
The Project Gutenberg EBook of Encyclopaedia Britannica, 11th Edition,
Volume 6, Slice 5, by Various
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
Title: Encyclopaedia Britannica, 11th Edition, Volume 6, Slice 5
"Clervaux" to "Cockade"
Author: Various
Release Date: March 27, 2010 [EBook #31793]
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK ENCYC. BRITANNICA, VOL 6 SL 5 ***
Produced by Marius Masi, Don Kretz, Juliet Sutherland and
the Online Distributed Proofreading Team at
https://www.pgdp.net
| Transcriber's note: |
A few typographical errors have been corrected. They
appear in the text like this, and the
explanation will appear when the mouse pointer is moved over the marked
passage. Sections in Greek will yield a transliteration
when the pointer is moved over them, and words using diacritic characters in the
Latin Extended Additional block, which may not display in some fonts or browsers, will
display an unaccented version. Links to other EB articles: Links to articles residing in other EB volumes will be made available when the respective volumes are introduced online. |
Articles in This Slice
CLERVAUX (clara vallis), a town in the northern province of Oesling, grand-duchy of Luxemburg, on the Clerf, a tributary of the Sûre. Pop. (1905) 866. In old days it was the fief of the de Lannoy family, and the present proprietor is the bearer of a name not less well known in Belgian history, the count de Berlaymont. The old castle of the de Lannoys exists, and might easily be restored, but its condition is now neglected and dilapidated. In 1798 the people of Clervaux specially distinguished themselves against the French in an attempt to resist the institution of the conscription. The survivors of what was called the Kloppel-krieg (the “cudgel war”) were shot, and a fine monument commemorates the heroism of the men of Clervaux.
CLETUS, formerly regarded as the name of one of the early successors of St Peter in the see of Rome, or, according to Epiphanius and Rufinus, as sharing the direction of the Roman Church with Linus during Peter’s lifetime. He has been identified beyond doubt with Anencletus (q.v.). See Père Colombier, in Rev. des questions hist. Ap. 1st, 1876, p. 413.
CLEVEDON, a watering-place in the northern parliamentary division of Somersetshire, England, on the Bristol Channel, 15½ m. W. of Bristol on a branch of the Great Western railway. Pop. of urban district (1901) 5900. The cruciform church of St Andrew has Norman and later portions; it is the burial-place of Henry Hallam the historian, and members of his family, including his sons Arthur and Henry. Clevedon Court is a remarkable medieval mansion, dating originally from the early part of the 14th century, though much altered in the Elizabethan and other periods. The house is considered to be the original of “Castlewood” in Thackeray’s Esmond; the novelist was acquainted with the place through his friendship with the Rev. William Brookfield and his wife, the daughter of Sir Charles Elton of Clevedon Court.
CLEVELAND, BARBARA VILLIERS, Duchess of (1641-1709), mistress of the English king Charles II., was the daughter of William Villiers, 2nd Viscount Grandison (d. 1643), by his wife Mary (d. 1684), daughter of Paul, 1st Viscount Bayning. In April 1659 Barbara married Roger Palmer, who was created earl of Castlemaine two years later, and soon after this marriage her intimacy with Charles II. began. The king was probably the father of her first child, Anne, born in February 1661, although the paternity was also attributed to one of her earliest lovers, Philip Stanhope, 2nd earl of Chesterfield (1633-1713). Mistress Palmer, as Barbara was called before her husband was made an earl, was naturally much disliked by Charles’s queen, Catherine of Braganza, but owing to the insistence of the king she was made a lady of the bedchamber to Catherine, and began to mix in the political intrigues of the time, showing an especial hatred towards Edward Hyde, earl of Clarendon, who reciprocated this feeling and forbad his wife to visit her. Her house became a rendezvous for the enemies of the minister, and according to Pepys she exhibited a wild paroxysm of delight when she heard of Clarendon’s fall from power in 1667. Whilst enjoying the royal favour Lady Castlemaine formed liaisons with various gentlemen, which were satirized in public prints, and a sharp quarrel which occurred between her and the king in 1667 was partly due to this cause. But peace was soon made, and her influence, which had been gradually rising, became supreme at court in 1667 owing to the marriage of Frances Stuart (la belle Stuart) (1648-1702) with Charles Stuart, 3rd duke of Richmond (1640-1672). Accordingly Louis XIV. instructed his ambassador to pay special attention to Lady Castlemaine, who had become a Roman Catholic in 1663.
In August 1670 she was created countess of Southampton and duchess of Cleveland, with remainder to her first and third sons, Charles and George Palmer, the king at this time not admitting the paternity of her second son Henry; and she also received many valuable gifts from Charles. An annual income of £4700 from the post office was settled upon her, and also other sums chargeable upon the revenue from the customs and the excise, whilst she obtained a large amount of money from seekers after office, and in other ways. Nevertheless her extravagance and her losses at gaming were so enormous that she was unable to keep up her London residence, Cleveland House, St James’s, and was obliged to sell the contents of her residence at Cheam. About 1670 her influence over Charles began to decline. She consoled herself meanwhile with lovers of a less exalted station in life, among them John Churchill, afterwards duke of Marlborough, and William Wycherley; by 1674 she had been entirely supplanted at court by Louise de Kéroualle, duchess of Portsmouth. Soon afterwards the duchess of Cleveland went to reside in Paris, where she formed an intrigue with the English ambassador, Ralph Montagu, afterwards duke of Montagu (d. 1709), who lost his position through some revelations which she made to the king. She returned to England just before Charles’s death in 1685. In July 1705 her husband, the earl of Castlemaine, whom she had left in 1662, died; and in the same year the duchess was married to Robert (Beau) Feilding (d. 1712), a union which was declared void in 1707, as Feilding had a wife living. She died at Chiswick on the 5th of October 1709.
Bishop Burnet describes her as “a woman of great beauty, but most enormously vicious and ravenous, foolish but imperious, ever uneasy to the king, and always carrying on intrigues with other men, while yet she pretended she was jealous of him.” Dryden addressed Lady Castlemaine in his fourth poetical Epistle in terms of great adulation, and Wycherley dedicated to her his first play, Love in a Wood. Her portrait was frequently painted by Sir Peter Lely and others, and many of these portraits are now found in various public and private collections. By Charles II. she had three sons and either one or two daughters. 501 She had also in 1686 a son by the actor Cardonnell Goodman (d. 1699), and one or two other daughters.
Her eldest son, Charles Fitzroy (1662-1730), was created in 1675 earl of Chichester and duke of Southampton, and became duke of Cleveland and earl of Southampton on his mother’s death. Her second son, Henry (1663-1690), was created earl of Euston in 1672 and duke of Grafton in 1675; by his wife Isabella, daughter of Henry Bennet, earl of Arlington, he was the direct ancestor of the later dukes of Grafton; he was the most popular and the most able of the sons of Charles II., saw a considerable amount of military service, and met his death through a wound received at the storming of Cork. Her third son, George (1665-1716), was created duke of Northumberland in 1683, and died without issue, after having served in the army. Her daughters were Anne (1661-1722), married in 1674 to Thomas Lennard, Lord Dacre (d. 1715), who was created earl of Sussex in 1684; Charlotte (1664-1718), married in 1677 to Edward Henry Lee, earl of Lichfield (d. 1716); and Barbara (1672-1737), the reputed daughter of John Churchill, who entered a nunnery in France, and became by James Douglas, afterwards 4th duke of Hamilton (1658-1712), the mother of an illegitimate son, Charles Hamilton (1691-1754).
The first husband of the duchess, Roger Palmer, earl of Castlemaine (1634-1705), diplomatist and author, was an ardent Roman Catholic, who defended his co-religionists in several publications. Having served in the war against Holland in 1665-67, he wrote in French an account of this struggle, which was translated into English and published by T. Price in London in 1671. Having been denounced by Titus Oates as a Jesuit, he was tried and acquitted, afterwards serving James II. as ambassador to Pope Innocent XI., a mission which led to a brief imprisonment after the king’s flight from England. Subsequently his Jacobite sympathies caused him to be suspected by the government, and his time was mainly spent either in prison or in exile. The earl died at Oswestry on the 21st of July 1705.
The title of duke of Cleveland, which had descended in 1709 to Charles Fitzroy, together with that of duke of Southampton, became extinct when Charles’s son William, the 2nd duke, died without issue in 1774. One of the first duke’s daughters, Grace, was married in 1725 to Henry Vane, 3rd Baron Barnard, afterwards earl of Darlington (d. 1758), and their grandson William Henry Vane (1766-1842) was created duke of Cleveland in 1833. The duke was succeeded in the title in turn by three of his sons, who all died without male issue; and consequently when Harry George, the 4th duke, died in 1891 the title again became extinct.
Previous to the creation of the dukedom of Cleveland there was an earldom of Cleveland which was created in 1626 in favour of Thomas, 4th Baron Wentworth (1591-1667), and which became extinct on his death.
CLEVELAND (or Cleiveland), JOHN (1613-1658), English poet and satirist, was born at Loughborough, where he was baptized on the 20th of June 1613. His father was assistant to the rector and afterwards vicar of Hinckley. John Cleveland was educated at Hinckley school under Richard Vines, who is described by Fuller as a champion of the Puritan party. In his fifteenth year he was entered at Christ’s College, Cambridge, and in 1634 was elected to a fellowship at St John’s. He took his M.A. degree in 1635, and was appointed college tutor and reader in rhetoric. His Latinity and oratorical powers were warmly praised by Fuller, who also commends the “lofty fancy” of his verse. He eagerly opposed the candidature of Oliver Cromwell as M.P. for Cambridge, and when the Puritan party triumphed there Cleveland, like many other Cambridge students, found his way (1643) to Oxford. His gifts as a satirist were already known, and he was warmly received by the king, whom he followed (1645) to Newark. In that year he was formally deprived of his Cambridge fellowship as a “malignant.” He was judge-advocate in the garrison at Newark, and under the governor defended the town until in 1646 Charles I. ordered the surrender of the place to Leslie; when there is a curious story that the Scottish general contemptuously dismissed him as a mere ballad-monger. He saw Charles’s error in giving himself into the hands of the Scots, and his indignation when they surrendered the king to the Parliament is expressed in the vigorous verses of “The Rebel Scot,” the sting of which survives even now. Cleveland wandered over the country depending on the alms of the Royalists for bread. He at length found a refuge at Norwich in the house of Edward Cooke, but in 1655 he was arrested as being of no particular occupation, and moreover a man whose great abilities “rendered him able to do the greater disservice.” He spent three months in prison at Yarmouth, but was released by order of Cromwell, to whom he addressed a manly appeal, in which he declared his fidelity to the royal house, pointing out at the same time that his poverty and inoffensiveness were sufficient assurance that his freedom was no menace to Cromwell’s government. He was released early in 1656, and seems to have renewed his wanderings, finding his way eventually to Gray’s Inn, where Aubrey says he and Samuel Butler had a “club” every night. There he died on the 29th of April 1658.
Cleveland’s poems were more highly esteemed than Milton’s by his contemporaries, and his popularity is attested by the very numerous editions of his works. His poems are therefore of great value as an index to the taste of the 17th century. His verse is frequently obscure and full of the far-fetched conceits of the “metaphysical” poets, none of whom surpassed the ingenuity of “Fuscara, or the Bee Errant.” His satires are vigorous personal attacks, the interest of which is, from the nature of the subject, often ephemeral; but the energy of his invective leaves no room for obscurity in such pieces as “Smectymnuus, or the Club Divines,” “Rupertismus” and “The Rebel Scot.”
Cleveland’s works are: “Character of a London Diurnal,” a broadside; Monumentum regale ... (1649), chiefly by Cleveland, containing three of his elegies on the king; “The King’s Disguise” (1646); “On the Memory of Mr Edward King,” in the collection of verse which also included Milton’s “Lycidas,” and many detached poems.
CLEVELAND, STEPHEN GROVER (1837-1908), president of the United States from 1885 to 1889, and again from 1893 to 1897, was born, the fifth in a family of nine children, in the village of Caldwell, Essex county, New Jersey, on the 18th of March 1837. His father, Richard F. Cleveland, a clergyman of the Presbyterian Church, was of good colonial stock, a descendant of Moses Cleveland, who emigrated from Ipswich, England, to Massachusetts in 1635. The family removed to Fayetteville, N.Y., and afterwards to Clinton, N.Y. It was intended that young Grover should be educated at Hamilton College, but this was prevented by his father’s death in 1852. A few years later he drifted westward with twenty-five dollars in his pocket, and the autumn of 1855 found him in a law office in the city of Buffalo. At the end of four years (1859), he was admitted to the bar.
In 1863 he was appointed assistant district attorney of Erie county, of which Buffalo is the chief city. This was his first 502 public office, and it came to him, like all later preferments, without any solicitation of his own. Two years later (1865) he was the Democratic candidate for district attorney, but was defeated. In 1869 Cleveland was nominated by the Democratic party for the office of sheriff, and, despite the fact that Erie county was normally Republican by a decisive majority, was elected. The years immediately succeeding his retirement from the office of sheriff in 1873 he devoted exclusively to the practice of law, coming to be generally recognized as one of the leaders of the western New York bar. In the autumn of 1881 he was nominated by the Democrats for mayor of Buffalo. The city government had been characterized by extravagance and maladministration, and a revolt of the independent voters at the polls overcame the usual Republican majority and Cleveland was elected. As mayor he attracted wide attention by his independence and business-like methods, and under his direction the various departments of the city government were thoroughly reorganized. His ability received further recognition when in 1882 he was nominated by his party as its candidate for governor. The Republican party in the state was at that time weakened by the quarrels between the “Stalwart” and “Halfbreed” factions within its ranks; and the Democrats were thus given an initial advantage which was greatly increased by the Republicans’ nomination for governor of Charles J. Folger (1818-1884), then secretary of the treasury. Secretary Folger was a man of high character and ability, who had been chief justice of the New York supreme court when placed in control of the treasury department by President Arthur in 1881. But the cry of Federal interference was raised as a result of the methods employed in securing his nomination, and this, together with the party division and the popularity of Cleveland, brought about Cleveland’s election by the unprecedented plurality of 192,854. As governor Cleveland’s course was marked by the sterling qualities that he had displayed in his other public positions. His appointees were chosen for their business qualifications. The demands of party leaders were made subordinate to public interests. He promoted the passage of a good civil service law. All bills passed by the legislature were subjected to the governor’s laborious personal scrutiny, and the veto power was used without fear or favour.
In 1884 the Democratic party had been out of power in national affairs for twenty-three years. In this year, however, the generally disorganized state of the Republican party seemed to give the Democrats an unusual opportunity. Upon a platform which called for radical reforms in the administrative departments, the civil service, and the national finances, Cleveland was nominated for president, despite the opposition of the strong Tammany delegation from his own state. The nominee of the Republican party, James G. Blaine (q.v.) of Maine, had received the nomination only after a contest in which violent personal animosities were aroused. The campaign that followed was one of the bitterest political contests in American history. The Republican party was still further weakened by the defection of a large body of independents, known as “Mugwumps.” The result was close, but Cleveland carried New York, and was elected, obtaining a majority in the electoral college of 219 to 182.
Cleveland’s first term was uneventful, but was marked by firmness, justice and steady adherence on his part to the principles which he deemed salutary to the nation. He was especially concerned in promoting a non-partisan civil service. Congress in 1883 had passed the “Pendleton Bill” (introduced by Senator George H. Pendleton) to classify the subordinate places in the service, and to make entrance to it, and promotion therein, depend upon competitive examination of applicants, instead of mere political influence. The first test of the efficiency and permanence of this law came with the shifting of political power at Washington. The new president stood firmly by the new law. It applied only to places of the rank of clerkships, but the president was authorized to add others to the classified service from time to time. He added 11,757 during his first term.
President Cleveland made large use of the veto power upon bills passed by Congress, vetoing or “pocketing” during his first term 413 bills, more than two-thirds of which were private pension bills. The most important bill vetoed was the Dependent Pension Bill, a measure of extreme profligacy, opening the door, by the vagueness of its terms, to enormous frauds upon the treasury. In 1887 there was a large and growing surplus in the treasury. As this money was drawn from the channels of business and locked up in the public vaults, the president looked upon the condition as fraught with danger to the commercial community and he addressed himself to the task of reducing taxation. About two-thirds of the public revenue was derived from duties on imports, in the adjustment of which the doctrine of protection to native industry had a large place. Cleveland attacked the system with great vigour in his annual message of 1887. He did not propose the adoption of free trade, but the administration tariff measure, known as the Mills Bill, from its introducer Congressman Roger Q. Mills (b. 1832) of Texas, passed the House, and although withdrawn owing to amendments in the Republican Senate, it alarmed and exasperated the protected classes, among whom were many Democrats, and spurred them to extraordinary efforts to prevent his re-election.
In the following year (1888), however, the Democrats renominated Cleveland, and the Republicans nominated Benjamin Harrison of Indiana. The campaign turned on the tariff issue, and Harrison was elected, receiving 233 electoral votes to 168 for Cleveland, who however received a popular plurality of more than 100,000. Cleveland retired to private life and resumed the practice of the law in New York. He had married on the 2nd of June 1886 Miss Frances Folsom, a daughter of a former law partner in Buffalo.
Congress had passed a law in 1878 requiring the treasury department to purchase a certain amount of silver bullion each month and coin it into silver dollars to be full legal tender. As no time had been fixed for this operation to cease, it amounted to an unlimited increase of a kind of currency that circulated at a nominal value much above its real value. Both political parties were committed to this policy, and strong passions were aroused whenever it was called in question. Cleveland had written a letter for publication before he became president, saying that a financial crisis of great severity must result if this coinage were continued, and expressing the hope that Congress would speedily put an end to it. In 1890 Congress, now controlled by the Republican party, passed the McKinley Bill, by which the revenues of the government were reduced by more than $60,000,000 annually, chiefly through a repeal of the sugar duties. At the same time expenditures were largely increased by liberal pension legislation, and the government’s purchase of silver bullion almost doubled by the provisions of the new Sherman Silver Purchase Act of 1890.
In 1892 Cleveland was nominated for president a third time in succession. President Harrison was nominated by the Republicans. Cleveland received 277 electoral votes and Harrison 145, and 22 were cast for James B. Weaver (b. 1833) of Iowa, the candidate of the “People’s” party. Cleveland’s second term embraced some notable events. The most important was the repeal of the silver legislation, which had been a growing menace for fifteen years. Nearly $600,000,000 of “fiat money” had been thrust into the channels of commerce in addition to $346,000,000 of legal tender notes that had been issued during the Civil War. A reserve of $100,000,000 of gold had been accumulated for the redemption of these notes. In April 1893 the reserve fell below this sum. President Cleveland called an extra session of Congress to repeal the Silver Law. The House promptly passed the repealing act. In the Senate there was a protracted struggle. The Democrats now had a majority of that body and they were more decidedly pro-silver than the Republicans. The president had undertaken to coerce his own party to do something against its will, and it was only by the aid of the Republican minority that the passage of the repealing bill was at last made possible (October 30th). The mischief, however, was not ended. The deficit in the treasury made it inevitable that the gold reserve should be used to meet current expenses. Holders of the government’s legal tender notes anticipating this fact presented them for redemption. Borrowing was 503 resorted to by the government. Bonds were issued and sold to the amount of $162,000,000. The business world was in a state of constant agitation. Bank failures were numerous and commercial distress widespread. Among the consequences of the panic was a reduction of wages in many employments, accompanied by labour troubles more or less serious. The centre of disturbance was the Pullman strike at Chicago (q.v.), whence the disorder extended to the Pacific coast, causing riot and bloodshed in many places. President Cleveland waited a reasonable time, as he conceived, for Governor Altgeld of Illinois to put an end to the disorder in that state. On the 6th of July 1894, despite Governor Altgeld’s protest, he directed the military forces of the United States to clear the way for trains carrying the mails. The rioters in and around Chicago were dispersed in a single day, and within a week the strike was broken.
Another important event was the action of the government as regards the question of arbitration between Great Britain and Venezuela (q.v.), in which Richard Olney, the secretary of state, played a somewhat aggressive part. On the 17th of December 1895 President Cleveland sent to Congress a special message calling attention to Great Britain’s action in regard to the disputed boundary line between British Guiana and Venezuela, and declaring the necessity of action by the United States to prevent an infringement of the Monroe Doctrine. Congress at once appropriated funds for an American commission to investigate the matter. The diplomatic situation became for the moment very acute, but after a short period of bellicose talk the common-sense of both countries prevailed. Negotiations with Great Britain ensued, and before the American special commission finished its work, Great Britain had agreed, November 1896, to arbitrate on terms which safeguarded the national dignity on both sides.
Cleveland’s independence was nowhere more strikingly shown during his second term than in his action in regard to the tariff legislation of his party in Congress. A tariff bill introduced in the House by William Lyne Wilson (1843-1900), of West Virginia, chairman of the Committee of Ways and Means, was so amended in the Senate, through the instrumentality of Senator Arthur Pue Gorman and a coterie of anti-administration democratic senators, that when the bill eventually came before him, although unwilling to veto it, the president signified his dissatisfaction with its too high rates by allowing it to become a law without his signature. Cleveland’s second administration began by vigorous action in regard to Hawaii; he at once withdrew from the Senate the annexation treaty which President Harrison had negotiated.
During his second term Cleveland added 44,004 places in the civil service to the classified list, bringing them within the rules of the merit system. This was a greater number than all that had been placed in the list before, and brought the whole number up to 86,932. Toward the end of his second term the president became very much out of accord with his party on the free-silver question, in consequence of which the endorsement of the administration was withheld by the Democratic national convention at Chicago in 1896. In the ensuing campaign the president and his cabinet, with the exception of Hoke Smith (b. 1855), secretary of the interior, who resigned, gave their support to Palmer and Buckner, the National, or “Sound Money” Democratic nominees.
Cleveland’s second term expired on the 4th of March 1897, and he then retired into private life, universally respected and constantly consulted, in the university town of Princeton, New Jersey, where he died on the 24th of June 1908. He was a trustee of Princeton University and Stafford Little lecturer on public affairs. Chosen in 1905 as a member of a committee of three to act as trustees of the majority of the stock of the Equitable Life Assurance Company, he promoted the reorganization and the mutualization of that company, and acted as rebate referee for it and for the Mutual and New York Life insurance companies. He published Presidential Problems (New York, 1904), made up in part of lectures at Princeton University, and Fishing and Hunting Sketches (1906).
CLEVELAND, a city and port of entry in the state of Ohio, U.S.A., and the county-seat of Cuyahoga county, the sixth largest city in the United States. It is on Lake Erie at the mouth of Cuyahoga river, about 260 m. N.E. of Cincinnati, 357 m. E. of Chicago, and 623 m. W. by N. of New York. Pop. (1890) 261,353; (1900) 381,768, of whom 124,631 were foreign-born, 288,591 were of foreign parentage (i.e. having one or both parents foreign-born), and 5988 were negroes; (1910) 560,663. Of the 124,631, who in 1900 were foreign-born, Germans were greatly predominant (40,648, or 32.6%), with the Bohemians (13,599, or 10.9%) and Irish (13,120, or 10.6%) next in importance, the Bohemians being later comers than the Irish.
The city commands pleasant views from its position on a plateau, which, at places on bluffs along the shore, has elevations of about 75 ft. above the water below, and rises gradually toward the S.E. to 115 ft. and on the extreme E. border to more than 200 ft. above the lake, or about 800 ft. above sea-level; the surface has, however, been cut deeply by the Cuyahoga, which here pursues a meandering course through a valley about ½ m. wide, and is also broken by several smaller streams. The city’s shore-line is more than 12 m. long. The city varies considerably in width, and occupies a total area of about 41 sq. m., much the greater part of which is E. of the river. The streets are of unusual width (varying from 60 ft. to 132 ft.); are paved chiefly with Medina dressed stone, brick and asphalt; and, like the parks, are so well shaded by maples, elms and other trees, that Cleveland has become known as the “Forest City.” The municipality maintains an efficient forestry department. About ½ m. from the lake and the same distance E. of the river is the Public Square, or Monumental Park, in the business centre of the city. Thence the principal thoroughfares radiate. The river is spanned with bridges, and its valley by two viaducts, the larger of which (completed in 1878 at a cost of more than $2,000,000), 3211 ft. long, 64 ft. wide, and 68 ft. above water, connects Superior Avenue on the E. with Detroit Avenue on the W. The Central Viaduct, finished in 1888, extends from Central Avenue to W. 14th Street, and there connects with a smaller viaduct across Walworth Run, the combined length of the two being about 4000 ft. Another viaduct (about 830 ft. long) crosses Kingsbury Run a short distance above its mouth. Lower Euclid Avenue (the old country road to Euclid, O., and Erie, Pa.) is given up to commercial uses; the eastern part of the avenue has handsome houses with spacious and beautifully ornamented grounds, and is famous as one of the finest residence streets in the country. Sections of Prospect Avenue, E. 40th, E. 93rd, E. 75th, E. 55th, W. 44th and E. 79th streets also have many fine residences. The principal business thoroughfares are Superior Avenue (132 ft. wide), the W. part of Euclid Avenue, and Ontario St. The manufacturing quarters are chiefly in the valley of the Cuyahoga, and along the railway tracks entering the city, chiefly on the E. side. In 1902 the city arranged for grouping its public buildings—in the so-called “Group Plan”—at a cost of $25,000,000. The court-house and city hall are on the bluff overlooking Lake Erie; 1000 ft. south are the Federal post-office and the public library. The Mall connecting the court-house and city hall with the post-office and library is 600 ft. wide; on one side of it is the grand music-hall, on the other a fine art gallery. The six granite buildings forming this quadrangle were built under the supervision of Arnold Brunner, a government architect, and of John M. Carrere and D. H. Burnham, 504 who planned the buildings at the Pan-American Exposition and the Chicago World’s Fair respectively. The city has, besides, numerous fine office buildings, including that of the Society for Savings (an institution in which each depositor is virtually a stockholder), the Citizens’, Rose, Williamson, Rockefeller, New England and Garfield buildings; and several beautiful churches, notably the Roman Catholic and Trinity cathedrals, the First Presbyterian (“Old Stone”), the Second Presbyterian, the First Methodist and Plymouth (Congregational) churches. The Arcade, between Euclid and Superior avenues, and the Colonial Arcade, between Euclid and Prospect avenues, are office and retail store buildings worthy of mention. The former, finished in 1889, is 400 ft. long, 180 ft. wide, and 140 ft. high, with a large interior court, overlooked by five balconies. The Colonial Arcade contains a hotel as well; it was finished in 1898. In the Public Square is a soldiers’ and sailors’ monument consisting of a granite shaft rising from a memorial room to a height of 125 ft., and surmounted with a figure of Liberty; in the same park, also, is a bronze statue of Moses Cleaveland, the founder of the city. On a commanding site in Lake View Cemetery is the Garfield Memorial (finished in 1890) in the form of a tower (165 ft. high), designed by George Keller and built mostly of Ohio sandstone; in the base is a chapel containing a statue of Garfield and several panels on which are portrayed various scenes in his life; his remains are in the crypt below the statue. A marble statue of Commodore Oliver H. Perry, erected in commemoration of his victory on Lake Erie in 1813, is in Wade Park, where there is also a statue of Harvey Rice (1800-1891), who reformed the Ohio public school system and wrote Pioneers of the Western Reserve (1882) and Sketches of Western Life (1888).
The parks contain altogether more than 1500 acres. A chain of parks connected by driveways follows the picturesque valley of Doan Brook on the E. border of the city. At the mouth of the brook and on the lake front is the beautiful Gordon Park of 122 acres, formerly the private estate of William J. Gordon but given by him to the city in 1893; from this extends up the Doan Valley the large Rockefeller Park, which was given to the city in 1896 by John D. Rockefeller and others, and which extends to and adjoins Wade Park (85 acres; given by J. H. Wade) in which are a zoological garden and a lake. Lake View Park along the lake shore contains only 10½ acres, but is a much frequented resting-place near the business centre of the city, and affords pleasant views of the lake and its commerce. Monumental Park is divided into four sections (containing about 1 acre each) by Superior Avenue and Ontario Street. Of the several cemeteries, Lake View (about 300 acres), on an elevated site on the E. border, is by far the largest and most beautiful, its natural beauty having been enhanced by the landscape gardener. Besides Garfield, John Hay and Marcus A. Hanna are buried here.
Education.—Cleveland has an excellent public school system. A general state law enacted in 1904 placed the management of school affairs in the hands of an elective council of seven members, five chosen at large and two by districts. This board has power to appoint a school director and a superintendent of instruction. The superintendent appoints the teaching force, the director all other employés; appointments are subject to confirmation by the board, and all employés are subject to removal by the executive officials alone. The “Cleveland plan,” in force in the public schools, minimizes school routine, red tape and frequent examinations, puts great stress on domestic and manual training courses, and makes promotion in the grammar schools depend on the general knowledge and development of the pupil, as estimated by a teacher who is supposed to make a careful study of the individual. In 1909 there were 8 high schools and 90 grammar schools in the city; more than $2,500,000 is annually expended by Cleveland on its public schools. Besides the public school system there are many parochial schools; the University school, with an eight years’ course; the Western Reserve University, with its medical school (opened in 1843), the Franklin T. Backus Law School (1892), the dental department (1892), Adelbert College (until 1882 the Western Reserve College, founded in 1826, at Hudson, Ohio), the College for Women (1888), and the Library school (1904); St Ignatius College (Roman Catholic, conducted by the Fathers of the Society of Jesus; incorporated 1890), which has an excellent meteorological observatory; St Mary’s theological seminary (Roman Catholic); the Case School of Applied Science, founded in 1880 by Leonard Case (1820-1880), and opened in 1881; the Cleveland College of Physicians and Surgeons (founded in 1863; from 1869 until 1896 the medical department of the University of Wooster; since 1896 a part of Ohio Wesleyan University, Delaware, Ohio), the Cleveland Homeopathic Medical College, the Cleveland School of Pharmacy, the Cleveland Art School, and a school for the deaf, dumb and blind. In 1907-1908 Western Reserve University had 193 instructors and 914 students (277 in Adelbert College; 269 in College for Women; 20 in graduate department; and 102 in medical, 133 in law, 75 in dental and 51 in Library school); and the Case School of Applied Science 40 instructors and 440 students. The public library contained 330,000 volumes in 1908, the Case library (subscription) 65,000 volumes, the Hatch library of Adelbert College about 56,000 volumes, the library of the Western Reserve Historical Society 22,500 volumes, and the Cleveland law library, in the court house, 20,000 volumes.
The city has a highly developed system of charitable and corrective institutions. A farm of more than 1600 acres, the Cleveland Farm Colony, 11 m. from the city, takes the place of workhouses, and has many cottages in which live those of the city’s poor who were formerly classed as paupers and were sent to poorhouses, and who now apply their labour to the farm and are relieved from the stigma that generally attaches to inmates of poorhouses. On the “farm” the city maintains an “infirmary village,” a tuberculosis sanatorium, a detention hospital, a convalescent hospital and houses of correction. On a farm 22 m. from the city is the Boyville Home (maintained in connexion with the juvenile court) for “incorrigible” boys. The “cottage” plan has been adopted; each cottage is presided over by a man and wife whom the boys call father and mother. The boys have a government of their own, elect their officials from among themselves, and inflict such punishment on any of their number as the boys deem merited. Besides the city, there are the Northern Ohio (for the insane, founded in 1855), the Cleveland general. Lake Side (endowed), St Alexis and the Charity hospitals (the last managed by Sisters of Charity). The Goodrich House (1897), the Hiram House and the Alta House are among the best equipped and most efficient social settlements in the country. Cleveland has also its orphan asylums, homes for the aged, homes for incurables, and day nurseries, besides a home for sailors, homes for young working women, and retreats for unfortunate girls. The various charity and benevolent institutions are closely bound together on a co-operative basis by the agency of the associated charities.
The principal newspapers of the city are the Plain Dealer (1841, independent), the Press (1878, independent), the Leader (1847, Republican), and the News (1889, Republican). Bohemian, Hungarian and German dailies are published.
Municipal Enterprise.—Municipal ownership has been a greater issue in Cleveland than in any other large city in the United States, chiefly because of the advocacy of Tom Loftin Johnson (born 1854), a street-railway owner, iron manufacturer, an ardent single-taxer, who was elected mayor of the city in 1901, 1903, 1905 and 1907. The municipality owns the water-works, a small electric-light plant, the garbage plant and bath houses. The city water is pumped to reservoirs, through a tunnel 9 ft. in diameter 60 ft. below the bottom of the lake, from an intake situated a distance of 26,500 ft. from the shore. The system has a delivery capacity of 80,000,000 gallons daily. The department serves about 70,000 consumers. All water is metered and sells for 40 cents per thousand cub. ft., or 5 barrels for 1 cent. The municipal electric-lighting plant does not seriously compete with the private lighting company. The municipal garbage plant (destructor) collects and reduces to fertilizer 100 tons of garbage per day. The sale of the fertilizer more than pays for the cost of reduction, and the only expense the city has is in collecting it. In the city’s six bath houses the average number of baths per day, 505 per house, in 1906, was 1165. The municipal street cleaning department cleans all streets by the wet process. To do this the city maintained (1906) 24 flushing wagons working 2 shifts of 8 hours each per day. A new street car company began operations on the 1st of November 1906, charging a 3 cent fare. The grants of this company were owned by the Forest City Railway Company and the property was leased to the Municipal Traction Company (on behalf of the public—the city itself not being empowered to own and operate street railways). In 1908 the Cleveland Electric Street Railway Corporation (capital $23,000,000), which owned most of the electric lines in the city, was forced to lease its property to the municipality’s holding company, receiving a “security franchise,” providing that under certain circumstances (e.g. if the holding company should default in its payment of interest) the property was to revert to the corporation, which was then to charge not more than twenty-five cents for six tickets. In October 1908, at a special election, the security franchise was invalidated, and the entire railway system was put in the hands of receivers. In 1909 Johnson was defeated. In 1910 a 25-year franchise was granted to the Cleveland Railway Company, under which a 3-cent fare is required if the company can earn 6% on that basis, and 4 cents (7 tickets for 25 cents) is the maximum fare, with a cent transfer charge, returned when the transfer is used.
Commerce.—To meet the demands of the rapidly increasing commerce the harbour has been steadily improved. In 1908 it consisted of two distinct parts, the outer harbour being the work of the federal government, and the inner harbour being under the control of the city. The outer harbour was formed by two breakwaters enclosing an area of 2 m. long and 1700 ft. wide; the main entrance, 500 ft. wide, lying opposite the mouth of the Cuyahoga river, 1350 ft. distant. The depth of the harbour ranges from 21 to 26 ft.; and by improving this entrance, so as to make it 700 ft. wide, and 1000 ft. farther from the shore, and extending the east breakwater 3 m., the capacity of the outer harbour has been doubled. The inner harbour comprises the Cuyahoga, the old river bed, and connecting slips. The channel at the mouth of the river (325 ft. wide) is lined on the W. side by a concrete jetty 1054 ft. long, and on the E. side by commercial docks. The river and old river bed furnish about 13 m. of safe dock frontage, the channel having been dredged for 6 m. to a depth of 21 ft. The commerce of the harbour of Cleveland in 1907 was 12,872,448 tons.
Cleveland’s rapid growth both as a commercial and as a manufacturing city is due largely to its situation between the iron regions of Lake Superior and the coal and oil regions of Pennsylvania and Ohio. Cleveland is a great railway centre and is one of the most important ports on the Great Lakes. The city is served by the Lake Shore & Michigan Southern; the New York, Chicago & St Louis; the Cleveland, Cincinnati, Chicago & St Louis; the Pennsylvania; the Erie; the Baltimore & Ohio; and the Wheeling & Lake Erie railways; by steamboat lines to the principal ports on the Great Lakes; and by an extensive system of inter-urban electric lines. Cleveland is the largest ore market in the world, and its huge ore docks are among its most interesting features; the annual receipts and shipments of coal and iron ore are enormous. It is also the largest market for fresh-water fish in America, and handles large quantities of lumber and grain. The most important manufactures are iron and steel, carriage hardware, electrical supplies, bridges, boilers, engines, car wheels, sewing machines, printing presses, agricultural implements, and various other commodities made wholly or chiefly from iron and steel. Other important manufactures are automobiles (value, 1905, $4,256,979) and telescopes. More steel wire, wire nails, and bolts and nuts are made here than in any other city in the world (the total value for iron and steel products as classified by the census was, in 1905, $42,930,995, and the value of foundry and machine-shop products in the same year was $18,832,487), and more merchant vessels than in any other American city. Cleveland is the headquarters of the largest shoddy mills in the country (value of product, 1905, $1,084,594), makes much clothing (1905, $10,426,535), manufactures a large portion of the chewing gum made in the United States, and is the site of one of the largest refineries of the Standard Oil Company. The product of Cleveland breweries in 1905 was valued at $3,986,059, and of slaughtering and meat-packing houses in the same year at $10,426,535. The total value of factory products in 1905 was $172,115,101, an increase of 36.4% since 1900; and between 1900 and 1905 Cleveland became the first manufacturing city in the state.
Government.—Since Cleveland became a city in 1836 it has undergone several important changes in government. The charter of that year placed the balance of power in a council composed of three members chosen from each ward and as many aldermen as there were wards, elected on a general ticket. From 1852 to 1891 the city was governed under general laws of the state which entrusted the more important powers to several administrative boards. Then, from 1891 to 1903, by what was practically a new charter, that which is known as the “federal plan” of government was tried; this centred power in the mayor by making him almost the only elective officer, by giving to him the appointment of his cabinet of directors—one for the head of each of the six municipal departments—and to each director the appointment of his subordinates. The federal plan was abandoned in 1903, when a new municipal code went into effect, which was in operation until 1909, when the Paine Law established a board of control, under a government resembling the old federal plan. (For laws of 1903 and 1909 see Ohio.) Few if any cities in the Union have, in recent years, been better governed than Cleveland, and this seems to be due largely to the keen interest in municipal affairs which has been shown by her citizens. Especially has this been manifested by the Cleveland Chamber of Commerce and by the Municipal Association, an organization of influential professional and business men, which, by issuing bulletins concerning candidates at the primaries and at election time, has done much for the betterment of local politics. The Cleveland Chamber of Commerce, an organization of 1600 leading business men, is a power for varied good in the city; besides its constant and aggressive work in promoting the commercial interests of the city, it was largely influential in the federal reform of the consular service; it studied the question of overcrowded tenements and secured the passage of a new tenement law with important sanitary provisions and a set minimum of air space; it urges and promotes home-gardening, public baths and play-grounds, and lunch-rooms, &c., for employés in factories; and it was largely instrumental in devising and carrying out the so-called “Group Plan” described above.
History.—A trading post was established at the mouth of the Cuyahoga river as early as 1786, but the place was not permanently settled until 1796, when it was laid out as a town by Moses Cleaveland (1754-1806), who was then acting as the agent of the Connecticut Land Company, which in the year before had purchased from the state of Connecticut a large portion of the Western Reserve. In 1800 the entire Western Reserve was erected into the county of Trumbull and a township government was given to Cleveland; ten years later Cleveland was made the seat of government of the new county of Cuyahoga, and in 1814 it was incorporated as a village. Cleveland’s growth was, however, very slow until the opening of the Ohio canal as far as Akron in 1827; about the same time the improvement of the harbour was begun, and by 1832 the canal was opened to the Ohio river. Cleveland thus was connected with the interior of the state, for whose mineral and agricultural products it became the lake outlet. The discovery of iron ore in the Lake Superior region made Cleveland the natural meeting-point of the iron ore and the coal from the Ohio, Pennsylvania and West Virginia mines; and it is from this that the city’s great commercial importance dates. The building of railways during the decade 1850-1860 greatly increased this importance, and the city grew with great rapidity. The growth during the Civil War was partly due to the rapid development of the manufacturing interests of the city, which supplied large quantities of iron products and of clothing to the Federal government. 506 The population of 1076 in 1830 increased to 6071 in 1840, to 17,034 in 1850, to 43,417 in i860, to 92,829 in 1870 and to 160,146 in 1880. Until 1853 the city was confined to the E. side of the river, but in that year Ohio City, which was founded in 1807, later incorporated as the village of Brooklyn, and in 1836 chartered as a city (under the name Ohio City), was annexed. Other annexations followed: East Cleveland in 1872, Newburg in 1873, West Cleveland and Brooklyn in 1893, and Glenville and South Brooklyn in 1905. In recent history the most notable events not mentioned elsewhere in this article were the elaborate celebration of the centennial of the city in 1896 and the street railway strike of 1899, in which the workers attempted to force a redress of grievances and a recognition of their union. Mobs attacked the cars, and cars were blown up by dynamite. The strikers were beaten, but certain abuses were corrected. There was a less violent street car strike in 1908, after the assumption of control by the Municipal Traction Company, which refused to raise wages according to promises made (so the employees said) by the former owner of the railway; the strikers were unsuccessful.
Authorities.—Manual of the City Council (1879); Annuals of the Cleveland Chamber of Commerce (1894- ); E. M. Avery, Cleveland in a Nutshell: An Historical and Descriptive Ready-reference Book (Cleveland, 1893); James H. Kennedy, A History of the City of Cleveland (Cleveland, 1896); C. A. Urann, Centennial History of Cleveland (Cleveland, 1896); C. Whittlesey, The Early History of Cleveland (Cleveland, 1867); C. E. Bolton, A Few Civic Problems of Greater Cleveland (Cleveland, 1897); “Plan of School Administration,” by S. P. Orth, in vol. xix. Political Science Quarterly (New York, 1904); Charles Snavely, A History of the City Government of Cleveland (Baltimore, 1902); C. C. Williamson, The Finances of Cleveland (New York, 1907); “The Government of Cleveland, Ohio,” by Lincoln Steffens, in McClure’s Magazine, vol. xxv. (New York, 1905); and C. F. Thwing, “Cleveland, the Pleasant City,” in Powell’s Historic Towns of the Western States (New York, 1901).
CLEVER, an adjective implying dexterous activity of mind or body, and ability to meet emergencies with readiness and adroitness. The etymology and the early history of the word are obscure. The earliest instance quoted by the New English Dictionary is in the Bestiary of c. 1200 (An Old English Miscellany, ed. R. Morris, 1872, E.E.T.S. 49)—“On the clothed the neddre (adder) is cof (quick) and the devel cliver on sinnes,” i.e. quick to seize hold of; this would connect the word with a M. Eng. “cliver” or “clivre,” a talon or claw (so H. Wedgwood, Dict. of Eng. Etym.). The ultimate original would be the root appearing in “claw,” “cleave,” “cling,” “clip,” &c., meaning to “stick to.” This original sense probably survives in the frequent use of the word for nimble, dexterous, quick and skilful in the use of the hands, and so it is often applied to a horse, “clever at his fences.” The word has also been connected with O. Eng. gléaw, wise, which became in M. Eng. gleu, and is cognate with Scottish gleg, quick of eye. As to the use of the word, Sir Thomas Browne mentions it among “words of no general reception in English but of common use in Norfolk or peculiar to the East Angle countries” (Tract. viii. in Wilkins’s ed. of Works, iv. 205). The earlier uses of the word seem to be confined to that of bodily dexterity. In this sense it took the place of a use of “deliver” as an adjective, meaning nimble, literally “free in action,” a use taken from Fr. delivre (Late Lat. deliberare, to set free), cf. Chaucer, Prologue to Cant. Tales, 84, “wonderly deliver and grete of strength,” and Romaunt of the Rose, 831, “Deliver, smert and of gret might.” It has been suggested that “clever” is a corruption of “deliver” in this sense, but this is not now accepted. The earliest use of the word for mental quickness and ability in the New English Dictionary is from Addison in No. 22 of The Freeholder (1716).
CLEVES (Ger. Cleve or Kleve), a town of Germany in the kingdom of Prussia, formerly the capital of the duchy of its own name, 46 m. N.W. of Düsseldorf, 12 m. E. of Nijmwegen, on the main Cologne-Amsterdam railway. Pop. (1900) 14,678. The town is neatly built in the Dutch style, lying on three small hills in a fertile district near the frontier of Holland, about 2 m. from the Rhine, with which it is connected by a canal (the Spoykanal). The old castle of Schwanenburg (formerly the residence of the dukes of Cleves), has a massive tower (Schwanenturm) 180 ft. high. With it is associated the legend of the “Knights of the Swan,” immortalized in Wagner’s Lohengrin. The building has been restored in modern times to serve as a court of justice and a prison. The collegiate church (Stiftskirche) dates from about 1340, and contains a number of fine ducal monuments. Another church is the Annexkirche, formerly a convent of the Minorites; this dates from the middle of the 15th century. The chief manufactures are boots and shoes, tobacco and machinery; there is also some trade in cattle. To the south and west of the city a large district is laid out as a park, where there is a statue to the memory of John Maurice of Nassau-Siegen (1604-1679), who governed Cleves from 1650 to 1679, and in the western part there are mineral wells with a pump room and bathing establishment. Owing to the beautiful woods which surround it and its medicinal waters Cleves has become a favourite summer resort.
The town was the seat of the counts of Cleves as early as the 11th century, but it did not receive municipal rights until 1242. The duchy of Cleves, which lay on both banks of the Rhine and had an area of about 850 sq. m., belonged before the year 1000 to a certain Rutger, whose family became extinct in 1368. It then passed to the counts of La Marck and was made a duchy in 1417, being united with the neighbouring duchies of Jülich and Berg in 1521. The Reformation was introduced here in 1533, but it was not accepted by all the inhabitants. The death without direct heirs of Duke John William in 1609 led to serious complications in which almost all the states of Europe were concerned; however, by the treaty of Xanten in 1614, Cleves passed to the elector of Brandenburg, being afterwards incorporated with the electorate by the great elector, Frederick William. The French held Cleves from 1757 to 1762 and in 1795 the part of the duchy on the left bank of the Rhine was ceded to France; the remaining portion suffered a similar fate in 1805. After the conclusion of peace in 1815 it was restored to Prussia, except some small portions which were given to the kingdom of Holland.
See Char, Geschichte des Herzogtums Kleve (Cleves, 1845); Velsen, Die Stadt Kleve (Cleves, 1846); R. Scholten, Die Stadt Kleve (Cleves, 1879-1881). For Anne of Cleves see that article.
CLEYNAERTS (Clenardus or Clénard), NICOLAS (1495-1542), Belgian grammarian and traveller, was born at Diest, in Brabant, on the 5th of December 1495. Educated at the university of Louvain, he became a professor of Latin, which he taught by a conversational method. He applied himself to the preparation of manuals of Greek and Hebrew grammar, in order to simplify the difficulties of learners. His Tabulae in grammaticen hebraeam (1529), Institutiones in linguam graecam (1530), and Meditationes graecanicae (1531) appeared at Louvain. The Institutiones and Meditationes passed through a number of editions, and had many commentators. He maintained a principle revived in modern teaching, that the learner should not be puzzled by elaborate rules until he has obtained a working acquaintance with the language. A desire to read the Koran led him to try to establish a connexion between Hebrew and Arabic. These studies resulted in a scheme for proselytism among the Arabs, based on study of the language, which should enable Europeans to combat the errors of Islam by peaceful methods. In prosecution of this object he travelled in 1532 to Spain, and after teaching Greek at Salamanca was summoned to the court of Portugal as tutor to Don Henry, brother of John III. He found another patron in Louis Mendoza, marquis of Mondexas, governor-general of Granada. There with the help of a Moorish slave he gained a knowledge of Arabic. He tried in vain to gain access to the Arabic MSS. in the possession of the Inquisition, and finally, in 1540, set out for Africa to seek information for himself. He reached Fez, then a flourishing seat of Arab learning, but after fifteen months of privation and suffering was obliged to return to Granada, and died in the autumn of 1542. He was buried in the Alhambra palace.
See his Latin letters to his friends in Belgium, Nicolai Clenardi, Peregrinationum ac de rebus machometicis epistolae elegantissimae (Louvain, 1550), and a more complete edition, Nic. Clenardi 507 Epistolarum libri duo (Antwerp, 1561), from the house of Plantin; also Victor Chauvin and Alphonse Roersch, “Étude sur la vie et les travaux de Nicolas Clénard” in Mémoires couronnés (vol. lx., 1900-1901) of the Royal Academy of Belgium, which contains a vast amount of information on Cleynaerts and an extensive bibliography of his works, and of notices of him by earlier commentators.
CLICHTOVE, JOSSE VAN (d. 1543), Belgian theologian, received his education at Louvain and at Paris under Jacques Lefèbvre d’Etaples. He became librarian of the Sorbonne and tutor to the nephews of Jacques d’Amboise, bishop of Clermont and abbot of Cluny. In 1519 he was elected bishop of Tournai, and in 1521 was translated to the see of Chartres. He is best known as a distinguished antagonist of Martin Luther, against whom he wrote a good deal. When Cardinal Duprat convened his Synod of Paris in 1528 to discuss the new religion, Clichtove was summoned and was entrusted with the task of collecting and summarizing the objections to the Lutheran doctrine. This he did in his Compendium veritatum ... contra erroneas Lutheranorum assertiones (Paris, 1529). He died at Chartres on the 22nd of September 1543.
CLICHY, or Clichy-la-Garenne, a town of northern France, in the department of Seine, on the right bank of the Seine, immediately north of the fortifications of Paris, of which it is a manufacturing suburb. Pop. (1906) 41,516. Its church was built in the 17th century under the direction of St Vincent de Paul, who had previously been curé of Clichy. Its industries include the manufacture of starch, rubber, oil and grease, glass, chemicals, soap, &c. Clichy, under the name of Clippiacum, was a residence of the Merovingian kings.
CLIFF-DWELLINGS, the general archaeological term for the habitations of primitive peoples, formed by utilizing niches or caves in high cliffs, with more or less excavation or with additions in the way of masonry. Two special sorts of cliff-dwelling are distinguished by archaeologists, (1) the cliff-house, which is actually built on levels in the cliff, and (2) the cavate house, which is dug out, by using natural recesses or openings. A great deal of attention has been given to the North American cliff-dwellings, particularly among the canyons of the south-west, in Arizona, New Mexico, Utah and Colorado, some of which are still used by Indians. There has been considerable discussion as to their antiquity, but modern research finds no definite justification for assigning them to a distinct primitive race, or farther back than the ancestors of the modern Pueblo Indians. The area in which they occur coincides with that in which other traces of the Pueblo tribes have been found. The niches which were utilized are often of considerable size, occurring in cliffs of a thousand feet high, and approached by rock steps or log-ladders.
See the article, with illustrations and bibliography, in the Handbook of American Indians (Washington, 1907).
CLIFFORD, the name of a famous English family and barony, taken from the village of Clifford in Herefordshire, although the family were mainly associated with the north of England.
Robert de Clifford (c. 1275-1314), a son of Roger de Clifford (d. 1282), inherited the estates of his grandfather, Roger de Clifford, in 1286; then he obtained through his mother part of the extensive land of the Viponts, and thus became one of the most powerful barons of his age. A prominent soldier during the reigns of Edward I. and Edward II., Clifford was summoned to parliament as a baron in 1299, won great renown at the siege of Carlaverock Castle in 1300, and after taking part in the movement against Edward II.’s favourite, Piers Gaveston, was killed at Bannockburn. His son Roger, the 2nd baron (1299-1322), shared in the rebellion of Thomas, earl of Lancaster, and was probably executed at York on the 23rd of March 1322. Robert’s grandson Roger, the 5th baron (1333-1389), and the latter’s son Thomas, the 6th baron (c. 1363-c. 1391), served the English kings on the Scottish borders and elsewhere. The same is true of Thomas, the 8th baron (1414-1455), who was killed at the first battle of St Albans in May 1455.
Thomas’s son John, the 9th baron (c. 1435-1461), was more famous. During the Wars of the Roses he fought for Henry VI., earning by his cruelties the name of the “butcher”; after the battle of Wakefield in 1460 he murdered Edmund, earl of Rutland, son of Richard, duke of York, exclaiming, according to the chronicler Edward Hall, “By God’s blood thy father slew mine; and so will I do thee and all thy kin.” Shakespeare refers to this incident in King Henry VI., and also represents Clifford as taking part in the murder of York. It is, however, practically certain that York was slain during the battle, and not afterwards like his son. Clifford was killed at Ferrybridge on the 28th of March 1461, and was afterwards attainted. His young son Henry, the 10th baron (c. 1454-1523), lived disguised as a shepherd for some years, hence he is sometimes called the “shepherd lord.” On the accession of Henry VII. the attainder was reversed and he received his father’s estates. He spent a large part of his time at Barden in Lancashire, being interested in astronomy and astrology. Occasionally, however, he visited London, and he fought at the battle of Flodden in 1513. This lord, who died on the 23rd of April 1523, is celebrated by Wordsworth in the poems “The white doe of Rylstone” and “Song at the feast of Brougham Castle.” Henry, the 11th baron, was created earl of Cumberland in 1525, and from this time until the extinction of the title in 1643 the main line of the Cliffords was associated with the earldom of Cumberland (q.v.).
Richard Clifford, bishop of Worcester and London under Henry IV. and Henry V., was probably a member of this family. This prelate, who was very active at the council of Constance, died on the 20th of August 1421.
On the death of George, 3rd earl of Cumberland, in 1605, the barony of Clifford, separated from the earldom, was claimed by his daughter Anne, countess of Dorset, Pembroke and Montgomery; and in 1628 a new barony of Clifford was created in favour of Henry, afterwards 5th and last earl of Cumberland. After Anne’s death in 1676 the claim to the older barony passed to her daughter Margaret (d. 1676), wife of John Tufton, 2nd earl of Thanet, and her descendants, whose title was definitely recognized in 1691. After the Tuftons the barony was held with intervening abeyances by the Southwells and the Russells, and to this latter family the present Lord De Clifford belongs.1
When the last earl of Cumberland died in 1643 the newer barony of Clifford passed to his daughter Elizabeth, wife of Richard Boyle, 2nd earl of Cork, and from the Boyles it passed to the Cavendishes, falling into abeyance on the death of William Cavendish, 6th duke of Devonshire, in 1858.
The barony of Clifford of Lanesborough was held by the Boyles from 1644 to 1753, and the Devonshire branch of the family still holds the barony of Clifford of Chudleigh, which was created in 1672.
1 The original writ of summons (1299) was addressed in Latin, Roberto domino de Clifford, i.e. Robert, lord of Clifford, and subsequently the barons styled themselves indifferently Lords Clifford or de Clifford, until in 1777 the 11th lord definitively adopted the latter form. The “De” henceforth became part of the name, having quite lost its earliest significance, and with unconscious tautology the barony is commonly referred to as that of De Clifford.
CLIFFORD, JOHN (1836- ), British Nonconformist minister and politician, son of a warp-machinist at Sawley, Derbyshire, was born on the 16th of October 1836. As a boy he worked in a lace factory, where he attracted the notice of the leaders of the Baptist community, who sent him to the academy at Leicester and the Baptist college at Nottingham to be educated for the ministry. In 1858 he was called to Praed Street chapel, Paddington (London), and while officiating there he attended University College and pursued his education by working at the British Museum. He matriculated at London University (1859), and took its B.A. degree (1861), B.Sc. (1862), M.A. (1864), and LL.B. (1866), and in 1883 he was given the honorary degree of D.D. by Bates College, U.S.A., being known therefrom as Dr Clifford. This degree, from an American college of minor academic status, afterwards led to sarcastic allusions, but Dr Clifford had not courted it, and his London University achievements were evidence enough of his intellectual equipment. At Praed Street chapel he gradually obtained a 508 large following, and in 1877 Westbourne Park chapel was opened for him. As a preacher, writer, propagandist and ardent Liberal politician, he became a power in the Nonconformist body. He was president of the London Baptist Association in 1879, of the Baptist Union in 1888 and 1899, and of the National Council of Evangelical Churches in 1898. His chief prominence in politics, however, dates from 1903 onwards in consequence of his advocacy of “passive resistance” to the Education Act of 1902. Into this movement he threw himself with militant ardour, his own goods being distrained upon, with those of numerous other Nonconformists, rather than that any contribution should be made by them in taxation for the purpose of an Education Act which in their opinion was calculated to support denominational religious teaching in the schools. The “passive resistance” movement, with Dr Clifford as its chief leader, had a large share in the defeat of the Unionist government in January 1906, and his efforts were then directed to getting a new act passed which should be undenominational in character. The rejection of Mr Birrell’s bill in 1906 by the House of Lords was accordingly accompanied by denunciations of that body from Dr Clifford and his followers; but as year by year went by, up to 1909, with nothing but failure on the part of the Liberal ministry to arrive at any solution of the education problem,—failure due now not to the House of Lords but to the inherent difficulties of the subject (see Education),—it became increasingly clear to the public generally that the easy denunciations of the act of 1902, which had played so large a part in the elections of 1906, were not so simple to carry into practice, and that a compromise in which the denominationalists would have their say would have to be the result. Meanwhile “passive resistance” lost its interest, though Dr Clifford and his followers continued to protest against their treatment.
CLIFFORD, WILLIAM KINGDON (1845-1879), English mathematician and philosopher, was born on the 4th of May 1845 at Exeter, where his father was a prominent citizen. He was educated at a private school in his native town, at King’s College, London, and at Trinity College, Cambridge, where he was elected fellow in 1868, after being second wrangler in 1867 and second Smith’s prizeman. In 1871 he was appointed professor of mathematics at University College, London, and in 1874 became fellow of the Royal Society. In 1875 he married Lucy, daughter of John Lane of Barbados. In 1876 Clifford, a man of high-strung and athletic, but not robust, physique, began to fall into ill-health, and after two voyages to the South, died during the third of pulmonary consumption at Madeira, on the 3rd of March 1879, leaving his widow with two daughters. Mrs W. K. Clifford soon earned for herself a prominent place in English literary life as a novelist, and later as a dramatist. Her best-known story, Mrs Keith’s Crime (1885), was followed by several other volumes, the best of which is Aunt Anne (1893); and the literary talent in the family was inherited by her daughter Ethel (Mrs Fisher Dilke), a writer of some charming verse.
Owing to his early death, Professor Clifford’s abilities and achievements cannot be fairly judged without reference to the opinion formed of him by his contemporaries. He impressed every one as a man of extraordinary acuteness and originality; and these solid gifts were set off to the highest advantage by quickness of thought and speech, a lucid style, wit and poetic fancy, and a social warmth which made him delightful as a friend and companion. His powers as a mathematician were of the highest order. It harmonizes with the concrete visualizing turn of his mind that, to quote Professor Henry Smith, “Clifford was above all and before all a geometer.” In this he was an innovator against the excessively analytic tendency of Cambridge mathematicians. In his theory of graphs, or geometrical representations of algebraic functions, there are valuable suggestions which have been worked out by others. He was much interested, too, in universal algebra, non-Euclidean geometry and elliptic functions, his papers “Preliminary Sketch of Bi-quaternions” (1873) and “On the Canonical Form and Dissection of a Riemann’s Surface” (1877) ranking as classics. Another important paper is his “Classification of Loci” (1878). He also published several papers on algebraic forms and projective geometry.
As a philosopher Clifford’s name is chiefly associated with two phrases of his coining, “mind-stuff” and the “tribal self.” The former symbolizes his metaphysical conception, which was suggested to him by his reading of Spinoza. “Briefly put,” says Sir F. Pollock, “the conception is that mind is the one ultimate reality; not mind as we know it in the complex forms of conscious feeling and thought, but the simpler elements out of which thought and feeling are built up. The hypothetical ultimate element of mind, or atom of mind-stuff, precisely corresponds to the hypothetical atom of matter, being the ultimate fact of which the material atom is the phenomenon. Matter and the sensible universe are the relations between particular organisms, that is, mind organized into consciousness, and the rest of the world. This leads to results which would in a loose and popular sense be called materialist. But the theory must, as a metaphysical theory, be reckoned on the idealist side. To speak technically, it is an idealist monism.” The other phrase, “tribal self,” gives the key to Clifford’s ethical view, which explains conscience and the moral law by the development in each individual of a “self,” which prescribes the conduct conducive to the welfare of the “tribe.” Much of Clifford’s contemporary prominence was due to his attitude towards religion. Animated by an intense love of truth and devotion to public duty, he waged war on such ecclesiastical systems as seemed to him to favour obscurantism, and to put the claims of sect above those of human society. The alarm was greater, as theology was still unreconciled with the Darwinian theory; and Clifford was regarded as a dangerous champion of the anti-spiritual tendencies then imputed to modern science.
His works, published wholly or in part since his death, are Elements of Dynamic (1879-1887); Seeing and Thinking, popular science lectures (1879); Lectures and Essays, with an introduction by Sir F. Pollock (1879); Mathematical Papers, edited by R. Tucker, with an introduction by Henry J. S. Smith (1882); and The Common Sense of the Exact Sciences, completed by Professor Karl Pearson (1885).
CLIFFORD OF CHUDLEIGH, THOMAS CLIFFORD, 1st Baron (1630-1673), English lord treasurer, a member of the ancient family of Clifford, descended from Walter de Clifford of Clifford Castle in Herefordshire, was the son of Hugh Clifford of Ugbrook near Exeter, and of Mary, daughter of Sir George Chudleigh of Ashton, Devonshire. He was born on the 1st of August 1630, matriculated in 1647 at Exeter College, Oxford, where he showed distinguished ability, supplicated for the B.A. degree in 1650, and entered the Middle Temple in 1648. He represented Totnes in the convention parliament and in that of 1661; and he joined the faction of young men who spoke “confidently and often,” and who sought to rise to power by attacking Clarendon. The chancellor, according to Burnet, had repulsed his advances on account of his Romanism, and Clifford accordingly offered his services to Arlington, whose steady supporter he now became.
On the 16th of February 1663 Clifford obtained the reversion of a tellership in the exchequer, and in 1664, on the outbreak of the Dutch war, was appointed commissioner for the care of the sick, wounded and prisoners, with a salary of £1200. He was knighted, and was present with James at the victory off Lowestoft over the Dutch on the 3rd of June 1665, was rewarded with the prize-ship “Patriarch Isaac,” and in August, under the earl of Sandwich, took a prominent part in the unsuccessful attempt to capture the Dutch East India fleet in Bergen harbour. In August he was appointed by Arlington’s influence ambassador with Henry Coventry to the north of Europe. Subsequently he served again with the fleet, was present with Albemarle at the indecisive fight on the 1st to the 4th of June 1666, and at the victory on the 25th of July. In October 1667 he was one of those selected by the Commons to prepare papers concerning the naval operations. He showed great zeal and energy in naval affairs, and he is described by Pepys as “a very fine gentleman, and much set by at court for his activity in going to sea and stoutness everywhere and stirring up and down.” He became the same year controller of the household and a privy councillor, 509 in 1667 a commissioner for the treasury, and in 1668 treasurer of the household. In the Commons he supported the court, opposing the bill for frequent parliaments in 1668 and the Coventry Act (see Coventry, Sir John) in 1670.
Clifford was an ardent Roman Catholic, a supporter of the royal prerogative and of the French alliance. He regarded with favour the plan of seeking French assistance in order to force Romanism and absolute government upon the country, and his complete failure to understand the real political position and the interests of the nation is reflected in the advice he was said to have given to Charles, to accept the pension from Louis, and “be the slave of one man rather than of 500.” As one of the Cabal ministry, therefore, he co-operated very zealously with the king in breaking through the Triple Alliance and in effecting the understanding with France. He was the only minister besides Arlington entrusted with the secret treaty of Dover of 1670, signing both this agreement and also the ostensible treaty imparted to all the members of the Cabal, and did his utmost to urge Charles to join France in the attack upon the Dutch, whom he detested as republicans and Protestants. In 1672, during the absence of Arlington and Coventry abroad, Clifford acted as principal secretary of state, and was chiefly responsible for the “stop of the exchequer,” and probably also for the attack upon the Dutch Smyrna fleet. He was appointed this year a commissioner to inquire into the settlement of Ireland. On the 22nd of April he was raised to the peerage as Baron Clifford of Chudleigh, and on the 28th of November, by the duke of York’s interest, he was made lord treasurer; his conduct to Arlington, whose claims to the office he had pretended to press, was, according to Evelyn, the only act of “real ingratitude” in his career. Arlington, however, quickly discovered a means of securing Clifford’s fall. The latter was strongly in favour of Charles’s policy of indulgence, and supported the declaration of this year, urging the king to overcome the resistance of parliament by a dissolution. Arlington advocated the contrary policy of concession, and after Charles’s withdrawal of the declaration gave his support to the Test Act of 1673. Clifford spoke with great vehemence against the measure, describing it as “monstrum horrendum ingens,” but his speech only increased the anti-Roman Catholic feeling in parliament and ensured the passing of the bill. In consequence Clifford, as a Roman Catholic, followed the duke of York into retirement. His resignation caused considerable astonishment, since he had never publicly professed his religion, and in 1671 had even built a new Protestant chapel at his home at Ugbrook. According to Evelyn, however, his conduct was governed by a promise previously given to James. He gave up the treasuryship and his seat in the privy council in June. On the 3rd of July 1673 he received a general pardon from the king. In August he said a last farewell to Evelyn, and in less than a month he died at Ugbrook. In Evelyn’s opinion the cause of death was suicide, but his suspicions do not appear to have received any contemporary support. Clifford was one of the worst advisers of Charles II., but a sincere and consistent one. Evelyn declares him “a valiant, uncorrupt gentleman, ambitious, not covetous, generous, passionate, a most constant, sincere friend.” He married Elizabeth, daughter of William Martin of Lindridge, Devonshire, by whom he had fifteen children, four sons and seven daughters surviving him. He was succeeded as 2nd baron by Hugh, his fifth, but eldest surviving son, the ancestor of the present Lord Clifford of Chudleigh.
CLIFTON, a suburb and residential district of Bristol, England, adjoining it on the west; 122 m. W. of London by the Great Western railway. The river Avon (q.v.) here runs in a gorge, followed closely by a railway on either side, and having several quarries, which have in a measure spoiled the beauty of its hanging woods. At a height of 245 ft. above high water Isambard Brunel’s famous suspension bridge bestrides this gorge. It was begun in 1832 and completed in 1864. It has a span of 702 ft., and its total weight is 1500 tons, and it is calculated to bear a burden of 9 tons per sq. in. The long famous hot springs of Clifton, to which, in fact, the town was indebted for its rise, issue from an aperture at the foot of St Vincent’s Rock, in the portion of Clifton known as Hotwells. The water has a temperature of about 76° F. A hydropathic establishment is attached to them. Immediately above the suspension bridge the Clifton Rocks railway ascends from the quays by the river-side to the heights above. The Clifton and Durdham Downs (both on the Gloucestershire side of the river), form the principal pleasure-grounds of Bristol. They lie high above the river, extend for some 5000 acres, and command a beautiful prospect over the city, with its picturesque irregular site and many towers, and over the surrounding well-wooded country.
Three ancient British earthworks bear witness to an early settlement on the spot, and a church was in existence as far back as the time of Henry II., when it was bestowed by William de Clyfton on the abbot of the Austin canons in Bristol; but there are no longer any architectural vestiges of an earlier date than the 18th century. Clifton gives name to a Roman Catholic bishopric. Of the churches the most important are St Andrew’s parish church; All Saints, erected in 1863 after the designs of G. E. Street, and remarkable for the width of its nave and the narrowness of its aisles; and the Roman Catholic pro-cathedral church of the Holy Apostles, with a convent and schools attached. Clifton College, a cluster of buildings in Gothic style, was founded in 1862 by a limited liability company, and takes rank among the principal modern English public schools. Down the river from Clifton is Shirehampton, a favourite resort from Bristol.
CLIM (or Clym) OF THE CLOUGH, a legendary English archer, a supposed companion of the Robin Hood band. He is commemorated in the ballad Adam Bell, Clym of the Cloughe and Wyllyam of Cloudeslee. The three were outlaws who had many adventures of the Robin Hood type. The oldest printed copy of this ballad is dated 1550.
CLIMACTERIC (from the Gr. κλιμακτήρ, the rung or step of a κλῖμαξ or ladder), a critical period in human life; in a medical sense, the period known as the “change of life,” marked in women by the menopause. Certain ages, especially those which are multiples of seven or nine, have been superstitiously regarded as particularly critical; thus the sixty-third and the eighty-first year of life have been called the “grand climacteric.” The word is also used, generally, of any turning-point in the history of a nation, a career or the like.
CLIMATE AND CLIMATOLOGY. The word clima (from Gr. κλίνειν, to lean or incline; whence also the English “clime,” now a poetical term for this or that region of the earth, regarded as characterized by climate), as used by the Greeks, probably referred originally either to the supposed slope of the earth towards the pole, or to the inclination of the earth’s axis. It was an astronomical or a mathematical term, not associated with any idea of physical climate. A change of clima then meant a change of latitude. The latter was gradually seen to mean a change in atmospheric conditions as well as in length of day, and clima thus came to have its present meaning. “Climate” is the average condition of the atmosphere. “Weather” denotes a single occurrence, or event, in the series of conditions which make up climate. The climate of a place is thus in a sense its average weather. Climatology is the study or science of climates.
Relation of Meteorology and Climatology.—Meteorology and climatology are interdependent. It is impossible to distinguish sharply between them. In a strict sense, meteorology deals with the physics of the atmosphere. It considers the various atmospheric phenomena individually, and seeks to determine their physical causes and relations. Its view is largely theoretical. When meteorology (q.v.) is considered in its broadest meaning, climatology is a subdivision of it. Climatology is largely descriptive. It aims at giving a clear picture of the interaction of the various atmospheric phenomena at any place on the earth’s surface. Climatology may almost be defined as geographical meteorology. Its main object is to be of practical service to man. Its method of treatment lays most emphasis on the elements which are most important to life. Climate and crops, climate and industry, climate and health, are subjects of vital interest to man.
The Climatic Elements and their Treatment.—Climatology has 510 to deal with the same groups of atmospheric conditions as those with which meteorology is concerned, viz. temperature (including radiation); moisture (including humidity, precipitation and cloudiness); wind (including storms); pressure; evaporation, and also, but of less importance, the composition and chemical, optical and electrical phenomena of the atmosphere. The characteristics of each of these so-called climatic elements are set forth in a standard series of numerical values, based on careful, systematic, and long-continued meteorological records, corrected and compared by well-known methods. Various forms of graphic presentation are employed to emphasize and simplify the numerical results. In Hann’s Handbuch der Klimatologie, vol i., will be found a general discussion of the methods of presenting the different climatic elements. The most complete guide in the numerical, mathematical and graphic treatment of meteorological data for climatological purposes is Hugo Meyer’s Anleitung zur Bearbeitung meteorologischer Beobachtungen für die Klimatologie (Berlin, 1891).
Climate deals first of all with average conditions, but a satisfactory presentation of a climate must include more than mere averages. It must take account, also, of regular and irregular daily, monthly and annual changes, and of the departures, mean and extreme, from the average conditions which may occur at the same place in the course of time. The mean minimum and maximum temperatures or rainfalls of a month or a season are important data. Further, a determination of the frequency of occurrence of a given condition, or of certain values of that condition, is important, for periods of a day, month or year, as for example the frequency of winds according to direction or velocity; or of different amounts of cloudiness; or of temperature changes of a certain number of degrees; the number of days with and without rain or snow in any month, or year, or with rain of a certain amount, &c. The probability of occurrence of any condition, as of rain in a certain month; or of a temperature of 32°, for example, is also a useful thing to know.
Solar Climate.—Climate, in so far as it is controlled solely by the amount of solar radiation which any place receives by reason of its latitude, is called solar climate. Solar climate alone would prevail if the earth had a homogeneous land surface, and if there were no atmosphere. For under these conditions, without air or ocean currents, the distribution of temperature at any place would depend solely on the amount of energy received from the sun and upon the loss of heat by radiation. And these two factors would have the same value at all points on the same latitude circle.
The relative amounts of insolation received at different latitudes and at different times have been carefully determined. The values all refer to conditions at the upper limit of the earth’s atmosphere, i.e. without the effect of absorption by the atmosphere. The accompanying figure (fig. 1), after Davis, shows the distribution of insolation in both hemispheres at different latitudes and at different times in the year. The latitudes are given at the left margin and the time of year at the right margin. The values of insolation are shown by the vertical distance above the plane of the two margins.
At the equator, where the day is always twelve hours long, there are two maxima of insolation at the equinoxes, when the sun is vertical at noon, and two minima at the solstices when the sun is farthest off the equator. The values do not vary much through the year because the sun is never very far from the zenith, and day and night are always equal. As latitude increases, the angle of insolation becomes more oblique and the intensity decreases, but at the same time the length of day rapidly increases during the summer, and towards the pole of the hemisphere which is having its summer the gain in insolation from the latter cause more than compensates for the loss by the former. The double period of insolation above noted for the equator prevails as far as about lat. 12° N. and S.; at lat. 15° the two maxima have united in one, and the same is true of the minima. At the pole there is one maximum at the summer solstice, and no insolation at all while the sun is below the horizon. On the 21st of June the equator has a day twelve hours long, but the sun does not reach the zenith, and the amount of insolation is therefore less than at the equinox. On the northern tropic, however, the sun is vertical at noon, and the day is more than twelve hours long. Hence the amount of insolation received at this latitude is greater than that received on the equinox at the equator. From the tropic to the pole the sun stands lower and lower at noon, and the value of insolation would steadily decrease with latitude if it were not for the increase in the length of day. Going polewards from the northern tropic on the 21st of June, the value of insolation increases for a time, because, although the sun is lower, the number of hours during which it shines is greater. A maximum value is reached at about lat. 43½° N. The decreasing altitude of the sun then more than compensates for the increasing length of day, and the value of insolation diminishes, a minimum being reached at about lat. 62°. Then the rapidly increasing length of day towards the pole again brings about an increase in the value of insolation, until a maximum is reached at the pole which is greater than the value received at the equator at any time. The length of day is the same on the Arctic circle as at the pole itself, but while the altitude of the sun varies during the day on the former, the altitude at the pole remains 23½° throughout the 24 hours. The result is to give the pole a maximum. On the 21st of June there are therefore two maxima of insolation, one at lat. 43½° and one at the north pole. From lat. 43½° N., insolation decreases to zero on the Antarctic circle, for sunshine falls more and more obliquely, and the day becomes shorter and shorter. Beyond lat. 66½° S. the night lasts 24 hours. On the 21st of December the conditions in southern latitudes are similar to those in the northern hemisphere on the 21st of June, but the southern latitudes have higher values of insolation because the earth is then nearer the sun.
 |
| From Davis’s Elementary Meteorology. |
| Fig. 1.—Distribution of Insolation over the Earth’s Surface. |
At the equinox the days are equal everywhere, but the noon sun is lower and lower with increasing latitude in both hemispheres until the rays are tangent to the earth’s surface at the poles (except for the effect of refraction). Therefore, the values of insolation diminish from a maximum at the equator to a minimum at both poles.
The effect of the earth’s atmosphere is to weaken the sun’s rays. The more nearly vertical the sun, the less the thickness of atmosphere traversed by the rays. The values of insolation at the earth’s surface, after passage through the atmosphere, have been calculated. They vary much with the condition of the air as to dust, clouds, water vapour, &c. As a rule, even when the sky is clear, about one-half of the solar radiation is lost during the day by atmospheric absorption. The great weakening of insolation at the pole, where the sun is very low, is especially noticeable. The following table (after Angot) shows the effect of the earth’s atmosphere (coefficient of transmission 0.7) upon the value of insolation received at sea-level.
Values of Daily Insolation at the Upper Limit of the Earth’s Atmosphere and at Sea-Level.
| Lat. | Upper Limit of Atmosphere. | Earth’s Surface. | ||||
| Equator. | 40°. | N. Pole. | Equator. | 40°. | N. Pole. | |
| Winter solstice | 948 | 360 | 0 | 552 | 124 | 0 |
| Equinoxes | 1000 | 773 | 0 | 612 | 411 | 0 |
| Summer solstice | 888 | 1115 | 1210 | 517 | 660 | 494 |
The following table gives, according to W. Zenker, the relative thickness of the atmosphere at different altitudes of the sun, and also the amount of transmitted insolation:
Relative Distances traversed by Solar Rays through the Atmosphere, and Intensities of Radiation per Unit Areas.
| Altitude of sun | 0° | 5° | 10° | 20° | 30° | 40° | 50° | 60° | 70° | 80° | 90° |
| Relative lengths of path through the atmosphere | 44.7 | 10.8 | 5.7 | 2.92 | 2.00 | 1.56 | 1.31 | 1.15 | 1.06 | 1.02 | 1.00 |
| Intensity of radiation on a surface normal to the rays | 0.0 | 0.15 | 0.31 | 0.51 | 0.62 | 0.68 | 0.72 | 0.75 | 0.76 | 0.77 | 0.78 |
| Intensity of radiation on a horizontal surface | 0.0 | 0.01 | 0.05 | 0.17 | 0.31 | 0.44 | 0.55 | 0.65 | 0.72 | 0.76 | 0.78 |
Physical Climate.—The distribution of insolation explains many of the large facts of temperature distribution, for example, the decrease of temperature from equator to poles; the double maximum of temperature on and near the equator; the increasing seasonal contrasts with increasing latitude, &c. But the regular distribution of solar climate between equator and poles which would exist on a homogeneous earth, whereby similar conditions prevail along each latitude circle, is very much modified by the unequal distribution of land and water; by differences of altitude; by air and ocean currents, by varying conditions of cloudiness, and so on. Hence the climates met with along the same latitude circle are no longer alike. Solar climate is greatly modified by atmospheric conditions and by the surface features of the earth. The uniform arrangement of solar climatic belts, arranged latitudinally, is interfered with, and what is known as physical climate results. According to the dominant control we have solar, continental and marine, and mountain climates. In the first-named, latitude is the essential; in the second and third, the influence of land or water; in the fourth, the effect of altitude.
Classification of the Zones by Latitude Circles.—It is customary to classify climates roughly into certain broad belts. These are the climatic zones. The five zones with which we are most familiar are the so-called torrid, the two temperate, and the two frigid zones. The torrid, or better, the tropical zone, naming it by its boundaries, is limited on the north and south by the two tropics of Cancer and Capricorn, the equator dividing the zone into two equal parts. The temperate zones are limited towards the equator by the tropics, and towards the poles by the Arctic and Antarctic circles. The two polar zones are caps covering both polar regions, and bounded on the side towards the equator by the Arctic and Antarctic circles.
These five zones are classified on purely astronomical grounds. They are really zones of solar climate. The tropical zone has the least annual variation of insolation. It has the maximum annual amount of insolation. Its annual range of temperature is very slight. It is the summer zone. Beyond the tropics the contrasts between the seasons rapidly become more marked. The polar zones have the greatest variation in insolation between summer and winter. They also have the minimum amount of insolation for the whole year. They may well be called the winter zones, for their summer is so short and cool that the heat is insufficient for most forms of vegetation, especially for trees. The temperate zones are intermediate between the tropical and the polar in the matter of annual amount and of annual variation of insolation. Temperate conditions do not characterize these zones as a whole. They are rather the seasonal belts of the world.
 |
| From Grundzüge der physischen Erdkunde, by permission of Veit & Co. |
| Fig. 2.—Supan’s Temperature Zones. |
Temperature Zones.—The classification of the zones on the basis of the distribution of sunshine serves very well for purposes of simple description, but a glance at any isothermal chart shows that the isotherms do not coincide with the latitude lines. In fact, in the higher latitudes, the former sometimes follow the meridians more closely than they do the parallels of latitude. Hence it has been suggested that the zones be limited by isotherms rather than by parallels of latitude, and that a closer approach be thus made to the actual conditions of climate. Supan1 (see fig. 2) has suggested limiting the hot belt, which corresponds to, but is slightly greater than, the old torrid zone, by the two mean annual isotherms of 68°—a temperature which approximately coincides with the polar limit of the trade-winds and with the polar limit of palms. The hot belt widens somewhat over the continents, chiefly because of the mobility of the ocean waters, whereby there is a tendency towards an equalization of the temperature between equator and poles in the oceans, while the stable lands acquire a temperature suitable to their own latitude. Furthermore, the unsymmetrical distribution of land in the low latitudes of the northern and southern hemispheres results in an unsymmetrical position of the hot belt with reference to the equator, the belt extending farther north than south of the equator. The polar limits of the temperate zones are fixed by the isotherm of 50° for the warmest month. Summer heat is more important for vegetation than winter cold, and where the warmest month has a temperature below 50°, cereals and forest trees do not grow, and man has to adjust himself to the peculiar climatic conditions in a very special way. The two polar caps are not symmetrical as regards the latitudes which they occupy. The presence of extended land masses in the high northern latitudes carries the temperature of 50° in the warmest month farther poleward there than is the case in the corresponding latitudes occupied by the oceans of the southern hemisphere, which warm less easily and are constantly in motion. Hence the southern cold cap, which has its equatorial limits at about lat. 50° S., is of much greater extent than the northern polar cap. The northern temperate belt, in which the great land areas lie, is much broader than the southern, especially over the continents. These temperature zones emphasize the natural conditions of climate more than is the case in any subdivision by latitude circles, and they bear a fairly close resemblance to the old zonal classification of the Greeks.
Classification of the Zones by Wind Belts.—The heat zones however, emphasize the temperature to the exclusion of such 512 important elements as wind and rainfall. So distinctive are the larger climatic features of the great wind belts of the world, that a classification of climates according to wind systems has been suggested.2 As the rain-belts of the world are closely associated with these wind systems, a classification of the zones by winds also emphasizes the conditions of rainfall. In such a scheme the tropical zone is bounded on the north and south by the margins of the trade-wind belts, and is therefore larger than the classic torrid zone. This trade-wind zone is somewhat wider on the eastern side of the oceans, and properly includes within its limits the equable marine climates of the eastern margins of the ocean basins, even as far north as latitude 30° or 35°. Most of the eastern coasts of China and of the United States are thus left in the more rigorous and more variable conditions of the north temperate zone. Through the middle of the trade-wind zone extends the sub-equatorial belt, with its migrating calms, rains and monsoons. On the polar margins of the trade-wind zone lie the sub-tropical belts, of alternating trades and westerlies. The temperate zones embrace the latitudes of the stormy westerly winds, having on their equator-ward margins the sub-tropical belts, and being somewhat narrower than the classic temperate zones. Towards the poles there is no obvious limit to the temperate zones, for the prevailing westerlies extend beyond the polar circles. These circles may, however, serve fairly well as boundaries, because of their importance from the point of view of insolation. The polar zones in the wind classification, therefore, remain just as in the older scheme.
Need of a Classification of Climates.—A broad division of the earth’s surface into zones is necessary as a first step in any systematic study of climate, but it is not satisfactory when a more detailed discussion is undertaken. The reaction of the physical features of the earth’s surface upon the atmosphere complicates the climatic conditions found in each of the zones, and makes further subdivision desirable. The usual method is to separate the continental (near sea-level) and the marine. An extreme variety of the continental is the desert; a modified form, the littoral; while altitude is so important a control that mountain and plateau climates are always grouped by themselves.
Marine or Oceanic Climate.—Land and water differ greatly in their behaviour regarding absorption and radiation. The former warms and cools readily, and to a considerable degree; the latter, slowly and but little. The slow changes in temperature of the ocean waters involve a retardation in the times of occurrence of the maxima and minima, and a marine climate, therefore, has a cool spring and a warm autumn, the seasonal changes being but slight. Characteristic, also, of marine climates is a prevailingly higher relative humidity, a larger amount of cloudiness, and a heavier rainfall than is found over continental interiors. All of these features have their explanation in the abundant evaporation from the ocean surfaces. In the middle latitudes the oceans have distinctly rainy winters, while over the continental interiors the colder months have a minimum of precipitation. Ocean air is cleaner and purer than land air, and is generally in more active motion.
Continental Climate.—Continental climate is severe. The annual temperature ranges increase, as a whole, with increasing distance from the oceans. The coldest and warmest months are usually January and July, the times of maximum and minimum temperatures being less retarded than in the case of marine climates. The greater seasonal contrasts in temperature over the continents than over the oceans are furthered by the less cloudiness over the former. Diurnal and annual changes of nearly all the elements of climate are greater over continents than over oceans; and this holds true of irregular as well as of regular variations. Fig. 3 illustrates the annual march of temperature in marine and continental climates. Bagdad, in Asia Minor (Bd.), and Funchal on the island of Madeira (M.) are representative continental and marine stations for a low latitude. Nerchinsk in eastern Siberia (N.) and Valentia in south-western Ireland (V.) are good examples of continental and marine climates of higher latitudes in the northern hemisphere. The data for these and the following curves were taken from Hann’s Lehrbuch der Meteorologie (1901).
Owing to the distance from the chief source of supply of water vapour—the oceans—the air over the larger land areas is naturally drier and dustier than that over the oceans. Yet even in the arid continental interiors in summer the absolute vapour content is surprisingly large, and in the hottest months the percentages of relative humidity may reach 20% or 30%. At the low temperatures which prevail in the winter of the higher latitudes the absolute humidity is very low, but, owing to the cold, the air is often damp. Cloudiness, as a rule, decreases inland, and with this lower relative humidity, more abundant sunshine and higher temperature, the evaporating power of a continental climate is much greater than that of the more humid, cloudier and cooler marine climate. Both amount and frequency of rainfall, as a rule, decrease inland, but the conditions are very largely controlled by local topography and by the prevailing winds. Winds average somewhat lower in velocity, and calms are more frequent, over continents than over oceans. The seasonal changes of pressure over the former give rise to systems of inflowing and outflowing, so-called continental, winds, sometimes so well developed as to become true monsoons. The extreme termperature changes which occur over the continents are the more easily borne because of the dryness of the air; because the minimum temperatures of winter occur when there is little or no wind, and because during the warmer hours of the summer there is the most air-movement.
 | |
| Fig. 3.—Annual March of Air Temperature. Influence of Land and Water. (After Angot.) | |
| M, Madeira. | V, Valentia. |
| Bd, Bagdad. | N, Nerchinsk. |
Desert Climate.—An extreme type of continental climate is found in deserts. Desert air is notably free from micro-organisms. The large diurnal temperature ranges of inland regions, which are most marked where there is little or no vegetation, give rise to active convectional currents during the warmer hours of the day. Hence high winds are common by day, while the nights are apt to be calm and relatively cool. Travelling by day is unpleasant under such conditions. Diurnal cumulus clouds, often absent because of the excessive dryness of the air, are replaced by clouds of blowing dust and sand. Many geological phenomena, and special physiographic types of varied kinds, are associated with the peculiar conditions of desert climate. The excessive diurnal ranges of temperature cause rocks to split and break up. Wind-driven sand erodes and polishes the rocks. When the separate fragments become small enough they, in their turn, are transported by the winds and further eroded by friction during their journey. Curious conditions of drainage result from the deficiency in rainfall. Rivers “wither” away, or end in sinks or brackish lakes.
513 Desert plants protect themselves against the attacks of animals by means of thorns, and against evaporation by means of hard surfaces and by a diminished leaf surface. The life of man in the desert is likewise strikingly controlled by the climatic peculiarities of strong sunshine, of heat, and of dust.
Plate I
 |
| ANNUAL DISTRIBUTION OF TEMPERATURE AND PRESSURE. (Click to enlarge.) |
Plate II
 |
| SEASONAL DISTRIBUTION OF TEMPERATURE AND PRESSURE. (Click to enlarge.) |
Coast or Littoral Climate.—Between the pure marine and the pure continental types the coasts furnish almost every grade of transition. Prevailing winds are here important controls. When these blow from the ocean, the climates are marine in character, but when they are off-shore, a somewhat modified type of continental climate prevails, even up to the immediate sea-coast. Hence the former have a smaller range of temperature; their summers are more moderate and their winters milder; extreme temperatures are rare; the air is damp, and there is much cloud. All these marine features diminish with increasing distance from the ocean, especially when there are mountain ranges near the coast. In the tropics, windward coasts are usually well supplied with rainfall, and the temperatures are modified by sea breezes. Leeward coasts in the trade-wind belts offer special conditions. Here the deserts often reach the sea, as on the western coasts of South America, Africa and Australia. Cold ocean currents, with prevailing winds along-shore rather than on-shore, are here hostile to rainfall, although the lower air is often damp, and fog and cloud are not uncommon.
Monsoon Climate.—Exceptions to the general rule of rainier eastern coasts in trade-wind latitudes are found in the monsoon regions, as in India, for example, where the western coast of the peninsula is abundantly watered by the wet south-west monsoon. As monsoons often sweep over large districts, not only coast but interior, a separate group of monsoon climates is desirable. In India there are really three seasons—one cold, during the winter monsoon; one hot, in the transition season; and one wet, during the summer monsoon. Little precipitation occurs in winter, and that chiefly in the northern provinces. In low latitudes, monsoon and non-monsoon climates differ but little, for summer monsoons and regular trade-winds may both give rains, and wind direction has slight effect upon temperature.
The winter monsoon is off-shore and the summer monsoon on-shore under typical conditions, as in India. But exceptional cases are found where the opposite is true. In higher latitudes the seasonal changes of the winds, although not truly monsoonal, involve differences in temperature and in other climatic elements. The only well-developed monsoons on the coast of the continents of higher latitudes are those of eastern Asia. These are off-shore during the winter, giving dry, clear and cold weather; while the on-shore movement in summer gives cool, damp and cloudy weather.
Mountain and Plateau Climate.—Both by reason of their actual height and because of their obstructive effects, mountains influence climate similarly in all the zones. Mountains as contrasted with lowlands are characterized by a decrease in pressure, temperature and absolute humidity; an increased intensity of insolation and radiation; usually a greater frequency of, and up to a certain altitude more, precipitation. At an altitude of 16,000 ft., more or less, pressure is reduced to about one-half of its sea-level value. The highest human habitations are found under these conditions. On high mountains and plateaus the pressure is lower in winter than in summer, owing to the fact that the atmosphere is compressed to lower levels in the winter and is expanded upwards in summer.
The intensity of insolation and of radiation both increase aloft in the cleaner, purer, drier and thinner air of mountain climates. The great intensity of the sun’s rays attracts the attention of mountain-climbers at great altitudes. The vertical decrease of temperature, which is also much affected by local conditions, is especially rapid during the warmer months and hours; mountains are then cooler than lowlands. The inversions of temperature characteristic of the colder months, and of the night, give mountains the advantage of a higher temperature then—a fact of importance in connexion with the use of mountains as winter resorts. At such times the cold air flows down the mountain sides and collects in the valleys below, being replaced by warmer air aloft. Hence diurnal and annual ranges of temperature on the mountain tops of middle and higher latitudes are lessened, and the climate in this respect resembles a marine condition. The times of occurrence of the maximum and minimum temperature are also much influenced by local conditions. Elevated enclosed valleys, with strong sunshine, often resemble continental conditions of large temperature range, and plateaus, as compared with mountains at the same altitude, have relatively higher temperatures and larger temperature ranges. Altitude tempers the heat of the low latitudes. High mountain peaks, even on the equator, can remain snow-covered all the year round.
No general law governs the variations of relative humidity with altitude, but on the mountains of Europe the winter is the driest season, and the summer the dampest. At well-exposed stations there is a rapid increase in the vapour content soon after noon, especially in summer. The same is true of cloudiness, which is often greater on mountains than at lower levels, and is usually at a maximum in summer, while the opposite is true of the lowlands in the temperate latitudes. One of the great advantages of the higher Alpine valleys in winter is their small amount of cloud. This, combined with their low wind velocity and strong insolation, makes them desirable winter health resorts. Latitude, altitude, topography and winds are the determining factors in controlling the cloudiness on mountains. In the rare, often dry, air of mountains and plateaus evaporation is rapid, the skin dries and cracks, and thirst is increased.
Rainfall usually increases with increasing altitude up to a certain point, beyond which, owing to the loss of water vapour, this increase stops. The zone of maximum rainfall averages about 6000 to 7000 ft. in altitude, more or less, in intermediate latitudes, being lower in winter and higher in summer. Mountains usually have a rainy and a drier side; the contrast between the two is greatest when a prevailing damp wind crosses the mountain, or when one slope faces seaward and the other landward. Mountains often provoke rainfall, and local “islands,” or better, “lakes,” of heavier precipitation result.
Mountains resemble marine climates in having higher wind velocities than continental lowlands. Mountain summits have a nocturnal maximum of wind velocity, while plateaus usually have a diurnal maximum. Mountains both modify the general, and give rise to local winds. Among the latter the well-known mountain and valley winds are often of considerable hygienic importance in their control of the diurnal period of humidity, cloudiness and rainfall, the ascending wind of daytime tending to give clouds and rain aloft, while the opposite conditions prevail at night.
Supan’s Climatic Provinces.—The broad classification of climates into the three general groups of marine, continental and mountain, with the subordinate divisions of desert, littoral and monsoon, is convenient for purposes of summarizing the interaction of the climatic elements under the controls of land, water and altitude. But in any detailed study some scheme of classification is needed in which similar climates in different parts of the world are grouped together, and in which their geographic distribution receives particular consideration. An almost infinite number of classifications might be proposed; or we may take as the basis of subdivision either the special conditions of one climatic element, or similar conditions of a combination of two or more elements. Or we may take a botanical or a zoological basis. Of the various classifications which have been suggested, that of Supan gives a very rational, simple and satisfactory scheme of grouping. In this scheme there are thirty-five so-called climatic provinces.3 It emphasizes the essentials of each climate, and serves to impress these essentials upon the mind by means of a compact, well-considered verbal summary in the case of each province described. Obviously, no classification of climates which is at all complete can approach the simplicity of the ordinary classification of the zones.
The Characteristics of the Torrid Zone.
General: Climate and Weather.—The dominant characteristic of the torrid zone is the simplicity and uniformity of its climatic features. The tropics lack the proverbial uncertainty and changeableness of the weather of higher latitudes. Weather and climate are essentially synonymous terms. Periodic phenomena, depending upon the daily and annual march of the sun, are dominant. Non-periodic weather changes are wholly subordinate. In special regions only, and at special seasons, is the regular sequence of weather temporarily interrupted by an occasional tropical cyclone. These cyclones, although comparatively infrequent, are notable features of the climate of the areas in which they occur, generally bringing very heavy rains. The devastation produced by one of these storms often affects the economic condition of the people in the district of its occurrence for many years.
Temperature.—The mean temperature is high, and very uniform over the whole zone. There is little variation during the year. The mean annual isotherm of 68° is a rational limit at the polar margins of the zone, and the mean annual isotherm of 80° encloses the greater portion of the land areas, as well as much of the tropical oceans. The warmest latitude circle for the year is not the equator, but latitude 10° N. The highest mean annual temperatures, shown by the isotherm of 85°, are in Central Africa, in India, the north of Australia and Central America, but, with the exception of the first, these areas are small. The temperatures average highest where there is little rain. In June, July and August there are large districts in the south of Asia and north of Africa with temperatures over 90°.
Over nearly all of the zone the mean annual range of temperature is less than 10°, and over much of it, especially on the oceans, it is less than 5°. Even near the margins of the zone the ranges are less than 25°, as at Calcutta, Hong-Kong, Río de Janeiro and Khartum. The mean daily range is usually larger than the mean annual. It has been well said that “night is the winter of the tropics.” Over an area covering parts of the Pacific and Indian Oceans from Arabia to the Caroline Islands and from Zanzibar to New Guinea, as well as on the Guiana coast, the minimum temperatures do not normally fall below 68°. Towards the margins of the zone, however, the minima on the continents fall to or even below 32°. Maxima of 115° and even over 120° occur over the deserts of northern Africa. A district where the mean maxima exceed 113° extends from the western Sahara to north-western India, and over Central Australia. Near the equator the maxima are therefore not as high as those in many so-called “temperate” climates. The tropical oceans show remarkably small variations in temperature. The “Challenger” results on the equator showed a daily range of hardly 0.7° in the surface water temperature, and P. G. Schott determined the annual range as 4.1° on the equator, 4.3° at latitude 10°, and 6.5° at latitude 20°.
The Seasons.—In a true tropical climate the seasons are not classified according to temperature, but depend on rainfall and the prevailing winds. The life of animals and plants in the tropics, and of man himself, is regulated very largely, in some cases almost wholly, by rainfall. Although the tropical rainy season is characteristically associated with a vertical sun, that season is not necessarily the hottest time of the year. It often goes by the name of winter for this reason. Towards the margins of the zone, with increasing annual ranges of temperature, seasons in the extra-tropical sense gradually appear.
Physiological Effects of Heat and Humidity.—Tropical heat is associated with high relative humidity except over deserts and in dry seasons. The air is therefore muggy and oppressive. The high temperatures are disagreeable and hard to bear. The “hot-house air” has an enervating effect. Energetic physical and mental action are often difficult or even impossible. The tonic effect of a cold winter is lacking. The most humid districts in the tropics are the least desirable for persons from higher latitudes; the driest are the healthiest. The most energetic natives are the desert-dwellers. The monotonously enervating heat of the humid tropics makes man sensitive to slight temperature changes. The intensity of direct insolation, as well as of radiation from the earth’s surface, may produce heat prostration and sunstroke. “Beware of the sun” is a good rule in the tropics.
Pressure.—The uniform temperature distribution in the tropics involves uniform pressure distribution. Pressure gradients are weak. The annual fluctuations are slight, even on the continents. The diurnal variation of the barometer is so regular and so marked that, as von Humboldt said, the time of day can be told within about twenty minutes if the reading of the barometer be known.
Winds and Rainfall.—Along the barometric equator, where the pressure gradients are weakest, is the equatorial belt of calms, variable winds and rains—the doldrums. This belt offers exceptionally favourable conditions for abundant rainfall, and is one of the rainiest regions of the world, averaging probably about 100 in. Here the sky is prevailingly cloudy; the air is hot and oppressive; heavy showers and thunderstorms are frequent, chiefly in the afternoon and evening. Here are the dense tropical forests of the Amazon and of equatorial Africa. This belt of calms and rains shifts north and south of the equator after the sun. In striking contrast are the easterly trade winds, blowing between the tropical high pressure belts and the equatorial belt of low pressure. Of great regularity, and contributing largely to the uniformity of tropical climates, the trades have long been favourite sailing routes because of the steadiness of the wind, the infrequency of storms, the brightness of the skies and the freshness of the air. The trades are subject to many variations. Their northern and southern margins shift north and south after the sun; at certain seasons they are interrupted, often over wide areas near their equatorward margins, by the migrating belt of equatorial rains and by monsoons; near lands they are often interfered with by land and sea breezes; in certain regions they are invaded by violent cyclonic storms. The trades, except where they blow on to windward coasts or over mountains, are drying winds. They cause the deserts of northern Africa and of the adjacent portions of Asia; of Australia, South Africa and southern South America. The monsoons on the southern and eastern coasts of Asia are the best known winds of their class. In the northern summer the south-west monsoon, warm and sultry, blows over the latitudes from about 10° N. to and beyond the northern tropic, between Africa and the Philippines, giving rains over India, the East Indian archipelago and the eastern coasts of China. In winter, the north-east monsoon, the normal cold-season outflow from Asia combined with the north-east trade, and generally cool and dry, covers the same district, extending as far north as latitude 30°. Crossing the equator, these winds reach northern Australia and the western islands of the South Pacific as a north-west rainy monsoon, while this region in the opposite season has the normal south-east trade. Other monsoons are found in the Gulf of Guinea and in equatorial Africa. Wherever they occur, they control the seasonal changes.
Tropical rains are in the main summer rains, coming when the normal trade gives way to the equatorial belt of rains, or when the summer monsoon sets in. There are, however, many cases of a rainy season when the sun is low, expecially on windward coasts in the trades. Tropical rains come usually in the form of heavy downpours and with a well-marked diurnal period, the maximum varying with the locality between noon and midnight. Local influences are, however, very important, and in many places night rainfall maxima are found.
Land and Sea Breezes.—The sea breeze is an important climatic feature on many tropical coasts. With its regular occurrence, and its cool, clean air, it serves to make many districts habitable for white settlers, and has deservedly won the name of “the doctor.” On not a few coasts, the sea breeze is a true prevailing wind. The location of dwellings is often determined by the exposure of a site to the sea breeze.
Thunderstorms.—Local thunderstorms are frequent in the humid portions of the tropics. They have a marked diurnal periodicity, find their best opportunity in the equatorial belt 515 of weak pressure gradients and high temperature, and are commonly associated with the rainy season, being most common at the beginning and end of the regular rains. In many places, thunderstorms occur daily throughout their season, with extraordinary regularity and great intensity.
Cloudiness.—Taken as a whole, the tropics are not favoured with such clear skies as is often supposed. Cloudiness varies about as does the rainfall. The maximum is in the equatorial belt of calms and rains, where the sky is always more or less cloudy. The minimum is in the trade latitudes, where fair skies as a whole prevail. The equatorial cloud belt moves north and south after the sun. Wholly clear days are very rare in the tropics generally, especially near the equator, and during the rainy season heavy clouds usually cover the sky. Wholly overcast, dull days, such as are common in the winter of the temperate zone, occur frequently only on tropical coasts in the vicinity of cold ocean currents, as on the coast of Peru and on parts of the west coast of Africa.
Intensity of Sky-Light and Twilight.—The light from tropical skies by day is trying, and the intense insolation, together with the reflection from the ground, increases the general dazzling glare under a tropical sun. During much of the time smoke from forest and prairie fires (in the dry season), dust (in deserts), and water-vapour give the sky a pale whitish appearance. In the heart of the trade-wind belts at sea the sky is of a deeper blue. Twilight within the tropics is shorter than in higher latitudes, but the coming on of night is less sudden than is generally assumed.
 |
| Fig. 4.—Annual march of temperature: equatorial type. A, Africa, interior; B, Batavia; J, Jaluit, Marshall Islands. |
Climatic Subdivisions.—The rational basis for a classification of the larger climatic provinces of the torrid zone is found in the general wind systems, and in their control over rainfall. Following this scheme there are: (1) the equatorial belt; (2) the trade-wind belts; (3) the monsoon belts. In each of these subdivisions there are modifications due to marine and continental influences. In general, both seasonal and diurnal phenomena are more marked in continental interiors than on the oceans, islands and windward coasts. Further, the effect of altitude is so important that another group should be added to include (4) mountain climates.
1. The Equatorial Belt.—Within a few degrees of the equator, and when not interfered with by other controls, the annual curve of temperature has two maxima following the two zenithal positions of the sun, and two minima at about the time of the solstices. This equatorial type of annual march of temperature is illustrated in the three curves for the interior of Africa, Batavia and Jaluit (fig. 4). The greatest range is shown in the curve for the interior of Africa; the curve for Batavia illustrates insular conditions with less range, and the oceanic type for Jaluit, Marshall Islands, gives the least range. This double maximum is not a universal phenomenon, there being many cases where but a single maximum occurs.
 | |
| Fig. 5.—Annual march of rainfall in the tropics. | |
| S.A, South Africa. | M, Mexico. |
| Q, Quito. | H, Hilo. |
| S.P., São Paulo. | P.D., Port Darwin. |
As the belt of rains swings back and forth across the equator after the sun, there should be two rainy seasons with the sun vertical, and two dry seasons when the sun is farthest from the zenith, and while the trades blow. These conditions prevail on the equator, and as far north and south of the equator (about 10°-12°) as sufficient time elapses between the two zenithal positions of the sun for the two rainy seasons to be distinguished from one another. In this belt, under normal conditions, there is therefore no dry season of any considerable duration. The double rainy season is clearly seen in equatorial Africa and in parts of equatorial South America. The maxima lag somewhat behind the vertical sun, coming in April and November, and are unsymmetrically developed, the first maximum being the principal one. The minima are also unsymmetrically developed, and the so-called “dry seasons” are seldom wholly rainless. This rainfall type with double maxima and minima has been called the equatorial type, and is illustrated in the following curves for South Africa and Quito (fig. 5). The monthly rainfalls are given in thousandths of the annual mean. The mean annual rainfall at Quito is 42.12 in. These double rainy and dry seasons are easily modified by other conditions, as by the monsoons of the Indo-Australian area, so that there is no rigid belt of equatorial rains extending around the world. In South America, east of the Andes, the distinction between rainy and dry seasons is often much confused. In this equatorial belt the cloudiness is high throughout the year, averaging .7 to .8, with a relatively small annual period. The curve following, E (fig. 6), is fairly typical, but the annual period varies greatly under local controls.
 |
| Fig. 6.—Annual march of cloudiness in the tropics. E, Equatorial type; M, Monsoon type. |
At greater distances from the equator than about 10° or 12° the sun is still vertical twice a year within the tropics, but the interval between these two dates is so short that the two rainy seasons merge into one, in summer, and there is also but one dry season, in winter. This is the so-called tropical type of rainfall, and is found where the trade belts are encroached upon by the equatorial rains during the migration of these rains into each hemisphere. It is illustrated in the curves for São Paulo, Brazil, and for the city of Mexico (fig. 5). The mean annual rainfall at São Paulo is 54.13 in. and at Mexico 22.99 in. The districts of tropical rains of this type lie along the equatorial margins of the torrid zone, outside of the latitudes of the equatorial type of rainfall. The rainy season becomes shorter with increasing distance from the equator. The weather of the opposite seasons is strongly contrasted. The single dry season lasts longer than either dry season in the equatorial belt, reaching eight months in typical cases, with the wet season lasting four months. The lowlands often become dry and parched during the long dry trade-wind season (winter) 516 and vegetation withers away, while grass and flowers grow in great abundance and all life takes on new activity during the time when the equatorial rainy belt with its calms, variable winds and heavy rains is over them (summer). The Sudan lies between the Sahara and the equatorial forests of Africa. It receives rains, and its vegetation grows actively, when the doldrum belt is north of the equator (May-August). But when the trades blow (December-March) the ground is parched and dusty. The Venezuelan llanos have a dry season in the northern winter, when the trade blows. The rains come in May-October. The campos of Brazil, south of the equator, have their rains in October-April, and are dry the remainder of the year. The Nile overflow results from the rainfall on the mountains of Abyssinia during the northward migration of the belt of equatorial rains.
 |
| Fig. 7.—Annual march of temperature: tropical type. W, Wadi Halfa; A, Alice Springs; H, Honolulu; J, Jamestown, St Helena; N, Nagpur. |
The so-called tropical type of temperature variation, with one maximum and one minimum, is illustrated in the accompanying curves for Wadi Halfa, in upper Egypt; Alice Springs, Australia; Nagpur, India; Honolulu, Hawaii; and Jamestown, St Helena (fig. 7). The effect of the rainy season is often shown in a displacement of the time of maximum temperature to an earlier month than the usual one.
