
in the Prairie Vole (Microtus ochrogaster)
Lawrence
1957
Editors: E. Raymond Hall, Chairman, Henry S. Fitch,
Harrison B. Tordoff
Volume 10, No. 4, pp. 129-161, 8 figs. in text, 6 tables
Published December 19, 1957
University of Kansas
Lawrence, Kansas
THE STATE PRINTING PLANT
TOPEKA, KANSAS
1957
29-5936
The prairie vole is by far the most abundant mammal on the University of Kansas Natural History Reservation and on grassland areas throughout northeastern Kansas. This vole therefore affects the vegetation, perhaps more than any other native vertebrate, and it is an important food source for most of the vertebrate predators. Since the Reservation was established, in 1948, more data have been accumulated concerning this vole than for any other species of animal there. From February, 1950, to February, 1954, a grid of live-traps at 50-foot intervals was set for several days each month in a three-acre field inhabited by voles, and the population of marked individuals was studied throughout the four-year period. From November, 1953, to June, 1956, a half-acre trap grid with 20-foot interval was used on an area adjoining the three-acre field. Other trap lines in somewhat different habitats were maintained for shorter periods as a basis for comparison. By June, 1956, a total of some 3550 voles had been caught and recorded 14,750 times in all. The present report is a preliminary attempt to analyze, in part, these extensive data, and is concerned with certain phases of the species' reproduction and growth that have bearing on the observed population changes from month to month and from year to year on the Reservation.
Through the studies of Jameson (1947) and Martin (1956), both made in the same general area as my own, and several earlier studies, the life history and ecology of the prairie vole are already well known. The present report, with much larger amounts of data, further clarifies certain phases of the ecology; and by using types of data not available to Jameson and Martin I have dealt with some topics not included in their reports.
Previous studies of growth in Microtus have been based almost entirely on weights. However, the weight of an individual vole may fluctuate widely over a short period, depending on pregnancy and parturition, length of time in a trap without food, availability of moisture, and other factors. In the course of my study, in 1954 [Pg 132] and 1955, and parts of 1953 and 1956, measurements of total length, in addition to weights, were recorded for most of the voles live-trapped.
To test the accuracy of measurements, successive readings were compared in individual voles that were already of large adult size and that presumably either had stopped growing or were growing so slowly that the gain was scarcely detectable in the relatively short periods involved. For 200 such readings 33 per cent were just the same as previous records for the same animals, 24 per cent deviated by 1 mm., 22 per cent deviated by 2 mm., 15 per cent by 3 mm., 4.5 per cent by 4 mm., .5 per cent by 5 mm., 1 per cent by 6 mm., and .5 per cent by 7 mm. On the average, successive measurements varied by 1.43 mm., somewhat less than one per cent of the adult vole's total length. Occasional errors of two to four per cent were easily eliminated because for the voles used for growth records, series of measurements were available, with clearly defined trends. The occasional readings that deviated from the general trend for the individual were discarded.
Measurements were recorded along with other data in the field at the point of capture. Obtaining a reasonably accurate measurement on a live and struggling vole required patience and practice. With the thumb and forefinger of the left hand, I grasped the vole by loose skin of the nape, and simultaneously grasped the tail at a point approximately three-fourths of the distance to the tip. Then, with gentle but steady pressure, I stretched the vole out to its full length, meanwhile manipulating a millimeter ruler with the free fingers, so that the vole was pressed against it, with the nose pad at the end of the ruler.
The total length measurement is considered the best index to over-all size. The relative tail-length varies slightly between individuals, averaging approximately 22 per cent of the total length. Individuals having broken tails, or having the distal parts of their tails missing, were not included. The total length can be measured with greater accuracy than can either the head-and-body length or the tail-length separately.
As compared with other mammals, voles are tolerant and somewhat social. That individuals are not mutually exclusive (territorially) in areas occupied was demonstrated on many occasions when more than one individual was caught simultaneously in the same live-trap. Injury of a vole by a trap-mate was a rare occurrence.
[Pg 133] Multiple captures often involved a female in oestrus and one or more males, or a female and her young, but other instances involved various combinations of sex and age groups. As many as five adults have been caught in a trap simultaneously at times when the population density was high. At such times, the meadow habitat is crossed by a maze of interconnecting surface runways and one runway may be traced continuously for 100 yards or more. Because each individual vole normally confines its activity to a small area, only a fraction of an acre, it is evident that individuals living at different places overlap in their home ranges, and also in the trailways followed in foraging. A high degree of tolerance is indicated. Where population is so sparse that the systems of surface runways comprise separate and isolated units, trapping experience has shown that one such system may harbor several or many individuals.
As direct observations on voles under natural conditions are rarely feasible, because of the animals' timidity, their utilization of concealing cover, and tendency to crepuscular habits, best evidence of social habits and underground life is based upon behavior of captive individuals. Many voles were kept in confinement for varying lengths of times, either singly or in association with others. Under such conditions there was sometimes sporadic fighting, but it was mainly defensive and serious injuries were rare. Two or more voles caught at a given spot regardless of whether they were found in the same trap simultaneously, or trapped separately within a short time, usually were completely tolerant of each other. When at rest in their container, such voles would huddle together in a corner or in a nest, if materials were provided, so that collectively they presented the minimum exposed surface. The intimacy and lack of antagonism displayed on such occasions, suggested that the voles were accustomed to living together amicably in the same nest chamber. In live-trapping, "double" captures in a single trap often involved the same two individuals. Such trap-mates were often male and female, and in many instances the female was not in breeding condition. That the voles are not monogamous in habits was demonstrated when the same female was often trapped in association with either of two males. Other trap associates taken together repeatedly often were two males, or two females. Voles that are nest mates or "neighbors" may tend to move about together in their foraging, or one confined in a trap may attract the other sufficiently to cause it to force an entrance by lifting the heavy door of a trap.
[Pg 134] When a new vole, caught at a different location, is added to a container in which one or more are already confined, there is mutual circumspection between the original occupants and the newcomer. At first, each vole is intimidated by movements of the other, and as a result, the original occupants huddle in their established corner while the newcomer cowers in the most remote part of the container. Gradually the voles become less timid and one may approach another slowly and cautiously, to sniff at it. The vole approached may react with a show of hostility which is largely defensive. In the characteristic posture of threat for defense, the vole crouches, or rears back on its haunches, with snout elevated and incisors prominently displayed. If the warning posture is unheeded, or if the vole is made unusually aggressive by having young to defend, or for some other reason, it attacks with a sudden forward lunge, striking the adversary simultaneously with both forefeet and with the incisors. The lunge is so rapid that when I have observed it, I have been unable to discern whether the attacker bit its opponent. The attack serves to force back the other animal, throwing it off balance and intimidating it. The attacked animal may dodge nimbly to avoid the lunge, but whether or not it is actually struck, it usually retreats, avoiding or postponing further hostilities. Voles that have been kept in containers for periods of hours or days tend to be more hostile and aggressive toward a newcomer than are those newly introduced. After series of meetings resulting from the exploratory behavior of the newcomer and the curiosity or normal activity of those longer confined, hostility gradually subsides. Within a few hours a newcomer is usually accepted, and thenceforth he huddles with other members of the group when at rest, and hostility is rarely evident.
This ready acceptance on short acquaintance of strange voles into the family or social group suggests that lack of territoriality extends even to the use of the nest burrows, and that groups of voles may share the same nest, huddling together and deriving mutual benefit from the association, such as warmth in cold weather. Schmidt (1931: 113), studying this vole in Clark County, Wisconsin, noted its colonial habits. He found isolated small mounds that were riddled with burrows, and little sign in intervening areas. At one mound he trapped two adult males, one adult female, and two young; at another mound, two adult males, two adult females, and four young were trapped. My individuals that were released from live-traps were on many occasions trailed by means of a stiff wire collar with spool of thread attached, to holes that presumably [Pg 135] were their home burrows. Data obtained in this manner indicated that ordinarily several or many individuals use the same burrow system. The histories of individual voles on the study area at the Reservation indicate shift of home base from time to time, usually for short distances within the area already included in the home range, but occasionally to new areas relatively remote from the original home range.
Severe fighting between adult prairie voles occurs at times. Occasionally, sharp squeaks accompanied by brisk rustling in the grass suggesting pursuit or conflict, are heard in their habitat. An unusually large adult male, long resident on a study area, suddenly lost weight and deteriorated in condition over a period of several days, then was found dead in a nest-box attached to a trap. Dissection revealed numerous punctures in the skin and flesh of the neck and back, probably made by the incisors of another vole. Extensive hemorrhage and swelling had occurred, and obviously these injuries were the cause of death.
