ends; median line complex; striĉ, 4 or 5 in 10 µ, slightly convergent or parallel at the ends, crossed by a band one-third as wide as the length of the striĉ. L. ? to 350 µ.
Blue clay. Fresh water.
Pl. 28, Fig. 1.
PINNULARIA DACTYLUS EHR.
Valve broad, linear, slightly gibbous in the middle; ends broad, rounded; median line not complex, sinuous; striĉ, 4 or 5 in 10 µ, crossed by a very broad band. L. ? to 300 µ.
Navicula gigas A. S.
Blue clay. Fresh water.
Pl. 28, Fig. 3.
Forms occur which are with difficulty assigned to either nobilis or dactylus.
PINNULARIA DACTYLUS VAR. DARIANA (A. S.) CL.
Valve linear-lanceolate, obtuse; axial area broad, less than one-third the width of the valve; striĉ, 6 in 10 µ, crossed by a broad band. L. 220 µ.
Absecon, N. J.
Pl. 29, Fig. 3.
PINNULARIA DACTYLUS VAR. DEMERARĈ CL.
Valve elliptical-lanceolate, with sub-cuneate ends; axial area lanceolate, broad in the middle; median line flexuose; striĉ radiate throughout, 6 in 10 µ. L. 150 µ.
Blue clay.
Pl. 29, Fig. 10.
PINNULARIA GENTILIS (DONK.) CL.
Valve linear, with rounded ends; axial area about one-fourth the diameter of the valve; striĉ radiate in the middle, convergent at the ends, 7 in 10 µ, crossed by a broad indistinct band.
Fresh water. Not common.
Pl. 29, Fig. 1.
PINNULARIA TRIGONOCEPHALA CL.
Valve linear, gibbous in the middle and at the cuneate ends; axial area wider between the middle and the ends, dilated to an elliptical space in the middle; striĉ, 6 in 10 µ. L. 145 µ.
Blue clay.
Pl. 29, Fig. 8.
PINNULARIA VIRIDIS NITZSCH
Valve linear-elliptical, with rounded ends; axial area narrow, widened in the middle; striĉ, 6 to 7 in 10 µ, crossed by a band as wide as one-third the length of the striĉ.
Common in fresh water.
Pl. 29, Fig. 2.
Quite variable in size. Approaches P. major by intermediate forms as in Fig. 9, Pl. 29.
PINNULARIA VIRIDIS VAR. FALLAX CL.
Valve linear, with rounded ends; axial area narrow, slightly widened in the middle; striĉ sometimes unilaterally interrupted, nearly parallel, 10 in 10 µ.
Elm, N. J.
Pl. 29, Fig. 4.
In Fig. 2, Pl. 30, a form is represented which corresponds closely to Navicula viridis var. B, of Wm. Smith. It is given as synonymous with var. fallax; it is bilaterally interrupted. Blue clay.
PINNULARIA VIRIDIS VAR. ?
Valve linear-elliptical, with rounded ends; axial area narrow, widened in the middle to a transverse fascia which is sometimes unilateral; striĉ, 14, in the middle, divergent, convergent at the ends and closer, crossed by a narrow band. L. 45-60 µ. Fascia sometimes absent or very narrow.
Northbrook, Pa.
Pl. 30, Fig. 17 (represents a form with wider area than usual).
PINNULARIA VIRIDIS VAR. CAUDATA N. VAR.
Valve elliptical-lanceolate, with sub-rostrate ends; axial area narrow, widened to an orbicular space in the middle; striĉ radiate in the middle, 11-12 in 10 µ, convergent and closer at the ends, crossed by a narrow band; median line with very long terminal fissures; terminal nodules noticeable because of the thickening of the edges of the terminal striĉ. L. 43 µ.
Fresh water, Newtown Square. Not common.
Pl. 30, Fig. 18.
PINNULARIA SOCIALIS (PALMER)
Valve linear, with rounded ends; axial area broad, one-third the width of the valve; striĉ slightly radiate in the middle, convergent at the ends, elsewhere parallel, 8 in 10 µ, crossed by an indistinct band about one-third the length of the striĉ. L. 60-120 µ.
This species, discovered by Mr. Palmer near Media, Pa., is remarkable for the grouping of the frustules "held with girdle sides together by a siliceous cementing of valve edges and enclosed in a common coleoderm." The usual number included in a group is four, but sometimes six or eight are noticed. The frustules adhere near their ends and are so firmly fastened that boiling in nitric acid and bichromate of potash for fifteen minutes will not separate them.
Navicula socialis Palmer (Proc. Acad. Nat. Sci., Phila., 1910, p. 460, Pl. 35).
Media, Pa.
Pl. 29, Fig. 5.
PINNULARIA ĈSTUARII CL.
Valve linear, with rounded ends; axial area broad, less than one-third the width of the valve; central area a transverse fascia; striĉ, 7 in 10 µ, parallel except at the ends where they are slightly convergent; median line flexuose, with short, terminal semicircular fissures. L. 85 µ.
Port Penn, Delaware River. Rare.
Pl. 29, Fig. 6.
Gracillimĉ
PINNULARIA MOLARIS (GRUN.) CL.
Valve very convex, linear, with sub-cuneate ends; axial area narrow, expanded in the middle to a transverse fascia reaching the margin; striĉ divergent in the middle, convergent at the ends, 16 in 10 µ. L. 60 µ.
Fresh water.
Pl. 29, Fig. 15.
PINNULARIA LEPTOSOMA GRUN.
Valve linear, rounded at the ends; axial area narrow; central area a broad transverse fascia; striĉ slightly divergent in the middle and convergent at the ends, 17 in 10 µ in the middle, closer at the ends. L. 56 µ.
Fresh water. Not common.
Pl. 30, Fig. 10.
Capitatĉ
PINNULARIA MESOLEPTA EHR.
Valve linear, with triundulate margins and capitate ends; axial area narrow, widened in the middle; striĉ divergent in the middle, convergent at the ends, about 12 in 10 µ. L. 34 µ.
Common in fresh water.
Pl. 29, Fig. 13.
PINNULARIA MESOLEPTA VAR. STAURONEIFORMIS GRUN.
Valve triundulate, capitate; axial area narrow, widened in the middle to a transverse fascia, broader at the margin; striĉ strongly divergent in the middle and convergent at the ends, 9-10 in 10 µ. L. 70 µ.
Pavonia, N. J., artesian well. Fresh water.
Pl. 30, Fig. 20.
PINNULARIA SUBCAPITATA GREG.
Valve linear or linear-elliptical, with sub-capitate ends; axial area distinct, widened to a transverse fascia in the middle; striĉ divergent in the middle, convergent at the ends, 13 in 10 µ. L. 32 µ.
Fresh water.
Pl. 29, Fig. 20.
PINNULARIA SUBCAPITATA VAR. PAUCISTRIATA GRUN.
Valve linear-elliptical, with rounded ends; axial area gradually widened into a broad, transverse fascia; striĉ divergent in the middle, convergent at the ends, 11-12 in 10 µ. L. 47 µ.
Fresh water.
Pl. 30, Fig. 16.
PINNULARIA TERMES (EHR.) A. S.
Valve linear, with concave margins and rostrate-capitate ends; axial area narrow, widened in the middle to an orbicular or sub-quadrate space; striĉ divergent in the middle, scarcely, if at all, convergent at the ends, 10 in 10 µ.
Pensauken, N. J., artesian well.
Pl. 29, Fig. 17.
This is, I believe, the form figured by Schmidt (Atlas, Pl. 45, Fig. 67). Cleve refers it to Pinnularia interrupta forma biceps, in which the central space is rhomboid.
PINNULARIA TERMES VAR. STAURONEIFORMIS V. H.
Valve linear, with concave margins and capitate-rostrate ends; axial area narrow, widened into a rhomboidal fascia, reaching the margin; striĉ, 10 in 10 µ, divergent in the middle, convergent at the ends.
Pinnularia interrupta forma stauroneiformis Cl.
Fresh water.
Pl. 29, Fig. 14.
PINNULARIA APPENDICULATA (AG.) CL.
Valve linear, with subcapitate ends; axial area narrow; central area a transverse fascia; striĉ divergent in the middle, convergent at the ends, 16 in 10 µ. L. 43 µ.
Fresh water. Marl pits, Lenola, N. J. (Palmer).
Pl. 29, Fig. 18.
PINNULARIA BRAUNII GRUN.
Valve linear-lanceolate, with capitate ends; axial area gradually widened toward the middle and expanded into a fascia reaching the margin; striĉ divergent in the middle, convergent at the ends, 11 in 10 µ. L. 52 µ.
Pensauken, N. J., artesian well.
Pl. 29, Fig. 16.
PINNULARIA MICROSTAURON (EHR.) CL.
Valve convex, linear, tapering to sub-cuneate or sub-rostrate ends; axial area very narrow; central area a broad fascia; striĉ divergent in the middle, convergent at the ends, 12 in 10 µ. L. 35 µ.
Pavonia, N. J., artesian well.
Pl. 29, Fig. 19.
This form does not exactly correspond to Cleve's diagnosis, as the ends are not broad. All species in the group Capitatĉ are quite variable.
Divergentes
PINNULARIA DIVERGENS VAR. ELLIPTICA GRUN.
Valve linear, with rounded ends; axial area widened in the middle to a transverse fascia; striĉ, 9 in 10 µ, divergent in the middle, convergent at the ends. L. 150 µ.
Fresh water. Not common in this locality.
Pl. 31, Fig. 13.
PINNULARIA CARDINALICULUS CL.
Valve linear, with rounded ends; axial area wide, less than one-third the width of the valve, expanded to a transverse fascia; striĉ divergent in the middle and slightly convergent at the ends, 9 in 10 µ. L. 97 µ.
Blue clay.
Pl. 30, Fig. 1.
As a rule, the median fissures in Pinnularia are turned inwards on the side of the longer edge of the terminal fissures, but not always. In this specimen the median fissures are turned slightly toward the side of the shorter edge of the terminal fissures.
PINNULARIA LEGUMEN EHR.
Valve linear, with more or less triundulate margins and broad, capitate ends; axial area less than one-fourth the width of valve, widened in the middle; striĉ strongly divergent in the middle and convergent at the ends, 10 in 10 µ. L. 84 µ.
Fresh water. May's Landing, N. J.
Pl. 30, Fig. 3.
PINNULARIA LEGUMEN VAR. ?
Valve as in type, but with a transverse fascia; striĉ, 10 in 10 µ, curved or bent near the ends. L. 84 µ.
This form is not var. florentina Grun.
May's Landing, N. J. (with the type).
Pl. 30, Fig. 4.
PINNULARIA BRÉBISSONII (KUETZ.) CL.
Valve linear-elliptical, with rounded ends; axial area narrow, widened into a transverse fascia which is usually broader at the ends; striĉ divergent in the middle, convergent at the ends, about 12 in 10 µ. L. 40-60 µ (Cl.).
Fresh water. Common.
Pl. 29, Fig. 12; Pl. 31, Fig. 11.
Variable in outline.
PINNULARIA MORMONORUM (GRUN.)
Valve linear, with rounded ends; striĉ divergent in the middle, convergent at the ends, 10 in 10 µ; axial area rhombic-lanceolate, widened to a fascia usually reaching the border. L. 62 µ.
Navicula mormonorum Grun.
Common near Willistown, Pa.
This form is regarded by Cleve as P. brébissonii, but the axial area appears to distinguish it. The valves are sometimes narrowed in the middle.
Pl. 29, Fig. 11.
Brevistriatĉ
PINNULARIA ACROSPHĈRIA (BRÉB.) CL.
Valve linear, gibbous in the middle and at the ends; axial area about half the width of the valve; median line with approximate central pores; median area punctate; striĉ nearly parallel, radiate at the ends, 9 in 10 µ. L. 32-180 µ (Cl.).
Blue clay. Recent, fresh water.
Pl. 30, Fig. 7.
PINNULARIA ACROSPHĈRIA VAR. TURGIDULA GRUN. ?
Valve strongly gibbous in the middle; ends rounded; striĉ, 12-13 in 10 µ. L. 54 µ.
Blue clay, Gloucester, N. J., artesian well.
Pl. 30, Fig. 8.
PINNULARIA BLANDITA N. SP.
Valve linear, gibbous in the middle, and with rounded ends; striĉ radiate in the middle, convergent at the ends, 13 in 10 µ; axial area about one-fourth the width of the valve, widened in the middle; median line with small semicircular terminal fissures. L. 65 µ.
Pavonia, N. J., artesian well. Rare.
Pl. 30, Fig. 25.
PINNULARIA PARVA (EHR.) CL. VAR. ?
Valve linear, tapering to the subcapitate ends; axial area broad, lanceolate; median line with approximate central pores and semicircular terminal fissures; striĉ slightly divergent in the middle and convergent at the ends, 12 in 10 µ. L. 58 µ.
Differs from the type in having finer striĉ.
Atco, N. J.
Pl. 30, Fig. 14.
PINNULARIA NODOSA FORMA CAPITATA CL.
Valve triundulate, with capitate ends; axial area about one-fourth the width of valve; striĉ parallel, convergent at the ends, 10 in 10 µ, sometimes interrupted in the middle. L. 47 µ.
Fresh water. Common.
Pl. 30, Figs. 15 and 19.
PINNULARIA POLYONCA (BRÉB.) LEWIS
Valve with triundulate margins, more inflated in the middle, with capitate ends; axial area very broad; striĉ marginal, short, 9 in 10 µ, divergent in the middle, convergent at the ends. L. 97 µ.
Kirkwood Pond, N. J.
Pl. 30, Fig. 21.
The description of Kuetzing (Species Algarum, p. 85), where he states that the margins are "triundulate, the median inflation larger, apices rounded-capitate," appears to sufficiently distinguish this species, which I believe to be the same as Brun's Navicula peripunctata, except that the form figured (Espèces Nouvelles, Pl. 16, Fig. 11) is interrupted in the middle, a common variation in these forms. Cleve makes Navicula polyonca Bréb. equal Pinnularia mesolepta, but at the same time he considers Lewis' form and also Brun's as equivalent to Navicula formica Ehr., and calls it Pinnularia nodosa var. formica Ehr. P. mesolepta has a narrower area than nodosa. I adhere to Lewis' identification, as in any case it is the form here figured and is nearly, if not quite, the same as Brun's species.
Distantes
PINNULARIA LATA (BRÉB.) WM. SM.
Valve linear-elliptical, broad; axial area broad, widened in the middle; striĉ slightly radiate in the middle, 3 in 10 µ; median line oblique, the terminal fissures hook-shaped. L. 86 µ.
Blue clay. Not uncommon.
Pl. 30, Fig. 23.
PINNULARIA BOREALIS EHR.
Valve linear, with rounded or sub-truncate ends; axial area about one-fourth the width of the valve, widened in the middle; median line with large hook-shaped terminal fissures; striĉ, 4 or 5 in 10 µ. L. 54 µ.
Blue clay. Occasional in fresh water in a smaller form. Specimens occur intermediate between P. lata and P. borealis.
Pl. 30, Fig. 22; Pl. 31, Fig. 12.
PINNULARIA BOREALIS VAR. SCALARIS (EHR.) CL.
Valve narrow, linear; axial area broad, widened into a transverse fascia; striĉ, 8 in 10 µ. L. 32 µ.
Fresh water.
Pl. 30, Fig. 24.
Tabellariĉ
PINNULARIA STOMATOPHORA (GRUN.) CL.
Valve linear, with rounded ends; axial area less than one-third the width of the valve, gradually widened in the middle to a transverse fascia; on each side of the central nodule is a lunate space; striĉ divergent in the middle, convergent at the ends, 13 in 10 µ; terminal fissures very long, bayonet shaped. L. 75 µ.
Cleve describes a variety continua as not interrupted. In some forms the fascia is marked by very faint, short striĉ on the margin.
Fresh water. Newtown Square.
Pl. 30, Fig. 12.
PINNULARIA GIBBA (KUETZ.) V. H.
Valve linear, tapering to the subcapitate ends; axial area dilated in the middle; striĉ, 10-11 µ, divergent in the middle, convergent at the ends. L. 80 µ.
Fresh water.
Pl. 30, Fig. 5.
PINNULARIA MESOGONGYLA (EHR.) CL.
Valve linear, gibbous in the middle, ends subcapitate; axial area narrow, widened in the middle to a large orbicular space; striĉ strongly divergent in the middle, convergent at the ends, 11 in 10 µ. L. 60 µ.
Fresh water. Common.
Pl. 30, Fig. 6.
PINNULARIA STAUROPTERA (GRUN.) CL.
Valve linear, with slightly triundulate margins tapering to the subcapitate ends; axial area more than one-third the width of the valve, slightly widened in the middle; median line with approximate central pores and semicircular terminal fissures; striĉ divergent in the middle, convergent at the ends, 11 in 10 µ. L. 82 µ.
May's Landing, N. J.
Pl. 30, Fig. 13.
Some of the forms are more triundulate than the specimen figured.
PINNULARIA STAUROPTERA VAR. INTERRUPTA CL.
Valve linear, tapering to the subcapitate ends; axial area broad, widened in the middle to a transverse fascia; striĉ divergent in the middle, convergent at the ends, 10 in 10 µ; median pores approximate. L. 118 µ.
Schuylkill River.
Pl. 30, Fig. 11.
PINNULARIA TABELLARIA (EHR.) CL.
Valve linear, gibbous in the middle and tapering to the subcapitate ends; axial area about one-third the width of the valve, widened in the middle; median line with approximate central pores and bayonet-shaped terminal fissures; striĉ sometimes unilaterally interrupted, divergent in the middle, strongly convergent at the ends, 9 in 10 µ. L. 138 µ.
Blue clay. Rare.
Pl. 30, Fig. 9.
The form here figured has coarser striĉ than in the type which is also usually more capitate.
P. legumen has triundulate margins, P. mesogongyla has an orbicular space, while P. gibba has the space widened. According to Cleve, P. gibba has approximate central pores, as has also P. mesogongyla. In what I have considered to be P. legumen, the central pores are more approximate than in the other two species mentioned. In fact, all of the three resemble each other closely, and are variously named by different authors. The form of P. gibba here figured, which may be P. stauroptera, is not the typical form of Wm. Smith, which has a narrow area and central space. There are, however, among the typical specimens in H. L. Smith's Type Slide No. 275, smaller valves which show a resemblance.
Marinĉ
PINNULARIA RECTANGULATA (GREG.) CL.
Valve linear, with abruptly rounded ends; axial area very narrow; central area large, somewhat quadrate; striĉ, 7-8 in 10 µ. L. 78 µ.
Navicula rectangulata Greg.
Shark River, N. J.
Pl. 29, Fig. 7.
(epithema, a cover or lid)
Frustules epiphytic, solitary, sometimes geminate, adherent on the ventral side at the ends; in zone view rectangular, sometimes tumid in the middle. Valve arcuate, having an interior costate stratum or transverse septa extending to the girdle, often detached, and an exterior valve surface with transverse rows of puncta. Central and terminal nodules not easily seen; in some species a true raphe is indicated.
The resemblance between Epithemia and Eunotia has been already mentioned. In the shape and striation of the valves there is an approach to Cymbella.
The genus is divided into two groups, one in which the costĉ alternate with double rows of puncta, as in E. turgida, and the other in which the rows of puncta are more than two.
The endochrome usually consists of a band lying along the ventral zone and extending in two flaps on the valves.
EPITHEMIA TURGIDA (EHR.) KUETZ.
Valve arcuate, with ends subcapitate; costĉ radiate, 4 in 10 µ, alternating with double rows of puncta. Median nodule central, the raphe curved toward the ventral edge which it closely follows.
Parasitic on algĉ. Very common in fresh water, especially in ponds. In the figure the valve is asymmetrical with respect to the transverse axis, an unusual condition.
Pl. 31, Fig. 14.
EPITHEMIA ARGUS KUETZ.
Valve with dorsal margin convex, and ventral margin nearly straight; ends rounded, constricted; costĉ robust, alternating with more than two rows of puncta; zone view rectangular, the thickened ends of the costĉ forming large nodules in a row along the edge of the valve next to the connecting zone.
Cystopleura argus (Ehr.) Kunze.
Common in fresh water.
Pl. 31, Figs. 15 and 21.
EPITHEMIA ARGUS VAR. ?
Valve strongly arcuate on the dorsal side and concave on the ventral; tapering to the rounded but not produced ends; costĉ at unequal distances, about 2 in 10 µ; granules in transverse rows, 8 in 10 µ. L. 100 µ.
Pensauken, N. J., artesian well.
Pl. 31, Fig. 16.
EPITHEMIA MUELLERI A. S. ?
Valve broad, convex, slightly arcuate, with obtuse, somewhat constricted apices; costĉ about 4 in 10 µ; striĉ, 12-14 in 10 µ; in zone view the outline is rectangular, slightly tumid in the middle. L. 78 µ.
Blue clay.
Pl. 31, Fig. 17.
EPITHEMIA ZEBRA VAR. PROBOSCIDEA (KUETZ.) GRUN.
Valve convex on the dorsal, concave on the ventral side; costĉ, 3-4 in 10 µ, slightly radiating; apices recurved, capitate.
Blue clay.
Pl. 31, Fig. 18.
EPITHEMIA GIBBERULA VAR. PRODUCTA GRUN.
Valve narrow, lunate, with produced and arcuate apices; costĉ radiate, 3-4 in 10 µ; striĉ, 16-18 in 10 µ, punctate. L. 58 µ, usually smaller.
Blue clay.
Pl. 31, Fig. 19.
EPITHEMIA MUSCULUS KUETZ.
Valve short, strongly arcuate on the dorsal, concave on the ventral side; apices slightly produced; costĉ radiate, about 5 in 10 µ; striĉ, 15 in 10 µ, punctate. L. 20-60 µ.
Shark River, N. J.
Pl. 31, Fig. 20.
EPITHEMIA MUSCULUS VAR. CONSTRICTA (BRÉB.) V. H.
Frustule elliptical, slightly constricted in the middle. Valve convex on the dorsal, straight on the ventral side; costĉ about 4 in 10 µ; striĉ about 18 in 10 µ, finely punctate. L. 45 µ.
Epithemia succinta Bréb.
New Rochelle, N. Y.
Pl. 31, Fig. 22.
(Rhopalodes, like a war club)
Frustule in zone view linear, linear-elliptical (in our species), or clavate. Valve reniform or lunate; a raphe, not visible in some species in the usual position of the valve, is found along the convex edge or keel. Median and terminal nodules, although very small, can be determined. The name is more appropriate to the African species which are clavate. Two species only are found in this locality.
The chief distinction between Epithemia and Rhopalodia is in the position of the raphe and the nodules. In R. gibba and R. ventricosa the costĉ are parallel and not radiate since the valves are not lunate.
Chromatophore a single band irregularly divided.
RHOPALODIA GIBBA (KUETZ.) MUELLER
Valve linear, arcuate on the dorsal, straight on the ventral side, reflexed at the extremities. Costĉ, 6-7 in 10 µ; striĉ about 14 in 10 µ. L. 80-200 µ.
Fresh water. Common.
Pl. 31, Fig. 23.
In this species the raphe and nodules can be seen only when the valve is examined at right angles to its usual position.
RHOPALODIA VENTRICOSA (KUETZ.) MUELLER
Valve gibbous in the middle on the dorsal side, straight on the ventral side, with reflexed apices; costĉ, 7 in 10 µ; striĉ, 14-16 in 10 µ. L. 40-100 µ.
The median nodule appears as a minute depression in the middle of the dorsal side. The two species usually occur together.
Epithemia gibba var. ventricosa Kuetz.
Pl. 31, Fig. 24.
The Surirelloideĉ are usually understood to include the genera Surirella, Podocystis, Cymatopleura and Campylodiscus, all of which resemble each other more or less, either in having a keel or markings like the divisions of the keel in Surirella and a median line, or pseudoraphe. The genus Nitzschia also has a keel, but it does not border each side of the valve as in Surirella, being found either near one margin or between it and the centre. Certain of the Surirellĉ are allied to the group Tryblionella of the Nitzschiĉ, while forms of Stenopterobia are distinguished with difficulty from the group Sigmata.
The following arrangement, therefore, is intended to include all genera having a keel or something which resembles it.
Hantzschia.—Valve asymmetrical; keels of the two valves opposite each other.