2. Trade-Wind Belts.—The trade belts near sea-level are characterized by fair weather, steady winds, infrequent light rains or even an almost complete absence of rain, very regular, although slight, annual and diurnal ranges of temperature, and a constancy and regularity of weather. The climate of the ocean areas in the trade-wind belts is indeed the simplest and most equable in the world, the greatest extremes over these oceans being found to leeward of the larger lands. On the lowlands swept over by the trades, beyond the polar limits of the equatorial rain belt (roughly between lats. 20° and 30°), are most of the great deserts of the world. These deserts extend directly to the water’s edge on the leeward western coasts of Australia, South Africa and South America.
The ranges and extremes of temperature are much greater over the continental interiors than over the oceans of the trade-wind belts. Minima of 32° or less occur during clear, quiet nights, and daily ranges of over 50° are common. The midsummer mean temperature rises above 90°, with noon maxima of 110° or more in the non-cloudy, dry air of a desert day. The days, with high, dry winds, carrying dust and sand, with extreme heat, accentuated by the absence of vegetation, are disagreeable, but the calmer nights, with active radiation under clear skies, are much more comfortable. The nocturnal temperatures are even not seldom too low for comfort in the cooler season, when thin sheets of ice may form.
While the trades are drying winds as long as they blow strongly over the oceans, or over lowlands, they readily become rainy if they are cooled by ascent over a mountain or highland. Hence the windward (eastern) sides of mountains or bold coasts in the trade-wind belts are well watered, while the leeward sides, or interiors, are dry. Mountainous islands in the trades, like the Hawaiian islands, many of the East and West Indies, the Philippines, Borneo, Ceylon, Madagascar, Teneriffe, &c., show marked differences of this sort. The eastern coasts of Guiana, Central America, south-eastern Brazil, south-eastern Africa, and eastern Australia are well watered, while the interiors are dry. The eastern highland of Australia constitutes a more effective barrier than that in South Africa; hence the Australian interior has a more extended desert. South America in the south-east trade belt is not well enclosed on the east, and the most arid portion is an interior district close to the eastern base of the Andes where the land is low. Even far inland the Andes again provoke precipitation along their eastern base, and the narrow Pacific coastal strip, to leeward of the Andes, is a very pronounced desert from near the equator to about lat. 30° S. The cold ocean waters, with prevailing southerly (drying) winds alongshore, are additional factors causing this aridity. Highlands in the trade belts are therefore moist on their windward slopes, and become oases of luxuriant plant growth, while close at hand, on the leeward sides, dry savannas or deserts may be found. The damp, rainy and forested windward side of Central America was from the earliest days of European occupation left to the natives, while the centre of civilization was naturally established on the more open and sunny south-western side.
The rainfall associated with the conditions just described is known as the trade type. These rains have a maximum in winter, when the trades are most active. In cases where the trade blows steadily throughout the year against mountains or bold coasts, as on the Atlantic coast of Central America, there is no real dry season. The curve for Hilo (mean annual rainfall 145.24 in.) on the windward side of the Hawaiian Islands, shows typical conditions (see fig. 5). The trade type of rainfall is often much complicated by the combination with it of the tropical type and of the monsoon type. In the Malay archipelago there are also complications of equatorial and trade rains; likewise in the West Indies.
3. Monsoon Belts.—In a typical monsoon region the rains follow the vertical sun, and therefore have a simple annual period much like that of the tropical type above described. This monsoon type of rainfall is well illustrated in the curve for Port Darwin (mean annual rainfall 62.72 in.), in Australia (see fig. 5). This summer monsoon rainfall results from the inflow of a body of warm, moist air from the sea upon a land area; there is a consequent retardation of the velocity of the air currents, as the result of friction, and an ascent of the air, the rainfall being particularly heavy where the winds have to climb over high lands. In India, the precipitation is heaviest at the head of the Bay of Bengal (where Cherrapunji, at the height of 4455 ft. in the Khasi Hills, has a mean annual rainfall of between 400 and 500 in.), along the southern base of the Himalayas (60 to 160 in.), on the bold western coast of the peninsula (80 to 120 in. and over), and on the mountains of Burma, (up to 160 in.). In the rain-shadow of the Western Ghats, the Deccan often suffers from drought and famine unless the monsoon rains are abundant and well distributed. The prevailing direction of the rainy monsoon wind in India is south-west; on the Pacific coast of Asia, it is south-east. This monsoon district is very large, including the Indian Ocean, Arabian Sea, Bay of Bengal, and adjoining continental areas; the Pacific coast of China, the Yellow and Japan seas, and numerous islands from Borneo to Sakhalin on the north and to the Ladrone Islands on the east. A typical temperature curve for a monsoon 517 district is that for Nagpur, in the Indian Deccan (fig. 7), and a typical monsoon cloudiness curve is given in fig. 6, the maximum coming near the time of the vertical sun, in the rainy season, and the minimum in the dry season.
In the Australian monsoon region, which reaches across New Guinea and the Sunda Islands, and west of Australia, in the Indian Ocean, over latitudes 0°-10° S., the monsoon rains come with north-west winds in the period between November and March or April.
The general rule that eastern coasts in the tropics are the rainiest finds exceptions in the case of the rainy western coasts in India and other districts with similar monsoon rains. On the coast of the Gulf of Guinea, for example, there is a small rainy monsoon area during the summer; heavy rains fall on the seaward slopes of the Cameroon Mountains. Gorée, lat. 15° N., on the coast of Senegambia, gives a fine example of a rainy (summer) and a dry (winter) monsoon. Numerous combinations of equatorial, trade and monsoon rainfalls are found, often creating great complexity. The islands of the East Indian archipelago furnish many examples of such curious complications.
4. Mountain Climate.—In the torrid zone altitude is chiefly important because of its effect in tempering the heat of the lowlands, especially at night. If tropical mountains are high enough, they carry snow all the year round, even on the equator, and the zones of vegetation may range from the densest tropical forest at their base to the snow on their summits. The highlands and mountains within the tropics are thus often sharply contrasted with the lowlands, and offer more agreeable and more healthy conditions for white settlement. They are thus often sought by residents from colder latitudes as the most attractive resorts. In India, the hill stations are crowded during the hot months by civilian and military officials. The climate of many tropical plateaus and mountains has the reputation of being a “perpetual spring.” Thus on the interior plateau of the tropical Cordilleras of South America, and on the plateaus of tropical Africa, the heat is tempered by the altitude, while the lowlands and coasts are very hot. The rainfall on tropical mountains and highlands often differs considerably in amount from that on the lowlands, and other features common to mountain climates the world over are also noted.
The Characteristics of the Temperate Zones.
General.—As a whole, the “temperate zones” are temperate only in that their mean temperatures and their physiological effects are intermediate between those of the tropics and those of the polar zones. A marked changeableness of the weather is a striking characteristic of these zones. Apparently irregular and haphazard, these continual weather changes, although they are essentially non-periodic, nevertheless run through a fairly systematic series. Climate and weather are by no means synonymous over most of the extra-tropical latitudes.
Temperature.—The mean annual temperatures at the margins of the north temperate zone differ by more than 70°. The ranges between the mean temperatures of hottest and coldest months reach 120° at their maximum in north-eastern Siberia, and 80° in North America. A January mean of -60° and a July mean of 95°, and maxima of over 120° and minima of -90°, occur in the same zone. Such great ranges characterize the extreme land climates. Under the influence of the oceans, the windward coasts have much smaller ranges. The annual ranges in middle and higher latitudes exceed the diurnal, the conditions of much of the torrid zone thus being exactly reversed. Over much of the oceans of the temperate zones the annual range is less than 10°. In the south temperate zone there are no extreme ranges, the maxima, slightly over 30°, being near the margin of the zone in the interior of South America, South Africa and Australia. In these same localities the diurnal ranges rival those of the north temperate zone.
The north-eastern Atlantic and north-western Europe are about 35° too warm for their latitude in January, while north-eastern Siberia is 30° too cold. The lands north of Hudson Bay are 25° too cold, and the waters of the Alaskan Bay 20° too warm. In July, and in the southern hemisphere, the anomalies are small. The lands which are the centre of civilization in Europe average too warm for their latitudes. The diurnal variability of temperature is greater in the north temperate zone than elsewhere in the world, and the same month may differ greatly in its character in different years. The annual temperature curve has one maximum and one minimum. In the continental type the times of maximum and minimum are about one month behind the dates of maximum and minimum insolation. In the marine type the retardation may amount to nearly two months. Coasts and islands have a tendency to a cool spring and warm autumn; continents, to similar temperatures in both spring and fall.
Pressure and Winds.—The prevailing winds are the “westerlies,” which are much less regular than the trades. They vary greatly in velocity in different regions and in different seasons, and are stronger in winter than in summer. They are much interfered with, especially in the higher northern latitudes, by seasonal changes of temperature and pressure over the continents, whereby the latter establish, more or less successfully, a system of obliquely outflowing winds in winter and of obliquely inflowing winds in summer. In summer, when the lands have low pressure, the northern oceans are dominated by great oval areas of high pressure, with outflowing spiral eddies, while in winter, when the northern lands have high pressure, the northern portions of the oceans develop cyclonic systems of inflowing winds over their warm waters. All these great continental and oceanic systems of spiralling winds are important climatic controls.
The westerlies are also much confused and interrupted by storms, whence their designation of stormy westerlies. So common are such interruptions that the prevailing westerly wind direction is often difficult to discern without careful observation. Cyclonic storms are most numerous and best developed in winter. Although greatly interfered with near sea-level by continental changes of pressure, by cyclonic and anticyclonic whirls, and by local inequalities of the surface, the eastward movement of the atmosphere remains very constant aloft. The south temperate zone being chiefly water, the westerlies are but little disturbed there by continental effects. Between latitudes 40° and 60° S. the “brave west winds” blow with a constancy and velocity found in the northern hemisphere only on the oceans, and then in a modified form. Storms, frequent and severe, characterize these southern hemisphere westerlies, and easterly wind directions are temporarily noted during their passage. Voyages to the west around Cape Horn against head gales, and in cold wet weather, are much dreaded. South of Africa and Australia, also, the westerlies are remarkably steady and strong. The winter in these latitudes is stormier than the summer, but the seasonal difference is less than north of the equator.
Rainfall.—Rainfall is fairly abundant over the oceans and also over a considerable part of the lands (30-80 in. and more). It comes chiefly in connexion with the usual cyclonic storms, or in thunderstorms. So great are the differences, geographic and periodic, in rainfall produced by differences in temperature, topography, cyclonic conditions, &c., that only the most general rules can be laid down. The equatorward margin of the temperate zone rains is clearly defined on the west coasts, at the points where the coast deserts are replaced by belts of light or moderate rainfall. Bold west coasts, on the polar side of lat. 40°, are very rainy (100 in. and more a year in the most favourable situations). The hearts of the continents, far from the sea, and especially when well enclosed by mountains, or when blown over by cool ocean winds which warm while crossing the land, have light rainfall (less than 10-20 in.). East coasts are wetter than interiors, but drier than west coasts. Winter is the season of maximum rainfall over oceans, islands and west coasts, for the westerlies are then most active, cyclonic storms are most numerous and best developed, and the cold lands chill the inflowing damp air. At this season, however, the low temperatures, high pressures, and tendency to outflowing winds over the continents are unfavourable to rainfall, and the interior land areas as a rule 518 then have their minimum. The warmer months bring the maximum rainfall over the continents. Conditions are then favourable for inflowing damp winds from the adjacent oceans; there is the best opportunity for convection; thunder-showers readily develop on the hot afternoons; the capacity of the air for water vapour is greatest. The marine type of rainfall, with a winter maximum, extends in over the western borders of the continents, and is also found in the winter rainfall of the sub-tropical belts. Rainfalls are heaviest along the tracks of most frequent cyclonic storms.
For continental stations the typical daily march of rainfall shows a chief maximum in the afternoon, and a secondary maximum in the night or early morning. The chief minimum comes between 10 A.M. and 2 P.M. Coast stations generally have a night maximum and a minimum between 10 A.M. and 4 P.M.
Humidity and Cloudiness.—S.A. Arrhenius gives the mean cloudiness for different latitudes as follows:—
| 70° N. | 60° | 50° | 40° | 30° | 20° | 10° | Eq. | 10° | 20° | 30° | 40° | 50° | 60° S. |
| 59 | 61 | 48 | 49 | 42 | 40 | 50 | 58 | 57 | 48 | 46 | 56 | 66 | 75 |
The higher latitudes of the temperate zones thus have a mean cloudiness which equals and even exceeds that of the equatorial belt. The amounts are greater over the oceans and coasts than inland. The belts of minimum cloudiness are at about lat. 30° N. and S. Over the continental interiors the cloudiest season is summer, but the amount is never very large. Otherwise, winter is generally the cloudiest season and with a fairly high mean annual amount.
The absolute humidity as a whole decreases as the temperature falls. The relative humidity averages 90%, more or less, over the oceans, and is high under the clouds and rain of cyclonic storms, but depends, on land, upon the wind direction, winds from an ocean or from a lower latitude being damper, and those from a continent or from a colder latitude being drier.
Seasons.—Seasons in the temperate zones are classified according to temperature; not, as in the tropics, by rainfall. The four seasons are important characteristics, especially of the middle latitudes of the north temperate zone. Towards the equatorial margins of the zones the difference in temperature between summer and winter becomes smaller, and the transition seasons weaken and even disappear. At the polar margins the change from winter to summer, and vice versa, is so sudden that there also the transition seasons disappear.
These seasonal changes are of the greatest importance in the life of man. The monotonous heat of the tropics and the continued cold of the polar zones are both depressing. Their tendency is to operate against man’s highest development. The seasonal changes of the temperate zones stimulate man to activity. They develop him, physically and mentally. They encourage higher civilization. A cold, stormy winter necessitates forethought in the preparation during the summer of clothing, food and shelter. Development must result from such conditions. In the warm, moist tropics life is too easy; in the cold polar zones it is too hard. Near the poles, the growing season is too short; in the moist tropics it is so long that there is little inducement to labour at any special time. The regularity, and the need, of outdoor work during a part of the year are important factors in the development of man in the temperate zones.
Weather.—An extreme changeableness of the weather, depending on the succession of cyclones and anticyclones, is another characteristic. For most of the year, and most of the zone, settled weather is unknown. The changes are most rapid in the northern portion of the north temperate zone, especially on the continents, where the cyclones travel fastest. The nature of these changes depends on the degree of development, the velocity of progression, the track, and other conditions of the disturbance which produces them. The particular weather types resulting from this control give the climates their distinctive character.
The types vary with the season and with the geographical position. They result from a combination, more or less irregular, of periodic diurnal elements, under the regular control of the sun; and of non-periodic cyclonic and anticyclonic elements. In summer, on land, when the Cyclonic element is weakest and the solar control is the strongest, the dominant types are associated with the regular changes from day to night. Daytime cumulus clouds; diurnal variation in wind velocity; afternoon thunderstorms, with considerable regularity, characterize the warmest months over the continents and present an analogy with tropical conditions. Cyclonic and anticyclonic spells of hotter or cooler, rainy or dry, weather, with varying winds differing in the temperatures and the moisture which they bring, serve to break the regularity of the diurnal types. In winter the non-periodic, cyclonic control is strongest. The irregular changes from clear to cloudy, from warmer to colder, from dry air to snow or rain, extend over large areas, and show little diurnal control. Spring and fall are transition seasons, and have transition weather types. The south temperate zone oceans have a constancy of non-periodic cyclonic weather changes through the year which is only faintly imitated over the oceans of the northern hemisphere. Winter types differ little from summer. The diurnal control is never very strong. Stormy weather prevails throughout the year although the weather changes are more frequent and stronger in the colder months.
Climatic Subdivisions.—There are fundamental differences between the north and south temperate zones. The latter zone is sufficiently individual to be given a place by itself. The marginal sub-tropical belts must also be considered as a separate group by themselves. The north temperate zone as a whole includes large areas of land, stretching over many degrees of latitude, as well as of water. Hence it embraces so remarkable a diversity of climates that no single district can be taken as typical of the whole. The simplest and most rational scheme for a classification of these climates is based on the fundamental differences which depend upon land and water, upon the prevailing winds, and upon altitude. Thus there are the ocean areas and the land areas. The latter are then subdivided into western (windward) and eastern (leeward) coasts, and interiors. Mountain climates remain as a separate group.
South Temperate Zone.—Because of the large ocean surface, the whole meteorological régime in the south temperate zone is more uniform than in the northern. The south temperate zone may properly be called “temperate.” Its temperature changes are small; its prevailing winds are stronger and steadier than in the northern hemisphere; its seasons are more uniform; its weather is prevailingly stormier, more changeable, and more under cyclonic control. The uniformity of the climatic conditions over the far southern oceans is monotonously unattractive. The continental areas are small, and develop to a limited degree only the more marked seasonal and diurnal changes which are characteristic of lands in general. The summers are less stormy than the winters, but even the summer temperatures are not high. Such an area as that of New Zealand, with its mild climate and fairly regular rains, is really at the margins of the zone, and has much more favourable conditions than the islands farther south. These islands, in the heart of this zone, have dull, cheerless and inhospitable climates. The zone enjoys a good reputation for healthfulness, which fact has been ascribed chiefly to the strong and active air movement, the relatively drier air than in corresponding northern latitudes, and the cool summers. It must be remembered, also, that the lands are mostly in the sub-tropical belt, which possesses peculiar climatic advantages, as will be seen.
Sub-tropical Belts: Mediterranean Climates.—At the tropical margins of the temperate zones are the so-called sub-tropical belts. Their rainfall regime is alternately that of the westerlies and of the trades. They are thus associated, now with the temperate and now with the torrid zones. In winter the equatorward migration of the great pressure and wind systems brings these latitudes under the control of the westerlies, whose frequent irregular storms give a moderate winter precipitation. 519 These winter rains are not steady and continuous, but are separated by spells of fine sunny weather. The amounts vary greatly.4 In summer, when the trades are extended polewards by the outflowing equatorward winds on the eastern side of the ocean anticyclones, mild, dry and nearly continuous fair weather prevails, with general northerly winds.
The sub-tropical belts of winter rains and dry summers are not very clearly defined. They are mainly limited to the western coasts of the continents, and to the islands off these coasts in latitudes between about 28° and 40°. The sub-tropical belt is exceptionally wide in the old world, and reaches far inland there, embracing the countries bordering on the Mediterranean in southern Europe and northern Africa, and then extending eastward across the Dalmatian coast and the southern part of the Balkan peninsula into Syria, Mesopotamia, Arabia north of the tropic, Persia and the adjacent lands. The fact that the Mediterranean countries are so generally included has led to the use of the name “Mediterranean climate.” Owing to the great irregularity of topography and outline, the Mediterranean province embraces many varieties of climate, but the dominant characteristics are the mild temperatures, except on the higher elevations, and the sub-tropical rains.
On the west coasts of the two Americas the sub-tropical belt of winter rains is clearly seen in California and in northern Chile, on the west of the coast mountain ranges. Between the region which has rain throughout the year from the stormy westerlies, and the districts which are permanently arid under the trades, there is an indefinite belt over which rains fall in winter. In southern Africa, which is controlled by the high pressure areas of the South Atlantic and south Indian oceans, the south-western coastal belt has winter rains, decreasing to the north, while the east coast and adjoining interior have summer rains, from the south-east trade. Southern Australia is climatically similar to South Africa. In summer the trades give rainfall on the eastern coast, decreasing inland. In winter the westerlies give moderate rains, chiefly on the south-western coast.
The sub-tropical climates follow the tropical high pressure belts across the oceans, but they do not retain their distinctive character far inland from the west coasts of the continents (except in the Mediterranean case), nor on the east coasts. On the latter, summer monsoons and the occurrence of general summer rains interfere, as in eastern Asia and in Florida.
 |
| Fig. 8.—Annual March of Rainfall: Sub-tropical Type. W.A, Western Australia: M, Malta. |
Strictly winter rains are typical of the coasts and islands of this belt. The more continental areas have a tendency to spring and autumn rains. The rainy and dry seasons are most marked at the equatorward margins of the belt. With increasing latitude, the rain is more evenly distributed through the year, the summer becoming more and more rainy until, in the continental interiors of the higher latitudes, the summer becomes the season of maximum rainfall. The monthly distribution of rainfall in two sub-tropical regions is shown in the accompanying curves for Malta and for Western Australia (fig. 8). In Alexandria the dry season lasts nearly eight months; in Palestine, from six to seven months; in Greece, about four months. The sub-tropical rains are peculiarly well developed on the eastern coast of the Atlantic Ocean.
The winter rains which migrate equatorward are separated by the Sahara from the equatorial rains which migrate poleward. An unusually extended migration of either of these rain belts may bring them close together, leaving but a small part, if any, of the intervening desert actually rainless. The Arabian desert occupies a somewhat similar position. Large variations in the annual rainfall may be expected towards the equatorial margins of the sub-tropical belts.
The main features of the sub-tropical rains east of the Atlantic are repeated on the Pacific coasts of the two Americas. In North America the rainfall decreases from Alaska, Washington and northern Oregon southwards to lower California, and the length of the summer dry season increases. At San Diego, six months (May-October) have each less than 5% of the annual precipitation, and four of these have 1%. The southern extremity of Chile, from about latitude 38°S. southward, has heavy rainfall throughout the year from the westerlies, with a winter maximum. Northern Chile is persistently dry. Between these two there are winter rains and dry summers. Neither Africa nor Australia extends far enough south to show the different members of this system well. New Zealand is almost wholly in the prevailing westerly belt. Northern India is unique in having summer monsoon rains and also winter rains, the latter from weak cyclonic storms which correspond with the sub-tropical winter rains.
 |
| Fig. 9.—Annual March of Temperature for selected Sub-tropical Stations. C, Cordoba; A, Auckland; Ba, Bermuda; Bd, Bagdad. |
From the position of the sub-tropical belts to leeward of the oceans, and at the equatorial margins of the temperate zones, it follows that their temperatures are not extreme. Further, the protection afforded by mountain ranges, as by the Alps in Europe and the Sierra Nevada in the United States, is an important factor in keeping out extremes of winter cold. The annual march and ranges of temperature depend upon position with reference to continental or marine influences. This is seen in the accompanying data and curves for Bagdad, Cordoba (Argentina), Bermuda and Auckland (fig. 9). The Mediterranean basin is particularly favoured in winter, not only in the protection against cold afforded by the mountains but also in the high temperature of the sea itself. The southern Alpine valleys and the Riviera are well situated, having good protection and a southern exposure. The coldest month usually has a mean temperature well above 32°. Mean minimum temperatures of about, and somewhat below, freezing occur in the northern portion of the district, and in the more continental localities minima a good deal lower have been observed. Mean maximum temperatures of about 95° occur in northern Italy, and of still higher degrees in the southern portions. Somewhat similar conditions obtain in the sub-tropical district of North America. Under the control of passing cyclonic storm areas, hot or cold winds, which often owe some of their special characteristics to the topography, bring into the sub-tropical belts, from higher or lower latitudes, unseasonably high or low temperatures. These winds have been given special names (mistral, sirocco, bora, &c.).
These belts are among the least cloudy districts in the world. The accompanying curve, giving an average for ten stations shows the small annual amount of cloud, the winter maximum and the marked summer minimum, in a typical sub-tropical 520 climate (fig. 10). The winter rains do not bring continuously overcast skies, and a summer month with a mean cloudiness of 10% is not exceptional in the drier parts of the sub-tropics.
 |
| Fig. 10.—Annual March of Cloudiness in a Sub-tropical Climate (Eastern Mediterranean). |
 | |
| Fig. 11.—Annual March of Temperature for Selected Stations in the Temperate Zones. | |
| S. I., Scilly Isles. | B, Blagovyeshchensk. |
| P, Prague. | Sa, Sakhalin. |
| C, Charkow. | T, Thorshavn. |
| S, Semipalatinsk. | Y, Yakutsk. |
| K, Kiakta. | |
With prevailing fair skies, even temperatures, and moderate rainfall, the sub-tropical belts possess many climatic advantages which fit them for health resorts. The long list of well-known resorts on the Mediterranean coast, and the shorter list for California, bear witness to this fact.
North Temperate Zone: West Coasts.—Marine climatic types are carried by the prevailing westerlies on to the western coasts of the continents, giving them mild winters and cool summers, abundant rainfall, and a high degree of cloudiness and relative humidity. North-western Europe is particularly favoured because of the remarkably high temperatures of the North Atlantic Ocean. January means of 40° to 50° in the British Isles and on the northern French coast occur in the same latitudes as those of 0° and 10° in the far interior of Asia. In July means 60° to 70° in the former contrast with 70° to 80° in the latter districts. The conditions are somewhat similar in North America. Along the western coasts of North America and of Europe the mean annual ranges are under 25°—actually no greater than some of those within the tropics. Irregular cyclonic temperature changes are, however, marked in the temperate zone, while absent in the tropics. The curves for the Scilly Isles and for Thorshavn, Faröe Islands, illustrate the insular type of temperature on the west coasts (fig. 11). The annual march of rainfall, with the marked maximum in the fall and winter which is characteristic of the marine regime, is illustrated in the curve for north-western Europe (fig. 12). On the northern Pacific coast of North America the distribution is similar, and in the southern hemisphere the western coasts of southern South America, Tasmania and New Zealand show the same type. The cloudiness and relative humidity average high on western coasts, with the maximum in the colder season.
 |
| Fig. 12.—Annual March of Rainfall: Temperate Zone. C.E. Central Europe; A. Northern Asia; N.A. Atlantic coast of North America; N.W.E. North-west Europe. |
The west coasts therefore, including the important climatic province of western Europe, and the coast provinces of north-western North America, New Zealand and southern Chile, have as a whole mild winters, equable temperatures, small ranges, and abundant rainfall, fairly well distributed through the year. The summers are relatively cool.
Continental Interiors.—The equable climate of the western coasts changes, gradually or suddenly, into the more extreme climates of the interiors. In Europe, where no high mountain ranges intervene, the transition is gradual, and broad stretches of country have the benefits of the tempering influence of the 521 Atlantic. In North America the change is abrupt, and comes on crossing the lofty western mountain barrier. The curves in fig. 11 illustrate well the gradually increasing continentality of the climate with increasing distance inland in Eurasia.
The continental interiors of the north temperate zone have the greatest extremes in the world. Towards the Arctic circle the winters are extremely severe, and January mean temperatures of -10° and -20° occur over considerable areas. At the cold pole of north-eastern Siberia a January mean of -60° is found. Mean minimum temperatures of -40° occur in the area from eastern Russia, over Siberia and down to about latitude 50° N. Over no small part of Siberia minimum temperatures below -70° may be looked for every winter. Thorshavn and Yakutsk are excellent examples of the temperature differences along the same latitude line (see fig. 11). The winter in this interior region is dominated by a marked high pressure. The weather is prevailingly clear and calm. The ground is frozen all the year round below a slight depth over wide areas. The extremely low temperatures are most trying when the steppes are swept by icy storm winds (buran, purga), carrying loose snow, and often resulting in loss of life. In the North American interior the winter cold is somewhat less severe. North American winter weather in middle latitudes is often interrupted by cyclones, which, under the steep poleward temperature gradient then prevailing, cause frequent, marked and sudden changes in wind direction and temperature over the central and eastern United States. Cold waves and warm waves are common, and blizzards resemble the buran or purga of Russia and Siberia. With cold northerly winds, temperatures below freezing are carried far south towards the tropic.
The January mean temperatures in the southern portions of the continental interiors average about 50° or 60°. In summer the northern continental interiors are warm, with July means of 60° and thereabouts. These temperatures are not much higher than those on the west coasts, but as the northern interior winters are much colder than those on the coasts, the interior ranges are very large. Mean maximum temperatures of 86° occur beyond the Arctic circle in north-eastern Siberia, and beyond latitude 60° in North America. In spite of the extreme winter cold, agriculture extends remarkably far north in these regions, because of the warm, though short, summers, with favourable rainfall distribution. The summer heat is sufficient to thaw the upper surface of the frozen ground, and vegetation prospers for its short season. At this time great stretches of flat surface become swamps. The southern interiors have torrid heat in summer, temperatures of over 90° being recorded in the south-western United States and in southern Asia. In these districts the diurnal ranges of temperature are very large, often exceeding 40°, and the mean maxima exceed 110°.
The winter maximum rainfall of the west coasts becomes a summer maximum in the interiors. The change is gradual in Europe, as was the change in temperature, but more sudden in North America. The curves for central Europe and for northern Asia illustrate these continental summer rains (see fig. 12). The summer maximum becomes more marked with the increasing continental character of the climate. There is also a well-marked decrease in the amount of rainfall inland. In western Europe the rainfall averages 20 to 30 in., with much larger amounts (reaching 80-100 in. and even more) on the bold west coasts, as in the British Isles and Scandinavia, where the moist Atlantic winds are deflected upwards, and also locally on mountain ranges, as on the Alps. There are small rainfalls (below 20 in.) in eastern Scandinavia and on the Iberian peninsula. Eastern Europe has generally less than 20 in., western Siberia about 15 in., and eastern Siberia about 10 in. In the southern part of the great overgrown continent of Asia an extended region of steppes and deserts, too far from the sea to receive sufficient precipitation, shut in, furthermore, by mountains, controlled in summer by drying northerly winds, receives less than 10 in. a year, and in places less than 5 in. In this interior district of Asia population is inevitably small and suffers under a condition of hopeless aridity.
The North American interior has more favourable rainfall conditions than Asia, because the former continent is not overgrown. The heavy rainfalls on the western slopes of the Pacific coast mountains correspond, in a general way, with those on the west coast of Europe, although they are heavier (over 100 in. at a maximum). The close proximity of the mountains to the Pacific, however, involves a much more rapid decrease of rainfall inland than is the case in Europe, as may be seen by comparing the isohyetal lines5 in the two cases. A considerable interior region is left with deficient rainfall (less than 10 in.) in the south-west. The eastern portion of the continent is freely open to the Atlantic and the Gulf of Mexico, so that moist cyclonic winds have access, and rainfalls of over 20 in. are found everywhere east of the 100th meridian. These conditions are much more favourable than those in eastern Asia. The greater part of the interior of North America has the usual warm-season rains. In the interior basin, between the Rocky and Sierra Nevada mountains, the higher plateaus and mountains receive much more rain than the desert lowlands. Forests grow on the higher elevations, while irrigation is necessary for agriculture on the lowlands. The rainfall here comes largely from thunderstorms.
In South America the narrow Pacific slope has heavy rainfall (over 80 in.). East of the Andes the plains are dry (mostly less than 10 in.). The southern part of the continent is very narrow, and is open to the east, as well as more open to the west owing to the decreasing height of the mountains. Hence the rainfall increases somewhat to the south, coming in connexion with passing cyclones. Tasmania and New Zealand have most rain on their western slopes.
 |
| Fig. 13.—Annual March of Cloudiness: Temperate Zones. E, Central Europe; A, Eastern Asia; M, mountain. |
In a typical continental climate the winter, except for radiation fogs, is very clear, and the summer the cloudiest season, as is well shown in the accompanying curve for eastern Asia (A, fig. 13). In a more moderate continental climate, such as that of central Europe (E, fig. 13), and much of the United States, the winter is the cloudiest season. In the first case the mean cloudiness is small; in the second there is a good deal of cloud all the year round.
East Coasts.—The prevailing winds carry the continental climates of the interiors off over the eastern coasts of the temperate zone lands, and even for some distance on to the adjacent oceans. The east coasts therefore have continental climates, with modifications resulting from the presence of the oceans to leeward, and are necessarily separated from the west coasts, with which they have little in common. On the west coasts of the north temperate lands the isotherms are far apart. On the east coasts they are crowded together. The east coasts share with the interiors large annual and cyclonic ranges of temperature. A glance at the isothermal maps of the world will show at once how favoured, because of its position to leeward of the warm North Atlantic waters, is western Europe as compared with eastern North America. A similar contrast, less marked, is seen in eastern Asia and western North America. In eastern Asia there is some protection, by the coast mountains, against the extreme cold of the interior, but in North America there is no such barrier, and severe cold winds sweep across the Atlantic coast states, even far to the south. Owing to the prevailing offshore winds, the oceans to leeward have relatively little effect.
As already noted, the rainfall increases from the interiors towards the east coasts. In North America the distribution through the year is very uniform, with some tendency to a summer maximum, as in the interior (N.A, fig. 12).
In eastern Asia the winters are relatively dry and clear, under 522 the influence of the cold offshore monsoon, and the summers are warm and rainy. Rainfalls of 40 in. are found on the east coasts of Korea, Kamchatka and Japan, while in North America, which is more open, they reach farther inland. Japan, although occupying an insular position, has a modified continental rather than a marine climate. The winter monsoon, after crossing the water, gives abundant rain on the western coast, while the winter is relatively dry on the lee of the mountains, on the east. Japan has smaller temperature ranges than the mainland.
Mountain Climates.—The mountain climates of the temperate zone have the usual characteristics which are associated with altitude everywhere. If the altitude is sufficiently great the decreased temperature gives mountains a polar climate, with the difference that the summers are relatively cool while the winters are mild owing to inversions of temperature in anticyclonic weather. Hence the annual ranges are smaller than over lowlands. At such times of inversion the mountain-tops often appear as local areas of higher temperature in a general region of colder air over the valleys and lowlands. The increased intensity of insolation aloft is an important factor in giving certain mountain resorts their deserved popularity in winter (e.g. Davos and Meran). Of Meran it has been well said that from December to March the nights are winter, but the days are mild spring. The diurnal ascending air currents of summer usually give mountains their maximum cloudiness and highest relative humidity in the warmer months, while winter is the drier and clearer season. This is shown in curve M, fig. 13. The clouds of winter are low, those of summer are higher. Hence the annual march of cloudiness on mountains is usually the opposite of that on lowlands.
Characteristics of the Polar Zones.
General.—The temperate zones merge into the polar zones at the Arctic and Antarctic circles, or, if temperature be used as the basis of classification, at the isotherms of 50° for the warmest month, as suggested by Supan. The longer or shorter absence of the sun gives the climate a peculiar character, not found elsewhere.
Beyond the isotherm of 50° for the warmest month forest trees and cereals do not grow. In the northern hemisphere this line is well north of the Arctic circle in the continental climate of Asia, and north of it also in north-western North America and in northern Scandinavia, but falls well south in eastern British America, Labrador and Greenland, and also in the North Pacific Ocean. In the southern hemisphere this isotherm crosses the southern extremity of South America, and runs fairly east and west around the globe there. The conditions of life are necessarily very specialized for the peculiar climatic features which are met with in these zones. There is a minimum of life, but more in the north polar than the south polar zone. Plants are few and lowly. Land animals which depend upon plant food must therefore likewise be few in number. Farming and cattle-raising cease. Population is small and scattered. There are no permanent settlements at all within the Antarctic circle. Life is a constant struggle for existence. Man seeks his food by the chase on land, but chiefly in the sea. He lives along, or near, the sea-coast. The interior lands, away from the sea, are deserted. Gales and snow and cold cause many deaths on land, and, especially during fishing expeditions, at sea. Under such hard conditions of securing food, famine is a likely occurrence.
In the arctic climate vegetation must make rapid growth in the short, cool summer. In the highest latitudes the summer temperatures are not high enough to melt snow on a level. Exposure is therefore of the greatest importance. Arctic plants grow and blossom with great rapidity and luxuriance where the exposure is favourable, and where the water from the melting snow can run off. The soil then dries quickly, and can be effectively warmed. Protection against cold winds is another important factor in the growth of vegetation. Over great stretches of the northern plains the surface only is thawed out in the warmer months, and swamps, mosses and lichens are found above eternally frozen ground. Direct insolation is very effective in high latitudes. Where the exposure is favourable, snow melts in the sun when the temperature of the air in the shade is far below freezing.
Arctic and antarctic zones differ a good deal in the distribution and arrangement of land and water around and in them. The southern zone is surrounded by a wide belt of open sea; the northern, by land areas. The northern is therefore much affected by the conditions of adjacent continental masses. Nevertheless, the general characteristics are apparently much the same over both, so far as is now known, the antarctic differing from the arctic chiefly in having colder summers and in the regularity of its pressure and winds. Both zones have the lowest mean annual temperatures in their respective hemispheres, and hence may properly be called the cold zones.
Temperature.—At the solstices the two poles receive the largest amounts of insolation which any part of the earth’s surface ever receives. It would seem, therefore, that the temperatures at the poles should then be the highest in the world, but as a matter of fact they are nearly or quite the lowest. Temperatures do not follow insolation in this case because much of the latter never reaches the earth’s surface; because most of the energy which does reach the surface is expended in melting the snow and ice of the polar areas; and because the water areas are large, and the duration of insolation is short.
A set of monthly isothermal charts of the north polar area, based on all available observations, has been prepared by H. Mohn and published in the volume on Meteorology of the Nansen expedition. In the winter months there are three cold poles, in Siberia, in Greenland and at the pole itself. In January the mean temperatures at these three cold poles are -49°, -40° and -40° respectively. The Siberian cold pole becomes a maximum of temperature during the summer, but the Greenland and polar minima remain throughout the year. In July the temperature distribution shows considerable uniformity; the gradients are relatively weak. A large area in the interior of Greenland, and one of about equal extent around the pole, are within the isotherm of 32°. For the year a large area around the pole is enclosed by the isotherm of -4°, with an isotherm of the same value in the interior of Greenland, but a local area of -7.6° is noted in Greenland, and one of -11.2° is centred at lat. 80° N. and long. 170° E.
The north polar chart of annual range of temperature shows a maximum range of about 120° in Siberia; of 80° in North America; of 75.6° at the North Pole, and of 72° in Greenland. The North Pole obviously has a continental climate. The minimum ranges are on the Atlantic and Pacific Oceans. The mean annual isanomalies show that the interior of Greenland has a negative anomaly in all months. The Norwegian sea area is 45° too warm in January and February. Siberia has +10.8° in summer, and -45° in January. Between Bering Strait and the pole there is a negative anomaly in all months. The influence of the Gulf Stream drift is clearly seen on the chart, as it is also on that of mean annual ranges.
For the North Pole Mohn gives the following results, obtained by graphic methods:—
Mean Temperatures at the North Pole.
| Jan. | Feb. | Mar. | Apr. | May. | June. | July. | Aug. | Sept. | Oct. | Nov. | Dec. | Year. |
| -41.8° | -41.8° | -31.0° | -18.4° | 8.6° | 28.4° | 30.2° | 26.6° | 8.6° | -11.2° | -27.4° | -36.4° | -8.9° |
It appears that the region about the North Pole is the coldest place in the northern hemisphere for the mean of the year, and that the interior ice desert of Greenland, together with the inner polar area, are together the coldest parts of the northern hemisphere in July. In January, however, Verkhoyansk, in north-eastern Siberia, just within the Arctic circle, has a mean temperature of about -60°, while the inner polar area and the northern interior of Greenland have only -40°. Thus far no minima as low as those of north-eastern Siberia have been recorded in the Arctic.
For the Antarctic our knowledge is still very fragmentary, 523 and relates chiefly to the summer months. Hann has determined the mean temperatures of the higher southern latitudes as follows:—6
Mean Temperatures of High Southern Latitudes.
| S. Lat. | 50° | 60° | 70° | 80° |
| Mean Annual | 41.9 | 28.4 | 11.3 | -3.6 |
| January | 46.9 | 37.8 | 30.6 | 20.3 |
| July | 37.2 | 18.3 | -8.0 | -24.7 |
From lat. 70° S. polewards, J. Hann finds that the southern hemisphere is colder than the northern. Antarctic summers are decidedly cold. The mean annual temperatures experienced have been in the vicinity of 10°, and the minima of an ordinary antarctic winter go down to -40° and below, but so far no minima of the severest Siberian intensity have been noted. The maxima have varied between 35° and 50°.
The temperatures at the South Pole itself furnish an interesting subject for speculation. It is likely that near the South Pole will prove to be the coldest point on the earth’s surface for the year, as the distribution of insolation would imply, and as the conditions of land and ice and snow there would suggest. The lowest winter and summer temperatures in the southern hemisphere will almost certainly be found in the immediate vicinity of the pole. It must not be supposed that the isotherms in the antarctic region run parallel with the latitude lines. They bend polewards and equatorwards at different meridians, although much less so than in the Arctic.
The annual march of temperature in the north polar zone, for which we have the best comparable data, is peculiar in having a much-retarded minimum in February or even in March—the result of the long, cold winter. The temperature rises rapidly towards summer, and reaches a maximum in July. Autumn is warmer than spring.
The continents do not penetrate far enough into the arctic zone to develop a pure continental climate in the highest latitudes. Verkhoyansk, in lat. 67° 6′ N., furnishes an excellent example of an exaggerated continental type for the margin of the zone, with an annual range of 120°. One-third as large a range is found on Novaya Zemlya. Polar climate as a whole has large annual and small diurnal ranges, but sudden changes of wind may cause marked irregular temperature changes within twenty-four hours, especially in winter. The smaller ranges are associated with greater cloudiness, and vice versa. The mean diurnal variability is very small in summer, and reaches its maximum in winter, about 7° in February, according to Mohn.
Pressure and Winds.—Owing to the more symmetrical distribution of land and water in the southern than in the northern polar area, the pressures and winds have a simpler arrangement in the former, and may be first considered. The rapid southward decrease of pressure, which is so marked a feature of the higher latitudes of the southern hemisphere on the isobaric charts of the world, does not continue all the way to the South Pole. Nor do the prevailing westerly winds, constituting the “circumpolar whirl,” which are so well developed over the southern portions of the southern hemisphere oceans, blow all the way home to the South Pole. The steep poleward pressure gradients of these southern oceans end in a trough of low pressure, girdling the earth at about the Antarctic circle. From here the pressure increases again towards the South Pole, where a permanent inner polar anticyclonic area is found, with outflowing winds deflected by the earth’s rotation into easterly and south-easterly directions. These easterly winds have been observed by the recent expeditions which have penetrated far enough south to cross the low-pressure trough. The limits between the prevailing westerlies and the outflowing winds from the pole (“easterlies”) vary with the longitude and migrate with the seasons. The change in passing from one wind system to the other is easily observed. This south polar anticyclone, with its surrounding low-pressure girdle, migrates with the season, the centre apparently shifting polewards in summer and towards the eastern hemisphere in winter. The outflowing winds from the polar anticyclones sweep down across the inland ice. Under certain topographic conditions, descending across mountain ranges, as in the case of the Admiralty Range in Victoria Land, these winds may develop high velocity and take on typical föhn characteristics, raising the temperature to an unusually high degree. Föhn winds are also known on both coasts of Greenland, when a passing cyclonic depression draws the air down from the icy interior. These Greenland föhn winds are important climatic elements, for they blow down warm and dry, raising the temperature even 30° or 40° above the winter mean, and melting the snow.
In the Arctic area the wind systems are less clearly defined and the pressure distribution is much less regular, on account of the irregular distribution of land and water. The isobaric charts published in the report of the Nansen expedition show that the North Atlantic low-pressure area is more or less well developed in all months. Except in June, when it lies over southern Greenland, this tongue-shaped trough of low pressure lies in Davis strait, to the south-west or west of Iceland, and over the Norwegian Sea. In winter it greatly extends its limits farther east into the inner Arctic Ocean, to the north of Russia and Siberia. The Pacific minimum of pressure is found south of Bering Strait and in Alaska. Between these two regions of lower pressure the divide extends from North America to eastern Siberia. This divide has been called by Supan the “Arktische Wind-scheide.” The pressure gradients are steepest in winter. At the pole itself pressure seems to be highest in April and lowest from June to September. The annual range is only about 0.20 in.
The prevailing westerlies, which in the high southern latitudes are so symmetrically developed, are interfered with to such an extent by the varying pressure controls over the northern continents and oceans in summer and winter that they are often hardly recognizable on the wind maps. The isobaric and wind charts show that on the whole the winds blow out from the inner polar basin, especially in winter and spring.
Rain and Snow.—Rainfall on the whole decreases steadily from equator to poles. The amount of precipitation must of necessity be comparatively slight in the polar zones, chiefly because of the small capacity of the air for water vapour at the low temperatures there prevailing; partly also because of the decrease, or absence, of local convectional storms and thunder-showers. Locally, under exceptional conditions, as in the case of the western coast of Norway, the rainfall is a good deal heavier. Even cyclonic storms cannot yield much precipitation. The extended snow and ice fields tend to give an exaggerated idea of the actual amount of precipitation. It must be remembered, however, that evaporation is slow at low temperatures, and melting is not excessive. Hence the polar store of fallen snow is well preserved: interior snowfields, ice sheets and glaciers are produced.
The commonest form of precipitation is naturally snow, the summer limit of which, in the northern hemisphere, is near the Arctic circle, with the exception of Norway. So far as exploration has yet gone into the highest latitudes, rain falls in summer, and it is doubtful whether there are places where all the precipitation falls as snow. The snow of the polar regions is characteristically fine and dry. At low polar temperatures flakes of snow are not found, but precipitation is in the form of ice spicules. The finest glittering ice needles often fill the air, even on clear days, and in calm weather, and gradually descending to the surface, slowly add to the depth of snow on the ground. Dry snow is also blown from the snowfields on windy days, interfering with the transparency of the air.
Humidity, Cloudiness and Fog.—The absolute humidity must be low in polar latitudes, especially in winter, on account of the low temperatures. Relative humidity varies greatly, and very low readings have often been recorded. Cloudiness seems to decrease somewhat towards the inner polar areas, after passing the belt of high cloudiness in the higher latitudes of the temperate zones. In the marine climates of high latitudes the summer, which is the calmest season, has the maximum cloudiness; the winter, with more active wind movement, is clearer. The 524 curve here given illustrates these conditions (fig. 14). The summer maximum is largely due to fogs, which are produced where warm, damp air is chilled by coming in contact with ice. They are also formed over open waters, as among the Faeroe Islands, for example, and open water spaces, in the midst of an ice-covered sea, are commonly detected at a distance by means of the “steam fog” which rises from them. Fogs are less common in winter, when they occur as radiation fogs, of no great thickness. The small winter cloudiness, which is reported also from the antarctic zone, corresponds with the low absolute humidity and small precipitation. The coasts and islands bathed by the warm waters of the Gulf Stream drift usually have a higher cloudiness in winter than in summer. The place of fog is in winter taken by the fine snow crystals, which often darken the air like fog when strong winds raise the dry snow from the surfaces on which it is lying. Cumulus cloud forms are rare, even in summer, and it is doubtful whether the cloud occurs at all in its typical development. Stratus is probably the commonest cloud of high latitudes, often covering the sky for days without a break. Cirrus cloud forms probably decrease polewards.
 |
| Fig. 14.—Annual March of Cloudiness in Polar Latitudes (marine type). |
Cyclones and Weather.—The prevailing westerlies continue up into the margins of the polar zones. Many of their cyclonic storms also continue on to the polar zones, giving sudden and irregular pressure and weather changes. The inner polar areas seem to be beyond the reach of frequent and violent cyclonic disturbance. Calms are more common; the weather is quieter and fairer; precipitation is less. Most of the observations thus far obtained from the Antarctic come from this marginal zone of great cyclonic activity, violent winds, and wet, disagreeable, inhospitable weather, and therefore do not show the features of the actual south polar climate.
During the three years of the “Fram’s” drift depressions passed on all sides of her, with a preponderance on the west. The direction of progression averaged nearly due east, and the hourly velocity 27 to 34 m., which is about that in the United States. For the higher latitudes, most of the cyclones must pass by on the equatorial side of the observer, giving “backing” winds in the northern hemisphere. The main cyclonic tracks are such that the wind characteristically backs in Iceland, and still more so in Jan Mayen and on the eastern coast of Greenland, these districts lying on the north and west of the path of progression. Frightful winter storms occasionally occur along the east coast of Greenland and off Spitzbergen.
For much of the year in the polar zones the diurnal control is weak or absent. The successive spells of stormy or of fine weather are wholly cyclonically controlled. Extraordinary records of storm and gale have been brought back from the far south and the far north. Wind direction and temperature vary in relation to the position of the cyclone. During the long dreary winter night the temperature falls to very low readings. Snowstorms and gales alternate at irregular short intervals with calmer spells of more extreme cold and clearer skies. The periods of greatest cold in winter are calm. A wind from any direction will bring a rise in temperature. This probably results from the fact that the cold is the result of local radiation, and a wind interferes with these conditions by importing higher temperatures, or by mixing upper and lower strata. During the long summer days the temperature rises well above the winter mean, and under favourable conditions certain phenomena, such as the diurnal variation in wind velocity, for example, give evidence of the diurnal control. But the irregular cyclonic weather changes continue, in a modified form. There is no really warm season. Snow still falls frequently. The summer is essentially only a modified winter, especially in the Antarctic. In summer clear spells are relatively warm, and winds bring lower temperatures. In spite of its lack of high temperatures, the northern polar summer, near the margins of the zone, has many attractive qualities in its clean, pure, crisp, dry air, free from dust and impurities; its strong insolation; its slight precipitation.
Twilight and Optical Phenomena.—The monotony and darkness of the polar night are decreased a good deal by the long twilight. Light from moon and stars, and from the aurora, also relieves the darkness. Optical phenomena of great variety, beauty and complexity are common. Solar and lunar haloes, and coronae, and mock suns and moons are often seen. Auroras seem to be less common and less brilliant in the Antarctic than in the Arctic. Sunset and sunrise colours within the polar zones are described as being extraordinarily brilliant and impressive.
Physiological Effects.—The north polar summer, as has been pointed out, in spite of its drawbacks, is in some respects a pleasant and healthful season. But the polar night is monotonous, depressing, repelling. Sir W. E. Parry said that it would be difficult to conceive of two things which are more alike than two polar winters. An everlasting uniform snow covering; rigidity; lifelessness; silence—except for the howl of the gale or the cracking of the ice. Small wonder that the polar night has sometimes unbalanced men’s minds. The first effects are often a strong desire for sleep, and indifference. Later effects have been sleeplessness and nervousness, tending in extreme cases to insanity; anaemia, digestive troubles. Extraordinarily low winter temperatures are easily borne if the air be dry and still. Zero weather seems pleasantly refreshing if clear and calm. But high relative humidity and wind—even a light breeze—give the same degree of cold a penetrating feeling of chill which may be unbearable. Large temperature ranges are endured without danger in the polar winter when the air is dry. When exposed to direct insolation the skin burns and blisters; the lips swell and crack. Thirst has been much complained of by polar explorers, and is due to the active evaporation from the warm body into the dry, relatively cold air. There is no doubt that polar air is singularly free from micro-organisms—a fact which is due chiefly to lack of communication with other parts of the world. Hence many diseases which are common in temperate zones, “colds” among them, are rare.
Changes of Climate.
Popular Belief in Climatic Change.—Belief in a change in the climate of one’s place of residence, within a few generations, and even within the memory of living men, is widespread. Evidence is constantly being brought forward of apparent climatic variations of greater or less amount which are now taking place. Thus we have many accounts of a gradual desiccation which seems to have been going on over a large region in Central Asia during historical times. In northern Africa certain ancient historical records have been taken by different writers to indicate a general decrease of rainfall during the last 3000 or more years. In his crossing of the Sahara between Algeria and the Niger, E. F. Gautier found evidence of a former large population. A gradual desiccation of the region is therefore believed to have taken place, but to-day the equatorial rain belt seems to be again advancing farther north, giving an increased rainfall. Farther south, several lakes have been reported as decreasing in size, e.g. Chad and Victoria; and wells and springs as running dry. In the Lake Chad district A. J. B. Chevalier reports the discovery of vegetable and animal remains which indicate an invasion of the Sudan by a Saharan climate. It is often held that a steady decrease in rainfall has taken place over Greece, Syria and other eastern Mediterranean lands, resulting in a gradual and inevitable deterioration and decay of their people.
What Meteorological Records show.—As concerns the popular impression regarding change of climate, it is clear at the start that no definite answer can be given on the basis of tradition or of general impression. The only answer of real value must be based on the records of accurate instruments, properly exposed and carefully read. When such instrumental records 525 are carefully examined, from the time when they were first kept, which in a few cases goes back about 150 years, there is found no good evidence of any progressive change in temperature, or in the amount of rain and snow. Even when the most accurate instrumental records are available, care must be taken to interpret them correctly. Thus, if a rainfall or snowfall record of several years at some station indicates an apparent increase or decrease in the amount of precipitation, it does not necessarily follow that this means a permanent, progressive change in climate, which is to continue indefinitely. It may simply mean that there have been a few years of somewhat more precipitation, and that a period of somewhat less precipitation is to follow.
Value of Evidence concerning Changes of Climate.—The body of facts which has been adduced as evidence of progressive changes of climate within historical times is not yet sufficiently large and complete to warrant any general correlation and study of these facts as a whole. But there are certain considerations which should be borne in mind in dealing with this evidence before any conclusions are reached. In the first place, changes in the distribution of certain fruits and cereals, and in the dates of the harvest, have often been accepted as undoubted evidence of changes in climate. Such a conclusion is by no means inevitable, for many changes in the districts of cultivation of various crops have naturally resulted from the fact that these same crops are in time found to be more profitably grown, or more easily prepared for market, in another locality. In France, C. A. Angot has made a careful compilation of the dates of the vintage from the 14th century down to the present time, and finds no support for the view so commonly held there that the climate has changed for the worse. At the present time, the average date of the grape harvest in Aubonne is exactly the same as at the close of the 16th century. After a careful study of the conditions of the date tree, from the 4th century, B.C., D. Eginitis concludes that the climate of the eastern portion of the Mediterranean basin has not changed appreciably during twenty-three centuries.
Secondly, a good many of the reports by explorers from little-known regions are contradictory. This shows the need of caution in jumping at conclusions of climatic change. An increased use of water for irrigation may cause the level of water in a lake to fall. Periodic oscillations, giving higher and then lower water, do not indicate progressive change in one direction. Many writers have seen a law in what was really a chance coincidence.
Thirdly, where a progressive desiccation seems to have taken place, it is often a question whether less rain is actually falling, or whether the inhabitants have less capacity and less energy than formerly. Is the change from a once cultivated area to a barren expanse the result of decreasing rainfall, or of the emigration of the former inhabitants to other lands? The difference between a country formerly well irrigated and fertile, and a present-day sandy, inhospitable waste may be the result of a former compulsion of the people, by a strong governing power, to till the soil and to irrigate, while now, without that compulsion, no attempt is made to keep up the work. A region of deficient rainfall, once thickly settled and prosperous, may readily become an apparently hopeless desert, even without the intervention of war and pestilence, if man allows the climate to master him. In many cases the reports of increasing dryness really concern only the decrease in the water supply from rivers and springs, and it is well known that a change in the cultivation of the soil, or in the extent of the forests, may bring about marked changes in the flow of springs and rivers without any essential change in the actual amount of rainfall.
Lastly, a region whose normal rainfall is at best barely sufficient for man’s needs may be abandoned by its inhabitants during a few years of deficient precipitation, and not again occupied even when, a few years later, normal or excessive rainfall occurs.
Periodic Oscillations of Climate: Sun-spot Period.—The discovery of a distinct eleven-year periodicity in the magnetic phenomena of the earth naturally led to investigations of similar periods in meteorology. The literature on this subject has assumed large proportions. The results, however, have not been satisfactory. The problem is difficult and obscure. Fluctuations in temperature and rainfall, occurring in an eleven-year period, have been made out for certain stations but the variations are slight, and it is not yet clear that they are sufficiently marked, uniform and persistent over large areas to make practical application of the periodicity in forecasting possible. In some cases the relation to sun-spot periodicity is open to debate; in others, the results are contradictory.
W. P. Köppen has brought forward evidence of a sun-spot period in the mean annual temperature, especially in the tropics, the maximum temperatures coming in the years of sun-spot minima. The whole amplitude of the variation in the mean annual temperatures, from sun-spot minimum to sun-spot maximum, is, however, only 1.3° in the tropics and a little less than 1° in the extra-tropics. More recently Nordmann (for the years 1870-1900) has continued Köppen’s investigation.
In 1872 C. Meldrum, then Director of the Meteorological Observatory at Mauritius, first called attention to a sun-spot periodicity in rainfall and in the frequency of tropical cyclones in the South Indian Ocean. The latter are most numerous in years of sun-spot maxima, and decrease in frequency with the approach of sun-spot minima. Poëy found later a similar relation in the case of the West Indian hurricanes. Meldrum’s conclusions regarding rainfall were that, with few exceptions, there is more rain in years of sun-spot maxima. S. A. Hill found it to be true of the Indian summer monsoon rains that there seems to be an excess in the first half of the cycle, after the sun-spot maximum. The winter rains of northern India, however, show the opposite relation; the minimum following, or coinciding with, the sun-spot maximum. Particular attention has been paid to the sun-spot cycle of rainfall in India, because of the close relation between famines and the summer monsoon rainfall in that country. Sir Norman Lockyer and Dr W. J. S. Lockyer have recently studied the variations of rainfall in the region surrounding the Indian Ocean in the light of solar changes in temperature. They find that India has two pulses of rainfall, one near the maximum and the other near the minimum of the sun-spot period. The famines of the last fifty years have occurred in the intervals between these two pulses, and these writers believe that if as much had been known in 1836 as is now known, the probability of famines at all the subsequent dates might have been foreseen.
Relations between the sun-spot period and various other meteorological phenomena than temperature, rainfall and tropical cyclones have been made the subject of numerous investigations, but on the whole the results are still too uncertain to be of any but a theoretical value. Some promising conclusions seem, however, to have been reached in regard to pressure variations, and their control over other climatic elements.
Brückner’s 35-Year Cycle.—Of more importance than the results thus far reached for the sun-spot period are those which clearly establish a somewhat longer period of slight fluctuations or oscillations of climate, known as the Brückner cycle, after Professor Brückner of Bern, who has made a careful investigation of the whole subject of climatic changes and finds evidence of a 35-year periodicity in temperature and rainfall. In a cycle whose average length is 35 years, there comes a series of years which are somewhat cooler and also more rainy, and then a series of years which are somewhat warmer and drier. The interval in some cases is twenty years; in others it is fifty. The average interval between two cool and moist, or warm and dry, periods is about 35 years. The mean amplitude of the temperature fluctuation, based on large numbers of data, is a little less than 2°. The fluctuations in rainfall are more marked in interiors than on coasts. The general mean amplitude is 12%, or, excluding exceptional districts, 24%. Regions whose normal rainfall is small are most affected.
The following table shows the dates and characters of Brückner’s periods:—
| Warm | 1746-1755 | 1791-1805 | 1821-1835 | 1851-1870 | .. |
| Dry | 1756-1770 | 1781-1805 | 1826-1840 | 1856-1870 | .. |
| Cold | 1731-1745 | 1756-1790 | 1806-1820 | 1836-1850 | 1871-1885 |
| Wet | 1736-1755 | 1771-1780 | 1806-1825 | 1841-1855 | 1871-1885 |
Interesting confirmation of Brückner’s 35-year period has been found by E. Richter in the variations of the Swiss glaciers, but as these glaciers differ in length, they do not all advance and retreat at the same time. The advance is seen during the cold and damp periods. Brückner has found certain districts in which the phases and epochs of the climatic cycle are exactly reversed. These exceptional districts are almost altogether limited to marine climates. There is thus a sort of compensation between oceans and continents. The rainier periods on the continents are accompanied by relatively low pressures, while the pressures are high and the period dry over the oceans and vice versa. The cold and rainy periods are also marked by a decrease in all pressure differences. It is obvious that changes in the general distribution of atmospheric pressures, over extended areas, are closely associated with fluctuations in temperature and rainfall. These changes in pressure distribution must in some way be associated with changes in the general circulation of the atmosphere, and these again must depend upon some external controlling cause or causes. W. J. S. Lockyer has called attention to the fact that there seems to be a periodicity of about 35 years in solar activity, and that this corresponds with the Brückner period.
It is clear that the existence of a 35-year period will account for many of the views that have been advanced in favour of a progressive change of climate. A succession of a few years wetter or drier than the normal is likely to lead to the conclusion that the change is permanent. Accurate observations extending over as many years as possible, and discussed without prejudice, are necessary before any conclusions are drawn. Observations for one station during the wetter part of a cycle should not be compared with observations for another station during the drier part of the same, or of another cycle.
There are evidences of longer climatic cycles than eleven or 35 years. Brückner calls attention to the fact that sometimes two of his periods seem to merge into one. E. Richter shows much the same thing for the Alpine glaciers. Evidence of considerable climatic changes since the last glacial period is not lacking. But as yet nothing sufficiently definite to warrant general conclusions has been brought forward.
Geological Changes in Climate.—Changes of climate in the geological past are known with absolute certainty to have taken place: periods of glacial invasion, as well as periods of more genial conditions. The evidence, and the causes of these changes have been discussed and re-discussed, by writers almost without number, and from all points of view. Changes in the intensity of insolation; in the sun itself; in the conditions of the earth’s atmosphere; in the astronomical relations of earth and sun; in the distribution of land and water; in the position of the earth’s axis; in the altitude of the land; in the presence of volcanic dust;—now cosmic, now terrestrial conditions—have been suggested, combated, put forward again. None of these hypotheses has prevailed in preference to others. No actual proof of the correctness of this or that theory has been brought forward. No general agreement has been reached.
Conclusion.—Without denying the possibility, or even the probability, of the establishment of the fact of secular changes, there is as yet no sufficient warrant for believing in considerable permanent changes over large areas. Dufour, after a thorough study of all available evidence, has concluded that a change of climate has not been proved. There are periodic oscillations of slight amount. A 35-year period is fairly well established, but is nevertheless of considerable irregularity, and cannot as yet be practically applied in forecasting. Longer periods are suggested, but not made out. As to causes, variations in solar activity are naturally receiving attention, and the results thus far are promising. But climate is a great complex, and complete and satisfactory explanations of all the facts will be difficult, perhaps impossible, to reach. At present, indeed, the facts which call for explanation are still in most cases but poorly determined, and the processes at work are insufficiently understood. Climate is not absolutely a constant. The pendulum swings to the right and to the left. And its swing is as far to the right as to the left. Each generation lives through a part of one, or two, or even three oscillations. A snapshot view of these oscillations makes them seem permanent. As Supan has well said, it was formerly believed that climate changes locally, but progressively and permanently. It is now believed that oscillations of climate are limited in time, but occur over wide areas.
Climate in the Treatment of Disease.—The most important qualities of the atmosphere in relation to health are (i.) the chemical composition, (ii.) the solids floating in it, (iii.) the mean and extreme temperatures, (iv.) the degree of humidity, (v.) the diathermancy, (vi.) the intensity of light, (vii.) the electrical conditions, (viii.) the density and pressure, and (ix.) the prevailing winds. Generally speaking, the relative purity of the air—i.e. absence of septic solid particles—is an important consideration; while cold acts as a stimulant and tonic, increasing the amount of carbon dioxide exhaled in the twenty-four hours. Different individuals, however, react both to heat and cold very differently. At health resorts, where the temperature may vary between 55° and 70° F., strong individuals gradually lose strength and begin to suffer from various degrees of lassitude; whereas a delicate person under the same conditions gains vigour both of mind and body, puts on weight, and is less liable to disease. And a corresponding intensity of cold acts in the reverse manner in each case. Thus a health resort with a moderate degree of heat is very valuable for delicate or elderly people, and those who are temporarily weakened by illness. Cold, however, when combined with wind and damp must be specially avoided by the aged, the delicate, and those prone to gouty and rheumatic affections. The moisture of the atmosphere controls the distribution of warmth on the earth, and is closely bound up with the prevailing winds, temperature, light and pressure. In dry air the evaporation from both skin and lungs is increased, especially if the sunshine be plentiful and the altitude high. In warm moist air strength is lost and there is a distinct tendency to intestinal troubles. In moist cold air perspiration is checked, and rheumatic and joint affections are very common. The main differences between mountain air and that of the plains depend on the former being more rarefied, colder, of a lower absolute humidity, and offering less resistance to the sun’s rays. As the altitude is raised, circulation and respiration are quickened, probably as an effort on the part of the organism to compensate for the diminished supply of oxygen, and somewhat more gradually the number of red blood corpuscles increases, this increase persisting for a considerable time after a return to lower ground. In addition to these changes there is a distinct tendency to diminished proteid metabolism, resulting in an increase of weight owing to the storage of proteid in the tissues. Thus children and young people whose development is not yet complete are especially likely to benefit by the impetus given to growth and the blood-forming organs, and the therapeutic value in their case rarely fails. For older people, however, the benefit depends on whether their organs of circulation and respiration 527 are sufficiently vigorous to respond to the increased demands on them. For anaemia, pulmonary tuberculosis, pleural thickening, deficient expansion of the lungs, neurasthenia, and the debility following fevers and malaria, mountain air is invaluable. But where there is valvular disease of the heart, or rapidly advancing disease of the lungs, it is to be avoided. Light, especially direct sunlight, is of primary importance, the lack of it tending to depression and dyspeptic troubles. Probably its germicidal power accounts for the aseptic character of the air of the Alps, the desert and other places.
Sir Hermann Weber has defined a “good” climate as that in which all the organs and tissues of the body are kept evenly at work in alternation with rest. Thus a climate with constant moderate variations in its principal factors is the best for the maintenance of health. But the best climate for an invalid depends on the particular weakness from which he may suffer. Pulmonary tuberculosis stands first in the importance of the effects of climate. The continuous supply of pure fresh air is the main desideratum, a cool climate being greatly superior to a tropical one. Exposure to strong winds is harmful, since it increases the tendency to cough and thus leads to loss of body temperature, which is in its turn made up at the expense of increased metabolism. A high altitude, from the purity and stimulating properties of the air, is of value to many mild or very early cases, but where the disease is extensive, where the heart is irritable, or where there is any tendency to insomnia, high altitudes are contra-indicated, and no such patient should be sent higher than some 1500 ft. Where the disease is of long standing, with much expectoration, or accompanied by albuminuria, the patient appears to do best in a humid atmosphere but little above the sea-level. The climate of Egypt is especially suitable for cases complicated with bronchitis or bronchiectasis, but is contra-indicated where there is attendant diarrhoea. Madeira and the Canaries are useful when emphysema is present or where there is much irritability of constitution. Bronchitis in young people is best treated by high altitudes, but in older patients by a moist mild climate, except where much expectoration is present.
The influence of atmospheric conditions on the functions of the nose is very marked. Within the ordinary ranges of humidity and temperature the nasal mucous membrane completely saturates the air with aqueous vapour before it reaches the pharynx. In cold and dry mountain climates there is a very free nasal secretion, far beyond what is needed for the saturation of the air; and at low levels the reverse action takes place, the nose becoming “stuffy.” The mechanism on which this depends is found in the erectile tissue, and anything favouring the engorgement of the veins, such as weak heart action, chronic bronchitis or kidney troubles, &c, leads to a corresponding turgidity of the nose and sinuses. In addition to barometric and other influences, it has been found that light produces collapse of this tissue, smoke having a similar effect. On this latter effect probably depends the fact that many asthmatics are better in a city like London than elsewhere, the smoke relieving the turgescence of the inferior turbinals of the nose. In the treatment of pathological nasal conditions, all cases of obstruction from whatsoever cause are best in a dry atmosphere, and where there is atrophy and a deficient flow of mucus in a moist atmosphere. If the mucous membrane is irritable a dry sheltered spot on a sandy soil and in the neighbourhood of pine trees is by far the best.
Scrofulous children, namely, those in whom the resistance to micro-organisms and their products is low, pre-eminently require sea air, and had better be educated at some seaside place. Where the child is very delicate, with small power of reaction, the winter should be passed on some mild coast resort. Gouty and rheumatic affections require a dry soil and warm dry climate, cold and moist winds being especially injurious.
For heart affections high altitudes are to be avoided, though some physicians make an exception of mitral cases where the compensation is good. Moderate elevations of 500 to 1500 ft. are preferable to the sea-level.
In diseases of the kidneys, a warm dry climate, by stimulating the action of the skin, lessens the work to be done by these organs, and thus is the most beneficial. Extremes of heat and cold and elevated regions are all to be avoided.
1 A. Supan, Grundzüge der physischen Erdkunde (Leipzig, 1896), 88-89. Also Atlas of Meteorology, Pl. 1.
2 W.M. Davis, Elementary Meteorology (Boston, 1894), pp. 334-335.
3 A. Supan, Grundzüge der physischen Erdkunde (3rd ed., Leipzig, 1903), pp. 211-214. Also Atlas of Meteorology, Pl. 1.
4 Approximately Lisbon has 28.60 in.; Madrid, 16.50; Algiers, 28.15; Nice, 33.00; Rome, 29.90; Ragusa, 63.90.
5 i.e. lines drawn on a map to connect all places having an equal rainfall.
6 Nature, lxxi. (Jan. 5, 1905), p. 221.
CLIMAX, JOHN (c. 525-600 A.D.), ascetic and mystic, also called Scholasticus and Sinaïtes. After having spent forty years in a cave at the foot of mount Sinai, he became abbot of the monastery. His life has been written by Daniel, a monk belonging to the monastery of Raithu, on the Red Sea. He derives his name Climax (or Climacus) from his work of the same name (Κλῖμαξ τοῦ Παραδείσου, ladder to Paradise), in thirty sections, corresponding to the thirty years of the life of Christ. It is written in a simple and popular style. The first part treats of the vices that hinder the attainment of holiness, the second of the virtues of a Christian.
CLIMBING1 FERN, the botanical genus Lygodium, with about twenty species, chiefly in the warmer parts of the Old World, of interest from its climbing habit. The plants have a creeping stem, on the upper face of which is borne a row of leaves. Each leaf has a slender stem-like axis, which twines round a support and bears leaflets at intervals; it goes on growing indefinitely. It is a favourite warm greenhouse plant.
1 The word “climb” (O.E. climban), meaning strictly to ascend (or similarly descend) by progressive self-impulsion, with some apparent degree of laborious effort and by means of contact with the surface traversed, is connected with the same root as in “cleave” and “cling.” For Alpine climbing, &c., see Mountaineering.
CLINCHANT, JUSTIN (1820-1881), French soldier, entered the army from St Cyr in 1841. From 1847 to 1852 he was employed in the Algerian campaigns, and in 1854 and 1855 in the Crimea. At the assault on the Malakoff (Sept. 8th, 1855) he greatly distinguished himself at the head of a battalion. During the 1859 campaign he won promotion to the rank of lieut.-colonel, and as a colonel he served in the Mexican War. He was made general of brigade in 1866, and led a brigade of the Army of the Rhine in 1870. His troops were amongst those shut up in Metz, and he passed into captivity, but soon escaped. The government of national defence made him general of division and put him at the head of the 20th corps of the Army of the East. He was under Bourbaki during the campaign of the Jura, and when Bourbaki attempted to commit suicide he succeeded to the command (Jan. 23rd, 1871), only to be driven with 84,000 men over the Swiss frontier at Pontarlier. In 1871 Clinchant commanded the 5th corps operating against the Commune. He was military governor of Paris when he died in 1881.
CLINIC; CLINICAL (Gr. κλίνη, a bed), an adjective strictly connoting association with the bedside, and so used in ecclesiology of baptism of the sick or dying, but more particularly in medicine to characterize its aspect as associated with practice on the living patient. Thus clinical experience is opposed to what is learnt from laboratory research or theoretical considerations. The substantive “clinic” is technically employed for a medical school or class where instruction is given in practical work as illustrated by the examination and treatment of actual cases of disease.
CLINKER. (1) (From an old Dutch word klinkaerd, from klinken, to ring), a hard paving brick, a brick with a vitrified surface, or a fused mass of brick; also the incombustible residue of coal, which occurs, half-fused into hard masses, in grates or furnaces; a fused mass of lava. (2) (From clinch, or clench, a common Teutonic word, meaning “to fasten together”), a term appearing usually in the form “clinker-built” as opposed to “cravel-built,” for a boat whose strakes overlap and are not fastened “flush.”
CLINOCLASITE, a rare mineral consisting of the basic copper arsenate (CuOH)3AsO4. It crystallizes in the monoclinic 528 system and possesses a perfect cleavage parallel to the basal plane; this cleavage is obliquely placed with respect to the prism faces of the crystal, hence the name clinoclase or clinoclasite, from Gr. κλίνειν, to incline, and κλᾶν, to break. The crystals are deep blue in colour, and are usually radially arranged in hemispherical groups. Hardness 2½-3; specific gravity 4.36. The mineral was formerly found with other copper arsenates in the mines of the St Day district of Cornwall. It has also been found near Tavistock in Devonshire, near Sayda (or Saida) in Saxony, and in the Tintic district of Utah. It is a mineral of secondary origin, having resulted by the decomposition of copper ores and mispickel in the upper part of mineral veins. The corresponding basic copper phosphate, (CuOH)3PO4, is the mineral pseudomalachite, which occurs as green botryoidal masses resembling malachite in appearance.
CLINTON, DE WITT (1769-1828), American political leader, was born on the 2nd of March 1769 at Little Britain, Orange county, New York. His father, James Clinton (1736-1812), served as a captain of provincial troops in the French and Indian War, and as a brigadier-general in the American army in the War of Independence, taking part in Montgomery’s attack upon Quebec in 1775, unsuccessfully resisting at Fort Montgomery, along the Hudson, in 1777 the advance of Sir Henry Clinton, accompanying General John Sullivan in 1779 in his expedition against the Iroquois in western New York, and in 1781 taking part in the siege of Yorktown, Virginia. De Witt Clinton graduated at Columbia College in 1786, and in 1790 was admitted to the bar. From 1790 to 1795 he was the private secretary of his uncle, George Clinton, governor of New York and a leader of the Republican party. He was a member of the New York assembly from January to April 1798, and in August of that year entered the state senate, serving until April 1802. He at once became a dominant factor in New York politics, and for the next quarter of a century he played a leading rôle in the history of the commonwealth. From 1801 to 1802 and from 1806 to 1807 he was a member of the Council of Appointment, and realizing the power this body possessed through its influence over the selection of a vast number of state, county and municipal officers, he secured in 1801, while his uncle was governor, the removal of a number of Federalist office-holders, in order to strengthen the Republican organization by new appointments. On this account Clinton has generally been regarded as the originator of the “spoils system” in New York; but he was really opposed to the wholesale proscription of opponents that became such a feature of American politics in later years. It was his plan to fill the more important offices with Republicans, as they had been excluded from appointive office during the Federalist ascendancy, and to divide the smaller places between the parties somewhat in accordance with their relative strength.1 In counties where the Federalists had a majority very few removals were made.
In 1802 Clinton became a member of the United States Senate, but resigned in the following year to become mayor of New York city, an office he held from 1803 to 1807, from 1808 to 1810, and from 1811 to 1815. During his mayoralty he also held other offices, being a member of the state senate from 1806 to 1811 and lieutenant-governor from 1811 to 1813. In 1812, after a congressional caucus at Washington had nominated Madison for a second term, the Republicans of New York, desiring to break up the so-called Virginia dynasty as well as the system of congressional nominations, nominated Clinton for the presidency by a legislative caucus. Opponents of a second war with Great Britain had revived the Federalist organization, and Federalists from eleven states met in New York and agreed to support Clinton, not on account of his war views, which were not in accord with their own, but as a protest against the policy of Madison. In the election Clinton received 89 electoral votes and Madison 128.
As a member of the legislature Clinton was active in securing the abolition of slavery and of imprisonment for debt, and in perfecting a system of free public schools. In 1810 he was a member of a commission to explore a route for a canal between Lake Erie and the Hudson river, and in 1811 he and Gouverneur Morris were sent to Washington to secure Federal aid for the undertaking, but were unsuccessful. The second war with Great Britain prevented any immediate action by the state, but in 1816 Clinton was active in reviving the project, and a new commission was appointed, of which he became president. His connexion with this work so enhanced his popularity that he was chosen governor by an overwhelming majority and served for two triennial terms (1817-1823). As governor he devoted his energies to the construction of the canal, but the opposition to his administration, led by Martin Van Buren and Tammany Hall, became so formidable by 1822 that he declined to seek a third term. His successful opponents, however, overreached themselves when in 1824 they removed him from the office of canal commissioner. This partisan action aroused such indignation that at the next election he was again chosen governor, by a large majority, and served from 1825 until his death. As governor he took part in the formal ceremony of admitting the waters of Lake Erie into the canal in October 1825, and thus witnessed the completion of a work which owed more to him than to any other man. Clinton died at Albany, N.Y., on the 11th of February 1828. In addition to his interest in politics and public improvements, he devoted much study to the natural sciences; among his published works are a Memoir on the Antiquities of Western New York (1818), and Letters on the Natural History and Internal Resources of New York (1822).
1 In 1801 a state convention adopted an amendment to the constitution giving the council an equal voice with the governor in the matter of appointments; but Clinton, who is often represented as the father of this movement, though chosen as a member of the convention, did not attend its meetings.
CLINTON, GEORGE (1739-1812), American soldier and political leader, was born at Little Britain, Ulster (now Orange) county, New York, on the 26th of July 1739. His father, Charles Clinton (1690-1773), who was born of English parents in Co. Longford, Ireland, emigrated to America in 1729, and commanded a regiment of provincial troops in the French and Indian War. The son went to sea at the age of sixteen, but, finding the sailor’s life distasteful, joined his father’s regiment and accompanied him as lieutenant in the expedition against Fort Frontenac in 1758. After the war he practised law in his native town and held a number of minor civil offices in Ulster county. From 1768 to 1775 he sat in the New York provincial assembly, and in the controversies with Great Britain zealously championed the colonial cause. In 1774 he was a member of the New York committee of correspondence, and in 1775 was chosen a member of the second Continental Congress. In December of this year he was appointed a brigadier-general of militia by the New York provincial congress, and in the following summer, being ordered by Washington to assist in the defence of New York, he left Philadelphia shortly after voting for the Declaration of Independence, but too soon to attach his signature to that document. He had also been chosen a deputy to the provincial congress (later the state convention) for 1776-1777, but his various other duties prevented his attendance.
General Clinton took part in the battle of White Plains (October 28th, 1776), and later was charged with the defence of the Highlands of the Hudson, where, with De Witt Clinton, in October 1777, he offered a firm but unsuccessful resistance to the advance of Sir Henry Clinton. In March of this year he had been appointed by Congress a brigadier general in the Continental army, and he thus held two commissions, as the state convention refused to accept his resignation as brigadier-general of militia. So great was Clinton’s popularity at this time that at the first election under the new state constitution he was chosen both governor and lieutenant-governor; he declined the latter office, and on the 30th of July 1777 entered upon his duties as governor, which were at first largely of a military nature. In 1780 he took the field and checked the advance of Sir John Johnson and the 529 Indians in the Mohawk Valley. In his administration Clinton was energetic and patriotic, and though not possessing the intellectual attainments of some of his New York contemporaries, he was more popular than any of them, as is attested by his service as governor for eighteen successive years (1777-1795), and for another triennial term from 1801 to 1804. In the elections of 1780, 1783 and 1786 he had no opponent. In 1800-1801 he was a member of the assembly. In the struggle in New York over the adoption of the Federal Constitution he was one of the leaders of the opposition, but in the state convention of 1788, over which he presided, his party was defeated, and the constitution was ratified. In national politics he was a follower of Thomas Jefferson, and in state politics he led the faction known as “Clintonians,” which was for a long time dominant. In 1789, 1792 and 1796 Clinton received a number of votes in the electoral college, but not a sufficient number to secure him the vice-presidency, which was then awarded to the recipient of the second highest number of votes. In 1804, however, after the method of voting had been changed, he was nominated for the vice-presidency by a Congressional caucaus, and was duly elected. In 1808 he sought nomination for the presidency, and was greatly disappointed when this went to Madison. He was again chosen as vice-president, however, and died at Washington before the expiration of his term, on the 20th of April 1812. He was buried in the Congressional Cemetery, from which in May 1908 his remains were transferred to Kingston, N.Y. His casting vote in the Senate in 1811 defeated the bill for the renewal of the charter of the Bank of the United States.
The Public Papers of George Clinton (6 vols., New York, 1899-1902) have been published by the state of New York.
CLINTON, SIR HENRY (c. 1738-1795), British general, was the son of admiral George Clinton (governor of Newfoundland and subsequently of New York), and grandson of the 6th earl of Lincoln. After serving in the New York militia, he came to England and joined the Coldstream Guards. In 1758 he became captain and lieutenant-colonel in the Grenadier Guards, and in 1760-62 distinguished himself very greatly as an aide-de-camp to Ferdinand of Brunswick in the Seven Years’ War. He was promoted colonel in 1762, and after the peace received the colonelcy of a regiment of foot, becoming major-general in 1772. From 1772 to 1784, thanks to the influence of his cousin, the 2nd duke of Newcastle, he had a seat in parliament, first for Boroughbridge and subsequently for Newark, but for the greater part of this time he was on active service in America in the War of Independence. He took part in the battles of Bunker Hill and Long Island, subsequently taking possession of New York. For his share in the battle of Long Island he was made a lieutenant-general and K.B. After Saratoga he succeeded Sir William Howe as commander-in-chief in North America. He had already been made a local general. He at once concentrated the British forces at New York, pursuing a policy of foraying expeditions in place of regular campaigns. In 1779 he invaded South Carolina, and in 1780 in conjunction with Admiral M. Arbuthnot won an important success in the capture of Charleston. Friction, however, was constant between him and Lord Cornwallis, his second in command, and in 1782, after the capitulation of Cornwallis at York town, he was superseded by Sir Guy Carleton. Returning to England, he published in 1783 his Narrative of the Campaign of 1781 in North America, which provoked an acrimonious reply from Lord Cornwallis. He was elected M.P. for Launceston in 1790, and in 1794 was made governor of Gibraltar, where he died on the 23rd of December 1795.
His elder son, Sir William Henry Clinton (1769-1846), entered the British army in 1784, and served in the campaigns of 1793-94 in the Low Countries. In 1796 he became aide-de-camp to the duke of York, and in 1799 he was entrusted with a mission to the Russian army in Italy, returning to the duke in time for the Dutch expedition of 1799. He was promoted colonel in 1801, and took part in the expedition which took possession of Madeira, which he governed up to 1802. His next important service was in 1807, when he went to Sweden on a military mission. Promoted major-general in 1808, he served from 1812 to 1814 in the Mediterranean and in Catalonia, and in the latter year he commanded against Marshal Suchet. He had become a lieutenant-general in 1813, and in 1815 he was made a G.C.B. He commanded the British troops in Portugal, 1826-28, and was promoted full general in 1830. He died at Cockenhatch, near Royston, Herts, on the 15th of February 1846.
The younger son, Sir Henry Clinton (1771-1829), entered the army in 1787 and saw some service with the Prussians in Holland in 1789. He served on the staff of the duke of York in 1793-94, becoming brevet-major in 1794, and lieutenant-colonel of a line regiment in 1796. In 1797-98 he was aide-de-camp to Lord Cornwallis in the Irish rebellion, and in 1799 he was sent with Lord William Bentinck to the Russian headquarters in Italy, being present at the Trebbia, at Novi, and in the fighting about the St Gotthard. During a short period of service in India Clinton distinguished himself at Laswari. He accompanied the Russian headquarters in the Austerlitz campaign, and was adjutant-general to his intimate friend, Sir John Moore, in the Corunna campaign of 1808-9. Promoted major-general in 1810, he returned to the Peninsula to fill a divisional command under Wellington in 1811. His division played a notable part in the capture of the forts at Salamanca and in the battle of Salamanca (1812), and he was given the local rank of lieutenant-general early in 1813. For his conduct at Vitoria he was made a K.B., and he took his part in the subsequent victories of the Nive, Orthes and Toulouse. At the end of the war he was made a lieutenant-general and inspector-general of infantry. Clinton commanded a division with distinction at Waterloo. He died on the 11th of December 1829.
CLINTON, HENRY FYNES (1781-1852), British classical scholar and chronologist, was born at Gamston in Nottinghamshire on the 14th of January 1781. He was descended from Henry, second earl of Lincoln; for some generations his family bore the name of Fynes, but his father resumed the older family name of Clinton in 1821. He was educated at Westminster school and Christ Church, Oxford, where he studied classical literature and history. From 1806 to 1826 he was M.P. for Aldborough. He died at Welwyn, Herts, where he had purchased the residence and estate of the poet Young, on the 24th of October 1852. His reading was extraordinarily methodical (see his Literary Remains). The value of his Fasti, which set classical chronology on a scientific basis, can scarcely be overestimated, even though subsequent research has corrected some of his conclusions.
His chief works are: Fasti Hellenici, the Civil and Literary Chronology of Greece from the 55th to the 124th Olympiad (1824-1851), including dissertations on points of Greek history and Scriptural chronology; and Fasti Romani, the Civil and Literary Chronology of Rome and Constantinople from the Death of Augustus to the Death of Heraclius (1845-1850). In 1851 and 1853 respectively he published epitomes of the above. The Literary Remains of H. F. Clinton (the first part of which contains an autobiography written in 1818) were edited by C. J. F. Clinton in 1854.
CLINTON, a city and the county-seat of Clinton county, Iowa, U.S.A., on the Mississippi river, in the extreme eastern part of the state. Pop. (1890) 13,619; (1900) 22,698 (5434 being foreign-born); (1905) 22,756; (1910) 25,577. The great increase during the decade 1890-1900 was partly due to the absorption by Clinton in 1895 of the city of Lyons (pop. in 1890, 5700). Clinton is served by the Chicago & North-Western (which has machine-shops here), the Chicago, Burlington & Quincy, the Chicago, Milwaukee & St Paul, and the Chicago, Rock Island & Pacific railways, and is connected with Davenport by an electric line. The river is spanned here by a railway bridge. A large portion of the city stands between the river and a series of bluffs. Clinton is the seat of Wartburg College (1869), a German Evangelical Lutheran institution, and of the Clinton Business College. Among the public buildings are the city hall, the court-house, the Federal building and the Carnegie library. As a manufacturing centre Clinton has considerable importance; among its manufactures are furniture, blinds, wire-cloth, papier-mâché goods, gas-engines, farm wagons, harness and saddlery, door locks, pressed brick, flour, and glucose products. There is also 530 a large sugar refinery. The value of the factory product in 1900 was $6,203,316; in 1905, $4,906,355. The American Protective Association (A.P.A.), a secret order opposed to Roman Catholicism, was formed here in 1887. The city was founded in 1855 by the Iowa Land Company, and was incorporated first in 1857, and again in 1867, this time under a general law of the state for the incorporation of cities. The county, from which the city took its name, was named in honour of De Witt Clinton.
CLINTON, a township of Worcester county, Massachusetts, U.S.A., in the central part of the state, on the Nashua river, about 15 m. N.N.E. of Worcester. Pop. (1890) 10,424; (1900) 13,667, of whom 5504 were foreign-born; (1910, U.S. census) 13,075. The township is traversed by the Boston & Maine, and New York, New Haven & Hartford railways. It contains 7 sq. m. of varied and picturesque hilly country on the E. slope of the highland water-parting between the Connecticut river and the Atlantic. There is charming scenery along the Nashua river, the chief stream. The S.W. corner of the township is now part of an immense water reservoir, the Wachusett dam and reservoir (excavated 1896-1905; circumference, 35.2 m.), on the S. branch of the Nashua, which will hold 63,000 million gallons of water for the supply of the metropolitan region around Boston. On this is situated the village of Clinton, which has large manufactories, among whose products are cotton and woollen fabrics, carpets, wire-cloth, iron and steel, and combs. The textile and carpet mills are among the most famous in the United States. In 1905 the total factory product of the township was valued at $5,457,865, the value of cotton goods, carpets and wire-work constituting about nine-tenths of the total. The prominence of the township as a manufacturing centre is due to Erastus Brigham Bigelow (1814-1879), one of the incorporators of the Massachusetts Institute of Technology, who devised power-looms for the weaving of a variety of figured fabrics,—coach-lace, counterpanes, ginghams, silk brocatel, tapestry carpeting, ingrain and Brussels carpets,—and revolutionized their manufacture. In 1843 he and his brother Horatio N. Bigelow established in Clinton the Lancaster Mills for the manufacture of ginghams. From 1845 to 1851 he perfected his loom for the weaving of Brussels and Wilton carpets, the greatest of his inventions; and he established the Bigelow Carpet Mills here. He also invented the loom for the weaving of wire-cloth. It is claimed that the first production in the United States of finished cotton cloths under one roof and under the factory system was not at Waltham in 1816, but at Clinton in 1813; neither place was the first to spin by power, nor the first to produce finished cloths without the factory system. The comb industry dates from the eighteenth century. The first of the modern textile mills were established in 1838 for the manufacture of coach-lace. Clinton was a part of Lancaster, now a small farming township (pop. in 1910, 2464), until 1850, when it was set off as an independent township. The earliest settlement goes back to 1645.
See A. E. Ford, History of the Origin of the Town of Clinton, Massachusetts, 1653-1865 (Clinton, 1896).
CLINTON, a city and the county-seat of Henry county, Missouri, U.S.A., on the Grand river, 87 m. S.E. of Kansas City. Pop. (1890) 4737; (1900) 5061 (470 being negroes); (1910) 4992. It is served by the St Louis & San Francisco, the Missouri, Kansas & Texas, and the Kansas City, Clinton & Springfield railways. The city is situated on the border of a rolling prairie about 770 ft. above the sea. The vicinity abounds in coal, but is principally agricultural, and Clinton’s chief interest is in trade with it. The principal manufactures are flour and pottery. Clinton was laid out in 1836 and was incorporated in 1865.
CLINTON, a village of Oneida county, New York, U.S.A., on the Oriskany Creek, about 9 m. S.W. of Utica. Pop. (1890) 1269; (1900) 1340; (1905) 1315; (1910) 1236. It is served by the New York, Ontario & Western railway, and is connected with Utica by an electric line. Several fine mineral springs in the vicinity have given Clinton some reputation as a health resort. There are iron mines, blast furnaces, and iron smelters. Clinton is the seat of Hamilton College (non-sectarian), which was opened as the Hamilton Oneida Academy in 1798, and was chartered under its present name in 1812. It was founded by the Rev. Samuel Kirkland (1741-1808), a missionary among the Oneida Indians; its corner-stone was laid by Baron Steuben; its shade trees were furnished by Thomas Jefferson; and its name was received from Alexander Hamilton, one of its early trustees. It had in 1907-1908 20 instructors, 178 students, and a library of 47,000 volumes and 30,000 pamphlets. At Clinton are also excellent minor schools. Litchfield Observatory is connected with the college, and was long in charge of the well-known astronomer, Christian H. F. Peters (1813-1890), who discovered here more than 40 asteroids and made extensive investigations concerning comets. The village was settled about 1786 by pioneers from New England, was named in honour of George Clinton, and was incorporated in 1842.
CLINTONITE, a group of micaceous minerals known as the “brittle micas.” Like the micas and chlorites, they are monoclinic in crystallization and have a perfect cleavage parallel to the flat surface of the plates or scales, but differ markedly from these in the brittleness of the laminae; they are also considerably harder, the hardness of chloritoid being as high as 6½ on Mohs’ scale. They differ chemically from the micas in containing less silica and no alkalis, and from the chlorites in containing much less water; in many respects they are intermediate between the micas and chlorites.
The following species are distinguished:—
Margarite is a basic calcium aluminium silicate, H2CaAl4Si2O12, and is classed by some authors as a lime-mica. It forms white pearly scales, and was at first known as pearl-mica and afterwards as margarite, from μαργαρίτης, a pearl. It is a characteristic associate of corundum, of which it is frequently an alteration product (facts which suggested the synonymous names corundellite and emerylite), and is found in the emery deposits of Asia Minor and the Grecian Archipelago, and with corundum at several localities in the United States.
Seybertite, Brandisite and Xanthophyllite are closely allied species consisting of basic magnesium, calcium and aluminium silicate, and have been regarded as isomorphous mixtures of a silicate (H2CaMg4Si3O12) and an aluminate (H2CaMgAl6O12). Seybertite (the original clintonite) occurs as reddish-brown to copper-red, brittle, foliated masses in metamorphic limestone at Amity, New York; brandisite as yellowish-green hexagonal prisms in metamorphic limestone in the Fassathal, Tirol; xanthophyllite as yellow folia and as distinct crystals (waluewite) in chloritic schists in the Urals.
Chloritoid has the formula H2(Fe,Mg)Al2SiO7. It forms tabular crystals and scales, with indistinct hexagonal outlines, which are often curved or bent and aggregated in rosettes. The colour is dark grey or green; a characteristic feature is the pleochroism, the pleochroic colours varying from yellowish-green to indigo-blue. Hardness, 6½; specific gravity, 3.4-3.6. It occurs as isolated scales scattered through schistose rocks and phyllites of dynamo-metamorphic origin. The ottrelites of the phyllites and ottrelite-schists of Ottrez and other localities in the Belgian Ardennes is a manganiferous variety of chloritoid, but owing to enclosed impurities the analyses differ widely from those of typical chloritoid.
CLISSON, OLIVIER DE (1336-1407), French soldier, was the son of the Olivier de Clisson who was put to death in 1343 on the suspicion of having wished to give up Nantes to the English. He was brought up in England, where his mother, Jeanne de Belleville, had married her second husband. On his return to Brittany he took arms on the side of de Montfort, distinguishing himself at the battle of Auray (1364), but in consequence of differences with Duke John IV. went over to the side of Blois. In 1370 he joined Bertrand du Guesclin, who had lately become constable of France, and followed him in all his campaigns against the English. On the death of du Guesclin Clisson received the constable’s sword (1380). He fought with the citizens of Ghent, defeating them at Roosebek (1382), later on commanded the army in Poitou and Flanders (1389), and made an unsuccessful attempt to invade England. On his return to Paris, in 1392, 531 an attempt was made to assassinate him by Pierre de Craon, at the instigation of John IV. of Brittany. In order to punish the latter, Charles VI., accompanied by the constable, marched on Brittany, but it was on this expedition that the king was seized with madness. The uncles of Charles VI. took proceedings against Clisson, so that he had to take refuge in Brittany. He was reconciled with John IV., and after the duke’s death, in 1399, he became protector of the duchy, and guardian of the young princes. He had gathered vast wealth before his death on the 23rd of April 1407.
CLISSON, a town of western France, in the department of Loire-Inférieure, prettily situated at the confluence of the Sèvre Nantaise and the Moine 17 m. S.E. of Nantes by rail. Pop. (1906) 2244. The town gave its name to the celebrated family of Clisson, of which the most famous member was Olivier de Clisson. It has the imposing ruins of their stronghold, parts of which date from the 13th century. The town and castle were destroyed in 1792 and 1793 during the Vendean wars. The sculptor F. F. Lemont afterwards bought the castle, and the town was rebuilt in the early part of the 19th century according to his plans. There are picturesque parks on the banks of the rivers. The Moine is crossed by an old Gothic bridge and by a fine modern viaduct.
CLITHEROE, a market town and municipal borough in the Clitheroe parliamentary division of Lancashire, England, 220 m. N.N.W. from London and 35 m. N. by W. from Manchester, on the Lancashire & Yorkshire railway. Pop. (1901) 11,414. It is finely situated in the valley of the Ribble, at the foot of Pendle Hill, a steep plateau-like mass rising to 1831 ft. The church of St Mary Magdalene, though occupying an ancient site, is wholly modernized. There are a grammar school, founded in 1554, and a technical school. On a rocky elevation commanding the valley stands the keep and other fragments of a Norman castle, but part of the site is occupied by a modern mansion. The industrial establishments comprise cotton-mills, print-works, paper-mills, foundries, and brick and lime works. The corporation consists of a mayor, 4 aldermen and 12 councillors. Area, 2385 acres.
Stonyhurst College, 5 m. S.W. of Clitheroe, is the principal establishment in England for Roman Catholic students. The Jesuits of St Omer, after emigrating to Bruges and Liége, were disorganized by the revolutionary troubles at the close of the 18th century, and a large body came to England, when Thomas Weld, in 1795, conferred his property of Stonyhurst upon them. The fine and extensive buildings, of which the nucleus is a mansion of the 17th century, contain a public school for boys and a house of studies for Jesuit ecclesiastics, while there is a preparatory school at a short distance. Every branch of study is prosecuted, the college including such institutions as an observatory, laboratories and farm buildings.
The Honour of Clitheroe, the name of which is also written Clyderhow and Cletherwoode, was first held by Roger de Poictou, who was almost certainly the builder of the castle, which was dismantled in 1649. He granted it to Robert de Lacy, in whose family it remained with two short intervals until it passed by marriage to Thomas, earl of Lancaster, in 1310. It formed part of the duchy of Lancaster till Charles II. at the Restoration bestowed it on General Monk, from whose family it descended through the house of Montague to that of Buccleuch. The Clitheroe Estate Company are the present lords of the Honour. The first charter was granted about 1283 to the burgesses by Henry de Lacy, second earl of Lincoln, confirming the liberties granted by the first Henry de Lacy, who is therefore sometimes said, although probably erroneously, to have granted a charter about 1147. The 1283 charter was confirmed by Edward III. in 1346, Henry V. in 1413-1414, Henry VIII. in 1542, and James I. in 1604. Of the fairs, those on December 7th to 9th and March 24th to 26th are held under a charter of Henry IV. in 1409. A weekly market has been held on Saturday since the Conqueror’s days. In 1558 the borough was granted two members of parliament, and continued to return them till 1832, when the number was reduced to one. Under the Redistribution Act of 1885 the borough was disfranchised. The municipal government was formerly vested in an in-bailiff and an out-bailiff elected annually from the in and out burgesses. A court-leet and court-baron used to be held half-yearly, but both are now obsolete. The present corporation governs under the Municipal Corporation Act (1837). There was a church or chapel here in early times, and a chaplain is mentioned in Henry II.’s reign.
CLITOMACHUS, Greek philosopher, was a Carthaginian originally named Hasdrubal, who came to Athens about the middle of the 2nd century B.C. at the age of twenty-four. He made himself well acquainted with Stoic and Peripatetic philosophy; but he studied principally under Carneades, whose views he adopted, and whom he succeeded as chief of the New Academy in 129 B.C. He made it his business to spread the knowledge of the doctrines of Carneades, who left nothing in writing himself. Clitomachus’ works were some four hundred in number; but we possess scarcely anything but a few titles, among which are De sustinendis assensionibus (Περὶ ἐποχῆς, “on suspension of judgment”) and Περὶ αἱρέσεων (an account of various philosophical sects). In 146 he wrote a treatise to console his countrymen after the ruin of their city, in which he insisted that a wise man ought not to feel grieved at the destruction of his country. Cicero highly commends his works and admits his own debt in the Academics to the treatise Περὶ ἐποχῆς. Parts of Cicero’s De Natura and De Divinatione, and the treatise De Fato are also in the main based upon Clitomachus.
See E. Wellmann in Ersch and Gruber’s Allgemeine Encyclopädie; R. Hirzel, Untersuchungen zu Ciceros philosophischen Schriften, i. (1877); Diog. Laërt. iv. 67-92; Cicero, Acad. Pr. ii. 31, 32, and Tusc.. iii. 22; and article Academy, Greek.
CLITUMNUS, a river in Umbria, Italy, which rises from a very abundant spring by the road between the ancient Spoletium and Trebia, 8 m. from the former, 4 m. from the latter, and after a short course through the territory of the latter town joins the Tinia, a tributary of the Tiber. The spring is well described by Pliny (Epist. viii. 8): it was visited by Caligula and by Honorius, and is still picturesque—a clear pool surrounded by poplars and weeping willows. The stream was personified as a god, whose ancient temple lay near the spring, and close by other smaller shrines; the place, therefore, occurs under the name Sacraria (the shrines) as a Roman post station. The building generally known as the Tempio di Clitunno, close to the spring, is, however, an ancient tomb, converted into a Christian church in the early middle ages, the decorative sculptures, which are obviously contemporary with those of S. Salvatore at Spoleto, belonging to the 4th or 6th century according to some authorities, to the 12th according to others.
See H. Grisar, Nuovo bullettino di archeologia cristiana (Rome, 1895) i. 127; A. Venturi, Storia dell’ arte italiana (Milan, 1904), iii. 903.
CLIVE, CAROLINE (1801-1873), English authoress, was born in London on the 24th of June 1801, the daughter of Mr Meysey-Wigley, M.P. for Worcester. She married, in 1840, the Rev. Archer Clive. She published, over the signature “V.,” eight volumes of poetry, but is best known as the author of Paul Ferroll (1855), a sensational novel, and Why Paul Ferroll killed his Wife (1860). She died on the 13th of July 1873, at Whitfield, Herefordshire.
CLIVE, CATHERINE [Kitty] (1711-1785), British actress, was born, probably in London, in 1711. Her father, William Raftor, an Irishman of good family but small means, had held a captain’s commission in the French army under Louis XIV. From her earliest years she showed a talent for the stage, and about 1728 became a member of the company at Drury Lane, of which Colley Cibber was then manager. Her first part was that of the page Ismenes (“with a song”) in the tragedy Mithridates. Shortly afterwards she married George Clive, a barrister and a relative of the 1st Lord Clive, but husband and wife soon separated by mutual consent. In 1731 she definitely established her reputation as a comic actress and singer in Charles Coffey’s farce-opera adaptation, The Devil to Pay, and from this time she was always a popular favourite. She acted little outside Drury Lane, where in 1747 she became one of the original 532 members of Garrick’s company. She took part, however, in some of the oratorios of Handel, whose friend she was. In 1769, having been a member of Garrick’s company for twenty-two years, she quitted the stage, and lived for sixteen years in retirement at a villa at Twickenham, which had been given her some time previously by her friend Horace Walpole. Mrs Clive had small claim to good looks, but as an actress of broad comedy she was unreservedly praised by Goldsmith, Johnson and Garrick. She had a quick temper, which on various occasions involved her in quarrels, and at times sorely tried the patience of Garrick, but her private life remained above suspicion, and she regularly supported her father and his family. She died at Twickenham on the 6th of December 1785. Horace Walpole placed in his garden an urn to her memory, bearing an inscription, of which the last two lines run:
|
“The comic muse with her retired And shed a tear when she expired.” |
See Percy Fitzgerald, Life of Mrs Catherine Clive (1888); W. R. Chetwood, General History of the Stage (1749); Thomas Davies, Memoirs of the Life of David Garrick (1784).
CLIVE, ROBERT CLIVE, Baron (1725-1774), the statesman and general who founded the empire of British India, was born on the 29th of September 1725 at Styche, the family estate, in the parish of Moreton Say, Market Drayton, Shropshire. We learn from himself, in his second speech in the House of Commons in 1773, that as the estate yielded only £500 a year, his father followed the profession of the law also. The Clives, or Clyves, were one of the oldest families in the county of Shropshire, having held the manor of that name in the reign of Henry II. One Clive was Irish chancellor of the exchequer under Henry VIII.; another was a member of the Long Parliament; Robert’s father for many years represented Montgomeryshire in parliament. His mother, to whom he was tenderly attached, and who had a powerful influence on his career, was a daughter, and with her sister Lady Sempill co-heir, of Nathaniel Gaskell of Manchester. Robert was their eldest son. With his five sisters, all of whom were married in due time, he ever maintained the most affectionate relations. His only brother survived to 1825.