Although it was not feasible to study the home life of the voles underground, clues were gained from those uncovered in runways and nests beneath large boards and strips of tarpaper, previously distributed for this purpose. Nests were constructed by the voles beneath several such pieces of tarpaper and runways appeared beneath all the pieces that were placed in habitat favorable to the voles. In summer, however, the high daytime temperatures beneath these shelters made them uninhabitable to the voles, and they were used mainly in spring. From February 15 to May 1, 1953, 14 voles were caught 19 times beneath five of the tarpaper strips, and many other voles that were seen beneath them escaped. Upon turning one of the strips I often discovered voles in close proximity. Sometimes two or more darted from the same nest. The disturbance of repeatedly raising the strips and exposing the voles' shelters soon caused them to desert the sites; consequently the information obtained by this means was limited.
There is sexual activity in every month of the year, but its incidence varies greatly from one season to another. As has been indicated by various authors, male voles reach sexual maturity later than females. It seems that ordinarily the availability of sexually active males is not a limiting factor, however. While males that are still well below average adult size produce mature spermatozoa, and are probably capable of breeding (Jameson, 1947: 145), certain [Pg 136] large old males may sire a disproportionately large percentage of the litters produced. Observations on males in confinement indicated that sexual activity tended to be directly proportional to the size of the testes. Occasional individuals, having much enlarged scrotal testes were more readily stimulated to sexual activity and more aggressive toward females than were those in which the testes were of more nearly typical size or abdominal or were smaller than normal. The combination of factors controlling size of testes is not well understood, but males having unusually large testes were caught most often when food supply was optimum, for instance after a period of heavy precipitation when an abundant supply of new grass provided succulent and nutritious food.
In confinement sexual activity was largely inhibited and attempts to establish a laboratory colony met with failure. Sexual activity was observed mainly in recently captured males, and their interest was aroused chiefly by females that had given birth to litters within a few hours previously. Oestrus is known to follow closely after parturition. Females found in live-traps with newborn young often were brought to the laboratory for observation. An apparent instance of hostility between rival males competing for an oestrus female was observed on September 2, 1950. The female was found in a trap with four newborn young, and since the young had not yet attached to her teats, she was temporarily returned to the trap after recording, to prevent desertion of the litter. Returning twenty minutes later I found another adult vole at this trap. It would suddenly emerge from dense grass nearby, and would move over the trap or around it, with jerky, halting movements, then would dart back under cover. The female emerged from the nest box into the trap runway, and sniffed at the other, and both pressed against the intervening wire barrier. There was gnawing on the wire by one or both. A third adult vole appeared. As it moved toward the trap, all three suddenly took alarm and darted back under cover, the female hiding in the trap nest box. In a few seconds they again appeared. The two outsiders, presumably both males, were not individually recognizable, but several times one was seen to dart at the other, chasing it away momentarily. They were seldom both in sight at once.
Males confined with post-partum females usually evinced sexual interest, following them about persistently and nuzzling their genitalia. The females, however, were often unreceptive perhaps because they were disturbed by strange surroundings and by the presence of their litters, so that they usually attempted to escape, [Pg 137] or to rebuff the male's attention. At first the female might flee, squeaking in protest at the male's pursuit. If he still continued to follow, she would turn on him, rearing back in the characteristic threatening pose, and would lunge at him, striking him sharply or driving him back. After such rebuff, males were usually intimidated or discouraged so that they temporarily or permanently abandoned their advances, and small males were more easily rebuffed than were larger individuals. On several occasions large males having enlarged testes were not readily rebuffed by females but continued to follow them. When the female turned upon him, such a male might lunge against her, throwing her off balance, and causing her to attempt to escape, and then continuing the pursuit until it ended in copulation or in more severe fighting. Although not accepted sexually, a rebuffed male might be readily accepted as a nest-mate, huddling along with the female and perhaps other individuals of both sexes. In huddling voles, the most frequently observed type of social behavior was grooming; one individual would slide its chin or muzzle through the other's fur with a stroking movement consisting of a series of rapid forward jerks and the stroking movements might continue for periods of minutes. The recipient of the grooming usually made no evident response indicative of either pleasure or displeasure. Often it seemed to be sleeping while the grooming was performed. Individuals of both sexes performed this grooming and the recipient might be of either the same sex or the opposite sex. This grooming may have some significance as a search for ectoparasites such as fleas, or mites that often infest the voles. However, after prolonged grooming by a companion, a vole's fur was of mussed and disarranged appearance. Although the grooming that occurs between voles that are resting in nests seems to have no direct significance as sexual behavior, somewhat similar actions constitute part of the mating pattern. A sexually aroused male overtaking a receptive female, slides his chin forward along her back with jerky, stroking movements. In some observed instances this behavior continued intermittently for several minutes before actual copulation. In some other instances it was almost lacking.
In female voles that are sexually quiescent, both those that have not yet attained breeding maturity, and those that have undergone regression after attainment of sexual maturity, the vaginal orifice is not evident. The canal is sealed externally by a membranous [Pg 138] layer of epithelium. Presence of a vaginal orifice indicates that the individual is in some active stage of the breeding cycle. The appearance of the orifice varies between different females, and it changes in the same female from day to day or even from hour to hour. Presumably these changes in the vaginal orifice are cyclical and are closely correlated with oestrus, but attempts to trace them were unsuccessful largely because the normal cycle was rapidly suppressed in captive voles, which soon became sexually quiescent. Individual voles living under natural conditions were not trapped with sufficient regularity to permit tracing the details of changes in their genitalia.
In those females having the vaginal orifice most developed, the margins are turgid and slightly inflamed. The circular opening gapes 1.0 to 1.5 mm. in diameter when the tail is raised. A female may remain in this condition for two days or more. Vaginal smears at this stage often showed nucleated cells characteristic of oestrus. Subsequently the margins of the orifice become less prominent and the opening becomes smaller. The dorsal and ventral walls adhere until an opening is no longer evident unless the adjacent skin is stretched.
In pregnancy the orifice is occasionally sealed, but usually is evident. It is, however, less prominent than in oestrus, and does not gape. The margins are less turgid than in oestrus, and the opening is in the form of a transverse slit through which the purplish epithelial lining of the dorsal wall of the vagina can be seen. After parturition, placentae and bloody discharge often are in evidence in the vaginal canal. Females that have not given birth to young recently may also have bloody mucous discharge. Its significance has not been determined. In females that are undergoing sexual regression, the margins of the vaginal orifice become shrunken and pale, and the orifice becomes partly or wholly sealed.
Bodenheimer and Sulman (1946:255) concluded from their study of Microtus guentheri that in this species, as in "the cat," "the rabbit," "the ferret," and a few other mammals, ovulation is induced by copulation, and that there is no regular vaginal cycle. Hoyte (1955:412) disagreed with these conclusions for other species of Microtus, as he trapped individuals of M. oeconomus that had recently ovulated without copulation (at least no sperm were found in the genital tracts). In M. ochrogaster oestrus seems to be controlled largely by the food supply, at least the incidence of perforate females was found to fluctuate irregularly tending to follow the trend of rainfall, and, probably in more direct correlation, the amount [Pg 139] of new grass present (see Table 1, and Martin, 1956:383-384). It therefore seems unlikely that in this species ovulation is dependent on copulation.
In females that have not yet produced young the teats are minute and well concealed in the fur, so that they are difficult to find, but in lactation they become conspicuous. In early lactation the teats are typically about 1 mm. in diameter and 2.5 mm. in length. As lactation progresses, they become thickened to nearly twice the original diameter. After lactation, as inversion occurs, they shrink to scabrous low prominences, 2 mm. to 3 mm. in diameter, surrounded by bare skin. There are three pairs of mammae, one pair pectoral and the other two abdominal. As mentioned by Jameson (1947:146), the pectoral mammae show little evidence of use in lactating prairie voles. Probably they are not used at all except in females with more than the four young in a litter accommodated by the abdominal mammae. As in various other rodents, the suckling young may cling to the female's teats and may be dragged over the ground as she moves about. When the female forages near the nest, she may drag the young with her instead of leaving them, but she can detach them instantly if she so desires. On many occasions females found in live-traps had young that were several days old clinging to their teats. In some instances young that had their eyes open may have followed the female into the trap and attached afterward.
In the region of my study the prairie vole breeds the year round, but the rate of breeding changes continually. There is no regularity in the trend of the breeding season from year to year. It is obvious that the species is responsive to environmental changes and is so well attuned that its breeding is speedily initiated or inhibited by changes to favorable or unfavorable weather. The incidence of breeding is highest when temperature is moderate and both water and foods of preferred sorts are plentiful.
