
The Project Gutenberg EBook of Ecological Observations on the Woodrat,
Neotoma floridana, by Henry S. Fitch and Dennis G. Rainey
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
Title: Ecological Observations on the Woodrat, Neotoma floridana
Author: Henry S. Fitch
Dennis G. Rainey
Release Date: August 29, 2010 [EBook #33566]
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK ECOLOGICAL OBSERVATIONS--WOODRAT ***
Produced by Chris Curnow, Joseph Cooper, Josephine Paolucci
and the Online Distributed Proofreading Team at
https://www.pgdp.net.
University of Kansas Publications
Museum of Natural History
Volume 8, No. 9, pp. 499-533, 3 figs.
June 12, 1956
University of Kansas
Lawrence
1956
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, A. Byron Leonard,
Robert W. Wilson
Volume 8, No. 9, pp. 499-533
Published June 12, 1956
University of Kansas
Lawrence, Kansas
PRINTED BY
FERD VOILAND, JR., STATE PRINTER
TOPEKA, KANSAS
1956
The eastern woodrat exerts important effects on its community associates by its use of the vegetation for food, by providing shelter in its stick houses for many other small animals, and by providing a food supply for certain flesh-eaters. In the course of our observations on this rodent on the University of Kansas Natural History Reservation, extending over an eight-year period, from February, 1948, to February, 1956, these effects have changed greatly as the population of woodrats has constantly changed in density, and in extent of the area occupied.
This report is concerned with the population of woodrats on the Reservation, the changes that the species has undergone, and the factors that have affected it. Our two sets of field data, used as a basis for this report, supplement each other and overlap little, either in time or space. Fitch's field work which covered approximately the western half of the Reservation, was begun in September, 1948, and was pursued most intensively in the autumn of 1948 and in 1949, with relatively small amounts of data obtained in 1950 and 1951 because of the great reduction in numbers of rats. Rainey's field work began in the spring of 1951 and was continued through 1954, concentrating on a colony in the extreme northwestern corner of the Reservation and on adjacent privately owned land. In actual numbers of rats live-trapped and for total number of records the two sets of data are comparable. Fitch's field work consisted chiefly of live-trapping while Rainey's relied also upon various other approaches to the woodrat's ecology. Rainey's findings were incorporated originally in a more comprehensive report (1956), from which short passages have been extracted that are most pertinent to the present discussion. Our combined data represent 258 woodrats (153 Fitch's and 105 Rainey's) caught a total of 1110 times (660 Rainey's and 450 Fitch's). Rainey's records pertain, in part, to woodrats outside the Reservation but within a few hundred yards, at most, of its boundaries.[Pg 502]
In the autumn of 1948 the population of woodrats was far below the level it had attained in 1947 or earlier, but the rats were still abundant and distributed throughout a variety of habitats. Almost every part of the woodland was occupied by at least a sparse population. Also, many rats lived beyond the limits of the woodland proper, in such places as deserted buildings, thickets, roadside hedges, and tangles of exposed tree roots along cut banks of gullies. All these situations are characterized by providing abundant cover, a limiting factor for this woodrat.
In 1947, when the population of woodrats was especially high, plant succession on the wooded parts of the Reservation may have been near the optimum stage for the rats. For some 80 years, since the time the land was first settled and prairie fires were brought under control, woody vegetation has been encroaching into areas that were formerly grassland.
About 1934 the University changed its policy with regard to treatment of the tract that was later made the Reservation. Up to that time, most of the area had been used as pasture and subjected to heavy grazing, but several fields had been fenced and cultivated. Under the new policy the hillsides and hilltop edges with open stands of various deciduous trees were enclosed with stock fences and protected from grazing. Successional trends were greatly altered. Woody vegetation, already favored by protection from the prairie fires originally important in the ecology of this region underwent further development as a result of protection from browsing. Thickets of shrubs and saplings sprang up throughout the woodland, forming a dense understory layer beneath the discontinuous canopy of the relatively scattered mature trees. The composition and density of the undergrowth varied markedly in different parts of the woodland. The parts that were formerly most open acquired the most dense understory. Blackberry, honey locust, osage orange, and prickly ash formed in places thorny tangles almost impenetrable to humans. This thicket stage reached its peak in density in the middle to late forties coinciding approximately with the time of maximum abundance of the rats. In the past eight years, under continued protection from burning, cutting and browsing, the forest has developed further; sizable trees 20 feet or more high and up to eight inches in trunk diameter have grown from seedlings during the period of protection.[Pg 503] An almost continuous canopy of foliage has developed, shading the understory and thinning it by killing shrubs and saplings. In those situations where the canopy is most dense, as on north slopes having stands of young hickory averaging twenty feet high, the understory is now largely lacking, but in other situations, particularly on south slopes, the understory thickets are still dense. On the whole, however, habitat conditions have become less favorable for the woodrat.
Within the woodland the population of woodrats was not evenly distributed even at its maximum density; only those situations that provided sufficient overhead shelter were occupied by woodrats. The hilltop limestone outcrop, which was the refugium of the survivors when the population was at low ebb, also supported the greatest concentration when the population was high. The number of individuals living along any particular stretch of ledge could be determined only by intensive live-trapping, whereas residences of individuals could be more readily identified in most other situations away from the ledge. Stick houses of woodrats are, characteristically, large and dome-shaped in woodland, but along the ledges they usually lacked this typical form and consisted of a much smaller accumulation of sticks, often merely filling a small crevice. Sticks carried into such places where they were partly or wholly protected from moisture and sunshine were much less subject to decay than those in more open situations, and remained long after the rats themselves were gone. Accumulations of droppings in depressions in rock surfaces beneath overhanging ledges likewise have lasted for many years. The rock outcrop provided a continuous travelway along the hilltops, and even parts that were not permanently occupied usually had some sign. The following types of situations were found to be especially favorable for occupancy: deep crevices beneath overhanging projections of the ledge; large flat boulders broken away from the main ledge; thick clumps of brush (usually fragrant sumac, Rhus trilobata) providing shelter and support for the house; logs fallen across the ledge providing support and protection for the house structure.
A second outcropping limestone stratum approximately 20 feet below the level of the hilltop was just as extensive as the upper outcrop, but it was little used by the rats because the exposed rock surface was more regular, lacking the jagged cracks and deep fissures of the hilltop outcrop; and it lacked the overhanging projections which provided overhead shelter for the rats along the[Pg 504] upper outcrop. More than ninety per cent of the rats that were recorded as associated with the outcrops were at the hilltop stratum.
Second in preference to the hilltop outcrop as a house site was the base of an osage orange tree in thick woods. This tree occurs throughout the woodland of the Reservation, having become established when the leaf canopy was more open, and the whole area was subject to grazing, with less development of the understory vegetation in the woodland. Houses were most often situated in those osage orange trees that had been cut one or more times, and had regenerated with spreading growth form, the multiple branching stems offering substantial support. Occasionally houses were built in crotches from two to six feet above ground.
Blackberry thickets also are favorable locations for houses. These thickets grew up mostly in fenced areas from which livestock were excluded, but where there was not dense shade—hilltop edges and level or gently sloping ground adjacent to creek banks. The houses were usually in densest parts of the thickets where they were almost inaccessible. Mats of dead canes more or less horizontal, with the live canes growing up through them, provided effective overhead protection, while the ground beneath was relatively open. Houses built in the thickets were so well concealed that they were usually not detected until after leaves were shed in autumn. In most cases the blackberry thickets were small and well isolated. Houses of the rats were sometimes unusually near together suggesting that these thickets provided especially favorable habitat conditions.
Hollow trees are often utilized, the accumulation of sticks for the house being largely inside the cavity. To be suitable for a house site, the snag must have an opening near ground level, and another higher on the trunk, providing emergency outlets in two directions. Most of the hollow trees utilized were black oaks (Quercus velutina).
In 1948 there were many houses in cut tops of trees left from small scale lumbering operations a few years earlier. The densely branched tops of elms, oaks and hickories had satisfied the requirement for support of the house and nearby shelter. The houses built in them were in open woodland well separated from otherwise favorable situations. By 1948 the tops were disintegrating and no longer provided effective shelter. The houses built in them were falling into disrepair and were not permanently inhabited but were often used temporarily by wandering individuals.[Pg 505]
Along cut banks of gullies where trees were partly undermined by erosion, the exposed, tangled root systems provided sites for occupancy. In these situations the accumulations of sticks were small and lacked the typical domed shape, consisting essentially of a lining to the cavity beneath the roots.
