
The Project Gutenberg EBook of North American Jumping Mice (Genus Zapus), by Philip H. Krutzsch This eBook is for the use of anyone anywhere at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org Title: North American Jumping Mice (Genus Zapus) Author: Philip H. Krutzsch Release Date: June 30, 2012 [EBook #40110] Language: English Character set encoding: ISO-8859-1 *** START OF THIS PROJECT GUTENBERG EBOOK NORTH AMERICAN JUMPING MICE *** Produced by Chris Curnow, Joseph Cooper, Tom Cosmas and the Online Distributed Proofreading Team at http://www.pgdp.net

![]()
University of Kansas Publications
Museum of Natural History
![]()
Volume 7, No. 4, pp. 349-472, 47 figures in text, 4 tables
![]() April 21, 1954
April 21, 1954
![]()
North American Jumping Mice
(Genus Zapus)
BY
PHILIP H. KRUTZSCH
University of Kansas
Lawrence
1954
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, A. Byron Leonard,
Robert W. Wilson
Volume 7, No. 4, pp. 349-472, 47 figures in text, 4 tables
Published April 21, 1954
University of Kansas
Lawrence, Kansas
PRINTED BY
FERD VOILAND, JR., STATE PRINTER
TOPEKA, KANSAS
1954
![]()
25-1128
The jumping mice (Genus Zapus) are widely distributed over northern North America, occurring as far north as the Arctic Circle and as far south as Georgia, Missouri, Oklahoma, New Mexico, Arizona, and central California. In some years these small rodents are locally common in moist places that are either grassy or weedy; the jumping mice are notable for the much enlarged hind legs and the exceptionally long tail.
Members of the Genus as a whole have received no serious comprehensive taxonomic attention in the 54 years since Preble’s (1899) revisionary work. In this time 15 new names have been proposed, mostly for subspecies, and only a few attempts have been made at grouping related named kinds.
In the present account it is aimed to record what is known concerning geographic distribution, taxonomically significant characters, and interrelationships of the known kinds as well as to provide means for recognizing the species and subspecies in the genus. In addition, attention is given to the probable center of origin of the subfamily Zapodinae and to the relationships and taxonomic positions of the genera Zapus, Napaeozapus, and Eozapus.
The present report is based on a study of approximately 3,600 specimens that were assembled at the Museum of Natural History of the University of Kansas or that were examined at other institutions. Most of these specimens are stuffed skins with skulls separate. Skulls without skins, skins without skulls, entire skeletons, and separately preserved bacula are included as a part of the total. Almost every specimen is accompanied by an attached label, which bears place and date of capture, name of collector, external measurements, and sex.
Specimens used in the study of geographic variation were arranged by season of capture and according to geographic location; then they were segregated as to sex, and, under each sex, by age. Next, individual variation was measured in comparable samples of like age, sex, season, and geographic origin. Finally, comparable materials were arranged geographically in order to determine variations of systematic significance.
The only external measurements used were total length, length of tail, and length of hind foot; these measurements were recorded by the collectors on the labels attached to the skins. Height of the ear was not used since it was not recorded by many of the collectors.
In order to determine which cranial structures showed the least individual variation but at the same time showed substantial geographic variation, a statistical analysis was made of the 30 measurements, of cranial structures, heretofore used in taxonomic work on Zapus. The following measurements of the skull showed the least individual variation but showed some geographic variation and therefore, were used in this study. See figs. 1-3 which show points between which measurements were taken:
Occipitonasal length.—From anteriormost projection of nasal bones to posteriormost projection of supraoccipital bone. a to a´
Condylobasal length.—Least distance from a line connecting posteriormost parts of exoccipital condyles to a line connecting anteriormost projections of premaxillary bones. b to n
Palatal length.—From anterior border of upper incisors to anteriormost point of postpalatal notch. b to b´
Incisive foramina, length.—From anteriormost point to posteriormost point of incisive foramina. c to c´
Incisive foramina, breadth.—Greatest distance across incisive foramina perpendicular to long axis of skull. f to f´
Zygomatic length.—From anteriormost point of zygomatic process of maxillary to posteriormost point of zygomatic process of squamosal. d to d´
Zygomatic breadth.—Greatest distance across zygomatic arches of cranium at right angles to long axis of skull. j to j´
Breadth of inferior ramus of zygomatic process of maxillary.—Greatest distance across inferior ramus of zygomatic process of maxillary taken parallel to long axis of skull. d to e
Palatal breadth at M3.—Greatest distance from inside margin of alveolus of right M3 to its opposite. g to g´
Palatal breadth at P4.—Same as above except taken at P4. g to g´
Mastoid breadth.—Greatest distance across mastoid bones perpendicular to long axis of skull. h to h´
Breadth of braincase.—Greatest distance across braincase taken perpendicular to long axis of skull. i to i´
Interorbital breadth.—Least distance across top of skull between orbits. k to k´
Length of maxillary tooth-row.—From anterior border of P4 to posterior border of M3. l to l´
Breadth of base zygomatic process of squamosal.—Greatest distance across base of zygomatic process of squamosal taken parallel to long axis of skull. m to m´
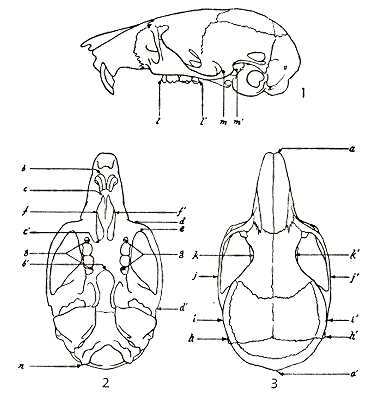
Figs. 1-3. Three views of the skull to show points between which measurements of the skull were taken. Based on Z. t. montanus, adult, female, No. 22165 KU, Cascade Divide, 6400 ft., Crater Lake Nat'l Park, Klamath County, Oregon. × 4.
The baculum has a characteristic size and shape according to the species, and the following significant measurements of the structure were taken:
Greatest length.—From posteriormost border of base to anteriormost point on tip.
Greatest breadth at base.—Greatest distance across base taken parallel to long axis of bone.
Greatest breadth at tip.—Greatest distance across tip taken parallel to long axis of bone.
In the descriptions of color the capitalized color terms refer to those in Ridgway (1912). Any color term that does not have the initial letter capitalized does not refer to any one standard.
In the description of the subspecies the two sexes are treated as one because no significant secondary sexual variation was found. Only fully adult specimens of age groups 3 to 5, as defined on pages 377 and 388, have been considered.
Unless otherwise indicated, specimens are in the University of Kansas Museum of Natural History. Those in other collections are identified by the following abbreviations:
| AMNH. | American Museum of Natural History. |
| CAS. | California Academy of Science. |
| CM. | Carnegie Museum. |
| Chic. AS. | Chicago Academy of Science. |
| Clev. MNH. | Cleveland Museum of Natural History. |
| LMH. | Collection of Lawrence M. Huey. |
| JKJ. | Collection of J. Knox Jones, Jr. |
| CMNH. | Colorado Museum of Natural History. |
| FM. | Chicago Museum of Natural History. |
| HM. | Hastings Museum, Hastings, Nebraska. |
| ISC. | Iowa State College. |
| MCZ. | Museum of Comparative Zoology. |
| MO. | University of Missouri Museum of Zoology. |
| MVZ. | Museum of Vertebrate Zoology, Berkeley, Calif. |
| NMC. | National Museum of Canada. |
| NGFP. | Nebraska Game, Forestation, and Parks Commission. |
| NCS. | North Carolina State College. |
| OHIO. | Ohio Wildlife Research Unit, Ohio State University. |
| OKLA. | Oklahoma Agricultural and Mechanical College. |
| PM. | Provincial Museum of British Columbia. |
| ROM. | Royal Ontario Museum of Zoology. |
| SDM. | San Diego Natural History Museum. |
| SITC. | Southern Illinois Teachers College. |
| USBS. | United States Biological Surveys Collection. |
| USNM. | United States National Museum. |
| UCM. | University of Colorado Museum. |
| UIM. | University of Illinois Museum of Natural History. |
| UM. | University of Michigan Museum of Zoology. |
| UU. | University of Utah Museum of Zoology. |
The species are arranged from least to most progressive, and the subspecies are arranged alphabetically.
The synonymy for each subspecies includes first a citation to the earliest available name then one citation to each name combination that has been applied to the subspecies and, finally, any other especially important references.
Marginal records of occurrence for each subspecies are shown on the maps by means of hollow circles and these localities are listed in clockwise order beginning with the northernmost locality. If more than one of these localities lies on the line of latitude that is northernmost for a given subspecies the western-most of these is recorded first. Marginal localities have been cited in a separate paragraph at the end of the section on specimens examined in the account of a subspecies. Localities that are not marginal are shown on the maps by solid black circles. Localities that could not be represented on the distribution map because of undue crowding or overlapping of symbols are italicized in the lists of specimens examined and in the lists of marginal records.
The localities of capture of specimens examined are recorded alphabetically by state or province, and then by county in each state or province. Within a county the specimens are recorded geographically from north to south. The word “County” is written out in full when the name of the county is written on the label of each specimen listed for that county, but the abbreviation “Co.” is used when one specimen or more here assigned to a given county lacks the name of the county on the label.
The following account has been made possible only by the kindness and cooperation of those persons in charge of the collections listed above. For the privilege of using the specimens in their care I am deeply grateful, as I am also to Prof. A. Byron Leonard for assistance with figures 35-37, to Dr. Rufus Thompson for figures 16-21, and to Mr. Victor Hogg who made all of the other illustrations. My wife, Dorothy Krutzsch, helped untiringly in assembling data, in typing the manuscript, and gave me continued encouragement. Finally, I am grateful to Professor E. Raymond Hall for guidance in the study and critical assistance in the preparation of the manuscript and to Professors Rollin H. Baker, Robert W. Wilson, and Robert E. Beer for valued suggestions.
The fossil record of the genus Zapus is scanty. All of the known fossils of it are lower jaws of Pleistocene Age. The Recent species Z. hudsonius was recorded by Cope (1871:86) in the Port Kennedy Cave fauna (pre-Wisconsinian) of Pennsylvania. Gidley and Gazin (1938:67) reported a single mandibular ramus bearing m1-m3 recovered from the Cumberland Cave (pre-Wisconsinian) of Maryland. The teeth are not typical of modern Zapus in that m1 and m2 are shorter crowned and m1 has a longer anterior lobe. Gidley and Gazin, nevertheless, considered their material insufficient for establishing a new species.
Two extinct species have been described: Zapus burti Hibbard (1941:215) from the Crooked Creek formation (= Meade formation of the State Geological Survey of Kansas) mid-Pleistocene of Kansas and Zapus rinkeri Hibbard (1951:351) from the Rexroad formation (= Blanco formation of the State Geological Survey of Kansas) of Blancan Age of Kansas. Both species resemble Zapus hudsonius, but differ from it in broader crowned more brachydont cheek-teeth. Z. rinkeri differs from Z. burti and Z. hudsonius by a more robust ramus, broader molars, and three instead of two internal re-entrant valleys posterior to the anterior loop on m1. The three species Z. rinkeri, Z. burti, and Z. hudsonius are in a structurally, as well as a geologically, progressive series. The trend in dentition is from broad, brachydont cheek-teeth to narrow, semi-hypsodont cheek-teeth.
The subfamily Zapodinae is known from Pliocene and Pleistocene deposits of North America and now occurs over much of northern North America and in Szechuan and Kansu, China. The living species occur among grasses and low herbs in damp or marshy places both in forested areas and in plains areas.
The early Pliocene Macrognathomys nanus Hall (1930:305), originally described as a Cricetid, is actually a Zapodid as shown by the structure of the mandibular ramus, shape of the incisors, and occlusal pattern of the cheek-teeth.
If Macrognathomys can be considered a member of the subfamily Zapodinae (possibly it is a sicistine) then it represents the oldest known member of this subfamily. Judging from the published illustrations, Macrognathomys seems to be structurally ancestral to the Mid Pliocene Pliozapus solus Wilson; the labial re-entrant folds are wider and shorter and on m2 and m3 fewer. The difference in stage of wear of the teeth in Macrognathomys and Pliozapus is a handicap in comparing the two genera but they are distinct. Wilson (1936:32) points out that Pliozapus clearly falls in the Zapodinae and stands in an ancestral position with respect to the structurally progressive series Eozapus, Zapus, and Napaeozapus. Nevertheless, Pliozapus cannot be considered as directly ancestral to Eozapus because of the progressive features in the dentition of Pliozapus. Wilson (1937:52) remarked that if Pliozapus is ancestral to Zapus and Napaeozapus, considerable evolution must have taken place in the height of crown and in the development of the complexity of the tooth pattern. In contrast to Wilson’s opinion, Stehlin and Schaub (1951:313) placed Pliozapus and Eozapus in the subfamily Sicistinae because certain elements in the occlusal pattern of the cheek-teeth are similar. I disagree with those authors and hold with Wilson; I consider Pliozapus and Eozapus in the subfamily Zapodinae. In dental pattern Pliozapus, as Wilson (1936:32) pointed out, resembles the Recent Eurasiatic sicistid, Sicista more than do Zapus or Napaeozapus. Nevertheless, from Sicista Wilson distinguishes Pliozapus and relates it to the subfamily Zapodinae by: "more oblique direction of protoconid-hypoconid ridge, anterior termination of this ridge at buccal portion of protoconid rather than between protoconid and metaconid as in Sicista; cusps more compressed into lophs; cheek-teeth somewhat broader; greater [357] development of metastylid; greater development of hypoconulid ridge, … absence of anteroconid…."
Eozapus is more closely related to Pliozapus than to either Zapus or Napaeozapus (Wilson, 1936:32) but all four genera are in the subfamily Zapodinae. Stehlin and Schaub (op. cit.:158 and 311) relate Eozapus to the subfamily Sicistinae on the basis of similarity in the occlusal pattern of the cheek-teeth of Eozapus and various sicistines. Stehlin and Schaub do not consider other structures such as the elongate hind limbs, the shape of malleus and incus, and the shape of the baculum, in which there is close resemblance to the Zapodinae. It is these structural similarities as well as those, pointed out by Wilson (loc. cit.), in dentition that leads me to place Eozapus in the subfamily Zapodinae. The early Pleistocene Zapus rinkeri Hibbard shows that the Zapus stage of development had already been achieved perhaps as early as the late Pliocene. Hibbard (1951:352) thought that Zapus rinkeri was not structurally intermediate between Pliozapus and any Recent species of Zapus; although the teeth of Z. rinkeri have the broader, shallower, re-entrant folds of Pliozapus, these teeth are higher crowned and have an occlusal pattern resembling that of the Recent species of Zapus. The middle Pleistocene species, Zapus burti Hibbard, progressed essentially to the structural level of the Recent Zapus hudsonius, but the molars were more brachydont, broader crowned, and their enamel folds less crowded. Pleistocene material of pre-Wisconsin age obtained from cave deposits in Pennsylvania and Maryland is most nearly like Zapus hudsonius. One such cave deposit in Maryland contained an example of the Recent genus Napaeozapus, indicating that its history dates from at least middle Pleistocene time.
The Asiatic Recent Genus, Eozapus, has not progressed much beyond the Pliocene stage in zapidine evolution if Pliozapus be taken as a standard; the North American Recent Genus Zapus essentially achieved its present form by early Pleistocene times, and the Recent Genus Napaeozapus achieved its more progressive structure by middle Pleistocene times.
Perhaps Pliozapus and Eozapus represent one phyletic line and Zapus and Napaeozapus a second line, both of which lines evolved from a pre-zapidine stock in the Miocene. As mentioned earlier, Wilson (1936) thinks that Pliozapus is not directly ancestral to Eozapus. Possibly these two genera diverged at an early date; nevertheless, they are closely related primitive forms.
Zapus and Napaeozapus closely resemble each other and both are [358] structurally advanced; Napaeozapus seems to have differentiated at a more rapid rate.
According to Simpson (1947), the occurrence of the same group of mammals on two different land masses is to be taken as prima facie evidence that migration has occurred. Keeping in mind then the present geographic distribution, unspecialized condition of the dentition of Eozapus, and its resemblance to the extinct Pliozapus known from North America but not from Asia, it may be that Eozapus descended from primitive stock of a North American jumping mouse that was forced to the periphery (across the Asiatic North American land bridge) by the more specialized zapidine stock.
Subsequently or perhaps during the migration of the pre-Eozapus stock the zapidine stock may have dispersed transcontinentally, occupying most of northern North America. The unprogressive Macrognathomys and Pliozapus line which remained in North America may have become extinct. Any such period of dispersal and climatic equilibrium ended when glaciers came to cover most of the northern part of the continent and the mammals living there were forced southward by the ice or remained in ice-free refugia within the glaciated area. Later, with melting and retreat of the ice, the jumping mice could have again spread enough to occupy the northern part of the continent. Such glaciation isolated segments of the population and aided their evolution into distinct species.
If it be assumed, as Matthew (1915) did and as Hooper (1952:200) later on the generic level did, that the region of origin and center of dispersal for a given group of animals is characterized by the presence of the most progressive forms, then southeastern Canada and the northeastern United States make up the area of origin and center of dispersal in relatively late time of the subfamily Zapodinae. This area is inhabited by Zapus hudsonius and Napaeozapus, the most progressive members of the subfamily.
As I visualize it, the evolution of the Zapodinae occurred in two stages: the first stage involved the movement of the primitive pre-Eozapus stock to Asia and the second stage involved the dispersal, isolation, and specialization in North America of the more progressive basic zapidine stock into the present genera Zapus and Napaeozapus.
The genus Zapus is one of three living genera in the subfamily Zapodinae. These genera Zapus and Napaeozapus from North [359] America and Eozapus from China have been variously considered as subgenera of the genus Zapus (Preble, 1899) or as three separate genera (Ellerman, 1940).

Figs. 4-15. Three views of the skull and a lateral view of the left lower jaw of each of the Recent genera of the subfamily Zapodinae. × 1.5.
Figs. 4-7. Eozapus s. vicinus, adult, male, No. 240762 USNM, Lanchow, Kansu, China.
Figs. 8-11. Zapus h. pallidus, adult, male, No. 240762 KU, 51/2 mi. N, 13/4 mi. E Lawrence, Douglas County, Kansas.
Figs. 12-15. Napaeozapus i. insignis, adult, male, No. 41109 KU, Shutsburg Rd., at Roaring Creek, 600 ft., Franklin County, Massachusetts.

Figs. 16-21. Occlusal views of upper and lower right cheek-teeth, of the three Recent genera of the subfamily Zapodinae. × 121/2.
Figs. 16 and 19. Eozapus s. vicinus, adult (age group 3), male, No. 240762 USNM, Lanchow, Kansu, China.
Figs. 17 and 20. Zapus h. alascensis, adult (age group 2), female, No. 29073 KU, E side Chilkat River, 9 mi. W and 4 mi. N Haines, Alaska.
Figs. 18 and 21. Napaeozapus i. insignis, adult (age group 3), male, No. 41109 KU, Shutsburg Rd., at Roaring Creek, 600 ft., Franklin County, Massachusetts.
Note especially the variation in complexity of occlusal pattern, width of re-entrant folds, and degree of tubercularity.
The remarkable similarity of the body form, post-cranial skeleton, mandibular rami, and general structure of the cranium of Zapus, Napaeozapus, and Eozapus indicate their relationship (see figs. 4-15); however, dissimilarity between the groups in the dentition (tooth number and occlusal pattern), bacula, and ear ossicles provides [361] basis for considering them distinct genera. As pointed out earlier, Zapus and Napaeozapus appear to be more closely related and progressive and the Asiatic Eozapus somewhat removed and less progressive.
Teeth.—According to the complexity in dental pattern and in number and size of the cheek-teeth, these genera can be arranged in a structurally progressive series with Eozapus showing the least complexity and Napaeozapus the most (see figs. 16-21). There are three distinct molar patterns; one is simple (Eozapus) and the others (Zapus and Napaeozapus) are more complex. The complexity is greatest in Napaeozapus, which is characterized by numerous additional flexures in the enamel and dentine. The simplicity of the molars of Eozapus is evident in the tuberculate rather than flat-crowned occlusal surface; the wide, simple, re-entrant bays; the small (or sometimes absent) anteroconid; and the essentially quadritubercular nature of the teeth. The molars of Zapus and Napaeozapus are flat crowned; however, Zapus has wider and fewer re-entrant bays, a smaller anteroconid, and less complexity in the occlusal pattern. The characteristics of the molar teeth would tend to indicate a close relationship between Zapus and Napaeozapus and to place Eozapus as primitive.
The absence of P4 in Napaeozapus would lead one to suspect that this genus has evolved at a more rapid rate than the historically older Zapus and Eozapus which still retain this structure. The small size of P4, even in the primitive Eozapus, indicates that it has long been of little use to the mouse. An even greater reduction of P4 in the more complex dentition of Zapus argues for complete loss of this tooth as the next step in specialization, such as is seen in the more progressive Napaeozapus. The following parallel columns show selected differences between the occlusal patterns of the cheek-teeth of the three genera:
Baculum.—The baculum (os penis) of Eozapus is known to me only from Vinogradov’s (1925) figures of the dorsal and lateral aspects. The proximal end (base) is laterally expanded, and the shaft tapers gradually toward the distal end where it expands abruptly into the spade-shaped tip. In lateral aspect the bone is relatively thick; it is curved downward slightly from the proximal end to the base of the tip where it curves upward to a rounded point.
The baculum of Zapus differs from that of Eozapus as follows: base less expanded horizontally; shaft slenderer; distal end less spade-shaped except in Z. trinotatus. The tip is less expanded [362] in Z. princeps and is still less so in Z. hudsonius. In Napaeozapus the tip is lanceolate, the base is narrow, and in lateral view the shaft is slender and curved (see figs. 22-31).
| Eozapus | Zapus | Napaeozapus | |
P4— |
Small |
Smaller |
Absent |
M1— |
Four wide labial re-entrant folds of equal length; paracone and metacone largest cusps; anterior cingulum large. |
Four moderately narrow labial re-entrant folds of unequal length; 1st and 3d longer than 2d, 4th shortest; paracone smaller than in Eozapus; metacone largest cusp; anterior cingulum small. |
Three narrow labial re-entrant folds of unequal length, 1st long, 2d and 3d shorter; paracone and metacone larger than in Zapus and Eozapus; anterior cingulum absent. |
M2— |
Four wide labial re-entrant folds; 2d short, others of equal length but longer than 2d; anterior and posterior cingula large; occlusal pattern simple. |
Four moderately narrow labial re-entrant folds of unequal length, 1st and 3d long, 2d and 4th short; anterior and posterior cingula moderately large; occlusal pattern moderately complex. |
Narrow labial re-entrant folds, variable in number, often as many as 6; anterior and posterior cingula small; occlusal pattern complex. |
M3— |
Three wide labial re-entrant folds of unequal length, 1st short, 2d and 3d long; anterior and posterior cingula low, small; occlusal pattern simple. |
Two moderately narrow labial re-entrant folds of equal length; anterior and posterior cingula moderately large; occlusal pattern moderately complex. |
Three narrow labial re-entrant folds of unequal length, 1st long, 2d and 3d short; anterior and posterior cingula large; occlusal pattern complex. |
m1— |
Anterior oblique re-entrant fold separating equal sized protoconid and metaconid cusps; 3 wide lingual re-entrant folds of equal length; anteroconid absent; occlusal pattern simple; mesoconid present. |
No anterior re-entrant fold; 4 moderately narrow lingual re-entrant folds of equal length, 1st joining 1st labial re-entrant fold, 4th joining 2d labial re-entrant fold; anteroconid well developed, encloses small lake; occlusal pattern moderately complex; mesoconid absent. |
No anterior re-entrant fold; narrow lingual re-entrant folds variable in number, often as many as 4; anteroconid well developed, encloses 1 or 2 small lakes; occlusal pattern complex; mesoconid absent. |
m2— |
Four wide lingual re-entrant folds of unequal length, 1st short, other 3 equal and long; anteroconid moderately large; occlusal pattern simple. |
Four moderately narrow lingual re-entrant folds, 1st and 2d long, 3d and 4th short, 1st joins 1st labial re-entrant fold and 4th joins 2d labial re-entrant fold; anteroconid large; occlusal pattern moderately complex. |
Narrow lingual re-entrant folds, variable in number, may be as many as 5; anteroconid large, encloses complex folds from 1st labial re-entrant fold; occlusal pattern complex. |
m3— |
Three wide lingual re-entrant folds of near equal length; anteroconid absent; occlusal pattern simple; 1 labial re-entrant fold. |
Narrow lingual re-entrant folds variable in number, as many as 3; anteroconid present; occlusal pattern complex; 2 labial re-entrant folds. |
Three moderately narrow lingual re-entrant folds of unequal length, 1st and 2d long, 3d short; anteroconid absent; occlusal pattern moderately complex; 1 labial re-entrant fold. |

Figs. 22-31. Dorsal and lateral views of the bacula of the Recent genera (and species of the genus Zapus) of the subfamily Zapodinae. × 10.
Figs. 22 and 27. Eozapus setchuanus (after Vinogradov, 1925:585).
Figs. 23 and 28. Zapus t. trinotatus, adult, No. 94596 MVZ, 11/4 mi. ENE Amboy, 350 ft., Clark County, Washington.
Figs. 24 and 29. Zapus p. princeps, adult, No. 20870 KU, 3 mi. S Ward, Boulder County, Colorado.
Figs. 25 and 30. Zapus h. pallidus, adult, No. 22954 KU, 4 mi. N, 13/4 mi. E Lawrence, Douglas County, Kansas.
Figs. 26 and 31. Napaeozapus i. insignis, adult, No. 41110 KU, Shutsburg Rd., at Roaring Creek, 600 ft., Franklin County, Massachusetts.
Ear ossicles.—The auditory ossicles are of three types which differ only slightly. These ossicles possibly are more conservative than some other structures because the ossicles are not so much affected by the molding influence of the environment.
Instances of variation in the auditory region in mammals in general are small, even at the family level; therefore, these differences [364] in the subfamily Zapodinae are offered as additional support for recognizing Eozapus, Zapus, and Napaeozapus as distinct genera. The distinctive features are chiefly in the malleus and incus; the stapes, however, differs slightly and, therefore, it too is described (see figs. 32-34).
In Eozapus the head of the malleus is narrow, oblong, and rounded dorsally and attaches to the body by a long, slender, abruptly recurved neck. The body is weakly pointed ventrally and rounded dorsally. A beaklike manubrium malleus composed of anterior projecting external and internal spines extends from the body to the tympanum. The incus has a dorsally rounded body with an anterior downward snoutlike projection with which the malleus articulates. The short limb of the incus is broad basally and narrows somewhat distally. The long limb is narrow and its articulating lenticular process is a flat circular structure. The limbs of the stapes are wide-spread and heavy. The neck is short and wide with a large circular articulating surface.
In Zapus the head of the malleus is angular with an anterior projecting point and is flattened in dorsal aspect. The neck is slender, elongate, and gently curved away from the long limb of the incus. The body is pointed dorsally and rounded ventrally, the reverse of the condition in Eozapus. There is a beaklike manubrium malleus composed of internal and external anteriorly projecting spines extending from the body to the tympanum as in Eozapus. The incus has a rounded body with a long angular limb articulating via a small lenticular process with the stapes. The short limb is narrow but does not taper distally as in Eozapus. The limbs of the stapes are relatively narrow, weak, and gently curved. The neck is longer and more slender than that of Eozapus.
In Napaeozapus the head and neck of the malleus resemble those of Zapus but are less robust. The body is more rounded dorsally, having the curved dorsal surface directed anteriorly rather than posteriorly (as in Zapus) and the lateral surface is nearly flat instead of curved as in the other genera. The manubrium resembles that of Eozapus and Zapus. The body of the incus is flattened dorsally but otherwise rounded. The long limb of the incus is angular and longer than that of Zapus. The short limb of the incus is broad at the base and tapers distally. The limbs of the stapes are narrow, weak, and abruptly curved. The neck is more slender and elongate than in Zapus.
In summary: Only the head and body of the malleus and the short and long limbs and body of the incus are sufficiently consistent [365] within a given group to be of taxonomic importance. The similarity in the morphology of these ossicles indicates a close relationship between all three genera. Zapus and Napaeozapus resemble one another more than either resembles Eozapus. The differences recorded are constant between the described groups and, therefore, are considered to be of taxonomic significance. The differences give basis for dividing the subfamily Zapodinae into the three genera Eozapus, Zapus, and Napaeozapus.
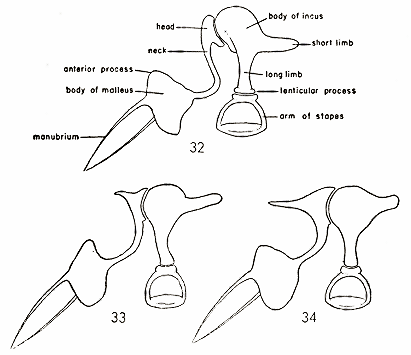
Figs. 32-34. Lateral views of the left ear ossicles (articulated) of the Recent genera of the subfamily Zapodinae. × 20.
Fig. 32. Eozapus s. vicinus, adult, male, No. 240762 USNM, Lanchow, Kansu, China.
Fig. 33. Zapus p. princeps, adult, male, No. 32858 KU, Medicine Wheel Ranch, 28 mi. E Lovell, Big Horn County, Wyoming.
Fig. 34. Napaeozapus i. insignis, adult, male, No. 9544 KU, 3 mi. W Base Station, Coos County, New Hampshire.
Distribution of and Speciation in the Genus Zapus
Many of the described kinds of the genus Zapus were initially named as distinct species (see Preble, 1899). Subsequently (see Hall, 1931), some of the nominal species were reduced to the rank of subspecies. Only three species in the genus Zapus are recognized in the following account. The concept of species adopted here is, in Mayr’s (1942:120) words, this: “Species are groups of actually or potentially interbreeding natural populations, which are reproductively isolated from other such groups.” The three species are [366] Z. trinotatus, Z. princeps, and Z. hudsonius. No hybridization is known where two occur together or where their ranges are adjacent. Each of these species has several geographically contiguous subspecies.
The three species of Zapus are closely related but are not equally progressive. If eastern North America is considered to be the region of origin and center of dispersal of Zapus (see pp. 368-369) the geographically distant species would be expected to be the least progressive, and such seems to be the case. Zapus trinotatus is geographically farthest removed and structurally least progressive. Zapus hudsonius occurs at the center of dispersal and is the most progressive structurally whereas Z. princeps is geographically and structurally intermediate. Structural progressiveness is postulated for the species that has the simplest (in this instance specialized) baculum and smallest fourth upper premolar. The phyletic branches of the genus Zapus possibly developed from geographic segments of a population radiating from the centrally located progressive group. On continental areas where a species with a wide and continuous range gives rise to several daughter species, geographic isolation is thought to be important in bringing about the formation of species. The unspecialized populations conceivably occupied an area west of the present Rocky Mountains and south of latitude 50°. From later Miocene times on, climatic and geological differentiation occurred in this area, and with the growth of geological barriers and differentiation of habitat these unspecialized populations may have been separated into two ecological groups, one inhabiting the more arid area between the present Rocky Mountains and the present Cascade Range and Sierra Nevada and the other group inhabiting the Pacific coastal region. Isolation of each of these groups probably was not complete. How far differentiation might have proceeded with incomplete isolation can only be guessed, but at least incipient differences probably were present and possibly the animals approached in character those found in these areas today in that the ecology of the region was much the same as now.
In the region between the Rocky Mountains and the present Cascade Range and the Sierra Nevada, the flora (in late Pliocene) became semidesert, which presumably made most of this region uninhabitable for jumping mice. The aridity probably induced local concentration into boreal montane islands, thus possibly displacing the populations of the two species that were in contact.
In Pleistocene times continental glaciation must have interrupted the contacts between the coastal, intermontane (the area between the present Rocky Mountains and the present Cascade Range and the Sierra Nevada), and northern and eastern groups of Zapus or mammals of any genus that occurred over all of this vast region. The advance of the ice southward would have increased opportunity for evolution by interposing barriers that isolated some populations. The populations possibly were re-established in interglacial periods and then were isolated again by another descent of glacial ice.
If a population occupied the unglaciated coastal region of Oregon and Washington it may have been separated from other populations to the north and east by an ice cap which covered most of the Cascade Range. The population occupying the intermountain region probably was isolated from the population to the north and west. The formation of glaciers presumably reduced the size of areas available to the populations occupying eastern North America, Alaska, and Canada with the result that they persisted only in areas south of the ice or in ice-free refugia (central and western Alaska) within the glaciated area. According to Axelrod (1948), the flora in the eastern United States during the Pleistocene furnished most of the stock for the revegetation of southern and subarctic Canada east of the Rocky Mountains. Eastern populations of Z. hudsonius (or its progenitors) probably followed the spread of this vegetation and, thus, extended their range into Canada where they crossbred with populations advancing south and east from the refugia in Alaska. Western montane floras, which extended north along the Rocky Mountains and the Cascade and Coast ranges, probably paved the path for a northward migration of populations of the intermountain Z. princeps (or its progenitors). Populations of Z. princeps moved eastward from the present Rocky Mountains, inhabiting the high plains of southern Canada and the north-central United States. In general, Zapus hudsonius occupies the region to the north and to the east of that inhabited by Zapus princeps; however, the ranges of the two meet and overlap in central and northern British Columbia and in the high plains area of southern Alberta, Saskatchewan, eastern Manitoba, eastern Montana, North Dakota, and northern South Dakota. In these places of overlap, owing to range expansion following the retreat of the ice, there is no sign of interbreeding, indicating that the populations have attained specific rank.
Populations of both Z. hudsonius and Z. princeps occur together at Indianpoint Lake, British Columbia. Specimens taken there are readily sorted into two groups; none is intermediate. The difference in size between these species there is especially marked; Z. p. saltator there is a large derivative of Z. princeps and Z. h. tenellus is a medium-sized Z. hudsonius.
Z. princeps minor and Z. hudsonius intermedius have been taken at several neighboring localities in North Dakota. Although these geographic races are more nearly of the same size (minor is a small subspecies of princeps and intermedius is a moderately large subspecies of hudsonius) they do not interbreed. Specimens of Z. p. minor and Z. h. intermedius have been obtained from an ecologically homogeneous area in the vicinity of Fort Totten and Devils Lake, North Dakota. Values obtained from several measurements of the skull and baculum allow for ready recognition of the two species. The populations from North Dakota are, however, not so widely divergent as are those populations from the area of contact in British Columbia. Perhaps the difference in the degree of distinction between the species at the two areas of contact is indicative of the length and completeness of geographic isolation between neighboring populations.
The ranges of Z. trinotatus and Z. hudsonius are not at present in contact, but the two species differ more strongly than do hudsonius and princeps or princeps and trinotatus. Therefore, trinotatus and hudsonius are here considered to be two distinct species.
As pointed out earlier in this discussion, the separation between the progenitors of Z. trinotatus and Z. princeps probably occurred when the present Cascade Range and the Sierra Nevada were being formed. From this time until Pleistocene glaciation an incomplete geographic isolation was in effect between the populations of the Pacific coast and the intermountain populations. Perhaps in the region north of the present Cascade Range there was moderate interbreeding between these populations and the transcontinental form. There may have been a similar zone of interbreeding along the crest of the present Cascades where the intermountain and coastal populations conceivably could have met. At least incipient characters probably were present when in Pleistocene time, continental glaciation further isolated the two populations. Since the retreat of the last ice (Wisconsin) the unprogressive coastal Z. trinotatus has expanded its range only slightly, reaching as far as southwestern British Columbia. It seems that ecological difference rather than [369] the barrier formed by the higher elevations is responsible for the limited expansion of range. The population of princeps has extended its range northward to the southern part of the Yukon Territory but does not occur in coastal southern British Columbia because that area already was occupied by Zapus trinotatus. The ranges of the two species meet and overlap in southwestern British Columbia. The species occur sympatrically in Manning Park where, according to Carl et al. (1952:77), they occupy the same range in the region of Allison Pass, Pinewoods, and Timberline Valley. These workers remark that no intergradation was apparent between individuals of the two species obtained in the same trap line.
I have examined material of both species from Allison Pass. There the species differ in color, in the shape of the skull, and in the size and shape of the baculum. Material from Timberline Valley, an area in which Carl et al. (loc. cit.) reported both species, here is assigned to Z. princeps. Where bacula have been preserved the identity of the species is instantly possible.
In summary: First, a population of jumping mice, possibly a monotypic genus, occurred over most of North America; then this population partly divided into Pacific northwest, intermountain (from the east slopes of the present Rocky Mountains to the east slopes of the present Cascade Range and the Sierra Nevada), and transcontinental (eastern and northern) groups with the least progressive groups peripheral; a further reduction or possibly a complete isolation of these populations followed owing to Pleistocene glaciation (especially in the Wisconsin period); and, finally, the present day contacts were established between these populations which by now have differentiated into species. Conceivably, Z. burti (Blancan age) and Z. rinkeri (mid Pleistocene) may represent stages in the development of Z. hudsonius.
Edward A. Preble’s (1899) early revisionary account of the genus Zapus provides an annotated list of the names which had been proposed for American jumping mice to that date. The present account supplies in chronological order the names proposed (including the new kinds described by Preble) in the 54 years since Preble’s revision. Detailed synonymies are given for each kind under the accounts of the subspecies.
1899 campestris (Zapus hudsonius) Preble, N. Amer. Fauna, 15:20, August 8, 1899, applies to the jumping mouse of southeastern Montana, and the Black Hills region of Wyoming and South Dakota.
1899 minor (Zapus princeps) Preble, N. Amer. Fauna, 15:23, August 8, 1899, originally applied to the jumping mouse of the prairies of Saskatchewan, but now includes populations of this species from the plains of Canada (southern Manitoba to Canadian Rockies) and northern United States (Montana, North and South Dakota).
1899 oregonus (Zapus princeps) Preble, N. Amer. Fauna, 15:24, August 8, 1899, originally applied to the jumping mouse of eastern Oregon, but now applies also to populations from southeastern Idaho, eastern and central Nevada, and extreme northeastern California.
1899 major (Zapus) Preble [= Zapus princeps oregonus], N. Amer. Fauna, 15:25, August 8, 1899, arranged as a subspecies of Zapus princeps by Hall, Univ. California Publ. Zool., 37:10, April 10, 1931; here considered a synonym of Zapus princeps oregonus.
1899 nevadensis (Zapus) Preble [= Zapus princeps oregonus], N. Amer. Fauna, 15:25, August 8, 1899, arranged as a subspecies of Zapus princeps by Hall, Univ. California Publ. Zool., 37:10, April 10, 1931; here considered a synonym of Zapus princeps oregonus.
1899 orarius (Zapus) Preble [= Zapus trinotatus orarius], N. Amer. Fauna, 15:29, August 8, 1899, applies to the animals from southwestern Marin County, California.
1911 luteus (Zapus) Miller [= Zapus princeps luteus], Proc. Biol. Soc. Washington, 24:253, December 23, 1911, applies to the jumping mouse in north-central and southern New Mexico and eastern Arizona.
1913 australis (Zapus luteus) Bailey [= Zapus princeps luteus], Proc. Biol. Soc. Washington, 26:129, May 21, 1913, was applied to the jumping mouse of southern New Mexico, but is here regarded as a synonym of luteus.
1920 eureka (Zapus trinotatus) Howell, Univ. California Publ. Zool., 21:229, May 20, 1920, applies to the jumping mouse of the humid coastal district of northern California.
1931 cinereus (Zapus princeps) Hall, Univ. California Publ. Zool., 37:7, April 10, 1931, applies to the jumping mouse of extreme northwest Utah and south-central Idaho.
1931 curtatus (Zapus princeps) Hall, Univ. California Publ. Zool., 37:7, April 10, 1931, applies to the jumping mouse of the Pine Forest Mountains, Humboldt County, Nevada.
1931 palatinus (Zapus princeps) Hall [= Zapus princeps oregonus], Univ. California Publ. Zool., 37:8, April 10, 1931, was applied to the jumping mouse of Lander and Nye counties, Nevada, but is here regarded as a synonym of oregonus.
1932 kootenayensis (Zapus princeps) Anderson, Ann. Rept. Nat. Mus. Canada for 1931:108, November 24, 1932, applies to the jumping mouse of southeastern and central British Columbia, northern Idaho, and eastern Washington.
1934 idahoensis (Zapus princeps) Davis, Jour. Mamm., 15:221, August 10, 1931, applies to populations in parts of British Columbia, Alberta, Idaho, Montana, and Wyoming.
1939 utahensis (Zapus princeps) Hall, Occas. papers Mus. Zool. Univ. Michigan, 296:3, November 2, 1934, applies to the jumping mouse of southeastern Idaho, western Wyoming, and eastern Utah.
1941 burti (Zapus) Hibbard, Univ. Kansas Publ., Bull. State Geol. Surv. Kansas, 38:214, July 14, 1941, refers to two fragmentary right rami of Pleistocene age (Borchers fauna) from Loc. No. 9, Meade County, Kansas.
1942 brevipes (Zapus hudsonius) Bole and Moulthrop [= Zapus hudsonius americanus], Sci. Publ. Cleveland Mus. Nat. Hist., 5:168, September 11, 1942, based on specimens from Bettsville, Seneca County, Ohio, which are inseparable from americanus that has priority.
1942 rafinesquei (Zapus hudsonius) Bole and Moulthrop [= Zapus hudsonius americanus], Sci. Publ. Cleveland Mus. Nat. Hist., 5:169, September 11, 1942, was applied to jumping mouse of southeastern Ohio but is here regarded as a synonym of americanus.
1943 ontarioensis (Zapus hudsonius) Anderson [= Zapus hudsonius canadensis], Ann. Rept. Provancher Soc. Nat. Hist., Quebec, 1942:52, September 7, 1943, was applied to animals from eastern Ontario but is here regarded as a synonym of canadensis.
1950 pallidus (Zapus hudsonius) Cockrum and Baker, Proc. Biol. Soc. Washington, 63:1, April 26, 1950, refers to the jumping mouse from Kansas, Missouri, Oklahoma, Nebraska, and south-central South Dakota.
1951 rinkeri (Zapus) Hibbard, Jour. Mamm., 32:351, August, 1951, refers to single incomplete right ramus of upper Pliocene age, Rexroad formation and fauna, from Loc. UM-UK-47, Fox Canyon, sec. 25, T. 34S, R. 30W, XI Ranch, Meade County, Kansas.
1953 intermedius (Zapus hudsonius) described as new on page 447 of this paper.
1953 preblei (Zapus hudsonius) described as new on page 452 of this paper.
External parts.—The total length, the length of the tail, and the length of the hind foot are useful to some extent in distinguishing species and subspecies. Geographic variation in these measurements is clinal in some species. For example, Zapus trinotatus, which inhabits the western coast of North America, decreases in size from the northern to the southern part of its range. There is considerable overlap in external measurements, in specimens of the same age, between the species Z. trinotatus and Z. princeps, but only slight overlap between Z. princeps and Z. hudsonius and between Z. trinotatus and Z. hudsonius. If all collectors measured external parts in the same way the measurements would be more useful for differentiating one species from another.
Pelage.—The pelage, both in its entirety and as individual hairs, provides taxonomic characters as has been pointed out by Moojen (1948:324) for the genus Proechimys, by Williams (1938:239) for the Insectivora, and by Hausman (1920:496) for several groups of [372] mammals. In addition to the sensory hairs, facial vibrissae, nasal hairs, and carpal vibrissae, there are three kinds of hairs in the normal coat of Zapus: guard hairs, overhairs, and underfur. The guard hairs and underfur differ in different species (see figs. 35-37).
The guard hairs taper at both ends, are elliptical in cross section, and are wider and longer than the other two kinds of hair. The bases of the guard hairs are grayish, and the amount of pigment gradually increases distally to a dark brownish or blackish shade. The guard hairs vary in greatest diameter from 96 microns to 168 microns, depending upon the species, and variation in diameter provides characters of taxonomic worth. No clinal variation in diameter of the guard hairs was detected. In Z. hudsonius the guard hairs average 115 microns (96-140) and are significantly narrower than those of Z. princeps and Z. trinotatus, which average 142 microns (130-168) and 141 microns (133-154), respectively. Pigmentation of the guard hairs contributes little information useful in separating the species of Zapus. All of the species have a prominent compounded medulla in which the pigment cells anastomose to form a labyrinthine column.
The individual hair of the underfur is cylindrical and tapers abruptly at each end; it is short, thin, flexible, and usually is bicolored on the back and sides of the mouse. The apical zone is yellow-brown (for example, Ochraceous-Buff) and the proximal part is whitish or grayish, which gradually darkens to near black subapically.
The width of a hair in the underfur is of no taxonomic significance, in that individual variation exceeds that between species.
The pattern of the pigment in the medulla of the hair, however, does vary specifically. Comparable samples from Z. trinotatus, Z. princeps, and Z. hudsonius of the same age, sex, and season reveal a pattern characteristic for each species (see figs. 35-37).
All species of Zapus agree closely in color pattern. A broad longitudinal dorsal band of some shade of yellow-brown flecked with black hairs is bordered by a lateral band of a lighter color usually containing fewer black hairs than on the dorsum. The underparts are usually white but are sometimes suffused with color resembling that on the sides. Between the white underparts and the darker color of the sides there is often a narrow, clear ochraceous stripe. Dorsal and lateral hairs are uniformly grayish-white at their bases; only the distal parts of the hairs are responsible for the external color of the animal.