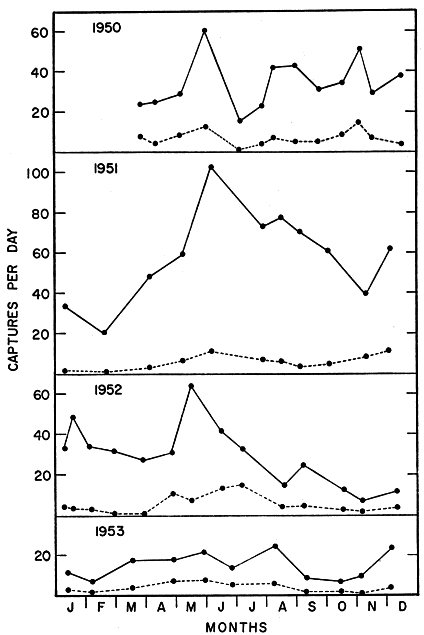
Tables 1 and 2 and Fig. 1, based on 11,109 records representing each month over a four-year period, show the changing trends from month to month. The perforate condition recorded in Table 1 may represent any of several stages in oestrus or pregnancy, but is regarded as a crude index of rate of breeding, since voles in the anoestrus stage lack the vaginal orifice. Highest percentages of perforate females occurred in the months of February, March, April, May, and June, while by far the lowest percentages were recorded in the drought summers of 1952 and 1953. Even in mid-winter a substantial proportion of the females trapped were perforate.
in the Monthly Samples From 1950 Through 1953.
| Jan. | Feb. | Mar. | Apr. | May | June | July | Aug. | Sept. | Oct. | Nov. | Dec. | |
| 1950 | .... | .... | 40.6 | 76.0 | 84.0 | 67.7 | 57.3 | 43.1 | 47.0 | 44.8 | 24.4 | 31.1 |
| 1951 | 27.3 | 47.7 | 38.5 | 41.9 | 40.0 | 41.5 | 45.5 | 52.2 | 56.5 | 48.9 | 45.0 | 45.0 |
| 1952 | 41.7 | 53.1 | 77.0 | 51.9 | 52.0 | 19.3 | 12.7 | 5.4 | 51.6 | 43.4 | 24.1 | 37.5 |
| 1953 | 33.3 | 72.9 | 50.0 | 73.0 | 58.2 | 16.6 | 15.4 | 31.3 | 56.2 | 60.0 | 61.5 | 41.6 |
in the Monthly Samples From 1950 Through 1953.
| Jan. | Feb. | Mar. | Apr. | May | June | July | Aug. | Sept. | Oct. | Nov. | Dec. | |
| 1950 | .... | .... | 5.8 | 8.0 | 21.0 | 13.3 | 57.3 | 43.8 | 40.4 | 45.2 | 7.0 | 0 |
| 1951 | 2.3 | 0 | 0 | 19.4 | 37.1 | 14.9 | 6.7 | 15.2 | 15.0 | 21.9 | 8.9 | 0 |
| 1952 | 0 | 10.4 | 22.6 | 22.6 | 29.5 | 16.5 | 7.9 | 10.8 | 20.3 | 18.9 | 3.3 | 0 |
| 1953 | 0 | 9.1 | 13.3 | 27.5 | 39.4 | 5.5 | 3.8 | 12.5 | 6.2 | 10.0 | 23.0 | 8.3 |
Usually pregnancy can be recognized only in the last week before birth of the litter, when the female's abdomen is noticeably distended by the enlarged fetuses. Palpating to detect embryos was not attempted because of the danger of injuring them or the female. Because gestation is of approximately three weeks duration, the figures in Table 2 represent roughly perhaps one-third, or a little less, of the adult females actually pregnant. At most times of year a substantial proportion of adult females (sometimes nearly all) are pregnant. Only in the winter (including March in 1951) were samples taken in which no recognizably pregnant females were found. Incidence of pregnancy was notably high in July, August, September, and October of 1950, May, 1951, May, 1952, and April and May, 1953. A high rate of breeding was not necessarily followed by an increase in the population. A relatively low rate of breeding was adequate to maintain the population level, provided that environmental factors remained favorable. Fig. 1 shows the average catch per day (with approximately 100 live-traps) over the four-year period, 1950 through 1953. The young (including all those weighing 30 grams or less, and corresponding roughly with the part of the population less than two months old) are shown separately. It is noteworthy that throughout [Pg 142] the entire period the ratio of young to adults tended to be fairly stable—usually fluctuating between ten and thirty per cent of the total catch. Ratios of young to adults were notably high in March and May, 1950; April, June and July, 1952; and April, May and June, 1953. Ratios of young were notably low in June and December, 1950; January, February, March, and June through October, 1951; January, February, and March, 1952; and November, 1953.
In Fig. 1 the catch per day of voles, varying from month to month, reflects chiefly the changing population density. However, other factors also have important effects on the catch. For example, bait acceptance is better in the winter when natural foods, especially greens, are scarce, with the result that a higher catch can be made with the same population density. Interference with the trap line by other animals also affected the catch of voles. In warm weather the traps were checked in both morning and evening, and the catch was correspondingly greater than it was in cool weather when the traps were checked only once daily. The ratios obtained of young to adult voles cannot be accepted at face value as the true ratios in the population, either. For the first several days of each trapping period, the voles caught were mostly adults previously marked and, presumably, conditioned to the grain bait. Later, young voles not previously recorded, came to the traps in increasing numbers. The young, being at first not conditioned to the bait, and also having relatively small home ranges, would generally be less well represented in the catch than would the adults.
In other species of Microtus, so far as known, a 21-day gestation period seems to be the rule (Bailey, 1924:528; Hamilton, 1941:13; Hatfield, 1935:264). M. ochrogaster seems to conform to this pattern, but the data obtained were meager, because breeding activity was usually inhibited in voles kept in confinement.
A female live-trapped on July 23, 1951, appeared to be in breeding condition. When trapped two days later, she had a copulatory plug, and 21 days after this she was found with a newborn litter in a trap. A female thought to have given birth to a litter between successive captures on July 20, and July 21, 1951 (on the basis of appearance of genitalia, and reduction in weight from 53 to 46 grams), appeared to have just completed parturition [Pg 143] when she was examined on August 10. A female that gave birth to a litter in confinement on May 18, 1954, bred and was released the same day. She was recorded as pregnant in the first week of June, but on June 7 was no longer pregnant. If this pregnancy terminated normally, a gestation of 20 days or less is indicated.
Greenwald (1956:221) suggested that in M. californicus, oestrus might occur in the period of lactation, because he found recently formed corpora lutea in lactating females. In the course of my field work on M. ochrogaster, I obtained precise or approximate dates of successive litters born at intervals of somewhat more than 21 days apart. In different females, intervals of 23, 23, 24, 26, and approximately 27 (between 26 and 28) days were recorded between successive litters. In four other females intervals between litters were known only approximately because one of two records was based on a capture in late pregnancy judged to be within two or three days of parturition. For these females, intervals of 23, 24, 24, and 26 days were recorded. From the trend of these records, it seems that females often became pregnant within a few days after birth of a litter. Pregnancy from post-partum oestrus would seem to be less frequent than pregnancies beginning a few days after birth of the previous litter, and within the period of lactation.
Jameson (1947:146) found an average of 3.4 young per litter in 58 litters of M. ochrogaster from northeastern Kansas, mostly from Douglas County. Martin (1956:386) recorded a somewhat lower mean of 3.18 ± 0.24 in 65 litters on the Reservation in 1950, 1951, and 1952. For a total of 82 litters recorded from 1950 through 1956, inclusive, I obtained an average of 3.37 ± .075 young per litter. Several litters that were recorded were excluded from this computation as in each instance there was reason to suspect that they were incomplete. These included instances of females found in traps with young several days old, females that may not have completed parturition when they were released with newborn young, and those litters that might have sustained losses through cannibalism by the mother or her trap-mates.
Mean numbers of young per litter were found to vary from year to year and from month to month, as shown by the following lists: 1950, 3.0 (13 litters); 1951, 3.5 (23 litters); 1952, 3.5 (11 litters); 1953, 3.4 (5 litters); 1954, 3.4 (15 litters); 1955, 4.1 (7 litters); 1956, 3.8 (5 litters); January 2.0 (1 litter); February 3.5 (4 litters); [Pg 144] March 4.5 (4 litters); April 3.9 (12 litters); May 3.3 (25 litters); June 3.0 (9 litters); July 2.7 (4 litters); August 2.9 (7 litters); September 2.8 (6 litters); October 3.4 (7 litters); November 5.0 (2 litters); December 4.0 (1 litter).