Two small buildings at the Reservation headquarters were accessible to woodrats and were utilized off and on throughout much of the period of this study, despite the fact that most other sites of occupation away from the hilltop outcrops were deserted in the same period. One small building used as a laboratory had an enclosed wooden box five feet square housing an electric water pump. The interior of this box was accessible to the rats from beneath the floor. Litter of sticks and stems and various food materials were carried in by the rats. The nest thus protected and enclosed was not surrounded by the usual accumulation of sticks. An old garage 30 feet from the laboratory building was also occupied, sometimes by a different individual. The nest and food stores were behind boards propped against the wall.
In October, 1948, live-trapping was begun on a heavily wooded slope facing northwest, and a ten-acre area was trapped rather thoroughly in the succeeding weeks. Because few traps were then available, this was the only area that was well sampled in 1948, although diffuse trapping was carried on over some 200 acres. On the ten-acre tract a total of 17 adult and subadult woodrats were caught, four along the hilltop rock outcrop, six along the gully at the bottom of the slope, and seven at intermediate levels on the slope. Judging from the many unoccupied houses, the population on this tract had been much higher before the study was begun. On the basis of this sample it seems that in 1947 a population of several hundred woodrats lived on the wooded parts of the square mile where the Reservation is located.
The abrupt reduction in the population of woodrats on the Reservation cannot be explained conclusively with available data. Probably weather played a major part, but other unknown factors must have been important also. It is certain that the population of woodrats was high, if not at an all-time peak, in 1947. In late February, 1948, when one of us (Fitch) first visited the area on a preliminary inspection trip (not concerned primarily with woodrats), houses of these rats were found to be unusually numerous[Pg 506] and those seen seemed to be occupied and well repaired. Possibly the population was drastically reduced within the next few weeks, as unseasonably cold and stormy weather occurred in early March. For the first 12 days of March, 1948, temperature averaged 20° below that of average March weather, and even colder than the average for January or February. A reading of -5°F. on March 11 set a new low locally for the month since records were begun in 1869. The record low temperatures were accompanied by 12.8 inches of snow. This spell of unusually severe weather in early March coincided with the period in which first litters of young usually are born, as most females breed in early February and the gestation period is in the neighborhood of five weeks. That most of these first-litter young may have been eliminated by the unfavorable extreme of weather at the most critical stage in the life cycle may be readily imagined although definite proof is lacking. However, the mortality must have extended beyond newborn young. Loss of first litters ordinarily would be compensated for by the end of the season, since a female usually breeds more than once in the course of a season. In any case, by autumn, when the actual field study of woodrats was initiated, many houses were already deserted and in disrepair. Although the rats were still moderately abundant, they were, seemingly, much below the population peak of the preceding year.
Further drastic reduction of adults and subadults took place in the winter of 1948-49. In the course of live-trapping operations from mid-October into early December, 51 individuals were caught and marked. Chiefly because of unfavorable weather conditions, field work was discontinued in mid-December, and live-trapping was not resumed until early March. Subsequently, only 12 of the woodrats previously marked could be recaptured, and the population had become noticeably sparse. Seemingly, more than three-fourths of the population present in late autumn had been eliminated in the interval. In January, weather was exceptionally severe; on the ninth and tenth the worst sleet storm in twelve years occurred. Sleet fell in small granules, while the temperature remained several degrees below freezing. Partial thawing on January 12, 13 and 14 was followed by a steady drizzling rain on the fifteenth. On the following day the temperature dropped to -7°F. Ice still remained from the sleet storm, and the slush again froze. On the night of January 18, there was one of the worst snow storms on record and temperature reached a low of 2°F. Exceptionally low[Pg 507] temperatures persisted through January 24, with more sleet on January 25. Ice from the earlier storm still remained. On January 30, the temperature dropped to -7° and a three-inch cover of snow still remained over the coat of ice. The month of January, 1949, had the heaviest precipitation in 81 years (5.09 inches) and a cover of ice remained for at least 21 days. There were other sleet storms of lesser proportions on February 2 and again on February 21.
Ordinarily sleet would not seriously damage woodrats living in houses in woodland habitats and less suitable hedge rows because it usually freezes as it falls and coats only the surface of the house. Gradual thawing would allow normal runoff without much penetration. Because the sleet during the storm described above did not form a glaze as it fell, the ice particles penetrated many houses. It has been observed many times that captive woodrats refused food that was frozen or were unable to eat it. Woodrats in live-traps in winter rapidly weaken unless a large supply of food is available. If food supplies became sealed over by ice, woodrats would have died by starvation or by falling an easy prey to predators. The rats were more accessible to several predators than were smaller mammals such as meadow voles which were difficult to obtain because of the coating of ice over the fields.
The decimated population surviving into the breeding season of 1949 failed to make substantial gains. In fact, during the following four-year period the general trend of the population over the Reservation as a whole seemed to be one of gradual further decline.
In November, 1949, the rats were almost gone from the area of north slope and hilltop in oak-hickory-elm woodland where the most intensive live-trapping and other field work had been done the previous year. The following descriptions of houses remaining on the area at that time give some idea of the habitat, and of the course of events correlated with the fluctuations in numbers of woodrats.
No. 1. At the hilltop outcrop, partly on a substrate of limestone boulders, built around an elm of two-foot DBH, which lent support to one side. A hackberry sapling one inch in stem diameter grew through the middle of the house, providing further support. The house was two feet high and six feet in diameter, and was in obvious disrepair, with a hole several inches in diameter in its top. It had been occupied in the autumn of 1948. It was constructed mainly of sticks, ranging in diameter from approximately one inch to straw size. Many of the sticks, from .4 to .5 inches in diameter and one to two feet long, seemingly would have been heavy burdens for a rat, although they were of light-weight wood, sumac and elm. Mixed with the sticks were quantities of dry leaves, bark, and chips of wood, all material appearing old and weathered. This house was in elm-oak-hickory woods 50 feet from a cultivated field on the hilltop to the east and south. To the north and west the escarpment sloped away abruptly. There was a coralberry thicket beneath the trees on the adjacent hilltop.

(A) Map of part of University of Kansas Natural History Reservation, showing first-capture sites for all woodrats live-trapped in the autumn of 1948. Because of the short time involved and the few traps available, much of the area shown was not thoroughly trapped. Woodrats were abundant, though much less so than in 1947, as shown by the large number of deserted houses.
(B) Map of woodrat study area, same as shown in (A), showing first-capture sites for all woodrats live-trapped in 1949. Woodrats were still moderately abundant, but much below the level of the previous year. Triangles indicate those capture sites not sampled in 1948.
(C) Map of woodrat study area, same as shown in (A), showing first-capture sites for all woodrats live-trapped in 1950. Numbers were medium-low, having undergone drastic reduction from the peak level. Triangles indicate those capture sites where trapping was not done in earlier years.
(D) Map of woodrat study area, same as shown in (A), showing first-capture sites for all woodrats live-trapped in 1951. The population was low, but had not yet reached its lowest ebb.
(E) Map of woodrat study area, same as shown in (A), showing first-capture sites for all woodrats live-trapped in 1952, when the population had declined to relatively low numbers and disappeared from much of its former habitat.
(F) Map of the 590-acre Natural History Reservation, showing the area where woodrats were studied.[Pg 510]
No. 2. On gently sloping hilltop edge 15 feet from the outcrop and escarpment, built around a forked walnut sapling having both trunks approximately five inches in diameter. The sapling, coming up through the center of the house at a 45° angle, evidently had been bent by the accumulated weight of the debris at an early stage of its growth, many years before. Trees were small in this part of the woods, with a well developed understory thicket of coralberry and sumac. This house approximately one foot high and six feet wide, was constructed mainly of sticks and was similar in composition to No. 1, but appeared considerably older with all the sticks blackened and rotten. In the autumn of 1948 this house was used by woodrats, but probably only as a temporary stopping place, because it was already in disrepair then.
