
BOYS' SECOND BOOK OF
INVENTIONS


BY RAY STANNARD BAKER
Author of
Boys' Book of Inventions, Seen in
Germany
FULLY ILLUSTRATED

NEW YORK
DOUBLEDAY, PAGE & COMPANY
MCMIX
Copyright, 1903, by
McCLURE, PHILLIPS & CO.
Published, November, 1903, N
| CHAPTER I | ||
| PAGE | ||
| The Miracle of Radium | 3 | |
| Story of the Marvels and Dangers of the New Element Discovered by Professor and Madame Curie. |
||
| CHAPTER II | ||
| Flying Machines | 27 | |
| Santos-Dumont's Steerable Balloons. | ||
| CHAPTER III | ||
| The Earthquake Measurer | 79 | |
| Professor John Milne's Seismograph. | ||
| CHAPTER IV | ||
| Electrical Furnaces | 113 | |
| How the Hottest Heat is Produced—Making Diamonds. | ||
| CHAPTER V | ||
| Harnessing the Sun | 153 | |
| The Solar Motor. | ||
| CHAPTER VI | ||
| The Inventor and the Food Problem | 173 | |
| Fixing of Nitrogen—Experiments of Professor Nobbe. | ||
| CHAPTER VII | ||
| Marconi and his Great Achievements | 207 | |
| New Experiments in Wireless Telegraphy. | ||
| CHAPTER VIII | ||
| Sea-Builders | 255 | |
| The Story of Lighthouse Building—Stone-Tower Lighthouses, Iron Pile Lighthouses, and Steel Cylinder Lighthouses. |
||
| CHAPTER IX | ||
| The Newest Electric Light | 293 | |
| Peter Cooper Hewitt and his Three Great Inventions — The Mercury Arc Light—The New Electrical Converter—The Hewitt Interrupter. |
||
| Page | |
| Guglielmo Marconi Frontispiece | |
| M. Curie Explaining the Wonders of Radium at the Sorbonne |
5 |
| Dr. Danlos Treating a Lupus Patient with Radium at the St. Louis Hospital, Paris |
13 |
| Radium as a Test for Real Diamonds | 19 |
| At the approach of Radium pure gems are thrown into great brilliancy, while imitations remain dull. |
|
| M. and Mme. Curie Finishing the Preparation of some Radium |
25 |
| M. Alberto Santos-Dumont | 29 |
| Severo's Balloon, the "Pax," which on its First Ascent at a Height of about 2,000 feet, Burst and Exploded, Sending to a Terrible Death both M. Severo and his Assistant |
33 |
| The Trial of Count Zeppelin's Air-Ship, July 2, 1900 | 37 |
| M. Santos-Dumont at Nineteen | 41 |
| M. Santos-Dumont's First Balloon (Spherical) | 43 |
| M. Santos-Dumont's Workshop | 45 |
| "Santos-Dumont No. 1" | 49 |
| Basket of "Santos-Dumont No. 1" | 52 |
| Showing propeller and motor. | |
| "Santos-Dumont No. 1" | 54 |
| Showing how it began to fold up in the middle. | |
| "Santos-Dumont No. 5" Rounding Eiffel Tower, July 13, 1901 |
57 |
| The Interior of the Aërodrome | 61 |
| Showing its construction, the inflated balloon, and the pennant with its mystic letters. |
|
| The Fall into the Courtyard of the Trocadero Hotel | 65 |
| "Santos-Dumont No. 5." | |
| "Santos-Dumont No. 6"—The Prize Winner | 69 |
| Air-Ship Pointing almost Vertically Upward | 73 |
| Falling to the Sea | 73 |
| Just Before the Air-Ship Lost all its Gas | 74 |
| Losing its Gas and Sinking | 74 |
| The Balloon Falling to the Waves | 75 |
| Boats Around the Ruined Air-Ship | 75 |
| Manœuvring Above the Bay at Monte Carlo | 77 |
| Professor John Milne | 80 |
| From a photograph by S. Suzuki, Kudanzaka, Tokio. | |
| Professor Milne's Sensitive Pendulum, or Seismograph, as it Appears Enclosed in its Protecting Box |
81 |
| The Sensitive Pendulum, or Seismograph, as it Appears with the Protecting Box Removed |
81 |
| Gifu, Japan, after the Earthquake of 1891 | 85 |
| This and the pictures following on pages 89, 101, 111, are from Japanese photographs reproduced in "The Great Earthquake in Japan, 1891," by John Milne and W. K. Burton. |
|
| The Work of the Great Earthquake of 1891 in Neo Valley, Japan |
89 |
| Diagram Showing Vertical and Horizontal Sections of the More Sensitive of Professor Milne's Two Pendulums, or Seismographs |
93 |
| Seismogram of a Borneo Earthquake that Occurred September 20, 1897 |
94 |
| Effect of the Great Earthquake of 1891 on the Nagara Gawa Railway Bridge, Japan |
101 |
| Pieces of a Submarine Cable Picked Up in the Gulf of Mexico in 1888 |
108 |
| The kinks are caused by seismic disturbances, and they show how much distortion a cable can suffer and still remain in good electrical condition, as this was found to be. |
|
| Record made on a Stationary Surface by the Vibrations of the Japanese Earthquake of July 19, 1891 |
111 |
| Showing the complicated character of the motion (common to most earthquakes), and also the course of a point at the centre of disturbance. |
|
| Table of Temperatures | 115 |
| Mr. E. G. Acheson, One of the Pioneers in the Investigation of High Temperatures |
125 |
| The Furnace-Room, where Carborundum is Made | 131 |
| "A great, dingy brick building, open at the sides like a shed." | |
| Taking Off a Crust of the Furnace at Night | 135 |
| The light is so intense that you cannot look at it without hurting the eyes. |
|
| The Interior of a Furnace as it Appears after the Carborundum has been Taken Out |
143 |
| Blowing Off | 147 |
| "Not infrequently gas collects, forming a miniature mountain, with a crater at its summit, and blowing a magnificent fountain of flame, lava, and dense white vapour high into the air, and roaring all the while in a most terrifying manner." |
|
| Side View of the Solar Motor | 155 |
| Front View of the Los Angeles Solar Motor | 159 |
| The Brilliant Steam Boiler Glistens in the Centre | 163 |
| The Rear Machinery for Operating the Reflector | 167 |
| Trees Growing in Water at Professor Nobbe's Laboratory |
187 |
| Experimenting with Nitrogen in Professor Nobbe's Laboratory |
191 |
| Mr. Charles S. Bradley | 198 |
| Mr. D. R. Lovejoy | 199 |
| Eight-Inch 10,000-Volt Arcs Burning the Air for Fixing Nitrogen |
200 |
| Machine for Burning the Air with Electric Arcs so as to Produce Nitrates |
201 |
| Marconi. The Sending of an Epoch-Making Message | 206 |
| January 18, 1903, marks the beginning of a new era in telegraphic communication. On that day there was sent by Marconi himself from the wireless station at South Wellfleet, Cape Cod, Mass., to the station at Poldhu, Cornwall, England, a distance of 3,000 miles, the message—destined soon to be historic—from the President of the United States to the King of England. |
|
| Preparing to Fly the Kite which Supported the Receiving Wire |
213 |
| Marconi on the extreme left. | |
| Mr. Marconi and his Assistants in Newfoundland: Mr. Kemp on the Left, Mr. Paget on the Right |
217 |
| They are sitting on a balloon basket, with one of the Baden-Powell kites in the background. |
|
| Marconi Transatlantic Station at Wellfleet, Cape Cod, Mass. |
229 |
| At Poole, England | 231 |
| Nearer View, South Foreland Station | 235 |
| Alum Bay Station, Isle of Wight | 237 |
| Marconi Room, S.S. Philadelphia | 241 |
| Transatlantic High Power, Marconi Station at Glace Bay, Nova Scotia |
247 |
| Work on the Smith Point Lighthouse Stopped by a Violent Storm |
254 |
| Just after the cylinder had been set in place, and while the workmen were hurrying to stow sufficient ballast to secure it against a heavy sea, a storm forced the attending steamer to draw away. One of the barges was almost overturned, and a lifeboat was driven against the cylinder and crushed to pieces. |
|
| Robert Stevenson, Builder of the Famous Bell Rock Lighthouse, and Author of Important Inventions and Improvements in the System of Sea Lighting |
256 |
| From a bust by Joseph, now in the library of Bell Rock Lighthouse. | |
| The Bell Rock Lighthouse, on the Eastern Coast of Scotland |
257 |
| From the painting by Turner. The Bell Rock Lighthouse was built by Robert Stevenson, grandfather of Robert Louis Stevenson, on the Inchcape Reef, in the North Sea, near Dundee, Scotland, in 1807-1810. |
|
| The Present Lighthouse on Minot's Ledge, near the Entrance of Massachusetts Bay, Fifteen Miles Southeast of Boston |
260 |
| "Rising sheer out of the sea, like a huge stone cannon, mouth upward."—Longfellow. |
|
| The Lighthouse on Stannard Rock, Lake Superior | 261 |
| This is a stone-tower lighthouse, similar in construction to the one built with such difficulty on Spectacle Reef, Lake Huron. |
|
| The Fowey Rocks Lighthouse, Florida |
264 |
| Fourteen-Foot Bank Light Station, Delaware Bay, Del. |
268 |
| The Great Beds Light Station, Raritan Bay, N. J. | 270 |
| A specimen of iron cylinder construction. | |
| A Storm at the Tillamook Lighthouse, in the Pacific, one mile out from Tillamook Head, Oregon |
275 |
| Saving the Cylinder of the Lighthouse at Smith Point, Chesapeake Bay, from being Swamped in a High Sea |
279 |
| When the builders were towing the unwieldy cylinder out to set it in position, the water became suddenly rough and began to fill it. Workmen, at the risk of their lives, boarded the cylinder, and by desperate labours succeeded in spreading sail canvas over it, and so saved a structure that had cost months of labour and thousands of dollars. |
|
| Great Waves Dashed Entirely Over Them, so that They had to Cling for Their Lives to the Air-Pipes |
285 |
| In erecting the Smith Point lighthouse, after the cylinder was set up, it had to be forced down fifteen and a half feet into the sand. The lives of the men who did this, working in the caisson at the bottom of the sea, were absolutely in the hands of the men who managed the engine and the air-compressor at the surface; and twice these latter were entirely deluged by the sea, but still maintained steam and kept everything running as if no sea was playing over them. |
|
| Peter Cooper Hewitt | 292 |
| With his interrupter. | |
| Watching a Test of the Hewitt Converter | 299 |
| Lord Kelvin in the centre. | |
| The Hewitt Mercury Vapour Light | 305 |
| The circular piece just above the switch button is one form of "boosting coil" which operates for a fraction of a second when the current is first turned on. The tube shown here is about an inch in diameter and several feet long. Various shapes may be used. Unless broken, the tubes never need renewal. |
|
| Testing a Hewitt Converter | 311 |
| The row of incandescent lights is used, together with a voltmeter and ammeter, to measure strength of current, resistance, and loss in converting. |
|
BOYS' SECOND BOOK OF
INVENTIONS
No substance ever discovered better deserves the term "Miracle of Science," given it by a famous English experimenter, than radium. Here is a little pinch of white powder that looks much like common table salt. It is one of many similar pinches sealed in little glass tubes and owned by Professor Curie, of Paris. If you should find one of these little tubes in the street you would think it hardly worth carrying away, and yet many a one of them could not be bought for a small fortune. For all the radium in the world to-day could be heaped on a single table-spoon; a pound of it would be worth nearly a million dollars, or more than three thousand times its weight in pure gold.
Professor and Madame Curie, who discovered radium, now possess the largest amount of any one, but there are small quantities in the hands of English and German scientists, and perhaps a dozen specimens in America, one owned by the American Museum of Natural History and several by Mr. W. J. Hammer, of New York, who was the first American to experiment with the rare and precious substance.
And perhaps it is just as well, at first, not to have too much radium, for besides being wonderful it is also dangerous. If a pound or two could be gathered in a mass it would kill every one who came within its influence. People might go up and even handle the white powder without at the moment feeling any ill-effects, but in a week or two the mysterious and dreadful radium influence would begin to take effect. Slowly the victim's skin would peel off, his body would become one great sore, he would fall blind, and finally die of paralysis and congestion of the spinal cord. Even the small quantities now in hand have severely burned the experimenters. Professor Curie himself has a number of bad scars on his hands and arms due to ulcers caused by handling radium. And Professor Becquerel, in journeying to London, carried in his waistcoat pocket a small tube of radium to be used in a lecture there. Nothing happened at the time, but about two weeks later Professor Becquerel observed that the skin under his pocket was beginning to redden and fall away, and finally a deep and painful sore formed there and remained for weeks before healing.
It is just as well, therefore, that scientists learn more about radium and how to handle and control it before too much is manufactured.
But the cost and danger of radium are only two of its least extraordinary features. Seen in the daylight radium is a commonplace white powder, but in the dark it glows like live fire, and the purer it is the more it glows. I held for a moment one of Mr. Hammer's radium tubes, and, the lights being turned off, it seemed like a live coal burning there in my hand, and yet I felt no sensation of heat. But radium really does give off heat as well as light—and gives it off continually without losing appreciable weight. And that is what seems to scientists a miracle. Imagine a coal which should burn day in and day out for hundreds of years, always bright, always giving off heat and light, and yet not growing any smaller, not turning to ashes. That is the almost unbelievable property of radium. Professor Curie has specimens which have thus been radiating light and heat for several years, with practically no loss of weight; and no small amount of light and heat either. Professor Curie has found that a given quantity of radium will melt its own weight of ice every hour, and continue doing so practically for ever. One of his associates has calculated that a fixed quantity of radium, after throwing out heat for 1,000,000,000 years, would have lost only one-millionth part of its bulk.
What is the reason for these extraordinary properties? Is it not "perpetual motion"? All the great scientists of the world have been trying in vain to answer these questions. Several theories have been advanced, of which I shall speak later, but none seems a satisfactory explanation. When we know more of radium perhaps we shall be better prepared to say what it really is, and we may have to unlearn many of the great principles of physics and chemistry which were seemingly settled for all time. Radium would seem, indeed, to defy the very law of the conservation of energy.
The practical mind at once sees radium in use as a new source of heat and light for mankind, a furnace that would never have to be fed or cleaned, a lamp that would glow perpetually—and the time may really come, the inventor having taken hold of the wonder that the scientist has produced, when many practical applications of the new element may be devised. At present, however, the scarcity and cost and danger of radium will keep it in the hands of the experimenter.
Another astonishing property of radium is its power of communicating some of its strange qualities to certain substances brought within its influence. Mr. Hammer kept his radium tubes for a time in a pasteboard box. This being broken, he removed the tubes and threw the pasteboard aside. Several days later, having occasion to turn off the lights in the laboratory, he found that the discarded box was glowing there in the dark. It had taken up some of the rays from the radium. Nearly everything that comes in contact with radium thus becomes "radio-active"—even the experimenter's clothes and hands, so that delicate instruments are disturbed by the invisible shine of the experimenter. Photographs can be taken with radium; it also makes the air around it a better conductor of electricity. And still more marvellous, besides being an agency for the destruction of life, as I shall show later, it can actually be used in other ways to prolong life, and the future may show many wonderful uses for it in the treatment of disease. Already, in Paris, several cases of lupus have been cured with it, and there is evidence that it will help to restore sight in certain cases of blindness. I held a tube of radium to my closed eye and was conscious of the sensation of light; the same sensation was present when the tube was held to my temple, thus showing that the radium has an effect on the optic nerve. A little blind girl in New York, who had never had the sensation of light, began to see a little after one treatment with radium, and experiments are still going on, but cautiously, for fear that injuries may result.
We now come to the fascinating story of the discovery and manufacture of radium. It has long been known that certain substances are phosphorescent; that is, under the proper conditions they glow without apparent heat. Everybody has seen "fox-fire" in the damp and decaying woods—a cold light which scientists have never been able to explain.
To M. Henri Becquerel of the French Institute is generally given the credit for having begun the real study of radio-activity, although, as in every great discovery and invention, many other scientists and practical electricians had paved the way by their investigations. In 1896 M. Becquerel was conducting some experiments with various phosphorescent substances. He exposed some salts of the metal uranium to the sunlight until they became phosphorescent, and then tried their effect upon a photographic plate.
It rained, and he put the plate away in a drawer for several days. When he developed it he was surprised to find on it a better image than sunlight would have made. And thus, by a sort of accident, he led up to the discovery of the Becquerel rays, so called.
Uranium is extracted from a metal or ore called uranite by mineralogists, and popularly known as pitch-blende. Every young college student who has studied geology or chemistry has heard of pitch-blende.
Two years after Becquerel's discovery of the radio-activity of uranium Professor Pierre Curie and Madame Curie, of Paris, made the discovery that some of the samples of pitch-blende which they had were much more powerful than any uranium that they had used.
Was there, then, something more powerful than uranium within the pitch-blende? They began to "boil down" the waste rock left at the uranium mines, and found a strange new element, related to uranium but different, to which Madame Curie gave the name polonium, after her native land, Poland.
Then they did some more boiling down, and succeeded in isolating an entirely new substance, and the most radio-active yet discovered—radium. Shortly after that Debierne discovered still another radio-active substance, to which he gave the name actinium.
Thus three new elements were added to the list of the world's substances, and the most wonderful of these is radium. In a day, almost, the Curies became famous in the scientific world, and many of the greatest investigators in the world—Lord Kelvin, Sir William Crookes, and others—took up the study of radium.
Very rarely have a man and woman worked together so perfectly as Professor Curie and his wife. Madame Curie was a Polish girl; she came to Paris to study, very poor, but possessed of rare talents. Her marriage with M. Curie was such a union as must have produced some fine result. Without his scientific learning and vivid imagination it is doubtful if radium would ever have been dreamed of, and without her determination and patience against detail it is likely the dream would never have been realised.
One of the chief problems to be met in finding the secrets of radium is the great difficulty and expense, in the first place, of getting any of the substance to experiment with. The Curies have had to manufacture all they themselves have used. In the first place, pitch-blende, which closely resembles iron in appearance, is not plentiful. The best of it comes from Bohemia, but it is also found in Saxony, Norway, Egypt, and in North Carolina, Colorado, and Utah. It appears in small lumps in veins of gold, silver, and mica, and sometimes in granite.
Comparatively speaking, it is easy to get uranium from pitch-blende. But to get the radium from the residues is a much more complicated task. According to Professor Curie, it is necessary to refine about 5,000 tons of uranium residues to get a kilogramme—or about 2.2 pounds—of radium.
It is hardly surprising, therefore, considering the enormous amount of raw material which must be handled, that the cost of this rare mineral should be high. It has been said that there is more gold in sea-water than radium in the earth. Professor Curie has an extensive plant at Ivry, near Paris, where the refuse dust brought from the uranium mines is treated by complicated processes, which finally yield a powder or crystals containing a small amount of radium. These crystals are sent to the laboratory of the Curies where the final delicate processes of extraction are carried on by the professor and his wife.
And, after all, pure metallic radium is not obtained. It could be obtained, and Professor Curie has actually made a very small quantity of it, but it is unstable, immediately oxidised by the air and destroyed. So it is manufactured only in the form of chloride and bromide of radium. The "strength" of radium is measured in radio-activity, in the power of emitting rays. So we hear of radium of an intensity of 45 or 7,000 or 300,000. This method of measurement is thus explained. Taking the radio-activity of uranium as the unit, as one, then a certain specimen of radium is said to be 45 or 7,000 or 300,000 times as intense, to have so many times as much radio-activity. The radium of highest intensity in this country now is 300,000, but the Curies have succeeded in producing a specimen of 1,500,000 intensity. This is so powerful and dangerous that it must be kept wrapped in lead, which has the effect of stopping some of the rays. Rock-salt is another substance which hinders the passage of the rays.
English scientists have devised a curious little instrument, called the spinthariscope, which allows one actually to see the emanations from radium and to realise as never before the extraordinary atomic disintegration that is going on ceaselessly in this strange metal. The spinthariscope is a small microscope that allows one to look at a tiny fragment of radium supported on a little wire over a screen.

Radium as a Test for Real Diamonds.
At the approach of Radium pure gems are thrown into great brilliancy, while imitations remain dull.
The experiment must be made in a darkened room after the eye has gradually acquired its greatest sensitiveness to light. Looking intently through the lenses the screen appears like a heaven of flashing meteors among which stars shine forth suddenly and die away. Near the central radium speck the fire-shower is most brilliant, while toward the rim of the circle it grows fainter. And this goes on continuously as the metal throws off its rays like myriads of bursting, blazing stars. M. Curie has spoken of this vision, really contained within the area of a two-cent piece, as one of the most beautiful and impressive he ever witnessed; it was as if he had been allowed to assist at the birth of a universe. Radium emits radiations, that is, it shoots off particles of itself into space at such terrific speed that 92,500 miles a second is considered a small estimate. Yet, in spite of the fact that this waste goes on eternally and at such enormous velocity, the actual loss sustained by the radium is, as I have said, infinitesimal.
We now come to one of the most interesting phases of the whole subject of radium—that is, the influence which its strange rays have upon animal life. Mr. Cleveland Moffett, to whom I am indebted for the facts of the following experiments, recently visited M. Danysz, of the Pasteur Institute in Paris, who has made some wonderful investigations in this branch of science. M. Danysz has tried the effect of radium on mice, rabbits, guinea-pigs, and other animals, and on plants, and he found that if exposed long enough they all died, often first losing their fur and becoming blind.
But the most startling experiment performed thus far at the Pasteur Institute is one undertaken by M. Danysz, February 3, 1903, when he placed three or four dozen little larvæ that live in flour in a glass flask, where they were exposed for a few hours to the rays of radium. He placed a like number of larvæ in a control-flask, where there was no radium, and he left enough flour in each flask for the larvæ to live upon. After several weeks it was found that most of the larvæ in the radium flask had been killed, but that a few of them had escaped the destructive action of the rays by crawling away to distant corners of the flask, where they were still living. But they were living as larvæ, not as moths, whereas in the natural course they should have become moths long before, as was seen by the control-flask, where the larvæ had all changed into moths, and these had hatched their eggs into other larvæ, and these had produced other moths. All of which made it clear that the radium rays had arrested the development of these little worms.
More weeks passed, and still three or four of the larvæ lived, and four full months after the original exposure one larva was still alive and wriggling, while its contemporary larvæ in the other jar had long since passed away as aged moths, leaving generations of moths' eggs and larvæ to witness this miracle, for here was a larva, venerable among his kind, that had actually lived through three times the span of life accorded to his fellows and that still showed no sign of changing into a moth. It was very much as if a young man of twenty-one should keep the appearance of twenty-one for two hundred and fifty years!
Not less remarkable than these are some recent experiments made by M. Bohn at the biological laboratories of the Sorbonne, his conclusions being that radium may so far modify various lower forms of life as to actually produce new species of "monsters," abnormal deviations from the original type of the species. Furthermore, he has been able to accomplish with radium what Professor Loeb did with salt solutions—that is, to cause the growth of unfecundated eggs of the sea-urchin, and to advance these through several stages of their development. In other words, he has used radium to create life where there would have been no life but for this strange stimulation.
So much for the wonders of radium. We seem, indeed, to be on the border-land of still more wonderful discoveries. Perhaps these radium investigations will lead to some explanation of that great question in science, "What is electricity?"—and that, who can say, may solve that profounder problem, "What is life?"
At present there are two theories as to the source of energy in radium, thus stated by Professor Curie:
"Where is the source of this energy? Both Madame Curie and myself are unable to go beyond hypotheses; one of these consists in supposing the atoms of radium evolving and transforming into another simple body, and, despite the extreme slowness of that transformation, which cannot be located during a year, the amount of energy involved in that transformation is tremendous.

