
The Project Gutenberg EBook of A Guide to the Scientific Knowledge of Things Familiar, by Ebenezer Cobham Brewer This eBook is for the use of anyone anywhere at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org Title: A Guide to the Scientific Knowledge of Things Familiar Author: Ebenezer Cobham Brewer Release Date: September 3, 2012 [EBook #40652] Language: English Character set encoding: UTF-8 *** START OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC KNOWLEDGE--THINGS FAMILIAR *** Produced by David Garcia, Marilynda Fraser-Cunliffe, Matthew Wheaton and the Online Distributed Proofreading Team at http://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)

LONDON:
JARROLD AND SONS, 47, ST. PAUL’S CHURCHYARD,
ALSO HAMILTON AND CO., SIMPKIN AND CO.,
AND WHITTAKER AND CO.
Of all science, none is more generally interesting than that which explains the common phenomena of life. We see that salt and snow are both white, a rose red, leaves green, and the violet a deep purple; but how few persons ever ask the reason why! We know that a flute produces a musical sound, and a cracked bell a discordant one—that fire is hot, ice cold, and a candle luminous—that water boils when subjected to heat, and freezes from cold; but when a child looks up into our face and asks us “why,”—how many times is it silenced with a frown, or called “very foolish for asking such silly questions!” The object of the present book is to explain about 2000 of these “silly questions” (which are often more easily asked than answered) in language so simple that a child may understand it, yet not so childish as to offend the scientific; and in order that the answers may be strictly correct, not only the most approved modern authors have been consulted, but the manuscript has been submitted sheet by sheet to the revision of two gentlemen of acknowledged reputation for scientific attainments. To the Rev. A. Bath Power, M. A. especially, great obligation is due, for a careful revision of the whole manuscript, for many excellent hints, and useful additions. In conclusion, so much diligence has been bestowed upon this little work for nearly ten years, so much useful information has been supplied by scientific friends, and so minute a revision has been made of every answer, that it is no presumption to express a hope that this “Guide to the Scientific Knowledge of Things Familiar” will become generally useful and acceptable, not only to the young, but to those advanced to maturer life.
In this work some questions occur more than once, because they serve to illustrate different principles; and whenever cognate questions occur, the answers have been rendered as similar as possible, in order to assist the memory of the learner.
| PART I.—HEAT. | ||
| PAGE | ||
| I. | The Sun a source of heat | 2 |
| II. | Electricity a source of heat | 3 |
| Thunder and lightning | 3-29 | |
| III. | Chemical action a source of heat | 30 |
| III.—Combustion | 36 | |
| IV.—Smoke and smoky chimneys | 59 | |
| V.—Lamps and candles | 74 | |
| VI.—Animal heat | 83 | |
| VII. | Mechanical action a source of heat | 95 |
| VII.—Percussion | 95 | |
| VIII.—Friction | 98 | |
| VIII.—Compression | 102 | |
| IX. | Effects of heat | 103 |
| X.—Expansion | 103 | |
| XI.—Liquefaction | 126 | |
| XI.—Vaporization (clouds) | 127 | |
| XII.—Evaporation | 156 | |
| XIII. | Communication of heat | 164 |
| XIII.—Conduction | 164 | |
| XIV.—Absorption | 184 | |
| XV.—Reflection | 192 | |
| XVI.—Radiation (dew) | 195 | |
| XVII.—Convection (boiling) | 231 | |
| PART II.—AIR. | ||
| XVIII. | Air | 240 |
| Rust | 257 | |
| Tarnish | 259 | |
| XIX. | Carbonic acid gas | 264 |
| Froth. Effervescence. Fermentation, &c. | 269 | |
| XX. | Carburetted hydrogen gas | 279 |
| Fire damp | 280 | |
| Safety lamp | 281 | |
| XXI. | Phosphuretted hydrogen gas | 283 |
| Ignis fatuus | 285 | |
| Ghosts | 286 | |
| XXII. | Wind | 287 |
| XXIII. | Barometer | 317 |
| Ten special Rules | 319 | |
| XXIV. | Snow. Hail. Rain | 331 |
| XXV. | Water | 342 |
| XXVI. | Ice | 349 |
| Frost | 357 | |
| Freezing mixtures | 360 | |
| XXVII. | Light | 363 |
| Reflection. Telescopes. Refraction | 386 | |
| Spectacles | 389 | |
| Rainbows | 394 | |
| Colour | 399 | |
| XXVIII. | Sound | 409 |
| Ear trumpets | 415 | |
| Echoes | 416 | |
| XXIX. | Miscellaneous | 419 |
| Attraction. Anti-putrescents. Sleep. Dreams. | 424 | |
| Glossary | 426 | |
| Index | 427 | |
PART I.
Q. What is heat?
A. The sensation of warmth.
Q. How is this sensation produced?
A. When we touch a substance of higher temperature than ourselves, the warmer substance keeps parting with its heat, till both are of equal temperature.
Q. What is that “stream of heat” called, which flows thus, from one body, to another?
A. Calo’ric. Caloric, therefore, is the matter of heat, which passes from body to body; but Heat is the sensation, of warmth, produced by the influx of Calo’ric.
Q. What are the four principal sources of heat?
A. 1.—The Sun. 2.—Electricity. 3.—Chemical Action: and 4.—Mechanical Action.
Q. What are the principal effects of heat?
A. Expansion, Liquefaction, Vaporization, and Ignition.
Q. What is the principal source of Heat?
A. The Sun.
Q. Why do burning glasses set fire to substances submitted to their power?
A. The rays of the sun, collected by the Burning Glass, are all bent to one point, called the “focus;” thus the heat and light, (which should be diffused over the whole glass,) being gathered together into one point, are very greatly increased.
Q. Why is there a dark rim round this focus?
A. Because the rays of light, which should have fallen there, are bent into the focus, and the space around, (being deprived of these rays) is accordingly darkened.
Q. Are all the rays bent into one point?
A. No, not quite all: and, therefore, the rim round the focus is only slightly shadowed.
Q. What is the second chief source of heat?
A. Electricity.
Q. What is lightning?
A. Lightning is only an Electric Spark, taken from the clouds.
Q. What causes the discharge of an electric cloud?
A. When a cloud, overcharged with electric fluid, approaches another which is under-charged, the fluid rushes from the former into the latter, till both have the same quantity.
Q. Is there any other cause of lightning, besides the one just mentioned?
A. Yes; sometimes mountains, trees, and steeples, will discharge a lightning cloud floating near; and sometimes electric fluid rushes out of the earth, into the clouds.
Q. What produces electricity in the clouds?
A. 1st—The evaporation from the earth’s surface.
2ndly—The chemical changes perpetually going on: and
3rdly—Currents of air of unequal temperature, excite electricity by friction, as they pass by each other.
Q. How high are the lightning-clouds from the earth?
A. Electrical clouds are the lowest of all clouds; they are rarely more than 700 yards above the ground; and sometimes, they actually touch the earth with one of their edges.
Q. How high are the clouds generally?
A. In a fine day, the clouds are often 4 or 5 miles above our head; but the average height of the clouds is from 1-1/2 to 2 miles.
Q. Why is lightning sometimes forked?
A. When the lightning-cloud is a long way off, the resistance of the air is so great, that the electrical current is diverted into a zig-zag course.
Q. Why does the resistance of the air make the lightning zig-zag?
A. As the lightning condenses the air, in the immediate advance of its path; it keeps flying from side to side, in order to pass where there is the least resistance.
Q. How does lightning condense the air in the immediate advance of its path?
A. The air is condensed by the rapidity of the lightning-flash.
Q. Why is forked lightning more dangerous than a straight flash?
A. Whatever resists the flash, diverts its course; and when terrestrial objects offer resistance to the current, they are in great danger of being destroyed.
Q. Why are there sometimes two flashes of forked lightning at the same moment?
A. Sometimes (in very severe storms) a flash of lightning will divide into two or more parts; and then each branch assumes the zig-zag form.
Q. Why is the flash sometimes quite straight?
A. When the lightning-cloud hovers near the earth, as the flash meets with very little resistance, it is not diverted; or (in other words) the flash is straight.
Q. What is the cause of sheet lightning?
A. It is only the reflection of distant flashes, not distinctly visible: and sometimes several flashes (from different clouds) intermingle, and form one vast blaze or sheet of lightning.
Q. Which form of lightning is the most dangerous?
A. The ball of fire is by far the most dangerous; and the zig-zag lightning is next in danger. Sheet lightning is not often attended with danger.
Q. Why are balls of fire so very dangerous?
A. Because (whenever they fall) much mischief is occasioned by their bursting, which they always do, with an explosion like that of a cannon.
Q. Do these balls of lightning ever run along the ground?
A. Yes; they often run a considerable way along the ground, then stop for a little time, and burst in numberless pieces: sometimes each of these pieces will explode; and at other times, the whole ball will burst at once, producing most mischievous consequences.
Q. What mischief will these balls of fire produce?
A. They will set houses and barns on fire; and kill all cattle and human beings, which happen to be in their course.
Q. Why does lightning sometimes kill men and beasts?
A. When the electric current passes through a man or beast, it produces so violent an action upon the nerves, that it destroys life.
Q. When is a person struck dead by lightning?
A. Only when his body forms a part of the lightning’s path: i. e. when the electric fluid (in its way to the earth) actually passes through his body.
Q. Why are men sometimes maimed by lightning?
A. Because lightning strikes with amazing force, whatever opposes it: and if a man stand in the way, it strikes him such a blow, as to maim him.
Q. What is thunder?
A. Lightning parts the air through which it passes; and when the parted air closes again, the noise made by the concussion, is called Thunder.
Q. Why does lightning part the air through which it passes? It does not part a rod of iron.
A. Iron is a conductor, and therefore allows the fluid to go freely through it: but air being a non-conductor, resists the lightning; which, therefore, rips it open, in order to pass through it.
Q. Why is thunder sometimes one vast crash?
A. When the lightning-cloud is near the earth, as the flash is straight,—the whole volume of air (through which it passes) collapses at once; and produces one unbroken sudden crash.
Q. What is meant by the air collapsing?
A. When the rent air closes again, it is said to collapse.
Q. Why is the peal sometimes an irregular mangling broken roar?
A. When the lightning-cloud is a long way off, as the flash is zigzag, the air does not collapse all at once; and as we hear the concussion of one part after another, the peal is broken, protracted, and irregular.
Q. Which part of the collapsing air do we hear first?
A. That part nearest the earth; then the strata above; and last of all, that in the immediate vicinity of the cloud.
Q. What is meant by “strata of air?”
A. If a board were laid upon the earth, and several other boards were piled upon it, this pile would represent strata of wood.
Q. How does this illustration apply to the air?
A. A layer of air covers the earth; another layer rests upon it; and thus layer is piled upon layer, for 50 miles in height. Each layer is a “stratum” of air; and the plural of stratum is strata.
Q. Why do we hear the collapsing of the air nearest the earth first?
A. Because sound takes a whole second of time to travel 380 yards; but the air is ripped from top to bottom instantaneously: if, therefore, the cloud were 1000 yards off, we should hear the collapsing of the lowest strata nearly three seconds, before we heard that in the immediate vicinity of the cloud.
Q. Why is the thunder sometimes like a deep growl?
A. When the storm is far distant, the thunder sounds like a deep growl.
Q. Does not scenery affect the sound of thunder?
A. Yes; the flatter the country, the more unbroken the peal: Mountain scenery breaks the peal, and makes it harsh and irregular.
Q. What is the cause of rolling thunder?
A. The rolling is produced by the reverberation of the thunder along the massive clouds.
Q. What is meant by the reverberation?
A. The echo.
Q. Why is a flash of lightning generally followed by a pouring rain?
A. The cloud collapses, as soon as the electric fluid has left it; and the water it contained is squeezed out.
Q. Why is a flash of lightning generally followed by a gust of wind?
A. The flash rent the air asunder through which it darted; and when the two parts collapse, a rapid motion is produced, which we call wind: the vibration of the thunder contributes also to agitate the air.
Q. What is meant by the “vibration of the thunder?”
A. The quivering motion it gives to the air, by its loud sound.
Q. Why is there no thunder to what is called summer lightning?
A. Because the lightning-clouds are so far off, that the sound of the thunder is lost, before it reaches the earth.
Q. Do thunder-bolts ever drop from the clouds?
A. No; the notion of thunder-bolts falling from the clouds, arises from the globular form, that is sometimes assumed by a flash of lightning.
Q. Why is the thunder often several moments after the flash?[1]
A. The flash travels nearly a million times faster than the thunder; if, therefore, the thunder has far to come, it will not reach the earth till a considerable time after the flash.
[1] The speed of lightning is so great, that it would go 480 times round the earth in one minute: whereas, thunder would go scarcely 13 miles in the same space of time.
Q. Can we not tell the distance of a thunder-cloud, by observing the interval which elapses between the flash and the peal?
A. Yes; the flash is instantaneous, but the thunder will take a whole second of time to travel 380 yards: hence, if the flash is 5 seconds before the thunder, the cloud is 1900 yards off.
(i. e. 380 × 5 = 1900 yards.)
Q. What places are most dangerous to be in, during a storm?
A. It is very dangerous to be near a tree, or lofty building; it is dangerous also, to be near a river, or any running water.
Q. Why is it dangerous to be near a tree, or lofty building, during a thunder-storm?
A. Because a tall pointed object, [Pg 13](like a tree or spire,) will frequently discharge a lightning-cloud; and then the electric fluid will pass down it, in its way to the earth.
Q. How can a tree or spire discharge a lightning-cloud?
A. A lightning-cloud (floating over a plain) may be too far off to be discharged by it; but as a tree, or spire, would shorten the distance between the cloud and its conductor, it might no longer be too far off a conductor to be discharged.
Q. Is not air a conductor of lightning?
A. No; dry air is not a conductor of lightning; and therefore, the flash rends it in twain, to get to some conductor.
Q. Why would it be dangerous to stand near a tree or spire, while lightning is passing down it?
A. Because the electric fluid (called lightning) always rushes down the outside of the tree or spire; and if any one were standing near, might pass through him, and kill or maim him.
Q. Does lightning go through the inside or outside of a tree?
A. It rolls down the outside of a tree; but passes through the inside of a man.
Q. Why does lightning pass down the outside of a tree?
A. Lightning always makes choice of the best conductors; and the outside of a tree is a better conductor than the inside.
Q. Why does lightning pass through the inside of a man?
A. As the fluids of the human body make a better conductor than the skin, therefore lightning passes through a man, and not down the skin.
Q. Why is it dangerous to be near a deep river, or any other running water, during a thunder-storm?
A. Because running water is a good conductor; and lightning always takes in its course the best conductors.
Q. Why is it dangerous for a man to be near water, in a thunder-storm?
A. Because the height of a man may be sufficient to discharge a cloud: and (if there were no taller object nigh) the lightning might make the man its conductor to the water.
Q. Why is it dangerous to ring church-bells during a thunder-storm?
A. For two reasons: 1st—Because the steeple may discharge the lightning-cloud, in consequence of its mere height.
2ndly—The swinging of the bells causes a current of air, which collects electric fluid.
Q. Why is it unsafe to run or drive fast during a thunder-storm?
A. The rapid motion of running causes a current of air, which collects electric fluid, and is often fatal.
Q. What parts of a dwelling are most dangerous during a thunder-storm?
A. The fire-place, (especially if the fire be lighted); the attics and cellar. It is also dangerous to sit close by the walls; to ring the bell; or to bar the shutters, during a thunder-storm.
Q. Why is it dangerous to sit before a fire, during a thunder-storm?
A. Because the heated air and soot are conductors of lightning; especially when connected with such excellent conductors as the stove, fender, and fire-irons.
Q. Why are the attics and cellar dangerous, during a thunder-storm?
A. Lightning sometimes passes from the clouds to the earth, and sometimes from the earth to the clouds; and therefore, the middle story of a house is always the safest to be in, during a thunder-storm.
Q. When does lightning pass from the earth to the clouds?
A. When the clouds are in a “negative” state of electricity.
Q. When does lightning pass from the clouds to the earth?
A. When the clouds are in a “positive” state of electricity.
Q. What is meant by the clouds being in a “positive state of electricity?”
A. When the clouds contain more electric fluid than they generally do, they are said to be in a positive state of electricity.
Q. What is meant by the clouds being in a “negative state of electricity?”
A. When the clouds contain less electric fluid than they ought to do, they are said to be in a negative state of electricity.
Q. Does the flash proceed from a negative or positive body?
A. Always from a positive body, or one over-burdened with electric fluid.
Q. When lightning flashes from the earth to the clouds, what is the flash called?
A. It is called the “returning stroke;” because the earth (being over-burdened with electric fluid) returns the surplus quantity to the clouds.
Q. Why is it dangerous to lean back against a wall during a thunder-storm?
A. Because the electric fluid sometimes runs down the wall of a house or room; and (as a man is a better conductor than a brick wall), would make him its path, and injure him.
Q. Why is it dangerous to ring a bell during a thunder-storm?
A. Bell-wire is an excellent conductor; and (if a person were to touch the bell-handle), the electric fluid, passing down the wire, might run through his hand and injure it.
Q. Why would the lightning run through a man touching a bell-handle?
A. Because the human body is a better conductor than the wall (between the bell-handle and the floor); and as lightning always chooses the best conductors for its path, it would (in this case) pass through the man, and injure him.
Q. Why is it dangerous to bar a shutter during a thunder-storm?
A. The iron shutter-bar is an excellent conductor; and (if a person were touching the bar), the electric fluid passing down it, might run from the bar through the person touching it, and injure him.
Q. Why is it dangerous to be in a crowd during a thunder-storm?
A. For two reasons. 1st—Because a mass of people form a better conductor than an individual: and
2ndly—The vapour from a crowd increases the danger of such a place.
Q. Why is a mass of bodies a better conductor than a single body?
A. Each living body is a conductor of electricity; and a connected mass of such conductors is more likely to be[Pg 19] struck, than a single individual.
Q. Why is the danger increased by the vapour which rises from a crowd?
A. Vapour is a conductor, and therefore, may determine the shock; especially when connected with so many living bodies.
Q. Why is a theatre dangerous, during a thunder-storm?
A. Because the crowd assembled there, and the great vapour arising from so many living bodies, render a theatre an excellent conductor of lightning.
Q. Why is a flock of sheep in greater danger than a smaller number?
A. Because each sheep is a conductor of lightning, and the greater the number, the better its conducting power; besides, the vapour arising from a flock of sheep increases its conducting power, and its danger.
Q. Why is a herd of cattle in danger during a storm?
A. 1st—The number of living bodies increases the conducting power of the animal fluids: and
2ndly—The vapour arising from a herd is also a good conductor.
Q. If a person be abroad in a thunder-storm, what place is the safest?
A. Any spot about 20 or 30 feet from some tall tree or building; unless that spot be near to running water.
Q. Why would it be safe to stand 20 or 30 feet from some tall tree, in a thunder-storm?
A. Because the lightning would always choose the tall tree as a conductor, rather than the shorter man; and he would not be sufficiently near the tree, to be injured by the electric current passing down it.
Q. If a person be in a carriage in a thunder-storm, in what way can he travel most safely?
A. He should not lean against the carriage; but sit upright, without touching any of the four sides.
Q. Why should not a person lean against the carriage in a storm?
A. Because the electric fluid might run down the sides of the carriage; and (if a person were leaning against the[Pg 21] sides), would make choice of him for a conductor, and perhaps destroy life.
Q. If a person be in a house during a thunder storm, what place is safest?
A. Any room in the middle story. The middle of the room is best; especially if you place yourself on a mattrass, bed, or hearth-rug.
Q. Why is the middle story of a house safest in a thunder-storm?
A. Because (even if the fluid struck the house), its strength would be exhausted before it reached the middle story.
Q. Why is the middle of the room more safe, than any other part of it, in a thunder-storm?
A. Because, if the lightning came into the room at all, it would come down the chimney or walls of the room; and therefore, the further distant from these, the better.
Q. Why is a mattrass bed, or hearth-rug a good security against injury from lightning?
A. Because they are all non-conductors; and, as lightning always takes in its course the best conductors, it would not select such things as these.
Q. Is it better to be wet or dry during a storm?
A. To be wet: if a person be in the open field, the best thing he can do, is to stand about 20 feet from some tree, and get completely drenched to the skin.
Q. Why is it better to be wet than dry?
A. Because the wet clothes would form a far better conductor than the fluids of our body; and, lightning would roll down the wet clothes, without touching our body at all.
Q. What is the safest thing a person can do to avoid injury from lightning?
A. He should draw his bedstead into the middle of his room, commit himself to the care of God, and go to bed; remembering that our Lord has said, “The very hairs of your head are all numbered.”
Q. What is a lightning-conductor?
A. A metal rod fixed in the earth, running up the whole height of a building, and rising in a point above it.
Q. What metal is the best for this purpose?
A. Stout copper wire.
Q. Why is copper wire better than iron?
A. 1st—Because copper is a better conductor than iron:
2ndly—It is not so easily fused or melted: and
3rdly—It is not so much injured by weather.
Q. What is the good of a lightning-conductor?
A. Metal wire is a most excellent conductor; and as the lightning makes choice of the best conductors, it would run down the metal wire, rather than the bricks of the building.
Q. How far will the beneficial influence of a lightning-conductor extend?
A. It will protect a circumference all round, the diameter of which is (at least) 4 times as long as that part of the rod, which rises above the building.
Q. Give me an example.
A. If the rod rise 2 feet above the house, it will protect the building for (at least) 8 feet all round.
Q. Why are not lightning-conductors more generally used?
A. Because they are often productive of more harm than good.
Q. How can lightning-conductors be productive of harm?
A. If the rod be broken by weather or accident, the electric fluid (being obstructed in its path) will rend the building into fragments.
Q. Is there any other evil to be apprehended from a lightning rod?
A. Yes; if the rod be not big enough to conduct the whole current to the earth, the lightning will fuse the metal, and greatly injure the building.
Q. How stout is it needful for the copper wire to be, that it may conduct the fluid safely to the earth?
A. It should be (at least) one inch in diameter.
Q. Why does lightning sometimes knock down houses and churches?
A. The steeple, or chimney is first struck; the lightning then darts to the iron bars and cramps employed in the building; and (as it darts from bar to bar) shatters to atoms the bricks and stones, which oppose its progress.
Q. Can you tell me how St. Bride’s Church (London) was nearly destroyed by lightning, about 100 years ago?
A. The lightning first struck the metal vane, and ran down the rod; it then darted to the iron cramps, employed to support the building; and (as it flew from bar to bar) smashed the stones of the church, which lay between.
Q. Why did the lightning fly about from place to place, and not pass down in a straight course?
A. Because it always takes in its course the best conductors; and will fly both right and left, in order to reach them.
Q. Why does lightning turn milk sour?
A. Lightning causes the gases of the air (through which it passes) to combine, and thus produces a poison, called nitric acid; some small portion of which, mixing with the milk, turns it sour.[2]
(N. B. Sometimes, the mere heat of the air, during the storm, turns milk sour.)
[2] The air is composed of two gases, called oxygen and hydrogen, mixed together, but not combined. If oxygen is combined with nitrogen, it produces five deadly poisons, viz.—nitrous oxide, nitric oxide, hyponitrous acid, nitrous acid, and nitric acid, according to the proportion of each gas in the combination.
Q. What is the difference between combining and mixing?
A. When different ingredients mingle [Pg 26]without undergoing any chemical change, they are said to be mixed; but when the natural properties of each are altered by the union, then those ingredients are said to be combined.
Q. Give me an example.
A. If different coloured sands be shaken together in a bottle, the various grains will mix together, but not combine: but if water be poured on quick lime, the water will combine with the lime, and not mix with it.
Q. Why are the different grains of sand said to be mixed, when they are shaken together?
A. Because they are mingled together, but the property of each grain remains the same as it was before.
Q. Why is water poured on lime, said to combine with it?
A. Because the properties, both of the water and the lime, are altered by the mixture: the lime alters the character of the water, and the water alters the character of the lime.
Q. Do oxygen and nitrogen combine, or only mix together, in common atmospheric air?
A. They only mix together, as grains of sand would do, when shaken in a bottle. When oxygen and nitrogen combine, they do not constitute air, but acid poisons.
Q. Why does lightning turn beer sour, although contained in a close cask?
A. If the beer be new, and the process of fermentation not complete, lightning will so accelerate the process, as to turn the liquor sour.
Q. Why is not old beer and strong porter made sour by lightning?
A. Because the fermentation is complete already; and, therefore, is not affected by electrical influence.
Q. Why is metal sometimes fused by lightning?
A. Because the dimension of the metal is too small, to afford a path for the electric current.
Q. Why does lightning purify the air?
A. For two reasons: 1st—Because the oxygen and nitrogen of the air combine,[3] and produce “nitric acid:”
2ndly—Because the agitation of the storm stirs up the air.
[3] The oxygen and hydrogen are not combined, but simply mixed in the ordinary air; but the lightning causes the mixed elements to combine.
Q. How does the production of nitric acid purify the air?
A. Nitric acid acts very powerfully in destroying exhalations, arising from putrid vegetable and animal matters.
Q. Why is lightning more common in summer and autumn, than in spring and winter?
A. The heat of summer and autumn produces great evaporation; and the conversion of water to vapour, always develops electricity.
Q. Why does a thunder-storm generally follow very dry weather, and rarely succeeds continued wet?
A. The clouds are always charged with electricity; but dry air (being a non-conductor), will not conduct the surplus fluid from the clouds to the earth: so it violently rends the dry air with a flash, in order to relieve the cloud, and reach the earth.
Q. What is the general direction of a thunder-storm?
A. Either from east to west; or else from north to south.
Q. Why is electricity excited by friction?
A. Electricity, like heat, exists in all matter; but is often in a latent state: friction disturbs it, and brings it into active operation. (see p. 31.)
Q. Why is a tree sometimes scorched by lightning, as if it had been set on fire?
A. Lightning scorches it by its own positive heat, just the same as fire would.
Q. Why is the bark of a tree often ripped quite off by a flash of lightning?
A. As the lightning runs down the tree, it develops the latent heat so rapidly, that it carries the bark of the tree along with it, while it seeks to escape.
Q. Why are boughs of trees broken off by lightning?
A. The mechanical force of lightning is very great; and when the flash strikes a tree, it will often break off the boughs by the force with which it strikes against it.
Q. Why is an electric shock felt most at the elbow joint?
A. Because the path of the fluid is obstructed by the joint: and the shock felt at the elbow is caused by the fluid leaping from one bone to another.
Q. What is the third chief source of heat?
A. Chemical Action.
Q. What is meant by chemical action being the source of heat?
A. Many things, when their chemical constitution is changed, (either by the abstraction of some of their gases, or by the combination of others not before united,) evolve heat, while the change is going on.
Q. Explain by illustration what you mean.
A. Water is cold, and sulphuric acid is cold; but if these two cold liquids be mixed together, they will produce boiling heat.
Q. Why will cold water, mixed with sulphuric acid, produce heat?
A. Because water (being lighter than sulphuric acid), is condensed by the heavier liquid; and its heat is squeezed out, as water from a sponge.
Q. Why does cold water, poured on lime, make it intensely hot?
A. The heat is evolved by the chemical action, produced by the cold water combining with the lime.
Q. Where does the heat come from?
A. It was in the water and lime before; but was in a latent state.
Q. Was there heat in the cold water and lime, before they were mixed together?
A. Yes. All bodies contain heat; the coldest ice, as well as the hottest fire.
Q. Is there heat even in ice?
Q. How do you know there is heat, if you cannot perceive it?
Q. What becomes of the 140°, which went into the ice to melt it?
A. It is hidden in the water; or (to speak more scientifically) it is stored up in a latent state.
Q. How much heat may be thus secreted or made latent?
A. All things contain a vast quantity of latent heat; but, as much as 1140° of heat may remain latent in water.
Q. How can 1140° of heat be added to water, without being perceptible to our feelings?
A. 1st—140° of heat are hidden in the water, when ice is melted by the sun or fire.
2ndly—1000° more of heat are secreted, when water is converted into steam. Thus, before ice is converted into steam, 1140° of heat become latent.[6]
[6] Thus, one pint of boiling water, (212° according to the thermometer,) will make 1800 pints of steam; but the steam is no hotter to the touch than boiling water, both are 212°: therefore, when water is converted into steam, 1000° of heat become latent. Hence, before ice is converted to steam, it must contain 1140° of latent heat.
Q. Can we be made to feel the heat of ice or snow?
A. Yes. Into a pint of snow put half as much salt; then plunge your hand into the liquid; and it will feel so intensely cold, that the snow itself will seem quite warm in comparison to it.
Q. Is salt and snow really colder than snow?
A. Yes, many degrees; and by [Pg 33]dipping your hand into the mixture first, and into snow afterwards, the mere snow will seem to be comparatively warm.
Q. What is fire?
A. Combustion is another instance of heat, arising from chemical action.
Q. What two things are essential to produce combustion?
A. Fuel and air.
Q. What are the elements of fuel?
A. As bread is a compound of flour, yeast, and salt; so fuel is a compound of hydrogen and carbon.
Q. What are the elements of atmospheric air?
A. The air is a compound of oxygen and nitrogen mixed together; in the proportion of five gallons of nitrogen, to one of oxygen.
Q. What is carbon?
A. The solid part of fuel. It abounds also in all animal bodies, earths, and minerals.
Q. Mention some different species of carbon.
A. Common charcoal, lamp-black, coke, black lead, and the diamond, are all varieties of carbon.
Q. What is hydrogen?
A. An inflammable gas. The gas used in our streets, is only the hydrogen gas driven out of coals by heat.
Q. What are the peculiar characteristics of hydrogen gas?
A. Though this gas itself will burn, yet a candle will not burn when immersed in it; nor can an animal live in it. Hydrogen gas is the lightest of all known substances.[7]
[7] Hydrogen gas may be made thus:—Put some pieces of zinc or iron filings into a glass: pour over them a little sulphuric acid (vitriol), diluted with twice the quantity of water; then cover the glass over for a few minutes, and hydrogen gas will be given off.
Exp. If a flame be put into the glass, an explosion will be made.
If the experiment be tried in a phial, which has a piece of tobacco-pipe run through the cork; and a light held a few moments to the top of the pipe, a flame will be made.
If a balloon be held over the phial, (so that the gas can inflate it,) the balloon will ascend in a very few minutes.
Q. What is oxygen?
A. A gas, much heavier than hydrogen; which gives brilliancy to flame, and is essential to animal life.[8]
[8] Oxygen gas is much more troublesome to make than hydrogen. The cheapest plan is to put a few ounces of manganese (called the black oxide of manganese) into an[Pg 35] iron bottle, furnished with a bent tube; set the bottle on a fire till it becomes red hot, and put the end of the tube into a pan of water. In a few minutes, bubbles will rise through the water; these bubbles are oxygen gas.
These bubbles may be collected thus:—Fill a common bottle with water; hold it topsy-turvy over the bubbles which rise through the pan, but be sure the mouth of the bottle be held in the water. As the bubbles rise into the bottle, the water will run out; and when all the water has run out, the bottle is full of gas. Cork the bottle while the mouth remains under water; set the bottle on its base; cover the cork with lard or wax, and the gas will keep till it be wanted.
N. B. The quickest way of making oxygen gas, is to rub together in a mortar half an ounce of oxide of copper, and half an ounce of chlorate of potassa. Put the mixture into a common oil flask, furnished with a cork which has a bent tube thrust through it. Heat the bottom of the flask over a candle or lamp; and when the mixture is red hot, oxygen gas will be given off. Note—the tube must be immersed in a pan of water, and the gas collected as before.
(Chlorate of potassa may be bought at any chemist’s; and oxide of copper may be procured by heating a sheet of copper red hot, and when cool, striking it with a hammer: the scales that peel off, are oxide of copper.)
Exp. Put a piece of red hot charcoal, (fixed to a bit of wire,) into your bottle of oxygen gas; and it will throw out most dazzling sparks of light.
Blow a candle out; and while the wick is still red, hold the candle (by a piece of wire,) in the bottle of oxygen gas; the wick will instantly ignite, and burn brilliantly.
(Burning sulphur emits a blue flame, when immersed in oxygen gas.)
Q. What is nitrogen?
A. Nitrogen is another invisible gas. It will not burn, like hydrogen; and [Pg 36]an animal cannot live in it: it abounds in animal and vegetable substances, and is the chief ingredient of the common air.[9]
[9] Nitrogen gas may easily be obtained thus:—Put a piece of burning phosphorus on a little stand, in a plate of water; and cover a bell glass over. (Be sure the edge of the glass stands in the water.) In a few minutes the air will be decomposed, and nitrogen alone remain in the bell glass.
(N.B. The white fume which will arise and be absorbed by the water in this experiment, is phosphoric acid; i. e. phosphorus combined with oxygen of the air.)
Q. Why is there so much nitrogen in the air?
A. In order to dilute the oxygen. If the oxygen were not thus diluted, fires would burn out, and life would be exhausted too quickly.
Q. What three elements are necessary to produce combustion?
A. Hydrogen gas, carbon, and oxygen gas; the two former in the fuel, and the last in the air which surrounds the fuel.
Q. What causes the combustion of the fuel?
A. The hydrogen gas of the fuel being set free, and excited by a piece of lighted paper, instantly unites with the oxygen of the air, and makes a yellow flame: this flame heats the carbon of the [Pg 37]fuel, which also unites with the oxygen of the air, and produces carbonic acid gas.
Q. What is carbonic acid gas?
A. Only carbon (or charcoal) combined with oxygen gas.
Q. Why does fire produce heat?
A. 1st—By liberating latent heat from the air and fuel: and
2ndly—By throwing into rapid motion the atoms of matter.
Q. How is latent heat liberated by combustion?
A. When the oxygen of the air combines with the hydrogen of the fuel, the two gases condense into water; and latent heat is squeezed out, as water from a sponge.
Q. How are the atoms of matter disturbed by combustion?
A. 1st—When hydrogen of fuel and oxygen of air condense into water, a vacuum is made; and the air is disturbed, as a pond would be, if a pail of water were taken out of it: and
2ndly—When the carbon of fuel and oxygen of air expand into carbonic acid[Pg 38] gas, the air is again disturbed, as it would be by the explosion of gunpowder.
Q. How does fire condense hydrogen and oxygen into water?
A. The hydrogen of fuel and oxygen of air (liberated by combustion) combining together, condense into water.
Q. How does fire expand carbon into carbonic acid gas?
A. The carbon of fuel and oxygen of air (combining together in combustion) expand into a gas, called carbonic acid.
Q. Why is a fire (after it has been long burning) red hot?
A. When coals are heated throughout, the carbon is so completely mixed with the oxygen of the air, that the whole surface is in a state of combustion, and therefore red hot.
Q. In a blazing fire, why is the upper surface of the coals black, and the lower surface red?
A. Carbon (being very solid) requires a great degree of heat to make it unite with the oxygen of the air. When fresh[Pg 39] coals are put on, their under surface is heated before the upper surface; and one is red (or in a state of combustion), while the other is black.
Q. Which burns the quicker, a blazing fire, or a red hot one?
A. A blazing fire burns out the fuel quickest.
Q. Why do blazing coals burn quicker than red hot ones?
A. In red hot coals, only the mere surface is in a state of combustion, because the carbon is solid; but in a blazing fire, (where the gases are escaping), the whole volume of the coal throughout is in a state of decomposition.
Q. What is smoke?
A. Unconsumed parts of fuel (principally carbon), separated from the solid mass, and carried up the chimney by the current of hot air.
Q. Why is there more smoke when coals are fresh added, than when they are red hot?
A. Carbon (being solid), requires a great degree of heat to make it unite with oxygen, (or, in other words, to bring it[Pg 40] into a state of perfect combustion): when coals are fresh laid on, more carbon is separated than can be reduced to combustion; and so it flies off in smoke.
Q. Why is there so little smoke with a red hot fire?
A. When a fire is red hot, the entire surface of the coals is in a state of combustion; so a very little flies off unconsumed, as smoke.
Q. Why are there dark and bright spots in a clear cinder fire?
A. Because the intensity of the combustion is greater in some parts of the fire, than it is in others.
Q. Why is the intensity of the combustion so unequal?
A. Because the air flies to the fire in various and unequal currents.
Q. Why do we see all sorts of grotesque figures in hot coals?
A. Because the intensity of combustion is so unequal, (owing to the gusty manner in which the air flies to the fuel; and the various shades of red, yellow, and white heat mingling with the black of the[Pg 41] unburnt coal), produce strange and fanciful resemblances.
Q. Why does paper burn more readily than wood?
A. Merely because it is of a more fragile texture; and, therefore, its component parts are more easily heated.
Q. Why does wood burn more readily than coal?
A. Because it is not so solid; and, therefore, its elemental parts are more easily separated, and made hot.
Q. When a fire is lighted, why is paper laid at the bottom, against the grate?
A. Because paper (in consequence of its fragile texture), so very readily catches fire.
Q. Why is wood laid on the top of the paper?
A. Because wood, (being more substantial), burns longer than paper; and, therefore, affords a longer contact of flame to heat the coals.
Q. Why would not paper do without wood?
A. Because paper burns out so rapidly, that it would not afford sufficient contact of flame to heat the coals to combustion.
Q. Why would not wood do without shavings, straw, or paper?
A. Because wood is too substantial to be heated into combustion, by the flame issuing from a mere match.
Q. Why would not the paper do as well, if placed on the top of the coals?
A. As every blaze tends upwards, if the paper were placed on the top of the fire, its blaze would afford no contact of flame to fuel lying below.
Q. Why should coal be placed above the wood?
A. As every flame tends upwards, if the wood were above the coal, the flame would not rise through the coal to heat it.
Q. Why is a fire kindled at the lowest bar of a grate?
A. As every flame tends upwards; when a flame is made at the bottom of a fire, it ascends through the fuel and heats it: whereas, if the fire were lighted from the top, the flame would not come into contact with the fuel piled below.
Q. Why does coal make such excellent fuel?
A. Because it is so very hard and compact, that it burns away very slowly.
Q. Why will cinders become red hot, quicker than coals?
A. Because they are more porous and less solid; and are, therefore, sooner reduced to a state of combustion.
Q. Why will not iron cinders burn?
A. Iron cinders are cinders saturated with oxygen; they are unfit for fuel, because they can imbibe no more oxygen, being saturated already.
Q. Why are cinders lighter than coals?
A. Because their vapour, gases, and volatile parts, have been driven off by previous combustion.
Q. Why will not stones do for fuel, as well as coals?
A. Because they contain no hydrogen (or inflammable gas) like coals.
Q. Why will not wet kindling light a fire?
A. 1st—Because the moisture of the wet kindling prevents the oxygen of the air from getting to the fuel to form it into carbonic acid gas: and
2ndly—The heat of the fire is perpetually drawn off, by the conversion of water into steam.
Q. Why does dry wood burn better than green?
A. 1st—Because no heat is carried away, by the conversion of water into steam: and
2ndly—The pores of dry wood are filled with air, which supply the fire with oxygen.
Q. Why do two pieces of wood burn better than one?
A. 1st—Because they help to entangle the heat of the passing smoke, and throw it on the fuel: and
2ndly—They help to entangle the air that passes over the fire, and create a kind of eddy or draught.
Q. Why does salt crackle when thrown into a fire?
A. Salt contains water; and the cracking of the salt is owing to the sudden conversion of the water into steam.
Q. Why will not wood or paper burn, if they are steeped in a solution of potash, phosphate of lime, or ammonia (hartshorn)?
A. Because any “al’kali” (such as potash) will arrest the hydrogen (as it escapes from the fuel), and prevent its combination with the oxygen of air.
Q. What is an al’kali?
A. The con’verse of an acid; as bitter is the con’verse of sweet, or insipid the con’verse of pungent.
Q. Why does a jet of flame sometimes burst into the room through the bars of a stove?
A. The iron bars conduct heat to the interior of some lump of coal: and its volatile gas (bursting through the weakest part) is kindled by the glowing coals over which it passes.
Q. Why is this jet sometimes of a greenish yellow colour?
A. When a lump of coals lies over the hot bars, or the coals below it are not red hot, the gas which bursts from the lump escapes unburnt, and is of a greenish colour.
Q. Why does the gas escape unburnt?
A. Because neither the bars nor coals (over which it passes) are red-hot.
Q. Why does a bluish flame sometimes flicker on the surface of hot cinders?
A. Gas from the hot coals at the bottom of the grate mixing with the carbon of the coals above, produces an inflammable gas (called carbonic oxide), which burns with a blue flame.
Q. Why is the flame of a good fire yellow?
A. Because both the hydrogen and carbon of the fuel are in a state of perfect combustion. It is the white heat of the carbon, which gives the pale yellow tinge to the flaming hydrogen.
Q. What is light?
A. Rapid undulations of a fluid called ether, striking on the eye.
Q. How does combustion make these undulations of light?
A. The atoms of matter (set in motion by heat) striking against this ether, produce undulations in it; as a stone thrown into a stream, would produce undulations in the water.
Q. How can undulations of ether produce light?
A. As sound is produced by undulations[Pg 47] of air striking on the ear; so light is produced by undulations of ether striking on the eye.
Q. What is ether?
A. A very subtile fluid, which pervades and surrounds every thing we see.
Q. Mention a simple experiment to prove that light is produced by rapid motion.
A. When a fiddle-string is jerked suddenly, its rapid vibration produces a grey light; and when a carriage wheel revolves very quickly, it sends forth a similar light.