No. 3. At edge of escarpment, 25 feet from No. 2, on a flat boulder approximately six feet long, three feet wide and one foot thick. The decaying and much flattened mass of sticks was mainly on top of the boulder, but also spilled over its edges. Fresh sign was noted at this house in the autumn of 1948, but the house was already in disrepair then, and seemingly it was used only as a stopping place.
No. 4. At the hilltop outcrop where an elm had fallen across it. The decaying log remaining was approximately 12 feet long and 15 inches thick. This log passed diagonally through the house, providing its main support. The house was approximately 39 inches high, its summit extending a little above the level of the top of the outcrop. The house was approximately seven feet wide along the outcrop. This house was somewhat intermediate between the typical dome-shaped stick piles that the rat builds in open situations and the formless accumulations of sticks with which some rats living in deep rock crevices line the entrances. Part of the accumulation was beneath the limestone boulders and outcropping slabs. Approximately half of the material used in the house consisted of sticks and the remainder of pieces of bark and chips of wood, mostly gathered from the fallen elm. This house had shrunken noticeably from decay and settling in the months since it was occupied, in the autumn of 1948. The house was surrounded by a thicket of fragrant sumac, dogwood, and hackberry saplings.
No. 5. At edge of a protruding boulder one foot thick at the hilltop outcrop of the west facing escarpment, and 100 feet back in the woods from the edge of a corn field, in undergrowth of dogwood, wild currant, and coralberry. The house consisted of a pile of rotten twigs, 3 inches deep and 30 inches wide on the upper side of the boulder, and a lining of similar material at the lower edge of the boulder, partly blocking the crevice beneath it. The twigs composing the house were old and rotten. However, a few dry but still green hackberry leaves were stored in the crevice beneath the boulder. In a bare space atop the boulder were several recent woodrat droppings, small and obviously produced by an immature individual, which, perhaps, had recently settled at this old house site.
No. 6. In hilltop woods, 30 feet from a corner adjoining a pasture and a corn field, at the base of an osage orange tree of one foot DBH, and also over a hollow cottonwood log one foot in diameter and three feet from the osage[Pg 511] orange tree. Suspended mats of grape and smilax vines, and the thorny, dead, lower branches of the tree provided additional shelter. The house was composed of sticks and twigs, mostly of osage orange, with spines still present; slabs of bark, wood chips, and dry leaves also made up part of it. Materials on the exterior of the house appeared old and weathered, but the house was conical and solid. Seven fresh corn cobs were on the house or near its base, suggesting that corn from the nearby field had figured importantly in the diet of the occupant. A well beaten path led from the base of the house alongside the log, to a large cottonwood tree 15 feet from the house. This evidence that the house was occupied was verified by live-trapping the occupant. Late in 1948, also, the house was occupied by another individual, but seemingly was deserted for a period of months thereafter.
No. 7. On upper part of north slope where a hickory seven inches in diameter had fallen across an old sunken log approximately one foot in diameter. The house, composed mainly of hickory twigs 1/4-inch to 1/2-inch in diameter, mixed with bark, wood chips, and leaves, was partly decayed, with no fresh sign and was in a thicket of greenbrier, saplings of hickory and hackberry, and cut tops of hickories. The top was flattened to less than four inches above the level of the supporting hickory log. There were large cavities in the side of the house. When first discovered in the autumn of 1948, this house was occupied by a subadult female rat, but she moved away permanently, and the house had been deserted for approximately a year when these observations were recorded.
No. 8. In middle of northwest slope, in thick branches of broken top of a black oak. This house had become flattened by decay and settling to form a mound approximately one foot high and five feet in diameter. Only the top protruded through the carpet of dry leaves. Once well protected and partly concealed by the branches and twigs of the oak top, this house was now fully exposed by the disintegration of the top. The house consisted chiefly of oak twigs. In October, 1948, a woodrat was live-trapped at this house, but probably it was a wanderer. The house had then already undergone much deterioration.
Some 56 species of animals that regularly prey on small vertebrates live on the Reservation. Many of the larger kinds may take woodrats occasionally. Because of size, habitat preferences and the time and manner of hunting, five species stand out as the more formidable enemies—the horned owl (Bubo virginianus), prairie spotted skunk (Spilogale putorius), long-tailed weasel (Mustela frenata), pilot black snake (Elaphe obsoleta) and timber rattlesnake (Crotalus horridus).
Throughout the study horned owls were common on the area, but their numbers were highest in 1948. Samples of pellet collections have shown that the cottontail is the staple food, being represented in almost every pellet. Various rodents also are important in the diet, the cotton rat, prairie vole, or white-footed mouse being[Pg 512] most prominent according to the time and place of collection. The woodrat is approximately optimum size for prey, and it constitutes one of the most preferred food sources. Remains of only two woodrats were found in the pellets examined, but at times when the pellets were collected woodrats were so scarce that they constituted only an insignificant percentage of the biomass of potential prey. On several occasions woodrats in live-traps were attacked by horned owls, as shown by the overturned and displaced trap and quantities of fine down adhering to them and to nearby objects. The horned owl lives in the same habitat as does the woodrat. In other regions woodrats are known to figure prominently in the diet of the horned owl. At the San Joaquin Experimental Range in California, for instance, N. fuscipes was found 240 times, more frequently than any other kind of prey, in 654 pellets of the horned owl, and this owl was shown to be the one most important natural enemy of the rat, although many kinds of carnivores, raptors and snakes also took toll from its populations. On the Reservation the population of horned owls has been fairly stable from year to year, with roughly one pair to 100 acres of woodland. Some territories have been maintained continuously throughout the eight-year period of observation, though changing to some extent in size, shape and area included. In 1948, when livestock grazed on the area, and the ground cover of herbaceous vegetation was relatively sparse, cottontails were much less abundant than they were later when the vegetation was protected. Small rodents including voles, cottonrats, and deer mice, were also less abundant then, and the numerous horned owls may have been supported in part by the high population of woodrats.
The spotted skunk may be an even more important enemy of the woodrat, although the evidence is circumstantial. No records of these skunks preying on woodrats have been found in the literature, nor were any such instances recorded by us except for attacks on woodrats confined in live-traps. This skunk is a formidable enemy of small and medium-sized rodents, as it can climb, dig, and squeeze through small openings. That it may prey on rat-sized rodents and may even be a limiting factor to their occurrence is well shown by Crabb's (1941:353) studies in Iowa. He found that Norway rats (Rattus norvegicus) ranked third in frequency (cottontail, mostly carrion, ranked first) in the winter food of the spotted skunk. Crabb observed that about farmyards and farm buildings where the skunks had been eliminated by persistent[Pg 513] persecution, rats were abundant, but that about others where the skunks were present, the rats were scarce or absent. On several occasions he noted that heavy populations of rats about farm buildings in summer and autumn nearly disappeared in winter if a skunk was in residence.
Sign of spotted skunk was noted frequently on various parts of the Reservation, especially along the hilltop ledges which were the best woodrat habitat. On several occasions skunks released from live-traps took shelter in woodrat houses which appeared to be unoccupied. According to a local fur dealer, C. W. Ogle, spotted skunks reached a peak of abundance in Douglas County in the winter of 1947-1948, and many pelts were brought in for sale then. The concentration of skunks may have had detrimental effect on the population of woodrats, especially when extremes of weather had already made conditions critical for them, as in early March, 1948, and in January, 1949, when snow and sleet made their usual food supply unavailable.
The long-tailed weasel is considered to be a potentially important enemy of the woodrat. Weasels have been seen on the Reservation on only a few occasions, but they may be more numerous than these records would indicate. Two were caught at the hilltop outcrop, at different times and places, in funnel traps put out to catch snakes. The weasel seems to prefer this rocky habitat, which is also favored by the woodrat. Because of its ferocity and willingness to attack relatively large prey, and because it is an agile climber and able to squeeze through any openings large enough to accommodate a woodrat, it would seem to be a formidable enemy.