M. and Mme. Curie Finishing the Preparation of some Radium.
"The second hypothesis consists in the supposition that radium is capable of capturing and utilising some radiations of unknown nature which cross space without our knowledge."
Among the inventors engaged in building flying machines the most famous, perhaps, is M. Santos-Dumont, whose thrilling adventures and noteworthy successes have given him world-wide fame. He was the first, indeed, to build a balloon that was really steerable with any degree of certainty, winning a prize of $20,000 for driving his great air-ship over a certain specified course in Paris and bringing it back to the starting-point within a specified time. Another experimenter who has had some degree of success is the German, Count Zeppelin, who guided a huge air-ship over Lake Geneva, Switzerland, in 1901.
Carl E. Myers, an American, an expert balloonist, has also built balloons of small size which he has been able to steer. And mention must also be made of M. Severo, the Frenchman, whose ship, Pax, exploded in the air on its first trip, dropping the inventor and his assistant hundreds of feet downward to their death on the pavements of Paris.
It will be most interesting and instructive to consider especially the work of Santos-Dumont, for he has been not only the most successful in making actual flights of any of the inventors who have taken up this great problem of air navigation, but his adventures have been most romantic and thrilling. In five years' time he has built and operated no fewer than ten great air-ships which he has sailed in various parts of Europe and in America. He has even crowned his experiences with more than one shipwreck in the air, an adventure by the side of which an ordinary sea-wreck is tame indeed, and he has escaped with his life as a result not only of good fortune but of real daring and presence of mind in the face of danger.
For an inventor, M. Santos-Dumont is a rather extraordinary character. The typical inventor—at least so we think—is poor, starts poor at least, and has a struggle to rise. M. Santos-Dumont has always had plenty of means. The inventor is always first a dreamer, we think. M. Santos-Dumont is first a thoroughly practical man, an engineer with a good knowledge of science, to which he adds the imagination of the inventor and the keen love and daring of the sportsman and adventurer, without which his experiments could never have been carried through.
It would seem, indeed, that nature had especially equipped M. Santos-Dumont for his work in aërial navigation. Supposing an inventor, having all the mental equipment of Santos-Dumont, the ideas, the energy, the means—supposing such a man had weighed two hundred pounds! He would have had to build a very large ship to carry his own weight, and all his problems would have been more complex, more difficult. Nature made Santos-Dumont a very small, slim, slight man, weighing hardly more than one hundred pounds, but very active and muscular. The first time I ever saw him, in Crystal Palace, London, where he was setting up one of his air-ships in a huge gallery, I thought him at first glance to be some boy, a possible spectator, who was interested in flying machines. His face, bare and shaven, looked youthful; he wore a narrow-brimmed straw hat and was dressed in the height of fashion. One would not have guessed him to be the inventor. A moment later he had his coat off and was showing his men how to put up the great fan-like rudder of the ship which loomed above us like some enormous Rugby football, and then one saw the power that was in him. Brazilian by nationality, he has a dark face, large dark eyes, an alertness of step and an energetic way of talking. His boyhood was spent on his father's extensive coffee plantation in Brazil; his later years mostly in Paris, though he has been a frequent visitor to England and America. He speaks Spanish, French, and English with equal fluency. Indeed, hearing his English one would say that he must certainly have had his training in an English-speaking country, though no one would mistake him in appearance for either English or American, for he is very much a Latin in face and form. One finds him most unpretentious, modest, speaking freely of his inventions, and yet never taking to himself any undue credit.

Severo's Balloon, the "Pax," which, on its First Ascent at a Height of about 2,000 feet, Burst and Exploded, Sending to a Terrible Death both M. Severo and his Assistant.
Santos-Dumont is still a very young man to have accomplished so much. He was born in Brazil, July 20, 1873. From his earliest boyhood he was interested in kites and dreamed of being able to fly. He says:
"I cannot say at what age I made my first kites; but I remember how my comrades used to tease me at our game of 'Pigeon flies'! All the children gather round a table, and the leader calls out: 'Pigeon flies! Hen flies! Crow flies! Bee flies!' and so on; and at each call we were supposed to raise our fingers. Sometimes, however, he would call out: 'Dog flies! Fox flies!' or some other like impossibility, to catch us. If any one should raise a finger, he was made to pay a forfeit. Now my playmates never failed to wink and smile mockingly at me when one of them called 'Man flies!' For at the word I would always lift my finger very high, as a sign of absolute conviction; and I refused with energy to pay the forfeit. The more they laughed at me, the happier I was."
Of course he read Jules Verne's stories and was carried away in imagination in that author's wonderful balloons and flying machines. He also devoured the history of aërial navigation which he found in the works of Camille Flammarion and Wilfrid de Fonvielle. He says, further:
"At an early age I was taught the principles of mechanics by my father, an engineer of the École Centrale des Arts et Manufactures of Paris. From childhood I had a passion for making calculations and inventing; and from my tenth year I was accustomed to handle the powerful and heavy machines of our factories, and drive the compound locomotives on our plantation railroads. I was constantly taken up with the desire to lighten their parts; and I dreamed of air-ships and flying machines. The fact that up to the end of the nineteenth century those who occupied themselves with aërial navigation passed for crazy, rather pleased than offended me. It is incredible and yet true that in the kingdom of the wise, to which all of us flatter ourselves we belong, it is always the fools who finish by being in the right. I had read that Montgolfière was thought a fool until the day when he stopped his insulters' mouths by launching the first spherical balloon into the heavens."
Upon going to Paris Santos-Dumont at once took up the work of making himself familiar with ballooning in all of its practical aspects. He saw that if he were ever to build an air-ship he must first know all there was to know about balloon-making, methods of filling with gas, lifting capacities, the action of balloons in the air, and all the thousand and one things connected with ordinary ballooning. And Paris has always been the centre of this information. He regards this preliminary knowledge as indispensable to every air-ship builder. He says:
"Before launching out into the construction of air-ships I took pains to make myself familiar with the handling of spherical balloons. I did not hasten, but took plenty of time. In all, I made something like thirty ascensions; at first as a passenger, then as my own captain, and at last alone. Some of these spherical balloons I rented, others I had constructed for me. Of such I have owned at least six or eight. And I do not believe that without such previous study and experience a man is capable of succeeding with an elongated balloon, whose handling is so much more delicate. Before attempting to direct an air-ship, it is necessary to have learned in an ordinary balloon the conditions of the atmospheric medium; to have become acquainted with the caprices of the wind, now caressing and now brutal, and to have gone thoroughly into the difficulties of the ballast problem, from the triple point of view of starting, of equilibrium in the air, and of landing at the end of the trip. To go up in an ordinary balloon, at least a dozen times, seems to me an indispensable preliminary for acquiring an exact notion of the requisites for the construction and handling of an elongated balloon, furnished with its motor and propeller."

M. Santos-Dumont's First Balloon (Spherical).
His first ascent in a balloon was made in 1897, when he was 24 years old, as a passenger with M. Machuron, who had then just returned from the Arctic regions, where he had helped to start Andrée on his ill-fated voyage in search of the North Pole. He found the sensations delightful, being so pleased with the experience that he subsequently secured a small balloon of his own, in which he made several ascents. He also climbed the Alps in order to learn more of the condition of the air at high altitudes.
In 1898 he set about experimentation in the building of a real air-ship or steerable balloon. Efforts had been made in this direction by former inventors, but with small success. As far back as 1852 Henri Gifford made the first of the familiar cigar-shaped balloons, trying steam as a motive power, but he soon found that an engine strong enough to propel the balloon was too heavy for the balloon to lift. That simple failure discouraged experimenters for a long time. In 1877 Dupuy de Lome tried steering a balloon by man power, but the man was not strong enough. In 1883 another Frenchman, Tissandier, experimented with electricity, but, as his batteries had to be light enough to be taken up in the balloon, they proved effective only in helping to weigh it down to earth again. Krebs and Renard, military aëronauts, succeeded better with electricity, for they could make a small circuit with their air-ship, provided only that no air was stirring. Enthusiasts cried out that the problem was solved, but the two aëronauts themselves, as good mathematicians, figured out that they would have to have a motor eight times more powerful than their own, and that without any increase in weight, which was an impossibility at that time.
Santos-Dumont saw plainly that none of these methods would work. What then was he to try? Why, simple enough: the petroleum motor from his automobile. The recent development of the motor-vehicle had produced a light, strong, durable motor. It was Santos-Dumont's first great claim to originality that he should have applied this to the balloon. He discovered no new principles, invented nothing that could be patented. The cigar-shaped balloon had long been used, so had the petroleum motor, but he put them together. And he did very much more than that. The very essence of success in aërial navigation is to secure light weight with great strength and power. The inventor who can build the lightest machine, which is also strong, will, other things being equal, have the greatest success. It is to Santos-Dumont's great credit that he was able to build a very light motor, that also gave a good horse-power, and a light balloon that was also very strong. The one great source of danger in using the petroleum motor in connection with a balloon is that the sparking of the motor will set fire to the inflammable hydrogen gas with which the balloon is filled, causing a terrible explosion. This, indeed, is what is thought to have caused the mortal mishap to Severo and his balloon. But Santos-Dumont was able to surmount this and many other difficulties of construction.
The inventor finally succeeded in making a motor—remarkable at that time—which, weighing only 66 pounds, would produce 3½ horse-power. It is easy to understand why a petroleum motor is such a power-producer for its size. The greater part of its fuel is in the air itself, and the air is all around the balloon, ready for use. The aëronaut does not have to take it up with him. That proportion of his fuel that he must carry, the petroleum, is comparatively insignificant in weight. A few figures will prove interesting. Two and one-half gallons of gasoline, weighing 15 pounds, will drive a 2½ horse-power autocycle 94 miles in four hours. Santos-Dumont's balloon needs less than 5⅓ gallons for a three hours' trip. This weighs but 37 pounds, and occupies a small cigar-shaped brass reservoir near the motor of his machine. An electric battery of the same horse-power would weigh 2,695 pounds.
Santos-Dumont tested his new motor very thoroughly by attaching it to a tricycle with which he made some record runs in and around Paris. Having satisfied himself that it was thoroughly serviceable he set about making the balloon, cigar-shaped, 82 feet long.
"To keep within the limit of weight," he says, "I first gave up the network and the outer cover of the ordinary balloon. I considered this sort of second envelope, holding the first within it, to be superfluous, and even harmful, if not dangerous. To the envelope proper I attached the suspension-cords of my basket directly, by means of small wooden rods introduced into horizontal hems, sewed on both sides along the stuff of the balloon for a great part of its length. Again, in order not to pass the 66 pounds weight, including varnish, I was obliged to choose Japan silk that was extremely fine, but fairly resisting. Up to this time no one had ever thought of using this for balloons intended to carry up an aëronaut, but only for little balloons carrying light registering apparatus for investigations in the upper air.

Basket of "Santos-Dumont No. 1."
Showing propeller and motor.
"I gave the order for this balloon to M. Lachambre. At first he refused to take it, saying that such a thing had never been made, and that he would not be responsible for my rashness. I answered that I would not change a thing in the plan of the balloon, if I had to sew it with my own hands. At last he agreed to sew and varnish the balloon as I desired."
After repeated trials of his motor in the basket—which he suspended in his workshop—and the making of a rudder of silk he was able, in September, 1898, to attempt real flying. But, after rising successfully in the air, the weight of the machinery and his own body swung beneath the fragile balloon was so great that while descending from a considerable height the balloon suddenly sagged down in the middle and began to shut up like a portfolio.
"At that moment," he said, "I thought that all was over, the more so as the descent, which had already become rapid, could no longer be checked by any of the usual means on board, where nothing worked.

"Santos-Dumont No. 1."
Showing how it began to fold up in the middle.
"The descent became a rapid fall. Luckily, I was falling in the neighborhood of the soft, grassy pélouse of the Longchamps race-course, where some big boys were flying kites. A sudden idea struck me. I cried to them to grasp the end of my 100-meter guide-rope, which had already touched the ground, and to run as fast as they could with it against the wind! They were bright young fellows, and they grasped the idea and the guide-rope at the same lucky instant. The effect of this help in extremis was immediate, and such as I had expected. By this manœuvre we lessened the velocity of the fall, and so avoided what would otherwise have been a terribly rough shaking up, to say the least. I was saved for the first time. Thanking the brave boys, who continued to aid me to pack everything into the air-ship's basket, I finally secured a cab and took the relic back to Paris."
His life was thus saved almost miraculously; but the accident did not deter him from going forward immediately with other experiments. The next year, 1899, he built a new air-ship called Santos-Dumont II., and made an ascension with it, but it dissatisfied him and he at once began with Santos-Dumont III., with which he made the first trip around the Eiffel Tower.
He now made ready to compete for the Deutsch prize of $20,000. The winning of this prize demanded that the trip from Saint-Cloud to the Eiffel Tower, around it and back to the starting place, a distance of some eight miles, should be made in half an hour. For this purpose he finished a much larger air-ship, Santos-Dumont V., in 1901. After a trial, made on July 12, which was attended by several accidents, the inventor decided to make a start early on the following morning, July 13. As early as four o'clock he was ready, and a crowd had begun to gather in the park.
At 6.20 the great sliding doors of the balloon-house were pushed open, and the massive inflated occupant was towed out into the open space of the park. The big pointed nose of the balloon and its fish-like belly resembled a shark gliding with lazy craft from a shadow into light waters. In the basket of the car stood the coatless aëronaut, who laughed and chatted like a boy with the crowd around him.
From the very first the conditions did not show themselves favourable for the attempt. The wind was blowing at the rate of six or seven yards a second. The change of temperature from the balloon-house to the cool morning air had somewhat condensed the hydrogen gas of the balloon, so that one end flapped about in a flabby manner. Air was pumped into the air reservoir, inside the balloon, but still the desired rigidity was not attained. But, more discouraging yet, when the motor was started, its continuous explosions gave to the practised ear signs of mechanical discord.
Nevertheless, Santos-Dumont, with his sleeves rolled up, fixed himself in his basket. His eye took a careful survey of the entire air-ship lest some preliminary had been overlooked. He counted the ballast bags under his feet in the basket, he looked to the canvas pocket of loose sand at either hand, then saw to his guide-rope.
There is a very great deal to look after in managing such a ship, and it requires a calm head and a steady hand to do it.
"Near the saddle on which I sat," he writes, "were the ends of the cords and other means for controlling the different parts of the mechanism—the electric sparking of the motor, the regulation of the carburetter, the handling of the rudder, ballast, and the shifting weights (consisting of the guide-rope and bags of sand), the managing of the balloon's valves, and the emergency rope for tearing open the balloon. It may easily be gathered from this enumeration that an air-ship, even as simple as my own, is a very complex organism; and the work incumbent on the aëronaut is no sinecure."
Several friends shook his hand, among them Mr. Deutsch. The place was very still as the man holding the guide-rope awaited the signal to let go. Then the little man in the basket above them raised his hands and shouted.

The Interior of the Aërodrome.
Showing its construction, the inflated balloon, and the pennant with its mystic letters.
At first it did not look like a race against time. The balloon rose sluggishly, and Santos-Dumont had to dump out bag after bag of sand, till finally the guide-rope was clear of the trees. All this gave him no opportunity to think of his direction, and he was drifting toward Versailles; but while yet over the Seine he pulled his rudder ropes taut. Then slowly, gracefully, the enormous spindle veered round and pointed its nose toward the Eiffel Tower. The fans spun energetically, and the air-ship settled down to business-like travelling. It marked a straight, decided line for its goal, then followed the chosen route with a considerable speed. Soon the chug-chugging of the motor could be heard no longer by the spectators, and the balloon and car grew smaller and smaller in its halo of light smoke. Those in the park saw only the screw and the rear of the balloon, like the stern of a steamer in dry dock. Before long only a dot remained against the sky. Gradually he came nearer again, almost returning to the park, but the wind drove him back across the river Seine. Suddenly the motor stopped, and the whole air-ship was seen to fall heavily toward the earth. The crowd raced away expecting to find Santos-Dumont dead and his air-ship a wreck. But they found him on his feet, with his hands in his pockets, reflectively looking up at his air-ship among the top branches of some chestnut trees in the grounds of Baron Edmund de Rothschild, Boulevard de Boulogne.
"This," he says, "was near the hôtel of Princesse Ysabel, Comtesse d'Eu, who sent up to me in my tree a champagne lunch, with an invitation to come and tell her the story of my trip.
"When my story was over, she said to me:
"'Your evolutions in the air made me think of the flight of our great birds of Brazil. I hope that you will succeed for the glory of our common country.'"
And an examination showed that the air-ship was practically uninjured.
So he escaped death a second time. Less than a month later he had a still more terrible mishap, best related in his own words. He says:
"And now I come to a terrible day—August 8, 1901. At 6.30 A.M., I started for the Eiffel Tower again, in the presence of the committee, duly convoked. I turned the goal at the end of nine minutes, and took my way back to Saint-Cloud; but my balloon was losing hydrogen through the automatic valves, the spring of which had been accidentally weakened; and it shrank visibly. All at once, while over the fortifications of Paris, near La Muette, the screw-propeller touched and cut the suspension-cords, which were sagging behind. I was obliged to stop the motor instantly; and at once I saw my air-ship drift straight back to the Eiffel Tower. I had no means of avoiding the terrible danger, except to wreck myself on the roofs of the Trocadero quarter. Without hesitation I opened the manœuvre-valve, and sent my balloon downward.
"At 32 metres (106 feet) above the ground, and with the noise of an explosion, it struck the roof of the Trocadero Hotels. The balloon-envelope was torn to rags, and fell into the courtyard of the hotels, while I remained hanging 15 metres (50 feet) above the ground in my wicker basket, which had been turned almost over, but was supported by the keel. The keel of the Santos-Dumont V. saved my life that day.
"After some minutes a rope was thrown down to me; and, helping myself with feet and hands up the wall (the few narrow windows of which were grated like those of a prison), I was hauled up to the roof. The firemen from Passy had watched the fall of the air-ship from their observatory. They, too, hastened to the rescue. It was impossible to disengage the remains of the balloon-envelope and suspension apparatus except in strips and pieces.
"My escape was narrow; but it was not from the particular danger always present to my mind during this period of my experiments. The position of the Eiffel Tower as a central landmark, visible to everybody from considerable distances, makes it a unique winning-post for an aërial race. Yet this does not alter the other fact that the feat of rounding the Eiffel Tower possesses a unique element of danger. What I feared when on the ground—I had no time to fear while in the air—was that, by some mistake of steering, or by the influence of some side-wind, I might be dashed against the Tower. The impact would burst my balloon, and I should fall to the ground like a stone. Though I never seek to fly at a great height—on the contrary, I hold the record for low altitude in a free balloon—in passing over Paris I must necessarily move above all its chimney-pots and steeples. The Eiffel Tower was my one danger—yet it was my winning-post!
"But in the air I have no time to fear. I have always kept a cool head. Alone in the air-ship, I am always very busy. I must not let go the rudder for a single instant. Then there is the strong joy of commanding. What does it feel like to sail in a dirigible balloon? While the wind was carrying me back to the Eiffel Tower I realised that I might be killed; but I did not feel fear. I was in no personal inconvenience. I knew my resources. I was excessively occupied. I have felt fear while in the air, yes, miserable fear joined to pain; but never in a dirigible balloon."
Even this did not daunt him. That very night he ordered a new air-ship, Santos-Dumont VI., and it was ready in twenty-two days. The new balloon had the shape of an elongated ellipsoid, 32 metres (105 feet) on its great axis, and 6 metres (20 feet) on its short axis, terminated fore and aft by cones. Its capacity was 605 cubic metres (21,362 cubic feet), giving it a lifting power of 620 kilos (1,362 pounds). Of this, 1,100 pounds were represented by keel, machinery, and his own weight, leaving a net lifting-power of 120 kilos (261 pounds).
On October 19, 1901, he made another attempt to round the Eiffel Tower, and was at last successful in winning the $20,000 prize. Following this great feat, Santos-Dumont continued his experiments at Monte Carlo, where he was wrecked over the Mediterranean Sea and escaped only by presence of mind, and he is still continuing his work.
The future of the dirigible balloon is open to debate. Santos-Dumont himself does not think there is much likelihood that it will ever have much commercial use. A balloon to carry many passengers would have to be so enormous that it could not support the machinery necessary to propel it, especially against a strong wind. But he does believe that the steerable balloon will have great importance in war time. He says:
"I have often been asked what present utility is to be expected of the dirigible balloon when it becomes thoroughly practicable. I have never pretended that its commercial possibilities could go far. The question of the air-ship in war, however, is otherwise. Mr. Hiram Maxim has declared that a flying machine in South Africa would have been worth four times its weight in gold. Henri Rochefort has said: 'The day when it is established that a man can direct an air-ship in a given direction and cause it to manœuvre as he wills ... there will remain little for the nations to do but to lay down their arms.'"

Falling to the Sea.

Losing its Gas and Sinking.

Boats Around the Ruined Air-Ship.
But such experiments as Santos-Dumont's, whether they result immediately in producing an air-ship of practical utility in commerce or not, have great value for the facts which they are establishing as to the possibility of balloons, of motors, of light construction, of air currents, and moreover they add to the world's sum total of experiences a fine, clean sport in which men of daring and scientific knowledge show what men can do.
Of all strange inventions, the earthquake recorder is certainly one of the most remarkable and interesting. A terrible earthquake shakes down cities in Japan, and sixteen minutes later the professor of earthquakes, in his quiet little observatory in England, measures its extent—almost, indeed, takes a picture of it. Actual waves, not unlike the waves of the sea blown up by a hurricane, have travelled through or around half the earth in this brief time; vast mountain ranges, cities, plains, and oceans have been heaved to their crests and then allowed to sink back again into their former positions. And some of these earthquake waves which sweep over the solid earth are three feet high, so that the whole of New York, perhaps, rises bodily to that height and then slides over the crest like a skiff on an ocean swell.

Professor John Milne.
From a photograph by S. Suzuki, Kudanzaka, Tokio.
At first glance this seems almost too strange and wonderful to believe, and yet this is only the beginning of the wonders which the earthquake camera—or the seismograph (earthquake writer, as the scientists call it)—has been disclosing.