Q. Does heat always produce light?
A. No: the heat of a stack of hay, or reeking dunghill, though very great, is not sufficient to produce light.
Q. Why is a yellow flame brighter than a red hot coal?
A. Because yellow rays always produce the greatest amount of light; though red rays produce the greatest amount of heat.
Q. Why is the light of a fire more intense sometimes than at others?
A. The intensity of fire-light depends upon the whiteness to which the carbon is reduced, by combustion. If the carbon be white hot, its combustion is perfect, and the light intense; if not, the light is obscured by smoke.
Q. Why will not cinders blaze, as well as fresh coals?
A. The flame of coals is made chiefly by hydrogen gas. As soon as this gas is consumed, the hot cinders produce only an invisible gas, called carbonic acid.
Q. Where does the hydrogen gas of a fire come from?
A. The fuel is decomposed (by combustion) into its simple elements, carbon and hydrogen gas. (see p. 33)
Q. Why does not a fire blaze on a frosty night, so long as it does upon another night?
A. The air (being very cold) rushes to the fire so rapidly, that the coals burn out faster, and the inflammable gas is sooner consumed.
Q. Why does a fire burn clearest on a frosty night?
A. Because the volatile gases are[Pg 49] quickly consumed; and the solid carbon plentifully supplied with air, to make it burn bright and intensely.
Q. Why does a fire burn more intensely in winter than in summer time?
A. Because the air is colder in winter, than in summer-time.
Q. How does the coldness of the air increase the heat of a fire?
A. For two reasons: 1st—Because cold air being more condensed than hot air, contains a greater body: and
2ndly—Cold air rushes more quickly to the fire, and supplies more oxygen.
Q. Why does the sun, shining on a fire, make it dull, and often put it out?
A. 1st—When the sun shines, the air is rarefied; and, therefore, flows more slowly to the fire.
2ndly—As the air is rarefied, even that which reaches the fire, affords less nourishment.
Q. Why does the air flow to the fire more tardily for being rarefied?
A. The greater the contrast (between the external air, and that which has[Pg 50] been heated by the fire) the more rapid will be the current of air towards that fire.
Q. Why does rarefied air afford less nourishment to fire, than cold air?
A. Because it is spread out, (like a piece of gold beaten into leaf); and as a square inch of gold leaf will not contain so much gold as a square inch of bullion—so, a square inch of rarefied air has less body, than a square inch of cold air.
Q. Why does a fire burn more fiercely in the open air?
A. 1st—Because the air out-of-doors is more dense, than the air in-doors: and
2ndly—Because air is more freely supplied to a fire out-of-doors.
Q. Why is the air out-of-doors more dense than that in-doors?
A. Because the circulation is more free; and as soon as any portion has been rarefied, it instantly escapes, and is supplied by colder currents.
Q. Why does not a fire burn so freely in a thaw, as in a frost?
A. During a thaw, the air is filled with vapour; and, both moves too slowly, and is too much diluted to nourish the fire.
Q. Why does a fire burn so fiercely in windy weather?
A. In windy weather the air is rapidly changed, and affords plentiful nourishment to the fire.
Q. Why do a pair of bellows get a fire up?
A. A pair of bellows, (like the wind), drives the air more rapidly to the fire; and the plentiful supply of oxygen soon makes the fire burn intensely.
Q. Why is a candle blown out by the breath, and not made more intense, like a fire?
A. As the flame of a candle is confined to a very small wick, it is severed from it by the breath; and (being unsupported) must go out.
Q. Why is a smouldering wick sometimes rekindled by blowing it?
A. The breath carries the air to it with great rapidity; and the oxygen of the air kindles the red hot wick, as it kindles charred wood.
Q. Why is not the red hot wick kindled by the air around it, without blowing it?
A. Because oxygen is not supplied with sufficient freedom, unless it be blown to the wick.
Q. When is this experiment most likely to succeed?
A. In frosty weather; because the air contains more oxygen then, being condensed by the cold.
Q. Why does a poker, laid across a dull fire, revive it?
A. For two reasons. 1st—Because the poker concentrates the heat, and therefore increases it: and
2ndly—Because the poker arrests the air which passes over the fire, and produces a draught.
Q. Why do several pieces of wood or coal burn better than one?
A. When there are two or three pieces of wood on a fire, the air (circulating round them) produces an eddy or draught, which draws up the fire.
Q. Why are stoves fixed on the floor of a room?
A. In order that the air, on the lower part of the room, may be heated by the fire.
Q. Would not the air of the lower part of a room be heated equally well, if the stoves were fixed higher up?
A. No; the heat of a fire has a very little effect upon the air below the level of the grate; and, therefore, every grate should be as near to the floor as possible.
Q. Why are our feet so cold when we sit close by a good fire?
A. As the fire consumes the air which passes over it, cold air rushes through the crevices of the doors and windows along the bottom of the room to supply the deficiency; and these currents of cold air, rushing constantly over our feet, deprive them of their warmth.
Q. If a piece of paper be laid flat on a clear fire, it will not blaze, but char. Why so?
A. The carbon of a clear fire, being sufficiently hot to unite with the oxygen of the air, produces carbonic acid gas,[Pg 54] which soon envelops the paper laid flat upon the cinders: but carbonic acid gas will not blaze.
Q. If you blow the paper, it will blaze immediately. Why so?
A. By blowing, or opening the door suddenly, the carbonic acid is dissipated, and the paper is instantly fanned into flame.
Q. Why does water extinguish a fire?
1st—Because the water forms a coating over the fuel, and keeps it from the air:
2ndly—The conversion of water into steam, draws off the heat of the burning fuel.
Q. Why does a little water make a fire fiercer, while a larger quantity of water puts it out?
A. Water is composed of oxygen and hydrogen; when, therefore, the fire can decompose the water into its simple elements, it serves for fuel to the flame.
Q. How can water serve for fuel to fire?
A. The hydrogen of the water will burn with a flame; and the oxygen of the water will increase the intensity of that flame.
Q. If a house be on fire, is too little water worse than no water at all?
A. Certainly. Unless the water be supplied so plentifully as to quench the fire, it will increase the intensity, like fuel.
Q. When will water extinguish fire?
A. When the supply is so rapid and abundant, that the fire cannot convert it into steam.
Q. Does not a very little water slacken the heat of fire?
A. Yes, till it is converted into steam; but then it increases the intensity of fire, and acts like fuel.
Q. Why does the wick of a candle (when the flame has been blown out) catch fire so readily?
A. As the wick is already very hot, a little extra heat will throw it into flame.
Q. Why does the extra heat revive the flame?
A. Because it again liberates the hydrogen of the tallow, and ignites it.
Q. Cannot wood be made to blaze without actual contact with fire?
A. Yes; if a piece of wood be held near the fire for a little time it will blaze, even though it does not touch the fire.
Q. Why will wood blaze, even if it does not touch the fire?
A. The heat of the fire drives out the hydrogen gas of the wood; which is inflamed by contact with the red-hot coals.
Q. Why will a neighbour’s house sometimes catch fire, though no flame of the burning house ever touches it?
A. The heat of the burning house sets at liberty the hydrogen gas of the neighbouring wood-work, which is ignited by the flames or red-hot bricks of the house on fire.
Q. What is coke?
A. Coal freed from its volatile gases, by the action of artificial heat.
Q. Why do arnott’s stoves sometimes smell so strong of sulphur?
A. The fire is made of coke, which contains sulphur; and, whenever the draught is not rapid enough to drive the sulphur up the flue, it is emitted into the room.
Q. What is meant by spontaneous combustion?
A. Ignition produced by the action of one uninflamed body on another.
Q. Give an example of spontaneous combustion.
A. Goods packed in a warehouse will often catch fire of themselves; especially such goods as cotton, flax, hemp, rags, &c.
Q. Why do such goods sometimes catch fire of themselves?
A. Because they are piled together in very great masses in a damp state or place.
Q. Why does this produce spontaneous combustion?
A. The damp produces decay or the decomposition of the goods, and the great heat of the piled-up mass makes the decaying goods ferment.
Q. How does this fermentation produce combustion?
A. During fermentation, carbonic acid gas is given off by the goods,—a slow combustion ensues,—till at length the whole pile bursts into flame.
Q. Why is the heat of a large mass of goods greater than that of a smaller quantity?
A. Because compression squeezes out[Pg 58] heat, as water is squeezed from a sponge; and as the goods of a large pile are greatly compressed, much of their latent heat is squeezed out.
Q. Why do hay-stacks sometimes catch fire of themselves?
A. Either because the hay was got up damp, or because rain has penetrated the stack.
Q. Why will a hay-stack catch fire if the hay be damp?
A. Damp hay soon decays, and undergoes a state of fermentation; during which, carbonic acid gas is given off, and the stack catches fire.
Q. Why does roasted coffee sometimes catch fire spontaneously?
A. The heat of coffee is greatly increased by being roasted; and the carbon of the coffee uniting with the oxygen of the air, produces carbonic acid gas, and bursts into flame.
Q. Why do old rags, used for cleaning lamps and candles, sometimes set a house on FIRE?
A. Because they very readily ferment, and (during fermentation) throw off exceedingly inflammable gases.
(N.B. Lamp-black mixed with linseed oil is more liable to spontaneous combustion, than anything that servants handle.)
Q. Why does smoke ascend the chimney?
A. As the air of the room passes over the fire, it becomes heated; and (being thus made lighter,) ascends the chimney, carrying the smoke with it.
Q. What is smoke?
A. Small particles of carbon, separated by combustion from the fuel, but not consumed.
Q. Why do smoke and steam curl, as they ascend?
A. Because they are moved in a right line, and then pushed on all sides; and this forces them into a circular motion.
Q. What are blacks?
A. When the hot air of the chimney has been cooled by the external air, it can no longer buoy up the solid smoke; so it falls to the earth in condensed flakes, called “blacks.”
Q. Why are there no blacks in the smoke of a railway engine?
A. The smoke of a railway engine consists chiefly of watery vapour, which dissolves in air, as sugar does in water; but the smoke of a common chimney consists of small fragments of unburnt fuel.
Q. Why does a “COPPER HOLE” DRAW up more fiercely than an open stove?
A. As the air, which supplies the copper hole, must pass through the furnace, it becomes exceedingly heated, and rushes up the chimney with great violence.
Q. What produces the roaring noise made by a copper-hole fire?
A. Air rushing rapidly through the crevices of the iron door, and up the chimney flue.
Q. Why is the roar less, if the copper-hole door be thrown open?
A. Because fresh air gets access to the fire more easily; and as the air is not so intensely heated, its motion is not so violent.
Q. Why do some chimneys smoke?
A. If fresh air is not admitted into a room, as fast as it is consumed by the fire, a current of air will rush down the chimney to supply the deficiency, and bring the smoke along with it.
Q. What prevents air being supplied, as fast as it is consumed by the fire?
A. Leather and curtains round the doors; sand-bags at the threshhold and on the window-frames; and other contrivances to keep out the draught.
Q. Why is it needful for cold fresh air to be so constantly supplied?
A. If water be taken with a pail out of a river, other water will rush towards the hole, as soon as the pail is lifted out; and if air be taken from a room, (as it is, when some of it goes up the chimney) other air will rush towards the void to fill it up.
Q. Why will it come down the chimney?
A. Because if doors and windows are all made air-tight, it can get to the room in no other way.
Q. What is the best remedy in such a case?
A. The speediest remedy is to open the door or window: but by far the best remedy is to carry a small tube from the hearth into the external air.
Q. Why is that the best remedy?
A. Because the fire will be plentifully supplied with air by the tube; the doors and windows may all remain air-tight; and we may enjoy a warm fireside, without the inconvenience of draughts and cold feet.
Q. Why is a chimney raised so high above the roof?
A. If it were not so, it would smoke; as all funnels do which are too short.
Q. What is meant by the funnel, or flue of a chimney?
A. That part of a chimney through which the smoke passes, is called the funnel, or flue.
Q. Why does a chimney smoke, if the funnel be very short?
A. Because the draught of a short flue is too slack to carry the smoke up the chimney.
Q. Why is the draught of a short flue more slack that that of a long one?
A. For many reasons. 1st—The fire is always dull and sluggish if the chimney be too short.
2ndly—The smoke rolls out of the chimney, before it has acquired its full velocity.
3rdly—The wind, rain, and air, have more influence over a short funnel, than over a long one.
Q. Why is the fire always dull and sluggish if the chimney-flue be very short?
A. Because the draught is so bad: and as the rarefied air passes up the chimney very tardily, fresh air flows as tardily towards the fire, to supply it with oxygen.
Q. On what does the intensity of fire depend?
A. The intensity of fire is always in proportion to the quantity of oxygen with which it is supplied.
Q. Why does not smoke acquire its full velocity in a short funnel?
A. Because the higher smoke ascends in a flue, (provided it be clear and hot) the faster it goes; (as a stone falls faster and faster the lower it descends): if, therefore, a funnel be very short, the smoke never acquires its full velocity.
Q. Does the draught of a chimney depend on the speed of the smoke through the flue?
A. Yes. The more quickly hot air flies up the chimney, the more quickly cold air will rush towards the fire to supply the place; and, therefore, the longer the flue, the greater the draught.
Q. Why is the draught of a long flue greater than that of a short one?
A. Because the higher smoke ascends, the faster it goes; (as a stone falls faster and faster, the nearer it approaches to the earth): if, therefore, a funnel be long, the smoke acquires great velocity, and the draught is great.
Q. If a chimney be too short, and cannot be lengthened, what is the best remedy to prevent smoking?
A. To contract the opening of the chimney contiguous to the stove.
Q. Why will a smaller opening against the stove prevent the smoking?
A. As all the air (which enters the chimney) must pass near the fire, it will become greatly heated, and rise rapidly through the funnel; and this increase of heat will compensate for the shortness of the flue.
Q. Why will a room smoke, if there be two fires in it?
A. Because the fiercer fire will exhaust the most air; and draw from the smaller one, to supply its demand.
Q. Why will a chimney smoke if there be a fire in two rooms communicating with each other?
A. Whenever the door between the two rooms is opened, air will rush from the chimney of the inferior fire, to supply the other; and both rooms will be filled with smoke.
Q. What is the remedy in this case?
A. Let a tube be carried from the hearth of each stove, into the external[Pg 66] air; and then each fire will be so well supplied, that neither will need to borrow from the other.
Q. Why do vestry chimneys so often smoke?
A. Because the wind (striking against the steeple) is reflected back; and tumbles down the vestry chimney, forcing the smoke into the room.
Q. what winds make vestry chimneys smoke?
A. Those from the north-east or south-east; according to the position of the vestry.
Q. Why will the eastern winds make vestries smoke, more than those from the west?
A. Because they strike against the steeple, and bound back to the vestry chimney: but western winds cannot rebound over the roof of a church.
(N. B. The steeple of a church is always due west, and the other end of the church due east; if, therefore, a western wind rebound, it would rebound to the west, or away from the church, and not towards it.)
Q. Why does a house in a valley very often smoke?
A. Because the wind (striking against[Pg 67] the surrounding hills) rebounds back again upon the chimney, and destroys its draught.
Q. What is the common remedy in both these cases?
A. To fix a cowl on the chimney top, to turn like a weather-cock, and present its back to the wind.
Q. Why will not a cowl always prevent a chimney smoking?
A. If the wind be strong, it will keep the opening of the cowl towards the steeple or hill; and then the reflected wind will blow into the cowl, and down the chimney.
Q. As a cowl is such a poor remedy, can any other be devised?
A. If the chimney flue can be carried higher than the steeple or hills, no wind can enter the flue.
Q. Why cannot the wind enter a chimney flue, if it be carried up higher than the steeple or hills?
A. Because the reflected wind would strike against the sides of the chimney-flue, and not pass over the opening at all.
Q. In what other cases will a chimney smoke?
A. If both door and chimney be placed on the same side of a room, the chimney will often smoke.
Q. Why will a chimney smoke, if the door and stove are both on the same side?
A. Because when the door is opened, a current of air will blow into the chimney-place, and drive the smoke into the room.
Q. What remedy can be applied to this evil?
A. The door must be set opposite to the chimney, or nearly so; and then the draught from the door will blow the smoke up the chimney, and not into the room.
Q. Why will a chimney smoke if it needs sweeping?
A. Because the obstruction in the chimney (presented by the loose soot, to the free passage of the smoke) delays its current, and prevents the draught.
Q. Why will a chimney smoke, if out of repair?
A. 1st—Because the loose mortar and bricks obstruct the smoke: and
2ndly—The cold air (oozing through the chinks) chills the air in the chimney, and prevents its ascent.
Q. Why will an arnott’s stove smoke, if the joints of the flue do not fit air-tight?
A. Because the cold air (which gets through the joints) chills the air in the flue, and prevents its ascent.
Q. Why does an old fashioned farm chimney-place so often smoke?
A. Because the opening is so very large, that much of the air which goes up the chimney, has never passed near the fire; and this cold air mixing with the other, so reduces its temperature, that it ascends very slowly, and the draught is destroyed.
Q. Why does a chimney smoke, if the draught be slack?
A. Because, unless the current of air up the chimney be very powerful, it cannot buoy the smoke up through the flue.
Q. If the opening of a chimney be too large, what remedy can be applied?
A. The chimney-place must be contracted.
Q. Why will contracting the chimney-place prevent its smoking?
A. As the air will then pass nearer the fire, it will be more heated, and fly up the chimney much faster.
Q. Why do almost all chimneys smoke in gusty weather?
A. The gust (blowing the air away from the top of the chimney) removes (for a time) all resistance to the smoke: but when the wind lulls again, the resistance of the air suddenly returns—the draught is checked—and a puff of smoke rushes into the room.
Q. What is the use of a chimney-pot?
A. When the opening of a chimney is large, the top must be contracted by a chimney-pot, in order to increase the draught.
Q. How does a chimney-pot increase the draught of a chimney?
A. As the same quantity of hot air has to escape through a much smaller opening, it must pass through more quickly.
Q. Why do tin blowers help to get a fire up?
A. Because they compel the air to go through the fire, and not over it; therefore the fire is well supplied with oxygen, and the draught greatly increased.
Q. Why does a tin blower increase the draught?
A. As all the air which enters the chimney has to pass through the fire, it is much hotter, and ascends the chimney very fast; and the faster the air flies up the chimney, the faster it rushes towards the fire also.
Q. Why does a parlour often smell disagreeably of soot in summer-time?
A. The air in the chimney (being colder than the air in the parlour) descends into the room, and leaves a disagreeable smell of soot behind.
Q. Why are the ceilings of public offices so black and filthy?
A. The heated air ascending, carries the dust and fine soot to the ceiling; where the hot air escapes through the plaster, and leaves the soot and dust behind.
Q. Why are some parts of the ceiling blacker and more filthy than others?
A. As the air cannot penetrate the thick joists of the ceiling, it passes by those parts, and deposits its soot and dust on those which are more penetrable.
Q. What is charcoal?
A. Wood which has been exposed to a red heat, till it has been deprived of all its gases and volatile parts.
Q. Why is a charcoal fire hotter than a wood fire?
A. Because so large a quantity of water has been abstracted from the fuel, by the red heat to which it has been already exposed.
Q. Why does charcoal remove the taint of meat?
A. Because it absorbs all odoriferous effluvia, whether they arise from putrefying animal or vegetable matter.
Q. Why is water purified by being filtered through charcoal?
A. Charcoal absorbs the impurities of the water, and removes all disagreeable tastes and smells, whether they arise from animal or vegetable matter.
Q. Why are water and wine casks charred inside?
A. Charring the inside of the cask reduces it to a kind of charcoal; and charcoal (by absorbing animal and vegetable impurities) keeps the liquor sweet and good.
Q. Why does a piece of burnt bread, steeped in impure water, make it fit to drink?
A. The surface of the bread is reduced to charcoal by being burnt; and the charcoal surface of the bread abstracts all the impurities of the water, and makes it palatable.
Q. Why should the toast and water, placed by the side of the sick, be made of burnt bread?
A. The surface of the bread being reduced to charcoal by being burnt, prevents the water from being affected by the impurities of the sick room.
Q. Why are timbers, which are to be exposed to damp, charred?
A. Charcoal undergoes no change by exposure to air and water; therefore timber will resist weather much longer, after it has been charred.
Q. Of what are oil, tallow, and wax composed?
A. Principally of carbon and hydrogen gas. The solid part is carbon, the volatile part is gas.
Q. What is carbon?
A. A solid substance, generally of a black colour; well known under the forms of charcoal, lamp-black, coke, black-lead, &c.
Q. What is hydrogen gas?
Q. Why does a candle burn when lighted?
A. The heat of the lighted wick decomposes the tallow into its elementary [Pg 75]parts of carbon and hydrogen; and the hydrogen of the tallow, combining with the oxygen of the air, produces flame.
Q. Why is the flame of a candle hot?
A. 1st—Because the flame liberates latent heat from the air and tallow: and
2ndly—It throws into rapid motion the atoms of matter.
Q. How is latent heat liberated by the flame of a candle?
A. When the hydrogen of the tallow and oxygen of the air combine, they condense into water; and much of their latent heat is squeezed out.
Q. How are the atoms of matter disturbed by the flame of a candle?
A. 1st—When the hydrogen of the tallow and oxygen of the air condense into water, a vacuum is made; and the air is disturbed, as a pond would be, if a pail of water were taken out.
2ndly—When the carbon of tallow and oxygen of the air expand into carbonic acid gas, the air is again disturbed; in a similar way as by the explosion of gunpowder.
Q. Why does the flame of a candle produce light?
A. The chemical changes made by combustion, excite undulations of ether, which (striking the eye) produce light. (see p. 46.)
Q. Why is the flame of a candle yellow?
A. Only the outer coat of the flame is yellow; the lower part of the flame is violet; and the inside of the flame is hollow.
Q. Why is the outside of the flame yellow?
A. Because the carbon of the tallow (being in a state of perfect combustion) is made white-hot.
Q. Why is the bottom part purple of the flame of a candle?
A. The bottom part of the flame is overladen with hydrogen, raised from the tallow by the burning wick; and this half-burnt gas gives a purple tinge to the flame.
Q. Why is the inside of the flame of a candle hollow?
A. Because it is filled with vapour, raised from the candle by the heat of the wick.
Q. Describe the different parts of the flame of a common candle.
A. The flame consists of three cones. The innermost cone is hollow; the intermediate cone of a dingy purple hue; and the outside cone is yellow.
Q. Why is the intermediate cone of a flame purple, as well as the bottom of the flame.
A. Because the gases are not in a state of perfect combustion; but contain an excess of hydrogen, which gives this cone a purple tinge.
Q. Why is not the middle cone in a state of perfect combustion, as well as the outer cone?
A. Because the outer cone prevents the oxygen of the air from getting freely to the middle of the cone; and without the free access of oxygen gas, there is no such thing as complete combustion.
Q. Why does the flame of a candle point upwards?
A. The flame heats the surrounding air, which (being hot) rapidly ascends, and drives the flame upwards at the same time.
Q. Why is the flame of a candle pointed at the top, like a cone?
A. The upper part of a flame is more volatile than the lower parts; and as it affords less resistance to the air, is reduced to a mere point.
Q. Why is the upper part of a flame more volatile than the lower parts?
A. The lower parts of the flame are laden with unconsumed gas and watery vapour; which present considerable resistance to the air.
Q. Why is the flame of a candle blown out by a puff of breath?
A. As the flame of a candle is attached to a very small wick, a puff of breath severs the flame from the wick; and it goes out for want of support.
Q. Why does the flame of a candle make a glass damp, which is held over it?
A. The hydrogen of the tallow combining with the oxygen of the air, produce a “watery vapour,” which is condensed by the cold glass held above the flame.
Q. Why does our hand, held above a candle, suffer from the heat of the flame so much more, than when it is placed below the flame, or on one side of it?
A. Because the hot gases and air (in their ascent) come in contact with the hand placed above the flame: but when the hand is placed below the flame, or on one side, it only feels heat from radiation.
Q. Why is a rush light extinguished so much more quickly than a cotton-wicked candle?
A. As the rush wick is smooth and hard, the mere motion of the air (produced by carrying the candle from one place to another,) is sufficient to sever the flame from the rush.
Q. Why is it more difficult to blow out a cotton wick?
A. The cotton wick is quite full of small threads or filaments, which help to hold the flame on the wick, like the roots of a tree.
Q. Why does an extinguisher put a candle out?
A. Because the air in the extinguisher is soon exhausted of its oxygen by the flame: and when there is no oxygen to support it, the flame goes out.
Q. Why does not a candle set fire to a piece of paper twisted into an extinguisher, and used as such?
A. 1st—Because the flame very soon exhausts the little oxygen contained in the paper extinguisher: and
2ndly—The flame invests the inside of the paper extinguisher with carbonic acid gas, which prevents it from blazing.
Q. Why is a long wick never upright?
A. Because it is bent by its own weight.
Q. Why is a long wick covered with an efflorescence at the top?
A. The knotty or flowery appearance of the top of a wick arises from an accumulation of particles partly separated, but still loosely hanging to the wick.
Q. Why is not the end of a long wick burnt off, as it hangs over the flames?
A. Because the length of the wick so diminishes the heat of the flame, that it is not hot enough to burn it off.
Q. Why do palmer’s metallic wicks never need snuffing?
A. The wick is divided into two parts, each of which bends outward to the outside of the flame; where the end is intensely heated, and separated from[Pg 81] the wick by the current of air up the candle.
Q. Why do common candles require to be snuffed?
A. Because the heat of the flame is not sufficient to consume the wick; and the longer the wick grows, the less heat the flame produces.
Q. Why do wax candles never need snuffing?
A. The wick of wax candles is made of very fine thread, which the heat of the flame is sufficient to consume: but the wick of tallow candles is made of coarse cotton, which is too substantial to be consumed by the heat of the flame, and must be cut off by snuffers.
Q. Why does a pin, stuck in a rush-light, extinguish it?
A. Because a pin (being a good conductor), carries away the heat of the flame from the wick, and prevents the combustion of the tallow.
Q. What is the smoke of a candle?
A. Solid particles of carbon separated from the wick and tallow, but not consumed.
Q. Why are some particles consumed and not others?
A. The combustion of the carbon depends upon its combining with the oxygen of the air: but as the outer surface of the flame prevents the access of air to the interior parts, therefore much of the carbon of those parts passes off in smoke.
Q. Why do lamps smoke?
A. Either because the wick is cut unevenly, or else because it is turned up too high.
Q. Why does a lamp smoke when the wick is cut unevenly?
A. 1st—Because the points of the jagged edge (being very easily separated from the wick,) load the flame with more carbon than it can consume: and
2ndly—As the heat of the flame is greatly diminished by these bits of wick, it is unable to consume even the usual quantity of smoke.
Q. Why does a lamp smoke when the wick is turned up too high?
A. Because more carbon is separated from the wick than can be consumed by the flame.
Q. Why do not “Argand burners” smoke?
A. Because a current of air passes through the middle of the flame; and therefore the carbon of the interior is consumed, as well as that in the outer coating of the flame.
Q. Why does a lamp-glass diminish the smoke of a lamp?
A. Because it both concentrates and reflects the heat of the flame; in consequence of which, the heat is so greatly increased, that very little carbon escapes unconsumed.
Q. What is the cause of animal heat?
A. Animal heat is produced by the combustion of hydrogen and carbon in the capillary veins.
Q. What are capillary veins?
A. Veins as small as hairs running all over the body; so called from the Latin word “capilla’ris” (like a hair).
Q. Do these capillary veins run all over the human body?
A. Yes. Whenever blood flows from a wound, some vein must be divided; and as you cannot insert a needle into any part of the body without bringing blood, therefore these little veins must run through every part of the human frame.
Q. How do hydrogen gas and carbon get into these very little veins?
A. The food we eat is converted into blood, and blood contains both hydrogen and carbon.
Q. How does combustion take place in the veins?
A. The carbon of the blood combines with the oxygen of the air we breathe, and forms into carbonic acid gas.
Q. What becomes of this carbonic acid gas formed in the human blood?
A. Some of it is thrown off by the breath; and the rest of it is absorbed by the blood, to keep up the animal heat.
Q. What is the cause of the combustion of fire?
A. The carbon of fuel unites with the oxygen of the air, and forms carbonic acid gas.
Q. What is the cause of the combustion of a candle or lamp?
A. The carbon of the oil or tallow unites with the oxygen of the air, and forms carbonic acid gas.
Q. What is the cause of spontaneous combustion?
A. The piled-up goods ferment from heat and damp; and (during fermentation) carbonic acid gas is formed, as in the two former cases.
Q. Does the heat of the human body arise from the same cause as the heat of fire?
A. Yes, precisely. The carbon of the blood, combining with the oxygen of air inhaled, produces carbonic acid gas, which is attended with combustion.
Q. If animal heat is produced by combustion, why does not the human body burn up like a coal or candle?
A. It actually does so. Every muscle, nerve, and organ of the body, actually[Pg 86] wastes away like a burning candle; and (being reduced to air and ashes) is rejected from the system as useless.
Q. If every bone, muscle, nerve, and organ, is thus consumed by combustion, why is not the body entirely consumed?
A. It would be so, unless the parts destroyed were perpetually renewed: but as a lamp will not go out, so long as it is supplied with fresh oil; neither will the body be consumed, so long as it is supplied with sufficient food.
Q. When a man is starved, what parts of the body go first?
A. First the fat, because it is the most combustible; then the muscles; last of all the brain; and then the man dies, like a candle which is burnt out.
Q. Why does want of sufficient nourishment often produce madness?
A. After the fat and muscles of the body have been consumed by animal combustion, the brain is next attacked; and (unless the patient dies) madness must ensue from starvation.
Q. Why does a man shrink when starved?
A. A starved man shrinks just as a fire does, unless it be supplied with sufficient fuel.
Q. What is the fuel of the body?
A. Food is the fuel of the body; and the carbon of the food mixing with the oxygen of the air, evolves heat in the same way that a fire or candle does.
Q. Why is every part of the body warm?
A. As the capillary veins run through every part of the human body, and the combustion of blood takes place in the capillary veins, therefore every part of the body is warm.
Q. Why does running make us warm?
A. When we run, we inhale air more rapidly; and the rapidity with which we inhale air fans the combustion of our body, as a pair of bellows quickens the flame of a common fire.
Q. How does inhaling air rapidly make the body feel warm?
A. As the combustion of the blood is more rapid, (in consequence of the introduction of more oxygen from the[Pg 88]air), therefore the blood is more heated, and every part of the body is warmer also.
Q. Why does hard work produce hunger?
A. Because it produces quicker respiration; by which means a larger amount of oxygen is introduced into the lungs, and the capillary combustion increased. Hunger is the notice (given by our body) to remind us, that our food-fuel must be replenished.
Q. Why does singing make us hungry?
A. Singing increases respiration; and as more oxygen is introduced into the lungs, our food-fuel is more rapidly consumed.
Q. Why does reading aloud make us feel hungry?
A. Reading aloud increases respiration; and as more oxygen is introduced into the lungs, our food-fuel is more rapidly consumed.
Q. Why do we feel more hungry in the day-time than in the night-time?
A. As we breathe more slowly during sleep, therefore, less oxygen is introduced into the lungs to consume our food-fuel.
Q. Why do we need warmer clothing by night than by day?
A. 1st—Because the night is generally colder than the day.
2ndly—As our respiration is slower, our animal combustion is slower also; in consequence of which, our bodies are more cold.
Q. Why do we perspire when very hot?
A. The pores of the body are like the safety valves of a steam-engine; when the heat of the body is too great, the combustible gas and grease flow out in perspiration, instead of burning in the blood.
Q. Why do persons feel lazy and averse to exercise, when they are half-starved or ill-fed?
A. Animal food contains great nourishment, and produces a desire for active occupations; but when the body is not supplied with strong food, this desire for muscular action ceases, and the person grows slothful.
Q. Why have persons, who follow hard out-of-doors occupations, more appetite than those who are engaged in sedentary pursuits?
A. Hard bodily labour in the open air causes much oxygen to be conveyed into the lungs by inspiration; the combustion of the food is carried on quickly; animal heat increased; and need for nutritious food more quickly indicated by craving hunger.
Q. Why have persons who follow sedentary pursuits less appetite than ploughmen and masons?
A. 1st—The air they inhale is not so pure, because its oxygen is partly exhausted: and
2ndly—Their respiration is neither so quick nor strong, and therefore the combustion of their food is carried on more slowly.
Q. Why do we like strong meat and greasy food when the weather is very cold?
A. Strong meat and grease contain large portions of hydrogen, which (when burned in the blood) produce a larger amount of heat than any other kind of food.
Q. Why do persons eat more food in cold weather, than in hot?
A. In cold weather the body requires more fuel to keep up the same amount of[Pg 91] animal heat; and as we put more coals on a fire on a cold day to keep our room warm, so we eat more food on a cold day to keep our body warm.
Q. Why does cold produce hunger?
A. 1st—The air contains more oxygen in cold weather; and as fires burn fiercer, so animal combustion is more rapid: and
2ndly—We are more active in cold weather; and increased respiration acts like a pair of bellows on the capillary combustion.
Q. Why does rapid digestion produce a craving appetite?
A. This is a wise providence to keep our bodies in health; in order that the body itself may not be consumed, it gives notice (by hunger) that the capillary fires need replenishing.
Q. Why do we feel a desire for activity in cold weather?
A. 1st—Because activity increases the warmth of the body, by fanning the combustion of the blood: and
2ndly—The strong food we eat creates a desire for muscular exertion.
Q. Why are the Esquimeaux so passionately fond of train oil and whale blubber?
A. Oil and blubber contain a very large amount of hydrogen, which is exceedingly combustible; and as these people live in climates of intense cold, the heat of their bodies is increased by the greasy nature of their food.
Q. Why do we feel a dislike to strong meat and greasy foods in very hot weather?
A. Strong meat and grease contain so much hydrogen, that they would make us intensely hot; and therefore we refuse them in hot weather.
Q. Why do we like fruits and vegetables so very much in hot weather?
A. Fruits and vegetables contain less carbon than meat, and therefore produce less blood: instead of blood, they combine into water as they are digested, and keep the body cool.
Q. Why do people say that fruits and vegetables cool the blood?
A. 1st—Because they deprive the blood of carbon, which is the chief cause of animal heat: and
2ndly—These gases coalesce into water, which greatly tempers the animal heat.
Q. Why do we feel lazy and averse to activity in very hot weather?
A. 1st—Because muscular activity would increase the heat of the body, by quickening the respiration: and
2ndly—The food we eat in hot weather, not being greasy, naturally abates our desire for bodily activity.
Q. Why do the inhabitants of tropical countries live chiefly upon rice and fruit?
A. Rice and fruit by digestion are mainly converted into water, and (by cooling the blood) prevent the tropical heat from feeling so oppressive.
Q. Why are poor people generally averse to cleanliness?
A. 1st—Cleanliness increases hunger; and as poor people are generally ill-fed, they are averse to cleanliness.
2ndly—Dirt is warm, (thus pigs who love warmth, are fond of dirt); and as poor people are generally ill-clad, they like the warmth of dirt.
Q. Why are poor people generally averse to ventilation?
A. 1st—Because ventilation increases the oxygen of the air,—the combustion of food,—and the cravings of appetite: and
2ndly—Ventilation cools the air of our rooms: poor people, therefore, (who are generally ill-clad) love the warmth of an ill-ventilated apartment.
Q. Why does flannel, &c. make us warm?
A. Flannel and warm clothing do not make us warm, but merely prevent the body from becoming cold.
Q. How does flannel, &c. prevent the body from becoming cold?
A. Flannel (being a bad conductor) will neither carry off the heat of the body into the cold air, nor suffer the cold of the air to come into contact with our warm bodies; and thus it is that flannel clothing keeps us warm.
Q. Why are frogs and fishes cold-blooded animals?
A. Because they consume so little air; and without a plentiful supply of air, combustion is so slow, that very little animal heat is evolved.
Q. Why is a dead body cold?
A. Air is no longer conveyed to the lungs after respiration has ceased; and, therefore, animal heat is no longer evolved by combustion.
Q. How is heat produced by mechanical action?
A. 1.—By Percussion. 2.—By Friction. 3.—By Condensation.
Q. What is meant by percussion?
A. The act of striking; as when a blacksmith strikes a piece of iron on his anvil with his hammer.
Q. Why does beating iron make it red-hot?
A. Beating the iron condenses the particles of the metal; and squeezes out its latent heat, as water from a sponge.
Q. Does cold iron contain heat?
A. Yes; every thing contains heat; but when a thing feels cold, its heat is latent.
Q. What is meant by latent heat?
A. Heat not perceptible to our feeling. When anything contains heat without feeling the hotter for it, that heat is called “latent.” (See p. 31.)
Q. Does cold iron contain latent heat?
A. Yes; and when a blacksmith compresses the particles of the iron by his hammer, he squeezes out this latent heat, and makes the iron red-hot.
Q. How did blacksmiths use to light their matches before the general use of lucifers?
A. They used to place a soft iron nail upon their anvil; strike it two or three times with a hammer; and the point became sufficiently hot to light a brimstone match.
Q. How can a nail (beaten by a hammer) ignite a brimstone match?
A. As the particles of the nail are compressed by the hammer, it cannot contain so much heat as it did before; so some of it flies out (as water flows from a sponge when it is squeezed).
Q. Why does striking a flint against a piece of steel produce a spark?
A. The blow condenses those parts of the flint and steel which strike together, and squeezes out their latent heat.
Q. How does this development of heat produce a spark?
A. A very small fragment (either of the steel or flint) is knocked off red-hot, and sets fire to the tinder on which it falls.
Q. Why is it needful to keep blowing the tinder with the breath?
A. Because blowing the tinder, drives the oxygen of the air towards it.
Q. Where does the oxygen of the air come from, which is blown to the lighted tinder?
A. The air itself is composed of two gases (nitrogen and oxygen) mixed together.
(Every 5 lbs. of common air contain 4 lbs. of nitrogen, and 1 lb. of oxygen.)
Q. What is the good of blowing oxygen gas to lighted tinder?
A. Oxygen gas supports combustion; and lighted tinder is quickened by the breath, in the same way as a dull fire is revived by a pair of bellows.
Q. Why do horses sometimes strike fire with their feet?
A. When iron horse-shoes strike against the flint-stones of the road, very small fragments (either of the shoe or stones) are knocked off red-hot, and look like sparks.
Q. What makes these fragments red-hot?
A. The percussion condenses the part struck, and squeezes out its latent heat.
Q. What is meant by friction?
A. The act of rubbing two things together; as the Indians rub two pieces of wood together to produce fire.
Q. How do the Indians produce fire, by merely rubbing two pieces of dry wood together?
A. They take a piece of dry wood (sharpened to a point), which they rub quickly up and down a flat piece, till a groove is made; and the saw-dust (collected in this groove) soon catches fire.
Q. Why does the saw-dust of the wood catch fire by rubbing?
A. The latent heat of the wood is developed by friction; because the particles of the wood are squeezed closer together, and the heat pours out, as water from a sponge.
(The best woods for this purpose are box-wood against mulberry, or laurel against poplar or ivy.)
Q. Do not carriage wheels sometimes catch fire?
A. Yes; if the wheels be dry,—or fit too tightly,—or revolve very rapidly,—they often catch fire.
Q. Why do wheels catch fire in such cases?
A. The friction of the wheels against the axle-tree is so great, that their latent heat is disturbed, and produces ignition.
Q. What is the use of greasing cart wheels?
A. The grease lessens the friction; and (by diminishing the friction) the latent heat is less disturbed.
Q. Why is the top of a mountain colder than the valley beneath, although it be two or three miles nearer to the sun?
A. 1st—Because the air on a mountain is less compressed, than the air in a valley.
2ndly—It is more rarefied: and
3rdly—It is less heated by reflection.
Q. Why is air colder on a mountain “because it is less compressed?”
A. As the air in a valley is more compressed (by the mass of air above) than that on the top of a mountain, therefore more heat runs out; just as more water runs from a sponge, the closer it is squeezed together.
Q. Why is a mountain-top colder than a valley, “because the air there is more rarefied?”
A. As the air is more rarefied, its heat is diffused over a larger space and is less intense; just as a candle would show less light in a large room, than in a small one.
Q. Why is a mountain-top colder than a valley, “because the air there is less heated by reflection?”
A. Air is not heated by the sun, but by reflection from the surface of the earth; and as there is no earth round a mountain-top to reflect heat, therefore the air there is intensely cold.
Q. Why does rubbing our hands and faces make them feel warm?
A. Chiefly because the friction excites the latent heat of our hands and faces, and makes it sensible to our feeling.
Q. When a man has been almost drowned, why is suspended animation restored by rubbing?
A. The vital heat of the body (which had become latent by the action of the water) is again developed by friction: and, as soon as this animal heat can be excited, the vital powers of the body are restored.
Q. Why do two pieces of ice (rubbed together) melt?
A. Ice contains 140 degrees of latent heat, and (when two pieces are rubbed[Pg 102] together) their particles are compressed, and this latent heat rolls out and melts the ice.
Q. Are not forests sometimes set on fire by friction?
A. Yes; when two branches or trunks of trees (blown about by the wind) rub violently against each other, their latent heat is developed, and sets fire to the forest.