Young Clive was the despair of his teachers. Sent from school to school, and for only a short time at the Merchant Taylors’ school, which then as now had a high reputation, he neglected his books for perilous adventures. But he was not so ignorant as his biographers represent. He could read Horace in after life; and he must have laid in his youth the foundation of that clear and vigorous English style which marked all his despatches, and made Lord Chatham declare of one of his speeches in the House of Commons that it was the most eloquent he had ever heard. From his earliest years, however, his ambition was to lead his fellows; but he never sacrificed honour, as the word was then understood, even to the fear of death. At eighteen he was sent out to Madras as a “factor” or “writer” in the civil service of the East India Company. The detention of the ship in Brazil for nine months enabled him to acquire the Portuguese language, which, at a time when few or none of the Company’s servants learned the vernaculars of India, he often found of use. For the first two years of his residence he was miserable. He felt keenly the separation from home; he was always breaking through the restraints imposed on young “writers”; and he was rarely out of trouble with his fellows, with one of whom he fought a duel. Thus early, too, the effect of the climate on his health began to show itself in those fits of depression during one of which he afterwards prematurely ended his life. The story is told of him by his companions, though he himself never spoke of it, that he twice snapped a pistol at his head in vain. His one solace was found in the governor’s library, where he sought to make up for past carelessness by a systematic course of study. He was just of age, when in 1746 Madras was forced to capitulate to Labourdonnais during the War of the Austrian Succession. The breach of that capitulation by Dupleix, then at the head of the French settlements in India, led Clive, with others, to escape from the town to the subordinate Fort St David, some 20 m. to the south. There, disgusted with the state of affairs and the purely commercial duties of an East Indian civilian, as they then were, Clive obtained an ensign’s commission.
At this time India was ready to become the prize of the first conqueror who to the dash of the soldier added the skill of the administrator. For the forty years since the death of the emperor Aurangzeb, the power of the Great Mogul had gradually fallen into the hands of his provincial viceroys or subadhars. The three greatest of these were the nawab of the Deccan, or south and central India, who ruled from Hyderabad, the nawab of Bengal, whose capital was Murshidabad, and the nawab or wazir of Oudh. The prize lay between Dupleix, who had the genius of an administrator, or rather intriguer, but was no soldier, and Clive, the first of a century’s brilliant succession of those “soldier-politicals,” as they are called in the East, to whom Great Britain owes the conquest and consolidation of its greatest dependency. Clive successively established British ascendancy against French influence in the three great provinces under these nawabs. But his merit lies especially in the ability and foresight with which he secured for his country, and for the good of the natives, the richest of the three, Bengal. First, as to Madras and the Deccan, Clive had hardly been able to commend himself to Major Stringer Lawrence, the commander of the British troops, by his courage and skill in several small engagements, when the peace of Aix-la-Chapelle (1748) forced him to return to his civil duties for a short time. An attack of the malady which so severely affected his spirits led him to visit Bengal, where he was soon to distinguish himself. On his return he found a contest going on between two sets of rival claimants for the position of viceroy of the Deccan, and for that of nawab of the Carnatic, the greatest of the subordinate states under the Deccan. Dupleix, who took the part of the pretenders to power in both places, was carrying all before him. The British had been weakened by the withdrawal of a large force under Admiral Boscawen, and by the return home, on leave, of Major Lawrence. But that officer had appointed Clive commissary for the supply of the troops with provisions, with the rank of captain. More than one disaster had taken place on a small scale, when Clive drew up a plan for dividing the enemy’s forces, and offered to carry it out himself. The pretender, Chanda Sahib, had been made nawab of the Carnatic with Dupleix’s assistance, while the British had taken up the cause of the more legitimate successor, Mahommed Ali. Chanda Sahib had left Arcot, the capital of the Carnatic, to reduce Trichinopoly, then held by a weak English battalion. Clive offered to attack Arcot in order to force Chanda Sahib to raise the siege of Trichinopoly. But Madras and Fort St David could supply him with only 200 Europeans and 300 sepoys. Of the eight officers who led them, four were civilians like Clive himself, and six had never been in action. His force had but three field-pieces. The circumstances that Clive, at the head of this handful, had been seen marching during a storm of thunder and lightning, frightened the enemy into evacuating the fort, which the British at once began to strengthen against a siege. Clive treated the great population of the city with so much consideration that they helped him, not only to fortify his position, but to make successful sallies against the enemy. As the days passed on, Chanda Sahib sent a large army under his son and his French supporters, who entered Arcot and closely besieged Clive in the citadel.
Macaulay gives the following brilliant account of the siege:—
“Raja Sahib proceeded to invest the fort, which seemed quite incapable of sustaining a siege. The walls were ruinous, the ditches dry, the ramparts too narrow to admit the guns, and the battlements too low to protect the soldiers. The little garrison had been greatly reduced by casualties. It now consisted of 120 Europeans and 200 sepoys. Only four officers were left, the stock of provisions was scanty, and the commander who had to conduct the defence under circumstances so discouraging was a young man of five and twenty, who had been bred as a book-keeper. During fifty days the siege went on, and the young captain maintained the defence with a firmness, vigilance and ability which would have done honour to the oldest marshal in Europe. The breach, however, increased day by day. Under such circumstances, any troops so scantily provided with officers might have been expected to show signs of insubordination; and the danger was peculiarly’ great in a force composed of men differing widely from each other in extraction, colour, language, 533 manners and religion. But the devotion of the little band to its chief surpassed anything that is related of the Tenth Legion of Caesar, or the Old Guard of Napoleon. The sepoys came to Clive, not to complain of their scanty fare, but to propose that all the grain should be given to the Europeans, who required more nourishment than the natives of Asia. The thin gruel, they said, which was strained away from the rice would suffice for themselves. History contains no more touching instance of military fidelity, or of the influence of a commanding mind. An attempt made by the governor of Madras to relieve the place had failed; but there was hope from another quarter. A body of 3000 Mahrattas, half soldiers, half robbers, under the command of a chief named Murari Rao had been hired to assist Mahommed Ali; but thinking the French power irresistible, and the triumph of Chanda Sahib certain, they had hitherto remained inactive on the frontiers of the Carnatic. The fame of the defence of Arcot roused them from their torpor; Murari Rao declared that he had never before believed that Englishmen could fight, but that he would willingly help them since he saw that they had spirit to help themselves. Raja Sahib learned that the Mahrattas were in motion, and it was necessary for him to be expeditious. He first tried negotiations—he offered large bribes to Clive, which were rejected with scorn; he vowed that if his proposals were not accepted, he would instantly storm the fort, and put every man in it to the sword. Clive told him, in reply, with characteristic haughtiness, that his father was a usurper, that his army was a rabble, and that he would do well to think twice before he sent such poltroons into a breach defended by English soldiers. Raja Sahib determined to storm the fort. The day was well suited to a bold military enterprise. It was the great Mahommedan festival, the Muharram, which is sacred to the memory of Husain, the son of Ali. Clive had received secret intelligence of the design, had made his arrangements, and, exhausted by fatigue, had thrown himself on his bed. He was awakened by the alarm, and was instantly at his post. The enemy advanced, driving before them elephants whose foreheads were armed with iron plates. It was expected that the gates would yield to the shock of these living battering-rams. But the huge beasts no sooner felt the English musket balls than they turned round and rushed furiously away, trampling on the multitude which had urged them forward. A raft was launched on the water which filled one part of the ditch. Clive perceiving that his gunners at that post did not understand their business, took the management of a piece of artillery himself, and cleared the raft in a few minutes. Where the moat was dry, the assailants mounted with great boldness; but they were received with a fire so heavy and so well directed, that it soon quelled the courage even of fanaticism and of intoxication. The rear ranks of the English kept the front ranks supplied with a constant succession of loaded muskets, and every shot told on the living mass below. The struggle lasted about an hour; 400 of the assailants fell; the garrison lost only five or six men. The besieged passed an anxious night, looking for a renewal of the attack. But when day broke, the enemy were no more to be seen. They had retired, leaving to the English several guns and a large quantity of ammunition.”
In India, we might say in all history, there is no parallel to this exploit of 1751 till we come to the siege of Lucknow in 1857. Clive, now reinforced, followed up his advantage, and Major Lawrence returned in time to carry the war to a successful issue. In 1754 the first of the Carnatic treaties was made provisionally, between T. Saunders, the Company’s resident at Madras, and M. Godeheu, the French commander, in which the English protégé, Mahommed Ali, was virtually recognized as nawab, and both nations agreed to equalize their possessions. When war again broke out in 1756, and the French, during Clive’s absence in Bengal, obtained successes in the northern districts, his efforts helped to drive them from their settlements. The Treaty of Paris in 1763 formally confirmed Mahommed Ali in the position which Clive had won for him. Two years after, the Madras work of Clive was completed by a firman from the emperor of Delhi, recognizing the British possessions in southern India.
The siege of Arcot at once gave Clive a European reputation. Pitt pronounced the youth of twenty-seven who had done such deeds a “heaven-born general,” thus endorsing the generous appreciation of his early commander, Major Lawrence. When the court of directors voted him a sword worth £700, he refused to receive it unless Lawrence was similarly honoured. He left Madras for home, after ten years’ absence, early in 1753, but not before marrying Miss Margaret Maskelyne, the sister of a friend, and of one who was afterwards well known as astronomer royal. All his correspondence proves him to have been a good husband and father, at a time when society was far from pure, and scandal made havoc of the highest reputations. In after days, when Clive’s uprightness and stern reform of the Company’s civil and military services made him many enemies, a biography of him appeared under the assumed name of Charles Carracioli, Gent. All the evidence is against the probability of its scandalous stories being true. Clive as a young man occasionally indulged in loose or free talk among intimate friends, but beyond this nothing has been proved to his detriment. After he had been two years at home the state of affairs in India made the directors anxious for his return. He was sent out, in 1756, as governor of Fort St David, with the reversion of the government of Madras, and he received the commission of lieutenant-colonel in the king’s army. He took Bombay on his way, and there commanded the land force which captured Gheria, the stronghold of the Mahratta pirate, Angria. In the distribution of prize money which followed this expedition he showed no little self-denial. He took his seat as governor of Fort St David on the day on which the nawab of Bengal captured Calcutta, and thither the Madras government at once sent him, with admiral Watson. He entered on the second period of his career.
Since, in August 1690, Job Charnock had landed at the village of Sutanati with a guard of one officer and 30 men, the infant capital of Calcutta had become a rich centre of trade. The successive nawabs or viceroys of Bengal had been friendly to it, till, in 1756, Suraj-ud-Dowlah succeeded his uncle at Murshidabad. His predecessor’s financial minister had fled to Calcutta to escape the extortion of the new nawab, and the English governor refused to deliver up the refugee. Enraged at this, Suraj-ud-Dowlah captured the old fort of Calcutta on the 20th of June, and plundered it of more than two millions sterling. Many of the English fled to ships and dropped down the river. The 146 who remained were forced into “the Black Hole” in the stifling heat of the sultriest period of the year. Only 23 came out alive. The fleet was as strong, for those days, as the land force was weak. Disembarking his troops some miles below the city, Clive marched through the jungles, where he lost his way owing to the treachery of his guides, but soon invested Fort William, while the fire of the ships reduced it, on the 2nd of January 1757. On the 4th of February he defeated the whole army of the nawab, which had taken up a strong position just beyond what is now the most northerly suburb of Calcutta. The nawab hastened to conclude a treaty, under which favourable terms were conceded to the Company’s trade, the factories and plundered property were restored, and an English mint was established. In the accompanying agreement, offensive and defensive, Clive appears under the name by which he was always known to the natives of India, Sabut Jung, or “the daring in war.” The hero of Arcot had, at Angria’s stronghold, and now again under the walls of Calcutta, established his reputation as the first captain of the time. With 600 British soldiers, 800 sepoys, 7 field-pieces and 500 sailors to draw them, he had routed a force of 34,000 men with 40 pieces of heavy cannon, 50 elephants, and a camp that extended upwards of four miles in length. His own account, in a letter to the archbishop of Canterbury, gives a modest but vivid description of the battle, the importance of which has been overshadowed by Plassey. In spite of his double defeat and the treaty which followed it, the madness of the nawab burst forth again. As England and France were once more at war, Clive sent the fleet up the river against Chandernagore, while he besieged it by land. After consenting to the siege, the nawab sought to assist the French, but in vain. The capture of their principal settlement in India, next to Pondicherry, which had fallen in the previous war, gave the combined forces prize to the value of £130,000. The rule of Suraj-ud-Dowlah became as intolerable to his own people as to the British. They formed a confederacy to depose him, at the head of which was Jafar Ali Khan, his commander-in-chief. Associating with himself Admiral Watson, Governor Drake and Mr Watts, Clive made a treaty in which it was agreed to give the office of viceroy of Bengal, Behar and Orissa to Jafar, who was to pay a million sterling to the Company for its losses in Calcutta and the cost of its troops, half a million to the British inhabitants of Calcutta, £200,000 to the native inhabitants, and £70,000 to its Armenian merchants. Up to this point all is clear. Suraj-ud-Dowlah was 534 hopeless as a ruler. His relations alike to his master, the merely titular emperor of Delhi, and to the people left the province open to the strongest. After “the Black Hole,” the battle of Calcutta, and the treachery at Chandernagore in spite of the treaty which followed that battle, the East India Company could treat the nawab only as an enemy. Clive, it is true, might have disregarded all native intrigue, marched on Murshidabad, and at once held the delta of the Ganges in the Company’s name. But the time was not ripe for this, and the consequences, with so small a force, might have been fatal. The idea of acting directly as rulers, or save under native charters and names, was not developed by events for half a century. The political morality of the time in Europe, as well as the comparative weakness of the Company in India, led Clive not only to meet the dishonesty of his native associate by equal dishonesty, but to justify his conduct by the declaration, years after, in parliament, that he would do the same again. It became necessary to employ the richest Bengali trader, Omichund, as an agent between Jafar Ali and the British officials. Master of the secret of the confederacy against Suraj-ud-Dowlah, the Bengali threatened to betray it unless he was guaranteed, in the treaty itself, £300,000. To dupe the villain, who was really paid by both sides, a second, or fictitious treaty, was shown him with a clause to this effect. This Admiral Watson refused to sign; “but,” Clive deponed to the House of Commons, “to the best of his remembrance, he gave the gentleman who carried it leave to sign his name upon it; his lordship never made any secret of it; he thinks it warrantable in such a case, and would do it again a hundred times; he had no interested motive in doing it, and did it with a design of disappointing the expectations of a rapacious man.” Such is Clive’s own defence of the one act which, in a long career of abounding temptations, was of questionable honesty.
The whole hot season of 1757 was spent in these negotiations, till the middle of June, when Clive began his march from Chandernagore, the British in boats, and the sepoys along the right bank of the Hugli. That river above Calcutta is, during the rainy season, fed by the overflow of the Ganges to the north through three streams, which in the hot months are nearly dry. On the left bank of the Bhagirathi, the most westerly of these, 100 m. above Chandernagore, stands Murshidabad, the capital of the Mogul viceroys of Bengal, and then so vast that Clive compared it to the London of his day. Some miles farther down is the field of Plassey, then an extensive grove of mango trees, of which enough yet remains, in spite of the changing course of the stream, to enable the visitor to realize the scene. On the 21st of June Clive arrived on the bank opposite Plassey, in the midst of that outburst of rain which ushers in the south-west monsoon of India. His whole army amounted to 1100 Europeans and 2100 native troops, with 9 field-pieces. The nawab had drawn up 18,000 horse, 50,000 foot and 53 pieces of heavy ordnance, served by French artillerymen. For once in his career Clive hesitated, and called a council of sixteen officers to decide, as he put it, “whether in our present situation, without assistance, and on our own bottom, it would be prudent to attack the nawab, or whether we should wait till joined by some country power?” Clive himself headed the nine who voted for delay; Major (afterwards Sir) Eyre Coote led the seven who counselled immediate attack. But, either because his daring asserted itself, or because, also, of a letter that he received from Jafar Ali, as has been said, Clive was the first to change his mind and to communicate with Major Eyre Coote. One tradition, followed by Macaulay, represents him as spending an hour in thought under the shade of some trees, while he resolved the issues of what was to prove one of the decisive battles of the world. Another, turned into verse by Sir Alfred Lyall, pictures his resolution as the result of a dream. However that may be, he did well as a soldier to trust to the dash and even rashness that had gained Arcot and triumphed at Calcutta, and as a statesman, since retreat, or even delay, would have put back the civilization of India for years. When, after the heavy rain, the sun rose brightly on the 22nd, the 3200 men and the 9 guns crossed the river and took possession of the grove and its tanks of water, while Clive established his headquarters in a hunting lodge, On the 23rd the engagement took place and lasted the whole day. Except the 40 Frenchmen and the guns which they worked, the enemy did little to reply to the British cannonade which, with the 39th Regiment, scattered the host, inflicting on it a loss of 500 men. Clive restrained the ardour of Major Kilpatrick, for he trusted to Jafar Ali’s abstinence, if not desertion to his ranks, and knew the importance of sparing his own small force. He lost hardly a white soldier; in all 22 sepoys were killed and 50 wounded. His own account, written a month after the battle to the secret committee of the court of directors, is not less unaffected than that in which he had announced the defeat of the nawab at Calcutta. Suraj-ud-Dowlah fled from the field on a camel, secured what wealth he could, and came to an untimely end. Clive entered Murshidabad, and established Jafar Ali in the position which his descendants have ever since enjoyed, as pensioners, but have not infrequently abused. When taken through the treasury, amid a million and a half sterling’s worth of rupees, gold and silver plate, jewels and rich goods, and besought to ask what he would, Clive was content with £160,000, while half a million was distributed among the army and navy, both in addition to gifts of £24,000 to each member of the Company’s committee, and besides the public compensation stipulated for in the treaty. It was to this occasion that he referred in his defence before the House of Commons, when he declared that he marvelled at his moderation. He sought rather to increase the shares of the fleet and the troops at his own expense, as he had done at Gheria, and did more than once afterwards, with prize of war. What he did take from the grateful nawab for himself was less than the circumstances justified from an Oriental point of view, was far less than was pressed upon him, not only by Jafar Ali, but by the hundreds of native nobles whose gifts Clive steadily refused, and was openly acknowledged from the first. He followed a usage fully recognized by the Company, although the fruitful source of future evils which he himself was again sent out to correct. The Company itself acquired a revenue of £100,000 a year, and a contribution towards its losses and military expenditure of a million and a half sterling. Such was Jafar Ali’s gratitude to Clive that he afterwards presented him with the quit-rent of the Company’s lands in and around Calcutta, amounting to an annuity of £27,000 for life, and left him by will the sum of £70,000, which Clive devoted to the army.
While busy with the civil administration, the conqueror of Plassey continued to follow up his military success. He sent Major Coote in pursuit of the French almost as far as Benares. He despatched Colonel Forde to Vizagapatam and the northern districts of Madras, where that officer gained the battle of Condore, pronounced by Broome “one of the most brilliant actions on military record.” He came into direct contact, for the first time, with the Great Mogul himself, an event which resulted in the most important consequences during the third period of his career. Shah Alam, when shahzada, or heir-apparent, quarrelled with his father Alam Gir II., the emperor, and united with the viceroys of Oudh and Allahabad for the conquest of Bengal. He advanced as far as Patna, which he besieged with 40,000 men. Jafar Ali, in terror, sent his son to its relief, and implored the aid of Clive. Major Caillaud defeated the prince’s army and dispersed it. Finally, at this period, Clive repelled the aggression of the Dutch, and avenged the massacre of Amboyna, on that occasion when he wrote his famous letter, “Dear Forde, fight them immediately; I will send you the order of council to-morrow.” Meanwhile he never ceased to improve the organization and drill of the sepoy army, after a European model, and enlisted into it many Mahommedans of fine physique from upper India. He refortified Calcutta. In 1760, after four years of labour so incessant and results so glorious, his health gave way and he returned to England. “It appeared,” wrote a contemporary on the spot, “as if the soul was departing from the government of Bengal.” He had been formally made governor of Bengal by the court of directors at a time when his nominal superiors in Madras sought to recall him to their help there. But he had discerned the importance of the province 535 even during his first visit to its rich delta, mighty rivers and teeming population. It should be noticed, also, that he had the kingly gift of selecting the ablest subordinates, for even thus early he had discovered the ability of young Warren Hastings, destined to be his great successor, and, a year after Plassey, made him resident at the nawab’s court.
In 1760, at thirty-five years of age, Clive returned to England with a fortune of at least £300,000 and the quit-rent of £27,000 a year, after caring for the comfort of his parents and sisters, and giving Major Lawrence, his old commanding officer, who had early encouraged his military genius, £500 a year. The money had been honourably and publicly acquired, with the approval of the Company. The amount might have been four times what it was had Clive been either greedy after wealth or ungenerous to the colleagues and the troops whom he led to victory. In the five years of his conquests and administration in Bengal, the young man had crowded together a succession of exploits which led Lord Macaulay, in what that historian termed his “flashy” essay on the subject, to compare him to Napoleon Bonaparte. But there was this difference in Clive’s favour, due not more to the circumstances of the time than to the object of his policy—he gave peace, security, prosperity and such liberty as the case allowed of to a people now reckoned at nearly three hundred millions, who had for centuries been the prey of oppression, while Napoleon’s career of conquest was inspired only by personal ambition, and the absolutism he established vanished with his fall. During the three years that Clive remained in England he sought a political position, chiefly that he might influence the course of events in India, which he had left full of promise. He had been well received at court, had been made Baron Clive of Plassey, in the peerage of Ireland, had bought estates, and had got not only himself, but his friends returned to the House of Commons after the fashion of the time. Then it was that he set himself to reform the home system of the East India Company, and began a bitter warfare with Mr Sulivan, chairman of the court of directors, whom in the end he defeated. In this he was aided by the news of reverses in Bengal. Vansittart, his successor, having no great influence over Jafar Ali Khan, had put Kasim Ali Khan, the son-in-law, in his place in consideration of certain payments to the English officials. After a brief tenure Kasim Ali had fled, had ordered Walter Reinhardt (known to the Mahommedans as Sumru), a Swiss mercenary of his, to butcher the garrison of 150 English at Patna, and had disappeared under the protection of his brother viceroy of Oudh. The whole Company’s service, civil and military, had become demoralized by gifts, and by the monopoly of the inland as well as export trade, to such an extent that the natives were pauperized, and the Company was plundered of the revenues which Clive had acquired for them. The court of proprietors, accordingly, who elected the directors, forced them, in spite of Sulivan, to hurry out Lord Clive to Bengal with the double powers of governor and commander-in-chief.
What he had done for Madras, what he had accomplished for Bengal proper, and what he had effected in reforming the Company itself, he was now to complete in less than two years, in this the third period of his career, by putting his country politically in the place of the emperor of Delhi, and preventing for ever the possibility of the corruption to which the British in India had been driven by an evil system. On the 3rd of May 1765 he landed at Calcutta to learn that Jafar Ali Khan had died, leaving him personally £70,000, and had been succeeded by his son, though not before the government had been further demoralized by taking £100,000 as a gift from the new nawab; while Kasim Ali had induced not only the viceroy of Oudh, but the emperor of Delhi himself, to invade Behar. After the first mutiny in the Bengal army, which was suppressed by blowing the sepoy ringleader from a gun, Major Munro, “the Napier of those times,” scattered the united armies on the hard-fought field of Buxar. The emperor, Shah Alam, detached himself from the league, while the Oudh viceroy threw himself on the mercy of the British. Clive had now an opportunity of repeating in Hindustan, or Upper India, what he had accomplished for the good of Bengal. He might have secured what are now called the United Provinces, and have rendered unnecessary the campaigns of Wellesley and Lake. But he had other work in the consolidation of rich Bengal itself, making it a base from which the mighty fabric of British India could afterwards steadily and proportionally grow. Hence he returned to the Oudh viceroy all his territory save the provinces of Allahabad and Kora, which he made over to the weak emperor. But from that emperor he secured the most important document in the whole of British history in India up to that time, which appears in the records as “firmaund from the King Shah Aalum, granting the dewany of Bengal, Behar and Orissa to the Company, 1765.” The date was the 12th of August, the place Benares, the throne an English dining-table covered with embroidered cloth and surmounted by a chair in Clive’s tent. It is all pictured by a Mahommedan contemporary, who indignantly exclaims that so great a “transaction was done and finished in less time than would have been taken up in the sale of a jackass.” By this deed the Company became the real sovereign rulers of thirty millions of people, yielding a revenue of four millions sterling. All this had been accomplished by Clive in the few brief years since he had avenged “the Black Hole” of Calcutta. This would be a small matter, or might even be a cause of reproach, were it not that the Company’s undisputed sovereignty proved, after a sore period of transition, the salvation of these millions. The lieutenant-governorship of Bengal since Clive’s time has grown so large and prosperous that in 1905 it was found advisable to divide it into two separate provinces. But Clive, though thus moderate and even generous to an extent which called forth the astonishment of the natives, had all a statesman’s foresight. On the same date he obtained not only an imperial charter for the Company’s possession in the Carnatic also, thus completing the work he began at Arcot, but a third firman for the highest of all the lieutenancies of the empire, that of the Deccan itself. This fact is mentioned in a letter from the secret committee of the court of directors to the Madras government, dated the 27th of April 1768. Still so disproportionate did the British force seem, not only to the number and strength of the princes and people of India, but to the claims and ambition of French, Dutch and Danish rivals, that Clive’s last advice to the directors, as he finally left India in 1767, was this: “We are sensible that, since the acquisition of the dewany, the power formerly belonging to the soubah of those provinces is totally, in fact, vested in the East India Company. Nothing remains to him but the name and shadow of authority. This name, however, this shadow, it is indispensably necessary we should seem to venerate.” On a wider arena, even that of the Great Mogul himself, the shadow was kept up till it obliterated itself in the massacre of English people in the Delhi palace in 1857; and Queen Victoria was proclaimed, first, direct ruler on the 1st of November 1858, and then empress of India on the 1st of January 1877.
Having thus founded the empire of British India, Clive’s painful duty was to create a pure and strong administration, such as alone would justify its possession by foreigners. The civil service was de-orientalized by raising the miserable salaries which had tempted its members to be corrupt, by forbidding the acceptance of gifts from natives, and by exacting covenants under which participation in the inland trade was stopped. Not less important were his military reforms. With his usual tact and nerve he put down a mutiny of the English officers, who chose to resent the veto against receiving presents and the reduction of batta at a time when two Mahratta armies were marching on Bengal. His reorganization of the army, on the lines of that which he had begun after Plassey, and which was neglected during his second visit to England, has since attracted the admiration of the ablest Indian officers. He divided the whole into three brigades, so as to make each a complete force, in itself equal to any single native army that could be brought against it. He had not enough British artillerymen, however, and would not make the mistake of his successors, who trained natives to work the guns, which were turned against the British 536 with such effect in 1857. It is sufficient to say that after the Mutiny the government returned to his policy, and not a native gunner is now to be found in the Indian army.
Clive’s final return to England, a poorer man than he went out, in spite of still more tremendous temptations, was the signal for an outburst of his personal enemies, exceeded only by that which the malice of Sir Philip Francis afterwards excited against Warren Hastings. Every civilian whose illicit gains he had cut off, every officer whose conspiracy he had foiled, every proprietor or director, like Sulivan, whose selfish schemes he had thwarted, now sought their opportunity. He had, with consistent generosity, at once made over the legacy of £70,000 from the grateful Jafar Ali, as the capital of what has since been known as “the Clive Fund,” for the support of invalided European soldiers, as well as officers, and their widows, and the Company had allowed 8% on the sum for an object which it was otherwise bound to meet. General John Burgoyne, of Saratoga memory, did his best to induce the House of Commons, in which Lord Clive was now member for Shrewsbury, to impeach the man who gave his country an empire, and the people of that empire peace and justice, and that, as we have seen, without blot on the gift, save in the matter of Omichund. The result, after the brilliant and honourable defences of his career which will be found in Almon’s Debates for 1773, was a compromise that saved England this time from the dishonour which, when Warren Hastings had to run the gauntlet, put it in the same category with France in the treatment of its public benefactors abroad. On a division the House, by 155 to 95, carried the motion that Lord Clive “did obtain and possess himself” of £234,000 during his first administration of Bengal; but, refusing to express an opinion on the fact, it passed unanimously the second motion, at five in the morning, “that Robert, Lord Clive, did at the same time render great and meritorious services to his country.” The one moral question, the one questionable transaction in all that brilliant and tempted life—the Omichund treaty—was not touched.
Only one who can personally understand what Clive’s power and services had been will rightly realize the effect on him, though in the prime of life, of the discussions through which he had been dragged. In the greatest of his speeches, in reply to Lord North, he said,—“My situation, sir, has not been an easy one for these twelve months past, and though my conscience could never accuse me, yet I felt for my friends who were involved in the same censure as myself.... I have been examined by the select committee more like a sheep-stealer than a member of this House.” Fully accepting that statement, and believing him to have been purer than his accusers in spite of temptations unknown to them, we see in Clive’s end the result merely of physical suffering, of chronic disease which opium failed to abate, while the worry and chagrin caused by his enemies gave it full scope. This great man, who did more for his country than any soldier till Wellington, and more for the people and princes of India than any statesman in history, died by his own hand on the 22nd of November 1774 in his fiftieth year.
The portrait of Clive, by Dance, in the council chamber of Government House, Calcutta, faithfully represents him. He was slightly above middle-size, with a countenance rendered heavy and almost sad by a natural fulness above the eyes. Reserved to the many, he was beloved by his own family and friends. His encouragement of scientific undertakings like Major James Rennell’s surveys, and of philological researches like Francis Gladwin’s, gained him to two honorary distinctions of F.R.S. and LL.D.
His son and successor Edward (1754-1839) was created earl of Powis in 1804, his wife being the sister and heiress of George Herbert, earl of Powis (1755-1801). He is thus the ancestor of the later earls of Powis, who took the name of Herbert instead of that of Clive in 1807.
See Sir A. J. Arbuthnot, Lord Clive (“Builders of Great Britain” series) (1899); Sir C. Wilson, Lord Clive (“English Men of Action” series) (1890); G. B. Malleson, Lord Clive (“Rulers of India” series) (1890); F. M. Holmes, Four Heroes of India (1892); C. Caraccioli, Life of Lord Clive(1775).
CLOACA, the Latin term given to the sewers laid to drain the low marshy grounds between the hills of Rome. The most important, which drained the forum, is known as the Cloaca Maxima and dates from the 6th century B.C. This was 10 ft. 6 in. wide, 14 ft. high, and was vaulted with three consecutive rings of voussoirs in stone, the floor being paved with polygonal blocks of lava.
CLOCK. The measurement of time has always been based on the revolution of the celestial bodies, and the period of the apparent revolution of the sun, i.e. the interval between two consecutive crossings of a meridian, has been the usual standard for a day. By the Egyptians the day was divided into 24 hours of equal length. The Greeks adopted a different system, dividing the day, i.e. the period from sunrise to sunset, into 12 hours, and also the night. Whence it followed that it was only at two periods in the year that the length of the hours during the day and night were uniform (see Calendar). In consequence, those who adopted the Greek system were obliged to furnish their water-clocks (see Clepsydra) with a compensating device so that the equal hours measured by those clocks should be rendered unequal, according to the exigencies of the season. The hours were divided into minutes and seconds, a system derived from the sexagesimal notation which prevailed before the decimal system was finally adopted. Our mode of computing time, and our angular measure, are the only relics of this obsolete system.
The simplest measure of time is the revolution of the earth round its axis, which so far as we know is uniform, perfectly regular, and has not varied in speed during any period of human observation. The time of such a revolution is called a sidereal day, and is divided into hours, minutes and seconds. The period of rotation of the earth is practically measured by observations of the fixed stars (see Time), the period between two successive transits of the same star across a meridian constituting the sidereal day. But as the axis of the earth slowly revolves round in a cone, whereby the phenomenon known as the precession of the equinoxes is produced, it follows that the astronomical sidereal day is not the true period of the earth’s rotation on its axis, but varies from it by less than a twenty millionth part, a fraction so small as to be inappreciable. But the civil day depends not on the revolution of the earth with regard to the stars, but on its revolution as compared with the position of the sun. Therefore each civil day is on the average longer than a sidereal one by nearly four minutes, or, to be exact, each sidereal day is to an average civil day as .99727 to 1, and the sidereal hour, minute and second are also shorter in like proportion. Hence a sidereal clock has a shorter, quicker-moving pendulum than an ordinary clock.
Ordinary civil time thus depends on the apparent revolution of the sun round the earth. As, however, this is not uniform, it is needful for practical convenience to give it an artificial uniformity. For this purpose an imaginary sun, moving round the earth with the average velocity of the real sun, and called the “mean” sun, is taken as the measure of civil time. The day is divided into 24 hours, each hour into 60 minutes, and each minute into 60 seconds. After that the sexagesimal division system is abandoned, and fractions of seconds are estimated in decimals.
A clock consists of a train of wheels, actuated by a spring or weight, and provided with a governing device which so regulates the speed as to render it uniform. It also has a mechanism by which it strikes the hours on a bell or gong (cp. Fr. cloche, Ger. Glocke, a bell; Dutch klok, bell, clock), whereas, strictly, a timepiece does not strike, but simply shows the time.
The earliest clocks seem to have come into use in Europe during the 13th century. For although there is evidence that they may have been invented some centuries sooner, yet until that date they were probably only curiosities. The first form they took was that of the balance clock, the invention of which is ascribed, but on very insufficient grounds, to Pope Silvester II. in A.D. 996. A clock was put up in a former clock tower at Westminster with some great bells in 1288, out of a fine imposed on a chief-justice who had offended the government, and the motto Discite justitiam, moniti, inscribed upon it. The bells were sold, 537 or rather, it is said, gambled away, by Henry VIII. In 1292 a clock in Canterbury cathedral is mentioned as costing £30, and another at St Albans, by R. Wallingford, the abbot in 1326, is said to have been such as there was not in all Europe, showing various astronomical phenomena. A description of one in Dover Castle with the date 1348 on it was published by Admiral W.H. Smyth (1788-1865) in 1851, and the clock itself was exhibited going, in the Scientific Exhibition of 1876. A very similar one, made by Henry de Vick for the French king Charles V. in 1379 was much like the common clocks of the 18th century, except that it had a vibrating balance instead of a pendulum. The works of one of these old clocks still exist in a going condition at the Victoria and Albert Museum. It came from Wells cathedral, having previously been at Glastonbury abbey.
 |
| Fig. 1.—Verge Escapement. |
These old clocks had what is called a verge escapement, and a balance. The train of wheels ended with a crown wheel, that is, a wheel serrated with teeth like those of a saw, placed parallel with its axis (fig. 1). These teeth, D, engaged with pallets CB, CA, mounted on a verge or staff placed parallel to the face of the crown wheel. As the crown wheel was turned round the teeth pushed the pallets alternately until one or the other slid past a tooth, and thus let the crown wheel rotate. When one pallet had slipped over a tooth, the other pallet caught a corresponding tooth on the opposite side of the wheel. The verge was terminated by a balance rod placed at right angles to it with a ball at each end. It is evident that when the force of any tooth on the crown wheel began to act on a pallet, it communicated motion to the balance and thus caused it to rotate. This motion would of course be accelerated, not uniformly, but according to some law dependent on the shape of the teeth and pallets. When the motion had reached its maximum, the tooth slipped past the pallet. The other pallet now engaged another tooth on the opposite side of the wheel. The motion of the balls, however, went on and they continued to swing round, but this time they were opposed by the pressure of the tooth. For a time they overcame that pressure, and drove the tooth back, causing a recoil. As, however, every motion if subjected to an adverse acceleration (i.e. a retardation) must come to rest, the balls stopped, and then the tooth, which had been forced to recoil, advanced in its turn, and the swing was repeated. The arrangement was thus very like a huge watch balance wheel in which the driving weight acted in a very irregular manner, not only as a driving force, but also as a regulating spring. The going of such clocks was influenced greatly by friction and by the oil on the parts, and never could be satisfactory, for the time varied with every variation in the swing of the balls, and this again with every variation of the effective driving force.
 |
| Fig. 2.—Galileo’s Escapement. |
The first great step in the improvement of the balance clock was a very simple one. In the 17th century Galileo had discovered the isochronism of the pendulum, but he made no practical use of it, except by the invention of a little instrument for enabling doctors to count their patients’ pulse-beats. His son, however, is supposed to have applied the pendulum to clocks. There is at the Victoria and Albert Museum a copy of an early clock, said to be Galileo’s, in which the pins on a rotating wheel kick a pendulum outwards, remaining locked after having done so till the pendulum returns and unlocks the next pin, which then administers another kick to the pendulum (fig. 2). The interest of the specimen is that it contains the germ of the chronometer escapement and free pendulum, which is possibly destined to be the escapement of the future.
The essential component parts of a clock are:—
1. The pendulum or time-governing device;
2. The escapement, whereby the pendulum controls the speed of going;
3. The train of wheels, urged round by the weight or main-spring, together with the recording parts, i.e. the dial, hands and hour motion wheels;
4. The striking mechanism.
 |
| Fig. 3.—Section of House Clock. |
The general construction of the going part of all clocks, except large or turret clocks, is substantially the same, and fig. 3 is a section of any ordinary house clock. B is the barrel with the cord coiled round it, generally 16 times for the 8 days; the barrel is fixed to its arbor K, which is prolonged into the winding square coming up to the face or dial of the clock; the dial is here shown as fixed either by small screws x, or by a socket and pin z, to the prolonged pillars p, p, which (4 or 5 in number) connect the plates or frame of the clock together, though the dial is commonly set on to the front plate by another set of pillars of its own. The great wheel G rides on the arbor, and is connected with the barrel by the ratchet R, the action of which is shown more fully in fig. 25. The intermediate wheel r in this drawing is for a purpose which will be described hereafter, and for the present it may be considered as omitted, and the click of the ratchet R as fixed to the great wheel. The great wheel drives the pinion c which is called the centre pinion, on the arbor of the centre wheel C, which goes through to the dial, and carries the long, or minute-hand; this wheel always turns in an hour, and the great wheel generally in 12 hours, by having 12 times as many teeth as the centre pinion. The centre wheel drives the “second wheel” D by its pinion d, and that again drives the scape-wheel E by its pinion e. If the pinions d and e have each 8 teeth or leaves (as the teeth of pinions are usually called), C will have 64 teeth and D 60, in a clock of which the scape-wheel turns in a minute, so that the seconds hand may be set on its arbor prolonged to the dial. A represents the pallets of the escapement, which will be described presently, and their arbor a goes through a large hole in the back plate near F, and its back pivot turns in a cock OFQ screwed on to the back plate. From the pallet arbor at F descends the crutch Ff, ending in the fork f, which embraces the pendulum P, so that as the pendulum vibrates, the crutch and the pallets necessarily vibrate with it. The pendulum is hung by a thin spring S from the cock Q, so that the bending point of the spring may be just opposite the end of the pallet arbor, and the edge of the spring as close to the end of that arbor as possible.
We may now go to the front (or left hand) of the clock, and 538 describe the dial or “motion-work.” The minute hand fits on to a squared end of a brass socket, which is fixed to the wheel M, and fits close, but not tight, on the prolonged arbor of the centre wheel. Behind this wheel is a bent spring which is (or ought to be) set on the same arbor with a square hole (not a round one as it sometimes is) in the middle, so that it must turn with the arbor; the wheel is pressed up against this spring, and kept there, by a cap and a small pin through the end of the arbor. The consequence is, that there is friction enough between the spring and the wheel to carry the hand round, but not enough to resist a moderate push with the finger for the purpose of altering the time indicated. This wheel M, which is sometimes called the minute-wheel, but is better called the hour-wheel as it turns in an hour, drives another wheel N, of the same number of teeth, which has a pinion attached to it; and that pinion drives the twelve-hour wheel H, which is also attached to a large socket or pipe carrying the hour hand, and riding on the former socket, or rather (in order to relieve the centre arbor of that extra weight) on an intermediate socket fixed to the bridge L, which is screwed to the front plate over the hour-wheel M. The weight W, which drives the train and gives the impulse to the pendulum through the escapement, is generally hung by a catgut line passing through a pulley attached to the weight, the other end of the cord being tied to some convenient place in the clock frame or seat-board, to which it is fixed by screws through the lower pillars.
 |
| Fig. 4. |
Pendulum.—Suppose that we have a body P (fig. 4) at rest, and that it is material, that is to say, has “mass.” And for simplicity let us consider it a ball of some heavy matter. Let it be free to move horizontally, but attached to a fixed point A by means of a spring. As it can only move horizontally and not fall, the earth’s gravity will be unable to impart any motion to it. Now it is a law first discovered by Robert Hooke (1635-1703) that if any elastic spring be pulled by a force, then, within its elastic limits, the amount by which it will be extended is proportional to the force. Hence then, if a body is pulled out against a spring, the restitutional force is proportional to the displacement. If the body be released it will tend to move back to its initial position with an acceleration proportioned to its mass and to its distance from rest. A body thus circumstanced moves with harmonic motion, vibrating like a stretched piano string, and the peculiarity of its motion is that it is isochronous. That is to say, the time of returning to its initial position is the same, whether it makes a large movement at a high velocity under a strong restitutional force, or a small movement at a lower velocity under a smaller restitutional force (see Mechanics). In consequence of this fact the balance wheel of a watch is isochronous or nearly so, notwithstanding variations in the amplitude of its vibrations. It is like a piano string which sounds the same note, although the sound dies away as the amplitude of its vibrations diminishes.
 |
| Fig. 5. |
A pendulum is isochronous for similar reasons. If the bob be drawn aside from D to C (fig. 5), then the restitutional force tending to bring it back to rest is approximately the force which gravitation would exert along the tangent CA, i.e.
| g cos ACW = g | BC | = g | displacement BC | . |
| OC | length of pendulum |
Since g is constant, and the length of the pendulum does not vary, it follows that when a pendulum is drawn aside through a small arc the force tending to bring it back to rest is proportional to the displacement (approximately). Thus the pendulum bob under the influence of gravity, if the arc of swing is small, acts as though instead of being acted on by gravity it was acted on by a spring tending to drag it towards D, and therefore is isochronous. The qualification “If the arc of swing is small” is introduced because, as was discovered by Christiaan Huygens, the arc of vibration of a truly isochronous pendulum should not be a circle with centre O, but a cycloid DM, generated by the rolling of a circle with diameter DQ = ½OD, upon a straight line QM. However, for a short distance near the bottom, the circle so nearly coincides with the cycloid that a pendulum swinging in the usual circular path is, for small arcs, isochronous for practical purposes.
 |
| Fig. 6. |
The formula representing the time of oscillation of a pendulum, in a circular arc, is thus found:—Let OB (fig. 6) be the pendulum, B be the position from which the bob is let go, and P be its position at some period during its swing. Put FC = h, and MC = x, and OB = l. Now when a body is allowed to move under the force of gravity in any path from a height h, the velocity it attains is the same as a body would attain falling freely vertically through the distance h. Whence if v be the velocity of the bob at P, v = √2gFM = √2g(h - x). Let Pp = ds, and the vertical distance of p below P = dx, then Pp = velocity at P × dt; that is, dt = ds/v. Also
| ds | = | 1 | = | 1 | , |
| dx | MP | √x(2l - x) |
whence
| dt = | ds | = | ldx | . | 1 | = | 1 | √ | l | . | dx | . | 1 | . |
| v | √x(2l - x) | √2g(h - x) | 2 | g | √x(p - x) | √1 - (x/2l) |
Expanding the second part we have
| dt = | 1 | √ | l | . | dx | . ( 1 + | x | + ... ) . |
| 2 | g | √x(h - x) | 4l |
If this is integrated between the limits of 0 and h, we have
| t = π √ | l | . ( 1 + | h | + ... ) , |
| g | 8l |
where t is the time of swing from B to A. The terms after the second may be neglected. The first term, π √l/g, is the time of swing in a cycloid. The second part represents the addition necessary if the swing is circular and not cycloidal, and therefore expresses the “circular error.” Now h = BC²/l = 2π²θ²l/360², where θ is half the angle of swing expressed in degrees; hence h/8l = θ²/52520, and the formula becomes
| t = π √ | l | ( 1 + | θ² | ) . |
| g | 52520 |
Hence the ratio of the time of swing of an ordinary pendulum of any length, with a semiarc of swing = θ degrees is to the time of swing of a corresponding cycloidal pendulum as 1 + θ²/52520 : 1. Also the difference of time of swing caused by a small increase θ′ in the semiarc of swing = 2θθ′ / 52520 second per second, or 3.3θθ′ seconds per day. Hence in the case of a seconds pendulum whose semiarc of swing is 2° an increase of .1° in this semiarc of 2° would cause the clock to lose 3.3 × 2 × 0.1 = .66 second a day.
Huygens proposed to apply his discovery to clocks, and since the evolute of a cycloid is an equal cycloid, he suggested the use of a flexible pendulum swinging between cycloidal cheeks. But this was only an example of theory pushed too far, because the friction on the cycloidal cheeks involves more error than they correct, and other disturbances of a higher degree of importance are left uncorrected. In fact the application of pendulums to clocks, though governed in the abstract by theory, has to be modified by experiment.
Neglecting the circular error, if L be the length of a pendulum and g the acceleration of gravity at the place where the pendulum is, then T, the time of a single vibration = π√(L/g). From this formula it follows that the times of vibration of pendulums are directly proportional to the square root of their lengths, and inversely proportional to the square root of the acceleration of gravity at the place where the pendulum is swinging. The value of g for London is 32.2 ft. per second per second, whence it results that the length of a pendulum for London to beat seconds of mean solar time = 39.14 in. nearly, the length of an astronomical pendulum to beat seconds of sidereal time being 38.87 in.
This length is calculated on the supposition that the arc of swing is cycloidal and that the whole mass of the pendulum is concentrated at a point whose distance, called the radius of oscillation, from the point of suspension of the pendulum is 39.14 in. From this it might be imagined that if a sphere, say of iron, were suspended from a light rod, so that its centre were 39.14 in. below its point of support, it would vibrate once per second. This, however, is not the case. For as the pendulum swings, the ball also tends to turn in space to and fro round a horizontal axis perpendicular to the direction of its motion. Hence the force stored up in the pendulum is expended, not only in making it swing, but also in causing the ball to oscillate to and fro through a small angle about a horizontal axis. We have therefore to consider not merely the vibrations of the rod, but the oscillations of the bob. The moment of the momentum of the system round the point of suspension, called its moment of inertia, is composed of the sum of the mass of each particle multiplied into the square of its distance from the axis of rotation. Hence the moment 539 of inertia of the body I = Σ(ma²). If k be defined by the relation Σ(ma²) = Σ(m) X k², then k is called the radius of gyration. If k be the radius of gyration of a bob round a horizontal axis through its centre of gravity, h the distance of its centre of gravity below its point of suspension, and k’ the radius of gyration of the bob round the centre of suspension, then k′² = h² + k². If l be the length of a simple pendulum that oscillates in the same time, then lh = k′² = h² + k². Now k can be calculated if we know the form of the bob, and l is the length of the simple pendulum = 39.14 in.; hence h, the distance of the centre of gravity of the bob below the point of suspension, can be found.
In an ordinary pendulum, with a thin rod and a bob, this distance h is not very different from the theoretical length, l = 39.14 in., of a simple theoretical pendulum in which the rod has no weight and the bob is only a single heavy point. For the effect of the weight of the rod is to throw the centre of oscillation a little above the centre of gravity of the bob, while the effect of the size of the bob is to throw the centre of oscillation a little down. In ordinary practice it is usual to make the pendulum so that the centre of gravity is about 39 in. below the upper free end of the suspension spring and leave the exact length to be determined by trial.
 |
| Fig. 7.—Section of Westminster Clock Pendulum. |
Since T = π√L/g, we have, by differentiating, dL/L = 2dT/T, that is, any small percentage of increase in L will correspond to double the percentage of increase in T. Therefore with a seconds pendulum, in order to make a second’s difference in a day, equivalent to 1/86,400 of the pendulum’s Regulation. rate of vibration, since there are 86,400 seconds in 24 hours, we must have a difference of length amounting to 2/86,400 = 1/43,200 of the length of the rod. This is 39.138/43,200 = .000906 in. Hence if under the pendulum bob be put a nut working a screw of 32 threads to the inch and having its head divided into 30 parts, a turn of this nut through one division will alter the length of the pendulum by .0009 in. and change the rate of the clock by about a second a day. To accelerate the clock the nut has always to be turned to the right, or as you would drive in a corkscrew and vice versa. But in astronomical and in large turret clocks, it is desirable to avoid stopping or in any way disturbing the pendulum; and for the finer adjustments other methods of regulation are adopted. The best is that of fixing a collar, as shown in fig. 7 at C, about midway down the rod, capable of having very small weights laid upon it, this being the place where the addition of any small weight produces the greatest effect, and where, it may be added, any moving of that weight up or down on the rod produces the least effect. If M is the weight of the pendulum and l its length (down to the centre of oscillation), and m a small weight added at the distance n below the centre of suspension or above the c.o. (since they are reciprocal), t the time of vibration, and -dt the acceleration due to adding m; then
| -dt | = | m | ( | n | - | n² | ) ; |
| t | 2M | l | l² |
from which it is evident that if n = l/2, then = dt/t = m/8M. But as there are 86400 seconds in a day, -dT, the daily acceleration, = 86400 dt, or 10800 m/M, or if m is the 10800th of the weight of the pendulum it will accelerate the clock a second a day, or 10 grains will do that on a pendulum of 15 ℔ weight (7000 gr. being = 1 ℔.), or an ounce on a pendulum of 6 cwt. In like manner if n = l/3 from either top or bottom, m must = M/7200 to accelerate the clock a second a day. The higher up the collar the less is the risk of disturbing the pendulum in putting on or taking off the regulating weights, but the bigger the weight required to produce the effect. The weights should be made in a series, and marked ¼, ½, 1, 2, according to the number of seconds a day by which they will accelerate; and the pendulum adjusted at first to lose a little, perhaps a second a day, when there are no weights on the collar, so that it may always have some weight on, which can be diminished or increased from time to time with certainty, as the rate may vary.
The length of pendulum rods is also affected by temperature and also, if they are made of wood, by damp. Hence, to ensure good time-keeping qualities in a clock, it is necessary (1) to make the rods of materials that are as little affected by such Compensation. influences as possible, and (2) to provide means of compensation by which the effective length of the rod is kept constant in spite of expansion or contraction in the material of which it is composed. Fairly good pendulums for ordinary use may be made out of very well dried wood, soaked in a thin solution of shellac in spirits of wine, or in melted paraffin wax; but wood shrinks in so uncertain a manner that such pendulums are not admissible for clocks of high exactitude. Steel is an excellent material for pendulum rods, for the metal is strong, is not stretched by the weight of the bob, and does not suffer great changes in molecular structure in the course of time. But a steel rod expands on the average lineally by .0000064 of its length for each degree F. by which its temperature rises; hence an expansion of .00009 in. on a pendulum rod of 39.14 in., that is .000023 of its length, will be caused by an increase of temperature of about 4° F., and that is sufficient to make the clock lose a second a day. Since the summer and winter temperatures of a room may differ by as much as 50° F., the going of a clock may thus be affected by an error of 12 seconds a day. With a pendulum rod of brass, which has a coefficient of expansion of .00001, a clock might gain one-third of a minute daily in winter as compared with its rate in summer. The coefficients of linear expansion per degree F. of some other materials used in making pendulums are as follows: white deal, .0000024; flint glass, .0000048; iron, .000007; lead, .000016; zinc, .000016; and mercury, .000033. The solid or cubical expansions of these bodies are three times the above quantities respectively.
The first method of compensating a pendulum was invented in 1722 by George Graham, who proposed to use a bob of mercury, taking advantage of the high coefficient of expansion of that metal. As now employed, the mercurial pendulum consists of a rod of steel terminating in a stirrup of the same metal on which rests a glass vessel full of mercury, having its centre of gravity about 39 in. below the point of suspension of the pendulum. For each Fahrenheit degree of temperature the centre of gravity of the bob is lowered by the expansion of the rod about 1⁄4000 of an inch. The glass vessel and the mercury in it have therefore to be so contrived, that their centre of gravity will rise 1⁄4000 in. per degree F. The glass having a small coefficient of expansion, the lateral expansion of the mercury will be checked by it, and this will help to raise the column. For the linear coefficient of expansion of glass is .0000048 per degree F., whence the sectional area of a glass vessel increases by .0000096 per degree F., and therefore the coefficient of vertical expansion of a column of mercury whose volumetric expansion coefficient is .0001 per degree F. is (.0001 - .0000096) = .0000904. Let x be the height of the vessel necessary to compensate a steel rod upon the bottom of which it rests. Then, the coefficient of expansion of steel being .0000066 per degree F., we have
| x | (.0000904 - .0000066) = .0000066 × 39.14, whence x = 6¼ in. |
| 2 |
It must, however, be remembered that the glass jar has some weight and that it does not rise by anything like the amount of the mercury. This tends to keep the centre of gravity down. So that the height of mercury of 6¼ in. will not be sufficient to effect the compensation, and about 6¾ to 7 in. will be required. Some authors specify 7 in.; this is when the diameter of the jar is small. A certain amount of negative compensation must also be deducted to allow for the changes of temperature in the air, as will presently be seen; this amounts in the case of mercury to about 1⁄5 in.
In consequence of the complication of all these calculations it is usual to allow about 6¾ to 7 in. of mercury in the glass vessel and to adjust the exact amount of mercury by trial.
Another very good form of mercurial pendulum was proposed by E. J. Dent; it consists of a cast-iron jar into the top of which the steel pendulum rod is screwed, having its end plunged into the mercury contained in the jar. By this means the mercury, jar and rod rapidly acquire the same temperature. This pendulum is less likely to break than the form just described. The depth of mercury required in an iron jar is stated by Lord Grimthorpe to be 8½ to 9 in. The reason why it is greater than it is when a glass jar is employed is that iron has a larger coefficient of expansion than glass, and that it is also heavier. In all cases, however, of mercury pendulums experiment seems to be the only ultimate test of the quantity of mercury required, for the results are so complicated by the behaviour of the oil and the barometric errors that at its best the regulation of a clock can only be ultimately a matter of scientifically guided compromise. A small amount of compensation of a purely experimental character is also allowed to compensate the changes which temperature effects on the suspension spring. This is sometimes made as much as 1⁄6 of the length correction.
As an alternative to the mercurial pendulum other systems have been employed. The “gridiron” pendulum consists of a group of alternate rods of steel and brass, so arranged that the expansion of the brass acts upwards and counteracts that of the steel downwards. It was invented in 1726 by John Harrison. Assuming that 9 rods are used—5 of steel and 4 of brass—their lengths may be as follows from pin to pin:—Centre steel rod 31.5 in.; 2 steel rods next the centre 24.5 in.; 2 steel rods farthest from centre 29.5 in.; from the lower end of outside steel rods to centre of bob 3 in.; total 89.5 in. Of the 4 brass rods the 2 outside ones are 26.87 in.; and the two inside ones 22.25 in.; total 49.12 in. Thus the expansion of 88½ in. of steel is counteracted by the expansion of 491⁄8 in. of brass. Everything depends, however, on the expansion coefficient of the steel and brass employed, the requirement in every case being that of total lengths of the brass and iron should be in proportion to the linear coefficients of expansion of those metals. The above figures 540 are for a very soft brass and steel. Thos. Reid, with more ordinary steel and brass, prescribed a ratio of 112 to 71, Lord Grimthorpe a ratio of 100 to 61. It is absolutely necessary to put the actual rods to be used for making the pendulum in a hot water bath, and measure their expansions with a microscope.
John Smeaton, taking advantage of a far greater expansion coefficient of zinc as compared with brass, proposed to use a steel rod with a collar at the bottom, on which rested a hard drawn zinc rod. From this rod hung a steel tube to which the bob was attached. The total length of the steel rod and of the steel tube down to the centre of the bob was made to the total length of the zinc tube, in the ratio of 5 to 2 (being the ratio of the expansions of zinc and steel); for a 39.14 in. pendulum we should therefore want a zinc tube equal in length to 2⁄3 (39.14) = 26¼ in. In practice the zinc tube is made about 27 in. long, and then gradually cut down by trial. In fact the weight of a heavy pendulum squeezes the zinc, and it is impossible by mere theory to determine what will be its behaviour. The zinc tube must be of rolled zinc, hard drawn through a die, and must not be cast. Ventilating holes must be made in suitable places in the steel tube and the collar on which it rests, to ensure that changes of temperature are rapidly communicated throughout the system.
A pendulum with a rod of dry varnished deal is tolerably compensated by a bob of lead or of zinc 10½ to 13 in. in height, resting on a nut at the bottom of the rod.
The old methods of pendulum compensation for heat may now be considered as superseded by the invention of “invar,” a combination of nickel and steel, due to Charles E. Guillaume, of the International Office of Weights and Measures at Invar. Sèvres near Paris. This alloy has a linear coefficient of expansion on the average of .000001 per degree centigrade, that is to say, only about 1⁄11 that of ordinary steel. Hence it can be easily compensated by means of brass, lead or any other suitable metal. Brass is usually employed. In the invar pendulum introduced into Great Britain by Mr Agar Baugh a departure is made from the previous practice of merely calculating the length of the compensator, fastening it to the lower part of the pendulum, and attaching it to the centre of the bob. In the case of these pendulums, accurate computations are made of the moments of inertia of every separate individual part. Thus, for instance, since an addition of volume due to the effect of heat to the upper part of the bob has a different effect upon the moment of inertia from that of an equal quantity added to the lower part of the bob, the bob is suspended not from its centre, but from a point about 1⁄10 in. below it, the distance varying according to the shape of the bob, so that the heat expansion of the bob may cause its centre of gravity to rise and compensate the effect of its increased moment of inertia. Again the suspension spring is measured for isochronism, and an alloy of steel prepared for it which does not alter its elasticity with change of temperature. Moreover, since rods of invar steel subjected to strain do not acquire their final coefficients of expansion and elasticity for some time, the invar is artificially “aged” by exposure to strain and heat.
These considerations serve as a guide in arranging for the compensation of the expansion of the rod and bob due to change of temperature. But they are not the only ones required; we have also to deal with changes due to the density of the air in which the pendulum is moving. A body suspended in a fluid loses in weight by an amount equal to the weight of the fluid displaced, whence it follows that a pendulum suspended in air has not the weight which ought truly to correspond to its mass. M remains constant while Mg is less than in a vacuum. If the density of the air remained constant, this loss of weight, being constant, could be allowed for and would make no difference to the time-keeping. The period of swing would only be a little increased over what it would be in vacuo. But the weight of a given volume of air varies both with the barometric pressure and also with temperature. If the bob be of type metal it weighs less in air than in a vacuum by about .000103 part, and for each 1° F. rise in temperature (the barometer remaining constant and therefore the pressure remaining the same), the variation of density causes the bob to gain .00000024 of its weight. This, of course, makes the pendulum go quicker. Since the time of vibration varies as the inverse square root of g, it follows that a small increment of weight, the mass remaining constant, produces a diminution of one half that increment in time of swing. Hence, then, a rise of temperature of 1° F. will produce a diminution in the time of swing of .00000012th part or .0104 second in a day. But in making this calculation it has been assumed that the mass moved remains unaltered by the temperature. This is not so. A pendulum when swinging sets in motion a volume of air dependent on the size of the bob, but in a 10 ℔ bob nearly equal to its own volume. Hence while the rise of 1° of temperature increases the weight by .00000012th part, it also decreases the mass by about the same proportion, and therefore the increase of period due to a rise of temperature of 1° F. will, instead of being .0104 second a day, be about .02 second. This must be compensated negatively by lengthening the pendulum by about .02⁄1000 in. for each degree of rise of temperature, which will require a piece of brass about 2 in. long. It follows, therefore, that with an invar rod having a linear expansion coefficient of .0000002 per degree F., which requires a piece of brass about .8 in. long to compensate it, the compensation which is to regulate both the expansion of the rod and also that of the air must be .8 in. - 2 in., or -1.2 in.; so that the bob must be hung downwards from a piece of brass nearly 11⁄5 in. in length. If the coefficient of expansion of the invar were .00000053 per degree F., then the two corrections, one for the expansion of the rod and the other for the expansion of the air, would just neutralize one another, and the pendulum rod would require no compensator at all. There are a number of other refinements which might be added, but which are too long for insertion here. By taking in all the sources of error of higher orders, it has been possible to calculate a pendulum so accurately that, when the clock is loaded with the weight sufficient to give the pendulum the arc of swing for which it is designed, a rate of error has been produced of only half a minute in a year. These refinements, however, are only required for clocks of precision; for ordinary clocks an invar pendulum with a lead bob and brass compensator is quite sufficient.
Invar pendulum rods are often made of steel with coefficients of expansion of about .0000012 linear per 1° C.; such a bob as this would require about 6.7 cm. of brass to compensate it, and, deducting 5 cm. of brass for the air compensation, this leaves about 1.7 cm. of positive compensation for the pendulum. But as has been said, the exact deduction depends on the shape and size of the bob, and the metal of which it is made. The diameters of the rods are 8 mm. for a 15 ℔ bob, 5 mm. for a 4 ℔ bob, and 12 to 15 mm. for a 60 ℔ bob. The bob is either a single cylinder or two cylinders with the rod between them. Lenticular and spherical bobs are not used. The great object is to allow the air ready access to all parts of the rod and compensator, so that they are all heated or cooled simultaneously. The bobs are usually made of a compound of lead, antimony, and tin, which forms a hard metal, free from bubbles and with a specific gravity of about 10. The usual weight of the bobs of the best pendulums for an ordinary astronomical clock is about 15 ℔. A greater weight than this is found liable to make the support of the pendulum rock and to put an undue strain on the parts, without any corresponding advantage. The rods used are all artificially aged, and have their heat expansion measured. No adjusting screw at the bottom is provided, the regulation being done by the addition of weights half way up the rod. An adjusting screw at the bottom has the disadvantage that it is impossible to know on which of the threads the rod is really resting; hence extra compensation may be introduced when not required. It is considered better that the supports of the bob should be rigid and invariable.
The effect of changes in the pressure of the air as shown by a barometer is too important to be omitted in the design of a good clock. But we do not propose to give more than a mere indication of the principles which govern compensation Barometrical error. for this effect, since the full discussion of the problem would be too protracted. We have seen that the action of the air in affecting the time of oscillation of a pendulum depends chiefly on the fact that its buoyancy makes the pendulum lighter, so that while the mass of the bob which has to be moved remains the same or nearly the same, the acceleration of gravity on it has less effect. A volume of air at ordinary temperature and pressure has, as has been said, .000103 the weight of an equal volume of type metal, whence it follows that the acceleration of gravity on a type metal bob in air is .999897 of the acceleration of gravity on the bob in vacuo. If, therefore, we diminish the value of g in the formula T = π√L/g by .000103, we shall have the difference of time of vibration of a type metal bob in air, as compared with its time in vacuo, and this, by virtue of the principle used when discussing the increase of time of oscillation due to increased pendulum lengths, is ½(.000103) second in one second, or about 4½ seconds in a day of 86,400 seconds. It follows that a barometric pressure of 30 in. causes a loss of 4½ seconds in the day, equivalent to .15 second per day for each inch of difference of the barometer. But, as has already been explained, the effect of the mass of the air transported with the pendulum must also be taken into account and therefore the above figures must be doubled or nearly doubled. A difference of 30 in. of barometric pressure would thus make a difference of 9 seconds per day in the rate of the pendulum, and the clock would lose about 1⁄3 of a second a day for each inch of rise of the barometer, the result being of the same magnitude as would be produced by a fall of temperature of 15° F. in the air. Either of these effects would require a shortening of the pendulum of 1⁄3000 in. This estimate is not far from the truth, for observations taken at various European observatories on various clocks, and collected by Jakob Hilfiker, give a mean of .15 second of retardation per day per centimetre of barometric pressure, or .37 second per day for each inch rise of the barometer.
In order to counteract variations in going which must thus obviously be produced by variations of barometrical pressure, attempts have been made purposely to disturb the isochronism of the pendulum, by making the arcs of vibration abnormally large. Again, the bob has been fitted with a piece of iron, which is subjected to the attraction of a piece of magnetized steel floating on the mercury in the open end of a barometer tube, so that when the barometer falls the attraction is increased and the pendulum retarded. Again, mercury barometers have been attached to pendulums. A simple method is to fix an aneroid barometer with about seven compartments on the pendulum about 5 to 6 in. below the suspension spring, 541 and to attach to the top of it a suitable weight which is lowered as the barometric pressure increases. One of the best methods of neutralizing the effects of variations of barometric pressure is to enclose the whole clock in an air-tight case, which may either be a large glass cylinder or a square case with a stout plate-glass front. This renders it independent of outside variations, whether of temperature or pressure, and keeps the density of the air inside the case uniform. If the case could be completely, or almost completely, exhausted of air, and kept so exhausted, of course the pendulum would experience the minimum of resistance and would have to be lengthened a little. But in practice it is impossible to secure the maintenance of a good vacuum without sealing up the case in such a way as to render repairs very difficult, and this plan is therefore rarely resorted to. What is usually done is to put the clock in a metal case covered with a thick sheet of plate glass bedded in india-rubber strips, and held down by an iron flanged lid or frame firmly fixed by means of small bolts. An air-pump is attached to the case, a turn-off tap being inserted, and by a few strokes the pressure of the air inside the case can be lowered to (say) 29 in., or a little below the usual barometric height at the place where the clock is. The difference of pressure being small, the tendency of air from outside to leak in is also small, and if the workmanship is good the inside pressure will remain unaltered for many days. In any case the difference produced by leakage will be small, and will not greatly affect the going of the clock. With care, and a daily or weekly touch of the pump, the pressure inside can be kept practically constant, and hence the atmospheric error will be eliminated. The cover has also incidentally the effect of keeping damp and fumes from the clock and thus preserving it from rust, especially if a vessel with quicklime or some hygroscopic material be put in the case.
Cases have considerable effect on the air, which moves with a pendulum and is flung off from it at each vibration; the going rate of a chronometer can be altered by removing the case. It is therefore desirable that cases enclosing pendulums should be roomy. Many people prefer to omit the air-tight case, and to keep a record of barometric, thermometric and hygrometric changes, applying corrections based on these to the times shown by the clock.
It was formerly usual to suspend pendulums by means of a single spring about ½ in. wide riveted with chops of metal. The upper chop had a pin driven through it, which rested in grooves so as to allow the pendulum to hang vertically. The Suspension of pendulums. best modern pendulums are now made with two parallel springs put a little less than an inch apart. The edges of the chops where the springs enter are slightly rounded so as to avoid too sharp bending of the springs. Suspension of pendulums on knife edges was tried by B. L. Vulliamy and others, but did not prove a success.
It was once thought that lenticular pendulum bobs resisted the air less than those of other shapes, but it was forgotten that their large surface offered more “skin friction.” They are now no longer used, nor are spheres on account of difficulty of construction. A cylinder is the best form of bob; it is sometimes rounded at the top and bottom.
Escapements.—The term escapement is applied to any arrangement by which, as the wheels rotate, periodic impulses are given to the pendulum, while at the same time the motion of the wheels is arrested until the vibration of the pendulum has been completed. It thus serves as a mechanism for both counting and impelling. Since the vibrations of a pendulum through small arcs are performed in times independent of the length of the arc, it follows that if a pendulum hanging at rest receive an impulse it will swing out and in again, and the time of its excursion outwards and of its return will remain the same whatever (within limits) be the arc of the swing, and whatever be the impulse given to it. If the impulse is big, it starts with a high velocity, but makes a larger excursion outwards, and the distance it has to travel counteracts its increase of speed, so that its time remains the same. Hence a pendulum, if free to swing outwards and in again, without impediment, will adapt the length of its swing to the impulse it has received, and any interference with it, as by the locking or unlocking of the escapement, will be far less deleterious to its isochronism when such interference occurs at the middle of its path rather than at the ends. It follows that the best escapement will be one which gives an impulse to the pendulum for a short period at the lowest point of its path, and then leaves it quite free to move as it chooses until the time comes for the next impulse.
But a pendulum is not quite truly isochronous, and has its time slightly affected by an increase of its arc; it is therefore desirable that the impulses given to it shall always be equal. If the escapement forms the termination of a clock-train impelled by a weight, the driving force of the escapement is apt to vary according to the friction of the wheels, while every change in temperature causes a difference in the thickness of the oil. It is therefore desirable, if possible, to secure uniformity of impulse—say, by causing the train of wheels to lift up a certain specified weight, and let it drop on the pendulum at regular intervals, or by some equivalent method.
The two requirements above stated have given rise respectively to what are known as detached escapements, and remontoires, which will be described presently. In the first place, however, it is desirable to describe the principal forms of escapement in ordinary use.
The balance escapement, which has been already mentioned, was in use before the days of pendulums. It was to a Balance escapement. balance escapement that Huygens applied the pendulum, by removing the weight from one arm and increasing the length of the other arm.
 |
| Fig. 8.—Anchor or Recoil Escapement. |
 |
| Fig. 9.—Dead Escapement. |
Very shortly afterwards R. Hooke invented the anchor or recoil escapement. This is represented in fig. 8, where a tooth of the escape-wheel is just escaping from the right pallet, and another tooth at the same time falls upon the left-hand pallet at Anchor escapement. some distance from its point. As the pendulum moves on in the same direction, the tooth slides farther up the pallet, thus producing a recoil, as in the crown-wheel escapement. The acting faces of the pallets should be convex. For when they are flat, and of course still more when they are concave, the points of the teeth always wear a hole in the pallets at the extremity of their usual swing, and the motion is obviously easier and therefore better when the pallets are made convex; in fact, they then approach more nearly to the “dead” escapement, which will be described presently. The effect of some escapements is not only to counteract the circular error, or the natural increase of the time of a pendulum as the arc increases, but to over-balance it by an error of the contrary kind. The recoil escapement does so; for it is almost invariably found that whatever may be the shape of these pallets, the clock loses as the arc of the pendulum falls off, and vice versa. It is unfortunately impossible so to arrange the pallets that the circular error may be thus exactly neutralized, because the escapement error depends, in a manner reducible to no law, upon variations in friction of the pallets themselves and of the clock train, which produce different effects; and the result is that it is impossible to obtain very accurate time-keeping from any clock of this construction. The point in which the anchor escapement was superior to all that had gone before, was that it would work well with a small arc of swing of the pendulum. The balance escapement, even when adapted to a pendulum, necessitated a swing of some 20°, and hence the circular error, that is to say, the deviation of the path from a true cycloid, was considerable. But with an anchor escapement the pendulum swing need be only 3° or 4°. On the other hand, it violates the conditions above laid down for a perfect escapement, inasmuch as the pendulum is never free, but at the end of its swing is still operated on by the escapement, which it causes to recoil.
To get rid of this defect the dead escapement, or, as the French call it, l’échappement à repos, was invented by G. Graham. It is represented in fig. 9. It will be observed that the teeth of the scape-wheel have their points set the opposite way Dead escapements. to those of the recoil escapement. The tooth B is here represented in the act of dropping on to the right-hand pallet as the 542 tooth A escapes from the left pallet. But instead of the pallet having a continuous face as in the recoil escapement, it is divided into two, of which BE on the right pallet, and FA on the left, are called the impulse faces, and BD, FG, the dead faces. The dead faces are portions of circles (not necessarily of the same circle), having the axis of the pallets C for their centre; and the consequence evidently is, that as the pendulum goes on, carrying the pallet still nearer to the wheel than the position in which a tooth falls on to the corner A or B of the impulse and the dead faces, the tooth still rests on the dead faces without any recoil, until the pendulum returns and lets the tooth slide down the impulse face, giving the impulse to the pendulum as it goes. In order to diminish the friction and the necessity for using oil as far as possible, the best clocks are made with jewels (sapphires are the best for the purpose) let into the pallets.
The pallets are generally made to embrace about one-third of the circumference of the wheel, and it is not at all desirable that they should embrace more; for the longer they are, the longer is the run of the teeth upon them, and the greater the friction. In some clocks the seconds hand moves very slowly and rests a very short time; this shows that the impulse is long in proportion to the arc of swing. In others the contrary is the case. A not uncommon proportion is that out of a total arc of swing of 3°, 2°, or about one degree on each side of the vertical, are occupied in receiving the impulse. In other words, the points F and A should subtend an angle of 2° at the centre C. It is not to be forgotten that the scape-wheel tooth does not overtake the face of the pallet immediately, on account of the moment of inertia of the wheel. The wheels of astronomical clocks, and indeed of all English house clocks, are generally made too heavy, especially the scape-wheel, which, by increasing the moment of inertia, causes a part of the work to be lost in giving blows, instead of being all used up in gentle pushes.
 |
| Fig. 10.—Pin-Wheel Escapement. |
A very useful form of the dead escapement, which is adopted in many of the best turret clocks, is called the “pin-wheel escapement.” Fig. 10 will sufficiently explain its action and construction. Its advantages are—that it does not require so much accuracy as the other; if a pin gets broken it is easily replaced, whereas in the other the wheel is ruined if the point of a tooth is injured; a wheel of given size will work with more pins than teeth, and therefore a train of less velocity will do, and that sometimes amounts to a saving of one wheel in the train, and a good deal of friction; and the blow on both pallets being downwards, instead of one up and the other down, the action is more steady; all which things are of more consequence in the heavy and rough work of a turret clock than in an astronomical one. It has been found expedient to make the dead faces not quite dead, but with a very slight recoil, which rather tends to check the variations of arc, and also the general disposition to lose time if the arc is increased; when so made the escapement is generally called “half-dead.”
In the dead escapement, during each excursion of the pendulum the repose surface of the pallets rubs against the points of the teeth of the scape-wheel. Thus the pendulum is subject to a constant retardation by friction. Curiously enough, this friction, which at first sight might appear a defect, is an advantage, and to a large extent accounts for the excellence of the escapement. For if the driving force of the clock is increased so that the impulse on the pallets is greater, the velocity of the pendulum is increased. But this very increase of the driving force causes a greater pressure of the teeth of the scape-wheel on the rest-faces of the pallets, and hence counteracts the increased drive of the pendulum by an increased frictional retardation. If the clock weight be enormously increased, the frictional retardation becomes increased relatively in a greater proportion than the drive, so that as the weight of the clock is increased the pendulum’s time of vibration is first diminished, until at last a neutral point is reached and finally the increased loading of the clock weight begins to make the time of vibration increase again. It is the neutral point which it is desirable to arrange for, and only trial and experience can so fit the shape and size of the pallets, scape-wheel and clock weight to one another, as to secure that a moderate variation of the driving power neither accelerates nor retards the motion of the pendulum, while at the same time such an arc of vibration is secured as shall be least subject to barometric error, and not have too great a circular error. The celebrated clockmaker B. L. Vulliamy (1780-1854) greatly improved Graham’s escapement by careful experiment, and other makers introduced further improvements into the shape of the scape-wheel and pallets, so that the best form of the deadbeat escapement is now fairly well determined and is given in books upon horology. For small clocks a little slope is given to the rest-faces so as to diminish the friction retardation. This is known as the half-dead escapement. The pin-wheel escapement, if properly constructed, is also “dead,” that is to say, the outward swing of the pendulum is unfettered except by the slight friction of the teeth against the dead faces of the pallets.
 |
| Fig. 11.—Riefler’s Escapement. |
In order to diminish the effect of the impact of the scape-wheel on the pallets, and of the crutch on the pendulum rod, the plan has been tried of making the crutch into an elastic spring. In theory this of course would not destroy the isochronism of the pendulum, for it would only be to apply upon the pendulum a force at right angles to the rod, and varying as the displacement. Hence any acceleration given by such a spring would, like the action of gravity, be harmonic, and it is an analytical principle that harmonic motions superposed on one another still remain harmonic. Hence, then, the action of a spring superadded upon the action of gravity on a pendulum still leaves the motion harmonic. But changes of temperature would affect the spring considerably. In the case of such a spring the repose faces of Graham’s escapement might be minimized and the escapement checked each side by a stop, so as to prevent the pallets from rubbing on the points of the scape-wheel. Graham’s escapement can, if well made, be arranged so as not to vary more than an average of 1⁄30 of a second from its mean daily rate, and this is so good a result that many people doubt whether further effort in the direction of inventing new escapements will result in any better form. Two adaptations of Graham’s escapement have been made, one by Clemens Riefler of Nesselwang, and the other by L. Strasser of Glashütte, Saxony, which give good results in practice. Riefler’s scheme is to mount the upper block, into which the suspension spring is fastened, upon knife edges, and rock it to and fro by the action of a modified Graham’s escapement, thus giving impulses to the pendulum. Fig. 11 shows the arrangement. PP are the agates upon which the knife edges CC rest. A is the anchor, RH the scape-wheels, and S the pallets.
Strasser’s clock is arranged on the same idea as that of Riefler, only that the rocking motion is given, not to the springs that carry the pendulum, but to a second pair of springs placed outside of them and parallel to them. The weight of the pendulum is therefore carried by an upper stationary block, but above that a second block is subjected to the rocking motion of the anchor. The general design is shown in fig. 12. The pallets are each formed of two stones, so contrived as to minimize the banging of the teeth of the scape-wheel. Both Riefler’s and Strasser’s clocks aim at haying a virtually free pendulum; in fact, they are in reality adaptations of the principle of the spring-clutch to Graham’s escapement. The weak point in both is the tampering with the suspension.
 |
| Fig. 12.—Strasser’s Escapement (Strasser & Rohde). |
The dead escapement is not, however, truly free. In order to make a free escapement it would be necessary to provide that as soon as the pendulum approached its centre position, some pin or projecting point upon it should free the Detached escapement. escapement wheel, a tooth of which should thus be enabled to leap upon the back of the pendulum, give it a short push, and then be locked until the pendulum had returned and again swung forward. An arrangement of this kind is shown in fig. 13. Let A be a block of metal fixed on the lower end of a pendulum rod. On the block let a small pall B be fastened, free to move round a 543 centre C and resting against a stop D. Let E be a 4-leaved scape-wheel, the teeth of which as they come round rest against the bent pall GFL at G. The pall is prevented from flying too far back by a pin H, and kept up to position by a very delicate spring K. As soon as the pendulum rod, moving from left to right, has arrived at the position shown in the figure, the pall B will engage the arm FL, force it forwards, and by raising G will liberate the scape-wheel, a tooth of which, M, will thus close upon the heel N of the block A, and urge it forward. As soon, however, as N has arrived at G the tooth M will slip off the block A and rest on the pall G, and the impulse will cease. The pendulum is now perfectly free or “detached,” and can swing on unimpeded as far as it chooses. On its return from right to left, the pall B slips over the pall L without disturbing it, and the pendulum is still free to make an excursion towards the left. On its return journey from left to right the process is again repeated. Such an escapement operates once every 2 seconds. One made on a somewhat similar plan was applied to a clock by Robert-Houdin, about 1830, and afterwards by Mr Haswell, and another by Sir George Airy. But the principle was already an old one, as may be seen from fig. 14, which was the work of an anonymous maker in the 18th century. A consideration of this escapement will show that it is only the application of the detached chronometer escapement to a clock.
| Fig. 13.—Free Escapement. | Fig. 14.—Free Escapement (old form). |
Even detached escapements, however, are not perfect. In order that an escapement should be perfect, the impulse given to the pendulum should be always exactly the same. It may be asked why, if the time of oscillation of the pendulum be independent of the amplitude of the arc of vibration, and hence of the impulse, it is necessary that the impulse should be uniform. The answer is that the arc of vibration not being a true cycloid, as it should be if true isochronism is to be secured, but being the arc of a circle, any change of amplitude of vibration produces a change of time in the swing given by the formula 3⁄2(a² - b²) = loss in seconds per day, where a and b are the semi-arcs of vibration estimated in degrees. Thus 10’ increase of arc in a swing of 4°, that is to say, .1 in. increase of arc in a total arc of 2½ in., produces an error of about a second a day. Now cold weather, by making the oil thick and thus clogging the wheels, will easily produce such a change of arc; dust will also make a change even though the clock weight, acted on by gravity, still exerts a uniform pull. Besides, if the clock has work to do of a varying amount—as when the hands of a turret clock are acted on by a heavy wind pressure tending sometimes to retard them, sometimes to drive them on—then it is clear that the impulses given by the scape-wheel to the pendulum may be very unequal, and that the arc of vibration of the pendulum may thus be seriously affected and its isochronism disturbed.
To abolish errors arising from the changes in the force driving the escapement, what is known as the “remontoire” system was adopted. It first came into use for watches, which was perhaps natural, seeing that the driving force of a watch Remontoire. is not a uniform weight like that of a clock, but depends on springs, which are far less trustworthy. The idea of a remontoire is to disconnect the escapement from the clock train, and to give the escapement a driving power of its own, acting as directly as possible on the pallets without the intervention of a clock-train containing many wheels. The escapement is thus as it were made into a separate clock, which of course needs repeated winding, and this winding is effected by the clock-train. From this it results that variations in the force transmitted by the clock-train merely affect the speed at which the “rewinding” of the escapement is effected, but do not affect the force exerted by the driving power of the escapement.
There are several modes of carrying out this plan. The first of them is simply to provide the scape-wheel with a weight or spring of its own, which spring is wound up by the clock-train as often as it runs down. Contrivances of this kind are Train remontoires. called train remontoires. In arranging such a remontoire it is obvious that the clock-train must be provided with a stop to prevent it from overwinding the scape-wheel weight or spring, and further, that there must be on the scape-wheel some sort of stud or other contrivance to release the clock-train as soon as the scape-wheel weight or spring has run down and needs rewinding. We believe the first maker of a large clock with a train remontoire was Thomas Reid of Edinburgh, who described his apparatus in his book on Horology (1819). The scape-wheel was driven by a small weight hung by a Huygens’s endless chain, of which one of the pulleys was fixed to the arbor, and the other rode upon the arbor, with the pinion attached to it, and the pinion was driven and the weight wound up by the wheel below (which we will call the third wheel), as follows. Assuming the scape-wheel to turn in a minute, its arbor has a notch cut half through it on opposite sides in two places near to each other; on the arbor of the wheel, which turns in ten minutes, suppose, there is another wheel with 20 spikes sticking out of its rim, but alternately in two different planes, so that one set of spikes can only pass through one of the notches in the scape-wheel arbor, and the other set only through the other. Whenever, then, the scape-wheel completes a half-turn, one spike is let go, and the third wheel is able to move, and with it the whole clock-train and the hands, until the next spike of the other set is stopped by the scape-wheel arbor; at the same time the pinion on that arbor is turned half round, winding up the remontoire weight, but without taking its pressure off the scape-wheel. Reid says that, so long as this apparatus was kept in good order, the clock went better than it did after it was removed in consequence of its getting out of order from the constant banging of the spikes against the arbor.
 |
| Fig. 15.—Gravity Train Remontoire. |
A clock at the Royal Exchange, London, was made in 1844 on the same principle, except that, instead of the endless chain, an internal wheel was used, with the spikes set on it externally, which is one of the modes by which an occasional secondary motion may be given to a wheel without disturbing its primary and regular motion. The following is a more simple arrangement of a gravity train remontoire, much more frequently used in principle. Let E in fig. 15 be the scape-wheel turning in a minute, and e its pinion, which is driven by the wheel D having a pinion d driven by the wheel C, which we may suppose to turn in an hour. The arbors of the scape-wheel and hour-wheel are distinct, their pivots meeting in a bush fixed somewhere between the wheels. The pivots of the wheel D are set in the frame AP, which rides on the arbors of the hour-wheel and scape-wheel, or on another short arbor between them. The hour-wheel also drives another wheel G, which again drives the pinion f on the arbor which carries the two arms fA, fB; and on the same arbor is set a fly with a ratchet, like a common striking fly, and the numbers of the teeth are so arranged that the fly will turn once for each turn of the scape-wheel. The ends of the remontoire arms fA, fB are capable of alternately passing the notches cut half through the arbor of the scape-wheel, as those notches successively come into the proper position at the end of every half-minute; as soon as that happens the hour-wheel raises the movable wheel D and its frame through a small angle; but, nevertheless, that wheel keeps pressing on the scape-wheel as if it were not moving, the point of contact of the wheel C and the pinion d being the fulcrum or centre of motion of the lever AdP. It will be observed that the remontoire arms fA, 544 fB have springs set on them to diminish the blow on the scape-wheel arbor, as it is desirable not to have the fly so large as to make the motion of the train, and consequently of the hands, too slow, to be distinct.
Another kind of remontoire is on the principle of one bevelled wheel lying between two others at right angles to it. The first of the bevelled wheels is driven by the train, and the third is fixed to the arbor of the scape-wheel; and the intermediate bevelled wheel, of any size, rides on its arbor at right angles to the other two arbors which are in the same line. The scape-wheel will evidently turn with the same average velocity as the first bevelled wheel, though the intermediate one may move up and down at intervals. The transverse arbor which carries it is let off and lifted a little at half-minute intervals, as in the remontoire just now described; and it gradually works down as the scape-wheel turns under its pressure, until it is freed again and lifted by the clock-train.
 |
| Fig. 16.—Spring Remontoire. |
In all these gravity remontoires, however, only the friction of the heavy parts of the train and the dial-work is got rid of, and the scape-wheel is still subject to the friction of the remontoire wheels, which, though much less than the other, is still something considerable. Accordingly, attempts have frequently been made to drive the scape-wheel by a spiral spring, like the mainspring of a watch. One of these was described in the 7th edition of this encyclopaedia; and Sir G. Airy invented another on the same principle, of which one specimen is still going well. One of the best forms of such a remontoire is shown in fig. 16, in which A, B, D, E, e, f are the same things as in fig. 15. But e, the scape-wheel pinion, is no longer fixed to the arbor, nor does it ride on the arbor, as had been the case in all the previous spring remontoires, thereby producing probably more friction than was saved in other respects; but it rides on a stud k, which is set in the clock frame. On the face of the pinion is a plate, of which the only use is to carry a pin h (and consequently its shape is immaterial), and in front of the plate is set a bush b, with a hole through it, of which half is occupied by the end of the stud k, to which the bush is fixed by a small pin, and the other half is the pivot-hole for the scape-wheel arbor. On the arbor is set the remontoire spring s (a moderate-sized musical-box spring is generally used), of which the outer end is bent into a loop to take hold of the pin h. In fact, there are two pins at h, one a little behind the other, to keep the coils of the spring from touching each other. Now, it is evident that the spring may be wound up half or a quarter of a turn at the proper intervals without taking the force off the scape-wheel, and also without affecting it by any friction whatever. When the scape-wheel turns in a minute, the letting-off would be done as before described, by a couple of notches in the scape-wheel arbor, through which the spikes A, B, as in fig. 15, would pass alternately. During the half-minute that the spring is running down the impulse on the pendulum constantly diminishes; but this error is small if the spring be properly shaped, and besides, being periodic, does not affect the average time-keeping of the clock. It would be inadmissible in astronomical clocks where each particular second has always to be true. In clocks with only three wheels in the train it is best to make the scape-wheel turn in two minutes. In that case four notches and four remontoire arms are required, and the fly makes only a quarter of a turn. Lord Grimthorpe made the following provision for diminishing the friction of the letting-off work. The fly pinion f has only half the number of teeth of the scape-wheel pinion, being a lantern pinion of 7 or 8, while the other is a leaved pinion of 14 or 16, and therefore the same wheel D will properly drive both, as will be seen hereafter. The scape-wheel arbor ends in a cylinder about 5⁄8 in. in diameter, with two notches at right angles cut in its face, one of them narrow and deep, and the other broad and shallow, so that a long and thin pin B can pass only through one, and a broad and short pin A through the other. Consequently, at each quarter of a turn of the scape-wheel, the remontoire fly, on which the pins A, B are set on springs, as in fig. 15, can turn half round. It is set on its arbor f by a square ratchet and click, which enables the spring to be adjusted to the requisite tension to obtain the proper vibration of the pendulum. A better construction, afterwards introduced, is to make the fly separate from the letting-off arms, whereby the blow on the cylinder is diminished, the fly being allowed to go on as in the gravity escapement. It should be observed, however, that even a spring remontoire requires a larger weight than the same clock without one; but as none of that additional force reaches the pendulum, that is of no consequence. The variation of force of the remontoire spring from temperature, as it only affects the pendulum through the medium of the dead escapement, is far too small to produce any appreciable effect; and it is found that clocks of this kind, with a compensated pendulum 8 ft. long, and weighing about 2 cwt., will not vary above a second a month, if the pallets are kept clean and well oiled. No turret clock without either a train remontoire or a gravity escapement will approach that degree of accuracy.
The introduction of this remontoire led to another very important alteration in the construction of large clocks. Hitherto it had always been considered necessary, with a view to diminish the friction as far as possible, to make the wheels of brass or gun-metal, with the teeth cut in an engine. The French clockmakers had begun to use cast iron striking parts, and cast iron wheels had been occasionally used in the going part of inferior clocks for the sake of cheapness; but they had never been used in any clock making pretensions to accuracy. But in consequence of the success of a clock shown in the 1851 Exhibition, it was determined by Sir G. Airy and Lord Grimthorpe (then E. Denison), who were jointly consulted by the Board of Works about the great Westminster clock in 1852, to alter the original requisition for gun-metal wheels there to cast iron. But cast iron wheels must drive cast iron pinions, for they will wear out steel.

| Fig. 17.—Mudge’s Gravity Escapement. | Fig. 18.—Bloxam’s Gravity Escapement. |
The next kind of remontoire still leaves the scape-wheel linked up with the clock-train, but makes it wind up the pallets which are held raised up till their action is wanted, when they are allowed to drop gently on the crutch or the pendulum rod. In this case the Gravity escapements. two arms of the anchor are usually divided and mounted on separate shafts so as to act independently. This idea was first started by Thomas Mudge (1717-1794) and Alexander Cumming (1733-1814). Mudge’s escapement is shown in fig. 17. The tooth A of the scape-wheel is resting against the stop or detent a at the end of the pallet CA, from the axis or arbor of which descends the half-fork CP to touch the pendulum. From the other pallet CB descends the other half-fork CO. The two arbors are set as near the point of suspension, or top of the pendulum spring, as possible. The pendulum, as here represented, must be moving to the right, and just leaving contact with the left pallet and going to take up the right one; as soon as it has raised that pallet a little it will evidently unlock the wheel and let it turn, and then the tooth B will raise the left pallet until it is caught by the stop b on that pallet, and then it will stay until the pendulum returns and releases it by raising that pallet still higher. Each pallet therefore descends with the pendulum to a lower point than that where it is taken up, and the difference between them is supplied by the lifting of each pallet by the clock, which does not act on the pendulum at all; so that the pendulum is independent of all variations of force and friction in the train. This escapement is said by Lord Grimthorpe, in his Rudimentary Treatise on Clocks, first published in 1850, to be liable to trip, the pallets being apt to be jerked by the pendulum, so that the teeth slip past the hook, and the wheel flies round. This, however, appears entirely a matter of construction. The really weak point is that while the impulses on the pendulum due to the gravitational fall of the arms are uniform, the force which has to be exercised by the pendulum in unlocking them from the scape-wheel varies with the pressure of the clock-train. Hence we miss the compensation which is so beautiful a result of Graham’s escapement. To avoid this, J. M. Bloxam, a barrister, proposed about the middle of the 19th century his legged gravity escapement (fig. 18). By this arrangement the parts of the scape-wheel which 545 lifted the gravity arms were brought as near to the axis of the scape-wheel as possible, while the locking arms were brought as far from the axis as possible so that the pressure should be light. The pallet arbors were cranked, to embrace the pendulum-spring, so that their centres of motion might coincide with that of the pendulum as nearly as possible—perhaps an unnecessary refinement; at least the three-legged and four-legged gravity escapements answer very well with the pallet arbors set on each side of the top of the spring. The size of the wheel determines the length of the pallets, as they must be at such an angle to each other that the radii of the wheel when in contact with each stop may be at right angles to the pallet arm; and therefore, for a wheel of this size, the depth of locking can only be very small. The pinion in Bloxam’s clock only raises the pallet through 40′ at each beat; i.e. the angle which we call γ, viz. the amplitude of the pendulum when it begins to lift the pallet, is only 20′; and probably, if it were increased to anything like a/√2, where a is the semiarc of swing, the escapement would trip immediately. The two broad pins marked E, F, are the fork-pins, and A and B are the stops. The clock which Bloxam had went very well; but it had an extremely fine train, with pinions of 18; and nobody else appears to have been able to make one to answer.
| Fig. 19.—Four-legged Gravity Escapement. | Fig. 20.—Double Three-legged Escapement. |
Bloxam’s escapement was modified in form by Lord Grimthorpe, his chief improvement being the addition of a fly vane, which, however, had previously been used for remontoires to steady the motion. He tried various modifications of construction, but finally adopted the “four-legged” and “double-three-legged” forms as being the most satisfactory, the former for regulators and the latter for large clocks. Fig. 19 is a back view of the escapement part of an astronomical clock with the four-legged wheel; seen from the front the wheel would turn the other way. The long locking teeth are made about 2 in. long from the centre, and the lifting pins, of which four point forwards while four other intermediate ones point backwards, are at not more than 1⁄30 of the distance between the centres EC, of the scape-wheel and pallets; or rather C is the top of the pendulum spring to which the pallets Cs, Cs′ converge, though the resultant of their action is a little below C. It is not worth while to crank them as Bloxam did, in order to make them coincide exactly with the top of the pendulum, as the friction of the beat pins on the pendulum is insignificant, and even then would not be quite destroyed. The pallets are not in the same plane, but one is behind and the other in front of the wheel, with one stop pointing backwards and the other forwards to receive the teeth alternately—it does not matter which; in this figure the stop s is behind and the stop s’ forward. The pendulum is now going to the right, and just beginning to lift the right pallet and free the stop s′; then the wheel will begin to turn and lift the other pallet by one of the pins which is now lowest, and which moves through 45° across the line of centres, and therefore lifts with very little friction. It goes on till the tooth now below s reaches s and is stopped there. Meanwhile the pallet Cs’ goes on with the pendulum as far as it may go, to the end of the arc which we have called α, starting from γ; but it falls with the pendulum again, not only to γ but to -γ on the other side of 0, so that the impulse is due to the weight of each pallet alternately falling through 2γ; and the magnitude of the impulse also depends on the obliqueness of the pallet on the whole, i.e. on the distance of its centre of gravity from the vertical through C. The fly KK′ is set on with a friction spring like the common striking-part fly, and should be as long as there is room for, length being much more effective than width.
The double three-legged gravity escapement, which was first used in the Westminster clock, is shown in fig. 20. The principle of it is the same as of the four-legs; but instead of the pallets being one behind and the other in front of the wheel, with two sets of lifting pins, there are two wheels ABC, abc, with the three lifting pins and the two pallets between them like a lantern pinion. One stop B points forward and the other A backward. The two wheels have their teeth set intermediately or 60° apart, though that is not essential, and the angle of 120° may be divided between them in any other proportions, as 70° and 50°, and in that way the pallets may be still more oblique than 30° from the vertical, which, however, is found enough to prevent tripping even if the fly gets loose, which is more likely to happen from carelessness in large clocks than in astronomical ones.
Of course the fly for those escapements in large clocks, with weights heavy enough to drive the hands in all weather, must be much larger than in small ones. For average church clocks with 1¼ sec. pendulum the legs of the scape-wheels are generally made 4 in. long and the fly from 6 to 7 in. long in each vane by 1¼ or 1½ wide. For 1½ sec. pendulums the scape-wheels are generally made 4½ radius. At Westminster they are 6 in.
Lord Grimthorpe considered that these escapements act better, especially in regulators, if the pallets do not fall quite on the lifting pins, but on a banking, or stop at any convenient place, so as to leave the wheel free at the moment of starting; just as the striking of a common house clock will sometimes fail to start unless the wheel with the pins has a little run before a pin begins to lift the hammer. The best way to manage the banking is to make the beat-pins long enough to reach a little way behind the pendulum, and let the banking be a thin plate of any metal screwed adjustably to the back of the case. This plate cannot well be shown in the drawings together with the pendulum, which, it may be added, should take up one pallet just when it leaves the other.
 |
| Fig. 21.—Chronometer Spring Remontoire. |
In chronometer spring remontoires the pendulum, as it goes by, flips a delicate spring and releases a small weight or spring which has been wound up in readiness by the action of the scape-wheel and which by leaping on to the pendulum gives it Chronometer spring remontoire. a push. One on this principle made about the middle of the 19th century by Robert Houdin is to be seen at the Conservatoire des Arts et Métiers. It is very complicated. The following is more simple. In fig. 21 a scape-wheel AB has 30 pins and 360 teeth. It is engaged with a fly vane EP mounted on a pinion of 12 teeth. Each pin as it passes raises an impulse arm CD which is hooked upon a detent K. A pall NM then engages the fly vane and prevents the scape-wheel from moving farther. The impulse arm being now set, as the plate F attached to the lower end of the pendulum flies past from left to right a pall G knocks aside the detent K, and allows a pin O projecting from the end of the impulse arm to fall upon an inclined pallet h, which is thus urged forward. As soon as the pallet has left the pin, the impulse arm in its further fall strikes N, which disengages the pall at P and allows the scape-wheel to move on and again wind up the impulse arm CD, which is then again locked by the detent K. On the return journey of the pendulum the light pall G, which acts the part of a chronometer spring, flips over the detent. The pallet is double sided, h and h′, so that if by chance the clock runs down while the pendulum swings from left to right the impulse arm will be simply raised and not smashed. It has a flat apex, on which the pin falls before descending. The impulse given depends on the weight of the impulse arm and may be varied at pleasure. The work done in unlocking the detent is invariable, as it depends on the pressure of the fly vane at P and is independent of the clock-train. The duration of the impulse is very short—only about 1⁄10 of the arc of swing. It is given exactly at the centre of the swing, and when not under impulse the pendulum is detached.
Clock Wheels.—Since, as we have seen, any increase in the arc of a pendulum is accompanied by a change in its going rate, 546 it is very desirable to keep the force which acts on the pendulum uniform. This in fact is the great object of the best escapements. Inasmuch as the impulse on the pendulum, derived from the work done by a falling weight or an unwinding spring, is transmitted through a train of wheels, it is desirable that that transmission should be as free from friction and as regular as possible. This involves care in the shaping of the teeth. The object to be aimed at is that as the wheel turns round the ratio of the power of the driver to that of the driven wheel (“runner” or “follower”) should never vary. That is to say, whether the back part of the tooth of the driver is acting on the tip of the tooth of the follower, or the tip of the driver is acting on the back part of the tooth of the follower, the leverage ratio shall always be uniform. For simplicity of manufacture the pinion wheels are always constructed with radial leaves, so that the surface of each tooth is a plane passing through the axis of the wheel. The semicircular rounding of the end of the tooth is merely ornamental. The question therefore is, suppose that it is desired by means of a tooth on a wheel to push a plane round an axis, what is the shape that must be given to that tooth in order that the leverage ratio may remain unaltered?

| Fig. 22.—Cam and Plane. | Fig. 23. |
If a curved surface, known as a “cam,” press upon a plane one, both being hinged or centred upon pivots A and B respectively (fig. 22), then the line of action and reaction at D, the point where they touch, will be perpendicular to their Epicycloidal teeth. surfaces at the point of contact—that is perpendicular to BD, and the ratio of leverage will obviously be AE:BD, or AC:CB. Hence to cause the leverage ratio of the cam to the plane always to remain unaltered, the cam must be so shaped that in any position the ratio AC:CB will remain unchanged. In other words the shape of the cam must be such that, as it moves and pushes BD before it, the normal at the point of contact must always pass through the fixed point C.
 |
| Fig. 24. |
If a circle PMB roll upon another circle SPT (fig. 23) any point M on it will generate an epicycloid MN. The radius of curvature of the curve at M will always be MP, for the part at M is being produced by rotation round the point P. It follows that a line from B to M will always be tangential to the epicycloid. If the epicycloid be a cam moving as a centre round the centre R (not shown in the figure) of the circle SPT, the leverage it will exert upon a plane surface BM moving round a parallel axis at B, will always be as BP to PR, that is, a constant; whence MN is the proper shape of a tooth to act on a pinion with radial arms and centred at B. In designing a pair of wheels to transmit motion, which is to be multiplied say 6 times in the transmission (about the usual ratio for clock wheels), if we take two circles (called the “pitch circles”) touching one another with radii as 1:6, then the circumference of the smaller will roll 6 times round that of the larger. The smaller wheel will have a number of teeth, say 8 to 16, each of them being sectors of the circle (fig. 24). If there are 16 teeth, then on the surface of the driving wheel there will be 96 teeth. Each of these teeth will be shaped as the curve of an epicycloid formed by the rolling on the big circle of a circle whose diameter is the radius of the pitch circle of the pinion. Points of the teeth so formed are cut off, so as to allow of the pinion having a solid core to support it, and gaps are made into the pitch circle to admit the rounded ends of the leaves of the pinon wheel. Thus a cog-wheel is shaped out.
Clock wheels are made of hard hammered brass cut out by a wheel cutting machine. This machine consists of a vertical spindle on the top of which the wheel to be cut is fixed on a firmly resisting plate of metal of slightly smaller diameter, so as to allow the wheel to overlap. A cutter with the edges most delicately ground to the exact shape of the gap between two teeth is caused to rotate 3000 - 4000 times a minute, and brought down upon the edge of the wheel. The shavings that come off are like fine dust, but the cutter is pushed on so as to plunge right through the rim of the wheel in a direction parallel to the axis. In this way one gap is cut. The vertical spindle is now rotated one division, by means of a dividing plate, and another tooth is cut, and so the operation goes on round the wheel.
It is not desirable in clocks that the pinion wheels which are driven should have too few teeth, for this throws all the work on a pair of surfaces before the centres and is apt to produce a grinding motion. Theoretically the more leaves a pinion has the better. Pinions can be made with leaves of thin steel watch-spring. In this case quite small pinions can have 20 leaves or more. The teeth in the driving wheels then become mere notches for which great accuracy of shape is not necessary. Such wheels are easy to make and run well. Lantern pinions are also excellent and are much used in American clocks. They are easy to make in an ordinary lathe. The cog-wheels must, however, be specially shaped to fit them. They consist of a number of round pins arranged in a circle round the axis of the wheel and parallel to it. The ends are secured in flanges like the wires of a squirrel cage. The teeth of cog-wheels engage them and thus drive the wheel round. They were much used at one time but are now falling out of favour again.
It is possible to make toothed wheels that drive with perfect uniformity by using for the curve of the teeth involutes of circles. These involutes are traced out by a point on a string that is gradually unwound from a circle. They are Involute teeth. in fact epicycloids traced by a rolling circle of infinite radius, i.e. a straight line. Involute teeth have the advantage that they roll on one another instead of sliding. When badly made they put considerable strain on the axes or shafts that carry them. Hence they have not been regarded with great favour by clockmakers.
By the pitch of a wheel is meant the number of teeth to the inch of circumference or diameter of the wheel; the former is called the circumferential pitch, the latter the diametral pitch. Thus if we say that a wheel has Pitch. 40 diametral pitch we mean that it has 40 teeth to each inch of diameter. The circumferential pitch is of course got by dividing the diametral pitch by π. Wheel-cutters are made for all sizes of pitches. If it were needed to make a pair of wheels the ratio of whose motion was say 6:1 and we determined to use a diametral pitch of 30 to the inch, that is teeth about 1⁄10 in. wide at the base, and if the smaller circle were to have 20 teeth, we should need a blank of a diameter of 20⁄30 + 2⁄30 = 22⁄30 in. for the smaller wheel, and one of 120⁄30 + 2⁄30 = 122⁄30 in. for the larger wheel which would have 120 teeth to the inch and be 4.06 in diameter to the tips of the teeth. The smaller toothed wheel would be .73 of an inch in diameter over all. The pitch circles of the wheels would be 2⁄3 and 4 in. respectively. For fine wheel work, where the driver is always much larger than the driven wheel, the epicycloidal tooth appears preferable, as it is generally considered to put less side strain on the pinion wheel. But the relative merits of the two systems have never been properly tested for clock work.
Going Barrels.—A clock which is capable of going accurately must have some contrivance to keep it going while it is being wound up. In the old-fashioned house clocks, which were wound up by merely pulling one of the strings, and in which one such winding served for both the going and striking parts, this was done by what is called the endless chain of Huygens, which consists of a string or chain with the ends joined together, and passing over two pulleys on the arbors of the great wheels, 547 with deep grooves and spikes in them, to prevent the chain from slipping. In one of the two loops or festoons which hang from the upper pulleys is a loose pulley without spikes, carrying the clock-weight, and in the other a small weight only heavy enough to keep the chain close to the upper pulleys. Now, suppose one of those pulleys to be on the arbor of the great wheel of the striking part, with a ratchet and click, and the other pulley fixed to the arbor of the great wheel of the going part; then (whenever the clock is not striking) the weight may be pulled up by pulling down that part of the string which hangs from the other side of the striking part; and yet the weight will be acting on the going part all the time. It would be just the same if the striking part and its pulley were wound up with a key, instead of the string being pulled, and also the same, if there were no striking part at all, but the second pulley were put on a blank arbor, except that in that case the weight would take twice as long to run down, supposing that the striking part generally requires the same weight × fall as the going part.
 |
| Fig. 25.—Harrison’s Going-Ratchet. |
This kind of going barrel, however, is evidently not suited to the delicacy of an astronomical clock; and Harrison’s going ratchet is now universally adopted in such clocks, and also in chronometers and watches for keeping the action of the train on the escapement during the winding. Fig. 25 (in which the same letters are used as in the corresponding parts of fig. 3) shows its construction. The click of the barrel-ratchet R is set upon another larger ratchet-wheel with its teeth pointing the opposite way, and its click rT is set in the clock frame. That ratchet is connected with the great wheel by a spring ss’ pressing against the two pins s in the ratchet and s’ in the wheel. When the weight is wound up (which is equivalent to taking it off), the click Tr prevents that ratchet from turning back or to the right; and as the spring ss’ is kept by the weight in a state of tension equivalent to the weight itself it will drive the wheel to the left for a short distance, when its end s is held fast, with the same force as if that end was pulled forward by the weight; and as the great wheel has to move very little during the short time the clock is winding, the spring will keep the clock going long enough.