Nitzschia.—Valve asymmetrical; keels not (usually) opposite each other.
Surirella.—Valve usually symmetrical; a keel on each border.
Cymatopleura.—Valve without an elevated keel, but with markings like those of Surirella; undulated in zone view.
Campylodiscus.—Valves saddle-shaped.
(named after C. A. Hantzsch)
Valve arcuate, with rostrate ends; keel puncta short, prolonged into costĉ or extending across the valve; median nodule rudimentary; the keels of the two valves opposite each other.
Distinguished from Nitzschia chiefly by the position of the keels. According to Mereschkowsky, however, two species of Nitzschia, N. lanceolata and N. spectabilis, show the same peculiarity.
Chromatophores four, two on each of the zones (Mereschkowsky).
HANTZSCHIA AMPHIOXYS (EHR.) GRUN.
Valve slightly arcuate, with rostrate apices; keel puncta, 8 in 10 µ; striĉ transverse, 16-18 in 10 µ, punctate. L. 60 µ.
Quite variable.
Fresh water.
Pl. 32, Fig. 9.
HANTZSCHIA AMPHIOXYS VAR. MAJOR GRUN.
Valve as in type, but the keel puncta are 5 in 10 µ and the striĉ are 11-12 in 10 µ. L. 71 µ.
H. amphioxys var. major Grun. is stated to be 120 µ in length. The present form is smaller but corresponds in puncta and striation. Van Heurck remarks that it approaches H. virgata.
Abundant in sand ripples on the beach at Cape May, N. J.
Pl. 39, Fig. 4.
Fig. 6, Pl. 39, is drawn from an authentic specimen of Wm. Smith's Nitzschia amphioxys, from England, and is introduced for comparison. The central nodule is not evident.
Fig. 3, Pl. 39, is from a specimen from an unknown locality. The keel puncta are 6 and the striĉ 16 in 10 µ.
HANTZSCHIA VIRGATA (ROPER) GRUN.
Valve arcuate on the dorsal side, nearly straight on the ventral side, with rostrate, recurved apices; keel puncta prolonged to one-third the width of the valve, 4 in 10 µ; transverse striĉ, 9-10 in 10 µ. L. 115 µ.
Shark River, N. J. (Kain).
I have not been able to find this form on our coast. The figure is drawn from a specimen from another locality.
Pl. 32, Fig. 23.
HANTZSCHIA MARINA (DONK.) GRUN.
Valve with dorsal margin slightly arcuate, ventral margin straight; apices rostrate and recurved; keel puncta, 6 in 10 µ, prolonged into costĉ across the entire valve; transverse striĉ, 12 in 10 µ, in double rows of alternating puncta between the costĉ. L. 106 µ.
Epithemia marina Donkin.
Along the coast.
Pl. 32, Fig. 22.
(named after Christian L. Nitzsch, of Halle)
Frustules usually free, sometimes enclosed in tubes or united into a filament. Valves keeled, the keels of the two valves usually diagonally opposite (see Hantzschia); keel puncta short or prolonged.
According to Mereschkowsky, there are at least two endochrome plates placed transversely on the zones; sometimes there are from four to six plates, in one species twenty granules and in another no trace of any endochrome whatever.
The following analysis is that of Grunow as given in Cleve and Grunow's "Arctic Diatoms," and adopted and illustrated by Van Heurck in his "Synopsis."
GROUPS
1. Tryblionella.—Keel very excentric, valve often folded; keel puncta indistinct, usually the same in number as the striĉ.
2. Panduriformes.—Valve broad, constricted in the middle, with more or less evident fold; keel very near the edge; keel puncta quite evident or apparently wanting.
3. Apiculatĉ.—Keel very near the edge; valve linear or somewhat narrower in the middle; striĉ on the longitudinal fold fainter than on the remaining surface, or wanting; puncta not in quincunx.
4. Pseudo-Tryblionella.—Keel more or less close to the edge; valve with a more or less deep longitudinal fold over which the striĉ are spread in the same way as over the remaining surface; keel puncta always distinct.
5. Circumsutĉ.—Valve with more or less wide longitudinal fold; keel very excentric; keel puncta quite evident; surface of valve irregularly punctate and also traversed by rows of delicate puncta which belong to a different layer of the valve.
6. Dubiĉ.—Like the group Pseudo-Tryblionella, but the valves are not so much folded; frustules sometimes narrowed in the middle. The separation of species is difficult and, in part, doubtful. Keel excentric.
7. Bilobatĉ.—Like the group Dubiĉ, but with more central keel and so forming a transition to the group Pseudo-Amphiprora; valves without longitudinal folds.
8. Pseudo-Amphiprora.—Valve with quite central, sharp keel, arcuate, without longitudinal fold; keel puncta always evident; frustule narrowed in the middle with more or less marked central nodule.
Includes two species not found in this locality.
9. Perrya.—Valve arched with very sharp central keel; not narrowed in the middle; keel puncta mostly on short or long lines which are sometimes interrupted.
Includes six species not found in this locality.
10. Epithemioideĉ.—Keel excentric; keel puncta extended into costĉ across the entire valve.
11. Grunowia.—As in the group Epithemioideĉ, except that the costĉ are shorter, not extending across the valve; keel very excentric.
12. Scalares.—Like Grunowia, but with sharper, somewhat excentric keel; transverse section of frustule quadrangular.
13. Insignes.—Like Scalares, but with more central keel so that many of the forms are near the group Perrya; frustule somewhat sigmoid.
14. Bacillaria.—Keel central or nearly so; valve somewhat arched; keel sharp, as in the group Insignes.
15. Vivaces.—Keel moderately excentric; valve, according to position, semi-lanceolate, with keel puncta in short rows, or lanceolate with quite central keel. The valves have in many positions a resemblance to Hantzschia, so that N. vivax frequently becomes confounded with a form of H. amphioxys. The median keel puncta are not distant and a central nodule is not evident as is the case in all species of Hantzschia.
16. Spathulatĉ.—Like the group Bacillaria, but usually with very delicate striated valves; keel in valve view usually bordered with two parallel lines.
17. Dissipatĉ.—Like Vivaces and Spathulatĉ, but with smaller central keel and without parallel lines. Valves usually small, very delicately striated; no central nodule.
18. Sigmoideĉ.—Keel quite central; no parallel lines; frustule sigmoid; valve without longitudinal furrow; keel puncta not extended; no central nodule evident.
19. Sigmata.—Like Sigmoideĉ, but with a more excentric keel.
20. Obtusĉ.—Like Sigmata, with a more or less excentric keel which has in the middle a small bending to the inside; middle keel puncta somewhat more distant than the others, and between them a central nodule evident.
21. Spectabiles.—Valve large, slightly arcuate, with excentric keel; no longitudinal folds; keel puncta somewhat extended over the valve but much less than in the group Insignes, and often scarcely perceptible.
22. Lineares.—Keel somewhat excentric, but less than in Spectabiles; frustule straight, sometimes a little constricted in the middle, so that a transition is shown to the groups Dubiĉ and Bilobatĉ. Valve without longitudinal fold; keel puncta round or somewhat angular, scarcely extended.
23. Lanceolatĉ.—Valve lanceolate, linear-lanceolate or rarely elliptical, with very excentric keel; not folded; keel puncta not extended.
24. Nitzschiella.—Valve with excentric keel and long, produced apices.
Tryblionella
NITZSCHIA TRYBLIONELLA HANTZSCH
Valve elliptical-lanceolate, with subacute apices; longitudinal fold well marked; striĉ coarse, transverse, 5 in 10 µ; indistinct puncta intermediate between the striĉ. L. 45 µ. Quite variable.
Blue clay.
Pl. 32, Fig. 8.
NITZSCHIA GRANULATA GRUN.
Valve elliptical or elliptical-lanceolate; striĉ in double rows, each row of three or four small puncta along the margin and rows of large puncta about 6 in 10 µ across the valve. L. 28-44 µ.
Blue clay. Along the coast.
Pl. 32, Fig. 3.
NITZSCHIA NAVICULARIS (BRÉB.) GRUN.
Valve elliptical-lanceolate, with acute apices; striĉ on one side a double row of large and small puncta, and on the other side radiate short rows of large puncta, 7 in 10 µ; middle of valve hyaline. L. 35-60 µ.
Blue clay. Not common.
Pl. 32, Fig. 4.
NITZSCHIA COMPRESSA (BAIL.)
Valve elliptical-lanceolate, sometimes acuminate; striĉ, 6 or 7 in 10 µ, coarsely punctate. L. 56 µ.
Pyxidicula compressa Bailey.
Nitzschia punctata (Wm. Sm.) Grun.
Tryblionella punctata Wm. Sm.
Common along the coast.
Pl. 39, Fig. 7.
Var. minor (H. L. Smith).—Valve acuminate; striĉ, 8 in 10 µ. L. 22 µ.
Pyxidicula compressa var. minor H. L. Smith, Type Slide No. 431.
Pl. 39, Fig. 8.
The smaller forms occur northward, while the larger are found southward. This is unquestionably Bailey's form, as indicated by his figure and by the fact that it is found everywhere along the coast. Wm. Smith's T. punctata is the same species, although the puncta are smaller.
Panduriformes
NITZSCHIA PANDURIFORMIS GREG.
Valve elliptical, constricted in the middle, with sub-cuneate apices; longitudinal fold, with a punctate longitudinal line; striĉ transverse and oblique, 15 in 10 µ; keel puncta, 6 in 10 µ. L. 108 µ.
Along the coast. More often found southward.
Pl. 39, Fig. 2.
NITZSCHIA PANDURIFORMIS VAR. MINOR GRUN.
Valve elliptical, constricted in the middle, with cuneate apices; keel puncta, 9 in 10 µ; striĉ in transverse and oblique lines about 20 in 10 µ; longitudinal fold bordered by a punctate line. L. 34 µ.
Pavonia, N. J., artesian well.
Pl. 32, Fig. 5.
The var. continua Grun. is reported as occurring in Shark River. It varies in having the longitudinal fold punctate. It is also usually smaller than var. minor.
Apiculatĉ
NITZSCHIA APICULATA (GREG.) GRUN.
Valve oblong-linear, with cuneate-apiculate apices; striĉ punctate, apparently interrupted or pervious, about 18 in 10 µ. L. 26 µ.
Chester River, Md.
Pl. 32, Fig. 6.
The puncta are continued across the valve, but are less distinct on the fold. The figure shows the entire frustule with the fold on each valve. The valves are sometimes slightly constricted.
NITZSCHIA ACUMINATA (WM. SM.) GRUN.
Valve linear, sometimes slightly constricted in the middle, with acuminate apices; longitudinal fold entirely without or with indistinct striĉ; keel puncta not evident; striĉ, 14-15 in 10 µ. L. 82 µ.
Port Penn, Delaware River.
Pl. 32, Fig. 13.
NITZSCHIA PLANA WM. SM.
Valve linear; apices acute, slightly constricted in the middle; longitudinal fold further from the keel than the margin, broad, with scattered puncta; striĉ subtle, irregular, interrupted, about 18 in 10 µ; keel puncta oblong, 3-6 in 10 µ. L. 100-170 µ.
Blue clay. Along the coast.
Pl. 32, Fig. 2.
Pseudo-Tryblionella
NITZSCHIA LITORALIS VAR. DELAWARENSIS GRUN.
Valve linear, with obtusely rounded cuneate ends, scarcely, if at all, constricted in the middle; longitudinal fold wide; keel puncta, 5 or 6 in 10 µ, sometimes confluent; striĉ obscure, about 21 in 10 µ. L. 75 µ.
Delaware River.
Pl. 32, Fig. 12.
This form is drawn from a slide of Christian Febiger containing an abundance of specimens from Delaware City, and marked "Nitzschia dubia."
Circumsutĉ
NITZSCHIA CIRCUMSUTA (BAIL.) GRUN.
Valve elliptical, sometimes more than 200 µ in length; longitudinal fold more or less conspicuous; keel puncta about 4 in 10 µ, the middle distant with the appearance of a nodule; striĉ irregular, subtle, finely punctate, frequently interrupted.
Surirella circumsuta Bail.
Tryblionella scutellum Wm. Sm.
Common in brackish water.
Pl. 32, Fig. 1.
Dubiĉ
NITZSCHIA DUBIA WM. SM.
Valve linear, scarcely, if at all, constricted in the middle, with cuneate, produced, apiculate apices, somewhat recurved; keel very excentric; puncta sometimes partly prolonged, about 9 in 10 µ; striĉ, 20-24 in 10 µ. L. 93 µ.
Reported from along the New Jersey coast. I have not seen it. It is generally regarded as fresh-water. Slides sometimes labelled N. dubia are in reality N. litoralis var. delawarensis.
Pl. 39, Fig. 5.
The figure is drawn from a specimen from another locality.
Bilobatĉ
NITZSCHIA BILOBATA WM. SM.
Valve linear-lanceolate, constricted in the middle, apiculate at the ends; keel puncta 6 in 10 µ, prolonged unequally across part of the valve, the two median sub-remote; striĉ, 16 in 10 µ. Frustule oblong, truncate, constricted in the middle. L. 120 µ.
Shark River, N. J., Chester River, Md.
Pl. 32, Figs. 10 and 11.
Epithemioideĉ
NITZSCHIA EPITHEMIOIDES GRUN.
Valve linear, with cuneate, rostrate apices; slightly constricted on the keel side; keel puncta, 8 or 9 in 10 µ, extending as costĉ across the valve; striĉ delicate, 22 in 10 µ. L. 47 µ.
Brackish water, Long Island Sound.
Pl. 32, Fig. 21.
Grunowia
NITZSCHIA TABELLARIA GRUN.
Valve rhomboidal, inflated in the middle; apices produced; keel puncta extend in costĉ across half of the valve, 7 in 10 µ; striĉ transverse, about 22 in 10 µ. L. 20 µ.
Dimerogramma sinuatum Thwaites.
Nitzschia sinuata var. tabellaria (Grun.) V. H.
Schuylkill River. Not common.
Pl. 32, Fig. 7.
Scalares
NITZSCHIA SCALARIS (EHR.) WM. SM.
Valve linear, with obtusely conical apices; costĉ transverse, extending more or less to one-third the width of the valve, 3 or 4 in 10 µ; striĉ, 9 or 10 in 10 µ, punctate. Length of valve quite variable, up to 480 µ (Cleve).
A well-known form, abundant in salt marshes and more or less brackish water.
Pl. 33, Fig. 6. (To the right of the figure is an outline of the valve reduced one-third.)
Insignes
NITZSCHIA INSIGNIS GREG.
Valve nearly linear or linear-lanceolate; apices broad, slightly produced, obtuse; keel puncta extended into short costĉ, 4 or 5 in 10 µ; striĉ about 14 in 10 µ. Length variable up to 400 µ.
Delaware Bay.
Pl. 33, Fig. 8.
Bacillaria
NITZSCHIA PAXILLIFER (O. F. MUELLER) HEIBERG
Frustules united in a filament, afterwards free; valve lanceolate with nearly central keel; keel puncta, 7-9 in 10 µ; striĉ about 21 in 10 µ. L. 110 µ.
Vibrio paxillifer O. F. Mueller.
Bacillaria paradoxa Gmelin.
Nitzschia paradoxa (Gmelin) Grun.
Brackish water or streams subject to its influence.
Pl. 33, Figs. 13 and 14.
Otto Frederick Mueller, in 1786, published at Copenhagen a work on "Infusorial Animalcules," including a description of a Vibrio which he named paxillifer, obviously alluding to the partially-extended frustules bearing at the end a tablet-like bundle. Two years later, Gmelin described the same form as Bacillaria paradoxa, a name still used. Heiberg, however, in 1863, placed the form under Nitzschia where it properly belongs and called it Nitzschia paxillifer (O. F. Mueller). I have adopted Heiberg's name.
Perhaps the most remarkable of all diatoms. Many species possess the power of motion, which, however, is evident only in the free frustule. In N. paxillifer, the movement of the frustules occurs without the loss of continuity or adherence to each other, so that, while at one time the adnate frustules form a narrow filament, like that of Fragilaria, at another {120}time they move laterally to their extreme length and form a thread of frustules adherent at their ends, later resuming their original position. The motion is repeated at intervals of from five to ten seconds. No satisfactory explanation of the movement has ever been made. In the filamentous form the frustules adhere to water-plants.
Vivaces
NITZSCHIA FLUMINENSIS GRUN.
Valve lanceolate, apices produced; keel puncta, 4-6 in 10 µ, partly extended in short costĉ; striĉ transverse, 14-15 in 10 µ, punctate; keel without a pseudo-nodule. L. 73 µ.
Common at Greenwich Point, Philadelphia.
Pl. 32, Fig. 16.
The form here figured is smaller than the type, which is from 130-160 µ in length.
Spathulatĉ
NITZSCHIA SPATHULATA BRÉB.
Frustule linear, truncate, dilated at the ends; zone with longitudinal folds; valve lanceolate, keel central; apices acute, with an elevated appendage; keel puncta, 5-6 in 10 µ; striĉ very fine. L. 56 µ.
Atlantic City and Cape May, N. J. (Lewis).
Pl. 40, Fig. 3.
Dissipatĉ
NITZSCHIA DISSIPATA (KUETZ.) GRUN.
Valve lanceolate, with sub-rostrate apices; keel excentric; keel puncta about 6 in 10 µ; striĉ, 14 in 10 µ. L. 20-40 µ.
Fresh and brackish water.
Pl. 40, Fig. 7.
Sigmoideĉ
NITZSCHIA MACILENTA GREG.
Frustule sigmoid, truncate at the ends; valve linear, with sub-acute apices and nearly central keel; keel with 5-6 puncta in 10 µ; striĉ obscure, about 25 to 28 (?) in 10 µ. Length variable, up to 490 µ.
As the valve is usually seen when the keel is on the margin, the outline (reduced one-third, shown to the left of the figure) is, as a rule, sigmoid.
Delaware Bay.
Pl. 33, Fig. 7.
NITZSCHIA VERMICULARIS (KUETZ.) HANTZSCH
Valve linear, sigmoid, attenuated toward the obtuse ends; keel puncta, 9 in 10 µ, quite distinct; striĉ very fine. L. 105 µ.
Fresh-water pools.
Pl. 32, Fig. 24; Pl. 33, Fig. 9.
SIGMATA
NITZSCHIA SIGMA (KUETZ.) WM. SM.
Frustule linear, sigmoid; valve linear, slightly sigmoid, tapering to the sub-acute apices; keel excentric, puncta, 8 in 10 µ; striĉ, 20-24 in 10 µ. L. to 250 µ.
Along the coast.
Pl. 39, Fig. 13.
NITZSCHIA SIGMATELLA GREG.
Valve linear, sigmoid, slightly attenuated toward the obtuse apices; keel excentric, puncta, 8-10 (?) in 10 µ; striĉ delicate, 25-30 in 10 µ. L. to 400 µ. The keel puncta are quite obscure.
Nitzschia curvula Wm. Sm.
Nitzschia sigma var. curvula (Wm. Sm.) De Toni.
Fresh water. Hammonton Pond; May's Landing, N. J.
Pl. 33, Figs. 4 and 5.
Gregory remarks that the keel puncta are seen in some specimens. In both of the forms figured I have counted 30 striĉ in 10 µ, but, after many examinations, I have not been quite certain about the keel puncta. The general appearance of the valves in any position is that of a Stenopterobia or Surirella anceps, with which it occurs.
NITZSCHIA CLAUSII HANTZSCH
Valve linear, slightly sigmoid, tapering to the sub-capitate ends; keel puncta, 11 in 10 µ; striĉ subtle. L. 40 µ.
Abundant in Ridley Creek, Delaware Co. (Palmer).
Pl. 32, Fig. 20.
Obtusĉ
NITZSCHIA OBTUSA WM. SM.
Frustule sigmoid, rounded at the ends; keel somewhat excentric, inflexed in the middle, the two median puncta distant; keel puncta, 5-6 in 10 µ; striĉ, 26 in 10 µ. L. to 300 µ.
Along the coast.
Pl. 39, Fig. 16.
NITZSCHIA OBTUSA VAR. FLEXELLA H. L. SMITH
Valve more attenuate at the ends than the type and smaller.
Pl. 39, Fig. 14.
NITZSCHIA OBTUSA VAR. SCALPELLIFORMIS GRUN.
Valve linear, with apices unilaterally truncate; keel excentric; keel puncta, 8 in 10 µ; striĉ, 26 in 10 µ. L. 48 µ.
Along the coast.
Pl. 32, Fig. 17.
Spectabiles
NITZSCHIA SPECTABILIS VAR. AMERICANA GRUN.
Frustule linear, slightly constricted in the middle, with sub-cuneate ends; valve linear, slightly arcuate, tapering to the sub-rostrate ends; keel excentric, keel puncta sometimes confluent, 4-6 in 10 µ, prolonged into short costĉ; striĉ distinct, 14 in the middle, 18 at the ends in 10 µ (but variable in different specimens). L. 186 µ.
Blue clay, especially at Tioga St.
Pl. 33, Fig. 3; Pl. 39, Fig. 1.
This is, probably, one of the most beautiful of the Nitzschiĉ. It sometimes, according to De Toni, reaches a length of 520 µ.
Grunow states that his variety is found in the S. Bridgeton deposit. In a slide of Mœller labelled "Bridgeton, Maine," I find specimens identical in every respect with the Philadelphia form.
Lineares
NITZSCHIA LINEARIS (AG.) WM. SM.
Valve linear, slightly inflexed in the middle; keel excentric; keel puncta, 8-9 in 10 µ, the two median distant; striĉ about 30 in 10 µ. Frustules in zone view narrowed toward the ends, truncate. L. 75 µ.
Very common in fresh water.
Pl. 32, Fig. 18. Fig. 20, Pl. 40, a transverse section of frustule.
Lanceolatĉ
NITZSCHIA PALEA (KUETZ.) WM. SM.
Valve linear-lanceolate, slightly rostrate at the apices; keel puncta, 10 in 10 µ, the median not distant; striĉ, 33-36 in 10 µ; zone view linear, with rounded ends. L. 25-65 µ.
Fresh water.
Pl. 32, Fig. 15.
NITZSCHIA AMPHIBIA GRUN.
Valve lanceolate, apices sometimes slightly produced, rounded; keel puncta, 8-9 in 10 µ; striĉ, 16 in 10 µ. L. 20-32 µ.
Fresh water.
Pl. 32, Figs. 14 and 25.
NITZSCHIA COMMUNIS RAB.
Frustule linear, slightly attenuated at the obtuse ends; valve elliptical-lanceolate, attenuated toward the obtuse ends; keel puncta, 12 in 10 µ; striĉ more than 30 in 10 µ. L. 35 µ.
Fresh water.
Pl. 32, Fig. 19.
NITZSCHIA INTERMEDIA HANTZSCH
Valve linear-lanceolate; keel puncta, 8 in 10 µ; striĉ about 24 in 10 µ. L. 100 µ.
Crum Creek. Not common.
Pl. 33, Fig. 2.
Nitzschiella
NITZSCHIA LONGISSIMA (BRÉB.) RALFS
Valve linear-lanceolate, with exceedingly long horns or beaks; keel puncta about 10 in 10 µ; striĉ about 16 in 10 µ. L. to 500 µ.
Shark River, N. J.
Pl. 33, Fig. 1.
Forma parva V. H.—Keel puncta, 10-12 in 10 µ. L. 70 µ.
East Park Reservoir, Philadelphia.
Pl. 33, Fig. 10.
Differs from N. closterium (Ehr.) Wm. Sm. in the keel puncta.
The type form occurs in brackish and salt water. The occurrence of the variety in fresh water is another instance of the finding of presumably brackish forms in the water supply of the city. If these cases prove to be unusual, it may be because of one of two reasons. The Schuylkill River, before the building of the dam at Fairmount, was tidal as far as the Falls of Schuylkill, and brackish influences, while not now existent, may have caused the growth of forms which now survive. Another reason may be that the opening of the locks at Fairmount Dam may cause a slight admission of brackish forms from tidal water below. The abundance of the brackish species appears to indicate that the first reason is the more plausible.
NITZSCHIA REVERSA WM. SM.
Valve lanceolate extended into beaks or horns curving in opposite directions; keel puncta not evident; striĉ, "20-26" in 10 µ. L. 70 µ.