These differences can be logically explained on the basis of changes in the average age of the breeding females in the population. On the average, with greater length, weight and age, females produced progressively larger litters, although individuals did not necessarily conform to this general trend. For 24 females recorded in 1954-1956 and measured within a few days of birth of their litters, average length was correlated with number of young as follows: 6 young, 163.5 mm.; 5 young, 158.0 mm.; 4 young, 157.7 mm.; 3 young, 154.6 mm.; 2 young, 160.5 mm.
For 48 other females, recorded in 1950-1953, that were not measured, but that were mostly assignable to broad age groups on the basis of their individual histories in the trapping records, the following well defined trend was demonstrated.
| Age or Size Group of Female |
Number of females in sample |
Average number of young per litter |
| More than one year old | 4 | 4.25 |
| 6 to 12 months old | 16 | 3.50 |
| Large (age indeterminate) | 9 | 3.44 |
| 2 to 5 months old | 9 | 2.90 |
| Small and medium (age indeterminate) | 10 | 2.80 |
It seems that the exceptionally high average numbers of young per litter in March and April result from the breeding females in those months being nearly all fully mature survivors of the previous year. In summer, when many females that are only a few weeks old become pregnant, the average litter declines to less than three young. The small average litter of 3.0 young for 1950 probably resulted from the fact that the population on the Reservation was then expanding rapidly in the newly favorable habitat created by one year's crop of vegetation after discontinuance of grazing, and had an unusually high percentage of breeding females that were not fully adult.
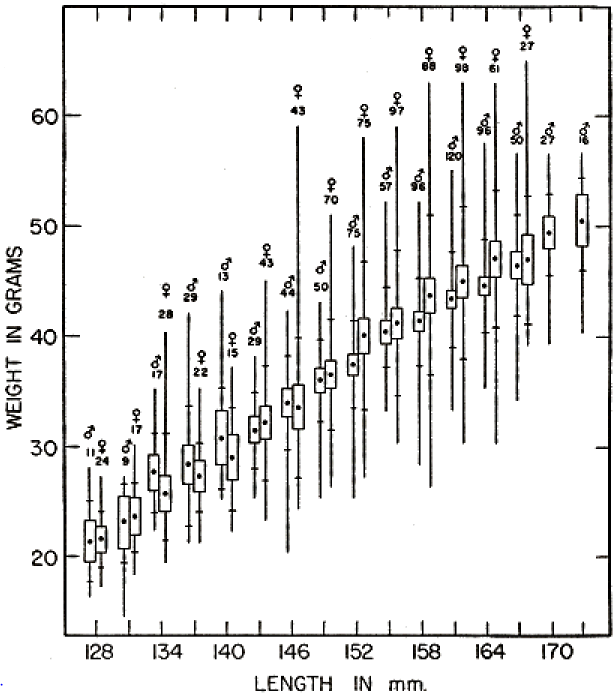
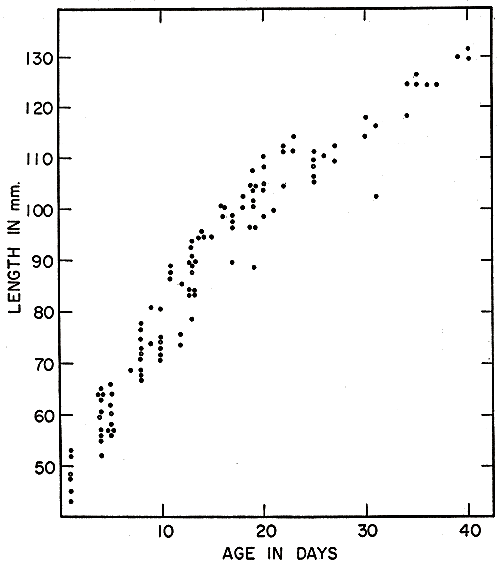
In four newborn young, total lengths, in mm., were 47, 45, 45, and 42. From the length-weight relationships shown in Fig. 2, it seems that a length of approximately 47 mm. is typical of newborn young of average weight. Martin (1956:388) found a mean weight of 2.8 ± 0.36 grams in sixteen newborn prairie voles from the Reservation. For a series of 67 other newborn voles representing 27 different litters in seven different years, I found an average of 2.9 ± .05 grams. Young ranged in weight from 3.8 to 2.0 grams. Weights of the newborn voles could not be correlated with season, size, age of females, or food conditions. However, a distinct trend toward larger size in those litters that contained fewer young was evident, as shown in Table 4.
| Known Young Per Litter |
Mean weight in grams |
Number of litters in sample |
Number of young in sample |
| 2 | 3.1 ± .09 | 7 | 13 |
| 3 | 3.0 ± .17 | 11 | 28 |
| 4 | 2.7 ± .22 | 6 | 17 |
| 5 | 2.6 ± .42 | 3 | 9 |
Voles less than 100 mm. in total length were seldom captured, because those less than this size are dependent on the female, and rarely venture far enough from the nest to be caught in a trap. A further difficulty in obtaining growth records on the smallest young is that of making accurate measurements. During their first few days they partially retain the fetal posture, usually lying on one side, with the head, body and tail flexed in an arc almost completed by the tail approximating the muzzle. Straightening the animal by stretching it and holding it with sufficient firmness to obtain a measurement might have involved injury to it. Therefore, in most instances the newborn voles examined were merely weighed or an approximate measurement was estimated without stretching the young to its full length.
Newborn voles were obtained when females that were caught in live-traps produced their litters before they were found and released. [Pg 146] In some instances, females caught while in late pregnancy were retained in the laboratory for a day or more until parturition occurred. Many of the newborn voles were marked by toe-clipping, according to the same system used for adults. Early growth was measured in some instances by keeping the female with her litter in confinement, measuring and weighing the young at intervals. In most instances, the female was released at the point of capture (presumably near her nest burrow) with the young clinging to her teats. For the young so released, the incidence of recovery was remarkably low, seeming to indicate that they were subject to decimating losses. Perhaps such losses are normal, at least on the study area where voles are live-trapped regularly. Holding of adults and partly grown young in live-traps ordinarily has no harmful effects on them, but the resultant separation of females from newly born litters may often result in death of the young either from hunger and exposure, or from attack by other voles and natural enemies.
During the first ten days the increase in length from an original 47 mm. is from three to four mm. per day. Figs. 2, 5, and 8 show length and weights of voles whose ages in days were definitely known because they were born in the laboratory, or in a live-trap after the female was caught there. Young voles marked at birth and released with the female were rarely recovered in the period of suckling, as they ordinarily remain in the nest burrow when the female ventures out to forage. Litters retained in the laboratory therefore have provided most of the records of growth in suckling young. Growth varied greatly between litters. It was not clearly correlated with size of female, size of young at birth, or number of young in litter, but probably was influenced by attentiveness of the female, her adjustment to captivity, and her productivity of milk. Within each litter there were usually persistent differences in development, but these were minor (except for those of occasional runts) compared with the differences between litters. In several litters of five young, one was usually smaller than the others at birth and therefore could not compete successfully with its litter mates, so that it never gained possession of a teat other than one of the pectoral pair, and always succumbed within a few days, after failing to gain weight as its litter mates did. The relatively few voles marked at birth and recovered after developing under natural conditions, did not deviate from the trend of those in confinement.
Females in confinement were attentive to young, and, soon after parturition, licked them clean and huddled over them protectively. Ordinarily, the newborn young soon attached to a teat, and spent a large part of its time attached during its early development. Females found in live-traps with their litters of young less than a day old, often had some or all of the young clinging to their teats. Females with newborn litters, when released from live-traps, always left without attempting to retrieve any young that were unattached. Such young usually were permanently deserted, but in some instances disappeared within an hour or less, perhaps rescued by the female returning for them.
Females with newborn young were made far more aggressive than most other voles by their tendency to protect their young from possible danger. In captivity such females usually took the offensive in attacking or rebuffing any other voles confined with them. Post-partum females obviously in oestrus were prevented from being fully receptive by their hostility toward males whose presence might endanger the young. Such a female has been seen to turn on a pursuing male and attack him viciously, several times within a few minutes, before copulation occurred. In captivity, at least, such attacks would soon discourage a male so that unless he was exceptionally active sexually, mating was prevented.