The pilot black snake (Elaphe obsoleta) is an important enemy of this woodrat on the Reservation and probably throughout the rat's geographic range except for the extreme western part. Although this snake occurs in every habitat of the Reservation, it has been found most often along rock outcrops of wooded hilltop edges in the type of habitat most favored by the rat. Most often pilot black snakes have attempted to escape into crevices of the outcrop. These snakes are also skillful climbers and often have escaped by climbing out of reach along branches or even vertical tree trunks. On several occasions these snakes have been found on or beside woodrat houses, or have escaped into them. Over a seven-year period 143 pilot black snakes have been recorded, 53 of which were adults.[Pg 514]
On September, 1948, a large pilot black snake found basking on a rock ledge, distended by a recent meal, was palped and contained a subadult female woodrat. On June 19, 1953, one of us, approaching a live-trap set under an overhanging rock ledge, saw a four-foot pilot black snake on top of it. The snake struck repeatedly at the rat in this trap, but was unable to reach it. At each stroke the rat would dash about the trap frantically.
These snakes hunt by stealth, and might catch woodrats by entering their nests, or by lying in wait along their runways, but are not quick enough to catch them in actual pursuit. Young in the nest would seem to be especially susceptible to predation by the pilot black snake. These snakes hunt by active prowling, either by night or by day, and much of their food consists of the helpless young of birds and mammals found in the nests. While only well-grown or adult pilot black snakes would be able to swallow an adult woodrat, any but first-year young probably would be able to overcome and swallow the small young. The female woodrat's habit of dragging the young attached to her teats as she flees from the house at any alarm must save many litters from predation by the pilot black snake. First litters of young, born in early March, are already well grown, and past the age of greatest susceptibility to predation before the snakes emerge from hibernation in late April or early May.
The timber rattlesnake is another potentially destructive enemy, but on the Reservation, and throughout much of its original range it is now relatively scarce. The genus Neotoma largely coincides in its over-all distribution with the genus Crotalus, of the rattlesnakes. For most kinds of woodrats, the larger species of rattlesnakes are among the chief natural enemies.
The timber rattlesnake has habitat preferences similar to those of the eastern woodrat. Of 30 timber rattlesnakes recorded on the Reservation over an eight-year period, all but one were at or near hilltop rock ledges in woodland. The woodrat is probably one of the most important prey species for the timber rattlesnake. Like the woodrat, the rattlesnake is mostly nocturnal in its activity. Unlike the pilot black snake, it hunts by lying in wait, striking prey which comes within range, and waiting for it to die from the venomous bite, rather than by active prowling. Therefore, it is probably less of a hazard to young in the nest than is the pilot black snake. Even young rattlesnakes too small to eat woodrats are potentially dangerous to them, as they may strike and kill any that come within range.[Pg 515]
Rainey (1956) listed many kinds of small animals that use the houses of the eastern woodrat and live in more or less commensal relationships with these rodents.
A situation unusually favorable for observing woodrats and their associates was discovered on the Reservation where, in July, 1948, two old strips of sheet metal, each covering an area of approximately 25 square feet, were used as shelter by a lactating female with three young. This was on a brushy slope just below an old quarry site. A rock pile and remains of an old rock wall were nearby. Woodrats had carried many sticks back under the metal strips, filling the spaces beneath their edges. There was a nest and a system of runways beneath the strips. In the following seven years this site was seldom deserted for long and was used by a succession of individuals. The strips of metal could be easily raised and then lowered into place with little disturbance. Because the situation was not entirely natural, the findings may not be typical of other rat houses. Animals found over a period of years beneath these metal strips include: several dozen each of the ring-necked snake (Diadophis punctatus), five-lined skink (Eumeces fasciatus), and ant-eating toad (Gastrophryne olivacea); several individuals each of cottontail (Sylvilagus floridanus), white-footed mouse (Peromyscus leucopus), short-tailed shrew (Blarina brevicauda), least shrew (Cryptotis parva), American toad (Bufo americanus), Great Plains skink (Eumeces obsoletus), pilot black snake (Elaphe obsoleta); and one each of bull snake (Pituophis catenifer), spotted king snake (Lampropeltis calligaster), red milk snake (L. triangulum), and timber rattlesnake (Crotalus horridus). The snakes which were potential predators on the rats seemed to be merely utilizing the shelter in these instances, but they may have been lying in wait for prey there.
Among mammals, the cottontail and the white-footed mouse are the most persistent users of the woodrat houses, especially those that are no longer occupied by the rats. On one occasion five white-footed mice were caught simultaneously in a trap set beside a house at the base of an osage orange tree. Subsequent trapping showed that this house was no longer occupied by a rat, but that the mice lived in it. Occupancy of such an old woodrat house by white-footed mice may continue long after abandonment of the house by the rat, even after the house has partly decayed and settled to a small part of its original volume.[Pg 516]
Cottontails often have their forms under the edges of houses, either occupied or deserted. These situations offer protection overhead and on three sides. Abandoned houses having one or more of the entrance holes enlarged, as by predators breaking through the side of the house to gain access to the nest, are especially well adapted for occupancy by the cottontail. The rabbit may make its form inside the house structure.
The opossum, also, finds the type of shelter that it requires in abandoned houses that have had the entrances sufficiently enlarged. On various occasions opossums or their remains have been found in such old houses, and opossums released from live-traps have been known to seek shelter in abandoned woodrat houses.
At the old quarry on the Reservation woodrat sign was especially abundant. A wooden bin approximately seven feet square, used to store crushed rock before quarrying operations were abandoned, was inhabited by one rat. At the base of a rock crusher on the top of a bank a few yards from the bin was an accumulation of sticks and other debris brought by woodrats. A rock wall at the top of the bank between the crusher and the bin had many crevices providing shelter for the rats, and projecting rocks were littered with their droppings. In the spring of 1949 the bin and rock crusher were removed, but at least one rat continued to live in the rock wall. In the summer of 1951 several tons of corn ruined in the flood were dumped on the top of the bank above the wall. By autumn, Norway rats, either brought in with the corn or attracted by it, had taken possession of the wall, evidently displacing the woodrats, which were no longer present. Although this Old World murid rat is much different from the woodrat in habits, it seemingly can compete with it and replace it where habitat conditions are otherwise favorable for both.
The woodrat is dependent on the stick houses that it constructs for shelter. For each individual the house constitutes a home base to which it is attached, and about which its movements revolve. The area within which routine daily movements are confined constitutes the home range, which is variable in size and shape. An individual may, and usually does, alter its home range over periods of time. The home range is somewhat nebulous because the rat may at any time move far beyond the small area to which its activities are largely confined. It may be motivated by sexual urge or other[Pg 517] voluntary wandering; it may be enticed by a food supply or some other specific attraction not available near its house; or it may be forcibly displaced by an intruder or may abandon in favor of an offspring.
An occupied house normally has several runways radiating from it. These are well worn paths, smoothed by use, and cleared of obstructions, and the rat tends to keep to them in its foraging expeditions. Usually a trail leads to a bush or tree showing evidence of heavy use by the rat. Ordinarily such a trail cannot be traced more than 30 feet from the house, and it seems that the most concentrated foraging occurs within this short radius. Experience in live-trapping has indicated that the distance covered by a woodrat in its normal foraging for food is ordinarily less than 75 feet in any direction from the house.
Usually the rats can be caught in traps only at their houses or nearby places that they frequent, as indicated by their sign. When travelling, woodrats make use of overhead cover as much as possible. Storing of food seems to be associated with the animal's reluctance to wander far from home. When a rat is gathering preferred food for storage the home range may be enlarged (or the animal may travel beyond the limits of its regular home range). In any case the rat may find it necessary to traverse an additional area in order to reach the food source. This may involve, in part, extension vertically, as when the rat obtains food from trees directly over the house. The home range is thus somewhat three-dimensional; both trails and feeding places are often above ground. Because of dependency on cover, woodrats do not forage randomly in all directions from the house.
Although the house and its immediate environs are defended as a territory by the occupant, possession may be soon relinquished. A woodrat may shift frequently from one house to another, especially if unoccupied houses are readily available. Because woodrats had undergone drastic reduction in numbers, as discussed on p. 505, unoccupied houses in various stages of disrepair were numerous throughout the woodland in 1948 and 1949, and the rats that were present then seemed especially inclined to wander. Even old houses that are collapsed and disintegrating may be used temporarily, or may be taken over and repaired. Houses that are in sites exceptionally favorable in that they provide food and shelter may be occupied more or less permanently, with a succession of woodrats over many generations.[Pg 518]
Shifts to new areas are perhaps most often motivated by a search for mates. Such shifts are, on the average, longer and more frequent in males. Males must range farther in search of females when numbers are low. On the other hand, when numbers are high and most of the best sites are occupied, newly independent young and displaced adults are forced to travel greater distances in search of homes. Some of the larger and more powerful males move far greater distances than smaller males. The longest distances recorded were mostly for large adult males in breeding condition. The average maximum distance between successive points of capture for 27 adult males was 345 feet. For 39 females (adults and subadults) the corresponding figure was 143 feet. The extremes for males were 0 to 1080 feet and for females, 0 to 650 feet. Of the 27 males, five moved the maximum distance in a single night. Most of the long movements by males did not constitute clear-cut shifts in home range, and many returned to their original locations.