Q. What is meant by compression?
A. The act of bringing parts nearer together; as a sponge is compressed by being squeezed in the hand.
Q. Cannot heat be evolved from common air merely by compression?
Q. Why will the tinder catch fire?
A. Because the air is compressed; and its latent heat being squeezed out, sets fire to the tinder at the bottom of the tube.
Q. What are the principal effects of heat?
A. 1.—Expansion. 2.—Liquefaction. 3.—Vaporization. 4.—Evaporation; and 5.—Ignition.
Q. Does heat expand the air?
A. Yes; if a bladder (partially filled with air) be tied up at the neck, and laid before the fire, the air will swell till the bladder bursts.
Q. Why will the air swell, if the bladder be laid before the fire?
A. Because the heat of the fire gets between the particles of air, and drives them further apart from each other; which causes the bladder to expand.
Q. Why do unslit chestnuts crack with a loud noise, when roasted?
A. Chestnuts contain a great deal of air, which is expanded by the heat of the fire; and, as the thick rind prevents the air from escaping, it violently bursts through, slitting the rind, and making a great noise.
Q. What occasions the loud crack or report which we hear?
A. 1st—The sudden bursting of the rind makes a report, in the same way as a piece of wood or glass would do, if snapped in two: and
2ndly—The escape of hot air from the chestnut makes a report also, in the same way as gunpowder, when it escapes from a gun.
Q. Why does the sudden bursting of the rind, or snapping of a piece of wood, make a report?
A. As the attraction of the parts is suddenly overcome, a violent jerk is given to the air; this jerk produces rapid undulations in the air, which (striking upon the ear) give the brain the sensation of sound.
Q. Why does the escape of air from the chestnut, or the explosion of gunpowder, produce a report?
A. Because a quantity of air (suddenly let loose) pushes against the air around, in order to make room for itself; and as the air of the chestnut slaps against the air of the room, a report is made, (as when I slap a book or table).
Q. If a chestnut be slit, it will not crack; why is this?
A. Because the heated air of the chestnut can freely escape through the slit in the rind.
Q. Why does an apple spit and spurt about, when roasted?
A. An apple contains a vast quantity of air, which (being expanded by the heat of the fire) bursts through the peel, carrying the juice of the apple along with it.
Q. Does an apple contain more air, in proportion, than a chestnut?
A. Yes, much more. There is as much condensed air in a common apple, as would fill a space 48 times as big as the apple itself.
Q. Where is all this quantity of air stowed in the apple?
A. The inside of an apple is made up of little cells (like a honey-comb), each of which contains a portion of the air.
Q. When an apple is roasted, why is one part made soft, while all the rest remains hard?
A. When an apple is roasted, the air in the cells next to the fire is expanded and flies out; the cells are broken, and their juices mixed together; so the apple collapses (from loss of air and juice), and feels soft in those parts.
Q. What is meant by the “apple collapsing?”
A. The plumpness gives way, and the apple becomes flabby and shrivelled.
Q. Why do sparks of fire start (with a crackling noise) from pieces of wood laid upon a fire?
A. The air in the wood (expanded by the heat), forces its way through the pores of the log; and carries along with it the covering of the pore, which resisted its passage.
Q. What is meant by the “pores of the wood?”
A. Very small holes in the wood, through which the sap circulates.
Q. What are the sparks of fire, which burst from the wood?
A. Very small pieces of wood red hot, separated from the log by the force of the air, as it bursts from its confinement.
Q. Why does deal make more snapping than any other wood?
A. The pores of deal are very large, and contain much more air than wood of a closer grain.
Q. Why does dry wood make more snapping than green wood?
A. In green wood the pores are filled with sap, and therefore contain very little air; but in dry wood the sap is dried up, and the pores are filled with air instead.
Q. Why does dry wood burn more easily than green or wet wood?
A. Because the pores of dry wood are filled with air, which supports combustion; but the pores of green or wet wood are filled with vapour, which extinguishes flame.
Q. Why does vapour extinguish flame?
1st—Because the coat of water (which wraps the fuel round) prevents the[Pg 108] oxygen of the air from getting to the fuel, to form into carbonic acid gas: and
2ndly—Heat is perpetually carried off, by the formation of the sap or water into steam.
(Carbonic acid gas is a compound of carbon and oxygen. The solid part of the fuel is carbon, and one of the gases of the air is oxygen.)
Q. What has carbonic acid gas to do with combustion?
A. Combustion is produced by the chemical action which takes place, while the carbon of fuel unites with the oxygen of air, and forms “carbonic acid gas.” (See p. 36.)
Q. Why do stones snap and fly about, when heated in the fire?
A. The air in the stones (expanded by the heat of the fire), meets with great resistance from the close texture of the stone; and, therefore, bursts forth with great violence, tearing the stone to atoms, and forcing the fragments into the room.
Q. Must not air be very strong, to shatter into atoms a hard stone?
A. Yes. All the dreadful effects of gunpowder are merely the results of the sudden expansion of air.
Q. When bottled ale and porter is set before a fire, why is the cork forced out sometimes?
A. If the bottle be not quite full, there will be air between the liquor and the cork; this air (expanded by the heat of the fire) forces out the cork.
Q. Why does ale or porter froth more, after it has been set before the fire?
A. The froth of ale or porter depends upon the pressure to which it is subjected; and as the air (between the liquor and the cork) is expanded by the heat, it presses against the liquor, and increases the quantity of froth.
Q. Why is the froth of ale and porter increased by pressure?
A. Because the liquor absorbs carbonic acid so long as it is under pressure; and the moment that the pressure is removed, the carbonic acid escapes in foam or froth.
Q. When a boy makes a balloon, and sets fire to the cotton or sponge (which has been steeped in spirits of wine), why is the balloon inflated, or blown out?
A. The air inside the balloon is expanded by the flame, till the whole balloon is blown out without a crumple.
Q. Why does the balloon rise, after it has been inflated by the expanded air?
A. The same quantity of air is expanded to three or four times its original volume; and is made so much lighter than common air, that even when all the paper, wire, and cotton are added, it is still lighter bulk for bulk.
Q. What is meant by being lighter “bulk for bulk?”
A. If the balloon be 3 square feet in size, it is lighter (when inflated) than 3 square feet of common air, and therefore floats through it; as a cork (at the bottom of a tub of water) would rise to the surface.
Q. Why does smoke rush up a chimney?
A. The heat of the fire expands the air in the chimney; and (being thus made lighter than the air around), it rises up the chimney, and carries the smoke in its current.
Q. Why has a long chimney a greater draught than a short one?
A. Because air rises faster and faster the higher it ascends in a chimney flue;[Pg 111] the same as a stone falls faster and faster the nearer it approaches to the ground.
Q. Why will a long chimney smoke, unless the fire be pretty fierce?
A. If the fire be not pretty fierce, its heat will not be sufficient to rarefy all the air in the chimney; and then the chimney will smoke.
Q. Why will the chimney smoke, if the fire be not big enough to heat all the air in the chimney flue?
A. Because the cold air (condensed in the upper part of the flue), will sink from its own weight, and sweep the ascending smoke back with it into the room.
Q. What is the use of a cowl upon a chimney-pot?
A. The cowl acts as a screen against the wind, to prevent it from blowing into the chimney.
Q. What harm would the wind do, if it were to blow into a chimney?
A. 1st—It would prevent the smoke from getting out: and
2ndly—The cold air (introduced into[Pg 112] the chimney by the wind) would fall down the flue, and drive the smoke with it back into the room.
Q. Why does a smoke-jack turn round in a chimney?
A. The current of hot air up the chimney, striking against the oblique vanes of the smoke-jack, drives them round and round; in the same way as the sails of a wind-mill are driven round by the wind.
Q. Why are some things solid, others liquid, and others gaseous?
A. As heat enters any substance, it drives its particles further asunder; and a solid (like ice) becomes a liquid; and a liquid (like water) becomes a gas.
Q. Why does water simmer before it boils?
A. The particles of water near the bottom of the kettle (being formed into steam sooner than the rest) shoot upwards; but are condensed again (as they rise) by the colder water, and produce what is called “simmering.”
Q. What is meant by simmering?
A. A gentle tremor or undulation on[Pg 113] the surface of the water. When water simmers, the bubbles collapse beneath the surface, and the steam is condensed to water again: but when water boils, the bubbles rise to the surface, and steam is thrown off.
Q. Why does a kettle sing when the water simmers?
A. Because the air (entangled in the water) escapes by fits and starts through the spout of the kettle; which makes a noise like a wind instrument, when it is blown into.
Q. Why does not a kettle sing, when the water boils?
A. As all the water is boiling hot, the steam meets with no impediment, but freely escapes in a continuous stream.
Q. When does a kettle sing most?
A. When it is set on a hob to boil.
Q. Why does a kettle sing more when it is set on the side of a fire, than when it is set in the midst of the fire?
A. When the kettle is set on the hob to boil, the heat is applied very partially: one side is hotter than the other, and therefore the steam is more entangled.
Q. Why does a kettle sing, when the boiling water begins to cool again?
A. Because the upper surface cools first; and the steam (still rising from the lower parts of the kettle) is again entangled, and escapes fitfully.
Q. Why does boiling water swell?
A. Water (like air) expands by heat. The heat of the fire drives the particles of water further apart from each other; and (as they are not packed so closely together) they take up more room; or (in other words) the water swells.
Q. What is meant when it is said, “that heat drives the particles of water further apart from each other.”
A. Water is composed of little globules, like very small grains of sand; the heat drives these particles away from each other; and (as they then require more room) the water swells.
Q. Why does boiling water bubble?
A. Water contains air; and (as the water is heated) the air is driven out, and raises a bubble in that part of the water which resists its escape.
Q. Why does a kettle sometimes boil over?
A. Liquids expand very much by heat; if, therefore, a kettle be filled with cold water, some of it must run over as soon as it is expanded by heat.
Q. But I have seen a kettle boil over, although it has not been filled full of water; how do you account for that?
A. If a fire be very fierce, the air is expelled so rapidly, that the bubbles are very numerous; and (towering one above the other) reach the top of the kettle, and fall over.
Q. Why is a pot, which is full to overflowing (while the water is boiling hot), nothing like full, when it has been taken off the fire for a short time?
A. When the water was swelled by boiling heat, it filled the pot even to overflowing; but as soon as the water is condensed by cold, it contracts again, and occupies a much less space.
Q. Why does the water of a kettle run out of the spout when it boils?
A. Because the steam cannot escape so fast as it is formed, and (being confined in the kettle) presses on the water[Pg 116] with great power, and forces it out of the spout.
Q. How can the pressure of steam on the surface of the water, force the water through the kettle-spout?
A. In the same manner as the pressure of air on the mercury of a barometer, forces the quicksilver up the glass tube.
Q. What causes the rattling noise so often made by the lid of a saucepan or boiler?
A. The steam (seeking to escape) forces up the lid of the boiler, and the weight of the lid causes it to fall back again: this being done frequently, produces a rattling noise.
Q. If the steam could not lift up the lid of the boiler, how would it escape?
A. If the lid fitted so tightly, that the steam could not raise it up, the boiler would burst into fragments, and the consequences might be fatal.
Q. When steam pours out from the spout of a kettle, the stream begins apparently half an inch off the spout; why does it not begin close to the spout?
A. Steam is really invisible; and the half-inch (between the spout and the[Pg 117] “stream of mist”) is the real steam, before it has been condensed by air.
Q. Why is not all the stream invisible, as well as that half-inch?
A. As the steam comes in contact with the colder air, the invisible particles (being condensed), roll one into another, and look like a thick mist.
Q. What becomes of the steam? for it soon vanishes.
A. After it is condensed into mist, it is dissolved by the air, and dispersed abroad as invisible vapour.
Q. And what becomes of the invisible vapour?
A. Being lighter than air, it ascends to the upper regions, where (being again condensed) it contributes to form clouds.
Q. Why does a metal spoon, left in a saucepan, retard the process of boiling?
A. The metal spoon (being an excellent conductor) carries off the heat from the water; and (as heat is carried off by the spoon) the water takes a longer time to boil.
Q. Why will a pot (filled with water) never boil, when immersed in another vessel full of water also?
A. Because water can never be heated above the boiling point: all the heat absorbed by the water after it boils, is employed in converting the water into steam.
Q. How does the conversion of water into steam prevent the inner pot from boiling?
A. The moment the water in the larger pot is boiling hot (or 212°), steam is formed, and carries off some of its heat; therefore, 212 degs. of heat can never pass through it, to raise the inner vessel to the same heat.
Q. Why do sugar, salt, &c. retard the process of boiling?
A. Because they have a tendency to fix water by chemical attraction; and therefore retard its conversion into steam.
Q. If you want water to boil, without coming in contact with the saucepan, what plan must you adopt?
A. Immerse the pot (containing the water you want to boil) in a saucepan containing strong brine, or sugar.
Q. Why would the inner vessel boil, if the outer vessel contained strong brine?
A. Though water boils at 212 degs. of heat, yet brine will not boil till raised to 218 or 220 degs. Therefore, 212 degs. of heat may easily pass through brine to raise the vessel immersed in it to boiling heat, before any of it is carried off by steam.
Q. Why will brine impart to another vessel more than 212°, and water not so much?
A. Because both liquids will impart heat till they boil, and then they can impart heat no longer.
Q. Why can they impart no extra heat after they boil?
A. Because all extra heat is spent in making steam. Hence water will not boil a vessel of water immersed in it, because it cannot impart to it 212 degs. of heat: but brine will, because it can impart more than 212 degs. of heat, without being converted itself into steam.
| Ether boils at | 104 degs. |
| Alcohol boils at | 173-1/2 degs. |
| Water boils at | 212 degs. |
| Water with one-fifth salt at | 219 degs. |
| Syrup boils at | 221 degs. |
| Oil of turpentine at | 304 degs. |
| Sulphuric acid at | 472 degs. |
| Linseed oil at | 640 degs. |
| &c. &c. |
Any liquid which boils at a lower degree can be made to[Pg 120] boil if immersed in a liquid which boils at a higher degree. Thus a cup of ether can be made to boil in a saucepan of water. A cup of water in a saucepan of brine or syrup. But a cup of water will not boil if immersed in ether; nor a cup of syrup in water.
Q. Why are clouds higher on a fine day?
A. 1st—Because the air (expanded by heat) drives them higher up: and
2ndly—The clouds themselves are lighter, and therefore more buoyant.
Q. Why are the clouds lighter on a fine day?
A. Because their mists are either absorbed by the dry air, or vapourized by the hot sun.
Q. Why is a cup put topsy-turvy into a fruit-pie?
A. Its principal use is to hold the crust up, and prevent it from sinking, when the cooked fruit gives away under it.
Q. Does not the cup prevent the fruit of the pie from boiling over?
A. No, by no means; it would rather tend to make it boil over, than otherwise.
Q. Why would the cup tend rather to make the fruit boil over?
A. As soon as the pie is put into the[Pg 121] oven, the air in the cup will begin to expand, and drive every particle of juice from under it; the pie dish, therefore, will have a cup-full less room to hold its fruit, than if the cup were taken out.
Q. If the juice is driven out of the cup, why is the cup always full of juice, when the pie is cut up?
A. Immediately the pie is drawn, the air in the cup begins to condense again, and occupy a smaller space; in consequence of which, there is no longer enough air to fill the cup, and so juice rushes in to fill up the deficiency.
Q. Why does juice rush into the cup, because the cup is not full of air?
A. As the external air presses upon the surface of the juice, it rushes into the cup unobstructed; as mercury rises through the tube of a barometer through similar pressure.
Q. Does heat expand every thing else besides air and water?
A. Yes; every thing (that man is acquainted with) is expanded by heat.
Q. Why does a cooper make his hoops red-hot, when he puts them on a tub?
A. 1st—As iron expands by heat, the hoops will be larger when they are red-hot; and will, therefore, fit more easily on the tub: and
2ndly—As iron contracts by cold, the hoops will shrink as they cool down, and girt the tub with a tighter grasp.
Q. Why does a wheelwright make his hoops red-hot, which he fixes on the nave of a wheel?
A. 1st—That they may fit on more easily: and
2ndly—That they may girt the nave more tightly.
Q. Why will the wheelwright’s hoop fit the nave more easily, because they are made red-hot?
A. As iron expands by heat, the hoops will be larger when they are hot; and (being larger) will go on the nave more easily.
Q. Why will the hoops, which have been put on hot, girt the nave more firmly?
A. As iron contracts by cold, the hoops will shrink as they cool down; and, therefore, girt the nave with a tighter grasp.
Q. Why does a farrier put the horse-shoe on hot?
A. That it may stick the closer, when it has contracted by cold.
Q. Why does a stove make a cracking noise, when a fire is very hot?
A. The iron stove expands by heat, and (as it swells) the parts rub both against each other, and against the bricks around, driving them further off; and this produces a cracking noise.
Q. Why does a stove make a similar cracking noise, when a large fire is taken down?
A. The iron stove contracts again,[Pg 124] as soon as the fire is removed; and (as it shrinks into a smaller space) the parts rub against each other again, and the bricks are again disturbed; and this produces a cracking noise.
Q. Why does the plaster round a stove crack and fall away?
A. When the fire is lighted, the iron-work (which expands more than the brick-work and plaster) pushes away the bricks and plaster: but when the fire is put out, the metal shrinks again, and leaves the “setting” behind.
Q. Why does the plaster fall away?
A. As a chink is left (between the “setting” and the stove), the plaster will frequently fall away from its own weight.
Q. What other cause contributes to bring the plaster down?
A. As the heat of the fire varies, the size of the iron stove varies also; and this swelling and perpetually contracting, keeps up such a constant disturbance about the plaster, that it cracks and falls off, leaving the fire-place very unsightly.
Q. Why does the mercury of a thermometer rise in hot weather?
A. Heat expands the metal; and as the metal is increased in bulk, it occupies a larger space, (or, in other words, rises higher in the tube.)
Q. Why is a glass broken, when hot water is poured into it?
A. Because the inside of the glass is expanded by the hot water, and not the outside; so the glass snaps for want of flexibility.
Q. Why is not the outside of the glass expanded by the hot water, as well as the inside?
A. Glass is a non-conductor of heat; and, therefore, breaks before the heat of the inner surface is conducted to the outside.
Q. Why does a glass snap, because the inner surface is hotter than the outer?
A. Glass is expanded by heat; and as the inner surface expands, it stretches the outer surface till it snaps.
Q. Why is a china cup broken, if hot water be poured over it, or into it?
A. China is a non-conductor; and, as the inner surface expands by the heat, before the outer one, it forms an arch, and pulls the parts of the cup asunder.
Q. Why does the bottom come off, if a glass beaker be set on a warm hob?
A. Glass is a non-conductor; and, as the bottom of the glass (from the warmth of the hot stove) expands, before the sides are heated, the two parts separate the one from the other.
Q. What is meant by liquefaction?
A. The state of being melted; as ice is melted by the heat of the sun.
Q. Why is ice melted by the heat of the sun?
Q. Why are metals melted by the heat of fire?
A. The heat of the fire (entering the solid metal) forces its particles asunder, till their attraction of cohesion is sufficiently overcome, to convert the solid metal to a liquid.
Q. Why is water converted to steam by the heat of fire?
A. The heat of the fire (entering the water) divides its globules into very minute bubbles, which (being made lighter than air) fly off from the surface in the form of steam.
Q. Why does not wood melt, like metal?
A. Because the heat of the fire decomposes the wood into gas, smoke, and ashes; and the different parts separate from each other.
Q. What is meant by vaporization?
A. The conversion of liquid into vapour; as water is converted into vapour by the heat of the sun.
Q. What are clouds?
A. Moisture evaporated from the earth, and collected in the upper regions of the air.
Q. What is the difference between a fog and a cloud?
A. Clouds and fogs differ only in one respect. Clouds are elevated above our heads: but fogs come in contact with the surface of the earth.
Q. If clouds are water, why do they float on the air?
A. 1st—The vapour of clouds is composed of very minute bubbles (called ves’cicles), which float like soap bubbles: and
2ndly—Warm air (between the bubbles) keeps them apart, and makes the mass lighter; and the currents of air (which constantly ascend from the warm earth) buoy them up.
Q. Why does vapour sometimes form into clouds, and sometimes rest upon the earth as mist or fog?
A. When the surface of the earth is warmer than the air, the vapour of the earth (being condensed by the chill air) becomes mist or fog. But when the air is warmer than the earth, the vapour rises through the air, and becomes cloud.
Q. Are all clouds alike?
A. No. They vary greatly in density, height, and colour.
Q. What is the chief cause of fog and clouds?
A. The changes of the wind.
Q. How can the changes of the wind affect the clouds?
A. If a cold current of wind blows suddenly over any region, it condenses the invisible vapour of the air into cloud or rain: but if a warm current of wind, blows over any region, it disperses the clouds, by absorbing their vapour.
Q. What countries are the most cloudy?
A. Those where the winds are most variable, as Britain.
Q. What countries are the least cloudy?
A. Those where the winds are not variable, as Egypt.
Q. What distance are the clouds from the earth?
A. Some thin light clouds are elevated above the highest mountain-top; some heavy ones touch the steeples, trees, and[Pg 130] even the earth: but the average height is between one and two miles.
(Streaky curling clouds, like hair, are often five or six miles high.)
Q. What clouds are the lowest?
A. Those that are most highly electrified: lightning clouds are rarely more than about 700 yards above the ground; and very often actually touch the earth with one of their edges.
Q. What is the thickness of the clouds?
A. Some clouds are 20 square miles in surface, and above a mile in thickness; while others are only a few yards or inches.
Q. How can persons ascertain the thickness of a cloud?
A. As the tops of high mountains are generally above the clouds; therefore, travellers (who climb the mountains) may pass quite through the clouds, into a clear blue firmament, when they may see the clouds beneath their feet.
Q. Why are the clouds so variable in shape?
A. The shape of clouds depends upon two things:—Their state of electricity, and the wind.
Q. How can electricity affect the shape of clouds?
A. If one cloud be full of electricity, and another not, they will be attracted to each other, and either coalesce,—diminish in size,—or vanish altogether.
Q. Which clouds assume the most fantastic shapes?
A. Those that are the most highly electrified.
Q. What effect have winds on the shape of clouds?
A. They sometimes absorb them entirely: sometimes increase their volume and density; and sometimes change the position of their parts.
Q. How can winds absorb clouds altogether?
A. A warm dry wind will convert the substance of the clouds into invisible vapour, and carry it in its own current.
Q. How can winds increase the bulk and density of clouds?
A. A cold current of wind will condense the invisible vapour of the air, and add it to the clouds as it passes by.
Q. How can winds change the shape of clouds by altering the position of their parts?
A. Because clouds are so voluble and light, that every breath of wind changes the position of those ves’cicles or bubbles.
Q. What are the general colours of the clouds?
A. White and grey, when the sun is above the horizon: but red, orange, and yellow, at sun-rise and sun-set.
The blue sky cannot be considered as clouds at all.
Q. Why are the last clouds of evening generally of a red tinge?
A. Because red rays are the least refrangible of all; and, therefore, are the last to disappear.
Q. What is meant by being “less refrangible”?
A. Being less able to be bent. Blue and green rays being very easily bent (by the resistance of the air) are thrown off from the horizon; but red rays not being bent back in the same way, give a tinge to the evening clouds.
Q. Why are morning clouds generally of a red tinge?
A. Because red rays are the least refrangible of all, and not being bent back by the air (like blue and green), strike upon the horizon, and give a tinge to the morning clouds.
Q. Why is not the reflection of clouds always alike?
A. Because their size, density, and situation in regard to the sun, vary perpetually; so that sometimes one colour is reflected, and sometimes another.
Q. What regulates the motion of the clouds?
A. The motion of the clouds is generally directed by the winds; but sometimes electricity will influence their motion also.
Q. How do you know that clouds move by other influences besides wind?
A. Because we often see in calm weather small clouds meeting each other from opposite directions.
Q. How do you know that electricity affects the motion of the clouds?
A. Because clouds often meet from opposite directions; and (after they have[Pg 134] discharged their opposite electricities into each other) vanish altogether.
Q. Into how many classes are the different sorts of clouds generally divided?
A. Into three classes:—viz. Simple, Intermediate, and Compound.
Q. How are simple clouds sub-divided?
A. 1.—Cirrus. 2.—Cum’ulus; and 3.—Stra’tus.
Q. What are cirrus clouds?
A. Clouds like fibres, loose hair, or thin streaks, are called cirrus clouds.
Q. Why are these clouds called cirrus?
A. From the Latin word, cirrus (“a lock of hair, or curl”): they are the most elevated of all clouds.
Q. What do cirrus clouds portend?
A. When the streamers point upwards, the clouds are falling, and rain is at hand: but when the streamers point downwards, expect easterly wind or drought.
Q. What are cum’ulus clouds?
A. Cum’ulus clouds are lumps like great sugar-loaves,—volumes of smoke,—or mountain towering over mountain.
Q. Why are these monster masses called cum’ulus clouds?
A. From the Latin word, cum’ulus (a mass or pile).
Q. What do cum’ulus clouds foreshow?
A. When these piles of cloud are fleecy, and sail against the wind, they indicate rain; but when their outline is very hard, and they come up with the wind, they foretell fine weather.
Cumulus clouds should be smaller towards evening than they are at noon. If they increase in size at sun-set, a thunder-storm may be expected in the night.
Q. What are stra’tus clouds?
A. Creeping mists, especially prevalent in a summer’s evening: these clouds rise at sun-set in low damp places, and are always nearer the earth, than any other sort of cloud.
Q. Why are these mists called stra’tus clouds?
A. From the Latin word, stra’tus (“laid low,” or “that which lies low”).
Q. How are the intermediate clouds sub-divided?
A. Into two sorts. 1.—The Cirro-Cum’ulus; and 2.—The Cirro-Stra’tus.
Q. What are cirro-cum’ulus clouds?
A. When cirrus clouds spring from a massy centre; or when heavy masses of cloud terminate at their edges in long streaks, or what are called “mares’ tails.”
A system of small round clouds may be called cirro-cum’ulus.
Q. What do cirro-cum’ulus clouds generally forebode?
A. Continued drought, or hot dry weather.
Q. What are cirro-stra’tus clouds?
A. They compose what is generally called a “mackarel sky.” This class of clouds always indicate rain and wind; hence the proverb—
Q. How are compound clouds sub-divided?
A. Compound clouds are also sub-divided into two sorts. 1.—The Cum’ulo-stra’tus; and 2.—The Nimbus.
Q. What is meant by cum’ulo-stra’tus clouds?
A. Those clouds which assume all[Pg 137] sorts of gigantic fancy forms; such as vast towers and rocks,—huge whales and dragons,—scenes of battle,—and cloudy giants. This class of clouds is the most romantic and strange of all.
Q. What do the cumulo-stratus clouds foretell?
A. A change of weather; either from fine to rain, or from rain to fine weather.
Q. What are nimbus clouds?
A. Nimbus is the Latin word for “clouds which bring a storm;” and all clouds from which rain falls are so named.
Q. What appearance takes place in the clouds at the approach of rain?
A. The cum’ulus cloud becomes stationary, and cirrus streaks settle upon it, forming cumulo-stratus clouds; which are black at first, but afterwards of a grey colour.
Q. Why do clouds gather round mountain-tops?
A. Because (as they float along) they dash against the mountains; and (being arrested in their motion) collect round the top.
Q. What is the use of clouds?
A. 1st—They act as screens to arrest the radiation of heat from the earth:
2ndly—They temper the heat of the sun’s rays: and
3rdly—They are the great store-houses of rain.
Q. Why is wind said to blow up the clouds?
A. When a dry wind travels over sea, and accumulates more vapour than the air can sustain, it relinquishes a part (as it flies along) in the form of clouds.
Q. Why does wind sometimes drive away the clouds?
A. When wind travels over dry climes or thirsty deserts, it becomes so dry itself, that it absorbs vapour from the clouds, and disperses them.
Q. What is the cause of a red sun-set?
A. Because the vapour of the air is not actually condensed into clouds, but only on the point of being condensed; in which state it bends the red rays of the sun towards the horizon, where they are reflected at sun-set.
Q. Why is a red sun-set an indication of a fine day to-morrow?
A. Because (notwithstanding the cold of sun-set) the vapours of the earth are not condensed into clouds. Our Lord referred to this prognostic in the following words: “When it is evening ye say, it will be fair weather, for the sky is red.” (Matt. xvi. 2.)
Q. What is the cause of a coppery yellow sun-set?
A. Because the vapour of the air is actually condensed into clouds; in which case it “refracts” (or bends) the yellow rays of the sun towards the horizon, where they are reflected at sun-set.
Q. Why is a yellow sunset an indication of wet?
A. Because the vapours of the air are already condensed into clouds; rain, therefore, may be shortly expected.
Q. What is the cause of a red sun-rise?
A. Vapour in the upper region of the air just on the point of being condensed.
Q. Why is a red and lowering sky at sunrise an indication of a wet day?
A. Because the higher regions of the air are laden with vapour, on the very point of condensation, which the rising sun cannot disperse. Hence our Lord’s observation, “In the morning (ye say) it will be foul weather to-day, for the sky is red and lowering.” (Matt. xvi. 3.)
Q. Why is a grey morning an indication of a fine day?
A. Because that air alone contiguous to the earth is damp and full of vapour. There are no vapours in the higher regions of the air to reflect red rays; and hence the morning-light looks grey.
Q. What difference (in the state of the air) is required, to make a grey and red sunrise?
A. In a grey sunrise, only that portion of air contiguous to the earth is filled with vapour; all the rest is clear and dry. But in a red sunrise the air in the upper regions is so full of vapour that the rising sun cannot disperse it.
Q. Why is a grey sunset an indication of wet?
A. If the air on the surface of the earth be very damp at sunset, it is a[Pg 141] proof that the air is saturated with vapour, and wet may be expected: hence the proverb—
Q. The proverb says, “A rainbow in the morning is the shepherd’s warning:” why is it so?
A. A rainbow can only be formed when the clouds (containing or dropping rain) are opposite the sun: a morning rainbow, therefore, is always in the west, and indicates that bad weather is on the road to us.
Q. Why does a rainbow in the west indicate that bad weather is on the road to us?
A. Because our heavy rains are usually brought by west or south-west winds; and, therefore, clouds which reflect the colour of the rainbow in the west, are coming up with the wind, bringing rain with them.
Q. The proverb says, “A rainbow at night, is the shepherd’s delight;” why is it so?
A. As a rainbow is always opposite to[Pg 142] the sun, therefore a rainbow at night is in the east, and indicates that bad weather is leaving us.
Q. Why does a rainbow in the east indicate that bad weather is leaving us?
A. As west and south-west winds bring rain, if the clouds have been driven from the west to the east, they have passed over us, and are going away from us.
Q. What is meant by an aurora borea’lis, or northern light?
A. A luminous white cloud in the north of the sky at night-time. Sometimes streaks of blue, purple, and red,—and sometimes flashes of light, are seen also.
In our island this phenomenon generally rises from a dark cloud (running from the north to the east and west) elevated about 10 or 20 degrees above the horizon: above this dark bed of clouds the luminous white light appears.
Q. What is the cause of the aurora borealis, or northern light?
A. Electricity in the clouds.
Q. Why is the aurora borealis generally a white light?
A. Because the electric fluid passes[Pg 143] through air extremely rarefied: and whenever electric fluid passes through air much rarefied, it always produces a white light.
Q. Why are there sometimes different colours in the aurora borealis, such as yellow, red, and purple?
A. Because the electric fluid passes through air of different densities. The most rarefied air produces a white light; the most dry air, red; and the most damp produces yellow streaks.
Q. Does the aurora borealis forbode fine weather or wet?
A. When its corruscations are very bright, it is generally followed by stormy moist unsettled weather.
Q. Why does a haze round the sun indicate rain?
A. Because the haze is caused by very fine rain falling in the upper regions of the air; when this is the case, a rain of 5 or 6 hours continuance, may be expected.
Q. Why is a halo round the moon a sure indication of rain?
A. Because the halo is caused by fine rain falling in the upper regions of the air. The larger the halo the nearer the rain-clouds, and the sooner may rain be expected.
Q. Why does a black mist bring wet weather?
A. The mist is black, because it is overshadowed by dense clouds or masses of vapour; and, therefore, it forebodes wet.
Q. Why does a white mist indicate fine weather?
A. The mist is white, because no clouds blacken it with their shadow; and (as the sky is cloudless) fine weather may be expected.
Q. Why do we feel almost suffocated in a hot cloudy night?
A. Because the heat of the earth (being unable to escape into the upper region of the air, in consequence of the clouds) floats, like a sea of heat, on the surface of the earth.
Q. Why do we feel more sprightly in a clear bright night?
A. Because the heat of the earth can readily escape into the upper regions of the air, and is not confined and pent-in by thick clouds.
Q. Why do we feel depressed in spirits on a wet murky day?
A. 1st—Because when the air is laden with vapour, it has less oxygen.
2ndly—The air being lighter than usual, does not balance the air in our body: and
3rdly—Moist air has a tendency to relax the nervous system.
Q. What is meant by the “air balancing the air” in our body?
A. The human body is filled with air of the same density as that around: if, therefore, we ascend into purer air, or descend into denser air, the balance is destroyed, and we feel oppressed and suffocated.
Q. Why do we feel oppressed and suffocated if the air around is not of the same density as that in our body?
A. If the air around be more dense, it will squeeze our body in by its weight:[Pg 146] if it be less dense, the air in our body will blow us out.
Q. Why do persons who ascend in balloons feel pain in their eyes, ears and chest?
A. Because the air in the upper regions is more rare than the air in their bodies; and (till the equilibrium is restored) great pain is felt in all the more sensitive parts of the body.
Q. Why do persons who descend in diving-bells feel pain in their eyes, ears and chest?
A. Because the air in the sea is more dense than the air in their bodies; and (till the equilibrium is restored) great pain is felt in all the more sensitive parts of the body.
Q. Why does the sea heave and sigh just previous to a storm?
A. The density of the air (just previous to a storm) is very suddenly diminished, but the air in the sea is not so quickly affected; therefore the sea heaves and sighs in its effort to restore an equilibrium.
Q. Why is the air so universally still just previous to a tempest?
A. Because the air is suddenly and very greatly rarefied; and (as the density of the air is diminished) its power to transmit sound is diminished also.
Q. How do you know that rarefied air cannot transmit sound so well as dense air?
A. Because the sound of a bell (in the receiver of an air-pump) cannot be heard at all, after the air has been partially exhausted; and a pistol fired on a high mountain would not sound louder than a common cracker.
Q. Why do we feel braced and light-hearted on a fine spring or frosty morning?
A. 1st—Because there is more oxygen in the air on a fine frosty morning, than there is on a wet day: and
2ndly—A brisk and frosty air has a tendency to brace the nervous system.
Q. Why do dogs and cats (confined to a room) feel lazy and drowsy at the approach of rain?
A. 1st—Because the air does not contain its full proportion of oxygen: and
2ndly—Because the damp relaxes their nervous system, and makes them drowsy.
Q. Why do horses neigh, cattle low, sheep bleat, and asses bray, at the approach of rain?
A. 1st—As the air does not contain its full proportion of oxygen, they feel a difficulty in breathing: and
2ndly—As damp relaxes their nerves, they feel languid and uneasy.
Q. Why do candles and fires burn with a bluer flame in wet weather?
A. As the air contains less oxygen in wet weather, the heat of fire is less intense: and the flame is blue, because the fuel is not thoroughly consumed.
Q. Why do hills, &c. appear larger in wet weather?
A. Because (when the air is laden with vapour) the rays of light are more dispersed, and produce a larger reflection; objects, therefore, seen at a distance, appear larger.
Q. Why do trees, &c. in wet weather appear further off than they really are?
A. Because the fog or mist diminishes the light reflected from the object; and as the object becomes more dim, it seems to be further off.
Q. Why does the sun seem larger when he sets and rises, than he does at noon?
A. Because the rays pass through more of the vapoury atmosphere which surrounds the earth; and this vapoury atmosphere acts like a magnifying glass.
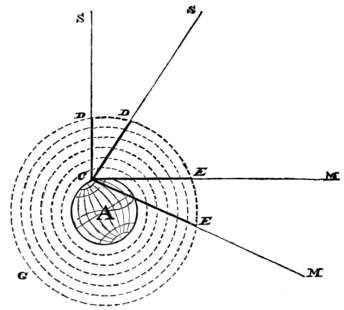
It is very manifest that the lines D C are shorter than the lines E C: if, therefore, A be the earth, and D G E the boundary of the atmosphere round the earth, then the rays M E C (at the horizon) will pass through more of the atmosphere, than the rays S D C, which are more elevated.
Q. Why does the moon appear larger at her rising and setting, than when above our heads?
A. Because the rays pass through more of the vapoury atmosphere which surrounds[Pg 150] the earth; and this vapoury atmosphere magnifies the moon, just like a magnifying glass.
Q. Why do cats rub their ears when it is likely to rain?
A. Either because the air is full of vapour, and its humidity (piercing between the hair of the cat) produces an itching sensation; or more probably, because the air is overcharged with electricity.
Q. How can the electricity of air produce a sensation of itching?
A. If the air is overcharged with electricity, the hair of the cat is overcharged also; and this makes her feel as if she were covered with cobwebs.
Q. Why does the cat keep rubbing herself?
A. Her hair will not lie smooth, but has a perpetual tendency to become turgid and ruffled; so the cat keeps rubbing her coat and ears, to smooth the hair down, and brush away the feeling of cobwebs.
Q. Why do our heads and skin itch before rain?
A. Probably because the air is overcharged with electricity; and, therefore, a sensation (like that of cobwebs) irritates the skin, and produces an itching.
Q. Why do we hear distant CLOCKS more distinctly when rain is near at hand?
A. Because the air is filled with vapour, and water is a better conductor of sound than dry air.
Q. Why do we hear church-bells further, just previous to rain?
A. Because the air is filled with vapour, and vapour is a better conductor of sound than dry air.
Q. Why do doors swell, when rain is at hand?
A. Because the air is filled with vapour, which (penetrating into the pores of the wood) forces the parts further apart, and swells the door.
Q. Why do doors shrink in dry weather?
A. Because the moisture is absorbed from the wood; and, as the particles are brought closer together, the size of the door is lessened, (or in other words, the wood shrinks).
Q. Why is the air filled with offensive smells previous to a coming rain?
A. Because the volatile parts, (which rise from dunghills, sewers, &c.), being laden with vapour, are unable to rise so readily, as when they are rarefied by a bright sun.
Q. Why do flowers smell sweeter and stronger just previous to rain?
A. Because the volatile parts (which constitute the perfume of flowers) are laden with vapour; and (being unable to rise) are confined to the lower regions of the air.
Q. Why do horses and other animals stretch out their necks, and snuff up the air, just previous to a fall of rain?
A. Because they smell the odour of plants and hay, and delight to snuff in their fragrance.
Q. Why does smoke fall when rain is at hand?
A. The air being less dense in wet weather, cannot buoy up smoke so readily, as when more dry and heavy.
Q. Why do swallows fly low when rain is at hand?
A. Because the insects (of which they are in pursuit) have fled from the cold upper regions of the air, to the warm air near the earth: and as their food is low, the swallows fly low.
Q. Why do these insects seek the lower regions of the air in wet weather, more than in fine weather?
A. Because they are forced downward, by some current of cold air which drives them down.
Q. Why does a downward current of cold air bring rain?
A. Because it condenses the warm vapour; which then descends in rain.
Q. The proverb says, “a single magpie in spring, foul weather will bring:” why is this the case?
A. In cold stormy weather, one magpie alone will leave its warm snug nest in search of food, while the other stays with the eggs or young ones; but in fine mild weather (when their brood will not be injured by cold) both the magpies will fly out together.
Q. Why is it unlucky for anglers to see a single magpie in spring?
A. Because when magpies fly abroad singly, the weather is cold and stormy; but when both birds fly out together, the weather is warm and mild, which is favourable for fishing.
Q. Why do sea gulls fly about the sea in fine weather?
A. Because they live upon fish, which are found near the surface of the sea in fine weather.
Q. Why may we expect stormy rains, when sea gulls assemble on the land?
A. Because the fish (on which they live) leave the surface of the sea in stormy weather, and go down too deep for the gulls to get at them; they are obliged, therefore, to feed on the worms and larvæ which are driven out of the ground at such times.
Q. Why does the petrel always fly to the sea during a storm?
A. Because the petrel lives upon sea insects, which are always to be found in abundance about the spray of swelling waves.
(The Petrel is a bird of the duck-kind, which lives in the[Pg 155] open sea. They run on the top of the sea, and are called Petrels, or rather Peter-els, from “St. Peter,” in allusion to his walking on the sea, to go to Jesus.)
Q. Why do candles and lamps spirt when rain is at hand?
A. Because the air is filled with vapour, and the humidity penetrates the wick; where (being formed into steam) it expands suddenly, and produces a little explosion.
Q. Why does a drop of water sometimes roll along a piece of hot iron without leaving the least trace?
A. If the iron be very hot indeed, the bottom of the drop is turned into vapour, before the drop can evaporate; and the vapour thus formed buoys the drop up, without allowing it to touch the iron at all.
Q. Why does it roll?
A. The current of air (which is always passing over the heated surface) drives it along.
Q. Why does a laundress put a little saliva on an ironing-box to know if it be hot enough?
A. If the saliva sticks to the box and[Pg 156] is evaporated, the box is not hot enough; but if the saliva runs along the box, it is.