The pelage of juveniles is usually finer and softer than the pelage of adults. The lateral and dorsal bands are not so conspicuously marked in young animals, and individual hairs are not so long or so wide as in adult animals.

Figs. 35-37. Photomicrographs of underhairs (middle third) from each of the species of the genus Zapus. × 500.
Fig. 35. Zapus t. orarius, adult, female, No. 20293 MVZ, 3 mi. W Inverness, 300 ft., Marin County, California.
Fig. 36. Zapus p. oregonus, adult, male, No. 47856 KU, Harrison Pass R. S., Ruby Mt’s, Elko County, Nevada.
Fig. 37. Zapus h. pallidus, adult, male, No. 22954 KU, 4 mi. N, 13/4 mi. E Lawrence, Douglas County, Kansas.
Preble (1899:7) and Howell (1920:226) remark as to the noticeable difference between pelages of spring and early fall. The pelage in spring is described as bright and fresh whereas that in fall is dull and worn. Actually both bright and worn pelages can occur in any one population at any one time. Some newly molted individuals are in fresh unworn pelage; some individuals, which are molting, are in ragged, worn pelage; and other individuals perhaps could be found to represent intermediate stages.
Variations from the normal color of the pelage are rare. Among more than 3,000 specimens of Zapus examined there were only 12 individuals (five Z. princeps, 6 Z. hudsonius, and 1 Z. trinotatus) that were abnormally colored. A single white spot was noted on each of 10 (5 Z. princeps, 4 Z. hudsonius, and 1 Z. trinotatus) of these individuals; the spots were on the dorsal, anterior half of the body. The skin beneath the patch of white hair was in each animal like that beneath the neighboring normally-pigmented hair. One specimen of Z. hudsonius (NMC No. 6669) is everywhere black, excepting the dorsal surface of the toes of the forefeet. Most of the individual hairs from various areas of the body are black for their [374] entire length; some, however, have non-pigmented silvery tips. One specimen of Z. hudsonius (KU No. 645) lacks any black; dorsally the pelage is nearest to Ochraceous-Buff and it is white on the venter. Individual hairs of the dorsal area are white for the basal two-thirds of their length (as compared to gray and brown in the animals with normal pigmentation) and near Ochraceous-Buff on the distal third (as compared to hairs which are dark brown tipped with Ochraceous-Buff). The feet and tail are white.
Molt.—The sequence of molt for Zapus has been ascertained from examination of the study skins. In all species of this genus there seems to be only one annual molt in adults. In the young of the year this molt occurs after August first and before hibernation. All individuals of a single population do not molt at any one time; females continue to molt later in the autumn than do the males; some individuals begin the molt as early as mid-June and others show molt as late as the end of October; approximately three weeks are required for an individual to complete its molt (Quimby, 1951:74); readiness for molt and early stages in molt can be detected (in museum specimens) by the greater thickness of the skin. Hairs lost accidentally are quickly replaced, regardless of the condition of the molt.
In Zapus hudsonius, new hair appears simultaneously on the anterior dorsal surface of the nose and on the mid-dorsal surface between the scapulae. The molt proceeds anteriorly from the shoulders and posteriorly from the nose. At the same time that the head is covered, new hair appears on the sides of the body from the forelegs to the cheeks. New pelage then appears posteriorly, and molt continues as a wave from these points over the sides and back with the rump receiving new hair last (see figs. 42 and 43).
In Zapus princeps new hair appears first on the mid-dorsal surface between the scapulae. From this starting point molt progresses anteriorly, laterally, and posteriorly. Progress over the head is rapid; the head receives its new hair sooner than the caudal region. Molt moves progressively nearer to the base of the tail and progressively nearer to the mid-ventral surface. The rump is the last area to complete its molt (see figs. 40 and 41).
The progress of molt in Z. princeps might be likened to the flow of a drop of paint on the curved surface of a ball where the paint flows in all directions but is speeded at one point and slowed at the opposite by a slight tilting of the ball from the horizontal.
In the species Zapus trinotatus new hair appears simultaneously [375] on the anterior, dorsal surface of the nose and on the mid-dorsal surface between the scapulae. In this respect the progress of molt of Z. trinotatus resembles that of Z. hudsonius. From these starting points molt progresses rapidly over the head, the molt moving anteriorly from the shoulders and posteriorly from the nose with the result that it covers the dorsal surface of the head; hair then appears on the cheeks and sides of the neck. The progress of molt on the remaining areas of the body is comparable to that of Z. princeps; molt progresses toward the tail and toward the mid-ventral line. The rump, as in Z. princeps, is the last area to complete its molt (see figs. 38 and 39).

Figs. 38-43. Diagrams showing differences in progress of molt in the three species of the genus Zapus. All approximately 1/2 natural size. Figs. 38, 40 and 42 lateral view. Figs. 39, 41 and 43 dorsal view.
Figs. 38 and 39. Zapus trinotatus.
Figs. 40 and 41. Zapus princeps.
Figs. 42 and 43. Zapus hudsonius.
Baculum.—The general shape and dimensions of the baculum (os penis) provide characters of taxonomic value for the species of Zapus (see figs. 23-25 and figs. 28-30).
Three measurements—length, transverse diameter at the base, and transverse diameter at the tip—are easily obtained and are diagnostic. The bacula of all species are somewhat curved. The measurement of length used by me does not represent the actual length of the bone, but instead the chords of the arcs involved.
Skull.—Some of the structures useful for separating taxonomic entities may have little or no biological significance to the animals [376] in nature. Characters mentioned by me are chosen simply for their significance taxonomically. The zygomata vary in degree of lateral bowing, being widely bowed in Z. princeps and Z. trinotatus, and less so in Z. hudsonius. Differences in zygomatic breadth owing to the degree of bowing are an aid in differentiating subspecies. The length of the skull from the occipital condyles to the tip of the longest nasal bone is useful in separating Z. hudsonius from Z. trinotatus and Z. princeps. The narrowness of the base of the zygomatic process of the squamosal is useful in distinguishing between Z. hudsonius and Z. princeps, but shows no variation of subspecific worth. The shape and dimensions of the incisive foramina provide specific and subspecific characters. The position of the anterior margin of the postpalatal notch, in relation to the last molars, provides subspecific characters in Z. princeps. In the species Z. princeps the median projection on the inferior ramus of the zygomatic process of the maxillary is absent in some subspecies, small in others, and large in some. Shape and inflation of the auditory bullae, shape of the pterygoid fossae, and shape of the nasals are useful in determining specific and subspecific relationships.
Teeth.—The alveolar length of the upper maxillary tooth-rows aids in distinguishing Z. hudsonius from Z. princeps and Z. trinotatus. Nearly parallel versus anteriorly divergent upper tooth-rows is a subspecific difference in Z. princeps. Variations in the dimensions of P4 and M1 aid in estimating the relationships of species. The occlusal pattern shows little variation and was of no use in separating species.
A knowledge of variation resulting from age, individual, or secondary sexual differences, as opposed to geographic variation between two or more populations of a single species is important in determining the reliability of taxonomic characters.
The largest population-sample of Zapus available to me for the study of nongeographic variation was 63 individuals from various localities in Keweenaw and Menominee counties, Michigan. Thirty-nine were females and 24 were males. It is on these specimens that this discussion is based.
Teeth.—The teeth provide a valuable standard for age determination in that they wear at a measurable rate. The molars erupt in sequence from front to back, and wear shows first on M1 and last on M3. The peglike permanent P4, of which I have not seen the deciduous precursor, receives wear at the same time that the molars are being worn. Wear proceeds at approximately the same rate in the teeth of both the upper jaws and lower jaws.
In order to be more nearly certain that specimens used in making racial comparisons were comparable as to age, six age-groups were established, from youngest to oldest. These groups were based on the degree of wear on the occlusal surface of the upper cheek-teeth, and are as follows: group 1, in which M1 and M2 have not reached full and equal height and show no occlusal wear, and M3 has not erupted or is just breaking through the alveolus; group 2, in which M1 and M2 have reached full and equal height and show slight wear, and M3 may be almost or quite equal in height to M1 and M2 and, when equal, sometimes shows slight wear; group 3, in which M1 and M2 show wear on all cusps but cusps are visible, and M3 shows slight wear; group 4, in which P4 shows slight wear, M1 has cusps and re-entrant folds between cusps mostly gone, M2 shows considerable wear but re-entrant folds are visible, and M3 has most re-entrant folds and cusps gone; group 5, in which P4 shows considerable wear, M1 has cusps completely worn away, M2 has re-entrant folds and cusps worn away, and M3 lacks occlusal pattern except for one or two lakes; group 6, in which all upper cheek-teeth are without occlusal pattern.
These groupings are based on continuously variable features, and, therefore, when the teeth are at certain stages of wear a specimen is difficult to place in one of two groups.
Age group 1 and 2 include juvenal and subadult animals. Animals of age groups 3 through 6 are considered adult. Individuals of age groups 3 through 5, including as they do the great majority of the adult population, were the only age classes used in measuring geographic variation.
Quimby’s (1951:69) data indicate that some mice produce litters at the age of approximately 2 months, when four-fifths grown. Therefore, sexual maturity is not always synonymous with morphological maturity.
Measurements of external parts.—Data presented here on Z. hudsonius are those recorded by Quimby (1951) on specimens from Anoka County, Minnesota, and those obtained by me from museum specimens from Menominee and Keweenaw counties, Michigan.
According to Quimby (1951:65-66) the mean length [= body length] for three newly born Z. hudsonius is 24.8 mm (24.0-25.5); at the end of the fourth week of growth the mean length averaged 64.4 mm and at the 13th week 77.6 mm. Rapid growth occurs during the first four weeks, with the mean length increasing approximately 2.6 times the size at birth. After the fourth week of development, growth proceeds at a slower rate; the mean length at 13 weeks is only 3.1 times greater than the mean length at birth.
In specimens assigned to age groups 1 and 2 the length of the body averaged 70 and 74.8 mm, respectively. The individuals of both groups are less than 13 weeks old if we assume that growth proceeds at the same rate in Michigan as it does in Minnesota.
In the specimens from Michigan of age groups 3, 4, 5, and 6 the average length of the body is 80.9, 83.7, 89.0, and 83.6, respectively.
According to Quimby (loc. cit.), the average length of the tail for three Z. hudsonius at birth was 9.2 mm. (8.5-10.0). During the first four weeks of development the tail grew rapidly and reached an average length of 92.0 mm, which was 10 times the length at birth. By the end of 13 weeks of development the average length of the tail for these three individuals was 119.6 mm or 12 times the average length at birth. The most rapid growth [378] was early in development: 80 per cent of the growth of the tail occurred during the first month, after which growth proceeded at a much slower rate.
Quimby (loc. cit.) records an average dimension of 4.7 mm (4.5-5.0) for the length of the hind foot in three newly born Z. hudsonius. The hind foot grew rapidly in length and by the fourth week had increased 5.6 times in its length and averaged 26.3 mm. Growth was much less rapid from the fourth to the thirteenth week when the hind foot averaged 27.7 mm, only five per cent more than in mice four weeks old. Assuming the average length of the hind foot of the adults to be 29.0 mm, the hind foot in individuals 13 weeks old is 96 per cent of the adult size.
According to Quimby (loc. cit.), the pinna of the ear at birth is small and folded over the external auditory meatus. The length of the ear increases proportionately more (29 per cent) than any other external dimension after the first four weeks of growth.
If the average length of the ear (measured from the crown) of adults is 14.7 mm, the animals from Michigan in age groups 1 and 2 are 91.8 per cent and 96.5 per cent as large as adults.
Table 1.—Average Dimensions (in Millimeters) for Specimens of Z. h. hudsonius of Various Ages (Specimens from Michigan).
| Age groups | 1 | 2 | 3 | 4 | 5 | 6 |
| No. examined | 4 |
13 |
33 |
12 |
3 |
3 |
| Body | 70.0 |
74.8 |
80.9 |
83.7 |
89.0 |
83.6 |
| Tail | 113.8 |
118.5 |
122.9 |
125.0 |
125.0 |
118.3 |
| Hind foot | 28.8 |
28.6 |
28.9 |
29.1 |
28.9 |
29.3 |
| Ear | 13.5 |
14.2 |
14.7 |
14.8 |
15.0 |
14.3 |
From these data, concerning growth of external parts, it seems that: growth is most rapid during the four weeks following parturition; specimens from Michigan, assigned to age groups 1 and 2 on the basis of tooth wear, are less fully developed and probably younger than mice from Minnesota, with a known age of 13 weeks; individuals with sufficient wear on the teeth to be placed in age group 3, if they were obtained in the late fall, may be young from the first litters of the year or, if they were obtained in early spring, may be at least one year old; individuals in age groups 4, 5, and 6 are at least one year old.
Skull.—The post-embryonic development of the skull is rapid. Animals in age groups 1 and 2 have skulls which average more than 80 per cent of the size that is here considered adult (an average size obtained from age groups 3, 4, and 5). The actual increase in size of certain cranial elements for various age groups is given in table 2.
In age group 1 the rostrum is relatively short as it is in Neotoma micropus (J. A. Allen, 1894:235) and juveniles of Peromyscus truei (Hoffmeister, 1951:7). The rostrum lengthens rapidly and there is a general increase in actual and relative size of the entire preorbital region; the increase after age group 3 is slower and of lesser magnitude. Changes with age in the size of the braincase are slight. In age group 1 the average depth of the braincase is 99.6 per cent of the adult size; the average breadth of the braincase is 98 per cent of the adult size, and the average width across the mastoid region is 96.4 per cent of the adult size. These dimensions indicate that the braincase reaches full size early. The zygomatic arch, however, undergoes change with age; there is a gradual increase in breadth owing to lateral bowing and a gradual lengthening which is in keeping with a general elongation of the skull anterior to the braincase.
The incisive foramina in age group 1 are short (4.0 mm), broad (2.2 mm in the middle), and taper to a point at each end. In age group 2 the foramina have elongated (4.2 mm) and are less pointed posteriorly, but there is no change in breadth. In age groups 3, 4, 5, and 6 the foramina become progressively longer (4.5 mm in age group 6), have a relatively constant breadth (2.2 mm), and become more nearly truncate anteriorly.
Table 2.—Average and Extreme Measurements (in Millimeters) of Skulls of Six Age-groups in Specimens of Zapus hudsonius from Michigan.
| Age groups | 1 | 2 | 3 | 4 | 5 | 6 |
| Number examined | 4 | 13 | 33 | 14 | 3 | 3 |
| Occipitonasal length | 20.5 20.0 21.2 |
21.2 20.8 21.8 |
22.0 21.5 23.2 |
22.7 21.8 23.4 |
22.9 22.7 23.3 |
23.0 22.4 23.7 |
| Mastoid breadth | 9.8 9.7 10.0 |
10.04 9.6 10.4 |
10.12 9.5 10.5 |
10.12 9.6 10.7 |
10.3 10.0 10.8 |
10.36 10.1 10.8 |
| Length of zygomatic arch | 8.07 8.0 8.2 |
9.02 8.5 9.3 |
9.07 8.5 9.4 |
9.25 9.2 9.4 |
9.5 9.5 9.5 |
9.35 9.1 9.6 |
| Breadth of palate at P4 | 3.36 3.3 3.5 |
3.33 3.1 3.4 |
3.37 3.1 3.8 |
3.44 3.1 3.7 |
3.66 3.6 3.7 |
3.45 3.4 3.5 |
| Breadth of palate at M3 | 2.4 2.3 2.6 |
2.55 2.3 2.7 |
2.66 2.3 3.2 |
2.74 2.5 3.0 |
3.11 3.0 3.2 |
2.77 2.6 2.9 |
| Palatal length | 8.67 8.4 9.1 |
8.98 8.8 9.2 |
9.38 9.3 9.8 |
9.59 9.0 10.0 |
9.73 9.5 9.9 |
9.8 9.6 10.1 |
| Distance from incisors to postpalatal notch | 8.53 8.4 8.7 |
8.98 8.5 8.5 |
9.08 9.0 9.8 |
9.68 9.2 10.0 |
9.73 9.5 9.9 |
9.80 9.6 10.1 |
| Interorbital breadth | 4.25 4.2 4.3 |
4.19 4.0 4.4 |
4.2 4.0 4.4 |
4.2 4.0 4.4 |
4.23 4.1 4.4 |
4.2 4.2 4.2 |
| Average length of upper molar series | 3.2 3.2 3.4 |
3.2 3.2 3.4 |
3.21 2.9 3.5 |
3.22 2.9 3.5 |
3.2 3.2 3.2 |
3.16 3.1 3.2 |
| Breadth of braincase | 9.5 9.3 9.7 |
9.58 9.2 9.7 |
9.61 9.1 10.0 |
9.68 9.3 10.0 |
9.83 9.5 10.2 |
9.63 9.3 9.9 |
| Zygomatic breadth | 10.33 10.0 10.7 |
10.49 10.4 10.9 |
10.55 10.1 11.2 |
10.80 10.7 11.2 |
11.0 10.5 11.5 |
11.25 11.2 11.3 |
| Condylobasal length | 16.9 16.6 17.1 |
18.33 17.4 19.2 |
18.80 18.2 19.5 |
19.33 18.5 19.9 |
19.6 19.4 19.8 |
19.9 19.5 20.3 |
Measurements of external parts in Zapus are more variable than are measurements of most parts of the skull. As Hoffmeister (1951:16) points out for Peromyscus truei, this variation in external features results in part from “the difficulties in accurately measuring soft parts of the anatomy” and also from inconsistencies on the part of collectors in making these measurements.
A comparison of coefficients of variation (see table 3) for cranial measurements between populations of like age and sex for the species Z. hudsonius, Z. princeps, and Z. trinotatus shows that variation of approximately the same degree is recorded in corresponding elements in all species; that is to say, structures which are most variable individually in Z. princeps are also most variable in Z. trinotatus and Z. hudsonius.
Individual variation in the occlusal pattern of the molariform teeth is slight. In several specimens, however, the re-entrant fold is absent from the lingual surface of M1. Teeth in addition to the normal number were recorded for five specimens. In all instances they are in the upper dentition and usually at the posterior end of the maxillary tooth-row. In each of four specimens (KU No. 34852, KU No. 32852, MVZ No. 52105, all Z. princeps, and USBS No. 22921, Z. hudsonius), there is only a single additional tooth. One individual (USBS No. 264388, Z. princeps) possessed two extra molars, one in each maxillary tooth-row. The extra teeth vary in size from those which are only slightly smaller than the adjacent normal molars to those which are simple, peglike structures. In four of the five animals the extra teeth are posterior to the normal M3; in the fifth (MVZ No. 52105) the added tooth is anteriormedial to M3.
Table 3.—Coefficients of Variation for Dimensions of Corresponding Parts of the Skull of Three Species of Zapus. The Specimens of Zapus hudsonius are from Menominee and Keweenaw counties, Michigan, the Zapus princeps are from the Vicinity of Encampment, Wyoming, and the Zapus trinotatus from Huntingdon, British Columbia.
| Species | Z. h. hudsonius | Z. p. princeps | Z. t. trinotatus |
| No. examined | 52 | 46 | 19 |
| Mastoid width | 2.85 | 1.98 | 2.21 |
| Occipitonasal length | 2.64 | 1.37 | 1.20 |
| Incisors to postpalatal notch | 3.02 | 2.56 | 2.56 |
| Interorbital constriction | 2.75 | 3.66 | 3.22 |
| Zygomatic breadth | 2.74 | 2.54 | 1.94 |
| Maxillary tooth-row | 4.50 | 4.44 | 3.82 |
The size and shape of certain cranial elements vary individually even between right and left sides of the same animal. The paired parietal bones in [381] some animals are nearly square and identical. In other animals these bones are approximately equal and straight on three sides with the fourth side forming an anterolateral projection; this projection may be slightly or greatly produced, and opposite elements in a single individual differ in this respect.
The interparietal also is variable; the lateral arms may be blunted and not included in the fusion of the squamosal, parietal, and occipital elements, or the interparietals may be elongated and fused with these elements. Posterior and anterior borders of the interparietal may be straight, produced anteriorly, produced posteriorly, or produced anteriorly and posteriorly.
There is frequently variation in the degree of taper of the nasals. They may be parallel sided, narrowed distally, or narrowed proximally. There is some variation in the degree of inflation, in the size, and in the shape of the frontal bones. The anterior surface of the postpalatal notch varies individually and may be truncate, anteriorly convex, or anteriorly concave.
Individual variation in the color of the pelage of animals that are in the same stage of molt or non-molt is by my observation slight. The presence of oil in the hair results in a false impression of sleekness and seemingly darker pigmentation. Abnormal white-spotting dorsally occurs as does yellow and melanistic coat color. These mutations are considered in the discussion concerning pelage.
In specimens of the two sexes from similar age groups of hudsonius from Michigan, the mean values for each measurement for the two sexes differ only slightly or are essentially the same (see table 4). In no species has secondary sexual variation been found to be greater than individual variation.
Table 4.—Mean Measurements for Adult Male and Female Z. hudsonius of Age Group 2 and Per Cent Difference of Females to Males (Specimens from Michigan).
| Sex | Male | Female | Per cent difference, females to males | |
| No. examined | 18 | 15 | ||
| Total length | 202.85 |
202.88 |
0.02% | larger |
| Hind foot | 122.85 |
122.10 |
0.60% | smaller |
| Mastoid width | 10.10 |
10.28 |
1.50% | larger |
| Occipitonasal length | 22.15 |
22.03 |
0.55% | smaller |
| Incisors to postpalatal notch | 9.39 |
9.33 |
0.64% | smaller |
| Zygomatic breadth | 10.47 |
10.57 |
0.95% | larger |
| Maxillary tooth-row length | 3.52 |
3.60 |
0.23% | larger |
| PAGE | |
| Zapus trinotatus | 385 |
| Zapus trinotatus eureka A. B. Howell | 389 |
| Zapus trinotatus montanus Merriam | 390 |
| Zapus trinotatus orarius Preble | 391 |
| Zapus trinotatus trinotatus Rhoads | 392 |
| Zapus princeps | 394 |
| Zapus princeps cinereus Hall | 399 |
| Zapus princeps curtatus Hall | 400 |
| Zapus princeps idahoensis Davis | 401 |
| Zapus princeps kootenayensis Anderson | 404 |
| Zapus princeps luteus Miller | 406 |
| Zapus princeps minor Preble | 407 |
| Zapus princeps oregonus Preble | 409 |
| Zapus princeps pacificus Merriam | 412 |
| Zapus princeps princeps Allen | 414 |
| Zapus princeps saltator Allen | 416 |
| Zapus princeps utahensis Hall | 418 |
| Zapus hudsonius | 420 |
| Zapus hudsonius acadicus (Dawson) | 432 |
| Zapus hudsonius alascensis Merriam | 435 |
| Zapus hudsonius americanus (Barton) | 436 |
| Zapus hudsonius campestris Preble | 441 |
| Zapus hudsonius canadensis (Davies) | 442 |
| Zapus hudsonius hudsonius (Zimmerman) | 443 |
| Zapus hudsonius intermedius Krutzsch | 447 |
| Zapus hudsonius ladas Bangs | 449 |
| Zapus hudsonius pallidus Cockrum and Baker | 450 |
| Zapus hudsonius preblei Krutzsch | 452 |
| Zapus hudsonius tenellus Merriam | 453 |
External characters.—Muriform in general appearance; forelimbs small, short; hind limbs greatly developed; hind feet long and narrow; tail tapering, attenuate, subcylindrical; head long and mouse-shaped; eyes small and situated midway between nose and ear; external ear somewhat longer than surrounding hair and provided with antitragal flap which can cover external auditory meatus, and in company with tragus completely close opening; upper lip without median groove; internal cheek-pouches well developed and opening at corners of mouth; mystacial vibrissae conspicuous; supercilliary vibrissae few; genal tuft absent; teats normally eight and arranged in pairs (one pectoral, two abdominal, and one inguinal); anterior and posterior pairs frequently undeveloped; general pelage coarse; color of pelage varies somewhat in different species but always follows single basic pattern of broad dorsal band of some shade of brown or brownish-yellow darkened with brownish-black, sides of a lighter tone and slightly streaked with brownish-black, underparts snow-white, sometimes suffused [383] with color of the sides and usually separated from color of sides by sharp line of clear brownish-yellow; backs of forefeet and hind feet grayish-white; tail distinctly bicolor, dark brown above and yellowish-white below; ears dark and narrowly edged with light color.
Cranial characters.—Skull short in relation to width, deep relative to other dimensions, somewhat convex; delicate, papery, without strong angularity; braincase relatively unexpanded; antorbital foramen obliquely oval and transmits masseter muscle of great size; foramen in inferior ramus of zygomatic process of maxillary for passage of superior maxillary branch of trigeminal nerve small; zygomata not wide-spreading; underside of zygoma nearly horizontal, upper edge anteriorly rises prominently owing to extension of jugal upward along maxillary; jugal and lachrymal in contact; one ramus of zygomatic process of maxilla arises directly above other; rostrum thick basally and relatively attenuate distally; ends of nasals project noticeably beyond incisors; premaxillaries develop strong alveolar plate separating superior incisors for half their length; palatal bones shortened posteriorly, free edge often concave; incisive foramina long, broad, and separated by bulbose (except at posterior end) bony septum; mastoid bullae absent; auditory bullae short and transversely placed; postorbital process never present; parietals nearly square, sometimes emarginate in front; angle of mandible flattened and bent inward; coronoid process weak, acute, and slopes strongly upward.
Dental characters.—Dental formula
I 1 | C O | P 1 | M 3 |
= 18; | |||||||
| , | , | , | |||||||||
i 1 | c o | p o | mv 3 |
upper incisors short, compressed, curved backward, and strongly grooved; lower incisors slender, curved backward, and ungrooved; both upper and lower incisors deep orange or yellow; four upper cheek-teeth present; premolar small, single rooted and, sometimes, non-functional; upper molars tri-rooted, sub-hypsodont, and with occlusal surface non-cuspidate (flat); enamel pattern, much complicated, consisting of one main re-entrant fold lingually and four re-entrant folds labially; three lower molars, bi-rooted, sub-hypsodont, flat crowned, with two outer and four inner re-entrant folds.
Postcranial characters.—Neck short and weak; atlas large; axis separate from atlas; remaining (5) cervical vertebrae also free; thoracic (12) and lumbar (7) vertebrae strongly built; posterior lumbars with enlarged neural and anteriorly directed transverse processes; sacral vertebrae (7) as in murids; caudal vertebrae variable in number (average 36); clavicle long, slender, uniformly curved, convex outwardly; scapula with supraspinous and infraspinous fossae of equal size; forelimbs short, approximately half as long as hind limbs; hind limbs elongate, slender; femur with third trochanter; tibia and fibula fused slightly distal to middle of former; five elongate, separate metatarsals (first and fifth subequal, shorter than others).
| A. | Baculum with tip spade-shaped and tip wider than 0.43 mm; underfur with medullary pattern rectangular, cuticular scales small; coronoid process of mandible long and slender, angle of divergence from condyle broad; angle of mandible turned in and wide; pterygoid fossae wide; skull broad in relation to length; premolars with crescentine fold on occlusal surface. | |
| Zapus trinotatus p. 385 | ||
| A´. | Baculum with tip lanceolate (not spade-shaped) and tip less than 0.43 mm wide; underfur with medullary pattern square or rectangular; but, if rectangular, cuticular scales large; coronoid process short and broad, angle of divergence from condyle narrow; angle of mandible turned inward and small to medium; pterygoid fossae usually narrow; skull not broad in relation to length; premolars without crescentine fold on occlusal surface. | |
| B (A´). | Baculum less than 5.1 mm in total length; guard hair averaging 115 micra in diameter; underfur with rectangular medullary pattern, cuticular scales large; skull small; incisive foramina shorter than 4.6 mm; condylobasal length averaging less than 20 mm; length of maxillary tooth-row averaging less than 3.7 mm; palatal breadth at M3 less than 4.2 mm. | |
| Zapus hudsonius p. 420 | ||
| B´. | Baculum more than 5.1 mm in total length; guard hair averaging more than 140 micra in diameter; underfur with square medullary pattern, cuticular scales moderately large; skull large; incisive foramina longer than 4.7 mm; condylobasal length more than 21 mm; maxillary tooth-row averaging more than 3.8 mm; palatal breadth at M3 more than 4.4 mm. | |
| Zapus princeps p. 394 | ||
Range.—From southwestern British Columbia southward through western Washington and Oregon and in the humid coastal district of California almost to the Golden Gate (see fig. 45).
Characters of the species: External.—Size medium to large (total length 221 mm to 238 mm); tail longer than head and body (131 mm to 149 mm) and bicolored, brown above, white to yellowish-white below; hind feet long (31 mm to 34 mm), grayish-white above; back various hues and tones of ochraceous and tawny; sides paler than back; lateral line separating sides from ventral surface usually distinct and bright; ventral coloration white, usually with suffusion of ochraceous; ears usually dark, sometimes flecked, and usually narrowly edged with color of sides; guard hairs average 141 microns (133u to 155u) in diameter; underhair with medullary pigment in narrow, hollow rectangles; cuticular scales of underhair smaller and more numerous than in other species.
Baculum.—Size large (total length 6.7 mm to 7.4 mm); base broad (0.7 mm to 0.9 mm); tip broad (0.44 mm to 0.57 mm); spade-shaped in dorsal aspect and tilted upward, gradually tapering to thin-edged tip; shaft rounded, straight.
Skull.—Large, broad and deep in relation to length; pterygoid fossa broad; anterior ramus of zygomatic process of maxillary relatively narrow; nasofrontal juncture relatively broad; coronoid process of mandible elongate. Upper premolars relatively large (averaging .70 mm in length and .75 mm in width), usually functional, occlusal surface with labial re-entrant fold forming crescentine loop incompletely enclosing single central cusp; m3 relatively large, elongated; m1 elongated, broadly rounded anteriorly.
There are four subspecies currently recognized, all of which are confined to the Pacific coastal region of North America (See fig. 45). The features that vary geographically are external size, color of pelage (shade and tone of upper parts and tint of lower parts), and dimensions of certain cranial structures (zygomata, braincase, incisive foramina, palatal bridge, auditory bullae, and pterygoid fossae).
External size is smallest in the southernmost geographic race (Z. t. orarius) and largest in the northernmost geographic race (Z. t. trinotatus). This decrease in size from north to south is clinal and is in keeping with Bergman’s Rule which postulates that within one species the smallest individuals occur in the warmer parts of its geographic range.

Fig. 45. Map showing distribution of Zapus trinotatus.
| 1. Z. t. eureka 2. Z. t. montanus 3. Z. t. orarius 4. Z. t. trinotatus |
Coloration of pelage is geographically variable. There is a gradual change in the color of the pelage from north to south. Animals obtained in the northern part of the geographic range of Z. trinotatus are generally darker dorsally (more tawny) with the ventral pelage usually pure white. Those individuals from the southern part of the geographic range of Z. trinotatus have the dorsal pelage lighter (more reddish and yellow-brown) and ventrally the pelage is usually heavily suffused with reddish-brown. The crania also vary geographically; they are largest in the northernmost part of the range of the species and smallest in the southernmost part.
Habitat.—On the Olympic Peninsula, Washington, in 1931 Svihla and Svihla (1933:132) found this species equally abundant in alpine meadows near timberline, in open grassy areas, and in tall meadow grass and low blueberry bushes. All of the mice were in wet marshy places. Bailey (1936:232) reported that in Oregon, these mice live in meadows, marshes, under ferns and weeds in the woods, or near mountain brooks and streams. Taylor (1922:221) found Zapus in moderately moist meadows in the Hudsonian Life-zone at Mt. Rainier, Washington, and Dice (1932:49) found them in deciduous forest and in open, grassy, or sphagnum bogs. Dice records it as common also among the alders and willows in high, open, grassy parks. Merriam (1897b:223) found Z. trinotatus abundantly in moist places grown-over with grass or weeds. Grass cuttings two to three inches long were left in small heaps at feeding sites and indicate the presence of these mice.
Behavior.—Svihla and Svihla (1933:131) write that the long tail of Z. trinotatus is used as a balancing organ when the mouse is in motion. A tailless mouse, attempting to escape, turned somersaults in the air and invariably landed on its back; the loss of its tail seemed to leave the mouse without compensation for the vigorous push of the hind legs. Dalquest (1948:371) noted that the jumping mouse sometimes walks on all fours, but ordinarily moves by means of short hops on the hind feet alone. When startled, jumping mice travel in bounds of six feet or more at a jump.
Zapus trinotatus, according to Bailey (1936:232) and Elliot (1899:261), is mainly nocturnal but occasionally is active in daylight.
Svihla and Svihla (op. cit.:132) heard captive animals make squeaking noises when fighting. On several occasions captive animals [388] made a drumming noise by rapidly beating the tail against a resonant body such as the bottom of a tin can.
Concerning hibernation, Bailey (loc. cit.) remarks that animals of this species in Oregon, become fat in early autumn and lay down excess adipose tissue under the skin, over the muscles, and in the abdominal cavity. Svihla and Svihla (op. cit.:133) noted that captives from the Olympic Peninsula, Washington, gained weight in September and October and became extremely fat. With the additional weight they were more listless and drowsy, often spending days curled up in the hibernating position with the head between the hind legs and the long tail curled completely over the head and body. Warmth aroused the animals to activity, but when the temperature dropped they again hibernated. Flahaut (1939:17) reported the discovery on February 23, 1939, at Henderson Inlet, South Bay, Thurston County, Washington, of two nest cavities inhabited by jumping mice that were hibernating. The nests, four inches apart and 30 inches below the surface of the ground, were approximately five inches in diameter and made of shredded paper. Both mice were dormant, covered by nesting materials and curled up in the aforementioned hibernating posture. Dalquest (1948: 371) writes that in the lowlands of Washington this species disappears by late July but that in the mountains it remains active until the middle of September. Edson (1932:56) records an individual taken on April 20 from its place of hibernation beneath the roots of a decaying stump. This animal quickly roused in the warm mid-afternoon sun but became dormant again when the temperature dropped to 45° F. It seems that animals near the end of hibernation become active on warm days and return to the torpid state on cold ones.
Enemies.—Little is recorded concerning enemies of Z. trinotatus, but Bailey (1936:233) lists owls and other nocturnal birds, weasels, skunks, and badgers as preying on this mouse. Smith and Hopkins (1937:191) found Z. t. orarius in barn owl pellets obtained in Elk Valley, Marin County, California.
Food.—Bailey (loc. cit.) remarks that in Oregon, these mice feed mainly on small seeds of grasses, small grains (wheat, barley, oats, and rye), and other plants. These seeds are obtained by cutting the stems, drawing the stems down and biting off lower sections until the seed-laden heads are reached. Bailey (op. cit.:234) found that trinotatus utilized also the seeds of the western skunk cabbage.
Near Seattle, Washington, according to Dalquest (loc. cit.), the principal food of Z. trinotatus was velvet grass (Holchus lanatus), [389] broad-leaved dock, and the seeds of other grasses. Dalquest reports also that the fruit of the blackberry (Rubus macropelatus) is eaten and that an occasional jumping mouse has its chin stained a deep purple by juice from these berries.
Reproduction.—There is normally a single litter of from four to eight young per year according to Bailey (loc. cit.). Newly born young have been described by Svihla and Svihla (1933:132) as follows: slightly smaller than newly born harvest mice (Reithrodontomys m. megalotis), average weight .8 grams, hairless (without even vibrissae visible), pink, eyes closed, ears folded, heads short and stubby, tails long (longer than those of newly born Peromyscus), and bodies surprisingly small (when compared with newly born Peromyscus maniculatus).
Zapus trinotatus eureka A. B. Howell, Univ. California Publ., Zool. 21:229, May 20, 1920.
Zapus trinotatus trinotatus, Preble, N. Amer. Fauna, 15:26, August 8, 1899 (part—the part from Crescent City and Carsons Camp, Mad River, California).
Zapus orarius Preble, N. Amer. Fauna, 15:29, August 8, 1899 (part—the part from Eureka and Carsons Camp, Mad River, California).
Type.—Female, adult, skin and skull, No. 11703, Mus. Vert. Zool.; Fair Oaks, Humboldt County, California; obtained on August 27, 1910, by Joseph S. Dixon, original No. 1743.
Range.—Northwestern coastal region of California, from Russian Gulch State Park, Mendocino County north to Trinidad, Humboldt County. Zonal range: humid Transition.
Description.—Size medium; color dull; back near Ochraceous-Buff with heavy admixture of black hairs, forming broad dorsal band; sides from near Ochraceous-Buff to near Ochraceous-Salmon, sometimes with heavy admixture of black hairs; lateral line usually distinct, sometimes blending with color of belly and side; ventral surface usually suffused with color of sides; tail bicolored, dark brown above, white to yellowish-white below; feet grayish-white above; ears dark, edged with color of sides; auditory bullae large; pterygoid fossae broad; incisive foramina relatively short; palatal bridge short; maxillary tooth-rows relatively short; narrow across zygomata; braincase narrow; interorbital region narrow; zygomatic arch relatively short.
Comparisons.—From Zapus trinotatus trinotatus, Z. t. eureka differs in: Size smaller; ventral surface with much greater suffusion of ochraceous; auditory bullae larger; pterygoid fossae relatively broader; frontal region less inflated; palatal bridge shorter; braincase narrower; narrow across zygomata; upper tooth-rows shorter.
For comparison with Zapus trinotatus orarius see account of that subspecies.
Remarks.—Howell (1920:230), without having examined the material, provisionally referred specimens from Requa and Crescent City, Del Norte County, California, to Z. t. eureka. I have studied [390] this material and find the specimens to be intermediate between Z. t. trinotatus and Z. t. eureka in cranial characters (zygomatic breadth, interorbital width, and breadth of braincase), but nearer Z. t. trinotatus in coloration (absence of ochraceous suffusion ventrally). They are here referred to Z. t. trinotatus. The zone of intergradation between Z. t. trinotatus and Z. t. eureka seems to extend from Requa, California, north to Gold Beach, Oregon, where other specimens intermediate between these two subspecies, have been obtained. These individuals are also referred to Z. t. trinotatus on the basis of cranial features and color.
Specimens examined.—Total, 42, all from California, distributed as follows: Humboldt Co.: Trinidad, 4 (SDM); Carsons Camp, Mad River, 3 (USBS); 3 mi. W Arcata, 5 (MVZ); 73/10 mi. E Bayside, 1 (MVZ); 12 mi. S Korbel, on Maple Creek, 2 (MVZ); Falk, 1 (MVZ); Carlotta, 1 (MVZ); F. B. Summer Redwoods, S Eureka, 1 (MVZ); Maple Creek, 1 mi. W junction Mad River, 12 (MVZ). Mendocino County: Mendocino City, 1 (MVZ); Albion River, 1/3 mi. E MacDonalds Ranch, 1 (MVZ); Russian Gulch State Park, 10 (MVZ).
Marginal records.—California: Trinidad; Russian Gulch State Park; Albion River, 1/3 mi. E MacDonalds Ranch; Mendocino City; Carlotta.
Zapus trinotatus montanus Merriam, Proc. Biol. Soc. Washington, 11:104, April 26, 1897; Bailey, N. Amer. Fauna, 55:234, August 29, 1936.
Zapus montanus, Preble, N. Amer. Fauna, 15:28, August 8, 1899.
Type.—Female, adult, skin and skull; No. 79863, U. S. Nat. Mus., Biol. Surv. Coll.; Crater Lake, Klamath County, Oregon; obtained on August 19, 1896, by Edward A. Preble, original No. 1388.
Range.—From Crater Lake, Klamath County, Oregon, northward along the Cascade Range into Hood River County, Oregon. Zonal range: Transition and Canadian.
Description.—Size medium; back near Ochraceous-Buff with admixture of black hair, resulting in a grizzled, broad, dorsal band; sides lighter than back, from near Ochraceous-Buff to near Pinkish-Cinnamon, and lined with black hair; lateral line distinct; underparts usually pure white, sometimes with slight suffusion of ochraceous on lower throat and upper chest; tail bicolored, brown above and yellowish-white below; ears dark, sometimes flecked with ochraceous, edged with yellowish-white; feet grayish-white above; braincase relatively narrow; zygomata relatively short; condylobasal length short; mastoid region relatively narrow; palatal bridge short; auditory bullae large; frontal region inflated; pterygoid fossae relatively narrow.
Comparison.—From Zapus trinotatus trinotatus, Z. t. montanus differs as follows: Size averaging smaller; sides more ochraceous, fewer black hairs; upper parts duller; skull smaller; zygomatic arch shorter, braincase relatively narrower; frontal region more inflated; pterygoid fossae relatively narrower; zygomata narrower.
Remarks.—The systematic status of Z. t. montanus has been in doubt. Several workers, for example, Howell (1920:227) and [391] Preble (1899:28), considered it to be a species, and others (Merriam, 1897a:104, Bailey, 1936:234) considered it to be a subspecies of Z. trinotatus. Z. montanus is here considered to be a subspecies of Z. trinotatus, because of the agreement of the two in size and shape of the baculum, diameter and pigment pattern of the hair, and the over-all proportions of the skull. In addition, animals from intermediate geographic areas are available and show actual intergradation.
Intergradation has been noted in specimens from North Santiam River, 3400 ft., Oregon. In color, in length of incisive foramina, in breadth of braincase, and in width of zygomata these specimens are intermediate between Zapus trinotatus montanus and Z. t. trinotatus, but in the sum-total of characters they are referable to the former. Specimens from Lost Creek R. S., 10 mi. SE McKenzie Bridge, are intermediate in color between Z. t. trinotatus and Z. t. montanus; they are referable to Z. t. montanus. The animals available from Brooks Meadow, 4300 ft., 9 mi. ENE Mt. Hood and the one from Mt. Hood, in color, in length of incisive foramina, and in mastoid width, closely approach Z. t. trinotatus from Skamania County, Washington, but in the sum-total of characters are nearest Z. t. montanus and are here referred to montanus.
Specimens examined.—Total, 35, all from Oregon, distributed as follows: Deschutes County: Tumalo Creek, 15 mi. W Bend, 6100 ft., 3 (MVZ). Douglas Co.: Diamond Lake, 1 (USBS). Hood River Co.: Brooks Meadow, 4300 ft., 9 mi. ENE Mt. Hood, 10 (MVZ); Mt. Hood, 1 (USBS). Klamath Co.: Crater Lake, 3 (MVZ); 1/2 mi. N Government Camp, 6700 ft., Munson Valley, Crater Lake Nat’l Park, 2 (MVZ); east slope Cascade Divide, 6400 ft., Crater Lake Nat’l Park, 2; Anna Creek, Mt. Mazama, 6000 ft., 2 (USBS). Lane Co.: Lost Creek R. S., 10 mi. SE McKenzie Bridge, 6 (USBS); Three Sisters, Alder Springs, 4300 ft., 2 (USBS). Linn County: North Santiam River, 3400 ft., 3 (MVZ).
Marginal records.—Oregon: Brooks Meadow, 4300 ft., 9 mi. ENE Mt. Hood; Tumalo Creek, 15 mi. W Bend, 6100 ft.; Anna Creek, Mt. Mazama, 6000 ft.; east slope Cascade Divide, 6400 ft., Crater Lake Nat’l Park; Diamond Lake; North Santiam River, 3400 ft.
Zapus orarius Preble, N. Amer. Fauna, 15:29, August 8, 1899.
Zapus pacificus Merriam, Proc. Biol. Soc. Washington, 11:104, April 26, 1897 (part—the part from Point Reyes, Marin County, California).
Zapus trinotatus orarius, Hooper, Miscl. Publ. Mus. Zool. Univ. Michigan, 59:67, January 12, 1944.
Type.—Male, adult, skin and skull, No. 250, collection of E. A. and O. Bangs (now in Mus. Comp. Zool.); Point Reyes, Marin County, California; obtained on May 14, 1893, by C. A. Allen, original No. 618.
Range.—Southern and western Marin County, California. Zonal range: Upper Sonoran areas that are moist yet safe from continuous inundation.
Description.—Size small; back dark ochraceous, usually overlaid with black hairs forming broad dorsal band; side lighter than back with admixture of black hairs; lateral line distinct, usually bright, near Ochraceous-Buff; under parts strongly suffused with ochraceous; tail bicolored, white to yellowish-white below and dark brown above; feet grayish-white above; ears dark, edged with yellowish-white or tan; skull small; zygomata narrow; braincase narrow; maxillary tooth-rows short; interorbital region narrow; incisive foramina short; palatal bridge relatively long; mastoid region relatively broad; occipitonasal length short.
Comparison.—From Zapus trinotatus eureka, Z. t. orarius differs in: Size smaller; color, dorsally and laterally, brighter, more ochraceous; skull averaging smaller in all measurements taken except length of palatal bridge, where it averages longer; auditory bullae smaller, less inflated; pterygoid fossae narrower.
Remarks.—Preble (1899:30) named this jumping mouse as a full species. Included in the specimens examined were animals from Eureka and Mad River, Humboldt County, California. Howell (1920:231) retained Z. orarius as a full species but restricted its range to Marin County, California, and referred material from northern California, including the animals from Eureka and Mad River, to a new subspecies (eureka) of the species Z. trinotatus. Howell (loc. cit.) suggested that Z. orarius had its closest affinity with Z. t. eureka but remarked that intergrading material was not available. Hooper (1944:68) arranged Z. orarius as a subspecies of Z. trinotatus and suggested that intergrades could be expected from geographically intermediate areas, for example, northern Sonoma County, California.