Cannibalism, involving destruction of the newborn, is probably an important factor in the population dynamics of the prairie vole. Only a small percentage of the young known to have been born on an area ever survived to be live-trapped; this small percentage was indirect evidence of decimating losses in the young. Under unfavorable conditions each of several females killed and ate her own litter, but the degree of provocation varied greatly among individuals. Females that gave birth to young in live-traps occasionally ate one or more of their newborn young, as evidenced by discarded remnants. Perhaps other instances passed unnoticed because no remnants were found. That need for food or moisture as well as psychological stress often motivated such cannibalism was suggested by the fact that surviving litter mates might be accepted and cared for by a female that had already eaten one or more of her young. Although cannibalism is most likely to occur within a few hours after birth of the young, they may be killed and eaten at any stage of development. One female that had probably [Pg 148] eaten one or more of her litter, soon after parturition, nursed the two survivors. When these were two weeks old, all were "pastured out" in a wire mesh cage in tall brome grass. When the supply of grass had become scarce (though some was still available), the female killed and partly ate both her remaining young.
One female was captured with three young attached that were several days old. The young were detached from the female's teats with great difficulty. When these young were returned to the female a few minutes later, after they had been measured, weighed and marked, she attacked them viciously, and within a few seconds had killed all of them by biting their heads. In this instance the dead young were not eaten, although they were temporarily left with the female.
Females with young have ample cause for their circumspective demeanor toward adult males, which are especially inclined to eat the newborn. A male engaged in sexual pursuit has been observed to grasp a young dangling behind the female, pull it from her teat, and pausing momentarily, nibble its head off, before continuing to follow the female. Like the genitalia of the post-partum female, the newborn young seem to have an odor that attracts and excites the male.
To a lesser degree, adult females also display marked interest in the newborn young of other individuals, which is liable to result in cannibalism. The incidence of cannibalism is affected by the condition, collectively, of the population of voles, and the availability of nutritious food and moisture. In periods of summer drought the grass becomes coarse and fibrous, and its protein content declines. Under such conditions many voles appear to be undernourished, and some are actually emaciated. Dehydration may be an important factor at times when dew is unavailable for drinking and the green vegetation remaining is exceptionally low in moisture content. Voles caught at such times and brought to the laboratory, drank avidly, and gained several grams soon after being offered water or succulence. Cannibalism by adults on newborn young in times of drought may be motivated by the acute need for moisture and nutritious food. In times of drought the birth rate is at low ebb.
Adult males have never been observed to display paternal solicitude toward young, but some individuals, kept with females and their litters, did not molest the young and were accepted by the females as members of the family group.
[Pg 149] Other things being equal, cannibalism involving the young might be expected to be greater at times of high population density. Then, young left in the nest by a female in the course of her foraging would more often encounter adults and partly grown young, both those that lived in the same burrow system and exploring intruders from other areas.
The eyes open at an age of nine or ten days. Then the young enter upon an exploratory period, when each wanders out of the nest, emerges from the burrow, and wanders through the adjacent surface runways in frequent short forays, sometimes following the female and sometimes alone. Such forays usually cover only a few inches at first, but as the young vole grows, becomes familiar with its surroundings, and takes more plant food, its sphere of activity gradually widens, and family ties are dissolved. Voles reared to an age of three weeks in the laboratory and then released, survived just as well if the female was not released with them demonstrating that they were fully capable of shifting for themselves at this age. In confinement, however, young voles of greater age continued to suckle and remained closely associated with the female. Females in confinement evinced much uneasiness because of their inability to evade the young when the latter were old enough to walk. The young then followed the female continually and suckled whenever she stopped or even while she moved about, unless she paused to remove them from her teats, but they would not remain detached for more than a few seconds. When a young followed the female away from the nest and then attached to a teat, the female after pulling the young from her teat, would usually carry it, grasped between her incisors, back to the nest and deposit it there. On one occasion a young vole caught in a live-trap was partly plucked and eventually killed by the female on the outside trying to pull it through the wire mesh.
On several occasions, young were successfully transferred from the mother to another lactating female in confinement, which accepted them as part of her own litter. Young, up to the time of weaning, appeared not to differentiate between the mother and other adult voles. They would follow any larger individual indiscriminately, and would huddle against it or nuzzle its undersurface searching for a teat.
The following notes are based upon many different litters, and give some idea of the sequence of events in their early development.
[Pg 150] Newborn: The skin is pinkish gray dorsally and pink ventrally. In profile, sparse and exceedingly fine hairs less than 1 mm. in length are discernible. The vibrissae are approximately 2 mm. long. The skin is thin and partly transparent, much wrinkled, with some deeper folds, notably one between the knee and the heel. The young lie on their sides making violent convulsive respiratory movements. When not attached to the female's teats, they may make faint squeaking sounds.
One day old: Little changed in appearance or behavior except that the dorsal surface has become darker because of growth of hair.
Two days old: Covering of fine brown hair readily discernible on dorsal surface; lower incisors protruding about .5 mm. from the gum; upper incisors have barely pierced the gum.
Four days old: Pale brown hair averaging about 1 mm. in length over the dorsal surface gives the young a sleek, seallike appearance. The young have gained greatly in muscular co-ordination. Part of the time they may still lie on their sides, but they are able also to gain an upright sprawling posture. In crawling, they are unsteady and often topple over on their sides after taking a few halting steps. They make frequent jerky lateral flexions of the body, probably to search for a teat. Their eyes and ears still are sealed shut.
Five days old: Young have changed but little in appearance since the preceding day, but they have become notably more active, with movements better co-ordinated. When placed on a level surface they can crawl briskly.
Eight days old: Young are able to stand erect, with bodies held clear of the ground, and they can even run, but the gait is slow and clumsy, and the forequarters and hind quarters are poorly co-ordinated, so that the voles tend to fall on their sides. The fur averages approximately 3 mm. in length.
Nine days old: At this stage all young have their eyes open or beginning to open.
Ten days old: All young of this age have their eyes open, but not to their fullest extent, and the eyes are still slitlike in appearance. The young have become rather gopherlike in appearance and gait. They walk briskly but unsteadily, with bodies held high off the ground. When handled, they struggle vigorously, and try to bite. These young are similar in size and appearance to the smallest voles caught in live-traps apart from their mothers.
Thirteen days old: Hair on back has grown to an average length of 8 mm. (shorter on ventral surface, head, and limbs).
[Pg 151] Seventeen days old: The young have become alert, and almost as quick in their movements as adults. They have molariform teeth, and are taking plant food. When a family group was examined, the young instantly detached from the female's teats and scattered. The hair on the back averages 10 mm. long and the vibrissae average 20 mm. long.
There is intense competition among the young of a litter, especially if the litter has more than the average number of young. In litters with more than four young, there is competition for the inguinal teats, since, in most females at least, the pectoral teats seem to have an inadequate milk supply. As a result, it is doubtful whether more than four young to a litter are ever able to survive. From the time their eyes open, the young compete actively. When litters in confinement were fed with fresh greens, there was nearly always quarrelsome squeaking and scuffling, as the young competed for food. At such times, they have been seen to chase and attack each other.
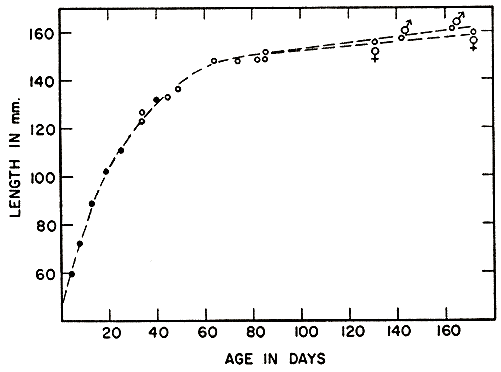
No individual vole was recaptured with sufficient regularity, from birth to maturity, to provide a complete growth curve. The curve in Fig. 7 is a composite based on all available records of voles that were recorded as making growth in length and were recaptured before they were fully grown, so that growth rates could be computed. The figure shows that growth is extremely rapid for the first three weeks, and thereafter slows gradually but steadily, until in individuals of adult size, the increment per day is much less than that in the small young.
Since rate of growth changes rapidly, with a slowing trend, only those young voles that were recaptured within a few weeks showed the approximate growth rate for any specific portion of the ontogenetic curve. Table 5 summarizes the records of 98 such young sorted into size groups representative of several stages in development. The slowing trend of growth in voles that are nearing subadult size is well shown by these records. Throughout the greater part of the growth curve no difference could be found in rate between the sexes. It is only after sexual maturity has been attained and growth has become relatively slow that males become noticeably larger than females. This tendency for continued growth in the adult males results in a much more marked disparity in size between the sexes in the oldest voles, as evident in Fig. 2.