The Sensitive Pendulum, or Seismograph, as it Appears with the Protecting Box Removed.
The earthquake professor who has worked such scientific magic is John Milne. He lives in a quaint old house in the little Isle of Wight, not far from Osborne Castle, where Queen Victoria made her home part of the year. Not long ago he was a resident of Japan and professor of seismology (the science of earthquakes) at the University of Tokio, where he made his first discoveries about earthquakes, and invented marvellously delicate machines for measuring and photographing them thousands of miles away. Professor Milne is an Englishman by birth, but, like many another of his countrymen, he has visited some of the strangest nooks and corners of the earth. He has looked for coal in Newfoundland; he has crossed the rugged hills of Iceland; he has been up and down the length of the United States; he has hunted wild pigs in Borneo; and he has been in India and China and a hundred other out-of-the-way places, to say nothing of measuring earthquakes in Japan. Professor Milne laid the foundation of his unusual career in a thorough education at King's College, London, and at the School of Mines. By fortunate chance, soon after his graduation, he met Cyrus Field, the famous American, to whom the world owes the beginnings of its present ocean cable system. He was then just twenty-one, young and raw, but plucky. He thought he was prepared for anything the world might bring him; but when Field asked him one Friday if he could sail for Newfoundland the next Tuesday, he was so taken with astonishment that he hesitated, whereupon Field leaned forward and looked at him in a way that Milne has never forgotten.
"My young friend, I suppose you have read that the world was made in six days. Now, do you mean to tell me that, if this whole world was made in six days, you can't get together the few things you need in four?"
And Milne sailed the next Tuesday to begin his lifework among the rough hills of Newfoundland. Then came an offer from the Japanese Government, and he went to the land of earthquakes, little dreaming that he would one day be the greatest authority in the world on the subject of seismic disturbances. His first experiments—and they were made as a pastime rather than a serious undertaking—were curiously simple. He set up rows of pins in a certain way, so that in falling they would give some indication as to the wave movements in the earth. He also made pendulums made of strings with weights tied at the end, and from his discoveries made with these elementary instruments, he planned earthquake-proof houses, and showed the engineers of Japan how to build bridges which would not fall down when they were shaken. So highly was his work regarded that the Japanese made him an earthquake professor at Tokio and supplied him with the means for making more extended experiments. And presently we find him producing artificial earthquakes by the score. He buried dynamite deep in the ground and exploded it by means of an electric button. The miniature earthquake thus produced was carefully measured with curious instruments of Professor Milne's invention. At first one earthquake was enough at any one time, but as the experiments continued, Professor Milne sometimes had five or six earthquakes all quaking together; and once so interested did he become that he forgot all about the destructive nature of earthquakes, and ventured too near. A ton or more of earth came crashing down around him, half burying him and smashing his instruments flat. All this made the Japanese rub their eyes with astonishment, and by and by the Emperor heard of it. Of course he was deeply interested in earthquakes, because there was no telling when one might come along and shake down his palace over his head. So he sent for Professor Milne, and, after assuring himself that these experimental earthquakes really had no serious intentions, he commanded that one be produced on the spot. So Professor Milne laid out a number of toy towns and villages and hills in the palace yard with a tremendous toy earthquake underneath. The Emperor and his gayly dressed followers stood well off to one side, and when Professor Milne gave the word the Emperor solemnly pressed a button, and watched with the greatest delight the curious way in which the toy cities were quaked to earth. And after that, this surprising Englishman, who could make earthquakes as easily as a Japanese makes a lacquered basket, was held in high esteem in Japan, and for more than twenty years he studied earthquakes and invented machines for recording them. Then he returned to his home in England, where he is at work establishing earthquake stations in various parts of the world, by means of which he expects to reduce earthquake measurement to an exact science, an accomplishment which will have the greatest practical value to the commercial interests of the world, as I shall soon explain.
But first for a glimpse at the curious earthquake measurer itself. To begin with, there are two kinds of instruments—one to measure near-by disturbances, and the second to measure waves which come from great distances. The former instrument was used by Professor Milne in Japan, where earthquakes are frequent; the latter is used in England. The technical name for the machine which measures distant disturbances is the horizontal pendulum seismograph, and, like most wonderful inventions, it is exceedingly simple in principle, yet doing its work with marvellous delicacy and accuracy.
In brief, the central feature of the seismograph is a very finely poised pendulum, which is jarred by the slightest disturbance of the earth, the end of it being so arranged that a photograph is taken of every quiver. Set a pendulum clock on the dining-table, jar the table, and the pendulum will swing, indicating exactly with what force you have disturbed the table. In exactly the same way the delicate pendulum of the earthquake measurer indicates the shaking of the earth.
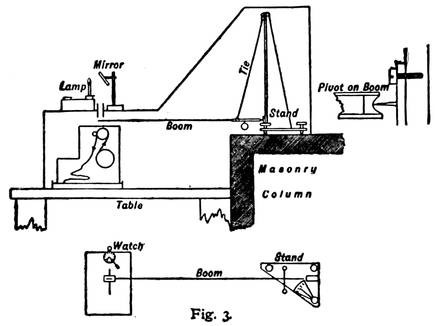
Diagram Showing Vertical and Horizontal Sections of the More Sensitive of Professor Milne's Two Pendulums, or Seismographs.
The accompanying diagram gives a very clear idea of the arrangement of the apparatus. The "boom" is the pendulum. It is customary to think of a pendulum as hanging down like that of a clock, but this is a horizontal pendulum. Professor Milne has built a very solid masonry column, reaching deep into the earth, and so firmly placed that nothing but a tremor of the hard earth itself will disturb it. Upon this is perched a firm metal stand, from the top of which the boom or pendulum, about thirty inches long, is swung by means of a "tie" or stay. The end of the boom rests against a fine, sharp pivot of steel (as shown in the little diagram to the right), so that it will swing back and forth without the least friction. The sensitive end of the pendulum, where all the quakings and quiverings are shown most distinctly, rests exactly over a narrow roll of photographic film, which is constantly turned by clockwork, and above this, on an outside stand, there is a little lamp which is kept burning night and day, year in and year out. The light from this lamp is reflected downward by means of a mirror through a little slit in the metal case which covers the entire apparatus. Of course this light affects the sensitive film, and takes a continuous photograph of the end of the boom. If the boom remains perfectly still, the picture will be merely a straight line, as shown at the extreme right and left ends of the earthquake picture on this page. But if an earthquake wave comes along and sets the boom to quivering, the picture becomes at once blurred and full of little loops and indentations, slight at first, but becoming more violent as the greater waves arrive, and then gradually subsiding. In the picture of the Borneo earthquake of September 20, 1897, taken by Professor Milne in his English laboratory, it will be seen that the quakings were so severe at the height of the disturbance that nothing is left in the photograph but a blur. On the edge of the picture can be seen the markings of the hours, 7.30, 8.30, and 9.30. Usually this time is marked automatically on the film by means of the long hand of a watch which crosses the slit beneath the mirror (as shown in the lower diagram with figure 3). The Borneo earthquake waves lasted in England, as will be seen, two hours fifty-six minutes and fifteen seconds, with about forty minutes of what are known as preliminary tremors. Professor Milne removes the film from his seismograph once a week—a strip about twenty-six feet long—develops it, and studies the photographs for earthquake signs.

Seismogram of a Borneo Earthquake that Occurred September 20, 1897.
Besides this very sensitive photographic seismograph Professor Milne has a simpler machine, not covered up and without lamp or mirror. In this instrument a fine silver needle at the end of the boom makes a steady mark on a band of smoked paper, which is kept turning under it by means of clockwork. A glance at this smoked-paper record will tell instantly at any time of day or night whether the earth is behaving itself. If the white line on the dark paper shows disturbances, Professor Milne at once examines his more sensitive photographic record for the details.
It is difficult to realise how very sensitive these earthquake pendulums really are. They will indicate the very minutest changes in the earth's level—as slight as one inch in ten miles. A pair of these pendulums placed on two buildings at opposite sides of a city street would show that the buildings literally lean toward each other during the heavy traffic period of the day, dragged over from their level by the load of vehicles and people pressing down upon the pavement between them. The earth is so elastic that a comparatively small impetus will set it vibrating. Why, even two hills tip together when there is a heavy load of moisture in a valley between them. And then when the moisture evaporates in a hot sun they tip away from each other. These pendulums show that.
Nor are these the most extraordinary things which the pendulums will do. G. K. Gilbert, of the United States Geological Survey, argues that the whole region of the great lakes is being slowly tipped to the southwest, so that some day Chicago will sink and the water outlet of the great fresh-water seas will be up the Chicago River toward the Mississippi, instead of down the St. Lawrence. Of course this movement is as slow as time itself—thousands of years must elapse before it is hardly appreciable; and yet Professor Milne's instruments will show the changing balance—a marvel that is almost beyond belief. Strangely enough, sensitive as this special instrument is to distant disturbances, it does not swerve nor quiver for near-by shocks. Thus, the blasting of powder, the heavy rumbling of wagons, the firing of artillery has little or no effect in producing a movement of the boom. The vibrations are too short; it requires the long, heavy swells of the earth to make a record.
Professor Milne tells some odd stories of his early experiences with the earthquake measurer. At one time his films showed evidences of the most horrible earthquakes, and he was afraid for the moment that all Japan had been shaken to pieces and possibly engulfed by the sea. But investigation showed that a little grey spider had been up to pranks in the box. The spider wasn't particularly interested in earthquakes, but he took the greatest pleasure in the swinging of the boom, and soon began to join in the game himself. He would catch the end of the boom with his feelers and tug it over to one side as far as ever he could. Then he would anchor himself there and hold on like grim death until the boom slipped away. Then he would run after it, and tug it over to the other side, and hold it there until his strength failed again. And so he would keep on for an hour or two until quite exhausted, enjoying the fun immensely, and never dreaming that he was manufacturing wonderful seismograms to upset the scientific world, since they seemed to indicate shocking earthquake disasters in all directions.
Mr. Cleveland Moffett, to whom I am indebted for much of the information contained in this chapter, tells how the reporters for the London papers rush off to see Professor Milne every time there is news of a great earthquake, and how he usually corrects their information. In June, 1896, for instance, the little observatory was fairly besieged with these searchers for news.
"This earthquake happened on the 17th," said they, "and the whole eastern coast of Japan was overwhelmed with tidal waves, and 30,000 lives were lost."
"That last is probable," answered Professor Milne, "but the earthquake happened on the 15th, not the 17th;" and then he gave them the exact hour and minute when the shocks began and ended.
"But our cables put it on the 17th."
"Your cables are mistaken."
And, sure enough, later despatches came with information that the destructive earthquake had occurred on the 15th, within half a minute of the time Professor Milne had specified. There had been some error of transmission in the earlier newspaper despatches.
Again, a few months later, the newspapers published cablegrams to the effect that there had been a severe earthquake at Kobe, with great injury to life and property.
"That is not true," said Professor Milne. "There may have been a slight earthquake at Kobe, but nothing that need cause alarm."
And the mail reports a few weeks later confirmed his reassuring statement, and showed that the previous sensational despatches had been grossly exaggerated.
Professor Milne is also the man to whose words cable companies lend anxious ear, for what he says often means thousands of dollars to them. Early in January, 1898, it was officially reported that two West Indian cables had broken on December 31, 1897.
"That is very unlikely," said Professor Milne; "but I have a seismogram showing that these cables may have broken at 11.30 A.M. on December 29, 1897." And then he located the break at so many miles off the coast of Haiti.
This sort of thing, which is constantly happening, would look very much like magic if Professor Milne had kept his secrets to himself; but he has given them freely to all the world.
Professor Milne has learned from his experiments that the solid earth is full of movements, and tremors, and even tides, like the sea. We do not notice them, because they are so slow and because the crests of the waves are so far apart. Professor Milne likes to tell, fancifully, how the earth "breathes." He has found that nearly all earthquake waves, whether the disturbance is in Borneo or South America, reach his laboratory in sixteen minutes, and he thinks that the waves come through the earth instead of around it. If they came around, he says, there would be two records—one from waves coming the short way and one from waves coming the long way round. But there is never more than a single record, so he concludes that the waves quiver straight through the solid earth itself, and he believes that this fact will lead to some important discoveries about the centre of our globe. Professor Milne was once asked how, if earthquake waves from every part of the earth reached his observatory in the same number of minutes, he could tell where the earthquake really was.
"I may say, in a general way," he replied, "that we know them by their signatures, just as you know the handwriting of your friends; that is, an earthquake wave which has travelled 3,000 miles makes a different record in the instruments from one that has travelled 5,000 miles; and that, again, a different record from one that has travelled 7,000 miles, and so on. Each one writes its name in its own way. It's a fine thing, isn't it, to have the earth's crust harnessed up so that it is forced to mark down for us on paper a diagram of its own movements?"
He took pencil and paper again, and dashed off an earthquake wave like this:

"There you have the signature of an earthquake wave which has travelled only a short distance, say 2,000 miles; but here is the signature of the very same wave after travelling, say, 6,000 miles:"

"You see the difference at a glance; the second seismogram (that is what we call these records) is very much more stretched out than the first, and a seismogram taken at 8,000 miles from the start would be more stretched out still. This is because the waves of transmission grow longer and longer, and slower and slower, the farther they spread from the source of disturbance. In both figures the point A, where the straight line begins to waver, marks the beginning of the earthquake; the rippling line AB shows the preliminary tremors which always precede the heavy shocks, marked C; and D shows the dying away of the earthquake in tremors similar to AB.
"Now, it is chiefly in the preliminary tremors that the various earthquakes reveal their identity. The more slowly the waves come, the longer it takes to record them, and the more stretched out they become in the seismograms. And by carefully noting these differences, especially those in time, we get our information. Suppose we have an earthquake in Japan. If you were there in person you would feel the preliminary tremors very fast, five or ten in a second, and their whole duration before the heavy shocks would not exceed ten or twenty seconds. But these preliminary tremors, transmitted to England, would keep the pendulums swinging from thirty to thirty-two minutes before the heavy shocks, and each vibration would occupy five seconds.
"There would be similar differences in the duration of the heavy vibrations; in Japan they would come at the rate of about one a second: here, at the rate of about one in twenty or forty seconds. It is the time, then, occupied by the preliminary tremors that tells us the distance of the earthquake. Earthquakes in Borneo, for instance, give preliminary tremors occupying about forty-one minutes, in Japan about half an hour, in the earthquake region east of Newfoundland about eight minutes, in the disturbed region of the West Indies about nineteen or twenty minutes, and so on. Thus the earthquake is located with absolute precision."
Most earthquakes occur in the deep bed of the ocean, in the vast valleys between ocean mountains, and the dangerous localities are now almost as well known as the principal mountain ranges of North America. There is one of these valleys, or ocean holes, off the west coast of South America from Ecuador down; there is one in the mid-Atlantic, about the equator, between twenty degrees and forty degrees west longitude: there is one at the Grecian end of the Mediterranean; one in the Bay of Bengal, and one bordering the Alps; there is the famous "Tuscarora Deep," from the Philippine Islands down to Java; and there is the North Atlantic region, about 300 miles east of Newfoundland. In the "Tuscarora Deep" the slope increases 1,000 fathoms in twenty-five miles, until it reaches a depth of 4,000 fathoms.

Pieces of a Submarine Cable Picked Up in the Gulf of Mexico in 1888.
The kinks are caused by seismic disturbances, and they show how much distortion a cable can suffer and still remain in good electrical condition, as this was found to be.
And this brings us to the consideration of one of the greatest practical advantages of the seismograph—in the exact location of cable breaks. Indeed, a large proportion of these breaks are the result of earthquakes. In a recent report Professor Milne says that there are now about twenty-seven breaks a year for 10,000 miles of cable in active use. Most of these are very costly, fifteen breaks in the Atlantic cable between 1884 and 1894 having cost the companies $3,000,000, to say nothing of loss of time. And twice it has happened in Australia (in 1880 and 1888) that the whole island has been thrown into excitement and alarm, the reserves being called out, and other measures taken, because the sudden breaking of cable connections with the outside world has led to the belief that military operations against the country were preparing by some foreign power. A Milne pendulum at Sydney or Adelaide would have made it plain in a moment that the whole trouble was due to a submarine earthquake occurring at such a time and such a place. As it was, Australia had to wait in a fever of suspense (in one case there was a delay of nineteen days) until steamers arriving brought assurances that neither Russia nor any other possibly unfriendly power had begun hostilities by tearing up the cables.
There have been submarine earthquakes in the Tuscarora, like that of June 15, 1896, that have shaken the earth from pole to pole; and more than once different cables from Java have been broken simultaneously, as in 1890, when the three cables to Australia snapped in a moment. And the great majority of breaks in the North Atlantic cables have occurred in the Newfoundland hollow, where there are two slopes, one dropping from 708 to 2,400 fathoms in a distance of sixty miles, and the other from 275 to 1,946 fathoms within thirty miles. On October 4, 1884, three cables, lying about ten miles apart, broke simultaneously at the spot. The significance of such breaks is greater when the fact is borne in mind that cables frequently lie uninjured for many years on the great level plains of the ocean bed, where seismic disturbances are infrequent.
The two chief causes of submarine earthquakes are landslides, where enormous masses of earth plunge from a higher to a lower level, and in so doing crush down upon the cable, and "faults," that is, subsidences of great areas, which occur on land as well as at the bottom of the sea, and which in the latter case may drag down imbedded cables with them.
It is in establishing the place and times of these breaks that Professor Milne's instruments have their greatest practical value; scientifically no one can yet calculate their value.

Record Made on a Stationary Surface by the Vibrations of the Japanese Earthquake of July 19, 1891.
Showing the complicated character of the motion (common to most earthquakes), and also the course of a point at the centre of disturbance.
In addition to the first instrument set up by Professor Milne in Tokio in 1883, which is still recording earthquakes, there are now in operation about twenty other seismographs in various parts of the world, so that earthquake information is becoming very accurate and complete, and there is even an attempt being made to predict earthquakes just as the weather bureau predicts storms. In any event Professor Milne's invention must within a few years add greatly to our knowledge of the wonders of the planet on which we live.
No feats of discovery, not even the search for the North Pole or Stanley's expeditions in the heart of Africa, present more points of fascinating interest than the attempts now being made by scientists to explore the extreme limits of temperature. We live in a very narrow zone in what may be called the great world of heat. The cut on the opposite page represents an imaginary thermometer showing a few of the important temperature points between the depths of the coldest cold and the heights of the hottest heat—a stretch of some 10,461 degrees. We exist in a narrow space, as you will see, varying from 100° or a little more above the zero point to a possible 50° below; that is, we can withstand these narrow extremes of temperature. If some terrible world catastrophe should raise the temperature of our summers or lower that of our winters by a very few degrees, human life would perish off the earth.
But though we live in such narrow limits, science has found ways of exploring the great heights of heat above us and of reaching and measuring the depths of cold below us, with the result of making many important and interesting discoveries.
I have written in the former "Boys' Book of Inventions" of that wonderful product of science, liquid air—air submitted to such a degree of cold that it ceases to be a gas and becomes a liquid. This change occurs at a temperature 312° below zero. Professor John Dewar, of England, who has made some of the most interesting of discoveries in the region of great cold, not only reached a temperature low enough to produce liquid air, but he succeeded in going on down until he could freeze this marvellous liquid into a solid—a sort of air ice. Not content even with this astonishing degree of cold, Professor Dewar continued his experiments until he could reduce hydrogen—that very light gas—to a liquid, at 440° below zero, and then, strange as it may seem, he also froze liquid hydrogen into a solid. From his experiments he finally concluded that the "absolute zero"—that is, the place where there is no heat—was at a point 461° below zero. And he has been able to produce a temperature, artificially, within a very few degrees of this utmost limit of cold.
Think what this absolute zero means. Heat, we know, like electricity and light, is a vibratory or wave motion in the ether. The greater the heat, the faster the vibrations. We think of all the substances around us as solids, liquids, and gases, but these are only comparative terms. A change of temperature changes the solid into the liquid, or the gas into the solid. Take water, for instance. In the ordinary temperature of summer it is a liquid, in winter it is a hard crystalline substance called ice; apply the heat of a stove and it becomes steam, a gas. So with all other substances. Air to us is an invisible gas, but if the earth should suddenly drop in temperature to 312° below zero all the air would fall in liquid drops like rain and fill the valleys of the earth with lakes and oceans. Still a little colder and these lakes and oceans would freeze into solids. Similarly, steel seems to us a very hard and solid substance, but apply enough heat and it boils like water, and finally, if the heat be increased, it becomes a gas.
Imagine, if you can, a condition in which all substances are solids; where the vibrations known as heat have been stilled to silence; where nothing lives or moves; where, indeed, there is an awful nothingness; and you can form an idea of the region of the coldest cold—in other words, the region where heat does not exist. Our frozen moon gives something of an idea of this condition, though probably, cold and barren as it is, the moon is still a good many degrees in temperature above the absolute zero.
Some of the methods of exploring these depths of cold are treated in the chapter on liquid air already referred to. Our interest here centres in the other extreme of temperature, where the heat vibrations are inconceivably rapid; where nearly all substances known to man become liquids and gases; where, in short, if the experimenter could go high enough, he could reach the awful degree of heat of the burning sun itself, estimated at over 10,000 degrees. It is in the work of exploring these regions of great heat that such men as Moissan, Siemens, Faure, and others have made such remarkable discoveries, reaching temperatures as high as 7,000, or over twice the heat of boiling steel. Their accomplishments seem the more wonderful when we consider that a temperature of this degree burns up or vaporises every known substance. How, then, could these men have made a furnace in which to produce this heat? Iron in such a heat would burn like paper, and so would brick and mortar. It seems inconceivable that even science should be able to produce a degree of heat capable of consuming the tools and everything else with which it is produced.
The heat vibrations at 7,000° are so intense that nickel and platinum, the most refractory, the most unmeltable of metals, burn like so much bee's-wax; the best fire-brick used in lining furnaces is consumed by it like lumps of rosin, leaving no trace behind. It works, in short, the most marvellous, the most incredible transformations in the substances of the earth.
Indeed, we have to remember that the earth itself was created in a condition of great heat—first a swirling, burning gas, something like the sun of to-day, gradually cooling, contracting, rounding, until we have our beautiful world, with its perfect balance of gases, liquids, solids, its splendid life. A dying volcano here and there gives faint evidence of the heat which once prevailed over all the earth.
It was in the time of great heat that the most beautiful and wonderful things in the world were wrought. It was fierce heat that made the diamond, the sapphire, and the ruby; it fashioned all of the most beautiful forms of crystals and spars; and it ran the gold and silver of the earth in veins, and tossed up mountains, and made hollows for the seas. It is, in short, the temperature at which worlds were born.
More wonderful, if possible, than the miracles wrought by such heat is the fact that men can now produce it artificially; and not only produce, but confine and direct it, and make it do their daily service. One asks himself, indeed, if this can really be; and it was under the impulse of some such incredulity that I lately made a visit to Niagara Falls, where the hottest furnaces in the world are operated. Here clay is melted in vast quantities to form aluminium, a metal as precious a few years ago as gold. Here lime and carbon, the most infusible of all the elements, are joined by intense heat in the curious new compound, calcium carbide, a bit of which dropped in water decomposes almost explosively, producing the new illuminating gas, acetylene. Here, also, pure phosphorus and the phosphates are made in large quantities; and here is made carborundum—gem-crystals as hard as the diamond and as beautiful as the ruby.
An extensive plant has also been built to produce the heat necessary to make graphite such as is used in your lead-pencils, and for lubricants, stove-blacking, and so on. Graphite has been mined from the earth for thousands of years; it is pure carbon, first cousin to the diamond. Ten years ago the possibility of its manufacture would have been scouted as ridiculous; and yet in these wonderful furnaces, which repeat so nearly the processes of creation, graphite is as easily made as soap. The marvel-workers at Niagara Falls have not yet been able to make diamonds—in quantities. The distinguished French chemist Moissan has produced them in his laboratory furnaces—small ones, it is true, but diamonds; and one day they may be shipped in peck boxes from the great furnaces at Niagara Falls. This is no mere dream; the commercial manufacture of diamonds has already had the serious consideration of level-headed, far-seeing business men, and it may be accounted a distinct probability. What revolution the achievement of it would work in the diamond trade as now constituted and conducted no one can say.
These marvellous new things in science and invention have been made possible by the chaining of Niagara to the wheels of industry. The power of the falling water is transformed into electricity. Electricity and heat are both vibratory motions of the ether; science has found that the vibrations known as electricity can be changed into the vibrations known as heat. Accordingly, a thousand horse-power from the mighty river is conveyed as electricity over a copper wire, changed into heat and light between the tips of carbon electrodes, and there works its wonders. In principle the electrical furnace is identical with the electric light. It is scarcely twenty years since the first electrical furnaces of real practical utility were constructed; but if the electrical furnaces to-day in operation at Niagara Falls alone were combined into one, they would, as one scientist speculates, make a glow so bright that it could be seen distinctly from the moon—a hint for the astronomers who are seeking methods for communicating with the inhabitants of Mars. One furnace has been built in which an amount of heat energy equivalent to 700 horse-power is produced in an arc cavity not larger than an ordinary water tumbler.
On reaching Niagara Falls, I called on Mr. E. G. Acheson, whose name stands with that of Moissan as a pioneer in the investigation of high temperatures. Mr. Acheson is still a young man—not more than forty-five at most—and clean-cut, clear-eyed, and genial, with something of the studious air of a college professor. He is pre-eminently a self-made man. At twenty-four he found a place in Edison's laboratory—"Edison's college of inventions," he calls it—and, at twenty-five, he was one of the seven pioneers in electricity who (in 1881-82) introduced the incandescent lamp in Europe. He installed the first electric-light plants in the cities of Milan, Genoa, Venice, and Amsterdam, and during this time was one of Edison's representatives in Paris.
"I think the possibility of manufacturing genuine diamonds," he said to me, "has dazzled more than one young experimenter. My first efforts in this direction were made in 1880. It was before we had command of the tremendous electric energy now furnished by the modern dynamo, and when the highest heat attainable for practical purposes was obtained by the oxy-hydrogen flame. Even this was at the service of only a few experimenters, and certainly not at mine. My first experiments were made in what I might term the 'wet way'; that is, by the process of chemical decomposition by means of an electric current. Very interesting results were obtained, which even now give promise of value; but the diamond did not materialise.
"I did not take up the subject again until the dynamo had attained high perfection and I was able to procure currents of great power. Calling in the aid of the 6,500 degrees Fahrenheit or more of temperature produced by these electric currents, I once more set myself to the solution of the problem. I now had, however, two distinct objects in view: first, the making of a diamond; and, second, the production of a hard substance for abrasive purposes. My experiments in 1880 had resulted in producing a substance of extreme hardness, hard enough, indeed, to scratch the sapphire—the next hardest thing to the diamond—and I saw that such a material, cheaply made, would have great value.
"My first experiment in this new series was of a kind that would have been denounced as absurd by any of the old-school book-chemists, and had I had a similar training, the probability is that I should not have made such an investigation. But 'fools rush in where angels fear to tread,' and the experiment was made."
This experiment by Mr. Acheson, extremely simple in execution, was the first act in rolling the stone from the entrance to a veritable Aladdin's cave, into which a multitude of experimenters have passed in their search for nature's secrets; for, while the use of the electrical furnace in the reduction of metals—in the breaking down of nature's compounds—was not new, its use for synthetic chemistry—for the putting together, the building up, the formation of compounds—was entirely new. It has enabled the chemist not only to reproduce the compounds of nature, but to go further and produce valuable compounds that are wholly new and were heretofore unknown to man. Mr. Acheson conjectured that carbon, if made to combine with clay, would produce an extremely hard substance; and that, having been combined with the clay, if it should in the cooling separate again from the clay, it would issue out of the operation as diamond. He therefore mixed a little clay and coke dust together, placed them in a crucible, inserted the ends of two electric-light carbons into the mixture, and connected the carbons with a dynamo. The fierce heat generated at the points of the carbons fused the clay, and caused portions of the carbon to dissolve. After cooling, a careful examination was made of the mass, and a few small purple crystals were found. They sparkled with something of the brightness of diamonds, and were so hard that they scratched glass. Mr. Acheson decided at once that they could not be diamonds; but he thought they might be rubies or sapphires. A little later, though, when he had made similar crystals of a larger size, he found that they were harder than rubies, even scratching the diamond itself. He showed them to a number of expert jewellers, chemists, and geologists. They had so much the appearance of natural gems that many experts to whom they were submitted without explanation decided that they must certainly be of natural production. Even so eminent an authority as Geikie, the Scotch geologist, on being told, after he had examined them, that the crystals were manufactured in America, responded testily: "These Americans! What won't they claim next? Why, man, those crystals have been in the earth a million years."
Mr. Acheson decided at first that his crystals were a combination of carbon and aluminium, and gave them the name carborundum. He at once set to work to manufacture them in large quantities for use in making abrasive wheels, whetstones, and sandpaper, and for other purposes for which emery and corundum were formerly used. He soon found by chemical analysis, however, that carborundum was not composed of carbon and aluminium, but of carbon and silica, or sand, and that he had, in fact, created a new substance; so far as human knowledge now extends, no such combination occurs anywhere in nature. And it was made possible only by the electrical furnace, with its power of producing heat of untold intensity.