In the commoner kind of turret clocks a more simple apparatus is used, which goes by the name of the bolt and shutter, because it consists of a weighted lever with a broad end, which shuts up the winding-hole. When it is lifted a spring-bolt attached to the lever, or its arbor, runs into the teeth of one of the wheels, and the weight of the lever keeps the train going until the bolt has run itself out of gear. Clocks are not always driven by weights. When accuracy is not necessary, but portability is desirable, springs are used. The old form of spring became weaker as it was unwound and necessitated the use of a device called a fusee or spiral drum. This apparatus will be found described in the article Watch.
Striking Mechanism.—There are two kinds of striking work used in clocks. The older of them, the locking-plate system, which is still used in most foreign clocks, and in turret clocks in England also, will not allow the striking of any hour to be either omitted or repeated, without making the next hour strike wrong; whereas in the rack system, which is used in all English house clocks, the number of blows to be struck depends merely on the position of a wheel attached to the going part, and therefore the striking of any hour may be omitted or repeated without deranging the following ones. We shall only describe the second of these, which is the more usual in modern timepieces.
Fig. 26 is a front view of a common English house clock with the face taken off, showing the repeating or rack striking movement. Here, as in fig. 3, M is the hour-wheel, on the pipe of which the minute-hand is set, N the reversed hour-wheel, and n its pinion, driving the 12-hour wheel H, on whose socket is fixed what is called the snail Y, which belongs to the striking work exclusively. The hammer is raised by the eight pins in the rim of the second wheel in the striking train, in the manner which is obvious.
 |
| Fig. 26.—Front view of common English House Clock. |
The hammer does not quite touch the bell, as it would jar in striking if it did, and prevent the full sound. The form of the hammer-shank at the arbor where the spring S acts upon it is such that the spring both drives the hammer against the bell when the tail T is raised, and also checks it just before it reaches the bell, the blow on the bell thus being given by the hammer having acquired momentum enough to go a little farther than its place of rest. Sometimes two springs are used, one for impelling the hammer, and the other for checking it. But nothing will check the chattering of a heavy hammer, except making it lean forward so as to act, partially at least, by its weight. The pinion of the striking-wheel generally has eight leaves, the same number as the pins; and as a clock strikes 78 blows in 12 hours, the great wheel will turn in that time if it has 78 teeth instead of 96, which the great wheel of the going part has for a centre pinion of eight. The striking-wheel drives the wheel above it once round for each blow, and that wheel drives a fourth (in which there is a single pin P), six, or any other integral number of turns, for one turn of its own, and that drives a fan-fly to moderate the velocity of the train by the resistance of the air, an expedient at least as old as De Vick’s clock in 1379.
The wheel N is so adjusted that, within a few minutes of the hour, the pin in it raises the lifting-piece LONF so far that that piece lifts the click C out of the teeth of the rack BKRV, which immediately falls back (helped by a spring near the bottom) as far as its tail V can go by reason of the snail Y, against which it falls; and it is so arranged that the number of teeth which pass the click is proportionate to the depth of the snail; and as there is one step in the snail for each hour, and it goes round with the hour-hand, the rack always drops just as many teeth as the 548 number of the hour to be struck. This drop makes the noise of “giving warning.” But the clock is not yet ready to strike till the lifting piece has fallen again; for, as soon as the rack was let off, the tail of the gathering pallet G, on the prolonged arbor of the third wheel, was enabled to pass the pin K of the rack on which it was pressing before, and the striking train began to move; but before the fourth wheel had got half round, its pin P was caught by the end of the lifting-piece, which is bent back and goes through a hole in the plate, and when raised stands in the way of the pin P, so that the train cannot go on till the lifting-piece drops, which it does exactly at the hour, by the pin N then slipping past it. Then the train is free; the striking wheel begins to lift the hammer, and the gathering pallet gathers up the rack, a tooth for each blow, until it has returned to the place at which the pallet is stopped by the pin K coming under it. In this figure the lifting-piece is prolonged to F, where there is a string hung to it, as this is the proper place for such a string when it is wanted for the purpose of learning the hour in the dark, and not (as it is generally put) on the click C; for if it is put there and the string is held a little too long, the clock will strike too many; and if the string accidentally sticks in the case, it will go on striking till it is run down—neither of which things can happen when the string is put on the lifting-piece.
The snail is sometimes set on a separate stud with the apparatus called a star-wheel and jumper. On the left side of the frame we have placed a lever x, with the letters st below it, and si above. If it is pushed up to si, the other end will come against a pin in the rack, and prevent it from falling, and will thus make the clock silent; and this is much more simple than the old-fashioned “strike and silent” apparatus, which we shall therefore not describe, especially as it is seldom used now.
If the clock is required to strike quarters, a third “part” or train of wheels is added on the right hand of the going part; and its general construction is the same as the hour-striking part; only there are two more bells, and two hammers so placed that one is raised a little after the other. If there are more quarter-bells than two, the hammers are generally raised by a chime-barrel, which is merely a cylinder set on the arbor of the striking-wheel (in that case generally the third in the train), with short pins stuck into it in the proper places to raise the hammers in the order required for the tune of the chimes. The quarters are usually made to let off the hour, and this connexion may be made in two ways. If the chimes are different in tune for each quarter, and not merely the same tune repeated two, three and four times, the repetition movement must not be used for them, as it would throw the tunes into confusion, but the old locking-plate movement, as in turret clocks; and therefore, if we conceive the hour lifting-piece connected with the quarter locking-plate, as it is with the wheel N, in fig. 26, it is evident that the pin will discharge the hour striking part as the fourth quarter finishes.
But where the repetition movement is required for the quarters, the matter is not quite so simple. The principle of it may shortly be described thus. The quarters themselves have a rack and snail, &c., just like the hours, except that the snail is fixed on one of the hour-wheels M or N, instead of on the twelve-hour wheel, and has only four steps in it. Now suppose the quarter-rack to be so placed that when it falls for the fourth quarter (its greatest drop), it falls against the hour lifting-piece somewhere between O and N, so as to raise it and the click C. Then the pin Q will be caught by the click Qq, and so the lifting-piece will remain up until all the teeth of the quarter-rack are gathered up; and as that is done, it may be made to disengage the click Qq, and so complete the letting off the hour striking part. This click Qq has no existence except where there are quarters.
The method in which an alarum is struck may be understood by reference to either of the recoil escapements (figs. 1 and 7). If a short hammer instead of a long pendulum be attached to the axis of the pallets, and the wheel be driven with sufficient force, it will evidently swing the hammer rapidly backwards and forwards; and the position and length of the hammer-head may be so adjusted as to strike a bell inside, first on one side and then on the other. As to the mode of letting off the alarum at the time required: if it was always to be let off at the same time all that would be necessary would be to set a pin in the twelve-hour wheel at the proper place to raise the lifting-piece which lets off the alarum at that time. But as the time must be capable of alteration, this discharging pin must be set in another wheel (without teeth), which rides with a friction-spring on the socket of the twelve-hour wheel, with a small movable dial attached to it, having figures so arranged with reference to the pin that whatever figure is made to come to a small pointer set as a tail to the hour hand, the alarum shall be let off at that hour.
The watchman’s or tell-tale clock, used when it is desired to make sure of a watchman being on the spot and awake all the night, is a clock with a set of spikes, generally 48 or 96, sticking out all round the dial, and a handle somewhere in the case, by pulling which one of the spikes which is opposite to it, or to some lever connected with it is pressed in. This wheel of spikes is carried round with the hour-hand, which in these clocks is generally a twenty-four hour one. It is evident that every spike which is seen still sticking out in the morning indicates that at the particular time to which that spike belongs the watchman was not there to push it in—or at any rate, that he did not. At some other part of their circuit, the inner ends of the pins are carried over a roller or an inclined plane which pushes them out again ready for business the next night. The time at which workmen arrive at their work may be recorded by providing each of them with a numbered key with which he stamps his number on a moving tape, on which also the time is marked by a clock.
Church and Turret Clocks.—Seeing that a clock—at least the going part of it—is a machine in which the only work to be done is the overcoming of its own friction and the resistance of the air, it is evident that when the friction and resistance are much increased it may become necessary to resort to expedients for neutralizing their effects, which are not required in a smaller machine with less friction. In a turret clock the friction is enormously increased by the great weight of all the parts; and the resistance of the wind, and sometimes snow, to the motion of the hands, further aggravates the difficulty of maintaining a constant force on the pendulum; and besides that, there is the exposure of the clock to the dirt and dust which are always found in towers, and of the oil to a temperature which nearly or quite freezes it all through the usual cold of winter. This last circumstance alone will generally make the arc of the pendulum at least half a degree more in summer than in winter; and inasmuch as the time is materially affected by the force which arrives at the pendulum, as well as the friction on the pallets when it does arrive there, it is evidently impossible for any turret clock of the ordinary construction, especially with large dials, to keep any constant rate through the various changes of temperature, weather and dirt to which it is exposed. Hence special precautions, such as the use of remontoires and gravity escapements, have to be observed in the design of large clocks that have any pretensions to accuracy, in order to ensure that the arc of the pendulum is not affected by external circumstances, such as wind-pressure on the hands or dirt in the wheel-train. But such have been the improvements effected in electric clocks, that rather than go to the trouble and expense required by such precautions, it appears far preferable to keep an accurate time-piece in some sheltered position and use it with a source of electricity to drive the hands of the large dial.
Electrical Clocks.—One of the first attempts to apply electricity to clocks was made by Alexander Bain in 1840-1850. About the same time Sir C. Wheatstone, R. L. Jones, C. Shepherd, Paul Garnier and Louis Bréguet invented various forms of electrical time-keepers. It is not proposed here to go into the history of these abortive attempts. Those who desire to follow them may consult Bain, An Account of Some Applications of the Electric Fluid to the Useful Arts (1843) and Short History of Electric Clocks (1852); Sir Charles Wheatstone, Trade Circular of the British Telegraph Manufactory; C. Shepherd, On the Application of Electro-magnetism as a Motor for Clocks (1851), and a list of 549 references in the Appendix to Tobler’s Die electrischen Uhren (Leipzig, 1883), and a list of books given by F. Hope Jones, Proc. Inst. Elec. Eng., 1900, vol. 29. The history of electrical clocks is a long and complicated matter, for there are some 600 or 700 patents for these clocks in Europe and America, some containing the germs of valuable ideas but most pure rubbish. All that can be done is to select one or two prominent types of each class and give a brief description of their general construction.
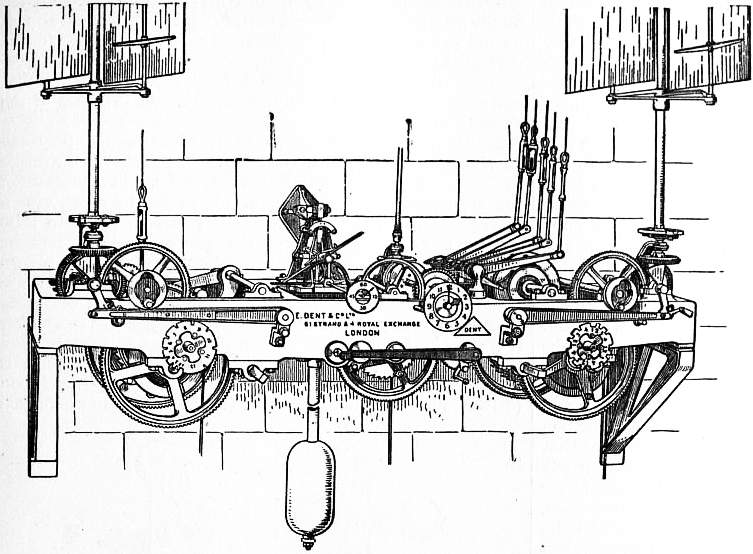 |
| Fig. 27.—Turret Clock for Hidalgo, Mexico, driving four 8 ft. dials. |
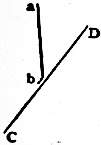 |
| Fig. 28. |
It is in the apparently simple matter of making and keeping the electrical contact that most of the systems of electrical time-keeping have failed, for want of attention to the essential conditions of the problem. In practice every metal is covered with a thin film of non-conducting oxide over which is another film of moisture, oil, dirt or air. Hence what is wanted is a good vigorous push of a blunted point or edge preferably obliquely upon a more or less yielding surface so as to get a rubbing action. Thus if the stiff spring a b (fig. 28) were stabbed down on the oblique surface C D a good contact would invariably result, provided that the metals employed were gold, platinum or some not easily oxidizable metal. Or again, if a mercury surface be simply touched with a pin, the slight sparking that is produced on making the current will soon form a little pile of dirty oxide at the point of entry, and the contact will frequently fail. If it be necessary to have a mercury contact, the pin must be well driven in below the surface of the mercury or else swept through it as an oar is swept through the water. Another form of electrical contact that acts well is a knife edge brought into contact with a series of fine elastic strips of metal laid parallel to one another like the fingers of a hand. The best metal for contacts, if they are to bear hard usage, is either silver or gold or a mixture of 40% iridium with 60% of platinum. A pressure of some 15 grammes, at least, is needful to secure a good contact.
As to the source of current for driving electrical clocks, if Leclanché cells be used they should preferably be kept in the open air under cover so as not to dry up. If direct electric current is available from electric light mains or the accumulators used for lighting a private house, so much the better. Of course the pressure of 50 or 100 volts used for lighting would be far too great for clock-driving, where only the pressure of a few volts is required. But it is easy by the insertion of suitable resistances, as for instance one or more incandescent lamps, to weaken down the pressure of the lighting system and make it available for electric clocks, bells or other similar purposes.
Electricity is applied to clocks in three main ways:—(1) in actuating timepieces which measure their own time and must therefore be provided with pendulums or balance wheels; (2) in reproducing on one or more dials the movements of the hands of a master clock, by the aid of electric impulses sent at regular intervals, say of a minute or a half-minute; and (3) in synchronizing ordinary clocks by occasional impulses sent from some accurate regulator at a distance.
Electrically driven timepieces may be divided under two heads:—(a) those in which the electric current drives either the pendulum or some lever which operates upon it, which lever or pendulum in turn drives the clock hands; and (b) those timepieces which are driven by a weight or spring which is periodically wound up by electricity—in fact electrical remontoires.
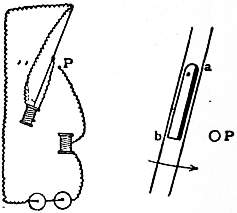 |
| Fig. 29.—Electrical Clock (faulty design). |
The simplest clock of the first character that could be imagined would be constructed by fastening an electromagnet with a soft iron core to the bottom of a pendulum, and causing it to be attracted as the pendulum swings by another electromagnet fixed vertically under it (fig. 29). As the pendulum approached the vertical and was say half an inch from its lowest point, the current would be switched on, and switched off as soon as the pendulum got to its lowest point. A very small attraction with this arrangement, probably about a grain weight, acting through the ½ in. would drive a heavy pendulum. A switch would have to be worked in connexion with the pendulum. A strip of ebonite with a small face of metal on the end of one side, such as a b (fig. 29) might be pivoted at one end on the pendulum with a weak spring to keep it where free along the rod. As the pendulum swung by this would be swept on its journey from left to right against a fixed pin P. This would complete the electric circuit down through the pendulum rod, round the coil on the bottom of the pendulum, through the switch into the pin P, thence through the fixed electromagnet, and so back to the battery. On the return journey no contact would be made because only the ebonite face of the switch would touch P. The pendulum would thus receive an impulse every other vibration. We have described this switch, not to advocate it, but to warn against its use. For the contact would be quite insufficient. In order that the switch might not unduly retard the pendulum it must be light, but this would make the pressure on P too light to be trustworthy. Moreover, the strength of the impulse would vary with the strength of the battery, and hence the arc would be repeatedly uneven.
 |
| Fig. 30.—Hipp Electrical Clock (Peyer, Favarger et Cie.). |
In contrast with this, let us consider a clock that is now giving excellent results at the Observatory of Neuchatel in Switzerland on Hipp’s system (La Pendule électrique de précision, Neuchatel, 1884 and 1891). The pendulum (fig. 30) consists of two rods of steel joined by four bridges, one just below the suspension spring, the next about 12 in. lower, the next about half way down, and the last supporting a glass vessel of mercury which forms the bob. On the third of them is placed an iron armature, 550 which works between the poles of an electromagnet fixed to the case, and by which the pendulum is actuated. The circuit is closed and broken by a flipper, which is swayed to and fro by a block fixed to the pendulum at the second bridge. As long as the flipper is merely swayed, no contact takes place, but when the arc of vibration of the pendulum is diminished the flipper does not clear the block but is caught by a nick in it, and forced downwards. In this way the circuit is closed. Fig. 31 is a diagram of the apparatus. When the block g attached to the pendulum catches and presses down the flipper s, the lever l l is rocked over, so that a contact is made at k, and the current which enters the lever l through the knife edge m, runs through the second lever n n, down through the knife edge o, to the battery, and through the electromagnet b which causes the armature a to be attracted. As the block g goes on and releases s, the lever l again falls upon the rest p, the lever n follows it a part of the way till it is stopped by the contact q; this shortcircuits the electromagnet and prevents to a large extent the formation of an induced current. It is claimed that sparking is by this method almost entirely avoided. It is only when s is caught in the notch of the block g that s is pressed down, so that the electric attraction only takes place every few vibrations. This ingenious arrangement makes the working of the clock nearly independent of the strength of the battery, for if the battery is strong the impulses are fewer and the average arc remains the same. The clock is enclosed in an airtight glass case so as to avoid barometric error. It was tested in 1905 at the Neuchâtel observatory. In winter in a room of a mean temperature of 35° F. it was ¼ sec. too slow, in summer when the temperature was 70°, it was ½ sec. too fast. In the succeeding winter it became .53 sec. too slow again, thus gaining a little in summer and losing in winter. Its average variation from its daily rate was, however, only .033 sec.
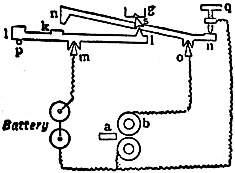 |
| Fig. 31.—Contact Arrangement of Hipp Clock. |
In another system originated by G. Froment, a small weight is raised by electricity and allowed to fall upon an arm sticking out at right angles to the pendulum in the plane of its motion, so as to urge it onwards. The weight is only allowed to rest on the arm during the downward swing of the pendulum. The method is not theoretically good, as the impulse is given at the end of the vibration of the pendulum instead of at its middle position.
In the clock invented by C. Féry (chef des travaux pratiques at the École de Physique et Chimie, Paris), an electric impulse is given at every vibration, not by a battery but by means of the uniform movement of an armature which is alternately pulled away from and pushed towards a permanent horseshoe magnet. Currents are thus induced in a bobbin of fine wire placed between the poles of the horseshoe magnet. The movements of the armature are produced by another horseshoe magnet actuated by the primary current from a battery which is turned on and off by the swinging of the pendulum. The energy of the induced current that drives the clock depends solely on the total movement of the armature, and is independent of whether that movement be executed slowly or rapidly, and therefore of the strength of the battery.
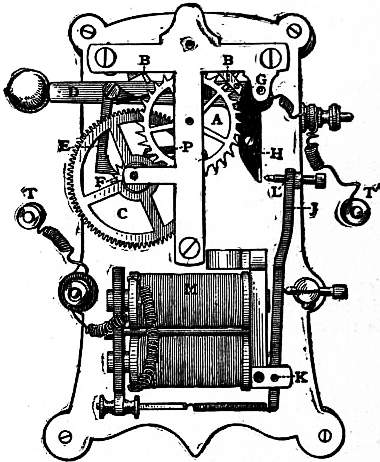 |
| Fig. 32.—Hope Jones Electrical Remontoire. |
Electrical remontoires possess great advantages if they can be made to operate with certainty. For they can be made to wind up a scape-wheel just as is done in the case of the arrangement shown in fig. 16 so as to constitute a spring remontoire, or better still they can be made to raise a weight as in the case of the gravity train remontoire (fig. 15) but without the complications of wheel-work shown in that contrivance. Of this type one of the best known is that of H. Chesters Pond. A mainspring fixed on the arbor of the hour wheel is wound up every hour by means of another toothed wheel riding loose on the same arbor and driven by a small dynamo, to which the other end of the mainspring is attached. As soon as the hour wheel has made one revolution (driven round by the spring), a contact switch is closed whereupon the dynamo winds up the spring again exactly as the train and fly wind up the spring in fig. 15. These clocks require a good deal of power, and not being always trustworthy seem to have gone out of use. A contrivance of this kind now in use is that patented by F. Hope Jones and G.B. Bowell, and is represented in fig. 32. A pendulum is driven by the scape-wheel A, and pallets B B in the usual way. The scape-wheel is driven by another wheel C which, in turn, is driven by the weighted lever D supported by click E engaging the ratchet wheel F. This lever is centred at G and has an extension H at right angles to it. J is an armature of soft iron pivoted at K and worked by the electromagnet M. D gradually falls in the act of driving the clock by turning the wheels C and A until the contact plate on the arm H meets with the contact screw L at the end of the armature J, thus completing the electrical circuit from terminal T to terminal T′ through the electromagnet M, and through any number of step-by-step dial movements which may be included in the same series circuit. The armature is then drawn towards the magnet with rapid acceleration, carrying the lever D with it. The armature is suddenly arrested by the poles of the magnet, but the momentum of the lever D carries it farther, and the click E engages another tooth of the ratchet F. A quick break of the circuit is thus secured, and the contact at L is a good one, first because the whole of the energy required to keep the clock going, or in other words the energy required to raise the lever D is 551 mechanically transmitted through its surfaces at each operation, and secondly, owing to the arrangement of the fulcrums at G and K which secure a rubbing contact. The duration of the contact is just that necessary to accomplish the work which has to be done, and it is remarkable that when used to operate large circuits of electrically propelled dials the duration accommodates itself to their exact requirements and the varying conditions of battery and self-induction. The ratchet wheel F is usually mounted loosely upon its arbor and is connected to the wheel C by means of a spiral spring, which in conjunction with the back-stop click P maintains the turning force on the wheelwork at the instant when the lever D is being raised.
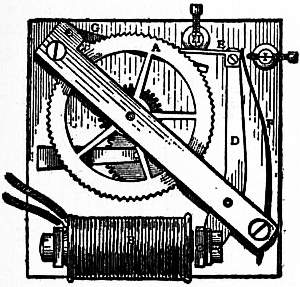 |
| Fig. 33.—Hope Jones’s Dial-driving Device. |
Electrically driven dials usually consist of a ratchet wheel driven by an electrically moved pall. Care has to be taken that the pushes of the pall do not cause the ratchet wheel to be impelled too far. The anchor escapement of a common grandfather’s clock can be made to drive the works by means of an electromagnet, the pendulum being removed. With a common anchor escapement the scape-wheel can be driven round by wagging the anchor to and fro. All then that is necessary is to fix a piece of iron on the anchor so that its weight pulls the anchor over one way, while an electromagnet pulls the iron the other. Impulses sent through the electromagnet will then drive the clock. If the clock is wound up in the ordinary way the motion will be so much helped that the electric current has very little to do, and thus may be very feeble. Fig. 33 shows the dial-driving device of Hope Jones’s clock. Each time that a current is sent by the master-clock, the electromagnet B attracts the pivoted armature C, and when the current ceases the lever D with the projecting arm E is driven back to its old position by the spring F, thus driving the wheel A forward one division. G is a back-stop click, and H, I, fixed stops.
It seems doubtful whether in large towns a number of dials could be electrically driven from a distance because of the large amount of power that would have to be transmitted. But for large buildings, such as hotels, they are excellent. One master-clock in the cellar will drive a hundred or so placed over the building. The master-clock may itself be driven by electricity, but it will require the services from time to time of some one to correct the time. Even this labour may be avoided if the master-clock is synchronized, and as synchronization requires but a small expenditure of force, it can be done over large areas. Hence the future of the clock seems to be a series of master-clocks, electrically driven, and synchronized one with another, in various parts of a city, from each of which a number of dials in the vicinity are driven. Electrical synchronization was worked out by Louis Bréguet and others, and a successful system was perfected in England by J.A. Lund. The leading principle of the best systems is at each hour to cause a pair of fingers or some equivalent device to close upon the minute hand and put it exactly to the hour. Other systems are designed to retard or to accelerate the pendulum, but the former appears the more practical method. There is probably a future before synchronization which will enable the services of a clockmaker to be largely dispensed with and relegate his work merely to keeping the instruments in repair.
Miscellaneous Clocks.—Some small clocks are made to go for a year. They have a heavy balance wheel of brass weighing about 2 1b and about 2½ in. in diameter, suspended from a point above its centre by a fine watch spring about 4 in. long. The crutch engages with the upper part of the spring, and as the balance wheel swings the pallets are actuated. The whole clock is but a large watch with a suspended balance wheel, oscillating once in about 8 seconds. Unless the suspension spring be compensated for temperature, such clocks gain very much in winter.
An ingenious method of driving a clock by water has been proposed. As the pendulum oscillates to one side, an arm on it rises and at last lightly touches a drop of water hanging from a very fine nozzle; this drop is taken off and carried away by the arm, to be subsequently removed by adhesion to an escape funnel placed below the arm. Hence at each double vibration of the pendulum part of the work done by a drop of water falling through a short distance is communicated to the pendulum, which is thus kept in motion as long as the water lasts. At this rate a gallon of water ought to drive the clock for 40 hours. Care of course must be taken to keep the water in the reservoir at a constant level, so that the drops formed shall be uniform.
If it were worth while, no doubt the oscillations of a pendulum working in a vacuum could be maintained by the communication and discharge at each oscillation of a slight charge of electricity; or again, heat might at each oscillation be communicated to a thermo-electric junction, and the resulting current used to drive the pendulum.
The expansions and contractions of metal rods under the influence of the changes of temperature which take place in the course of each night and day have also been employed to keep a clock wound up, and if there were any need for it no doubt a small windmill rotating at the top of a tower would easily keep a turret clock fully wound, by a simple arrangement which would gear the going barrel of the clock to the wind vane motion, whenever the weight had fallen too low, and release it when the winding up was completed. Even a smoke jack would do the same office for a kitchen clock.
The methods of driving astronomical telescopes by means of clockwork will be found in the article Telescope. Measurements of small intervals of time are performed by means of chronographs which in principle depend on the use of isochronous vibrating tuning-forks in place of pendulums. In practice it is needful in most cases that an observer should intervene in time measurements, although perhaps by means of a revolving photographic film a transit of the sun might be timed with extraordinary accuracy. But if the transit of a star across a wire is to be observed, there is no mode at present in use of doing so except by the use of the human eye, brain and hand. Hence in all such observations there is an element of personal error. Unfortunately we cannot apply a microscope to time as we can to space and make the cycle of events that takes place in a second last say for five minutes so as to time them truly. By personal observations the divisions of a second cannot in general be made more accurately than to 1⁄10 or 1⁄15 of a second. The most rapid music player does not strike a note more than 10 or 12 times in a second. It is only in case of recurring phenomena that we can make personal observations more accurate than this by taking the mean of a large number of observations, and allowing for personal error. For the purpose of determining longitude at sea accuracy to 1⁄30 of a second of time would find the place to about 20 yards. It seems to follow that the extent to which astronomical clocks can be made accurate, viz. to 1⁄30 of a second average variation from their mean daily rate, or one two-and-a-half millionth of 24 hours, is a degree of accuracy sufficient for present purposes, and it seems rather doubtful whether mechanical science will in the case of clocks be likely to reach a much higher figure.
In the 17th century it was a favourite device to make a clock show sidereal time as well as mean solar time. The length of the sidereal day is to the mean solar day as .99727 to 1, and various attempts have been made by trains of wheels to obtain this relation—but all are somewhat complicated.
Magical clocks are of several kinds. One that was in vogue about 1880 had a bronze figure on the top with outstretched arm holding in its hand the upper part of the spring of a pendulum, 552 about 10 in. long. The pendulum had apparently no escapement and the puzzle was how it was maintained in motion. It was impossible to detect the mystery by the aid of the eye alone; the truth, however, was that the whole figure swung to and fro at each oscillation of the pendulum, to an amount of 1⁄400 of an inch on the outside rim of the base. A movement of 1⁄400 of an inch per half second of time is imperceptible; it would be equivalent to perception of motion of the minute hand of a clock about 6 in. in diameter, which is almost impossible. The connexion of the figure to the anchor of the escapement was very complicated, but clocks of the kind kept fair time. A straw, poised near the end on a needle and with the short end united by a thread to the bronze figure, makes the motion apparent at once and discloses the trick. Another magical clock consists of two disks of thin sheet glass mounted one close behind the other, one carrying the minute hand and the other the hour hand. The disks rest on rollers which rotate and turn them round. The front and back of the movable disks are covered by other disks of glass surrounded by a frame, so that the whole looks simply like a single sheet of glass mounted in a frame, in the centre of which the hands rotate, without any visible connexion with the works of the clock.
Clocks have been made with a sort of balance wheel consisting of a thread with a ball at the end which winds backwards and forwards spirally round a rod. In others a swing or see-saw is attached to the pendulum, or a ship under canvas is made to oscillate in a heavy sea. In others the time is measured by the fall of a ball down an inclined plane, the time of fall being given by the formula t = √(2s/g sin a), where s is the length of the incline and a the inclination. But friction so modifies the result as to render experiment the only mode of adjusting such a clock. Sometimes a clock is made to serve as its own weight, as for instance when a clock shaped like a monkey is allowed to slide down a rope wound round the going barrel. Or the clock is made of a cylindrical shape outside and provided with a weighted arm instead of a going barrel; on being put upon an incline, it rolls down, and the fall supplies the motive power.
Clocks are frequently provided with chimes moved exactly like musical boxes, except that the pins in the barrel, instead of flipping musical combs, raise hammers which fall upon bells. The driving barrel is let off at suitable intervals. The cuckoo clock is a pretty piece of mechanism. By the push of a wire given to the body of the bird, it is bent forward, the wings and tail are raised and the beak opened. At the same time two weighted bellows measuring about 1 × 2 in. are raised and successively let drop. These are attached to small wooden organ pipes, one tuned a fifth above the other, which produce the notes. Phonographs are also attached to clocks, by which the hours are called instead of rung.
Clocks are also constructed with conical pendulums. It is a property of the conical pendulum that if swung round, the time of one complete revolution is the same as that of the double vibration of a pendulum equal in length to the vertical distance of the bob of the conical pendulum below its point of support. It follows that if the driving force of such a pendulum can be kept constant (as it easily can by an electric contact which is made at every revolution during which it falls below a certain point) the clock will keep time; or friction can be introduced so as to reduce the speed whenever the pendulum flies round too fast and hence the bob rises. Or again by suitable arrangements the bob may be made to move in certain curves so as to be isochronous. Plans of this kind are employed rather to drive telescopes, phonographs and other machines requiring uniform and steady movement.
Comical and performing clocks were very popular in the 15th and 16th centuries. One at Basel in Switzerland was arranged so as gradually to protrude a long tongue as the pendulum vibrated. It is still to be seen there in the museum. The famous clock at Strassburg, originally constructed in 1574, remade in 1842, displays a whole series of scenes, including processions of the apostles and other persons, and a cock that crows. A fine clock at Venice has two rather stiff bronze giants that strike the hours.
Clocks with complicated movements representing the positions of the heavenly bodies and the days of the week and month, allowance being made for leap year, were once the delight of the curious. Repeating clocks, which sounded the hours when a string was pulled, were once popular. The string simply raised the lifting piece and let the clock strike as the hands would do when they came to the hour. This was of use in the old days when the only mode of striking a light at night was with a flint and steel, but lucifer matches and the electric light have rendered these clocks obsolete.
 |
| Fig. 34.—Curve of Variation of daily rate. |
Testing Clocks.—The average amount by which a clock gains or loses is called its mean or average daily rate. A large daily rate of error is no proof that a clock is a bad one, for it might be completely removed by pendulum adjustment. What is required is that the daily rate shall be uniform, that is, that the clock shall not be gaining (or losing) more on one day than on another, or at one period of the same day than at another. In fig. 34 A B is a curve in which the abscissae represent intervals of time, the ordinates the number of seconds at any time by which the clock is wrong. The curve C D is one in which the ordinates are proportional to the tangents of the angles of inclination of the curve A B to the axis of x, that is dy/dx. Whenever the line A B is horizontal, C D cuts the axis of x. In a clock having no variation in its daily rate the curve A B would become a straight line, though it might be inclined to the axis of x, and C D, also a straight line, would be parallel to the axis of x, though it might not coincide with it. In a clock set to exact time and having no variations of daily rate, both the curves would be straight lines and would coincide with the axis of x. The curve C D, known as the curve of variation of daily rate, will generally be found to follow changes of day and night, and of temperature, and the fluctuations of the barometer and hygrometer; it is the curve which reveals the true character of the clock. Hence in testing a clock two things have to be determined: first, the daily rate of error, and second, the average variations from that daily rate, in other words the irregularities of going. To test a clock well six months’ or a year’s trial is needed, and it is desirable to have it subjected to considerable changes of temperature.
Decorative Aspects.—In art the clock occupies a position of considerable distinction, and antique examples are prized and collected as much for the decorative qualities of their cases as for the excellence of their time-keeping. French and English cabinet-makers have especially excelled, although in entirely different ways, in the making of clock cases. The one aimed at comely utility, often made actually beautiful by fit proportion and the employment of finely grained woods; the other sought a bold and dazzling splendour in which ornament overlay material. It was not in either country until the latter part of the 17th century that the cabinet-maker’s opportunity came. The bracket or chamber clock gave comparatively little scope to the worker in wood—in its earlier period, indeed, it was almost invariably encased in brass or other metal; and it was not until the introduction of the long pendulum swinging in a small space that it became customary to encase clocks in decorative woodwork. The long or “grandfather” clock dates from about the fourth quarter of the 17th century—what is, perhaps, the earliest surviving English dated specimen is inscribed with the date 1681. Originally it was a development 553 of the dome-shaped bracket clock, and in the older examples the characteristic dome or canopy is preserved. The first time-keepers of this type had oaken cases—indeed oak was never entirely abandoned; but when walnut began to come into favour a few years later that beautifully marked wood was almost invariably used for the choicest and most costly specimens. Thus in 1698 the dean and chapter of St Paul’s cathedral paid the then very substantial price of £14 for an inlaid walnut long-cased eight-day clock to stand in one of the vestries. The rapidity with which the new style came into use is suggested by the fact that while very few long clocks can be certainly dated before 1690, between that year and the end of the century there are many examples. Throughout the 18th century they were made in myriads all over England, and since they were a prized possession it is not surprising that innumerable examples have survived. Vary as they may in height and girth, in wood and dial, they are all essentially alike. In their earlier years their faces were usually of brass engraved with cherubs’ heads or conventional designs, but eventually the less rich white face grew common. There are two varieties—the eight-day and the thirty-hour. The latter is but little esteemed, notwithstanding that it is often as decorative as the more expensive clock. The favourite walnut case of the late 17th and early 18th century gave place in the course of a generation to mahogany, which retained its primacy until the introduction of cheaper clocks brought about the supersession of the long-cased variety. Many of these cases were made in lacquer when that material was in vogue; satinwood and other costly foreign timbers were also used for bandings and inlay. The most elegant of the “grandfather” cases are, however, the narrow-waisted forms of the William and Mary period in walnut inlay, the head framed in twisted pilasters. Long clocks of the old type are still made in small numbers and at high prices; they usually contain chimes. During the later period of their popularity the heads of long clocks were often filled in with painted disks representing the moon, by which its course could be followed. Such conceits as ships moving on waves or time with wings were also in favour. The northern parts of France likewise produced tall clocks, usually in oaken cases; those with Louis Quinze shaped panels are often very decorative. French love of applied ornament was, however, generally inimical to the rather uncompromising squareness of the English case, and the great Louis Quinze and Louis Seize cabinetmakers made some magnificent and monumental clocks, many of which were “long” only as regards the case, the pendulum being comparatively short, while sometimes the case acted merely as a pedestal for a bracket-clock fixed on the top. These pieces were usually mounted very elaborately in gilt bronze, cast and chased, and French bracket and chamber clocks were usually of gilded metal or marble, or a combination of the two; this essentially late 18th-century type still persists. English bracket clocks contemporary with them were most frequently of simple square or arched form in mahogany. The “grandfather” case was also made in the Low Countries, of generous height, very swelling and bulbous.
CLODIA, VIA, an ancient high-road of Italy. Its course, for the first 11 m., was the same as that of the Via Cassia; it then diverged to the N.N.W. and ran on the W. side of the Lacus Sabatinus, past Forum Clodii and Blera. At Forum Cassii it may have rejoined the Via Cassia, and it seems to have taken the same line as the latter as far as Florentia (Florence). But beyond Florentia, between Luca (Lucca) and Luna, we find another Forum Clodii, and the Antonine Itinerary gives the route from Luca to Rome as being by the Via Clodia—wrongly as regards the portion from Florentia southwards, but perhaps rightly as regards that from Luca to Florentia. In that case the Clodius whose name the road bears, possibly C. Clodius Vestalis (c. 43 B.C.), was responsible for the construction of the first portion and of that from Florentia to Luca (and Luna), and the founder of the two Fora Clodii. The name seems, in imperial times, to have to some extent driven out that of the Cassia, and both roads were administered, with other minor roads, by the same curator.
CLODIUS,1 PUBLIUS (c. 93-52 B.C.), surnamed Pulcher, Roman politician. He took part in the third Mithradatic war under his brother-in-law Lucius Licinius Lucullus, but considering himself treated with insufficient respect, he stirred up a revolt; another brother-in-law, Q. Marcius Rex, governor of Cilicia, gave him the command of his fleet, but he was captured by pirates. On his release he repaired to Syria, where he nearly lost his life during a mutiny instigated by himself. Returning to Rome in 65, he prosecuted Catiline for extortion, but was bribed by him to procure acquittal. There seems no reason to believe that Clodius was implicated in the Catilinarian conspiracy; indeed, according to Plutarch (Cicero, 29), he rendered Cicero every assistance and acted as one of his body-guard. The affair of the mysteries of the Bona Dea, however, caused a breach between Clodius and Cicero in December 62. Clodius, dressed as a woman (men were not admitted to the mysteries), entered the house of Caesar, where the mysteries were being celebrated, in order to carry on an intrigue with Caesar’s wife. He was detected and brought to trial, but escaped condemnation by bribing the jury. Cicero’s violent attacks on this occasion inspired Clodius with the desire for revenge. On his return from Sicily (where he had been quaestor in 61) he renounced his patrician rank, and, having with the connivance of Caesar been adopted by a certain P. Fonteius, was elected tribune of the people (10th of December 59). His first act was to bring forward certain laws calculated to secure him the popular favour. Corn, instead of being sold at a low rate, was to be distributed gratuitously once a month; the right of taking the omens on a fixed day and (if they were declared unfavourable) of preventing the assembly of the comitia, possessed by every magistrate by the terms of the Lex Aelia Fufia, was abolished; the old clubs or gilds of workmen were re-established; the censors were forbidden to exclude any citizen from the senate or inflict any punishment upon him unless he had been publicly accused and condemned. He then contrived to get rid of Cicero (q.v.) and the younger Cato (q.v.), who was sent to Cyprus as praetor to take possession of the island and the royal treasures. Cicero’s property was confiscated by order of Clodius, his house on the Palatine burned down, and its site put up to auction. It was purchased by Clodius himself, who, not wishing to appear in the matter, put up some one to bid for him. After the departure of Caesar for Gaul, Clodius became practically master of Rome with the aid of armed ruffians and a system of secret societies. In 57 one of the tribunes proposed the recall of Cicero, and Clodius resorted to force to prevent the passing of the decree, but was foiled by Titus Annius Milo (q.v.), who brought up an armed band sufficiently strong to hold him in check. Clodius subsequently attacked the workmen who were rebuilding Cicero’s house at the public cost, assaulted Cicero himself in the street, and set fire to the house of Q. Cicero. In 56, when curule aedile, he impeached Milo for public violence (de vi), when defending his house against the attacks of Clodius, and also charged him with keeping armed bands in his service. Judicial proceedings were hindered by outbreaks of disturbance, and the matter was finally dropped. In 53, when Milo was a candidate for the consulship, and Clodius for the praetorship, the rivals collected armed bands and fights took place in the streets of Rome, and on the 20th of January 52 Clodius was slain near Bovillae.
His sister, Clodia, wife of Q. Caecilius Metellus Celer, was notorious for her numerous love affairs. It is now generally admitted that she was the Lesbia of Catullus (Teuffel-Schwabe, Hist. of Roman Lit., Eng. tr., 214, 3). For her intrigue with M. Caelius Rufus, whom she afterwards pursued with unrelenting 554 hatred and accused of attempting to poison her, see Cicero, Pro Caelio, where she is represented as a woman of abandoned character.
1 It is suggested (W. M. Lindsay, The Latin Language, p. 41) that he changed his name Claudius into the plebeian form Clodius, in order to gain the favour of the mob.
CLOGHER, a market village of Co. Tyrone, Ireland, in the south parliamentary division, on the Clogher Valley light railway. Pop. (1901) 225. It gives name to dioceses of the Church of Ireland and the Roman Catholic Church, but the seat of the Roman Catholic bishop is at Monaghan, with the cathedral. The Protestant cathedral, dedicated to St Macartin, dates from the 18th and early 19th century, but St Macartin (c. 500) was a disciple of St Patrick, and it is said that St Patrick himself founded a bishopric here. The name is derived from the Irish cloch, a pillar stone, such as were worshipped and regarded as oracles in many parts of pagan Ireland; the stone was preserved as late as the 15th century in the cathedral, and identity is even now claimed for a stone which lies near the church.
CLOISTER (Lat. claustrum; Fr. cloître; Ital. chiostro; Span. claustro; Ger. Kloster). The word “cloister,” though now restricted to the four-sided enclosure, surrounded with covered ambulatories, usually attached to coventual and cathedral churches, and sometimes to colleges, or by a still further limitation to the ambulatories themselves, originally signified the entire monastery. In this sense it is of frequent occurrence in earlier English literature (e.g. Shakespeare, Meas. for Meas. i. 3, “This day my sister should the cloister enter”), and is still employed in poetry. The Latin claustrum, as its derivation implies, primarily denoted no more than the enclosing wall of a religious house, and then came to be used for the whole building enclosed within the wall. To this sense the German “Kloster” is still limited, the covered walks, or cloister in the modern sense, being called “Klostergang,” or “Kreuzgang.” In French the word cloître retains the double sense.
In the special sense now most common, the word “cloister” denotes the quadrilateral area in a monastery or college of canons, round which the principal buildings are ranged, and which is usually provided with a covered way or ambulatory running all round, and affording a means of communication between the various centres of the ecclesiastical life, without exposure to the weather. According to the Benedictine arrangement, which from its suitability to the requirements of monastic life was generally adopted in the West, one side of the cloister was formed by the church, the refectory occupying the side opposite to it, that the worshippers might have the least annoyance from the noise or smell of the repasts. On the eastern side the chapter-house was placed, with other apartments belonging to the common life of the brethren adjacent to it, and, as a common rule, the dormitory occupied the whole of the upper story. On the opposite or western side were generally the cellarer’s lodgings, with the cellars and store-houses, in which the provisions necessary for the sustenance of the confraternity were housed. In Cistercian monasteries the western side was usually occupied by the “domus conversorum,” or lodgings of the lay-brethren, with their day-rooms and workshops below, and dormitory above. The cloister, with its surrounding buildings, generally stood on the south side of the church, to secure as much sunshine as possible. A very early example of this disposition is seen in the plan of the monastery of St Gall (see Abbey, fig. 3). Local requirements, in some instances, caused the cloister to be placed to the north of the church. This is the case in the English cathedrals, formerly Benedictine abbeys, of Canterbury, Gloucester and Chester, as well as in that of Lincoln. Other examples of the northward situation are at Tintern, Buildwas and Sherborne. Although the covered ambulatories are absolutely essential to the completeness of a monastic cloister, a chief object of which was to enable the inmates to pass from one part of the monastery to another without inconvenience from rain, wind, or sun, it appears that they were sometimes wanting. The cloister at St Albans seems to have been deficient in ambulatories till the abbacy of Robert of Gorham, 1151-1166, when the eastern walk was erected. This, as was often the case with the earliest ambulatories, was of wood covered with a sloping roof or “penthouse.” We learn from Osbern’s account of the conflagration of the monastery of Christ Church, Canterbury, 1067, that a cloister with covered ways existed at that time, affording communication between the church, the dormitory and the refectory. We learn from an early drawing of the monastery of Canterbury that this cloister was formed by an arcade of Norman arches supported on shafts, and covered by a shed roof. A fragment of an arcaded cloister of this pattern is still found on the eastern side of the infirmary-cloister of the same foundation. This earlier form of cloister has been generally superseded in England by a range of windows, usually unglazed, but sometimes, as at Gloucester, provided with glass, lighting a vaulted ambulatory, of which the cloisters of Westminster Abbey, Salisbury and Norwich are typical examples. The older design was preserved in the South, where “the cloister is never a window, or anything in the least approaching to it in design, but a range of small elegant pillars, sometimes single, sometimes coupled, and supporting arches of a light and elegant design, all the features being of a character suited to the place where they are used, and to that only” (Fergusson, Hist. of Arch. i. p. 610). As examples of this description of cloister, we may refer to the exquisite cloisters of St John Lateran, and St Paul’s without the walls, at Rome, where the coupled shafts and arches are richly ornamented with ribbons of mosaic, and those of the convent of St Scholastica at Subiaco, all of the 13th century, and to the beautiful cloisters at Arles, in southern France; those of Aix, Fontfroide, Elne, &c., are of the same type; as also the Romanesque cloisters at Zürich, where the design suffers from the deep abacus having only a single slender shaft to support it, and at Laach, where the quadrangle occupies the place of the “atrium” of the early basilicas at the west end, as at St Clement’s at Rome, and St Ambrose at Milan. Spain also presents some magnificent cloisters of both types, of which that of the royal convent of Huelgas, near Burgos, of the arcaded form, is, according to Fergusson, “unrivalled for beauty both of detail and design, and is perhaps unsurpassed by anything in its age and style in any part of Europe.” Few cloisters are more beautiful than those of Monreale and Cefalu in Sicily, where the arrangement is the same, of slender columns in pairs with capitals of elaborate foliage supporting pointed arches of great elegance of form.
All other cloisters are surpassed in dimensions and in sumptuousness of decoration by the “Campo Santo” at Pisa. This magnificent cloister consists of four ambulatories as wide and lofty as the nave of a church, erected in 1278 by Giovanni Pisano round a cemetery composed of soil brought from Palestine by Archbishop Lanfranchi in the middle of the 12th century. The window-openings are semicircular, filled with elaborate tracery in the latter half of the 15th century. The inner walls are covered with frescoes invaluable in the history of art by Orcagna, Simone Memmi, Buffalmacco, Benozzo Gozzoli, and other early painters of the Florentine school. The ambulatories now serve as a museum of sculpture. The internal dimensions are 415 ft. 6. in. in length, 137 ft. 10 in. in breadth, while each ambulatory is 34 ft. 6. in. wide by 46 ft. high.
The cloister of a religious house was the scene of a large part of the life of the inmates of a monastery. It was the place of education for the younger members, and of study for the elders. A canon of the Roman council held under Eugenius II., in 826, enjoins the erection of a cloister as an essential portion of an ecclesiastical establishment for the better discipline and instruction of the clerks. Peter of Blois (Serm. 25) describes schools for the novices as being in the west walk, and moral lectures delivered in that next the church. At Canterbury the monks’ 555 school was in the western ambulatory, and it was in the same walk that the novices were taught at Durham (Willis, Monastic Buildings of Canterbury, p. 44; Rites of Durham, p. 71). The other alleys, especially that next the church, were devoted to the studies of the elder monks. The constitutions of Hildemar and Dunstan enact that between the services of the church the brethren should sit in the cloister and read theology. For this purpose small studies, known as “carrols,” i.e. a ring or enclosed space, were often found in the recesses of the windows. Of this arrangement there are examples at Gloucester, Chester and elsewhere. The use of these studies is thus described in the Rites of Durham:—“In every wyndowe” in the north alley “were iii pewes or carrells, where every one of the olde monkes had his carrell severally by himselfe, that when they had dyned they dyd resorte to that place of cloister, and there studyed upon their books, every one in his carrell all the afternonne unto evensong tyme. This was there exercise every daie.” On the opposite wall were cupboards full of books for the use of the students in the carrols. The cloister arrangements at Canterbury were similar to those just described. New studies were made by Prior De Estria in 1317, and Prior Selling (1472-1494) glazed the south alley for the use of the studious brethren, and constructed “the new framed contrivances, of late styled carrols” (Willis, Mon. Buildings, p. 45). The cloisters were used not for study only but also for recreation. The constitutions of Archbishop Lanfranc, sect. 3, permitted the brethren to converse together there at certain hours of the day. To maintain necessary discipline a special officer was appointed under the title of prior claustri. The cloister was always furnished with a stone bench running along the side. It was also provided with a lavatory, usually adjacent to the refectory, but sometimes standing in the central area, termed the cloister-garth, as at Durham. The cloister-garth was used as a place of sepulture, as well as the surrounding alleys. The cloister was in some few instances of two stories, as at Old St Paul’s, and St Stephen’s chapel, Westminster, and occasionally, as at Wells, Chichester and Hereford, had only three alleys, there being no ambulatory under the church wall.
The larger monastic establishments had more than one cloister; there was usually a second connected with the infirmary, of which there are examples at Westminster Abbey and at Canterbury; and sometimes one giving access to the kitchen and other domestic offices.
The cloister was not an appendage of monastic houses exclusively. It was also attached to colleges of secular canons, as at the cathedrals of Lincoln, Salisbury, Wells, Hereford and Chichester, and formerly at St Paul’s and Exeter. It is, however, absent at York, Lichfield, Beverley, Ripon, Southwell and Wimborne. A cloister forms an essential part of the colleges of Eton and Winchester, and of New College and Magdalen at Oxford, and was designed by Wolsey at Christ Church. These were used for religious processions and lectures, for ambulatories for the studious at all times, and for places of exercise for the inmates generally in wet weather, as well as in some instances for sepulture.
For the arrangements of the Carthusian cloisters, as well as for some account of those appended to the monasteries of the East, see Abbey.
CLONAKILTY, a seaport and market town of Co. Cork, Ireland, in the south parliamentary division, at the head of Clonakilty Bay, 33 m. S.W. of Cork on a branch of the Cork, Bandon & South Coast railway. Pop. of urban district (1901), 3098. It was brought into prosperity by Richard Boyle, first earl of Cork, and was granted a charter in 1613; but was partly demolished on the occasion of a fight between the English and Irish in 1641. It returned two members to the Irish parliament until the union. In the 18th century there was an extensive linen industry. The present trade is centred in brewing, corn-milling, yarn and farm-produce. The harbour-mouth is obstructed by a bar, and there is a pier for large vessels at Ring, a mile below the town. The fisheries are of importance. A ruined church on the island of Inchdorey, and castles on Galley Head, at Dunnycove, and at Dunowen, together with a stone circle, are the principal antiquities in the neighbourhood.
CLONES, a market town of Co. Monaghan, Ireland, in the north parliamentary division, 64½ m. S.W. by W. from Belfast, and 93½ m. N.W. from Dublin by the Great Northern railway, on which system it is an important junction, the lines from Dublin, from Belfast, from Londonderry and Enniskillen, and from Cavan converging here. Pop. of urban district (1901), 2068. The town has a considerable agricultural trade, and there are corn mills and manufactures of agricultural implements. A former lace-making industry is extinct. The market-place, called the Diamond, occupies the summit of the slight elevation on which the town is situated. Clones was the seat of an abbey founded in the 6th century by St Tighernach (Tierney), to whom the Protestant parish church is dedicated. Remains of the abbey include a nave and tower of the 12th century, and a curious shrine formed out of a great block of red sandstone. Other antiquities are a round tower of rude masonry, 75 ft. high but lacking the cap; a rath, or encampment, and an ancient market cross in the Diamond.
CLONMACNOISE, one of the most noteworthy of the numerous early religious settlements in Ireland, on the river Shannon, in King’s county, 9 m. S. of Athlone. An abbey was founded here by St Kieran in 541, which as a seat of learning gained a European fame, receiving offerings, for example, from Charles the Great, whose companion Alcuin the scholar received part of his education from the great teacher Colcu at Conmacnoise. Several books of annals were compiled here, and the foundation became the seat of a bishopric, but it was plundered and wasted by the English in 1552, and in 1568 the diocese was united with that of Meath. The most remarkable literary monument of Clonmacnoise is the Book of the Dun Cow, written about 1100, still preserved (but in an imperfect form) by the Royal Irish Academy, and containing a large number of romances. It is a copy of a much earlier original, which was written on the skin of a favourite cow of St Kieran, whence the name of the work. The full title of the foundation is the “Seven Churches of Clonmacnoise,” and remains of all these are extant. The Great Church, though rebuilt by a chief named McDermot, in the 14th century, retains earlier remains in a fine west doorway; the other churches are those of Fineen, Conor, St Kieran, Kelly, Melaghlin and Dowling. There are two round towers; O’Rourke’s, lacking the roof, but occupying a commanding situation on rising ground, is dated by Petrie from the early 10th century, and stands 62 ft. in height; and McCarthy’s, attached to Fineen’s church, which is more perfect, but rather shorter, and presents the unusual feature of a doorway level with the ground, instead of several feet above it as is customary. There are three crosses, of which the Great Cross, made of a single stone and 15 ft. in height, is splendidly carved, with tracery and inscriptions. It faces the door of the Great Church, and is of the same date. A large number of inscribed stones dating from the 9th century and after are preserved in the churches. There are further remains of the Castle and Episcopal palace, a fortified building of the 14th century, and of a nunnery of the 12th century. In the neighbourhood are seen striking examples of the glacial phenomenon of eskers, or gravel ridges.
CLONMEL, a municipal borough and the county town of Co. Tipperary, Ireland, in the east parliamentary division, 112 m. S.W. from Dublin on a branch from Thurles of the Great Southern & Western railway, which makes a junction here with the Waterford and Limerick line of the same company. Pop. (1901) 10,167. Clonmel is built on both sides of the Suir, and also occupies Moore and Long Islands, which are connected with the mainland by three bridges. The principal buildings are the parish church, two Roman Catholic churches, a Franciscan friary, two convents, an endowed school dating from 1685, and the various county buildings. The beauty of the environs, and especially of the river, deserves mention; and their charm is enhanced by the neighbouring Galtee, Knockmealdown and other mountains, among which Slievenaman (2364 ft.) is conspicuous. A woollen manufacture was established in 1667, and was extensively carried on until the close of the 18th century. The 556 town contains breweries, flour-mills and tanneries, and has a considerable export trade in grain, cattle, butter and provisions. It stands at the head of navigation for barges on the Suir. It was the centre of a system, established by Charles Bianconi (1786-1875) in 1815 and subsequently, for the conveyance of travellers on light cars, extending over a great part of Leinster, Munster and Connaught. It is governed by a mayor and corporation, which, though retained under the Local Government (Ireland) Act of 1898, has practically the status of an urban district council. By the same act a part of the town formerly situated in county Waterford was added to county Tipperary. It was a parliamentary borough, returning one member, until 1885; having returned two members to the Irish parliament until the union.
The name, Cluain mealla, signifies the Vale of Honey. In 1269 the place was chosen as the seat of a Franciscan friary by Otho de Grandison, the first English possessor of the district; and it frequently comes into notice in the following centuries. In 1641 it declared for the Roman Catholic party, and in 1650 it was gallantly defended by Hugh O’Neill against the English under Cromwell. Compelled at last to capitulate, it was completely dismantled, and was never again fortified. Remains of the wall are seen in the churchyard, and the West Gate still stands in the main street.
CLOOTS, JEAN BAPTISTE DU VAL DE GRÂCE, Baron von (1755-1794), better known as Anacharsis Cloots, a noteworthy figure in the French Revolution, was born near Cleves, at the castle of Gnadenthal. He belonged to a noble Prussian family of Dutch origin. The young Cloots, heir to a great fortune, was sent at eleven years of age to Paris to complete his education. There he imbibed the theories of his uncle the Abbé Cornelius de Pauw (1739-1799), philosopher, geographer and diplomatist at the court of Frederick the Great. His father placed him in the military academy at Berlin, but he left it at the age of twenty and traversed Europe, preaching his revolutionary philosophy as an apostle, and spending his money as a man of pleasure. On the breaking out of the Revolution he returned in 1789 to Paris, thinking the opportunity favourable for establishing his dream of a universal family of nations. On the 19th of June 1790 he appeared at the bar of the Assembly at the head of thirty-six foreigners; and, in the name of this “embassy of the human race,” declared that the world adhered to the Declaration of the Rights of Man and of the Citizen. After this he was known as “the orator of the human race,” by which title he called himself, dropping that of baron, and substituting for his baptismal names the pseudonym of Anacharsis, from the famous philosophical romance of the Abbé Jean Jacques Barthélemy. In 1792 he placed 12,000 livres at the disposal of the Republic—“for the arming of forty or fifty fighters in the sacred cause of man against tyrants.” The 10th of August impelled him to a still higher flight; he declared himself the personal enemy of Jesus Christ, and abjured all revealed religions. In the same month he had the rights of citizenship conferred on him; and, having in September been elected a member of the Convention, he voted the king’s death in the name of the human race, and was an active partisan of the war of propaganda. Excluded at the instance of Robespierre from the Jacobin Club, he was soon afterwards implicated in an accusation levelled against the Hébertists. His innocence was manifest, but he was condemned, and guillotined on the 24th of March 1794.
Cloots’ main works are: La Certitude des preuves du mahométisme (London, 1780), published under the pseudonym of Ali-Gur-Ber, in answer to Bergier’s Certitude des preuves du christianisme; L’Orateur du genre humain, ou Dépêches du Prussien Cloots au Prussien Herzberg (Paris, 1791), and La République universelle (1792).
CLOQUET, a city of Carlton county, Minnesota, U.S.A., on the St Louis river, 28 m. W. by S. of Duluth. Pop. (1890) 2530; (1900) 3072; (1905, state census) 6117, of whom 2755 were foreign-born (716 Swedes, 689 Finns, 685 Canadians, 334 Norwegians); (1910) 7031. Cloquet is served by the Northern Pacific, the Great Northern, the Duluth & North-Eastern, and (for freight only) the Chicago, Milwaukee & St Paul railways. The river furnishes good water-power, and the city has various manufactures, including lumber, paper, wood pulp, match blocks and boxes. The first mill was built in 1878, and the village was named from the French word claquet (sound of the mill). Cloquet was incorporated as a village in 1883 and was chartered as a city in 1903.
CLOSE, MAXWELL HENRY (1822-1903), Irish geologist, was born in Dublin in 1822. He was educated at Weymouth and at Trinity College, Dublin, where he graduated in 1846; and two years later he entered holy orders. For a year he was curate of All Saints, Northampton; from 1849 to 1857 he was rector of Shangton in Leicestershire; and then for four years he was curate of Waltham-on-the-Wolds. In 1861, on the death of his father, he returned to Dublin, and while giving his services to various churches in the city, devoted himself almost wholly to literary and scientific pursuits, and especially to the glacial geology of Ireland, on which subject he became an acknowledged authority. His paper, read before the Geological Society of Ireland in 1866, on the “General Glaciation of Ireland” is a masterly description of the effects of glaciation, and of the evidence in favour of the action of land-ice. Later on he discussed the origin of the elevated shell-bearing gravels near Dublin, and expressed the view that they were accumulated by floating ice when the land had undergone submergence. He was for a time treasurer of the Royal Irish Academy, an active member of the Royal Dublin Society, and president in 1878 of the Royal Geological Society of Ireland. Astronomy and physics, as well as the ancient language and antiquities of Ireland, attracted his attention. He died in Dublin on the 12th of September 1903.
CLOSE (from Lat. clausum, shut), a closed place or enclosure. In English law, the term is applied to a portion of land, enclosed or not, held as private property, and to any exclusive interest in land sufficient to maintain an action for trespass quare clausum fregit. The word is also used, particularly in Scotland, of the entry or passage, including the common staircase, of a block of tenement houses, and in architecture for the precincts of a cathedral or abbey.
The adjective “close” (i.e. closed) is found in several phrases, such as “close time” or “close season” (see Game Laws); close borough, one of which the rights and privileges were enjoyed by a limited class (see Borough); close rolls and writs, royal letters, &c., addressed to particular persons, under seal, and not open to public inspection (see Record; Chancery; Letters Patent). From the sense of “closed up,” and so “confined,” comes the common meaning of “near.”
CLOSURE (Fr. clôture), the parliamentary term for the closing of debate according to a certain rule, even when certain members are anxious to continue the debate. (See Parliament: Procedure.)
CLOT, ANTOINE BARTHÉLEMY (1793-1868), French physician, known as Clot Bey, was born at Grenoble on the 7th of November 1793, and graduated in medicine and surgery at Montpellier. After practising for a time at Marseilles he was made chief surgeon to Mehemet Ali, viceroy of Egypt. At Abuzabel, near Cairo, he founded a hospital and schools for all branches of medical instruction, as well as for the study of the French language; and, notwithstanding the most serious religious difficulties, instituted the study of anatomy by means of dissection. In 1832 Mehemet Ali gave him the dignity of bey without requiring him to abjure his religion; and in 1836 he received the rank of general, and was appointed head of the medical administration of the country. In 1849 he returned to Marseilles, though he revisited Egypt in 1856. He died at Marseilles on the 28th of August 1868. His publications included: Relation des épidémies de choléra qui ont régné à l’Heggiaz, 557 à Suez, et en Égypte (1832); De la peste observée en Égypte (1840); Aperçu général sur l’Égypte (1840); Coup d’œil sur la peste et les quarantaines (1851); De l’ophthalmie (1864).
CLOTAIRE (Chlothachar), the name of four Frankish kings.
Clotaire I. (d. 561) was one of the four sons of Clovis. On the death of his father in 511 he received as his share of the kingdom the town of Soissons, which he made his capital, the cities of Laon, Noyon, Cambrai and Maastricht, and the lower course of the Meuse. But he was very ambitious, and sought to extend his domain. He was the chief instigator of the murder of his brother Clodomer’s children in 524, and his share of the spoils consisted of the cities of Tours and Poitiers. He took part in the various expeditions against Burgundy, and after the destruction of that kingdom in 534 obtained Grenoble, Die and some of the neighbouring cities. When Provence was ceded to the Franks by the Ostrogoths, he received the cities of Orange, Carpentras and Gap. In 531 he marched against the Thuringi with his brother Theuderich (Thierry) I., and in 542 with his brother Childebert against the Visigoths of Spain. On the death of his great-nephew Theodebald in 555, Clotaire annexed his territories; and on Childebert’s death in 558 he became king of all Gaul. He also ruled over the greater part of Germany, made expeditions into Saxony, and for some time exacted from the Saxons an annual tribute of 500 cows. The end of his reign was troubled by internal dissensions, his son Chram rising against him on several occasions. Following Chram into Brittany, where the rebel had taken refuge, Clotaire shut him up with his wife and children in a cottage, to which he set fire. Overwhelmed with remorse, he went to Tours to implore forgiveness at the tomb of St Martin, and died shortly afterwards.
Clotaire II. (d. 629) was the son of Chilperic I. On the assassination of his father in 584 he was still in his cradle. He was, however, recognized as king, thanks to the devotion of his mother Fredegond and the protection of his uncle Gontran, king of Burgundy. It was not until after the death of his cousin Childebert II. in 595 that Clotaire took any active part in affairs. He then endeavoured to enlarge his estates at the expense of Childebert’s sons, Theodebert, king of Austrasia, and Theuderich II., king of Burgundy; but after gaining a victory at Laffaux (597), he was defeated at Dormelles (600), and lost part of his kingdom. After the war between Theodebert and Theuderich and their subsequent death, the nobles of Austrasia and Burgundy appealed to Clotaire, who, after putting Brunhilda to death, became master of the whole of the Frankish kingdom (613). He was obliged, however, to make great concessions to the aristocracy, to whom he owed his victory. By the constitution of the 18th of October 614 he gave legal force to canons which had been voted some days previously by a council convened at Paris, but not without attempting to modify them by numerous restrictions. He extended the competence of the ecclesiastical tribunals, suppressed unjust taxes and undertook to select the counts from the districts they had to administer. In 623 he made his son Dagobert king of the Austrasians, and gradually subdued all the provinces that had formerly belonged to Childebert II. He also guaranteed a certain measure of independence to the nobles of Burgundy, giving them the option of having a special mayor of the palace, or of dispensing with that officer. These concessions procured him a reign of comparative tranquillity. He died on the 18th of October 629, and was buried at Paris in the church of St Vincent, afterwards known as St Germain des Prés.
Clotaire III. (652-673) was a son of King Clovis II. In 657 he became the nominal ruler of the three Frankish kingdoms, but was deprived of Austrasia in 663, retaining Neustria and Burgundy until his death.
Clotaire IV. (d. 719) was king of Austrasia from 717 to 719.
CLOTH, properly a covering, especially for the body, clothing, then the material of which such a covering is made; hence any material woven of wool or hair, cotton, flax or vegetable fibre. In commercial usage, the word is particularly applied to a fabric made of wool. The word is Teutonic, though it does not appear in all the branches of the language. It appears in German as Kleid, dress (Kleidung, clothing), and in Dutch as kleed. The ultimate origin is unknown; it may be connected with the root kli- meaning to stick, cling to, which appears in “clay,” “cleave” and other words. The original meaning would be either that which clings to the body, or that which is pressed or “felted” together. The regular plural of “cloth” was “clothes,” which is now confined in meaning to articles of clothing, garments, in which sense the singular “cloth” is not now used. For that word, in its modern sense of material, the plural “cloths” is used. This form dates from the beginning of the 17th century, but the distinction in meaning between “cloths” and “clothes” is a 19th-century one.
CLOTHIER, a manufacturer of cloth, or a dealer who sells either the cloth or made-up clothing. In the United States the word formerly applied only to those who dressed or fulled cloth during the process of manufacture, but now it is used in the general sense, as above.
CLOTILDA, SAINT (d. 544), daughter of the Burgundian king Chilperic, and wife of Clovis, king of the Franks. On the death of Gundioc, king of the Burgundians, in 473, his sons Gundobald, Godegesil and Chilperic divided his heritage between them; Chilperic apparently reigning at Lyons, Gundobald at Vienne and Godegesil at Geneva. According to Gregory of Tours, Chilperic was slain by Gundobald, his wife drowned, and of his two daughters, Chrona took the veil and Clotilda was exiled. This account, however, seems to have been a later invention. At Lyons an epitaph has been discovered of a Burgundian queen, who died in 506, and was most probably the mother of Clotilda. Clotilda was brought up in the orthodox faith. Her uncle Gundobald was asked for her hand in marriage by the Frankish king Clovis, who had just conquered northern Gaul, and the marriage was celebrated about 493. On this event many romantic stories, all more or less embroidered, are to be found in the works of Gregory of Tours and the chronicler Fredegarius, and in the Liber historiae Francorum. Clotilda did not rest until her husband had abjured paganism and embraced the orthodox Christian faith (496). With him she built at Paris the church of the Holy Apostles, afterwards known as Ste Geneviève. After the death of Clovis in 511 she retired to the abbey of St Martin at Tours. In 523 she incited her sons against her uncle Gundobald and provoked the Burgundian war. In the following year she tried in vain to protect the rights of her grandsons, the children of Clodomer, against the claims of her sons Childebert I. and Clotaire I., and was equally unsuccessful in her efforts to prevent the civil discords between her children. She died in 544, and was buried by her husband’s side in the church of the Holy Apostles.
CLOUD (from the same root, if not the same word, as “clod,” a word common in various forms to Teutonic languages for a mass or lump; it is first applied in the usual sense in the late 13th century; the Anglo-Saxon clūd is only used in the sense of “a mass of rock,” wolcen being used for “cloud”), a mass of condensed vapour hanging in the air at some height from the earth.
Classification of Clouds.—The earliest serious attempt to name the varieties of cloud was made by J.B. Lamarck in 1801, but he only used French terms, and those were not always happily chosen. The field was therefore still clear when in 1803 Luke Howard published, in Tilloch’s Philosophical Magazine, an entirely independent scheme in which the terms were all Latin, and were applied with such excellent judgment that his system remains as the broad basis of those in use to-day. He recognized three primary types of cloud—Cirrus, Cumulus and Stratus—and four derivative or compound forms,—Cirro-cumulus, Cirro-stratus, Cumulo-stratus and Cumulo-cirro-stratus or Nimbus.
His own definitions were:—
(1) Cirrus.—Parallel, flexuous or diverging fibres, extensible in any or all directions.
(2) Cumulus.—Convex or conical heaps, increasing upward from a horizontal base.
(3) Stratus.—A widely-extended continuous horizontal sheet, increasing from below.
(4) Cirro-cumulus.—Small, well-defined, roundish masses, in close horizontal arrangement.
(5) Cirro-stratus.—Horizontal or slightly inclined masses, attenuated towards a part or the whole of their circumferences, bent downward, or undulated, separate or in groups consisting of small clouds having these characters.
(6) Cumulo-stratus.—The cirro-stratus blended with the cumulus, and either appearing intermixed with the heaps of the latter or superadding a widespread structure to its base.
(7) Cumulo-cirro-stratus, or nimbus.—The rain-cloud: a cloud or system of clouds from which rain is falling. It is a horizontal sheet, above which the cirrus spreads, while the cumulus enters it laterally and from beneath.
This system was universally adopted, and apart from some ambiguity in the definitions of cumulo-stratus and nimbus, it was sufficiently detailed for many purposes, such as the general relations between clouds and the movements of the barometer. When, however, such questions as the mode of origin of particular forms of cloud came to be investigated, it was at once felt that Howard’s classes were too wide, and something much more detailed was required. The result has been the promulgation from time to time of revised schemes, most of these being based on Howard’s work, and differing from him by the introduction of new terms or of subdivisions of his types. Some of these new terms have come more or less into use, such as A. Poëy’s pallium to signify a uniform sheet, but as a general rule the proposals were not accompanied by a clear enough exposition of their precise meaning for others to be quite sure of the author’s intention. Other writers not appreciating how fully Howard’s names had become established, boldly struck out on entirely new lines. The most important of these were probably those due respectively to (1) Poëy, published in the Annuaire de la société météorologique de France, 1865, (2) M. l’Abbé Maze, published in the Mémoires du congrès météorologique international, 1889, and (3) Frederic Gaster, Quart. Jour. R. Meteorological Society, 1893. In all of these Howard’s terms are used, but the systems were much more elaborate, and the verbal descriptions sometimes difficult to follow.
In his book Cloudland (1894) Clement Ley published a novel system. He grouped all clouds under four heads, in accordance with the mode in which he believed them to be formed.
| I. Clouds of Radiation. | |
| Nebula | Fog. |
| Nebula Stillans | Wet fog. |
| Nebula Pulverea | Dust fog. |
| II. Clouds of Interfret. | |
| Nubes Informis | Scud. |
| Stratus Quietus | Quiet cloud. |
| Stratus Lenticularis | Lenticular cloud. |
| Stratus Maculosus | Mackerel cloud. |
| Stratus Castellatus | Turret cloud. |
| Stratus Precipitans | Plane shower. |
| III. Clouds of Inversion. | |
| Cumulo-rudimentum | Rudiment. |
| Cumulus | Heap cloud. |
| Cumulo-stratus | Anvil cloud. |
| Cumulo-stratus Mammatus | Tubercled anvil cloud. |
| Cumulo-nimbus | Shower cloud. |
| Cumulo-nimbus Nivosus | Snow shower. |
| Cumulo-nimbus Grandineus | Hail shower. |
| Cumulo-nimbus Mammatus | Festooned shower cloud. |
| Nimbus | Rainfall cloud. |
| Nimbus nivosus | Snowfall. |
| Nimbus grandineus | Hailfall. |
| IV. Clouds of Inclination. | |
| Nubes Fulgens | Luminous cloud. |
| Cirrus | Curl cloud. |
| Cirro-filum | Gossamer cloud. |
| Cirro-velum | Veil cloud. |
| Cirro-macula | Speckle cloud. |
| Cirro-velum Mammatum.1 | Draped veil cloud. |
It will be seen that Ley’s scheme is really an amplification of Howard’s. The term “Interfret” is defined as the interaction of horizontal currents of different velocities. Inversion is a synonym for vertical convection, and Inclination is used to imply that such clouds consist of sloping lines of falling ice particles.
While Ley had been finishing his work and seeing it through the press, H. Hildebrand-Hildebrandsson and R. Abercromby had devised another modification which differed from Howard’s chiefly by the introduction of a new class, which they distinguished by the use of the prefix Alto. This scheme was formally adopted by the International Meteorological Conference held at Munich in 1891, and a committee was appointed to draw up an atlas showing the exact forms typical of each variety considered. Finally in August 1894 a small sub-committee consisting of Messrs H. Hildebrand-Hildebrandsson, A. Riggenbach-Burckhardt and Teisserenc de Bort was charged with the task of producing the atlas. Their task was completed in 1896, and meteorologists were at last supplied with a fairly detailed scheme, and one which was adequately illustrated, so that there could be no doubt of the authors’ meaning. It is as follows:—
The International Classification.
(a) Separate or globular masses (most frequently seen in dry weather).
(b) Forms which are widely extended, or completely cover the sky (in wet weather).
A. Upper clouds, average altitude 9000 metres.2
a. 1. Cirrus.
b. 2. Cirro-stratus.
B. Intermediate clouds, between 3000 m. and 7000 m.
a. 3. Cirro-cumulus.
4. Alto-cumulus.
b. 5. Alto-stratus.
C. Lower clouds, 2000 m.
a. 6. Strato-cumulus.
b. 7. Nimbus.
D. Clouds of Diurnal Ascending Currents.
a. 8. Cumulus, apex 1800 m., base 1400 m.
b. 9. Cumulo-nimbus, apex 3000 m. to 8000 m., base 1400 m.
E. High Fogs, under 1000 m.
10. Stratus.
Explanations.
1. Cirrus (Ci.).—Detached clouds, delicate and fibrous-looking, taking the form of feathers, generally of a white colour, sometimes arranged in belts which cross a portion of the sky in great circles and by an effect of perspective, converge towards one or two points of the horizon (the Ci.-S. and the Ci.-Cu. often contribute to the formation of these belts). See Plate, fig. 1.
2. Cirro-stratus (Ci.-S.).—A thin, whitish sheet, at times completely covering the sky, and only giving it a whitish appearance (it is then sometimes called cirro-nebula), or at others presenting, more or less distinctly, a formation like a tangled web. This sheet often produces halos around the sun and moon. See fig. 2.
3. Cirro-cumulus (Ci.-Cu.).—Small globular masses, or white flakes without shadows, or having very slight shadows, arranged in groups and often in lines. See fig. 3.
4. Alto-cumulus (A.-Cu.).—Largish globular masses, white or greyish, partially shaded, arranged in groups or lines, and often so closely packed that their edges appear confused. The detached masses are generally larger and more compact (changing to S.-Cu.) at the centre of the group; at the margin they form into finer flakes (changing to Ci.-Cu.). They often spread themselves out in lines in one or two directions. See fig. 4.
5. Alto-stratus (A.-S.).—A thick sheet of a grey or bluish colour, showing a brilliant patch in the neighbourhood of the sun or moon, and without causing halos, sometimes giving rise to coronae. This form goes through all the changes like Cirro-stratus, but according to measurements made at Upsala, its altitude is one-half as great. See fig. 5.
6. Strato-cumulus (S.-Cu.).—Large globular masses or rolls of dark cloud, frequently covering the whole sky, especially in winter, and occasionally giving it a wavy appearance. The layer is not, as a rule, very thick, and patches of blue sky are often seen through intervening spaces. All sorts of transitions between this form and Alto-cumulus are seen. It may be distinguished from nimbus by its globular or rolled appearance, and also because it does not bring rain. See fig. 6.

7. Nimbus (N.), Rain Cloud.—A thick layer of dark clouds, without shape and with ragged edges, from which continued rain or snow generally falls. Through openings in these clouds an upper layer of cirro-stratus or alto-stratus may almost invariably be seen. If the layer of nimbus separates up into shreds, or if small loose clouds are visible floating at a low level, underneath a large nimbus they may be described as fracto-nimbus (Scud of sailors). See fig. 9.
8. Cumulus (Cu.) (Wool-pack Clouds).—Thick clouds of which the upper surface is dome-shaped and exhibits protuberances while the base is horizontal. These clouds appear to be formed by a diurnal ascensional movement which is almost always observable. When the cloud is opposite the sun, the surfaces usually presented to the observer have a greater brilliance than the margins of the protuberances. When the light falls aslant, these clouds give deep shadows, but if they are on the same side as the sun they appear dark, with bright edges. See fig. 7.
The true cumulus has clear superior and inferior limits. It is often broken up by strong winds, and the detached portions undergo continual changes. These altered forms may be distinguished by the name of Fracto-cumulus.
9. Cumulo-nimbus (Cu.-N.); The Thunder-cloud; Shower-cloud.—Heavy masses of clouds, rising in the form of mountains, turrets or anvils, generally having a sheet or screen of fibrous appearance above (false cirrus) and underneath, a mass of cloud similar to nimbus. From the base there generally fall local showers of rain or snow (occasionally hail or soft hail). Sometimes the upper edges have the compact form of cumulus, rising into massive peaks round which the delicate false cirrus floats, and sometimes the edges themselves separate into a fringe of filaments similar to that of cirrus. This last form is particularly common in spring showers. See fig. 10.
The front of thunderclouds of wide extent frequently presents the form of a large bow spread over a portion of the sky which is uniformly brighter in colour.
10. Stratus (S.).—A horizontal sheet of lifted fog. When this sheet is broken up into irregular shreds by the wind, or by the summits of mountains, it may be distinguished by the name of Fracto-stratus. See fig. 8.
The scheme also provides that where a stratus or nimbus takes a lumpy form, this fact shall be described by the adjective cumuliformis, and if its base shows downward projecting bosses the word mammato is prefixed.
Issued as it has been with the authority of an international congress of specialists, this scheme has been generally accepted, and must be regarded as the orthodox system, and for the great majority of observations it is quite detailed enough. But it does not give universal satisfaction. Cirrus clouds, for instance, exhibit many forms, and these so diverse that they must be due to very different causes. Hence for the minuter study of cloud forms a more elaborate scheme is still needed.
Hence in 1896 H. H. Clayton of the Blue Hill observatory, Massachusetts, published in the Annals of the astronomical observatory of Harvard College a highly detailed scheme in which the International types and a number of subdivisions were grouped under four classes—stratiforms or sheet clouds; cumuliforms or woolpack clouds; flocciforms, including strato-cumulus, alto-cumulus and cirro-cumulus; and cirriforms or hairy clouds. The International terms are embodied and the special varieties are distinguished by the use of prefixes such as tracto-cirrus or cirrus bands, grano-cirro-cumulus or granular cirrus, &c.
Again in 1904 F. L. Obenbach of the Cleveland observatory devised a different system, published in the annual report, in which the International types are preserved, but each is subdivided into a number of species. In the absence of any atlas to define the precise meaning of the descriptions given, neither of these American schemes has come into general use.
Further proposals were put forward by A. W. Clayden in Cloud Studies (1905). His scheme accepts the whole of the International names which he regards as the cloud genera, and suggests specific Latin names for the chief varieties, accompanying the descriptions by photographs. The proposed scheme is as follows.
| Genus. | Species. | |
| Cirrus | Cirro-nebula | Cirrus haze. |
| Cirro-filum | Thread cirrus. | |
| Cirrus Excelsus | High Cirrus | |
| Cirrus Ventosus | Windy Cirrus | |
| Cirrus Nebulosus | Hazy Cirrus | |
| Cirrus Caudatus | Tailed Cirrus | |
| Cirrus Vittatus | Ribbon Cirrus | |
| Cirrus Inconstans | Change Cirrus | |
| Cirrus Communis | Common Cirrus | |
| Cirro-stratus | Communis | Common Ci. S. |
| Nebulosus | Hazy Ci. S. | |
| Vittatus | Ribbon Ci. S. | |
| Cumulosus | Flocculent Ci.-S. | |
| Cirro-cumulus | Cirro-macula | Speckle cloud. |
| Nebulosus | Hazy Ci. cu. | |
| Alto-clouds | Alto-stratus | |
| Alto-stratus maculosus | Mackerel sky. | |
| Alto-stratus fractus | ||
| Alto-strato-cumulus | ||
| Alto-cumulus informis | ||
| Alto-cumulus nebulosus | ||
| Alto-clouds | Alto-cumulus castellatus | Turret cloud. |
| Alto-cumulus glomeratus | High ball cumulus. | |
| Alto-cumulus communis | ||
| Alto-cumulus stratiformis | Flat alto-cum. | |
| Stratus | Stratus maculosus | |
| Stratus maculosus radius | Roll cloud. | |
| Stratus maculosus lenticularis | Fall cloud. | |
| Strato-cumulus | ||
| Cumulus | Cumulus minor | Small cumulus. |
| Cumulus major | Large cumulus. | |
| Cumulo-nimbus | Storm cloud. |
The term nimbus is to be applied to any cloud from which rain is falling, but if the true form of the cloud is visible the term should be used as a qualifying adjective. The prefix fracto- or the adjective fractus should be used when the cloud is undergoing disintegration or appears ragged or broken. Mammato- is used in the ordinary sense, and finally undatus or waved is to be added to the name of any cloud showing a wave-like or rippled structure.
CLOUDBERRY, Rubus Chamaemorus, a low-growing creeping herbaceous plant, with stem not prickly, and with simple obtusely lobed leaves and solitary white flowers, resembling those of the blackberry, but larger—one inch across,—and with stamens and pistils on different plants. The orange-yellow fruit is about half an inch long and consists of a few large drupes with a pleasant flavour. The plant occurs in the mountainous parts of Great Britain, and is widely distributed through the more northerly portions of both hemispheres. In northern Denmark and Sweden the fruit is gathered in large quantities and sold in the markets.
CLOUD-BURST, a sudden and violent storm of rain. The name probably originated from the idea that the clouds were solid masses full of water that occasionally burst with disastrous results. A whirlwind passing over the sea sometimes carries the water upwards in a whirling vortex; passing over the land its motion is checked and a deluge of water falls. Occasionally on high lands far from the sea violent storms occur, with rain that seems to descend in sheets, sweeping away bridges and culverts and tearing up roads and streets, being due to great and rapid condensation and vortical whirling of the resulting heavy clouds (see Meteorology).
CLOUDED LEOPARD (Felis nebulosa or macroscelis), a large arboreal cat from the forests of south-east Asia, Sumatra, Java, Borneo and Formosa. This cat, often called the clouded tiger, is beautifully marked, and has an elongated head and body, long tail and rather short limbs. The canine teeth are proportionately longer than in any other living cat. Little is known of the habits of the clouded leopard, but it preys on small mammals and birds, and rarely comes to the ground. The native Malay name is Arimaudahan (“tree-tiger”). The species is nearly related to the small Indian marbled cat (F. marmorata), and Fontaniers cat (F. tristis) of Central Asia.
CLOUET, FRANÇOIS (d. 1572), French miniature painter. The earliest reference to him is the document dated December 1541 (see Clouet, Jean), in which the king renounces for the benefit of the artist his father’s estate which had escheated to the crown as the estate of a foreigner. In it the younger Janet is said to have “followed his father very closely in the science of his art.” Like his father, he held the office of groom of the chamber and painter in ordinary to the king, and so far as salary is concerned, he started where his father left off. A long list of drawings contains those which are attributed to this artist, but we still lack perfect certainty about his works. There is, however, more to go upon than there was in the case of his father, 560 as the praises of François Clouet were sung by the writers of the day, his name was carefully preserved from reign to reign, and there is an ancient and unbroken tradition in the attribution of many of his pictures. There are not, however, any original attestations of his works, nor are any documents known which would guarantee the ascriptions usually accepted. To him are attributed the portraits of Francis I. at the Uffizi and at the Louvre, and various drawings relating to them. He probably also painted the portrait of Catherine de’ Medici at Versailles and other works, and in all probability a large number of the drawings ascribed to him were from his hand. One of his most remarkable portraits is that of Mary, queen of Scots, a drawing in chalks in the Bibliothèque Nationale, and of similar character are the two portraits of Charles IX. and the one at Chantilly of Marguerite of France. Perhaps his masterpiece is the portrait of Elizabeth of Austria in the Louvre.
He resided in Paris in the rue de Ste Avoye in the Temple quarter, close to the Hôtel de Guise, and in 1568 is known to have been under the patronage of Claude Gouffier de Boisy, Seigneur d’Oiron, and his wife Claude de Baune. Another ascertained fact concerning François Clouet is that in 1571 he was “summoned to the office of the Court of the Mint,” and his opinion was taken on the likeness to the king of a portrait struck by the mint. He prepared the death-mask of Henry II., as in 1547 he had taken a similar mask of the face and hands of Francis I., in order that the effigy to be used at the funeral might be prepared from his drawings; and on each of these occasions he executed the painting to be used in the decorations of the church and the banners for the great ceremony.
Several miniatures are believed to be his work, one very remarkable portrait being the half-length figure of Henry II. in the collection of Mr J. Pierpont Morgan. Another of his portraits is that of the duc d’Alençon in the Jones collection at South Kensington, and certain representations of members of the royal family which were in the Hamilton Palace collection and the Magniac sale are usually ascribed to him. He died on the 22nd of December 1572, shortly after the massacre of St Bartholomew, and his will, mentioning his sister and his two illegitimate daughters, and dealing with the disposition of a considerable amount of property, is still in existence. His daughters subsequently became nuns.
His work is remarkable for the extreme accuracy of the drawing, the elaborate finish of all the details, and the exquisite completeness of the whole portrait. He must have been a man of high intelligence, and of great penetration, intensely interested in his work, and with considerable ability to represent the character of his sitter in his portraits. His colouring is perhaps not specially remarkable, nor from the point of style can his pictures be considered specially beautiful, but in perfection of drawing he has hardly any equal.
CLOUET, JEAN (d. c. 1541). French miniature painter, generally known as JANET. The authentic presence of this artist at the French court is first to be noted in 1516, the second year of the reign of Francis I. By a deed of gift made by the king to the artist’s son of his father’s estate, which had escheated to the crown, we learn that he was not actually a Frenchman, and never even naturalized. He is supposed to have been a native of the Low Countries, and probably his real name was Clowet. His position was that of groom of the chamber to the king, and he received a stipend at first of 180 livres and later of 240. He lived several years in Tours, and there it was he met his wife, who was the daughter of a jeweller. He is recorded as living in Tours in 1522, and there is a reference to his wife’s residence in the same town in 1523, but in 1529 they were both settled in Paris, probably in the neighbourhood of the parish of Ste Innocent, in the cemetery of which they were buried. He stood godfather at a christening on the 8th of July 1540, but was no longer living in December 1541, and therefore died between those two dates.
His brother, known as Clouet de Navarre, was in the service of Marguérite d’Angoulême, sister of Francis I., and is referred to in a letter written by Marguérite about 1529. Jean Clouet had two children, François and Catherine, who married Abel Foulon, and left one son, who continued the profession of François Clouet after his decease. Jean Clouet was undoubtedly a very skilful portrait painter, but it must be acknowledged without hesitation that there is no work in existence which has been proved to be his. There is no doubt that he painted a portrait of the mathematician, Oronce Finé, in 1530, when Finé was thirty-six years old, but the portrait is now known only by a print. Janet is generally believed, however, to have been responsible for a very large number of the wonderful portrait drawings now preserved at Chantilly, and at the Bibliothèque Nationale, and to him is attributed the portrait of an unknown man at Hampton Court, that of the dauphin Francis, son of Francis I. at Antwerp, and one other portrait, that of Francis I. in the Louvre.
Seven miniature portraits in the Manuscript of the Gallic War in the Bibliothèque Nationale (13,429) are attributed to Janet with very strong probability, and to these may be added an eighth in the collection of Mr J. Pierpont Morgan, and representing Charles de Cossé, Maréchal de Brissac, identical in its characteristics with the seven already known. There are other miniatures in the collection of Mr Morgan, which may be attributed to Jean Clouet with some strong degree of probability, inasmuch as they closely resemble the portrait drawings at Chantilly and in Paris which are taken to be his work. In his oil paintings the execution is delicate and smooth, the outlines hard, the texture pure, and the whole work elaborately and very highly finished in rich, limpid colour. The chalk drawings are of remarkable excellence, the medium being used by the artist with perfect ease and absolute sureness, and the mingling of colour being in exquisite taste, the modelling exceedingly subtle, and the drawing careful, tender and emphatic. The collection of drawings preserved in France, and attributed to this artist and his school, comprises portraits of all the important persons of the time of Francis I. In one album of drawings the portraits are annotated by the king himself, and his merry reflections, stinging taunts or biting satires, add very largely to a proper understanding of the life of his time and court. Definite evidence, however, is still lacking to establish the attribution of the best of these drawings and of certain oil paintings to the Jean Clouet who was groom of the chambers to the king.
CLOUGH, ANNE JEMIMA (1820-1892), English educationalist, was born at Liverpool on the 20th of January 1820, the daughter of a cotton merchant. She was the sister of Arthur Hugh Clough, the poet. When two years old she was taken with the rest of the family to Charleston, South Carolina. It was not till 1836 that she returned to England, and though her ambition was to write, she was occupied for the most part in teaching. Her father’s failure in business led her to open a school in 1841. This was carried on until 1846. In 1852, after making some technical studies in London and working at the Borough Road and the Home and Colonial schools, she opened another small school of her own at Ambleside in Westmorland. Giving this up some ten years later, she lived for a time with the widow of her brother Arthur Hugh Clough—who had died in 1861—in order that she might educate his children. Keenly interested in the education of women, she made friends with Miss Emily Davies, Madame Bodichon, Miss Buss and others. After helping to found the North of England council for promoting the higher education of women, she acted as its secretary from 1867 to 1870 and as its president from 1873 to 1874. When it was decided to open a house for the residence of women students at Cambridge, Miss Clough was chosen as its first principal. 561 This hostel, started in Regent Street, Cambridge, in 1871 with five students, and continued at Merton Hall in 1872, led to the building of Newnham Hall, opened in 1875, and to the erection of Newnham College on its present basis in 1880. Miss Clough’s personal charm and high aims, together with the development of Newnham College under her care, led her to be regarded as one of the foremost leaders of the women’s educational movement. She died at Cambridge on the 27th of February 1892. Two portraits of Miss Clough are at Newnham College, one by Sir W. B. Richmond, the other by J. J. Shannon.
CLOUGH, ARTHUR HUGH (1819-1861), English poet, was born at Liverpool on the 1st of January 1819. He came of a good Welsh stock by his father, James Butler Clough, and of a Yorkshire one by his mother, Anne Perfect. In 1822 his father, a cotton merchant, moved to the United States, and Clough’s childhood was spent mainly at Charleston, South Carolina, much under the influence of his mother, a cultivated woman, full of moral and imaginative enthusiasm. In 1828 the family paid a visit to England, and Clough was left at school at Chester, whence he passed in 1829 to Rugby, then under the sway of Dr Thomas Arnold, whose strenuous views on life and education he accepted to the full. Cut off to a large degree from home relations, he passed a somewhat reserved and solitary boyhood, devoted to the well-being of the school and to early literary efforts in the Rugby Magazine. In 1836 his parents returned to Liverpool, and in 1837 he went with a scholarship to Balliol College, Oxford. Here his contemporaries included Benjamin Jowett, A. P. Stanley, J. C. Shairp, W. G. Ward, Frederick Temple and Matthew Arnold.
Oxford, in 1837, was in the full swirl of the High Church movement led by J. H. Newman. Clough was for a time carried away by the flood, and, although he recovered his equilibrium, it was not without an amount of mental disturbance and an expenditure of academic time, which perhaps accounted for his failure to obtain more than a second class in his final examination. He missed a Balliol fellowship, but obtained one at Oriel, with a tutorship, and lived the Oxford life of study, speculation, lectures and reading-parties for some years longer. Gradually, however, certain sceptical tendencies with regard to the current religious and social order grew upon him to such an extent as to render his position as an orthodox teacher of youth irksome, and in 1848 he resigned it. The immediate feeling of relief showed itself in buoyant, if thoughtful, literature, and he published poems both new and old. Then he travelled, seeing Paris in revolution and Rome in siege, and in the autumn of 1849 took up new duties as principal of University Hall, a hostel for students at University College, London. He soon found that he disliked London, in spite of the friendship of the Carlyles, nor did the atmosphere of Unitarianism prove any more congenial than that of Anglicanism to his critical and at bottom conservative temper. A prospect of a post in Sydney led him to engage himself to Miss Blanche Mary Shore Smith, and when it disappeared he left England in 1852, and went, encouraged by Emerson, to Cambridge, Massachusetts. Here he remained some months, lecturing and translating Plutarch for the book-sellers, until in 1853 the offer of an examinership in the Education Office brought him to London once more. He married, and pursued a steady official career, diversified only by an appointment in 1856 as secretary to a commission sent to study certain aspects of foreign military education. At this, as at every period of his life, he enjoyed the warm respect and admiration of a small circle of friends, who learnt to look to him alike for unselfish sympathy and for spiritual and practical wisdom. In 1860 his health began to fail. He visited first Malvern and Freshwater, and then the East, France and Switzerland, in search of recovery, and finally came to Florence, where he was struck down by malaria and paralysis, and died on the 13th of November 1861. Matthew Arnold wrote upon him the exquisite lament of Thyrsis.
Shortly before he left Oxford, in the stress of the Irish potato-famine, Clough wrote an ethical pamphlet addressed to the undergraduates, with the title, A Consideration of Objections against the Retrenchment Association at Oxford (1847). His Homeric pastoral The Bothie of Toper-na-Fuosich, afterwards rechristened Tober-na-Vuolich (1848), was inspired by a long vacation after he had given up his tutorship, and is full of socialism, reading-party humours and Scottish scenery. Ambarvalia (1849), published jointly with his friend Thomas Burbidge, contains shorter poems of various dates from 1840, or earlier, onwards. Amours de Voyage, a novel in verse, was written at Rome in 1849; Dipsychus, a rather amorphous satire, at Venice in 1850; and the idylls which make up Mari Magno, or Tales on Board, in 1861. A few lyric and elegiac pieces, later in date than the Ambarvalia, complete the tale of Clough’s poetry. His only considerable enterprise in prose was a revision of the 17th century translation of Plutarch by Dryden and others, which occupied him from 1852, and was published as Plutarch’s Lives (1859).
No part of Clough’s life was wholly given up to poetry, and he probably had not the gift of detachment necessary to produce great literature in the intervals of other occupations. He wrote but little, and even of that little there is a good deal which does not aim at the highest seriousness. He never became a great craftsman. A few of his best lyrics have a strength of melody to match their depth of thought, but much of what he left consists of rich ore too imperfectly fused to make a splendid or permanent possession. Nevertheless, he is rightly regarded, like his friend Matthew Arnold, as one of the most typical English poets of the middle of the 19th century. His critical instincts and strong ethical temper brought him athwart the popular ideals of his day both in conduct and religion. His verse has upon it the melancholy and the perplexity of an age of transition. He is a sceptic who by nature should have been with the believers. He stands between two worlds, watching one crumble behind him, and only able to look forward by the sternest exercise of faith to the reconstruction that lies ahead in the other. On the technical side, Clough’s work is interesting to students of metre, owing to the experiments which he made, in the Bothie and elsewhere, with English hexameters and other types of verse formed upon classical models.
CLOUTING, the technical name given to a light plain cloth used for covering butter and farmers’ baskets, and for dish and pudding cloths. The same term is often given to light cloths of the nursery diaper pattern.
CLOVELLY, a fishing village in the Barnstaple parliamentary division of Devonshire, England, 11 m. W.S.W. of Bideford. Pop. (1901) 621. It is a cluster of old-fashioned cottages in a unique position on the sides of a rocky cleft in the north coast; its main street resembles a staircase which descends 400 ft. to the pier, too steeply to allow of any wheeled traffic. Thick woods shelter it on three sides, and render the climate so mild that fuchsias and other delicate plants flourish in midwinter. All Saints’ church, restored in 1866, is late Norman, containing several monuments to the Carys, lords of the manor for 600 years. The surrounding scenery is famous for its richness of colour, especially in the grounds of Cary Court, and along “The Hobby,” a road cut through the woods and overlooking the sea. Clovelly is described by Dickens in A Message from the Sea.
CLOVER, in botany, the English name for plants of the genus Trifolium, from Lat. tres, three, and folium, a leaf, so called from the characteristic form of the leaf, which has three leaflets (trifoliate), hence the popular name trefoil. It is a member of the family Leguminosae, and contains about three hundred species, found chiefly in north temperate regions, but also, like other north temperate genera, on the mountains in 562 the tropics. The plants are small annual or perennial herbs with trifoliate (rarely 5- or 7-foliate) leaves, with stipules adnate to the leaf-stalk, and heads or dense spikes of small red, purple, white, or rarely yellow flowers; the small, few-seeded pods are enclosed in the calyx. Eighteen species are native in Britain, and several are extensively cultivated as fodder-plants. T. pratense, red or purple clover, is the most widely cultivated.
This plant, either sown alone or in mixture with rye-grass, has for a long time formed the staple crop for soiling; and so long as it grew freely, its power of shooting up again after repeated mowings, the bulk of crop thus obtained, its palatableness to stock and feeding qualities, the great range of soils and climate in which it grows, and its fitness either for pasturage or soiling, well entitled it to this preference. Except on certain rich calcareous clay soils, it has now, however, become an exceedingly precarious crop. The seed, when genuine, which unfortunately is very often not the case, germinates as freely as ever, and no greater difficulty than heretofore is experienced in having a full plant during autumn and the greater part of winter; but over most part of the country, the farmer, after having his hopes raised by seeing a thick cover of vigorous-looking clover plants over his field, finds to his dismay, by March or April, that they have either entirely disappeared, or are found only in capricious patches here and there over the field. No satisfactory explanation of this “clover-sickness” has yet been given, nor any certain remedy, of a kind to be applied to the soil, discovered. One important fact is, however, now well established, viz. that when the cropping of the land is so managed that clover does not recur at shorter intervals than eight years, it grows with much of its pristine vigour. The knowledge of this fact now determines many farmers in varying their rotation so as to secure this important end. At one time there was a somewhat prevalent belief that the introduction of beans into the rotation had a specific influence of a beneficial kind on the clover when it came next to be sown; but the true explanation seems to be that the beans operate favourably only by the incidental circumstance of almost necessarily lengthening the interval betwixt the recurrences of clover.
When the four-course rotation is followed, no better plan of managing this process has been yet suggested than to sow beans, pease, potatoes or tares, instead of clover, for one round, making the rotation one of eight years instead of four. The mechanical condition of the soil seems to have something to do with the success or failure of the clover crop. We have often noticed that headlands, or the converging line of wheel-tracks near a gateway at which the preceding root crop had been carted from a field, have had a good take of clover, when on the field generally it had failed. In the same way a field that has been much poached by sheep while consuming turnips upon it, and which has afterwards been ploughed up in an unkindly state, will have the clover prosper upon it, when it fails in other cases where the soil appears in far better condition. If red clover can be again made a safe crop, it will be a boon indeed to agriculture. Its seeds are usually sown along with a grain crop, any time from the 1st of February to May, at the rate of 12 ℔ to 20 ℔ per acre when not combined with other clovers or grasses.
Italian rye-grass and red clover are now frequently sown in mixture for soiling, and succeed admirably. It is, however, a wiser course to sow them separately, as by substituting the Italian rye-grass for clover, for a single rotation, the farmer not only gets a crop of forage as valuable in all respects, but is enabled, if he choose, to prolong the interval betwixt the sowings of clover to twelve years, by sowing, as already recommended, pulse the first round, Italian rye-grass the second, and clover the third.
These two crops, then, are those on which the arable-land farmer mainly relies for green forage. To have them good, he must be prepared to make a liberal application of manure. Good farm-yard dung may be applied with advantage either in autumn or spring, taking care to cart it upon the land only when it is dry enough to admit of this being done without injury. It must also be spread very evenly so soon as emptied from the carts. But it is usually more expedient to use either guano, nitrate of soda, or soot for this purpose, at the rates respectively of 2 cwt., 1 cwt. and 20 bushels. If two or more of these substances are used, the quantities of each will be altered in proportion. They are best also to be applied in two or three portions at intervals of fourteen to twenty days, beginning towards the end of December, and only when rain seems imminent or has just fallen.
When manure is broadcast over a young clover field, and presently after washed in by rain, the effect is identical with that of first dissolving it in water, and then distributing the dilution over the surface, with this difference, namely, that the first plan costs only the price of the guano, &c, and is available at any time and to every one, whereas the latter implies the construction of tanks and costly machinery.
T. incarnatum, crimson or Italian clover, though not hardy enough to withstand the climate of Scotland in ordinary winters, is a most valuable forage crop in England. It is sown as quickly as possible after the removal of a grain crop at the rate of 18 ℔ to 20 ℔ per acre. It is found to succeed better when only the surface of the soil is stirred by the scarifier and harrow than when a ploughing is given. It grows rapidly in spring, and yields an abundant crop of green food, peculiarly palatable to live stock. It is also suitable for making into hay. Only one cutting, however, can be obtained, as it does not shoot again after being mown.
T. repens, white or Dutch clover, is a perennial abundant in meadows and good pastures. The flowers are white or pinkish, becoming brown and deflexed as the corolla fades. T. hybridum, Alsike or Swedish clover, is a perennial which was introduced early in the 19th century and has now become naturalized in Britain. The flowers are white or rosy, and resemble those of the last species. T. medium, meadow or zigzag clover, a perennial with straggling flexuous stems and rose-purple flowers, is of little agricultural value. Other British species are: T. arvense, hare’s-foot trefoil, found in fields and dry pastures, a soft hairy plant with minute white or pale pink flowers and feathery sepals; T. fragiferum, strawberry clover, with densely-flowered, globose, rose-purple heads and swollen calyxes; T. procumbens, hop trefoil, on dry pastures and roadsides, the heads of pale yellow flowers suggesting miniature hops; and the somewhat similar T. minus, common in pastures and roadsides, with smaller heads and small yellow flowers turning dark brown. The last named is the true shamrock. Specimens of shamrock and other clovers are not infrequently found with four leaflets, and, like other rarities, are considered lucky. Calvary clover is a member of the closely allied genus Medicago—M. Echinus, so called from the curled spiny pod; it has small heads of yellow clover-like flowers, and is a native of the south of France.
CLOVES, the dried, unexpanded flower-buds of Eugenia caryophyllata, a tree belonging to the natural order Myrtaceae. They are so named from the French word clou, on account of their resemblance to a nail. The clove tree is a beautiful evergreen which grows to a height of from 30 to 40 ft., having large oval leaves and crimson flowers in numerous groups of terminal clusters. The flower-buds are at first of a pale colour and gradually become green, after which they develop into a bright red, when they are ready for collecting. Cloves are rather more than half an inch in length, and consist of a long cylindrical calyx, terminating in four spreading sepals, and four unopened petals which form a small ball in the centre. The tree is a native of the small group of islands in the Indian Archipelago called the Moluccas, or Spice Islands; but it was long cultivated by the Dutch in Amboyna and two or three small neighbouring islands. Cloves were one of the principal Oriental spices that early excited the cupidity of Western commercial communities, having been the basis of a rich and lucrative trade from an early part of the Christian era. The Portuguese, by doubling the Cape of Good Hope, obtained possession of the principal portion of the clove trade, which they continued to hold for nearly a century, when, in 1605, they were expelled 563 from the Moluccas by the Dutch. That power exerted great and inhuman efforts to obtain a complete monopoly of the trade, attempting to extirpate all the clove trees growing in their native islands, and to concentrate the whole production in the Amboyna Islands. With great difficulty the French succeeded in introducing the clove tree into Mauritius in the year 1770; subsequently the cultivation was introduced into Guiana, Brazil, most of the West Indian Islands and Zanzibar. The chief commercial sources of supply are now Zanzibar and its neighbouring island Pemba on the East African coast, and Amboyna. Cloves are also grown in Java, Sumatra, Réunion, Guiana and the West India Islands.