Although animals from intermediate geographic areas still are not available to show actual intergradation, I concur with Hooper (loc. cit.) and arrange Z. orarius as a subspecies of Z. trinotatus. The close relationship of Z. orarius to Z. trinotatus is evident; certain diagnostic characters, held in common, are the shape and size of the os penis, the diameter and pigment pattern of the hair, and the general configuration of the skull.
Interbreeding in the wild between Z. t. orarius and Z. t. eureka probably does not take place, because these subspecies are separated by terrain unsuited to jumping mice.
Specimens examined.—Total, 29, all from California, distributed as follows: Marin County (MVZ): 3 mi. W Inverness, 300 ft., 14; 5 mi. NNE Point Reyes Lighthouse, 12; W end Elk Valley, 10 ft., 1; West Portal, Fort Barry, 2.
Marginal records.—California: 3 mi. W Inverness, 300 ft.; West Portal, Fort Barry.
Zapus trinotatus Rhoads, Proc. Acad. Nat. Sci. Philadelphia, 1894:42, January 15, 1895.
Jaculus hudsonius, Baird, Repts. Expl. and Surv. 111, 8 (pt. 1): 433, July 14, 1858 (part—the part from Washington).
Zapus hudsonius, Coues, Bull. U. S. Geol. and Geog. Surv. of the Territories, 2nd ser., No. 5:260, 1877 (part—the part from Steilacoom [Pierce County], Washington).
Zapus imperator Elliot, Field Columbian Mus., publ. 30, zool. ser., 1:228, February 1, 1899, type from Siegs Ranch, Elwah River, Clallam County, Washington.
Zapus princeps trinotatus, Dalquest, Univ. Kansas Publ. Mus. Nat. Hist., 2:371, April 9, 1948.
Type.—Male, adult, skin and skull, No. 360, S. N. Rhoads Coll.; Lulu Island, mouth of Frazer River, British Columbia; obtained on May 31, 1892, by S. N. Rhoads (type in Philadelphia Acad. Nat. Sci.).
Range.—Pacific coastal region from Requa, Del Norte County, California, north in Oregon west of the Cascades, and in Washington including the Cascades; to southwestern British Columbia.
Description.—Size large; back from near Ochraceous-Buff to near Tawny with admixture of black hair forming broad dorsal band; sides lighter than back from near Ochraceous-Buff to near Tawny; lateral line usually distinct; belly white, sometimes with faint suffusion of ochraceous on lower throat and upper chest; tail bicolored, brown above, white to yellowish-white below; ears dark, sometimes flecked with color of sides, edged with ochraceous; feet grayish-white above; palatal bridge relatively short; incisive foramina relatively long; condylobasal region long; zygomatic width great; braincase relatively broad; distance from incisors to postpalatal notch relatively great.
Comparisons.—For comparisons with Zapus trinotatus montanus and Zapus trinotatus eureka see accounts of those subspecies.
Remarks.—This subspecies retains most of its diagnostic characters throughout nearly all parts of its geographic range. Intergradation occurs between Z. t. eureka and Z. t. trinotatus in extreme southwestern Oregon and northwestern California (see account of Z. t. eureka). Intergrades between Z. t. montanus and Z. t. trinotatus have been commented on in the account of Z. t. montanus. Specimens from Eugene, Oregon, according to Bailey (1936:232), show affinity to Z. t. montanus but are considered by him to be Z. t. trinotatus.
Specimens examined.—Total, 238, distributed as follows:
British Columbia: Alta Lake, on Pac. Gt. Eastern Ry., 2600 ft., 5 (MVZ); Okanagan, 1 (FM); Vedder Crossing, 4 (1 MVZ, 3 PM); Chilliwack Valley, 2 (NMC); 18 mi. S Chilliwack, 1 (MVZ); Cultus Lake, 2 (NMC); Lihumption Park, 4500-4800 ft., 12 (NMC); Seymour Mtn., 4000 ft., 8 (1 MVZ, 7 PM); Cariboo, 2 (FM); Sumas, 8 (1 MVZ, 7 FM); Huntingdon, 40 (NMC); Parnassus Creek, Black Tusk Meadow, 5200 ft., 1 (PM); Howe Sound, Brackendale, 2 (NMC); Stanley Park, Vancouver, 1 (PM); Allison Pass, Manning Park, 1 (PM); Manning Park, 2 (PM).
California: Del Norte Co.: Crescent City, 11 (6 FM, 5 USBS); Requa, 4 (FM).
Oregon: Benton County: 3 mi. N Corvallis, 2. Clatsop County: Old Fort Clatsop, 100 ft., 11 (MVZ); 71/2 mi. S Cannon Beach, 50 ft., 1 (MVZ). Columbia County: 7 mi. SE Rainier, 100 ft., 11 (MVZ). Curry County: Gold Beach, 3 (FM). Douglas County: Gardiner, 7 (5 MVZ, 2 FM). Lane County: Sutton Lake, 6 mi. N Florence, 1 (MVZ). Lincoln County: Delake, 3 (2 MVZ); Newport, 2 (MVZ). Multnomah County: Portland, Council Crest, 950 ft., 1 [394] (MVZ). Tillamook Co.: Tillamook, 1 (MVZ); 9 mi. S Tillamook, 1 (MVZ); Netarts, 3 (SDM); Blaine, 3 (MVZ). Washington County: 181/2 mi. NW Portland, 1300 ft., 5 (MVZ).
Washington: Clallam County: Deer Lake, 3800 ft., 3. Clarke County: 31/2 mi. E and 11/2 N Amboy, 3500 ft., 3 (MVZ); 11/2 mi. ENE Amboy, 3500 ft., 13 (MVZ); 31/2 mi. E and 5 mi. N Yacolt, 500 ft., 1 (MVZ); 11/2 mi. W Yacolt, 800 ft., 11 (MVZ). Cowlitz County: 6 mi. NE Kelso, 4 (MVZ); 4 mi. E mouth Kalama River, 5 (MVZ). King County: Lakeridge Tract, S end Forest Ave., Lake Washington, 2 (MVZ); Seattle 2 (MVZ); Snoqualmie Pass, 5 (MVZ). Mason County: Potlatch, 2 (MVZ). Pacific County: 11/2 mi. N Chinook, 10 ft., 1 (MVZ); 31/2 mi. SE Chinook, 10 ft., 5 (MVZ). Pierce Co.: 5 mi. E Tacoma, 4 (MVZ); Puyallup, 3 (1 MVZ, 2 FM); Mt. Rainier, 1 (MVZ); 3 mi. E Ashford, 1 (LMH). Skamania County: Ice Caves, 2800 ft., 5 mi. WSW Guler, 1 (MVZ). Thurston County: Boston Harbor, 5 (CAS). Wahkiakum County: 4 mi. E Skamokawa, 5 (MVZ). Whatcom County: Baker Lake, 2 (MVZ).
Marginal records.—British Columbia: Okanagan; Manning Park. Washington: Baker Lake; Snoqualmie Pass; Mt. Rainier; Ice Caves, 2800 ft., 5 mi. WSW Gulch. Oregon: Portland, Council Crest, 950 ft. California: Requa; Crescent City. Oregon: Gold Beach; Gardiner; Sutton Lake, 6 mi. N Florence; Newport; Netarts; Old Fort Clatsop, 100 ft. Washington: 31/2 mi. SE Chinook, 10 ft.; Deer Lake, 3800 ft. British Columbia: Stanley Park, Vancouver; Alta Lake, 2600 ft.
Range.—The Rocky Mountains region from Yukon south into Arizona and New Mexico; westward through eastern Oregon and through the Cascades and Sierra Nevada of California; eastward in the northern Great Plains to extreme eastern parts of the Dakotas (see fig. 46).
Characters of the species: External.—Size medium to large (total length 216 mm to 247 mm); tail longer than head and body (129 mm to 148 mm) and bicolored, pale brown to grayish-brown above, white to yellowish-white below; hind feet long (31 mm to 34 mm), grayish-white above; back variable from yellowish-gray to salmon-brown and ochraceous; sides paler than back; lateral line usually present but sometimes indistinct or entirely absent (when present usually clear Ochraceous-Buff); ventral coloration white, usually suffused with ochraceous; ears usually dark, sometimes flecked and usually narrowly edged with light color; guard hairs average 142 microns (130u to 168u) in diameter; underhair with medullary pigment in form of hollow squares; cuticular scales of underhair larger and fewer than in other species.
Baculum.—Size medium (total length 5.6 mm to 6.6 mm); base moderately broad (0.7 mm to 0.8 mm); tip narrow (0.26 mm to 0.31 mm) rounded and dished out in dorsal aspect, blunted; shaft rounded, slightly sinusoidal, recurved at tip.
Skull.—Large, not exceptionally broad and deep in relation to length; rostrum broad but tapering; pterygoid fossa moderately narrow; anterior ramus of zygomatic process usually broad; incisive foramina usually broadly rounded and elongate; auditory bullae usually moderately inflated; coronoid process of mandible relatively short. Upper premolars of medium size (averaging .55 mm in length and .50 mm in breadth), sometimes functional, with occlusal surface normally divided by single shallow re-entrant fold; m1 relatively short, narrow anteriorly.
Fig. 46. Distribution of Zapus princeps.
Guide to subspecies
| 1. | Z. p. cinereus | 7. | Z. p. oregonus | ||
| 2. | Z. p. curtatus | 8. | Z. p. pacificus | ||
| 3. | Z. p. idahoensis | 9. | Z. p. princeps | ||
| 4. | Z. p. kootenayensis | 10. | Z. p. saltator | ||
| 5. | Z. p. luteus | 11. | Z. p. utahensis | ||
| 6. | Z. p. minor |
There are 11 subspecies recognized, most of which are in the mountains of the western United States and southwestern Canada. There is geographic variation in color, relative proportions of external parts (tail, hind feet, head, and body), and shape and size of the skull.
Three basic types of coloration occur in Z. princeps, as pointed out by Hall (1931:9). Yellow-sided dark-backed jumping mice exemplified by kootenayensis, idahoensis, and utahensis are found to the eastward in the Rocky Mountains. Reddishbrown-sided, brown-backed jumping mice typified by luteus and pacificus are found to the westward in the Sierra Nevada and in New Mexico and Arizona; mice with yellowish-buff or pinkish-buff-sides and light backs are the subspecies, cinereus, curtatus, and oregonus, that occur in the intervening Great Basin.
External dimension as a whole decreases from north to south, although not uniformly. For example, the smallest individuals are of the southernmost geographic subspecies (Z. p. luteus), but the largest are of the subspecies (Z. p. utahensis) that is near the geographic center of the range for the species. In the skull there is geographic variation in the length and shape of the zygomata, size and shape of the incisive foramina, alignment of maxillary tooth-rows, size and shape of auditory bullae, position of the postpalatal notch in relation to M3, and the presence or absence and size of the medial projection on the inferior ramus of the zygomatic process of the maxillary.
Habitat.—Zapus princeps occurs most commonly adjacent to streams where grasses and herbs are in lush growth. It frequents mountain meadows neighboring small streams and is often taken from alder, aspen, or stands of willow, where the moist ground supports a heavy undergrowth of herbs. Davis (1939:330) found these mice in heavy herbage along a small stream bordered by quaking aspen near Victor, Teton County, Idaho. They were found along streams bordered by willow, rose, alder, huckleberry, sedges, and herbs of various kinds at Alturas Lake, Mill Creek, and at the head of the Pahsimeroi River. Linsdale (1938:195) found jumping mice in the Toyabe Mountains, Nevada, near the streamsides or in seepy areas close to the streams where associated vegetation included rose, willow, wild peach, sage, grasses, and herbs. In the Uinta Mountains, [397] Utah, R. D. Svihla (1931:264) obtained them from willows along streams in mountain parks. Borell and Ellis (1934:37) in the Ruby Mountains, Nevada, found jumping mice to be common in heavy vegetation along streams. Louise Kellogg (1916:369) obtained jumping mice in northern California; all were near water, in grassy meadows, or under alders where vegetation was dense.
Zapus princeps is locally abundant, but its numbers seem to vary considerably from year to year as well as seasonally. Early autumn, when young of the year are abroad, seems to be the period of greatest abundance. Moore (1928:154) remarks that runways were plainly marked and well strewn with four-inch pieces of brome-grass. Davis (1939:334) notes that Z. princeps has runways, and found that sections, four inches long, of cut grass piled in runways was good evidence of the presence of the mouse.
Behavior.—In reference to locomotion of Z. princeps, Davis (loc. cit.) writes, “In rapid progression jumping mice move by a series of zigzag hops. One young of the year found in tall grass near Victor made horizontal leaps of approximately three feet. The zigzag course was difficult for me to follow, and I was led to wonder if this mode of locomotion were not advantageous to the mice in eluding animals that would do them harm.” Hollister (1912:26) remarked that princeps, when startled, sometimes jumps five to six feet at a bound. Concerning the swimming ability of Z. princeps, Bailey (1936:233) quotes from Hollister’s notes, “While I was walking around the grassy border of a small pond one jumped out at my feet and struck in the water like a frog, which at first it was thought to be, until it was seen swimming across the pond on the surface of the water … he certainly handled himself as if perfectly at home and swam with little effort and great speed over the still surface of the pond.” Davis (1939:334) obtained two individuals at Mill Creek, Idaho, in traps placed on artificial islands of stones in the middle of the creek where the water was about six inches deep. He speculated that the only way the mice could have reached the traps was by swimming. Grinnell, Dixon, and Linsdale (1930:531) record an individual which was seen hopping in the inch-deep water of a small stream at Lake Helen, California.
According to Hollister (1912:26) and Davis (1939:335), jumping mice are for the most part nocturnal, but occasionally they are seen by day in tall grass.
Little is recorded concerning the hibernation of Z. princeps. What data are available suggest that, starting in July, these animals [398] accumulate a heavy layer of fat on the inside of the skin with especially large amounts in the inguinal region. By August or early September, animals are excessively fat, and the start of hibernation is dependent then upon the arrival of a heavy cold snap. Grinnell, Dixon, and Linsdale (1930:531), in their study of the vertebrates of the Lassen Peak region of California noted that the latest activity by these mice was September 13. As regards the time of onset of hibernation in Idaho, Davis (1939:336) states that, “I know of no records of capture later than September and infer that hibernation begins in that month or the next.” Bailey (1932:227) writes that in New Mexico, animals obtained on September 20 were very fat, probably were ready to hibernate at the first cold wave, and had winter nests in burrows well underground.
Enemies.—Bailey (loc. cit.) lists hawks, owls, and weasels as natural predators on Z. princeps. Stanford (1931:362) records the garter snake (Thamnophis) as a predator of jumping mice. A large snake of this genus obtained by him regurgitated two jumping mice a few hours after its capture. Grinnell, Dixon, and Linsdale (1937:232) report that on Parker Creek, in California, H. C. Bryant frightened a weasel that dropped a freshly killed jumping mouse. Crowe (1943:407) reported Cuterebra fly larvae in the inguinal region of a Z. princeps obtained at Invermere, British Columbia. Several mice of this species taken at Moccasin Lake, 19 mi. W and 4 mi. N of Lander, 10,000 ft., Fremont County, Wyoming, were heavily infested with mites of the family Laelaptidae.
Food.—In early September in central Utah, Moore (1928:154) found only a white, starchy, glutinous paste in stomachs of six Z. princeps and only traces of a brown seed coat in a seventh. The main seeds eaten seemed to be from an introduced brome-grass which was abundant in the vicinity of capture. Bailey (1932:226) wrote of Z. princeps in New Mexico, that “In feeding they cut down the tall grass, beginning at the bottom and cutting the stem at intervals as high as they can reach until the seed part of the grass is brought down.” He (op. cit.:227) remarked that the food was almost entirely seeds of grass and grasslike plants and that the stomach contents almost always were perfectly clean white dough from the shelled kernels of small seeds.
Reproduction.—Females with embryos have been collected from late May to mid-July and lactating individuals until late August. Possibly there is only one litter per season as Davis (1939:336) suggests is the case in Idaho.
Embryos in 25 pregnant females averaged 5 (2-7). The mammae of the female are arranged in four pairs (two abdominal, one pectoral, and one inguinal).
Z. princeps builds a grass nest on the ground which is placed under cover of vegetation or surface litter. Bailey (1932:227) writes that in New Mexico jumping mice of this species use fibers of grass to construct a ball-shaped nest. The nest usually has one opening but sometimes there are two. In the Ruby Mountains, of Nevada, Borell and Ellis (1934:37) found the globular nests of this mouse on the ground in tall grass.
Zapus princeps cinereus Hall, Univ. California Publ. Zool., 37:7, April 10, 1931.
Type.—Female, adult, skin and skull; No. 45422, Mus. Vert. Zool.; Pine Canyon, 6600 feet altitude, Raft River Mountains, 17 mi. northwest Kelton, Boxelder County, Utah; obtained on July 14, 1930, by Annie M. Alexander; original No. 689.
Range.—Raft River Mt’s in northwestern Utah and in isolated mountains in southern Idaho. See fig. 46. Zonal range: Transition and Canadian.
Description.—Size, medium; back with broad mid-dorsal band, varying from pale brown mixed with Pinkish-Buff to dark brown mixed with Warm-Buff or Ochraceous-Buff; sides varying from near Pinkish-Buff to near Ochraceous-Buff; ventral surface white to base of hairs, not suffused with other color; tail bicolored, pale brown above and white to yellowish-white below; ears dark, edged with white or yellowish-white; upper teeth divergent anteriorly; auditory bullae small; skull relatively long; zygomata relatively weak and not widely bowed; nasals wide posteriorly; pterygoid fossae relatively narrow.
Comparisons.—From Zapus princeps nevadensis, Z. p. cinereus differs as follows: Size averaging smaller; entire coloration lighter; zygomata not so widely bowed; incisive foramina not so wide posteriorly; auditory bullae smaller; nasals wider posteriorly; pterygoid fossae narrower.
From Zapus princeps idahoensis, Z. p. cinereus can be distinguished by: generally paler color; smaller auditory bullae; broader interorbital region; anteriorly diverging tooth-rows; narrower pterygoid fossae.
For comparison with Zapus princeps utahensis see account of that subspecies.
Remarks.—Davis (1939:343) writes that “since cinereus was described from nine specimens, only two of which are near adult, one cannot place much value on the coloration ascribed to it by Hall (1931:7).” I examined the type series and found, as did Davis (loc. cit.), that the type is much lighter and grayer than is a near adult paratype, which was obtained the same day; however, I do not concur with Davis (loc. cit.) that specimens from Mt. Harrison, 10 mi. S Albion, Idaho, which are darker and much more ochraceous [400] than the paratype, necessarily are more nearly typically colored. These individuals, judged by cranial characters, are more nearly typical of cinereus but show intergradation with Z. p. idahoensis in their darker and more ochraceous pelage.
Durrant (1952:387) found that the gray color of Z. p. cinereus was not diagnostic in separating Z. p. cinereus from Z. p. utahensis, because gray animals are also found in Z. p. utahensis. Specimens from Camp Tendoy, Pocatello, Idaho, are intermediate in color and in cranial characters as between Z. p. idahoensis and Z. p. cinereus, but here are referred to Z. p. cinereus. Whitlow and Hall (1933:268) compared these individuals with specimens of Z. p. princeps and Z. p. cinereus, finding them intermediate but in the aggregate of several differential characters better referred to the latter.
Specimens examined.—Total, 35, distributed as follows:
Idaho: Bannock County: Camp Tendoy, Pocatello, 2 (MVZ). Cassia County: Mt. Harrison, 10 mi. S Albion, 16 (MVZ).
Utah: Boxelder Co.: south fork of George Creek, 5 mi. SE Yost, Raft River Mts., 6700 ft., 1 (UU); George Creek, 7 mi. SE Yost, Raft River Mts., 6500 ft., 6 (UU); Pine Canyon, 6600 ft., 17 mi. NW Kelton, Raft River Mts., 8 (MVZ); Pine Creek, 3 mi. N Rosette, Raft River Mts., 6100 ft., 2 (UU).
Marginal records.—Idaho: Camp Tendoy, Pocatello. Utah: Pine Creek, 3 mi. N Rosette, Raft River Mts., 6100 ft. Idaho: Mt. Harrison, 10 mi. S Albion.
Zapus princeps curtatus Hall, Univ. California Publ. Zool., 37:7, April 10, 1931.
Zapus princeps oregonus, Taylor, Univ. California Publ. Zool., 7:281, June 24, 1911.
Type.—Female, adult, skin and skull, No. 7991, Mus. Vert. Zool.; head of Big Creek, 8000 feet altitude, Pine Forest Mountains, Humboldt County, Nevada; obtained on June 30, 1909, by Walter P. Taylor and C. H. Richardson, original No. 777 of W. P. T.
Range.—Pine Forest Mt’s, Humboldt County, Nevada. See fig. 46. Zonal range: Transition and Canadian.
Description.—Size medium; back pale near Light Ochraceous-Buff with admixture of black hair forming dark dorsal band; sides lighter than back; lateral line faintly indicated; ventral surface white; tail bicolored, grayish-white to yellowish-white below and pale brown above; ears dark, edged with yellowish-white; feet grayish-white above; palatal bridge short; tooth-rows almost parallel; mastoid region of skull relatively narrow; incisive foramina wide posteriorly; narrow across zygomata; nasals relatively narrow posteriorly.
Comparisons.—For comparison with Zapus princeps oregonus see account of that subspecies.
Remarks.—This jumping mouse, which was described from the Pine Forest Mountains, closely resembles Zapus princeps oregonus but differs in lighter color, slightly smaller body, less divergent [401] tooth-rows, shorter palate, and narrower skull across the mastoid region.
The Pine Forest Mountains are isolated from neighboring boreal regions by a belt of the Upper Sonoran Life-zone, which is inhospitable to Zapus; therefore, intergrades between Z. p. oregonus and Z. p. curtatus are not known and probably do not exist. Nevertheless, Z. p. curtatus shows close affinity with Z. p. oregonus, as indicated by Taylor (1911:281), and I agree with Hall (1931:7) that the relationships of Z. p. curtatus are best expressed by arranging it as a subspecies of Zapus princeps.
Specimens examined.—Total, 18, all from Nevada, distributed as follows: Humboldt County: Pine Forest Mts.; Alder Creek, 6000 ft., 2 (MVZ); head of Big Creek, 8000 ft., 14 (MVZ); Leonard Creek, 6500 ft., 2 (MVZ); Meadow, 1 (MVZ).
Marginal records.—Nevada: Pine Forest Mts., Alder Creek; Meadow.
Zapus princeps idahoensis Davis, Jour. Mamm., 15:221, August 10, 1931.
Jaculus hudsonius, Allen, Bull. Essex Inst., 6:61, April, 1874 (part—the part in Carbon County, Wyoming).
Zapus hudsonius, Merriam, N. Amer. Fauna, 5:72-73, July 30, 1891.
Zapus princeps princeps, Preble, N. Amer. Fauna, 15:22-23, August 8, 1899 (part).
Type.—Male, adult, skin and skull; No. 54845, Mus. Vert. Zool.; 5 mi. E Warm Lake, 7000 ft., Valley County, Idaho; obtained on July 9, 1932, by Robert T. Orr; original No. 660.
Range.—From Banff, Alberta, southward through extreme southwestern Alberta and extreme southwestern British Columbia, most of the panhandle of Idaho, Kamiak Butte in eastern Washington, western Montana, and western Wyoming (Green, Wind River and Absoroka ranges of the Rocky Mt’s). See fig. 46.
Description.—Size, medium; back from near Clay Color to near Warm Buff, usually overlaid with black hairs forming broad dorsal band; sides lighter than back; lateral line indistinct or wanting; belly pure white, occasionally faintly tinged with Ochraceous-Buff; tail indistinctly bicolored, tan to grayish-white below and pale brown above; hind feet grayish-white above; ears dark, edged with white or yellowish-white; postpalatal notch anterior to posterior border of last molars; proximal part of inferior ramus of zygomatic process of maxillary relatively narrow and usually without enlarged median projection; auditory bullae well inflated; incisive foramina relatively narrow.
Comparisons.—From Zapus princeps kootenayensis, Z. p. idahoensis differs as follows: Size averaging larger; upper parts with greater suffusion of ochraceous, not grayish or dusty; skull larger; incisive foramina longer and relatively wider; zygomatic breadth averaging greater; nasals broader at tips; auditory bullae more inflated.
From Zapus princeps oregonus, Z. p. idahoensis differs in: Size averaging smaller; upper parts generally more suffused with black hairs, on the average more yellowish with less ochraceous; skull smaller; incisive foramina narrower [402] (breadth less, instead of more, than 52 per cent of length); palatal bridge shorter; zygomatic arch shorter; pterygoid fossae narrower.
From Zapus princeps utahensis, Z. p. idahoensis can be distinguished by: Size less; color slightly darker; skull averaging smaller in zygomatic breadth, least interorbital constriction, and occipitonasal length; palate narrower; upper tooth-rows nearly parallel as opposed to diverging anteriorly.
From Zapus princeps minor, Z. p. idahoensis differs in: Size larger; color of underparts less ochraceous; lateral line indistinct or wanting; skull averaging larger in all measurements taken except that the two subspecies are approximately same in least interorbital constriction, length of zygomatic arch, and distance from anterior face of incisors to postpalatal notch; nasals, in profile, straight instead of with proximal third depressed; postpalatal notch anterior to posterior face of last molar, instead of even with, or usually posterior to, same.
From Zapus princeps saltator, Z. p. idahoensis differs as follows: Size averaging slightly larger; color darker, being less ochraceous and more yellow dorsally and laterally; auditory bullae more inflated; zygomatic arches less bowed laterally; incisive foramina narrower.
For comparison with Zapus princeps princeps and Zapus princeps cinereus see accounts of those subspecies.
Remarks.—Intergradation occurs at almost all of the places where the range of Z. p. idahoensis is known to touch that of any other geographic race. Nevertheless, each of the populations studied has characters which make this subspecies recognizable as a taxonomic unit, although its characters are not yet stabilized even in the central part of its range.
Among named subspecies of Zapus princeps, Zapus p. idahoensis most closely resembles Zapus princeps kootenayensis, its nearest geographic neighbor to the north. Three specimens from 2 mi. NE Weippe, 3000 ft., Idaho, are best referred to Z. p. idahoensis but show relationship to Z. p. kootenayensis in size and shape of the tympanic bullae. The relationship of individuals from Idaho, here referred to Z. p. idahoensis, from Glidden Lakes, Enaville, Cascade Creek, and 13 mi. E and 5 mi. N Coeur d’Alene, is discussed in the account of Z. p. kootenayensis. British Columbian specimens from Newgate and Crows Nest Pass, 4450 ft., as well as Albertan specimens from Crows Nest Pass and various places in Waterton Lake Park, resemble Z. p. kootenayensis in color but cranially are more nearly like Z. p. idahoensis.
Intergradation with Zapus princeps oregonus was noted by Davis (1939:340) in a specimen from Cedar Mountain in Idaho. I have not seen this individual which he referred to Z. p. idahoensis but have seen a specimen from the N Fork of Potlatch River (15 mi. SE Cedar Mt.), which, in color, closely resembles Z. p. oregonus but cranially (shape of incisive foramina, size, and inflation of auditory bullae) is more nearly like Z. p. idahoensis to which it is referred. [403] Davis (loc. cit.) indicates that specimens from summit of Smith Mt., from 1 mi. N Bear Creek R. S., from 1/2 mi. E Black Lake, and from 3 mi. W Payette Lake, Idaho, are in an area of intergradation between Z. p. oregonus and Z. p. idahoensis, but he referred them to Z. p. idahoensis on the basis of cranial characters and length of hind foot. Seven specimens from Alturas Lake, 7000 ft., Idaho, were likewise so allocated by Davis (loc. cit.). I concur with him and in addition refer the following intermediate individuals from Idaho to Z. p. idahoensis: New Meadow, 1; Warren, 1; Perkins Lake, 7000 ft., Sawtooth Nat’l Forest, 1; Prairie Creek, 12 mi. W Ketchum, 2400 ft., 3. All are more nearly like Z. p. oregonus in color but cranially they show more resemblance to Z. p. idahoensis.
In the eastern part of the range of Z. p. idahoensis, intergradation occurs with Zapus princeps minor, as at 15 mi. S Heath, N Fork Flat Willow Creek, Big Snowy Mt’s, Montana. Specimens from there have the lateral line enlarged and the maximum seen in this species of Ochraceous color ventrally. The pterygoid fossae are large and the bullae are reduced as in Z. p. minor, but in the sum total of the characters the mice more closely resemble Z. p. idahoensis. At Lewistown, 7 mi. NE Judith Mt’s, Lime Kiln Gulch, Montana, the animals are colored as are Z. p. minor but cranially are like Z. p. idahoensis to which they are referred. Specimens from the Highwood Mt’s, Montana, also are intergrades; they have a relatively distinct lateral line as in Z. p. minor but show no ventral suffusion of Ochraceous; they have large bullae, nasals that are straight in lateral profile and other cranial characters of Z. p. idahoensis to which they are here referred.
A single specimen from Kamiak Butte, Whitman County, Washington, has been referred to Z. p. idahoensis by Dalquest (1948:373). I have not seen this individual, but, on geographic grounds, it is likely to be of this subspecies.
Specimens examined.—Total, 342, distributed as follows:
Alberta: Boom Creek, 5600 ft., 27 mi. W Banff, 2 (NMC); Banff, Cascade Basin, 2 (NMC); Bryant Creek, Banff Park, 1 (NMC); Spray River, 7 mi. Cabin, Banff Park, 3 (NMC); Crows Nest Pass, 2 (NMC); Waterton Lakes Park, 16 (NMC); Linnets Pond, Waterton Lakes Park, 4 (NMC); Bertha Creek, Waterton Lakes Park, 8 (NMC).
British Columbia: Vermilion Crossing, Kootenay, 1 (ROM); Paradise Mine, 3 (PM); Crows Nest Pass, 4450 ft., 3 (NMC); Newgate, 10 (NMC).
Idaho: Adam Co.: 1/2 mi. E Black Lake, 6800 ft., 8; summit of Smith Mtn., 7500 ft., 9 (3 MVZ); 1 mi. N Bear Creek R. S., SW Slope Smith Mtn., 5400 ft., 13; New Meadows, 1 (USBS); 3 mi. W Payette, 5400 ft., 4 (MVZ). Blaine County: Perkins Lake, 7000 ft., Sawtooth Nat’l Forest, 1; Alturas Lake, 7000 ft., 3 (MVZ); Prairie Creek, 12 mi. NW Ketchum, 2400 ft., 3. Clearwater County: 2 mi. NE Weippe, 3000 ft., 3 (MVZ). Custer County: Loon Creek R. S., 6000 ft., Challis Nat’l Forest, 2; Head Pahsimeroi River, 2 (MVZ); [404] Mill Creek, 14 mi. WSW Challis, 8370 ft., 1 (MVZ). Fremont County: 7 mi. W West Yellowstone, 7000 ft., 3; 17 mi. E and 4 mi. N of Ashton, 6275 ft., 9 (MVZ). Idaho Co.: Packers Meadow, near state line, South Lobo Hot Springs, 5150 ft., 7 (USBS); Warren, 1 (USBS). Kootenai Co.: 13 mi. E and 5 mi. N Coeur d’Alene, 5; Cascade Creek, 36 mi. E Coeur d’Alene, Coeur d’Alene Nat’l Forest, 1 (USBS). Latah Co.: N Fork Potlatch River, 1 (USBS). Lemhi County: Salmon River Mts., 3 (USBS). Shoshone Co.: Enaville 1 (USBS); Glidden Lakes, 5700 ft., 4 (MVZ). Valley County: 5 mi. E Warm Lake, 7000 ft., 6 (MVZ); 5 mi. W Cape Horn, 7000 ft., Sawtooth Range, 1 (MVZ).
Montana: Beaverhead County: Birch Creek, 18 mi. NE Dillon, 7100 ft., 14 (MVZ). Carbon Co.: Pryor Mts., 1 (USBS); 2 mi. E Shriver, 6500 ft., 6 (MVZ). Cascade Co.: Neihart, 1 (USBS). Chouteau Co.: Upper Muddy, 1 (USBS); Highwood Mts., 2 (USBS). Fergus Co.: Lime Kiln Gulch, 7 mi. NE Judith Mts., 3 (USBS); 15 mi. S Heath, N Fork Flat Willow Creek, 8 (USBS); 10 mi. W Tyler, N Fork Flat Willow Creek, 1 (USBS); Crystal Lake, 6000 ft., Big Snowy Mts., 3 (UM). Flathead Co.: Waterton Lake, 1 (USBS); Crosley Lake, Glacier Nat’l Park, 1 (USBS); Paola, 1 (USBS); Summit, 2 (USBS); 1 mi. W and 2 mi. S Summit, 5000 ft., 12. Gallatin Co.: 4 mi S Logan, Camas Creek, Big Belt Mts., 5 (USBS); Gallatin Gateway, 5 (SDM); west fork West Gallatin River, 6500 ft., 6 (USBS). Glacier Co.: Babb, 1 (LMH); 21/2 mi. W and 11/2 mi. S Babb, 4700 ft., 1; Many Glaciers, 4900 ft., Glacier Nat’l Park, 5 (MVZ); 6 mi. S St. Marys, 6500 ft., 1; St. Marys Lake, 7 (USBS); McDermit Lake, 1 (USBS); Blackfoot Agency, 1 (USBS). Golden Valley County: Swimming Woman Canyon, 3/4 mi. S Fergus County line, Big Snowy Mts., 4 (UM). Judith Basin Co.: Little Belt Mts., Dry Wolf Creek, 20 mi. SW Stanford, 4 (USBS); 13 mi. W Buffalo, 1 (USBS). Madison Co.: 12 mi. SW Alder, Hinch Creek, Ruby Mts., 2 (USBS). Meagher Co.: 16 mi. N White Sulphur Springs, Little Belt Mts., 7 (USBS). Park County: West Boulder Creek, 18 mi. SE Livingston, 1 (USBS); Emigrant Gulch, 3 mi. SE Chico, 6500 ft., 4 (USBS); 2 mi. NE Cooke, 8000 ft., 22 (MVZ). Ravalli County: 3 mi. SW Florence, 3700 ft., 1; 6 mi. E Hamilton, 3700 ft., 1. Sanders Co.: Prospect Creek, near Thompson, 1 (USBS). Sweet Grass Co.: near head of Big Timber Creek, 5200 ft., Crazy Mts., 11 (USBS); Brannin Ranch, Sweet Grass Creek Canyon, 6 (UM); Big Timber, 1 (USBS). Teton County: 171/2 mi. W and 61/2 mi. N Agusta, 5100 ft., 2.
Wyoming: Fremont County: Moccasin Lake, 19 mi. W and 4 mi. N of Lander, 10,000 ft., 1; 231/2 mi. S and 5 mi. W Lander, 8600 ft., 4. Park County: 311/2 mi. N and 36 mi. W Cody, 6900 ft., 7; 28 mi. N and 30 mi. W Cody, 7200 ft., 1; 161/4 mi. N and 17 mi. W Cody, 5625 ft., 14; 2 mi. S and 42 mi. W Cody, 6400 ft., 5; 12 mi. W Wapiti, 6 (LMH); 25 mi. S and 28 mi. W Cody, 6350 ft., 5. Sublette County: E end Island Lake, 10,600 ft., 3 mi. S Fremont Park, 1; N side Halfmoon Lake, 7900 ft., 3; W end Halfmoon Lake, 7900 ft., 2; 10 mi. NE Pinedale, 8000 ft., 1; 5 mi. E and 8 mi. N Pinedale, 7500 ft., 1; 3 mi. E and 5 mi. N Pinedale, 7500 ft., 4; 19 mi. W and 2 mi. S Big Piney, 7700 ft., 3.
Marginal records.—Alberta: Boom Creek, 5600 ft., 27 mi. W Banff; Crows Nest Pass; Waterton Lakes Park. Montana: Highwood Mts.; 15 mi. S Heath, N Fork Flat Willow Creek; 2 mi. E Shriver, 6500 ft. Wyoming: 231/2 mi. S and 51/2 mi. W Lander, 8600 ft.; 10 mi. W and 2 mi. S Big Piney, 7700 ft. Idaho: 7 mi. W West Yellowstone, 7000 ft.; Prairie Creek, 12 mi. NW Ketchum, 2400 ft.; 5 mi. W Warm Lake, 7000 ft.; 1 mi. N Bear Creek R. S., SW slope Smith Mtn., 5400 ft.; N Fork Potlatch River; 13 mi. E and 5 mi. N Coeur d’Alene. British Columbia: Newgate; Vermilion Crossing, Kootenay.
Zapus princeps kootenayensis Anderson, Ann. Rept. Nat. Mus. Canada for 1931:108, November 24, 1932.
Zapus princeps princeps, Preble, N. Amer. Fauna, 15:23, August 8, 1899 (part).
Type.—Adult female, skin and skull, No. 10,020, Nat. Mus. Canada; near summit of Green Mountain, head of Murphy Creek, about 10 miles north of [405] Rossland, West Kootenay district, British Columbia, at about, 6000 ft.; latitude 49° 13′ north, longitude 117° 52′ west; obtained on July 18, 1929, by R. M. Anderson, original No. 24.
Range.—From Glacier in the Selkirk Range, British Columbia, south to 5 mi. W Cocolalla, Bonner County, Idaho, west and north to Sullivan Lake, Pend Oreille County, Washington; and northwestward to Manning Park on the eastern summit of the Cascade Range in British Columbia. See fig. 46.
Description.—Size, medium; color moderately dark; upper parts noticeably dull and dusty; broad dorsal band of dull Ochraceous-Buff to near Warm Buff sprinkled with black hair to a varying degree, resulting in two color phases (dark has more black hair; Ochraceous phase or Warm Buff phase has more brown hair); sides paler than back owing to fewer black hairs; lateral line, when present, narrow and dull; ventral surface pure white; tail bicolored, pale brown above, yellowish-white to dull white below; ears dark with narrow white or yellowish-white edgings; feet white above; skull narrow across zygomata; incisive foramina narrow; bullae moderately inflated; nasals narrow at tips; postpalatal notch anterior to posterior face of last molars; braincase moderately narrow; zygomatic arch short.
Comparisons.—From Zapus princeps saltator, Z. p. kootenayensis differs as follows: Upper parts generally dull with less ochraceous; sides with more yellow, less ochraceous; lateral line wanting or not bright; skull averaging slightly smaller; incisive foramina smaller and narrower posteriorly; small medium projection on inferior ramus of the zygomatic process of maxillary frequently present instead of absent; pterygoid fossae shorter and narrower.
For comparison with Zapus princeps idahoensis see account of that subspecies.
Remarks.—This subspecies is paler and averages smaller than either of the subspecies with adjoining geographic ranges. There is intergradation with Zapus princeps idahoensis in color, shape and size of incisive foramina, and in the shape of the nasals in Idaho-taken specimens from Glidden Lakes and Enaville. These individuals are thought to be Z. p. idahoensis. Specimens from the same state taken at Cascade Creek and 13 mi. E and 5 mi. N Coeur d’Alene show intergradation in color, size and inflation of bullae, configuration of nasals, and shape of the vomer between Zapus princeps idahoensis and Z. p. kootenayensis. The majority of characters studied show these animals to be referable to Z. p. idahoensis.
Specimens from Monashee Pass, 4000 ft., British Columbia, show relationship to Zapus princeps saltator in the posteriorly wide incisive foramina, in the narrow vomer, and, in some individuals, in the increased amount of ochraceous, dorsally and laterally. The majority of characters studied show these animals to be referable to Z. p. kootenayensis.
The animals available from Glacier, British Columbia, are in color more nearly like Z. p. saltator and cranially combine the characters of Z. p. idahoensis, Z. p. saltator, and Z. p. kootenayensis. [406] The sum total of their characters places them with Z. p. saltator. Anderson (1932:108) remarks on the disparity of size between the two sexes of Z. p. kootenayensis, stating that females are considerably larger than males. I have examined most of the material used in the original description and find that animals of like age in the two sexes show no significant size difference. Anderson (loc. cit.) seems to have compared young males with adult females.
Specimens examined.—Total, 68, distributed as follows:
British Columbia: Manning Park, 3 (PM); Good Fellow Creek, Manning Park, 1 (PM); Mt. Beaver Valley, 6300 ft., Manning Park, 1 (PM); Timberline Valley, 6500 ft., 3 (PM); Allison Pass, 1 mi. E Manning Park, 1 (PM); Monashee Pass, 4000 ft., 13 (PM); Hope-Princeton Summit, 5500 ft., 1 (NMC); Hedley, Stirling Creek, 1 (NMC); Anarchist Mts., 1 (PM); Fairview-Keremeos Summit, 5 (NMC); Westbridge, 2 (NMC); Midway, 2 (NMC); Green Mtn., near Rossland, 6000 ft., 12 (11 NMC, 1 MVZ); Mt. Old Glory, 7000 ft., Rossland, 5 (4 NMC, 1 MVZ); Rossland, 5800 ft., 12 (11 NMC, 1 MVZ); Camp 6, Meadow Creek, 7 mi. SE of Yahk, 1 (NMC).
Idaho: Bonner County: 5 mi. W Cocolalla, 3500 ft., 2 (MVZ). Boundary County: 4 mi. W Meadow Creek, 3000 ft., 2 (MVZ).
Marginal records.—British Columbia: Monashee Pass, 4000 ft.; Camp 6, Meadow Creek, 7 mi. SE Yahk. Idaho: 4 mi. W Meadow Creek, 3000 ft.; 5 mi. W Cocolalla, 3500 ft. British Columbia: Hope-Princeton Summit, 5500 ft.; Manning Park.
Zapus luteus, Miller, Proc. Biol. Soc. Washington, 24:253, December 23, 1911.
Zapus luteus australis, Bailey, Proc. Biol. Soc. Washington, 26:132, May 21, 1913. Type from Socorro, Socorro County, New Mexico.
Type.—Female, adult, skin and skull, No. 133601, U. S. Nat. Mus. Biol. Surv. Coll., Espanola, 5000 ft., Rio Arriba Co., New Mexico; obtained on June 24, 1904, by McClure Surber, original No. 162.
Range.—White Mt’s of southern Apache County and northern Greenlee County, Arizona; in New Mexico, from the Sacramento Mt’s, Otero County, northward to the San Juan Mt’s, Rio Arriba County. See fig. 46. Zonal range: Lower Sonoran (1 individual), Upper Sonoran, Transition, and Canadian.
Description.—Size, small; back near Ochraceous-Buff, having black hair interspersed; mid-dorsal band not always well marked; sides Ochraceous-Buff with fine admixture of black hair; lateral line blending with Ochraceous-Buff of sides, not distinct; ventral surface white to base of hairs, in some cases lightly suffused with color of sides; tail indistinctly bicolored, tan to grayish-white below and brown above; hind feet grayish-white above; ears brownish, narrowly edged with Ochraceous-Buff; skull small; antorbital foramina relatively large; interorbital region broad; inferior ramus of the zygomatic process of the maxillary broad, often with medial projection; incisive foramina narrow posteriorly becoming broadly rounded anteriorly; palatal bridge relatively long; pterygoid fossae narrow; zygomatic arches relatively robust; nasals tapering at each end.
Comparisons.—From Zapus princeps princeps, Z, p. luteus differs as follows: Size, smaller; color lighter, more Ochraceous-Buff; ears lighter, edged with Ochraceous-Buff as compared with white or yellowish-white; lateral line indistinct or wanting as opposed to distinct; dorsal stripe not well defined; interorbital [407] region broader; antorbital foramina relatively larger; zygomatic arches more robust; nasals tapering at each end as opposed to parallel sided; auditory bullae smaller, less inflated.
Remarks.—The characters of this subspecies are relatively stable throughout most of its geographic range. Hall and Davis (1934:56) remarked that their material from the White Mountains of Arizona answered precisely to Miller’s original description (1911:253) of the species, and my examination of these and other specimens from that area indicates the same thing except that the specimens average slightly darker mid-dorsally than those from New Mexico.
Zapus luteus australis, based on a single individual taken in a riparian thicket along the Rio Grande at Socorro, New Mexico, is referable to Z. p. luteus. The diagnostic characters, referred to in the original description, are as follows: Small, slender, and very narrow skull; especially narrow braincase; slender rostrum; and light dentition. These are expressions of age, rather than of geographic variation, in that the individual is a subadult (young of the year). The color, which is paler than in adults of Z. p. luteus, is almost identical with that of a subadult (No. 205585 USBS) from Alpine, Arizona. I can see no basis for recognition of Z. p. australis and the name, therefore, is placed as a synonym of Z. p. luteus.
Four specimens from 4 mi. NE El Rito, 7000 ft., New Mexico, show intergradation, in the shape of the nasals and incisive foramina, in the robustness of the zygomatic arch, and in the breadth of the braincase with a specimen of Zapus princeps princeps from Tierra Amarilla, New Mexico. In color and in external measurements as well as in other cranial characters they closely agree with typical Z. p. luteus and are here referred to the latter.
Specimens examined.—Total, 49, distributed as follows:
Arizona: Apache Co.: North Fork White River, White Mts., 24 (SDM); Alpine, 8500 ft., 6 (USBS); West Fork Black River, 7700 ft., 8 (MVZ); Greenlee County: Hannagan Creek, 8200 ft., 2 (MVZ).