Q. Why is the box hotter if the saliva runs along the box, than if it adheres to it till it is evaporated?
A. If the saliva runs along the box, the iron is hot enough to convert the bottom of the drop of spittle into vapour; but if the saliva will not roll, the box is not hot enough to convert the bottom of the drop of spittle into vapour.
Q. What is meant by evaporation?
A. The dissipation of liquid by its being converted into vapour.
Q. What effects are produced by evaporation?
A. The liquid vaporized absorbs heat from the body whence it issues; and the body deprived of the liquid by evaporation, loses heat thereby.
Q. If you wet your finger in your mouth, and hold it up in the air, why does it feel cold?
A. The saliva quickly evaporates; and (as it evaporates) absorbs heat from the finger, which makes it feel cold.
Q. If you bathe your temples with ether, why does it allay inflammation and feverish heat?
A. Ether very rapidly evaporates; and (as it evaporates) absorbs heat from the burning head, producing a sensation of cold.
Q. Why is ether better for this purpose than water?
A. Because it requires less heat to convert it into vapour; and therefore it evaporates much more quickly.
(Ether is converted into steam with 104 degs. of heat, but water requires 212 degs. of heat to convert it into steam.)
Q. Why does ether very greatly relieve a scald or burn?
A. Because it evaporates very rapidly; and (while it is converted into vapour) carries off the heat of the burn.
Q. Why do we feel so cold when we have wet feet or clothes?
A. As the wet of our shoes or clothes[Pg 158] evaporates, it keeps absorbing heat from the body, which makes it feel cold.
Q. Why do wet feet or clothes give us “cold?”
A. Because the evaporation absorbs heat from the body so abundantly, that it is lowered below its natural standard; and therefore health is injured.
Q. Why is it dangerous to sleep in a damp bed?
A. Because the heat of the body is continually absorbed in converting the damp of the sheets into vapour; and as heat is abstracted from the body, its temperature is reduced below the healthy standard.
Q. Why do we not feel the same sensation of cold, if we throw a macintosh over our wet clothes?
A. The macintosh prevents evaporation, because the steam cannot escape through the air-tight fabric; and (as the wet cannot evaporate from the clothes) no heat is absorbed from our bodies.
Q. Why do not sailors get cold, who are so often wet all day with sea-water?
A. The salt of the sea retards evaporation; and (as the heat of the body is drawn off very gradually) the sensation of cold is prevented.
Q. Why does sprinkling a hot room with water cool it?
A. The heat of the room causes a rapid evaporation of the sprinkled water; and as the water evaporates, it absorbs heat from the room, and cools it.
Q. Why does watering the streets and roads cool them?
A. The hot streets and roads part with their heat to promote the evaporation of the water sprinkled on them.
Q. Why does a shower of rain seem to cool the air in summer-time?
A. The earth (being wet with the rain) parts with its heat to promote evaporation; and as the earth is cooled, it cools the air also.
Q. Why is linen dried by being exposed to the wind?
A. The air (blowing over the linen) promotes evaporation, by removing the vapour from the surface of the wet linen, as soon as it is formed.
Q. Why is linen dried sooner in the open air, than in a confined room?
A. Because the particles of vapour are more rapidly removed from the surface of the linen by evaporation.
Q. Why are wet summers generally succeeded by cold winters?
A. Because the great evaporation (carried on through the wet summer) reduces the temperature of the earth lower than usual, and produces cold.
Q. Why is england warmer than it used to be, when agues were so common?
A. Because it is better drained and better cultivated.
Q. Why does draining land promote warmth?
A. Because it diminishes evaporation; in consequence of which less heat is abstracted from the earth.
Q. Why does cultivation increase the warmth of a country?
A. 1st—Because hedges and belts of trees are multiplied;
2ndly—Because the land is better drained;
3rdly—Because the land is dug and ploughed; and
4thly—Because the vast forests are cut down.
Q. Why do hedges and belts of trees promote warmth?
A. Because they retard evaporation, by keeping off the wind.
Q. If belts of trees promote warmth, why do forests produce cold?
A. 1st—Because they detain and condense the passing clouds:
2ndly—They prevent the access of both wind and sun:
3rdly—The soil of forests is always covered with long damp grass, rotting leaves, and thick brushwood: and
4thly—There are always many hollows in every forest full of stagnant water.
Q. Why do long grass and rotting leaves promote cold?
A. Because they are always damp; and the evaporation which they promote, is constantly absorbing heat from the earth beneath.
Q. Why do digging and ploughing help to make a country warm?
A. Digging and ploughing help to[Pg 162] pulverize the soil, by admitting air into it, and this increases its mean temperature.
Q. Why are France and Germany warmer now, than when the vine would not ripen there?
A. Chiefly because their vast forests have been cut down; and the soil is better drained and cultivated.
Q. What becomes of the water of ponds and tubs in summer-time?
A. Ponds and tubs in summer-time are often left dry, because their water is evaporated by the air.
Q. How is this evaporation produced and carried on?
A. The air contains heat, and changes the surface of the water into vapour; this vapour (blending with the air) is soon wafted away; while fresh portions of air blow over the water, and produce a similar evaporation; till the pond or tub is left quite dry.
Q. Why are the wheels of some machines kept constantly wet with water?
A. To carry off the heat (arising from the rapid motion of the wheels) by evaporation, as soon as it is developed.
Q. Why is mould hardened by the sun?
A. Because (when the moisture of the mould has been evaporated by the sun) the earthy particles come into closer contact, and the mass becomes more solid.
Q. Show the wisdom of god in this arrangement.
A. If the soil did not become crusty and hard in dry weather, the heat and drought would penetrate the soil, and kill both seeds and roots.
Q. Why is tea cooled faster in a saucer than in a cup?
A. Because evaporation is increased by increasing the surface; and as tea in a saucer presents a much larger surface to the air, its heat is more rapidly carried off by evaporation.
(The subject of “convection” will be treated of in a future chapter, and would scarcely be understood in this place.)
Q. Why is not the vapour of the sea salt?
A. Because the salt is always left behind, by the process of evaporation.
Q. Why does a white crust appear (in hot weather) upon clothes wetted by sea water?
A. The white crust is the salt of the water left on the clothes by evaporation.
Q. Why does this white crust always disappear in wet weather?
A. In wet weather the moisture of the air dissolves the salt; and, therefore, it no longer remains visible.
Q. Why should not persons, who take violent exercise, wear very thick clothing?
A. When the heat of the body is increased by exercise, perspiration reduces the heat (by evaporation) to a healthy standard: as thick clothing prevents this evaporation, and confines the heat and perspiration to the body, it is injurious to health.
Q. How is heat communicated from one body to another?
A. 1. By Conduction. 2. By Absorption. 3. By Reflection. 4. By Radiation: and 5. By Convection.
Q. What is meant by conduction of heat?
A. Heat communicated from one body to another, by actual contact.
Q. Why does a piece of wood (blazing at one end) not feel hot at the other end?
A. Wood is a bad conductor of heat; and, therefore, heat does not traverse freely through it: hence, though one end of a stick be blazing-hot, the other end may be quite cold.
Q. Why do some things feel so much colder than others?
A. Principally because they are better conductors; and, therefore, draw off the heat from our body (which touches them) so much faster.
Q. What are the best conductors of heat?
A. Dense solid bodies, such as metal and stone.
Q. Which metals are the most rapid conductors of heat?
A. Silver is the best conductor, then copper, then gold or tin, then iron, then zinc, and then lead.
Q. What are the worst conductors of heat?
A. All light and porous bodies, such as hair, fur, wool, charcoal, and so on.
Q. Why are cooking vessels so often furnished with wooden handles?
A. Wood is not a good conductor, like metal; and, therefore, many vessels (which are exposed to the heat of the fire) have wooden handles, lest they should burn our hands when we take hold of them.
Q. Why is the handle of a metal tea-pot made of wood?
A. As wood is a bad conductor, the heat of the boiling water is not so quickly conveyed to the wooden handle, nor so quickly poured into the hand by it, as when the handle is made of metal.
Q. Why would a metal handle burn the hand of the tea-maker?
A. As metal is an excellent conductor, the heat of the boiling water rushes quickly into the metal handle, and into the hand that touches it.
Q. How do you know that a metal handle would be hotter than a wooden one?
A. By touching the metal collar into which the wooden handle is fixed: though the wooden handle is quite cold, this metal collar is intensely hot.
Q. Why do persons use paper or woollen kettle-holders to take hold of a kettle with?
A. Paper and woollen are both very bad conductors of heat; and, therefore, the heat of the kettle does not readily pass through them to the hand.
Q. Does the heat of the boiling kettle never get through the woollen or paper kettle-holder?
A. Yes; but though the kettle-holder became as hot as the kettle itself, it would never feel so hot.
Q. Why would not the kettle-holder feel so hot as the kettle, when it really is of the same temperature?
A. Because (being a very bad conductor) it disposes of its heat so slowly, that it is scarcely perceptible; but metal (being an excellent conductor) disposes of its heat so quickly, that the sudden influx is painful.
Q. Why then does hot metal feel so much more intensely warm than hot wool?
A. Because it gives out a much greater quantity of heat in the same space of time; and the influx of heat is, therefore, more perceptible.
Q. Why does money in our pocket feel so hot, when we stand before a fire?
A. Metal is an excellent conductor; and, therefore, becomes rapidly heated. For the same reason it becomes rapidly cold, when it comes in contact with a body colder than itself.
Q. Why does a pump-handle feel intensely cold in winter?
A. As metal is an excellent conductor, when the hot hand touches the cold pump-handle, the heat passes rapidly from the hand into the iron; and this rapid loss of heat produces a sensation of intense coldness.
Q. Is the iron handle of the pump really colder than the wooden pump itself?
A. No; every inanimate substance (exposed to the same temperature) possesses the same degree of heat.
Q. Why then does the iron handle seem so much colder than the wooden pump?
A. Merely because the iron is a better conductor; and, therefore, draws off the heat from our hand much more rapidly than wood does.
Q. Why does a stone or marble hearth feel to the feet so much colder than a carpet or hearth-rug?
A. Because stone and marble are good conductors, but woollen carpets and hearth-rugs are very bad conductors.
Q. Why does the stone hearth make our feet cold?
A. As soon as the hearth-stone has absorbed a portion of heat from our foot, it instantly disposes of it, and calls for a fresh supply; till the hearth-stone has become of the same temperature as the foot placed upon it.
Q. Do not the woollen carpet and hearth-rug, also, conduct heat from the human body?
A. Yes; (but being very bad conductors) they convey the heat away so slowly, that it is scarcely perceptible.
Q. Is the cold hearth-stone and warm carpet then of the same temperature?
A. Yes; everything in the room is[Pg 170] really of the same temperature; but some feel colder than others because they are better conductors.
Q. How long will the hearth-stone feel cold to the feet resting on it?
A. Till the feet and the hearth-stone are both of the same temperature; and then the sensation of cold in the hearth-stone will go off.
Q. Why would not the hearth-stone feel cold, when it is of the same temperature as our feet?
A. Because the heat would no longer rush out of our feet into the hearth-stone, in order to produce an equilibrium.
Q. Why does the hearth-stone (when the fire is lighted) feel so much hotter than the hearth-rug?
A. The hearth-stone is an excellent conductor; and, therefore, parts with its heat more readily than the woollen hearth-rug; which (being a very bad conductor) parts with its heat reluctantly.
Q. Why does parting with heat rapidly make the hearth-stone feel warm?
A. As the heat of the stone rushes[Pg 171] quickly into our foot, it raises its temperature so suddenly, that we cannot help perceiving the increase of heat.
Q. Why does the non-conducting power of the hearth-rug prevent its feeling so hot as it really is?
A. Because it parts with its heat so slowly and gradually, that we scarcely perceive its transmission into our feet.
Q. When we plunge our hands into a basin of water, why does it produce a sensation of cold?
A. Though the water (in which we wash) is really warmer than the air of our bed-room; yet because it is a better conductor, it feels colder.
Q. Why does the conducting power of water make it feel colder than the air, though in reality it is warmer?
A. Because it abstracts heat from our hands so rapidly, that we feel its loss; but the air abstracts heat so very slowly, that its gradual loss is hardly perceptible.
Q. Is water a good conductor of heat?
A. No; no liquid is a good conductor of heat; but yet water is a much better conductor than air.
Q. Why is water a better conductor of heat than air?
A. Because it is less subtile; and the conducting power of any substance depends upon its solidity, or the closeness of its particles.
Q. How do you know that water is not a good conductor of heat?
A. Because water may be made to boil at its surface, without imparting sufficient heat to melt ice a quarter of an inch below the boiling surface.
Q. Why are not liquids good conductors of heat?
A. Because the heat (which should be transmitted) produces evaporation, and flies off in the vapour.
Q. Why does a poker (resting on the fender) feel so much colder than the hearth-rug, which is further off the fire?
A. The poker (being an excellent conductor) draws heat from the hand much more quickly than the rug, which is a bad conductor: and, therefore, (though both are equally warm) the poker seems to be much colder.
Q. Why are hot bricks (wrapped in cloth) employed in cold weather to keep the feet warm?
A. Bricks are bad conductors of heat, and cloth or flannel still worse: therefore a hot brick (wrapped in flannel) will retain its heat a very long time.
Q. Why is a tin pan (filled with hot water) employed as a foot warmer?
A. Because polished tin (being a bad radiator of heat) keeps hot a very long time; and warms the feet resting upon it.
Q. What is meant by being a “bad radiator of heat?”
A. To radiate heat is to throw off heat by rays, as the sun; a polished tin pan does not throw off the heat of boiling water from its surface, but keeps it in.
Q. Why is the tin foot-warmer covered with flannel?
A. 1st—To prevent the perspiration of the foot from taking off the polish of the tin:
2ndly—Flannel is a very bad conductor; and, therefore, helps to keep the tin hot longer: and
3rdly—If the feet were not protected, the conducting surface of the tin would feel painfully hot.
Q. What harm would it be if the polish of the tin were injured by the perspiration of our feet?
A. Polished tin throws off its heat very slowly; but dull, scratched, painted, or dirty tin, throws off its heat very quickly: if, therefore, the tin foot-warmer were to lose its polish, it would get cold in a much shorter time.
Q. Why are furnaces and stoves (where much heat is required) built of porous brick?
A. As bricks are bad conductors, they prevent the escape of heat: and are, therefore, employed where great heat is required.
Q. Why are furnace doors, &c. frequently covered with a paste of clay and sand?
A. Because this paste is a very bad conductor of heat; and, therefore, prevents the escape of heat from the furnace.
Q. If a stove be placed in the middle of a room, should it be made of bricks or iron?
A. A stove in the middle of a room should be made of iron; because iron[Pg 175] is an excellent conductor, and rapidly communicates its heat to the air around.
Q. Why does the Bible say, that God “giveth snow like wool?”
A. As snow is a very bad conductor of heat, it protects vegetables and seeds from the frost and cold.
Q. How does the non-conducting power of snow protect vegetables from the frost and cold?
A. As snow is a bad conductor, it prevents the heat of the earth from being drawn off by the cold air which rests upon it.
Q. Why are woollens and furs used in cold weather for clothing?
A. Because they are very bad conductors of heat; and, therefore, prevent the warmth of the body from being drawn off by the cold air.
Q. Do not woollens and furs actually impart heat to the body?
A. No; they merely prevent the heat of the body from escaping.
Q. Where would the heat escape to, if the body were not wrapped in wool or fur?
A. The heat of the body would fly[Pg 176] off into the air; for the cold air (coming into contact with our body) would gradually draw away its heat, till it was as cold as the air itself.
Q. What then is the principal use of clothing in winter-time?
A. To keep the body air-tight; and prevent the external air (or wind) from coming into contact with it, to absorb its heat.
Q. Why are beasts covered with fur, hair, or wool?
A. Because fur, hair, and wool are very slow conductors of heat; and (as dumb animals cannot be clad like human beings) God has given them a robe of hair or wool, to keep them warm.
Q. Why are birds covered with down or feathers?
A. Because down and feathers are very bad conductors of heat; and (as birds cannot be clad like human beings) God has given them a robe of feathers to keep them warm.
Q. Why are wool, fur, hair, or feathers such slow conductors of heat?
A. Because a great quantity of air lurks entangled between their fibres; and air is a very bad conductor of heat.
Q. If air be a bad conductor of heat, why should we not feel as warm without clothing, as when we are wrapped in wool and fur?
A. Because the air (which is cooler than our body) is never at rest; and, therefore, fresh particles (perpetually passing over our body) keep drawing off the heat little by little.
Q. Why does the ceaseless change of air tend to decrease the warmth of a naked body?
A. Thus:—the air which cases the body absorbs as much heat from it as it can, while it remains in contact; it is then blown away, and makes room for a fresh coat of air, which does the same.
Q. Does the air (which encases a naked body) become by contact as warm as the body itself?
A. It would do so, if it remained motionless; but as it remains only a very short time, it absorbs as much heat as it can in the time, and passes on.
Q. Why do we feel colder in windy weather, than in a calm day?
A. Because (in windy weather) the particles of air pass over us more rapidly; and every fresh particle takes from us some portion of heat.
Q. Show the wisdom of God in making the air a bad conductor.
A. If air were a good conductor (like iron and stone) the heat would be drawn so rapidly from our body, that we must be chilled to death. Similar evils would be felt also by all the animal and vegetable world.
Q. Does not the bad conducting power of air enable persons to judge whether an egg be new or stale?
A. Yes; touch your tongue against the shell at the larger end; if it feels warm to the tongue, the egg is stale; if not, it is new-laid.
Q. Why will the shell of a stale egg feel warm to the tongue?
A. Between the shell and the “white of the egg” there is a small quantity of air, which expands in a stale egg, from the shrinking of the white.
Q. Why does the expansion of air (at the end of an egg) make it feel warm to the tongue?
A. As air is a very bad conductor, the more air an egg contains, the less heat will be drawn from the tongue when it touches the shell.
Q. Why do ladies fan themselves in summer, to make their faces cool?
A. The fan puts the air in motion, and makes it pass more rapidly over their face; and (as the temperature of the air is always lower than that of the human face) each puff of air carries off some portion of heat from the face.
Q. Does fanning the air make the air itself cooler?
A. No; fanning makes the air hotter and hotter.
Q. Why does fanning the air increase its heat?
A. By causing the air continually to absorb heat from the human body which it passes over.
Q. If fanning makes the air hotter, how can it make a person feel cooler?
A. Fanning makes the air hotter, but the face cooler; because it keeps taking the heat out of the face, and giving it to the air.
Q. Why is broth cooled by blowing it?
A. The breath causes a rapid change of air to pass over the broth; and (as the air is not so hot as the broth) it keeps absorbing heat, and thus makes the broth cooler and cooler.
Q. Would not the air absorb heat from the broth just as well without blowing?
A. No; air is a very bad conductor; unless, therefore, the change be rapid, the air nearest the surface of the broth would soon become as hot as the broth itself.
Q. But would not the hot air part with its heat instantly to the circumjacent air?
A. No; not instantly. Air is so bad a conductor, that it parts with its heat very slowly: unless, therefore, the air be kept in continual motion, it would cool the broth very slowly indeed.
Q. Why does wind generally feel cool?
A. Wind is only air in motion; and the more quickly the air passes over our body, the more rapidly it absorbs the heat therefrom.
Q. Why does air absorb heat more quickly by being set in motion?
A. Because every fresh gust of air absorbs a fresh portion of heat; and the more rapid the succession of gusts, the greater will be the quantity of air absorbed.
Q. If the air were hotter than our body, would the wind feel cool?
A. No; if the air were hotter than our body, it would feel insufferably hot.
Q. Why would the air feel intensely hot, if it were warmer than our blood?
A. Because then the wind would add to the heat of our body, instead of diminishing it.
Q. Is the air ever as hot as the human body?
A. Not in this country: in the hottest summer’s day, the air is always 10 or 12 degrees cooler than the human body.
Q. Is the earth a good conductor of heat?
A. No; the power of conducting heat depends upon the continuity of matter; if the particles of which a thing is composed are not continuous, they have very little power to conduct heat.
Q. Why is the earth (below the surface) warmer in winter than the surface itself?
A. Because the earth is a bad conductor of heat; and, therefore (although the ground be frozen) the frost never penetrates above an inch or two below the surface.
Q. Why is the earth (below the surface) cooler in summer than the surface itself?
A. Because the earth is a bad conductor of heat; and, therefore, (although the surface be scorched with the burning sun) the intense heat cannot penetrate to the roots of the plants and trees.
Q. Shew the wisdom of God in making the earth a bad conductor.
A. If the heat and cold could penetrate the earth (as freely as the heat of a fire penetrates iron), the springs would be dried up in summer and frozen in winter, and all vegetation would perish.
Q. Why is water from a spring so cool in summer?
A. As the earth is a bad conductor, the burning rays of the sun can penetrate only a few inches below the surface; in consequence of which, the springs of water are not affected.
Q. Why is it cool under a shady tree in a hot summer’s day?
A. 1st—Because the overhanging foliage screens off the rays of the sun:
2ndly—As the rays of the sun are warded off, the air (beneath the tree) is not heated by the reflection of the earth: and
3rdly—The leaves of trees, being non-conductors, allow no heat to penetrate through them.
Q. Why do the laplanders wear skins, with the fur inwards?
A. The dry skin prevents the wind from penetrating to their body; and as the fur contains a quantity of air between its hairs (which soon becomes heated by the body) the Laplander is clad in a case of hot air, impervious to the cold and wind.
Q. Why does a linen shirt feel colder than a cotton one?
A. Linen is a much better conductor than cotton; and, therefore, (as soon as it touches the body) it more rapidly draws away the heat, and produces a sensation of cold.
Q. Why is the face cooled by wiping the temples with a fine cambric handkerchief?
A. The fine fibres of the cambric have a strong capillary attraction for moisture; and are excellent conductors of heat: thus the moisture and heat are both abstracted from the face, and a sensation of coolness is produced.
“Capillary attraction,” i. e. the attraction of a thread or hair. The wick of a candle is wet with grease, because the melted tallow runs up the cotton from capillary attraction.
Q. Why would not a cotton handkerchief do as well?
A. The coarse fibres of cotton have much less capillary attraction, and are nothing like such good conductors as linen: and, therefore, wiping the face with a cotton handkerchief, increases the sensation of warmth.
Q. What is the difference between conducting heat, and absorbing heat?
A. To conduct heat, is to transmit it[Pg 185] from one body to another through a conducting medium: to absorb heat, is to suck it up, as a sponge sucks up water.
Q. Give me an example.
A. Black cloth absorbs, but does not conduct heat: thus, if black cloth be laid in the sun, it will absorb the rays very rapidly; but if one end of the black cloth be made hot, it would not conduct the heat to the other end.
Q. Are good conductors of heat, good absorbers also?
A. No; every good conductor of heat is a bad absorber of it; and no good absorber of heat can be a good conductor also.
Q. Is iron a good absorber of heat?
A. No; iron is a good conductor, but a very bad absorber of heat.
Q. Why do the fender and fire-irons (which lie upon it) remain cold, although they are before a good fire?
A. Because the metal fender and fire-irons have very little capacity for absorbing heat; although they are soon made hot (by conduction), when placed in contact with the hot fire or stove.
Q. Why does a kettle boil faster, when the bottom and back are covered with soot?
A. The black soot absorbs heat very quickly from the fire, and the metal conducts it to the water.
Q. Why will not a new kettle boil so fast as an old one?
A. Because the bottom and sides of a new kettle are clean and bright; but in an old kettle are covered with soot.
Q. Why would the kettle be slower boiling, if the bottom and back were clean and bright?
A. Bright metal does not absorb heat, but reflect it (i. e. throw the heat back again); and as the heat is thrown off from the surface of bright metal, therefore, a new kettle is longer boiling.
Q. Why do we wear white linen and a black outer dress, if we want to be warm?
A. The black outer dress quickly absorbs heat from the sun, and conveys it to the body; and the white linen (being a bad absorbent) abstracts no heat from the warm body.
Q. Why do persons wear white dresses in summer time?
A. White throws off the heat of the sun by reflection, and is, therefore, a very bad absorbent of heat; in consequence of which, it never becomes so hot from the scorching sun as dark colours do.
Q. Why do not persons wear white dresses in winter time?
A. White will not absorb heat, like black and other dark colours; and, therefore, white dresses are not so warm as dark ones.
Q. What colours are warmest for dresses?
A. For outside garments black is the warmest, and then such colours as approach nearest to black (as dark blue and green). White is the coldest colour for external clothing.
Q. Why are dark colours (for external wear) so much warmer than light ones?
A. Because dark colours absorb heat from the sun more abundantly than light ones.
Q. How can you prove that dark colours are warmer than light ones?
A. If a piece of black cloth and a piece of white were laid upon snow,[Pg 188] in a few hours the black cloth will have melted the snow beneath; whereas the white cloth will have produced little or no effect upon it at all.
N. B. The darker any colour is, the warmer it is, because it is a better absorbent of heat. The order may be thus arranged:—1. Black (warmest of all).—2. Violet.—3. Indigo.—4. Blue.—5. Green.—6. Red.—8. Yellow: and 9. White (coldest of all).
Q. Why are black kid gloves so hot in summer time?
A. 1st—Because the black absorbs the solar heat: and
2ndly—The kid will not allow the heat of the hand to escape through the glove.
Q. Why are lisle thread gloves so cool in summer time?
A. 1st—Because thread absorbs the perspiration of the hands: and
2ndly—It conducts away the heat of our hot hands.
Q. Are Lisle thread gloves absorbents of heat?
A. As Lisle thread gloves are generally of a grey or lilac colour, they do not absorb solar heat.
Q. Why is a plate-warmer made of un-painted bright tin?
A. Bright tin reflects (or throws back) the heat, which issues from the fire in rays; and (by reflecting the heat upon the meat) assists greatly in roasting it.
Q. Why would not the tin reflector do as well if it were painted?
A. If the tin reflector were painted, it would be utterly spoiled, because it would then absorb heat, and not reflect it at all. A plate-warmer should be kept very clean, bright, and free from all scratches.
Q. Why should a reflector be kept so very clean and free from scratches?
A. If a reflector be spotted, dull, or scratched, it will absorb heat, instead of reflecting it; and, therefore, would be of no use whatsoever as a reflector.
Q. Why does hoar-frost remain on tombstones, long after it has melted from the grass and gravel-walks of a church-yard?
A. Tomb-stones being white, will not absorb heat, like the darker grass and gravel; and, therefore, the white tombstones (being so much colder) retain the hoar-frost after it has melted from other things.
Q. If black absorbs heat, why have those who live in hot climates black skins, and not white skins (which would not absorb heat at all)?
A. Though the black skin of the negro absorbs heat more plentifully than the white skin of a European, yet the blackness prevents the sun from blistering or scorching it.
Q. How is it known that the black colour prevents the sun from either blistering or scorching the skin?
A. If you put a white glove on one hand, and a black glove on the other (when the sun is burning hot), the hand with the white glove will be scorched, but not the other.
Q. Which hand will feel the hotter?
A. The hand with the black glove will feel the hotter, but it will not be scorched by the sun; whereas the hand with the white glove (though much cooler) will be severely scorched.
Q. Why does the black skin of a negro never scorch or blister with the hot sun?
A. Because the black colour absorbs the heat,—conveys it below the surface[Pg 191] of the skin, and converts it to sensible heat and perspiration.
Q. Why does the white European skin blister and scorch when exposed to the hot sun?
A. Because the white will not absorb the heat; and, therefore, the hot sun rests on the surface of the skin, and scorches it.
Q. Why has a negro black eyes?
A. The black colour of a negro’s eyes defends them from the strong light of the tropical sun. If a negro’s eyes were not black, the sun would scorch them, and every negro would be blind.
Q. Why is water kept cooler (in summer time) in a bright tin pot, than in an earthen one?
A. Because bright metal will not absorb the heat of the summer sun, like an earthen vessel.
Q. Why is boiling water kept hot in a bright tin vessel longer, than in an earthen one?
A. Because bright tin will not suffer the heat of the boiling water to escape in rays, as an earthen vessel does.
Q. What is meant by reflecting heat?
A. To reflect heat, is to throw it back in rays from the surface of the reflecting body, towards the place from whence it came.
Q. What are the best reflectors of heat?
A. All bright surfaces, and light colours.
Q. Are good absorbers of heat good reflectors also?
A. No; those things which absorb heat best, reflect heat worst; and those which reflect heat worst, absorb it best.
Q. Why are those things which absorb heat unable to reflect it?
A. Because if any thing sucks in heat like a sponge, it cannot throw it off from its surface; and if any thing throws off heat from its surface, it cannot drink it in.
Q. Why are reflectors always made of light-coloured and highly polished metal?
A. Because light coloured and highly polished metal makes the best of all reflectors.
Q. Why do not plate-warmers blister and scorch the wood behind?
A. Because the bright tin front throws the heat of the fire back again, and will not allow it to penetrate to the wood behind.
Q. If metal be such an excellent conductor of heat, how can it reflect heat, or throw it off?
A. Polished metal is a conductor of heat, only when that heat is communicated by actual contact; but whenever heat falls upon bright metal in rays, it is reflected back again, and the metal remains quite cool.
Q. What is meant by “heat falling upon metal in rays,” and not “by contact”?
A. If a piece of tin were thrust into a fire, it would be in actual contact with the fire; but if it be held before a fire, the heat of the fire falls upon it in rays.
Q. What is the use of the tin screen or reflector used in roasting?
A. The tin reflector throws the heat[Pg 194] of the fire back upon the meat; and, therefore, assists the process of roasting and helps to keep the kitchen cool.
Q. How does a tin reflector tend to keep the kitchen cool?
A. Because it confines the heat to the hearth, and prevents it from being dispersed throughout the kitchen.
Q. Why does a lamp glass diminish the smoke of a lamp?
A. As glass is a reflector, it reflects the heat of the lamp back upon the flame; in consequence of which, less carbon escapes unconsumed (as smoke).
Q. Why are shoes hotter for being dusty?
A. 1st—Because dust absorbs heat: and
2ndly—As it destroys the blackness of our shoes, it prevents them from throwing off the heat of our feet in rays.
Q. Why can we not see into the road or street, when a candle is lighted in a room?
A. Glass is a reflector; and, therefore, throws the rays of the candle back into the room, and thus prevents our seeing into the road or street.
Q. Why can persons in the dark street see into a room (lighted by a candle or lamp)?
A. The pupil of the eye expands greatly, when persons are in the dark; and, therefore, when any one in the dark street looks into a light room, his dilated pupil sees every thing distinctly.
Q. Why does it always freeze on the top of a mountain?
A. Air is heated by the reflection of the earth, and not by the rays of the sun; and, as there is no earth round a mountain-top to reflect heat, therefore, it remains intensely cold.
Q. What is meant by radiation?
A. Radiation means the emission of rays: thus the sun radiates both light and heat; that is, it emits rays of light and heat in all directions.
Q. When is heat radiated from one body to another?
A. When the two bodies are separated by a non-conducting medium: thus the sun radiates heat towards the earth, because the air comes between (which is a very bad conductor).
Q. On what does radiation depend?
A. On the roughness of the radiating surface: thus if metal be scratched, its radiating power is increased, because the heat has more points to escape from.
Q. Does a fire radiate heat?
A. Yes; and because burning fuel emits rays of heat, therefore we feel warm when we stand before a fire.
Q. Why does our face feel uncomfortably hot, when we approach a fire?
A. Because the fire radiates heat upon the face; which (not being covered) feels the effect immediately.
Q. Why does the fire catch the face more than the rest of the body?
A. The rest of the body is covered with clothing, which (being a bad conductor of heat) prevents the same sudden[Pg 197] and rapid transmission of heat to the skin.
Q. Do those substances which radiate heat, absorb heat also?
A. Yes. Those substances which radiate most, also absorb most heat: and those which radiate least, also absorb the least heat.
Q. Does any thing else radiate heat, besides the sun and fire?
A. Yes; all things radiate heat in some measure, but not equally well.
Q. What things radiate heat the next best to the sun and fire?
A. All dull and dark substances are good radiators of heat; but all light and polished substances are bad radiators of heat.
Q. Why does a polished metal tea-pot make better tea than a black earthen one?
A. As polished metal is a very bad radiator of heat, it keeps the water hot much longer; and the hotter the water is, the better it “draws” the tea.
Q. Why will not a dull black tea-pot make good tea?
A. Because the heat of the water flies off so quickly through the dull black surface of the tea-pot, that the water is rapidly cooled, and will not “draw” the tea.
Q. Do not pensioners, and most aged cottagers, prefer the little black earthen tea-pot to the bright metal one?
A. Yes; because they set it on the hob “to draw;” in which case, the little black tea-pot will make the best tea.
Q. Why will a black tea-pot make better tea than a bright metal one, if it be set upon the hob to draw?
A. Because the black tea-pot will absorb heat plentifully from the fire, and keep the water boiling hot: whereas, a bright metal tea-pot (set upon the hob) would throw off the heat by reflection.
Q. Then sometimes a black earthen tea-pot is the best, and sometimes a bright metal one?
A. Yes; when the tea-pot is set on the hob “to draw,” the black earth is the best, because it absorbs heat: but when the tea-pot is not set on the hob, the bright metal is the best, because it[Pg 199] radiates heat very slowly, and therefore keeps the water hot.
Q. Why does a saucepan which has been used, boil quicker than a new one?
A. Because the bottom and back are covered with soot; and the black soot rapidly absorbs the heat of the glowing coals.
Q. Why should the front and lid of a saucepan be clean and bright?
A. As they do not come in contact with the fire, they cannot absorb heat; and (being bright) they will not suffer the heat to escape by radiation.
Q. In what state should a saucepan be, in order that it may boil quickly?
A. All those parts which come in contact with the fire should be covered with soot, to absorb heat; but all the rest of the saucepan should be as bright as possible, to prevent the escape of heat by radiation.
Q. Why is it said that “Saturday’s kettle boils the fastest?”
A. Because on Saturday the front and top of the kettle are generally cleaned[Pg 200] and polished; but the bottom and back of the kettle are never cleaned.
Q. Why should not the bottom and back of a kettle be cleaned and polished?
A. Because they come in contact with the fire, and (while they are covered with black soot) absorb heat freely from the burning coals.
Q. Why should the front and top of a kettle be clean and well polished?
A. Because polished metal will not radiate heat; and, therefore, (while the front and top of the kettle are well polished) the heat is kept in, and not suffered to escape by radiation.
Q. Why is the inside of a kettle and saucepan white?
A. White will not radiate heat: if, therefore, the inside of a boiler be white, the liquor in it is kept hot much longer.
Q. Why is the bottom of a kettle nearly cold, when the water is boiling hot?
A. Black soot is a very bad conductor of heat; and, therefore, the heat of the boiling water is some considerable time, before it gets through the soot[Pg 201] which adheres to the bottom of the kettle.
Q. Why is the lid of a kettle so intensely hot, when the water boils?
A. The bright metal lid of the kettle is an admirable conductor of heat; and, therefore, the heat from the boiling water pours into our hand the moment we touch it.
Q. Show the benefit of smoke in cooking.
A. The carbon of the fuel (which flies off in smoke) naturally blackens all culinary vessels set upon the fire to boil, and thus renders them fit for use.
(“Culinary vessels” are vessels used in kitchens for cooking, as saucepans, boilers, kettles, &c.)
Q. How does smoke make culinary vessels fit for use?
A. If it were not for the smoke, (which gathers round a kettle or saucepan) heat would not be absorbed, and the process of boiling would be greatly retarded.
Q. Why is boiling water kept hot best in a bright metal pot?
A. Because bright metal being a[Pg 202] bad radiator will not throw off the heat of the boiling water from its surface.
Q. Why is water kept cold in summer-time in a bright metal pot, better than in an earthen vessel?
A. Because bright metal will not absorb heat from the hot air, like an earthen vessel; in consequence of which, the water is kept cooler.
Q. Why are dinner-covers made of bright tin or silver?
A. Light-coloured and highly-polished metal is a very bad radiator of heat; and, therefore, bright tin or silver will not allow the heat of the cooked food to escape through the cover by radiation.
Q. Why should a meat-cover be very brightly polished?
A. If the cover be dull or scratched it will absorb heat from the hot food beneath it; and (instead of keeping it hot) will make it cold.
Q. Why should a silver meat-cover be plain, and not chased?
A. If the cover be chased, it will[Pg 203] absorb the heat of the food covered by it; and instead of keeping it hot, will make it cold by absorption.
Q. What is dew?
A. Dew is the vapour of the air condensed, by coming in contact with bodies colder than itself.
Q. Why is the ground sometimes covered with dew?
A. The earth is more heated by solar rays than the air, during the day; but at night, the earth parts with more heat than the air, and becomes (in consequence) 5 or 10 degrees colder.
Q. How does the earth being colder than the air account for the deposition of dew?
A. As soon as the air touches the cold earth, its warm vapour is chilled, and condensed into dew.
Q. Why is the surface of the ground colder in a fine clear night, than in a cloudy one?
A. On a fine clear star-light night, heat radiates from the earth freely, and is lost in open space: but on a cloudy night, the clouds arrest the process of radiation.
Q. Why is dew deposited only on a fine clear night?
A. Because, when the night is clear and fine, the surface of the ground radiates heat most freely; and (being cooled down by this loss of heat) chills the vapour of the air into dew.
Q. Why is there no dew on a dull cloudy night?
A. The clouds arrest the radiation of heat from the earth; and (as the heat cannot freely escape) the surface is not sufficiently cooled down to chill the vapour of the air into dew.
Q. Why is a cloudy night warmer than a fine one?
A. Because the clouds prevent the radiation of heat from the earth; and, therefore, the surface of the earth remains warmer on a dull cloudy night.
Q. Why is dew most abundant in situations most exposed?
A. Because the radiation of heat is not arrested by houses, trees, hedges, or any other thing.
Q. Why is there scarcely any dew under a shady tree?
A. The shady head of the tree both arrests the radiation of heat from the earth, and also radiates some of its own heat towards the earth; and, therefore, the ground (underneath a tree) is not sufficiently cooled down to chill the vapour of the air into dew.
Q. Why is there never much dew at the foot of walls and hedges?
A. 1st—Because the wall or hedge acts as a screen, to arrest the radiation of heat from the earth: and
2ndly—The wall or hedge also radiates some portion of heat towards the earth.
Q. How do these things prevent the deposition of dew?
A. As the ground (beneath a wall, tree, or hedge) is not cooled by the radiation of heat, it remains of the same temperature as the air above it; in consequence of which, the vapours of the air are not chilled by it into dew.
Q. Why is there little or no dew beneath a flower-awning, although that awning be open on all four sides?
A. 1st—Because the awning arrests the radiation of heat from the ground beneath: and
2ndly—It radiates some of its own heat downwards; in consequence of which, the ground beneath an awning is not sufficiently cooled down to chill the vapour of air into dew.
Q. How can a thin covering of bass or even muslin protect trees from frost?
A. Because any covering prevents the radiation of heat from the tree; and if the tree be not cooled down by radiation, the vapour of the air will not be frozen as it comes in contact with it.
Q. Why is the bass or canvass itself (which covers the tree) always drenched with dew?
A. The bass or canvass covering radiates heat both upwards and downwards; and is, therefore, so cooled down, that it readily chills all the vapour of the air (which passes over it) into dew.
Q. Why does snow at the foot of a hedge or wall melt sooner, than in an open field?
A. Because the hedge or wall radiates heat into the snow beneath, which melts it.
Q. Why is there no dew after a windy night?
A. 1st—Because the wind evaporates the moisture, as fast as it is deposited; and
2ndly—It disturbs the radiation of heat, and diminishes the deposition of dew thereby.
Q. Why are VALLEYS & HOLLOWS often thickly covered with dew, although they are sheltered?
A. The surrounding hills prevent the repose of air (in the valleys) from being disturbed; but do not overhang and screen them, so as to arrest their radiation.
Q. Why does dew fall more abundantly on some things than upon others?
A. Because some things radiate heat more freely than others, and therefore become much cooler in the night.
Q. Why are things which radiate heat most freely, always the most thickly covered with dew?
A. Because the vapour of the air is chilled into dew, the moment it comes in contact with them.
Q. What kind of things radiate heat most freely?
A. Grass, wood, and the leaves of plants, radiate heat very freely: but polished metal, smooth stones, and woollen cloth, part with their heat very tardily.
Q. Do the leaves of all plants radiate heat equally well?
A. No. Rough woolly leaves (like those of a holly-hock) radiate heat much more freely, than the hard smooth polished leaves of a common laurel.
Q. Shew the wisdom of God in making grass, the leaves of trees, and all vegetables, excellent radiators of heat.
A. As vegetables require much moisture, and would often perish without a plentiful deposit of dew, God wisely made them to radiate heat freely, so as to chill the vapour (which touches them) into dew.
Q. Will polished metal, smooth stones, and woollen cloth, readily collect dew?
A. No. While grass and the leaves of plants are completely drenched with[Pg 209] dew, a piece of polished metal, or of woollen cloth (lying on the same spot) will be almost dry.
Q. Why would polished metal and woollen cloth be dry, while grass and leaves are drenched with dew?
A. Because the polished metal and woollen cloth part with their heat so slowly, that the vapour of the air is not chilled into dew as it passes over them.