Brackish water. Abundant in Duck Creek, Delaware River.
Pl. 33, Fig. 11.
NITZSCHIA ACICULARIS (KUETZ.) WM. SM.
Valve lanceolate, with beaks or horns about half the length of the median part of the valve; keel puncta, 18 in 10 µ; striĉ exceedingly delicate, "about 40 in 10 µ." L. 45 µ.
Fresh water. Darby Creek.
Pl. 33, Fig. 12.
(homoios, like, and clados, a branch)
Frustules like Nitzschia, but enclosed in branching or simple tubes.
HOMŒOCLADIA FILIFORMIS WM. SM.
Frustule linear, tumid in the middle, obtuse at the ends; valve linear-lanceolate, with somewhat acute apices; keel central or nearly so; keel puncta, 8 in 10 µ; striĉ delicate. L. 108 µ.
Fresh and brackish water. Newark, N. J.
Pl. 33, Fig. 15.
(named after Dr. Suriray, a physician of Havre)
Valve linear, elliptical or ovate; pseudoraphe linear or lanceolate; a marginal keel forming wings or alĉ seen in zone view; costĉ short or reaching the pseudoraphe, frequently with intercostal striĉ more or less evident.
The genus is divided by Grunow according to the length and form of the costĉ. I include Stenopterobia.
Section 1.—Costĉ of nearly equal width throughout, reaching the pseudoraphe.
Section 2.—Costĉ short or marginal.
Section 3.—Costĉ dilated at the margin, attenuated toward the pseudoraphe.
Section 4.—Valve having the appearance of Nitzschia, with inconspicuous alĉ (Stenopterobia).
The endochrome consists of two laminate chromatophores, one on each valve.
The auxospores are single, originating from the union of two frustules (H. L. Smith).
Section 1
SURIRELLA BISERIATA (EHR.) BRÉB.
Valve lanceolate, subacute at the ends; costĉ robust, about 2 in 10 µ, parallel in the middle, radiate at the ends; pseudoraphe narrow. L. 100 µ.
Surirella bifrons Ehr.
Fresh water.
Pl. 39, Fig. 12; Pl. 35, Fig. 2 (smaller form).
SURIRELLA LINEARIS WM. SM.
Valve linear, with cuneate ends, slightly constricted in the middle; costĉ parallel, 2-3 in 10 µ. L. 90 µ.
Fresh water.
Pl. 35, Fig. 8.
SURIRELLA AMPHIOXYS WM. SM.
Valve oblong-linear, with cuneate ends; pseudoraphe narrow; costĉ, 3-4 in 10 µ; striĉ, 14-16 in 10 µ, somewhat radiate. L. 34-54 µ.
Surirella mœlleriana Grun.
Fresh and brackish water. Common along the coast.
Pl. 35, Figs. 12 and 13.
SURIRELLA ROBUSTA EHR.
Valve linear-ovate; pseudoraphe wide; alĉ prominent; costĉ wide, 1ĵ in 10 µ. Frustule in zone view clavate. L. 200-365 µ.
Fresh water.
Pl. 36, Fig. 2.
SURIRELLA SPLENDIDA (EHR.) KUETZ.
Valve ovate; costĉ, 1½ to 2 in 10 µ; pseudoraphe linear, narrow. L. 125-200 µ.
Fresh water.
Pl. 35, Fig. 3.
S. splendida is smaller than S. robusta and wider in proportion, but, as intermediate forms occur, it is difficult to distinguish between them.
SURIRELLA ELEGANS EHR.
Valve ovate, rounded at one end and acute at the other; pseudoraphe lanceolate, narrow; costĉ, 1½ in 10 µ; striĉ subtle, 22 in 10 µ. Frustule in zone view cuneate. L. 180-220 µ.
Fresh water.
Pl. 36, Fig. 1.
SURIRELLA STRIATULA TURPIN
Valve broad, obovate or elliptical, rounded at each end; costĉ, 1ĵ in 10 µ, curved at the ends; striĉ, 14 in 10 µ. Frustule in zone view cuneate; marginal alĉ quite robust. L. 100-160 µ.
Blue clay. Brackish water.
Pl. 34, Fig. 1.
In the specimen figured, the outline is exactly elliptical, although the species is usually conical at one end.
SURIRELLA GEMMA EHR.
Valve ovate or ovate-elliptical, rounded at each end, sometimes asymmetrical along the longitudinal axis; pseudoraphe very narrow; costĉ distant, at irregular intervals, about 2 in 10 µ, somewhat radiate, reaching the pseudoraphe; striĉ, 20 in 10 µ, punctate. Frustule in zone view cuneate. L. 70-120 µ.
Along the coast.
Pl. 36, Fig. 4.
SURIRELLA TENERA GREG.
Valve ovate; pseudoraphe narrow, well-defined; costĉ indistinct, 2½ in 10 µ, their margins invisible; striĉ about 14 in 10 µ, punctate, more evident near the margin. L. 90 µ.
Surirella diaphana Bleisch.
Pavonia, N. J., artesian well.
Pl. 35, Fig. 6.
The figure is that of the var. nervosa A. S. (Atlas, Pl. 23, Fig. 15), which differs from the type in having the position of the costĉ indicated by scattered puncta.
Section 2
SURIRELLA GUATIMALENSIS EHR.
Valve ovate; pseudoraphe very narrow and indistinct; costĉ short, marginal, 2-2½ in 10 µ, absent from the rounded end. L. 120 µ.
Surirella cardinalis Kitton.
Smith's Island, Delaware River.
Pl. 36, Fig. 5.
SURIRELLA OVALIS BRÉB.
Valve ovate; costĉ short, marginal, radiate, 3-6 in 10 µ, often unequal; central area ovate, indistinctly costate; striĉ scarcely visible, about 18 in 10 µ; pseudoraphe narrow. L. 45-93 µ.
Surirella davidsonii A. S.
Fresh or brackish water.
Pl. 35, Fig. 5; Pl. 39, Fig. 11.
The smaller specimen is from the Delaware River, and the larger from the Hudson River.
SURIRELLA CRUMENA BRÉB.
Valve nearly orbicular; costĉ short, marginal, radiate; pseudoraphe narrow, indistinct; central area indistinctly costate, sometimes interrupted.
On account of the extreme confusion in the names of many forms which appear to be variations of S. ovalis, I have followed Van Heurck in retaining the original names as specific. De Toni gives S. crumena as a variety of S. ovalis.
Fresh and brackish water. Quite common in the Delaware River.
Pl. 35, Fig. 4.
SURIRELLA PINNATA WM. SM.
Valve ovate or oblong-ovate; costĉ reaching the linear pseudoraphe, about 6 in 10 µ. L. 40 µ.
Surirella ovalis var. pinnata (Wm. Sm.) De Toni.
S. pinnata is the type of a number of small forms usually found together, including S. panduriformis, S. angusta and S. minuta.
Fresh water. Media (Palmer).
Pl. 36, Fig. 7; Fig. 9 (abnormal).
Var. minuta, a small form of S. pinnata, occurs with the type.
SURIRELLA PANDURIFORMIS WM. SM.
Valve linear-oblong, with rounded ends, more or less constricted in the middle; otherwise as in S. pinnata. L. 54 µ.
Fresh water.
Pl. 36, Fig. 6.
SURIRELLA ANGUSTA KUETZ.
Valve linear, with cuneate ends; otherwise as in S. pinnata.
Fresh water.
Pl. 36, Fig. 8.
S. pinnata, S. panduriformis, and S. angusta have a narrow central area, and differ from S. ovalis which has short costĉ.
SURIRELLA OBLONGA EHR. ?
Valve elliptical-lanceolate, with obtuse ends; costĉ, marginal, 2½ in 10 µ; median area granulate; pseudoraphe narrow, lanceolate, scarcely visible; striĉ about 18 in 10 µ. L. 60 µ.
Blue clay. Rare.
Pl. 35, Fig. 9.
This has the outline and appearance of S. oblonga Ehr. (Mik. Pl. 15, Fig. 48), but the costĉ are closer.
SURIRELLA RECEDENS A. S.
Valve ovate; costĉ, 2-2½ in 10 µ; pseudoraphe narrow, not reaching the ends of the valve; intercostal spaces more evident near the middle. L. 50 µ.
Blue clay. Not uncommon.
Pl. 35, Fig. 7.
SURIRELLA CRUCIATA A. S.
Valve ovate; pseudoraphe very narrow; costĉ, 2 in 10 µ; the outline of several of the median costĉ strongly emphasized, while the other costĉ are indistinct. L. 54 µ.
Blue clay.
Pl. 35, Fig. 10.
SURIRELLA GRACILIS GRUN.
Valve linear, with sub-cuneate ends, slightly constricted in the middle; pseudoraphe very narrow; costĉ, 6-7 in 10 µ; transverse striĉ about 26 in 10 µ, punctate. L. 75 µ.
According to De Toni (p. 598), this form is a Nitzschia. It has, however, a narrow pseudoraphe.
Pavonia, N. J., artesian well. Rare.
Pl. 35, Fig. 11.
Section 3
SURIRELLA FASTUOSA EHR.
Valve ovate; costĉ about 1-2 in 10 µ, dilated at the margin and contracting at about one-fourth the distance toward the middle; area, ovate-lanceolate; pseudoraphe, narrow and indistinct; intercostate striĉ more evident near the margin, 19 in 10 µ, becoming again evident in a narrow band about one-half the distance to the pseudoraphe. L. 50-120 µ.
Along the coast. More common southward.
Pl. 35, Fig. 1.
SURIRELLA FEBIGERII LEWIS
Valve ovate-lanceolate; costĉ about 2½ in 10 µ with punctate interspaces extending half the distance toward the median hyaline area, which is divided longitudinally on each side of the narrow pseudoraphe by two longitudinal bands composed of short, transverse, irregular, punctate lines.
Along the coast.
Pl. 36, Fig. 3.
Section 4 (Stenopterobia)
SURIRELLA ANCEPS LEWIS
Frustule linear, straight or nearly so; valve sigmoid with rounded apices; costĉ marginal, nearly obsolete; striĉ distinct, about 15 in 10 µ; pseudoraphe wide. L. to 320 µ.
Hammonton Pond and Tom's River, N. J.
Pl. 34, Fig. 2.
SURIRELLA INTERMEDIA LEWIS
Frustule linear, straight, widened at the truncate ends; valve linear, sigmoid, tapering to the sub-acute ends; costĉ about 5 in 10 µ; striĉ about 20 in 10 µ. L. variable.
Hammonton Pond, N. J.
Pl. 34, Fig. 3; Pl. 39, Fig. 9 (zone view).
This, perhaps, is forma sub-acuta Fricke.
Fig. 7, Pl. 34, is probably a small form of S. intermedia, from Willistown, Pa. It resembles a Nitzschia.
SURIRELLA DELICATISSIMA LEWIS
Frustule linear, rounded at the ends; valve linear-lanceolate, sometimes very slightly constricted in the middle, with acute apices; costĉ, 5 in 10 µ; striĉ about 20 in 10 µ; pseudoraphe well defined, lanceolate. L. to 90 µ.
Fresh water. Newtown Square.
Pl. 34, Figs. 5 and 6 (small forms).
SURIRELLA ARCTISSIMA A. S.
Valve linear, tapering to the sub-acute ends; costĉ marginal, 5 in 10 µ; striĉ, 18 in 10 µ; pseudoraphe not evident. L. 184 µ.
May's Landing, N. J.
Pl. 34, Fig. 4.
Fig. 10, Pl. 39, is a small form from Newtown Square, Pa., in which the length is 86 µ, the costĉ 5 and the striĉ 16 in 10 µ.
(pous, a foot, and cystis, a bag)
Frustules cuneate, similar to Surirella, but attached by short stipes to other algĉ; valve obovate.
PODOCYSTIS ADRIATICA KUETZ.
Valve nearly symmetrical, obovate, with transverse costĉ about 4 in 10 µ, alternating with double rows of coarse puncta; median line distinct, linear. L. 43 µ.
Podocystis americana Bail.
Hell Gate, N. Y.
Pl. 40, Fig. 6.
(cuma, a wave, and pleura, a side)
Valve elliptical; surface transversely undulate, with short, marginal costĉ. Frustule in zone view linear, with undulated sides.
Auxospore formation as in Surirella.
CYMATOPLEURA SOLEA (BRÉB.) WM. SM.
Valve oblong, with cuneate apices, constricted in the middle; costĉ about 6 in 10 µ; striĉ, 10 in 10 µ; pseudoraphe scarcely visible. L. 50-300 µ.
Blue clay. Common in the Hudson River.
Pl. 34, Figs. 8 and 9.
CYMATOPLEURA ELLIPTICA (BRÉB.) WM. SM.
Valve elliptical; marginal costĉ short, 3 in 10 µ; striĉ delicate, 18 in 10 µ; undulations four or more. L. 70-140 µ.
Blue clay.
Pl. 37, Fig. 1.
Forma spiralis.—Valve ovate, swelled into curved ridges at the lower end, with a contraction of the valve.
Port Penn, Delaware River.
Pl. 37, Fig. 2.
CYMATOPLEURA MARINA LEWIS
Frustule linear, with numerous undulations, ends apiculate; valve linear-lanceolate, with acute ends; striĉ transverse, punctate at unequal intervals, from 16-18 in 10 µ. L. 43 µ.
East River, N. Y.
Pl. 37, Figs. 3 and 4.
Lewis states that the ends are more or less truncate. I do not find them so.
(campulos, curved like a saddle)
Valve orbicular or sub-orbicular, with costĉ or punctate rays converging from the circumference toward the hyaline centre, which sometimes appears like a pseudoraphe. Frustule of two saddle-shaped valves at right angles to each other. The zone view may be of almost any shape according to position.
Endochrome consists of two bands, each lining the inner surface of each valve. Auxospore and conjugation unknown.
CAMPYLODISCUS ECHENEIS EHR.
Valve sub-orbicular, saddle-shaped; costĉ indistinct, short, marginal; rows of round or elongated puncta converge toward the lanceolate, hyaline median space. Diam. 80-140 µ.
Campylodiscus argus Bail.
Blue clay. Reservoir at Thompson and Twenty-sixth Sts., Phila.
Pl. 37, Fig. 6.
This form, usually considered as brackish and marine, is occasionally found in fresh water. According to Deby, it is fossil in the "Champlain deposit of N. A."
CAMPYLODISCUS HIBERNICUS EHR.
Valve irregularly orbicular; costĉ, 40-60, about 2 in 10 µ, wide at the margin and attenuated toward the centre which is somewhat quadrate; the radials rough with minute apiculi.
Pensauken, N. J., artesian well.
Pl. 37, Fig. 5.
Collection and Preparation of Diatoms
It is assumed that every student of the Diatomaceĉ has a general knowledge of the collection, preparation, mounting and examination of material. For the novice, however, the following methods, used by the author for many years, may be of service.
Collection of Fresh-water Material.—The yellow film on the inside of aquaria always contains small species. Stems of water-plants near the shores of ponds and the submerged roots, the brownish coating of rocks in streams and water-falls, fountains, and water-troughs, are prolific. At all times of the year, some diatoms may be found in a thin layer upon the mud of rivers or creeks. In the spring, brown patches of mud, filled with bubbles, floating near the shore in ponds, or coming down with the current in rivers, are rich in various forms. Within the limits assigned to our district, I have made collections in the following localities: Schuylkill River, including the region near Fairmount Dam, several reservoirs and the water-supply; the Wissahickon and Fairmount Park, Darby, Crum and Ridley Creeks, the Neshaminy and the Brandywine; meadow pools and rivulets near the city; the upper Delaware, the Water Gap and numerous cascades northward; the Shawangunk Mountains and the Poconos; many parts of New Jersey along the coast; the Pine Barren region, the Hammonton, Atsion and Kirkwood Ponds and the swamps near Atco.
In the collection of fresh-water material, it is well to be provided with a number of small bottles. Take a handful of the water-plants or algĉ, and squeeze the material into the bottles, or, lacking a bottle, wrap it in paper. With a small forceps it is possible to detach minute quantities of a pure gathering which may not need further preparation beyond burning to a red heat on the cover-glass before mounting. A malacca cane, with extending rod to which may be screwed a bottle, net, spoon or hook, is useful on a long trip. If it is impossible to separate the thin film of diatoms from the mud in the bed of streams, dip up the surface mud with one bottle, allow to settle a few minutes, then pour off the supernatant liquid, which will be comparatively free from sand, into another bottle. It must be confessed, however, that the mud in streams near Philadelphia contains a large quantity of fine mica which, in some instances, it is impossible to remove.
Collection of Marine Material.—Shell scrapings, the stomachs of fish, marine algĉ, especially the brown and red algĉ, the hulls of vessels, mud from anchors and dredgings, are all sources which may prove valuable. In the sand ripples, after the tide recedes, a yellowish-brown deposit will be noticed. This should be taken up carefully with a spoon and placed in a bottle; the sand will settle at once and a very pure gathering will be held in suspension in the water. Such collections may be made along the entire coast of New Jersey on sunny days in summer. In salt meadows near Absecon and Hackensack, large quantities of diatoms, including Pleurosigma, may be obtained in the yellow scum floating on the surface.
The Blue Clay Deposit.—The blue clay occurs as a pre- or post-glacial deposit in the bed of the ancient Delaware River, and, at depths varying usually from fifteen to forty feet below the surface, has been obtained from artesian wells at Pavonia, Pensauken and Gloucester, N. J., also at Port Penn on the Delaware, and especially from the dredgings {132}made by the removal of Smith's Island opposite the city. In the city proper, it may be stated briefly that material may be found in a stratum of very light blue clay at a depth varying from twenty to sixty feet in many places south of Arch St. east of Broad St., and also along the beds of ancient rivulets near Tioga St., at Sixteenth St., and in certain other places which were probably subject to tidal overflow. One of the best collections was made along the bank of the Schuylkill at the east end of Walnut St. Bridge, at a depth of thirteen feet below the surface. Excavations for the Reading Terminal and the Subway and several buildings, as the Bingham House, have furnished numerous specimens.
Cleaning the Material.—Some gatherings may be so pure as to be ready for mounting when treated with dilute alcohol and oil of cloves. If, when gathered, the diatoms are immersed in a saturated solution of picric acid for several days, they may be stained with carmine or methylene blue, or whatever may be required to emphasize the contents of the frustules, including the endochrome and the pyrenoids. After staining, pass as rapidly as expedient through the treatment with dilute alcohol and oil of cloves, and mount in benzol balsam, avoiding heat. A hot solution of mercuric bichloride is sometimes used for the preservation of the endochrome, although washing is needed before mounting. For the particular stain considered best for certain details of structure, it will be advisable to consult works on Micro-Chemistry or Heinzerling (l. c.). The stains of most importance are carmine, methylene blue, hĉmatoxylin, gold chloride and Bismarck brown.
Whatever method may be used in staining, the identification of forms is impossible, in most cases, unless the valves are carefully cleaned and the cell-contents destroyed. For this purpose provide a casserole holding from five to eight ounces, an iron tripod stand with alcohol lamp, several six-inch test-tubes, preferably those with a standard base, fitted with pure rubber corks. Take the material as free from twigs, dead leaves, sand, and other matter as possible, place it in the casserole, and add about the same quantity of nitric acid. Boil for twenty minutes and then add about half a teaspoonful of powdered bichromate of potash, stirring with a glass rod. Then take a beaker-glass partly filled with water and pour into it slowly the liquid which has been allowed to cool a short time, whirling the casserole to cause the concentration of sand in the centre. Allow the material to settle for half an hour or longer, according to the amount of diatoms and their size. Pour off the water, add more water, and place in a test-tube. Repeat the decantation, shaking the test-tube, closed with a rubber cork, vigorously each time. From time to time whirl the diatoms in the casserole and throw away the sand collected in the centre. By repeating the decantation, shaking and whirling, the deposit will be found to consist almost entirely of diatoms. It may be necessary to repeat the boiling in the acid and bichromate. If, however, any detritus other than sand is noted, boil in sulphuric acid and add from time to time minute pinches of powdered chlorate of potash, being careful to protect the eyes by holding a piece of glass before them; otherwise the explosions which occur are likely to throw some of the boiling acid into the eyes and destroy the sight. The material, when clean, should be white or, in the case of Synedra, yellowish. It is quite easy to construct a box fitted with the proper apparatus for boiling and provided with a glass door for observation, and a method of introducing the chlorate of potash through a small aperture or tube. The box may be placed in the garden or fastened outside of a window so that the poisonous fumes may be carried off.
An excellent method, in the case of larger forms, is to boil the material already cleaned by the acid in water to which a few shavings of coarse brown soap are added. The difference in density will hold in suspension any flocculent matter, and while many of the smaller {133}forms will not settle, the others will be perfectly cleaned. When satisfied with the cleaning, preserve the stock material in part alcohol and, in using, pour into a smaller bottle the amount required, replace the dilute alcohol with distilled water, and mount as directed. It often happens that gatherings are made consisting almost entirely of sand. Attempts at cleaning in the usual way will cause the loss of nearly all of the diatoms. In this case, after the material has been treated with acid until nothing remains but sand and a few diatoms, the mechanical finger must be used.
In the cleaning of marine deposits, various methods may be required. In the case of partly siliceous species, washing in pure water repeatedly is all that can be done. The larger and heavier diatoms may be separated from the sand by elutriation or by whirling in a casserole, by rocking in a shallow dish the shape of a watch crystal, or by pouring slowly over a strip of plate-glass at least two feet in length inclined at an angle of thirty degrees. The sand will cling to the glass, while the greater portion of the diatoms will run off. Where particles of shells or foraminifera are present, a preliminary boiling in hydrochloric acid is advisable. In all marine gatherings, the salt should first be washed out before proceeding with the cleaning.
For hardened masses of clay and for fossil deposits, it is necessary to boil in carbonate of soda and follow with the acid treatment. Citric acid and acetate of potash used alternately in boiling may be tried. Soaking for a time in acetate of potash and allowing the material to deliquesce for a week before further process, has proved successful in some instances. The repetition of several methods and the gentle breaking of the harder masses with the point of a needle will disintegrate almost any diatomaceous earth, but, as a last resort for refractory deposits, boil in pure water, add a piece of caustic potash about the size of a pea, continue the boiling not more than thirty seconds longer, and pour instantly into dilute hydrochloric acid; otherwise the diatoms will be destroyed. Afterwards proceed with the usual treatment.
Slides and Covers.—Take half an ounce of No. 1 covers, circles, and place them in a wide-mouthed bottle. Add a portion of the following mixture (Dr. Carl Seiler's formula):
| Bichromate of potash | 2 oz. |
| Sulphuric acid | 3 fl. oz. |
| Water | 25 fl. oz. |
Shake the bottle in order that the surfaces of the covers may be fully exposed to the action of the acid, and set aside for several hours. Decant the solution, add water repeatedly until all traces of the mixture are removed, and keep the circles in the bottle in fifty-per cent. alcohol. When needed, take out a circle with forceps and dry on a linen cloth.
The slides may be treated in the same way, or they may be easily prepared by immersion in a solution of washing soda, and then washed and dried. This process may be used in cleaning the balsam or styrax from old slides.
Preparation of Strewn Mounts.—Place several covers on the mounting stand. With a dipping tube, cover each circle with distilled water, and add a small drop of the prepared diatoms, being careful to avoid any vibration of the stand. Heat the stand until small bubbles begin to appear, remove the lamp, and allow the water to evaporate. If the above method is carefully followed, the diatoms will be deposited in an even layer, provided the material is not too dense. Take a slide, centre it, and place a small amount of styrax on the centre. Invert the prepared cover, and gently place it upon the styrax. Heat the slide {134}on the mounting stand until the styrax bubbles and then allow to cool. If bubbles still remain, heat again until they disappear. It is well to mount several slides more than required, as some may be imperfect.