The average distance between points of first and last captures for 72 subadult and adult males was 165 feet. A similar figure for 72 subadult and adult females was 133 feet. Of the males 23.7 per cent were at the same place at the first and last captures; for females the percentage was 36.1. These figures are from the combined data of our trapping records, but the trends differed sharply in the two sets of records. In Fitch's records, movements averaged longer and difference between the sexes was much less: 189 feet for 41 males and 178 feet for 42 females. Corresponding figures from Rainey's records were: 141 feet for 31 males and 74 feet for 30 females. In Fitch's field work, opportunities to record exceptionally long movements obviously were better because the trap line encompassed a larger area, approximately half a square mile, whereas Rainey's live-trapping was concentrated on relatively small areas. The reason for the greater vagility of females in Fitch's records is less evident. However, the data were obtained within the period of drastic population reduction, at a time when there were numerous empty houses throughout the woodland, facilitating travel, and shifts from one home range to another where conditions were, temporarily at least, more favorable. Rainey found that the females in the small colony in woodland where he trapped, moved much less than did those that lived along the hilltop outcrop, which provided a natural travel route.
Following are several examples of males and females with long histories showing individual variation in frequency and distance of movements.[Pg 519]
Males
(1.) First captured October 14, 1951, and last captured 327 days later on September 6, 1952. He was taken 12 times. For the first seven captures (October 14, 1951, to July 15, 1952), no movements were recorded. In the following seven days he moved 367 feet. Within the next 21 days he returned to within 114 feet of the site of original capture. Less than one month later he was caught for the last time, at this same site.
(2.) This large male was captured twelve times over a period of 827 days (March 16, 1952, to June 21, 1954). He tended to wander more than other males and was absent from the trapping area from early 1952 to May 1953. One round trip made in a two-weeks period, amounted to a linear distance of 1894 feet if the rat followed natural cover. The return trip of 947 feet was the greatest distance traversed in a single night in any of the woodrats we recorded. Other movements between successive captures were: 722, 397, 356, 293, 253 and 144 feet (the latter shift made three different times). Sexual urge probably motivated most of his wandering, since numbers of females were low.
(3.) For this male the span of records was 143 days, with 18 captures. For the first eight recaptures, extending over a period of 39 days, he was still at the original location. Four days later he had moved 120 feet and was visiting a female. A week later he returned. In the following month he was recorded as making two more moves, of 115 feet and 215 feet. He was last recorded at the hilltop outcrop.
(4.) The records of this male extended over 465 days, with 13 captures. For the entire period only one movement, of 163 feet, was recorded. Twelve of the 13 captures were at the same house.
(5.) This male was captured 16 times over a span of 130 days. After the second capture he moved 144 feet along the outcrop and was caught there for the next 14 times, having developed a "trap habit."
(6.) This male was in the area 210 days (13 captures) and shifted his range. He was first captured on August 17, 1952, at a house at the rock fence 433 feet from the outcrop. Between this date and October 12, 1952, he moved to the outcrop and established residence in a vacant house. He was recorded as making six more moves, the longest of which was only 40 feet.
(7.) This male was first caught in June, 1949, as a juvenile probably between two and three months old (weighing 96 grams) and hence probably still at the maternal house. In September, grown to adult size, he was caught twice, still at this same place. In October, November, December, and in February, 1950, he was caught 11 times at eight places all within a 90-foot radius of his original location. In April, 1950, he was caught at points 550 feet WSW and 700 feet SW. In October he was caught within 150 feet of the original location. In November, 1950, and in March and April, 1951, he was caught four times at a place 900 feet SW from his original location.
(8.) This subadult male was first caught at the hilltop outcrop on October 4, 1949. Two days later he had moved 160 feet north along the outcrop. A month later he had shifted 600 feet south; in three more days 1040 feet north. On November 15 he was 105 feet south of the November 8 location; on November 16, he had moved 70 feet north. On November 17 he had moved 900 feet back south, but had returned on the 18th to the November 16 location. On[Pg 520] November 22, he had again shifted 900 feet south. All capture sites were at the hilltop outcrop.
(9.) This male was caught as a juvenile (75 grams) on October 8, 1950. On November 9 he had moved 220 feet, from the lower outcrop to the upper, and he was recaptured at or near this same site on November 10, 28 and 29, and on January 11 and February 9, 1951. On November 21, 1951, grown to maximum adult size, he was caught at a new location 1080 feet from the original.
(10.) This male was caught as a subadult twice at the same place on November 30 and December 14. By the following autumn he had shifted to a new location 180 feet south along the outcrop, and he was caught there on September 22 and October 18, 1951, and on January 20 and February 2, 1952.
Females
(11.) This female was captured 27 times over a span of 211 days. She moved back and forth considerably between two houses 40 feet apart but made only one substantial movement of 245 feet; at this time she was in breeding condition. Nearly seven months after the first capture she was seen for the last time only 16 feet from the original site of capture. It was assumed she fell prey to spotted skunks which were raiding traps.
(12.) First captured on March 24, 1951, she remained on the area 105 days in which period she was live-trapped 25 times. Sixty per cent of the total captures were at the same house and the longest movement recorded was only 56 feet. She was last caught in a trap 25 feet from the site of original capture.
(13.) This young adult remained at her house at the rock fence approximately four months. In this period she was captured 11 times. On March 16, 1952, she had moved 410 feet to a house at the eastern section of outcrop, probably searching for a male. She was never seen again.
(14.) This subadult female moved from the site of original capture to a house 253 feet away on the same outcrop. She was probably in search of a new home when caught the first time. She was recorded at another house 40 feet away on one occasion.
(15.) Over a span of 90 days and 15 captures this female was not recorded as making any movement. She was living in one of the woodland houses. Mature males were numerous in the area and she was visited by at least two.
(16.) This female was also living in the woodland section and was first caught on March 30, 1952, in one of the less favorable houses. She was trapped 17 times over a period of 85 days. One movement of 68 feet to a new home site was recorded, but the area of foraging probably did not change. She was caught here four times and then disappeared.
(17.) This female was first trapped as a subadult on October 5, 1948, at a house in brush on the upper part of a north slope. On November 24 she had shifted 590 feet to the bottom of the slope and was living in the recess beneath an undermined honey locust on a gully bank. On November 25 she was caught in a similar situation 100 feet farther east along the gully bank. She was recaptured at the gully on November 26 and 30, December 1, 3, 22, and March 8 and 9, and in all she shifted six times between the two gully-bank dens.[Pg 521]
(18.) This female was first trapped as an adult on November 18, 1948, in a gully-bank den. She was recaptured at this same place a year later, on November 18 and 30, 1949. On February 19, 1950, she was caught at a hollow sycamore 650 feet farther up the gully, and she was recaptured there on February 25 and April 7, and on June 15, 1951. On August 6, 1951, she was caught at a house in a thicket on the gully bank, between the first and second locations and 150 feet from the latter.
(19.) This female was recorded only twice; on October 15, 1948, she was at a hilltop rock outcrop. On July 14, 1950, she had moved 1480 feet and was living in a rock pile at the base of the slope, near the same hollow sycamore where female No. 18 had been caught.
(20.) This female was first caught as an adult on April 5, 1950, at a large boulder of a hillside rock outcrop. On October 7, 1950, she had shifted 110 feet to a house at an osage orange tree on the hilltop rock outcrop. On November 9 she was back at the first location and on November 28 she had moved 70 feet south along the hillside outcrop. On January 11 and February 9, 1951, she was back at the original location. On November 9, and 21, 1951, she was again at the site 70 feet south, and was still there at her last capture on February 3, 1952.