Q. Why is a gravel walk almost dry, when a grass plat is covered thick with dew?
A. Grass, (being a good radiator) throws off its heat very freely; but gravel (being a very bad radiator) parts with its heat very reluctantly.
Q. Is that the reason why grass is saturated with dew, and the gravel is not?
A. Yes. When the vapour of warm air comes in contact with the cold grass, it is instantly chilled into dew; but (as the gravel is not so cold as the grass) the vapour of air is not so freely condensed as it passes over the gravel.
Q. Why does dew rarely fall upon hard rocks and barren lands?
A. Rocks and barren lands are so compact and hard, that they can neither absorb nor radiate much heat; and (as their temperature varies but very little) very little dew distils upon them.
Q. Why does dew fall more abundantly on cultivated soils, than on barren lands?
A. Because cultivated soils (being loose and porous) absorb heat freely during the day, and radiate it by night; and (being much cooled by the rapid radiation of heat) as the vapour of the air passes over them, it is plentifully condensed into dew.
Q. Shew the wisdom of God in this arrangement.
A. Every plant and inch of land which needs the moisture of dew, is adapted to collect it; but not a single drop even of dew is wasted, where its refreshing moisture is not required.
Q. Shew the wisdom of God in making polished metal and woollen cloth bad radiators of heat.
A. If polished metal collected dew as easily as grass, it could never be kept dry,[Pg 211] and free from rust. Again, if woollen garments collected dew as readily as the leaves of trees, we should be often soaking wet, and subject to constant colds.
Q. Shew how this affords a beautiful illustration of Gideon’s miracle, recorded in the book of Judges, VI. 37, 38.
A. The fleece of wool (which is a very bad radiator of heat) was soaking wet with dew: when the grass (which is a most excellent radiator) was quite dry.
Q. Was not this contrary to the laws of nature?
A. Yes; and was, therefore, a plain demonstration of the power of God, who could change the very nature of things at his will.
Q. Why do our clothes feel damp, after walking in a fine evening in spring or autumn?
A. Because the vapour (condensed by the cold earth) lights upon them, like dew.
Q. Why are windows often covered with thick mist, and the frames wet with standing water?
A. The temperature of the external air always falls at sun-set, and chills[Pg 212] the window-glass, with which it comes in contact.
Q. How does this account for the mist and water on a window?
A. As the warm vapour of the room touches the cold glass, it is chilled and condensed into mist; and the mist (collecting into drops) rolls down the window-frame in little streams of water.
Q. Does the glass of a window cool down more rapidly than the air of the room itself?
A. Yes; because the air is kept warm by fires, and the animal heat of the people in the room; in consequence of which, the air of a room suffers very little diminution of heat from the setting of the sun.
Q. Whence arises the vapour of a room?
A. 1st—The very air of the room contains vapour:
2ndly—The breath and insensible perspiration of the inmates increase this vapour: and
3rdly—Hot dinners, the steam of tea, &c. contribute to increase it still more.
Q. What is meant by “the insensible perspiration?”
A. From every part of the human body an insensible and invisible perspiration issues all night and day; not only in the hot weather of summer, but also in the coldest day of winter.
Q. If the perspiration be both insensible and invisible, how is it known that there is any such perspiration?
A. If you put your naked arm into a clean dry glass cylinder, the perspiration of your arm will soon condense on the glass, like mist.
Q. Why are carriage windows very soon covered with thick mist?
A. The warm vapour of the carriage is condensed the moment it touches the cold glass, and covers it over with a thick mist.
Q. Why is the glass window cold enough to condense the vapour of the carriage?
A. Because the inside of the carriage is much warmer than the outside, and the glass window is made cold by contact with the external air.
Q. Where does the warm vapour of the carriage come from?
A. The warm breath and insensible perspiration of the persons riding in the carriage, load the air of it with warm vapour.
Q. What is the cause of the pretty frost-work seen on bed-room windows in winter-time?
A. The breath and insensible perspiration of the sleeper (coming in contact with the ice-cold window) is frozen by the cold glass, and forms those beautiful appearances seen in our bed-rooms in a winter morning.
Q. Why is the glass of a window colder than the walls of a room?
A. Glass is a very excellent radiator; and, therefore, most rapidly parts with its heat.
Q. Why is a tumbler of cold water made quite dull with mist, when brought into a room full of people?
A. Because the hot vapour of the room (coming in contact with the cold tumbler) is condensed upon it; and changes its invisible and gaseous form for that of a thick mist.
Q. Why is a glass made quite dull, by laying a hot hand upon it?
A. The insensible perspiration of the hot hand is condensed upon the cold glass, and thus made perceptible.
Q. Why are wine-glasses made quite dull when they are brought into a room full of company?
A. The hot vapour of the room (coming in contact with the cold wine-glasses) is condensed upon them, and covers them with vapour like dew.
Q. Why does this misty appearance go off after a little time?
A. Because the glass becomes of the same temperature as the air of the room, and will no longer chill the vapour which touches it, and condense it into mist.
Q. Why is a wine-glass (brought out of a cellar into the air) covered with a thick mist in summer-time?
A. The vapour of the hot air is condensed by the cold glass, and covers it as a thick mist.
Q. Why does breathing on a glass make it quite dull?
A. Because the hot breath is condensed by the cold glass; and, therefore, covers it with a thick mist.
Q. Why do walls stand thick with wet in a sudden thaw?
A. The walls (being thick) cannot change their temperature so fast as the thin air can; and, therefore, they retain their cold after the thaw has set in.
Q. How does retaining their cold account for their being so wet?
A. As the vapour of the warm air touches the cold wall, it is chilled and condensed into water, which sticks to the wall, and sometimes trickles down in little streams.
Q. Why does a thick well-built house contract more damp of this kind, than an ordinary one?
A. Because the walls are much thicker; and (if the frost has penetrated far into the bricks) it takes a long time to reduce them to the same temperature as the air.
Q. Why are banisters, &c. damp after a thaw?
A. The wooden banister (being made of some very close-grained, varnished wood) cannot change its temperature so fast as the air; and, therefore, remains cold some time after the thaw has set in.
Q. How does this account for the banisters being damp?
A. The vapour of the warm air (coming in contact with the cold banister) is chilled, and condensed into water upon it.
Q. Why is our breath visible in winter and not in summer?
A. In winter the coldness of the air condenses our breath into visible vapour; but in summer the air is not cold enough to condense it into visible vapour.
Q. Why are our hair and the brim of our hat often covered with little drops of pearly dew in winter-time?
A. The breath (issuing from our mouth and nose) is condensed into drops, as it comes in contact with our cold hair or hat; and (being condensed) hangs there in little dew-drops.
Q. Why does the steam of a railway boiler often pour down, like fine rain, when the steam is “let off?”
A. The steam from the steam-pipe (when the air is cold) is condensed by contact with the chill air, and falls like fine rain.
Q. Why is there less dew when the wind is easterly, than when the wind is westerly?
A. Easterly winds cross the continent of Europe, and, (as they pass over land) are dry and arid; but westerly winds cross the Atlantic Ocean; and (as they pass over water) are moist and full of vapour.
Q. How does the dryness of an eastern wind prevent dew-falls?
A. As the easterly winds are dry, they imbibe the moisture of the air; and, therefore, there is very little left to be condensed into dew.
Q. How does the moistness of a western wind promote dew-falls?
A. As the westerly winds are saturated with vapour, they require a very little reduction of heat to cause a copious deposition of dew.
Q. When is dew most copiously distilled?
A. After a hot day in summer or autumn, with the wind in the west.
Q. Why is dew distilled most copiously after a hot day?
A. Because the surface of the earth radiates heat very freely at sunset; and (becoming thus much colder than the air) chills its vapour, and condenses it into dew.
Q. Does not air radiate heat, as well as the earth and its various plants?
A. No. The air never radiates heat, nor is the air itself made hot by the rays of the sun.
Q. How is the air made hot or cold?
A. By convection of hot or cold currents.
Q. What is meant by “convection of hot and cold currents?”
A. The air (which is heated by the surface of the earth) ascends, warming the air through which it passes. Other air (being warmed in a similar way) also ascends, carrying heat; till all the air is made hot.
Q. Is the air made cold in a similar way?
A. Yes. The air resting on the earth is made cold by contact: this cold air makes the air above it cold; and cold currents or winds shake the whole together, till all becomes of one temperature.
Q. Why is meat very subject to taint on a moon-light night?
A. In a bright moon-light night, meat radiates heat very freely; and is, therefore, soon covered with dew, which produces rapid decomposition.
Q. Why do plants grow rapidly in moon-light nights?
A. In bright moon-light nights rapid radiation is carried on, and dew is plentifully deposited on young plants, which conduces much to their growth and vigour.
Q. Why is evening dew injurious to health?
A. Because the condensed vapours are always laden with noxious exhalations from the earth: this is especially the case in marshy countries.
Q. Is honey-dew a similar thing to dew?
A. No. Honey-dew is a sweet liquid shed by a very small insect (called the aphis), and deposited in autumn on the under surface of favourite leaves.
Q. Does honey-dew injure leaves, or do them good?
A. It injures them very much, because it fills the pores of the leaf with a thick clammy liquid; and, therefore, prevents the leaf from transpiring and absorbing.
Q. What effect has honey-dew upon the appearance of a leaf?
A. After a little time, the leaf (being smothered and starved) begins to turn a dingy yellow.
Q. Are not ants very fond of honey-dew?
A. Yes; and they crawl up the loftiest trees, in order to obtain it.
Q. What is the cause of mist (or earth-fog)?
A. If the night has been very calm, a rapid radiation of heat has taken place in the earth; in consequence of which, the air (resting on the earth) is made so cold, that its vapour is chilled, and condensed into a thick mist.
Q. Why does not the mist become dew?
A. Because the chill of the air is so rapid, that vapour is condensed faster than it can be deposited; and (covering the earth in a mist) prevents any further radiation of heat from the earth.
Q. When the earth can no longer radiate heat upwards, does it continue to condense the vapour of the air?
A. No; the air (in contact with the earth) becomes about equal in temperature with the surface of the earth itself; for which reason, the mist is not condensed into dew, but remains floating above the earth as a thick cloud.
Q. Why does this mist seem to rise higher and higher, and yet remain quite as dense below as before?
A. The air resting on the earth is first chilled, and chills the air resting on it; the air which touches this new layer of mist being also condensed, layer is added to layer; and the mist seems to be rising, when (in fact) it is only deepening.
Q. Why does mist and dew vanish as the sun rises?
A. Because the condensed vapour is[Pg 223] again rarefied by the heat of the sun, and separated into invisible particles.
Q. Why is a dew-drop round?
A. Because every part of the drop is equally balanced; and, therefore, there is no cause why one part of the drop should be further from the centre than another.
Q. Why is the dew-drop on a broad leaf sometimes flattened?
A. Whenever two or more drops of dew roll together, they make one large spheroid (or flattened drop).
Q. Why will DEW-DROPS ROLL ABOUT CABBAGE-PLANTS, POPPIES, &c. without wetting the surface?
A. The leaves of cabbages and poppies are covered with a very fine powder; and the dew-drop rolls over this fine powder, as a drop of rain over dust, without wetting the surface.
Q. Why does not the drop of rain wet the dust over which it rolls?
A. Because it is driven from grain to grain by capillary repulsion.
Q. Why does not the dew-drop wet the powder of the cabbage-plant?
A. Because it is driven from grain to grain by capillary repulsion.
Q. Why will dew-drops roll over roses, &c. without wetting their petals?
A. The leaves of a rose contain an essential oil, which prevents them from absorbing the dew immediately.
Q. Why can a swan or duck dive under water without being wetted?
A. Because their feathers are covered with an oily secretion, which repels the water.
Q. What is the cause of mist?
A. When currents of air from land mix with currents of air from water, the currents from the water are condensed into mist by the colder currents blowing from the land.
Q. Why are the currents of air from the land colder than those blowing over water?
A. Because the earth radiates heat very freely, and (being greatly cooled down) cools the air also which comes in contact with it.
Q. Why is not the air, which passes over water, so cool as that which passes over land?
A. Because water does not cool down at sun-set, so fast as the land does; and, therefore, the air in contact with it is warmer.
Q. Why does not water cool down so fast as land?
A. 1st—Because the surface of water is perpetually changing, and as fast as one surface is made cold, another is presented: and
2ndly—The moment water is made cold it sinks, and warmer portions of water rise to occupy its place: therefore, before the surface of water is cooled, the whole volume must be made cold; which is not the case with land.
Q. What is the cause of a “pea-soup” london fog?
A. These fogs (which occur generally in the winter time) are occasioned thus:—Some current of air (being suddenly cooled) descends into the warm streets, preventing the rise of the smoke, and forcing it back in a mass towards the earth.
Q. Why are there not always fogs every night?
A. Because the air will always hold in solution a certain quantity of vapour, (which varies according to its temperature): and when the air is not saturated with vapour, it may be condensed without parting with it.
Q. Why are there ever fogs at night?
A. If the air be pretty well saturated with vapour during the day, as soon as its capacity for holding vapour is lessened by the cold night, it deposits some of the superabundant vapour in the form of dew or fog.
Q. Why is there very often a fog over marshes and rivers at night-time?
A. The air of marshes is almost always near saturation; and, therefore, the least depression of temperature, will compel it to relinquish some part of its moisture in dew or fog.
Q. What is the difference between dew and rain?
A. In dew, the condensation is made near the earth’s surface:
In rain, the drops fall from a considerable height; but the cause of both is[Pg 227] the same, viz.—cold condensing the vapour of the air, when it is near the point of saturation.
Q. Why does mist and fog vanish at sunrise?
A. Because the condensed particles are again changed into invisible vapour, by the heat of the sun.
Q. What is the difference between a mist and fog?
A. Mist is generally applied to vapours condensed on marshes, rivers, and lakes.
Fog is generally applied to vapours condensed on land, especially if those vapours are laden with smoke.
Q. What is the reason why condensed vapour sometimes forms into clouds, and sometimes into fog?
A. If the surface of the earth be hotter than the air, then the vapour of the earth (being chilled by the cold air) becomes fog: but if the air be hotter than the earth, the vapour rises through the air, and becomes cloud.
Q. If cold air produces fog, why is it not foggy on a frosty morning?
A. 1st—Because less vapour is formed on a frosty day; and
2ndly—The vapour is frozen upon the ground before it can rise from the earth, and becomes hoar-frost.
Q. Why are fogs more general in autumn than in spring?
A. In spring the earth is not so hot as it is in autumn. In autumn the earth is generally warmer than the air; and, therefore, the vapour (issuing from the earth) is condensed into fog by the chill air.
Q. Why are fogs more common in valleys than on hills?
A. 1st—Because valleys contain more moisture than hills: and
2ndly—They are not exposed to so much wind, (which dissipates the vapour).
Q. How does wind dissipate fogs?
A. Either by blowing them away; or else by dissolving them into vapour again.
Q. What is hoar-frost?
A. There are two sorts of hoar-frost: 1.—Frozen dew: and 2.—Frozen fog.
Q. What is the cause of the ground hoar-frost, or frozen dew?
A. Very rapid radiation of heat from the earth; in consequence of which, the surface is so cooled down, that it freezes the dew condensed upon it.
Q. Why is hoar-frost seen only after a very clear night?
A. Unless the night has been very clear indeed, the earth will not have thrown off heat enough by radiation, to freeze the vapour condensed upon its surface.
Q. Why does hoar-frost very often cover the ground and trees, when the water of rivers is not frozen?
A. Hoar-frost is not the effect of cold in the air, but the cold of the earth (produced by excessive radiation); in consequence of which, the dew (condensed upon it) is frozen.
Q. Why is the hoar-frost upon grass and vegetables much thicker than that upon lofty trees?
A. Because the air (resting on the surface of the ground) is much colder after sun-set, than the air higher up;[Pg 230] in consequence of which, more vapour is condensed and frozen there.
Q. Why is the air (resting on the surface of the earth) colder than that in the higher regions?
A. Because the earth radiates more heat than the leaves of lofty trees; and, therefore, condenses and freezes the vapour of the air more rapidly.
Q. Why are evergreens often frost-bitten, when lofty trees are not?
A. Evergreens do not rise far above the surface of the earth; and (as the air contiguous to the earth is much colder than that in the higher regions) therefore, the low evergreen is often frost-bitten, when the lofty tree is uninjured.
Q. Why are tomb-stones covered with hoar-frost, long after it has melted from every object around?
A. White is a very bad absorbent of solar heat; and, therefore, the white tomb-stone remains too cold to thaw the frost congealed upon its surface.
Q. Why is there little or no hoar-frost under shrubs and shadowy trees?
A. 1st—Because the leafy shrubs[Pg 231] and trees arrest the process of radiation from the earth: and
2ndly—Shrubs and trees radiate a little heat towards the earth; and, therefore, the ground beneath is never cold enough to congeal the little dew which rests upon it.
Q. What is the cause of that hoar-frost which arises from frozen fog?
A. The thick fog (which invested the earth during the night) is condensed by the cold frost of early morning, and congealed upon every object with which it comes in contact.
Q. What is meant by the convection of heat?
Q. Are liquids good conductors of heat?
A. No; liquids are bad conductors; and are, therefore, made hot by convection.
Q. Why are liquids bad conductors of heat?
A. Because heat converts a liquid into steam, and flies off with the vapour, instead of being conducted through the liquid.
Q. Explain how water is made hot?
A. The water nearest the fire is first heated, and (being heated) rises to the top; other cold water succeeds, is also heated, and rises in turn; and this interchange keeps going on, till all the water boils.
Q. Why is water in such continual ferment, when it is boiling?
A. This commotion is mainly produced by the ascending and descending currents of hot and cold water.
The escape of air from the water contributes also to increase this agitation.
Q. How do these two currents pass each other?
A. The hot ascending current passes close by the metal sides of the kettle; while the cold descending current passes down the centre.
Q. Why does boiling water bubble?
A. The bubbles are portions of steam (formed at the bottom of the vessel) which rise to the surface, and escape into the air.
Q. Why does a kettle run over, when the water boils?
A. As the heat insinuates itself between the particles of water, it drives them asunder; and (as the particles of water are driven apart from each other) the same vessel will no longer hold the expanded water, and some runs over.
Q. Why does a kettle sing, when it is about to boil?
A. Water contains a great deal of air, which (being expanded by the heat of the fire) escapes by fits through the spout of the kettle; which sings in the same way as a trumpet does, when a person blows in it.
Q. Why does water boil?
A. Boiling is the effect of a more violent escape of air from the heated water; when, therefore, the air is not permitted to escape, water will never boil.
Q. Why is heat applied to the bottom, and not to the top of a kettle?
A. Because the heated water always ascends to the surface, heating the water through which it passes: if, therefore, heat were applied to the top of a vessel, the water below the surface would never be heated.
Q. As the lower part of a grate is made red-hot by the fire above, why would not the water boil, if fire were applied to the top?
A. The iron of a grate is an excellent conductor; and, therefore, if one part be heated, the heat is conducted to every other part: but water is a very bad conductor, and will not diffuse heat in a similar way.
Q. How do you know that water is a bad conductor of heat?
A. When a blacksmith immerses[Pg 235] his red-hot iron in a tank of water, the water which surrounds the red-hot iron is made boiling hot, but the water below the surface remains quite cold.
Q. If you wish to cool liquids, where should the cold be applied?
A. To the top of the liquid; because the cold portion will always descend, and allow the warmer parts to come in contact with the cooling substance.
Q. Does boiling water get hotter by being kept on the fire?
A. No; not if the steam be suffered to escape.
Q. Why does not boiling water get hotter, if the steam be suffered to escape?
A. Because as fast as the water boils, it is converted into steam; and the steam carries away the additional heat, as fast as it is communicated.
Q. Is steam visible or invisible?
A. Steam is invisible; but when it comes in contact with the air (being condensed into small drops) it instantly becomes visible.
Q. How do you know that steam is invisible?
A. If you look at the spout of a boiling kettle, you will find that the steam (which issues from the spout) is always invisible for about half an inch; after which, it becomes visible.
Q. Why is the steam invisible for only half an inch, and not either all invisible or all visible?
A. The air is not able to condense the steam as it first issues from the spout, but when it spreads and comes in contact with a larger volume of air, the invisible steam is readily condensed into visible drops.
Q. Why is our breath visible in winter-time?
A. Because it is condensed by the cold air into small drops, which are visible to the eye.
Q. Why do steam-engines sometimes burst?
A. Steam is very elastic; and this elasticity increases in a greater proportion than the heat which produces it; unless, therefore, some vent be freely allowed, the steam heaves and swells, till it bursts the vessel which confined it.
Q. What becomes of the steam, after it has been condensed?
A. It is dissolved by the air, and forms a part of its invisible vapour.
Q. Is air a good conductor?
A. No; air is a very bad conductor, and is heated (like water) by convection.
Q. How is a room warmed by a stove?
A. The air nearest the fire is made hot first; the cold air descends, is heated also, and rises in turn; and this goes on, till all the air of the room is warmed.
Q. Why are fires placed on the floor of a room, and not towards the ceiling?
A. As heated air always ascends, if the fire were not near the floor, the lower part of the air (which we want to be the warmest) would never be benefited by the fire at all.
Q. If you take a poker out of the fire, and hold the hot end downwards, why is the handle so intensely hot?
A. Because the hot end of the poker heats the air around it, and this hot air (in its ascent) scorches the poker, and the hand which holds it.
Q. How should a red-hot poker be carried so as not to burn our fingers?
A. With the hot end upwards; because then the air (heated by the poker) would not pass over our hand to scorch it.
Q. Why is a poker (resting on the fender) cold; but if it leans against the stove, intensely warm?
A. The poker is an excellent conductor; while, therefore, it rests against the hot stove, the heat of the stove is conducted into the poker; but when it rests on the fender, it does not come in contact with the hot stove.
Q. Why does it feel so cold, when it rests on the fender?
A. Not being so warm as our hand, it imbibes the heat from it with such rapidity, that our loss of heat is palpable, and produces the sensation of coldness.
Q. Why are flues (which are carried through a church or room) always blackened with black lead?
A. In order that the heat of the flue may be more readily diffused throughout the room. Black lead radiates heat more freely than any other known substance.
Q. Why do country people touch the thick end of an egg with their tongue, to know if it be stale or not?
A. The thick end of an egg always contains a little air (between the shell and the white); but, when the egg is stale, the white shrinks, and the air expands.
Q. How can the tongue tell from this, whether the egg be stale or fresh laid?
A. As air is a very bad conductor, if the egg be stale, it will feel much warmer to the tongue, than if it be new-laid.
Q. Why will the big end of an egg feel warmer to the tongue, because it contains more air?
A. As air is a bad conductor, it will draw off the heat of the tongue very slowly, and, therefore, appear warm; but when there is only a very little air in the egg (as the white is a pretty good conductor), the heat of the tongue will be more rapidly drawn off, and the egg appear colder.
Q. Why is the large end of an egg cracked, when put into a saucepan to boil?
A. To let the air out; if the large end were not cracked, the air (expanded by the heat) would enter the white of the egg, and give it an offensive taste.
Q. Of what is atmospheric air composed?
A. Principally of two gases, oxygen and nitrogen; mixed together in the following proportion: viz. 1 part of oxygen, to 4 parts of nitrogen.
Q. What are the uses of the oxygen of the air?
A. It is the oxygen of the air which supports combustion, and sustains life.
Q. What is meant when it is said, that the oxygen of the air “supports combustion?”
A. It means this; that it is the oxygen of the air which makes fuel burn.
Q. How does the oxygen of the air make fuel burn?
A. The fuel being decomposed (by heat) into hydrogen and carbon; the carbon combines with the oxygen of the air, and produces combustion.
Q. What does the combination of carbon and oxygen produce?
Q. What becomes of the hydrogen of the fuel?
A. Hydrogen (being very inflammable) burns with a blaze, and is the cause of the flame which is produced by combustion. (see p.34).
Q. What becomes of the nitrogen of the air, amidst all these changes and combinations?
A. The nitrogen of the air escapes, and is absorbed by the leaves of grass, trees, and various other vegetables.
Q. What is meant when it is said, that oxygen “sustains life”?
A. It means this: if a person could not inhale oxygen, he would die.
Q. What good does this inspiration of oxygen do?
A. 1st—It gives vitality to the blood: and
2ndly—It is the cause of animal heat.
Q. How is food converted into blood?
A. After it is swallowed, it is dissolved in the stomach into a grey pulp; it then passes into the intestines, and is converted by the “bile” into a milky substance (called chyle).
Q. What becomes of the milky substance, called chyle?
A. It is absorbed by the vessels called “lacteals,” and poured into the veins on the left side of the neck.
Q. What becomes of the chyle after it is poured into the veins?
A. It then mingles with the blood, and is itself converted into blood.
Q. How does the oxygen we inhale mingle with the blood?
A. The oxygen of the air mingles with the blood in the lungs, and converts it into a bright red colour.
Q. What colour is the blood before it is oxydized in the lungs?
A. A dark purple. The oxygen turns it to a bright red.
Q. Why are persons so pale who live in close rooms and cities?
A. The blood derives its redness from the oxygen of the air inhaled; but, as the air in close rooms and cities is not fresh, it is deficient in oxygen, and cannot turn the blood to a beautiful bright red.
Q. Why are persons who live in the open air and in the country, of a ruddy complexion?
A. As the blood derives its bright red colour from the oxygen of the air inhaled, therefore, country-people (who inhale fresh air) are more ruddy than citizens.
Q. Why is not the air in cities so fresh as that in the country?
A. Because it is impregnated with the breath of its numerous inhabitants, the odour of its sewers, the smoke of its fires, and many other impurities.
Q. How does the combination of oxygen with the blood produce animal heat?
Q. What becomes of the nitrogen of the air, after the oxygen enters the blood?
A. The nitrogen is exhaled, and taken up by the leaves of trees and other vegetables. (see p.35).
Q. Why does the vitiated air (after the oxygen has been absorbed) come out of the mouth, and not sink into the stomach?
A. The vitiated air (being heated by the heat of the body) ascends naturally, and passes by the heavier fresh air (which we inhale) without obstruction or injury.
Q. If (both in combustion and in respiration) the oxygen of the air is consumed, and the nitrogen rejected—Why are not the proportions of the air destroyed?
A. Because the upper surface of vegetable leaves (during the day) gives out oxygen and absorbs nitrogen, and thus the proper balance is perpetually restored.
Q. Show how God has made animal and vegetable life dependent on each other?
A. Animals require oxygen to keep them alive, and draw it from the air by inspiration; the upper surface of leaves (all day long) gives out oxygen, and thus supplies the air with the very gas required by man and other animals.
Q. Do not animals exhale the very gas needed by vegetables?
A. Yes; animals reject the nitrogen of the air (as not suited to the use of animal life), but vegetables absorb it, as it is the food they live on; and thus the vegetable world restores the equilibrium of the air, disturbed by man and other animals.
Q. Is air a good conductor?
A. No; air is a very bad conductor.
Q. How is air heated?
A. By “convective currents.”
Q. What are meant by “convective currents?”
A. When a portion of air is heated, it rises upward in a current, carrying the heat with it: other colder air succeeds, and (being heated in a similar way)[Pg 246] ascends also; and these are called convective currents.
(“Convective currents;” so called from the Latin words, cum-vectus (carried with) because the heat is “carried with” the current.)
Q. Is air heated by the rays of the sun?
A. No; air is not heated (in any sensible degree) by the action of the sun’s rays passing through it.
Q. Why then is the air hotter on a sunny day, than on a cloudy one?
A. On a fine day, the sun heats the surface of the earth, and the air (resting on the earth) is heated by contact; as soon as it is heated it ascends, and other air succeeding is heated in a similar way, till all is heated by convection.
Q. If air be a bad conductor, why does hot iron get cold, by being exposed to the air?
A. A piece of hot iron exposed to the air, is made cold—1st—By “convection;” and
2ndly—By “radiation.”
Q. How is hot iron (exposed to the air) made cold by convection?
A. The air around the iron (being intensely heated by contact) rapidly ascends, carrying some of its heat with it:[Pg 247] other air succeeds, absorbs more heat, ascends, and gives place to that which is colder; till the hot iron is cooled completely down.
Q. How is hot iron cooled by radiation?
A. While the heat of the iron is being carried off by “convection,” it is throwing off heat (on all sides) by radiation.
Q. What is meant by radiation?
A. Heat emitted (in all directions) from any surface, by innumerable rays.
Q. Why is broth cooled by being left exposed to the air?
A. Hot broth throws off some heat by radiation; but it is mainly cooled down by convection.
Q. How is hot broth cooled down by convection?
A. The air resting on the hot broth (being heated) ascends; colder air succeeding absorbs more heat, and ascends also; and this process is repeated, till the broth is made cool.
Q. Why is hot tea and broth cooled faster, for being stirred about?
A. 1st—The agitation assists the liquor in bringing its hottest particles to the surface:
2ndly—The action of stirring agitates the air, and brings it quicker to the broth or tea: and
3rdly—As the hottest particles are more rapidly brought into contact with the air, therefore convection is more rapid.
Q. Why is hot tea, &c. cooled more rapidly by blowing it?
A. Because the heated air is blown more rapidly away; in consequence of which, cold air more rapidly succeeds to absorb heat from the surface of the tea or broth.
Q. If a shutter be closed in the day-time, the stream of light (piercing through the crevice) seems in constant agitation. Why is this?
A. The air (in the sun-beam piercing through the shutter-crevice) is more heated, than that in its neighbourhood; the convective current, therefore, is distinctly seen, where little motes and particles of dust are thrown into agitation by the violence of the current.
Q. Why is the gallery of a church or theatre hotter than the aisle or pit?
A. The hot air ascends from the bottom to the top of the room, and cold air (from the doors and windows) flies to the bottom to supply its place.
Q. Why does a crowded room produce head-ache?
A. Because we breathe air vitiated by the crowd.
Q. How does a crowd vitiate the air of a room?
A. Whenever we breathe, the elements of the air are separated in the lungs, some of the oxygen is absorbed by the blood, and some of it is converted into carbonic acid gas, and exhaled with the nitrogen.
Q. Is all the nitrogen rejected by the lungs?
A. Yes; all the nitrogen of the air is always exhaled.
Q. What is carbonic acid gas?
A. As carbon has a very great affinity for oxygen, therefore, whenever they are exposed to heat, they combine,[Pg 250] and form carbonic acid gas (or what is vulgarly called fixed air).
Q. Is carbonic acid gas wholesome?
A. No; it is quite fatal to animal life; and whenever it is inhaled, it acts like a narcotic poison, (producing drowsiness which ends in death).
Q. Why is a crowded room unwholesome?
A. Because the oxygen of the air is either absorbed by the lungs, or substituted for carbonic acid gas, which is a noxious poison.
Q. Mention the historical circumstances, so well known in connection with the “Black Hole of Calcutta.”
A. In the reign of George II, the Raja (or Prince) of Bengal[12] marched suddenly to Calcutta to drive the English from the country; as the attack was unexpected, the English were obliged to submit, and 146 persons were taken prisoners.
Q. What became of these prisoners?
A. They were driven into a place about 18 feet square, and 15 or 16 feet in height, with only two small grated windows. 123 of the prisoners died in one night; and (of the 23 who survived) the larger portion died of putrid fevers, after they were liberated in the morning.
Q. Why were 123 persons suffocated in a few hours, from confinement in this close hot prison-hole?
A. Because the oxygen of the air was soon consumed by so many lungs, and its place supplied by carbonic acid exhaled by the hot breath.
Q. Why do persons in a crowded church feel drowsy?
A. 1st—Because the crowded congregation inhale a large portion of the oxygen of the air, which alone can sustain vitality and healthy action: and
2ndly—The air of the church is impregnated with carbonic acid gas, which (being a strong narcotic) produces drowsiness in those who inhale it.
Q. Why did the captives in the black hole die sleeping?
A. 1st—Because the absence of oxygen quickly affects the vital functions, depresses the nervous energies, and produces a lassitude which ends in death: and
2ndly—The carbonic acid gas inhaled by the captives (being a narcotic poison) would also produce drowsiness and death.
Q. Why do persons, who are so much in the open air, enjoy the best health?
A. Because the air they inhale is much more pure.
Q. Why is country air more pure than the air in cities?
A. 1st—Because there are fewer inhabitants to vitiate the air:
2ndly—There are more trees to restore the equilibrium of the vitiated air: and
3rdly—The free circulation of air keeps it pure and wholesome (in the same way as running streams are pure and wholesome, while stagnant waters are the contrary).
Q. Why does the scantiness of a country population render the country air more pure?
A. Because the fewer the inhabitants,[Pg 253] the less carbonic acid will be exhaled; and thus country people will inhale pure oxygen, instead of air impregnated with the narcotic poison, called carbonic acid gas.
Q. Why do trees and flowers help to make country air wholesome?
A. Because trees and flowers absorb the carbonic acid generated by the lungs of animals, putrid substances, and other noxious exhalations.
Q. Why is the air of cities less wholesome than country air?
A. 1st—Because there are more inhabitants to vitiate the air:
2ndly—The sewers, drains, bins, and filth of a city, very greatly vitiate the air:
3rdly—The streets and alleys prevent a free circulation: and
4thly—Besides all this, there are fewer trees to absorb the excess of carbonic acid gas, and restore the equilibrium.
Q. Why are persons who live in close rooms and crowded cities, generally sickly?
A. Because the air they breathe is not pure, but is both defective in oxygen, and impregnated with carbonic acid gas.
Q. Where does the carbonic acid of close rooms and cities come from?
A. From the lungs of the inhabitants, the sewers, drains, and so on: besides, trees and gardens are not numerous enough to absorb the noxious gas as fast as it is generated.
Q. What becomes of the carbonic acid of crowded cities?
A. Some of it is absorbed by vegetables, and the rest is blown away by the wind, and diffused through the whole volume of the air.
Q. Does not this constant diffusion of carbonic acid affect the purity of the whole air?
A. No; because after it is thus diffused, it is carried to various lands, and absorbed in its passage by the vegetable world.
Q. Why do persons who ascend in balloons feel intense pain in their eyes and ears?
A. Because the air of the upper regions is more rarefied than the air on the earth; and the air inside their bodies (seeking to become of the same rarity) bursts through their eyes and ears, producing an intense pain.
Q. Why is it often painful, and difficult to breathe, on a mountain top?
A. Because the pressure of air on the mountain top is not so great as on the plain; and the air inside our bodies (seeking to become of the same rarity) bursts through the pores of the body, and produces great pain.
Q. Why do we feel oppressed just previous to a storm?
A. Because the air is greatly rarefied by heat and vapour; and the air inside us (seeking to become of the same rarity) produces an oppressive and suffocating feeling.
Q. Why do divers suffer great pain in their eyes and ears under water?
A. Because the air at the bottom of the sea is more dense than the air on the surface; and while the air inside the diver’s body is settling into the same density, he feels oppressed with pain, especially in the ears.
Q. Why is this pain felt especially about the ears of a diver?
A. The ear is fitted with a small[Pg 256] membrane called the drum (or tympanum), through which the dense air bursts, and the rupture very often produces incurable deafness.
Q. Why do our corns ache just previous to rain?
A. Previous to rain, the density of air is greatly lowered (as every one knows from the fall of the barometer); in consequence of an unequal pressure, our feet swell; but the hard corn, not being elastic, is painfully stretched and pressed.
(Some of this pain is due to electricity.)
Q. Why do cellars feel warm in winter?
A. As the external air has not free access into cellars, they remain at a pretty even temperature, which (in winter time) is about 10 degrees warmer than the external air.
Q. Why do cellars feel cold in summer time?
A. As the external air has not free access into cellars, they remain at a pretty even temperature, which (in summer time) is about 10 degrees colder than the external air.
Q. Why does lightning strike the oak-tree more frequently than any other tree?
A. 1st—Because the grain of the oak, being closer than that of any other tree, renders it a better conductor: and
2ndly—The sap of the oak contains a large quantity of iron in solution, which is a most admirable conductor of lightning.
Q. Why does air rust iron?
A. The oxygen of the air combines with the surface of the iron, and produces oxide of iron, which is generally called rust.
This rust is a species of combustion.
Q. Why does hot iron scale and peel off, when struck with a hammer?
A. The oxygen of the air very readily unites with the surface of the hot iron, and forms a metallic oxide (or rust) which scales off when struck with a hammer.
Q. Does iron rust in dry air?
A. No; iron undergoes no change in dry air.
Q. Why do stoves and fire-irons become rusty, in rooms which are not occupied?
A. Because the air is damp; and moist air oxidizes (or rusts) iron and steel.
Q. In what part of the year is it most difficult to keep stoves and fire-irons bright?
A. In autumn and winter; because the capacity of the air for holding water being on the decrease, its vapour is deposited on every-thing with which it comes in contact.
Q. Why does greasing iron prevent its becoming rusty?
A. Because grease prevents the humidity of air from coming in contact with the surface of the iron.
Q. Why do not stoves rust so frequently as pokers and tongs?
A. Because stoves are generally covered with plumbago, or black lead.
Q. What is plumbago, or black lead?
A. A mixture of charcoal and iron filings.
A most excellent varnish to prevent rust is made of 1 pint of fat oil varnish, mixed with 5 pints of highly rectified spirits of turpentine, rubbed on the iron or steel with a piece of sponge. This varnish may be applied to bright stoves and even mathematical instruments, without injuring their delicate polish.
Q. Why does ornamental steel (of a purple or lilac colour) rust more readily than polished white steel?
A. Because the lilac tinge is produced by partial oxidation; and the process which forms rust has, therefore, already commenced.
Q. How can lilac steel be kept free from rust?
A. By keeping it in a very dry place; for then no additional oxygen will come in contact with it, to increase its amount of rust.
Q. Do any other metals (besides iron) combine rapidly with oxygen?
A. Yes; copper, lead, mercury, and even silver to some extent.
Q. Why does copper tarnish?
A. The tarnish of copper is caused by its oxidation; that is, the oxygen of the air combines with the surface of the copper, and instead of rusting it, covers it with a dark tarnish.
Q. Why does lead lose its brightness, and become dull and of a darker hue, by being exposed to the air?
A. The vapour of the air combines[Pg 260] with the lead, and oxidizes its surface; but instead of becoming rusty, the surface becomes dull, and of a darker colour.
Q. Why is it difficult to keep silver bright?
A. Because the vapour of the air oxidizes its surface, and tarnishes it.
Q. Why do silver tea-pots and spoons tarnish more quickly than silver ore or bullion?
A. Because alloy (of some baser metal) is used to make it more hard and lasting; and this alloy oxidizes more quickly than silver itself.
Q. Why does German silver turn a dingy yellow in a few hours?
A. German silver has a great affinity for oxygen, and shows its oxidation by a sickly yellow tarnish, instead of rust.
Q. If quicksilver (or mercury) is tarnished like copper and lead,—Why does it preserve its brilliancy in barometers and thermometers?
A. Because air is excluded from it, and no moisture comes in contact with it to oxidize (or tarnish it).
Q. Is gold affected by the atmosphere?
A. Not readily: gold will never[Pg 261] combine with oxygen of itself, (or without aid).
Q. Which of the metals is capable of resisting oxidation altogether?
A. Plat’inum; in consequence of which, the graduated arcs of delicate instruments for observation are made of plat’inum instead of any other metal.
Q. Why is plat’inum used for the graduated arcs of delicate mathematical instruments, instead of any other metal?
A. Because it will never oxidize; but retain its bright surface in all weathers free from both rust and tarnish.
Q. Before plat’inum was discovered, which of the metals was employed for the same purpose?
A. Gold.
Platinum, (a white metal), so called from “plata,” the Spanish word for silver. It was first introduced into England by Mr. Wood, (A. D. 1749) from South America.
Q. For what other scientific purposes is plat’inum now used?
A. For crucibles in which acids are employed, and for galvanic batteries.
Q. Why are crucibles (in which acids are employed) made of plat’inum?
A. Because the acid would act upon[Pg 262] other metals, or upon glass, and prevent the experimenter’s success.
Q. Which of the metals have the greatest affinity to oxygen?
A. Those called potassium and sodium.
Potassium and sodium derive their names from potash and soda. Potassa is the oxide of potassium; and soda is the oxide of sodium.
Q. How is the affinity of potassium and sodium for oxygen shewn?
A. They decompose water the moment they are brought into contact with it.
Q. What effect has potassium on water?
A. It catches fire the moment it is thrown into water, and burns with a vivid flame, which is still further increased by the combustion of hydrogen separated from the water.
(N.B. Water is composed of oxygen and hydrogen; and potassium separates the two gases.)
Q. What effect has sodium on water?
A. It does not take fire as potassium does, but undergoes very rapid oxidation.
Q. Is the furr of kettles an oxide?
A. No; the furr (or deposit of boiling water) is a precipitate of lime[Pg 263] and mineral salt, separated from the water by the process of boiling.
Q. Is not this furr of boiling water often dangerous?
A. Yes; especially in tubular boilers, such as those employed in railways.
Q. Why is this furr especially troublesome in railway engines?
A. Because it is a bad conductor of heat; in consequence of which, it hinders the evaporating effect of the fire, and prevents the economy of fuel.
Q. Why is this furr especially dangerous in railway engines?
A. Because when it is deposited in the boilers, they are likely to become over-heated; and then explosion will take place from the sudden generation of highly elastic steam.