New Mexico: Otero Co.: 12 mi. E Cloudcroft, 7500 ft., 2 (USBS). Rio Arriba Co.: 4 mi. NE of El Rito, 7000 ft., 4; Espanola, 5000 ft., 2 (USBS). Socorro Co.: Socorro, 1 (USBS).
Marginal records.—New Mexico: 4 mi. N El Rito, 7000 ft.; Espanola, 5000 ft.; 12 mi. E Cloudcroft, 7500 ft. Arizona: Hannagan Creek, 8200 ft.; W. Fork Black River, 7700 ft.; N. Fork White River, White Mts. New Mexico: Socorro.
Zapus princeps minor Preble, N. Amer. Fauna, 15:23, August 8, 1899.
Zapus hudsonius campestris, Bailey, N. Amer. Fauna, 49:117, January 8, 1927 (part).
Type.—Adult female, skin and skull, No. 73673, U. S. Nat. Mus. Biol. Surv. Coll., Wingard, near Carlton House, Saskatchewan; obtained on July 23, 1895, by J. Alden Loring, original No. 3123.
Range.—Most of southern half of Saskatchewan and Alberta, northeastern Montana southeastward to Aweme, Manitoba, and Webster, South Dakota. See fig. 46. Zonal range: Transition, Hudsonian, and Canadian.
Description.—Size, small; back dark, usually with a distinct mid-dorsal band of black mixed with Warm Buff; sides lighter, more yellowish, but always with an admixture of black hairs; lateral line distinct, near Ochraceous-Buff, ventral surface characteristically suffused with Ochraceous-Buff; tail bicolored, grayish-white to yellowish-white below and pale brown above; hind feet grayish-white above; ears dark, edged with white or yellowish-white; skull small; postpalatal notch often anterior to posterior part of molars; inferior ramus of zygomatic process of maxillary often with well developed medial projection; auditory bullae flattened; nasals narrower anteriorly and proximal third depressed; base of zygomatic process of squamosal broad.
Comparisons.—From Zapus princeps princeps, Z. p. minor differs as follows: Size averaging smaller in all measurements taken, except least interorbital constriction which is approximately the same; color dorsally and laterally more yellowish, less Ochraceous-Buff; ventrally greater suffusion of Ochraceous-Buff.
For comparison with Zapus princeps idahoensis see account of that subspecies.
Remarks.—This geographic race is notably stable and retains most of its diagnostic characters throughout nearly all parts of its range. Intergradation occurs with Zapus princeps idahoensis at various localities in Montana, as is described in more detail in the account of idahoensis. Crowe (1943:406) gives evidence of intergradation between Zapus princeps idahoensis and Z. p. minor in specimens from Entrance in western Alberta. Crowe (loc. cit.) described these individuals as intermediate in color (lateral line present, under parts washed with buff, sides and dorsal stripe rich in ochraceous), and in cranial characters (smaller skulls, anteriorly narrower nasals, shorter more deflected rostrum, and higher cranium); but he considered them closer to Z. p. minor.
A skin without skull from Kananaskis Valley, Alberta, shows intergradation between Z. p. idahoensis and Z. p. minor. This individual is like Z. p. idahoensis in dorsal and lateral coloring, but is nearer Z. p. minor in ventral coloring and in the presence of a distinct lateral line. External measurements provide basis for tentatively assigning the skin to Z. p. minor.
Specimens examined.—Total, 118, distributed as follows:
Alberta: 4 mi. N Marinville, 2; Blindman River, 1 (USBS); Camrose, 1 (ROM); Red Deer River, 1 (USBS); Didsbury, Little Red Deer River, 1 (ROM); Kananaskis Valley, 7000 ft., 1 (ROM); High River, 2 (ROM); Lodge Creek, 2 (NMC).
Manitoba: Shoal Lake, 6 (NMC); Oak Lake, 4 (NMC); Aweme, 7 (6 ROM; 1 USBS).
Montana: Chouteau County: Eagle Creek, 25 mi. SE Big Sandy, 3 (UM). Hill Co.: Fort Assiniboine, 1 (USBS); Bear Paw Mt’s, 20 mi. SE Fort Assiniboine, [409] 4 (USBS); head Eagle Creek, Bear Paw Mt’s, 7 (UM). Valley Co.: Glasgow, 1 (USBS).
North Dakota: Benson Co.: 4 mi. W Leeds, 1400 ft., 2; 2 mi. W Fort Totten, 1400 ft., 13; Fort Totten, 4 (USBS). Bottineau Co.: 48/10 mi. N Bottineau, 2100 ft., 2; 31/2 mi. N Bottineau, 1920 ft., 2; 21/10 mi. N Bottineau, 1800 ft., 3; Bottineau, 1 (USBS). Dickey Co.: Oakes, 3 (USBS). Grand Forks Co.: Larimore, 3 (USBS). Montrail Co.: 6 mi. N Lostwood, 2 (USBS). Nelson Co.: Stump Lake, 1 (USBS). Richland Co.: Lidgerwood, 1 (USBS); 4 mi. S Blackner, (USBS). Rolette Co.: St. John, 1 (USBS). Sargent County: 71/5 mi. E and 11/5 mi. S Oakes, 1200 ft., 6; 3 mi. W Cayuga, 1000 ft., 2. Walsh Co.: Grafton, 2. Ward Co.: Minot, 3 (CMNH). Williams Co.: Grinnell, 2 (USBS); Buford, 2 (USBS).
Saskatchewan: Wingard, near Carlton House, 2 (USBS); Fort Carlton, 1 (MVZ); Indian Head, 2 (USBS); Cypress Hills, N Maple Creek, 18 (NMC); Battle Creek, 1 (NMC).
South Dakota: Day Co.: Webster, 1 (Chic. AS).
Marginal records.—Saskatchewan: Wingard, near Carlton House; Fort Carlton. Manitoba: Shoal Lake; Aweme. North Dakota: Larimore; 4 mi. S Blackner. South Dakota: Webster. North Dakota: Oakes; Grinnell. Montana: Eagle Creek, 25 mi. SE Big Sandy. Alberta: High River; Kananaskis Valley, 2000 ft.; Red Deer River; Blindman River; 4 mi. N Marinville.
Zapus princeps oregonus Preble, N. Amer. Fauna, 15:24, August 8, 1899.
Zapus major Preble, N. Amer. Fauna, 15:24, August 8, 1899, type from Warner Mt’s, Lake County, Oregon.
Zapus princeps major, Hall, Univ. California Publ. Zool., 37:10, April 10, 1931.
Zapus nevadensis Preble, N. Amer. Fauna, 15:25, August 8, 1899, type from Ruby Mt’s, Elko County, Nevada.
Zapus princeps nevadensis, Hall, Univ. California Publ. Zool., 37:10, April 10, 1931.
Zapus princeps palatinus Hall, Univ. California Publ. Zool., 37:8, April 10, 1931, type from Wisconsin Creek, 7800 ft., Toyabe Mt’s, Nye County, Nevada.
Zapus princeps princeps, Anthony, Bull. Amer. Mus. Nat. Hist., 33:17, March 17, 1913.
Type.—Male, adult, skin and skull; No. 78156, U. S. Nat. Mus. Biol. Surv. Coll.; Elgin, Blue Mountains, Union Co., Oregon; obtained on May 29, 1896, by Edward A. Preble, original No. 959.
Range.—Southeastern Washington, eastern Oregon east of Cascades, northeastern California, central and northeastern Nevada, and southwestern Idaho. See fig. 46. Zonal range: Transition and Canadian.
Description.—Size large; back from near Light Ochraceous-Buff to near Cinnamon-Buff, usually overlaid with black hairs forming broad dorsal band, which in some individuals is almost black; sides lighter than back, from near Light Pinkish-Cinnamon to near Cinnamon-Buff and Ochraceous-Buff, often with black hairs interspersed; lateral line faintly marked or wanting; belly pure white; tail bicolored, grayish-brown above and grayish-white to yellowish-white below; ears dark, edged with color of sides; palatal bridge long; interorbital region broad; inferior ramus of zygomatic process of maxillary usually with median projection; auditory bullae relatively small; incisive foramina greatly enlarged posteriorly; tooth-rows divergent anteriorly; nasals narrow posteriorly.
Comparisons.—From Zapus princeps curtatus, Z. p. oregonus differs as follows: Size averaging larger; upper parts darker; tooth-rows more divergent [410] anteriorly; palatal bridge longer; mastoid region broader; incisive foramina relatively wider posteriorly.
For comparisons with Zapus princeps cinereus, Zapus princeps pacificus and Zapus princeps idahoensis see accounts of those subspecies.
Remarks.—The coloration in Z. p. oregonus varies somewhat from north to south. In the northern part of the range the average coloration of the upper parts is darker with more ochraceous on the sides. To the southward the upper parts are progressively paler and the sides are near Light Pinkish-Cinnamon. Because of this variation of color, and because of the small samples available to workers in the past, three populations of this subspecies have been named as distinct. However, with the large amount of additional material now available, the supposed diagnostic characters of these “forms” prove to be within the range of individual variations of each of several populations of which large samples are available.
Zapus major Preble (1899:24) was described as having zygomata short, palate broad and long, incisive foramina large and elliptical, and color dark. Some specimens of Z. p. oregonus, from nearly all parts of its geographic range, show these same characters. Resemblances in anteriorly divergent tooth-rows, broad interorbital region, small auditory bullae, and posteriorly narrow nasals, are additional reasons for placing Z. major as a synonym of Z. p. oregonus.
Zapus nevadensis Preble (1899:25), here considered a synonym of Z. p. oregonus, was described as having: auditory bullae small, posterior border of the palate usually convex anteriorly, palatal bridge long, and color pale. These characters, however, are within the range of individual variation of Zapus p. oregonus. Similarities such as tooth-rows diverging anteriorly, nasals narrow posteriorly, interorbital region broad, and incisive foramina enlarged posteriorly are added reasons for placing Z. nevadensis as a synonym of Z. p. oregonus.
Zapus princeps palatinus Hall (1931:8) was described as having: palatal bridge long, incisive foramina wide posteriorly, posterior border of palate straight or convex posteriorly, and color pale. These characteristics are to be found in some individuals in most populations of Z. p. oregonus. Additional well marked cranial similarities, such as small auditory bullae, broad interorbital region, and nasals narrow posteriorly offer additional evidence as to the close relationship of Z. p. palatinus and Z. p. oregonus. Hall (loc. cit.), with a small sample available to him for comparative purposes (14 specimens of Z. p. palatinus and 12 specimens of Z. p. nevadensis), [411] was impressed by the condition of the palate in Z. p. palatinus and wrote: “the generally straight, or even posteriorly convex, posterior border of the palate seems to be unique among described forms of Zapus. The name palatinus is given in allusion to this structural feature.” With more than 300 specimens of Z. p. oregonus available for study I find that a straight or posteriorly convex posterior border of the palate occurs in more than 50 per cent of the individuals examined. Specimens displaying this described palatal condition are known from all parts of the range of Z. p. oregonus, but do occur in a higher percentage of specimens in the area ascribed by Hall (loc. cit.) to the range of Z. p. palatinus.
Intergradation with Zapus princeps idahoensis and Zapus princeps cinereus is discussed in the accounts of those subspecies.
Specimens examined.—Total, 340, distributed as follows:
California: Modoc Co.: Buck Creek R. S., 1 (CAS); Willow Ranch, 4 (CAS); Sugar Hill, 5000 ft., 1 (MVZ); Goose Lake Meadows, near Sugar Hill, 4 (MVZ); Parker Creek, Warner Mts., 5500 ft., 18 (MVZ); Dry Creek, Warner Mts., 4800 ft., 3 (MVZ) east face Warner Peak, Warner Mts., 8700 ft., 1 (MVZ); 5 mi. NW Eagle Peak, 7000 ft., 5 (MVZ); Lassen Creek, 1 (SDM); Happy Camp, 1 (CAS).
Idaho: Boise Co.: Bald Mtn. R. S., Boise Nat’l Forest, 10 mi. S. Idaho City, 7400 ft., 2 (USBS). Elmore Co.: Trail Creek, Boise Nat’l Forest, 2 (USBS). Washington County: 1 mi. NE Heath, SW Slope Cuddy Mtn., 4000 ft., 20 (5 MVZ).
Nevada: Elko County: 6 mi. SW Mountain City, Cobb Creek, 6500-6550 ft., 44 (MVZ); summit between heads of Copper and Coon creeks, Jarbidge Mts., 18 (9 MVZ); head of Ackler Creek, 6800 ft., 2: Steel Creek, 7000 ft., 11 (4 MVZ); summit of Secret Pass, 6200 ft., 8; south fork Long Creek, 7830 ft., 4; Harrison Pass R. S., Green Mtn., Canyon, 6050 ft., 12. Eureka County: 4 mi. S Tonkin, Denay Creek, Roberts Mt’s, 1 (MVZ). Humboldt County: Martin Creek R. S., 1 (MVZ); 13 mi. N Paradise Valley, 6700 ft., 19 (MVZ). Lander County: Kingston R. S., 7500 ft., 4 (MVZ). Nye County: Wisconsin Creek, 7000 ft., 12 (MVZ). White Pine County: Willow Creek, 2 mi. S Elko County line, Ruby Mts., 6500 ft., 24 (2 MVZ).
Oregon: Baker Co.: East Pine Creek, 21/2 mi. NE Cornucopia, 6 (USBS); McEwen, 2 (USBS); Bourne, 7 (USBS). Clackamas County: Marks Creek, 12 mi. N of Howard, 2 (USBS); Howard, 2 (USBS). Crook County: Ochoco R. S., 4000 ft., 4 (MVZ). Grant Co.: Austin, 2 (USBS); Cold Spring, 4900 ft., 8 mi. E Austin, 4 (MVZ); Beech Creek, 1 (USBS); Strawberry Mts., 6 (USBS); north fork Malheur River, 21 mi. SE Prairie City, 5000 ft., 21 (MVZ). Harney Co.: 10 mi. N. Harney, 1 (USBS); Steen Mts., Keiger Gorge, 6900 ft., 6 (USBS); Diamond, 4300 ft., 2 (USBS). Jefferson Co.: Foley Creek, 12 mi. E Hay Creek, 1 (USBS). Klamath Co.: Fort Klamath, 1 (USBS). Lake Co.: Silver Creek, 7000 ft., Yamsey Mts., 1 (USBS); 2 mi. E Lakeview, 5200 ft., 3 (MVZ). Malheur Co.: Jordan Valley, 4200 ft., 1 (USBS). Umatilla Co.: Meacham, 1 (USBS). Union County: Elgin, 2 (USBS). Wallowa Co.: Paradise, 10 mi. N Horse Creek, 7000 ft., 1 (USBS); Minam Lake, 1 (USBS); 16 mi. S and 3 mi. E Lostine, 5500 ft., 9 (MVZ); west fork Wallowa River, 5000 ft., 21/2 mi. above Wallowa Lake, 1 (FM); near Wallowa Lake, 4500 ft., 3 (FM). Wheeler County: 11 mi. W and 7 mi. S Mitchell, 4850 ft., 20 (MVZ).
Washington: Asotin Co.: Anatone, 3300 ft., 1 (USBS). Columbia County: Twin Buttes, 25 mi. SE Dayton, Blue Mts., 2 (MVZ); Stayawhile Spring, 5150 ft., 4 (MVZ).
Marginal records.—Washington: Anatone, 3300 ft. Oregon: East Pine Creek, 21/2 mi. NE Cornucopia. Idaho: 1 mi. NE Heath, SW slope Cuddy Mtn., 4000 ft.; Bald Mtn., R. S., Boise Nat’l Forest, 10 mi. S. Idaho City, 7400 ft.; Trail Creek, Boise Nat’l Forest. Nevada: Harrison Pass R. S., Ruby Mts.; Steel Creek, 7000 ft.; Wisconsin Creek, 7000 ft.; 13 mi. N Paradise Valley, 6700 ft. California: Lassen Creek; Buck Creek R. S. Oregon: Fort Klamath; Howard; Meacham. Washington: Twin Buttes, 25 mi. SE Dayton, Blue Mts.
Zapus pacificus Merriam, Proc. Biol. Soc. Washington, 11:104, April 26, 1897; Preble, N. Amer. Fauna, 15:30, August 8, 1899.
Jaculus hudsonius, Baird, Repts. Expl. and Surv. 111 8 (pt. 1):433, July 14, 1858 (part—the part from Canoe Creek, California).
Zapus alleni Elliot, Field Columbian Mus., publ. 27, zool. ser., 1:212, April 19, 1898, type from Pyramid Peak, Lake Tahoe, El Dorado County, California.
Zapus trinotatus alleni, Elliot, Field Columbian Mus. Publ. 91, zool. ser., 3:315, July 5, 1904; Preble, N. Amer. Fauna, 15:27, August 8, 1899.
Zapus pacificus alleni, Howell, Univ. California Publ. Zool., 21:232, May 20, 1920.
Zapus trinotatus pacificus, Bailey, N. Amer. Fauna, 55:233, August 29, 1936.
Zapus princeps alleni, Hall, Mammals of Nevada; Univ. California Press, Berkeley, California, 579, July 1, 1946.
Type.—Male, subadult, skin and skull, No. 80445, U. S. Nat. Mus. Biol. Surv. Coll.; Prospect, Rogue River Valley, Jackson Co., Oregon; obtained on August 29, 1896, by Edward A. Preble, original No. 1454.
Range.—Sierra Nevada Mt’s, from Kern Peak, Tulare County, California, northeastward to Mt. Rose, Washoe County, Nevada, then northwestward through the Trinity and Salmon mountains, California, to the upper Rogue River Valley, Oregon, thence southwestward to South Yolla Bolly Mt’n, Tehama County, California. See fig. 46. Zonal range: Transition, Canadian, and Hudsonian.
Description.—Size medium; color bright; back near Ochraceous-Buff with admixture of black hair forming dark dorsal band; sides bright Ochraceous-Buff with fine admixture of black hair; lateral line blending with color of sides or wanting or indistinct; ventral surface white; tail bicolored, grayish-brown above, yellowish-white below, in some specimens with white tip; feet grayish-white above; ears dark, edged with Ochraceous Buff; braincase relatively narrow; incisive foramina relatively short; pterygoid fossae usually broad; proximal part of inferior ramus of zygomatic process of maxillary broad; postpalatal notch usually broadly rounded; auditory bullae relatively small and flattened; nasals parallel sided; maxillary tooth-row short; interorbital region moderately broad.
Comparison.—From Zapus princeps oregonus, Z. p. pacificus differs in being brighter in all pigmented areas; more ochraceous and less yellow laterally; dorsally more ochraceous and less black; size averaging smaller; maxillary tooth-rows shorter; auditory bullae less inflated and smaller; interorbital region averaging narrower; palatal bridge averaging shorter; incisive foramina shorter and posteriorly narrower; nasals parallel rather than narrowed posteriorly.
Remarks.—Original describers considered both Z. pacificus and Z. alleni as specifically distinct from Z. trinotatus. Merriam (1897a:104) named Z. pacificus and gave the following diagnostic characters: [413] short rostrum and nasals; small auditory bullae; basioccipital broad between bullae. Elliot (1898:212) named Z. alleni and ascribed to it the following diagnostic characters: cranium long and narrow; nasals same breadth for entire length; palate wide; pterygoid fossae wide posteriorly; auditory bullae small; basisphenoid and basioccipital wide; upper tooth-rows short. Preble (1899:27) considered Z. alleni to be a subspecies of the species Z. trinotatus, remarking that the skulls are similar to those of Z. trinotatus but smaller with much smaller bullae; in coloration the animals are lighter above and without fulvous below. Preble remarked that the skull of Z. alleni differs so greatly from that of Z. montanus that comparison was not required. Preble (op. cit.:30) treated Z. pacificus as a full species. Howell (1920:233) considered Z. pacificus and Z. alleni to be subspecies of Z. pacificus. Howell (loc. cit.) pointed out size, cranial, and color similarities between the two, and remarked that pacificus is clearly distinct from Z. montanus, its nearest geographic neighbor. Hall (1946:578) arranged Z. alleni as a subspecies of Z. princeps, although not on grounds wholly satisfactory to him because actual intergrades between alleni and neighboring races of princeps were not available.
I here consider Z. alleni to be synonymous with Z. pacificus; the latter is a subspecies of Z. princeps. Certain diagnostic characters, such as the shape and size of the os penis, the diameter and pigment pattern of the hair, the over-all proportions of the skull, and the size and shape of the teeth indicate that alleni and princeps belong to the same species, even though animals from intermediate geographic areas are not available to show actual intergradation.
The diagnostic characters referred to in the original description of Z. alleni, as given earlier in this account, agree with characters of specimens of Z. p. pacificus. Howell (1920:233) remarks that, in coloration and length of foot, typical alleni differs but slightly from pacificus. Howell (loc. cit.) noted, as I also have, that there are slight cranial differences in specimens from various parts of the range of Z. p. pacificus; these variations are somewhat clinal in nature, cranial dimensions showing a slight increase from south to north. The largest animals occur in western Tehama, Trinity, and Siskiyou counties, California. Samples from various localities in Jackson County, Oregon, are slightly smaller than these, but are larger than specimens from the southern Sierra Nevada.
Specimens examined.—Total, 264, distributed as follows:
California: Alpine County: Carson River, 1/4 mi. SW Woodfords, 5700 ft., 3 (MVZ); Diamond Valley, 5500 ft., 1 mi. SE Woodfords, 6 (MVZ); Faith Valley, [414] 1 (MVZ). El Dorado County: Glen Alpine Creek, near Fallen Leaf Lake, 6600 ft., 8 (MVZ); 1 mi. W Fyffe, 1 (MVZ); Fresno County: Hume, 1 (MVZ). Mariposa County: Chinquapin, 6700 ft., Yosemite Nat’l Park, 12 (MVZ); E fork Indian Canyon, 7300 ft., 8 (MVZ); Merced Grove, Big Trees, 7 (MVZ); 1 mi. E Merced Lake, 5 (MVZ); near Mono Meadow, Yosemite Nat’l Park, 4 (MVZ); near Mt. Hoffman, 8100 ft., Yosemite Nat’l Park, 5 (MVZ); Porcupine Flat, 8100 ft., Yosemite Nat’l Park, 9 (MVZ); Yosemite Creek, Yosemite Valley, 7 (MVZ); foot Yosemite Falls, Yosemite Nat’l Park, 8 (MVZ). Mono County: Walker Lake, 8000 ft., 5 (MVZ); Swager Canyon, 7800 ft., 3; Mono Lake P. O. 6500 ft., 4 (MVZ). Placer Co.: Truckee River, Squaw Creek, 1 (SDM); W bank Truckee River, 1 (MVZ). Plumas County: Rich Gulch, 3850 ft., 11 mi. W and 8 mi. N Quincy, 2 (MVZ). Shasta County: Warner Creek, 8000 ft., Lassen Peak, 6 (MVZ). Siskiyou Co.: Donomore Meadow, 5800 ft., 15 mi. W Hilt, 7 (MVZ); Poker Flat, 5000 ft., 12 mi. NW Happy Camp, 7 (MVZ); Little Shasta, 1 (USBS); Siskiyou Mts., 6000 ft., 2 (USBS); Sisson, 1 (SDM); Mt. Shasta, 6500 ft., 6 (MVZ). Salmon River Divide, 2 (MVZ); S fork Salmon River, 5000 ft., 7 (MVZ). Tehama County: 2 mi. W Black Butte, on Lassen Rd., 6800 ft., 5 (MVZ); 2 mi. E Mineral, 5200 ft., 2 (MVZ); 2 mi. S Yolla Bolly Mtn., 11 (MVZ). Trinity Co.: N fork Coffee Creek, 4500 ft., 34 (MVZ); Canyon Creek, 4 (USBS); 8 mi. NE Hyampon, 2900 ft., 1 (MVZ); 3 mi. NNW Mad River Bridge, 2900 ft., South Fork Mtn., 5 (MVZ); 11/2 mi. N Mad River Bridge, 3000 ft., South Fork Mtn., 6 (MVZ); 1 mi. SW North Yolla Bolly Mtn., 14 (11 MVZ); 1/2 mi. S South Yolla Bolly Mtn., 3 (MVZ). Tulare County: Jordan Hot Springs, Sierra Nevada Mts., 6700 ft., 9 (MVZ); Sherman Creek, Sequoia Nat’l Park, 1 (MVZ); Tokopah Valley, 7000 ft., Sequoia Nat’l Park, 1 (MVZ); 2 mi. E Kern Peak, 9300 ft., Sierra Nevada Mts., 1 (MVZ). Tuolumne County: head Lyle Canyon, Yosemite Nat’l Park, 10,000 ft., 9 (MVZ); Tuolumne Meadows, 8600 ft., Yosemite Nat’l Park, 1 (MVZ).
Nevada: Douglas County: 1/2 mi. E Zephyr Cove, Lake Tahoe, 6400 ft., 1 (MVZ). Ormsby County: S end Marlette Lake, 8000 ft., 2 (MVZ); 1/2 mi. S Marlette Lake, 8150 ft., 3 (MVZ). Washoe County: 1/2 mi. S Mt Rose, 9500 ft., 3 (2 MVZ); 3 mi. S Mt. Rose, 8500 ft., 3 (MVZ).
Oregon: Jackson Co.: Prospect, 3 (2 USBS, 1 MVZ); W slope Grizzly Peak, 4600 ft., 1 (USBS); Siskiyou, 1 (USBS); Longs Camp, N base Ashland Peak, 3300 ft., 1 (USBS).
Marginal records.—Oregon: Prospect. Nevada: 3 mi. S Mt. Rose, 8500 ft.; 1/2 mi. E Zephyr Cove, Lake Tahoe, 6400 ft. California: Mono Lake P. O., 6500 ft.; 2 mi. E Kern Peak, 9300 ft., Sierra Nevada Mts.; Rich Gulch, 3850 ft., 11 mi. W and 8 mi. N Quincy; Warner Creek, 8000 ft., Lassen Peak; 2 mi. S Yolla Bolly Mtn.; 8 mi. NE Hyampon, 2900 ft.; Siskiyou Mts., 6000 ft.; Poker Flat, 5000 ft., 12 mi. NW Happy Camp.
Zapus princeps J. A. Allen, Bull. Amer. Mus. Nat. Hist., 5:71-72, April 28, 1893; Preble, N. Amer. Fauna, 15:23, August 8, 1899.
Type.—Female, adult, skin and skull; No. 5260/4140, Amer. Mus. Nat. Hist.; Florida, La Plata County, Colorado; obtained on June 27, 1892, by Charles P. Rowley.
Range.—Sierra Madre, Medicine Bow, Laramie, and Big Horn mountains of Wyoming southward through Colorado into the Taos and San Juan mountains in northern New Mexico. See fig. 46. Zonal range: Transition, Canadian and Hudsonian.
Description.—Size, medium; back dark usually with broad mid-dorsal band of black mixed with Warm Buff or Ochraceous-Buff; sides light (Warm Buff) but varying to Ochraceous-Buff, always with admixture of black hair; lateral line distinct and broad, varying from Light Ochraceous-Buff to Ochraceous-Buff; [415] ventral surface white to base of hairs, frequently suffused with Ochraceous-Buff; tail indistinctly bicolored, tan to grayish-white below and pale brown above; hind feet grayish-white above; ears edged with white or yellowish-white; skull medium; large medial projection on inferior ramus of zygomatic process of maxillary; palate moderately long; postpalatal notch usually broadly rounded and posterior to posterior part of last molar; proximal part of inferior ramus of zygomatic process of maxillary broad; pterygoid fossae broad; auditory bullae moderately inflated.
Comparisons.—From Zapus princeps luteus, Z. p. princeps differs as follows: Total length, tail and hind foot longer; color darker, being less ochraceous; ears darker, edged with white or yellowish-white instead of Ochraceous-Buff; lateral line more distinct; skull larger, except least interorbital breadth which is smaller; auditory bullae larger, more inflated; pterygoid fossae larger; incisive foramina broader, longer, and posteriorly more truncate; nasals broader, tapering less distally.
From Zapus princeps idahoensis, Z. p. princeps differs in: Size larger; darker with more Ochraceous-Buff; lateral line much more distinct; underparts frequently suffused with Ochraceous-Buff rather than seldom so; skull larger as regards length of palatal bridge, length of zygomatic arch, and width of proximal part of inferior ramus of zygomatic process of maxillary; pterygoid fossae broader; medial projection on inferior ramus of zygomatic process of maxillary large instead of reduced or absent; postpalatal notch usually anterior to, or on a plane with, posterior face of last molars rather than posterior to same.
Remarks.—This subspecies retains most of its diagnostic characters in all parts of its geographic range. An individual from the type locality, Florida, Colorado, resembles Zapus princeps luteus in color, but cranially is most nearly like Z. p. princeps. A specimen from Tierra Amarilla, New Mexico, a locality 25 miles north of, and in homogeneous habitat with, El Rito, New Mexico, from which specimens of Z. p. luteus are known, shows resemblance to the latter in some cranial characters (see account of Zapus princeps luteus) but is most nearly like Z. p. princeps to which it is referred.
Animals from Medicine Wheel Ranch, 9000 ft., 28 mi. E Lovell, Wyoming, which are here referred to Z. p. princeps, show intergradation with Zapus princeps idahoensis, being similar in size of pterygoid fossae, breadth of postpalatal notch, and in size and degree of inflation of the auditory bullae, but differ in color and in other cranial characters. Specimens from 2 mi. E Shriver, 6500 ft., Montana, which lack the distinct lateral line and ventral suffusion of Ochraceous-Buff, are here referred to Z. p. idahoensis.
Specimens examined.—Total, 344, distributed as follows:
Colorado: Archuleta County: upper Navajo River, 5 (CMNH); Navajo River, 6 (CMNH). Boulder Co.: 121/2 mi. S Estes Park, 2; 3 mi. S Ward, 3; Gold Hill, 1 (USBS); 7 mi. NW Nederland’s, 2 (UM); 3 mi. E Pine Cliff, 3 (CMNH). Chaffee County: 11/2 mi. S Monarch, 10,500 ft., 2 (OKLA). Conejos Co.: Antonito, 1 (USBS); 5 mi. S and 24 mi. W Antonito, 9600 ft., 2. Costilla Co.: 7 mi. SE Russell, 9200 ft., 1 (MVZ); Fort Garland, 6 (USBS). [416] El Paso County: Minnehaha, Half Way, 5 (UM). Grand Co.: Rocky Mtn. Nat’l Park, 5 (UM). Gunnison County: Gothic, 10 (8 OKLA; 2 USBS); Major Creek, foot of Monarch Pass, 1 (OKLA). Jackson Co.: Arapahoe Pass, Rabbit Ear Mts., 1 (USBS). La Plata Co.: 7 mi. N Florida, Florida River, 7146 ft., 8 (MVZ); Florida, 6500 ft., 11 (1 FM; 9 AMNH). Larimer Co.: Elkhorn, 7000 ft., 1 (USBS); 191/2 mi. W and 21/2 mi. S Loveland, 7300 ft., 3. Mineral Co.: Wasson Ranch, Creede, 1; 3 mi. E Creede, 1; 23 mi. S and 11 mi. E Creede, 9300 ft., 5. Rio Blanco Co.: 91/2 mi. SW Pagoda Peak, 7700 ft., 5; Meeker, 1 (USBS). Rio Grande County: Rock Creek Camping Area, 1 (OKLA). Saguache Co.: Saguache Park, Cochetopa Forest, 1 (USBS); 22 mi. W Saguache, 1 (MVZ); 20 mi. S Saguache, Cochetopa Pass, 1 (USBS). San Juan County: 61/2 mi. SW Silverton, 4.
New Mexico: Rio Arriba Co.: Tierra Amarilla, 1 (USBS). Taos Co.: Hondo Canyon, 8200 ft., west slope Taos Mts., 1 (USBS); east slope Taos Mts., 8800 ft., 1 (USBS).
Wyoming: Albany County: 32 mi. N and 121/2 mi. E Laramie, 6080 ft., 1; 30 mi. N and 10 mi. E Laramie, 6760 ft., 1; 29 mi. N and 83/4 mi. E Laramie, 6420 ft., 6; 2 mi. S Browns Peak, 10,600 ft., 2; 3 mi. ESE Browns Peak, 10,000 ft., 8; 8 mi. E and 4 mi. S Laramie, 8600 ft., 2; 8 mi. E and 6 mi. S Laramie, 8500 ft., 1; 1 mi. ESE Pole Mtn., 8350 ft., 2; 11/2 mi. ESE Pole Mtn., 8200 ft., 1; 2 mi. SE Pole Mtn., 8300 ft., 3; Centennial, 8120 ft., 1. Big Horn County: Medicine Wheel Ranch, 9000 ft., 28 mi. E Lovell, 36; 12 mi. E and 2 mi. N Shell, 7500 ft., 13; 17 mi. E and 3 mi. S Shell, 9000 ft., 1; 171/2 mi. E and 41/2 mi. S Shell, 9100 ft., 6. Carbon County: Bridgers Pass, 18 mi. SW Rawlins, 7500 ft., 6; Lake Marie, Medicine Bow Nat’l Forest, 10,400 ft., 6; 14 mi. E and 6 mi. S Saratoga, 5; 10 mi. N and 10 mi. E Encampment, 8000 ft., 1; 10 mi. N and 12 mi. E Encampment, 7200 ft., 2; 10 mi. N and 14 mi. E Encampment, 8000 ft., 28; 9 mi. N and 3 mi. E Encampment, 2; 8 mi. N and 8 mi. E Encampment, 8900 ft., 1; 8 mi. N and 14 mi. E Encampment, 8400 ft., 5; 8 mi. N and 141/2 mi. E Encampment, 8100 ft., 12; 8 mi. N and 16 mi. E Encampment, 8400 ft., 6; 8 mi. N and 22 mi. E Encampment, 10,000 ft., 1; 8 mi. N and 191/2 mi. E Savery, 8800 ft., 12; 8 mi. N and 20 mi. E Savery, 8800 ft., 1; 71/2 mi. N and 18 mi. E Savery, 8400 ft., 2; 71/2 mi. N and 181/2 mi. E Savery, 8400 ft., 1; 7 mi. N and 18 mi. E Savery, 8400 ft., 2; 6 mi. N and 131/2 mi. E Savery, 8400 ft., 6; 6 mi. N and 14 mi. E Savery, 8350 ft., 6; 4 mi. N and 8 mi. E Savery, 7300 ft., 1. Converse County: 21 mi. S and 24 mi. W Douglas, 7400 ft., 6; 21 mi. S and 241/2 mi. W Douglas, 7400 ft., 3; 211/2 mi. S and 241/2 mi. W Douglas, 7600 ft., 15; 221/2 mi. S and 241/2 mi. W Douglas, 7600 ft., 1; 23 mi. S and 25 mi. W Douglas, 7800 ft., 7. Johnson County: 61/2 mi. W and 2 mi. S Buffalo, 5700 ft., 4; 51/2 mi. W and 11/2 mi. S Buffalo, 5520 ft., 3; 51/2 mi. W and 1 mi. S Buffalo, 4800 ft., 1; 1 mi. W and 4/5 mi. S Buffalo, 4800 ft., 1. Laramie County: 5 mi. W and 1 mi. N Horse Creek P. O., 3. Natrona County: 2 mi. W and 7 mi. S Casper, 6370 ft., 2. Washakie County: 9 mi. E and 5 mi. N Tensleep, 7400 ft., 2; 9 mi. E and 4 mi. N Tensleep, 7000 ft., 5.
Marginal records.—Wyoming: Medicine Wheel Ranch, 9000 ft., 28 mi. E Lovell; 21 mi. S and 24 mi. W Douglas, 7400 ft.; 5 mi. W and 1 mi. N Horse Creek P. O. Colorado: Gold Hill; Minnehaha. New Mexico: E slope Taos Mts.; Tierra Amarilla. Colorado: Florida; 61/2 mi. SW Silverton; Meeker. Wyoming: Bridgers Pass, 18 mi. W Rawlins, 7500 ft.
Zapus saltator J. A. Allen, Bull. Amer. Mus. Nat. Hist., 12:3-4, March 4, 1899; Preble, N. Amer. Fauna, 15:31, August 8, 1899.
Zapus princeps, Preble, N. Amer. Fauna, 15:23, August 8, 1899 (part—the part from Glacier, British Columbia).
Zapus hudsonius, Kermode and Anderson, Rep. Prov. Mus. Nat. Hist. for 1913:21, 1914.
Zapus princeps saltator, Hall, Univ. California Publ. Zool., 37:10, April 10, 1931.
Type.—Female, subadult, skin and skull, No. 14408, Amer. Mus. Nat. Hist.; Telegraph Creek, British Columbia; obtained on August 23, 1897, by A. J. Stone.
Range.—Southern Yukon and southeastern Alaska south in British Columbia, to Bella Coola Inlet and Glacier. See fig. 46. Zonal range: Canadian and Hudsonian.
Description.—Size medium; back near Ochraceous-Buff, overlaid with black hairs forming dark dorsal band thickly flecked with ochraceous; sides lighter than back; lateral line usually distinct; belly pure white, sometimes faintly suffused with Ochraceous-Buff; tail bicolored, dark above and grayish-white below; hind feet grayish-white above; ears dark, edged with yellowish-white or Ochraceous-Buff; incisive foramina long, broad posteriorly; palatal bridge relatively short; postpalatal notch anterior to posterior border of last molars; proximal part of inferior ramus of zygomatic process of maxillary without enlarged median projection; zygomatic arch short.
Comparisons.—For comparison with Zapus princeps kootenayensis and Zapus princeps idahoensis see accounts of those subspecies.
Remarks.—The geographic range of Z. p. saltator, as here understood, includes several localities heretofore considered to be within the geographic ranges of neighboring subspecies. Specimens from Indianpoint Lake, 15 mi. N of Barkerville, British Columbia, for example, which Hall (1934:379) considered nearer Z. p. princeps, are here referred to Z. p. saltator, with which they closely agree in cranial measurements and color of pelage. One individual from Glacier, British Columbia, thought to be Z. p. princeps by Preble (1899:32), is here considered to show intergradation between Z. p. kootenayensis and Z. p. saltator but is more nearly like Z. p. saltator to which it is here referred. Intergradation between Zapus princeps idahoensis and Z. p. saltator is noted, in color and in shape and size of the incisive foramina, in a specimen from Vermilion Crossing, Kootenay, British Columbia. The majority of cranial characters show these animals to be referable to Z. p. idahoensis. Specimens from Mt. Revelstoke, 3400 ft., British Columbia, show intergradation in shape of auditory bullae, in breadth of pterygoid fossae, and in shape and size of antorbital foramina between Z. p. idahoensis and Z. p. saltator. Resemblance in pelage and in the majority of cranial characters indicates that these specimens are best referred to Z. p. saltator.
Specimens examined.—Total, 187, distributed as follows:
Alaska: Taku River, 1 (MVZ).
British Columbia: Atlin, 7 (6 CAS; 1 PM); Deep Creek, 60 mi. above Telegraph Creek, 1 (USBS); Sawmill Lake, near Telegraph Creek, 6 (MVZ); junction 4 mi. N Telegraph Creek, 1 (ROM); McDame Post, Dease River, 1 (USBS); Stikine River, at Glenora, 28 (MVZ); Kispiox Valley, 23 mi. N Hazelton, 3 (MVZ); 9-mi. Mtn., 4500 ft., NE Hazelton, 1 (MVZ); Hazelton, 959 ft., 20 (MVZ); Bear River, 7 mi. N Bear Lake, 1 (USBS); Charlie Lake, Fort St. John, 1 (PM); Moose River, 2 (PM); Tupper Creek, 7 (PM); Babine, 2 (USBS); Port Simpson, 3 (USBS); 12 mi. N Summit Lake, Alaska Highway, 3300 ft., 3 (NMC); Giscome, 1 (USBS); Ootsa Lake, 3 (PM); Inverness, mouth Skeena River, 1 (USBS); W end Eutsuk Lake, 1 (PM); Wapiti, [418] head of Middle Branches River, 1 (USBS); Hagensborg, 15 (NMC); Stuie, Cariboo Mtn., 4700 ft., 2 (NMC); Rainbow Mts., Mt. Brilliant, 5000 ft., 10 (NMC); N 7 Wistaria P. O., 13 (NMC); Mt. McLean, Lillooet, 1 (PM); Mt. Robson P. O., Mt. Robson Park, 1 (MVZ); Indianpoint Lake, 15 mi. NE Barkerville, 42 (29 MVZ; 18 PM); Cottonwood P. O., 2 (MVZ); Mt. Revelstoke, 3400 ft., 6 (PM); Glacier, 1 (ROM).
Yukon: Rose River, mile 95 on Canol Road, 1 (NMC).
Marginal records.—Yukon: Rose River, mile 95 on Canol Road, British Columbia; McDame Post, Dease River; Charlie Lake, Fort St. John; Tupper Creek; Wapiti, head of Middle Branches River; Mt. Robson P. O., Mt. Robson Park; Mt. Revelstoke, 3400 ft.; Cottonwood P. O.; Rainbow Mts., Mt. Brilliant, 5000 ft.; Inverness, mouth Skeena River. Alaska: Taku River. British Columbia: Atlin.
Zapus princeps utahensis Hall, Occ. papers, Mus. Zool., Univ. Michigan, 296:3, November 2, 1934.
Jaculus Hudsonius, J. A. Allen, Bull. Essex Inst., 6:65, April, 1874 (part—the part concerning Great Salt Lake Valley, Utah).
Zapus princeps princeps, Wolfe, Jour. Mamm., 91:154, May 9, 1928.
Zapus princeps idahoensis, Davis, Recent Mammals of Idaho, Caxton Printers, Caldwell, Idaho, p. 341, April 5, 1939 (part—the part from southeast Idaho).
Type.—Female, adult, skin and skull; No. 59153, Museum of Zoology, University of Michigan; Beaver Creek, 19 mi. S Manila, Daggett County, Utah; obtained on July 16, 1928, by A. and R. D. Svihla, original No. 176.
Range.—Southeastern Idaho and extreme western Wyoming (Teton, Snake, and Uinta Mt’s) southward through Uinta, Wasatch, Oquirrh, and Beaver Mt’s of Utah. See fig. 46. Zonal range: Transition, Canadian, and Hudsonian.
Description.—Size, large; back from Cinnamon-Buff to Warm Buff overlaid with black hairs; sides lighter with less admixture of black hairs; lateral line indistinct, sometimes wanting; tail bicolored, brownish-black above, white to yellowish-white beneath; feet grayish-white above; ventral surface white to base of hairs; ears dark, edged with white to yellowish-white; skull large; palatal bridge relatively short; upper tooth-rows diverging anteriorly; occipitonasal length great; interorbital region broad; zygomata widely bowed; postpalatal notch anterior to posterior face of last molars; mastoid width great.
Comparisons.—From Zapus princeps princeps, Z. p. utahensis differs in: color dorsally and laterally less ochraceous, lacking broad lateral line; skull larger in every part measured, excepting length of palatal bridge and breadth of palate at M3; zygomata more bowed; upper tooth-rows more divergent anteriorly; postpalatal notch anterior to posterior border of last molars.
Compared with Zapus princeps cinereus, Z. p. utahensis differs as follows: Size averaging larger; upper parts darker, Cinnamon-Buff not Pinkish-Buff; incisive foramina wider posteriorly; palate wider; zygomata more robust.
For comparison with Zapus princeps idahoensis see account of that subspecies.
Remarks.—Zapus princeps utahensis most closely resembles the several subspecies in the Great Basin in its large size, widely bowed zygomata, and posteriorly broadened incisive foramina. Intergradation between Z. p. utahensis and Zapus princeps cinereus, geographically the nearest of the Great Basin subspecies, is not [419] known. Intergradation in color and cranial characters occurs between Zapus princeps idahoensis and Z. p. utahensis in specimens from 17 mi. E and 4 mi. N of Ashton, Idaho. All these specimens are, however, referable to Z. p. idahoensis. Animals from 9 mi. SE Irwin and from 3 mi. SW Victor, Idaho, resemble Z. p. utahensis in most differential characters (dorsally ochraceous, lateral line more distinct, incisive foramina large, palate broad anteriorly, auditory bullae less inflated), and are here referred to Z. p. utahensis. A series of specimens from the head of Crow Creek, Idaho, were considered by Davis (1939:340) to be intergrades between Z. p. idahoensis and Z. p. utahensis; he thought that the specimens were more nearly like Z. p. utahensis in color, but cranially (80 per cent in average ratio of anterior width of palate to posterior width of palate), more nearly like Z. p. idahoensis, to which subspecies he referred them. I have examined these specimens and find them to be more nearly like Z. p. utahensis not only in color but in cranial characters as well. For example, the average ratio obtained by me for anterior width of palate to posterior width of palate is 72 per cent, rather than 80 per cent as given by Davis (loc. cit.). Other cranial characters, size of the incisive foramina, shape of the foramen magnum, and shape of the auditory bullae, indicate relationship with Z. p. utahensis to which they are here referred. Two immature individuals from Strawberry Creek, 20 mi. E Preston, Idaho, considered to be Z. p. idahoensis by Davis (op. cit.:341), also are here referred to Z. p. utahensis.
Specimens examined.—Total, 178, distributed as follows:
Idaho: Bonnerville County: 9 mi. SE Irwin, 6400 ft., 3. Caribou Co.: Head Crow Creek, Preuss Mts., 7500 ft., 6 (USBS). Franklin County: Strawberry Creek, 20 mi. NE Preston, 6700 ft., 2 (MVZ). Teton County: 3 mi. SE Victor, 6 (MVZ).