The Furnace-Room, where Carborundum is Made.
"A great, dingy brick building, open at the sides like a shed."
In order to get a clear understanding of the actual workings of the electrical furnace, I visited the plant where Mr. Acheson makes carborundum. The furnace-room is a great, dingy brick building, open at the sides like a shed. It is located only a few hundred yards from the banks of the Niagara River and well within the sound of the great falls. Just below it, and nearer the city, stands the handsome building of the Power Company, in which the mightiest dynamos in the world whir ceaselessly, day and night, while the waters of Niagara churn in the water-wheel pits below. Heavy copper wires carrying a current of 2,200 volts lead from the power-house to Mr. Acheson's furnaces, where the electrical energy is transformed into heat.
There are ten furnaces in all, built loosely of fire-brick, and fitted at each end with electrical connections. And strange they look to one who is familiar with the ordinary fuel furnace, for they have no chimneys, no doors, no drafts, no ash-pits, no blinding glow of heat and light. The room in which they stand is comfortably cool. Each time a furnace is charged it is built up anew; for the heat produced is so fierce that it frequently melts the bricks together, and new ones must be supplied. There were furnaces in many stages of development. One had been in full blast for nearly thirty hours, and a weird sight it was. The top gave one the instant impression of the seamy side of a volcano. The heaped coke was cracked in every direction, and from out of the crevices and depressions and from between the joints of the loosely built brick walls gushed flames of pale green and blue, rising upward, and burning now high, now low, but without noise beyond a certain low humming. Within the furnace—which was oblong in shape, about the height of a man, and sixteen feet long by six wide—there was a channel, or core, of white-hot carbon in a nearly vaporised state. It represented graphically in its seething activity what the burning surface of the sun might be—and it was almost as hot. Yet the heat was scarcely manifest a dozen feet from the furnace, and but for the blue flames rising from the cracks in the envelope, or wall, one might have laid his hand almost anywhere on the bricks without danger of burning it.

Taking Off a Crust of the Furnace at Night.
The light is so intense that you cannot look at it without hurting the eyes.
In the best modern blast-furnaces, in which the coal is supplied with special artificial draft to make it burn the more fiercely, the heat may reach 3,000 degrees Fahrenheit. This is less than half of that produced in the electrical furnace. In porcelain kilns, the potters, after hours of firing, have been able to produce a cumulative temperature of as much as 3,300 degrees Fahrenheit; and this, with the oxy-hydrogen flame (in which hydrogen gas is spurred to greater heat by an excess of oxygen), is the very extreme of heat obtainable by any artificial means except by the electrical furnace. Thus the electrical furnace has fully doubled the practical possibilities in the artificial production of heat.
Mr. Fitzgerald, the chemist of the Acheson Company, pointed out to me a curious glassy cavity in one of the half-dismantled furnaces. "Here the heat was only a fraction of that in the core," he said. But still the fire-brick—and they were the most refractory produced in this country—had been melted down like butter. The floors under the furnace were all made of fire-brick, and yet the brick had run together until they were one solid mass of glassy stone. "We once tried putting a fire-brick in the centre of the core," said Mr. Fitzgerald, "just to test the heat. Later, when we came to open the furnace, we couldn't find a vestige of it. The fire had totally consumed it, actually driving it all off in vapour."
Indeed, so hot is the core that there is really no accurate means of measuring its temperature, although science has been enabled by various curious devices to form a fairly correct estimate. The furnace has a provoking way of burning up all of the thermometers and heat-measuring devices which are applied to it. A number of years ago a clever German, named Segar, invented a series of little cones composed of various infusible earths like clay and feldspar. He so fashioned them that one in the series would melt at 1,620 degrees Fahrenheit, another at 1,800 degrees, and so on up. If the cones are placed in a pottery kiln, the potter can tell just what degree of temperature he has reached by the melting of the cones one after another. But in Mr. Acheson's electrical furnaces all the cones would burn up and disappear in two minutes. The method employed for coming at the heat of the electrical furnace, in some measure, is this: a thin filament of platinum is heated red hot—1,800 degrees Fahrenheit—by a certain current of electricity. A delicate thermometer is set three feet away, and the reading is taken. Then, by a stronger current, the filament is made white hot—3,400 degrees Fahrenheit—and the thermometer moved away until it reads the same as it read before. Two points in a distance-scale are thus obtained as a basis of calculation. The thermometer is then tried by an electrical furnace. To be kept at the same marking it must be placed much farther away than in either of the other instances. A simple computation of the comparative distances with relation to the two well-ascertained temperatures gives approximately, at least, the temperature of the electrical furnace. Some other methods are also employed. None is regarded as perfectly exact; but they are near enough to have yielded some very interesting and valuable statistics regarding the power of various temperatures. For instance, it has been found that aluminium becomes a limpid liquid at from 4,050 to 4,320 degrees Fahrenheit, and that lime melts at from 4,940 to 5,400 degrees, and magnesia at 4,680 degrees.
There are two kinds of electrical furnaces, as there are two kinds of electric lights—arc and incandescent. Moissan has used the arc furnace in all of his experiments, but Mr. Acheson's furnaces follow rather the principle of the incandescent lamp. "The incandescent light," said Mr. Fitzgerald, "is produced by the resistance of a platinum wire or a carbon filament to the passage of a current of electricity. Both light and heat are given off. In our furnace, the heat is produced by the resistance of a solid cylinder or core of pulverised coke to the passage of a strong current of electricity. When the core becomes white hot it causes the materials surrounding it to unite chemically, producing the carborundum crystals."
The materials used are of the commonest—pure white sand, coke, sawdust, and salt. The sand and coke are mixed in the proportions of sixty to forty, the sawdust is added to keep the mixture loose and open, and the salt to assist the chemical combination of the ingredients. The furnace is half filled with this mixture, and then the core of coke, twenty-one inches in diameter, is carefully moulded in place. This core is sixteen feet long, reaching the length of the furnace, and connecting at each end with an immense carbon terminal, consisting of no fewer than twenty-five rods of carbon, each four inches square and nearly three feet long. These terminals carry the current into the core from huge insulated copper bars connected from above. When the core is complete, more of the carborundum mixture is shovelled in and tramped down until the furnace is heaping full.
Everything is now ready for the electric current. The wires from the Niagara Falls power-plant come through an adjoining building, where one is confronted, upon entering, with this suggestive sign:
DANGER
2,200 Volts.
Tesla produces immensely higher voltages than this for laboratory experiments, but there are few more powerful currents in use in this country for practical purposes. Only about 2,000 volts are required for executing criminals under the electric method employed in New York; 400 volts will run a trolley-car. It is hardly comfortable to know that a single touch of one of the wires or switches in this room means almost certain death. Mr. Fitzgerald gave me a vivid demonstration of the terrific destructive force of the Niagara Falls current. He showed me how the circuit was broken. For ordinary currents, the breaking of a circuit simply means a twist of the wrist and the opening of a brass switch. Here, however, the current is carried into a huge iron tank full of salt water. The attendant, pulling on a rope, lifts an iron plate from the tank. The moment it leaves the water, there follow a rumbling crash like a thunder-clap, a blinding burst of flame, and thick clouds of steam and spray. The sight and sound of it make you feel delicate about interfering with a 2,200-volt current.
This current is, indeed, too strong in voltage for the furnaces, and it is cut down, by means of what were until recently the largest transformers in the world, to about 100 volts, or one-fourth the pressure used on the average trolley line. It is now, however, a current of great intensity—7,500 ampères, as compared with the one-half ampère used in an incandescent lamp; and it requires eight square inches of copper and 400 square inches of carbon to carry it.
Within the furnace, when the current is turned on, a thousand horse-power of energy is continuously transformed into heat. Think of it! Is it any wonder that the temperature goes up? And this is continued for thirty-six hours steadily, until 36,000 "horse-power hours" are used up and 7,000 pounds of the crystals have been formed. Remembering that 36,000 horse-power hours, when converted into heat, will raise 72,000 gallons of water to the boiling point, or will bring 350 tons of iron up to a red heat, one can at least have a sort of idea of the heat evolved in a carborundum furnace.
When the coke core glows white, chemical action begins in the mixture around it. The top of the furnace now slowly settles, and cracks in long, irregular fissures, sending out a pungent gas which, when lighted, burns lambent blue. This gas is carbon monoxide, and during the process nearly six tons of it are thrown off and wasted. It seems, indeed, a somewhat extravagant process, for fifty-six pounds of gas are produced for every forty of carborundum.
"It is very distinctly a geological condition," said Mr. Fitzgerald; "crystals are not only formed exactly as they are in the earth, but we have our own little earthquakes and volcanoes." Not infrequently gas collects, forming a miniature mountain, with a crater at its summit, and blowing a magnificent fountain of flame, lava, and dense white vapour high into the air, and roaring all the while in a most terrifying manner. The workmen call it "blowing off."