Ordinarily each house that is in use harbors only a single woodrat. To a greater degree than any other kind of mammal on this area woodrats show intraspecific intolerance. On various occasions when captives were placed in the same or adjacent cages, they focused their attention on each other with evident hostility, the more powerful or aggressive individuals attacking or pursuing. Several times the confinement of two rats in the same live-trap or cage resulted in the death of the weaker individual, and seemingly this is the normal outcome unless the attacked rat is able to escape. On various other occasions two or more rats have been caught in the same trap simultaneously but in every instance these were either: a pair of adults, the female appearing to be in oestrus; a lactating female and one or more of her young; or young less than half-grown, that were obviously litter mates. Older woodrats, especially males, often have their ears torn and punctured from fighting.
Territoriality involves, primarily, defense of the house itself. An individual that ventures into an occupied house may be quickly routed by the occupant even though the latter is smaller. Chasing has been observed occasionally, but it is doubtful whether any individual is able consistently to defend the entire area over which it forages. Because each rat spends most of its time within the shelter of its house, an intruder might venture onto its home range unchallenged and undetected, so long as it did not enter the nest cavity.[Pg 522]
An adult female was live-trapped on October 14, 1951, beside her house at the outcrop. As soon as she was released, she disappeared within the house. After approximately two minutes, a soft, high pitched whine was heard and immediately another woodrat dashed into view closely followed by the female. The chase continued for several seconds in the vicinity of the house, but the woodrat being chased soon left the area via the outcrop. Probably this intruder had moved into the house in the night while the female was in the trap.
On June 17, 1952, an adult male was found in a live-trap set at one of the brush pile houses in the woodland area. This house was occupied by an adult female. He ran into the house after release, and immediately there was a loud squeal. He ran outside and paused under some limbs approximately 15 feet from the house, and remained there for 15 minutes before clipping off an ironweed 12 inches long, which he carried to the house. He did not enter the house but stopped beneath overhanging sticks at the edge, eating leaves from the plant. He made another attempt to enter the house but loud squeals and rustling followed and he returned to the ironweed plant and was still eating when observations were halted. In another instance, squeals and rustling indicated that the occupant and intruder were in combat.
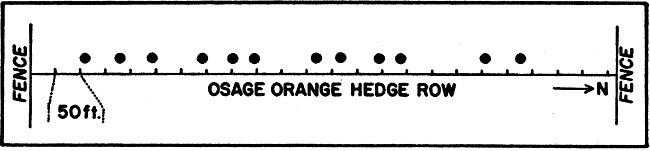 Fig. 2. Diagram illustrating spacing (due to
territoriality or intolerance of the rats) in twelve woodrat houses in a
hedge row extending south from south boundary of the Reservation at the
middle.
Fig. 2. Diagram illustrating spacing (due to
territoriality or intolerance of the rats) in twelve woodrat houses in a
hedge row extending south from south boundary of the Reservation at the
middle.
Although home ranges may overlap to some extent, intraspecific intolerance tends to maintain a certain minimum interval between houses. The arrangement of twelve houses along a hedge row 1170 feet long is diagrammatically represented in Figure 2. The average interval was 78.5 feet (minimum 42; maximum 171). The habitat was uniform. Home ranges probably overlap somewhat, and the spacing is the expression of the need for an otherwise unoccupied area in which there is sufficient space to live. Because individuals tend to fight whenever they meet, there is probably a psychological[Pg 523] tendency for sequestration which results in spacing of houses and reduces social contact thereby avoiding a depletion of energy that would be detrimental to the population. Whereas condition of the hedge row determines whether or not it will be inhabited by woodrats, length determines the number of occupants. The spacing of houses in a hedge row must be attributed to something other than restriction of sites because the number of sites available always exceeds the number that are in use. Although rock outcrops situated in areas of uniform habitat have not been observed to the extent that hedge rows have, a similar spacing seems to exist and the sites available for houses always exceed the actual number found. This behavior pattern limits the number of houses and is probably advantageous to the species through preventing overcrowding and possible critical depletion of the food supply.
Eleven of the young that weighed 100 grams or less when originally captured and were presumably still living at the mothers' houses, were recaptured repeatedly over periods of weeks or months, providing a limited amount of information regarding dispersal. They followed no definite pattern. In seven instances (five males and two females) the young stayed on at the house beyond the age when they were completely independent of the female. In at least two instances the female was known to have moved away while the young remained. One female shifted to a house 58 feet from the one where she had reared her litter of two, and was accompanied by the young male, while the young female stayed on in possession of the maternal house. Two months later this young female was caught at a house 90 feet away, and an adult male was in possession of her former house. One young male shifted to a house 220 feet from his original home and remained there several months, but was recaptured once back at the original location. Another male made a series of moves over a period of weeks and finally settled in a house 490 feet from his first home. One male who stayed in the maternal house all summer, and reached adult size there, later moved several times, and was last recorded 900 feet away. One young female shifted 110 feet. In several instances juveniles appeared abruptly in houses known to have been unoccupied previously, and some of these houses were in poor repair. These young had wandered from their maternal houses, for unknown reasons. On one occasion a young woodrat was caught in a mouse trap set in a meadow, a habitat into which adult woodrats would scarcely be expected to venture.[Pg 524]
Rainey (1956) has listed 31 food plants that are used by the woodrat in northeastern Kansas. He has emphasized that each rat usually obtains its food from plants growing in the immediate vicinity of its house, and that individuals thus differ greatly in their feeding, according to the local vegetation. Therefore, with a sufficiently large number of observations, the list of food plants might be greatly expanded, to include most of the local flora, with the exception of the relatively few kinds that have developed strongly repellent properties rendering them unpalatable to herbivores in general.
At the quarry where one or more woodrats usually lived beneath metal strips, as described previously (under the heading of "Commensals"), the situation seemed to be especially favorable, despite the fact that the metal offered no insulation from extremes of heat in summer and cold in winter. Perhaps the rat had an alternative nest among nearby boulders, to use when temperature was unendurable beneath the metal.
The rat itself, the stored food, and other details of its home life, could be observed with a minimum of disturbance by raising one side of the metal strip momentarily, then carefully lowering it into place. The following observations made in the summer and autumn of 1948 give some idea of the range of food plants stored at any one time and the change as the season progresses.
July 12: Bundles of leaves of carrion-flower (Smilax herbacea); 15 green pods of honey locust (Gleditsia triacanthos) with seeds eaten out; several green fruits of osage orange (Maclura pomifera), and several seeds of coffee-tree (Gymnocladus dioica).
July 24: Bundles of green leaves of osage orange and carrion-flower; many pods of honey locust.
August 30: Three large clusters of the fruits of pokeberry (Phytolacca americana).
October 20: Many small clusters of grapes (Vitis vulpina) judged to weigh perhaps one pound in all; several old pods of coffee-tree and a few berries of dogwood (Cornus Drummondi) and of pokeberry; a pile of small acorns of chinquapin oak (Quercus prinoides); dry seed heads of grass (Bromus inermis and B. japonicus).
December 22: Many twigs of bittersweet (Celastrus scandens) with fruits still attached; several seed heads of sunflower (Helianthus annuus); a few acorns of chinquapin oak; fragments of the fruit of osage orange; cured bundles of trefoil (Desmodium glutinosum), carrion-flower, and tickle grass (Panicum capillare).
Although the eastern woodrat is relatively unspecialized in its[Pg 525] feeding habits, a few species of favored food plants probably make up the greater part of its diet. In northeastern Kansas, at present, osage orange probably is by far the most important single species. Despite the fact that its aromatic leaves and fruits are somewhat repellent to insects and some other animals, they are well liked by woodrats, and provide a year-round food supply to those individuals having houses in or near the trees. Honey locust similarly provides thorny shelter for house sites, while the foliage, the seeds, and the bark of twigs and trunks are eaten. In houses that are situated near honey locusts, the large, heavy seed pods are sometimes stored by the hundreds. Old pods are often used in substitution for sticks as building material in the house. Nevertheless, honey locust is used relatively little as compared with osage orange. Other plants that figure most importantly in the diet include bittersweet, fox grape, pokeberry and horse nettle (Solanum carolinense).