Q. Why cannot railway engines be fed with brackish water?
A. Because brackish water contains mineral salt, which makes a much larger deposit of furr, than that which contains only vegetable matter.
Q. What is choke damp?
A. Carbonic acid gas accumulated at the bottom of wells and pits, which renders them noxious, and often fatal.
Q. Why is not this carbonic acid taken up by the air, and diffused, as it is in cities?
A. Because (being heavier than common air) it cannot rise from the well or pit; and no wind can get to it to blow it away.
Q. Is carbonic acid wholesome?
A. No; it is fatal to animal life, when inhaled through the mouth; acting on the stomach, as a narcotic poison (i. e.. a poison which produces death from drowsiness).
Q. How can any one know, if a place be infested with carbonic acid gas?
A. If a pit or well contain carbonic acid, a candle (let down into it) will be[Pg 265] instantly extinguished. The rule, therefore, is this—Where a candle will burn, a man can live; but what will extinguish a candle, will also destroy life.
Q. Why does a miner lower a candle into a mine, before he descends?
A. Because the candle will be extinguished, if the mine contains carbonic acid gas: but if the candle is not extinguished, the mine is safe, and the man may fearlessly descend.
Q. Why are persons sometimes killed, by leaning over beer vats?
A. Vats (where beer has been made) contain a large quantity of carbonic acid gas, produced by the “vinous fermentation” of the beer; and when a man incautiously leans over a beer vat, and inhales the carbonic acid, he is immediately killed thereby.
Q. Why are persons often killed, who enter beer vats to clean them?
A. Carbonic acid (being heavier than atmospheric air) often rests upon the bottom of a vat: when, therefore, a person enters the vat, and stoops to clean the[Pg 266] bottom, he inhales the pernicious gas, which kills him.
Q. Why are the jungles of Jarva and Hindostan so fatal to life?
A. Because vast quantities of carbonic acid are thrown off by decaying vegetables; and (as the wind cannot penetrate the thick brushwood to blow it away) it settles there, and destroys animal life.
Q. Why are persons sometimes killed by having a charcoal fire in their bed-rooms?
A. When charcoal is burned, the carbon of the charcoal unites with the oxygen of the air, and forms carbonic acid gas, which is a narcotic poison.
Q. Why does the carbonic acid gas of a charcoal fire rise and disperse itself about the room; whereas the carbonic acid gas of a beer vat settles near the floor?
A. The carbonic acid gas of a charcoal fire is heated by the combustion of the fuel, and rises; but the carbonic acid gas of a beer vat is not heated, and, therefore, rests on the bottom of the vat.
Q. Why do persons throw lime into bins to prevent their offensive smell, in summer time?
A. Bins contain large quantities of[Pg 267] carbonic acid gas, which readily combines with lime, and produces “carbonate of lime,” which is entirely free from all offensive odour.
Q. Why do persons throw lime into sewers in summer time?
A. Sewers (like bins) contain large quantities of carbonic acid, which readily combines with lime, and produces carbonate of lime; and thus the offensive gas of the sewer is neutralized.
Q. Can carbonic acid be removed in any way besides by lime?
A. Yes; water thrown into a pit will disperse the carbonic acid.
Q. What effect has water on carbonic acid gas?
A. Water (under pressure) absorbs carbonic acid gas; and parts with it (when the pressure is removed) in the form of effervescence.
Q. Why does aerated water effervesce, when the cork is removed?
A. While the cork was fastened down, the water absorbed the carbonic acid; but the moment the pressure is[Pg 268] removed (by taking out the cork) the gas is given out with effervescence.
Q. Why does soda water effervesce?
A. Soda water contains 8 times its own bulk of carbonic acid gas, which makes its escape in effervescence, the moment that the cork is removed.
Q. Why does ginger pop fly about in froth, when the string of the cork is cut?
A. All vinous fermentation produces carbonic acid gas. While the cork is fast, the water of the liquor absorbs the carbonic acid; but the moment that the pressure is removed, the gas is given off in effervescence.
Q. Why does bottled ale froth, more than draught ale?
A. Because the pressure is greater in a bottle than in a tub which is perpetually tapped: and effervescence is always produced in proportion to the pressure.
Q. Why does bottled ale and porter become “lively” and frothy by being set before the fire?
A. The heat of the fire expands the air (between the liquid and the cork),[Pg 269] and as this air expands, it presses the liquid down, which causes effervescence.
Q. What produces the froth of bottled porter?
A. The carbonic acid gas, produced by its vinous fermentation; which is absorbed by the liquor so long as the bottle is well corked, but is given off in froth as soon as the pressure of the cork is removed.
Q. What gives the pleasant acid taste to soda water, ginger beer, champagne, and cider?
A. The presence of carbonic acid, generated by fermentation, and liberated by effervescence when the pressure of the cork is removed.
Q. Why does fresh spring water sparkle, when poured from one vessel to another?
A. Because fresh spring and pump water contain carbonic acid; and it is the presence of this gas which makes the water sparkle.
Q. What is the fermentation of beer and wine?
A. The production of carbonic acid gas and al’cohol.
Q. How is carbonic acid gas produced by fermentation?
A. Malt and fruit both contain sugar; and sugar consists of carbon, oxygen, and hydrogen. In fermentation, a part of the carbon and oxygen of the sugar escape, in the form of carbonic acid gas.
Carbonic acid gas is a compound of carbon and oxygen, in the following proportions:—3 lbs. of carbon and 8 lbs. of oxygen will form 11 lbs. of carbonic acid gas. Now, 100 lbs. of white sugar contains 43 lbs. of carbon; 50 lbs. of oxygen; and 7 lbs. of hydrogen.
Q. How is al’cohol produced by fermentation?
A. The hydrogen of the sugar combines with the residue of the oxygen and carbon to form “al’cohol.”
Q. What is al’cohol?
A. Al’cohol is the spirit of wine or beer, obtained by fermentation.
(100 gallons of alcohol consist of 38 gallons of oxygen; 43-1/2 of carbon; 15 of hydrogen; and 3-1/2 of nitrogen.)
Q. Why is barley malted?
A. Because germination is produced by the artificial heat; and in germination the starch of the grain is converted into sugar.
Q. How is barley malted?
A. The barley is moistened with water, and heaped up; by which means, great heat is produced, which makes the barley sprout.
Q. Why is not the barley suffered to grow, as well as sprout?
A. Plants in the germ contain more sugar than in any other state; as soon as the germ puts forth shoots, the sugar of the plant is consumed, to support the shoot.
Q. How is barley prevented from shooting, in the process of malting?
A. The barley is put into a kiln as soon as it sprouts; and the heat of the kiln checks or destroys the young shoot.
Q. Why is yeast put into beer to make it work?
A. Yeast supplies the beer with nitrogen, which is one of the ingredients of alcohol.
Alcohol consists of oxygen, carbon, and hydrogen, (obtained from the sugar of malt), and nitrogen, (obtained from yeast).
Q. Why is it not needful to put yeast into wine?
A. Because fruit contains carbon, hydrogen, oxygen, and nitrogen, in the form of “gluten;” and, therefore, ferments spontaneously.
(Gluten is explained fully in the Appendix.—Turn to the word in the Index.)
Q. Does not malt contain carbon, hydrogen, oxygen, and nitrogen, as well as fruit?
A. No; the sugar of malt contains carbon, hydrogen, and oxygen, but no nitrogen; in consequence of which, yeast (which contains nitrogen) is added to the wort.
Q. Why do not grapes ferment while they hang on the vine?
A. 1st—Because the skin lets out the water of the pulp, which causes the grapes to shrivel and dry up: and
2ndly—The skin prevents the admission of oxygen into the pulp, from the air without.
Q. What is the froth or scum of fermented liquors?
A. Carbonic acid gas, which (being heavier than common air) settles on the top of the liquor, in the form of scum.
Q. Why does a small piece of raw meat, or a few raisins improve flat beer?
A. 1st—Because they supply it with nitrogen to form it into al’cohol.
2ndly—As the raw meat, &c. putrifies, it gives off carbonic acid gas into the beer, which gives it “life.”
Q. Why is beer flat, if the cask be open too long?
A. Because too much of the carbonic acid gas (produced by fermentation) is suffered to escape.
Q. How is the carbonic acid gas of beer generated?
A. The saccharine (or sugar) of the malt is converted by fermentation into carbonic acid gas and alcohol.
Q. Why does beer turn flat, if the vent peg be left out of the tub?
A. Because the carbonic acid gas escapes through the vent hole.
Q. Why will not beer run out of the tub, till the vent peg is taken out?
A. When the tap is turned, air rushes through the tap into the bottom of the tub, and holds the liquor in.
The upward pressure of air is illustrated by the
following simple experiment:—Fill a wine-glass with water; cover the top of the glass with a piece of writing paper; turn the glass topsy turvy, and the water will not run out. The paper is used merely to give the air a medium sufficiently dense to act against.
Q. Why does the beer run freely, immediately the vent peg is taken out?
A. As soon as the vent peg is taken out, air rushes through the vent hole at the top of the tub,—presses the liquor down, and forces it through the tap.
Q. Why does liquor flow reluctantly out of a bottle held upside down?
A. Because the upward pressure of the air prevents the liquor from flowing out.
Q. Why should a bottle be held obliquely, in order to be emptied of its liquor?
A. Because air will then flow into the bottle, and help the liquor out by balancing the upward pressure.
Q. Why does wine (poured from a bottle quickly) spirt about without going into the decanter?
A. The liquor fills the top of the decanter (like a cork), and leaves no room for the air inside to escape; therefore, the decanter (being full of air) refuses to admit the wine.
Q. Why is beer made stale, by being exposed to the air?
A. Because air absorbs its carbonic acid, which gave it “life.”
Q. Why is porter made stale, by being exposed to the air?
A. Because air absorbs its carbonic acid, which gave it “life.”
Q. Why does the effervescence of soda water and ginger beer so soon go off?
A. Because air absorbs the carbonic acid, which produced the effervescence.
Q. Why is boiled water flat and insipid?
A. Because the whole of the carbonic acid is expelled by boiling, and absorbed by the air.
Q. Why does water become flat and insipid, after it has been drawn some time?
A. Because air absorbs its carbonic acid; and when its carbonic acid is absorbed, the water is flat and insipid.
Q. Why should spring water (used for washing) be exposed to the air?
A. Spring water contains carbonic acid; but (by being exposed to the air) this carbonic acid is absorbed, and the water becomes more soft.
Q. Why does yeast make bread light?
A. Flour contains a small portion of saccharine matter (or sugar); and the yeast (mixing with this) produces fermentation, as it does in brewing.
Q. How does fermentation make the dough rise?
A. During fermentation, carbonic acid gas is evolved; but the sticky texture of the dough will not allow it to escape, so it forces up little bladders all over the dough.
Q. Why is dough placed before the fire?
A. 1st—Because the heat of the fire increases the fermentation: and
2ndly—It expands the gas which is confined in the little bladders; in consequence of which, the bladders are blown up larger, and the dough becomes lighter and more porous.
Q. Why is bread heavy, if the dough be removed from the fire?
A. Because the dough gets cold, and then the air in the bladders condenses,—the paste falls,—and the bread is close and heavy.
Q. Whence does the heat of fire arise?
A. The carbon of fuel (when heated) combines with the oxygen of the air, and produces carbonic acid gas: again, the hydrogen of the fuel combining with other portions of oxygen, condenses into water; by which chemical actions heat is evolved.
Q. Whence does the heat of our own body arise?
A. The carbon of the blood combines with the oxygen of the air inhaled, and produces carbonic acid gas; which produces heat in a way similar to burning fuel.
Q. Whence does the heat of a dunghill arise?
A. The straw, &c. of the dunghill undergoes fermentation as it decays: the fermentation produces carbonic acid gas, and heat is evolved by a species of combustion (as in the two former cases).
Q. What changes do vegetables undergo from putrefaction?
A. The hydrogen of the vegetables combines with the oxygen of the air, and forms water: again, the carbon of the[Pg 278] vegetables combines with oxygen of the air, and forms carbonic acid gas. Putrefaction, therefore, is only another species of combustion.
Q. What changes do animal bodies undergo from putrefaction?
A. The same as vegetables, with this addition—they give out ammonia, sulphur, and phosphorus also; which causes the offensive smell of putrefying animal bodies.
Q. Why is lime heated by a kiln?
A. All marl and chalk abound in carbonic acid; and (when heated by a fire) the carbonic acid flies off in gas, producing great heat.
Q. What is mortar?
A. Lime mixed with sand and water.
Q. What is lime?
A. Lime-stone burnt produces lime.
Q. Why is the lime-stone burnt, in order to make it into lime?
A. The fire expels the carbonic acid, and converts the hard lime-stone into a loose powder.
Q. Why does mortar become hard, after a few days?
A. Because the lime re-imbibes the carbonic acid of the air, which was expelled by fire; and the loose powder again becomes as hard as the original lime-stone.
Q. Why is mortar adhesive?
A. When the carbonic acid is expelled, the hard lime-stone is converted into a loose powder, which (being mixed with sand and water) becomes a soft and sticky plaster; but, as soon as it is placed between bricks, it imbibes carbonic acid again, and hardens into lime-stone.
Q. What is choke-damp?
Q. What is marsh-gas or fire-damp?
A. Carburetted hydrogen gas accumulated on marshes, in stagnant waters, and coal pits; it is frequently called “inflammable air.”
Q. What is carburetted hydrogen gas?
A. Carbon combined with hydrogen.
Q. How may carburetted hydrogen gas be procured on marshes?
A. By stirring the mud at the bottom of any stagnant pool, and collecting the gas (as it escapes upwards) in an inverted glass vessel.
Q. What is coal gas?
A. Carburetted hydrogen extracted from coals, by the heat of fire.
Q. Why is carburetted hydrogen gas called fire-damp, or inflammable air?
A. Because it very readily catches fire and explodes, when a light is introduced to it.
Q. Why is carburetted hydrogen gas frequently called marsh gas?
A. Because it is generated in meadows and marshes from putrefying vegetable substances. (See ignis fatuus, p. 285).
Q. What gas is evolved by the wick of a burning candle?
A. Carburetted hydrogen gas: that is, the carbon and hydrogen of the tallow combine into a gas from the heat of the flame; and this gas is carburetted hydrogen, or inflammable air.
Q. Why do coal-mines so frequently explode?
A. Because the carburetted hydrogen gas (which is generated in these mines by the coals) explodes, when a light is incautiously introduced.
Q. How can miners see in the coal-pits, if they may never introduce a light?
A. Sir Humphrey Davy invented a lantern for the use of miners, called “the Safety Lamp,” which may be used without danger.
Q. Who was Sir Humphrey Davy?
A. A very clever chemist, born in Cornwall. (1778—1829).
Q. What kind of thing is the safety lamp?
A. It is a kind of lantern covered with a fine gauze wire, instead of glass or horn.
Q. How does this fine gauze wire prevent an explosion in the coal mine?
A. 1st—Because flame will never pass through fine gauze wire: and
2ndly—Though the wire get red-hot, it will not ignite the gas; for carburetted hydrogen gas can be ignited only by flame.
(N. B. The interstices of the gauze wire must not exceed the 7th of an inch in diameter.)
Q. Why will not flame pass through very fine wire-gauze?
A. Because the metal wire is a very rapid conductor of heat; and when the flame of burning gas in the lamp reaches the wire gauze, the heat (which is needful to produce flame) is conducted away by the wire, and the flame is extinguished.
Q. Does the gas of the coal-pit get through the wire gauze into the lantern?
A. Yes; but the inflammable gas[Pg 283] ignites and burns inside the lamp: as soon, however, as this is the case, the miner is in danger, and should withdraw.
Q. Why is the miner in danger, if the gas ignites and burns in the inside of the safety-lamp?
A. Because the heat of the burning gas will soon destroy the wire gauze, and then the flame (being free) will set fire to the mine.
Q. From what does the very offensive effluvia of church-yards arise?
A. From a gas called phosphuretted hydrogen; which is phosphorus combined with hydrogen gas.
Q. What is phosphorus?
A. A pale amber-coloured substance,[Pg 284] resembling wax in appearance. The word is derived from two Greek words, which mean “to produce or carry light.” (φῶς-φέρεινφῶς).
Q. How is phosphorus obtained?
A. By heating bones to a white heat; by which means the animal matter and charcoal are consumed, and what is left is called “phosphate of lime.”
Q. How is phosphate of lime converted into phosphorus?
A. It is reduced to powder, and mixed with sulphuric acid; which (being heated and filtered) is converted into phosphorus.
Q. Of what are lucifer matches made?
A. Of phosphorus; and above 250 thousand lbs. of phosphorus are used every year in London alone, merely for the manufacture of lucifer matches.
Q. Why does a putrefying dead body smell so offensively?
A. From the phosphuretted hydrogen gas, which always arises from putrefying animal substances.
The escape of ammonia and sulphur contributes also to this offensive effluvia.
Q. What is the cause of the ignis fatuus, Jack o’Lantern, or Will o’the Wisp?
A. This luminous appearance (which haunts meadows, bogs, and marshes) arises from the gas of putrefying animal and vegetable substances; especially decaying fish.
Q. What gases arise from these putrefying substances?
A. Phosphuretted hydrogen gas from putrefying animal substances: and
Carburetted hydrogen, (or inflammable gas) from fermenting vegetable matters.
Some persons erroneously think that the Aurora Borealis, or Northern Lights, may be attributed to the same gases, burning in the upper regions of the air.
Q. How are these gases ignited on bogs and meadows?
A. By the electricity of the air, the rays of the sun, some accidental spark, the lamp of some traveller, or in some similar way.
And sometimes from the spontaneous combustion of some dung-heaps, &c. in the locality.
Q. Why does an ignis fatuus or Will o’the Wisp fly from us when we run to meet it?
A. When we run towards an ignis[Pg 286] fatuus, we produce a current of air, which drives the light gas forwards.
Q. Why does an ignis fatuus run after us, when we flee from it in fright?
A. When we run away from the ignis fatuus, we produce a current in the way we run, which attracts the light inflammable gas in the same course.
Q. Is not a kind of Jack o’Lantern sometimes produced by an insect?
A. Yes; a swarm of luminous insects sometimes passes over a meadow, and produces an appearance exactly like that of the ignis fatuus.
Q. May this meteoric appearance be attributed to any other cause, besides those mentioned?
A. Yes; if many horses, sheep, pigs, or oxen, are pastured on a meadow, the animal vapour arising from them (strongly electrified by the air) will ignite, and produce a luminous appearance.
Q. May not many ghost stories have risen from some ignis fatuus lurking about church-yards?
A. Perhaps all the ghost stories (which deserve any credit at all) have[Pg 287] arisen from the ignited gas of church-yards lurking about the tombs, to which fear has added its own creations.
Q. What is wind?
A. Wind is air in motion.
Q. What puts the air in motion, so as to produce wind?
A. The principal causes are the variations of heat and cold, produced by the succession of day and night, and the four seasons.
Q. What effect has heat upon the air?
A. Heat rarefies the air, and causes it to expand.
Q. How do you know that heat causes the air to expand?
A. If a bladder half full of air (tied tight round the neck), were laid before a[Pg 288] fire, the heat of the fire would expand the air so much, that the bladder would soon be entirely inflated; (in this case, the air in the bladder is expanded to twice its original bulk, by the heat of the fire).
Q. What effect is produced upon air by rarefaction?
A. It causes the air to ascend through colder strata, as a cork (put at the bottom of a basin of water) would ascend through the water.
Q. How do you know that rarefied air ascends?
A. When a boy sets fire to the cotton of his balloon, the flame heats the air inside the balloon; and the air becomes so light, that it ascends, and carries the balloon with it.
Q. What effect is produced upon air by cold?
A. Air is condensed by cold, or squeezed into a smaller compass; in consequence of which, it becomes heavier, and descends towards the ground.
Q. How do you know that air is condensed by cold?
A. After the bladder is fully inflated, (by lying before the fire), if it be taken away from the fire, the bladder will collapse, and show that it is not half full.
Q. What is meant by the bladder “collapsing?”
A. The skin will become wrinkled, shrivelled, and flabby, because there is not sufficient air inside to fill it out.
Q. How do you know that condensed air will descend?
A. As soon as the cotton of the balloon is burnt out, the air inside becomes cold again, and the balloon falls to the earth.
Q. Does the sun heat the air as it does the earth?
A. No; the air is not heated by the rays of the sun, because air (like water) is a very bad conductor.
Q. How is the air heated?
A. By convection, thus:—The sun heats the earth, and the earth heats the air resting upon it; the air thus heated rises, and is succeeded by other air, which[Pg 290] is heated in a similar way, till all is warmed by “convective currents.”
Q. What is meant by “convective currents of air?”
A. Streams of air heated by the earth, which rise upwards and carry heat with them, are called “convective currents” of hot air.
Q. Is the air in a room in perpetual motion, as the air abroad is?
A. Yes; there are always two currents of air in the room we occupy, one of hot air flowing out of the room, and another of colder air flowing into the room.
Q. How do you know, that there are these two currents of air in every occupied room?
A. If I hold a lighted candle near the crevice at the top of the door, the flame will be blown outward (towards the hall); but if I hold the candle at the bottom of the door, the flame will be blown inwards (into the room).
Q. Why would the flame be blown outwards (towards the hall), if the candle were held at the top of the door?
A. Because as the air of the room is[Pg 291] warmed by the fire, &c., it ascends; and (floating about the upper part of the room) some of it escapes through the crevice at the top of the door, and thus produces a current of air outwards (into the hall).
Q. Why would the flame be blown inwards (into the room), if the candle were held at the bottom of the door?
A. Because after the warm air of the room has ascended to the ceiling, or made its escape into the hall, &c., a partial vacuum is made at the bottom of the room; and cold air (from the hall) rushes under the door to supply the void.
Q. What is meant by a “partial vacuum being made, at the bottom of the room?”
A. A vacuum means a place from which the air has been taken: and a “partial vacuum” means, a place from which a part of its air has been taken away. Thus when the air on the floor ascends to the ceiling, a partial vacuum is made on the floor.
Q. And how is the vacuum filled UP again?
A. It is filled up by colder air, which[Pg 292] rushes (under the door, and through the window crevices) into the room.
Q. Give me an illustration.
A. If I dip a pail into a pond and fill it with water, a hole (or vacuum) is made in the pond as big as the pail; but the moment I draw the pail out, the hole is filled up by the water around.
Q. Show how this illustration applies.
A. The heated air which ascends from the bottom of a room, is as much taken away, as the water in the pail; and (as the void was instantly supplied by other water in the pond) so the void of air is supplied by a current from without.
Q. What is the cause of wind?
A. The sun heats the earth, and the earth heats the air resting upon it; as the warm air ascends, the void is filled up by a rush of cold air to the place, and this rush of air we call wind.
Q. Does the wind always blow?
A. Yes; there is always some motion in the air; but the violence of the motion is perpetually varying.
Q. Why is there always some motion in the air?
A. As the earth is always turning round, the vertical rays of the sun are always varying.
Q. What do you mean by “the vertical rays of the sun?”
A. The rays made at noon-day: when the sun is in a direct line above any place, his rays are said to be “vertical” to that place.
Q. How are the vertical rays of the sun always varying?
A. Suppose the brass meridian of a globe to represent the vertical rays of the sun; as you turn the globe round, different parts of it will pass under the brass rim, in constant succession.
Q. And is it noon-day to the place over which the sun is vertical?
A. Yes; as each place passes under the brass meridian, it is noon-day to one half, and mid-night to the other.
Q. Show how this rotation of the earth affects the air.
A. If we suppose the brass meridian[Pg 294] to be the vertical sun, the whole column of air beneath will be heated by the noon-day rays; that part which the sun has left, will become gradually colder and colder; and that part to which the sun is approaching, will grow constantly warmer and warmer.
Q. Then there are three qualities of air about this spot?
A. Yes; the air over the place which has passed the meridian is cooling: the air under the vertical sun is the hottest; and the air which is over the place about to pass under the meridian, is increasing in heat.
Q. How does this variety in the heat of air produce wind?
A. The air always seeks to preserve an equilibrium; so the cold air rushes to the void, made by the upward current of the warmer air.
Q. Why does not the wind always blow one way, following the direction of the sun?
A. Because the direction of the wind is subject to perpetual interruptions from hills and valleys, deserts and seas.
Q. How can hills and mountains alter the course of the wind?
A. Suppose a wind, blowing from the north, comes to a mountain, as it cannot pass through it, it must either rush back again, or fly off at one side (as a marble when it strikes against a wall).
Q. Do mountains affect the wind in any other way?
A. Yes; many mountains are capped with snow, and the warm air is condensed as it comes in contact with them; but as soon as the temperature of the wind is changed, its direction may be changed also.
Suppose A B C to be three columns of air. A, the column of air which is cooling down; B, the column to which the sun is vertical; and C, the column which is to be heated next. In this case the cold air of A will rush towards B C, because the air of B and C is hotter than A. But suppose now C to be a snow-capped mountain. As the hot air of B reaches C, it is chilled; and (being now colder than the air behind) it rushes back again towards A, instead of following the sun.
Q. How can the ocean affect the direction of the wind?
A. When the ocean rolls beneath the vertical sun, the water is not made so hot as the land; and (as another change of[Pg 296] temperature is produced) another obstacle is offered to the uniform direction of the wind.
Q. Why is not the water of the sea made so hot by the vertical sun, as the surface of the land?
A. 1st—Because the evaporation of the sea is greater than that of the land:
2ndly—The waters are never still: and
3rdly—The rays of the sun strike into the water, and are not reflected from its surface, as they are by land.
Q. Why does the evaporation of the sea prevent its surface from being heated by the vertical sun?
A. As water absorbs heat by being converted into vapour; the surface of the sea is continually losing heat by evaporation.
Q. How does the motion of the sea prevent its surface from being heated by the vertical sun?
A. As one portion is heated it rolls away, and is succeeded by another; and this constant motion prevents one part of the sea from being heated more than another.
Q. How is the wind affected by the sea?
A. When air from the hot earth reaches the sea, it is often condensed, and either rushes back again, or else its violence is very greatly abated.
Q. Do clouds affect the wind?
A. Yes. As passing clouds screen the direct heat of the sun from the earth, they diminish the rarefication of the air also: and this is another cause why neither the strength nor direction of the wind is uniform.
Q. Would the winds blow regularly from east to west, if these obstructions were removed?
A. Without doubt they would. If the whole earth were covered with water, the winds would always follow the sun, and blow from east to west. Their irregularity is owing to the interspersion of sea and land, and the irregularities of the earth’s surface.
Q. Do winds never blow regularly?
A. Yes; in those parts of the world, where these obstructions do not exist; as on the Atlantic and Pacific Ocean, the winds are pretty uniform.
Q. What are the winds, which blow over the Atlantic and Pacific Ocean, called?
A. They are called “Trade Winds.”
Q. Why are they called trade winds?
A. Because (as they blow uniformly in one direction) they are very convenient to those who carry on trade by means of these oceans.
Q. In what direction do the trade winds blow?
A. That in the northern hemisphere blows from the north-east: that in the southern hemisphere from the south-east.
Q. Why do they not blow from the full north and south?
A. Because the polar current, combining with the equatorial current, give the wind a new direction.
Q. What is the cause of the equatorial current?
A. The rotation of the earth upon its axis.
Q. What is the cause of the polar current?
A. As the heat in the torrid zone is always greatest, and at the poles the least, therefore a constant current of air rushes from the poles towards the equator.
Q. How does the combination of these two currents give a new direction to them both?
A. When these currents of air meet at the equator, they clash together, and fly off in a new direction.
Q. Do trade winds blow from the north-east and south-east all the year round?
A. Yes, in the open sea; that is, in the Atlantic and Pacific Oceans for about 30 degs. each side of the equator.
Q. Do the trade winds blow uniformly from north-east and south-east in the indian ocean?
A. No; nor yet in those parts of the Atlantic and Pacific which verge on the land.
Q. Why do not the trade winds blow uniformly from north-east and south-east in the indian ocean?
A. Because when Arabia, Persia, India, and China, are exposed to the enormous heat of their summer sun, the air is so rarefied, that the colder air from the south pole rushes towards these nations, and not to the equator; in consequence of which, a south-west wind is produced for six months of the year.
Q. How does it blow for the other 6 months?
A. When the sun has left the northern side of the equator for the southern, then the southern part of the torrid zone is most heated; and the cold air from the north (rushing towards the southern tropic) is diverted to the north-east, where it continues for the other six months of the year.
Q. What are the six-month trade winds called?
A. They are called monsoons; and blow from the north-east from September to April, and from the south-west for the other six months of the year.
Q. Have we any regular winds in England?
A. No; our island (having a continent on one side, and a sea on the other) has a most variable climate.
Q. Have the winds in England no general direction throughout the year?
A. We generally find that easterly winds prevail during the spring of the year, and westerly winds are most common in the summer and autumn.
S-West winds are most frequent in July and August. N-East winds in January, March, April, May, June; and most seldom in July, September, and December.
Q. When are the winds in England generally the highest?
A. The winds in December and January are generally the highest. Those in February and November the next; and those in August and September the least boisterous.
Q. Why are the winds of Europe generally highest in December and January?
A. Because the sun is furthest south in those months; and (as the heat in these northern regions rapidly decreases) the contrast between our temperature and that of the torrid zone is greater in December and January, than in any other two months throughout the year.
Q. Why does this contrast of heat increase the violence of the winds?
A. As the air always seeks to preserve an equilibrium, therefore the greater the contrast, the more violent will be the rush of air to equalize the two volumes.
Q. Why are the winds in Europe generally the most placid during the months of September and August?
A. August and September are our[Pg 302] warmest months, when we approach nearer to the heat of the torrid zone than in any other two months; therefore, the air (to and from the equator) moves with less velocity in our northern hemisphere.
Q. Show the goodness and wisdom of God in the constant tendency of air to equilibrium.
A. If the cool air of the polar regions did not rush into the torrid zone, it would become so hot, that no human being could endure it. If (on the other hand) the hot air from the torrid zone did not modify the polar regions, they would soon become insufferably cold.
Q. Why are east winds in England generally dry?
A. Because, as they come over the vast continents of Asia and Europe, they absorb very little water.
Q. Why does their imbibing so little water make them dry winds?
A. Being thirsty when they reach our island, they readily imbibe moisture from the air and clouds; and, therefore, bring dry weather.
Q. Why is the north wind in England generally cold?
A. The north wind comes from the polar regions, over mountains of snow, and seas of ice; in consequence of which, it is very cold.
Q. Why are north winds in England generally dry and biting?
A. As they come from regions colder than our own, they are warmed by the heat of our island; and (as their temperature is raised) they absorb moisture from every thing they touch; in consequence of which, they are both dry and parching.
Q. Why is the south wind generally warm in England?
A. The south wind comes over the hot sandy deserts of Africa, and is heated by the land it traverses.
Q. Why does the south wind often bring us rain?
A. The south wind (being much heated by the hot sands of Africa) imbibes water very plentifully, as it passes over the Mediterranean Sea and British Channel.
Q. Why does the saturation of the south wind cause rain?
A. As soon as it reaches our cold climate, it is condensed, and its vapour is squeezed out (as water from a wet sponge).
Q. Why are west winds in England generally rainy?
A. The west winds come over the Atlantic Ocean, and are laden with vapour: if, therefore, they meet with the least chill, some of the vapour is squeezed out.
Q. Why is a fine clear day sometimes overcast in a few minutes?
A. Because some sudden change of temperature has condensed the vapour of the air into clouds.
Q. Why are clouds sometimes dissipated quite as suddenly?
A. Because some dry wind (blowing over the clouds) has imbibed their moisture, and carried it off in invisible vapour.
Q. Why does a south-west wind bring us rain?
A. As it comes from the torrid zone, and crosses the ocean, the hot wind is[Pg 305] laden with vapour; and as some of the heat escapes (as soon as it reaches our northern island) the vapour is condensed, and precipitated as rain.
Q. Why does a north-east wind rarely bring rain?
A. As it comes from a climate colder than our own, its capacity for imbibing vapour is increased when it reaches our island; in consequence of which, it dries the air, dispels the clouds, and promotes evaporation.
Q. Why does wind sometimes bring rain, and sometimes fine weather?
A. If the wind be colder than the clouds, it will condense their vapour into rain: if the wind be warmer than the clouds, it will dissolve them, and cause them to disappear.
Q. Why are March winds dry?
A. Because they generally blow from the east or north-east; and, therefore, sweep over the continent of Europe.
Q. What is the use of March winds?
A. They dry the soil (which is saturated with the floods of February), break[Pg 306] up the heavy clods, and fit the land for the seeds which are committed to it.
Q. Why does “March come in like a lion?”
A. Because it comes in with blustering east winds, which are essential to dry the soil, which would otherwise rot the seed committed to it.
Q. Why does “March go out like a lamb?”
A. Because the water (evaporated by the high winds) falls again in showers to fertilize the earth, and breaks the violence of the winds.
Q. Why is it said that “A bushel of March dust is worth the king’s ransom?”
A. Because it indicates that there has been a continuance of dry weather; and unless March be dry, the seed will rot in the wet soil.
Q. Why is it said “A dry cold March never begs bread?”
A. Because the dry cold winds of March prepare the soil for seeds, which germinate, and produce fruit in the autumn.
Q. Why is it said that “A wet March makes a sad autumn?”
A. Because, if March be wet, so much of the seed rots in the ground, that the autumn crops are spoiled.
Q. Why is it said that “March flowers make no summer bowers?”
A. Because, if the spring be very mild, vegetation gets too forward, and is pinched by the nightly frosts, so as to produce neither fruits nor flowers.
Q. Why is it said “A late spring makes a fruitful year?”
A. Because if the vegetation of spring be backward, the frosty nights will do no harm; for the fruits and flowers will not put forth their tender shoots, till the nights become too warm to injure them.
Q. Why is it said that “April showers bring May flowers?”
A. Before seeds can germinate, three things are essential:—Darkness, Heat, and Moisture. April showers supply the principal nourishment on which seeds depend for existence.
Q. Does rain-water possess any fertilizing properties besides that of mere moisture[Pg 308]?
A. Yes; rain-water contains “ammonia,” to which much of its fertilizing power may be attributed.
(Ammonia is a compound of nitrogen and hydrogen. Common hartshorn is only ammonia and water.)
Q. Why has God made November a very rainy month?
A. Because the rain hastens the putrefaction of the fallen leaves, and this makes the earth fertile.
Q. Why is there more rain from September to March than from March to September?
A. From September to March, the temperature of the air is constantly decreasing; on which account, its capacity for holding vapour is on the decrease, and the vapour is precipitated as rain.
Q. Why is there less rain from March to September, than from September to March?
A. From March to September, the temperature of the air is constantly increasing; on which account, its capacity for holding vapour is on the increase, and very little is precipitated as rain.
Q. Why is the rising sun in summer accompanied with a breeze?
A. Because the heat of the rising[Pg 309] sun stops the radiation of heat from the earth, and warms its surface.
Q. How does this warmth produce a breeze?
A. The air (resting on the earth’s surface) is warmed by contact, ascends upwards, and colder air rushes in to fill up the void, which is the cause of the morning breeze.
Q. Why is there often an evening breeze during the summer months?
A. The earth radiates heat at sun-set, and the air is cooled down quickly by contact: this condensation causes a motion in the air, which is the evening breeze.
Q. Why are tropical islands always subject to a sea-breeze every morning (i. e.. a breeze blowing from the sea to the land)?
A. The solar rays are unable to heat the surface of the sea as they do the earth; therefore, the air resting on the earth is more heated than the air resting on the sea; and the colder sea air blows inland to restore the equilibrium.
Q. Why is the land breeze unhealthy?
A. Because it is frequently loaded with exhalations from putrefying animal and vegetable substances.
Q. Why is the sea breeze fresh and healthy?
A. Because it passes over the fresh sea, and is not laden with noxious exhalations.
It is healthy, therefore, to walk on the sea-beach before ten o’clock in the morning; but unhealthy after sun-set.
Q. Why is there generally a fresh breeze from the sea (in English watering places) during the summer and autumn mornings?
A. As the land is more heated by the sun than the sea; therefore, air resting on the land is hotter than air resting on the sea; in consequence of which, cooler sea air glides inland, to restore the equilibrium.
Q. Why does the sea breeze feel cool?
A. As the sun cannot make the surface of the sea so hot as the surface of the land; therefore, the air which blows from the sea, feels cooler than the air of the land.
Q. Why are tropical islands subject to a land breeze every evening (i. e.. a breeze blowing from the land towards the sea)?
A. The surface of land cools down faster (after sun-set) than the surface of the sea: in consequence of which, the air of the cold land is condensed, sinks down, and spreads itself into the warmer sea air, causing the land breeze.
Q. Why is the land breeze cool?
A. As the surface of the land is cooled at sun-set quicker than the surface of the sea; therefore, the seaman feels the air from the land to be chill.
Q. Why is the temperature of islands more equable than that of continents?
A. Because the water around the island absorbs the extreme heat of summer, and gives out heat to mitigate the extreme cold of winter.
Q. Why does the sea round an island give out heat in winter?
A. Unless the sea be frozen (which is rarely the case), it is warmer than the frozen land; and, therefore, the warmth of the sea air (mixing with the cold land air) helps to mitigate the intense cold.
Q. Why are there waves in the sea?
A. The wind (acting on the surface of the sea) piles up ridges of water, which leave behind an indentation: as the water on all sides rushes to fill up this indentation, the disturbance spreads on all sides, and billow rolls after billow.
Q. Why does wind in England generally feel cold?
A. Because a constantly changing surface comes in contact with our body, to draw off its heat.
Q. Why is a room (even without a fire) generally warmer than the open air?
A. As the air in a room is not subject to much change, it soon becomes of the same temperature as our skin, and no longer feels cold.
Q. Why do we generally feel colder out-of-doors?
A. Because the air (which surrounds us) is always changing; and as fast as one portion of air has become warmer by contact with our body, another colder portion surrounds us to absorb more heat.
Q. Why are hot foods made cool by blowing them?
A. Blowing causes the air (which covers the hot food) to change more rapidly; in consequence of which, the hot air is quickly blown away, and gives place to fresh cold air.
Q. Why do ladies fan themselves in hot weather?
A. By the action of the fan, fresh particles of air are perpetually brought in contact with the face, and every fresh particle of air absorbs some heat from the skin.
Q. Does the fan cool the air?
A. No; it makes the air hotter, by imparting to it the heat out of our face: but it cools the face blown upon, by transferring its heat to the air.
Q. Is the air in summer time ever so hot as our bodies?
A. No, not in England. In the hottest day in summer, the air of England is 15 or 20 degrees cooler than the human body.
Q. How fast does wind travel?
A. A gentle breeze goes at about the rate of 5 miles an hour. A high[Pg 314] wind from 20 to 60. A hurricane from 80 to 100 miles an hour.
Q. How is the velocity of winds ascertained?
A. By observing the velocity of the clouds, and by an instrument for the purpose.
This instrument is called an Anemometer.
Q. How is the velocity of the clouds ascertained?
A. By observing the speed of their shadow along the ground; which is found in a high wind to vary from 20 to 60 miles an hour.
Q. Why is there always a strong draught through the keyhole of a door?
A. As the air of the room we occupy is warmer than the air in the hall, therefore the cold hall air rushes through the keyhole into the room, and causes a draught.
Q. Why is there always a strong draught under the door, and through the crevice on each side?
A. The cold air rushes from the hall under the door, &c. into the room, to[Pg 315] supply the void caused in the room (by the escape of warm air up the chimney, &c.)
Q. Why is there always a draught through the window crevices?
A. The external air (being colder than the air of the room we occupy) rushes through the window crevices to supply the deficiency, caused by the escape of air up the chimney, &c.
Q. Why is there more draught if you open the lower sash of a window, than if you open the UPPER sash?
A. If the lower sash be open, the cold external air will rush more freely into the room; but if the upper sash be open the heated air of the room will rush out; and (of course) there will be less draught.
Q. By which means is the room better ventilated, by opening the lower or the upper sash?
A. A room is better ventilated by opening the upper sash; because the hot vitiated air (which always ascends towards the ceiling) can better escape.
Q. By which means is a hot room more quickly cooled—By opening the upper or the lower sash?
A. A hot room is cooled more quickly by opening the lower sash; because the cold air can enter more freely by an under current, than by one higher up.
Q. Why does wind dry damp linen?
A. Because dry wind (like a dry sponge) imbibes the particles of vapour from the surface of the linen, as fast as they are formed.
Q. Which is the hottest place in a church, chapel, or theatre?
A. The gallery.
Q. Why is the gallery of all public places hotter than the lower parts of the building?
A. Because the heated air of the room ascends, and all the cold air (which can enter through the doors and windows) keeps to the floor, till it has become heated.
Q. Why do plants often grow out of walls and towers?
A. Because sometimes the wind blows the seed there with the dust; and sometimes birds (flying over) drop the seed which they had formerly eaten.
Q. What is a barometer?
A. A weather-glass, or instrument to show the changes of the weather, by marking the variations in the weight of air.
Q. What is a thermometer?
A. An instrument to show how hot or cold anything is.
Q. What is the difference between a thermometer and a barometer?
A. In a thermometer the mercury is sealed up from the air:
In a barometer the mercury is left exposed (or open) to the air.