Preparation of Selected Mounts.—Take a slide, place a minute quantity of beeswax on two places at a distance apart nearly equal to the diameter of the cover used. Place a cover on the wax and press it down flat, or sufficiently to keep it in position. Dip a fine needle into the following cement:
| Glacial acetic acid | 12 drachms |
| Gelatine | 02 drachms |
| Alcohol | 01 drachm |
This is made by adding the acid to the gelatine in a water-bath and then the alcohol, and filtering. Apply the moistened needle to the centre of the cover and spread as small a quantity as possible in a thin layer. Now place the slide upon the turn table, centre it with respect to the position of the gelatine, and with the finest sable brush draw a circle about a tenth of an inch in diameter around the gelatine in water-color (Windsor), blue or vermilion, or in India ink. Instead of the water-color, a circle of tin-foil the size of the cover and pierced with a hole in the centre may be used, but the colored circle is to be preferred, as, when brought into view, it indicates exactly the focus required for observing the diatom.
The bottle containing the cleaned material, which has been kept in water and alcohol, should be refilled with distilled water and well shaken, when a small portion may be taken up with a dipping tube and evenly distributed over a portion of a slide and then dried. By the use of a mechanical finger, fitted with a small piece of finely spun glass attached by wax to the holder of the finger, when the microscope is focussed until the glass thread touches the diatom selected, it will adhere to the thread. Raise the body of the microscope, remove the slide containing the spread material, or move it to another part of the stage, and place the slide with the prepared cover in the same position. Now carefully lower the body-tube of the instrument until the diatom rests upon the gelatine, breathe gently upon it, remove the cover from the slide, invert it over another slide containing a drop of styrax and proceed by heating to mount as before. The size of the diatom, the amount of gelatine, and several other factors, will enter into the question of success or failure. I have, however, employed the above method and have mounted thousands of slides of selected diatoms successfully. It is necessary to avoid any air current which will cause the diatom to fall from the thread. On very cold days the glass thread sometimes becomes electrified and the diatoms will not stick; on sultry days in August in our locality the diatoms will stick too closely.
By the same method, slides of arranged diatoms can be made using a glass circle properly marked with lines in the eye-piece. Care should be taken to use glass threads more or less in proportion to the size of the diatoms. A cat's whisker is preferred by some to the glass thread. It has the advantage of not breaking, but unless it is quite short it is too flexible. If the point of the thread becomes covered with gelatine, lower it into a minute drop of water upon a separate slide, and by moving it about it will be cleaned. The diatom itself may be washed in the same way, if it is not too small.
Instruments Required.—For collecting, in order to determine the quality of the find, any simple lens of fifteen to twenty diameters is sufficient. A Stanhope is quite useful {135}although difficult to obtain, while an achromatic triplet of sufficient power will probably be all that is necessary. For selecting with the mechanical finger, an objective of two-thirds-inch focus is the most convenient, but for determining species a one-fifth-inch is needed, an immersion objective being essential for minute forms.
No particular form of microscope is required. Any instrument having standard parts, inclination of the body to the axis, a sub-stage condenser and movable stage, will prove serviceable in nearly all investigations. For critical work, measurement of striĉ and location of specimens on the slide, the large models of Bausch and Lomb leave nothing to be desired. One smaller instrument may be used for rapid examination and for selection with the mechanical finger. If the stage is supplied with a vernier, the diatoms can be located rapidly and recorded for future reference. The Zentmayer Army Hospital stand with mechanical stage is excellent. The Continental stands, convenient for laboratory work, especially in the examination of bacteria, are not so serviceable as the larger stands of American and English make. The stand especially designed by Dr. Henri Van Heurck, the celebrated Belgian naturalist, is, without doubt, admirably suited to the investigation of the Diatomaceĉ. In the form of the Circuit Stage as made by Watson and Sons, of London, supplied with proper condenser and mechanical stage with vernier attachment, it has been used in the preparation of the present work with much satisfaction.
The drawings have all been made with an Abbé camera lucida, a 3 mm. objective and a No. 10 eye-piece, producing a magnification of about 800 diameters. All illustrations are from actual specimens in my cabinet or, in a few instances, from slides sent me by friends. In the measurement of striĉ and puncta, the number in ten microns is stated, and will be found to be approximately correct in most of the drawings, except when the number is in excess of twenty in ten microns, in which case it is impossible to represent the markings accurately on figures of the magnification adopted. All drawings are from specimens in this locality, except in a few cases mentioned in the text.
(SYNONYMS IN ITALICS)
| PAGE | |
| Achnanthes, | 58 |
| brevipes Ag., | 59 |
| coarctata (Bréb.) Grun., | 59 |
| danica (Floegel) Grun., | 60 |
| exigua Grun., | 59 |
| inflata (Kuetz.) Grun., | 59 |
| lanceolata (Bréb.) Grun., | 59 |
| linearis forma curta H.L.S., | 59 |
| longipes Ag., | 58 |
| subsessilis Kuetz., | 59 |
| Actinella, | 54 |
| punctata Lewis, | 54 |
| Actinocyclus, | 26 |
| barkleyi var. aggregata Rattr., | 27 |
| ellipticus var. delawarensis n. var., | 27 |
| moniliformis Ralfs, | 27 |
| Actinoptychus, | 24 |
| cellulosa Ehr., | 24 |
| heliopelta Grun. var.?, | 25 |
| omphalopelta Ehr., | 24 |
| undulatus (Kuetz.) Ralfs, | 24 |
| vulgaris var. interrupta n. var., | 24 |
| Amphipleura, | 78 |
| pellucida Kuetz., | 78 |
| rutilans (Trentepohl) Cl., | 78 |
| Amphiprora, | 68 |
| alata Kuetz., | 68 |
| conspicua Grev., | 68 |
| lepidoptera Greg., | 69 |
| ornata Bail., | 68 |
| paludosa Wm. Sm., | 68 |
| pulchra Bail., | 68 |
| Amphitetras, | |
| antediluviana Ehr., | 32 |
| tessellata Shad., | 32 |
| Amphora, | 65 |
| acuta Greg., | 66 |
| angusta var. eulensteinii Grun., | 67 |
| aponina Kuetz., | 66 |
| arenaria Donk., | 67 |
| areolata Grun., | 66 |
| coffĉiformis (Ag.) Kuetz., | 66 |
| crassa Greg., | 65 |
| eulensteinii A.S., | 67 |
| gigantea var. fusca A.S., | 65 |
| insecta Grun., | 69 |
| lĉvis Greg., | 66 |
| lineolata Ehr., | 66 |
| mucronata H.L.S., | 69 |
| obtusa Greg., | 67 |
| ocellata var. cingulata Cl., | 67 |
| ostrearia Bréb., | 66 |
| ovalis (Bréb.) Kuetz., | 65 |
| var. libyca (Ehr.) Cl., | 65 |
| var. pediculus (Kuetz.) Cl., | 65 |
| plicata Greg., | 66 |
| porcellus Kitton, | 66 |
| proteus Greg., | 65 |
| quadrata Bréb., | 66 |
| robusta Greg., | 65 |
| salina Wm. Sm., | 66 |
| vitrĉa Cl., | 66 |
| Anomœoneis, | 80 |
| follis (Ehr.) Cl., | 80 |
| serians Bréb., | 80 |
| sphĉrophora (Kuetz.) Cl., | 80 |
| Anorthoneis, | 56 |
| excentrica (Donk.) Grun., | 56 |
| Asterionella, | 50 |
| formosa Hass., | 50 |
| inflata Heib., | 50 |
| Attheya, | 38 |
| decora West, | 38 |
| Aulacodiscus, | 26 |
| argus (Ehr.) A.S., | 26 |
| Auliscus, | 28 |
| cĉlatus Bail., | 29 |
| pruinosus Bail., | 28 |
| punctatus Bail., | 28 |
| sculptus (Wm. Sm.) Ralfs, | 29 |
| spinosus Christian, | 29 |
| Auricula, | 69 |
| insecta (Grun.) Cl., | 69 |
| mucronata (H.L.S.) Per., | 69 |
| Bacillaria, | 119 |
| paradoxa Gmelin, | 119 |
| Biddulphia, | 31 |
| alternans (Bail.) V. H., | 33 |
| antediluviana (Ehr.) V. H., | 32 |
| biddulphiana (Smith), | 31 |
| favus (Ehr.) V. H., | 31 |
| granulata Roper, | 32 |
| lĉvis Ehr., | 33 |
| pulchella Gray., | 31 |
| reticulum (Ehr.), | 33 |
| rhombus (Ehr.) Wm. Sm., | 32 |
| smithii (Ralfs) V. H., | 32 |
| turgida (Ehr.) Wm. Sm., | 32 |
| Brébissonia, | 79 |
| bœckii (Kuetz.) Grun., | 79 |
| palmerii n. sp., | 80 |
|
Caloneis, |
81 |
| brevis var. vexans Grun., | 82 |
| formosa (Greg.) Cl., | 82 |
| liber (Wm. Sm.) Cl., | 81 |
| permagna (Bail.) Cl., | 82 |
| var. lewisiana n. var., | 82 |
| powellii (Lewis) Cl., | 83 |
| silicula (Ehr.) Cl., | 81 |
| var. inflata (Grun.) Cl., | 81 |
| trinodis (Lewis), | 81 |
| wardii Cl., | 82 |
| Campylodiscus, | 129 |
| argus Bail., | 130 |
| echeneis Ehr., | 130 |
| hibernicus Ehr., | 130 |
| Cerataulus | |
| smithii Ralfs, | 32 |
| turgidus Ehr., | 32 |
| Cocconeis, | 57 |
| dirupta Greg., | 58 |
| pediculus Ehr., | 57 |
| pellucida Grun., | 58 |
| placentula Ehr., | 57 |
| var. lineata (Ehr.) V. H., | 58 |
| scutellum Ehr., | 57 |
| var. ornata Grun., | 57 |
| Cocconema | |
| asperum Ehr., | 61 |
| Colletonema | |
| neglectum Thwaites, | 95 |
| vulgaris Thwaites, | 77 |
| Conferva | |
| biddulphiana Smith, | 31 |
| flocculosa Roth, | 36 |
| moniliformis Mueller, | 16 |
| nummuloides Dillw., | 16, 17 |
| rutilans Trentepohl, | 78 |
| Coscinodiscus, | 21 |
| argus Ehr., | 23 |
| asteromphalus Ehr., | 23 |
| var. omphalantha Grun., | 23 |
| biangulatus A. S., | 23 |
| denarius A. S., | 22 |
| excentricus Ehr., | 21 |
| var. perpusilla Grun., | 21 |
| lewisianus Grev., | 24 |
| lineatus Ehr., | 21 |
| marginatus Ehr., | 22 |
| minor Wm. Sm., | 14 |
| nitidulus Grun., | 21 |
| nitidus Greg., | 21 |
| oculus-iridus Ehr., | 23 |
| polyacanthus Grun., | 22 |
| radiatus Ehr., | 23 |
| striatus Kuetz., | 19 |
| subaulacodiscoidalis Rattr., | 23 |
| subtilis Ehr., | 21 |
| velatus Ehr., | 22 |
| Creswellia | |
| turris Grev., | 18 |
| Cyclotella, | 19 |
| antiqua Wm. Sm., | 20 |
| comta (Ehr.) Kuetz., | 20 |
| dallasiana Wm. Sm., | 19 |
| kuetzingiana Wm. Sm., | 19 |
| meneghiniana Kuetz., | 19 |
| var. stelligera Cl. and Grun., | 20 |
| var. stellulifera Cl. and Grun., | 20 |
| operculata (Ag.) Kuetz., | 20 |
| scotica Kuetz., | 18 |
| striata (Kuetz.) Grun., | 19 |
| stylorum (Br.?) V. H., | 20 |
| Cymatopleura, | 129 |
| elliptica (Bréb.) Wm. Sm., | 129 |
| marina Lewis, | 129 |
| solea (Bréb.) Wm. Sm., | 129 |
| Cymbella, | 60 |
| affinis Kuetz., | 61 |
| amphicephala Nĉgeli, | 61 |
| aspera (Ehr.) Cl., | 61 |
| cistula (Hempr.) Kirchn., | 62 |
| cuspidata Kuetz., | 60 |
| cymbiformis (Kuetz.) Bréb., | 62 |
| ehrenbergii Kuetz., | 60 |
| excisa (Kuetz.) De Toni, | 61 |
| gastroides Kuetz., | 61 |
| gracilis (Rab.) Cl., | 64 |
| heteropleura (Ehr.) Kuetz., | 60 |
| lacustris (Ag.) Cl., | 64 |
| lanceolata (Ehr.) Kirchn., | 62 |
| mexicana (Ehr.) A. S., | 62 |
| naviculiformis Auerswald, | 60 |
| parva (Wm. Sm.) Cl., | 61 |
| philadelphica n. sp., | 63 |
| prostrata (Berk.) Cl., | 63 |
| rhomboidea n. sp., | 63 |
| sinuata Greg., | 61 |
| triangulum (Ehr.) Cl., | 63 |
| tumida (Bréb.) V. H., | 62 |
| turgida (Greg.) Cl., | 63 |
| var.?, | 63 |
| ventricosa Kuetz., | 62 |
| Diatoma, | 41 |
| anceps (Ehr.) Kirchn., | 42 |
| arcuatum Lyng., | 35 |
| biddulphianum Ag., | 31 |
| hiemale (Lyng.) Heib., | 42 |
| marinum Lyng., | 37 |
| vulgare Bory., | 42 |
| var. elongatum (Ag.), | 42 |
| var. grande (Wm. Sm.) Grun., | 42 |
| Dictyoneis, | 78 |
| marginata var. commutata Cl., | 79 |
| var. maxima n. var., | 79 |
| var. typica Cl., | 78 |
| Dimerogramma, | 46 |
| marinum (Greg.) Ralfs, | 46 |
| minus (Greg.) Ralfs, | 47 |
| sinuatum Thwaites, | 119 |
| surirella (Ehr.) Grun., | 46 |
|
Diploneis, |
84 |
| campylodiscus (Grun.) Cl., | 86 |
| crabro Ehr. var.?, | 85 |
| var. expleta (A. S.) Cl., | 85 |
| var. pandura (Bréb.) Cl., | 85 |
| var. pandurella Cl.?, | 85 |
| elliptica (Kuetz.) Cl., | 84 |
| var. minutissima Grun., | 85 |
| excentrica n. sp., | 85 |
| fusca var. delicata (A. S.) Cl., | 85 |
| gemmata (Grev.) Cl., | 86 |
| gruendleri (A. S.) Cl., | 85 |
| oculata (Bréb.) Cl., | 86 |
| puella (Schum.) Cl., | 85 |
| smithii (Bréb.) Cl., | 84 |
| Ditylum, | 30 |
| intricatum (West) Grun., | 30 |
| Echinella | |
| circularis Grev., | 40 |
| flabellata Carm., | 39 |