Rainey (op. cit.) mentioned that captive woodrats would eat meat, both cooked and raw, and on one occasion he found remains of a cicada on a house under circumstances suggesting that this insect had been eaten by a rat. In the course of trapping for opossums and small carnivores, woodrats were caught on many occasions by Fitch in traps baited with animal material exclusively—miscellaneous meat scraps, canned dog-food, bacon grease, or carcasses of small vertebrates. In fact, such baits seemed to be even more attractive than the grain, seeds, peanut butter and raisins that had been used customarily to bait the traps set for woodrats. However, such meat baits could be used effectively only in cold weather, because of rapid spoilage and interference by insects at higher temperatures.
On one occasion an adult pilot black snake found dead on the road, a recent traffic victim, was brought to the Reservation headquarters for examination and was left overnight in the garage. On the following morning the carcass of the snake was found to have been dragged a short distance and gnawed; a quantity of flesh was eaten at an exposed wound on the neck. Woodrat tracks were thickly imprinted on the dusty soil around the snake. The adult male woodrat that lived in the garage had evidently spent much time moving about the carcass and over it, and feeding upon it. It seemed remarkable that this individual was not deterred from feeding on the snake by an instinctive fear of one of its chief natural enemies.
Although the eastern woodrat's food consists mostly of vegetation,[Pg 526] the strong tendency noted to feed upon flesh when it is available suggests that these rodents may, occasionally at least, prey upon helpless young of small vertebrates that are readily available to them. Nestling birds, either on the ground or in low trees, and young mice in nests that are accessible, might tempt the rat to indulge in predation.
Reproductive activity continues to some extent throughout the year except in late autumn and early winter. Presence of a vaginal orifice was used as an indication of sexual activity. In most instances the orifice was not indicative of actual oestrus, as it persisted through the preceding and following stages of an oestrus cycle. In anoestrus the orifice is sealed, the genitalia are reduced in size and the skin in the genital region is white. Immature females, and adults during most of the winter, are in this quiescent condition. Onset of the breeding season in late winter is relatively abrupt, and seemingly is a photoperiodic response. Breeding may begin in late January, and most females are in breeding condition within the first half of February. In oestrus the genitalia are enlarged and discolored and the vaginal orifice is prominent and gaping. By February most females born the previous season have matured, and breeding involves the entire population, except possibly for retarded young and individuals suffering from disease, injury or malnutrition. Rainey (1956) recorded an average of 2.3 young per litter.
Number of litters normally produced in the course of a season by an adult female is unknown, but most mature females examined within the period February to September inclusive were in some stage of the breeding cycle. It is obvious that the females which are successful in rearing their litters produce at least two litters annually, and probably some produce three litters. When entire litters are lost at an early age, to predation, or other causes, productivity is much increased, with perhaps only short intervals between pregnancies.
The smallest female having a vaginal orifice weighed 160 grams, but in most instances somewhat larger size is attained before the onset of oestrus. Judging from the average growth rate of immature females (Fig. 3), most probably attain sexual maturity at an age of five to six months unless this age is reached in the winter period of sexual quiescence. Rainey (op. cit.) found no clear cut instances of young maturing in time to breed before their first winter. He[Pg 527] concluded, tentatively, that in most instances sexual maturity is not attained until the spring of the year following that in which the rat is born. However, the evidence was inconclusive because few of the young marked survived to maturity. In late summer and early autumn, the latter third of the breeding season, newly matured young of the year, born in early spring, may be the most productive group. Young conceived at the beginning of the breeding season, and born in early March, would normally reach adult size and breeding maturity in August. For example, a young female first caught on June 15, 1951, weighed only 150 grams, but by August 10 she had gained to 220 grams (probably in pregnancy) and had a vaginal orifice. Of 35 adult and subadult females examined by Fitch in October, eleven had a vaginal orifice, the latest on October 18. Of these eleven showing signs of breeding, four at least had not yet produced litters, judging from the undeveloped condition of their mammae, and others that showed evidence of recent lactation probably included young of the year that had bred in August or September. One female gave birth to a litter in a trap on the night of October 6, 1950. Of 32 adult and subadult females recorded by Fitch in November, all were sexually quiescent, with the possible exception of one having a partially open vagina on November 10. All females taken in December, and most of those taken in January, also were sexually quiescent. January 20 was the earliest recorded date for a female with a vaginal orifice. Females examined in February mostly were perforate and many of them appeared to be in oestrus. One female trapped on February 19, 1950, weighed only 140 grams and was still imperforate. Another, weighing 200 grams on February 3, 1952, still was imperforate, but by February 27 she was perforate and appeared to be in oestrus. An adult female that appeared to be in oestrus on February 3, 1952, was imperforate on February 10.
At birth woodrats weigh approximately 10 grams or a little more. In a litter born in captivity and kept by Rainey, the average gain amounted to a little more than 1.5 grams per day during the first two months, but in the third month it was somewhat less. As this was an unusually large litter, of five young, one more than the female's teats could accommodate, their growth may have been a little less rapid than in most of those under natural conditions. At an age of three months they averaged approximately 120 grams. The three males consistently exceeded the two females.[Pg 528]
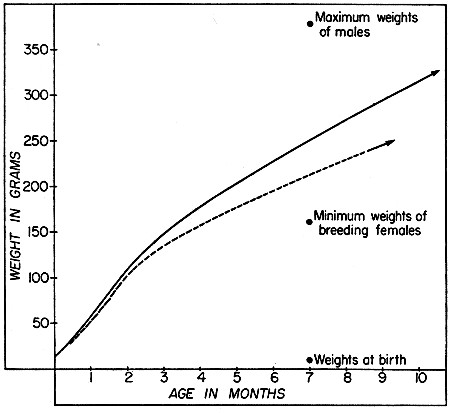 Fig. 3. Typical growth curves for male and female
woodrats; early stages are based on the litter of a captive female,
later stages on average gains of recaptured juveniles and subadults,
excluding those that seemed to be stunted. Solid line represents males
and broken line represents females.
Fig. 3. Typical growth curves for male and female
woodrats; early stages are based on the litter of a captive female,
later stages on average gains of recaptured juveniles and subadults,
excluding those that seemed to be stunted. Solid line represents males
and broken line represents females.
Young weighing less than 100 grams are rarely caught in live-traps. Four young, all males, first caught at an average weight of 80 grams, gained on the average, 1.39 grams per day over intervals that averaged 44 days. Six other young males first caught while in the weight range of 100 to 149 grams, were recaptured after intervals of 17 to 45 days and they had gained, on the average, .92 grams per day. The corresponding figure for four young females in the same size range was .71 grams per day. In seven young males in the weight range 150 to 250 grams, that were caught after intervals averaging 66 days, the gain in weight amounted to .83 grams per day. In seven females in the range 150 to 199 grams, gains averaged only .68 grams per day. Fully grown females that are not pregnant weigh, most typically, a little less than 250 grams while fully grown adult males average a little more than 300 grams. Growth rate and adult weight both[Pg 529] are influenced to a large extent by season and even more by individual differences. The underlying causes are obscure in most instances, but individual rats that are still short of adult size may stop growing for periods of months, and some individuals grow much more rapidly than others. One male that weighed 108 grams when he was first caught on July 3, 1951, was estimated to have been born in early May. He was last captured 152 days later on December 2, 1951, and by then his weight was 300 grams, representing an increase of 1.2 grams per day. Another male that weighed only 75 grams when he was caught on October 8, 1950, may have been less than two months old then. By November 21, 1951, at a probable age of 15 months, he weighed 350 grams having attained almost the maximum size. Other exceptionally large individuals were known to be less than two years old, while those rats that survived longest on the study areas did not much exceed average adult size. These records seem to show that exceptionally large woodrats are usually not those of advanced age, but are individuals which have grown most rapidly through fortuitous circumstances, probably depending upon both innate and environmental factors.
None of the woodrats handled was excessively fat, nor were any emaciated. The habit of keeping on hand stores of food at all seasons perhaps obviates the necessity for storing quantities of fat. Seasonal trends in weight vary among individuals, and are not wholly consistent from year to year. Rainey found that in late autumn and winter, rats steadily gain weight reaching a peak in late February or March. However, in the winters of 1948-49 and 1949-50, Fitch found that most rats lost weight and hardly any, even those that were short of adult size, made gains.