Q. If the mercury of the thermometer be sealed up from the air, how can the air affect it?
A. The heat of the air passing through the glass tube into the mercury, causes it to expand more or less, and rise in the tube accordingly.
Q. Why is the tube of a barometer left open?
A. That the air may press upon it freely; and as this pressure is more or less, the mercury rises or falls in the tube.
Q. How can weather be affected by the weight of the air?
A. When air is warm or moist, it is lighter than usual:
When it is cold or dry, it is heavier: and as a barometer marks whether the air be light or heavy, it indicates these changes.
Q. How can you tell (by looking at a barometer) what kind of weather it will be?
A. Because the mercury in the tube rises and falls, as the air becomes lighter or heavier: and we can generally tell by the weight of the air, what kind of weather to expect.
Q. Does the weight of the air vary much?
A. Yes; the atmosphere in England varies as much as one-tenth part more or less.
Q. What is the chief use of a barometer?
A. To warn sailors how to regulate their ships, before squalls come on.
Q. How can a barometer warn sailors to regulate their ships?
A. As the barometer will tell when wind, rain, or storm is at hand, the sailor can make his ship trim before it overtakes him.
Q. Are there any rules which can be depended on?
A. Yes; there are ten special rules to direct us how to know the changes of weather, by marking the mercury of a barometer.
Q. What is the 1ST SPECIAL RULE in regard to the barometer?
A. The barometer is highest of all during a long frost; and it generally rises with a north-east wind.
Q. Why is the barometer highest of all during a long frost?
A. Because long frost condenses the air very greatly; and the more air is condensed, the greater is its pressure on the mercury of the barometer.
Q. Why does the barometer generally rise with a north-east wind?
A. Because north-east winds make[Pg 320] the air both cold and dry: the air, therefore, is both condensed, and without vapour.
Q. What is the 2ND SPECIAL RULE in regard to the barometer?
A. The barometer is lowest of all during a thaw which follows a long frost: it generally falls with south and western winds.
Q. Why does the barometer fall lowest of all at the breaking up of a long frost?
A. 1st—Because the air (which had been much dried by the frost) absorbs the moisture of the fresh warm current of wind from the south or south-west: and
2ndly—The air (which had been much condensed by the frost) is suddenly expanded by the warm wind which is introduced.
Q. Why does the barometer fall very low with south and west winds?
A. Because south and west winds come heavily laden with vapour; and vaporized air is lighter than dry air.
Q. What effect has wind on the mercury?
A. All winds make the barometer[Pg 321] drop, except eastern winds: those winds which blow from the south, and south-west make it drop the lowest.
Q. Why do winds generally make the mercury of a barometer drop?
A. Wind is caused by a partial vacuum in some parts of the globe; and as the air rushes in to supply this deficiency, its general pressure is lessened, and the barometer falls.
Q. What is the 3RD SPECIAL RULE in regard to the barometer?
A. While the barometer stands above 30°, the air must be very dry or very cold, or perhaps both, and no rain may be expected.
Q. Why will there be no rain if the air be very dry?
A. If the air be very dry it will absorb moisture, and not part with what it has in rain.
Q. Why will there be no rain if the air be very cold?
A. If the air be very cold it is so much condensed, that it has already parted with as much moisture as it can spare.
Q. What is the 4TH SPECIAL RULE in regard to the barometer?
A. When the barometer stands very low indeed, there is never much rain, although a fine day will seldom occur at such times.
Q. What kind of weather will it be when the barometer is unusually low?
A. There will be short heavy showers, with sudden squalls of wind from the west.
Q. Why will there be very little rain if the barometer be unusually low?
A. Because the air must be very warm, or very moist, or perhaps both.
Q. Why will there be little or no rain, if the air be very warm?
A. If the air be very warm it will have a tendency to imbibe more moisture, and not to part with what it has.
Q. Why will there be little or no rain if the air be moist, and the barometer remains very low?
A. If the air be ever so moist, rain will never fall till cold air has been introduced to condense the vapour; and the moment that the cold air is introduced, the barometer will rise.
Q. What is the 5TH SPECIAL RULE in regard to the barometer?
A. In summer-time (after a long continuance of fair weather) the barometer will fall gradually for 2 or 3 days before rain comes; but if the fall of the mercury be very sudden, a thunder-storm is at hand.
Q. What is the 6TH SPECIAL RULE in regard to the barometer?
A. When the sky is cloudless, and seems to promise fair weather, if the barometer be low, the face of the sky will soon be suddenly overcast.
Q. What is the 7TH SPECIAL RULE in regard to the barometer?
A. Dark dense clouds will pass over without rain, when the barometer is high; but if the barometer be low, it will often rain without any gathering of clouds.
Q. What is the 8TH SPECIAL RULE in regard to the barometer?
A. The higher the barometer, the greater is the probability of fair weather.
Q. Why is the barometer high in fine weather?
A. Because the air contains but very little vapour. The drier the air, the higher does the mercury of the barometer rise.
Q. What is the 9TH SPECIAL RULE in regard to the barometer?
A. When the mercury is in a rising state, fine weather is at hand; but when the mercury is in a sinking state, foul weather is near.
Q. Why does the mercury rise at the approach of fine weather?
A. Because the air is becoming more dry, and therefore its pressure is greater.
Q. Why does the mercury sink at the approach of foul weather?
A. Because the air is laden with vapour, or disturbed by wind.
Q. Why does vapour in the air make the mercury sink?
A. Because vaporized air is lighter than dry air, and therefore its pressure is less on the mercury of the barometer.
Q. What is the 10TH SPECIAL RULE in regard to the barometer?
A. If (in frosty weather) it begins to snow, the barometer generally rises to 32°, where it remains as long as the snow continues to fall; if, after this, the weather clear up, you may expect very severe cold.
Q. How can you know if the mercury of the barometer be rising?
A. If it be convex (i. e. higher in the middle than at the sides;) it is in a rising state.
Q. How can you tell if the mercury of the barometer be about to fall?
A. If it be concave (i. e. hollow in the middle) it is in a falling state.
Q. Why is the mercury convex when it is rising?
A. The sides of the mercury rub against the glass tube, and are delayed by it, so that the middle part rises faster than the sides.
Q. Why is the mercury concave when it is falling?
A. The sides of the mercury rub against the glass tube, and are delayed by it, so that the middle part sinks faster than the sides.
Q. What effect does a thunder-storm produce on the weather?
A. Thunder is generally preceded by hot weather, and followed by cold and showery weather.
Q. What effect does a sudden change produce on the weather?
A. A great and sudden change (either from hot to cold, or from cold to hot) is generally followed by rain within 24 hours.
Q. Why is a sudden change from hot to cold followed by rain?
A. The cold condenses the air and its vapour; which, being condensed and squeezed out, falls in rain.
Q. Why is a sudden change from cold to hot followed by rain?
A. Because the air is quickly saturated with moisture; and as soon as night comes on, the temperature is lowered again, and some of the abundant moisture falls in rain.
Q. Why is the air quickly saturated with moisture, when heat succeeds rapidly from cold?
A. Because the evaporation (which[Pg 327] was checked by the cold) is carried on very rapidly, in consequence of the diminished pressure of the air.
(N. B. The less the pressure of the air, the more rapidly it evaporates moisture.)
Q. When does the barometer vary most?
A. In winter time.
Q. Why does the barometer vary more in winter than in summer time?
A. Because the difference of temperature between the torrid and temperate zones is so great, that the state of the air is perpetually disturbed by their mixing together.
Q. When does the barometer vary least?
A. In summer time.
Q. Why does the barometer vary less in summer than in winter time?
A. Because the temperature of our island is so nearly equal to that of the torrid zone, that its state is not much disturbed by interchange of currents.
Q. What effect has wind on the barometer?
A. North and east winds make the mercury rise; all other winds make it sink; but south and west winds make it sink lower than any other winds.
Q. Have heat and cold any effect on the barometer?
A. No, not of themselves; but because cold weather is generally either dry, or rough with north-east winds, therefore the mercury rises in cold weather; and because warm weather is often moist or fanned by south-west winds, therefore, the mercury sinks.
Q. Why is the mercury of a barometer lower in the torrid than in the frigid zones?
A. Because the warm air of the torrid zone contains much more vapour than the condensed air of the frigid zone; and the moister the air, the less is its pressure.
Q. In what months is the barometer highest?
A. In May and August; next to these, in June, March, September, and April.
Q. In what months is the barometer lowest?
A. In November and February; then in October, July, December, and January.
Q. What are the driest months?
A. March and June; then May and August; then April and November.
Q. What are the wettest months?
A. October and February; then July and September; then January and December.
Q. Why is there less wet from March to August, than there is from August to March?
A. Because the heat is constantly increasing; and the capacity of the air to absorb and retain moisture increases likewise.
Q. Why is there more wet from August to March, than there is from March to August?
A. Because the heat is constantly decreasing, and the capacity of the air to retain moisture decreases also; so that (although it often rains) yet the air is always on the point of saturation.
Q. Why does the mercury of a barometer rise in a frost?
A. Because frost condenses the air; and condensed air is heavier than rarefied air.
Q. Why does the mercury of a barometer fall in a thaw?
A. Because the air is both warmer (or more rarefied), and also filled with vapour.
Q. What does a sudden rise or fall of the barometer indicate?
A. If the rise be sudden, fine weather will not continue long:
If the fall be sudden, foul weather will not continue long.
Q. What sort of weather may we expect if the barometer be very fluctuating?
A. If the mercury fluctuates much, the weather will be very changeable and unsettled.
The fall of the barometer.
In very hot weather, the fall of the mercury denotes thunder.
Except in very hot weather, the sudden falling of the barometer denotes high wind.
In frosty weather, the fall of the barometer denotes thaw.
If wet weather happens soon after the fall of the barometer, expect but little of it.
In wet weather if the barometer falls, expect much wet.
In fair weather, if the barometer falls much and remains low, expect much wet in a few days, and probably wind.
N. B. The barometer sinks lowest of all for wind and rain together, next to that for wind (except it be an east or north-east wind).
The rise of the barometer.
In winter the rise of the barometer presages frost.
In frosty weather, the rise of the barometer presages snow.
If fair weather happens soon after the rise of the barometer, expect but little of it.
In wet weather, if the mercury rises high and remains so, expect continued fine weather in a day or two.
In wet weather, if the mercury rises suddenly very high, fine weather will not last long.
N. B. The barometer rises highest of all for north and east winds; for all other winds it sinks.
If the barometer be unsettled.
If the motion of the mercury be unsettled, expect unsettled weather.
If it stand at “much rain” and rise to “changeable,” expects fair weather of short continuance.
If it stand at “fair” and fall to “changeable,” expect foul weather.
N. B. Its motion upwards indicates the approach of fine weather: its motion downwards indicates the approach of foul weather.
Q. What is snow?
A. The condensed vapour of the air frozen, and precipitated to the earth.
Q. What is the cause of snow?
A. When the air is nearly saturated with vapour, and condensed by a current[Pg 332] of air below freezing point, some of the vapour is squeezed out, and frozen into snow.
A few years ago, some fishermen (who wintered at Nova-Zembla), after they had been shut up in a hut for several days, opened the window, and the cold external air rushing in, instantly condensed the air of the hut, and the vapour (which was squeezed out) fell on the floor in a shower of snow.
Q. Why does snow fall in winter time?
A. Because the sun’s rays are too oblique to heat the surface of the earth; and (as the earth has no heat to radiate into the air) the air is very cold.
Q. What is sleet?
A. When flakes of snow (in their descent) pass through a bed of air above freezing point, they melt; and fall to the earth as half-melted snow or sleet.
Q. What is the use of snow?
A. To keep the earth warm, and to nourish it.
Q. How can snow keep the earth warm?
A. Because it is a very bad conductor; in consequence of which, the earth which is covered with snow, very rarely descends below freezing point, even when the air is 15 or 20 degrees colder.
Q. Why is snow a bad conductor of heat and cold?
A. Because air is confined and entangled between the crystals, and air is a very bad conductor; when, therefore, the earth is covered with snow, it cannot throw off its heat by radiation.
Q. Tell me the words of the psalmist (cxlvii. 16.) respecting snow, and explain what he means.
A. The Psalmist says—“The Lord giveth snow like wool:” and he means not only that snow is as white as wool, but that it is also as warm as wool.
Q. Why is wool warm?
A. Because air is entangled between the fibres of the wool, and air is a bad conductor.
Q. Why is snow warm?
A. Because air is entangled between the crystals of the snow, and air is a bad conductor.
Q. Why does snow nourish the earth?
A. Because it supplies it with moisture for a considerable time; which penetrates slowly into the soil, and insinuates itself through every clod, ridge, and furrow.
Q. Why is there no snow in summer time?
A. No snow reaches the general surface of the earth in summer time, because the heat of the earth melts it in its descent.
Q. Why are some mountains always covered with snow?
A. 1st—Because the air is more rarefied; and rarefied air abstracts heat which it holds in a latent state:
2ndly—As the mountain top is not surrounded by earth to radiate heat into the air; therefore, the snow is not melted in its descent, but falls on the mountain, and lies there.
Q. Why is snow white?
A. Snow is formed of an infinite number of very minute crystals and prisms, which reflect all the colours of the rays of light; and these colours uniting before they meet the eye, cause snow to appear white.
Q. What is hail?
A. Rain, which has passed in its descent through some cold bed of air, and has been frozen into drops of ice.
Q. Why is one bed of air colder than another?
A. This is frequently caused by electricity in the air, unequally distributed.
Q. Why is hail frequently accompanied with thunder and lightning?
A. 1st—Because the congelation of water into hail disturbs the electricity of the air: and
2ndly—The friction (produced by the fall of hail) excites it still more.
Q. Why does hail fall generally in summer and autumn?
A. 1st—Because the air is more highly electrified in summer and autumn: and
2ndly—The vapours (being rarefied) ascend to the more elevated regions, where the cold is greater than it is nearer the earth.
Q. What two things are essential to cause HAIL?
A. Two strata of clouds having opposite electricities, and two currents of wind. The lower cloud (being negative) is the one precipitated.
Q. What is rain?
A. The vapour of the clouds or air condensed, and precipitated to the earth.
Q. Why is the vapour of the air or clouds precipitated?
A. When the air is saturated with vapour, if a cold current condenses it, it is no longer able to hold all its vapour in solution, and some of it is squeezed out, and falls as rain.
Q. Why does rain fall in drops?
A. The vapoury particles in their descent attract each other; and those which are sufficiently near, unite and form into a drop.
Q. Why does not the cold of night always cause rain?
A. When the air is not near saturation (although condensed by the chill of evening), it will still be able to hold its vapour in solution.
Q. Why does a passing cloud often drop rain?
A. Because the cloud (travelling about on the wind) comes into contact with something that chills it; and its[Pg 337] vapour being squeezed out, falls to the earth as rain.
Q. Why are rain-drops sometimes much larger than at other times?
A. When the rain-cloud is floating near the earth, the drops are large, because such a cloud is much more dense than one which is more elevated.
The size of the rain-drop is increased according to the rapidity with which the vapours are condensed.
Q. Does not wind sometimes increase the size of rain-drops?
A. Yes; by blowing two or more drops into one.
Q. Why do clouds fall in rainy weather?
A. 1st—Because the clouds are heavy with abundant vapour: and
2ndly—As the density of the air is diminished, it is less able to buoy the clouds up.
Q. How do you know that the density of the air is diminished in rainy weather?
A. Because the mercury of a barometer falls.
Q. Why is rain-water more fertilizing than pump-water?
A. Because it contains a compound of hydrogen and nitrogen (called ammonia), which is a very excellent food for young plants.
Q. Why is November made by God to be a rainy month?
A. Because rain hastens the putrefaction of the fallen leaves by causing fermentation.
Q. Why does rain purify the air?
A. 1st—Because it beats down the noxious exhalations collected in the air, and dissolves them:
2ndly—It mixes the air of the upper regions with that of the lower regions: and
3rdly—It washes the earth, and sets in motion the stagnant sewers and ditches.
Q. Why are mountainous countries more rainy than flat ones?
A. The air (striking against the side of the mountains) is carried up the inclined plane, and brought in contact with the cold air of the higher regions, by which it is condensed, and its vapour squeezed out.
Q. Why does a sponge swell when it is wetted?
A. Because the water penetrates the pores of the sponge, and drives the particles of the sponge further from each other; in consequence of which, the bulk of the sponge is greatly increased.
Q. Why do fiddle-strings snap in wet weather?
A. Because the moisture of the air (penetrating the string) causes it to swell; and (as the cord thickens) its tension is increased, and the string snaps.
Q. Why does paper pucker when it is wetted?
A. Because the moisture (penetrating the paper) drives its particles further apart; and (as the moisture is absorbed unequally by the paper) some parts are more enlarged than others; in consequence of which, the paper blisters or puckers.
Q. Why do the weather toys called capu’chins lift the cowl over the figures in wet weather, and remove it in dry?
A. The cowl of the capu’chin is[Pg 340] fastened to a piece of cat-gut. When the weather is wet, the moisture swells the cat-gut and it is shortened, by which means the cowl is pulled up; but in dry weather, the string is loosened, and the cowl falls down.
Q. In another weather toy, the man comes out in wet weather, and the lady in fine:—Why is this?
A. The two figures are attached to a piece of cat-gut in such a manner, that when the cat-gut is shortened by moisture, it pulls the man out; but when it is loose, the woman falls out by her own weight.
Q. Why are wet stockings difficult to pull on?
A. The moisture (by penetrating the threads of the stockings) causes them to shrink in size.
Q. What is the most rainy spot in England?
A. Keswick (in Cumberland); and then Kendal (a market town in Westmoreland).
(In Keswick, about 63 inches of rain fall in a year. In Kendal, 58; Manchester, 38; Liverpool, 34; Dublin and Cambridge, 25; Lincoln, 24; London, 21; and in Paris, only 18.)
Q. In which part of the day does the most rain fall?
A. More rain falls by night than by day; because the cold night condenses the air, and diminishes its capacity for holding vapour in solution.
Q. Does more rain fall in summer or in winter time?
A. There are more rainy days from September to March; but heavier rains between March and September.
Q. Why are there more rainy days from September to March, than from March to September?
A. Because the temperature of the air is constantly decreasing, and its capacity for vapour decreases also; in consequence of which, it is perpetually obliged to part with some of its vapour in rain.
Q. In what part of the world does rain fall most abundantly?
A. Near the equator; and the quantity of rain decreases as we approach the poles.
Q. Why does more rain fall at the equator than at the poles?
A. Because the contrast between the night and day is very great. The hot air absorbs moisture very abundantly during the day; and when the cold night condenses the air, it is unable to retain the moisture imbibed, and some of it falls in rain.
Q. What is water?
A. Water is composed of two gases, oxygen and hydrogen.
(In 9 lbs. of water, 8 are oxygen, and 1 is hydrogen.)
Q. Why is water fluid?
A. Because its particles are kept separate by latent heat; but when a certain quantity of this latent heat is driven out, water becomes solid, and is called ice.
Q. How can water be converted into a gas?
A. By increasing its latent heat, the particles, of water are again subdivided into invisible steam.
Q. Why is pump water called hard water?
A. Because it is laden with foreign matters, and will not readily dissolve substances immersed in it.
Q. What makes pump-water hard?
A. Because when it filters through the earth, it becomes impregnated with sulphate of lime, and many other impurities from the earths and minerals with which it comes in contact.
Q. Why is it difficult to wash our hands clean with hard water?
A. Because the soda of the soap combines with the sulphuric acid of the hard water, and the oil of the soap with the lime, and float in flakes on the top of the water.
N.B. Sulphate of lime consists of sulphuric acid and lime.
Q. Why is it difficult to wash in salt water?
A. Because salt water contains muriatic acid; and the soda of soap combines[Pg 344] with the muriatic acid of the salt water, and produces a cloudiness.
Q. Why does a black hat turn red at the sea side?
A. The muriatic acid of the sea-water disturbs the gallic acid of the black dye, and turns it red.
Q. Of what is soap made?
A. Of kelp (or the ashes of sea-weed dried and burnt in a pit) mixed with oil or fat.
Yellow Soap is made of whale-oil, soda, and resin. Soft soap is made of oil and potash. Hard soap of oil and soda.
Q. Why does water clean dirty linen?
A. Because the oxygen of the water attaches itself to the stains of the linen, and dissolves them; as oxalic acid dissolves ink spots.
Q. Why does soap greatly increase the cleansing power of water?
A. 1st—Because soap increases the oxygen of the water: and
2ndly—It neutralizes the grease of the things washed.
Q. Why is rain water soft?
A. Because it has not come in contact with earths and minerals.
Q. Why is it more easy to wash with soft water than with hard?
A. Because it unites freely with the soap, dissolving it instead of decomposing it, as hard water does.
Q. Why do wood ashes make hard water soft?
A. 1st—Because the carbonic acid of the wood ashes combines with the sulphate of lime in the hard water, and converts it into chalk: and
2ndly—The sulphuric acid of the water combines with the potash of the wood ashes, and prevents it from neutralizing the oily matter of the soap.
Q. Why has rain water such an unpleasant smell, when it is collected in a rain water tub or tank?
A. Because it is impregnated with decomposed organic matter, washed from roofs, trees, or the casks in which it is collected.
Q. Why does water melt sugar?
A. Because very minute particles of[Pg 346] water insinuate themselves into the pores of the sugar, and force the crystals apart from each other.
Q. Why does water melt salt?
A. Because very minute particles of water insinuate themselves into the pores of the salt, and force the crystals apart from each other.
Q. Why does melted sugar or salt give a flavour to the water?
A. Because the sugar or salt (being disunited into very minute pieces) floats about the water, and mixes with every part.
Q. Why does hot water melt sugar and salt quicker than cold water?
A. 1st—Because the heat of the water entering the pores of the sugar or salt, opens a passage for the water: and
2ndly—The particles of hot water being smaller than those of cold, can more readily penetrate the pores of salt or sugar.
Q. Why is sea-water salt?
A. 1st—Because it contains mines of salt at the bottom of its bed:
2ndly—It is impregnated with bituminous matter, which is brackish: and
3rdly—It contains many putrid substances, which increase its brackishness.
Q. Why is not rain-water salt, although most of it is evaporated from the sea?
A. Because salt will not evaporate; and, therefore, when sea-water is turned to vapour, its salt is left behind.
Q. Why does stagnant water putrefy?
A. Because leaves, plants, insects, &c. are decomposed in it.
Q. Why is stagnant water full of worms, eels, &c.?
A. Because numberless insects lay their eggs in the leaves and plants which float on the surface; these eggs are soon hatched, and produce swarms of worms, eels, and insects.
Q. Why are flowing waters free from these impurities?
A. 1st—Because the motion of running water prevents its fermentation:
2ndly—It dissolves the putrid substances which happen to fall into it: and
3rdly—It casts on the bank (by its[Pg 348] current) such substances as it cannot dissolve.
Q. Why does running water oscillate and whirl in its current?
A. 1st—Because it impinges against its banks, and is perpetually diverted from its forward motion: and
2ndly—Because the centre of a river flows faster than its sides.
Q. Why do the sides of a river flow more tardily than its centre?
A. Because they rub against the banks, and are delayed in their current thereby.
Q. Why does soapy water bubble?
A. Because the soap makes the water tenacious, and prevents the bubbles from bursting as soon as they are formed.
Q. Why will not water bubble without soap?
A. Because it is not tenacious enough to hold together the bubbles that are formed.
Q. When soap bubbles are blown from a pipe, why do they ascend?
A. Because they are filled with warm breath, which is lighter than air.
Q. What is ice?
A. Frozen Water. When the air is reduced to 32 degrees of heat, water will no longer remain in a fluid state.
Q. Why is solid ice lighter than water?
A. Because water expands by freezing; and as the bulk is increased, the gravity must be less.
Nine cubic inches of water become ten when frozen.
Q. Why do ewers break in a frosty night?
A. Because the water in them freezes; and as the water is expanded by frost, it bursts the ewers to make room for its increased volume.
Q. Why does it not expand upwards (like boiling water), and run over?
A. Because the surface is first frozen, and the frozen surface acts as a plug, which is more difficult to burst than the earthen ewer itself.
Q. Why do tiles, stones, and rocks often split in winter?
A. Because the moisture (which they imbibed) freezes, and by its expansion splits the solid mass.
Q. In winter time, foot-marks and wheel-ruts are often covered with an icy net-work, through the interstices of which the soil is clearly seen,—Why does the water freeze in net-work?
A. The water in these hollows froze first at the sides of the foot-prints: other crystals gradually shot across the water, and would have covered the whole surface, had not the earth absorbed the water before it had time to freeze.
Q. In winter time these foot-marks and wheel-ruts are sometimes covered with a perfect sheet of ice, and not an icy net-work,—Why is this?
A. The air being colder and the earth harder (than in the former case), the entire surface of the foot-print is frozen over, before the earth can draw the water in.
Q. Why is not the ice solid in these ruts?—why is there only a very thin film or net-work of ice?
A. Because the earth absorbs the water, and leaves the icy film behind.
Q. Does not water expand by heat as well as cold?
A. Yes; it expands as soon as it is more than 42 degrees till it boils, and then it flies off in steam.
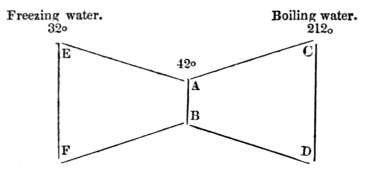
Here A B measures the bulk of a portion of water at 42
degrees.
It goes on increasing in bulk to C D, when it boils. It also goes on
increasing in bulk to E F, when it freezes.
Q. Why do water-pipes frequently burst in frosty weather?
A. Because the water in them freezes; and as the water expands by frost, it bursts the pipes to make room for its increased volume.
Q. When does water begin to expand from cold?
A. Water (which is wisely ordained[Pg 352] by God to be an exception to a very general rule) contracts till it is reduced to 42 degrees, and then it expands till it freezes.
(Water freezes at 32°.)
Q. Why does water expand when it freezes?
A. Because it is converted into solid crystals, which do not fit close, like the particles of water.
Q. Why is the water at the bottom of a river never frozen?
A. Because when water is colder than 42 degrees, it instantly ascends to the surface; and (if it freezes) floats there till it is melted.
(When a river is frozen, the water below the surface is never less than 42°.)
Q. Show the wisdom of God in this wonderful exception to a general law.
A. If ice were heavier than water, it would sink; and a river would soon become a solid block of ice, which could never be dissolved.
Q. Why does not the cold ice on the surface of a river chill the water beneath, and make it freeze?
A. 1st—Water is a very bad conductor, and is heated or chilled by convection only:
2ndly—If the ice on the surface were to communicate its coldness to the water beneath, the water beneath must communicate its heat to the ice, and the ice would instantly melt: and
3rdly—The ice on the surface acts as a shield to prevent the cold air from penetrating the river to freeze it below the mere crust.
Q. Why does water freeze at the surface first?
A. Because the surface is in contact with the air, and the air carries away its heat.
Q. Why does the coat of ice grow thicker and thicker, if the frost continues?
A. Because the heat of the water (immediately below the frozen surface) passes through the pores of the ice into the cold air.
Q. Why then are not whole rivers frozen (layer by layer) till they become solid ice?
A. Because water is so slow a con[Pg 354]ductor, that our frosts never continue long enough to convert a whole river into a solid mass of ice.
Q. Why does not running water freeze so fast as still water?
A. 1st—Because the motion of the current dissolves the crystals as fast as they are formed; and
2ndly—The heat of the under surface is more freely distributed to the upper surface by the rolling water.
Q. When running water is frozen, why is the ice generally very rough?
A. Because little flakes of ice are first formed and carried down the stream, till they meet some obstacle to stop them; other flakes of ice (impinging against them) are arrested in like manner; and the edges of the different flakes overlapping each other, make the surface rough.
Q. Why do some parts of a river freeze less than others?
A. Because springs issue from the bottom, and (as they bubble upwards) thaw the ice, or make it thin.
Q. When persons fall into a river in winter time, why does the water feel remarkably warm?
A. Because the frosty air is at least 10 or 12 degrees colder than the water.
(The water below the surface is at least 42°; but the air 32°, or even less.)
Q. Why is shallow water frozen quicker than deep water?
A. Because (as the whole volume of water must be cooled to 42 degrees before the surface can be frozen) it will take a longer time to cool down a deep bed of water than a shallow one.
Q. Why is sea-water rarely frozen?
A. 1st—Because the mass of water is so great that it requires a very long time to cool the whole volume down to 42 degrees:
2ndly—The ebb and flow of the sea interfere with the cooling influence of the air: and
3rdly—Salt never freezes till the surface is cooled down at least 25 degrees below the freezing point.
Q. Why do some lakes rarely if ever freeze?
A. 1st—Because they are very deep:
2ndly—Because their water is supplied by springs, which bubble from the bottom.
Q. Why does the depth of the water retard its freezing?
A. As the whole volume of water must be reduced to 42 degrees before the surface will freeze, the deeper the water, the longer it will be before the whole volume is thus reduced.
Q. Why do springs at the bottom of a lake prevent its freezing?
A. Because they keep continually sending forth fresh water, which prevents the lake from being reduced to the necessary degree of coldness.
Q. Why is it colder in a thaw than in a frost?
A. When frozen water is thawed, it absorbs heat from the air and objects around to melt its ice, in consequence of which the cold is greatly increased.
Q. Why is it warmer in a frost than in a thaw?
A. When water freezes it gives out its latent heat, in order that it may be converted into solid ice; and as much heat is liberated from the water into the air, we feel warmer.
Q. Why does salt dissolve ice?
A. Water freezes at 32°, but salt and water will not freeze till the air is 25° colder: if, therefore, salt be added to frozen water it becomes liquid, unless the thermometer stands below 7°, (which it never does in our island).
Q. Will any thing do instead of salt?
A. Yes; any acid, such as sulphuric, nitric, &c.
Q. Why are salt and snow mixed together, colder than snow?
A. When salt is mixed with snow, it dissolves the crystals into a fluid; and whenever a solid is converted to a liquid, heat is absorbed, and the cold made more intense.
Q. Why does frost make the earth crack?
A. During the warm weather the earth absorbed abundance of moisture, which the winter freezes: and (as water expands by frost) the expanding water thrusts the particles of earth apart from each other, and leaves a chink or crack behind.
Q. Show the wisdom of God in this arrangement.
A. These cracks in the earth let in the air, the dew and rain, and many gases favourable to vegetation.
Q. Why does the earth crumble in spring?
A. In spring the ice of the clods dissolves, and the particles of earth (which had been held apart by the expanded ice) are left unsupported, and tumble into minute parts (because their cement is dissolved).
Q. Why does mortar crumble away in frost?
A. If the mortar was not dried in the warm weather, its moisture freezes, expands, and thrusts the particles of the mortar away from each other; but (as soon as the frost goes) the water condenses and leaves the mortar full of cracks and chinks.
Q. Why does stucco peel from a wall in frosty weather?
A. If the stucco was not dried in the warm weather, its moisture freezes, expands, and thrusts its particles away from the wall; but as soon as the water condenses again by the thaw, the stucco[Pg 359] (being unsupported) falls by its own weight.
Q. Why cannot bricklayers and plasterers work in frosty weather?
A. Because the bricks and plaster would start from their position as soon as the frost came and expanded the mortar.
Q. Why do bricklayers cover their work with straw in spring and autumn?
A. Because straw is a non-conductor, and prevents the mortar of their new work from freezing during the cold nights of spring and autumn.
Q. Why are water-pipes often covered with stall-litter in winter time?
A. Because straw (being a non-conductor) prevents the water of the pipes from freezing, and the pipes from bursting.
Q. Why are delicate trees covered with straw in WINTER?
A. Because straw (being a non-conductor) prevents the sap of the tree from being frozen.
Q. Can water be frozen in any way besides by frosty weather?
A. Yes; in very many ways. For example—a bottle of water wrapped in cotton, and frequently wetted with ether, will soon freeze.
Q. Why would water freeze if the bottle were kept constantly wetted with ether?
A. Because evaporation would carry off the heat of the water, and reduce it to freezing point.
Q. Why does ether freeze under the receiver of an air-pump, when the air is exhausted?
A. Because evaporation is very greatly increased by the diminution of atmospheric pressure; and the ether freezes by evaporation.
FREEZING MIXTURES.
1. If nitre be dissolved in water, the heat of the liquid will be reduced 16 degrees.
2. If 5 oz. of nitre, and 5 of sal-ammoniac (both finely powdered) be dissolved in 19 oz. of water, the heat of the liquid will be reduced 40 degrees.
3. If 3 lbs. of snow be added to 1 lb. of salt, the mixture will fall to 0° (or 32 degrees below freezing point).
The two following are the coldest mixtures yet known:—
1. Mix 3 lbs. of muriate of lime with 1 lb. of snow.
2. Mix 5 lbs. of diluted sulphuric acid with 4 lbs. of snow.
Q. Why is it more easy to swim in the sea than in a river?
A. Because the specific gravity of salt water is greater than that of fresh, and therefore it buoys up the swimmer better.
Q. How do cooks ascertain if their brine be salt enough for pickling?
A. They put an egg into their brine. If the egg sinks the brine is not strong enough, if the egg floats it is.
Q. Why will the egg sink if the brine be not strong enough for pickling?
A. As an egg is heavier than water, it will sink if immersed therein; but if as much salt be added as the water can dissolve, the egg will float.
Q. Why will the egg float in strong brine?
A. Because the specific gravity of salt and water is greater than that of water only.
Q. Why do persons sink in water when they are unskilful swimmers?
A. 1st—Because (in their floundering about) they take in water at their nose and mouth, which makes them heavier:
2ndly—Fear contracts the body; and[Pg 362] as the body is compressed by fear into a smaller compass, it becomes heavier: and
3rdly—The water and fear take away the breath; and when the breath is taken from the body, its bulk is reduced, and it becomes heavier.
Q. Why can quadrupeds swim more easily than man?
A. 1st—Because the trunk of a quadruped is lighter than water, and this is the greatest part of them:
2ndly—The position of a beast in water is a natural one.
Q. Why is it more difficult for a man to swim than for a beast?
A. Because the head and limbs of a man (like those of a beast) are heavier than water, and these compose more than half his body:
2ndly—The position of a man in water is unnatural to him.
Q. Why can fat men swim more easily than spare men?
A. Fat is lighter than water; and the fatter a man is, the more buoyant will he be.
Q. How are fishes able to ascend to the surface of water?
A. Fishes have an air-bladder near their abdomen: when this bladder is filled with air, the fish increases in size; and (being lighter) ascends through the water to its surface.
Q. How are fishes able to dive in a minute to the bottom of a stream?
A. They expel the air from their air-bladder; in consequence of which, their size is diminished, and they sink instantly.
Q. What is light?
Q. How fast does light travel?
A. Light travels so fast, that it would go eight times round the earth, while a person counts “one.”
Q. Does all light travel equally fast?
A. Yes; the light of the sun, or the light of a candle, or the light from houses, trees, and fields.
Q. Where does the light of houses, trees, and fields come from?
A. The light of the sun (or of some lamp or candle) is reflected from their surfaces.
Q. Why are some surfaces brilliant like glass and steel, and others dull like lead?
A. Those surfaces which reflect the most light, are the most brilliant; and those which absorb light are dull.
Q. What is meant by reflecting light?
A. Throwing the rays of light back again, from the surface on which they light.
Q. What is meant by absorbing light?
A. Letting the rays of light sink below the surface which they touch, so as not to be seen.
Q. Why can a thousand persons see the same object at the same time?
A. Because it throws off from its surface an infinite number of rays in all directions; and one person sees one portion of these rays, and another person another.
Q. Why is the eye pained by a sudden light?
A. Because the pupil of the eye is burdened with rays, before it has had time to contract.
Q. Why does it give us pain, if a candle be brought suddenly towards our bed at night time?
A. In the dark the pupils of the eyes dilate very much, in order to admit more rays. When a candle is brought before them, the enlarged pupil is overladen with rays, and feels pained.
Q. Why can we bear the candle-light after a few moments?
A, Because the pupil contracts again almost instantly, and adjusts itself to the quantity of light which falls upon it.
Q. Why can we see nothing, when we leave a well-lighted room, and go into the dark road or street?
A. Because the pupil (which contracted in the bright room) does not dilate instantaneously; and the contracted pupil is not able to collect rays enough (from the dark road or street) to enable us to see before us.
Q. Why do we see better, when we get used to the dark?
A. Because the pupil dilates again, and is able to gather together more rays; in consequence of which, we see more distinctly.
Q. If we look at the sun for a few moments, why do all other things appear dark?
A. Because the pupil of the eye (which was very much contracted by looking at the sun) is too small to collect sufficient rays from other objects, to enable us to distinguish their colours. (See “accidental colours.”)
Q. If we watch a bright fire for a few moments, why does the room seem dark?
A. Because the pupil of the eye (which was very much contracted by looking at the fire) is too small to collect sufficient rays from the objects around, to enable us to distinguish their colours.
Q. Why can we see the proper colour of every object again, after a few minutes?
A. Because the pupil dilates again, and accommodates itself to the light around.
Q. Why can tigers, cats, and owls see in the dark?
A. Because they have the power of enlarging the pupil of their eyes, so as to collect several scattered rays of light; in consequence of which, they can see distinctly when it is not light enough for us to see any thing at all.
Q. Why do cats and owls sleep almost all day?
A. As the pupil of their eyes is very broad, daylight fatigues them; so they close their eyes for relief.
Q. Why do cats keep winking, when they sit before a fire?
A. As the pupil of their eyes is very broad, the light of the fire pains them; and they keep shutting their eyes to relieve the sensation of too much light.
Q. Why do tigers, cats, owls, &c. prowl by night for prey?
A. As these animals cannot see distinctly in strong daylight, they sleep during the day: and as they can see clearly in the dark, they prowl then for prey.
Q. Why do glow-worms glisten by night only?
A. Because the light of day is so much stronger, that it eclipses the feeble light of a glow-worm; in consequence of which, glow-worms are invisible by day.
Q. Why can we not see the stars in the day-time?
A. Because the light of day is so powerful, that it eclipses the feeble light of the stars: in consequence of which, they are invisible by day.
Q. Why can we see the stars even at mid-day, from the bottom of a deep well?
A. As the rays of the sun never come directly over a well, but the rays of the stars do; therefore the light from those stars (in such a situation) is more clear than the light of the sun.
Q. What is the use of two eyes, since they present only one image of any object?
A. The use of two eyes is to increase the light, or take in more rays of light from the object looked at, in order that it may appear more distinct.
Q. Why do we not see things double, with two eyes?
A. 1st—Because the axis of both eyes is turned to one object; and, therefore, the same impression is made on the ret´ina of each eye.
2ndly—The nerves (which receive the impression) have one point of union, before they reach the brain.
Q. Why do we see ourselves in a glass?
A. The rays of light from our face strike against the surface of the glass, and (instead of being absorbed) are reflected, or sent back again to our eye.
Q. Why are the rays of light reflected by a mirror?
A. Because they cannot pass through the impenetrable metal with which the back of the glass is covered; so they rebound back, just as a marble would do if it struck against a wall.
Q. When a marble is rolled towards a wall, what is that path through which it runs called?
A. The line of the angle of incidence.
Q. When a marble rebounds back again, what is the path it then describes called?
A. The line of the angle of reflection.
Q. When the light of our face goes to the glass, what is the path through which it goes called?
A. The line of the angle of incidence.
Q. When the light of our face is reflected back again from the mirror, what is this returning path called?
A. The line of the angle of reflection.
Q. Why does our reflection in a mirror seem to approach us as we walk towards it, and to retire from us as we retire?
A. Because the line of the angle of incidence is always equal to the line and angle of reflection.
Here CA, EA and DB, FB are the lines of the angle of incidence; and GA, KA and HB, LB are the lines of the angle of reflection. When the arrow is at CD, its shadow will appear at GH, because the line CA=GA and the angle CAB=angle GAB, &c.; and the same may be said about the point D.
Q. Why can a man see his whole person reflected in a little mirror not 6 inches in length?
A. Because the line of the angle of incidence is always equal to the line and angle of reflection.
Take the last figure—CD is much larger than the mirror AB; but the head of the arrow C is reflected obliquely behind the mirror to G; and the barb D appears at H.—Why? Because the line CA=AG and the angle CAB=angle GAB, &c. The same may be said of the point D.
Q. Why does a shadow in water always appear topsy-turvy?
A. Because the line of the angle of incidence is always equal to the line and angle of reflection.

Here the arrow-head A strikes the water at F, and is
reflected to D; and the barb B strikes the water at E, and is reflected
to C.
If a spectator stands at G, he will see the reflected lines CE and DF,
produced as far as G.
It is very plain that the more elevated object A will strike the water,
and be projected from it more perpendicularly than the point B, and
therefore the shadow will seem inverted.
Q. When we see our shadow in water, why do we seem to stand on our head?
A. Because the line of the angle of incidence is always equal to the line and angle of reflection.
Suppose our head to be at A, and our feet at B; then the shadow of our head will be seen at D, and the shadow of our feet at C. (See last figure.)
Q. Why do windows seem to blaze at sun-rise and sun-set?
A. Because glass is a good reflector of light; and the rays of the sun (striking against the window glass) are reflected, or thrown back.