| Average lengths in mm. at beginning and end of growth period |
Average length, in days, of growth periods |
Average increment per day in mm. |
Total, and number of each sex in sample |
| 97.0 to 126.6 | in 16.8 | 1.76 | 5 (1 ♂, 4 ♀ ♀) |
| 103.3 to 127.3 | in 14.9 | 1.61 | 9 (3 ♂ ♂, 6 ♀ ♀) |
| 107.5 to 123.4 | in 11.0 | 1.44 | 8 (5 ♂ ♂, 3 ♀ ♀) |
| 114.0 to 132.3 | in 17.5 | 1.05 | 6 (5 ♂ ♂, 1 ♀) |
| 118.5 to 136.0 | in 19.7 | .88 | 6 (3 ♂ ♂, 3 ♀ ♀) |
| 122.1 to 135.8 | in 16.2 | .85 | 15 (5 ♂ ♂, 10 ♀ ♀) |
| 129.3 to 145.5 | in 22.8 | .71 | 4 (all ♂ ♂) |
| 130.6 to 146.1 | in 19.8 | .78 | 12 (all ♀ ♀) |
| 139.8 to 147.5 | in 29.5 | .26 | 10 (all ♂ ♂) |
| 141.2 to 148.8 | in 26.2 | .29 | 23 (all ♀ ♀) |
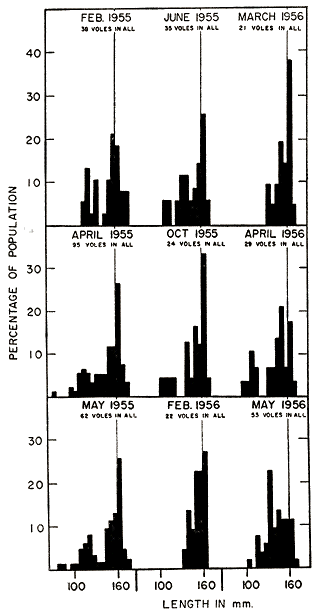
Martin (1956:389) stated that growth in young prairie voles was, in general, most rapid in the period April-May-June and least rapid in mid-winter. However, his data were based entirely on weights. The high incidence of pregnancy in the larger young females in spring and early summer may have caused the trend. Measurements taken by me of lengths do not bear out the idea of more rapid growth in the spring and summer, but, indeed, show the opposite. In most instances, voles of comparable sizes made significantly more rapid growth in the colder half of the year (mid-October to mid-March) than in the warmer half. Dividing the young voles in eight size groups and separating each group into comparable summer and winter samples, I found more rapid average growth in the summer sample in only two instances. These [Pg 155] deviations from the general trend probably resulted from inadequately small sizes of some samples. On the average, the growth rate in summer was 92 per cent of that in winter.

Greenwald (1956: 220) found that in females of Microtus californicus some individuals are extremely precocious sexually, and might, at an age of as little as two weeks, produce corpora lutea and have sperm in the uterus. Greenwald mentioned one perforate female which weighed only 10 grams, but most reached a weight of at least 30 grams before their first pregnancies. The sterile cycles passed through earlier seemed to represent a "tuning-up" stage before establishment of the pituitary-gonad relationship.
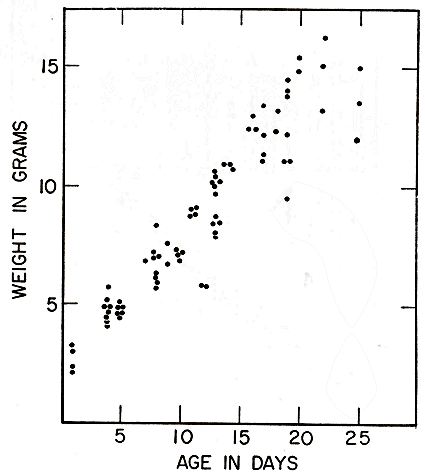
Although females of M. ochrogaster are much less precocious in their manifestations of puberty, they may become perforate [Pg 157] well before impregnation can occur, and seem to pass through sterile cycles before becoming pregnant. The 18 smallest females recognized as being pregnant were of the following over-all lengths, in mm.: 149, 149, 149, 148, 148, 148, 147, 146, 145, 145, 144, 144, 143, 143, 143, 142, 135, and 134. As pregnancy is ordinarily recognized only in the last four days the females must have been impregnated from 20 to 17 days earlier—when they were in most instances 7 to 11 weeks old and 135 to 145 mm. in length. The two smallest individuals, recorded as pregnant at 135 and 134 mm., must, if they were of typical size for their age, have become pregnant at an age of approximately one month, when they were only 119 and 122 mm. in length. The smallest lactating females (some of them pregnant also) were recorded at lengths of 149, 148, 148, 147, 147, 146, 144, 144, 143, 143, and 142 mm. Occasionally females of less than 120 mm. were found to be perforate, and seemingly had begun oestral cycles. Records of a female of definitely known age, typical of many of the same size in her development, are cited below:
March 19, 1956 Born in captivity.
April 7, 1956 (19 days old) Released on study area at site of mother's capture; length 102 mm., weight 11.1 gms.
[Pg 158] April 15, 1956 (27 days old) Recaptured; perforate with a copulatory plug; length 113 mm., weight 13.4 gms.
April 27, 1956 (39 days old) Recaptured; imperforate; length 131 mm., weight 24.3 gms.
May 12, 1956 (54 days old) Recaptured; perforate and in late pregnancy; length 146 mm.
May 25, 1956 (67 days old) Recaptured; imperforate, in an advanced state of lactation; length 150 mm., weight 33 gms.
When captured on May 12, at an age of 54 days, this female appeared to be within two or three days of parturition, and hence [Pg 159] must have become pregnant at an age of approximately 35 or 36 days. Pregnancy in the more precocious females probably occurs at a length of approximately 130 mm. and an age of a little less than 40 days. Such females are still growing so rapidly that by the time their litters are born, they have grown to more than 140 mm.
Table 6 is a summarization of 73 records of individuals that made substantial growth as adults, after they were marked and measured. These records show the slowing trend of growth with advanced age. Also, they show the wide range of individual variation in growth rate, and difference between the sexes. With advanced age, growth in females lags behind that in males to an increasing extent. Exceptionally large individuals, of either sex, are many months old, but some individuals live to be a year old or more without growing much beyond average adult size. The average growth rate of more than 1 mm. per day in young has slowed to less than .1 mm. per day, on the average, in adults exceeding 160 mm., and has slowed to less than .05 mm. per day, on the average, in those exceeding 165 mm.
| Size Group Length in mm. | Estimated age, in days | Number in sample | ||
| Average | Maximum | Minimum | ||
| 171 to 175 | ♂ 435 | ..... | ..... | 1 |
| ♀ 324 | 338 | 310 | 2 | |
| All 361 | 435 | 310 | 3 | |
| 166 to 170 | ♂ 304 | 523 | 179 | 9 |
| ♀ 398 | 597 | 158 | 6 | |
| All 346 | 597 | 158 | 15 | |
| 161 to 165 | ♂ 227 | 465 | 104 | 15 |
| ♀ 257 | 394 | 134 | 18 | |
| All 243 | 465 | 104 | 33 | |
| 156 to 160 | ♂ 188 | 349 | 107 | 12 |
| ♀ 187 | 284 | 93 | 11 | |
| All 188 | 349 | 93 | 23 | |
The prairie vole is non-territorial and somewhat social. Several or many individuals of both sexes and various sizes may use the same system of surface runways and burrows and even the same nest. In general, members of such a group are mutually tolerant. A strange vole may provoke some hostility at first, but may soon be accepted as a member of a new group. Consequently, there are frequent shifts from one home base to another. Sexual relations are probably more or less promiscuous, although a male and female may rest and travel together in a semi-permanent association. In confinement only those males having markedly enlarged scrotal testes showed interest in females that were in oestrus. Post-partum females especially were eagerly pursued by such males. Anoestrus females are imperforate, and a vaginal orifice is present only during an active oestral cycle or in pregnancy. The perforate condition therefore, is a crude index of breeding activity in the population. In adult females the ratio of those that were perforate usually fluctuated between one-fourth and three-fourths of the total. Only in severe summer drought did the numbers decline below 24 per cent. Normally, breeding continues the year around, but it is temporarily inhibited in unusually cold weather or drought. The highest incidence of pregnancy normally is in late spring and early summer. The ratio of juveniles in the population from month to month and year to year is far more stable than the actual population density.
Gestation is 21 days or a little less. The mean litter is 3.37 ± .075 young. Three is the most frequent number per litter, with four, two, and five in that order of frequency. Larger and older females have more young per litter, on the average. Average size is greater in those litters having fewer young. At birth, young are between 40 and 50 mm. in length (typically, 47 mm.), and weigh 2.9 ± .05 grams.