Utah: Beaver County: Puffer Lake, 1 (UU). Daggett County: junction Deep Creek and Carter Creek, 7900 ft., 2 (UU). Duchesne Co.: Currant Creek, Uinta Forest, 2 (USBS). Morgan Co.: exact locality not given, 1 (UU). Rich County: 12 mi. SW Woodruff, 1 (MVZ). Salt Lake County: Lambs Canyon, 2 mi. above Parleys Canyon, 7000 ft., 1 (UU); head Lambs Canyon, 9000 ft., 3 (UU); Salamander Lake and Lambs Canyon, 9000 ft., 11 (UU); “The Firs,” Mill Creek Canyon, 2 (UU); Brighton, Silver Lake P. O., 8700 ft., Cottonwood Canyon, 1 (UU); Brighton, Big Cottonwood Canyon, 8000 ft., 1 (UU); 1 mi. above Alta, 4 (UU); Butterfield Canyon, approximately 5 mi. above Butterfield Tunnel, 3 (UU). Sanpete Co.: 8 mi. E Fairview and 5 mi. S Mammoth R. S., Manti Nat’l Forest, 9000 ft., 1 (USBS); Baldy R. S., Manti Nat’l Forest, 1 (UU); Ephraim, 8850 ft., 1 (USBS). Summit County: Henrys Fork, Uinta Mts., 8000 ft., 4 (UU); 14 mi. S and 2 mi. E Robertson, 9300 ft., 3. Uintah County: 21 mi. W and 15 mi. N Vernal, 10,050 ft., 1. Utah County: Payson Lake, 8300 ft., 12 mi. SE Payson, Mt. Nebo, 12 (UU); 1 mi. E Payson Lake, 8300 ft., Mt. Nebo, 3 (UU). Wasatch County: Provo River, 3 mi. N Soapstone R. S., Wasatch Nat’l Forest, 1 (UU).
Wyoming: Lincoln County: 3 mi. N and 11 mi. E Alpine, 5650 ft., 37. Teton County: 1/4 mi. E Moran, 6700 ft., 4; Bar B. G. Ranch, 6500 ft., 21/2 mi. NE Moose, 11; [420] Moose, 6225 ft., 1. Uinta County: 2 mi. E Robertson, 7200 ft., 1; 9 mi. S Robertson, 8000 ft., 21; 9 mi. S and 21/2 mi. E Robertson, 8000 ft., 1; 91/2 mi. S and 1 mi. W Robertson, 8600 ft., 2; 10 mi. S and 1 mi. W Robertson, 8700 ft., 18; 101/2 mi. S and 2 mi. E Robertson, 8900 ft., 1; 13 mi. S and 1 mi. E Robertson, 9000 ft., 4; 5 mi. E Lonetree, 1 (ROM).
Marginal records.—Wyoming: 1/4 mi. E Moran, 6700 ft.; 2 mi. E Robertson, 7200 ft. Utah: junction Deep Creek and Carter Creek, 7900 ft.; Paradise Park, 21 mi. W and 15 mi. N Vernal, 10,500 ft.; Ephraim, 8500 ft.; Puffer Lake; Payson Lake, 8300 ft., 12 mi. SE Payson, Mt. Nebo; Butterfield Canyon, approximately 5 mi. above Butterfield Tunnel. Idaho: Strawberry Creek, 20 mi. NE Preston, 6700 ft.; 3 mi. SW Victor.
Range.—From Pacific Coast of Alaska eastward to Atlantic Coast; from northern limit of tree-growth south into central Colorado and northeastern parts of Oklahoma and Georgia. See fig. 47.

Fig. 47. Distribution of Zapus hudsonius.
Guide to subspecies
| 1. | Z. h. acadicus | 7. | Z. h. intermedius | |
| 2. | Z. h. alascensis | 8. | Z. h. ladas | |
| 3. | Z. h. americanus | 9. | Z. h. pallidus | |
| 4. | Z. h. campestris | 10. | Z. h. preblei | |
| 5. | Z. h. canadensis | 11. | Z. h. tenellus | |
| 6. | Z. h. hudsonius |
Externals.—Size small to medium (total length 188 mm to 216 mm); tail longer than head and body (112 mm to 134 mm) and bicolored, pale brown to brownish-black above, white to yellowish-white below; hind feet long (28 mm [421] to 31 mm), grayish-white above; back ochraceous to dark brown; sides paler than back with dark hair interspersed; lateral line usually present but sometimes indistinct or entirely absent (when present usually clear Ochraceous-Buff); ventral coloration white, sometimes with suffusion of ochraceous; guard hairs average 115 microns (96u to 140u) in diameter; underhair with pigment pattern in form of hollow, narrow rectangles; cuticular scales of underhair large and fewer than those of the underfur of Z. trinotatus, but underhair of Z. hudsonius otherwise resembles that of Z. trinotatus.
Baculum.—Size small (total length 4.5 mm to 4.9 mm); base medium in width (0.64 mm to 0.72 mm); tip narrow (0.24 mm to 0.26 mm) and dished out in dorsal aspect, blunted; shaft rounded, curving gently upward at tip.
Skull.—Small to medium and relatively narrow in relation to length; rostrum pointed and short; mastoid region relatively narrow; incisive foramina short; base of zygomatic process of squamosal narrow; coronoid process of mandible short, relatively weak. Upper premolar usually small (averaging .30 mm in length and .35 mm in breadth) sometimes functional (most often so in old adults), occlusal surface divided by single shallow re-entrant fold, which in worn teeth forms centrally located lake; tooth-row short as compared to that of other species; individual cheek-teeth usually smaller than those of other species; lower cheek-teeth shorter and narrower than those of other species; angle of mandible strongly inflected.
The species Z. hudsonius is divisible into 11 subspecies based on differences in color, relative proportions of the tail, hind feet, body, and size and shape of parts of the skull (zygomata, braincase, incisive foramina, auditory bullae, pterygoid fossae, rostrum, and interorbital breadth).
Color of the pelage varies, as a general rule, from dark-backed, dull-sided individuals in the northern parts of the geographic range of the species to light-backed, bright-sided individuals in the southern parts of the range.
Individuals from the southernmost geographic races (Z. h. americanus and Z. h. pallidus) are the smallest for the species and those from the northernmost subspecies (Z. h. alascensis) are the largest. One subspecies, Z. h. campestris, from the central part of the range of the species, however, seems to be out of the cline. This form inhabits the eastern foothills of the Rocky Mountains and is a robust animal approaching Z. princeps in size.
Seemingly there is no clinal variation in the several qualitative features of the cranium, for instance in the shape of the auditory bullae, shape of the incisive foramina, and shape of the postpalatal notch. On the other hand, the dimensions of the entire skull show that the larger crania are of the northernmost subspecies and the smaller of the southernmost subspecies.
Habitat.—Zapus hudsonius occurs in low undergrowth usually of grasses or forbs or both, in open coniferous forests, deciduous hardwood groves, or in stands of tall shrubs and low trees, but most frequently in open, moist areas.
Quimby (1951:75) notes that jumping mice were more common in the moist lowlands than in the drier uplands. More were in the open type lowlands than in the forested type, and these mice favored habitats normally bordered by small streams affording moist to semi-aquatic living conditions. The reports of Goodwin (1924:255), Christian (1936:416), G. S. Miller (1899:329), Cory (1912:249), Lyon (1936:277), Stoner (1918:123), and others, although concerning widely different parts of North America, indicate that Z. hudsonius selects habitats in vegetation of like form, even though different assemblages of plant species may be involved.
An average of 11.91 mice per acre was recorded by Quimby (1951:91) from a study plot at Itasca Park, Clearwater County, Minnesota. He gives the monthly population densities per acre for Z. hudsonius at Centerville, Anoka County, Minnesota, as follows: June 2.78, July 3.57, August 3.10, and September 1.81. Blair’s (1940:248) data on bi-monthly population density per acre for Z. hudsonius on the Edwin S. George Reserve, Livingston County, Michigan, are remarkably similar, when adjusted on a monthly basis, to those obtained by Quimby (loc. cit.). Blair’s (loc. cit.) monthly population densities per acre are as follows: June 3.90, July 3.85, August 3.10, and September 2.00. Townsend (1935:90) estimated population densities per acre for Z. hudsonius in central New York state, at 11 to 72 individuals. As Quimby (1951:92) points out, Townsend’s figures are probably too high, as commonly is the case when the moving quadrat technique is used because animals from neighboring areas enter the trapped area to take over the niches made available by their predecessors’ removal.
The population of Z. hudsonius may vary considerably from year to year as well as seasonally. Blair (1940:249) found notably fewer jumping mice on the George Reserve in 1938 than in 1939. Quimby (1951:94) found the numbers of Zapus to be highly variable and thought that there was a rapid turnover. Young animals were not caught until July when 25 per cent were either juveniles, young, or subadults; from this time on these age classes increased to a high of sixty-one per cent in September. Quimby (loc. cit.) found that separating the individuals into their proper age classes was more [423] difficult in September, since the young from early litters are adultlike in appearance. His data indicate as he remarked, “That the over-wintering adults are, for the most part, gradually replaced by the young of the year as the summer progresses.”
The sexes in Z. hudsonius vary only slightly from a one to one ratio. Quimby (1951:63) found a sex ratio of 110 females to 100 males and Blair (1940:245) records a sex ratio of 113 males to 100 females. Townsend (1935:42) records a sex ratio in central New York of 155 males to 100 females. Such a wide variation from a one to one ratio suggest that the moving quadrat technique, which Townsend (1935:90) employed in obtaining his data, may be, in some way unknown to me, more selective for the males.
Behavior.—The saltatorial powers of Z. hudsonius are well developed and often have been described in the literature. Stoner (1918:123) remarks that, “When disturbed hudsonius moves away by a series of leaps … the distance traversed in one of these leaps is from six to eight feet.”; Cory (1912:249) observed these mice to make surprisingly long leaps, and, according to him, a distance of 10 feet is by no means unusual; Handley and Patton (1947:49) credit these animals with jumping eight to ten feet at a single bound; Hamilton (1935:190) remarked that he noted an average of not more than four to six feet per jump; Townsend (1935:91) observed one individual make jumps of about two feet; and Harper (1932:29) records a jumping mouse leaping for distances of two to three feet. Quimby (1951:72) notes that he had never seen one jump farther than three feet. He found that the greatest jumps occurred initially and normally covered a distance of two to three feet; subsequent leaps were shorter but more rapid. A jumping mouse in full retreat progressed by jumps of about one foot.
Statements concerning the gait of Z. hudsonius are not in agreement but the consensus of opinion is that these animals when unfrightened progress by a series of hops of one to six inches, or, occasionally, with a slow creeping motion while the animal is on all fours. When frightened, however, their progress is by long bounds; the mice make a series of two or three such leaps to the nearest protective cover, and then sit motionless until pursued.
Concerning the use of the tail as a balancing organ, G. S. Miller (1899:330) describes the behavior of a jumping mouse from which the tail had been severed by the sickle of a mowing machine. "When I approached, it made violent efforts to escape, but the moment it was launched in the air, its body, deprived of its balancing [424] power, turned end over end so that it was as likely as not to strike the ground facing the direction from which it had come."
Riparian animals such as Z. hudsonius need enter the water to escape from enemies or perhaps in search of food. Zapus hudsonius can and does swim. Hamilton (1935:190) found it to be a strong swimmer capable of remaining in the water for from four to five minutes. According to Hamilton (loc. cit.), when the mouse is swimming the head is held high, the tail is arched near its middle, and only the hind limbs are employed in propulsion. According to Sheldon (1938:327), Philip Allan, in northern Minnesota, saw many Z. hudsonius swimming three or four inches under the surface of the water. The mice swam upstream and only the hind legs were employed in the swimming movements. N. A. Preble (1944:200), at Archer’s Pond, 3 miles southeast of Center, Ossipee County, New Hampshire, observed a jumping mouse swimming rapidly under water toward another portion of the shore 30 or 40 feet away. The mouse, swimming less than a foot beneath the surface, was vigorously using both forefeet and hind feet, but the long tail trailing limply behind, contributed in no way to the animal’s movements. Quimby (1951:72) released five of the mice, one at a time, in the open water of a lake. He followed alongside in a boat and observed that, “In all instances the animals proved to be excellent swimmers both on and underneath the surface. The methods of progression were similar to land movements; i. e., the limbs were employed differently at various times depending upon the speed. When first placed in water they moved rapidly by lunges produced by sweeping strokes of the hind limbs employed simultaneously. This movement was accomplished similarly to the long jumps made on land … Following the first excited lunges, they settled down to a steadier and slower gait using all four limbs one at a time. The anterior part of the body was held high in the water … When approached too closely, they attempted to escape by diving. The maximum distance noted was about four feet … One was able to swim vigorously for approximately three minutes after which it tired greatly and was in danger of drowning.”
As concerns digging ability, Goodwin (1935:148) reports that Z. hudsonius makes its own burrows; these are short and close to the surface in the summer but longer, deeper, and below the frost-line in winter. Two captives used their forefeet and nails in digging a tunnel in the foot of soil that Goodwin (loc. cit.) had placed in their cage. Quimby (1951:72) remarks that captives excavate soil by means of the front feet and throw the soil out behind; as the [425] burrow deepened the hind feet were also utilized to throw the loose soil out of the burrow.
Zapus hudsonius climbs; Sheldon (1934:293) observed captive animals to climb over small evergreen trees in their cages. They moved with surprising sureness and agility, chasing each other among the branches or sitting for several minutes at a time on one of the limbs. Hamilton (1935:190) found that the mice ran over limbs and brush which were placed in their outdoor enclosure.
Ordinarily Z. hudsonius is nocturnal, appearing in the early dusk and remaining active until pre-dawn. Occasional individuals are abroad in daylight hours. Sheldon (1934:293) found in Nova Scotia that Z. hudsonius is most active from early dusk through the night, but that it may be abroad in daylight as well. Her statements are based on trapping results, field observations, and observations made on captive individuals. Quimby (1951:73) found that Z. hudsonius in Michigan is mostly nocturnal; however, he saw mice on a few occasions in the daytime. Diurnal activity seems to be increased in cloudy or damp weather; Quimby (loc. cit.) almost invariably trapped more of these mice on cloudy, damp days than on other days.
This jumping mouse usually is silent but does utter various sounds. Sheldon (1934:295) records squeaking and clucking noises. Quimby (1951:73) records the clucking noise described by Sheldon (loc. cit.) and mentions also the squeaking and suckling sounds produced by the small young. This mouse is most vociferous when young or when about to go into hibernation. Sheldon (1938:327) writes that Z. hudsonius makes a drumming noise by vibrating the tail against dry leaves.
Many data are available concerning the hibernation of Z. hudsonius. In general it seems necessary for the mice to put on a certain amount of fat preparatory to hibernation. This fat is deposited in a thin layer over the inside of the skin, over the back, and in the body cavities. The thickest deposits are in and about the inguinal region.
Quimby (1951:83) noted that gain in weight was accelerated in a brief period prior to entrance into hibernation. This relationship of rapid gain in weight to hibernation allows a person to estimate the date of hibernation. Cold weather seems to hasten hibernation, but less so than the correct physiologic condition which is foreshadowed by a rapid gain in weight. For example, Quimby’s (1951:84) data reveal that mice that were moved to a heated room gained weight and hibernated in a fashion similar to those in unheated [426] surroundings. Hamilton (1935:193) states that, “It seems necessary for the mouse to lay on a certain amount of fat before it is capable of hibernation.” Hamilton (loc. cit.) reported that 18 specimens of Z. hudsonius taken [presumably in an active state] near Ithaca, New York, on November 13, were without a trace of fat.
Data that are available concerning the hibernation sites of Z. hudsonius show that almost invariably these mice seek shelter in burrows beneath the surface of the ground and there construct nests of grass, leaves, or some other vegetation. Nicholson (1937:103) found a hibernating Z. hudsonius on the George Reserve, Livingston County, Michigan, on October 20. The mouse was in a nest, composed of 10 to 12 damp elm leaves, in a sand bank two feet three inches vertically and three feet nine inches horizontally from the surface. On April 11, 1948, Schwartz (1951:228) found five nests (three with occupants) of Z. hudsonius at Jefferson City, Cole County, Missouri. All nests were one foot beneath the surface of a pile of coal-ash, which was about three and one-half feet high and five feet in diameter. The nests were spherical, approximately four inches in diameter and consisted of dried oak leaves and bits of dried grass. Grizzell (1949:74) found two hibernating jumping mice at the Patuxent Research Refuge, Laurel, Maryland, in January, 1948. The mice were in separate woodchuck dens; one mouse was 40 inches below the surface and the other was 26 inches below the surface. The mice were curled up in the center of masses of dead leaves, and thus, were well insulated against the cold. On April 29, 1944, at Ithaca, New York, Eadie (1949:307) uncovered a hibernating jumping mouse. The nest, about the size of a baseball, was compactly made of fine grasses and was 10 inches below the surface of the ground in a mound of earth that was approximately six by four feet at the base and three feet high.
From the foregoing reports on hibernation sites it is evident that well drained areas are utilized. Sheldon (1934:300) remarks that the burrows used for hibernating are dug in a bank or some place from which the rain water and melted snow probably drains off.
Eadie (1949:307), Grizzell (1949:75), Sheldon (1934:299), Schwartz (1951:228), and Sheldon (1938:331) all agree that the hibernating mouse rolls up into a ball-like shape (resting on its head and pelvis) with the head between the hind legs, the nose against the lower belly, the forefeet curled on the chest, and the tail curled around the head and body.
A marked loss of weight occurs immediately after hibernation [427] begins, and then reduction in weight is slow and regular. (See Hamilton, 1935:194 and Quimby, 1951:84.)
Sheldon (1934:297) cites a letter from Vernon Bailey in which he remarks on the necessity of abundant moisture and saturate air for hibernating jumping mice. Bailey writes “… they will awaken at times famished for water and will drink and drink before going back to sleep.”
Hamilton (1935:195) thinks that in the Ithaca area of New York these mice probably leave their winter quarters in the second half of April and that in southern New York and Long Island they emerge considerably earlier. Quimby (1951:82) and Bernard Bailey (1929:163) report that males appear earlier in the spring than do the females. Quimby (loc. cit.), by recording the sequence and dates of phenological events and appearance of Zapus in several years, was able to predict fairly accurately the time of emergence of Zapus in a succeeding year. In Minnesota, jumping mice emerged late compared to other hibernating rodents.
Enemies.—V. Bailey (1927:119) reports that A. K. Fisher found 50 skulls of Zapus in barn owl pellets taken from the towers of the Smithsonian Institution, Washington, D. C. Dearborn (1932:32) reported mink as having fed on jumping mice. Surface (1906:197) records taking a Zapus from the stomach of a rattlesnake. Pearson and Pearson (1947:138) found remains of Z. hudsonius in pellets of barn owls. Quimby (1951:74) reports two cases of predation on Z. hudsonius; one was by a northern pike, Esox lucius Linnaeus and the other was by a weasel, Mustela sp. Vergeer (1948:91) collected a green frog, Rana clamitans Latreille, which had eaten a jumping mouse.
Quimby (1951:74) frequently found the fleas, Megabothris quirini Rothschild, and Megabothris wagneri (Baker), and occasionally a larval tick, Dermocenter variabilis (Say), on Z. hudsonius. Sheldon (1934:296) remarks that captive animals are burdened with numerous fleas. Hamilton (1935:191) removed a louse from a jumping mouse. One mouse had a hole in the throat and three others had holes in the inguinal region; presumably bot-flies had emerged from these holes. Test (1943:507) found a single Cuterebra larva in the inguinal region of a Z. hudsonius, and Sheldon (1938:328) found Z. hudsonius infested by larvae of Cuterebra fontinella Clark. Here, as in other cases, these larvae were found immediately below the skin. Erickson (1938:252) examined 18 Z. hudsonius obtained in Minnesota, and found that three were [428] parasitized. He found a bot-fly larva, Cuterebra sp., nematodes of the genera Subulura and Spirocerca, and a fluke of the genus Notocotylus.
Food.—Quimby (1951:85-86) studied the food preferences, by presenting to caged Z. hudsonius the plants and invertebrate animals normally available to these mice in nature, and indicates that in general, the starchy fruits of the Gramineae and the less fleshy fruits of various groups of plants are more heavily utilized than other plant materials. His observations indicate that these rodents are highly insectivorous and that they consume many insects under natural conditions. Goodwin (1935:148) reports that the stomach contents of several individuals obtained at South Woodstock, Connecticut, consisted exclusively of blackberries, and that others had subsisted principally on cranberries. Hamilton (1935:197) remarks that seeds are the favored food but that berries, nuts, fruits of various kinds, roots, and insects are also utilized. Stoner (1918:123) writes that the food in cultivated areas of Iowa is various grains as well as grass and weed seeds; in wooded places the mice feed on seeds and nuts of trees. Vernon Bailey (1927:118) states that the examination of a great many stomachs of these jumping mice [in North Dakota] revealed nothing “but the fine white pulp of carefully shelled, well-masticated seeds. Generally these are from grasses, although grain and a variety of other plant seeds are eaten.” Schmidt (1931:116) examined the stomach contents of several Z. hudsonius taken in Clark County, Wisconsin, and in most stomachs found the remains of finely chewed roots; however, two from Hewett had eaten several geometrid caterpillars.
Lyon (1938:279), Stoner (1918:123), and J. W. Bailey (1946:263) present information which indicates that Z. hudsonius stores food in its nests or burrows. Possibly these mice awaken at intervals from hibernation and eat.
“These rodents characteristically seize the material to be eaten with the front feet and devour it while reclining on their haunches. The following observation of a caged animal is typical of their feeding habits. The mouse selected a head of yellow foxtail, Setaria glauca (Weig.) Stuntz, from several in the cage, separated it by gnawing through the supporting stem, seized it with the front feet, held it up to the mouth and began to gnaw at one end, stripping all parts from the rachis. The grass head was slowly rotated and shifted sideways until nothing remained but the rachis which was discarded. Actually the seeds were the only parts eaten …” [429] (Quimby, 1951:73). Sheldon (1934:294) remarks that Z. hudsonius eats from a squatting position and holds the piece of food in the forepaws. The mouse seems to bite off a seed, and then, holding it in the forepaws, transfers it to the mouth.
According to Sheldon (op. cit.:295) and Quimby (loc. cit.), caged jumping mice drink water. When drinking, the mouths of the mice are in contact with the water, but neither observer determined whether the mice lapped or sucked the water. Sheldon (loc. cit.) observed these mice passing stems of long grass through their mouths as though to squeeze out moisture, and thought that the mice obtain most of their required moisture from green plants.
Reproduction.—The breeding season begins shortly after the jumping mice emerge from hibernation in the spring, and reproduction continues until a few weeks before they hibernate in the autumn. The extent of the breeding period probably varies geographically and possibly seasonally. For example, Quimby’s (op. cit.:70) information suggests that the 1947 period of parturition occurred between June 15 and August 30 in the area of Centerville, Minnesota. In Michigan, Blair (1940:246) found a peak of breeding activity in spring and another in late summer with little activity in the intervening midsummer. Brimley (1923:263) records a female in North Carolina, with eight embryos on June 13, 1895, and another with seven embryos on September 17, 1891, indicating a strong possibility of two litters per year there. Vernon Bailey (1927:118) records young born in May or June in North Dakota and thinks that there is time for only one litter per year. Petrides (1948:76) captured a female on September 22, 1944, at Athens, Georgia, that gave birth to six young on September 29. This late parturition date indicates a longer breeding season in the southeastern part of the range of Z. hudsonius.
The gestation period of nonlactating, caged Z. hudsonius, Quimby (1951:63) thinks, “is approximately 18 days … [but] gestation is prolonged in lactating females.”
Data from museum labels indicate that embryos in 62 pregnant females averaged 5.4 (2-8) per female. Quimby (1951:67) found the average number of embryos per female for 14 females taken in Minnesota, to be 5.3 and that litters of young found in nests averaged 5.8. Sheldon (1938:330) reports two litters of seven young each and one of four young for Z. hudsonius in Vermont. Petrides (1948:76) records a litter of six young for Z. hudsonius in Georgia. Brimley (1923:263) records one lot of seven and one lot of eight [430] embryos for Z. hudsonius in North Carolina. Vernon Bailey (1923:120) reports six embryos for a female of Z. hudsonius taken in Washington, D. C. Ivor (1934:8) obtained a litter of five young Z. hudsonius from Erindale, Peel County, Ontario. Hamilton (1935:195) records litters of two, four, and five young and embryo counts of four, two, four, and four for Z. hudsonius in New York.
There seems to be two litters per year. According to Quimby (1951:69), “most adult females breed soon after emergence from hibernation and produce the first litters within a month. The remaining females do not breed immediately but produce the first litter,” he says, “in the second month after emergence.” Both early-breeding females and late-breeding females produce at least 2 litters per year. Those that breed early may have 3 litters.
The appearance and development of growing young of Z. hudsonius in successive weeks is described by Quimby (1951:65). Newborn young are pink and hairless except for microscopic vibrissae. The eyes and external auditory meatus are closed, and the pinnae are folded. The toes are fleshy and clawless; the tail is short in relation to the length of the body. The average weight was .78 grams. The average measurements of three from different litters are: total length, 34 mm; tail, 9.2 mm; hind foot, 4.7 mm. The young are helpless but capable of emitting a high pitched squeaking sound which is audible for several feet.
In the first week of growth the vibrissae become visible to the naked eye, the body changes to flesh color, the dorsal parts become dark gray, the pinna unfolds and is black tipped, and the claws appear. The young now are able to crawl and make a suckling noise, but they are not yet able to support themselves on their legs.
In the second week of development, tawny yellow hair appears on the back and spreads onto the sides. Sparse hair of a lighter color appears on the belly, backs of the feet, and outer surfaces of the legs. Vibrissae are now prominent. The eyes are still closed, but a crack down the center of each is visible by the 13th day. Claws have grown, the longest measuring 1.5 mm. The incisors erupt on approximately the 13th day, those in the lower jaw appearing slightly before those in the upper jaw, and all are white. Activity is increased; nevertheless the young still crawl, make suckling notes, and squeak.
In the third week of development the mice are covered with hair; darker hair appears dorsally; and vibrissae continue rapid growth. The external auditory meatus begins to open on about the 19th day and young react to sound on the 20th. The incisors now [431] are 1 mm long and the claws 1.5 mm long. Young are able to support themselves on their legs, walk, and make one inch hops.
In the fourth week the juvenal pelage is replaced by adult pelage. The eyes open between the 22nd and 25th days. The color of the incisors changes from white to yellowish-orange as in the adults. P4, M1, M2, m1 and m2 have emerged from the maxillary and dentary bones; M3 and m3 have not yet erupted. A mouse 33 days old had all teeth well developed. By the end of the 4th week the young, except for size, are adultlike and capable of independent existence.
The greatest increase in dimensions of the body is in the first four weeks. A slowing down of growth is simultaneous with weaning.
Other workers, Sheldon (1938:330), Petrides (1948:76), and Ivor (1934:8) also describe the appearance of the young.
Summer nesting sites are usually on the surface of the ground. Jumping mice characteristically construct a globular nest of grass but will utilize other vegetation if grasses are not available. Nests are usually concealed under rocks, logs, bushes, or grass and can be entered by a hole at one side.
Sheldon (1938:328) described a nest of Z. hudsonius found on the ground near the edge of a small hay field. The nest was globular, not more than four inches in outside diameter and two inches in inside diameter; it was closely woven of fine, dry grass and bits of moss. Another nest found in the same field measured 11.5 inches in circumference at the base and six inches in circumference over the top. The inside width and length each was three inches, and the inside height was 3.5 inches. Vernon Bailey (1927:118) remarks that summer nests are placed on the surface of the ground well concealed under grass or other vegetation. He describes the nest as “neat little balls of fine grass with a tiny opening at one side and a soft lining in the central chamber.” Cory (1912:249) reports that summer nests are concealed behind rocks or under bushes and thick grass. The nests are round and four or five inches in diameter with an entrance hole at one side. Goodwin (1935:148) examined a nest made entirely of straight, narrow leaves of grass. Ivor (1934:8) found one made of finely shredded jute sacking. Quimby (1951:80) describes several nests: one in the center of a rotten willow log was lined with small pieces of pulpy wood; another was in the rotted wood and debris, at ground level, inside a large, red oak (this globular nest composed of grasses, plant fibers, and rootlets measured six inches in diameter). Another nest was composed of a pile of [432] wood pulp, leaves of oaks, and grasses; this nest was in a hollow root detached from a willow tree.
The mean home range of males, of Z. hudsonius in Minnesota, according to Quimby (1951:86), was 2.70 plus or minus .50 acres; this was significantly larger than the mean home range of females, 1.57 plus or minus .27 acres. According to Quimby (loc. cit.), the size and shape of the home range is influenced by the general features of the terrain, density and type of cover, and land use in the immediate area. Quimby (1951:94) remarked that the home range of the jumping mouse is relatively unstable and Blair (1940:247) stated that the home ranges of both sexes generally overlapped the ranges of other members of the same species and sex. The average size of the home range for Z. hudsonius in Michigan was .89 plus or minus .11 acres for males and .92 plus or minus .11 acres for females.
Meriones acadicus Dawson, Edinburgh New Philos. Jour., new ser., 3:2, 1856.
Meriones labradorius, Dawson, Edinburgh New Philos. Jour., new ser., 3:2, 1856.
Jaculus hudsonius, Baird, Rept. Expl. and Surv…., 8 (pt. 1):433, July 14, 1858 (part—the part from Nova Scotia, Vermont, and New York).
Zapus hudsonius, Coues, Bull. U. S. Geol. and Geog. surv. of the territories, 2nd ser., No. 5:260, 1877 (part—the part from Nova Scotia, Vermont, and New York); Preble, N. Amer. Fauna, 15:17, August 8, 1899 (part—the part from New Brunswick, Nova Scotia, Maine, New Hampshire, Vermont, Massachusetts, and northeastern New York).
Zapus hudsonius canadensis, Batchelder, Proc. New England Zool. Club, 1:5, February 8, 1899 (part—the part from Keene Valley in Essex County of New York, and Orivell in Vermont); Anderson, Ann. Rept. Provancher Soc. Nat. Hist., Quebec, 1941:35-37, July 14, 1942 (part—the part from the tip of the Gaspé Peninsula in Quebec, New Brunswick, Maine, New Hampshire, Vermont, and New York).
Zapus hudsonius hardyi, Batchelder, Proc. New England Zool. Club, 1:6, February 8, 1899, type from Mt. Desert Island, Hancock County, Maine; Bole and Moulthrop, Sci. Publ. Cleveland Mus. Nat. Hist., 5:165, September 11, 1947 (part—but excluding Pennsylvania and Ohio).
Zapus hudsonius acadicus, Anderson, Ann. Rept. Provancher Soc. Nat. Hist., Quebec, 1941:38, July 14, 1942.
Type.—No type specimen designated. Subspecies characterized from specimens obtained in Nova Scotia.
Range.—Gaspé Peninsula of Quebec, New Brunswick, Nova Scotia, Prince Edward Island, Maine, New Hampshire, Vermont, Massachusetts, northern Connecticut and northeastern New York. See fig. 47. Zonal range: Transition and Canadian.
Description.—Size medium; back from near Ochraceous-Tawny to near Yellow-Ocher with heavy admixture of black-tipped hair, the dorsal band distinct against color of sides; sides lighter than back and from near Cinnamon-Buff to near Ochraceous-Buff lined with black-tipped hair; lateral line usually [433] faintly marked but sometimes distinct and clear Warm-Buff; underparts white, sometimes suffused with color of sides; tail distinctly bicolored, brownish-black above and yellowish-white to grayish-white below; ears dark, edged with color of sides; feet grayish-white above; pterygoid fossae relatively narrow; zygomata relatively long and broad; auditory bullae relatively narrow, usually with depression on anterior surface; mastoid region relatively narrow; inferior arm of zygomatic process of maxillary relatively narrow.
Comparisons.—From Zapus hudsonius canadensis, Z. h. acadicus differs in: Size averaging larger; upper parts usually less brownish and more ochraceous, sides and flanks being more ochraceous and less yellowish; zygomata relatively longer; pterygoid fossae relatively narrower; auditory bullae relatively narrower and usually with depression on anterior surface.
From Zapus hudsonius americanus, Z. h. acadicus differs as follows: Size larger; color darker on upper parts, flanks duller (less ochraceous); underparts white, much less frequently suffused with color of sides; ears dark, usually without flecks of ochraceous; general appearance of pelage not so brightly colored; zygomata longer; condylobasal length greater; mastoid region relatively broader; bullae larger, more inflated and usually with depression on anterior surface; maxillary tooth-row relatively longer.
For comparison with Zapus hudsonius ladas see account of that subspecies.
Remarks.—Specimens from various localities in Nova Scotia, Prince Edward Island and New Brunswick are essentially similar. Anderson (1942:38) revived the name Z. h. acadicus for jumping mice from these areas, correctly considering them to be distinct from Z. h. canadensis, the geographic race immediately to the west.
In the size and shape of the auditory bullae, length of the zygomata, breadth of the pterygoid fossae, and general color of the pelage the populations from Nova Scotia and New Brunswick are essentially indistinguishable from material of Zapus hudsonius hardyi from Maine. Thus, Z. h. hardyi must fall as a synonym of the earlier proposed name Z. h. acadicus.
Bole and Moulthrop (1942:165) applied the name Z. h. hardyi (= acadicus) to the mice inhabiting a large area from coastal Maine and central New Hampshire through southern New England, New York, northwestern Pennsylvania, and northeastern Ohio. I agree with Bole and Moulthrop (loc. cit.) that the population of Zapus hudsonius from Maine, New Hampshire, west-central and northern New England are different from neighboring subspecies and are referable to Z. h. acadicus, but find that material from extreme southern Massachusetts, Connecticut, southern New York, northwestern Pennsylvania, and northeastern Ohio is best referred to Zapus hudsonius americanus (see account of that subspecies).
Intergradation between Z. h. americanus and Z. h. acadicus is indicated by specimens from Berlin, Rensselaer County, New York. [434] In color of ears, length of zygomata, and size and shape of the incisive foramina these specimens are more nearly like Z. h. americanus but in size and shape of the auditory bullae, breadth of the mastoid region, and general appearance of the pelage they are more nearly like Z. h. acadicus and are here referred to acadicus. Specimens from Glenville, Schenectady County, New York, are intermediate in cranial characters between Z. h. americanus and Z. h. acadicus but in color are best referred to the latter. Specimens from 1 mi. S Ayer, Worchester County, Massachusetts, are like Z. h. americanus in their short zygomata, narrow mastoid region and suffusion of the underparts; nevertheless, in the shape of the auditory bullae, breadth of the pterygoid fossae, and greater condylobasal length the specimens are more nearly like Z. h. acadicus which they are here considered to be. Animals from Essex and Wilmington, Essex County, Massachusetts, are like Z. h. americanus in external size and in the size and shape of the auditory bullae; but they are more nearly like Z. h. acadicus in most cranial characters and in the general color of the pelage and are here assigned to Z. h. acadicus.
Specimens from Keene Valley, Essex County, New York, considered by Batchelder (1899:4) to be Z. h. canadensis, are in color, length of the zygomata, and size and shape of the auditory bullae more nearly like Z. h. acadicus to which subspecies they are here assigned. A specimen from Orwell, Addison County, Vermont, that Batchelder (op. cit.:5) referred to Z. h. canadensis is more nearly like Z. h. acadicus in the shape of the auditory bullae, length of the zygomata, and color of the pelage, and is here referred to Z. h. acadicus. Specimens from western New Brunswick, referred to Z. h. canadensis by Anderson (1942:37), are more nearly like Z. h. acadicus. Specimens from Ste. Anne des Monts, Gaspé Peninsula, Quebec, are intermediate between Z. h. canadensis and Z. h. acadicus in color and size and also in the shape of the auditory bullae but are best referred to Z. h. acadicus.
Zapus hudsonius acadicus as here understood is a relatively wide-ranging subspecies. Populations at the southern periphery of its range are difficult to separate from populations at the northern periphery of the range of Z. h. americanus. These two geographic races represent opposite extremes of a clinal gradient and, as would be expected, geographic intermediates are morphologically similar.
Specimens examined.—Total, 156, distributed as follows:
Maine: Aroostock County: Madawaska, 6 (MCZ). Hancock County: Mount Desert Island, 9 (6 MCZ, 3 UM). Piscataquis County: Mount Katahdin, 1 (USNM); Sebec Lake, 4 (USBS); Katahdin Lake, 1 (USBS). Sagadahoc Co.: Small Point Beach, 1 (Clev. MNH). Somerset County: east [435] branch Penobscot River, 2 (USBS). Washington County: Columbia Falls, 1 (USBS).
Massachusetts: Essex County: Essex, 4 (Clev. MNH); Wilmington, 4 (3 USBS, 1 USNM). Worchester County: Lunenberg, 2 (USBS); 1 mi. S Ayer, 2 (MVZ); 2 mi. N Gilbertville, 1.
New Brunswick: Charlotte County: 6 mi. N St. Andrews, 2 (NMC); 5 mi. N St. Andrews, 4 (NMC). Carleton County: Debec, 1 (MVZ). Gloucester County: Dalhousie, 2 (MVZ); Miramichi Road, 15 mi. from Bathurst, 4 (NMC); Youghall, 3 (NMC). Madawaska County: Baker Lake, 2 (NMC); 9 mi. NE Edmundston, 4 (NMC); 5 mi. N St. Leonard, 5 (NMC). Victoria Co.: Tobique Point, 1 (AMNH). York County: Queensbury, 1 (USBS).
New Hampshire: Carroll County: Intervale, 1 (UM); Ossipee, 4 (3 USBS); 2 mi. S Ossipee, 12 (2 USNM). Coos County: Nathan Pond, 1 (UM); Fabyans-Bretton Woods, Dartmouth Brook, 2 (UM); Fabyans, 1 (USNM); 3 mi. W Base Station, 1; Mt. Washington, 1 (MVZ); Pinkham Notch, 1900 ft., 1 (USNM). Grafton County: Franconia Notch, Profile Lake, 1 (UM); Lebanon, 3 (UM). Strafford Co.: 1 mi. E Durham, 1 (UM).
New York: Essex Co.: Keene Valley, 5 (MCZ); Keene Heights, 5 (MCZ); Minerva, 1700 ft., 1 (AMNH). Herkimer County: Northwood, 7 (AMNH). Rensselaer Co.: Berlin, 8 (AMNH). Schenectady County: Glenville, 1 (USBS). Warren County: Lake George, 5 (USBS). Washington County: Patterns Mills, 1 (USBS).
Nova Scotia: Annapolis Co.: Bear River, 7 (NMC); Lake Kedgemakooge, 5 (UM); 2 mi. S Milford, 1 (AMNH). Kings Co.: Black River Dist., 1 (NMC); no exact locality, 1 (NMC). Shelburne County: Doctors Cove, N Barrington Passage, 1 (NMC); Barrington Passage, 4 (NMC).
Prince Edward Island: no exact locality, 1 (USBS).
Quebec: Ste. Anne des Monts, 1 (AMNH).
Vermont: Addison County: Orwell, 1 (MCZ); Lamville County: Mt. Mansfield, 2 (USBS). Windham County: Whitingham, 2 (AMNH).
Marginal records.—Quebec: Ste. Anne des Monts. New Brunswick: Dalhousie. Prince Edward Island. Nova Scotia: Black River District; Doctors Cove, N Barrington Passage. Maine: Columbia Falls; Small Point Beach. Massachusetts: Wilmington; 2 mi. N Gilbertville. New York: Berlin; North Wood; Keene Valley. Maine: E branch Penobscot River. New Brunswick: Baker Lake.
Zapus hudsonius alascensis Merriam, Proc. Biol. Soc. Washington, 2:223, July 15, 1897.
Zapus hudsonius hudsonius, Osgood, N. Amer. Fauna, 24:37, November 23, 1904.
Type.—Male, adult, skin and skull, No. 73584, U. S. Nat. Mus., Biol. Surv. Coll.; Yakutat Bay, Alaska; obtained on July 5, 1895, by Clark P. Streator, original No. 4660.
Range.—Alaska Peninsula, coastal section of mainland of southern and southeastern Alaska including Revillagigedo Island; also southwestern Yukon. See fig. 47. Zonal range: Canadian and Hudsonian.
Description.—Size large; back from near Ochraceous-Tawny to near Dresden Brown, sometimes darkened with black tipped hair usually with darker mid-dorsal area forming a band; sides lighter than back and from near Ochraceous-Tawny to near Clay Color; lateral line usually distinct, of clear Ochraceous-Buff; belly white, frequently with a slight suffusion of Ochraceous-Buff; tail bicolored, brownish to brownish-black above, white to yellowish-white below; ears dark, edged and flecked on the inner surface with color of sides; feet grayish-white [436] above; auditory bullae broad and moderately inflated; pterygoid fossae relatively broad; incisive foramina relatively long, zygomata relatively long and broadly bowed; mastoid region relatively broad; distance from incisors to postpalatal notch relatively great; occipitonasal length relatively great.
Comparisons.—From Zapus hudsonius tenellus, Z. h. alascensis differs as follows: Size larger; upper parts darker, less ochraceous; sides duller, less ochraceous more tawny; incisive foramina averaging longer; mastoid region broader; occipitonasal length greater; zygomata wider-spreading and longer; condylobasal length averaging greater; auditory bullae less broadly rounded; and distance from incisors to postpalatal notch averaging greater.
For comparison with Zapus hudsonius hudsonius see account of that subspecies.
Remarks.—Zapus hudsonius alascensis is a fairly well marked subspecies retaining most of its characters throughout its range. Variation is noted in specimens from the southwest end of Dezadeash Lake, 2400 ft., Yukon Territory, and seems to be the result of intergradation between Zapus hudsonius hudsonius and Z. h. alascensis. These animals are like Z. h. hudsonius in the shape of the auditory bullae but are otherwise more nearly like Z. h. alascensis to which they are here assigned. Alaskan specimens from 7 mi. SSE Haines, and from a point 9 mi. W and 4 mi. N Haines average slightly larger than Z. h. alascensis in most measurements taken; however, in coloration they more nearly agree with Z. h. alascensis than with Z. h. hudsonius or Z. h. tenellus the geographic ranges of which adjoin that of Z. h. alascensis.
Specimens examined.—Total, 56, distributed as follows:
Alaska: Cook Inlet, Tyonek, 1 (USBS); head Chalitna River, 2 (USBS); Lake Clark, 4 (USBS); east side Chilkat River, 100 ft., 9 mi. W and 4 mi. N Haines, 8; Yakutat, 3 (USBS); Lake Iliamma, 1 (USBS); Lake Aleknagik, 1 (USBS); Kokwok, 1 (USBS); Nushagak River, 3 (USBS); Chilkat Peninsula, 10 ft., 7 mi. SSE Haines, 18; Nushagak, 3 (USBS); Chignik Bay, 1 (USBS); Portage Cove, Revillagigedo, 1 (MVZ); Izembek Bay, 1 (USBS); Frosty Peak, 1 (USBS).
British Columbia: west end Kelsall Lake, 2900 ft., 1; Stonehouse Creek, 51/2 mi. W junction Stonehouse Creek and Kelsall River, 4.
Yukon: SW end Dezadeash Lake, 2400 ft., 2.
Marginal records.—Alaska: Lake Aleknagik; head Chalitna River. Yukon: SW end Dezadeash Lake, 2400 ft. Alaska: E side Chilkat River, 100 ft., 9 mi. W and 4 mi. N Haines; Portage Cove, Revillagigedo Island; Yakutat; Cook Inlet, Tyonek; Chignik Bay; Frosty Peak.
Dipus americanus Barton, Trans. Amer. Philos. Soc., 4:115, 1799.
Jaculus americanus Wagler, Nat. Syst. Amphibien, 23, 1830.
Meriones microcephalus Harlan, Proc. Zool. Soc. London, p. 1, 1839, based on two specimens from "the farm of Mr. Beck, in Philadelphia County, a few miles northeast of the city [= Philadelphia, Pennsylvania]."
Jaculus hudsonius, Baird, Repts. Expl. and Surv. 111, 8 (pt. 1): 433, July 14, 1858 (part—the part from Massachusetts, Connecticut, New York, New Jersey, and Pennsylvania).
Zapus hudsonius, Coues, Bull. U. S. Geol. and Geog. Surv. of the territories, 2nd ser. No. 5:260, 1877 (part—the part from Massachusetts, Connecticut, New York, and Pennsylvania); Preble, N. Amer. Fauna, 15:17, August 8, 1899 (part—the part from Peterboro and Waterville, New York, southeastern Massachusetts, Connecticut, New Jersey, Pennsylvania, West Virginia, Maryland, North Carolina, and Ohio).
Zapus hudsonius americanus, Batchelder, Proc. New England Zool. Club, 1:6, February 8, 1899; Preble, N. Amer. Fauna, 15:19, August 8, 1899.
Zapus hudsonius hardyi, Bole and Moulthrop, Sci. Publ. Cleveland Mus. Nat. Hist., 5:165, September 11, 1942 (part—the part from New York, Ohio, and Pennsylvania).
Zapus hudsonius brevipes Bole and Moulthrop, Sci. Publ. Cleveland Mus. Nat. Hist., 5:168, September 11, 1942, type from Bettsville, Seneca County, Ohio.
Zapus hudsonius rafinesquei Bole and Moulthrop, Sci. Publ. Cleveland Mus. Nat. Hist., 5:169, September 11, 1942 (part—the part from southeastern Ohio), type from Cat Run, extreme southeastern Belmont County, Ohio.