The following records of a male born in the spring of 1949 show rapid growth and attainment of adult size in his first summer, cessation of growth during the winter, and resumption of growth, with attainment of near-maximum size the following spring.
| June 16, 1949 | 96 gms. |
| September 26, 1949 | 230 gms. |
| September 27, 1949 | 230 gms. |
| October 18, 1949 | 260 gms. |
| October 27, 1949 | 250 gms. |
| October 29, 1949 | 220 gms. |
| November 8, 1949 | 235 gms. |
| November 15, 1949 | 245 gms. |
| November 24, 1949 | 240 gms. |
| November 26, 1949 | 240 gms. |
| November 30, 1949 | 240 gms. |
| December 20, 1949 | 260 gms. |
| February 18, 1950 | 230 gms. |
| April 5, 1950 | 290 gms. |
| April 7, 1950 | 300 gms. |
| October 7, 1950 | 320 gms. |
| November 29, 1950 | 345 gms. |
| March 23, 1951 | 340 gms. |
Another example, showing winter cessation of growth in a male at even smaller size is shown below. This was in the winter of 1950-1951.
| November 9 | 145 gms. |
| November 28 | 175 gms. |
| November 29 | 165 gms. |
| January 10 | 180 gms. |
| January 11 | 175 gms. |
| March 1 | 225 gms. |
| March 23 | 200 gms. |
The longest span of records for an individual woodrat recorded was 991 days in a female, already adult when she was first caught on November 18, 1948. Other relatively long spans of records were: 827 days in a male, adult when first caught on March 16, 1952; 754 days in a female, also adult when first captured; 649 days in a male first captured as a juvenile; 465 days in a male, adult when first captured; 409 days in a male, juvenile when first captured; 399 days in a female, juvenile when first captured; 395 days in a female, adult when first captured; 390 days in a female, adult when first captured; 366 days in a male, adult when first captured. Of these eleven individuals (six females and five males) whose records cover more than a year, eight were already adult when first caught. These eleven rats represent only 4.3 per cent of the total number captured. Our study was made at a time when populations were shrinking and disappearing, and obviously individual spans would have been longer if we had been working with a stable population. In most instances the spans of our records represent only small parts of the life spans of the individuals involved. Nevertheless, our records emphasize the potentially greater longevity of the woodrat as contrasted with the various smaller rodents living in the same area. Of several thousand individuals of the genera Mus, Zapus, Reithrodontomys, Peromyscus, Sigmodon, and especially Microtus, none is known to have survived so long as two years, and only a few individuals are known to have survived so long as one year after being marked.
Plant succession resulting from land use practices created habitat conditions especially favorable for woodrats in the late nineteen forties in northeastern Kansas, and particularly on the University of Kansas Natural History Reservation. With protection from prairie fires, woody vegetation had encroached onto areas that were formerly grassland, and, later, fencing against livestock permitted dense thickets of undergrowth to develop. In this region the woodrat[Pg 531] usually lives in a forest habitat, and requires for its house sites places that are especially well sheltered, as in matted thickets of undergrowth, root tangles exposed along eroded gully banks, hollow stumps or tree trunks, bases of thorny trees with multiple trunks for support, thick tops of fallen trees, or, especially, rock outcrops with deep crevices.
At the time of their maximum population density in or about 1947, woodrats probably averaged several per acre on the woodland parts of the Reservation. In the autumn of 1948, 17 were caught on the ten-acre tract of woodland that was live-trapped most intensively. By then, however, the population had already undergone drastic reduction, as shown by the fact that there were many unoccupied and disintegrating houses throughout the woodland. While the time and manner of mortality was not definitely determined, circumstantial evidence suggests that the downward trend began in early March, 1948, when record low temperatures and unusually heavy snowfall coincided with the time when parturition normally occurs. The rigorous weather conditions then may have been injurious, not only to the newborn litters but to the females comprising the breeding stock. Nevertheless, the population remained moderately high through 1948, but by early spring of 1949 more than three-fourths of the adults and subadults present in late autumn had been eliminated. Again, unusually severe winter weather seemed to be the underlying cause, as in January precipitation was the heaviest on record in 81 years, with penetrating sleet storms, persistent ice glaze, and occasional brief thawing followed by sudden drops to extremely low temperature.
After the drastic reduction in the winter of 1948-49, the population did not recover. Although no further sudden reductions due to extremes of weather were noted, the trend seemed to be one of gradual, progressive decline throughout the following period of years. Deterioration of the habitat, as the developing forest shaded out undergrowth, and inroads of certain predators may have been important in preventing recovery of the population. Many kinds of predatory mammals, hawks, owls, and snakes probably take woodrats occasionally, but the spotted skunk, long-tailed weasel, horned owl, timber rattlesnake and pilot black snake are considered to be by far the most important predators because of their habits and prey preferences. Few actual records of predation on woodrats were obtained because of their scarcity during most of the period covered by our study.[Pg 532]
Of the animals which share the woodrat's habitat, many small mammals, reptiles, amphibians, and invertebrates use its houses and live in a somewhat commensal relationship.
Woodrats are somewhat territorial, each defending its house and an indefinite surrounding area against intrusion by others. Houses tend to be spaced at intervals of at least 40 feet; occasionally they are closer together. Most foraging for food is done within 75 feet of the house. However, woodrats often wander far beyond the limits of the usual home range. On the average, males travel more frequently and more widely than females, and the larger and older males travel more than the smaller and younger. Search for mates provides the chief motivation for wandering. Extent of wandering is controlled to a large degree by availability of natural travelways, such as rock ledges, by shelters for temporary stopping places, such as old deserted houses, and by population density of the rats themselves.
Food of the eastern woodrat consists chiefly of vegetation; many kinds of leaves, fruits, and seeds are eaten. For many individuals foliage and seeds of the osage orange are the staple; hedge rows and dense trees of osage orange provide favorable sites for the houses. Woodrats are attracted to meat baits, and have been known to feed on flesh of carcasses, even on one of the pilot black snake which is a predator on the rat.
Woodrats are born blind, naked, and helpless, at a weight approximately four per cent of the adult female's. They gain at a rate of at least 1.5 grams per day in the first two months. When they have reached a weight of 100 grams, the gain averages somewhat less than one gram per day, but individual variation is great. Males gain more rapidly than females, especially in the later stages of growth, as adult weight is greater by approximately one-fourth in the male. Some individuals grow to maximum adult size at an age of one year. Unusually large individuals are not necessarily those that are unusually old. Longevity is greater in woodrats than in most smaller rodents. One female of adult size when first trapped was last captured 991 days later when she must have been well over three years old, and others are known to have survived more than two years even though populations were shrinking so that few of the rats were able to survive for their normal life span.
Crabb, W. D.
1941. Food habits of the prairie spotted skunk in southeastern Iowa. Jour. Mamm., 22:349-364.
Fitch, H. S.
1947. Predation by owls in the Sierran foothills of California. Condor, 49:137-151.
Rainey, D. G.
1956. Eastern woodrat, Neotoma floridana: natural history and ecology. Univ. Kansas Publ. Mus. Nat. Hist., 8: No. 10, in press.
End of the Project Gutenberg EBook of Ecological Observations on the
Woodrat, Neotoma floridana, by Henry S. Fitch and Dennis G. Rainey
*** END OF THIS PROJECT GUTENBERG EBOOK ECOLOGICAL OBSERVATIONS--WOODRAT ***
***** This file should be named 33566-h.htm or 33566-h.zip *****
This and all associated files of various formats will be found in:
https://www.gutenberg.org/3/3/5/6/33566/
Produced by Chris Curnow, Joseph Cooper, Josephine Paolucci
and the Online Distributed Proofreading Team at
https://www.pgdp.net.
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License (available with this file or online at
https://gutenberg.org/license).
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS' WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTIBILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation web page at https://www.pglaf.org.
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Its 501(c)(3) letter is posted at
https://pglaf.org/fundraising. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at
809 North 1500 West, Salt Lake City, UT 84116, (801) 596-1887, email
business@pglaf.org. Email contact links and up to date contact
information can be found at the Foundation's web site and official
page at https://pglaf.org
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit https://pglaf.org
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including including checks, online payments and credit card
donations. To donate, please visit: https://pglaf.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For thirty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
https://www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.