At an age of nine days the young have their eyes open, and they may be weaned at an age of approximately three weeks. Young suckle chiefly from the four abdominal teats. The pectoral mammae seem to be inadequately developed, with the result that in exceptionally large litters of five, six or seven young, usually no more than four survive. Until weaning the young spend much of their time attached to the female's teats. She may even drag them behind as she forages. Females that have suckling young [Pg 161] become much less tolerant of other voles. Attacks on young, and cannibalism, are common. Adult males, especially, are liable to eat the newborn young. The acquisition of cannibalistic habits by individuals, and seasonal lack of adequately nutritious plant foods may result in the killing off of young in such numbers that the population level is held down.
In young females sterile oestral cycles often begin at about the time of weaning. Earliest pregnancies occur when females are approximately one month old, but most are several weeks older before they become pregnant. Rate of growth declines steadily from a length increment of approximately 2 mm. per day in voles less than two weeks old to an increment of approximately one-fourth mm. per day in subadults. Growth rate is highly variable among individuals at all stages, and especially in those that have attained adult size. Even adults tend to gain in length, slowly, as well as in weight, and the largest individuals are all many months old.
26-7561
MUSEUM OF NATURAL HISTORY
Institutional libraries interested in publications exchange may obtain this series by addressing the Exchange Librarian, University of Kansas Library, Lawrence, Kansas. Copies for individuals, persons working in a particular field of study, may be obtained by addressing instead the Museum of Natural History, University of Kansas, Lawrence, Kansas. There is no provision for sale of this series by the University Library, which meets institutional requests, or by the Museum of Natural History, which meets the requests of individuals. Nevertheless, when individuals request copies from the Museum, 25 cents should be included, for each separate number that is 100 pages or more in length, for the purpose of defraying the costs of wrapping and mailing.
* An asterisk designates those numbers of which the Museum's supply (not the Library's supply) is exhausted. Numbers published to date, in this series, are as follows:
| Vol. 1. | Nos. 1-26 and index. Pp. 1-638, 1946-1950. | |
| *Vol. 2. | (Complete) Mammals of Washington. By Walter W. Dalquest. Pp. 1-444, 140 figures in text. April 9, 1948. | |
| Vol. 3. | *1. | The avifauna of Micronesia, its origin, evolution, and distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June 12, 1951. |
| *2. | A quantitative study of the nocturnal migration of birds. By George H. Lowery, Jr. Pp. 361-472, 47 figures in text. June 29, 1951. | |
| 3. | Phylogeny of the waxwings and allied birds. By M. Dale Arvey. Pp. 473-530, 49 figures in text, 13 tables. October 10, 1951. | |
| 4. | Birds from the state of Veracruz, Mexico. By George H. Lowery, Jr., and Walter W. Dalquest. Pp. 531-649, 7 figures in text, 2 tables. October 10, 1951. | |
| Index. Pp. 651-681. | ||
| *Vol. 4. | (Complete) American weasels. By E. Raymond Hall. Pp. 1-466, 41 plates, 31 figures in text. December 27, 1951. | |
| Vol. 5. | 1. | Preliminary survey of a Paleocene faunule from the Angels Peak area, New Mexico. By Robert W. Wilson. Pp. 1-11, 1 figure in text. February 24, 1951. |
| 2. | Two new moles (Genus Scalopus) from Mexico and Texas. By Rollin H. Baker. Pp. 17-24. February 28, 1951. | |
| 3. | Two new pocket gophers from Wyoming and Colorado. By E. Raymond Hall and H. Gordon Montague. Pp. 25-32. February 28, 1951. | |
| 4. | Mammals obtained by Dr. Curt von Wedel from the barrier beach of Tamaulipas, Mexico. By E. Raymond Hall. Pp. 33-47, 1 figure in text. October 1, 1951. | |
| 5. | Comments on the taxonomy and geographic distribution of some North American rabbits. By E. Raymond Hall and Keith R. Kelson. Pp. 49-58. October 1, 1951. | |
| 6. | Two new subspecies of Thomomys bottae from New Mexico and Colorado. By Keith R. Kelson. Pp. 59-71, 1 figure in text. October 1, 1951. | |
| 7. | A new subspecies of Microtus montanus from Montana and comments on Microtus canicaudus Miller. By E. Raymond Hall and Keith R. Kelson. Pp. 73-79. October 1, 1951. | |
| 8. | A new pocket gopher (Genus Thomomys) from eastern Colorado. By E. Raymond Hall. Pp. 81-85. October 1, 1951. | |
| 9. | Mammals taken along the Alaskan Highway. By Rollin H. Baker. Pp. 87-117, 1 figure in text. November 28, 1951. | |
| *10. | A synopsis of the North American Lagomorpha. By E. Raymond Hall. Pp. 119-202, 68 figures in text. December 15, 1951. | |
| 11. | A new pocket mouse (Genus Perognathus) from Kansas. By E. Lendell Cockrum. Pp. 203-206. December 15, 1951. | |
| 12. | Mammals from Tamaulipas, Mexico. By Rollin H. Baker. Pp. 207-218. December 15, 1951. | |
| 13. | A new pocket gopher (Genus Thomomys) from Wyoming and Colorado. By E. Raymond Hall. Pp. 219-222. December 15, 1951. | |
| 14. | A new name for the Mexican red bat. By E. Raymond Hall. Pp. 223-226. December 15, 1951. | |
| 15. | Taxonomic notes on Mexican bats of the Genus Rhogeëssa. By E. Raymond Hall. Pp. 227-232. April 10, 1952. | |
| 16. | Comments on the taxonomy and geographic distribution of some North American woodrats (Genus Neotoma). By Keith R. Kelson. Pp. 233-242. April 10, 1952. | |
| 17. | The subspecies of the Mexican red-bellied squirrel, Sciurus aureogaster. By Keith R. Kelson. Pp. 243-250, 1 figure in text. April 10, 1952. | |
| 18. | Geographic range of Peromyscus melanophrys, with description of new subspecies. By Rollin H. Baker. Pp. 251-258, 1 figure in text. May 10, 1952. | |
| 19. | A new chipmunk (Genus Eutamias) from the Black Hills. By John A. White. Pp. 259-262. April 10, 1952. | |
| 20. | A new piņon mouse (Peromyscus truei) from Durango, Mexico. By Robert B. Finley, Jr. Pp. 263-267. May 23, 1952. | |
| 21. | An annotated checklist of Nebraskan bats. By Olin L. Webb and J. Knox Jones, Jr. Pp. 269-279. May 31, 1952. | |
| 22. | Geographic variation in red-backed mice (Genus Clethrionomys) of the southern Rocky Mountain region. By E. Lendell Cockrum and Kenneth L. Fitch. Pp. 281-292, 1 figure in text. November 15, 1952. | |
| 23. | Comments on the taxonomy and geographic distribution of North American microtines. By E. Raymond Hall and E. Lendell Cockrum. Pp. 293-312. November 17, 1952. | |
| 24. | The subspecific status of two Central American sloths. By E. Raymond Hall and Keith R. Kelson. Pp. 313-337. November 21, 1952. | |
| 25. | Comments on the taxonomy and geographic distribution of some North American marsupials, insectivores, and carnivores. By E. Raymond Hall and Keith R. Kelson. Pp. 319-341. December 5, 1952. | |
| 26. | Comments on the taxonomy and geographic distribution of some North American rodents. By E. Raymond Hall and Keith R. Kelson. Pp. 343-371. December 15, 1952. | |
| 27. | A synopsis of the North American microtine rodents. By E. Raymond Hall and E. Lendell Cockrum. Pp. 373-498, 149 figures in text. January 15, 1953. | |
| 28. | The pocket gophers (Genus Thomomys) of Coahuila, Mexico. By Rollin H. Baker. Pp. 499-514, 1 figure in text. June 1, 1953. | |
| 29. | Geographic distribution of the pocket mouse, Perognathus fasciatus. By J. Knox Jones, Jr. Pp. 515-526, 7 figures in text. August 1, 1953. | |