| paradoxa Lyng., | 39 |
| Encyonema, | 62 |
| Epithemia, | 111 |
| argus Kuetz., | 111 |
| var.?, | 111 |
| gibba var. ventricosa Kuetz., | 113 |
| gibberula var. producta Grun., | 112 |
| marina Donk., | 114 |
| muelleri A. S.?, | 111 |
| musculus Kuetz., | 112 |
| var. constricta (Bréb.) V. H., | 112 |
| succincta Bréb., | 112 |
| turgida (Ehr.) Kuetz., | 111 |
| zebra var. proboscidea (Kuetz.) Grun., | 112 |
| Eunotia, | 51 |
| bactriana Ehr., | 54 |
| biceps Ehr., | 53 |
| bidentula Wm. Sm., | 54 |
| bigibba Greg., | 53 |
| formica Ehr. var.?, | 54 |
| gracilis (Ehr.) Rab., | 51 |
| hemicyclus (Ehr.) Ralfs, | 53 |
| incisa Greg., | 52 |
| luna Ehr., | 52 |
| lunaris (Ehr.) Grun., | 53 |
| major (Wm. Sm.) Rab., | 51 |
| nymanniana Grun., | 51 |
| pectinalis (Kuetz.), | 52 |
| var. solierolii (Kuetz.), | 52 |
| var. undulata Ralfs, | 52 |
| var. ventricosa Grun., | 52 |
| prĉrupta Ehr., | 53 |
| var. bidens Grun., | 53 |
| robusta Ralfs, | 53 |
| veneris Kuetz., | 52 |
| Eunotogramma, | 33 |
| lĉve Grun., | 33 |
| Euodia, | 34 |
| gibba Bail., | 34 |
| Eupodiscus, | 28 |
| argus (Ehr.) Wm. Sm., | 26 |
| radiatus Bail., | 28 |
| radiatus Wm. Sm., | 32 |
| Fragilaria, | 44 |
| arctica Grun., | 44 |
| capucina var. mesolepta Rab., | 45 |
| construens (Ehr.) Grun., | 45 |
| harrisonii (Wm. Sm.) Grun., | 45 |
| linearis Cstr., | 45 |
| parasitica (Wm. Sm.), | 45 |
| undata Wm. Sm., | 44 |
| virescens Ralfs, | 44 |
| Frustulia, | 77 |
| acuminata Kuetz., | 76 |
| interposita (Lewis) De Toni, | 78 |
| lewisiana (Grev.) De Toni, | 77 |
| rhomboides (Ehr.) De Toni, | 77 |
| var. amphipleuroides Grun., | 77 |
| var. saxonica Rab., | 77 |
| vulgaris (Thwaites) De Toni, | 77 |
| Gaillonella, | 16 |
| crenulata Ehr., | 15 |
| granulata Ehr., | 15 |
| moniliformis Bail., | 16 |
| nummuloides (Dillw.) Bory., | 16 |
| sulcata Ehr., | 15 |
| Glœonema, | 63 |
| triangulum Ehr., | 63 |
| Gomphoneis, | 70 |
| herculaneum (Ehr.) Cl., | 70 |
| mamilla (Ehr.) Cl., | 70 |
| Gomphonema, | 70 |
| acuminatum, | 71 |
| var. coronata (Ehr.) Cl., | 71 |
| var. trigonocephala (Ehr.) Cl., | 71 |
| var. turris (Ehr.) Cl., | 71 |
| var. turris (Ehr.) Cl.?, | 71 |
| ĉquale Greg., | 72 |
| angustatum Kuetz., | 72 |
| augur Ehr., | 72 |
| brasiliense var. demerarĉ Grun.?, | 73 |
| capitatum Ehr., | 72 |
| capitatum var. herculaneum Ehr., | 70 |
| constrictum Ehr., | 72 |
| geminatum Lyng., | 71 |
| insigne Greg., | 71 |
| intricatum Kuetz., | 72 |
| lanceolatum var. insignis (Greg.) Cl., | 71 |
| montanum Schum., | 71 |
| olivaceum Lyng., | 73 |
| parvulum var. micropus (Kuetz.) Cl., | 73 |
| sarcophagus Greg., | 72 |
| sphĉrophorum Ehr., | 72 |
| subclavatum var. montana Schum., | 71 |
| tinctum Ag., | 40 |
| ventricosum Greg., | 73 |
| Grammatophora, | 36 |
| angulosa var. hamulifera (Kuetz.) Grun., | 37 |
| islandica Ehr., | 37 |
|
marina (Lyng.) Kuetz., |
37 |
| var. subtilissima (Bail.) V. H., | 37 |
| serpentina Ralfs, | 37 |
| subtilissima Bail., | 37 |
| Gyrosigma, | 75 |
| acuminatum (Kuetz.) Cl., | 76 |
| attenuatum (Kuetz.) Cl., | 75 |
| balticum (Ehr.) Cl., | 75 |
| var. similis (Grun.) Cl., | 76 |
| fasciola (Ehr.) Cl., | 77 |
| hippocampus (Ehr.), | 75 |
| kuetzingii (Grun.) Cl., | 76 |
| parkeri var. stauroneioides Grun., | 75 |
| prolongatum (Wm. Sm.) Cl., | 76 |
| scalproides (Rab.) Cl., | 76 |
| simile (Grun.), | 76 |
| spencerii var. nodifera Grun., | 76 |
| strigilis (Wm. Sm.) Cl., | 76 |
| Hantzschia, | 113 |
| amphioxys (Ehr.) Grun., | 113 |
| var. major Grun., | 114 |
| marina (Donk.) Grun., | 114 |
| virgata (Roper) Grun., | 114 |
| Himantidium | |
| pectinate Kuetz., | 52 |
| Homœocladia, | 123 |
| filiformis Wm. Sm., | 123 |
| Hyalodiscus, | 17 |
| radiatus var. arctica Grun., | 17 |
| scoticus (Kuetz.) Grun., | 18 |
| stelliger Bail., | 17 |
| subtilis Bail., | 18 |
| Licmophora, | 38 |
| baileyi (Ehr.) Grun., | 40 |
| ehrenbergii (Kuetz.) Grun., | 40 |
| flabellata (Carm.) Ag., | 39 |
| gracilis (Ehr.) Grun., | 39 |
| var. elongata (Kuetz.) De Toni, | 39 |
| lyngbyei (Kuetz.) Grun., | 40 |
| ovulum Mer., | 39 |
| paradoxa (Lyng.) Ag., | 39 |
| splendida Wm. Sm., | 39 |
| tincta (Ag.) Grun., | 40 |
| Lysigonium, | 16 |
| moniliforme (Muell.) Link, | 16 |
| nummuloides (Lyng.) O'Meara, | 17 |
| varians (Ag.) De Toni, | 17 |
| Mastogloia, | 86 |
| angulata Lewis, | 87 |
| apiculata Wm. Sm., | 87 |
| braunii Grun., | 87 |
| elegans Lewis, | 87 |
| exigua Lewis, | 87 |
| kinsmanii Lewis, | 87 |
| lanceolata Thwaites, | 87 |
| smithii Thwaites, | 87 |
| Meloseira, | 14 |
| borreri Grev., | 17 |
| crenulata (Ehr.) Kuetz., | 15 |
| distans (Ehr.) Kuetz., | 14 |
| gowenii A. S., | 15 |
| granulata (Ehr.) Ralfs, | 15 |
| nivalis Wm. Sm., | 14 |
| nummuloides Ag., | 16 |
| roeseana Rab., | 15 |
| var. epidendron (Ehr.) Grun., | 15 |
| sulcata Kuetz., | 15 |
| undulata (Ehr.) Kuetz., | 15 |
| varians Ag., | 17 |
| Meridion, | 40 |
| circulare (Grev.) Ag., | 40 |
| constrictum Ralfs, | 41 |
| Micromega | |
| ramosissimum Ag., | 95 |
| Navicula, | 89 |
| affinis Ehr., | 83 |
| americana Ehr., | 98 |
| amphibola Cl., | 92 |
| amphigomphus Ehr., | 83 |
| anglica Ralfs, | 96 |
| angulata Quek., | 74 |
| ardua Mann, | 96 |
| arenaria Donk., | 95 |
| atomus Nĉgeli, | 100 |
| bacillum Ehr., | 98 |
| baltica Ehr., | 75 |
| brasiliensis var. bicuneata Cl., forma constricta, | 92 |
| crucigera (Wm. Sm.) Cl., | 100 |
| cryptocephala Kuetz., | 97 |
| cuspidata Kuetz., | 100 |
| var. ambigua (Ehr.) Cl., | 100 |
| cyprinus (Wm. Sm.), | 95 |
| delawarensis Grun., | 92 |
| dicephala Wm. Sm., | 96 |
| digito-radiata var. cyprinus (Ehr.?) Wm. Sm., | 95 |
| elegans Wm. Sm., | 101 |
| var. cuspidata Cl., | 101 |
| firma Kuetz., | 84 |
| fischeri A. S., | 90 |
| follis Ehr., | 80 |
| fuchsii Pant., | 91 |
| gastrum Ehr., | 96 |
| gigas A. S., | 103 |
| globiceps Lagerstedt, | 96 |
| gracilis var. schizonemoides (Ehr.) V. H., | 95 |
| grevillei (Ag.) Cl., | 99 |
| hasta Pant., | 97 |
| var. punctata n. var., | 97 |
| hennedyi Wm. Sm., | 93 |
| var. circumsecta Grun., | 93 |
| var. manta A. S., | 93 |
| hippocampus Ehr., | 75 |
| hitchcockii Ehr., | 84 |
| humerosa Bréb., | 91 |
| var. elongata Pant., | 91 |
| var. fuchsii (Pant.) Cl., | 91 |
|
humilis Donk., |
96 |
| hungarica var. capitata (Ehr.) Cl., | 96 |
| inflexa Greg., | 96 |
| integra Wm. Sm., | 99 |
| interposita Lewis, | 78 |
| iridis Ehr., | 84 |
| irrorata Grev., | 93 |
| lacustris Greg., | 92 |
| lanceolata var. arenaria (Donk.) Cl., | 95 |
| latissima Greg., | 90 |
| var. elongata (Pant.) Cl., | 91 |
| libellus Greg., | 99 |
| limosa Donk., | 81 |
| longa (Greg.) Ralfs, | 97 |
| lyra Ehr., | 93 |
| var. dilatata A. S., | 93 |
| var. ehrenbergii Cl., | 93 |
| var.?, | 93 |
| maculata (Bail.) Cl., | 90 |
| marginata Lewis, | 78 |
| marina Ralfs, | 92 |
| minima Grun., | 98 |
| mormonorum Grun., | 107 |
| mutica Kuetz., | 97 |
| oblonga Kuetz., | 97 |
| oculata Bréb., | 86 |
| palpebralis Bréb., | 101 |
| pennata A. S., | 96 |
| peregrina Ehr., | 94 |
| pinnata Pant.?, | 96 |
| placenta Ehr., | 94 |
| prĉtexta Ehr., | 92 |
| producta Wm. Sm., | 83 |
| punctata var. asymmetrica Lagerstedt, | 92 |
| punctulata Wm. Sm., | 92 |
| pupula var. bacillarioides Grun., | 98 |
| pusilla Wm. Sm., | 91 |
| var. subcapitata n. var., | 91 |
| pygmĉa Kuetz., | 94 |
| radiosa Kuetz., | 94 |
| ramosissima (Ag.) Cl., | 95 |
| rectangulata Greg., | 110 |
| reinhardtii Grun., | 95 |
| rhyncocephala Kuetz., | 97 |
| salinarum Grun., | 95 |
| semen Ehr., | 98 |
| silicula Ehr., | 81 |
| socialis Palmer, | 104 |
| spectabilis var. emarginata Cl., | 94 |
| sphĉrophora Kuetz., | 80 |
| spicula (Hickie) Cl., | 100 |
| trochus Kuetz., | 80 |
| tumida (Bréb.) Cl., | 99 |
| viridula var. rostellata Kuetz., | 95 |
| yarrensis Grun., | 101 |
| Neidium, | 83 |
| affine (Ehr.) Pfitzer, | 83 |
| var. amphirhyncus (Ehr.) Cl., | 83 |
| var. genuina forma maxima Cl., | 83 |
| var. genuina forma minor Cl., | 83 |
| amphigomphus (Ehr.) Pfitzer, | 83 |
| hitchcockii (Ehr.) Cl., | 84 |
| iridis (Ehr.) Cl., | 84 |
| productum (Wm. Sm.) Cl., | 83 |
| Nitzschia, | 114 |
| acicularis (Kuetz.) Wm. Sm., | 123 |
| acuminata (Wm. Sm.) Grun., | 117 |
| amphibis Grun., | 122 |
| amphioxys Wm. Sm., | 114 |
| apiculata (Greg.) Grun., | 117 |
| bilobata Wm. Sm., | 118 |
| circumsuta (Bail.) Grun., | 118 |
| clausii Hantzsch, | 121 |
| communis Rab., | 122 |
| compressa Bail., | 116 |
| var. minor H. L. S., | 116 |
| curvula Wm. Sm., | 121 |
| dissipata (Kuetz.) Grun., | 120 |
| dubia Wm. Sm., | 118 |
| epithemioides Grun., | 118 |
| fluminensis Grun., | 120 |
| granulata Grun., | 116 |
| insignis Greg., | 119 |
| intermedia Hantzsch, | 122 |
| linearis (Ag.) Wm. Sm., | 122 |
| litoralis var. delawarensis Grun., | 118 |
| longissima (Bréb.) Ralfs, | 123 |
| forma parva V. H., | 123 |
| macilenta Greg., | 120 |
| navicularis (Bréb.) Grun., | 116 |
| obtusa Wm. Sm., | 121 |
| var. flexella H. L. S., | 121 |
| var. scalpelliformis Grun., | 121 |
| palea (Kuetz.) Wm. Sm., | 122 |
| panduriformis Greg., | 117 |
| var. minor Grun., | 117 |
| paradoxa (Gmelin) Grun., | 119 |
| paxillifer (O. F. Mueller) Heib., | 119 |
| plana Wm. Sm., | 117 |
| punctata (Wm. Sm.) Grun., | 116 |
| reversa Wm. Sm., | 123 |
| scalaris (Ehr.) Wm. Sm., | 119 |
| sigma (Kuetz.) Wm. Sm., | 121 |
| var. curvula (Wm. Sm.) De Toni, | 121 |
| sigmatella Greg., | 121 |
| sinuata var. tabellaria (Grun.) V. H., | 119 |
| spathulata Bréb., | 120 |
| spectabilis var. americana Grun., | 122 |
| tabellaria Grun., | 119 |
| tryblionella Hantzsch, | 116 |
| vermicularis (Kuetz.) Hantzsch, | 120 |
| Odontidium | |
| parasiticum Wm. Sm., | 45 |
| tabellaria Wm. Sm., | 45 |
| Opephora, | 43 |
| pacifica (Grun.) Petit, | 43 |
| pinnata var. lanceolata n. var., | 44 |
| schwartzii (Grun.) Petit, | 43 |
|
Orthosira |
|
| orichalcea Wm. Sm., | 15 |
| punctata Wm. Sm., | 15 |
| spinosa Grev., | 15 |
| Paralia | |
| marina Heib., | 15 |
| sulcata (Ehr.) Cl., | 15 |
| Pinnularia, | 101 |
| acrosphĉria (Bréb.) Cl., | 108 |
| var. turgidula Grun.?, | 108 |
| ĉstuarii Cl., | 105 |
| appendiculata (Ag.) Cl., | 106 |
| blandita n. sp., | 108 |
| borealis Ehr., | 109 |
| var. scalaris (Ehr.) Cl., | 109 |
| braunii Grun., | 106 |
| brébissonii (Kuetz.) Cl., | 107 |
| cardinaliculus Cl., | 107 |
| cyprinus Wm. Sm., | 95 |
| dactylus Ehr., | 103 |
| var. dariana (A. S.) Cl., | 103 |
| var. demerarĉ Cl., | 103 |
| divergens var. elliptica Grun., | 107 |
| gentilis (Donk.) Cl., | 103 |
| gibba (Kuetz.) V. H., | 109 |
| interrupta forma stauroneiformis Cl., | 106 |
| lata (Bréb.) Wm. Sm., | 109 |
| legumen Ehr., | 107 |
| var.?, | 107 |
| leptosoma Grun., | 105 |
| major (Kuetz.) Wm. Sm., | 102 |
| var. pulchella n. var., | 102 |
| mesogongyla (Ehr.) Cl., | 109 |
| mesolepta Ehr., | 105 |
| var. stauroneiformis Grun., | 105 |
| microstauron (Ehr.) Cl., | 106 |
| molaris (Grun.) Cl., | 105 |
| mormonorum Grun., | 107 |
| nobilis Ehr., | 103 |
| nodosa forma capitata Cl., | 108 |
| parva (Ehr.) Cl., | 108 |
| permagna Bail., | 82 |
| polyonca (Bréb.) Lewis, | 108 |
| rectangulata (Greg.) Cl., | 110 |
| socialis (Palmer), | 104 |
| stauroptera (Grun.) Cl., | 110 |
| var. interrupta forma stauroneiformis Cl., | 110 |
| stomatophora (Grun.) Cl., | 109 |
| subcapitata Greg., | 105 |
| var. paucistriata Grun., | 106 |
| tabellaria (Ehr.) Cl., | 110 |
| termes (Ehr.) A. S., | 106 |
| var. stauroneiformis V. H., | 106 |
| trigonocephala Cl., | 103 |
| viridis Nitzsch, | 104 |
| var. caudata n. var., | 104 |
| var. fallax Cl., | 104 |
| var.?, | 104 |
| Plagiogramma, | 42 |
| obesum Grev., | 43 |
| pygmĉum Grev., | 43 |
| tessellatum Grev., | 43 |
| wallichianum Grev., | 43 |
| Pleurosigma, | 73 |
| ĉstuarii Bréb., | 74 |
| affine var. fossilis Grun., | 74 |
| angulatum (Quekett) Cl., | 74 |
| balticum (Ehr.) Wm. Sm., | 75 |
| formosum Wm. Sm., | 73 |
| hippocampus (Ehr.) Wm. Sm., | 75 |
| naviculaceum Bréb., | 74 |
| normanii var. fossilis Grun., | 74 |
| obscurum Wm. Sm., | 74 |
| rigidum Wm. Sm., | 75 |
| simile Grun., | 76 |
| spencerii var. acutiuscula Grun., | 76 |
| var. kuetzingii Grun., | 76 |
| strigosum Wm. Sm., | 74 |
| virginiacum H. L. S., | 74 |
| Podocystis, | 128 |
| adriatica Kuetz., | 129 |
| americana Bail., | 129 |
| Podosira | |
| hormoides Wm. Sm., | 18 |
| maculata Wm. Sm., | 17 |