Cloves as they come into the market have a deep brown colour, a powerfully fragrant odour, and a taste too hot and acrid to be pleasant. When pressed with the nail they exude a volatile oil with which they are charged to the unusual proportion of about 18%. The oil is obtained as a commercial product by submitting the cloves with water to repeated distillation. It is, when new and properly prepared, a pale yellow or almost colourless fluid, becoming after some time of a brown colour; and it possesses the odour and taste peculiar to cloves. The essential oil of cloves—the Oleum Caryophylli of the British Pharmacopoeia—is a mixture of two substances, one of which is oxidized, whilst the other is not. Eugenol, or eugenic acid, C10H12O2, is the chief constituent. It is capable of forming definite salts. The other constituent is a hydrocarbon C15H24, of which the distilling point differs from that of eugenol, and which solidifies only with intense cold. Oil of cloves is readily soluble in alcohol and ether, and has a specific gravity of about 1.055. Its dose is ½-3 minims. Besides this oil, cloves also contain two neutral bodies, eugenin and caryophyllin, the latter of which is an isomer of camphor. They are of no practical importance. The British Pharmacopoeia contains an infusion of cloves (Infusum Caryophylli), of which the strength is 1 part in 40 of boiling water and the dose ½-1 oz. Cloves are employed principally as a condiment in culinary operations, in confectionery, and in the preparation of liqueurs. In medicine they are tonic and carminative, but they are little used except as adjuncts to other substances on account of their flavour, or with purgatives to prevent nausea and griping. The essential oil forms a convenient medium for using cloves for flavouring purposes, it possesses the medicinal properties characteristic of a volatile oil, and it is frequently employed to relieve toothache. Oil of cloves is regarded by many dental surgeons as the most effective local anaesthetic they possess in cases where it is desired, before cutting a sensitive tooth for the purpose of filling it, to lower the sensibility of the dentine. For this purpose the cavity must be exposed to cotton wool saturated with the oil for about ten days.
CLOVIO, GIORGIO GIULIO (1498-1578), Italian painter, by birth a Croat and by profession a priest, is said to have learned the elements of design in his own country, and to have studied afterwards with intense diligence at Rome under Giulio Romano, and at Verona under Girolamo de’ Libri. He excelled in historical pieces and portraits, painting as for microscopical examination, and yet contriving to handle his subjects with great force and precision. His book of twenty-six pictures representing the procession of Corpus Domini, in Rome, was the work of nine years, and the covers were executed by Benvenuto Cellini. The British Museum has his twelve miniatures of the victories of the emperor Charles V. In the Vatican library is preserved a manuscript life of Frederick, duke of Urbino, superbly illustrated by Clovio, who is facile princeps among Italian miniaturists. He was called Macedo, or Macedone, to connect him with his supposed Macedonian ancestry.
CLOVIS [Chlodovech] (c. 466-511), king of the Salian Franks, son of Childeric I., whom he succeeded in 481 at the age of fifteen. At that date the Salian Franks had advanced as far as the river Somme, and the centre of their power was at Tournai. On the history of Clovis between the years 481 and 486 the records are silent. In 486 he attacked Syagrius, a Roman general who, after the fall of the western empire in 476, had carved out for himself a principality south of the Somme, and is called by Gregory of Tours “rex Romanorum.” After being defeated by Clovis at the battle of Soissons, Syagrius sought refuge with the Visigothic king Alaric II., who handed him over to the conqueror. Henceforth Clovis fixed his residence at Soissons, which was in the midst of public lands, e.g. Berny-Rivière, Juvigny, &c. The episode of the vase of Soissons1 has a legendary character, and all that it proves is the deference shown by the pagan king to the orthodox clergy. Clovis undoubtedly extended his dominion over the whole of Belgica Secunda, of which Reims was the capital, and conquered the neighbouring cities in detail. Little is known of the history of these conquests. It appears that St Geneviève defended the town of Paris against Clovis for a long period, and that Verdun-sur-Meuse, after a brave stand, accepted an honourable capitulation thanks to St Euspitius. In 491 some barbarian troops in the service of Rome, Arboruchi (Άρμόρνχοι), Thuringians, and even Roman soldiers who could not return to Rome, went over to Clovis and swelled the ranks of his army.
In 493 Clovis married a Burgundian princess, Clotilda, niece of Gundobald and Godegesil, joint kings of Burgundy. This princess was a Christian, and earnestly desired the conversion of her husband. Although Clovis allowed his children to be baptized, he remained a pagan himself until the war against the Alemanni, who at that time occupied the country between the Vosges, and the Rhine and the neighbourhood of Lake Constance. By pushing their incursions westward they came into collision with Clovis, who marched against them and defeated them in the plain of the Rhine. The legend runs that, in the thickest of the fight, Clovis swore that he would be converted to the God of Clotilda if her God would grant him the victory. After subduing a part of the Alemanni, Clovis went to Reims, where he was baptized by St Remigius on Christmas day 496, together with three thousand Franks. The story of the phial of holy oil (the Sainte Ampoule) brought from heaven by a white dove for the baptism of Clovis was invented by Archbishop Hincmar of Reims three centuries after the event.
The baptism of Clovis was an event of very great importance. From that time the orthodox Christians in the kingdom of the Burgundians and Visigoths looked to Clovis to deliver them from their Arian kings. Clovis seems to have failed in the case of Burgundy, which was at that time torn by the rivalry between Godegesil and his brother Gundobald. Godegesil appealed for help to Clovis, who defeated Gundobald on the banks of the Ouche near Dijon, and advanced as far as Avignon (500), but had to retire without being able to retain any of his conquests. Immediately after his departure Gundobald slew Godegesil at Vienne, and seized the whole of the Burgundian kingdom. Clovis was more fortunate in his war against the Visigoths. Having completed the subjugation of the Alemanni in 506, he marched against the Visigothic king Alaric II. in the following year, in spite of the efforts of Theodoric, king of the Ostrogoths, to prevent the war. After a decisive victory at Vouillé near Poitiers, in which Clovis slew Alaric with his own hand, the whole of the kingdom of the Visigoths as far as the Pyrenees was added to the Frankish empire, with the exception of Septimania, which, together with Spain, remained in possession of Alaric’s grandson Amalaric, and Provence, which was seized by Theodoric and annexed to Italy. In 508 Clovis received at Tours the insignia of the consulship from the eastern emperor, Anastasius, but the title was purely honorific. The last years of his life Clovis spent in Paris, which he made the capital of his kingdom, and where he built the church of the Holy Apostles, known later as the church of St Geneviève. By murdering the petty Frankish 564 kings who reigned at Cambrai, Cologne and other residences, he became sole king of all the Frankish tribes. He died in 511.
Clovis was the true founder of the Frankish monarchy. He reigned over the Salian Franks by hereditary right; over the other Frankish tribes by reason of his kinship with their kings and by the choice of the warriors, who raised him on the shield; and he governed the Gallo-Romans by right of conquest. He had the Salic law drawn up, doubtless between the years 486 and 507; and seems to have been represented in the cities by a new functionary, the graf, comes, or count. He owed his success in great measure to his alliance with the church. He took the property of the church under his protection, and in 511 convoked a council at Orleans, the canons of which have come down to us. But while protecting the church, he maintained his authority over it. He intervened in the nomination of bishops, and at the council of Orleans it was decided that no one, save a son of a priest, could be ordained clerk without the king’s order or the permission of the count.
1 The story is as follows. The vase had been taken from a church by a Frankish soldier after the battle of Soissons, and the bishop had requested Clovis that it might be restored. But the soldier who had taken it refused to give it up, and broke it into fragments with his francisca, or battle-axe. Some time afterwards, when Clovis was reviewing his troops, he singled out the soldier who had broken the vase, upbraided him for the neglect of his arms, and dashed his francisca to the ground. As the man stooped to pick it up, the king clove his skull with the words: “Thus didst thou serve the vase of Soissons.”
CLOWN (derived by Fuller, in his Worthies, from Lat. colonus, a husbandman; but apparently connected with “clod” and with similar forms in Teutonic and Scandinavian languages), a rustic, boorish person; the comic character in English pantomime, always dressed in baggy costume, with face whitened and eccentrically painted, and a tufted wig. The character probably descends from representations of the devil in medieval miracle-plays, developed partly through the stage rustics and partly through the fools or jesters (also called clowns) of the Elizabethan drama. The whitened face and baggy costume indicate a connexion also with the continental Pierrot. The prominence of the clown in pantomime (q.v.) is a comparatively modern development as compared with that of Harlequin.
CLOYNE, a small market town of Co. Cork, Ireland, in the east parliamentary division, 15 m. E.S.E. of the city of Cork. Pop. (1901) 827. It gives its name to a Roman Catholic diocese, the cathedral of which is at Queenstown. Cloyne was the seat of a Protestant diocese until 1835, when it was united to that of Cork. It was originally a foundation of the 6th century. The cathedral church, dedicated to its founder St Colman, a disciple of St Finbar of Cork, is a plain cruciform building mainly of the 14th century, with an earlier oratory in the churchyard. It contains a few handsome monuments to its former bishops, but until 1890, when a monument was erected, had nothing to preserve the memory of the illustrious Dr George Berkeley, who held the see from 1734 to 1753. Opposite the cathedral is a very fine round tower 100 ft. in height, though the conical roof has long been destroyed. The Roman Catholic church is a spacious building of the early 19th century. The town was several times plundered by the Danes in the 9th century; it was laid waste by Dermot O’Brien in 1071, and was burned in 1137. In 1430 the bishopric was united to that of Cork; in 1638 it again became independent, and in 1660 it was again united to Cork and Ross. In 1678 it was once more declared independent, and so continued till 1835. The name, Cluain-Uamha, signifies “the meadow of the cave,” from the curious limestone caves in the vicinity. The Pipe Roll of Cloyne, compiled by Bishop Swaffham in 1364, is a remarkable record embracing a full account of the feudal tenures of the see, the nature of the impositions, and the duties the puri homines Sancti Colmani were bound to perform at a very early period. The roll is preserved in the record office, Dublin. It was edited by Richard Caulfield in 1859.
CLUB (connected with “clump”), (1) a thick stick, used as a weapon, or heavy implement for athletic exercises (“Indian club,” &c.); (2) one of the four suits of playing-cards,—the translation of the Spanish basto—represented by a black trefoil (taken from the French, in which language it is trèfle); (3) a term given to a particular form of association of persons. It is to this third sense that this article is devoted.
By the term “club,” the most general word for which is in Gr. ἑταιρία, in Lat. sodalitas, is here meant an association within the state of persons not united together by any natural ties of kinship, real or supposed. Modern clubs are dealt with below, and we begin with an account of Greek and Roman clubs. Such clubs are found in all ancient states of which we have any detailed knowledge, and seem to have dated in one form or another from a very early period. It is not unreasonable to suppose, in the absense of certain information, that the rigid system of groups of kin, i.e. family, gens, phratria, &c., affording no principle of association beyond the maintenance of society as it then existed, may itself have suggested the formation of groups of a more elastic and expansive nature; in other words, that clubs were an expedient for the deliverance of society from a too rigid and conservative principle of crystallization.
Greek.—The most comprehensive statement we possess as to the various kinds of clubs which might exist in a single Greek state is contained in a law of Solon quoted incidentally in the Digest of Justinian (47.22), which guaranteed the administrative independence of these associations provided they kept within the bounds of the law. Those mentioned (apart from demes and phratries, which were not clubs as here understood) are associations for religious purposes, for burial, for trade, for, privateering (ἐπὶ λείαν), and for the enjoyment of common meals. Of these by far the most important are the religious clubs, about which we have a great deal of information, chiefly from inscriptions; and these may be taken as covering those for burial purposes and for common meals, for there can be no doubt that all such unions had originally a religious object of some kind. But we have to add to Solon’s list the political ἑταιρίαι which we meet with in Athenian history, which do not seem to have always had a religious object, whatever their origin may have been; and it may be convenient to clear the ground by considering these first.
In the period between the Persian and Peloponnesian wars we hear of hetairies within the two political parties, oligarchic and democratic; Themistocles is said (Plut. Aristides, 2) to have belonged to one, Pericles’ supporters seem to have been thus organized (Plut. Per. 7 and 13), and Cimon had a hundred hetairoi devoted to him (Plut. Cim. 17). These associations were used, like the collegia sodalicia at Rome (see below), for securing certain results at elections and in the law-courts (Thuc. viii. 54), and were not regarded as harmful or illegal. But the bitterness of party struggles in Greece during the Peloponnesian War changed them in many states into political engines dangerous to the constitution, and especially to democratic institutions; Aristotle mentions (Politics, p. 1310a) a secret oath taken by the members of oligarchic clubs, containing the promise, “I will be an enemy to the people, and will devise all the harm I can against them.” At Athens in 413 b.c. the conspiracy against the democracy was engineered by means of these clubs, which existed not only there but in the other cities of the empire (Thuc. viii. 48 and 54), and had now become secret conspiracies (συνωμοσίαι) of a wholly unconstitutional kind. On this subject see Grote, Hist. of Greece, v. 360; A.H.J. Greenidge, Handbook of Greek Constitutional History, 208 foll.
Passing over the clubs for trade or plunder mentioned in Solon’s law, of which we have no detailed knowledge, we come to the religious associations. These were known by several names, especially thiasi, eranoi and orgeones, and it is not possible to distinguish these from each other in historical times, though they may have had different origins. They had the common object of sacrifice to a particular deity; the thiasi and orgeones seem to be connected more especially with foreign deities whose rites were of an orgiastic character. The organization of these societies is the subject of an excellent treatise by Paul Foucart (Les Associations religieuses chez les Grecs, Paris, 1873), still indispensable, from which the following particulars are chiefly drawn. For the greater part of them the evidence consists of 565 inscriptions from various parts of Greece, many of which were published for the first time by Foucart, and will be found at the end of his book.
The first striking point is that the object of all these associations is to maintain the worship of some foreign deity, i.e. of some deity who was not one of those admitted and guaranteed by the state—the divine inhabitants of the city, as they may be called. For all these the state made provision of priests, temples, sacrifices, &c.; but for all others these necessaries had to be looked after by private individuals associated for the purpose. The state, as we see from the law of Solon quoted above, made no difficulty about the introduction of foreign worships, provided they did not infringe the law and were not morally unwholesome, and regarded these associations as having all the rights of legal corporations. So we find the cult of deities such as Sabazius, Mater Magna (see Great Mother of the Gods) and Attis, Adonis, Isis, Serapis, Mēn Tyrannos, carried on in Greek states, and especially in seaports like the Peiraeus, Rhodes, Smyrna, without protest, but almost certainly without moral benefit to the worshippers. The famous passage in Demosthenes (de Corona, sect. 259 foll.) shows, however, that the initiation at an early age in the rites of Sabazius did not gain credit for Aeschines in the eyes of the best men. We are not surprised to find that, in accordance with the foreign character of the cults thus maintained, the members of the associations are rarely citizens by birth, but women, freedmen, foreigners and even slaves. Thus in an inscription found by Sir C. Newton at Cnidus, which contains a mutilated list of members of a thiasos, one only out of twelve appears to be a Cnidian citizen, four are slaves, seven are probably foreigners. Hence we may conclude that these associations were of importance, whether for good or for evil, in organizing and encouraging the foreign population in the cities of Greece.
The next striking fact is that these associations were organized, as we shall also find them at Rome, in imitation of the constitution of the city itself. Each had its law, its assembly, its magistrates or officers (i.e. secretary, treasurer) as well as priests or priestesses, and its finance. The law regulated the conditions of admission, which involved an entrance fee and an examination (δοκιμασία) as to character; the contributions, which had to be paid by the month, and the steps to be taken to enforce payment, e.g. exclusion in case of persistent neglect of this duty; the use to be made of the revenues, such as the building or maintenance of temple or club-house, and the cost of crowns or other honours voted by the assembly to its officers. This assembly, in accordance with the law, elected its officers once a year, and these, like those of the state itself, took an oath on entering office, and gave an account of their stewardship at the end of the year. Further details on these points of internal government will be found in Foucart’s work (pp. 20 foll.), chiefly derived from inscriptions of the orgeones engaged in the cult of the Mother of the Gods at the Peiraeus. The important question whether these religious associations were in any sense benefit clubs, or relieved the sick and needy, is answered by him emphatically in the negative.
As might naturally be supposed, the religious clubs increased rather than diminished in number and importance in the later periods of Greek history, and a large proportion of the inscriptions relating to them belong to the Macedonian and Roman empires. One of the most interesting, found in 1868, belongs to the 2nd century A.D., viz. that which reveals the worship of Mēn Tyrannos at Laurium (Foucart, pp. 119 foll.). This Phrygian deity was introduced into Attica by a Lycian slave, employed by a Roman in working the mines at Laurium. He founded the cult and the eranos which was to maintain it, and seems also to have drawn up the law regulating its ritual and government. This may help us to understand the way in which similar associations of an earlier age were instituted.
Roman.—At Rome the principle of private association was recognized very early by the state; sodalitates for religious purposes are mentioned in the XII. Tables (Gaius in Digest, 47. 22. 4), and collegia opificum, or trade gilds, were believed to have been instituted by Numa, which probably means that they were regulated by the jus divinum as being associated with particular worships. It is difficult to distinguish between the two words collegium and sodalitas; but collegium is the wider of the two in meaning, and may be used for associations of all kinds, public and private, while sodalitas is more especially a union for the purpose of maintaining a cult. Both words indicate the permanence of the object undertaken by the association, while a societas is a temporary combination without strictly permanent duties. With the societates publicanorum and other contracting bodies of which money-making was the main object, we are not here concerned.
The collegia opificum ascribed to Numa (Plut. Numa, 17) include gilds of weavers, fullers, dyers, shoemakers, doctors, teachers, painters, &c., as we learn from Ovid, Fasti, iii. 819 foll., where they are described as associated with the cult of Minerva, the deity of handiwork; Plutarch also mentions flute-players, who were connected with the cult of Jupiter on the Capitol, and smiths, goldsmiths, tanners, &c. It would seem that, though these gilds may not have had a religious origin as some have thought, they were from the beginning, like all early institutions, associated with some cult; and in most cases this was the cult of Minerva. In her temple on the Aventine almost all these collegia had at once their religious centre and their business headquarters. When during the Second Punic War a gild of poets was instituted, this too had its meeting-place in the same temple. The object of the gild in each case was no doubt to protect and advance the interests of the trade, but on this point we have no sufficient evidence, and can only follow the analogy of similar institutions in other countries and ages. We lose sight of them almost entirely until the age of Cicero, when they reappear in the form of political clubs (collegia sodalicia or compitalicia) chiefly with the object of securing the election of candidates for magistracies by fair or foul means—usually the latter (see esp. Cic. pro Plancio, passim). These were suppressed by a senatusconsultum in 64 B.C., revived by Clodius six years later, and finally abolished by Julius Caesar, as dangerous to public order. Probably the old trade gilds had been swamped in the vast and growing population of the city, and these, inferior and degraded both in personnel and objects, had taken their place. But the principle of the trade gild reasserts itself under the Empire, and is found at work in Rome and in every municipal town, attested abundantly by the evidence of inscriptions. Though the right of permitting such associations belonged to the government alone, these trade gilds were recognized by the state as being instituted “ut necessariam operam publicis utilitatibus exhiberent” (Digest, 50. 6. 6). Every kind of trade and business throughout the Empire seems to have had its collegium, as is shown by the inscriptions in the Corpus from any Roman municipal town; and the life and work of the lower orders of the municipales are shadowed forth in these interesting survivals. The primary object was no doubt still to protect the trade; but as time went on they tended to become associations for feasting and enjoyment, and more and more to depend on the munificence of patrons elected with the object of eliciting it. Fuller information about them will be found in G. Boissier, La Religion romaine d’Auguste aux Antonins, ii. 286 foll., and S. Dill, Roman Society from Nero to Marcus Aurelius, pp. 264 foll. How far they formed a basis or example for the gilds of the early middle ages is a difficult question which cannot be answered here (see Gilds); it is, however, probable that they gradually lost their original business character, and became more and more associations for procuring the individual, lost as he was in the vast desert of the empire, some little society and enjoyment in life, and the certainty of funeral rites and a permanent memorial after death.
We may now return to the associations formed for the maintenance of cults, which were usually called sodalitates, though the word collegium was also used for them, as in the case of the college of the Arval Brothers (q.v.). Of the ancient Sodales Titii nothing is known until they were revived by Augustus; but it seems probable that when a gens or family charged with 566 the maintenance of a particular cult had died out, its place was supplied by a sodalitas (Marquardt, Staatsverwaltung, iii. 134). The introduction of new cults also led to the institution of new associations; thus in 495 B.C. when the worship of Minerva was introduced, a collegium mercatorum was founded to maintain it, which held its feast on the dies natalis (dedication day) of the temple (Liv. ii. 27. 5); and in 387 the ludi Capitolini were placed under the care of a similar association of dwellers on the Capitoline hill. In 204 B.C. when the Mater Magna was introduced from Pessinus (see Great Mother of the Gods) a sodalitas (or sodalitates) was instituted which, as Cicero tells us (de Senect. 13. 45) used to feast together during the ludi Megalenses. All such associations were duly licensed by the state, which at all times was vigilant in forbidding the maintenance of any which it deemed dangerous for religious or political reasons; thus in 186 B.C. the senate, by a decree of which part is preserved (C.I.L. i. 43), made all combination for promoting the Bacchic religious rites strictly illegal. But legalized sodalitates are frequent later; the temple of Venus Genetrix, begun by Julius and finished by Augustus, had its collegium (Pliny, N.H. ii. 93), and sodalitates were instituted for the cult of the deified emperors Augustus, Claudius, &c.
We thus arrive by a second channel at the collegia of the empire. Both the history of the trade gilds and that of the religious collegia or sodalitates conduct us by a course of natural development to that extraordinary system of private association with which the empire was honeycombed.
As has been already said of the trade gilds, the main objects of association seem to have been to make life more enjoyable and to secure a permanent burial-place; and of these the latter was probably the primary or original one. It was a natural instinct in the classical as in the pre-classical world to wish to rest securely after death, to escape neglect and oblivion. This is not the place to explain the difficulties which the poorer classes in the Roman empire had to face in satisfying this instinct; but since the publication of the Corpus Inscriptionum has made us familiar with the conditions of the life of these classes, there can be no doubt that this was always a leading motive in their passion for association. In the year A.D. 133 under Hadrian this instinct was recognized by law, i.e. by a senatusconsultum which has fortunately come down to us. It was engraved at the head of their own regulations by a collegium instituted for the worship of Diana and Antinous at Lanuvium, and runs thus: ”Qui stipem menstruam conferre volent in funera, in id collegium coëant, neque sub specie ejus collegii nisi semel in mense coëant conferendi causa unde defuncti sepeliantur” (C.I.L. xiv. 2112). From the Digest, 47. 22. 1, the locus classicus on this subject, we learn that this was a general law allowing the founding of funerary associations, provided that the law against illicit collegia were complied with, and it was natural that from that time onwards such collegia should spring up in every direction. The inscription of Lanuvium, together with many others (for which see the works of Boissier and Dill already cited), has given us a clear idea of the constitution of these colleges. Their members were as a rule of the humblest classes of society, and often included slaves; from each was due an entrance fee and a monthly subscription, and a funeral grant was made to the heir of each member at his death in order to bury him in the burying-place of the college, or if they were too poor to construct one of their own, to secure burial in a public columbarium. The instinct of the Roman for organization is well illustrated in the government of these colleges. They were organized on exactly the same lines as the municipal towns of the empire; their officers were elected, usually for a year, or in the case of honorary distinctions, for life; as in a municipal town, they were called quinquennales, curatores, praefecti, &c., and quaestors superintended the finances of the association. Their place of meeting, if they were rich enough to have one, was called schola and answered the purpose of a club-house; the site or the building was often given them by some rich patron, who was pleased to see his name engraved over its doorway. Here we come upon one of those defects in the society of the empire which seem gradually to have sapped the virility of the population—the desire to get others to do for you what you are unwilling or unable to do for yourself. The patroni increased in number, and more and more the colleges acquired the habit of depending on their benefactions, while at the same time it would seem that the primary object of burial became subordinate to the claims of the common weal. It may also be asserted with confidence, as of the Greek clubs, that these collegia rarely or never did the work of our benefit clubs, by assisting sick or infirm members; such objects at any rate do not appear in the inscriptions. The only exceptions seem to be the military collegia, which, though strictly forbidden as dangerous to discipline, continued to increase in number in spite of the law. The great legionary camps of the Roman province of Africa (Cagnat, L’Armée romaine, 457 foll.) have left us inscriptions which show not only the existence of these clubs, but the way in which their funds were spent; and it appears that they were applied to useful purposes in the life of a member as well as for his burial, e.g. to travelling expenses, or to his support after his discharge (see especially C.I.L. viii. 2552 foll.).
As the Roman empire became gradually impoverished and depopulated, and as the difficulty of defending its frontiers increased, these associations must have been slowly extinguished, and the living and the dead citizen alike ceased to be the object of care and contribution. The sudden invasion of Dacia by barbarians in A.D. 166 was followed by the extinction of one collegium which has left a record of the fact, and probably by many others. The master of the college of Jupiter Cernenius, with the two quaestors and seven witnesses, attest the fact that the college has ceased to exist. “The accounts have been wound up, and no balance is left in the chest. For a long time no member has attended on the days fixed for meetings, and no subscriptions have been paid” (Dill, op. cit. p. 285). The record of similar extinctions in the centuries that followed, were they extant, would show us how this interesting form of crystallization, in which the well-drilled people of the empire displayed an unusual spontaneity, gradually melted away and disappeared (see further Gilds and Charity and Charities).
Modern Clubs.—The word “club,” in its modern sense of an association to promote good-fellowship and social intercourse, is not very old, only becoming common in England at the time of The Tatler and The Spectator (1709-1712). It is doubtful whether its use originated in its meaning of a knot of people, or from the fact that the members “clubbed” together to pay the expenses of their meetings. The oldest English clubs were merely informal periodic gatherings of friends for the purpose of dining or drinking together. Thomas Occleve (temp. Henry IV.) mentions such a club called La Court de Bone Compaignie, of which he was a member. John Aubrey (writing in 1659) says: “We now use the word clubbe for a sodality in a tavern.” Of these early clubs the most famous was the Bread Street or Friday Street Club, originated by Sir Walter Raleigh, and meeting at the Mermaid Tavern. Shakespeare, Beaumont, Fletcher, Selden and Donne were among the members. Another such club was that which met at the Devil Tavern near Temple Bar; and of this Ben Jonson is supposed to have been the founder.
With the introduction of coffee-drinking in the middle of the 17th century, clubs entered on a more permanent phase. The coffee-houses of the later Stuart period are the real originals of the modern club-house. The clubs of the late 17th and early 18th century type resembled their Tudor forerunners in being oftenest associations solely for conviviality or literary coteries. But many were confessedly political, e.g. The Rota, or Coffee Club (1659), a debating society for the spread of republican ideas, broken up at the Restoration, the Calves Head Club (c. 1693) and the Green Ribbon Club (1675) (q.v.). The characteristics of all these clubs were: (1) no permanent financial bond between 567 the members, each man’s liability ending for the time being when he had paid his “score” after the meal; (2) no permanent club-house, though each clique tended to make some special coffee-house or tavern their headquarters. These coffee-house clubs soon became hotbeds of political scandal-mongering and intriguing, and in 1675 Charles II. issued a proclamation which ran, “His Majesty hath thought fit and necessary that coffee houses be (for the future) put down and suppressed,” owing to the fact “that in such houses divers false, malitious and scandalous reports are devised and spread abroad to the Defamation of his Majesty’s Government and to the Disturbance of Peace and Quiet of the Realm.” So unpopular was this proclamation that it was almost instantly found necessary to withdraw it, and by Anne’s reign the coffee-house club was a feature of England’s social life.
From the 18th-century clubs two types have been evolved. (1) The social and dining clubs, permanent institutions with fixed club-house. The London coffee-house clubs in increasing their members absorbed the whole accommodation of the coffee-house or tavern where they held their meetings, and this became the club-house, often retaining the name of the original keeper, e.g. White’s, Brooks’s, Arthur’s, Boodle’s. The modern club, sometimes proprietary, i.e. owned by an individual or private syndicate, but more frequently owned by the members who delegate to a committee the management of its affairs, first reached its highest development in London, where the district of St James’s has long been known as “Clubland”; but the institution has spread all over the English-speaking world. (2) Those clubs which have but occasional or periodic meetings and often possess no club-house, but exist primarily for some specific object. Such are the many purely athletic, sports and pastimes clubs, the Jockey Club, the Alpine, chess, yacht and motor clubs. Then there are literary clubs, musical and art clubs, publishing clubs; and the name of “club” has been annexed by a large group of associations which fall between the club proper and mere friendly societies, of a purely periodic and temporary nature, such as slate, goose and Christmas clubs, which are not required to be registered under the Friendly Societies Act.
Thus it is seen that the modern club has little in common with its prototypes in the 18th century. Of those which survive in London the following may be mentioned: White’s, originally established in 1698 as White’s Chocolate House, became the headquarters of the Tory party, but is to-day no longer political. Brooks’s (1764), originally the resort of the Whigs, is no longer strictly associated with Liberalism. Boodle’s (1762) had a tradition of being the resort of country gentlemen, and especially of masters of foxhounds. Arthur’s (1765), originally an offshoot of White’s, has always been purely social. The Cocoa Tree (1746) also survives as a social resort. Social clubs, without club-houses, are represented by the Literary Club (“The Club”), founded in 1764 by Sir Joshua Reynolds and Dr Johnson, and such recent institutions as the Johnson Club, Ye Sette of Odd Volumes (founded by Bernard Quaritch) and many others.
The number of regularly established clubs in London is now upwards of a hundred. Of these the more important, with the dates of their establishment, are: Army and Navy (1837); Athenaeum (1824), founded by Sir Walter Scott and Thomas Moore “for the association of individuals known for their scientific or literary attainments, artists of eminence in any class of the fine arts, and noblemen and gentlemen distinguished as liberal patrons of science, literature or the arts”; Bachelors’ (1881); Carlton (1832), the chief Conservative club; City Carlton (1868); Conservative (1840); Constitutional (1883); Devonshire (1875); East India United Service (1849); Garrick (1831), “for the general patronage of the drama, for bringing together the supporters of the drama, and for the formation of a theatrical library with works on costume”; Guards (1813); Junior Athenaeum (1864); Junior Carlton (1864); Marlborough (1869); National Liberal (1882); Oriental (1824); Oxford and Cambridge (1830); Reform (1837), formerly the Liberal headquarters; Savage (1857); St James’s (1857), diplomatic; Travellers’ (1819), for which a candidate must have “travelled out of the British Islands to a distance of at least 500 m. from London in a direct line”; Turf (1868); Union (1822); United Service (1815); Wellington (1885); Windham (1828). Almost every interest, rank and profession has its club. Thus there is a Press Club, a Fly-Fishers’ Club, a Gun Club, an Authors’, a Farmers’, a Lawyers’ (the Eldon) and a Bath Club. Of the purely women’s clubs the most important are the Alexandra (1884), the Empress (1897), Lyceum (1904) and Ladies’ Army & Navy (1904); while the Albemarle and the Sesame have a leading place among clubs for men and women. Of political clubs having no club-house, the best known are the Cobden (Free Trade, 1866); the Eighty (Liberal, 1880) and the United (Unionist, 1886). There are clubs in all important provincial towns, and at Edinburgh the New Club (1787), and in Dublin the Kildare Street (1790), rival those of London.
The mode of election of members varies. In some clubs the committee alone have the power of choosing new members. In others the election is by ballot of the whole club, one black ball in ten ordinarily excluding. In the Athenaeum, whilst the principle of election by ballot of the whole club obtains, the duty is also cast upon the committee of annually selecting nine members who are to be “of distinguished eminence in science, literature or the arts, or for public services,” and the rule makes stringent provision for the conduct of these elections. On the committee of the same club is likewise conferred power to elect without ballot princes of the blood royal, cabinet ministers, bishops, the speaker of the House of Commons, judges, &c.
The affairs of clubs are managed by committees constituted of the trustees, who are usually permanent members, and of ordinarily twenty-four other members, chosen by the club at large, one-third of whom go out of office annually. These committees have plenary powers to deal with the affairs of the club committed to their charge, assembling weekly to transact current business and audit the accounts. Once a year a meeting of the whole club is held, before which a report is laid, and any action taken thereupon which may be necessary. (See J. Wertheimer, The Law relating to Clubs, 1903; and Sir E. Carson on Club law, in vol. iii. of The Laws of England, 1909.)
Previous to 1902 clubs in England had not come within the purview of the licensing system. The Licensing Act of 1902, however, remedied that defect, and although it was passed principally to check the abuse of “clubs” being formed solely to sell intoxicating liquors free from the restrictions of the licensing acts, it applied to all clubs in England and Wales, of whatever kind, from the humblest to the most exalted Pall Mall club. The act required the registration of every club which occupied any premises habitually used for the purposes of a club and in which intoxicating liquor was supplied to members or their guests. The secretary of every club was required to furnish to the clerk to the justices of the petty sessional division a return giving (a) the name and objects of the club; (b) the address of the club; (c) the name of the secretary; (d) the number of members; (e) the rules of the club relating to (i.) the election of members and the admission of temporary and honorary members and of guests; (ii.) the terms of subscription and entrance fee, if any; (iii.) the cessation of membership; (iv.) the hours of opening and closing; and (v.) the mode of altering the rules. The same particulars must be furnished by a secretary before the opening of a new club. The act imposed heavy penalties for supplying and keeping liquor in an unregistered club. The act gave power to a court of summary jurisdiction to strike a club off the register on complaint in writing by any person on any of various grounds, e.g. if its members numbered less than twenty-five; if there was frequent drunkenness on the premises; if persons were habitually admitted as members without forty-eight hours’ interval between nomination and admission; if the supply of liquor was not under the control of the members or the committee, &c. The Licensing (Scotland) Act 1903 made Scottish clubs liable to registration in a similar manner.
In no other country did club-life attain such an early perfection 568 as in England. The earliest clubs on the European continent were of a political nature. These in 1848 were repressed in Austria and Germany, and the modern clubs of Berlin and Vienna are mere replicas of their English prototypes. In France, where the term cercle is most usual, the first was Le Club Politique (1782), and during the Revolution such associations proved important political forces (see Jacobins, Feuillants, Cordeliers). Of the modern purely social clubs in Paris the most notable are The Jockey Club (1833) and the Cercle de la Rue Royale.
In the United States clubs were first established after the War of Independence. One of the first in date was the Hoboken Turtle Club (1797), which still survives. Of the modern clubs in New York the Union (1836) is the earliest, and other important ones are the Century (1847), Union League (1863), University (1865), Knickerbocker (1871), Lotus (1870), Manhattan (1865), and Metropolitan (1891). But club-life in American cities has grown to enormous proportions; the number of excellent clubs is now legion, and their hospitality has become proverbial. The chief clubs in each city are referred to in the topographical articles.
Walter Arnold, Life and Death of the Sublime Society of Beefsteaks (1871); John Aubrey, Letters of Eminent Persons (2 vols.); C. Marsh, Clubs of London, with Anecdotes of their Members, Sketches of Character and Conversation (2 vols., 1832); Notes and Queries, 3rd series, vols. 1, 9, 10; W. H. Pyne, Wine and Walnuts (2 vols., 1823); Admiral Smyth, Sketch of the Use and Progress of the Royal Society Club (1860); John Timbs, Club Life of London, with Anecdotes of Clubs, Coffee-Houses and Taverns (2 vols., 1866), and History of Clubs and Club Life (1872); Th. Walker, The Original, fifth edition, by W. A. Guy (1875); The Secret History of Clubs of all Descriptions by Ned Ward (1709); Complete and Humourous Account of all the Remarkable Clubs and Societies in the Cities of London and Westminster, by Ned Ward (7th edition, 1756); The London Clubs; their Anecdotes, History, Private Rules and Regulations (12mo, 1853); Rev. A. Hume, Learned Societies and Printing Clubs (1847); J. Strang, Glasgow and its Clubs (1857); A. F. Leach, Club Cases (1879); Col. G. J. Ivey, Clubs of the World (1880); J. Wertheimer, Law relating to Clubs (1885); L. Fagan, The Reform Club (1887); F. G. Waugh, Members of the Athenaeum Club (privately printed 1888).
CLUB-FOOT (talipes), the name given to deformities of the foot, some of which are congenital, others acquired—the latter being chiefly due to infantile paralysis. Talipes equinus is that form in which the heel does not touch the ground, the child resting on the toes. In talipes varus the foot is turned inwards and shortened, the inner edge of the foot is raised, and the child walks on the outer edge. These two conditions are often combined, the heel being drawn up and the foot twisted inward; the name given to the twofold deformity is talipes equino-varus. It is the most usual congenital form. In talipes calcaneus the toes are pointed upwards and the foot rests on the heel. This is always an acquired (paralytic) deformity.
The treatment of congenital club-foot, which is almost invariably varus or equino-varus, should be begun as soon as ever the abnormal condition of the foot is recognized. The nurse should be shown how to twist and coax the foot into the improved position, and should so hold it in her hand many times a day. And thus by daily, or, one might almost say, hourly manipulations, much good may be accomplished without distress to the infant. If after weeks or months of these measures insufficient progress has been made, the subcutaneous division of a tendon or two, or of some tendons and ligaments may be necessary, the foot being subsequently fixed up in the improved position in plaster of Paris. If these subcutaneous operations also prove disappointing, or if after their apparently successful employment the foot constantly relapses into the old position, a more radical procedure will be required. Of the many procedures which have been adopted there is, probably, none equal to that of free transverse incision introduced by the late Dr A. M. Phelps of New York. By this “open method” the surgeon sees exactly what structures are at fault and in need of division—skin, fasciae tendons, ligaments; everything, in short, which prevented the easy rectification of the deformity. After the operation, the foot is fixed, without any strain, in an over-corrected position, between plaster of Paris splints. By the adoption of this method the old instrument of torture known as “Scarpa’s shoe” has become obsolete, as have also some of those operations which effected improvement of the foot by the removal of portions of the bony arch. Phelps’s operation removes the deformity by increasing the length of the concave border of the foot rather than by shortening the convex borders as in cuneiform osteotomy; it is a levelling up, not a levelling down.
Talipes valgus is very rare as a congenital defect, but is common enough as a result of infantile paralysis and as such is apt to be combined with the calcanean variety. “Flat-foot” is sometimes spoken of as spurious talipes valgus; it is due to the bony arches of the foot being called upon to support a weight beyond their power. The giving way of the arches may be due to weakness of the muscles, tendons or ligaments—probably of all three. It is often met with in feeble and flabby children, and in nurses, waiters, policemen and others whose feet grow tired from much standing. Exercises on tip-toe, especially with a skipping rope, massage, rest and tonic treatment will give relief, and shoes or boots may be supplied with the heel and sole thickened along the inner borders so that the weight may be received along the strong outer border of the foot. When the flat-footed individual stands it should be upon the outer borders of his feet, or better still, when convenient, on tip-toe, as this posture strengthens those muscles of the leg which run into the sole of the foot and hold up the bony arches. In certain extreme cases the surgeon wrenches the splay feet into an inverted position and fixes them in plaster of Paris, taking off the casing every day for the purpose of massage and exercises.
Flat-foot is often associated with knock-knee in children and young adults who are the subject of rickets.
Morton’s Disease.—In some cases of flat-foot the life of the individual is made miserable by neuralgia at the root of the toes, which comes on after much standing or walking, the distress being so great that, almost regardless of propriety, he is compelled to take off his boot. The condition is known as Morton’s disease or metatarsalgia. The pain is due to the nerves of the toes (which come from the sole of the foot) being pressed upon by the rounded ends of the long bones of the foot near the web of the toes. It does not generally yield to palliative measures (though rest of the foot and a change to broad-toed, easy boots may be helpful), and the only effectual remedy is resection of the head of one of the metatarsal bones, after which relief is complete and permanent.
For paralytic club-foot, in which distressing corns have been developed over the unnatural prominences upon which the sufferer has been accustomed to walk, the adoption of the most promising conservative measures are usually disappointing, and relief and happiness may be obtainable only after the performance of Syme’s amputation through the ankle-joint.
CLUE, or Clew (O. Eng. cluwe), originally a ball of thread or wool, the thread of life, which, according to the fable, the Fates spin for every man. The ordinary figurative meaning, a piece of evidence leading to discovery, or a sign pointing to the right track, is derived from the story of Theseus, who was guided through the labyrinth by the ball of thread held by Ariadne.
CLUENTIUS HABITUS, AULUS, of Larinum in Samnium, the hero of a Roman cause célèbre. In 74 B.C. he accused his stepfather Statius Albius Oppianicus of an attempt to poison him; had it been successful, the property of Cluentius would have fallen to his mother Sassia. Oppianicus and two others were condemned, and some years later Oppianicus died in exile. But the verdict was looked upon with suspicion, and it was known for a fact that one of the jurymen had received a large sum of money for distribution amongst his colleagues. The result was the degradation of Cluentius himself and several of the jurymen. In 66, Sassia induced her stepson Oppianicus to charge Cluentius with having caused the elder Oppianicus to be poisoned while in exile. On this occasion the defence was undertaken by Cicero in the extant speech Pro Cluentio. In the end Cluentius was acquitted. Cicero afterwards boasted openly that he had thrown dust in the eyes of the jury (Quintilian, Instit. ii. 17. 21, who quotes this speech more than any other). His efforts are chiefly devoted to proving that the condemnation 569 of the elder Oppianicus was just and in no way the result of the jury having been bribed by Cluentius; only a small portion of the end of the speech deals with the specific charge. It was generally believed that the verdict in the former trial was an unfair one; and this opinion was most prejudicial to Cluentius. But even if it could be shown that Cluentius had bribed the jurymen, this did not prove that he had poisoned Oppianicus, although it supplied a sufficient reason for wishing to get him out of the way. The speech delivered by Cicero on this occasion is considered one of his best.
Editions of the speech by W. Y. Fausset (1887), W. Ramsay (1883); see also H. Nettleship, Lectures and Essays (1885).
CLUMP, a word common to Teutonic languages, meaning a mass, lump, group or cluster of indefinite form, as a clump of grass or trees. The word is used of a wooden and clumsy shoe, made out of one piece of wood, worn by German peasants, and by transference is applied to the thick extra sole added to heavy boots for rough wear. Shoemakers speak of “clumping” a boot when it is mended by having a new sole fastened by nails and not sewn by hand to the old sole.
CLUNES, a borough of Talbot county, Victoria, Australia, 97½ m. by rail N.W. of Melbourne. Pop. (1901) 2426. It is the centre of an agricultural, pastoral and mining district, in which gold was first discovered in 1851. It lies in a healthy and picturesque situation at an elevation of 1081 ft. An annual agricultural exhibition and large weekly cattle sales are held in the town.
CLUNY, or Clugny, a town of east central France, in the department of Saône-et-Loire, on the left bank of the Grosne, 14 m. N.W. of Mâcon by road. Pop. (1906) 3105. The interest of the town lies in its specimens of medieval architecture, which include, besides its celebrated abbey, the Gothic church of Notre-Dame, the church of St Marcel with its beautiful Romanesque spire, portions of the ancient fortifications, and a number of picturesque houses belonging to the Romanesque, Gothic and Renaissance periods. The chief remains of the abbey (see Abbey) are the ruins of the basilica of St Peter and the abbot’s palace. The church was a Romanesque building, completed early in the 12th century, and until the erection of St Peter at Rome was the largest ecclesiastical building in Europe. It was in great part demolished under the First Empire, but the south transept, a high octagonal tower, the chapel of Bourbon (15th century), and the ruins of the apse still remain. In 1750 the abbey buildings were largely rebuilt and now contain a technical school. Part of the site of the church is given up to the stabling of a government stud. The abbot’s palace, which belongs to the end of the 15th century, serves as hôtel-de-ville, library and museum. The town has quarries of limestone and building-stone, and manufactures pottery, leather and paper.
A mere village at the time when the abbey was founded (910), Cluny gradually increased in importance with the development of the religious fraternity, and in 1090 received a communal charter from the abbot St Hugh. In 1471 the town was taken by the troops of Louis XI. In 1529 the abbey was given “in commendam” to the family of Guise, four members of which held the office of abbot during the next hundred years. The town and abbey suffered during the Wars of Religion of the 16th century, and the abbey was closed in 1790. The residence erected in Paris at the end of the 15th century by the abbots Jean de Bourbon and Jacques d’Amboise, and known as the Hôtel de Cluny (see House, Plate I., fig. 6), is occupied by the du Sommerard collection; but the Collège de Cluny founded in 1269 by the abbot Yves de Vergy, as a theological school for the order, is no longer in existence.
The Order of Cluniac Benedictines.—The Monastery of Cluny was founded in 910 by William I. the Pious, count of Auvergne and duke of Guienne (Aquitaine). The first abbot was Berno, who had under his rule two monasteries in the neighbourhood. Before his death in 927 two or three more came under his control, so that he bequeathed to his successor the government of a little group of five or six houses, which became the nucleus of the order of Cluny. Berno’s successor was Odo: armed with papal privileges he set to work to make Cluny the centre of a revival and reform among the monasteries of France; he also journeyed to Italy, and induced some of the great Benedictine houses, and among them St Benedict’s own monasteries of Subiaco and Monte Cassino, to receive the reform and adopt the Cluny manner of life. The process of extension, partly by founding new houses, partly by incorporating old ones, went on under Odo’s successors, so that by the middle of the 12th century Cluny had become the centre and head of a great order embracing 314 monasteries—the number 2000, sometimes given, is an exaggeration—in all parts of Europe, in France, Italy, the Empire, Lorraine, Spain, England, Scotland, Poland, and even in the Holy Land. And the influence of Cluny extended far beyond the actual order: many monasteries besides Monte Cassino and Subiaco adopted its customs and manner of life without subjecting themselves to its sway; and of these, many in turn became the centres of reforms which extended Cluny ideas and influences over still wider circles: Fleury and Hirsau may be mentioned as conspicuous examples. The gradual stages in the growth of the Cluny sphere of influence is exhibited in a map [VI. C.] in Heussi and Mulert’s Handatlas zur Kirchengeschichte, 1905.
When we turn to the inner life of Cluny, we find that the decrees of Aix-la-Chapelle, which summed up the Carolingian movement for reform (see Benedictines), were taken as the basis of the observance. Field work and manual labour were given up; and in compensation the tendency initiated by Benedict of Aniane, to prolong and multiply the church services far beyond the canonical office contemplated by St Benedict, was carried to still greater extremes, so that the services came to occupy nearly the whole day. The lessons at the night office became so lengthy that, e.g., the Book of Genesis was read through in a week; and the daily psalmody, between canonical office and extra devotions, exceeded a hundred psalms (see Edm. Bishop, Origin of the Primer, Early English Text Soc., Original Series, No. 109).
If its influence on the subsequent history of monastic and religious life and organization be considered, the most noteworthy feature of the Cluny system was its external polity, which constituted it a veritable “order” in the modern sense of the word, the first that had existed since that of Pachomius (see Monasticism). All the houses that belonged, either by foundation or incorporation, to the Cluny system were absolutely subject to Cluny and its abbot, who was “general” in the same sense as the general of the Jesuits or Dominicans, the practically absolute ruler of the whole system. The superiors of all the subject houses (usually priors, not abbots) were his nominees; every member of the order was professed by his permission, and had to pass some of the early years of his monastic life at Cluny itself; the abbot of Cluny had entire control over every one of the monks—some 10,000, it is said; it even came about that he had the practical appointment of his successor. For a description and criticism of the system, see F. A. Gasquet, Sketch of Monastic Constitutional History, pp. xxxii-xxxv (the Introduction to 2nd ed. (1895) of the English trans. of the Monks of the West); here it must suffice to say that it is the very antithesis of the Benedictine polity (see Benedictines).
The greatness of Cluny is really the greatness of its early abbots. If the short reign of the unworthy Pontius be excepted, Cluny was ruled during a period of about 250 years (910-1157) by a succession of seven great abbots, who combined those high qualities of character, ability and religion that were necessary for so commanding a position; they were Berno, Odo, Aymard, Majolus (Maieul), Odilo, Hugh, Peter the Venerable. Sprung from noble families of the neighbourhood; educated to the highest level of the culture of those times; endowed with conspicuous ability and prudence in the conduct of affairs; enjoying the consideration and confidence of popes and sovereigns; employed again and again as papal legates and imperial ambassadors; taking part in all great movements of ecclesiastical and temporal politics; refusing the first sees in Western Christendom, the cardinalate, and the papacy itself: 570 they ever remained true to their state as monks, without loss of piety or religion. Four of them, indeed, Odo, Maieul, Odilo and Hugh, are venerated as saints.
In the movement associated with the name of Hildebrand the influence of Cluny was thrown strongly on the side of religious and ecclesiastical reform, as in the suppression of simony and the enforcing of clerical celibacy; but in the struggle between the Papacy and the Empire the abbots of Cluny seem to have steered a middle course between Guelfs and Ghibellines, and to have exercised a moderating influence; St Hugh maintained relations with Henry IV. after his excommunication, and probably influenced him to go to Canossa. Hildebrand himself, though probably not a monk of Cluny, was a monk of a Cluniac monastery in Rome; his successor, Urban II., was actually a Cluny monk, as was Paschal II. It may safely be said that from the middle of the 10th century until the middle of the 12th, Cluny was the chief centre of religious influence throughout Western Europe, and the abbot of Cluny, next to the pope, the most important and powerful ecclesiastic in the Latin Church.
Everything at Cluny was on a scale worthy of so great a position. The basilica, begun 1089 and dedicated 1131, was, until the building of the present St Peter’s, the largest church in Christendom, and was both in structure and ornamentation of unparalleled magnificence. The monastic buildings were gigantic.
During the abbacy of Peter the Venerable (1122-1157) it became clear that, after a lapse of two centuries, a renewal of the framework of the life and a revival of its spirit had become necessary. Accordingly he summoned a great chapter of the whole order whereat the priors and representatives of the subject houses attended in such numbers that, along with the Cluny community, the assembly consisted of 1200 monks. This chapter drew up the 76 statutes associated with Peter’s name, regulating the whole range of claustral life, and solemnly promulgated as binding on the whole Cluniac obedience. But these measures did not succeed in saving Cluny from a rapid decline that set in immediately after Peter’s death. The monarchical status of the abbot was gradually curtailed by the holding of general chapters at fixed periods and the appointment of a board of definitors, elected by the chapter, as a permanent council for the abbot. Owing to these restrictions and still more to the fact that the later abbots were not of the same calibre as the early ones, their power and influence waned, until in 1528 (if not in 1456) the abbey fell into “commendam.” The rise of the Cistercians and the mendicant orders were contributory causes, and also the difficulties experienced in keeping houses in other countries subject to a French superior. And so the great system gradually became a mere congregation of French houses. Of the commendatory abbots the most remarkable were Cardinals Richelieu and Mazarin, who both initiated attempts to introduce reforms into the Cluny congregation, the former trying to amalgamate it with the reformed congregation of St Maur, but without effect. Martène tells us that in the early years of the 18th century in the monastery of Baume, one of Berno’s original group of Cluny houses—indeed the parent house of Cluny itself—no one was admitted as a monk who had not sixteen quarterings in his coat of arms. A reform movement took root in the Cluny congregation, and during the last century of its existence the monks were divided into two groups, the Reformed and the Unreformed, living according to different laws and rules, with different superiors, and sometimes independent, and even rival, general chapters. This most unhappy arrangement hopelessly impaired the vitality and work of the congregation, which was finally dissolved and suppressed in 1790, the church being deliberately destroyed.
Cluniac houses were introduced into England under the Conqueror. The first foundation was at Barnstaple; the second at Lewes by William de Warenne, in 1077, and it counted as one of the “Five Daughters of Cluny.” In quick succession followed Thetford, Montacute, Wenlock, Bermondsey, and in Scotland, Paisley; a number of lesser foundations were made, and offshoots from the English houses; so that the English Cluniac dependencies in the 13th century amounted to 40. It is said that in the reign of Edward III. they transmitted to Cluny annually the sum of £2000, equivalent to £60,000 of our money. Such a drain on the country was naturally looked on with disfavour, especially during the French wars; and so it came about that as “alien priories” they were frequently sequestered by the crown. As the communities came to be composed more and more of English subjects, they tended to grow impatient of their subjection to a foreign house, and began to petition parliament to be naturalized and to become denizen. In 1351 Lewes was actually naturalized, but a century later the prior of Lewes appears still as the abbot of Cluny’s vicar in England. Though the bonds with Cluny seem to have been much relaxed if not wholly broken, the Cluniac houses continued as a separate group up to the dissolution, never taking part in the chapters of the English Benedictines. At the end there were eight greater and nearly thirty lesser Cluniac houses: for list see Table in F. A. Gasquet’s English Monastic Life; and Catholic Dictionary, art. “Cluny.”
CLUSERET, GUSTAVE PAUL (1823-1900), French soldier and politician, was born at Paris. He was an officer in the garde mobile during the revolution of 1848. He took part in several expeditions in Algeria, joined Garibaldi’s volunteers in 1860, and in 1861 resigned his commission to take part in the Civil War in America. He served under Frémont and McClellan, and rose to the rank of general. Then, joining a band of Irish adventurers, he went secretly to Ireland, and participated in the Fenian insurrection (1866-67). He escaped arrest on the collapse of the movement, but was condemned to death in his absence. On his return to France he proclaimed himself a Socialist, opposed militarism, and became a member of the Association Internationale des travailleurs, a cosmopolitan Socialist organization, known as the “Internationale.” On the proclamation of the Third Republic in 1871 he set to work to organize the social revolution, first at Lyons and afterwards at Marseilles. His energy, his oratorical gifts, and his military experience gave him great influence among the working classes. On the news of the communist rising of the 18th of March 1871 he hastened to Paris, and on the 16th of April was elected a member of the commune. Disagreements with the other communist leaders led to his arrest on the 1st of May, on a false charge of betraying the cause. On the 24th of the same month the occupation of Paris by the Versailles troops restored him to liberty, and he succeeded in escaping from France. He did not return to the country till 1884. In 1888 and 1889 he was returned as a deputy to the chamber by Toulon. He died in 1900. Cluseret published his Mémoires (of the Commune) at Paris in 1887-1888.
CLUSIUM (mod. Chiusi, q.v.), an ancient town of Italy, one of the twelve cities of Etruria, situated on an isolated hill at the S. end of the valley of the Clanis (China). It was according to Roman tradition one of the oldest cities of Etruria and indeed of all Italy, and, if Camars (the original name of the town, according to Livy) is rightly connected with the Camertes Umbri, its foundation would go back to pre-Etruscan times. It first appears in Roman history at the end of the 7th century B.C., when it joined the other Etruscan towns against Tarquinius Priscus, and at the end of the 6th century B.C. it placed itself, 571 under its king Lars Porsena, at the head of the attempt to re-establish the Tarquins in Rome. At the time of the invasion of the Gauls in 391 B.C., on the other hand, Clusium was on friendly terms with Rome; indeed, it was the action of the Roman envoys who had come to intercede for the people of Clusium with the Gauls, and then, contrary to international law, took part in the battle which followed, which determined the Gauls to march on Rome. Near Clusium too, according to Livy (according to Polybius ii. 19. 5, ἐν τῆ Καμερτίων χώρᾳ, i.e. in Umbria near Camerinum), a battle occurred in 296 B.C. between the Gauls and Samnites combined, and the Romans; a little later the united forces of Clusium and Perusia were defeated by the Romans. The precise period at which Clusium came under Roman supremacy is, however, uncertain, though this must have happened before 225 B.C., when the Gauls advanced as far as Clusium. In 205 B.C. in the Second Punic War we hear that they promised ship timber and corn to Scipio. The Via Cassia, constructed after 187 B.C., passed just below the town. In the first civil war, Papirius Carbo took up his position here, and two battles occurred in the neighbourhood. Sulla appears to have increased the number of colonists, and a statue was certainly erected in his honour here. In imperial times we hear little of it, though its grain and grapes were famous. Christianity found its way into Clusium as early as the 3rd century, and the tombstone of a bishop of A.D. 322 exists. In A.D. 540 it is named as a strong place to which Vitiges sent a garrison of a thousand men.
Of pre-Roman or Roman buildings in the town itself there are few remains, except for some fragments of the Etruscan town walls composed of rather small rectangular blocks of travertine, built into the medieval fortifications. Under it, however, extends an elaborate system of rock-cut passages, probably drains. The chief interest of the place lies in its extensive necropolis, which surrounds the city on all sides. The earliest tombs (tombe a pozzo, shaft tombs) are previous to the beginning of Greek importation. Of tombe a fosso there are none, and the next stage is marked by the so-called tombe a ziro, in which the cinerary urn (often with a human head) is placed in a large clay jar (ziro, Lat. dolium). These belong to the 7th century B.C., and are followed by the tombe a camera, in which the tomb is a chamber hewn in the rock, and which can be traced back to the beginning of the 6th century B.C. From one of the earliest of these came the famous François vase; another is the tomb of Poggio Renzo, or della Scimmia (the monkey), with several chambers decorated with archaic paintings. The most remarkable group of tombs is, however, that of Poggio Gaiella, 3 m. to the N., where the hill is honeycombed with chambers in three storeys (now, however, much ruined and inaccessible), partly connected by a system of passages, and supported at the base by a stone wall which forms a circle and not a square—a fact which renders impossible its identification with the tomb of Porsena, the description of which Pliny (Hist. Nat. xxxvi. 91) has copied from Varro. Other noteworthy tombs are those of the Granduca, with a single subterranean chamber carefully constructed in travertine, and containing eight sarcophagi of the same material; of Vigna Grande, very similar to this; of Colle Casuccini (the ancient stone door of which is still in working order), with two chambers, containing paintings representing funeral rites; of Poggio Moro and Valdacqua, in the former of which the paintings are almost destroyed, while the latter is now inaccessible.
A conception of the size of the whole necropolis may be gathered from the fact that nearly three thousand Etruscan inscriptions have come to light from Clusium and its district alone, while the part of Etruria north of it as far as the Arno has produced barely five hundred. Among the later tombs bilingual inscriptions are by no means rare, and both Etruscan and Latin inscriptions are often found in the same cemeteries, showing that the use of the Etruscan language only died out gradually. A large number of the inscriptions are painted upon the tiles which closed the niches containing the cinerary urns. The urns themselves are small, often of terra-cotta, originally painted, though the majority of them have lost their colour, and rectangular in shape. This style of burial seems peculiar to a district which E. Bormann (Corp. Inscr. Lat. xi., Berlin, 1887, p. 373) defines as a triangle formed by the Clanis (with the lakes of Chiusi and Montepulciano, both small, shallow and fever-breeding), on the E., the villages of Cetona, Sarteano, Castelluccio and Monticchiello on the W., and Montepulciano and Acquaviva on the N. In Roman times the territory of Clusium seems to have extended as far as Lake Trasimene. The local museum contains a valuable and important collection of objects from the necropolis, including some specially fine bucchero, sepulchral urns of travertine, alabaster and terra-cotta, painted vases, stone cippi with reliefs, &c.
Two Christian catacombs have been found near Clusium, one in the hill of S. Caterina near the railway station, the inscriptions of which seem to go back to the 3rd century, another 1 m. to the E. in a hill on which a church and monastery of S. Mustiola stood, which goes back to the 4th century, including among its inscriptions one bearing the date A.D. 303, and the tombstone of L. Petronius Dexter, bishop of Clusium, who died in A.D. 322. The total number of inscriptions known in Clusium is nearly 3000 Etruscan (Corp. Inscr. Etrusc., Berlin, 475-3306) and 500 Latin (Corp. Inscr. Lat. xi. 2090-2593). To the W. and N.W. of Chiusi—at Cetona, Sarteano, Chianciano and Montepulciano—Etruscan cemeteries have been discovered; the objects from them formed, in the latter half of the 19th century, interesting local collections described by Dennis, which have since mostly passed to larger museums or been dispersed.
CLUWER (Cluver, Cluvier, Cluverius), PHILIP (1580-1623), German geographer and historian, was born at Danzig in 1580. After travelling in Germany and Poland (where he learnt Polish), he began the study of law at Leiden, but he soon turned his attention to history and geography, which were then taught there by Joseph Scaliger. After campaigning in Bohemia and Hungary, suffering imprisonment, and travelling in England, Scotland and France, he finally settled in Holland, where (after 1616) he received a regular pension from Leiden Academy. In 1611 he began to publish his works. He died at Leiden in 1623. His principal writings are: Germania Antiqua (1616), Siciliae Antiquae libri duo, Sardinia et Corsica Antiqua (1619), and the posthumous Italia Antiqua (1624) and Introductio in Universam Geographiam (1629).
CLYDE, COLIN CAMPBELL, Baron (1792-1863), British soldier, was born at Glasgow on the 20th of October 1792. He received his education at the Glasgow high school, and when only sixteen years of age obtained an ensigncy in the 9th foot, through the influence of Colonel Campbell, his maternal uncle. The youthful officer had an early opportunity of engaging in active service. He fought under Sir Arthur Wellesley at Vimiera, took part in the retreat of Sir John Moore, and was present at the battle of Corunna. He shared in all the fighting of the Peninsular campaigns, and was severely wounded while leading a storming-party at the attack on San Sebastian. He was again wounded at the passage of the Bidassoa, and compelled to return to England, when his conspicuous gallantry was rewarded by promotion without purchase. Campbell held a command in the American expedition of 1814; and after the peace of the following year he devoted himself to studying the theoretical branches of his profession. In 1823 he quelled the negro insurrection in Demerara, and two years later obtained his majority by purchase, In 1832 he became lieutenant-colonel of the 98th foot, and with that regiment rendered distinguished service in the Chinese War of 1842. Campbell was next employed in the Sikh War of 1848-49, under Lord Gough. At Chillianwalla, where he was wounded, and at the decisive victory of Gujrat, his skill and valour largely contributed to the success of the British arms; and his “steady coolness and military precision” were highly praised in official despatches. He was made a K.C.B. in 1849, and specially named in the thanks of parliament.
After rendering important services in India Sir Colin Campbell 572 returned home in 1853. Next year the Crimean War broke out, and he accepted the command of the Highland brigade, which formed part of the duke of Cambridge’s division. The brigade and its leader distinguished themselves very greatly at the Alma; and with his “thin red line” of Highlanders he repulsed the Russian attack on Balaklava. At the close of the war Sir Colin was promoted to be knight grand cross of the Bath, and elected honorary D.C.L. of Oxford. His military services, however, had as yet met with tardy recognition; but, when the crisis came, his true worth was appreciated. The outbreak of the Indian Mutiny (q.v.) called for a general of tried experience; and on the 11th of July 1857 the command was offered to him by Lord Palmerston. On being asked when he would be ready to set out, the veteran replied, “Within twenty-four hours.” He was as good as his word; he left England the next evening, and reached Calcutta on the 13th of August. After spending upwards of two months in the capital to organize his resources, he started for the front on the 27th of October, and on the 17th of November relieved Lucknow for the second time. Sir Colin, however, considered Lucknow a false position, and once more abandoned it to the rebels, retaking it in March 1858. He continued in charge of the operations in Oudh until the embers of the revolt had died away. For these services he was raised to the peerage, in 1858, as Lord Clyde; and, returning to England in the next year, he received the thanks of both Houses of Parliament and a pension of £2000 a year. He died on the 14th of August 1863.
Though not a great general, and lacking in the dash which won England so many victories in India, Lord Clyde was at once a brave soldier and a careful and prudent leader. The soldiers whom he led were devotedly attached to him; and his courteous demeanour and manly independence of character won him unvarying respect.
See Sir Owen Tudor Burne, Clyde and Strathnairn (“Rulers of India” series, 1891); and L. Shadwell, Life of Colin Campbell, Lord Clyde (1881).
CLYDE (Welsh, Clwyd, “far heard,” “strong,” the Glotta of Tacitus), the principal river of Lanarkshire, Scotland. It is also the name of the estuary which forms the largest and finest firth on the west coast.
1. The River.—Daer Water, rising in Gana Hill (2190 ft.) on the borders of Lanarkshire and Dumfriesshire, after a course of 10½ m., and Potrail Water, rising 3 m. farther W. in the same hilly country (1928 ft.), after running N.N.E. for 7 m., unite 3½ m. S. of Elvanfoot to form the Clyde, of which they are the principal headstreams, though many mountain burns in these upland regions are also contributory. The old rhyme that “Annan, Tweed and Clyde rise a’ out o’ ae hillside” is not true, for Little Clyde Burn here referred to, rising in Clyde Law (2190 ft.), is only an affluent and not a parent stream. From the junction of the Daer and Potrail the river pursues a direction mainly northwards for several miles, winding eastwards around Tinto Hill, somewhat north-westerly to near Carstairs, where it follows a serpentine course westwards and then southwards. From Harperfield, a point about 4 m. above Lanark, it assumes a north-westerly direction, which, roughly, it maintains for the rest of its course as a river, which is generally held to end at Dumbarton, where it merges in the Firth. Its principal tributaries on the right are the Medwin (16 m. long), entering near Carnwath, the Mouse (15 m.), joining it at Lanark, the South Calder (16 m.) above Bothwell, the North Calder (12 m.) below Uddingston, the Kelvin (21 m.) at Glasgow, and the Leven (7 m.) at Dumbarton. The chief left-hand affluents are the Elvan (8 m.), entering at Elvanfoot, the Duneaton (19 m.), joining a few miles above Roberton, the Garf (6½ m.) below Lamington, the Douglas (20 m.) above Bonnington, the Nethan (12 m.) at Crossford, the Avon (28 m.) at Hamilton, the Rotten Calder (10 m.) near Newton, and the Cart (1 m.), formed by the junction of the Black Cart (9 m.) and the White Cart (19 m.), below Renfrew.
The total length of the Clyde from the head of the Daer to Dumbarton is 106 m., and it drains an area estimated at 1481 sq. m. It is thus the third longest river in Scotland (being exceeded by the Spey and Tay), but in respect of the industries on its lower banks, and its sea-borne commerce, it is one of the most important rivers in the world. Near Lanark it is broken by the celebrated Falls, four in number, which are all found within a distance of 3¾ m. Bonnington Linn, the most graceful, 2 m. above Lanark, is divided into two parts by a mass of tree-clad rocks in mid-stream, and has a height of 30 ft. From this spot the river runs for half a mile through a rugged, red sandstone gorge till it reaches Corra Linn, the grandest of the Falls, where in three leaps, giving it the aspect of a splendid cascade, it makes a descent of 84 ft., which, however, it accomplishes during flood at a single bound. Almost ¾ m. below Corra Linn, Dundaff Linn is reached, a fall of only 10 ft. Farther down, 1¾ m. below Lanark, at Stonebyres Linn, reproducing the characteristic features of Corra Linn, the river descends in ordinary water in three leaps, and in flood in one bold drop of 80 ft. Within this space of 3¾ m. the river effects a total fall of 230 ft., or 611⁄3 ft. in the mile. From Stonebyres Linn to the sea the fall is practically 4 ft. in every mile. The chief villages and towns on or close to the river between its source and Glasgow are Crawford, Lamington, New Lanark, Lanark, Hamilton, Bothwell, Blantyre and Uddingston. At Bowling (pop. 1018)—the point of transhipment for the Forth and Clyde Canal—the river widens decidedly, the fairway being indicated by a stone wall continued seawards as far as Dumbarton. Dunglass Point, near Bowling, is the western terminus of the wall of Antoninus, or Grim’s Dyke; and in the grounds of Dunglass Castle, now a picturesque fragment, stands an obelisk to Henry Bell (1767-1830), the pioneer of steam navigation in Europe.
As far down as the falls the Clyde remains a pure fishing stream, but from the point at which it begins to receive the varied tribute of industry, its water grows more and more contaminated, and at Glasgow the work of pollution is completed. Towards the end of the 18th century the river was yet fordable at the Broomielaw in the heart of Glasgow, but since that period, by unexampled enterprise and unstinted expenditure of money, the stream has been converted into a waterway deep enough to allow liners and battleships to anchor in the harbour (see Glasgow).
Clydesdale, as the valley of the upper Clyde is called, begins in the district watered by headstreams of the river, the course of which in effect it follows as far as Bothwell, a distance of 50 m. It is renowned for its breed of cart-horses (specifically known as Clydesdales), its orchards, fruit fields and market gardens, its coal and iron mines.
2. The Firth.—From Dumbarton, where the firth is commonly considered to begin, to Ailsa Craig, where it ends, the fairway measures 64 m. Its width varies from 1 m. at Dumbarton to 37 m. from Girvan to the Mull of Kintyre. The depth varies from a low-tide minimum of 22 ft. in the navigable channel at Dumbarton to nearly 100 fathoms in the Sound of Bute and at other points. The Cumbraes, Bute and Arran are the principal islands in its waters. The sea lochs all lie on the Highland shore, and comprise Gare Loch, Loch Long, Loch Goil, Holy Loch, Loch Striven, Loch Riddon and Loch Fyne. The only rivers of any importance feeding the Firth are the Ayrshire streams, of which the chief are the Garnock, Irvine, Ayr, Doon and Girvan. The tide ascends above Glasgow, where its farther rise is barred by a weir. The head-ports are Glasgow, Port Glasgow, Greenock, Ardrossan, Irvine, Troon, Ayr and Campbeltown. In addition to harbour lights, beacons on rocks, and light-ships, there are lighthouses on Ailsa Craig, Sanda, Davaar, Pladda, Holy Isle, and Little Cumbrae, and at Turnberry Point, Cloch Point and Toward Point. The health and holiday resorts on the lochs, islands and mainland coast are numerous.
CLYDEBANK, a police burgh of Dumbartonshire, Scotland, on the right bank of the Clyde, 6 m. from Glasgow. Pop. (1891) 10,014; (1901) 21,591. There are stations at Yoker, Clydebank, Kilbowie and Dalmuir, all comprised within the burgh since 1886, served by both the North British and the Caledonian railways. In 1875 the district was almost purely rural, but since that date flourishing industries have been planted in the different 573 parts. At Clydebank are large shipbuilding yards and engineering works; at Yoker there is some shipbuilding and a distillery; at Kilbowie the Singer Manufacturing Company have an immense factory, covering nearly 50 acres and giving employment to many thousands of operatives; at Dalmuir are the building and repairing yards of the Clyde Navigation Trust. The important Rothesay Dock, under this trust, was opened by the prince and princess of Wales in April 1907. The municipality owns a fine town hall and buildings. Part of the parish extends across the Clyde into the shire of Renfrew.
CNIDUS (mod. Tekir), an ancient city of Caria in Asia Minor, situated at the extremity of the long peninsula that forms the southern side of the Sinus Ceramicus or Gulf of Cos. It was built partly on the mainland and partly on the Island of Triopion or Cape Krio, which anciently communicated with the continent by a causeway and bridge, and now by a narrow sandy isthmus. By means of the causeway the channel between island and mainland was formed into two harbours, of which the larger, or southern, now known as Port Freano, was further enclosed by two strongly-built moles that are still in good part entire. The extreme length of the city was little less than a mile, and the whole intramural area is still thickly strewn with architectural remains. The walls, both insular and continental, can be traced throughout their whole circuit; and in many places, especially round the acropolis, at the N.E. corner of the city, they are remarkably perfect. Our knowledge of the site is largely due to the mission of the Dilettanti Society in 1812, and the excavations executed by C. T. Newton in 1857-1858; but of recent years it has become a frequent calling station of touring steamers, which can still lie safely in the southern harbour. The agora, the theatre, an odeum, a temple of Dionysus, a temple of the Muses, a temple of Aphrodite and a great number of minor buildings have been identified, and the general plan of the city has been very clearly made out. The most famous statue by the elder Praxiteles, the Aphrodite, was made for Cnidus. It has perished, but late copies exist, of which the most faithful is in the Vatican gallery. In a temple-enclosure C. T. Newton discovered a fine seated statue of Demeter, which now adorns the British Museum; and about 3 m. south-east of the city he came upon the ruins of a splendid tomb, and a colossal figure of a lion carved out of one block of Pentelic marble, 10 ft. in length and 6 in height, which has been supposed to commemorate the great naval victory of Conon over the Lacedaemonians in 394 B.C. Among the minor antiquities obtained from the city itself, or the great necropolis to the east, perhaps the most interesting are the leaden κατάδεσμοι, or imprecationary tablets, found in the temple of Demeter, and copied in facsimile in the appendix to the second volume of Newton’s work. Peasants still find numerous antiquities, and the site would certainly repay more thorough excavation.
Cnidus was a city of high antiquity and probably of Lacedaemonian colonization. Along with Halicarnassus and Cos, and the Rhodian cities of Lindus, Camirus and Ialysus it formed the Dorian Hexapolis, which held its confederate assemblies on the Triopian headland, and there celebrated games in honour of Apollo, Poseidon and the nymphs. The city was at first governed by an oligarchic senate, composed of sixty members, known as ἀμνήμονες, and presided over by a magistrate called an ἀρεστήρ; but, though it is proved by inscriptions that the old names continued to a very late period, the constitution underwent a popular transformation. The situation of the city was favourable for commerce, and the Cnidians acquired considerable wealth, and were able to colonize the island of Lipara, and founded the city of Corcyra Nigra in the Adriatic. They ultimately submitted to Cyrus, and from the battle of Eurymedon to the latter part of the Peloponnesian War they were subject to Athens. In 394 B.C. Conon fought off the port the battle which destroyed Spartan hegemony. The Romans easily obtained their allegiance, and rewarded them for help given against Antiochus by leaving them the freedom of their city. During the Byzantine period there must still have been a considerable population; for the ruins contain a large number of buildings belonging to the Byzantine style, and Christian sepulchres are common in the neighbourhood. Eudoxus, the astronomer, Ctesias, the writer on Persian history, and Sostratus, the builder of the celebrated Pharos at Alexandria, are the most remarkable of the Cnidians mentioned in history.
See C. T. Newton and R. P. Pullen, Hist. of Discoveries at Halicarnassus, Cnidus, &c. (1863).
CNOSSUS, Knossos, or Gnossus, an ancient city of Crete, on the left bank of the Caeratus, a small stream which falls into the sea on the north side of the island. The city was situated about 3 m. from the coast, and, according to the old traditions, was founded by Minos, king of Crete. The locality was associated with a number of the most interesting legends of Greek mythology, particularly with those which related to Jupiter, who was said to have been born, to have been married, and to have been buried in the vicinity. Cnossus was also assigned as the site of the labyrinth in which the Minotaur was confined. The truth behind these legends has been revealed in recent years by the excavations of Dr Evans. As the historical city was peopled by Dorians, the manners, customs and political institutions of its inhabitants were all Dorian. Along with Gortyna and Cydonia, it held for many years the supremacy over the whole of Crete; and it always took a prominent part in the civil wars which from time to time desolated the island. When the rest of Crete fell under the Roman dominion, Cnossus shared the same fate, and became a Roman colony. Aenesidemus, the sceptic philosopher, and Chersiphron, the architect of the temple of Diana at Ephesus, were natives of Cnossus.
The Site.—As the excavations at Cnossus are discussed at length in the article Crete, it must suffice here briefly to enumerate the more important. The chief building is the Great Palace, the so-called “House of Minos,” the excavation of which by Arthur Evans dates from 1900: a number of rooms lying round the central paved court, oriented north and south, have been identified, among them being the throne-room with some well-preserved wall paintings and a small bathroom attached, in the north-west quarter a larger bathroom and a shrine, and residential chambers in the south and east. The latter part of the palace is composed of a number of private rooms and halls, and is especially remarkable for its skilful drainage and water-supply systems.
In 1907 excavations on the south side of the palace showed that the plan was still incomplete, and a southern cryptoporticus, and outside it a large south-west building, probably an official residence, were discovered. Of special interest was a huge circular cavity under the southern porch into which the sub-structures of the palace had been sunk. This cavity was filled with rubbish, sherds, &c., the latest of which was found to date as far back as the beginning of the Middle Minoan age, and the later work of 1908 only proved (by means of a small shaft sunk through the débris) that the rock floor was 52 ft. below the surface. The first attempt to reach the floor by a cutting in the hill-side proved abortive, but the operations of 1910 led to a successful result. The cavity proved to be a great reservoir approached by a rock-cut staircase and of Early Minoan date.
In 1904-1905 a paved way running due west from the middle of the palace was excavated, and found to lead to another building described as the “Little Palace” largely buried under an olive grove. The first excavations showed that this building was on the same general plan and belonged to the same period as the “House of Minos,” though somewhat later in actual date (17th century B.C.). Large halls, which had subsequently been broken up into smaller apartments, were found, and among a great number of other artistic remains one seal-impression of special interest showing a one-masted ship carrying a thorough-bred horse—perhaps representing the first importation of horses into Crete. A remarkable shrine with fetish idols was also discovered. The sacred Double-Axe symbol is prominent, as in the greater palace. By the end of 1910 the excavation of this smaller palace was practically completed. It was found to cover an area of more than 9400 ft. with a frontage of more than 130 ft., and had five stone staircases. One object of special interest found 574 in the course of excavation is a black steatite vessel in the form of a bull’s head. The modelling is of a very high order, and the one eye which remains perfect is cut out of rock crystal, with the pupil and iris marked by colours applied to the lower face of the crystal.
The work of excavation in the palace has been complicated by the necessity of propping up walls, floors and staircases. In some instances it has been found necessary to replace the original wooden pillars by pillars of stone. Again in the “Queen’s Megaron” in the east wing of the Great Palace it was found that the exposure of the remains to the violent extremes of Cretan weather must soon prove fatal to them. It was therefore decided to restore the columns and part of the wall, and to roof over the whole area.
COACH (through the Fr. coche, originally from the Magyar kocsi, an adjective from the Hungarian place named Kocs, between Raab and Buda, i.e. the sort of vehicle used there in the 15th century), a large kind of carriage for passengers (see Carriage). As a general term it is used (as in “coach-building”) for all carriages, and also in combination with qualifying attributes for particular forms (stage-coach, mail-coach, mourning-coach, hackney-coach, &c.); but the typical coach involves four wheels, springs and a roof. The stage-coach, with seats outside and in, was a public conveyance which was known in England from the 16th century, and before railways the stage-coaches had regular routes (stages) all over the country; through their carrying the mails (from 1784) the term “mail-coach” arose. Similar vehicles were used in America and on the European continent. The diligence, though not invariably with four horses, was the Continental analogue for public conveyance, with other minor varieties such as the Stellwagen and Eilwagen.
The driving of coaches with four horses was a task in which a considerable amount of skill was required,1 and English literature is full of the difficulties and humours of “the road” in old days. A form of sport thus arose for enterprising members of the nobility and gentry, and after the introduction of railways made the mail-coach obsolete as a matter of necessity, the old sport of coaching for pleasure still survived, though only to a limited extent. The Four-in-hand Club was started in England in 1856 and the Coaching Club in 1870, as the successors of the old Bensington Driving Club (1807-1852), and Four-Horse Club (1808-1829); and in America the New York Coaching Club was founded in 1875. But coaching remains the sport of the wealthier classes, although in various parts of England (e.g. London to Brighton, and in the Lake district), in America, and in Europe, public coaches still have their regular times and routes for those who enjoy this form of travel. The earliest railway vehicles for passengers were merely the road coaches of the period adapted to run on rails, and the expression “coaching traffic” is still used in England to denote traffic carried in passenger trains.
Of coaches possessing a history the two best known in the United Kingdom are the king’s state coach, and that of the lord mayor of London. The latter is the oldest, having been built, or at least first used, for the procession of Sir Charles Asgil, lord mayor elect, in November 1757. The body of this vehicle is not supported by springs, but hung on leather straps; and the whole structure is very richly loaded with ornamental carving, gilding and paint-work. The different panels and the doors contain various allegorical groups of figures representing suitable subjects, and heraldic devices painted in a spirited manner. The royal state coach, which is described as “the most superb carriage ever built,” was designed by Sir William Chambers, the paintings on it were executed by Cipriani, and the work was completed in 1761. During the later part of Queen Victoria’s reign it was hardly ever seen, but on the accession of Edward VII. the coach was once more put in order for use on state occasions. The following is an official description of this famous coach:—
“The whole of the carriage and body is richly ornamented with laurel and carved work, beautifully gilt. The length, 24 ft.; width, 8 ft. 3 in.; height, 12 ft.; length of pole, 12 ft. 4 in.; weight, 4 tons. The carriage and body of the coach is composed as follows:—Of four large tritons, who support the body by four braces, covered with red morocco leather, and ornamented with gilt buckles, the two figures placed in front of the carriage bear the driver, and are represented in the action of drawing by cables extending round their shoulders, and the cranes and sounding shells to announce the approach of the monarch of the ocean; and those at the back carry the imperial fasces, topped with tridents. The driver’s foot-board is a large scallop shell, ornamented with bunches of reeds and other marine plants. The pole represents a bundle of lances; the splinter bar is composed of a rich moulding, issuing from beneath a voluted shell, and each end terminating in the head of a dolphin; and the wheels are imitated from those of the ancient triumphal chariot. The body of the coach is composed of eight palm-trees, which, branching out at the top, sustain the roof; and four angular trees are loaded with trophies allusive to the victories obtained by Great Britain during the late glorious war, supported by four lions’ heads. On the centre of the roof stand three boys, representing the genii of England, Scotland and Ireland, supporting the imperial crown of Great Britain, and holding in their hands the sceptre, sword of state, and ensigns of knighthood; their bodies are adorned with festoons of laurel, which fall from thence towards the four corners. The panels and doors are painted with appropriate emblematical devices, and the linings are of scarlet velvet richly embossed with national emblems.”
See the Badminton Driving, by the duke of Beaufort (1888); Rogers’s Manual of Driving (Philadelphia, 1900); and “Nimrod’s” Essays on the Road (1876).
1 The idea of “driving” was responsible for the use of the term “coach” and “coaching” to mean a tutor or trainer, for examinations or athletic contests.
COAHUILA, a northern frontier state of Mexico, bounded N. and N.E. by Texas, U.S.A., E. by Nuevo León, S. by San Luis Potosi and Zacatecas, and W. by Durango and Chihuahua. Area, 63,569 sq.m.; pop. (1895) 237,815; (1900) 296,938. Its surface is a roughly broken plateau, traversed N.W. to S.E. by several ranges of mountains and sloping gently toward the Rio Grande. The only level tract of any size in the state is the Bolsón de Mapimí, a great depression on the western side which was long considered barren and uninhabitable. It is a region of lakes and morasses, of arid plains and high temperatures, but experiments with irrigation toward the end of the 19th century were highly successful and considerable tracts have since been brought under cultivation. In general the state is insufficiently watered, the rainfall being light and the rivers small. The rivers flow eastward to the Rio Grande. The climate is hot and dry, and generally healthy. Stock-raising was for a time the principal industry, but agriculture has been largely developed in several localities, among the chief products of which are cotton—Coahuila is the principal cotton-producing state in Mexico—Indian corn, wheat, beans, sugar and grapes. The Parras district in the southern part of the state has long been celebrated for its wines and brandies. The mineral wealth of the state is very great, and the mining industries, largely operated with foreign capital, are important. The mineral products include silver, lead, coal, copper, and iron. The mining operations are chiefly centred in the Sierra Mojada, Sierra Carmen, and in the Santa Rosa valley. The modern industrial development of the state is due to the railway lines constructed across it during the last quarter of the 19th century, and to the investment of foreign capital in local enterprises. The first Spanish settlement in the region now called Coahuila was at Saltillo in 1586, when it formed part of the province of Nueva Viscaya. Later it became the province of Nueva Estremadura under the Spanish régime, and in 1824, under the new republican organization, it became the state of Coahuila and included Texas and Nuevo León. Later in the same year Nuevo León was detached, but Texas remained a part of the state until 1835. The capital of the state is Saltillo; Monclova was the capital from 1833 to 1835. Among the more important towns are Parras (pop. 6476 in 1900), 98 m. W. by N. of Saltillo in a rich grape-producing district, Ciudad Porfirio Diaz, and Monclova (pop. 6684 in 1900), 105 m. N. by W. of Saltillo, on the Mexican International railway.
COAL. In its most general sense the term “coal” includes all varieties of carbonaceous minerals used as fuel, but it is now usual in England to restrict it to the particular varieties of such minerals occurring in the older Carboniferous formations. On the continent of Europe it is customary to consider coal as divisible into two great classes, depending upon differences of colour, namely, brown coal, corresponding to the term “lignite” used in England and France, and black or stone coal, which is equivalent to coal as understood in England. Stone coal is also a local English term, but with a signification restricted to the substance known by mineralogists as anthracite. In old English writings the terms pit-coal and sea-coal are commonly used. These have reference to the mode in which the mineral is obtained, and the manner in which it is transported to market.
The root kol is common to all the Teutonic nations, while in French and other Romance languages derivatives of the Latin carbo are used, e.g. charbon de terre. In France and Belgium, however, a peculiar word, houille, is generally used to signify mineral coal. This word is supposed to be derived from the Walloon hoie, corresponding to the medieval Latin hullae. Littré suggests that it may be related to the Gothic haurja, coal. Anthracite is from the Greek ἄνθραξ, and the term lithanthrax, stone coal, still survives, with the same meaning, in the Italian litantrace.
It must be borne in mind that the signification now attached to the word coal is different from that which formerly obtained when wood was the only fuel in general use. Coal then meant the carbonaceous residue obtained in the destructive distillation of wood, or what is known as charcoal, and the name collier was applied indifferently to both coal-miners and charcoal-burners.
The spelling “cole” was generally used up to the middle of the 17th century, when it was gradually superseded by the modern form, “coal.” The plural, coals, seems to have been used from a very early period to signify the broken fragments of the mineral as prepared for use.
Coal is an amorphous substance of variable composition, and therefore cannot be as strictly defined as a crystallized or definite mineral can. It varies in colour from a light brown in the newest lignites to a pure black, often with Physical properties. a bluish or yellowish tint in the more compact anthracite of the older formations. It is opaque, except in exceedingly thin slices, such as made for microscopic investigation, which are imperfectly transparent, and of a dark brown colour by transmitted light. The streak is black in anthracite, but more or less brown in the softer varieties. The maximum hardness is from 2.5 to 3 in anthracite and hard bituminous coals, but considerably less in lignites, which are nearly as soft as rotten wood. A greater hardness is due to the presence of earthy impurities. The densest anthracite is often of a semi-metallic lustre, resembling somewhat that of graphite. Bright, glance or pitch coal is another brilliant variety, brittle, and breaking into regular fragments of a black colour and pitchy lustre. Lignite and cannel are usually dull and earthy, and of an irregular fracture, the latter being much tougher than the black coal. Some lignites are, however, quite as brilliant as anthracite; cannel and jet may be turned in the lathe, and are susceptible of taking a brilliant polish. The specific gravity is highest in anthracite and lowest in lignite, bituminous coals giving intermediate values (see Table I.). As a rule, the density increases with the amount of carbon, but in some instances a very high specific gravity is due to intermixed earthy matters, which are always denser than even the densest form of coal substance.
Coal is never definitely crystalline, the nearest approach to such a structure being a compound fibrous grouping resembling that of gypsum or arragonite, which occurs in some of the steam coals of South Wales, and is locally known as “cone in cone,” but no definite form or arrangement can be made out of the fibres. Usually it occurs in compact beds of alternating bright and dark bands in which impressions of leaves, woody fibre and other vegetable remains are commonly found. There is generally a tendency in coals towards cleaving into cubical or prismatic blocks, but sometimes the cohesion between the particles is so feeble that the mass breaks up into dust when struck. These peculiarities of structure may vary very considerably within small areas; and the position of the divisional planes or cleats with reference to the mass, and the proportion of small coal or slack to the larger fragments when the coal is broken up by cutting-tools, are points of great importance in the working of coal on a large scale.
The divisional planes often contain small films of other minerals, the commonest being calcite, gypsum and iron pyrites, but in some cases zeolitic minerals and galena have been observed. Salt, in the form of brine, is sometimes present in coal. Hydrocarbons, such as petroleum, bitumen, paraffin, &c., are also found occasionally in coal, but more generally in the associated sandstones and limestones of the Carboniferous formation. Gases, consisting principally of light carburetted hydrogen or marsh gas, are often present in considerable quantity in coal, in a dissolved or occluded state, and the evolution of these upon exposure to the air, especially when a sudden diminution of atmospheric pressure takes place, constitutes one of the most formidable dangers that the coal miner has to encounter.
The classification of the different kinds of coal may be considered from various points of view, such as their chemical composition, their behaviour when subjected to heat or when burnt, and their geological position and Classification. origin. They all contain carbon, hydrogen, oxygen and nitrogen, forming the carbonaceous or combustible portion, and some quantity of mineral matter, which remains after combustion as a residue or “ash.” As the amount of ash varies very considerably in different coals, and stands in no relation to the proportion of the other constituents, it is necessary in forming a chemical classification to compute the results of analysis after deduction of the ash and hygroscopic water. Examples of analyses treated in this manner are furnished in the Anthracite. last column of Table I., from which it will be seen that the nearest approach to pure carbon is furnished by anthracite, which contains above 90%. This class of coal burns with a very small amount of flame, producing intense local heat and no smoke. It is especially used for drying hops and malt, and in blast furnaces where a high temperature is required, but it is not suited for reverberatory furnaces.
The most important class of coals is that generally known as bituminous, from their property of softening or undergoing an apparent fusion when heated to a temperature far below that at which actual combustion takes place. Bituminous coals. This term is founded on a misapprehension of the nature of the occurrence, since, although the softening takes place at a low temperature, still it marks the point at which destructive distillation commences, and hydrocarbons both of a solid and gaseous character are formed. That nothing analogous to bitumen exists in coals is proved by the fact that the ordinary solvents for bituminous substances, such as bisulphide of carbon and benzol, have no effect upon them, as would be the case if they contained bitumen soluble in these re-agents. The term is, however, a convenient one, and one whose use is almost a necessity, from its having an almost universal currency among coal miners. The proportion of carbon in bituminous coals may vary from 80 to 90%—the amount being highest as they approach the character of anthracite, and least in those which are nearest to lignites. The amount of hydrogen is from 4½ to 6%, while the oxygen may vary within much wider limits, or from about 3 to 14%. These variations in composition are attended with corresponding differences in qualities, which are distinguished by special names. Thus the semi-anthracitic coals of South Wales are known as “dry” or “steam coals,” being especially valuable for use in marine steam-boilers, as they burn more readily than anthracite and with a larger amount of flame, while giving out a great amount of heat, and practically without producing smoke. Coals richer in hydrogen, on the other hand, are more useful for burning in open fires—smiths’ forges and furnaces—where a long flame is required.
The excess of hydrogen in a coal, above the amount necessary 576 to combine with its oxygen to form water, is known as “disposable” hydrogen, and is a measure of the fitness of the coal Gas coal. for use in gas-making. This excess is greatest in what is known as cannel coal, the Lancashire kennel or candle coal, so named from the bright light it gives out when burning. This, although of very small value as fuel, commands a specially high price for gas-making. Cannel is more compact and duller than ordinary coal, and can be wrought in the lathe and polished.
Table I.—Elementary Composition of Coal (the figures denote the amounts per cent).
| Composition exclusive of Water, Sulphur and Ash. | |||||||||||
| Localities. | Specific Gravity. |
Carbon. | Hydrogen. | Oxygen. | Nitrogen. | Sulphur. | Ash. | Water. | Carbon. | Hydrogen. | O. and N. |
| Anthracite. | |||||||||||
| 1. South Wales | 1.392 | 90.39 | 3.28 | 2.98 | 0.83 | 0.91 | 1.61 | 2.00 | 93.54 | 3.39 | 3.82 |
| 2. Pennsylvania | 1.462 | 90.45 | 2.43 | 2.45 | .. | .. | 4.67 | .. | 94.89 | 2.54 | 2.57 |
| 3. Peru | .. | 82.70 | 1.41 | 0.85 | 10.35 | 3.75 | 0.94 | 97.34 | 1.66 | 1.00 | |
| Bituminous Steam and Coking Coal. | |||||||||||
| 4. Risca, South Wales | .. | 75.49 | 4.73 | 6.78 | 1.21 | 10.67 | 1.12 | 86.78 | 5.43 | 7.79 | |
| 5. Aberdare, South Wales | .. | 86.80 | 4.25 | 3.06 | 0.83 | 4.40 | 0.66 | 92.24 | 4.51 | 3.25 | |
| 6. Hartley, Northumberl’d | .. | 78.65 | 4.65 | 13.36 | 0.55 | 2.49 | .. | 80.67 | 4.76 | 14.5 | |
| 7. Dudley, Staffordshire | 1.278 | 78.57 | 5.29 | 12.88 | 1.84 | 0.39 | 1.03 | 1.13 | 79.70 | 5.37 | 14.9 |
| 8. Stranitzen, Styria | .. | 79.90 | 4.85 | 12.75 | 0.64 | 0.20 | 1.66 | .. | 81.45 | 4.92 | 13.63 |
| Cannel or Gas Coal. | |||||||||||
| 9. Wigan, Lancashire | 1.276 | 80.07 | 5.53 | 8.08 | 2.12 | 1.50 | 2.70 | 0.91 | 85.48 | 5.90 | 8.62 |
| 10. Boghead, Scotland | .. | 63.10 | 8.91 | 7.25 | 0.96 | 19.78 | .. | 79.61 | 11.24 | 9.15 | |
| 11. (Albertite) Nova Scotia | .. | 82.67 | 9.14 | 8.19 | .. | .. | .. | 82.67 | 9.14 | 8.19 | |
| 12. (Tasmanite) Tasmania | 1.18 | 79.34 | 10.41 | 4.93 | 5.32 | .. | .. | 83.80 | 10.99 | 5.21 | |
| Lignite and Brown Coal. | |||||||||||
| 13. Cologne | 1.100 | 63.29 | 4.98 | 26.24 | .. | 8.49 | .. | 66.97 | 5.27 | 27.76 | |
| 14. Bovey Tracy, Devonshire | .. | 66.31 | 5.63 | 22.86 | 0.57 | 2.36 | 2.36 | .. | 69.53 | 5.90 | 24.57 |
| 15. Trifail, Styria | .. | 50.72 | 5.34 | 33.18 | 2.80 | 0.90 | 7.86 | .. | 55.11 | 5.80 | 39.09 |
These properties are most highly developed in the substance known as jet, which is a variety of cannel found in the lower oolitic strata of Yorkshire, and is almost entirely used for ornamental purposes, the whole quantity produced near Whitby, together with a further supply from Spain, being manufactured into articles of jewellery at that town.
When coal is heated to redness out of contact with the air, the more volatile constituents, water, hydrogen, oxygen, and nitrogen are in great part expelled, a portion of the carbon being also volatilized in the form of hydrocarbons Caking coals. and carbonic oxide,—the greater part, however, remaining behind, together with all the mineral matter or ash, in the form of coke, or, as it is also called, “fixed carbon.” The proportion of this residue is greatest in the more anthracitic or drier coals, but a more valuable product is yielded by those richer in hydrogen. Very important distinctions—those of caking or non-caking—are founded on the behaviour of coals when subjected to the process of coking. The former class undergo an incipient fusion or softening when heated, so that the fragments coalesce and yield a compact coke, while the latter (also called free-burning) preserve their form, producing a coke which is only serviceable when made from large pieces of coal, the smaller pieces being incoherent and of no value. The caking property is best developed in coals low in oxygen with 25 to 30% of volatile matters. As a matter of experience, it is found that caking coals lose that property when exposed to the action of the air for a lengthened period, or by heating to about 300° C., and that the dust or slack of non-caking coal may, in some instances, be converted into a coherent coke by exposing it suddenly to a very high temperature, or compressing it strongly before charging it into the oven.
Lignite or brown coal includes all varieties which are intermediate in properties between wood and coals of the older formations. A coal of this kind is generally to be distinguished by its brown colour, either in mass or in Lignite. the blacker varieties in the streak. The proportion of carbon is comparatively low, usually not exceeding 70%, while the oxygen and hygroscopic water are much higher than in true coals. The property of caking or yielding a coherent coke is usually absent, and the ash is often very high. The specific gravity is low when not brought up by an excessive amount of earthy matter. Sometimes it is almost pasty, and crumbles to powder when dried, so as to be susceptible of use as a pigment, forming the colour known as Cologne earth, which resembles umber or sepia. In Nassau and Bavaria woody structure is very common, and it is from this circumstance that the term lignite is derived. The best varieties are black and pitchy in lustre, or even bright and scarcely to be distinguished from true coals. These kinds are most common in Eastern Europe. Lignites, as a rule, are generally found in strata of a newer geological age, but there are many instances of perfect coals being found in such strata.
By the term “ash” is understood the mineral matter remaining unconsumed after the complete combustion of the carbonaceous portion of a coal. According to Couriot (Annales de la société géologique de Belgique, vol. xxiii. Ash of coal. p. 105) the stratified character of the ash may be rendered apparent in an X-ray photograph of a piece of coal about an inch thick, when it appears in thin parallel bands, the combustible portion remaining transparent. It may also be rendered visible if a smooth block of free-burning coal is allowed to burn away quickly in an open fire, when the ash remains in thin grey or yellow bands on the surface of the block. The composition of the ashes of different coals is subject to considerable variation, as will be seen by Table II.
The composition of the ash of true coal approximates to that of a fire-clay, allowance being made for lime, which may be present either as carbonate or sulphate, and for sulphuric acid. Sulphur is derived mainly from iron Sulphur in coal. pyrites, which yields sulphates by combustion. An indication of the character of the ash of a coal is afforded by its colour, white ash coals being generally freer from sulphur than those containing iron pyrites, which yield a red ash. There are, however, several striking exceptions, as for instance in the anthracite from Peru, given in Table I., which contains more than 10% of sulphur, and yields but a very small percentage of a white ash. In this coal, as well as in the lignite of Tasmania, known as white coal or Tasmanite, the sulphur occurs in organic combination, but is so firmly held that it can only be very partially expelled, even by exposure to a very high and continued heating out of contact with the air. An anthracite occurring in connexion with the old volcanic rocks of Arthur’s Seat, Edinburgh, which contains a large amount of sulphur in proportion to the 577 ash, has been found to behave in a similar manner. Under ordinary conditions, from 1⁄8 to 1⁄4 of the whole amount of sulphur in a coal is volatilized during combustion, the remaining 3⁄4 to 7⁄8 being found in the ash.
Table II.—Composition of the Ashes of Coals.
| Silica. | Alumina. | Ferric Oxide. | Lime. | Magnesia. | Potash. | Sulphuric Acid. | Phosphoric Acid. |
Total. | |
| True Coals. | |||||||||
| Dowlais, South Wales | 39.64 | 39.20 | 11.84 | 1.81 | 2.58 | .. | .. | 3.01 | 98.08 |
| Ebbw Vale, South Wales | 53.00 | 35.01 | .. | 3.94 | 2.20 | .. | 4.89 | 0.88 | 99.92 |
| Königsgrube, Silesia | 55.41 | 18.95 | 16.06 | 3.21 | 1.87 | 2.05 | 1.73 | 0.36 | 99.64 |
| Ohio | 44.60 | 41.10 | 7.40 | 3.61 | 1.28 | 1.82 | 0.59 | 0.29 | 100.69 |
| Lignites. | |||||||||
| Helmstadt, Saxony | 17.27 | 11.57 | 5.57 | 23.67 | 2.58 | 2.64 | 33.83 | .. | 97.13 |
| Edeléney, Hungary | 36.01 | 23.07 | 5.05 | 15.62 | 3.64 | 2.38 | 12.35 | .. | 98.12 |
The amount of water present in freshly raised coals varies very considerably. It is generally largest in lignites, which may sometimes contain 30% or even more, while in the Water in coal. coals of the coal measures it does not usually exceed from 5 to 10%. The loss of weight by exposure to the atmosphere from drying may be from ½ to ¾ of the total amount of water contained.
Table III.—Composition of Fuels (assuming Carbon = 100).
| Carbon. | Hydrogen. | Oxygen. | Disposable Hydrogen. | |
| Wood | 100 | 12.18 | 83.07 | 1.80 |
| Peat | 100 | 9.85 | 55.67 | 2.89 |
| Lignite | 100 | 8.37 | 42.42 | 3.07 |
| Thick Coal, S. Staffordshire | 100 | 6.12 | 21.23 | 3.47 |
| Hartley Steam Coal | 100 | 5.91 | 18.32 | 3.62 |
| South Wales Steam Coal | 100 | 4.75 | 5.28 | 4.09 |
| American Anthracite | 100 | 2.84 | 1.74 | 2.63 |
Coal is the result of the transformation of woody fibre and other vegetable matter by the elimination of oxygen and hydrogen in proportionally larger quantity than carbon, so that the percentage of the latter element Origin of Coal. is increased in the manner shown in Table III., given by J. Percy, the mineral matter being also changed by the removal of silica and alkalis and the substitution of substances analogous in composition to fire-clay. The causes and methods of these changes are, however, not very exactly defined. According to the elaborate researches of B. Renault (Bulletin de la Société de l’Industrie minérale, 3 ser. vol. xiii. p. 865), the agents of the transformation of cellulose into peaty substances are saprophytic fungi and bacterial ferments. As the former are only active in the air while the latter are anaerobic, the activity of either agent is conditioned by variation in the water level of the bog. The ultimate term of bacterial activity seems to be the production of ulmic acid, containing carbon 65.31 and hydrogen 3.85%, which is a powerful antiseptic. By the progressive elimination of oxygen and hydrogen, partly as water and partly as carbon dioxide and marsh gas, the ratios of carbon to oxygen and hydrogen in the rendered product increase in the following manner:—
| C : H | C : O | |
| Cellulose | 7.2 | 0.9 |
| Peat | 9.8 | 1.8 |
| Lignite, imperfect | 12.2 | 2.4 |
| Lignite, perfect | 12.6 | 3.6 |
The resulting product is a brown pasty or gelatinous substance which binds the more resisting parts of the plants into a compact mass. The same observer considers Boghead coal, kerosene shale and similar substances used for the production of mineral oils to be mainly alteration products of gelatinous fresh water algae, which by a nearly complete elimination of oxygen have been changed to substances approximating in composition to C2H3 and C3H5, where C : H = 7.98 and C : O + N = 46.3. In cannel coals the prevailing constituents are the spores of cryptogamic plants, algae being rare or in many cases absent. By making very thin sections and employing high magnification (1000-1200 diameters), Renault has been enabled to detect numerous forms of bacilli in the woody parts preserved in coal, one of which, Micrococcus carbo, bears a strong resemblance to the living Cladothrix found in trees buried in peat bogs. Clearer evidence of their occurrence has, however, been found in fragments of wood fossilized by silica or carbonate of lime which are sometimes met with in coal seams.
The subsequent change of peaty substance into coal is probably due to geological causes, i.e. chemical and physical processes similar to those that have converted ordinary sediments into rock masses. Such changes seem, however, to have been very rapidly accomplished, as pebbles of completely formed coal are commonly found in the sandstones and coarser sedimentary strata alternating with the coal seams in many coalfields.
The variation in the composition of coal seams in different parts of the same basin is a difficult matter to explain. It has been variously attributed to metamorphism, consequent upon igneous intrusion, earth movements and other kinds of geothermic action, greater or less loss of volatile constituents during the period of coaly transformation, conditioned by differences of permeability in the enclosing rocks, which is greater for sandstones than for argillaceous strata, and other causes; but none of these appears to be applicable over more than limited areas. According to L. Lemière, who has very fully reviewed the relation of composition to origin in coal seams (Bulletin de la Société de l’Industrie minérale, 4 ser. vol. iv. pp. 851 and 1299, vol. v. p. 273), differences in composition are mainly original, the denser and more anthracitic varieties representing plant substance which has been more completely macerated and deprived of its putrescible constituents before submergence, or of which the deposition had taken place in shallow water, more readily accessible to atmospheric oxidizing influences than the deeper areas where conditions favourable to the elaboration of compounds richer in hydrogen prevailed.
The conditions favourable to the production of coal seem therefore to have been—forest growth in swampy ground about the mouths of rivers, and rapid oscillation of level, the coal produced during subsidence being covered up by the sediment brought down by the river forming beds of sand or clay, which, on re-elevation, formed the soil for fresh growths, the alternation being occasionally broken by the deposit of purely marine beds. We might therefore expect to find coal wherever strata of estuarine origin are developed in great mass. This is actually the case; the Carboniferous, Cretaceous and Jurassic systems (qq.v.) contain coal-bearing strata though in unequal degrees,—the first being known as the Coal Measures proper, while the others are of small economic value in Great Britain, though more productive in workable coals on the continent of Europe. The Coal Measures which form part of the Palaeozoic or oldest of the three great geological divisions are mainly confined to the countries north of the equator. Mesozoic coals are more abundant in the southern hemisphere, while Tertiary coals seem to be tolerably uniformly distributed irrespective of latitude.