Q. Why do not windows reflect the noon-day rays also?
A. They do, but the reflection is not seen.
Q. Why is the reflection of the rising and setting sun seen in the window, and not that of the noon-day sun?
A. As the angle of incidence always[Pg 373] equals the angle of reflection, therefore the rays of the noon-day sun enter the glass too perpendicularly for their reflection to be seen.

Here AB represents a ray of the noon-day sun striking the
window at B; its reflection will be at C:
But DB (a ray of the rising or setting sun) will be reflected to E (the
eye of the spectator).
Q. Why can we not see the reflection of the sun in a well, during the day-time?
A. Because the rays of the sun fall so obliquely, that they never reach the surface of the water at all, but strike against the brick sides.
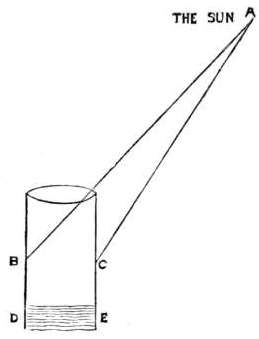
Let BDEC be the well, and DE the water.
The ray AB strikes against the brick-work inside the well; and
The ray AC strikes against the brick-work outside the well.
None will ever touch the water DE.
Q. Why do we see the moon reflected in a well very often?
A. As the rays of the moon are not so oblique as those of the sun, they will often reach the water. (See next figure.)
Q. Why are the stars reflected in a well, although the sun is not?
A. As the rays of the stars are not[Pg 375] so oblique as those of the sun, they will often reach the water.
Here the moon's rays AB, AC, both strike the water DE, and are reflected by it.
Q. In a sheet of water at noon, the sun appears to shine upon only one spot, and all the rest of the water seems dark,—Why is this?
A. Because the rays (which fall at various degrees of obliquity on the water) are reflected at similar angles; but as only those which meet the eye of the spectator are visible, all the sea will appear dark but that one spot.

Here of the rays SA, SB, and SC, only the ray SC meets
the eye of the spectator D.
The spot C, therefore, will appear luminous to the spectator D, but no
other spot of the water ABC.
Q. At night the moon seems to be reflected from only one spot of a lake of water, while all the rest seems dark,—Why is this?
A. Because the rays (which fall at various degrees of obliquity on the lake) are reflected at similar angles; but as only those which enter the eye of the spectator will be visible, all the water will appear dark but that one spot. (See last figure.)
Q. Why are more stars visible from a mountain, than from a plain?
A. As the air absorbs and diminishes light, the higher we ascend, the less light will be absorbed.
Q. Why does the sun seem larger at his rise and set, than it does at noon?
A. Because the earth is surrounded by air, which acts like a magnifying glass; and when the sun is near the horizon (as its rays pass through more of this air), it is more magnified.
Here SC represents a ray of the sun at noon, and MC a ray
of the sun near the horizon. DEG represents the air or atmosphere around
the earth.
Because EC is longer than DC, therefore the rays of the sun at M pass
through more air than the rays of the sun at S, and the sun is more
magnified.
Q. Why does the rising and setting moon appear so much larger, than after it is risen higher above our heads?
A. Because the earth is surrounded by air, which acts like a magnifying glass; and when the moon is near the horizon (as its rays pass through more of this air) it is more magnified. (See last figure.)
Q. When candles are lighted, we cannot see into the street or road,—Why is this?
A. 1st—Because glass is a reflector, and throws the candle-light back into the room again; and
2ndly—The pupil of the eye (which has become contracted by the light of the room) is too small to collect rays enough from the dark street, to enable us to see into it.
Q. Why can’t persons in the street see into a well-lighted room?
A. Because the pupil of their eyes is much dilated by the dark, and cannot[Pg 379] collect from the window sufficient rays to enable them to see into the room.
Q. Why do we often see the fire reflected in our parlour window in winter time?
A. Because glass is a good reflector; and the rays of the fire (striking against the window-glass) are reflected back into the room again.
Q. Why do we often see the shadow of our candles in the window, while we are sitting in our parlour?
A. Because the rays of the candle (striking against the glass) are reflected back into the room: and the darker the night, the clearer the reflection.
Q. Why is this reflection more clear, if the external air be dark?
A. Because the reflection is not then eclipsed by the brighter rays of the sun striking on the other side of the window.
Q. Why is the shadow of an object (thrown on the wall) larger and larger, the closer any object be held to the candle?
A. Because the rays of light diverge (from the flame of a candle) in straight lines, like lines drawn from the centre of a circle.
Here the arrow A held close to the candle, will cast the shadow BF on the wall: while the same arrow held at C, would cast only the little shadow D E.
Q. When we enter a long avenue of trees, why does the avenue seem to get narrower and narrower till it appears to meet?
A. Because the further the trees are off, the more acute will be the angle that any two will make with our eye.
Here the width between the trees A and B will seem to be as great as the line AB: But the width between the trees C and D will seem to be no more than EF.
Q. In a long straight street, why do the houses seem to approach nearer and nearer as they are more distant?
A. Because the more distant the houses are, the more acute will be the angle which any two make with our eye.
Thus in the last figure—
If A and B were two houses at the top of the street, the street would seem to be as wide as the line A B:
And if C and D were two houses at the bottom of the street, the street at the bottom would seem to be no wider than E F.
Q. In an avenue of trees, why do they seem to be smaller as their distance increases?
A. Because the further the trees are off, the more acute will be the angle made by their perpendicular height with our eye.
Here the first tree A B will appear the height of the line A B; but the last tree C D will appear only as high as the line E F.
Q. In a long straight street, why do the houses seem to be smaller and smaller the further they are off?
A. Because the further any house is off, the more acute will be the angle made by its perpendicular height with our eye.
Thus in the last figure—
If A B be a house at the top of the street, its perpendicular height will be that of the line A B.
If C D be a house at the bottom of the street, its perpendicular height will appear to be that of E F.
Q. Why does a man on the top of a mountain or church spire seem to be no bigger than a crow?
A. Because the angle made by the perpendicular height of the man (at that distance) with our eye, is no bigger than the perpendicular height of a crow close by.

Let AB be a man on a distant mountain or spire, and CD a
crow close by:
The man will appear only as high as the line CD, which is the height of
the crow.
Q. Why does the moon appear to us so much bigger than the stars, though in fact it is a great deal smaller?
A. Because the moon is very much nearer to us than any of the stars.
Let AB represent a fixed star, and CD the moon.
AB, though much the larger body, will appear no bigger than EF; whereas
the moon (CD) will appear as big as the line CD to the spectator G.
The moon is 240,000 miles from the earth, not quite a quarter of a
million of miles. The nearest fixed stars are 20,000,000,000,000.
(i. e.. 20 billions.)
If a ball went 500 miles an hour, it would reach the moon in twenty
days: but it would not reach the nearest fixed star in 4,500,000 years.
Had it begun, therefore, when Adam was created, it would be no further
on its journey than a coach (which has to go from the bottom of Cornwall
to the top of Scotland) after it has past about three-quarters of a
mile.
Q. Why does the moon (which is a sphere) appear to be a flat surface?
A. It is so far off, that we cannot distinguish any difference between the length of the rays which issue from the[Pg 384] edge, and those which issue from the centre.
The rays AD and CD appear to be no longer than the ray
BD; but if all the rays seem of the same length, the part B will not
seem to be nearer to us than A and C, and therefore ABC will look like a
flat or straight line.
The rays AD and CD are 240,000 miles long.
The ray BD is 238,910 miles long.
Q. Why do the sun and stars (which are spheres) appear to be flat surfaces?
A. Because they are such an immense way off, that we can discern no difference of length between the rays which issue from the edge, and those which issue from the centre of these bodies.
The rays AD and CD appear no longer than BD; and as B appears to be no nearer than A or C, therefore ABC must all seem equally distant; and ABC will seem a flat or straight line. (See last figure.)
Q. Why does distance make an object invisible?
A. Because the angle (made by the perpendicular height of the distant object with our eye) is so very acute, that one line of the angle merges in the other.

Here the tree AD would not be visible to the spectator C, even if he were to approach as far as B; because no visible perpendicular can be inserted between the two lines AC, DC, till after the point B is past; when the tree will appear like a very little speck.
Q. Why do telescopes enable us to see objects invisible to the naked eye?
A. Because they concentrate several rays within the tube of the telescope, and bend them upon the mirror or lens, which acts as a magnifying glass.
Q. When a ship (out at sea) is approaching the shore, why do we see the small masts before we see the bulky hull?
A. Because the earth is round, and the curve of the sea hides the hull from our eyes, after the tall masts have become visible.
Here only that part of the ship above the line AC can be seen by the spectator A; the rest of the ship is hidden by the swell of the curve DE.
Q. What is meant by refraction?
A. The bending of a ray of light, as it passes from one medium to another.
Q. How is a ray of light bent, as it passes from one medium to another?
A. When a ray of light passes into a denser medium, it is bent towards the perpendicular. When it passes into a rarer medium, it is bent from the perpendicular.
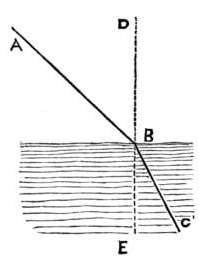
Suppose DE to be a perpendicular line.
If AB (a ray of light,) enters the water, it will be bent towards the
perpendicular to C.
If (on the other hand) CB (a ray of light) emerges from the water, it
would be bent away from the perpendicular towards A.
Q. Why does a spoon (in a glass of water) always appear bent?
A. Because as the light of the spoon emerges from the water, it is refracted.
And the spoon looks like ABC. (See the last figure.)
Q. Why does a river always appear more shallow than it really is?
A. Because the light of the bottom of the river is refracted as it emerges out of the water: and (as a stick is not so long when it is bent, as it is when it is straight) so the river seems less deep than it really is.
Q. How much deeper is a river than it seems to be?
A. One-third. If, therefore, a river seems only 4 feet deep, it is really 6 feet deep.
N. B. Many boys get out of their depth in bathing, in consequence of this deception. Remember, a river is always one-third deeper than it appears to be:—thus, if a river seems to be 4 feet deep, it is in reality 6 feet deep, and so on.
Q. Why do fishes always seem to be nearer the surface of a river than they really are?
A. Because the rays of light from the fish are refracted as they emerge from the[Pg 388] eye: and (as a bent stick is not so far from end to end as a straight one) so the fishes appear nearer our eye than they really are.
Q. Why are some persons near-sighted?
A. Because the COR´NEA of their eye is so prominent, that the image of distant objects is reflected before it reaches the ret’ina; and, therefore, is not distinctly seen.
N.B. The cor´nea shields the crystalline lens, and is more or less convex according to the lens which it covers.
Q. What is meant by the “cor’nea of the eye?”
A. All the outside of the visible part of the eye-ball.
The curve A B C is called the cor'nea.
If this curve be too prominent (or convex), the eye is near-sighted.
If too flat (or concave), the eye is far-sighted.
Q. What is meant by the “ret’ina of the eye?”
A. The net-work which lines the back of the eye, is so called.
The net-work ABC is called the ret'ina, and the
projecting part DEF is called the cor'nea.
Q. What sort of glasses do near-sighted persons wear?
A. If the cor’nea be too convex (or projecting), the person must wear double concave glasses, to counteract it.
Q. What is meant by “double concave glasses?”
A. Glasses hollowed in on both sides.

The figure A is double concave, or concave on both
sides.
Q. What is meant by the “image of objects being reflected before it reaches the ret’ina?”
A. If the cor’nea be too convex, the image of a distant object is reflected (on the vitreous humours of the eye) before it reaches the ret’ina.

Thus the image is reflected at DE, instead of on ABC (the
ret'ina).
Q. What is the use of double concave spectacle glasses?
A. Near-sighted spectacles cast the reflection further back; and the image (being thrown upon the ret’ina) becomes visible.
Q. Why are old people far-sighted?
A. Because the humours of their eyes are dried up by age, and the cor’nea sinks in, or becomes flattened.
Q. Why does the flattening of the cor’nea prevent persons seeing objects which are near?
A. As the cor’nea is too flat, the image of any near object is formed behind the ret’ina of the eye, and is not seen at all.
The reflection is made at DE, instead of at ABC (the
retina).
Q. What sort of glasses do old people wear?
A. As their cor’nea is not sufficiently convex, they must use double convex[Pg 391] glasses, to enable them to see objects near at hand.
Q. What sort of glasses are double convex spectacle-glasses?
A. Glasses which curve outwards on both sides.

The figure A is double convex, or convex on both sides.
Q. What is the use of double convex spectacle-glasses?
A. As the image of near objects is reflected behind the ret’ina, these double convex glasses shorten the focus of the eye, and bring the image into the eye (upon the ret’ina).
Q. Why do near-sighted persons bring objects close to the eye, in order to see them?
A. As the distance between the front and back of their eye is too great, distant objects are reflected before they reach the ret’ina; therefore, near-sighted persons bring the objects closer, in order that the reflection may be cast further back, (to reach the ret’ina).
Q. Why do old people hold objects further off, in order to see them better?
A. As the distance between the front and back of their eye is not great enough, the reflection of near objects is thrown beyond the ret’ina; therefore, they hold objects a long way off, in order to bring their images forward (so as to cast it on the ret’ina).
Q. Why are hawks able to see such an immense way off?
A. Because they have a muscle in the eye which enables them to flatten their cor’nea, by drawing back the crystalline lens.
This muscle is called the “marsupium.”
Q. Why can hawks not only see such a long way off, but also objects within half-an-inch of their eye?
A. Because their eyes are furnished with a broad circular rim which confines the action of this muscle, and throws the cor’nea forward.
Q. Into how many parts may a ray of light be divided?
A. Into three parts: Blue, Yellow, and Red.
N.B. These 3 colours, by combination, make seven. 1.—Red. 2.—Red and yellow form orange. 3.—Yellow. 4.—Yellow and blue make green. 5.—Blue. 6 and 7.—Shades of blue called indigo and violet.
Q. How is it known, that a ray of light consists of several different colours?
A. Because, if a ray of light be cast upon a triangular piece of glass (called a prism), it will be distinctly divided into seven colours: 1.—Red; 2.—Orange; 3.—Yellow; 4.—Green; 5.—Blue; 6.—Indigo; and 7.—Violet.
Q. Why does a prism divide a ray of light into various colours?
A. Because all these colours have different refractive powers. Red is refracted least, and blue the most; therefore, the blue colour of the ray will be bent to the top of the prism, and the red will remain at the bottom.

Here the ray AB received on a prism, would have the blue part bent up to C; the yellow part to D; and the red part no further than E.
Q. What is meant by the refraction of a ray?
A. Bending it from its straight line.
Thus the ray AB of the last figure is refracted at B into three courses, C, D, and E.
Q. What is the cause of a rainbow?
A. When the clouds opposite the sun are very dark, and rain is still falling from them, the rays of the bright sun are divided by the rain-drops, as they would be by a prism.

Let A, B, and C be three drops of rain; SA, SB, and SC
three rays of the sun. SA is divided into the 3 colours; the blue and
yellow are bent above the eye D, and the red enters it.
br />
The ray SB is divided into the three colours; the
blue is bent above the eye, and the red falls below the
eye D; but the yellow enters it.
The ray SC is also divided into the three colours.
The blue (which is bent most) enters the eye;
and the other two fall below it. Thus the eye sees
the blue of C, and all drops in the position of C;
the yellow of B, and of all drops in the position of
B; and the red of A, &c.; and thus it sees a
rainbow.
Q. Does every person see the same colours from the same drops?
A. No; no two persons see the same rainbow.
To another spectator the rays from SB might be red instead of yellow; the ray from SC, yellow; and the blue might be reflected from some drop below C. To a third person the red may issue from a drop above A, and then A would reflect the yellow, and B the blue, and so on.
Q. Why are there often two rainbows at one and the same time?
A. In one rainbow we see the rays of the sun entering the rain-drops at the top, and reflected to the eye from the bottom.
In the other rainbow, we see the rays of the sun entering the rain-drops at the bottom, and reflected to the top, whence they reach the eye.

Here the ray SA strikes the drop at A,—is refracted or bent to B,—is then reflected to C, where it is refracted again, and reaches the eye of the spectator.

Here the ray SB strikes the drop at B,—is refracted to A,—is then reflected to C,—is again reflected to D, when it is again refracted or bent till it reaches the eye of the spectator.
Q. Why are the colours of the second bow all reversed?
A. Because in one bow we see the[Pg 397] rays which enter at the top of the raindrops, refracted from the bottom:
But in the other bow we see the rays which enter at the bottom of the raindrops (after two reflections), refracted from the top.
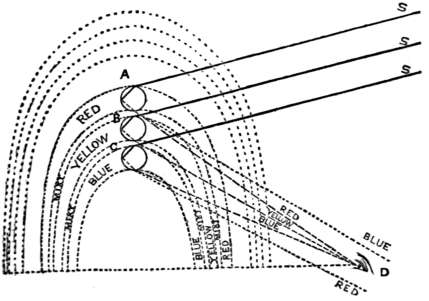
Here A, B, C, represent three drops of rain in the
primary (or inner) rainbow.
The least refracted line is red, and blue the most.
So the red (or least refracted rays) of all the drops in the position
of A,—the yellow of those in the position of B,—and the blue (or the
most refracted rays) of the lowest drops, all meet the eye D, and form
a rainbow to the spectator.
The reason why the primary bow exhibits the stronger colours is
this—because the colours are seen after one reflection and two
refractions; but the colours of the secondary (or upper) rainbow undergo
two reflections and three refractions.
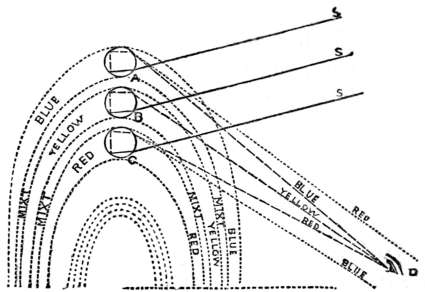
Here also the least refracted ray is red, and the most refracted blue (as in the former case); but the position of each is reversed.
Q. Why does a soap bubble exhibit such variety of colours?
A. The changing colour of the bubble depends upon the changing thickness of the film through which the ray passes.
Q. How does the thickness of the film affect the colour of the soap bubble?
A. Because different degrees of thickness produce different angles of refraction, and, therefore, different colours reach the eye.
Q. Why is the soap bubble so constantly changing its thickness?
A. As the bubble is suspended, the water keeps running down from the top to the bottom of the bubble, till the crown becomes so thin as to burst.
Q. Why are the late evening clouds red?
A. Because red rays (being the least refrangible) are the last to disappear.

Here it will be seen that the red ray PA, being reflected on the horizon at A, will be visible to us; but the yellow and blue rays will be hidden by the curve of the earth.
Q. Why are the early morning clouds red?
A. Because red rays (being the least refrangible) are the first to appear.
See last figure.—It is evident that PA (the red rays) will be reflected on the horizon before either the yellow or blue ones.
Q. What becomes of the blue and yellow rays?
A. They are refracted below the horizon, and are soon made invisible by the curve of the earth. (See last figure.)
Q. Why are the edges of clouds more luminous than their centres?
A. Because the body of vapour is thinnest at the edges of the clouds.
Q. What is the cause of morning and evening twilight?
A. When the sun is below the horizon, the rays (which strike upon the atmosphere or clouds) are bent down towards the earth, and produce a little light called twilight.
See figure on p. 399.—Here the rays of PA will give some light.
Q. Why is a ray of light composed of various colours?
A. If solar light were of one colour only, all objects would appear of that one colour (or else black.)
Q. Why are some things of one colour, and some of another?
A. As every ray of light is composed of all the colours of the rainbow, some things reflect one of these colours, and some another.
Q. Why do some things reflect one colour, and some another?
A. Because the surface of things is so differently constructed, both physically and chemically; and, therefore, some things reflect one ray; some two rays; some all the rays; and some none.
Q. What mainly determines the colour of any object?
A. The fluid or gas either in the body, or on its surface.
N. B. Nitrogen gives green,—Oxygen gives red,—Hydrogen gives blue colours.
Q. Why does dying a silk, &c. change its colour?
A. Because the materials used in dyeing alter the chemical construction of the substance dyed.
Q. Why is a rose red?
A. Because the surface of a rose absorbs the blue and yellow rays of light, and reflects only the red ones.
Q. Why does a rose absorb the yellow and blue rays, and reflect the red?
A. Because the action of the sun’s rays on the oxygen (accumulated in the[Pg 402] petals) produces an acid which turns them red.
The leaves which compose a flower, are called petals.
Q. Why is a violet blue?
A. Because the surface of the violet absorbs the red and yellow rays of the sun, and reflects the blue only.
Q. Why do violets absorb the red and yellow rays, and reflect the blue?
A. Because the petals of the violet contain an alkali, which gives them a purple tinge.
Q. Why is a primrose yellow?
A. Because the surface of the primrose absorbs the blue and red rays of solar light, and reflects the yellow ones.
All plants which have much alkali in their ash, have blue or yellow flowers.
Those which have acid in their ash, have orange, pink, or red flowers.
N. B. Anti-acids (like soda) are called alkalis.
Q. Why are some things black?
A. Because they absorb all the rays of light, and reflect none.
Q. Why are some things white?
A. Because they absorb none of the rays of light, but reflect them all.
Q. Why are coals black?
A. Because they absorb all the rays of the sun which impinge upon them, and stifle their reflection.
Q. Why is snow white?
A. Snow consists of a vast number of crystals (or small prisms), which separate the rays into their elemental colours; but as these crystals are very numerous, the colours unite again before they meet the eye, and appear white.
N. B. The combination of all colours makes white.
Q. Why is sugar white?
A. Sugar consists of a vast number of small crystals, which separate the rays into their elemental colours; but as these crystals are very numerous, the colours unite again before they meet the eye, and appear white.
Q. Why is salt white?
A. Salt consists of a vast number of small crystals, which reflect the various rays of light from different points of the salt; and as these colours unite before they meet the eye, the salt appears to be white.
N. B. The combination of all colours makes white.
Q. Why are the leaves of plants green?
A. Because the carbon of the leaves is a bluish olive, and the sap and tissue of the cells, yellow; when, therefore, the yellow sap flows into the blue carbon, it produces a green leaf.
Q. Why are leaves a light green in spring?
A. Because the young leaves of spring have more sap than carbon; and, therefore, the yellow of the green prevails.
Q. Why are leaves a yellowish brown in autumn?
A. Because the carbon of the leaves is dying away, and the yellow tinge of the tissue and falling sap prevails over the blue.
Q. Why are plants a pale yellow when kept in the dark?
A. Solar light is essential for the production of carbon; and as plants kept in the dark lose their carbon, they lose the blue colour which should convert their yellow sap to green.
Q. Why are potatoes yellow?
A. Potatoes are grown underground, and, therefore, contain very little carbon[Pg 405] (or blue colour); hence the yellow sap of the potato is not converted to green by carbon.
Q. Why are potatoes (which grow exposed to the air and light) green?
A. Because the sun-light increases their carbon; which (mingling with the yellow sap) turns the potato green.
Q. Why is it dangerous to sleep in a room which contains living plants?
A. Because they exhale carbon in the dark in the form of carbonic acid gas, which is destructive to animal life.
Q. Why are some things (like glass) transparent?
A. In transparent bodies (like glass) all the rays of light emerge on the opposite side.
Q. Why are some things shining and splendid?
A. Those objects which reflect the most rays are the most splendid; and those which absorb them most, are dull.
Q. Why are deserts so dazzling in summer time?
A. Because each separate grain of[Pg 406] sand reflects the rays of the sun like a mirror.
Q. If you move a stick (burnt at one end) round pretty briskly, it seems to make a circle of fire,—Why is this?
A. Because the eye retains the image of any bright object, after the object itself is withdrawn; and as the spark of the stick returns before the image has faded from the eye, therefore, it seems to form a complete circle.
Q. If separate figures (as a man and a horse) be drawn on separate sides of a card, and the card twisted quickly, the man seems to be seated on the horse,—Why is this?
A. Because the image of the horse remains upon the eye till the man appears.
The Thaumatrope is constructed on this principle.
Q. Why do the stars twinkle?
A. Fixed stars are so far off, that their rays of light do not strike upon the eye in a continuous flow, but at intervals: when their rays reach the eye, the star becomes visible, and then is obscured till the next batch of rays arrive; and this perpetually occurring, makes a kind of twinkling.
Q. If we look at a red-hot fire for a few minutes, why does every thing seem tinged with a bluish green colour?
A. Because bluish green is the “accidental colour” of red: and if we fix our eye upon any colour whatsoever, when we turn aside, we see every object tinged with its accidental colour.
Q. If we wear blue glasses, (when we take them off,) every thing appears tinged with orange,—Why is this?
A. Because orange is the “accidental colour” of blue: and if we look through blue glasses, we shall see its “accidental colour,” when we lay our glasses aside.
Q. If we look at the sun for a few moments, every thing seems tinged with a violet colour,—Why is this?
A. Because violet is the “accidental colour” of yellow light; and as the sun is yellow, we shall see its “accidental colour” blue, when we turn from gazing at it.
Q. Does not the dark shadow (which seems to hang over every thing after we turn from looking at the sun) arise from our eyes being dazzled?
A. Partly so: the pupil of the eye is very much contracted by the brilliant light[Pg 408] of the sun, and does not adjust itself immediately to the feebler light of terrestrial objects; but, independent of this, the “accidental colour” of the sun being dark violet, would tend to throw a shadow upon all things. (See p. 366.)
Q. Why is black glass for spectacles the best for wear?
A. Because white is the accidental colour of black; and if we wear black glasses, every thing will appear in white light, when we take them off.
Q. Why does every thing seem shadowed with a black mist, when we take off our common spectacles?
A. Because the glasses are white, and black being its “accidental colour,” every thing appears in a black shade, when we lay our glasses down.
The accidental colour of red is bluish green.
The accidental colour of orange is blue.
The accidental colour of violet is yellow.
The accidental colour of of black is white.
And the converse of this is true:—
The accidental colour of bluish green is red.
The accidental colour of of blue is orange.
The accidental colour of of yellow is violet.
The accidental colour of of white is black.
(The law of an accidental colour is this—The accidental colour is always half the spectrum. Thus, if we take half the length of the spectrum by a pair of compasses, and fix one leg in any colour, the other leg will hit upon its accidental colour.)
N. B. The spectrum means the seven colours—Red, orange, yellow, green, blue, indigo, and violet, divided into seven equal bands, and placed side by side in the order just mentioned.
Q. What is sound?
A. The vibration of some sonorous substance produces motion in the air called sound waves, which strike upon the drum of the ear, and give the sensation of sound.
Q. What are musical sounds?
A. Regular and uniform successions of vibrations, which are always pleasing to the ear.
Q. How fast does sound travel?
A. About 13 miles in a minute, or 1142 feet in a second of time.
Q. How fast does light travel?
A. Light would go 8 times round the whole earth, while sound is going its 13 miles.
Q. Why are some things sonorous, and others not?
A. The sonorous quality of any substance depends upon its hardness and elasticity.
Q. Why are copper and iron sonorous, and not lead?
A. Copper and iron are hard and elastic; but as lead is neither hard nor yet elastic, it is not sonorous.
Q. Of what is bell-metal made?
A. Of copper and tin in the following proportions:—In every 5 pounds of bell-metal, there should be 1 lb. of tin, and 4 lbs. of copper.
Q. Why is this mixture of tin and copper used for bell-metal?
A. Because it is much harder and more elastic than either of the pure metals.
Q. Why is the sound of a bell stopped by touching the bell with our finger?
A. The weight of the finger stops the vibrations of the bell; and as soon as the bell ceases to vibrate, it ceases to make sound-waves in the air.
Q. Why does a split bell make a hoarse disagreeable sound?
A. The split of the bell causes a double vibration; and as the sound-waves clash and jar, they impede each other’s motion, and produce discordant sounds.
Q. Why does a fiddle-string give a musical sound?
A. The bow drawn across the string causes it to vibrate, and this vibration of the string sets in motion the sound-waves of the air, and produces musical notes.
Q. Why does a drum sound?
A. The parchment head of the drum vibrates from the blow of the drum-stick, and sets in motion the sound-waves of the air.
Q. Why do musical glasses give sounds?
A. Because the glasses vibrate as[Pg 412] soon as they are struck, and set in motion the sound-waves of the air.
Q. Why do flutes, &c. produce musical sounds?
A. The breath of the performer causes the air in the flute to vibrate, and sets in motion the sound-waves of the air.
Q. Why do piano-fortes produce musical sounds?
A. The keys of the piano (being struck with the finger) lift up a little hammer which knocks against a string; and the vibration thus produced, sets in motion the sound-waves of the air.
Q. Why are some notes bass and some treble?
A. Slow vibrations produce bass or deep sounds; whereas, quick vibrations produce shrill or treble sounds.
Q. Why is an instrument flat when the strings are unstrung?
A. Because the vibrations are too slow; in consequence of which, the sounds produced are not shrill or sharp enough.
Q. Why can persons living a mile or two from
a town hear the bells of the town-church some times, and not at others?
A. Fogs, rain, and snow, obstruct the passage of sound; but when the air is cold and clear, sound is propagated more easily.
Q. Why can we not hear sounds (as distant church bells) in rainy weather, so well as in fine weather?
A. Because the falling rain interferes with the undulations of the sound-waves, and breaks them up.
Q. Why can we not hear sounds (as distant church bells) in snowy weather, so well as in fine weather?
A. Because the falling snow interferes with the undulations of the sound-waves, and stops their progress.
Q. Why can we hear distant clocks most distinctly in clear cold weather?
A. Because the air is most uniform then: there are not two currents of air (one up and one down) to interrupt the sound-waves.
Q. Why can persons hear the voices of men in conversation for a mile distant, near the poles, in winter time?
A. Because the air is very cold and very clear; in consequence of which, there are not two currents of air (one up and one down) to interrupt the sound-waves.
Captain Ross heard the voices of his men in conversation, a mile and a half from the spot where they stood.
Q. Why are not sounds (such as distant church bells) heard so distinctly on a hot day as in frosty weather?
A. Because there are two currents of air; the current of hot air ascending from the earth, and the current of colder air falling towards the earth; and these two currents break up the sound-waves.
Q. Why can we not hear sounds (such as distant clocks) so distinctly in a thick mist or haze, as in a clear night?
A. Because the mist diminishes the velocity of the sound-waves, and (by overburdening them with vapour) limits their length.
Q. Why do we hear sounds better by night than by day?
A. 1st—Night air is more uniform, because the ascending currents of air[Pg 415] (raised by the action of the sun’s rays) cease as the evening advances; and
2ndly—Night is more still from the suspension of business, and the cessation of the hum of men.
Q. How should partition walls be made to prevent the voices in adjoining rooms from being heard?
A. The space between the laths (or canvass) should be filled with shavings or saw-dust; and then no sound would ever pass from one room to another.
Q. Why would shavings or saw-dust prevent the transmission of sound from room to room?
A. Because there would be several different media for the sound to pass through: 1st—the air;
2ndly—the laths and paper;
3rdly—the saw-dust or shavings;
4thly—the air again: and every variety diminishes the strength of the sound-waves.
Q. Why can deaf people hear through an ear trumpet?
A. The ear trumpet restrains the spread of the voice, and limits the diameter of the sound-waves; in con[Pg 416]sequence of which, their strength is increased.
Q. Why are mountains so noiseless and quiet?
A. Because the air of mountains is very rarefied; and as the air becomes rarefied, sound becomes less intense.
Q. How do you know that the rarety of air diminishes the intensity of sound?
A. If a bell be rung in the receiver of an air-pump, the sound becomes fainter and fainter as the air is exhausted, till at last it is quite inaudible.
Q. What is the cause of echo?
A. Whenever a sound-wave strikes against any obstacle (such as a wall or hill), it is reflected (or thrown back); and this reflected sound is called an echo.
The same laws govern echo as light. (See p. 370.)
Q. What places are most famous for echoes?
A. Caverns, grottoes, and ruined abbeys; the areas of antique halls; the windings of long passages; the aisles of cathedral churches; mountains, and ice-bergs.
Q. Why are caverns, grottoes, and ruined abbeys famous for echoes?
A. 1st—Because the sound-waves cannot pass beyond the cavern or grotto, and must flow back:
2ndly—The return waves (being entangled by the cavern) are detained for a short time, and come deliberately to the ear.
Q. Why are antique halls, winding passages, and cathedral aisles famous for echoes?
A. Because the sound-waves cannot flow freely forward, but strike against the winding walls perpetually, and are beaten back.
Q. Why are mountains and ice-bergs famous for echoes?
A. Because they present a barrier to the sound-waves which they cannot pass; and are sufficiently elastic to throw them back.
Q. Why do not the walls of a room or church produce echo?
A. Because sound travels with such velocity, that the echo is blended with the original sound, and produce but one impression on the ear.
Sound travels 13 miles in a minute.
Q. Why do very large buildings (as cathedrals), often reverberate the voice of the speaker?
A. Because the walls are so far off from the speaker, that the echo does not get back in time to blend with the original sound; and, therefore, each is heard separately.
Q. Why do some echoes repeat only one syllable?
A. The further the echoing body is distant, the more sound it will reflect. If, therefore, the echoing body be near, it will repeat but one syllable.
Q. Why does an echo sometimes repeat two or more syllables?
A. Because the echoing body is far off; and, therefore, there is time for one reflection to pass away before another reaches the ear.
Q. Why do windows rattle when carts pass by a house?
A. 1st—Glass is sonorous; and the air communicates its vibrations to the glass, which echoes the same sound: and
2ndly—The window-frame is shaken by the sound-waves impinging against the window, and contributes to the noise.
Q. Why do the bubbles in a cup of tea range round the sides of the cup?
A. Because the cup attracts them.
Q. Why do all the little bubbles tend towards the large ones?
A. Because the large bubbles (being the superior masses) attract them.
Q. Why do the bubbles of a cup of tea follow a tea-spoon?
A. Because the tea-spoon attracts them.
Q. Why are the sides of a pond covered with leaves, while the middle of the pond is quite clear?
A. Because the shore attracts the leaves to itself.
Q. Why do all fruits, &c. (when severed from the tree) fall to the earth?
A. Because the earth attracts them.
Q. Why do persons (who water plants) very
often pour the water into the saucer, and not over the plants?
A. Because the water in the saucer is supped up by the mould (through the hole at the bottom of the flower-pot), and is transferred to the stem and leaves of the plant by capillary attraction, (See p. 84).
Q. Why is vegetation on the margin of a river more luxuriant than in an open field?
A. Because the porous earth on the bank sups up water to the roots of the plants by capillary attraction.
Q. Why is a lump of sugar (left at the bottom of a cup) so long in melting?
A. Because as it melts, it makes the tea above it heavier; and (so long as it remains at the bottom) is surrounded by tea fully saturated with sugar; in consequence of which, the same portions of liquid will hold no more sugar in solution.
Q. Why does the lump of sugar melt more quickly when stirred about?
A. Because fresh portions of unsaturated tea keep coming in contact with the lump, and soon dissolve it.
Q. Why does a piece of sugar (held in a spoon at the top of our tea) melt very rapidly?
A. Because as the tea becomes sweetened, it descends to the bottom of the cup by its own gravity; and fresh portions of unsweetened tea are brought constantly into contact with the sugar, till the lump is entirely dissolved.
Q. How can a sick room be kept free from unhealthy effluvia?
A. Vinegar boiled with myrrh, or camphor, sprinkled in a sick room, will entirely correct putridity.
Q. Why does lime destroy the offensive smells of bins, sewers, &c.?
A. Because it combines with the carbonic acid of these places, and converts it into carbonate of lime, which is entirely free from smell.
Q. Why does chloride of lime fumigate a sick room?
A. Because the chlorine absorbs the hydrogen of the stale air; and by this means removes both the offensive smell and the infection of a sick room.
Q. How can the taint of meat be removed?
A. Either by washing with pyroligneous acid,—covering it for a few hours with common charcoal,—or by putting a few lumps of charcoal into the water in which it is boiled.
Q. Why do these things destroy the taint of meat?
A. Because they combine with the putrescent particles, and neutralize their offensive taste and smell.
Q. Why should bed-rooms, cottages, hospitals, and stables, be washed occasionally with lime-white?
A. Because the lime is very caustic, and removes all organic matters adhering to the walls.
Q. How can mouldiness be prevented?
A. The perfume of any essential oil will prevent mouldiness from ink, paste, preserves, &c.
Alum, salt of amber, borax, nitre, salt, camphor, charcoal, and pyroligneous acid, are all excellent antiseptics.
Salt, corrosive sublimates, copperas, and alum, all arrest the decay of timber. (See p. 426.)
Q. Why will strong Souchong tea poison flies?
A. Because it produces prussic acid, which destroys their nervous system.
Q. Why is strong green tea unwholesome?
A. Because it contains prussic acid, which destroys the nervous system.
Q. Why is a dead man taller than a living man?
A. Because at death the cartilages are relaxed. So, also, after a night’s rest, a man is taller than when he went to bed.
Q. What is sleep?
A. Sleep is the rest of the brain and nervous system.
Q. Why can we not see, when we are asleep with our eyes open?
A. Because the “RET´INA of the eye” is inactive and at rest.
Q. Why can we not hear in sleep?
A. Because the drum or “tympanum of the ear” is placid and at rest.
Q. Why can we not taste when we are asleep?
A. Because the nerves at the end of the tongue (called papillæ) are inactive and at rest.
Q. Why can we not feel when we are asleep?
A. Because the ends of the nerves (called papillæ), situated in the skin, are inactive and at rest.
Q. Why have persons in sleep no will of their own, but may be moved at the will of any one?
A. Because the “cerebellum” (or posterior part of the brain) is inactive and at rest.
Q. Why have dreamers no power of judgment or reason?
A. Because the “cerebrum” (or front of the brain) is inactive and at rest.
Q. Why are dreams such foolish and inconsistent things?
A. Because the “pineal gland” is acting without the brain; and the faculty of thinking exists in the “pineal gland,” but the faculty of judgment in the “cerebrum of the brain.”
The cerebrum of the brain occupies the top and front of the skull. The pineal gland is a small conical gland (about the size of a pea) in the brain.
Q. Why do some persons lose all power of sensation?
A. Because the “cerebrum” (or front of their brain) has been injured.
Q. Why are many persons idiots?
A. Because the “cerebellum of the brain” has been removed by some accident, or injured by some disease.
The cerebellum is all the posterior part of the brain.
Q. Why does a person feel when he is touched?
A. The ends of certain nerves (called PAPILLÆ) situated in the skin erect themselves when touched, and produce a nervous sensation called feeling.
Q. Why are persons able to taste different flavours?
A. Because the “PAPILLÆ” of the tongue and palate erect themselves when food touches them, and produce a nervous sensation called taste.
Q. Why do very old people lose the power of volition, sensation, and thought?
A. Because their brain ossifies; and as the “cerebrum” (or front of the brain) goes, they lose the power of sensation and reason; and as the “cerebellum” (or posterior part of the brain) goes, they lose the power of volition.
Q. Why are old people unable to walk?
A. Because their muscles become rigid.
Acetic Acid, commonly called Distilled Vinegar.
Citric commonly called Juice of Lemons.
Nitric commonly called Aqua Fortis.
Oxalic commonly called Salt of Lemons.
Sulphuric commonly called Oil of Vitriol.
Sulphate of Lime called Plaster of Paris.
Sulphate of Magnesia called Epsom Salts.
Sulphate of Soda called Glauber Salts.
Sulphate ofZinc called White Vitriol.
Nitrate of Silver called Lunar Caustic.
Acetate of Copper called Verdigris.
Muriate of Soda called Table Salt.
Tartrate of Potash called Tartar Emetic.
Carbonate of Ammonia called Smelling Salts.
Carbonate of Lime called Chalk, Marble, &c.
Super-acetate of Lead called Sugar of Lead.
Oxide of Lead called Goulard.
Sublimates are chemical preparations, the basis of which is quicksilver. In corrosive sublimates, the quicksilver is extinguished, either by vitriol, potter’s clay, or some other ingredient.
Sublimation is a similar process to distillation; only solids (such as metals) are employed, instead of liquids.
Thus the fine blue used by painters is a sublimate, and made thus:—Take 2 parts of quicksilver, 3 flower of brimstone, 8 sal ammoniac; and (having ground them) put them with the quicksilver into a glass retort, luted at the bottom; place the retort in a sand-heat; and (when the moisture is given off) you will have a splendid blue sublimate for painting.
FINIS
JARROLD AND SONS, PRINTERS, NORWICH.
Transcriber’s Notes:
Archaic and inconsistent punctuation and spelling retained.
Inconsistent question formats were regularized.
End of the Project Gutenberg EBook of A Guide to the Scientific Knowledge of
Things Familiar, by Ebenezer Cobham Brewer
*** END OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC KNOWLEDGE--THINGS FAMILIAR ***
***** This file should be named 40652-h.htm or 40652-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/4/0/6/5/40652/
Produced by David Garcia, Marilynda Fraser-Cunliffe, Matthew
Wheaton and the Online Distributed Proofreading Team at
http://www.pgdp.net (This file was produced from images
generously made available by The Internet Archive)
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation information page at www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at 809
North 1500 West, Salt Lake City, UT 84116, (801) 596-1887. Email
contact links and up to date contact information can be found at the
Foundation's web site and official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For forty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.