Type.—No type specimen designated. Dipus americanus was characterized from jumping mice obtained by Barton near the Schuylkill River, a few miles from Philadelphia, Pennsylvania.
Range.—Southeastern United States and lower peninsula of Michigan; east of central Indiana; from central New York and Massachusetts southward to northern Georgia. See fig. 47. Zonal range: Austroriparian (Lower Austral), Carolinian (Upper Austral), Alleghanian (Transition), and Canadian.
Description.—Size small; back from near Light Ochraceous-Buff to near Ochraceous-Buff with admixture of black-tipped hair forming distinct dorsal band; sides bright, lighter than back from near Light Ochraceous-Buff to near Ochraceous-Buff; lateral line usually distinct and of color of sides; underparts white, sometimes with slight suffusion of color of sides; tail bicolored, brown to brownish-black above, yellowish-white to grayish-white below; ears narrowly edged and heavily flecked with color of sides; feet white to grayish-white above; skull short; braincase relatively narrow; incisive foramina relatively broad; skull relatively narrow across zygomata; interorbital region relatively broad; distance from incisors to postpalatal notch relatively short; auditory bullae relatively small.
Comparisons.—Compared with Zapus hudsonius canadensis, Z. h. americanus differs as follows: Smaller; paler (in a sense brighter because more ochraceous and less tawny); skull smaller; auditory bullae narrower, less inflated; incisive foramina relatively more bowed; condylobasal length averaging less.
From Zapus hudsonius intermedius, Z. h. americanus differs as follows: Smaller; color brighter, more ochraceous, less yellow; braincase relatively narrower; auditory bullae usually smaller; incisive foramina broader; inferior ramus of zygomatic process of maxillary usually with median projection; interorbital region averaging broader.
For comparison with Zapus hudsonius acadicus see account of that subspecies.
Remarks.—Intergradation with Zapus hudsonius acadicus occurs in southeastern New York as indicated by a series of 25 specimens from Peterboro. They resemble Z. h. acadicus in width of the mastoid [438] region and relatively longer tooth-row, but in the size and shape of the auditory bullae, width of the pterygoid fossae, and lighter, brighter, color of the sides they are more nearly like Z. h. americanus to which they are here referred.
Intergradation between Z. h. americanus and Z. h. acadicus is indicated also by specimens from Lawyersville and Schoharie, New York. In animals from both localities the length of the zygomata and the breadth of the mastoid region are more nearly as in Z. h. acadicus, but in size and shape of the auditory bullae, over-all length of the skull, color of the ears, and general color of the pelage they are more nearly like Z. h. americanus to which they are here referred.
Specimens from western Pennsylvania, judged to be Z. h. hudsonius by Preble (1899:17), and those from northwestern Pennsylvania and northeastern Ohio, allocated to Z. h. hardyi (= acadicus) by Bole and Moulthrop (1942:165), are more nearly like Z. h. americanus in size and shape of the auditory bullae, short zygomata, relatively narrow mastoid region, and color of pelage.
Specimens from the lower peninsula of Michigan, northeastern Indiana, and northwestern Ohio, described by Bole and Moulthrop (op. cit.:168) as belonging to a new subspecies (Zapus hudsonius brevipes), are to me indistinguishable from most specimens of Z. h. americanus. The characters which Bole and Moulthrop (loc. cit.) ascribe to Z. h. brevipes—color bright Ochraceous-Buff, tail and hind feet short, and skull narrow—are also those of Z. h. americanus.
Specimens from various localities in southeastern Ohio, all within the range ascribed by Bole and Moulthrop (op. cit.:169) to Zapus hudsonius rafinesquei, are indistinguishable from specimens of Z. h. americanus from eastern Tennessee, West Virginia, North Carolina, and Maryland. Zapus hudsonius rafinesquei (at least that part from southeastern Ohio) is indistinguishable from Z. h. americanus and therefore is synonymized under Z. h. americanus.
Specimens from Lagrange County, Indiana, show intergradation between Zapus hudsonius intermedius and Z. h. americanus in the color of the pelage but are more nearly like Z. h. americanus to which they are here referred. One from Porter County, Indiana, is more nearly like Z. h. intermedius in size and shape of the bullae and in breadth of the pterygoid fossae but in color and degree of lateral bowing of the zygomata is better placed with Z. h. americanus.
Z. h. americanus is a wide ranging subspecies. Animals at the northern periphery of the range (lower peninsula of Michigan to the west and southeastern Massachusetts to the east) are largest and darkest; to the southward there is a progressive reduction in size and a change to a lighter, brighter color. Animals from Maryland, Virginia, and North Carolina are more nearly average representatives of the subspecies than are those from the region of the type locality.
A jumping mouse allegedly of this subspecies has been recorded by Coleman (1941:91) from Caesars Head, 300 ft., South Carolina. This specimen and one from Athens, Georgia, provide the southeasternmost record-stations of occurrence for the species Z. hudsonius.
Specimens examined.—Total, 318, distributed as follows:
Connecticut: Hartford County: Windsor, 1 (USBS); East Hartford, 2 (MCZ). Litchfield County: Sharon, 3 (AMNH); Macedonia Park, 2 (AMNH). Middlesex County: Clinton, 1 (AMNH). Windham County: South Woodstock, 10 (AMNH); Pomfret, near Hampton line, 1.
Georgia: Clarke Co.: Athens, 1 (USBS).
Indiana: Lagrange Co.: no exact locality, 2 (UM). Porter Co.: Mineral Springs, 1 (FM); no exact locality, 1 (FM).
Maryland: Anne Arundel County: Patuxent Research Refuge, 1 (USBS). Charles County: no exact locality, 1 (USBS). Garrett Co.: Finzel, 6 mi. N Frostburg, 1 (USBS). Montgomery County: Sandy Springs, 2 (USBS); Kensington, 1 (USNM); Cabin John Bridge, 2 (1 USBS; 1 USNM). Prince Georges County: Laurel, 8 (USNM); Branchville, 1 (USBS); College Park, 1. Worchester County: Assateague, 5 mi. S Ocean City, 1 (USBS).
Massachusetts: Barnstable County: West Falmouth, 1 (USBS). Bristol County: Raynham, 1 (Clev. MNH). Dukes County: Martha’s Vineyard, 1 (USBS); West Chop, Martha’s Vineyard, 1 (Clev. MNH). Nantucket County: Nantucket Island, 1 (USNM). Plymouth County: Middleboro, 1 (USNM); Plymouth, 1 (UM); Marshfield, 6 (USBS); Wareham, 3 (1 Clev. MNH; 2 UM).
Michigan: Alcona Co.: 2 mi. S Harrisville, 2 (UM). Allegan Co.: near junction Swan Creek and Kalamazoo River, 3 (UM). Berrien Co.: Warren Woods, 2 (UM); Three Oaks, 1 (UM). Charlevoix Co.: Thumb Lake, 1 (UM); Section 1 Norwood Township, 1 (UM); Boyne Falls, 12 (UM); 2 mi. S Boyne Falls, 2 (UM). Cheboygan Co.: Douglas Lake, 2 (UM). Clinton Co.: 2 mi. SE DeWitt, 1 (UM). Emmet Co.: Maple River, near Douglas Lake, 1 (UM). Huron Co.: Rush Lake, 1 (UM). Kalamazoo Co.: no exact locality, 1 (UM). Lake Co.: 1 mi. NW Chase, 1 (UM). Livingston Co.: George Reserve, Pinckney, 2 (UM); Upper Whitewood Lake, 1 (UM); Whitmore Lake, 1 (UM); Portage Lake, 3 (UM). Mason Co.: 9 mi. N Ludington, 1 (UM). Midland Co.: Sanford, 1 (UM). Montmorency Co.: T. 32N, R. 1E, Sec. 30, 1 (UM). Muskegon Co.: 4 mi. NW North Muskegon, 2 (UM). Oakland Co.: Bloomfield, 1 (UM); no exact locality, 1 (UM). Otsego Co.: Pigeon River, 1 (UM); T. 32N, R. 1W, Sec. 25, 1 (UM); Waters, 1. Roscommon Co.: T. 24N, R. 2W, Sec. 2, 1 (UM). Shiawassee Co.: 1/2 mi. NE Byron, 5 (UM); 1/4 mi. S Byron, 2 (UM); 2 mi. SE Byron, 1 (UM); 3 mi. SW Byron, 1 (UM). Van Buren Co.: Van Auken Lake, 1 (UM). Washtenaw County: Whitmore Lake, 1 (UM); 2 mi. W Cherry Hill, 1 (UM); Ann Arbor, 7 (UM); 2 mi. E Ann Arbor, 2 (UM); Willow Run Village, 1 (UM).
New Jersey: Bergen County: Harrington Park, 1 (AMNH); Englewood, 1 (USNM). Cape May County: Mays Landing, 3 (Clev. MNH). Morris County: Mendham, 1 (AMNH). Ocean County: Tuckerton, 3 (USBS).
New York: Broome Co.: 5 mi. N Binghamton, 2 (USNM). Cayuga County: E Aurora, 1 (USBS). Greene County: Catskills, 4 (USNM); Kaaterskill Junction, 1 (USNM). Madison County: Peterboro, 25 (2 MCZ; 19 USNM; 4 Clev. MNH). Nassau County: Locust Grove, 3 (USNM). Orange Co.: Cranberry Pond, 840 ft., Highland, 2 (USNM). Otsego County: Lake Charlotte, 1 (AMNH). Queens County: Woodside, Long Island, 1 (USNM); near Forest Hills, Long Island, 1 (AMNH); Ray Nu Beach, Long Island, 1 (USNM). Rockland County: Tappan, 1 (AMNH). Schoharie County: Lawyersville, 1 (AMNH); Schoharie, 1 (AMNH). Suffolk County: Montauk Point, Long Island, 8 (USBS). Tioga County: Owego, 1 (USBS). Westchester Co.: Bedford, 1 (AMNH).
North Carolina: Buncombe County: Weaverville, 1 (AMNH). Cherokee Co.: Martin Creek, 2 (UM). Mitchell County: Roan Mountain, 2 (USBS). Wake County: Raleigh, 5 (3 USNM; 1 UM; 1 NCS).
Ohio: Carroll Co.: Carrollton, 2 (UM). Cuyahoga County: Big Creek, Brookside Park, 1 (Clev. MNH); Dover, 1 (Clev. MNH); Rocky River Metr. Park, 3 (Clev. MNH); North Olmstead, 1 (Clev. MNH). Erie Co.: Milan, 1 (Clev. MNH); Mill Hollow, Vermilion River, 1 (Clev. MNH). Lake Co.: Holden Arboretum, 3 (Clev. MNH). Meigs Co.: Portland Station, 1 (Clev. MNH). Seneca Co.: Bettsville, 4 (Clev. MNH); Old Fort Seneca, 4 (Clev. MNH); Corners, 1 (Clev. MNH). Wayne Co.: Wooster, 1 (UM); Craighton, 1 (UM).
Pennsylvania: Beaver Co.: 1 mi. NE Darlington, 1 (CM); 2 mi. E Industry, 1 (CM); 4 mi. E Frankfort, 2 (CM). Bedford Co.: 1 mi. NE Osterburg, 1 (CM). Berks Co.: 2 mi. W Strausstown, 1 (USNM). Bradford Co.: 21/2 mi. NNW Wyalusing, 2 (CM). Bucks Co.: 2 mi. N New Britain, 1 (CM). Butler Co.: Thorn Creek, 4 mi. S Butler, 4 (CM); 2 mi. E Middle Lancaster, 1 (CM); Orphans Home, 2 mi. E Mars, 2 (CM). Cambria Co.: 21/2 mi. S Patton, 1750 ft., 1 (CM); 51/2 mi. NE Ebensburg, 1 (CM). Centre Co.: 2, mi. E Snowshoe, 2 (CM). Chester Co.: 2 mi. S West Chester, 1 (CM). Clinton Co.: Tamarack, 9 mi. NNW Renovo, 1 (CM). Crawford Co.: Pymatuning Lake, 3 (Clev. MNH). Erie Co.: 41/2 mi. SW [town of] North East; 2 (CM); East Springfield, 1 (CM). Fulton Co.: 11/2 mi. NE Warfordsburg, 580 ft., 1 (CM). Huntington Co.: 61/2 mi. S Shade Gap, 2 (CM). Indiana Co.: 1/2 mi. E Indiana, 1320 ft., 2 (CM). Lebanon Co.: 11/2 mi. SE Cornwall, 800 ft., 1 (CM). Mercer Co.: 21/2 mi. W Mercer, 2 (CM); 5 mi. S Mercer, 1 (CM). Monroe Co.: Pocene Lake, 1(CM). Pike Co.: Bruce Lake, 1 (CM). Potter Co.: Woodcock Run, 71/2 mi. WSW Ulysses, 2 (CM). Sommerset County: 4 mi. SW Somerset, 2100 ft., 2 (CM); New Lexington, 1 (USBS). Susquehanna Co.: 10 mi. NNW Montrose, 1 (CM). Union Co.: Glen Iron, 2 (CM). Warren Co.: Bensons Swamp, 5 mi. E Columbus, 1 (USNM); Miles Run, 5 mi. NW Pittsfield, 1 (CM); 11/2 mi. N Pittsfield, 1 (CM); 21/2 mi. N Kinzua, 2 (CM); 2 mi. N Kinzua, 1 (CM).
Tennessee: Carter Co.: 3 mi. SSW Roan Mountain (town), 2900 ft., 1 (UM).
Virginia: Amelia Co.: Amelia, 1 (UM). Elizabeth City County: Near Hampton, 2 (UM). Fairfax County: Fall Church, 4 (2 USNM; 2 USBS); opposite Plummers Island, Maryland, 1 (USNM). Highland Co.: Laurel Park, 9 mi. NNW Monterey, 3100 ft., 4 (UM). Nelson Co.: no exact locality, 5 (USNM). Norfolk County: Deep Creek, 1 (USBS). Page Co.: no exact locality, 1 (USNM). Smyth Co.: Sugar Grove, 1 (UM); 1/2 mi. E Konnarock, 2800 ft., 1 (UM). Washington Co.: Konnarock, 2900 ft., 1 (UM).
Washington D. C.: Chevy Chase, 1 (USBS); no exact locality, 4 (3 USNM; 1 USBS).
West Virginia: Monongalia Co.: Morgantown, 6.
Marginal records.—Michigan: Douglas Lake; Bloomfield. New York: E Aurora; Peterboro; Catskills. Connecticut: Sharon; South Woodstock. Massachusetts: [441] Middleboro. New Jersey: Tuckerton. Maryland: Assateague, 5 mi. S Ocean City. North Carolina: Raleigh. Georgia: Athens. Indiana: Mineral Springs. Michigan: 9 mi. N Ludington.
Zapus hudsonius campestris Preble, N. Amer. Fauna, 15:20, August 8, 1899.
Type.—Male, adult, No. 65872 U. S. Nat. Mus., Biol. Surv. Coll.; Bear Lodge Mt’s [Crook County], Wyoming; obtained on June 21, 1894, by B. H. Dutcher, original No. 600.
Range.—Southeastern Montana, southwestern South Dakota, and northeastern Wyoming. See fig. 47. Zonal range: Transition.
Description.—Size large; back from near Ochraceous-Tawny to near Ochraceous-Buff with admixture of black tipped hair forming distinct dorsal band; sides lighter than back, from near Ochraceous-Buff to near Yellow Ocher with black hair interspersed; lateral line usually distinct, of clear Ochraceous-Buff; belly white, usually with moderate suffusion of Ochraceous-Buff; tail bicolored, brownish to brownish-black above, grayish-white to yellowish-white below; ears dark, edged with Ochraceous-Buff; feet grayish-white above; auditory bullae large, well inflated; incisive foramina long and usually truncate at posterior border; pterygoid fossae broad; zygomata relatively wide-spread and long; large medial projection on inferior ramus of zygomatic process of maxillary; condylobasal length and occipitonasal length relatively great; mastoid region and palatal region relatively broad; interparietal bone usually broad.
Comparisons.—From Zapus hudsonius pallidus, Z. h. campestris differs as follows: Coloration darker (more black and yellow but less orange); averaging larger in all measurements taken except in least interorbital constriction and distance from incisors to postpalatal notch which are slightly larger and breadth across zygomatic arches which is same; zygomatic arch heavier; incisive foramina larger; interparietal bone broader.
Compared with Zapus hudsonius intermedius, Z. h. campestris differs as follows: Coloration more tawny and ochraceous, less yellow; auditory bullae averaging larger, more inflated; condylobasal length averaging greater; zygomata averaging more wide-spread and longer; distance from incisors to postpalatal notch averaging longer; mastoid region broader; incisive foramina longer and more truncate posteriorly.
From Zapus hudsonius hudsonius, Z. h. campestris differs as follows: Size larger; color lighter, more ochraceous, less tawny; occipitonasal length averaging greater; mastoid region broader; zygomata averaging longer; zygomatic arch more widely bowed; distance from incisors to postpalatal notch averaging longer; incisive foramina longer; auditory bullae broader, more inflated.
For comparison with Zapus hudsonius preblei see account of that subspecies.
Remarks.—Animals from the Black Hills of South Dakota and Wyoming are thought of as most characteristic of this geographic race. Intergradation is noted with Zapus hudsonius pallidus and is discussed in the account of that subspecies.
Specimens examined.—Total, 66, distributed as follows:
Montana: Big Horn County: Rotten Grass Creek, north base Big Horn Mts., 2 (USBS); Little Big Horn River, 2 mi. from Wyoming line, 1 (USBS).
South Dakota: Custer County: Custer, 3 (USNM); Bull Springs, 6 (Clev. MNH); Beaver Creek, Wind Cave Nat’l Park, 1 (UM); Wind Cave Nat’l Park [442] Game Ranch, Cold Spring Creek, Wind Cave Nat’l Park, 2 (UM); Pennington County: Rapid Creek, 2 mi. W Pactola, 4800 ft., 3 (UM); Castle Creek, R. 2E, T. 1N, 6500 ft., 3 (UM); Nelsons Place, 3 mi. SE Hill City, 6 (UM); Palmer Gulch, 4 mi. SE Hill City, 3 (UM); Palmer Gulch, 9 (FM); no definite locality, 4 (UM).
Wyoming: Crook County: Devils Tower, flood plain Belle Fourche River, 3350 ft., 1 (USBS); Bear Lodge Mts., 4 (USBS); 15 mi. N Sundance, Black Hills Nat’l Forest, 5500 ft., 2; 3 mi. NW Sundance, 5900 ft., 17; Sundance, 2 (USBS). Weston Co.: 11/2 mi. E Buckhorn, 6150 ft., 5.
Marginal records.—Montana: Rotten Grass Creek, N base Big Horn Mts. South Dakota: Nelsons Place, 3 mi. SE Hill City; Wind Cave Nat’l Park Game Ranch, Cold Spring Creek. Wyoming: 11/2 mi. E Buckhorn, 6150 ft.
Dipus canadensis Davies, Trans. Linn. Soc. London, 4:157, 1798.
Zapus hudsonius hudsonius, Preble, N. Amer. Fauna, 15:17, August 8, 1899 (part—the part from Ontario).
Zapus hudsonius canadensis, Batchelder, Proc. New England Zool. Club, 1:5, February 8, 1899 (part—the part from Quebec); Anderson, Rept. Provancher Soc. Nat. Hist., Quebec, 1941:35-37, July 14, 1942 (part—the part from Quebec excepting the Gaspé Peninsula).
Zapus hudsonius ontarioensis Anderson, Ann. Rept. Provancher Soc. Nat. Hist., Quebec, 1942:59, September 7, 1943, type from Pancake Bay (Batchawana Bay) southeast end of Lake Superior, Algoma District, about 40 miles northeast of Sault Ste-Marie, Ontario.
Type.—No type specimen designated, subspecies characterized on the basis of two specimens obtained by Major General Thomas Davies within a few miles of the city of Quebec.
Range.—Eastern Ontario and western Quebec from Hudson Bay southward to the Great Lakes and into northwestern New York. See fig. 47. Zonal range: Transition and Canadian.
Description.—Size medium; back from near Clay Color to near Cinnamon-Buff with admixture of black hair usually forming a dorsal band; sides from near Clay Color to near Cinnamon-Buff and lighter than back; lateral line usually distinct, and clear Cinnamon-Buff; belly white, sometimes with slight suffusion of Cinnamon-Buff mid-ventrally; tail bicolored, brownish to brownish-black above, grayish-white to yellowish-white below; ears dark, sometimes flecked with color of the sides, edged with Cinnamon-Buff; feet grayish-white above; auditory bullae large, relatively broad and flat; incisive foramina relatively short and narrow, widest posteriorly; zygomata not widely bowed outward; mastoid region relatively wide; frontal region well inflated; nasals relatively narrow, short, and parallel sided.
Comparisons.—From Zapus hudsonius hudsonius, Z. h. canadensis differs as follows: Upper parts generally dull averaging lighter, less black tipped hair; sides also lighter with less suffusion of dark hair; frontal region more inflated; mastoid region averaging broader; auditory bullae broader; distance from incisors to postpalatal notch averaging slightly longer.
For comparison with Zapus hudsonius acadicus, Zapus hudsonius ladas, and Zapus hudsonius americanus see accounts of those subspecies.
Remarks.—Bole and Moulthrop (1942:165) refer 2 specimens from Elba, New York, to Z. h. hardyi (= acadicus); they are more nearly like Z. h. canadensis in size and shape of the auditory bullae [443] and general color of the pelage. A specimen from Spectacle Pond, New York, has the narrow pterygoid fossae and relatively narrow auditory bullae of Z. h. acadicus and the relatively short, narrow incisive foramina, inflated frontal region, and color of Z. h. canadensis to which the specimen is here referred. Intergradation is noted also in animals from Schreiber, Ontario. They resemble Zapus hudsonius hudsonius in their darker coloration and shape of auditory bullae but in the remainder of the characters studied resemble Z. h. canadensis to which they are referred. Specimens from Notre Dame de la Dore and 1/2 mi. N Mistassini Post, Quebec, in size and shape of the auditory bullae and in width of the pterygoid fossae, closely approach Z. h. ladas but in color, distinct dorsal band, and in narrower zygomata are all nearest Z. h. canadensis to which subspecies they are here referred.
Zapus hudsonius ontarioensis Anderson (1942:59) from eastern Ontario was based chiefly, in comparison with Z. h. canadensis, upon, “dorsal stripe less distinct and sides somewhat duller yellowish with more admixture of blackish hairs.” Examination of 68 of the 69 specimens from the type locality shows that 58 are subadult and in subadult pelage. Individuals which are adult are indistinguishable in color of pelage and in cranial features from comparable material from southern Quebec. Z. h. ontarioensis is, therefore, considered to be a synonym of Z. h. canadensis.
Specimens examined.—Total, 123, distributed as follows:
New York: Franklin Co.: Spectacle Pond, Brighton Township, 2 (AMNH). Genesee Co.: Elba, 2 (Clev. MNH).
Ontario: Schreiber, 2 (NMC); Franz, 5 (MVZ); Pancake Bay, Algoma District, 68 (NMC); Maclennan, Algoma District, 3 (ROM); Cache Lake, Algonquin Park, 1 (MVZ); Experimental Farm, Ottawa, 1 (NMC); Dows Swamp, Ottawa, 1 (NMC); Apple Hill, 1 (NMC); Clear Lake, Arden, 1 (NMC); Athens, 1 (NMC); Aurora, 4 (Clev. MNH); Pattageville, Toronto, 1; Lorne Park, Toronto, 1 (NMC); Credit, 2 (NMC); Pickering, 1 (MVZ); Preston, 1 (NMC); St. Thomas, 1 (NMC).
Quebec: Notre Dame de la Dore, 3 (NMC); 1/2 mi. N Mistassini Post, 1 (NMC); Lake Albanel, 1 (NMC); St. Felicien, 3 (NMC); Valcartier, 8 (NMC); Kiamika Lake, 4 (NMC); Ste. Veronique, 2 (NMC); Val Jalbert, 2 (NMC); St. Methode, 1 (NMC).
Marginal records.—Quebec: 1/2 mi. N Mistassini Post; Valcartier. New York: Spectacle Pond, Brighton Township; Elba. Ontario: St. Thomas; Pancake Bay, Algoma Dist.; Franz; Schreiber. Quebec: Kiamika Lake.
Dipus hudsonius Zimmerman, Geog. Geschichte d Menschen u. vierfussigen Thiere, 2:358, 1780.
Dipus labradorius Kerr, Animal Kingdom:276 (based on the Labrador Jerboid Rat of Pennant, 1781—but Preble, N. Amer. Fauna, 15:11, August 8, 1899, states that the specimen came from Hudson Bay), 1792.
Gerbillus canadensis, Desmarest, Mammalogie, 2:321, 1822.
Gerbillus labradorius, Harlan, Fauna Amer., p. 157, 1825.
Meriones labradorius, Richardson, Fauna Boreali-Americana, 1:144, 1829.
Jaculus labradorius Wagner, Suppl. Schreber’s Saugthiere, 3:294, 1843.
Zapus hudsonius hudsonius, Preble, N. Amer. Fauna, 15:15, August 8, 1899 (part—the part from Northwest Territory, Ontario, Michigan, northern Wisconsin and northern Minnesota).
Zapus hudsonius alascensis, Osgood, N. Amer. Fauna, 19:38, October 6, 1900.
Type.—Type specimen not known to be in existence; from Hudson Bay, locality now considered to be Fort Severn, Ontario (see Anderson, 1942:37).
Range.—Central Alaska southeastward to central Ontario, northern Minnesota, northern Wisconsin, and upper peninsula of Michigan. See fig. 47. Zonal range: Hudsonian, Canadian, and into Transition.
Description.—Size medium; back dark, from near Tawny-Olive to near Cinnamon with heavy admixture of black hair forming dorsal band; sides lighter than back and from near Tawny-Olive to near Cinnamon, sometimes with admixture of black hair giving sides streaked appearance; lateral line usually distinct, clear Ochraceous-Buff; underparts white, sometimes with slight suffusion of Ochraceous-Buff; tail bicolored, brown to brownish-black above, grayish-white to yellowish-white below; ears dark, usually edged with ochraceous; feet grayish-white above; incisive foramina relatively short and broadly rounded; zygomata relatively short; braincase relatively broad; auditory bullae flat, long, and relatively broad; pterygoid fossae relatively narrow; nasals relatively broad and short.
Comparisons.—From Zapus hudsonius alascensis, Z. h. hudsonius differs as follows: upper parts generally darker, more black tipped hair; sides darker with greater suffusion of dark hair; lateral line brighter, more distinct; size averaging smaller; zygomatic arches less bowed outward; distance from incisors to postpalatal notch shorter; zygomata shorter; occipitonasal length less; mastoid region narrower.
From Zapus hudsonius intermedius, Z. h. hudsonius differs in: color darker, more tawny dorsally; sides averaging darker, more black-tipped hairs; size averaging larger; braincase averaging broader; distance from incisors to postpalatal notch averaging slightly shorter; zygomata averaging longer; mastoid region averaging broader; incisive foramina averaging shorter.
From Zapus hudsonius tenellus, Z. h. hudsonius differs as follows: upper parts averaging darker; tail averaging shorter; condylobasal length averaging more; braincase averaging broader; auditory bullae broader and less inflated; interparietal averaging broader; incisive foramina more broadly rounded and averaging longer.
For comparison with Zapus hudsonius canadensis and Zapus hudsonius campestris see accounts of those subspecies.
Remarks.—Preble (1899:16) had available for study five specimens of Zapus hudsonius hudsonius from Hudson Bay. Four were preserved in alcohol and one as an incomplete skin (prepared from an alcoholic specimen). All were unreliable for comparative purposes owing to the effects of the preservative. Preble, therefore, (loc. cit.) selected as a fairly typical sample a series of specimens from Tower, St. Louis County, Minnesota; these formed the basis of [445] comparison between Z. h. hudsonius and other subspecies of Zapus hudsonius. Now that additional material (well prepared skins and skulls) is available from the Hudson Bay region and from other localities in northern and western Canada it is evident that the specimens from Tower, although here considered to be Z. h. hudsonius, are not typical Z. h. hudsonius but are intergrades between hudsonius and specimens of Zapus hudsonius intermedius. Comparisons made in the present account are based on specimens from the vicinity of Hudson Bay (Fort Severen, Ontario, York Factory, Shamatawa River, and Robinson Portage, Manitoba). These individuals are considered typical of this subspecies. With these new data available the range of Z. h. hudsonius is now understood to include all of the region from eastern Alaska to the northern parts of Minnesota, Wisconsin, and Michigan.
Intergradation between Zapus hudsonius canadensis and Z. h. hudsonius is noted in specimens from 30 mi. NE Port Arthur and also in those from Silver Islet, Thunder Cape, Ontario. These individuals resemble Z. h. canadensis in size and shape of the auditory bullae and in the shape of the nasals, but in their darker coloration, broadly rounded incisive foramina, and relatively narrow pterygoid fossae they are more nearly like Z. h. hudsonius to which they are here referred.
Specimens from Minaki, Ontario, are tending toward Zapus hudsonius intermedius in lighter coloration but in the size and shape of the auditory bulla, size and shape of the incisive foramina, and in the width of the pterygoid fossae they are more nearly like Z. h. hudsonius to which they are here referred. Specimens from various localities in Menominee County, Michigan, are like Z. h. intermedius in shape of the incisive foramina and shape of the postpalatal notch, but in color of pelage, size and shape of the auditory bullae, and breadth of the pterygoid fossae they closely resemble Z. h. hudsonius.
In Wisconsin, intergradation occurs in color and in cranial characters in specimens from Mercer, Solon Spring, and in a single individual from Basswood Lake. All these specimens, however, are best referable to Z. h. hudsonius.
Specimens from one mile southwest of Fairbanks and from Fairbanks, Alaska, show intergradation with Zapus hudsonius alascensis in coloration (more brown, less black), but in small size, short, broadly rounded incisive foramina, and in size and shape of the auditory bullae are nearest to Z. h. hudsonius to which they are here assigned.
Intergradation with Zapus hudsonius alascensis is noted also in specimens from McIntyre Creek, Yukon. They are like Z. h. alascensis in the size and shape of the auditory bullae and in the more elongate incisive foramina, but in the coloration, size of the pterygoid fossae, and breadth of the braincase are more nearly like Z. h. hudsonius and are here referred to this geographic race.
In British Columbia, in specimens from 1 mi. NW junction of Irons Creek and Laird River as well as in those from Hot Springs, 3 mi. WNW junction of Trout River and Laird River, and in those from 1/4 mi. S of the junction of the same rivers, three way intergradation occurs. These animals are like Zapus hudsonius alascensis in color and in length of tail. They agree with Zapus hudsonius tenellus in shape of nasals. In degree of inflation of auditory bullae, in length and width of incisive foramina, and in shape of pterygoid fossae they are as in Z. h. hudsonius to which they are here assigned.
Specimens examined.—Total, 230, distributed as follows:
Alaska: Fairbanks, 1 (USNM); 1 mi. SW Fairbanks, 440 ft., 1.
Alberta: Conibear Lake, Wood Buffalo Park, 1 (NMC); Assineau River, 1920 ft., 10 mi. E and 1 mi. N Kinuso, 1; Mountain Rapid, Athabasca River, 1 (USBS); Brule Rapid, Athabasca River, 1 (USBS); 25 mi. above Pelican Rapid, Athabasca River, 1 (USBS); Lac la Nonne, 7 (NMC); Swift Current, Athabasca River, 1 (USBS); junction Lac la Biche River and Athabasca River, 1 (USBS); 30 mi. above Athabasca Landing, Athabasca River, 1 (USBS).
British Columbia: 1 mi. NW junction Irons Creek and Laird River, 3; Hot Springs, 3 mi. WNW junction Trout River and Laird River, 1; 1/4 mi. S junction Trout River and Laird River, 1.
Manitoba: York Factory, 2 (USBS); Shamatawa River, 1 (USBS); Oxford House, 15 (USBS); Robinson Portage, 4 (USBS); Echamamish, 1 (USBS); Norway House, 1 (USBS); Swan River, 1 (NMC); Bird, 1 (NMC); Aimie Lake, 2 (NMC); Albert’s Lake, Flin Flon, 2 (NMC); Portage La Prairie Prov., Delta, 1 (UM).
MacKenzie District: Fort Resolution, 3 (USBS); Fort Smith, 3 (USBS).
Michigan: Chippewa Co.: Marquette Nat’l Forest, 4; no exact locality, 2. Gogebic Co.: Mud Lake, 1/4 mi. SE Thousand Island Lake, 2. Keweenaw Co.: Lake Manganese, 1 mi. SSE Copper Harbor, 5 (UM); 21/5 mi. SE Copper Harbor, 8 (UM); 5 mi. E Eagle Harbor, 6 (UM); E end Lake Upson, 3 (UM); Bete Grise, 5 (UM). Marquette County: Michigamme, 3 (2 USBS). Menominee Co.: 8 mi. N Hermansville, 6 (UM); 6 mi. NW Banat, 8 (UM); 5 mi. SW Banat, 8 (UM); 8 mi. SW Banat, 2 (UM); 7 mi. E Stephenson, 3 (UM); 8 mi. WSW Stephenson, 2 (UM); 10 mi. W Stephenson, 2 (UM); 13 mi. WSW Stephenson, 2 (UM); 5 mi. N Menominee, 2 (UM).
Minnesota: Lake Co.: Splitrock River, 2 (UM); St. Louis County: Tower, 27 (USBS).
Ontario: Fort Severn, Kenora District, 6 (ROM); Minaki, 7 (MVZ); 30 mi. NE Port Arthur, 6 (UM); Silver Islet, Thunder Bay District, 4 (NMC); 20 mi. SW Fort Williams, 3 (UM); 20 mi. SE Fort Williams, 1 (UM).
Saskatchewan: Emma Lake, 3 (ROM).
Wisconsin: Bayfield County: Herbster, 4 (USBS); Brinks Camp, Washburn, 1 (AMNH); Basswood Lake, 10 mi. SE Iron River, 1 (USBS). Douglas County: Solon Springs, 9 (USBS). Forest County: Crandon, 1 (USBS). Iron County: Mercer, 2 (USBS). Oneida County: Crescent Lake, 2 (USBS). Vilas County: Mamie Lake, 2 (USBS); Lake St. Germain, 9 (USBS).
Yukon: Lake Lebarge, 3 (USBS); Forks of MacMillian River, 1 (USBS); McIntyre Creek, 2250 ft., 3 mi. NW Whitehorse, 4.
Marginal records.—Alaska: Fairbanks. MacKenzie: Ft. Resolution. Manitoba: York Factory. Ontario: Fort Severn, Kenora District; Silver Islet, Thunder Bay Dist. Michigan: Marquette Nat’l Forest; 5 mi. N Menominee. Wisconsin: Crandon; Solon Springs. Minnesota: Tower. Manitoba: Portage la Prairie Prov., Delta. Saskatchewan: Emma Lake. Alberta: 30 mi. above Athabasca Landing, Athabasca River; Lac la Nonne. British Columbia: 1 mi. NW junction Irons Creek and Laird River. Yukon: McIntyre Creek, 2250 ft., 3 mi. NW Whitehorse; Lake Lebarge.
Type.—Male, adult, No. 83400, Univ. Michigan Mus. Zool.; Ridgeway, Winneshiek County, Iowa; obtained on July 22, 1939, by S. A. Hoslett, original No. 517.
Range.—Eastern Montana, North Dakota, probably northern South Dakota, all but northern parts of Minnesota and Wisconsin, Iowa, Illinois, southwestern Indiana, and western Kentucky. See fig. 47. Zonal range: Upper Austral (Upper Sonoran and Carolinian) and Transition (Alleghanian and Transition).
Description.—Size medium; back from near Warm Buff to near Ochraceous-Buff with admixture of hair tipped with black or dark brown usually forming distinct, broad, dorsal band; sides lighter, from near Warm Buff to near Ochraceous-Buff with sparse mixture of dark-tipped hairs; lateral line often poorly marked but when present of clear Ochraceous-Buff; belly white, sometimes with slight suffusion of color of sides; tail bicolored, grayish-brown to brownish-black above, white to grayish-white or yellowish-white below; ears dark, narrowly edged with color of sides; feet white to grayish-white above; tail relatively short; lateral margins of nasals parallel; auditory bullae relatively short, broadly rounded, and moderately inflated; incisive foramina relatively long and narrow; pterygoid fossae relatively narrow; zygomata relatively long; inferior ramus of zygomatic process of maxillary frequently lacking a median projection.
Comparisons.—From Zapus hudsonius pallidus, Z. h. intermedius differs as follows: Coloration duller, not so bright, more yellow or buff and less bright Ochraceous-Buff; interorbital region averaging narrower; incisive foramina averaging longer and narrower; condylobasal length averaging greater; braincase averaging broader; mastoid region averaging broader.
For comparisons with Zapus hudsonius hudsonius, Zapus hudsonius campestris, and Zapus hudsonius americanus see accounts of those subspecies.
Remarks.—Zapus hudsonius intermedius has a large geographic range. There is some variation detectable when individuals from widely separate localities are compared, but where there is much variation it is obviously the result of intergradation. All characters differentiating Z. h. intermedius from any contiguous subspecies are not present in every specimen even in the type series. Nevertheless, a certain series of cranial characters (narrow incisive foramina, short rounded auditory bullae, parallel lateral margins of nasals and narrow pterygoid fossae) is diagnostic.
Animals obtained from extreme southwestern Indiana and from eastern Illinois approach Z. h. americanus in color and in shape of [448] the incisive foramina, but in the shape of the nasals, width of the pterygoid fossae and breadth of the zygomata are most nearly like Z. h. intermedius to which they are here referred. Specimens from Lake and Kane counties, Illinois, also show affinity with Z. h. americanus in color, but cranially are most nearly like Z. h. intermedius and are assigned to that subspecies.
Two specimens from southern Illinois (Perry County) are intergrades between Z. h. pallidus and Z. h. intermedius. Cockrum and Baker (1950:3) mentioned that these individuals showed evidence of intergradation with Z. h. pallidus in color of the pelage and the breadth of the least interorbital constriction. In other characters the specimens are most nearly like Z. h. intermedius to which they are here referred. Animals from Lyon County, Iowa, also show intergradation between Z. h. pallidus and Z. h. intermedius. These individuals are most nearly like Z. h. pallidus in interorbital breadth of the skull but in other characters agree with Z. h. intermedius and, therefore, are referred to that subspecies.
Intergradation between Z. h. campestris and Z. h. intermedius is noted in a specimen from 7 mi. NE Glendive, Montana. This individual has the larger, broader, auditory bullae and more widely bowed incisive foramina of Z. h. campestris, but in color, in smaller external size, and in the majority of cranial characters it is best referred to Z. h. intermedius.
Specimens from the north-central periphery of the geographic range of Z. h. intermedius (northern Minnesota and Wisconsin) on the average are darker, have longer auditory bullae, wider bowed incisive foramina, and (some specimens) a slightly wider pterygoid fossa than is normal in more southern populations. This deviation from the norm is interpreted as intergradation between Z. h. hudsonius and Z. h. intermedius. Individuals from Burnett, Price, and Oconto counties, Wisconsin, and those from Cass and southern Clearwater counties, Minnesota, show such intergradation but are here considered to be Z. h. intermedius.
Specimens examined.—Total, 199, distributed as follows:
Illinois: Coles Co.: Fox Ridge State Park, 1 (UIM). Fulton Co.: 1/2 mi. N Norris, 2 (UIM); 3 mi. N Canton, 1 (UIM); 21/2 mi. N Canton, 2 (UIM); 2 mi. NW Canton, 3 (UIM); 2 mi. W Canton, 1 (UIM); 3 mi. SW Monterey, 1 (UIM). Jo Daviess Co.: near Galen, 3 (FM). Kane Co.: Sugar Grove, 1 (Chic. AS). Lake Co.: Fox Lake, 4 (FM); Pistake Bay, 1 (FM). Perry Co.: 6 mi. S Pinckneyville (near Pyatt), 2 (SITC). Vermilion Co.: Kickapoo State Park, 2 (UIM); Jordan Creek, 3 mi. NE Fairmont, 5 (UIM).
Indiana: Owen Co.: La Fayette, 1 (USNM). Parks Co.: Turkey Run State Park, 2 (1 UM; 1 UIM). Posey Co.: Hovey Lake, 1 (UM); New Harmony, 2 (Clev. MNH); no exact locality, 2 (UM). Sullivan Co.: no exact locality, 1 (UM).
Iowa: Dickinson Co.: Camp Forester, E Okeboji Lake, 3 (ISC). Emmet Co.: Fort Defiance State Park, 1 (ISC). Hamilton Co.: Little Wall Lake, Jewell, 6 (ISC). Ida Co.: Arthur, 1 (ISC). Lyon Co.: Elgin Township, Sec. 35, 2 (ISC); Riverside Township, Sec. 28, 1 (ISC). Palo Alto Co.: Ruthven, 1 (ISC). Sioux Co.: Ireton, 1 (UM). Story Co.: Ames, 1 (ISC). Winneshiek Co.: Decorah, 3 (UM); Ridgeway, 11 (UM); Conover, 3 (UM).
Kentucky: Lyon Co.: no exact locality, 1 (USNM).
Montana: Dawson Co.: Yellowstone River, 7 mi. NE Glendive, 2000 ft., 1 (MVZ).
Minnesota: Cass County: Cass Lake, 7 (USBS). Clearwater Co.: Itasca Park, Biological Station, 5 (UM). Grant Co.: 3 mi. NW Barrett, 1 (UM). Jackson Co.: 4 mi. E Heron Lake, 1 (UM). Ottertail Co.: 5 mi. NW Vergas, 8 (UM); 4 mi. NW Ashley, 1430 ft., 2. Ramsey Co.: St. Paul, 1 (UM). Sherburne County: Elk River, 23 (2 UM; 6 MVZ; 3 USBS). Winona County: La Crescent, 3 (USBS).
North Dakota: Cass County: Fargo, 1 (USBS). Dickey County: Ludden, 1 (USBS); Ellendale, 1 (USBS). Kidder County: Pettibone, 3 (Chic. AS). La Moure County: La Moure, 1 (USBS). Oliver County: Fort Clark, 3 (USBS). Pembina County: Pembina, 2 (USNM). Ramsey County: Devils Lake, 3 (USBS). Ramson County: Lisbon, 1 (USBS). Richland County: Wahpeton, 2 (USBS); 5 mi. NE Fairmont, Sioux River, 5 (USBS); Blackner, 2 (USBS). Rolette County: Fish Lake, 2 (USBS). Sioux County: Cannon Ball, 4 (USBS). Williams Co.: Grinnell, 2 (USBS).
Wisconsin: Burnett County: Danbury, 1 (USBS). Chippewa County: Holcombe, 3 (USBS). Clark County: Withee, 4 (USBS); Worden Township, 2 (USBS). Crawford County: Lynxville, 1 (USBS). Dane Co.: Madison, 2 (OHIO). Dodge Co.: Horicorn Refuge, 2 (USBS). Juneau County: Mather, 1 (USBS). Marathon Co.: Rib Hill, 8 (USBS). Oconto County: Lakewood, 1 (USBS). Portage County: Stevens Point, 3 (USBS). Price County: Ogema, 2 (USBS). Rock County: Milton, 1 (USBS). Sauk County: Devils Lake, 1 (USBS). Sheboygan County: 8 mi. SW Mellen, 1 (USBS); Elkhart Lake, 1 (USBS). Walworth County: Delavan, Fosters Bridge, 1 (USBS); Turtle Lake, 1 (USBS). Wood Co.: Thorp Township, 2 (AMNH); Hewett Township, 4 (AMNH).
Marginal records.—North Dakota: Fish Lake; Pembina. Wisconsin: Danbury; Ogema; Lakewood. Illinois: Fox Lake. Indiana: La Fayette; New Harmony. Illinois: 6 mi. S Pinckneyville (near Pyatt). Iowa: Ames; Arthur; Ireton. Montana: Yellowstone River, 7 mi. NE Glendive, 2000 ft. North Dakota: Grinnell.
Zapus hudsonius ladas Bangs, Proc. New England Zool. Club, 1:10, February 28, 1899.
Type.—Female, adult, skin and skull, No. 4169, E. A. and O. Bangs Coll. (now in Mus. Comp. Zool.); Rigoulette, Hamilton Inlet, Labrador; obtained on July 18, 1895, by C. H. Goldthwaite.
Range.—Eastern Quebec north of Gulf of St. Lawrence, Labrador, and Newfoundland. See fig. 47. Zonal range: Canadian and Hudsonian.
Description.—Size medium; back relatively dark, near Ochraceous-Tawny with admixture of black-tipped hair; dorsal band relatively wide but not sharply defined against color of sides; side lighter than back, from near Ochraceous-Tawny to near Cinnamon and lined with black-tipped hair; lateral line distinct of clear Cinnamon-Buff or Light Ochraceous-Buff; underparts white, often suffused with Ochraceous-Buff; tail distinctly bicolored, dark brown to black above and yellowish-white to grayish-white below; ears dark, usually flecked with Tawny Ochraceous and edged with ochraceous; feet grayish-white above; [450] incisive foramina relatively short and broad; pterygoid fossae relatively broad; auditory bullae broad and well inflated; mastoid region relatively broad; zygomata relatively short; inferior arm of zygomatic process of maxillary relatively broad.