The nature of the Coal Measures will be best understood by 578 considering in detail the areas within which they occur in Britain, together with the rocks with which they are most intimately associated. The commencement of the Carboniferous period is Sequences of carboniferous strata. marked by a mass of limestones known as the Carboniferous or Mountain Limestone, which contains a large assemblage of marine fossils, and has a maximum thickness in S.W. England and Wales of about 2000 ft. The upper portion of this group consists of shales and sandstones, known as the Yoredale Rocks, which are highly developed in the moorland region between Lancashire and the north side of Yorkshire. These are also called the Upper Limestone Shale, a similar group being found in places below the limestone, and called the Lower Limestone Shale, or, in the north of England, the Tuedian group. Going northward the beds of limestone diminish in thickness, with a proportional increase in the intercalated sandstones and shales, until in Scotland they are entirely subordinate to a mass of coal-bearing strata, which forms the most productive members of the Scotch coalfields. The next member of the series is a mass of coarse sandstones, with some slates and a few thin coals, known as the Millstone Grit, which is about equally developed in England and in Scotland. In the southern coalfields it is usually known by the miners’ name of “Farewell rock,” from its marking the lower limit of possible coal working. The Coal Measures, forming the third great member of the Carboniferous series, consist of alternations of shales and sandstones, with beds of coal and nodular ironstones, which together make up a thickness of many thousands of feet—from 12,000 to 14,000 ft. when at the maximum of development. They are divisible into three parts, the Lower Coal Measures, the middle or Pennant, a mass of sandstone containing some coals, and the Upper Coal Measures, also containing workable coal. The latter member is marked by a thin limestone band near the top, containing Spirorbis carbonarius, a small marine univalve.
The uppermost portion of the Coal Measures consists of red sandstone so closely resembling that of the Permian group, which are next in geological sequence, that it is often difficult to decide upon the true line of demarcation between the two formations. These are not, however, always found together, the Coal Measures being often covered by strata belonging to the Trias or Upper New Red Sandstone series.
The areas containing productive coal measures are usually known as coalfields or basins, within which coal occurs in more or less regular beds, also called seams or veins, which can often be followed over a considerable length of country without change of character, although, like all stratified rocks, their continuity may be interrupted by faults or dislocations, also known as slips, hitches, heaves or troubles.
The thickness of coal seams varies in Great Britain from a mere film to 35 or 40 ft.; but in the south of France and in India masses of coal are known up to 200 ft. in thickness. These very thick seams are, however, rarely constant in character for any great distance, being found commonly to degenerate into carbonaceous shales, or to split up into thinner beds by the intercalation of shale bands or partings. One of the most striking examples of this is afforded by the thick or ten-yard seam of South Staffordshire, which is from 30 to 45 ft. thick in one connected mass in the neighbourhood of Dudley, but splits up into eight seams, which, with the intermediate shales and sandstones, are of a total thickness of 400 ft. in the northern part of the coalfield in Cannock Chase. Seams of a medium thickness of 3 to 7 ft. are usually the most regular and continuous in character. Cannel coals are generally variable in quality, being liable to change into shales or black-band ironstones within very short horizontal limits. In some instances the coal seams may be changed as a whole, as for instance in South Wales, where the coking coals of the eastern side of the basin pass through the state of dry steam coal in the centre, and become anthracite in the western side.
The most important European coalfields are in Great Britain, Belgium and Germany. In Great Britain there is the South Welsh field, extending westward from the march of Monmouthshire to Kidwelly, and northward to Merthyr Tydfil. A midland group of coalfields extends from south Lancashire to the West Geographical distribution of coalfields. Riding of Yorkshire, the two greatest industrial districts in the country, southward to Warwickshire and Staffordshire, and from Nottinghamshire on the east to Flintshire on the west. In the north of England are the rich field of Northumberland and Durham, and a lesser field on the coast of Cumberland (Whitehaven, &c.). Smaller isolated fields are those of the Forest of Dean (Gloucestershire) and the field on either side of the Avon above Bristol. Coal has also been found in Kent, in the neighbourhood of Dover. In Scotland coal is worked at various points (principally in the west) in the Clyde-Forth lowlands. In Belgium the chief coal-basins are those of Hainaut and Liége. Coal has also been found in an extension northward from this field towards Antwerp, while westward the same field extends into north-eastern France. Coal is widely distributed in Germany. The principal field is that of the lower Rhine and Westphalia, which centres in the industrial region of the basin of the Ruhr, a right-bank tributary of the Rhine. In the other chief industrial region of Germany, in Saxony, Zwickau and Lugau, are important mining centres. In German Silesia there is a third rich field, which extends into Austria (Austrian Silesia and Galicia), for which country it forms the chief home source of supply (apart from lignite). Part of the same field also lies within Russian territory (Poland) near the point where the frontiers of the three powers meet. Both in Germany and in Austria-Hungary the production of lignite is large—in the first-named especially in the districts about Halle and Cologne; in the second in north-western Bohemia, Styria and Carniola. In France the principal coalfield is that in the north-east, already mentioned; another of importance is the central (Le Creusot, &c.) and a third, the southern, about the lower course of the Rhone. Coal is pretty widely distributed in Spain, and occurs in several districts in the Balkan peninsula. In Russia, besides the Polish field, there is an important one south of Moscow, and another in the lower valley of the Donetz, north of the Sea of Azov. The European region poorest in coal (proportionately to area) is Scandinavia, where there is only one field of economic value—a small one in the extreme south of Sweden.
In Asia the Chinese coalfields are of peculiar interest. They are widely distributed throughout China Proper, but those of the province of Shansi appear to be the richest. Proportionately to their vast extent they have been little worked. In a modified degree the same is true of the Indian fields; large supplies are unworked, but in several districts, especially about Raniganj and elsewhere in Bengal, workings are fully developed. Similarly in Siberia and Japan there are extensive supplies unworked or only partially exploited. Those in the neighbourhood of Semipalatinsk may be instanced in the first case and those in the island of Yezo in the second. In Japan, however, several smaller fields (e.g. in the island of Kiushiu) are more fully developed. Coal is worked to some extent in Sumatra, British North Borneo, and the Philippine Islands.
In the United States of America the Appalachian mountain system, from Pennsylvania southward, roughly marks the line of the chief coal-producing region. This group of fields is followed in importance by the “Eastern Interior” group in Indiana, Illinois and Kentucky, and the “Western Interior” group in Iowa, Missouri and Kansas. In Arkansas, Oklahoma and Texas, and along the line of the Rocky Mountains, extensive fields occur, producing lignite and bituminous coal. The last-named fields are continued northward in Canada (Crow’s Nest Pass field, Vancouver Island, &c.). There is also a group of coalfields on the Atlantic seaboard of the Dominion, principally in Nova Scotia. Coal is known at several points in Alaska, and there are rich but little worked deposits in Mexico.
In the southern countries coal-production is insignificant compared with that in the northern hemisphere. In South America coal is known in Venezuela, Colombia, Peru, northern Chile, Brazil (chiefly in the south), and Argentina (Parana, the extreme south of Patagonia, and Tierra del Fuego), but in no 579 country are the workings extensive. Africa is apparently the continent poorest in coal, though valuable workings have been developed at various points in British South Africa, e.g. at Kronstad, &c., in Cape Colony, at Vereeniging, Boksburg and elsewhere in the Transvaal, in Natal and in Swaziland. Australia possesses fields of great value, principally in the south-east (New South Wales and Victoria), and in New Zealand considerable quantities of coal and lignite are raised, chiefly in South Island.
The following table, based on figures given in the Journal of the Iron and Steel Institute, vol. 72, will give an idea of the coal production of the world:—
Table IV.
| Europe:— | Tons. | |
| United Kingdom | 1905 | 236,128,936 |
| Germany, coal | ” | 121,298,167 |
| Germany, lignite | ” | 52,498,507 |
| France | ” | 35,869,497 |
| Belgium | ” | 21,775,280 |
| Austria, coal | ” | 12,585,263 |
| Austria, lignite | ” | 22,692,076 |
| Hungary, coal | 1904 | 1,031,501 |
| Hungary, lignite | ” | 5,447,283 |
| Spain | 1905 | 3,202,911 |
| Russia | 1904 | 19,318,000 |
| Holland | ” | 466,997 |
| Bosnia, lignite | 1905 | 540,237 |
| Rumania, lignite | 1903 | 110,000 |
| Servia | 1904 | 183,204 |
| Italy, coal and lignite | 1905 | 412,916 |
| Sweden | ” | 322,384 |
| Greece, lignite | 1904 | 466,997 |
| Asia:— | ||
| India | 1905 | 8,417,739 |
| Japan | 1903 | 10,088,845 |
| Sumatra | 1904 | 207,280 |
| Africa:— | ||
| Transvaal | 1904 | 2,409,033 |
| Natal | 1905 | 1,129,407 |
| Cape Colony | 1904 | 154,272 |
| America:— | ||
| United States | 1905 | 350,821,000 |
| Canada | 1904 | 7,509,860 |
| Mexico | ” | 700,000 |
| Peru | 1905 | 72,665 |
| Australasia:— | ||
| New South Wales | 1905 | 6,632,138 |
| Queensland | ” | 529,326 |
| Victoria | ” | 153,135 |
| Western Australia | ” | 127,364 |
| Tasmania | ” | 51,993 |
| New Zealand | ” | 1,585,756 |
The questions, what is the total amount of available coal in the coalfields of Great Britain and Ireland, and how long it may be expected to last, have frequently been discussed since the early part of the 19th century, and particular Coal resources of Great Britain. attention was directed to them after the publication of Stanley Jevons’s book on The Coal Question in 1865. In 1866 a royal commission was appointed to inquire into the subject, and in its report, issued in 1871, estimated that the coal resources of the country, in seams of 1 ft. thick and upwards situated within 4000 ft. of the surface, amounted to 90,207,285,398 tons. A second commission, which was appointed in 1901 and issued its final report in 1905, taking 4000 ft. as the limit of practicable depth in working and 1 ft. as the minimum workable thickness, and after making all necessary deductions, estimated the available quantity of coal in the proved coalfields of the United Kingdom as 100,914,668,167 tons. Although in the years 1870-1903 the amount raised was 5,694,928,507 tons, this later estimate was higher by 10,707,382,769 tons than that of the previous commission, the excess being accounted for partly by the difference in the areas regarded as productive by the two commissions, and partly by new discoveries and more accurate knowledge of the coal seams. In addition it was estimated that in the proved coalfields at depths greater than 4000 ft. there were 5,239,433,980 tons, and that in concealed and unproved fields, at depths less than 4000 ft. there were 39,483,844,000 tons, together with 854,608,307 tons in that part of the Cumberland coalfield beyond 5 m. and within 12 m. of high-water mark, and 383,024,000 tons in the South Wales coalfield under the sea in St Bride’s Bay and part of Carmarthen Bay.
In Table V. below column I. shows the quantity of coal still remaining unworked in the different coalfields at depths not exceeding 4000 ft. and in seams not less than 1 ft. thick, as estimated by seven district commissioners; column II. the total estimated reductions on account of loss in working due to faults and other natural causes in seams and of coal required to be left for barriers, support of surface buildings, &c.; and column III. the estimated net available amount remaining unworked.
Table V.
| District. | Coalfield. | I. | II. | III. |
| A. | South Wales and Monmouthshire | 33,443,000,339 | 6,972,003,760 | 26,470,996,579 |
| Somersetshire and part of Gloucestershire | No details | No details | 4,198,301,099 | |
| Forest of Dean | 305,928,137 | 47,394,690 | 258,533,447 | |
| B. | North Stafford | 5,267,833,074 | 89,782,727 | 4,368,050,347 |
| South Stafford | 1,953,627,435 | 538,179,363 | 1,415,448,072 | |
| Warwickshire | 1,448,804,556 | 321,822,653 | 1,126,981,903 | |
| Leicestershire | 2,467,583,205 | 642,124,654 | 1,825,458,551 | |
| Shropshire | 369,174,620 | 48,180,921 | 320,993,699 | |
| C. | Lancashire | 5,349,554,437 | 1,111,046,710 | 4,238,507,727 |
| Cheshire | 358,998,172 | 87,165,901 | 291,832,271 | |
| North Wales | 2,513,026,200 | 776,558,371 | 1,736,467,829 | |
| D. | Yorkshire | No details | No details | 19,138,006,395 |
| Derby and Notts | No details | No details | 7,360,725,100 | |
| E. | Northumberland | 7,040,348,127 | 1,530,722,486 | 5,509,625,641 |
| Cumberland | 2,188,938,830 | 661,230,025 | 1,527,708,805 | |
| Durham | 6,607,700,522 | 1,336,584,176 | 5,271,116,346 | |
| F. | Scotland | 21,259,767,661 | 5,579,311,305 | 15,681,456,356 |
| G. | Ireland | No details | No details | 174,458,000 |
As regards the duration of British coal resources, the commissioners reported (1905):—
“This question turns chiefly upon the maintenance or the variation of the annual output. The calculations of the last Coal Commission as to the future exports and of Mr Jevons as to the future annual consumption make us hesitate to prophesy how long our coal resources are likely to last. The present annual output is in round numbers 230 million tons, and the calculated available resources in the proved coalfields are in round numbers 100,000 million tons, exclusive of the 40,000 million tons in the unproved coalfields, which we have thought best to regard only as probable or speculative. For the last thirty years the average increase in the output has been 2½% per annum, and that in the exports (including bunkers) 4½% per annum. It is the general opinion of the District Commissioners that owing to physical considerations it is highly probable that the present rate of increase of the output of coal can long continue—indeed, they think that some districts have already attained their maximum output, but that on the other hand the developments in the newer coalfields will possibly increase the total output for some years.
In view of this opinion and of the exhaustion of the shallower collieries we look forward to a time, not far distant, when the rate of increase of output will be slower, to be followed by a period of stationary output, and then a gradual decline.”
According to a calculation made by P. Frech in 1900, on the basis of the then rate of production, the coalfields of central France, central Bohemia, the kingdom of Saxony, the Prussian province of Saxony and the north of England, would be exhausted in 100 to 200 years, the other British coalfields, the Waldenburg-Schatzlar and that of the north of France in 250 years, those of Saarbrücken, Belgium, Aachen and Westphalia in 600 to 800 years, and those of Upper Silesia in more than 1000 years.
Coal-Mining.
The opening and laying out, or, as it is generally called, “winning,” of new collieries is rarely undertaken without a preliminary examination Preliminary trial of coalworkings. of the character of the strata by means of borings, either for the purpose of determining the 580 number and nature of the coal seams in new ground, or the position of the particular seam or seams which it is proposed to work in extensions of known coalfields.
 |
| Fig. 1.—Proving by Boreholes. |
The principle of proving a mineral field by boring is illustrated by fig. 1, which represents a line direct from the dip to the rise of the field, the inclination of the strata being one in eight. No. 1 bore is commenced at the dip, and reaches a seam of coal A, at 40 fathoms; at this depth it is considered proper to remove nearer to the outcrop so that lower strata may be bored into at a less depth, and a second bore is commenced. To find the position of No. 2, so as to form a continuous section, it is necessary to reckon the inclination of the strata, which is 1 in 8; and as bore No. 1 was 40 fathoms in depth, we multiply the depth by the rate of inclination, 40 × 8 = 320 fathoms, which gives the point at which the coal seam A should reach the surface. But there is generally a certain depth of alluvial cover which requires to be deducted, and which we call 3 fathoms, then (40 - 3 = 37) × 8 = 296 fathoms; or say 286 fathoms is the distance that the second bore should be placed to the rise of the first, so as to have, for certain, the seam of coal A in clear connexion with the seam of coal B. In bore No. 3, where the seam B, according to the same system of arrangement, should have been found at or near the surface, another seam C is proved at a considerable depth, differing in character and thickness from either of the preceding. This derangement being carefully noted, another bore to the outcrop on the same principle is put down for the purpose of proving the seam C; the nature of the strata at first is found to agree with the latter part of that bored through in No. 3, but immediately on crossing the dislocation seen in the figure it is changed and the deeper seam D is found.
The evidence therefore of these bores (3 and 4) indicates some material derangement, which is then proved by other bores, either towards the dip or the outcrop, according to the judgment of the borer, so as to ascertain the best position for sinking pits. (For the methods of boring see Boring.)
The working of coal may be conducted either by means of levels or galleries driven from the outcrop in a valley, or by shafts or pits sunk from the surface. In the early days of coal-mining, open working, or quarrying from Methods of working. the outcrop of the seams, was practised to a considerable extent; but there are now few if any places in England where this can be done. In 1873 there could be seen, in the thick coal seams of Bengal, near Raniganj, a seam about 50 ft. thick laid bare, over an area of several acres, by stripping off a superficial covering varying from 10 to 30 ft., in order to remove the whole of the coal without loss by pillars. Such a case, however, is quite exceptional. The operations by which the coal is reached and laid out for removal are known as “winning,” the actual working or extraction of the coal being termed “getting.” In fig. 2 A B is a cross cut level, by which the seams of coal 1 and 2 are won, and C D a vertical shaft by which the seams 1, 2 and 3 are won. When the field is won by the former method, the coal lying above the level is said to be “level-free.” The mode of winning by level is of less general application than that by shafts, as the capacity for production is less, owing to the smaller size of roadways by which the coal must be brought to the surface, levels of large section being expensive and difficult to keep open when the mine has been for some time at work. Shafts, on the other hand, may be made of almost any capacity, owing to the high speed in drawing which is attainable with proper mechanism, and allow of the use of more perfect arrangements at the surface than can usually be adopted at the mouth of a level on a hill-side. A more cogent reason, however, is to be found in the fact that the principal coalfields are in flat countries, where the coal can only be reached by vertical sinking.
The methods adopted in driving levels for collieries are generally similar to those adopted in other mines. The ground is secured by timbering, or more usually by arching in masonry or brick-work. Levels like that in fig. 2, which are driven across the stratification, or generally anywhere not in coal, are known as “stone drifts.” The sinking of colliery shafts, however, Sinking of shafts. differs considerably from that of other mines, owing to their generally large size, and the difficulties that are often encountered from water during the sinking. The actual coal measure strata, consisting mainly of shales and clays, are generally impervious to water, but when strata of a permeable character are sunk through, such as the magnesian limestone of the north of England, the Permian sandstones of the central counties, or the chalk and greensand in the north of France and Westphalia, special methods are required in order to pass the water-bearing beds, and to protect the shaft and workings from the influx of water subsequently. Of these Tubbing. methods one of the chief is the plan of tubbing, or lining the excavation with an impermeable casing of wood or iron, generally the latter, built up in segments forming rings, which are piled upon each other throughout the whole depth of the water-bearing strata. This method necessitates the use of very considerable pumping power during the sinking, as the water has to be kept down in order to allow the sinkers to reach a water-tight stratum upon which the foundation of the tubbing can be placed. This consists of a heavy cast iron ring, known as a wedging crib, or curb, also fitted together in segments, which is lodged in a square-edged groove cut for its reception, tightly caulked with moss, and wedged into position. Upon this the tubbing is built up in segments, of which usually from 10 to 12 are required for the entire circumference, the edges being made perfectly true. The thickness varies according to the pressure expected, but may be taken at from ¾ to 1½ in. The inner face is smooth, but the back is strengthened with angle brackets at the corners. A small hole is left in the centre of each segment, which is kept open during the fitting to prevent undue pressure upon any one, but is stopped as soon as the circle is completed. In the north of France and Belgium wooden tubbings, built of polygonal rings, were at one time in general use. The polygons adopted were of 20 or more sides approximating to a circular form.
 |
| Fig. 2.—Shaft and Level. |
The second principal method of sinking through water-bearing ground is by compressed air. The shaft is lined with a cylinder of wrought iron, within which a tubular chamber, provided with doors above and below, known as an Pneumatic sinking. air-lock, is fitted by a telescopic joint, which is tightly packed so as to close the top of the shaft air-tight. Air is then forced into the inclosed space by means of a compressing engine, until the pressure is sufficient to oppose the flow of water into the excavation, and to drive out any that may collect in the bottom of the shaft through a pipe which is carried through the air-sluice to the surface. The miners work in the bottom in the same manner as divers in an ordinary diving-bell. Access to the surface is obtained through the double doors of the air-sluice, 581 the pressure being reduced to that of the external atmosphere when it is desired to open the upper door, and increased to that of the working space below when it is intended to communicate with the sinkers, or to raise the stuff broken in the bottom. This method has been adopted in various sinkings on the continent of Europe.
The third method of sinking through water-bearing strata is that of boring, adopted by Messrs Kind & Chaudron in Belgium and Germany. For this purpose a horizontal bar armed with vertical cutting chisels is used, which cuts Shaft boring. out the whole section of the shaft simultaneously. In the first instance, a smaller cutting frame is used, boring a hole from 3 to 5 ft. in diameter, which is kept some 50 or 60 ft. in advance, so as to receive the detritus, which is removed by a shell pump of large size. The large trepan or cutter weighs about 16 tons, and cuts a hole of from 9 to 15 ft. in diameter. The water-tight lining may be either a wrought iron tube, which is pressed down by jack screws as the borehole advances, or cast iron tubbing put together in short complete rings, in contradistinction to the old plan of building them up of segments. The tubbing, which is considerably less in diameter than the borehole, is suspended by rods from the surface until a bed suitable for a foundation is reached, upon which a sliding length of tube, known as the moss box, bearing a shoulder, which is filled with dried moss, is placed. The whole weight of the tubbing is made to bear on the moss, which squeezes outwards, forming a completely water-tight joint. The interval between the back of the tubbing and the sides of the borehole is then filled up with concrete, which on setting fixes the tubbing firmly in position. With increase in depth, however, the thickness and weight of the cast iron tubbing in a large shaft become almost unmanageable; in one instance, at a depth of 1215 ft., the bottom rings in a shaft 14½ ft. in diameter are about 4 in. thick, which is about the limit for sound castings. It has therefore been proposed, for greater depths, to put four columns of tubbings of smaller diameters, 8½ and 5½ ft., in the shaft, and fill up the remainder of the boring with concrete, so that with thinner and lighter castings a greater depth may be reached. This, however, has not as yet been tried. Another extremely useful method of sinking through water-bearing ground, introduced by Messrs A. & H. T. Poetsch in 1883, and originally applied to shafts passing through quicksands above brown coal seams, has been applied with advantage in opening new pits through the secondary and tertiary strata above the coal measures in the north of France and Belgium, some of the most successful examples being those at Lens, Anzin and Vicq, in the north of France basin. In this system the soft ground or fissured water-bearing rock is rendered temporarily solid by freezing the contained water within a surface a few feet larger in diameter than the size of the finished shaft, so that the ground may be broken either by hand tools or blasting in the same manner as hard rock. The miners are protected by the frozen wall, which may be 4 or 5 ft. thick. The freezing is effected by circulating brine (calcium chloride solution) cooled to 5° F. through a series of vertical pipes closed at the bottom, contained in boreholes arranged at equal distances apart around the space to be frozen, and carried down to a short distance below the bottom of the ground to be secured. The chilled brine enters through a central tube of small diameter, passes to the bottom of the outer one and rises through the latter to the surface, each system of tubes being connected above by a ring main with the circulating pumps. The brine is cooled in a tank filled with spiral pipes, in which anhydrous ammonia, previously liquefied by compression, is vaporized in vacuo at the atmospheric temperature by the sensible heat of the return-current of brine, whose temperature has been slightly raised in its passage through the circulating tubes. When hard ground is reached, a seat is formed for the cast iron tubbing, which is built up in the usual way and concreted at the back, a small quantity of caustic soda being sometimes used in mixing the concrete to prevent freezing. In an application of this method at Vicq, two shafts of 12 and 16.4 ft. diameter, in a covering of cretaceous strata, were frozen to a depth of 300 ft. in fifty days, the actual sinking and lining operations requiring ninety days more. The freezing machines were kept at work for 200 days, and 2191 tons of coal were consumed in supplying steam for the compressors and circulating pumps.
The introduction of these special methods has considerably simplified the problem of sinking through water-bearing strata. Some of the earlier sinkings of this kind, when pumps had to be depended on for keeping down the water, were conducted at great cost, as, for instance, at South Hetton, and more recently Ryhope, near Sunderland, through the magnesian limestone of Durham.
The size and form of colliery shafts vary in different districts. In the United States and Scotland rectangular pits secured by timber framings are still common, but the tendency is now generally to make them round, 20 ft. being about Size of shafts. the largest diameter employed. In the Midland counties, from 7 to 9 ft. is a very common size, but larger dimensions are adopted where a large production is required. Since the accident at Hartley colliery in 1862, caused by the breaking of the pumping-engine beam, which fell into the shaft and blocked it up, whereby the whole of the men then at work in the mine were starved to death, it has been made compulsory upon mine-owners in the United Kingdom to have two pits for each working, in place of the single one divided by walls or brattices which was formerly thought sufficient. The use of two independent connexions—whether separate pits or sections of the same pit, between the surface and the workings—is necessary for the service of the ventilation, fresh air from the surface being carried down one, known as the “downcast,” while the foul or return air of the mine rises through the other or “upcast” pit back to the surface. In a heavily-watered mine it is often necessary to establish a special engine-pit, with pumps permanently fixed, or a division of one of the pits may be devoted to this purpose. The pumps, placed close to the point where the water accumulates, may be worked by an engine on the surface by means of heavy reciprocating rods which pass down the shaft, or by underground motors driven by steam, compressed air or electricity.
Where the water does not accumulate very rapidly it is a common practice to allow it to collect in a pit or sump below the working bottom of the shaft, and to draw it off in a water tub or “hoppet” by the main engine, when the latter is not employed in raising coal.
The laying out of a colliery, after the coal has been won, by sinkings or levels, may be accomplished in various ways, according to the nature of the coal, its thickness and dip, and the extent of ground to be worked. In the South Laying out workings. Staffordshire and other Midland coalfields, where only shallow pits are required, and the coals are thick, a pair of pits may be sunk for a very few acres, while in the North of England, on the other hand, where sinking is expensive, an area of some thousands of acres may be commanded from the same number of pits. In the latter case, which represents the most approved practice, the sinking is usually placed about the centre of the ground, so that the workings may radiate in every direction from the pit bottom, with the view of employing the greatest number of hands to advantage. Where a large area cannot be commanded, it is best to sink to the lowest point of the field for the convenience of drawing the coal and water which become level-free in regard to the pit. Where properties are much divided, it is always necessary to maintain a thick barrier of unwrought coal between the boundary of the mine and the neighbouring workings, especially if the latter are to the dip. If a prominent line of fault crosses the area it may usually be a convenient division of the fields into sections or districts. The first process in laying out the workings consists in driving a gallery on the level along the course of the coal seam, which is known as a “dip head level,” and a lower parallel one, in which the water collects, known as a “lodgment level.” Galleries driven at right angles to these are known as a “dip” or “rise headings,” according to their position above or below the pit bottom. In Staffordshire the main levels are also known as 582 “gate roads.” To secure the perpendicularity of the shaft, it is necessary to leave a large mass or pillar of the seam untouched around the pit bottom. This pillar is known in Scotland as the “pit bottom stoop.” The junction of the levels with the pit is known as the “pit eye”; it is usually of an enlarged section, and lined with masonry or brick-work, so as to afford room for handling the wagons or trams of coal brought from the working faces. In this portion of the pit are generally placed the furnaces for ventilation, and the boilers required for working steam engines underground, as well as the stables and lamp cabin.
The removal of the coal after the roads have been driven may be effected in many different ways, according to the custom of the district. These may, however, all be considered as modifications of two systems, viz. pillar work Method of working coal. and long-wall work. In the former which is also known as “post and stall” or “bord and pillar” in the north of England, “pillar and stall” in South Wales, and “stoop and room” in Scotland, the field is divided into strips by numerous openings driven parallel to the main rise headings, called “bords” or “bord gates,” which are again divided by cutting through them at intervals, so as to leave a series of Pillar working. pillars arranged chequer-wise over the entire area. These pillars are left for the support of the roof as the workings advance, so as to keep the mine open and free from waste. In the oldest form of this class of working, where the size of the pillar is equal to the width of the stall or excavation, about ¾ of the whole seam will be removed, the remainder being left in the pillars. A portion of this may be got by the process known as robbing the pillars, but the coal so obtained is liable to be very much crushed from the pressure of the superincumbent strata. This crushing may take place either from above or below, producing what are known as “creeps” or “sits.”
 |
| Fig 3.—“Creeps” in Coal-Mines. |
A coal seam with a soft pavement and a hard roof is the most subject to a “creep.” The first indication is a dull hollow sound heard when treading on the pavement or floor, probably occasioned by some of the individual layers parting from each other as shown at a fig. 3; the succeeding stages of creep are shown at b, c, d, f, and g, in the same figure; the last being the final stage, when the coal begins to sustain the pressure from the overlying strata, in common with the disturbed pavement.
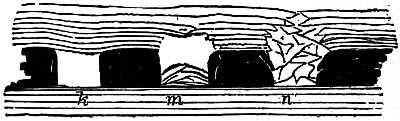 |
| Fig. 4.—“Sits” in Mines. |
“Sits” are the reverse of creeps; in the one case the pavement is forced up, and in the other the roof is forced or falls down, for want of proper support or tenacity in itself. This accident generally arises from an improper size of pillars; some roofs, however, are so difficult to support that sits take place where the half of the coal is left in pillars. Fig. 4 will convey a general idea of the appearance of sits,—k, m, n showing different stages.
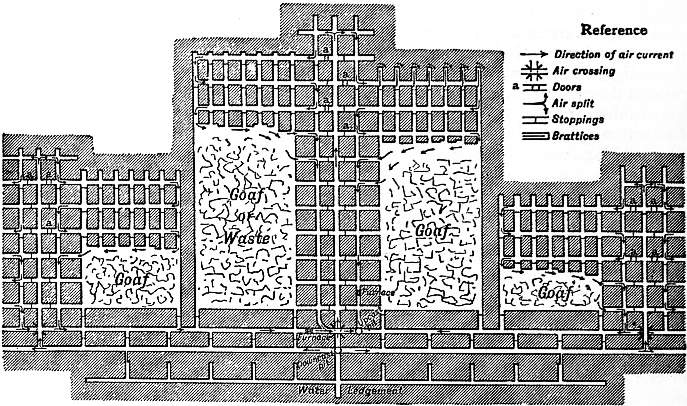 |
| Fig. 5.—Pillar Working. |
The modern method of pillar working is shown in fig. 5. In the Northumberland steam coal district, where it is carried out in the most perfect manner, the bords are 5 to 6 yds. in width, while the pillars are 22 yds. broad and 30 yds. long, which are subsequently got out on coming back. In the same figure is also shown the method of working whole coal and pillars at the same time, a barrier of two or three ranges of pillars or a rib of solid coal being left between the working in the solid and those in the pillars. The space from which the entire quantity of coal has been removed is known in different districts as the “goaf,” “gob,” or “waste.”
 |
| Fig. 6.—Lancashire method of working Coal. |
Fig. 6 represents the Lancashire system of pillar working. The area is laid out by two pairs of level drifts, parallel to each other, about 150 yds. apart, which are carried to the boundary. About 100 yds. back from the boundary a communication is made between these levels, from which other levels are driven forward, dividing the coal into ribs of about 25 or 30 yds. wide, which are then cut back by taking off the coal in slices from the level towards the rise in breadths of about 6 yds. By this method the whole of the coal is got backwards, the main roads being kept in solid coal; the intermediate levels not being driven till they are wanted, a greater amount of support is given, and the pillars are less crushed than is usual in pillar working.
In the South Wales system of working, cross headings are driven from the main roads obliquely across the rise to get a sufficiently easy gradient for horse roads, and from these the stalls are opened out with a narrow entrance, in order to 583 leave support on either side of the road, but afterwards widening to as great a breadth as the seam will allow, leaving pillars of a minimum thickness. The character of such workings is very irregular in plan, and as the ventilation is attended with considerable difficulty, it is now becoming generally superseded by more improved methods.
 |
| Fig. 7.—Long-wall method of working Coal in Derbyshire. |
The second great principle of working is that known as long-wall or long-work, in which the coal is taken away either in broad faces from roads about 40 or 50 yds. apart and parallel to each other, or along curved faces between roads Long-wall working. radiating from the pit bottom—the essential feature in both cases being the removal of the whole of the coal at once, without first sub-dividing it into pillars, to be taken away at a second working. The roof is temporarily supported by wooden props or pack walling of stone, for a sufficient breadth along the face to protect the workmen, and allow them to work together behind. The general character of a long-wall working is shown in fig. 7, which represents an area of about 500 acres of the bottom hard steam coal at Shipley in Derbyshire. The principal road extends from the shafts southward; and on both sides of it the coal has been removed from the light-shaded area by cutting it back perpendicularly towards the boundaries, along faces about 50 yds. in length, those nearest to the shaft being kept in advance of those farther away, producing a step-shaped outline to the face of the whole coal. It will be seen that by this method the whole of the seam, with the exception of the pillars left to protect the main roadways, is removed. The roads for drawing the coal from the working faces to the shaft are kept open by walling through the waste or goaf produced by the fall of the unsupported roof. The straight roads are the air-ways for carrying pure air from the down-cast shaft to the working faces, while the return air passes along the faces and back to the up-cast by the curved road. The above is the method of working long-wall forward, i.e. taking the coal in advance from the pit towards the boundary, with roads kept open through the gob. Another method consists in driving towards the boundary, and taking the coal backward towards the shafts, or working homeward, allowing the waste to close up without roads having to be kept open through it. This is of course preferable, but is only applicable where the owner of the mine can afford to expend the capital required to reach the limit of the field in excess of that necessary when the raising of coal proceeds pari passu with the extension of the main roads. Fig. 6 is substantially a modification of this kind of long-wall work. South Yorkshire method. Fig. 8 represents a method of working practised in the South Yorkshire district, known as bords and banks. The field is divided by levels and headings into rectangular banks, while from the main levels bords or wickets about 30 yds. wide, separated from each other by banks of about the same width, are carried forward in long-wall work, as shown on the left side of the figure, the waste being carefully packed behind so as to secure the ventilation. When these have been worked up to the extremity, as shown on the right side, the intermediate bank is removed by working backward towards the level. This system, therefore, combines both methods of long-wall working, but it is not generally applicable, owing to the difficulty of ventilation, due to the great length of air-way that has to be kept open around the waste on each bank.
The relative advantages of the different methods may be generally stated as follows. Long-wall work is best suited for thin coals, and those having a good roof, i.e. one that gives way gradually and fills up the excavation made by removing the coal without scaling off suddenly and falling into the working faces, when practically the whole of the coal may be removed. Against these advantages must be placed the difficulties attending the maintenance of roads through the goaves, and in some cases the large proportion of slack to round or large coal obtained. Pillar working, in the whole coal, is generally reputed to give a more advantageous proportion of round coal to slack, the latter being more abundantly produced on the removal of the pillars, but as these form only a small portion of the whole seam, the general yield is more advantageous than in the former method. The ventilation of pillar working is often attended with difficulty, and the coal is longer exposed to the influence of the air, a point of importance in some coals, which deteriorate in quality when exposed to a hot damp atmosphere. The great increase in the size of the pillars in the best modern collieries worked upon this principle has, however, done much to approximate the two systems to an equality in other respects.
Where the whole of the coal is removed at once there is less chance of surface damage, when the mines are deep, than with pillar workings. A notable instance of this was afforded at Newstead, Notts, where the ruined front of Newstead Abbey was lowered several feet without any injury to the structure.
 |
| Fig. 8.—Bords and Banks. |
The working of very thick seams presents certain special peculiarities, owing to the difficulties of supporting the roof in the excavated portions, and supplying fresh air to the workings. The most typical example of this kind of Working thick seams. working in England is afforded by the thick coal of South Staffordshire, which consists of a series of closely associated coal seams, varying from 8 to 12 or 13, divided from each other by their partings, but making together one great bed of from 25 to 40 ft. or more in thickness. The partings together do not amount to more than 2 or 3 ft. The method of working which has been long in use is represented in fig. 9. The main level or gate road is driven in the benches coal, or lower part of the seam, while a smaller drift for ventilation, called an air heading, is carried above it in one of the upper beds called the slipper coal. From the gate road a heading called a bolt-hole is opened, and extended into a large rectangular chamber, known as a “side of work,” large pillars being left at regular intervals, besides smaller ones or cogs. The order in which the coal is cut is shown in the dotted and numbered squares in the figure. The coal is first cut to the top of the slipper coal from below, after which the upper portion is either broken down by wedging or falls of itself. The working of these upper portions is exceedingly 584 dangerous, owing to the great height of the excavations, and fatal accidents from falls of roof are in consequence more common in South Staffordshire than in any other coalfield in this country. The air from the down-cast shaft enters from the gate road, and passes to the up-cast through the air heading above. About one-half of the total coal (or less) is obtained in the first working; the roof is then allowed to fall, and when the gob is sufficiently consolidated, fresh roads are driven through it to obtain the ribs and pillars left behind by a second or even, in some cases, a third working. The loss of coal by this method is very considerable, besides great risk to life and danger from fire. It has, therefore, been to some extent superseded by the long-wall method, the upper half being taken at the first working, and removed as completely as possible, working backwards from the boundaries to the shaft. The lower half is then taken in the same manner, after the fallen roof has become sufficiently consolidated to allow the mine to be re-opened.
 |
| Fig. 9.—South Staffordshire method of working Thick Coal. |
In the working of thick seams inclined at a high angle, such as those in the south of France, and in the lignite mines of Styria and Bohemia, the method of working in horizontal slices, about 12 or 15 ft. thick, and filling up the excavation with broken rock and earth from the surface, is now generally adopted in preference to the systems formerly used. At Monceaux les Mines, in France, a seam 40 ft. thick, and dipping at an angle of 20°, is worked in the following manner. A level is driven in a sandstone forming the floor, along the course of the coal, into which communications are made by cross cuts at intervals of 16 yds., which are driven across to the roof, dividing up the area to be worked into panels. These are worked backwards, the coal being taken to a height of 20 ft., the opening being packed up with stone sent down from the surface. As each stage is worked out, the floor level is connected with that next below it by means of an incline, which facilitates the introduction of the packing material. Stuff containing a considerable amount of clay is found to be the best suited for the purpose of filling, as it consolidates readily under pressure.
In France and Germany the method of filling the space left by the removal of the coal with waste rock, quarried underground or sent down from the surface, which was originally used in connexion with the working of thick inclined seams by the method of horizontal slices, is now largely extended to long-wall workings on thin seams, and in Westphalia is made compulsory where workings extend below surface buildings, and safety pillars of unwrought coal are found to be insufficient. With careful packing it is estimated that the surface subsidence will not exceed 40% of the thickness of the seam removed, and will usually be considerably less. The material for filling may be the waste from earlier workings stored in the spoil banks at the surface; where there are blast furnaces in the neighbourhood, granulated slag mixed with earth affords excellent packing. In thick seams packing adds about 5d. per ton to the cost of the coal, but in thinner seams the advantage is on the other side.
In some anthracite collieries in America the small coal or culm and other waste are washed into the exhausted workings by water which gives a compact mass filling the excavation when the water has drained away. A modification of this method, which originated in Silesia, is now becoming of importance in many European coalfields. In this the filling material, preferably sand, is sent down from the surface through a vertical steel pipe mixed with sufficient water to allow it to flow freely through distributing pipes in the levels commanding the excavations to be filled; these are closed at the bottom by screens of boards sufficiently close to retain the packing material while allowing the water to pass by the lower level to the pumping-engine which returns it to the surface.
 |
| Fig. 10.—Long-wall working-face—Plan and Section. |
The actual cutting of the coal is chiefly performed by manual labour, the tool employed being a sharp-pointed double-armed pick, which is nearly straight, except when required for use in hard rock, when the arms are made with an Methods of cutting coal. inclination or “anchored.” The terms pike, pick, mandril and slitter are applied to the collier’s pick in different districts, the men being known as pikemen or hewers. In driving levels it is necessary to cut grooves vertically parallel to the walls, a process known as shearing; but the most important operation is that known as holing or kirving, which consists in cutting a notch or groove in the floor of the seam to a depth of about 3 ft., measured back from the face, so as to leave the overhanging part unsupported, which then either falls of its own accord within a few hours, or is brought down either by driving wedges along the top, or by blasting. The process of holing in coal is one of the severest kinds of human labour. It has to be performed in a constrained position, and the miner lying on his side has to cut to a much greater height, in order to get room to carry the groove in to a sufficient depth, than is required to bring the coal down, giving rise to a great waste in slack as compared with machine work. This is sometimes obviated by holing in the beds below the coal, or in any portion of a seam of inferior quality that may not be worth working. This loss is proportionately greater in thin than in thick seams, the same quantity being cut to waste in either case. The method of cutting coal on the long-wall system is seen in fig. 10, representing the working at the Shipley colliery. The coal is 40 in. thick, with a seam of fire-clay and a roof of black shale; about 6 in. of the upper part, known as the roof coal, not being worth working, is left behind. A groove of triangular section of 30 in. base and 9 in. high is cut along the face, inclined timber props being placed at intervals to support the overhanging portion until the required length is cut. These are then removed, and the coal is allowed to fall, wedges or blasting being employed when necessary. The roof of the excavation is supported as the coal is removed, by packing up the waste material, and by a double row of props, 2 ft. from each other, placed temporarily along the face. These are placed 5 ft. apart, the props of the back row alternating with those in front. 585 The props used are preferably of small oak or English larch, but large quantities of fir props, cut to the right length, are also imported from the north of Europe. As the work proceeds onwards, the props are withdrawn and replaced in advance, except those that may be crushed by the pressure or buried by sudden falls of the roof.
In Yorkshire hollow square pillars, formed by piling up short blocks of wood or chocks, are often used instead of props formed of a single stem.
In securing the roof and sides of coal workings, malleable iron and steel are now used to some extent instead of timber, although the consumption of the latter material is extremely large. As a substitute for timber props at the face, pieces of steel joists, with the web cut out for a short distance on either end, with the flanges turned back to give a square bearing surface, have been introduced. In large levels only the cap pieces for the roof are made of steel joists, but in smaller ones complete arches made of pieces of rails fish-jointed at the crown are used. In another system introduced by the Mannesmann Tube Company the prop is made up of weldless steel tubes sliding telescopically one within the other, which are fixed at the right height by a screw clamp capable of carrying a load of 15 to 16 tons. These can be most advantageously used on thick seams 6 to 10 ft. or upwards. For shaft linings steel rings of H or channel section supported by intermediate struts are also used, and cross-bearers or buntons of steel joists and rail guides are now generally substituted for wood.
When the coal has been under-cut for a sufficient length, the struts are withdrawn, and the overhanging mass is allowed to fall during the time that the workmen are out of the pit, or it may be brought down by driving wedges, or if it be of a compact character a blast in a borehole near the roof may be required. Sometimes, but rarely, it happens that it is necessary to cut vertical grooves in the face to determine the limit of the fall, such limits being usually dependent upon the cleet or divisional planes in the coal, especially when the work is carried perpendicular to them or on the end.
The substitution of machinery for hand labour in cutting coal has long been a favourite problem with inventors, the earliest plan being that of Michael Meinzies, in 1761, who proposed to work a heavy pick underground by power Coal-cutting machines. transmitted from an engine at the surface, through the agencies of spear-rods and chains passing over pulleys; but none of the methods suggested proved to be practically successful until the general introduction of compressed air into mines furnished a convenient motive power, susceptible of being carried to considerable distances without any great loss of pressure. This agent has been applied in various ways, in machines which either imitate the action of the collier by cutting with a pick or make a groove by rotating cutters attached to an endless chain or a revolving disk or wheel. The most successful of the first class, or pick machines, that of William Firth of Sheffield, consists essentially of a horizontal pick with two cutting arms placed one slightly in advance of the other, which is swung backwards and forwards by a pair of bell crank levers actuated by a horizontal cylinder engine mounted on a railway truck. The weight is about 15 cwt. At a working speed of 60 yds. per shift of 6 hours, the work done corresponds to that of twelve average men. The width of the groove cut is from 2 to 3 in. at the face, diminishing to 1½ in. at the back, the proportion of waste being very considerably diminished as compared with the system of holing by hand. The use of this machine has allowed a thin seam of cannel, from 10 to 14 in. in thickness, to be worked at a profit, which had formerly been abandoned as too hard to be worked by hand-labour. Pick machines have also been introduced by Jones and Levick, Bidder, and other inventors, but their use is now mostly abandoned in favour of those working continuously.
In the Gartsherrie machine of Messrs Baird, the earliest of the flexible chain cutter type, the chain of cutters works round a fixed frame or jib projecting at right angles from the engine carriage, an arrangement which makes it necessary to cut from the end of the block of coal to the full depth, instead of holing into it from the face. The forward feed is given by a chain winding upon a drum, which hauls upon a pulley fixed to a prop about 30 yds. in advance. This is one of the most compact forms of machine, the smaller size being only 20 in. high. With an air pressure of from 35 to 40 ℔. per sq. in., a length of from 300 to 350 ft. of coal is holed, 2 ft. 9 in. deep, in the shift of from 8 to 10 hours. The chain machine has been largely developed in America in the Jeffrey, Link Bell, and Morgan Gardner coal cutters. These are similar in principle to the Baird machine, the cutting agent being a flat link chain carrying a double set of chisel points, which are drawn across the coal face at the rate of about 5 ft. per second; but, unlike the older machines, in which the cutting is done in a fixed plane, the chain with its motor is made movable, and is fed forward by a rack-and-pinion motion as the cutting advances, so that the cut is limited in breadth (3½ to 4 ft.), while its depth may be varied up to the maximum travel (8 ft.) of the cutting frame. The carrying frame, while the work is going on, is fixed in position by jack-screws bearing against the roof of the seam, which, when the cut is completed, are withdrawn, and the machine shifted laterally through a distance equal to the breadth of the cut and fixed in position again. The whole operation requires from 8 to 10 minutes, giving a cutting speed of 120 to 150 sq. ft. per hour. These machines weigh from 20 to 22 cwt., and are mostly driven by electric motors of 25 up to 35 h.p. as a maximum. By reason of their intermittent action they are only suited for use in driving galleries or in pillar-and-stall workings.
 |
| Fig. 11.—Winstanly & Barker’s Coal-cutting Machine—Plan. |
A simple form of the saw or spur wheel coal-cutting machine is that of Messrs Winstanly & Barker (fig. 11), which is driven by a pair of oscillating engines placed on a frame running on rails in the usual way. The crank shaft carries a pinion which gears into a toothed wheel of a coarse pitch, carrying cutters at the ends of the teeth. This wheel is mounted on a carrier which, being movable about its centre by a screw gearing worked by hand, gives a radial sweep to the cutting edges. When at work it is slowly turned until the carrier is at right angles to the frame, when the cut has attained the full depth. The forward motion is given by a chain winding upon a crab placed in front, by which it is hauled slowly forward. With 25 ℔ pressure it will hole 3 ft. deep, at the rate of 30 yds. per hour, the cut being only 2¾ in. high, but it will only work on one side of the carriage. This type has been greatly improved and now is the most popular machine in Great Britain, especially in long-wall workings. W. E. Garforth’s Diamond coal cutter, one of the best known, undercuts from 5½ to 6 ft. In some instances electric motors have been substituted for compressed-air engines in such machines.
Another class of percussive coal-cutters of American origin is represented by the Harrison, Sullivan and Ingersoll-Sergeant machines, which are essentially large rock-drills without turning gear for the cutting tool, and mounted upon a pair of wheels placed so as to allow the tool to work on a forward slope. When in use the machine is placed upon a wooden platform inclining 586 towards the face, upon which the miner lies and controls the direction of the blow by a pair of handles at the back of the machine, which is kept stationary by wedging the wheels against a stop on the platform. These machines, which are driven by compressed air, are very handy in use, as the height and direction of the cut may be readily varied; but the work is rather severe to the driver on account of the recoil shock of the piston, and an assistant is necessary to clear out the small coal from the cut, which limits the rate of cutting to about 125 sq. ft. per hour.
Another kind of application of machinery to coal mining is that of Messrs Bidder & Jones, which is intended to replace the use of blasting for bringing down the coal. It consists of a small hydraulic press, which forces a set of expanding Coal-wedging machines. bits or wedges into a bore-hole previously bored by a long screw augur or drill, worked by hand, the action of the press being continued until a sufficient strain is obtained to bring down the coal. The arrangement is, in fact, a modification of the plug and feather system used in stone quarrying for obtaining large blocks, but with the substitution of the powerful rending force of the hydraulic press for hand-power in driving up the wedges. This apparatus has been used at Harecastle in North Staffordshire, and found to work well, but with the disadvantage of bringing down the coal in unmanageably large masses. A method of wedging down coal sufficiently perfected to be of general application would add greatly to the security of colliers.
The removal of the coal broken at the working face to the pit bottom may in small mines be effected by hand labour, but more generally it is done by horse or mechanical traction, upon railways, the “trams” or “tubs,” as the pit Underground conveyance. wagons are called, being where possible brought up to the face. In steeply inclined seams passes or shoots leading to the main level below are sometimes used, and in Belgium iron plates are sometimes laid in the excavated ground to form a slide for the coal down to the loading place. In some instances travelling belts or creepers have been adopted, which deliver the coal with a reduced amount of breakage, but this application is not common. The capacity of the trams varies with the size of the workings and the shaft. From 5 to 7 cwt. are common sizes, but in South Wales they are larger, carrying up to one ton or more. The rails used are of flat bottomed or bridge section varying in weight from 15 to 25 ℔ to the yd.; they are laid upon cross sleepers in a temporary manner, so that they can be easily shifted along the working faces, but are carefully secured along main roads intended to carry traffic continuously for some time. The arrangement of the roads at the face is shown in the plan, fig. 10. In the main roads to the pit when the distance is not considerable horse traction may be used, a train of 6 to 15 vehicles being drawn by one horse, but more generally the hauling or, as it is called in the north of England, the leading of the trains of tubs is effected by mechanical traction.
In a large colliery where the shafts are situated near the centre of the field, and the workings extend on all sides, both to the dip and rise, the drawing roads for the coal may be of three different kinds—(1) levels driven at right angles to the dip, suitable for horse roads, (2) rise ways, known as jinny roads, jig-brows, or up-brows, which, when of sufficient slope, may be used as self-acting planes, i.e. the loaded waggons may be made to pull back the empty ones to the working faces, and (3) dip or down-brows, requiring engine power. A road may be used as a self-acting or gravitating incline when the gradient is 1 in 30 or steeper, in which case the train is lowered by a rope passing over a pulley or brake drum at the upper end, the return empty train being attached to the opposite end of the rope and hauled up by the descending load. The arrangements for this purpose vary, of course, with the amount of work to be done with one fixing of the machinery; where it is likely to be used for a considerable time, the drum and brake are solidly constructed, and the ropes of steel or iron wire carefully guided over friction rollers, placed at intervals between the rails to prevent them from chafing and wearing out on the ground. Where the load has to be hauled up a rising gradient, underground engines, driven by steam or compressed air or electric motors, are used. In some cases steam generated in boilers at the surface is carried in pipes to the engines below, but there is less loss of power when compressed air is sent down in the same way. Underground boilers placed near the up-cast pit so that the smoke and gases help the ventilating furnace have been largely used but are now less favourably regarded than formerly. Water-pressure engines, driven by a column of water equal to the depth of the pit, have also been employed for hauling. These can, however, only be used advantageously where there are fixed pumps, the fall of water generating the power resulting in a load to be removed by the expenditure of an equivalent amount of power in the pumping engine above that necessary for keeping down the mine water.
The principal methods in which power can be applied to underground traction are as follows:—
1. Tail rope system.
2. Endless chain system.
3. Endless rope system on the ground.
4. Endless rope system overhead.
The three last may be considered as modifications of the same principle. In the first, which is that generally used in Northumberland and Durham, a single line of rails is used, the loaded tubs being drawn “out bye,” i.e. towards the shaft, and the empty ones returned “in bye,” or towards the working faces, by reversing the engine; while in the other systems, double lines, with the rope travelling continuously in the same direction, are the rule. On the tail rope plan the engine has two drums worked by spur gearing, which can be connected with, or cast loose from, the driving shaft at pleasure. The main rope, which draws out the loaded tubs, coils upon one drum, and passes near the floor over guide sheaves placed about 20 ft. apart. The tail rope, which is of lighter section than the main one, is coiled on the second drum, passes over similar guide sheaves placed near the roof or side of the gallery round a pulley at the bottom of the plane, and is fixed to the end of the train or set of tubs. When the load is being drawn out, the engine pulls directly on the main rope, coiling it on to its own drum, while the tail drum runs loose paying out its rope, a slight brake pressure being used to prevent its running out too fast. When the set arrives out bye, the main rope will be wound up, and the tail rope pass out from the drum to the end and back, i.e. twice the length of the way; the set is returned in bye, by reversing the engine, casting loose the main, and coupling up the tail drum, so that the tail rope is wound up and the main rope paid out. This method, which is the oldest, is best adapted for ways that are nearly level, or when many branches are intended to be worked from one engine, and can be carried round curves of small radius without deranging the trains; but as it is intermittent in action, considerable engine-power is required in order to get up the required speed, which is from 8 to 10 m. per hour. From 8 to 10 tubs are usually drawn in a set, the ways being often from 2000 to 3000 yds. long. In dip workings the tail rope is often made to work a pump connected with the bottom pulley, which forces the water back to the cistern of the main pumping engine in the pit.
For the endless chain system, which is much used in the Wigan district, a double line of way is necessary, one line for full and the other for empty tubs. The chain passes over a pulley driven by the engine, placed at such a height as to allow it to rest upon the tops of the tubs, and round a similar pulley at the far end of the plane. The forward edge of the tub carries a projecting pin or horn, with a notch into which the chain falls which drags the tub forward. The road at the outer end is made of a less slope than the chain, so that on arrival the tub is lowered, clears the pin, and so becomes detached from the chain. The tubs are placed on at intervals of about 20 yds., the chain moving continuously at a speed of from 2½ to 4 m. per hour. This system presents the greatest advantages in point of economy of driving power, especially where the gradients are variable, but is expensive in first cost, and is not well suited for curves, and branch roads cannot be worked continuously, as a fresh set of pulleys worked by bevel gearing is required for each branch.
The endless rope system may be used with either a single or 587 double line of way, but the latter is more generally advantageous. The rope, which is guided upon sheaves between the rails, is taken twice round the head pulley. It is also customary to use a stretching pulley to keep the rope strained when the pull of the load diminishes. This is done by passing a loop at the upper end round a pulley mounted in a travelling frame, to which is attached a weight of about 15 cwt. hanging by a chain. This weight pulls directly against the rope; so if the latter slacks, the weight pulls out the pulley frame and tightens it up again. The tubs are usually formed into sets of from 2 to 12, the front one being coupled up by a short length of chain to a clamping hook formed of two jaws moulded to the curve of the rope which are attached by the “run rider,” as the driver accompanying the train is called. This system in many respects resembles the tail rope, but has the advantage of working with one-third less length of rope for the same length of way.
The endless rope system overhead is substantially similar to the endless chain. The wagons are attached at intervals by short lengths of chain lapped twice round the rope and hooked into one of the links, or in some cases the chains are hooked into hempen loops on the main rope. In mines that are worked from the outcrop by adits or day levels traction by locomotives driven by steam, compressed air or electricity is used to some extent. The most numerous applications are in America.
One of the most important branches of colliery work is the management of the ventilation, involving as it does the supply of fresh air to the men working in the pit, as well as the removal of inflammable gases that may be given Ventilation. off by the coal. This is effected by carrying through the workings a large volume of air which is kept continually moving in the same direction, descending from the surface by one or more pits known as intake or downcast pits, and leaving the mine by a return or upcast pit. Such a circulation of air can only be effected by mechanical means when the workings are of any extent, the methods actually adopted being—(1) The rarefaction of the air in the upcast pit by a furnace placed at the bottom; and (2) Exhaustion by machinery at the surface. The former plan, being the older, has been most largely used, but is becoming replaced by some form of machine.
The usual form of ventilating furnace is a plain fire grate placed under an arch, and communicating with the upcast shaft by an inclined drift. It is separated from the coal by a narrow passage walled and arched in brickwork on both-sides. The size of the grate varies with the requirements of the ventilation, but from 6 to 10 ft. broad and from 6 to 8 ft. long are usual dimensions. The fire should be kept as thin and bright as possible, to reduce the amount of smoke in the upcast. When the mine is free from gas, the furnace may be worked by the return air, but it is better to take fresh air directly from the downcast by a scale, or split, from the main current. The return air from fiery workings is never allowed to approach the furnace, but is carried into the upcast by a special channel, called a dumb drift, some distance above the furnace drift, so as not to come in contact with the products of combustion until they have been cooled below the igniting point of fire-damp. Where the upcast pit is used for drawing coal, it is usual to discharge the smoke and gases through a short lateral drift near the surface into a tall chimney, so as to keep the pit-top as clear as possible for working. Otherwise the chimney is built directly over the mouth of the pit.
Mechanical ventilation may be effected either by direct exhaustion or centrifugal displacement of the air to be removed. In the first method reciprocating bells, or piston machines, or rotary machines of varying capacity like gas-works exhausters, are employed. They were formerly used on a very large scale in Belgium and South Wales, but the great weight of the moving parts makes it impossible to drive them at the high speed called for by modern requirements, so that centrifugal fans are now generally adopted instead. An early and very successful machine of this class, the Guibal fan, is represented in fig. 12. The fan has eight arms, framed together of wrought iron bars, with diagonal struts, so as to obtain rigidity with comparative lightness, carrying flat close-boarded blades at their extremities. It revolves with the smallest possible clearance in a chamber of masonry, one of the side walls being perforated by a large round hole, through which the air from the mine is admitted to the centre of the fan. The lower quadrant of the casing is enlarged spirally, so as to leave a narrow rectangular opening at the bottom, through which the air is discharged into a chimney of gradually increasing section carried to a height of about 25 ft. The size of the discharge aperture can be varied by means of a flexible wooden shutter sliding in a groove in a cast iron plate, curved to the slope of the casing. By the use of the spiral guide casing and the chimney the velocity of the effluent air is gradually reduced up to the point of final discharge into the atmosphere, whereby a greater useful effect is realized than is the case when the air streams freely from the circumference with a velocity equal to that of the rotating fan. The power is applied by steam acting directly on a crank at one end of the axle, and the diameter of the fan may be 40 ft. or more.
 |
| Fig. 12.—Guibal Fan. |
The Waddle fan, represented in fig. 13, is an example of another class of centrifugal ventilator, in which a close casing is not used, the air exhausted being discharged from the circumference directly into the atmosphere. It consists of a hollow sheet iron drum formed by two conoidal tubes, united together by numerous guide blades, dividing it up into a series of rectangular tubes of diminishing section, attached to a horizontal axle by cast iron bosses and wrought iron arms. The tubes at their smallest part are connected to a cast iron ring, 10 ft. in diameter, but at their outer circumference they are only 2 ft. apart. The extreme diameter is 25 ft.
 |
| Fig. 13.—Waddle Fan. |
By the adoption of more refined methods of construction, especially in the shape of the intake and discharge passages for the air and the forms of the fan blades, the efficiency of the ventilating fan has been greatly increased so that the dimensions can be much reduced and a higher rate of speed adopted. Notable examples are found in the Rateau, Ser and Capell fans, and where an electric generating station is available electric motors can be advantageously used instead of steam.
The quantity of air required for a large colliery depends upon the number of men employed, as for actual respiration from 588 100 to 200 cub. ft. per minute should be allowed. In fiery mines, however, a very much larger amount must be provided in order to dilute the gas to the point of safety. Distribution of air underground. Even with the best arrangements a dangerous increase in the amount of gas is not infrequent from the sudden release of stored-up masses in the coal, which, overpowering the ventilation, produce magazines of explosive material ready for ignition when brought in contact with the flame of a lamp or the blast of a shot. The management of such places, therefore, requires the most constant vigilance on the part of the workmen, especially in the examination of the working places that have been standing empty during the night, in which gas may have accumulated, to see that they are properly cleared before the new shift commences.
The actual conveyance or coursing of the air from the intake to the working faces is effected by splitting or dividing the current at different points in its course, so as to carry it as directly as possible to the places where it is required. In laying out the mine it is customary to drive the levels or roads in pairs, communication being made between them at intervals by cutting through the intermediate pillar; the air then passes along one and returns by the other. As the roads advance other pillars are driven through in the same manner, the passages first made being closed by stoppings of broken rock, or built up with brick and mortar walls, or both. When it is desired to preserve a way from one road or similar class of working to another, double doors placed at sufficient intervals apart to take in one or more trams between them when closed are used, forming a kind of lock or sluice. These are made to shut air-tight against their frames, so as to prevent the air from taking a short cut back to the upcast, while preserving free access between the different districts without following the whole round of the air-ways. The ventilation of ends is effected by means of brattices or temporary partitions of thin boards placed midway in the drift, and extending to within a few feet of the face. The air passes along one side of the brattice, courses round the free end, and returns on the other side. In many cases a light but air-proof cloth, specially made for the purpose, is used instead of wood for brattices, as being more handy and more easily removed. In large mines where the air-ways are numerous and complicated, it often happens that currents travelling in opposite directions are brought together at one point. In these cases it is necessary to cross them. The return air is usually made to pass over the intake by a curved drift carried some distance above in the solid measures, both ways being arched in brickwork, or even in some cases lined with sheet iron so as to ensure a separation not likely to be destroyed in case of an explosion (see figs. 5 and 8). The use of small auxiliary blowing ventilators underground, for carrying air into workings away from the main circuits, which was largely advocated at one time, has lost its popularity, but a useful substitute has been found in the induced draught produced by jets of compressed air or high-pressure water blowing into ejectors. With a jet of 1⁄200 in. area, a pipe discharging 12⁄3 gallon of water per minute at 165 ℔ pressure per sq. in., a circulation of 850 cub. ft. of air per minute was produced at the end of a level, or about five times that obtained from an equal volume of air at 60 ℔ pressure. The increased resistance, due to the large extension of workings from single pairs of shafts, the ventilating currents having often to travel several miles to the upcast, has led to great increase in the size and power of ventilating fans, and engines from 250 to 500 H.P. are not uncommonly used for such purposes.
The lighting of underground workings in collieries is closely connected with the subject of ventilation. In many of the smaller pits in the Midland districts of England, and generally in South Staffordshire, the coals are sufficiently Lighting. free from gas, or rather the gases are not liable to become explosive when mixed with air, to allow the use of naked lights, candles being generally used. Oil lamps are employed in many of the Scotch collieries, and are almost universally used in Belgium and other European countries. The buildings near the pit bottom, such as the stables and lamp cabin, and even the main roads for some distance, are often in large collieries lighted with gas brought from the surface, or in some cases the gas given off by the coal is used for the same purpose. Where the gases are fiery, the use of protected lights or safety lamps (q.v.) becomes a necessity.
The nature of the gases evolved by coal when freshly exposed to the atmosphere has been investigated by several chemists, more particularly by Lyon Playfair and Ernst von Meyer. The latter observer found the gases given off Composition of gas evolved by coal. by coal from the district of Newcastle and Durham to contain carbonic acid, marsh gas or light carburetted hydrogen (the fire-damp of the miner), oxygen and nitrogen. A later investigation, by J. W. Thomas, of the gases dissolved or occluded in coals from South Wales basin shows them to vary considerably with the class of coal. The results given below, which are selected from a much larger series published in the Journal of the Chemical Society, were obtained by heating samples of the different coals in vacuo for several hours at the temperature of boiling water:—
| Quality. | Colliery. | Volume per ton in cub. ft. |
Composition in Volumes per cent. | |||
| Carbonic Acid. | Oxygen. | Marsh gas. | Nitrogen. | |||
| Bituminous | Cwm Clydach | 19.72 | 5.44 | 1.05 | 63.76 | 29.75 |
| ” | Lantwit | 14.34 | 9.43 | 2.25 | 31.95 | 56.34 |
| Steam | Navigation | 89.62 | 13.21 | 0.49 | 81.64 | 4.66 |
| Anthracite | Bonville’s Court | 198.95 | 2.62 | .. | 93.13 | 4.25 |
In one instance about 1% of hydride of ethyl was found in the gas from a blower in a pit in the Rhondda district, which was collected in a tube and brought to the surface to be used in lighting the engine-room and pit-bank. The gases from the bituminous house coals of South Wales are comparatively free from marsh gas, as compared with those from the steam coal and anthracite pits. The latter class of coal contains the largest proportion of this dangerous gas, but holds it more tenaciously than do the steam coals, thus rendering the workings comparatively safer. It was found that, of the entire volume of occluded gas in an anthracite, only one-third could be expelled at the temperature of boiling water, and that the whole quantity, amounting to 650 cub. ft. per ton, was only to be driven out by a heat of 300° C. Steam coals being softer and more porous give off enormous volumes of gas from the working face in most of the deep pits, many of which have been the scene of disastrous explosions.
The gases evolved from the sudden outbursts or blowers in coal, which are often given off at a considerable tension, are the most dangerous enemy that the collier has to contend with. They consist almost entirely of marsh gas, with only a small quantity of carbonic acid, usually under 1%, and from 1 to 4% of nitrogen.
Fire-damp when mixed with from four to twelve times its volume of atmospheric air is explosive; but when the proportion is above or below these limits it burns quietly with a pale blue flame.
The danger arising from the presence of coal dust in the air of dry mines, with or without the addition of fire-damp, has, since it was first pointed out by Professor W. Galloway, been made the subject of special inquiries in the Coal dust. principal European countries interested in coal mining; and although certain points are still debatable, the fact is generally admitted as one calling for special precautions. The conclusions arrived at by the royal commission of 1891, which may be taken as generally representative of the views of British colliery engineers, are as follows:—
1. The danger of explosion when gas exists in very small quantities is greatly increased by the presence of coal dust.
2. A gas explosion in a fiery mine may be intensified or indefinitely propagated by the dust raised by the explosion itself.
3. Coal dust alone, without any gas, may cause a dangerous 589 explosion if ignited by a blown-out shot; but such cases are likely to be exceptional.
4. The inflammability of coal dust varies with different coals, but none can be said to be entirely free from risk.
5. There is no probability of a dangerous explosion being produced by the ignition of coal dust by a naked light or ordinary flame.
Danger arising from coal dust is best guarded against by systematically sprinkling or watering the main roads leading from the working faces to the shaft, where the dust falling from the trams in transit is liable to accumulate. This may be done by water-carts or hose and jet, but preferably by finely divided water and compressed air distributed from a network of pipes carried through the workings. This is now generally done, and in some countries is compulsory, when the rocks are deficient in natural moisture. In one instance the quantity of water required to keep down the dust in a mine raising 850 tons of coal in a single shift was 28.8 tons, apart from that required by the jets and motors. The distributing network extended to more than 30 m. of pipes, varying from 3½ in. to 1 in. in diameter.
In all British coal-mines, when gas in dangerous quantities has appeared within three months, and in all places that are dry and dusty, blasting is prohibited, except with “permitted” explosives, whose composition and properties have been examined at the testing station at Safety explosives. the Royal Arsenal, Woolwich. A list of those sanctioned is published by the Home Office. They are mostly distinguished by special trade names, and are mainly of two classes—those containing ammonium nitrate and nitrobenzene or nitronaphthalene, and those containing nitroglycerin and nitrocellulose, which are essentially weak dynamites. The safety property attributed to them is due to the depression of the temperature of the flame or products of explosion to a point below that necessary to ignite fire-damp or coal dust in air from a blown-out shot. New explosives that are found to be satisfactory when tested are added to the list from time to time, the composition being stated in all cases.
Methods for enabling miners to penetrate into workings where the atmosphere is totally irrespirable have come into use for saving life after explosions and for repairing shafts and pit-work under water. The aerophore of A. Aerophores. Galibert was in its earlier form a bag of about 12 cub. ft. capacity containing air at a little above atmospheric pressure; it was carried on the back like a knapsack and supplied the means of respiration. The air was continually returned and circulated until it was too much contaminated with carbonic acid to be further used, a condition which limited the use of the apparatus to a very short period. A more extended application of the same principle was made in the apparatus of L. Denayrouze by which the air, contained in cylinders at a pressure of 300 to 350 ℔ per sq. in., was supplied for respiration through a reducing valve which brought it down nearly to atmospheric pressure. This apparatus was, however, very heavy and became unmanageable when more than an hour’s supply was required. The newer forms are based upon the principle, first enunciated by Professor Theodor Schwann in 1854, of carrying compressed oxygen instead of air, and returning the products of respiration through a regenerator containing absorptive media for carbonic acid and water, the purified current being returned to the mouth with an addition of fresh oxygen. The best-known apparatus of this class is that developed by G. A. Meyer at the Shamrock colliery in Westphalia, where a body of men are kept in systematic training for its use at a special rescue station. This corps rendered invaluable service at the exploring and rescue operations after the explosion at Courrières in March 1906, the most disastrous mining accident on record, when 1100 miners were killed. A somewhat similar apparatus called the “weg,” after the initials of the inventor, is due to W. E. Garforth of Wakefield. In another form of apparatus advantage is taken of the property possessed by sodium-potassium peroxide of giving off oxygen when damped; the residue of caustic soda and potash yielded by the reaction is used to absorb the carbonic acid of the expired air. Experiments have also been made with a device in which the air-supply is obtained by the evaporation of liquid air absorbed in asbestos.
Underground fires are not uncommon accidents in coal-mines. In the thick coal workings in South Staffordshire the slack left behind in the sides of work is especially liable to fire from so-called spontaneous combustion, due to the rapid oxidization that is set up when finely divided coal is brought in contact with air. The best remedy in such cases is to prevent the air from gaining access to the coal by building a wall round the burning portion, which can in this way be isolated from the remainder of the working, and the fire prevented from spreading, even if it cannot be extinguished. When the coal is fired by the blast of an explosion it is often necessary to isolate the mine completely by stopping up the mouths of the pits with earth, or in extreme cases it must be flooded with water or carbonic acid before the fire can be brought under. There have been several instances of this being done in the fiery pits in the Barnsley district, notably at the great explosion at the Oaks colliery in 1866, when 360 lives were lost.
The drawing or winding of the coal from the pit bottom to the surface is one of the most important operations Methods of winding. in coal mining, and probably the department in which mechanical appliances have been brought to the highest state of development.
The different elements making up the drawing arrangements of a colliery are—(1) the cage, (2) the shaft or pit fittings, (3) the drawing-rope, (4) the engine and (5) the surface arrangements. The cage, as its name implies, consists Cage. of one or more platforms connected by an open framework of vertical bars of wrought iron or steel, with a top bar to which the drawing-rope is attached. It is customary to have a curved sheet iron roof or bonnet when the cage is used for raising or lowering the miners, to protect them from injury by falling materials. The number of platforms or decks varies considerably; in small mines only a single one may be used, but in the larger modern pits two-, three- or even four-decked cages are used. The use of several decks is necessary in old pits of small section, where only a single tram can be carried on each. In the large shafts of the Northern and Wigan districts the cages are made about 8 ft. long and 3½ ft. broad, being sufficient to carry two large trams on one deck. These are received upon a railway made of two strips of angle iron of the proper gauge for the wheels, and are locked fast by a latch falling over their ends. At Cadeby Main with four-decked cages the capacity is eight 10-cwt. tubs or 4 tons of coal.
The guides or conductors in the pit may be constructed of wood, in which case rectangular fir beams, about 3 by 4 in., are used, attached at intervals of a few feet to buntons or cross-beams built into the lining of the pit. Two guides are required for each cage; they may be placed opposite to each other, either on the long or short sides—the latter being preferable. The cage is guided by shoes of wrought iron, a few inches long and bell-mouthed at the ends, attached to the horizontal bars of the framing, which pass loosely over the guides on three sides, but in most new pits rail guides of heavy section are used. They are applied on one side of the cage only, forming a complete vertical railway, carried by iron cross sleepers, with proper seats for the rails instead of wooden buntons; the cage is guided by curved shoes of a proper section to cover the heads of the rails. Rigid guides connected with the walling of the pit are probably the best and safest, but they have the disadvantage of being liable to distortion, in case of the pit altering its form, owing to irregular movements of the ground, or other causes. Wooden guides being of considerable size, block up a certain portion of the area of the pit, and thus offer an impediment to the ventilation, especially in upcast shafts, where the high temperature, when furnace ventilation is used, is also against their use. In the Lancashire and the Midland districts wire-rope guides have been introduced to a very considerable extent, with a view of meeting the above objections. These are simply wire-ropes, from ¾ to 1½ in. in diameter, hanging from a cross-bar connected with the pit-head framing at the surface, and attached to a similar 590 bar at the bottom, which are kept straight by a stretching weight of from 30 cwt. to 4 tons attached to the lower bar. In some cases four guides are used—two to each of the long sides of the cage; but a more general arrangement is to have three—two on one side, and the third in an intermediate position on the opposite side. Many colliery managers, however, prefer to have only two opposite guides, as being safer. The cage is connected by tubular clips, made in two pieces and bolted together, which slide over the ropes. In addition to this it is necessary to have an extra system of fixed guides at the surface and at the bottom, where it is necessary to keep the cage steady during the operations of loading and landing, there being a much greater amount of oscillation during the passage of the cage than with fixed guides. For the same reason it is necessary to give a considerable clearance between the two lines of guides, which are kept from 15 to 18 in. apart, to prevent the possibility of the two cages striking each other in passing. With proper precautions, however, wire guides are perfectly safe for use at the highest travelling speed.
The cage is connected with the drawing-rope by short lengths of chain from the corners, known as tackling chains, gathered into a central ring to which the rope is attached. Round steel wire-ropes, about 2 in. in diameter, are Cage. now commonly used; but in very deep pits they are sometimes tapered in section to reduce the dead weight lifted. Flat ropes of steel or iron wire were and are still used to a great extent, but round ones are now generally preferred. In Belgium and the north of France flat ropes of aloe fibre (Manila hemp or plantain fibre) are in high repute, being considered preferable by many colliery managers to wire, in spite of their great weight. A rope of this class for a pit 1200 metres deep, tapered from 15.6 in. to 9 in. in breadth and from 2 in. to 11⁄8 in. in thickness, weighed 14.3 tons, and another at Anzin, intended to lift a gross load of 15 tons from 750 metres, is 22½ in. broad and 3 in. thick at the drum end, and weighs 18 tons. Tapered round ropes, although mechanically preferable, are not advantageous in practice, as the wear being greater at the cage end than on the drum it is necessary to cut off portions of the former at intervals. Ultimately also the ropes should be reversed in position, and this can only be done with a rope of uniform section.
The engines used for winding or hoisting in collieries are usually direct-acting with a pair of horizontal cylinders coupled directly to the drum shaft. Steam at high pressure exhausting into the atmosphere is still commonly used, Winding engines. but the great power required for raising heavy loads from deep pits at high speeds has brought the question of fuel economy into prominence, and more economical types of the two-cylinder tandem compound class with high initial steam pressure, superheating and condensing, have come in to some extent where the amount of work to be done is sufficient to justify their high initial cost. One of the earliest examples was erected at Llanbradack in South Wales in 1894, and they have been somewhat extensively used in Westphalia and the north of France. In a later example at the Bargold pit of the Powell Duffryn Steam Coal Company a mixed arrangement is adopted with horizontal high-pressure and vertical low-pressure cylinders. This engine draws a net load of 55 tons of coal from a depth of 625 yds. in 45 seconds, the gross weight of the four trams, cage and chains, and rope, with the coal, being 20 tons 12 cwt. The work of the winding engine, being essentially of an intermittent character, can only be done with condensation when a central condenser keeping a constant vacuum is used, and even with this the rush of steam during winding may be a cause of disturbance. This difficulty may be overcome by using Rateau’s arrangement of a low-pressure turbine between the engine and the condenser. The accumulator, which is similar in principle to the thermal storage system of Druitt Halpin, is a closed vessel completely filled with water, which condenses the excess of steam during the winding period, and becoming superheated maintains the supply to the turbine when the main engine is standing. The power so developed is generally utilized in the production of electricity, for which there is an abundant use about large collieries.
The drum, when round ropes are used, is a plain broad cylinder, with flanged rims, and cased with soft wood packing, upon which the rope is coiled; the breadth is made sufficient to take the whole length of the rope at two laps. One drum is usually fixed to the shaft, while the other is loose, with a screw link or other means of coupling, in order to be able to adjust the two ropes to exactly the same length, so that one cage may be at the surface when the other is at the bottom, without having to pay out or take up any slack rope by the engine.
For flat ropes the drum or bobbin consists of a solid disk, of the width of the rope fixed upon the shaft, with numerous parallel pairs of arms or horns, arranged radially on both sides, the space between being just sufficient to allow the rope to enter and coil regularly upon the preceding lap. This method has the advantage of equalizing the work of the engine throughout the journey, for when the load is greatest, with the full cage at the bottom and the whole length of rope out, the duty required in the first revolution of the engine is measured by the length of the smallest circumference; while the assistance derived from gravitating action of the descending cage in the same period is equal to the weight of the falling mass through a height corresponding to the length of the largest lap, and so on, the speed being increased as the weight diminishes, and vice versa. The same thing can be effected in a more perfect manner by the use of spiral or scroll drums, in which the rope is made to coil in a spiral groove upon the surface of the drum, which is formed by the frusta of two obtuse cones placed with their smaller diameters outwards. This plan, though mechanically a very good one, has certain defects, especially in the possibility of danger resulting from the rope slipping sideways, if the grooves in the bed are not perfectly true. The great size and weight of such drums are also disadvantages, as giving rather unmanageable dimensions in a very deep pit. In some cases, therefore, a combined form is adopted, the body of the drum being cylindrical, and a width equal to three or four laps conical on either side.
Counterbalance chains for the winding engines are used in the collieries of the Midland districts of England. In this method a third drum is used to receive a heavy flat link chain, shorter than the main drawing-ropes, the end of which hangs down a special or balance pit. At starting, when the full load is to be lifted, the balance chain uncoils, and continues to do so until the desired equilibrium between the working loads is attained, when it is coiled up again in the reverse direction, to be again given out on the return trip.
In Koepe’s method the drum is replaced by a disk with a grooved rim for the rope, which passes from the top of one cage over the guide pulley, round the disk, and back over the second guide to the second cage, and a tail rope, passing round a pulley at the bottom of the shaft, connects the bottoms of the cages, so that the dead weight of cage, tubs and rope is completely counterbalanced at all positions of the cages, and the work of the engine is confined to the useful weight of coal raised. Motion is communicated to the rope by frictional contact with the drum, which is covered through about one-half of the circumference. This system has been used in Nottinghamshire, and at Sneyd, in North Staffordshire. In Belgium it was tried in a pit 940 metres deep, where it has been replaced by flat hempen ropes, and is now restricted to shallower workings. In Westphalia it is applied in about thirty different pits to a maximum depth of 761 metres.
A novelty in winding arrangements is the substitution of the electromotor for the steam engine, which has been effected in a few instances. In one of the best-known examples, the Zollern colliery in Westphalia, the Koepe system is used, the winding disk being driven by two motors of 1200 H.P. each on the same shaft. Motion is obtained from a continuous-current generator driven by an alternating motor with a very heavy fly-wheel, a combination known as the Ilgner transformer, which runs continuously with a constant draught on the generating station, the extremely variable demand of the winding engine during the acceleration period being met by the energy stored in the fly-wheel, which runs at a very high speed. This 591 arrangement works admirably as regards smoothness and safety in running, but the heavy first cost and complication stand in the way of its general adoption. Nevertheless about 60 electric winding engines were at work or under construction in May 1906.
The surface arrangements of a modern deep colliery are of considerable extent and complexity, the central feature being the head gear or pit frame carrying the guide pulleys which lead the winding ropes from the axis of the pit Surface arrangements. to the drum. This is an upright frame, usually made in wrought iron or steel strutted by diagonal thrust beams against the engine-house wall or other solid abutments, the height to the bearings of the guide pulleys being from 80 to 100 ft. or more above the ground level. This great height is necessary to obtain head-room for the cages, the landing platforms being usually placed at some considerable height above the natural surface. The pulleys, which are made as large as possible up to 20 ft. in diameter to diminish the effect of bending strains in the rope by change in direction, have channelled cast iron rims with wrought iron arms, a form combining rigidity with strength, in order to keep down their weight.
To prevent accidents from the breaking of the rope while the cage is travelling in the shaft, or from over-winding when in consequence of the engine not being stopped in time the cage may be drawn up to the head-gear pulleys (both of which are unhappily not uncommon), various forms of safety catches and disconnecting hooks have been adopted. The former contrivances consist essentially of levers or cams with toothed surfaces or gripping shoes mounted upon transverse axes attached to the sides of the cage, whose function is to take hold of the guides and support the cage in the event of its becoming detached from the rope. The opposite axes are connected with springs which are kept in compression by tension of the rope in drawing but come into action when the pull is released, the side axes then biting into wooden guides or gripping those of steel bars or ropes. The use of these contrivances is more common in collieries on the continent of Europe, where in some countries they are obligatory, than in England, where they are not generally popular owing to their uncertainty in action and the constant drag on the guides when the rope slacks.
For the prevention of accidents from over-winding, detaching hooks are used. These consist essentially of links formed of a pair of parallel plates joined by a central bolt forming a scissors joint which is connected by chain links to the cage below and the winding-rope above. The outer sides of the link are shaped with projecting lugs above. When closed by the load the width is sufficient to allow it to enter a funnel-shaped guide on a cross-bar of the frame some distance above the bank level, but on reaching the narrower portion of the guide at the top the plates are forced apart which releases the ropes and brings the lugs into contact with the top of the cross-bar which secures the cage from falling.
Three principal patterns, those of King, Ormerod and Walker, are in use, and they are generally efficient supposing the speed of the cage at arrival is not excessive. To guard against this it is now customary to use some speed-checking appliance, independent of the engine-man, which reduces or entirely cuts off the steam supply when the cage arrives at a particular point near the surface, and applies the brake if the load is travelling too quickly. Maximum speed controllers in connexion with the winding indicator, which do not allow the engine to exceed a fixed rate of speed, are also used in some cases, with recording indicators.
When the cage arrives at the surface, or rather the platform forming the working top above the mouth of the pit, it is received upon the keeps, a pair of hinged gratings which are kept in an inclined position over the pit-top by counter-balance Striking and screening. weights, so that they are pushed aside to allow the cage to pass upwards, but fall back and receive it when the engine is reversed. The tubs are then removed or struck by the landers, who pull them forward on to the platform, which is covered with cast iron plates; at the same time empty ones are pushed in from the opposite side. The cage is then lifted by the engine clear of the keeps, which are opened by a lever worked by hand, and the empty tubs start on the return trip. When the cage has several decks, it is necessary to repeat this operation for each, unless there is a special provision made for loading and discharging the tubs at different levels. An arrangement of this kind for shifting the load from a large cage at one operation was introduced by Fowler at Hucknall, in Leicestershire, where the trains are received into a framework with a number of platforms corresponding to those of the cage, carried on the head of a plunger movable by hydraulic pressure in a vertical cylinder. The empty tubs are carried by a corresponding arrangement on the opposite side. By this means the time of stoppage is reduced to a minimum, 8 seconds for a three-decked cage as against 28 seconds, as the operations of lowering the tubs to the level of the pit-top, discharging, and replacing them are performed during the time that the following load is being drawn up the pit.
In the United Kingdom the drawing of coal is generally confined to the day shift of eight hours, with an output of from 100 to 150 tons per hour, according to the depth, capacity of coal tubs, and facilities for landing and changing tubs. With Fowler’s hydraulic arrangement 2000 tons are raised 600 yds. in eight hours. In the deeper German pits, where great thicknesses of water-bearing strata have to be traversed, the first establishment expenses are so great that in order to increase output the shaft is sometimes provided with a complete double equipment of cages and engines. In such cases the engines may be placed in line on opposite sides of the pit, or at right angles to each other. It is said that the output of single shafts has been raised by this method to 3500 and 4500 tons in the double shift of sixteen hours. It is particularly well suited to mines where groups of seams at different depths are worked simultaneously. Some characteristic figures of the yield for British collieries in 1898 are given below:—
| Albion Colliery, South Wales | 551,000 tons in a year for one shaft and one engine. |
| Silksworth Colliery, Northumberland | 535,000 tons in a year for shaft 580 yds. deep, two engines. |
| Bolsover Colliery, Derby | 598,798 tons in 279 days, shaft 365 yds. deep. |
| Denaby Main Colliery, Yorkshire | 629,947 tons in 281 days, maximum per day 2673 tons. |
At Cadeby Main colliery near Doncaster in 1906, 3360 tons were drawn in fourteen hours from one pit 763 yds. deep.
The tub when brought to the surface, after passing over a weigh-bridge where it is weighed and tallied by a weigher specially appointed for the purpose by the men and the owner jointly, is run into a “tippler,” a cage turning about a horizontal axis which discharges the load in the first half of the rotation and brings the tub back to the original position in the second. It is then run back to the pit-bank to be loaded into the cage at the return journey.
Coal as raised from the pit is now generally subjected to some final process of classification and cleaning before being despatched to the consumer. The nature and extent of these operations vary with the character of the coal, which if hard and free from shale partings may be finished by simple screening into large and nut sizes and smaller slack or duff, with a final hand-picking to remove shale and dust from the larger sizes. But when there is much small duff, with intermixed shale, more elaborate sizing and washing plant becomes necessary. Where hand-picking is done, the larger-sized coal, separated by 3-in. bar screens, is spread out on a travelling band, which may be 300 ft. long and from 3 to 5 wide, and carried past a line of pickers stationed along one side, who take out and remove the waste as it passes by, leaving the clean coal on the belt. The smaller duff is separated by vibrating or rotating screens into a great number of sizes, which are cleaned by washing in continuous current or pulsating jigging machines, where the lighter coal rises to the surface and is removed by a stream of water, while the heavier waste falls and is discharged at a lower level, or through a valve at the bottom of the machine. The larger or “nut” sizes, from ¼ in. upwards, are washed on plain sieve plates, but for finer-grained duff the sieve is covered with a bed of broken felspar lumps about 3 in. 592 thick, forming a kind of filter, through which the fine dirt passes to the bottom of the hutch. The cleaned coal is carried by a stream of water to a bucket elevator and delivered to the storage bunkers, or both water and coal may be lifted by a centrifugal pump into a large cylindrical tank, where the water drains away, leaving the coal sufficiently dry for use. Modern screening and washing plants, especially when the small coal forms a considerable proportion of the output, are large and costly, requiring machinery of a capacity of 100 to 150 tons per hour, which absorbs 350 to 400 H.P. In this, as in many other cases, electric motors supplied from a central station are now preferred to separate steam-engines.
Anthracite coal in Pennsylvania is subjected to breaking between toothed rollers and an elaborate system of screening, before it is fit for sale. The largest or lump coal is that which remains upon a riddle having the bars 4 in. apart; the second, or steamboat coal, is above 3 in.; broken coal includes sizes above 2½ or 2¾ in.; egg coal, pieces above 2¼ in. sq.; large stove coal, 1¾ in.; small stove, 1 to 1½ or 11⁄3 in.; chestnut coal, 2⁄3 to ¾ in.; pea coal, ½ in.; and buckwheat coal, 1⁄3 in. The most valuable of these are the egg and stove sizes, which are broken to the proper dimensions for household use, the larger lumps being unfit for burning in open fire-places. In South Wales a somewhat similar treatment is now adopted in the anthracite districts.
With the increased activity of working characteristic of modern coal mining, the depth of the mines has rapidly increased, and at the present time the level of 4000 ft., formerly Depth of working. assumed as the possible limit for working, has been nearly attained. The following list gives the depths reached in the deepest collieries in Europe in 1900, from which it will be seen that the larger number, as well as the deepest, are in Belgium:—
| Metres. | Ft. | ||
| Saint Henriette, Cie des Produits, Flenu, | Belgium | 1150 | 3773 |
| Viviers Gilly | ” | 1143 | 3750 |
| Marcinelle, No. 11, Charleroi | ” | 1075 | 3527 |
| Marchienne, No. 2, Charleroi | ” | 1065 | 3494 |
| Agrappe, Mons | ” | 1060 | 3478 |
| Pendleton dip workings | Lancashire | 1059 | 3474 |
| Sacré Madame, Charleroi | Belgium | 1055 | 3461 |
| Ashton Moss dip workings | Lancashire | 1024 | 3360 |
| Ronchamp, No. 11 pit | France | 1015 | 3330 |
| Viernoy, Anderlues | Belgium | 1006 | 3301 |
| Astley Pit, Dukinfield, dip workings | Cheshire | 960 | 3150 |
| Saint André, Poirier, Charleroi | Belgium | 950 | 3117 |
The greatest depth attained in the Westphalian coal is at East Recklinghausen, where there are two shafts 841 metres (2759 ft.) deep.
The subject of the limiting depth of working has been very fully studied in Belgium by Professor Simon Stassart of Mons (“Les Conditions d’exploitation à grande profondeur en Belgique,” Bulletin de la Société de l’Industrie minérale, 3 ser., vol. xiv.), who finds that no special difficulty has been met with in workings above 1100 metres deep from increased temperature or atmospheric pressure. The extreme temperatures in the working faces at 1150 metres were 79° and 86° F., and the maximum in the end of a drift, 100°; and these were quite bearable on account of the energetic ventilation maintained, and the dryness of the air. The yield per man on the working faces was 4.5 tons, and for the whole of the working force underground, 0.846 tons, which is not less than that realized in shallower mines. From the experience of such workings it is considered that 1500 metres would be a possible workable depth, the rock temperature being 132°, and those of the intake and return galleries, 92° and 108° respectively. Under such conditions work would be practically impossible except with very energetic ventilation and dry air. It would be scarcely possible to circulate more than 120,000 to 130,000 cub. ft. per minute under such conditions, and the number of working places would thus be restricted, and consequently the output reduced to about 500 tons per shift of 10 hours, which could be raised by a single engine at the surface without requiring any very different appliances from those in current use.
In the United Kingdom the ownership of coal, like that of other minerals, is in the proprietor of the soil, and passes with it, except when specially reserved in the sale. Coal lying under the sea below low-water mark belongs to the Ownership of coal. crown, and can only be worked upon payment of royalties, even when it is approached from shafts sunk upon land in private ownership. In the Forest of Dean, which is the property of the crown as a royal forest, there are certain curious rights held by a portion of the inhabitants known as the Free Miners of the Forest, who are entitled to mine for coal and iron ore, under leases, known as gales, granted by the principal agent or gaveller representing the crown, in tracts not otherwise occupied. This is the only instance in Great Britain of the custom of free coal-mining under a government grant or concession, which is the rule in almost every country on the continent of Europe.
The working of collieries in the United Kingdom is subject to the provisions of the Coal Mines Regulation Act 1887, as amended by several minor acts, administered by inspectors Coal Mines Regulation Act. appointed by the Home Office, and forming a complete disciplinary code in all matters connected with coal-mining. An important act was passed in 1908, limiting the hours of work below ground of miners. For a detailed account of these various acts see the article Labour Legislation.
Coal-mining is unfortunately a dangerous occupation, more than a thousand deaths from accident being reported Accidents. annually by the inspectors of mines as occurring in the collieries of the United Kingdom.
The number of lives lost during the year 1906 was, according to the inspectors’ returns:—
| From explosions | 54 |
| From falls of ground | 547 |
| From other underground accidents | 328 |
| From accidents in shafts | 65 |
| From surface accidents | 135 |
| —— | |
| Total 1129 |
The principal sources of danger to the collier, as distinguished from other miners, are explosions of fire-damp and falls of roof in getting coal; these together make up about 70% of the whole number of deaths. It will be seen that the former class of accidents, though often attended with great loss of life at one time, are less fatal than the latter.
Authorities.—The most important new publication on British coal is that of the royal commission on coal supplies appointed in 1901, whose final report was issued in 1905. A convenient digest of the evidence classified according to subjects was published by the Colliery Guardian newspaper in three quarto volumes in 1905-1907, and the leading points bearing on the extension and resources of the different districts were incorporated in the fifth edition (1905) of Professor Edward Hull’s Coal Fields of Great Britain. The Report of the earlier royal commission (1870), however, still remains of great value, and must not be considered to have had its conclusions entirely superseded. In connexion with the re-survey in greater detail of the coalfields by the Geological Survey a series of descriptive memoirs were undertaken, those on the North Staffordshire and Leicestershire fields, and nine parts dealing with that of South Wales, having appeared by the beginning of 1908.
An independent work on the coal resources of Scotland under the title of the Coalfields of Scotland, by R. W. Dixon, was published in 1902.
The Rhenish-Westphalian coalfield was fully described in all details, geological, technical and economic, in a work called Die Entwickelung des niederrheinisch-westfälischen Steinkohlen Bergbaues in der zweiten Hälfte des 19ten Jahrhunderts (also known by the short title of Sammelwerk) in twelve quarto volumes, issued under the auspices of the Westphalian Coal Trade Syndicate (Berlin, 19O2-1905).
The coalfields of the Austrian dominions (exclusive of Hungary) are described in Die Mineralkohlen Österreichs, published at Vienna by the Central Union of Austrian mineowners. It continues the table of former official publications in 1870 and 1878, but in much more detail than its predecessors.
Systematic detailed descriptions of the French coalfields appear from time to time under the title of Études sur les gîtes minéraux de la France from the ministry of public works in Paris.
Much important information on American coals will be found in the three volumes of Reports on the Coal Testing Plant at the St Louis Exhibition, published by the United States Geological Survey in 1906. A special work on the Anthracite Coal Industry of the United States, by P. Roberts, was published in 1901.
The most useful general work on coal mining is the Text Book of Coal Mining, by H. W. Hughes, which also contains detailed 593 bibliographical lists for each division of the text. The 5th edition appeared in 1904.
Current progress in mining and other matters connected with coal can best be followed by consulting the abstracts and bibliographical lists of memoirs on these subjects that have appeared in the technical journals of the world contained in the Journal of the Institute of Mining Engineers and that of the Iron and Steel Institute. The latter appears at half-yearly intervals and includes notices of publications up to about two or three months before the date of its publication.
COALBROOKDALE, a town and district in the Wellington parliamentary division of Shropshire, England. The town has a station on the Great Western railway, 160 m. N.W. from London. The district or dale is the narrow and picturesque valley of a stream rising near the Wrekin and following a course of some 8 m. in a south-easterly direction to the Severn. Great ironworks occupy it. They were founded in 1709 by Abraham Darby with the assistance of Dutch workmen, and continued by his son and descendants. Father and son had a great share in the discovery and elaboration of the use of pit-coal for making iron, which revolutionized and saved the English iron trade. The father hardly witnessed the benefits of the enterprise, but the son was fully rewarded. It is recorded that he watched the experimental filling of the furnace ceaselessly for six days and nights, and that, just as fatigue was overcoming him, he saw the molten metal issuing, and knew that the experiment had succeeded.
The third Abraham Darby built the famous Coalbrookdale iron bridge over the Severn, which gives name to the neighbouring town of Ironbridge, which with a portion of Coalbrookdale is in the parish of Madeley (q.v.). Fine wrought iron work is produced, and the school of art is well known. There are also brick and tile works.
COAL-FISH (Gadus virens), also called green cod, black pollack, saith and sillock, a fish of the family Gadidae. It has a very wide range, which nearly coincides with that of the cod, although of a somewhat more southern character, as it extends to both east and west coasts of the North Atlantic, and is occasionally found in the Mediterranean. It is especially common in the north, though rarely entering the Baltic; it becomes rare south of the English Channel. Unlike the cod and haddock, the coal-fish is, to a great extent, a surface-swimming fish, congregating together in large schools, and moving from place to place in search of food; large specimens (3 to 3½ ft. long), however, prefer deep water, down to 70 fathoms. The flesh is not so highly valued as that of the cod and haddock. The lower jaw projects more or less beyond the upper, the mental barble is small, sometimes rudimentary, the vent is below the posterior half of the first dorsal fin, and there is a dark spot in the axil of the pectoral fin.
COALING STATIONS. Maritime war in all ages has required that the ships of the belligerents should have the use of sheltered waters for repairs and for replenishment of supplies. The operations of commerce from the earliest days demanded natural harbours, round which, as in the conspicuous instance of Syracuse, large populations gathered. Such points, where wealth and resources of all kinds accumulated, became objects of attack, and great efforts were expended upon their capture. As maritime operations extended, the importance of a seaboard increased, and the possession of good natural harbours became more and more advantageous. At the same time, the growing size of ships and the complexity of fitments caused by the development of the sailing art imposed new demands upon the equipment of ports alike for purposes of construction and for repairs; while the differentiation between warships and the commercial marine led to the establishment of naval bases and dockyards provided with special resources. From the days when the great sailors of Elizabeth carried war into distant seas, remote harbours began to assume naval importance. Expeditionary forces required temporary bases, such as Guantanamo Bay, in Cuba, which was so utilized by Admiral Vernon in 1741. As outlying territories began to be occupied, and jurisdiction to be exercised over their ports, the harbours available for the free use of a belligerent were gradually reduced in number, and it became occasionally necessary to take them by force. Thus, in 1782, the capture of Trincomalee was an object of sufficient importance to justify special effort, and Suffren gained a much-needed refuge for his ships, at the same time compelling his opponent to depend upon the open roadstead of Madras, and even to send ships to Bombay. In this case a distant harbour acquired strategic importance, mainly because sheltered waters, in the seas where Hughes and Suffren strove for naval supremacy, were few and far between. A sailing man-of-war usually carried from five to six months’ provisions and water for 100 to 120 days. Other needs required to be met, and during the wars of the French Revolution it was usual, when possible, to allow ships engaged in blockade to return to port every five or six weeks “to refresh.” For a sailing fleet acting on the offensive, a port from which it could easily get to sea was a great advantage. Thus Raleigh protested against the use of closely landlocked harbours. “Certain it is,” he wrote, “that these ships are purposely to serve His Majesty and to defend the kingdom from danger, and not to be so penned up from casualitie as that they should be less able or serviceable in times of need.” Nelson for this reason made great use of Maddalena Bay, in Sardinia, and was not greatly impressed with the strategic value of Malta in spite of its fine natural harbour. The introduction of steam gave rise to a new naval requirement—coal—which soon became vital. Commerce under steam quickly settled down upon fixed routes, and depots of coal were established to meet its needs. Coaling stations thus came into existence by a natural process, arising from the exigencies of trade, and began later to supply the needs of navies.
For many years there was no inquiry into the war requirements of the British fleet as regards coal, and no attempt to regularize or to fortify the ports at which it was stored. Successful naval war had won for Great Britain many British coaling stations. points of vantage throughout the world, and in some cases the strategic value of ports had been proved by actual experience. The extreme importance of the Cape of Good Hope, obscured for a time after the opening of the Suez Canal, was fully realized in sailing days, and the naval conditions of those days to some extent determined the choice of islands and harbours for occupation. There does not, however, appear to have been any careful study of relative strategic values. Treaties were occasionally drafted by persons whose geographical knowledge was at fault, and positions were, in some cases, abandoned which ought to have been retained, or tenaciously held when they might have been abandoned. It was left to the personal exertions of Sir Stamford Raffles to secure such a supremely important roadstead as that of Singapore for the empire. Although, therefore, the relative values of positions was not always recognized, Great Britain obtained as a legacy from sailing days a large number of harbours admirably adapted for use as coaling stations. Since the dawn of the era of steam, she has acquired Aden, Perim, Hong-Kong, North Borneo, Fiji, part of New Guinea, Fanning Island, and many other islands in the Pacific, while the striking development of Australia and New Zealand has added to the long roll of British ports. The coaling stations, actual and potential, of the empire are unrivalled in number, in convenience of geographical distribution, and in resources. Of the numerous British ports abroad which contained coal stores, only the four so-called “fortresses”—Gibraltar, Malta, Halifax and Bermuda—were at first fortified as naval stations after the introduction of rifled ordnance. The term fortress is a misnomer in every case except Gibraltar, which, being a peninsula separated only by a neck of neutral ground from the territory of a foreign power, exists under fortress conditions. Large sums were expended on these places with little regard to principles, and the defences of Bermuda, which were very slowly constructed, are monuments of misapplied ingenuity.
In 1878 great alarm arose from strained relations with Russia. Rumours of the presence of Russian cruisers in many waters, and of hostile projects, were readily believed, although the Russian navy, which had just shown itself unable Carnarvon Commission. to face that of Turkey, would at this period have been practically powerless. Widespread fears for the security of coaling stations led to the appointment of a strong 594 royal commission, under the presidency of the earl of Carnarvon, which was instructed to inquire into and report upon the protection of British commerce at sea. This was the first attempt to formulate any principles, or to determine which of the many ports where coal was stored should be treated as coaling stations essential for the purposes of war. The terms of the reference to the commission were ill-conceived. The basis of all defence of sea-borne commerce is a mobile navy. It is the movement of commerce upon the sea during war, not its security in port, that is essential to the British empire, and a navy able to protect commerce at sea must evidently protect ports and coaling stations. The first object of inquiry should, therefore, have been to lay down the necessary standard of naval force. The vital question of the navy was not referred to the royal commission, and the four fortresses were also strangely excluded from its purview. It followed inevitably that the protection of commerce was approached at the wrong end, and that the labours of the commission were to a great extent vitiated by the elimination of the principal factor. Voluminous and important evidence, which has not been made public, was, however, accumulated, and the final report was completed in 1881. The commissioners recalled attention to the extreme importance of the Cape route to the East; they carefully examined the main maritime communications of the empire, and the distribution of trade upon each; they selected certain harbours for defence, and they obtained from the War Office and endorsed projects of fortification in every case; lastly, they condemned the great dispersion of troops in the West Indies, which had arisen in days when it was a political object to keep the standing army out of sight of the British people, and had since been maintained by pure inadvertence. Although the principal outcome of the careful inquiries of the commission was to initiate a great system of passive defence, the able reports were a distinct gain. Some principles were at last formulated by authority, and the information collected, if it had been rendered accessible to the public, would have exercised a beneficial influence upon opinion. Moreover, the commissioners, overstepping the bounds of their charter, delivered a wise and statesmanlike warning as to the position of the navy.
Meanwhile, the impulse of the fears of 1878 caused indifferent armaments to be sent to Cape Town, Singapore and Hong-Kong, there to be mounted after much delay in roughly designed works. At the same time, the great colonies of Australasia began to set about the defence of their ports with commendable earnestness. There is no machinery for giving effect to the recommendations of a royal commission, and until 1887, when extracts were laid before the first colonial Conference, the valuable report was veiled in secrecy. After several years, during which Lord Carnarvon persistently endeavoured to direct attention to the coaling stations, the work was begun. In 1885 a fresh panic arose out of the Panjdeh difficulty, which supplied an impetus to the belated proceedings. Little had then been accomplished and the works were scarcely completed before the introduction of long breech-loading guns rendered their armaments obsolete.
The fortification of the coaling stations for the British empire is still proceeding on a scale which, in some cases, cannot easily be reconciled with the principles laid down by the president of the cabinet committee of defence. At the Guildhall, London, on the 3rd of December 1896, the duke of Devonshire stated that “The maintenance of sea supremacy has been assumed as the basis of the system of imperial defence against attack from over the sea. This is the determining factor in fixing the whole defensive policy of the empire.” It was, however, he added, necessary to provide against “the predatory raids of cruisers”; but “it is in the highest degree improbable that this raiding attack would be made by more than a few ships, nor could it be of any permanent effect unless troops were landed.” This is an unexceptionable statement of the requirements of passive defence in the case of the coaling stations of the British empire. Their protection must depend primarily on the navy. Their immobile armaments are needed to ward off a raiding attack, and a few effective guns, well mounted, manned by well-trained men, and kept in full readiness, will amply suffice.