| 30. | A new subspecies of wood rat (Neotoma mexicana) from Colorado. By Robert B. Finley, Jr. Pp. 527-534, 2 figures in text. August 15, 1953. | |
| 31. | Four new pocket gophers of the genus Cratogeomys from Jalisco, Mexico. By Robert J. Russell. Pp. 535-542. October 15, 1953. | |
| 32. | Genera and subgenera of chipmunks. By John A. White. Pp. 543-561, 12 figures in text. December 1, 1953. | |
| 33. | Taxonomy of the chipmunks, Eutamias quadrivittatus and Eutamias umbrinus. By John A. White. Pp. 563-582, 6 figures in text. December 1, 1953. | |
| 34. | Geographic distribution and taxonomy of the chipmunks of Wyoming. By John A. White. Pp. 584-610, 3 figures in text. December 1, 1953. | |
| 35. | The baculum of the chipmunks of western North America. By John A. White. Pp. 611-631, 19 figures in text. December 1, 1953. | |
| 36. | Pleistocene Soricidae from San Josecito Cave, Nuevo Leon, Mexico. By James S. Findley. Pp. 633-639. December 1, 1953. | |
| 37. | Seventeen species of bats recorded from Barro Colorado Island, Panama Canal Zone. By E. Raymond Hall and William B. Jackson. Pp. 641-646. December 1, 1953. | |
| Index. Pp. 647-676. | ||
| *Vol. 6. | (Complete) Mammals of Utah, taxonomy and distribution. By Stephen D. Durrant. Pp. 1-549, 91 figures in text, 30 tables. August 10, 1952. | |
| Vol. 7. | *1. | Mammals of Kansas. By E. Lendell Cockrum. Pp. 1-303, 73 figures in text, 37 tables. August 25, 1952. |
| 2. | Ecology of the opossum on a natural area in northeastern Kansas. By Henry S. Fitch and Lewis L. Sandidge. Pp. 305-338, 5 figures in text. August 24, 1953. | |
| 3. | The silky pocket mice (Perognathus flavus) of Mexico. By Rollin H. Baker. Pp. 339-347, 1 figure in text. February 15, 1954. | |
| 4. | North American jumping mice (Genus Zapus). By Philip H. Krutzsch. Pp. 349-472, 47 figures in text, 4 tables. April 21, 1954. | |
| 5. | Mammals from Southeastern Alaska. By Rollin H. Baker and James S. Findley. Pp. 473-477. April 21, 1954. | |
| 6. | Distribution of Some Nebraskan Mammals. By J. Knox Jones, Jr. Pp. 479-487. April 21, 1954. | |
| 7. | Subspeciation in the montane meadow mouse, Microtus montanus, in Wyoming and Colorado. By Sydney Anderson. Pp. 489-506, 2 figures in text. July 23, 1954. | |
| 8. | A new subspecies of bat (Myotis velifer) from southeastern California and Arizona. By Terry A. Vaughn. Pp. 507-512. July 23, 1954. | |
| 9. | Mammals of the San Gabriel mountains of California. By Terry A. Vaughn. Pp. 513-582, 1 figure in text, 12 tables. November 15, 1954. | |
| 10. | A new bat (Genus Pipistrellus) from northeastern Mexico. By Rollin H. Baker. Pp. 583-586. November 15, 1954. | |
| 11. | A new subspecies of pocket mouse from Kansas. By E. Raymond Hall. Pp. 587-590. November 15, 1954. | |
| [Pg iii] | 12. | Geographic variation in the pocket gopher, Cratogeomys castanops, in Coahuila, Mexico. By Robert J. Russell and Rollin H. Baker. Pp. 591-608. March 15, 1955. |
| 13. | A new cottontail (Sylvilagus floridanus) from northeastern Mexico. By Rollin H. Baker. Pp. 609-612. April 8, 1955. | |
| 14. | Taxonomy and distribution of some American shrews. By James S. Findley. Pp. 613-618. June 10, 1955. | |
| 15. | The pigmy woodrat, Neotoma goldmani, its distribution and systematic position. By Dennis G. Rainey and Rollin H. Baker. Pp. 619-624, 2 figs. in text. June 10, 1955. | |
| Index. Pp. 625-651. | ||
| Vol. 8. | 1. | Life history and ecology of the five-lined skink, Eumeces fasciatus. By Henry S. Fitch. Pp. 1-156, 26 figs. in text. September 1, 1954. |
| 2. | Myology and serology of the Avian Family Fringillidae, a taxonomic study. By William B. Stallcup. Pp. 157-211, 23 figures in text, 4 tables. November 15, 1954. | |
| 3. | An ecological study of the collared lizard (Crotaphytus collaris). By Henry S. Fitch. Pp. 213-274, 10 figures in text. February 10, 1956. | |
| 4. | A field study of the Kansas ant-eating frog, Gastrophryne olivacea. By Henry S. Fitch. Pp. 275-306, 9 figures in text. February 10, 1956. | |
| 5. | Check-list of the birds of Kansas. By Harrison B. Tordoff. Pp. 307-359, 1 figure in text. March 10, 1956. | |
| 6. | A population study of the prairie vole (Microtus ochrogaster) in northeastern Kansas. By Edwin P. Martin. Pp. 361-416, 19 figures in text. April 2, 1956. | |
| 7. | Temperature responses in free-living amphibians and reptiles of northeastern Kansas. By Henry S. Fitch. Pp. 417-476, 10 figures in text, 6 tables. June 1, 1956. | |
| 8. | Food of the crow, Corvus brachyrhynchos Brehm, in south-central Kansas. By Dwight Platt. Pp. 477-498, 4 tables. June 8, 1956. | |
| 9. | Ecological observations on the woodrat, Neotoma floridana. By Henry S. Fitch and Dennis G. Rainey. Pp. 499-533, 3 figures in text. June 12, 1956. | |
| 10. | Eastern woodrat, Neotoma floridana: Life history and ecology. By Dennis G. Rainey. Pp. 535-646, 12 plates, 13 figures in text August 15, 1956. | |
| Index. Pp. 647-675. | ||
| Vol. 9. | 1. | Speciation of the wandering shrew. By James S. Findley. Pp. 1-68, 18 figures in text. December 10, 1955. |
| 2. | Additional records and extension of ranges of mammals from Utah. By Stephen D. Durrant, M. Raymond Lee, and Richard M. Hansen. Pp. 69-80. December 10, 1955. | |
| 3. | A new long-eared myotis (Myotis evotis) from northeastern Mexico. By Rollin H. Baker and Howard J. Stains. Pp. 81-84. December 10, 1955. | |
| 4. | Subspeciation in the meadow mouse, Microtus pennsylvanicus, in Wyoming. By Sydney Anderson. Pp. 85-104, 2 figures in text. May 10, 1956. | |
| 5. | The condylarth genus Ellipsodon. By Robert W. Wilson. Pp. 105-116, 6 figures in text. May 19, 1956. | |
| 6. | Additional remains of the multituberculate genus Eucosmodon. By Robert W. Wilson. Pp. 117-123, 10 figures in text. May 19, 1956. | |
| 7. | Mammals of Coahulia, Mexico. By Rollin H. Baker. Pp. 125-335, 75 figures in text. June 15, 1956. | |
| 8. | Comments on the taxonomic status of Apodemus peninsulae, with description of a new subspecies from North China. By J. Knox Jones, Jr. Pp. 337-346, 1 figure in text, 1 table. August 15, 1956. | |
| 9. | Extensions of known ranges of Mexican bats. By Sydney Anderson. Pp. 347-351. August 15, 1956. | |
| 10. | A new bat (Genus Leptonycteris) from Coahulia. By Howard J. Stains. Pp. 353-356. January 21, 1957. | |
| 11. | A new species of pocket gopher (Genus Pappogeomys) from Jalisco, Mexico. By Robert J. Russell. Pp. 357-361. January 21, 1957. | |
| More numbers will appear in volume 9. | ||
| Vol. 10. | 1. | Studies of birds killed in nocturnal migration. By Harrison B. Tordoff and Robert M. Mengel. Pp. 1-44, 6 figures in text, 2 tables. September 12, 1956. |
| 2. | Comparative breeding behavior of Ammospiza caudacuta and A. maritima. By Glen E. Woolfenden. Pp. 45-75, 6 plates, 1 figure. December 20, 1956. | |
| 3. | The forest habitat of the University of Kansas Natural History Reservation. By Henry S. Fitch and Ronald R. McGregor. Pp. 77-127, 2 plates, 7 figures in text, 4 tables. December 31, 1956. | |
| 4. | Aspects of reproduction and development in the prairie vole (Microtus ochrogaster). By Henry S. Fitch. Pp. 129-161, 8 figures in text, 6 tables. December 19, 1957. | |
| More numbers will appear in volume 10. | ||
With the exception of the typographical corrections listed below and minor corrections not noted here, the text presented here is the same as in the original printed version. The list of UKMNH publications was compiled at the end of the article's text.
| Page(s) | Correction | |
| 129, 130, ii | This publication: 4 tables ⇒ 6 tables | |
| 138 | cyle ⇒ cycle |