| Podosphenia | |
| baileyi (Edw.) Lewis, | 40 |
| ehrenbergii Kuetz., | 40 |
| lyngbyei Kuetz., | 40 |
| Polymyxus, | 25 |
| coronalis L. W. Bail., | 25 |
| Pseudauliscus, | 29 |
| radiatus (Bail.) Rattr., | 29 |
| spinosus (Christian) Rattr., | 29 |
| Pyxidicula, | 19 |
| compressa Bail., | 116 |
| var. minor H. L. S., | 116 |
| cruciata Ehr., | 19 |
| radiata O'Meara, | 17 |
| Rhabdonema, | 35 |
| adriaticum Kuetz., | 36 |
| arcuatum (Lyng.) Kuetz., | 35 |
| minutum Kuetz., | 36 |
| Rhaphoneis, | 46 |
| amphiceros Ehr., | 46 |
| var. rhombica Grun., | 46 |
| belgica var. intermedia Grun., | 46 |
| Rhipidophora | |
| elongata Kuetz., | 39 |
| paradoxa Kuetz., | 39 |
| Rhoicosphenia, | 56 |
| curvata (Kuetz.) Grun., | 56 |
| Rhopalodia, | 112 |
| gibba (Kuetz.) Mueller, | 112 |
| ventricosa (Kuetz.) Mueller, | 113 |
| Schizonema | |
| cruciger Wm. Sm., | 100 |
| dillwynii Wm. Sm., | 78 |
|
grevillei Ag., |
99 |
| smithii Kuetz., | 95 |
| Scoliopleura | |
| tumida (Bréb.) V. H., | 99 |
| Scoliotropis, | 69 |
| latestriata var. amphora Cl., | 69 |
| Stauroneis, | 88 |
| acuta Wm. Sm., | 89 |
| americana A. S., | 89 |
| anceps Ehr., | 88 |
| var. amphicephala (Kuetz.) Cl., | 88 |
| var. gracilis (Ehr.) Cl., | 88 |
| crucicula (Grun.) Cl., | 89 |
| exilis Kuetz., | 59 |
| frickei var. angusta n. var., | 88 |
| legumen Ehr., | 89 |
| maculata Bail., | 90 |
| phœnicenteron Ehr., | 88 |
| salina Wm. Sm., | 89 |
| smithii Grun., | 89 |
| spicula Hickie, | 100 |
| Staurosira | |
| construens Ehr., | 45 |
| Stephanopyxis, | 18 |
| appendiculata Ehr., | 18 |
| corona (Ehr.) Grun., | 18 |
| turris (Grev.) Ralfs, | 18 |
| Striatella, | 37 |
| interrupta (Ehr.) Heib., | 38 |
| unipunctata (Lyng.) Ag., | 38 |
| Surirella, | 124 |
| amphioxys Wm. Sm., | 124 |
| anceps Lewis, | 128 |
| angusta Kuetz., | 127 |
| arctissima A. S., | 128 |
| bifrons Ehr., | 124 |
| biseriata (Ehr.) Bréb., | 124 |
| cardinalis Kitton, | 126 |
| circumsuta Bail., | 118 |
| cruciata A. S., | 127 |
| crumena Bréb., | 126 |
| davidsonii A. S., | 126 |
| delicatissima Lewis, | 128 |
| diaphana Bleisch, | 125 |
| elegans Ehr., | 125 |
| fastuosa Ehr., | 127 |
| febigerii Lewis, | 128 |
| gemma Ehr., | 125 |
| gracilis Grun., | 127 |
| guatimalensis Ehr., | 126 |
| intermedia Lewis, | 128 |
| linearis Wm. Sm., | 124 |
| mœlleriana Grun., | 124 |
| oblonga Ehr.?, | 127 |
| ovalis Bréb., | 126 |
| var. pinnata (Wm. Sm.) De Toni, | 126 |
| panduriformis Wm. Sm., | 126 |
| pinnata Wm. Sm., | 126 |
| var. minuta Grun., | 126 |
| recedens A. S., | 127 |
| robusta Ehr., | 124 |
| splendida (Ehr.) Kuetz., | 125 |
| striatula Turpin, | 125 |
| tenera Greg., | 125 |
| Synedra, | 47 |
| acus Kuetz., | 48 |
| affinis Kuetz., | 50 |
| var. parva (Kuetz.) V. H., | 50 |
| var. tabulata (Ag.) V. H., | 50 |
| biceps (Kuetz.) A. S., | 48 |
| capitata Ehr., | 48 |
| danica Kuetz., | 48 |
| fulgens (Grev.) Wm. Sm., | 50 |
| goulardi Bréb., | 48 |
| gracilis Kuetz., | 50 |
| oxyrhynchus var. undulata Grun., | 48 |
| pulchella (Ralfs) Kuetz., | 48 |
| var. abnormis Macchiati?, | 48 |
| var. flexella n. var., | 49 |
| radians Kuetz., | 49 |
| ulna (Nitzsch) Ehr., | 47 |
| vaucheriĉ var. parvula (Kuetz.) Rab., | 49 |
| Tabellaria, | 36 |
| fenestrata (Lyng.), | 36 |
| flocculosa (Roth) Kuetz., | 36 |
| Terpsinoë, | 34 |
| americana (Bail.) Ralfs, | 34 |
| novĉ-cĉsareĉ Boyer, | 34 |
| Tessella | |
| interrupta Ehr., | 38 |
| Trachyneis, | 79 |
| aspera var. intermedia Grun., | 79 |
| Trachysphenia, | 47 |
| australis Petit, | 47 |
| Triceratium | |
| alternans Bail., | 33 |
| favus Ehr., | 31 |
| obtusum Br., | 33 |
| pileotus Ehr., | 30 |
| punctatum Br., | 33 |
| sculptum Shad., | 33 |
| Trinacria, | 30 |
| pileolus (Ehr.) Grun., | 30 |
| Tripodiscus | |
| argus Ehr., | 26 |
| Tropidoneis, | 68 |
| lepidoptera (Greg.) Cl., | 69 |
| Tryblionella | |
| punctata Wm. Sm., | 116 |
| scutellum Wm. Sm., | 118 |
| Vibrio | |
| paxillifer O. F. Mueller, | 119 |
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| MELOSEIRA | ||
| 3-4 | Meloseira roeseana var. epidendron (Ehr.) Grun. | 15 |
| 5-6 | Meloseira roeseana Rab. | 15 |
| 8-9 | Meloseira distans (Ehr.) Kuetz. | 14 |
| 10 | Meloseira granulata (Ehr.) Ralfs | 15 |
| 11-12 | Meloseira sulcata Kuetz. | 15 |
| 15-16-17 | Meloseira undulata (Ehr.) Kuetz. | 15 |
| GAILLONELLA | ||
| 13-14 | Gaillonella nummuloides (Dillw.) Bory | 16 |
| LYSIGONIUM | ||
| 7 | Lysigonium moniliforme (Muell.) Link. | 16 |
| 18-19 | Lysigonium varians (Ag.) De Toni | 17 |
| HYALODISCUS | ||
| 20 | Hyalodiscus scoticus (Kuetz.) Grun. | 18 |
| 21 | Hyalodiscus radiatus var. arctica Grun. | 17 |
| 22 | Hyalodiscus stelliger Bail. | 17 |
| Note.—The figures in all of the plates, except when otherwise noted, are magnified 800 diameters. | ||
 |
||
|
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| STEPHANOPYXIS | ||
| 1-2 | Stephanopyxis turris (Grev.) Ralfs | 18 |
| 3 | Stephanopyxis corona (Ehr.) Grun. | 18 |
| CYCLOTELLA | ||
| 4 | Cyclotella meneghiniana var. stelligera Cl. and Grun. | 20 |
| 5-6 | Cyclotella operculata (Ag.) Kuetz. | 20 |
| 7 | Cyclotella comta (Ehr.) Kuetz. | 20 |
| 8 | Cyclotella meneghiniana Kuetz. | 19 |
| 9 | Cyclotella striata (Kuetz.) Grun. | 19 |
| 10 | Cyclotella stylorum (Br.?) V. H. | 20 |
| 11 | Cyclotella antiqua Wm. Sm. | 20 |
| 12 | Cyclotella meneghiniana var. stellulifera Cl. and Grun. | 20 |
| COSCINODISCUS | ||
| 13 | Coscinodiscus denarius A. S. | 22 |
| 14 | Coscinodiscus excentricus Ehr. | 21 |
| 15-17 | Coscinodiscus subtilis Ehr. | 21 |
| 16 | Coscinodiscus asteromphalus Ehr. | 23 |
| 18 | Coscinodiscus nitidus Greg. | 21 |
| 19 | Coscinodiscus nitidulus Grun. | 21 |
| 20 | Coscinodiscus excentricus var. perpusilla Grun. ? | 21 |
 |
||
|
 |
|||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| COSCINODISCUS—Continued | ||
| 1-11 | Coscinodiscus radiatus Ehr. | 23 |
| 2 | Coscinodiscus velatus Ehr. | 22 |
| 3 | Coscinodiscus biangulatus A. S. | 23 |
| 4 | Coscinodiscus subaulacodiscoidalis Rattr. | 23 |
| 5 | Coscinodiscus lewisianus Grev. | 24 |
| 7 | Coscinodiscus argus Ehr. | 23 |
| 8 | Coscinodiscus lineatus Ehr. | 21 |
| 9 | Coscinodiscus marginatus Ehr. | 22 |
| 10 | Coscinodiscus oculus-iridis Ehr. | 23 |
| ACTINOCYCLUS | ||
| 6 | Actinocyclus ellipticus var. delawarensis n. var. | 27 |
 |
||
|
 |
||||||||||||||||||||||||||||||
| Fig. | Page | |
| ACTINOPTYCHUS | ||
| 1-4-6 | Actinoptychus undulatus (Kuetz.) Ralfs. | 24 |
| 2 | Actinoptychus undulatus (inner stratum) | 24 |
| 3 | Actinoptychus heliopelta Grun. var.? | 25 |
| 5 | Actinoptychus vulgaris var. interrupta n. var. | 24 |
| POLYMYXUS | ||
| 7 | Polymyxus coronalis L. W. Bail. | 25 |
| AULACODISCUS | ||
| 8 | Aulacodiscus argus (Ehr.) A. S. | 26 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| EUODIA | ||
| 1 | Euodia gibba Bail. | 34 |
| POLYMYXUS | ||
| 2 | Polymyxus coronalis L. W. Bail., zone view | 25 |
| EUPODISCUS | ||
| 3 | Eupodiscus radiatus Bail. | 28 |
| AULISCUS | ||
| 4 | Auliscus cĉlatus Bail. | 29 |
| 5 | Auliscus sculptus (Wm. Sm.) Ralfs | 29 |
| 6 | Auliscus punctatus Bail. | 28 |
| 7 | Auliscus (intermediate form between A. cĉlatus and A. sculptus) | 29 |
| 8 | Auliscus pruinosus Bail. | 28 |
| PSEUDAULISCUS | ||
| 9 | Pseudauliscus radiatus (Bail.) Rattr. | 29 |
| 10 | Pseudauliscus spinosus (Christian) Rattr. | 29 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| ACTINOCYCLUS | ||
| 1 | Actinocyclus barkleyi var. aggregata Rattr. | 27 |
| 2 | Actinocyclus moniliformis Ralfs. | 27 |
| BIDDULPHIA | ||
| 3 | Biddulphia antediluviana (Ehr.) V. H. | 32 |
| 5 | Biddulphia reticulum (Ehr.) | 33 |
| 6 | Biddulphia favus (Ehr.) V. H. | 31 |
| 7-8 | Biddulphia alternans (Bail.) V. H. | 33 |
| TRINACRIA | ||
| 9 | Trinacria pileolus (Ehr.) Grun. | 30 |
| DITYLUM | ||
| 4 | Ditylum intricatum (West) Grun. | 30 |
| TERPSINOË | ||
| 10 | Terpsinoë americana (Bail.) Ralfs. | 34 |
| 11 | Terpsinoë novĉ-cĉsareĉ Boyer | 34 |
 |
||
|
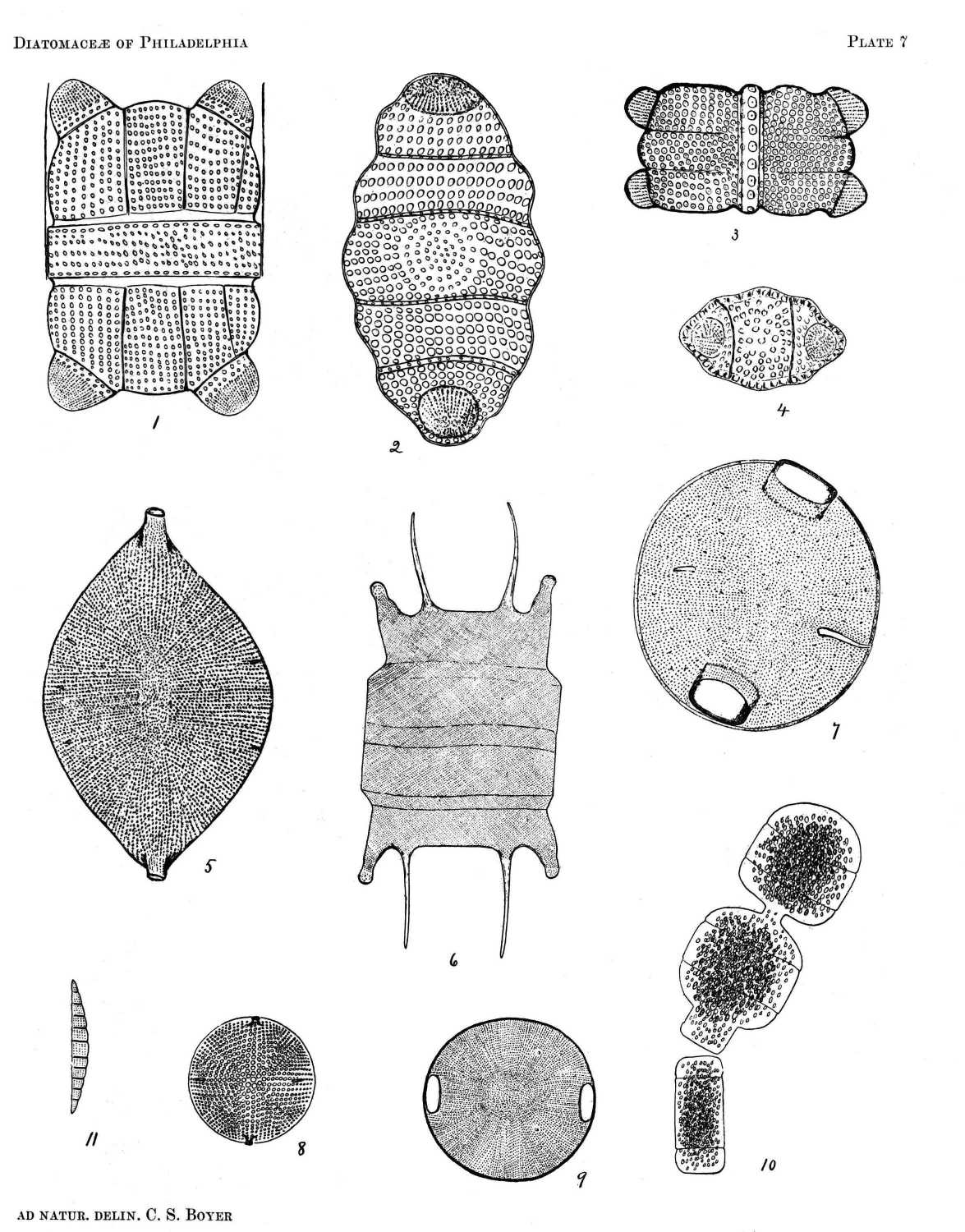 |
|||||||||||||||||||||||||||||||||
| Fig. | Page | |
| BIDDULPHIA | ||
| 1-2-3-4 | Biddulphia biddulphiana (Smith) | 31 |
| 5 | Biddulphia rhombus (Ehr.) Wm. Sm. | 32 |
| 6 | Biddulphia granulata Roper | 32 |
| 7 | Biddulphia turgida (Ehr.) Wm. Sm. | 32 |
| 8 | Biddulphia smithii (Ralfs) V. H. | 32 |
| 9 | Biddulphia lĉvis Ehr. | 33 |
| 10 | Biddulphia lĉvis Ehr. Sporangial frustules (260 diam.) | 33 |
| EUNOTOGRAMMA | ||
| 11 | Eunotogramma lĉve Grun. | 33 |
 |
||
|
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| RHABDONEMA | ||
| 1-2-3 | Rhabdonema arcuatum (Lyng.) Kuetz. | 35 |
| 4-5-6 | Rhabdonema adriaticum Kuetz. | 36 |
| 7 | Rhabdonema minutum Kuetz. | 36 |
| TABELLARIA | ||
| 8-9-10 | Tabellaria flocculosa (Roth) Kuetz. | 36 |
| 11-12 | Tabellaria fenestrata (Lyng.) Kuetz. | 36 |
| GRAMMATOPHORA | ||
| 13-14 | Grammatophora marina var. subtilissima (Bail.) V. H. | 37 |
| 15-16 | Grammatophora angulosa var. hamulifera (Kuetz.) Grun. | 37 |
| 17-18 | Grammatophora marina (Lyng.) Kuetz. | 37 |
| 19-20 | Grammatophora islandica Ehr. | 37 |
| 21 | Grammatophora serpentina Ralfs. | 37 |
| STRIATELLA | ||
| 22-23 | Striatella unipunctata (Lyng.) Ag. | 38 |
| 24 | Striatella interrupta (Ehr.) Heib. | 38 |
| ATTHEYA | ||
| 25 | Attheya decora West | 38 |
 |
||
|
 |
|||||||||||||||||||||||||||||||||
| Fig. | Page | |
| LICMOPHORA | ||
| 1-2 | Licmophora flabellata (Carm.) Ag. | 39 |
| 3-4 | Licmophora lyngbyei Kuetz. | 40 |
| 5 | Licmophora ehrenbergii (Kuetz.) Grun. | 40 |
| 6-7 | Licmophora paradoxa (Lyng.) Ag. | 39 |
| 8-9 | Licmophora ovulum Mer. | 39 |
| 10 | Licmophora baileyi (Edw.) Grun. | 40 |
| 11 | Licmophora gracilis (Ehr.) Grun. | 39 |
| 12-13 | Licmophora gracilis var. elongata (Kuetz.) De Toni | 39 |
| 14-15 | Licmophora tincta (Ag.) Grun. | 40 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| MERIDION | ||
| 1-2-3 | Meridion circulare (Grev.) Ag. | 40 |
| DIATOMA | ||
| 4 | Diatoma vulgare var. grande (Wm. Sm.) Grun. | 42 |
| 5-6 | Diatoma anceps (Ehr.) Kirchn. | 42 |
| 7-8 | Diatoma hiemale (Lyng.) Heib. | 42 |
| 9-10 | Diatoma vulgare Bory. | 42 |
| PLAGIOGRAMMA | ||
| 11 | Plagiogramma tessellatum Grev. | 43 |
| 12 | Plagiogramma obesum Grev. | 43 |
| 13 | Plagiogramma pygmĉum Grev. | 43 |
| 14 | Plagiogramma wallichianum Grev. | 43 |
| EUNOTOGRAMMA | ||
| 15 | Eunotogramma lĉve Grun. | 33 |
| OPEPHORA | ||
| 16-19 | Opephora schwartzii (Grun.) Petit. | 43 |
| 17 | Opephora pinnata var. lanceolata n. var. | 44 |
| 18 | Opephora pacifica (Grun.) Petit. | 43 |
| FRAGILARIA | ||
| 20-21 | Fragilaria virescens Ralfs. | 44 |
| 22-23 | Fragilaria arctica Grun. | 44 |
| 24-25-27-28-29 | Fragilaria undata Wm. Sm. | 44 |
| 26 | Fragilaria undata Wm. Sm., var.? | 44 |
| 30 | Fragilaria construens (Ehr.) Grun. | 45 |
| 31 | Fragilaria harrisonii (Wm. Sm.) Grun. | 45 |
| 34 | Fragilaria capucina var. mesolepta Rab. | 45 |
| 35 | Fragilaria parasitica (Wm. Sm.) | 45 |
| 36 | Fragilaria sp. ? | 45 |
| 37 | Fragilaria linearis Cstr. | 45 |
| RHAPHONEIS | ||
| 38 | Rhaphoneis amphiceros Ehr. | 46 |
| 39-40 | Rhaphoneis amphiceros var. rhombica Grun. | 46 |
| 41 | Rhaphoneis belgica var. intermedia Grun. | 46 |
| SYNEDRA | ||
| 32-33 | Synedra radians Kuetz. | 49 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| SYNEDRA—Continued | ||
| 1-5-6 | Synedra ulna (Nitzsch) Ehr. Sporangial | 47 |
| 2 | Synedra danica Kuetz. | 48 |
| 3 | Synedra biceps (Kuetz.) A. S. | 48 |
| 4-7-11 | Synedra ulna (Nitzsch) Ehr. | 47 |
| 8 | Synedra capitata Ehr. | 48 |
| 9-18 | Synedra acus Kuetz. | 48 |
| 10 | Synedra fulgens (Grev.) Wm. Sm. | 50 |
| 12-13 | Synedra goulardi Bréb. | 48 |
| 14-15-16 | Synedra pulchella (Ralfs) Kuetz. | 48 |
| 17 | Synedra pulchella var. abnormis Macchiati? | 48 |
 |
||
|
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| SYNEDRA—Continued | ||
| 1 | Synedra oxyrhynchus var. undulata Grun. | 48 |
| 2 | Synedra pulchella var. flexella n. var. | 49 |
| 3 | Synedra affinis Kuetz. | 50 |
| 4 | Synedra affinis var. tabulata (Ag.) V. H. | 50 |
| 5-6 | Synedra vaucheriĉ var. parvula (Kuetz.) Rab. | 49 |
| 7 | Synedra affinis var. parva (Kuetz.) V. H. | 50 |
| 8 | Synedra radians (Kuetz.) H. L. S. | 49 |
| DIMEROGRAMMA | ||
| 9-10 | Dimerogramma marinum (Greg.) | 46 |
| 11 | Dimerogramma surirella (Ehr.) Grun. | 46 |