Blowing Off.
"Not infrequently gas collects, forming a miniature mountain, with a crater at its summit, and blowing a magnificent fountain of flame, lava, and dense white vapour high into the air, and roaring all the while in a most terrifying manner."
At the end of thirty-six hours the current is cut off, and the furnace is allowed to cool, the workmen pulling down the brick as rapidly as they dare. At the centre of the furnace, surrounding the core, there remains a solid mass of carborundum as large in diameter as a hogshead. Portions of this mass are sometimes found to be composed of pure, beautifully crystalline graphite. This in itself is a surprising and significant product, and it has opened the way directly to graphite-making on a large scale. An important and interesting feature of the new graphite industry is the utilisation it has effected of a product from the coke regions of Pennsylvania which was formerly absolute waste.
To return to carborundum: when the furnace has been cooled and the walls torn away, the core of carborundum is broken open, and the beautiful purple and blue crystals are laid bare, still hot. The sand and the coke have united in a compound nearly as hard as the diamond and even more indestructible, being less inflammable and wholly indissoluble in even the strongest acids. After being taken out, the crystals are crushed to powder and combined in various forms convenient for the various uses for which it is designed.
I asked Mr. Acheson if he could make diamonds in his furnaces. "Possibly," he answered, "with certain modifications." Diamonds, as he explained, are formed by great heat and great pressure. The great heat is now easily obtained, but science has not yet learned nature's secret of great pressure. Moissan's method of making diamonds is to dissolve coke dust in molten iron, using a carbon crucible into which the electrodes are inserted. When the whole mass is fluid, the crucible and its contents are suddenly dashed into cold water or melted lead. This instantaneous cooling of the iron produces enormous pressure, so that the carbon is crystallised in the form of diamond.
But whatever it may or may not yet be able to do in the matter of diamond-making, there can be no doubt that the possibilities of the electrical furnace are beyond all present conjecture. With American inventors busy in its further development, and with electricity as cheap as the mighty power of Niagara can make it, there is no telling what new and wonderful products, now perhaps wholly unthought-of by the human race, it may become possible to manufacture, and manufacture cheaply.
It seems daring and wonderful enough, the idea of setting the sun itself to the heavy work of men, producing the power which will help to turn the wheels of this age of machinery.
At Los Angeles, Cal., I went out to see the sun at work pumping water. The solar motor, as it is called, was set up at one end of a great enclosure where ostriches are raised. I don't know which interested me more at first, the sight of these tall birds striding with dignity about their roomy pens or sitting on their big yellow eggs—just as we imagine them wild in the desert—or the huge, strange creation of man by which the sun is made to toil. I do not believe I could have guessed the purpose of this unique invention if I had not known what to expect. I might have hazarded the opinion that it was some new and monstrous searchlight: beyond that I think my imagination would have failed me. It resembled a huge inverted lamp-shade, or possibly a tremendous iron-ribbed colander, bottomless, set on its edge and supported by a steel framework. Near by there was a little wooden building which served as a shop or engine-house. A trough full of running water led away on one side, and from within came the steady chug-chug, chug-chug of machinery, apparently a pump. So this was the sun-subduer! A little closer inspection, with an audience of ostriches, very sober, looking over the fence behind me and wondering, I suppose, if I had a cracker in my pocket, I made out some other very interesting particulars in regard to this strange invention. The colander-like device was in reality, I discovered, made up of hundreds and hundreds (nearly 1,800 in all) of small mirrors, the reflecting side turned inward, set in rows on the strong steel framework which composed the body of the great colander. By looking up through the hole in the bottom of the colander I was astonished by the sight of an object of such brightness that it dazzled my eyes. It looked, indeed, like a miniature sun, or at least like a huge arc light or a white-hot column of metal. And, indeed, it was white hot, glowing, burning hot—a slim cylinder of copper set in the exact centre of the colander. At the top there was a jet of white steam like a plume, for this was the boiler of this extraordinary engine.
"It is all very simple when you come to see it," the manager was saying to me. "Every boy has tried the experiment of flashing the sunshine into his chum's window with a mirror. Well, we simply utilise that principle. By means of these hundreds of mirrors we reflect the light and heat of the sun on a single point at the centre of what you have described as a colander. Here we have the cylinder of steel containing the water which we wish heated for steam. This cylinder is thirteen and one-half feet long and will hold one hundred gallons of water. If you could see it cold, instead of glowing with heat, you would find it jet black, for we cover it with a peculiar heat-absorbing substance made partly of lampblack, for if we left it shiny it would re-reflect some of the heat which comes from the mirrors. The cold water runs in at one end through this flexible metallic hose, and the steam goes out at the other through a similar hose to the engine in the house."
Though this colander, or "reflector," as it is called, is thirty-three and one-half feet in diameter at the outer edge and weighs over four tons, it is yet balanced perfectly on its tall standards. It is, indeed, mounted very much like a telescope, in meridian, and a common little clock in the engine-room operates it so that it always faces the sun, like a sunflower, looking east in the morning and west in the evening, gathering up the burning rays of the sun and throwing them upon the boiler at the centre. In the engine-house I found a pump at work, chug-chugging like any pump run by steam-power, and the water raised by sun-power flowing merrily away. The manager told me that he could easily get ten horse-power; that, if the sun was shining brightly, he could heat cold water in an hour to produce 150 pounds of steam.
The wind sometimes blows a gale in Southern California, and I asked the manager what provision had been made for keeping this huge reflector from blowing away.
"Provision is made for varying wind-pressures," he said, "so that the machine is always locked in any position, and may only be moved by the operating mechanism, unless, indeed, the whole structure should be carried away. It is designed to withstand a wind-pressure of 100 miles an hour. It went through the high gales of the November storm without a particle of damage. One of the peculiar characteristics of its construction is that it avoids wind-pressure as much as possible."
The operation of the motor is so simple that it requires very little human labour. When power is desired, the reflector must be swung into focus—that is, pointed exactly toward the sun—which is done by turning a crank. This is not beyond the power of a good-sized boy. There is an indicator which readily shows when a true focus is obtained. This done, the reflector follows the sun closely all day. In about an hour the engine can be started by a turn of the throttle-valve. As the engine is automatic and self-oiling, it runs without further attention. The supply of water to the boiler is also automatic, and is maintained at a constant height without any danger of either too much or too little water. Steam-pressure is controlled by means of a safety-valve, so that it may never reach a dangerous point. The steam passes from the engine to the condenser and thence to the boiler, and the process is repeated indefinitely.
Having now the solar motor, let us see what it is good for, what is expected of it. Of course when the sun does not shine the motor does not work, so that its usefulness would be much curtailed in a very cloudy country like England, for instance; but here in Southern California and in all the desert region of the United States and Mexico, to say nothing of the Sahara in Africa, where the sun shines almost continuously, the solar motor has its greatest sphere of usefulness, and, indeed, its greatest need; for these lands of long sunshine, the deserts, are also the lands of parched fruitlessness, of little water, so that the invention of a motor which will utilise the abundant sunshine for pumping the much-needed water has a peculiar value here.
The solar motor is expected to operate at all seasons of the year, regardless of all climatic conditions, with the single exception of cloudy skies. Cold makes no difference whatever. The best results from the first model used in experimental work at Denver were obtained at a time when the pond from which the water was pumped was covered with a thick coating of ice. But, of course, the length of the solar day is longer in the summer, giving more heat and more power. The motor may be depended upon for work from about one hour and a half after sunrise to within half an hour of sunset. In the summer time this would mean about twelve hours' constant pumping.
Think what such an invention means, if practically successful, to the vast stretches of our arid Western land, valueless without water. Spread all over this country of Arizona, New Mexico, Southern California, and other States are thousands of miles of canals to bring in water from the rivers for irrigating the deserts, and there are untold numbers of wind-mills, steam and gasoline pumps which accomplish the same purpose more laboriously. Think what a new source of cheap power will do—making valuable hundreds of acres of desert land, providing homes for thousands of busy Americans. Indeed, a practical solar motor might make habitable even the Sahara Desert. And it can be used in many other ways besides for pumping water. Threshing machines might be run by this power, and, converted into electricity and saved up in storage batteries, it might be used for lighting houses, even for cooking dinners, or in fact for any purpose requiring power.
These solar motors can be built at no great expense. I was told that ten-horse-power plants would cost about $200 per horse-power, and one-hundred-horse-power plants about $100 per horse-power. This would include the entire plant, with engine and pump complete. When it is considered that the annual rental of electric power is frequently $50 per horse-power, whether it is used or not, it will be seen that the solar motor means a great deal, especially in connection with irrigation enterprises.
And the time is coming—long-headed inventors saw it many years ago—when some device for the direct utilisation of the sun's heat will be a necessity. The world is now using its coal at a very rapid rate; its wood, for fuel purposes, has already nearly disappeared, so that, within a century or two, new ways of furnishing heat and power must be devised or the human race will perish of cold and hunger. Fortunately there are other sources of power at hand; the waterfalls, the Niagaras, which, converted into electricity, may yet heat our sitting-rooms and cook our dinners. There is also wind-power, now used to a limited extent by means of wind-mills. But greater than either of these sources is the unlimited potentiality of the tides of the sea, which men have sought in vain to harness, and the direct heat of the sun itself. Some time in the future these will be subdued to the purpose of men, perhaps our main dependence for heat and power.
When we come to think of it, the harnessing of the sun is not so very strange. In fact, we have had the sun harnessed since the dawn of man on the earth, only indirectly. Without the sun there would be nothing here—no men, no life. Coal is nothing but stored-up, bottled sunshine. The sunlight of a million years ago produced forests, which, falling, were buried in the earth and changed into coal. So when we put coal in the cook-stove we may truthfully say that we are boiling the kettle with million-year-old sunshine. Similarly there would be no waterfalls for us to chain and convert into electricity, as we have chained Niagara, if the sun did not evaporate the waters of the sea, take it up in clouds, and afterward empty the clouds in rain on the mountain-tops from whence the water tumbles down again to the sea. So no wind would blow without the sun to work changes in the air.
In short, therefore, we have been using the sunlight all these years, hardly knowing it, but not directly. And think of the tremendous amount of heat which comes to the earth from the sun. Every boy has tried using a burning-glass, which, focusing a few inches of the sun's rays, will set fire to paper or cloth.
Professor Langley says that "the heat which the sun, when near the zenith, radiates upon the deck of a steamship would suffice, could it be turned into work without loss, to drive her at a fair rate of speed."
The knowledge of this enormous power going to waste daily and hourly has inspired many inventors to work on the problem of the solar motor. Among the greatest of these was the famous Swedish engineer, John Ericsson, who invented the iron-clad Monitor. He constructed a really workable solar motor, different in construction but similar in principle to the one in California which I have described. In 1876 Ericsson said:
"Upon one square mile, using only one-half of the surface and devoting the rest to buildings, roads, etc., we can drive 64,800 steam-engines, each of 100 horse-power, simply by the heat radiating from the sun. Archimedes, having completed his calculation of the force of a lever, said that he could move the earth. I affirm that the concentration of the heat radiated by the sun would produce a force capable of stopping the earth in its course."
A firm believer in the truth of his theories, he devoted the last fifteen years of his life and $100,000 to experimental work on his solar engine. For various reasons Ericsson's invention was not a practical success; but now that modern inventors, with their advancing knowledge of mechanics, have turned their attention to the problem, and now that the need of the solar motor is greater than ever before, especially in the world's deserts, we may look to see a practical and successful machine. Perhaps the California motor may prove the solution of the problem; perhaps it will need improvements, which use and experience will indicate; perhaps it may be left for a reader of these words to discover the great secret and make his fortune.
No lad of to-day, ambitious to become a scientist or inventor, reading of all the wonderful and revolutionising discoveries and inventions of recent years, need fear for plenty of new problems to solve in the future. No, the great problems have not all been solved. We have the steam-engine, the electric motor, the telegraph, the telephone, the air-ship, but not one of them is perfect, not one that does not bring to the attention of inventors scores of entirely new problems for solution. The further we advance in science and mechanics the further we see into the marvels of our wonderful earth and of our life, and the more there is for us to do.
As population increases and people become more intelligent there is a constant demand for new things, new machinery which will enable the human race to move more rapidly and crowd more work and more pleasure into our short human life. One man working to-day with machinery can accomplish as much as many men of a hundred years ago; he can live in a house that would then have been a palace; enjoy advantages of education, amusement, luxury, that would then have been possible only to kings and princes.
And the very greatest of all the problems which the inventors and scientists of coming generations must solve is the question—seemingly commonplace—of food.
We who live in this age of plenty can hardly realise that food could ever be a problem. But far-sighted scientists have already begun to look forward to the time when there will be so many people on the earth that the farms and fields will not supply food for every one. It is a well-known fact that the population of the world is increasing enormously. Think how America has been expanding; a whole continent overrun and settled almost within a century and a half! Nearly all the land that can be successfully farmed has already been taken up, and the land in some of the older settled localities, like Virginia and the New England States, has been so steadily cropped that it is failing in fertility, so that it will not raise as much as it would years ago. In Europe no crop at all can be raised without quantities of fertiliser.
While there was yet new country to open up, while America and Australia were yet virgin soil, there was no immediate cause for alarm; but, as no less an authority than Sir William Crookes pointed out a few years ago in a lecture before the British Association, the new land has now for the most part been opened and tamed to the plough or utilised for grazing purposes. And already we are hearing of worn-out land in Dakota—the paradise of the wheat producer. The problem, therefore, is simple enough: the world is reaching the limits of its capacity for food production, while the population continues to increase enormously: how soon will starvation begin? Sir William Crookes has prophesied, I believe, that the acute stage of the problem will be reached within the next fifty years, a time when the call of the world for food cannot be supplied. If it were not for our coming inventors and scientists it would certainly be a gloomy outlook for the human race.
But science has already foreseen this problem. When Sir William Crookes gave his address he based his arguments on modern agricultural methods; he did not look forward into the future, he did not show any faith in the scientists and inventors who are to come, who are now boys, perhaps. He did not even take cognisance of the work that had already been done. For inventors and scientists are already grappling with this problem of food.
In a nutshell, the question of food production is a question of nitrogen.
This must be explained. A crop of wheat, for instance, takes from the soil certain elements to help make up the wheat berry, the straw, the roots. And the most important of all the elements it takes is nitrogen. When we eat bread we take this nitrogen that the wheat has gathered from the soil into our own bodies to build up our bones, muscles, brains. Each wheat crop takes more nitrogen from the soil, and finally, if this nitrogen is not given back to the earth in some way, wheat will no longer grow in the fields. In other words, we say the farm is "worn out," "cropped to death." The soil is there, but the precious life-giving nitrogen is gone. And so it becomes necessary every year to put back the nitrogen and the other elements which the crop takes from the soil. This purpose is accomplished by the use of fertilisers. Manure, ground bone, nitrates, guano, are put in fields to restore the nitrogen and other plant foods. In short, we are compelled to feed the soil that the soil may feed the wheat, that the wheat may feed us. You will see that it is a complete circle—like all life.
Now, the trouble, the great problem, lies right here: in the difficulty of obtaining a sufficient amount of fertiliser—in other words, in getting food enough to keep the soil from nitrogen starvation. Already we ship guano—the droppings of sea-birds—from South America and the far islands of the sea to put on our lands, and we mine nitrates (which contain nitrogen) at large expense and in great quantities for the same purpose. And while we go to such lengths to get nitrogen we are wasting it every year in enormous quantities. Gunpowder and explosives are most made up of nitrogen—saltpetre and nitro-glycerin—so that every war wastes vast quantities of this precious substance. Every discharge of a 13-inch gun liberates enough nitrogen to raise many bushels of wheat. Thus we see another reason for the disarmament of the nations.
A prediction has been made that barely thirty years hence the wheat required to feed the world will be 3,260,000,000 bushels annually, and that to raise this about 12,000,000 tons of nitrate of soda yearly for the area under cultivation will be needed over and above the 1,250,000 tons now used by mankind. But the nitrates now in sight and available are estimated good for only another fifty years, even at the present low rate of consumption. Hence, even if famine does not immediately impend, the food problem is far more serious than is generally supposed.
Now nitrogen, it will be seen, is one of the most precious and necessary of all substances to human life, and it is one of the most common. If the world ever starves for the lack of nitrogen it will starve in a very world of nitrogen. For there is not one of the elements more common than nitrogen, not one present around us in larger quantities. Four-fifths of every breath of air we breathe is pure nitrogen—four-fifths of all the earth's atmosphere is nitrogen.
But, unfortunately, most plants are unable to take up nitrogen in its gaseous form as it appears in the air. It must be combined with hydrogen in the form of ammonia or in some nitrate. Ammonia and the nitrates are, therefore, the basis of all fertilisers.
Now, the problem for the scientist and inventor takes this form: Here is the vast store-house of life-giving nitrogen in the air; how can it be caught, fixed, reduced to the purpose of men, spread on the hungry wheat-fields? The problem, therefore, is that of "fixing" the nitrogen, taking the gas out of the air and reducing it to a form in which it can be handled and used.
Two principal methods for doing this have already been devised, both of which are of fascinating interest. One of these ways, that of a clever American inventor, is purely a machinery process, the utilisation of power by means of which the nitrogen is literally sucked out of the air and combined with soda so that it produces nitrate of soda, a high-class fertiliser. The water power of Niagara Falls is used to do this work—it seems odd enough that Niagara should be used for food production!
The other method, that of a hard-working German professor, is the cunning utilisation of one of nature's marvellous processes of taking the nitrogen from the air and depositing it in the soil—for nature has its own beautiful way of doing it. I will describe the second method first because it will help to clear up the whole subject and lead up to the work of the American inventor and his extraordinary machinery.
Nearly every farmer, without knowing it, employs nature's method of fixing nitrogen every year. It is a simple process which he has learned from experience. He knows that when land is worn out by overcropping with wheat or other products which draw heavily on the earth's nitrogen supply certain crops will still grow luxuriantly upon the worn-out land, and that if these crops are left and ploughed in, the fertility of the soil will be restored, and it will again produce large yields of wheat and other nitrogen-demanding plants. These restorative crops are clover, lupin, and other leguminous plants, including beans and peas. Every one who is at all familiar with farming operations has heard of seeding down an old field to clover and then ploughing in the crop, usually in the second year.
The great importance of this bit of the wisdom of experience was not appreciated by science for many years. Then several German experimenters began to ask why clover and lupin and beans should flourish on worn-out land when other crops failed. All of these plants are especially rich in nitrogen, and yet they grew well on soil which had been robbed of its nitrogen. Why was this so?
It was a hard problem to solve, but science was undaunted. Botanists had already discovered that the roots of the leguminous plants—that is, clover, lupin, beans, peas, and so on—were usually covered with small round swellings, or tumors, to which were given the name nodules. The exact purpose of these swellings being unknown, they were set down as a condition, possibly, of disease, and no further attention was paid to them until Professor Hellriegel, of Burnburg, in Anhalt, Germany, took up the work. After much experimenting, he made the important discovery that lupins which had nodules would grow in soil devoid of nitrogen, and that lupins which had no nodules would not grow in the same soil. It was plain, therefore, that the nodules must play an important, though mysterious, part in enabling the plant to utilise the free nitrogen of the air. That was early in the '80s. His discovery at once started other investigators to work, and it was not long before the announcement came—and it came, curiously enough, at a time when Dr. Koch was making his greatest contributions to the world's knowledge of the germ theory of disease—that these nodules were the result of minute bacteria found in the soil. Professor Beyerinck, of Münster, gave the bacteria the name Radiocola.
It was at this time that Professor Nobbe took up the work with vigour. If these nodules were produced by bacteria, he argued that the bacteria must be present in the soil; and if they were not present, would it not be possible to supply them by artificial means? In other words, if soil, say worn-out farm-soil or, indeed, pure sand like that of the sea-shore could thus be inoculated, as a physician inoculates a guinea-pig with diphtheria germs, would not beans and peas planted there form nodules and draw their nourishment from the air? It was a somewhat startling idea, but all radically new ideas are startling; and, after thinking it over, Professor Nobbe began, in 1888, a series of most remarkable experiments, having as their purpose the discovery of a practical method of soil inoculation. He gathered the nodule-covered roots of beans and peas, dried and crushed them, and made an extract of them in water. Then he prepared a gelatine solution with a little sugar, asparagine, and other materials, and added the nodule-extract. In this medium colonies of bacteria at once began to grow—bacteria of many kinds. Professor Nobbe separated the Radiocola—which are oblong in shape—and made what is known as a "clear culture," that is, a culture in gelatine, consisting of billions of these particular germs, and no others. When he had succeeded in producing these clear cultures he was ready for his actual experiments in growing plants. He took a quantity of pure sand, and, in order to be sure that it contained no nitrogen or bacteria in any form, he heated it at a high temperature three different times for six hours, thereby completely sterilising it. This sand he placed in three jars. To each of these he added a small quantity of mineral food—the required phosphorus, potassium, iron, sulphur, and so on. To the first he supplied no nitrogen at all in any form; the second he fertilised with saltpetre, which is largely composed of nitrogen in a form in which plants may readily absorb it through their roots; the third of the jars he inoculated with some of his bacteria culture. Then he planted beans in all three jars, and awaited the results, as may be imagined, somewhat anxiously. Perfectly pure sterilised water was supplied to each jar in equal amounts and the seeds sprouted, and for a week the young shoots in the three jars were almost identical in appearance. But soon after that there was a gradual but striking change. The beans in the first jar, having no nitrogen and no inoculation, turned pale and refused to grow, finally dying down completely, starved for want of nitrogenous food, exactly as a man would starve for the lack of the same kind of nourishment. The beans in the second jar, with the fertilised soil, grew about as they would in the garden, all of the nourishment having been artificially supplied. But the third jar, which had been jealously watched, showed really a miracle of growth. It must be remembered that the soil in this jar was as absolutely free of nitrogen as the soil in the first jar, and yet the beans flourished greatly, and when some of the plants were analysed they were found to be rich in nitrogen. Nodules had formed on the roots of the beans in the third or inoculated jar only, thereby proving beyond the hope of the experimenter that soil inoculation was a possibility, at least in the laboratory.
With this favourable beginning Professor Nobbe went forward with his experiments with renewed vigour. He tried inoculating the soil for peas, clover, lupin, vetch, acacia, robinia, and so on, and in every case the roots formed nodules, and although there was absolutely no nitrogen in the soil, the plants invariably flourished. Then Professor Nobbe tried great numbers of difficult test experiments, such as inoculating the soil with clover bacteria and then planting it with beans or peas, or vice versa, to see whether the bacteria from the nodules of any one leguminous plant could be used for all or any of the others. He also tried successive cultures; that is, bean bacteria for beans for several years, to see if better results could be obtained by continued use. Even an outline description of all the experiments which Professor Nobbe made in the course of these investigations would fill a small volume, and it will be best to set down here only his general conclusions.
These wonderful nitrogen-absorbing bacteria do not appear in all soil, although they are very widely distributed. So far as known they form nodules only on the roots of a few species of plants. In their original form in the soil they are neutral—that is, not especially adapted to beans, or peas, or any one particular kind of crop. But if clover, for instance, is planted, they straightway form nodules and become especially adapted to the clover plant, so that, as every farmer knows, the second crop of clover on worn-out land is much better than the first. And, curiously enough, when once the bacteria have become thoroughly adapted to one of the crops, say beans, they will not affect peas or clover, or only feebly.
Another strange feature of the life of these little creatures, which has a marvellous suggestion of intelligence, is their activities in various kinds of soil. When the ground is very rich—that is, when it contains plenty of nitrogenous matter—they are what Professor Nobbe calls "lazy." They do not readily form nodules on the roots of the plants, seeming almost to know that there is no necessity for it. But when once the nitrogenous matter in the soil begins to fail, then they work more sharply, and when it has gone altogether they are at the very height of activity. Consequently, unless the soil is really worn out, or very poor to begin with, there is no use in inoculating it—it would be like "taking owls to Athens," as Professor Nobbe says.
Having thus proved the remarkable efficacy of soil inoculation in his laboratory and greenhouses, where I saw great numbers of experiments still going forward, Professor Nobbe set himself to make his discoveries of practical value. He gave to his bacteria cultures the name "Nitragen"—spelled with an "a"—and he produced separate cultures for each of the important crops—peas, beans, vetch, lupin, and clover. In 1894 the first of these were placed on the market, and they have had a steadily increasing sale, although such a radical innovation as this, so far out of the ordinary run of agricultural operation, and so almost unbelievably wonderful, cannot be expected to spread very rapidly. The cultures are now manufactured at one of the great commercial chemical laboratories on the river Main. I saw some of them in Professor Nobbe's laboratory. They come in small glass bottles, each marked with the name of the crop for which it is especially adapted. The bottle is partly filled with the yellow gelatinous substance in which the bacteria grow. On the surface of this there is a mossy-like growth, resembling mould. This consists of innumerable millions of the little oblong bacteria. A bottle costs about fifty cents and contains enough bacteria for inoculating half an acre of land. It must be used within a certain number of weeks after it is obtained, while it is still fresh. The method of applying it is very simple. The contents of the bottle are diluted with warm water. Then the seeds of the beans, clover, or peas, which have previously been mixed with a little soil, are treated with this solution and thoroughly mixed with the soil. After that the mass is partially dried so that the seeds may be readily sown. The bacteria at once begin to propagate in the soil, which is their natural home, and by the time the beans or peas have put out roots they are present in vast numbers and ready to begin the active work of forming nodules. It is not known exactly how the bacteria absorb the free nitrogen from the air, but they do it successfully, and that is the main thing. Many German farmers have tried Nitragen. One, who was sceptical of its virtues, wrote to Professor Nobbe that he sowed the bacteria-inoculated seeds in the form of a huge letter N in the midst of his field, planting the rest in the ordinary way. Before a month had passed that N showed up green and big over all the field, the plants composing it being so much larger and healthier than those around it.
The United States Government has recently been experimenting along the same lines and has produced a new form of dry preparation of the bacteria in some cakes somewhat resembling a yeast-cake.
The possibilities of such a discovery as this seem almost limitless. Science predicts the exhaustion of nitrogen and consequent failure of the food supply, and science promptly finds a way of making plants draw nitrogen from the boundless supplies of the air. The time may come when every farmer will send for his bottles or cakes of bacteria culture every spring as regularly as he sends for his seed, and when the work of inoculating the soil will be a familiar agricultural process, with discussions in the farmers' papers as to whether two bottles or one is best for a field of sandy loam with a southern exposure. Stranger things have happened. But it must be remembered, also, that the work is in its infancy as yet, and that there are vast unexplored fields and innumerable possibilities yet to fathom.
Wonderful as this discovery is, and much as it promises in the future, its efficacy, as soon as it becomes generally known, is certain to be overestimated, as all new discoveries are. Professor Nobbe himself says that it has its own limited serviceability. It will produce a bounteous crop of beans in the pure sand of the sea-shore if (and this is an important if) that sand also contains enough of the mineral substances—phosphorus, potassium, and so on—and if it is kept properly watered. A man with a worn-out farm cannot go ahead blindly and inoculate his soil and expect certain results. He must know the exact disease from which his land is suffering before he applies the remedy. If it is deficient in the phosphates, bacteria cultures will not help it, whereas if it is deficient in nitrogen, bacteria are just what it needs. And so agricultural education must go hand in hand with the introduction of these future preservers of the human race. It is safe to say that by the time there is a serious failure of the earth's soil for lack of nitrogen, science, with this wonderful beginning, will have ready a new system of cultivation, which will gradually, easily, and perfectly take the place of the old.
Before leaving this wonderful subject of soil inoculation, a word about Professor Nobbe himself will surely be of interest. I visited his laboratory and saw his experiments.
Tharandt, in Saxony, where Professor Nobbe has carried on his investigations for over thirty years, is a little village set picturesquely among the Saxon hills, about half an hour's ride by railroad from the city of Dresden. Here is located the Forest Academy of the Kingdom, with which Professor Nobbe is prominently connected, and here also is the agricultural experiment station of which he is director. He has been for more than forty years the editor of one of the most important scientific publications in Germany; he is chairman of the Imperial Society of Agricultural Station Directors, and he has been the recipient of many honours.
We now come to a consideration of the other method—the fixing of nitrogen by machinery: a practical problem for the inventor.
Every one has noticed the peculiar fresh smell of the air which follows a thunderstorm; the same pungent odour appears in the vicinity of a frictional electric machine when in operation. This smell has been attributed to ozone, but it is now thought that it may be due to oxides of nitrogen; in other words, the electric discharges of lightning or of the frictional machine have burned the air—that is, combined the nitrogen and oxygen of the air, forming oxides of nitrogen.

Mr. Charles S. Bradley.

Mr. D. R. Lovejoy.
The fact that an electric spark will thus form an oxide of nitrogen has long been known, but it remained for two American inventors, Mr. Charles S. Bradley and Mr. D. R. Lovejoy, of Niagara Falls, N. Y., to work out a way by inventive genius for applying this scientific fact to a practical purpose, thereby originating a great new industry. I shall not attempt here to describe the long process of experimentation which led up to the success of their enterprise. Here was their raw material all around them in the air; their problem was to produce a large number of very hot electric flames in a confined space or box so that air could be passed through, rapidly burned, and converted into oxides of nitrogen (nitric oxides and peroxides), which could afterward be collected. They took the power supplied by the great turbine wheels at Niagara Falls and produced a current of 10,000 volts, a pressure far above anything ever used before for practical purposes in this country. This was led into a box or chamber of metal six feet high and three feet in diameter—the box having openings to admit the air. By means of a revolving cylinder the electric current is made to produce a rapid continuance of very brilliant arcs, exactly like the glaring white arc of the arc-lamp, only much more intense, a great deal hotter. The air driven in through and around these hot arcs is at once burned, combining the oxygen and nitrogen of which it is composed and producing the desired oxides of nitrogen. These are led along to a chamber where they are combined with water, producing nitric or nitrous acid; or if the gases are brought into contact with caustic potash, saltpetre is the result; if with caustic soda, nitrate of soda is the product—a very valuable fertiliser. And the inventors have been able to produce these various results at an expense so low that they can sell their output at a profit in competition with nitrates from other sources, thus giving the world a new source of fertiliser at a moderate price.

Eight-Inch 10,000-Volt Arcs Burning the Air for Fixing Nitrogen.
In this way the power of Niagara has become a factor in the food question, a defence against the ultimate hunger of the human race. And when we think of the hundreds of other great waterfalls to be utilised, and with our growing knowledge of electricity this utilisation will become steadily cheaper, easier, it would seem that the inventor had already found a way to help the farmer. Then there is the boundless power of the tides going to waste, of the direct rays of the sun utilised by some such sun motor as that described in another chapter of this book, which in time may be called to operate upon the boundless reservoir of nitrogen in the air for helping to produce the future food for the human race.