Comparison.—From Zapus hudsonius acadicus, which Z. h. ladas closely resembles, it differs in: Color darker, dorsal band much less distinct, underparts more frequently suffused with Ochraceous-Buff; auditory bullae relatively broader and more inflated; pterygoid fossae broader; zygomata averaging shorter; incisive foramina relatively shorter; inferior arm of zygomatic process of maxillary relatively broader.
From Zapus hudsonius canadensis, Z. h. ladas differs as follows: Color darker, more richly tawny, dorsal band less distinct; auditory bullae relatively shorter, more inflated; pterygoid fossae averaging broader; zygomata averaging broader; incisive foramina averaging longer.
Remarks.—This subspecies retains all of its diagnostic characters throughout nearly all parts of its geographic range. Specimens from Nova Scotia are like Z. h. ladas in their darker color and less distinct dorsal band, but in the remainder of their characters they are distinct and best referable to Z. h. acadicus.
Zapus h. ladas, with its relatively large size, poorly defined dorsal band, and broad, well inflated auditory bullae, is one of the better marked subspecies of the species Zapus hudsonius.
Specimens examined.—Total, 41, distributed as follows:
Labrador: Mahkovik, 1 (USNM); Etagaulet Bay, Lake Melvikl, 2 (USNM); 3 mi. above mouth of Naskaupi River, 1 (USNM); Northwest River, 6, (USNM); Cartwright, 1 (USBS); Muskrat Falls, Hamilton River, 1 (USNM); Hamilton River, Flour Lake, 3 (USNM); Hawke Harbor, 4 (USNM); Goose Bay, 3 (USNM); Niger Sound, Islet Bay, 1 (USNM); Red Bay, 5 (USNM); Mecklenburg Harbor, 2 (USNM); Mary Harbor, 1 (USNM).
Newfoundland: Hare Harbor, 3 (USNM).
Quebec: northwest Ungava, 1 (NMC); Moise Bay, 5 (NMC); Trout Lake, near Moise Bay, 1 (NMC).
Marginal records.—Labrador: Mahkovik; Red Bay. Newfoundland: Hare Harbor. Quebec: Trout Lake, near Moise Bay; northwest Ungava.
Zapus hudsonius pallidus Cockrum and Baker, Proc. Biol. Soc. Washington, 63:1, April 26, 1950.
Jaculus hudsonius, Baird, Repts. Expl. and Surv. 111, 8 (pt. 1):433, July 14, 1858 (part—the part from Platte River, Nebraska, and Cass County, Missouri).
Zapus hudsonius, Coues, Bull. U. S. Geol. and Geog. Surv. of the Territories, 2nd ser. No. 5:260, 1877 (part—the part from Platte River, Nebraska).
Zapus hudsonius campestris Preble, N. Amer. Fauna, 15:20, August 8, 1899 (part—the part from Columbus in Nebraska and Jackson County in Missouri).
Type.—Male, adult, No. 22953, Univ. Kansas Mus. Nat. Hist.; NW corner sec. 4, T. 12S, R. 20E, 51/2 mi. N, 13/4 mi. E Lawrence, Douglas County, Kansas; obtained on May 4, 1948, by E. Lendell Cockrum and Rollin H. Baker, original No. 916 of Cockrum.
Range.—Southern South Dakota, Nebraska, Kansas, Missouri, and northeastern Oklahoma. See fig. 47. Zonal range: Upper Austral (Upper Sonoran and Carolinian).
Description.—Size small; back near Cinnamon-Buff with admixture of dark-tipped hair forming distinct, broad, dorsal band; sides bright Cinnamon-Buff with sparse mixture of dark-tipped hair; lateral line usually distinct, of clear Cinnamon-Buff; belly white, sometimes with suffusion of color of sides, tail bicolored, brownish to brownish-black above, grayish-white to yellowish-white below; ears dark, narrowly edged with color of sides; feet white to grayish-white above; mastoid region relatively narrow; maxillary tooth-row relatively short; zygomata relatively short; zygomatic arch relatively broad; interorbital region relatively broad; auditory bullae relatively small and narrow; lateral margins of nasals not constricted posteriorly.
Comparisons.—From Zapus hudsonius preblei, Z. h. pallidus differs as follows: Coloration brighter and richer, more buff, less black; zygomatic arch more broadly bowed; condylobasal length averaging less; braincase narrower; interorbital region broader; incisive foramina shorter.
For comparisons with Zapus hudsonius pallidus and Zapus hudsonius intermedius see accounts of those subspecies.
Remarks.—The characters that distinguish this jumping mouse from neighboring kinds are relatively stable throughout most of its geographic range. Zapus hudsonius pallidus is one of the best defined subspecies of Z. hudsonius.
One specimen from Batesland, South Dakota, is referred to Z. h. pallidus but shows evidence of intergradation with Zapus hudsonius campestris in the shape of the nasals, incisive foramina, and in breadth of the zygomatic arch. An animal from 3 mi. NE Ponca, Nebraska, is intermediate between Z. h. pallidus and Zapus hudsonius intermedius in size and shape of the auditory bullae and in the breadth of the pterygoid fossae, but since this individual shows more resemblance to Z. h. pallidus in coloration and in the majority of cranial characters it is here referred to Z. h. pallidus. Specimens from Beemer, Nebraska, show an intergrading tendency toward Zapus hudsonius intermedius in the reduced lateral bowing of the zygomatic arch and in shorter zygomata. Since these individuals resemble Z. h. pallidus in the majority of characters they are referred to that race. An individual of Z. h. pallidus from Pevely, Missouri, is to some extent an intergrade with Z. h. intermedius of neighboring southern Illinois. Two individuals of Z. h. pallidus from Mohawk Park, Oklahoma, are darker dorsally than, but otherwise similar to, specimens from the type locality.
Zapus hudsonius pallidus seems to be the terminus of a cline; this is a southward trend toward smaller size and lighter, brighter color. There is a similar clinal tendency in the jumping mice in [452] eastern North America, and Z. h. americanus from North Carolina, pronouncedly resembles Z. h. pallidus from Kansas.
Specimens examined.—Total, 44, distributed as follows:
Kansas: Brown Co.: Horton, 1. Douglas Co.: Sec. 8, T. 123, R. 20E, 51/2 mi. N, 13/4 mi. E Lawrence, 10; 5 mi. N and 11/2 mi. E Lawrence, 3; Robinson Farm, 5 mi. N and 3 mi. E Lawrence, 2; 4 mi. N, 21/2 mi. E Lawrence, 1; Lakeview, 2; 71/2 mi. SW Lawrence, 1. Greenwood Co.: 1/2 mi. S Hamilton, 1.
Missouri: Cole Co.: Jefferson City, 2 (MO). Jackson Co.: no exact locality, 1 (USBS). Jefferson County: Pevely, 1 (USBS).
Nebraska: Blaine Co.: Dismal River, at Thomas-Blaine County line, 1 (NGFP). Boyd Co.: 2 mi. E and 15 mi. S Spencer, 1. Buffalo Co.: Platte Meadows, Kearney, 1 (HM). Butler Co.: 5 mi E Rising City, 1. Cherry Co.: Niobrara River, 18 mi. NW Kennedy, 1; Ballard Marsh, 20 mi. S Valentine, 1 (JKJ); Pony Lake Headquarters, Valentine Nat’l Wildlife Refuge, 1 (JKJ). Colfax Co.: 2 mi. S Schuyler, 1 (JKJ). Cuming County: Beemer, 4 (USBS). Dixon Co.: 3 mi. NE Ponca, 1. Platte County: Columbus, 1 (USBS). Richardson Co.: 5 mi. SE Rulo, 1 (NGFP).
Oklahoma: Tulsa Co.: Mohawk Park, 2 (UM).
South Dakota: Bennett Co.: Batesland, 1 (FM).
Marginal records.—South Dakota: Batesland. Nebraska: 3 mi. NE Ponca; Beemer; 5 mi. SE Rulo. Missouri: Pevely. Oklahoma: Mohawk Park. Kansas: 1/2 mi. S Hamilton. Nebraska: Platte Meadows, Kearney; Ballard Marsh, 20 mi. S Valentine; Niobrara River, 18 mi. NW Kennedy.
Type.—Male, adult, No. 73085, U. S. Nat. Mus., Biol. Surv. Coll.; Loveland, Larimer County, Colorado; obtained on July 23, 1895, by E. A. Preble, original No. 435.
Range.—Southeastern Wyoming and north-central Colorado. See fig. 47. Zonal range: Transition.
Description.—Size medium; color dull, back from near Clay Color to near Tawny-Olive with admixture of black hair forming poorly defined dorsal band; sides lighter than back from near Clay Color to near Cinnamon-Buff; lateral line distinct and clear Ochraceous-Buff; belly white, sometimes with faint wash of clear Ochraceous-Buff; tail bicolored, brownish to light brownish-black above, grayish-white to yellowish-white below; ears dark, narrowly edged with color of sides; feet grayish-white above; incisive foramina relatively narrow and elongate; auditory bullae moderately inflated; pterygoid fossae relatively broad; postpalatal notch broadly rounded; interorbital region relatively narrow; zygomatic arch not widely bowed; frontal region well inflated; distance from incisors to postpalatal notch relatively short.
Comparisons.—Among named subspecies, Zapus hudsonius preblei most closely resembles Z. h. campestris. From topotypes of Z. h. campestris, Z. h. preblei differs as follows: Upper parts generally dull, averaging lighter, less black-tipped hair; dorsal band less distinct; sides duller; averaging smaller in most cranial measurements taken; least interorbital constriction narrower; auditory bullae smaller, less well inflated; incisive foramina narrower, not truncate posteriorly; frontal region usually more inflated.
From Zapus hudsonius pallidus, Z. h. preblei differs as follows: Upper parts generally duller (less ochraceous); dorsal band less distinct; sides paler (not bright Ochraceous-Buff); zygomatic arch less widely bowed; least interorbital constriction narrower; occipitonasal length averaging greater; distance from [453] incisors to postpalatal notch averaging less; incisive foramina longer, proportionally less widely bowed; auditory bullae longer; pterygoid fossae averaging broader.
Remarks.—No evidence of intergradation with any other geographic race was noted. To the east the range of Z. h. preblei is separated from that of Z. h. pallidus (western Kansas and southwestern Nebraska), by several hundred miles of mixed and short grass prairie. Much of this area is unsuitable to jumping mice but local marshy places might be inhabited. Much territory inhospitable to Zapus intervenes also between the ranges of Z. h. preblei and Z. h. campestris. This area (northern Platte, Goshen, eastern Converse, Niobrara, and southern Weston counties, Wyoming) is chiefly rolling hills and short grass prairie and, like that to the east, is only locally suitable for Zapus. If jumping mice do occur in suitable places in these intervening areas it is to be expected that they will show intergradation between the subspecies concerned.
Zapus hudsonius preblei, on the basis of 11 specimens, agrees most closely in size and color with Z. h. campestris; there is much less resemblance between Z. h. preblei and Z. h. pallidus.
An adult from Springhill, 12 mi. N Laramie Peak, is typically Z. h. preblei as is one from Cheyenne.
Although specimens of Z. h. preblei are few (4 adult, 7 non-adults), the differences between this and neighboring named kinds is considerable.
Specimens examined: Total, 11, distributed as follows:
Colorado: Boulder County: 3 mi. E Boulder, 1 (UCM); 5 mi. E Boulder, 1 (UCM); south of Boulder (no exact locality), 1 (UCM). Jefferson County: Semper, 1. Larimer County: Loveland, 2 (USBS).
Wyoming: Albany County: Springhill, 12 mi. N Laramie Peak, 6300 ft., 3 (USBS). Laramie County: Cheyenne, 1 (USNM). Platte County: Chugwater, 1 (Clev. MNH).
Marginal records.—Wyoming: Springhill, 12 mi. N Laramie Peak, 6300 ft.; Chugwater; Cheyenne. Colorado: Loveland; Semper.
Zapus tenellus Merriam, Proc. Biol. Soc. Washington, 11:103, April 26, 1897.
Zapus hudsonius tenellus, Hall, Univ. California Publ. Zool., 40:377, November 5, 1934.
Zapus hudsonius hudsonius, Baker, Univ. Kansas Publ., Mus. Nat. Hist., 5:111, November 28, 1951 (part—the part from E side Minaker River, 1 mi. W Trutch and 3 mi. N Fort St. John, British Columbia).
Type.—Female, young adult, skin and skull, No. 66932 U. S. Nat. Mus., Biol. Surv. Coll.; Kamloops, British Columbia; obtained on August 25, 1894, by Clark P. Streator, original No. 4196.
Range.—British Columbia. See fig. 47. Zonal range: Canadian and Hudsonian.
Description.—Size medium; back from near Clay Color (brighter) to near Cinnamon-Buff with admixture of black tipped hairs forming a weakly defined dorsal band; sides lighter than back from near dull Ochraceous-Buff to near Cinnamon-Buff frequently with admixture of dark-tipped hairs; lateral line usually distinct, of clear Ochraceous-Buff; belly white sometimes with slight suffusion of Ochraceous-Buff; tail bicolored, brownish to brownish-black above, white or grayish-white to yellowish-white below; ears dark, edged and flecked on inner surface with color of sides; feet grayish-white above; auditory bullae relatively narrow, moderately inflated, elongate when viewed from below, anterior edge slightly concave; incisive foramina relatively short; braincase relatively narrow; vertical depth of skull at junction of frontals and nasals relatively great; nasals relatively narrow; pterygoid fossae moderately broad; zygomata relatively short.
Comparisons.—For comparisons with Zapus hudsonius hudsonius and Zapus hudsonius alascensis see accounts of those subspecies.
Remarks.—Merriam (1897a:103) named this jumping mouse as a full species, mentioning that the skull is similar in size and characters to that of Zapus hudsonius but that externally these animals differed in coloration and length of the tail. Hall (1934:377) treated Z. tenellus as a subspecies of Z. hudsonius. He observed that the difference between Z. tenellus, Z. h. alascensis, and Z. h. hudsonius was of the same degree, and, even though intergrading material was not known to him, he considered tenellus only subspecifically distinct from Z. hudsonius. Hall (loc. cit.) tentatively referred to Z. h. tenellus specimens from Indianpoint Lake, 15 mi. NE Barkerville, Cottonwood P. O., and Hazelton, British Columbia. I have seen and compared with the type of Z. tenellus all specimens examined by Hall and agree with him that they are best referred to Z. h. tenellus. Since 1934, several additional localities in British Columbia have yielded specimens. Those from Minaker River and Fort St. John are intermediate in dorsal coloration and in size and shape of the auditory bullae between Zapus hudsonius hudsonius and Z. h. tenellus but in all other characters are most nearly like Z. h. tenellus to which they are here assigned. These intergrades constitute additional evidence that Z. tenellus and Z. hudsonius are only subspecies of a single species.
Specimens examined.—Total, 17, all from British Columbia, distributed as follows: east side Minaker River, 1 mi. W Trutch, 1; Hazelton, 959 ft., 2 (MVZ); 5 mi. W and 3 mi. N Fort St. John, 1; Indianpoint Lake, 15 mi. NE Barkerville, 5 (MVZ); Cottonwood P. O., 3 (MVZ); S end Swan Lake, Vernon, 1200 ft., 2 (MVZ); Kamloops, 3 (USBS).
Marginal records.—British Columbia.—E side Minaker River, 1 mi. W Trutch; 5 mi. W and 3 mi. N Fort St. John; S end Swan Lake, Vernon, 1200 ft.; Kamloops; Hazelton, 959 ft.
Table 5.—Cranial Measurements (in Millimeters) of Zapus.
| Number examined, ♂♂ plus ♀♀ | Breadth of braincase | Condylobasal length | Interorbital breadth | Mastoidal breadth | Length of maxillary tooth-row | Occipitonasal length | Palatal length | Zygomatic breadth | Zygomatic length |
Zapus trinotatus eureka, Big Lagoon, California. |
|||||||||
| 13 mean | 10.3 | 21.4 | 4.2 | 10.9 | 3.9 | 24.5 | 10.4 | 12.5 | 9.5 |
| max | 10.6 | 22.1 | 4.4 | 11.1 | 4.0 | 25.5 | 11.5 | 13.2 | 10.0 |
| min | 10.0 | 20.7 | 3.9 | 10.0 | 3.7 | 24.0 | 10.5 | 12.1 | 9.0 |
Zapus trinotatus montanus, Crater Lake, Oregon. |
|||||||||
| 10 mean | 10.3 | 20.7 | 4.5 | 10.6 | 4.0 | 24.3 | 10.5 | 12.1 | 9.5 |
| max | 10.5 | 21.5 | 4.7 | 11.1 | 4.1 | 24.8 | 10.8 | 12.6 | 9.8 |
| min | 10.2 | 20.3 | 4.2 | 10.2 | 3.7 | 23.5 | 10.1 | 11.8 | 9.0 |
Lost Cr. R. S., 10 mi. SE McKenzie Bridge, Oregon. |
|||||||||
| 5 mean | 10.3 | 20.9 | 4.5 | 10.7 | 3.9 | 24.2 | 10.4 | 12.1 | 9.0 |
| max | 10.5 | 21.3 | 4.6 | 10.9 | 4.1 | 24.6 | 10.7 | 12.2 | 9.0 |
| min | 10.2 | 20.4 | 4.4 | 10.5 | 3.8 | 23.5 | 10.0 | 12.0 | 9.1 |
Zapus trinotatus orarius, 3 mi. W Inverness, 800 ft., California. |
|||||||||
| 12 mean | 9.9 | 20.4 | 3.8 | 10.8 | 3.7 | 22.6 | 10.0 | 12.1 | 9.3 |
| max | 10.2 | 20.9 | 4.1 | 11.0 | 3.8 | 23.5 | 10.6 | 12.5 | 9.8 |
| min | 9.6 | 19.9 | 3.7 | 10.4 | 3.6 | 21.7 | 9.8 | 11.9 | 8.5 |
Zapus trinotatus trinotatus, Old Fort Clatsop, 100 ft., Oregon. |
|||||||||
| 8 mean | 10.3 | 21.1 | 4.4 | 10.7 | 3.8 | 24.3 | 10.6 | 12.3 | 9.0 |
| max | 10.6 | 21.8 | 4.8 | 11.0 | 3.9 | 25.0 | 11.0 | 12.7 | 10.0 |
| min | 10.0 | 20.2 | 4.0 | 10.4 | 3.7 | 23.6 | 10.0 | 11.9 | 9.2 |
Cayuse Meadow, 3800 ft., 31/2 mi. SW Steamboat Mt’n, Wash. |
|||||||||
| 10 mean | 10.4 | 21.5 | 4.5 | 11.1 | 3.9 | 24.6 | 10.7 | 12.6 | 9.7 |
| max | 10.6 | 22.2 | 4.8 | 11.4 | 4.1 | 25.4 | 11.1 | 12.8 | 10.0 |
| min | 10.2 | 20.6 | 3.8 | 10.8 | 3.8 | 23.7 | 10.2 | 12.2 | 9.3 |
Snoqualmie Pass, Washington. |
|||||||||
| 5 mean | 10.6 | 21.6 | 4.6 | 11.1 | 4.0 | 24.8 | 10.9 | 12.8 | 9.0 |
| max | 10.7 | 22.2 | 4.8 | 11.5 | 4.2 | 25.6 | 11.2 | 13.2 | 10.0 |
| min | 10.4 | 21.2 | 4.4 | 10.9 | 3.8 | 24.0 | 10.5 | 12.5 | 9.5 |
Alta Lake, 2200 ft., British Columbia. |
|||||||||
| 5 mean | 10.6 | 21.6 | 4.3 | 11.3 | 4.1 | 24.7 | 10.8 | 12.7 | 9.0 |
| max | 10.9 | 21.9 | 4.6 | 11.5 | 4.3 | 25.0 | 10.9 | 13.1 | 9.0 |
| min | 10.5 | 21.2 | 4.2 | 11.0 | 3.9 | 24.4 | 10.7 | 12.3 | 9.6 |
Zapus princeps cinereus, Raft River Mt’s, Utah. [456] |
|||||||||
| 4 mean | 10.5 | 21.5 | 5.0 | 11.1 | 4.1 | 24.5 | 10.9 | 12.6 | 9.0 |
| max | 11.0 | 22.5 | 5.1 | 11.4 | 4.3 | 25.0 | 11.6 | 12.9 | 10.0 |
| min | 10.3 | 21.0 | 4.6 | 10.7 | 3.9 | 24.0 | 10.4 | 12.3 | 9.4 |
Mt. Harrison, 10 mi. S Albion, Idaho. |
|||||||||
| 13 mean | 10.5 | 21.4 | 4.8 | 10.9 | 4.1 | 24.9 | 10.9 | 12.4 | 10.1 |
| max | 10.7 | 22.0 | 5.1 | 11.4 | 4.2 | 25.5 | 11.4 | 12.8 | 10.8 |
| min | 10.2 | 20.9 | 4.5 | 10.5 | 3.9 | 24.3 | 10.2 | 11.7 | 9.8 |
Zapus princeps curtatus, Pine Forest Mt’s, Nevada. |
|||||||||
| 11 mean | 10.5 | 21.1 | 4.7 | 10.7 | 4.2 | 24.6 | 10.9 | 12.2 | 9.7 |
| max | 10.6 | 21.8 | 5.0 | 11.0 | 4.4 | 25.3 | 11.4 | 12.5 | 10.0 |
| min | 10.2 | 20.5 | 4.2 | 10.5 | 3.9 | 24.0 | 10.5 | 11.9 | 9.4 |
Zapus princeps idahoensis, several localities near Cody, Wyoming. |
|||||||||
| 24 mean | 10.5 | 21.4 | 4.6 | 11.0 | 4.0 | 24.7 | 10.9 | 12.6 | 9.6 |
| max | 11.2 | 22.6 | 5.2 | 11.4 | 4.2 | 25.6 | 11.5 | 13.3 | 10.2 |
| min | 9.9 | 20.6 | 4.3 | 10.5 | 3.9 | 24.1 | 10.2 | 12.0 | 9.3 |
5 mi. E Warm Lake, 7000 ft., Idaho. |
|||||||||
| 4 mean | 10.2 | 21.2 | 4.3 | 11.0 | 4.1 | 24.4 | 10.7 | 12.3 | 9.0 |
| max | 10.3 | 21.8 | 4.4 | 11.1 | 4.2 | 24.8 | 11.0 | 12.6 | 9.0 |
| min | 10.0 | 20.7 | 4.2 | 10.8 | 4.0 | 23.9 | 10.3 | 12.0 | 9.1 |
Summit Smith Mt’n, 7500 ft., Idaho. |
|||||||||
| 9 mean | 10.2 | 21.3 | 4.4 | 10.9 | 4.1 | 24.5 | 10.7 | 12.1 | 9.0 |
| max | 10.5 | 22.5 | 4.6 | 11.3 | 4.3 | 25.2 | 11.2 | 12.6 | 10.0 |
| min | 10.0 | 20.8 | 4.0 | 10.5 | 3.9 | 23.9 | 10.3 | 11.8 | 9.1 |
2 mi. NE Cooke, 8500 ft., Montana. |
|||||||||
| 6 mean | 10.4 | 21.1 | 4.4 | 10.9 | 4.0 | 24.3 | 10.5 | 12.5 | 9.0 |
| max | 10.6 | 21.8 | 4.5 | 11.5 | 4.1 | 25.4 | 11.1 | 12.9 | 10.0 |
| min | 10.2 | 20.3 | 4.3 | 10.7 | 3.8 | 23.5 | 9.9 | 12.0 | 9.0 |
Birch Cr., 18 mi. NE Dillon, 7100 ft., Montana. |
|||||||||
| 11 mean | 10.3 | 21.3 | 4.3 | 11.0 | 4.0 | 24.5 | 10.8 | 12.6 | 9.6 |
| max | 10.6 | 22.2 | 4.6 | 11.6 | 4.3 | 25.5 | 11.5 | 13.0 | 9.9 |
| min | 9.7 | 20.7 | 4.0 | 10.6 | 3.8 | 23.7 | 10.3 | 12.4 | 9.2 |
Zapus princeps idahoensis, Waterton Lakes Park, Alberta. [457] |
|||||||||
| 5 mean | 10.6 | 21.4 | 4.6 | 10.7 | 4.1 | 24.5 | 10.8 | 12.2 | 9.0 |
| max | 10.9 | 22.1 | 4.7 | 11.0 | 4.2 | 25.2 | 11.0 | 12.3 | 10.0 |
| min | 10.3 | 21.0 | 4.4 | 10.4 | 4.0 | 24.2 | 10.6 | 11.8 | 9.3 |
Zapus princeps kootenayensis, near Rossland, British Columbia. |
|||||||||
| 10 mean | 10.2 | 20.5 | 4.4 | 10.6 | 4.0 | 23.7 | 10.4 | 11.9 | 9.2 |
| max | 10.8 | 21.0 | 4.8 | 10.9 | 4.2 | 24.4 | 10.7 | 12.2 | 9.5 |
| min | 9.7 | 20.0 | 4.1 | 10.0 | 3.8 | 23.2 | 9.9 | 11.4 | 9.0 |
Zapus princeps luteus, White Mt’s, Arizona. |
|||||||||
| 20 mean | 10.1 | 20.3 | 4.9 | 10.7 | 3.9 | 23.8 | 10.4 | 11.9 | 9.7 |
| max | 10.4 | 21.1 | 5.1 | 11.2 | 4.0 | 24.8 | 10.9 | 12.6 | 10.2 |
| min | 9.6 | 19.1 | 4.3 | 10.2 | 3.6 | 22.5 | 9.5 | 11.1 | 8.9 |
Espanola, 5000 ft., New Mexico. |
|||||||||
| 3 mean | 9.8 | 19.8 | 4.7 | 10.5 | 3.7 | 23.4 | 9.9 | 11.5 | 9.0 |
| max | 10.0 | 20.1 | 4.8 | 10.6 | 3.8 | 23.7 | 10.3 | 11.6 | 9.0 |
| min | 9.7 | 19.5 | 4.5 | 10.3 | 3.6 | 22.9 | 9.6 | 11.4 | 9.4 |
Zapus princeps minor, 2 mi. W Fort Totten, 1400 ft., No. Dakota. |
|||||||||
| 11 mean | 9.9 | 20.5 | 4.5 | 10.6 | 3.7 | 23.7 | 10.5 | 11.8 | 9.6 |
| max | 10.1 | 20.8 | 4.8 | 10.8 | 3.8 | 24.2 | 10.7 | 12.1 | 9.9 |
| min | 9.7 | 20.0 | 4.4 | 10.4 | 3.6 | 23.4 | 10.2 | 11.4 | 9.3 |
Near Bottineau, North Dakota. |
|||||||||
| 4 mean | 10.1 | 20.9 | 4.6 | 10.6 | 3.8 | 24.1 | 10.8 | 12.3 | 10.0 |
| max | 10.2 | 21.3 | 4.7 | 10.9 | 3.8 | 24.5 | 10.9 | 12.5 | 10.0 |
| min | 10.1 | 20.6 | 4.5 | 10.4 | 3.7 | 23.8 | 10.7 | 12.1 | 9.9 |
Head Eagle Cr., Bear Paw Mt’s, Montana. |
|||||||||
| 7 mean | 10.0 | 20.8 | 4.6 | 10.7 | 3.8 | 24.2 | 10.7 | 12.1 | 9.0 |
| max | 10.5 | 21.3 | 4.7 | 10.9 | 4.0 | 24.7 | 11.1 | 12.5 | 10.0 |
| min | 9.8 | 20.3 | 4.4 | 10.5 | 3.6 | 23.2 | 10.3 | 11.9 | 9.7 |
N Maple Cr., Cypress Hills, Saskatchewan. |
|||||||||
| 10 mean | 10.1 | 21.2 | 4.6 | 10.7 | 3.9 | 24.5 | 10.9 | 12.3 | 9.8 |
| max | 10.4 | 21.7 | 4.9 | 10.8 | 4.0 | 24.8 | 11.3 | 12.7 | 10.0 |
| min | 9.9 | 20.4 | 4.4 | 10.5 | 3.6 | 24.0 | 10.5 | 11.8 | 9.6 |
Zapus princeps oregonus, Parker Cr., Warner Mt’s, 5500 ft., Cal. [458] |
|||||||||
| 12 mean | 10.6 | 21.6 | 4.7 | 11.1 | 4.2 | 25.0 | 11.1 | 12.6 | 10.2 |
| max | 11.0 | 22.2 | 4.9 | 11.6 | 4.4 | 25.7 | 11.4 | 13.0 | 10.9 |
| min | 10.2 | 21.2 | 4.3 | 10.8 | 4.0 | 24.5 | 10.7 | 12.4 | 9.9 |
Cobb Cr., 6 mi. SW Mt’n City, Nevada. |
|||||||||
| 12 mean | 10.7 | 21.6 | 5.0 | 11.2 | 4.1 | 25.0 | 11.2 | 12.6 | 10.0 |
| max | 11.1 | 22.1 | 5.2 | 11.4 | 4.3 | 25.7 | 11.8 | 13.0 | 10.3 |
| min | 10.5 | 21.0 | 4.6 | 10.7 | 3.8 | 24.4 | 10.9 | 12.2 | 9.5 |
Wisconsin Cr., 8000 ft., Nevada. |
|||||||||
| 10 mean | 10.6 | 21.6 | 4.9 | 11.2 | 4.0 | 24.8 | 11.1 | 12.4 | 9.5 |
| max | 10.8 | 22.2 | 5.0 | 11.5 | 4.2 | 25.2 | 11.4 | 12.8 | 9.6 |
| min | 10.3 | 21.2 | 4.6 | 10.8 | 3.9 | 24.1 | 10.6 | 12.2 | 9.3 |
North Fork Malheur River, 21 mi. SE Prairie City, 5000 ft., Ore. |
|||||||||
| 10 mean | 10.6 | 21.5 | 4.7 | 11.3 | 4.2 | 24.8 | 11.0 | 12.7 | 9.9 |
| max | 11.2 | 22.3 | 5.2 | 11.6 | 4.4 | 26.2 | 11.7 | 13.2 | 10.9 |
| min | 10.0 | 20.8 | 4.3 | 10.9 | 4.0 | 23.5 | 10.5 | 12.4 | 9.7 |
Zapus princeps pacificus, North Fork Coffee Cr., 4500 ft., Calif. |
|||||||||
| 8 mean | 10.4 | 21.5 | 4.7 | 10.9 | 3.9 | 24.8 | 11.1 | 12.5 | 10.0 |
| max | 10.8 | 22.4 | 5.0 | 11.4 | 4.0 | 25.2 | 11.4 | 13.2 | 10.0 |
| min | 10.0 | 20.7 | 4.5 | 10.7 | 3.8 | 24.0 | 10.5 | 12.0 | 9.6 |
Jackson Lake, 5900 ft., California. |
|||||||||
| 7 mean | 10.4 | 21.5 | 4.5 | 11.1 | 4.0 | 24.9 | 11.0 | 12.6 | 10.0 |
| max | 10.6 | 22.1 | 4.6 | 11.5 | 4.1 | 25.5 | 11.4 | 12.9 | 10.0 |
| min | 10.1 | 20.7 | 4.3 | 10.5 | 3.8 | 23.5 | 10.0 | 12.2 | 9.6 |
Head of Lyle Canyon, 9700 ft., California. |
|||||||||
| 7 mean | 10.3 | 20.8 | 4.7 | 10.6 | 3.8 | 24.0 | 10.5 | 12.3 | 9.0 |
| max | 10.5 | 21.8 | 5.0 | 11.0 | 4.0 | 24.6 | 10.8 | 12.7 | 10.0 |
| min | 10.0 | 20.0 | 4.5 | 10.2 | 3.5 | 23.0 | 10.0 | 11.8 | 9.0 |
Zapus princeps princeps, Florida, Colorado. |
|||||||||
| 7 mean | 10.2 | 21.4 | 4.6 | 10.5 | 3.7 | 24.9 | 11.1 | 12.3 | 9.0 |
| max | 10.5 | 22.3 | 4.7 | 11.3 | 3.8 | 25.4 | 11.4 | 12.5 | 10.0 |
| min | 9.7 | 20.7 | 4.3 | 9.8 | 3.5 | 23.9 | 10.9 | 11.9 | 9.3 |
Zapus princeps princeps, Half Way, Colorado. [459] |
|||||||||
| 6 mean | 10.1 | 21.7 | 4.6 | 10.8 | 3.9 | 24.9 | 11.0 | 12.3 | 9.0 |
| max | 10.3 | 22.0 | 4.8 | 11.1 | 4.0 | 25.8 | 11.3 | 12.7 | 10.0 |
| min | 10.0 | 21.2 | 4.5 | 10.5 | 3.8 | 24.2 | 10.7 | 12.1 | 9.6 |
8 mi. N, 191/2 mi. E Savery, Wyoming. |
|||||||||
| 11 mean | 10.2 | 21.2 | 4.5 | 10.9 | 3.9 | 24.5 | 10.8 | 12.2 | 9.7 |
| max | 10.5 | 21.8 | 4.7 | 11.1 | 4.1 | 25.0 | 11.1 | 12.5 | 10.2 |
| min | 10.0 | 20.8 | 4.2 | 10.6 | 3.7 | 23.7 | 10.5 | 12.0 | 9.3 |
| 211/2 mi. S, 241/2 mi. W Douglas, 7600 ft., Wyoming. | |||||||||
| 11 mean | 10.1 | 21.2 | 4.6 | 10.9 | 4.0 | 24.6 | 10.8 | 12.3 | 9.8 |
| max | 10.4 | 21.9 | 4.9 | 11.2 | 4.1 | 25.0 | 11.2 | 12.8 | 10.1 |
| min | 9.9 | 20.7 | 4.5 | 10.8 | 3.8 | 24.2 | 10.5 | 12.0 | 9.5 |
Medicine Wheel Ranch, 28 mi. E Lovell, 9000 ft., Wyoming. |
|||||||||
| 20 mean | 10.3 | 21.5 | 4.7 | 11.2 | 4.0 | 24.7 | 11.0 | 12.6 | 10.0 |
| max | 10.6 | 22.2 | 4.9 | 11.5 | 4.2 | 25.3 | 11.4 | 12.9 | 10.4 |
| min | 10.0 | 20.7 | 4.4 | 10.8 | 3.8 | 24.1 | 10.6 | 12.2 | 9.8 |
Zapus princeps saltator, Stikine River at Glenora, British Columbia. |
|||||||||
| 17 mean | 10.4 | 21.3 | 4.4 | 10.9 | 4.1 | 24.3 | 10.7 | 12.5 | 9.6 |
| max | 10.7 | 22.2 | 4.5 | 11.4 | 4.5 | 25.0 | 11.4 | 13.0 | 10.0 |
| min | 9.8 | 20.5 | 4.1 | 10.6 | 3.8 | 23.3 | 10.3 | 12.0 | 9.3 |
Hazelton, 959 ft., British Columbia. |
|||||||||
| 15 mean | 10.4 | 21.6 | 4.5 | 11.0 | 4.0 | 24.6 | 10.9 | 12.5 | 9.8 |
| max | 10.8 | 22.3 | 4.7 | 11.6 | 4.2 | 25.5 | 11.4 | 12.9 | 10.0 |
| min | 9.9 | 20.7 | 4.2 | 10.7 | 3.8 | 23.7 | 10.5 | 11.7 | 9.4 |
Zapus princeps utahensis, near Robertson, 8700 ft., Wyoming. |
|||||||||
| 15 mean | 10.7 | 22.0 | 5.0 | 11.2 | 4.1 | 25.4 | 11.1 | 13.2 | 9.9 |
| max | 11.1 | 22.6 | 5.1 | 11.6 | 4.2 | 26.4 | 11.7 | 14.0 | 10.3 |
| min | 10.3 | 21.0 | 4.7 | 10.8 | 3.9 | 24.6 | 10.8 | 12.4 | 9.6 |
3 mi. N and 11 mi. E Alpine, 5650 ft., Wyoming. |
|||||||||
| 17 mean | 10.6 | 22.1 | 4.7 | 11.3 | 4.1 | 25.3 | 11.3 | 13.0 | 9.9 |
| max | 11.0 | 23.0 | 4.9 | 11.7 | 4.3 | 26.2 | 11.8 | 13.5 | 10.5 |
| min | 10.3 | 21.3 | 4.4 1 | 0.8 | 4.0 | 24.2 | 10.7 | 12.1 | 9.5 |
Zapus princeps utahensis, Salamander Lake and Lambs Canyon, 9000 ft., Utah. [460] |
|||||||||
| 9 mean | 10.9 | 22.0 | 4.8 | 11.2 | 4.1 | 25.2 | 11.1 | 13.1 | 10.0 |
| max | 11.3 | 22.4 | 5.0 | 11.3 | 4.3 | 25.9 | 11.4 | 13.3 | 10.0 |
| min | 10.7 | 21.5 | 4.5 | 11.0 | 3.9 | 24.6 | 10.7 | 12.6 | 9.7 |
Zapus hudsonius acadicus, vicinity of St. Andrews, New Brunswick. |
|||||||||
| 4 mean | 9.6 | 19.8 | 4.1 | 10.2 | 3.5 | 23.0 | 10.1 | 10.8 | 9.0 |
| max | 9.8 | 19.9 | 4.2 | 10.3 | 3.7 | 23.1 | 10.2 | 11.1 | 9.0 |
| min | 9.2 | 19.7 | 3.9 | 10.0 | 3.3 | 22.8 | 9.9 | 10.4 | 9.2 |
Sebec Lake, Maine. |
|||||||||
| 3 mean | 9.6 | 19.5 | 4.3 | 10.0 | 3.5 | 23.0 | 10.0 | 10.7 | 9.0 |
| max | 9.8 | 19.8 | 4.5 | 10.1 | 3.6 | 23.3 | 10.1 | 11.3 | 9.0 |
| min | 9.4 | 19.0 | 4.0 | 9.7 | 3.4 | 22.6 | 9.9 | 9.9 | 9.0 |
2 mi. S Center Ossipee, New Hampshire. |
|||||||||
| 10 mean | 9.6 | 19.8 | 4.2 | 10.1 | 3.5 | 23.3 | 10.0 | 10.8 | 9.3 |
| max | 10.0 | 20.5 | 4.6 | 10.4 | 3.6 | 24.0 | 10.3 | 11.0 | 9.9 |
| min | 9.2 | 18.9 | 3.8 | 9.7 | 3.2 | 22.3 | 9.6 | 10.6 | 8.8 |
Berlin, New York. |
|||||||||
| 6 mean | 9.5 | 19.5 | 4.3 | 10.1 | 3.5 | 22.9 | 9.8 | 10.8 | 9.0 |
| max | 9.9 | 20.6 | 4.4 | 10.6 | 3.7 | 23.8 | 10.6 | 11.3 | 9.0 |
| min | 9.2 | 18.8 | 4.2 | 9.5 | 3.4 | 21.8 | 9.3 | 10.4 | 8.6 |
Lake Kedgemakooge, Nova Scotia. |
|||||||||
| 5 mean | 9.5 | 19.9 | 4.2 | 10.3 | 3.5 | 23.4 | 10.2 | 11.3 | 9.0 |
| max | 9.7 | 20.1 | 4.3 | 10.4 | 3.5 | 23.5 | 10.4 | 11.4 | 9.0 |
| min | 9.3 | 19.7 | 4.0 | 10.3 | 3.4 | 23.3 | 9.9 | 11.0 | 9.3 |
Zapus hudsonius alascensis, Lake Clark, Alaska. |
|||||||||
| 2 mean | 9.5 | 19.3 | 4.3 | 10.0 | 3.6 | 22.8 | 9.9 | 10.7 | 9.0 |
| max | 9.6 | 19.6 | 4.4 | 10.0 | 3.6 | 23.0 | 9.9 | 10.7 | 9.0 |
| min | 9.4 | 19.1 | 4.2 | 9.9 | 3.5 | 22.6 | 9.9 | 10.7 | 9.2 |
Frosty Peak, Yakutat Bay, Alaska. |
|||||||||
| 3 mean | 9.8 | 19.7 | 4.2 | 10.3 | 3.6 | 23.5 | 10.1 | 10.8 | 9.0 |
| max | 10.0 | 20.0 | 4.5 | 10.6 | 3.7 | 24.2 | 10.4 | 11.1 | 9.0 |
| min | 9.6 | 19.0 | 4.0 | 9.8 | 3.5 | 22.5 | 9.6 | 10.2 | 9.2 |
Zapus hudsonius alascensis, 7 mi. SSE Haines, 10 ft., Alaska. [461] |
|||||||||
| 14 mean | 9.9 | 20.0 | 4.2 | 10.4 | 3.6 | 23.7 | 10.2 | 11.0 | 9.4 |
| max | 10.1 | 20.5 | 4.4 | 10.8 | 3.7 | 24.6 | 10.7 | 11.4 | 9.8 |
| min | 9.8 | 19.5 | 4.0 | 10.2 | 3.4 | 23.0 | 9.8 | 10.4 | 9.0 |
SW end Dezadeash Lake, Yukon. |
|||||||||
| 2 mean | 10.0 | 19.8 | 4.5 | 10.5 | 3.6 | 23.5 | 10.2 | 11.3 | 9.0 |
| max | 10.1 | 20.1 | 4.5 | 10.5 | 3.6 | 23.8 | 10.4 | 11.3 | 9.0 |
| min | 9.8 | 19.5 | 4.4 | 10.5 | 3.5 | 23.2 | 10.0 | 11.2 | 9.5 |
Zapus hudsonius americanus, Boyne Falls, Michigan. |
|||||||||
| 8 mean | 9.5 | 18.7 | 4.1 | 9.7 | 3.3 | 22.0 | 9.5 | 11.0 | 9.0 |
| max | 9.8 | 19.4 | 4.3 | 10.0 | 3.4 | 23.2 | 10.0 | 11.4 | 9.0 |
| min | 9.3 | 18.3 | 3.8 | 9.4 | 3.0 | 21.5 | 9.0 | 10.3 | 9.0 |
Ann Arbor, Michigan. |
|||||||||
| 3 mean | 9.5 | 18.6 | 4.2 | 9.9 | 3.3 | 22.0 | 9.5 | 10.9 | 9.0 |
| max | 9.6 | 18.8 | 4.4 | 10.0 | 3.3 | 22.4 | 9.8 | 11.0 | 9.0 |
| min | 9.4 | 18.5 | 3.8 | 9.8 | 3.2 | 21.9 | 9.3 | 10.8 | 8.9 |
Montauk Point, L. I., New York. |
|||||||||
| 2 mean | 9.4 | 18.8 | 4.3 | 9.2 | 3.5 | 22.2 | 9.7 | 10.6 | 8.0 |
| max | 9.6 | 19.1 | 4.4 | 9.2 | 3.5 | 22.5 | 9.8 | 10.7 | 8.0 |
| min | 9.2 | 18.4 | 4.2 | 9.2 | 3.4 | 21.9 | 9.5 | 10.5 | 8.9 |
Mays Landing, New Jersey. |
|||||||||
| 2 mean | 9.4 | 18.8 | 4.4 | 9.8 | 3.4 | 22.1 | 9.6 | 10.9 | 8.0 |
| max | 9.5 | 18.8 | 4.4 | 9.8 | 3.5 | 22.2 | 9.7 | 11.0 | 8.0 |
| min | 9.3 | 18.7 | 4.4 | 9.8 | 3.2 | 22.0 | 9.5 | 10.8 | 8.2 |
Laurel, Maryland. |
|||||||||
| 3 mean | 9.1 | 18.6 | 4.3 | 9.6 | 3.3 | 22.0 | 9.5 | 10.6 | 8.0 |
| max | 9.3 | 18.9 | 4.5 | 9.7 | 3.3 | 22.0 | 9.7 | 10.8 | 8.0 |
| min | 8.9 | 18.2 | 4.1 | 9.5 | 3.3 | 21.9 | 9.2 | 10.4 | 8.6 |
Hampton, Virginia. |
|||||||||
| 3 mean | 9.0 | 18.8 | 4.1 | 9.7 | 3.3 | 21.9 | 9.4 | 10.6 | 9.0 |
| max | 9.3 | 18.9 | 4.1 | 9.8 | 3.3 | 22.0 | 9.6 | 11.1 | 9.0 |
| min | 8.6 | 18.5 | 4.0 | 9.6 | 3.2 | 21.8 | 9.2 | 10.0 | 8.9 |
Zapus hudsonius americanus, Raleigh, North Carolina. [462] |
|||||||||
| 3 mean | 9.2 | 18.8 | 4.0 | 9.9 | 3.4 | 22.4 | 9.6 | 10.9 | 8.0 |
| max | 9.6 | 19.7 | 4.2 | 10.4 | 3.5 | 23.0 | 9.9 | 10.9 | 9.0 |
| min | 8.8 | 17.9 | 3.9 | 9.4 | 3.2 | 21.8 | 9.4 | 10.8 | 8.5 |
Zapus hudsonius campestris, 3 mi. NW Sundance, 5900 ft., Wyo. |
|||||||||
| 19 mean | 9.7 | 19.9 | 4.3 | 10.4 | 3.6 | 23.2 | 10.0 | 11.1 | 9.5 |
| max | 10.0 | 20.8 | 4.5 | 10.9 | 3.8 | 24.2 | 10.5 | 11.8 | 10.0 |
| min | 9.4 | 19.2 | 3.8 | 10.1 | 3.4 | 22.4 | 9.5 | 10.7 | 9.2 |
Palmer Gulch, Black Hills, South Dakota. |
|||||||||
| 11 mean | 9.8 | 20.2 | 4.3 | 10.5 | 3.7 | 23.4 | 10.1 | 11.4 | 9.6 |
| max | 10.2 | 21.4 | 4.5 | 11.1 | 3.9 | 24.9 | 10.9 | 12.0 | 10.2 |
| min | 9.5 | 19.0 | 4.2 | 10.1 | 3.5 | 21.9 | 9.5 | 10.7 | 9.0 |
Zapus hudsonius canadensis, St. Methode, Quebec. |
|||||||||
| 4 mean | 9.6 | 19.2 | 4.3 | 10.1 | 3.6 | 22.6 | 9.8 | 10.9 | 9.0 |
| max | 9.9 | 19.7 | 4.5 | 10.2 | 3.7 | 23.5 | 10.1 | 11.2 | 9.0 |
| min | 9.1 | 18.5 | 3.9 | 9.9 | 3.3 | 21.7 | 9.5 | 10.7 | 8.7 |
Pancake Bay, Algoma District, Ontario. |
|||||||||
| 11 mean | 9.6 | 18.8 | 4.1 | 10.0 | 3.5 | 22.2 | 9.6 | 10.4 | 9.2 |
| max | 10.0 | 19.4 | 4.4 | 10.3 | 3.6 | 22.8 | 9.7 | 10.6 | 9.7 |
| min | 9.2 | 18.3 | 3.8 | 9.6 | 3.3 | 21.8 | 9.2 | 9.8 | 8.6 |
Franz, Ontario. |
|||||||||
| 5 mean | 9.8 | 19.4 | 4.2 | 10.3 | 3.5 | 22.6 | 9.8 | 10.7 | 9.0 |
| max | 10.0 | 19.8 | 4.3 | 10.5 | 3.6 | 23.2 | 10.2 | 11.1 | 9.0 |
| min | 9.6 | 18.9 | 4.1 | 10.2 | 3.4 | 22.1 | 9.6 | 10.4 | 8.8 |
Zapus hudsonius hudsonius, Fort Severn, Ontario. |
|||||||||
| 4 mean | 9.9 | 19.3 | 4.2 | 9.9 | 3.5 | 22.7 | 9.6 | 10.9 | 9.0 |
| max | 10.1 | 19.7 | 4.3 | 10.1 | 3.6 | 23.3 | 9.8 | 11.0 | 9.0 |
| min | 9.8 | 19.0 | 4.0 | 9.7 | 3.4 | 22.0 | 9.3 | 10.7 | 9.0 |
Oxford House, Manitoba. |
|||||||||
| 6 mean | 9.6 | 19.1 | 4.4 | 9.9 | 3.5 | 22.3 | 9.7 | 10.4 | 9.0 |
| max | 9.9 | 19.8 | 4.6 | 10.1 | 3.7 | 23.1 | 10.0 | 10.8 | 9.0 |
| min | 9.3 | 18.7 | 4.2 | 9.6 | 3.3 | 21.7 | 9.5 | 9.8 | 8.8 |
Zapus hudsonius hudsonius, Emma Lake, Saskatchewan. [463] |
|||||||||
| 2 mean | 9.8 | 19.4 | 4.3 | 9.9 | 3.6 | 22.7 | 9.8 | 10.9 | 9.0 |
| max | 9.8 | 19.5 | 4.3 | 9.9 | 3.6 | 22.9 | 9.9 | 10.9 | 9.0 |
| min | 9.8 | 19.3 | 4.2 | 9.9 | 3.5 | 22.4 | 9.6 | 10.9 | 8.9 |
Lac la Nonne, Alberta. |
|||||||||
| 4 mean | 9.8 | 19.1 | 4.2 | 10.0 | 3.4 | 22.4 | 9.7 | 10.5 | 9.0 |
| max | 9.8 | 19.4 | 4.2 | 10.0 | 3.5 | 22.6 | 9.9 | 10.5 | 9.0 |
| min | 9.7 | 18.8 | 4.1 | 9.9 | 3.3 | 22.2 | 9.6 | 10.5 | 8.9 |