If the command of the sea is lost, large expeditionary forces can be brought to bear upon coaling stations, and their security will thus depend upon their mobile garrisons, not upon their passive defences. In any case, where coal is stored on shore, it cannot be destroyed by the fire of a ship, and it can only be appropriated by landing men. A small force, well armed and well handled, can effectually prevent a raid of this nature without any assistance from heavy guns. In war, the possession of secure coal stores in distant ports may be a great advantage, but it will rarely suffice for the needs of a fleet engaged in offensive operations, and requiring to be accompanied or met at prearranged rendezvous by colliers from which coal can be transferred in any sheltered Modern conditions. waters. In the British naval manœuvres of 1892, Admiral Sir Michael Seymour succeeded in coaling his squadron at sea, and by the aid of mechanical appliances this is frequently possible. In the Spanish-American War of 1898 some coaling was thus accomplished; but Guantanamo Bay served the purpose of a coaling station during the operations against Santiago. Watering at sea was usually carried out by means of casks in sailing days, and must have been almost as difficult as coaling. As, however, it is certainty of coaling in a given time that is of primary importance, the utilization of sheltered waters as improvised coaling stations is sure to be a marked feature of future naval wars. Although coaling stations are now eagerly sought for by all powers which cherish naval ambitions, the annexation of the Hawaiian Islands by the United States being a case in point, it is probable that they will play a somewhat less important part than has been assumed. A fleet which is able to assert and to maintain the command of the sea, will not find great difficulty in its coal supply. Moreover, the increased coal endurance of ships of war tends to make their necessary replenishment less frequent. On the other hand, the modern warship, being entirely dependent upon a mass of complex machinery, requires the assistance of workshops to maintain her continuous efficiency, and unless docked at intervals suffers a material reduction of speed. Prolonged operations in waters far distant from home bases will therefore be greatly facilitated in the case of the Power which possesses local docks and means of executing repairs. Injuries received in action, which might otherwise disable a ship during a campaign, may Secondary bases. thus be remedied. During the hostilities between France and China in 1884, the French ship “La Galissonnière” was struck by a shell from one of the Min forts, which, though failing to burst, inflicted serious damage. As, by a technical fiction, a state of war was not considered to exist, the “La Galissonnière” was repaired at Hong-Kong and enabled again to take the sea. Local stores of reserve ammunition and of spare armaments confer evident advantages. Thus, independently of the question of coal supply, modern fleets employed at great distances from their bases require the assistance of ports furnished with special resources, and a power like Japan with well-equipped naval bases in the China Sea, and possessing large sources of coal, occupies, for that reason, a favoured position in regard to naval operations in the Far East. As the term “coaling station” refers only to a naval need which can often be satisfied without a visit to any port, it appears less suitable to modern conditions than “secondary base.” Secondary bases, or coaling stations, when associated with a powerful mobile navy, are sources of maritime strength in proportion to the services they can render, and to their convenience of geographical position. In the hands of an inferior naval power, they may be used, as was Mauritius in 1809-1810, as points from which to carry on operations against commerce; but unless situated near to trade routes, which must be followed in war, they are probably less useful for this purpose than in sailing days, since convoys can now be more effectively protected, and steamers have considerable latitude of courses. Isolated ports dependent on sea-borne resources, and without strong bodies of organized fighting men at their backs are now, as always, hostages offered to the power which obtains command of the sea.
COALITION (Lat. coalitio, the verbal substantive of coalescere, to grow together), a combination of bodies or parts into one 595 body or whole. The word is used, especially in a political sense, of an alliance or temporary union for joint action of various powers or states, such as the coalition of the European powers against France, during the wars of the French Revolution; and also of the union in a single government of distinct parties or members of distinct parties. Of the various coalition ministries in English history, those of Fox and North in 1782, of the Whigs and the Peelites, under Lord Aberdeen in 1852-1853, and of the Liberal Unionists and Conservatives in Lord Salisbury’s third ministry in 1895, may be instanced.
COAL-TAR, the black, viscous, sometimes semi-solid, fluid of peculiar smell, which is condensed together with aqueous “gas liquor” when the volatile products of the destructive distillation of coal are cooled down. It is also called “gas-tar,” because it was formerly exclusively, and even now is mostly, obtained as a by-product in the manufacture of coal-gas, but the tar obtained from the modern coke-ovens, although not entirely identical with gas-tar, resembles it to such an extent that it is worked up with the latter, without making any distinction in practice between the two kinds. Some descriptions of gas-tar indeed differ very much more than coke-oven tar from pure coal-tar, viz. those which are formed when bituminous shale or other materials, considerably deviating in their nature from coal, are mixed with the latter for the purpose of obtaining gas of higher illuminating power.
It may be generally said that for the purpose of tar-distillers the tar is all the more valuable the less other materials than real coal have been used by the gas-maker. All these materials—bog-head shale, bituminous lignite and so forth—by destructive distillation yield more or less paraffinoid oils, which render the purification of the benzols very difficult and sometimes nearly impossible for the purposes of the manufacturer of coal-tar colours.
Neither too high nor too low a temperature should have been observed in gas-making in order to obtain a good quality of tar. Since in recent times most gas retorts have been provided with heating arrangements based on the production of gaseous fuel from coke, which produce higher temperatures than direct firing and have proved a great economy in the process of gas-making itself, the tar has become of decidedly inferior quality for the purposes of the tar-distillers, and in particular yields much less benzol than formerly.
Entirely different from gas-tar is the tar obtained as a by-product from those (Scottish) blast furnaces which are worked with splint-coal. This tar contains very little aromatic hydrocarbons, and the phenols are of quite a different character from those obtained in the working of gas-tar. The same holds good of oil-gas tars and similar substances. These should not be worked up like gas-tars.
The ordinary yield of tar in the manufacture of coal-gas is between 4 and 5% of the weight of the coal. Rather more is obtained when passing the gas through the apparatus of E. Pelouze and P. Audouin, where it is exposed to several shocks against solid surfaces, or by carrying on the process at the lowest possible temperature, as proposed by H. J. Davis, but this “carbonizing process” can only pay under special circumstances, and is probably no longer in practical use.
All coal-tars have a specific gravity above that of water, in most cases between 1.12 and 1.20, but exceptionally up to 1.25. The heavier tars contain less benzol than the lighter tars, and more “fixed carbon,” which remains behind when the tars are exhausted of benzol and is a decidedly objectionable constituent. All tars also mechanically retain a certain quantity of water (or rather gas-liquor), say, 4% on the average, which is very obnoxious during the process of distillation, as it leads to “bumping,” and therefore ought to be previously removed by prolonged settling, preferably at a slightly elevated temperature, which makes the tar more fluid. The water then rises to the top, and is removed in the ordinary way or by special “separators.”
The tar itself is a mixture of exceedingly complex character. The great bulk of its constituents belongs to the class of “aromatic” hydrocarbons, of very different composition and degrees of volatility, beginning with the simplest and most volatile, benzene (C6H6), and ending with an entirely indistinguishable mass of non-volatile bodies, which compose the pitch left behind in the tar-stills. The hydrocarbons mostly belong to the benzene series CnH2n-6, the naphthalene series CnH2n-12, and the anthracene and phenanthrene series CnH2n-18. Small quantities of “fatty” (“aliphatic”) hydrocarbons are never absent, even in pure tars, and are found in considerable quantities when shales and similar matters have been mixed with the coal in the gas-retorts. They belong mostly to the paraffins CnH2n+2, and the olefines CnH2n. The “asphalt” or soluble part of the pitch is also a mixture of hydrocarbons, of the formula CnH2n; even the “carbon,” left behind after treating the pitch with all possible solvents is never pure carbon, but contains a certain quantity of hydrogen, although less than any of the volatile and soluble constituents of the tar.
Besides the hydrocarbons, coal-tar contains about 2% of the simpler phenols CnH2n-7OH, the best known and most valuable of which is the first of the series, carbolic acid (q.v.) C6H5OH, besides another interesting oxygenized substance, cumarone C8H6O. The phenols, especially the carbolic acid, are among the more valuable constituents of coal-tar. Numerous sulphur compounds also occur in coal-tar, some of which impart to it their peculiar nauseous smell, but they are of no technical importance or value.
Still more numerous are the nitrogenated compounds contained in coal-tar. Most of these are of a basic character, and belong to the pyridine and the quinoline series. Among these we find a somewhat considerable quantity of aniline, which, however, is never obtained from the tar for commercial purposes, as its isolation in the pure state is too difficult. The pyridines are now mostly recovered from coal-tar, but only in the shape of a mixture of all members of the series which is principally employed for denaturing alcohol. Some of these nitrogenated compounds possess considerable antiseptic properties, but on the whole they are only considered as a contamination of the tar-oils.
Applications of Coal-Tar in the Crude State.—Large quantities of coal-tar are employed for various purposes without submitting it to the process of distillation. It is mostly advisable to dehydrate the tar as much as possible for any one of its applications, and in some cases it is previously boiled in order to remove its more volatile constituents.
No preparation whatever is needed if the tar is to be used as fuel, either for heating the gas-retorts or for other purposes. Its heating-value is equal to the same weight of best coal, but it is very difficult to burn it completely without producing a great deal of evil-smelling smoke. This drawback has been overcome by employing the same means as have been found suitable for the combustion of the heavy petroleum residues, called “masut,” viz. converting the tar into a fine spray by means of steam or compressed air. When the gas-maker cannot conveniently or profitably dispose of his tar for other purposes, he burns it by the above means under his retorts.
Several processes have also been patented for producing illuminating gas from tar, the most notable of which is the Dinsmore process. This process has been adversely criticized by very competent gas-makers, and no great success can be expected in this line.
Coal-tar is very much employed for painting wood, iron, brickwork, or stone, as a preventive against the influence of weather or the far more potent action of corrosive chemicals. This, of course, can be done only where appearance is no object, for instance in chemical works, where all kinds of erections and apparatus are protected by this cheap kind of paint. Coal-tar should not be used for tarring the woodwork and ropes of ships, a purpose for which only wood-tar has been found suitable.
One of the most considerable outlets for crude tar is in the manufacture of roofing-felt. This industry was introduced in Germany upwards of a hundred years ago, even before coal-tar was available, and has reached a very large extension both in that country and in the United States, where most of the gas-tar seems to be devoted to this purpose. In the United Kingdom 596 it is much less extensive. For this manufacture a special fabric is made from pure woollen fibre, on rolls of about 3 ft. width and of considerable length. The tar must be previously dehydrated, and is preferably deprived of its more volatile portions by heating in a still. It is heated in an iron pan to about 90° or 100°C.; the fabric is drawn through it by means of rollers which at the same time squeeze out the excess of tar; on coming out of these, the tarred felt is covered with a layer of sand on both sides by means of a self-acting apparatus; and is ultimately wound round wooden rolls, in which state it is sent out into the trade. This roofing-felt is used as a cheap covering, both by itself and as a grounding for tiles or slates. In the former case it must be kept in repair by repainting with tar from time to time, a top covering of sand or small gravel being put on after every coat of paint.
Coal-tar is also employed for the manufacture of lamp-black. This is done by burning the tar in ovens, connected with brick-chambers in which the large quantity of soot, formed in this process, deposits before the gases escape through the chimney. Numerous patents have been taken out for more efficiently collecting this soot. Most of it is employed without further manipulation for the manufacture of electric carbons, printing inks, shoe-blacking, patent leather and so forth. A finer quality of lamp-black, free from oily and empyreumatic parts, is obtained by calcining the soot in closed iron pots at a red heat.
Distillation of Coal-Tar.—Much more important than all applications of crude coal-tar is the industry of separating its constituents from it in a more or less pure form by fractional distillation, mostly followed by purifying processes. Most naturally this industry took its rise in Great Britain, where coal-gas was invented and made on a large scale before any other nation took it up, and up to this day both the manufacture of coal-gas and the distillation of the tar, obtained as a by-product thereof, are carried out on a much larger scale in that than in any other country. The first attempts in this line were made in 1815 by F. C. Accum, and in 1822 by Dr G. D. Longstaff and Dr Dalston. At first the aim was simply to obtain “naphtha,” used in the manufacture of india-rubber goods, for burning in open lamps and for some descriptions of varnish; the great bulk of the tar remained behind and was used as fuel or burned for the purpose of obtaining lamp-black.
It is not quite certain who first discovered in the coal-naphtha the presence of benzene (q.v.), which had been isolated from oil-gas by M. Faraday as far back as 1825. John Leigh claims to have shown coal-tar benzene and nitro-benzene made from it at the British Association meeting held at Manchester in 1842, but the report of the meeting says nothing about it, and the world in general learned the presence of benzene in coal-tar only from the independent discovery of A. W. Hofmann, published in 1845. And it was most assuredly in Hofmann’s London laboratory that Charles Mansfield worked out that method of fractional distillation of the coal-tar and of isolating the single hydrocarbons which laid the foundation of that industry. His patent, numbered 11,960 and dated November 11th, 1847, is the classical land-mark of it. About the same time, in 1846, Brönner, at Frankfort, brought his “grease-remover” into the trade, which consisted of the most volatile coal-tar oils, of course not separated into the pure hydrocarbons; he also sold water-white “creosote” and heavy tar-oils for pickling railway timbers, and used the remainder of the tar for the manufacture of roofing-felt. The employment of heavy oils for pickling timber had already been patented in 1838 by John Bethell, and from this time onward the distillation of coal-tar seems to have been developed in Great Britain on a larger scale, but the utilization of the light oils in the present manner naturally took place only after Sir W. H. Perkin, in 1856, discovered the first aniline colour which suddenly created a demand for benzene and its homologues. The isolation of carbolic acid from the heavier oils followed soon after; that of naphthalene, which takes place almost automatically, went on simultaneously, although the uses of this hydrocarbon for a long time remained much behind the quantities which are producible from coal-tar, until the manufacture of synthetic indigo opened out a wide field for it. The last of the great discoveries in that line was the preparation of alizarine from anthracene by C. Graebe and C. T. Liebermann, in 1868, soon followed by patents for its practical manufacture by Sir W. H. Perkin in England, and by Graebe, Liebermann and H. Caro in Germany.
The present extension of the industry of coal-tar distilling can be only very roughly estimated from the quantity of coal-tar produced in various countries. Decidedly at the head is Great Britain, where about 700,000 tons are produced per annum, most of which probably finds its way into the tar-distilleries, whilst in Germany and the United States much less gas-tar is produced and a very large proportion of it is used for roofing-felt and other purposes.
We shall now give an outline of the processes used in the distillation of tar.
Dehydration.—The first operation in coal-tar distilling is the removal of the mechanically enclosed water. Some water is chemically combined with the bases, phenols, &c., and this, of course, cannot be removed by mechanical means, but splits off only during the distillation itself, when a certain temperature has been reached. The water mechanically present in the tar is separated by long repose in large reservoirs. Very thick viscous tars are best mixed with thinner tars, and the whole is gently heated by coils of pipes through which the heated water from the oil-condensers is made to flow. Sometimes special “tar-separators” are employed, working on the centrifugal principle. The water rises to the top and is worked up like ordinary gas-liquor. More water is again separated during the heating-up of the tar in the still itself, and can be removed there by a special overflow.
 |
| Fig. 1.—Tar-Still (sectional elevation).1 |
Tar-Stills.—The tar is now pumped into the tar-still, fig. 1. This is usually, as shown, an upright wrought-iron cylinder, with an arched top, and with a bottom equally vaulted upwards for the purpose of increasing the heating surface and of raising the level of the pitch remaining at the end of the operation above the fire-flues. The fuel is consumed on the fire-grate a, and, after having traversed the holes bb in the annular wall e built below the still, the furnace gases are led around the still by means of the flue d, whence they pass to the chimney. Cast-iron necks are provided in the top for the outlet of the vapours, for a man-hole, supply-pipe, thermometer-pipe, safety valve, and for air and steam-pipes reaching down to the bottom and branching out into a number of distributing 597 arms. Near the top there is an overflow pipe which comes into action on filling the still. In the lowest part of the bottom there is a running-off valve or tap. In some cases (but only exceptionally) a perpendicular shaft is provided, with horizontal arms, and chains hanging down from these drag along the bottom for the purpose of keeping it clean and of facilitating the escape of the vapours. This arrangement is quite unnecessary where the removal of the vapours is promoted by the injection of steam, but this steam must be carefully dried beforehand, or, better, slightly superheated, in order to prevent explosions, which might be caused by the entry of liquid water into the tar during the later stages of the work, when the temperature has arisen far above the boiling-point of water. The steam acts both by stirring up the tar and by rapidly carrying off the vapours formed in distillation. The latter object is even more thoroughly attained by the application of a vacuum, especially during the later stage of distillation. For this purpose the receivers, in which the liquids condensed in the cooler are collected, are connected with an air pump or an ejector, by which a vacuum of about 4 in., say 1⁄8 atmosphere, is made which lowers the boiling process by about 80° C.; this not merely hastens the process, but also produces an improvement of the quality and yield of the products, especially of the anthracene, and, moreover, lessens or altogether prevents the formation of coke on the still-bottom, which is otherwise very troublesome.
Most manufacturers employ ordinary stills as described. A few of them have introduced continuously acting stills, of which that constructed by Frederic Lennard has probably found a wider application than any of the others. They all work on the principle of gradually heating the tar in several compartments, following one after the other. The fresh tar is run in at one end and the pitch is run out from the other. The vapours formed in the various compartments are separately carried away and condensed, yielding at one and the same time those products which are obtained in the ordinary stills at the different periods of the distillation. Although in theory this continuous process has great advantages over the ordinary style of working, the complication of the apparatus and practical difficulties arising in the manipulation have deterred most manufacturers from introducing it.
The tar-stills are set in brickwork in such a manner that there is no over-heating of their contents. For this purpose the fire-grate is placed at a good distance from the bottom or even covered by a brick arch so that the flame does not touch the still-bottom at all and acts only indirectly, but the sides of the still are always directly heated. The fire-flue must not be carried up to a greater height than is necessary to provide against the overheating of any part of the still not protected inside by liquid tar, or, at the end of the operation, by liquid pitch. The outlet pipe is equally protected against overheating and also against any stoppage by pitch solidifying therein. The capacity of tar-stills ranges from 5 to 50 tons. They hold usually about 10 tons, in which case they can be worked off during one day.
The vapours coming from the still are condensed in coolers of various shapes, one of which is shown in figs. 2 and 3. The cooling-pipes are best made of cast-iron, say 4 in. wide inside and laid so as to have a continuous fall towards the bottom. A steam-pipe (b) is provided for heating the cooling water, which is necessary during the later part of the operation to prevent the stopping up of the pipes by the solidification of the distillates. A cock (a) allows steam to be injected into the condensing worm in order to clear any obstruction.
 |
| Fig. 2.—Condensing Worm (Plan) |
The cooling-pipe is at its lower end connected with receivers for the various distillates in such a manner that by the turning of a cock the flow of the distillates into the receivers can be changed at will. In a suitable place provision is made for watching the colour, the specific gravity, and the general appearance of the distillates. At the end of the train of apparatus, and behind the vacuum pump or ejector, when one is provided, there is sometimes a purifier for the gases which remain after condensation; or these gases are carried back into the fire, in which case a water-trap must be interposed to prevent explosions.
Distillation of the Tar.—The number of fractions taken during the distillation varies from four to six. Sometimes a first fraction is taken as “first runnings,” up to a temperature of 105° C. in the still, and a second fraction as “light oil,” up to 210° C., but more usually these two are not separated in the first distillation, and the first or “light oil” fraction then embraces everything which comes over until the drops no longer float on, but show the same specific gravity as water. The specific gravity of this fraction varies from 0.91 to 0.94. The next fraction is the “middle oil” or “carbolic oil,” of specific gravity 1.01, boiling up to 240° C.; it contains most of the carbolic acid and naphthalene. The next fraction is the “heavy oil” or “creosote oil,” of specific gravity 1.04. Where the nature of the coals distilled for gas is such that the tar contains too little anthracene to be economically recovered, the creosote-oil fraction is carried right to the end; but otherwise, that is in most cases, a last fraction is made at about the temperature 270° C., above which the “anthracene oil” or “green oil” is obtained up to the finish of the distillation.
 |
| Fig. 3.—Condensing Worm (side elevation). |
During the light-oil period the firing must be performed very cautiously, especially where the water has not been well removed, to prevent bumping and boiling over. It has been observed that, apart from the water, those tars incline most to boiling over which contain an unusual quantity of “fixed carbon.” During this period cold water must be kept running through the cooler. The distillate at once separates into water (gas-liquor) and light oil, floating at the top. Towards the end of this fraction the distillation seems to cease, in spite of increasing the fires, and a rattling noise is heard in the still. This is caused by the combined water splitting off from the bases and phenols and causing slight explosions in the tar.
As soon as the specific gravity approaches 1.0, the supply of cold water to the cooler is at least partly cut off, so that the temperature of the water rises up to 40° C. This is necessary because otherwise some naphthalene would crystallize out and plug up the pipes. If a little steam is injected into the still during this period no stoppage of the pipes need be feared in any case, but this must be done cautiously.
When the carbolic oil has passed over and the temperature in the still has risen to about 240° C., the distillate can be run freely by always keeping the temperature in the cooler at least up to 40° C. The “creosote oil” which now comes over often separates a good deal of solid naphthalene on cooling.
The last fraction is made, either when the thermometer indicates 270° C., or when “green grease” appears in the distillate, or simply by judging from the quantity of the distillate. What comes over now is the “anthracene oil.” The firing may cease towards the end as the steam (with the vacuum) will finish the work by itself. The water in the cooler should now approach the boiling-point.
The point of finishing the distillation is different in various places and for various objects. It depends upon the fact whether soft or hard pitch is wanted. The latter must be made where it has to be sold at a distance, as soft pitch cannot be easily carried during the warmer season in railway trucks and not at all in ships, where it would run into a single lump. Hard pitch is also always made where as much anthracene as possible is to be obtained. For hard pitch the distillation is carried on as far as practicable without causing the residue in the still to “coke.” The end cannot be judged by the thermometer, but by the appearance and quantity of the distillate 598 and its specific gravity. If carried too far, not merely is coke formed, but the pitch is porous and almost useless, and the anthracene oil is contaminated with high-boiling hydrocarbons which may render it almost worthless as well. Hard pitch proper should soften at 100° C., or little above.
Where the distillation is to stop at soft pitch it is, of course, not carried up to the same point, but wherever the pitch can be disposed of during the colder season or without a long carriage, even the hard pitch is preferably softened within the still by pumping back a sufficient quantity of heavy oil, previously deprived of anthracene. This makes it much easier to discharge the still. When the contents consist of soft pitch they are run off without much trouble, but hard pitch not merely emits extremely pungent vapours, but is mostly at so high a temperature that it takes fire in the air. Hard pitch must, therefore, always be run into an iron or brick cooler where it cools down out of contact with air, until it can be drawn out into the open pots where its solidification is completed.
Most of the pitch is used for the manufacture of “briquettes” (“patent fuel”), for which purpose it should soften between 55° and 80° C. according to the requirements of the buyer. In Germany upwards of 50,000 tons are used annually in that industry; much of it is imported from the United Kingdom, whence also France and Belgium are provided. Apart from the softening point the pitch is all the more valued the more constituents it contains which are soluble in xylene. The portion insoluble in this is denoted as “fixed carbon.” If the briquette manufacturer has bought the pitch in the hard state he must himself bring it down to the proper softening point by re-melting it with heavy coal-tar oils.
We now come to the treatment of the various fractions obtained from the tar-stills. These operations are frequently not carried out at the smaller tar-works, which sell their oils in the crude state to the larger tar-distillers.
Working up of the Light-Oil Fraction.—The greatest portion of the light-oil fraction consists of aromatic hydrocarbons, about one-fifth being naphthalene, four-fifths benzene and its homologues, in the proportion of about 100 benzene, 30 toluene, 15 xylenes, 10 trimethylbenzenes, 1 tetramethylbenzene. Besides these the light-oil contains 5-15% phenols, 1-3% bases, 0.1 sulphuretted compounds, 0.2-0.3% nitriles, &c. It is usually first submitted to a preliminary distillation in directly fired stills, similar to the tar-stills, but with a dephlegmating head. Here we obtain (1) first runnings (up to O.89 spec. grav.), (2) heavy benzols (up to O.95), (3) carbolic oil (up to 1.00). The residue remaining in the still (chiefly naphthalene) goes to the middle-oil fraction.
The “first runnings” are now “washed” in various ways, of which we shall describe one of the best. The oil is mixed with dilute caustic soda solution, and the solution of phenols thus obtained is worked up with that obtained from the next fractions. After this follows a treatment with dilute sulphuric acid (spec. grav. 1.3), to extract the pyridine bases, and lastly with concentrated sulphuric acid (1.84), which removes some of the aliphatic hydrocarbons and “unsaturated” compounds. After this the crude benzol is thoroughly washed with water and dilute caustic soda solution, until its reaction is neutral. The mixing of the basic, acid and aqueous washing-liquids with the oils is performed by compressed air, or more suitably by mechanical stirrers, arranged on a perpendicular, or better, a horizontal shaft. Precisely the same treatment takes place with the next fraction, the “heavy benzols,” and the oils left behind after the washing operations now go to the steam-stills. The heaviest hydrocarbons are sometimes twice subjected to the operation of washing.
The washed crude benzols are now further fractionated by distillation with steam. The steam-stills are in nearly all details on the principle of the “column apparatus” employed in the distillation of alcoholic liquids, as represented in fig. 4. They are usually made of cast iron. The still itself is either an upright or a horizontal cylinder, heated by a steam-coil, of a capacity of from 1000 to 2000 gallons. The superposed columns contain from 20 to 50 compartments of a width of 2½ or 3 ft. The vapours pass into a cooler, and from this the distillate runs through an apparatus, where the liquor can be seen and tested, into the receivers. The latter are so arranged that the water passing over at the same time is automatically removed. This is especially necessary, because the last fraction is distilled by means of pure steam.
The fractions made in the steam distillation vary at different works. In some places the pure hydrocarbons are net extracted and here only the articles called: “90 per cent. benzol,” “50 per cent. benzol,” “solvent naphtha,” “burning naphtha” are made, or any other commercial articles as they are ordered. The expression “per cent.” in this case does not signify the percentage of real benzene, but that portion which distills over up to the temperature of 100° C., when a certain quantity of the article is heated in glass retorts of a definite shape, with the thermometer inserted in the liquid itself. By the application of well-constructed rectifying-columns and with proper care it is, however, possible to obtain in this operation nearly pure benzene, toluene, xylene, and cumene (in the two last cases a mixture of the various isomeric hydrocarbons). These hydrocarbons contain only a slight proportion of thiophene and its isomers, which can be removed only by a treatment with fuming sulphuric acid, but this is only exceptionally done.
 |
| Fig. 4.—Benzol Still (elevation). |
Sometimes the pyridine bases are recovered from the tarry acid which is obtained in the treatment of the light oil with sulphuric acid, and which contains from 10 to 30% of bases, chiefly pyridine and its homologues with a little aniline, together with resinous substances. The latter are best removed by a partial precipitation with ammonia, either in the shape of gas or of concentrated ammoniacal liquor. This reagent is added until the acid reaction has just disappeared and a faint smell of pyridine is perceived. The mixture is allowed to settle, and it then separates into two layers. The upper layer, containing the impurities, is run off; the lower layer, containing the sulphates of ammonia and of the pyridine bases, is treated with ammonia in excess, where it separates into a lower aqueous layer of ammonium sulphate solution and an oil, consisting of crude pyridine. This is purified by fractionation in iron stills and distillation over caustic soda. Most of it is used for denaturing spirit of wine in Germany, for which purpose it is required to contain 90% of bases boiling up to 140° C. (see Alcohol).
Working up of the Middle-Oil Fraction (Carbolic Oil Fraction).—Owing to its great percentage of naphthalene (about 40%) this fraction is solid or semi-solid at ordinary temperatures. Its specific gravity is about 1.2; its colour may vary from light yellow to dark brown or black. In the latter case it must be re-distilled before further treatment. On cooling down, about four-fifths of the naphthalene crystallizes out on standing from three to ten days. The crystals are freed from the mother oils by draining and cold or hot pressing; they are then washed at 100° C. with concentrated sulphuric acid, afterwards with water and re-distilled or sublimed. About 10,000 tons of naphthalene are used annually in Germany, mostly for the manufacture of many azo-colours and of synthetic indigo.
The oils drained from the crude naphthalene are re-distilled and worked for carbolic acid and its isomers. For this purpose the oil is washed with a solution of caustic soda, of specific gravity 1.1; the solution thus obtained is treated with sulphuric acid or with carbon dioxide, and the crude phenols now separated are fractionated in a similar manner as is done in the case of crude benzol. The pure phenol crystallizes out and is again distilled in iron stills with a silver head and cooling worm; the remaining oils, consisting mainly of cresols, are sold as “liquid carbolic acid” or under other names.
Most of the oil which passes as the “creosote-oil fraction” is sold in the crude state for the purpose of pickling timber. It is at the ordinary temperature a semi-solid mixture of about 20% crystallized hydrocarbons (chiefly naphthalene), and 80% of a dark brown, nauseous smelling oil, of 1.04 spec. grav., and boiling between 200° and 300° C. The liquid portion contains phenols, bases, and a great number of hydrocarbons. Sometimes it is redistilled, when most of the naphthalene passes over in the first fraction, between 180° and 230° C., and crystallizes out in a nearly pure state. The oily portion remaining behind, about 60% of this distillate, contains about 30% phenols and 3% bases. It has highly disinfectant properties and is frequently converted into special disinfectants, e.g. by mixing it with four times its volume of slaked lime, which yields “disinfectant powder” for stables, railway cars, &c. Mixtures 599 of potash soaps (soft soaps) with this oil have the property of yielding with water emulsions which do not settle for a long time and are found in the trade as “creolin,” “sapocarbol,” “lysol,” &c.
That description of creosote oil which is sold for the purpose of pickling railway sleepers, telegraph posts, timber for the erection of wharves and so forth, must satisfy special requirements which are laid down in the specifications for tenders to public bodies. These vary to a considerable extent. They always stipulate (1) a certain specific gravity (e.g. not below 1.035 and not above 1.065); (2) certain limits of boiling points (e.g. to yield at most 3% up to 150°, at most 30% between 150° and 255°, and at least 85% between 150° and 355°); (3) a certain percentage of phenols, as shown by extraction with caustic soda solution, say 8 to 10%.
Much of this creosote oil is obtained by mixing that which has resulted in the direct distillation of the tar with the liquid portion of the anthracene oils after separating the crude anthracene (see below). It is frequently stipulated that the oil should remain clear at the ordinary temperature, say 15° C., which means that no naphthalene should crystallize out.
Working up the Anthracene Oil Fraction.—The crude oil boils between 280° and 400° C. It is liquid at 60° C., but on cooling about 6 to 10% of crude anthracene separates as greenish-yellow, sandy crystals, containing about 30% of real anthracene, together with a large percentage of carbazol and phenanthrene. This crystallization takes about a week. The crude anthracene is separated from the mother oils by filter presses, followed by centrifugals or by hot hydraulic presses. The liquid oils are redistilled, in order to obtain more anthracene, and the last oils go back to the creosote oil, or are employed for softening the hard pitch (vide supra). The crude anthracene is brought up to 50 or 60, sometimes to 80%, by washing with solvent naphtha, or more efficiently with the higher boiling portion of the pyridine bases. The naphtha removes mostly only the phenanthrene, but the carbazol can be removed only by pyridine, or by subliming or distilling the anthracene over caustic potash. The whole of the anthracene is sold for the manufacture of artificial alizarine.
Bibliography.—The principal work on Coal-tar is G. Lunge’s Coal-tar and Ammonia (3rd ed., 1900). Consult also G. P. Sadtler, Handbook of Industrial Organic Chemistry (1891), and the article “Steinkohlentheer,” Kraemer and Spreker, in Encyklopädisches Handbuch der technischen Chemie (4th ed., 1905, viii. 1).
1 The illustrations in this article are from Prof. G. Lunge’s Coal Tar and Ammonia, by permission of Friedrich Vieweg u. Sohn.
COALVILLE, a town in the Loughborough parliamentary division of Leicestershire, England, 112 m. N.N.W. from London. Pop. of urban district (1901) 15,281. It is served by the Midland railway, and there is also a station (Coalville East) on the Nuneaton-Loughborough branch of the London & North-Western railway. This is a town of modern growth, a centre of the coal-mining district of north Leicestershire. There are also iron foundries and brick-works. A mile north of Coalville is Whitwick, with remains of a castle of Norman date, while to the north again are slight remains of the nunnery of Gracedieu, founded in 1240, where, after its dissolution, Francis Beaumont, the poet-colleague of John Fletcher, was born about 1586. In the neighbourhood is the Trappist abbey of Mount St Bernard, founded in 1835, possessing a large domain, with buildings completed from the designs of A. W. Pugin in 1844.
COAST (from Lat. costa, a rib, side), the part of the land which meets the sea in a line of more or less regular form. The word is sometimes applied to the bank of a river or lake, and sometimes to a region (cf. Gold Coast, Coromandel Coast) which may include the hinterland. If the coast-line runs parallel to a mountain range, such as the Andes, it has usually a more regular form than when, as in the rias coast of west Brittany, it crosses the crustal folds. Again, a recently elevated coast is more regular than one that has been long exposed to wave action. A recently depressed coast will show the irregularities that were impressed upon the surface before submergence. Wave erosion and the action of marine currents are the chief agents in coast sculpture. A coast of homogeneous rock exposed to similar action will present a regular outline, but if exposed to differential action it will be embayed where that action is greatest. A coast consisting of rocks of unequal hardness or of unequal structure will present headlands, “stacks” and “needles” of hard rocks, and bays of softer or more loosely aggregated rocks, when the wave and current action is similar throughout. The southern shore-line of the Isle of Wight and the western coast of Wales are simple examples of this differential resistance. In time the coast becomes “mature” and its outline undergoes little change as it gradually recedes, for the hard rock being now more exposed is worn away faster, but the softer rock more slowly because it is protected in the bays and re-entrants.
COAST DEFENCE, a general term for the military and naval protection and defence of a coast-line, harbours, dockyards, coaling-stations, &c., against serious attack by a strong naval force of the enemy, bombardment, torpedo boat or destroyer raids, hostile landing parties, or invasion by a large or small army. The principal means employed by the defender to cope with these and other forms of attack which may be expected in time of war or political crisis are described below. See also for further details Navy; Army; Fortification and Siege-craft; Ammunition; Ordnance; Submarine Mines; Torpedo. The following is a general description of modern coast defences as applied in the British service.
No system of coast defence is of any value which does not take full account of the general distribution of sea-power and the resultant strength of the possible hostile forces. By resultant strength is meant the balance of one side over the other, for it is now generally regarded as an axiom that two opposing fleets must make their main effort in seeking one another, and that the force available for attack on coast defences will be either composed of such ships as can be spared from the main engagement, or the remnant of the hostile fleet after it has been victorious in a general action.
Coast defences are thus the complement and to some extent the measure of naval strength. It is often assumed that this principle was neglected in the large scheme of fortification associated in England with the name of Lord Palmerston, but it is at least arguable that the engineers responsible for the details of this scheme were dependent then as now on the naval view of what was a suitable naval strength. Public opinion has since been educated to a better appreciation of the necessity for a strong navy, and, as the British navy has increased, the scale of coast defences required has necessarily waned. Such a change of opinion is always gradual, and it is difficult to name an exact date on which it may be said that modern coast defence, as practised by British engineers, first began.
An approximation may, however, be made by taking the bombardment of Alexandria (1881) as being the parting of the ways between the old and the modern school. At that time the British navy, and in fact all other navies, had not really emerged from the stage of the wooden battleships. Guns were still muzzle-loaders, arranged mainly in broadsides, and protected by heavy armour; sails were still used as means of propulsion; torpedoes, net defence, signalling, and search-lights quite undeveloped.
At this time coast defences bore a close resemblance to the ships—the guns were muzzle-loaders, arranged in long batteries like a broadside, often in two tiers. The improvement of rifled ordnance had called for increased protection, and this was found first by solid constructions of granite, and latterly by massive iron fronts. Examples of these remain in Garrison Fort, Sheerness, and in Hurst Castle at the west end of the Solent. The range of guns being then relatively short, it was necessary to place forts at fairly close intervals, and where the channels to be defended could not be spanned from the shore, massive structures with two or even three tiers of guns, placed as close as on board ship and behind heavy armour, were built up from the ocean bed. On both sides the calibre and weight of guns were increasing, till the enormous sizes of 80 and 100 tons were used both ashore and afloat.
The bombardment of Alexandria established two new principles, or new applications of old principles, by showing the value of concealment and dispersion in reducing the effect of the fire of the fleet. On the old system, two ships firing at one another or ships firing at an iron-fronted fort shot “mainly into the brown”; if they missed the gun aimed at, one to the right or left was likely to be hit; if they missed the water-line, the upper works were in danger. At Alexandria, however, the Egyptian guns were scattered over a long line of shore, and it was soon found that with the guns and gunners available, hits could only be obtained by running in to short range and dealing with one gun at a time.
This new principle was not at once recognized, for systems 600 die hard, and much money and brains were invested in the then existing system. But a modern school was gradually formed; a small group of engineer officers under the headship of Sir Andrew Clarke, the then inspector-general of fortifications, took the matter up, and by degrees the new views prevailed and the modern school of coast defence came into being between 1881 and 1885. Meanwhile important changes had been developing in the gun, the all-important weapon of coast defence, changes due mainly to the gradual supersession of the muzzle-loader by the breech-loader. The latter gave the advantages of quicker loading and more protection for the gun detachment over and above the technical improvements in the gun itself, which gave higher muzzle velocity, greater striking effect and longer effective range.
All this reacted on the general scheme of coast defence by enabling the number of guns to be reduced and the distance between forts increased. On the other hand, the ships, too, gained increased range and increased accuracy of fire, so that it became necessary in many cases to advance the general line of the coast defences farther from the harbour or dockyard to be defended, in order to keep the attackers out of range of the objective.
Another change resulted from an improvement in the method of mounting. Even in the older days discussion had arisen freely on the relative merits of barbette and casemate mounting. In the former the gun fires over a parapet, giving a larger field of view to the gun-layer, and a larger field of fire for the gun, with, however, more exposure for the detachment. The latter gives a restricted view and greater safety to the layer, but unless the casemate takes the form of a revolving turret, the arc of fire is very limited.
An important advantage of the barbette system is its cheapness, and thus in order to obtain with it concealment, suggestions were made for various forms of mounting which would allow of the gun, under the shock of recoil, disappearing behind the parapet to emerge only when loaded and ready for the next round. A mounting of this description for muzzle-loading guns, designed by Colonel Moncrieff, was actually in use in the defences of Alexandria and in H.M.S. “Téméraire.”
But with the increased charges and length of breech-loading guns, a further change was desirable, and after some trials a system of disappearing mountings (see Ordnance: Garrison Mountings) was adopted into the British service.
A word must be now said on the size of gun finally adopted. At first muzzle-loaders figured largely in the British defences, even though these were planned on modern ideas; and even in 1906 muzzle-loading guns still existed and were counted as part of the defences. The sizes of these guns varied from the 32- or 64-pounder, of which the nomenclature depends on the weight of the shell, to the 7-in., 9-in., 10-in., 11-in., 12.5- and finally 17.25-in., the size indicating the calibre. Such a multiplication of sizes was due to gradual improvements in the science of gun manufacture, each advance being hailed as the last word to be said on the subject, and each in turn being rapidly made obsolete by something bigger and better. But with the improvements in gun design which followed the introduction of breech-loaders, the types used in coast defence were gradually narrowed down to two, the 9.2-in. and the 6-in. guns. Of these, the 9.2-in. was considered powerful enough to attack armour at any practical range, while the 6-in. gun was introduced to deal with lightly armed vessels at shorter ranges where 9.2-in. guns were unnecessarily powerful.
A few larger guns of 10-in. calibre have actually been used, but though the British navy has now sealed a 12-in. 50-ton gun as the stock size for battleships, for the heavy armament of the coast defences the War Office remain faithful to the 9.2-in. calibre, preferring to develop improvements rather in the direction of more rapid fire and higher muzzle velocity.
The 6-in. has also been retained and is extensively used for the smaller ports, where attack by powerful vessels is for various reasons considered improbable.
The design of the forts to contain the guns necessarily varied with the type of defence adopted, and the duties which the forts had to fulfil. These duties may be said to be twofold, first to facilitate the service of the guns, and secondly to protect the guns and their detachments from damage by fire from ships, or by close attack from landing parties. The service of the gun is provided for by a system of cartridge and shell magazines (see Ammunition), well protected from fire and suitably arranged. The shelters for the gun detachments must be bomb-proof and fitted with some arrangements for comfort and sanitation. Formerly it was the custom to provide living accommodation for the full garrison in casemates inside each fort, but it is now considered better to provide barrack accommodation in the vicinity and to occupy forts in peace only by a few caretakers. The shelters in the fort itself can thus be kept at the minimum required when actually manning the guns. The protection of the guns and magazines against bombardment is provided, in the British service, mainly by an earthen parapet over a substantial roof or wall of concrete, but immediately round the gun an “apron” of concrete is necessary to withstand the shock of discharge or “blast.”
It has been already mentioned that in the old designs a large number of guns was put in each fort, but with dispersion and improved gun power this number was much reduced. At first the type of fort adopted was for four guns, of which the two in the centre were heavy and the two on the flank of medium power. Such a design was good from the point of view of the engineer; it gave an economical grouping of magazines and shelters and was easily adapted to varying sites, and the smaller guns helped the larger by covering their flanks both towards the sea and also over the land approaches. But from the point of view of the artillery officer the arrangement was faulty, for when the guns are too much separated, ranging has to be carried out separately for each gun. On the other hand, two guns of the same calibre placed near one another can be fought simultaneously and form what is known as a “group.” In the typical 4-gun battery described above, the flank guns had to be fought independently, which was wasteful of officers and staff. Further, in a battery of more than two guns the arc of fire of the centre guns is much restricted by that of the guns on either flank.
For these reasons it is now generally recognized that new works should be designed for only two guns of the same calibre, though 3- or 4-gun batteries are occasionally used in special circumstances.
Protection of the gun detachments against infantry attack is best provided by a line of infantry posts outside and on the flanks of the gun batteries, but as small parties may evade the outposts, or the latter may be driven in, it is necessary to place round each fort a line of obstacles sufficient to protect the guns against a rush and to cover the infantry while it rallies. This obstacle was formerly a wet or dry ditch, with escarp, counterscarp and flanking galleries; but with the new design of parapet a simpler form of obstacle was adopted. This was obtained by carrying down and forward the slope of the parapet to a point well below the level of the surrounding ground, and then placing a stout fence at the foot of the parapet and concealed from view. It is in fact the old principle of the sunk fence, and has this further advantage, that the fence, being visible from the parapet, can be kept under fire by men posted between the guns without any special flanking galleries.
Occasionally two or more batteries are placed inside one line of obstacles, but usually each 2-gun battery is complete in itself.
Cases arise, e.g. with sites on the top of a cliff, where no obstacle is required; in such places the parapet merges into the surrounding ground.
In old days the parapet was shaped with well-defined edges and slopes. Now the parapet slopes gently down to the front and is rounded at the sides, so as to present no definite edge or angle to the enemy, and concealment is furthered by allowing grass or small scrub to grow over the parapet and round the guns. In order to obtain complete concealment from view the background behind the guns must be carefully studied from the 601 point of view of the attack. Sites on the sky-line, and marked contrasts of colour or shape, should be avoided. In some cases extensive planting, amounting to landscape gardening, is justified. This is most easily arranged in the tropics, where plant growth is rapid. In all cases the guns and their mountings should be coloured to blend with the background and thus avoid hard lines and shadows.
Any change of principle such as that of 1885 involves improvements both in guns and their adjuncts. Of these latter the most important was the position-finder designed by Colonel Watkin. This instrument in its simplest form, when the observer is following a ship through the telescope of the instrument, draws on a chart the track of the ship, so that the exact bearing and distance of the latter can be ascertained at any time and communicated to the guns by electrical and other dials, &c. The position-finder may be some distance from the guns it serves, and connected with them by electric cable. The guns can then be placed well under cover and in many cases out of sight of the target, giving a measure of protection which cannot be obtained with any system of direct laying over sights. This instrument has been applied on a high site to control guns placed low, or where guns are so placed as to be liable to obscuration by fog or mist the position-finder can be placed below the fog-line. In either case direct laying is provided for as an alternative. In some defences batteries equipped with old pattern 9-in. muzzle-loading guns, mounted as howitzers for long-range firing, have been placed in folds in the ground so as to be quite invisible from the sea and therefore invulnerable. Such batteries are fought entirely by the position-finder.
The next adjunct to coast defences is the submarine mine. In Great Britain the first submarine mining company dates from 1873, and from that date mining defences were gradually installed both at home and abroad; but the modern system of mining, which for twenty years was maintained at British ports, really started into full life under the impetus of Sir A. Clarke, about the same year (1885) in which we have dated the commencement of the modern coast defence system.
With the increased speed of warships, a method of attack on fortifications was evolved by running past the main defences and either taking them in reverse, or disregarding them and attacking the dockyard or other objective at short range. This was made more possible at most defended ports by the pushing forward of the defences which has been already alluded to, and it is especially dangerous where dockyards or towns are situated some way up a river or estuary, so that once the defences are passed there is a large stretch of water (e.g. the Thames, the Solent, and Cork harbour) in which the enemy can manoeuvre. In such cases there are two possible forms of defence, first by arranging for gun-fire behind the main gun position, usually called the defence of inner waters, and secondly by placing in the entrance and under the fire of the main gun defence some form of obstruction to detain ships under fire. This obstruction can be passive (booms, chains, rows of piles or sunken ships) or active (mines or torpedoes). Passive obstructions are only effective against comparatively small craft, and at important ports mines are the only efficient obstruction which can be used against large vessels.
After some years of experiment, English engineers adopted two main classes of mines, called “observation” and “contact” mines (see Submarine Mines). Both were fired by electricity, which was applied only at the moment a hostile ship was within the dangerous zone of a mine. In the observation mines the moment of applying the electric current was ascertained by a position-finder, which, tracing a ship’s course on a chart, made an electrical connexion at the moment the ship was over a mine. These mines were placed so as to be well below the bottom of any ships afloat and were used in channels which it was desired to leave open for the entrance of friendly vessels. Contact mines, which are moored a few feet below the surface of the water, are fired after certain electrical connexions have been made in a firing room on shore by the ship itself striking against the mine. These are used in waters which it is intended to deny to friend and foe. Except in narrow waters where the whole width of the channel was required for friendly traffic, contact mines were generally used to limit the width of the channel to the minimum consistent with the amount of friendly traffic which would use the port in war. It will be readily understood that by bending this channel and disclosing its exact position only to special pilots, a very complete measure of security against surprise would be obtained. In English ports the practical importance of allowing free ingress for friendly traffic overruled all other considerations, and the friendly channels were always straight and coincided with some part of the usual fairway channel. They were also carefully marked by lightships and buoys.
A variation of the submarine mine is the Brennan torpedo, purchased by the British government about 1890. This differs from the torpedo used on board ship, mainly by the fact that the engine-power which drives it is on shore and connected with the torpedo by two strong wires. These wires are wound out of the torpedo by the engine, and by varying the strain on the two wires very accurate control of the steering can be obtained. This torpedo shares with the submarine mine the disadvantages that it must wait for the enemy to venture within its range, and with all other forms of defence (except contact mines), that it is made useless by fog or rain. As compared with a mine it has the advantage of being unaffected by tide or depth, and of forming no obstruction to traffic, except when actually in action. It was installed at the principal ports only.
The system of defence hitherto described is thus a main gun defence of 9.2-in. and 6-in. guns pushed well forward, assisted by position-finders, mine-fields and torpedo stations, and with some gun defence of inner waters. Subject to improvements in patterns of guns and mountings—of which the most important has been the substitution of barbette mounting and shield for the recoil mounting described above—this system held the field up to 1905, when, partly as a result of the experience of the Russo-Japanese War, and partly owing to the alteration of the naval balance of power due to the destruction of the Russian fleet, both the scale and system of defence were very considerably modified.
We can now consider another branch of defence, which was evolved pari passu with the automobile torpedo, and was therefore almost non-existent in 1885. In this year the boats specially built for carrying torpedoes were little more than launches, but in the next five years was developed the type of first-class torpedo boat. This, while seaworthy, was limited as to its radius of action by the small amount of coal it would carry. But with a possibly hostile coast only a few hours’ steam away, and with foreign harbours thronged with torpedo craft, it became necessary for the British government especially to consider this form of attack and its antidote. It was obvious that in daytime and in clear weather such an attack would have little chance of success, also that in no circumstances would torpedo boats be able to damage fixed defences. Their best chance was attack by night, and the only form of attack was that referred to above as “running past,” that is, an attempt to evade the defences and to attack ships or docks inside. The light draught of torpedo boats and their comparative invisibility favoured this form of attack.
To meet it the first requirement was some form of illumination of the defended channel. Experiments in the attack and defence of defended harbours took place at Gosport in 1879 and 1880, at Milford Haven in 1885, at Berehaven (by the royal navy) in 1886, at Langston Harbour in 1887, and a series at the Needles entrance of the Isle of Wight up to 1892. During the course of these experiments various methods of illumination were tried, but by far the best was found to be the light from an electric arc-lamp of high power projected by powerful reflectors. At first these were used as concentrated beams forming a pencil of light with an angular opening of about 2° to 3°. Such a beam directed at an incoming ship gives effective illumination up to a mile or more from the source of light, but has the disadvantage that it must be moved so as to follow the ship’s movements. 602 Each beam thus lights only one ship at a time, and the movements of several beams crossing and recrossing have a very confusing effect, with the consequent risk that a proportion of the attacking vessels may slip through unnoticed.
An alternative method of using electric lights is to arrange the projector so that the light comes out in a fan (generally of 30° divergence). Two or three such lights are usually placed side by side, forming an illuminated fan of considerable divergence. These fans are now used for the main defence, with in front of them one or more search-lights to warn the defences of the approach of ships. There is some loss of range when using these fans as compared with search-lights, but by occupying both sides of a channel and placing the defences against torpedo boats at the narrowest point, an effective illumination can be obtained in moderate weather.
Heavy guns can, of course, be fired against torpedo boats, but their rate of fire is relatively slow, and at first they had also the disadvantage of using black powder, the smoke of which obscured the lights.
A small quick-firing gun using smokeless powder was seen to be a necessity. At first the 6-pounder was adopted as the stock size supplemented by machine guns for close range, but soon afterwards it became necessary to reconsider the scale of anti-torpedo boat defences, owing first to the increased size of first-class torpedo boats, and secondly to the introduction of a new type of vessel, the torpedo boat destroyer. The increased size of torpedo boats, and improved arrangements for the distribution of coal on board, made these boats practically proof against 6-pounder guns and necessitated the introduction of the 12-pounder. The torpedo boat destroyer, originally introduced to chase and destroy torpedo boats, not only justified its existence by checking the construction of more torpedo boats, but in addition became itself a sea-going torpedo craft, and thus increased the menace to defended ports and also the area over which this form of attack would be dangerous.
This development was met by an increased number of 12-pounder guns, assisted in the more important places by 4.7-in. (and latterly 4-in.) guns, and also by an increased number of lights, both guns and lights increasing at some places nearly fourfold. But even with the best possible arrangement of this form of defence, the possibility of interference by fog, mist or rain introduces a considerable element of uncertainty.
About the same time, and largely on account of the demand for better and quicker firing, the “automatic sight” was introduced (see Ordnance: Garrison; and Sights). In this, a development of the principle of the position-finder, the act of bringing an object into the field of the auto-sight automatically lays the gun. In order to take full advantage of this, the ammunition was made up into a cartridge with powder and shell in one case to allow of the quickest possible loading. It may be added that the efficiency of the auto-sight depends on the gun being a certain height above the water, and that therefore the rise and fall of tide has to be allowed for in setting the sight.
In view of the possible interference by fog it was thought wise at an early stage to provide, towards the rear of the defences, some form of physical obstacle behind which ships could lie in safety. Such an obstacle had been designed in the early days by the Royal Engineers and took the form of a “boom” of baulks of timber secured by chains. Such booms were limited in size by considerations of expense and were only partially successful. About 1892 the British navy took the matter up and began experiments on a larger scale, substituting wire hawsers for chains and using old gunboats to divide the booms up into sections of convenient length. The result was that booms were definitely adopted as an adjunct of coast defence. Their place is behind the lighted area, but within reach of some of the anti-torpedo boat batteries.
Other forms of obstacle to torpedo boat attack, based on a modification of contact mines or a combination of mines and passive obstructions, have been tried but never definitely adopted, though some form of under-water defence of this description seems necessary to meet attack by submarines.
We may now summarize the anti-torpedo boat defences. These are, first, an outpost or look-out line of electric search-lights, then a main lighted area composed of fixed lights with which there are a considerable number of 12-pounder or 4-in. Q.F. guns fitted with auto-sights, and behind all this, usually at the narrowest part of the entrance, the boom.
Once coast defences are designed and installed, little change is possible during an attack, so that the operation of fighting a system of defence, such as we have considered above, is mainly a matter of peace training of gun-crews, electric light men and look-outs, coupled with careful organization. To facilitate the transmission of order and intelligence, a considerable system of telephonic and other electrical communication has been established. This may be considered under the three heads of (1) orders, (2) intelligence, (3) administration.
The communication of orders follows the organization adopted for the whole fortress. Each fortress is commanded by a fortress commander, who has a suitable staff. This officer sends orders to commanders of artillery, engineers, and infantry. The artillery officer in charge of a group of batteries is called a “fire commander”; his command is generally confined to such batteries as fire over the same area of water and can mutually support one another. Thus there may be several fire commanders at a defended port. Anti-torpedo boat batteries are not in a fire command, and are connected to the telephone system for intelligence only and not for orders. The engineers require orders for the control of electric lights or Brennan torpedo. The officer in charge of a group of lights or of a torpedo station is called a director. Though receiving orders direct from the fortress commander, he has also to co-operate with the nearest artillery commander. The infantry are posted on the flanks of the fixed defences, or on the land front. They are divided into suitable groups, each under a commanding officer, who communicates with the fortress commander. In large fortresses the area is divided into sections, each including some portion of the artillery, engineers, and infantry defence. In such cases the section commanders receive orders from the fortress commander and pass them on to their subordinates.
The intelligence system includes communication with the naval signal stations in the vicinity, one of which is specially selected for each port as the warning station and is directly connected to some part of the defences. Another part of the intelligence system deals with the arrangements for examining all ships entering a harbour. This is usually effected by posting in each entrance examination vessels, which are in communication by signal with a battery or selected post on shore. Any points on shore which can see the approaches are connected by a special alarm circuit, mainly for use in case of torpedo boat attack.
The administrative system of telephones is used for daily routine messages. These usually take the form of telephone lines radiating from a central exchange. In many stations the same lines may be used for command and administration, or intelligence and command, but at the larger stations each class of line is kept distinct.
COASTGUARD, a naval force maintained in Great Britain and Ireland to suppress smuggling, aid shipwrecked vessels and serve as a reserve to the navy. The coastguard was originally designed to prevent smuggling. Before 1816 this duty was entrusted to the revenue cutters, and to a body of “riding officers,” mounted men who were frequently supported by detachments of dragoons. The crews of the cutters and the riding officers were under the authority of the custom house in London, and were appointed by the treasury. On the conclusion of the war with Napoleon in 1815 it was resolved to take stricter precautions against smuggling. A “coast blockade” was established in Kent and Sussex. The “Ramillies” (74) was stationed in the Downs and the “Hyperion” (42) at Newhaven. A number of half-pay naval lieutenants were appointed to these vessels, but were stationed with detachments of men and boats at the Martello towers erected along the coast as a defence against French invasion. They were known as the “preventive 603 water guard” or the “preventive service.” The crews of the boats were partly drawn from the revenue cutters, and partly hired from among men of all trades. The “coast blockade” was extended to all parts of the coast. The revenue cutters and the riding officers continued to be employed, and the whole force was under the direction of the custom house. The whole was divided into districts under the command of naval officers. In 1822 the elements of which the preventive water guard was composed were consolidated, and in 1829 it was ordered that only sailors or fishermen should be engaged as boatmen. In 1830 the whole service consisted of 50 revenue cutters, fine vessels of 150 and 200 tons, of the “preventive boats,” and the riding officers. In 1831, during the administration of Sir James Graham, the service was transferred to the admiralty, though the custom house flag was used till 1857. After 1840 the men were drilled “in the common formations,” mainly with a view to being employed for the maintenance of order and in support of the police, in case of Chartist or other agitations. But in 1845 the first steps were taken to utilize the coastguard as a reserve to the navy. The boatmen were required to sign an engagement to serve in the navy if called upon. In May 1857 the service was transferred entirely to the admiralty, and the coastguard became a part of the navy, using the navy flag. The districts were placed under captains of the navy, known as district captains, in command of ships stationed at points round the coast. Since that year the coastguard has been recruited from the navy, and has been required to do regular periods of drill at sea, on terms laid down by the admiralty from time to time. It has, in fact, been a form of naval reserve.
COASTING, usually called tobogganing (q.v.) in Europe, the sport of sliding down snow or ice-covered hills or artificial inclines upon hand-sleds, or sledges, provided with runners shod with iron or steel. It is uncertain whether the first American sleds were copied from the Indian toboggans, but no sled without runners was known in the United States before 1870, except to the woodsmen of the Canadian border. American laws have greatly restricted, and in most places prohibited, the practice, once common, of coasting on the highways; and the sport is mainly confined to open hills and artificial inclines or chutes. Two forms of hand-sled are usual in America, the original “clipper” type, built low with long, pointed sides, originally shod with iron but since 1850 with round steel runners; and the light, short “girls’ sled,” with high skeleton sides, usually flat shod. There is also the “double-runner,” or “bob-sled,” formed of two clipper sleds joined by a board and steered by ropes, a wheel or a cross-bar, and seating from four to ten persons.
In Scandinavia several kinds of sled are common, but that of the fishermen, by means of which they transport their catch over the frozen fjords, is the one used in coasting, a sport especially popular in the neighbourhood of Christiania, where there are courses nearly 3 m. in length. This sled is from 4 to 6 ft. long, with skeleton sides about 7 in. high, and generally holds three persons. It is steered by two long sticks trailing behind. On the ice the fisherman propels his sled by means of two short picks. The general Norwegian name for sledge is skijälker, the primitive form being a kind of toboggan provided with broad wooden runners resembling the ski (q.v.). In northern Sweden and Finland the commonest form of single sled is the Sparkstottinger, built high at the back, the coaster standing up and steering by means of two handles projecting from the sides.
Coasting in its highest development may be seen in Switzerland, at the fashionable winter resorts of the Engadine, where it is called tobogganing. The first regular races there were organized by John Addington Symonds, who instituted an annual contest for a challenge cup, open to all comers, over the steep post-road from Davos to Klosters, the finest natural coast in Switzerland, the sled used being the primitive native Schlittli or Handschlitten, a miniature copy of the ancient horse-sledge. Soon afterwards followed the construction of great artificial runs, the most famous being the “Cresta” at St Moritz, begun in 1884, which is about 1350 yds. in length, its dangerous curves banked up like those of a bicycle track. On this the annual “Grand National” championship is contested, the winner’s time being the shortest aggregate of three heats. In 1885 and the following year the native Schlittli remained in use, the rider sitting upright facing the goal, and steering either with the heels or with short picks. In 1887 the first American clipper sled was introduced by L. P. Child, who easily won the championship for that year on it. The sled now used by the contestants is a development of the American type, built of steel and skeleton in form. With it a speed of over 70 m. an hour has been attained. The coaster lies flat upon it and steers with his feet, shod with spiked shoes, to render braking easier, and helped with his gloved hands. The “double-runner” has also been introduced into Switzerland under the name of “bob-sleigh.”
COATBRIDGE, a municipal and police burgh, having the privileges of a royal burgh, of Lanarkshire, Scotland. Pop. (1891) 15,212; (1901) 36,991. It is situated on the Monkland Canal, 8 m. E. of Glasgow, with stations on the Caledonian and North British railways. Until about 1825 it was only a village, but since then its vast stores of coal and iron have been developed, and it is now the centre of the iron trade of Scotland. Its prosperity was largely due to the ironmaster James Baird (q.v.), who erected as many as sixteen blast-furnaces in the immediate neighbourhood between 1830 and 1842. The industries of Coatbridge produce malleable iron, boilers, tubes, wire, tinplates and railway wagons, tiles, fire-bricks and fire-clay goods. There are two public parks in the town, and its public buildings include a theatre, a technical school and mining college, hospitals, and the academy and Baird Institute at Gartsherrie. Janet Hamilton, the poetess (1795-1873), spent most of her life at Langloan—now a part of Coatbridge—and a fountain has been erected to her memory near the cottage in which she lived. For parliamentary purposes the town, which became a municipal burgh in 1885, is included in the north-west division of Lanarkshire. About 4 m. west by south lies the mining town of Baillieston (pop. 3784), with a station on the Caledonian railway. It has numerous collieries, a nursery and market garden.
COATESVILLE, a borough of Chester county, Pennsylvania, U.S.A., on the west branch of Brandywine Creek, 39 m. W. of Philadelphia. Pop. (1890) 3680; (1900) 5721 (273 foreign-born); (1910) 11,084. It is served by the Pennsylvania and the Philadelphia & Reading railways, and interurban electric lines. For its size the borough ranks high as a manufacturing centre, iron and steel works, boiler works, brass works, and paper, silk and woollen mills being among its leading establishments. Its water-works are owned and operated by the municipality. Named in honour of Jesse Coates, one of its early settlers, it was settled about 1800, and was incorporated in 1867.
COATI, or Coati-Mundi, the native name of the members of the genus Nasua, of the mammalian family Procyonidae. They are easily recognized by their long body and tail, and elongated, upturned snout; from which last feature the Germans call them Rüsselbären or “snouted bears.” In the white-nosed coati, a native of Mexico and Central America, the general hue is brown, but the snout and upper lip are white, and the tail is often banded. In the red coati, ranging from Surinam to Paraguay, the tail is marked with from seven to nine broad fulvous or rufous rings, alternating with black ones, and tipped with black. Coatis are gregarious and arboreal in habit, and feed on birds, eggs, lizards and insects. They are common pets of the Spaniards in South America. (See Carnivora.)
COB, a word of unknown origin with a variety of meanings, which the New English Dictionary considers may be traced to the notions of something stout, big, round, head or top. In “cobble,” e.g. in the sense of a round stone used in paving, the same word may be traced. The principal uses of “cob” are for a stocky strongly built horse, from 13 to 14 hands high, a small round loaf, 604 a round lump of coal, in which sense “cobble” is also used, the fruiting spike of the maize plant, and a large nut of the hazel type, more commonly known as the cob-nut.
“Cobbler,” a patcher or mender of boots and shoes, is probably from a different root. It has nothing to do with an O. Fr. coubler, Mod. coupler, to fasten together. In “cobweb,” the web of the spider, the “cob” represents the older cop, coppe, spider, cf. Dutch spinnekop.
COBALT (symbol Co, atomic weight 59), one of the metallic chemical elements. The term “cobalt” is met with in the writings of Paracelsus, Agricola and Basil Valentine, being used to denote substances which, although resembling metallic ores, gave no metal on smelting. At a later date it was the name given to the mineral used for the production of a blue colour in glass. In 1735 G. Brandt prepared an impure cobalt metal, which was magnetic and very infusible. Cobalt is usually found associated with nickel, and frequently with arsenic, the chief ores being speiss-cobalt, (Co,Ni,Fe)As2, cobaltite (q.v.), wad, cobalt bloom, linnaeite, Co3S4, and skutterudite, CoAs3. Its presence has also been detected in the sun and in meteoric iron. For the technical preparation of cobalt, and its separation from nickel, see Nickel. The metal is chiefly used, as the oxide, for colouring glass and porcelain.
Metallic cobalt may be obtained by reduction of the oxide or chloride in a current of hydrogen at a red heat, or by heating the oxalate, under a layer of powdered glass. As prepared by the reduction of the oxide it is a grey powder. In the massive state it has a colour resembling polished iron, and is malleable and very tough. It has a specific gravity of 8.8, and it melts at 1530° C. (H. Copaux). Its mean specific heat between 9° and 97° C. is 0.10674 (H. Kopp). It is permanent in dry air, but in the finely divided state it rapidly combines with oxygen, the compact metal requiring a strong heating to bring about this combination. It decomposes steam at a red heat, and slowly dissolves in dilute hydrochloric and sulphuric acids, but more readily in nitric acid. Cobalt burns in nitric oxide at 150° C. giving the monoxide. It may be obtained in the pure state, according to C. Winkler (Zeit. für anorg. Chem., 1895, 8, p. 1), by electrolysing the pure sulphate in the presence of ammonium sulphate and ammonia, using platinum electrodes, any occluded oxygen in the deposited metal being removed by heating in a current of hydrogen.
Three characteristic oxides of cobalt are known, the monoxide, CoO, the sesquioxide, Co2O3, and tricobalt tetroxide, Co3O4; besides these there are probably oxides of composition CoO2, Co8O9, Co6O7 and Co4O5. Cobalt monoxide, CoO, is prepared by heating the hydroxide or carbonate in a current of air, or by heating the oxide Co3O4 in a current of carbon dioxide. It is a brown coloured powder which is stable in air, but gives a higher oxide when heated. On heating in hydrogen, ammonia or carbon monoxide, or with carbon or sodium, it is reduced to the metallic state. It is readily soluble in warm dilute mineral acids forming cobaltous salts. Cobaltous hydroxide, Co(OH)2, is formed when a cobaltous salt is precipitated by caustic potash in the absence of air. A blue basic salt is precipitated first, which, on boiling, rapidly changes to the rose-coloured hydroxide. It dissolves in acids forming cobaltous salts, and on exposure to air it rapidly absorbs oxygen, turning brown in colour. A. de Schulten (Comptes Rendus, 1889, 109, p. 266) has obtained it in a crystalline form; the crystals have a specific gravity of 3.597, and are easily soluble in warm ammonium chloride solution. Cobalt sesquioxide, Co2O3, remains as a dark-brown powder when cobalt nitrate is gently heated. Heated at 190-300° in a current of hydrogen it gives the oxide Co3O4, while at higher temperatures the monoxide is formed, and ultimately cobalt is obtained. Cobaltic hydroxide, Co(OH)3, is formed when a cobalt salt is precipitated by an alkaline hypochlorite, or on passing chlorine through water containing suspended cobaltous hydroxide or carbonate. It is a brown-black powder soluble in hydrochloric acid, chlorine being simultaneously liberated. This hydroxide is soluble in well cooled acids, forming solutions which contain cobaltic salts, one of the most stable of which is the acetate. Cobalt dioxide, CoO2, has not yet been isolated in the pure state; it is probably formed when iodine and caustic soda are added to a solution of a cobaltous salt. By suspending cobaltous hydroxide in water and adding hydrogen peroxide, a strongly acid liquid is obtained (after filtering) which probably contains cobaltous acid, H2CoO3. The barium and magnesium salts of this acid are formed when baryta and magnesia are fused with cobalt sesquioxide. Tricobalt tetroxide, Co3O4, is produced when the other oxides, or the nitrate, are heated in air. By heating a mixture of cobalt oxalate and sal-ammoniac in air, it is obtained in the form of minute hard octahedra, which are not magnetic, and are only soluble in concentrated sulphuric acid.
The cobaltous salts are formed when the metal, cobaltous oxide, hydroxide or carbonate, are dissolved in acids, or, in the case of the insoluble salts, by precipitation. The insoluble salts are rose-red or violet in colour. The soluble salts are, when in the hydrated condition, also red, but in the anhydrous condition are blue. They are precipitated from their alkaline solutions as cobalt sulphide by sulphuretted hydrogen, but this precipitation is prevented by the presence of citric and tartaric acids; similarly the presence of ammonium salts hinders their precipitation by caustic alkalis. Alkaline carbonates give precipitates of basic carbonates, the formation of which is also retarded by the presence of ammonium salts. For the action of ammonia on the cobaltous salts in the presence of air see Cobaltammines (below). On the addition of potassium cyanide they give a brown precipitate of cobalt cyanide, Co(CN)2, which dissolves in excess of potassium cyanide to a green solution.
Cobalt chloride, CoCl2, in the anhydrous state, is formed by burning the metal in chlorine or by heating the sulphide in a current of the same gas. It is blue in colour and sublimes readily. It dissolves easily in water, forming the hydrated chloride, CoCl2·6H2O, which may also be prepared by dissolving the hydroxide or carbonate in hydrochloric acid. The hydrated salt forms rose-red prisms, readily soluble in water to a red solution, and in alcohol to a blue solution. Other hydrated forms of the chloride, of composition CoCl2·2H2O and CoCl2·4H2O have been described (P. Sabatier, Bull. Soc. Chim. 51, p. 88; Bersch, Jahresb. d. Chemie, 1867, p. 291). Double chlorides of composition CoCl2·NH4Cl·6H2O; CoCl2·SnCl4·6H2O and CoCl2·2CdCl2·12H2O are also known. By the addition of excess of ammonia to a cobalt chloride solution in absence of air, a greenish-blue precipitate is obtained which, on heating, dissolves in the solution, giving a rose-red liquid. This solution, on standing, deposits octahedra of the composition CoCl2·6NH3. These crystals when heated to 120° C. lose ammonia and are converted into the compound CoCl2·2NH3 (E. Frémy). The bromide, CoBr2, resembles the chloride, and may be prepared by similar methods. The hydrated salt readily loses water on heating, forming at 100° C. the hydrate CoBr2·2H2O, and at 130° C. passing into the anhydrous form. The iodide, CoI2, is produced by heating cobalt and iodine together, and forms a greyish-green mass which dissolves readily in water forming a red solution. On evaporating this solution the hydrated salt CoI2·6H2O is obtained in hexagonal prisms. It behaves in an analogous manner to CoBr2·6H2O on heating.
Cobalt fluoride, CoF2·2H2O, is formed when cobalt carbonate is evaporated with an excess of aqueous hydrofluoric acid, separating in rose-red crystalline crusts. Electrolysis of a solution in hydrofluoric acid gives cobaltic fluoride, CoF3.
Sulphides of cobalt of composition Co4S3, CoS, Co3S4, Co2S3 and CoS2 are known. The most common of these sulphides is cobaltous sulphide, CoS, which occurs naturally as syepoorite, and can be artificially prepared by heating cobaltous oxide with sulphur, or by fusing anhydrous cobalt sulphate with barium sulphide and common salt. By either of these methods, it is obtained in the form of bronze-coloured crystals. It may be prepared in the amorphous form by heating cobalt with sulphur dioxide, in a sealed tube, at 200° C. In the hydrated condition it is formed by the action of alkaline sulphides on cobaltous salts, or by precipitating cobalt acetate with sulphuretted hydrogen (in the absence of free acetic acid). It is a black amorphous powder soluble in concentrated sulphuric and hydrochloric acids, and when in the moist state readily oxidizes on exposure.
Cobaltous sulphate, CoSO4·7H2O, is found naturally as the mineral bieberite, and is formed when cobalt, cobaltous oxide or carbonate are dissolved in dilute sulphuric acid. It forms dark red crystals isomorphous with ferrous sulphate, and readily soluble in water. By dissolving it in concentrated sulphuric acid and warming the solution, the anhydrous salt is obtained. Hydrated sulphates of composition CoSO4·6H2O, CoSO4·4H2O and CoSO4·H2O are also known. The heptahydrated salt combines with the alkaline sulphates to form double sulphates of composition CoSO4·M2SO4·6H2O (M = K, NH4, &c.).
The cobaltic salts corresponding to the oxide Co2O3 are generally unstable compounds which exist only in solution. H. Marshall (Proc. Roy. Soc. Edin. 59, p. 760) has prepared cobaltic sulphate Co2(SO4)3·18H2O, in the form of small needles, by the electrolysis of cobalt sulphate. In a similar way potassium and ammonium cobalt alums have been obtained. A cobaltisulphurous acid, probably H6[(SO3)6·Co2] has been obtained by E. Berglund (Berichte, 1874, 7, p. 469), in aqueous solution, by dissolving ammonium cobalto-cobaltisulphite (NH4)2Co2[(SO3)6·Co2]·14H2O in dilute hydrochloric or nitric acids, or by decomposition of its silver salt with hydrochloric acid. The ammonium cobalto-cobaltisulphite is prepared by saturating an air-oxidized ammoniacal solution of cobaltous chloride with sulphur dioxide. The double salts containing the metal in the cobaltic form are more stable than the corresponding single salts, and of these potassium cobaltinitrite, Co2(NO2)6·6KNO2·3H2O, is best known. It may be prepared by the addition of potassium nitrite to an acetic acid solution of cobalt chloride. The yellow precipitate obtained is washed with a solution 605 of potassium acetate and finally with dilute alcohol. The reaction proceeds according to the following equation: 2CoCl2 + 10KNO2 + 4HNO2 = Co2(NO2)6·6KNO2 + 4KCl + 2NO + 2H2O (A. Stromeyer, Annalen, 1855, 96, p. 220). This salt may be used for the separation of cobalt and nickel, since the latter metal does not form a similar double nitrite, but it is necessary that the alkaline earth metals should be absent, for in their presence nickel forms complex nitrites containing the alkaline earth metal and the alkali metal. A sodium cobaltinitrite is also known.
Cobalt nitrate, Co(NO3)2·6H2O, is obtained in dark-red monoclinic tables by the slow evaporation of a solution of the metal, its hydroxide or carbonate, in nitric acid. It deliquesces in the air and melts readily on heating. By the addition of excess of ammonia to its aqueous solution, in the complete absence of air, a blue precipitate of a basic nitrate of the composition 6CoO·N2O5·5H2O is obtained.
By boiling a solution of cobalt carbonate in phosphoric acid, the acid phosphate CoHPO4·3H2O is obtained, which when heated with water to 250° C. is converted into the neutral phosphate Co3(PO4)2·2H2O (H. Debray, Ann. de chimie, 1861, [3] 61, p. 438). Cobalt ammonium phosphate, CoNH4PO4·12H2O, is formed when a soluble cobalt salt is digested for some time with excess of a warm solution of ammonium phosphate. It separates in the form of small rose-red crystals, which decompose on boiling with water.
Cobaltous cyanide, Co(CN)2·3H2O, is obtained when the carbonate is dissolved in hydrocyanic acid or when the acetate is precipitated by potassium cyanide. It is insoluble in dilute acids, but is readily soluble in excess of potassium cyanide. The double cyanides of cobalt are analogous to those of iron. Hydrocobaltocyanic acid is not known, but its potassium salt, K4Co(CN)6, is formed when freshly precipitated cobalt cyanide is dissolved in an ice-cold solution of potassium cyanide. The liquid is precipitated by alcohol, and the washed and dried precipitate is then dissolved in water and allowed to stand, when the salt separates in dark-coloured crystals. In alkaline solution it readily takes up oxygen and is converted into potassium cobalticyanide, K3Co(CN)6, which may also be obtained by evaporating a solution of cobalt cyanide, in excess of potassium cyanide, in the presence of air, 8KCN + 2Co(CN)2 + H2O + O = 2K3Co(CN)6 + 2KHO. It forms monoclinic crystals which are very soluble in water. From its aqueous solution, concentrated hydrochloric acid precipitates hydrocobalticyanic acid, H3Co(CN)6, as a colourless solid which is very deliquescent, and is not attacked by concentrated hydrochloric and nitric acids. For a description of the various salts of this acid, see P. Wesselsky, Berichte, 1869, 2, p. 588.
Cobaltammines. A large number of cobalt compounds are known, of which the empirical composition represents them as salts of cobalt to which one or more molecules of ammonia have been added. These salts have been divided into the following series:—
Diammine Series, [Co(NH3)2]X4M. In these salts X = NO2 and M = one atomic proportion of a monovalent metal, or the equivalent quantity of a divalent metal.
Triammine Series, [Co(NH3)3]X3. Here X = Cl, NO3, NO2, ½SO4, &c.
Tetrammine Series. This group may be divided into the
Praseo-salts [R2Co(NH3)4]X, where X = Cl.
Croceo-salts [(NO2)2Co(NH3)4]X, which may be considered as a subdivision of the praseo-salts.
Tetrammine purpureo-salts [RCo(NH3)4·H2O]X2.
Tetrammine roseo-salts [Co(NH3)4·(H2O)2]X3.
Fuseo-salts [Co(NH3)4]OH·X2.
Pentammine Series.
Pentammine purpureo-salts [R·Co(NH3)5]X2 where X = Cl, Br, NO3, N02, ½SO4, &c.
Pentammine roseo-salts [Co(NH3)5·H2O]X2.
Hexammine or Luteo Series [Co(NH3)6]X3.
The hexammine salts are formed by the oxidizing action of air on dilute ammoniacal solutions of cobaltous salts, especially in presence of a large excess of ammonium chloride. They form yellow or bronze-coloured crystals, which decompose on boiling their aqueous solution. On boiling their solution in caustic alkalis, ammonia is liberated. The pentammine purpureo-salts are formed from the luteo-salts by loss of ammonia, or from an air slowly oxidized ammoniacal cobalt salt solution, the precipitated luteo-salt being filtered off and the filtrate boiled with concentrated acids. They are violet-red in colour, and on boiling or long standing with dilute acids they pass into the corresponding roseo-salts.
The pentammine nitrito salts are known as the xanthocobalt salts and have the general formula [NO2·Co·(NH3)5]X2. They are formed by the action of nitrous fumes on ammoniacal solutions of cobaltous salts, or purpureo-salts, or by the mutual reaction of chlorpurpureo-salts and alkaline nitrites. They are soluble in water and give characteristic precipitates with platinic and auric chlorides, and with potassium ferrocyanide. The pentammine roseo-salts can be obtained from the action of concentrated acids, in the cold, on air-oxidized solutions of cobaltous salts. They are of a reddish colour and usually crystallize well; on heating with concentrated acids are usually transformed into the purpureo-salts. Their alkaline solutions liberate ammonia on boiling. They give a characteristic pale red precipitate with sodium pyrophosphate, soluble in an excess of the precipitant; they also form precipitates on the addition of platinic chloride and potassium ferrocyanide. For methods of preparation of the tetrammine and triammine salts, see O. Dammer’s Handbuch der anorganischen Chemie, vol. 3 (containing a complete account of the preparation of the cobaltammine salts). The diammine salts are prepared by the action of alkaline nitrites on cobaltous salts in the presence of much ammonium chloride or nitrate; they are yellow or brown crystalline solids, not very soluble in cold water.
The above series of salts show striking differences in their behaviour towards reagents; thus, aqueous solutions of the luteo chlorides are strongly ionized, as is shown by their high electric conductivity; and all their chlorine is precipitated on the addition of silver nitrate solution. The aqueous solution, however, does not show the ordinary reactions of cobalt or of ammonia, and so it is to be presumed that the salt ionizes into [Co(NH3)6] and 3Cl′. The purpureo chloride has only two-thirds of its chlorine precipitated on the addition of silver nitrate, and the electric conductivity is much less than that of the luteo chloride; again in the praseo-salts only one-third of the chlorine is precipitated by silver nitrate, the conductivity again falling; while in the triammine salts all ionization has disappeared. For the constitution of these salts and of the “metal ammonia” compounds generally, see A. Werner, Zeit. für anorg. Chemie, 1893 et seq., and Berichte, 1895, et seq.; and S. Jörgensen, Zeit. für anorg. Chemie, 1892 et seq.
The oxycobaltammines are a series of compounds of the general type [Co2O3·H2(NH3)10]X4 first observed by L. Gmelin, and subsequently examined by E. Frémy, W. Gibbs and G. Vortmann (Monatshefte für Chemie, 1885, 6, p. 404). They result from the cobaltammines by the direct taking up of oxygen and water. On heating, they decompose, forming basic tetrammine salts.
The atomic weight of cobalt has been frequently determined, the earlier results not being very concordant (see R. Schneider, Pog. Ann., 1857, 101, p. 387; C. Marignac, Arch. Phys. Nat. [2], 1, p. 373; W. Gibbs, Amer. Jour. Sci. [2], 25, p. 483; J. B. Dumas, Ann. Chim. Phys., 1859 [3], 55, p. 129; W. J. Russell, Jour. Chem. Soc., 1863, 16, p. 51). C. Winkler, by the analysis of the chloride, and by the action of iodine on the metal, obtained the values 59.37 and 59.07, whilst W. Hempel and H. Thiele (Zeit. f. anorg. Chem., 1896, II, p. 73), by reducing cobalto-cobaltic oxide, and by the analysis of the chloride, have obtained the values 58.56 and 58.48. G. P. Baxter and others deduced the value 58.995 (O = 16).
Cobalt salts may be readily detected by the formation of the black sulphide, in alkaline solution, and by the blue colour they produce when fused with borax. For the quantitative determination of cobalt, it is either weighed as the oxide, Co3O4, obtained by ignition of the precipitated monoxide, or it is reduced in a current of hydrogen and weighed as metal. For the quantitative separation of cobalt and nickel, see E. Hintz (Zeit. f. anal. Chem., 1891, 30, p. 227), and also Nickel.
COBALTITE, a mineral with the composition CoAsS, cobalt sulpharsenide. It is found as granular to compact masses, and frequently as beautifully developed crystals, which have the same symmetry as the isomorphous mineral pyrites, being cubic with parallel hemihedrism. The usual forms are the cube, octahedron and pentagonal dodecahedron {210}. The colour is silver-white with a reddish tinge, and the lustre brilliant and metallic, hence the old name cobalt-glance; the streak is greyish-black. The mineral is brittle, and possesses distinct cleavages parallel to the faces of the cube; hardness 5½; specific gravity 6.2. The brilliant crystals from Tunaberg in Sodermanland and Håkansboda in Vestmanland, Sweden, and from Skutterud near Drammen in Norway are well known in mineral collections. The cobalt ores at these localities occur with pyrites and chalcopyrite as bands in gneiss. Crystals have also been found at Khetri in Rajputana, and under the name sehta the mineral is used by Indian jewellers for producing a blue enamel on gold and silver ornaments. Massive cobaltite has been found in small amount in the Botallack mine, Cornwall. A variety containing much iron replacing cobalt, and known as ferrocobaltite (Ger. Stahlkobalt), occurs at Siegen in Westphalia.
COBÁN, or Santo Domingo de Cobán, the capital of the department of Alta Vera Paz in central Guatemala; about 90 m. N. of the city of Guatemala, on the Cojabón, a left-hand tributary of the Polochic. Pop. (1905) about 31,000. The town is built in a mountainous and fertile district, and consists chiefly of adobe Indian cottages, surrounded by gardens of flowering shrubs. More modern houses have been erected for the foreign residents, among whom the Germans are numerically predominant. In the chief square of the town stands a 16th-century Dominican church, externally plain, but covered internally with curious Indian decorations. The municipal offices, formerly a college for priests, are remarkable for their handsome but 606 disproportionately large gateway in Renaissance style. Despite the want of a railway, Cobán has a flourishing trade in coffee and cinchona; cocoa, vanilla and sugar-cane are also cultivated, and there are manufactures of rum, cotton fabrics, soap and cigars. The prosperity of the town is largely due to the industry of the Quecchi, Kacchi or Kakchi Indians who form the majority of the inhabitants.
Cobán was founded in the 16th century by Dominican monks under Fray Pedro de Angulo, whose portrait is preserved in the church. In honour of the emperor Charles V. (1500-1558), Cobán received the name of Ciudad Imperial (which soon became obsolete), together with a coat of arms and other privileges belonging to a Spanish city of the first class.
COBAR, a mining town of Robinson county, New South Wales, Australia, 459 m. N.W. by W. of Sydney by rail. Pop. (1901) 3371. The district of which Cobar is the centre abounds in minerals of all kinds, but copper and gold are those most extensively worked. The Great Cobar copper-mine is the most important in the state, and there are a number of successful gold-mines. In addition to the mining, the district produces large quantities of wool. Cobar is a municipality, as also is the adjacent township of Gladstone, with a mining population.
COBB, HOWELL (1815-1868), American political leader, was born at Cherry Hill, Jefferson county, Georgia, on the 7th of September 1815. He graduated from Franklin College (University of Georgia) in 1834, and two years later was admitted to the bar. From 1837 to 1840 he was solicitor-general for the western circuit of his state; from 1843 to 1851 and from 1855 to 1857 he was a member of the National House of Representatives, becoming Democratic leader in that body in 1847, and serving as speaker in 1849-1851; from 1851 to 1853 he was governor of his state; and from March 1857 to December 1860 he was secretary of the treasury in President Buchanan’s cabinet. He was president of the convention of the seceded states which drafted a constitution for the Confederacy. In 1861 he was appointed colonel of a regiment and two years later was made a major-general. He died in New York on the 9th of October 1868. He sided with President Jackson on the question of nullification; was an efficient supporter of President Polk’s administration during the Mexican War; and was an ardent advocate of slavery extension into the Territories, but when the Compromise of 1850 had been agreed upon he became its staunch supporter as a Union Democrat, and on that issue was elected governor of Georgia by a large majority. In 1860, however, he ceased to be a Unionist, and became a leader of the secession movement. From the close of the war until his death he vigorously opposed the Reconstruction Acts.
COBBETT, WILLIAM (1766-1835), English politician and writer, was born near Farnham in Surrey, according to his own statement, on the 9th of March 1766. He was the grandson of a farm-labourer, and the son of a small farmer; and during his early life he worked on his father’s farm. At the age of sixteen, inspired with patriotic feeling by the sight of the men-of-war in Portsmouth harbour, he thought of becoming a sailor; and in May 1783, having, while on his way to Guildford fair, met the London coach, he suddenly resolved to accompany it to its destination. He arrived at Ludgate Hill with exactly half-a-crown in his pocket, but an old gentleman who had travelled with him invited him to his house, and obtained for him the situation of copying clerk in an attorney’s office. He greatly disliked his new occupation; and rejecting all his father’s entreaties that he would return home, he went down to Chatham early in 1784 with the intention of joining the marines. By some mistake, however, he was enlisted in a regiment of the line, which rather more than a year after proceeded to St John’s, New Brunswick. All his leisure time during the months he remained at Chatham was devoted to reading the contents of the circulating library of the town, and getting up by heart Lowth’s English Grammar. His uniform good conduct, and the power of writing correctly which he had acquired, quickly raised him to the rank of corporal, from which, without passing through the intermediate grade of sergeant, he was promoted to that of sergeant-major. In November 1791 he was discharged at his own request, and received the official thanks of the major and the general who signed his discharge. In February 1792 Cobbett married the daughter of a sergeant-major of artillery, whom he had met some years before in New Brunswick. But his liberty was threatened in consequence of his bringing a charge of peculation against certain officers in his old regiment, and he went over to France in March, where he studied the language and literature. In his absence, the inquiry into his charges ended in an acquittal.
In September he crossed to the United States, and supported himself at Wilmington, Delaware, by teaching English to French emigrants. Among these was Talleyrand, who employed him, according to Cobbett’s story, not because he was ignorant of English, but because he wished to purchase his pen. Cobbett made his first literary sensation by his Observations on the Emigration of a Martyr to the Cause of Liberty, a clever retort on Dr Priestley, who had just landed in America complaining of the treatment he had received in England. This pamphlet was followed by a number of papers, signed “Peter Porcupine,” and entitled Prospect from the Congress Gallery, the Political Censor and the Porcupine’s Gazette. In the spring of 1796, having quarrelled with his publisher, he set up in Philadelphia as bookseller and publisher of his own works. On the day of opening, his windows were filled with prints of the most extravagant of the French Revolutionists and of the founders of the American Republic placed side by side, along with portraits of George III., the British ministers, and any one else he could find likely to be obnoxious to the people; and he continued to pour forth praises of Great Britain and scorn of the institutions of the United States, with special abuse of the French party. Abuse and threats were of course in turn showered upon him, and in August 1797, for one of his attacks on Spain, he was prosecuted, though unsuccessfully, by the Spanish ambassador. Immediately on this he was taken up for libels upon American statesmen, and bound in recognizances to the amount of $4000, and shortly after he was prosecuted a third time for saying that Dr Benjamin Rush, who was much addicted to blood-letting, killed nearly all the patients he attended. The trial was repeatedly deferred, and was not settled till the end of 1799, when he was fined $5000. After this last misfortune, for a few months Cobbett carried on a newspaper called the Rushlight; but in June 1800 he set sail for England.
At home he found himself regarded as the champion of order and monarchy. Windham invited him to dinner, introduced him to Pitt, and begged him to accept a share in the True Briton. He refused the offer and joined an old friend, John Morgan, in opening a book shop in Pall Mall. For some time he published the Porcupine’s Gazette, which was followed in January 1802 by the Weekly Political Register. In 1801 appeared his Letters to Lord Hawkesbury (afterwards earl of Liverpool) and his Letters to the Rt. Hon. Henry Addington, in opposition to the proposed peace of Amiens. On the conclusion of the peace (1802) Cobbett made a still bolder protest; he determined to take no part in the general illumination, and—assisted by the sympathy of his wife, who, being in delicate health, removed to the house of a friend—he carried out his resolve, allowing his windows to be smashed and his door broken open by the angry mob. The letters to Addington are among the most polished and dignified of Cobbett’s writings; but by 1803 he was once more revelling in personalities. The government of Ireland was singled out for wholesale attack; and a letter published in the Register remarked of Hardwicke, the lord-lieutenant, that the appointment was like setting the surgeon’s apprentice to bleed the pauper patients. For this, though not a word had been uttered against Hardwicke’s character, Cobbett was fined £500; and two days after the conclusion of this trial a second commenced, at the suit of Plunkett, the solicitor-general for Ireland, which resulted in a similar fine. About this time he began to write in support of Radical views; and to cultivate the friendship of Sir Francis Burdett, from whom he received considerable sums of money, and other favours, for which he gave no very grateful return. In 1809 he was once more in the most serious trouble. 607 He had bitterly commented on the flogging of some militia, because their mutiny had been repressed and their sentence carried out by the aid of a body of German troops, and in consequence he was fined £1000 and imprisoned for two years. His indomitable vigour was never better displayed. He still continued to publish the Register, and to superintend the affairs of his farm; a hamper containing specimens of its produce and other provisions came to him every week; and he amused himself with the company of some of his children and with weekly letters from the rest. On his release a public dinner, presided over by Sir F. Burdett, was held in honour of the event. He returned to his farm at Botley in Hampshire, and continued in his old course, extending his influence by the publication of the Twopenny Trash, which, not being periodical, escaped the newspaper stamp tax. Meanwhile, however, he had contracted debts to the amount of £34,000 (for it is said that, notwithstanding the aversion he publicly expressed to paper currency, he had carried on his business by the aid of accommodation bills to a very large amount); and early in 1817 he fled to the United States. But his pen was as active as ever; from Long Island his MS. for the Register was regularly sent to England; and it was here that he wrote his clear and interesting English Grammar, of which 10,000 copies were sold in a month.
His return to England was accompanied by his weakest exhibition—the exhuming and bringing over of the bones of Thomas Paine, whom he had once heartily abused, but on whom he now wrote a panegyrical ode. Nobody paid any attention to the affair; the relics he offered were not purchased; and the bones were reinterred.
Cobbett’s great aim was now to obtain a seat in the House of Commons. He calmly suggested that his friends should assist him by raising the sum of £5000; it would be much better, he said, than a meeting of 50,000 persons. He first offered himself for Coventry, but failed; in 1826 he was by a large number of votes last of the candidates for Preston; and in 1828 he could find no one to propose him for the office of common councillor. In 1830, that year of revolutions, he was prosecuted for inciting to rebellion, but the jury disagreed, and soon after, through the influence of one of his admirers, Mr Fielden, who was himself a candidate for Oldham, he was returned for that town. In the House his speeches were listened to with amused attention. His position is sufficiently marked by the sneer of Peel that he would attend to Mr Cobbett’s observations exactly as if they had been those of a “respectable member”; and the only striking part of his career was his absurd motion that the king should be prayed to remove Sir Robert Peel’s name from the list of the privy council, because of the change he had proposed in the currency in 1819. In 1834 Cobbett was again member for Oldham, but his health now began to give way, and in June 1835 he left London for his farm, where he died on the 16th of that month.
Cobbett’s account of his home-life makes him appear singularly happy; his love and admiration of his wife never failed; and his education of his children seems to have been distinguished by great kindliness, and by a good deal of healthy wisdom, mingled with the prejudices due to the peculiarities of his temper and circumstances. Cobbett’s ruling characteristic was a sturdy egoism, which had in it something of the nobler element of self-respect. A firm will, a strong brain, feelings not over-sensitive, an intense love of fighting, a resolve to get on, in the sense of making himself a power in the world—these are the principal qualities which account for the success of his career. His opinions were the fruits of his emotions. It was enough for him to get a thorough grasp of one side of a question, about the other side he did not trouble himself; but he always firmly seizes the facts which make for his view, and expresses them with unfailing clearness. His argument, which is never subtle, has always the appearance of weight, however flimsy it may be in fact. His sarcasm is seldom polished or delicate, but usually rough, and often abusive, while coarse nicknames were his special delight. His style is admirably correct and always extremely forcible.
Cobbett’s contributions to periodical literature occupy 100 volumes, twelve of which consist of the papers published at Philadelphia between 1794 and 1800, and the rest of the Weekly Political Register, which ended only with Cobbett’s death (June 1835). An abridgment of these works, with notes, was published by his sons, John M. Cobbett and James P. Cobbett. Besides this he published An Account of the Horrors of the French Revolution, and a work tracing all these horrors to “the licentious politics and infidel philosophy of the present age” (both 1798); A Year’s Residence in the United States; Parliamentary History of England from the Norman Conquest to 1800 (1806); Cottage Economy; Roman History; French Grammar and English Grammar, both in the form of letters; Geographical Dictionary of England and Wales; History of the Regency and Reign of George IV., containing a defence of Queen Caroline, whose cause he warmly advocated (1830-1834); Life of Andrew Jackson, President of the United States (1834); Legacy to Labourers; Legacy to Peel; Legacy to Parsons (1835), an attack on the secular claims of the Established Church; Doom of Tithes; Rural Rides (1830; new ed. 1885), an account of his tours on horse-back through England, full of admirable descriptive writing; Advice to Young Men and Women; Cobbett’s Corn (1828); and History of the Protestant Reformation in England and Ireland (1824-1827), in which he defends the monasteries, Queen Mary and Bonner, and attacks the Reformation, Henry VIII., Elizabeth and all who helped to bring it about, with such vehemence that the work was translated into French and Italian, and extensively circulated among Roman Catholics.
In 1798 Cobbett published in America an account of his early life, under the title of The Life and Adventures of Peter Porcupine; and he left papers relating to his subsequent career. His life has been written by R. Huish (1835), E. Smith (1878), and E. I. Carlyle (1904). See also the annotated edition of the Register (1835).
COBBOLD, THOMAS SPENCER (1828-1886), English man of science, was born at Ipswich in 1828, a son of the Rev. Richard Cobbold (1797-1877), the author of the History of Margaret Catchpole. After graduating in medicine at Edinburgh in 1851, he was appointed lecturer on botany at St Mary’s hospital, London, in 1857, and also on zoology and comparative anatomy at Middlesex hospital in 1861. From 1868 he acted as Swiney lecturer on geology at the British Museum until 1873, when he became professor of botany at the Royal Veterinary College, afterwards filling a chair of helminthology which was specially created for him at that institution. He died in London on the 20th of March 1886. His special subject was helminthology, particularly the worms parasitic in man and animals, and as a physician he gained a considerable reputation in the diagnosis of cases depending on the presence of such organisms. His numerous writings include Entozoa (1864); Tapeworms (1866); Parasites (1879); Human Parasites (1882); and Parasites of Meat and Prepared Flesh Food (1884).
COBDEN, RICHARD (1804-1865), English manufacturer and Radical politician, was born at a farmhouse called Dunford, near Midhurst, in Sussex, on the 3rd of June 1804. The family had been resident in that neighbourhood for many generations, occupied partly in trade and partly in agriculture. Formerly there had been in the town of Midhurst a small manufacture of hosiery with which the Cobdens were connected, though all trace of it had disappeared before the birth of Richard. His grandfather was a maltster in that town, an energetic and prosperous man, almost always the bailiff or chief magistrate, and taking rather a notable part in county matters. But his father, forsaking that trade, took to farming at an unpropitious time. He was amiable and kind-hearted, and greatly liked by his neighbours, but not a man of business habits, and he did not succeed in his farming enterprise. He died when his son Richard was a child, and the care of the family devolved upon the mother, who was a woman of strong sense and of great energy of character, and who, after her husband’s death, left Dunford and returned to Midhurst.
The educational advantages of Richard Cobden were not very ample. There was a grammar school at Midhurst, which at one time had enjoyed considerable reputation, but which had fallen into decay. It was there that he had to pick up such rudiments of knowledge as formed his first equipment in life, but from his earliest years he was indefatigable in the work of self-cultivation. When fifteen or sixteen years of age he went to London to the warehouse of Messrs Partridge & Price, in Eastcheap, one of the partners being his uncle. His relative, 608 noting the lad’s passionate addiction to study, solemnly warned him against indulging such a taste, as likely to prove a fatal obstacle to his success in commercial life. But the admonition was unheeded, for while unweariedly diligent in business, he was in his intervals of leisure a most assiduous student. During his residence in London he found access to the London Institution, and made ample use of its large and well-selected library.
When he was about twenty years of age he became a commercial traveller, and soon became eminently successful in his calling. But never content to sink into the mere trader, he sought to introduce among those he met on the “road” a higher tone of conversation than usually marks the commercial room, and there were many of his associates who, when he had attained eminence, recalled the discussions on political economy and kindred topics with which he was wont to enliven and elevate the travellers’ table. In 1830 Cobden learnt that Messrs Fort, calico printers at Sabden, near Clitheroe, were about to retire from business, and he, with two other young men, Messrs Sheriff and Gillet, who were engaged in the same commercial house as himself, determined to make an effort to acquire the succession. They had, however, very little capital among them. But it may be taken as an illustration of the instinctive confidence which Cobden through life inspired in those with whom he came into contact, that Messrs Fort consented to leave to these untried young men a large portion of their capital in the business. Nor was their confidence misplaced. The new firm had soon three establishments,—one at Sabden, where the printing works were, one in London and one in Manchester for the sale of their goods. This last was under the direct management of Cobden, who, in 1830 or 1831, settled in the city with which his name became afterwards so closely associated. The success of this enterprise was decisive and rapid, and the “Cobden prints” soon became known through the country as of rare value both for excellence of material and beauty of design. There can be no doubt that if Cobden had been satisfied to devote all his energies to commercial life he might soon have attained to great opulence, for it is understood that his share in the profits of the business he had established amounted to from £8000 to £10,000 a year. But he had other tastes, which impelled him irresistibly to pursue those studies which, as Bacon says, “serve for delight, for ornament and for ability.” Prentice, the historian of the Anti-Corn-Law League, who was then editor of the Manchester Times, describes how, in the year 1835, he received for publication in his paper a series of admirably written letters, under the signature of “Libra,” discussing commercial and economical questions with rare ability. After some time he discovered that the author of these letters was Cobden, whose name was until then quite unknown to him.
In 1835 he published his first pamphlet, entitled England, Ireland and America, by a Manchester Manufacturer. It attracted great attention, and ran rapidly through several editions. It was marked by a breadth and boldness of views on political and social questions which betokened an original mind. In this production Cobden advocated the same principles of peace, non-intervention, retrenchment and free trade to which he continued faithful to the last day of his life. Immediately after the publication of this pamphlet, he paid a visit to the United States, landing in New York on the 7th of June 1835. He devoted about three months to this tour, passing rapidly through the seaboard states and the adjacent portion of Canada, and collecting as he went large stores of information respecting the condition, resources and prospects of the great western republic. Soon after his return to England he began to prepare another work for the press, which appeared towards the end of 1836, under the title of Russia. It was mainly designed to combat a wild outbreak of Russophobia which, under the inspiration of David Urquhart, was at that time taking possession of the public mind. But it contained also a bold indictment of the whole system of foreign policy then in vogue, founded on ideas as to the balance of power and the necessity of large armaments for the protection of commerce. While this pamphlet was in the press, delicate health obliged him to leave England, and for several months, at the end of 1836 and the beginning of 1837, he travelled in Spain, Turkey and Egypt. During his visit to Egypt he had an interview with Mehemet Ali, of whose character as a reforming monarch he did not bring away a very favourable impression. He returned to England in April 1837. From that time Cobden became a conspicuous figure in Manchester, taking a leading part in the local politics of the town and district. Largely owing to his exertions, the Manchester Athenaeum was established, at the opening of which he was chosen to deliver the inaugural address. He became a member of the chamber of commerce, and soon infused new life into that body. He threw himself with great energy into the agitation which led to the incorporation of the city, and was elected one of its first aldermen. He began also to take a warm interest in the cause of popular education. Some of his first attempts in public speaking were at meetings which he convened at Manchester, Salford, Bolton, Rochdale and other adjacent towns, to advocate the establishment of British schools. It was while on a mission for this purpose to Rochdale that he first formed the acquaintance of John Bright, who afterwards became his distinguished coadjutor in the free-trade agitation. Nor was it long before his fitness for parliamentary life was recognized by his friends. In 1837, the death of William IV. and the accession of Queen Victoria led to a general election. Cobden was candidate for Stockport, but was defeated, though not by a large majority.
In 1838 an anti-Corn-Law association was formed at Manchester, which, on his suggestion, was afterwards changed into a national association, under the title of the Anti-Corn-Law League (see Corn Laws). Of that famous association Cobden was from first to last the presiding genius and the animating soul. During the seven years between the formation of the league and its final triumph, he devoted himself wholly to the work of promulgating his economic doctrines. His labours were as various as they were incessant—now guiding the councils of the league, now addressing crowded and enthusiastic meetings of his supporters in London or the large towns of England and Scotland, now invading the agricultural districts and challenging the landlords to meet him in the presence of their own farmers, to discuss the question in dispute, and now encountering the Chartists, led by Feargus O’Connor. But whatever was the character of his audience he never failed, by the clearness of his statements, the force of his reasoning and the felicity of his illustrations, to make a deep impression on the minds of his hearers.
In 1841, Sir Robert Peel having defeated the Melbourne ministry in parliament, there was a general election, when Cobden was returned for Stockport. His opponents had confidently predicted that he would fail utterly in the House of Commons. He did not wait long, after his admission into that assembly, in bringing their predictions to the test. Parliament met on the 19th of August. On the 24th, in course of the debate on the Address, Cobden delivered his first speech. “It was remarked,” says Miss Martineau, in her History of the Peace, “that he was not treated in the House with the courtesy usually accorded to a new member, and it was perceived that he did not need such observance.” With perfect self-possession, which was not disturbed by the jeers that greeted some of his statements, and with the utmost simplicity, directness and force, he presented the argument against the corn-laws in such a form as startled his audience, and also irritated some of them, for it was a style of eloquence very unlike the conventional style which prevailed in parliament.
From that day he became an acknowledged power in the House, and though addressing a most unfriendly audience, he compelled attention by his thorough mastery of his subject, and by the courageous boldness with which he charged the ranks of his adversaries. He soon came to be recognized as one of the foremost debaters on those economical and commercial questions which at that time so much occupied the attention of parliament; and the most prejudiced and bitter of his opponents were fain to acknowledge that they had to deal with a man whom the most practised and powerful orators of their party found it hard to cope with, and to whose eloquence, indeed, the great statesman 609 in whom they put their trust was obliged ultimately to surrender. On the 17th of February 1843 an extraordinary scene took place in the House of Commons. Cobden had spoken with great fervour of the deplorable suffering and distress which at that time prevailed in the country, for which, he added, he held Sir Robert Peel, as the head of the government, responsible. This remark, when it was spoken, passed unnoticed, being indeed nothing more than one of the commonplaces of party warfare. But a few weeks before, Mr Drummond, who was Sir Robert Peel’s private secretary, had been shot dead in the street by a lunatic. In consequence of this, and the manifold anxieties of the time with which he was harassed, the mind of the great statesman was no doubt in a moody and morbid condition, and when he arose to speak later in the evening, he referred in excited and agitated tones to the remark, as an incitement to violence against his person. Sir Robert Peel’s party, catching at this hint, threw themselves into a frantic state of excitement, and when Cobden attempted to explain that he meant official, not personal responsibility, they drowned his voice with clamorous and insulting shouts. But Peel lived to make ample and honourable amend for this unfortunate ebullition, for not only did he “fully and unequivocally withdraw the imputation which was thrown out in the heat of debate under an erroneous impression,” but when the great free-trade battle had been won, he took the wreath of victory from his own brow, and placed it on that of his old opponent, in the following graceful words:—“The name which ought to be, and will be associated with the success of these measures, is not mine, or that of the noble Lord (Russell), but the name of one who, acting I believe from pure and disinterested motives, has, with untiring energy, made appeals to our reason, and has enforced those appeals with an eloquence the more to be admired because it was unaffected and unadorned; the name which ought to be chiefly associated with the success of these measures is the name of Richard Cobden.” Cobden had, indeed, with unexampled devotion, sacrificed his business, his domestic comforts and for a time his health to the public interests. His friends therefore felt, at the close of that long campaign, that the nation owed him some substantial token of gratitude and admiration for those sacrifices. No sooner was the idea of such a tribute started than liberal contributions came from all quarters, which enabled his friends to present him with a sum of £80,000. Had he been inspired with personal ambition, he might have entered upon the race of political advancement with the prospect of attaining the highest official prizes. Lord John Russell, who, soon after the repeal of the corn laws, succeeded Sir Robert Peel as first minister, invited Cobden to join his government. But he preferred keeping himself at liberty to serve his countrymen unshackled by official ties, and declined the invitation. He withdrew for a time from England. His first intention was to seek complete seclusion in Egypt or Italy, to recover health and strength after his long and exhausting labours. But his fame had gone forth throughout Europe, and intimations reached him from many quarters that his voice would be listened to everywhere with favour, in advocacy of the doctrines to the triumph of which he had so much contributed at home. Writing to a friend in July 1846, he says—“I am going to tell you of a fresh project that has been brewing in my brain. I have given up all idea of burying myself in Egypt or Italy. I am going on an agitating tour through the continent of Europe.” Then, referring to messages he had received from influential persons in France, Prussia, Austria, Russia and Spain to the effect mentioned above, he adds:—“Well, I will, with God’s assistance during the next twelve months, visit all the large states of Europe, see their potentates or statesmen, and endeavour to enforce those truths which have been irresistible at home. Why should I rust in inactivity? If the public spirit of my countrymen affords me the means of travelling as their missionary, I will be the first ambassador from the people of this country to the nations of the continent. I am impelled to this by an instinctive emotion such as has never deceived me. I feel that I could succeed in making out a stronger case for the prohibitive nations of Europe to compel them to adopt a freer system than I had here to overturn our protection policy.” This programme he fulfilled. He visited in succession France, Spain, Italy, Germany and Russia. He was received everywhere with marks of distinction and honour. In many of the principal capitals he was invited to public banquets, which afforded him an opportunity of propagating those principles of which he was regarded as the apostle. But beside these public demonstrations he sought and found access in private to many of the leading statesmen, in the various countries he visited, with a view to indoctrinate them with the same principles. During his absence there was a general election, and he was returned (1847) for Stockport and for the West Riding of Yorkshire. He chose to sit for the latter.