MARCONI.
The Sending of an Epoch-Making Message.
January 18, 1903, marks the beginning of a new era in telegraphic communication. On that day there was sent by Marconi himself from the wireless station at South Wellfleet, Cape Cod, Mass., to the station at Poldhu, Cornwall, England, a distance of 3,000 miles, the message—destined soon to be historic—from the President of the United States to the King of England.
No invention of modern times, perhaps, comes so near to being what we call a miracle as the new system of telegraphy without wires. The very thought of communicating across the hundreds of miles of blue ocean between Europe and America with no connection, no wires, nothing but air, sunshine, space, is almost inconceivably wonderful. A few years ago the mere suggestion of such a thing would have been set down as the wildest flight of imagination, unbelievable, perfectly impossible. And yet it has come to pass!
Think for a moment of sitting here on the shore of America and quietly listening to words sent through space across some 3,000 miles of ocean from the edge of Europe! A cable, marvellous as it is, maintains a real connection between speaker and hearer. We feel that it is a road along which our speech can travel; we can grasp its meaning. But in telegraphing without wires we have nothing but space, poles with pendent wires on one side of the broad, curving ocean, and similar poles and wires (or perhaps only a kite struggling in the air) on the other—and thought passing between!
I have told in the first "Boys' Book of Inventions" of Guglielmo Marconi's early experiments. That was a chapter of uncertain beginnings, of great hopes, of prophecy. This is the sequel, a chapter of achievement and success. What was only a scientific and inventive novelty a few years ago has become a great practical enterprise, giving promise of changing the whole world of men, drawing nations more closely together, making us near neighbours to the English and the Germans and the French—in short, shrinking our earth. There may come a time when we will think no more of sending a Marconigram, or an etheragram, or whatever is to be the name of the message by wireless telegraphy, to an acquaintance in England than we now think of calling up our neighbour on the telephone.
Every one will recall the astonishment that swept over the country in December, 1901, when there came the first meagre reports of Marconi's success in telegraphing across the Atlantic Ocean between England and Newfoundland. At first few would believe the reports, but when Thomas A. Edison, Graham Bell, and other great inventors and scientists had expressed their confidence in Marconi's achievement, the whole country, was ready to hail the young inventor with honours. And his successes since those December days have been so pronounced—for he had now sent messages both ways across the Atlantic and at much greater distances—have more than borne out the promise then made. Wireless telegrams can now be sent directly from the shore of Massachusetts to England, and ocean-going ships are being rapidly equipped with the Marconi apparatus so that they can keep in direct communication with both continents during every day of the voyage. On some of the great ships a little newspaper is published, giving the world's news as received from day to day.
It was the good fortune of the writer to arrive in St. John's, Newfoundland, during Mr. Marconi's experiments in December, 1901, only a short time after the famous first message across the Atlantic had been received. Three months later it was also the writer's privilege to visit the Marconi station at Poldhu, in Cornwall, England, from which the message had been sent, Mr. Marconi being then planning his greater work of placing his invention on a practical basis so that his company could enter the field of commercial telegraphy. It was the writer's fortune to have many talks with Mr. Marconi, both in America and in England, to see him at his experiments, and to write some of the earliest accounts of his successes. The story here told is the result of these talks.
Mr. Marconi kept his own counsel regarding his plans in coming to Newfoundland in December, 1901. He told nobody, except his assistants, that he was going to attempt the great feat of communicating across the Atlantic Ocean. Though feeling very certain of success, he knew that the world would not believe him, would perhaps only laugh at him for his great plans. The project was entirely too daring for public announcement. Something might happen, some accident to the apparatus, that would cause a delay; people would call this failure, and it would be more difficult another time to get any one to put confidence in the work. So Marconi very wisely held his peace, only announcing what he had done when success was assured.
Mr. Marconi landed at St. John's, Newfoundland, on December 6, 1901, with his two assistants, Mr. Kemp and Mr. Paget.
He set up his instruments in a low room of the old barracks on Signal Hill, which stands sentinel at the harbour mouth half a mile from the city of St. John's. So simple and easily arranged is the apparatus that in three days' time the inventor was prepared to begin his experiments. On Wednesday, the 11th, as a preliminary test of the wind velocity, he sent up one of his kites, a huge hexagonal affair of bamboo and silk nine feet high, built on the Baden-Powell model: the wind promptly snapped the wire and blew the kite out to sea. He then filled a 14-foot hydrogen balloon, and sent it upward through a thick fog bank. Hardly had it reached the limit of its tetherings, however, when the aërial wire on which he had depended for receiving his messages fell to the earth, the balloon broke away, and was never seen again. On Thursday, the 12th, a day destined to be important in the annals of invention, Marconi tried another kite, and though the weather was so blustery that it required the combined strength of the inventor and his assistants to manage the tetherings, they succeeded in holding the kite at an elevation of about 400 feet. Marconi was now prepared for the crucial test. Before leaving England he had given detailed instructions to his assistants for the transmission of a certain signal, the Morse telegraphic S, represented by three dots (...), at a fixed time each day, beginning as soon as they received word that everything at St. John's was in readiness. This signal was to be clicked out on the transmitting instruments near Poldhu, Cornwall, the southwestern tip of England, and radiated from a number of aërial wires pendent from masts 210 feet high. If the inventor could receive on his kite-wire in Newfoundland some of the electrical waves thus produced, he knew that he held the solution of the problem of transoceanic wireless telegraphy. He had cabled his assistants to begin sending the signals at three o'clock in the afternoon, English time, continuing until six o'clock; that is, from about 11.30 to 2.30 o'clock in St. John's.
At noon on Thursday (December 12, 1901) Marconi sat waiting, a telephone receiver at his ear, in a room of the old barracks on Signal Hill. To him it must have been a moment of painful stress and expectation. Arranged on the table before him, all its parts within easy reach of his hand, was the delicate receiving instrument, the supreme product of years of the inventor's life, now to be submitted to a decisive test. A wire ran out through the window, thence to a pole, thence upward to the kite which could be seen swaying high overhead. It was a bluff, raw day; at the base of the cliff 300 feet below thundered a cold sea; oceanward through the mist rose dimly the rude outlines of Cape Spear, the easternmost reach of the North American Continent. Beyond that rolled the unbroken ocean, nearly 2,000 miles to the coast of the British Isles. Across the harbour the city of St. John's lay on its hillside wrapped in fog: no one had taken enough interest in the experiments to come up here through the snow to Signal Hill. Even the ubiquitous reporter was absent. In Cabot Tower, near at hand, the old signalman stood looking out to sea, watching for ships, and little dreaming of the mysterious messages coming that way from England. Standing on that bleak hill and gazing out over the waste of water to the eastward, one finds it difficult indeed to realise that this wonder could have become a reality. The faith of the inventor in his creation, in the kite-wire, and in the instruments which had grown under his hand, was unshaken.

Mr. Marconi and his Assistants in Newfoundland: Mr. Kemp on the Left, Mr. Paget on the Right.
They are sitting on a balloon basket, with one of the Baden-Powell kites in the background.
"I believed from the first," he told me, "that I would be successful in getting signals across the Atlantic."
Only two persons were present that Thursday noon in the room where the instruments were set up—Mr. Marconi and Mr. Kemp. Everything had been done that could be done. The receiving apparatus was of unusual sensitiveness, so that it would catch even the faintest evidence of the signals. A telephone receiver, which is no part of the ordinary instrument, had been supplied, so that the slightest clicking of the dots might be conveyed to the inventor's ear. For nearly half an hour not a sound broke the silence of the room. Then quite suddenly Mr. Kemp heard the sharp click of the tapper as it struck against the coherer; this, of course, was not the signal, yet it was an indication that something was coming. The inventor's face showed no evidence of excitement. Presently he said:
"See if you can hear anything, Kemp."
Mr. Kemp took the receiver, and a moment later, faintly and yet distinctly and unmistakably, came the three little clicks—the dots of the letter S, tapped out an instant before in England. At ten minutes past one, more signals came, and both Mr. Marconi and Mr. Kemp assured themselves again and again that there could be no mistake. During this time the kite gyrated so wildly in the air that the receiving wire was not maintained at the same height, as it should have been; but again, at twenty minutes after two, other repetitions of the signal were received.
Thus the problem was solved. One of the great wonders of science had been wrought. But the inventor went down the hill toward the city, now bright with lights, feeling depressed and disheartened—the rebound from the stress of the preceding days. On the following afternoon, Friday, he succeeded in getting other repetitions of the signal from England, but on Saturday, though he made an effort, he was unable to hear anything. The signals were, of course, sent continuously, but the inventor was unable to obtain continuous results, owing, as he explains, to the fluctuations of the height of the kite as it was blown about by the wind, and to the extreme delicacy of his instruments, which required constant adjustment during the experiments.
Even now that he had been successful, the inventor hesitated to make his achievement public, lest it seem too extraordinary for belief. Finally, after withholding the great news for two days, certainly an evidence of self-restraint, he gave out a statement to the press, and on Sunday morning the world knew and doubted; on Monday it knew more and believed. Many, like Mr. Edison, awaited the inventor's signed announcement before they would credit the news. Sir Cavendish Boyle, the Governor of Newfoundland, reported at once to King Edward; and the cable company which has exclusive rights in Newfoundland, alarmed at an achievement which threatened the very existence of its business, demanded that he desist from further experiments within its territory, truly an evidence of the belief of practical men in the future commercial importance of the invention. It is not a little significant of the increased willingness of the world, born of expanding knowledge, to accept a new scientific wonder, that Mr. Marconi's announcement should have been so eagerly and so generally believed, and that the popular imagination should have been so fired with its possibilities. One cannot but recall the struggle against doubt, prejudice, and disbelief in which the promoters of the first transatlantic cable were forced to engage. Even after the first cable was laid (in 1858), and messages had actually been transmitted, there were many who denied that it had ever been successfully operated, and would hardly be convinced even by the affidavits of those concerned in the work. But in the years since then, Edison, Bell, Röntgen, and many other famous inventors and scientists have taught the world to be chary of its disbelief. Outside of this general disposition to friendliness, however, Marconi on his own part had well earned the credit of the careful and conservative scientist; his previous successes made it the more easy to credit his new achievement. For, as an Englishman (Mr. Flood Page), in defending Mr. Marconi's announcement, has pointed out, the inventor has never made any statement in public until he has been absolutely certain of the fact; he has never had to withdraw any statement that he has made as to his progress in the past. And these facts unquestionably carried great weight in convincing Mr. Edison, Mr. Graham Bell, and others of equal note of the literal truth of his report. It was astonishing how overwhelmingly credit came from every quarter of the world, from high and low alike, from inventors, scientists, statesmen, royalty. Before Marconi left St. John's he was already in receipt of a large mail—the inevitable letters of those who would offer congratulations, give advice, or ask favours. He received offers to lecture, to write articles, to visit this, that, and the other place—and all within a week after the news of his success. The people of the "ancient colony" of Newfoundland, famed for their hospitality, crowned him with every honour in their power. I accompanied Mr. Marconi across the island on his way to Nova Scotia, and it seemed as if every fisher and farmer in that wild country had heard of him, for when the train stopped they came crowding to look in at the window. From the comments I heard, they wondered most at the inventor's youthful appearance. Though he was only twenty-seven years old, his experience as an inventor covered many years, for he began experimenting in wireless telegraphy before he was twenty. At twenty-two he came to London from his Italian home, and convinced the British Post-Office Department that he had an important idea; at twenty-three he was famous the world over.
Following this epoch-making success Mr. Marconi returned to England, where he continued most vigorously the work of perfecting his invention, installing more powerful transmitters, devising new receivers, all the time with the intention of following up his Newfoundland experiments with the inauguration of a complete system of wireless transmission between America and Europe. In the latter part of the year 1902 he succeeded in opening regular communication between Nova Scotia and England, and January 18, 1903, marked another epoch in his work. On that day there was sent by Marconi himself from the wireless station at South Wellfleet, Cape Cod, Mass., to the station at Poldhu, Cornwall, England, a distance of 3,000 miles, the message—destined to be historic—from the President of the United States to the King of England.
It will be interesting to know something of the inventor himself. He is somewhat above medium height, and, though of a highly strung temperament, he is deliberate in his movements. Unlike the inventor of tradition, he dresses with scrupulous neatness, and, in spite of being a prodigious worker, he finds time to enjoy a limited amount of club and social life. The portrait published with this chapter, taken at St. John's a few days after the experiments, gives a very good idea of the inventor's face, though it cannot convey the peculiar lustre of his eyes when he is interested or excited—and perhaps it makes him look older than he really is. One of the first and strongest impressions that the man conveys is that of intense nervous activity and mental absorption; he has a way of pouncing upon a knotty question as if he could not wait to solve it. He talks little, is straightforward and unassuming, submitting good-naturedly, although with evident unwillingness, to being lionised. In his public addresses he has been clear and sensible; he has never written for any publication; nor has he engaged in scientific disputes, and even when violently attacked he has let his work prove his point. And he has accepted his success with calmness, almost unconcern; he certainly expected it. The only elation I saw him express was over the attack of the cable monopoly in Newfoundland, which he regarded as the greatest tribute that could have been paid his achievement. During all his life, opposition has been his keenest spur to greater effort.
Though he was born and educated in Italy, his mother was of British birth, and he speaks English as perfectly as he does Italian. Indeed, his blue eyes, light hair, and fair complexion give him decidedly the appearance of an Englishman, so that a stranger meeting him for the first time would never suspect his Italian parentage. His parents are still living, spending part of their time on their estate in Italy and part of the time in London. One of the first messages conveying the news of his success at St. John's went to them. He embarked in experimental research because he loved it, and no amount of honour or money tempts him from the pursuit of the great things in electricity which he sees before him. Besides being an inventor, he is also a shrewd business man, with a clear appreciation of the value of his inventions and of their possibilities when generally introduced. What is more, he knows how to go about the task of introducing them.
No sooner had Marconi announced the success of his Newfoundland experiments than critics began to raise objections. Might not the signals which he received have been sent from some passing ship fitted with wireless-telegraphy apparatus? Or, might they not have been the result of electrical disturbances in the atmosphere? Or, granting his ability to communicate across seas, how could he preserve the secrecy of his messages? If they were transmitted into space, why was it not possible for any one with a receiving instrument to take them? And was not his system of transmission too slow to make it useful, or was it not rendered uncertain by storms? And so on indefinitely. An acquaintance with some of the principles which Marconi considers fundamental, and on which his work has been based, will help to clear away these objections and give some conception of the real meaning and importance of the work at St. John's and of the plans for the future development of the inventor's system.
In the first place, Mr. Marconi makes no claim to being the first to experiment along the lines which led to wireless telegraphy, or the first to signal for short distances without wires. He is prompt with his acknowledgment to other workers in his field, and to his assistants. Professor S. F. B. Morse, the inventor of telegraphy; Dr. Oliver Lodge and Sir William Preece, of England; Edison, Tesla, and Professors Trowbridge and Dolbear, of America, and others had experimented along these lines, but it remained for Marconi to perfect a system and put it into practical working order. He took the coherer of Branley and Calzecchi, the oscillator of Righi, he used the discoveries of Henry and Hertz, but his creation, like that of the poet who gathers the words of men in a perfect lyric, was none the less brilliant and original.

Marconi Transatlantic Station at
South Wellfleet, Cape Cod, Mass.
In its bare outlines, Marconi's system of telegraphy consists in setting in motion, by means of his transmitter, certain electric waves which, passing through the ether, are received on a distant wire suspended from a kite or mast, and registered on his receiving apparatus. The ether is a mysterious, unseen, colourless, odourless, inconceivably rarefied something which is supposed to fill all space. It has been compared to a jelly in which the stars and planets are set like cherries. About all we know of it is that it has waves—that the jelly may be made to vibrate in various ways. Etheric vibrations of certain kinds give light; other kinds give heat; others electricity. Experiments have shown that if the ether vibrates at the inconceivable swiftness of 400 billions of waves a second we see the colour red, if twice as fast we see violet, if more slowly—perhaps 230 millions to the second, and less—we have the Hertz waves used by Marconi in his wireless-telegraphy experiments. Ether waves should not be confounded with air waves. Sound is a result of the vibration of the air; if we had ether and no air, we should still see light, feel heat, and have electrical phenomena, but no sound would ever come to our ears. Air is sluggish beside ether, and sound waves are very slow compared with ether waves. During a storm the ether brings the flash of the lightning before the air brings the sound of thunder, as every one knows.
Electricity is, indeed, only another name for certain vibrations in the ether. We say that electricity "flows" in a wire, but nothing really passes except an etheric wave, for the atoms composing the wire, as well as the air and the earth, and even the hardest substances, are all afloat in ether. Vibrations, therefore, started at one end of the wire travel to the other. Throw a stone into a quiet pond. Instantly waves are formed which spread out in every direction; the water does not move, except up and down, yet the wave passes onward indefinitely. Electric waves cannot be seen, but electricians have learned how to incite them, to a certain extent how to control them, and have devised cunning instruments which register their presence.
Electrical waves have long been harnessed by the use of wires for sending communications; in other words, we have had wire telegraphy. But the ether exists outside of the wire as well as within; therefore, having the ether everywhere, it must be possible to produce waves in it which will pass anywhere, as well through mountains as over seas, and if these waves can be controlled they will evidently convey messages as easily and as certainly as the ether within wires. So argued Mr. Marconi. The difficulty lay in making an instrument which would produce a peculiar kind of wave, and in receiving and registering this wave in a second apparatus located at a distance from the first. It was, therefore, a practical mechanical problem which Marconi had to meet. Beginning with crude tin boxes set up on poles on the grounds of his father's estate in Italy, he finally devised an apparatus from which a current generated by a battery and passing in brilliant sparks between two brass balls was radiated from a wire suspended on a tall pole. By shutting off and turning on this peculiar current, by means of a device similar to the familiar telegrapher's key, the waves could be so divided as to represent dashes and dots, and spell out letters in the Morse alphabet. This was the transmitter. It was, indeed, simple enough to start these waves travelling through space, to jar the etheric jelly, so to speak; but it was far more difficult to devise an apparatus to receive and register them. For this purpose Marconi adopted a device invented by an Italian, Calzecchi, and improved by a Frenchman, M. Branley, called the coherer, and the very crux of the system, without which there could be no wireless telegraphy. This coherer, which he greatly improved, is merely a little tube of glass as big around as a lead-pencil, and perhaps two inches long. It is plugged at each end with silver, the plugs nearly meeting within the tube. The narrow space between them is filled with finely powdered fragments of nickel and silver, which possess the strange property of being alternately very good and very bad conductors of electrical waves. The waves which come from the transmitter, perhaps 2,000 miles away, are received on a suspended kite-wire, exactly similar to the wire used in the transmitter, but they are so weak that they could not of themselves operate an ordinary telegraph instrument. They do, however, possess strength enough to draw the little particles of silver and nickel in the coherer together in a continuous metal path. In other words, they make these particles "cohere," and the moment they cohere they become a good conductor for electricity, and a current from a battery near at hand rushes through, operates the Morse instrument, and causes it to print a dot or a dash; then a little tapper, actuated by the same current, strikes against the coherer, the particles of metal are jarred apart or "decohered," becoming instantly a poor conductor, and thus stopping the strong current from the home battery. Another wave comes through space, down the suspended kite-wire, into the coherer, there drawing the particles again together, and another dot or dash is printed. All these processes are continued rapidly, until a complete message is ticked out on the tape. Thus Mr. Kemp knew when he heard the tapper strike the coherer that a signal was coming, though he could not hear the click of the receiver itself. And this is in bare outline Mr. Marconi's invention—this is the combination of devices which has made wireless telegraphy possible, the invention on which he has taken out more than 132 patents in every civilised country of the world. Of course his instruments contain much of intricate detail, of marvellously ingenious adaptation to the needs of the work, but these are interesting chiefly to expert technicians.

Nearer View of
South Foreland Station.

Alum Bay Station
Isle of Wight.
In his actual transoceanic experiments of December, 1901, Mr. Marconi's transmitting station in England was fitted with twenty masts 210 feet high, each with its suspended wire, though not all of them were used. A current of electricity sufficient to operate some 300 incandescent lamps was used, the resulting spark being so brilliant that one could not have looked at it with the unshaded eye. The wave which was thus generated had a length of about a fifth of a mile, and the rate of vibration was about 800,000 to the second. Following the analogy of the stone cast in the pond with the ripples circling outward, these waves spread from the suspended wires in England in every direction, not only westward toward the cliff where Marconi was flying his kite, but eastward, northward, and southward, so that if some of Mr. Marconi's assistants had been flying kites, say on the shore of Africa, or South America, or in St. Petersburg, they might possibly, with a corresponding receiver, have heard the identical signals at the same instant. In his early experiments Marconi believed that great distances could not be obtained without very high masts and long, suspended wires, the greater the distance the taller the mast, on the theory that the waves were hindered by the curvature of the earth; but his later theory, substantiated by his Newfoundland experiments, is that the waves somehow follow around the earth, conforming to its curve, and the next station he establishes in America will not be set high on a cliff, as at St. John's, but down close to the water on level land. His Newfoundland experiments have also convinced him that one of the secrets of successful long-distance transmission is the use of a more powerful current in his transmitter, and this he will test in his next trials between the continents.
And now we come to the most important part of Mr. Marconi's work, the part least known even to science, and the field of almost illimitable future development. This is the system of "tuning," as the inventor calls it, the construction of a certain receiver so that it will respond only to the message sent by a certain transmitter. When Marconi's discoveries were first announced in 1896, there existed no method of tuning, though the inventor had its necessity clearly in mind. Accordingly the public inquired, "How are you going to keep your messages secret? Supposing a warship wishes to communicate with another of the fleet, what is to prevent the enemy from reading your message? How are private business despatches to be secured against publicity?" Here, indeed, was a problem. Without secrecy no system of wireless telegraphy could ever reach great commercial importance, or compete with the present cable communication. The inventor first tried using a parabolic copper reflector, by means of which he could radiate the electric waves exactly as light—which, it will be borne in mind, is only another kind of etheric wave—is reflected by a mirror. This reflector could be faced in any desired direction, and only a receiver located in that direction would respond to the message. But there were grave objections to the reflector; an enemy might still creep in between the sending and receiving stations, and, moreover, it was found that the curvature of the earth interfered with the transmission of reflected messages, thereby limiting their usefulness to short distances.
In passing, however, it may be interesting to note one extraordinary use for this reflecting system which the inventor now has in mind. This is in connection with lighthouse work. Ships are to be provided with reflecting instruments which in dense fog or storms can be used exactly as a searchlight is now employed on a dark night to discover the location of the lighthouses or lightships. For instance, the lighthouse, say, on some rocky point on the New England coast would continually radiate a warning from its suspended wire. These waves pass as readily through fog and darkness and storm as in daylight. A ship out at sea, hidden in fog, has lost its bearings; the sound of the warning horn, if warning there is, seems to come first from one direction, then from another, as sounds do in a fog, luring the ship to destruction. If now the mariner is provided with a wireless reflector, this instrument can be slowly turned until it receives the lighthouse warning, the captain thus learning his exact location; if in distress, he can even communicate with the lighthouse. Think also what an advantage such an equipment would be to vessels entering a dangerous harbour in thick weather. This is one of the developments of the near future.
The reflector system being impracticable for long-distance work, Mr. Marconi experimented with tuning. He so constructed a receiver that it responds only to a certain transmitter. That is, if the transmitter is radiating 800,000 vibrations a second, the corresponding receiver will take only 800,000 vibrations. In exactly the same way a familiar tuning fork will respond only to another tuning fork having exactly the same "tune," or number of vibrations per second. And Mr. Marconi has now succeeded in bringing this tuning system to some degree of perfection, though very much work yet remains to be done. For instance, in one of his English experiments, at Poole in England, he had two receivers connected with the same wire, and tuned to different transmitters located at St. Catherine's Point. Two messages were sent, one in English and one in French. Both were received at the same time on the same wire at Poole, but one receiver rolled off its message in English, the other in French, without the least interference. And so when critics suggested that the inventor may have been deceived at St. John's by messages transmitted from ocean liners, he was able to respond promptly:
"Impossible. My instrument was tuned to receive only from my station in Cornwall."
Indeed, the only wireless-telegraph apparatus that could possibly have been within hundreds of miles of Newfoundland would be one of the Marconi-fitted steamers, and the "call" of a steamer is not the letter "S," but "U."
The importance of the new system of tuning can hardly be overestimated. By it all the ships of a fleet can be provided with instruments tuned alike, so that they may communicate freely with one another, and have no fear that the enemy will read the messages. The spy of the future must be an electrical expert who can slip in somehow and steal the secret of the enemy's tunes. Great telegraph companies will each have its own tuned instruments, to receive only its own messages, and there may be special tunes for each of the important governments of the world. Or perhaps (for the system can be operated very cheaply) the time will even come when the great banking and business houses, or even families and friends, will each have its own wireless system, with its own secret tune. Having variations of millions of different vibrations, there will be no lack of tunes. For instance, the British navy may be tuned to receive only messages of 700,000 vibrations to the second, the German navy 1,500,000, the United States Government 1,000,000, and so on indefinitely.
Tuning also makes multiplex wireless telegraphy a possibility; that is, many messages may be sent or received on the same suspended wire. Supposing, for instance, the operator was sending a hurry press despatch to a newspaper. He has two transmitters, tuned differently, connected with his wire. He cuts the despatch in two, sends the first half on one transmitter, and the second on the other, thereby reducing by half the time of transmission.
A sort of impression prevails that wireless telegraphy is still largely in the uncertain experimental stage; but, as a matter of fact, it has long since passed from the laboratory to a wide commercial use. Its development since Mr. Marconi's first paper was read, in 1896, and especially since the first message was sent from England to France across the Channel in March, 1899, has been astonishingly rapid. Most of the ships of the great navies of Europe and all the important ocean liners are now fitted with the "wireless" instruments. The system has been recently adopted by the Lloyds of England, the greatest of shipping exchanges. It is being used on many lightships, and the New York Herald receives daily reports from vessels at sea, communicated from a ship station off Nantucket. Were there space to be spared, many incidents might be told showing in what curious and wonderful ways the use of the "wireless" instruments has saved life and property, to say nothing of facilitating business.
And it cannot now be long before a regular telegraph business will be conducted between Massachusetts and England, through the new stations. Mr. Marconi informed me that he would be able to build and equip stations on both sides of the Atlantic for less than $150,000, the subsequent charge for maintenance being very small. A cable across the Atlantic costs between $3,000,000 and $4,000,000, and it is a constant source of expenditure for repairs. The inventor will be able to transmit with single instruments about twenty words a minute, and at a cost ridiculously small compared with the present cable tolls. He said in a speech delivered at a dinner given him by the Governor at St. John's that messages which now go by cable at twenty-five cents a word might be sent profitably at a cent a word or less, which is even much cheaper than the very cheapest present rates in America for messages by land wires. It is estimated that about $400,000,000 is invested in cable systems in various parts of the world. If Marconi succeeds as he hopes to succeed, much of the vast network of wires at the bottom of the world's oceans, represented by this investment, will lose its usefulness. It is now the inventor's purpose to push the work of installation between the continents as rapidly as possible, and no one need be surprised if the year 1902 sees his system in practical operation. Along with this transatlantic work he intends to extend his system of transmission between ships at sea and the ports on land, with a view to enabling the shore stations to maintain constant communication with vessels all the way across the Atlantic. If he succeeds in doing this, there will at last be no escape for the weary from the daily news of the world, so long one of the advantages of an ocean voyage. For every morning each ship, though in mid-ocean, will get its bulletin of news, the ship's printing-press will strike it off, and it will be served hot with the coffee. Yet think what such a system will mean to ships in distress, and how often it will relieve the anxiety of friends awaiting the delayed voyager.
Mr. Marconi's faith in his invention is boundless. He told me that one of the projects which he hoped soon to attempt was to communicate between England and New Zealand. If the electric waves follow the curvature of the earth, as the Newfoundland experiments indicate, he sees no reason why he should not send signals 6,000 or 10,000 miles as easily as 2,000.
Then there is the whole question of the use of wireless telegraphy on land, a subject hardly studied, though messages have already been sent upward of sixty miles overland. The new system will certainly prove an important adjunct on land in war-time, for it will enable generals to signal, as they have done in South Africa, over comparatively long distances in fog and storm, and over stretches where it might be impossible for the telegraph corps to string wires or for couriers to pass on account of the presence of the enemy.