1 mi. NW Junct. Irons Cr. and Laird River, British Columbia. |
|||||||||
| 3 mean | 9.6 | 19.0 | 4.3 | 9.9 | 3.5 | 22.2 | 9.7 | 10.6 | 9.0 |
| max | 9.6 | 19.3 | 4.3 | 10.1 | 3.5 | 22.6 | 9.8 | 10.9 | 9.0 |
| min | 9.5 | 18.7 | 4.3 | 9.7 | 3.4 | 21.7 | 9.5 | 10.3 | 8.9 |
Zapus hudsonius intermedius, Blackner, North Dakota. |
|||||||||
| 2 mean | 10.1 | 19.6 | 4.3 | 10.0 | 3.6 | 22.7 | 9.9 | 11.1 | 9.0 |
| max | 10.1 | 20.0 | 4.4 | 10.4 | 3.7 | 23.2 | 9.9 | 11.4 | 9.0 |
| min | 10.0 | 19.1 | 4.1 | 9.8 | 3.4 | 22.2 | 9.8 | 10.8 | 9.0 |
Cannon Ball, North Dakota. |
|||||||||
| 2 mean | 9.8 | 19.1 | 4.4 | 10.1 | 3.6 | 22.0 | 9.5 | 11.0 | 9.0 |
| max | 9.9 | 19.1 | 4.4 | 10.2 | 3.7 | 22.4 | 9.8 | 11.3 | 9.0 |
| min | 9.6 | 19.0 | 4.3 | 9.9 | 3.4 | 21.8 | 9.2 | 10.7 | 9.0 |
Elk River, Minnesota. |
|||||||||
| 8 mean | 9.5 | 19.2 | 4.2 | 9.9 | 3.4 | 22.5 | 9.6 | 10.6 | 9.0 |
| max | 9.7 | 19.6 | 4.3 | 10.0 | 3.6 | 23.0 | 9.9 | 11.0 | 9.0 |
| min | 9.2 | 18.9 | 4.0 | 9.9 | 3.3 | 22.1 | 9.4 | 10.2 | 9.0 |
E Okeboji Lake, Iowa. |
|||||||||
| 3 mean | 9.6 | 19.3 | 4.2 | 9.9 | 3.4 | 22.2 | 9.3 | 10.2 | 9.0 |
| max | 9.8 | 19.3 | 4.2 | 10.0 | 3.4 | 22.2 | 9.3 | 10.2 | 9.0 |
| min | 9.4 | 19.3 | 4.2 | 9.8 | 3.4 | 22.2 | 9.2 | 10.2 | 9.0 |
Turkey Run State Park, Indiana. |
|||||||||
| 2 mean | 9.5 | 18.9 | 4.2 | 9.6 | 3.5 | 22.3 | 9.8 | 10.4 | 9.0 |
| max | 9.6 | 18.9 | 4.4 | 9.6 | 3.6 | 22.4 | 9.8 | 10.8 | 9.0 |
| min | 9.4 | 18.9 | 4.0 | 9.6 | 3.3 | 22.2 | 9.8 | 10.0 | 9.0 |
Zapus hudsonius intermedius, Jordan Cr., 3 mi. NE Fairmont, Ill. [464] |
|||||||||
| 5 mean | 9.6 | 19.4 | 4.1 | 10.1 | 3.6 | 22.6 | 10.0 | 10.7 | 9.2 |
| max | 9.9 | 19.8 | 4.2 | 10.3 | 3.8 | 23.4 | 10.5 | 11.6 | 9.6 |
| min | 9.3 | 18.9 | 4.0 | 10.0 | 3.4 | 21.9 | 9.5 | 10.3 | 8.9 |
Rib Hill, Wisconsin. |
|||||||||
| 5 mean | 9.6 | 19.0 | 4.3 | 10.1 | 3.4 | 22.6 | 9.7 | 10.8 | 9.0 |
| max | 10.0 | 19.8 | 4.4 | 10.5 | 3.6 | 23.7 | 10.2 | 11.2 | 9.3 |
| min | 9.4 | 18.4 | 4.1 | 9.7 | 3.2 | 21.9 | 9.4 | 10.3 | 8.7 |
Lake St. Germain, Wisconsin. |
|||||||||
| 5 mean | 9.7 | 18.9 | 4.1 | 10.1 | 3.5 | 22.5 | 9.6 | 10.5 | 9.0 |
| max | 9.9 | 19.5 | 4.3 | 10.2 | 3.6 | 23.2 | 10.0 | 10.8 | 9.3 |
| min | 9.5 | 18.4 | 3.9 | 9.8 | 3.3 | 21.8 | 9.0 | 10.2 | 8.5 |
Zapus hudsonius ladas, Northwest River, Labrador. |
|||||||||
| 6 mean | 9.5 | 19.0 | 4.2 | 10.2 | 3.6 | 22.4 | 9.7 | 10.9 | 9.0 |
| max | 9.6 | 20.0 | 4.4 | 10.3 | 3.6 | 23.2 | 10.2 | 11.0 | 9.4 |
| min | 9.3 | 18.4 | 4.0 | 10.0 | 3.5 | 21.5 | 9.4 | 10.6 | 8.8 |
Moisie Bay, Labrador. |
|||||||||
| 4 mean | 9.8 | 19.1 | 4.3 | 10.0 | 3.4 | 22.8 | 9.6 | 11.0 | 9.1 |
| max | 9.8 | 19.7 | 4.4 | 10.0 | 3.5 | 23.5 | 10.1 | 11.3 | 9.8 |
| min | 9.7 | 18.6 | 4.1 | 9.9 | 3.3 | 22.1 | 9.3 | 10.8 | 8.9 |
Zapus hudsonius pallidus, Mohawk Park, Oklahoma. |
|||||||||
| 2 mean | 9.7 | 18.4 | 4.3 | 9.9 | 3.7 | 21.5 | 9.3 | 11.0 | 8.7 |
| max | 10.1 | 18.8 | 4.3 | 9.9 | 3.7 | 21.8 | 9.4 | 11.0 | 8.7 |
| min | 9.3 | 18.0 | 4.3 | 9.8 | 3.7 | 21.1 | 9.2 | 11.0 | 8.6 |
vicinity of Lawrence, Kansas. |
|||||||||
| 10 mean | 9.2 | 18.8 | 4.4 | 9.8 | 3.4 | 21.6 | 9.7 | 10.9 | 9.0 |
| max | 9.5 | 19.4 | 4.8 | 10.2 | 3.6 | 22.4 | 10.1 | 11.6 | 9.4 |
| min | 8.9 | 18.1 | 4.0 | 9.3 | 3.3 | 21.0 | 9.0 | 10.3 | 8.8 |
2 mi. S Schuyler, Nebraska. |
|||||||||
| 1 | 9.2 | 18.5 | 4.4 | 9.5 | 3.3 | 21.5 | 9.6 | 10.4 | 9.1 |
| [465] | Zapus hudsonius pallidus, Valentine National Wildlife Refuge, Nebraska. |
||||||||
| 1 | 10.0 | 19.6 | 4.5 | 10.5 | 3.5 | 22.9 | 10.0 | 11.6 | 9.3 |
Zapus hudsonius preblei, Loveland, Colorado. |
|||||||||
| 2 mean | 9.6 | 18.5 | 4.1 | 10.0 | 3.6 | 22.3 | 9.6 | 10.4 | 9.1 |
| max | 9.6 | 19.0 | 4.2 | 10.0 | 3.6 | 22.4 | 9.8 | 10.6 | 9.1 |
| min | 9.5 | 18.0 | 3.9 | 10.0 | 3.6 | 22.2 | 9.4 | 10.2 | 9.1 |
Spring Hill, 12 mi. N Laramie Peak, 6300 ft., Wyoming. |
|||||||||
| 1 | 9.8 | 19.2 | 4.1 | 10.2 | 3.6 | 22.8 | 10.2 | 10.7 | 9.3 |
Zapus hudsonius tenellus, Hazelton, 959 ft., British Columbia. |
|||||||||
| 2 mean | 9.6 | 19.4 | 4.4 | 10.1 | 3.5 | 22.9 | 9.6 | 10.9 | 9.2 |
| max | 9.6 | 19.5 | 4.4 | 10.2 | 3.5 | 23.0 | 9.6 | 10.9 | 9.2 |
| min | 9.6 | 19.3 | 4.3 | 10.0 | 3.4 | 22.7 | 9.6 | 10.8 | 9.2 |
Cottonwood P. O., British Columbia. |
|||||||||
| 2 mean | 9.5 | 19.5 | 4.4 | 10.1 | 3.5 | 22.8 | 9.7 | 10.8 | 9.2 |
| max | 9.6 | 19.6 | 4.5 | 10.2 | 3.5 | 22.8 | 9.8 | 10.9 | 9.3 |
| min | 9.3 | 19.4 | 4.2 | 10.0 | 3.4 | 22.7 | 9.6 | 10.7 | 9.1 |
S end Swan Lake, British Columbia. |
|||||||||
| 2 mean | 9.4 | 19.7 | 4.1 | 10.0 | 3.6 | 22.9 | 10.1 | 10.9 | 9.5 |
| max | 9.4 | 19.7 | 4.2 | 10.2 | 3.6 | 23.2 | 10.2 | 10.9 | 9.6 |
| min | 9.3 | 19.6 | 4.0 | 9.8 | 3.5 | 22.6 | 10.0 | 10.8 | 9.4 |
Indianpoint Lake, 15 mi. NE Barkerville, British Columbia. |
|||||||||
| 4 mean | 9.6 | 18.9 | 4.1 | 9.9 | 3.4 | 21.9 | 9.5 | 10.8 | 9.0 |
| max | 9.6 | 19.6 | 4.2 | 10.0 | 3.6 | 23.0 | 9.8 | 11.3 | 9.4 |
| min | 9.4 | 18.3 | 4.0 | 9.8 | 3.3 | 21.3 | 9.1 | 10.2 | 8.8 |
Allen, J. A.
1894. Cranial variation in Neotoma micropus due to growth and individual differentiation. Bull. Amer. Mus. Nat. Hist., 6:233-246, 1 pl., August 3, 1894.
Anderson, R. M.
1932. Five new mammals from British Columbia. Ann. Rept. 1931, Nat. Mus. Canada: 99-119, 1 pl., November 24, 1932.
1942. Six additions to the list of Quebec mammals with descriptions of four new forms. Ann. Rept. Prov. Soc. Nat. Hist. for 1941:31-42, July 14, 1942.
Axelrod, D. I.
1948. Climate and evolution in western North America during Middle Pliocene time. Evol., 2:127-144, July 2, 1948.
Bailey, B.
1929. Mammals of Sherburne County, Minnesota. Jour. Mamm., 10:153-164, May 9, 1929.
Bailey, J. W.
1946. The mammals of Virginia. Williams Printing Company, Richmond, Virginia, xii + 416 pp., 96 figs. in text, December, 1946.
Bailey, V.
1923. Mammals of the District of Columbia. Proc. Biol. Soc. Washington, 36:103-138, May 1, 1923.
1927. A biological survey of North Dakota. N. Amer. Fauna, 49:vi + 226 pp., 1 map, 21 pls., 8 figs. in text, January 8, 1927.
1932. Mammals of New Mexico. N. Amer. Fauna, 53:1-412, 22 pls., 58 figs. in text, March 1, 1932.
1936. The mammals and life zones of Oregon. N. Amer. Fauna, 55:1-416, 1 map, 52 pls., 102 figs. in text, August 29, 1936.
Batchelder, C. F.
1899. Some unrecognized jumping mice of the genus Zapus. Proc. New England Zool. Club, 1:3-7, February 8, 1899.
Blair, F. W.
1940. Home ranges and populations of the jumping mouse. Amer. Midland Nat., 23:244-250, 1 table, January, 1940.
Bole, B. P., Jr., and Moulthrop, P. N.
1942. The Ohio Recent mammal collection in the Cleveland Museum of Natural History. Scientific Publs., Cleveland Mus. Nat. Hist., 5:83-181, September 11, 1942.
Borell, A., and Ellis, R.
1934. Mammals of the Ruby Mountains region of northeastern Nevada. Jour. Mamm., 15:12-44, 6 pls., 1 fig. in text, 4 tables, February 15, 1934.
Brimley, C. S.
1923. Breeding dates of small mammals at Raleigh, North Carolina. Jour. Mamm., 4:263-264, November 1, 1923.
Carl, G. C., Guiguet, C. J., and Hardy, G. A.
1952. A natural history survey of the Manning Park area British Columbia. Occ. Papers British Columbia Prov. Mus., 9:1-130, 22 figs. in text, July, 1952.
Christian, J. J.
1936. Mammals caught in post holes. Jour. Mamm., 17:416, November 16, 1936.
Cockrum, E. L., and Baker, R. H.
1950. A new jumping mouse (genusi Zapus) from Kansas. Proc. Biol. Soc. Washington, 63:1-4, 1 fig. in text, April 26, 1950.
Coleman, R. H.
1941. Zapus hudsonius americanus in South Carolina. Jour. Mamm., 22:91, February 14, 1941.
Cope, E. D.
1871. Preliminary report on the Vertebrata discovered in the Port Kennedy Bone Cave. Proc. American Philos. Soc., 12:73-102, 20 figs., April 7, 1871.
Cory, C. B.
1912. The mammals of Illinois and Wisconsin. Field Mus. Nat. Hist. Publ. 153, zool. ser., 11:1-505, many unnumbered pls., figs. and maps in text, 1912.
Crowe, P. E.
1943. Notes on some mammals of the southern Canadian Rocky Mountains. Bull. Amer. Mus. Nat. Hist., 80:391-410, 4 pls., 1 fig. in text, February 4, 1943.
Dalquest, W. W.
1948. Mammals of Washington. Univ. Kansas Publ., Mus. Nat. Hist., 2:1-444, 140 figs. in text, April 9, 1948.
Davis, W. B.
1939. The Recent mammals of Idaho. The Caxton Printers, Caldwell, Idaho, 444 pp., 2 full page half tones, 33 figs. in text, April 5, 1939.
Dearborn, N.
1932. Foods of some predatory fur-bearing animals of Michigan. Univ. Michigan School of Forestry and Conservation, Bull. 1:1-52, 8 figs., 22 charts, 10 maps, 1932.
Dice, L. R.
1932. Mammals collected by F. M. Gaige in 1919 at Lake Cushman and vicinity, Olympic Peninsula, Washington. Murrelet, 13:47-49, May, 1932.
Durrant, S. D.
1952. Mammals of Utah, taxonomy and distribution. Univ. Kansas Publ., Mus. Nat. Hist., 6, 1-549, 91 figs. in text, 30 tables, August 10, 1952.
Eadie, R. W. 1949. Hibernating meadow jumping mouse. Jour. Mamm., 30:307-308, August 17, 1949.
Edson, J. M.
1932. Hibernation of the northwest jumping mouse. Murrelet, 13:55-56, May, 1932.
Ellerman, J. R.
1940. The families and genera of living Rodents. British Mus. (Nat. Hist.), Vol. 1, pp. xxvi + 689, 189 figs., June 8, 1940.
Elliot, D. G.
1898. Lists of species of mammals principally rodents obtained by W. W. Price, Dr. S. E. Meek, G. R. Cherrie, and E. S. Thompson in the states of Iowa, Wyoming, Montana, Idaho, Nevada, and California with descriptions of new species. Field Columbian Mus. Publ. 27, zool. ser. 1:193-221, March, 1898.
1899. Catalogue of mammals from the Olympic Mountains, Washington with descriptions of new species. Field Columbian Mus. Publ. 32, zool. ser., 1:241-276, 21 pls., 13 unnumbered figs. in text, March, 1899.
Erickson, A. B.
1938. Parasites of some Minnesota rodents. Jour. Mamm., 19:252-253, May 12, 1938.
Flahaut, M. R.
1939. Unusual location of hibernating jumping mice. Murrelet, 20:17-18, 1 unnumbered pl., April 30, 1939.
Gidley, I. W., and Gazin, C. L.
1938. The Pleistocene vertebrate fauna from Cumberland Cave, Maryland. U. S. Nat. Mus. Bull., 171:1-93, 50 figs., 25 tables, January 25, 1938.
Goodwin, G. G.
1924. Mammals of the Gaspé Peninsula, Quebec. Jour. Mamm., 5:246-257, 2 pls., November 15, 1924.
1935. The mammals of Connecticut. Bull. Connecticut State Geol. and Nat. Hist. Surv., 53:1-221, 33 pls., 19 figs. in text, 1935.
Grinnell, J., Dixon, J., and Linsdale, J. M.
1930. Vertebrate natural history of a section of northern California through the Lassen Peak Region. Univ. California Publ. Zool., 35:v + 594, 181 figs. in text, October 10, 1930.
1937. Fur-bearing mammals of California…. Univ. California Press, 2 vols., xii + 375, pls. 1-7, figs. 1-138, xiv + 377-777, pls. 8-13, figs. 139-345, July 22, 1937.
Grizzell, R. A., Jr.
1949. Hibernating jumping mice in woodchuck dens. Jour. Mamm., 30:74-75, February 14, 1949.
Hall, E. R.
1930. Rodents and lagomorphs from the Later Tertiary of Fish Lake Valley, Nevada. Univ. California Publ. Bull. Dept. Geol. Sci., 19:295-312, 1 pl., 29 figs. in text, November 25, 1930.
1931. Critical comments on mammals from Utah with descriptions of new forms from Utah, Nevada and Washington. Univ. California Publ. Zool., 37:1-13, April 10, 1931.
1934. Mammals collected by T. T. and E. B. McCabe in the Bowron Lake Region of British Columbia. Univ. California Publ. Zool., 40:363-386, 1 fig. in text, November 5, 1934.
1946. Mammals of Nevada. Univ. California Press, Berkeley, xi + 710, colored frontispiece, 11 pls., 485 figs. in text, plus unnumbered silhouettes and maps, July 1, 1946.
Hall, E. R., and Davis, W. B.
1934. Notes on Arizona rodents. Proc. Biol. Soc. Washington, 47:51-56, 1 fig. in text, February 9, 1934.
Hamilton, W. J.
1935. Habits of jumping mice. Amer. Midland Nat., 16:187-200, 1 pl., 2 figs. in text, 1935.
Handley, C. O., Jr., and Patton, C. P.
1947. Wild mammals of Virginia. Commonwealth of Virginia, Comm. Game and Inland Fisheries, Richmond, vi + 220 pp., frontispiece, 103 figs. in text, 1947.
Harper, F.
1932. Mammals of the Athabaska and Great Slave lakes region. Jour. Mamm., 13:19-36, 3 pls., February 9, 1932.
Hausman, L. A.
1920. Structural characteristics of the hair of mammals. Amer. Nat., 54:496-523, 7 pls., November-December, 1920.
Hibbard, C. W.
1941. The Borchers Fauna a new Pleistocene interglacial fauna from Meade County, Kansas. Univ. Kansas Publ. State Geol. Surv. Kansas, Bull., 38:197-220, 2 pls., July 14, 1941.
1951. A new jumping mouse from the Upper Pliocene of Kansas. Jour. Mamm., 32:351-352, 1 fig. in text, August 23, 1951.
Hoffmeister, D. F.
1951. A taxonomic and evolutionary study of the piñon mouse, Peromyscus truei. Illinois Biol. Monog., 21:ix + 104 pp., 5 pls., 24 figs. in text, 7 tables, November 12, 1951.
Hollister, N.
1912. Mammals of the Alpine Club expedition to the Mount Robson Region. Alpine Club of Canada, Spec. No.:1-44, 13 pls. in text, 1912.
Hooper, E. T.
1944. San Francisco Bay as a factor influencing speciation in rodents. Univ. Michigan, Mus. Zool., Miscl. Publ., 59:1-89, 5 pls., 18 maps, January 12, 1944.
1952. A systematic review of the harvest mice (genus Reithrodontomys) of Latin America. Miscl Publ. Mus. Zool. Univ. Michigan, 77:1-255, 9 pls., 24 figs. in text, 7 tables, 12 maps, January 16, 1952.
Howell, A. B.
1920. A study of the California jumping mice of the genus Zapus. Univ. California Publ. Zool., 21:225-238, 1 fig. in text, May 20, 1920.
Ivor, H. R.
1934. Notes on the rearing of captive young meadow jumping mice. Canadian Field-Nat., 48:8-10, January 15, 1934.
Kellogg, L.
1916. Report upon mammals and birds found in portions of Trinity, Siskiyou and Shasta counties, California. Univ. California Publ. Zool., 12:335-398, 4 pls., 1 fig., January 27, 1916.
Linsdale, J. M.
1938. Environmental responses of vertebrates in the Great Basin. Amer. Midland Nat., 19:1-206, 12 figs. in text, January, 1938.
Lyon, M. W., Jr.
1938. Mammals of Indiana. Amer. Midland Nat., 17:1-384, 125 figs. in text, 85 maps, January, 1936.
Matthew, W. D.
1915. Climate and evolution. New York Acad. Sci., 24:171-318, 33 figs., 17 tables, February 18, 1915.
Mayr, E.
1942. Systematics and the origin of species from the viewpoint of a zoologist. Columbia Univ. Press, New York, xiv + 334 pp., 29 figs., 1942.
Merriam, C. H.
1897a. Three new jumping mice (Zapus) from the Northwest. Proc. Biol. Soc. Washington, 11:103-104, April 26, 1897.
1897b. Mammals of Mount Mazama, Oregon. Mazama, 1:204-230, 10 pls., 1 map, October, 1897.
Miller, G. S., Jr.
1899. Preliminary list of New York mammals. Bull. New York State Mus., 6:273-390, November 18, 1899.
1911. A new jumping mouse from New Mexico. Proc. Biol. Soc. Washington, 24:253-254, December 23, 1911.
Moojen, J.
1948. Speciation in the Brazilian spiny rats (genus Proechimys, family Echimyidae). Univ. Kansas Publ. Mus. Nat. Hist., 1:301-406, 140 figs. in text, 1 table, December 10, 1948.
Moore, A. W.
1928. Zapus princeps princeps in Utah. Jour. Mamm., 9:154-155, May 9, 1928.
Nicholson, A. J.
1937. A hibernating jumping mouse. Jour. Mamm., 18:103, February 11, 1937.
Pearson, O. P., and Pearson, A. K.
1947. Owl predation in Pennsylvania, with notes on the small mammals of Delaware County. Jour. Mamm., 28:137-147, 1 fig., 3 tables, June 1, 1947.
Petrides, G. A.
1948. The jumping mouse in Georgia. Jour. Mamm., 29:75-76, February 13, 1948.
Preble, E. A.
1899. Revision of the jumping mice of the genus Zapus. N. Amer. Fauna, 15:1-42, 1 pl., 4 figs. in text, August 8, 1899.
Preble, N. A.
1944. A swimming jumping mouse. Jour. Mamm., 25:200-201, May 26, 1944.
Quimby, D. C.
1951. The life history and ecology of the jumping mouse, Zapus hudsonius. Ecol. Monog., 21:61-95, 14 figs. in text, 7 tables, January, 1951.
Ridgway, R.
1912. Color standards and color nomenclature. Washington, D. C., privately printed, iv + 44 pp., 53 pls., 1912.
Schmidt, F. J. W.
1931. Mammals of western Clark County, Wisconsin. Jour. Mamm., 12:99-117, 1 map, May 14, 1931.
Schwartz, C. W.
1951. A new record of Zapus hudsonius in Missouri and notes on its hibernation. Jour. Mamm., 32:227-228, May 21, 1951.
Sheldon, C.
1934. Studies of the life histories of Zapus and Napaeozapus in Nova Scotia. Jour. Mamm., 15:290-300, November 15, 1934.
1938. Vermont jumping mice of the genus Zapus. Jour. Mamm., 19:324-332, 4 figs. in text, August 18, 1938.
Simpson, G. G.
1947. Holarctic mammalian faunas and continental relationships during the Cenozoic. Bull. Geol. Soc. America, 58:613-688, 6 figs. in text, 9 tables, July, 1947.
Smith, C. F., and Hopkins, C. L.
1937. Notes on the barn owls of the San Francisco Bay Region. Condor, 39:189-191, September 15, 1937.
Stanford, J. S.
1931. Notes on small mammals of Utah. Jour. Mamm., 12:356-363, November 11, 1931.
Stehlin, H. G., and Schaub, S.
1951. Die Trigondontie der simplicidentaten Nager. Schweizerischen Paleont. Abhandl., Basel, 67:1-385, 620 text figuren.
Stoner, D.
1918. The rodents of Iowa. Iowa Geol. Surv., Bull., 5:1-172, 37 figs. in text, 1918.
Surface, H. A.
1906. The serpents of Pennsylvania. Monthly Bull. Div. Zool., Pennsylvania State Dept. Agric., 4:113-208, pls. 15-42, 23 figs. in text, August and September, 1906.
Svihla, A., and Svihla, R. D.
1933. Notes on the jumping mouse Zapus trinotatus trinotatus Rhoads. Jour. Mamm., 14:131-134, May 15, 1933.
Svihla, R. D.
1931. Mammals of the Uinta Mountain Region. Jour. Mamm., 12:256-266, 1 pl., 1 fig. in text, August 24, 1931.
Taylor, W. P.
1911. Mammals of the Alexander Nevada Expedition of 1909. Univ. California Publ. Zool., 7:205-307, 2 figs. in text, June 24, 1911.
1922. A distributional and ecological study of Mount Rainier, Washington. Ecol., 3:213-236, 4 figs. in text, July, 1922.
Test, F. H., and Test, A.
1943. Incidence of dipteran parasitosis in populations of small mammals. Jour. Mamm., 24:506-508, November 20, 1943.
Townsend, M. T.
1935. Studies on some of the small mammals of central New York. Roosevelt Wildlife Annals, 4(No. 1):1-120, 8 pls., 22 figs. in text, 35 tables, 4 maps, December, 1935.
Vergeer, T.
1948. Frog catches mouse in natural environment. Turtox News, 26:91, March, 1948.
Vinogradov, B. S.
1925. On the structure of the external genitalia in Dipodidae and Zapodidae (Rodentia) as a classificatory character. Proc. Zool. Soc. London, Part 1:577-585, 6 pls., 1925.
Whitlow, W. B., and Hall, E. R.
1933. Mammals of the Pocatello Region of southeastern Idaho. Univ. California Publ. Zool., 40:235-276, 3 figs. in text, September 30, 1933.
Williams, C. S.
1938. Aids to the identification of mole and shrew hairs with general comments on hair structure and hair determination. Jour. Wildl. Mgt., 2:239-250, 1 pl., 9 figs. in text, October, 1943.
Wilson, R. W.
1936. A Pliocene rodent fauna from Smiths Valley, Nevada. Carnegie Inst. Publ., 473:15-34, 2 pls., May 21, 1936.
1937. Pliocene rodents of western North America. Carnegie Inst. Publ., 487:21-73, 2 figs. in text, July 23, 1937.
Transmitted October 9, 1953.
UNIVERSITY OF KANSAS PUBLICATIONS, MUSEUM OF NATURAL HISTORY
Institutional libraries interested in publications exchange may obtain this series by addressing the Exchange Librarian, University of Kansas Library, Lawrence, Kansas. Copies for individuals, persons working in a particular field of study, may be obtained by addressing instead the Museum of Natural History, University of Kansas, Lawrence, Kansas. There is no provision for sale or this series by the University Library which meets institutional requests, or by the Museum of Natural History which meets the requests of individuals. However, when individuals request copies from the Museum, 25 cents should be included, for each separate number that is 100 pages or more in length, for the purpose of defraying the costs of wrapping and mailing.
* An asterisk designates those numbers of which the Museum’s supply (not the Library’s supply) is exhausted. Numbers published to date, in this series, are as follows:
| Vol. 1. | 1. | The pocket gophers (Genus Thomomys) of Utah. By Stephen D. Durrant. Pp. 1-82, 1 figure in text. August 15, 1946. |
| 2. | The systematic status of Eumeces pluvialis Cope, and noteworthy records of other amphibians and reptiles from Kansas and Oklahoma. By Hobart M. Smith. Pp. 85-89. August 15, 1946. | |
| 3. | The tadpoles of Bufo cognatus Say. By Hobart M. Smith. Pp. 93-96, 1 figure in text. August 15, 1946. | |
| 4. | Hybridization between two species of garter snakes. By Hobart M. Smith. Pp. 97-100. August 15, 1946. | |
| 5. | Selected records of reptiles and amphibians from Kansas. By John Breukelman and Hobart M. Smith. Pp. 101-112. August 15, 1946. | |
| 6. | Kyphosis and other variations in soft-shelled turtles. By Hobart M. Smith. Pp. 117-124, 3 figures in text. July 7, 1947. | |
| 7. | Natural history of the prairie vole (Mammalian Genus Microtus). By E. W. Jameson, Jr. Pp. 125-151, 4 figures in text. October 6, 1947. | |
| 8. | The postnatal development of two broods of great horned owls (Bubo virginianus). by Donald F. Hoffmeister and Henry W. Setzer. Pp. 157-173, 5 figures in text. October 6, 1947. | |
| 9. | Additions to the list of the birds of Louisiana. By George H. Lowery, Jr. Pp. 177-192. November 7, 1947. | |
| 10. | A check-list of the birds of Idaho. By M. Dale Arvey. Pp. 193-216. November 29, 1947. | |
| 11. | Subspeciation in pocket gophers of Kansas. By Bernardo Villa-R. and E. Raymond Hall. Pp. 217-236, 2 figures in text. November 29, 1947. | |
| 12. | A new bat (Genus Myotis) from Mexico. By Walter W. Dalquest and E. Raymond Hall. Pp. 237-244, 6 figures in text. December 10, 1947. | |
| 13. | Tadarida femorosacca (Merriam) in Tamaulipas, Mexico. By Walter W. Dalquest and E. Raymond Hall. Pp. 245-248, 1 figure in text. December 10, 1947. | |
| 14. | A new pocket gopher (Thomomys) and a new spiny pocket mouse (Liomys) from Michoacán, Mexico. By E. Raymond Hall and Bernardo Villa-R. Pp. 249-256, 6 figures in text. July 26, 1948. | |
| 15. | A new hylid frog from eastern Mexico. By Edward H. Taylor. Pp. 257-264, 1 figure in text. August 16, 1948. | |
| 16. | A new extinct emydid turtle from the Lower Pliocene of Oklahoma. By Edwin C. Galbreath. Pp. 265-280, 1 plate. August 16, 1948. | |
| 17. | Pliocene and Pleistocene records of fossil turtles from western Kansas and Oklahoma. By Edwin C. Galbreath. Pp. 281-284. August 16, 1948. | |
| 18. | A new species of heteromyid rodent from the Middle Oligocene of northeastern Colorado with remarks on the skull. By Edwin C. Galbreath. Pp. 285-300, 2 plates. August 16, 1948. | |
| 19. | Speciation in the Brazilian spiny rats (Genus Proechimys, Family Echimyidae). By João Moojen. Pp. 301-406, 140 figures in text. December 10, 1948. | |
| 20. | Three new beavers from Utah. By Stephen D. Durrant and Harold S. Crane. Pp. 407-417, 7 figures in text. December 24, 1948. | |
| 21. | Two new meadow mice from Michoacán, Mexico. By E. Raymond Hall. Pp. 423-427, 6 figures in text. December 24, 1948. | |
| 22. | An annotated check list of the mammals of Michoacán, Mexico. By E. Raymond Hall and Bernardo Villa-R. Pp. 431-472, 2 plates, 1 figure in text. December 27, 1949. | |
| 23. | Subspeciation in the kangaroo rat, Dipodomys ordii. By Henry W. Setzer. Pp. 473-573, 27 figures in text, 7 tables. December 27, 1949. | |
| 24. | Geographic range of the hooded skunk, Mephitis macroura, with description of a new subspecies from Mexico. By E. Raymond Hall and Walter W. Dalquest. Pp. 575-580, 1 figure in text. January 20, 1950. | |
| 25. | Pipistrellus cinnamomeus Miller 1902 referred to the Genus Myotis. By E. Raymond Hall and Walter W. Dalquest. Pp. 581-590, 5 figures in text. January 20, 1950. | |
| 26. | A synopsis of the American bats of the Genus Pipistrellus. By E. Raymond Hall and Walter W. Dalquest. Pp. 591-602, 1 figure in text. January 20, 1950. | |
| Index. Pp. 605-638. | ||
| *Vol. 2. | (Complete) Mammals of Washington. By Walter W. Dalquest. Pp. 1-444, 140 figures in text. April 9, 1948. | |
| Vol. 3. | *1. | The avifauna of Micronesia, its origin, evolution, and distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June 12, 1951. |
| *2. | A quantitative study of the nocturnal migration of birds. By George H. Lowery, Jr. Pp. 361-472, 47 figures in text. June 29, 1951. | |
| 3. | Phylogeny of the waxwings and allied birds. By M. Dale Arvey. Pp. 473-530, 49 figures in text, 13 tables. October 10, 1951. | |
| 4. | Birds from the state of Veracruz, Mexico. By George H. Lowery, Jr. and Walter W. Dalquest. Pp. 531-649, 7 figures in text, 2 tables. October 10, 1951. | |
| Index. Pp. 651-681. | ||
| *Vol. 4. | (Complete) American weasels. By E. Raymond Hall. Pp. 1-466, 41 plates, 31 figures in text. December 27, 1951. | |
| Vol. 5. | 1. | Preliminary survey of a Paleocene faunule from the Angels Peak area, New Mexico. By Robert W. Wilson. Pp. 1-11, 1 figure in text. February 24, 1951. |
| 2. | Two new moles (Genus Scalopus) from Mexico and Texas. By Rollin H. Baker. Pp. 17-24. February 28, 1951. | |
| 3. | Two new pocket gophers from Wyoming and Colorado. By E. Raymond Hall and H. Gordon Montague. Pp. 25-32. February 28, 1951. | |
| 4. | Mammals obtained by Dr. Curt von Wedel from the barrier beach of Tamaulipas, Mexico. By E. Raymond Hall. Pp. 33-47, 1 figure in text. October 1, 1951. | |
| 5. | Comments on the taxonomy and geographic distribution of some North American rabbits. By E. Raymond Hall and Keith R. Kelson. Pp. 49-58. October 1, 1951. | |
| 6. | Two new subspecies of Thomomys bottae from New Mexico and Colorado. By Keith R. Kelson. Pp. 59-71, 1 figure in text. October 1, 1951. | |
| 7. | A new subspecies of Microtus montanus from Montana and comments on Microtus canicaudus Miller. By E. Raymond Hall and Keith R. Kelson. Pp. 73-79. October 1, 1951. | |
| 8. | A new pocket gopher (Genus Thomomys) from eastern Colorado. By E. Raymond Hall. Pp. 81-85. October 1, 1951. | |
| 9. | Mammals taken along the Alaskan Highway. By Rollin H. Baker. Pp. 87-117, 1 figure in text. November 28, 1951. | |
| *10. | A synopsis of the North American Lagomorpha. By E. Raymond Hall. Pp. 119-202, 68 figures in text. December 15, 1951. | |
| 11. | A new pocket mouse (Genus Perognathus) from Kansas. By E. Lendell Cockrum. Pp. 203-206. December 15, 1951. | |
| 12. | Mammals from Tamaulipas, Mexico. By Rollin H. Baker. Pp. 207-218. December 15, 1951. | |
| 13. | A new pocket gopher (Genus Thomomys) from Wyoming and Colorado. By E. Raymond Hall. Pp. 219-222. December 15, 1951. | |
| 14. | A new name for the Mexican red bat. By E. Raymond Hall. Pp. 223-226. December 15, 1951. | |
| 15. | Taxonomic notes on Mexican bats of the Genus Rhogeëssa. By E. Raymond Hall. Pp. 227-232. April 10, 1952. | |
| 16. | Comments on the taxonomy and geographic distribution of some North American woodrats (Genus Neotoma). By Keith R. Kelson. Pp. 233-242. April 10, 1952. | |
| 17. | The subspecies of the Mexican red-bellied squirrel, Sciurus aureogaster. By Keith R. Kelson. Pp. 243-250, 1 figure in text. April 10, 1952. | |
| 18. | Geographic range of Peromyscus melanophrys, with description of new subspecies. By Rollin H. Baker. Pp. 251-258, 1 figure in text. May 10, 1952. | |
| 19. | A new chipmunk (Genus Eutamias) from the Black Hills. By John A. White. Pp. 259-262. April 10, 1952. | |
| 20. | A new piñon mouse (Peromyscus truei) from Durango, Mexico. By Robert B. Finley, Jr. Pp. 263-267. May 23, 1952. | |
| 21. | An annotated check-list of Nebraskan bats. By Olin L. Webb and J. Knox Jones, Jr. Pp. 269-279. May 31, 1952. | |
| 22. | Geographic variation in red-backed mice (Genus Clethrionomys) of the southern Rocky Mountain region. By E. Lendell Cockrum and Kenneth L. Fitch. Pp. 281-292, 1 figure in text. November 15, 1952. | |
| 23. | Comments on the taxonomy and geographic distribution of North American microtines. By E. Raymond Hall and E. Lendell Cockrum. Pp. 293-312. November 17, 1952. | |
| 24. | The subspecific status of two Central American sloths. By E. Raymond Hall and Keith R. Kelson. Pp. 313-337. November 21, 1952. | |
| 25. | Comments on the taxonomy and geographic distribution of some North American marsupials, insectivores, and carnivores. By E. Raymond Hall and Keith R. Kelson. Pp. 319-341. December 5, 1952. | |
| 26. | Comments on the taxonomy and geographic distribution of some North American rodents. By E. Raymond Hall and Keith R. Kelson. Pp. 343-371. December 15, 1952. | |
| 27. | A synopsis of the North American microtine rodents. By E. Raymond Hall and E. Lendell Cockrum. Pp. 373-498, 149 figures in text. January 15, 1953. | |
| 28. | The pocket gophers (Genus Thomomys) of Coahuila, Mexico. By Rollin H. Baker. Pp. 499-514, 1 figure in text. June 1, 1953. | |
| 29. | Geographic distribution of the pocket mouse, Perognathus fasciatus. By J. Knox Jones, Jr. Pp. 515-526, 7 figures in text. August 1, 1953. | |
| 30. | A new subspecies of wood rat (Neotoma mexicana) from Colorado. By Robert B. Finley, Jr. Pp. 527-534, 2 figures in text. August 15, 1953. | |
| 31. | Four new pocket gophers of the genus Cratogeomys from Jalisco, Mexico. By Robert J. Russell. Pp. 535-542. October 15, 1953. | |
| 32. | Genera and subgenera of chipmunks. By John A. White. Pp. 543-561, 12 figures in text. December 1, 1953. | |
| 33. | Taxonomy of the chipmunks, Eutamias quadrivittatus and Eutamias umbrinus. By John A. White. Pp. 563-582, 6 figures in text. December 1, 1953. | |
| 34. | Geographic distribution and taxonomy of the chipmunks of Wyoming. By John A. White. Pp. 584-610, 3 figures in text. December 1, 1953. | |
| 35. | The baculum of the chipmunks of western North America. By John A. White. Pp. 611-631, 19 figures in text. December 1, 1953. | |
| 36. | Pleistocene Soricidae from San Josecito Cave, Nuevo Leon, Mexico. By James S. Findley. Pp. 633-639. December 1, 1953. | |
| 37. | Seventeen species of bats recorded from Barro Colorado Island, Panama Canal Zone. By E. Raymond Hall and William B. Jackson. Pp. 641-646. December 1, 1953. | |
| Index. Pp. 647-676. | ||
| *Vol. 6. | (Complete) Mammals of Utah, taxonomy and distribution. By Stephen D. Durrant. Pp. 1-549, 91 figures in text, 30 tables. August 10, 1952. | |
| Vol. 7. | *1. | Mammals of Kansas. By E. Lendell Cockrum. Pp. 1-303, 73 figures in text, 37 tables. August 25, 1952. |
| 2. | Ecology of the opossum on a natural area in northeastern Kansas. By Henry S. Fitch and Lewis L. Sandidge. Pp. 305-338, 5 figures in text. August 24, 1953. | |
| 3. | The silky pocket mice (Perognathus flavus) of Mexico. By Rollin H. Baker. Pp. 339-347, 1 figure in text. February 15, 1954. | |
| 4. | North American jumping mice (Genus Zapus). By Philip H. Krutzsch. Pp. 349-472, 47 figures in text, 4 tables. April 21, 1954. | |
| More numbers will appear in Volume 7. | ||
Transcriber’s Notes
All obvious typos corrected. In Table 5 on the Swan Lake row, the Mean value for the Palatal length was corrected to 10.1 mm as there were only two values averaged (10.0 and 10.2). Abbreviation inconsistencies for mountain(s) were retained. Where a publication name contains an alternate spelling of a word, it was retained (example, Athabaska). The author Bernardo Villa-Ramirez is sometimes listed with the hyphen and sometimes without. For consistancy, they were standardized with a hyphen.
Typographical Corrections
End of the Project Gutenberg EBook of North American Jumping Mice (Genus
Zapus), by Philip H. Krutzsch
*** END OF THIS PROJECT GUTENBERG EBOOK NORTH AMERICAN JUMPING MICE ***
***** This file should be named 40110-h.htm or 40110-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/4/0/1/1/40110/
Produced by Chris Curnow, Joseph Cooper, Tom Cosmas and
the Online Distributed Proofreading Team at
http://www.pgdp.net
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation information page at www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at 809
North 1500 West, Salt Lake City, UT 84116, (801) 596-1887. Email
contact links and up to date contact information can be found at the
Foundation's web site and official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For forty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.