| 12-13-14 | Dimerogramma minus (Greg.) Ralfs. | 47 |
| TRACHYSPHENIA | ||
| 15 | Trachysphenia australis Petit. | 47 |
| ACTINELLA | ||
| 16-17-18 | Actinella punctata Lewis. | 54 |
| ASTERIONELLA | ||
| 19-20-21 | Asterionella formosa Hass. | 50 |
| 22 | Asterionella inflata Heib. | 50 |
| EUNOTIA | ||
| 23 | Eunotia hemicyclus (Ehr.) Ralfs | 53 |
| 24-25 | Eunotia lunaris (Ehr.) Grun. | 53 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| EUNOTIA—Continued | ||
| 1-2 | Eunotia major (Wm. Sm.) Rab. | 51 |
| 3 | Eunotia gracilis (Ehr.) Rab. | 51 |
| 4 | Eunotia major (Wm. Sm.) Rab. (intermediate form) | 51 |
| 5 | Eunotia prĉrupta Ehr. | 53 |
| 6-7 | Eunotia pectinalis (Kuetz.) | 52 |
| 8-10 | Eunotia pectinalis var. undulata Ralfs | 52 |
| 9 | Eunotia pectinalis var. solierolii (Kuetz.) | 52 |
| 11 | Eunotia luna Ehr. var.? | 52 |
| 12 | Eunotia pectinalis var. ventricosa Grun. | 52 |
| 13 | Eunotia robusta Ralfs (E. scalaris Ehr.) | 53 |
| 14 | Eunotia robusta Ralfs (E. prioritis Ehr.) | 53 |
| 15 | Eunotia robusta Ralfs (E. decadon Ehr.) | 53 |
| 16 | Eunotia robusta Ralfs (E. octodon Ehr.) | 53 |
| 17-22 | Eunotia robusta Ralfs (E. heptodon Ehr.) | 53 |
| 18 | Eunotia bactriana Ehr. | 54 |
| 19 | Eunotia prĉrupta var. bidens Grun. | 53 |
| 20 | Eunotia bidentula Wm. Sm. | 54 |
| 21 | Eunotia robusta Ralfs (E. diadema Ehr.) | 53 |
| 23 | Eunotia prĉrupta Ehr. var.? | 53 |
| 24 | Eunotia robusta Ralfs (E. triodon Ehr.) | 53 |
| 25 | Eunotia robusta Ralfs (E. tetraodon Ehr.) | 53 |
| 26 | Eunotia formica Ehr. var.? | 54 |
| 27 | Eunotia biceps Ehr. | 53 |
| 28-29 | Eunotia sp.? | 54 |
| 30-31 | Eunotia veneris Kuetz. | 52 |
| 32 | Eunotia nymanniana Grun. | 51 |
 |
||
|
 |
|||||||||||||||||||||||||||||||||
| Fig. | Page | |
| AMPHIPRORA | ||
| 1-2 | Amphiprora pulchra Bail. | 68 |
| 3 | Amphiprora alata Kuetz. | 68 |
| 4 | Amphiprora conspicua Grev. | 68 |
| 5 | Amphiprora paludosa Wm. Sm. | 68 |
| 6-7 | Amphiprora ornata Bail. | 68 |
| TROPIDONEIS | ||
| 8-9 | Tropidoneis lepidoptera (Greg.) Cleve. | 69 |
| SCOLIOTROPIS | ||
| 10-11 | Scoliotropis latestriata var. amphora Cleve. | 69 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| AMPHORA | ||
| 1 | Amphora robusta Greg. | 65 |
| 3 | Amphora crassa Greg. | 65 |
| 4 | Amphora obtusa Greg. | 67 |
| 5-6-19 | Amphora proteus Greg. | 65 |
| 7 | Amphora ovalis (Bréb.) Kuetz. | 65 |
| 8-18 | Amphora coffĉiformis (Ag.) Kuetz. | 66 |
| 9-10 | Amphora lineolata Ehr. | 66 |
| 11 | Amphora areolata Grun. | 66 |
| 12-21 | Amphora ostrearia Bréb. | 66 |
| 13 | Amphora lĉvis Greg. | 66 |
| 14-15 | Amphora ocellata var. cingulata Cleve. | 67 |
| 16 | Amphora angusta var. culensteinii Grun. | 67 |
| 17 | Amphora arenaria Donk. | 67 |
| 20 | Amphora acuta Greg. | 66 |
| AURICULA | ||
| 2 | Auricula mucronata (H. L. Smith) Peragallo | 69 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| ACHNANTHES | ||
| 1-2 | Achnanthes longipes Ag. | 58 |
| 3 | Achnanthes brevipes Ag. | 59 |
| 4-5-6 | Achnanthes subsessilis Kuetz. | 59 |
| 7-8 | Achnanthes inflata (Kuetz.) Grun. | 59 |
| 9 | Achnanthes coarctata (Bréb.) Grun. | 59 |
| 10-11-12 | Achnanthes lanceolata (Bréb.) Grun. | 59 |
| 13 | Achnanthes danica (Floegel) Grun. (lower valve) | 60 |
| 14-15 | Achnanthes exigua Grun. | 59 |
| 16-17 | Achnanthes linearis forma curta H. L. Smith | 59 |
| COCCONEIS | ||
| 18 | Cocconeis scutellum var.? | 57 |
| 19-20 | Cocconeis placentula Ehr. | 57 |
| 21 | Cocconeis scutellum Ehr. (upper valve) | 57 |
| 22 | Cocconeis dirupta Greg. (lower valve) | 58 |
| 23-24 | Cocconeis pediculus Ehr. | 57 |
| 25-26 | Cocconeis pellucida Grun. | 58 |
| 27-28 | Cocconeis scutellum var. ornata Grun. | 57 |
| 29 | Cocconeis placentula var. lineata (Ehr.) V. H. | 58 |
| ANORTHONEIS | ||
| 30-31 | Anorthoneis excentrica (Donk.) Grun. | 56 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| FRUSTULIA | ||
| 1 | Frustulia lewisiana (Grev.) De Toni | 77 |
| 2 | Frustulia rhomboides (Ehr.) De Toni | 77 |
| 3 | Frustulia rhomboides var. amphipleuroides Grun. | 77 |
| 4 | Frustulia vulgaris (Thwaites) De Toni | 77 |
| 5 | Frustulia interposita (Lewis) De Toni | 78 |
| 6 | Frustulia rhomboides var. saxonica (Rab.) De Toni | 77 |
| BREBISSONIA | ||
| 7 | Brébissonia bœckii (Kuetz.) Grun. | 79 |
| 8 | Brébissonia palmerii n. sp. | 80 |
| AMPHIPLEURA | ||
| 9 | Amphipleura pellucida Kuetz. | 78 |
| 10-11 | Amphipleura rutilans (Trentepohl) Cl. | 78 |
| ANOMŒONEIS | ||
| 12 | Anomœoneis serians (Bréb.) Cl. | 80 |
| 13 | Anomœoneis serians forma minor | 80 |
| 14 | Anomœoneis follis (Ehr.) Cl. | 80 |
| TRACHYNEIS | ||
| 15 | Trachyneis aspera var. intermedia Grun. | 70 |
| MASTOGLOIA | ||
| 16 | Mastogloia kinsmanii Lewis | 87 |
| 17 | Mastogloia angulata Lewis | 87 |
| 18 | Mastogloia lanceolata Thwaites | 87 |
| 19 | Mastogloia smithii Thwaites | 87 |
| 20 | Mastogloia elegans Lewis | 87 |
| 21-22-23 | Mastogloia apiculata Wm. Sm. | 87 |
| 24 | Mastogloia exigua Lewis | 87 |
 |
||
|
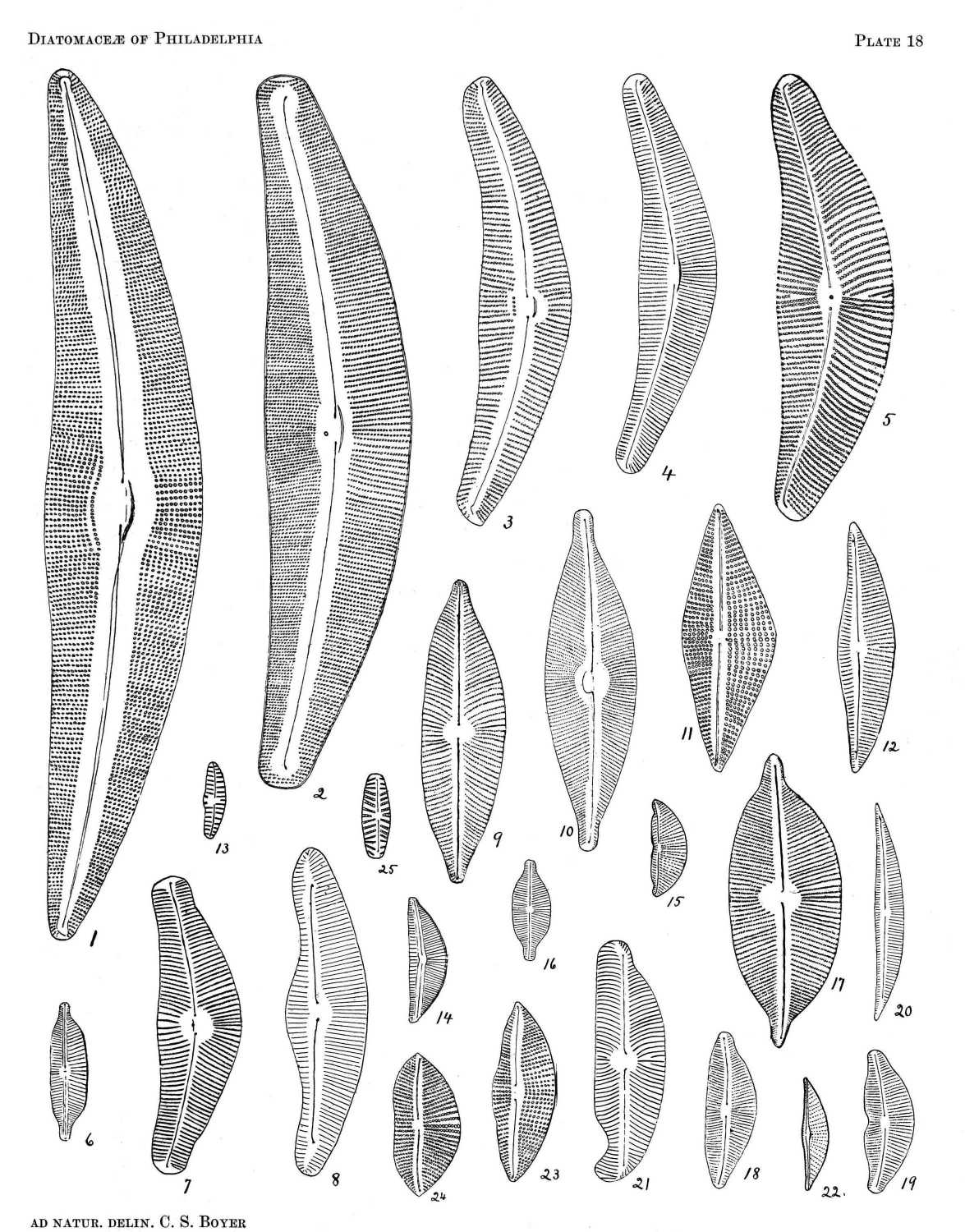 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| CYMBELLA | ||
| 1 | Cymbella aspera (Ehr.) Cl. | 61 |
| 2 | Cymbella cymbiformis (Kuetz.) Bréb. | 62 |
| 3 | Cymbella cistula (Hempr.) Kirchn. | 62 |
| 4 | Cymbella lanceolata (Ehr.) Kirchn. | 62 |
| 5 | Cymbella mexicana (Ehr.) A. S. | 62 |
| 6 | Cymbella naviculiformis Auerswald. | 60 |
| 7 | Cymbella tumida (Bréb.) V. H. | 62 |
| 8 | Cymbella philadelphica n. sp. | 63 |
| 9 | Cymbella ehrenbergii Kuetz. | 60 |
| 10 | Cymbella heteropleura (Ehr.) Kuetz. | 60 |
| 11 | Cymbella rhomboidea n. sp. | 63 |
| 12 | Cymbella turgida (Greg.) Cl. var.? | 63 |
| 13 | Cymbella sinuata Greg. | 61 |
| 14 | Cymbella ventricosa Kuetz. | 62 |
| 15-19 | Cymbella excisa (Kuetz.) De Toni. | 61 |
| 16 | Cymbella amphicephala Nĉgeli. | 61 |
| 17 | Cymbella cuspidata Kuetz. | 60 |
| 18 | Cymbella affinis Kuetz. | 61 |
| 20 | Cymbella gracilis (Rab.) Cl. | 64 |
| 21 | Cymbella prostrata (Berk.) Cl. | 63 |
| 22 | Cymbella ventricosa Kuetz.? | 62 |
| 23 | Cymbella turgida (Greg.) Cl. | 63 |
| 24 | Cymbella triangulum (Ehr.) Cl. | 63 |
| 25 | Cymbella lacustris (Ag.) Cl. | 64 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| GOMPHONEIS | ||
| 1 | Gomphoneis mamilla (Ehr.) Cl. | 70 |
| 2 | Gomphoneis herculaneum (Ehr.) Cl. | 70 |
| GOMPHONEMA | ||
| 3 | Gomphonema montanum Schum. | 71 |
| 4 | Gomphonema geminatum Lyng. | 71 |
| 5 | Gomphonema acuminatum var. turris (Ehr.) Cl. | 71 |
| 6-12 | Gomphonema lanceolatum var. insignis (Greg.) Cl. | 71 |
| 7 | Gomphonema acuminatum var. coronata (Ehr.) Cl. | 71 |
| 8 | Gomphonema constrictum Ehr. | 72 |
| 9-10 | Gomphonema sphĉrophorum Ehr. | 72 |
| 11 | Gomphonema acuminatum var. turris (Ehr.) Cl.? | 71 |
| 13 | Gomphonema ventricosum Greg. | 73 |
| 14 | Gomphonema intricatum Kuetz. | 72 |
| 15 | Gomphonema ĉquale Greg. | 72 |
| 16 | Gomphonema sarcophagus Greg. | 72 |
| 17 | Gomphonema parvulum var. micropus (Kuetz.) Cl. | 73 |
| 18-19 | Gomphonema angustatum Kuetz. | 72 |
| 20 | Gomphonema acuminatum var. trigonocephala (Ehr.) Cl. | 71 |
| 21 | Gomphonema augur Ehr. | 72 |
| 22 | Gomphonema capitatum Ehr. | 72 |
| 23 | Gomphonema olivaceum Lyng. | 73 |
| 24 | Gomphonema brasiliense var. demerarĉ Grun.? | 73 |
| RHOICOSPHENIA | ||
| 25-26-27 | Rhoicosphenia curvata (Kuetz.) Grun. | 56 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| DICTYONEIS | ||
| 1 | Dictyoneis marginata var. maxima n. var. | 79 |
| 2 | Dictyoneis marginata var. commutata Cleve. | 79 |
| 3 | Dictyoneis marginata var. typica Cleve. | 78 |
| DIPLONEIS | ||
| 4 | Diploneis crabro var. pandura (Bréb.) Cl. | 85 |
| 6 | Diploneis campylodiscus (Grun.) Cl. | 86 |
| 7-8 | Diploneis gruendleri (A. S.) Cl. | 85 |
| 9 | Diploneis crabro Ehr. var.? | 85 |
| 10 | Diploneis excentrica n. sp. | 85 |
| 11 | Diploneis fusca var. delicata (A. S.) Cl. | 85 |
| 12 | Diploneis puella (Schum.) Cl. | 85 |
| 13 | Diploneis crabro var. pandurella Cl.? | 85 |
| 14 | Diploneis elliptica (Kuetz.) Cl. | 84 |
| 15 | Diploneis crabro var. expleta (A. S.) Cl. | 85 |
| 16 | Diploneis geminata (Grev.) Cl. | 86 |
| 17 | Diploneis smithii (Bréb.) Cl. | 84 |
| NAVICULA | ||
| 5 | Navicula lyra Ehr. var.? | 93 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| CALONEIS | ||
| 1 | Caloneis permagna (Bail.) Cl. | 82 |
| 2 | Caloneis permagna var. lewisiana n. var. | 82 |
| 3 | Caloneis silicula (Ehr.) Cl. | 81 |
| 4 | Caloneis silicula var. inflata (Grun.) Cl. | 81 |
| 5 | Caloneis brevis var. vexans (Grun.) Cl. | 82 |
| 6-7 | Caloneis wardii Cl. | 82 |
| 8 | Caloneis trinodis (Lewis) | 81 |
| 9 | Caloneis trinodis (Lewis) var.? | 81 |
| 10 | Caloneis powellii (Lewis) Cl. | 83 |
| 18 | Caloneis formosa (Greg.) Cl. | 82 |
| NEIDIUM | ||
| 11 | Neidium affine (Ehr.) Pfitzer | 83 |
| 12 | Neidium affine var. genuina forma minor Cl. | 83 |
| 13 | Neidium affine var. amphirhyncus (Ehr.) Cl. | 83 |
| 14 | Neidium amphigomphus (Ehr.) Pfitzer. | 83 |
| 15 | Neidium hitchcockii (Ehr.) Cl. | 84 |
| 16 | Neidium productum (Wm. Sm.) Cl. | 83 |
| 17 | Neidium iridus (Ehr.) Cl. | 84 |
 |
||
|
 |
||||||||||||||||||||||||||||||
| Fig. | Page | |
| PLEUROSIGMA | ||
| 1 | Pleurosigma strigosum Wm. Sm. | 74 |
| 2 | Pleurosigma rigidum Wm. Sm. | 75 |
| 3 | Pleurosigma angulatum (Quekett) Cl. | 74 |
| 4 | Pleurosigma obscurum Wm. Sm. | 74 |
| 5 | Pleurosigma formosum Wm. Sm. | 73 |
| 6 | Pleurosigma naviculaceum Bréb. | 74 |
| 7 | Pleurosigma ĉstuarii Bréb. | 74 |
| 8 | Pleurosigma virginiacum H. L. Smith | 74 |
 |
||
|
 |
|||||||||||||||||||||||||||||||||
| Fig. | Page | |
| GYROSIGMA | ||
| 1 | Gyrosigma strigilis (Wm. Sm.) Cl. | 76 |
| 2 | Gyrosigma balticum (Ehr.) Cl. | 75 |
| 3 | Gyrosigma hippocampus (Ehr.) | 75 |
| 4 | Gyrosigma simile (Grun.) | 76 |
| 5 | Gyrosigma acuminatum (Kuetz.) Cl. | 76 |
| 6 | Gyrosigma scalproides (Rab.) Cl. | 76 |
| 7 | Gyrosigma parkeri var. stauroneioides Grun. | 75 |
| 8 | Gyrosigma spencerii var. nodifera Grun. | 76 |
| 9 | Gyrosigma fasciola (Ehr.) Cl. | 77 |
 |
||
|
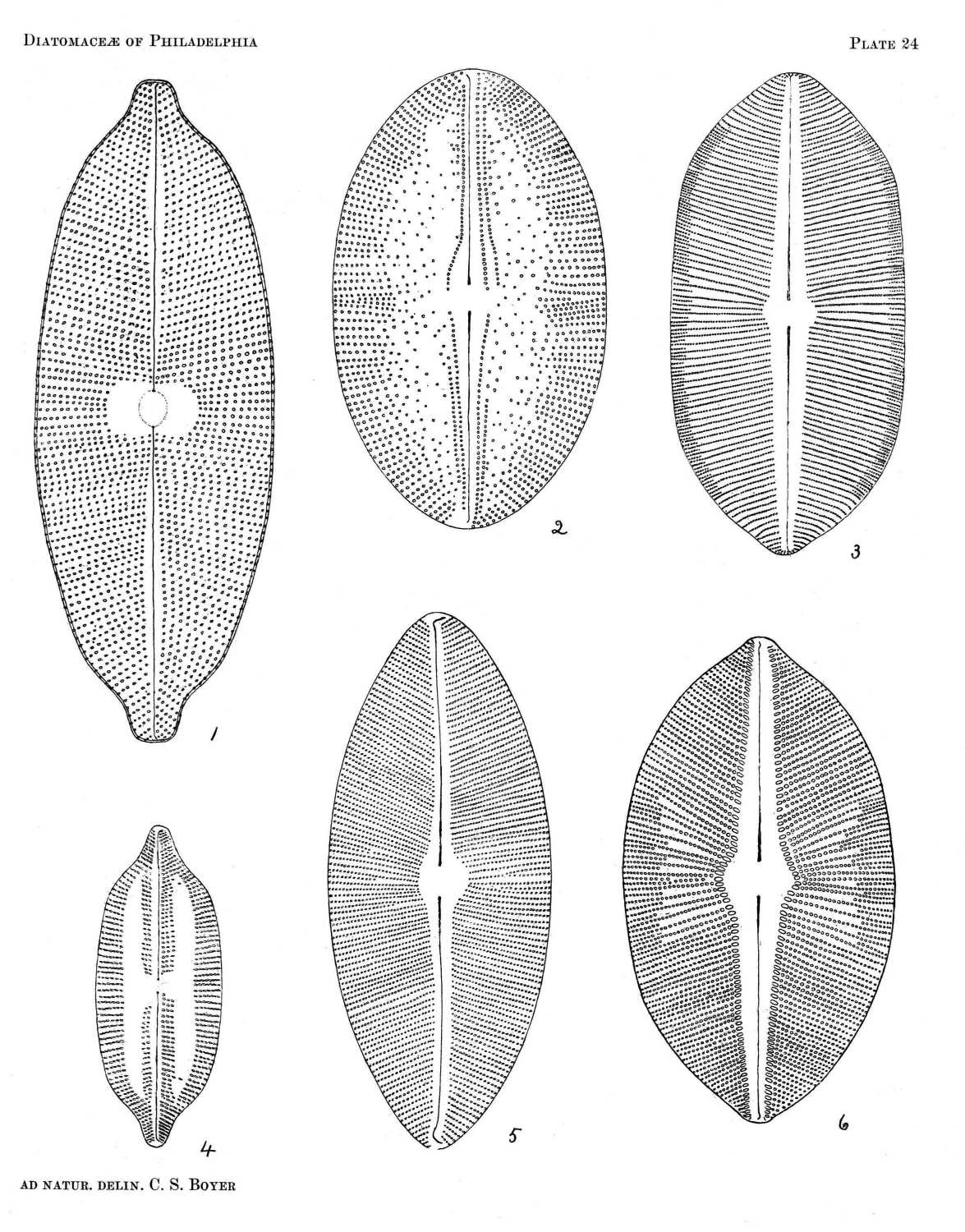 |
||||||||||||||||||||||||
| Fig. | Page | |
| NAVICULA | ||
| 1 | Navicula maculata (Bail.) Cl. | 90 |
| 2 | Navicula prĉtexta Ehr. | 92 |
| 3 | Navicula latissima Greg. | 90 |
| 4 | Navicula irrorata Grev. | 93 |
| 5 | Navicula latissima var. elongata (Pant.) Cl. | 91 |
| 6 | Navicula fuchsii Pant. | 91 |
 |
||
|
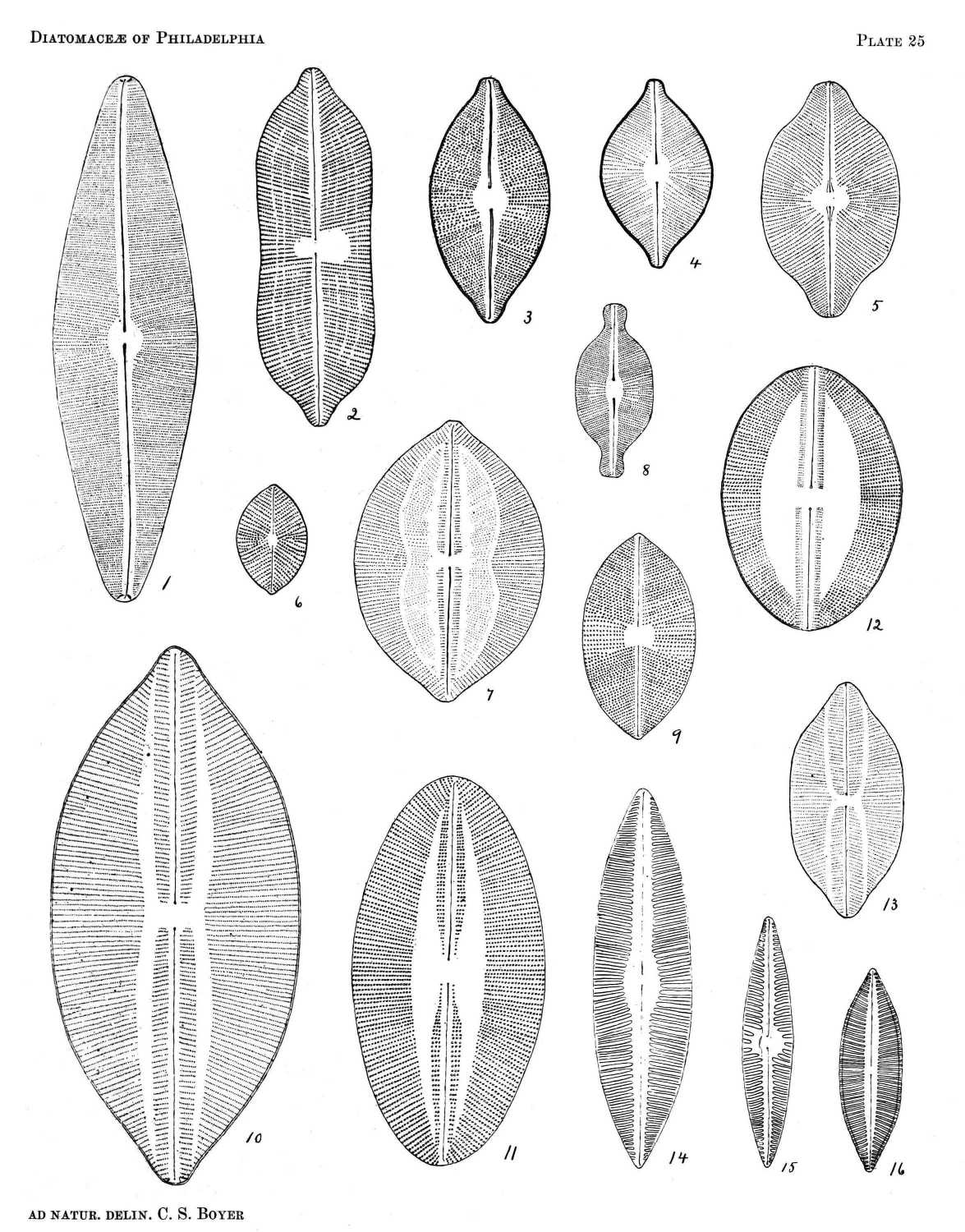 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| NAVICULA | ||
| 1 | Navicula tumida (Bréb.) Cl. | 99 |
| 2 | Navicula brasiliensis var. bicuneata Cl. forma constricta. | 92 |
| 3 | Navicula delawarensis Grun. | 92 |
| 4-6 | Navicula pusilla Wm. Sm. | 91 |
| 5 | Navicula humerosa Bréb. | 91 |
| 7 | Navicula spectabilis var. emarginata Cl. | 94 |
| 8 | Navicula pusilla var. subcapitata n. var. | 91 |
| 9 | Navicula punctulata Wm. Sm. | 92 |
| 10 | Navicula lyra Ehr. | 93 |
| 11 | Navicula hennedyi var. manca A. S. | 93 |
| 12 | Navicula hennedyi Wm. Sm. | 93 |
| 13 | Navicula lyra var. dilatata A. S. | 93 |
| 14 | Navicula yarrensis Grun. | 101 |
| 15 | Navicula yarrensis Grun. (smaller form) | 101 |
| 16 | Navicula yarrensis Grun. var.? | 101 |
 |
||
|
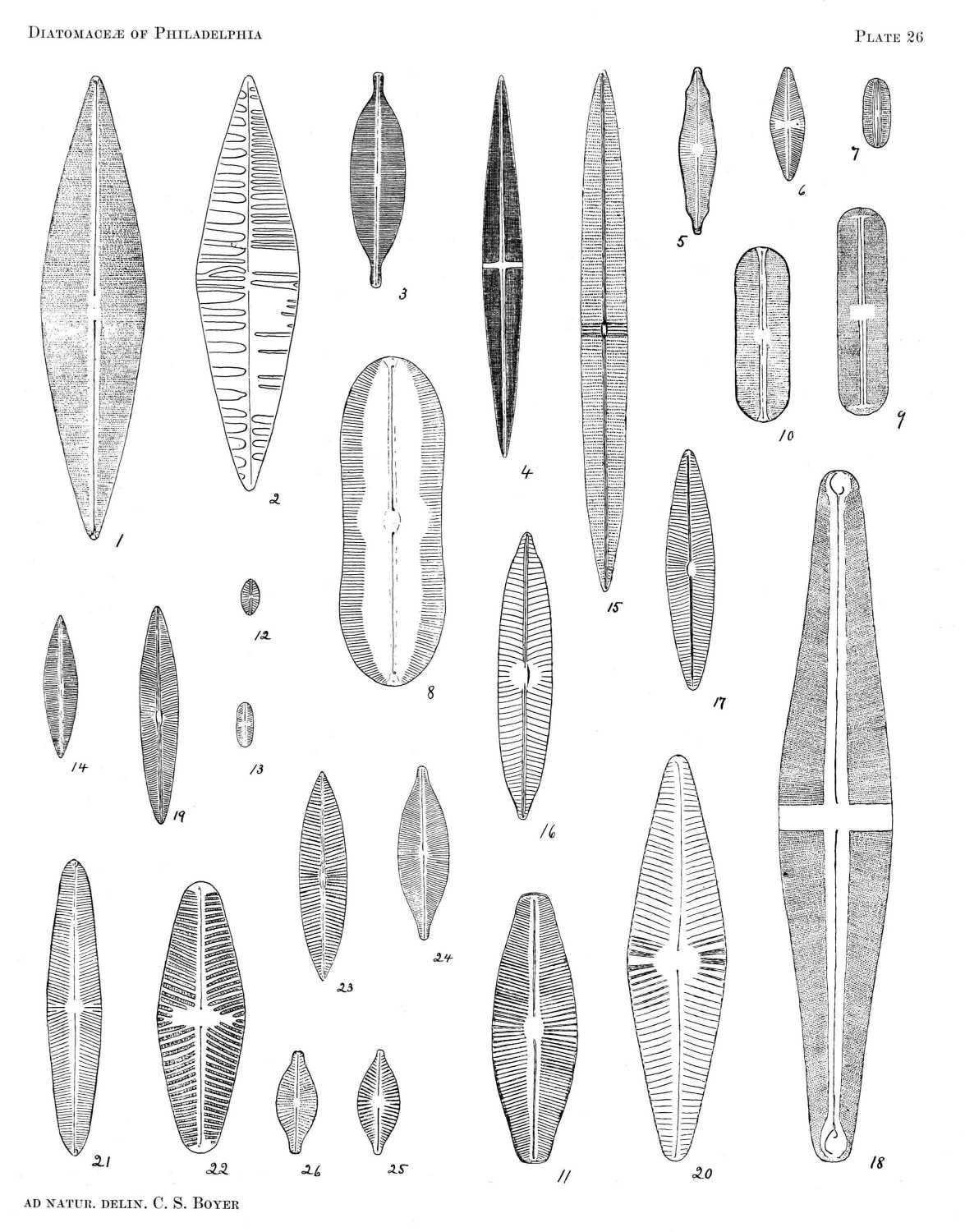 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| NAVICULA | ||
| 1-2 | Navicula cuspidata Kuetz. | 100 |
| 3 | Navicula cuspidata var. ambigua (Ehr.) Cl. | 100 |
| 4 | Navicula spicula (Hickie) Cl. | 100 |
| 5 | Navicula integra Wm. Sm. | 99 |
| 6 | Navicula mutica Kuetz. | 97 |
| 8 | Navicula americana Ehr. | 98 |
| 9 | Navicula pupula var. bacillarioides Grun. | 98 |
| 10 | Navicula bacillum Ehr. | 98 |
| 11 | Navicula semen Ehr. | 98 |
| 12 | Navicula atomus Nĉgeli. | 100 |
| 13 | Navicula minima Grun. | 98 |
| 14 | Navicula ramosissima (Ag.) Cl. | 95 |
| 15 | Navicula crucigera (Wm. Sm.) Cl. | 100 |
| 16 | Navicula viridula var. rostellata Kuetz. | 95 |
| 17 | Navicula radiosa Kuetz. | 94 |
| 19 | Navicula gracilis var. schizonemoides (Ehr.) V. H. | 95 |
| 20 | Navicula peregrina Ehr. | 94 |
| 21 | Navicula cyprinus (Wm. Sm.) | 95 |
| 22 | Navicula reinhardtii Grun. | 95 |
| 23 | Navicula lanceolata var. arenaria (Donk.) Cl. | 95 |
| 24 | Navicula salinarum Grun. | 95 |
| 25 | Navicula gastrum Ehr. | 96 |
| 26 | Navicula anglica Ralfs. | 96 |
| DIPLONEIS | ||
| 7 | Diploneis oculata (Bréb.) Cl. | 86 |