Work on the Smith Point Lighthouse Stopped by a Violent Storm.
Just after the cylinder had been set in place, and while the workmen were hurrying to stow sufficient ballast to secure it against a heavy sea, a storm forced the attending steamer to draw away. One of the barges was almost overturned, and a lifeboat was driven against the cylinder and crushed to pieces.
A sturdy English oak furnished the model for the first of the great modern lighthouses. A little more than one hundred and forty years ago John Smeaton, maker of odd and intricate philosophical instruments and dabbler in mechanical engineering, was called upon to place a light upon the bold and dangerous reefs of Eddystone, near Plymouth, England. John Smeaton never had built a lighthouse; but he was a man of great ingenuity and courage, and he knew the kind of lighthouse not to build; for twice before the rocks of Eddystone had been marked, and twice the mighty waves of the Atlantic had bowled over the work of the builders as easily as they would have overturned a skiff. Winstanley, he of song and story, designed the first of these structures, and he and all his keepers lost their lives when the light went down; the other, the work of John Rudyerd, was burned to the water's edge, and one of the keepers, strangely enough, died from the effects of melting lead which fell from the roof and entered his open mouth as he gazed upward. Both of these lighthouses were of wood, and both were ornamented with balconies and bay-windows, which furnished ready holds for the rough handling of the wind.

Robert Stevenson, Builder of the Famous Bell Rock Lighthouse, and Author of Important Inventions and Improvements in the System of Sea Lighting.
From a bust by Joseph, now in the library of Bell Rock Lighthouse.

The Bell Rock Lighthouse, on the Eastern Coast of Scotland.
From the painting by Turner. The Bell Rock Lighthouse was built by Robert Stevenson, grandfather of Robert Louis Stevenson, on the Inchcape Reef, in the North Sea, near Dundee, Scotland, in 1807-1810.
John Smeaton walked in the woods and thought of all these problems. He tells quaintly in his memoirs how he observed the strength with which an oak-tree bore its great weight of leaves and branches; and when he built his lighthouse, it was wide and flaring at the base, like the oak, and deeply rooted into the sea-rock with wedges of wood and iron. The waist was tapering and cylindrical, bearing the weight of the keeper's quarters and the lantern as firmly and jauntily as the oak bears its branches. Moreover, he built of stone, to avoid the possibility of fire, and he dovetailed each stone into its neighbour, so that the whole tower would face the wind and the waves as if it were one solid mass of granite. For years Smeaton's Eddystone blinked a friendly warning to English mariners, serving its purpose perfectly, until the Brothers of Trinity saw fit to build a larger tower in its place.
In England the famous lighthouses of Bell Rock, built by Robert Stevenson, Skerryvore, and Wolf Rock are all stone towers; and in our own country, Minot's Ledge, off Boston Harbour, more difficult of construction than any of them, Spectacle Reef light in Lake Huron, and Stannard Rock light in Lake Superior are good examples of Smeaton's method of building.

The Present Lighthouse on Minot's Ledge, near the Entrance of Massachusetts Bay, Fifteen Miles Southeast of Boston.
"Rising sheer out of the sea, like a huge stone cannon, mouth upward."—Longfellow.
The mighty stone tower still remains for many purposes the most effective method of lighting the pathways of the sea, but it is both exceedingly difficult to build, and it is very expensive. Within comparatively recent years busy inventors have thought out several new plans for lighthouses, which are quite as wonderful and important in their way as wireless telegraphy and the telephone are in the realm of electricity.
One of these inventions is the iron-pile or screw-pile lighthouse, and the other is the iron cylinder lighthouse. I will tell the story of each of them separately.

The Lighthouse on Stannard Rock, Lake Superior.
This is a stone-tower lighthouse, similar in construction to the one built with such difficulty on Spectacle Reef, Lake Huron.
The skeleton-built iron-pile lighthouse bears much the same relation to the heavy stone tower lighthouse that a willow twig bears to a great oak. The latter meets the fury of wind and wave with stern resistance, opposing force to force; the former conquers its difficulties by avoiding them.
A completed screw-pile lighthouse has the odd appearance of a huge, ugly spider standing knee-deep in the sea. Its squat body is the home of the keeper, with a single bright eye of light at the top, and its long spindly legs are the iron piles on which the structure rests. Thirty years ago lighthouse builders were much pleased with the ease and apparent durability of the pile light. An Englishman named Mitchell had invented an iron pile having at the end a screw not unlike a large auger. By boring a number of these piles deep into the sand of the sea-bottom, and using them as the foundation for a small but durable iron building, he was enabled to construct a lighthouse in a considerable depth of water at small expense. Later builders have used ordinary iron piles, which are driven into the sand with heavy sledges. Waves and tides pass readily through the open-work of the foundation, the legs of the spider, without disturbing the building overhead. For Southern waters, where there is no danger of moving ice-packs, lighthouses of this type have been found very useful, although the action of the salt water on the iron piling necessitates frequent repairs. More than eighty lights of this description dot the shoals of Florida and adjoining States. Some of the oldest ones still remain in use in the North, notably the one on Brandywine shoal in Delaware Bay; but it has been found necessary to surround them with strongly built ice-breakers.
Two magnificent iron-pile lights are found on Fowey Rocks and American Shoals, off the coast of Florida, the first of which was built with so much difficulty that its story is most interesting.

The Fowey Rocks Lighthouse,
Florida.
Fowey Reef lies five miles from the low coral island of Soldier Key. Northern storms, sweeping down the Atlantic, brush in wild breakers over the reef and out upon the little key, often burying it entirely under a torrent of water. Even in calm weather the sea is rarely quiet enough to make it safe for a vessel of any size to approach the reef. The builders erected a stout elevated wharf and store-house on the key, and brought their men and tools to await the opportunity to dart out when the sea was at rest and begin the work of marking the reef. Before shipment, the lighthouse, which was built in the North, was set up, complete from foundation to pinnacle, and thoroughly tested.
At length the workmen were able to remain on the reef long enough to build a strong working platform twelve feet above the surface of the water, and set on iron-shod mangrove piles. Having established this base of operations in the enemy's domain, a heavy iron disk was lowered to the reef, and the first pile was driven through the hole at its centre. Elaborate tests were made after each blow of the sledge, and the slightest deviation from the vertical was promptly rectified with block and tackle. In two months' time nine piles were driven ten feet into the coral rock, the workmen toiling long hours under a blistering sun. When the time came to erect the superstructure, the sea suddenly awakened and storm followed storm, so that for weeks together no one dared venture out to the reef. The men rusted and grumbled on the narrow docks of the key, and work was finally suspended for an entire winter. At the very first attempt to make a landing in the spring, a tornado drove the vessels far out of their course. But a crew was finally placed on the working platform, with enough food to last them several weeks, and there they stayed, suspended between the sea and the sky, until the structure was complete. This lighthouse cost $175,000.
The famous Bug Light of Boston and Thimble Light of Hampton Roads, Va., are both good examples of the iron-pile lighthouse.
Now we come to a consideration of iron cylinder lighthouses, which are even more wonderful, perhaps, than the screw-piles, and in constructing them the sea-builder touches the pinnacle of his art.
Imagine a sandy shoal marked only by a white-fringed breaker. The water rushes over it in swift and constantly varying currents, and if there is a capful of wind anywhere on the sea, it becomes an instant menace to the mariner. The shore may be ten or twenty miles away, so far that a land-light would only lure the seaman into peril, instead of guiding him safely on his way. A lightship is always uncertain; the first great storm may drive it from its moorings and leave the coast unprotected when protection is most necessary. Upon such a shoal, often covered from ten to twenty feet with water, the builder is called upon to construct a lighthouse, laying his foundation in shifting sand, and placing upon it a building strong enough to withstand any storm or the crushing weight of wrecks or ice-packs.
It was less than twenty years ago that sea-builders first ventured to grapple with the difficulties presented by these off-shore shoals. In 1881 Germany built the first iron cylinder lighthouse at Rothersand, near the mouth of the Weser River, and three years later the Lighthouse Establishment of the United States planted a similar tower on Fourteen-Foot Banks, over three miles from the shores of Delaware Bay, in twenty feet of water. Since then many hitherto dangerous shoals have been marked by new lighthouses of this type.

Fourteen-Foot Bank Light Station,
Delaware Bay, Del.
When a builder begins a stone tower light on some lonely sea-rock, he says to the sea, "Do your worst. I'm going to stick right here until this light is built, if it takes a hundred years." And his men are always on hand in fair weather or foul, dropping one stone to-day and another to-morrow, and succeeding by virtue of steady grit and patience. The builder of the iron cylinder light pursues an exactly opposite course. His warfare is more spirited, more modern. He stakes his whole success on a single desperate throw. If he fails, he loses everything: if he wins, he may throw again. His lighthouse is built, from foundation caisson to lantern, a hundred or a thousand miles away from the reef where it is finally to rest. It is simply an enormous cast-iron tube made in sections or courses, each about six feet high, not unlike the standpipe of a village water-works. The builder must set up this tube on the shoal, sink it deep into the sand bottom, and fill it with rocks and concrete mortar, so that it will not tip over. At first such a feat would seem absolutely impossible; but the sea-builder has his own methods of fighting. With all the material necessary to his work, he creeps up on the shoal and lies quietly in some secluded harbour until the sea is calmly at rest, suspecting no attack. Then he darts out with his whole fleet, plants his foundation, and before the waves and the wind wake up he has established his outworks on the shoal. The story of the construction of one of these lighthouses will give a good idea of the terrible difficulties which their builders must overcome.
Not long ago W. H. Flaherty, of New York, built such a lighthouse at Smith's Point, in Chesapeake Bay. At the mouth of the Potomac River the opposing tides and currents have built up shoals of sand extending eight or ten miles out into the bay. Here the waves, sweeping in from the open Atlantic, sometimes drown the side-lights of the big Boston steamers. The point has a grim story of wrecks and loss of life; in 1897 alone, four sea-craft were driven in and swamped on the shoals. The Lighthouse Establishment planned to set up the light just at the edge of the channel, and 120 miles south of Baltimore.

The Great Beds Light Station,
Raritan Bay, N. J.
A specimen of iron cylinder
construction.
Eighty thousand dollars was appropriated for doing the work. In August, 1896, the contractors formally agreed to build the lighthouse for $56,000, and, more than that, to have the lantern burning within a single year.
By the last of September a huge, unwieldy foundation caisson was framing in a Baltimore shipyard. This caisson was a bottomless wooden box, 32 feet square and 12 feet high, with the top nearly as thick as the height of a man, so that it would easily sustain the weight of the great iron cylinder soon to be placed upon it. It was lined and caulked, painted inside and out to make it air-tight and water-tight, and then dragged out into the bay, together with half an acre of mud and dock timbers. Here the workmen crowned it with the first two courses of the iron cylinder—a collar 30 feet in diameter and about 12 feet high. Inside of this a second cylinder, a steel air-shaft, five feet in diameter, rose from a hole in the centre of the caisson, this providing a means of entrance and exit when the structure should reach the shoal.
Upon the addition of this vast weight of iron and steel, the wooden caisson, although it weighed nearly a hundred tons, disappeared completely under the water, leaving in view only the great black rim of the iron cylinder and the top of the air-shaft.
On April 7th of the next year the fleet was ready to start on its voyage of conquest. The whole country had contributed to the expedition. Cleveland, O., furnished the iron plates for the tower; Pittsburg sent steel and machinery; South Carolina supplied the enormous yellow-pine timbers for the caisson; Washington provided two great barge-loads of stone; and New York City contributed hundreds of tons of Portland cement and sand and gravel, it being cheaper to bring even such supplies from the North than to gather them on the shores of the bay.
Everything necessary to the completion of the lighthouse and the maintenance of the eighty-eight men was loaded aboard ship. And quite a fleet it made as it lay out on the bay in the warm spring sunshine. The flagship was a big, double-deck steamer, 200 feet over all, once used in the coastwise trade. She was loaded close down to her white lines, and men lay over her rails in double rows. She led the fleet down the bay, and two tugs and seven barges followed in her wake like a flock of ducklings. The steamer towed the caisson at the end of a long hawser.
In three days the fleet reached the lighthouse site. During all of this time the sea had been calm, with only occasional puffs of wind, and the builders planned, somewhat exultantly, to drop the caisson the moment they arrived.
But before they were well in sight of the point, the sea awakened suddenly, as if conscious of the planned surprise. A storm blew up in the north, and at sunset on the tenth of April the waves were washing over the top of the iron cylinder and slapping it about like a boy's raft. A few tons of water inside the structure would sink it entirely, and the builder would lose months of work and thousands of dollars.
From a rude platform on top of the cylinder two men were working at the pumps to keep the water out. When the edge of the great iron rim heaved up with the waves, they pumped and shouted; and when it went down, they strangled and clung for their lives.
The builder saw the necessity of immediate assistance. Twelve men scrambled into a life-boat, and three waves later they were dashed against the rim of the cylinder. Here half of the number, clinging like cats to the iron plates, spread out a sail canvas and drew it over the windward half of the cylinder, while the other men pulled it down with their hands and teeth and lashed it firmly into place. In this way the cylinder shed most of the wash, although the larger waves still scuttled down within its iron sides. Half of the crew was now hurried down the rope-ladders inside the cylinder, where the water was nearly three feet deep and swashing about like a whirlpool. They all knew that one more than ordinarily large wave would send the whole structure to the bottom; but they dipped swiftly, and passed up the water without a word. It was nothing short of a battle for life. They must keep the water down, or drown like rats in a hole. They began work at sunset, and at sunrise the next morning, when the fury of the storm was somewhat abated, they were still at work, and the cylinder was saved.
The swells were now too high to think of planting the caisson, and the fleet ran into the mouth of the Great Wicomico River to await a more favourable opportunity. Here the builders lay for a week. To keep the men busy some of them were employed in mixing concrete, adding another course of iron to the cylinder, and in other tasks of preparation. The crew was composed largely of Americans and Irishmen, with a few Norwegians, the ordinary Italian or Bohemian labourer not taking kindly to the risks and terrors of such an expedition. Their number included carpenters, masons, iron-workers, bricklayers, caisson-men, sailors, and a host of common shovellers. The pay varied from twenty to fifty cents an hour for time actually worked, and the builders furnished meals of unlimited ham, bread, and coffee.
On April 17th, the weather being calmer, the fleet ventured out stealthily. A buoy marked the spot where the lighthouse was to stand. When the cylinder was exactly over the chosen site, the valves of two of the compartments into which it was divided were quickly opened, and the water poured in. The moment the lower edge of the caisson, borne downward by the weight of water, touched the shoal, the men began working with feverish haste. Large stones were rolled from the barges around the outside of the caisson to prevent the water from eating away the sand and tipping the structure over.
In the meantime a crew of twenty men had taken their places in the compartments of the cylinder still unfilled with water. A chute from the steamer vomited a steady stream of dusty concrete down upon their heads. A pump drenched them with an unceasing cataract of salt water. In this terrible hole they wallowed and struggled, shovelling the concrete mortar into place and ramming it down. Every man on the expedition, even the cooks and the stokers, was called upon at this supreme moment to take part in the work. Unless the structure could be sufficiently ballasted while the water was calm, the first wave would brush it over and pound it to pieces on the shoals.

Saving the Cylinder of the Lighthouse at Smith Point, Chesapeake Bay, from being Swamped in a High Sea.
When the builders were towing the unwieldy cylinder out to set it in position, the water became suddenly rough and began to fill it. Workmen, at the risk of their lives, boarded the cylinder, and by desperate labours succeeded in spreading sail canvas over it, and so saved a structure that had cost months of labour and thousands of dollars.
After nearly two hours of this exhausting labour the captain of the steamer suddenly shouted the command to cast away.
The sky had turned black and the waves ran high. All of the cranes were whipped in, and up from the cylinder poured the shovellers, looking as if they had been freshly rolled in a mortar bed. There was a confused babel of voices and a wild flight for the steamer. In the midst of the excitement one of the barges snapped a hawser, and, being lightened of its load, it all but turned over in a trough of the sea. The men aboard her went down on their faces, clung fast, and shouted for help, and it was only with difficulty that they were rescued. One of the life-boats, venturing too near the iron cylinder, was crushed like an egg-shell, but a tug was ready to pick up the men who manned it.
So terrified were the workmen by the dangers and difficulties of the task that twelve of them ran away that night without asking for their pay.
On the following morning the builder was appalled to see that the cylinder was inclined more than four feet from the perpendicular. In spite of the stone piled around the caisson, the water had washed the sand from under one edge of it, and it had tipped part way over. Now was the pivotal point of the whole enterprise. A little lack of courage or skill, and the work was doomed.
The waves still ran high, and the freshet currents from the Potomac River poured past the shoals at the rate of six or seven miles an hour. And yet one of the tugs ran out daringly, dragging a barge-load of stone. It was made fast, and although it pitched up and down so that every wave threatened to swamp it and every man aboard was seasick, they managed to throw off 200 tons more of stone around the base of the caisson on the side toward which it was inclined. In this way further tipping in that direction was prevented, and the action of the water on the sand under the opposite side soon righted the structure.
Beginning on the morning of April 21st the entire crew worked steadily for forty-eight hours without sleeping or stopping for meals more than fifteen minutes at a time. When at last they were relieved, they came up out of the cylinder shouting and cheering because the foundation was at last secure.
The structure was now about thirty feet high, and filled nearly to the top with concrete. The next step was to force it down 15½ feet into the hard sand at the bottom of the bay, thus securing it for ever against the power of the waves and the tide. An air-lock, which is a strongly built steel chamber about the size of a hogshead, was placed on top of the air-shaft, the water in the big box-like caisson at the bottom of the cylinder was forced out with compressed air, and the men prepared to enter the caisson.
No toil can compare in its severity and danger with that of a caisson worker. He is first sent into the air-lock, and the air-pressure is gradually increased around him until it equals that of the caisson below; then he may descend. New men often shout and beg pitifully to be liberated from the torture. Frequently the effect of the compressed air is such that they bleed at the ears and nose, and for a time their heads throb as if about to burst open.
In a few minutes these pains pass away, the workers crawl down the long ladder of the air-shaft and begin to dig away the sand of the sea-bottom. It is heaped high around the bottom of a four-inch pipe which leads up the air-shaft and reaches out over the sea. A valve in the pipe is opened and the sand and stones are driven upward by the compressed air in the caisson and blown out into the water with tremendous force. As the sand is mined away, the great tower above it slowly sinks downward, while the subterranean toilers grow sallow-faced, yellow-eyed, become half deaf, and lose their appetites.
When Smith's Point Light was within two feet of being deep enough the workmen had a strange and terrible adventure.
Ten men were in the caisson at the time. They noticed that the candles stuck along the wall were burning a lambent green. Black streaks, that widened swiftly, formed along the white-painted walls. One man after another began staggering dizzily, with eyes blinded and a sharp burning in the throat. Orders were instantly given to ascend, and the crew, with the help of ropes, succeeded in escaping. All that night the men lay moaning and sleepless in their bunks. In the morning only a few of them could open their eyes, and all experienced the keenest torture in the presence of light. Bags were fitted over their heads, and they were led out to their meals.