When Cobden returned from the continent he addressed himself to what seemed to him the logical complement of free trade, namely, the promotion of peace and the reduction of naval and military armaments. His abhorrence of war amounted to a passion. Throughout his long labours in behalf of unrestricted commerce he never lost sight of this, as being the most precious result of the work in which he was engaged,—its tendency to diminish the hazards of war and to bring the nations of the world into closer and more lasting relations of peace and friendship with each other. He was not deterred by the fear of ridicule or the reproach of Utopianism from associating himself openly, and with all the ardour of his nature, with the peace party in England. In 1849 he brought forward a proposal in parliament in favour of international arbitration, and in 1851 a motion for mutual reduction of armaments. He was not successful in either case, nor did he expect to be. In pursuance of the same object, he identified himself with a series of remarkable peace congresses—international assemblies designed to unite the intelligence and philanthropy of the nations of Christendom in a league against war—which from 1848 to 1851 were held successively in Brussels, Paris, Frankfort, London, Manchester and Edinburgh.
On the establishment of the French empire in 1851-1852 a violent panic took possession of the public mind. The press promulgated the wildest alarms as to the intentions of Louis Napoleon, who was represented as contemplating a sudden and piratical descent upon the English coast without pretext or provocation. By a series of powerful speeches in and out of parliament, and by the publication of his masterly pamphlet, 1793 and 1853, Cobden sought to calm the passions of his countrymen. By this course he sacrificed the great popularity he had won as the champion of free trade, and became for a time the best-abused man in England. Immediately afterwards, owing to the quarrel about the Holy Places which arose in the east of Europe, public opinion suddenly veered round, and all the suspicion and hatred which had been directed against the emperor of the French were diverted from him to the emperor of Russia. Louis Napoleon was taken into favour as England’s faithful ally, and in a whirlwind of popular excitement the nation was swept into the Crimean War. Cobden, who had travelled in Turkey, and had studied the condition of that country with great care for many years, discredited the outcry about maintaining the independence and integrity of the Ottoman empire which was the battle-cry of the day. He denied that it was possible to maintain them, and no less strenuously denied that it was desirable even if it were possible. He believed that the jealousy of Russian aggrandizement and the dread of Russian power were absurd exaggerations. He maintained that the future of European Turkey was in the hands of the Christian population, and that it would have been wiser for England to ally herself with them rather than with the doomed and decaying Mahommedan power. “You must address yourselves,” he said in the House of Commons, “as men of sense and men of energy, to the question—what are you to do with the Christian population? for Mahommedanism cannot be maintained, and I should be sorry to see this country fighting for the maintenance of Mahommedanism.... You may keep Turkey on the map of Europe, you may call the country by the name of Turkey if you like, but do not think you can keep up the Mahommedan rule in the country.” The torrent of popular sentiment in favour of war 610 was, however, irresistible; and Cobden and Bright were overwhelmed with obloquy.
At the beginning of 1857 tidings from China reached England of a rupture between the British plenipotentiary in that country and the governor of the Canton provinces in reference to a small vessel or lorcha called the “Arrow,” which had resulted in the English admiral destroying the river forts, burning 23 ships belonging to the Chinese navy and bombarding the city of Canton. After a careful investigation of the official documents, Cobden became convinced that those were utterly unrighteous proceedings. He brought forward a motion in parliament to this effect, which led to a long and memorable debate, lasting over four nights, in which he was supported by Sydney Herbert, Sir James Graham, Gladstone, Lord John Russell and Disraeli, and which ended in the defeat of Lord Palmerston by a majority of sixteen. But this triumph cost him his seat in parliament. On the dissolution which followed Lord Palmerston’s defeat, Cobden became candidate for Huddersfield, but the voters of that town gave the preference to his opponent, who had supported the Russian War and approved of the proceedings at Canton. Cobden was thus relegated to private life, and retiring to his country house at Dunford, he spent his time in perfect contentment in cultivating his land and feeding his pigs.
He took advantage of this season of leisure to pay another visit to the United States. During his absence the general election of 1859 occurred, when he was returned unopposed for Rochdale. Lord Palmerston was again prime minister, and having discovered that the advanced liberal party was not so easily “crushed” as he had apprehended, he made overtures of reconciliation, and invited Cobden and Milner Gibson to become members of his government. In a frank, cordial letter which was delivered to Cobden on his landing in Liverpool, Lord Palmerston offered him the presidency of the Board of Trade, with a seat in the Cabinet. Many of his friends urgently pressed him to accept; but without a moment’s hesitation he determined to decline the proposed honour. On his arrival in London he called on Lord Palmerston, and with the utmost frankness told him that he had opposed and denounced him so frequently in public, and that he still differed so widely from his views, especially on questions of foreign policy, that he could not, without doing violence to his own sense of duty and consistency, serve under him as minister. Lord Palmerston tried good-humouredly to combat his objections, but without success.
But though he declined to share the responsibility of Lord Palmerston’s administration, he was willing to act as its representative in promoting freer commercial intercourse between England and France. But the negotiations for this purpose originated with himself in conjunction with Bright and Michel Chevalier. Towards the close of 1859 he called upon Lord Palmerston, Lord John Russell and Gladstone, and signified his intention to visit France and get into communication with the emperor and his ministers, with a view to promote this object. These statesmen expressed in general terms their approval of his purpose, but he went entirely on his own account, clothed at first with no official authority. On his arrival in Paris he had a long audience with Napoleon, in which he urged many arguments in favour of removing those obstacles which prevented the two countries from being brought into closer dependence on one another, and he succeeded in making a considerable inpression on his mind in favour of free trade. He then addressed himself to the French ministers, and had much earnest conversation, especially with Rouher, whom he found well inclined to the economical and commercial principles which he advocated. After a good deal of time spent in these preliminary and unofficial negotiations, the question of a treaty of commerce between the two countries having entered into the arena of diplomacy, Cobden was requested by the British government to act as their plenipotentiary in the matter in conjunction with Lord Cowley, their ambassador in France. But it proved a very long and laborious undertaking. He had to contend with the bitter hostility of the French protectionists, which occasioned a good deal of vacillation on the part of the emperor and his ministers. There were also delays, hesitations and cavils at home, which were more inexplicable. He was, moreover, assailed with great violence by a powerful section of the English press, while the large number of minute details with which he had to deal in connexion with proposed changes in the French tariff, involved a tax on his patience and industry which would have daunted a less resolute man. But there was one source of embarrassment greater than all the rest. One strong motive which had impelled him to engage in this enterprise was his anxious desire to establish more friendly relations between England and France, and to dispel those feelings of mutual jealousy and alarm which were so frequently breaking forth and jeopardizing peace between the two countries. This was the most powerful argument with which he had plied the emperor and the members of the French government, and which he had found most efficacious with them. But while he was in the midst of the negotiations, Lord Palmerston brought forward in the House of Commons a measure for fortifying the naval arsenals of England, which he introduced in a warlike speech pointedly directed against France, as the source of danger of invasion and attack, against which it was necessary to guard. This produced irritation and resentment in Paris, and but for the influence which Cobden had acquired, and the perfect trust reposed in his sincerity, the negotiations would probably have been altogether wrecked. At last, however, after nearly twelve months’ incessant labour, the work was completed in November 1860. “Rare,” said Mr Gladstone, “is the privilege of any man who, having fourteen years ago rendered to his country one signal service, now again, within the same brief span of life, decorated neither by land nor title, bearing no mark to distinguish him from the people he loves, has been permitted to perform another great and memorable service to his sovereign and his country.”
On the conclusion of this work honours were offered to Cobden by the governments of both the countries which he had so greatly benefited. Lord Palmerston offered him a baronetcy and a seat in the privy council, and the emperor of the French would gladly have conferred upon him some distinguished mark of his favour. But with characteristic disinterestedness and modesty he declined all such honours.
Cobden’s efforts in furtherance of free trade were always subordinated to what he deemed the highest moral purposes—the promotion of peace on earth and goodwill among men. This was his desire and hope as respects the commercial treaty with France. He was therefore deeply disappointed and distressed to find the old feeling of distrust still actively fomented by the press and some of the leading politicians of the country. In 1862 he published his pamphlet entitled The Three Panics, the object of which was to trace the history and expose the folly of those periodical visitations of alarm as to French designs with which England had been afflicted for the preceding fifteen or sixteen years.
When the Civil War threatened to break out in the United States, Cobden was deeply distressed. But after the conflict became inevitable his sympathies were wholly with the North, because the South was fighting for slavery. His great anxiety, however, was that the British nation should not be committed to any unworthy course during the progress of that struggle. And when relations with America were becoming critical and menacing in consequence of the depredations committed on American commerce by vessels issuing from British ports, he brought the question before the House of Commons in a series of speeches of rare clearness and force.
For several years Cobden had been suffering severely at intervals from bronchial irritation and a difficulty of breathing. Owing to this he had spent the winter of 1860 in Algeria, and every subsequent winter he had to be very careful and confine himself to the house, especially in damp and foggy weather. In November 1864 he went down to Rochdale and delivered a speech to his constituents—the last he ever delivered. That effort was followed by great physical prostration, and he determined not to quit his retirement at Midhurst until spring had fairly set in. But in the month of March there were discussions 611 in the House of Commons on the alleged necessity of constructing large defensive works in Canada. He was deeply impressed with the folly of such a project, and he was seized with a strong desire to go up to London and deliver his sentiments on the subject. He left home on the 21st of March, and caught a chill. He recovered a little for a few days after his arrival in London; but on the 29th there was a relapse, and on the 2nd of April 1865 he expired peacefully at his apartments in Suffolk Street.
On the following day there was a remarkable scene in the House of Commons. When the clerk read the orders of the day Lord Palmerston rose, and in impressive and solemn tones declared “it was not possible for the House to proceed to business without every member recalling to his mind the great loss which the House and country had sustained by the event which took place yesterday morning.” He then paid a generous tribute to the virtues, the abilities and services of Cobden, and he was followed by Disraeli, who with great force and felicity of language delineated the character of the deceased statesman, who, he said, “was an ornament to the House of Commons and an honour to England.” Bright also attempted to address the House, but, after a sentence or two delivered in a tremulous voice, he was overpowered with emotion, and declared he must leave to a calmer moment what he had to say on the life and character of the manliest and gentlest spirit that ever quitted or tenanted a human form.
In the French Corps Législatif, also, the vice-president, Forçade la Roquette, referred to his death, and warm expressions of esteem were repeated and applauded on every side. “The death of Richard Cobden,” said M. la Roquette, “is not alone a misfortune for England, but a cause of mourning for France and humanity.” Drouyn de Lhuys, the French minister of foreign affairs, made his death the subject of a special despatch, desiring the French ambassador to express to the government “the mournful sympathy and truly national regret which the death, as lamented as premature, of Richard Cobden had excited on that side of the Channel.” “He is above all,” he added, “in our eyes the representative of those sentiments and those cosmopolitan principles before which national frontiers and rivalries disappear; whilst essentially of his country, he was still more of his time; he knew what mutual relations could accomplish in our day for the prosperity of peoples. Cobden, if I may be permitted to say so, was an international man.”
He was buried at West Lavington church, on the 7th of April. His grave was surrounded by a large crowd of mourners, among whom were Gladstone, Bright, Milner Gibson, Charles Villiers and a host besides from all parts of the country. In 1866 the Cobden Club was founded in London, to promote free-trade economics, and it became a centre for political propaganda on those lines; and prizes were instituted in his name at Oxford and Cambridge.
Cobden had married in 1840 Miss Catherine Anne Williams, a Welsh lady, and left five surviving daughters, of whom Mrs Cobden-Unwin (wife of the publisher Mr Fisher Unwin), Mrs Walter Sickert (wife of the painter) and Mrs Cobden-Sanderson (wife of the well-known artist in bookbinding), afterwards became prominent in various spheres, and inherited their father’s political interest. His only son died, to Cobden’s inexpressible grief, at the age of fifteen, in 1856.
The work of Cobden, and what is now called “Cobdenism,” has in recent years been subjected to much criticism from the newer school of English economists who advocate a “national policy” (on the old lines of Alexander Hamilton and Friedrich List) as against his cosmopolitan ideals. But it remains the fact that his success with the free-trade movement was for years unchallenged, and, that the leaps and bounds with which English commercial prosperity advanced after the repeal of the corn-laws were naturally associated with the reformed fiscal policy, so that the very name of protectionism came to be identified with all that was not merely heterodox but hateful. The tariff reform movement in England started by Mr Chamberlain (q.v.) had the result of giving new boldness to the opponents of Manchesterism, and the whole subject once more became controversial (see Free Trade; Corn Laws; Protection; Tariff; Economics). Cobden has left a deep mark on English history, but he was not himself a “scientific economist,” and many of his confident prophecies were completely falsified. As a manufacturer, and with the circumstances of his own day before him, he considered that it was “natural” for Great Britain to manufacture for the world in exchange for her free admission of the more “natural” agricultural products of other countries. He advocated the repeal of the corn-laws, not essentially in order to make food cheaper, but because it would develop industry and enable the manufacturers to get labour at low but sufficient wages; and he assumed that other countries would be unable to compete with England in manufactures under free trade, at the prices which would be possible for English manufactured products. “We advocate,” he said, “nothing but what is agreeable to the highest behests of Christianity—to buy in the cheapest market, and sell in the dearest.” He believed that the rest of the world must follow England’s example: “if you abolish the corn-laws honestly, and adopt free trade in its simplicity, there will not be a tariff in Europe that will not be changed in less than five years” (January 1846). His cosmopolitanism—which makes him in the modern Imperialist’s eyes a “Little Englander” of the straitest sect—led him to deplore any survival of the colonial system and to hail the removal of ties which bound the mother country to remote dependencies; but it was, in its day, a generous and sincere reaction against popular sentiment, and Cobden was at all events an outspoken advocate of an irresistible British navy. There were enough inconsistencies in his creed to enable both sides in the recent controversies to claim him as one who if he were still alive would have supported their case in the altered circumstances; but, from the biographical point of view, these issues are hardly relevant. Cobden inevitably stands for “Cobdenism,” which is a creed largely developed by the modern free-trader in the course of subsequent years. It becomes equivalent to economic laisser-faire and “Manchesterism,” and as such it must fight its own corner with those who now take into consideration many national factors which had no place in the early utilitarian individualistic régime of Cobden’s own day.
COBET, CAREL GABRIEL (1813-1889), Dutch classical scholar, was born at Paris on the 28th of November 1813, and educated at the Hague Gymnasium and the university of Leiden. In 1836 he won a gold medal for an essay entitled Prosopographia Xenophontea, a brilliant characterization of all the persons introduced into the Memorabilia, Symposium and Oeconomicus of Xenophon. His Observationes criticae in Platonis comici reliquias (1840) revealed his remarkable critical faculty. The university conferred on him an honorary degree, and recommended him to the government for a travelling pension. The ostensible purpose of his journey was to collate the texts of Simplicius, which, however, engaged but little of his time. He contrived, however, to make a careful study of almost every Greek manuscript in the Italian libraries, and returned after five years with an intimate knowledge of palaeography. In 1846 he married, and in the same year was appointed to an extraordinary professorship at Leiden. His inaugural address, De Arte interpretandi Grammatices et Critices Fundamentis innixa, has been called the most perfect piece of Latin prose written in the 19th century. The rest of his life was passed uneventfully at Leiden. In 1856 he became joint editor of Mnemosyne, a philological review, which he soon raised to a leading position among classical journals. He contributed to it many critical notes and emendations, which were afterwards collected in book form under the titles Novae Lectiones, Variae Lectiones and Miscellanea Critica. In 1875 he took a prominent part at the Leiden Tercentenary, and impressed all his hearers by his wonderful facility in Latin improvisation. In 1884, when his health was failing, he retired as emeritus professor. He died on the 26th of October 1889. Cobet’s special weapon as a critic 612 was his consummate knowledge of palaeography, but he was no less distinguished for his rare acumen and wide knowledge of classical literature. He has been blamed for rashness in the emendation of difficult passages, and for neglecting the comments of other scholars. He had little sympathy for the German critics, and maintained that the best combination was English good sense with French taste. He always expressed his obligation to the English, saying that his masters were three Richards—Bentley, Porson and Dawes.
COBHAM, a village in the Medway parliamentary division of Kent, England, 4 m. W. of Rochester. The church (Early English and later, and restored by Sir G.G. Scott) is famous for its collection of ancient brasses, of which thirteen belonging to the years 1320-1529 commemorate members of the Brooke and Cobham families. There are some fine oak stalls and some tilting armour of the 14th century in the chancel. Cobham college, containing 20 almshouses, took the place, after the dissolution, of a college for priests founded by Sir John de Cobham in the 14th century. The present mansion of Cobham Hall is mainly Elizabethan. The picture gallery contains a fine collection of works by the great masters, Italian, Dutch and English.
The Cobham family was established here before the reign of King John. In 1313 Henry de Cobham was created Baron Cobham, but on the execution of Sir John Oldcastle (who had been summoned to parliament, jure uxoris, as Baron Cobham) in 1417, the barony lay dormant till revived in 1445 by Edward, son of Sir Thomas Brooke and Joan, grand-daughter of the 3rd Baron Cobham. In 1603 Henry Brooke, Lord Cobham, was arraigned for participation in the Raleigh conspiracy, and spent the remainder of his life in prison, where he died in 1618. With him the title expired, and Cobham Hall was granted to Lodowick Stewart, duke of Lennox, passing subsequently by descent and marriage to the earls of Darnley. The present Viscount Cobham (cr. 1718) belongs to the Lyttelton family (see Lyttelton, 1st Baron).
COBIJA, or Puerto La Mar (the official title given to it by the Bolivian government), a port and town of the Chilean province of Antofagasta, about 800 m. N. of Valparaiso. It is the oldest port on this part of the coast, and was for a time the principal outlet for a large mining district. It was formerly capital of the Bolivian department of Atacama and the only port possessed by Bolivia, but the seizure of that department in 1879 by Chile and the construction of the Antofagasta and Oruro railway deprived it of all importance, and its population, estimated at 6000 in 1858, has fallen to less than 500. Its harbour is comparatively safe but lacks landing facilities. Smelting for neighbouring mines is still carried on, and some of its former trade remains, but the greater part of it has gone to Tocopilla and Antofagasta. The town occupies a narrow beach between the sea and bluffs, and was greatly damaged by an earthquake and tidal wave in 1877.
COBLE (probably of Celtic origin, and connected with the root ceu or cau, hollow; cf. Welsh ceubol, a ferry-boat), a flat-bottomed fishing-boat, with deep-lying rudder and lug-sail, used off the north-east coast of England.
COBLENZ (Koblenz), a city and fortress of Germany, capital of the Prussian Rhine Province, 57 m. S.E. from Cologne by rail, pleasantly situated on the left bank of the Rhine at its confluence with the Mosel, from which circumstance it derived its ancient name Confluentes, of which Coblenz is a corruption. Pop. (1885) 31,669; (1905) 53,902. Its defensive works are extensive, and consist of strong modern forts crowning the hills encircling the town on the west, and of the citadel of Ehrenbreitstein (q.v.) on the opposite bank of the Rhine. The old city was triangular in shape, two sides being bounded by the Rhine and Mosel and the third by a line of fortifications. The last were razed in 1890, and the town was permitted to expand in this direction. Immediately outside the former walls lies the new central railway station, in which is effected a junction of the Cologne-Mainz railway with the strategical line Metz-Berlin. The Rhine is crossed by a bridge of boats 485 yds. long, by an iron bridge built for railway purposes in 1864, and, a mile above the town, by a beautiful bridge of two wide and lofty spans carrying the Berlin railway referred to. The Mosel is spanned by a Gothic freestone bridge of 14 arches, erected in 1344, and also by a railway bridge.
The city, down to 1890, consisted of the Altstadt (old city) and the Neustadt (new city) or Klemenstadt. Of these, the Altstadt is closely built and has only a few fine streets and squares, while the Neustadt possesses numerous broad streets and a handsome frontage to the Rhine. In the more ancient part of Coblenz are several buildings which have an historical interest. Prominent among these, near the point of confluence of the rivers, is the church of St Castor, with four towers. The church was originally founded in 836 by Louis the Pious, but the present Romanesque building was completed in 1208, the Gothic vaulted roof dating from 1498. In front of the church of St Castor stands a fountain, erected by the French in 1812, with an inscription to commemorate Napoleon’s invasion of Russia. Not long after, the Russian troops occupied Coblenz; and St Priest, their commander, added in irony these words—“Vu et approuvé par nous, Commandant Russe de la Vitte de Coblence: Janvier 1er, 1814.” In this quarter of the town, too, is the Liebfrauenkirche, a fine church (nave 1250, choir 1404-1431) with lofty late Romanesque towers; the castle of the electors of Trier, erected in 1280, which now contains the municipal picture gallery; and the family house of the Metternichs, where Prince Metternich, the Austrian statesman, was born in 1773. In the modern part of the town lies the palace (Residenzschloss), with one front looking towards the Rhine, the other into the Neustadt. It was built in 1778-1786 by Clement Wenceslaus the last elector of Trier, and contains among other curiosities some fine Gobelin tapestries. From it some pretty gardens and promenades (Kaiserin Augusta Anlagen) stretch along the bank of the Rhine, and in them is a memorial to the poet Max von Schenkendorf. A fine statue to the empress Augusta, whose favourite residence was Coblenz, stands in the Luisen-platz. But of all public memorials the most striking is the colossal equestrian statue of the emperor William I., erected by the Rhine provinces in 1897, standing on a lofty and massive pedestal, at the point where the Rhine and Mosel meet. Coblenz has also handsome law courts, government buildings, a theatre, a museum of antiquities, a conservatory of music, two high grade schools, a hospital and numerous charitable institutions. Coblenz is a principal seat of the Mosel and Rhenish wine trade, and also does a large business in the export of mineral waters. Its manufactures include pianos, paper, cardboard, machinery, boats and barges. It is an important transit centre for the Rhine railways and for the Rhine navigation.
Coblenz (Confluentes, Covelenz, Cobelenz) was one of the military posts established by Drusus about 9 b.c. Later it was frequently the residence of the Frankish kings, and in 860 and 922 was the scene of ecclesiastical synods. At the former of these, held in the Liebfrauenkirche, took place the reconciliation of Louis the German with his half-brother Charles the Bald. In 1018 the city, after receiving a charter, was given by the emperor Henry II. to the archbishop of Trier (Treves), and it remained in the possession of the archbishop-electors till the close of the 18th century. In 1249-1254 it was surrounded with new walls by Archbishop Arnold II. (of Isenburg); and it was partly to overawe the turbulent townsmen that successive archbishops built and strengthened the fortress of Ehrenbreitstein (q.v.) that dominates the city. As a member of the league of the Rhenish cities which took its rise in the 13th century, Coblenz attained to great prosperity; and it continued to advance till the disasters of the Thirty Years’ War occasioned a rapid decline. After Philip Christopher, elector of Trier, had surrendered Ehrenbreitstein to the French the town received an imperial garrison (1632), which was soon, however, expelled by the Swedes. They in their turn handed the city over to the French, but the imperial forces succeeded in retaking it by 613 storm (1636). In 1688 it was besieged by the French under Marshal de Boufflers, but they only succeeded in bombarding the Altstadt into ruins, destroying among other buildings the old merchants’ hall (Kaufhaus), which was restored in its present form in 1725. In 1786 the elector of Trier, Clement Wenceslaus of Saxony, took up his residence in the town, and gave great assistance in its extension and improvement; a few years later it became, through the invitation of his minister, Ferdinand, Freiherr von Duminique, one of the principal rendezvous of the French émigrés. This drew down upon the archbishop-elector the wrath of the French republicans; in 1794 Coblenz was taken by the Revolutionary army under Marceau (who fell during the siege), and, after the peace of Lunéville, it was made the chief town of the Rhine and Mosel department (1798). In 1814 it was occupied by the Russians, by the congress of Vienna it was assigned to Prussia, and in 1822 it was made the seat of government of the Rhine province.
COBOURG, the capital of Northumberland county, Ontario, Canada, on Lake Ontario and the Grand Trunk railway; 70 m. E.N.E. of Toronto. Pop. (1901) 4239. It has a large, safe harbour, and steamboat communication with St Lawrence and Lake Ontario ports. It contains car-works, foundries, and carpet and woollen factories, and is a summer resort, especially for Americans. Victoria University, formerly situated here, was removed to Toronto in 1890.
 |
| Head of Cobra. |
COBRA (Naja tripudians), a poisonous Colubrine snake, belonging to the family Elapidae, known also as the hooded snake, cobra di capello or naga. In this genus the anterior ribs are elongated, and by raising and bringing forward these, the neck can be expanded at will into a broad disk or hood. It possesses two rows of palatine teeth in the upper jaw, while the maxillary bones bear the fangs, of which the anterior one only is in connexion with the poison gland, the others in various stages of growth remaining loose in the surrounding flesh until the destruction of the poison fang brings the one immediately behind to the front, which then gets anchylosed to the maxillary bone, and into connexion with the gland secreting the poison, which in the cobra is about the size of an almond. Behind the poison fangs there are usually one or two ordinary teeth. The cobra attains a length of nearly 6 ft. and a girth of about 6 in.
The typical cobra is yellowish to dark-brown, with a black and white spectacle-mark on the back of the hood, and with a pair of large black and white spots on the corresponding under-surface. There are, however, many varieties, in some of which the spectacle markings on the hood are wanting. The cobra may be regarded as nocturnal in its habits, being most active by night, although not unfrequently found in motion during the day. It usually conceals itself under logs of wood, in the roofs of huts and in holes in old walls and ruins, where it is often come upon inadvertently, inflicting a death wound before it has been observed. It feeds on small quadrupeds, frogs, lizards, insects and the eggs of birds, in search of which it sometimes ascends trees. When seeking its prey it glides slowly along the ground, holding the anterior third of its body aloft, with its hood distended, on the alert for anything that may come in its way. “This attitude,” says Sir J. Fayrer, “is very striking, and few objects are more calculated to inspire awe than a large cobra when, with his hood erect, hissing loudly and his eyes glaring, he prepares to strike.” It is said to drink large quantities of water, although like reptiles in general it will live for many months without food or drink. The cobra is oviparous; and its eggs, which are from 18 to 25 in number, are of a pure white colour, somewhat resembling in size and appearance the eggs of the pigeon, but sometimes larger. These it leaves to be hatched by the heat of the sun. It is widely distributed, from Transcaspia to China and to the Malay Islands, and is found in all parts of India, from Ceylon to the Himalayas up to about 8000 ft. above the level of the sea.
Closely allied is N. haje, the common hooded cobra of all Africa, the Spy-slange, i.e. spitting snake of the Boers.
The cobra is justly regarded as one of the most deadly of the Indian Thanatophidia. Many thousand deaths are caused annually by this unfortunately common species, but it is difficult to obtain accurate statistics. The bite of a vigorous cobra will often prove fatal in a few minutes, and as there is no practicable antidote to the poison, it is only in rare instances that such mechanical expedients as cauterizing, constriction or amputation can be applied with sufficient promptitude to prevent the virus from entering the circulation. Owing to a small reward offered by the Indian government for the head of each poisonous snake, great numbers of cobras have been destroyed; but only low-caste Hindus will engage in such work, the cobra being regarded by the natives generally with superstitious reverence, as a divinity powerful to injure, and therefore to be propitiated; and thus oftentimes when found in their dwellings this snake is allowed to remain, and is fed and protected. “Should fear,” says Sir J. Fayrer, “and perhaps the death of some inmate bitten by accident, prove stronger than superstition, it may be caught, tenderly handled, and deported to some field, where it is released and allowed to depart in peace, not killed” (Thanatophidia of India). Great numbers, especially of young cobras, are killed by the adjutant birds and by the mungoos—a small mammal which attacks it with impunity, apparently not from want of susceptibility to the poison, but by its dexterity in eluding the bite of the cobra. Mere scratching or tearing does not appear to be sufficient to bring the poison from the glands; it is only when the fangs are firmly implanted by the jaws being pressed together that the virus enters the wound, and in those circumstances it has been shown by actual experiment that the mungoos, like all other warm-blooded animals, succumbs to the poison. In the case of reptiles, the cobra poison takes effect much more slowly, while it has been proved to have no effect whatever on other venomous serpents.
In the Egyptian hieroglyphics the cobra occurs constantly with the body erect and hood expanded; its name was ouro, which signifies “king,” and the animal appears in Greek literature as ouraios and basiliscus. With the Egyptian snake-charmers of the present day the cobra is as great a favourite as with their Hindu colleagues. They pretend to change the snake into a rod, and it appears that the supple snake is made stiff and rigid by a strong pressure upon its neck, and that the animal does not seem to suffer from this operation, but soon recovers from the cataleptic fit into which it has been temporarily thrown.
The cobra is the snake usually exhibited by the Indian jugglers, who show great dexterity in handling it, even when not deprived of its fangs. Usually, however, the front fang at least is extracted, the creature being thus rendered harmless until the succeeding tooth takes its place, and in many cases all the fangs, with the germs behind, are removed—the cobra being thus rendered innocuous for life. The snake charmer usually plays a few simple notes on the flute, and the cobra, apparently delighted, rears half its length in the air and sways its head and body about, keeping time to the music.
The cobra, like almost all poisonous snakes, is by no means aggressive, and when it gets timely warning of the approach of man endeavours to get out of his way. It is only when trampled upon inadvertently, or otherwise irritated, that it attempts to use its fangs. It is a good swimmer, often crossing broad rivers, and probably even narrow arms of the sea, for it has been met with at sea at least a quarter of a mile from land.
COBURG, a town of Germany, the twin capital with Gotha of the duchy of Saxe-Coburg-Gotha, on the left bank of the Itz, an affluent of the Regen, on the southern slope of the Frankenwald, the railway from Eisenach to Lichtenfels, and 40 m. S.S.E. of Gotha. Pop. (1905) 22,489. The town is for the most part 614 old, and contains a number of interesting buildings. The ducal palace, known as the Ehrenburg, is a magnificent building, originally erected on the site of a convent of bare-footed friars by Duke John Ernest in 1549, renovated in 1698, and restored in 1816 by Duke Ernest I. It contains a vast and richly decorated hall, the court church and a fine picture gallery. In the gardens are the mausoleum of Duke Francis (d. 1806) and his wife, a bronze equestrian statue of Duke Ernest II. and a fountain in commemoration of Duke Alfred (duke of Edinburgh). In the market square are the medieval Rathaus, the government buildings, and a statue of Prince Albert (consort of Queen Victoria), by William Theed the younger (1804-1891). In the Schloss-platz are the Edinburgh Palace (Palais Edinburg), built in 1881, the theatre and an equestrian statue of Duke Ernest I. Among the churches the most remarkable is the Moritzkirche, with a lofty tower. The educational establishments include a gymnasium, founded in 1604 by Duke John Casimir (d. 1633) and thus known as the Casimirianum, a commercial, an agricultural and other schools. The Zeughaus (armoury) contains the ducal library of 100,000 volumes, and among other public buildings may be mentioned the Augustenstift, formerly the seat of the ministerial offices, and the Marstall (royal mews). On a commanding eminence above the town is the ancient castle of Coburg, dating from the 11th century (see below). In 1781 it was turned into a penitentiary and lunatic asylum, but in 1835-1838 was completely restored, and now contains a natural history museum. The most interesting room in this building is that which was occupied by Luther in 1530, where the surroundings may have inspired, though (as is now proved) he did not compose, the famous hymn, Ein’ feste Burg ist unser Gott; the bed on which he slept, and the pulpit from which he preached in the old chapel are shown. Coburg is a place of considerable industry, the chief branches of the latter being brewing, manufactures of machinery, colours and porcelain, iron-founding and saw-milling; and there is an important trade in the cattle reared in the neighbourhood. Among various places of interest in the vicinity are the ducal residences of Callenberg and Rosenau, in the latter of which Albert, Prince Consort, was born in 1819; the castle of Lauterburg; and the village of Neuses, with the house of the poet J. M. F. Rückert, who died here in 1866, and on the other side of the river the tomb of the poet Moritz August von Thümmel (1738-1817).
The town of Coburg, first mentioned in a record of 1207, owed its existence and its name to the castle, and in the 15th and 16th centuries was of considerable importance as a halting-place on the great trade route from Nuremberg via Bamberg to the North. In 1245 the castle became the seat of the elder branch of the counts of Henneberg (Coburg-Schmalkalden). The countships of Coburg and Schmalkalden passed by the marriage of Jutta, daughter of Hermann I. (d. 1290), to Otto V. of Brandenburg, whose grandson John, however, sold them to Henry VIII. of Henneberg, his brother-in-law. Henry’s daughter Catherine (d. 1397) married Frederick III. of Meissen, and so brought the castle, town and countship into the possession of the Saxon house of Wettin. In 1549 Duke John Ernest of Saxony made Coburg his residence and turned the old castle into a fortress strong enough to stand a three years’ siege (1632-1635) during the Thirty Years’ War. In 1641 Coburg fell to the dukes of Saxe-Altenburg. In 1835 it became the residence of the dukes of Saxe-Coburg. For the princes of the house of Coburg see Wettin And Saxe-Coburg.
COCA, or Cuca (Erythroxylon coca), a plant of the natural order Erythroxylaceae, the leaves of which are used as a stimulant in the western countries of South America.1 It resembles a blackthorn bush, and grows to a height of 6 or 8 ft. The branches are straight, and the leaves, which have a lively green tint, are thin, opaque, oval, more or less tapering at the extremities. A marked characteristic of the leaf is an areolated portion bounded by two longitudinal curved lines one on each side of the midrib, and more conspicuous on the under face of the leaf. Good samples of the dried leaves are uncurled, are of a deep green on the upper, and a grey-green on the lower surface, and have a strong tea-like odour; when chewed they produce a sense of warmth in the mouth, and have a pleasant, pungent taste. Bad specimens have a camphoraceous smell and a brownish colour, and lack the pungent taste. The flowers are small, and disposed in little clusters on short stalks; the corolla is composed of five yellowish-white petals, the anthers are heart-shaped, and the pistil consists of three carpels united to form a three-chambered ovary. The flowers are succeeded by red berries. The seeds are sown in December and January in small plots (almacigas) sheltered from the sun, and the young plants when from 1½ to 2 ft. in height are placed in holes (aspi), or, if the ground is level, in furrows (uachos) in carefully-weeded soil. The plants thrive best in hot, damp situations, such as the clearings of forests; but the leaves most preferred are obtained in drier localities, on the sides of hills. The leaves are gathered from plants varying in age from one and a half to upwards of forty years. They are considered ready for plucking when they break on being bent. The first and most abundant harvest is in March, after the rains; the second is at the end of June, the third in October or November. The green leaves (matu) are spread in thin layers on coarse woollen cloths and dried in the sun; they are then packed in sacks, which, in order to preserve the quality of the leaves, must be kept from damp.
In the Kew Bulletin for January 1889 is an account of the history and botany of the plant, which has been so long under cultivation in South America that its original home is doubtful. As the result of this cultivation numerous forms have arisen. The writer distinguishes from the typical Peruvian form with pointed leaves a variety novo-granatense, from New Granada, which has smaller leaves with a rounded apex. The plant is now cultivated in the West Indies, India, Ceylon, Java and elsewhere. It has been estimated that coca is used by about 8,000,000 of the human race, being consumed in Bolivia, Peru, Ecuador, Colombia and Rio Negro. In Peru the Indians carry a leathern pouch (the chuspa or huallqui) for the leaves, and a supply of pulverized unslaked lime, or a preparation of the ashes of the quinoa plant (Chenopodium Quinoa), called llipta or llucta. Three or four times a day labour is suspended for chacchar or acullicar, as the mastication of coca is termed. The leaves, deprived of their stalks, are chewed and formed into a ball (acullico) in the mouth; a small quantity of the lime or llipta is then applied to the acullico to give it a proper relish. Two or three ounces of coca are thus daily consumed by each Indian.
Coca was used by the Peruvian Indians in the most ancient times. It was employed as an offering to the sun, or to produce smoke at the great sacrifices; and the priests, it was believed, must chew it during the performance of religious ceremonies, otherwise the gods would not be propitiated. Coca is still held in superstitious veneration among the Peruvians, and is believed by the miners of Cerro de Pasco to soften the veins of ore, if masticated and thrown upon them.
The composition of different specimens of coca leaves is very inconstant. Besides the important alkaloid cocaine (q.v.), occurring to the extent of about O.2% in fresh specimens, there are several other alkaloids. The preparations of coca leaves are incompatible with certain drugs which might often be prescribed in combination with them, such as salts of mercury, menthol and mineral acids, which latter decompose cocaine into benzoic acid and ecgonine.
Coca leaves and preparations of them have no external action. Internally their action is similar to that of opium, though somewhat less narcotic, and causing a dilatation of the pupil of the eye instead of a contraction. When masticated, the leaves first cause a tingling in the tongue and mucous membrane of the mouth, owing to a stimulation of the nerves of common sensation, and then abolish taste owing to a paralysis of the terminals of the gustatory nerves. They have a definite anaesthetic action 615 upon the mucous membrane of the stomach, from which there come in large part those organic sensations which we interpret as hunger. Hence it is possible, under the influence of coca, to go without food or consciousness of needing it, for as long a period as three days. The drug is not a food, however, as its composition and history in the body clearly show, and the individual who comfortably fasts under its influence nevertheless shows all the physical signs of starvation, such as loss of weight. In small doses coca stimulates the intestinal peristalsis and thus is an aperient, but in large doses it paralyses the muscular coat of the bowel, causing constipation, such as is constantly seen in coco-maniacs, and in those inhabitants of Peru and the adjacent countries who take it in excess or are markedly susceptible to its influence.
The injection of coca leaves has a very remarkable effect upon the higher tracts of the nervous system—an effect curiously contrary to that produced by their chief ingredient upon the peripheral parts of the nervous apparatus. The mental power is, at any rate subjectively, enhanced in marked degree. In the absence of extended experiments in psychological laboratories, such as have been conducted with alcohol, it is not possible to say whether the apparent enhancement of the intellect is an objectively demonstrable fact. The physical power is unquestionably increased, such muscular exercises as are involved in ascending mountains being made much easier after the chewing of an ounce or so of these leaves. Excess in coca-chewing leads in many cases to great bodily wasting, mental failure, insomnia, weakness of the circulation and extreme dyspepsia. For other pharmacological characters and the therapeutic employments of coca see Cocaine.
1 Garcilasso de la Vega, writing of the plant, says that it is called cuca by the Indians, coca by the Spaniards; and Father Blas Valera states that the leaves are called cuca both by Indians and Spaniards (The Royal Commentaries of the Yncas, 1609-1617; trans, by C. R. Markham, Hakluyt Soc., 1871). See also, on the name cuca, Christison, Brit. Med. Journ., April 29, 1876, p. 527.
COCAINE, C17H21NO4, an alkaloid occurring to the extent of about 1% in the leaves of Erythroxylon coca (see above). It is associated with many other alkaloids: cinnamyl cocaine, C19H23NO4; α-truxilline, (C19H23NO4)2; β-truxilline, (C19H23NO4)2; benzoylecgonine, C16H19NO2; tropa-cocaine, C15H19NO2; hygrine, C8H15NO; cuscohygrine, C13H24NO2. These substances, which may be collectively termed “cocaines,” are all derivatives of ecgonine (q.v.). Cocaine is benzoylmethyl ecgonine. It crystallizes from alcohol in prisms, which are sparingly soluble in water. Its solution has a bitter taste, alkaline reaction, and is laevorotatory. Its use as a local anaesthetic (see Anaesthesia) makes it the most valuable of the coca alkaloids, and it is much used in ophthalmic practice. Applied to the conjunctiva it causes anaesthesia, dilatation of the pupil, diminution of the intraocular tension, and some interference with accommodation. The conversion of the mixture obtained by extracting coca-leaves into cocaine is effected by saponifying the esters into ecgonine and the respective acids, and then benzoylating and methylating the ecgonine. Homologues of cocaine—ethylbenzoylecgonine, &c.—have been prepared; they closely resemble natural cocaine. Cinnamyl cocaine is cinnamylmethylecgonine, i.e. cocaine in which the benzoyl group is replaced by the cinnamyl group. α- and β-truxillines, named from their isolation from a coca of Truxillo (Peru), are two isomeric alkaloids which hydrolyse to ecgonine, methyl alcohol, and two isomeric acids, the truxillic acids, C18H16O4. The alkaloids are therefore methyl truxillylecgonines. The truxillic acids have been studied by K. Liebermann and his students (Ber., vols. 21-27, and 31), and are diphenyl tetramethylene dicarboxylic acids.
COCANADA, or Coconada, a town of British India, in the Godavari district of Madras, on the coast in the extreme north of the Godavari delta, about 315 m. N. of Madras. Pop. (1901) 48,096, showing an increase of 18% in the decade. As the administrative headquarters of the district, and the chief port on the Coromandel coast after Madras, Cocanada was formerly of considerable importance, but its shipping trade has declined, owing to the silting of the anchorage, and to the construction of the railway. It is connected by navigable channels with the canal system of the Godavari delta, and by a branch line with Samalkot on the East Coast railway. The anchorage is an open roadstead, with two lighthouses. The chief exports are rice, cotton, sugar and oilseeds. Mills have been established for cleaning rice. The town contains a second-grade college, a high school, and a literary association.
COCCEIUS [strictly Koch], JOHANNES (1603-1669), Dutch theologian, was born at Bremen. After studying at Hamburg and Franeker, where Sixtinus Amama was one of his teachers, he became in 1630 professor of biblical philology at the “Gymnasium illustre” in his native town. In 1636 he was transferred to Franeker, where he held the chair of Hebrew, and from 1643 the chair of theology also, until 1650, when he succeeded Fr. Spanheim the elder as professor of theology at Leiden. He died on the 4th of November 1669. His chief services as an oriental scholar were in the department of Hebrew philology and exegesis. As one of the leading exponents of the “covenant” or “federal” theology, he spiritualized the Hebrew scriptures to such an extent that it was said that Cocceius found Christ everywhere in the Old Testament and Hugo Grotius found him nowhere. He taught that before the Fall, as much as after it, the relation between God and man was a covenant. The first covenant was a “Covenant of Works.” For this was substituted, after the Fall, the “Covenant of Grace,” to fulfil which the coming of Jesus Christ was necessary. He held millenarian views, and was the founder of a school of theologians who were called after him Cocceians. His theology was founded entirely on the Bible, and he did much to promote and encourage the study of the original text. In one of his essays he contends that the observance of the Sabbath, though expedient, is not binding upon Christians, since it was a Jewish institution. His most distinguished pupil was the celebrated Campeius Vitringa. His most valuable work was his Lexicon et Commentarius Sermonis Hebraici et Chaldaici (Leiden, 1669), which has been frequently republished; his theology is fully expounded in his Summa Doctrinae de Foedere et Testamento Dei (1648).
His collected works were published in 12 folio volumes (Amsterdam, 1673-1675). See Herzog-Hauck, Realencyklopädie.
COCCIDIA, an important order of Sporozoa Ectospora, parasites possessing certain very distinctive characters. With one or two possible exceptions, they are invariably intracellular during the entire trophic life of the individual. They always attack tissue-cells, usually of an epithelium, and never blood-corpuscles. Correlated with the advanced degree of parasitism, there is a complete absence of specialization or differentiation of the cell-body, and the trophozoite is quite incapable of any kind of movement. In all cases, so far as known, the life-cycle is digenetic, an asexual generation (produced by schizogony) alternating with a sexual one (gametogony). After conjugation of two highly-differentiated gametes has taken place, a resistant oocyst is formed, which provides for the dispersal of the species; inside this sporogony (spore- and sporozoite-formation) goes on.
Hake (1839) was, perhaps, the first to describe a Coccidian, but he regarded the parasites as pathological cell-products. In 1845 N. Lieberkühn pointed out the resemblances to Gregarines, with which organisms he considered History. Coccidia to be allied. A year later, H. Kloss proved the existence of similar parasites in the snail, and attempted to construct their life-history; this form was subsequently named Klossia helicina by A. Schneider. The asexual part of the life-cycle was first described by Th. Eimer in 1870, for a Coccidian infesting the mouse, which was afterwards elevated by Schneider into a distinct genus Eimeria. The generic name Coccidium was introduced by R. Leuckart in 1879, for the parasite of the rabbit. It was many years, however, before the double character of the life-cycle was realized, and the ideas of L. and R. Pfeiffer, who first suggested the possibility of an alternation of generations, for a long time found no favour. In the first decade of the 20th century great progress was accomplished, thanks largely to the researches of F. Schaudinn and M. Siedlecki, who first demonstrated the occurrence of sexual conjugation in the group; and the Coccidian life-history is now one of the best known among Sporozoa.
Coccidia appear to be confined1 to four great phyla, Vertebrates, 616 Molluscs, Arthropods and Annelids; the first named group furnishes by far the most hosts, the parasites being frequently met with in domestic animals, both birds and mammals. Following Habitat: effects on host. from the casual method of infection, the epithelium of the gut or of its appendages (e.g. the liver [Plate I., fig. 1]) is a very common seat of the parasitic invasion. But in many cases Coccidia are found in other organs, to which they are doubtless carried by lymphatic or circulatory channels. In Molluscs, they often occur in the kidneys (fig. 2); in Insects, they are met with as “coelomic” parasites, the fat-bodies, pericardial cells, &c., being a favourite habitat; even the testis is not free from their attentions in one or two instances, though the ovary appears always immune.
The parasite invariably destroys its host-cell completely. The latter is at first stimulated to abnormal growth and activity and becomes greatly hypertrophied, the nucleus also undergoing karyolytic changes (fig. 4). The fatty materials elaborated by the host-cell are rapidly used up by the Coccidian, as nourishment; and at length the weakened and disorganized cell is no longer able to assimilate but dies and is gradually absorbed by the parasite, becoming reduced to a mere enclosing skin or envelope. In some cases (ex. Cyclospora caryolytica of the mole) the parasite is actually intranuclear, the nucleus becoming greatly swollen and transformed into a huge vacuole containing it.
The effects of a Coccidian infection upon the host as a whole depend largely upon the extent to which endogenous multiplication of the parasites takes place. On the one hand, schizogony may be so limited in extent as not to cause appreciable injury to the host. This seems to be often the case in forms infecting Molluscs and Arthropods. On the other hand, where schizogony is rapid and prolonged, the results are often serious. For, although any one individual only causes the death of a single host-cell, yet the number of the parasites may be so enormously increased by this means, that the entire affected epithelium may be overrun and destroyed. Thus are occasioned grave attacks of coccidiosis, characterized by severe enteritis and diarrhoea, which may end fatally. In the case of the Vertebrates, secondary causes, resulting from the stoppage of the bile ducts, also help to produce death. There is, however, one factor in the endangered animal’s favour. Schizogony cannot go on indefinitely; it has a limit, dependent upon the supply of host-cells, and consequently of nutriment, available. As this shows signs of becoming exhausted, by the rapid multiplication of the parasites, the latter begin to make preparations for the exogenous cycle, inaugurated by gametogony. When conjugation has taken place and sporogony is begun, the danger to the host is at an end. So that, if the acute stage of the disease is once successfully passed, the regenerative capacity of the epithelium may be able to restore something like equilibrium to the deranged metabolism in time to prevent collapse.
Coccidium schubergi, parasitic in the intestine of a centipede (Lithobius forficatus), may be taken as an example of a Coccidian life-history (see Schaudinn, 1900): some of the more important variations exhibited by other forms will be Morphology and life-history. noted afterwards. The trophozoite, or actively-growing parasite, is an oval or rounded body (fig. 3, I.). The general cytoplasm shows no differentiation into ectoplasm and endoplasm; it is uniformly alveolar in character. The nucleus is relatively large, and possesses a distinct membrane and a well-marked reticulum in which are embedded grains of chromatin. Its most conspicuous feature is the large deeply-staining karyosome, which consists of the greater part of the chromatin of the nucleus intimately bound up with a plastinoid basis. When fully grown, the trophozoite (now a schizont) undergoes schizogony. Its nucleus divides successively to form a number of nuclei, which travel to the periphery, and there become more or less regularly disposed (fig. 3, II. and III.). The protoplasm in the neighbourhood of each next grows out, as a projecting bud, carrying the nucleus with it. In this manner are formed a number of club-shaped bodies, the merozoites, which are at length set free from the parent-body (IV.), leaving a certain amount of residual cytoplasm behind. By the rupture of the disorganized host-cell,2 the fully-formed merozoites are liberated into the intestinal lumen, and seek out fresh epithelial cells. Each is more or less sickle-shaped, and capable of active movements. Once inside a new host-cell, the merozoite grows to a schizont again.
After this course has been repeated several times, gametogony sets in, the trophozoites growing more slowly and becoming the parent-cells of the sexual elements (gametocytes), either male individuals (microgametocytes) or female ones (megagametocytes). A microgametocyte (fig. 3, VI. ♂) is characterized by its dense but finely reticular or alveolar cytoplasm, very different from the loose structure of that of a schizont. The male elements (microgametes) are formed in a manner essentially comparable to that in which the formation of merozoites takes place. Although the details of the nuclear changes and divisions vary somewhat, the end-result is similar, a number of little nuclear agglomerations being evenly distributed at the surface (VII. ♂) Each of these elongates considerably, becoming comma-shaped and projecting from the gametocyte. Nearly all the body of the male gamete (VIII. ♂) consists of chromatin, the cytoplasm only forming a very delicate zone or envelope around the nucleus. From the cytoplasm two long fine flagella grow out, one of which originates at the anterior end, the other, apparently, at the hinder end, acting as a rudder; but it is probable that this also is developed at the anterior end and attached to the side of the body. By means of their flagella the numerous microgametes break loose from the body of the microgametocyte and swim away in search of a female element.
A megagametocyte (VI. ♀) is distinguished by its rather different shape, being more like a bean than a sphere until ripe for maturation, and by the fact that it stores up in its cytoplasm quantities of reserve nutriment in the form of rounded refringent plastinoid grains. Each female gametocyte gives rise to only a single female element (megagamete), after a process of nuclear purification. The karyosome is expelled from the nucleus into the cytoplasm, where it breaks up at once into fragments (VII. ♀). Meanwhile the gametocyte is becoming spherical, and its changes in shape aid in setting it free from the shrivelled host-cell. The fragments of the karyosome, which are, as it were, squeezed out to the exterior, exert a powerful attraction upon the microgametes, many of which swarm round the now mature megagamete. The female nucleus (pronucleus) approaches the surface of the cell (VIII. ♀), and at this spot a little clear cytoplasmic prominence arises (cone of reception). On coming into contact with this protuberance (probably attracted to it by the female pronucleus), a microgamete adheres. Partly by its own movements and partly by the withdrawal of the cone of attraction, the male penetrates into the female element and fertilization is accomplished. Only one microgamete can thus pass into the megagamete, for immediately its entry is effected a delicate membrane is secreted around the copula (zygote), which effectually excludes other less fortunate ones. This membrane rapidly increases in thickness and becomes the oocyst (IX.), and the copula is now ready to begin sporogony.
Sporogony goes on indifferently either inside the host or after the cyst has been passed out with the faeces to the exterior. The definitive nucleus of the zygote (resulting from the intimate fusion of the male and female pronuclei, by means of a somewhat elaborate “fertilization-spindle” [X.]) gives rise by successive direct divisions to four nuclei (XII.), around which the protoplasm becomes segregated; these segments form the four sporoblasts. Around each sporoblast two membranes are successively secreted (exospore and endospore), which constitute the sporocyst (XIII.); the sporocyst and its contents forming the spore. The nucleus of each spore next divides, again directly, and this is followed by the division of the cytoplasm. As a final result, each of the four spores contains two germs (sporozoites), and a certain amount of residual protoplasm (fig. 3, XIV.); this latter encloses a viscid, vacuole-like body, which aids in the subsequent dehiscence of the sporocyst. On being eaten by a fresh host, the wall of the oocyst is dissolved at a particular region by the digestive juices, which are thus enabled to reach the spores and cause the rupture of the sporocysts. As the result of instructive experiments, Metzner has shown that it is the pancreatic and not the gastric juice by which this liberation of the germs is effected. The liberated sporozoites creep out and proceed to infect the epithelial cells. The sporozoites (XV.) are from 15-20 µ long by 4-6 µ wide; they are fairly similar to merozoites in form, structure and behaviour, the chief point of distinction being that they have no karyosome in the nucleus (cf. above).
Plate I.
 |
| Fig. 1.—SECTION THROUGH RABBIT’S LIVER, INFECTED WITH COCCIDIUM CUNICULI. (AFTER THOMA.) |
 |
| Fig. 2.—KLOSSIA HELICINA, FROM KIDNEY OF HELIX HORTENSIS. |
a, Portion of a section of the kidney showing normal epithelial cells containing concretions (c), and enlarged epithelial cells containing the parasite (k) in various stages; b, cyst of the Klossia containing sporoblasts; c, cyst with ripe spores, each enclosing four sporozoites and a patch of residual protoplasm. (From Wasielewski, after Balbiani.)
 |
| Fig. 3.—THE LIFE-CYCLE OF COCCIDIUM SCHUBERGI, SCHAUD. (PAR. LITHOBIUS FORFICATUS). (FROM MINCHIN, AFTER SCHAUDINN.) |
I.-IV represents the schizogony, commencing with infection of an epithelial cell by a sporozoite or merozoite. After stage IV the development may start again at stage I, as indicated by the arrows; or it may go on to the formation of gametocytes (V). V-VIII represents the sexual generation. The line of development, hitherto single (I-IV) becomes split into two lines—male (VI ♂, VII ♂, VIII ♂), and female (VI ♀, VII ♀, VIII ♀), culminating in the highly differentiated micro- and mega-gametes. By conjugation these two lines are again united. IX, X, show the formation of the zygote by fusion of the nuclei of the gametes. XI-XV, sporogony. H.C, host-cell; N, its nucleus; mz, merozoite; szt, schizont; ky, karyosome (or fragments of same); n.n, daughter-nuclei of schizont; pl.gr, plastinoid grains; ooc, oocyst; n.zyg, zygote-nucleus (segmentation-nucleus); sp.m, spore-membrane (sporocyst); rp, residual protoplasm of oocyst (“reliquat kystal”); rp.sp, residual protoplasm of spore (“reliquat sporal”); sp.z, sporozoite.
 |
| Fig. 4.—PHASES OF CARYOTROPHA MESNILII, SIEDL. (PAR. POLYMNIA NEBULOSA). |
a, Young schizont in a cluster of spermatogonia; the host-cell (represented granulated) and two of its neighbours are greatly hypertrophied, with very large nuclei, and have fused into a single mass containing the parasite (represented clear, with a thick outline). The other spermatogonia are normal. b, Intracellular schizont divided up into schizontocytes (c), each schizontocyte giving rise to a cluster of merozoites arranged as a “corps en barillet”; spg, spermatogonia; h.c, host-cell; N, nucleus of host-cell or cells; n, nucleus of parasite; szc, schizontocyte; mz, merozoites; r.b, residual bodies of the schizontocytes. (From Minchin, after Siedlecki.)
Plate II.
 |
| Fig. 5.—SCHIZOGONY OF ADELEA OVATA, A. SCHN. (PAR. LITHOBIUS FORFICATUS). |
a-c, ♀ generation; d-f, ♂ generation. a, Full-grown ♀ schizont (megaschizont), with a large nucleus (n) containing a conspicuous karyosome (ky). b, Commencement of schizogony; the nucleus has divided up to form a number of daughter-nuclei (d.n). The karyosome of stage a has broken up into a great number of daughter-karyosomes, each of which forms at first the centre of one of the star-shaped daughter-nuclei; but in a short time the daughter-karyosomes become inconspicuous. c, Completion of schizogony; the ♀ schizont has broken up into a number of megamerozoites (♀ mz) implanted on a small quantity of residual protoplasm (r.p.). Each ♀ merozoite has a chromatic nucleus (n) without a karyosome. d, Full-grown ♂ schizont (microschizont), with nucleus (n), karyosome (ky), and a number of characteristic pigment-granules (p.gr). e, Commencement of schizogony. The nucleus is dividing up into a number of daughter-nuclei (d.n), each with a conspicuous karyosome (ky). f, Completion of schizogony. The numerous micro-merozoites (♂ mz) have each a nucleus with a conspicuous karyosome (ky) at one pole, and the protoplasm contains pigment-granules (p.gr) near the nucleus, on the side farthest from the karyosome. (From Minchin, after Siedlecki.)
 |
| Fig. 6.—ASSOCIATION AND CONJUGATION IN ADELEA OVATA. |
a, Young microgametocyte (♂ gamc.) attached to a megagametocyte (♀ gamc.). The nucleus of the microgametocyte gives rise to 4 daughter-nuclei (c) which become (d) 4 microgametes (♀ gam.). e, One of the microgametes penetrates the megagamete, which forms a fertilization-spindle composed of male and female chromatin (♂ and ♀ chr.). The other 3 microgametes and the residual protoplasm of the microgametocyte (r.p.) perish. The karyosome of the megagamete has disappeared, as such. f, Union of the chromatin of both elements, to produce the zygote-nucleus (n.zyg.). (From Minchin, after Siedlecki.)
 |
| Fig. 7.—SPORES OF VARIOUS COCCIDIAN GENERA. |
a, Minchinia chitonis (E.R.L.), (par. (Chiton); b, Diaspora hydatidea, Léger (par. Polydesmus); c, Echinospora labbei, Léger (par. Lithobius mutabilis); d, Goussia motellae, Labbé; e, Diplospora (Hyaloklossia), lieberkuhni (Labbé), (par. Rana esculenta); f, Crystallospora crystalloides (Thél.), (par. Motella tricirrata). (From Minchin; b and c after Léger, the others after Labbé.)
 |
| Fig. 8.—SPOROGONY AND SPORE-GERMINATION IN BARROUSSIA ORNATA, A. SCH., FROM THE GUT OF NEPA CINERA. |
a, Oocyst with sporoblasts; b, oocyst with ripe spores; c, a spore highly magnified, showing the single sporozoite bent on itself; d, the spore has split along its outer coat or epispore, but the sporozoite is still enclosed in the endospore; e, the sporozoite, freed from the endospore, is emerging; f, the sporozoite has straightened itself out and is freed from its envelopes. (From Wasielewski, after A. Schneider.)
Comparing the life-cycle of other Coccidia with that just described, a greater or less degree of modification is frequently met with. In the process of schizogony two orders of division sometimes occur; the parent-schizont first divides up into a varying number of rounded daughter-schizonts (schizontocytes), each of which gives rise, in the usual manner, to a cluster of merozoites,3 which thus constitute a second order of cells. Siedlecki (1902) has found this to be the case in Caryotropha mesnilii (fig. 4), and Woodcock (1904) has shown that it is most probably really the same process which Smith and Johnson (1902) mistook for sporogony when originally describing their Coccidian of the mouse, Klossiella. In Caryotropha, a perfectly similar state of affairs is seen in the formation of microgametes from the microgametocyte; this is additionally interesting as showing that this process is neither more nor less than male schizogony.
Coming to the sexual generation, considerable variation is met with as regards the period in the life-history when sexual differentiation first makes its appearance. Sexuality may become evident at the very beginning of schizogony, as, e.g. in Adelea ovata (Siedlecki, 1899), where the first-formed schizonts (those developed from the sporozoites) are differentiated into male and female (micro-and mega-schizonts) (see Plate II., fig. 5). Correspondingly, the merozoites, to which they give rise, are also different (micro-and mega-merozoites). In one or two cases sexuality appears even earlier in the cycle, and has thus been carried still farther back.
The Coccidia, as a whole, have not developed the phenomenon of association of the sexual individuals prior to gamete-formation which is so characteristic of Gregarines. Their method of endeavouring to secure successful sporulation, and thus the survival of the species, has been rather by the extreme specialization of the sexual process. In place of many female elements, which the primitive or ancestral forms may be assumed to have had,4 there is always, save possibly for one exception,5 only a single relatively huge megagamete formed, which offers a comparatively easy goal for one of the many microgametes. Nevertheless in the effort to render fertilization absolutely certain, a few Coccidia have acquired (secondarily) the power of associating; a state of things which enables those forms, moreover, to effect an economy in the number of male gametes, only three or four being developed. Instances are seen in Adelea mesnili (Perez, 1903), A. ovata (fig. 6), and Klossia helicina (Siedlecki, 1899). It is very interesting to note that, in the two last cases, unless this association of the microgametocyte with the megagametocyte occurs, neither can the former produce male elements (microgametes) nor can the female individual maturate and become ready for fertilization. (Concerning this question of association see also Gregarines.)
In sporogony, great variation is seen with respect to the number of spores and sporozoites formed; and, as in Gregarines, these characters are largely used for purposes of classification, under which heading they are better considered. Usually, the spores (fig. 7) are quite simple in outline, and not produced into spines or processes; exceptions are found, however, in a few instances (e.g. Minchinia chitonis). In one case (Coccidium mitrarium), the oocyst itself, instead of being spherical, is curiously shaped like a mitre.
The life-history as a whole is invariably undergone in a single host, i.e. there is no alternation of true hosts.6 Schaudinn, in his work on the Coccidia of Lithobius (1900), showed that the oocysts expelled with the faeces may be eaten by wood-lice (Oniscus), but when this happens they pass through the intestine of the wood-louse unaltered, the latter not being an intermediate host but merely a carrier.
The order Coccidiidea is divided into four families, characterized by the number of sporocysts (if any) found in the oocyst.
Classification. Fam. Asporocystidae, Léger. No sporozoites are formed in the oocyst, the sporozoites being unenclosed (gymnospores).
Genus, Légerella, Mesnil. This genus actually conforms to Aimé Schneider’s original definition of Eimeria, which was founded on what were really the schizogonous generations of other forms, then thought to be distinct. In view of the great confusion attending the use of this name, however, Mesnil (1900) has suggested the new one here adopted. Two species known, L. nova and L. testiculi, both from different species of Glomeris, a Myriapod; the former inhabits the Malpighian tubules, the latter the testis.
Fam. Disporocystidae, Léger. The oocyst contains 2 spores.
Genus 1. Cyclospora, A. Schneider. Spores dizoic, i.e. with two sporozoites. C. glomericola, from the intestinal epithelium of Glomeris, and C. caryolytica, from the intestinal epithelium of the mole, intranuclear.
Genus 2. Diplospora, Labbé. Spores tetrazoic. D. lacazei, from many birds, is the best-known species; and others have been described from different Sauropsida. D. lieberkühni is an interesting form occurring in the kidneys of the frog, which it reaches by way of the circulation.
Genus 3. Isospora, Schn. Spores polyzoic. Founded for I. rara, parasitic in the black slug (Limax cinereo-niger). Many authors consider that Schneider was mistaken in attributing many sporozoites to this form, and would unite with it the genus Diplospora.
Fam. Tetrasporocystidae, Léger. The oocyst contains 4 spores.
Genus 1. Coccidium,7 Leuckart. The spores are dizoic and the sporocysts rounded or oval. A very large number of species are known, mostly from Vertebrate hosts. C. cuniculi (= C. oviforme) from the rabbit (intestine and diverticula), but also occurring sometimes in other domestic animals; C. falciformis, from the mouse; C. faurei from sheep; and C. schubergi, from Lithobius (a centipede), are among the best-known forms. All of them may cause disastrous epidemics of coccidiosis.
Genus 2. Paracoccidium, Laveran and Mesnil. This genus is distinguished from Coccidium by the fact that the sporocysts become dissolved up in the oocyst, thus leaving the 8 sporozoites unenclosed, recalling the condition in Légerella. P. prevoti, unique species, from the frog’s intestine.
Genus 3. Crystallospora, Labbé. Spores also dizoic, but having the form of a double pyramid. C. crystalloides from a fish, Motella tricirrata.
Genus 4. Angeiocystis, Brasil. Apparently 6 sporozoites, but the only species, A. audouiniae, has only been briefly described; from a Polychaete (Audouinia).
Fam. Polysporocystidae, Léger. The oocyst contains numerous spores.
There are several genera with monozoic spores, characterized by variations in the form and structure of the sporocysts, e.g. Barroussia, Schn. (fig. 8), Echinospora, Léger, and Diaspora, Léger; most of these forms are from Myriapods.
Genus Adelea, Schn. Dizoic spores; sporocysts round or oval, plain. Several species are included in this well-known genus, among them being A. ovata, A. mesnili, A. dimidiata; most of them are parasitic in Insects or Myriapods.
Genus Minchinia, Labbé. Dizoic spores; the sporocysts are produced at each pole into a long filament. M. chitonis, from the liver of Chiton (Mollusca).
Genus Klossia, Schn. The spores are tetrazoic (or perhaps polyzoic). K. helicina from the kidney of various land-snails is the best-known form. Usually said to have 5 to 6 spores, but Mesnil considers that the normal number is 4, as is the case in another species, K. soror.
Genus Caryotropha, Siedlecki. Many spherical spores (about 20) 618 each with 12 sporozoites. C. mesnilii, unique species, from the spermatogonial (testis) cells of Polymnia (a Polychaete). An interesting point in the schizogony is the formation of schizontocytes (see above).
A Coccidian parasitic in the kidneys of the mouse has been described by Smith and Johnson (1902) and named by them Klossiella, on the ground that it possessed many spores, each with about 20 sporozoites. Woodcock has shown, however, that the authors were in all probability dealing with a similar modification of schizogony to that which obtains in Caryotropha. The sporogony of this form (and hence its systematic position) remains at present, therefore, quite unknown.
There are several doubtful or insufficiently known genera, e.g. Bananella, Goussia, Hyaloklossia, Gonobia, Pfeifferella and Rhabdospora, many of which probably represent only schizogonous generations of other forms. (For information concerning these see Labbé, 1897.)
Lastly it remains to mention the extremely interesting forms parasitic in Cephalopods. For some years these have provided a fruitful source of discussion to systematists. Here it may be stated simply that their systematic position and nomenclature were thought to have been finally settled by the researches of Jacquemet (1903) and Lühe (1902) in the following terms:—
Genus Eucoccidium. Lühe (syn. Légerina Jacq.), Coccidia possessing polysporous oocysts and lacking schizogony, parasitic in Cephalopods. Two well-known species: E. eberthi (Labbé), (=Benedenia seu Klossia e. seu octopiana), parasitic in Sepia, which is tri- or tetra-zoic; and E. octopianum (Schn.), (syn. Benedenia seu Klossia o.) from Octopus, which is polyzoic, having 10 to 12 sporozoites. In both forms cysts containing megaspores and megasporozoites, and others containing microspores and microsporozoites are found, considered as representing sexual differentiation thrown back to the very earliest stages of the life-cycle.
Quite recently much additional light has been thrown upon our knowledge of these parasites, including a new one, E. jacquemeti. Moroff (1906) has shown that not one but many megagametes are formed, and fertilized by the microgametes. For this reason he regards them as Gregarines rather than Coccidia. Further, Léger and Duboscq (1906) have found that the characteristic coelomic parasites (Aggregata) of Crustacea, generally regarded as gymnosporous Gregarines (i.e. Gregarines in which the sporozoites are naked) constitute in reality nothing more or less than a schizogonous generation of these Cephalopodan parasites, which have thus an alternation of true hosts. The ripe sporocysts from the Cephalopod are eaten by a particular crab (e.g. Portunus or Inachus, according to the parasite), the sporozoites are liberated and traverse the mucous membrane of the intestine, coming to rest in the surrounding lymphatic layer. Here a large “cyst” is formed, projecting into the body-cavity, the contents of which give rise to a great number of merozoites. On the crab being devoured by the right species of Cephalopod, the merozoites doubtless give rise to the sexual generation again.
As the name Aggregata is much the older, and as, moreover, there is no longer any reason to retain that of Eucoccidium, these parasites must in future receive the former generic appellation. With regard to the various specific names, however, they remain quite unsettled until the life-history is properly worked out in different cases (see also Gregarines).
It seems to the writer a much more open question than Moroff and Léger and Duboscq apparently suppose, whether these parasites are to be relegated to the Gregarines. For undoubtedly they have many Coccidian features, and on the other hand they differ in many ways from Gregarines. The chief feature of agreement with the latter order is the possession of many female gametes. As already said, there can be little doubt that this was the condition in the Coccidian ancestor, and it is by no means impossible that one or two forms existing at the present day remain primitive in that respect. On the other hand, the advanced character of the parasitism (the parasites remaining intracellular up to and including gamete-formation); the entire lack of the characteristic feature of association; the schizogony, which is only a very rare occurrence in Gregarines, and which, in the present case, strongly suggests the process in Caryotropha and Klossiella; and, last but not least, the varying number of the sporozoites (3 in one form, 10-15 in others), which is very different from the almost constant number (8) in Gregarines, are all characters in which these forms agree with Coccidia and not with Gregarines. Having regard to these points, the writer is inclined, for the present, to consider Aggregata as an offshoot rather from the Coccidian than from the Gregarine branch of the Ectosporan tree.
Bibliography.—The following are some of the important papers dealing with the order:—G. Bonnet-Eymard, “Sur l’Évolution de l’Eimeria nova, Schneider,” C.R. Soc. Biol. 52, p. 659, 1900; L. Brasil, “Sur une Coccidie nouvelle, &c.,” C.R.Ac. Sci. 139, p. 645, 1904; L. Cuénot, “Légerella testiculi n. sp., &c.,” Arch. Zool. exp. (N. et R.), (3) 10, p. 49, 6 figs., 1902; M. Jacquemet, “Sur la systématique des Coccidies des Céphalopodes,” Arch. Protistenk. 2 p., 190, 1903; A. Labbé, “Recherches zoologiques, cytologiques et biologiques sur les Coccidies,” Arch. zool. exp. (3), 4, p. 517, 3 pls., 1897; A. Laveran, “Sur les modes de réproduction d’Isospora lacazei,” C.R. Soc. Biol. 50, p. 1139, 1898; A. Laveran and F. Mesnil, “Sur deux Coccidies intestinales de la Rana esculenta,” op. cit. 54, p. 857, 9 figs., 1902; A. Laveran and F. Mesnil, “Sur la Coccidie trouvée dans le rein de la Rana esculenta, &c.,” C.R.Ac. Sci. 135, p. 82, 10 figs., 1902; A. Laveran and F. Mesnil, “Sur quelques Protozoaires parasites d’une tortue, &c.” t. c. p. 609, 14 figs., 1902; L. Léger, “Sur une nouvelle Coccidie à microgamètes ciliés,” op. cit., 127, p. 418, 1898; L. Léger, “Sur la morphologie et le développement des microgamètes des Coccidies,” Arch. zool. exp. (N. et R.) (3), 6, 1898; L. Léger, “Essai sur la classification des Coccidies, &c.,” Ann. Mus. Nat. Hist., Marseille (2), Bull. i. p. 71, 4 pls., 1898; L. Léger “Sur la présence d’une Coccidie coelomique chez Olocrates, &c.,” Arch. zool. exp. (N. et R.) (3), 8, p. i., 1900; L. Léger, “Sur le genre Eimeria et la classification des Coccidies,” C.R. Soc. Biol. 52, p. 575, 1900; L. Léger and O. Duboscq, “Recherches sur les Myriapodes de Corse et leurs parasites,” Arch. zool. exp. (4), 1, p. 307, 24 figs., 1903; L. Léger and O. Duboscq, “Sur l’évolution des Grégarines gymnosporées des Crustacés,” C.R.Ac. Sci. 142, p. 1225, 1906; L. Léger and O. Duboscq, “L’Évolution d’une Aggregata de la seiche chez le Portunus depurator,” C.R. Soc. Biol. 6o, p. 1001, 1906; M. Lühe, “Über Geltung und Bedeutung der Gattungsnamen Eimeria und Coccidium,” C.B. Bakter (1) 31 Orig, p. 771, 1902; C.B. Bakter, “Die Coccidien-Literatur der letzten vier Jahre,” Zool. Centrlbl. 10, 45 pp., 1903; F. Mesnil, “Sur la conservation du nom générique Eimeria, &c.,” C.R. Soc. Biol. 52, p. 603, 1900; F. Mesnil, “Les Travaux récents sur les Coccidies,” Bull. Inst. Pasteur, i. pp. 473, 505, 1903; R. Metzner, “Untersuchungen an Coccidium cuniculi,” Arch. Protistenk. 2, p. 13, pl. ii. 1903; G. Moussu and G. Marotel, “La Coccidiose du mouton et son parasite,” Arch. Parasitol. 6, p. 82, 10 figs., 1902; T. Moroff, “Sur l’évolution des prétendues Coccidies des Céphalopodes,” C.R.Ac. Sci. 142, p. 652, 1906; C. Perez, “Le Cycle évolutif de l’Adelea mesnili, &c.,” Arch. Protistenk. 2, p. 1, pl. 1, 1903; F. Schaudinn, “Untersuchungen über den Generationswechsel bei Coccidien,” Zool. Jahrbücher (Anat.) 13, p. 197, 4 pls., 1900; F. Schaudinn, “Studien über krankheitserregende Protozoen—I. Cyclospora caryolytica, &c.,” Arb. kais. Gesundh.-amte, 18, p. 378, 2 pls., 1902; M. Siedlecki, “Réproduction sexuée ... chez ... Coccidium proprium,” C.R. Soc. Biol. 50, p. 664, figs., 1898; M. Siedlecki, “Étude cytologique ... de la Coccidie de la seiche, &c.,” Ann. Inst. Pasteur, 12, p. 799, 3 pls., 1898; M. Siedlecki, “Étude cytologique ... de Adelea ovata,” op. cit. 13, p. 169, 3 pls., 1899; M. Siedlecki, “Cycle évolutif de la Caryotropha mesnilii, &c.,” Bull. Ac. Cracovie, p. 561, 5 figs., 1902; T. Smith and H. P. Johnson, “On a Coccidian (Klossiella muris, gen. et spec. nov.), &c.,” J. exp. Med. 6, p. 303, 3 pls., 1902; H.M. Woodcock, “Notes on Sporozoa, I. On Klossiella muris, &c.,” Q.J. micr. Sci. 48, p. 153, 2 figs., 1904.
1 A curious organism, parasitic in a gregarine, has lately been described by Dogiel as a coccidian, and termed Hyalosphaera.
2 It is important to note that in schizogony there is never any cyst or cyst-membrane formed around the parasite.
3 The merozoites are frequently arranged like the staves of a barrel—whence the term barillet, which is frequently used.
4 In Cyclospora, Schaudinn (1902) has noted certain abnormal cases of the persistence and further multiplication of the “reduction-nuclei” of the female element (i.e. the nuclear portions given off during maturation), followed by multiple fertilization. This occurrence points strongly to the conclusion that there were originally many female gametes (cf. also the sporoblasts of Gregarines).
5 The remarkable forms parasitic in Cephalopods (of late known as Eucoccidium), if still ranked with the Coccidia, furnish an exception (see below).
6 Again with the exception of Eucoccidium.
7 Purists in systematic nomenclature maintain that this name should be relinquished in favour of Eimeria, since the latter was the first legitimate generic name given to a Coccidian. But one reason against the use of Eimeria has been stated already (it should be used for E. (Légerella) nova, if anywhere); and in addition, the word Coccidium and its important derivatives are now so universally established that it would be little short of ridiculous to displace them.
COCCULUS INDICUS, the commercial name for the dried fruits of Anamirta Cocculus (natural order Menispermaceae), a large climbing shrub, native to India. It contains a bitter poisonous principle, picrotoxin, used in small doses to control the night sweats of phthisis. It was formerly known as Levant nut and Levant shell, owing to the fact that it was brought to Europe by way of the Levant.
COCHABAMBA, a central department of Bolivia, occupying the eastern angle of the great Bolivian plateau, bounded N. by the department of El Beni, E. by Santa Cruz, S. by Chuquisaca and Potosi, and W. by Potosi, Oruro and La Paz. Area, 23,328 sq. m.; pop. (1900) 328,163. Its average elevation is between 8000 and 10,000 ft., and its mean temperature ranges from 50° to 60° F., making it one of the best climatic regions in South America. The rainfall is moderate and the seasons are not strongly marked, the difference being indicated by rainfall rather than by temperature. The rainy season is from November to February. Cochabamba is essentially an agricultural department, although its mineral resources are good and include deposits of gold, silver and copper. Its temperate climate favours the production of wheat, Indian corn, barley and potatoes, and most of the fruits and vegetables of the temperate zone. Coca, cacáo, tobacco and most of the fruits and vegetables of the tropics are also produced. Its forest products include rubber and cinchona. Lack of transportation facilities, however, have been an insuperable obstacle to the development of any industry beyond local needs except those of cinchona and rubber. Sheep and cattle thrive in this region, and an experiment with silkworms gave highly successful results. The population is chiefly of the Indian and mestizo types, education is in a backward state, and there are no manufactures other than those of the domestic stage, the natives making many articles of wearing apparel and daily use in their own homes. Rough highways and 619 mule-paths are the only means of communication, but a projected railway from Cochabamba (city) to Oruro, 132 m., promises to bring this isolated region into touch with the commercial world. The department is divided into nine provinces, but there is no effective local government outside the municipalities. The capital is Cochabamba; other important towns are Punata, Tarata, Totora, Mizque and Sacába.
COCHABAMBA, a city of Bolivia, capital of the department of the same name and of the province of Cercado, situated on the Rocha, a small tributary of the Guapay river, in lat. 17° 27′ S. and long. 65° 46′ W. Pop. (1900) 21,886, mostly Indians and mestizos. The city stands in a broad valley of the Bolivian plateau, 8400 ft. above sea-level, overshadowed by the snow-clad heights of Tunari and Larati, 291 m. north-north-west of Sucre and 132 m. east-north-east of Oruro, with both of which places it is connected by rough mountain roads. A subsidized stage-coach line runs to Oruro. A contract for a railway between the two cities was made in 1906, connecting with the Antofagasta and Arica lines. The climate is mild and temperate, and the surrounding country fertile and cultivated. Cochabamba is often described as the most progressive city of Bolivia, but it has been held back by its isolated situation. The warehouses of the city are well supplied with foreign goods, and trade is active in spite of high prices. The city is provided with telegraphic communication via Oruro, and enjoys a large part of the Amazon trade through some small river ports on tributaries of the Mamoré. The city is regularly laid out, and contains many attractive residences surrounded by gardens. It is an episcopal city (since 1847), containing many churches, four conventual establishments, and a missionary college of the “Propaganda Fide” for the conversion of Indians. The city has a university and two colleges, but they are poorly equipped and receive very little support from the government. Cochabamba was founded in the 16th century, and for a time was called Oropesa. It took an active part in the “war of independence,” the women distinguishing themselves in an attack on the Spanish camp in 1815, and some of them being put to death in 1818 by the Spanish forces. In 1874 the city was seized and partly destroyed by Miguel Aguirre, but in general its isolated situation has been a protection against the disorders which have convulsed Bolivia since her independence.
COCHEM, a town of Germany, in the Prussian Rhine province on the Mosel, and 30 m. W. of Coblenz by the railway to Trier, which above the town enters the longest tunnel (2½ m.) in Germany. Pop. 3500. It is romantically situated in the deep and winding valley of the Mosel, at the foot of a hill surrounded by a feudal castle dating from 1051, which has been restored in its former style. There is a considerable trade in wines.
COCHERY, LOUIS ADOLPHE (1819-1900), French statesman, was born at Paris. After studying law he soon entered politics, and was on the staff of the ministry of justice after the revolution of February 1848. From the coup d’état of 1851 to May 1869 he devoted himself to journalism. Then, elected deputy by the department of the Loiret, he joined the group of the Left Centre, and was a supporter of the revolution of the 4th of September 1870. His talent in finance won him a distinguished place in the chamber. From 1879 till 1885 he was minister of posts and telegraphs, and in January 1888 he was elected to the senate. He died in 1900.
His son, Georges Charles Paul, born in 1855, was in his father’s department from 1879 till 1885, deputy from 1885, five times president of the Budget Commission, minister of finance (1895-1898) and vice-president of the chamber (1898-1902), and again finance minister in the Briand Cabinet, 1909.
COCHIN, DENYS MARIE PIERRE AUGUSTIN (1851- ), French politician, was born at Paris. He studied law, was elected to the chamber of deputies in 1893, and gradually became one of the leaders and principal orators of the Conservative party. He opposed the project of the income-tax in 1894, the revision of the Dreyfus case in 1899, and the separation of the church and state in 1905. He is known as an author by his works, L’Évolution de la vie (1895); Le Monde extérieur (1895); Contre les barbares (1899); Ententes et ruptures (1905).
COCHIN, a feudatory state of southern India, in political subordination to Madras, with an area of 1361 sq. m. It is bounded on the N. by British Malabar, on the E. by British Malabar, Coimbatore and Travancore, on the S. by Travancore, and on the W. by British Malabar and the Arabian Sea. Isolated from the main territory, and situated to the north-east of it, lies the major portion of the Chittore taluk, entirely surrounded by British territory. The whole state may be divided into three well-defined regions or zones: (1) the eastern zone, consisting of broken forested portions of the Western Ghats, which, gradually decreasing in height, merge into (2) the central belt, comprising the uplands and plains that dip towards the lagoons or “backwaters” along the coast (see Cochin, town), beyond which lies (3) the western zone, forming the littoral strip. The low belt which borders on the seas and the backwaters is by nature flat and swampy, but has in the course of ages become enriched by the work of man. On leaving the seaboard, an undulating country is found, diversified with grassy flats, naked hills and wooded terraces, intersected by numerous torrents and rapids, and profusely dotted with homesteads, orchards and cultivated fields, up to the very foot of the Ghats. Here the landscape, now on a grander scale, embraces great forests which form a considerable source of wealth. Of the total area of the state the forests and lagoons cover nearly 605 and 16 sq. m. respectively.
In 1901 the population was 812,025, showing an increase of 12% in the decade. More than one-fifth are Christians, mostly Syrians and Roman Catholics. The revenue is estimated at £153,000, subject to a tribute of £13,000. During recent years the financial condition of the state has been flourishing. The principal products are rice, cocoanuts, timber, cardamoms, pepper and a little coffee. Salt is manufactured along the coast. The capital is Ernakulam, but the raja resides at Tripunthora. The principal commercial centre is Mattancheri, adjoining the British town of Cochin. The chief means of communication is by boat along the backwaters; but in 1902 a metre-gauge line was constructed by the Madras railway at the expense of the state to connect Ernakulam with Shoranur.
History.—What is now the native state of Cochin formed, until about the middle of the 9th century A.D., part of the ancient Chera or Kerala kingdom (see Kerala). Its port of Kodungalur (Kranganur, the ancient Muziris), at the mouth of the Periyar, was from early times one of the chief centres for the trade between Europe and India; and it was at Malankara, near Kodungalur, that the apostle Thomas is traditionally said to have landed. The history of Cochin is, however, like that of the Kerala kingdom generally, exceedingly obscure previous to the arrival of the Portuguese. The rajas of Cochin, who are of pure Kshatriya blood, claim descent from the Chera king Cheraman Perumal, the last of his race to rule the vast tract from Gokarn in North Kanara to Cape Comorin. About the middle of the 9th century this king, according to tradition, resigned his kingdom, embraced Islam, and went on pilgrimage to Arabia, where he died. Towards the end of the century the Chera kingdom was overrun and dismembered by the Cholas. It was in 1498 that Vasco da Gama reached the Malabar coast; and in 1502 the Portuguese were allowed to settle in the town of Cochin, where they built a fort and began to organize trade with the surrounding country. By the end of the century their influence had become firmly established, largely owing to the effective aid they had given to the rajas of Cochin in their wars with the Zamorin of Calicut. The Syrian Christians, forming at that time a large proportion of the population, now felt the weight of Portuguese ascendancy; in 1599 Menezes, the archbishop of Goa, held a synod at Udayamperur (Diamper), a village 12 m. south-east of Cochin, at which their tenets were pronounced heretical and their service-books purged of all Nestorian phrases. In 1663, however, Portuguese domination came to an end with the capture of Cochin by the Dutch, whose ascendancy continued for about a hundred years. In 1776 Hyder Ali of Mysore invaded the 620 state and forced the raja to acknowledge his suzerainty and pay tribute. In 1791 Tippoo, son of Hyder Ali, ceded the sovereignty to the British, who entered into a treaty with the raja by which he became their vassal and paid an annual tribute of a lakh of rupees. On the 17th of October 1809, in consequence of an attempt of the hereditary chief minister Paliyath Achan, in 1808, to raise an insurrection against the British without his master’s knowledge, a fresh treaty was made, by which the raja undertook to hold no correspondence with any foreign state and to admit no foreigners to his service without the sanction of the British government, which, while undertaking to defend the raja’s territories against all enemies, reserved the right to dismantle or to garrison any of his fortresses. In 1818 the tribute, raised to 2½ lakhs in 1808, was permanently fixed at 2 lakhs. Since then, under the rule of the rajas, the state has greatly advanced in prosperity, especially under that of H. H. Sir Sri Rama Varma (b. 1852), who succeeded in 1895, was made a K.C.S.I. in 1897, and G.C.S.I. in 1903.
COCHIN, a town of British India, in the district of Malabar, Madras. Pop. (1901) 19,274. The town lies at the northern extremity of a strip of land about 12 m. in length, but in few places more than a mile in breadth, which is nearly insulated by inlets of the sea and estuaries of streams flowing from the Western Ghats. These form the Cochin backwaters, which consist of shallow lagoons lying behind the beach-line and below its level. In the monsoon the Cochin backwaters are broad navigable channels and lakes; in the hot weather they contract into shallows in many places not 2 ft. deep. The town of Cochin is about a mile in length by half a mile in breadth. Its first European possessors were the Portuguese. Vasco da Gama founded a factory in 1502, and Albuquerque built a fort, the first European fort in India, in 1503. The British made a settlement in 1634, but retired when the Dutch captured the town in 1663. Under the Dutch the town prospered, and about 1778 an English traveller described it as a place of great trade, “a harbour filled with ships, streets crowded with merchants, and warehouses stored with goods from every part of Asia and Europe, marked the industry, the commerce, and the wealth of the inhabitants.” In 1795 Cochin was captured from the Dutch by the British, and in 1806 the fortifications and public buildings were blown up by order of the authorities. The explosion destroyed much private property, and for a long time seriously affected the prosperity of the town. Considerable sea-borne trade is still carried on. A lighthouse stands on the ruins of the old fort. The chief exports are cocoanut products, for the preparation of which there are factories, and tea; and the chief import is rice. Cochin is the only port south of Bombay in which large ships can be built.
COCHIN-CHINA,1 a French colony in the extreme south of French Indo-China. The term formerly included the whole Annamese empire—Tongking, Annam, and Lower Cochin-China, but it now comprises only the French colony, which corresponds to Lower Cochin-China, and consists of the six southern provinces of the Annamese empire annexed by France in 1862 and 1867. Cochin-China is bounded W. by the Gulf of Siam, N.W. and N. by Cambodia, E. by Annam, and S.E. by the China Sea. Except along part of the north-west frontier, where the canal of Vinh-Thé divides it from Cambodia, its land-limits are conventional. Its area is about 22,000 sq. m.
In 1901 the population numbered 2,968,529, of whom 4932 were French (exclusive of French troops, who numbered 2537), 2,558,301 Annamese, 231,902 Cambodians, 92,075 Chinese, 42,940 savages (Min Huong), the rest being Asiatics of other nationalities, together with a few Europeans other than French.
Geography.—Cochin-China consists chiefly of an immense plain, flat and monotonous, traversed by the Mekong and extending from Ha-Tien in the west to Baria in the east, and from Bien-Hoa in the north-east to the southern point of the peninsula of Ca-Mau in the south-west. The last spurs of the mountains of Annam, which come to an end at Cape St Jacques, extend over parts of the provinces of Tay-Ninh, Bien-Hoa and Baria in the north-east and east of the colony, but nowhere exceed 2900 ft. in height; low hills are found in the north-western province of Chau-Doc. Cochin-China is remarkable for the abundance of its waterways. The Mekong divides at Pnom-Penh in Cambodia into two arms, the Fleuve supérieur and the Fleuve inférieur, which, pursuing a course roughly parallel from north-west to south-east, empty into the China Sea by means of the numerous channels of its extensive delta. From June to October the inundations of the Mekong cover most of the country, portions of which, notably the Plaine des Joncs in the north and a large tract of the peninsula of Ca-Mau, are little else than marshes. Besides a great number of small coastal streams there are four other rivers of secondary importance, all of which water the east of the colony, viz. the Don-Nai, which rising in the Annamese mountains flows west, then abruptly south, reaching the sea to the west of Cape St Jacques; the Saigon river, which flowing from north-west to south-east passes Saigon, the capital of the colony, 12 m. below which it unites with the Don-Nai; and the two Vaicos, which join the Don-Nai close to its mouth. These rivers flow into the sea through numerous winding channels, forming a delta united by canals to that of the Mekong. The waterways of Cochin-China communicate by means of natural or artificial channels (arroyos), facilitating transport and aiding in the uniform distribution of the inundation to which the country owes its fertility. Canals from Chau-Doc to Ha-Tien and from Long Xuyen to Rach-Gia join the Mekong with the Gulf of Siam. East of Cape St Jacques the mountains of Annam come down close to the sea; west of that point, as far as the southern headland of Ca-Mau, the coast-line of Cochin-China runs north-east to south-west for about 160 m. in a straight line broken only by the mouths of the Don-Nai and Mekong. From Cape Ca-Mau to Rach-Gia it runs north for a distance of 120 m., then north-west as far as Ha-Tien, where the boundary line between it and Cambodia meets the sea.
Climate and Fauna.—The climate of the country is warm, humid, and very trying to Europeans. The wet season, during which heavy rain falls almost daily, lasts from April to October, coinciding with the south-west monsoon. The hottest period lasts from the middle of April to the middle of June, the thermometer during that time often reaching 94° F., and never descending below 86°. The forest regions of Cochin-China harbour the tiger, panther, leopard, tiger-cat, ichneumon, wild boar, deer, buffalo, rhinoceros and elephant, as well as many varieties of monkeys and rats. Of birds some species of parrakeet, the “mandarin” blackbird, and the woodcock are not found in the rest of Indo-China. Duck, teal, cranes and other aquatic birds abound in the delta. Venomous reptiles are numerous, and the Mekong contains crocodiles.
Agriculture and Industries.—The cultivation of the rice-fields, which cover large extents of the plains of Cochin-China, is by far the chief industry of the colony. Pepper is grown in considerable quantities in the districts of Ha-Tien and Bien-Hoa, and sugarcanes, coffee, cotton, tobacco and jute are also produced. The buffalo, used both for transport and in the rice-fields, and swine, the flesh of which forms an important element in the native diet, are the principal domestic animals. Oxen and cows are of secondary importance and the climate is unsuitable for sheep; horses of a small breed are used to some extent. The chief industrial establishments are those for the decortication of rice at Saigon and Cholon; they are in the hands of the Chinese, by whom most of the trade in the colony is conducted. Sugar-making, the distillation of rice-spirit, silk-weaving, fishing and the preparation of a fish-sauce (nuoc-mam) made from decayed fish, and the manufacture of salt from sea-water and of lime are carried on in many localities.
Commerce.—Rice is the chief article of export, dried or salted fish, pepper and cotton ranking next in order of value. Imports include woven goods, metals, ironware, machinery, tea, wines and spirits, mineral oils, opium, paper, and arms and powder. The ports of Saigon and Mytho are accessible to the largest vessels, and are connected by a railway (see Indo-China, French). The roadsteads of Rach-Gia, Ca-Mau, and Ha-Tien can accommodate only vessels of low tonnage. In 1905 exports 621 reached a value of £3,816,000, and imports a value of £4,834,000 (not including treasure and transit trade).
Government and Administration.—Cochin-China is administered by a lieutenant-governor under the authority of the governor-general of Indo-China. He is assisted by the conseil colonial numbering sixteen members, six of whom are French citizens elected by the French, six natives elected by the natives, the other four being members of the chamber of commerce of Saigon and the conseil privé. The conseil colonial, besides its advisory functions, discusses and votes the budget, determines the nature of the taxes, has supreme control over the tariffs, and extensive powers in the administration of colonial domains. The conseil privé is a deliberative body under the presidency of the lieutenant-governor, composed of colonial officials together with two native members. The colony is divided into four circumscriptions (Saigon, My-Tho, Vinh-Long, Bassac), at the head of each of which is an inspector of native affairs. These are subdivided into twenty provinces, each administered by an administrator of native affairs by whose side is the provincial council consisting of natives and occupied with the discussion of ways and means and questions of public works. The provinces are divided into cantons and subdivided into communes. The commune forms the basis of the native social system. Its assembly of notables or municipal council forms a sort of oligarchy, the members of which themselves elect individuals from among the more prominent inhabitants to fill vacancies. The notables elect the provincial councillors in the proportion, usually, of one to every canton, and their delegates elect the chief of the canton, who voices the wishes of the natives to the government. Local administration, e.g. supervision of markets, policing, land-transfer, &c., are carried on by a mayor and two assistants, to whom the municipal council delegates its powers. The same body draws up the list of males liable to the poll-tax and of the lands liable to land-tax, these being the chief sources of revenue. There are French tribunals of first instance in nine of the chief towns of the colony, and in four of these there are criminal courts. These administer justice in accordance both with French law and, in the case of natives, with Annamese law, which has been codified for the purpose. Saigon has two chambers of the court of appeal of French Indo-China and a tribunal of commerce. Primary instruction is given in some six hundred schools. Cochin-China is represented in the French chamber by a deputy. The capital is Saigon (q.v.); of the other towns, Cholon (q.v.), My-Tho, Vinh-Long and Chau-Doc are of importance.
In 1904 the budget receipts amounted to £495,241 (as compared with £474,545 in 1899). To this sum the land and poll-tax and other direct taxes contributed £374,630. The main heads of expenditure, of which the total was £467,328, were as follows:—
| Government | £87,271 |
| Administration | 62,725 |
| Public Works | 40,454 |
| Transport | 38,173 |
| Public Instruction | 36,009 |
| Topography and Surveying | 32,036 |
History.—The Khmer kingdom (see Cambodia), at its zenith from the 9th to the 12th centuries, included a large portion of the modern colony of Cochin-China, the coastal portion and perhaps the eastern region being under the dominion of the empire of Champa, which broke up during the 15th century. This eastern region was occupied in the 17th century by the Annamese, who in the 18th century absorbed the western provinces. From this period the history of Cochin-China follows that of Annam (q.v.) till 1867, when it was entirely occupied by the French and became a French colony. In 1887 it was united with Cambodia, Annam and Tongking to form the Indo-Chinese Union (see Indo-China, French).
1 See also Indo-China, French; and Annam.
COCHINEAL, a natural dye-stuff used for the production of scarlet, crimson, orange and other tints, and for the preparation of lake and carmine. It consists of the females of Coccus cacti, an insect of the family Coccidae of the order Hemiptera, which feeds upon various species of the Cactaceae, more especially the nopal plant, Opuntia coccinellifera, a native of Mexico and Peru. The dye was introduced into Europe from Mexico, where it had been in use long before the entrance of the Spaniards in the year 1518, and where it formed one of the staple tributes to the crown for certain districts. In 1523 Cortes received instructions from the Spanish court to procure it in as large quantities as possible. It appears not to have been known in Italy so late as the year 1548, though the art of dyeing then flourished there. Cornelius van Drebbel, at Alkmaar, first employed cochineal for the production of scarlet in 1650. Until about 1725 the belief was very prevalent that cochineal was the seed of a plant, but Dr Martin Lister in 1672 conjectured it to be a kind of kermes, and in 1703 Antony van Leeuwenhoek ascertained its true nature by aid of the microscope. Since its introduction cochineal has supplanted kermes (Coccus ilicis) over the greater part of Europe.
The male of the cochineal insect is half the size of the female, and, unlike it, is devoid of nutritive apparatus; it has long white wings, and a body of a deep red colour, terminated by two diverging setae. The female is apterous, and has a dark-brown plano-convex body; it is found in the proportion of 150 to 200 to one of the male insect. The dead body of the mother insect serves as a protection for the eggs until they are hatched. Cochineal is now furnished not only by Mexico and Peru, but also by Algiers and southern Spain. It is collected thrice in the seven months of the season. The insects are carefully brushed from the branches of the cactus into bags, and are then killed by immersion in hot water, or by exposure to the sun, steam, or the heat of an oven—much of the variety of appearance in the commercial article being caused by the mode of treatment. The dried insect has the form of irregular, fluted and concave grains, of which about 70,000 go to a pound. Cochineal has a musty and bitterish taste. There are two principal varieties—silver cochineal, which has a greyish-red colour, and the furrows of the body covered with a white bloom or fine down; and black cochineal, which is of a dark reddish brown, and destitute of bloom. Granilla is an inferior kind, gathered from uncultivated plants. The best crop is the first of the season, which consists of the unimpregnated females; the later crops contain an admixture of young insects and skins, which contain proportionally little colouring matter.
The black variety of cochineal is sometimes sold for silver cochineal by shaking it with powdered talc or heavy-spar; but these adulterations can be readily detected by means of a lens. The duty in the United Kingdom on imported cochineal was repealed in 1845.
Cochineal owes its tinctorial power to the presence of a substance termed cochinealin or carminic acid, C17H18O10, which may be prepared from the aqueous decoction of cochineal. Cochineal also contains a fat and wax; cochineal wax or coccerin, C30H60(C31H61O3)2, may be extracted by benzene, the fat is a glyceryl myristate C3H5(C14H27O2)3.
COCHLAEUS, JOHANN (1479-1552), German humanist and controversialist, whose family name was Dobneck, was born of poor parents in 1479 at Wendelstein (near Nuremberg), whence his friends gave him the punning surname Cochlaeus (spiral), for which he occasionally substituted Wendelstinus. Having received some education at Nuremberg from the humanist Heinrich Grieninger, he entered (1504) the university of Cologne. In 1507 he graduated, and published under the name of Wendelstein his first piece, In musicam exhortatorium. He left Cologne (May 1510) to become schoolmaster at Nuremberg, where he brought out several school manuals. In 1515 he was at Bologna, hearing (with disgust) Eck’s famous disputation against usury, and associating with Ulrich von Hutten and humanists. He took his doctor’s degree at Ferrara (1517), and spent some time in Rome, where he was ordained priest. In 1520 he became dean of the Liebfrauenkirche at Frankfort, where he first entered the lists as a controversialist against the party of Luther, developing that bitter hatred to the Reformation which animated his forceful but shallow ascription of the movement to the meanest motives, due to a quarrel between the Dominicans and Augustinians. Luther would not meet him in discussion at Mainz in 1521. He was present at the diets of Worms, Regensburg, Spires and 622 Augsburg. The peasants’ war drove him from Frankfort; he obtained (1526) a canonry at Mainz; in 1529 he became secretary to Duke George of Saxony, at Dresden and Meissen. The death of his patron (1539) compelled him to take flight. He became canon (September 1539) at Breslau, where he died on the 10th of January 1552. He was a prolific writer, largely of overgrown pamphlets, harsh and furious. His more serious efforts retain no permanent value. With humanist convictions, he had little of the humanist spirit. We owe to him one of the few contemporary notices of the young Servetus.
See C. Otto, Johannes Cochlaeus, der Humanist (1874); Haas, in I. Goschler’s Dict. encydopéd. de la théol. cath. (1858); Brecher, in Allgemeine deutsche Biographie (1876); T. Kolde, in A. Hauck’s Realencyklopädie für prot. Theol. u. Kirche (1898).
COCK, EDWARD (1805-1802), British surgeon, was born in 1805. He was a nephew of Sir Astley Cooper, and through him became at an early age a member of the staff of the Borough hospital in London, where he worked in the dissecting room for thirteen years. Afterwards he became in 1838 assistant surgeon at Guy’s, where from 1849 to 1871 he was surgeon, and from 1871 to 1892 consulting surgeon. He rose to be president of the College of Surgeons in 1869. He was an excellent anatomist, a bold operator, and a clear and incisive writer, and though in lecturing he was afflicted with a stutter, he frequently utilized it with humorous effect and emphasis. From 1843 to 1849 he was editor of Guy’s Hospital Reports, which contain many of his papers, particularly on stricture of the urethra, puncture of the bladder, injuries to the head, and hernia. He was the first English surgeon to perform pharyngotomy with success, and also one of the first to succeed in trephining for middle meningeal haemorrhage; but the operation by which his name is known is that of opening the urethra through the perinaeum (see Guy’s Hospital Reports, 1866). He died at Kingston in 1892.
COCKADE (Fr. cocarde, in 16th century coquarde, from coq, in allusion probably to the cock’s comb), a knot of ribbons or a rosette worn as a badge, particularly now as part of the livery of servants. The cockade was at first the button and loop or clasp which “cocked” up the side of an ordinary slouch hat. The word first appears in this sense in Rabelais in the phrase “bonnet à la coquarde,” which is explained by Cotgrave (1611) as a “Spanish cap or fashion of bonnet used by substantial men of yore ... worne proudly or peartly on th’ one side.” The bunch of ribbons as a party badge developed from this entirely utilitarian button and loop. The Stuarts’ badge was a white rose, and the resulting white cockade figured in Jacobite songs after the downfall of the dynasty. William III.’s cockade was of yellow, and the House of Hanover introduced theirs of black, which in its present spiked or circular form of leather is worn in England to-day by the royal coachmen and grooms, and the servants of all officials or members of the services. At the battle of Sheriffmuir in the reign of George I. the English soldiers wore a black rosette in their hats, and in a contemporary song are called “the red-coat lads wi’ black cockades.” At the outbreak of the French Revolution of 1789, cockades of green ribbon were adopted. These afterwards gave place to the tricolour cockade, which is said to have been a mixture of the traditional colours of Paris (red and blue) with the white of the Bourbons, the early Revolutionists being still Royalists. The French army wore the tricolour cockade until the Restoration. To-day each foreign nation has its special coloured cockade. Thus the Austrian is black and yellow, the Bavarian light blue and white, the Belgian black, yellow and red, French the tricolour, Prussian black and white, Russian green and white, and so on, following usually the national colours. Originally the wearing of a cockade, as soon as it had developed into a badge, was restricted to soldiers, as “to mount a cockade” was “to become a soldier.” There is still a trace of the cockade as a badge in certain military headgears in England and elsewhere. Otherwise it has become entirely the mark of domestic service. The military cocked hat, the lineal descendant of the bonnet à la coquarde, became the fashion in France during the reign of Louis XV.
See Genealogical Magazine, vols. i.-iii. (London, 1897-1899); Racinet, La Costume historique (6 vols., Paris, 1888).
End of the Project Gutenberg EBook of Encyclopaedia Britannica, 11th
Edition, Volume 6, Slice 5, by Various
*** END OF THIS PROJECT GUTENBERG EBOOK ENCYC. BRITANNICA, VOL 6 SL 5 ***
***** This file should be named 31793-h.htm or 31793-h.zip *****
This and all associated files of various formats will be found in:
https://www.gutenberg.org/3/1/7/9/31793/
Produced by Marius Masi, Don Kretz, Juliet Sutherland and
the Online Distributed Proofreading Team at
https://www.pgdp.net
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License (available with this file or online at
https://gutenberg.org/license).
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH F3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS' WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTIBILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation web page at https://www.pglaf.org.
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Its 501(c)(3) letter is posted at
https://pglaf.org/fundraising. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at
809 North 1500 West, Salt Lake City, UT 84116, (801) 596-1887, email
business@pglaf.org. Email contact links and up to date contact
information can be found at the Foundation's web site and official
page at https://pglaf.org
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit https://pglaf.org
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including including checks, online payments and credit card
donations. To donate, please visit: https://pglaf.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For thirty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
https://www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.