| STAURONEIS | ||
| 18 | Stauroneis frickei var. angusta n. var. | 88 |
 |
||
|
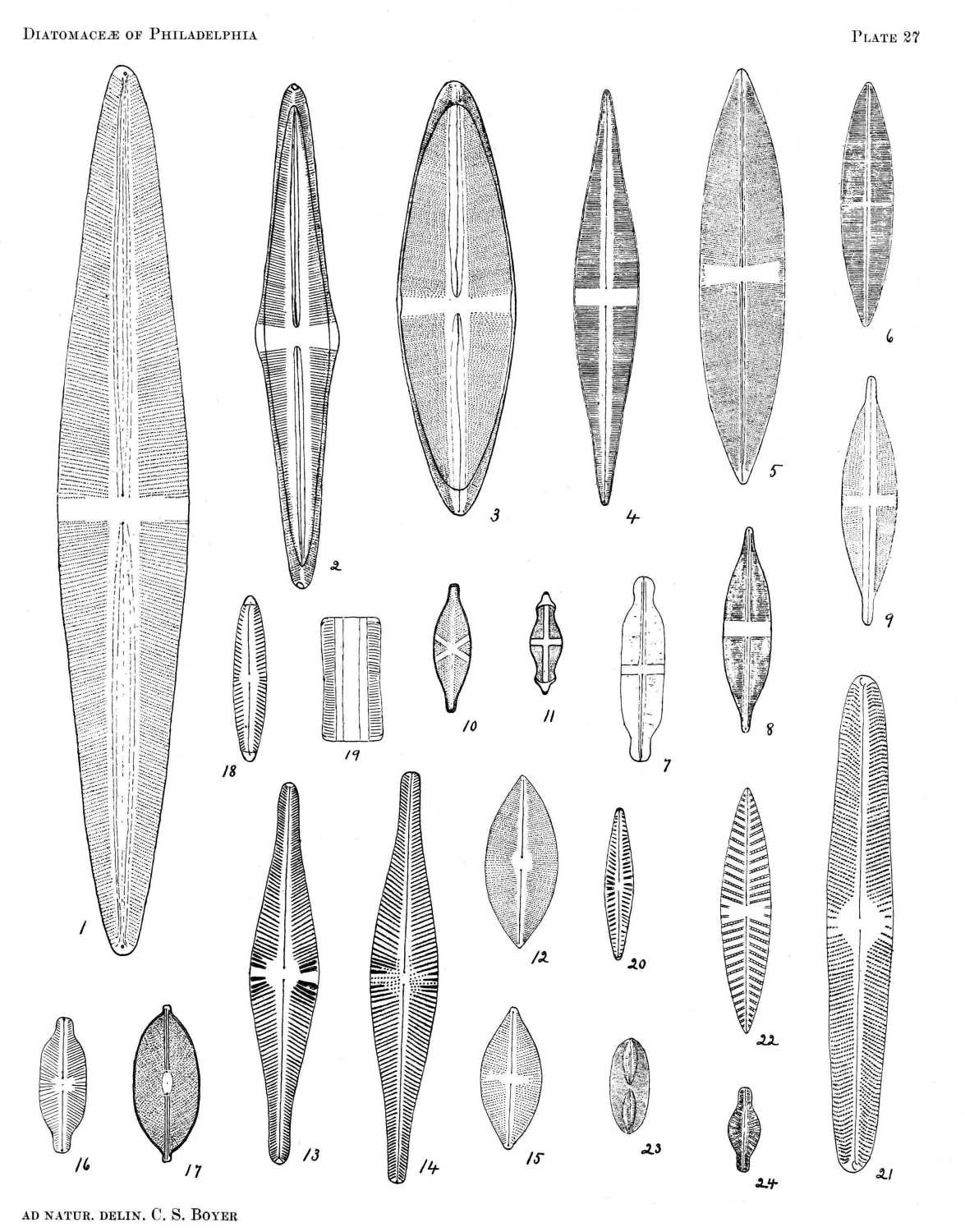 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| STAURONEIS—Continued | ||
| 1 | Stauroneis phœnicenteron Ehr. | 88 |
| 2 | Stauroneis acuta Wm. Sm. | 89 |
| 3 | Stauroneis americana A. S. | 89 |
| 4 | Stauroneis anceps var.? | 88 |
| 5 | Stauroneis anceps var. gracilis (Ehr.) Cl. | 88 |
| 6 | Stauroneis salina Wm. Sm. | 89 |
| 7 | Stauroneis anceps var. amphicephala (Kuetz.) Cl. | 88 |
| 8 | Stauroneis anceps var.? | 88 |
| 9 | Stauroneis anceps var.? | 88 |
| 10 | Stauroneis crucicula (Grun.) Cl. | 89 |
| 11 | Stauroneis smithii Grun. | 89 |
| NAVICULA | ||
| 12 | Navicula lacustris Greg. | 92 |
| 13 | Navicula hasta Pant. | 97 |
| 14 | Navicula hasta var. punctata n. var. | 97 |
| 15 | Navicula punctata var. asymmetrica Lagerstedt | 92 |
| 16 | Navicula dicephala Wm. Sm. | 96 |
| 17 | Navicula placenta Ehr. | 94 |
| 18-19 | Navicula inflexa Greg. | 96 |
| 20 | Navicula pinnata Pant.? | 96 |
| 21 | Navicula oblonga Kuetz. | 97 |
| 22 | Navicula pennata A. S. | 96 |
| 23 | Navicula pygmĉa Kuetz. | 94 |
| 24 | Navicula humilis Donk. | 96 |
 |
||
|
 |
||||||||||||||||||
| Fig. | Page | |
| PINNULARIA | ||
| 1 | Pinnularia nobilis Ehr. | 103 |
| 2 | Pinnularia major var. pulchella n. var. | 102 |
| 3 | Pinnularia dactylus Ehr. | 103 |
| 4 | Pinnularia major (Kuetz.) Wm. Sm. | 102 |
 |
||
|
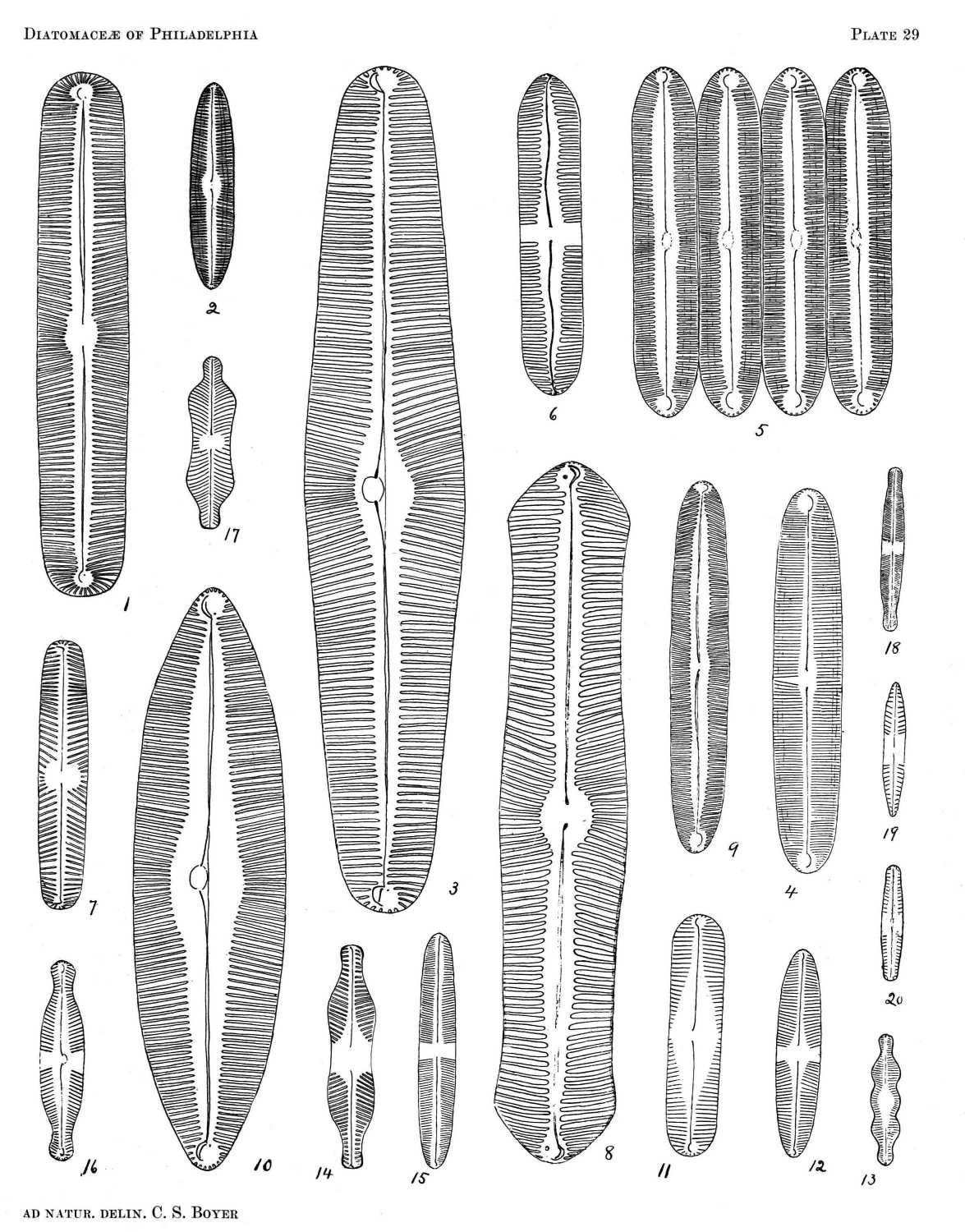 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| PINNULARIA—Continued | ||
| 1 | Pinnularia gentilis (Donk.) Cl. | 103 |
| 2 | Pinnularia viridis Nitzsch. | 104 |
| 3 | Pinnularia dactylus var. dariana (A. S.) Cl. | 103 |
| 4 | Pinnularia viridis var. fallax Cl. | 104 |
| 5 | Pinnularia socialis Palmer | 104 |
| 6 | Pinnularia ĉstuarii Cl. | 105 |
| 7 | Pinnularia rectangulata (Greg.) Cl. | 110 |
| 8 | Pinnularia trigonocephala Cl. | 103 |
| 9 | Pinnularia major (Kuetz.) Wm. Sm. (small form near P. viridis) | 102 |
| 10 | Pinnularia dactylus var. demerarĉ Cl. | 103 |
| 11 | Pinnularia mormonorum (Grun.) | 107 |
| 12 | Pinnularia brébissonii (Kuetz.) Cl. | 107 |
| 13 | Pinnularia mesolepta Ehr. | 105 |
| 14 | Pinnularia termes var. stauroneiformis V. H. | 106 |
| 15 | Pinnularia molaris (Grun.) Cl. | 105 |
| 16 | Pinnularia braunii Grun. | 106 |
| 17 | Pinnularia termes (Ehr.) A. S. | 106 |
| 18 | Pinnularia appendiculata (Ag.) Cl. | 106 |
| 19 | Pinnularia microstauron (Ehr.) Cl. var.? | 106 |
| 20 | Pinnularia subcapitata Greg. | 105 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| PINNULARIA—Continued | ||
| 1 | Pinnularia cardinaliculus Cl. | 107 |
| 2 | Pinnularia viridis var. fallax Cl.? (var. B., Wm. Sm.?). | 104 |
| 3 | Pinnularia legumen Ehr. | 107 |
| 4 | Pinnularia legumen var.? | 107 |
| 5 | Pinnularia gibba (Kuetz.) V. H. | 109 |
| 6 | Pinnularia mesogongyla (Ehr.) Cl. | 109 |
| 7 | Pinnularia acrosphĉria (Bréb.) Cl. | 108 |
| 8 | Pinnularia acrosphĉria var. turgidula Grun. | 108 |
| 9 | Pinnularia tabellaria (Ehr.) Cl. var.? | 110 |
| 10 | Pinnularia leptosoma Grun. | 105 |
| 11 | Pinnularia stauroptera var. interrupta Cl. | 110 |
| 12 | Pinnularia stomatophora (Grun.) Cl. | 109 |
| 13 | Pinnularia stauroptera (Grun.) Cl. | 110 |
| 14 | Pinnularia parva (Ehr.) Cl. var.? | 108 |
| 15-19 | Pinnularia nodosa forma capitata Cl. | 108 |
| 16 | Pinnularia subcapitata var. paucistriata Grun. | 105 |
| 17 | Pinnularia viridis Nitzsch var. | 104 |
| 18 | Pinnularia viridis var. caudata n. var. | 104 |
| 20 | Pinnularia mesolepta var. stauroneiformis Grun. | 105 |
| 21 | Pinnularia polyonca (Bréb.) Lewis. | 108 |
| 22 | Pinnularia borealis Ehr. | 109 |
| 23 | Pinnularia lata (Bréb.) Wm. Sm. | 109 |
| 24 | Pinnularia borealis var. scalaris (Ehr.) Cl. | 109 |
| 25 | Pinnularia blandita n. sp. | 108 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| NAVICULA | ||
| 1 | Navicula elegans Wm. Sm. | 101 |
| 2 | Navicula elegans var. cuspidata Cl. | 101 |
| 3-4 | Navicula grevillei (Ag.) Cl. | 99 |
| 5 | Navicula libellus Greg. | 99 |
| 6-7 | Navicula palpebralis Bréb. | 101 |
| 8 | Navicula rhyncocephala Kuetz. | 97 |
| 9 | Navicula cryptocephala Kuetz. | 97 |
| 10 | Navicula longa (Greg.) Ralfs. | 97 |
| PINNULARIA | ||
| 11 | Pinnularia brébissonii (Kuetz.) Cl. | 107 |
| 12 | Pinnularia borealis Ehr. | 109 |
| 13 | Pinnularia divergens var. elliptica Grun. | 107 |
| EPITHEMIA | ||
| 14 | Epithemia turgida (Ehr.) Kuetz. | 111 |
| 15-21 | Epithemia argus Kuetz. | 111 |
| 16 | Epithemia argus var.? | 111 |
| 17 | Epithemia muelleri A. S. | 111 |
| 18 | Epithemia zebra var. proboscidea (Kuetz.) Grun. | 112 |
| 19 | Epithemia gibberula var. producta Grun. | 112 |
| 20 | Epithemia musculus Kuetz. | 112 |
| 22 | Epithemia musculus var. constricta (Bréb.) V. H. | 112 |
| RHOPALODIA | ||
| 23 | Rhopalodia gibba (Kuetz.) Mueller | 112 |
| 24 | Rhopalodia ventricosa (Kuetz.) Mueller | 113 |
 |
||
|
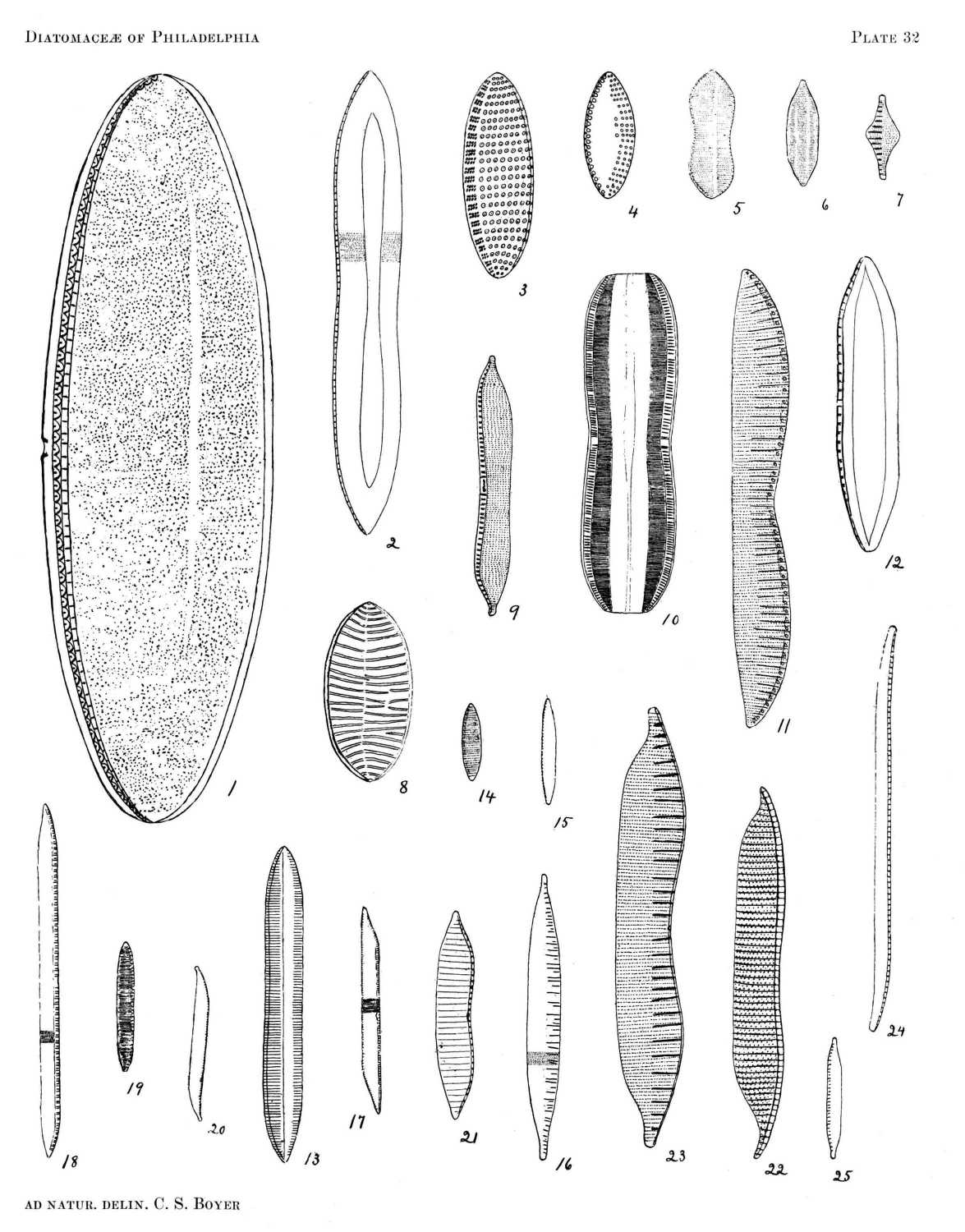 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| NITZSCHIA | ||
| 1 | Nitzschia circumsuta (Bail.) Grun. | 118 |
| 2 | Nitzschia plana Wm. Sm. | 117 |
| 3 | Nitzschia granulata Grun. | 116 |
| 4 | Nitzschia navicularis (Bréb.) Grun. | 116 |
| 5 | Nitzschia panduriformis var. minor Grun. | 117 |
| 6 | Nitzschia apiculata (Greg.) Grun. | 117 |
| 7 | Nitzschia tabellaria Grun. | 119 |
| 8 | Nitzschia tryblionella Hantzsch | 116 |
| 10-11 | Nitzschia bilobata Wm. Sm. | 118 |
| 12 | Nitzschia litoralis var. delawarensis Grun. | 118 |
| 13 | Nitzschia acuminata (Wm. Sm.) Grun. | 117 |
| 14-25 | Nitzschia amphibia Grun. | 122 |
| 15 | Nitzschia palea (Kuetz.) Wm. Sm. | 122 |
| 16 | Nitzschia fluminensis Grun. | 120 |
| 17 | Nitzschia obtusa var. scalpelliformis Grun. | 121 |
| 18 | Nitzschia linearis (Ag.) Wm. Sm. | 122 |
| 19 | Nitzschia communis Rab. | 122 |
| 20 | Nitzschia clausii Hantzsch. | 121 |
| 21 | Nitzschia epithemioides Grun. | 118 |
| 24 | Nitzschia vermicularis (Kuetz.) Wm. Sm. | 120 |
| HANTZSCHIA | ||
| 9 | Hantzschia amphioxys (Ehr.) Grun. | 113 |
| 22 | Hantzschia marina (Donk.) Grun. | 114 |
| 23 | Hantzschia virgata (Roper) Grun. | 114 |
 |
||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| NITZSCHIA | ||
| 1 | Nitzschia longissima (Bréb.) Ralfs | 123 |
| 2 | Nitzschia intermedia Hantzsch | 122 |
| 3 | Nitzschia spectabilis var. americana Grun. | 122 |
| 4-5 | Nitzschia sigmatella Greg. | 121 |
| 6 | Nitzschia scalaris (Ehr.) Wm. Sm. | 119 |
| 7 | Nitzschia macilenta Greg. | 120 |
| 8 | Nitzschia insignis Greg. | 119 |
| 9 | Nitzschia vermicularis (Kuetz.) Hantzsch | 120 |
| 10 | Nitzschia longissima forma parva V. H. | 123 |
| 11 | Nitzschia reversa Wm. Sm. | 123 |
| 12 | Nitzschia acicularis (Kuetz.) Wm. Sm. | 123 |
| 13-14 | Nitzschia paxillifer (O. F. Mueller) Heib. | 119 |
| HOMŒOCLADIA | ||
| 15 | Homœocladia filiformis Wm. Sm. | 123 |
 |
||
|
 |
||||||||||||||||||||||||||||||
| Fig. | Page | |
| SURIRELLA | ||
| 1 | Surirella striatula Turpin | 125 |
| 2 | Surirella anceps Lewis | 128 |
| 3 | Surirella intermedia Lewis | 128 |
| 4 | Surirella arctissima A. S. | 128 |
| 5-6 | Surirella delicatissima Lewis | 128 |
| 7 | Surirella intermedia Lewis forma minor? | 128 |
| CYMATOPLEURA | ||
| 8-9 | Cymatopleura solea (Bréb.) Wm. Sm. | 129 |
 |
||
|
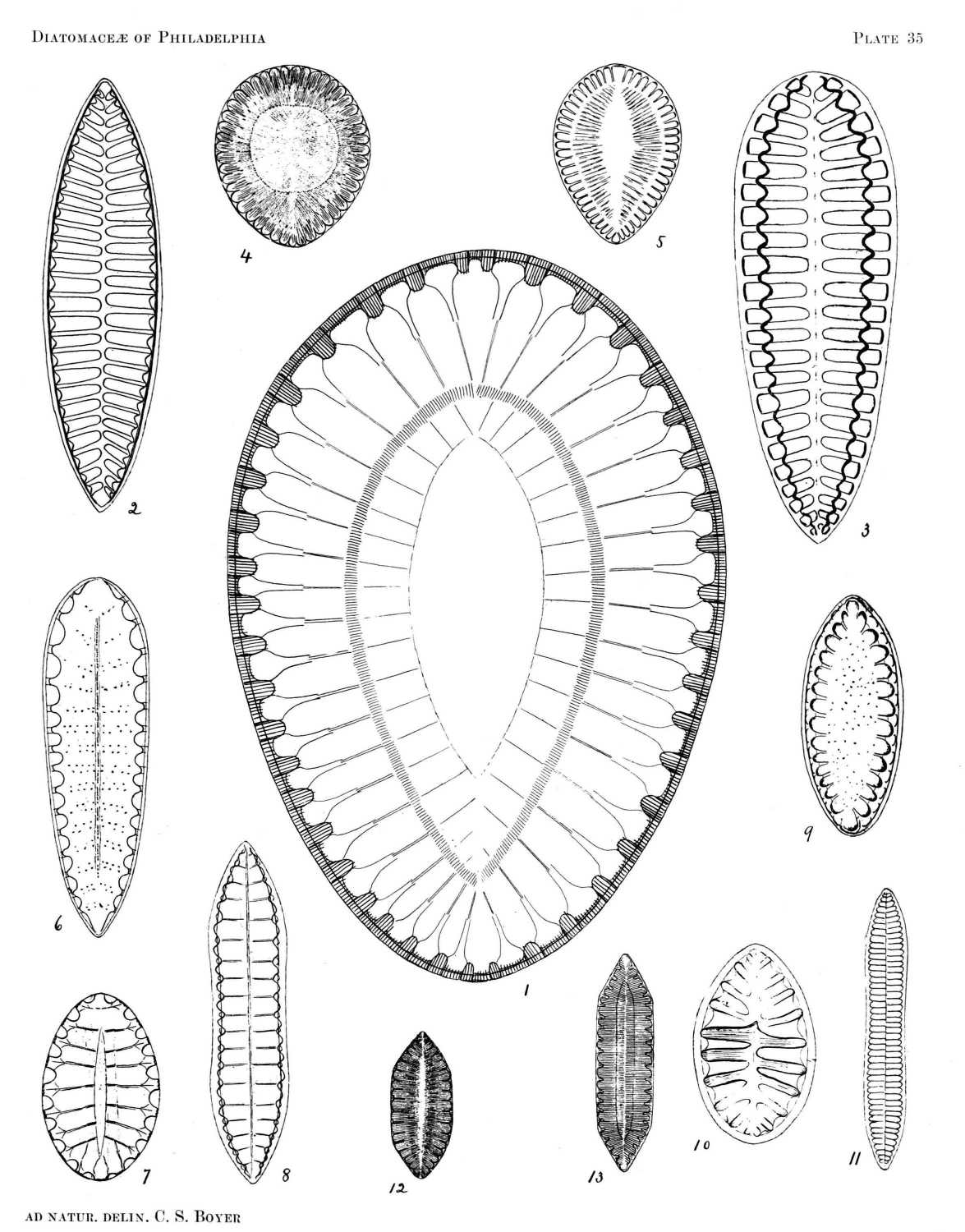 |
||||||||||||||||||||||||||||||||||||||||||
| Fig. | Page | |
| SURIRELLA | ||
| 1 | Surirella fastuosa Ehr. | 127 |
| 2 | Surirella biseriata (Ehr.) Bréb. | 124 |
| 3 | Surirella splendida (Ehr.) Kuetz. | 125 |
| 4 | Surirella crumena Bréb. | 126 |
| 5 | Surirella ovalis Bréb. | 126 |
| 6 | Surirella tenera Greg. | 125 |
| 7 | Surirella recedens A. S. | 127 |
| 8 | Surirella linearis Wm. Sm. | 124 |
| 9 | Surirella oblonga Ehr.? | 127 |
| 10 | Surirella cruciata A. S. | 127 |
| 11 | Surirella gracilis Grun. | 127 |
| 12-13 | Surirella amphioxys Wm. Sm. | 124 |
 |
||
|
 |
||||||||||||||||||||||||||||||
| Fig. | Page | |
| SURIRELLA—Continued | ||
| 1 | Surirella elegans Ehr. | 125 |
| 2 | Surirella robusta Ehr. | 124 |
| 3 | Surirella febigerii Lewis | 128 |
| 4 | Surirella gemma Ehr. | 125 |
| 5 | Surirella guatimalensis Ehr. | 126 |
| 6 | Surirella panduriformis Wm. Sm. | 126 |
| 7-9 | Surirella pinnata Wm. Sm. | 126 |
| 8 | Surirella angusta Kuetz. | 127 |
 |
||
|
 |
||||||||||||||||||||||||
| Fig. | Page | |
| CYMATOPLEURA | ||
| 1 | Cymatopleura elliptica (Bréb.) Wm. Sm. | 129 |
| 2 | Cymatopleura elliptica forma spiralis | 129 |
| 3-4 | Cymatopleura marina Lewis | 129 |
| CAMPYLODISCUS | ||
| 5 | Campylodiscus hibernicus Ehr. | 130 |
| 6 | Campylodiscus echeneis Ehr. | 130 |
 |
||
|
 |
| Fig. | Page | |
| 1 | Amphora gigantea var. fusca A. S. | 65 |
| 2 | Meloseira crenulata (Ehr.) Kuetz. | 15 |
| 3-4 | Licmophora baileyi (Edw.) Grun. | 40 |
| 5 | Coscinodiscus polyacanthus Grun. | 22 |
| 6-7 | Ditylum intricatum (West) Grun. | 30 |
| 8 | Pyxidicula cruciata Ehr. | 19 |
| 9 | Gyrosigma scalproides (Rab.) Cl. | 76 |
| 10 | Coscinodiscus asteromphalus var. omphalantha (Ehr.) Grun. | 23 |
| 11 | Rhabdonema minutum Kuetz. | 36 |
| 12 | Gyrosigma kuetzingii (Grun.) Cl. | 76 |
| 13 | Gyrosigma prolongatum (Wm. Sm.) Cl. | 76 |
| 14 | Cymbella parva (Wm. Sm.) Cl. | 61 |
| 15 | Gomphoneis herculaneum (Ehr.) Cl. (zone view) | 70 |
| 16 | Cymbella ventricosa Kuetz. | 62 |
| 17-18 | Eunotia sp. (abnormal?) | 54 |
 |
||
|
 |
| Fig. | Page | |
| 1 | Nitzschia spectabilis var. americana Grun. (zone view) | 122 |
| 2 | Nitzschia panduriformis Greg. | 117 |
| 3 | Hantzschia amphioxys (Ehr.) Grun. | 113 |
| 4 | Hantzschia amphioxys var. major Grun. | 114 |
| 5 | Nitzschia dubia Wm. Sm. | 118 |
| 6 | Nitzschia amphioxys Wm. Sm. | 114 |
| 7 | Nitzschia compressa (Bail.) | 116 |
| 8 | Nitzschia compressa var. minor H. L. Smith | 116 |
| 9 | Surirella intermedia Lewis (zone view) | 128 |
| 10 | Surirella arctissima A. S. forma minor | 128 |
| 11 | Surirella ovalis Bréb. | 126 |
| 12 | Surirella biseriata (Ehr.) Bréb. | 124 |
| 13 | Nitzschia sigma (Kuetz.) Wm. Sm. | 121 |
| 14 | Nitzschia obtusa var. flexella H. L. Smith | 121 |
| 15 | Stauroneis legumen Ehr. | 89 |
| 16 | Nitzschia obtusa Wm. Sm. | 121 |
 |
||
|
 |
| Fig. | Page | |
| 1 | Caloneis liber (Wm. Sm.) Cl. | 81 |
| 2 | Anomœoneis sphĉrophora (Kuetz.) Cl. | 80 |
| 3 | Nitzschia spathulata Bréb. | 120 |
| 4 | Stauroneis ? abnormal | 89 |
| 5 | Navicula ? abnormal | 101 |
| 6 | Podocystis adriatica Kuetz. | 129 |
| 7 | Nitzschia dissipata (Kuetz.) Grun. | 120 |
| 8 | Cymbella ventricosa Kuetz. (zone view) | 62 |
| 9 | Navicula radiosa Kuetz. (zone view) | 94 |
| 10 | Detail of Rhabdonema arcuatum (Lyng.) Kuetz. | 35 |
| 11 | Diatoma anceps (Ehr.) Kirchn. (containing chromataphores) | 42 |
| 12 | Coscinodiscus asteromphalus Ehr. (trans. section, after Pelletan) | 23 |
| 13-14-15 | Transverse section (diagram) of Pinnularia showing straight, oblique and grooved raphes | 101 |
| 16 | Transverse section (diagram) of Biddulphia favus showing inner punctate stratum (after Deby) | 31 |
| 17 | Transverse (ideal) section of Surirella | 124 |
| 18-19 | Transverse (ideal) section of Pinnularia, before and after division | 101 |
| 20 | Transverse section of Nitzschia linearis (Ag.) Wm. Sm. | 122 |
| 21 | Transverse section (diagram) of Navicula | 89 |
| 22 | Transverse section (diagram) of Cymbella | 60 |
| 23 | Transverse section (diagram) of Amphora | 65 |
 |
||
End of the Project Gutenberg EBook of The Diatomaceae of Philadelphia and
Vicinity, by Charles Sumner Boyer
*** END OF THIS PROJECT GUTENBERG EBOOK DIATOMACEAE ***
***** This file should be named 44569-h.htm or 44569-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/4/4/5/6/44569/
Produced by Charlene Taylor, Bryan Ness, Keith Edkins and
the Online Distributed Proofreading Team at
http://www.pgdp.net (This file was produced from images
generously made available by The Internet Archive)
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation information page at www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at 809
North 1500 West, Salt Lake City, UT 84116, (801) 596-1887. Email
contact links and up to date contact information can be found at the
Foundation's web site and official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For forty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.