Great Waves Dashed Entirely Over Them, so that They had to Cling for Their Lives to the Air-Pipes.
In erecting the Smith Point lighthouse, after the cylinder was set up, it had to be forced down fifteen and a half feet into the sand. The lives of the men who did this, working in the caisson at the bottom of the sea, were absolutely in the hands of the men who managed the engine and the air-compressor at the surface; and twice these latter were entirely deluged by the sea, but still maintained steam and kept everything running as if no sea was playing over them.
That afternoon Major E. H. Ruffner, of Baltimore, the Government engineer for the district, appeared with two physicians. An examination of the caisson showed that the men had struck a vein of sulphuretted hydrogen gas.
Here was a new difficulty—a difficulty never before encountered in lighthouse construction. For three days the force lay idle. There seemed no way of completing the foundation. On the fourth day, after another flooding of the caisson, Mr. Flaherty called for volunteers to go down the air-shaft, agreeing to accompany them himself—all this in the face of the spectacle of thirty-five men moaning in their bunks, with their eyes burning and blinded and their throats raw. And yet fourteen men stepped forward and offered to "see the work through."
Upon reaching the bottom of the tower they found that the flow of gas was less rapid, and they worked with almost frantic energy, expecting every moment to feel the gas griping in their throats. In half an hour another shift came on, and before night the lighthouse was within an inch or two of its final resting-place.
The last shift was headed by an old caisson-man named Griffin, who bore the record of having stood seventy-five pounds of air-pressure in the famous Long Island gas tunnel. Just as the men were ready to leave the caisson the gas suddenly burst up again with something of explosive violence. Instantly the workmen threw down their tools and made a dash for the air-shaft. Here a terrible struggle followed. Only one man could go up the ladder at a time, and they scrambled and fought, pulling down by main force every man who succeeded in reaching the rounds. Then one after another they dropped in the sand, unconscious.
Griffin, remaining below, had signalled for a rope. When it came down, he groped for the nearest workman, fastened it around his body, and sent him aloft. Then he crawled around and pulled the unconscious workmen together under the air-shaft. One by one he sent them up. The last was a powerfully built Irishman named Howard. Griffin's eyes were blinded, and he was so dizzy that he reeled like a drunken man, but he managed to get the rope around Howard's body and start him up. At the eighteen-inch door of the lock the unconscious Irishman wedged fast, and those outside could not pull him through. Griffin climbed painfully up the thirty feet of ladder and pushed and pulled until Howard's limp body went through. Griffin tried to follow him, but his numbed fingers slipped on the steel rim, and he fell backward into the death-hole below. They dropped the rope again, but there was no response. One of the men called Griffin by name. The half-conscious caisson-man aroused himself and managed to tie the rope under his arms. Then he, too, was hoisted aloft, and when he was dragged from the caisson, more dead than alive, the half-blinded men on the steamer's deck set up a shout of applause—all the credit that he ever received.
Two of the men prostrated by the gas were sent to a hospital in New York, where they were months in recovering. Another went insane. Griffin was blind for three weeks. Four other caisson-men came out of the work with the painful malady known as "bends," which attacks those who work long under high air-pressure. A victim of the "bends" cannot straighten his back, and often his legs and arms are cramped and contorted. These terrible results will give a good idea of the heroism required of the sea-builder.
Having sunk the caisson deep enough the workmen filled it full of concrete and sealed the top of the air-shaft. Then they built the light-keeper's home, and the lantern was ready for lighting. Three days within the contract year the tower was formally turned over to the Government.
And thus the builders, besides providing a warning to the hundreds of vessels that yearly pass up the bay, erected a lasting monument to their own skill, courage, and perseverance. As long as the shoal remains the light will stand. In the course of half a century, perhaps less, the sea-water will gnaw away the iron of the cylinder, but there will still remain the core of concrete, as hard and solid as the day on which it was planted.
It is fitting that work which has drawn so largely upon the highest intellectual and moral endowments of the engineer and the builder should not serve the selfish interests of any one man, nor of any single corporation, nor even of the Government which provided the means, but that it should be a gift to the world at large. Other nations, even Great Britain, which has more at stake upon the seas than any other country, impose regular lighthouse taxes upon vessels entering their harbours; but the lights erected by the United States flash a free warning to any ship of any land.
It is indeed a great moment when an inventor comes to the announcement of a new and epoch-making achievement. He has been working for years, perhaps, in his laboratory, struggling along unknown, unheard of, often poor, failing a hundred times for every achieved success, but finally, all in a moment, surprising the secret which nature has guarded so long and so faithfully. He has discovered a new principle that no one has known before, he has made a wonderful new machine—and it works! What he has done in his laboratory for himself now becomes of interest to all the world. He has a great message to give. His patience and perseverance through years of hard work have produced something that will make life easier and happier for millions of people, that will open great new avenues for human effort and human achievement, build up new fortunes; often, indeed, change the whole course of business affairs in the world, if not the very channels of human thought. Think what the steam-engine has done, and the telegraph, and the sewing-machine! All this wonder lies to-day in the brain of the inventor; to-morrow it is a part of the world's treasure.
Such a moment came on an evening in January, 1902, when Peter Cooper Hewitt, of New York City—then wholly unknown to the greater world—made the announcement of an invention of such importance that Lord Kelvin, the greatest of living electricians, afterward said that of all the things he saw in America the work of Mr. Hewitt attracted him most.
On that evening in January, 1902, a curious crowd was gathered about the entrance of the Engineers' Club in New York City. Over the doorway a narrow glass tube gleamed with a strange blue-green light of such intensity that print was easily readable across the street, and yet so softly radiant that one could look directly at it without the sensation of blinding discomfort which accompanies nearly all brilliant artificial lights. The hall within, where Mr. Hewitt was making the first public announcement of his discovery, was also illuminated by the wonderful new tubes. The light was different from anything ever seen before, grateful to the eyes, much like daylight, only giving the face a curious, pale-green, unearthly appearance. The cause of this phenomenon was soon evident; the tubes were seen to give forth all the rays except red—orange, yellow, green, blue, violet—so that under its illumination the room and the street without, the faces of the spectators, the clothing of the women lost all their shades of red; indeed, changing the very face of the world to a pale green-blue. It was a redless light. The extraordinary appearance of this lamp and its profound significance as a scientific discovery at once awakened a wide public interest, especially among electricians who best understood its importance. Here was an entirely new sort of electric light. The familiar incandescent lamp, the invention of Thomas A. Edison, though the best of all methods of illumination, is also the most expensive. Mr. Hewitt's lamp, though not yet adapted to all the purposes served by the Edison lamp, on account of its peculiar colour, produces eight times as much light with the same amount of power. It is also practically indestructible, there being no filament to burn out; and it requires no special wiring. By means of this invention electricity, instead of being the most costly means of illumination, becomes the cheapest—cheaper even than kerosene. No further explanation than this is necessary to show the enormous importance of this invention.
Mr. Hewitt's announcement at once awakened the interest of the entire scientific world and made the inventor famous, and yet it was only the forerunner of two other inventions equally important. Once discover a master-key and it often unlocks many doors. Tracing out the principles involved in his new lamp, Mr. Hewitt invented:
A new, cheap, and simple method of converting alternating electrical currents into direct currents.
An electrical interrupter or valve, in many respects the most wonderful of the three inventions.
Before entering upon an explanation of these discoveries, which, though seemingly difficult and technical, are really simple and easily understandable, it will be interesting to know something of Mr. Hewitt and his methods of work and the genesis of the inventions.
Mr. Hewitt's achievements possess a peculiar interest for the people of this country. The inventor is an American of Americans. Born to wealth, the grandson of the famous philanthropist, Peter Cooper, the son of Abram S. Hewitt, one of the foremost citizens and statesmen of New York, Mr. Hewitt might have led a life of leisure and ease, but he has preferred to win his successes in the American way, by unflagging industry and perseverance, and has come to his new fortune also like the American, suddenly and brilliantly. As a people we like to see a man deserve his success! The same qualities which made Peter Cooper one of the first of American millionaires, and Abram S. Hewitt one of the foremost of the world's steel merchants, Mayor of New York, and one of its most trusted citizens, have placed Mr. Peter Cooper Hewitt among the greatest of American inventors and scientists. Indeed, Peter Cooper and Abram S. Hewitt were both inventors; that is, they had the imaginative inventive mind. Peter Cooper once said:
"I was always planning and contriving, and was never satisfied unless I was doing something difficult—something that had never been done before, if possible."
The grandfather built the first American locomotive; he was one of the most ardent supporters of Cyrus Field in the great project of an Atlantic cable, and he was for a score of years the president of a cable company. His was the curious, constructive mind. As a boy he built a washing machine to assist his overworked mother; later on he built the first lawnmower and invented a process for rolling iron, the first used in this country; he constructed a torpedo-boat to aid the Greeks in their revolt against Turkish tyranny in 1824. He dreamed of utilising the current of the East River for manufacturing power; he even experimented with flying machines, becoming so enthusiastic in this labour that he nearly lost the sight of an eye through an explosion which blew the apparatus to pieces.
It will be seen, therefore, that the grandson comes naturally by his inclinations. It was his grandfather who gave him his first chest of tools and taught him to work with his hands, and he has always had a fondness for contriving new machines and of working out difficult scientific problems. Until the last few years, however, he has never devoted his whole time to the work which best pleased him. For years he was connected with his father's extensive business enterprise, an active member, in fact, of the firm of Cooper, Hewitt & Co., and he has always been prominent in the social life of New York, a member of no fewer than eight prominent clubs. But never for a moment in his career—he is now forty-two years old, though he looks scarcely thirty-five—has he ceased to be interested in science and mechanics. As a student in Stevens Institute, and later in Columbia College, he gave particular attention to electricity, physics, chemistry, and mechanics. Later, when he went into business, his inventive mind turned naturally to the improvement of manufacturing methods, with the result that his name appears in the Patent Records as the inventor of many useful devices—a vacuum pan, a glue clarifier, a glue cutter and other glue machinery. He worked at many sorts of trades with his own hands—machine-shop practice, blacksmithing, steam-fitting, carpentry, jewelry work, and other work-a-day employments. He was employed in a jeweller's shop, learning how to make rings and to set stones; he managed a steam launch; he was for eight years in his grandfather's glue factory, where he had practical problems in mechanics constantly brought to his attention. And he was able to combine all this hard practical work with a fair amount of shooting, golfing, and automobiling.
Most of Mr. Hewitt's scientific work of recent years has been done after business hours—the long, slow, plodding toil of the experimenter. There is surely no royal road to success in invention, no matter how well a man may be equipped, no matter how favourably his means are fitted to his hands. Mr. Hewitt worked for seven years on the electrical investigations which resulted in his three great inventions; thousands of experiments were performed; thousands of failures paved the way for the first glimmer of success.
His laboratory during most of these years was hidden away in the tall tower of Madison Square Garden, overlooking Madison Square, with the roar of Broadway and Twenty-third Street coming up from the distance. Here he has worked, gradually expanding the scope of his experiments, increasing his force of assistants, until he now has an office and two workshops in Madison Square Garden and is building a more extensive laboratory elsewhere. Replying to the remark that he was fortunate in having the means to carry forward his experiments in his own way, he said:
"The fact is quite the contrary. I have had to make my laboratory pay as I went along."
Mr. Hewitt chose his problem deliberately, and he chose one of the most difficult in all the range of electrical science, but one which, if solved, promised the most flattering rewards.
"The essence of modern invention," he said, "is the saving of waste, the increase of efficiency in the various mechanical appliances."
This being so, he chose the most wasteful, the least efficient of all widely used electrical devices—the incandescent lamp. Of all the power used in producing the glowing filament in the Edison bulb, about ninety-seven per cent. is absolutely wasted, only three per cent. appearing in light. This three per cent. efficiency of the incandescent lamp compares very unfavourably, indeed, with the forty per cent. efficiency of the gasoline engine, the twenty-two per cent. efficiency of the marine engine, and the ninety per cent. efficiency of the dynamo.

The Hewitt Mercury Vapour Light.
The circular piece just above the switch button is one form of "boosting coil" which operates for a fraction of a second when the current is first turned on. The tube shown here is about an inch in diameter and several feet long. Various shapes may be used. Unless broken, the tubes never need renewal.
Mr. Hewitt first stated his problem very accurately. The waste of power in the incandescent lamp is known to be due largely to the conversion of a considerable part of the electricity used into useless heat. An electric-lamp bulb feels hot to the hand. It was therefore necessary to produce a cool light; that is, a light in which the energy was converted wholly or largely into light rays and not into heat rays. This, indeed, has long been one of the chief goals of ambition among inventors. Mr. Hewitt turned his attention to the gases. Why could not some incandescent gas be made to yield the much desired light without heat?
This was the germ of the idea. Comparatively little was known of the action of electricity in passing through the various gases, though the problem involved had long been the subject of experiment, and Mr. Hewitt found himself at once in a maze of unsolved problems and difficulties.
"I tried many different gases," he said, "and found that some of them gave good results—nitrogen, for instance—but many of them produced too much heat and presented other difficulties."
Finally, he took up experiments with mercury confined in a tube from which the air had been exhausted. The mercury arc, as it is called, had been experimented with years before, had even been used as a light, although at the time he began his investigations Mr. Hewitt knew nothing of these earlier investigations. He used ordinary glass vacuum tubes with a little mercury in the bottom which he had reduced to a gas or vapour under the influence of heat or by a strong current of electricity. He found it a rocky experimental road; he has called invention "systematic guessing."
"I had an equation with a large number of unknown quantities," he said. "About the only thing known for a certainty was the amount of current passing into the receptacle containing the gas, and its pressure. I had to assume values for these unknown quantities in every experiment, and you can understand what a great number of trials were necessary, using different combinations, before obtaining results. I presume thousands of experiments were made."
Many other investigators had been on the very edge of the discovery. They had tried sending strong currents through a vacuum tube containing mercury vapour, but had found it impossible to control the resistance. One day, however, in running a current into the tube Mr. Hewitt suddenly recognised certain flashes; a curious phenomenon. Always it is the unexpected thing, the thing unaccounted for, that the mind of the inventor leaps upon. For there, perhaps, is the key he is seeking. Mr. Hewitt continued his experiments and found that the mercury vapour was conducting. He next discovered that when once the high resistance of the cold mercury was overcome, a very much less powerful current found ready passage and produced a very brilliant light: the glow of the mercury vapour. This, Mr. Hewitt says, was the crucial point, the genesis of his three inventions, for all of them are applications of the mercury arc.
Thus, in short, he invented the new lamp. By the use of what is known to electricians as a "boosting coil," supplying for an instant a very powerful current, the initial resistance of the cold mercury in the tube is overcome, and then, the booster being automatically shut off, the current ordinarily used in incandescent lighting produces an illumination eight times as intense as the Edison bulb of the same candle-power. The mechanism is exceedingly simple and cheap; a button turns the light on or off; the remaining apparatus is not more complex than that of the ordinary incandescent light. The Hewitt lamp is best used in the form of a long horizontal tube suspended overhead in a room, the illumination filling all the space below with a radiance much like daylight, not glaring and sharp as with the Edison bulb. Mr. Hewitt has a large room hung with green material and thus illuminated, giving the visitor a very strange impression of a redless world. After a few moments spent here a glance out of the window shows a curiously red landscape, and red buildings, a red Madison Square, the red coming out more prominently by contrast with the blue-green of the light.
"For many purposes," said Mr. Hewitt, "the light in its present form is already easily adaptable. For shopwork, draughting, reading, and other work, where the eye is called on for continued strain, the absence of red is an advantage, for I have found light without the red much less tiring to the eye. I use it in my own laboratories, and my men prefer it to ordinary daylight."
In other respects, however, its colour is objectionable, and Mr. Hewitt has experimented with a view to obtaining the red rays, thereby producing a pure white light.
"Why not put a red globe around your lamp?" is a common question put to the inventor. This is an apparently easy solution of the difficulty until one is reminded that red glass does not change light waves, but simply suppresses all the rays that are not red. Since there are no red rays in the Hewitt lamp, the effect of the red globe would be to cut off all the light.
But Mr. Hewitt showed me a beautiful piece of pink silk, coloured with rhodimin, which, when thrown over the lamp, changes some of the orange rays into red, giving a better balanced illumination, although at some loss of brilliancy. Further experiments along this line are now in progress, investigations both with mercury vapour and with other gases.
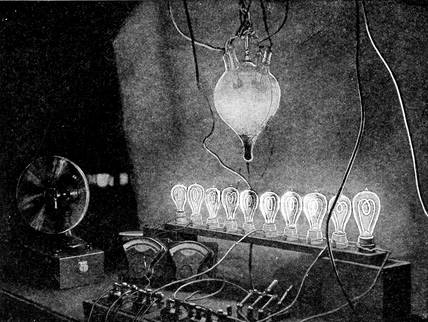
Testing a Hewitt Converter.
The row of incandescent lights is used, together with a voltmeter and an ammeter, to measure strength of current, resistance, and loss in converting.
Mr. Hewitt has found that the rays of his new lamp have a peculiar and stimulating effect on plant growth. A series of experiments, in which seeds of various plants were sown under exactly the same conditions, one set being exposed to daylight and one to the mercury gaslight, showed that the latter grew much more rapidly and luxuriantly. Without doubt, also, these new rays will have value in the curing of certain kinds of disease.
Further experimentation with the mercury arc led to the other two inventions, the converter and the interrupter. And first of the converter:
Hewitt's Electrical Converter.—The converter is simplicity itself. Here are two kinds of electrical currents—the alternating and the direct. Science has found it much cheaper and easier to produce and transmit the alternating current than the direct current. Unfortunately, however, only the direct currents are used for such practical purposes as driving an electric car or automobile, or running an elevator, or operating machine tools or the presses in a printing-office, and they are preferable for electric lighting. The power of Niagara Falls is changed into an alternating current which can be sent at high pressure (high voltage) over the wires for long distances, but before it can be used it must, for some purposes, be converted into a direct current. The apparatus now in use is cumbersome, expensive, and wasteful.
Mr. Hewitt's new converter is a mere bulb of glass or of steel, which a man can hold in his hand. The inventor found that the mercury bulb, when connected with wires carrying an alternating current, had the curious and wonderful property of permitting the passage of the positive half of the alternating wave when the current has started and maintained in that direction, and of suppressing the other half; in other words, of changing an alternating current into a direct current. In this process there was a loss, the same for currents of all potentials, of only 14 volts. A three-pound Hewitt converter will do the work of a seven-hundred-pound apparatus of the old type; it will cost dollars where the other costs hundreds; and it will save a large proportion of the electricity wasted in the old process. By this simple device, therefore, Mr. Hewitt has in a moment extended the entire range of electrical development. As alternating currents can be carried longer distances by using high pressure, and the pressure or voltage can be changed by the use of a simple transformer and then changed into a direct current by the converter at any convenient point along the line, therefore more waterfalls can be utilised, more of the power of coal can be utilised, more electricity saved after it is generated, rendering the operating of all industries requiring power so much cheaper. Every electric railroad, every lighting plant, every factory using electricity, is intimately concerned in Mr. Hewitt's device, for it will cheapen their power and thereby cheapen their products to you and to me.
Hewitt's Electrical Interrupter.—The third invention is in some respects the most wonderful of the three. Technically, it is called an electric interrupter or valve. "If a long list of present-day desiderata were drawn up," says the Electrical World and Engineer, "it would perhaps contain no item of more immediate importance than an interrupter which shall be ... inexpensive and simple of application." This is the view of science; and therefore this device is one upon which a great many inventors, including Mr. Marconi, have recently been working; and Mr. Hewitt has been fortunate in producing the much-needed successful apparatus.
The chief demand for an interrupter has come from the scores of experimenters who are working with wireless telegraphy. In 1894 Mr. Marconi began communicating through space without wires, and it may be said that wireless telegraphy has ever since been the world's imminent invention. Who has not read with profound interest the news of Mr. Marconi's success, the gradual increases of his distances? Who has not sympathised with his effort to perfect his devices, to produce a tuning apparatus by means of which messages flying through space could be kept secret? And here at last has come the invention which science most needed to complete and vitalise Marconi's work. By means of Mr. Hewitt's interrupter, the simplicity of which is as astonishing as its efficiency, the whole problem has been suddenly and easily solved.
Mr. Hewitt's new interrupter may, indeed, be called the enacting clause of wireless telegraphy. By its use the transmission of powerful and persistent electrical waves is reduced to scientific accuracy. The apparatus is not only cheap, light, and simple, but it is also a great saver of electrical power.
The interrupter, also, is a simple device. As I have already shown, the mercury vapour opposes a high resistance to the passage of electricity until the current reaches a certain high potential, when it gives way suddenly, allowing a current of low potential to pass through. This property can be applied in breaking a high potential current, such as is used in wireless telegraphy, so that the waves set up are exactly the proper lengths, always accurate, always the same, for sending messages through space. By the present method an ordinary arc or spark gap—that is, a spark passing between two brass balls—is employed in sending messages across the Atlantic. Marconi uses a spark as large as a man's wrist, and the noise of its passage is so deafening that the operators are compelled to wear cotton in their ears, and often they must shield their eyes from the blinding brilliancy of the discharges. Moreover, this open-air arc is subject to variations, to great losses of current, the brass balls become eroded, and the accuracy of the transmission is much impaired. All this is obviated by the cheap, simple, noiseless, sparkless mercury bulb.
"What I have done," said Mr. Hewitt, "is to perfect a device by means of which messages can be sent rapidly and without the loss of current occasioned by the spark gap. In wireless telegraphy the trouble has been that it was difficult to keep the sending and the receiving instruments attuned. By the use of my interrupter this can be accomplished."
And the possibilities of the mercury tube—indeed, of incandescent gas tubes in general—have by no means been exhausted. A new door has been opened to investigators, and no one knows what science will find in the treasure-house—perhaps new and more wonderful inventions, perhaps the very secret of electricity itself. Mr. Hewitt is still busily engaged in experimenting along these lines, both in the realm of abstract science and in that of practical invention. He is too careful a scientist, however, to speak much of the future, but those who are most familiar with his methods of work predict that the three inventions he has already announced are only forerunners of many other discoveries.
The chief pursuit of science and invention in this day of wonders is the electrical conquest of the world, the introduction of the electrical age. The electric motor is driving out the steam locomotive, the electric light is superseding gas and kerosene, the waterfall must soon take the place of coal. But certain great problems stand like solid walls in the way of development, part of them problems of science, part of mechanical efficiency. The battle of science is, indeed, not unlike real war, charging its way over one battlement after another, until the very citadel of final secret is captured. Mr. Hewitt with his three inventions has led the way over some of the most serious present barriers in the progress of technical electricity, enabling the whole industry, in a hundred different phases of its progress, to go forward.
THE END
[1]: In the first "Boys' Book of Inventions," the author devoted a chapter entitled "Through the Air" to the interesting work of the inventors of flying machines who have experimented with aëroplanes; that is, soaring machines modelled after the wings of a bird. The work of Professor S. P. Langley with his marvellous Aërodrome, and that of Hiram Maxim and of Otto Lilienthal, were given especial consideration. In the present chapter attention is directed to an entirely different class of flying machines—the steerable balloons.
Transcriber's Note:
Obvious punctuation errors have been silently repaired.
Inconsistencies, for example in hyphenation and spelling, have been retained.
Page 182: "Burnburg" is actually called "Bernburg".